EU Says Merck's Covid-19 Pill Can Be Used in Some Cases, Is Reviewing Pfizer's
20 November 2021 - 3:46AM
Dow Jones News
By Giulia Petroni
The European Medicines Agency on Friday advised that Merck &
Co's and Ridgeback Biotherapeutics' experimental Covid-19 capsules
can be used for the treatment of adults not requiring oxygen
support and at increased risk of severe illness.
The European Union's drug regulator said the medicine, which is
known as Lagevrio, should be administered within five days of first
symptoms and taken twice a day for five days.
Lagevrio is not currently authorized for use in the EU.
"EMA issued this advice to support national authorities who may
decide on possible early use of the medicine prior to marketing
authorization, for example in emergency-use settings, in light of
rising rates of infection and deaths due to Covid-19 across the
EU," it said.
The EMA also said it is currently reviewing data on the use of
Pfizer's Covid-19 pill Paxlovid in order to provide
recommendations.
The data comes from a study that analyzes the oral treatment in
non-hospitalized patients with mild-to-moderate Covid-19 infections
who were at high risk of progressing to severe disease.
According to the regulator, preliminary results shows that
Paxlovid reduced the risk of hospitalization or death compared with
placebo when treatment was given within three or five days of first
symptoms.
Write to Giulia Petroni at giulia.petroni@wsj.com
(END) Dow Jones Newswires
November 19, 2021 11:31 ET (16:31 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
Pfizer (NYSE:PFE)
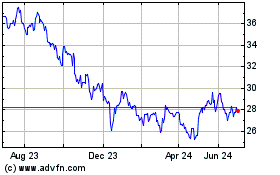
Historical Stock Chart
From Mar 2024 to Apr 2024
Pfizer (NYSE:PFE)
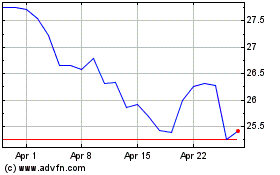
Historical Stock Chart
From Apr 2023 to Apr 2024