As filed with the Securities and Exchange Commission
on November 22, 2024
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-3
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
MUSTANG BIO, INC.
(Exact name of registrant as specified in its charter)
Delaware
(State or other jurisdiction of
incorporation or organization) |
47-3828760
(I.R.S. Employer
Identification No.) |
377 Plantation Street
Worcester, Massachusetts 01605
(781) 652-4500
(Address, including zip code, and telephone number,
including
area code, of registrant’s principal executive offices)
Manuel Litchman, M.D.
President and Chief Executive Officer
377 Plantation Street
Worcester, Massachusetts 01605
(781) 652-4500
(Name, address, including zip code, and telephone
number, including area code, of agent for service)
With a copy to:
Rakesh Gopalan
Joseph Walsh
Troutman Pepper Hamilton Sanders LLP
301 S. College Street, 34th Floor
Charlotte, NC 28202
(704) 998-4050
Approximate date of commencement of proposed
sale to the public: From time to time after the effective date of this registration statement.
If
the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check
the following box: ¨
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415
under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check
the following box: x
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act,
please check the following box and list the Securities Act registration statement number of the earlier effective registration statement
for the same offering. ¨
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box
and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If
this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become
effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ¨
If
this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register
additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following
box. ¨
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,”
and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ |
Accelerated filer ¨ |
Non-accelerated filer x |
Smaller reporting company x |
Emerging growth company ¨ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ¨
The Registrant hereby amends this registration
statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment that
specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the
Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission,
acting pursuant to said Section 8(a), may determine.
The information in this preliminary
prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities
and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and is not soliciting an offer
to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject
To Completion, Dated November 22, 2024
Preliminary Prospectus

34,767,934 Shares of Common Stock underlying certain Common Warrants
and Placement Agent Warrants
This prospectus relates to the resale by the selling
stockholders (the “Selling Stockholders”) identified in this prospectus under the section “The Selling Stockholders,”
or their pledgees, donees, transferees or other successors in interest, from time to time, of shares of our common stock, par value $0.0001
per share, comprised of (i) 16,877,638 shares of common stock (the “Series B-1 Warrant Shares”) issuable upon the
exercise of our outstanding Series B-1 Warrants to purchase common stock (the “Series B-1 Warrants”), (ii) 16,877,638
shares of common stock (the “Series B-2 Warrant Shares”) issuable upon exercise of our outstanding Series B-2 Warrants
to purchase common stock (the “Series B-2 Warrants”), and (iii) 1,012,658 shares of common stock (the “Placement
Agent Warrant Shares” and together with the Series B-1 Warrant Shares and the Series B-2 Warrant Shares, the “Warrant
Shares”) issuable upon the exercise of outstanding Placement Agent Warrants to purchase common stock (the “Placement Agent
Warrants” and together with the Series B-1 Warrants and Series B-2 Warrants, the “Warrants”). The Warrants
were issued to the Selling Stockholders in a private placement transaction (the “Private Placement”) that closed on October 25,
2024. For additional information about the Private Placement, see the section of this prospectus entitled “The Private Placement”
for more information. We are registering the resale of the shares of common stock issuable upon exercise of the Warrants (the “Warrant
Shares”) on behalf of the Selling Stockholders to satisfy certain registration rights that we have granted to the Selling Stockholders.
The Series B-1 Warrants and Series B-2
Warrants have an exercise price of $0.27 per share. The Placement Agent Warrants have an exercise price of $0.2963 per share. The Series B-1
Warrants and the Placement Agent Warrants will become exercisable on or after the date on which stockholder approval is obtained (the
“Stockholder Approval Date”) with respect to the issuance of the Series B-1 Warrant Shares and the Placement Agent Warrant
Shares until the five (5) year anniversary thereafter. The Series B-2 Warrants will become exercisable on or after the Stockholder
Approval Date with regard to the issuance of the Series B-2 Warrant Shares until the twelve (12) month anniversary thereafter.
The Selling Stockholders may resell or dispose
of the Warrant Shares, or interests therein, at fixed prices, at prevailing market prices at the time of sale or at prices negotiated
with purchasers, to or through underwriters, broker-dealers, agents, or through any other means described in the section of this prospectus
titled “Plan of Distribution.” The Selling Stockholders will bear the costs of commissions and discounts, if any, attributable
to the sale or disposition of the Warrant Shares, or interests therein, held by the Selling Stockholders. We will bear all costs, expenses
and fees in connection with the registration of the offer and sale of the Warrant Shares under the Securities Act of 1933, as amended
(the “Securities Act”). We will not receive any of the proceeds from the sale of the Warrant Shares by the Selling Stockholders.
However, we will receive the proceeds of any cash exercise of the Warrants. See the section of this prospectus entitled “Use
of Proceeds” for more information.
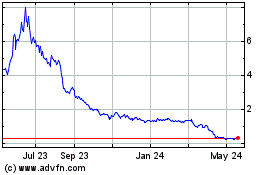
Our common stock is listed on the Nasdaq Capital
Market under the symbol “MBIO.” On November 21, 2024, the last reported sale price of our common stock was $0.2079
per share. You are urged to obtain current market quotations for our common stock.
Investing
in our securities involves risks. You should review carefully the risks and uncertainties described under the heading “Risk
Factors” contained in this prospectus and under similar headings in the other documents that are incorporated by
reference into this prospectus, as described on page 14 of this prospectus.
Neither
the Securities and Exchange Commission (the “SEC”) nor any state securities commission has approved
or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a
criminal offense.
The date of this prospectus is ,
2024
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus provides you with a general description
of the common stock that may be resold by the Selling Stockholders. In certain circumstances, we may provide a prospectus supplement that
will contain specific information about the terms of a particular offering by the Selling Stockholders. We also may provide a prospectus
supplement to add information to, or update or change information contained in, this prospectus. To the extent there is a conflict between
the information contained in this prospectus and any prospectus supplement, you should rely on the information in the prospectus supplement,
provided that if any statement in one of these documents is inconsistent with a statement in another document having a later date — for
example, a document incorporated by reference in this prospectus or any prospectus supplement — the statement in the
later-dated document modifies or supersedes the earlier statement.
You should read both this prospectus and any applicable
prospectus supplement together with the additional information about our company to which we refer you in the sections of this prospectus
titled “Where You Can Find More Information” and “Incorporation of Certain Documents by Reference.”
You should rely only on the information contained in or incorporated by reference into this prospectus and any prospectus supplement.
Neither we nor the Selling Stockholders have authorized any dealer, salesperson or other person to provide you with different or inconsistent
information. You should not assume that the information in this prospectus or any prospectus supplement is accurate as of any date other
than the date on the front of those documents or that any document incorporated by reference is accurate as of any date other than its
filing date. You should not consider this prospectus to be an offer or solicitation relating to the common stock in any jurisdiction in
which such an offer or solicitation relating to the common stock is not authorized. Furthermore, you should not consider this prospectus
to be an offer or solicitation relating to the common stock if the person making the offer or solicitation is not qualified to do so,
or if it is unlawful for you to receive such an offer or solicitation.
Unless otherwise indicated, information contained
or incorporated by reference in this prospectus concerning our industry, including our general expectations and market opportunity, is
based on information from our own management estimates and research, as well as from industry and general publications and research, surveys
and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry
and assumptions based on such information and knowledge, which we believe to be reasonable. In addition, assumptions and estimates of
our and our industry’s future performance are necessarily uncertain due to a variety of factors, including those described in section
of this prospectus titled “Risk Factors.”
These and other factors could cause our future
performance to differ materially from our assumptions and estimates. For investors outside the United States: we have not done anything
that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is
required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves
about, and observe any restrictions relating to, the offering of our securities and the distribution of this prospectus outside the United
States.
Unless the context indicates otherwise, when we
refer to “Mustang,” “we,” “our,” “us” and the “Company” in this prospectus,
we mean Mustang Bio, Inc., unless otherwise specified. When we refer to “you,” we mean the potential holders of the applicable
series of common stock.
This
prospectus contains references to trademarks, trade names and service marks belonging to other entities. Solely for convenience, trademarks,
trade names and service marks referred to in this prospectus may appear without the ® or TM symbols, but such
references are not intended to indicate, in any way, that the applicable licensor will not assert, to the fullest extent under applicable
law, its rights to these trademarks and trade names. We do not intend our use or display of other entities’ trade names,
trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other entities.
FORWARD-LOOKING STATEMENTS
This prospectus contains predictive or “forward-looking
statements” within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of
current or historical fact contained in this prospectus, including statements that express our intentions, plans, objectives, beliefs,
expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions are
forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,”
“expect,” “intend,” “may,” “plan,” “predict,” “project,” “will,”
“should,” “would” and similar expressions, as they relate to us, are intended to identify forward-looking statements.
These statements are based on current expectations,
estimates and projections made by management about our business, our industry and other conditions affecting our financial condition,
results of operations or business prospects. These statements are not guarantees of future performance and involve risks, uncertainties
and assumptions that are difficult to predict. Therefore, actual outcomes and results may differ materially from what is expressed or
forecasted in, or implied by, the forward-looking statements due to numerous risks and uncertainties. Factors that could cause such outcomes
and results to differ include, but are not limited to, risks and uncertainties arising from:
| |
· |
expectations for increases or decreases in expenses; |
| |
· |
expectations for the clinical and pre-clinical development, manufacturing, regulatory approval, and commercialization of our pharmaceutical product candidates or any other products we may acquire or in-license; |
| |
· |
use of clinical research centers and other contractors; |
| |
· |
expectations for incurring capital expenditures to expand our research and development and manufacturing capabilities; |
| |
· |
expectations for generating revenue or becoming profitable on a sustained basis; |
| |
· |
expectations or ability to enter into marketing and other partnership agreements; |
| |
· |
expectations or ability to enter into product acquisition and in-licensing transactions; |
| |
· |
expectations or ability to build our own commercial infrastructure to manufacture, market and sell our product candidates, if approved; |
| |
· |
expectations for the acceptance of our product candidates, if approved, by doctors, patients or payors; |
| |
· |
ability to compete against other companies and research institutions; |
| |
· |
ability to attract, hire and retain qualified personnel, including the impact of our recently announced reduction in work force; |
| |
· |
ability to secure adequate protection for our intellectual property; |
| |
· |
ability to attract and retain key personnel; |
| |
· |
ability to obtain reimbursement for our products, if approved; |
| |
· |
estimates of the sufficiency of our existing cash and cash equivalents and investments to finance our operating requirements, including expectations regarding the value and liquidity of our investments; |
| |
· |
stock price and the volatility of the equity markets; |
| |
· |
expected losses; and |
| |
· |
expectations for future capital requirements. |
Any forward-looking statements speak only as of
the date on which they are made, and we undertake no obligation to publicly update or revise any forward-looking statements to reflect
events or circumstances that may arise after the date of this prospectus, except as required by applicable law. Investors should evaluate
any statements made by us in light of these important factors.
PROSPECTUS SUMMARY
This summary highlights selected information
from this prospectus and does not contain all of the information that may be important to you in making an investment decision. This summary
is qualified in its entirety by the more detailed information included elsewhere in this prospectus and/or incorporated by reference herein.
Before making your investment decision with respect to our securities, you should carefully read this entire prospectus, including the
information in our filings with the SEC incorporated by reference into this prospectus.
Our Business
Overview and Product Candidate Development
We are a clinical-stage biopharmaceutical company
focused on translating today’s medical breakthroughs into potential cures for difficult-to-treat cancers. We aim to acquire rights
to these technologies by licensing or otherwise acquiring an ownership interest in the technologies, funding their research and development
and eventually either out-licensing or bringing the technologies to market.
Our pipeline is currently focused on CAR T therapies
for oncology and combination treatment with an oncolytic virus. For these therapies we have partnered with world class research institutions,
including the City of Hope National Medical Center (“COH” or “City of Hope”), Fred Hutchinson Cancer Center (“Fred
Hutch”), and Nationwide Children’s Hospital (“Nationwide”).
CAR T Therapies
Our pipeline of CAR T therapies is being developed
under exclusive licenses from several world class research institutions. Our strategy is to license these technologies, support preclinical
and clinical research activities by our partners and transfer the underlying technology to our or our contract manufacturer’s cell
processing facility in order to conduct our own clinical trials.
We are developing CAR T therapy for hematologic
malignancies in partnership with Fred Hutch targeting CD20 (MB-106). In May 2021, we announced that the U.S. Food and Drug Administration
(“FDA”) accepted our IND Application for MB-106. As of September 30, 2024, approximately 40 patients have been treated
in an ongoing Phase 1 clinical trial sponsored by Fred Hutch (ClinicalTrials.gov Identifier: NCT03277729), and approximately 20 patients
have been treated in the Phase 1 clinical trial sponsored by us (ClinicalTrials.gov Identifier: NCT05360238). In 2023, we received Safety
Review Committee approval to continue dose escalation in all three active arms of the ongoing Mustang-sponsored phase 1 trial. We presented
the latest results, demonstrating a favorable safety profile, complete response rate, and durability, from the ongoing Mustang-sponsored
Phase 1 trial at the 2023 American Society of Hematology Annual Meeting.
