This prospectus relates to the public offering of units (“Units”), with each Unit consisting of one share of the Company’s common stock, par value $0.01 per share, and one warrant (the “Warrants”) to purchase one share of common stock at a price of $4.25 per share. The Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. The shares of common stock and Warrants are immediately separable and will be issued separately in this offering. Each Warrant offered hereby is immediately exercisable on the date of issuance and will expire five years from the date of issuance.
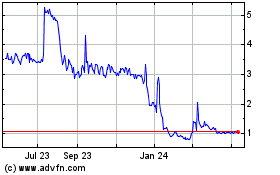
Through August 8, 2022, our common stock was quoted on the OTCPK, one of the OTC Markets Group over-the-counter markets, under the trading symbol “AIMD.” On August 5, 2022, the closing sale price for our common stock was $10.50 (after giving effect to our reverse stock split at a ratio of 1-for-15). As of August 9, 2022, our common stock and Warrants are listed on the Nasdaq Capital Market, or Nasdaq under the symbols “AIMD” and “AIMDW,” respectively.
Unless otherwise noted, the share and per share information in this prospectus reflects, other than in our financial statements and the notes thereto, a reverse stock split of our authorized and outstanding common stock at a ratio of 1-for-15 which became effective at 8:00pm ET on August 8, 2022.
Upon completion of this offering, assuming that the underwriters do not exercise their over-allotment or representative’s warrants, and none of our Warrants or other outstanding warrants or options to purchase our common stock are exercised, Ainos Inc., a Cayman Islands company, our principal shareholder and its affiliates will beneficially own approximately 68.4% of our common stock (or approximately 68% of our common stock if the over-allotment option is exercised) and we will be a “controlled company” within the meaning of the listing rules of The Nasdaq Stock Market LLC.
We have granted the underwriters a 45-day option to purchase up to an additional 117,000 shares of common stock at a purchase price of $4.24 per share and/or up to an additional 117,000 Warrants at a purchase price of $0.01 per Warrant, less in each case, the underwriting discount, solely to cover over-allotments, if any.
The underwriters expect to deliver the Units offered hereby to purchasers on or about August 11, 2022, subject to customary closing conditions.
The date of this prospectus is August 8, 2022.
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form S-1 that we filed with the SEC under the Securities Act. This prospectus does not contain all of the information included in the registration statement. For further information, we refer you to the registration statement, including its exhibits, filed with the SEC. Statements contained in this prospectus about the contents of any document are not necessarily complete. If SEC rules require that a document be filed as an exhibit to the registration statement, please see such document for a complete description of these matters. You should carefully read this prospectus, together with the additional information described under the headings “Where You Can Find More Information.”
Neither we nor the underwriter have authorized anyone to provide you with any information or to make any representations other than that contained in this prospectus or in any free writing prospectus we may authorize to be delivered or made available to you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. Neither we nor the underwriter are making an offer to sell securities in any jurisdiction in which the offer or sale is not permitted. The information in this prospectus is accurate only as of the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our shares of common stock and the information in any free writing prospectus that we may provide to you in connection with this offering is accurate only as of the date of that free writing prospectus. Our business, financial condition, results of operations and prospects may have changed since those dates.
For investors outside the United States: We have not and the underwriter has not, done anything that would permit this offering, or possession or distribution of this prospectus, in any jurisdiction where action for that purpose is required, other than in the United States. Persons who come into possession of this prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus applicable to those jurisdictions.
Unless otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate, including our general expectations and market position, market opportunity and market share, is based on information from our own management estimates and research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. In addition, assumptions and estimates of our and our industry’s future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause our future performance to differ materially from our assumptions and estimates. See “Cautionary Notice Regarding Forward-Looking Statements.”
This prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been, or will be, filed or incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the heading “Where You Can Find More Information.”
All product and company names are trademarks of their respective owners. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Throughout this prospectus, the terms “we,” “us,” “our,” and “our Company” and “the Company” refer to Ainos, Inc., a Texas corporation.
| | PROSPECTUS SUMMARY This summary highlights certain information about us, this offering and selected information contained elsewhere in this prospectus. Because this is only a summary, it does not contain all of the information that may be important to you or that you should consider before investing in our common stock. You should read the entire prospectus carefully, especially the information under “Risk Factors” set forth in this prospectus and the information included in any prospectus supplement or free writing prospectus that we have authorized for use in connection with this offering. This prospectus contains forward-looking statements, based on current expectations and related to future events and our future financial performance, that involve risks and uncertainties. Our actual results may vary materially from those discussed in the forward-looking statements as a result of various factors, including, without limitation, those set forth under “Risk Factors,” as well as other matters described in this prospectus. See “Cautionary Notice Regarding Forward-Looking Statements.” Except as otherwise indicated, all information in this prospectus assumes no exercise of the Underwriter’s over-allotment option to purchase additional Units from us in this offering. Overview We are a diversified medtech company focused on the development of novel point-of-care testing (POCT), low-dose interferon therapeutics and synthetic RNA (SRNA)-driven preventative medicine. Since inception, we have focused on the research of low-dose non-injectable interferon therapeutics. We have recently expanded our product candidates into POCT devices in order to diversify corporate revenue streams. Our POCT devices are based on core technologies involving COVID-19 POCT products and other POCT products that detect volatile organic compounds (VOC) emitted by the human body. We believe our core technologies empower a telehealth-enabled future for precision medicine with abilities to test anywhere, strengthen the immune system and expedite development of novel precision treatments. We believe the following attributes differentiate us from other diversified life science companies: | |
| | · | Intuitive Point-of-Care Testing | |
| | · | Telehealth-Friendly Rapid Testing | |
| | · | AI Powered VOC Testing Platform | |
| | · | Decades of Proprietary Interferon Clinical Research | |
| | · | Capital-Efficient Business Model | |
| | · | Outsourced Manufacturing | |
| | · | Global Distribution Partnership | |
| | Our product portfolio includes the following product and product candidates: COVID-19 Antigen Rapid Test Kit and Cloud-based Test Management Apps. Our cloud-based test management platform is comprised of the Ainos COVID-19 Antigen Rapid Test Kit, a personal management application, or app, and an enterprise management app. We anticipate our management apps will allow individuals and/or organizations to seamlessly manage tests, trace infections, and share results. As the first commercialized product we sell, we currently market the Ainos COVID-19 Antigen Rapid Test Kit in Taiwan under emergency use authorization (EUA) for healthcare professional use and self-test use issued by the Taiwan Federal and Drug Administration (TFDA) to TCNT, the product manufacturer. We expect TCNT to apply for EUA authorization from the U.S. Food and Drug Administration (“FDA”) sometime in the second half of 2022. COVID-19 Nucleic Acid Test Kit. This solution consists of a color-changing assay and a portable test equipment. The assay is compatible with most standard Polymerase Chain Reaction (PCR) machines and delivers test results within 40 minutes. We also expect to offer portable, low-cost test equipment intended to help medical professionals quickly scale testing capacity. Upon TCNT receiving regulatory approval, we will market the product under the Ainos brand name, with TCNT manufacturing the product. The candidate is currently under EUA review by the TFDA and we expect TCNT to submit for EUA review by the FDA sometime in the second half of 2022. VOC POCT - Ainos Flora. The Ainos Flora device is designed to perform a non-invasive test for female vaginal health and certain sexually transmitted diseases (STDs) including chlamydia, gonorrhea and trichomoniasis, within a few minutes. We expect Ainos Flora will provide convenient, discreet, rapid testing in a point-of-care setting which will allow women to self-test at home. We intend to collaborate with TCNT to conduct clinical trials in Taiwan, after which we expect TCNT will submit applications for TFDA approval for marketing in Taiwan and FDA 510(k) clearance for marketing in the U.S. sometime in the second half of 2022. | |
| | VOC POCT - Ainos Pen. Our Ainos Pen device is a cloud-connected, multi-purpose, portable breath analyzer that is intended to monitor health conditions including oral, gastrointestinal, liver, and renal health within minutes. We expect consumers to be empowered to share their self-test results with their physicians through in-person and telehealth medical consultations. We intend to explore commercialization opportunities with third-party collaborators as a consumer health device sometime in the second half of 2022. VOC POCT - CHS430. The CHS430 device is intended to provide non-invasive testing for ventilator-associated pneumonia within 10 minutes. We plan to be the exclusive sales agent for CHS430, pursuant to our Product Development Agreement with our co-developer, TCNT. We intend to collaborate with TCNT to conduct clinical trials in Taiwan, after which we expect TCNT to submit applications for TFDA approval for marketing in Taiwan and FDA 510(k) clearance for marketing in the U.S. in 2023. Very Low-Dose Oral Interferon Alpha (VELDONA). VELDONA is a low-dose oral interferon alpha (IFN-α) formulation based on our nearly four decades of research on IFN-α’s potential treatment applications. Our leading product candidate is a low-dose oral treatment for COVID-19. We have conducted a parallel study based on VELDONA alone and joint study with Innopharmax, Inc. We recently completed our animal studies and subsequently we plan to initiate Phase 2/3 clinical trials for the VELDONA-only program sometime in mid-2022 in Taiwan. We also plan to advance our VELDONA development efforts for disease indications such as thrombocytopenia and Sjögren’s syndrome in 2023. Synthetic RNA (SRNA). We are developing a SRNA technology platform in Taiwan. Our initial focus is to develop a potential COVID-19 mRNA vaccine platform using the full-length spike or the receptor-binding domain (RBD) gene sequence of the alpha and delta variants as reference sequences. We plan to continue developing these technologies with the goal of initiating clinical trials in Taiwan in 2023. Growth Strategy Key elements of our growth strategy include innovating and expand our applications; creating multiple revenue streams; driving ecosystem adoption; expanding our installed base; broadening our global footprint; increasing value-added services; and scaling production with manufacturing partners. We expect to employ several core growth strategies: | |
| | · | Execute rollout of our multi-staged technology roadmap | |
| | · | Launch POCTs through a staged regulatory approval approach | |
| | · | Build an AI Nose technology platform | |
| | · | Establish a VOC analytics service | |
| | · | Implement a focused approach on the development of low-dose interferon therapeutics | |
| | · | Invest in SRNA research | |
| | · | Expand global footprint through distributors | |
| | · | Work with select manufacturers | |
| | Recent Developments The following are highlights of recent major corporate milestones that we believe will serve as catalysts for us to develop and commercialize a multi-faceted product portfolio pipeline over the next several years: In order to facilitate our diversification strategy through development and commercialization of an expanded base of product offerings we secured a strategic investor, Ainos Inc., a Cayman Islands company focused on the development of intellectual property and patent assets for point-of-care testing diagnostics (“Ainos KY”), in early 2021. In exchange for majority interest in our company, we acquired an intellectual property portfolio from Ainos KY. See “Our Corporate History and Structure.” Through this relationship, we have implemented several strategic initiatives over the past year to augment our product development pipeline and achieve a strengthened financial position. | |
| | In June 2021, we became the master sales and marketing agent for the Ainos COVID-19 Antigen Rapid Test Kit and Ainos COVID-19 Nucleic Acid Test Kit. Since then we began marketing Ainos COVID-19 Antigen Rapid Test Kit in Taiwan. We appointed Inabata as a non-exclusive worldwide distributor of our products candidates upon commercialization under a five year distribution agreement commencing November 2021. We also entered into a Memorandum of Understanding effective November 2021 with Inabata’s subsidiary, Taiwan Inabata Sangyo Co., Ltd. (“Taiwan Inabata”), under which Taiwan Inabata will coordinate business development, working capital, logistics, and coordination of procurement of raw materials in support of our products upon commercialization. In December 2021, we entered into a strategic relationship with InnoPharmax, Inc., a biopharmaceutical company focused on new oral drug formulations, to jointly develop and promote an orally administered CICCT for the treatment of COVID-19 and potentially other viral infections. During 2021, Ainos KY provided us $3,000,000 principal amount of working capital advances in exchange for notes convertible at Ainos KY’s election into shares of our common stock at a conversion price of $3.00 per share, subject to adjustment. Ainos KY intends to convert the notes into shares of common stock immediately prior to completion of this offering See “Certain Relationships and Related Party Transactions” in this prospectus for more information. In January 2022, we acquired additional intellectual property and equipment assets from Ainos KY including technical know-how, medical device manufacturing, testing and office equipment in Taiwan and hired certain of Ainos KY’s R&D personnel in exchange for a $26,000,000 convertible note (the “APA Convertible Note”). The APA Convertible Note will automatically convert into shares of our common stock immediately prior to the closing of this offering at a conversion price equal to 80% of the per Unit public offering price. See “Our Corporate History and Structure.” In March 2022, we issued a non-convertible note to Ainos KY in the principal amount of $800,000, at a 1.85% per annum interest rate, with a maturity date of February 28, 2023. We intend to use the proceeds from the note for working capital. From March 28 to April 11, 2022, we issued non-interest bearing convertible notes in the aggregate principal amount of $1,400,000 due on March 30, 2027 (the “March 2027 Convertible Notes”) to certain investors, including a note in the principal amount of $500,000 to ASE Test Inc., a minority owner of Ainos KY. The March 2027 Convertible Notes will automatically convert into shares of our common stock immediately prior to the closing of this offering at a conversion price equal to 80% of the per Unit public offering price. Listing on the Nasdaq Capital Market Through August 8, 2022, our common stock was quoted on the OTCPK, one of the OTC Markets Group over-the-counter markets, under the trading symbol “AIMD.” As of August 9, 2022, our common stock and Warrants are listed on the Nasdaq Capital Market, or Nasdaq under the symbols “AIMD” and “AIMDW,” respectively. . Reverse Stock Split We previously obtained the written consent of the holder of a majority of the outstanding shares of our common stock to effect an amendment to our restated certificate of incorporation to effect a reverse stock split of our common stock at a ratio to be determined by our Board prior to the effective time of the amendment of not more than 1-for-50. The reverse stock split will not impact the number of authorized shares of common stock which will remain at 300,000,000 shares. In connection with the offering to which this registration statement on Form S-1 and prospectus relate, our Board determined to effect a reverse stock split of our common stock at a ratio of 1-for 15 which became effective at 8:00pm ET on August 8, 2022. Unless otherwise noted, the share and per share information in this prospectus reflects, other than in our financial statements and the notes thereto, a reverse stock split of our authorized and outstanding common stock at a ratio of 1-for-15. | |
| | Our Corporate History and Structure Ainos, Inc. (f/k/a Amarillo Biosciences, Inc.), is a Texas corporation formed on June 26, 1984. Under our former name of Amarillo Biosciences, Inc., we completed an initial public offering on the Nasdaq SmallCap Market in August 1996 and have traded on the U.S. over-the-counter market since October 1999. On October 31, 2013, we filed a voluntary petition for reorganization under Chapter 11 of the United States bankruptcy code. We emerged from bankruptcy on January 23, 2015. In 2017, we created a Taiwan branch office, Ainos Inc. Taiwan Branch (USA) (“Taiwan Branch”), to conduct research and development, including clinical trials, in Taiwan. The Taiwan Branch also serves as an operational hub to access Asian markets. In December 2020, we entered into a securities purchase agreement with Ainos KY which closed on April 15, 2021. Pursuant to the securities purchase agreement, we acquired exclusive rights and ownership of certain of Ainos KY’s intellectual property and patent assets in the areas of VOC sensing and diagnostics. We issued 8,333,333 shares of our common stock to Ainos KY, resulting in Ainos KY owning approximately 70.3% of our outstanding shares. As part of the transaction, we also increased our authorized common stock to 300,000,000 shares and changed our name to Ainos Inc. In November 2021, we entered into an asset purchase agreement with Ainos KY, which closed on January 30, 2022, pursuant to which we acquired additional intellectual property assets of Ainos KY in the area of COVID-19 diagnostics and VOCs in exchange for a non-interest bearing convertible note in the principal amount of $26,000,000. The note will convert into shares of common stock immediately prior to closing of the offering at a conversion price equal to 80% of the per Unit offering price. Ainos KY currently holds 48.5% of our outstanding shares and following conversion of the convertible notes held by Ainos KY and the issuance and sale of the shares in this offering, will own 61.4% of our then outstanding shares. The following diagram illustrates our current corporate structure: 
| |
| | Controlled Company Upon the completion of this offering, Ainos KY, our principal shareholder, and its affiliates will beneficially own approximately 68.4% of our common stock (approximately 68.0% if the over-allotment option is exercised in full) and we will be a “controlled company” within the meaning of the listing rules of The Nasdaq Stock Market LLC. As long as our principal shareholder owns at least 50% of the voting power of our Company, we are a “controlled company” as defined under Nasdaq Listing Rules. As a controlled company, we are permitted to rely on certain exemptions from Nasdaq’s corporate governance rules, including: | |
| | · | an exemption from the rule that a majority of our board of directors must be independent directors; | |
| | · | an exemption from the rule that the compensation of our chief executive officer must be determined or recommended solely by independent directors; and | |
| | · | an exemption from the rule that our director nominees must be selected or recommended solely by independent directors. | |
| | The majority of our Board consists of non-independent directors. Although we have elected to be deemed a controlled company our Compensation Committee is composed of two independent directors and our Audit Committee is composed of three independent directors of which two members are qualified as an “audit committee expert” as defined by the SEC. We do not have a Nominations and Corporate Governance Committee and our Board is responsible for nominations and corporate governance. Pursuant to our Corporate Governance Policies the Board is responsible for filling Board vacancies and nominations of candidates to the Board. As a result, you will not have the same protection afforded to shareholders of companies that are subject to these corporate governance requirements. Implications of Being a Smaller Reporting Company We are a “smaller reporting company” as defined in the Securities Exchange Act of 1934, as amended, or the Exchange Act, and have elected to take advantage of certain of the scaled disclosures available to smaller reporting companies. Accordingly we may provide less public disclosure than larger public companies, including the inclusion of only two years of audited consolidated financial statements and only two years of management’s discussion and analysis of financial condition and results of operations disclosure and the inclusion of reduced disclosure about our executive compensation arrangements. As a smaller reporting company, we are also exempt from compliance with the auditor attestation requirements pursuant to the Sarbanes-Oxley Act. . As a result, the information that we provide to our shareholders may be different than you might receive from other public reporting companies in which you hold equity interests. We will continue to be a “smaller reporting company” until we have $250 million or more in public float (based on our common stock) measured as of the last business day of our most recently completed second fiscal quarter or, in the event we have no public float or a public float (based on our common stock) that is less than $700 million, annual revenues of $100 million or more during the most recently completed fiscal year. Going Concern Our independent registered public accounting firm, PWR CPA, LLP, has expressed doubt in their audit opinion for our financial statements for the year ended December 31, 2021 about our ability to continue as a going concern. We have generated minimal revenue and have an accumulated deficit totaling $10,108,916 since inception. In order to obtain the necessary capital to sustain operations, management’s plans include, among other things, the possibility of pursuing new equity sales and/or making additional debt borrowings, There can be no assurances, however, that we y will be successful in obtaining additional financing, or that such financing will be available on favorable terms, if at all. If we are unable to obtain financing in the amounts and on terms deemed acceptable, the business and future success may be adversely affected and we may cease operations. | |
| | Corporate Information Our principal executive offices are located at 8880 Rio San Diego Drive, Ste. 800, San Diego, CA 92108, and our telephone number is (858) 869-2986. We maintain a website at www.ainos.com. Information contained on or accessible through our website is not, and should not be considered, part of, or incorporated by reference into, this prospectus. Summary Risk Factors Participating in this offering involves substantial risk. Our ability to execute our strategy is also subject to certain risks. You should carefully consider all of the information set forth in this prospectus and, in particular, should evaluate the specific factors set forth under the heading “Risk Factors” in deciding whether to invest in our securities. These risks include, but are not limited to, the following: Risks related to our limited operating history, financial position, and need for additional capital | |
| | · | We have a history of operating losses that are expected to continue for the foreseeable future, and we are unable to predict the extent of future losses, or whether we will generate significant revenues or achieve or sustain profitability. | |
| | · | Our revenue for at least the near term will almost exclusively depend on sales of our COVID-19 test kits until we can develop, obtain regulatory clearance or other appropriate authorization for, and commercialize additional product candidates. | |
| | · | We have generated very little revenue from product sales and may never become profitable. | |
| | · | We need to raise additional capital to operate our business. If we fail to obtain the capital necessary to fund our operations, we will be unable to continue or complete our product development. | |
| | · | We may be unable to access the capital markets and even if we can raise additional funding, we may be required to do so on terms that are dilutive to you. | |
| | · | Our operating results may fluctuate significantly, which will make our future results difficult to predict and could cause our results to fall below expectations. | |
| | | | |
| | Risks related to product development and regulatory process | |
| | | | |
| | · | We and/or our collaboration partner have conducted and intend to conduct clinical trials for selected product candidates at sites outside the United States, and for any of our product candidates for which we seek approval in the United States, the FDA may not accept data from trials conducted in such locations or may require additional U.S.-based trials. | |
| | · | Our long-term prospects depend in part upon discovering, developing and commercializing additional products, including diagnostic testing devices, which may fail in development or suffer delays that adversely affect their commercial viability. | |
| | · | Even if a current or future product candidate, including medical device products, receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success | |
| | · | We, or TCNT, may not obtain approval for our product candidates in any jurisdictions. | |
| | · | Even if we are able to commercialize any product candidates, such products may become subject to unfavorable pricing regulations or third-party coverage and reimbursement policies, which would harm our business. | |
| | · | Any disruption in our research and development facilities could adversely affect our business, financial condition and results of operations. | |
| | · | If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of any approved products. | |
| | · | Our business and operations would be adversely affected in the event that our computer systems or those of our partners, contract research organizations, contractors, consultants or other third parties we work with were to suffer system failures, cyber-attacks, loss of data or other security incidents. | |
| | | |
| | Risks related to reliance on third parties | |
| | | |
| | · | We may form or seek strategic partnerships or enter into additional licensing arrangements in the future, and we may not realize the benefits of such alliances or licensing arrangements. | |
| | · | Our employees, independent contractors, consultants, commercial or strategic partners, principal investigators or CROs may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading, which could have a material adverse effect on our business. | |
| | | |
| | Risks related to intellectual property, patents, and data privacy | |
| | | |
| | · | Intellectual property rights vary across foreign jurisdictions, and we may not be able to protect our intellectual property rights throughout the world. | |
| | · | If we and our collaborators are unable to obtain and maintain sufficient patent and other intellectual property protection for our product candidates and technology, our competitors could develop and commercialize products and technology similar or identical to ours, and we may not be able to compete effectively in our market or successfully commercialize any product candidates we may develop. | |
| | · | We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time-consuming and unsuccessful, and issued patents directed towards our technology and product candidates could be found invalid or unenforceable if challenged. | |
| | · | Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time. | |
| | · | If we are unable to protect the confidentiality of our trade secrets, our business and competitive position could be harmed. | |
| | · | Changes in patent laws could diminish the value of patents in general, thereby impairing our ability to protect our product candidates. | |
| | | |
| | Risks related to related to our business | |
| | | |
| | · | We will need to increase the size of our Company and may not effectively manage our growth. | |
| | · | Our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel. | |
| | · | The POCT market is extremely competitive and rapidly evolving, making it difficult to evaluate our business and future prospects. | |
| | · | Our business entails a significant risk of product liability and if we are unable to obtain sufficient insurance coverage such inability could have an adverse effect on our business and financial condition. | |
| | · | Our business activities may be subject to the U.S. Foreign Corrupt Practices Act, or the FCPA, and similar anti-bribery and anti-corruption laws of other countries in which we operate, including Taiwan, as well as U.S. and certain foreign export controls, trade sanctions, and import laws and regulations. Compliance with these legal requirements could limit our ability to compete in foreign markets and subject us to liability if we violate them. | |
| | | |
| | Risks related to our securities | |
| | | |
| | · | An active trading market for our common stock may not develop and the market price of our common stock could be volatile. | |
| | · | We do not intend to pay dividends for the foreseeable future and, as a result, our ability to achieve a return on your investment will depend on appreciation in the price of our common stock. | |
| | · | Our controlling shareholder, executive officers, directors, and their affiliates will continue to exercise significant influence over our Company after this offering, which will limit your ability to influence corporate matters and could delay or prevent a change in corporate control. | |
| | · | As a “controlled company” under the rules of the Nasdaq Capital Market, we may choose to exempt our company from certain corporate governance requirements that could have an adverse effect on our public shareholders. | |
| | · | We have acquired, and may in the future acquire, assets and technologies as part of our business strategy. | |
| | · | If we acquire companies or technologies in the future, they could prove difficult to integrate, disrupt our business, dilute stockholder value, and adversely affect our operating results and the value of our common stock. | |
| | · | Any failure to maintain effective internal control over financial reporting could harm us. | |
| | · | Our issuance of additional capital stock in connection with financings, acquisitions, investments, our 2021 Stock Incentive Plan or otherwise will dilute all other stockholders. | |
| | · | Our management has broad discretion in the use of proceeds from our offering and our use may not produce a positive rate of return. | |
| | | | |
| The Offering |
| | | | | |
| | Units being offered by us........................................................ | | 780,000 Units. Each Unit consists of one share of common stock and one Warrant to purchase one share of common stock. The Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. The common stock and Warrants will be immediately separable and will be issued separately in this offering. | |
| | | | | |
| | Offering Price | | $4.25 per Unit. | |
| | | | | |
| | Common Stock Outstanding Before this Offering: | | 9,625,287 shares of common stock | |
| | | | | |
| | Total shares of common stock outstanding immediately after this offering | | 19,478,423 shares of common stock (or 19,595,423 shares of common stock if the underwriters exercise their over-allotment option in full, and assuming in each case, no exercise of the Warrants). | |
| | | | | |
| | Description of the Warrants | | The Warrants will have an exercise price of $4.25 per share of common stock, will be immediately exercisable and will expire five years from the date of issuance. Each Warrant is exercisable for one share of common stock, subject to adjustment in the event of stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our common stock. A holder may not exercise any portion of a Warrant to the extent that the holder, together with its affiliates and any other person or entity acting as a group, would own more than 4.99% of our outstanding shares of common stock after exercise, as such ownership percentage is determined in accordance with the terms of the Warrants, except that upon notice from the holder to us, the holder may waive such limitation up to a percentage, not in excess of 9.99%. This prospectus also relates to the offering of the common stock issuable upon exercise of the Warrants. To better understand the terms of the Warrants, you should carefully read the “Description of Capital Stock” section of this prospectus. You should also read the form of Warrant, which is filed as an exhibit to the registration statement that includes this prospectus. | |
| | | | | |
| | Over-Allotment Option | | Pursuant to the underwriting agreement, we granted to the underwriter an option, exercisable within 45 days after the closing of this offering to acquire up to an additional 117,000 shares of common stock and/or up to an additional 117,000 Warrants, in each case, solely for the purpose of covering over-allotments, if any. | |
| | | | | |
| | Representative’s Warrants | | We will issue to the representative of the underwriters as compensation, upon closing of this offering, the Representative’s Warrants entitling the representative to purchase a number of shares of common stock equal to 5% of the aggregate number of shares of common stock issued in this offering, including shares of common stock issued pursuant to the exercise of the over-allotment option at an exercise price of $4.68 per share (110% of the price per Unit offered hereby). The Representative’s Warrants will have a term of five years and may be exercised at any time and from time to time, in whole or in part, during the four and one half year period commencing 180 days from the effective date of the registration statement of which this prospectus is a part. This prospectus also relates to the offering of shares of common stock issuable upon exercise of the Representative’s Warrants. | |
| | | | | |
| | Lock-up | | We, each of our officers, directors and 5% or more holders of our outstanding common stock as of the effective date of this prospectus (and all holders of securities exercisable for or convertible into shares of common stock) have agreed to enter into customary “lock-up” agreements in favor of the underwriters pursuant to which such persons and entities have agreed, not to offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any securities of the Company without the prior written consent of Maxim Group LLC, (excluding the issuance of shares of common stock upon the exercise of currently outstanding equity awards under the our employee benefit plans) for a period of 180 days from the effective date of this prospectus. See “Underwriting” for additional information. | |
| | | | | |
| | Use of Proceeds | | We estimate that we will receive net proceeds of approximately $2.1 million from our sale of Units in this offering (or $2.6 million if the underwriters exercise their over-allotment in full) after deducting underwriting discounts and estimated offering expenses payable by us. We intend to use the net proceeds of this offering for product commercialization, technology development, commercial scale up of our activities, clinical trials and working capital and other general corporate purposes. See “Use of Proceeds.” | |
| | | | | |
| | Nasdaq Symbol | | Through August 8, 2022, our common stock was quoted on the OTCPK, one of the OTC Markets Group over-the-counter markets, under the trading symbol “AIMD.” As of August 9, 2022, our common stock and Warrants are listed on the Nasdaq Capital Market, or Nasdaq under the symbols “AIMD” and “AIMDW,” respectively | |
| | | | | |
| | Risk Factors | | Investing in our securities involves a high degree of risk. You should carefully review and consider “Risk Factors” beginning on page 11 of this prospectus. | |
| | | | | |
| | Transfer Agent, Registrar and Warrant Agent | | Our transfer agent and registrar for our common stock and our warrant agent for the Warrants is American Stock Transfer & Trust Company, LLC. | |
| | | | | |
| | Dividend Policy | | We have never declared or paid any cash dividends on our common stock. We do not anticipate paying any cash dividends in the foreseeable future. | |
| | | | | |
| | Assumptions Used Throughout This Prospectus Unless otherwise stated in this prospectus, the total number of shares of common stock outstanding after this offering is based on 9,625,287 shares outstanding following the reverse stock split, and includes the issuance of 9.073,137 shares of common stock upon the conversion of the APA Convertible Note, the March 2027 Convertible Notes and $3,000,000 aggregate principal amount of convertible notes plus accrued interest thereon held by Ainos KY immediately prior to completion of this offering. Unless otherwise stated in this prospectus, the total number of shares of common stock outstanding excludes the following: | |
| | | |
| | · | 144,644 shares of common stock issuable upon the conversion of outstanding convertible promissory notes at a weighted average conversion price of approximately $2.70 per share, which are not converting concurrently with the consummation of this offering; | |
| | · | 30,174 shares of common stock issuable upon the exercise of outstanding warrants at an exercise price of $4.05 per share; | |
| | · | 36,666 shares of common stock issuable upon the exercise of outstanding equity awards under our 2018 Officers, Directors, Employees and Consultants Nonqualified Stock Option Plan at a weighted average exercise price of $5.70 per share; | |
| | · | 1,333,333 shares of common stock reserved for future issuance under our 2021 Stock Incentive Plan; | |
| | · | 50,000 shares of common stock reserved for future issuance under our 2021 Employee Stock Purchase Plan; | |
| | · | 39,000 shares of common stock, underlying the Representative’s Warrants to be issued to the representative of the underwriters at an exercise price of $4.68 per share; and | |
| | · | 780,000 shares of our common stock underlying the Warrants to be issued in this offering. | |
| | Unless otherwise indicated, this prospectus reflects and assumes the following: | |
| | | | |
| | · | no exercise of outstanding options or warrants described above; | |
| | · | no exercise of the Warrants; and | |
| | · | no exercise by the underwriter of its over-allotment option. | |
| | | | |
RISK FACTORS
Investing in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below in addition to the other information contained in this prospectus and any prospectus supplement before deciding whether to invest in shares of our common stock. If any of the following risks occur, our business, financial condition or operating results could be harmed. In that case, the trading price of our common stock could decline and you may lose part or all of your investment. In the opinion of management, the risks discussed below represent the material risks known to us. Additional risks and uncertainties not currently known to us or that we currently deem immaterial may also impair our business, financial condition and operating results and adversely affect the market price of our common stock.
Risks related to our limited operating history, financial position, and need for additional capital
We have a history of operating losses that are expected to continue for the foreseeable future, and we are unable to predict the extent of future losses, or whether we will generate significant revenues or achieve or sustain profitability.
We are focused on product development and have generated minimal revenue to date. Additionally, we expect to continue to incur operating losses until we are able to commercialize or license our products. These operating losses have adversely affected and are likely to continue to adversely affect our working capital, total assets and stockholders’ deficit. We have generated operating losses of $2,083,062 and $522,109 in the three months ended March 31, 2022 and 2021, respectively. As of December 31, 2021, we had net operating loss carryforwards of approximately $20,747,517 for federal income tax purposes expiring in 2022 through 2041. We expect to make substantial expenditures and incur increasing operating costs in the future and our accumulated deficit will increase significantly as we expand development and clinical trial activities for our product candidates. Because of the risks and uncertainties associated with product development, we are unable to predict the extent of any future losses, whether we will ever generate significant revenues or if we will ever achieve or sustain profitability.
We believe that our cash on hand, along with the anticipated net proceeds from the sale of products and additional financing, including this offering, will enable us to fund our operations over the medium term based on our current plan. We are dependent on obtaining, and are continuing to pursue, necessary funding from outside sources, including obtaining additional funding from the issuance of securities in order to continue our operations. Without adequate funding, we may not be able to meet our obligations. The successful commercialization of any of our products will require us to perform a variety of functions, including:
| | · | continuing to undertake preclinical and clinical development; |
| | · | engaging in the development of product candidate formulations and manufacturing processes; |
| | · | interacting with the applicable regulatory authorities and pursuing other required steps for regulatory approval; |
| | · | engaging with payors and other pricing and reimbursement authorities; |
| | · | submitting marketing applications to and receiving approval from the applicable regulatory authorities; and |
| | · | manufacturing the applicable products and product candidates in accordance with regulatory requirements and, if ultimately approved, conducting sales and marketing activities in accordance with health care, Taiwan Food and Drug Administration (the “TFDA”), the FDA, and similar foreign regulatory authority laws and regulations. |
Our revenue for at least the near term will almost exclusively depend on sales of the Ainos COVID-19 test kits until we can develop, obtain regulatory clearance or other appropriate authorization for, and commercialize additional product candidates.
We expect that sales of the Ainos COVID-19 Antigen Rapid Test Kits will account for majority of our revenue until at least such time as we can commercialize additional tests or other products. As a result, our ability to execute our growth strategy and become profitable in the near term will depend upon consumer adoption of the Ainos COVID-19 Antigen Rapid Test Kits. We currently have a very small number of customers for the Ainos COVID-19 Antigen Rapid Test Kits in Taiwan. We may not be able to successfully acquire new customers in a timely manner or at all. If we are unable to expand our customer base, we may not be able to increase our revenue. Adoption and use of the Ainos COVID-19 Antigen Rapid Test Kits will depend on several factors, including, but not limited to the accuracy, affordability and ease of use of our product as compared to other products and products that compete with the Ainos COVID-19 Antigen Rapid Test Kits.
Because we expect virtually all of our revenue for at least the near term to be generated from sales of the Ainos COVID-19 Antigen Rapid Test Kits in Taiwan, the failure of the Ainos COVID-19 Antigen Rapid Test Kits to gain market acceptance or retain regulatory authorization under our EUA in Taiwan may have a material adverse effect on our business, operating results and financial condition.
We have generated very little revenue from product sales and may never become profitable.
Our ability to generate product sales and achieve profitability depends on our ability, alone or with collaborative partners, to successfully complete the development of, and obtain the regulatory approvals necessary to commercialize our current and future product candidates. Our product candidates will require additional clinical, manufacturing, and non-clinical development, regulatory approval, commercial manufacturing arrangements, establishment of a commercial organization, significant marketing efforts, and further investment before we generate significant product sales.
We cannot assure you that we will meet our timelines for our development programs, which may be delayed or not completed for a number of reasons. Our ability to generate future revenues from product sales depends heavily on our, or our collaborators’, ability to successfully:
| | · | complete research and obtain favorable results from preclinical and clinical development of our current and future product candidates, including addressing any clinical holds that may be placed on our development activities by regulatory authorities; |
| | · | seek and obtain regulatory and marketing approvals for any of our product candidates for which we complete clinical trials, as well as their manufacturing facilities; |
| | · | launch and commercialize any of our product candidates for which we obtain regulatory and marketing approval by establishing a sales force, marketing, and distribution infrastructure or, alternatively, collaborating with a commercialization partner; |
| | · | qualify for coverage and establish adequate reimbursement by government and third-party payors for any of our product candidates for which we obtain regulatory and marketing approval; |
| | · | develop, maintain, and enhance a sustainable, scalable, reproducible, and transferable manufacturing process for the product candidates we may develop; |
| | · | establish and maintain supply and manufacturing capabilities or capacities internally or with third parties that can provide adequate, in both amount and quality, products, and services to support clinical development and the market demand for any of our product candidates for which we obtain regulatory and marketing approval; |
| | · | obtain market acceptance of current or any future product candidates and effectively compete to establish market share; |
| | · | maintain a continued acceptable safety and efficacy profile of our product candidates following launch; |
| | · | address competing technological and market developments; |
| | · | implement internal systems and infrastructure, as needed; |
| | · | negotiate favorable terms in any collaboration, licensing, or other arrangements into which we may enter and performing our obligations in such collaborations; |
| | · | maintain, protect, enforce, defend, and expand our portfolio of intellectual property rights, including patents, trade secrets, and know-how; |
| | · | avoid and defend against third-party interference, infringement, and other intellectual property claims; and |
| | · | attract, hire, and retain qualified personnel. |
Even if one or more of our current and future product candidates are approved for commercial sale, we anticipate incurring significant costs associated with commercializing any approved product candidate. Our expenses could increase beyond our expectations if we are required by the TFDA, the FDA or other regulatory authorities to perform clinical and other studies in addition to those that we currently anticipate. If we are required to conduct additional clinical trials or other testing of our product candidates that we develop beyond those that we currently expect, if we are unable to successfully complete clinical trials of our product candidates or other testing, if the results of these trials or tests are not positive or are only modestly positive, or if there are safety concerns, we may be delayed in obtaining marketing approval for our product candidates, not obtain marketing approval at all, or obtain more limited approvals. Even if we are able to generate revenues from the sale of any approved product candidates, we may not become profitable and may need to obtain additional funding to continue operations.
Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of the Company and could impair our ability to raise capital, maintain our research and development efforts, expand our business or continue our operations. A decline in the value of our Company also could cause you to lose all or part of your investment.
Our business, operations and clinical development plans and timelines and supply chain could be adversely affected by the effects of epidemics, including the ongoing COVID-19 pandemic.
Our business could be adversely affected by health epidemics wherever we have clinical trial sites or other business operations. In addition, health epidemics could cause significant disruption in the operations of third-party manufacturers, contract research organizations and other third parties upon whom we rely. For example, the COVID-19 pandemic has presented a substantial public health and economic challenge around the world and is affecting employees, patients, communities and business operations, as well as the U.S. economy and financial markets. Many geographic regions have imposed, or in the future may impose, “shelter-in-place” orders, quarantines or similar orders or restrictions to control the spread of COVID-19. These measures may negatively impact productivity, disrupt our business and delay our clinical programs and timelines, the magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course. These and similar, and perhaps more severe, disruptions in our operations could negatively impact our business, operating results and financial condition.
We are dependent on a worldwide supply chain for products to be used in our clinical trials and, if approved by the regulatory authorities, for commercialization. Quarantines, shelter-in-place and similar government orders, or the expectation that such orders, shutdowns or other restrictions could occur, whether related to COVID-19 or other infectious diseases, could impact personnel at third-party manufacturing facilities in the United States and other countries, or the availability or cost of materials, which could disrupt our supply chain. For example, any manufacturing supply interruption of any product candidate could adversely affect our ability to conduct ongoing and future clinical trials of such product candidate. In addition, closures of transportation carriers and modal hubs could materially impact our clinical development and any future commercialization timelines.
If our relationships with our suppliers or other vendors are terminated or scaled back as a result of the COVID-19 pandemic or other health epidemics, we may not be able to enter into arrangements with alternative suppliers or vendors or do so on commercially reasonable terms or in a timely manner. Switching or adding additional suppliers or vendors involves substantial cost and requires management time and focus. In addition, there is a natural transition period when a new supplier or vendor commences work. As a result, delays could generally occur, which could adversely impact our ability to meet our desired clinical development and any future commercialization timelines. See “Risks Related to Our Dependence on Third Parties.”
In addition, our clinical trials have been and may continue to be affected by the COVID-19 pandemic. Clinical site initiation and patient enrollment may be delayed due to prioritization of hospital resources toward the COVID-19 pandemic or concerns among patients about participating in clinical trials during a pandemic and public health measures imposed by the respective national governments of countries in which the clinical sites are located. Some patients may have difficulty following certain aspects of clinical trial protocols if quarantines impede patient movement or interrupt healthcare services. Similarly, our inability to successfully recruit and retain patients and principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19 or experience additional restrictions by their institutions, city or state governments could adversely impact our clinical trial operations.
We are continuing to monitor the potential impact of the pandemic, but we cannot be certain of the future impact on our business, financial condition, results of operations and prospects. Depending on developments relating to the pandemic, including the emergence of new variants, the pandemic may affect our ability to initiate and complete research studies, delay the initiation of our future research studies, disrupt regulatory activities or have other adverse effects on our business, results of operations, financial condition and prospects.
The global pandemic of COVID-19 continues to evolve rapidly. The ultimate impact of the COVID-19 pandemic or a similar health epidemic is highly uncertain and subject to change. We do not yet know the full extent of potential delays or impacts on our business, our clinical trials, healthcare systems or the global economy as a whole. However, these effects could have a material impact on our operations, and we will continue to monitor the COVID-19 situation closely.
We need to raise additional capital to operate our business. If we fail to obtain the capital necessary to fund our operations, we will be unable to continue or complete our product development.
We are a company primarily focused on product development and have generated little product revenues to date. Until, and if, we receive approval from the TFDA, FDA and other regulatory authorities for our product candidates, we cannot sell our products and will not have product revenues. We had cash and cash equivalents of approximately $1,871,349 as of March 31, 2022, and we will need to continue to seek capital from time to time to continue to capitalize the development and commercialization of our product candidates and to acquire and develop other product candidates. Our actual capital requirements will depend on many factors. For instance, our business or operations may change in a manner that would consume available funds more rapidly than anticipated and substantial additional funding may be required to maintain operations, fund expansion, develop new or enhanced products, acquire complementary products, business or technologies or otherwise respond to competitive pressures and opportunities, such as a change in the regulatory environment or a change in COVID-19 treatment modalities. If we experience unanticipated cash requirements, we may need to seek additional sources of financing, which may not be available on favorable terms, if at all.
However, we may not be able to secure funding when we need it or on favorable terms. If we cannot raise adequate funds to satisfy our capital requirements, we will have to delay, scale-back or eliminate our research and development activities, clinical studies or future operations, we may be unable to complete planned nonclinical studies and clinical trials or obtain approval of our product candidates from the TFDA, FDA and other regulatory authorities. In addition, we could be forced to discontinue product development, reduce or forego sales and marketing efforts and attractive business opportunities, reduce overhead, or discontinue operations. We may also be required to obtain funds through arrangements with collaborators, which arrangements may require us to relinquish rights to certain technologies or products that we otherwise would not consider relinquishing, including rights to future product candidates or certain major geographic markets. We may further have to license our technology to others. This could result in sharing revenues which we might otherwise retain for ourselves. Any of these actions may harm our business, financial condition and results of operations.
The amount of capital we may need depends on many factors, including the progress, timing and scope of our product development programs; the progress, timing and scope of our nonclinical studies and clinical trials; the time and cost necessary to obtain regulatory approvals; the time and cost necessary to further develop manufacturing processes and arrange for contract manufacturing; our ability to enter into and maintain collaborative, licensing and other commercial relationships; and our partners’ commitment of time and resources to the development and commercialization of our products.
We may be unable to access the capital markets and even if we can raise additional funding, we may be required to do so on terms that are dilutive to you.
The capital markets have been unpredictable in the recent past for unprofitable companies such as ours. The amount of capital that a company such as ours is able to raise often depends on variables that are beyond our control. As a result, we cannot assure you that we will be able to secure financing on terms attractive to us, or at all. If we are able to consummate a financing arrangement, the amount raised may not be sufficient to meet our future needs. If adequate funds are not available on acceptable terms, or at all, our business, results of operations, financial condition and our continued viability will be materially adversely affected.
Our operating results may fluctuate significantly, which will make our future results difficult to predict and could cause our results to fall below expectations.
Our quarterly and annual operating results may fluctuate significantly, which will make it difficult for us to predict our future results. These fluctuations may occur due to a variety of factors, many of which are outside of our control and may be difficult to predict, including:
| | · | the timing, cost of, and level of investment in, research, development and commercialization activities, which may change from time to time; |
| | · | the timing and status of enrollment for our clinical trials; |
| | · | the timing of regulatory approvals, if any, in the United States and internationally; |
| | · | the timing of expanding our operational, financial and management systems and personnel, including personnel to support our clinical development, quality control, manufacturing and commercialization efforts and our operations as a public company; |
| | · | the cost of manufacturing, as well as building out our supply chain, which may vary depending on the quantity produced, and the terms of any agreements we enter into with third-party suppliers; |
| | · | the timing and amount of any milestone, royalty or other payments due under any current or future collaboration or license agreement; |
| | · | coverage and reimbursement policies with respect to any future approved products, and potential future drugs that compete with our products; |
| | · | the timing and cost to establish a sales, marketing, medical affairs and distribution infrastructure to commercialize any products for which we may obtain marketing approval and intend to commercialize on our own or jointly with current or future collaborators; |
| | · | expenditures that we may incur to acquire, develop or commercialize additional products and technologies; |
| | · | the level of demand for any future approved products, which may vary significantly over time; |
| | · | future accounting pronouncements or changes in accounting principles or our accounting policies; and |
| | · | the timing and success or failure of nonclinical studies and clinical trials for our product candidates or competing product candidates, or any other change in the competitive landscape of our industry, including consolidation among our competitors or collaboration partners. |
The cumulative effects of these factors could result in large fluctuations and unpredictability in our quarterly and annual operating results. As a result, comparing our operating results on a period-to-period basis may not be meaningful. Investors should not rely on our past results as an indication of our future performance.
Risks related to product development and regulatory process
We are early in our development efforts of some of our product candidates, and our business is dependent on the successful development of our current and future product candidates. If we or our collaboration partner are unable to advance our current or future product candidates through clinical trials, obtain marketing approval and ultimately commercialize any product candidates we develop, or experience significant delays in doing so, our business will be materially harmed.
Our product candidates are in different stages of clinical development. Our current and future product candidates may never achieve expected levels of efficacy or an acceptable safety profile. Our use of clinically validated targets to pursue treatments does not guarantee efficacy or safety or necessarily reduce the risk that our current or future product candidates will not achieve expected levels of efficacy or an acceptable safety profile.
The success of our business, including our ability to finance our Company and generate revenue from products in the future will depend heavily on the successful development and eventual commercialization of our product candidates, which may never occur. Our current product candidates, and any future product candidates we develop, will require additional nonclinical and clinical development, management of clinical, nonclinical and manufacturing activities, marketing approval in the United States and other markets, obtaining sufficient manufacturing supply for both clinical development and commercial production, building of a commercial organization, and substantial investment and significant marketing efforts before we generate any revenues from product sales.
As a company, we have limited experience in preparing, submitting and prosecuting regulatory filings. In addition, we are reliant on TCNT to pursue and obtain regulatory approval for certain product candidates. If we do not receive regulatory approvals for current or future product candidates, or if TCNT chooses not to pursue or is unable to obtain regulatory approval for certain product candidates, we may not be able to continue our operations. Even if we, or TCNT, successfully obtain regulatory approval to market a product candidate, our revenue will depend, in part, upon the size of the markets in the territories for which we gain regulatory approval and have commercial rights, as well as the availability of competitive products, third-party reimbursement and adoption by physicians.
We, either individually or through TCNT, plan to seek regulatory approval to commercialize our product candidates both in the United States and in select foreign countries. While the scope of regulatory approval in other countries is generally similar to that in the United States, in order to obtain separate regulatory approval in other countries we must comply with numerous and varying regulatory requirements of such countries. We may be required to expend significant resources to obtain regulatory approval and to comply with ongoing regulations in these jurisdictions.
The success of our current and future product candidates will depend on many factors, which may include the following:
| | · | sufficiency of our financial and other resources to complete the necessary nonclinical studies and clinical trials, and our ability to raise any additional required capital on acceptable terms, or at all; |
| | · | the timely and successful completion of nonclinical studies and clinical trials for which the TFDA, FDA, or any comparable foreign regulatory authority, agree with the design, endpoints, or implementation ; |
| | · | receipt of regulatory approvals or authorizations to conduct future clinical trials or other studies beyond those planned to support approval of our product candidates; |
| | · | successful enrollment and completion of clinical trials; |
| | · | successful data from our clinical program that supports an acceptable risk-benefit profile of our product candidates in the intended populations; |
| | · | timely receipt and maintenance of marketing approvals from applicable regulatory authorities; |
| | · | establishing, scaling up and scaling out, either alone or with third-party manufacturers, cGMP compliant manufacturing capabilities of clinical supply for our clinical trials and commercial manufacturing (including licensure), if any of our product candidates are approved; |
| | · | entry into collaborations to further the development of our product candidates in select indications or geographies; |
| | · | obtaining and maintaining regulatory exclusivity for our product candidates as well as establishing competitive positioning amongst other therapies; and |
| | · | successfully launching commercial sales of our product candidates and obtaining and maintaining healthcare coverage and reimbursement from third party payors, if approved. |
If we are not successful with respect to one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully obtain regulatory approval of or commercialize the product candidates we develop, which would materially harm our business. If we do not receive marketing approvals for our current or future product candidates, we may not be able to continue our operations. Even if regulatory approvals are obtained, we may never be able to successfully commercialize any products. Accordingly, we cannot provide assurances that we will be able to generate sufficient revenue through the sale of products to continue our business.
If the FDA or other regulatory bodies do not approve TCNT’s Emergency Use Authorization (EUA) submissions or revoke or terminate the EUAs or other regulatory authorizations for Ainos COVID-19 test kits, we will be required to stop commercialization of COVID-19 test kits in those relevant markets unless we, or our manufacturing collaborators, can obtain 510(k) or other clearance or approval for our COVID-19 test and its currently authorized uses.
We understand Taiwan Carbon Nano Technology (“TCNT”), our product co-developer, manufacturing collaborator and affiliated company, intends to submit EUAs to the FDA for Ainos COVID-19 Antigen Rapid Test Kits. The terms of the TCNT Agreement do not contractually obligate TCNT to submit EUAs to the FDA, or to obtain marketing authorization from the FDA, or any other regulatory authority. If for any reason TCNT does not maintain or obtain marketing authorizations, our business, financial condition, results of operations and future prospects could be materially adversely affected. Moreover, we cannot predict if TCNT’s submission will be approved or, if approved, how long either of the EUAs will remain in effect, and TCNT may not receive advance notice from the TFDA or FDA regarding revocation of either or both of their EUAs. If the EUAs are terminated or TCNT’s submissions are not approved, we will be required to cease commercialization of Ainos COVID-19 Antigen Rapid Test Kits in the United States, unless and until TCNT has obtained marketing authorization from the FDA through another regulatory pathway, possibly requiring us to obtain a 510(k) or other marketing authorization from the FDA for the Ainos COVID-19 Antigen Rapid Test Kits. Changing policies and regulatory requirements could limit, delay or prevent further commercialization of Ainos COVID-19 Antigen Rapid Test Kits and could materially adversely impact our business, financial condition, results of operations and future prospects.
Clinical product development involves a lengthy and expensive process, with uncertain outcomes. We may experience delays in completing, or ultimately be unable to complete, the development and commercialization of our current and future product candidates, which could result in increased costs to us, delay or limit our ability to generate revenue and adversely affect our business, financial condition, results of operations and prospects.
To obtain the requisite regulatory approvals to commercialize any of our product candidates, we must demonstrate that our products are safe and effective in humans. Clinical trials are expensive and can take many years to complete, and their outcomes are inherently uncertain. We may experience delays in completing current and future clinical trials. We may also experience numerous unforeseen events prior to, during, or as a result of our nonclinical studies or clinical trials that could delay or prevent our ability to receive marketing approval or commercialize the product candidates we develop, including:
| | · | regulators, Institutional Review Boards (IRBs) or ethics committees may not authorize us to conduct the clinical study; |
| | · | we may experience delays due to challenges with third-party contractors and contract research organizations (CROs), including negotiating agreement terms, compliance with regulatory requirements, compliance with clinical trial protocols; |
| | · | it may be difficult to enroll a sufficient number of suitable patients, or enrollment may be slower than we anticipate or participants may drop out of these clinical trials or fail to return for post-treatment follow-up at a higher rate than we anticipate; |
| | · | the supply or quality of materials for product candidates we develop or other materials necessary to conduct clinical trials may be insufficient or inadequate; and |
| | · | we may experience disruptions by man-made or natural disasters or public health pandemics or epidemics or other business interruptions, including the current COVID-19 pandemic and future outbreaks of the disease. |
We could encounter delays if a current or future clinical trial is suspended or terminated by us, by TCNT, by the TFDA, FDA or other regulatory authorities and/or review boards. Such authorities may impose such a suspension or termination due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the TFDA, FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues, failure to demonstrate a benefit from using a product, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of marketing approval of our product candidates.
If termination or delays are experienced in the completion of any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of these product candidates may be delayed. In addition, any delays in completing our clinical trials will likely increase our costs, slow down our product candidate development and approval process and impact our ability to commence product sales and generate revenues. Significant clinical trial delays could also allow our competitors to bring products to market before we do, shorten any periods during which we may have the exclusive right to commercialize our product candidates, impair our ability to commercialize our product candidates and harm our business and results of operations.
Any of these occurrences may harm our business, financial condition and prospects significantly. Delays in clinical product development present material uncertainty and risk with respect to our clinical trials, business, and financial condition.
We and our collaboration partners have conducted and intend to conduct clinical trials for selected product candidates at sites outside the United States, and for any of our product candidates for which we seek approval in the United States, the FDA may not accept data from trials conducted in such locations or may require additional U.S.-based trials.
We and our collaboration partners have conducted and plan to continue to conduct, clinical trials outside the United States, particularly in Taiwan. Although the FDA may accept data from clinical trials conducted outside the United States, acceptance of these data is subject to certain conditions imposed by the FDA. There can be no assurance that the FDA will accept data from trials conducted outside of the United States. If the FDA does not accept the data from any clinical trials that we or our collaboration partners conduct outside the United States, it would likely result in the need for additional clinical trials, which would be costly and time-consuming and delay or permanently halt our ability to develop and market these or other product candidates in the United States. In other jurisdictions, for instance, in Taiwan, there is a similar risk regarding the acceptability of clinical trial data conducted outside of that jurisdiction.
Our long-term prospects depend in part upon discovering, developing and commercializing additional products, including POCT testing devices, which may fail in development or suffer delays that adversely affect their commercial viability.
Our future operating results are dependent on our ability to successfully discover, develop, obtain regulatory approval for and commercialize product candidates, including POCT testing devices, beyond those we currently have in development. The success of a product candidate is unknown and initial product development success may not result in a viable commercial product. The product development process may require changes in manufacturing methods and formulation/design or additional validation testing. We may also make changes as we work to optimize our manufacturing processes, but we cannot be sure that even minor changes in our processes will result in products that are safe and effective or that will be approved for commercial sale. If a product candidate fails to develop as expected, or we experience additional and/or unforeseen development costs and/or delays, we could face additional costs and/or loss of expected future revenue, which would adversely affect our current financial position and future prospects may be adversely affected.
Even if we complete the necessary nonclinical studies and clinical trials, the marketing approval process is expensive, time consuming and uncertain, which may prevent us or any of our future collaboration partners from obtaining approvals for the commercialization of our current product candidates and any other product candidate we develop.
