AS
FILED WITH THE SECURITIES AND EXCHANGE COMMISSION ON NOVEMBER 16, 2021
REGISTRATION
NO. 333-260588
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
S-3/A
(Amendment No.1)
REGISTRATION
STATEMENT UNDER THE SECURITIES ACT OF 1933
ABVC
BIOPHARMA, INC.
(Exact
name of registrant as specified in its charter)
Nevada
(State
or other jurisdiction of
incorporation
or organization)
26-0014658
I.R.S.
Employer Identification Number
44370
Old Warm Springs Blvd.
Fremont,
CA 94538
Tel:
(510) 668-0881
(Address,
including zip code, and telephone number, including area code of registrant’s principal executive offices)
Howard
Doong
Chief
Executive Officer
ABVC
BioPharma, Inc.
44370
Old Warm Springs Blvd.
Fremont,
CA 94538
Tel:
(510) 668-0881
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
Louis
Taubman, Esq.
Hunter
Taubman Fischer & Li LLC
800
Third Avenue, Suite 2800
New
York, NY 10022
(212)
202-6380
Approximate
date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If
the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check
the following box: ☐
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plants, check the following
box: ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.
☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective
upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ☐
If
this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional
securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”,
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer
|
☐
|
Accelerated filer
|
☐
|
|
Non-accelerated filer
|
☒
|
Smaller reporting company
|
☒
|
|
|
Emerging growth company
|
☐
|
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of Securities Act. ☐
CALCULATION
OF REGISTRATION FEE
|
Title of each class of Securities to be registered
|
|
|
Amount to be
registered(1)
|
|
|
|
Proposed maximum offering price per unit
|
|
|
|
Proposed maximum aggregate offering price(2)
|
|
|
|
Amount of registration fee(3)
|
|
|
Common stock, par value $0.001 per share
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Preferred stock, par value $0.001 per share
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Warrants(4)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Subscription Rights
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Debt Securities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Units(5)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
$
|
50,000,000
|
|
|
$
|
4,635.00
|
|
|
(1)
|
There
are being registered hereunder such indeterminate number of securities of each identified class of securities up to a proposed aggregate
offering price not to exceed $50,000,000. The securities registered also include such indeterminate prices and numbers of securities
as may be issued upon conversion of or exchange for or exercise of any securities registered hereunder, including under any applicable
anti-dilution provisions.
|
|
|
|
|
(2)
|
In
no event will the aggregate offering price of all securities issued from time to time pursuant to this registration statement exceed
$50,000,000.
|
|
|
|
|
(3)
|
Calculated
pursuant to Rule 457(o) under the Securities Act. The total amount is being paid herewith.
|
|
|
|
|
(4)
|
Includes
warrants to purchase common stock and warrants to purchase preferred stock.
|
|
|
|
|
(5)
|
Any
of the securities registered hereunder may be sold separately, or as units with other securities registered hereby. We will determine
the proposed maximum offering price per unit when we issue the above listed securities. The proposed maximum per unit and aggregate
offering prices per class of securities will be determined from time to time by the registrant in connection with the issuance by
the registrant of the securities registered under this registration statement and is not specified as to each class of security pursuant
to General Instruction II.D of Form S-3 under the Securities Act.
|
The
registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the
registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date
as the Commission, acting pursuant to said Section 8(a), may determine.
The
information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement
relating to these securities that has been filed with the Securities and Exchange Commission is effective. This prospectus is not an
offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not
permitted.
(Subject
to Completion, Dated November 16, 2021)
PROSPECTUS
$50,000,000
ABVC
BioPharma, Inc.
Common
Stock
Preferred
Stock
Warrants
Subscription
Rights
Debt
Securities
Units
We may from time to time, in one
or more offerings at prices and on terms that we will determine at the time of each offering, sell common stock, preferred stock, warrants,
or a combination of these securities, or units, for an aggregate offering price of up to $50,000,000. This prospectus describes the general
manner in which our securities may be offered using this prospectus. Each time we offer and sell securities, we will provide you with
a prospectus supplement that will contain specific information about the terms of that offering. Any prospectus supplement may also add,
update, or change information contained in this prospectus. You should carefully read this prospectus and the applicable prospectus supplement
as well as the documents incorporated or deemed to be incorporated by reference in this prospectus before you purchase any of the securities
offered hereby.
This
prospectus may not be used to offer and sell securities unless accompanied by a prospectus supplement.
Our common stock, par value
$.001 per share, is currently listed on the Nasdaq Capital Market under the symbol “ABVC”. As of the date of this prospectus,
none of the other securities that we may offer by this prospectus is listed on any national securities exchange or automated quotation
system.
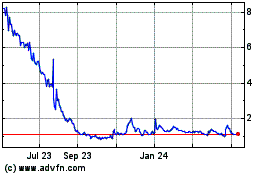
As of November 16, 2021, the aggregate
market value of the voting and non-voting common equity held by non-affiliates was $39,921,901.08, based on the closing price of $3.32
on November 12, 2021. We have not offered any securities pursuant to General Instruction I.B.6 of Form S-3 during the 12 calendar months
prior to and including the date of this prospectus.
Pursuant to General Instruction
I.B.6 of Form S-3, in no event will we sell our common stock in a public primary offering with a value exceeding more than one-third
of our public float in any 12-month period so long as our public float remains below $75,000. We have not offered any securities pursuant
to General Instruction I.B.6 of Form S-3 during the 12 calendar months prior to and including the date of this prospectus.
The
securities offered by this prospectus involve a high degree of risk. See “Risk Factors” beginning on page 3, in addition
to Risk Factors contained in the applicable prospectus supplement.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
We
may offer the securities directly or through agents or to or through underwriters or dealers. If any agents or underwriters are involved
in the sale of the securities their names, and any applicable purchase price, fee, commission or discount arrangement between or among
them, will be set forth, or will be calculable from the information set forth, in an accompanying prospectus supplement. We can sell
the securities through agents, underwriters or dealers only with delivery of a prospectus supplement describing the method and terms
of the offering of such securities. See “Plan of Distribution.”
This prospectus is dated November 16, 2021
Table
of Contents
You
should rely only on the information contained or incorporated by reference in this prospectus or any prospectus supplement. We have not
authorized anyone to provide you with information different from that contained or incorporated by reference into this prospectus. If
any person does provide you with information that differs from what is contained or incorporated by reference in this prospectus, you
should not rely on it. No dealer, salesperson or other person is authorized to give any information or to represent anything not contained
in this prospectus. You should assume that the information contained in this prospectus or any prospectus supplement is accurate only
as of the date on the front of the document and that any information contained in any document we have incorporated by reference is accurate
only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus or any prospectus
supplement or any sale of a security. These documents are not an offer to sell or a solicitation of an offer to buy these securities
by anyone in any jurisdiction in which such offer or solicitation is not authorized, or in which the person is not qualified to do so
or to any person to whom it is unlawful to make such offer or solicitation.
ABOUT
THIS PROSPECTUS
This prospectus is part of a registration
statement that we filed with the Securities and Exchange Commission, or SEC, using a “shelf” registration process. Under this
shelf registration process, we may sell any combination of the securities described in this prospectus in one of more offerings up to
a total dollar amount of proceeds of $50,000,000. This prospectus describes the general manner in which our securities may be offered
by this prospectus. Each time we sell securities, we will provide a prospectus supplement that will contain specific information about
the terms of that offering. The prospectus supplement may also add, update or change information contained in this prospectus or in documents
incorporated by reference in this prospectus. The prospectus supplement that contains specific information about the terms of the securities
being offered may also include a discussion of certain U.S. Federal income tax consequences and any risk factors or other special considerations
applicable to those securities. To the extent that any statement that we make in a prospectus supplement is inconsistent with statements
made in this prospectus or in documents incorporated by reference in this prospectus, you should rely on the information in the prospectus
supplement. You should carefully read both this prospectus and any prospectus supplement together with the additional information described
under “Where You Can Find More Information” before buying any securities in this offering.
Unless
the context otherwise requires, references to “we,” “our,” “us,” “ABVC” or the “Company”
in this prospectus mean ABVC BIOPHARMA, INC., a Nevada corporation, on a consolidated basis with its wholly-owned subsidiaries, as applicable.
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and the documents and information incorporated by reference in this prospectus include forward-looking statements within the
meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act
of 1934, as amended, or the Exchange Act. These statements are based on our management’s beliefs and assumptions and on information
currently available to our management. Such forward-looking statements include those that express plans, anticipation, intent, contingency,
goals, targets or future development and/or otherwise are not statements of historical fact.
All
statements in this prospectus and the documents and information incorporated by reference in this prospectus that are not historical
facts are forward-looking statements. We may, in some cases, use terms such as “anticipates,” “believes,” “could,”
“estimates,” “expects,” “intends,” “may,” “plans,” “potential,”
“predicts,” “projects,” “should,” “will,” “would” or similar expressions
or the negative of such items that convey uncertainty of future events or outcomes to identify forward-looking statements. “Common
Stock” is the Common Stock of ABVC Biopharma, Inc., par value US$0.001 per share.
Forward-looking
statements are made based on management’s beliefs, estimates and opinions on the date the statements are made and we undertake
no obligation to update forward-looking statements if these beliefs, estimates and opinions or other circumstances should change, except
as may be required by applicable law. Although we believe that the expectations reflected in the forward-looking statements are reasonable,
we cannot guarantee future results, levels of activity, performance or achievements.
