UNITED STATES
SECURITIES AND
EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant
to Section 13
or 15(d) of the Securities
Exchange
Act
of 1934
Date of Report (Date of earliest event reported):
March 4, 2024
AKARI
THERAPEUTICS, PLC
(Exact Name
of Registrant as Specified in Its Charter)
| England and Wales |
|
001-36288 |
|
98-1034922 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
22 Boston Wharf Road FL 7
Boston, MA |
02210 |
| (Address of Principal Executive Offices) |
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (929)
274-7510
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| x |
Written communications
pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ¨ |
Soliciting material pursuant
to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ¨ |
Pre-commencement communications
pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ¨ |
Pre-commencement communications
pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of
the Act:
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on
which
registered |
| American Depository Shares each representing 2000 Ordinary Shares |
|
AKTX |
|
The Nasdaq Stock Market LLC |
| Ordinary Shares, par value $0.0001 per share* |
|
True |
|
The Nasdaq Stock Market LLC |
*Trading, but only in connection with the American
Depositary Shares(ADS).
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2
of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ¨
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 1.01 | Entry into Material Definitive Agreement |
Merger Agreement
On March 4, 2024, Akari Therapeutics,
Plc, a public company limited by shares incorporated in England and Wales ("Akari"), entered into an Agreement and Plan
of Merger (the "Merger Agreement") with Peak Bio, Inc. ("Peak Bio") and Pegasus Merger Sub, Inc.,
a Delaware corporation and a wholly-owned subsidiary of Akari ("Merger Sub"), pursuant to which, upon the terms and subject
to the conditions thereof, Merger Sub will be merged with and into Peak Bio (the "Merger"), with Peak Bio surviving the
Merger as a wholly-owned subsidiary of Akari.
Pursuant to the Merger Agreement, and upon the
terms and subject to the conditions thereof, at the effective time of the Merger (the “Effective Time”), each issued
and outstanding share of Company common stock, par value $0.0001 per share (the “Peak Common Stock") (other than (x) shares
of Peak Common Stock held by Peak Bio as treasury stock, or shares of Peak Common Stock owned by Akari, Merger Sub or any direct or indirect
wholly-owned subsidiaries of Akari and (y) Dissenting Shares (as defined in the Merger Agreement), will be converted into the right
to receive Akari American Depositary Shares (“Akari ADSs”) representing a number of Akari ordinary shares, par value
$0.0001 per share (the “Akari Ordinary Shares”) equal to an exchange ratio calculated in accordance with the Merger
Agreement (the “Exchange Ratio”), each such share duly and validly issued against the deposit of the requisite number
of Akari Ordinary Shares in accordance with the Deposit Agreement (as defined in the Merger Agreement). The Exchange Ratio will be calculated
such that the total number of shares of Akari ADSs to be issued as merger consideration for the Peak Common Stock will be expected to
be, upon issuance, approximately 50% of the outstanding shares of Akari ADSs (provided, certain adjustments to this ratio will be made
in respect of the net cash, as determined in accordance with the Merger Agreement, of each of Akari and Peak Bio at the close of business
one business day prior to the anticipated consummation of the Merger). The Merger Agreement provides that, under certain circumstances,
additional Akari ADSs may be issued to the holders of shares of Peak Common Stock following the consummation of the Merger equal to an
exchange ratio calculated in accordance with the Merger Agreement (the “Additional Exchange Ratio”).
At the Effective Time, each warrant to purchase
capital stock of Peak Bio (“Peak Warrant”) outstanding immediately prior to the Effective Time will be converted into
and exchangeable for warrants to purchase a number of Akari Ordinary Shares or Akari ADSs, as determined by Akari (each, an “Adjusted
Warrant”), on substantially similar terms and subject to substantially similar conditions as were applicable to such Peak Warrant
immediately prior to the Effective Time, except (i) for terms rendered inoperative by reason of the transactions contemplated by
the Merger Agreement, (ii) as provided in the following sentence and (iii) such amendments to the terms of the Adjusted Warrants
as are necessary to comply with applicable Law (as defined in the Merger Agreement). The number of Akari Ordinary Shares (or the number
of Akari Ordinary Shares underlying Akari ADSs, as applicable) subject to each Adjusted Warrant will be equal to the number of shares
of Peak Common Stock issuable upon exercise of such Peak Warrant immediately prior to the Effective Time multiplied by the Exchange Ratio,
with any fractional Akari Ordinary Shares or Akari ADSs rounded down to the nearest whole Akari Ordinary Share or Akari ADS, as applicable,
and the exercise price with respect to each Akari Ordinary Share (or each Akari Ordinary Share underlying Akari ADSs, as applicable)
underlying such Adjusted Warrant will be equal to the exercise price of such Peak Warrant immediately prior to the Effective Time divided
by the Exchange Ratio. The grant of the Adjusted Warrants will be effected as of the Effective Time, or as soon thereafter as is reasonably
practicable, taking into account Parent’s administrative procedures. The Adjusted Warrants will be further adjusted, if applicable,
to give effect to the impact of the Additional Exchange Ratio.
Each option to acquire shares of Peak Common
Stock (“Peak Option”) that is outstanding and unexercised immediately prior to the Effective Time, whether or not
vested, will be assumed and converted into an option to purchase a number of Akari ordinary shares or Akari ADSs, as determined by Akari
(each, an “Adjusted Option”). The number of Akari Ordinary Shares (or the number of Akari Ordinary Shares underlying
Akari ADSs, as applicable) subject to the Adjusted Option will be equal to the product of (i) the total number of shares of Peak
Common Stock subject to such Peak Option immediately prior to the Effective Time multiplied by (ii) the Exchange Ratio, with any
fractional Akari Ordinary Shares or Akari ADSs rounded down to the nearest whole Akari Ordinary Share or Akari ADS, as applicable, and
the exercise price per share of each Adjusted Option will be equal to the exercise price of such Peak Option immediately prior to the
Effective Time divided by the Exchange Ratio. The Adjusted Options will be further adjusted, if applicable, to give effect to the impact
of the Additional Exchange Ratio.
The Merger Agreement contains customary representations,
warranties and covenants given by Akari, Peak Bio and Merger Sub. The Merger Agreement also contains customary pre-closing covenants,
including covenants by each of the parties relating to conduct of their respective business prior to the closing of the Merger. In
addition, the parties have agreed to use their respective commercially reasonable efforts to take all actions necessary, proper or advisable
to complete the Merger and the other transactions contemplated by the Merger Agreement, including making any required regulatory filings
with respect to the Merger as promptly as reasonably practicable, except that neither Akari nor Peak Bio is required to divest any assets
or businesses of Peak Bio, Akari or any of their respective affiliates and subsidiaries.
The Merger Agreement
also provides that, from the earlier of the Effective Time of the Merger and termination of the Merger Agreement, each of Akari and Peak
Bio is subject to certain restrictions on its ability to solicit acquisition proposals from third parties, to provide information to
third parties and to engage in discussions with third parties regarding acquisition proposals, subject to customary exceptions. In addition,
the board of directors of each of Akari and Peak Bio are required to recommend that their respective shareholders or stockholders vote
in favor of the Merger, subject to exceptions for superior proposals and other situations where failure to effect a recommendation change
would be inconsistent with such board’s fiduciary duties.
Consummation of the Merger is subject to various
conditions, including, among others, (i) approval of the Merger Agreement and Merger by Peak Bio stockholders, (ii) Akari’s
shareholders authorizing Akari’s board of directors to allot all Akari ordinary shares to be issued in connection with the Merger
(to be represented by Akari ADSs), (iii) the absence of any law or order prohibiting consummation of the Merger, (iv) Akari’s
Registration Statement on Form S-4 (to be issued in connection with the Merger) having been declared effective, (v) the Akari
ADSs issuable to Peak Bio stockholders having been authorized for listing on Nasdaq, (vi) accuracy of the other party’s representations
and warranties (subject to certain materiality standards set forth in the Merger Agreement), (vii) compliance by the other party
in all material respects with such other party’s obligations under the Merger Agreement; (viii) the absence of a material
adverse effect on the other party, (ix) the other party’s net cash being greater than negative $13,500,000 and (x) the
PIPE Investment (as defined in the Merger Agreement) shall have been consummated simultaneously with, and conditioned only upon, the
occurrence of the closing, and shall result in net proceeds to Akari of at least $10,000,000.
Either Akari or Peak Bio may terminate the Merger
Agreement under certain circumstances, including if (i) the Merger is not completed by September 4, 2024, (ii) the
other party's board of directors withdraws, modifies or qualifies its recommendation in favor of the transactions contemplated by the
Merger Agreement or approves or recommends an alternative transaction or (iii) Akari’s or Peak Bio’s board of directors,
as applicable, resolves to enter into a definitive agreement with respect to a superior proposal prior to obtaining approval of the Akari
ADS issuance or Merger, as applicable, from Akari’s shareholders or Peak Bio’s stockholders, as applicable. The Merger Agreement
also provides that under certain specified circumstances of termination described in the Merger Agreement, Akari or Peak Bio, as applicable,
will be required to pay a termination fee equal to $300,000 and reimburse the other party for expenses related to the transaction up
to $1.5 million.
Additional
Information
The foregoing is a general description of the
Merger and Merger Agreement; it does not purport to be complete and is qualified in its entirety by reference to the Merger Agreement,
which is attached as Exhibit 2.1 of this Current Report on Form 8-K and incorporated herein by reference.
The Merger Agreement, which is attached as Exhibit 2.1,
has been described above to provide investors and Akari shareholders with information regarding the terms of the Merger Agreement and
is not intended to modify or supplement any factual disclosures about Akari, Merger Sub or Peak Bio or any of their respective affiliates.
The representations, warranties and covenants contained in the Merger Agreement were made only for the purposes of the Merger Agreement,
were made as of specific dates, were made solely for the benefit of the parties to the Merger Agreement and may not have been intended
to be statements of fact, but rather, as a method of allocating risk and governing the contractual rights and relationships among the
parties to the Merger Agreement. In addition, such representations, warranties and covenants may have been qualified by certain disclosures
not reflected in the text of the Merger Agreement and may apply standards of materiality and other qualifications and limitations in
a way that is different from what may be viewed as material by Akari’s shareholders or Peak Bio’s stockholders. In reviewing
the representations, warranties and covenants contained in the Merger Agreement or any descriptions thereof in this summary, it is important
to bear in mind that such representations, warranties and covenants or any descriptions were not intended by the parties to the Merger
Agreement to be characterizations of the actual state of facts or conditions of Akari, Merger Sub or Peak Bio or any of their respective
affiliates. Moreover, information concerning the subject matter of the representations and warranties may change after the date of the
Merger Agreement, which subsequent information may or may not be fully reflected in public disclosures. For the foregoing reasons, the
representations, warranties and covenants or any descriptions of those provisions should not be read alone and should instead be read
in conjunction with the other information contained in the reports, statements and filings that Akari and Peak Bio publicly file with
the SEC.
Voting Agreements
Concurrently with the
Merger Agreement, Akari and Peak Bio entered into voting and support agreements (the “Voting Agreements”) with certain
shareholders of Akari (the “Akari Shareholders”), and certain stockholders of Peak Bio (the “Peak Stockholders”
and, together with the Akari Shareholders, the “Supporting Holders”). The Supporting Holders have agreed to, among
other things, vote their shares in favor of the Merger Agreement and the Merger or the issuance of Akari Ordinary Shares in connection
therewith, as applicable, in accordance with the recommendation of the respective boards of directors of Akari and Peak Bio.
As of March 1,
2024, the Akari Shareholders beneficially owned an aggregate of approximately 39.51% of the outstanding Akari Ordinary Shares. As of
March 1 2024, the Peak Stockholders beneficially owned an aggregate of approximately 39.3% of the outstanding shares of Peak Common
Stock.
The Voting Agreements
will terminate at the earliest to occur of (a) the Effective Time, (b) receipt of approval of the Supporting Holders, as applicable,
and (c) such date and time as the Merger Agreement is validly terminated.
The foregoing description of the Voting Agreements
does not purport to be complete and is subject to, and qualified in its entirety by, the form of the Voting Agreements, the forms of
which are filed as Exhibits 10.1 and 10.2 hereto and are incorporated herein by reference.
| Item 7.01 | Regulation FD Disclosure |
On March 5, 2024, Akari and Peak Bio issued
a joint press release announcing the execution of the Merger Agreement. The press release is attached as Exhibit 99.1 hereto.
The information furnished under this Item 7.01,
including Exhibit 99.1, shall not be deemed "filed" for purposes of Section 18 of the Securities Exchange Act of 1934,
as amended (the "Exchange Act"), or otherwise subject to the liabilities under that section and shall not be deemed to
be incorporated by reference into any filing under the Securities Act of 1933 or the Exchange Act, except as expressly set forth by specific
reference in such filing. In addition, Exhibit 99.1 contains statements intended as "forward-looking statements" that are
subject to the cautionary statements about forward-looking statements set forth in such exhibit.
| Item 9.01 | Exhibits and Financial Statements |
(d) Exhibits.
EXHIBIT
NUMBER |
|
DESCRIPTION |
| |
|
| 2.1* |
|
Agreement
and Plan of Merger, dated as of March 4, 2024, by and among Akari Therapeutics, Plc, Peak Bio, Inc. and Pegasus Merger Sub, Inc.
|
| 10.1 |
|
Form of
Voting and Support Agreement, dated as of March 4, 2024, by and among Akari, and certain stockholders of Peak Bio. |
| 10.2 |
|
Form of
Voting and Support Agreement, dated as of March 4, 2024, by and among Peak Bio and certain shareholders of Akari. |
| 99.1 |
|
Joint
Press Release of Akari and Peak Bio, dated March 5, 2024. |
| 99.2 |
|
Email to Employees, dated March 5, 2024 |
| 99.3 |
|
Email to Investors, dated March 5, 2024 |
| 99.4 |
|
Investor Presentation, dated March 5, 2024 |
| 104 |
|
Cover
Page Interactive Data File (embedded within the Inline XBRL document). |
| |
* |
Exhibits
and schedules to this Exhibit have been omitted in accordance with Item 601(b)(2) of Regulation S-K. The registrant agrees
to furnish supplementally a copy of all omitted exhibits and schedules to the SEC upon its request. |
Forward-looking Statements
This current report relates to the proposed transaction
pursuant to the terms of the Merger Agreement, by and among Akari, Pegasus Merger Sub, Inc., and Peak Bio and includes express or
implied forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E
of the Exchange Act, about the proposed transaction between Peak Bio and Akari and the operations of the combined company that involve
risks and uncertainties relating to future events and the future performance of Akari and Peak Bio. Actual events or results may differ
materially from these forward-looking statements. Words such as “will,” “could,” “would,” “should,”
“expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,”
“predict,” “project,” “potential,” “continue,” “future,” “opportunity”
“will likely result,” “target,” variations of such words, and similar expressions or negatives of these words
are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words.
Examples of such forward-looking statements include, but are not limited to, express or implied statements regarding: the Merger and
related matters, including, but not limited to, satisfaction of closing conditions to the proposed transaction, prospective performance
and opportunities with respect to Akari or Peak Bio, post-closing operations and the outlook for the companies’ businesses; Akari’s,
Peak Bio’s or the combined company’s targets, plans, objectives or goals for future operations, including those related to
Akari’s and Peak Bio’s product candidates, research and development, product candidate introductions and product candidate
approvals as well as cooperation in relation thereto; projections of or targets for revenues, costs, income (or loss), earnings per share,
capital expenditures, dividends, capital structure, net financials and other financial measures; future economic performance, future
actions and outcome of contingencies such as legal proceedings; and the assumptions underlying or relating to such statements.
These statements are based on Akari’s and
Peak Bio’s current plans, estimates and projections. By their very nature, forward-looking statements involve inherent risks and
uncertainties, both general and specific. A number of important factors, including those described in this communication, could cause
actual results to differ materially from those contemplated in any forward-looking statements. Factors that may affect future results
and may cause these forward-looking statements to be inaccurate include, without limitation: uncertainties as to the timing for completion
of the proposed transaction; uncertainties as to Peak Bio’s and/or Akari’s ability to obtain the approval of Akari’s
shareholders or Peak Bio’s stockholders required to consummate the proposed transaction; the possibility that competing offers
will be made by third parties; the occurrence of events that may give rise to a right of one or both of Akari and Peak Bio to terminate
the Merger Agreement; the possibility that various closing conditions for the proposed transaction may not be satisfied or waived on
a timely basis or at all, including the possibility that a governmental entity may prohibit, delay, or refuse to grant approval, if required,
for the consummation of the proposed transaction (or only grant approval subject to adverse conditions or limitations); the difficulty
of predicting the timing or outcome of consents or regulatory approvals or actions, if any; the possibility that the proposed transaction
may not be completed in the time frame expected by Akari and Peak Bio, or at all; the risk that Akari and Peak Bio may not realize the
anticipated benefits of the proposed transaction in the time frame expected, or at all; the effects of the proposed transaction on relationships
with Akari’s or Peak Bio’s employees, business or collaboration partners or governmental entities; the ability to retain
and hire key personnel; potential adverse reactions or changes to business relationships resulting from the announcement or completion
of the proposed transaction; significant or unexpected costs, charges or expenses resulting from the proposed transaction; the potential
impact of unforeseen liabilities, future capital expenditures, revenues, costs, expenses, earnings, synergies, economic performance,
indebtedness, financial condition and losses on the future prospects, business and management strategies for the management, expansion
and growth of the combined business after the consummation of the proposed transaction; potential negative effects related to this announcement
or the consummation of the proposed transaction on the market price of Akari’s American Depositary Shares or Peak Bio’s common
stock and/or Akari’s or Peak Bio’s operating or financial results; uncertainties as to the long-term value of Akari’s
American Depositary Shares (and the ordinary shares represented thereby), including the dilution caused by Akari’s issuance of
additional American Depositary Shares (and the ordinary shares represented thereby) in connection with the proposed transaction; unknown
liabilities related to Akari or Peak Bio; the nature, cost and outcome of any litigation and other legal proceedings involving Akari,
Peak Bio or their respective directors, including any legal proceedings related to the proposed transaction; risks related to global
as well as local political and economic conditions, including interest rate and currency exchange rate fluctuations; potential delays
or failures related to research and/or development of Akari’s or Peak Bio’s programs or product candidates; risks related
to any loss of Akari’s or Peak Bio’s patents or other intellectual property rights; any interruptions of the supply chain
for raw materials or manufacturing for Akari or Peak Bio’s product candidates, the nature, timing, cost and possible success and
therapeutic applications of product candidates being developed by Akari, Peak Bio and/or their respective collaborators or licensees;
the extent to which the results from the research and development programs conducted by Akari, Peak Bio, and/or their respective collaborators
or licensees may be replicated in other studies and/or lead to advancement of product candidates to clinical trials, therapeutic applications,
or regulatory approval; uncertainty of the utilization, market acceptance, and commercial success of Akari’s or Peak Bio’s
product candidates, and the impact of studies (whether conducted by Akari, Peak Bio or others and whether mandated or voluntary) on any
of the foregoing; unexpected breaches or terminations with respect to Akari’s or Peak Bios’s material contracts or arrangements;
risks related to competition for Akari’s or Peak Bio’s product candidates; Akari’s or Peak Bio’s ability to successfully
develop or commercialize Akari’s or Peak Bio’s product candidates; Akari’s, Peak Bio’s, and their collaborators’
abilities to continue to conduct current and future developmental, preclinical and clinical programs; potential exposure to legal proceedings
and investigations; risks related to changes in governmental laws and related interpretation thereof, including on reimbursement, intellectual
property protection and regulatory controls on testing, approval, manufacturing, development or commercialization of any of Akari’s
or Peak Bio’s product candidates; unexpected increase in costs and expenses with respect to the potential transaction or Akari’s
or Peak Bio’s business or operations; and risks and uncertainties related to epidemics, pandemics or other public health crises
and their impact on Akari’s and Peak Bio’s respective businesses, operations, supply chain, patient enrollment and retention,
preclinical and clinical trials, strategy, goals and anticipated milestones. While the foregoing list of factors presented here is considered
representative, no list should be considered to be a complete statement of all potential risks and uncertainties. There can be no assurance
that the proposed transaction or any other transaction described above will in fact be consummated in the manner described or at all.
A more complete description of these and other material risks can be found in Akari’s and Peak Bios’s respective filings
with the U.S. Securities and Exchange Commission (the “SEC”), including each of their Annual Reports on Form 20-F and
10-K, respectively, for the year ended December 31, 2022, subsequent periodic reports, and other documents that may be filed from
time to time with the SEC. These risks, as well as other risks associated with the proposed transaction, will be more fully discussed
in the joint proxy statement/prospectus that will be included in the registration statement on Form S-4 that will be filed with
the SEC in connection with the proposed transaction, which joint proxy statement/prospectus will be mailed or otherwise disseminated
to Akari’s shareholders and Peak Bio’s stockholders when it becomes available.
Any forward-looking statements speak only as of the date of this communication
and are made based on the current beliefs and judgments of Akari’s and Peak Bio’s management, and the reader is cautioned
not to rely on any forward-looking statements made by Akari or Peak Bio. Unless required by law, neither Akari nor Peak Bio is under
no duty and undertakes no obligation to update or revise any forward-looking statement after the distribution of this document, including
without limitation any financial projection or guidance, whether as a result of new information, future events or otherwise.
No Offer or Solicitation
This current report is not intended to and shall
not constitute an offer to subscribe for, buy or sell or the solicitation of an offer to subscribe for, buy or sell any securities, or
a solicitation of any vote or approval, nor shall there be any sale of, or offer to sell or buy, securities in any jurisdiction in which
such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction.
This communication is for informational purposes only. No offering of securities shall be made, except by means of a prospectus meeting
the requirements of Section 10 of the U.S. Securities Act of 1933, as amended, and otherwise in accordance with applicable law.
Additional Information and Where to Find It
In connection with the proposed transaction,
Akari and Peak Bio expect to file with the SEC a Registration Statement on Form S-4. The Registration Statement on Form S-4
will include a prospectus of Akari and a joint proxy statement of Akari and Peak Bio, and each party may also file other documents regarding
the proposed transaction with the SEC. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ CAREFULLY THE REGISTRATION STATEMENT ON FORM S-4,
JOINT PROXY STATEMENT/PROSPECTUS AND OTHER RELEVANT DOCUMENTS FILED OR WILL BE FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS
THERETO AND ANY DOCUMENTS INCORPORATED BY REFERENCE THEREIN, IN THEIR ENTIRETY IF AND WHEN THEY BECOME AVAILABLE BECAUSE THEY CONTAIN
OR WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION, RELATED MATTERS AND THE PARTIES TO THE PROPOSED TRANSACTION.
You may obtain a free copy of the Registration
Statement on Form S-4, joint proxy statement/prospectus and other relevant documents (if and when they become available) that are
or will be filed with the SEC for free at the SEC’s website at www.sec.gov. Copies of the documents filed with the SEC by Akari
will be available free of charge on Akari’s website at http://investor.akaritx.com/ or by contacting Akari’s Investor Relations
Department at http://investor.akaritx.com/investor-resources/contact-us. Copies of the documents filed with the SEC by Peak Bio will
be available free of charge on Peak Bio’s website at https://peak-bio.com/investors or by contacting Peak Bio’s Investor
Relations Department at https://peak-bio.com/contact.
Participants in the Solicitation
Akari, Peak Bio and their respective directors and executive officers
and other members of management and employees may be deemed to be participants in the solicitation of proxies in respect of the proposed
transaction. Information about the directors and executive officers of Akari, including a description of their direct or indirect interests,
by security holdings or otherwise, is set forth in Akari’s Annual Report on Form 20-F for the year ended December 31,
2022 filed with the SEC on May 1, 2023, subsequent quarterly and current reports on Form 10-Q and -K, respectively, and other
documents that may be filed from time to time with the SEC. Information about the directors and executive officers of Peak Bio, including
a description of their direct or indirect interests, by security holdings or otherwise, is set forth in Peak Bio’s proxy statement
for its 2022 Special Meeting of Stockholders, which was filed with the SEC on October 19, 2022, the Annual Report on Form 10-K
for the year ended December 31, 2022 filed with the SEC on June 29, 2023, subsequent quarterly and current reports on Form 10-Q
and Form 8-K, respectively, and other documents that may be filed from time to time with the SEC. Other information regarding the
participants in the proxy solicitations and a description of their direct and indirect interests, by security holdings or otherwise,
will be contained in the joint proxy statement/prospectus included in the Registration Statement on Form S-4 and other relevant
materials to be filed with the SEC regarding the proposed transaction when such materials become available. Security holders, potential
investors and other readers should read the joint proxy statement/prospectus, included in the Registration Statement on Form S-4
carefully when it becomes available before making any voting or investment decision. You may obtain free copies of these documents from
Akari or Peak Bio using the sources indicated above.
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934,
the Registrant has caused this Report to be signed on its behalf by the undersigned thereunto duly authorized.
| |
Akari
Therapeutics, Plc |
| |
|
|
| Date: March 5, 2024 |
By: |
/s/ Rachelle Jacques |
| |
Name: |
Rachelle
Jacques |
| |
Title: |
Chief
Executive Officer |
Exhibit 2.1
Execution Version
AGREEMENT AND PLAN OF MERGER
BY AND
AmONG
AKARI
THERAPEUTICS, PLC,
PEGASUS
MERGER SUB, INC.
AND
PEAK BIO, INC.