Any current or future product candidates, including medical device products, we may develop and the activities associated with their development and commercialization, including their design, testing, manufacture, recordkeeping, labeling, storage, approval, advertising, promotion, sale, and distribution, are subject to comprehensive regulation by the FDA and other regulatory authorities in the United States and by comparable authorities in Taiwan and other countries. Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate in a given jurisdiction. It is possible that some of our current or future product candidates will not obtain regulatory approval in the jurisdiction we are targeting. We have limited experience in filing and supporting the applications necessary to gain marketing approvals, but we expect to rely on third-party CROs or regulatory consultants to assist us in this process. Securing regulatory approval requires the submission of extensive applications to the various regulatory authorities. Product candidates we develop may not be effective or may prove to have adverse characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use.
The process of obtaining marketing approvals, in Taiwan, the United States and other jurisdictions, is expensive, may take many years, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity, and novelty of the product candidates involved. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. The FDA and comparable authorities in other countries may refuse to accept any application or may decide that our data are insufficient for approval and require additional nonclinical, clinical or other studies. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments. If we experience delays in obtaining marketing approval or if we fail to obtain marketing approval of any current or future product candidates we may develop, the commercial prospects for those product candidates may be harmed, and our ability to generate revenues will be materially impaired.
The results of our earlier research and development and clinical trials for our VELDONA platform may not be replicable or current, may not be safe and effective in combination with other treatments as planned in our COVID-19 product plans, and may not be sufficient to support the authorization or approval of our COVID-19 drug candidate.
Since inception, we have primarily focused on the research and development of our Very Low Dose Interferon (VELDONA) drug platform. Our proprietary drug therapeutic, VELDONA, is a low-dose oral interferon alpha (IFN-α) formulation based on nearly four decades of research on IFN-α’s broad treatment applications. Our earlier research indicates VELDONA delivers equal or superior efficacy to that of high-dose IFN-α without the associated adverse effects. Going forward, we plan to advance our development efforts with a particular focus on key disease indications such as thrombocytopenia, Sjögren’s syndrome and influenza, including COVID-19. We recently formed a strategic relationship with InnoPharmax, Inc. to jointly develop and market CICCT for the treatment of COVID-19 and other potential viral infections. We also initiated a parallel study for the COVID-19 oral treatment solely based on VELDONA. Based on our animal studies, we intend to advance the VELDONA-only program as our candidate for the COVID-19 oral treatment. Due to the dates of our earlier research and development and clinical trials for our VELDONA platform the results may not be replicable or current and may not be sufficient to support the authorization or approval of our COVID-19 drug candidate. Our plans for our COVID-19 drug candidate may not be safe and effective in combination with other treatments including our VELDONA-only or CICCT product plans.
Even if a current or future product candidate, including medical device products, receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success.
If any current or future product candidate we develop receives marketing approval, whether as a single agent or in combination with other therapies, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors, and others in the medical community, or such participants may prefer existing treatment options. If the product candidates we develop, including medical device products, do not achieve an adequate level of market acceptance, we may not generate expected levels of revenues associated with such products, which may prevent those products from becoming profitable. The degree of market acceptance of any product candidate, if approved for commercial sale, will depend on a number of factors, including:
| | · | efficacy and potential advantages compared to alternative tools; |
| | · | the ability to offer our products, if approved, for sale at competitive prices; |
| | · | convenience and ease of use; |
| | · | the willingness of the target market to adopt new technologies; and |
| | · | the strength of marketing and distribution support. |
The total addressable market opportunity for our current and future products may be much smaller than we estimate.
Our estimates of the total addressable market for our product candidates are based on internal and third-party estimates as well as a number of significant assumptions. Market opportunity estimates and growth forecasts included in this prospectus are subject to significant uncertainty and are based on assumptions and estimates. These estimates, which have been derived from a variety of sources, including market research and our own internal estimates, may prove to be incorrect. Further, the continued development of, and approval or authorizations for, vaccines and therapeutic treatments may affect these market opportunity estimates. Our market opportunity may also be limited by new POCT tests or other products that enter the market. If any of our estimates prove to be inaccurate, the market opportunity for platform and products could be significantly less than we estimate. If this turns out to be the case, our potential for growth may be limited and our business and future prospects may be materially adversely affected.
We may not obtain approval for our product candidates in any jurisdictions.
Approval of a product candidate in one jurisdiction by a regulatory authority, such as the FDA, does not ensure approval of such product candidate by regulatory authorities in other countries or jurisdictions. Commercialization of our product candidates will be subject to the regulatory requirements governing marketing authorization in the jurisdiction in which they are sold.
Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and more onerous than, those in Taiwan and the United States, including additional nonclinical studies or clinical trials. In many countries outside Taiwan and United States, a product candidate must be approved for reimbursement before it can be approved for sale in that country. In some cases, the price that we intend to charge for any product candidates, if approved, is also subject to approval. For example, obtaining approval for our product candidates in the European Union (the EU) from the European Commission following the opinion of the EMA, would be a lengthy and expensive process. The EMA may limit the indications for which the product may be marketed, require extensive warnings on the product labeling or require expensive and time-consuming additional clinical trials or reporting as conditions of approval. Approval of certain product candidates outside of Taiwan and the United States, particularly those that target diseases that are more prevalent outside of the United States, will be particularly important to the commercial success of such product candidates. Obtaining regulatory approvals in various jurisdictions and complying with the regulatory requirements of multiple jurisdictions could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our product candidates in certain countries.
Even if we are able to commercialize any product candidates, such products may become subject to unfavorable pricing regulations or third-party coverage and reimbursement policies, which would harm our business.
The regulations that govern marketing approvals, pricing and reimbursement for new products vary widely from country to country. Some countries require approval of the sale price of a product before it can be marketed. In many countries, the pricing review period begins after marketing approval is granted. As a result, we might obtain marketing approval for a product candidate in a particular country, but then be subject to price regulations that delay our commercial launch of the product candidate. Adverse pricing limitations may hinder our ability to recoup our investment in one or more product candidates, even if our product candidates obtain marketing approval. Our ability to successfully commercialize any product candidates, whether as a single agent or in combination, will also depend in part on the extent to which coverage and reimbursement for these product candidates and related treatments is available from government authorities, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, and establish reimbursement levels. It is difficult to predict at this time what government authorities and third-party payors may decide with respect to coverage and reimbursement for our programs (if approved).
A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities, particularly in the European Union, and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular products and requiring substitutions of generic products and/or biosimilars. Reimbursement may impact the demand for, or the price of, any product candidate for which we obtain marketing approval. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of any approved products.
We face an inherent risk of product liability as a result of the clinical testing of product candidates and will face an even greater risk if we commercialize any products. For example, we may be sued if any product candidate we develop is found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of any approved products. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| | · | decreased demand for any approved product; |
| | · | injury to our reputation; |
| | · | withdrawal of clinical trial participants; |
| | · | initiation of investigations by regulators; |
| | · | costs to defend litigation; |
| | · | a diversion of management’s time and our resources; |
| | · | substantial monetary payments to trial participants or patients; |
| | · | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| | · | loss of revenue; |
| | · | exhaustion of any available insurance and our capital resources; |
| | · | adverse effects to our results of operations and business; |
| | · | the inability to commercialize any product candidate; and |
| | · | a decline in our share price. |
Our inability to obtain sufficient product liability insurance at an acceptable cost or at all to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop, alone or with collaboration partners.
Additionally, insurance coverage is increasingly expensive. We may not be able to maintain insurance, including product liability insurance at a reasonable cost or in an amount adequate to satisfy any liability that may arise, if at all, that could have an adverse effect on our business and financial condition. Our product liability insurance policy contains various exclusions, and we may be subject to a product liability claim for which we have no coverage. We may have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. Similar challenges to obtaining coverage and reimbursement will apply to companion POCTs that we or our collaborators may develop. Even if our agreements with current or future collaborators entitle us to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
Our employees, independent contractors, principal investigators, consultants, commercial partners, and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of employee fraud or other misconduct. We cannot ensure that our compliance controls, policies, and procedures will in every instance protect us from acts committed by our employees, agents, contractors, or collaborators that would violate the laws or regulations of the jurisdictions in which we operate, including, without limitation, employment, foreign corrupt practices, trade restrictions and sanctions, environmental, competition, and patient privacy and other privacy laws and regulations. Misconduct by employees could include failures to comply with FDA regulations, provide accurate information to the FDA, comply with manufacturing standards we may establish, comply with federal and state healthcare fraud and abuse laws and regulations, report financial information or data accurately, or disclose unauthorized activities to us, or similar requirements and laws of regulatory authorities in other jurisdictions. In particular, sales, marketing, and business arrangements in the healthcare industry in the US and other jurisdictions are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing, and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, labeling, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations.
If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a material and adverse effect on our business, financial condition, results of operations and prospects, including the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, individual imprisonment, disgorgement of profits, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional reporting or oversight obligations if we become subject to a corporate integrity agreement or other agreement to resolve allegations of noncompliance with the law, and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and pursue our strategy.
Any disruption in our research and development facility could adversely affect our business, financial condition and results of operations.
Our facility may be affected by natural or man-made disasters. We are vulnerable to damage from other types of disasters, including power loss, attacks from extremist organizations, fires, floods, and similar events. If our facilities are affected by a natural or man-made disaster, we may be forced to curtail our operations and/or rely on third-parties to perform some or all of our research and development activities. Although we believe we possess adequate insurance in light of our current operations, such insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all. In the future, we may choose to expand our operations in either our existing facilities or in new facilities.
Our business and operations would be adversely affected in the event that our computer systems or those of our partners, contract research organizations, contractors, consultants or other third parties we work with were to suffer system failures, cyber-attacks, loss of data or other security incidents.
Despite the implementation of security measures, our computer systems, as well as those of our partners, contract research organizations, contractors, consultants, law and accounting firms and other third parties we work with, may sustain damage from computer viruses, unauthorized access, data breaches, phishing attacks, ransomware attacks, denial-of-service attacks, cybercriminals, natural disasters, terrorism, war and telecommunication and electrical failures. We rely on our partners and third-party providers to implement effective security measures and identify and correct for any such failures, deficiencies or breaches. The risks of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments and cyber-terrorists, have increased significantly and are becoming increasingly difficult to detect.
If a failure, accident or security breach were to occur and cause interruptions in our operations, or the operations of our partners or third-party providers, it could result in a misappropriation of confidential information, including our intellectual property or financial information or clinical trial participant personal data, a material disruption or delay in our drug development programs, and/or significant monetary losses. For example, the loss of preclinical or clinical trial data from completed, ongoing or planned trials, or chemistry, manufacturing and controls data for our product candidates could result in delays in regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Any such breach, loss or compromise of clinical trial participant personal data may also subject us to civil fines and penalties under the privacy laws of the European Union or other countries as well as state and federal privacy laws in the United States.
Risks related to reliance on third parties
We rely substantially on Taiwan Carbon Nano Technology (TCNT), an affiliate of our Company, to co-develop products.
We rely substantially, and intend to continue to rely substantially on, TCNT to co-develop pharmaceutical, medical and preventive medicine related products for which we are designated as TCNT’s exclusive sales agent. Our Product Development Agreement (TCNT Agreement) with TCNT is effective until mid-2026.
Any termination or loss of rights under the TCNT Agreement would harm our ability to commercialize, sell and distribute product candidates, which in turn would have a material adverse effect on our business, operating results and prospects. If we were to lose our rights under the TCNT Agreement, we believe it would be difficult for us to find an alternative development partner. In addition, TCNT is not contractually obligated to obtain regulatory approvals for our product candidates. To the extent TCNT or the alternative manufacturer has not secured applicable regulatory approvals, we would have to expend significant resources to obtain regulatory approvals that may never be obtained or require several years to obtain, which could significantly delay commercialization. We may be unable to raise additional capital to fund our operations during this extended time on terms acceptable to us or at all. In addition, if we were to commercialize product candidates and later experience manufacturing delays as a result of a dispute with TCNT or otherwise, the supply of our products could be harmed.
In addition, the manufacture of medical devices and pharmaceutical products is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. It may be difficult to predict the cost of manufacturing our products. TCNT may not be able to manufacture our products at expected prices. There may also be unforeseen occurrences that increase our costs, such as increased prices of the components of our products, changes to labor costs or less favorable terms with third-party suppliers or contract manufacturing partners.
Furthermore, quarantines, shelter-in-place and similar government orders related to COVID-19 or other infectious diseases, or the perception that such orders, shutdowns or other restrictions on the conduct of business operations could occur, could impact personnel at TCNT’s facilities. Further, TCNT may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or unstable political environments. If TCNT were to encounter any of these difficulties, or otherwise fail to comply with its contractual obligations, our ability to commercialize our products would be jeopardized.
We currently have limited sales and marketing capabilities. If we are unable to expand our sales and marketing capabilities on our own or through third parties, or are delayed in establishing these capabilities, we will be unable to successfully commercialize our product candidates, if approved, or generate product revenue.
We currently have limited sales and marketing capabilities. To commercialize our product candidates, if approved, in the United States and other jurisdictions we seek to enter, we must expand our marketing, sales, distribution, managerial and other non-technical capabilities or make arrangements with third parties to perform these services, and we may not be successful in doing so. There are significant risks involved in building and managing a sales organization, including our ability to hire, retain and incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel, and effectively manage a geographically dispersed sales and marketing team. Any failure or delay in the development of our internal sales, marketing, distribution and pricing/reimbursement/access capabilities would impact adversely the commercialization of these products.
To commercialize our products, we also intend to leverage the commercial infrastructure of our preferred distributor in Japan and non-exclusive worldwide distributor outside Japan, Inabata, which will provide us with resources and expertise in certain areas that are greater than we could initially build ourselves. We may choose to collaborate with additional third parties in various countries that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems. If we are unable to enter into such arrangements on acceptable terms or at all, we may not be able to successfully commercialize our product candidates, especially in other countries where we currently do not have a foreign legal presence. The inability to commercialize successfully our product candidates, either on our own or through collaborations with one or more third parties, would harm our business, financial condition, operating results and prospects.
Our employees, independent contractors, consultants, commercial or strategic partners, principal investigators or CROs may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees, independent contractors, consultants, commercial partners, principal investigators, contract manufacturing organizations or CROs could include intentional, reckless, negligent, or unintentional failures to comply with TFDA or FDA regulations, comply with applicable fraud and abuse laws, provide accurate information to the TFDA or FDA, properly calculate pricing information required by federal programs, report financial information or data accurately or disclose unauthorized activities to us. This misconduct could also involve the improper use or misrepresentation of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter this type of misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. Moreover, it is possible for a whistleblower to pursue a False Claims Act case against us even if the government considers the claim unmeritorious and declines to intervene, which could require us to incur costs defending against such a claim. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, financial condition, results of operations, stock price and prospects, including the imposition of significant fines or other sanctions.
We may form or seek strategic partnerships in the future, and we may not realize the benefits of such alliances or licensing arrangements.
From time to time, we may form or seek strategic partnerships, create joint ventures or collaborations or enter into licensing arrangements with third parties that we believe will complement or augment our development and commercialization efforts with respect to our product candidates and any future product candidates that we may develop. Any such relationships may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our management and business. These relationships also may result in a delay in the development of our product candidates if we become dependent upon the other party and such other party does not prioritize the development of our product candidates relative to its other development activities. Additionally, any joint ventures, collaborations, or licensing arrangements would be subject to the same product candidate development and compliance risks and obligations as we would be if we were to develop the product candidate on our own. Should any third party with which we enter into any of these arrangements not comply with the applicable regulatory requirements, we or they may be subject to regulatory enforcement action and we or they may be delayed or prevented from obtaining marketing approval for the applicable product candidate.
In addition, we face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangement for our product candidates because they may be deemed to be at too early of a stage of development for collaborative effort, and third parties may not view our product candidates as having the requisite potential to demonstrate safety and efficacy. If we license products or acquire businesses, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations and company culture. Any licensed products or acquired businesses may also subject us to the risk of regulatory enforcement should the product or business not be compliant with applicable regulatory requirements. We cannot be certain that, following a strategic transaction or licensing arrangement, we will achieve the revenue or specific net income that justifies such a transaction.
Risks related to intellectual property, patents, and data privacy
Intellectual property rights vary across foreign jurisdictions, and we may not be able to protect our intellectual property rights throughout the world.
We cannot assure you that any intellectual property rights that we currently have or may receive can be successfully asserted in the future or that they will not be invalidated, circumvented or challenged. In addition, the laws of some foreign countries do not protect proprietary rights to the same extent, as do the laws of the United States. Our means of protecting any proprietary rights we may receive in the United States or abroad may not be adequate. Filing, prosecuting, maintaining, defending and enforcing patents on our product candidates in all countries throughout the world would be prohibitively expensive. The requirements for patentability may differ in certain countries, particularly developing countries, and the breadth of patent claims allowed can be inconsistent. In addition, the laws of some foreign countries may not protect our intellectual property rights to the same extent as the laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patents to develop their own products and may export otherwise infringing products to territories where we have patents, but enforcement rights are not as strong as those in the United States. These products may compete with our product candidates and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
We do not have patent rights in certain foreign countries in which a market may exist in the future. Moreover, in foreign jurisdictions where we do have patent rights, proceedings to enforce such rights could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, and our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Thus, we may not be able to stop a competitor from marketing and selling in foreign countries products that are the same as or similar to our product.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of some countries do not favor the enforcement or protection of patents, trade secrets and other intellectual property, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our intellectual property and proprietary rights generally.
Many foreign countries, including some EU countries, India, Japan, and China, have compulsory licensing laws under which a patent owner may be compelled under specified circumstances to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In those countries, we may have limited remedies if patents are infringed or if we are compelled to grant a license to a third party, which could materially diminish the value of the applicable patents and limit our potential revenue opportunities. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license, which could adversely affect our business, financial condition, results of operations and prospects.
If we and our collaborators are unable to obtain and maintain sufficient patent and other intellectual property protection for our product candidates and technology, our competitors could develop and commercialize products and technology similar or identical to ours, and we may not be able to compete effectively in our market or successfully commercialize any product candidates we may develop.
Our success depends in significant part on our ability and the ability of our current or future collaborators and licensors to obtain, maintain, enforce and defend patents and other intellectual property rights with respect to our product candidates and technology and to operate our business without infringing, misappropriating, or otherwise violating the intellectual property rights of others. If we and our current or future collaborators and licensors are unable to obtain and maintain sufficient intellectual property protection for our product candidates or other future product candidates that we may identify, or if the scope of the intellectual property protection obtained is not sufficiently broad, our competitors and other third parties could develop and commercialize product candidates similar or identical to ours, and our ability to successfully commercialize our product candidates and other product candidates that we may pursue may be impaired.
The process of applying for patent protection itself is time consuming and expensive and we cannot assure you that we have prepared or will be able to prepare, file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. In addition, our patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. It is possible that defects of form in the preparation or filing of our patents or patent applications may exist, or may arise in the future, for example, with respect to proper priority claims, inventorship, claim scope or patent term adjustments. We can provide no assurance that any of our current or future patent applications will result in issued patents or that any issued patents will provide us with any competitive advantage. We cannot be certain that there is no invalidating prior art of which we and the patent examiner are unaware or that our interpretation of the relevance of prior art is correct. Failure to obtain issued patents could have a material adverse effect on our ability to develop and commercialize our product candidates. Even if our patent applications do issue as patents, third parties may be able to challenge the validity and enforceability of our patents on a variety of grounds, including that such third party’s patents and patent applications have an earlier priority date, and if such challenges are successful we may be required to obtain one or more licenses from such third parties, or be prohibited from commercializing our product candidates. We may not be able to obtain these licenses on acceptable or commercially reasonable terms, if at all, or these licenses may be non-exclusive, which could result in our competitors using the same intellectual property.
We seek to protect our proprietary positions by, among other things, filing patent applications in the United States and in relevant foreign jurisdictions related to our current product candidates and other future product candidates that we may identify. Obtaining, maintaining, defending and enforcing pharmaceutical patents is costly, time consuming and complex, and we may not be able to file and prosecute all necessary or desirable patent applications, or maintain, enforce and license any patents that may issue from such patent applications, at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Moreover, under certain of our license or collaboration agreements, we may not have the right to control the preparation, filing, prosecution and maintenance of patent applications, or to maintain the rights to patents licensed to or from third parties.
Although we enter into confidentiality agreements with parties who have access to confidential or patentable aspects of our research and development output, such as our employees, collaborators, CROs, contract manufacturers, consultants, advisors and other third parties, any of these parties may breach these agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection. Further, we may not be aware of all third-party intellectual property rights potentially relating to our product candidates. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing or, in some cases, not at all. Therefore, we cannot know with certainty whether we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions.
The patent position of biotech companies generally is highly uncertain, involves complex legal, technological and factual questions and has, in recent years, been the subject of much debate and litigation throughout the world. The subject matter claimed in a patent application can be significantly reduced or eliminated before the patent issues, if at all, and its scope can be reinterpreted or narrowed after issuance. Therefore, our pending and future patent applications may not result in patents being issued in relevant jurisdictions that protect our product candidates, in whole or in part, or that effectively prevent others from commercializing competitive product candidates, and even if our patent applications issue as patents in relevant jurisdictions, they may not issue in a form that will provide us with any meaningful protection for our product candidates or technology, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Additionally, our competitors may be able to circumvent our patents by challenging their validity or by developing similar or alternative product candidates or technologies in a non-infringing manner. The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in the United States and abroad. An adverse determination in any such submission, proceeding or litigation could result in loss of exclusivity or ability to sell our products free from infringing the patents of third parties, patent claims being narrowed, invalidated or held unenforceable, in whole or in part, and limitation of the scope or duration of the patents directed to our product candidates, all of which could limit our ability to stop others from using or commercializing similar or identical product candidates or technology to compete directly with us, without payment to us, or result in our inability to manufacture or commercialize product candidates or approved products (if any) without infringing third-party patent rights. In addition, if the breadth or strength of the claims of our patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates, or could have a material adverse effect on our ability to raise funds necessary to continue our research programs or clinical trials. Such proceedings also may result in substantial cost and require significant time from our scientists and management, even if the eventual outcome is favorable to us.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time-consuming and unsuccessful, and issued patents directed towards our technology and product candidates could be found invalid or unenforceable if challenged.
Competitors and other third parties may infringe or otherwise violate our issued patents or other intellectual property or the patents or other intellectual property of our licensors and collaborators. In addition, our patents or the patents of our licensors and collaborators may become involved in inventorship or priority disputes. To counter infringement or other unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. Significantly, our pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. Our ability to enforce patent rights also depends on our ability to detect infringement. It may be difficult to detect infringers who do not advertise the components or methods that are used in connection with their products and services. Moreover, it may be difficult or impossible to obtain evidence of infringement in a competitor’s or potential competitor’s product or service. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents or that our patents are invalid or unenforceable. In a patent infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology. An adverse result in any litigation proceeding could put one or more of our owned or licensed patents at risk of being invalidated, held unenforceable or interpreted narrowly. We may find it impractical or undesirable to enforce our intellectual property against some third parties.
If we were to initiate legal proceedings against a third party to enforce a patent directed to our product candidates, or one of our future product candidates, the defendant could counterclaim that our patent is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, non-enablement or insufficient written description. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO or made a misleading statement during prosecution. Third parties may also raise similar claims before the USPTO or an equivalent foreign body, even outside the context of litigation. Such proceedings could result in the revocation of, cancellation of, or amendment to our patents in such a way that they no longer cover our technology or any product candidates that we may develop. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent rights directed towards the applicable product candidates or technology related to the patent rendered invalid or unenforceable. Such a loss of patent rights would materially harm our business, financial condition, results of operations and prospects.
Interference and/or derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be materially harmed if the prevailing party does not offer us a license on commercially reasonable terms. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Some of our competitors are larger than we are and have substantially greater resources. They are, therefore, likely to be able to sustain the costs of complex patent litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing, misappropriating or otherwise violating our intellectual property. Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims could result in substantial costs and diversion of management resources, which could harm our business. In addition, the uncertainties associated with litigation could compromise our ability to raise the funds necessary to continue our clinical trials, continue our internal research programs, or in-license needed technology or other product candidates. There could also be public announcements of the results of the hearing, motions, or other interim proceedings or developments. If securities analysts or investors perceive those results to be negative, it could cause the price of shares of our common stock to decline. Any of the foregoing events could harm our business, financial condition, results of operation and prospects.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. In addition, periodic maintenance fees on issued patents often must be paid to the USPTO and foreign patent agencies over the lifetime of the patent. While an unintentional lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we fail to maintain the patents and patent applications covering our product or procedures, we may not be able to stop a competitor from marketing products that are the same as or similar to our product and technologies.
Patents have a limited lifespan. The actual protection afforded by a patent varies from country to country, and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent. Various extensions including patent term extension and patent term adjustment may be available, but the availability of such extensions, and the protections they afford, are limited. Even if patents covering our product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products, including biosimilars and generics. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting our product candidates might expire before or shortly after we or our partners commercialize those candidates. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
Our patent portfolio encompasses over 24 different patent families with various forms of patent protection sought per family in at least one of Taiwan, China, United States, Japan, Germany and Europe. Depending on the jurisdiction and the type of patent protections available, we have sought to obtain “invention patents,” “utility model patents” and/or “design patents.” In all instances, the issued patent may lapse or expire prematurely, if renewal/maintenance fees are not timely paid.
Although the patentability requirements vary among different jurisdictions, invention patents (or utility patents as referred to in the U.S.) generally protect something new, useful and non-obvious and undergo an extensive examination prior to issuance. The base term of a U.S. utility patent is 20 years from the filing date of the earliest-filed non-provisional patent application from which the patent claims priority. Similarly, other jurisdictions recognize a patent term of 20 years from the priority date for invention patents. As of January 31, 2022 we own 22 invention patents in the following jurisdictions: Taiwan (13), U.S. (4), Japan (3) and China (2). We also license one Japanese patent owned by TCNT. These patents expire between February 2033 and May 2040. Additionally, we have 14 pending invention patents in Taiwan (2), China (3), U.S. (3), Europe (3) and Japan (3).
Utility model patents are somewhat similar to an invention patent, but are generally cheaper to obtain and maintain, have a shorter term (10 years), shorter grant lag, and less stringent patentability requirements. The U.S. does not offer this form of patent protection. Our portfolio includes 12 granted utility model patents in the following jurisdictions: Taiwan (5), China (2), Japan (3) and Germany (2). These patents will expire between October 2029 and April 2031. We also currently have one pending utility model patent application in China.
Design patents generally protect the ornamental design of something that has a practical utility and have a term of between 15-20 years, depending on the jurisdiction. We have five granted design patents in the following jurisdictions: Taiwan (3), China (1) and Europe (1), which expire between September 2026 and April 2041. We also have one pending design patent in Japan. We license one Chinese design patent owned by TCNT.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position could be harmed.
In addition to seeking patents for our technologies and product candidates, we also rely on trade secret protection, as well as confidentiality agreements, non-disclosure agreements and invention assignment agreements with our employees, consultants and third-parties, to protect our know-how and other confidential and proprietary information, especially where we do not believe patent protection is appropriate or obtainable.
It is our policy to require our employees, corporate collaborators, outside scientific collaborators, CROs, contract manufacturers, consultants, advisors, and other third parties to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements generally provide that all confidential information concerning our business or financial affairs developed by or made known to an individual or entity during the course of that party’s relationship with us is to be kept confidential and not disclosed to third parties, except in certain specified circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual, and that are related to our current or planned business or research and development or made during normal working hours, on our premises or using our equipment or proprietary information, are our exclusive property. In the case of consultants and other third-party service providers, the agreements provide us with certain rights to all inventions arising from the services provided to us by those individuals or entities. However, we cannot guarantee that we have entered into such agreements with each party that may have or have had access to our trade secrets or proprietary technologies and processes. Additionally, the assignment of intellectual property rights may not be self-executing, or assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property. We may not be able to obtain adequate remedies for any breaches of such agreements. Ultimately, enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time-consuming, and the outcome is unpredictable.
In addition to contractual measures, we try to protect the confidential nature of our proprietary information through other appropriate precautions, such as physical and technological security measures. However, trade secrets and know-how can be difficult to protect. These measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and any recourse we might take against this type of misconduct may not provide an adequate remedy to protect our interests fully. In addition, our trade secrets may be independently developed by others in a manner that could prevent us from receiving legal recourse. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, or if any of that information was independently developed by a competitor, our competitive position could be harmed.
In addition, courts inside and outside the United States are sometimes less willing or unwilling to protect trade secrets. If we choose to go to court to stop a third party from using any of our trade secrets, we may incur substantial costs and we cannot guarantee a successful outcome. Even if we are successful, these types of lawsuits may consume significant amounts of our time and other resources. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our competitors or are in breach of non-competition or non-solicitation agreements with our competitors.
We could in the future be subject to claims that we or our employees have inadvertently or otherwise used or disclosed alleged trade secrets or other proprietary information of former employers, competitors, or other third parties. Although we endeavor to ensure that our employees and consultants do not use the intellectual property, proprietary information, know-how or trade secrets of others in their work for us, we may in the future be subject to claims that we caused an employee to breach the terms of his or her non-competition or non-solicitation agreement, or that we or these individuals have, inadvertently or otherwise, used or disclosed the alleged trade secrets or other proprietary information of a former employer or competitor. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and could be a distraction to management. If our defense to those claims fails, in addition to paying monetary damages, a court could prohibit us from using technologies or features that are essential to our product, if such technologies or features are found to incorporate or be derived from the trade secrets or other proprietary information of the former employers or other third parties. An inability to incorporate technologies or features that are important or essential to our product may prevent us from selling our product. In addition, we may lose valuable intellectual property rights or personnel. Moreover, any such litigation or the threat thereof may adversely affect our ability to hire employees or contract with independent sales representatives. A loss of key personnel or their work product could hamper or prevent our ability to commercialize our product.
Changes in U.S. patent law, or laws in other countries, could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other pharmaceutical and biotech companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the pharmaceutical industry involve a high degree of technological and legal complexity. Therefore, obtaining and enforcing pharmaceutical patents is costly, time consuming and inherently uncertain. Changes in either the patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property and may increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. We cannot predict the breadth of claims that may be allowed or enforced in our patents or in our licensor’s patents. In addition, Congress or other foreign legislative bodies may pass patent reform legislation that is unfavorable to us. For example, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty regarding our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the U.S. federal courts, the USPTO, or similar authorities in foreign jurisdictions, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and the patents we might obtain or license in the future. Additionally, the application and interpretation of China’s intellectual property rights laws and the procedures and standards for granting patents, copyrights, know-how or other intellectual property rights in China are still evolving and are uncertain, and we cannot assure you that PRC courts or regulatory authorities would agree with our analysis. If we were found to have violated the intellectual property rights of others, we may be subject to liability and penalties for our infringement activities or may be prohibited from using such intellectual property, and we may incur licensing fees or be forced to develop alternatives of our own. As a result, our business and results of operations may be materially and adversely affected.
Risks related to related to our business
Our financial statements disclose that there is substantial doubt regarding our ability to continue as a going concern, in which case you could lose your investment.
Our independent registered public accounting firm, PWR CPA, LLP, has expressed doubt in their audit opinion for our financial statements for the year ended December 31, 2021 about our ability to continue as a going concern. We have generated minimal revenue and have an accumulated deficit totaling $10,108,916 since inception. In order to obtain the necessary capital to sustain operations, management’s plans include, among other things, the possibility of pursuing new equity sales and/or making additional debt borrowings. There can be no assurances, however, that we will be successful in obtaining additional financing, or that such financing will be available on favorable terms, if at all. If we are unable to obtain financing in the amounts and on terms deemed acceptable, the business and future success may be adversely affected and we may cease operations.
We will need to increase the size of our Company and may not effectively manage our growth.
Our success will depend upon growing our business and our employee base. Over the next twelve months, we plan to add additional employees to assist us with research and development and our commercialization efforts. Our future growth, if any, may cause a significant strain on our management, and our operational, financial and other resources. Our ability to manage our growth effectively will require us to implement and improve our operational, financial and management systems and to expand, train, manage and motivate our employees. These demands may require the hiring of additional management personnel and the development of additional expertise by management. Any increase in resources devoted to research and product development without a corresponding increase in our operational, financial and management systems could have a material adverse effect on our business, financial condition, and results of operations.
Our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on the research and development, clinical, financial, operational and other business expertise of our executive officers, as well as the other principal members of our management, scientific and clinical teams. Although we have entered into employment agreements with our executive officers, each of them may terminate their employment with us at any time. We do not maintain “key person” insurance for any of our executives or other employees. Recruiting and retaining qualified scientific, clinical, manufacturing, accounting, legal and sales and marketing personnel will also be critical to our success.
The loss of the services of our executive officers or other key employees could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain marketing approval of and commercialize products. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. Our success as a public company also depends on implementing and maintaining internal controls and the accuracy and timeliness of our financial reporting. If we are unable to continue to attract and retain high quality personnel, our ability to pursue our growth strategy will be limited.
The point-of-care testing (POCT) market is extremely competitive and rapidly evolving, making it difficult to evaluate our business and future prospects.
The market for POCT testing is extremely competitive. Further, the POCT testing industry, as well as the manner in which healthcare services are delivered more broadly, is currently experiencing rapid change, technological and scientific breakthroughs, new product introductions and enhancements and evolving industry standards, as well as the emergence of telehealth and other changes in the way healthcare services are delivered. All of these factors could affect the degree to which our products gain market acceptance or approval or result in our products being less marketable or becoming obsolete. Our future success will depend on our ability to successfully compete with established and new market participants and to keep pace with scientific and technological changes and the evolving needs of customers and the healthcare marketplace.
We will be required to continuously enhance our products and develop new tests to keep pace with evolving standards of care. If we do not update our products to keep pace with technological and scientific advances, our products could become obsolete and sales of our products could decline or fail to grow as expected.
Many of our current or potential competitors, either alone or with their collaboration partners, have significantly greater financial resources and expertise than we do in research and development, manufacturing, obtaining regulatory clearances and approvals and regulatory compliance, and sales and distribution. Mergers and acquisitions involving POCT testing or other healthcare companies may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies or customer networks. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize POCT products or services that are more accurate, more convenient to use or more cost-effective than our products. Our competitors also may obtain FDA or other regulatory clearance or approval for their products more rapidly than we may obtain clearance or able to enter a particular market.
Further, some of our competitors’ products may be sold at prices that may be lower than our pricing, which could adversely affect our sales or force us to reduce our prices, which could harm our revenue, operating income or market share. If we are unable to compete successfully, we may be unable to increase or sustain our revenue or achieve profitability and our future growth prospects may be materially harmed.
Central labs continue to represent the most significant portion of the diagnostic testing market, and as a result we will be competing against very large and well-established lab companies such as Quest Diagnostics, Inc. and Laboratory Corporation of America. These companies have also expanded beyond centralized laboratory testing into home sample collection. In addition, we also face intense competition from other companies that develop or already have POCT tests.
To remain competitive, we will need to develop improvements to our products and other offerings. We cannot assure you that we will be able to successfully compete in the marketplace or develop and commercialize new tests or improvements to our products and other offerings on a timely basis. Our competitors may develop and commercialize competing or alternative products or services and improvements faster than we are able to do so, which would negatively affect our ability to increase or sustain our revenue or achieve profitability and could materially adversely affect our future growth prospects.
Our business activities are subject to the Foreign Corrupt Practices Act (the “FCPA”), and similar anti-bribery and anti-corruption laws of other countries in which we operate, including Taiwan, as well as U.S. and certain foreign export controls, trade sanctions, and import laws and regulations. Compliance with these legal requirements could limit our ability to compete in foreign markets and subject us to liability if we violate them.
Our business activities are subject to the FCPA and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we operate. The FCPA generally prohibits companies and their employees and third party intermediaries from offering, promising, giving or authorizing the provision of anything of value, either directly or indirectly, to a non-U.S. government official in order to influence official action or otherwise obtain or retain business. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls. There is no certainty that all of our employees, agents or contractors, or those of our affiliates, will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers or our employees, disgorgement, and other sanctions and remedial measures, and prohibitions on the conduct of our business. Any such violations could include prohibitions on our ability to offer our products in one or more countries and could materially damage our reputation, our brand, our international activities, our ability to attract and retain employees and our business, prospects, operating results and financial condition.
In addition, our products and technology may be subject to applicable foreign export controls, trade sanctions and import laws and regulations. Governmental regulation of the import or export of our products and technology, or our failure to obtain any required import or export authorization for our products, when applicable, could harm our international sales and adversely affect our revenue. Compliance with applicable regulatory requirements regarding the export of our products may create delays in the introduction of our products in international markets or, in some cases, prevent the export of our products to some countries altogether. If we fail to comply with export and import regulations and such economic sanctions, penalties could be imposed, including fines and/or denial of certain export privileges. Moreover, any new export or import restrictions, new legislation or shifting approaches in the enforcement or scope of existing regulations, or in the countries, persons, or products targeted by such regulations, could result in decreased use of our products by, or in our decreased ability to export our products to existing or potential customers with international operations. Any decreased use of our products or limitation on our ability to export or sell access to our products would likely adversely affect our business.
Recent geopolitical issues, conflicts and other global events could adversely affect our results of operations and financial condition.
Because a substantial portion of our business is conducted outside of the United States, our business is subject to global political issues and conflicts. Such political issues and conflicts could have a material adverse effect on our results of operations and financial condition if they escalate in areas in which we do business. In addition, changes in and adverse actions by governments in foreign markets in which we do business could have a material adverse effect on our results of operations and financial condition. For example, the recent and continuing conflict arising from the invasion of Ukraine by Russia could adversely impact macroeconomic conditions, give rise to regional instability and result in heightened economic tariffs, sanctions and import-export restrictions from the U.S. and the international community in a manner that adversely affects us, including to the extent that any such actions cause material business interruptions, restrict our ability to conduct business with certain suppliers or vendors, utilize the banking system, or repatriate cash.
We face risks associated with increased political uncertainty.
The recent invasion of Ukraine by Russia and the sanctions, bans and other measures taken by governments, organizations and companies against Russia and certain Russian citizens in response thereto has increased the political uncertainty in Europe and has strained the relations between Russia and a significant number of governments, including the U.S. The duration and outcome of this conflict, any retaliatory actions taken by Russia and the impact on regional or global economies are unknown, but could have a material adverse effect on our business, financial condition and results of our operations.
In the U.S., the change in the U.S. government to the Biden administration has resulted in uncertainty regarding potential changes in regulations, fiscal policy, social programs, domestic and foreign relations and international trade policies. In addition, potential changes in relationships among the U.S. and China and other countries including Taiwan could have significant impacts on global trade and regional economic conditions, among other things. In addition, changes in the relationships between the U.S. and its neighbors, such as Mexico, could have significant, potentially negative, impacts on commerce. Further, anti-American sentiment could harm the reputation and success of U.S. companies doing business abroad.
Our ability to respond to these developments or comply with any resulting new legal or regulatory requirements, including those involving economic and trade sanctions, could increase our costs of doing business, reduce our financial flexibility and otherwise have a material adverse effect on our business, financial condition and results of our operations.
Risks related to our securities
An active trading market for our securities may not develop and the market price of our securities could be volatile.
Through August 8, 2022, our common stock was quoted on the OTCPK, one of the OTC Markets Group over-the counter markets. As of August 9, 2022, our common stock and Warrants are listed on the Nasdaq Capital Market, or Nasdaq under the symbols “AIMD” and “AIMDW,” respectively. . Even though our securities were approved for listing on the Nasdaq Capital Market, an active trading market for our securities may not develop or be sustained. In the absence of an active trading market for our securities, the ability of our stockholders to sell their securities could be limited.
The trading market for our securities in the future could be subject to wide fluctuations in response to several factors, including, but not limited to:
| | · | actual or anticipated variations in our results of operations; |
| | · | our ability or inability to generate revenues or profit; |
| | · | the number of shares in our public float; and |
| | · | increased competition. |
Furthermore, our stock price may be impacted by factors that are unrelated or disproportionate to our operating performance. These market fluctuations, as well as general economic, political and market conditions, such as recessions, interest rates or international currency fluctuations may adversely affect the market price of our common stock. Additionally, moving forward we anticipate having a limited number of shares in our public float, and as a result, there could be extreme fluctuations in the price of our securities.
We do not intend to pay dividends for the foreseeable future and, as a result, our ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We have not declared or paid any cash dividends on our capital stock in 2022, and we do not intend to pay any cash dividends in the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our Board of Directors and may be restricted by the terms of any then-current credit facility. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investments.
We have acquired, and may in the future acquire, assets and technologies as part of our business strategy. If we acquire companies or technologies in the future, they could prove difficult to integrate, disrupt our business, dilute stockholder value, and adversely affect our operating results and the value of our common stock.
As part of our business strategy, we may acquire, enter into joint ventures with, or make investments in complementary or synergistic companies, services, and technologies in the future. Acquisitions and investments involve numerous risks, including without limitation:
| | · | difficulties in identifying and acquiring products, technologies, proprietary rights or businesses that will help our business; |
| | · | difficulties in integrating operations, technologies, services, and personnel; |
| | · | diversion of financial and managerial resources from existing operations; |
| | · | the risk of entering new development activities and markets in which we have little to no experience; |
| | · | risks related to the assumption of known and unknown liabilities; |
| | · | risks related to our ability to raise sufficient capital to fund additional operating activities; and |
| | · | the issuance of our securities as partial or full payment for any acquisitions and investments could result in material dilution to our existing stockholders. |
Upon closing of the Securities Purchase Agreement transaction with Ainos KY in April 2021, the Company acquired numerous patent assets. Additionally, upon closing of the Asset Purchase Agreement with Ainos KY in January 2022, we acquired additional patent assets and equipment. If we fail to integrate the patent assets and equipment into our operations, or if we fail to properly evaluate other acquisitions or investments, we may not achieve the anticipated benefits of any such acquisitions, we may incur costs in excess of what we anticipate, and management resources and attention may be diverted from other necessary or valuable activities.
Any failure to maintain effective internal control over financial reporting could harm us.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with U.S. generally accepted accounting principles. If our management is unable to conclude that we have effective internal control over financial reporting, or to certify the effectiveness of such controls, or if material weaknesses in our internal controls are identified in the future, we could have difficulty in timely and accurately reporting our financial results and could be subject to regulatory scrutiny and a loss of public confidence, any of which could have a material adverse effect on our business and our stock price. Our management has concluded there were deficiencies in the design and implementation of our internal controls as of December 31, 2021, including limited staff resources and the need to expand the segregation of duties. Pursuant to our remediation plan, during the first quarter of 2022 we hired a full-time accounting assistant dedicated to financial reporting and internal controls, delegated specific roles and responsibilities to individuals comprising our management team relating to data compilation, preparation of reports, and oversight of key reporting elements, designated senior management for review and oversight of our reports, and further integrated our Audit Committee and Board for final approvals of our reports. If we are unable to implement our remediation plan or adequately remediate the deficiencies identified or otherwise fail to maintain adequate financial and management personnel, processes and controls, we may not be able to manage our business effectively or accurately report our financial performance on a timely basis, which could cause a decline in our common stock price and adversely affect our results of operations and financial condition.
Our issuance of additional capital stock in connection with financings, acquisitions, investments, our 2021 Stock Incentive Plan or otherwise will dilute all other stockholders.
We may need to raise additional capital through equity and debt financings in order to fund our operations. If we raise capital through equity financings in the future, that will result in dilution to all other stockholders. We also expect to grant equity awards to employees, directors, and consultants under our 2021 Stock Incentive Plan. As part of our business strategy, we may acquire or make investments in complementary companies, products, or technologies and issue equity securities to pay for any such acquisition or investment. These, and any additional such issuances of capital stock will cause stockholders to experience significant dilution of their ownership interests and the per-share value of our common stock to decline.
A decline in the price of our common stock could affect our ability to raise working capital and adversely impact our ability to continue operations.
A prolonged decline in the price of our common stock could result in a reduction in the liquidity of our common stock and a reduction in our ability to raise capital. A decline in the price of our common stock could be especially detrimental to our liquidity, our operations and strategic plans. Such reductions may force us to reallocate funds from other planned uses and may have a significant negative effect on our business plan and operations, including our ability to develop new services and continue our current operations. If our common stock price declines, we can offer no assurance that we will be able to raise additional capital or generate funds from operations sufficient to meet our obligations. If we are unable to raise sufficient capital in the future, we may not be able to have the resources to continue our normal operations.
The Warrants offered by this prospectus may not have any value.
The Warrants offered by this prospectus will be exercisable for five years from the date of initial issuance at an initial exercise price of $4.25 per share of common stock. There can be no assurance that the market price of our common stock will ever equal or exceed the exercise price of the Warrants. In the event that our common stock price does not exceed the exercise price of the Warrants during the period when the Warrants are exercisable, the Warrants may not have any value.
Holders of Warrants do not entitle the holder to any rights as common stockholders until the holder exercises the Warrants for shares of our common stock.
Until you acquire shares of our common stock upon exercise of your Warrants, your Warrants will not provide you any rights as a common stockholder. Upon exercise of your Warrants, you will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
If we are not able to comply with the applicable continued listing requirements or standards of the Nasdaq Capital Market, Nasdaq could delist our securities.
Through August 8, 2022, our common stock was quoted on the OTCPK, one of the OTC Markets Group over-the counter markets. As of August 9, 2022, our common stock and Warrants are listed on the Nasdaq Capital Market, or Nasdaq under the symbols “AIMD” and “AIMDW,” respectively. Although, after giving effect to this offering we expect to meet the minimum initial listing standards set forth in the Nasdaq Listing Standards, we cannot assure you that our securities will be, or will continue to be, listed on The Nasdaq Capital Market in the future. In order to maintain that listing, we must satisfy minimum financial and other continued listing requirements and standards, including those regarding director independence and independent committee requirements, minimum stockholders’ equity, minimum share price, and certain corporate governance requirements. We may not be able to comply with the applicable listing standards and Nasdaq could delist our securities as a result.
We cannot assure you that our common stock and Warrants, if delisted from The Nasdaq Capital Market, will be listed on another national securities exchange. If our securities are delisted by The Nasdaq Capital Market, our common stock would likely trade on the OTCQB where an investor may find it more difficult to sell our securities or obtain accurate quotations as to the market value of our common stock.
Techniques employed by short sellers may drive down the market price of the common stock.
Short selling is the practice of selling securities that the seller does not own but rather has borrowed from a third party with the intention of buying identical securities back at a later date to return to the lender. The short seller hopes to profit from a decline in the value of the securities between the sale of the borrowed securities and the purchase of the replacement shares, as the short seller expects to pay less in that purchase than it received in the sale.
As it is in the short seller’s interest for the price of the security to decline, many short sellers publish, or arrange for the publication of, negative opinions regarding the relevant issuer and its prospects to create negative market momentum and generate profits for themselves after selling a security short. These short attacks have, in the past, led to selling of shares in the market.
It is not clear what effect such negative publicity could have on us. If we were to become the subject of any unfavorable allegations, whether such allegations are proven to be true or untrue, we could have to expend significant resources to investigate such allegations and/or defend ourselves.
While we would strongly defend against any such short seller attacks, we may be constrained in the manner in which we can proceed against the relevant short seller by principles of freedom of speech, applicable state law or issues of commercial confidentiality. Such a situation could be costly and time-consuming, and could distract our management from growing our business. Even if such allegations are ultimately proven to be groundless, allegations against us could severely impact our business, and any investment in the common stock could be greatly reduced or even rendered worthless.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, the market price for the common stock and trading volume could decline.
The trading market for our securities will depend in part on the research and reports that securities or industry analysts publish about us or our business. If research analysts do not establish and maintain adequate research coverage or if one or more of the analysts who covers us downgrades our securities or publishes inaccurate or unfavorable research about our business, the market price for our securities would likely decline. If one or more of these analysts ceases coverage of our company or fails to publish reports on us regularly, we could lose visibility in the financial markets, which, in turn, could cause the market price or trading volume for the common stock to decline.
Our management will have broad discretion over the use of any net proceeds from this offering and you may not agree with how we use the proceeds, and the proceeds may not be invested successfully.
Our management will have broad discretion as to the use of any net proceeds from this offering and could use them for purposes other than those contemplated at the time of this offering and in ways that do not necessarily improve our results of operations or enhance the value of our common stock. Accordingly, you will be relying on the judgment of our management with regard to the use of any proceeds from the exercise of warrants on a cash basis in this offering and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. The proceeds could be invested in a way that does not yield a favorable, or any, return for you.
Our certificate of formation and the Texas Business Combination Law contain anti-takeover provisions that could adversely affect the rights of holders of our common stock.
Our certificate of formation contains provisions to limit the ability of others to acquire control of our company or cause us to engage in change-of-control transactions. These provisions could deprive our shareholders of an opportunity to sell their shares at a premium over prevailing market prices by discouraging third parties from seeking to obtain control of our company in a tender offer or similar transaction.
Our board of directors has the authority to issue shares of preferred stock in one or more series and to fix their designations, powers and preferences, including dividend rights, conversion rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be greater than the rights associated with our common stock. Shares of preferred stock could be issued quickly with terms calculated to delay or prevent a change in control of our company or make removal of management more difficult. If our board of directors decides to issue preferred stock, the price of our common stock may fall and the voting and other rights of the holders of our common stock may be materially adversely affected.
Vacancies and newly created seats on our board may be filled only by a majority of the directors then in office. Only our Board may determine the number of directors on our board. The inability of stockholders to determine the number of directors or to fill vacancies or newly created seats on our Board makes it more difficult to change the composition of our board, but these provisions promote a continuity of existing management.
In addition, we are subject to the provisions of the Texas Business Combination Law. That law provides that a Texas corporation may not engage in specified types of business combinations, including mergers, consolidations and asset sales, with a person, or an affiliate or associate of that person, who is an “affiliated shareholder”, for a period of three years from the date that person became an affiliated shareholder, subject to certain exceptions. An “affiliated shareholder” is generally defined as the holder of 20% or more of the corporation’s voting shares. The law’s prohibitions do not apply if the business combination or the acquisition of shares by the affiliated shareholder was approved by the corporation’s board before the affiliated shareholder became an affiliated shareholder; or the business combination was approved by the affirmative vote of the holders of at least two-thirds of the outstanding voting shares of the corporation not beneficially owned by the affiliated shareholder, at a meeting of shareholders called for that purpose, not less than six months after the affiliated shareholder became an affiliated shareholder. The Texas Business Combination Law may have the effect of inhibiting a non-negotiated merger or other business combination involving our Company, even if that event would be beneficial to our shareholders.
USE OF PROCEEDS
We estimate that our net proceeds from this offering will be approximately $2.1 million, based on the sale of the maximum amount of Units in this offering at an assumed public offering price of $4.25 per Unit (or $2.6 million if the underwriters exercise their over-allotment option in full) after deducting the underwriter fees and estimated offering expenses payable by us. These estimates exclude the proceeds, if any, from the exercise of the Warrants sold in this offering. If all of the Warrants sold in this offering were to be exercised in cash at an exercise price of $4.25 per share, we would receive additional net proceeds of approximately $3.3 million. We cannot predict when or if these Warrants will be exercised.
Each $1.00 increase or decrease in the assumed public offering price of $4.25 per Unit would increase or decrease, respectively, our net proceeds by approximately $0.7 million, assuming the maximum number of Units offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriter fees and estimated offering expenses payable by us. We may also increase or decrease the number of Units we are offering. An increase or decrease of 50,000 in the number of Units we are offering would increase or decrease, respectively, the net proceeds from this offering, after deducting underwriter fees and estimated offering expenses payable by us, by approximately $0.2 million, assuming the assumed public offering price stays the same.
We intend to use the net proceeds of this offering for product commercialization, technology development, commercial scale up of our activities, clinical trials and working capital and other general corporate purposes.
Our expected use of net proceeds from the offering represents our current intentions based upon our present plans and business condition. Investors are cautioned, however, that expenditures may vary substantially from these uses. Investors will be relying on the judgment of our management, who will have broad discretion regarding the application of the proceeds of this offering. The amounts and timing of our actual expenditures will depend upon numerous factors, including the amount of cash generated by our operations, the amount of competition and other operational factors. We may find it necessary or advisable to use portions of the proceeds from this offering for other purposes.
DIVIDEND POLICY
We have not paid any dividends on our common stock to date. Any payment of cash dividends on our common stock in the future will be dependent upon the amount of funds legally available, our earnings, if any, our financial condition, our anticipated capital requirements and other factors that the Board may think are relevant. However, we currently intend for the foreseeable future to follow a policy of retaining all of our earnings, if any, to finance the development and expansion of our business and, therefore, do not expect to pay any dividends on our common stock during such time.
CAPITALIZATION
The following table presents the number of our issued and outstanding shares of common stock and our cash and cash equivalents and capitalization as of March 31, 2022 (on a post-split basis):
| | · | on an actual basis; |
| | · | on a pro forma basis to give effect to (i) the $26,000,000 Convertible Note issued on January 30, 2022 in connection with the Asset Purchase Agreement between the Company and Ainos KY dated November 18, 2021 (the “APA Convertible Note”) and the conversion of the APA Convertible Note into 7,647,058 shares of common stock concurrently with the consummation of this offering at a conversion price of $3.40 per share (which represents a 20% discount to the public offering price of $4.25 per Unit); (ii) the March 2027 Convertible Notes issued between March 28 and April 11, 2022 and the conversion of the March 2027 Convertible Notes into 411,760 shares of common stock concurrently with the consummation of this offering (which represents a 20% discount to the public offering price of $4.25 per Unit); and (iii) the conversion of $3,000,000 principal amount of convertible notes plus accrued interest thereon into 1,014,319 shares of common stock concurrently with the consummation of this offering at a conversion price of $3.00 per share; and |
| | · | on a pro forma, as adjusted basis to give effect to (i) the pro forma adjustment set forth above, and (ii) the issuance and sale by us of 780,000 Units in this offering based on the public offering price of $4.25 per Unit, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us and the receipt by us of the proceeds of such sale. |
You should read the information in this table together with our financial statements and the related notes included elsewhere in this prospectus and the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of this prospectus.
| | | As of March 31, 2022 | |
| | | Actual | | | Pro Forma | | | Pro Forma As Adjusted | |
| | | | | | (unaudited) | | | (unaudited) | |
| (in thousands, except share and per share data) | | | | | | | | | |
| Cash and cash equivalents | | $ | 1,871,349 | | | | 2,371,349 | | | | 4,466,349 | |
| Convertible notes payable | | | | | | | | | | | | |
| APA Convertible Note | | $ | 26,000,000 | | | | - | | | | - | |
| March 2027 Convertible Notes | | $ | 900,000 | | | | - | | | | - | |
| Other convertible notes - principal and accrued interest | | $ | 3,416,067 | | | | 373,108 | | | | 373,108 | |
| | | $ | 30,316,067 | | | | 373,108 | | | | 373,108 | |
| Stockholders’ equity (deficit): | | | | | | | | | | | | |
| Preferred stock, $0.01 par value, 10,000,000 shares authorized, 0 shares issued and outstanding, actual, pro forma and pro forma, as adjusted | | | - | | | | - | | | | - | |
| Common Stock, $0.01 par value, 300,000,000 shares authorized, 9,625,287 issued and outstanding, actual; 300,000,000 shares authorized, 18,698,423 shares issued and outstanding, pro forma; 300,000,000 shares authorized, 19,478,423 shares issued and outstanding, pro forma as adjusted | | $ | 96,253 | | | $ | 186,985 | | | $ | 194,785 | |
| Additional paid-in capital | | $ | 20,247,413 | | | $ | 50,599,640 | | | $ | 52,686,840 | |
| Accumulated deficit | | $ | (12,208,811 | ) | | $ | (12,228,578 | ) | | $ | (12,228,578 | ) |
| Translation Adjustment | | $ | (52,259 | ) | | | (52,259 | ) | | | (52,259 | ) |
| Total stockholders’ (deficit) equity | | $ | 8,082,596 | | | $ | 38,505,788 | | | $ | 40,600,788 | |
| Total capitalization | | $ | 38,398,662 | | | $ | 38,878,896 | | | $ | 40,973,895 | |
The number of shares of common stock that will be outstanding both before and immediately after this offering is based on shares outstanding as of March 31, 2022 (on a post-split basis) and excludes:
| | · | 144,644 shares of common stock issuable upon the conversion of outstanding convertible promissory notes at a weighted average conversion price of approximately $2.70 per share, which are not converting concurrently with the consummation of this offering; |
| | · | 30,174 shares of common stock issuable upon the exercise of outstanding warrants at an exercise price of $4.05 per share; |
| | · | 36,666 shares of common stock issuable upon the exercise of outstanding equity awards under our 2018 Officers, Directors, Employees and Consultants Nonqualified Stock Option Plan at a weighted average exercise price of $5.70 per share; |
| | · | 1,333,333 shares of common stock reserved for future issuance under our 2021 Stock Incentive Plan; |
| | · | 50,000 shares of common stock reserved for future issuance under our 2021 Employee Stock Purchase Plan; |
| | · | 780,000 shares of common stock issuable upon the exercise of the Warrants issued as part of the Units issued in this offering at an exercise price of $4.25 per share (which shall not be less than 100% of the public offering price of each Unit); and |
| | · | 39,000 shares of our common stock issuable upon the exercise of the Representative’s Warrants to be issued to the representative of the underwriters in this offering at an exercise price of $4.68 per share. |
DILUTION
If you invest in our securities in this offering, your ownership interest will be diluted immediately to the extent of the difference between the public offering price per share of common stock included in each Unit (attributing no value to the Warrants) and the pro forma as-adjusted net tangible book value per share after this offering.