ABOUT
ABVC
Overview
ABVC
BioPharma, Inc., formerly known as American BriVision (Holding) Corporation, a Nevada corporation, through the Company’s operating
entity, American BriVision Corporation (“BriVision”), which was incorporated in July 2015 in the State of Delaware, engages
in biotechnology to fulfill unmet medical needs and focuses on the development of new drugs and medical devices derived from plants.
BriVision develops its pipeline by carefully tracking new medical discoveries or medical device technologies in research institutions
in the Asia-Pacific region. Pre-clinical, disease animal model and Phase I safety studies are examined closely by the Company to identify
drugs that BriVision believes demonstrate efficacy and safety. Once a drug appears to be a good candidate for development and ultimately
commercialization, BriVision licenses the drug or medical device from the original researchers and begins to introduce the drugs clinical
plan to highly respected principal investigators in the United States, Australia and Taiwan to conduct a Phase II clinical trial. At
present, clinical trials for the Company’s drugs and medical devices are being conducted at such world-famous institutions as Memorial
Sloan Kettering Cancer Center (“MSKCC”) and MD Anderson Cancer Center. BriVision had no predecessor operations prior to its
formation on July 21, 2015.
Medicines
derived from plants have a long history of relieving or preventing many diseases and, typically, have exhibited fewer side effects than
drugs developed from animals or chemical ingredients. Perhaps the most famous example is aspirin, which evolved from a compound found
in the bark and leaves of the willow tree and was later marketed by Bayer starting in 1899. Aspirin has very few serious side effects
and has proven to be one of the most successful drugs in medical history. Some 50 years later, scientists identified anticancer compounds
in the rosy periwinkle, which Eli Lilly subsequently produced for the treatment of leukemia and Hodgkins disease. Other well-known examples
of successful botanical drugs include the cancer-fighting Taxol, isolated from the Pacific yew tree.
The
Company develops its pipeline by carefully tracking new medical discoveries or medical device technologies in research institutions in
the Asia-Pacific region. Pre-clinical, disease animal model and Phase I safety studies are examined closely by the Company’s scientists
and other specialists known to the Company to identify drugs that it believes demonstrate efficacy and safety based on the Company’s
internal qualifications. Once a drug is shown to be a good candidate for further development and ultimately commercialization, BriVision
licenses the drug or medical device from the original researchers and begins to introduce the drugs clinical plan to highly respected
principal investigators in the United States, Australia and Taiwan. In almost all cases, we have found that research institutions in
each of those countries are eager to work with the Company to move forward with Phase II clinical trials.
Currently,
institutions that have or are now conducting phase II clinical trials in partnership with ABVC include:
|
|
●
|
Drug:
ABV-1504, Major Depressive Disorder (MDD), Phase II completed. NCE drug Principal Investigators:
Charles DeBattista M.D. and Alan F. Schatzberg, MD, Stanford University Medical Center, Cheng-Ta
Li, MD, Ph.D – Taipei Veterans General Hospital
|
|
|
●
|
Drug:
ABV-1505, Adult Attention-Deficit Hyperactivity Disorder (ADHD), Phase II Part 1 completed.
NCE drug Principal Investigators: Keith McBurnett, Ph.D. and Linda Pfiffner, Ph.D., University
of California San Francisco (UCSF), School of Medicine
|
|
|
●
|
Drug:
ABV-1601, Major Depression in Cancer Patients, Phase I/II, NCE drug Principal Investigator:
Scott Irwin, MD, Ph.D. – Cedars Sinai Medical Center (CSMC)
|
|
|
●
|
Drug:
ABV-1703, Advanced Inoperable or Metastatic Pancreatic Cancer, Phase II, NCE drug Principal
Investigator: Andrew E. Hendifar, MD – Cedars Sinai Medical Center (CSMC)
|
The
following trials are expected to begin in the fourth quarter of 2021 and first quarter of 2022:
|
|
●
|
Medical
Device: ABV-1701, Vitargus® in vitrectomy surgery, Pivotal Study in Australia, Principal
Investigator: Andrew Chang, MD, Ph.D., Sydney Eye Hospital, Australia
|
|
|
●
|
Drug:
ABV-1501, A Phase I/II, Open Label Study to Evaluate the Safety and Efficacy of BLEX 404
Oral Liquid Combined with Docetaxel Monotherapy in Patients with Stage IV or Recurrent Breast
Cancer Patients
|
Upon
successful completion of the Phase II trial, the Company will seek a partner – a large pharmaceutical company – to complete
a Phase III study, submit the New Drug Application (NDA), and commercialize the drug upon approval by the FDA and Taiwan FDAs. The Company
expects to seek its first commercialization partner in 2021 for Vitargus, its vitreous substitute that helps to maintain a round shape
and retinal location during vitrectomy surgery.
Another
part of the Company’s business is conducted by BioKey, a wholly owned subsidiary, that is engaged in a wide range of services,
including, API characterization, pre-formulation studies, formulation development, analytical method development, stability studies,
IND/NDA/ANDA/510K submissions, and manufacturing clinical trial materials (phase I through phase III) and commercial manufacturing.
On
February 8, 2019, the Company, BioLite Holding, Inc. (“BioLite”), BioKey, Inc. (“BioKey”), BioLite Acquisition
Corp., a direct wholly-owned subsidiary of the Company and BioKey Acquisition Corp., a direct wholly-owned subsidiary of the Company
completed the business combination pursuant to that certain Agreement and Plan of Merger dated January 31, 2018, pursuant to which the
Company acquired BioLite and BioKey via issuing shares of the Company’s Common Stock to the shareholders of BioLite and BioKey.
As a result, BioLite and BioKey became two wholly-owned subsidiaries of the Company on February 8, 2019. The Company issued an aggregate
of 104,558,777 shares of Common Stock (prior to the reverse stock split in 2019) to the shareholders of both BioLite and BioKey under
a registration statement on Form S-4 (file number 333-226285), which became effective by operation of law on or about February 5, 2019.
BioLite
was incorporated under the laws of the State of Nevada on July 27, 2016, with 500,000,000 shares authorized, par value $0.0001. BioLite’s
key subsidiaries include BioLite BVI, Inc. (“BioLite BVI”) that was incorporated in the British Virgin Islands on September
13, 2016 and BioLite, Inc. (“BioLite Taiwan”), a Taiwanese corporation that was founded in February 2006. BioLite Taiwan
has been in the business of developing new drugs for over ten years.
BioLite
and BioLite BVI are holding companies and have not carried out substantive business operations of their own.
In
January 2017, BioLite, BioLite BVI, BioLite Taiwan, and certain shareholders of BioLite Taiwan entered into a share purchase / exchange
agreement (the “BioLite Share Purchase / Exchange Agreement”). Pursuant to the BioLite Share Purchase / Exchange Agreement,
the shareholder participants to the BioLite Share Purchase / Exchange Agreement sold their equity in BioLite Taiwan and used the proceeds
from such sales to purchase shares of Common Stock of BioLite at the same price per share, resulting in their owning the same number
of shares of Common Stock as they owned in BioLite Taiwan. Upon closing of the Share Purchase/ Exchange Agreement in August 2017, BioLite
owns, via BioLite BVI, approximately 73% of BioLite Taiwan. The other shareholders who did not enter this Share Purchase/ Exchange Agreement
retain their equity ownership in BioLite Taiwan.
BioKey
was incorporated on August 9, 2000 in the State of California. It is engaged primarily in research and development, manufacturing, and
distribution of generic drugs and nutraceuticals with strategic partners. BioKey provides a wide range of services, including, API characterization,
pre-formulation studies, formulation development, analytical method development, stability studies, IND/NDA/ANDA/510K submissions, and
manufacturing clinical trial materials (phase 1 through phase 3) and commercial manufacturing. It also licenses out its technologies
and initiates joint research and development processes with other biotechnology, pharmaceutical, and nutraceutical companies.
Our
principal executive offices are located at 44370 Old Warm Springs Blvd, Fremont, CA 94538. Our telephone number is: (510) 668-0881.
RISK
FACTORS
Investing
in our securities involves a high degree of risk. Before making an investment decision, you should consider carefully the risks, uncertainties
and other factors described in our most recent Annual Report on Form 10-K, as supplemented and updated by subsequent quarterly reports
on Form 10-Q and current reports on Form 8-K that we have filed or will file with the SEC, which are incorporated by reference into this
prospectus.
Our
business, affairs, prospects, assets, financial condition, results of operations and cash flows could be materially and adversely affected
by these risks. For more information about our SEC filings, please see “Where You Can Find More Information.”
USE
OF PROCEEDS
Unless
otherwise indicated in a prospectus supplement, we intend to use the net proceeds from the sale of the securities under this prospectus
for general corporate purposes, including for general working capital purposes, which may include the repayment of outstanding debt.