_____________________________
Dated as of March 4, 2024
_____________________________
Table
of Contents
| 1.4 | Directors and Officers of the Surviving Corporation |
3 |
| 1.6 | Post-Merger Operations |
3 |
| Section 2 CONVERSION
OF SECURITIES |
3 |
| 2.1 | Conversion of Capital Stock |
3 |
| 2.2 | Exchange of Certificates and Book-Entry Shares |
4 |
| 2.4 | Company Warrants and Company Compensatory Awards |
7 |
| 2.6 | Additional Company Merger Shares |
9 |
| Section 3 REPRESENTATIONS
AND WARRANTIES OF THE COMPANY |
9 |
| 3.1 | Organization, Standing and Corporate Power |
9 |
| 3.2 | Corporate Authorization |
10 |
| 3.3 | Governmental Authorization |
10 |
| 3.7 | SEC Filings and the Sarbanes-Oxley Act |
13 |
| 3.8 | Information Supplied |
16 |
| 3.9 | Absence of Certain Changes |
16 |
| 3.10 | No Undisclosed Liabilities |
16 |
| 3.11 | Compliance with Laws and Court Orders |
16 |
| 3.12 | Material Contracts |
16 |
| 3.15 | Intellectual Property |
20 |
| 3.17 | Employee Benefit Plans |
24 |
| 3.18 | Employment Matters |
25 |
| 3.19 | Environmental Matters |
26 |
| 3.20 | Regulatory Matters; Compliance |
27 |
| 3.21 | Healthcare Regulatory; Compliance |
28 |
| 3.23 | Anti-Corruption; Global Trade Control Laws |
29 |
| 3.25 | Brokers and Finder’s Fees |
30 |
| 3.26 | Opinion of the Financial Advisor |
31 |
| | 3.28 | No Other Representations; No Reliance; Waiver |
31 |
| Section 4 REPRESENTATIONS
AND WARRANTIES OF PARENT AND MERGER SUB |
32 |
| 4.1 | Organization, Standing and Corporate Power |
32 |
| 4.2 | Corporate Authorization |
32 |
| 4.3 | Governmental Authorization |
33 |
| 4.7 | SEC Filings and the Sarbanes-Oxley Act |
36 |
| 4.8 | Information Supplied |
38 |
| 4.9 | Absence of Certain Changes |
38 |
| 4.10 | No Undisclosed Liabilities |
38 |
| 4.11 | Compliance with Laws and Court Orders |
39 |
| 4.12 | Material Contracts |
39 |
| 4.15 | Intellectual Property |
42 |
| 4.17 | Employee Benefit Plans |
46 |
| 4.18 | Employment Matters |
48 |
| 4.19 | Environmental Matters |
48 |
| 4.20 | Regulatory Matters; Compliance |
49 |
| 4.21 | Healthcare Regulatory; Compliance |
51 |
| 4.23 | Anti-Corruption; Global Trade Control Laws |
52 |
| 4.25 | Brokers and Finder’s Fees |
53 |
| 4.26 | Opinion of the Financial Advisor |
53 |
| 4.28 | Ownership and Operations of Merger Sub |
53 |
| 4.29 | No Other Representations; No Reliance; Waiver |
53 |
| Section 5 COVENANTS
AND AGREEMENTS |
54 |
| 5.1 | Conduct of the Company’s Business |
54 |
| 5.2 | Conduct of Parent Business |
57 |
| 5.3 | No Solicitation by the Company |
61 |
| 5.4 | No Solicitation by Parent |
64 |
| Section 6 ADDITIONAL
COVENANTS AND AGREEMENTS |
66 |
| 6.1 | Registration Statement; Proxy Statement/Prospectus |
66 |
| 6.2 | Meetings of Stockholders |
67 |
| 6.3 | Access to Information |
68 |
| 6.5 | Regulatory Filings; Commercially Reasonable Efforts |
69 |
| 6.6 | Notification of Certain Matters |
71 |
| 6.7 | Transaction Litigation |
71 |
| 6.9 | Director and Officer Liability |
71 |
| 6.10 | Stock Exchange De-Listing and Deregistration |
72 |
| 6.11 | Stock Exchange Listing |
73 |
| 6.12 | Section 16 Matters |
73 |
| 6.13 | Company’s Auditors |
73 |
| 6.15 | Integration Planning |
73 |
| 6.18 | Determination of Exchange Ratio |
74 |
| Section 7 CONDITIONS
PRECEDENT TO THE OBLIGATION OF PARTIES TO CONSUMMATE THE MERGER |
76 |
| 7.1 | Conditions to Obligations of Each Party to Effect the Merger |
76 |
| 7.2 | Additional Conditions to the Obligations of Parent and Merger Sub |
76 |
| 7.3 | Additional Conditions to the Obligations of the Company |
77 |
| 7.4 | Frustration of Closing Conditions |
78 |
| Section 8 TERMINATION,
AMENDMENT AND WAIVER |
78 |
| 8.2 | Effect of Termination; Termination Fee |
80 |
| 8.4 | Notice of Termination |
81 |
| Section 9 MISCELLANEOUS |
82 |
| 9.5 | Binding Effect; No Assignment; No Third-Party Beneficiaries |
83 |
| 9.6 | Counterparts and Signature |
84 |
| 9.8 | Submission to Jurisdiction; Waiver |
84 |
| 9.10 | No Waiver; Remedies Cumulative |
85 |
| 9.11 | Waiver of Jury Trial |
85 |
| Section 10 DEFINITIONS |
85 |
| 10.1 | Certain Definitions |
85 |
| 10.2 | Other Definitional and Interpretative Provisions |
101 |
EXHIBITS
| Exhibit A: |
Form of Company Voting Agreement |
| Exhibit B: |
Form of Parent Voting Agreement |
Index of Defined Terms
| Section |
| |
| AAA |
6.18(e) |
| Accounting Firm |
6.18(e) |
| Action |
3.13 |
| Adjusted Option |
2.4(b) |
| Adjusted Warrant |
2.4(a) |
| Agreement |
Preamble |
| Antitrust Laws |
10.1 |
| Anti-Corruption Laws |
3.23(a) |
| Assignee |
9.5(a) |
| Bankruptcy and Equity Exception |
3.2(a) |
| Book-Entry Share |
2.1(c) |
| Business Day |
10.1 |
| Capitalization Date |
3.5(a) |
| Cash Determination Time |
6.18(a) |
| Certificate |
2.1(c) |
| Certificate of Merger |
1.2 |
| Chairman Appointment |
1.6 |
| Closing |
1.3 |
| Closing Date |
1.3 |
| Code |
2.4(b) |
| Collective Bargaining Agreements |
3.18(b) |
| Company |
Preamble |
| Company Acquisition Proposal |
10.1 |
| Company Adverse Recommendation Change |
5.3(c) |
| Company Board |
Recitals |
| Company Charter |
10.1 |
| Company Charter Documents |
3.1(c) |
| Company Common Stock |
Recitals |
| Company Disclosure Letter |
Section 3 |
| Company Equity Plan |
10.1 |
| Company Financial Advisors |
3.25 |
| Company Foreign Plan |
10.1 |
| Company Furnished Document |
3.7(a) |
| Company Insurance Policies |
3.22(a) |
| Company Intellectual Property |
3.15(a) |
| Company Intervening Event |
10.1 |
| Company Leased Real Property |
3.14(b) |
| Company Material Adverse Effect |
10.1 |
| Company Material Contracts |
3.12(a) |
| Company Net Cash Schedule |
6.18(a) |
| Company Option |
2.4(b) |
| Company Permits |
3.20(a) |
| Company Permitted Liens |
10.1 |
| Company Plan |
10.1 |
| Company Preferred Stock |
3.5(a) |
| Company Real Property Lease |
3.14(b) |
| Company Recommendation |
3.2(b) |
| Section |
| |
| Company Registered Intellectual Property |
3.15(b) |
| Company Regulatory Agency |
3.20(a) |
| Company Representatives |
5.3(a) |
| Company Related Persons |
3.28 |
| Company SEC Documents |
3.7(a) |
| Company Securities |
5.1(b)(ii) |
| Company Stockholder Approval |
3.2(a) |
| Company Stockholders Meeting |
6.2(a) |
| Company Superior Proposal |
10.1 |
| Company Systems |
3.15(o) |
| Company Voting Agreement |
Recitals |
| Company Warrant |
10.1 |
| Confidentiality Agreement |
10.1 |
| Contract |
10.1 |
| Copyrights |
10.1 |
| Deposit Agreement |
10.1 |
| Determination Date |
6.18(a) |
| Dispute Notice |
6.18(c) |
| Dissenting Shares |
2.3 |
| DGCL |
Recitals |
| EDGAR |
Section 3 |
| Effective Time |
1.2 |
| Employee Benefit Plan |
10.1 |
| Environmental Claim |
10.1 |
| Environmental Laws |
10.1 |
| Environmental Liability |
10.1 |
| Environmental Permits |
10.1 |
| Equity Interest |
10.1 |
| ERISA |
10.1 |
| ERISA Affiliate |
10.1 |
| Exchange Act |
10.1 |
| Exchange Agent |
2.2(a) |
| Exchange Fund |
2.2(a) |
| Exchange Ratio |
10.1 |
| FCPA |
10.1 |
| FDA |
3.20(a) |
| FDCA |
3.20(a) |
| Form S-4 |
3.8 |
| GAAP |
10.1 |
| Global Trade Control Laws |
10.1 |
| Government Official |
10.1 |
| Governmental Authority |
10.1 |
| Hazardous Materials |
10.1 |
| Healthcare Laws |
10.1 |
| HSR Act |
10.1 |
| Inbound IP Agreements |
3.15(a) |
| Indebtedness |
10.1 |
| Indemnified Party |
6.9(a) |
| Intellectual Property |
10.1 |
| Section |
| |
| Intentional Breach |
10.1 |
| IP Agreements |
3.15(g) |
| IP Governmental Authority |
10.1 |
| IRS |
3.17(a) |
| Knowledge of Parent |
10.1 |
| Knowledge of the Company |
10.1 |
| Law |
10.1 |
| Lien |
10.1 |
| material weakness |
3.7(g) |
| Maximum Premium |
6.9(c) |
| Merger |
1.1(a) |
| Merger Sub |
Preamble |
| Merger Sub Common Stock |
2.1 |
| Minimum Amount |
6.16 |
| Nasdaq |
4.2 |
| Net Cash Schedule |
6.18(b) |
| OFAC |
10.1 |
| Owned Company Intellectual Property |
10.1 |
| Outbound IP Agreements |
3.15(g) |
| Parent |
Preamble |
| Parent Acquisition Proposal |
10.1 |
| Parent ADSs |
Recitals |
| Parent Adverse Recommendation Change |
5.4(c) |
| Parent Board |
Recitals |
| Parent Charter Documents |
4.1(c) |
| Parent Disclosure Letter |
Section 4 |
| Parent Furnished Documents |
4.7(a) |
| Parent Inbound IP Agreements |
4.15(f) |
| Parent Insurance Policies |
4.22(a) |
| Parent Intellectual Property |
4.15(a) |
| Parent Intervening Event |
10.1 |
| Parent IP Agreements |
4.15(g) |
| Parent Leased Real Property |
4.14(b) |
| Parent Material Adverse Effect |
10.1 |
| Parent Material Contracts |
4.12(a) |
| Parent Net Cash Schedule |
6.18(b) |
| Parent Options |
4.5(b) |
| Parent Ordinary Shares |
Recitals |
| Parent Permits |
4.20(a) |
| Parent Permitted Liens |
10.1 |
| Parent Plan |
10.1 |
| Parent Real Property Lease |
4.14(b) |
| Parent Recommendation |
4.2(b) |
| Parent Registered Intellectual Property |
4.15(b) |
| Parent Regulatory Agency |
4.20(a) |
| Parent Related Person |
3.28 |
| Parent Representatives |
5.4(a) |
| Parent RSUs |
4.5(b) |
| Parent SEC Documents |
4.7(a) |
| Section |
| |
| Parent Securities |
5.2(b)(ii) |
| Parent Shareholder Approval |
4.2(a) |
| Parent Shareholder |
Recitals |
| Parent Shareholders Meeting |
6.2(b) |
| Parent Superior Proposal |
10.1 |
| Parent Systems |
4.15(o) |
| Parent Voting Agreement |
Recitals |
| Patents |
10.1 |
| Per Share Merger Consideration |
2.1(c) |
| Person |
10.1 |
| PHSA |
3.20(a) |
| PIPE Investment |
6.16 |
| Pre-Closing Period |
5.1(a) |
| Preparing Party |
6.18(c) |
| Proxy Statement/Prospectus |
3.8 |
| Redomiciliation |
6.17 |
| Release |
10.1 |
| Response Date |
6.18(c) |
| Restraints |
7.1(c) |
| Restricted Market |
10.1 |
| Restricted Party |
10.1 |
| Reviewing Party |
6.18(c) |
| Rights Agent |
Recitals |
| Sarbanes-Oxley Act |
10.1 |
| SEC |
10.1 |
| Securities Act |
10.1 |
| Software |
10.1 |
| Transaction Litigation |
6.7 |
| Subscription Agreement |
6.16 |
| Subsidiary |
10.1 |
| Surviving Corporation |
1.1(a) |
| Takeover Laws |
3.27 |
| Tax |
10.1 |
| Tax Return |
10.1 |
| Tax Sharing Agreements |
10.1 |
| Termination Date |
8.1(d)(i) |
| Termination Fee |
10.1 |
| third party |
10.1 |
| third-party Intellectual Property |
10.1 |
| Trade Secrets |
10.1 |
| Trademarks |
10.1 |
| Treasury Regulation |
10.1 |
| Union |
3.18(b) |
AGREEMENT AND PLAN OF MERGER
THIS AGREEMENT AND PLAN OF
MERGER (this “Agreement”), dated as of March 4, 2024, is among Akari Therapeutics, Plc (“Parent”),
a public company limited by shares incorporated in England and Wales, Pegasus Merger Sub, Inc. (“Merger Sub”),
a Delaware corporation and a wholly owned subsidiary of Parent, and Peak Bio, Inc. (the “Company”), a Delaware
corporation.
R E C I T A L S
WHEREAS,
the Board of Directors of Parent (the “Parent Board”) and the Board of Directors of the Company (the “Company
Board”) have determined that a business combination between Parent and the Company presents the opportunity for their
respective companies to achieve long-term financial and strategic benefits and accordingly have determined to effect a business combination
upon the terms and conditions set forth in this Agreement;
WHEREAS,
the Parent Board and the Company Board propose to effect such business combination, pursuant to which Merger Sub will merge with
and into the Company, with the Company surviving as a wholly owned subsidiary of Parent, as more fully provided in this Agreement;
WHEREAS, the Board of Directors
of Merger Sub has approved this Agreement and the transactions contemplated hereby, including the Merger, and has resolved to recommend
that the sole stockholder of Merger Sub adopt this Agreement, in accordance with the General Corporation Law of the State of Delaware
(the “DGCL”) and upon the terms and subject to the conditions set forth herein;
WHEREAS, the Company Board
has (i) determined that this Agreement and the transactions contemplated hereby, including the Merger, are advisable, fair to and
in the best interests of, the Company and the holders of outstanding shares of the common stock, par value $0.0001 per share, of the
Company (the “Company Common Stock”), (ii) approved, adopted and declared advisable this Agreement and the transactions
contemplated hereby, including the Merger and (iii) subject to the terms and conditions of this Agreement, has resolved to recommend
that the holders of shares of Company Common Stock adopt this Agreement;
WHEREAS,
the Parent Board has (i) determined that this Agreement and the transactions contemplated by this Agreement, including the
Merger, are advisable, fair to and in the best interests of the holders of ordinary shares, nominal value $0.0001 per share, of Parent
(the “Parent Ordinary Shares”), as a whole, with each Parent American Depository Share representing two thousand (2,000)
Parent Ordinary Shares (“Parent ADSs”) legally issued in accordance with the Deposit Agreement (such holders of Parent
Ordinary Shares, including Parent Ordinary Shares represented by Parent ADSs, collectively, the “Parent Shareholders”),
(ii) approved, adopted and declared advisable this Agreement and the transactions contemplated hereby, including the Merger and
(iii) subject to the terms and conditions of this Agreement, has resolved to recommend that the Parent Shareholders authorize the
Parent Board to allot all Parent Ordinary Shares to be issued in connection with the Merger and approve the issuance of Parent Ordinary
Shares to be represented by Parent ADSs in connection with the Merger as provided in Section 2;
WHEREAS, concurrently with
the execution and delivery of this Agreement, and as a condition and inducement to Parent’s willingness to enter into this Agreement,
certain stockholders of the Company have executed and delivered a voting agreement in the form set forth in Exhibit A attached
hereto, dated as of the date hereof, by and between Parent and such stockholders (the “Company Voting Agreement”);
WHEREAS, concurrently with
the execution and delivery of this Agreement, and as a condition and inducement to the Company’s willingness to enter into this
Agreement, certain shareholders of Parent have executed and delivered a voting agreement in the form set forth in Exhibit B
attached hereto, dated as of the date hereof, by and between the Company and such shareholders (the “Parent Voting Agreement”);
and
WHEREAS, Parent, Merger Sub
and the Company desire to make certain representations, warranties, covenants and agreements in connection with the Merger and the other
transactions contemplated hereby.
NOW, THEREFORE, in consideration
of the foregoing and the respective representations, warranties covenants and agreements set forth herein, the parties hereto agree as
follows:
Section 1
THE MERGER
1.1 The
Merger.
(a) Subject
to the terms and conditions of this Agreement, at the Effective Time, the Company and Merger Sub shall consummate a merger (the “Merger”),
in accordance with the DGCL, pursuant to which (i) Merger Sub shall be merged with and into the Company and the separate corporate
existence of Merger Sub shall thereupon cease, (ii) the Company shall be the surviving corporation in the Merger (the “Surviving
Corporation”) and shall continue to be governed by the laws of the State of Delaware, (iii) the corporate existence of
the Company, with all its rights, privileges, immunities, powers and franchises, shall continue unaffected by the Merger and (iv) the
Surviving Corporation shall succeed to and assume all the rights and obligations of Merger Sub and the Company in accordance with the
DGCL. As a result of the Merger, the Surviving Corporation shall become a wholly owned subsidiary of Parent.
(b) At
the Effective Time, by virtue of the Merger, the Certificate of Incorporation of the Surviving Corporation, as in effect immediately
prior to the Effective Time, shall be amended and restated in its entirety to read as the Certificate of Incorporation of Merger Sub
in effect immediately prior to the Effective Time, except that all references therein to Merger Sub shall be deemed to be references
to the Surviving Corporation, until thereafter changed or amended as provided therein or by applicable Law.
(c) At
the Effective Time, by virtue of the Merger, the By-Laws of the Surviving Corporation, as in effect immediately prior to the Effective
Time, shall be amended and restated in their entirety to read as the By-Laws of Merger Sub in effect immediately prior to the Effective
Time, except that all references therein to Merger Sub shall be deemed to be references to the Surviving Corporation, until thereafter
changed or amended as provided therein or by applicable Law.
1.2 Effective
Time. Parent, Merger Sub and the Company shall cause a certificate of merger with respect to the Merger (the “Certificate
of Merger”) to be filed on the Closing Date or on such other date as Parent and the Company may agree, with the Secretary of
State of the State of Delaware as provided in the DGCL. The Merger shall become effective at such time as the Certificate of Merger is
duly filed with the Secretary of State of the State of Delaware or such later time and date as may be agreed by Parent and the Company
in writing and specified in the Certificate of Merger, and such time on such date is referred to herein as the “Effective Time”.
1.3 Closing.
The closing of the Merger (the “Closing”) shall take place as early as practicable on a date to be specified by the
parties hereto, which shall be no later than the third (3rd) Business Day after satisfaction or valid waiver of all of the
conditions to Closing set forth in Section 7, except for any such conditions that by their nature may only be satisfied at
the Closing, but subject to the satisfaction or waiver of such conditions at the Closing (the “Closing Date”), by
electronic exchange of deliverables, unless another date, time or place is agreed to in writing by the parties hereto.
1.4 Directors
and Officers of the Surviving Corporation. The directors of Merger Sub immediately prior to the Effective Time shall, from and after
the Effective Time, be the directors of the Surviving Corporation, and the officers of Merger Sub immediately prior to the Effective
Time shall, from and after the Effective Time, be the officers of the Surviving Corporation, in each case until their respective successors
shall have been duly elected, designated or qualified, or until their earlier death, resignation or removal in accordance with the Surviving
Corporation's Certificate of Incorporation and By-Laws.
1.5 Subsequent
Actions. At and after the Effective Time, the Merger shall have the effects set forth in the DGCL. If at any time after the Effective
Time the Surviving Corporation shall determine, in its sole discretion, or shall be advised, that any deeds, bills of sale, instruments
of conveyance, assignments, assurances or any other actions or things are necessary or desirable to vest, perfect or confirm of record
or otherwise in the Surviving Corporation its right, title or interest in, to or under any of the rights, properties or assets of either
the Company or Merger Sub acquired or to be acquired by the Surviving Corporation as a result of, or in connection with, the Merger or
otherwise to carry out this Agreement, then the officers and directors of the Surviving Corporation shall be authorized to execute and
deliver, in the name and on behalf of either the Company or Merger Sub, all such deeds, bills of sale, instruments of conveyance, assignments
and assurances and to take and do, in the name and on behalf of each such corporation or otherwise, all such other actions and things
as may be necessary or desirable to vest, perfect or confirm any and all right, title or interest in, to and under such rights, properties
or assets in the Surviving Corporation or otherwise to carry out this Agreement.
1.6 Post-Merger
Operations. The Parent Board shall take all necessary corporate action to cause the following to occur as of the Effective Time:
(a) the directors constituting the Parent Board shall consist of seven (7) directors, with three (3) directors designated
by Parent, three (3) directors designated by the Company (provided, that, one such designee shall be the non-executive chairman
as set forth on Section 1.6(b) of the Parent Disclosure Letter) and one (1) director designated by Parent and the Company
by mutual agreement, subject to such individuals’ ability and willingness to serve and (b) the non-executive chairman of the
Parent Board be designated in accordance with the provisions of Section 1.6(b) of the Parent Disclosure Letter (the “Chairman
Appointment”), subject to such individual’s ability and willingness to serve. In the event any designee becomes unable
or unwilling to serve as a director on the Parent Board or chairman of Parent as of the Effective Time, a replacement for such designee
shall be determined by Parent and the Company by mutual agreement. Parent shall take all necessary actions to procure resignations of
directors of the Parent Board in a form reasonably acceptable to the Company (the “Resignations”) such that, at the
Effective Time, the Parent Board is determined in accordance with this Section 1.6.
Section 2
CONVERSION OF SECURITIES
2.1 Conversion
of Capital Stock. As of the Effective Time, by virtue of the Merger and without any action on the part of the holders of any shares
of Company Common Stock or any shares of common stock of Merger Sub (“Merger Sub Common Stock”):
(a) Merger
Sub Common Stock and Surviving Corporation Stock. Each issued and outstanding share of Merger Sub Common Stock immediately prior
to the Effective Time shall be converted into and become one share of the Surviving Corporation with the rights, powers and privileges
set forth in the Certificate of Incorporation and the By-Laws of the Surviving Corporation.
(b) Cancellation
of Treasury Stock and Parent-Owned Stock. All shares of Company Common Stock that are held by the Company as treasury stock and any
shares of Company Common Stock owned by Parent, Merger Sub or any other direct or indirect wholly owned subsidiary of Parent shall automatically
be cancelled and retired and shall cease to exist, and no consideration shall be delivered in exchange therefor.
(c) Conversion
of Shares of Company Common Stock. Each issued and outstanding share of Company Common Stock (other than shares of Company Common
Stock to be cancelled in accordance with Section 2.1(b) and Dissenting Shares) shall be converted into the right to
receive Parent ADSs representing a number of Parent Ordinary Shares equal to the Exchange Ratio, each such share duly and validly issued
against the deposit of the requisite number of Parent Ordinary Shares in accordance with the Deposit Agreement (the “Per Share
Merger Consideration”) plus, if applicable, any Additional Per Share Merger Consideration payable in accordance with
Section 2.6; provided, that after taking into account all of the Certificates and Book-Entry Shares delivered by or
on behalf of any holder, the number of Parent Ordinary Shares deposited in accordance with the Deposit Agreement and the Parent ADSs
issued to such holder shall, in each case, be rounded down to the nearest whole Parent Ordinary Share or Parent ADS, as applicable, and
no fractional Parent Ordinary Shares shall be deposited and no fractional Parent ADSs shall be issued. From and after the Effective Time,
all such shares of Company Common Stock shall no longer be outstanding and shall automatically be cancelled and retired and shall cease
to exist, and each holder of a certificate (a “Certificate”) or book-entry share (a “Book-Entry Share”)
that immediately prior to the Effective Time represented outstanding shares of Company Common Stock shall cease to have any rights with
respect thereto, except the right to receive the Per Share Merger Consideration (plus, if applicable, any Additional Per Share
Merger Consideration payable in accordance with Section 2.6) and any dividends or other distributions declared by the Company
Board having a record date prior to the Effective Time which remain unpaid as of the Effective Time, without interest thereon, together
with any dividends or other distributions to which holders thereof are entitled pursuant to Section 2.2(c), upon the surrender
of such Certificate or Book-Entry Share in accordance with Section 2.2.
(d) Adjustments.
If at any time during the period between the date of this Agreement and the Effective Time (or, in the case of the Additional Per Share
Merger Consideration, the applicable date of determination pursuant to Section 2.6), any change in the issued shares of Company
Common Stock, Parent Ordinary Shares or Parent ADSs, as the case may be, shall occur as a result of any reclassification, stock split
(including a reverse stock split), combination, exchange, readjustment, stock dividend or stock distribution or any similar event, the
Per Share Merger Consideration, the Additional Per Share Merger Consideration (if any) and any other similarly dependent items (including
any amounts payable pursuant to Section 2.4) shall be equitably adjusted to provide to the holders of shares of Company Common
Stock, Company Options, Company Warrants and other awards under the Company Equity Plan the same economic effect as contemplated by this
Agreement prior to such action; provided, that nothing in this Section 2.1(d) shall be deemed to permit any party hereto
to take any action that is prohibited under either Section 5.1(b) or 5.2(b) or that is not otherwise permitted
by this Agreement.
2.2 Exchange
of Certificates and Book-Entry Shares.
(a) Exchange
Agent. Prior to the Effective Time, Parent shall designate a bank or trust company reasonably acceptable to the Company to act as
agent for the holders of shares of Company Common Stock in connection with the Merger (the “Exchange Agent”) and to
receive the consideration to which holders of shares of Company Common Stock shall become entitled pursuant to Section 2.1(c).
Parent shall, at or prior to the Closing, (i) deposit with the Exchange Agent Parent ADSs evidencing or (ii) provide the Exchange
Agent an uncertificated Parent ADS book-entry representing the aggregate number of Parent ADSs that are issuable pursuant to Section 2.1(c) (such
Parent ADSs, together with any distributions or dividends with respect thereto as provided in Section 2.2(c), being hereinafter
referred to as the “Exchange Fund”).
(b) Exchange
Procedures.
(i) As
promptly as practicable after the Effective Time (but in no event later than five (5) Business Days following the Effective
Time), the Exchange Agent shall mail to each holder of record of a Certificate representing shares of Company Common Stock, whose
shares were converted pursuant to Section 2.1(c) into the right to receive the Per Share Merger Consideration:
(i) a letter of transmittal (which shall specify that delivery shall be effected, and risk of loss and title to a Certificate
shall pass, only upon delivery of such Certificate to the Exchange Agent and shall be in such form and have such other provisions as
Parent may reasonably specify); and (ii) instructions for effecting the surrender of the Certificates in exchange for payment
of the Per Share Merger Consideration plus, if applicable, any Additional Per Share Merger Consideration payable in
accordance with Section 2.6. Upon surrender of a Certificate for cancellation to the Exchange Agent, together with such
letter of transmittal, duly executed and properly completed, the holder of such Certificate shall be entitled to receive in exchange
therefor the Per Share Merger Consideration (plus, if applicable, any Additional Per Share Merger Consideration payable in
accordance with Section 2.6) for each share of Company Common Stock formerly represented by such Certificate, and the
Certificate so surrendered shall forthwith be cancelled. Until surrendered as contemplated by this Section 2.2, each
Certificate shall be deemed at any time after the Effective Time to represent only the right to receive the Per Share Merger
Consideration as contemplated by this Section 2.2 plus, if applicable, any Additional Per Share Merger Consideration
payable in accordance with Section 2.6 and shall not evidence any interest in, or any right to exercise the rights of a
stockholder or other equity holder of, the Company or the Surviving Corporation. In the event of a transfer of ownership of shares
of Company Common Stock that is not registered in the transfer records of the Company, the issuance of Parent ADSs or book-entries
permitting the proper number of Parent ADSs, together with a check for any cash to be paid upon due surrender of the Certificate,
shall be made to such transferee (after giving effect to any required Tax withholdings as provided in Section 2.5) if
the Certificate formerly representing such shares is presented to the Exchange Agent, accompanied by all documents reasonably
required to evidence and effect such transfer and to evidence that any and all transfer and other Taxes required by reason of the
issuance to such transferee have been paid or are not applicable.
(ii) Notwithstanding
anything to the contrary in this Agreement, any holder of Book-Entry Shares shall not be required to deliver a Certificate or an executed
letter of transmittal to the Exchange Agent to receive the Per Share Merger Consideration that such holder is entitled to receive pursuant
to this Section 2 plus, if applicable, any Additional Per Share Merger Consideration payable in accordance with Section 2.6.