We are also developing CAR T therapy for solid
tumors in partnership with COH targeting IL13Rα2 (MB-101). In addition, we have partnered with Nationwide for a herpes simplex
virus type 1 (“HSV-1”) oncolytic virus (MB-108) in order to enhance the activity of MB-101 for the treatment of patients
with high-grade malignant brain tumors. The Phase 1 clinical trial sponsored by COH for MB-101 (ClinicalTrials.gov Identifier: NCT02208362)
has completed the treatment phase and patients continue to be assessed for long-term safety. A Phase 1 clinical trial sponsored by the
University of Alabama at Birmingham for MB-108 (ClinicalTrials.gov Identifier: NCT03657576) began during the third quarter of 2019. In
October 2023, we announced that the FDA accepted our IND application for the combination of MB-101 and MB-108 – which is referred
to as MB-109 – for the treatment of patients with IL13Rα2+ relapsed or refractory glioblastoma (“GBM”) and high-grade
astrocytoma.
MB-106 (CD20-targeted CAR T cell therapy)
In the first quarter of 2024, we completed a
successful End-of-Phase 1 meeting with the FDA regarding a potential pivotal Phase 2 single-arm clinical trial for the treatment of WM.
Per the discussions, the FDA agreed with the proposed overall design of the pivotal trial for Waldenstrom macroglobulinemia (“WM”) at the recommended dose of 1 x 107 CAR-T
cells/kg and requested only minimal modifications to the study protocol. No additional nonclinical studies are expected prior to Phase
2 or a Biologics License Application filing. Due to limited resources, and as a result of the reduction in work force described below,
we do not expect to initiate our pivotal Phase 2 single-arm clinical trial of MB-106 for the treatment of WM trial in 2024. Subject to
available funds, we intend to rely on third party service providers to conduct study and manufacturing services to advance our priority
potential product candidates.
Also in the first quarter of 2024, we completed
enrollment of the indolent lymphoma arm in our multicenter Phase 1 trial. The tenth and final patient enrolled on that arm was a patient
with follicular lymphoma (FL) who achieved a complete response following treatment with 1 x 107 CAR-T cells/kg. As a result,
the overall complete response rate for FL in the Phase 1 portion of this trial was sustained at 100% (N=6), with no occurrence of CRS
above grade 1 and no ICANS of any grade, despite not using prophylactic tocilizumab or dexamethasone.
In March 2024, we announced plans to collaborate
with Fred Hutch for a proof-of-concept Phase 1 investigator-sponsored clinical trial evaluating MB-106 in autoimmune diseases.
In March 2024, we were granted the Regenerative
Medicine Advanced Therapy (“RMAT”) designation by the FDA for the treatment of relapsed or refractory CD20 positive WM and
FL, based on potential improvement in response as seen in clinical date to date. Drugs eligible for RMAT designation are those intended
to treat, modify, reverse or cure a serious or life-threatening disease or condition, and that present preliminary clinical evidence indicating
the drug has the potential to address unmet medical needs for such disease or condition. RMAT designation provides regenerative medicine
advanced therapy products with the same benefits to expedite the development and review of a marketing application that are available
to drugs that receive Breakthrough Therapy Designation. These advantages include timely advice and interactive communications with FDA,
as well as proactive and collaborative involvement by senior FDA managers and experienced review and regulatory health project management
staff. A product designated as an RMAT also may be eligible for other FDA-expedited programs, such as Priority Review. The FDA also may
conduct a rolling review of products in its expedited programs, reviewing portions of a marketing application before the complete application
is submitted.
In June 2024, we announced that updated data
for MB-106 in the Phase 1/2 Fred Hutch investigator-sponsored trial showed a favorable safety and efficacy profile in 10 patients with
WM. There was an overall response rate of 90% with durable responses observed, including three complete responses (“CR”),
two very good partial responses, and four partial responses (“PR”). One of the patients who achieved a CR remained in remission
for 31 months, with an immunoglobulin M (IgM) level that decreased rapidly to the normal range after treatment with MB-106 and remained
normal since. Patients had a median of nine prior lines of therapy, and only one patient started additional anti-WM treatment after being
treated with MB-106. From a safety perspective, CRS occurred in nine patients: five patients with grade 1 and four patients with grade
2. One patient experienced grade 1 ICANS. No grade 3 or 4 CRS or grade 2, 3 or 4 ICANS was observed, despite dose escalation.
In May 2024, we informed the clinical
sites participating in the Mustang-sponsored Phase 1/2 study in non-Hodgkin lymphoma and chronic lymphocytic leukemia,
MB106-CD20-001, that we had decided to close the trial. In June 2024, we similarly informed the clinical sites participating in
the Mustang-sponsored Long-term Follow-up Study in Patients Previously Treated with Mustang Bio, Inc. CAR-T Cell
Investigational Products, MB100-OBS-001, that we had decided to close that trial. As a result, further clinical development of
MB-106 is currently focused solely on autoimmune diseases unless funding and resources become available to restart the program for
hematologic malignancies. Planning for the aforementioned Phase 1 investigator-sponsored clinical trial in autoimmune diseases is in
progress, with initiation of the trial planned for 2025.
MB-109 (Combination of MB-101 CAR T Therapy
with MB-108 Oncolytic Virus Therapy for Malignant Brain Tumors)
In
October 2023, we received a safe-to-proceed “approval” from the FDA for our MB-109 IND application allowing us to initiate
a Phase 1, open-label, non-randomized, multicenter study of MB-109 in patients with IL13Ra2+
recurrent GBM and high-grade astrocytoma. In this Phase 1 clinical study, we intend to evaluate the combination of CAR-T cells (MB-101)
and the herpes simplex virus type 1 oncolytic virus (MB-108) in patients with IL13Ra2+
high-grade gliomas. The design of this study involves first a lead-in cohort, wherein patients are treated with MB-101 alone without
prior MB-108 administration. After successful confirmation of the safety profile of MB-101 alone, the study will then investigate increasing
doses of intratumorally administered MB-108 followed by dual intratumoral (ICT) and intraventricular (ICV) administration of MB-101.
On November 7, 2024, we announced that the
FDA granted Orphan Drug Designation to Mustang for MB-108, an HSV-1 oncolytic virus, for the treatment of malignant glioma. The Orphan
Drug Designation provides certain incentives, such as tax credits toward the cost of clinical trials upon approval and prescription drug
user fee waivers. If a product receives Orphan Drug Status from the FDA, that product is entitled to seven years of market exclusivity
for the disease in which it has Orphan Drug designation, which is independent from intellectual property protection. Due to limited resources,
we do not currently expect to initiate this study until such time, if any, that additional resources become available to us.
To date,
we have not received approval for the sale of any of our product candidates in any market and, therefore, have not generated any product
sales from our product candidates. In addition, we have incurred substantial operating losses since our inception, and expect to continue
to incur significant operating losses for the foreseeable future and may never become profitable.
Private Placement
On October 24, 2024, we entered into an inducement
offer letter agreement (the “Inducement Letter”) with an institutional accredited investor (the “Investor”) which
held certain outstanding (i) Series A-1 Warrants to purchase up to an aggregate of 16,877,638 shares of common stock, (ii) Series A-2
Warrants to purchase up to an aggregate of 16,877,638 shares of common stock, and (iii) Series A-3 Warrants to purchase up to
an aggregate of 16,877,638 shares of common stock, originally issued to the Investor on May 2, 2024 (the “May 2024 Warrants”).
The May 2024 Warrants had an exercise price of $0.237 per share.
Pursuant to the Inducement Letter, the Investor
agreed to exercise in full, for cash, the Series A-3 Warrants (the “Existing Warrants”) at the exercise price of $0.237
per share in consideration for our agreement to issue in a private placement (x) new Series B-1 Warrants to purchase Series B-1
Warrant Shares and (y) new Series B-2 Warrants to purchase Series B-2 Warrant Shares. In addition, we issued to H.C. Wainwright &
Co., LLC (“H.C. Wainwright,” together with the Investor, the “Holders”) or its designees Placement Agent Warrants
to purchase Placement Agent Warrant Shares. The Placement Agent Warrants have the same terms as the Series B-1 Warrants, except that
the Placement Agent Warrants have an exercise price equal to $0.2963 per share. The Holders (as defined below) are the Selling Stockholders
identified in this prospectus under the section heading titled, “The Selling Stockholders.”
The transactions contemplated by the Inducement
Letter closed on October 25, 2024 (the “Closing Date”). We received aggregate gross proceeds of approximately $4 million
from the exercise of the Existing Warrants by the Investor, before deducting placement agent fees and other expenses payable by us.
In the Inducement Letter, we agreed to file a
registration statement providing for the resale of the Warrant Shares. Accordingly, as required by the Inducement Letter, the registration
statement of which this prospectus forms a part relates to the offer and resale of the Warrant Shares issuable to the Selling Stockholders
upon the exercise of the Warrants. See the section of this prospectus entitled “The Private Placement” for more information.
Recent Developments
Non-Compliance
with Nasdaq Continued Listing Requirements
On March 13,
2024, we received a deficiency letter (the “Letter”) from the Listing Qualifications Department (the “Staff”)
of The Nasdaq Stock Market LLC (“Nasdaq”) notifying us that we were not in compliance with the minimum stockholders’
equity requirement for continued listing on the Nasdaq Capital Market under Nasdaq Listing Rule 5550(b)(1) (the “Equity
Rule”). The Equity Rule requires companies listed on the Nasdaq Capital Market to maintain stockholders’ equity of at
least $2.5 million (or, in the alternative, a market value of listed securities of $35 million or net income from continued operations
of $500,000 in the most recently completed fiscal year or in two of the last three most recently completed fiscal years). As of December 31,
2023, we reported stockholders’ equity of $123,000, and as of September 30, 2024, we reported stockholders’ deficit of
approximately $8.7 million.
The Letter
had no immediate effect on our continued listing on Nasdaq, subject to our compliance with the other continued listing requirements. In
accordance with the Nasdaq Listing Rules, we were provided 45 calendar days, or until April 29, 2024, to submit a plan to regain
compliance with the Equity Rule (the “Compliance Plan”). We submitted our Compliance Plan on April 29, 2024, and
the Staff granted our request for an extension of 180 calendar days through September 9, 2024, to regain compliance with the Equity
Rule. We were unable to demonstrate compliance with the Equity Rule by September 9, 2024.
On September 10,
2024, the Staff formally notified us that it had determined to delist our securities from Nasdaq
based upon our continued non-compliance with the Equity Rule unless we timely request a hearing before the Nasdaq Hearings Panel
(the “Panel”). On September 17, 2024, we requested a hearing before the Panel, which
stayed any further action by Nasdaq at least pending completion of the hearing and the expiration of any extension that may be granted
by the Panel to us following the hearing.
On
May 16, 2024, we received a notice (the “Second Letter”) from the Staff of Nasdaq indicating that the bid price of our
common stock had closed below $1.00 per share for 31 consecutive business days and, as a result, we were not in compliance with Nasdaq
Listing Rule 5550(a)(2), which sets forth the minimum bid price requirement for continued listing on the Nasdaq Capital Market (the
“Bid Price Rule”). The Second Letter from Nasdaq had no immediate effect on the listing of our common stock on Nasdaq. Pursuant
to Nasdaq Listing Rule 5810(c)(3)(A), we were afforded a 180-calendar day grace period, or until November 12, 2024, to regain
compliance with the Bid Price Rule. Compliance can be achieved by evidencing a closing bid price of at least $1.00 per share for a minimum
of ten consecutive business days (but generally not more than 20 consecutive business days) during the 180-calendar day grace period.
The
hearing before the Panel occurred on October 29, 2024. By decision dated November 8, 2024, the Panel granted our request for
an extension to evidence compliance with all applicable criteria for continued listing on the Nasdaq Capital Market, including the Bid
Price Rule, through January 31, 2025, and the Equity Rule through February 18, 2025. We are considering all available
options that may enable us to timely evidence compliance with the continued listing criteria and maintain our listing on Nasdaq. There
can be no assurance that we will be successful in our efforts to maintain the listing of our common stock on the Nasdaq Capital Market.
Corporate Information
We are a majority-controlled subsidiary of Fortress
Biotech, Inc. We were incorporated under the laws of the State of Delaware on March 13, 2015. Our principal executive offices
are located at 377 Plantation Street, Worcester, Massachusetts 01605, and our telephone number is 781-652-4500. We maintain a website
on the Internet at www.mustangbio.com and our e-mail address is info@mustangbio.com. Information on our website, or any other website,
is not incorporated by reference in this prospectus. We have included our website address in this prospectus solely as an inactive textual
reference.
Implications of Being a Smaller Reporting Company
We are a smaller reporting company as defined
in the Exchange Act. We may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able
to take advantage of these scaled disclosures for so long as (i) the market value of our voting and non-voting common stock held
by non-affiliates is less than $250 million measured on the last business day of our second fiscal quarter or (ii) our annual revenue
is less than $100 million during the most recently completed fiscal year and the market value of our voting and non-voting common stock
held by non-affiliates is less than $700 million measured on the last business day of our second fiscal quarter. Specifically, as a smaller
reporting company, we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Reports
on Form 10-K and have reduced disclosure obligations regarding executive compensation, and if we are a smaller reporting company
with less than $100 million in annual revenue, we would not be required to obtain an attestation report on internal control over financial
reporting issued by our independent registered public accounting firm.
