FDA Panel to Meet Dec. 10 to Discuss Pfizer/BioNTech Covid-19 Vaccine
21 November 2020 - 10:43AM
Dow Jones News
By Josh Beckerman
The U.S. Food and Drug Administration said the potential
Covid-19 vaccine from Pfizer Inc. and BioNTech SE will be discussed
at a Dec. 10 meeting of the Vaccines and Related Biological
Products Advisory Committee.
The FDA intends to make background materials available to the
public no later than two business days before the meeting. The
agency said it "recognizes that transparency and dialogue are
critical for the public to have confidence in Covid-19
vaccines."
On Friday, the companies filed an application for an emergency
use authorization.
The FDA said it "has been preparing for the review of EUAs for
Covid-19 vaccines for several months and stands ready to do so as
soon as an EUA request is submitted. While we cannot predict how
long the FDA's review will take, the FDA will review the request as
expeditiously as possible, while still doing so in a thorough and
science-based manner."
The agency plans to livestream the meeting on its YouTube,
Facebook and Twitter channels.
Write to Josh Beckerman at josh.beckerman@wsj.com
(END) Dow Jones Newswires
November 20, 2020 18:28 ET (23:28 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
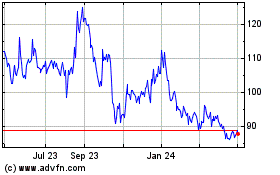
BioNTech (NASDAQ:BNTX)
Historical Stock Chart
From Mar 2024 to Apr 2024
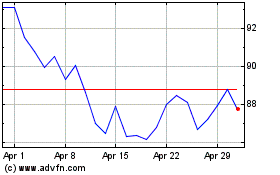
BioNTech (NASDAQ:BNTX)
Historical Stock Chart
From Apr 2023 to Apr 2024