In lieu thereof, each holder of record of one or more Book-Entry Shares whose shares of Company Common Stock were converted into the
right to receive the Per Share Merger Consideration plus, if applicable, any Additional Per Share Merger Consideration payable
in accordance with Section 2.6 shall upon receipt by the Exchange Agent of an “agent’s message” in customary
form (or such other evidence, if any, as the Exchange Agent may reasonably request), be entitled to receive, and Parent shall cause the
Exchange Agent to pay and deliver as promptly as reasonably practicable after the Effective Time, the Per Share Merger Consideration
plus, if applicable, any Additional Per Share Merger Consideration payable in accordance with Section 2.6, in each
case, in respect of each such share of Company Common Stock, and the Book-Entry Shares of such holder shall forthwith be cancelled.
(c) Distributions
with Respect to Unexchanged Shares. All Parent ADSs to be issued pursuant to the Merger (and all Parent Ordinary Shares represented
thereby) shall be deemed issued and outstanding as of the Effective Time; provided that no dividends or other distributions with
respect to Parent ADSs (or Parent Ordinary Shares represented thereby) with a record date after the Effective Time shall be paid to the
former holder of any Company Common Stock until such holder shall surrender such shares in accordance with this Section 2.2.
Subject to the effect of applicable Law: (i) at the time of the surrender of any such shares of Company Common Stock for exchange
in accordance with the provisions of this Section 2.2, there shall be paid to the surrendering holder, without interest,
the amount of dividends or other distributions declared by the Parent Board (having a record date after the Effective Time but on or
prior to surrender and a payment date on or prior to surrender) not theretofore paid with respect to the number of whole Parent ADSs
that such holder is entitled to receive; and (ii) at the appropriate payment date and without duplicating any payment made under
clause (i) above, there shall be paid to the surrendering holder, without interest, the amount of dividends or other distributions
(having a record date after the Effective Time but on or prior to surrender and a payment date subsequent to surrender) payable with
respect to the number of whole Parent ADSs that such holder receives.
(d) Transfer
Books; No Further Ownership Rights in Shares of Company Common Stock. At the Effective Time, the stock transfer books of the Company
shall be closed and thereafter there shall be no further registration of transfers of shares of Company Common Stock on the records of
the Company. From and after the Effective Time, the holders of Certificates or Book-Entry Shares evidencing ownership of shares of Company
Common Stock outstanding immediately prior to the Effective Time shall cease to have any rights with respect to such shares of Company
Common Stock, except as otherwise provided for herein or by applicable Law. If, after the Effective Time, Certificates are presented
to the Surviving Corporation for any reason, they shall be cancelled and exchanged as provided in this Section 2.2(d).
(e) Treatment
of Fractional Parent ADSs. Notwithstanding any other provision of this Agreement, no fractional Parent ADSs shall be issued in exchange
for any Company Common Stock or in respect of any Adjusted Warrant or Adjusted Option, and no holder of any of the foregoing shall be
entitled to receive a fractional Parent ADS. Furthermore, no holder of a fractional share of Company Common Stock, if any, shall receive
or be entitled to receive any aggregate consideration with respect to such fractional share. No scrip representing fractional Parent
ADSs or book-entry credit of the same shall be issued in the Merger and, except as provided in this Section 2.2(e), no dividend
or other distribution, stock split or interest shall relate to any such fractional share, and such fractional share shall not entitle
the owner thereof to vote or to any other rights of a Parent Shareholder or to any other aggregate consideration. The number of Parent
ADSs to which a former holder of Company Common Stock, Company Warrants or Company Options is entitled under the terms hereof shall (after
taking into account all of the Certificates and Book-Entry Shares delivered by or on behalf of such holder), be rounded down to the nearest
whole number of Parent ADSs.
(f) Termination
of Exchange Fund; No Liability. Any portion of the Exchange Fund deposited with the Exchange Agent that remains undistributed to
holders of Certificates or Book-Entry Shares as of twelve (12) months after the Effective Time shall be delivered to the Parent (subject
to abandoned property, escheat or similar Law). Notwithstanding the foregoing, none of Parent, the Surviving Corporation, the Exchange
Agent or any other Person shall be liable to any holder of a Certificate or Book-Entry Share for Per Share Merger Consideration or Additional
Per Share Merger Consideration delivered to a Governmental Authority pursuant to any applicable abandoned property, escheat or similar
Law. If Certificates and Book-Entry Shares are not surrendered prior to the fifth (5th) anniversary of the Closing Date (or
such earlier date immediately prior to such time as such amounts would otherwise escheat to or become property of any Governmental Authority),
unclaimed Per Share Merger Consideration or Additional Per Share Merger Consideration payable with respect to such shares of Company
Common Stock shall, to the extent permitted by applicable Law, become the property of Parent or as otherwise determined by Parent, free
and clear of all claims or interest of any Person previously entitled thereto.
(g) Lost
Certificates. If any Certificate shall have been lost, stolen or destroyed, upon the making of an affidavit of that fact by the Person
claiming such Certificate to be lost, stolen or destroyed and, if required by Parent, the posting by such Person of a bond in such customary
amount as Parent may reasonably direct as indemnity against any claim that may be made against it or the Surviving Corporation with respect
to such Certificate, the Exchange Agent shall issue in exchange for such lost, stolen or destroyed Certificate the applicable Per Share
Merger Consideration or, if applicable, the Additional Per Share Merger Consideration with respect thereto.
2.3 Dissenting
Shares. Notwithstanding any provision in this Agreement to the contrary, any share of Company Common Stock outstanding as of immediately
prior to the Effective Time and held by a holder who has not voted in favor of the Merger or consented thereto in writing and who has
properly demanded appraisal for such share in accordance with Section 262 of the DGCL (such shares of Company Common Stock, collectively,
“Dissenting Shares”) will not be converted into the right to receive the Per Share Merger Consideration or Additional
Per Share Merger Consideration. At the Effective Time, all Dissenting Shares will no longer be outstanding and automatically will be
cancelled and will cease to exist, and, except as otherwise provided by applicable Laws, each holder of Dissenting Shares will cease
to have any rights with respect to the Dissenting Shares, other than such rights as are granted under Section 262 of the DGCL. Holders
of such Dissenting Shares will be entitled to receive payment for the appraised value of such Dissenting Shares as determined in accordance
with Section 262 of the DGCL; provided, however, that if, after the Effective Time, such holder fails to perfect,
withdraws or loses the right to appraisal, each such Dissenting Share will be treated as if it had been converted as of the Effective
Time into the right to receive the Per Share Merger Consideration plus, if applicable, any Additional Per Share Merger Consideration,
in each case without interest thereon, upon surrender of such shares of Company Common Stock in the manner provided in Section 2.2.
The Company will give Parent prompt notice of any demands received by the Company for appraisal of shares and withdrawals of any such
demand, and any other communications delivered to the Company pursuant to or in connection with Section 262 of the DGCL, and Parent
shall have the opportunity to participate in all negotiations and proceedings with respect to such demands (including settlement offers).
The Company shall not, except with the prior written consent of Parent, make any payment, or offer or agree to make any payment, with
respect to any demands for appraisal or offer to settle or settle any such demands.
2.4 Company
Warrants and Company Compensatory Awards.
(a) At
the Effective Time, each Company Warrant outstanding immediately prior to the Effective Time shall be converted into and exchangeable
for warrants to purchase a number of Parent Ordinary Shares or Parent ADSs, as determined by Parent (each, an “Adjusted Warrant”),
on substantially similar terms and subject to substantially similar conditions as were applicable to such Company Warrant immediately
prior to the Effective Time, except (i) for terms rendered inoperative by reason of the transactions contemplated by this Agreement,
(ii) as provided in the following sentence and (iii) such amendments to the terms of the Adjusted Warrants as are necessary
to comply with applicable Law. The number of Parent Ordinary Shares (or the number of Parent Ordinary Shares underlying Parent ADSs,
as applicable) subject to each Adjusted Warrant shall be equal to the number of shares of Company Common Stock issuable upon exercise
of such Company Warrant immediately prior to the Effective Time multiplied by the Exchange Ratio, with any fractional Parent Ordinary
Shares or Parent ADSs rounded down to the nearest whole Parent Ordinary Shares or Parent ADS, as applicable, and the exercise price with
respect to each Parent Ordinary Share (or each Parent Ordinary Share underlying Parent ADSs, as applicable) underlying such Adjusted
Warrant shall be equal to the exercise price of such Company Warrant immediately prior to the Effective Time divided by the Exchange
Ratio. The grant of the Adjusted Warrants shall be effected as of the Effective Time, or as soon thereafter as is reasonably practicable,
taking into account Parent’s administrative procedures. The Adjusted Warrants shall be further adjusted, if applicable, in accordance
with the terms of this Section 2.4(a) (mutatis mutandis) to give effect to the impact of the Additional Exchange
Ratio pursuant to Section 2.6.
(b) Immediately
prior to the Effective Time, each option to acquire shares of Company Common Stock (each such option, a “Company Option”)
that is then outstanding and unexercised, whether or not vested, shall be assumed and converted into an option to purchase a number of
Parent Ordinary Shares or Parent ADSs, as determined by Parent (each, an “Adjusted Option”), on the same terms and
subject to the same conditions as were applicable to such Company Option immediately prior to the Effective Time, except for terms rendered
inoperative by reason of the transactions contemplated by this Agreement, such other administrative or ministerial changes as in the
reasonable determination of Parent are appropriate to conform the administration of the Adjusted Options with other awards under Parent’s
equity plans, and except as provided in the following sentence. The number of Parent Ordinary Shares (or the number of Parent Ordinary
Shares underlying Parent ADSs, as applicable) subject to the Adjusted Option shall be equal to the product of (i) the total number
of shares of Company Common Stock subject to such Company Option immediately prior to the Effective Time multiplied by (ii) the
Exchange Ratio, with any fractional Parent Ordinary Shares or Parent ADSs rounded down to the nearest whole Parent Ordinary Share or
Parent ADS, as applicable. The exercise price per share of such Adjusted Option shall be equal to the quotient of (A) the exercise
price per share subject to such Company Option immediately prior to the Effective Time divided by (B) the Exchange Ratio, with any
fractional cents rounded up to the nearest whole cent. The exercise price with respect to each Parent Ordinary Share (or each Parent
Ordinary Share underlying Parent ADSs, as applicable) underlying any such Adjusted Option and the number of Parent Ordinary Shares (or
Parent Ordinary Shares underlying Parent ADSs, as applicable) relating to any such Adjusted Option shall be determined in a manner consistent
with the requirements of Section 409A of the Internal Revenue Code of 1986, as amended (the “Code”), and the
applicable regulations promulgated thereunder; and, in the case of any Company Option to which Section 422 of the Code applies,
the exercise price per share of any such Adjusted Option and the number of Parent Ordinary Shares or Parent ADSs, as applicable, relating
to any such Adjusted Option shall be determined in accordance in a manner that satisfies the requirements of Section 424(a) of
the Code. The Adjusted Options shall be further adjusted, if applicable, in accordance with the terms of this Section 2.4(b) (mutatis
mutandis) to give effect to the impact of the Additional Exchange Ratio pursuant to Section 2.6.
(c) As
of the Effective Time, the Company Equity Plan shall terminate and all rights under any provision of any other plan, program or arrangement
providing for the issuance or grant of any Equity Interest or other interest in respect of the capital stock of the Company shall be
cancelled without consideration payable therefor, except to the extent provided in this Section 2.4.
(d) Prior
to the Effective Time, the Company Board (or the appropriate committee of the Company Board) shall adopt such resolutions and
shall take such other actions as are required to approve the transactions contemplated by this Section 2.4. Prior to adopting
any such resolutions, the Company shall provide Parent with a reasonable opportunity to review and comment upon such resolutions and
shall consider any comments from Parent thereon in good faith.
(e) Parent
shall file and cause to be effective as of no later than thirty (30) days following the Closing Date an effective registration
statement under the Securities Act on Form S-8 or other applicable form under the Securities Act relating to Parent Ordinary
Shares to be represented by Parent ADSs issuable with respect to all Adjusted Options and Parent shall use its commercially
reasonable efforts to maintain the effectiveness of such registration statement(s) for so long as such Adjusted Options remain
outstanding.
2.5 Withholding.
Parent (or, as directed by Parent, the Company or the Surviving Corporation) and any other applicable withholding agent shall be entitled
to deduct and withhold, or cause the Exchange Agent to deduct and withhold, from any amounts payable or otherwise deliverable pursuant
to this Agreement to any holder or former holder of shares of Company Common Stock, Company Options, Parent ADSs or Parent Ordinary Shares
such amounts as are required to be deducted or withheld therefrom under the Code or any provision of applicable Tax Law or under any
other applicable legal requirement. To the extent such amounts are so deducted or withheld and remitted to the applicable Governmental
Authority, such amounts shall be treated for all purposes under this Agreement as having been paid to the Person with respect to which
such deduction and withholding was made.
2.6 Additional
Company Merger Shares. If any Parent Licensing Deal Revenue or Company Licensing Deal Revenue is actually received in cash by Parent
or the Surviving Corporation within one hundred and twenty (120) days following the Closing Date, and the amounts of such Parent Licensing
Deal Revenue and/or Company Licensing Deal Revenue so received would result in a positive number of Additional Company Merger Shares
(as determined in accordance with the calculation set forth in the definition thereof), then in addition to the Per Share Merger Consideration,
each share of Company Common Stock shall have the right to receive an additional number of Parent ADSs (the “Additional Per
Share Merger Consideration”) representing a number of Parent Ordinary Shares equal to the quotient obtained by dividing (a) such
number of Additional Company Merger Shares by (b) the Company Outstanding Shares (the “Additional Exchange Ratio”).
As promptly as practicable after the final determination of the Additional Per Share Merger Consideration and the Additional Exchange
Ratio pursuant to this Section 2.6, Parent shall deposit into the Exchange Fund a number of Parent ADSs representing the
aggregate Additional Per Share Merger Consideration issuable pursuant to this Section 2.6 and shall take all action necessary
to cause the Exchange Agent to issue such Additional Per Share Merger Consideration in accordance with the procedures set forth in Section 2.2(b).
Section 3
REPRESENTATIONS AND WARRANTIES OF THE COMPANY
Except (i) as expressly
disclosed in the Company SEC Documents filed with or furnished to the SEC by the Company and publicly available on the SEC’s Electronic
Data Gathering Analysis and Retrieval System (“EDGAR”), in each case, prior to the date of this Agreement (but, in
each case, excluding any risk factor disclosures contained under the heading “Risk Factors,” any disclosure of risks included
in any “forward-looking statements” disclaimer or any other statements that are similarly non-specific or predictive or forward-looking
in nature) or (ii) as set forth in the disclosure letter delivered by the Company to Parent (the “Company Disclosure Letter”)
prior to the execution of this Agreement, which Company Disclosure Letter identifies items of disclosure by reference to a particular
section or subsection of this Agreement (provided, however, that any information set forth in one section or subsection
of the Company Disclosure Letter also shall be deemed to apply to each other section and subsection of this Agreement to which its applicability
is reasonably apparent from the text of the disclosure), the Company hereby represents and warrants to Parent and Merger Sub as follows:
3.1 Organization,
Standing and Corporate Power.
(a) Each
of the Company and its subsidiaries is a corporation or other legal entity duly organized and validly existing under the Laws of the
jurisdiction of its incorporation, formation or organization, as the case may be, and has all requisite corporate, partnership or similar
power and authority necessary to own, lease and operate all of its properties and assets and to carry on its business as currently conducted,
except for such failures to be duly organized or validly existing or to have corporate, partnership or similar power or authority that
would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect.
(b) Each
of the Company and its subsidiaries is duly licensed or qualified to do business and is in good standing (or equivalent status, to the
extent such concept exists) in each jurisdiction in which the nature of the business currently conducted by it or the character or location
of the properties and assets currently owned or leased by it makes such licensing or qualification necessary, except where the failure
to be so licensed, qualified or in good standing (or equivalent status) would not reasonably be expected, individually or in the aggregate,
to have a Company Material Adverse Effect.
(c) The
Company has made available to Parent true and complete copies of the Company Charter and by-laws of the Company (together, the “Company
Charter Documents”) in each case, as amended to the date of this Agreement. The Company Charter Documents and organizational
or governing documents of each of its subsidiaries are in full force and effect and the Company is not in violation of any of the provisions
of the Company Charter Documents and none of the Company’s subsidiaries is in violation of any of the provisions of its organizational
or governing documents except, in each case, where such failures or violations would not reasonably be expected, individually or in the
aggregate, to have a Company Material Adverse Effect.
3.2 Corporate
Authorization.
(a) The
Company has all necessary corporate power and authority to execute and deliver this Agreement and all other agreements and documents
contemplated hereby to which it is a party and, subject to obtaining the Company Stockholder Approval, to perform its obligations
hereunder and to consummate the transactions contemplated hereby. The execution, delivery and performance by the Company of this
Agreement, and the consummation by it of the transactions contemplated hereby, have been duly authorized and adopted by the Company
Board. Except for (i) obtaining the affirmative vote of the holders of a majority of the issued and outstanding shares of
Company Common Stock in favor of the adoption of this Agreement and the Merger (the “Company Stockholder
Approval”) and (ii) filing the Certificate of Merger with the Secretary of State of the State of Delaware, no other
corporate action or proceeding on the part of the Company is necessary to authorize the execution, delivery and performance by the
Company of this Agreement and the consummation by it of the transactions contemplated hereby. This Agreement has been duly executed
and delivered by the Company and, assuming due authorization, execution and delivery of this Agreement by the other parties hereto,
constitutes a legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms,
except that such enforceability (A) may be limited by bankruptcy, insolvency, fraudulent transfer, reorganization, moratorium
and other similar Laws of general application affecting or relating to the enforcement of creditors’ rights generally and
(B) is subject to general principles of equity, whether considered in a proceeding at Law or in equity (clauses (A) and
(B) together, the “Bankruptcy and Equity Exception”).
(b) At
a meeting duly called and held, the Company Board, by resolutions duly adopted at such meeting (which resolutions have not as of the
date hereof been subsequently rescinded, modified or withdrawn), has (i) unanimously determined that the terms of the Merger and
the other transactions contemplated hereby are advisable, fair to and in the best interests of the Company and its stockholders, (ii) unanimously
approved, adopted and declared advisable this Agreement and the transactions contemplated hereby, (iii) unanimously resolved, subject
to Section 5.3(c), to recommend that the Company’s stockholders adopt this Agreement and the transactions contemplated
hereby (the “Company Recommendation”) and (iv) directed that this Agreement and the transactions contemplated
hereby be submitted to the Company’s stockholders for adoption.
3.3 Governmental
Authorization. Except for (a) filings required under, and compliance with other applicable requirements of, (i) the Securities
Act, the Exchange Act, and any other applicable federal securities Laws, (ii) state securities or “blue sky” Laws and
(iii) the rules and regulations of the OTC Markets Group applicable with respect to its OTC Pink Market and (b) the filing
of the Certificate of Merger with the Secretary of State of the State of Delaware pursuant to the DGCL, no consents or approvals of,
or filings with, any Governmental Authority are necessary for the execution and delivery of this Agreement by the Company and the consummation
by the Company of the transactions contemplated hereby, other than such other consents, approvals or filings that, if not obtained, made
or given, would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect. The Company
does not engage in any activities that would require a mandatory filing pursuant to the United Kingdom’s National Security and
Investment Act 2021 (including any related or ancillary regulations) as a result of the transactions contemplated by this Agreement.
3.4 No
Conflict. Neither the execution and delivery of this Agreement by the Company nor the consummation by the Company of the Merger or
the other transactions contemplated hereby, nor compliance by the Company with any of the provisions of this Agreement, shall (a) assuming
that the Company Stockholder Approval is obtained, conflict with or violate the Company Charter Documents, (b) assuming that the
consents, approvals and filings referred to in Section 3.3 and the Company Stockholder Approval are obtained and made, violate
any Restraint or Law applicable to the Company or any of its subsidiaries, or (c) violate, breach, result in the loss of any benefit
under, conflict with any provision of, or constitute a default (or an event which, with the notice or lapse of time, or both, would constitute
a default) under, result in the termination of or a right of termination or cancellation under, cause any payment under or accelerate
the performance required by, or result in the creation of any Lien (other than a Company Permitted Lien) upon the respective properties
or assets, of the Company or any of its subsidiaries under, any Company Material Contract, except in the case of clauses (b) and
(c) as would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect.
3.5 Capitalization.
(a) As
of the close of business on February 29, 2024 (the “Capitalization Date”), the authorized capital stock of the
Company consisted of (i) 60,000,000 shares of Company Common Stock, of which 22,632,843 shares were issued and outstanding and no
shares were held in the treasury of the Company and (ii) 10,000,000 shares of the Company’s undesignated preferred stock,
par value $0.0001 per share (“Company Preferred Stock”), of which no shares were issued and outstanding. There are
no other classes of capital stock of the Company authorized or issued and outstanding. All issued and outstanding shares of the capital
stock of the Company are duly authorized, validly issued, fully paid and non-assessable, and no class of capital stock is entitled to
preemptive rights.
(b) As
of the Capitalization Date, the Company has reserved 4,150,470 shares of Company Common Stock for issuance pursuant to the Company Equity
Plan. As of the Capitalization Date, there were outstanding (i) Company Options to acquire 1,394,808 shares of Company Common Stock
and (ii) Company Warrants to acquire 9,911,397 shares of Company Common Stock. Section 3.5(b) of the Company Disclosure
Letter sets a true and complete list as of the Capitalization Date of the outstanding Company Options and Company Warrants, including,
with respect to each Company Option and Company Warrant, the number of shares of Company Common Stock issuable thereunder or with respect
thereto, the holder thereof and the exercise price (if any), and the Company has granted no other such awards since the Capitalization
Date and prior to the date of this Agreement.
(c) From
the close of business on the Capitalization Date through the date of this Agreement, there have been no issuances of shares of Company
Common Stock, Company Preferred Stock or any other Equity Interests of the Company other than issuances of shares of Company Common Stock
pursuant to the exercise of Company Options, in each case, outstanding as of the Capitalization Date under the Company Equity Plan. Except
as set forth in this Section 3.5, as of the close of business on the Capitalization Date the Company has not granted any
other Equity Interests or any other rights to a third party to acquire capital stock from the Company. Section 3.5(c) of
the Company Disclosure Letter sets forth a true and complete list, as of the Capitalization Date, of each outstanding Company Option
and, with respect to each such Company Option, (i) the number of shares of Company Common Stock subject to such Company Option,
(ii) the vesting schedule thereof, including any accelerated vesting provisions, (iii) the status of the Company Option as
an incentive stock option within the meaning of Section 422 of the Code, (iv) the name of the holder, (v) the date of
grant, (vi) the expiration date and, (vii) the exercise price thereof. Not later than five (5) Business Days prior to
the Effective Time, the Company shall update Section 3.5(c) of the Company Disclosure Letter as of the date of such update
and provide such updated schedule to Parent. The Company has made available true and complete copies of the Company Equity Plan, all
forms of award agreements thereunder and any agreement for any award under the Company Equity Plan that does not conform in all material
respects to the form agreements under the Company Equity Plan. No Company Option has been granted with a per share exercise price that
is less than the fair market value of a share of Company Common Stock on the date such Company Option was granted. Each Company Option
was granted in accordance with the terms of the applicable Company Equity Plan and applicable Laws. The Company has the requisite power
and authority, in accordance with the Company Equity Plan, the applicable award agreements and any other applicable Contract, to take
the actions contemplated by Section 2.4.
(d) As
of the close of business on the Capitalization Date, no bonds, debentures, notes or other Indebtedness of the Company having the right
to vote (or convertible into or exercisable for securities having the right to vote) on any matters on which holders of capital stock
of the Company may vote are issued or outstanding.
(e) As
of the date of this Agreement, (i) there are no outstanding obligations of the Company to repurchase, redeem or otherwise acquire
any shares of capital stock of the Company or any of its subsidiaries except for purchases, redemptions or other acquisitions of capital
stock or other securities (A) required by the terms of the Company Equity Plan, (B) in order to pay Taxes or satisfy withholding
obligations in respect of such Taxes in connection with awards under the Company Equity Plan or otherwise, or (C) as required by
the terms of, or necessary for the administration of, any plans, arrangements or agreements existing on the date of this Agreement and
set forth on Section 3.5(e) of the Company Disclosure Letter between the Company or any of its subsidiaries and any director
or employee of the Company or any of its subsidiaries, (ii) there are no outstanding stock-appreciation rights, security-based performance
units, restricted stock units, “phantom” stock or other security rights or other agreements, arrangements or commitments
of any character (contingent or otherwise) to which the Company is a party, in each case pursuant to which any Person is entitled to
receive any payment from the Company based in whole or in part on the value of any capital stock of the Company (other than under the
Company Equity Plan), and (iii) there are no outstanding obligations of the Company to accelerate the vesting of any Equity Interests
of the Company under any provision of the Company Equity Plan or any Contract or other agreement evidencing any outstanding Company Option.
(f) Except
for the Company Voting Agreements, as of the date of this Agreement, there are no outstanding obligations of the Company (i) restricting
the transfer of, (ii) affecting the voting rights of, (iii) requiring the sales, issuance, repurchase, redemption or disposition
of, or containing any right of first refusal with respect to, (iv) requiring the registration for sale of or (v) granting any
preemptive or anti-dilutive rights with respect to any shares of Company Common Stock, Company Preferred Stock or other Equity Interests
in the Company.
3.6 Subsidiaries.
(a) Other
than the subsidiaries of the Company, the Company does not own or control, directly or indirectly, any membership interest, partnership
interest, joint venture interest, other Equity Interest or any other capital stock of any Person, and there are no silent partnerships,
sub-partnerships and/or similar rights with respect to the Company or any subsidiary of the Company.
(b) All
outstanding shares of capital stock, voting securities or other Equity Interests of each subsidiary of the Company are duly authorized,
validly issued, fully paid and non-assessable (where such concept is applicable under applicable Law) and all such securities are owned
beneficially and of record by the Company or another wholly-owned subsidiary of the Company free and clear of all Liens (other than Company
Permitted Liens). As of the date of this Agreement, other than the Company Voting Agreements, there are no outstanding obligations of
any subsidiary of the Company (i) restricting the transfer of, (ii) affecting the voting rights of, (iii) requiring the
sales, issuance, repurchase, redemption or disposition of, or containing any right of first refusal with respect to, (iv) requiring
the registration for sale of or (v) granting any preemptive or anti-dilutive rights with respect to any shares of Equity Interests
in any subsidiary of the Company.
(c) There
are no (i) outstanding options or other rights of any kind which obligate the Company or any of its subsidiaries to issue, transfer,
sell or deliver any shares of capital stock, voting securities or other Equity Interests of any subsidiary of the Company or any securities
or obligations convertible into, exchangeable or exercisable for any shares of capital stock, voting securities or other Equity Interests
of a subsidiary of the Company or (ii) other options, calls, warrants or other rights, agreements, arrangements or commitments relating
to the capital stock, voting securities or other Equity Interests of any subsidiary of the Company to which the Company or any of its
subsidiaries is a party.
(d) Section 3.6(d) of
the Company Disclosure Letter sets forth, as of the date hereof, for each of the Company’s subsidiaries and joint ventures: (i) its
jurisdiction of organization, (ii) its authorized capital stock or other Equity Interests, (iii) the number of its outstanding
shares of capital stock or other Equity Interests and type(s) of such outstanding shares of capital stock or other Equity Interests
and (iv) the record owner(s) thereof. Except for the ownership of Equity Interests in the Company’s subsidiaries and
investments in marketable securities and cash equivalents, none of the Company or any of its subsidiaries owns directly or indirectly
any Equity Interest in any Person, or has any obligation or has made any commitment to acquire any such Equity Interest, to provide funds
to, or to make any investment (in the form of a loan, capital contribution or otherwise) in, any of its subsidiaries or any other Person
that is or would reasonably be expected to be, individually or in the aggregate, material to the Company and its subsidiaries, taken
as a whole.