THE OFFERING
| Issuer: |
|
Mustang Bio, Inc. |
| |
|
|
| Securities Offered by Selling Stockholders: |
|
34,767,934 shares of common stock issuable upon exercise of the Warrants. |
| |
|
|
| Shares of Common Stock Outstanding Prior to this Offering: |
|
46,934,863 shares as of November 15, 2024 |
| |
|
|
| Shares of Common Stock Outstanding Assuming Exercise of All Warrants(1): |
|
81,702,797 shares |
| |
|
|
| Terms of the Offering |
|
The Selling Stockholders will determine when and how they will sell the common stock offered in this prospectus, as described in the section of this prospectus titled “Plan of Distribution.” |
| |
|
|
| Use of Proceeds: |
|
We will not receive any proceeds from the sale of the common stock by the Selling Stockholders in this offering. However, we will receive the proceeds of any cash exercise of the Warrants. We intend to use the net proceeds from any cash of the Warrants for working capital and general corporate purposes. See “Use of Proceeds.” |
| |
|
|
| Risk Factors: |
|
See “Risk Factors” incorporated by reference into this prospectus from our most current Annual Report on Form 10-K and subsequent Quarterly Reports on Form 10-Q for a discussion of certain factors you should carefully consider before deciding to invest in shares of our common stock. |
| |
|
|
| Nasdaq Capital Market Symbol: |
|
MBIO |
(1) The number of shares of common stock to be outstanding after
this offering is based on 46,934,863 shares of our common stock outstanding as of November 15, 2024, and excludes:
| |
· |
60,957,004 shares of common stock issuable upon exercise of outstanding warrants having a weighted-average exercise price of $0.29 per share; |
| |
|
|
| |
· |
11,473 shares of common stock issuable upon the vesting and settlement of outstanding restricted stock units; |
| |
|
|
| |
· |
76,112 shares of common stock issuable upon the vesting and exercise of outstanding stock options; |
| |
|
|
| |
· |
56,359 shares of our common stock issuable upon conversion of the Class A common stock, at the holders’ election; |
| |
|
|
| |
· |
16,666 shares of our common stock issuable upon conversion of the Class A Preferred Stock, at the holders’ election; |
| |
|
|
| |
· |
397,723 shares of common stock reserved for issuance and available for future grant under our 2016 Incentive Plan; |
| |
|
|
| |
· |
338,315 shares of our common stock reserved for future issuance under the Mustang Bio, Inc. 2019 Employee Stock Purchase Plan, as amended, plus any future increases, including annual automatic evergreen increases, in the number of shares of common stock reserved for issuance thereunder; and |
| |
· |
11,128,000 shares of our common stock being held in abeyance for the benefit of certain of the Selling Stockholders due to certain beneficial ownership limitations. |
Except as otherwise indicated, all information
in this prospectus supplement assumes no exercise of the outstanding stock options or warrants and no settlement of the restricted stock
units described above.
RISK FACTORS
Investing
in our common stock involves a high degree of risk. Our business is influenced by many factors that are difficult to predict, involve
uncertainties that may materially affect actual results and are often beyond our control. We have identified a number of these factors
under the heading “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2023,
as supplemented by our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2024, June 30, 2024, and September 30, 2024, which is incorporated by reference in this prospectus, as well as in other information included or incorporated by reference in
this prospectus and any prospectus supplement. You should consider carefully these risks and uncertainties before deciding to invest
in our common stock. If any of the risks identified as risk factors in the incorporated documents were to materialize, our business,
financial condition, results of operations, and future growth prospects could be materially and adversely affected. In that event, the
market price of our common stock could decline, and you could lose part of or all of your investment in our common stock. See the section
of this prospectus titled “Where You Can Find More Information.”
A sale of a substantial number of shares
of common stock by the Selling Stockholders could cause the price of our common stock to decline.
The Warrant Shares represent
a large number of shares of our common stock, and, following the effectiveness of the Registration Statement of which this prospectus
forms a part and the issuance of the Warrant Shares upon exercise of the Warrants to the Selling Stockholders (or their permitted designees),
such shares of common stock may be sold by the Selling Stockholders in the public market without restriction. If the Selling Stockholders
sell, or the market perceives that our stockholders intend to sell for various reasons, substantial amounts of the Warrant Shares in the
public market, the price of our common stock may decline. Additionally, such conditions may make it more difficult for us to sell equity
or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
USE OF PROCEEDS
We will not receive any proceeds from the sale
of the Warrant Shares covered by this prospectus and any accompanying prospectus supplement. All proceeds from the sale of the Warrant
Shares will be for the respective accounts of the Selling Stockholders named herein. However, we will receive the proceeds of any cash
exercise of the Warrants. We intend to use the net proceeds from any cash exercise of the Warrants for working capital and general corporate
purposes.
We will bear all other costs, fees and expenses
incurred in effecting the registration of the offering and sale of the Warrant Shares covered by this prospectus and any accompanying
prospectus supplement, including, without limitation, all registration and filing fees, Nasdaq listing fees and fees and expenses of our
counsel and our accountants, in accordance with the terms of the Inducement Letters. The Selling Stockholders will pay any discounts,
commissions, and fees of underwriters, selling brokers, dealer managers or similar securities industry professionals incurred by the Selling
Stockholders in disposing of the Warrant Shares covered by this prospectus.
DETERMINATION OF OFFERING PRICE
The prices at which the shares of common stock
covered by this prospectus may actually be sold will be determined by the prevailing public market price for shares of our common stock
or be negotiations between the Selling Stockholders and buyers of our common stock in private transactions or as otherwise described in
“Plan of Distribution.”
THE PRIVATE PLACEMENT
On October 24, 2024, we entered into the
Inducement Letter with the Investor in connection with the Existing Warrants. The Existing Warrants had an exercise price of $0.237 per
share.
Pursuant to the Inducement Letter, the Investor
agreed to exercise for cash the Existing Warrants at an exercise price of $0.237 per share in partial consideration for our agreement
to issue in a private placement (x) the Series B-1 Warrants to purchase up to 16,877,638 shares of common stock and (y) Series B-2
Warrants to purchase up to 16,877,638 shares of common stock. The Warrants were issued on the Closing Date. We received aggregate gross
proceeds of approximately $4 million from the exercise of the Existing Warrants by the Investor, before deducting placement agent fees
and other expenses payable by us.
Pursuant to the Inducement Letter, we agreed to
file a registration statement on Form S-3 providing for the resale of the Warrant Shares (the “Resale Registration Statement”)
within 30 days of the date of the Inducement Letter, and to use best efforts to cause the Resale Registration Statement to be declared
effective by the SEC within 90 days following the date of the Inducement Letter and to keep the Resale Registration Statement effective
at all times until the Holders no longer own any Warrants or Warrant Shares. We have filed the registration statement of which this prospectus
forms a part pursuant to the Inducement Letter.
We engaged H.C. Wainwright to act as our
exclusive agent in connection with the transactions summarized above and paid H.C. Wainwright a cash fee equal to 7.0% of the aggregate
gross proceeds from the exercise of the Existing Warrants at the exercise price. In addition, we (i) reimbursed H.C. Wainwright for
$50,000 of the fees and expenses of H.C. Wainwright’s legal counsel and other of its out-of-pocket expenses, (ii) reimbursed
H.C. Wainwright for its non-accountable expenses in the amount of $25,000, and (iii) paid a management fee equal to 1.0% of the gross
proceeds raised. We also issued to H.C. Wainwright or its designees the Placement Agent Warrants to purchase 1,012,658 Placement
Agent Warrant Shares. The Placement Agent Warrants have the same terms as the Series B-1 Warrants, except that the Placement Agent
Warrants have an exercise price equal to $0.2963 per share.
THE SELLING STOCKHOLDERS
We have prepared this prospectus to allow the
Selling Stockholders or certain of their pledgees, donees, transferees or other successors in interest to sell or otherwise dispose of,
from time to time, the Warrant Shares issuable upon exercise of the Warrants.
The Warrants held by the Selling Stockholders
contain limitations which prevent the holder from exercising such Warrants if such exercise would cause any of the Selling Stockholders,
together with certain related parties, to beneficially own a number of shares of common stock which would exceed 4.99% (or, at the election
of the holder, 9.99%) of our then outstanding shares of common stock following such exercise, excluding for the purposes of such determination,
shares of common stock issuable upon exercise of the Warrants which have not been exercised. The number of shares in the second
and fourth columns in the table below do not reflect this limitation.
In connection with certain registration rights
that we granted to the Selling Stockholders pursuant to the Inducement Letters, we filed with the SEC a registration statement on Form S-3,
of which this prospectus forms a part, with respect to the resale or other disposition of the Warrant Shares offered by this prospectus
from time to time on Nasdaq, in privately negotiated transactions or otherwise. We have agreed to prepare and file amendments and supplements
to the registration statement to the extent necessary to keep the registration statement effective for the period of time required under
our agreement with the Selling Stockholders.
All information with respect to the Selling Stockholders’
ownership of the Warrant Shares has been furnished by or on behalf of the Selling Stockholders and is as of November 15, 2024. The
percentage ownership data is based on 46,934,863 shares of common stock issued and outstanding as of November 15, 2024. We believe,
based on information supplied by the Selling Stockholders, that except as may otherwise be indicated in the table below, the Selling Stockholders
and their affiliates listed in any footnote to the table below have sole voting and dispositive power with respect to the Shares reported
as beneficially owned by them.
The Selling Stockholders may sell some, all or
none of the Warrant Shares. We do not know how long the Selling Stockholders will hold the Warrant Shares before selling them, and we
currently have no agreements, arrangements or understandings with the Selling Stockholders regarding the sale or other disposition of
any of the Warrant Shares. The Warrant Shares may be offered and sold from time to time by the Selling Stockholders pursuant to this prospectus.
Because the Selling Stockholders may sell some
or all of the Warrant Shares included in this prospectus, and because there are currently no agreements, arrangements or understandings
with respect to the sale of any of the common stock, no estimate can be given as to the number of Warrant Shares available for resale
hereby that will be held by the Selling Stockholders in the future. In addition, the Selling Stockholders may have sold, transferred or
otherwise disposed of, or may sell, transfer or otherwise dispose of, at any time and from time to time, the Warrant Shares it holds in
transactions exempt from the registration requirements of the Securities Act after the date on which they provided the information set
forth in the table below. We have, therefore, assumed for the purposes of the following table, that the Selling Stockholders will sell
all of the Warrant Shares beneficially owned by them and their affiliates listed in any footnote to the table below that are covered by
this prospectus.
Selling Stockholders Information:
| Name of Selling Stockholder | |
Number of
Shares
of Common
Stock
Beneficially
Owned
Immediately
Prior
to the
Offering | | |
Maximum
Number
of Shares of
Common
Stock
Being
Offered for
Resale
Under this
Prospectus | | |
Number of
Shares
of Common
Stock Beneficially
Owned After the
Maximum
Offered
Shares are
Sold (1) | | |
Percentage of
Outstanding
Shares of
Common
Stock
Beneficially
Owned
Immediately
Following
the Sale
of
Shares(1)(2)(3) | |
|
| Armistice Capital, LLC(4) | |
| 90,454,758 | | |
| 33,755,276 | | |
| 56,699,482 | | |
| 42.22 | % |
|
| Noam Rubenstein(5) | |
| 1,106,983 | | |
| 318,987 | | |
| 787,996 | | |
| 1.64 | % |
|
| Craig Schwabe(5) | |
| 120,185 | | |
| 34,177 | | |
| 86,008 | | |
| * | % |
|
| Michael Vasinkevich(5) | |
| 2,283,535 | | |
| 649,367 | | |
| 1,634,168 | | |
| 3.32 | % |
|
| Charles Worthman(5) | |
| 35,612 | | |
| 10,127 | | |
| 25,485 | | |
| * | % |
|
* less than 1 percent.
| (1) |
Assumes the Selling Stockholders sell all of the shares of common stock being offered by this prospectus. |
| |
|
| (2) |
Percentage calculated based upon the assumption that the Selling Stockholders sell all of the shares of common stock offered by this prospectus. |
| |
|
| (3) |
Assumes the full exercise of the Warrants held by each respective Selling Stockholder, without regard to any beneficial ownership limitations on such exercises. |
| |
|
| (4) |
Consists of: (i) 14,225,970 shares of our common stock, of which 11,128,000 shares are held by us in abeyance for the benefit of the Selling Stockholder, (ii) 42,473,512 shares of our common stock underlying previously issued warrants, and (iii) 33,755,276 shares of our common stock being offered by this prospectus. The securities are directly held by Armistice Capital Master Fund Ltd., a Cayman Islands exempted company (the “Master Fund”), and may be deemed to be beneficially owned by: (i) Armistice Capital, LLC (“Armistice Capital”), as the investment manager of the Master Fund; and (ii) Steven Boyd, as the Managing Member of Armistice Capital. The warrants are subject to a beneficial ownership limitation of 4.99% or 9.99%, as applicable, which limitation restricts the holder from exercising that portion of the warrants that would result in such holder and its affiliates owning, after exercise, a number of shares of common stock in excess of the beneficial ownership limitation. The address of Armistice Capital Master Fund Ltd. is c/o Armistice Capital, LLC, 510 Madison Avenue, 7th Floor, New York, NY 10022. |
| |
|
| (5) |
Each of these Selling Stockholders is affiliated with H.C. Wainwright & Co., LLC, a registered broker dealer with a registered address of H.C. Wainwright & Co., LLC, 430 Park Ave, 3rd Floor, New York, NY 10022, and has sole voting and dispositive power over the securities held. The number of shares beneficially owned prior to this offering consist of shares of common stock issuable upon exercise of placement agent warrants, which were received as compensation. Each of these Selling Stockholders acquired the Placement Agent Warrants in the ordinary course of business and, at the time the Placement Agent Warrants were acquired, each of these Selling Stockholders had no agreement or understanding, directly or indirectly, with any person to distribute such securities. |
DESCRIPTION OF SECURITIES BEING REGISTERED
When used herein, the terms
“Company,” “we,” “our,” and “us” refer to Mustang Bio, Inc.
