AvroBio Gets Rare Pediatric Disease Designation for AVR-RD-04 Cystinosis Treatment
20 September 2022 - 9:54PM
Dow Jones News
By Chris Wack
AvroBio Inc. said Tuesday that the U.S. Food and Drug
Administration has granted rare pediatric disease designation to
AVR-RD-04, an investigational gene therapy for the treatment of
cystinosis.
The FDA's Rare Pediatric Disease Designation and Voucher Program
is intended to help the development of new drugs and biologics for
the prevention and treatment of rare pediatric diseases.
Companies that receive approval for a new drug application or
biologics license application for a rare pediatric disease may be
eligible to receive a voucher for a priority review of a subsequent
marketing application for a different product. The priority review
voucher may be used by the company or sold to a third party.
Cystinosis is a life-threatening disease that causes progressive
multiorgan damage that affects about 1,600 people in the U.S.,
Europe and Japan. AVR-RD-04 is designed to genetically modify
patients' own hematopoietic stem cells to express the gene encoding
cystinosin, the protein that is critically deficient in people
living with cystinosis, AvroBio said.
The company said preliminary data from an ongoing Phase 1/2
clinical trial suggest that this approach is well-tolerated, with
no adverse events related to the drug product reported to date.
AvroBio shares were up 11% to 89 cents in premarket trading.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
September 20, 2022 07:39 ET (11:39 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
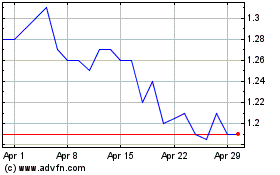
AVROBIO (NASDAQ:AVRO)
Historical Stock Chart
From Mar 2024 to Apr 2024
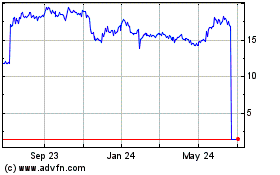
AVROBIO (NASDAQ:AVRO)
Historical Stock Chart
From Apr 2023 to Apr 2024