As of March 31, 2022, we had a historical net tangible book value (deficit) of ($28,131,427), or approximately ($2.92) per share of common stock based on 9,625,287 shares of common stock outstanding as of such date. Our historical net tangible book value (deficit) per share represents our total tangible assets less total liabilities divided by the number of shares of common stock outstanding as of March 31, 2022.
As of March 31, 2022, our pro forma net tangible book value was $2,291,765, or $0.12 per share of common stock. Pro forma net tangible book value per share represents our total tangible assets less total liabilities, divided by the number of shares of common stock outstanding as of March 31, 2022, after giving effect to the conversion, immediately prior to the completion of this offering of (i) the APA Convertible Note into 7,647,058 shares of our common stock, a 20% discount to the public offering price of $4.25 per Unit, (ii) the March 2027 Convertibles Notes into 411,760 shares of our common stock, at a 20% discount to the public offering price of $4.25 per Unit and (iii) the $3,000,000 principal amount of convertible notes plus accrued interest thereon into 1,014,319 shares of our common stock at a conversion price of $3.00 per share.
After giving effect to the sale of 780,000 shares of common stock included in each Unit in this offering at a public offering price of $4.25 per share of common stock included in each Unit, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, and assuming no exercise of the Warrants issued as part of the Units, our pro forma as adjusted net tangible book value as of March 31, 2022 would have been approximately $4.4 million, or approximately $0.23 per share. This represents an immediate increase in net tangible book value of approximately $0.10 per share to our existing security holders and an immediate dilution in as-adjusted net tangible book value of approximately $4.02 per share to purchasers of shares in this offering (attributing no value to the Warrants), as illustrated by the following table:
| Public offering price per share | | $ | 4.25 | |
| Historical net tangible book value (deficit) per share as of March 31, 2022 | | $ | (2.92 | ) |
| Increase in net tangible book value per share attributable to the pro forma adjustments described above | | | 3.05 | |
| Pro forma net tangible book value per share as of March 31, 2021, before giving effect to this offering | | $ | 0.12 | |
| Increase in pro forma net tangible book value per share attributable to new investors participating in this offering | | $ | 0.10 | |
| Pro forma as adjusted net tangible book value per share after this offering | | $ | 0.23 | |
| Dilution in pro forma as adjusted net tangible book value per share to new investors participating in this offering | | $ | 4.02 | |
If the underwriters exercise their option to purchase additional Units in full, our pro forma as adjusted net tangible book value per share after this offering would be $0.25, representing an immediate increase in pro forma as adjusted net tangible book value per share of $0.13 to existing stockholders and immediate dilution in pro forma as adjusted net tangible book value per share of $4.00 to new investors purchasing shares of common stock in this offering, at a public offering price of $4.25 per Unit and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The following table summarizes, as of March 31, 2022, on the pro forma as adjusted basis described above, the total number of shares of common stock purchased from us on an as converted to common stock basis, the total consideration paid or to be paid and the average price per share paid or to be paid by existing stockholders and by new investors in this offering at an public offering price of $4.25 per Unit, before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. As the table shows, new investors purchasing shares of common stock in this offering will pay an average price per share lower than our existing stockholders paid.
| | | Shares Purchased | | | Total Consideration | | | Weighted- Average Price | |
| | | Number | | | Percentage | | | Amount | | | Percentage | | | Per Share | |
| Existing stockholders(1) | | | 18,698,423 | | | | 96 | % | | $ | 82,309,491 | | | | 96 | % | | | 4.40 | |
| New investors | | | 780,000 | | | | 4 | % | | $ | 3,315,000 | | | | 4 | % | | | 4.25 | |
| Total | | | 19,478,423 | | | | 100 | % | | | 85,624,491 | | | $ | 100 | % | | | 4.40 | |
| (1) | The presentation in this table regarding ownership by existing stockholders does not give effect to any purchases that existing stockholders may make through this offering. |
The table above assumes no exercise of the underwriters option to purchase additional Units in this offering. If the underwriters exercise their option to purchase additional shares of common stock in full, the number of shares of our common stock held by existing stockholders would be reduced to 95% of the total number of shares of our common stock outstanding after this offering, and the number of shares of common stock held by new investors purchasing shares of common stock in this offering would be increased to 5% of the total number of shares of our common stock outstanding after this offering.
To the extent that outstanding options or warrants outstanding as of the date of this prospectus, have been or may be exercised or other shares issued, investors participating in this offering may experience further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
The number of shares of common stock that will be outstanding both before and immediately after this offering excludes:
| | · | 144,644 shares of common stock issuable upon the conversion of outstanding convertible promissory notes at a weighted average conversion price of approximately $2.70 per share, which are not converting concurrently with the consummation of this offering; |
| | · | 30,174 shares of common stock issuable upon the exercise of outstanding warrants at an exercise price of $4.05 per share; |
| | · | 36,666 shares of common stock issuable upon the exercise of outstanding equity awards under our 2018 Officers, Directors, Employees and Consultants Nonqualified Stock Option Plan at a weighted average exercise price of $5.70 per share; |
| | · | 1,333,333 shares of common stock reserved for future issuance under our 2021 Stock Incentive Plan; |
| | · | 50,000 shares of common stock reserved for future issuance under our 2021 Employee Stock Purchase Plan; |
| | · | 39,000 shares of common stock issuable upon the exercise of the Representative’s Warrants to be issued to the representative of the underwriters at an exercise price of $4.68 per share (which shall not be less than 100% of the public offering price of each Unit); and |
| | · | 780,000 shares of our common stock issuable upon the exercise of the Warrants issued as part of the Units in this offering at an exercise price of $4.25 per share. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
Overview
We are engaged in developing medical technologies for point-of-care (POCT) testing and safe and novel medical treatment for a broad range of disease indications. Since our inception in 1984, we have concentrated our resources on business planning, raising capital, research and clinical development activities for our programs, securing related intellectual property and commercialization of proprietary therapeutics using low-dose non-injectable interferon (IFN). In addition to our core IFN technology, we are committed to developing a diversified healthcare business portfolio to include medical devices and consumer healthcare products.
Although we have historically been involved in extensive pharmaceutical research and development of low-dose oral interferon as a therapeutic, we are prioritizing the commercialization of medical devices as part of our diversification strategy. Since the beginning of 2021, we have acquired significant intellectual property from our majority shareholder, Ainos KY, to expand our potential product portfolio into Volatile Organic Compounds (VOC) and COVID-19 POCTs. This includes 51 issued and pending patents related to VOC technologies and 3 issued patents for COVID-19 POCT products. We expect our underlying intellectual property to enable us to expedite the commercialization of our medical device pipeline, beginning with Ainos-branded COVID-19 POCT product candidates.
Our Portfolio of Products
Our portfolio of products is currently comprised of the following:
| | · | COVID-19 Antigen Rapid Test Kit and Ainos’ Cloud-based Test Management Apps. Our cloud-based test management platform is comprised of the Ainos COVID-19 antigen rapid test kit (“Ainos COVID-19 Antigen Rapid Test Kit”), a personal management application, or app, and an enterprise management app. We anticipate our management apps will allow individuals and/or organizations to seamlessly manage tests, trace infections, and share results. As the first commercialized COVID-19 product we sell, we currently market the Ainos COVID-19 Antigen Rapid Test Kit in Taiwan under emergency use authorization (EUA) for healthcare professional use and self-test use issued by the Taiwan Federal and Drug Administration (TFDA). We market the Ainos COVID-19 Antigen Rapid Test Kit, with manufacturing by TCNT, our product codeveloper. See “Item 1 - Business - Strategic Investment and Business Development with Ainos KY”. We expect TCNT to apply for EUA authorization from the U.S. Food and Drug Administration (FDA) sometime in the second half of 2022 and, if a EUA is granted by the FDA, we intend to market this product in the U.S. |
| | | |
| | · | COVID-19 Nucleic Acid Test Kit. This test consists of a color-changing assay and portable test equipment. The assay is compatible with most standard Polymerase Chain Reaction (PCR) machines and delivers test results within 40 minutes, which is faster than the typical PCR test turnaround time which can vary. In addition to our assay’s compatibility with most PCR equipment, we will also offer portable, low-cost test equipment intended to help medical professionals quickly scale testing capacity. Upon TCNT receiving regulatory approval, we will market the product under the Ainos brand name, with TCNT manufacturing the product. See “Item 1 - Business - Strategic Investment and Business Development with Ainos KY.” The product is currently under EUA review by the TFDA and we expect TCNT to submit for EUA review by the FDA sometime in the second half of 2022. |
| | | |
| | · | VOC POCT - Ainos Flora. Our Ainos Flora device is designed to perform a non-invasive test for female vaginal health and certain sexually transmitted diseases (“STDs”) including chlamydia, gonorrhea and trichomoniasis, within a few minutes. We expect Ainos Flora will provide convenient, discreet, rapid testing in a point-of-care setting which will allow women to self-test at home. We intend to work with TCNT to conduct clinical trials in Taiwan, after which we expect TCNT to submit applications for TFDA approval for marketing in Taiwan and FDA 510(k) clearance for marketing in the U.S. sometime in the second half of 2022. |
| | · | VOC POCT - Ainos Pen. Our Ainos Pen device is a cloud-connected, multi-purpose, portable breath analyzer that is intended to monitor health conditions including oral, gastrointestinal, liver, and renal health within minutes. We expect consumers to be empowered to share their self-test results with their physicians through in-person and telehealth medical consultations. We intend to explore commercialization opportunities with third-party collaborators as a consumer health device sometime in the second half of 2022. |
| | | |
| | · | VOC POCT - CHS430. The CHS430 device is intended to provide non-invasive testing for ventilator-associated pneumonia within 10 minutes. We plan to be the exclusive sales agent for CHS430, pursuant to our Product Development Agreement with our co-developer, TCNT. We intend to collaborate with TCNT to conduct clinical trials in Taiwan, after which we expect TCNT to submit applications for TFDA approval for marketing in Taiwan and FDA 510(k) clearance for marketing in the U.S. in 2023. |
| | | |
| | · | Very Low-Dose Oral Interferon Alpha (VELDONA). VELDONA is a low-dose oral interferon alpha (IFN-α) formulation based on our nearly four decades of research on IFN-α’s potential treatment applications. Our leading product candidate is a low-dose oral treatment for COVID-19. We have conducted a parallel study based on VELDONA alone and joint study with Innopharmax, Inc. We recently completed our animal studies and subsequently we plan to initiate Phase 2/3 clinical trials for the VELDONA-only program sometime in mid-2022 in Taiwan. We also plan to advance our VELDONA development efforts for disease indications such as thrombocytopenia and Sjögren’s syndrome in 2023. |
| | | |
| | · | Synthetic RNA (SRNA). We are developing a SRNA technology platform in Taiwan. Our initial focus is to develop a potential COVID-19 mRNA vaccine platform using the full-length spike or the receptor-binding domain (RBD) gene sequence of the alpha and delta variants as reference sequences. We plan to continue developing these technologies with the goal of initiating clinical trials in Taiwan in 2023. |
An integral part of our operating strategy is to create multiple revenue streams through commercializing our product portfolio and leveraging our intellectual property patents, including potentially out-licensing or forming strategic relationships to develop our medical devices, consumer healthcare products and low-dose interferon therapeutics.
In 2022, we are prioritizing the commercialization of our POCT devices, beginning with TCNT seeking EUA authorizations for the COVID-19 POCT product candidates. and plans to commercialize our other POCT product candidates. As a general strategy for our other POCT product candidates, we plan to conduct clinical trials in Taiwan and use the data to apply for TFDA approval and FDA clearance via the 510(k) or comparable pathway. If the products are approved, we plan to work with third-party distributors to market our products in countries where we receive regulatory approval and to seek various business relationships with other medtech companies to market our products. At the same time, we plan to initiate clinical trials in Taiwan for the COVID-19 oral treatment and continue our SRNA development programs over the course of this year.
Our ability to generate product revenue sufficient to achieve profitability will depend on further successful development and commercialization of one or more of our current or future product candidates and programs. We anticipate our POCT products candidates to potentially generate organic cash flows to support our business while we invest in our other pipeline projects. We expect to continue to incur significant expenses for the next few years as we advance our product candidates through preclinical development, clinical trials and regulatory approval. In addition, if we obtain marketing approval for any of our product candidates, we expect to incur significant commercialization expenses related to product manufacturing, marketing, sales and distribution, and legal and regulatory compliance. We may also incur expenses in connection with strategic relationships for the development of additional product candidates. Furthermore, we expect to continue to incur costs associated with operating as a public company, including significant legal, accounting, investor relations and other expenses.
Until we can generate significant revenue from product sales, if ever, we expect to finance our operations with business revenues and proceeds from external sources. We may pursue additional funding that may include our entry into or expansion of borrowing arrangements; research and development incentive payments, government grants, co-financing from pharmaceutical companies and other corporate sources; and potential future collaboration agreements with pharmaceutical companies or other third parties. We may be unable to raise additional funds or enter into such other agreements or arrangements when needed on favorable terms. If we fail to raise capital or enter into such agreements as, and when, needed, we may have to significantly delay, scale back or discontinue the development and commercialization, potential in-licenses or acquisitions plans for one or more of our product candidates.
We are unable to predict the timing or amount of unexpected expenses or when or if we will be able to achieve or maintain profitability due to the numerous risks and uncertainties associated with product development and related legal regulatory requirements. In the event that we are eventually able to generate additional product sales, those sales may not be sufficient to become profitable. If we fail to become profitable or are unable to sustain profitability on a continuing basis, we may be unable to continue our operations at planned levels and be forced to reduce or terminate our operations.
As of March 31, 2022, we had available cash and cash equivalents of approximately $1,871,349. We anticipate business revenues and further potential financial support from outside sources to fund our operations over the next twelve months. We have based this estimate on assumptions that may prove to be wrong and we could exhaust our available capital resources sooner than we expect. See “- Liquidity and Capital Resources” for additional information. To finance our continuing operations, we will need to raise additional capital, which cannot be assured.
Strategic Investment and Business Development with Ainos KY
In order to facilitate our diversification strategy through development and commercialization of an expanded base of product offerings we secured a strategic investor, Ainos KY. In December 2020, we entered into a Securities Purchase Agreement pursuant to which, on April 15, 2021, in exchange for 6,666,666 shares of our common stock, we acquired intellectual property assets from Ainos KY valued at approximately $20,000,000. Through this relationship, we have implemented several strategic initiatives over the past year to augment our product development pipeline and achieve a strengthened financial position.
The following are highlights of recent major corporate milestones that we believe will serve as catalysts for us to develop and commercialize a multi-faceted product portfolio pipeline over the next several years:
| | · | On June 14, 2021, we became the master sales and marketing agent for the Ainos COVID-19 Antigen Rapid Test Kit and Ainos COVID-19 Nucleic Acid Test Kit. We generated $592,042 in revenue from sales of the Ainos COVID-19 Antigen Rapid Test Kit in Taiwan in 2021 and $87,200 for the three months ended March 31, 2022. |
| | | |
| | · | On November 18, 2021, Ainos KY agreed to increase its strategic investment to further bolster our POCT business through an Asset Purchase Agreement pursuant to which we acquired additional intellectual property and equipment assets valued at approximately $26,000,000 including technical know-how, medical device manufacturing, testing and office equipment in Taiwan and hired certain of Ainos KY’s R&D personnel. As payment for the assets, in January 2022, we issued to Ainos KY a non-interest bearing Convertible Note in the principal amount of $26,000,000. See “- Liquidity and Capital Resources” for additional information. |
| | | |
| | · | On December 7, 2021, we entered into a strategic relationship with InnoPharmax, Inc., a biopharmaceutical company focused on new oral drug formulations, to jointly develop an orally administered CICCT for the treatment of COVID-19 and potentially other viral infections. The CICCT initiative leverages our interferon clinical data on influenza and integrates our interferon therapeutic platform, VELDONA, and InnoPharmax’s antiviral drug, GemOral. We also initiated a parallel study for the COVID-19 oral treatment solely based on VELDONA. Based on our animal studies, we intend to advance the VELDONA-only program as our candidate for the COVID-19 oral treatment. |
Impact of COVID-19 on Our Business
The COVID-19 pandemic presented us an opportunity to grow our business in 2021. We began selling and recording product revenue for Ainos COVID-19 Antigen Rapid Test Kits in June 2021 after TCNT obtained TFDA EUA in the same month. Between June and December 2021, we generated $592,042 of revenues from the Ainos COVID-19 Antigen Rapid Test Kit and $87,200 for the three months ended March 31, 2022.
.
In 2021, substantially all of our operating revenue came from the sale of the Ainos COVID-19 Antigen Rapid Test Kits in Taiwan. Substantially all of our operating revenue in 2022 to date has come from the sale of the Ainos COVID-19 Antigen Rapid Test Kits in Taiwan. According to Taiwan Centers for Disease Control (Taiwan CDC), COVID-19 infection rates totaled 17,029 as of March 31, 2022. As a result, the total revenues we generated from the Ainos COVID-19 Antigen Rapid Test Kit in the first quarter of 2022 was $87,200, which was less than we anticipated. We intend to broaden our market reach if TCNT successfully obtains regulatory clearance in the U.S. or other countries. COVID-19 cases have since increased in Taiwan since April 2022 and we recently began marketing Ainos COVID-19 Antigen Rapid Test Kits for the home-use segment in Taiwan. Accordingly, we expect our test kit sales to increase in the near-term.
We believe affordable, easy-to-use, rapid COVID-19 testing will continue to be in demand at least in the near-term. We anticipate our management apps, when used with the Ainos COVID-19 Antigen Rapid Test Kit, will allow individuals and organizations to effectively manage tests, trace infections, and share results. We also anticipate the Ainos COVID-19 Nucleic Acid Test Kit can help medical professionals quickly scale testing capacity if the product receives regulatory clearance.
We are continuing to monitor the potential impact of the pandemic, but we cannot be certain the future impact on our business, financial condition, results of operations and prospects. Depending on developments relating to the pandemic, including the emergence of new variants, the pandemic may affect our ability to initiate and complete research studies, delay the initiation of our future research studies, disrupt regulatory activities or have other adverse effects on our business, results of operations, financial condition and prospects.
Results of Operations
The following table sets forth the significant components of our results of operations for the periods presented.
| | | Three months ended | | | | |
| | | March 31 (unaudited) | | | Year ended December 31, | |
| | | 2022 | | | 2021 | | | 2021 | | | 2020 | |
| Revenues | | | 87,200 | | | | 2,121 | | | | 594,563 | | | | 16,563 | |
| Cost of revenues | | | (41,078 | ) | | | (1,249 | ) | | | (184,181 | ) | | | (11,277 | ) |
| Gross margin | | | 46,122 | | | | 872 | | | | 410,382 | | | | 5,286 | |
| | | | | | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development expenses | | | 1,577,454 | | | | - | | | | 1,920,645 | | | | 389 | |
| Selling, general and administrative expenses | | | 551,730 | | | | 522,981 | | | | 2,357,163 | | | | 1,445,332 | |
| Total operating expenses | | | 2,129,184 | | | | 522,981 | | | | 4,277,808 | | | | 1,445,721 | |
| | | | | | | | | | | | | | | | | |
| Operating loss | | | (2,083,062 | ) | | | (522,109 | ) | | | (3,867,426 | ) | | | (1,440,435 | ) |
| | | | | | | | | | | | | | | | | |
| Non-operating income and expenses | | | | | | | | | | | | | | | | |
| Interest expense, net | | | (16,687 | ) | | | (11,897 | ) | | | (18,689 | ) | | | (10,185 | ) |
| Other Losses | | | (146 | ) | | | - | | | | (2,547 | ) | | | (3 | ) |
| | | | (16,833 | ) | | | (11,897 | ) | | | (21,236 | ) | | | (10,188 | ) |
| Net loss | | | (2,099,895 | ) | | | (534,006 | ) | | | (3,888,661 | ) | | | (1,450,623 | ) |
Comparison of the three months ended March 31, 2022 and 2021
The following table summarizes our results of operations for the three months ended March 31, 2022 and 2021:
| | | Three months | | | | |
| | | ended March 31, | | | Change | |
| | | 2022 | | | 2021 | | | Amount | | | % | |
| Revenues | | | 87,200 | | | | 2,121 | | | | 85,079 | | | | 4,011 | % |
| Cost of revenues | | | (41,078 | ) | | | (1,249 | ) | | | (39,829 | ) | | | 3,189 | % |
| Gross margin | | | 46,122 | | | | 872 | | | | 45,250 | | | | 5,189 | % |
| | | | | | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development expenses | | | 1,577,454 | | | | - | | | | 1,577,454 | | | | - | % |
| Selling, general and administrative expenses | | | 551,730 | | | | 522,981 | | | | 28,749 | | | | 6 | % |
| | | | 2,129,184 | | | | 522,981 | | | | 1,606,203 | | | | 307 | % |
| | | | | | | | | | | | | | | | | |
| Operating loss | | | (2,083,062 | ) | | | (522,109 | ) | | | (1,560,953 | ) | | | 299 | % |
| | | | | | | | | | | | | | | | | |
| Non-operating income and expenses | | | | | | | | | | | | | | | | |
| Interest expense, net | | | (16,687 | ) | | | (11,897 | ) | | | (4,790 | ) | | | 40 | % |
| Other Losses | | | (146 | ) | | | - | | | | (146 | ) | | | - | % |
| | | | (16,833 | ) | | | (11,897 | ) | | | (4,936 | ) | | | 41 | % |
| Net loss | | | (2,099,895 | ) | | | (534,006 | ) | | | (1,565,889 | ) | | | 293 | % |
Revenues, Costs and Gross Margins
We reported $87,200 in revenue in the three months ended March 31, 2022 from product sales of the Ainos COVID-19 Antigen Rapid Test Kits. Revenue from product sales in the three months ended March 31, 2021 was $2,121 generated from sales of liposomal nutraceuticals. The cost of sales relating to product sales in the three months ended March 31, 2022 was $41,078 compared to $1,249 in the three months ended March 31, 2021. Gross profit from product sales in the three months ended March 31, 2022 was $46,122 as compared to $872 in the three months ended March 31, 2021. Gross profits generated from product sales increased by $45,250 between the three months ended March 31, 2022 and the same quarter in the previous year. In 2022, we discontinued sales of liposomal nutraceuticals and instead plans to focus on sales of POCTs, including the Ainos COVID-19 Antigen Test Kit.
Research and Development (R&D) Expenses
R&D expenses in the three months ended March 31, 2022 were $1,577,454 mainly consisting of amortization expense of intellectual property assets, staffing and co-development research. There were no R&D expenses during the three months ended March 31, 2021. We expect that our R&D expenses will increase over time as we further develop our POCT and other product candidates. In addition to increasing our in-house R&D staffing, we also contribute R&D funding under our co-development agreements with Taiwan Carbon Nano Technology (“TCNT”), our manufacturing collaborator and our affiliate company for POCT products, and InnoPharmax, Inc., to jointly develop and promote an orally administered CICCT for the treatment of COVID-19 and potentially other viral infections.
Selling, General and Administrative Expenses
Selling, general and administrative expenses were $551,730 and $522,981 in the three months ended March 31, 2022 and the three months ended March 31, 2021, respectively. The $28,749 (6%) increase was largely due to increased expenses associated with staffing and consulting.
Operating Loss
Our operating loss was $2,083,062 and $522,109 in the three months ended March 31, 2022 and the three months ended March 31, 2021, respectively, reflecting a $1,560,953 (299%) increase in operating losses between the reporting periods. As stated in our discussion about R&D expenses above, our operating losses are mainly attributable to additional R&D expenses in line with our product development initiatives.
Interest Expense
In the three months ended March 31, 2022, interest expense was $16,687 as compared to $11,897 in the three months ended March 31, 2021, due to accrued interest for convertible notes and other debt notes we issued.
Net Loss
Net loss attributable to our shareholders of common stock was $2,099,895, in the three months ended March 31, 2022as compared to $534,006 in the three months ended March 31, 2021, resulting in a $1,565,889 (293%) increase in net losses attributable to our shareholders of common stock. The net losses are attributable to increased R&D expenses in line with our product development plans.
Comparison of the year ended December 31, 2021 and 2020
The following table summarizes our results of operations for the years ended December 31, 2021 and 2020:
| | | Years ended | | | | |
| | | December 31 | | | Change | |
| | | 2021 | | | 2020 | | | Amount | | | % | |
| Revenues | | | 594,563 | | | | 16,563 | | | | 578,000 | | | | 3,490 | % |
| Cost of revenues | | | (184,181 | ) | | | (11,277 | ) | | | (172,904 | ) | | | 1,533 | % |
| Gross margin | | | 410,382 | | | | 5,286 | | | | 405,096 | | | | 7,664 | % |
| | | | | | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development expenses | | | 1,920,645 | | | | 389 | | | | 1,920,256 | | | | 493,639 | % |
| Selling, general and administrative expenses | | | 2,357,163 | | | | 1,445,332 | | | | 911,831 | | | | 63 | % |
| | | | 4,277,808 | | | | 1,445,721 | | | | 2,832,087 | | | | 196 | % |
| | | | | | | | | | | | | | | | | |
| Operating loss | | | (3,867,426 | ) | | | (1,440,435 | ) | | | (2,426,991 | ) | | | 168 | % |
| | | | | | | | | | | | | | | | | |
| Non-operating income and expenses | | | | | | | | | | | | | | | | |
| Interest expense, net | | | (18,689 | ) | | | (10,185 | ) | | | (8,504 | ) | | | 83 | % |
| Other Losses | | | (2,547 | ) | | | (3 | ) | | | (2,544 | ) | | | 84,800 | % |
| | | | (21,236 | ) | | | (10,188 | ) | | | (11,048 | ) | | | 108 | % |
| Net loss | | | (3,888,661 | ) | | | (1,450,623 | ) | | | (2,438,038 | ) | | | 168 | % |
Revenues, Costs and Gross Margins
Net revenues for 2021 and 2020 were $594,563 and $16,563, respectively. In 2021, we substantially increased revenues by 3,490%, primarily because we began selling the Ainos COVID-19 Antigen Rapid Test Kits in June 2021 in Taiwan. Substantially all revenues in 2021 were from the sale of the test kits. All revenues in 2020 were from sales of nutraceutical products.
Cost of revenues for 2021 and 2020 were $184,181 and $11,277 respectively, representing a 1,533% increase year-over-year. The increase was primarily attributable to increased business activity relating to the test kits. Gross profits rose to $410,382 from $5,286, an improvement of $405,096, or 7,664%. Gross margin for 2021 rose to 69% from 32% for 2020, driven by improved product sales resulting from our Ainos COVID-19 Antigen Rapid Test Kits.
Research and Development (R&D) Expenses
Research and development expenses for 2021 and 2020 were $1,920,645 and $389, an increase of 493,639% and $1,920,256, respectively. The increase is associated with higher R&D personnel costs and more expense for technology development and technical license fees. The main components include: costs associated with increased R&D personnel ($214,712), costs associated with technology development (approximately $277,000) and costs associated with intellectual property, including amortization of acquired intellectual properties ($1,264,865). Following the strategic investment by Ainos KY, since August 2021 we have increased research and development staffing to develop our product candidates including POCTs, VELDONA and SRNA research.
Selling, General and Administrative Expenses
Selling, general and administration expense increased to $2,357,163 in 2021 from $1,445,332 in 2020, an increase of 63% and $912,000, respectively. Selling expense increased by approximately $191,000 or 100% due to increased sales and marketing staffing (approximately $76,000) and fees to distributors (approximately $103,000). Administrative expense increased by about 50% amounting to approximately $721,000. Our expenses increased primarily due to increased professional service fees related to the Ainos KY Transaction and corporate governance matters (approximately $298,000) and the increased amortization of acquired intellectual properties (approximately $725,000). The increased administrative expenses were offset by a decrease of personnel expense (approximately $350,000) associated with departure of former directors and officers.
Operating Loss
While our gross profits improved by $405,096 in 2021 compared to $5,286 in 2020, we incurred additional operating expenses as we continue to invest resources to execute our growth strategy and product roadmap. As a result, our operating loss in 2021 increased to $3,867,426 from $1,440,435 in 2020.
Interest Expense
Interest expense was $18,689 in 2021 compared to $10,185 in 2020, due to accrued interest for convertible and other debt notes issued by the Company.
Net Loss
Net loss attributable to common stock shareholders was $3,888,661 in 2021 compared to $1,450,623 in 2020, resulting in a $2,438,038 (168%) increase in net losses attributable to our shareholder of common stock. The net losses are attributable to increased R&D expenses, selling, general and administrative expenses in line with the Company’s product development plans.
Liquidity and Capital Resources
As of March 31, 2022 and December 31, 2020, we have cash and cash equivalents of $1,871,349 and $22,245, respectively, representing an increase of $1,849,104. The increase is primarily attributable to financing activities and revenues from sales of the Ainos COVID-19 Antigen Rapid Test Kit in Taiwan.
Major Transactions in 2021 and the three months ended March 31, 2022
The following is a summary of our major transactions in 2021 and the three months ended March 31, 2022.
Securities Purchase Agreement
On April 15, 2021, we entered into a Securities Purchase Agreement with Ainos KY (SPA). Pursuant to the Securities Purchase Agreement, we issued 8,333,333 shares of common stock at $3.00 per share to Ainos KY in exchange for certain patent assignments, increased its authorized common stock to 300,000,000 shares, and changed the Company’s name to “Ainos, Inc.” Immediately after the consummation of the transaction, Ainos KY owned approximately 70.30% of the Company’s issued and outstanding shares of common stock.
Ainos COVID-19 Test Kit Sales and Marketing Agreement with Ainos KY
On June 14, 2021, we entered into an exclusive agreement with Ainos KY to serve as the master sales and marketing agent for the Ainos COVID-19 Antigen Rapid Test Kit and Ainos COVID-19 Nucleic Acid Test Kit, which were developed by our codeveloper TCNT. On June 7, 2021, the TFDA issued an emergency use authorization to TCNT for the Ainos COVID-19 Antigen Rapid Test Kit, which are being marketed and sold and under the Ainos brand in Taiwan. As TCNT secures regulatory authorizations from other foreign regulatory agencies, we expect to partner with regional distributors to promote sales in other strategic markets. We purchased $183,444 of Ainos COVID-19 Antigen Rapid Test Kit inventory from TCNT for the year ended December 31, 2021 and $386,412 associated with finished goods, raw materials and manufacturing fees for COVID-19 antigen rapid test kits for the three months ended March 31, 2022.
Ainos - TCNT Product Development
On August 1, 2021, we entered into a five-year Product Development Agreement with TCNT. Pursuant to the agreement, both parties will endeavor to work together to co-develop pharmaceutical, medical and preventive medicine related products, with Ainos being the exclusive sales agent. Under the agreement, we will bear the cost associated with product development and TCNT will make accessible its personnel and facilities. Under the agreement, we incurred product development expenses totaling $205,883 for the year ended December 31, 2021 and accrued payable of $65,156 as of December 31, 2021. We incurred $167,422 in product development expenses in the three months ended March 31, 2022.
Asset Purchase Agreement
On November 18, 2021, we entered into an Asset Purchase Agreement with Ainos KY, as amended by an Amended and Restated Asset Purchase Agreement dated as of January 29, 2022 (the “Asset Purchase Agreement”). Pursuant to the Asset Purchase Agreement, we acquired intellectual property and manufacturing, testing, and office equipment for a total purchase price of $26,000,000. Pursuant to the Asset Purchase Agreement, we also agreed to hire certain employees of Ainos KY on terms equal to the compensation arrangements undertaken by Ainos KY. From and after the closing, we have no responsibility, duty or liability with respect to any employee benefit plans of Ainos KY.
Under the Asset Purchase Agreement, we issued the APA Convertible Note, a non-interest bearing convertible promissory note in the principal amount of $26,000,000, to Ainos KY. The principal sum of the APA Convertible Note is payable in cash on January 30, 2027, although we may prepay in whole or in part without penalty. If not earlier repaid, the APA Convertible Note will be converted into shares of our common stock or such other securities or property for which the APA Convertible Note may become convertible, immediately prior to the closing of any public offering of our common stock as result of which our common stock will be listed on a U.S. stock exchange. The conversion price, subject to certain adjustments, will be 80% of the public offering price of such offering. We incurred $26,000,000 as account payable related to the Asset Purchase Agreement as we booked the related assets in 2021.
Other Liquidity and Capital Resource Factors
We incurred net operating outflow of $1,389,889 for the three months ended March 31, 2022 and $1,249,977 for the year ended December 31, 2021. While our revenues grew in the three months ended March 31, 2022 and the year ended December 31, 2021, the amortization and depreciation expense of intellectual property, increased staffing, and expenditure in research and developments increased our total expenses and resulted in higher net losses ($2,099,895 and $3,888,661, respectively). The total amortization and depreciation expenses were $1,168,773 and $2,044,447, respectively. Inventory purchases also increased due to upcoming anticipated sales activities, by $337,805 and $0, respectively.
Our net cash from financing activities increased to $1,644,884 for the three months ended March 31, 2022 and $3,154,373 for the year ended December 31, 2021, mainly due to the issuance of additional convertible notes and other notes payable. Our total convertible notes payable and other notes payable increased by $1,650,000 for the three months ended March 31, 2022, compared to an increase of $236,854 for the three months ended March 31, 2021; and an increase of $3,101,420 for the year ended December 31, 2021 and $120,000 for the year ended December 31, 2020.
In 2021, in accordance with the SPA, Ainos KY provided us $3,000,000 in working capital advances. In exchange for the advances, we issued convertible notes in the aggregate principal amount of $3,000,000 with an interest rate of 1.85% per annum. The notes have maturity dates ranging from October 2021 to June 2022. They are convertible at Ainos KY’s election into shares of our common stock at a conversion price of $3.00 per share, subject to adjustment. We understand Ainos KY intends to convert these notes plus accrued interest thereon immediately prior to closing of this offering.
Between January 2016 and April 2021, we issued multiple convertible promissory notes to Dr. Stephen Chen, our former Chairman, CEO and CFO, for deferred compensation and working capital loans. The total principal of these convertible promissory notes decreased to $376,526 in December 2021 from $670,991 in 2020. In 2021, Mr. Chen assigned certain notes and the assigned notes were subsequently converted into our common shares.
On March 4, 2022, we issued a non-convertible note to Ainos KY in the principal amount of $800,000, at a 1.85% per annum interest rate, with a maturity date of February 28, 2023. We intend to use the proceeds from the note for working capital.
On March 17, 2022, we executed a Promissory Note Extension with Ainos KY dated March 17, 2022. Pursuant to the agreement, the due dates for all convertible notes issued by us to Ainos KY in 2021 was extended to February 28, 2023. The total unpaid principal amount for such notes is $3,000,000.
From March 28 to March 31, 2022, we issued the non-interest bearing March 2027 Convertible Notes (each, a “March 2027 Convertible Note”) in the aggregate principal amount of $900,000 to certain investors. On April 11, 2022, we issued another March 2027 Convertible Note in the principal amount of $500,000 to ASE Test Inc., a minority owner of Ainos KY. The March 2027 Convertible Notes will be automatically converted into shares of our common stock immediately prior to the closing of this offering at a conversion price equal to 80% of the per Unit public offering price.
As of the three months ended March 31, 2022, we had negative working capital, incurred recurring losses and recurring negative cash flow from operating activities, and an increase in our cumulative liabilities. Accordingly, there is substantial doubt as to our ability to continue as a going concern. See Note 1 to the Financial Statements included herein for more information. Our ability to continue operations depends on our success in generating and increasing revenues as well as our ability to secure external financing, when necessary, to fund strategic objectives. We expect to continue to incur losses for the immediate future and will need additional equity or debt financing until we can achieve profitability and positive cash flows from operating activities.
We anticipate net proceeds from this offering, business revenues and further potential financial support from our majority shareholder, Ainos KY, or others, to fund our operations over the next twelve months. However, there can be no assurance that we will be successful in our efforts to make us profitable. If these efforts are not successful, we may need to raise additional capital through the issuance of equity securities, debt financings or other sources in order to further implement our business plan or we will need to reevaluate our business plan and operating budgets.
In 2022 we intend to focus on commercializing our POCT medical devices and developing our COVID-19 oral treatment program. Our near-term liquidity requirements will include expenses for clinical trials, regulatory clearances, and marketing to commercialize our POCT devices, including the Ainos COVID-19 Nucleic Acid Test Kit, the Ainos Flora, the Ainos Pen and our COVID-19 oral treatment program. We also intend to increase staffing for general administration, marketing and technology development purposes.
In 2023 and beyond, we intend to invest in research and development and clinical trial spending to advance our VELDONA development efforts for disease indications such as thrombocytopenia and Sjögren’s syndrome. We also plan on investing in clinical trials and regulatory approval for the CHS430 device, in collaboration with TCNT, and clinical trial expenses for our SRNA program.
Critical Accounting Policies
For a discussion of accounting policies considered to be critical given they involve estimates and judgments made by management and are important for our investors’ understanding of our operating results and financial condition, see the Notes to the Financial Statements included elsewhere in this document.
Off-balance Sheet Arrangements
We have no off-balance sheet arrangements as of March 31, 2022.
DESCRIPTION OF BUSINESS
Overview
Ainos is a diversified medtech company focused on the development of novel point-of-care testing (POCT), low-dose interferon therapeutics and synthetic RNA (SRNA)-driven preventative medicine. Since inception, we have focused on the research of low-dose non-injectable interferon therapeutics. We have recently expanded our product candidates into POCTs in order to diversify corporate revenue streams. Our POCT devices are based on core technologies involving COVID-19 POCT products and other POCT products that detect volatile organic compounds (VOCs) emitted by the human body. We anticipate that our POCT product sales will be our main source of revenue in the near-term. We also plan to introduce a subscription-based analytics service to our customers as a potential long-term source of revenues. We believe our core technologies potentially empower a telehealth-enabled future for precision medicine with abilities to test anywhere, strengthen the immune system and expedite the development of precision treatments.
In our opinion, COVID-19 reveals that our healthcare system is too location dependent, inconvenient, and outcome centric. Long-term healthcare changes need a coherent and synergistic approach to seamlessly extend more testing from hospitals to consumers, strengthen the immune system, and popularize precision medicine. We believe our three key technologies will develop synergies for a telehealth-enabled future for precision medicine. We enable healthcare stakeholders to test anywhere and anytime, to strengthen the immune system and to expedite the development of precision treatments and tests.
Our Key Differentiators
We believe the following attributes differentiate us from other diversified life science companies:
| | · | Intuitive Point-of-Care Testing. Ainos POCTs are intended to modernize the way individuals and healthcare providers access testing at home, at work or in a clinical setting. They are developed for optimal efficiency in a universal setting; one that is rapid, portable, connected and which enables consumers to control their health data. Users need only take a simple test and our test kits then reduce the need for time-consuming hospital visits and off-site laboratory testing. |
| | | |
| | · | Telehealth-Friendly Rapid Testing. Ainos POCTs provide a simple and effective solution that can deliver reliable results within minutes and wirelessly deliver those results to the telehealth community. We believe our tests provide a better, more convenient user experience compared to traditional lab tests by delivering accurate results from our highly portable, handheld devices. |
| | | |
| | · | AI Powered VOC Testing Platform. Our VOC POCTs employ an AI algorithm platform to analyze digitally profiled VOC biomarkers which we collect and digitally profile as “Smell IDs.” As the AI algorithm is populated with more Smell IDs, we believe our VOC POCTs will continue to improve. Additionally, as more VOC biomarkers are uncovered, we believe the AI platform will allow us to rapidly develop other non-invasive methods of early disease detection. As the AI matrix advances in development, our goal is to build and facilitate personalized health data based on Smell IDs. |
| | | |
| | · | Decades of Proprietary Interferon Clinical Research. Ainos’ core drug platform, Very Low-Dose Oral Interferon Alpha (VELDONA), is based on more than 30 years of clinical research on low-dose non-injectable IFN-α, which has shown to be safe and effective in clinical trials for treatment of many diseases. Our platform technology was designed to be flexible so that it can potentially be adapted to treat a broad range of disease indications. Our experience and in-depth knowledge of interferons allow us to quickly pivot into specific need indications such as the proposed COVID-19 oral treatment combination. |
| | | |
| | · | Capital-Efficient Business Model. Medical innovation continues to advance and rapidly change. We believe companies need to stay nimble in order to quickly address any unforeseen industry dynamics. We have constructed our infrastructure to be capital efficient by choosing Taiwan as our R&D and operating center. Taiwan has been a key center of the global technology supply chain and is also home to high-caliber engineers, scientists and healthcare professionals. Maintaining operations in Taiwan allows us to access top-quality talent while staying capital efficient, further enhancing our ability to offer quality, affordable, consumer-friendly products. |
| | | |
| | · | Outsourced Manufacturing. We engaged Taiwan-based Taiwan Carbon Nano Technology Corporation (“TCNT”), our affiliated company and product co-developer, as our primary manufacturing partner for all of our products. TCNT possesses International Organization for Standardization (“ISO”) and Good Manufacturing Practice (“GMP”) certifications and healthcare facility quality management systems meeting ISO 13485 standards, saving us the time and resources required to establish our own infrastructure. We have also entered into a five-year Product Development Agreement with TCNT to increase our R&D and manufacturing capabilities. |
| | | |
| | · | Global Distribution Partnership. To increase our sales, marketing and distribution capabilities, we appointed Inabata, a Japanese corporation (“Inabata”), as a non-exclusive worldwide distributor for all of our products. Additionally, we named Inabata our “preferred distributor” for customers based in Japan. We also entered into a Memorandum of Understanding with Inabata’s subsidiary, Taiwan Inabata, under which Taiwan Inabata will coordinate business development, working capital, logistics, and coordination of procurement of raw materials in support of all of our products. |
Our Product Portfolio
As more fully described in the “OUR PRODUCTS” section of this Prospectus, our portfolio of products includes:
| | · | COVID-19 Antigen Rapid Test Kit and Cloud-based Test Management Apps. Our cloud-based test management platform is comprised of Ainos COVID-19 Antigen Rapid Test Kit, a personal application, or app, and an enterprise app. We anticipate our management apps will allow individuals and/or organizations to seamlessly manage tests, trace infections, and share results. As the first commercialized COVID-19 product we sell, we currently market the Ainos COVID-19 Antigen Rapid Test Kit in Taiwan under emergency use authorization (EUA) issued by the Taiwan Federal and Drug Administration (TFDA) to TCNT. We market the Ainos COVID-19 Antigen Rapid Test Kit, with manufacturing by TCNT, our product codeveloper. We expect TCNT to apply for EUA authorization from the U.S. Food and Drug Administration (FDA) sometime in the second half of 2022 and, if a EUA is granted by the FDA, we intend to market this product in the U.S. |
| | | |
| | · | COVID-19 Nucleic Acid Test Kit. This solution consists of a color-changing assay and a portable test equipment. The assay is compatible with most standard Polymerase Chain Reaction (PCR) machines and delivers test results within 40 minutes, which is faster than the typical PCR test turnaround time which can vary between a few hours to several days. In addition to the assay’s compatibility with most PCR equipment, we will also offer portable, low-cost test equipment intended to help medical professionals quickly scale testing capacity. Upon TCNT receiving regulatory approval, we will market the product under the Ainos brand name, with TCNT manufacturing the product. The product is currently under EUA review by the TFDA and we expect TCNT to submit for EUA review by the FDA sometime in the second half of 2022. |
| | | |
| | · | VOC POCT - Ainos Flora. Our Ainos Flora device is designed to perform a non-invasive test for female vaginal health and certain sexually transmitted diseases (STDs) including chlamydia, gonorrhea and trichomoniasis, within a few minutes. We expect Ainos Flora will provide convenient, discreet, rapid testing in a point-of-care setting which will allow women to self-test at home. We intend to work with TCNT to conduct clinical trials in Taiwan, after which we expect TCNT to submit applications for TFDA approval for marketing in Taiwan and FDA 510(k) clearance for marketing in the U.S. sometime in the second half of 2022. |
| | | |
| | · | VOC POCT - Ainos Pen. Our Ainos Pen device is a cloud-connected, multi-purpose, portable breath analyzer that is intended to monitor health conditions including oral, gastrointestinal, liver, and renal health within minutes. We expect consumers to be empowered to share their self-test results with their physicians through in-person and telehealth medical consultations. We intend to explore commercialization opportunities with third-party collaborators as a consumer health device sometime in the second half of 2022. |
| | | |
| | · | VOC POCT - CHS430. The CHS430 device is intended to provide non-invasive testing for ventilator-associated pneumonia within 10 minutes, as compared to current standard of care invasive culture tests that typically take more than two days to provide results. We plan to be the exclusive sales agent for CHS430, pursuant to our Product Development Agreement with our co-developer, TCNT, who will manufacture the product. We intend to work with TCNT to conduct clinical trials in Taiwan, after which we expect TCNT to submit applications for TFDA approval for marketing in Taiwan and FDA 510(k) clearance for marketing in the U.S. in 2023. |
| | · | Very Low-Dose Oral Interferon Alpha (VELDONA). VELDONA is a low-dose oralIFN-α formulation based on our nearly four decades of research on IFN-α’s potential treatment applications. Our leading product candidate is a low-dose oral treatment for COVID-19. We have conducted a parallel study based on VELDONA alone and a joint study with Innopharmax, Inc. We recently completed our animal testing phase, and we plan to initiate Phase 2/3 clinical trials for the VELDONA-only program sometime in mid-2022 in Taiwan. We also plan to advance our VELDONA development efforts for disease indications such as thrombocytopenia and Sjögren’s syndrome in 2023. |
| | | |
| | · | Synthetic RNA (SRNA). We are developing an SRNA technology platform in Taiwan. Our initial focus is to develop a potential COVID-19 mRNA vaccine platform using the full-length spike or the receptor-binding domain (RBD)gene sequence of the alpha and delta variants as reference sequences. We plan to continue developing these technologies with the goal of initiating clinical trials in Taiwan in 2023. |
Our Strategy
Focus on Commercialization
We are prioritizing the commercialization of medical devices beginning with our COVID-19 POCT product candidates. We initiated sales of Ainos COVID-19 Antigen Rapid Test Kit in June 2021 in Taiwan under an EUA issued to TCNT by the TFDA and we understand that TCNT, our manufacturing partner and product co-developer, plans to submit an application for U.S. EUA for healthcare professional uses sometime in the second half of 2022. TCNT’s regulatory application of Ainos Nucleic Acid Test Kit is currently under Taiwan EUA review and we understand that TCNT plans to submit an application for U.S. EUA review sometime in the second half of 2022.
On a parallel track, we are preparing commercialization plans for the VOC POCT product candidates. As a general strategy, we, in collaboration with TCNT, plan to conduct clinical trials in Taiwan and use the data to apply for TFDA approval and FDA clearance via the 510(k) pathway. Our goal is to work with TCNT in submitting for the approval of the Ainos Flora device sometime in the second half of 2022 and CHS430 device in 2023. We also intend to explore commercialization opportunities for the consumer-grade Ainos Pen sometime in the second half of 2022.
Advance Our Therapeutic Programs in Targeted Indications
For our COVID-19 oral program for VELDONA, we completed our animal testing in June 2022. Subsequently, we plan to conduct a combined Phase 2/3 clinical trial for the VELDONA-only product candidate. Pending positive clinical trial results, we plan to apply for an EUA from the U.S. FDA via the 505(b)(2) regulatory pathway.
Our programs for COVID-19 antiviral treatment leverage our completed clinical trials of VELDONA for the treatment of influenza. We have also conducted Phase 2 and Phase 3 clinical trials of VELDONA for the treatment of thrombocytopenia and Sjögren’s syndrome, respectively. We plan to leverage these previous clinical trials to advance our development of VELDONA in 2023, including a modified Phase 2 study for the thrombocytopenia program and a larger-scale Phase 3 study for Sjögren’s syndrome.
For our SRNA research program, we intend to conduct a Phase 1 clinical trial of our mRNA vaccine platform in Taiwan in 2023. Thereafter, we plan to assess the optimal strategy based on results of our clinical trial.
AINOS PRODUCT ROADMAP

We believe we are well positioned to telehealth’s market opportunity
The COVID-19 pandemic has fundamentally changed the medical industry. A digital transformation of healthcare is underway to provide consumers access to quality healthcare with reduced physical contact. As a result, telehealth adoption has substantially accelerated. Since the onset of the COVID-19 pandemic, telehealth utilization in the U.S. has risen to levels 38 times higher than pre-pandemic, according to McKinsey & Company.
Healthcare models are evolving from purely “virtual urgent care” to a broader range of services enabling long-term virtual care, the integration of telehealth with other virtual health solutions and hybrid online/offline care models, all of which aim at improving convenience, access, outcomes and affordability.
Going forward, we expect consumers to demand a greater variety of rapid point-of-care testing to manage their health. Therefore, we anticipate an increased need for rapid, convenient, low-cost, cloud-connected diagnostics for different infections or diseases. According to a Fortune Business Insights report (“Telehealth Market Size, Share & COVID-19 Impact Analysis, By Type (Products and Services), By Application (Telemedicine, Patient Monitoring, Continuous Medical Education, and Others), By Modality (Real-time (Synchronous), Store-and-forward (Asynchronous), and Remote Patient Monitoring), By End-User (Hospital Facilities, Homecare, and Others), and Regional Forecast, 2021-2028”), the global telehealth market is projected to grow from $90.4 billion in 2021 to $636.38 billion by 2028, at a 32.1% compound annual growth rate (CAGR).
We believe we are well-positioned to capture this opportunity, as our POCT products, when combined with our management app, are intended to provide convenient, portable and cloud-enabled rapid testing that improves the patient experience.
Growth Strategy
Key elements of our growth strategy include innovating and expanding our applications; creating multiple revenue streams; driving ecosystem adoption; expanding our installed base; broadening our global footprint; increasing value-added services; and scaling production with manufacturing partners. We expect to employ several core growth strategies:
| | · | Execute rollout of our multi-staged technology roadmap. We have established a multi-staged product roadmap through 2024 that begins with the commercialization of our COVID-19 POCT product candidates, followed by the VOC POCT product candidates. By 2024, we expect to achieve clinical trial milestones for our VELDONA and SRNA programs. |
| | | |
| | · | Launch POCTs through a staged regulatory approval approach. We, by collaborating with TCNT, intend to conduct clinical trials for our product candidates in Taiwan and thereafter apply for regulatory authorizations or approvals in Taiwan and the U.S. Upon regulatory clearances, we expect to partner with regional distributors to promote sales in those strategic markets. Taiwan ranks as the world’s third most efficient healthcare system as measured by the Bloomberg’s 2020 Healthcare Efficiency Index and 13th overall in the Foundation for Research on Equal Opportunity’s 2021 World Index of Healthcare Innovation. We believe that Taiwan’s high-quality and cost-effective healthcare system provides an excellent foundation for our clinical trial needs. |
| | · | Build an AI Nose technology platform. The AI Nose platform is the key building block of our VOC POCT product candidates. We intend to build our proprietary AI analytical models of VOC “Smell IDs” for application toward a wide range of diseases and continue to improve the sensitivity and performance of our proprietary digital nose sensors. |
| | | |
| | · | Establish a VOC analytics service. As more Smell IDs are collected from individuals by our VOC POCT products we envision building personalized medical profiles based on this data. As a long-term plan, we intend to establish a VOC analytics service by leveraging the AI Nose platform and offering this as a subscription-based VOC analytic service to our customers. As we train our VOC AI algorithm with more Smell IDs, we anticipate to offer further value-added services. |
| | | |
| | · | Implement a focused approach on the development of low-dose interferon therapeutics. Since inception, we have amassed a library of interferon clinical data based on more than 60 clinical trials across various disease indications conducted globally by us and our development partners in several jurisdictions including the U.S., Canada, Germany, Poland, Japan, Taiwan, Thailand, the Philippines and Kenya. Going forward, we intend to take a more focused approach, prioritizing our development efforts on certain target indications that we believe have the strongest potential for commercialization, starting with the study for low-dose oral treatment for COVID-19. |
| | | |
| | · | Invest in SRNA research. We see tremendous long-term potential in SRNA as the clinical application of mRNAs is proving to be successful in expressing functional protein antigens and inducing protective antibodies against the SARS-CoV-2 virus. Our short-term goal is to develop a next generation COVID-19 mRNA vaccine platform with the initial aim of completing a Phase 1 clinical trial in 2024. We then intend to explore various strategic business opportunities including out-licensing. |
| | | |
| | · | Expand global footprint through distributors. To increase our sales and marketing reach, we have designated Inabata as our non-exclusive global distributor and as the preferred distributor for the Japan market for all our products. Inabata operates 60 offices in 17 countries, including the USA, Mexico, Brazil, France, Germany, the United Kingdom, Turkey, Japan, China, South Korea, India, Indonesia and Malaysia. Our relationship with Inabata enables us to potentially reach customers across multiple countries. We also engage several regional distributors and will continue to ramp up our distribution capabilities accordingly to expand product reach into more countries. |
| | | |
| | · | Working with select manufacturers. By outsourcing all product manufacturing operations, we employ an asset-light manufacturing model that allows us to dedicate capital resources towards product innovation and business development. Currently, we collaborate manufacturing with our affiliated company, TCNT. |
OUR PRODUCTS
Product pictures other than pictures of the Ainos COVID-19 Antigen Rapid Test Kit represent the potential product design only. The only product currently being marketed is the Ainos COVID-19 Antigen Rapid Test Kit.
| AINOS COVID-19 POCT: ANTIGEN RAPID TEST KIT | 
|
| AINOS APPS: COVID-19 TEST MANAGEMENT FOR INDIVIDUALS AND ENTERPRISE | 
|
The Challenge: Non-Standardized Maintenance of Test Results
We recognize that antigen test kits have been vital in testing for COVID-19 on a massive scale and at low cost. Most EUA-approved test kits claim nearly identical value propositions, featuring greater than 95% accuracies and visual results within 15 minutes. We thus believe that achieving a competitive advantage based on testing technology alone is unlikely.