Capital Stock
We are authorized to issue 200,000,000 shares
of common stock, par value of $0.0001 per share, of which 1,000,000 shares are designated as Class A common stock, and 2,000,000
of preferred stock, $0.0001 par value per share, of which 250,000 are designated as Class A Preferred Stock.
Common Stock
The holders of common stock are entitled to one
vote per share held.
As of November 15, 2024, there were 46,934,863
shares of our common stock outstanding held by 70 stockholders of record.
The undesignated preferred stock may be issued
from time to time in one or more series. Our board of directors is authorized to determine or alter the dividend rights, dividend rate,
conversion rights, voting rights, rights and terms of redemption (including sinking fund provisions, if any), the redemption price or
prices, the liquidation preferences and other designations, powers, preferences and relative, participating, optional or other special
rights, if any, and the qualifications, limitations and restrictions granted to or imposed upon any wholly unissued series of preferred
stock, and to fix the number of shares of any series of preferred stock (but not below the number of shares of any such series then outstanding).
Class A Common Stock
The holders of Class A common stock are entitled
to the number of votes equal to the number of whole shares of common stock into which the shares of Class A Common Shares held by
such holder are convertible. For a period of ten years from issuance, the holders of the Class A common stock have the right to appoint
one member of the Board of Directors of Mustang. To date, the holders of Class A common stock have not yet appointed such director.
Class A Preferred Stock
The Class A Preferred Stock is identical
to undesignated common stock other than as to voting rights, conversion rights, and the PIK dividend right.
The holders of the outstanding shares of Class A
Preferred Stock receive on each January 1 (each a “PIK Dividend Payment Date”) after the original issuance date of the
Class A Preferred Stock until the date all outstanding Class A Preferred Stock is converted into common stock or redeemed (and
the purchase price is paid in full), pro rata per share dividends paid in additional fully paid and non-assessable shares of common stock
such that the aggregate number of shares of common stock issued pursuant to such PIK dividend is equal to 2.5% of the Corporation’s
fully-diluted outstanding capitalization on the date that is one business day prior to any PIK Dividend Payment Date. In the event the
Class A Preferred Stock converts into common stock, the holders shall receive all PIK dividends accrued through the date of such
conversion. No dividend or other distribution shall be paid, or declared and set apart for payment (other than dividends payable solely
in capital stock on the capital stock) on the shares of common stock until all PIK dividends on the Class A Preferred Stock shall
have been paid or declared and set apart for payment. All dividends are non-cumulative.
On any matter presented to the stockholders for
their action or consideration at any meeting of stockholders (or by written consent of stockholders in lieu of meeting), each holder of
outstanding shares of Class A Preferred Stock shall be entitled to cast for each share of Class A Preferred Stock held by such
holder as of the record date for determining stockholders entitled to vote on such matter, the number of votes that is equal to one and
one-tenth (1.1) times a fraction, the numerator of which is the sum of (A) the number of shares of outstanding common stock and (B) the
whole shares of common stock in to which the shares of outstanding Class A common stock and the Class A Preferred Stock are
convertible, and the denominator of which is number of shares of outstanding Class A Preferred Stock. Thus, the Class A Preferred
Stock will at all times constitute a voting majority.
Each share of Class A Preferred Stock is
convertible, at the option of the holder, into one fully paid and nonassessable share of common stock, subject to certain adjustments.
If the Company, at any time effects a subdivision or combination of the outstanding common stock (by any stock split, stock dividend,
recapitalization, reverse stock split or otherwise), the applicable conversion ratio in effect immediately before that subdivision is
proportionately decreased or increased, as applicable, so that the number of shares of common stock issuable on conversion of each share
of Class A Preferred Stock shall be increased or decreased, as applicable, in proportion to such increase or decrease in the aggregate
number of shares of common stock outstanding. Additionally, if any reorganization, recapitalization, reclassification, consolidation or
merger involving the Company occurs in which the common stock (but not the Class A Preferred Stock) is converted into or exchanged
for securities, cash or other property, then each share of Class A Preferred Stock becomes convertible into the kind and amount of
securities, cash or other property which a holder of the number of shares of common stock of the Company issuable upon conversion of one
share of the Class A Preferred Stock immediately prior to such reorganization, recapitalization, reclassification, consolidation
or merger would have been entitled to receive pursuant to such transaction.
Additional Features
Other features of our capital stock include:
| |
· |
Dividend Rights. The holders of outstanding shares of our common stock, including Class A common stock, are entitled to receive dividends out of funds legally available at the times and in the amounts that our Board of Directors may determine. All dividends are non-cumulative. |
| |
· |
Voting Rights. The holders of our common stock are entitled to one vote for each share of common stock held on all matters submitted to a vote of the stockholders, including the election of directors. Our certificate of incorporation and bylaws do not provide for cumulative voting rights. |
| |
· |
No Preemptive or Similar Rights. The holders of our common stock have no preemptive, conversion, or subscription rights, and there are no redemption or sinking fund provisions applicable to our common stock. |
| |
· |
Right to Receive Liquidation Distributions. Upon our liquidation, dissolution, or winding-up, the assets legally available for distribution to our stockholders would be distributable ratably among the holders of our common stock, including Class A common stock, outstanding at that time after payment of other claims of creditors, if any. |
| |
· |
Fully Paid and Non-Assessable. All of the outstanding shares of our common stock, including Class A common stock, and the Class A Preferred Stock are duly issued, fully paid and non-assessable. |
Anti-Takeover Effects of Various Provisions of Delaware Law and
Mustang Bio’s Certificate of Incorporation and Bylaws
Provisions of the General
Corporation Law of the State of Delaware (“DGCL”) and our Certificate of Incorporation and Bylaws could make it more difficult
to acquire the Company by means of a tender offer, a proxy contest or otherwise, or to remove incumbent officers and directors. These
provisions, including those summarized below, may encourage certain types of coercive takeover practices and takeover bids.
Delaware
Anti-Takeover Statute. In general, Section 203 of the DGCL prohibits a publicly held Delaware corporation
from engaging in a “business combination” with an “interested stockholder” for a period of three years following
the time the person became an interested stockholder, unless the business combination or the acquisition of shares that resulted in a
stockholder becoming an interested stockholder is approved in a prescribed manner. Generally, a “business combination” includes
a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. Generally, an “interested
stockholder” is a person who, together with affiliates and associates, owns (or within three years prior to the determination of
interested stockholder status did own) 15% or more of a corporation’s voting stock. However, our Certificate of Incorporation provides
that we are not subject to the anti-takeover provisions of Section 203 of the DGCL.
Removal.
Subject to the rights of any holders of any outstanding series of our preferred stock, stockholders may remove our directors with or
without cause, by a vote of the stockholders. Removal will require the affirmative vote of holders of a majority of our voting stock.
Size
of Board and Vacancies. Our Bylaws provide that the number of directors be fixed exclusively by the board of directors.
Any vacancies may only be filled by a majority of the remaining directors, even if less than a quorum is present, or by a sole remaining
director. Any director appointed to fill a vacancy on our board of directors will be appointed until the next annual meeting and until
his or her successor has been elected and qualified.
Requirements
for Advance Notification of Stockholder Nominations and Proposals. Our Bylaws establish advance notice procedures
with respect to stockholder proposals and nomination of candidates for election as directors other than nominations made by or at the
direction of its board of directors or a committee of our board of directors.
Undesignated
Preferred Stock. Our board of directors is authorized to issue up to 2,000,000 shares of preferred stock without
additional stockholder approval, which preferred stock could have voting rights or conversion rights that, if exercised, could adversely
affect the voting power of the holders of common stock. The issuance of shares of preferred stock may have the effect of delaying, deferring
or preventing a change in control of the Company without any action by the Company’s stockholders.
Limitation on Liability of Directors and Indemnification
of Directors and Officers
Elimination
of Liability of Directors. The DGCL authorizes corporations to limit or eliminate the personal liability of directors
to corporations and their stockholders for monetary damages for breaches of directors’ fiduciary duties as directors, and our Certificate
of Incorporation includes such an exculpation provision. Our Certificate of Incorporation provides that, to the fullest extent permitted
by the DGCL, no director will be personally liable to us or to our stockholders for monetary damages for breach of fiduciary duty as a
director except for liability (i) for any breach of the director’s duty of loyalty to the Company or its stockholders, (ii) for
acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under Section 174
of the DGCL, or (iv) for any transaction from which the director derived any improper personal benefit. While our Certificate of
Incorporation provides directors with protection from awards for monetary damages for breaches of their duty of care, it does not eliminate
this duty. Accordingly, our Certificate of Incorporation has no effect on the availability of equitable remedies such as an injunction
or rescission based on a director’s breach of his or her duty of care. The provisions apply to an officer of Mustang Bio only if
he or she is a director of Mustang Bio and is acting in his or her capacity as director, and do not apply to officers of Mustang Bio who
are not directors.
Indemnification
of Directors, Officers and Employees. Our Bylaws require us to indemnify any person who was or is a party or is threatened
to be made a party to, or was otherwise involved in, a legal proceeding by reason of the fact that he or she is or was a director, officer
or employee or agent of Mustang Bio or, while a director, officer or employee of Mustang Bio, or is or was serving at the request of Mustang
Bio as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses
(including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection
with such action, suit or proceeding if he or she acted in good faith and in a manner he reasonably believed to be in or not opposed to
the best interests of the Mustang Bio and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or
her conduct was unlawful. The termination of any action, suit or proceeding by judgment, order, settlement, conviction or upon a plea
of nolo contendere or its equivalent, would not, of itself, create a presumption that the person did not act in good faith and
in a manner which he or she reasonably believed to be in or not opposed to the best interests of Mustang Bio and, with respect to any
criminal action or proceeding, had reasonable cause to believe that his conduct was unlawful. We are authorized under our Bylaws
to carry directors’ and officers’ insurance protecting us, any director, officer or employee or agent of ours or, against
any expense, liability or loss, whether or not we have the power to indemnify the person under the DGCL. We may, to the extent authorized
from time to time, indemnify any of our agents to the fullest extent permitted with respect to directors, officers and employees in our
Bylaws.
The limitation of liability
and indemnification provisions in our Certificate of Incorporation and Bylaws may discourage stockholders from bringing a lawsuit against
our directors for breach of fiduciary duty. These provisions also may reduce the likelihood of derivative litigation against our directors
and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. By its terms, the indemnification
provided for in our Bylaws is not exclusive of any other rights that the indemnified party may be or become entitled to under any law,
agreement, vote of stockholders or directors, provisions of our Certificate of Incorporation or Bylaws or otherwise. Any amendment, alteration
or repeal of our Bylaws’ indemnification provisions is, by the terms of our Bylaws, prospective only and will not adversely affect
the rights of any indemnity in effect at the time of any act or omission occurring prior to such amendment, alteration or repeal.
Series B-1 Warrants and Series B-2 Warrants Issued in
October 2024
Exercisability
Each Series B-1 Warrant and Series B-2
Warrant has an exercise price equal to $0.27 per share. The Series B-1 Warrants are immediately exercisable on or after
the Closing Date until the five-year anniversary of the Closing Date. The Series B-2 Warrants are immediately exercisable on or after
the Closing Date until the 12 month anniversary of the Closing Date. The exercise price and number of the Series B-1 Warrants
and the Series B-2 Warrant issuable upon exercise of the Series B-1 Warrants and the Series B-2 Warrant are subject to
appropriate adjustment in the event of stock dividends, stock splits, subsequent rights offerings, pro rata distributions, reorganizations,
or similar events affecting the common stock and the exercise price.
Exercise Limitation
The Series B-1 Warrants and the Series B-2
Warrants are exercisable, at the option of each holder, respectively, in whole or in part, by delivering a duly executed exercise
notice accompanied by payment in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless
exercise discussed below). A holder (together with their affiliates) may not exercise any portion of their Series B-1 Warrants and
the Series B-2 Warrants to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the
outstanding common stock immediately after exercise, except that upon prior notice from the holder, the holder may increase or decrease
the amount of ownership of outstanding stock after exercising their Series B-1 Warrants and the Series B-2 Warrants up to 9.99%
of the number of shares of common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined
in accordance with the terms of the Series B-1 Warrants and the Series B-2 Warrants, provided that any increase will not be
effective until 61 days following notice to the Company.
Exercise Price
The exercise price per whole share of common stock
purchasable upon exercise of the Series B-1 and Series B-2 Warrants is $0.27. The exercise price is subject to appropriate adjustment
in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting
our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Cashless Exercise
If, at the time a holder exercises its Series B-1
Warrants and the Series B-2 Warrant, a registration statement registering the resale of the underlying shares of common stock by
the holder, under the Securities Act is not then effective or available, then in lieu of making the cash payment otherwise contemplated
to be made upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either
in whole or in part), the net number of shares of common stock determined according to a formula set forth in the Series B-1 Warrants
and the Series B-2 Warrant.
Transferability
Subject to applicable laws, the Series B-1
Warrants and the Series B-2 Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing
The Series B-1 Warrants and the Series B-2
Warrants are not listed on any securities exchange or nationally recognized trading system.
