AnaptysBio Says JEMPERLI Drug Gets FDA Approval
23 April 2021 - 7:19AM
Dow Jones News
By Adriano Marchese
AnaptysBio Inc. said Thursday that the U.S. Food and Drug
Administration approved GlaxoSmithKline PLC's biologics license
application for JEMPERLI, a treatment for endometrial cancer.
The clinical-stage biotechnology company said JEMPERLI, also
known as dostarlimab-gxly, was generated by AnaptysBio and
developed by TESARO Inc.--now a part of GSK--under a collaboration
agreement.
AnaptysBio received a $20 million milestone payment as part of
the approval.
A Biologics License Application for JEMPERLI--a treatment for
patients with mismatch repair deficient recurrent or advanced
endometrial cancer--is needed to obtain permission for distribution
across U.S. states.
"This event provides important validation for our proprietary
SHM antibody discovery platform and provides significant potential
future milestone and royalty revenue to support AnaptysBio's
growth," President and Chief Executive Hamza Suria said.
Write to Adriano Marchese at adriano.marchese@wsj.com
(END) Dow Jones Newswires
April 22, 2021 17:04 ET (21:04 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
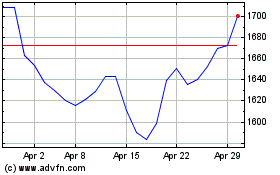
Gsk (LSE:GSK)
Historical Stock Chart
From Mar 2024 to Apr 2024
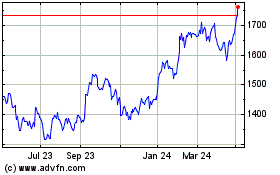
Gsk (LSE:GSK)
Historical Stock Chart
From Apr 2023 to Apr 2024