UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
For the month of May 2024
Commission File No. 001-39621
OPTHEA LIMITED
(Translation of registrant’s name into English)
Level 4
650 Chapel Street
South Yarra, Victoria, 3141
Australia
(Address of registrant’s principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
EXHIBIT INDEX
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereto duly authorized.
|
|
|
|
OPTHEA LIMITED |
|
(Registrant) |
|
|
|
|
By: |
/s/ Frederic Guerard |
|
Name: |
Frederic Guerard |
|
Title: |
Chief Executive Officer |
Date: 05/28/2024
Exhibit 99.1

ASX, Nasdaq and Media Release
28 May 2024
Opthea Completes Enrollment in Pivotal Phase 3 Clinical Program with Sozinibercept in Wet AMD
Phase 3 program enrolled 1,984 patients across COAST and ShORe trials
Trials designed to replicate superiority in visual outcomes demonstrated in Phase 2b
Topline data from both pivotal trials expected in mid-CY2025
MELBOURNE, Australia and PRINCETON, N.J., May 28, 2024 -- Opthea Limited (ASX/NASDAQ: OPT “Opthea”, the “Company”), a clinical-stage biopharmaceutical company developing novel therapies to treat highly prevalent and progressive retinal diseases, including wet age-related macular degeneration (wet AMD), today announced that it has now completed enrollment in both the COAST and ShORe trials constituting its pivotal Phase 3 clinical program. This program is designed to assess the safety and superior efficacy of sozinibercept in combination with standard-of-care anti-VEGF-A therapies compared to standard of care alone for the treatment of patients with wet AMD.
“Today marks the achievement of a key milestone for the sozinibercept global Phase 3 clinical trial program that brings us closer to our goal of improving visual outcomes for patients with wet AMD,” said Arshad M. Khanani, MD, MA, FASRS, Chief Medical Advisor of Opthea. “In a large Phase 2b clinical trial of 366 treatment-naïve wet AMD patients, sozinibercept demonstrated strong clinical evidence of superior visual outcomes in combination with ranibizumab. These data formed the basis for this large, global Phase 3 clinical program.”
Opthea’s Phase 3 clinical program consists of two multicenter, double-masked, randomized, sham-controlled trials COAST (Combination OPT-302 with Aflibercept Study) and ShORe (Study of OPT-302 in combination with Ranibizumab), which enrolled 1,984 treatment-naïve wet AMD patients in total (998 patients in COAST; 986 patients in ShORe), making it one of the largest Phase 3 programs in wet AMD. Both Phase 3 trials combine sozinibercept with standard-of-care anti-VEGF-A therapy to assess the efficacy and safety of intravitreal 2.0 mg sozinibercept in combination with 2.0 mg aflibercept (COAST), or 0.5 mg ranibizumab (ShORe), compared to standard of care alone. The primary endpoint for both trials is the mean change in Best Corrected Visual Acuity (BCVA) from baseline to week 52. Both trials are also evaluating the safety and tolerability over a two-year period.
“Sozinibercept is the only late-stage asset in development in over 15 years that is targeting better visual outcomes for wet AMD patients in combination with standard-of-care anti-VEGF-A therapies,” said Frederic Guerard, PharmD, Chief Executive Officer of Opthea. “We are excited about the potential of sozinibercept to transform the current treatment paradigm, with pivotal 52-week topline data expected in mid-CY2025 to support a potential BLA submission.”
About Sozinibercept
Sozinibercept (OPT-302) is a soluble form of vascular endothelial growth factor receptor 3 (VEGFR-3) expressed as an immunoglobulin G1 (IgG1) Fc-fusion protein. It binds and neutralizes the activity of VEGF-C and VEGF-D on their endogenous receptors,
VEGFR-2 and VEGFR-3. VEGF-C and VEGF-D are known to independently stimulate retinal angiogenesis and vascular leakage and permeability, while VEGF-A inhibition can also lead to the upregulation of VEGF-C and VEGF-D. Research shows that the targeted inhibition of VEGF-C and VEGF-D with sozinibercept can prevent blood vessel growth and vascular leakage, which both contribute to the pathophysiology of retinal diseases, including wet AMD.
About Opthea’s Clinical Development Program
In Opthea’s prospective, randomized and controlled Phase 2b trial including 366 treatment-naïve wet AMD patients, sozinibercept was administered in combination with standard-of-care ranibizumab for the treatment of wet AMD. Sozinibercept combination therapy met the pre-specified primary efficacy endpoint of a statistically superior gain in visual acuity at 24 weeks, compared to ranibizumab alone. In addition, secondary outcomes were positive with the combination therapy, including more patients gaining vision of 10 or more letters, improved anatomy, with a reduction in swelling and vascular leakage, and a favorable safety profile. These statistically significant results were published in Ophthalmology in February 2023.
The Company is currently conducting two concurrent pivotal Phase 3 multicenter, double-masked, randomized, sham-controlled clinical trials, COAST (Combination OPT-302 with Aflibercept Study) and ShORe (Study of OPT-302 in combination with Ranibizumab), designed to assess the safety and superior efficacy of sozinibercept combination therapy versus standard-of-care anti-VEGF-A for the treatment of wet AMD. Opthea’s Phase 3 clinical trial program is designed to support a broad label and, if successful, sozinibercept has the potential to be approved for use in combination with any anti-VEGF-A for the treatment of all wet AMD patients. Sozinibercept has received Fast Track Designation from the U.S. FDA for the treatment of wet AMD. To learn more about Opthea’s Phase 3 clinical trial program, please visit ClinicalTrials.gov for COAST, NCT04757636, and ShORe, NCT04757610.
About Opthea
Opthea (ASX/NASDAQ:OPT) is a biopharmaceutical company developing novel therapies to address the unmet need in the treatment of highly prevalent and progressive retinal diseases, including wet age-related macular degeneration (wet AMD) and diabetic macular edema (DME).
Opthea’s lead product candidate, sozinibercept, is being evaluated in two pivotal
Phase 3 clinical trials (COAST, NCT04757636, and ShORe, NCT04757610) for use in combination with standard-of-care anti-VEGF-A monotherapies to improve overall efficacy and deliver superior vision gains compared to standard-of-care anti-VEGF-A agents. To learn more, visit our website and follow us on Xand LinkedIn.
Inherent risks of Investment in Biotechnology Companies
There are a number of inherent risks associated with the development of pharmaceutical products to a marketable stage. The lengthy clinical trial process is designed to assess the safety and efficacy of a drug prior to commercialization and a significant proportion of drugs fail one or both of these criteria. Other risks include uncertainty of patent protection and proprietary rights, whether patent applications and issued patents will offer adequate protection to enable product development, the obtaining of necessary drug regulatory authority approvals and difficulties caused by the rapid advancements in technology. Companies such as Opthea are dependent on the success of their research and development projects and on the ability to attract funding to support these activities. Investment in research and development projects cannot be assessed on the same fundamentals as trading and manufacturing enterprises. Therefore, investment in companies specializing in drug development must be regarded as highly speculative. Opthea strongly recommends that professional investment advice be sought prior to such investments.
Forward-looking Statements