Fundamental Transactions
If at any time the Series B-1 Warrants or
the Series B-2 Warrants are outstanding, the Company, either directly or indirectly, in one or more related transactions, effects
a Fundamental Transaction (as defined in the Series B-1 Warrants and the Series B-2 Warrants), a holder of such warrants is
entitled to receive the number of shares of common stock of the successor or acquiring corporation, or of the Company if the Company is
the surviving corporation, and any additional consideration receivable as a result of the Fundamental Transaction by such holder of the
number of shares of common stock for which the Series B-1 Warrants and the Series B-2 Warrants are exercisable immediately prior
to the Fundamental Transaction. As an alternative, the holder may, at their option, in the event of a Fundamental Transaction, exercisable
at any time concurrently with, or within 30 days after, the consummation of the Fundamental Transaction (or, if later, the date of the
public announcement of the applicable fundamental transaction), cause the Company to purchase the unexercised portion of the Series B-1
Warrants and the Series B-2 Warrants from the holder, respectively, by paying to the holder, as applicable, an amount of cash equal
to the Black Scholes Value (as defined in the Series B-1 Warrants and the Series B-2 Warrants) of the remaining unexercised
portion of the Series B-1 Warrants or the Series B-2 Warrants on the date of the consummation of such Fundamental Transaction.
Rights as a Stockholder
Except as otherwise provided in the Series B-1
Warrants and the Series B-2 Warrants, or by virtue of the holder’s ownership of shares of common stock, such holder does not
have the rights or privileges of a holder of common stock, including any voting rights, until such holder exercises such holder’s
Series B-1 Warrants or Series B-2 Warrants. The Series B-1 Warrants and the Series B-2 Warrants provide that the holders
of the Series B-1 Warrants and the Series B-2 Warrants have the right to participate in certain distributions or dividends paid
on shares of common stock.
Waivers and Amendments
The Series B-1 Warrants and the Series B-2
Warrants may be modified or amended, or the provisions of the Series B-1 Warrants or the Series B-2 Warrants waived, with the
Company’s and the holder’s written consent.
Governing Law
The Series B-1 Warrants and the Series B-2
Warrants are governed by New York law.
Placement Agent Warrants Issued October 2024
Exercisability
Each Placement Agent Warrant has an exercise price
equal to $0.2963 per share. The Placement Agent Warrants became immediately exercisable upon issuance and will remain exercisable
until the five-year anniversary of the Closing Date. The exercise price and number of the Placement Agent Warrants issuable upon exercise
of the Placement Agent Warrants are subject to appropriate adjustment in the event of stock dividends, stock splits, subsequent rights
offerings, pro rata distributions, reorganizations, or similar events affecting the common stock and the exercise price.
Exercise
The Placement Agent Warrants are exercisable,
at the option of each holder, respectively, in whole or in part, by delivering a duly executed exercise notice accompanied by payment
in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise discussed below).
A holder (together with their affiliates) may not exercise any portion of their Placement Agent Warrants to the extent that the holder
would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding common stock immediately after exercise, except
that upon prior notice from the holder, the holder may increase or decrease the amount of ownership of outstanding stock after exercising
their Placement Agent Warrants up to 9.99% of the number of shares of common stock outstanding immediately after giving effect to the
exercise, as such percentage ownership is determined in accordance with the terms of the Placement Agent Warrants, provided that any
increase will not be effective until 61 days following notice to the Company.
Exercise Price
The exercise price per whole share of common stock
purchasable upon exercise of the Placement Agent Warrants is $0.2963. The exercise price is subject to appropriate adjustment in the event
of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common
stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Cashless Exercise
If, at the time a holder exercises its Placement
Agent Warrants, a registration statement registering the resale of the underlying shares of common stock by the holder, under the Securities
Act is not then effective or available, then in lieu of making the cash payment otherwise contemplated to be made upon such exercise in
payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part), the
net number of shares of common stock determined according to a formula set forth in the Placement Agent Warrants.
Transferability
Subject to applicable laws, the Placement Agent
Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing
The Placement Agent Warrants are not listed on
any securities exchange or nationally recognized trading system.
Fundamental Transactions
If at any time the Placement Agent Warrants are
outstanding, the Company, either directly or indirectly, in one or more related transactions, effects a Fundamental Transaction (as defined
in the Placement Agent Warrants), a holder of such warrants is entitled to receive the number of shares of common stock of the successor
or acquiring corporation, or of the Company if the Company is the surviving corporation, and any additional consideration receivable as
a result of the Fundamental Transaction by such holder of the number of shares of common stock for which the Placement Agent Warrants
are exercisable immediately prior to the Fundamental Transaction. As an alternative, the holder may, at their option, in the event of
a Fundamental Transaction, exercisable at any time concurrently with, or within 30 days after, the consummation of the Fundamental Transaction
(or, if later, the date of the public announcement of the applicable fundamental transaction), cause the Company to purchase the unexercised
portion of the Placement Agent Warrants from the holder, respectively, by paying to the holder, as applicable, an amount of cash equal
to the Black Scholes Value (as defined in the Placement Agent Warrants) of the remaining unexercised portion of the Placement Agent Warrants
on the date of the consummation of such Fundamental Transaction.
Rights as a Stockholder
Except as otherwise provided in the Placement
Agent Warrants, or by virtue of the holder’s ownership of shares of common stock, such holder does not have the rights or privileges
of a holder of common stock, including any voting rights, until such holder exercises such holder’s Placement Agent Warrants. The
Placement Agent Warrants provide that the holders of the Placement Agent Warrants have the right to participate in certain distributions
or dividends, other than cash, paid on shares of common stock.
Waivers and Amendments
The Placement Agent Warrants may be modified or
amended, or the provisions of the Placement Agent Warrants waived, with the Company’s and the holder’s written consent.
Governing Law
The Placement Agent Warrants are governed by New
York law.
CERTAIN U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following discussion summarizes certain U.S.
federal income tax considerations of the acquisition, ownership and disposition of the common stock offered by this prospectus but does
not purport to be a complete analysis of all potential tax effects. This discussion does not address effects of other U.S. federal tax
laws, such as estate and gift tax laws, or of state, local, non-U.S. or other tax considerations that may be relevant to a purchaser or
holder of the common stock in light of their particular circumstances. This discussion is based on the U.S. Internal Revenue Code of 1986,
as amended (the “Code”), the Treasury regulations promulgated thereunder, judicial decisions, and published rulings and administrative
pronouncements of the U.S. Internal Revenue Service (the “IRS”), in each case as of the date hereof. These authorities may
change, possibly with retroactive effect, or may be subject to differing interpretations that may adversely affect a holder of the common
stock. There can be no assurance the IRS or a court will not take a contrary position to that discussed below regarding the acquisition,
ownership and disposition of the common stock.
This discussion is limited to holders that hold
the common stock as a capital asset within the meaning of Section 1221 of the Code (generally property held for investment). This
discussion does not describe all of the U.S. federal income tax consequences that may be relevant to a holder in light of its particular
circumstances, including the impact of the alternative minimum tax and of the Medicare contribution tax on net investment income. In addition,
it does not address consequences for holders subject to special rules, including without limitation:
| |
● |
U.S. expatriates and former citizens or long-term residents of the United States; |
| |
● |
persons holding the common stock as part of a hedge, straddle, conversion, or other integrated transaction; |
| |
● |
banks, insurance companies, and other financial institutions; |
| |
● |
brokers, dealers or traders in securities; |
| |
● |
“controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| |
● |
S corporations or entities or arrangements that are treated as partnerships for U.S. federal income tax purposes (and investors therein); |
| |
● |
tax-exempt organizations or governmental organizations; |
| |
● |
real estate investment trusts or regulated investment companies; |
| |
● |
U.S. persons whose functional currency is not the U.S. dollar; |
| |
● |
persons subject to special tax accounting rules; |
| |
● |
persons who hold or receive the common stock pursuant to the exercise of any employee stock option or otherwise as compensation; |
| |
● |
persons deemed to sell our common stock under the constructive sale provisions of the Code; |
| |
● |
tax-qualified retirement plans, individual retirement accounts or other tax-deferred accounts; and |
| |
● |
“qualified foreign pension funds” as defined in Section 897(l)(2) of the Code. |
If a partnership (or any other entity treated
as a partnership for U.S. federal income tax purposes) holds the common stock, the U.S. federal income tax treatment of a partner of that
partnership generally will depend upon the status of the partner and the activities of the partnership. If you are a partnership or a
partner of a partnership holding the common stock, you should consult your tax advisors as to the particular U.S. federal income tax consequences
of holding and disposing of the common stock.
This discussion is for informational purposes
only and is not tax advice. You should consult your own independent tax advisor concerning the application of the U.S. federal income
tax laws to your particular circumstances as well as any tax consequences for the acquisition, ownership, or disposition of the common
stock arising under other U.S. federal tax laws and the laws of any state, local or non-U.S. tax jurisdiction or under any applicable
income tax treaty.
For purposes of this discussion, a “U.S. holder” is a beneficial
owner of our common stock that, for U.S. federal income tax purposes, is or is treated as:
| |
● |
an individual who is a citizen or resident of the United States; |
| |
● |
a corporation created or organized under the laws of the United States, any state thereof, or the District of Columbia; |
| |
● |
an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| |
● |
a trust that (i) is subject to the primary supervision of a U.S. court and the control of one or more “United States persons” (within the meaning of Section 770 1(a)(30) of the Code) or (ii) has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
A “non-U.S. holder” is any beneficial owner of our common
stock that is not a U.S. holder.
U.S. Holders
Distributions in General
If distributions are made with respect to the
common stock, such distributions will be treated as dividends to the extent of our current or accumulated earnings and profits as determined
under the Code. Subject to customary conditions and limitations, dividends will be eligible for the dividends-received deduction in the
case of U.S. holders that are (or are treated for U.S. federal income tax purposes) as corporations. Dividends paid to non-corporate U.S.
holders generally will qualify for taxation at preferential rates if those holders meet certain holding period and other applicable requirements.
Dividends received by non-corporate U.S. holders may also be subject to the additional 3.8% tax on net investment income. Any portion
of a distribution that exceeds our current and accumulated earnings and profits will first be applied to reduce a U.S. holder’s
tax basis in the common stock, but not below zero. Distributions in excess of our current and accumulated earnings and profits and in
excess of a U.S. holder’s tax basis in its shares will be taxable as gain from the disposition of the common stock, the tax treatment
of which is discussed below.
Extraordinary Dividends
Dividends that exceed certain thresholds in relation
to a U.S. holder’s tax basis in the common stock could be characterized as “extraordinary dividends” under Section 1059
of the Code. Corporate U.S. holders that have held our common stock for two years or less before the dividend announcement date and that
receive an extraordinary dividend will generally be required to reduce their tax basis in the stock by the nontaxed portion of the dividend
due to the dividends-received deduction. If the amount of reduction exceeds the U.S. holder’s tax basis in the stock, the excess
will be taxable as gain from the disposition of the stock, the tax treatment of which is discussed below. Non-corporate U.S. holders that
receive an extraordinary dividend will be required to treat any losses on the sale of our common stock as long-term capital losses to
the extent of the extraordinary dividends such U.S. holders receive that qualify for taxation as the preferential rates discussed above
under “— Distributions in General.” U.S. holders are urged to consult their tax advisors with respect to
the eligibility for and amount of any dividend received deduction and the application of Section 1059 of the Code to any dividends
they receive.
Disposition of Common Stock by Sale, Exchange or Redemption
Upon any sale or disposition (other than certain
redemptions, as discussed below) of the Common Stock, a U.S. holder generally will recognize capital gain or loss equal to the difference
between the amount realized by the U.S. holder and the U.S. holder’s adjusted tax basis in the Common Stock. Such capital gain or
loss will be long-term capital gain or loss if the U.S. holder’s holding period for the Common Stock is longer than one year. Non-corporate
U.S. holders may be eligible for preferential tax rates on long-term capital gains but also may be subject to the additional 3.8% tax
on net investment income. The deductibility of capital losses is subject to limitations.
A redemption of the Common Stock will be treated
as a sale or exchange described in the preceding paragraph if the redemption, based on the facts and circumstances, is treated for U.S.
federal income tax purposes as (i) a “complete termination” of your interest in the Common Stock, (ii) a “substantially
disproportionate” redemption of your Common Stock, or (iii) is “not essentially equivalent to a dividend”, each
within the meaning of Section 302 of the Code. In determining whether any of these tests has been met, you must take into account
not only the Common Stock and other equity interests that you actually own but also other equity interests that you constructively own
under U.S. federal income tax rules.
If you meet none of the alternative tests described
above, the redemption will be treated as a distribution subject to the rules described under “—Distributions In General.”
If a redemption of the Common Stock is treated as a distribution that is taxable as a dividend, you are urged to consult your tax advisor
regarding the allocation of your tax basis as between the redeemed and remaining shares of Common Stock.
Information Reporting and Backup Withholding
We or an applicable withholding agent will report
to our U.S. holders and the IRS the amount of dividends (including deemed dividends) paid during each year and the amount of any tax withheld
with respect to the Common Stock. Certain non-corporate U.S. holders may be subject to U.S. backup withholding at a rate of 28% on payments
of dividends on the Common Stock unless the holder furnishes the payor or its agent with a taxpayer identification number, certified under
penalties of perjury, and certain other information or otherwise establishes an exemption from backup withholding. Backup withholding
tax is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against
a U.S. holder’s U.S. federal income tax liability, provided the U.S. holder timely furnishes the required information to the IRS.