DILUTION
If
required, we will set forth in a prospectus supplement the following information regarding any material dilution of the equity interests
of investors purchasing securities in an offering under this prospectus:
|
|
●
|
the
net tangible book value per share of our equity securities before and after the offering;
|
|
|
|
|
|
|
●
|
the
amount of the increase in such net tangible book value per share attributable to the cash payments made by purchasers in the offering;
and
|
|
|
|
|
|
|
●
|
the
amount of the immediate dilution from the public offering price which will be absorbed by such purchasers.
|
DESCRIPTION
OF SECURITIES TO BE REGISTERED
We
may issue from time to time, in one or more offerings the following securities:
|
|
●
|
shares
of common stock;
|
|
|
●
|
shares
of preferred stock;
|
|
|
●
|
warrants
exercisable for debt securities, common stock or preferred stock;
|
|
|
●
|
rights
to purchase any of such securities; and
|
|
|
●
|
units
of debt securities, common stock, preferred stock or warrants, in any combination.
|
This
prospectus contains a summary of the material general terms of the various securities that we may offer. The specific terms of the securities
will be described in a prospectus supplement, information incorporated by reference or related free writing prospectus, which may be
in addition to or different from the general terms summarized in this prospectus. Where applicable, the prospectus supplement, information
incorporated by reference or related free writing prospectus will also describe any material United States federal income tax considerations
relating to the securities offered and indicate whether the securities offered are or will be listed on any securities exchange. The
summaries contained in this prospectus and in any prospectus supplements, information incorporated by reference or related free writing
prospectus may not contain all of the information that you would find useful. Accordingly, you should read the actual documents relating
to any securities sold pursuant to this prospectus.
The
terms of any particular offering, the initial offering price and the net proceeds to us will be contained in the prospectus supplement,
information incorporated by reference or free writing prospectus, relating to such offering.
Common
Stock
As of the date hereof, there are
28,609,388 shares of our Common Stock issued and outstanding. Holders of Common Stock are entitled to cast one vote for each share on
all matters submitted to a vote of shareholders, including the election of directors. The holders of Common Stock are entitled to receive
ratably such dividends, if any, as may be declared by the Board out of funds legally available therefore. Such holders do not have any
preemptive or other rights to subscribe for additional shares. All holders of Common Stock are entitled to share ratably in any assets
for distribution to shareholders upon the liquidation, dissolution or winding up of the Company, subject to prior distribution rights
of preferred stock then outstanding. There are no conversions, redemptions or sinking fund provisions applicable to the Common Stock.
All outstanding shares of Common Stock are fully paid and non-assessable.
Preferred
Stock
As
of the date hereof, there is no preferred stock outstanding, including the class of Series A Convertible Preferred Stock. Pursuant to
the articles of incorporation of the Company, the Board of Directors is expressly granted the authority to issue preferred stock up to
20,000,000 shares and prescribe its designations. On June 28, 2019, the Company filed a certificate of designation (the “Series
A COD”) of Series A Convertible Preferred Stock (the “Series A Stock”) with the Secretary of the State of Nevada, pursuant
to which the Company designated 3,500,000 shares of preferred stock as Series A Stock, par value of $0.001 per share.
The
following description of the Series A Stock is not complete. The Company’s Board of Directors has the authority, without further
action by the shareholders, to issue shares of preferred stock in one or more other series and to fix the rights, preferences, privileges
and restrictions granted to or imposed upon the preferred stock. Any or all of these rights may be greater than the rights of the Company’s
Common Stock. These descriptions are qualified in their entirety by reference to the Company’s Articles of Incorporation, as amended,
and the certificate of designation relating to each such series.
Dividends
We
have never declared or paid any cash dividends on our capital stock, and we do not currently intend to pay any cash dividends on our
capital stock in the foreseeable future. We currently intend to retain all available funds and any future earnings to support operations
and to finance the growth and development of our business. Any future determination to pay dividends will be made at the discretion of
our Board, subject to applicable laws and will depend upon, among other factors, our results of operations, financial condition, contractual
restrictions and capital requirements. Our future ability to pay cash dividends on our capital stock may also be limited by the terms
of any debt instruments or preferred securities issued in the future.
Conversion
Rights
Each
share of Series A Stock is initially convertible at any time at the option of the holders into one share of Common Stock and automatically
converts into one share of Common Stock (the “Conversion Ratio”) on its four-year anniversary of issuance and without the
payment of additional consideration by the holder thereof.
No
fractional shares shall be issued upon conversion of Series A Stock into Common Stock and no payment. In lieu of delivering fractional
shares, we will pay to the holder, to the extent permitted by law, an amount in cash equal to the current fair market value of such fractional
share as determined in good faith by our Board.
Voting
Rights
Holders
of shares of the Series A Stock vote on an as converted basis with the holders of our Common Stock.
Holders
of shares of Series A Stock were entitled to receive a cash dividend at the per annum rate of an amount equal to the product of 5% multiplied
by the public offering price per share of the shares that were to be sold pursuant to the registration statement on Form S-1 (File No.
333-228387) initially filed on November 14, 2018, but has since been withdrawn. Therefore, the Series A Stock is not eligible for dividends
at this time.
Conversion
Rights
Each
share of Series A Stock is initially convertible at any time at the option of the holders into one share of Common Stock and automatically
converts into one share of Common Stock (the “Conversion Ratio”) on its four-year anniversary of issuance and without the
payment of additional consideration by the holder thereof.
No
fractional shares shall be issued upon conversion of Series A Stock into Common Stock and no payment. In lieu of delivering fractional
shares, we will pay to the holder, to the extent permitted by law, an amount in cash equal to the current fair market value of such fractional
share as determined in good faith by our Board.
No
Maturity, Sinking Fund or Mandatory Redemption
The
Series A Stock has no maturity date and we are not required to redeem the Series A Stock at any time. However, we may choose to convert
all the outstanding shares of the Series A Stock into our Common Stock at the same Conversion Ratio at any time, provided that we have
prepaid and distributed all the dividend accrued and to be accrued at the end of the four-year period since issuance thereof. Accordingly,
the Series A Stock will remain outstanding until automatically converted to Common Stock on the four-year anniversary of issuance, unless
the holders of the Series A Convertible Preferred Stock or we choose to convert the Series A Stock into the Common Stock. The Series
A Stock is also not subject to any sinking fund.
Description
of Rights
We
may issue rights to purchase our securities. The rights may or may not be transferable by the persons purchasing or receiving the rights.
In connection with any rights offering, we may enter into a standby underwriting or other arrangement with one or more underwriters or
other persons pursuant to which such underwriters or other persons would purchase any offered securities remaining unsubscribed for after
such rights offering. Each series of rights will be issued under a separate rights agent agreement to be entered into between us and
one or more banks, trust companies or other financial institutions, as rights agent that we will name in the applicable prospectus supplement.
The rights agent will act solely as our agent in connection with the rights and will not assume any obligation or relationship of agency
or trust for or with any holders of rights certificates or beneficial owners of rights.
The
prospectus supplement relating to any rights that we offer will include specific terms relating to the offering, including, among other
matters:
|
|
●
|
the
date of determining the security holders entitled to the rights distribution;
|
|
|
●
|
the
aggregate number of rights issued and the aggregate amount of securities purchasable upon
exercise of the rights;
|
|
|
●
|
the
conditions to completion of the rights offering;
|
|
|
●
|
the
date on which the right to exercise the rights will commence and the date on which the rights
will expire; and
|
|
|
●
|
any
applicable federal income tax considerations.
|
Each
right would entitle the holder of the rights to purchase for cash the principal amount of securities at the exercise price set forth
in the applicable prospectus supplement. Rights may be exercised at any time up to the close of business on the expiration date for the
rights provided in the applicable prospectus supplement. After the close of business on the expiration date, all unexercised rights will
become void.
If
less than all of the rights issued in any rights offering are exercised, we may offer any unsubscribed securities directly to persons
other than our security holders, to or through agents, underwriters or dealers or through a combination of such methods, including pursuant
to standby arrangements, as described in the applicable prospectus supplement.
Description
of Warrants
We
may issue warrants to purchase our Common Stock or preferred shares. Warrants may be issued independently or together with any other
securities that may be sold by us pursuant to this prospectus or any combination of the foregoing and may be attached to, or separate
from, such securities. To the extent warrants that we issue are to be publicly-traded, each series of such warrants will be issued under
a separate warrant agreement to be entered into between us and a warrant agent. While the terms we have summarized below will apply generally
to any warrants that we may offer under this prospectus, we will describe in particular the terms of any series of warrants that we may
offer in more detail in the applicable prospectus supplement and any applicable free writing prospectus. The terms of any warrants offered
under a prospectus supplement may differ from the terms described below.
We
will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from another
report that we file with the SEC, the form of the warrant and/or warrant agreement, if any, which may include a form of warrant certificate,
as applicable that describes the terms of the particular series of warrants we may offer before the issuance of the related series of
warrants. We may issue the warrants under a warrant agreement that we will enter into with a warrant agent to be selected by us. The
warrant agent will act solely as our agent in connection with the warrants and will not assume any obligation or relationship of agency
or trust for or with any registered holders of warrants or beneficial owners of warrants. The following summary of material provisions
of the warrants and warrant agreements is subject to, and qualified in its entirety by reference to, all the provisions of the form of
warrant and/or warrant agreement and warrant certificate applicable to a particular series of warrants. We urge you to read the applicable
prospectus supplement and any related free writing prospectus, as well as the complete form of warrant and/or the warrant agreement and
warrant certificate, as applicable, that contain the terms of the warrants.
