Coherus BioSciences Gets FDA OK for Udenyca Injector
27 December 2023 - 8:43AM
Dow Jones News
By Ben Glickman
Coherus BioSciences has received approval from the U.S. Food and
Drug Administration for an on-body injector of Udenyca, its cancer
treatment.
The Redwood City, Calif.-based cancer-treatment developer's
Udenyca Onbody was previously rejected by the FDA due to issues at
a third-party filler. The company resubmitted its application weeks
after.
Coherus said Tuesday that it expected Udenyca to be available
commercially in the first quarter of 2024.
Udenyca is a cancer treatment administered the day after
chemotherapy to reduce infections.
Write to Ben Glickman at ben.glickman@wsj.com
(END) Dow Jones Newswires
December 26, 2023 16:28 ET (21:28 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
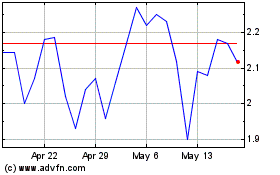
Coherus BioSciences (NASDAQ:CHRS)
Historical Stock Chart
From Apr 2024 to May 2024
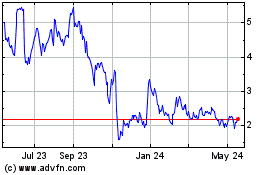
Coherus BioSciences (NASDAQ:CHRS)
Historical Stock Chart
From May 2023 to May 2024