Arecor Therapeutics Investigational New Drug Application Cleared by FDA, Phase 1 Trial Set
09 September 2021 - 5:57PM
Dow Jones News
By Joe Hoppe
Arecor Therapeutics PLC said Thursday that the U.S. Food and
Drug Administration has cleared its investigational new drug, or
IND, application for AT247, its proprietary rapid insulin treatment
for diabetes.
The London-listed biopharmaceutical company--backed by Unilever
PLC--said the application supports a Phase 1 clinical trial in the
U.S. in around 24 participants with type 1 diabetes. The trial will
be a double blind, randomized, threeway crossover study, comparing
AT247 with two other market-leading insulin treatments, NovoRapid
and Fiasp.
The aim of the drug is to accelerate insulin absorption,
post-injection, to enable more effective management of blood
glucose levels for people living with diabetes, and it has the
potential to significantly improve post-prandial glucose
control--avoiding both hypo-and hyperglycemic episodes.
Data from a European Phase 1 clinical study showed the drug
exhibited earlier insulin appearance, exposure and offset, with an
earlier glucose-lowering effect than NovoRapid and Fiasp. The data
also suggests the drug can also facilitate a fully closed loop
artificial pancreas, a potentially life changing treatment
option.
Shares at 0722 GMT were up 16.0 pence, or 6.8%, at 252.0
pence.
Write to Joe Hoppe at joseph.hoppe@wsj.com
(END) Dow Jones Newswires
September 09, 2021 03:42 ET (07:42 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
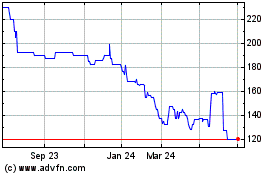
Arecor Therapeutics (LSE:AREC)
Historical Stock Chart
From Mar 2024 to Apr 2024
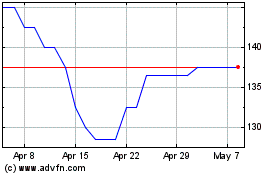
Arecor Therapeutics (LSE:AREC)
Historical Stock Chart
From Apr 2023 to Apr 2024