The
particular terms of any issue of warrants will be described in the prospectus supplement relating to the issue. Those terms may include:
|
|
●
|
the
title of the warrants;
|
|
|
●
|
the
price or prices at which the warrants will be issued;
|
|
|
●
|
the
designation, amount and terms of the securities or other rights for which the warrants are
exercisable;
|
|
|
●
|
the
designation and terms of the other securities, if any, with which the warrants are to be
issued and the number of warrants issued with each other security;
|
|
|
●
|
the
aggregate number of warrants;
|
|
|
●
|
any
provisions for adjustment of the number or amount of securities receivable upon exercise
of the warrants or the exercise price of the warrants;
|
|
|
●
|
the
price or prices at which the securities or other rights purchasable upon exercise of the
warrants may be purchased;
|
|
|
●
|
if
applicable, the date on and after which the warrants and the securities or other rights purchasable
upon exercise of the warrants will be separately transferable;
|
|
|
●
|
a
discussion of any material U.S. federal income tax considerations applicable to the exercise
of the warrants;
|
|
|
●
|
the
date on which the right to exercise the warrants will commence, and the date on which the
right will expire;
|
|
|
●
|
the
maximum or minimum number of warrants that may be exercised at any time;
|
|
|
●
|
information
with respect to book-entry procedures, if any; and
|
|
|
●
|
any
other terms of the warrants, including terms, procedures and limitations relating to the
exchange and exercise of the warrants.
|
Exercise
of Warrants
Each
warrant will entitle the holder of warrants to purchase the number of Common Stock or preferred shares of the relevant class or series
at the exercise price stated or determinable in the prospectus supplement for the warrants. Warrants may be exercised at any time up
to the close of business on the expiration date shown in the applicable prospectus supplement, unless otherwise specified in such prospectus
supplement. After the close of business on the expiration date, if applicable, unexercised warrants will become void. Warrants may be
exercised in the manner described in the applicable prospectus supplement. When the warrant holder makes the payment and properly completes
and signs the warrant certificate at the corporate trust office of the warrant agent, if any, or any other office indicated in the prospectus
supplement, we will, as soon as possible, forward the securities or other rights that the warrant holder has purchased. If the warrant
holder exercises less than all of the warrants represented by the warrant certificate, we will issue a new warrant certificate for the
remaining warrants. If we so indicate in the applicable prospectus supplement, holders of the warrants may surrender securities as all
or part of the exercise price for warrants.
Prior
to the exercise of any warrants to purchase Common Stock or preferred shares of the relevant class or series, holders of the warrants
will not have any of the rights of holders of Common Stock or preferred shares purchasable upon exercise, including the right to vote
or to receive any payments of dividends or payments upon our liquidation, dissolution or winding up on the Common Stock or preferred
shares purchasable upon exercise, if any.
Outstanding
Warrants
As of the date of this prospectus,
we have 545,182 and 5,238,425 options and warrants, respectively of the Company outstanding.
Description
of Units
The
following description, together with the additional information we may include in any applicable prospectus supplement, summarizes the
material terms and provisions of the units that we may offer under this prospectus. While the terms we have summarized below will apply
generally to any units that we may offer under this prospectus, we will describe the particular terms of any series of units in more
detail in the applicable prospectus supplement and any related free writing prospectus. The terms of any units offered under a prospectus
supplement may differ from the terms described below. However, no prospectus supplement will fundamentally change the terms that are
set forth in this prospectus or offer a security that is not registered and described in this prospectus at the time of its effectiveness.
We
will file as an exhibit to the registration statement of which this prospectus is a part, or will incorporate by reference from another
report we file with the SEC, the form of unit agreement that describes the terms of the series of units we may offer under this prospectus,
and any supplemental agreements, before the issuance of the related series of units. The following summaries of material terms and provisions
of the units are subject to, and qualified in their entirety by reference to, all the provisions of the unit agreement and any supplemental
agreements applicable to a particular series of units. We urge you to read the applicable prospectus supplement and any related free
writing prospectus, as well as the complete unit agreement and any supplemental agreements that contain the terms of the units.
We
may issue units comprised of Common Stock or preferred shares and warrants in any combination. Each unit will be issued so that the holder
of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations
of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the
unit may not be held or transferred separately, at any time or at any time before a specified date. If we offer any units, certain terms
of that series of units will be described in the applicable prospectus supplement, including, without limitation, the following, as applicable:
|
|
●
|
the
title of the series of units;
|
|
|
●
|
identification
and description of the separate constituent securities comprising the units;
|
|
|
●
|
the
price or prices at which the units will be issued;
|
|
|
●
|
the
date, if any, on and after which the constituent securities comprising the units will be
separately transferable, if applicable;
|
|
|
●
|
any
provisions for the issuance, payment, settlement, transfer or exchange of the units or of
the securities comprising the units;
|
|
|
●
|
a
discussion of certain United States federal income tax considerations applicable to the units;
and
|
|
|
●
|
any
other material terms of the units and their constituent securities.
|
The
provisions described in this section, as well as those described under “Description of Share Capital – Common Stock and Preferred
Shares” and “Description of Warrants” will apply to each unit and to any Common Stock, preferred shares or warrant
included in each unit, respectively.
Issuance
in Series
We
may issue units in such amounts and in numerous distinct series as we determine.
Enforceability
of Rights by Holders of Units
We
may enter into unit agreements with a unit agent. Each unit agent will act solely as our agent under the applicable unit agreement and
will not assume any obligation or relationship of agency or trust with any holder of any unit. A single bank or trust company may act
as unit agent for more than one series of units. A unit agent will have no duty or responsibility in case of any default by us under
the applicable unit agreement or unit, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make
any demand upon us. Any holder of a unit may, without the consent of the related unit agent or the holder of any other unit, enforce
by appropriate legal action its rights as holder under any security included in the unit.
We,
the unit agents and any of their agents may treat the registered holder of any unit certificate as an absolute owner of the units evidenced
by that certificate for any purpose and as the person entitled to exercise the rights attaching to the units so requested, despite any
notice to the contrary.
Description
of Debt Securities
The
following description, together with the additional information we include in any applicable prospectus supplements, summarizes the material
terms and provisions of the debt securities that we may offer under this prospectus. While the terms we have summarized below will generally
apply to any future debt securities we may offer under this prospectus, we will describe the particular terms of any debt securities
that we may offer in more detail in the applicable prospectus supplement. The terms of any debt securities we offer under a prospectus
supplement may differ from the terms we describe below. As of the date of this prospectus, we have no outstanding registered debt securities.
We
may issue notes under senior or subordinated indentures or, separately, without the use of an indenture. If we issue senior or subordinated
notes without the use of an indenture, we will issue such senior or subordinated notes directly to the purchasers of such senior or subordinated
notes.
If
we issue senior notes under a senior indenture, we will enter into such subordinated indenture with the trustee to be named in such senior
indenture. If we issue subordinated notes under a subordinated indenture, we will enter into such subordinated indenture with the trustee
to be named in such subordinated indenture. We will file as exhibits to the registration statement of which this prospectus is a part,
or will incorporate by reference from another report that we file with the SEC, the form of such notes and indentures, if any, that describes
the terms of the particular note we may offer under this prospectus, and any supplement agreements, before the issuance of the related
note. We use the term “indentures” to refer to both the senior indenture and the subordinated indenture.
The
indentures will be qualified under the Trust Indenture Act of 1939. References to the Trust Indenture Act of 1939 include
all amendments thereto. We use the term “debenture trustee” to refer to either the senior trustee or the subordinated trustee,
as applicable.
The
following summaries of material provisions of the senior notes, the subordinated notes and the indentures are subject to, and qualified
in their entirety by reference to, all the provisions of the indenture applicable to a particular series of debt securities, and all
supplements thereto. We urge you to read the applicable prospectus supplements related to the debt securities that we sell under this
prospectus, as well as the complete indentures that contain the terms of the debt securities. Except as we may otherwise indicate, the
terms of the senior and the subordinated indentures are identical.
The
statements and descriptions in this prospectus or in any prospectus supplement regarding provisions of debt securities and any indentures
are summaries of these provisions, do not purport to be complete and are subject to, and are qualified in their entirety by reference
to, all of the provisions of the debt securities and the indentures (including any amendments or supplements we may enter into from time
to time which are permitted under the debt securities or any indenture).