3.7 SEC
Filings and the Sarbanes-Oxley Act.
(a) All
of the reports, statements, schedules, forms and other documents filed or required to be filed by the Company with the SEC (such
reports, statements, schedules, forms and other documents filed by the Company and those filed by the Company subsequent to the date
hereof, collectively, and in each case including all exhibits and schedules thereto and documents incorporated by reference therein,
the “Company SEC Documents”) and all of the reports, statements, schedules, forms and other documents furnished
or required to be furnished by the Company to the SEC (such reports, statements, schedules, forms and other documents furnished by
the Company and those furnished by the Company subsequent to the date hereof, collectively, the “Company Furnished
Documents”), in each case in respect of reporting periods commencing on or after January 1, 2021 (including any
notice required under Section 13(r) of the Exchange Act) have been timely filed or furnished, as applicable. As of their
respective filing dates, such Company SEC Documents and Company Furnished Documents complied, or, if not yet filed or furnished,
shall comply, in all material respects with applicable Law, including the Securities Act, the Exchange Act and the Sarbanes-Oxley
Act, and none of such Company SEC Documents or Company Furnished Documents as of their respective filing dates contained, and no
Company SEC Document or Company Furnished Document as of their respective filing date shall contain, any untrue statement of a
material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements
therein, in light of the circumstances under which they were made, not misleading. The Company has made available to Parent copies
of all comment letters received by the Company from the SEC in respect of reporting periods commencing on or after January 1,
2021 and relating to such Company SEC Documents and Company Furnished Documents, together with all written responses of the Company
thereto, other than such comment letters or responses available on EDGAR as of the date of this Agreement. As of the date of this
Agreement, there are no outstanding or unresolved comments received from the SEC staff with respect to the Company SEC Documents or
Company Furnished Documents. To the Knowledge of the Company, as of the date hereof, there are no internal or third party inquiries
or investigations regarding accounting practices of the Company or otherwise regarding the Company.
(b) All
of the audited consolidated financial statements and unaudited consolidated interim financial statements of the Company included in the
Company SEC Documents complied at the time they were filed in all material respects with the applicable accounting requirements and the
published rules and regulations of the SEC with respect thereto in effect at the time of filing, were prepared in accordance with
GAAP (except as may be indicated in the notes thereto), applied on a consistent basis during the periods involved (except as may be indicated
in the notes thereto) and fairly present in all material respects the consolidated financial position of the Company and its consolidated
subsidiaries as of the dates thereof and the consolidated results of their operations and cash flows for the periods then ended (subject,
in the case of the financial statements for any quarter of the current fiscal year, to normal year-end audit adjustments).
(c) Neither
the Company nor any of its subsidiaries is a party to, or has any commitment to become a party to, any joint venture, off-balance sheet
partnership or any similar Contract (including any Contract or arrangement relating to any transaction or relationship between or among
the Company and any of its subsidiaries, on the one hand, and any unconsolidated Affiliate, on the other hand), including any structured
finance, special purpose or limited purpose entity or other Person, or any “off-balance sheet arrangements” (as defined in
Item 303(a) of Regulation S-K of the SEC), where the result, purpose or effect of such Contract is to avoid disclosure of any material
transaction involving, or material liabilities of, the Company or any of its subsidiaries in the Company’s or any of its subsidiaries’
published financial statements or any Company SEC Documents.
(d) Each
of the principal executive officer of the Company and the principal financial officer of the Company (or each former principal executive
officer of the Company and each former principal financial officer of the Company, as applicable) has made all certifications required
by Rule 13a-14 or 15d-14 under the Exchange Act and Sections 302 and 906 of the Sarbanes-Oxley Act, in each case, with respect to
the Company SEC Documents, and the statements contained in such certifications were true and complete on the date such certifications
were made. For purposes of this Agreement, “principal executive officer” and “principal financial officer” shall
have the meanings given to such terms in the Sarbanes-Oxley Act. No executive officer of the Company has failed to make the certifications
required of him or her under Section 302 or 906 of the Sarbanes-Oxley Act with respect to any Company SEC Document, except as disclosed
in certifications filed with the Company SEC Documents. Since January 1, 2021 through the date of this Agreement, (i) neither
the Company nor any of the Company’s subsidiaries have, nor, to the Knowledge of the Company, has any director or executive officer
of the Company or any of the Company’s subsidiaries, received any material complaint, allegation, assertion or claim, that the
Company or any of its subsidiaries has engaged in improper, illegal or fraudulent accounting or auditing practices, and (ii) to
the Knowledge of the Company, no attorney representing the Company or any of its subsidiaries, whether or not employed by the Company
or any of its subsidiaries, has reported evidence of a material violation of securities Laws, breach of fiduciary duty or similar violation
by the Company or any of its officers, directors, employees or agents to the Company Board or any committee thereof or to any director
or officer of the Company.
(e) The
Company has established and maintains a system of “internal control over financial reporting” (as defined in Rules 13a-15(f) and
15d-15(f) promulgated by the SEC under the Exchange Act) sufficient to provide reasonable assurance that (i) transactions are
executed in accordance with management’s general or specific authorizations; (ii) transactions are recorded as necessary to
permit preparation of financial statements in conformity with GAAP and to maintain asset accountability; (iii) access to assets
is permitted only in accordance with management’s general or specific authorization; and (iv) the recorded accountability
for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences.
(f) The
Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act),
as required by Rules 13a-15(a) and 15d-15(a) of the Exchange Act, are reasonably designed to ensure that all information
required to be disclosed by the Company in the reports it files or submits under the Exchange Act is made known to the chief executive
officer and the chief financial officer of the Company by others within the Company to allow timely decisions regarding required disclosure
as required under the Exchange Act and is recorded, processed, summarized and reported within the time periods specified by the SEC’s
rules and forms. The Company has evaluated the effectiveness of the Company’s disclosure controls and procedures and, to the
extent required by applicable Law, presented in any applicable Company SEC Document that is a report on Form 10-K or Form 10-Q,
or any amendment thereto, its conclusions about the effectiveness of the disclosure controls and procedures as of the end of the period
covered by such report or amendment based on such evaluation.
(g) Since
January 1, 2021, the Company has not received any oral or written notification of any (x) “significant deficiency”
or (y) “material weakness” in the Company’s internal controls over financial reporting. There is no outstanding
“significant deficiency” or “material weakness” which the Company’s independent accountants certify has
not been appropriately and adequately remedied by the Company. For purposes of this Agreement, the terms “significant deficiency”
and “material weakness” shall have the meanings assigned to them in Auditing Standard No. 5 of the Public Company Accounting
Oversight Board.
(h) The
Company is in compliance in all material respects with all current listing and corporate governance requirements of the OTC Markets
Group applicable with respect to its OTC Pink Market, and is in compliance in all material respects with all rules, regulations and
requirements of the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act and the SEC. Except as
permitted by the Exchange Act, including Sections 13(k)(2) and (3), since January 1, 2021, neither the Company nor
any of its subsidiaries has made, arranged, modified (in any material way), or forgiven personal loans to any executive officer or
director of the Company. Since January 1, 2021, to the Knowledge of the Company, no employee of the Company or any of its
subsidiaries has provided or is providing information to any law enforcement agency or Governmental Authority regarding the
commission or possible commission of any crime or the violation or possible violation of any applicable legal requirements of the
type described in Section 806 of the Sarbanes-Oxley Act by the Company or any of its subsidiaries.
3.8 Information
Supplied. The information relating to the Company and its subsidiaries in the proxy statement to be provided to the Company’s
stockholders in connection with the Company Stockholders Meeting and prospectus relating to the Parent ADSs (or the Parent Ordinary Shares
represented thereby) to be offered pursuant to this Agreement and the Merger (such proxy statement and prospectus and any amendment thereof
or supplement thereto, the “Proxy Statement/Prospectus”) and the registration statement on Form S-4 (of which
the Proxy Statement/Prospectus shall form a part) with respect to the issuance of the Parent ADSs (or the Parent Ordinary Shares represented
thereby) in the Merger (such registration statement together with the amendments and supplements thereto, the “Form S-4”)
and any other documents filed or furnished with or to the SEC pursuant to the Securities Act or the Exchange Act, in each case in connection
with the Merger shall not, on the date the Form S-4 is declared effective (and any amendment or supplement thereto), the date the
Proxy Statement/Prospectus is mailed to the Company’s stockholders and at the time of the Company Stockholder Meeting, contain
any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make
the statements therein, in the light of the circumstances under which they are made, not misleading. No representation is made by the
Company with respect to statements made in the Proxy Statement/Prospectus, the Form S-4 or any other document filed or furnished
with or to the SEC or pursuant to the Securities Act or the Exchange Act based on information supplied by Parent expressly for inclusion
therein.
3.9 Absence
of Certain Changes. Since September 30, 2023 through the date hereof, the Company and each of its subsidiaries have conducted
their respective businesses in the ordinary course consistent with past practices in all material respects and there has not been (a) any
event, occurrence, development or state of circumstances, facts or condition in such period that has had or would reasonably be expected,
individually or in the aggregate, to have a Company Material Adverse Effect or (b) any action taken by the Company or any of its
subsidiaries that, if taken during the period from the date of this Agreement through the Effective Time without Parent’s consent,
would constitute a breach of Section 5.1(b).
3.10 No
Undisclosed Liabilities. Except (a) as and to the extent disclosed or reserved against on any balance sheet of the Company that
is included in the Company SEC Documents; (b) as incurred after the date thereof in the ordinary course of business consistent with
past practice, (c) arising out of or in connection with this Agreement or the transactions contemplated hereby; or (d) liabilities
arising in the ordinary course of business in connection with the performance of obligations of the Company and its subsidiaries under
Company contracts in effect as of the date hereof (other than those liabilities resulting from a breach thereof by the Company or any
of its subsidiaries) the Company does not have any liabilities or obligations of any nature, whether known or unknown, absolute, accrued,
contingent or otherwise and whether due or to become due, in each case required by GAAP to be reflected or reserved against in the consolidated
balance sheet of the Company and its subsidiaries (or disclosed in the notes to such balance sheet).
3.11 Compliance
with Laws and Court Orders. Since January 1, 2021, the Company and its subsidiaries are and have been in compliance with all
Laws applicable to them, any of their properties or other assets or any of their respective businesses or operations, except where any
such failure to be in compliance would not, individually or in the aggregate, reasonably be expected to have a Company Material Adverse
Effect. To the Knowledge of the Company, as of the date hereof, no investigation or review by any Governmental Authority with respect
to the Company or any of its subsidiaries is pending or threatened except for any investigations or reviews that would not, individually
or in the aggregate, reasonably be expected to have a Company Material Adverse Effect.
3.12 Material
Contracts.
(a) As
of the date of this Agreement, none of the Company, any of its subsidiaries or their respective properties or other assets is a party
to or bound by any Contract (other than Company Plans):
(i) pursuant
to which the Company, any of its subsidiaries or any other party thereto has material continuing obligations, rights or interests and
including annual payments by the Company and its subsidiaries of $100,000 or more relating to the research, development, clinical trial,
distribution, supply, manufacture, marketing or co-promotion of, or collaboration with respect to, any product candidate for which the
Company or any of its subsidiaries is currently engaged in research or development, including but not limited to: (A) material manufacture
or supply services or material Contracts with contract research organizations for clinical trials-related services; (B) material
transfer Contracts for pre-clinical products or clinical products of the Company or any of its subsidiaries with commercial, pharmaceutical
or biotechnology companies; (C) Contracts involving the payment of royalties or other amounts calculated based upon the revenues
or income of the Company or any of its subsidiaries or income or revenues related to any clinical product candidate of the Company or
any of its subsidiaries; and (D) Contracts pursuant to which the Company has minimum purchase or “most favored nation”
obligations;
(ii) that
contains any non-compete or exclusivity provision or limits or purports to limit, curtail or restrict the ability of the Company or any
of its subsidiaries (or which following the consummation of the Merger and the other transactions contemplated hereby would reasonably
be expected to limit the ability of the Surviving Corporation) in a manner that is material to the business of the Company and its subsidiaries,
taken as a whole, as currently conducted (A) to compete in any line of business, in any geographic area or with any Person and (B) to
sell to or purchase from any other Person;
(iii) that
requires or permits the Company, or any successor to, or acquirer of, the Company, to make any payment to another Person, or requires
the consent of another Person, in each case in connection with a change of control of the Company or gives another Person a right to
receive or elect to receive a change of control payment;
(iv) that
is a joint-venture or partnership agreement or other similar agreement or arrangement;
(v) that
(A) relates to the disposition or acquisition by the Company or its subsidiaries of a material amount of assets or equity interests
in any Person (1) after the date of this Agreement, other than the sale of inventory in the ordinary course of business consistent
with past practice, or (2) which contains any ongoing obligations (including sale of inventory, indemnification, purchase price
adjustment, “earn-out” or other contingent obligations) that are still in effect that are reasonably likely to result in
claims in excess of $50,000 or (B) pursuant to which the Company or its subsidiaries will acquire or dispose of any material ownership
interest in any other person or other business enterprise other than the Company’s subsidiaries;
(vi) that
is a loan or credit agreement, indenture, note or other Contract or instrument relating to or evidencing Indebtedness for borrowed money
(including any guarantee thereto) or any Contract pursuant to which Indebtedness for borrowed money may be incurred or guaranteed, including
any Contract that is a financial derivatives master agreement or confirmation, or futures account opening agreement and/or brokerage
statement, evidencing financial hedging or similar trading activities;
(vii) that
is a mortgage, pledge, security agreement, deed of trust, capital lease or similar agreement that creates or grants a Lien on any material
property or asset of the Company or any of its subsidiaries, in each case involving annual payments of more than $100,000;
(viii) that
is a Collective Bargaining Agreement;
(ix) that
is a Contract providing for the issuance or sale of any equity securities of the Company or any of its subsidiaries;
(x) That
is a settlement agreement, or agreement entered into in connection with a settlement agreement, corporate integrity agreement, consent
decree, deferred prosecution agreement, or other similar type of agreement with any Governmental Authority or any other Person that has
existing or contingent performance obligations;
(xi) that
is a Contract granting a right of first refusal or first negotiation to any third party over any material assets of the Company;
(xii) that
is a Contract, including any ancillary or subagreements thereto, with any contract research organization or other agreement, including
any ancillary or subagreements thereto, with a third party which is conducting one or more clinical studies on behalf of the Company
or its subsidiaries and is reasonably expected to require payment of more than $50,000 within twelve (12) months prior to or after the
date of this Agreement;
(xiii) involves
the use or license by the Company or its subsidiaries of any material Software used by the Company or its subsidiaries as presently conducted
(other than non-customized Software subject to shrink-wrap, click-wrap and off-the-shelf or commercially available Software);
(xiv) is
an IP Agreement of the type set forth in Section 3.15(f) or 3.15(g) of the Company Disclosure Letter or involves the joint
development of products or technology with a third party that is material to the Company and its subsidiaries, taken as a whole; or
(xv) that
is any Contract that is a “material contract” (as such term is defined in Item 601(b)(10) of Regulation S-K of the SEC).
(xvi) All
Contracts, arrangements, commitments or understandings described in this Section 3.12(a), together with each Company Real
Property Lease, shall be collectively referred to as the “Company Material Contracts.”
(b) Except,
in each case, as has not been and would not reasonably be expected to be, individually or in the aggregate, material to the Company and
its subsidiaries, taken as a whole, as of the date hereof, (i) each of the Company Material Contracts is valid, binding and in full
force and effect with respect to the Company and its subsidiaries party thereto and, to the Knowledge of the Company, each other party
thereto and enforceable, in all material respects, in accordance with its terms by the Company and its subsidiaries party thereto (subject
to the Bankruptcy and Equity Exception); (ii) the Company and each of its subsidiaries has performed all material obligations required
to be performed by them under the Company Material Contracts to which they are parties; (iii) to the Knowledge of the Company, each
other party to a Company Material Contract has performed all material obligations required to be performed by it under such Company Material
Contract and (iv) no party to any Company Material Contract has given the Company or any of its subsidiaries written notice of its
intention to cancel, terminate, change the scope of rights under or fail to renew any Company Material Contract and neither the Company
nor any of its subsidiaries, nor, to the Knowledge of the Company, any other party to any Company Material Contract, has repudiated in
writing any material provision thereof. Neither the Company nor any of its subsidiaries has knowledge of, or has received written notice
of, any violation or default under any Company Material Contract or any other Contract to which it is a party or by which it or any of
its material properties or assets is bound, except for violations or defaults that have not been and would not reasonably be expected
to be, individually or in the aggregate, material to the Company and its subsidiaries, taken as a whole. True, unredacted and complete
copies of all of the Company Material Contracts have been made available to Parent.
3.13 Litigation.
There is no (nor since January 1, 2021 have there been any) material complaint, claim, action, charge, suit, arbitration, mediation,
investigation or proceeding (each, an “Action”) (excluding external investigations of which the Company has no Knowledge)
pending or, to the Knowledge of the Company, threatened, to which the Company or any of its subsidiaries are or were a party. There are
no material outstanding judgments, writs, injunctions, decrees or orders of any Governmental Authority against or binding on the Company
or its subsidiaries. There are no internal investigations or internal inquiries that, since January 1, 2021, have been conducted
by or at the direction of the Company Board (or any committee thereof) concerning any financial, accounting or other misfeasance or malfeasance
issues.
3.14 Properties.
(a) Neither
the Company nor any of its subsidiaries owns or has ever owned any real property.
(b) Section 3.14(b) of
the Company Disclosure Letter sets forth a true and complete list of all real property leased, subleased or otherwise occupied by the
Company or any of its subsidiaries as tenant, subtenant or occupant as of the date of this Agreement and material to the business of
the Company and its subsidiaries, taken as a whole (collectively, the “Company Leased Real Property”). No Company
Real Property Lease is subject to any Lien, including without limitation, any right to the use or occupancy of any Company Leased Real
Property, other than Company Permitted Liens. Each Company Real Property Lease constitutes the entire agreement between the parties thereto
with respect to the Company Leased Real Property leased thereunder, and is, with respect to the Company or the applicable subsidiary
of the Company, a valid and subsisting agreement in full force and effect and constitutes a valid, binding and enforceable obligation
of the Company or the applicable subsidiary of the Company, subject to the Bankruptcy and Equity Exception. The Company has not received
any written notice of termination or cancellation of or of a breach or default under any Company Real Property Lease that remains uncured
as of the date of this Agreement nor, to the Knowledge of the Company, has any event occurred which, with notice or lapse of time or
both, would constitute a breach or default under any such Company Real Property Lease, or permit the termination or cancellation of any
such Company Real Property Lease. With respect to the Company Leased Real Property, Section 3.14(b) of the Company Disclosure
Letter also contains a true and complete list as of the date hereof of all agreements under which the Company or any of its subsidiaries
is, as of the date hereof, the landlord, sublandlord, tenant, subtenant or occupant that have not been terminated or expired as of the
date hereof and are material to the business of the Company and its subsidiaries, taken as a whole (each a “Company Real Property
Lease”). The Company has heretofore made available to Parent true and complete copies of the Company Real Property Leases.
(c) With
respect to each of the Company Leased Real Properties, neither the Company nor any of its subsidiaries has exercised or given any notice
of exercise of any option or right of first offer or right of first refusal to purchase, expand, renew or terminate contained in the
Company Real Property Leases.
(d) Neither
the Company nor any of its subsidiaries has received written notice of any proceedings in eminent domain, condemnation or other similar
proceedings that are pending, and the Company has not received written notice threatening any such proceedings, in each case, affecting
any material portion of the Company Leased Real Property. Neither the Company nor any of its subsidiaries has received written notice
of the existence of any outstanding writ, injunction, decree, order or judgment or of any pending proceeding pertaining to or affecting
any material portion of the Company Leased Real Property. As of the date hereof, none of the material improvements located on any parcel
of Company Leased Real Property that is material to the business of the Company and its subsidiaries, taken as whole, has been damaged
by a fire or other casualty and not been restored and repaired either (i) to substantially the same condition they were in prior
to such event or (ii) to a condition necessary for the use of the Company in the ordinary course.
(e) To
the Knowledge of the Company, there are no conditions or defects, latent or otherwise, to the Company Leased Real Property that would,
individually or in the aggregate, reasonably be expected to have a Company Material Adverse Effect.
(f) None
of the Company’s or its subsidiaries’ current use of the Company Leased Real Property violates any restrictive covenant of
record that affects any of the Company Leased Real Property or any applicable Laws, in each case to the extent the same would reasonably
be expected to have a Company Material Adverse Effect.
3.15 Intellectual
Property.
(a) The
Company, or its subsidiaries, owns, is licensed under agreements that are in full force and effect, or, to the Knowledge of the Company,
otherwise has the right to use all Patents, Trademarks, Trade Secrets, Copyrights and all other Intellectual Property (including biological
materials), all registrations of any of the foregoing, or applications therefor, that are material to the Company’s business as
presently conducted (collectively, and along with the Company Registered Intellectual Property, the “Company Intellectual Property”).
The Company and its subsidiaries possess sufficient rights pursuant to written agreements to use all material Company Intellectual Property
not owned by the Company or its subsidiaries as such Company Intellectual Property are used in the Company’s business as presently
conducted. Except as otherwise indicated in Section 3.15(a) of the Company Disclosure Letter, the Company or its subsidiaries
is the sole and exclusive owner of all rights, title and interests in and to the Owned Company Intellectual Property, and, to the Knowledge
of the Company, all Owned Company Intellectual Property is free and clear of all Liens (other than Company Permitted Liens).
(b) Section 3.15(b) of
the Company Disclosure Letter sets forth as of the date hereof a true and complete list of all Patents, Trademarks that are trademark
registration, applications and material common law marks, and registered Copyrights that are (i) owned (or purported to be owned)
by the Company and its subsidiaries, (ii) exclusively licensed to the Company or its subsidiaries whereby ‘all substantial
rights’ are licensed to the Company or its subsidiaries, or (iii) that are non-exclusively licensed to the Company or its
subsidiaries and for which the Company or its subsidiaries controls prosecution thereof ((i), (ii), and (iii) are collectively,
the “Company Registered Intellectual Property”), indicating for each (as applicable) the name of the current record
owner(s), the applicable jurisdictions and the application or registration numbers, the registration date, and current status. The Company
Registered Intellectual Property owned by the Company or its subsidiaries, and, to the Knowledge of the Company, all other Company Registered
Intellectual Property, is subsisting and in full force and effect and has not been abandoned or adjudged invalid or unenforceable (other
than such Company Registered Intellectual Property that has expired, lapsed or been abandoned). All Company Registered Intellectual Property
which has been issued, granted or registered is, to the Company’s Knowledge, not invalid or unenforceable. Section 3.15(b) of
the Company Disclosure Letter also sets forth, as of the date of this Agreement, a list of all internet domain names with respect to
which the Company or its subsidiaries is the registrant and any social media handles registered by the Company or its subsidiaries.
(c) With
respect to the material items of Company Registered Intellectual Property, the Company has maintained them in the ordinary course consistent
with reasonable business practices. To the Knowledge of the Company, each of the Company’s or its subsidiaries’ owned Patents
(excluding invention disclosures) that are material to the Company and its subsidiaries properly identifies each and every inventor
of the claims thereof as determined in accordance with the laws of the jurisdiction in which such Patent was issued or such Patent application
is pending. The named inventors of each of the Company’s, or its subsidiaries’, owned Patents that are material to the Company
and its subsidiaries have assigned the applicable inventions for the Company’s, or its subsidiaries’, owned Patents to the
Company, or its subsidiaries, respectively, and the inventor assignments have been recorded with the USPTO as applicable except where
failure to do so would not be material. To the Knowledge of the Company and except as would not be material, all assignments to the Company
or its subsidiaries of the Company Registered Intellectual Property owned by the Company, or its subsidiaries, respectively, are valid
and enforceable.
(d) To
the Knowledge of the Company and except as would not be material, since January 1, 2021, no third party has infringed upon, misappropriated,
violated, or asserted any competing claim of right to use or own any of the Owned Company Intellectual Property or Company Registered
Intellectual Property that is exclusively licensed to the Company, or one of its subsidiaries. There is no litigation, opposition, interference,
inventorship challenge, refusal, cancellation, or proceeding pending, or asserted or threatened in writing, against the Company or its
subsidiaries concerning the validity, registrability, enforceability, duration, scope, priority, ownership or other violation of any
Company Owned Intellectual Property or Registered Intellectual Property exclusively licensed to the Company, or one of its subsidiaries
except where the proceeding is not material; this representation does not apply to office actions in the ordinary course of prosecution.
Since January 1, 2021, neither the Company nor its subsidiaries or its subsidiaries’ respective representatives have sent
or otherwise made in writing any assertion to any third party regarding any material alleged or suspected infringement, misappropriation,
dilution or violation of any Company Registered Intellectual Property.
(e) To
the Knowledge of the Company, the conduct of the business of the Company or its subsidiaries, as conducted since January 1, 2021,
and as contemplated to be conducted, has not interfered with, infringed upon, misappropriated, diluted, or otherwise violated, the Intellectual
Property of third parties in a manner that has or would reasonably be expected to result in a material liability to the Company and its
subsidiaries, taken as a whole. No claim or action alleging infringement, misappropriation, dilution, or other violation of any third
party Intellectual Property is pending or, to the Knowledge of the Company, threatened in writing against the Company, its subsidiaries
or, to the Knowledge of the Company, any other Person who is entitled to be indemnified, defended, held harmless or reimbursed by the
Company or its subsidiaries with respect to such claim or action that in each case has or would reasonably be expected to result in a
material liability to the Company and its subsidiaries, taken as a whole. Since January 1, 2021, neither the Company nor its subsidiaries
has received any written notice (or, to the Knowledge of Company, any non-written notice) from any third party alleging or threatening
that the operation of the business of the Company and its subsidiaries as conducted since January 1, 2021 infringes or otherwise
violates the Intellectual Property of such third party, including, but not limited to, any invitation to license that would reasonably
be construed as notice of infringement, any claim that the Company or its subsidiaries must license, or any claim that the Company must
refrain from using any Intellectual Property, where the allegation, if true, would reasonably be expected to result in a material liability
to the Company and its subsidiaries, taken as a whole.
(f) Section 3.15(f) of
the Company Disclosure Letter sets forth as of the date hereof a true and complete list of all agreements to which the Company or any
of its subsidiaries is a party that are material to the business of Company and its subsidiaries (taken as a whole) under which the Company
or its subsidiaries has been granted an exclusive or non-exclusive license under any Company Intellectual Property from a third party
(other than nondisclosure agreements, material transfer agreements or non-exclusive licenses and other agreements entered into in the
ordinary course of business) (“Inbound IP Agreements”).
(g) Section 3.15(g) of
the Company Disclosure Letter sets forth as of the date hereof a true and complete list of all agreements to which the Company or any
of its subsidiaries is a party that are material to the business of Company and its subsidiaries (taken as a whole) under which the Company
or its subsidiaries has (i) granted an exclusive or non-exclusive license or covenant not to sue under any Owned Company Intellectual
Property to a third party (other than nondisclosure agreements and material transfer agreements and other agreements entered into in
the ordinary course), (ii) assigned (or agreed to assign) any Owned Company Intellectual Property to a third party (other than agreements
entered into in the ordinary course), (iii) granted any third party an option or other right to obtain any such license, covenant
not to sue, or assignment (other than agreements entered into in the ordinary course), or (iv) covenanted not to pursue patent protection
with respect to any invention or technology other than agreements entered into in the ordinary course (“Outbound IP Agreements”
and together with the Inbound IP Agreements, the “IP Agreements”). The Company has provided Parent with true and correct
copies of all IP Agreements.
