Alzamend Neuro Partners With Biorasi to Conduct a First-in-Human, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Phase I/IIA Clinical Trial
05 January 2023 - 12:00AM
Business Wire
Study to Assess the Safety, Tolerability, and Efficacy of
Autologous Amyloid Beta Mutant Peptide-Pulsed Dendritic Cells
(ALZN002) in Subjects With Mild-to-Moderate Dementia of the
Alzheimer's Type
Alzamend Neuro, Inc. (Nasdaq: ALZN) (“Alzamend”), an
early clinical-stage biopharmaceutical company focused on
developing novel products for the treatment of Alzheimer’s disease
(“Alzheimer’s”), bipolar disorder, major depressive disorder
(“MDD”) and post-traumatic stress disorder (“PTSD”),
today announced that it is partnering with Biorasi as its contract
research organization (“CRO”) to conduct a first-in-human,
randomized, double-blind, placebo-controlled, parallel group, phase
I/IIA study to assess the safety, tolerability, and efficacy of
autologous amyloid beta mutant peptide-pulsed dendritic cells
(ALZN002) in subjects with mild-to-moderate dementia of the
Alzheimer's type.
The purpose of this trial will be to assess the safety,
tolerability, and efficacy of multiple ascending doses of ALZN002
compared with that of placebo in 20‑30 subjects with
mild-to-moderate dementia of the Alzheimer’s type. Also, the trial
will be designed to determine the optimal dosage of ALZN002 for
treatment of patients with Alzheimer’s in a larger Phase IIB
efficacy and safety clinical trial (ALZN002-02), which Alzamend
expects to initiate within three months of receiving data from the
initial trial. ALZN002 is a patented method using a mutant-peptide
sensitized cell as a cell-based therapeutic vaccine that seeks to
restore the ability of a patient’s immunological system to combat
Alzheimer’s.
“Biorasi is a global, award-winning, full-service CRO with a
plethora of experience with immunotherapeutic vaccines,” said
Stephan Jackman, Chief Executive Officer of Alzamend. “We are
thrilled to add Biorasi to the team and have full confidence in
their ability to conduct our Phase I/IIA clinical trial of ALZN002.
With the Miller School of Medicine, Interdisciplinary Stem Cell
Institute at the University of Miami manufacturing the vaccine as
announced on December 28, 2022, Biorasi’s appointment to the team
completes all logistics encompassing our ALZN002 clinical trial. We
are advancing the process and expect to initiate this study by the
end of March 2023.”
About ALZN002
ALZN002 is a proprietary “active” immunotherapy product, which
means it is produced by each patient’s immune system. It consists
of autologous dendritic cells (“DCs”), which are activated
white blood cells taken from each individual patient that are then
engineered outside of the body to attack Alzheimer’s-related
amyloid-beta proteins. These DCs are pulsed with a novel
amyloid-beta peptide (E22W) designed to bolster the ability of the
patient’s immune system to combat Alzheimer’s; the goal of this
treatment approach is to foster tolerance to treatment for safety
purposes while stimulating the immune system to reduce the brain’s
beta-amyloid protein burden, resulting in reduced Alzheimer’s signs
and symptoms.
The ALZN002 DC treatment is, by definition, an
individual-patient-specific therapy since these autologous DCs are
administered to the same patient from whom they were removed. Each
patient will undergo leukapheresis, i.e., removal and return to the
body of white blood cells. This procedure will isolate each
patient’s peripheral blood monocytes from the obtained white blood
cells. These are subsequently differentiated outside the body into
DCs that are engineered to induce immunogenicity (search and
destroy capability) towards amyloid, the protein associated with
Alzheimer’s in the patient’s body, but to be otherwise tolerated as
natural to the body to avoid adverse side effects.
Compared to passive immunization treatment approaches that use
foreign blood products (such as monoclonal antibodies), active
immunization with ALZN002 is anticipated to offer a more robust and
long-lasting effect on the clearance of amyloid. This is expected
to provide a safe and effective treatment for Alzheimer’s sufferers
that requires considerably less frequent treatment visits compared
to passive immunity approaches.
About Alzamend Neuro
Alzamend is an early clinical-stage biopharmaceutical company
focused on developing novel products for the treatment of
Alzheimer’s, bipolar disorder, MDD and PTSD. Our mission is to
rapidly develop and market safe and effective treatments. Our
current pipeline consists of two novel therapeutic drug candidates,
AL001 - a patented ionic cocrystal technology delivering lithium
via a therapeutic combination of lithium, proline and salicylate,
and ALZN002 - a patented method using a mutant-peptide sensitized
cell as a cell-based therapeutic vaccine that seeks to restore the
ability of a patient’s immunological system to combat Alzheimer’s.
Both of our product candidates are licensed from the University of
South Florida Research Foundation, Inc. pursuant to royalty-bearing
exclusive worldwide licenses.
Forward-Looking Statements
This press release contains “forward looking statements” within
the meaning of Section 27A of the Securities Act of 1933, as
amended, and Section 21E of the Securities Exchange Act of 1934, as
amended. These forward-looking statements generally include
statements that are predictive in nature and depend upon or refer
to future events or conditions, and include words such as
“believes,” “plans,” “anticipates,” “projects,” “estimates,”
“expects,” “intends,” “strategy,” “future,” “opportunity,” “may,”
“will,” “should,” “could,” “potential,” or similar expressions.
Statements that are not historical facts are forward-looking
statements. Forward-looking statements are based on current beliefs
and assumptions that are subject to risks and uncertainties.
Forward-looking statements speak only as of the date they are made,
and Alzamend undertakes no obligation to update any of them
publicly in light of new information or future events. Actual
results could differ materially from those contained in any
forward-looking statement as a result of various factors. More
information, including potential risk factors, that could affect
Alzamend’s business and financial results are included in
Alzamend’s filings with the U.S. Securities and Exchange
Commission. All filings are available at www.sec.gov and on
Alzamend’s website at www.Alzamend.com.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20230104005428/en/
Email: Info@Alzamend.com or call: 1-844-722-6333
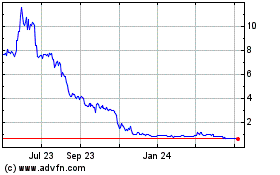
Alzamend Neuro (NASDAQ:ALZN)
Historical Stock Chart
From Mar 2024 to Apr 2024
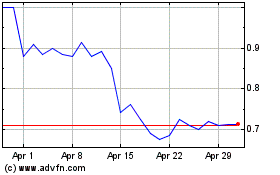
Alzamend Neuro (NASDAQ:ALZN)
Historical Stock Chart
From Apr 2023 to Apr 2024