General
The
terms of each series of debt securities will be established by or pursuant to a resolution of our board of directors and set forth or
determined in the manner provided in an officers’ certificate or by a supplemental indenture. Debt securities may be issued in
separate series without limitation as to aggregate principal amount. We may specify a maximum aggregate principal amount for the debt
securities of any series. In addition, the particular terms of each series of debt securities will be described in a prospectus supplement
relating to such series, including any pricing supplement. The prospectus supplement will set forth, among other things:
|
|
●
|
the
principal amount being offered, and, if a series, the total amount authorized and the total
amount outstanding;
|
|
|
●
|
any
limit on the amount that may be issued;
|
|
|
●
|
whether
or not we will issue the series of debt securities in global form and, if so, the terms and
who the depositary will be;
|
|
|
●
|
whether
and under what circumstances, if any, we will pay additional amounts on any debt securities
held by a person who is not a U.S. person for tax purposes, and whether we can redeem the
debt securities if we have to pay such additional amounts;
|
|
|
●
|
the
annual interest rate, which may be fixed or variable, or the method for determining the rate,
the date interest will begin to accrue, the dates interest will be payable and the regular
record dates for interest payment dates or the method for determining such dates;
|
|
|
●
|
the
terms of the subordination of any series of subordinated debt, if applicable;
|
|
|
●
|
the
place where payments will be payable;
|
|
|
●
|
restrictions
on transfer, sale or other assignment, if any;
|
|
|
●
|
our
right, if any, to defer payment of interest and the maximum length of any such deferral period;
|
|
|
●
|
the
date, if any, after which, the conditions upon which, and the price at which we may, at our
option, redeem the series of debt securities pursuant to any optional or provisional redemption
provisions, and any other applicable terms of those redemption provisions;
|
|
|
●
|
the
date, if any, on which, and the price at which we are obligated, pursuant to any mandatory
sinking fund or analogous fund provisions or otherwise, to redeem, or at the holder’s
option to purchase, the series of debt securities and the currency or currency unit in which
the debt securities are payable;
|
|
|
●
|
whether
the indenture will restrict our ability and/or the ability of our subsidiaries to, among
other things:
|
|
|
●
|
incur
additional indebtedness;
|
|
|
●
|
issue
additional securities;
|
|
|
●
|
pay
dividends and make distributions in respect of our capital stock and the capital stock of
our subsidiaries;
|
|
|
●
|
place
restrictions on our subsidiaries’ ability to pay dividends, make distributions or transfer
assets;
|
|
|
●
|
make
investments or other restricted payments;
|
|
|
●
|
sell
or otherwise dispose of assets;
|
|
|
●
|
enter
into sale-leaseback transactions;
|
|
|
●
|
engage
in transactions with stockholders and affiliates;
|
|
|
●
|
issue
or sell stock of our subsidiaries; or
|
|
|
●
|
effect
a consolidation or merger;
|
|
|
●
|
whether
the indenture will require us to maintain any interest coverage, fixed charge, cash flow-based,
asset-based or other financial ratios;
|
|
|
●
|
information
describing any book-entry features;
|
|
|
●
|
provisions
for a sinking fund purchase or other analogous fund, if any;
|
|
|
●
|
whether
the debt securities are to be offered at a price such that they will be deemed to be offered
at an “original issue discount” as defined in paragraph (a) of Section 1273 of
the Internal Revenue Code;
|
|
|
●
|
the
procedures for any auction and remarketing, if any;
|
|
|
●
|
the
denominations in which we will issue the series of debt securities, if other than denominations
of $1,000 and any integral multiple thereof;
|
|
|
●
|
if
other than dollars, the currency in which the series of debt securities will be denominated;
and
|
|
|
●
|
any
other specific terms, preferences, rights or limitations of, or restrictions on, the debt
securities, including any events of default that are in addition to those described in this
prospectus or any covenants provided with respect to the debt securities that are in addition
to those described above, and any terms that may be required by us or advisable under applicable
laws or regulations or advisable in connection with the marketing of the debt securities.
|
Conversion
or Exchange Rights
We
will set forth in the prospectus supplement the terms on which a series of debt securities may be convertible into or exchangeable for
Common Stock, preferred shares or other securities of ours or a third party, including the conversion or exchange rate, as applicable,
or how it will be calculated, and the applicable conversion or exchange period. We will include provisions as to whether conversion or
exchange is mandatory, at the option of the holder or at our option. We may include provisions pursuant to which the number of our securities
or the securities of a third party that the holders of the series of debt securities receive upon conversion or exchange would, under
the circumstances described in those provisions, be subject to adjustment, or pursuant to which those holders would, under those circumstances,
receive other property upon conversion or exchange, for example in the event of our merger or consolidation with another entity.
Consolidation, Merger or Sale
The indentures in the forms
initially filed as exhibits to the registration statement of which this prospectus is a part do not contain any covenant that restricts
our ability to merge or consolidate, or sell, convey, transfer or otherwise dispose of all or substantially all of our assets. However,
any successor of ours or the acquirer of such assets must assume all of our obligations under the indentures and the debt securities.
If the debt securities are
convertible for our other securities, the person with whom we consolidate or merge or to whom we sell all of our property must make provisions
for the conversion of the debt securities into securities that the holders of the debt securities would have received if they had converted
the debt securities before the consolidation, merger or sale.
Events of Default Under the Indenture
The following are events of
default under the indentures in the forms initially filed as exhibits to the registration statement with respect to any series of debt
securities that we may issue:
|
|
●
|
if we fail to pay interest when due and payable and our failure continues for 90 days and the time for
payment has not been extended or deferred;
|
|
|
|
|
|
|
●
|
if we fail to pay the principal, sinking fund payment or premium, if any, when due and payable and the
time for payment has not been extended or delayed;
|
|
|
|
|
|
|
●
|
if we fail to observe or perform any other covenant contained in the debt securities or the indentures,
other than a covenant specifically relating to another series of debt securities, and our failure continues for 90 days after we receive
notice from the debenture trustee or holders of at least 25% in aggregate principal amount of the outstanding debt securities of the applicable
series; and
|
|
|
|
|
|
|
●
|
if specified events of bankruptcy, insolvency or reorganization occur.
|
If an event of default with
respect to debt securities of any series occurs and is continuing, other than an event of default specified in the last bullet point above,
the debenture trustee or the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series,
by notice to us in writing, and to the debenture trustee if notice is given by such holders, may declare the unpaid principal of, premium,
if any, and accrued interest, if any, due and payable immediately. If an event of default specified in the last bullet point above occurs
with respect to us, the principal amount of and accrued interest, if any, of each issue of debt securities then outstanding shall be due
and payable without any notice or other action on the part of the debenture trustee or any holder.
The holders of a majority
in principal amount of the outstanding debt securities of an affected series may waive any default or event of default with respect to
the series and its consequences, except defaults or events of default regarding payment of principal, premium, if any, or interest, unless
we have cured the default or event of default in accordance with the indenture. Any waiver shall cure the default or event of default.
Subject to the terms of the
indentures, if an event of default under an indenture shall occur and be continuing, the debenture trustee will be under no obligation
to exercise any of its rights or powers under such indenture at the request or direction of any of the holders of the applicable series
of debt securities, unless such holders have offered the debenture trustee reasonable indemnity. The holders of a majority in principal
amount of the outstanding debt securities of any series will have the right to direct the time, method and place of conducting any proceeding
for any remedy available to the debenture trustee, or exercising any trust or power conferred on the debenture trustee, with respect to
the debt securities of that series, provided that:
|
|
●
|
the direction so given by the holder is not in conflict with any law or the applicable indenture; and
|
|
|
|
|
|
|
●
|
subject to its duties under the Trust Indenture Act of 1939, the debenture trustee need not take
any action that might involve it in personal liability or might be unduly prejudicial to the holders not involved in the proceeding.
|
A
holder of the debt securities of any series will only have the right to institute a proceeding under the indentures or to appoint a receiver
or trustee, or to seek other remedies if:
|
|
●
|
the holder has given written notice to the debenture trustee of a continuing event of default with respect to that series;
|
|
|
|
|
|
|
●
|
the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series have made written request,
and such holders have offered reasonable indemnity, to the debenture trustee to institute the proceeding as trustee; and
|
|
|
|
|
|
|
●
|
the debenture trustee does not institute the proceeding and does not receive from the holders of a majority in aggregate principal
amount of the outstanding debt securities of that series other conflicting directions within 90 days after the notice, request and offer.
|
These limitations do not apply
to a suit instituted by a holder of debt securities if we default in the payment of the principal, premium, if any, or interest on, the
debt securities.
We will periodically file
statements with the debenture trustee regarding our compliance with specified covenants in the indentures.
Modification of Indenture; Waiver
We and the debenture trustee
may change an indenture without the consent of any holders with respect to specific matters, including:
|
|
●
|
to fix any ambiguity, defect or inconsistency in the indenture;
|
|
|
|
|
|
|
●
|
to comply with the provisions described above under “Consolidation, Merger or Sale”;
|
|
|
|
|
|
|
●
|
to comply with any requirements of the SEC in connection with the qualification of any indenture under
the Trust Indenture Act of 1939;
|
|
|
|
|
|
|
●
|
to evidence and provide for the acceptance of appointment by a successor trustee;
|
|
|
|
|
|
|
●
|
to provide for uncertificated debt securities and to make all appropriate changes for such purpose;
|
|
|
|
|
|
|
●
|
to add to, delete from, or revise the conditions, limitations and restrictions on the authorized amount,
terms or purposes of issuance, authorization and delivery of debt securities or any series, as set forth in the indenture;
|
|
|
|
|
|
|
●
|
to provide for the issuance of and establish the form and terms and conditions of the debt securities
of any series as provided under “General” to establish the form of any certifications required to be furnished pursuant to
the terms of the indenture or any series of debt securities, or to add to the rights of the holders of any series of debt securities;
|
|
|
|
|
|
|
●
|
to add to our covenants such new covenants, restrictions, conditions or provisions for the protection
of the holders, to make the occurrence, or the occurrence and the continuance, of a default in any such additional covenants, restrictions,
conditions or provisions an event of default, or to surrender any of our rights or powers under the indenture; or
|
|
|
|
|
|
|
●
|
to change anything that does not materially adversely affect the interests of any holder of debt securities
of any series.