Non-U.S. Holders
Distributions
If distributions are made with respect to the
common stock, such distributions will be treated as dividends to the extent of our current or accumulated earnings and profits as determined
under the Code and may be subject to withholding as discussed below. Any portion of a distribution that exceeds our current and accumulated
earnings and profits will first be applied to reduce the Non-U.S. holder’s basis in the common stock, but not below zero. If the
distribution exceeds our current and accumulated earnings and profits and the Non-U.S. holder’s basis, the excess will be treated
as gain from the disposition of the common stock, the tax treatment of which is discussed below.
In addition, if we are classified as a U.S. real
property holding corporation (a “USRPHC”) within the meaning of Section 897(c) of the Code and any distribution
exceeds our current and accumulated earnings and profits, we will need to satisfy our withholding requirements either by (a) treating
the entire distribution (even if in excess of earnings and profits) as a dividend subject to the withholding rules described below
and withhold at a minimum rate of 15% or such lower rate as may be specified by an applicable income tax treaty for distributions from
a USRPHC; or (b) treating (i) only the amount of the distribution equal to our reasonable estimate of our current and accumulated
earnings and profits as a dividend subject to the withholding rules in the following paragraph; and (ii) the excess portion
of the distribution as subject to withholding at a rate of 15% (or such lower rate as may be specified by an applicable income tax treaty),
as if such excess were the result of a sale of shares in a USRPHC, with a credit generally allowed against the Non-U.S. holder’s
U.S. federal income tax liability for the tax withheld from such excess. We believe that we currently are not a USRPHC, and we do not
expect to become a USRPHC for the foreseeable future (see discussion of USRPHCs below under “— Disposition of Common
Stock, Including Redemptions”).
Dividends (including amounts distributed by a
USRPHC and subject to withholding as dividends per the preceding paragraph) paid to a Non-U.S. holder of the common stock will be subject
to withholding of U.S. federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. However,
dividends that are treated as being effectively connected with the conduct of a trade or business by the Non-U.S. holder within the United
States (and, where a tax treaty applies, are attributable to a permanent establishment maintained by the Non-U.S. holder in the United
States) are not subject to this withholding tax, provided that certain certification and disclosure requirements are satisfied including
completing IRS Form W-8ECI (or other applicable form). Instead, such dividends are subject to U.S. federal income tax on a net income
basis in the same manner as if the Non-U.S. holder were a United States person (as defined under the Code), unless an applicable income
tax treaty provides otherwise. Any such effectively connected dividends received by a foreign corporation may be subject to an additional
“branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty.
A Non-U.S. holder of the common stock who wishes
to claim the benefit of an applicable treaty rate and avoid backup withholding for dividends, as discussed below, will be required to
(i) complete IRS Form W-8BEN or Form W-8BEN-E (or other applicable form) and certify under penalty of perjury that such
holder is not a United States person as defined under the Code and is eligible for treaty benefits, or (ii) if the common stock is
held through certain foreign intermediaries, satisfy the relevant certification requirements of applicable Treasury regulations. A Non-U.S.
holder of the common stock eligible for a reduced rate of U.S. withholding tax pursuant to an income tax treaty may obtain a refund of
any excess amounts withheld by timely filing an appropriate claim for refund with the U.S. Internal Revenue Service.
Disposition of common stock, Including Redemptions
Any gain realized by a Non-U.S. holder on the
disposition of the common stock generally will not be subject to U.S. federal income or withholding tax unless:
| |
● |
the gain is effectively connected with the conduct of a trade or business by the Non-U.S. holder in the United States (and, if required by an applicable income tax treaty, attributable to a permanent establishment maintained by the Non-U.S. holder in the United States); |
| |
|
|
| |
● |
the Non-U.S. holder is an individual who is present in the United States for 183 days or more in the taxable year of disposition, and certain other conditions are met; or |
| |
|
|
| |
● |
we are or have been a USRPHC, as defined in Section 897(c) of the Code, and a Non-U.S. holder owned directly or pursuant to applicable attribution rules at any time during the five-year period ending on the date of disposition more than 5% of the common stock — assuming that the common stock is regularly traded on an established securities market, within the meaning of Section 897(c)(3) of the Code. |
A Non-U.S. holder described in the first bullet
point immediately above will generally be subject to tax on the gain derived from the sale under regular graduated U.S. federal income
tax rates in the same manner as if the Non-U.S. holder were a United States person as defined under the Code, and, if it is a corporation,
may also be subject to branch profits tax equal of 30% (generally applicable to its effectively connected earnings and profits) or at
such lower rate as may be specified by an applicable income tax treaty.
An individual Non-U.S. holder described in the
second bullet point immediately above will be subject to a flat 30% tax (or at such reduced rate as may be provided by an applicable tax
treaty) on the gain derived from the sale, which may be offset by U.S. source capital losses, even if the individual is not considered
a resident of the United States for U.S. federal income tax purposes.
A Non-U.S. holder described in the third bullet
point above will be subject to U.S. federal income tax under regular graduated U.S. federal income tax rates with respect to the gain
realized in the same manner as if the Non-U.S. holder were a United States person as defined under the Code. A corporation is a USRPHC
if it is a U.S. corporation and the fair market value of its U.S. real property interests equals or exceeds 50% of the sum of the fair
market value of its worldwide real property interests and its other assets used or held for use in a trade or business. We believe that
we currently are not a USRPHC for U.S. federal income tax purposes, and we do not expect to become a USRPHC for the foreseeable future.
Our common stock is listed on the Nasdaq Capital Market and we believe that, for as long as we continue to be so listed, our common stock
will be treated as regularly traded on an established securities market. However, if we become a USRPHC and our common stock is regularly
traded on an established securities market, a Non-U.S. holder generally will be subject to U.S. federal income tax on any gain from the
disposition of such stock if such Non-U.S. holder has owned or is deemed to have owned more than 5% of our common stock, at any time within
the shorter of the five-year period preceding the disposition or such holder’s holding period for such stock.
If a Non-U.S. holder is subject to U.S. federal
income tax on any sale, exchange, redemption (except as discussed below), or other disposition of the common stock, the Non-U.S. holder
will recognize capital gain or loss equal to the difference between the amount realized by the Non-U.S. holder and the Non-U.S. holder’s
adjusted tax basis in the common stock. Such capital gain or loss will be long-term capital gain or loss if the Non-U.S. holder’s
holding period for the common stock is longer than one year. A Non-U.S. holder should consult its own independent tax advisors with respect
to applicable tax rates and netting rules for capital gains and losses. Certain limitations exist on the deduction of capital losses
by both corporate and non-corporate taxpayers.
If a Non-U.S. holder is subject to U.S. federal
income tax on any disposition of the common stock, a redemption of shares of the common stock will be a taxable event. If the redemption
is treated as a sale or exchange, instead of as a dividend, a Non-U.S. holder generally will recognize capital gain or loss, equal to
the difference between the amount of cash received and fair market value of any property received and the Non-U.S. holder’s adjusted
tax basis in the common stock redeemed (except that to the extent that any cash received is attributable to any accrued but unpaid dividends),
and such capital gain or loss will be long-term capital gain or loss if the Non-U.S. holder’s holding period for such common stock
exceeds one year. A payment made in redemption of the common stock may be treated as a dividend (subject to taxation as discussed above
under “—Disposition of Common Stock, Including Redemptions”), rather than as payment in exchange for the
common stock, in the same circumstances discussed above under “— Disposition of Common Stock, Including Redemptions.”
Each Non-U.S. holder of the common stock should consult its own independent tax advisors to determine whether a payment made in redemption
of the common stock will be treated as a dividend or as payment in exchange for the common stock.
Information reporting and backup withholding.
We must annually report to the IRS and to each
Non-U.S. holder the amount of dividends (including constructive dividends) paid to such Non-U.S. holder and the tax withheld with respect
to such dividends, regardless of whether withholding was required. Copies of the information returns reporting such dividends and withholding
may also be made available under the provisions of an applicable tax treaty or agreement with the tax authorities in the country in which
the non-U.S. holder resides. U.S. backup withholding will generally apply to the payment of dividends to non-U.S. holders unless such
non-U.S. holders furnish to the payor an IRS Form W-8BEN or Form W-8BEN-E (or other applicable form) or otherwise establish
an exemption.
Payment by a U.S. office of a broker of the proceeds
of a sale of shares of our common stock is subject to both backup withholding and information reporting unless the non-U.S. holder, or
beneficial owner thereof, as applicable, certifies that it is a non-U.S. holder on Form W-8BEN or Form W-8BEN-E (or other suitable
substitute or successor form), or otherwise establishes an exemption. Subject to certain exceptions, backup withholding and information
reporting generally will not apply to a payment of proceeds from the sale of shares of our common stock if such sale is effected through
a foreign office of a broker, provided that the broker does not have certain U.S. connections. Any amount withheld under the backup withholding
rules from a payment to a non-U.S. holder is allowable as a credit against such holder’s U.S. federal income tax liability
(if any), which may entitle the holder to a refund if in excess of such liability, provided that the holder timely provides the required
information to the IRS. Non-U.S. holders are urged to consult their own tax advisers regarding the application of backup withholding in
their particular circumstances and the availability of and procedure for obtaining an exemption from backup withholding under current
Treasury Regulations.
Foreign Account Tax Compliance Act.
Sections 1471 to 1474 of the Code (such sections,
and the Treasury Regulations and administrative guidance issued thereunder, commonly referred to as FATCA) impose a 30% U.S. withholding
tax on certain “withholdable payments” made to a “foreign financial institution” or a “nonfinancial foreign
entity.” “Withholdable payments” include payments of dividends and the gross proceeds from a disposition of certain
property (such as shares of our common stock), if such disposition occurs after December 31, 2018. In general, if a holder is a “foreign
financial institution” (which includes investment entities such as hedge funds and private equity funds), the 30% withholding tax
will apply to withholdable payments made to such holder, unless such holder enters into an agreement with the U.S. Department of Treasury
to collect and provide substantial information regarding its U.S. account holders, including certain account holders that are foreign
entities with U.S. owners, and to withhold 30% on certain “pass-through payments.” If such holder is a “non-financial
foreign entity,” FATCA also generally will impose a withholding tax of 30% on withholdable payments made to such holder unless the
holder provides the withholding agent with a certification that it does not have any “substantial United States owners” or
a certification identifying its direct and indirect substantial United States owners. Intergovernmental agreements between the United
States and a holder’s resident country may modify some of the foregoing requirements.
Although withholding under FATCA would also have
applied to payments of gross proceeds from the sale or other disposition of the common stock on or after January 1, 2019, Treasury
Regulations proposed in late 2018 eliminate FATCA withholding on payments of gross proceeds entirely. Taxpayers generally may rely on
these proposed Treasury Regulations until final Treasury Regulations are issued.
We will not pay any additional amounts to holders
of the common stock in respect of any amounts withheld. Non-U.S. holders should consult their own tax advisers with respect to the U.S.
federal income tax consequences of FATCA on their ownership and disposition of shares of our common stock.
Documentation that holders provide in order to
be treated as FATCA compliant may be reported to the IRS and other tax authorities, including information about a holder’s identity,
its FATCA status and if applicable, its direct and indirect U.S. owners. Prospective investors should consult their tax advisers about
how information reporting and the possible imposition of withholding tax under FATCA may apply to their investment in the common stock.
PLAN OF DISTRIBUTION
The Selling Stockholders, which as used herein
includes certain donees, pledgees, transferees or other successors-in-interest selling Warrant Shares or interests in Warrant Shares received
after the date of this prospectus from the Selling Stockholders as a gift, pledge, partnership distribution or other transfer, may, from
time to time, sell, transfer or otherwise dispose of any or all of their Shares or interests in Warrant Shares on any stock exchange,
market or trading facility on which the shares are traded or in private transactions. These dispositions may be at fixed prices, at prevailing
market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale,
or at negotiated prices.
The Selling Stockholders may use any one or more
of the following methods when disposing of shares or interests therein:
| |
● |
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| |
● |
block trades in which the broker-dealer will attempt to sell the shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction; |
| |
● |
purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| |
● |
an exchange distribution in accordance with the rules of the applicable exchange; |
| |
● |
privately negotiated transactions; |
| |
● |
short sales effected after the date the registration statement of which this prospectus is a part is declared effective by the SEC; |
| |
● |
through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| |
● |
broker-dealers may agree with the Selling Stockholders to sell a specified number of such shares at a stipulated price per share; |
| |
● |
a combination of any such methods of sale; and |
| |
● |
any other method permitted by applicable law. |
The Selling Stockholders may transfer the Warrant
Shares in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial
owners for purposes of this prospectus.
In connection with the sale of our common stock
or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or other financial institutions,
which may in turn engage in short sales of the common stock in the course of hedging the positions they assume. The Selling Stockholders
may also sell shares of our common stock short and deliver these securities to close out its short positions, or loan or pledge the common
stock to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other transactions
with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to
such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial
institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The aggregate proceeds to the Selling Stockholders
from the sale of the Warrant Shares offered by them will be the sale price of the Warrant Shares less discounts or commissions, if any.
The Selling Stockholders reserve the right to accept and, together with its agents from time to time, to reject, in whole or in part,
any proposed purchase of Warrant Shares to be made directly or through agents. We will not receive any of the proceeds from this offering.
The Selling Stockholders also may resell all or
a portion of the shares in open market transactions in reliance upon Rule 144 under the Securities Act, provided that it meets the
criteria and conform to the requirements of that rule.
The Selling Stockholders and any underwriters,
broker-dealers or agents that participate in the sale of the common stock or interests therein may be “underwriters” within
the meaning of Section 2(11) of the Securities Act. Any discounts, commissions, concessions or profit they earn on any resale of
the shares may be underwriting discounts and commissions under the Securities Act. If any of the Selling Stockholders is an “underwriter”
within the meaning of Section 2(11) of the Securities Act, it will be subject to the prospectus delivery requirements of the Securities
Act.