When individuals conduct COVID-19 or other tests at home or in a clinical setting, the test results are typically paper-based which does not facilitate on-going monitoring of health conditions.
Enterprises that need to keep track of testing compliance and conduct contact-tracing face even greater monitoring challenges, such as complying with health care privacy regulations, maintaining up-to-date testing results and engaging in outreach to large numbers of participants.
In our opinion, helping communities store and report test results and assisting organizations in tracing infections are critical tasks as countries seek to reopen their economies. Unfortunately, few COVID-19 antigen test kits in the market facilitate these aims.
Our Solutions: Antigen Rapid Test Kit with Cloud-based Test Management Apps For Individuals and Enterprises
Ainos COVID-19 Antigen Rapid Test Kit: Quick Result, On-Kit Data Matrix Code for Easy Result Sharing
We currently sell and market the Ainos COVID-19 Antigen Rapid Test Kit in Taiwan under an EUA for healthcare professional use and self-test use issued to TCNT by the TFDA. Similar to other commercially available COVID-19 antigen tests, the Ainos COVID-19 Antigen Rapid Test Kit is a lateral flow immunoassay intended for the qualitative detection of SARS-CoV-2 directly from samples of nasopharyngeal specimens. Like other antigen tests, the Ainos COVID-19 Antigen Rapid Test Kit provides an alternative to expensive and time-consuming laboratory tests: A quick swab captures samples for testing and the test kit delivers results within 15 minutes, with a two-line or one-line indicator reporting positive or negative test results, respectively.
Unlike other competitive products, however, the Ainos COVID-19 Antigen Rapid Test Kit incorporates a cloud-based test management and tracking function, which can be accessed through a data matrix code, enabling both individuals and enterprises to store and track test results. We believe that integrating health care monitoring with consumer devices differentiates our test kit as a bundled product and service.
Ainos Test Management Apps
We developed a comprehensive, end-to-end, cloud-based platform compatible with iOS and Android mobile phone platforms that allows individuals and organizations to manage tests and effectively trace infections. Our platform consists of a personal management app and an enterprise management app that work with the Ainos COVID-19 Antigen Rapid Test Kit. Our professional-use test kit carries a data matrix code that stores the test kit’s unique serial number. Our companion personal app reads the data matrix code to authenticate users during testing. When a user completes the test, the user scans the kit’s datamatrix code again to store test results as the user’s “digital health pass.” We currently market the apps with the professional kits in Taiwan only and we plan to promote in other regions if the Ainos COVID-19 Antigen Rapid Test Kit is approved to market in other countries.
For Individuals: Because personal privacy is important, the personal management app includes:
| | · | Secure log-in: Each user logs in with an email address and mobile phone number and authenticates his or her identity with a PIN number. |
| | | |
| | · | Double authentication: Each test kit carries a data matrix code that stores the kit’s unique serial number. When users log into the app, they are instructed to take a photo and then asked to scan the data matrix code before and after testing. This establishes a double authentication protocol for reliable testing. |
| | · | Digital health pass: Our app displays the unique I.D. number of the test kit associated with the test result, the date of the test, a QR code, and the user’s picture. We call this report a “digital health pass.” |
| | | |
| | · | Test result management: The app stores all past tests results performed by our test kit and uploads test results to our secure cloud server. If the test kit is purchased by an organization that uses our enterprise management app, the organization can see the user’s testing history, which facilitates remote tracing of COVID-19. |
| | | |
| | · | Filter visitors at entry: The app can scan another person’s digital health pass. Organizations and business owners can use this function at any point of entry, creating a safe environment for gathering. |
For Enterprises: The enterprise management app features a centralized, cloud-based COVID-19 test management platform designed for public and private institutions to centrally manage and trace testing. It helps track testing results across an organization and features:
| | · | Secure log-in: Access control for different levels of administrators. |
| | | |
| | · | Tracking statistics: Tabulating number of tests performed and positive / negative test results, test kit inventory and date-range filters. |
If selected (and permitted by local government regulations), our cloud-based management system does not include personal identifiers and has added security features.
Performance of Our Technology
MacKay Memorial Hospital, a medical center in Taiwan, conducted a clinical study in August 2021 that evaluated the performance of the Ainos COVID-19 Antigen Rapid Test Kit against Abbott’s Alinity mRT-PCR platform. Samples were taken from 151 COVID-19 suspected patients, comprised of 31 positive cases and 120 negative cases. Ainos COVID-19 Antigen Rapid Test Kit delivered 96.8% sensitivity and 100% specificity.
| | Number of samples with reference result of: | |
| Number of samples with Ainos result of: | Positive | Negative | Total |
| Positive | 30 | 0 | 30 |
| Negative | 1 | 120 | 121 |
| Total | 31 | 120 | 151 |
| Sensitivity | 96.8% | - | - |
| Specificity | - | 100% | - |
The Market
According to a report by Grand View Research (“COVID-19 Antigen Test Market Size, Share & Trends Analysis Report By Product & Service (Reagents & Kits, Platforms), By End Use (Clinics & Hospitals, Home Care), By Region, And Segment Forecasts, 2021 - 2027”), the global COVID-19 antigen test market was valued at $5.3 billion in 2020 and is expected to expand at a CAGR of 6.7% from 2021 to 2027. According to Grand View Research, antigen tests have gained traction for its ease-of-use, user friendliness and rapid result, and thus, antigen tests help accelerate patient admission and treatment processes, leading to improved patient outcomes. We recognize that new variants such as Omicron are causing new infections. We also expect COVID-19 to remain endemic for the foreseeable future. We also believe affordable, easy-to-use, rapid COVID-19 testing will continue to be in demand at least in the near-term.
We acknowledge the global demand for COVID-19 antigen test kits will be difficult to predict. To counter this uncertainty, we are building a diversified product portfolio including our VOC POCT product candidates, and our VELDONA and SRNA programs.
Our Regulatory Strategy: Authorizations for Professional Clinical Use
The Ainos COVID-19 Antigen Rapid Test Kit received the CE marking in December 2020, TFDA EUA for healthcare professional use in June 2021 and TFDA EUA for self-test use in June 2022. We expect TCNT, our manufacturing partner for the Ainos COVID-19 Antigen Rapid Test Kit, to submit an EUA application to the FDA for healthcare professional use sometime in the second half of 2022.
Our Implementation Strategy
We began marketing the Ainos COVID-19 Antigen Rapid Test Kit in Taiwan in June 2021. We generated $592,042 in test kit revenues for the year ended December 31, 2021 and $87,200 for the quarter ended March 31, 2022. In 2022, we plan to intensify our sales efforts in Taiwan and we also expect to implement global sales efforts primarily through our distributors including Inabata once the Ainos COVID-19 Antigen Rapid Test Kit secures regulatory clearance or approvals in the U.S. and other countries.
| AINOS COVID-19 POCT: NUCLEIC ACID TEST KIT | 

|
The Challenge: Conventional Molecular Testing is Time-Consuming and Complex
COVID-19 testing methods generally fall into three categories: molecular tests, antigen tests and antibody tests. Molecular tests feature the best accuracy, but rely on centralized laboratory testing and face complex sample transportation, logistics and throughput challenges. As a result, molecular test results can take from a few hours to several days, require highly trained technicians to operate complex instruments and are generally expensive. These factors inhibit the rollout of testing capacity at the scale needed to meet current global demand. Additionally, effectively tracking molecular test results is difficult, as reporting is often conducted manually.
Our Solution: Rapid PCR-Compatible Assay With Portable, Low-Cost Equipment
The Ainos COVID-19 Nucleic Acid Test Kit consists of a color-changing assay and a portable, low-cost test equipment. The assay is compatible with either standard PCR machines or our portable, low-cost equipment,. We offer the following functionalities to our customers and when combined, we believe that they enable accurate, fast, cost-effective molecular-based COVID-19 testing management:
| | · | Intuitive test results. Our assay displays test results by color, permitting easy interpretation of the test results. Yellow indicates a person has tested positive for COVID-19, while pink indicates a negative test result. |
| | | |
| | · | Quick results. Our assay delivers test results in 30-40 minutes, significantly faster than a traditional test that may take from a few hours to several days. |
| | | |
| | · | Compatible with most standard PCR machines. We have designed our assay to be compatible with most standard PCR machines. As a result, healthcare stakeholders can quickly deploy our assay without investing in new equipment. |
| | | |
| | · | Portable, low-cost equipment. The RT-LAMP assay tests COVID-19 at a constant temperature. This allows us to develop a portable test device. As a result, we can deliver PCR-comparable performance with portability and at lowcost. In contrast, standard PCR tests operate at multiple temperature changes, which requires complex and large machines. |
Performance of Our Technology
The Ainos COVID-19 Nucleic Acid Test Kit is based on our proprietary reverse transcription loop-mediated isothermal amplification (RT-LAMP) technology, which enables a fast test cycle. Our RT-LAMP assay simultaneously amplifies and detects targeted SARS-CoV-2 genes without the thermal cycling steps required by traditional PCR tests.
The Institute of Preventive Medicine, National Defense Medical Center of Taiwan (NDMC) tested the Ainos COVID-19 Nucleic Acid Test Kit in accordance with Taiwan’s Reference Document for the Application of Nucleic Acid Test Reagents for Emergency Use in Response to the Novel Coronavirus (COVID-19). The NDMC tested a total of 10 clinical specimens and 30 mock specimens. The 10 clinical specimens included five positive samples and five negative samples. The 30 mock specimens included 15 positive mock specimens and 15 negative mock specimens. NDMC compared the test results of Ainos’ test kit and that of LabTurbo’s AIO COVID-19 RNA Testing Kit, which has received an EUA from the TFDA. The comparison test results reflect that Ainos COVID-19 Nucleic Acid Test Kit’s performance was in 100% agreement with the 10 clinical specimens and 30 mock specimens tested by the LabTurbo test kit.
COMPARISON TEST RESULT AGAINST 10 CLINICAL SPECIMENS
| Subject’s profile | Type of specimen | Ainos COVID-19 Nucleic Acid Test Kit result | LabTurboTM AIO COVID-19 RNA Testing Kit result | Comparison of the results of the two methods |
| 5 positive samples: male and female between 30-68 years old | Respiratory swab (in VTM) | positive | positive | consistent |
| 5 negative samples: Male and female between 35 to 56 years old | Respiratory swab (in VTM) | negative | not detected | consistent |
COMPARISON TEST AGAINST 30 MOCK SPECIMENS
| Mock specimen number | Nucleic acid amount of COVID-19 (copies/ reaction) | Ainos COVID-19 Nucleic Acid Test Kit result | LabTurbo AIO COVID-19 RNA Testing Kit result | Comparison of the results |
| Specimen 1-30 | 0 | negative | not detected | consistent |
| Specimen 31-60 | 50-200 | positive | positive | consistent |
The Market
The COVID-19 diagnostics market mainly consists of molecular, antigen and antibody tests. The global market size for COVID-19 diagnostics is expected to grow from $116 billion in 2022 to $284 billion in 2029, growing at 13.6% CAGR, according to a report by Polaris Market Research (“COVID-19 Diagnostics Market Share, Size, Trends, Industry Analysis Report, By Mode; By Product & Service Type; By Sample Type; By Test Type; By End Use; By Region; Segment Forecast, 2022 - 2029”). We believe we are positioned to capture the molecular and antigen markets with our COVID-19 POCT portfolio. Going forward, market growth will highly depend on how infections evolve and the rate of vaccination across countries.
Our Regulatory Strategy
TCNT, our manufacturing partner, submitted test results for the Ainos COVID-19 Nucleic Acid Test Kit to the TFDA for EUA review. We expect TCNT to initiate EUA application to the FDA sometime in the second half of 2022. Depending on the results of the TFDA and FDA reviews, we will evaluate other markets.
Our Implementation Strategy
We intend to work with strategic partners, including our global distributor, Inabata, to market in Taiwan and the U.S. if we receive the applicable regulatory authorizations. Subject to obtaining authorizations in other countries, we intend to market our product in low-income and developing countries that face severe challenges in controlling the spread of COVID-19. In addition to experiencing limited vaccine availability, some countries also lack the infrastructure and capital required to deploy molecular testing at scale.
We plan to focus on the sale of our proprietary assays to test sites with installed PCR machines and makers of PCR diagnostics. We anticipate that our small and affordable test machine will help our target customers to increase testing capacity.
| AINOS VOC POCT: AI NOSE TECHNOLOGY | 
|
The Challenge: Laboratory VOC Tests Are Complex And Expensive
The analysis of VOC biomarkers is a powerful, non-invasive POCT option for disease detection and health monitoring. VOCs are gaseous molecules that exist throughout the human body. Cellular metabolic activity, bacteria and viruses all produce VOCs within the body.
VOCs derived from human breath can reflect metabolic changes caused by diseases and possibly serve as biomarkers of diseases such as lung cancer. As an example, the urea breath test for helicobacter pylori (H. pylori) infection is a well-established VOC clinical application, and is considered the gold standard for H. pylori testing. Urea breath test diagnostics companies include Otsuka Pharmaceutical Co., Ltd., Meridian Bioscience Inc, Paladin Labs Inc. and Avanos Medical, Inc.
VOC analytic instruments include gas chromatography mass spectrometry (GC-MS), chemiluminescence and optical absorption spectroscopy systems. The most common method, GC-MS, is highly accurate and sensitive. However, GC-MS is impractical in clinical settings for a variety of reasons. The equipment is expensive, immobile and requires a highly-trained operator. GC-MS also requires complex preprocessing of the target samples.
Our Solution: VOC POCTs Powered by Our AI Nose Technology
Our VOC POCT product candidates are a series of telehealth-friendly POCTs that are designed to detect VOCs of our target indications within minutes. We implement a “digital nose,” or more generally, gas sensors, to detect VOCs of our target disease indications and a trained AI algorithm to analyze digital profiles of VOC biomarkers for rapid disease testing and health monitoring. In contrast to GC-MS, we believe the digital nose is a more-scalable enabler of VOC diagnostics because digital nose sensors can be made small and at low cost through semiconductor manufacturing technology.
Performance of Our Technology
Our AI Nose technology is the key enabler of the VOC POCTs. The technological pillars underlying the AI Nose include the digital nose sensor array, our proprietary VOC AI algorithm, Smell ID and our extensive know-how of VOC medical device engineering. Our technology digitally profiles each VOC as a Smell ID, and as more Smell IDs are collected from individuals, we anticipate we will facilitate the ability to build personalized medical treatment based on this data.
AI Nose is comprised of an array of gas sensors that electronically mimics the human nose and the advancement in semiconductor manufacturing technologies allow digital noses to be made small, and at low cost. When combined with an AI algorithm, the digital nose is designed to directly detect components of different VOCs present in a gas mixture, eliminating the need for complex preprocessing steps.
With the help of algorithms and other electronic components such as microprocessors and wireless technology, the digital nose is designed to convert a VOC profile into unique digital signals that can be understood by a variety of devices, which could enable the digital nose to be quickly developed into many easy-to-use, affordable, portable, cloud-connected POCTs for a wide range of diseases. We believe these potential new tools could facilitate new telehealth services that are currently unavailable.
There are currently three main gas sensor detection methods: optical, electrochemical and metal oxide semiconductor (MOS). We believe MOS is the best option for POCTs and consumer electronics, as MOS technology can be easily manufactured using various semiconductor process technologies, which enables MOS-type sensors to be small and inexpensive.
The Market: Background of Gas Sensor Market
The gas sensor market has historically been focused on industrial applications, such as the detection of harmful gases. In the medical and healthcare sector, gas sensors are used in critical care equipment such as ventilators, oxygen concentrators and other patient care systems. The adoption of gas sensors in consumer electronics remains novel but we believe it presents a significant high-volume opportunity.
We anticipate that the demand for gas sensors will increase as the result of two potential drivers. First, we believe gas sensors can be adopted in POCTs as a way to non-invasively test for infections and to monitor health conditions. We believe the inconvenience associated with conventional tests have limited the market potential for our target indications such as vaginitis. Second, we anticipate there is a long-term opportunity in integrating gas sensors in smartphones and wearable devices to facilitate continuous health and air quality monitoring.
As we populate our AI algorithms with more Smell IDs and prioritize efforts on healthcare monitoring and disease testing, we believe we may also apply our AI Nose technology to non-medical uses in the future.
Our Implementation Strategy: Development of AI Nose Module
While our VOC POCTs currently employ third-party digital nose sensors, we are developing an integrated multi-element digital nose sensor module called the AI Nose Module. We are optimizing our product design and discussing production capacity with our manufacturing partners. We intend to initially incorporate AI Nose Modules into our VOC POCTs to enhance performance. We may sell the AI Nose Module to other device makers if we source sufficient production capacity and the business opportunity develops.
The sensor chip’s structure utilizes a complex semiconductor manufacturing process called microelectromechanical systems (MEMS). Each module can work alone or in powerful multi-sensor arrays. We have designed the module to be small enough so that it can be adopted into portable devices. We believe it is an excellent companion technology for medtech innovators to launch the next generation of healthcare devices.
| AINOS VOC POCT: FLORA Women’s Health And STDs | 
|
The Challenge: Disadvantages of standard invasive vaginitis and STD diagnosis
Invasive sampling is essential in the diagnosis of vaginitis and certain STDs, such as gonorrhea, chlamydia and trichomoniasis. Pelvic exams are conducted primarily in an outpatient setting as they require the trained operation of specialized instruments.
There are various common STD test options, including point-of-care tests, such as pH tests and whiff tests, and reference laboratory tests, such as culture tests and pelvic exams. Conventional testing methods, including pelvic exams, have several disadvantages. While the pelvic test/whiff test is quick, the wait time at the outpatient clinic is often long. The pelvic test and pH test cannot by themselves provide a definitive diagnosis, and laboratory culture tests can take a few hours to a few days to complete.
These shortcomings have two critical implications. First, long wait times at crowded hospitals and clinics can amplify patients’ anxiety levels. This could discourage patients with STD-suspected infections from getting tested or cause delays in seeking medical help. In turn, this may increase the risk of patients with mild symptoms unknowingly contributing to the spread of STDs. Second, the shortcomings imply that the size of the STD testing market could be larger than reported by market research data.
Our Solution: Ainos Flora - discreet, non-invasive testing in your hands
The iF-award winning Ainos Flora is designed to empower patients to effortlessly, non-invasively and quickly test their vaginal health at a point-of-care setting. Through specialized digital nose and AI pattern recognition, Ainos Flora is designed to detect vaginal infection (including bacterial vaginosis and fungus infection) and common STDs (including chlamydia, gonorrhea and trichomoniasis) in minutes.
Ainos Flora is a portable, handheld device that enables remote monitoring by designated medical professionals. Patients can store test results in a mobile app. Flora is designed to give women back control of monitoring their personal health with the following clinical benefits:
| | · | Convenient. Ainos Flora’s form factor is designed to allow easy self-testing or clinical testing. |
| | | |
| | · | Non-invasive and no skin contact. The digital nose sensors enable Ainos Flora to non-invasively gather and analyze a pathogen’s VOC profile. |
| | | |
| | · | Results within minutes. The digital nose sensor and AI algorithm work together to deliver test results in about one minute. In contrast, a culture test can take days. |
| | | |
| | · | Remote monitor. We intend to design a complementary mobile app that wirelessly receives test results from Ainos Flora and uploads results to our secure cloud server. A patient’s designated physician can then download the test results and remotely prescribe treatment. The process is designed to avoid potentially long wait times, while acquiring and sharing test results discreetly. |
Performance of Our Technology
We have tested Ainos Flora and our AI algorithm against VOCs from lab-grown bacteria samples. We tested against pathogens, including Escherichia Coli (E. Coli), Candida Albicans (CA) and Group B Streptococcus (GBS), as well as against standard air to assess our algorithm’s accuracy. The results from our November 2021 study conducted in Taiwan are provided below. The vertical axis of the following chart represents the actual VOC sample. The horizontal axis represents the algorithm’s prediction. Our algorithm correctly identified 19 of the 20 air samples; 29 of the 30 E. Coli samples, 20 of the 24 GBS samples and 27 of the 31 CA samples. The aggregated test accuracy is approximately 90.5%. We aim to improve Ainos Flora’s performance as we repeat this process to train our AI.
Accuracy of Ainos Flora against VOC of Lab-grown Bacteria Samples

The Market
We believe that, with Ainos Flora, there is a significant opportunity to potentially enable an easy-to-use POCT for the self-care market for screening for bacterial vaginosis and sexually transmitted infections or diseases. According to the U.S. Centers for Disease Control and Prevention (“CDC”), bacterial vaginosis, a condition that happens when there is too much of certain bacteria in the vagina, is the most common vaginal condition in women ages 15-44. The prevalence of bacterial vaginosis in the U.S. is estimated to be 21.2 million and increases with the number of lifetime sexual partners. Bacterial vaginosis can increase a women’s chance of getting an STD.
STDs cost the U.S. healthcare system billions each year. The CDC estimated in 2018 that one in five people in the U.S. had a sexually transmitted infection (including HIV/AIDS), amounting to nearly 68 million infections. In 2018, an estimated $1.1 billion in direct medical costs were attributable to chlamydia, gonorrhea and syphilis. The CDC recommends that women with a medical history and relevant symptoms should be screened for STDs.
Chlamydia is the most frequently reported bacterial STD in the U.S. The CDC estimated that there were approximately four million infections in 2018. Most people with infections have no symptoms and do not seek testing so it is difficult to fully estimate the scope of cases.
Gonorrhea is the second most common bacterial STD in the U.S. The CDC estimates that that approximately 1.6 million new infections occurred in the U.S. in 2018. Many infections are asymptomatic, so reported cases may only capture a fraction of the true incidences of the disease.
The CDC estimates that there were more than 2 million new trichomoniasis infections in the U.S. in 2018. Trichomoniasis infections are more common in women than in men. Only about 30% of persons with infections develop any symptoms. Infections can increase the risk of getting or spreading other sexually transmitted infections.
The global STD diagnostics market size was valued at $151 billion in 2021 and is expected to reach about $256 billion by 2028, growing at a CAGR of 8.3% from 2022 to 2028, according to a report from Zion Market Research (“Sexually Transmitted Diseases (STD) Diagnostics Market- By Type (Chlamydia Testing, Syphilis Testing, Gonorrhea Testing, Herpes Simplex Virus Testing, Human Papilloma Virus Testing, And Human Immunodeficiency Virus Testing), By Testing Devices (Laboratory Equipment And Point Of Care Equipment): Global Industry Perspective, Comprehensive Analysis, And Forecast, 2022-2028.”). According to the report, growth drivers to the market include a rise in public awareness, an increase in persons having living relationships with many partners and people having sexual encounters with more than one partner resulting in sexually transmitted diseases. According to the report, a rise in point-of-care testing is expected to create new growth for the market.
According to a report from Fortune Business Insights (“Sexually Transmitted Diseases Testing Market Size, Share and Global Trend By Disease (Chlamydia, Syphilis, Gonorrhea, Herpes Simplex Virus, Human Papillomavirus, Human Immunodeficiency Virus, Chancroid, Others), By Location (Laboratory, Point of Care), By Device (Laboratory Devices, Point of Care (POC) Devices) and Geography Forecast till 2022-2029”), North America and Europe accounted for the highest share of the market, while Asia Pacific is expected to show significant growth. In Southeast Asia, vaginitis, including bacterial vaginosis, is the most common disease in women of reproductive age with a prevalence rate of 23-39%.
Our Regulatory Strategy
We believe that Taiwan’s high-quality, cost-effective medical community is an ideal location to conduct clinical trials. Following clinical trials, we, by collaborating with TCNT, plan to apply for approval in Taiwan as well as 510(k) clearance with the FDA and, if received, to apply for certification in other countries with FDA-approved data.
Our Implementation Strategy:
We believe Ainos Flora can serve both the professional use and self-care markets. We intend to commercialize the professional segment by working with third parties, including potentially incumbent women’s health diagnostics makers. We understand that incumbent women’s health diagnostics companies include Quest Diagnostics Incorporated, Hologic, Inc., Abbott Laboratories, Siemens AG, F. Hoffmann-La Roche Ltd., Cardinal Health, Inc., and Becton, Dickinson and Company.
If successful, we believe this would pave the way for increased adoption in the self-care segment. We believe our potential customers include digital health companies such as Hims & Hers Health, Inc., Flo Health Inc, and Ava AG. We estimate a substantial global market opportunity for Ainos Flora, based on the assumption of a global population of 2.5 billion women between the ages of 15-64 and the CDC’s estimated prevalence rate of 29.2% of vaginitis, gonorrhea and trichomoniasis cases. We anticipate a larger market size in the U.S. if we successfully penetrate the at-home self-care segment.
| AINOS VOC POCT: AINOS PEN HEALTH MONITORING | 
|
The Challenge: Shortcomings of Conventional Breath Analyzers
Conventional breath analyzers are typically single-purpose and primarily designed to test for legal compliance issues such as alcohol and drug use. Thus, the market for breath analyzers is currently limited.
Long-term and regular monitoring of health conditions typically require multiple medical appointments, various invasive and clinical tests, and costly and time-consuming lab testing. Even with the recent increase in availability of telehealth, remote consultations are limited to oral interviews and video and photographic examinations.
Our Solution: Ainos Pen - A Multipurpose Telehealth Breath Analyzer In Your Pocket
The Ainos Pen is a multi-purpose, cloud-connected breath analyzer intended for routine health and air quality monitoring. By enabling self-care at home, we anticipate that Ainos Pen will enable new non-invasive health monitoring applications. Ainos Pen securely stores test data (i.e. Smell ID) in a mobile app with the ability to upload test data to a cloud server for access by designated healthcare providers. Through telehealth and AI analytics, patients can provide comprehensive and long-term health information for their healthcare providers.
Ainos Pen measures VOCs from human breath in minutes including carbon monoxide (CO), alcohol (ETOH), hydrogen sulfide (H2S), and ammonia (NH3). Potential applications include monitoring alcohol and tobacco consumption, halitosis, and gastrointestinal, liver and renal health. As we further develop our AI analytical database, we plan to broaden the Ainos Pen’s application to additional health conditions. Ainos Pen also measures air quality VOCs including total VOC (TVOC), carbon monoxide (CO) and estimated carbon dioxide (eCO2).
Performance of Our Technology
We have tested Ainos Pen and our AI algorithm against human breath VOCs under different conditions in order to sequence our AI algorithm for a real-world environment. We are repeating this process to train our algorithm with the goal of enhancing Ainos Pen’s performance.
The following tables show users’ breath tests conducted in Taiwan with the Ainos Pen following the consumption of a glass of beer. Ainos Pen also measured the surrounding air before the breath test. We repeated the test at varied time intervals for 10 consecutive cycles to record the change of our target VOCs. We labeled the measured VOC level from A+ to D, with A+ being very low and D being very high. As shown below, the subjects’ breath ETOH after a glass of beer immediately rose to B and then normalized to A+ over time. Breath H2S was first measured at A+ and then returned to B as the ETOH level dropped over the cycle. The CO and CO2 levels for the surrounding air were consistent throughout the cycle.
Our study indicates that Ainos Pen is able to capture the change in VOC levels. Going forward, we plan to conduct broader trials in Taiwan to train our algorithm and further improve performance.
Table: Breath Test At Normal Air vs. After One Glass of Beer
| | Breath | Air quality |
| | | NH3 | ETOH | H2S | CO2 | CO |
| Cycle | Interval | Normal Breath | After Beer | Normal Breath | After Beer | Normal Breath | After Beer | Normal Breath | After Beer | Normal Breath | After Beer |
| 0 | 0 min | A | A+ | A+ | B | B | A+ | A+ | A+ | A+ | A+ |
| 1 | 1 min | A | A+ | A+ | B | B | A+ | A+ | A+ | A+ | A+ |
| 2 | 2 min | A | A+ | A+ | A | B | A+ | A+ | A+ | A+ | A+ |
| 3 | 3 min | A | A+ | A+ | A | B | A+ | A+ | A+ | A+ | A+ |
| 4 | 4 min | A | A+ | A+ | A | B | A+ | A+ | A+ | A+ | A+ |
| 5 | 5 min | A | A+ | A+ | A | B | A+ | A+ | A+ | A+ | A+ |
| 6 | 5 min | A | A+ | A+ | A | B | A+ | A+ | A+ | A+ | A+ |
| 7 | 4 min | A | A+ | A+ | A | B | A+ | A+ | A+ | A+ | A+ |
| 8 | 3 min | A | A+ | A+ | A | B | A+ | A+ | A+ | A+ | A+ |
| 9 | 2 min | A | A+ | A+ | A | B | A+ | A+ | A+ | A+ | A+ |
| 10 | 1 min | A | A | A+ | A+ | B | B | A+ | A+ | A+ | A+ |
The Market
The breath analyzer market was valued at $550 million in 2021 and is projected to reach $1.99 billion by 2028, growing at a CAGR of 18.1% from 2021 to 2028, according to a recent report by Vantage Market Research (“Breath Analyzer Market by Technology (Fuel Cell Technology, Semiconductor Oxide Sensor Technology, Infrared Spectroscopy), by Application (Drug Abuse Detection, Alcohol Detection, Other Applications (Medical Applications etc.), by Region (North America, Europe, Asia Pacific, Latin America) - Global Industry Assessment (2016 - 2021) & Forecast (2022 - 2028)”). The market is composed of breath analyzers used for alcohol detection, drug detection and health monitoring.
Based on the health indications associated with our target VOCs, we believe the Ainos Pen may be applicable to the following markets:
Halitosis: Halitosis (or bad breath) affects 15% to 60% of the human population. Halitosis can be formed by VOCs including hydrogen sulfide produced by bacteria in the oral cavity.
Kidney health: The CDC estimates that chronic kidney disease (CKD) affects 15% of the U.S. population, or 37 million people. Research shows that breath ammonia is a useful biomarker in monitoring kidney function. Early identification of impaired kidney function is crucial in delaying the progress of CKD. CKD can be asymptomatic at the early stages and thus patients commonly go undiagnosed in the disease’s early stages, even in developed countries.
Our Regulatory Strategy:
We intend to market the Ainos Pen as a consumer-grade health monitoring device and therefore do not expect to require regulatory approvals and/or clearances.
Our Implementation Strategy:
The Ainos Pen can be sold through two primary channels. We may focus on direct sales to consumers. In addition, we plan to focus on sales through potential relationships with consumer healthcare product distributors, telehealth providers, consumer electronics companies seeking to diversify into healthcare, general healthcare providers, and incumbent breath analyzer companies. We understand that incumbent breath analyzer companies include BACtrack, Hound Labs, Inc., AK GlobalTech Corp., PRC-Saltillo, Drägerwerk AG & Co. KgaA, Intoximeters, and Quest Products, Inc. Leveraging our proprietary Smell ID analytic AI, we plan to offer Smell ID analytics services to our target customers, helping them to quickly analyze health data.
| AINOS VOC POCT: CHS430 VENTILATOR-ASSOCIATED PNEUMONIA (VAP) | 
|
The Challenge: Current VAP Diagnosis Is Too Slow And Complex When Every Minute Counts
Ventilator-associated pneumonia (VAP) is one of the most common infections in intensive care units (ICUs). Reported prevalence varies widely from 5% to 40% (Papazian, L., Klompas, M., & Luyt, C. E. (2020)). Ventilator-associated pneumonia in adults: a narrative review. Intensive care medicine, 46(5), 888-906. Causes of VAP include prolonged duration of mechanical ventilation or endotracheal intubation. The estimated mortality of VAP is around 10%, with higher mortality rates in surgical ICU patients and in patients with mid-range severity scores at admission. VAP imposes a significant economic burden. A cost evaluation in the United States estimated that the attributable cost of VAP to be $40,144 per patient per ICU stay. (Papazian, L., Klompas, M., & Luyt, C. E. (2020)). Ventilator-associated pneumonia in adults: a narrative review. Intensive care medicine, 46(5), 888-906.
In our opinion, the current diagnosis of VAP is inefficient in method and speed, which complicates treatment and prevention and affects patient outcomes. The first step of diagnosis relies on clinical evaluation (e.g., evaluation of symptoms, use of a Clinical Pulmonary Infection Score (CPIS) and chest x-rays) and culture analysis. However, no single clinical criterion is sufficient to provide a definitive diagnosis. Recent guidelines do not recommend CPIS due to its low specificity and chest x-rays are considered to be inconclusive. Moreover, clinical signs and radiographic infiltrates are not specific to VAP.
The second step of diagnosis is to perform microbiological sampling for culture analysis. Culture analysis is a complex and slow process that increases the risk of antibiotics overuse. Performing culture analysis requires trained clinicians while sampling is often invasive and poses a threat to ICU patients who are already physically and emotionally vulnerable.
Culture test results are often delayed, and during the wait time, healthcare providers may prescribe broad-spectrum antibiotics empirically, which may lead to poor patient outcomes. The prescription of broad-spectrum antibiotics prior to a diagnosis raises the risk of antibiotic resistance.
Our Solution: CHS430 - VAP Testing Within 10 Minutes
The Ainos CHS430 is a patented portable breath analyzer that connects into to the y-connector of a respirator and detects VAP within 10 minutes. It is designed to screen VAP infections and the likely causation pathogen, subject to further clinical validation. The sensor array inside the device is designed to be periodically replaced. The device also requires regular maintenance. CHS430 is intended for use by medical professionals, particularly in critical care centers and ICUs. We believe the device may serve as a rapid screening tool and enables faster, better decision making by critical care providers. Key benefits include:
| | · | Quick results. CHS430 completes self-cleaning and testing within 10 minutes, faster than a culture test. |
| | | |
| | · | Non-invasive testing. The device non-invasively analyzes a patient’s breath VOCs, improving quality of care and avoiding the cross-contamination risk associated with invasive sampling. |
| | | |
| | · | Easy-to-use. Connecting CHS430 to the y-connector requires minimal training. CHS430 self-cleans and begins testing with the simple press of a button. The device can monitor the patient in real-time at customizable intervals. |
| | | |
| | · | Better treatment options. Faster bacteria identification allows doctors to select the right treatment path more quickly than culture analysis. |
| | | |
| | · | Improves outcome. Early identification leads to better patient outcomes. Treating VAP requires the right antibiotic for the right strain at the right time and mistakes can result in harm to patients. Early detection reduces the risk of mortality or disease metastasis. |
Performance of Our Technology
In a validation study conducted in Taiwan in 2018, CHS430 tested breath samples of 217 patients and delivered 127 true positive tests, nine false positive tests, seven false negative tests and 74 true negative tests. The implied sensitivity was 94.8% and specificity was 89.2%. As we continue to train our algorithm for CHS430, we expect CHS430’s performance will improve. As a next step, we plan to improve our algorithm to identify pathogens for VAPs more accurately.
2018 TAIWAN VALIDATION STUDY
| Total patients | True Positive (TP) | False Positive (FP) | False Negative (FN) | True Negative (TN) |
| 217 | 127 | 9 | 7 | 74 |
| Accuracy | 92.6% |
| Sensitivity | 94.8% |
| Specificity | 89.2% |
Our Regulatory Strategy
CHS430 is a CE-approved Class I medical device. It is classified as a Class III device by the TFDA and as a Class II device by the Chinese National Medical Products Administration. We plan to conduct further clinical trials for the product, likely in Taiwan in 2023, and, if successful, to apply for TFDA and FDA 510(k) clearance as well as relevant certification in other regions.
The Market
We assume ventilated patients are the potential users of CHS430. Ventilated patients are commonly found in ICUs, critical care centers, ambulatory surgical centers and nursing homes. Therefore, we associate this product’s market size with the number of ICU and critical care beds worldwide. Market research of mechanical ventilators supports our assumption. According to a report by Global Markets Insight, the intensive care segments account for more than 80% of the mechanical ventilators market (Mechanical Ventilators Market Size By Product (Intensive Care Ventilators {High-end, Mid-end, Basic-end}, Portable Ventilators), By Interface (Invasive Ventilation, Non-invasive Ventilation {CPAP, BiPAP, APAP}), By Ventilator Type (Adult Ventilators, Neonatal Ventilators), By Application (Resuscitation, Homecare Applications, Emergency/Transport, Sleep Apnea Therapy, Anesthesiology, Clinical Applications), By Mode (Combined Mode Ventilator, Control Mode Ventilator, Pressure Mode Ventilator), By End-use (Hospitals, Ambulatory Surgical Centers, Homecare), COVID-19 Impact Analysis, Regional Outlook, Application Development Potential, Competitive Market Share & Forecast, 2021 - 2027). Based on estimated ICU bed density and country demographics, we estimate 339,129 ICU beds in the world’s top 10 economies.
Estimated ICU Or Critical Care Beds In Crucial Economies
| Country | ICU bed per 100,000 population | Country population (100,000 persons) | ICU beds (estimated) |
| USA | 34.7 | 3,310 | 114,858 |
| China | 5.0 | 14,393 | 71,966 |
| Japan | 7.3 | 1,265 | 9,233 |
| Germany | 29.2 | 838 | 24,465 |
| India | 2.3 | 13,869 | 31,899 |
| UK | 6.6 | 679 | 4,480 |
| France | 11.6 | 653 | 7,572 |
| Brazil | 25.0 | 2,311 | 57,775 |
| Italy | 12.5 | 605 | 7,558 |
| Canada | 24.6 | 379 | 9,323 |
| Total | | | 339,129 |
Our Implementation Strategy
The CHS430 device is intended to be an optimal companion solution with mechanical ventilators. Accordingly, we would look to develop relationships with leading mechanical ventilator companies or other companies whose business strategies are in line with ours. Current mechanical ventilator companies include Becton, Dickinson and Company, Koninklijke Philips N.V., Hamilton Medical AG, Fisher & Paykel Healthcare, Limited, Draegerwerk AG CO. KgaA, Medtronic PLC, GE Healthcare, Smiths Group PLC, and ResMed Inc.
| LOW DOSE INTERFERON: Oral treatment for COVID-19 | 
|
The Challenge: The Cytokine Storm and Imbalanced Immunity Caused By COVID-19 Infection
COVID-19 infection delays the production of interferons and unbalances the body’s innate immunity response. Imbalanced immunity promotes the production of inflammatory cytokines which can cause necrotic changes in the lungs as a result of the continuous accumulation of immune cells in the lungs, known as a “cytokine storm.” The cytokine storm causes severe inflammation and necrosis and is associated with a high IL-6/IFN ratio. Laboratory studies have shown that the normal type I interferon response is suppressed after infection with SARS-CoV-2, the virus that causes COVID-19. In addition, previous studies of hospitalized patients with COVID-19 demonstrated reduced production of interferons in response to SARS-CoV-2 infection in many patients, which was associated with more severe symptoms of COVID-19.
Our Solution: Low-dose Oral Treatment based on VELDONA
We anticipate a low-dose oral therapy based on VELDONA may increase the secretion of interferons in the human body, thereby inhibiting the replication of the coronavirus and enhancing immunoreaction.
Interferons, the body’s first line of defense, protect the body by interfering with viral replication and stimulating the immune system. IFN-α is also known to have various antitumor activities including the direct induction of cytotoxicity and the activation of natural killer (NK) cells and antibody-dependent cell-mediated cytotoxicity. We believe it can effectively inhibit virus replication and enhance immune response, while reducing the secretion of pro-inflammatory hormones to avoid severe inflammatory response and cytokine storm.
Performance of Our Technology
Findings from our recent animal study for using VELDONA as an oral treatment for COVID-19 conducted in Taiwan are as follows:
| | · | In this COVID-19 antiviral efficacy study, VELDONA was orally administered to Delta variant-infected hamsters once a day. The test group hamsters (the “VELDONA Group”) maintained more body weight than the control group (the “Control Group”) and recovered at days post-infection (“DPI”) 0-4, then subsequently stabilized. In addition, on Day 6 of infection, VELDONA reduced the viral load in the VELDONA Group hamsters by up to 551.16 times in the nasal cavity; and up to 720,510.66 times in the lungs. Combined with physiological properties and pathological findings, the results show that VELDONA has a protective effect on the lung organs caused by SARS-CoV-2 by regulating the immune response. It also allows infected hamsters to return to a normal physiological state more quickly. |
| | | |
| | · | The VELDONA Group showed positive improvements on pathological indicators for moderate infection caused by COVID-19, including mixed-cellular inflammation, peribronchial infiltration, and perivascular infiltration: 1) at Day 6, some VELDONA Group hamsters showed 20-30% inflammation, compared to Control Group’s 40-50% inflammation rate; 2) viral qPCR showed the VELDONA Group had less virus load compared to the Control Group. |
Body weight change

Viral load in the nasal cavity

Viral load in the lungs

Lung pathology examination

Lung pathology examination with qPCR

Our previous animal study and Phase 2 clinical trials for VELDONA form the foundation of the upcoming COVID-19 oral treatment research, including:
| | · | “Low-Dose Oral Interferon Alpha As Prophylaxis Against Viral Respiratory Illness: A Double-Blind, Parallel Controlled Trial during an Influenza Pandemic Year” (2013). This Phase 2 study investigated the use of VELDONA in preventing Acute Respiratory Illnesses (ARI). We enrolled two hundred healthy adults aged 18-75 years, where the participants completed weekly health data questionnaires to monitor symptoms and impact of respiratory illness. Serum samples were tested for antibodies against influenza and other common respiratory viruses. Our post hoc analysis of participant subgroups identified significant reductions in the incidence of ARI reported by males, those aged 50 years or above and those who received the 2009 seasonal influenza vaccine as shown below in bottom left of the following figure. IFN-α prophylaxis had a significant impact on the reporting of moderate-to-severe feverishness by the study population. Seropositive participants in the IFN-α group were more likely to report asymptomatic or mild symptoms compared with those in the placebo group who were more likely to report stronger symptoms. |
Prophylaxis with 150 IU IFN-α significantly reduced the incidence
in males, those aged 50 years and above and those who had
received seasonal influenza vaccine

| | Source: Bennett AL, Smith DW, Cummins MJ, Jacoby PA, Cummins JM, Beilharz MW. Low-dose oral interferon alpha as prophylaxis against viral respiratory illness: a double-blind, parallel controlled trial during an influenza pandemic year. Influenza Other Respir Viruses. 2013;7(5):854-862. Doi:10.1111/irv.12094 | |
| | · | “Protection From Lethal Influenza Virus Challenge By Oral Type 1 Interferon” (2007). In this animal study, we identified an optimal oral low-dose of IFN-α that when delivered daily as a prophylactic therapy protected C57BL/6J mice from a lethal challenge with mouse adapted human influenza virus A/PR/8/34 (H1N1). VELDONA was orally administered to C57BL/6J mice once a day. After seven days, the mice were infected with human influenza virus (H1N1 virus) and the control group mice were given placebo for seven consecutive days. For comparison, the oral low-dose VELDONA group significantly reduced (P<0.05) virus replication in the lungs of mice and reduced death. These results provide strong support for the application of low-dose type 1 IFN-α pretreatment for human influenza control. |
Oral Interferon Protects Mice from a Fatal Flu Challenge

| | Source: Beilharz MW, Cummins JM, Bennett AL. Protection from lethal influenza virus challenge by oral type 1 interferon. Biochem Biophys Res Commun. 2007;355(3):740-744. Doi:10.1016/j.bbrc.2007.02.019 | |
Our Regulatory Strategy
Building on our previous studies, we have recently completed our animal studies in Taiwan. Based on the results of our June 2022 animal studies, we intend to initiate a combined Phase 2/3 clinical trial sometime in the second half of 2022. We subsequently plan to submit for FDA regulatory approval in the U.S. via the 505(b)(2) pathway.
The Market
We anticipate our COVID-19 oral treatment programs will position us to address the global COVID-19 oral antiviral drug market in the short-term. According to a report by Airfinity, the COVID-19 oral antiviral market is expected to reach $32.5 billion in 2022 and antiviral pills are playing a vital role as the COVID-19 pandemic moves to endemic. Antiviral pills are easier to produce and distribute, and face less hesitancy than vaccines, and thus we believe it is likely that we will see another race to secure limited supply in 2022.
Our Implementation Strategy
If we obtain approval from relevant regulators, we may incur commercialization expenses related to product manufacturing, marketing, sales and distribution. In order to facilitate commercialization, we may pursue additional funding from outside sources including, but not limited to, our entry into, or expansion of, new licensing arrangements with other corporate sources that have both global and regional product distribution capabilities. We may also choose to partner with companies whose core competencies and strategies are in line with ours.
| LOW DOSE INTERFERON: VELDONA T-2b Thrombocytopenia therapeutic | 
|
The Challenge:
Thrombocytopenia is a disorder caused by low platelet levels leading to bruising and excessive bleeding, commonly associated with certain cancers, cancer treatments, autoimmune diseases, and hepatitis C.
Our Solution: VELDONA T-2b
Our previous studies demonstrate that VELDONA can potentially be used as a prophylactic to reduce the likelihood of a relapse of hepatitis C and reverse thrombocytopenia caused by certain diseases, treatments, viruses and autoimmune disorders. For example, given that low-dose IFN-α could exert immune-modulating effects and effectively increase platelet counts, it can possibly contribute to reversing the immunosuppressive tumor microenvironment and prevent some of the dangerous side-effects of chemotherapy such as thrombocytopenia. We plan to update our previous trials and seek regulatory approvals for the VELDONA T-2b pharmaceutical product.
Performance of Our Technology
For over thirty years we have researched low-dose interferon and potential applications for various disease indications. In connection with thrombocytopenia and hepatitis C relapse, we and our research partner Cytopharm published “A Double-Blind Randomized Controlled Study to Evaluate the Efficacy of Low Dose Oral Interferon-alpha in Preventing Hepatitis C Relapse” (2014) based on a Phase 2 clinical trial of 169 hepatitis C patients conducted at Chang Gung Hospital in Taiwan that found VELDONA potentially able to reduce the relapse rate of hepatitis C and also improve low platelet recovery.
The 2014 study investigated the efficacy of low-dose IFN-α in preventing hepatitis C relapse where 169 genotype 1b chronic hepatitis C patients having achieved end-of-therapy virological clearance were randomized to receive our VELDONA IFN-α lozenge 500 IU/day (n = 59), 1,500 IU/day (n = 53) or placebo (n = 57) for 24 weeks. Patients were stratified by fibroindex into four subgroups where it was found that in patients within the mild fibrosis subgroup (fibroindex between 1.4 and 1.7), relapse occurred in 1 out of 12 (8.3%) 500 IU-group patients versus 9 out of 21 (42.9%) patients in the other groups (P = 0.05). The study concluded that at 500 IU/day, oral IFN-α exerted a borderline suppression effect of virological relapse in chronic hepatitis C patients with mild liver fibrosis.
We discovered that platelet count recovered more rapidly in patients in the low-dose group and, thus, this treatment potentially reduced the risk of gastrointestinal hemorrhage suggesting a possible clinical role. One of the major side effects for pegylated high-dose interferon treatment is the induction of thrombocytopenia where platelet count typically decreases progressively until the end of treatment, followed by a slow recovery. In 158 patients receiving at least four weeks of oral interferon, significantly higher platelet count was found at the end of trial in the 500 IU group (P = 0.003). In thrombocytopenic patients, a significantly expedited recovery of platelet count was found in the 500 IU group (P = 0.002). No drug-related severe adverse events were reported.
Progressive increase of platelet count in patients receiving at least
four weeks of oral interferon

| | Black circle, low dose 500 IU/day; purple triangle, high dose 1,500 IU/ day; blue diamond, placebo. Vertical lines, standard deviation | |
| | | |
| | Source: Lee CM, Chen CY, Chien RN, et al. A double-blind randomized controlled study to evaluate the efficacy of low-dose oral interferon-alpha in preventing hepatitis C relapse. J Interferon Cytokine Res. 2014;34(3):187-194. Doi:10.1089/jir.2013.0074 | |
In connection with these studies, Ainos received four thrombocytopenia-related patents.

Our Regulatory Strategy
Moving forward, we intend to leverage our previous clinical trial experience to prepare a well-designed protocol to test the effectiveness of low-dose IFN-α in treating thrombocytopenia and determine the best path to continue further clinical development, which we currently plan to initiate in 2023.
The Market
According to a report offered on Research and Markets (“Thrombocytopenia- Market Insight, Epidemiology and Market Forecast - 2030”), the market size of thrombocytopenia treatment in the seven major markets (United States, United Kingdom, Germany, Italy, France, Spain, and Japan), also referred to as “7MM,” was approximately $4.1 billion in 2020 and expected to grow at a CAGR of 4.69% for the study period of 2018-2030. The United States, at $2.3 billion in 2020, accounts for the highest market size of thrombocytopenia. We expect global prevalence of thrombocytopenia, commonly associated with certain cancers, cancer treatments, autoimmune diseases, and hepatitis C, will continue to drive the market for thrombocytopenia treatment.
Currently, there are several marketed drugs in this space, including Nplate (Romiplostim, AMG-531) from Amgen and Promacta (eltrombopag) from Novartis, with the expected launch of other therapies where the thrombocytopenia market might experience significant growth due to the increase in the patient pool and availability of products.
Our Implementation Strategy
If we obtain approval from the relevant regulators, we expect to incur commercialization expenses related to product manufacturing, marketing, sales and distribution. In order to facilitate commercialization we may pursue additional funding from outside sources including, but not limited to, our entry into, or expansion of, new licensing arrangements with other corporate sources that have both global and regional product distribution capabilities. We may also choose to partner with larger pharmaceutical companies whose core competencies and strategies are in line with ours.
| LOW DOSE INTERFERON: VELDONA SS-2b Sjögren’s syndrome therapeutic | 
|
The Challenge:
Sjögren’s syndrome is a chronic, autoimmune, systemic, inflammatory disorder of unknown cause. It is characterized by dryness of the eyes, mouth, and other mucous membranes where the condition often accompanies other immune system disorders. There is currently no known cure. Many of the currently available treatments for Sjögren’s syndrome either only relieve symptoms or are considered ineffective in many patients.
Our Solution: VELDONA SS-2b
We are further evaluating our clinical trials that show IFN-α given at low dosage by the oromucosal route can significantly increase unstimulated whole saliva (UWS) flow in patients with primary Sjögren’s syndrome without causing significant adverse events. We plan to update previous trials and seek regulatory approvals for the VELDONA SS-2b pharmaceutical product.
Performance of Our Technology
As part of our longstanding research on low-dose interferon, we published “Treatment of Primary Sjögren’s Syndrome with Low-Dose Human Interferon Alfa Administered by the Oromucosal Route: Combined Phase III Results” (2003). In this study we tested the safety and efficacy of low-dose IFN-α in the treatment of salivary hypofunction and dry mouth symptoms in primary Sjögren’s syndrome patients.
The combined results were reported from two Phase 3 clinical trials in which a total of 497 subjects with primary Sjögren’s syndrome received 150 IU of IFN-α or matching placebo three times per day for 24 weeks by the oromucosal route. Subjects given IFN-α had a significantly (P = 0.01) greater mean increase in UWS flow, compared with subjects given the placebo. In IFN-α patients, increases in UWS correlated positively and significantly with improvements noted in seven out of eight symptoms associated with oral and ocular dryness. The co-primary endpoints of stimulated whole saliva flow and oral dryness were not significantly improved in the IFN-α group relative to the placebo; however, dryness symptoms improved in both groups. No significant differences were found between the groups with respect to overall adverse event incidence or severity.
IFNα group had a significant increase (*P = 0.01) in saliva output at week 24 compared with placebo

| | Source: Cummins MJ, Papas A, Kammer GM, Fox PC. Treatment of primary Sjögren’s syndrome with low-dose human interferon alfa administered by the oromucosal route: combined phase III results. Arthritis Rheum. 2003;49(4):585-593. Doi:10.1002/art.11199 | |
The study concluded that IFN-α given at low dosage by the oromucosal route can significantly increase UWS flow in patients with primary Sjögren’s syndrome without causing significant adverse events.
Our Regulatory Strategy
We conducted two Phase 3 trials in 2003. We plan to leverage our previous research studies and reference previous FDA correspondence and guidance to design an optimal protocol to advance our late-stage trials, likely beginning in 2023.
The Market
According to research from DataM Intelligence, the global Sjögren’s syndrome market will reach $2 billion by 2024 and is expected to grow at a CAGR of 3.3% during the forecasting period of 2022-2029. Other factors seen as driving the market include a growing patient population of diseases that can cause Sjögren’s syndrome such as lupus and rheumatoid arthritis, where the lack of a cure and increasing awareness may create increased demand for advanced treatments. Currently, North America is the largest market for this disease indication due to the rising prevalence of autoimmune diseases and advanced testing and screening. Companies in this space include Bristol-Myers Squibb, Novartis AG, Allergan Inc., Otsuka Pharmaceutical Co., Ltd., Santen Pharmaceutical Co., Ltd., Argentis Pharmaceuticals, LLC, Auven Therapeutics, Bridge Pharma Inc. and Cellzome GmbH.
Our Implementation Strategy
If we obtain approval from relevant regulators, we may incur commercialization expenses related to product manufacturing, marketing, sales and distribution. In order to facilitate commercialization we may pursue additional funding from outside sources including, but not limited to, our entry into, or expansion of, new licensing arrangements with other corporate sources that have both global and regional product distribution capabilities. We may also choose to partner with larger pharmaceutical companies whose core competencies and strategies are in line with ours.
SRNA RESEARCH INITIATIVE
Our Research Program
We are establishing a synthetic RNA (SRNA) technology platform capable of producing messenger RNA (mRNA) and/or small interfering RNA (siRNAs) for potential vaccine development and disease treatment. Based on this platform, our short-term goal is to research and develop second generation COVID-19 mRNA vaccines. Our long-term goals are to develop therapeutic RNA drugs featuring the use of mRNA and siRNA as genetic modulators for effective control of aberrant immune or genetic disorders and to develop rapid tests for cancer, immune system problems and rare diseases.
Status of Our Technology
Our short-term goal focuses on developing a second generation SARS-CoV-2 vaccine platform using a synthetic mRNA-based platform. We plan to use the full-length spike gene sequence of the British alpha variant (lineage B1.1.7) and the Indian delta variant (lineage B.1.617.2) as reference sequences to generate mRNA vaccines with six proline mutations, subject to our capital resources. We also plan to adopt sequence modifications and codon optimization of the mRNA vaccines. We will test vaccine delivery with a liposome of known formulation and evaluate the immunization through intramuscular (systemic) and nasal (mucosal) routs in mice. We anticipate the research will indicate a T helper 1 (Th1) biased immune response with potent neutralization antibody and effector CD8 T cell response. In addition, relevant recombinant spike proteins will be produced in Baculovirus expression systems. These proteins can be used as alternative immunogens, immunostimulatory adjuvants, or antigens to develop therapeutic monoclonal antibodies.
We have accomplished a design of a plasmid (pEF-Vacc1) for universal viral gene expression in eukaryotic cells. This pEF-Vacc1 harbored a transgene cassette that is driven by EF1α and T7 promoter and is suitable for massive in vivo or in vitro mRNA expression. The mRNA was devised with modified gene sequence and regulatory elements to evade innate immune attacks, which was composed of a modified cap structure, a 5’ untranslated region (UTR), a 3’ UTR, an open reading frame encoding a sequence-modified spike gene of a SARS-CoV-2 delta strain, and a poly-A tail. A control plasmid pEF-eGFP expressing fluorescent eGFP had been confirmed for its feasibility in expressing protein antigens in Lung A549 cells.