|
In addition, under the indentures,
the rights of holders of a series of debt securities may be changed by us and the debenture trustee with the written consent of the holders
of at least a majority in aggregate principal amount of the outstanding debt securities of each series that is affected. However, we and
the debenture trustee may only make the following changes with the consent of each holder of any outstanding debt securities affected:
|
|
●
|
extending the fixed maturity of the series of debt securities;
|
|
|
|
|
|
|
●
|
reducing the principal amount, reducing the rate of or extending the time of payment of interest, or reducing
any premium payable upon the redemption of any debt securities; or
|
|
|
|
|
|
|
●
|
reducing the percentage of debt securities, the holders of which are required to consent to any amendment,
supplement, modification or waiver.
|
Discharge
Each indenture provides that
we can elect to be discharged from our obligations with respect to one or more series of debt securities, except that the following obligations,
among others survive until the maturity date or the redemption date:
|
|
●
|
register the transfer or exchange of debt securities of the series;
|
|
|
|
|
|
|
●
|
replace stolen, lost or mutilated debt securities of the series;
|
|
|
|
|
|
|
●
|
maintain paying agencies;
|
|
|
|
|
|
|
●
|
hold monies for payment in trust; and
|
|
|
|
|
|
|
●
|
appoint any successor trustee;
|
and
the following obligations survive the maturity date or the redemption date:
|
|
●
|
recover excess money held by the debenture trustee; and
|
|
|
|
|
|
|
●
|
compensate and indemnify the debenture trustee.
|
As more fully set forth in
the indentures, in order to exercise our rights to be discharged, we must either deliver for cancellation all securities of a series to
the debenture trustee or must deposit with the debenture trustee money or government obligations sufficient to pay all the principal of,
any premium, if any, and interest on, the debt securities of the series on the dates payments are due.
Form, Exchange and Transfer
We will issue the debt securities
of each series only in fully registered form without coupons and, unless we otherwise specify in the applicable prospectus supplement,
in denominations of $1,000 and any integral multiple thereof. The indentures provide that we may issue debt securities of a series in
temporary or permanent global form and as book-entry securities that will be deposited with, or on behalf of, The Depository Trust Company,
New York, New York, known as DTC, or another depositary named by us and identified in a prospectus supplement with respect to that series.
At the option of the holder,
subject to the terms of the indentures and the limitations applicable to global securities described in the applicable prospectus supplement,
the holder of the debt securities of any series can exchange the debt securities for other debt securities of the same series, in any
authorized denomination and of like tenor and aggregate principal amount.
Subject to the terms of the
indentures and the limitations applicable to global securities set forth in the applicable prospectus supplement, holders of the debt
securities may present the debt securities for exchange or for registration of transfer, duly endorsed or with the form of transfer endorsed
thereon duly executed if so required by us or the security registrar, at the office of the security registrar or at the office of any
transfer agent designated by us for this purpose. Unless otherwise provided in the debt securities that the holder presents for transfer
or exchange, we will make no service charge for any registration of transfer or exchange, but we may require payment of any taxes or other
governmental charges.
We will name in a board resolution
the security registrar, and any transfer agent in addition to the security registrar, that we initially designate for any debt securities.
We may at any time designate additional transfer agents or rescind the designation of any transfer agent or approve a change in the office
through which any transfer agent acts, except that we will be required to maintain a transfer agent in each place of payment for the debt
securities of each series.
If we elect to redeem the
debt securities of any series, we will not be required to:
|
|
●
|
issue, register the transfer of, or exchange any debt securities of any series being redeemed in part
during a period beginning at the opening of business 15 days before the day of mailing of a notice of redemption of any debt securities
that may be selected for redemption and ending at the close of business on the day of the mailing; or
|
|
|
|
|
|
|
●
|
register the transfer of or exchange any debt securities so selected for redemption, in whole or in part,
except the unredeemed portion of any debt securities we are redeeming in part.
|
Information Concerning the Debenture Trustee
The debenture trustee, other
than during the occurrence and continuance of an event of default under an indenture, undertakes to perform only those duties as are specifically
set forth in the applicable indenture. Upon an event of default under an indenture, the debenture trustee must use the same degree of
care as a prudent person would exercise or use in the conduct of his or her own affairs. Subject to this provision, the debenture trustee
is under no obligation to exercise any of the powers given it by the indentures at the request of any holder of debt securities unless
it is offered reasonable security and indemnity against the costs, expenses and liabilities that it might incur.
Payment and Paying Agents
Unless we otherwise indicate
in the applicable prospectus supplement, we will make payment of the interest on any debt securities on any interest payment date to the
person in whose name the debt securities, or one or more predecessor securities, are registered at the close of business on the regular
record date for the interest.
We will name in the applicable
board resolution any other paying agents that we initially designate for the debt securities of a particular series. We will maintain
a paying agent in each place of payment for the debt securities of a particular series.
All money we pay to a paying
agent or the debenture trustee for the payment of the principal of or any premium or interest on any debt securities that remains unclaimed
at the end of two years after such principal, premium or interest has become due and payable will be repaid to us, and the holder of the
debt security thereafter may look only to us for payment thereof.
Governing Law
The indentures and the debt
securities will be governed by and construed in accordance with the laws of the State of New York, except to the extent that the Trust
Indenture Act of 1939 is applicable.
Subordination of Subordinated Debt Securities
The subordinated debt securities
will be subordinate and junior in priority of payment to certain of our other indebtedness to the extent described in a prospectus supplement.
The indentures in the forms initially filed as exhibits to the registration statement of which this prospectus is a part do not limit
the amount of indebtedness that we may incur, including senior indebtedness or subordinated indebtedness, and do not limit us from issuing
any other debt, including secured debt or unsecured debt.
Anti-Takeover Provisions
Certain provisions
of Nevada law, our Articles of Incorporation and our Bylaws may have the effect of delaying, deferring or discouraging another person
from acquiring control of the Company.
Nevada Law
Nevada’s “combinations
with interested stockholders” statutes (NRS 78.411 through 78.444, inclusive) prohibit specified types of business “combinations”
between certain Nevada corporations and any person deemed to be an “interested stockholder” for two years after such person
first becomes an “interested stockholder” unless the corporation’s board of directors approves the combination (or the
transaction by which such person becomes an “interested stockholder”) in advance, or unless the combination is approved by
the board of directors and sixty percent of the corporation’s voting power not beneficially owned by the interested stockholder,
its affiliates and associates. Furthermore, in the absence of prior approval certain restrictions may apply even after such two-year period.
For purposes of these statutes, an “interested stockholder” is any person who is (1) the beneficial owner, directly or indirectly,
of ten percent or more of the voting power of the outstanding voting shares of the corporation, or (2) an affiliate or associate of the
corporation and at any time within the two previous years was the beneficial owner, directly or indirectly, of ten percent or more of
the voting power of the then-outstanding shares of the corporation. The definition of the term “combination” is sufficiently
broad to cover most significant transactions between a corporation and an “interested stockholder.”
Nevada’s “acquisition
of controlling interest” statutes (NRS 78.378 through 78.3793, inclusive) contain provisions governing the acquisition of a controlling
interest in certain Nevada corporations. These “control share” laws provide generally that any person that acquires a “controlling
interest” in certain Nevada corporations may be denied voting rights, unless a majority of the disinterested stockholders of the
corporation elects to restore such voting rights. These laws would apply to us if we were to have 200 or more stockholders as of the record
date (at least 100 of whom have had addresses in Nevada appearing on our stock ledger at all times during the 90 days immediately preceding
such date) and do business in the State of Nevada directly or through an affiliated corporation, unless the Articles of Incorporation
or Bylaws in effect on the tenth day after the acquisition of a controlling interest provide otherwise. These laws provide that a person
acquires a “controlling interest” whenever a person acquires shares of a subject corporation that, but for the application
of these provisions of the NRS, would enable that person to exercise (1) one-fifth or more, but less than one-third, (2) one-third or
more, but less than a majority or (3) a majority or more, of all of the voting power of the corporation in the election of directors.
Once an acquirer crosses one of these thresholds, shares which it acquired in the transaction taking it over the threshold and within
the 90 days immediately preceding the date when the acquiring person acquired or offered to acquire a controlling interest become “control
shares” to which the voting restrictions described above apply.
In addition, NRS 78.139 also
provides that directors may resist a change or potential change in control if the board of directors determines that the change is opposed
to, or not in, the best interests of the corporation.
The effect of these statutes
may be to potentially discourage parties interested in taking control of the Company from doing so if it cannot obtain the approval of
our board of directors.
Board of Directors Vacancies
Generally, under NRS 78.335,
one or more of the incumbent directors may be removed from office by the vote of stockholders representing two-thirds or more of the voting
power of the issued and outstanding stock entitled to vote. In addition, if a court of competent jurisdiction, or other governmental entity
or regulatory agency with authority over the corporation requires, without providing any other reasonable and practicable alternative,
that any specified director cease to be a director in order for the corporation to obtain, or avoid the suspension, conditioning or revocation
of, any permit, license, registration, franchise, finding of suitability or similar authorization or approval required for the conduct
of all or any material portion of the business of the corporation or any of its affiliates taken as a whole and such requirement is not
appealable or has otherwise become final after declination or exhaustion of all appeals therefrom, then that specified director may be
removed as a director by not less than a majority of the voting power of the other directors, even if less than a quorum, acting at a
meeting and not by written consent and without a vote of the stockholders. The Articles of Incorporation and Bylaws authorize the Board
to fill vacant directorships, although the shareholders can fill vacancies in certain situations.