(h) Section 3.15(h) of
the Company Disclosure Letter sets forth as of the date hereof all license, collaboration, or other agreements to which the Company or
any of its subsidiaries is a party that are material to the business of Company and its subsidiaries (taken as a whole) under which the
Company owes and pays material royalties or makes other material financial payments to third parties in connection with the sale of products
and services. Except as set forth in Section 3.15(h) of the Company Disclosure Letter, neither the Company nor its subsidiaries,
in the Contracts to which any of them are a party, has agreed to, nor has an obligation to pay any third party royalties or payments
in connection with the sale of products and services where the royalties or payments are material to the business of the Company and
its subsidiaries (taken as a whole).
(i) Except
as would not have a Company Material Adverse Effect, the consummation of the Merger shall not under any IP Agreements result in any:
(i) the termination by a third party of any IP Agreement, (ii) the release from escrow of any material Owned Company Intellectual
Property, or (iii) the grant to any other Person of any license or other right to Owned Company Intellectual Property.
(j) To
the Knowledge of the Company, none of the activities of the employees of the Company or its subsidiaries violates any agreement or
arrangement which any such employees have with former employers in any matter that would reasonably be expected to result in
material liability to Company and its subsidiaries, taken as a whole. All current and former employees and consultants who
contributed to the discovery or development of any material Owned Company Intellectual Property did so pursuant to written
agreements assigning all rights therein to the Company or its subsidiaries that do not vest with the Company and its subsidiaries
initially by operation of law (other than non-assignable moral rights).
(k) To
the Knowledge of the Company, each current or former employee, contractor or consultant of the Company or its subsidiaries who has proprietary
knowledge of or information relating to Trade Secrets of the Company or its subsidiaries has executed and delivered to the Company or
its subsidiaries an agreement or agreements restricting such Person’s right to use and disclose such information or Trade Secret
of the Company or its subsidiaries except where failure to do so would not be material.
(l) No
settlements, injunctions, forbearances to sue, consents, judgments, orders or similar obligations to which the Company or its subsidiaries
is party: (i) restrict the use, exploitation, assertion or enforcement of any material Owned Company Intellectual Property or exclusively
licensed Intellectual Property anywhere in the world consistent with past practices; (ii) restrict in any material manner consistent
with past practices the conduct of the business of the Company, its subsidiaries or any of its respective employees as presently conducted;
or (iii) grant third parties any material or exclusive rights (including field and territory-limited rights) under any material
Owned Company Intellectual Property or material exclusively licensed Intellectual Property.
(m) The
Company and its subsidiaries have exercised reasonable business discretion to protect their rights in their Trade Secrets and other confidential
information, in each case that are material to the business of the Company and its subsidiaries, taken as a whole.
(n) No
government funding nor government, academic or non-profit research facilities or personnel were used, directly or indirectly, to develop
or create, in whole or in part, any of the material Owned Company Intellectual Property, or, to the Knowledge of the Company, any other
material Company Intellectual Property.
(o) Except
as would not reasonably be expected to have a Company Material Adverse Effect: (i) to the Knowledge of the Company, the Software,
hardware, databases, websites, computer equipment, servers, telecommunication systems, networks, interfaces, platforms, systems and other
information technology or related infrastructure that are owned, operated, leased, used in or necessary for the conduct of the business
of the Company or its subsidiaries, including such information technology or related infrastructure obtained or licensed from a vendor
carrying out activities on behalf of the Company or its subsidiaries (collectively, the “Company Systems”) are lawfully
owned, leased, or licensed by the Company or its subsidiaries, and are reasonably sufficient for the conduct of their respective businesses
as presently conducted, (ii) since January 1, 2021, there have been no failures, breakdowns, continued substandard performance
or other adverse events affecting any such Company Systems that have caused a substantial disruption or substantial interruption in or
to the use of such Company Systems or the conduct of the business of the Company as presently conducted and remain unresolved or unaddressed,
and (iii) to the Knowledge of the Company, since January 1, 2021, there have not been any material incidents of unauthorized
access or other security breaches of the Company Systems, and (iv) to the Knowledge of the Company, the Company Systems do not contain
any viruses or other unauthorized, malicious disabling code that would reasonably be expected to (x) significantly disrupt or materially
and adversely affect the functionality or integrity of any Company System, or (y) enable or assist any Person to access Company
Systems without proper authorization. To the Knowledge of the Company, the Company Systems do not contain any “back door,”
“time bomb,” “Trojan horse,” “worm,” “drop-dead device,” “virus,” malware
or other Software routines or components intentionally designed to permit unauthorized access to, maliciously disable, maliciously encrypt,
or erase Software, hardware, or data that would reasonably be expected to materially disrupt the business of the Company or its subsidiaries,
taken as a whole. To the Knowledge of Company, the Company and its subsidiaries are not in material breach of any of their Contracts
relating to Company Systems. Since January 1, 2021, the Company and its subsidiaries have not been, to the Knowledge of the Company,
audited under any Contract pursuant to which they use any third party system, nor received any written notice of intent to conduct any
such audit.
3.16 Taxes.
(a) The
Company and each of its subsidiaries have prepared and duly and timely filed (taking into account any extension of time within which
to file) all income and other material Tax Returns required to be filed by any of them, and all such filed Tax Returns are true, correct
and complete in all material respects.
(b) Except
as would not have a Company Material Adverse Effect, the Company and each of its subsidiaries:
(i) have
complied with all applicable Laws, rules, and regulations relating to the payment and withholding of Taxes with respect to amounts owing
to any employee, independent contractor, stockholder, creditor or third party within the time and in the manner prescribed by Law;
(ii) have
not waived any statute of limitations with respect to any Taxes or agreed to any extension of time with respect to any Tax assessment
or deficiency, which waiver or extension is currently effective, other than in connection with an extension of time for filing a Tax
Return and the Company has identified to Parent in writing any such Tax Return to which an extension has been filed outside of the ordinary
course of business and the relevant Tax Return is yet to be filed;
(iii) have
no pending or threatened audits, examinations, or assessments (or other similar proceedings initiated by a Governmental Authority) in
respect of Taxes or Tax matters to which the Company is a party;
(iv) are
not and have not been a party to any Tax Sharing Agreement (other than an agreement exclusively between or among the Company and its
subsidiaries or among the Company’s subsidiaries) pursuant to which it may have any obligation to make any payments for Taxes after
the Effective Time and have no liability for Taxes of any Person (other than the Company or any of its subsidiaries) under Treasury Regulations
Section 1.1502-6 (or any similar provision of state, local, or non-U.S. Law) or as transferee or successor;
(v) have
no Liens for Taxes upon any property or assets of the Company or any of its subsidiaries, other than Company Permitted Liens described
in clause (i) of the definition thereof;
(vi) have
not entered into any “closing agreement” under section 7121 of the Code, or other similar agreement with a Governmental Authority
in respect of Taxes that remains in effect, and no request for a ruling, relief, advice, or any other item that relates to the Taxes
or Tax Returns of the Company or any of its subsidiaries is currently pending with any Governmental Authority, and no such ruling, relief
or advice has even been obtained; and
(vii) do
not participate and have not participated in a “listed transaction” within the meaning of Treasury Regulations Section 1.6011-4(b).
(c) Each
of the Company and its subsidiaries is, and always has been, treated for U.S. federal income Tax purposes as set forth on 3.16(c) of
the Company Disclosure Letter.
3.17 Employee
Benefit Plans.
(a) Section 3.17(a) of
the Company Disclosure Letter sets forth a true and complete list, as of the date of this Agreement, of each material Company Plan. With
respect to each material Company Plan, the Company has made available to Parent, as applicable, (i) the plan document (or, with
respect to any unwritten Company Plan, a written description thereof), (ii) the most recent annual report (Form 5500) prepared
in connection with any such Company Plan, (iii) the most recent determination or opinion letter, if any, from the Internal Revenue
Service of the United States (the “IRS”) for any Company Plan that is intended to qualify pursuant to Section 401(a) of
the Code, (iv) the most recent actuarial or valuation report, (vii) any material communications with any Governmental Authority
since January 1, 2021, and (viii) the most recent nondiscrimination testing results.
(b) Each
Company Plan and trust that is intended to be qualified under Section 401(a) of the Code is covered by a currently effective,
favorable determination letter, or is established on a pre-approved form of plan document that is covered by a favorable advisory or
opinion letter, or has pending or has time remaining in which to file an application for such determination from the IRS, and, to the
Knowledge of the Company, (i) no revocation of any such determination, advisory, or opinion letter has been threatened by any Governmental
Authority, and (ii) no circumstances exist that could reasonably be expected to result in the loss of such qualified status under
Section 401(a) of the Code or material liability to the Company.
(c) No
Company Plan is, and neither the Company nor any of its ERISA Affiliates sponsors, maintains or contributes (or is required to contribute)
to, or has ever sponsored, maintained or contributed (or been required to contribute) to (i) any employee benefit plan that is or
was subject to Title IV of ERISA, Section 412 of the Code or Section 302 of ERISA, (ii) a “multiemployer plan”
(as defined in Section 3(37) of ERISA), (iii) any “funded welfare benefit plan” (within the meaning of Section 419
of the Code), (iv) any “multiple employer plan” (within the meaning of Section 210 of ERISA or Section 413(c) of
the Code), or (v) any “multiple employer welfare arrangement” (as defined in Section 3(40) of ERISA), and neither
the Company nor any of its ERISA Affiliates has ever incurred any liability under Title IV of ERISA that has not been paid in full.
(d) Each
Company Plan has been established, operated, administered, and maintained in all material respects in compliance with its terms and in
all material respects with the requirements of applicable Laws, including ERISA and the Code.
(e) Neither
the Company nor any of its subsidiaries has any liability in respect of post-retirement health, medical or life insurance benefits for
any retired, former or current employee, officer, director or other service provider of the Company or any of its subsidiaries (or any
dependent or beneficiary thereof) except coverage or benefits as required under Section 4980B of the Code or any other applicable
Laws at the participant’s sole expense.
(f) Except
as set forth in Section 3.17(f) of the Company Disclosure Letter, neither the execution of this Agreement nor the consummation
of the transactions contemplated by this Agreement shall (either alone or together with a termination of employment or other event),
(i) entitle any current or former employee, officer, director or other service provider of the Company or any of its subsidiaries
to severance pay or any other payment or benefit, whether under any Company Plan or otherwise, (ii) accelerate the time of payment
or vesting or trigger any payment of funding (through a grantor trust or otherwise) of compensation or benefits under, or increase the
amount payable or trigger any other obligation pursuant to, any Company Plan, (iii) increase the amount payable under any Company
Plan or (iv) result in the payment or provision of an “excess parachute payment” as defined in Section 280G of
the Code to any “disqualified individual” (as defined in Section 280G of the Code) of the Company or any of its subsidiaries.
No Company Plan or other agreement with any employee provides for a “gross-up” or similar payment in respect of any Taxes
that may become payable under Section 409A or Section 4999 of the Code.
(g) There
is no material Action pending against or, to the Knowledge of the Company, threatened against, any Company Plan before any Governmental
Authority, other than routine claims for benefits. No Company Plan is, or in the past six (6) years has been, the subject of an
investigation, examination or audit by a Governmental Authority or is the subject of an application or filing under, or is a participant
in, an amnesty, voluntary compliance, self-correction, or similar program sponsored by any Governmental Authority.
(h) Each
Company Foreign Plan has been registered and maintained in all material respects in compliance with its terms and in all material respects
with the requirements of applicable Laws and in good standing with applicable regulatory authorities. No Company Foreign Plan is a defined
benefit plan (as defined in ERISA, whether or not subject to ERISA).
3.18 Employment
Matters.
(a) True
and complete information as to the name, current job title, exempt or non-exempt classification for purposes of FLSA and state wage
and hour laws, and compensation for all current employees of the Company and its subsidiaries has been provided to Parent. No
current employee of the Company or any of its subsidiaries, (i) has given notice of termination of employment or otherwise
disclosed plans to terminate employment with the Company or any of its subsidiaries within the twelve (12) month period following
the date hereof, (ii) is employed under a nonimmigrant work visa or other work authorization that is limited in duration, or
(iii) has been the subject of any sexual harassment, sexual assault, sexual discrimination or other misconduct allegations
during his or her tenure at the Company or any of its subsidiaries.
(b) Neither
the Company nor any of its subsidiaries is a party to or is bound by, or is currently negotiating, any collective bargaining agreement,
labor-related agreement, or other Contract (a “Collective Bargaining Agreement”) with any labor union, works council,
or other employee representative body (a “Union”). Neither the Company nor any of its subsidiaries is the subject
of an Action asserting that the Company or any such subsidiary has committed an unfair labor practice (within the meaning of the National
Labor Relations Act). For the last three (3) years, no Union or group of Company employees has made a pending demand for recognition
or certification, and, to the Knowledge of the Company, there are no representation or certification proceedings or petitions seeking
a representation proceeding presently pending or, to the Knowledge of the Company, threatened to be brought or filed with the National
Labor Relations Board, any other Governmental Authority. To the Knowledge of the Company, since January 1, 2021, there have been
no Union organizing activities with respect to any employees of the Company or any of its subsidiaries. There is no, and since January 1,
2021 there has not been, any work slowdown, lockout, work stoppage, picketing, strike, or other material labor dispute or collective
labor action involving the Company or any of its subsidiaries pending or, or to the Knowledge of the Company, threatened. No notice,
consent or consultation obligations with respect to any employees of the Company or any of its subsidiaries, or any Union, shall be a
condition precedent to, or triggered by, the execution of this Agreement or the consummation of the transactions contemplated by this
Agreement.
(c) Except
as would not be reasonably expected, individually or in the aggregate, to have a Company Material Adverse Effect, the Company and each
of its subsidiaries is, and since January 1, 2021 has been, in compliance with all applicable Laws and Contracts, relating to employment,
including but not limited to employment practices, labor, compensation, discrimination, harassment, workplace safety, retaliation, immigration,
whistleblowing, employee leave, paid time off, benefits, wages and hours, terms and conditions of employment, unemployment insurance,
workers’ compensation, the termination of employment, the proper classification of employees as exempt or nonexempt from overtime
pay requirements and the proper classification of individuals as independent contractors or employees, unemployment insurance, collective
dismissals, and the Worker Adjustment and Retraining Notification Act (and any applicable similar foreign, state or local Laws).
3.19 Environmental
Matters.
(a) Except
as would not be reasonably expected, individually or in the aggregate, to have a Company Material Adverse Effect:
(i) to
the Knowledge of the Company, there is no pending or threatened Environmental Claim or Environmental Liability regarding the Company
or any of its subsidiaries or any property currently, or formerly owned, operated or leased by the Company or its subsidiaries;
(ii) with
respect to real property that is currently leased or operated by the Company and its subsidiaries, and to the Knowledge of the Company,
with respect to real property that was formerly owned, leased or operated by the Company or its subsidiaries, there have been no Releases
of Hazardous Materials at or from any of such real properties that has caused environmental contamination at any location that is reasonably
likely to result in an obligation of the Company or any subsidiary to investigate or remediate such environmental contamination pursuant
to applicable Environmental Law or contractual agreement or otherwise result in any Environmental Claim or Environmental Liability;
(iii) neither
(A) the Company or any subsidiary thereof (B) nor to the Knowledge of the Company any entity previously owned by the Company
or any subsidiary thereof, has transported or arranged for the treatment, storage, handling, disposal or transportation of any Hazardous
Material at or to any third-party location that is reasonably likely to result in an Environmental Claim or Environmental Liability;
(iv) neither
the Company nor any subsidiary thereof has, either expressly or by operation of applicable Law, assumed or undertaken, or agreed to assume
or undertake, responsibility for any liability or obligation of any other Person arising under or relating to Environmental Laws;
(v) to
the Knowledge of the Company, the Company has provided Parent with (a) environmental site assessments and substantially similar
evaluations reasonably available and in its possession respecting material environmental conditions at properties currently leased or
used by the Company or its subsidiaries and (b) the most recent written compliance audit reasonably available in its possession
for current operating industrial facilities; and
(vi) to
the Knowledge of the Company, there are no other activities, conditions or circumstances that would be reasonably likely to result in
any material Environmental Claim or Environmental Liability.
(b) Except
as would not, individually or in the aggregate, reasonably be expected to have a Company Material Adverse Effect, to the Knowledge of
the Company, the Company and each of its subsidiaries are, and since January 1, 2021 have been, in compliance with all Environmental
Laws (which compliance includes, but is not limited to, possession of all Environmental Permits required under applicable Environmental
Laws, and compliance with the terms and conditions thereof).
3.20 Regulatory
Matters; Compliance.
(a) The
Company or its subsidiaries hold all material licenses, Permits, franchises, variances, registrations, exemptions, orders and other governmental
authorizations, consents, approvals, and clearances, and have submitted notices to, all Governmental Authorities, including all authorizations
under the Federal Food, Drug and Cosmetic Act of 1938, as amended (the “FDCA”), the Public Health Service Act of 1944,
as amended (the “PHSA”), and the regulations of the U.S. Food and Drug Administration (the “FDA”)
promulgated thereunder, and any other Governmental Authority that regulates the quality, identity, strength, purity, safety, efficacy
or manufacturing of the Company’s Products (any such Governmental Authority, a “Company Regulatory Agency”)
necessary for the lawful operation of the businesses of the Company or any of its subsidiaries as currently conducted (the “Company
Permits”), and as of the date hereof, all such Company Permits are valid and in full force and effect. There has not occurred
any material violation of, default (with or without notice or lapse of time or both) under, or event giving to others any right of termination,
amendment or cancellation of, with or without notice or lapse of time or both, any Company Permit. The Company and its subsidiaries are
in compliance in all material respects with the terms of all Company Permits, and no event has occurred that, to the Knowledge of the
Company, would reasonably be expected to result in the revocation, cancellation, non-renewal or material adverse modification of any
Company Permit. Since January 1, 2021, neither the Company nor its subsidiaries has received written notice of any pending or threatened
claim, suit, proceeding, hearing, enforcement, audit, investigation, arbitration or other action from the FDA or other Company Regulatory
Agency alleging that any operation or activity of the Company or any of its subsidiaries is in violation of any applicable Law.
(b) Since
January 1, 2021, all of the Company’s and its subsidiaries’ Products that are subject to the jurisdiction of the FDA
or other Company Regulatory Agencies have been manufactured, imported, exported, processed, developed, labeled, stored, and tested by
or on behalf of the Company or any of its subsidiaries in all material respects in compliance with all applicable requirements under
any Permit or Law, including applicable statutes and implementing regulations administered or enforced by the FDA or other Company Regulatory
Agency. Since January 1, 2021, all applications, submissions, notifications, information and data utilized by the Company or its
subsidiaries as the basis for, or submitted by or, to the Knowledge of the Company, on behalf of the Company or any of its subsidiaries
in connection with, any and all requests for Company Permits relating to the Company or any of its subsidiaries when submitted to the
FDA or other Company Regulatory Agency, were true, complete and correct, in all material respects, as of the date of submission, and
any updates, changes, corrections or modification to such applications, submissions, notifications, information and data required under
applicable Laws have been submitted to the FDA or other Company Regulatory Agency.
(c) Since
January 1, 2021, neither the Company, nor any of its subsidiaries, have committed any act, made any statement or failed to make
any statement that would reasonably be expected to provide a basis for the FDA or any other Company Regulatory Agency to invoke its policy
with respect to “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities,” or other similar Laws. Neither
the Company nor or any of its subsidiaries nor, to the Knowledge of the Company, any of their respective officers, employees, contractors,
suppliers or other entities or individuals performing research or work on behalf of the Company or any of its subsidiaries has been subject
to any kind of consent decree, individual integrity agreement, deferred prosecution agreement, or other similar form of agreement with
any Governmental Authority or convicted of any crime or engaged in any conduct that has resulted, or would reasonably be expected to
result, in a material debarment or exclusion under applicable Law, including, without limitation, 21 U.S.C. Section 335a. No claims,
actions, proceedings or, to the Knowledge of the Company, investigations that would reasonably be expected to result in such a material
debarment or exclusion are pending or threatened in writing against the Company or any of its subsidiaries or any of their respective
officers, employees, contractors, suppliers or other entities or individuals performing research or work on behalf of the Company or
any of its subsidiaries.
(d) Since
January 1, 2021, none of the Company, any of its subsidiaries, or, to the Knowledge of the Company, any of their respective contract
manufacturers for Products, has received any FDA Form 483, warning letter, untitled letter, or other similar correspondence or written
notice from the FDA or any other Company Regulatory Agency alleging or asserting material noncompliance with any applicable Laws or Company
Permits with respect to any Product of the Company or any of its subsidiaries.
(e) Since
January 1, 2021, all studies, tests and preclinical studies being conducted by the Company or any of its subsidiaries, or in which
the Company, any of its subsidiaries or any Product has participated, have been and are being conducted in compliance in all material
respects with applicable Laws, including the applicable requirements of Good Laboratory Practices, to the extent any such study or test
is required to be conducted in compliance with Good Laboratory Practices.
(f) Since
January 1, 2021, all studies, tests and preclinical and clinical trials being conducted by the Company or any of its subsidiaries,
or in which the Company, any of its subsidiaries or any Product or Product candidate has participated, have been and are being conducted
in compliance in all material respects with applicable Laws, including the applicable requirements of Good Laboratory Practices or Good
Clinical Practices. Since January 1, 2021, neither the Company or any of its subsidiaries have received any written notices, correspondence
or other communication from any institutional review board, the FDA or any other Company Regulatory Agency, recommending or requiring
the termination, suspension, or material modification of any ongoing or planned clinical trials conducted by, or on behalf of, the Company
or any of its subsidiaries, other than any comments on study design provided by the FDA as part of any pre-Investigational New Drug Application
activities, including any pre-Investigational New Drug Application meetings.
3.21 Healthcare
Regulatory; Compliance.
(a) The
Company and its subsidiaries is, and at all times since January 1, 2021 has been, in compliance in all material respects with all
applicable Healthcare Laws and, as of the date of this Agreement, there is no Action pending, received by or threatened orally or in
writing against the Company or its subsidiaries related to such Healthcare Laws.
(b) Neither
the Company nor its subsidiaries has engaged in an unlawful or unauthorized practice of medicine or other professionally licensed activities
through any web sites sponsored or operated, or formerly sponsored or operated, by the Company or its subsidiaries.
(c) The
Company has implemented and has in place a compliance program that conforms to and materially ensures compliance with applicable Healthcare
Laws and industry standards.
(d) No
Person has filed against the Company an action relating to the Company under any federal or state whistleblower statute, including under
the False Claims Act of 1863 (31 U.S.C. § 3729 et seq.).
(e) Since
January 1, 2021, the Company and its subsidiaries have made and kept books, records, and accounts which, in reasonable detail, accurately
and fairly reflect the transactions and dispositions of the assets of the Company and each of its subsidiaries.
3.22 Insurance.
(a) Except
as would not, individually or in the aggregate, reasonably be expected to have a Company Material Adverse Effect, each insurance policy
under which the Company or any of its subsidiaries is an insured or otherwise the principal beneficiary of coverage (collectively, the
“Company Insurance Policies”) is in full force and effect and all related premiums have been paid to date. The Company has
made available to Parent true, unredacted and complete copies of the Company Insurance Policies.
(b) Except
as would not, individually or in the aggregate, reasonably be expected to have a Company Material Adverse Effect, the Company Insurance
Policies are reasonable and customary in coverage, scope and size of premiums based on the activities of the Company as conducted and
as contemplated to be conducted as of the date of this Agreement.
(c) The
Company and its subsidiaries are in compliance with the terms and conditions of the Company Insurance Policies, except for any non-compliance
as would not reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect.
(d) Neither
the Company nor any of its subsidiaries is in material breach or default (including any such breach or default with respect to the payment
of premiums or the giving of notice under any such policy) under any Company Insurance Policy, and, to the Knowledge of the Company,
no event has occurred which, with notice or lapse of time, would constitute such breach or default, or permit termination or modification,
under such policy. Except as would not, individually or in the aggregate, reasonably be expected to have a Company Material Adverse Effect,
no insurance claims made by the Company or any of its subsidiaries has been questioned, denied or disputed.
3.23 Anti-Corruption;
Global Trade Control Laws.
(a) Since
January 1, 2018, neither the Company, nor its subsidiaries, nor any of the Company’s or its subsidiaries’ respective
current or former officers, directors, or, to the Knowledge of the Company, any representative acting on behalf of the Company or its
subsidiaries, including any of their respective officers, directors, or employees, has violated, to the extent applicable, the FCPA,
the U.S. Travel Act, the U.K. Bribery Act 2010, Laws implementing the Organisation for Economic Co-operation and Development Convention
on Combating Bribery of Foreign Public Officials in International Business Transactions or any other Law, rule or regulation relating
to anti-corruption or anti-bribery (the “Anti-Corruption Laws”), including by unlawfully directly or indirectly offering,
promising, providing, or authorizing the provision of any money, property, contribution, gift, entertainment or other thing of value
to any Person, so as to influence official action, to secure an improper advantage, or to encourage the recipient to breach a duty of
good faith or loyalty or the policies of their employer.
(b) Neither
the Company, nor its subsidiaries, nor, to the Knowledge of the Company, any representative acting at the direction of the Company or
its subsidiaries (i) is under external or internal investigation for (A) any violation of the Anti-Corruption Laws, (B) any
alleged irregularity, misstatement or omission arising under or relating to any Contract between such Person and any Governmental Authority,
or any instrumentality thereof or (C) any unlawful contribution, gift, bribe, rebate, payoff, influence payment, kickback or other
payment or the provision of anything of value, directly or indirectly, to a Government Official, (ii) has received any notice or
other written communication from any Governmental Authority with respect to any actual, alleged or potential violation of, or failure
to comply with, any Anti-Corruption Laws, or (iii) is the subject of any internal complaint, audit or review process with respect
to allegations of potential violation of the Anti-Corruption Laws.
(c) The
Company and its subsidiaries maintain policies and procedures designed to ensure compliance with the Anti-Corruption Laws.
(d) Neither
the Company, nor its subsidiaries, nor any director, officer or employee of any of the Company or its subsidiaries, is, or since January 1,
2018 has been, (i) a Restricted Party or (ii) majority owned or controlled by a Restricted Party.
(e) The
Company and its subsidiaries are, and since January 1, 2018 have been, in compliance in all material respects with all Global Trade
Control Laws, which includes possession of and compliance in all material respects with all licenses, permits, variances, registrations,
exemptions, orders, consents, approvals, clearances, and other authorizations required by Global Trade Control Laws and submission of
required notices or reports to all Governmental Authorities that are concerned with such Global Trade Control Laws.
(f) Since
January 1, 2018, neither the Company nor its subsidiaries has directly or indirectly engaged in any business with, or used, directly
or indirectly, any corporate funds to contribute to or finance the activities of, any Restricted Party or in or with any Restricted Market
and is not currently doing so. The Company acknowledges that activities under this Agreement shall not (i) be in a Restricted Market;
(ii) involve individuals ordinarily resident in a Restricted Market; or (iii) include companies, organizations, or governmental
entities from or located in a Restricted Market.
(g) To
the Knowledge of the Company, (i) since January 1, 2018, neither the Company nor its subsidiaries has been the subject of
any investigations, reviews, audits or inquiries by a Governmental Authority related to Global Trade Control Laws, and (ii) as
of the date hereof, no investigation, review, audit, or inquiry of or to the Company or its subsidiaries by any Governmental
Authority with respect Global Trade Control Laws is pending or threatened.
3.24 CFIUS.
Neither the Company nor its subsidiaries is a U.S. business that (i) produces, designs, tests, manufactures, fabricates, or develops
one or more “critical technologies”; (ii) performs the functions as set forth in column 2 of Appendix A to 31 C.F.R.