We have also preliminarily determined the formulation of liposome particles (LNPs), which will be synthesized for testing their delivery efficiency in cell lines (in vitro) and animal models (in vivo). Briefly, in-vitro transcribed mRNAs will be mixed with LNPs that are formulated by ionizable lipids, PEG-lipids, and helper lipids to investigate antigen expression and presentation of the mRNA-LNPs in transfected cells. The mRNA-LNPs will also be delivered into mice through intramuscular/intravenous/intranasal immunization to examine the safety, delivery efficacy and immunogenicity of the mRNA-LNPs. The humoral and cellular immunity generated by mRNA-LNPs will be analyzed through antigen-stimulated immune assays. Mice with or without immunization will be challenged with SARS-CoV-2 variants to assess the protective efficacy of the mRNA-LNPs. We anticipate that the mRNA-LNPs may elicit protective neutralizing antibodies and generate potent immunological memory against SARS-CoV-2 variants in a mouse model with a Th1-biased immune response and without an antibody-dependent pathogenic enhancement effect in the lungs.
Collectively, the mRNA-LNPs as generated by the SRNA platform are expected to have four specific features as well as versatile applications in the future. First, mRNA entrapped in PEG-lipids-containing LNPs may retain its integrity in the bloodstream. Second, all biodegradable lipids in LNPs may not cause liver damage. Third, the spike mRNA sequence is codon-optimized to endow abundant antigen expression in human cells for essential immune stimulation. Fourth, the transgene cassette can be quickly displaced for any genes through several enzymatic cleavage sites that are introduced between gene elements of the pEF-Vacc1. The successful development of the SARS-CoV-2 mRNA vaccine will facilitate the establishment of other synthetic RNA technology platforms for disease therapy, either by creating mRNAs or siRNAs with functional relevance in tumor suppression or immune modulation.
Our Regulatory Strategy
We plan to conduct preclinical animal studies in the second half of 2022, and, if successful, a subsequent Phase 1 clinical trial for our mRNA vaccine platform in Taiwan in 2023. Thereafter, we plan to assess the optimal strategy based on the trial’s results.
The Market
mRNA-based vaccines have gained prominence for preventing SARS-CoV-2 due to their underlying advantages, which include high efficacy, safety, reliability, reduced manufacturing time and the ability to protect individuals against infections of new SARS-CoV-2 mutant strains.
We believe the market for mRNA therapeutics will continue to grow after the CDC expressed a clinical preference for individuals to receive an mRNA COVID-19 vaccine in December 2021.
Our Implementation Strategy
We plan to focus on the research and development of the technology. In addition, we intend to explore strategic partnership opportunities upon completion of Phase 1 clinical trial(s).
Our Manufacturing
We believe our asset-light manufacturing business model allows us to dedicate resources to technology innovation and business development, and we believe our suppliers in Asia allow us to stay cost competitive.
Manufacturing for POCT, VELDONA and SRNA products. TCNT is our primary manufacturing partner. TCNT will manufacture our products and, if necessary, subcontract to other companies. On August 1, 2021, we entered into a Product Development Agreement with TCNT. Pursuant to the agreement, TCNT will manufacture our medical and diagnostic products. TCNT operates its facilities in conformance with a variety of International Organization for Standardization (ISO) and Good Manufacturing Practice (GMP) certifications, with most of our healthcare facilities’ quality management systems meeting ISO 13485.
Manufacturing for AI Nose module. Based on our design of the AI Nose module and our co-developed manufacturing process, TCNT plans to subcontract to other semiconductor manufacturers. TCNT plans to outsource production of the sensor chips to specialized contract manufacturers. Following their production, the sensor chips are expected to be shipped to another type of contract manufacturer, an outsourced semiconductor assembly and testing company, or “OSAT” company, that assembles our sensors into a highly miniaturized module and tests the functionality. Following assembly and testing, the final product will be shipped to our customers.
Material Contracts
Asset Purchase Agreement
On November 18, 2021, we entered into an Asset Purchase Agreement with Ainos KY, our majority shareholder. Pursuant to the agreement, upon closing, we will acquire intellectual property assets and certain manufacturing, testing, and office equipment for a total purchase price of $26,000,000. As part of the agreement, we agreed to hire certain employees of Ainos KY who are responsible for research and development of the intellectual property assets and/or equipment on terms at least equal to the compensation arrangements undertaken by the Seller. The purchase price was paid at closing by a convertible note. On January 29, 2022, we entered into an Amended and Restated Asset Purchase Agreement to amend certain provisions of the Asset Purchase Agreement.
On January 30, 2022, we issued to Ainos KY the APA Convertible Note, a non-interesting bearing convertible promissory note in the principal amount of $26,000,000, upon closing of the Asset Purchase Agreement. The principal sum of the APA Convertible Note is payable in cash on January 30, 2027, although we may prepay the APA Convertible Note in whole or in part without penalty. If not earlier repaid, the APA Convertible Note will be converted into shares of common stock, or such other securities or property for which the APA Convertible Note may become convertible, immediately prior to the closing of any public offering of our common stock as result of which our common stock will be listed on a U.S. stock exchange. The conversion price, subject to certain adjustments, will be 80% of the per Unit offering price of the offering.
Product Development Agreements
On August 1, 2021, we entered into a five-year product development agreement with TCNT, the majority shareholder of Ainos KY. Pursuant to the agreement both parties will endeavor to develop pharmaceutical, medical and preventive medicine related products. Under the agreement we are designated as the exclusive sales agent of these resulting products. Under the agreement we will be responsible for product development expense and TCNT will contribute personnel and facilities. Both parties will jointly own an equal proportion of the intellectual property rights arising from the product co-development cooperation.
On December 7, 2021, we entered into the InnoPharmax Agreement to develop and promote CICCT for the treatment of COVID-19 and potentially other viral infections. Pursuant to the agreement, we will provide VELDONA, its low-dose oral interferon alpha, and InnoPharmax will provide GemOral, its oral formulation of gemcitabine. The parties will share the preclinical research costs and if the preclinical research demonstrates significant results and academic clinical trials show safety and efficacy, then we are obligated to conduct clinical trials to obtain regulatory approval or EUA. The Company will pay for all such clinical trials and is responsible for all marketing activities if the combination product is approved and InnoPharmax will provide technical support. The parties will equally split all profits, calculated as the net sales, which is defined as revenues after deducting returns and discounts, and then further deducting direct manufacturing costs (including cost of raw materials, the cost of transportation of raw materials, and the cost of manufacturing and packaging by contract manufacturers), packaging costs, shipping costs (including reference to the cost of shipping the drugs from the manufacturing facilities to one of the designated countries or warehouses), insurance, customs duties, tariffs, excise tax, and value-added tax. In the event that either party licenses the product regionally, which may only occur with the consent of both parties, the parties will share all royalties equally, except that the licensing party will receive a five percent upfront payment as a broker fee. Ainos will be responsible for global sales, marketing, shipping and delivery, payment collection, insurance, and after-sales service and InnoPharmax responsible for quality control of research and development, disease indication development, and manufacturing. The parties agree to jointly own any new patent generated as a result of the agreement, and both parties agree to share the application and maintenance costs. The term of the InnoPharmax Agreement is twenty years from signing the agreement and the parties agreed to negotiate an extension six months prior to the expiration. The agreement may be terminated by either party for failure of the other party to commence its obligations related to the clinical trials within one year after signing the agreement after providing notice and one additional year to commence such obligations. The validity and interpretation of the InnoPharmax Agreement is in accordance with the laws of the Republic of China.
COVID-19 Test Kit Sales and Marketing Agreement
On June 14, 2021, we entered into an exclusive agreement with Ainos KY to serve as the master sales and marketing agent for the Ainos COVID-19 Antigen Rapid Test Kit and the Ainos COVID-19 Nucleic Acid Test Kit, which were developed by our co-developer TCNT. On June 7, 2021, the TFDA issued an EUA to TCNT for the Ainos COVID-19 Antigen Rapid Test Kit, which is being marketed and sold under the Ainos brand in Taiwan. As TCNT secures regulatory authorizations from foreign regulatory agencies, we expect to partner with regional distributors to promote sales in other strategic markets.
Distribution Agreements
We appointed Inabata as a non-exclusive worldwide distributor of our products under a five-year Distribution Agreement commencing November 1, 2021. Additionally, Inabata was named as a “preferred distributor” to customers based in Japan with certain sales and marketing rights for direct sales to customers and sub-distributors in Japan. Each party reserved certain rights to supply products to their respective existing and potential customers. Under the Agreement, Ainos is responsible for providing technical support and its products and Inabata is responsible for sales and marketing of Ainos’ products including its AI Nose module, Ainos Pen, Ainos Flora and CHS430 medical devices, all of Ainos’ pharmaceutical lines and SRNA products.
We also entered into a Memorandum of Understanding effective November 29, 2021 with Inabata’s subsidiary, Taiwan Inabata, under which Taiwan Inabata will coordinate business development, working capital, logistics, and coordination of procurement of raw materials in support of Ainos’ products including its AI Nose module, Ainos Pen, Ainos Flora and CHS430 medical devices, all of Ainos’ pharmaceutical lines and SRNA products.
Intellectual Property
Our commercial success depends in part on our ability to obtain and maintain patent and other proprietary protection for our commercially important technology, inventions and know-how; to defend and enforce our patents; to operate without infringing, misappropriating or violating the proprietary rights of others; and to prevent others from infringing, misappropriating or violating our proprietary rights. We rely on a combination of patent, copyright, trademark and trade secret laws and confidentiality and invention assignment agreements to protect our intellectual property rights. We also rely on know-how and continuing technological innovation to develop and maintain our competitive position. Notwithstanding these efforts, we cannot be sure that patents will be granted with respect to any patent applications we have filed or may license or file in the future, and we cannot be sure that any patents we own or license or patents that may be licensed or granted to us in the future will not be challenged, invalidated, or circumvented or that such patents will be commercially useful in protecting our test kits and technology. For more information regarding the risks related to our intellectual property, please see “Risk Factors-Risks Related to Our Intellectual Property.”
Our patent portfolio encompasses over 25 different patent families with various forms of patent protection sought per family in at least one of Taiwan, China, United States, Japan and Europe. Depending on the jurisdiction and the type of patent protections available, we have sought to obtain “invention patents,” “utility model patents” and/or “design patents.” Although the patentability requirements vary among different jurisdictions, invention patents (or utility patents as referred to in the U.S.) generally protect something new, useful and non-obvious and undergo an extensive examination prior to issuance. The base term of a U.S. utility patent is 20 years from the filing date of the earliest-filed non-provisional patent application from which the patent claims priority. Similarly, other jurisdictions recognize a patent term of 20 years from the priority date for invention patents.
As of January 31, 2022 we own 22 invention patents in the following jurisdictions: Taiwan (13), U.S. (4), Japan (3) and China (2). We also license one Japanese patent from TCNT pursuant to a July 1, 2020 patent license agreement between TCNT and Ainos KY, which was assigned to us pursuant to a Patent Assignment Agreement dated April 15, 2021. Specifically, we exclusively license Japan Patent Publication No. JP 3692811 B2 titled “Medical Ventilator Capable of Analyzing Infection and Bacteria of Pneumonia Via Gas Identification,” relating to our VOC sensing and diagnostics technologies. The license term is from January 1, 2021 through December 31, 2028 and permits the production, use and sale of the licensed products. The license from TCNT was subject to a NTD 3 million per year royalty for the term of the license. We are not obligated to pay royalties as Ainos KY paid the royalty in full for the term of the agreement on March 30, 2021. These patents expire between February 2033 and May 2040. Additionally, we have 14 pending invention patents in Taiwan (2), China (3), U.S. (3), Europe (3) and Japan (3).
Utility model patents are somewhat similar to an invention patent, but are generally cheaper to obtain and maintain, have a shorter term (10 years), shorter grant lag, and less stringent patentability requirements. The U.S. does not offer this form of patent protection. Our portfolio includes 12 granted utility model patents in the following jurisdictions: Taiwan (5), China (2), Japan (3) and Germany (2). These patents will expire between October 2029 and April 2031. We also currently have one pending utility model patent application in China.
Design patents generally protect the ornamental design of something that has a practical utility and have a term of between 15-20 years, depending on the jurisdiction. We have six (6) granted design patents in the following jurisdictions: Taiwan (3), China (1) and Europe (1), which expire between September 2026 and April 2041. We also license one Chinese design patent from TCNT pursuant to a July 15, 2020 patent license agreement between TCNT and Ainos KY, which was assigned to us pursuant to a April 15, 2021 Patent Assignment Agreement. Specifically, we exclusively license PRC patent publication no. CN 304042244 S, titled “Gas Detector,” relating to our VOC sensing and diagnostics technologies. The license term is from January 1, 2021 through December 31, 2028 and permits the production, use and sale of the licensed products. The license from TCNT was subject to a NTD 3 million per year royalty for the term of the license. We are not obligated to pay royalties as Ainos KY paid the royalty in full for the term of the agreement on March 30, 2021.
The majority of the above granted and pending patents protect our VOC sensing and diagnostics technologies relating to the development and manufacturing of point-of-care products that include diagnostic testing for pneumonia, vaginal infection, and H. pylori bacterial infection. In addition, we own three Taiwanese patents for our COVID-19 products (one design patent and two utility model patents). Three of our granted U.S. patents and one Taiwanese patent generally relate to our VELDONA product and are directed to methods of treating thrombocytopenia by orally administering interferon alpha.
In addition to patent protection, we rely on know-how and trade secrets, and careful monitoring of our proprietary information to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. We have also registered the trademark VELDONA in Taiwan.
Employees And Human Capital Resources
As of March 31, 2022, we had 42 full-time employees. As research and development is our core, 23 of our total employees work in research and development. Our employees are primarily located in Taiwan with some of our employees located in California. None of our employees are represented by a labor union or are a party to a collective bargaining agreement and we believe that we have good relations with our employees. We plan to continue expanding our manpower in research development, sales and marketing, and general operation to support our business programs.
Our human capital objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and additional employees. The principal purposes of our equity incentive plans are to attract, retain and motivate selected employees, consultants and directors through the granting of stock-based compensation awards.
Government Regulation
Regulation of Medical Devices in Taiwan
Our product candidates and operations are subject to the Taiwan Medical Devices Act and its implementation regulations (collectively the “Taiwan MDA”), which govern the development, design, pre-clinical and clinical research, manufacturing, safety, efficacy, labeling, packaging, storage, installation, servicing, recordkeeping, premarket clearance or approval, import, export, adverse event reporting, advertising, promotion, marketing and distribution of medical devices. Under the Taiwan MDA, medical devices, depending on the degree of risk associated with each medical device and the extent of manufacturer and regulatory control needed to provide reasonable assurance of its safety and effectiveness, will be subject to differentiated levels of review and examination by the TFDA before marketing the device. Unless an exemption applies, each medical device requires either (a) an approval granted by the TFDA or (b) a registration with the TFDA before launching distribution or marketing in Taiwan. The latter is a simplified premarket review process applicable to some medical devices classified as “lower risk level” items listed in the TFDA announcement. Our product candidates are not on the list of “lower risk level” and the approval of the TFDA will be required for us to launch distribution or marketing of such products in Taiwan.
Additionally, the TFDA may grant EUAs to allow commercial distribution of medical devices intended to address the public health emergency during public emergencies. The TFDA needs to assess the potential effectiveness of such medical devices on a case-by-case basis using a risk-benefit analysis and will require the submission of pre-clinical studies and clinical trials. The TFDA may also revise or revoke a granted EUA if the circumstances justifying such granting no longer exist, the criteria for its granting of EUA are no longer met, or other circumstances make a revision or revocation appropriate to protect the public health or safety. TCNT received an EUA for the Ainos COVID-19 Antigen Rapid Test Kit, which we distribute, from the TFDA on June 7, 2021.
Concerning the post-marketing regulatory requirements, a company engaging in medical devices business will be required to follow stringent design, testing, control, documentation, and other quality assurance procedures during all aspects of the design and manufacturing process and report to the TFDA when the device it markets has or may have caused or contributed to a death or serious injury. The TFDA also has broad discretion to take compliance and enforcement actions, such as requiring a safety surveillance report to be submitted regularly for review, ordering corrections, and conducting on-site inspection if it has any regulatory concerns. Failure to comply with applicable requirements under the MDA may subject a device and/or manufacturers, including us, to a variety of administrative sanctions, such as the TFDA’s refusal to approve pending premarket applications, mandatory product recalls, import detentions, business suspension or license/listing cancellation, administrative fines up to NT$50 million, product seizures and destruction, civil monetary penalties and/or criminal prosecution and criminal penalties up to NT$50 million. Any company engaging in medical devices business may additionally be subject to ten times the criminal fines for each violation made by its authorized representative and/or employees.
Personal Data Protection Laws in Taiwan
Under the Taiwan Personal Data Protection Act (“PDPA”), each individual or governmental or non-governmental agency, including our affiliate in Taiwan, should be subject to certain requirements and restrictions for collecting, processing or using personal data. The definition of “personal data” is extended to cover a broad scope, including name, birthday, ID, special features, fingerprints, marriage status, family, education, occupation, medical records, medical history, genetic information, sex life, health examination report, criminal records, contact information, financial status, social activities, and any other data which is sufficient to directly or indirectly identify a specific person. Due to the nature of the use of medical devices, our operation and the operation of our partners might collect, process, or use the data pertaining to a person’s medical records, healthcare, and genetics (collectively, sensitive data), which is subject to stricter scrutiny. Generally, we can only obtain such sensitive data when the person consents in writing or electronically. Furthermore, in September 2021, the TFDA published the draft bill of Regulations Governing Security Measures of the Personal Information File for the Business of Wholesale of Medical Devices and Retail Sale of Medical Apparatus authorized under the PDPA, which requires medical devices wholesalers and retailers to adopt necessary data security/protection measures and establish prevention and reporting mechanisms in relation to any data breach. The draft bill also empowers the TFDA to conduct regular inspections and audits. If we fail to comply with the PDPA, we may be subject to serious punishment for civil claims, criminal offenses and administrative liabilities: the ceiling of the aggregate compensation amount for damages payable in a single case will be up to NT$200 million or the actual value of loss arising from our violation provided the amount of actual value of such loss is higher than NT$200 million; the defendant may be subject to an imprisonment of up to five years; and the penalty for administrative liabilities will be up to NT$500,000 for each violation, and may be imposed consecutively if such violation continues.
Regulation of Medical Devices in the United States
Our product candidates and operations are subject to extensive and ongoing regulation by the FDA under the Federal Food, Drug, and Cosmetic Act of 1938 and its implementing regulations, collectively referred to as the FDCA, as well as other federal and state regulatory bodies in the United States. The laws and regulations govern, among other things, product design and development, pre-clinical and clinical testing, manufacturing, packaging, labeling, storage, record keeping and reporting, clearance or approval, marketing, distribution, promotion, import and export and post-marketing surveillance.
The FDA regulates the development, design, pre-clinical and clinical research, manufacturing, safety, efficacy, labeling, packaging, storage, installation, servicing, recordkeeping, premarket clearance or approval, import, export, adverse event reporting, advertising, promotion, marketing and distribution of medical devices in the United States to ensure that medical devices distributed domestically are safe and effective for their intended uses and otherwise meet the requirements of the FDCA. Failure to comply with applicable requirements may subject a device and/or its manufacturer to a variety of administrative sanctions, such as FDA refusal to approve pending premarket applications, issuance of warning letters, mandatory product recalls, import detentions, civil monetary penalties, and/or judicial sanctions, such as product seizures, injunctions, and criminal prosecution.
FDA Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device commercially distributed in the United States requires either FDA clearance of a 510(k) premarket notification, approval of a premarket approval (“PMA”) or grant of a de novo request for classification. During public emergencies, the FDA also may grant EUAs to allow commercial distribution of devices intended to address the public health emergency. Under the FDCA, medical devices are classified into one of three classes–Class I, Class II or Class III–depending on the degree of risk associated with each medical device and the extent of manufacturer and regulatory control needed to provide reasonable assurance of its safety and effectiveness.
Class I devices include those with the lowest risk to the patient and are those for which safety and effectiveness can be reasonably assured by adherence to the FDA’s “general controls” for medical devices. Some Class I or low risk devices also require premarket clearance by the FDA through the 510(k) premarket notification process described below.
Class II devices are moderate risk devices that require premarket review and clearance by the FDA through the 510(k) premarket notification process, though certain Class II devices are exempt from this premarket review process. Unless a specific exemption applies, 510(k) premarket notification submissions are subject to user fees. If the FDA determines that the device, or its intended use, is not substantially equivalent to a legally marketed device, the FDA will place the device, or the particular use of the device, into Class III, and the device sponsor must then fulfill more rigorous premarketing requirements.
Class III devices include devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices and devices deemed not substantially equivalent to a predicate device following a 510(k) submission. Submission and FDA approval of a PMA application is required before marketing of a Class III device. As with 510(k) submissions, unless an exemption applies, PMA submissions are subject to user fees.
Emergency Use Authorization
In emergency situations, such as a pandemic, the FDA has the authority to allow unapproved medical products or unapproved uses of cleared or approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused by chemical, biological, radiological, or nuclear warfare threat agents when there are no adequate, approved, and available alternatives.
Under this authority, the FDA may issue an EUA for an unapproved device if the following four statutory criteria have been met: (1) a serious or life-threatening condition exists; (2) evidence of effectiveness of the device exists; (3) a risk-benefit analysis shows that the benefits of the product outweigh the risks; and (4) no other alternatives exist for diagnosing, preventing, or treating the disease or condition.
Once granted, an EUA will remain in effect and generally terminate on the earlier of (1) the determination by the Secretary of the U.S. Department of Health and Human Services (“HHS”) that the public health emergency has ceased or (2) a change in the approval status of the product such that the authorized use(s) of the product are no longer unapproved. After the EUA is no longer valid, the product is no longer considered to be legally marketed and one of the FDA’s non-emergency premarket pathways would be necessary to resume or continue distribution of the subject product.
The FDA also may revise or revoke an EUA if the circumstances justifying its issuance no longer exist, the criteria for its issuance are no longer met, or other circumstances make a revision or revocation appropriate to protect the public health or safety.
On January 31, 2020, the Secretary of the HHS issued a declaration of a public health emergency related to COVID-19. On February 4, 2020, HHS determined that COVID-19 represents a public health emergency that has a significant potential to affect national security or the health and security of U.S. citizens living abroad and, subsequently, declared on March 24, 2020, that circumstances exist to justify the authorization of emergency use of medical devices, including alternative products used as medical devices, during the COVID-19 pandemic, subject to the terms of any authorization as issued by the FDA. On February 29, 2020, the FDA issued an immediately in effect guidance with policy specific to development of in vitro diagnostic tests during the COVID-19 public health emergency. This guidance was updated on March 16, 2020, May 4, 2020 and May 11, 2020.
510(k) Clearance Marketing Pathway
Our current products are Class II and, but for the immediate ability to seek an EUA, would be subject to premarket notification and clearance under section 510(k) of the FDCA. To obtain 510(k) clearance for a medical device, an applicant must submit to the FDA a 510(k) submission demonstrating that the proposed device is “substantially equivalent” to a legally marketed device, known as a “predicate device.” A showing of substantial equivalence sometimes, but not always, requires clinical data. Once the 510(k) submission is accepted for review, by regulation, the FDA has 90 calendar days to review and issue a determination. As a practical matter, clearance may take and often takes longer. Upon review, the FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence. In addition, the FDA collects user fees for certain medical device submissions and annual fees and for medical device establishments. For fiscal year 2020, the standard user fee for a 510(k) premarket notification application is $11,594.
Before the FDA will accept a 510(k) submission for substantive review, the FDA will first assess whether the submission satisfies a minimum threshold of acceptability. If the FDA determines that the 510(k) submission is incomplete, the FDA will issue a “Refuse to Accept” letter which generally outlines the information the FDA believes is necessary to permit a substantive review and to reach a determination regarding substantial equivalence. An applicant must submit the requested information within 180 days before the FDA will proceed with additional review of the submission.
If the FDA agrees that the device is substantially equivalent to a predicate device currently on the market, it will grant 510(k) clearance to commercially market the device. If the FDA determines that the device is “not substantially equivalent” to a previously cleared device, for example, due to a finding of a lack of a predicate device, that the device has a new intended use or different technological characteristics that raise different questions of safety or effectiveness when the device is compared to the cited predicate device, the device is automatically designated as a Class III device. The device sponsor must then fulfill more rigorous PMA requirements, or can request a risk-based classification determination for the device in accordance with the “de novo” process, which is a route to market for novel medical devices that are low to moderate risk and are not substantially equivalent to a predicate device. If the FDA determines that the information provided in a 510(k) submission is insufficient to demonstrate substantial equivalence to the predicate device, the FDA generally identifies the specific information that needs to be provided so that the FDA may complete its evaluation of substantial equivalence, and such information may be provided within the time allotted by the FDA or in a new 510(k) submission should the original 510(k) submission have been withdrawn.
After a device receives 510(k) marketing clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, will require a new 510(k) marketing clearance or, depending on the modification, PMA approval. The determination as to whether or not a modification could significantly affect the device’s safety or effectiveness is initially left to the manufacturer using available FDA guidance. Many minor modifications today are accomplished by a “letter to file” in which the manufacturer documents the rationale for the change and why a new 510(k) submission is not required. However, the FDA may review such letters to file to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing and recall the modified device until 510(k) marketing clearance or PMA approval is obtained. The manufacturer may also be subject to significant regulatory fines or penalties.
Over the last several years, the FDA has proposed reforms to its 510(k) clearance process, and such proposals could include increased requirements for clinical data and a longer review period, or could make it more difficult for manufacturers to utilize the 510(k) clearance process for their products. For example, in November 2018, FDA officials announced forthcoming steps that the FDA intends to take to modernize the premarket notification pathway under Section 510(k) of the FDCA. Among other things, the FDA announced that it planned to develop proposals to drive manufacturers utilizing the 510(k) pathway toward the use of newer predicates. These proposals included plans to potentially sunset certain older devices that were used as predicates under the 510(k) clearance pathway, and to potentially publish a list of devices that have been cleared on the basis of demonstrated substantial equivalence to predicate devices that are more than 10 years old. In May 2019, the FDA solicited public feedback on these proposals. The FDA requested public feedback on whether it should consider certain actions that might require new authority, such as whether to sunset certain older devices that were used as predicates under the 510(k) clearance pathway. These proposals have not yet been finalized or adopted, and the FDA may work with Congress to implement such proposals through legislation.
PMA Approval Pathway
A PMA must be submitted to the FDA if the device cannot be cleared through the 510(k) process. The PMA must be supported by extensive data, including data from pre-clinical studies and clinical trials. Review may take anywhere from 180 days to several years. An advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will generally conduct a pre-approval inspection of the applicant or its third-party manufacturers’ or suppliers’ manufacturing facility or facilities to ensure compliance with the QSR.
The FDA will approve the new device for commercial distribution if it determines that the data and information in the PMA constitute valid scientific evidence and that there is reasonable assurance that the device is safe and effective for its intended use(s). The FDA may approve a PMA with post-approval conditions, including some form of post-market surveillance. Failure to comply with the conditions of approval can result in material adverse enforcement action, including withdrawal of the approval.
None of our test kits are currently approved under a PMA, nor are we currently seeking approval under a PMA for our COVID-19 test kit. However, we may in the future develop devices which will require the approval of a PMA.
De novo Classification
Medical device types that the FDA has not previously classified as Class I, II or III are automatically classified into Class III regardless of the level of risk they pose. To market low to moderate risk medical devices that are automatically placed into Class III due to the absence of a predicate device, a manufacturer may request a de novo down-classification on the basis that the device presents low or moderate risk, rather than requiring the submission and approval of a PMA application. In the event the FDA determines the data and information submitted demonstrate that general controls or general and special controls are adequate to provide reasonable assurance of safety and effectiveness, the FDA will grant the de novo request for classification. When the FDA grants a de novo request for classification, the device is granted marketing authorization and further can serve as a predicate for future devices of that type, through a 510(k) premarket notification.
We are not currently seeking a de novo classification for the Ainos COVID-19 Antigen Rapid Test Kit or any device in development.
Clinical Trials
Clinical trials are typically required to support a PMA, oftentimes for a de novo request for classification, and are sometimes required to support a 510(k) submission. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with the FDA’s investigational device exemption (“IDE”), regulations which govern investigational device labeling, prohibit promotion of the investigational device, and specify an array of recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. The clinical trials must be approved by, and conducted under the oversight of, an Institutional Review Board (“IRB”) for each clinical site. If an IDE application is approved by the FDA and one or more IRBs, clinical trials may begin at a specific number of investigational sites with a specific number of patients, as approved by the FDA.
If the device is considered a “non-significant risk,” IDE submission to the FDA is not required. Instead, only approval from the IRB overseeing the investigation at each clinical trial site is required. After a trial begins, we, the FDA or the IRB could suspend or terminate a clinical trial at any time for various reasons.
Post-market Regulation
After a device is cleared or approved for marketing, numerous and pervasive regulatory requirements continue to apply. These include:
| | · | Establishment registration and device listing with the FDA; |
| | · | QSR requirements, which require manufacturers and contract manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation, and other quality assurance procedures during all aspects of the design and manufacturing process; |
| | · | Labeling regulations and FDA prohibitions against the promotion of investigational products, or “off-label” uses of cleared or approved products; |
| | · | Clearance or approval of product modifications to 510(k)-cleared devices that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared devices; |
| | · | Medical device reporting regulations, which require that a manufacturer report to the FDA if a device it markets may have caused or contributed to a death or serious injury, or has malfunctioned and the device or a similar device that it markets would be likely to cause or contribute to a death or serious injury, if the malfunction were to recur; and |
| | · | Post-market surveillance activities and regulations, which apply when deemed by the FDA to be necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
The FDA has broad regulatory compliance and enforcement powers. If the FDA determines that we failed to comply with applicable regulatory requirements, it can take a variety of compliance or enforcement actions, which may result in any of the following sanctions:
| | · | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| | · | customer notifications for repair, replacement, or refunds; |
| | · | recall, withdrawal, administrative detention, or seizure of our test kits; |
| | · | operating restrictions or partial suspension or total shutdown of production; |
| | · | refusal of or delay in granting our requests for 510(k) clearance or PMA approval of new test kits or modified test kits; |
| | · | withdrawing 510(k) clearance or PMA approvals that are already granted; |
| | · | refusal to grant export approval for our test kits; or |
| | · | criminal prosecution. |
Health Insurance Portability and Accountability Act
We are subject to compliance with the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Healthcare Information Technology for Economic and Clinical Health Act of 2009 (“HIPAA”), which, among other things, established federal protection for the privacy and security of protected health information (“PHI”). The HIPAA privacy regulations protect PHI by limiting its use and disclosure, giving patients the right to access certain information about them, and limiting most disclosures of PHI to the minimum amount necessary to accomplish an intended purpose. The HIPAA security standards require the adoption of administrative, physical, and technical safeguards and the adoption of written security policies and procedures.
In addition, various states, such as California and Massachusetts, have implemented similar privacy and security laws and regulations. The interplay of federal and state laws may be subject to varying interpretations by courts and government agencies, creating complex compliance issues. The compliance requirements of these laws, including additional breach reporting requirements, and the penalties for violation vary widely, and new privacy and security laws in this area are evolving. Requirements of these laws and penalties for violations vary widely.
Failure to comply with HIPAA, the Healthcare Information Technology for Economic and Clinical Health Act of 2009 or their implementing regulations, and similar state laws, may result in significant penalties, including civil, criminal and administrative penalties, fines, imprisonment and exclusion from participation in federal or state healthcare programs, and the curtailment or restructuring of our operations.
U.S. Federal, State and Foreign Fraud and Abuse Laws
The U.S. federal and state governments have enacted, and actively enforce, a number of laws to address fraud and abuse in federal healthcare programs. Our business is subject to compliance with these laws.
Anti-Kickback Statutes
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully soliciting, offering, receiving or paying remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward either the referral of an individual, or the purchase, order, arrangement for, or recommendation of, items or services for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare or Medicaid. Many states have adopted laws similar to the federal Anti-Kickback Statute. Some of these state prohibitions apply to referral of recipients for healthcare products or services reimbursed by any source, not only government healthcare programs, and may apply to payments made directly by the patient.
Government officials have focused their enforcement efforts on the marketing of healthcare services and products, among other activities, and recently have brought cases against companies, and certain individual sales, marketing and executive personnel, for allegedly offering unlawful inducements to potential or existing customers in an attempt to procure their business.
Federal False Claims Laws
The FCA prohibits any person or entity, among other things, to knowingly present, or cause to be presented, a false or fraudulent claim for payment of government funds and knowingly making, using or causing to be made or used, a false record or statement to get a false claim paid or to avoid, decrease or conceal an obligation to pay money to the federal government. The qui tam provisions of the FCA allow a private individual to bring actions on behalf of the federal government alleging that the defendant has violated the FCA and to share in any monetary recovery. In addition, various states have enacted false claims laws analogous to the FCA, and many of these state laws apply where a claim is submitted to any third-party payor and not only a federal healthcare program.
Violations of the FCA may result in treble damages, significant mandatory penalties, and civil monetary penalties, and violators may be subject to exclusion from participation in federal healthcare programs such as Medicare and Medicaid. Many medical device manufacturers and healthcare companies have reached substantial financial settlements with the federal government for a variety of alleged improper activities and have entered into corporate integrity agreements with the HHS Office of the Inspector General (“OIG”), under which the companies undertake certain compliance, certification and reporting obligations, to avoid exclusion from federal health care program.
Our activities, including those relating to the reporting of discount and rebate information and other information affecting federal, state and third-party reimbursement of our test kits (once approved) and the sale and marketing of our test kits (once approved), may be subject to scrutiny under the federal Anti-Kickback Statute and the FCA. We are also subject to other criminal federal laws that prohibit making false or fictitious claims and false statements to the federal government.
HIPAA Fraud Statute
HIPAA, among other things, imposes criminal liability for knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and creates federal criminal laws that prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal healthcare Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation.
Open Payments
The federal Physician Payments Sunshine Act, implemented as the Open Payments Program, requires certain manufacturers of drugs, medical devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program to report annually to the Centers of Medicare & Medicaid Services (“CMS”) information related to payments and other “transfers of value” to physicians, and teaching hospitals, and requires applicable manufacturers to report annually ownership and investment interests held by physicians and their immediate family members. Beginning in 2022, applicable manufacturers will also be required to report information and transfers of value provided (beginning in 2021) to physician assistants, nurse practitioners, clinical nurse specialists, certified nurse anesthetists, and certified nurse-midwives. Failure to submit timely, accurate and complete reports may result in substantial monetary penalties. We are subject to the Open Payments Program and the information we disclose may lead to greater scrutiny, which may result in modifications to established practices and additional costs. Additionally, similar reporting requirements have also been enacted on the state level domestically, and an increasing number of countries worldwide either have adopted or are considering similar laws requiring transparency of interactions with healthcare professionals.
Foreign Corrupt Practices Act
The FCPA prohibits any U.S. individual or business from paying, offering or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring them to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, if any, and to devise and maintain an adequate system of internal accounting controls for international operations.
U.S. Health Reform
Changes in healthcare policy could increase our costs, decrease our revenue and impact sales of and reimbursement for our current and future products once approved. The United States and some foreign jurisdictions are considering or have enacted a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our test kits profitably once approved. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality or expanding access. Current and future legislative proposals to further reform healthcare or reduce healthcare costs may limit coverage of or lower reimbursement for the procedures associated with the use of our test kits once approved. The cost containment measures that payors and providers are instituting and the effect of any healthcare reform initiative implemented in the future could impact our revenue from the sale of our test kits once approved.
We believe that there will continue to be proposals by legislators at both the federal and state levels, regulators and third-party payors to reduce costs and potentially affect individual healthcare benefits. Certain of these changes could impose additional limitations on the rates we will be able to charge for our current and future products or the amounts of reimbursement available for our current and future products from governmental agencies or third-party payors.
Data privacy and security
Numerous state, federal and foreign laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality and security of personal information, including health-related information. In the United States, numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws and regulations that govern the collection, use, disclosure, and protection of health-related and other personal information could apply to our operations or the operations of our partners. For example, HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and regulations implemented thereunder, imposes privacy, security and breach notification obligations on certain health care providers, health plans, and health care clearinghouses, known as covered entities, as well as their business associates and their subcontractors that perform certain services that involve creating, receiving, maintaining or transmitting individually identifiable health information for or on behalf of such covered entities. Entities that are found to be in violation of HIPAA as the result of a breach of unsecured PHI, a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. Further, entities that knowingly obtain, use, or disclose individually identifiable health information maintained by a HIPAA covered entity in a manner that is not authorized or permitted by HIPAA may be subject to criminal penalties.
Further, various states, such as California and Massachusetts, have implemented similar privacy laws and regulations, such as the California Confidentiality of Medical Information Act, that impose restrictive requirements regulating the use and disclosure of health information and other personally identifiable information, and the California Consumer Privacy Act, which came into effect on January 1, 2020, creates new data privacy rights for users. These laws and regulations are not necessarily preempted by HIPAA, particularly if a state affords greater protection to individuals than HIPAA. Where state laws are more protective, we may have to comply with the stricter provisions. In addition to fines and penalties imposed upon violators, some of these state laws also afford private rights of action to individuals who believe their personal information has been misused. The interplay of federal and state laws may be subject to varying interpretations by courts and government agencies, creating complex compliance issues for us and our clients, and potentially exposing us to additional expense, adverse publicity and liability. Further, as regulatory focus on privacy issues continues to increase and laws and regulations concerning the protection of personal information expand and become more complex, these potential risks to our business could intensify. Changes in laws or regulations associated with the enhanced protection of certain types of sensitive data, such as PHI or personally identifiable information along with increased demands for enhanced data security infrastructure, could greatly increase our costs of providing our services, decrease demand for our services, reduce our revenue and/or subject us to additional risks.
Even when HIPAA does not apply, according to the Federal Trade Commission (FTC), violating consumers’ privacy rights or failing to take appropriate steps to keep consumers’ personal information secure may constitute unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Individually identifiable health information is considered sensitive data that merits stronger safeguards.
In addition, certain state and non-U.S. laws, such as the General Data Protection Regulation (GDPR) govern the privacy and security of health information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. For example, California recently enacted legislation, the California Consumer Privacy Act (CCPA), which went into effect January 1, 2020. The CCPA, among other things, creates new data privacy obligations for covered companies and provides new privacy rights to California residents, including the right to opt out of certain disclosures of their information. The CCPA also creates a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach. Further, the California Privacy Rights Act (CPRA) was recently voted into law by California residents. The CPRA significantly amends the CCPA, and imposes additional data protection obligations on covered companies doing business in California, including additional consumer rights processes and opt outs for certain uses of sensitive data. It also creates a new California data protection agency specifically tasked to enforce the law, which would likely result in increased regulatory scrutiny of California businesses in the areas of data protection and security. The substantive requirements for businesses subject to the CPRA will go into effect on January 1, 2023, and become enforceable on July 1, 2023. In Europe, the GDPR went into effect in May 2018 and introduces strict requirements for processing the personal data of individuals within the European Economic Area (EEA). In addition, the GDPR increases the scrutiny of transfers of personal data from clinical trial sites located in the EEA to the United States and other jurisdictions that the European Commission does not recognize as having “adequate” data protection laws. Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater. Additionally, following the United Kingdom’s withdrawal from the European Union and the EEA, companies have to comply with the GDPR and the GDPR as incorporated into United Kingdom national law, the latter regime having the ability to separately fine up to the greater of £17.5 million or 4% of global turnover. The relationship between the United Kingdom and the European Union in relation to certain aspects of data protection law remains unclear, for example around how data can lawfully be transferred between each jurisdiction, which may introduce further compliance risk.
Legal Proceedings
From time to time, we may become involved in legal proceedings arising in the ordinary course of our business. Regardless of outcome, litigation can have an adverse impact on us due to defense and settlement costs, diversion of management resources, negative publicity, reputational harm and other factors, and there can be no assurances that favorable outcomes will be obtained. We are currently not a party to any material legal proceedings.
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following table sets forth the name, age and position held by each of our executive officers and directors as of the date of this prospectus. Directors are elected for a period of one year and thereafter serve until the next annual meeting at which their successors are duly elected by the stockholders.
| Name | | Age | | Position |
| | | | | |
| Chun-Hsien Tsai | | 52 | | Chairman of the Board of Directors, President and Chief Executive Officer |
| Hui-Lan Wu | | 61 | | Chief Financial Officer |
| Lawrence K. Lin | | 53 | | Executive Vice President of Operations |
| Chun-Jung Tsai | | 50 | | Director |
| Ting-Chuan Lee | | 38 | | Director |
| Chung-Yi Tsai | | 45 | | Director |
| Wen-Han Chang | | 58 | | Director |
| Yao-Chung Chiang | | 68 | | Director |
| Pao-Sheng Wei | | 64 | | Director |
Executive Officers
Chun-Hsien Tsai. Mr. Tsai has served as our Chairman of the Board of Directors, President, Chief Executive Officer, and as a Director since April 15, 2021. From April 15, 2021 until August 11, 2021, he also served as Chief Financial Officer. He concurrently serves as the Chief Executive Officer and Director of Ainos KY, the Chairman of the Board of Directors and Chief Executive Officer of TCNT, and Chief Executive Officer and Director of AI Nose Corporation, a clinical development company and an affiliate of the Company. Mr. Tsai has served as Chief Executive Officer and as a member of the Board of Directors of TCNT since 2012 (and as Chairman and Chief Executive Officer since July 2018), as a Director and President of Ainos KY since 2017, and as a Director and Chief Executive Officer of AI Nose Corporation since 2016. In his capacity as the Chief Executive Officer of TCNT, Mr. Tsai oversaw the completion of the world’s first carbon nanotube reactor. Mr. Tsai also currently serves as a member of the Taiwan Energy Storage Alliance and a member of the China Alternative Energy Association. Mr. Tsai owns more than 150 patents. Mr. Chun-Hsien Tsai is the brother of Mr. Chung-Yi Tsai and Mr. Chun-Jung Tsai. He is also the husband of Ms. Ting-Chuan Lee.
Hui-Lan Wu. Ms. Wu has served as our Chief Financial Officer since August 11, 2021. She has nearly 30 years of accounting, audit and management consulting experience. Before joining Ainos, Ms. Wu was a partner at KPMG Taiwan until January 2020, where she provided audit services to private and public companies in the technology, medical and chemical material sectors. Ms. Wu was the Chief Technical Officer of Mutual Benefit Consulting Co., Ltd, a finance consulting company, from April to September 2020, and Chief Financial Officer of FiduciaEdge Technologies Co., Ltd, a computing service company, from October 2020 to April 2021. She has mentored startup companies at the Center of Industry Accelerator and Patent Strategy at the National Yang Ming Chiao Tung University, and iLab Accelerator in Taiwan. Ms. Wu has devoted herself to promote impact investing in Taiwan. She received her Executive MBA from National Yang Ming Chiao Tung University and is a Certified Public Accountant in Taiwan and China.
Lawrence K. Lin. Mr. Lin has served as Executive Vice President of Operations since August 1, 2021. Prior to his appointment, Mr. Lin provided us with consulting services until August 2021, as the sole member of i2China Management Group, LLC (“i2China”), and served as Executive Advisor to the previous CEO and Chairman of the Company. Mr. Lin provided executive management consulting for Aquahelio Resources, LLC, an unaffiliated company, from 2014 to 2018. Mr. Lin brings more than 30 years of global cross-border strategic management consulting and financial investment experience at leading institutional corporates, such as Andersen Consulting, Salomon Smith Barney, and Credit Suisse First Boston. Mr. Lin has managed investment assets across several geographical locations, including the U.S., China and Taiwan, and advised on many private capital and structured public equity transactions for issuers in real estate, healthcare and consumer sectors. He spent nearly 15 years as an entrepreneur managing an independent Shanghai-based advisory and merchant banking practice where he completed numerous corporate acquisition and investment financing advisory mandates. Mr. Lin has a dual MBA in Finance & International Business from New York University- Stern School of Business.
Employee Directors
Chun-Jung Tsai. Mr. Tsai has served as a member of our Board of Directors since April 2021. He also manages our sales team. Mr. Tsai is also a member of the Board of Directors of Ainos KY. He concurrently serves as a Director of Ainos KY (a position held since 2019). He has served as Executive Director of TCNT since 2012. In his capacity as the Executive Director of TCNT, Mr. Tsai oversaw the completion of the world’s first carbon nanotube reactor. Mr. Chun-Jung Tsai is the brother of Mr. Chun-Hsien Tsai and Mr. Chung-Yi Tsai and brother-in-law of Ms. Ting-Chuan Lee.
Ting-Chuan Lee. Ms. Lee has served as a member of our Board of Directors since April 2021. She also manages the Company’s corporate development team. She has served as a director on the Board of Directors of TCNT since 2012, and served as a manager at TCNT from 2012 to 2021. From 2012 to 2018, Ms. Lee served as the Chairman of the Board of Directors of TCNT. Ms. Lee has served as Chairman of the Board of Directors of AI Nose Corporation since 2016. Ms. Lee holds a master’s degree of science from the National Taiwan University and a bachelor’s of science degree from the National Cheung Kung University. Ms. Lee is the spouse of Mr. Chung-Hsien Tsai and sister-in-law of Chung-Yi Tsai and Chun-Jun Tsai.
Non-Employee Directors
Chung-Yi Tsai. Mr. Tsai has served as a member of our Board of Directors since April 2021. He has also served as a member of the Board of Directors of TCNT since July 2012. He has served as the Executive Business Manager in the Automotive Business Unit of Maxim Integrated since November 2019. From October 2013 through November 2019, Mr. Tsai served as a Senior Product Marketing Manager in the Battery & Optical Business Unit of Intersil Corporation. In such roles, Mr. Tsai is an experienced executive in the technology hardware business. Mr. Chung-Yi Tsai is the brother of Mr. Chun-Hsien Tsai and Mr. Chun-Jung Tsai and brother-in-law of Ms. Ting-Chuan Lee.
Wen-Han Chang. Mr. Chang has served as a member of our Board of Directors since April 2021 and has served as the chairperson of our Compensation Committee and a member of our Audit Committee since August 2021. He also currently serves as the President of the Taiwan Society of Health Intelligent Medical Technology Development, the President of the Childhood Burn Foundation of R.O.C, the President of the Taiwan Society of Health Technology and Intelligence Medicine, and the Executive Director of the Taiwan Society of Geriatric Emergency & Critical Care Medicine. In addition, Mr. Chang is an Honorary President of the Taiwan Society of Engineering Technology and Practical Medicine, a Director on the board of directors of the Taiwan Society of Emergency Medicine, a Director on the board of directors of the Taiwan Society of Emergency Management Medicine, and a Supervisor of the Emergency and Critical Care Medicine Society. From September 2015 to May 2019, Mr. Chang served as a Vice President General at Mackay Memorial Hospital.
Yao-Chung Chiang. Mr. Chiang has served as a member of our Board of Directors since April 2021 and has served as a member of our Audit Committee since August 2021. He is also the current Chairman of the Board of Directors of the Taiwan High Speed Rail Corporation and is an independent director for Radiant Opto-Electronics Corporation and Tyntek Corporation, each a publicly-traded company on the Taiwan Stock Exchange. Mr. Chiang has previously served as the Chairman of the Board of Directors for the China Steel Chemical Corporation, Kaohsiung Rapid Transit Corporation, China Steel Corporation and China Airlines. Mr. Chiang holds a Ph.D. in Mechanical Engineering from the University of Wisconsin-Madison and a master’s degree in Mechanical Engineering from the National Cheung Kung University.
Pao-Sheng Wei. Mr. Wei was appointed on June 15, 2022 as a member of our Board of Directors, Chairperson of the Audit Committee of our Board of Directors and an Audit Committee Financial Expert, and as a member of the Compensation Committee. He also is an Independent Director of Nuvoton Technology Corporation from June 2022 to present, which is not affiliated with the Company. Prior to joining the Board, Mr. Wei was Chairman of KGI Bank, a subsidiary of China Development Financial Holding Company from September 2014 to June 2022, when he retired as Chairman. He also served as Chairman of the Taiwan offices of AIG Investments, AIG General Insurance, KGI Securities, respectively. In addition to his executive leadership in banking, securities, and insurance, Mr. Wei was a securities regulator as the Division Director of Corporate Finance of the Securities and Futures Bureau of the Financial Supervisory Commission, R.O.C. (Taiwan). Mr. Wei earned his MBA from the George Washington University in Washington D.C., USA in 1991.
The Company’s directors are elected at the annual meeting of shareholders to hold office until the annual meeting of shareholders for the ensuing year or until their successors have been duly elected and qualified.
Officers are elected by the Board of Directors and serve at the discretion of the Board.
Audit Committee
Our Audit Committee consists of Mr. Wen-Han Chang, Mr. Yao-Chung Chiang and Mr. Pao-Sheng Wei, each of whom has been determined to be “independent” under applicable independence standards, the Board of Director’s Corporate Governance Policies and the Charter of our Audit Committee. Mr. Wei currently serves as Chairperson of our Audit Committee. Our Board has determined that each of the members of the Audit Committee is able to read and understand fundamental financial statements, including our balance sheet, statement of operations and cash flow statement.
In addition, our Board has determined that Mr. Wen-Han Chang and Mr. Yao-Chung Chiang qualify as “audit committee financial experts” as defined by applicable SEC regulations. The Board reached its conclusion as to Mr. Chang’s and Mr. Chiang’s qualification based on, among other things:
| | · | Mr. Chang served as vice president of a medical center in Taiwan and chairperson of many medical associations and foundations with executive oversight of complex financial transactions and accounting and audit compliance matters. |
| | · | Mr. Chiang served as Chairman of multiple publicly-listed companies in Taiwan, including Taiwan High Speed Rail Corporation, China Airlines, China Steel Corporation, and China Steel Chemical Corporation. Mr. Chiang has extensive experience in financial oversight. |
The duties and responsibilities of the Audit Committee are set forth in its charter, which may be found on the Corporate Governance page of our Investor Relations website, and include the following:
| | · | selecting our independent registered public accounting firm and reviewing its qualifications, independence and performance; |
| | · | reviewing the audit plans of our internal auditors and any significant reports prepared by our internal auditors as well as management’s responses; |
| | · | in consultation with management and the Company’s internal and external auditors, reviewing our guidelines and policies with respect to risk assessment, risk management and internal financial and disclosure controls; and |
| | · | reviewing any material written communications between the independent registered public accounting firm and management, including any management or internal control letter issued or proposed to be issued by the independent registered public accounting firm and management’s response, if any. |
From time to time, we may engage an independent internal control auditor who consults with the Company on its existing internal controls and possible changes or augmentations to those controls.
Compensation Committee
Our Compensation Committee currently consists of Mr. Wen-Han Chang and Mr. Pao-Sheng Wei. Mr. Chang currently serves as the Chairperson of our Compensation Committee. The duties and responsibilities of the Compensation Committee are set forth in its charter, which may be found on the Corporate Governance page of our Investor Relations website, and include the following:
| | · | reviewing, modifying (as needed) and approving the salary, variable compensation, equity compensation and any other compensation and terms of employment of our Chief Executive Officer; |
| | · | reviewing and approving corporate performance goals, the structure and method for determining the terms of overall executive variable compensation or other compensatory plans, the method of determination of individual goals for executives and other senior management, and the payment of individual executive variable compensation to the extent such variable compensation contains a discretionary component; and |
| | · | reviewing, modifying (as needed) and approving our overall compensation plans and structure, including our overall compensation philosophy. |
Director Independence
Ainos KY controls, and is expected to continue to control, a majority of the voting power of the common stock. As a result, Ainos KY is able to elect all of the directors of the Company. Our Board of Directors has determined that Mr. Chang, Mr. Chiang and Mr. Wei are “independent directors” as determined under the standards of the Nasdaq Stock Market and under applicable SEC rules. The Board has adopted Nasdaq’s standards as its metric for director independence.
Code of Business Conduct and Ethics
We adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officers or persons performing similar functions. A copy of the code is posted on our corporate website at www.ainos.com, was included as Exhibit 5.05 in our Form 8-K filed with the SEC on August 26, 2021, is attached hereto as Exhibit 14.1 and is incorporated herein by this reference. In addition, we intend to post on our website all disclosures that are required by law or listing standards concerning any amendments to, or waivers from, any provision of the code. The information contained in, or accessible through, our website does not constitute a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
EXECUTIVE COMPENSATION
As a “smaller reporting company” under SEC rules, our named executive officers during the fiscal year January 1, 2021 through December 31, 2021 (collectively, the “Named Executive Officers”) were as follows:
| | · | Mr. Chun-Hsien Tsai, Chairman, President & Chief Executive Officer; |
| | · | Ms. Hui-Lan Wu, Chief Financial Officer; |
| | · | Mr. Lawrence K. Lin, Executive Vice President of Operations; |
| | · | Dr. Stephen T. Chen, our former Chairman of the Board, President, Chief Executive Officer and Chief Financial Officer; and |
| | · | Bernard Cohen, our former Vice President - Administration |
No other executive officers received total annual compensation during the fiscal year ended December 31, 2021 in excess of $100,000.
Summary Compensation Table
The following table sets forth certain information relating to the total compensation earned for services rendered to us in all capacities by our Named Executive Officer:
| Name and Principal Position | | Year | | Salary ($) | | | Bonus ($) | | | Stock Awards ($) | | | Options Awards ($) | | | Total ($) | |
| Chun-Hsien Tsai(1) Chairman of the Board & Chief Executive Officer | | 2021 | | | 45,044 | | | | 18,014 | | | | - | | | | - | | | | 63,058 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Hui-Lan Wu(2) Chief Financial Officer | | 2021 | | | 41,449 | | | | 11,034 | | | | - | | | | - | | | | 52,483 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Lawrence K. Lin(3) EVP Operations | | 2021 | | | 60,000 | | | | - | | | | - | | | | 468,950 | | | | 528,950 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Dr. Stephen T. Chen(4) | | 2021 | | | 975 | | | | - | | | | 33,333 | | | | - | | | | 34,308 | |
| Former Chairman of the Board, President, Chief Executive Officer and Chief Financial Officer | | 2020 | | | 240,975 | | | | - | | | | 100,000 | | | | - | | | | 340,975 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Bernard Cohen(5) | | 2021 | | | 18,846 | | | | - | | | | 3,167 | | | | - | | | | 22,013 | |
| Former Vice President - Administration | | 2020 | | | 71,085 | | | | - | | | | 12,000 | | | | - | | | | 83,085 | |
| | (1) | Mr. Chun-Hsieh Tsai was appointed Chairman of the Board, Chief Executive Officer and President, effective as of April 15, 2021 |
| | (2) | Ms. Hui-Lan Wu was appointed Chief Financial Officer, effective as of August 11, 2021 |
| | (3) | Mr. Lawrence K. Lin was appointed Executive Vice-President of Operations, effective as of August 1, 2021. Mr. Lin indirectly owns 30,174 warrants issued on November 25, 2020 to i2China, of which Mr. Lin is the sole member; a non-convertible note issued to i2China on January 1, 2020 with a principal amount of $84,000; and convertible notes issued to i2China with a total principal and accrued interest amount of $103,342 that were converted into 27,557 shares of common stock on December 27, 2021 at a conversion price of $3.75 per share. |
| | (4) | Dr. Chen resigned as Chairman of the Board, President, Chief Executive Officer and Chief Financial Officer, effective as of April 15, 2021. |
| | (5) | Mr. Cohen resigned as Vice President - Administration, effective as of April 15, 2021 |
Employment Arrangements
Effective April 15, 2021, our Board appointed Mr. Chun-Hsien Tsai to serve as Chief Executive Officer. Pursuant to an Offer Letter dated September 29, 2021, Mr. Tsai received a monthly salary of NT$250,000, a year-end bonus of two months’ salary, and a variable compensation based on our profit targets decided by our Compensation Committee, and payable as 10-30% of total annual compensation in the form of cash, securities and/or other discretionary remuneration. Other benefits, including labor insurance, health insurance and other benefits, were based on local regulations and our policies.