Cumulative Voting
The NRS does not permit stockholders
to cumulate their votes other than in the election of directors, and then the company’s articles of incorporation must specifically
provide for it. . The Company’s Articles of Incorporation as currently in effect are silent as to whether cumulative voting in the
election of directors is permitted; however, the Company’s current bylaws allow for cumulative voting, so long as a shareholder
has given notice prior to commencement of the voting of the shareholder’s intention to cumulate votes. If any shareholder has given
such a notice, then every shareholder entitled to vote may cumulate votes for candidates in nomination either (i) by giving one candidate
a number of votes equal to the number of directors to be elected multiplied by the number of votes to which that shareholder’s shares
are normally entitled or (ii) by distributing the shareholder’s votes on the same principle among any or all of the candidates,
as the shareholder thinks fit. The candidates receiving the highest number of affirmative votes, up to the number of directors to be elected,
shall be elected; votes against any candidate and votes withheld shall have no legal effect.
Transfer Agent and Registrar
The transfer agent and registrar
for our common stock is Vstock Transfer, LLC.
18 Lafayette Place, Woodmere,
NY 11598
(212) 828-8436
https://www.vstocktransfer.com/
PLAN OF DISTRIBUTION
We may sell the securities
offered through this prospectus (i) to or through underwriters or dealers, (ii) directly to purchasers, including our affiliates, (iii)
through agents, or (iv) through a combination of any these methods. The securities may be distributed at a fixed price or prices, which
may be changed, market prices prevailing at the time of sale, prices related to the prevailing market prices, or negotiated prices. The
prospectus supplement will include the following information:
|
|
●
|
the terms of the offering;
|
|
|
|
|
|
|
●
|
the names of any underwriters or agents;
|
|
|
|
|
|
|
●
|
the name or names of any managing underwriter or underwriters;
|
|
|
|
|
|
|
●
|
the purchase price of the securities;
|
|
|
|
|
|
|
●
|
any over-allotment options under which underwriters may purchase additional securities from us;
|
|
|
●
|
the net proceeds from the sale of the securities;
|
|
|
|
|
|
|
●
|
any delayed delivery arrangements;
|
|
|
|
|
|
|
●
|
any underwriting discounts, commissions and other items constituting underwriters’ compensation;
|
|
|
|
|
|
|
●
|
any offering price;
|
|
|
|
|
|
|
●
|
any discounts or concessions allowed or reallowed or paid to dealers;
|
|
|
|
|
|
|
●
|
any commissions paid to agents; and
|
|
|
|
|
|
|
●
|
any securities exchange or market on which the securities may be listed.
|
Sale Through Underwriters or Dealers
Only underwriters named in
the prospectus supplement are underwriters of the securities offered by the prospectus supplement. If underwriters are used in the sale,
the underwriters will acquire the securities for their own account, including through underwriting, purchase, security lending or repurchase
agreements with us. The underwriters may resell the securities from time to time in one or more transactions, including negotiated transactions.
Underwriters may sell the securities in order to facilitate transactions in any of our other securities (described in this prospectus
or otherwise), including other public or private transactions and short sales. Underwriters may offer securities to the public either
through underwriting syndicates represented by one or more managing underwriters or directly by one or more firms acting as underwriters.
Unless otherwise indicated in the prospectus supplement, the obligations of the underwriters to purchase the securities will be subject
to certain conditions, and the underwriters will be obligated to purchase all the offered securities if they purchase any of them. The
underwriters may change from time to time any offering price and any discounts or concessions allowed or reallowed or paid to dealers.
If dealers are used in the
sale of securities offered through this prospectus, we will sell the securities to them as principals. They may then resell those securities
to the public at varying prices determined by the dealers at the time of resale. The prospectus supplement will include the names of the
dealers and the terms of the transaction.
We will provide in the applicable
prospectus supplement any compensation we will pay to underwriters, dealers or agents in connection with the offering of the securities,
and any discounts, concessions or commissions allowed by underwriters to participating dealers.
Direct Sales and Sales Through Agents
We may sell the securities
offered through this prospectus directly. In this case, no underwriters or agents would be involved. Such securities may also be sold
through agents designated from time to time. The prospectus supplement will name any agent involved in the offer or sale of the offered
securities and will describe any commissions payable to the agent. Unless otherwise indicated in the prospectus supplement, any agent
will agree to use its reasonable best efforts to solicit purchases for the period of its appointment.
We may sell the securities
directly to institutional investors or others who may be deemed to be underwriters within the meaning of the Securities Act with respect
to any sale of those securities. The terms of any such sales will be described in the prospectus supplement.
Delayed Delivery Contracts
If the prospectus supplement
indicates, we may authorize agents, underwriters or dealers to solicit offers from certain types of institutions to purchase securities
at the offering price under delayed delivery contracts. These contracts would provide for payment and delivery on a specified date in
the future. The contracts would be subject only to those conditions described in the prospectus supplement. The applicable prospectus
supplement will describe the commission payable for solicitation of those contracts.
Market Making, Stabilization and Other Transactions
Unless the applicable prospectus
supplement states otherwise, other than our common stock, all securities we offer under this prospectus will be a new issue and will have
no established trading market. We may elect to list offered securities on an exchange or in the over-the-counter market. Any underwriters
that we use in the sale of offered securities may make a market in such securities, but may discontinue such market making at any time
without notice. Therefore, we cannot assure you that the securities will have a liquid trading market.
Any underwriter may also engage
in stabilizing transactions, syndicate covering transactions and penalty bids in accordance with Rule 104 under the Securities Exchange
Act. Stabilizing transactions involve bids to purchase the underlying security in the open market for the purpose of pegging, fixing or
maintaining the price of the securities. Syndicate covering transactions involve purchases of the securities in the open market after
the distribution has been completed in order to cover syndicate short positions.
Penalty bids permit the underwriters
to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member are purchased in a
syndicate covering transaction to cover syndicate short positions. Stabilizing transactions, syndicate covering transactions and penalty
bids may cause the price of the securities to be higher than it would be in the absence of the transactions. The underwriters may, if
they commence these transactions, discontinue them at any time.
General Information
Agents, underwriters, and
dealers may be entitled, under agreements entered into with us, to indemnification by us against certain liabilities, including liabilities
under the Securities Act. Our agents, underwriters, and dealers, or their affiliates, may be customers of, engage in transactions with
or perform services for us, in the ordinary course of business.
LEGAL MATTERS
Unless otherwise indicated
in the applicable prospectus supplement, the validity of the securities offered by this prospectus, and any supplement thereto, will be
passed upon for us by Hunter Taubman Fischer & Li LLC, New York, NY. The legality of the securities for any underwriters, dealers
or agents will be passed upon by counsel as may be specified in the applicable prospectus supplement.
EXPERTS
KCCW Accountancy Corp. (“KCCW”),
an independent registered public accounting firm, audited our financial statements included in our Annual Report on Form 10-K for the
year ended December 31, 2020 and 2019, as set forth in their report included therein, which are incorporated by reference in this prospectus
and elsewhere in the registration statement. Our financial statements are incorporated by reference in reliance on KCCW’s report,
given on their authority as experts in accounting and auditing.
Material Changes
None.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We file annual, quarterly
and special reports, along with other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s
website at http://www.sec.gov; you can also find our filings on our company website: www.abvcpharma.com. You may also read and copy any
document we file at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330
for further information on the Public Reference Room.
This prospectus is part of
a registration statement on Form S-3 that we filed with the SEC to register the securities offered hereby under the Securities Act of
1933, as amended. This prospectus does not contain all of the information included in the registration statement, including certain exhibits
and schedules. You may obtain the registration statement and exhibits to the registration statement from the SEC at the address listed
above or from the SEC’s internet site.
INFORMATION INCORPORATED BY REFERENCE
The Securities and Exchange
Commission allows us to incorporate by reference the information we file with them under certain conditions, which means that we can disclose
important information to you by referring you to those documents. The information incorporated by reference is considered to be a part
of this prospectus and any information that we file subsequent to this prospectus with the Securities and Exchange Commission will automatically
update and supersede this information. The documents we are incorporating by reference are as follows:
|
|
(a)
|
the Company’s Annual Report on Form 10-K for the year ended December 31, 2020;
|
|
|
(b)
|
the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2021;
|
|
|
(c)
|
the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2021;
|
|
|
(d)
|
the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2021;
|
|
|
(e)
|
the Company’s Current Reports on Form 8-K filed on October 8, 2021;
|
|
|
(f)
|
the Company’s Current Reports on Form 8-K filed on August 5, 2021;
|
|
|
(g)
|
the Company’s Current Reports on Form 8-K/A filed on June 8, 2021;
|
|
|
|
|
|
|
(h)
|
the Company’s Current Reports on Form 8-K/A filed on May 3, 2021;
|
|
|
|
|
|
|
(i)
|
the Company’s Current Reports on Form 8-K filed on February 11, 2021;
|
|
|
|
|
|
|
(j)
|
the Company’s Current Reports on Form 8-K filed on January 7, 2021;
|
|
|
|
|
|
|
(k)
|
the Company’s Current Reports on Form 8-K filed on January 8, 2021; and
|
|
|
(l)
|
the description of the Common Stock, $0.001 par value per share, contained in the Registrant’s registration statement on Form 8-A filed with the Commission on August 2, 2021 pursuant to Section 12(b) of the Exchange Act and all amendments or reports filed by us for the purpose of updating those descriptions.
|
All documents filed by us
pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the initial filing date of this prospectus, through the date
declared effective, until the termination of the offering of securities contemplated by this prospectus shall be deemed to be incorporated
by reference into this prospectus. These documents that we file later with the Securities and Exchange Commission and that are incorporated
by reference in this prospectus will automatically update information contained in this prospectus or that was previously incorporated
by reference into this prospectus. You will be deemed to have notice of all information incorporated by reference in this prospectus as
if that information was included in this prospectus.