Part 800 with respect to “covered investment critical infrastructure”; or (iii) maintains or collects, directly
or indirectly, “sensitive personal data” of U.S. citizens, in each case as such terms in quotation marks are defined in the
Defense Production Act of 1950, as amended, including all implementing regulations thereof.
3.25 Brokers
and Finder’s Fees. Except for River Corporate Advisors, LLC (the “Company Financial Advisor”), no broker,
investment banker, financial advisor or other Person is entitled to any broker’s, finder’s or financial advisor’s fee
or commission in connection with the transactions contemplated hereby based upon arrangements made by or on behalf of the Company or
any of its subsidiaries. Prior to the date hereof, the Company has provided Parent with an unredacted copy of each engagement letter
between the Company and the Company Financial Advisor, pursuant to which the Company Financial Advisor would be entitled to any payment
relating to the Merger and any other transactions contemplated by this Agreement. The Company Financial Advisor’s estimated fees
and expenses in connection with the transactions contemplated hereby are disclosed in Section 3.24 of the Company Disclosure
Letter.
3.26 Opinion
of the Financial Advisor. The Company Financial Advisor has delivered to the Company Board its opinion, dated on or about the date hereof, to the effect that, as
of such date and based upon and subject to the various assumptions, qualifications and limitations set forth therein, the Consideration
(as defined in such opinion) to be received by holders of shares of Company Common Stock pursuant to the terms of this Agreement is fair,
from a financial point of view, to such holders of shares of Company Common Stock. The opinion of the Company Financial Advisor has not
been withdrawn, revoked or modified.
3.27 Antitakeover
Laws. The Company Board has duly taken all actions so that no “fair price,” “control share acquisition,”
“business combination” or other similar anti-takeover statute or regulation enacted under state or federal Laws in the United
States (including under the DGCL) or the United Kingdom (collectively, “Takeover Laws”) shall prohibit the execution,
delivery or performance of or compliance with this Agreement, the Merger or the other transactions contemplated hereby. The Company has
no “rights plan”, “rights agreement” or “poison pill” in effect.
3.28 No
Other Representations; No Reliance; Waiver. The Company represents, warrants, acknowledges and agrees that none of Parent, Merger
Sub, any of their Affiliates or shareholders or any of their respective Representatives (collectively, the “Parent Related Persons”)
makes or has made any representation or warranty, either express or implied, as to the accuracy or completeness of any information provided
or made available to the Company, any of its Affiliates or shareholders or any of their respective Representatives (collectively, “Company
Related Persons”) or any other Person in connection with this Agreement, the Company Voting Agreements, the Merger or any of
the other transactions contemplated by this Agreement or with respect to any projections, forecasts, estimates, plans or budgets of future
revenues, expenses or expenditures, future results of operations, future cash flows or future financial condition, or any component of
the foregoing, or any other forward looking information, of Parent, Merger Sub or any of their Affiliates, and no Company Related Person
has relied on any information or statements made or provided (or not made or provided) to any Company Related Person other than the representations
and warranties of the Parent and Merger Sub expressly set forth in Section 4 of this Agreement (as qualified by the Parent
Disclosure Letter) and any certificate delivered pursuant to Section 7.
Section 4
REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER
SUB
Except (i) as expressly
disclosed in the Parent SEC Documents filed with or furnished to the SEC by Parent and publicly available on EDGAR, in each case, prior
to the date of this Agreement (but, in each case, excluding any risk factor disclosures contained under the heading “Risk Factors,”
any disclosure of risks included in any “forward-looking statements” disclaimer or any other statements that are similarly
non-specific or predictive or forward-looking in nature) or (ii) as set forth in the disclosure letter delivered by Parent to the
Company (the “Parent Disclosure Letter”) prior to the execution of this Agreement, which Parent Disclosure Letter
identifies items of disclosure by reference to a particular section or subsection of this Agreement (provided, however,
that any information set forth in one section or subsection of the Parent Disclosure Letter also shall be deemed to apply to each other
section and subsection of this Agreement to which its applicability is reasonably apparent from the text of the disclosure), Parent and
Merger Sub jointly and severally represent and warrant to the Company as follows:
4.1 Organization,
Standing and Corporate Power.
(a) Each
of Parent and its subsidiaries is a corporation or other legal entity duly organized and validly existing under the Laws of the jurisdiction
of its incorporation, formation or organization, as the case may be, and has all requisite corporate, partnership or similar power and
authority necessary to own, lease and operate all of its properties and assets and to carry on its business as currently conducted, except
for such failures to be duly organized or validly existing or to have corporate, partnership or similar power or authority that would
not reasonably be expected, individually or in the aggregate, to have a Parent Material Adverse Effect.
(b) Each
of Parent and its subsidiaries is duly licensed or qualified to do business and is in good standing (or equivalent status, to the extent
such concept exists) in each jurisdiction in which the nature of the business currently conducted by it or the character or location
of the properties and assets currently owned or leased by it makes such licensing or qualification necessary, except where the failure
to be so licensed, qualified or in good standing (or equivalent status) would not reasonably be expected, individually or in the aggregate,
to have a Parent Material Adverse Effect.
(c) Parent
has made available to the Company true and complete copies of the articles of association of Parent (the “Parent Charter Documents”),
as amended to the date of this Agreement. The Parent Charter Documents and organizational or governing documents of each of its subsidiaries
are in full force and effect and Parent is not in violation of any of the provisions of the Parent Charter Documents and none of Parent’s
subsidiaries is in violation of any of the provisions of its organizational or governing documents except, in each case, where such failures
or violations would not reasonably be expected, individually or in the aggregate, to have a Parent Material Adverse Effect. The UK Panel
on Takeovers and Mergers has confirmed to Parent that Parent is not subject to the UK City Code on Takeovers and Mergers (the “Takeover
Code”) and there have been no subsequent changes in Parent’s circumstances that would result in Parent having its central
management and control in the United Kingdom for the purposes of the Takeover Code.
4.2 Corporate
Authorization.
(a) Each
of Parent and Merger Sub has all necessary corporate power and authority to execute and deliver this Agreement and all other agreements
and documents contemplated hereby to which it is a party and, subject to obtaining Parent Shareholder Approval and approval of this Agreement
by Parent, as the sole stockholder of Merger Sub, to perform its obligations hereunder and to consummate the transactions contemplated
hereby. The execution, delivery and performance by Parent and Merger Sub of this Agreement, and the consummation by them of the transactions
contemplated hereby, have been duly authorized and adopted by the Parent Board and the board of directors of Merger Sub, respectively.
Except for (i) obtaining the affirmative vote of the majority of the votes cast by Parent Shareholders present and entitled to vote
(A) approving the issuance of Parent Ordinary Shares to be represented by Parent ADSs in connection with the Merger, (B) approving
the Chairman Appointment and (C) any other resolutions required by Law or the rules and regulations of the Nasdaq Capital Market
(“Nasdaq”) or other listing authority (the “Parent Shareholder Approval”), (ii) obtaining
the approval of this Agreement by Parent as the sole stockholder of Merger Sub and (iii) filing the Certificate of Merger with the
Secretary of State of the State of Delaware, no other corporate action or proceeding on the part of Parent or Merger Sub is necessary
to authorize the execution, delivery and performance by Parent of this Agreement and the consummation by it of the transactions contemplated
hereby. This Agreement has been duly executed and delivered by Parent and Merger Sub and, assuming due authorization, execution and delivery
of this Agreement by the other parties hereto, constitutes a legal, valid and binding obligation of Parent and Merger Sub, enforceable
against such parties in accordance with its terms, except that such enforceability may be limited by the Bankruptcy and Equity Exception.
The Parent Ordinary Shares to be issued in connection with the Merger (and to be represented by Parent ADSs delivered to holders of Company
Common Stock) will be issued fully-paid, free from all and any rights of pre-emption to which the members of the Parent may be entitled
(whether arising by virtue of the United Kingdom’s Companies Act 2006 or otherwise) and will be allotted in reliance on the exception
pursuant to section 565 of the United Kingdom’s Companies Act 2006.
(b) At
a meeting duly called and held, the Parent Board, by resolutions duly adopted at such meeting (which resolutions have not as of the date
hereof been subsequently rescinded, modified or withdrawn), has (i) unanimously determined that the terms of the Merger and the
other transactions contemplated hereby are advisable, fair to and in the best interests of Parent Shareholders as a whole, (ii) unanimously
approved, adopted and declared advisable this Agreement and the transactions contemplated hereby, (iii) unanimously resolved, subject
to Section 5.4(c), to recommend that the Parent Shareholders approve (A) the issuance of Parent Ordinary Shares
represented by Parent ADSs to be issued in connection with the Merger and (B) the Chairman Appointment (the “Parent Recommendation”)
and (iv) directed that (A) the issuance of Parent Ordinary Shares represented by Parent ADSs in connection with the Merger
and (B) the Chairman Appointment be submitted to the Parent Shareholders for approval. The board of directors of Merger Sub has
adopted resolutions (A) determining that the terms of the Merger and the other transactions contemplated by this Agreement are advisable,
fair to and in the best interests of Merger Sub and Parent, as its sole stockholder, (B) approving this Agreement, the Merger and
the other transactions contemplated by this Agreement and (C) recommending that Parent, as sole stockholder of Merger Sub, approve
this Agreement and directing that this Agreement be submitted to Parent, as sole stockholder of Merger Sub, for approval. The Parent
and Merger Sub do not engage in any activities that would require a mandatory filing pursuant to the United Kingdom’s National
Security and Investment Act 2021 (including any related or ancillary regulations) as a result of the transactions contemplated by this
Agreement.
4.3 Governmental
Authorization. Except for (a) filings required under, and compliance with other applicable requirements of, (i) the Securities
Act, the Exchange Act, and any other applicable federal securities Laws, (ii) state securities or “blue sky” Laws and
(iii) the rules and regulations of Nasdaq and (b) the filing of the Certificate of Merger with the Secretary of State
of the State of Delaware pursuant to the DGCL, no consents or approvals of, or filings with, any Governmental Authority are necessary
for the execution and delivery of this Agreement by Parent or Merger Sub and the consummation by Parent and Merger Sub of the transactions
contemplated hereby, other than such other consents, approvals or filings that, if not obtained, made or given, would not reasonably
be expected, individually or in the aggregate, to have a Parent Material Adverse Effect.
4.4 No
Conflict. Neither the execution and delivery of this Agreement by Parent nor the consummation by Parent of the Merger or the other
transactions contemplated hereby, nor compliance by Parent with any of the provisions of this Agreement, shall (a) assuming that
the Parent Shareholder Approval is obtained, conflict with or violate the Parent Charter Documents, (b) assuming that the consents,
approvals and filings referred to in Section 4.3 and the Parent Shareholder Approval are obtained and made, violate any Restraint
or Law applicable to Parent or any of its subsidiaries, or (c) violate, breach, result in the loss of any benefit under, conflict
with any provision of, constitute a default (or an event which, with the notice or lapse of time, or both, would constitute a default)
under, or result in the termination of or a right of termination or cancellation under, cause any payment under or accelerate the performance
required by, or result in the creation of any Lien (other than a Parent Permitted Lien) upon the respective properties or assets, of
Parent or any of its subsidiaries under, any Parent Material Contract, except in the case of clauses (b) and (c) as would not
reasonably be expected, individually or in the aggregate, to have a Parent Material Adverse Effect.
4.5 Capitalization.
(a) As
of the close of business on the Capitalization Date, (i) the issued share capital of Parent consisted of 13,206,163,523 Parent Ordinary
Shares, of which no Parent Ordinary Shares were held in the treasury, and (ii) there were 6,600,922 Parent ADSs issued and outstanding.
All issued and outstanding Parent Ordinary Shares are duly authorized, validly issued and fully paid, and holders of such Parent Ordinary
Shares are not entitled to preemptive rights, except pursuant to the Companies Act 2006.
(b) As
of the Capitalization Date, the Parent has reserved 765,819,200 Parent Ordinary Shares for issuance pursuant to a Parent Plan. As of
the Capitalization Date, there were (i) outstanding Parent options to acquire 651,237,400 Parent Ordinary Shares (“Parent
Options”), (ii) 414,106,700 Parent Ordinary Shares underlying awards of restricted stock units with respect to Parent
Ordinary Shares (“Parent RSUs”) and (iii) outstanding Parent Warrants to acquire 4,337,221,500 Parent Ordinary
Shares. Section 4.5(b) of the Parent Disclosure Letter sets a true and complete list as of the Capitalization Date of the outstanding
Parent Options, Parent RSUs and Parent Warrants, including, with respect to each Parent Option and Parent Warrant, the number of Parent
Ordinary Shares issuable thereunder or with respect thereto, the holder thereof and the exercise price (if any), and Parent has granted
no other such awards since the Capitalization Date and prior to the date of this Agreement.
(c) From
the close of business on the Capitalization Date through the date of this Agreement, there have been no issuances of Parent Ordinary
Shares or any other Equity Interests of Parent other than issuances of Parent Ordinary Shares pursuant to the exercise of Parent Options
or the settlement of Parent RSUs outstanding as of the Capitalization Date under a Parent Plan. Except as set forth in this Section 4.5,
as of the close of business on the Capitalization Date, Parent has not granted any other Equity Interests or any other rights to a third
party to acquire capital stock from Parent or any Parent ADSs. Section 4.5(c) of the Parent Disclosure Letter sets forth a
true and complete list, as of the Capitalization Date, of each outstanding Parent Option and each Parent RSU and, with respect to each
such Parent Option or Parent RSU, to the extent applicable, (i) the number of Parent Ordinary Shares subject to such Parent Option,
(ii) the vesting schedule thereof, including any accelerated vesting provisions, (iii) the status of the Parent Option as an
incentive stock option within the meaning of Section 422 of the Code, (iv) the name of the holder, (v) the date of grant,
(vi) the expiration date and, (vii) the exercise price thereof. Not later than five (5) Business Days prior to the Effective
Time, Parent shall update Section 4.5(c) of the Parent Disclosure Letter as of the date of such update and provide such updated
schedule to the Company. Parent has made available true and complete copies of the Parent Plan, all forms of award agreements thereunder
and any agreement for any award under the Parent Plan that does not conform in all material respects to the form agreements under the
Parent Plan. No Parent Option has been granted with a per share exercise price that is less than the fair market value of a Parent Ordinary
Share on the date such Parent Option was granted. Each Parent Option or Parent RSU was granted in accordance with the terms of the applicable
Parent Plan and applicable Laws. Parent has the requisite power and authority, in accordance with a Parent Plan, the applicable award
agreements and any other applicable Contract, to take the actions contemplated by Section 2.4.
(d) As
of the close of business on the Capitalization Date, no bonds, debentures, notes or other Indebtedness of Parent having the right to
vote (or convertible into or exercisable for securities having the right to vote) on any matters on which holders of capital stock of
Parent may vote are issued or outstanding.
(e) As
of the date of this Agreement, (i) there are no outstanding obligations of Parent to repurchase, redeem or otherwise acquire any
Parent Ordinary Shares or any shares of capital stock of its subsidiaries except for purchases, redemptions or other acquisitions of
capital stock or other securities (A) required by the terms of a Parent Plan, (B) in order to pay Taxes or satisfy withholding
obligations in respect of such Taxes in connection with awards under a Parent Plan or otherwise, or (C) as required by the terms
of, or necessary for the administration of, any plans, arrangements or agreements existing on the date hereof and set forth on Section 4.5(e) of
the Parent Disclosure Letter between Parent or any of its subsidiaries and any director or employee of Parent or any of its subsidiaries,
(ii) there are no outstanding stock-appreciation rights, security-based performance units, restricted stock units, “phantom”
stock or other security rights or other agreements, arrangements or commitments of any character (contingent or otherwise) to which Parent
is a party, in each case, pursuant to which any Person is entitled to receive any payment from Parent based in whole or in part on the
value of any capital stock of Parent (other than under a Parent Plan), and (iii) there are no outstanding obligations of Parent
to accelerate the vesting of any Equity Interests of Parent under any provision of any Parent Plan or any Contract or other agreement
evidencing any outstanding Parent Option or Parent RSU.
(f) Except
for the Parent Voting Agreements, as of the date of this Agreement, there are no outstanding obligations of Parent (i) restricting
the transfer of, (ii) affecting the voting rights of, (iii) requiring the sales, issuance, repurchase, redemption or disposition
of, or containing any right of first refusal with respect to, (iv) requiring the registration for sale of or (v) granting any
preemptive or anti-dilutive rights with respect to any Parent Ordinary Shares or other Equity Interests in Parent.
4.6 Subsidiaries.
(a) Other
than the subsidiaries of the Parent, the Parent does not own or control, directly or indirectly, any membership interest, partnership
interest, joint venture interest, other Equity Interest or any other capital stock of any Person, and there are no silent partnerships,
sub-partnerships and/or similar rights with respect to the Parent or any subsidiary of the Parent.
(b) All
outstanding shares of capital stock, voting securities or other Equity Interests of each subsidiary of the Parent are duly authorized,
validly issued, fully paid and non-assessable (where such concept is applicable under applicable Law) and all such securities are owned
beneficially and of record by the Parent or another wholly-owned subsidiary of the Parent free and clear of all Liens (other than Parent
Permitted Liens). As of the date of this Agreement, other than the Parent Voting Agreements, there are no outstanding obligations of
any subsidiary of the Parent (i) restricting the transfer of, (ii) affecting the voting rights of, (iii) requiring the
sales, issuance, repurchase, redemption or disposition of, or containing any right of first refusal with respect to, (iv) requiring
the registration for sale of or (v) granting any preemptive or anti-dilutive rights with respect to any shares of Equity Interests
in any subsidiary of the Parent.
(c) There
are no (i) outstanding options or other rights of any kind which obligate the Parent or any of its subsidiaries to issue, transfer,
sell or deliver any shares of capital stock, voting securities or other Equity Interests of any subsidiary of the Parent or any securities
or obligations convertible into, exchangeable or exercisable for any shares of capital stock, voting securities or other Equity Interests
of a subsidiary of the Parent or (ii) other options, calls, warrants or other rights, agreements, arrangements or commitments relating
to the capital stock, voting securities or other Equity Interests of any subsidiary of the Parent to which the Parent or any of its subsidiaries
is a party.
(d) Section 4.6(d) of
the Parent Disclosure Letter sets forth, as of the date hereof, for each of the Parent’s subsidiaries and joint ventures: (i) its
jurisdiction of organization, (ii) its authorized capital stock or other Equity Interests, (iii) the number of its outstanding
shares of capital stock or other Equity Interests and type(s) of such outstanding shares of capital stock or other Equity Interests
and (iv) the record owner(s) thereof. Except for the ownership of Equity Interests in the Parent’s subsidiaries and investments
in marketable securities and cash equivalents, none of the Parent or any of its subsidiaries owns directly or indirectly any Equity Interest
in any Person, or has any obligation or has made any commitment to acquire any such Equity Interest, to provide funds to, or to make
any investment (in the form of a loan, capital contribution or otherwise) in, any of its subsidiaries or any other Person that is or
would reasonably be expected to be, individually or in the aggregate, material to the Parent and its subsidiaries, taken as a whole.
4.7 SEC
Filings and the Sarbanes-Oxley Act.
(a) All
of the reports, statements, schedules, forms and other documents filed or required to be filed by Parent with the SEC (such reports,
statements, schedules, forms and other documents filed by Parent and those filed by Parent subsequent to the date hereof, collectively,
and in each case including all exhibits and schedules thereto and documents incorporated by reference therein, the “Parent SEC
Documents”) and all of the reports, statements, schedules, forms and other documents furnished or required to be furnished
by Parent to the SEC (such reports, statements, schedules, forms and other documents furnished by Parent and those furnished by Parent
subsequent to the date hereof, collectively, the “Parent Furnished Documents”), in each case in respect of reporting
periods commencing on or after January 1, 2021 (including any notice required under Section 13(r) of the Exchange Act)
have been timely filed or furnished, as applicable. As of their respective filing dates, such Parent SEC Documents and Parent Furnished
Documents complied, or, if not yet filed or furnished, shall comply, in all material respects with applicable Law, including the Securities
Act, the Exchange Act and the Sarbanes-Oxley Act, and none of such Parent SEC Documents or Parent Furnished Documents as of their
respective filing dates contained, and no Parent SEC Document or Parent Furnished Document as of their respective filing date shall contain,
any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make
the statements therein, in light of the circumstances under which they were made, not misleading. Parent has made available to the Company
copies of all comment letters received by Parent from the SEC in respect of reporting periods commencing on or after January 1,
2021 and relating to such Parent SEC Documents and Parent Furnished Documents, together with all written responses of Parent thereto,
other than such comment letters or responses available on EDGAR as of the date of this Agreement. As of the date of this Agreement, there
are no outstanding or unresolved comments received from the SEC staff with respect to Parent SEC Documents or Parent Furnished Documents.
To the Knowledge of Parent, as of the date hereof, there are no internal or third party inquiries or investigations regarding accounting
practices of Parent or otherwise regarding Parent.
(b) All
of the audited consolidated financial statements and unaudited consolidated interim financial statements of Parent included in Parent
SEC Documents complied at the time they were filed in all material respects with the applicable accounting requirements and the published
rules and regulations of the SEC with respect thereto in effect at the time of filing, were prepared in accordance with GAAP (except
as may be indicated in the notes thereto), applied on a consistent basis during the periods involved (except as may be indicated in the
notes thereto) and fairly present in all material respects the consolidated financial position of Parent and its consolidated subsidiaries
as of the dates thereof and the consolidated results of their operations and cash flows for the periods then ended (subject, in the case
of the financial statements for any quarter of the current fiscal year, to normal year-end audit adjustments).
(c) Neither
Parent nor any of its subsidiaries is a party to, or has any commitment to become a party to, any joint venture, off-balance sheet partnership
or any similar Contract (including any Contract or arrangement relating to any transaction or relationship between or among Parent and
any of its subsidiaries, on the one hand, and any unconsolidated Affiliate, on the other hand), including any structured finance, special
purpose or limited purpose entity or other Person, or any “off-balance sheet arrangements” (as defined in Item 303(a) of
Regulation S-K of the SEC), where the result, purpose or effect of such Contract is to avoid disclosure of any material transaction involving,
or material liabilities of, Parent or any of its subsidiaries in Parent’s or any of its subsidiaries’ published financial
statements or any Parent SEC Documents.
(d) Each
of the principal executive officer of Parent and the principal financial officer of Parent (or each former principal executive officer
of Parent and each former principal financial officer of Parent, as applicable) has made all certifications required by Rule 13a-14
or 15d-14 under the Exchange Act and Sections 302 and 906 of the Sarbanes-Oxley Act, in each case, with respect to Parent SEC Documents,
and the statements contained in such certifications were true and complete on the date such certifications were made. No executive officer
of Parent has failed to make the certifications required of him or her under Section 302 or 906 of the Sarbanes-Oxley Act with
respect to any Parent SEC Document, except as disclosed in certifications filed with Parent SEC Documents. Since January 1, 2021
through the date of this Agreement, (i) neither Parent nor any of Parent’s subsidiaries have, nor, to the Knowledge of Parent,
has any director or executive officer of Parent or any of Parent’s subsidiaries, received any material complaint, allegation, assertion
or claim, that Parent or any of its subsidiaries has engaged in improper, illegal or fraudulent accounting or auditing practices, and
(ii) to the Knowledge of Parent, no attorney representing Parent or any of its subsidiaries, whether or not employed by Parent or
any of its subsidiaries, has reported evidence of a material violation of securities Laws, breach of fiduciary duty or similar violation
by Parent or any of its officers, directors, employees or agents to the Parent Board or any committee thereof or to any director or officer
of Parent.
(e) Parent
has established and maintains a system of “internal control over financial reporting” (as defined in Rules 13a-15(f) and
15d-15(f) promulgated by the SEC under the Exchange Act) sufficient to provide reasonable assurance that (i) transactions are
executed in accordance with management’s general or specific authorizations; (ii) transactions are recorded as necessary to
permit preparation of financial statements in conformity with GAAP and to maintain asset accountability; (iii) access to assets
is permitted only in accordance with management’s general or specific authorization; and (iv) the recorded accountability
for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences.
(f) Parent’s
disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act), as required by Rules 13a-15(a) and
15d-15(a) of the Exchange Act, are reasonably designed to ensure that all information required to be disclosed by Parent in the
reports it files or submits under the Exchange Act is made known to the chief executive officer and the chief financial officer of Parent
by others within Parent to allow timely decisions regarding required disclosure as required under the Exchange Act and is recorded, processed,
summarized and reported within the time periods specified by the SEC’s rules and forms. Parent has evaluated the effectiveness
of Parent’s disclosure controls and procedures and, to the extent required by applicable Law, presented in any applicable Parent
SEC Document that is a report on Form 10-K or Form 10-Q, or any amendment thereto, its conclusions about the effectiveness
of the disclosure controls and procedures as of the end of the period covered by such report or amendment based on such evaluation.
(g) Since
January 1, 2021, Parent has not received any oral or written notification of any (x) “significant deficiency” or
(y) “material weakness” in Parent’s internal controls over financial reporting. There is no outstanding “significant
deficiency” or “material weakness” which Parent’s independent accountants certify has not been appropriately
and adequately remedied by Parent. For purposes of this Agreement, the terms “significant deficiency” and “material
weakness” shall have the meanings assigned to them in Auditing Standard No. 5 of the Public Company Accounting Oversight Board.
(h) Parent
is in compliance in all material respects with all current listing and corporate governance requirements of Nasdaq, and is in compliance
in all material respects with all rules, regulations and requirements of the United Kingdom’s Companies Act 2006, the Sarbanes-Oxley
Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act and the SEC. Except as permitted by the Exchange Act, including Sections
13(k)(2) and (3), since January 1, 2021, neither Parent nor any of its subsidiaries has made, arranged, modified (in any material
way), or forgiven personal loans to any executive officer or director of Parent. Since January 1, 2021, to the Knowledge of Parent,
no employee of Parent or any of its subsidiaries has provided or is providing information to any law enforcement agency or Governmental
Authority regarding the commission or possible commission of any crime or the violation or possible violation of any applicable legal
requirements of the type described in Section 806 of the Sarbanes-Oxley Act by Parent or any of its subsidiaries.
4.8 Information
Supplied. The information relating to Parent and its subsidiaries included in the Proxy Statement/Prospectus, the Form S-4,
and any other documents filed or furnished with or to the SEC pursuant to the Securities Act or the Exchange Act in each case in connection
with the Merger shall not, on the date the Form S-4 is declared effective (and any amendment or supplement thereto), the date the
Proxy Statement/Prospectus is mailed to the Company’s stockholders, and at the time of the Company Stockholder Meeting, contain
any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make
the statements therein, in the light of the circumstances under which they are made, not misleading. No representation is made by Parent
with respect to statements made in the Proxy Statement/Prospectus, the Form S-4 or any other document filed or furnished with or
to the SEC or pursuant to the Securities Act or the Exchange Act based on information supplied by the Company expressly for inclusion
therein.
4.9 Absence
of Certain Changes. Since December 31, 2022 through the date hereof, Parent and each of its subsidiaries have conducted their
respective businesses in the ordinary course consistent with past practices in all material respects and there has not been (a) any
event, occurrence, development or state of circumstances, facts or condition in such period that has had or would reasonably be expected,
individually or in the aggregate, to have a Parent Material Adverse Effect or (b) any action taken by Parent or any of its subsidiaries
that, if taken during the period from the date of this Agreement through the Effective Time without the Company’s consent, would
constitute a breach of Section 5.2(b).