Pursuant to an Employment Agreement dated March 17, 2022 (the “Tsai Agreement”), Mr. Tsai receives a basic monthly salary of NT$247,600 and NT$2,400 meal allowance. The Tsai Agreement also includes certain incentive compensation including a target year-end bonus equal to two months of Mr. Tsai’s base monthly salary, a variable reward target of 10% to 100% of Mr. Tsai’s annual salary based on our profitability and Mr. Tsai’s work performance, and a grant of 333,333 units of Restricted Stock Units, to be made subject to and upon shareholder approval of the 2021 Stock Incentive Plan with a vesting date of September 30, 2022.
Effective August 11, 2021, our Board appointed Ms. Hui-Lan Wu to serve as Chief Financial Officer. Pursuant to an Offer Letter dated September 29, 2021, Ms. Wu received a monthly salary of NT$230,000, a year-end bonus of 2 months’ salary, and a variable compensation based on our profit targets decided by our Compensation Committee, and payable as 10-30% of total annual compensation in the form of cash, securities and/or other discretionary remuneration. Other benefits, including labor insurance, health insurance and other benefits, were to be based on local regulations and our policies.
Pursuant to an Employment Agreement dated March 17, 2022 (the “Wu Agreement”), Ms. Wu receives a basic monthly salary of NT$227,600 and NT$2,400 meal allowance. The Wu Agreement also includes certain incentive compensation including a target year-end bonus equal to two months of Ms. Wu’s base monthly salary, a variable reward target of 10% to 100% of the annual salary based on our profitability and Ms. Wu’s work performance, and a grant of 133,333 units of Restricted Stock Units, to be made subject to and upon shareholder approval of the 2021 Stock Incentive Plan with a vesting date of September 30, 2022.
Effective August 1, 2021, we entered into an employment contract with Mr. Lawrence K. Lin in connection with his election as Executive Vice President of Operations (the “LL Agreement”). The LL Agreement is effective for three years and may be extended for an additional years on the same terms and conditions upon mutual agreement. Under the LL Agreement, Mr. Lin will receive a monthly salary of $12,000, vesting stock options for 33,333 shares in our 2018 Officers, Directors, Employees, and Consultants Nonqualified Stock Option Plan, and a bonus of 666 shares of our common stock upon our successful listing on a Major National Exchange (as defined in the LL Agreement), and normal and customary benefits available to our employees. Mr. Lin is the sole member of i2China, a consultant previously engaged by us. Mr. Lin indirectly owns 30,174 warrants issued on November 25, 2020 to i2China; and a non-convertible note issued to i2China on January 1, 2020 with a principal amount of $84,000. On December 27, 2021, convertible notes issued to i2China with a total principal and accrued interest amount of $103,342 were converted into 27,557 shares of common stock at a conversion price of $3.75 per share.
We previously hired Dr. Stephen T. Chen under an employment contract for the period January 1, 2018 through December 31, 2020 (“Prior Chen Contract”). On January 1, 2021 an employment agreement for a 3-month term was executed reflecting the same material terms and conditions of the Prior Chen Contract which includes (i) a $240,000 annual salary, (ii) $100,000 in shares of our common stock payable quarterly based on the average share price of the closing quotes for the one month preceding issuance (referred to in the table as “Other Compensation”), (iii) certain employee benefits available to our employees, and (iv) reimbursement of expenses made on our behalf. We and Dr. Chen also executed a Settlement Agreement and Mutual General Release made effective December 24, 2020 covering any employment-related claims arising under the Prior Chen Contract. We also entered into an Intellectual Property Assignment Agreement with Dr. Chen made effective January 19, 2021 whereby Dr. Chen has assigned all right, title, and interest to certain patents, trademarks, and other intellectual property created or developed during Dr. Chen’s employment with us.
We previously hired Mr. Cohen under an employment contract for the period January 1, 2018 through December 31, 2020 (“Prior Cohen Contract”). On January 1, 2021, an employment agreement for a 3-month term was executed reflecting the same material terms and conditions of the Prior Cohen Contract which includes, (i) a $70,000 annual salary, (ii) $1,000 per month in Company shares paid monthly based on the average share price of the closing quotes for the one month preceding issuance (referred to in the table as “Other Compensation”), (iii) certain employee benefits available to the Company’s employees, and (iv) reimbursement of expenses made on behalf of the Company. The Company and Mr. Cohen also executed a Settlement Agreement and Mutual General Release made effective December 24, 2020 covering any employment-related claims arising under the Prior Cohen Contract. The Company and Mr. Cohen also entered into an Intellectual Property Assignment Agreement made effective January 19, 2021 whereby Mr. Cohen has assigned all right, title, and interest to certain patents, trademarks, and other intellectual property created or developed during Mr. Cohen’s employment with the Company.
Stock Options Plans
2018 Employee Stock Option Plan
On September 26, 2018, the Board adopted the Company 2018 Employee Stock Option Plan (the “2018-ESOP”), formerly referred to as the “Amarillo Biosciences, Inc., 2018 Employee Stock Option Plan” in prior filings. The 2018-ESOP provides for the grant of Qualified Incentive Stock Options to our employees. The Board, in its adoption of the 2018-ESOP, directed the Officers to submit the 2018-ESOP to the shareholders for ratification and approval at the next scheduled shareholders meeting. Failure of the ratification and approval of the 2018-ESOP within one year of the effective date renders the qualified options to become nonqualified options for purposes of the U.S. Internal Revenue Code. A stockholders meeting was not convened within the one year period and, as a result, any qualified options automatically became non-qualified options effective September 26, 2019.
The 2018-ESOP is administered by the Board or by a committee of directors appointed by the Board (the “Compensation Committee”) as constituted from time to time. The maximum number of shares of common stock which may be issued under the 2018-ESOP is 66,666 shares which will be reserved for issuance upon exercise of options.
The option price per share of common stock deliverable upon the exercise of an incentive stock option is 100% of the fair market value of a share on the date of grant. The option price is $5.70 per share and the options are exercisable during a period of ten years from the date of grant, where the options vest 20% annually over five years, commencing one year from date of grant.
Effective as of October 6, 2021, with the adoption by the Board of the 2021 SIP, no further awards may be granted under the 2018-NQSOP. As of December 31, 2021, options to acquire 36,666 shares of common stock remained outstanding.
2018 Officers, Directors, Employees, and Consultants Nonqualified Stock Option Plan
On September 26, 2018, the Board adopted the Company 2018 Officers, Directors, Employees, and Consultants Nonqualified Stock Option Plan (the “2018-NQSOP”), formerly referred to as the “Amarillo Biosciences, Inc., 2018 Officers, Directors, Employees, and Consultants Nonqualified Stock Option Plan” in prior filings. The 2018-NQSOP provides for the grant of nonqualified incentive stock options to employees. The 2018-NQSOP is administered by the Board or by the Compensation Committee as constituted from time to time. The maximum number of shares of common stock which may be issued under the 2018-NQSOP is 266,666 which will be reserved for issuance upon exercise of options. The option price for the nonqualified options is $5.70 exercisable for a period of ten years, with a vesting period of five years at 20% per year commencing one year from date of grant.
Effective as of October 6, 2021, with the adoption by the Board of the 2021 SIP, no further awards may be granted under the 2018-NQSOP. As of December 31, 2021, options to acquire 36,666 shares of common stock remained outstanding.
2021 Employee Stock Purchase Plan
On October 6, 2021, the Board approved the 2021 Employee Stock Purchase Plan (the “2021 ESPP” or “Plan”). The purpose of the 2021 ESPP is to provide an opportunity for eligible employees of the company and its designated companies (as defined in the Plan) to purchase common stock at a discount through voluntary contributions, thereby attracting, retaining and rewarding such persons and strengthening the mutuality of interest between such persons and our stockholders. The Company intends for offerings under the Plan to qualify as an “employee stock purchase plan” under Section 423 of the Code; provided, that the Plan administrator may also authorize the grant of rights under offerings that are not intended to comply with the requirements of Section 423, pursuant to any rules, procedures, agreements, appendices, or sub-plans adopted by the administrator. Subject to adjustments as provided in the Plan, the maximum number of shares of common stock that may be issued under the Plan may not exceed 50,000 shares. Such shares may be authorized but unissued shares, treasury shares or shares purchased in the open market. The Plan was approved by our stockholders and became effective as of June 20, 2022. The Plan will continue in effect until it expires on the tenth anniversary of the effective date of the Plan, unless terminated earlier.
2021 Stock Incentive Plan
On October 6, 2021, the Board approved the 2021 Stock Incentive Plan (the “2021 SIP” or “Plan”). The purpose of the 2021 SIP is to provide a means through which the Company, and the other members of the Company Group, defined by Section 2(n) of the Plan as the Company and its subsidiaries, and any other affiliate of the Company designated as a member of the Company Group by the Committee, may attract and retain key personnel, and to provide a means whereby directors, officers, employees, consultants and advisors of the Company and the other members of the Company Group can acquire and maintain an equity interest in the Company, or be paid incentive compensation measured by reference to the value of common stock, thereby strengthening their commitment to the interests of the Company Group and aligning their interests with those of our stockholders. The types of awards that may be granted from the Plan include individually or collectively, any Incentive Stock Option, Nonqualified Stock Option, Stock Appreciation Right, Restricted Stock, Restricted Stock Unit, Dividend Equivalent Rights and Other Equity-Based Award granted under the Plan. The Plan became effective as of June 20, 2022 following shareholder approval. The expiration date of the Plan, on and after which date no awards may be granted, will be the tenth anniversary of the date of Board approval of the Plan, provided, however, that such expiration will not affect awards then outstanding, and the terms and conditions of the Plan will continue to apply to such Awards. The aggregate number of shares which may be issued pursuant to awards under the Plan is 1,333,333 shares of common stock (the “Plan Share Reserve”), subject to adjustments as provided in the Plan. Each Award granted under the Plan will reduce the Plan Share Reserve by the number of shares underlying the award. No more than 666,666 shares may be issued in the aggregate pursuant to the exercise of incentive stock options granted under the Plan. The maximum number of shares subject to awards granted during a single fiscal year to any non-employee director, taken together with any cash fees paid to such director during the fiscal year, will not exceed $600,000 in total value (calculating the value of any such awards based on their grant date fair value for financial reporting purposes).
Outstanding Equity Awards at Fiscal Year-End
| Name and Principal Position | | No. of securities underlying unexercised options (#) exercisable | | | No. of securities underlying unexercised options (#) unexercisable | | | Equity incentive plan awards: number of securities underlying unexercised unearned option (#) | | | Option exercise price ($) | | | Option expiration date | |
| Chun-Hsien Tsai Chairman of the Board & Chief Executive Officer | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | |
| Hui-Lan Wu Chief Financial Officer | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | |
| Lawrence K. Lin(1) EVP Operations | | | - | | | | 33,333 | | | | - | | | | 5.07 | | | | 07/31/31 | |
| | | | | | | | | | | | | | | | | | | | | |
| Dr. Stephen T. Chen | | | - | | | | - | | | | - | | | | - | | | | - | |
| Former Chairman of the Board, President, Chief Executive Officer and Chief Financial Officer | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Bernard Cohen | | | - | | | | - | | | | - | | | | - | | | | - | |
| Former VP Administration | | | | | | | | | | | | | | | | | | | | |
| | (1) | Mr. Lawrence K. Lin indirectly owns 30,174 warrants issued on November 25, 2020 to i2China, of which Mr. Lin is the sole member; a non-convertible note issued to i2China on January 1, 2020 with a principal amount of $84,000; and convertible notes issued to i2China with a total principal and accrued interest amount of $103,342 that were converted into 27,557 shares of common stock on December 27, 2021 at a conversion price of $3.75 per share. |
On August 1, 2021, Lawrence Lin was hired as our Executive Vice President of Operations and in recognition for Mr. Lin’s prior services he was granted non-qualified stock options for 33,333 shares in our voting common stock under the 2018-NQSOP that will vest over a period of 3 years from August 1, 2021 through July 31, 2024. In accordance to the 2018-NQSOP, the stock options are exercisable at $5.70 per share within 10 years of August 1, 2021.
Non-Employee Director Compensation Policy (the “2021 NEDCP”)
On September 28, 2021, our Board of Directors adopted the Company’s Non-Employee Director Compensation Policy (the “2021 NEDCP” or “Policy”). On appointment to the Board, and without any further action of the Board or Compensation Committee of the Board, at the close of business on the day of such appointment, each Non-Employee Director will automatically receive an award of 22,000 restricted stock units (“RSUs”) over common stock (the “Appointment Grant”). The Appointment Grant will vest in three equal annual installments, with the first installment vesting on the last day of the six-month period commencing on the grant date and each subsequent installment vesting on the last day of the six-month period commencing on the next two subsequent anniversaries of the grant date, subject to the Director’s continuous service with us on each applicable vesting date. The RSUs will be granted pursuant to 2021 SIP, following shareholder approval of the plan, and will be subject to such other provisions set forth in the agreement evidencing the award of the RSUs, in the form adopted from time to time by the Board or the Compensation Committee of the Board. The directors elected to the Board prior to shareholder approval of the SIP 2021 will receive their initial grants of RSUs following shareholder approval and effectiveness of the 2021 SIP.
In addition to the RSU grants, each member of the Board of who is not an employee of the Company or any of subsidiaries will receive the cash compensation set forth below for service on the Board. The annual cash compensation amounts will be payable in equal quarterly installments, in arrears following the end of each quarter in which the service occurred, pro-rated for any partial months of service. All annual cash fees are vested upon payment.
| | 1. | Annual Board Service Retainer: |
| | | (a) | All Eligible Directors: $12,000 |
| | | (b) | Chairperson of the Board: $14,000 |
| | 2. | Annual Committee Chair Service Retainer: |
| | | (a) | Chairperson of the Audit Committee: $7,000 |
| | | (b) | Chairperson of the Compensation Committee: $4,500 |
| | 3. | Annual Committee Member Service Retainer: |
| | | (a) | Member of the Audit Committee: $4,000 |
| | | (b) | Member of the Compensation Committee: $3,000 |
Directors receive $1,000 compensation for attendance at directors’ meetings and $250 for regularly scheduled teleconference meetings. There were no regularly scheduled meetings during 2021 for which the attendance or teleconference fee was due to directors. The Board enacted corporate actions through written consent and special meetings.
Director Compensation for Last Fiscal Year
The following table summarizes the total compensation we paid to our non-employee directors for the fiscal year ended December 31, 2021:
| Name(1) | | Fees Earned or Paid in Cash ($) | | | Stock Awards ($) | | | Option Awards ($) | | | Total ($) | |
| Wen-Han Chang | | | 14,659 | | | | - | | | | - | | | | 14,659 | |
| Yao-Chung Chiang | | | 11,441 | | | | - | | | | - | | | | 11,441 | |
| Hsiu-Chen Chiu(2) | | | 15,732 | | | | - | | | | - | | | | 15,732 | |
| Chung-Yi Tsai | | | 8,581 | | | | - | | | | - | | | | 8,581 | |
| Yasushi Chikagami(3) | | | - | | | | - | | | | - | | | | - | |
| Daniel Fisher(3) | | | - | | | | - | | | | - | | | | - | |
| Nicholas Moren(3) | | | - | | | | - | | | | - | | | | - | |
| Edward L. Morris(3) | | | - | | | | - | | | | - | | | | - | |
| Dr. Beatrice Liu(3) | | | - | | | | - | | | | - | | | | - | |
| | (1) | Mr. Chun-Hsien Tsai, Chairman of the Board, is also Chief Executive Officer and does not receive additional compensation for this service on the Board. |
| | (2) | Ms. Hsiu-Chen Chiu resigned from the Board of Directors as of June 15, 2022. |
| | (3) | Mr. Chikagami, Mr. Fisher, Mr. Moren, Mr. Morris and Ms. Liu resigned from the Board, effective as of April 15, 2021. |
Pursuant to the 2021-NEDCP, four non-employee directors of the Company received 27,500 RSUs pursuant to the Appointment Grant on July 28, 2022 . The RSUs were granted pursuant to the 2021 SIP, following shareholder approval of the plan, and are subject to such other provisions set forth in the agreement evidencing the award of the RSUs, in the form adopted from time to time by the Board or the Compensation Committee of the Board.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following is a description of transactions since January 1, 2019 or any currently proposed transaction in which we are, were or will be a participant and in which any related party has, had or will have a direct or indirect material interest and for which the amount of the transaction exceeded or exceeds $120,000 or 1% of the average of our total assets as of the end of the last two completed fiscal years. A related party is defined as any of our directors, executive officers or holders of more than 5% of our outstanding capital stock, or any immediate family member of, or person sharing the household with, any of these individuals or entities.
For information on our compensation arrangements, including employment, termination of employment and with our directors and executive officers, see the sections titled “Management” and “Executive Compensation.”
Transactions with Ainos KY
Securities Purchase Agreement
On April 15, 2021, we consummated the Securities Purchase Agreement with Ainos KY, which focused on advanced testing devices and artificial intelligence consumer healthcare solutions. Pursuant to the Securities Purchase Agreement, we issued 6,666,666 shares of common stock at $3.000 per share to Ainos KY in exchange for certain patent assignments, increased our authorized common stock to 300,000,000 shares and changed our name from “Amarillo Biosciences, Inc.” to “Ainos, Inc.” Immediately after the consummation of the transaction and the issuance of the shares, Ainos KY’s ownership in the Company amounted to approximately 70.30% of the issued and outstanding shares of common stock of the Company.
Ainos COVID-19 Test Kits Sales and Marketing Agreement with Ainos KY
On June 14, 2021, we entered into an exclusive agreement to serve as the master sales and marketing agent for the Ainos COVID-19 Antigen Rapid Test Kit and COVID-19 Nucleic Acid Test Kit with Ainos KY which was developed by TCNT. On June 7, 2021, the TFDA approved emergency use authorization to TCNT for the Ainos COVID-19 Antigen Rapid Test Kit that will be sold and marketed under the “Ainos” brand in Taiwan. As TCNT secures regulatory authorizations from foreign regulatory agencies, the Company expects to partner with regional distributors to promote sales in other strategic markets. The Company purchased Ainos COVID-19 Antigen Rapid Test Kit inventory from TCNT in the amount of $183,444 for the year ended December 31, 2021 and $0 in 2020. The Company incurred costs associated with finished goods, raw materials and manufacturing fees for Covid-19 antigen rapid test kits from TCNT pursuant to a Sales and Marketing Agreement totaling $386,412 for the three months ended March 31, 2022. There were no purchases from TCNT during the same period last year.
Asset Purchase Agreement
On November 18, 2021, we entered into the Asset Purchase Agreement (the “Asset Purchase Agreement”) with Ainos KY, our majority shareholder, as modified by an Amended and Restated Asset Purchase Agreement dated as of January 29, 2022 (the “Amended Asset Purchase Agreement”). We closed the transaction on January 30, 2022.
Pursuant to the Asset Purchase Agreement, we acquired certain intellectual property assets (the “IP Assets”) and certain manufacturing, testing, and office equipment (the “Equipment”) for a total purchase price of $26,000,000 (the “Purchase Price”). The Purchase Price included $24,886,023 of for intangible intellectual property assets and $1,113,977 in equipment. As payment of the Purchase Price, at the closing on January 30, 2022, the Company issued to Ainos KY the APA Convertible Note, a convertible promissory note in the principal amount of $26,000,000.
As part of the Asset Purchase Agreement, we agreed to hire certain employees of Ainos KY who are responsible for research and development of the IP Assets and/or Equipment on terms at least equal to the compensation arrangements undertaken by Ainos KY. We did not assume any responsibility or liability with respect to any employee benefit plans of Ainos KY.
The principal sum of the APA Convertible Note is payable in cash on January 30, 2027, although we may prepay the APA Convertible Note in whole or in part without penalty. The APA Convertible Note is non-interest bearing. The APA Convertible Note will be automatically converted into shares of our common stock immediately prior to the closing of this offering at a conversion price equal to 80% of the per Unit public offering price. For more information, please refer to the disclosure in Notes 2, 3 and 12 of the 2021 Financial Statements included elsewhere in this prospectus.
Working Capital Advances
In 2021, Ainos KY provided working capital advances in the form of convertible note financing in the aggregate amount of $3,000,000. The working capital convertible notes issued in 2021 bear interest at the AFR short-term rate of 1.85% and may be convertible in whole or in part at a conversion price of $3.00 per share, subject to adjustment. On March 17, 2022, we executed a Promissory Note Extension with Ainos KY dated March 17, 2022, pursuant to which the due dates for the convertible notes issued in 2021 to Ainos were extended to February 28, 2023.
In March 2022, Ainos KY provided us a working capital advance in the form of a non-convertible note financing in the principal amount of $800,000, at a 1.85% per annum interest rate, with a maturity date of February 28, 2023.
As of April 11, 2022, there is $3,800,000 outstanding principal amount of indebtedness owed to Ainos KY, including $3,000,000 relating to convertible notes.
| Date of Issuance | Maturity Date | Interest Rate | Conversion Rate | Initial Principal Amount | Interest Payments | Principal Payments | Principal Outstanding as of April 11, 2022 | Largest aggregate amount of principal outstanding |
| 4/27/2021 | 2/28/2023(1) | 1.85% | $3.00 | 15,000 | - | - | 15,000 | 15,000 |
| 5/5/2021 | 2/28/2023(1) | 1.85% | $3.00 | 20,000 | - | - | 20,000 | 20,000 |
| 5/25/2021 | 2/28/2023(1) | 1.85% | $3.00 | 30,000 | - | - | 30,000 | 30,000 |
| 5/28/2021 | 2/28/2023(1) | 1.85% | $3.00 | 35,000 | - | - | 35,000 | 35,000 |
| 6/9/2021 | 2/28/2023(1) | 1.85% | $3.00 | 300,000 | - | - | 300,000 | 300,000 |
| 6/21/2021 | 2/28/2023(1) | 1.85% | $3.00 | 107,000 | - | - | 107,000 | 107,000 |
| 7/2/2021 | 2/28/2023(1) | 1.85% | $3.00 | 54,000 | - | - | 54,000 | 54,000 |
| 9/1/2021 | 2/28/2023(1) | 1.85% | $3.00 | 120,000 | - | - | 120,000 | 120,000 |
| 9/28/2021 | 2/28/2023(1) | 1.85% | $3.00 | 300,000 | - | - | 300,000 | 300,000 |
| 11/10/2021 | 2/28/2023(1) | 1.85% | $3.00 | 50,000 | - | - | 50,000 | 50,000 |
| 11/25/2021 | 2/28/2023(1) | 1.85% | $3.00 | 450,000 | - | - | 450,000 | 450,000 |
| 11/29/2021 | 2/28/2023(1) | 1.85% | $3.00 | 300,000 | - | - | 300,000 | 300,000 |
| 12/29/2021 | 2/28/2023(1) | 1.85% | $3.00 | 1,219,000 | - | - | 1,219,000 | 1,219,000 |
| 3/4/2022 | 2/28/2023 | 1.85% | NA | 800,000 | - | - | 800,000 | 800,000 |
| (1) | Due date extended to February 28, 2023, pursuant to a Promissory Note Extension dated March 17, 2022. |
Transactions with TCNT
On August 1, 2021, we entered into the TCNT Agreement, a five-year product development agreement, with TCNT. Pursuant to the TCNT Agreement both parties will endeavor to work together to develop pharmaceutical, medical and preventive medicine related products, with the Company being the exclusive sales agent. The Company will bear the expenses of the product development and TCNT will make its personnel and facilities accessible. Under the TCNT Agreement, both parties will jointly own the intellectual property rights of all research results of the co-development collaboration. In connection with the TCNT Agreement, the Company incurred product development expenses totaling $167,422 for the three months ended March 31, 2022, and $205,883 for the year ended December 31,2021. As of March 31,2022 and December 31,2021, the related expense payable were $109,131, and $65,156, respectively.
TCNT is the supplier manufacturer of the Ainos COVID-19 Antigen Rapid Test Kits. We incurred costs associated with finished goods, raw materials and manufacturing fees for Covid-19 antigen rapid test kits from TCNT pursuant to a Sales and Marketing Agreement, totaling $386,412 for the three months ended March 31 and $183,444 for the year ended December 31, 2021. There were no purchases from TCNT during 2021. So long as we continue to sell Ainos COVID-19 Antigen Rapid Test Kits, we expect to continue to purchase inventory from TCNT.
TCNT is the majority shareholder of Ainos KY.
Transactions with Dr. Stephen T. Chen
In 2021, Dr. Stephen T. Chen, our former Chief Executive Officer, deferred compensation and provided working capital to us in the form of convertible note and non-convertible note financing in the amounts of $69,025 and $145,395, respectively. We paid Dr. Chen $150,000 in 2021 to reduce the outstanding balance of a non-convertible note. In 2020, Dr. Chen deferred compensation and provided working capital to us in the form of convertible note and non-convertible note financing in the amounts of $239,966 and $134,010, respectively. As of April 11, 2022, the aggregate principal amount of outstanding notes to Dr. Chen is $505,931, including $376,526 relating to convertible notes.
The convertible notes bear interest at the AFR short-term rate of the note’s issuance month, with an interest rate ranging from 0.65% to 1.85%, and may be convertible in whole or in part at a conversion price of $2.55, $2.85, and $3.75 per share, as applicable. A sole non-convertible note bears interest at the AFR short-term rate of 0.13%.
On September 1, 2021, Dr. Stephen T. Chen and i2China Management Group, LLC (“i2China”) (together, the “Payees”), agreed to waive their rights pertaining to the conditional term “Annual Interest Rate on Matured, Unpaid Amounts: 10% per annum, compounded annually of Convertible Notes” in regards to interest charged on unpaid amounts following maturity for all of their respective notes. The Company and the Payees agree that the originally agreed annual interest rate will continue to be valid for any unpaid amounts after maturity. Interest waived totaled $45,875.
As of April 11, 2022, the aggregate principal amount of outstanding notes to Dr. Chen is $505,931, including $376,526 relating to convertible notes.
| Date of Issuance | Maturity Date | Interest Rate | Conversion Rate | Initial Principal Amount | Interest Payments | Principal Payments | Principal Outstanding as of April 11, 2022(1) | Largest aggregate amount of principal outstanding | |
|
| 1/30/2016 | on demand | 0.75% | $2.55 | 114,026 | - | - | 114,026 | 114,026 | |
| 3/18/2016 | on demand | 0.65% | $2.85 | 262,500 | - | - | 262,500 | 262,500 | |
| 9/1/2019 | 9/1/2020 | 1.85% | $3.75 | 39,620 | 1,671 | 39,620 | -(1) | 39,620 | |
| 12/1/2019 | 12/31/2020 | 1.61% | $3.75 | 14,879 | 490 | 14,879 | -(1) | 14,879 | |
| 1/1/2020 | 1/1/2021 | 1.85% | $3.75 | 216,600 | 6,049 | 216,600 | -(1) | 216,600 | |
| 1/1/2020 | 1/2/2021 | 1.60% | $3.75 | 23,366 | 430 | 23,366 | -(1) | 23,366 | |
| 1/1/2020 | 4/14/2021 | 0.13% | N/A | 279,405 | - | 150,000 | 129,405 | 279,405 | |
| 1/1/2021 | 4/1/2021 | 1.85% | $3.75 | 59,025 | 955 | 59,025 | -(1) | 59,025 | |
| 4/1/2021 | 5/1/2021 | 1.85% | $3.75 | 10,000 | 131 | 10,000 | -(1) | 10,000 | |
| (1) | On December 2021, Dr. Chen assigned notes to another investor for cash consideration. The investor subsequently converted the notes into shares of the Company’s common stock. |
Transactions with ASE Test Inc. and ASE Technology Holding
We sold Ainos COVID-19 Antigen Rapid Test Kits to ASE Technology Holding, totaling $209,468 for the year ended December 31, 2021 and $81,100 for the three months ended March 31, 2022. We anticipate further sales of Ainos COVID-19 Antigen Rapid Test Kits to ASE Technology Holding in the future.
On April 11, 2022, we issued to ASE Test Inc., a minority owner of Ainos KY, a convertible note in the principal amount of $500,000 due on March 30, 2027, although we may prepay the March 2027 Convertible Notes in whole or in part without penalty (the “ASE Note”). The ASE Note is non-interest bearing. The convertible note will automatically convert into shares of our common stock immediately prior to the closing of any public offering of our common stock as a result of which our common stock will be listed on a U.S. stock exchange. The conversion price, subject to certain adjustments, will be 80% of the initial public offering price of the offering.
ASE Test Inc. holds more than 10% of the voting rights in Ainos KY and ASE Technology Holding is the sole owner of ASE Test Inc.
VOTING SECURITIES AND PRINCIPAL HOLDERS
The following table sets forth certain information regarding beneficial ownership of our common stock as of August 8, 2022:
| | · | by each person, or group of affiliated persons, known by us to own beneficially 5% or more of any class of our voting securities; |
| | · | by each of our Named Executive Officers; |
| | · | by each of our directors; and |
| | · | by all our current executive officers and directors as a group. |
As of August 8, 2022, there were 9,625,287 shares of common stock issued and outstanding.
Beneficial ownership is determined in accordance with the rules and regulations of the SEC and includes voting or investment power with respect to our common stock. Shares of common stock subject to stock options, convertible notes and debentures and warrants that are currently exercisable or convertible, or exercisable or convertible within 60 days of August 8, 2022, are deemed to be outstanding for purposes of computing the percentage ownership of that person but are not treated as outstanding for computing the percentage ownership of any other person. Unless indicated below, the persons and entities named in the table have sole voting and sole investment power with respect to all shares beneficially owned, subject to community property laws where applicable.
Except as otherwise indicated, the address of each stockholder is c/o Ainos, Inc. at 8880 Rio San Diego Drive, Suite 800, San Diego, California, 92108.
| Name of Beneficial Owner | | Shares of Common Stock Beneficially Owned Prior to the Offering | | | Percentage of Shares of Common Stock Beneficially Owned Prior to the Offering | | | Shares of Common Stock Beneficially Owned Following the Offering | | | Percentage of Shares of Common Stock Beneficially Owned Following the Offering | |
| | | | | | | | | | | | | |
| Security Ownership of Certain Beneficial Owners: | | | | | | | | | | | | |
| Ainos Inc. (“Ainos KY”) (1) | | | 6,426,119 | | | | 60.40 | % | | | 14,073,177 | | | | 77.42 | % |
| Dr. Stephen T. Chen(2) | | | 667,365 | | | | 6.83 | % | | | 667,365 | | | | 3.40 | % |
| | | | | | | | | | | | | | | | | |
| Security Ownership of Management and Directors: | | | | | | | | | | | | | | | | |
| Chun-Hsien Tsai(3) | | | 355,598 | | | | 3.57 | % | | | 355,598 | | | | 1.79 | |
| Hui-Lan Wu(4) | | | 148,733 | | | | 1.52 | % | | | 163,438 | | | * | |
| Lawrence K. Lin(5) | | | 74,613 | | | * | | | | 74,613 | | | * | |
| Chung-Yi Tsai(8) | | | - | | | * | | | | - | | | * | |
| Chun-Jung Tsai | | | 7,599 | | | * | | | | 7,599 | | | * | |
| Ting-Chuan Lee | | | - | | | * | | | | - | | | * | |
| Wen-Han Chang(6)(8) | | | 266,666 | | | | 2.77 | % | | | 266,666 | | | | 1.37 | % |
| Yao-Chung Chiang(7)(8) | | | 10,000 | | | * | | | | 10,000 | | | * | |
| Pao-Sheng Wei (9) | | | - | | | * | | | | - | | | * | |
| Executive officers and directors as a group - 9 persons | | | 863,209 | | | | 8.82 | % | | | 877,914 | | | | 4.47 | % |
* Represents less than one percent.
| | (1) | Ainos Inc. (Ainos KY), a Cayman Islands company, has a total beneficial ownership of 6,426,119 shares through the following: (i) 4,664,705 shares owned by Ainos KY; (ii) 1,014,319 shares issuable upon note conversions based on principal outstanding and accrued interest thereon through August 8, 2022; and (iii) 747,095 shares subject to a Voting Agreement dated December 9, 2021 with Stephen T. Chen, Virginia M. Chen, the Stephen T. Chen and Virginia M. Chen Living Trust, dated April 12, 2018, and Hung Lan Lee. Ainos KY’s beneficial ownership may fluctuate due to the effect of this Voting Agreement with Chen and Lee which grants Chen and Lee authority to sell or transfer 25% of their respective shares in private transactions (including sales, gifts, or any other transfers) annually. Ainos KY intends to convert its $3,000,000 principal amount of convertible notes plus accrued interest into 1,014,319 shares prior to the completion of the Offering. The information in the table above assumes shares of common stock to be issued upon the conversion of the APA Convertible Note and the March 2027 Convertible Notes immediately prior to the closing of, and at exchange price equal to 80% of the public offering price of our common stock as result of which our common stock will be listed on the Nasdaq Capital Market. TCNT owns a majority of the outstanding voting securities of Ainos KY and, accordingly, may be deemed, for purposes of Section 13(d) of the Exchange Act, to share beneficial ownership of the shares held by Ainos KY. TCNT’s address is 10F-2, No. 66, Shengyi 5th Rd., Zhubei City, Hsinchu County 30261, Taiwan (R.O.C.). |
| | | |
| | (2) | Dr. Stephen T. Chen has a total beneficial ownership of 667,365 shares through the following: (i) 301,864 shares under Stephen T Chen & Virginia M Chen TTEES, Stephen T & Virginia M Chen Living Trust, DTD 04/12/2018; (ii) 129,215 shares owned by Stephen T. Chen individually, (iii) 39,661 shares owned by STC International, Inc., of which Dr. Chen is the majority owner and serves as Chairman, President and a Board member; (iv) 2,632 shares owned by ACTS Biosciences, Inc., of which Dr. Chen serves as Chairman and a Board member; (v) 49,349 shares owned by Virginia M. Chen IRA, Dr. Chen’s spouse; and (vi) 144,644 shares reserved for note conversions based on principal and accrued interest through March 31, 2022, beneficially owned by Dr. Chen exercisable on demand. |
| | | |
| | (3) | Chun-Hsien Tsai has a total beneficial ownership of 355,598 shares which includes 333,333 RSUs that will fully vest on September 30, 2022. |
| | | |
| | (4) | Hui-Lan Wu has a total beneficial ownership of 148,733 shares which includes 133,333 RSUs that will fully vest on September 30, 2022. Ms. Wu owns 11,200 shares of common stock directly and an additional 4,200 shares of common stock indirectly through her daughter, Ms. Yun-Han Liao. The information in the table above assumes shares of common stock to be issued upon the conversion of the March 2027 Convertible Note in the aggregate principal amount of $50,000 owned indirectly through Ms. Yun-Han Liao. |
| | | |
| | (5) | Lawrence K. Lin has a total beneficial ownership of 74,613 shares through the following: (i) 16,881 shares under Mr. Lin’s name; (ii) 27,558 shares beneficially owned by i2China, of which he is the sole member; and (iii) 30,174 shares reserved for warrants, beneficially owned by i2China on demand. |
| | | |
| | (6) | Wen-Han Chang owns 266,666 shares of common stock indirectly through his spouse, Chien-Hsuan Huang. |
| | | |
| | (7) | Yao-Chung Chiang owns 10,000 shares of common stock indirectly through his spouse, Hsiu-Hwei Tsai Chiang. |
| | | |
| | (8) | Excludes 7,333 RSUs to non-employee directors that have yet to vest within 60 days of August 8, 2022. |
| | | |
| | (9) | The Board appointed Mr. Pao-Sheng Wei as a Director of the Board on June 15, 2022, following the resignation of former Director, Hsiu-Chen Chiu. |
DESCRIPTION OF CAPITAL STOCK AND SECURITIES BEING OFFERED
The following summarizes the material terms and provisions of our capital stock and securities we are offering. For the complete terms of our capital stock, please refer to our certificate of formation and bylaws that are filed as exhibits to the registration statement of which this prospectus is a part. For the complete terms of the Warrants, please refer to the form of Warrant Agency Agreement and form of Warrant that are filed as exhibits to the registration statement of which this prospectus forms a part. The summary description of our capital stock below is qualified in its entirety by reference to our certificate of formation and bylaws.
General
As of the date of this prospectus, our authorized capital stock consists of 300,000,000 shares of common stock, par value $0.01 per share, and 10,000,000 shares of preferred stock, par value $0.01 per share. As of August 8, 2022, there were 9,625,287 shares of our common stock issued and outstanding. No shares of preferred stock have been issued or are outstanding.
Common Stock
Holders of our common stock are entitled to one vote for each share of common stock held of record for the election of directors and on all matters submitted to a vote of stockholders. Holders of our common stock are entitled to receive dividends ratably, if any, as may be declared by the Board out of legally available funds, subject to any preferential dividend rights of any preferred stock then outstanding. In the event of our dissolution, liquidation or winding up, holders of our common stock are entitled to share ratably in our net assets legally available after the payment of all of our debts and other liabilities, subject to the liquidation preferences of any preferred stock then outstanding. Holders of our common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future. All outstanding shares of our common stock are fully paid and non-assessable.
Preferred Stock
Under the terms of the certificate of formation, our Board of Directors is authorized to direct us to issue shares of preferred stock in one or more series without stockholder approval. Our Board of Directors has the discretion to determine the rights, powers, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock.
The purpose of authorizing our Board of Directors to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions, future financings and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or could discourage a third party from seeking to acquire, a majority of the outstanding voting stock. Additionally, the issuance of preferred stock may adversely affect the holders of common stock by restricting dividends on the common stock, diluting the voting power of the common stock or subordinating the liquidation rights of the common stock. As a result of these or other factors, the issuance of preferred stock could have an adverse impact on the market price of the common stock.
Business Combinations under Texas Law
A number of provisions of Texas law, our Certificate of Formation and Bylaws could make it more difficult for the acquisition of the Company by means of a tender offer, a proxy contest or otherwise and the removal of incumbent officers and directors. These provisions are intended to discourage coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of the Company to negotiate first with our Board.
We are subject to the provisions of Title 2, Chapter 21, Subchapter M of the Texas Business Organizations Code (the “Texas Business Combination Law”). That law provides that a Texas corporation may not engage in specified types of business combinations, including mergers, consolidations and asset sales, with a person, or an affiliate or associate of that person, who is an “affiliated shareholder”, for a period of three years from the date that person became an affiliated shareholder, subject to certain exceptions (described below). An “affiliated shareholder” is generally defined as the holder of 20% or more of the corporation’s voting shares. The law’s prohibitions do not apply if the business combination or the acquisition of shares by the affiliated shareholder was approved by the Board of Directors of the corporation before the affiliated shareholder became an affiliated shareholder; or the business combination was approved by the affirmative vote of the holders of at least two-thirds of the outstanding voting shares of the corporation not beneficially owned by the affiliated shareholder, at a meeting of shareholders called for that purpose, not less than six months after the affiliated shareholder became an affiliated shareholder.
Because we have more than 100 of record shareholders, we are considered an “issuing public corporation” for purposes of this law. The Texas Business Combination Law does not apply to the following:
| | · | the business combination of an issuing public corporation: where the corporation’s original charter or bylaws contain a provision expressly electing not to be governed by the Texas Business Combination Law; or that adopts an amendment to its charter or bylaws, by the affirmative vote of the holders, other than affiliated shareholders, of at least two-thirds of the outstanding voting shares of the corporation, expressly electing not to be governed by the Texas Business Combination Law and so long as the amendment does not take effect for 18 months following the date of the vote and does not apply to a business combination with an affiliated shareholder who became affiliated on or before the effective date of the amendment; |
| | | |
| | · | a business combination of an issuing public corporation with an affiliated shareholder that became an affiliated shareholder inadvertently, if the affiliated shareholder divests itself, as soon as possible, of enough shares to no longer be an affiliated shareholder and would not at any time within the three-year period preceding the announcement of the business combination have been an affiliated shareholder but for the inadvertent acquisition; |
| | | |
| | · | a business combination with an affiliated shareholder who became an affiliated shareholder through a transfer of shares by will or intestacy and continuously was an affiliated shareholder until the announcement date of the business combination; or |
| | | |
| | · | a business combination of a corporation with its wholly owned Texas subsidiary if the subsidiary is not an affiliate or associate of the affiliated shareholder other than by reason of the affiliated shareholder’s beneficial ownership of voting shares of the corporation. |
Neither our Certificate of Formation nor our Bylaws contain any provision expressly providing that we will not be subject to the Texas Business Combination Law. The Texas Business Combination Law may have the effect of inhibiting a non-negotiated merger or other business combination involving the Company, even if that event would be beneficial to our shareholders.
Anti-Takeover Provisions of Our Articles of Formation and Bylaws
Our Articles of Formation and Bylaws contain various provisions intended to promote the stability of our stockholder base and render more difficult certain unsolicited or hostile attempts to take over the Company, that could disrupt the Company, divert the attention of our directors, officers and employees and adversely affect the independence and integrity of our business. These provisions include:
Restated Certificate of Formation
Undesignated Preferred Stock. Our Board has the ability to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of us. These and other provisions may have the effect of deferring hostile takeovers or delaying changes in control or management of our company.
Board Vacancies Filled Only by Majority of Directors. Vacancies and newly created seats on our Board may be filled only by a majority of the directors then in office. Only our Board may determine the number of directors on our board. The inability of stockholders to determine the number of directors or to fill vacancies or newly created seats on our Board makes it more difficult to change the composition of our Board, but these provisions promote a continuity of existing management.
No Cumulative Voting. Our certificate of formation expressly prohibits cumulative voting in the election of directors.
Authorized but Unissued Shares - Our Board may cause the Company to issue authorized but unissued shares of common stock in the future without stockholders’ approval. These additional shares may be utilized for a variety of corporate purposes, including future public offerings to raise additional capital, corporate acquisitions and employee benefit plans. The existence of authorized but unissued shares of common stock could render more difficult or discourage an attempt to obtain control of a majority of common stock by means of a proxy contest, tender offer, merger or otherwise.
Transfer Agent and Registrar
Our transfer agent and registrar for our capital stock is American Stock Transfer & Trust Company, LLC. The transfer agent’s address is 6201 15th Ave., Brooklyn, NY 11219, and its telephone number is (800) 937-5449.
Nasdaq Symbol
Through August 8, 2022, our common stock was quoted on the OTCPK, one of the OTC Markets Group over-the counter markets. As of August 9, 2022, our common stock and Warrants are listed on the Nasdaq Capital Market, or Nasdaq under the symbols “AIMD” and “AIMDW,” respectively. .
Warrants Offered in this Offering
The following summary of certain terms and provisions of the Warrants offered hereby is not complete and is subject to, and qualified in its entirety by the provisions of the form of Warrant, which is filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the form of Warrant.
The Warrants issued in this offering entitle the registered holders to purchase common stock at a price of $4.25 per share, subject to adjustment as discussed below, immediately following the issuance of such Warrants and terminating at 5:00 p.m., New York City time, five years after the closing of this offering.
The exercise price and number of shares of common stock issuable upon exercise of the Warrants may be adjusted in certain circumstances, including in the event of a stock dividend or recapitalization, reorganization, merger or consolidation. However, the Warrants will not be adjusted for issuances of shares of common stock at prices below its exercise price.
Exercisability. The Warrants are exercisable immediately upon issuance and at any time up to the date that is five years from the date of issuance. The Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of common stock purchased upon such exercise. Each Warrant entitles the holder thereof to purchase one share of common stock. Warrants are not exercisable for a fraction of a share and may only be exercised into whole numbers of shares. In lieu of fractional shares, we will, pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price and round down to the nearest whole share. Unless otherwise specified in the Warrant, the holder will not have the right to exercise the Warrants, in whole or in part, if the holder (together with its affiliates) would beneficially own in excess of 4.99% (or 9.99% at the holder’s election) of the number of our shares of common stock outstanding immediately after giving effect to the exercise, as such percentage is determined in accordance with the terms of the Warrant. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon at least 61 days’ prior notice from the holder to us.
Exercise Price. The initial exercise price per share of common stock purchasable upon exercise of the Warrants is$4.25 per, and is subject to adjustments for stock splits, reclassifications, subdivisions, and other similar transactions. In addition to the exercise price per share of common stock, and other applicable charges and taxes are due and payable upon exercise.
Cashless Exercise. If we fail to maintain the effectiveness of the registration statement and current prospectus relating to the common shares issuable upon exercise of the Warrants the holders of the Warrants shall have the right to exercise the Warrants solely via a cashless exercise feature provided for in the Warrants, until such time as there is an effective registration statement and current prospectus. Upon a cashless exercise, the holder would be entitled to receive a number of shares of common stock in accordance with certain formula set forth in the Warrant.
Delivery of shares. We shall deliver the common stock underlying the Warrants to the holders exercising such Warrants by no later than 5:00 P.M. New York City time on the second trading day following the Warrants’ exercise date, provided the funds in payment of the exercise price for such Warrants have cleared on the trading day following the exercise date.
No Fractional Shares. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of the Warrants, and the number of Warrants will be rounded to the nearest whole number.
Authorized Shares. During the period the Warrants are outstanding, we will reserve from our authorized and unissued common stock a sufficient number of shares to provide for the issuance of common stock underlying the warrants upon the exercise of the Warrants.
Warrant Agent; Global Certificate. The warrant agent for the Warrants will be American Stock Transfer & Trust Company LLC. The Warrants will be issued in registered form under a warrant agency agreement between a warrant agent and us. The Warrants will initially be represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company (“DTC”), and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Transferability. Subject to applicable laws, the Warrants may be transferred at the option of the holders upon surrender of the Warrants to the warrant agent, together with the appropriate instruments of transfer.
Nasdaq Symbol. As of August 9, 2022, our common stock and Warrants are listed on the Nasdaq Capital Market, or Nasdaq under the symbols “AIMD” and “AIMDW,” respectively.
Adjustments; Fundamental Transaction. The exercise price and the number of shares underlying the Warrants are subject to appropriate adjustment in the event of stock splits, stock dividends on our common shares, stock combinations or similar events affecting our common shares. In addition, in the event we consummate a merger or consolidation with or into another person or other reorganization event in which our common shares are converted or exchanged for securities, cash or other property, or we sell, lease, license, assign, transfer, convey or otherwise dispose of all or substantially all of our assets or we or another person acquire 50% or more of our outstanding common shares (each, a Fundamental Transaction), then following such Fundamental Transaction the holders of the Warrants will be entitled to receive upon exercise of the Warrants the same kind and amount of securities, cash or property which the holders would have received had they exercised the Warrants immediately prior to such Fundamental Transaction. Any successor to us or surviving entity will assume the obligations under the warrants. Additionally, as more fully described in the Warrant, in the event of certain Fundamental Transactions, the holders of the Warrants will be entitled to receive consideration in an amount equal to the Black Scholes value of the Warrants on the date of consummation of such transaction.
Rights as a Shareholder. Except by virtue of such holder’s ownership of our common stock, the holder of a Warrant does not have rights or privileges of a shareholder, including any voting rights, until the holder exercises such Warrant.
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES
The following is a discussion of material U.S. federal income tax consequences generally applicable to the acquisition, ownership, and disposition of Units issued pursuant to this offering and the components thereof. This discussion does not address tax consequences other than those pertaining to U.S. federal income taxation. For example, this discussion does not address any consequences relating to estate or gift taxation, the alternative minimum tax, or the Medicare tax on investment income. Nor does this discussion address any aspects of U.S. state or local or non-U.S. taxation. This discussion applies only to holders that hold our Units and the components thereof as “capital assets” within the meaning of Section 1221 of the U.S. Internal Revenue Code of 1986, as amended (the “Code”). This discussion does not address all aspects of U.S. federal income taxation that may be relevant to holders in light of their particular circumstances or status, including:
| | · | financial institutions or financial services entities; |
| | · | broker-dealers; |
| | · | S corporations; |
| | · | partnerships (including entities or arrangements treated as partnerships for U.S. federal income tax purposes); |
| | · | taxpayers that are subject to the mark-to-market accounting rules; |
| | · | tax-exempt entities; |
| | · | governments or agencies or instrumentalities thereof; |
| | · | insurance companies; |
| | · | regulated investment companies or real estate investment trusts; |
| | · | expatriates or former long-term residents or citizens of the United States; |
| | · | persons for whom our common stock constitute “qualified small business stock” within the meaning of Section 1202 of the Code; |
| | · | persons that hold our securities as part of a straddle, constructive sale, hedging, conversion or other integrated or similar transaction; |
| | · | persons subject to the alternative minimum tax; |
| | · | persons whose functional currency is not the U.S. dollar; |
| | · | controlled foreign corporations; |
| | · | accrual method taxpayers that file applicable financial statements as described in Section 451(b) of the Code; or |
| | · | passive foreign investment companies. |
If a partnership (or any entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds Units or the components thereof, the tax treatment of such partnership and a person treated as a partner of such partnership will generally depend on the status of the partner and the activities of the partnership. Partnerships holding any Units or the components thereof and persons that are treated as partners of such partnerships should consult their tax advisors.
This discussion is based on the Code, Treasury Regulations promulgated under the Code, and judicial and administrative interpretations thereof, all as of the date hereof. U.S. tax law is subject to change, which change could apply retroactively and could affect the tax considerations described herein. We have not and do not intend to seek any ruling from the U.S. Internal Revenue Service (the “IRS”) regarding any U.S. federal income tax considerations described herein. There can be no assurance that the IRS will not take positions inconsistent with the considerations discussed below or that any such positions would not be sustained by a court.
EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH PROSPECTIVE INVESTOR OF THE ACQUISITION, OWNERSHIP, AND DISPOSITION OF UNITS AND THE COMPONENTS THEREOF, INCLUDING THE EFFECTS OF U.S. FEDERAL, STATE AND LOCAL, AND NON-U.S. TAX LAWS.
CHARACTERIZATION OF UNITS
Characterization of Units
Although the characterization of Units for U.S. federal income tax purposes is not entirely clear, we intend to take the position that each Unit is not a single integrated instrument for U.S. federal income tax purposes because each Unit is immediately separable into its component parts, which consist of a share of our common stock and a Warrant. Accordingly, we intend to take the position that the acquisition, ownership, or disposition of a Unit is characterized for U.S. federal income tax purposes as the acquisition, ownership, or disposition of the underlying common stock and Warrant. The remainder of this discussion assumes that the characterization of Units described above will be respected for U.S. federal income tax purposes.
Allocation of Purchase Price Between Components of Each Unit
For U.S. federal income tax purposes, each holder that acquires a Unit pursuant to this offering should allocate the purchase price paid by such holder for such Unit between the underlying common stock and Warrant based on their relative fair market values at the time of issuance. The amount of purchase price allocated to each share of common stock and each Warrant should establish the applicable holder’s initial tax basis in such share of common stock and such Warrant. We do not intend to advise holders of Units with respect to the relative fair market values of the component parts of each Unit. Each holder is accordingly urged to consult its tax advisor with respect to the allocation of purchase price among the components of each Unit.
U.S. HOLDERS
As used herein, a “U.S. Holder” is a beneficial owner of our common stock or Warrants (as applicable) that is, for U.S. federal income tax purposes:
| | · | an individual citizen or resident of the United States, |
| | · | a corporation (or other entity that is treated as a corporation for U.S. federal income tax purposes) that is created or organized (or treated as created or organized) in or under the laws of the United States or any state thereof or the District of Columbia, |
| | · | an estate whose income is subject to U.S. federal income tax regardless of its source, or |
| | · | a trust if (1) a U.S. court can exercise primary supervision over the administration of such trust and one or more U.S. persons have the authority to control all substantial decisions of the trust or (2) it has a valid election in place to be treated as a U.S. person. |
Distributions on Our Common Stock
We currently intend for the foreseeable future to follow a policy of retaining all of our earnings, if any, to finance the development and expansion of our business and, therefore, do not expect to make any distributions on our common stock during such time. If we do make distributions with respect to our common stock, a U.S. Holder generally should be required to include in gross income as a dividend the amount of any cash distribution or the fair market value of any other property distributed with respect to shares of our common stock, to the extent the distribution is paid out of our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts treated as a dividend that we pay to a U.S. Holder that is a taxable corporation may qualify for a dividends received deduction, provided certain holding period and other requirements are satisfied. Amounts treated as a dividend that we pay to a non-corporate U.S. Holder may be taxed as “qualified dividend income” at preferential tax rates accorded to long-term capital gains, subject to certain exceptions and provided certain holding period and other requirements are satisfied. Distributions in excess of current and accumulated earnings and profits should generally constitute a return of capital that is applied against and that reduces (not below zero) the U.S. Holder’s adjusted tax basis in its shares of our common stock. Any remaining excess should generally be treated as gain realized on the sale or other disposition of our common stock and should generally be treated as described below under “-U.S. Holders-Sale, Exchange, or Other Taxable Disposition of Our Common Stock or Warrants.”
Sale, Exchange, or Other Taxable Disposition of Our Common Stock or Warrants
Upon a sale, exchange, or other taxable disposition of our common stock or Warrants, a U.S. Holder generally should recognize capital gain or loss equal to the difference between the amount realized on such sale, exchange, or other taxable disposition and the U.S. Holder’s adjusted tax basis in the applicable shares of our common stock, or Warrants. Any such capital gain or loss generally should be long-term capital gain or loss if the U.S. Holder’s holding period for the shares of our common stock or Warrants so disposed of exceeds one year. Long-term capital gains recognized by non-corporate U.S. Holders may be eligible to be taxed at reduced rates. The deductibility of capital losses is subject to limitations.
Exercise or Lapse of a Warrant
A U.S. Holder generally should not recognize taxable gain or loss as a result of the acquisition of shares of our common stock upon exercise of a Warrant for cash in the amount of its exercise price. The U.S. Holder’s tax basis in the share of our common stock received upon exercise of the Warrant generally should be an amount equal to the sum of the U.S. Holder’s tax basis in the Warrant and the exercise price of such Warrant. It is unclear whether a U.S. Holder’s holding period for the shares of our common stock received upon exercise of the Warrant will commence on the date of exercise of the Warrant or the day following the date of exercise of the Warrant. In either case, the holding period should not include the period during which the U.S. Holder held the Warrant. If a Warrant is allowed to lapse unexercised, a U.S. Holder generally should recognize a capital loss equal to such U.S. Holder’s tax basis in the Warrant. The deductibility of capital losses is subject to limitations.
Possible Constructive Distributions
The terms of each Warrant provide in certain circumstances for an adjustment to the exercise price of the Warrant or an increase in the shares of our common stock issuable upon exercise. An adjustment made pursuant to a bona fide, reasonable, adjustment formula which has the effect of preventing dilution generally is not taxable. The U.S. Holders of the Warrants would, however, be treated as receiving a constructive distribution from us if, for example, the adjustment increases the U.S. Holder’s proportionate interest in our assets or earnings and profits (e.g., through a decrease to the exercise price or an increase in the number of shares of our common stock that would be obtained upon exercise) as a result of a distribution of cash or other property with respect to shares of our common stock. Such a constructive distribution to a U.S. Holder of Warrants would generally be treated as if such U.S. Holder had received a cash distribution from us in an amount equal to the fair market value of such increased interest, taxed under principles similar to those described in “-U.S. Holders-Distributions on Our Common Stock.” The rules governing constructive distributions as a result of certain adjustments with respect to a Warrant are complex. U.S. Holders are urged to consult their tax advisors on the potential existence and tax consequences of any such constructive distribution with respect to a Warrant.
