Atossa Appoints Life Sciences Financial and Operations Industry Veteran Greg Weaver as Chief Financial Officer
01 June 2023 - 11:10PM

Atossa Therapeutics, Inc. (Nasdaq: ATOS), a clinical stage
biopharmaceutical company developing innovative proprietary
medicines to address significant unmet needs in oncology with a
current focus on breast cancer, today announces the appointment of
Greg Weaver as the Company’s new Chief Financial Officer (CFO). Mr.
Weaver succeeds Kyle Guse.
A short video interview with Mr. Weaver about his background and
vision for Atossa can be found here:
https://youtu.be/G6ZzHLh-qsQ
Mr. Weaver brings more than 30 years of life sciences, financial
and operations experience to the Company. Recently, he served as
CFO of privately held BioIntelliSense, a commercial stage med-tech
company focused on remote patient monitoring. Before this, he was
the CFO of atai Life Sciences (Nasdaq: ATAI), where he led a
successful IPO, managed the acquisition of multiple mental health
therapeutics assets, and raised more than $500 million to advance
multiple clinical and discovery programs. In addition, Mr. Weaver
previously served as Chief Financial Officer of Eloxx
Pharmaceuticals, Inc. (NASDAQ: ELOX), where he led their reverse
merger and Nasdaq listing. During his career, Mr. Weaver has
managed M&A transactions involving oncology products, medical
device technologies, and other biotech assets.
Earlier in his career, Mr. Weaver held roles on the executive
teams of several oncology-focused, public biotech companies,
including ILEX Oncology, which was acquired by Genzyme in
2005, Celsion Corporation and Poniard Pharmaceuticals, Inc. He has
been involved with development programs that led to multiple FDA
approvals and product launches. In addition to serving on the
Atossa board since 2013, Mr. Weaver is a director of Rejuveron Life
Sciences and BioIntelliSense. He also serves on the not-for-profit
board of HarborPath. He began his career as a CPA with Arthur
Andersen.
“We are excited to welcome Greg as our CFO and look forward to
drawing from his broad experience leading biotech companies and
partnering innovative products and programs within the
biotechnology industry,” said Steven Quay, MD, PhD, Atossa’s
President and Chief Executive Officer. “Greg is a trusted colleague
that I and other members of the Atossa board have had the pleasure
of working alongside in previous board and management roles. Greg
has served on the board of Atossa since the beginning, and I am
confident that his expertise in life sciences corporate strategy,
financing and business development will be a vital addition to
Atossa as our programs progress in later stages of clinical
development. On behalf of the entire leadership team, we are
excited to work with Greg to achieve our shared goal of
meaningfully improving the current standard of care for cancer
patients. We also want to thank our outgoing CFO Kyle Guse for his
service over the past years. His partnership and contributions have
been invaluable and key to the success of our Company to date. We
wish him the best in his next role.”
“I’m excited to join Atossa’s executive team as we continue to
advance the company’s Phase 2 clinical trials in breast cancer and
mammographic breast density,” said Mr. Weaver. “Atossa is at a
critical stage in its development with key clinical data readouts
expected over the next year and with a strong multi-year cash
position. I look forward to working on behalf of our shareholders,
partners and patients to fully capitalize on the value of
(Z)-endoxifen and to fundamentally change the breast cancer
paradigm.”
About (Z)-Endoxifen(Z)-endoxifen is the most
active metabolite of the FDA approved Selective Estrogen Receptor
Modulator (SERM), tamoxifen. Studies by others have demonstrated
that the therapeutic effects of tamoxifen are driven in a
concentration-dependent manner by (Z)-endoxifen. In addition to its
potent anti-estrogen effects, (Z)-endoxifen at higher
concentrations has been shown to target PKCβ1, a known oncogenic
protein.
Atossa is developing a proprietary oral formulation of
(Z)-endoxifen that does not require liver metabolism to achieve
therapeutic concentrations and is encapsulated to bypass the
stomach as acidic conditions in the stomach convert a greater
proportion of (Z)-endoxifen to the inactive (E)-endoxifen. Atossa’s
(Z)-endoxifen has been shown to be well tolerated in Phase 1
studies and in a small Phase 2 study of women with breast cancer.
We are currently studying (Z)-endoxifen in three Phase 2 studies:
one in healthy women with measurable breast density and two other
studies including the EVANGELINE study in women with ER+/HER2-
breast cancer. Atossa’s (Z)-endoxifen is protected by three issued
U.S. patents and numerous pending patent applications.
About Atossa TherapeuticsAtossa Therapeutics,
Inc. is a clinical-stage biopharmaceutical company developing
innovative medicines in areas of significant unmet medical need in
oncology with a current focus on breast cancer. For more
information, please visit www.atossatherapeutics.com
CONTACTS:Greg WeaverChief Financial
Officergreg.weaver@atossainc.com
Eric Van ZantenVP, Investor and Public
Relations610-529-6219eric.vanzanten@atossainc.com
FORWARD LOOKING STATEMENTSForward-looking
statements in this press release, which Atossa undertakes no
obligation to update, are subject to risks and uncertainties that
may cause actual results to differ materially from the anticipated
or estimated future results, including the risks and uncertainties
associated with any variation between interim and final clinical
results, actions and inactions by the FDA, the outcome or timing of
regulatory approvals needed by Atossa including those needed to
commence studies of (Z)-endoxifen, lower than anticipated rate of
patient enrollment, estimated market size of drugs under
development, the safety and efficacy of Atossa’s products,
performance of clinical research organizations and investigators,
obstacles resulting from proprietary rights held by others such as
patent rights, whether reduction in breast density or in Ki-67 or
any other result from a neoadjuvant study is an approvable endpoint
for (Z)-endoxifen, whether Atossa can complete acquisitions, and
other risks detailed from time to time in Atossa’s filings with the
Securities and Exchange Commission, including without limitation
its periodic reports on Form 10-K and 10-Q, each as amended and
supplemented from time to time.
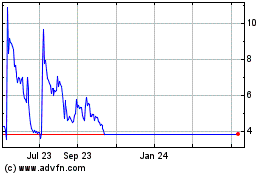
Eloxx Pharmaceuticals (NASDAQ:ELOX)
Historical Stock Chart
From Oct 2024 to Dec 2024
Eloxx Pharmaceuticals (NASDAQ:ELOX)
Historical Stock Chart
From Dec 2023 to Dec 2024