To the extent required, the shares of our common
stock to be sold, the names of the Selling Stockholders, the respective purchase prices and public offering prices, the names of any
agents, dealer or underwriter, any applicable commissions or discounts with respect to a particular offer will be set forth in an accompanying
prospectus supplement or, if appropriate, a post-effective amendment to the registration statement that includes this prospectus.
In order to comply with the securities laws of
some states, if applicable, the Warrant Shares may be sold in these jurisdictions only through registered or licensed brokers or dealers.
In addition, in some states the Warrant Shares may not be sold unless it has been registered or qualified for sale or an exemption from
registration or qualification requirements is available and is complied with.
We have advised the Selling Stockholders that
the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of shares in the market and to the activities
of the Selling Stockholders and their affiliates. In addition, to the extent applicable we will make copies of this prospectus (as it
may be supplemented or amended from time to time) available to the Selling Stockholders for the purpose of satisfying the prospectus delivery
requirements of the Securities Act. The Selling Stockholders may indemnify any broker-dealer that participates in transactions involving
the sale of the shares against certain liabilities, including liabilities arising under the Securities Act.
We have agreed to indemnify the Selling Stockholders
against liabilities, including liabilities under the Securities Act and the Exchange Act, relating to the registration of the shares offered
by this prospectus.
We have agreed with the Selling Stockholders to
keep the registration statement, of which this prospectus constitutes a part, effective at all times until no Selling Stockholder owns any
Warrants or Warrant Shares.
Our common stock is listed on the Nasdaq Capital
Market under the symbol “MBIO.”
LEGAL MATTERS
Troutman Pepper Hamilton Sanders LLP, Charlotte,
North Carolina, will pass upon the validity of the securities being offered by this prospectus. Additional legal matters may be passed
upon for us or any underwriters, dealers or agents, by counsel that we will name in the applicable prospectus supplement.
EXPERTS
The financial statements of Mustang Bio, Inc.
as of December 31, 2023 and 2022, and for each of the years in the two-year period ended December 31, 2023, have been incorporated
by reference herein and in the registration statement in reliance upon the report of KPMG LLP, independent registered public accounting
firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing. The audit report covering
the December 31, 2023, financial statements contains an explanatory paragraph that states the Company’s expectation to generate
operating losses and negative operating cash flows in the future, and the need for additional funding to support its planned operations
raise substantial doubt about its ability to continue as a going concern. The financial statements do not include any adjustments that
might result from the outcome of that uncertainty.
WHERE YOU CAN FIND MORE INFORMATION
We file reports and proxy statements with the
SEC. These filings include our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K
and proxy statements on Schedule 14A, as well as any amendments to those reports and proxy statements, which are available free of charge
through our website as soon as reasonably practicable after we file them with, or furnish them to, the SEC. Our Internet website address
is www.mustangbio.com. Our website and the information contained on, or that can be accessed through, the website will not be deemed
to be incorporated by reference in, and are not considered part of, this prospectus. You should not rely on any such information in making
your decision whether to purchase our securities. The SEC also maintains a website at www.sec.gov that contains reports, proxy and information
statements and other information regarding us and other issuers that file electronically with the SEC.
We have filed with the SEC a registration statement
on Form S-3 under the Securities Act relating to the securities being offered by this prospectus. This prospectus, which constitutes
part of that registration statement, does not contain all of the information set forth in the registration statement or the exhibits and
schedules which are part of the registration statement. For further information about us and the securities offered, see the registration
statement and the exhibits and schedules thereto. Statements contained in this prospectus regarding the contents of any contract or any
other document to which reference is made are not necessarily complete, and, in each instance where a copy of a contract or other document
has been filed as an exhibit to the registration statement, reference is made to the copy so filed, each of those statements being qualified
in all respects by the reference.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate by reference”
into this prospectus the information we file with the SEC in other documents which means that we can disclose important information to
you by referring you to those documents instead of having to repeat the information in this prospectus. The information incorporated by
reference is considered to be part of this prospectus, and later information that we file with the SEC will automatically update and supersede
such information. We incorporate by reference the documents listed below and any future information filed (rather than furnished) with
the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act between the date of this prospectus and the date all securities
to which this prospectus relates have been sold or the offering is otherwise terminated and also the date of the initial registration
statement and prior to effectiveness of the registration statement, provided, however, that we are not incorporating any information furnished
under Item 2.02 or Item 7.01 of any Current Report on Form 8-K.
| |
a) |
Our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 11, 2024; |
| |
|
|
| |
b) |
Our Quarterly Reports on Form 10-Q, for the quarterly periods ended March 31, 2024, June 30, 2024, and September 30, 2024, filed with the SEC on May 15, 2024, August 13, 2024, and November 8, 2024, respectively; |
| |
|
|
| |
c) |
Our Current Reports on Form 8-K, filed with the SEC on January 4, 2024, January 25, 2024, February 14, 2024, March 15, 2024, March 29, 2024, April 12, 2024, May 2, 2024, May 21, 2024, June 6, 2024, June 24, 2024, July 3, 2024, September 13, 2024, October 25, 2024, November 6, 2024, and November 15, 2024; and |
| |
|
|
| |
e) |
The description of our common stock included in our registration statements on Form 8-A12B, filed with the SEC on August 21, 2017, and any amendment or report filed for the purpose of further updating such description. |
We will furnish without charge to any person (including
any beneficial owner) a copy of any or all of the documents incorporated by reference, including exhibits to these documents, upon written
or oral request. Direct your request to: Corporate Secretary, 377 Plantation Street, Worcester, Massachusetts 01605, or (781) 652-4500.
A statement contained in a document incorporated by reference into this prospectus shall be deemed to be modified or superseded for purposes
of this prospectus to the extent that a statement contained in this prospectus, any prospectus supplement or in any other subsequently
filed document which is also incorporated in this prospectus modifies or replaces such statement. Any statements so modified or superseded
shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.

Mustang Bio, Inc.
34,767,934 Shares of Common Stock underlying
certain Common Warrants and Placement Agent Warrants
PROSPECTUS
, 2024
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 14. Other Expenses of Issuance and Distribution
The following table indicates the expenses to
be incurred in connection with the offering described in this registration statement, other than underwriting discounts and commissions,
all of which will be paid by us. All amounts are estimated except the SEC registration fee.
| | |
Amount | |
| SEC registration fee | |
$ | 1,144 | |
| Accounting fees and expenses | |
$ | 25,000 | |
| Legal fees and expenses | |
$ | 100,000 | |
| Miscellaneous fees and expenses | |
$ | 3,856 | |
| Total expenses | |
$ | 130,000 | |
Item 15. Indemnification of Directors and Officers
Under the General Corporation Law of the State
of Delaware (“DGCL”), a corporation may include provisions in its certificate of incorporation that will relieve its directors
of monetary liability for breaches of their fiduciary duty to the corporation, except under certain circumstances, including a breach
of the director’s duty of loyalty, acts or omissions of the director not in good faith or which involve intentional misconduct or
a knowing violation of law, the approval of an improper payment of a dividend or an improper purchase by the corporation of stock or any
transaction from which the director derived an improper personal benefit. The Company’s Amended and Restated Certificate of Incorporation,
as amended, eliminates the personal liability of directors to the Company or its stockholders for monetary damages for breach of fiduciary
duty as a director with certain limited exceptions set forth in the DGCL.
Section 145 of the DGCL grants to corporations
the power to indemnify each officer and director against liabilities and expenses incurred by reason of the fact that he or she is or
was an officer or director of the corporation if he or she acted in good faith and in a manner he or she reasonably believed to be in
or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause
to believe his or her conduct was unlawful. The Company’s Amended and Restated Certificate of Incorporation, as amended, and Amended
and Restated Bylaws, as amended, provide for indemnification of each officer and director of the Company to the fullest extent permitted
by the DGCL. Section 145 of the DGCL also empowers corporations to purchase and maintain insurance on behalf of any person who is
or was an officer or director of the corporation against liability asserted against or incurred by him in any such capacity, whether or
not the corporation would have the power to indemnify such officer or director against such liability under the provisions of Section 145
of the DGCL.
Item 16. Exhibits and Financial Statement
Schedules
The exhibits to the Registration Statement are
listed in the Exhibit Index attached hereto and incorporated by reference herein.
EXHIBIT INDEX
| Exhibit No. |
|
Description |
|
Form |
|
File
Number |
|
Date |
|
Exhibit No. |
|
Filed
Herewith |
| 1.1 |
|
At Market Issuance Sales Agreement, dated July 27, 2018, between the Company, B. Riley FBR, Inc., Cantor Fitzgerald & Co., National Securities Corporation, and Oppenheimer & Co. Inc. |
|
8-K |
|
001-38191 |
|
July 27, 2018 |
|
1.1 |
|
|
| 1.2 |
|
Amendment No. 1 to At Market Issuance Sales Agreement, dated July 20, 2020, between the Company, B. Riley FBR, Inc., Cantor Fitzgerald & Co., National Securities Corporation and Oppenheimer & Co. Inc. |
|
8-K |
|
001-38191 |
|
July 24, 2020 |
|
1.2 |
|
|
| 1.3 |
|
Amendment No. 2 to At Market Issuance Sales Agreement, dated December 31, 2020, between the Company, B. Riley Securities, Inc., Cantor Fitzgerald & Co., National Securities Corporation, Oppenheimer & Co. Inc. and H.C. Wainwright & Co., LLC. (incorporated by reference to the Exhibit 1.1 of the Registrant’s Current Report on Form 8-K (file No. 001-38191) filed with the SEC on December 31, 2020). |
|
8-K |
|
001-38191 |
|
December 31, 2020 |
|
1.1 |
|
|
| 1.4 |
|
Amendment No. 3 to At Market Issuance Sales Agreement, dated April 14, 2023, between the Registrant B. Riley Securities, Inc., Cantor Fitzgerald & Co. and H.C. Wainwright & Co., LLC |
|
8-K |
|
001-38191 |
|
April 20, 2023 |
|
1.1 |
|
|
| 1.5 |
|
At the Market Offering Agreement, dated May 31, 2024, by and between the Company and H.C. Wainwright & Co., LLC |
|
8-K |
|
001-38191 |
|
June 6, 2024 |
|
1.1 |
|
|
| 2.1# |
|
Asset Purchase Agreement, dated May 18, 2023, between the Company and uBriGene (Boston) Biosciences, Inc. |
|
8-K |
|
001-38191 |
|
May 22, 2023 |
|
1.1 |
|
|
| 2.2 |
|
First Amendment to Asset Purchase Agreement, dated June 29, 2023, between the Company and uBriGene (Boston) Biosciences, Inc. |
|
8-K |
|
001-38191 |
|
June 30, 2023 |
|
2.2 |
|
|
| 2.3 |
|
Second Amendment to Asset Purchase Agreement, dated July28, 2023, between the Company and uBriGene (Boston) Biosciences, Inc. |
|
8-K |
|
001-38191 |
|
July 31, 2023 |
|
2.3 |
|
|
| 3.1 |
|
Amended and Restated Certificate of Incorporation of Mustang Bio, Inc. (formerly Mustang Therapeutics, Inc.), dated July 26, 2016 |
|
10-12G |
|
000-5568 |
|
July 28, 2016 |
|
3.1 |
|
|
| 3.2 |
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation of Mustang Bio, Inc., dated June 14, 2018 |
|
10-Q |
|
001-38191 |
|
August 13, 2018 |
|
3.1 |
|
|
| 3.3 |
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation of Mustang Bio, Inc., dated September 30, 2019 |
|
8-K |
|
001-38191 |
|
September 30, 2019 |
|
3.1 |
|
|
| 3.4 |
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation of Mustang Bio, Inc., dated December 4, 2020 |
|
8-K |
|
001-38191 |
|
December 4, 2020 |
|
3.1 |
|
|
| 3.5 |
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation of Mustang Bio, Inc., dated June 17, 2021 |
|
8-K |
|
001-38191 |
|
June 22, 2021 |
|
3.1 |
|
|
| 3.6 |
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation of Mustang Bio, Inc., dated July 5, 2022 |
|
8-K |
|
001-38191 |
|
July 7, 2022 |
|
3.1 |
|
|
| 3.7 |
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation of Mustang Bio, Inc., dated April 3, 2023 |
|
8-K |
|
001-38191 |
|
April 3, 2023 |
|
3.1 |
|
|
| 3.8 |
|
Amended and Restated Bylaws of Mustang Bio, Inc. |
|
8-K |
|
001-38191 |
|
April 3, 2023 |
|
3.2 |
|
|
| 4.1 |
|
Specimen certificates evidencing shares of common stock, Class A common stock and Class A preferred stock |
|
10-12G |
|
000-5568 |
|
July 28, 2016 |
|
4.1 |
|
|
| 4.2 |
|
Form of Warrant Agreement |
|
10-12G |
|
000-5568 |
|
July 28, 2016 |
|
4.2 |
|
|
| 4.3 |
|
Common Stock Warrant issued by Mustang Bio, Inc. to NSC Biotech Venture Fund I, LLC, dated July 5, 2016 |
|
10-12G |
|
000-5568 |
|
July 28, 2016 |
|
10.5 |
|
|
| 4.4 |
|
Warrant to Purchase Common Stock issued to Runway Growth Finance Corp., dated March 4, 2022 |
|
8-K |
|
001-3891 |
|
March 8, 2022 |
|
4.1 |
|
|
| 4.5 |
|
Form of October Pre-funded Warrant |
|
8-K |
|
001-38191 |
|
October 30, 2023 |
|
4.1 |
|
|
| 4.6 |
|
Form of October Warrant |
|
8-K |
|
001-38191 |
|
October 30, 2023 |
|
4.2 |
|
|
| 4.7 |
|
Form of October Wainwright Warrant |
|
8-K |
|
001-38191 |
|
October 30, 2023 |
|
4.3 |
|
|
| 4.8 |
|
Form of May 2024 Pre-Funded Warrant |
|
8-K |
|
001-38191 |
|
May 2, 2024 |
|
4.1 |
|
|
| 4.9 |
|
Form of May 2024 Series A-1, A-2, and A-3 Warrant |
|
8-K |
|
001-38191 |
|
May 2, 2024 |
|
4.2 |
|
|
| 4.10 |
|
Form of May 2024 Placement Agent Warrant |
|
8-K |
|
001-38191 |
|
May 2, 2024 |
|
4.3 |
|
|
| 4.11 |
|
Form of June 2024 Pre-Funded Warrant |
|
8-K |
|
001-38191 |
|
June 24, 2024 |
|
4.1 |
|
|
| 4.12 |
|
Form of June 2024 Warrant |
|
8-K |
|
001-38191 |
|
June 24, 2024 |
|
4.2 |
|
|
| 4.13 |
|
Form of June 2024 Wainwright Warrant |
|
8-K |
|
001-38191 |
|
June 24, 2024 |
|
4.3 |
|
|
| 4.14 |
|
Form of Series B-1 Warrant |
|
8-K |
|
001-38191 |
|
October 25, 2024 |
|
4.1 |
|
|
| 4.14 |
|
Form of Series B-2 Warrant |
|
8-K |
|
001-38191 |
|
October 25, 2024 |
|
4.2 |
|
|
| 4.14 |
|
Form of Placement Agent Warrant |
|
8-K |
|
001-38191 |
|
October 25, 2024 |
|
4.3 |
|
|
| 5.1 |
|
Opinion of Troutman Pepper Hamilton Sanders LLP |
|
|
|
|
|
|
|
|
|
X |
Item 17. Undertakings
| (a) |
The undersigned registrant hereby undertakes: |
| |
(1) |
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| |
(i) |
To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| |
(ii) |
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; |
| |
(iii) |
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; provided, however, that paragraphs (i), (ii) and (iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the SEC by the registrant pursuant to Section 13 or Section 15(d) of the Exchange Act that are incorporated by reference in the registration statement, or, is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement. |
| |
(2) |
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| |
(3) |
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| |
(4) |
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: |
| |
(A) |
Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and |
| |
(B) |
Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by Section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date. |
| |
(5) |
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| |
(i) |
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| |
(ii) |
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| |
(iii) |
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| |
(iv) |
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| (b) |
The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefits plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (c) |
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act of 1933, as amended, and will be governed by the final adjudication of such issue. |
| (d) |
The undersigned registrant hereby undertakes that: |
| |
(1) |
For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| |
(2) |
For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
SIGNATURES
Pursuant to the requirements of the Securities
Act of 1933, the Registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3
and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City
of Worcester, Commonwealth of Massachusetts, on November 22, 2024.