Information Reporting and Backup Withholding
U.S. backup withholding and information reporting requirements may apply to distributions on our common stock, constructive distributions on Warrants, and the receipt of proceeds from the sale, exchange, or other disposition of our common stock or Warrants. Backup withholding generally should not apply, however, to a U.S. Holder who furnishes a correct taxpayer identification number and makes other required certifications, or who is otherwise exempt from backup withholding and establishes such exempt status. Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. Holder’s U.S. federal income tax liability, and a U.S. Holder generally may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for refund with the IRS and furnishing any required information.
NON-U.S. HOLDERS
As used herein, a “non-U.S. Holder” is a beneficial owner of our common stock or Warrant (as applicable) that is not a U.S. Holder.
Distributions on Our Common Stock
We currently intend for the foreseeable future to follow a policy of retaining all of our earnings, if any, to finance the development and expansion of our business and, therefore, do not expect to make any distributions on our common stock during such time. If we do make distributions with respect to our common stock, any such distribution made to a non-U.S. Holder with respect to our common stock should generally constitute a dividend for U.S. federal income tax purposes to the extent paid out of our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Provided that any such dividend is not effectively connected with such non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, attributable to a U.S. permanent establishment or fixed base maintained by such non-U.S. Holder), such dividend should generally be subject to withholding tax from the gross amount of the dividend at a rate of 30%, unless such non-U.S. Holder is eligible for a reduced rate of withholding tax under an applicable income tax treaty and provides proper certification of its eligibility for such reduced rate (usually on an IRS Form W-8BEN or W-8BEN-E, as applicable). Any distribution not constituting a dividend should generally be treated first as reducing (not below zero) the non-U.S. Holder’s adjusted tax basis in our common stock and then, to the extent such distribution exceeds the non-U.S. Holder’s adjusted tax basis, as gain realized from the sale or other disposition of our common stock, which should generally be treated as described below under “-Non-U.S. Holders-Sale, Exchange or Other Taxable Disposition of Our Common Stock or Warrants.”
Dividends paid by us to a non-U.S. Holder that are effectively connected with such non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, attributable to a U.S. permanent establishment or fixed base maintained by such non-U.S. Holder) should generally not be subject to U.S. withholding tax, provided such non-U.S. Holder complies with certain certification and disclosure requirements (usually by providing an IRS Form W-8ECI). Instead, such dividends should generally be subject to U.S. federal income tax, net of certain deductions, at the same graduated individual or corporate rates applicable to U.S. Holders. If the non-U.S. Holder is a corporation, dividends that are effectively connected income may also be subject to a “branch profits tax” at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty).
Sale, Exchange, or Other Taxable Disposition of Our Common Stock or Warrants
A non-U.S. Holder generally should not be subject to U.S. federal income tax on gain realized from a sale, exchange, or other disposition of our common stock or Warrants unless:
| | (i) | such non-U.S. Holder is an individual who was present in the United States for 183 days or more in the taxable year of such disposition and certain other requirements are met, in which case any gain realized will generally be subject to a flat 30% U.S. federal income tax; |
| | | |
| | (ii) | the gain is effectively connected with a trade or business of such non-U.S. Holder in the United States (and, if required by an applicable income tax treaty, attributable to a U.S. permanent establishment or fixed base maintained by such non-U.S. Holder), in which case such gain will be subject to U.S. federal income tax, net of certain deductions, at the same graduated individual or corporate rates applicable to U.S. Holders, and any such gain of a non-U.S. Holder that is a corporation may be subject to an additional “branch profits tax” at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty); or |
| | | |
| | (iii) | subject to certain exceptions discussed below, we are or have been a U.S. real property holding corporation (a “USRPHC”) at any time during the shorter of the five-year period preceding such disposition and such non-U.S. Holder’s holding period, in which case (a) gain recognized by such non-U.S. holder on the sale, exchange, or other disposition of our common stock or Warrants should generally be subject to tax at generally applicable U.S. federal income tax rates and (b) a buyer of our common stock or Warrants from such non-U.S. Holder may be required to withhold U.S. federal income tax at a rate of 15% of the amount realized upon such disposition. |
For purposes of item (iii) immediately above, we will generally be classified as a USRPHC if the fair market value of our “United States real property interests” equals or exceeds 50% of the sum of the fair market value of our worldwide real property interests and our other assets used or held for use in a trade or business, as determined for U.S. federal income tax purposes. Although there can be no assurance, we believe that we are not currently a USRPHC and we do not anticipating becoming a USRPHC. Even if we are or become a USRPHC, a non-US Holder should generally not be subject to U.S. federal income tax under the rules discussed in item (iii) with respect to gain realized on a sale or other disposition of our common stock if (A) our common stock is considered to be regularly traded on an established securities market and (B) such non-U.S. Holder has not owned and is not deemed to have owned more than 5% of our common stock at any time during the shorter of the five-year period preceding such disposition and such non-U.S. Holder’s holding period.
Exercise or Lapse of a Warrant
The U.S. federal income tax treatment of a non-U.S. Holder’s exercise of a Warrant generally should correspond to the U.S. federal income tax treatment of the exercise of a Warrant held by a U.S. Holder, as described above under “-U.S. Holders-Exercise or Lapse of a Warrant.” If a Warrant is allowed to lapse unexercised, a non-U.S. Holder generally should recognize a loss equal to such U.S. Holder’s tax basis in the Warrant. However, subject to additional conditions and restrictions, a non-U.S. Holder generally cannot utilize a loss recognized upon expiration of a Warrant to reduce the non-U.S. Holder’s U.S. federal income tax liability unless the loss is effectively connected with the non-U.S. Holder’s conduct of a trade or business within the United States (and, if an income tax treaty applies, is attributable to a U.S. permanent establishment or fixed base maintained by the non-U.S. Holder) or if the loss is treated as U.S.-source and incurred by a non-U.S. Holder that is an individual during a taxable year in which the non-U.S. Holder is present in the United States for at least 183 days.
Possible Constructive Distributions
As described above under “-U.S. Holders-Possible Constructive Distributions,” certain adjustments with respect to the Warrants may give rise to a constructive distribution, the consequences of which to a non-U.S. Holder would be similar to those described above under “-Non-U.S. Holders-Distributions on Our Common Stock.”
Information Reporting and Backup Withholding
U.S. backup withholding and information reporting requirements may apply to distributions on our common stock, constructive distributions on Warrants, and the receipt of proceeds from the sale or disposition of our common stock, or Warrants. A non-U.S. Holder may have to comply with certification procedures to establish that it is not a U.S. person for U.S. federal income tax purposes, to otherwise establish an exemption from information reporting and backup withholding requirements, or to claim a reduced rate of withholding under an applicable income tax treaty. Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a non-U.S. Holder’s U.S. federal income tax liability, and a non-U.S. Holder generally may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for refund with the IRS and furnishing any required information.
Foreign Account Tax Compliance Act
Sections 1471 through 1474 of the Code and the Treasury Regulations and administrative guidance promulgated thereunder (commonly referred as the “Foreign Account Tax Compliance Act” or “FATCA”) generally impose withholding at a rate of 30% in certain circumstances on dividends in respect of securities (including our common stock) which are held by or through certain foreign financial institutions (including investment funds), unless any such institution (i) enters into, and complies with, an agreement with the IRS to, among other things, report, on an annual basis, information with respect to interests in, and accounts maintained by, the institution that are owned by certain U.S. persons and by certain non-U.S. entities that are wholly or partially owned by U.S. persons and to withhold on certain payments, or (ii) if allowed under an intergovernmental agreement between the United States and an applicable foreign country, reports such information to its local tax authority, which may exchange such information with the U.S. authorities. An intergovernmental agreement between the United States and an applicable foreign country may modify these requirements. Accordingly, the disclosure of the ownership of the entity through which our securities (including our common stock) is held will affect the determination of whether such withholding and reporting is required. Similarly, dividends in respect of our common stock held by an investor that is a non-financial non-U.S. entity that does not qualify under certain exceptions will generally be subject to withholding at a rate of 30%, unless such entity either (i) certifies to the applicable withholding agent that such entity does not have any “substantial United States owners” or (ii) provides certain information regarding the entity’s “substantial United States owners,” which may in turn be provided to the U.S. Department of Treasury. All holders should consult their tax advisors regarding the possible implications of FATCA on their investment in our common stock and Warrants.
UNDERWRITING
Maxim Group LLC (“Maxim”) is acting as lead managing underwriter, sole book running manager and representative of the underwriters in the offering. Subject to the terms and conditions stated in the underwriting agreement dated the date of this prospectus, the underwriters have agreed to purchase, and we have agreed to sell to the underwriters, the number of Units set forth below.
| Underwriter | | Number of Units | |
| Maxim Group LLC | | | 585,000 | |
| Brookline Capital Markets, a division of Arcadia Securities, LLC | | | 195,000 | |
| Total | | | 780,000 | |
The underwriting agreement provides that the obligations of the underwriter to purchase the Units included in this offering are subject to approval of legal matters by counsel and to other conditions. The underwriter is obligated to purchase all of such Units (not including the shares of common stock and Warrants covered by the over-allotment option described below) if they purchase any of the Units.
Units sold by the underwriter to the public will initially be offered at the initial public offering price set forth on the cover of this prospectus. Any Units sold by the underwriters to securities dealers may be sold at a discount from the initial public offering price not to exceed $0.14875 per Unit. If all the Units are not sold at the initial offering price, the underwriter may change the offering price and the other selling terms.
Pursuant to the underwriting agreement, we granted to the underwriter an option, exercisable within 45 days after the closing of this offering to acquire up to an additional 117,000 shares of common stock and/or up to an additional 117,000 Warrants, solely for the purpose of covering over-allotments, if any. If the underwriter exercises all or any part of this option, it will purchase shares of common stock and/or Warrants covered by the option at $4.24 per share of common stock and $0.01 per Warrant, less, in each case, the underwriting discount.
Underwriting discounts and commissions
The following table shows the per Unit price and total underwriting discounts and commissions we will pay in connection with this offering. These amounts are shown assuming both no exercise and full exercise of the underwriters’ over-allotment option:
| | | Per Unit | | | Total without O/A Option | | | Total with O/A Option | |
| Public Unit price | | $ | 4.25 | | | $ | 3,315,000 | | | $ | 3,812,250 | |
| Underwriting discount (7.5%) | | $ | 0.31875 | | | $ | 248,625 | | | $ | 285,919 | |
| Proceeds, before expenses, to us | | $ | 3.93125 | | | $ | 3,066,375 | | | $ | 3,526,331 | |
We have also agreed to reimburse the underwriter for its expenses in connection with this offering, including all reasonable fees and expenses of the underwriters’ legal counsel, up to $150,000. We have paid $25,000 to Maxim as an advance to be applied towards reasonable out-of-pocket expenses (the “Advance”). Any portion of the Advance shall be returned back to us to the extent not actually incurred.
We estimate the total expenses of this offering which will be payable by us, excluding the underwriting discount and the underwriter’s expenses payable by us, will be approximately $0.8 million.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make because of any of those liabilities.
Representative’s Warrants
We have agreed to issue to the Representative (or its permitted assignees) Warrants to purchase up to a total 5% of the shares of common stock sold in the offering (including 5% of any shares of common stock purchased upon exercise of the over-allotment option). The Representative’s Warrants will have a term of five years from the date of the commencement of sales related to this offering and will have an exercise price equal to 110% of the public offering price of the combination of shares and warrants set forth on the cover of this prospectus. The Representative’s Warrants may be exercised on a cashless basis. The Representative’s Warrants are not redeemable by us. This prospectus also covers the sale of the Representative’s Warrants and the shares of common stock underlying such warrants. The Representative’s Warrants and the underlying securities have been deemed compensation by FINRA, and are therefore subject to FINRA Rule 5110(g)(1). In accordance with FINRA Rule 5110(g)(1), neither the Representative’s Warrants nor any securities issued upon exercise of the Representative’s Warrants may be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of such securities by any person for a period of 180 days immediately following the date of effectiveness or commencement of sales of the offering pursuant to which the Representative’s Warrants are being issued, except the transfer of any security: (i) by operation of law or by reason of reorganization of our company; (ii) to any FINRA member firm participating in this offering and the officers or partners thereof, if all securities so transferred remain subject to the lock-up restriction described above for the remainder of the time period; (iii) if the aggregate amount of our securities held by either an underwriter or a related person do not exceed 1% of the securities being offered; (iv) that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund, and participating members in the aggregate do not own more than 10% of the equity in the fund; or (v) the exercise or conversion of any security, if all securities received remain subject to the lock-up restriction set forth above for the remainder of the time period. The Representative’s Warrants will provide for cashless exercise. The Representative’s Warrants will contain provisions for one demand registration of the sale of the underlying shares of common stock at our expense, an additional demand registration at the Warrant holders’ expense, and unlimited “piggyback” registration rights for a period of three years after the effective date of this prospectus at our expense.
Lock-up Agreements
We, each of our officers, directors and holders of 5% of more of our outstanding common stock as of the effective date of this prospectus (and all holders of securities exercisable for or convertible into shares of common stock) have agreed to enter into customary “lock-up” agreements in favor of Maxim pursuant to which such persons and entities have agreed, for a period of six months from the effective date of this prospectus, that they shall neither offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any securities of the Company without Maxim’s prior written consent, (excluding the issuance of shares of common stock upon the exercise of currently outstanding equity awards under the Company’s employee benefit plans).
Maxim may in its sole discretion and at any time without notice release some or all of the shares subject to lock-up agreements prior to the expiration of the lock-up period. When determining whether or not to release shares from the lock-up agreements, the representative will consider, among other factors, the security holder’s reasons for requesting the release, the number of shares for which the release is being requested and market conditions at the time.
Right of First Refusal
We have granted Maxim a right of first refusal, for a period of 18 months from the commencement of sales of this offering, to act as lead managing underwriter and sole book runner or minimally as a co-lead manager and joint-book runner and/or co-lead placement agent with at least 75.0% of the economics for any and all future public or private equity, equity-linked or debt (excluding commercial bank debt) offerings during such 18 month period of the Company, or any successor to or any subsidiary of the Company.
Stabilization, Short Positions and Penalty Bids
The representatives may engage in stabilizing transactions, short sales and purchases to cover positions created by short sales, and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of the common stock, in accordance with Regulation M under the Exchange Act:
| | · | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. |
| | | |
| | · | A short position involves a sale by the underwriters of Units in excess of the number of Units the underwriters are obligated to purchase in the offering, which creates the syndicate short position. This short position may be either a covered short position or a naked short position. In a covered short position, the number of Units involved in the sales made by the underwriters in excess of the number of Units they are obligated to purchase is not greater than the number of Units that they may purchase by exercising their option to purchase additional Units. In a naked short position, the number of Units involved is greater than the number of Units in their option to purchase additional Units. The underwriters may close out any short position by either exercising their option to purchase additional Units and/or purchasing Units in the open market. In determining the source of Units to close out the short position, the underwriters will consider, among other things, the price of the Units available for purchase in the open market as compared to the price at which they may purchase Units through their option to purchase additional Units. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the Units in the open market after pricing that could adversely affect investors who purchase in the offering. |
| | | |
| | · | Syndicate covering transactions involve purchases of the common stock in the open market after the distribution has been completed in order to cover syndicate short positions. |
| | | |
| | · | Penalty bids permit the representatives to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result, the price of the common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on the Nasdaq Capital Market or otherwise and, if commenced, may be discontinued at any time.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of the common stock. In addition, neither we nor any of the underwriters make any representation that the representatives will engage in these stabilizing transactions or that any transaction, once commenced, will not be discontinued without notice.
Electronic Distribution
A prospectus in electronic format may be made available on the Internet sites or through other online services maintained by one or more of the underwriters and/or selling group members participating in this offering, or by their affiliates. In those cases, prospective investors may view offering terms online and, depending upon the particular underwriter or selling group member, prospective investors may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of shares for sale to online brokerage account holders. Any such allocation for online distributions will be made by the representatives on the same basis as other allocations.
Other than the prospectus in electronic format, the information on any underwriter’s or selling group member’s web site and any information contained in any other web site maintained by an underwriter or selling group member is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriter or selling group member in its capacity as underwriter or selling group member and should not be relied upon by investors.
Other Relationships
The underwriter and certain of its affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriter and certain of its affiliates have, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for the issuer and its affiliates, for which they received or may in the future receive customary fees and expenses.
In the ordinary course of their various business activities, the underwriters and certain of their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of the issuer or its affiliates. If the underwriters or their affiliates have a lending relationship with us, they routinely hedge their credit exposure to us consistent with their customary risk management policies. The underwriters and their affiliates may hedge such exposure by entering into transactions which consist of either the purchase of credit default swaps or the creation of short positions in our securities or the securities of our affiliates, including potentially the shares of common stock offered hereby. Any such credit default swaps or short positions could adversely affect future trading prices of the shares of common stock offered hereby. The underwriters and certain of their affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Offer Restrictions Outside the United States
No action has been taken in any jurisdiction (except in the United States) that would permit a public offering of the securities the possession, circulation or distribution of this prospectus or any other material relating to us or the securities in any jurisdiction where action for that purpose is required. Accordingly, the securities may not be offered or sold, directly or indirectly, and neither this prospectus nor any other material or advertisements in connection with the securities may be distributed or published, in or from any country or jurisdiction except in compliance with any applicable laws, rules and regulations of any such country or jurisdiction.
Australia. This prospectus:
| | · | does not constitute a product disclosure document or a prospectus under Chapter 6D.2 of the Corporations Act 2001 (Cth) (the “Corporations Act”); |
| | | |
| | · | has not been, and will not be, lodged with the Australian Securities and Investments Commission (“ASIC”), as a disclosure document for the purposes of the Corporations Act and does not purport to include the information required of a disclosure document under Chapter 6D.2 of the Corporations Act; |
| | | |
| | · | does not constitute or involve a recommendation to acquire, an offer or invitation for issue or sale, an offer or invitation to arrange the issue or sale, or an issue or sale, of interests to a “retail client” (as defined in section 761G of the Corporations Act and applicable regulations) in Australia; and |
| | | |
| | · | may only be provided in Australia to select investors who are able to demonstrate that they fall within one or more of the categories of investors, or Exempt Investors, available under section 708 of the Corporations Act. |
The securities may not be directly or indirectly offered for subscription or purchased or sold, and no invitations to subscribe for or buy the securities Units may be issued, and no draft or definitive offering memorandum, advertisement or other offering material relating to any securities may be distributed in Australia, except where disclosure to investors is not required under Chapter 6D of the Corporations Act or is otherwise in compliance with all applicable Australian laws and regulations. By submitting an application for the securities, you represent and warrant to us that you are an Exempt Investor.
As any offer of securities under this prospectus will be made without disclosure in Australia under Chapter 6D.2 of the Corporations Act, the offer of those securities for resale in Australia within 12 months may, under section 707 of the Corporations Act, require disclosure to investors under Chapter 6D.2 if none of the exemptions in section 708 applies to that resale. By applying for the securities, you undertake to us that you will not, for a period of 12 months from the date of issue of the securities, offer, transfer, assign or otherwise alienate those securities to investors in Australia except in circumstances where disclosure to investors is not required under Chapter 6D.2 of the Corporations Act or where a compliant disclosure document is prepared and lodged with ASIC.
This prospectus contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Canada. The securities may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Cayman Islands. This prospectus does not constitute a public offer of the securities, whether by way of sale or subscription, in the Cayman Islands. Securities have not been offered or sold, and will not be offered or sold, directly or indirectly, in the Cayman Islands.
Dubai International Financial Centre (“DIFC”). This prospectus relates to an Exempt Offer in accordance with the Markets Rules 2012 of the Dubai Financial Services Authority (the “DFSA”). This prospectus is intended for distribution only to persons of a type specified in the Markets Rules 2012 of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus supplement nor taken steps to verify the information set forth herein and has no responsibility for this prospectus. The securities to which this prospectus relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the securities offered should conduct their own due diligence on the securities. If you do not understand the contents of this prospectus you should consult an authorized financial advisor.
In relation to its use in the DIFC, this prospectus is strictly private and confidential and is being distributed to a limited number of investors and must not be provided to any person other than the original recipient, and may not be reproduced or used for any other purpose. The interests in the securities may not be offered or sold directly or indirectly to the public in the DIFC.
European Economic Area. In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a Relevant Member State), with effect from and including the date on which the Prospectus Directive was implemented in that Relevant Member State (the Relevant Implementation Date), an offer of the securities to the public may not be made in that Relevant Member State prior to the publication of a prospectus in relation to the securities which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the Prospectus Directive, except that, with effect from and including the Relevant Implementation Date, an offer of securities may be made to the public in that Relevant Member State at any time:
| | · | to any legal entity which is a qualified investor as defined under the Prospectus Directive; |
| | | |
| | · | to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive); or |
| | | |
| | · | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities described in this prospectus shall result in a requirement for the publication by us of a prospectus pursuant to Article 3 of the Prospectus Directive. |
For the purposes of the above paragraph, the expression “an offer of the securities to the public” in relation to any ordinary share in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe the securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State. The expression Prospectus Directive means Directive 2003/71/EC (and any amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State) and includes any relevant implementing measure in each Relevant Member State, and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Hong Kong. The securities may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules promulgated thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the securities may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to securities which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules promulgated thereunder.
Israel. The securities offered by this prospectus has not been approved or disapproved by the Israeli Securities Authority, or ISA, nor have such securities been registered for sale in Israel. The securities may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Japan. Securities have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948, as amended) and, accordingly, will not be offered or sold directly or indirectly in Japan or to, or for the benefit of any Japanese person or to others, for re-offering or re-sale directly or indirectly in Japan or to any Japanese person, except in each case pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Securities and Exchange Law of Japan and any other applicable laws, rules and regulations of Japan. For purposes of this paragraph, “Japanese person” means any person resident in Japan, including any corporation or other entity organized under the laws of Japan.
Kuwait. Unless all necessary approvals from the Kuwait Ministry of Commerce and Industry required by Law No. 31/1990 “Regulating the Negotiation of Securities and Establishment of Investment Funds,” its Executive Regulations and the various Ministerial Orders issued pursuant thereto or in connection therewith, have been given in relation to the marketing and sale of the securities, these may not be marketed, offered for sale, nor sold in the State of Kuwait. Neither this prospectus (including any related document), nor any of the information contained therein is intended to lead to the conclusion of any contract of whatsoever nature within Kuwait.
Malaysia. No prospectus or other offering material or document in connection with the offer and sale of the securities has been or will be registered with the Securities Commission of Malaysia (the “Commission”) for the Commission’s approval pursuant to the Capital Markets and Services Act 2007. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the securities may not be circulated or distributed, nor may the securities be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Malaysia other than (i) a closed end fund approved by the Commission; (ii) a holder of a Capital Markets Services License; (iii) a person who acquires the securities, as principal, if the offer is on terms that the securities may only be acquired at a consideration of not less than RM250,000 (or its equivalent in foreign currencies) for each transaction; (iv) an individual whose total net personal assets or total net joint assets with his or her spouse exceeds RM3 million (or its equivalent in foreign currencies), excluding the value of the primary residence of the individual; (v) an individual who has a gross annual income exceeding RM300,000 (or its equivalent in foreign currencies) per annum in the preceding twelve months; (vi) an individual who, jointly with his or her spouse, has a gross annual income of RM400,000 (or its equivalent in foreign currencies), per annum in the preceding twelve months; (vii) a corporation with total net assets exceeding RM10 million (or its equivalent in a foreign currencies) based on the last audited accounts; (viii) a partnership with total net assets exceeding RM10 million (or its equivalent in foreign currencies); (ix) a bank licensee or insurance licensee as defined in the Labuan Financial Services and Securities Act 2010; (x) an Islamic bank licensee or takaful licensee as defined in the Labuan Financial Services and Securities Act 2010; and (xi) any other person as may be specified by the Commission; provided that, in the each of the preceding categories (i) to (xi), the distribution of the securities is made by a holder of a Capital Markets Services License who carries on the business of dealing in securities. The distribution in Malaysia of this prospectus is subject to Malaysian laws. This prospectus does not constitute and may not be used for the purpose of public offering or an issue, offer for subscription or purchase, invitation to subscribe for or purchase any securities requiring the registration of a prospectus with the Commission under the Capital Markets and Services Act 2007.
People’s Republic of China. This prospectus may not be circulated or distributed in the PRC and the securities may not be offered or sold, and will not offer or sell to any person for re-offering or resale directly or indirectly to any resident of the PRC except pursuant to applicable laws, rules and regulations of the PRC. For the purpose of this paragraph only, the PRC does not include Taiwan and the special administrative regions of Hong Kong and Macau.
Qatar. In the State of Qatar, the offer contained herein is made on an exclusive basis to the specifically intended recipient thereof, upon that person’s request and initiative, for personal use only and shall in no way be construed as a general offer for the sale of securities to the public or an attempt to do business as a bank, an investment company or otherwise in the State of Qatar. This prospectus and the underlying securities have not been approved or licensed by the Qatar Central Bank or the Qatar Financial Centre Regulatory Authority or any other regulator in the State of Qatar. The information contained in this prospectus shall only be shared with any third parties in Qatar on a need to know basis for the purpose of evaluating the contained offer. Any distribution of this prospectus by the recipient to third parties in Qatar beyond the terms hereof is not permitted and shall be at the liability of such recipient.
Saudi Arabia. This prospectus may not be distributed in the Kingdom of Saudi Arabia except to such persons as are permitted under the Offers of Securities Regulations issued by the Capital Market Authority. The Capital Market Authority does not make any representation as to the accuracy or completeness of this prospectus, and expressly disclaims any liability whatsoever for any loss arising from, or incurred in reliance upon, any part of this prospectus. Prospective purchasers of the securities offered hereby should conduct their own due diligence on the accuracy of the information relating to the securities. If you do not understand the contents of this prospectus you should consult an authorized financial adviser.
Singapore. This prospectus or any other offering material relating to the securities has not been registered as a prospectus with the Monetary Authority of Singapore under the Securities and Futures Act, Chapter 289 of Singapore, or the SFA. Accordingly, (a) the securities have not been, and will not be, offered or sold or made the subject of an invitation for subscription or purchase of such securities in Singapore, and (b) this prospectus or any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the securities have not been and will not be circulated or distributed, whether directly or indirectly, to the public or any member of the public in Singapore other than (i) to an institutional investor as specified in Section 274 of the SFA, (ii) to a relevant person (as defined in Section 275 of the SFA) and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the securities are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| | (a) | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| | | |
| | (b) | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the securities pursuant to an offer made under Section 275 of the SFA except: |
| | | (a) | to an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA; |
| | | | |
| | | (b) | where no consideration is or will be given for the transfer; |
| | | | |
| | | (c) | where the transfer is by operation of law; |
| | | | |
| | | (d) | as specified in Section 276(7) of the SFA; or |
| | | | |
| | | (e) | as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore. |
Switzerland. The securities will not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or SIX, or on any other stock exchange or regulated trading facility in Switzerland. This prospectus has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this prospectus nor any other offering or marketing material relating to our company or the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this prospectus will not be filed with, and the offer of the securities will not be supervised by, the Swiss Financial Market Supervisory Authority, and the offer of the securities has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (the “CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of the securities.
Taiwan. The securities have not been and will not be registered with the Financial Supervisory Commission of Taiwan pursuant to relevant securities laws and regulations and may not be sold, issued or offered within Taiwan through a public offering or in circumstances which constitutes an offer within the meaning of the Securities and Exchange Act of Taiwan that requires a registration or approval of the Financial Supervisory Commission of Taiwan. No person or entity in Taiwan has been authorized to offer, sell, give advice regarding or otherwise intermediate the offering and sale of the securities in Taiwan.
United Arab Emirates. The securities have not been offered or sold, and will not be offered or sold, directly or indirectly, in the United Arab Emirates, except: (i) in compliance with all applicable laws and regulations of the United Arab Emirates; and (ii) through persons or corporate entities authorized and licensed to provide investment advice and/or engage in brokerage activity and/or trade in respect of foreign securities in the United Arab Emirates. The information contained in this prospectus does not constitute a public offer of securities in the United Arab Emirates in accordance with the Commercial Companies Law (Federal Law No. 8 of 1984 (as amended)) or otherwise and is not intended to be a public offer and is addressed only to persons who are sophisticated investors.
United Kingdom. This prospectus is only being distributed to and is only directed at, and any offer subsequently made may only be directed at: (i) persons who are outside the United Kingdom; (ii) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”); or (iii) high net worth companies, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons falling within (1)-(3) together being referred to as “relevant persons”). The securities are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire the securities will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this prospectus or any of its contents.
LEGAL MATTERS
The validity of the common stock offered by this prospectus will be passed upon for us by Baker & McKenzie LLP, Houston, TX. Certain legal matters in connection with the offering will be passed upon for the underwriter by Loeb & Loeb LLP, New York, New York.
EXPERTS
The financial statements of the Company at December 31, 2021 and 2020, and for each of the two years then ended appearing in this prospectus have been audited by PWR CPA, LLP, an independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 under the Securities Act with the SEC with respect to this offering. This prospectus was filed as a part of that registration statement but does not contain all of the information contained in the registration statement and exhibits. Reference is thus made to the omitted information. Statements made in this prospectus are summaries of the material terms of contracts, agreements and documents and are not necessarily complete; however, all information we considered material has been disclosed. Reference is made to each exhibit for a more complete description of the matters involved and these statements are qualified in their entirety by the reference. The SEC also maintains a web site (http://www.sec.gov) that contains this filed registration statement, reports and other information regarding us that we have filed electronically with the SEC. For more information pertaining to our company and this offering, reference is made to the registration statement.
INDEX TO FINANCIAL STATEMENTS
Audited Financial Statements of Ainos, Inc.
| Condensed Consolidated Balance Sheets– March 31, 2022 and December 31, 2021 (unaudited) | | F-2 | |
| Condensed Consolidated Statements of Operations – Three Months Ended March 31, 2022 and 2021 (unaudited) | | F-3 | |
| Condensed Consolidated Statements of Comprehensive Loss – Three Months Ended March 31, 2022 and 2021 (unaudited) | | F-4 | |
| Condensed Consolidated Statements of Stockholders’ Equity (Deficit) – Three Months Ended March 31, 2022 and 2021 (unaudited) | | F-5 | |
| Condensed Consolidated Statements of Cash Flows – Three Months Ended March 31, 2022 and 2021 (unaudited) | | F-6 | |
| Notes to Financial Statements (unaudited) | | F-7 | |
| | | | |
| Report of Independent Registered Public Accounting Firm | | F-12 | |
| Balance Sheets as of December 31, 2021 and 2020 | | F-14 | |
| Statement of Operations for years ended December 31, 2021 and 2020 | | F-15 | |
| Statement of Stockholders’ Equity (Deficit) for years ended December 31, 2021 and 2020 | | F-16 | |
| Statement of Cash Flows for years ended December 31, 2021 and 2020 | | F-17 | |
| Notes to Consolidated Financial Statements | | F-18 | |
Ainos, Inc.
Condensed Consolidated Balance Sheets
(Unaudited)
| | | March 31, | | | December 31, | |
| | | 2022 | | | 2021 | |
| Assets | | | | | | |
| Current assets: | | | | | | |
| Cash and cash equivalents | | $ | 1,871,349 | | | $ | 1,751,499 | |
| Inventory | | | 337,805 | | | | - | |
| Other current assets | | | 813,905 | | | | 466,198 | |
| Total current assets | | | 3,023,059 | | | | 2,217,697 | |
| Intangible assets, net | | | 36,214,023 | | | | 37,329,191 | |
| Property and equipment, net | | | 1,419,584 | | | | 1,187,702 | |
| Other assets | | | 79,598 | | | | 87,571 | |
| Total assets | | $ | 40,736,264 | | | $ | 40,822,161 | |
| | | | | | | | | |
| Liabilities and Stockholders’ Equity | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Convertible notes payable | | $ | 3,376,526 | | | $ | 3,376,526 | |
| Notes payable | | | 1,013,405 | | | | 213,405 | |
| Accrued expenses and others current liabilities | | | 1,339,585 | | | | 1,004,868 | |
| Payables - related party | | | - | | | | 26,000,000 | |
| Total current liabilities | | | 5,729,516 | | | | 30,594,799 | |
| Long term liabilities: | | | | | | | | |
| Convertible notes payable - noncurrent | | | 26,900,000 | | | | - | |
| Operating lease liabilities - noncurrent | | | 24,152 | | | | 30,255 | |
| Total long term liabilities | | | 26,924,152 | | | | 30,255 | |
| Total liabilities | | | 32,653,668 | | | | 30,625,054 | |
| | | | | | | | | |
| Stockholders’ equity | | | | | | | | |
| Preferred stock, $0.01 par value; 10,000,000 shares | | | | | | | | |
| authorized; none issued | | | | | | | | |
| Common stock, $0.01 par value; 300,000,000 shares | | | | | | | | |
| authorized as of March 31, 2022 and December 31, | | | | | | | | |
| 2021; 144,379,308 shares issued and outstanding as | | | | | | | | |
| of March 31, 2022 and December 31, 2021 | | | 96,252 | | | | 96,252 | |
| Additional paid-in capital | | | 20,247,414 | | | | 20,203,971 | |
| Accumulated deficit | | | (12,208,811 | ) | | | (10,108,916 | ) |
| Translation adjustment | | | (52,259 | ) | | | 5,800 | |
| Total stockholders’ equity | | | 8,082,596 | | | | 10,197,107 | |
| Total liabilities and stockholders’ equity | | $ | 40,736,264 | | | $ | 40,822,161 | |
See accompanying notes to condensed consolidated financial statements.
Ainos, Inc.
Condensed Consolidated Statements of Operations
(Unaudited)
| | | Three Months Ended March 31, | |
| | | 2022 | | | 2021 | |
| Revenues | | $ | 87,200 | | | $ | 2,121 | |
| Cost of revenues | | | (41,078 | ) | | | (1,249 | ) |
| Gross margin | | | 46,122 | | | | 872 | |
| Operating expenses: | | | | | | | | |
| Research and development expenses | | | 1,577,454 | | | | - | |
| Selling, general and administrative expenses | | | 551,730 | | | | 522,981 | |
| Total operating expenses | | | 2,129,184 | | | | 522,981 | |
| Operating loss | | | (2,083,062 | ) | | | (522,109 | ) |
| Non-operating income and expenses, net | | | | | | | | |
| Interest expenses, net | | | (16,687 | ) | | | (11,897 | ) |
| Other losses | | | (146 | ) | | | - | |
| Total non-operating income and expenses, net | | | (16,833 | ) | | | (11,897 | ) |
| Net loss | | $ | (2,099,895 | ) | | $ | (534,006 | ) |
| | | | | | | | | |
| Basic and diluted net loss per average share available to common shareholders | | $ | (0.01 | ) | | $ | (0.01 | ) |
| | | | | | | | | |
| Weighted average common shares outstanding- basic and diluted | | | 144,379,308 | | | | 42,066,172 | |
See accompanying notes to condensed consolidated financial statements.
Ainos, Inc.
Condensed Consolidated Statements of Comprehensive Loss
(Unaudited)
| | | Three Months Ended March 31, | |
| | | 2022 | | | 2021 | |
| Net loss | | $ | (2,099,895 | ) | | $ | (534,006 | ) |
| Other comprehensive loss: | | | | | | | | |
| Translation adjustment | | | (58,059 | ) | | | - | |
| Comprehensive loss | | $ | (2,157,954 | ) | | $ | (534,006 | ) |
See accompanying notes to condensed consolidated financial statements.
Ainos, Inc.
Condensed Consolidated Statements of Stockholders’ Equity (Deficit)
For the three months ended March 31, 2022 and 2021
(Unaudited)
| | | Preferred Stock | | | Common Stock | | | Additional Paid in | | | Accumulated | | | Other comprehensive | | | Total Stockholders’ Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | loss | | | (Deficit) | |
| Balance at December 31, 2021 | | | - | | | $ | - | | | | 144,379,308 | | | $ | 1,443,793 | | | $ | 18,856,430 | | | $ | (10,108,916 | ) | | $ | 5,800 | | | $ | 10,197,107 | |
| Share-based compensation | | | - | | | | - | | | | | | | | | | | | 43,443 | | | | | | | | | | | | 43,443 | |
| Net loss | | | - | | | | - | | | | | | | | | | | | | | | | (2,099,895 | ) | | | | | | | (2,099,895 | ) |
| Translation Adjustment | | | | | | | | | | | | | | | | | | | | | | | | | | | (58,059 | ) | | | (58,059 | ) |
| Balance at March 31, 2022 | | | - | | | $ | - | | | | 144,379,308 | | | $ | 1,443,793 | | | $ | 18,899,873 | | | $ | (12,208,811 | ) | | $ | (52,259 | ) | | $ | 8,082,596 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2020 | | | - | | | $ | - | | | | 42,066,172 | | | | 420,662 | | | | 4,961,315 | | | | (6,220,255 | ) | | | | | | | (838,278 | ) |
| Share-based compensation | | | | | | | | | | | | | | | | | | | 94,105 | | | | | | | | | | | | 94,105 | |
| Net loss | | | | | | | | | | | | | | | | | | | | | | | (534,006 | ) | | | | | | | (534,006 | ) |
| Balance at March 31, 2021 | | | - | | | $ | - | | | | 42,066,172 | | | $ | 420,662 | | | $ | 5,055,420 | | | $ | (6,754,261 | ) | | $ | - | | | $ | (1,278,179 | ) |
See accompanying notes to condensed consolidated financial statements.
Ainos, Inc.
Condensed Consolidated Statements of Cash Flows
(Unaudited)
| | | Three months ended March 31, | |
| | | 2022 | | | 2021 | |
| Cash flows from operating activities: | | | | | | |
| Net loss | | $ | (2,099,895 | ) | | $ | (534,006 | ) |
| Adjustments to reconcile net loss to net cash used in operating. activities: | | | | | | | | |
| Depreciation and amortization | | | 1,168,773 | | | | 3,905 | |
| Share-based compensation expense | | | 43,443 | | | | 94,105 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Inventory | | | (337,805 | ) | | | 3,024 | |
| Other current assets | | | (297,707 | ) | | | 18,254 | |
| Accrued expenses and others current liabilities | | | 133,302 | | | | 202,981 | |
| Net cash used in operating activities | | | (1,389,889 | ) | | | (211,737 | ) |
| Cash flows from investing activities: | | | | | | | | |
| Acquisition of property and equipment | | | (135,899 | ) | | | - | |
| Net cash used in investing activities | | | (135,899 | ) | | | - | |
| Cash flows from financing activities | | | | | | | | |
| Proceeds from convertible notes payable | | | 850,000 | | | | 236,854 | |
| Proceeds from notes payable | | | 800,000 | | | | - | |
| Payments of other notes payable | | | - | | | | (37,985 | ) |
| Payments of lease liabilities | | | (5,116 | ) | | | - | |
| Net cash provided by financing activities | | | 1,644,884 | | | | 198,869 | |
| Net change in cash | | | 119,096 | | | | (12,868 | ) |
| Effect from foreign currency exchange | | | 754 | | | | - | |
| Cash and cash equivalents at beginning of period | | | 1,751,499 | | | | 22,245 | |
| Cash and cash equivalents at end of period | | | 1,871,349 | | | | 9,377 | |
| | | | | | | | | |
| Supplemental disclosures of noncash financing and investing activities | | | | | | | | |
| Issuance of convertible notes for payables - related party | | | 26,000,000 | | | | - | |
| Receivable of convertible notes issued | | | 50,000 | | | | - | |
| Net change in equipment payable | | | 202,002 | | | | - | |
See accompanying notes to condensed consolidated financial statements.
Ainos, Inc.
Notes to Financial Statements
(Unaudited)
1. Organization and Business. Ainos, Inc., a Texas corporation formerly known as Amarillo Biosciences, Inc. (the “Company”, “we” or “us”), is engaged in developing medical technologies for point-of-care (“POCT”) testing and safe and novel medical treatment for a broad range of disease indications. Since our inception in 1984, we have concentrated our resources on business planning, raising capital, research and clinical development activities for our programs, securing related intellectual property and commercialization of proprietary therapeutics using low-dose non-injectable interferon (“IFN”). In addition to our core IFN technology, we are committed to developing a diversified healthcare business portfolio to include medical devices and consumer healthcare products. Although we have historically been involved in extensive pharmaceutical research and development of low-dose oral interferon as a therapeutic, we are prioritizing the commercialization of medical devices as part of our diversification strategy. Since the beginning of 2021, we have acquired significant intellectual property from our majority shareholder, Ainos, Inc., a Cayman Islands corporation (“Ainos KY”), to expand our potential product portfolio into Volatile Organic Compounds (“VOC”) POCTs and COVID-19 POCTs. We expect our underlying intellectual property to enable us to expedite the commercialization of our medical device pipeline, beginning with the Ainos-branded COVID-19 POCT product candidates.
2. Basis of presentation. The accompanying consolidated financial statements, which should be read in conjunction with the audited financial statements and footnotes included in the Company’s Form 10-K/A for the year ended December 31, 2021, as filed with the Securities and Exchange Commission on April 15, 2022 have been prepared in accordance with the Generally Accepted Accounting Principles (“GAAP”) for interim financial information. Accordingly, they do not include all of the information and footnotes required by for audited financial statements. In the opinion of management, all adjustments (consisting only of normal recurring adjustments) considered necessary for a fair presentation have been included. Operating results for the three months ended March 31, 2022, are not necessarily indicative of the results that may be expected for the full year ending December 31, 2022.
3. Financial Condition. These financial statements have been prepared in accordance with GAAP, on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has not yet achieved sustained operating income, and its operations are funded primarily from related-party convertible debt and equity financings. However, losses are anticipated in the ongoing development of its business and there can be no assurance that the Company will be able to achieve or maintain profitability.
The continuing operations of the Company and the recoverability of the carrying value of assets is dependent upon the ability of the Company to obtain necessary financing to fund its working capital requirements, and upon future profitable operations. The accompanying financial statements do not include any adjustments relative to the recoverability and classification of asset carrying amounts or the amount and classification of liabilities that might result from the outcome of this uncertainty.
There can be no assurance that capital will be available as necessary to meet the Company’s working capital requirements or, if the capital is available, that it will be on terms acceptable to the Company. The issuances of additional equity securities by the Company may result in dilution in the equity interests of its current stockholders. Obtaining commercial loans, assuming those loans would be available, will increase the Company’s liabilities and future cash commitments. If the Company is unable to obtain financing in the amounts and on terms deemed acceptable, the business and future success may be adversely affected and the Company may cease operations. These factors raise substantial doubt regarding our ability to continue as a going concern.
4. Common Stock. We have 300,000,000 shares of voting common shares authorized for issuance. On March 31, 2022, a total of 163,987,550 shares of common stock were either issued (144,379,308), reserved for conversion of convertible debt to stock (17,285,625), reserved for future issuance of RSUs for non-employee directors (1,320,000), held for future exercise of stock options (550,000) and shares reserved for warrant conversion (452,617). We also have $26.9 million outstanding in convertibles notes which are convertible into shares of common stock upon and at a conversion price equal to 80% of the offering price of any public offering as a result of which the Company’s common stock is listed on a national exchange.
We have not paid any dividends to our common stock shareholders to date, and have no plans to do so in the immediate future.
5. Preferred Stock. We have 10,000,000 shares of preferred stock authorized for issuance. No shares of preferred stock were outstanding as of March 31, 2022.
6. Current Convertible Notes Payable and Other Notes Payable. As of March 31, 2022 and December 31, 2021, the amount of convertible and other notes payable totaled $4,389,931 and $3,589,931, respectively. The details of the convertible notes payable and other notes payable are shown in the table below:
| Payee | No. | Effective Date | Due Date | From Effective | Following Maturity | Conversion Rate | Issuing Purpose | As of 12/31/2021 | Addition | Payment | As of 3/31/2022 | Accrued Interest |
| Current Convertible Notes Payable: |
| Stephen Chen | #1.16 | 1/30/2016 | Payable on demand | 0.75% | N/A | $ 0.17 | working capital | 114,026 | - | - | 114,026 | 6,050 |
| Stephen Chen | #2.16 | 3/18/2016 | Payable on demand | 0.65% | N/A | $ 0.19 | working capital | 262,500 | - | - | 262,500 | 10,298 |
| | | | | | | | 376,526 | - | - | 376,526 | 16,348 |
| Ainos KY | #12.21 | 4/27/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 15,000 | - | - | 15,000 | 257 |
| Ainos KY | #13.21 | 5/5/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 20,000 | - | - | 20,000 | 335 |
| Ainos KY | #14.21 | 5/25/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 30,000 | - | - | 30,000 | 471 |
| Ainos KY | #15.21 | 5/28/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 35,000 | - | - | 35,000 | 545 |
| Ainos KY | #16.21 | 6/9/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 300,000 | - | - | 300,000 | 4,486 |
| Ainos KY | #17.21 | 6/21/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 107,000 | - | - | 107,000 | 1,535 |
| Ainos KY | #18.21 | 7/2/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 54,000 | - | - | 54,000 | 744 |
| Ainos KY | #19.21 | 9/1/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 120,000 | - | - | 120,000 | 1,289 |
| Ainos KY | #20.21 | 9/28/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 300,000 | - | - | 300,000 | 2,798 |
| Ainos KY | #21.21 | 11/10/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 50,000 | - | - | 50,000 | 357 |
| Ainos KY | #22.21 | 11/25/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 450,000 | - | - | 450,000 | 2,851 |
| Ainos KY | #23.21 | 11/29/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 300,000 | - | - | 300,000 | 1,840 |
| Ainos KY | #24.21 | 12/29/2021 | 2/28/2023 (1) | 1.85% | N/A | $ 0.20 | working capital | 1,219,000 | - | - | 1,219,000 | 5,684 |
| | | | | | | | | 3,000,000 | - | - | 3,000,000 | 23,192 |
| Total convertible notes payable- related parties | 3,376,526 | - | - | 3,376,526 | 39,540 |
| Non-Convertible Notes Payable: | | | | | | | | | | |
| Stephen Chen | #9.21 | 1/1/2021 | 4/14/2021 | 0.13% | N/A | N/A | working capital | 129,405 | - | - | 129,405 | 354 |
| Ainos KY | #26.22 (2) | 3/4/2022 | 3/31/2023 | 1.85% | N/A | N/A | working capital | - | 800,000 | - | 800,000 | 1,135 |
| | | Non-convertible notes payable-related party | 129,405 | 800,000 | - | 929,405 | 1,489 |
| i2 China | #8b.20 | 1/1/2020 | 1/1/2021 | 1.85% | N/A | N/A | consulting fee | 84,000 | - | - | 84,000 | 3,527 |
| | | | Non-Convertible Notes payable- non-related party | 84,000 | | | 84,000 | 3,527 |
| | | | Total non-convertible notes payable | 213,405 | 800,000 | - | 1,013,405 | 5,016 |
| | Total convertible and non-convertible | 3,589,931 | 800,000 | - | 4,389,931 | 44,556 |
Notes:
(1) On March 17, 2022, we executed a Promissory Note Extension Agreement with Ainos KY in which the due dates for certain convertible notes enumerated as #12.21 to #24.21 issued by the Company to Ainos KY were extended to February 28, 2023. The total unpaid principal for these extended period convertible notes amount to $3,000,000 in the aggregate.
(2) On March 11, 2022, the Board approved a Non-Convertible Note dated March 4, 2022 in favor of Ainos KY with a principal amount of $800,000, interest of 1.85% per annum on unpaid principal and accrued interest, and a maturity date of February 28, 2023. The Note includes standard provisions for notice, default, and remedies for default.
All of the aforementioned convertible promissory notes and other notes payable are unsecured and due on demand upon maturity. The Company may prepay the notes in whole or in part at any time. The holder of convertible notes has the option to convert some or all of the unpaid principal and accrued interest to our common voting stock.
The total interest expense of convertible notes payable and other notes payable for the three months ended March 31, 2022 and as of December 31 2021 was $15,883 and $11,897 respectively; the cumulative related accrued interest as of March 31, 2022 and December 31, 2021 were $44,556 and $28,673, respectively.
7. Non-Current Convertible Notes Payable. As of March 31, 2022 and December 31, 2021, the amount of non-current convertible notes payable was $26,900,000 and $0, respectively.
On January 30, 2022, we issued to Ainos KY a Convertible Promissory Note in the principal amount of $26,000,000 (the “APA Convertible Note”) for the Asset Purchase Transaction as more particularly described below in Item 8 in these Notes to Financial Statements. The principal sum of the APA Convertible Note is payable in cash on January 30, 2027, although we may prepay the APA Convertible Note in whole or in part without penalty. The APA Convertible Note is noninterest bearing. If not earlier repaid, the APA Convertible Note will be converted into shares of our common stock or such other securities or property for which the APA Convertible Note may become convertible, immediately prior to the closing of any public offering of our common stock as a result of which our common stock will be listed on a U.S. stock exchange. The conversion price, subject to certain adjustments, will be 80% of the initial public offering price of the offering.
Convertible Note Offering Pursuant to Regulation S
The Company issued Convertible Notes pursuant to certain Convertible Note Purchase Agreements under Regulation S. The transactions are more particularly described below:
| | · | $50,000 Convertible Note issued on March 31, 2022 to Yun-Han Liao. The purchaser is the daughter of Wu Hui-Lan, the Company’s Chief Financial Officer (the “Liao Convertible Note”). |
| | | |
| | · | $850,000 aggregate Convertible Notes issues on March 28, 2022 to Chih-Cheng Tsai, Ming-Hsien Lee, Yu-Yuan Hsu, and Top Calibre Corporation, a British Virgin Islands company (collectively the “Regulation S Notes”). |
| | | |
| | · | The Liao Convertible Note and the Regulation S Notes are collectively referred to as the “Convertible Notes”. |
The Principal Amount of the Convertible Notes are payable in cash on March 30, 2027, although the Company may prepay the Convertible Notes in whole or in part without penalty. The Convertible Notes are non-interest bearing. If not earlier repaid, the Convertible Notes will be converted into shares of common stock, $0.01 par value per share of the Company, or such other securities or property for which the Convertible Notes may become convertible, immediately prior to the closing of any public offering of the Company’s common stock as result of which the Company’s common stock will be listed on a U.S. stock exchange. The conversion price, subject to certain adjustments, will be eighty percent (80%) of the initial public offering price of the offering.
8. Related Party Transactions. The following is a summary of related party transactions that met our disclosure threshold for the three months ended March 31, 2022 and 2021:
Asset Purchase Agreement
Ainos KY and the Company entered into an Asset Purchase Agreement dated as of November 18, 2021 (the “Asset Purchase Agreement”), as modified by an Amended and Restated Asset Purchase Agreement dated as of January 29, 2022 (the “Amended Asset Purchase Agreement”). Pursuant to the Asset Purchase Agreement, the Company acquired certain intellectual property assets and certain manufacturing, testing, and office equipment for a total purchase price of $26,000,000. Pursuant to the Asset Purchase Agreement, the Company agreed to hire certain employees of Ainos KY who are responsible for research and development of the IP Assets and/or Equipment on terms at least equal to the compensation arrangements undertaken by Ainos KY. From and after the closing, we will have no responsibility, duty or liability with respect to any employee benefit plans of Ainos KY. As payment of the purchase price, we issued to Ainos KY a Convertible Promissory Note in the principal amount of $26,000,000 upon closing on January 30, 2022 (the “APA Convertible Note”). Refer to Note 7 of the Notes to Financial Statements for more information.
Related Party Working Capital
All convertible and other notes payable were issued either as a result of financing or deferred compensation provided by related parties. As of March 31, 2022 and December 31, 2021, the convertible and non-convertible notes payable for related parties totaled $4,355,931 and $3,505,931, respectively. Refer to Note 6 and 7 of the Notes to Financial Statements for more information.
Purchase related to COVID-19 Antigen Rapid Test Kits
We incurred costs associated with finished goods, raw materials and manufacturing fees for Covid-19 antigen rapid test kits from TCNT pursuant to a Sales and Marketing Agreement, totaling $386,412 for the three months ended March 31, 2022. There were no purchases from TCNT during the same period last year.
Product Co-development Agreement
Pursuant to the five-year product co-development agreement effective on August 1, 2021 with TCNT (the “Product Co-Development Agreement”) we incurred development expenses totaling $167,422 for the three months ended March 31, 2022 of which $109,131 is in accrued payable as of March 31, 2022.
Promissory Note Extension Agreement
On March 17, 2022, we executed a Promissory Note Extension Agreement with Ainos KY in which the due dates for certain convertible notes enumerated as #12.21 to #24.21 issued by the Company to Ainos KY were extended to February 28, 2023 (the “Promissory Note Extension Agreement”). The total unpaid principal for these extended period convertible notes amount to $3,000,000 in the aggregate. Refer to Footnote 1 of Note 6 of the Notes to Financial Statements for more information.
9. Subsequent Events.
On April 11, 2022, we issued to ASE Test Inc., a minority owner of Ainos KY, a convertible note in the principal amount of $500,000 due on March 30, 2027 (the “ASE Note”). The convertible note will automatically convert into shares of our common stock immediately prior to the closing of any public offering of our common stock as a result of which our common stock will be listed on a U.S. stock exchange. The conversion price, subject to certain adjustments, will be 80% of the initial public offering price of the offering.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Ainos, Inc. (formerly Amarillo Bioscience, Inc.)
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Ainos, Inc. (formerly Amarillo Biosciences, Inc.) (the Company) as of December 31, 2021 and 2020, and the related statements of operations, stockholders equity (deficit), and cash flows for each of the years in the two-year period ended December 31, 2021, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 1 to the financial statements, the Company’s absence of significant revenues, recurring losses from operations, and its need for additional financing in order to fund its projected loss in 2021 raise substantial doubt about its ability to continue as a going concern. These 2020 financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has negative working capital at December 31, 2021, has incurred recurring losses and recurring negative cash flow from operating activities, and has an accumulated deficit which raises substantial doubt about its ability to continue as a going concern. Management’s plans concerning these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Critical Audit Matters
Critical audit matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/PWR CPA, LLP
Houston, Texas
PCAOB #6686
We have served as the Company’s auditor since 2020.
Houston, Texas
March 18, 2022
Ainos, Inc.
Balance Sheets
| | | December 31, | |
| | | 2021 | | | 2020 | |
| Assets | | | | | | |
| Current assets: | | | | | | |
| Cash and cash equivalents | | $ | 1,751,499 | | | $ | 22,245 | |
| Other current assets | | | 466,198 | | | | 54,168 | |
| Total current assets | | | 2,217,697 | | | | 76,413 | |
| Intangible assets, net | | | 37,329,191 | | | | 180,628 | |
| Property and equipment, net | | | 1,187,702 | | | | 3,249 | |
| Other assets | | | 87,571 | | | | - | |
| Total assets | | $ | 40,822,161 | | | $ | 260,290 | |
| | | | | | | | | |
| Liabilities and Stockholders’ Equity (Deficit) | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Convertible notes payable | | $ | 3,376,526 | | | $ | 734,991 | |
| Notes payable | | | 213,405 | | | | 218,010 | |
| Accrued expenses and other current liabilities | | | 1,004,868 | | | | 145,567 | |
| Payables (related party) | | | 26,000,000 | | | | - | |
| Total current liabilities | | | 30,594,799 | | | | 1,098,568 | |
| Operating lease liabilities- non-current | | | 30,255 | | | | - | |
| Total liabilities | | | 30,625,054 | | | | 1,098,568 | |
| | | | | | | | | |
| Stockholders’ Equity (Deficit) | | | | | | | | |
| Preferred stock, $0.01 par value: | | | | | | | | |
| Authorized shares - 10,000,000, | | | | | | | | |
| Issued and outstanding shares - 0 at December 31, 2021 and 2020, respectively | | | - | | | | - | |
| Common stock, $0.01 par value: | | | | | | | | |
| Authorized shares - 300,000,000, and 100,000,000, | | | | | | | | |
| Issued and outstanding shares - 144,379,308 and 42,066,172 at December 31, 2021 and 2020, respectively | | | 1,443,793 | | | | 420,662 | |
| Additional paid-in capital | | | 18,856,430 | | | | 4,961,315 | |
| Accumulated deficit | | | (10,108,916 | ) | | | (6,220,255 | ) |
| Translation adjustment | | | 5,800 | | | | - | |
| Total stockholders’ equity (deficit) | | | 10,197,107 | | | | (838,278 | ) |
| Total liabilities and stockholders’ equity (deficit) | | $ | 40,822,161 | | | $ | 260,290 | |
The accompanying notes are an integral part of these financial statements.
