Pfizer, BioNTech Covid-19 Vaccine Recommended for Children Under 5 Years
20 October 2022 - 3:35AM
Dow Jones News
By Denny Jacob
Pfizer Inc. and BioNTech SE on Wednesday said regulators
recommended marketing authorization for a Covid-19 vaccine as a
three-dose series for children ages 6 months to 4 years old.
The European Medicines Agency's Committee for Medicinal Products
for Human Use recommended marketing authorization for a 3-ug dose
of Comirnaty. The European Commission will review the committee's
recommendation and is expected to make a final decision soon, the
companies said.
The U.S. Food and Drug Administration granted emergency use
authorization of the original Pfizer-BioNTech Covid-19 vaccine as a
three 3-ug dose series in this age group in June.
Pfizer and BioNTech said they also are in discussions with
health authorities regarding a regulatory pathway for potential
authorization of a different Covid-19 vaccine for use in children
under 5 years of age.
Write to Denny Jacob at denny.jacob@wsj.com
(END) Dow Jones Newswires
October 19, 2022 12:20 ET (16:20 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
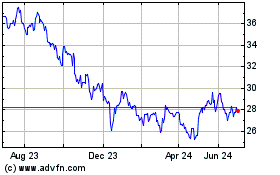
Pfizer (NYSE:PFE)
Historical Stock Chart
From Mar 2024 to Apr 2024
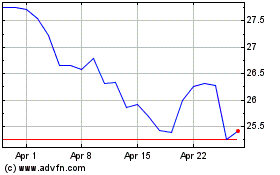
Pfizer (NYSE:PFE)
Historical Stock Chart
From Apr 2023 to Apr 2024