Report of Foreign Issuer Pursuant to Rule 13a-16 or 15d-16 (6-k)
08 September 2022 - 9:01PM
Edgar (US Regulatory)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
September 8, 2022
(Commission File No. 001-38475)
ASLAN PHARMACEUTICALS LIMITED
(REG. NO. 289175)
(Translation of registrant’s name into English)
CAYMAN ISLANDS
(Jurisdiction of incorporation or organisation)
3 Temasek Avenue
Level 18 Centennial Tower
Singapore 039190
(Address of registrant’s principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F Form 40-F
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (1):
Yes No
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (7):
Yes No
ASLAN Pharmaceuticals presents new data on eblasakimab in multiple posters at the 31st Annual European Academy of Dermatology and Venereology (EADV) Congress
On September 7, 2022, ASLAN Pharmaceuticals Limited (the “Company”) announced the presentation of new data on eblasakimab in multiple posters at the 31st Annual European Academy of Dermatology and Venereology (EADV) Congress. Key points include:
• Data presented at EADV for the first time show eblasakimab suppresses downstream inflammatory biomarkers of atopic dermatitis, continuing 4-6 weeks after the last dose
• Notable improvements in quality-of-sleep measures, with fewer patients reporting sleep disturbance on eblasakimab
• Eblasakimab significantly reduced P-NRS (itch) scores and improvements continued throughout 8-week course of treatment across all dose cohorts
Further information is set out in the press release attached hereto as Exhibit 99.1 and which is incorporated by reference herein.
The information contained in this Form 6-K is hereby incorporated by reference into the Company’s Registration Statement on Form F-3 (File
No. 333-234405), Registration Statement on Form F-3 (File No. 333-252575), Registration Statement on Form F-3 (File No. 333-254768), RegistrationStatement on Form S-8 (File No. 333-252118) and Registration Statement on Form S-8 (File No. 333-263843).
Forward Looking Statements
This Form 6-K contains forward-looking statements. These statements are based on the current beliefs and expectations of the management of ASLAN Pharmaceuticals Limited and/or its affiliates (the "Company"). These forward-looking statements may include, but are not limited to, statements regarding the Company’s business strategy and clinical development plans; the Company’s plans to develop and commercialize eblasakimab; the safety and efficacy of eblasakimab; the Company’s plans and expected timing with respect to clinical trials, clinical trial enrolment and clinical trial results for eblasakimab; and the potential of eblasakimab as a first-in-class treatment for atopic dermatitis. The Company’s estimates, projections and other forward-looking statements are based on management's current assumptions and expectations of future events and trends, which affect or may affect the Company’s business, strategy, operations, or financial performance, and inherently involve significant known and unknown risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of many risks and uncertainties, which include, unexpected safety or efficacy data observed during preclinical or clinical studies; clinical site activation rates or clinical trial enrolment rates that are lower than expected; the impact of the COVID-19 pandemic or the ongoing conflict between Ukraine and Russia on the Company’s business and the global economy; general market conditions; changes in the competitive landscape; and the Company’s ability to obtain sufficient financing to fund its strategic and clinical development plans. Other factors that may cause actual results to differ from those expressed or implied in such forward-looking statements are described in the Company’s US Securities and Exchange Commission filings and reports (Commission File No. 001- 38475), including the Company’s Annual Report on Form 20-F filed with the US Securities and Exchange Commission on March 25, 2022. All statements other than statements of historical fact are forward-looking statements. The words “believe,” “may,” “might,” “could,” “will,” “aim,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “plan,” or the negative of those terms, and similar expressions that convey uncertainty of future events or outcomes are intended to identify estimates, projections, and other forward-looking statements. Estimates, projections, and other forward-looking statements speak only as of the date they were made, and, except to the extent required by law, the Company undertakes no obligation to update or review any estimate, projection, or forward-looking statement.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereto duly authorized.
|
|
|
|
ASLAN PHARMACEUTICALS LIMITED |
(Registrant) |
|
|
By: |
/s/ Kiran Kumar Asarpota |
Name: |
Kiran Kumar Asarpota |
Title: |
Chief Operating Officer |
Date: September 08, 2022
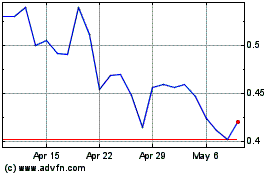
ASLAN Pharmaceuticals (NASDAQ:ASLN)
Historical Stock Chart
From Mar 2024 to Apr 2024
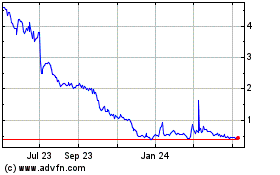
ASLAN Pharmaceuticals (NASDAQ:ASLN)
Historical Stock Chart
From Apr 2023 to Apr 2024