| |
Mustang Bio, Inc. |
| |
|
|
| |
By: |
/s/ Manuel Litchman, M.D. |
| |
|
Manuel Litchman, M.D. |
| |
|
Chief Executive Officer and President |
POWER OF ATTORNEY
KNOW ALL BY THESE PRESENTS, that each person whose
signature appears below constitutes and appoints Manuel Litchman, M.D. and Peter Carney, and each of them, as his or her true and lawful
attorneys-in-fact and agents, each with the full power of substitution, for him or her and in his or her name, place or stead, in any
and all capacities, to sign any and all amendments to this registration statement (including post-effective amendments), and to sign any
registration statement for the same offering covered by this registration statement that is to be effective upon filing pursuant to Rule 462(b) promulgated
under the Securities Act, and all post-effective amendments thereto, and to file the same, with exhibits thereto and other documents in
connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them,
full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises,
as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact
and agents, or their or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities
Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
|
Title |
|
Date |
| /s/ Manuel Litchman, M.D. |
|
President, Chief Executive Officer and Director |
|
|
| Manuel Litchman, M.D. |
|
(Principal Executive Officer, and Principal Financial and Accounting Officer) |
|
November 22, 2024 |
| |
|
|
|
|
| /s/ Michael S. Weiss |
|
Chairman of the Board of Directors and Executive |
|
|
| Michael S. Weiss |
|
Chairman |
|
November 22, 2024 |
| |
|
|
|
|
| /s/ Adam Chill |
|
|
|
|
| Adam Chill |
|
Director |
|
November 22, 2024 |
| |
|
|
|
|
| /s/ Neil Herskowitz |
|
|
|
|
| Neil Herskowitz |
|
Director |
|
November 22, 2024 |
| |
|
|
|
|
| /s/ David Jin |
|
|
|
|
| David Jin |
|
Director |
|
November 22, 2024 |
| |
|
|
|
|
| /s/ Lindsay A, Rosenwald, M.D. |
|
|
|
|
| Lindsay A. Rosenwald, M.D. |
|
Director |
|
November 22, 2024 |
| |
|
|
|
|
| /s/ Michael Zelefsky, M.D. |
|
|
|
|
| Michael Zelefsky, M.D. |
|
Director |
|
November 22, 2024 |
Exhibit 5.1
Troutman Pepper Hamilton Sanders LLP
301 S College Street, Suite 3400
Charlotte, NC 28202 |
 |
| troutman.com |
|
November 22, 2024
Mustang Bio, Inc.
377 Plantation Street
Worcester, Massachusetts 01605
| |
Re: |
Securities
Registered under Registration Statement on Form S-3 |
Ladies and Gentlemen:
We have acted as counsel to
Mustang Bio, Inc., a Delaware corporation (the “Company”), in connection with the Company’s registration
statement on Form S-3 (the “Registration Statement”), including the prospectus that is part of the Registration
Statement (the “Prospectus”), filed on the date hereof, with the U.S. Securities and Exchange Commission (the
“Commission”), under the Securities Act of 1933, as amended (the “Securities Act”). The Registration
Statement relates to the registration of the resale by the selling stockholders named in the Prospectus (the “Selling Stockholders”)
of up to an aggregate of 34,767,934 shares of the Company’s common stock, par value $0.0001 per share (the “Common Stock”),
comprised of (i) 16,877,638 shares of Common Stock issuable upon exercise of outstanding Series B-1 Warrants to purchase Common
Stock (the “Series B-1 Warrants”) previously issued to certain of the Selling Stockholders (the “Series B-1
Warrant Shares”), (ii) 16,877,638 shares of Common Stock issuable upon the exercise of outstanding Series B-2 Warrants
to purchase Common Stock (the “Series B-2 Warrants”) previously issued to certain of the Selling Stockholders
(the “Series B-2 Warrant Shares”) and (iii) 1,012,658 shares of Common Stock issuable upon the exercise
of outstanding placement agent warrants (the “Placement Agent Warrants,” and together with the Series B-1 Warrants
and the Series B-2 Warrants, the “Warrants”) to purchase Common Stock previously issued to certain of the Selling
Stockholders (the “Placement Agent Warrant Shares,” and together with the Series B-1 Warrant Shares and the Series B-2
Warrant Shares, the “Shares”). The Shares are described in the Registration Statement and may be sold from time to
time by the Selling Stockholders.
This opinion letter is being
furnished in accordance with the requirements of Item 16 of Form S-3 and Item 601(b)(5)(i) of Regulation S-K promulgated under
the Securities Act. Capitalized terms used and not defined herein shall have the meanings assigned to them in the Registration Statement.
In connection with this opinion,
we have reviewed the corporate proceedings taken by the Company with respect to the issue and sale, and registration of the resale of,
the Shares. We have also examined and relied upon originals or copies of such corporate records, documents, agreements or other instruments
of the Company, and such certificates and records of public officials, and such other documents, as we have deemed necessary or appropriate
in connection herewith, including, but not limited to, the Company’s Amended and Restated Certificate of Incorporation, as amended,
the Company’s Amended and Restated Bylaws and the Series B-1 Warrants, Series B-2 Warrants and Placement Agent Warrants
pursuant to an exercise of which the Shares are issuable. As to all matters of fact (including, without limitation, factual conclusions
and characterizations and descriptions of purpose, intention or other state of mind) we have relied entirely upon a certificate of an
officer of the Company, and have assumed, without independent inquiry, the accuracy of that certificate.
| |
 |
| |
|
In rendering this opinion,
we have assumed the genuineness and authenticity of all signatures on the original documents; the legal capacity of all natural persons;
the authenticity of all documents submitted to us as originals; the conformity to originals of all documents submitted to us as certified
or photocopies; the accuracy and completeness of all documents and records reviewed by us; the accuracy, completeness and authenticity
of certificates issued by any governmental official, office or agency and the absence of change in the information contained therein
from the effective date of any such certificate; and the due authorization, execution and delivery of all documents where authorization,
execution and delivery are prerequisites to the effectiveness of such documents, except that we make no such assumption with respect
to the Company.
Our opinion is expressed only
with respect to the General Corporation Law of the State of Delaware. We are not opining as to the applicability thereto, or the effect
thereon, of the laws of any other jurisdiction or, in the case of Delaware, any other laws, or as to matters of municipal law or the
laws of any local agencies within any states (including “blue sky” or other state securities laws).
Based upon the foregoing, we
are of the opinion that, as of the date hereof, the Shares have been duly authorized and, when issued and delivered in the manner described
in the Registration Statement upon the valid exercise of the Warrants, will be validly issued, fully paid and non-assessable.
We assume no obligation to
supplement this opinion if any applicable law changes after the date hereof or if we become aware of any fact that might change the opinion
expressed herein after the date hereof.
We hereby consent to the filing
of this opinion as a part of the Registration Statement and to the reference of our firm under the caption “Legal Matters”
in the Prospectus. In giving such consent, we do not hereby admit that we are in the category of persons whose consent is required under
Section 7 of the Securities Act or the rules and regulations of the Commission. Except as otherwise set forth herein, this
opinion may not be used, circulated, quoted or otherwise referred to for any purpose or relied upon by any other person without the express
written permission of this firm.
| |
Very
truly yours, |
| |
|
| |
/s/ TROUTMAN PEPPER HAMILTON
SANDERS LLP |
| |
Troutman
Pepper Hamilton Sanders LLP |
Exhibit 23.1
 |
KPMG LLP |
| |
Two Financial Center
60 South Street
Boston, MA 02111 |
Consent of Independent Registered
Public Accounting Firm
We consent to the use of our report dated March 11,
2024, with respect to the financial statements of Mustang Bio, Inc., incorporated herein by reference, and to the reference to our firm
under the heading “Experts” in the prospectus.
/s/ KPMG LLP
Boston, Massachusetts
November 22, 2024
KPMG LLP, a Delaware limited liability
partnership and a member firm of
the KPMG global organization of independent
member firms affiliated with
KPMG International Limited, a private
English company limited by guarantee.
Exhibit 107
Calculation of Filing Fee Tables
Form S-3
(Form Type)
Mustang
Bio, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered Securities
|
Security
Type |
|
Security
Class
Title |
|
Fee
Calculation
Rule(1) |
|
Amount
Registered(2) |
|
|
Proposed
Maximum
Offering
Price
Per Unit(1) |
|
|
|
Maximum
Aggregate
Offering
Price(1) |
|
Fee Rate |
|
Amount of
Registration Fee |
|
| Equity |
|
Common stock, par value $0.0001 per share |
|
|
457(c) |
|
|
34,767,934(3) |
|
|
$ |
0.215 |
(2) |
|
$ |
7,475,105.81 |
|
|
0.00015310 |
|
$ |
1,144.44 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total Offering Amounts |
|
|
|
|
|
|
|
|
|
|
|
|
$ |
7,475,105.81 |
|
|
|
|
$ |
1,144.44 |
|
| Total Fee Offsets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
| Net Fee Due |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
1,144.44 |
|
(1) Pursuant to Rule 457(c) under
the Securities Act, and solely for the purpose of calculating the registration fee, the proposed maximum offering price per share is the
average of the high and low prices reported for the registrant’s common stock quoted on the Nasdaq Capital Market on November 20,
2024.
(2) Pursuant to Rule 416(a) under the Securities Act,
this registration statement also covers an indeterminate number of additional shares as may be issuable as a result of stock splits, stock
dividends or similar transactions.
(3) Represents 34,767,934 shares of common stock issuable upon
the exercise of (i) common stock purchase warrants to purchase one share of common stock at an exercise price of $0.27, issued to
certain selling stockholders on October 25, 2024, and (ii) common stock purchase warrants to purchase one share of common stock
at an exercise price of $0.2963, issued to the Placement Agent or its designees on October 25, 2024.
Mustang Bio (NASDAQ:MBIO)
Historical Stock Chart
From Jan 2025 to Feb 2025
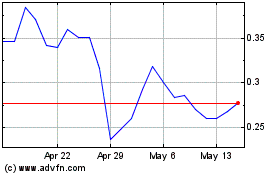
Mustang Bio (NASDAQ:MBIO)
Historical Stock Chart
From Feb 2024 to Feb 2025