Avidity Biosciences Gets FDA Fast-Track Designation for AOC 1020
19 January 2023 - 2:05AM
Dow Jones News
By Colin Kellaher
Avidity Biosciences Inc. on Wednesday said the U.S. Food and
Drug Administration granted fast-track designation to its AOC 1020
for the treatment of facioscapulohumeral muscular dystrophy.
The San Diego biopharmaceutical company said AOC 1020 is
currently in a Phase 1/2 study in adults with the rare, hereditary
muscle-weakening condition, adding that it plans to share data from
a preliminary assessment of about half of study participants in the
first half of 2024.
The FDA's fast-track program is designed to facilitate the
development and expedite the review of treatments for serious or
potentially life-threatening illnesses with high unmet medical
needs.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
January 18, 2023 09:50 ET (14:50 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
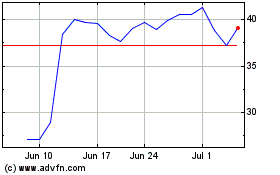
Avidity Biosciences (NASDAQ:RNA)
Historical Stock Chart
From Mar 2024 to Apr 2024
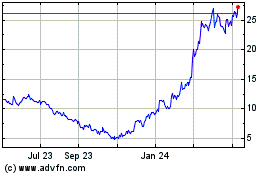
Avidity Biosciences (NASDAQ:RNA)
Historical Stock Chart
From Apr 2023 to Apr 2024