This ASX announcement contains certain forward-looking statements, including within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. The words “expect”, “believe,” “should”, “could”, “may”, “will”, “plan” and other similar expressions are intended to identify forward-looking statements. Forward-looking statements in this ASX announcement include statements regarding expectations on the outcomes of Opthea’s Phase 3 clinical trials of sozinibercept, the potential for sozinibercept as a combination therapy to be the first therapy to achieve superior visual outcomes over
anti-VEGF-A monotherapy and improve vision outcomes for patients with wet AMD, the top-line data for Opthea’s Phase 3 clinical trials of sozinibercept, the market opportunity for sozinibercept, and the future performance of Opthea. Forward-looking statements, opinions and estimates provided in this ASX announcement are based on assumptions and contingencies which are subject to change without notice, as are statements about market and industry trends, which are based on interpretations of current conditions. Forward-looking statements are provided as a general guide only and should not be relied upon as an indication or guarantee of future performance. They involve known and unknown risks and uncertainties and other factors, many of which are beyond the control of Opthea and its directors and management and may involve significant elements of subjective judgment and assumptions as to future events that may or may not be correct. These statements may be affected by a range of variables which could cause actual results or trends to differ materially, including but not limited to future capital requirements, the development, testing, production, marketing and sale of drug treatments, regulatory risk and potential loss of regulatory approvals, ongoing clinical studies to demonstrate sozinibercept safety, tolerability and therapeutic efficacy, additional analysis of data from Opthea’s Phase 3 clinical trials, clinical research organization and labor costs, intellectual property protections, and other factors that are of a general nature which may affect the future operating and financial performance of the Company including risk factors set forth in Opthea’s Annual Report on Form 20-F filed with the U.S. Securities and Exchange Commission (the “SEC”) on September 28, 2023, Opthea’s 2024 Half Year Report included as an exhibit to the Form 6-K filed with the SEC on February 29, 2024, and other future filings with the SEC. Actual results, performance or achievement may vary materially from any projections and forward-looking statements and the assumptions on which those statements are based. Subject to any continuing obligations under applicable law or any relevant ASX listing rules, Opthea disclaims any obligation or undertaking to provide any updates or revisions to any forward-looking statements in this ASX announcement to reflect any change in expectations in relation to any forward-looking statements or any change in events, conditions or circumstances on which any such statement is based, except as otherwise required by applicable law.

Authorized for release to ASX by Fred Guerard, CEO
|
|
Investor Inquiries |
Join our email database to receive program updates: |
PJ Kelleher LifeSci Advisors LLC Email: pkelleher@lifesciadvisors.com Phone: 617-430 7579 Media Inquiries Silvana Guerci-Lena NorthStream Global Partners silvana@nsgpllc.com |
Tel: +61 (0) 3 9826 0399 Email: info@opthea.com Web: www.opthea.com Source: Opthea Limited |
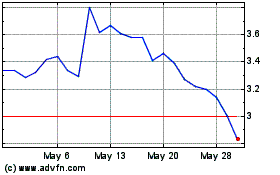
Opthea (NASDAQ:OPT)
Historical Stock Chart
From May 2024 to Jun 2024
Opthea (NASDAQ:OPT)
Historical Stock Chart
From Jun 2023 to Jun 2024