4.10 No
Undisclosed Liabilities. Except (a) as and to the extent disclosed or reserved against on any balance sheet of Parent that is
included in the Parent SEC Documents; (b) as incurred after the date thereof in the ordinary course of business consistent with
past practice, (c) arising out of or in connection with this Agreement or the transactions contemplated hereby; or (d) liabilities
arising in the ordinary course of business in connection with the performance of obligations of Parent and its subsidiaries under Parent
contracts in effect as of the date hereof (other than those liabilities resulting from a breach thereof by Parent or any of its subsidiaries)
Parent does not have any liabilities or obligations of any nature, whether known or unknown, absolute, accrued, contingent or otherwise
and whether due or to become due, in each case required by GAAP to be reflected or reserved against in the consolidated balance sheet
of Parent and its subsidiaries (or disclosed in the notes to such balance sheet).
4.11 Compliance
with Laws and Court Orders. Since January 1, 2021, Parent and its subsidiaries are and have been in compliance with all Laws
applicable to them, any of their properties or other assets or any of their respective businesses or operations, except where any such
failure to be in compliance would not, individually or in the aggregate, reasonably be expected to have a Parent Material Adverse Effect.
To the Knowledge of Parent, as of the date hereof, no investigation or review by any Governmental Authority with respect to Parent or
any of its subsidiaries is pending or threatened except for any investigations or reviews that would not, individually or in the aggregate,
reasonably be expected to have a Parent Material Adverse Effect.
4.12 Material
Contracts.
(a) As
of the date of this Agreement, none of Parent, any of its subsidiaries or their respective properties or other assets is a party to or
bound by any Contract (other than Parent Plans):
(i) pursuant
to which the Parent, any of its subsidiaries or any other party thereto has material continuing obligations, rights or interests and
including annual payments by the Parent and its subsidiaries of $100,000 or more relating to the research, development, clinical trial,
distribution, supply, manufacture, marketing or co-promotion of, or collaboration with respect to, any product candidate for which the
Parent or any of its subsidiaries is currently engaged in research or development, including but not limited to: (A) material manufacture
or supply services or material Contracts with contract research organizations for clinical trials-related services; (B) material
transfer Contracts for pre-clinical products or clinical products of the Company or any of its subsidiaries with commercial, pharmaceutical
or biotechnology companies; (C) Contracts involving the payment of royalties or other amounts calculated based upon the revenues
or income of the Parent or any of its subsidiaries or income or revenues related to any clinical product candidate of the Parent or any
of its subsidiaries; and (D) Contracts pursuant to which the Parent has minimum purchase or “most favored nation” obligations;
(ii) that
contains any non-compete or exclusivity provision or limits or purports to limit, curtail or restrict the ability of the Parent or any
of its subsidiaries (or which following the consummation of the Merger and the other transactions contemplated hereby would reasonably
be expected to limit the ability of the Surviving Corporation) in a manner that is material to the business of the Parent and its subsidiaries,
taken as a whole, as currently conducted (A) to compete in any line of business, in any geographic area or with any Person and (B) to
sell to or purchase from any other Person;
(iii) that
requires or permits Parent, or any successor to, or acquirer of, the Parent, to make any payment to another Person, or requires the consent
of another Person, in each case in connection with a change of control of Parent or gives another Person a right to receive or elect
to receive a change of control payment;
(iv) that
is a joint-venture or partnership agreement or other similar agreement or arrangement;
(v) that
(A) relates to the disposition or acquisition by Parent or its subsidiaries of a material amount of assets or equity interests in
any Person (1) after the date of this Agreement, other than the sale of inventory in the ordinary course of business consistent
with past practice, or (2) which contains any ongoing obligations (including sale of inventory, indemnification, purchase price
adjustment, “earn-out” or other contingent obligations) that are still in effect that are reasonably likely to result in
claims in excess of $50,000 or (B) pursuant to which Parent or its subsidiaries will acquire or dispose of any material ownership
interest in any other person or other business enterprise other than the Parent’s subsidiaries;
(vi) that
is a loan or credit agreement, indenture, note or other Contract or instrument relating to or evidencing Indebtedness for borrowed money
(including any guarantee thereto) or any Contract pursuant to which Indebtedness for borrowed money may be incurred or guaranteed, including
any Contract that is a financial derivatives master agreement or confirmation, or futures account opening agreement and/or brokerage
statement, evidencing financial hedging or similar trading activities;
(vii) that
is a mortgage, pledge, security agreement, deed of trust, capital lease or similar agreement that creates or grants a Lien on any material
property or asset of the Parent or any of its subsidiaries, in each case involving annual payments of more than $100,000;
(viii) that
is a Collective Bargaining Agreement;
(ix) that
is a Contract providing for the issuance or sale of any equity securities of the Company or any of its subsidiaries;
(x) That
is a settlement agreement, or agreement entered into in connection with a settlement agreement, corporate integrity agreement, consent
decree, deferred prosecution agreement, or other similar type of agreement with any Governmental Authority or any other Person that has
existing or contingent performance obligations;
(xi) that
is a Contract granting a right of first refusal or first negotiation to any third party over any material assets of the Parent;
(xii) that
is a Contract, including any ancillary or subagreements thereto, with any contract research organization or other agreement, including
any ancillary or subagreements thereto, with a third party which is conducting one or more clinical studies on behalf of the Parent or
its subsidiaries and is reasonably expected to require payment of more than $50,000 within twelve (12) months prior to or after the date
of this Agreement;
(xiii) involves
the use or license by the Parent or its subsidiaries of any material Software used by the Parent or its subsidiaries as presently conducted
(other than non-customized Software subject to shrink-wrap, click-wrap and off-the-shelf or commercially available Software);
(xiv) is
a Parent IP Agreement of the type set forth in Section 4.15(f) or Section 4.15(g) of the Parent Disclosure Letter
or involves the joint development of products or technology with a third party that is material to Parent and its subsidiaries, taken
as a whole; or
(xv) that
is any Contract that is a “material contract” (as such term is defined in Item 601(b)(10) of Regulation S-K of the SEC).
(xvi) All
Contracts, arrangements, commitments or understandings described in this Section 4.12(a), together with each Parent Real
Property Lease, shall be collectively referred to as the “Parent Material Contracts.”
(b) Except,
in each case, as has not been and would not reasonably be expected to be, individually or in the aggregate, material to Parent and
its subsidiaries, taken as a whole, as of the date hereof, (i) each of the Parent Material Contracts is valid, binding and in
full force and effect with respect to Parent and its subsidiaries party thereto and, to the Knowledge of Parent, each other party
thereto and enforceable, in all material respects, in accordance with its terms by Parent and its subsidiaries party thereto;
(ii) Parent and each of its subsidiaries has performed all material obligations required to be performed by them under the
Parent Material Contracts to which they are parties; (iii) to the Knowledge of Parent, each other party to a Parent Material
Contract has performed all material obligations required to be performed by it under such Parent Material Contract and (iv) no
party to any Parent Material Contract has given Parent or any of its subsidiaries written notice of its intention to cancel,
terminate, change the scope of rights under or fail to renew any Parent Material Contract and neither Parent nor any of its
subsidiaries, nor, to the Knowledge of Parent, any other party to any Parent Material Contract, has repudiated in writing any
material provision thereof. Neither Parent nor any of its subsidiaries has knowledge of, or has received written notice of, any
violation or default under any Parent Material Contract or any other Contract to which it is a party or by which it or any of its
material properties or assets is bound, except for violations or defaults that have not been and would not reasonably be expected to
be, individually or in the aggregate, material to Parent and its subsidiaries, taken as a whole. True, unredacted and complete
copies of all of the Parent Material Contracts have been made available to the Company.
4.13 Litigation.
There is no (nor since January 1, 2021 has there been any) Action (excluding external investigations of which Parent has no Knowledge)
pending or, to the Knowledge of Parent, threatened, to which Parent or any of its subsidiaries are or were a party. There are no material
outstanding judgments, writs, injunctions, decrees or orders of any Governmental Authority against or binding on Parent or its subsidiaries.
There are no internal investigations or internal inquiries that, since January 1, 2021, have been conducted by or at the direction
of the Parent Board (or any committee thereof) concerning any financial, accounting or other misfeasance or malfeasance issues.
4.14 Properties.
(a) Neither
the Parent nor any of its subsidiaries owns or has ever owned any real property.
(b) Section 4.14(b) of
the Parent Disclosure Letter sets forth a true and complete list of all real property leased, subleased or otherwise occupied by the
Parent or any of its subsidiaries as tenant, subtenant or occupant as of the date of this Agreement and material to the business of the
Parent and its subsidiaries, taken as a whole (collectively, the “Parent Leased Real Property”). No Parent Real Property
Lease is subject to any Lien, including without limitation, any right to the use or occupancy of any Parent Leased Real Property, other
than Parent Permitted Liens. Each Parent Real Property Lease constitutes the entire agreement between the parties thereto with respect
to the Parent Leased Real Property leased thereunder, and is, with respect to the Parent or the applicable subsidiary of the Parent,
a valid and subsisting agreement in full force and effect and constitutes a valid, binding and enforceable obligation of the Parent or
the applicable subsidiary of the Parent, subject to the Bankruptcy and Equity Exception. The Parent has not received any written notice
of termination or cancellation of or of a breach or default under any Parent Real Property Lease that remains uncured as of the date
of this Agreement nor, to the Knowledge of Parent, has any event occurred which, with notice or lapse of time or both, would constitute
a breach or default under any such Parent Real Property Lease, or permit the termination or cancellation of any such Parent Real Property
Lease. With respect to the Parent Leased Real Property, Section 4.14(b) of the Parent Disclosure Letter also contains a true
and complete list as of the date hereof of all agreements under which the Parent or any of its subsidiaries is, as of the date hereof,
the landlord, sublandlord, tenant, subtenant or occupant that have not been terminated or expired as of the date hereof and are material
to the business of the Parent and its subsidiaries, taken as a whole (each, a “Parent Real Property Lease”). The Parent
has heretofore made available to Parent true and complete copies of the Parent Real Property Leases.
(c) With
respect to each of the Parent Leased Real Properties, neither the Parent nor any of its subsidiaries has exercised or given any notice
of exercise of any option or right of first offer or right of first refusal to purchase, expand, renew or terminate contained in the
Parent Real Property Leases.
(d) Neither
the Parent nor any of its subsidiaries has received written notice of any proceedings in eminent domain, condemnation or other similar
proceedings that are pending, and the Parent has not received written notice threatening any such proceedings, in each case, affecting
any material portion of the Parent Leased Real Property. Neither the Parent nor any of its subsidiaries has received written notice of
the existence of any outstanding writ, injunction, decree, order or judgment or of any pending proceeding pertaining to or affecting
any material portion of the Parent Leased Real Property. As of the date hereof, none of the material improvements located on any parcel
of Parent Leased Real Property that is material to the business of the Parent and its subsidiaries, taken as whole, has been damaged
by a fire or other casualty and not been restored and repaired either (i) to substantially the same condition they were in prior
to such event or (ii) to a condition necessary for the use of the Parent in the ordinary course.
(e) To
the Knowledge of Parent, there are no conditions or defects, latent or otherwise, to the Parent Leased Real Property that would, individually
or in the aggregate, reasonably be expected to have a Parent Material Adverse Effect.
(f) None
of the Parent’s or its subsidiaries’ current use of the Parent Leased Real Property violates any restrictive covenant of
record that affects any of the Parent Leased Real Property or any applicable Laws, in each case to the extent the same would reasonably
be expected to have a Parent Material Adverse Effect.
4.15 Intellectual
Property.
(a) The
Parent, or its subsidiaries, owns, is licensed under agreements that are in full force and effect, or, to the Knowledge of Parent,
otherwise has the right to use all Patents, Trademarks, Trade Secrets, Copyrights and all other Intellectual Property (including
biological materials), all registrations of any of the foregoing, or applications therefor, that are material to Parent’s
business as presently conducted (collectively, and along with the Parent Registered Intellectual Property, the “Parent
Intellectual Property”). The Parent and its subsidiaries possess sufficient rights pursuant to written agreements to use
all material Parent Intellectual Property not owned by the Parent or its subsidiaries as such Parent Intellectual Property are used
in the Parent’s business as presently conducted. Except as otherwise indicated in Section 4.15(a) of the Parent
Disclosure Letter, the Parent or its subsidiaries is the sole and exclusive owner of all rights, title and interests in and to the
Owned Parent Intellectual Property, and to the Knowledge of Parent, all Owned Parent Intellectual Property is free and clear of all
Liens (other than Parent Permitted Liens).
(b) Section 4.15(b) of
the Parent Disclosure Letter sets forth as of the date hereof a true and complete list of all Patents, Trademarks that are trademark
registration, applications and material common law marks, and registered Copyrights that are (i) owned (or purported to be owned)
by the Parent and its subsidiaries, (ii) exclusively licensed to the Parent or its subsidiaries whereby ‘all substantial rights’
are licensed to the Parent or its subsidiaries, or (iii) that are non-exclusively licensed to the Parent or its subsidiaries and
for which the Parent or its subsidiaries controls prosecution thereof ((i), (ii), and (iii) are collectively, the “Parent
Registered Intellectual Property”), indicating for each (as applicable) the name of the current record owner(s), the applicable
jurisdictions and the application or registration numbers, the registration date, and current status. The Parent Registered Intellectual
Property owned by the Parent or its subsidiaries, and, to the Knowledge of Parent, all other Parent Registered Intellectual Property,
is subsisting and in full force and effect and has not been abandoned or adjudged invalid or unenforceable (other than such Parent Registered
Intellectual Property that has expired, lapsed or been abandoned). All Parent Registered Intellectual Property which has been issued,
granted or registered is, to the Parent’s Knowledge, not invalid or unenforceable. Section 4.15(b) of the Parent Disclosure
Letter also sets forth, as of the date of this Agreement, a list of all internet domain names with respect to which the Parent or its
subsidiaries is the registrant and any social media handles registered by the Parent or its subsidiaries.
(c) With
respect to the material items of Parent Registered Intellectual Property, the Parent has maintained them in the ordinary course consistent
with reasonable business practices. To the Knowledge of the Parent, each of the Parent’s or its subsidiaries’ owned Patents
(excluding invention disclosures) that are material to the Parent and its subsidiaries properly identifies each and every inventor
of the claims thereof as determined in accordance with the laws of the jurisdiction in which such Patent was issued or such Patent application
is pending. The named inventors of each of the Parent’s, or its subsidiaries’, owned Patents that are material to the Parent
and its subsidiaries have assigned the applicable inventions for the Parent’s, or its subsidiaries’, owned Patents to the
Parent, or its subsidiaries, respectively, and the inventor assignments have been recorded with the USPTO as applicable except where
failure to do so would not be material. To the Knowledge of the Parent and except as would not be material, all assignments to the Parent
or its subsidiaries of the Parent Registered Intellectual Property owned by the Parent, or its subsidiaries, respectively, are valid
and enforceable.
(d) To
the Knowledge of Parent and except as would not be material, since January 1, 2021, no third party has infringed upon, misappropriated,
violated, or asserted any competing claim of right to use or own any of the Owned Parent Intellectual Property or Parent Registered Intellectual
Property that is exclusively licensed to the Parent, or one of its subsidiaries. There is no litigation, opposition, interference, inventorship
challenge, refusal, cancellation, or proceeding pending, or asserted or threatened in writing, against the Parent or its subsidiaries
concerning the validity, registrability, enforceability, duration, scope, priority, ownership or other violation of any Parent Owned
Intellectual Property or Registered Intellectual Property exclusively licensed to the Parent, or one of its subsidiaries except where
the proceeding is not material; this representation does not apply to office actions in the ordinary course of prosecution. Since January 1,
2021, neither the Parent nor its subsidiaries or its subsidiaries’ respective representatives have sent or otherwise made in writing
any assertion to any third party regarding any material alleged or suspected infringement, misappropriation, dilution or violation of
any Parent Registered Intellectual Property.
(e) To
the Knowledge of Parent, the conduct of the business of the Parent or its subsidiaries, as conducted since January 1, 2021, and
as contemplated to be conducted, has not interfered with, infringed upon, misappropriated, diluted, or otherwise violated, the Intellectual
Property of third parties in a manner that has or would reasonably be expected to result in a material liability to the Parent and its
subsidiaries, taken as a whole. No claim or action alleging infringement, misappropriation, dilution, or other violation of any third
party Intellectual Property is pending or, to the Knowledge of the Parent, threatened in writing against the Parent, its subsidiaries
or, to the Knowledge of the Parent, any other Person who is entitled to be indemnified, defended, held harmless or reimbursed by the
Parent or its subsidiaries with respect to such claim or action that in each case has or would reasonably be expected to result in a
material liability to the Parent and its subsidiaries, taken as a whole. Since January 1, 2021, neither the Parent nor its subsidiaries
has received any written notice (or, to the Knowledge of Parent, any non-written notice) from any third party alleging or threatening
that the operation of the business of the Parent and its subsidiaries as conducted since January 1, 2021 infringes or otherwise
violates the Intellectual Property of such third party, including, but not limited to, any invitation to license that would reasonably
be construed as notice of infringement, any claim that the Parent or its subsidiaries must license, or any claim that the Parent must
refrain from using any Intellectual Property, where the allegation, if true, would reasonably be expected to result in a material liability
to the Parent and its subsidiaries, taken as a whole.
(f) Section 4.15(f) of
the Parent Disclosure Letter sets forth as of the date hereof a true and complete list of all agreements to which the Parent or any of
its subsidiaries is a party that are material to the business of Parent and its subsidiaries (taken as a whole) under which the Parent
or its subsidiaries has been granted an exclusive or non-exclusive license under any Parent Intellectual Property from a third party
(other than nondisclosure agreements, material transfer agreements or non-exclusive licenses and other agreements entered into in the
ordinary course of business) (“Parent Inbound IP Agreements”).
(g) Section 4.15(g) of
the Parent Disclosure Letter sets forth as of the date hereof a true and complete list of all agreements to which the Parent or any of
its subsidiaries is a party that are material to the business of Parent and its subsidiaries (taken as a whole) under which the Parent
or its subsidiaries has (i) granted an exclusive or non-exclusive license or covenant not to sue under any Owned Parent Intellectual
Property to a third party (other than nondisclosure agreements and material transfer agreements and other agreements entered into in
the ordinary course), (ii) assigned (or agreed to assign) any Owned Parent Intellectual Property to a third party (other than agreements
entered into in the ordinary course), (iii) granted any third party an option or other right to obtain any such license, covenant
not to sue, or assignment (other than agreements entered into in the ordinary course), or (iv) covenanted not to pursue patent protection
with respect to any invention or technology other than agreements entered into in the ordinary course (“Parent Outbound IP Agreements”
and together with the Parent Inbound IP Agreements, the “Parent IP Agreements”). The Parent has provided Company with
true and correct copies of all Parent IP Agreements.
(h) Section 4.15(h) of
the Parent Disclosure Letter sets forth as of the date hereof all license, collaboration, or other agreements to which the Parent or
any of its subsidiaries is a party that are material to the business of Parent and its subsidiaries (taken as a whole) under which the
Parent owes and pays material royalties or makes other material financial payments to third parties in connection with the sale of products
and services. Except as set forth in Section 4.15(h) of the Parent Disclosure Letter, neither the Parent nor its subsidiaries,
in the Contracts to which any of them are a party, has agreed to, nor has an obligation to pay any third party royalties or payments
in connection with the sale of products and services where the royalties or payments are material to the business of the Parent and its
subsidiaries (taken as a whole).
(i) Except
as would not have a Parent Material Adverse Effect, the consummation of the Merger shall not under any Parent IP Agreements result in
any: (i) the termination by a third party of any Parent IP Agreement, (ii) the release from escrow of any material Owned Parent
Intellectual Property, or (iii) the grant to any other Person of any license or other right to Owned Parent Intellectual Property.
(j) To
the Knowledge of Parent, none of the activities of the employees of the Parent or its subsidiaries violates any agreement or arrangement
which any such employees have with former employers in any matter that would reasonably be expected to result in material liability to
Parent and its subsidiaries, taken as a whole. All current and former employees and consultants who contributed to the discovery or development
of any material Owned Parent Intellectual Property did so pursuant to written agreements assigning all rights therein to the Parent or
its subsidiaries that do not vest with the Parent and its subsidiaries initially by operation of law (other than non-assignable moral
rights).
(k) To
the Knowledge of Parent, each current or former employee, contractor or consultant of the Parent or its subsidiaries who has proprietary
knowledge of or information relating to Trade Secrets of the Parent or its subsidiaries has executed and delivered to the Parent or its
subsidiaries an agreement or agreements restricting such Person’s right to use and disclose such information or Trade Secret of
the Parent or its subsidiaries except where failure to do so would not be material.
(l) No
settlements, injunctions, forbearances to sue, consents, judgments, orders or similar obligations to which the Parent or its subsidiaries
is party: (i) restrict the use, exploitation, assertion or enforcement of any material Owned Parent Intellectual Property or exclusively
licensed Intellectual Property anywhere in the world consistent with past practices; (ii) restrict in any material manner consistent
with past practices the conduct of the business of the Parent, its subsidiaries or any of its respective employees as presently conducted;
or (iii) grant third parties any material or exclusive rights (including field and territory-limited rights) under any material
Owned Parent Intellectual Property or material exclusively licensed Intellectual Property.
(m) The
Parent and its subsidiaries have exercised reasonable business discretion to protect their rights in their Trade Secrets and other confidential
information, in each case that are material to the business of Parent and its subsidiaries, taken as a whole.
(n) No
government funding nor government, academic or non-profit research facilities or personnel were used, directly or indirectly, to develop
or create, in whole or in part, any of the material Owned Parent Intellectual Property, or, to the Knowledge of the Parent, any other
material Parent Intellectual Property.
(o) Except
as would not reasonably be expected to have a Parent Material Adverse Effect: (i) to the Knowledge of Parent, the Software, hardware,
databases, websites, computer equipment, servers, telecommunication systems, networks, interfaces, platforms, systems and other information
technology or related infrastructure that are owned, operated, leased, used in or necessary for the conduct of the business of the Parent
or its subsidiaries, including such information technology or related infrastructure obtained or licensed from a vendor carrying out
activities on behalf of the Parent or its subsidiaries (collectively, the “Parent Systems”) are lawfully owned, leased,
or licensed by the Parent or its subsidiaries, and are reasonably sufficient for the conduct of their respective businesses as presently
conducted, (ii) since January 1, 2021, there have been no failures, breakdowns, continued substandard performance or other
adverse events affecting any such Parent Systems that have caused a substantial disruption or substantial interruption in or to the use
of such Parent Systems or the conduct of the business of the Parent as presently conducted and remain unresolved or unaddressed, and
(iii) to the Knowledge of Parent, since January 1, 2021, there have not been any material incidents of unauthorized access
or other security breaches of the Parent Systems, and (iv) to the Knowledge of Parent, the Parent Systems do not contain any viruses
or other unauthorized, malicious disabling code that would reasonably be expected to (x) significantly disrupt or materially and
adversely affect the functionality or integrity of any Parent System, or (y) enable or assist any Person to access Parent Systems
without proper authorization. To the Knowledge of Parent, the Parent Systems do not contain any “back door,” “time
bomb,” “Trojan horse,” “worm,” “drop-dead device,” “virus,” malware or other Software
routines or components intentionally designed to permit unauthorized access to, maliciously disable, maliciously encrypt, or erase Software,
hardware, or data that would reasonably be expected to materially disrupt the business of the Parent or its subsidiaries, taken as a
whole. To the Knowledge of Parent, the Parent and its subsidiaries are not in material breach of any of their Contracts relating to Parent
Systems. Since January 1, 2021, the Parent and its subsidiaries have not been, to the Knowledge of the Parent, audited under any
Contract pursuant to which they use any third party system, nor received any written notice of intent to conduct any such audit.
4.16 Taxes.
(a) The
Parent and each of its subsidiaries have prepared and duly and timely filed (taking into account any extension of time within which to
file) all income and other material Tax Returns required to be filed by any of them, and all such filed Tax Returns are true, correct
and complete in all material respects.
(b) Except
as would not have a Parent Material Adverse Effect, Parent and each of its subsidiaries:
(i) have
complied with all applicable Laws, rules, and regulations relating to the payment and withholding of Taxes with respect to amounts owing
to any employee, independent contractor, stockholder, creditor or third party within the time and in the manner prescribed by Law;
(ii) have
not waived any statute of limitations with respect to any Taxes or agreed to any extension of time with respect to any Tax assessment
or deficiency, which waiver or extension is currently effective, other than in connection with an extension of time for filing a Tax
Return and Parent has identified to the Company in writing any such Tax Return to which an extension has been filed outside of the ordinary
course of business and the relevant Tax Return is yet to be filed;
(iii) have
no pending or threatened audits, examinations, or assessments (or other similar proceedings initiated by a Governmental Authority) in
respect of Taxes or Tax matters to which the Parent is a party;
(iv) are
not and have not been a party to any Tax Sharing Agreement (other than an agreement exclusively between or among the Parent and its subsidiaries
or among the Parent’s subsidiaries) pursuant to which it may have any obligation to make any payments for Taxes after the Effective
Time and have no liability for Taxes of any Person (other than the Parent or any of its subsidiaries) under Treasury Regulations Section 1.1502-6
(or any similar provision of state, local, or non-U.S. Law) or as transferee or successor;
(v) have
no Liens for Taxes upon any property or assets of the Parent or any of its subsidiaries, other than Parent Permitted Liens described
in clause (i) of the definition thereof;
(vi) have
not entered into any “closing agreement” under section 7121 of the Code, or other similar agreement with a Governmental Authority
in respect of Taxes that remains in effect, and no request for a ruling, relief, advice, or any other item that relates to the Taxes
or Tax Returns of the Parent or any of its subsidiaries is currently pending with any Governmental Authority, and no such ruling, relief
or advice has even been obtained; and
(vii) do
not participate and have not participated in a “listed transaction” within the meaning of Treasury Regulations Section 1.6011-4(b).
(c) Each
of the Parent and its subsidiaries is, and always has been, treated for U.S. federal income Tax purposes as set forth on 4.16(c) of
the Parent Disclosure Letter.
4.17 Employee
Benefit Plans.
(a) Section 4.17(a) of
the Parent Disclosure Letter sets forth a true and complete list, as of the date of this Agreement, of each material Parent Plan. With
respect to each material Parent Plan, Parent has made available to the Company, as applicable, (i) the plan document (or, with respect
to any unwritten Parent Plan, a written description thereof), (ii) the most recent annual report (Form 5500) prepared in connection
with any such Parent Plan, (iii) the most recent determination or opinion letter, if any, from the IRS for any Parent Plan that
is intended to qualify pursuant to Section 401(a) of the Code, (iv) the most recent actuarial or valuation report, (vii) any
material communications with any Governmental Authority since January 1, 2021, and (viii) the most recent nondiscrimination
testing results.
(b) Each
Parent Plan and trust that is intended to be qualified under Section 401(a) of the Code is covered by a currently effective,
favorable determination letter, or is established on a pre-approved form of plan document that is covered by a favorable advisory or
opinion letter, or has pending or has time remaining in which to file an application for such determination from the IRS, and, to the
Knowledge of Parent, (i) no revocation of any such determination, advisory, or opinion letter has been threatened by any Governmental
Authority, and (ii) no circumstances exist that could reasonably be expected to result in the loss of such qualified status under
Section 401(a) of the Code or material liability to Parent.