Ainos, Inc.
Statements of Operations
| | | Years ended December 31, | |
| | | 2021 | | | 2020 | |
| Revenues | | $ | 594,563 | | | $ | 16,563 | |
| Cost of revenues | | | (184,181 | ) | | | (11,277 | ) |
| Gross margin | | | 410,382 | | | | 5,286 | |
| | | | | | | | | |
| Operating expenses: | | | | | | | | |
| Research and development expenses | | | 1,920,645 | | | | 389 | |
| Selling, general and administrative expenses | | | 2,357,163 | | | | 1,445,332 | |
| Total operating expenses | | | 4,277,808 | | | | 1,445,721 | |
| | | | | | | | | |
| Operating loss | | | (3,867,426 | ) | | | (1,440,435 | ) |
| | | | | | | | | |
| Non-operating income (expenses), net | | | | | | | | |
| Interest expense, net | | | (18,689 | ) | | | (10,185 | ) |
| Other losses | | | (2,547 | ) | | | (3 | ) |
| Total non-operating income (expenses), net | | | (21,236 | ) | | | (10,188 | ) |
| Net loss | | $ | (3,888,661 | ) | | $ | (1,450,623 | ) |
| | | | | | | | | |
| Basic and diluted net loss per average share available to common shareholders | | $ | (0.03 | ) | | $ | (0.04 | ) |
| | | | | | | | | |
| Weighted average common shares outstanding - basic and diluted | | | 113,270,245 | | | | 40,730,934 | |
The accompanying notes are an integral part of these financial statements.
Ainos, Inc.
Statements of Stockholders’ Equity (Deficit)
Years Ended December 31, 2021 and 2020
| | | Preferred Stock | | | Common Stock | | | Additional Paid in | | | Accumulated | | | Translation | | | Total Stockholders’ Equity | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | Adjustment | | | (Deficit) | |
| Balance December 31, 2020 | | | - | | | $ | - | | | | 42,066,172 | | | $ | 420,662 | | | $ | 4,961,315 | | | $ | (6,220,255 | ) | | $ | - | | | $ | (838,278 | ) |
| Conversion of convertible notes into common stock | | | | | | | | | | | 1,905,321 | | | | 19,053 | | | | 457,276 | | | | - | | | | - | | | | 476,329 | |
| Issuance of common stock for intangible assets | | | - | | | | - | | | | 100,000,000 | | | | 1,000,000 | | | | 19,000,000 | | | | - | | | | - | | | | 20,000,000 | |
| Issuance of stock for compensation | | | - | | | | - | | | | 237,415 | | | | 2,374 | | | | 158,604 | | | | - | | | | - | | | | 160,978 | |
| Issuance of stock for options | | | - | | | | - | | | | 170,400 | | | | 1,704 | | | | 63,048 | | | | - | | | | - | | | | 64,752 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Share-based compensation | | | - | | | | - | | | | - | | | | - | | | | (10,683 | ) | | | - | | | | - | | | | (10,683 | ) |
| Acquisition of intangible assets and equipment | | | - | | | | - | | | | - | | | | - | | | | (5,773,130 | ) | | | - | | | | - | | | | (5,773,130 | ) |
| Net Loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | (3,888,661 | ) | | | - | | | | (3,888,661 | ) |
| Translation adjustment | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 5,800 | | | | 5,800 | |
| Balance December 31, 2021 | | | - | | | $ | - | | | | 144,379,308 | | | $ | 1,443,793 | | | $ | 18,856,430 | | | $ | (10,108,916 | ) | | $ | 5,800 | | | $ | 10,197,107 | |
| Balance December 31, 2019 | | | - | | | $ | - | | | | 40,516,351 | | | $ | 405,164 | | | $ | 4,207,786 | | | $ | (4,769,632 | ) | | $ | - | | | $ | (156,682 | ) |
| Issuance of stock for compensation | | | - | | | | - | | | | 1,149,821 | | | | 11,498 | | | | 283,192 | | | | - | | | | - | | | | 294,690 | |
| Issuance of stock for cash | | | - | | | | - | | | | 400,000 | | | | 4,000 | | | | 96,000 | | | | - | | | | - | | | | 100,000 | |
| Share-based compensation | | | - | | | | - | | | | - | | | | - | | | | 374,337 | | | | - | | | | - | | | | 374,337 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | (1,450,623 | ) | | | - | | | | (1,450,623 | ) |
| Balance at December 31, 2020 | | | - | | | $ | - | | | | 42,066,172 | | | $ | 420,662 | | | $ | 4,961,315 | | | $ | (6,220,255 | ) | | $ | - | | | $ | (838,278 | ) |
The accompanying notes are an integral part of these financial statements.
Ainos, Inc.
Statements of Cash Flows
| | | Year Ended December 31 | |
| | | 2021 | | | 2020 | |
| Cash flows from Operating Activities: | | | | | | |
| Net loss | | $ | (3,888,661 | ) | | $ | (1,450,623 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation and amortization | | | 2,044,447 | | | | 14,698 | |
| Share-based compensation expense | | | (10,683 | ) | | | 374,337 | |
| Stock issued for compensation | | | 160,978 | | | | 150,440 | |
| Loss on disposal of property, plant and equipment | | | 2,223 | | | | - | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Inventory | | | 3,024 | | | | 1,107 | |
| Prepaid expense and other current assets | | | (415,054 | ) | | | (19,020 | ) |
| Accounts payable and accrued expenses | | | 794,174 | | | | 429,510 | |
| Contract liabilities | | | 59,575 | | | | - | |
| Net cash used in operating activities | | | (1,249,977 | ) | | | (499,551 | ) |
| Cash flows from Investing Activities: | | | | | | | | |
| Patent purchases | | | - | | | | (7,243 | ) |
| Acquisition of property, plant and equipment | | | (143,792 | ) | | | - | |
| Increase in refundable deposits and others | | | (36,725 | ) | | | - | |
| Net cash used in investing activities | | | (180,517 | ) | | | (7,243 | ) |
| Cash flows from Financing Activities: | | | | | | | | |
| Proceeds from convertible note payable - related party | | | 3,106,025 | | | | 120,000 | |
| Payments of other note payable | | | (4,605 | ) | | | - | |
| Proceeds from exercise of stock options | | | 64,752 | | | | - | |
| Payments of lease liabilities | | | (11,799 | ) | | | - | |
| Net cash provided by financing activities | | | 3,154,373 | | | | 120,000 | |
| Net change in cash | | | 1,723,879 | | | | (386,794 | ) |
| Effect from foreign currency exchange | | | 5,375 | | | | - | |
| Cash and cash equivalents at beginning of period | | | 22,245 | | | | 409,039 | |
| Cash and cash equivalents at end of period | | $ | 1,751,499 | | | $ | 22,245 | |
| Supplemental Cash Flow Information | | | | | | | | |
| Cash paid for interest | | $ | 19,380 | | | $ | 855 | |
| Supplemental disclosures of noncash financing and investing activities: | | | | | | | | |
| Stock issued for subscription | | $ | - | | | $ | 100,000 | |
| Stock issued for patent cost and accrued liabilities | | $ | - | | | $ | 144,250 | |
| Conversion of convertible notes and accrued interest into common stock | | $ | 476,329 | | | $ | - | |
| ROU leased assets | | $ | 62,903 | | | $ | - | |
| Acquisition of intangible assets and equipment | | $ | 40,226,870 | | | | - | |
| Decrease of additional paid in capital | | $ | 5,773,130 | | | | - | |
| Issuance of common stock and increase of current liability | | $ | 46,000,000 | | | | - | |
The accompanying notes are an integral part of these financial statements.
Ainos, Inc.
Notes to Financial Statements
December 31, 2021 and 2020
1. Organization and Summary of Significant Accounting Policies
Organization and Business
We are engaged in developing medical technologies for point-of-care (“POCT”) testing and safe and novel medical treatment for a broad range of disease indications. Since our inception in 1984, we have concentrated our resources on business planning, raising capital, research and clinical development activities for our programs, securing related intellectual property and commercialization of proprietary therapeutics using low-dose non-injectable interferon (“IFN”). In addition to our core IFN technology, we are committed to developing a diversified healthcare business portfolio to include medical devices and consumer healthcare products.
Although we have historically been involved in extensive pharmaceutical research and development of low-dose oral interferon as a therapeutic, we are prioritizing the commercialization of medical devices as part of our diversification strategy. Since the beginning of 2021, we have acquired significant intellectual property from our majority shareholder, Ainos KY, to expand our potential product portfolio into Volatile Organic Compounds (“VOC”) and COVID-19 POCTs. This includes 51 issued and pending patents related to VOC technologies and 3 issued patents for COVID-19 POCT products. We expect our underlying intellectual property to enable us to expedite the commercialization of our medical device pipeline, beginning with Ainos-branded COVID-19 POCT product candidates.
Basis of Accounting
The basis is United States generally accepted accounting policies (“U.S. GAAP”).
Going Concern
These financial statements have been prepared in accordance with United States generally accepted accounting principles (“U.S. GAAP”) applicable to a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has generated minimal revenue and has an accumulated deficit totaling $10,108,916 since inception. These factors, among others, indicate that there is substantial doubt about the Company’s ability to continue as a going concern within one year from the issuance date of this filing.
In order to obtain the necessary capital to sustain operations, management’s plans include, among other things, the possibility of pursuing new equity sales and/or making additional debt borrowings, There can be no assurances, however, that the Company will be successful in obtaining additional financing, or that such financing will be available on favorable term, if at all. Obtaining commercial loans, assuming those loans would be available, will increase the Company’s liabilities and future cash commitments. If the Company is unable to obtain financing in the amounts and on terms deemed acceptable, the business and future success may be adversely affected and the Company may cease operations. These factors raise substantial doubt regarding our ability to continue as a going concern. The accompanying financial statements do not include any adjustments relative to the recoverability and classification of asset carrying amounts or the amount and classification of liabilities that might result from the outcome of this uncertainty.
Fair Value of Financial Instruments
Under the Financial Account Standards Board Accounting Standards Codification (“FASB ASC”), we are permitted to elect to measure financial instruments and certain other items at fair value, with the change in fair value recorded in earnings. We elected not to measure any eligible items using the fair value option. Consistent with the Fair Value Measurement Topic of the FASB ASC, we implemented guidelines relating to the disclosure of our methodology for periodic measurement of our assets and liabilities recorded at fair market value.
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. A three-tier fair value hierarchy prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1 measurements) and the lowest priority to unobservable inputs (level 3 measurements). These tiers include:
| | · | Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets; |
| | · | Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and |
| | · | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one more significant inputs or significant value drivers are unobservable. |
Our Level 1 assets and liabilities primarily include our cash and cash equivalents. Valuations are obtained from readily available pricing sources for market transactions involving identical assets or liabilities. The carrying amounts of accounts receivable, prepaid expense, accounts payable, accrued liabilities, advances from investors, and notes payable approximate fair value due to the immediate or short-term maturities of these financial instruments.
Stock-Based Compensation
Stock-based compensation expense is recorded in accordance with FASB ASC Topic 718, Compensation - Stock Compensation, for stock and stock options awarded in return for services rendered. The expense is measured at the grant-date fair value of the award and recognized as compensation expense on a straight-line basis over the service period, which is the vesting period. The Company has adopted the simplified method to account for forfeitures of employee awards as they occur and as a result, we will record compensation cost assuming all option holders will complete the requisite service period. If an employee forfeits an award because they fail to complete the requisite service period, we will reverse compensation cost previously recognized in the period the award is forfeited.
Cash and Cash Equivalents
The Company classifies investments as cash equivalents if the original maturity of an investment is three months or less.
Revenue Recognition
We account for revenue from contracts with customers in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) No. 2014-09, “Revenue from Contracts with Customers (“Topic 606”).” The unit of account in Topic 606 is a performance obligation, which is a promise in a contract to transfer to a customer either a distinct good or service (or bundle of goods or services) or a series of distinct goods or services provided at a point in time or over a period of time. Topic 606 requires that a contract’s transaction price, which is the amount of consideration to which an entity expects to be entitled in exchange for transferring promised goods or services to a customer, is to be allocated to each performance obligation in the contract based on relative standalone selling prices and recognized as revenue when (point in time) or as (over time) the performance obligation is satisfied.
Total revenues include sales of products to customers, net of discounts or allowances, if any, and include freight and delivery costs billed to customers. Revenues for product sales are recognized when control of the promised good is transferred to unaffiliated customers, typically when finished products are shipped. Shipping costs are deemed fulfillment costs and are not recognized as a separate performance obligation.
Allowance for Doubtful Accounts
The Company establishes an allowance for doubtful accounts to ensure trade and notes receivable are not overstated due to non-collectability. The Company’s allowance is based on a variety of factors, including age of the receivable, significant one-time events, historical experience, and other risk considerations. The Company had no material accounts receivable and no allowance at December 31, 2021 or 2020.
Inventory
Inventories are stated at the lower of cost or market. Cost is determined on a first-in, first-out basis. The Company continually assesses the appropriateness of inventory valuations giving consideration to slow-moving, non-saleable, out-of-date or close-dated inventory.
Property and Equipment
Property and equipment are stated on the basis of historical cost less accumulated depreciation. Depreciation is provided using the straight-line method over the two to seven year estimated useful lives of the assets.
Patents and Patent Expenditures
The Company holds patent license agreements and maintains patents that are owned by the Company. All patent license agreements remain in effect over the life of the underlying patents. Accordingly, the patent license fee is being amortized over the estimated life of the patent using the straight-line method. Patent fees and legal fees associated with the issuance of new owned patents are capitalized and amortized over the estimated 8 to 20 year life of the patent. The Company continually evaluates the amortization period and carrying basis of patents to determine whether subsequent events and circumstances warrant a revised estimated useful life or impairment in value. No patent costs were written off for the years ended December 31, 2021, or December 31, 2020.
Income Taxes
The asset and liability approach is used to account for income taxes by recognizing deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities. The Company records a valuation allowance to reduce the deferred tax assets to the amount that is more likely than not to be realized.
Research and Development
Internal research and development (“R&D”) costs are expensed as incurred. Clinical trial costs incurred by third parties are expensed as the contracted work is performed. Where contingent milestone payments are due to third parties under research and development collaborations, prior to regulatory approval, the payment obligations are expensed when the milestone results are achieved. Payments made to third parties subsequent to regulatory approval are capitalized as intangible assets and amortized to cost of products sold over the remaining useful life of the related product.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Basic and Diluted Net Income (Loss) Per Share
The basic earnings (loss) per share is calculated by dividing the Company’s net income (loss) available to common shareholders by the weighted average number of common shares issued and outstanding during the year. The diluted earnings (loss) per share is calculated by dividing the Company’s net income (loss) available to common shareholders by the diluted weighted average number of shares outstanding during the year. The diluted weighted average number of shares outstanding is the basic weighted number of shares adjusted as of the first year for any potentially dilutive debt or equity.
As of December 31, 2021, potentially dilutive shares are not included in the calculation of fully diluted net loss per share as the effect with a net loss would be antidilutive.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to significant concentration of credit risk consist principally of cash. The Company has cash balances in a single U.S. financial institution which, from time to time, could exceed the federally insured limit of $250,000. The Company maintains multiple accounts in its Taiwan Branch office which help to mitigate risk. Our bank deposits in Taiwan are insured by the Central Deposit Insurance Corp. (“CDIC”) with an insured limit of NT$3,000,000 per account.
No loss has been incurred related to the aforementioned concentration of cash.
Recent Accounting Pronouncements
There have been no new accounting pronouncements issued or adopted during the year ended December 31, 2021 that are of significance to us.
2. Property and Equipment, net
Property and equipment are stated at cost less accumulated depreciation and consist of the following at December 31, 2021 and 2020:
| | | December 31, | |
| | | 2021 | | | 2020 | |
| Machinery and equipment | | $ | 938,047 | | | $ | - | |
| Furniture and fixture | | | 47,960 | | | | 107,549 | |
| Construction in process | | | 232,729 | | | | - | |
| Total cost | | | 1,218,736 | | | | 107,549 | |
| Less: accumulated depreciation | | | (31,034 | ) | | | (104,300 | ) |
| Property and equipment, net | | $ | 1,187,702 | | | $ | 3,249 | |
Depreciation expense for the year ended December 31, 2021 and 2020 was $31,395 and $1,820, respectively. Construction in process represents assets that are not available for their intended use as of the balance sheet date.
Net property and equipment were $1,187,702 and $3,249 as of December 31, 2021 and 2020, respectively. We acquired $944,152 of machinery and equipment from Ainos KY pursuant to the Asset Purchase Agreement entered in November 2021.
3. Intangible assets, net
Intangible assets are stated at cost less accumulated amortization and consist of the following at December 31, 2021 and 2020:
| | | December 31, | |
| | | 2021 | | | 2020 | |
| Patents and technology | | $ | 39,371,317 | | | $ | 245,898 | |
| Less: accumulated amortization | | | (2,042,126 | ) | | | (65,270 | ) |
| Patents and technology, net | | $ | 37,329,191 | | | $ | 180,628 | |
Amortization expense amounted to $2,000,302 for the year ended December 31, 2021 and $12,878 for the year ended December 31, 2020 respectively, and is included in R&D, selling, general and administrative expenses.
Patents were $37,329,191 and $180,628 as of December 31, 2021 and 2020 respectively. We acquired intellectual properties related to VOC and COVID-19 technologies from Ainos KY pursuant to a Securities Purchase Agreement dated December 24, 2020, by and between the Company (under its former name “Amarillo Biosciences, Inc.”) and Ainos KY (the “Securities Purchase Agreement”) and the Asset Purchase Agreement.
Estimated future amortization expense is as follows:
| 2022 | | | 4,522,141 | |
| 2023 | | | 4,522,141 | |
| 2024 | | | 4,534,493 | |
| 2025 | | | 4,522,141 | |
| 2026 | | | 4,521,973 | |
| Thereafter | | | 14,706,301 | |
| Total expense | | $ | 37,329,191 | |
4. Convertible Notes Payable and Other Notes Payable
All convertible and other notes payable were issued either as a result of financing or deferred compensation provided by executives of the Company. As of December 31, 2021 and December 31, 2020, convertible and other notes payable totaled $3,589,931 and $953,001, respectively; including notes payable for related parties totaling $3,505,931 and $805,001, respectively. Refer to disclosure in Note 5 below.
The details of the convertible notes payable and other notes payable are shown in the table below:
| Payee | No. | Effective Date | Due Date | From Effective | Following Maturity | Conversion Rate | Issuing Purpose | 1/1/2021 | Addition | Payment | 12/31/2021 | Accrued Interest |
| Convertible notes payable: | | | | | | | | | | |
| Stephen Chen | #1.16 | 1/30/2016 | Payable on demand | 0.75% | NA | $ 0.17 | working capital | 114,026 | | | 114,026 | 5,839 |
| Stephen Chen | #2.16 | 3/18/2016 | Payable on demand | 0.65% | NA | $ 0.19 | working capital | 262,500 | | | 262,500 | 9,878 |
| Stephen Chen | #3.19 | 912019 | 9/1/2020 | 1.85% | 10% | $ 0.25 | salary | 39,620 | | (39,620) | 0 | 0 |
| Stephen Chen | #4.19 | 1212019 | 12/31/2020 | 1.61% | 10% | $ 0.25 | working capital | 14,879 | | (14,879) | 0 | 0 |
| Stephen Chen | #6.20 | 112020 | 1/1/2021 | 1.85% | 10% | $ 0.25 | salary | 216,600 | | (216,600) | 0 | 0 |
| Stephen Chen | #7.20 | 112020 | 1/2/2021 | 1.60% | 10% | $ 0.25 | working capital | 23,366 | | (23,366) | 0 | 0 |
| Stephen Chen | #10.21 | 112021 | 4/1/2021 | 1.85% | 1.85% | $ 0.25 | salary | | 59,025 | (59,025) | 0 | 0 |
| Stephen Chen | #11.21 | 412021 | 5/1/2021 | 1.85% | 10% | $ 0.25 | salary | | 10,000 | (10,000) | 0 | 0 |
| | | | | | | | 670,991 | 69,025 | (363,490) | 376,526 | 15,717 |
| Ainos KY | #12.21 | 4/27/2021 | 10/27/2021 | 1.85% | NA | $ 0.20 | working capital | | 15,000 | | 15,000 | 189 |
| Ainos KY | #13.21 | 5/5/2021 | 11/5/2021 | 1.85% | NA | $ 0.20 | working capital | | 20,000 | | 20,000 | 243 |
| Ainos KY | #14.21 | 5/25/2021 | 11/25/2021 | 1.85% | NA | $ 0.20 | working capital | | 30,000 | | 30,000 | 335 |
| Ainos KY | #15.21 | 5/28/2021 | 11/28/2021 | 1.85% | NA | $ 0.20 | working capital | | 35,000 | | 35,000 | 385 |
| Ainos KY | #16.21 | 6/9/2021 | 12/9/2021 | 1.85% | NA | $ 0.20 | working capital | | 300,000 | | 300,000 | 3,117 |
| Ainos KY | #17.21 | 6/21/2021 | 12/21/2021 | 1.85% | NA | $ 0.20 | working capital | | 107,000 | | 107,000 | 1,047 |
| Ainos KY | #18.21 | 7/2/2021 | 1/2/2022 | 1.85% | NA | $ 0.20 | working capital | | 54,000 | | 54,000 | 498 |
| Ainos KY | #19.21 | 912021 | 3/1/2022 | 1.85% | NA | $ 0.20 | working capital | | 120,000 | | 120,000 | 742 |
| Ainos KY | #20.21 | 9/28/2021 | 3/28/2022 | 1.85% | NA | $ 0.20 | working capital | | 300,000 | | 300,000 | 1,429 |
| Ainos KY | #21.21 | 11102021 | 5102022 | 1.85% | NA | $ 0.20 | working capital | | 50,000 | | 50,000 | 129 |
| Ainos KY | #22.21 | 11252021 | 11/25/2022 | 1.85% | NA | $ 0.20 | working capital | | 450,000 | | 450,000 | 798 |
| Ainos KY | #23.21 | 11/29/2021 | 5/29/2022 | 1.85% | NA | $ 0.20 | working capital | | 300,000 | | 300,000 | 471 |
| Ainos KY | #24.21 | 12292021 | 6/29/2022 | 1.85% | NA | $ 0.20 | working capital | | 1,219,000 | | 1,219,000 | 124 |
| | | | | | | | 0 | 3,000,000 | 0 | 3,000,000 | 9,507 |
| | | | Total convertible notes payable- related parties | 670,991 | 3,069,025 | (363,490) | 3,376,526 | 25,224 |
| i2 China | #5.19 | 9/1/2019 | 9/1/2020 | 1.85% | 10% | $ 0.25 | consulting fee | 16,000 | | (16,000) | 0 | 0 |
| i2 China | #8a.20 | 1/1/2020 | 1/1/2021 | 1.85% | 10% | $ 0.25 | consulting fee | 48,000 | | (48,000) | 0 | 0 |
| i2 China | #11.21 | 112020 | 4/1/2021 | 1.85% | 10% | $ 0.25 | consulting fee | | 37,000 | (37,000) | 0 | 0 |
| | | | Total convertible notes payable- non-related party | 64,000 | 37,000 | (101,000) | 0 | 0 |
| | | | Total Convertible notes payable | 734,991 | 3,106,025 | (464,490) | 3,376,526 | 25,224 |
| Notes payable: | | | | | | | | | | |
| Stephen Chen | #9.21 | 1/1/2021 | 4/14/2021 | 0.13% | 10% | NA | working capital | 134,010 | 145,395 | (150,000) | 129,405 | 312 |
| | | | Notes payable-related party | 134,010 | 145,395 | (150,000) | 129,405 | 312 |
| i2 China | #8b.20 | 1/1/2020 | 1/1/2021 | 1.85% | 10% | NA | consulting fee | 84,000 | | | 84,000 | 3,137 |
| | | | Notes payable- non-related party | 84,000 | 0 | 0 | 84,000 | 3,137 |
| | | | Total notes payable | 218,010 | 145,395 | (150,000) | 213,405 | 3,449 |
| | | | Total convertible and non-convertible | 953,001 | 3,251,420 | (614,490) | 3,589,931 | 28,673 |
All of the aforementioned convertible promissory notes and other notes payable are unsecured and due on demand upon maturity. The Company may prepay the notes in whole or in part at any time. The Payee has the option to convert some or all of the unpaid principal and accrued interest to our common voting stock.
The convertible promissory notes are convertible on demand. The following convertible notes due to Stephen T. Chen - Notes 3.19, 4.19, 6.20, 7.20, 10.21, and 11.21 -- with a total principal and accrued interest amount of $372,988 were assigned by the holder to Top Calibre Corporation, a British Virgin Islands corporation, and subsequently converted in common stock of our company at a conversion price of $0.25 per share on December 27, 2021. No convertible notes were assigned in 2020.
During 2021, the Company received funding from Dr. Stephen T. Chen and Ainos KY totaling $214,420 and $3,000,000, respectively. Amounts owed to Dr. Stephen T. Chen of $150,000 were repaid. In 2020, the Company received funding from Dr. Stephen T. Chen totaling $373,976.
Note holders, i2China Management Group, LLC (“i2China”) and Dr. Stephen T. Chen (together the “Payees”), agreed to waive their rights pertaining to the conditional term “Annual Interest Rate on Matured, Unpaid Amounts: 10% per annum, compounded annually of Convertible Notes” in regards to interest charged on unpaid amounts following maturity for all of their respective notes. The Company and the Payees agree that the originally agreed annual interest rate will continue to be valid for any unpaid amounts after maturity. The amended terms of the above convertible notes and other notes payable were made during on September 1, 2021. Interest waived totaled $45,875.
The total interest expense for 2021 and 2020 totaled $21,727 and $10,702 respectively; the cumulative related accrued interest as of December 31, 2021 and 2020 were $28,673 and $24,196, respectively.
5. Related Party Transactions
The following is a summary of related party transactions in 2021 and 2020 to which we have been a participant in which the amount involved exceeded or will exceed the lesser of $120,000 or 1% of the average of our total assets as of December 31, 2021 and 2020, and in which any of our directors, executive officers or holders of more than 5% of our capital stock, or any of our directors, executive officers or holders of more than 5% of our outstanding capital stock, or any immediate family member of, or person sharing the household with, any of these individuals or entities, had or will have a direct or indirect material interest, other than compensation arrangements which are described in Part II, Item 5 “Market for the Registrant’s Common Equity and Related Shareholder Matters, and Issuer Purchases of Equity Securities,” and Part III, Item 11 “Executive Compensation.”
| Name of the related party | | Relationship | | Description |
| Taiwan Carbon Nano Technology Corporation (“TCNT”) | | Affiliated company | | TCNT is the majority shareholder of Ainos KY |
| | | | | |
| Ainos, Inc. (Cayman Island) (“Ainos KY”) | | Affiliated company | | Ainos KY is the majority shareholder of the Company |
| | | | | |
| ASE Technology Holding | | Affiliated company | | Sole owner of ASE Test Inc. which is Ainos KY’s board member and has more than 10% of the voting rights in Ainos KY |
| | | | | |
| Dr. Stephen T. Chen | | Ainos’ former Chairman, President, CEO and CFO | | Shareholder with more than 5% of the Company voting rights in 2021 and 2020 |
Purchase of intangible assets and equipment
Securities Purchase Agreement
On April 15, 2021, we consummated the Securities Purchase Agreement with Ainos KY. Pursuant to the Securities Purchase Agreement, we issued 100,000,000 shares of common stock at $0.20 per share to Ainos KY in exchange for certain patent assignments, increased its authorized common stock to 300,000,000 shares, and changed the Company’s name to “Ainos, Inc.” Immediately after the consummation of the transaction Ainos KY owned approximately 70.30% of the Company’s issued and outstanding shares of common stock.
Asset Purchase Agreement
On November 18, 2021, we entered into an Asset Purchase Agreement as modified by an Amended and Restated Asset Purchase Agreement dated as of January 29, 2022 (the “Asset Purchase Agreement”) with Ainos KY. We closed the transaction on January 30, 2022. See Notes 2, 3, and 12 for a discussion of the transaction.
Related Party Financing
All convertible and other notes payable were issued either as a result of financing or deferred compensation provided by shareholders. As of December 31, 2021 and 2020, the convertible notes payable and non-convertible notes payable for related parties totaled $3,505,931 and $805,001, respectively. Refer to Note 4 of the Notes to Financial Statements, which are incorporated herein by this reference, for more information.
Other transactions
COVID-19 Test Kits Sales and Marketing Agreement with Ainos KY
On June 14, 2021, we entered into an exclusive agreement with Ainos KY to serve as the master sales and marketing agent for the Ainos COVID-19 antigen rapid test kit and COVID-19 nucleic acid test kits which are manufactured by TCNT. On June 7, 2021, the TFDA issued an emergency use authorization to TCNT for the Ainos COVID-19 antigen rapid test kit that will be sold and marketed under the “Ainos” brand in Taiwan. As TCNT secures regulatory authorizations from foreign regulatory agencies, we expect to partner with regional distributors to promote sales in other strategic markets. We purchased $183,444 of COVID-19 antigen rapid test kit inventory from TCNT for the year ended December 31, 2021 and $0 in 2020.
Ainos - TCNT Product Development
On August 1, 2021, we entered into a five-year product development agreement with TCNT. Pursuant to the agreement both parties will endeavor to work together to develop pharmaceutical, medical and preventive medicine related products, with the Company being the exclusive sales agent. We will bear the cost associated with product development and TCNT will make accessible its personnel and facilities. Both parties shall each jointly own the intellectual property rights of all research results of the co-development collaboration. As a result, we incurred product development expenses totaling $205,883 as of December 31, 2021 of which $65,156 is in accrued payable as of December 31, 2021 and $0 in product development expenses in 2020.
COVID-19 Antigen Rapid Test Kits Sales
We sold Covid-19 antigen rapid test kits to ASE Technology Holding totaling $209,468 for the year ended December 31, 2021 and $0 in 2020.
6. Common Stock
We have 300,000,000 shares of voting common shares authorized for issuance. As of December 31, 2021, a total of 163,915,625 shares of common stock were either issued (144,379,308), reserved for conversion of convertible debt to stock (17,213,700), reserved for future issuance of RSUs for non-employee directors (1,320,000), held for future exercise of stock options (550,000) and shares reserved for warrant conversion (452,617).
From January 1, 2021 to December 31, 2021, we granted common stock to the following:
| | · | On April 7, 2021, we issued 48,077 shares of common stock to Stephen T. Chen and/or Stephen T. Chen and Virginia M. Chen, Trustees, Stephen T. & Virginia M. Chen Living Trust Dated April 12, 2018 (Chen) as partial compensation payable for the period January 1, 2021 through March 31, 2021 under the Employment Agreement by and between the Company and Chen effective January 1, 2021 (“Chen Agreement”). |
| | | |
| | · | On April 7, 2021, we issued 5,769 shares of common stock to Bernard Cohen (“Cohen”) as partial compensation payable for the period January 1, 2021 through March 31, 2021 under the Employment Agreement by and between the Company and Cohen effective January 1, 2021 (“Cohen Agreement”). |
| | | |
| | · | On April 7, 2021, we issued 11,538 shares of common stock to Lawrence Lin (“Lin”) as compensation payable for the period January 1, 2021 through March 31, 2021 under the Consulting Agreement by and between the Company and Lin’s company, i2China Management Group, LLC, effective April 15, 2018 (“Lin Agreement”), as amended and made effective on January 1, 2020 (“Lin Amendment”). |
| | | |
| | · | On April 7, 2021, we issued 109,038 shares of common stock to John Junyong Lee as compensation payable for the period January 1, 2021 through March 31, 2021 under the Legal Retainer Agreement by and between the Company and Lee effective June 21, 2019 (“Lee Agreement”). |
| | | |
| | · | On April 15, 2021, we consummated the Securities Purchase Agreement and issued 100,000,000 shares of common stock at $0.20 per share to Ainos KY in exchange for certain patent assignments. |
| | | |
| | · | On June 30, 2021, we issued 5,342 shares of common stock as compensation payable for the period April 1, 2021 through April 15, 2021 under the Chen Agreement as amended by Amendment No. 2 that extended the termination date to April 15, 2021. |
| | | |
| | · | On June 30, 2021, we issued 107 shares of common stock to Bernard Cohen as compensation payable for the period April 1, 2021 through April 5, 2021 under the Cohen Agreement as amended by Amendment No. 1 that extended the termination date to April 5, 2021. |
| | | |
| | · | On June 30, 2021, we issued 3,846 shares of common stock to Lawrence Lin as compensation payable for the period April 1, 2021 through June 30, 2021 under the Lin Agreement and Lin Amendment. |
| | | |
| | · | On June 30, 2021, we issued 21,926 shares of common stock to John Junyong Lee as compensation payable for the period April 1, 2021 through June 30, 2021 under the Lee Agreement. |
| | | |
| | · | On July 30, 2021, we issued 20,000 shares of voting common stock to Ya-Ju (“Maggie Wang”), previously a branch manager of the Company’s Taiwan branch office. The Company received payment of $7,600 ($0.38 per share) in accordance to a Stock Option Agreement under the Company’s 2018 Employee Stock Option Plan. |
| | | |
| | · | On July 30, 2021, we issued 150,400 shares of voting common stock to Daniel Fisher, previously a Company board director. The Company received payment of $57,152 ($0.38 per share) in accordance to a Stock Option Agreement under the Company’s 2018 Officers, Directors, Employees and Consultants Nonqualified Stock Option Plan. |
| | | |
| | · | On December 27, 2021, we issued 1,491,953 shares of common stock to Top Calibre Corporation (“TCC”) resulting from an assignment of convertible promissory notes from Dr. Stephen T. Chen to TCC under that certain Assignment Agreement by and between Dr. Stephen T. Chen and TCC, dated December 15, 2021 (“TCC Agreement”). Convertible promissory notes #3.19, #4.19, #6.20, #7.20, #10.21 and #11.21 were exercised at its entirety at a strike price of $0.25 per share based on a combined aggregate principal and accrued interest amount of $372,988. |
| | | |
| | · | On December 27, 2021, we issued 413,368 shares of common stock to i2China Management Group LLC (“i2China”) resulting from a notice of demand from i2China to initiate the conversion of convertible promissory notes #5.19, #8.20a, and #11 exercised at its entirety at a strike price of $0.25 per share based on a combined aggregate principal and accrued interest amount of $103,342. |
| | · | On December 27, 2021, we issued 2,946 shares of common stock to Lawrence Lin as compensation payable for the period July 1, 2021 through August 1, 2021 under the Lin Agreement and Lin Amendment. |
| | | |
| | · | On December 27, 2021, we issued 28,826 shares of common stock to John Junyong Lee as compensation payable for the period July 1, 2021 through September 31, 2021 under the Lee Agreement. |
We did not pay any dividends to its common stock shareholders in 2021 and has no plans to do so in the immediate future.
7. Preferred Stock
We have 10,000,000 shares of preferred stock authorized for issuance.
No shares of preferred stock were outstanding as of December 31, 2021 and 2020 and none are outstanding as of the date of this report.
8. Stock Option and Stock Plans
2018 Employee Stock Option Plan (the “2018-ESOP”)
On September 26, 2018, the Board adopted the Company 2018 Employee Stock Option Plan (the “2018-ESOP”), formerly referred to as the “Amarillo Biosciences, Inc., 2018 Employee Stock Option Plan” in prior filings. The 2018-ESOP provides for the grant of Qualified Incentive Stock Options to the Company’s employees. The Board, in its adoption of the 2018-ESOP, directed the Officers to submit the 2018-ESOP to the shareholders for ratification and approval at the next scheduled shareholders meeting. Failure of the ratification and approval of the 2018-ESOP within one year of the effective date renders the qualified options to become nonqualified options for purposes of the U.S Internal Revenue Code. A stockholders meeting was not convened within the one year period and, as a result, any qualified options automatically became non-qualified options effective September 26, 2019.
The 2018-ESOP is administered by the Board or by a committee of directors appointed by the Board (the “Compensation Committee”) as constituted from time to time. The maximum number of shares of common stock which may be issued under the 2018-ESOP is 1,000,000 shares which will be reserved for issuance upon exercise of options.
The option price per share of common stock deliverable upon the exercise of an incentive stock option is 100% of the fair market value of a share on the date of grant. The option price is $0.38 per share and the options are exercisable during a period of ten years from the date of grant, where the options vest 20% annually over five years, commencing one year from date of grant.
Effective as of October 6, 2021, with the adoption by the Board of the 2021 SIP, no further awards may be granted under the 2018-ESOP. As of December 31, 2021, options to acquire 550,000 shares of common stock remained outstanding.
2018 Officers, Directors, Employees, and Consultants Nonqualified Stock Option Plan (the “2018-NQSOP”)
On September 26, 2018, the Board adopted the Company 2018 Officers, Directors, Employees, and Consultants Nonqualified Stock Option Plan (the “2018-NQSOP”), formerly referred to as the “Amarillo Biosciences, Inc., 2018 Officers, Directors, Employees, and Consultants Nonqualified Stock Option Plan” in prior filings. The 2018-NQSOP provides for the grant of nonqualified incentive stock options to employees. The 2018-NQSOP is administered by the Board or by the Compensation Committee as constituted from time to time. The maximum number of shares of common stock which may be issued under the 2018-NQSOP is 4,000,000 which will be reserved for issuance upon exercise of options. The option price for the nonqualified options is $0.382 exercisable for a period of ten years, with a vesting period of five years at 20% per year commencing one year from date of grant.
Effective as of October 6, 2021, with the adoption by the Board of the 2021 SIP, no further awards may be granted under the 2018-NQSOP. As of December 31, 2021, options to acquire 550,000 shares of common stock remained outstanding.
Equity Compensation Plans Information:
| Stock Plans 1 | | Issue Date Range | | Total Options Authorized | | | Options Issued | | | Options Remaining2 | |
| 2018 Employee Stock Option Plan3, 4 | | 9/26/18 - 9/26/28 | | | 1,000,000 | | | | 950,000 | | | | 0 | |
| 2018 Officers, Directors, Employees, and Consultants Nonqualified Stock Option Plan3 | | 9/26/18 - 9/26/28 | | | 4,000,000 | | | | 4,495,000 | 5 | | | 0 | |
____________
1 The Board of Directors has approved all stock, stock option and stock warrant issuances.
2 Effective October 6, 2021, no further stock option issuance from 2018-ESOP and 2018-NQSOP as per provision in newly adopted 2021 Stock Incentive Plan.
3 Details of the option plans are also disclosed in Financial Statements footnote 8, Stock Options and Stock Plans.
4 On September 26, 2019, all qualified options under the 2018-ESOP became non-qualified options since the 2018-ESOP was not ratified by the Company’s shareholders within one year of adoption.
5 3,844,600 non-qualified options were forfeited as of July 15, 2021, while an additional 500,000 non-qualified options were reissued on August 1, 2021.
A summary of option activity for the years ended December 31, 2020 and December 31, 2021 are presented below.
| Date | | Number of Options 1Qualified | | | Number of Options Nonqualified | | | Weighted Average Exercise Price | | | Weighted Average Remaining Contractual Term | | | Aggregate Intrinsic Value | |
| Balance December 31, 2019 | | | 850,000 | | | | 3,807,000 | | | $ | 0.38 | | | 8 years | | | | - | |
| Exercised | | | - | | | | - | | | | - | | | | - | | | | - | |
| Expired or Forfeited | | | - | | | | - | | | | - | | | | - | | | | - | |
| Balance December 31, 2020 | | | 850,000 | | | | 3,807,000 | | | $ | 0.38 | | | 7 years | | | | | |
| Granted 2021 | | | - | | | | 500,000 | | | $ | 0.38 | | | 10 years | | | | | |
| Exercised | | | 20,000 | | | | 150,400 | | | $ | 0.38 | | | | - | | | | - | |
| Expired or Forfeited | | | 780,000 | | | | 3,656,600 | | | $ | 0.38 | | | | - | | | | - | |
| Balance December 31, 2021 | | | 50,000 | | | | 500,000 | | | $ | 0.38 | | | 9.54 years | | | | - | |
| Vested as of December 31, 2021 | | | 30,000 | | | | 0 | | | $ | 0.38 | | | 6.74 years | | | | - | |
1 Because the 2018 Employee Stock Option Plan was not ratified by the Company’s shareholders, the qualified options became non-qualified on September 26, 2019. These totals remain separated since the two different plans are still in existence.
The Company used the Black-Scholes option pricing model to value the option awards with the following assumptions applied: (1) Volatility - 276%; (2) Term - 5 years was chosen although the full option term is 10 years to be more commensurate with the 5-year vesting portion of the plan; (3) Discount - 2.96%.
____________
2 See footnote 4 above.
As of December 31, 2021, there is $410,022 in unrecognized option expense that will be recognized over the next 2.58 years.
2021 Employee Stock Purchase Plan
On September 28, 2021, the Board approved the 2021 Employee Stock Purchase Plan (the “2021 ESPP” or “Plan”). The purpose of the 2021 ESPP is to provide an opportunity for eligible employees of the company and its designated companies (as defined in the Plan) to purchase common stock at a discount through voluntary contributions, thereby attracting, retaining and rewarding such persons and strengthening the mutuality of interest between such persons and the Company’s stockholders. The Company intends for offerings under the Plan to qualify as an “employee stock purchase plan” under Section 423 of the Code; provided, that the Plan administrator may also authorize the grant of rights under offerings that are not intended to comply with the requirements of Section 423, pursuant to any rules, procedures, agreements, appendices, or sub-plans adopted by the administrator. Subject to adjustments as provided in the Plan, the maximum number of shares of common stock that may be issued under the Plan may not exceed 750,000 shares. Such shares may be authorized but unissued shares, treasury shares or shares purchased in the open market. The Plan is be subject to approval by the Company’s stockholders within twelve months after the date of Board approval. The Plan will become effective on the date that stockholder approval is obtained, and will continue in effect until it expires on the tenth anniversary of the effective date of the Plan, unless terminated earlier.
2021 Stock Incentive Plan
On September 28, 2021, the Board approved the 2021 Stock Incentive Plan (the “2021 SIP” or “Plan”). The purpose of the 2021 SIP is to provide a means through which the Company, and the other members of the Company Group, defined by Section 2(n) of the Plan as the Company and its subsidiaries, and any other affiliate of the Company designated as a member of the Company Group by the Committee, may attract and retain key personnel, and to provide a means whereby directors, officers, employees, consultants and advisors of the Company and the other members of the Company Group can acquire and maintain an equity interest in the Company, or be paid incentive compensation measured by reference to the value of common stock, thereby strengthening their commitment to the interests of the Company Group and aligning their interests with those of the Company’s stockholders. The types of awards that may be granted from the Plan include individually or collectively, any Incentive Stock Option, Nonqualified Stock Option, Stock Appreciation Right, Restricted Stock, Restricted Stock Unit, Dividend Equivalent Rights and Other Equity-Based Award granted under the Plan. The Plan will be effective upon shareholder approval. The expiration date of the Plan, on and after which date no awards may be granted, will be the tenth anniversary of the date of Board approval of the Plan, provided, however, that such expiration will not affect awards then outstanding, and the terms and conditions of the Plan will continue to apply to such Awards. The aggregate number of shares which may be issued pursuant to awards under the Plan is 20,000,000 shares of Common Stock (the “Plan Share Reserve”), subject to adjustments as provided in the Plan. The number of shares underlying any award granted under 2018 ESOP or 2018 NQSOP (the “Prior Plans”) that expires, terminates or is canceled or forfeited for any reason whatsoever under the terms of the Prior Plans, will increase the Plan Share Reserve. Each Award granted under the Plan will reduce the Plan Share Reserve by the number of shares underlying the award. No more than 10,000,000 shares may be issued in the aggregate pursuant to the exercise of incentive stock options granted under the Plan. The maximum number of shares subject to awards granted during a single fiscal year to any non-employee director, taken together with any cash fees paid to such director during the fiscal year, will not exceed $600,000 in total value (calculating the value of any such awards based on their grant date fair value for financial reporting purposes).
9. Warrants
As of December 31, 2021, there is only one warrant certificate outstanding between the Company and i2China Management Group, LLC, deemed for the purposes of related party transactions to be a related party of the company from August 1, 2021 to December 1, 2021, effective from November 25, 2020 until November 25, 2025. The warrant entitles the holder to purchase 452,617 shares of common stock at an exercise price of $0.27 per share. The warrant was valued at $68,349 and will be expensed over sixty (60) months. The Company used the Black-Scholes option pricing model to value the warrants with the following assumptions applied: (1) Volatility - 201%; (2) Term - 5 years (3) Discount Rate - 0.11%.
No warrants were exercised in 2020 or 2021.
10. Income Taxes
The Company accounts for income taxes under FASB Accounting Standard Codification ASC 740, Income Taxes. ASC 740 requires use of the liability method. ASC 740 provides that deferred tax assets and liabilities are recorded based on the differences between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes, referred to as temporary differences. Deferred tax assets and liabilities at the end of each period are determined using the currently enacted tax rates applied to taxable income in the periods in which the deferred tax assets and liabilities are expected to be settled or realized.
Income tax expense (benefit) attributable to income from continuing operations differed from the amounts computed by applying the U.S. Federal income tax of 21% to pretax income from continuing operations as a result of the following:
| | | December 31, | |
| | | 2021 | | | 2020 | |
| Provision (benefit) at statutory rate | | $ | (816,000 | ) | | $ | (305,000 | ) |
| Permanent differences | | | - | | | | 1,000 | |
| Temporary differences | | | 206,000 | | | | 79,000 | |
| Change in valuation allowance | | | 610,000 | | | | 225,000 | |
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and deferred tax liabilities at December 31, 2021 and 2020, are presented below:
| | | December 31, | |
| | | 2021 | | | 2020 | |
| Deferred tax assets: | | | | | | |
| Net operating loss carryforward | | $ | 4,357,000 | | | $ | 4,328,270 | |
| Other assets | | | 248,000 | | | | 217,000 | |
| Deferred tax assets | | | 4,605,000 | | | | 4,545,270 | |
| | | | | | | | | |
| Deferred tax liabilities: | | | - | | | | - | |
| Net deferred tax assets | | | 4,605,000 | | | | 4,545,270 | |
| Valuation allowance | | | (4,605,000 | ) | | | (4,545,270 | ) |
At December 31, 2021, we estimate net operating loss carryforwards of approximately $20,747,517 for federal income tax purposes expiring in 2022 through 2041. The ability of the Company to utilize these carryforwards may be difficult and directly dependent upon many factors outside of our control, including, but not limited to, changes in the legal and regulatory framework and the operational and corporate structure of the Company and shareholders, or sales or transfers of stock by or among shareholders. For example, when the Company has experienced a change of control as defined in the relevant provisions of the Internal Revenue Code of 1986, as amended, the use of any existing tax attributes would be severely limited. Also, obtaining value from the tax attributes is a function our return to profitable operations and the timeframe of that return. While we believe it is possible, there is no assurance that the Company will return to profitability in the future.
As of December 31, 2021, the Company had open tax years of 2020, 2019 and 2018 which are subject to examination by tax authorities.
11. Commitments and Contingencies
Lease and contract commitment
Our executive and administrative offices in the U.S. are located at 8880 Rio San Diego Drive, Suite 800, San Diego, CA 92108. The lease term began on April 1, 2021 as a semi-annual term and automatically renewed currently as a month-to-month renewal agreement.
Our Taiwan branch office is located in New Taipei City, Taiwan (“R.O.C.”) under a three-year office lease contract from June 2021 to May 2024. The office space is 1,250 square feet. We also have staff at a product development facility of approximately 8,517 square feet located in Miaoli County, Taiwan, pursuant to our Product Development Agreement with TCNT.
We have several construction work related contracts to build out our office and lab facilities in Taiwan. As of December 31, 2021, the total contract amount and outstanding contract amount for construction in progress were approximately US$670,000 and US$464,000, respectively.
Litigation
We not at this time involved in any legal proceedings.
Officer Compensation
Effective April 15, 2021, our Board appointed Mr. Chun-Hsien Tsai to serve as Chief Executive Officer. Mr. Tsai will receive a monthly salary of 250,000 New Taiwan Dollars (equivalent to approximately $8,929), a year-end bonus of two months’ salary, and a variable compensation based on Company profit targets decided by the Company’s Compensation Committee, and payable as 10-30% of total annual compensation in the form of cash, securities and/or other discretionary remuneration. An initial equity grant to Mr. Tsai will be determined by the Compensation Committee at a later date. Other benefits, including labor insurance, health insurance and other benefits, will be based on local regulations and the Company’s policies.
Effective August 11, 2021, our Board appointed Ms. Hui-Lan (“Celia”) Wu to serve as Chief Financial Officer. Ms. Wu will receive a monthly salary of 230,000 New Taiwan Dollars (equivalent to approximately $8,214), a year-end bonus of 2 months’ salary, and a variable compensation based on Company profit targets decided by the Company’s Compensation Committee, and payable as 10-30% of total annual compensation in the form of cash, securities and/or other discretionary remuneration. An initial equity grant to Ms. Wu will be determined by the Compensation Committee at a later date. Other benefits, including labor insurance, health insurance and other benefits, will be based on local regulations and the Company’s policies.
Effective August 1, 2021, we entered into an employment contract with Mr. Lawrence K. Lin in connection with his election as Executive Vice President of Operations (the “LL Agreement”). The LL Agreement is effective for three years and may be extended for additional years on the same terms and conditions upon mutual agreement. Under the LL Agreement, Mr. Lin will receive a monthly salary of $12,000, vesting stock options for 500,000 shares in the Company’s 2018 Officers, Directors, Employees and Consultants Non-Qualified Stock Option Plan, and a bonus of 10,000 shares in the Company’s common stock upon the Company’s successful listing on a Major National Exchange (as defined in the LL Agreement), and normal and customary benefits available to the Company’s employees. Mr. Lin is the sole member of i2China Management Group, LLC (“i2China”), a consultant previously engaged by the Company. Mr. Lin indirectly owns 452,617 warrants issued on November 25, 2020 to i2China; a non-convertible note issued to i2China on January 1, 2020 with a principal amount of $84,000; and convertible notes issued to i2China with a total principal and accrued interest amount of $103,342, that were converted into 413,368 shares of common stock on December 27, 2021 at a conversion price of $0.25 per share.
We previously hired Dr. Stephen T. Chen under an employment contract for the period January 1, 2018 through December 31, 2020 (“Prior Chen Contract”). On January 1, 2021 an employment agreement for a 3-month term was executed reflecting the same material terms and conditions of the Prior Chen Contract which includes (i) a $240,000 annual salary, (ii) $100,000 in Company shares payable quarterly based on the average share price of the closing quotes for the one month preceding issuance (referred to in the table as “Other Compensation”), (iii) certain employee benefits available to the our employees, and (iv) reimbursement of expenses made on behalf of the Company. The Company and Dr. Chen also executed a Settlement Agreement and Mutual General Release made effective December 24, 2020 covering any employment-related claims arising under the Prior Chen Contract. The Company and Dr. Chen also entered into an Intellectual Property Assignment Agreement made effective January 19, 2021 whereby Dr. Chen has assigned all right, title, and interest to certain patents, trademarks, and other intellectual property created or developed during Dr. Chen’s employment with the Company.
We previously hired Mr. Cohen under an employment contract for the period January 1, 2018 through December 31, 2020 (“Prior Cohen Contract”). On January 1, 2021 an employment agreement for a 3-month term was executed reflecting the same material terms and conditions of the Prior Cohen Contract which includes, (i) a $70,000 annual salary, (ii) $1,000 per month in Company shares paid monthly based on the average share price of the closing quotes for the one month preceding issuance (referred to in the table as “Other Compensation”), (iii) certain employee benefits available to the Company’s employees, and (iv) reimbursement of expenses made on behalf of the Company. The Company and Mr. Cohen also executed a Settlement Agreement and Mutual General Release made effective December 24, 2020 covering any employment-related claims arising under the Prior Cohen Contract. The Company and Mr. Cohen also entered into an Intellectual Property Assignment Agreement made effective January 19, 2021 whereby Mr. Cohen has assigned all right, title, and interest to certain patents, trademarks, and other intellectual property created or developed during Mr. Cohen’s employment with the Company.
12. Subsequent Events
Asset Purchase Agreement
Ainos KY and the Company entered into an Asset Purchase Agreement dated as of November 18, 2021 as modified by an Amended and Restated Asset Purchase Agreement dated as of January 29, 2022 (the “Asset Purchase Agreement”). Pursuant to the Asset Purchase Agreement, the Company acquired certain intellectual property assets (the “IP Assets”) and certain manufacturing, testing, and office equipment (the “Equipment”) for a total purchase price of $26,000,000.
Pursuant to the Asset Purchase Agreement, the Company agreed to hire certain employees of Ainos KY (the “Employees”) who are responsible for research and development of the IP Assets and/or Equipment on terms at least equal to the compensation arrangements undertaken by Ainos KY. From and after the closing, we will have no responsibility, duty or liability with respect to any employee benefit plans of Ainos KY.
As payment of the purchase price, we issued to Ainos KY a Convertible Promissory Note in the principal amount of $26,000,000 (the “Convertible Note”) upon closing on January 30, 2022.
The principal sum of the Convertible Note is payable in cash on January 30, 2027, although we may prepay the Convertible Note in whole or in part without penalty. The Convertible Note is noninterest bearing. If not earlier repaid, the Convertible Note will be converted into shares of our common stock or such other securities or property for which the Convertible Note may become convertible, immediately prior to the closing of any public offering of our common stock as a result of which our common stock will be listed on a U.S. stock exchange. The conversion price, subject to certain adjustments, will be 80% of the initial public offering price of the offering.
On March 11, 2022, the Board approved a Non-Convertible Note dated March 4, 2022 in favor of Ainos KY with a principal amount of $800,000, interest of 1.85% per annum on unpaid principal and accrued interest, and a maturity date of February 28, 2023. The Note includes standard provisions for notice, default, and remedies for default. Ainos KY is the Company’s majority and controlling shareholder.
On March 17, 2022, we executed a Promissory Note Extension with Ainos KY dated March 17, 2022. Pursuant to the Agreement, the due dates for certain convertible notes enumerated as #12.21 to #24.21 issued by the Company to Ainos KY was extended to February 28, 2023. As of December 31, 2021 the total unpaid principal amount of $3,000,000, along with $9,507 in accrued interest were owed and outstanding to Ainos KY.
PROSPECTUS

780,000 Units each consisting of
One Share of Common Stock
and
One Warrant to purchase one Share of Common Stock
Sole Book Running Manager
Maxim Group LLC
Co-Manager
Brookline Capital Markets
a division of Arcadia Securities, LLC
August 8, 2022
Ainos (NASDAQ:AIMD)
Historical Stock Chart
From Mar 2024 to Apr 2024
Ainos (NASDAQ:AIMD)
Historical Stock Chart
From Apr 2023 to Apr 2024