We will provide to any person,
including any beneficial owner, to whom this prospectus is delivered, a copy of any or all of the information that has been incorporated
by reference in this prospectus but not delivered with this prospectus (excluding exhibits, unless the exhibits are specifically incorporated),
at no cost to the requesting party, upon request to us in writing or by telephone using the following information:
Howard Doong
Chief Executive Officer
ABVC BioPharma, Inc.
44370 Old Warm Springs Blvd.
Fremont, CA 94538
Tel: (510) 668-0881
Disclosure of Commission Position on Indemnification
for Securities Act Liabilities.
Insofar as indemnification
for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing
provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed
in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other
than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the
successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the
securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit
to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities
Act and will be governed by the final adjudication of such issue.
ABVC BioPharma, Inc.
$50,000,000
Common Stock
Preferred Stock
Debt Securities
Warrants
Rights
Units
November 16, 2021
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 14. Other Expenses of Issuance and Distribution.
The following table sets forth
the costs and expenses payable by the registrant in connection with this offering, other than underwriting commissions and discounts,
all of which are estimated except for the SEC registration fee.
|
Securities and Exchange Commission registration fee
|
|
$
|
4,635
|
|
|
Accounting fees and expenses
|
|
$
|
[●
|
]
|
|
Legal fees and expenses
|
|
$
|
[●
|
]
|
|
Miscellaneous expenses
|
|
$
|
*
|
|
|
Total
|
|
$
|
*
|
|
|
|
*
|
Estimated expenses are not presently known because they depend
upon, among other things, the number of offerings that will be made pursuant to this registration statement, the amount and type of securities
being offered and the timing of such offerings; we cannot compute the total until the exact expenses are known.
|
Item 15. Indemnification of Directors and
Officers.
As authorized by Section 78.751
of the Nevada Revised Statutes, we may indemnify our officers and directors against expenses incurred by such persons in connection with
any threatened, pending or completed action, suit or proceedings, whether civil, criminal, administrative or investigative, involving
such persons in their capacities as officers and directors, so long as such persons acted in good faith and in a manner which they reasonably
believed to be in our best interests. If the legal proceeding, however, is by or in our right, the director or officer may not be indemnified
in respect of any claim, issue or matter as to which he is adjudged to be liable for negligence or misconduct in the performance of his
duty to us unless a court determines otherwise.
Under Nevada law, corporations
may also purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director or officer
(or is serving at our request as a director or officer of another corporation) for any liability asserted against such person and any
expenses incurred by him in his capacity as a director or officer. These financial arrangements may include trust funds, self-insurance
programs, guarantees and insurance policies.
Additionally, our bylaws,
provide that we shall indemnify our directors and officers to the fullest extent not prohibited by the Nevada Revised Statutes. We also
have power to indemnify our employees and other agents as set forth in the Nevada Revised Statutes.
Our bylaws also provide that
we are required to advance to any person who was or is a party or is threatened to be made a party to any threatened, pending or completed
action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he is or was a director
or officer, of the corporation, or is or was serving at the request of the Company as a director or executive officer of another corporation,
partnership, joint venture, trust or other enterprise, prior to the final disposition of the proceeding, promptly following request therefor,
all expenses incurred by any director or officer in connection with such proceeding upon receipt of an undertaking by or on behalf of
such person to repay said amounts if it should be determined ultimately that such person is not entitled to be indemnified under the bylaws
or otherwise.
Neither our bylaws nor our
Articles of Incorporation, as amended, include any specific indemnification provisions for our officers or directors against liability
under the Securities Act. Additionally, insofar as indemnification for liabilities arising under the Securities Act may be permitted to
directors, officers and controlling persons of the Company pursuant to the foregoing provisions, or otherwise, the Company has been advised
that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities
Act and is, therefore, unenforceable.
Item 16. Exhibits.
The following documents are
filed as exhibits to this registration statement, including those exhibits incorporated herein by reference to a prior filing under the
Securities Act or the Exchange Act, as indicated in parentheses:
|
*
|
Filed herewith.
|
|
|
|
|
**
|
To be filed by amendment or as an exhibit to a filing with the SEC under Section 13 or 15(d) of the Securities Exchange Act of 1934 and incorporated by reference in connection with the offering of securities to the extent required for any such offering.
|
|
|
|
|
***
|
Incorporated by reference to the Registration Statement on Form S-3
filed on October 29, 2021.
|
|
|
|
|
+
|
To be filed pursuant to Rule 305(b)(2) of the Trust Indenture Act, if applicable.
|
Item 17. Undertakings
|
(a)
|
The undersigned registrant hereby undertakes:
|
|
|
(1)
|
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
|
|
|
(i)
|
To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
|
|
|
|
|
|
|
(ii)
|
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
|
|
|
|
|
|
|
(iii)
|
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
|
|
|
|
|
provided, however, Paragraphs (a)(1)(i),
(a)(1)(ii) and (a)(1)(iii) of this section do not apply if the registration statement is on Form S-3 or Form F-3 and the information required
to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by
the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the
registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
|
|
(2)
|
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
|
|
|
(3)
|
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
|
|
|
(4)
|
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
|
(A) Each prospectus filed
by the registrant pursuant to Rule 424(b)(3)shall be deemed to be part of the registration statement as of the date the filed prospectus
was deemed part of and included in the registration statement; and
(B) Each prospectus required
to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an
offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the
Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form
of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the
prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date
shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which
that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made
in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration
statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that
was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately
prior to such effective date; or
|
|
(5)
|
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
|
(i) Any preliminary prospectus
or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus
relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other
free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided
by or on behalf of the undersigned registrant; and
(iv) Any other communication
that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) That for purposes of determining any liability
under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the
Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section
15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a
new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed
to be the initial bona fide offering thereof.
(c) To supplement the prospectus, after the expiration
of any subscription period, to set forth the results of any subscription offer, the transactions by the underwriters during any subscription
period, the amount of unsubscribed securities to be purchased by the underwriters, and the terms of any subsequent reoffering thereof.
If any public offering by the underwriters is to be made on terms differing from those set forth on the cover page of the prospectus,
a post-effective amendment will be filed to set forth the terms of such offering.
(d) Insofar as indemnification for liabilities
arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to
the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission
such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for
indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer
or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer
or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the
matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification
by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements
of the Securities Act of 1933, ABVC BioPharma, Inc. certifies that it has reasonable grounds to believe that it meets all of the requirements
for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized,
in Taiwain on November 16, 2021.
|
|
ABVC BioPharma, Inc.
|
|
|
|
|
|
|
By:
|
/s/ Howard Doong
|
|
|
|
Howard Doong
|
|
|
|
President and Chief Executive Officer
|
POWER OF ATTORNEY
KNOW BY ALL MEN BY THESE PRESENTS
that each person whose signature appears below hereby constitutes and appoints Howard Doong as his true and lawful attorney-in-fact and
agent with full power of substitution and re-substitution, to act, without the other, for him and in his name, place and stead, in any
and all capacities, to sign any or all amendments (including post-effective amendments) to this Registration Statement, including any
subsequent registration statement for the same offering that may be filed under Rule 462(b), and to file the same, with all exhibits thereto,
and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents
full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises,
as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact
and agents, or any of them, their substitute may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements
of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates
indicated.
|
/s/ Howard Doong
|
|
President and Chief Executive Officer
|
|
November 16, 2021
|
|
Howard Doong
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Eugene Jiang
|
|
Chairman of the Board of Directors and Chief Business Officer
|
|
November 16, 2021
|
|
Eugene Jiang
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Chihliang An
|
|
Chief Financial Officer
|
|
November 16, 2021
|
|
Chihliang An
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Yen-Hsin Chou
|
|
Director
|
|
November 16, 2021
|
|
Yen-Hsin Chou
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Shin-Yu Miao
|
|
Director
|
|
November 16, 2021
|
|
Shin-Yu Miao
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Tsang-Ming Jiang
|
|
Director
|
|
November 16, 2021
|
|
Tsang-Ming Jiang
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Norimi Sakamoto
|
|
Director
|
|
November 16, 2021
|
|
Norimi Sakamoto
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Tsung-Shann Jiang
|
|
Chief Strategy Officer and Director
|
|
November 16, 2021
|
|
Tsung-Shann Jiang
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Chang-Jen Jiang
|
|
Director
|
|
November 16, 2021
|
|
Chang-Jen Jiang
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Yoshinobu Odaira
|
|
Director
|
|
November 16, 2021
|
|
Yoshinobu Odaira
|
|
|
|
|
II-5
ABVC BioPharma (NASDAQ:ABVC)
Historical Stock Chart
From Mar 2024 to Apr 2024
ABVC BioPharma (NASDAQ:ABVC)
Historical Stock Chart
From Apr 2023 to Apr 2024