(c) No
Parent Plan is, and neither Parent nor any of its ERISA Affiliates sponsors, maintains or contributes (or is required to contribute)
to, or has ever sponsored, maintained or contributed (or been required to contribute) to (i) any employee benefit plan that is or
was subject to Title IV of ERISA, Section 412 of the Code or Section 302 of ERISA, (ii) a “multiemployer plan”
(as defined in Section 3(37) of ERISA), (iii) any “funded welfare benefit plan” (within the meaning of Section 419
of the Code), (iv) any “multiple employer plan” (within the meaning of Section 210 of ERISA or Section 413(c) of
the Code), or (v) any “multiple employer welfare arrangement” (as defined in Section 3(40) of ERISA), and neither
Parent nor any of its ERISA Affiliates has ever incurred any liability under Title IV of ERISA that has not been paid in full.
(d) Each
Parent Plan has been established, operated, administered, and maintained in all material respects in compliance with its terms and in
all material respects with the requirements of applicable Laws, including ERISA and the Code.
(e) Neither
Parent nor any of its subsidiaries has any liability in respect of post-retirement health, medical or life insurance benefits for any
retired, former or current employee, officer, director or other service provider of Parent or any of its subsidiaries (or any dependent
or beneficiary thereof) except coverage or benefits as required under Section 4980B of the Code or any other applicable Laws at
the participant’s sole expense.
(f) Except
as set forth in Section 4.17(f) of the Parent Disclosure Letter, neither the execution of this Agreement nor the
consummation of the transactions contemplated by this Agreement shall (either alone or together with a termination of employment or
other event), (i) entitle any current or former employee, officer, director or other service provider of the Company or any of
its subsidiaries to severance pay or any other payment or benefit, whether under any Parent Plan or otherwise, (ii) accelerate
the time of payment or vesting or trigger any payment of funding (through a grantor trust or otherwise) of compensation or benefits
under, or increase the amount payable or trigger any other obligation pursuant to, any Parent Plan, (iii) increase the amount
payable under any Parent Plan or (iv) result in the payment or provision of an “excess parachute payment” as
defined in Section 280G of the Code to any “disqualified individual” (as defined in Section 280G of the Code)
of Parent or any of its subsidiaries. No Parent Plan or other agreement with any employee provides for a “gross-up” or
similar payment in respect of any Taxes that may become payable under Section 409A or Section 4999 of the Code.
(g) There
is no material Action pending against or, to the Knowledge of Parent, threatened against, any Parent Plan before any Governmental Authority,
other than routine claims for benefits. No Parent Plan is, or in the past six (6) years has been, the subject of an investigation,
examination or audit by a Governmental Authority or is the subject of an application or filing under, or is a participant in, an amnesty,
voluntary compliance, self-correction, or similar program sponsored by any Governmental Authority.
(h) Each
Parent Foreign Plan has been registered and maintained in all material respects in compliance with its terms and in all material respects
with the requirements of applicable Laws and in good standing with applicable regulatory authorities. No Parent Foreign Plan is a defined
benefit plan (as defined in ERISA, whether or not subject to ERISA).
4.18 Employment
Matters.
(a) True
and complete information as to the name, current job title, exempt or non-exempt classification for purposes of FLSA and state wage and
hour laws (or any foreign equivalent), and compensation for all current employees of the Parent and its subsidiaries has been provided
to Parent. No current employee of the Parent or any of its subsidiaries, (i) has given notice of termination of employment or otherwise
disclosed plans to terminate employment with the Parent or any of its subsidiaries within the twelve (12) month period following the
date hereof, (ii) is employed under a nonimmigrant work visa or other work authorization that is limited in duration, or (iii) has
been the subject of any sexual harassment, sexual assault, sexual discrimination or other misconduct allegations during his or her tenure
at the Parent or any of its subsidiaries.
(b) Neither
the Parent nor any of its subsidiaries is a party to or is bound by, or is currently negotiating, a Collective Bargaining Agreement with
any Union. Neither the Parent nor any of its subsidiaries is the subject of an Action asserting that the Parent or any such subsidiary
has committed an unfair labor practice (within the meaning of the National Labor Relations Act). For the last three (3) years, no
Union or group of Parent employees has made a pending demand for recognition or certification, and, to the Knowledge of Parent, there
are no representation or certification proceedings or petitions seeking a representation proceeding presently pending or, to the Knowledge
of Parent, threatened to be brought or filed with the National Labor Relations Board, any other Governmental Authority. To the Knowledge
of Parent, since January 1, 2021, there have been no Union organizing activities with respect to any employees of the Parent or
any of its subsidiaries. There is no, and since January 1, 2021 there has not been, any work slowdown, lockout, work stoppage, picketing,
strike, or other material labor dispute or disputes or collective labor action involving the Parent or any of its subsidiaries pending
or, or to the Knowledge of Parent, threatened. No notice, consent or consultation obligations with respect to any employees of the Parent
or any of its subsidiaries, or any Union, shall be a condition precedent to, or triggered by, the execution of this Agreement or the
consummation of the transactions contemplated by this Agreement.
(c) Except
as would not be reasonably expected, individually or in the aggregate, to have a Parent Material Adverse Effect, Parent and each of its
subsidiaries is, and since January 1, 2021 has been, in compliance with all applicable Laws and Contracts, relating to employment,
including but not limited to employment practices, labor, compensation, discrimination, harassment, workplace safety, retaliation, immigration,
whistleblowing, employee leave, paid time off, benefits, wages and hours, terms and conditions of employment, unemployment insurance,
workers’ compensation, termination of employment, the proper classification of employees as exempt or nonexempt from overtime pay
requirements and the proper classification of individuals as independent contractors or employees, unemployment insurance, collective
dismissals, and the Worker Adjustment and Retraining Notification Act (and any applicable similar foreign, state or local Laws).
4.19 Environmental
Matters.
(a) Except
as would not be reasonably expected, individually or in the aggregate, to have a Parent Material Adverse Effect:
(i) to
the Knowledge of Parent, there is no pending or threatened Environmental Claim or Environmental Liability regarding the Parent or any
of its subsidiaries or any property currently, or formerly owned, operated or leased by the Parent or its subsidiaries;
(ii) with
respect to real property that is currently leased or operated by the Parent and its subsidiaries, and to the Knowledge of Parent, with
respect to real property that was formerly owned, leased or operated by the Parent or its subsidiaries, there have been no Releases of
Hazardous Materials at or from any of such real properties that has caused environmental contamination at any location that is reasonably
likely to result in an obligation of the Parent or any subsidiary to investigate or remediate such environmental contamination pursuant
to applicable Environmental Law or contractual agreement or otherwise result in any Environmental Claim or Environmental Liability;
(iii) neither
(A) the Parent or any subsidiary thereof (B) nor to the Knowledge of Parent any entity previously owned by the Parent or any
subsidiary thereof, has transported or arranged for the treatment, storage, handling, disposal or transportation of any Hazardous Material
at or to any third-party location that is reasonably likely to result in an Environmental Claim or Environmental Liability;
(iv) neither
the Parent nor any subsidiary thereof has, either expressly or by operation of applicable Law, assumed or undertaken, or agreed to assume
or undertake, responsibility for any liability or obligation of any other Person arising under or relating to Environmental Laws;
(v) to
the Knowledge of Parent, the Parent has provided Parent with (a) environmental site assessments and substantially similar evaluations
reasonably available and in its possession respecting material environmental conditions at properties currently leased or used by the
Parent or its subsidiaries and (b) the most recent written compliance audit reasonably available in its possession for current operating
industrial facilities; and
(vi) to
the Knowledge of Parent, there are no other activities, conditions or circumstances that would be reasonably likely to result in any
material Environmental Claim or Environmental Liability.
(b) Except
as would not, individually or in the aggregate, reasonably be expected to have a Parent Material Adverse Effect, to the Knowledge of
Parent, the Parent and each of its subsidiaries are, and since January 1, 2021 have been, in compliance with all Environmental Laws
(which compliance includes, but is not limited to, possession of all Environmental Permits required under applicable Environmental Laws,
and compliance with the terms and conditions thereof).
4.20 Regulatory
Matters; Compliance.
(a) Parent
or its subsidiaries hold all material licenses, Permits, franchises, variances, registrations, exemptions, orders and other governmental
authorizations, consents, approvals, and clearances, and have submitted notices to, all Governmental Authorities, including all authorizations
under the FDCA, the PHSA, and the regulations of the FDA promulgated thereunder, and any other Governmental Authority that regulates
the quality, identity, strength, purity, safety, efficacy or manufacturing of Parent’s Products (any such Governmental Authority,
a “Parent Regulatory Agency”) necessary for the lawful operation of the businesses of Parent or any of its subsidiaries
as currently conducted (the “Parent Permits”), and as of the date hereof, all such Parent Permits are valid and in
full force and effect. There has not occurred any material violation of, default (with or without notice or lapse of time or both) under,
or event giving to others any right of termination, amendment or cancellation of, with or without notice or lapse of time or both, any
Parent Permit. Parent and its subsidiaries are in compliance in all material respects with the terms of all Parent Permits, and no event
has occurred that, to the Knowledge of Parent, would reasonably be expected to result in the revocation, cancellation, non-renewal or
material adverse modification of any Parent Permit. Since January 1, 2021, neither Parent nor its subsidiaries has received written
notice of any pending or threatened claim, suit, proceeding, hearing, enforcement, audit, investigation, arbitration or other action
from the FDA or other Parent Regulatory Agency alleging that any operation or activity of Parent or any of its subsidiaries is in violation
of any applicable Law.
(b) Since
January 1, 2021, all of Parent’s and its subsidiaries’ Products that are subject to the jurisdiction of the FDA or other
Parent Regulatory Agencies have been manufactured, imported, exported, processed, developed, labeled, stored, and tested by or on behalf
of Parent or any of its subsidiaries in all material respects in compliance with all applicable requirements under any Permit or Law,
including applicable statutes and implementing regulations administered or enforced by the FDA or other Parent Regulatory Agency. Since
January 1, 2021, all applications, submissions, notifications, information and data utilized by Parent or its subsidiaries as the
basis for, or submitted by or, to the Knowledge of Parent, on behalf of Parent or any of its subsidiaries in connection with, any and
all requests for Parent Permits relating to Parent or any of its subsidiaries when submitted to the FDA or other Parent Regulatory Agency,
were true, complete and correct, in all material respects, as of the date of submission, and any updates, changes, corrections or modification
to such applications, submissions, notifications, information and data required under applicable Laws have been submitted to the FDA
or other Parent Regulatory Agency.
(c) Since
January 1, 2021, neither Parent, nor any of its subsidiaries, have committed any act, made any statement or failed to make any statement
that would reasonably be expected to provide a basis for the FDA or any other Parent Regulatory Agency to invoke its policy with respect
to “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities,” or other similar Laws. Neither the Company
nor or any of its subsidiaries nor, to the Knowledge of Parent, any of their respective officers, employees, contractors, suppliers or
other entities or individuals performing research or work on behalf of Parent or any of its subsidiaries has been subject to any kind
of consent decree, individual integrity agreement, deferred prosecution agreement, or other similar form of agreement with any Governmental
Authority or convicted of any crime or engaged in any conduct that has resulted, or would reasonably be expected to result, in a material
debarment or exclusion under applicable Law, including, without limitation, 21 U.S.C. Section 335a. No claims, actions, proceedings
or, to the Knowledge of Parent, investigations that would reasonably be expected to result in such a material debarment or exclusion
are pending or threatened in writing against Parent or any of its subsidiaries or any of their respective officers, employees, contractors,
suppliers or other entities or individuals performing research or work on behalf of Parent or any of its subsidiaries.
(d) Since
January 1, 2021, none of Parent, any of its subsidiaries, or, to the Knowledge of Parent, any of their respective contract manufacturers
for Products, has received any FDA Form 483, warning letter, untitled letter, or other similar correspondence or written notice
from the FDA or any other Parent Regulatory Agency alleging or asserting material noncompliance with any applicable Laws or Parent Permits
with respect to any Product of Parent or any of its subsidiaries.
(e) Since
January 1, 2021, all studies, tests and preclinical studies being conducted by Parent or any of its subsidiaries, or in which Parent,
any of its subsidiaries or any Product has participated, have been and are being conducted in compliance in all material respects with
applicable Laws, including the applicable requirements of Good Laboratory Practices, to the extent any such study or test is required
to be conducted in compliance with Good Laboratory Practices.
(f) Since
January 1, 2021, all studies, tests and preclinical and clinical trials being conducted by Parent or any of its subsidiaries, or
in which Parent, any of its subsidiaries or any Product or Product candidate has participated, have been and are being conducted in compliance
in all material respects with applicable Laws, including the applicable requirements of Good Laboratory Practices or Good Clinical Practices.
Since January 1, 2021, neither Parent nor any of its subsidiaries have received any written notices, correspondence or other communication
from any institutional review board, the FDA or any other Parent Regulatory Agency, recommending or requiring the termination, suspension,
or material modification of any ongoing or planned clinical trials conducted by, or on behalf of, Parent or any of its subsidiaries,
other than any comments on study design provided by the FDA as part of any pre-Investigational New Drug Application activities, including
any pre-Investigational New Drug Application meetings.
4.21 Healthcare
Regulatory; Compliance.
(a) Parent
and its subsidiaries is, and at all times since January 1, 2021 has been, in compliance in all material respects with all applicable
Healthcare Laws and, as of the date of this Agreement, there is no Action pending, received by or threatened orally or in writing against
Parent or its subsidiaries related to such Healthcare Laws.
(b) Neither
Parent nor its subsidiaries has engaged in an unlawful or unauthorized practice of medicine or other professionally licensed activities
through any web sites sponsored or operated, or formerly sponsored or operated, by Parent or its subsidiaries.
(c) Parent
has implemented and has in place a compliance program that conforms to and materially ensures compliance with applicable Healthcare Laws
and industry standards.
(d) No
Person has filed against Parent an action relating to Parent under any federal or state whistleblower statute, including under the False
Claims Act of 1863 (31 U.S.C. § 3729 et seq.).
(e) Since
January 1, 2021, Parent and its subsidiaries have made and kept books, records, and accounts which, in reasonable detail, accurately
and fairly reflect the transactions and dispositions of the assets of Parent and each of its subsidiaries.
4.22 Insurance.
(a) Except
as would not, individually or in the aggregate, reasonably be expected to have a Parent Material Adverse Effect, each insurance policy
under which the Parent or any of its subsidiaries is an insured or otherwise the principal beneficiary of coverage (collectively, the
“Parent Insurance Policies”) is in full force and effect and all related premiums have been paid to date. Parent has
made available to Parent true, unredacted and complete copies of the Parent Insurance Policies.
(b) Except
as would not, individually or in the aggregate, reasonably be expected to have a Parent Material Adverse Effect, the Parent Insurance
Policies are reasonable and customary in coverage, scope and size of premiums based on the activities of Parent as conducted and as contemplated
to be conducted as of the date of this Agreement.
(c) Parent
and its subsidiaries are in compliance with the terms and conditions of the Parent Insurance Policies, except for any non-compliance
as would not reasonably be expected, individually or in the aggregate, to have a Parent Material Adverse Effect.
(d) Neither
Parent nor any of its subsidiaries is in material breach or default (including any such breach or default with respect to the payment
of premiums or the giving of notice under any such policy) under any Parent Insurance Policy, and, to the Knowledge of Parent, no event
has occurred which, with notice or lapse of time, would constitute such breach or default, or permit termination or modification, under
such policy. Except as would not, individually or in the aggregate, reasonably be expected to have a Parent Material Adverse Effect,
no insurance claims made by Parent or any of its subsidiaries has been questioned, denied or disputed.
4.23 Anti-Corruption;
Global Trade Control Laws.
(a) Since
January 1, 2018, neither Parent, nor its subsidiaries, nor any of Parent’s or its subsidiaries’ respective current or
former officers, directors, or, to the Knowledge of Parent, any representative acting on behalf of Parent or its subsidiaries, including
any of their respective officers, directors, or employees, has violated, to the extent applicable, any Anti-Corruption Laws, including
by unlawfully directly or indirectly offering, promising, providing, or authorizing the provision of any money, property, contribution,
gift, entertainment or other thing of value to any Person, so as to influence official action, to secure an improper advantage, or to
encourage the recipient to breach a duty of good faith or loyalty or the policies of their employer.
(b) Neither
Parent, nor its subsidiaries, nor, to the Knowledge of Parent, any representative acting at the direction of Parent or its subsidiaries
(i) is under external or internal investigation for (A) any violation of the Anti-Corruption Laws, (B) any alleged irregularity,
misstatement or omission arising under or relating to any Contract between such Person and any Governmental Authority, or any instrumentality
thereof or (C) any unlawful contribution, gift, bribe, rebate, payoff, influence payment, kickback or other payment or the provision
of anything of value, directly or indirectly, to a Government Official, (ii) has received any notice or other written communication
from any Governmental Authority with respect to any actual, alleged or potential violation of, or failure to comply with, any Anti-Corruption
Laws, or (iii) is the subject of any internal complaint, audit or review process with respect to allegations of potential violation
of the Anti-Corruption Laws.
(c) Parent
and its subsidiaries maintain policies and procedures designed to ensure compliance with the Anti-Corruption Laws.
(d) Neither
Parent, nor its subsidiaries, nor any director, officer or employee of any of Parent or its subsidiaries, is, or since January 1,
2018 has been, (i) a Restricted Party or (ii) majority owned or controlled by a Restricted Party.
(e) Parent
and its subsidiaries are, and since January 1, 2018 have been, in compliance in all material respects with all Global Trade Control
Laws, which includes possession of and compliance in all material respects with all licenses, permits, variances, registrations, exemptions,
orders, consents, approvals, clearances, and other authorizations required by Global Trade Control Laws and submission of required notices
or reports to all Governmental Authorities that are concerned with such Global Trade Control Laws.
(f) Since
January 1, 2018, neither Parent nor its subsidiaries has directly or indirectly engaged in any business with, or used, directly
or indirectly, any corporate funds to contribute to or finance the activities of, any Restricted Party or in or with any Restricted Market
and is not currently doing so. Parent acknowledges that activities under this Agreement shall not (i) be in a Restricted Market;
(ii) involve individuals ordinarily resident in a Restricted Market; or (iii) include companies, organizations, or governmental
entities from or located in a Restricted Market.
(g) To
the Knowledge of Parent, (i) since January 1, 2018, neither Parent nor its subsidiaries has been the subject of any investigations,
reviews, audits or inquiries by a Governmental Authority related to Global Trade Control Laws, and (ii) as of the date hereof, no
investigation, review, audit, or inquiry of or to Parent or its subsidiaries by any Governmental Authority with respect Global Trade
Control Laws is pending or threatened.
4.24 CFIUS.
Neither Parent nor its subsidiaries is a U.S. business that (i) produces, designs, tests, manufactures, fabricates, or develops
one or more “critical technologies”; (ii) performs the functions as set forth in column 2 of Appendix A to 31 C.F.R.
Part 800 with respect to “covered investment critical infrastructure”; or (iii) maintains or collects, directly
or indirectly, “sensitive personal data” of U.S. citizens, in each case as such terms in quotation marks are defined in the
Defense Production Act of 1950, as amended, including all implementing regulations thereof.
4.25 Brokers
and Finder’s Fees. Except for as set forth on Section 4.25 of the Parent Disclosure Letter, no broker, investment banker,
financial advisor or other Person is entitled to any broker’s, finder’s or financial advisor’s fee or commission in
connection with the transactions contemplated hereby based upon arrangements made by or on behalf of Parent or any of its subsidiaries.
Prior to the date hereof, Parent has provided the Company with an unredacted copy of each engagement letter between Parent and the Parent
Financial Advisor, pursuant to which the Parent Financial Advisor would be entitled to any payment relating to the Merger and any other
transactions contemplated by this Agreement. The Parent Financial Advisor’s estimated fees and expenses in connection with the
transactions contemplated hereby are disclosed in Section 4.25 of the Parent Disclosure Letter.
4.26 Opinion
of the Financial Advisor. The Parent Financial Advisor has delivered to the Parent Board its opinion, dated on or about the date
hereof, to the effect that, as of the date of such opinion and based upon and subject to the assumptions, factors, qualifications, limitations
and other matters set forth therein, the Per Share Merger Consideration is fair, from a financial point of view, to the holders of Parent
Ordinary Shares (including holders of Parent ADSs). The opinion of the Parent Financial Advisor has not been withdrawn, revoked or modified.
4.27 Antitakeover
Laws. The Parent Board has duly taken all actions so that no Takeover Laws shall prohibit the execution, delivery or performance
of or compliance with this Agreement, the Merger or the other transactions contemplated hereby. Parent has no “rights plan”,
“rights agreement” or “poison pill” in effect.
4.28 Ownership
and Operations of Merger Sub. Parent owns, and at the Effective Time shall own, beneficially and of record, all of the outstanding
capital stock of Merger Sub either directly or indirectly through one or more of its wholly-owned subsidiaries. Merger Sub was formed
solely for the purpose of engaging in the transactions contemplated hereby, has engaged in no other business activities, has not incurred
any material obligations or liabilities except pursuant to this Agreement and has conducted its operations only as contemplated by this
Agreement.
4.29 No
Other Representations; No Reliance; Waiver. Each of Parent and Merger Sub represents, warrants, acknowledges and agrees that none
of the Company Related Persons makes or has made any representation or warranty, either express or implied, as to the accuracy or completeness
of any information provided or made available to the Parent Related Persons or any other Person in connection with this Agreement, the
Parent Voting Agreements, the Merger or any of the other transactions contemplated by this Agreement or with respect to any projections,
forecasts, estimates, plans or budgets of future revenues, expenses or expenditures, future results of operations, future cash flows
or future financial condition, or any component of the foregoing, or any other forward looking information, of the Company or any of
its Affiliates, and no Parent Related Person has relied on any information or statements made or provided (or not made or provided) to
any Parent Related Person other than the representations and warranties of the Company expressly set forth in Section 3 of
this Agreement (as qualified by the Company Disclosure Letter) and any certificate delivered pursuant to Section 7.
Section 5
COVENANTS AND AGREEMENTS
5.1 Conduct
of the Company’s Business.
(a) The
Company covenants and agrees as to itself and its subsidiaries that, from the date of this Agreement until the earlier of the Effective
Time and termination of this Agreement in accordance with Section 8.1 (the “Pre-Closing Period”), except
(i) as required or specifically permitted by any other provision of this Agreement (or as expressly set forth in Section 5.1(a) of
the Company Disclosure Letter), (ii) as required by applicable Law or (iii) with Parent’s prior written consent (such
consent not to be unreasonably withheld, conditioned or delayed), the Company and its subsidiaries shall conduct their respective businesses
in all material respects in the ordinary course of business consistent with past practice and, to the extent consistent therewith, use
their commercially reasonable efforts to (A) keep in effect casualty, product liability, workers’ compensation, property damage,
business interruption and other insurance policies in coverage amounts substantially similar to those in effect on the date of this Agreement,
(B) preserve the Company’s business organization and maintain its existing relations and goodwill with suppliers, distributors,
creditors, lessors, consultants, regulators and business partners, and (C) preserve and protect the material Company Intellectual
Property.
(b) Negative
Covenants Pending Closing. Except as required or specifically permitted by this Agreement (or as expressly set forth in Section 5.1(b) of
the Company Disclosure Letter) or as required by applicable Law, from the date of this Agreement until the earlier of the Effective Time
and termination of this Agreement in accordance with Section 8.1, unless Parent otherwise consents in advance in writing
(such consent not to be unreasonably withheld, conditioned, or delayed), neither the Company nor any of its subsidiaries shall or may:
(i) amend
the Company Charter Documents or the organizational or governing documents of any of the Company’s subsidiaries;
(ii) (A) issue,
deliver, sell, grant, dispose of, pledge or otherwise encumber any shares of capital stock of any class or any other Equity Interest
of the Company or any of its direct or indirect subsidiaries (the “Company Securities”), or any rights, warrants,
options, calls, commitments or any other agreements of any character to purchase or acquire any Company Securities, or any securities
or rights convertible into, exchangeable or exercisable for, or evidencing the right to subscribe for, any Company Securities, in each
case to or in favor of a Person other than the Company or a wholly owned subsidiary of the Company, provided that the Company may issue
shares of Company Common Stock solely upon the exercise of Company Options that are outstanding on the date of this Agreement in accordance
with their terms as of the date of this Agreement or in accordance with the terms of any Contract in effect as of the date of this Agreement;
(B) redeem, purchase or otherwise acquire any outstanding Company Securities, or any rights, warrants, options, calls, commitments,
convertible securities or any other agreements of any character to acquire any Company Securities, except in connection with the exercise
of Company Options that are outstanding on the date of this Agreement and in accordance with their terms as of the date of this Agreement;
(C) adjust, split, combine, subdivide or reclassify any Company Securities; (D) enter into, amend or waive any of the rights
under any Contract with respect to the sale or repurchase of any Company Securities; or (E) except as expressly required by the
terms of this Agreement, amend (including by reducing an exercise price or extending a term) or waive any of its rights under any agreement
evidencing any outstanding Company Options;
(iii) directly
or indirectly acquire or agree to acquire in any transaction any Equity Interest in, or business of, any firm, corporation, partnership,
company, limited liability company, trust, joint venture, association or other entity or division thereof or the purchase (including
by license, collaboration or joint development agreement) directly or indirectly of any properties or assets (other than purchases of
supplies and inventory in the ordinary course of business consistent with the Company’s past practice);
(iv) sell,
pledge, dispose of, transfer, lease, license, mortgage or otherwise encumber or subject to any Lien (including pursuant to a sale leaseback
transaction or an asset securitization transaction) (other than a Company Permitted Lien) any properties, rights or assets (including
securities of the Company and its subsidiaries but excluding the Company Intellectual Property), except dispositions of obsolete assets
or expired inventory;
(v) (A) incur,
create, assume or otherwise become liable for any Indebtedness for borrowed money (including the issuance of any debt security and the
assumption or guarantee of obligations of any Person) (or enter into a “keep well” or similar agreement), except for Indebtedness
that does not exceed $1,000,000 in the aggregate or (B) issue or sell any debt securities or options, warrants, calls or other rights
to acquire any debt securities of the Company, except trade credit or trade payables in the ordinary course of business;
(vi) declare,
set aside, make or pay any dividend or other distribution, whether payable in cash, stock, property or otherwise, in respect of the Company
Common Stock, or Equity Interests of any non-wholly owned subsidiary of the Company;
(vii) other
than as required by applicable Law or in accordance with the terms of any Contract or Company Plan set forth in Section 5.1(b)(vii) of
the Company Disclosure Letter, (A) increase the compensation or benefits (including severance benefits) of any current or former
employees, officers, directors or other service providers of the Company or its subsidiaries; (B) make any new equity or equity-based
awards to any current or former employees, officers, directors or other service providers of the Company or its subsidiaries; (C) take
any action to accelerate the vesting or payment, or prefund or in any other way secure the payment of, compensation or benefits under
any Company Plan; (D) enter into, negotiate, establish, amend, extend or terminate any Company Plan (including any arrangement that
would be a Company Plan if in effect on the date hereof) or any Collective Bargaining Agreement; or (E) change any actuarial or
other assumptions used to calculate funding obligations with respect to any Company Plan or to change the manner in which contributions
to such plans are made or the basis on which such contributions are determined, except insofar as may be required by GAAP, applicable
Law or regulatory guidelines;
(viii) communicate
in a writing that is intended for broad dissemination to the Company’s (or any of its subsidiary’s) employees regarding compensation,
benefits or other treatment they will receive following the Merger, unless any such communication is expressly permitted by this Agreement
(in which case, the Company shall provide Parent with prior notice of, and the opportunity to review and comment upon, any such communications);
(ix) make
any material changes in financial accounting methods, principles or practices (or change an annual accounting period), except insofar
as may be required by GAAP, applicable Law or regulatory guidelines;
(x) write
up, write down or write off the book value of any material assets, except to the extent required by GAAP;
(xi) release,
compromise, assign, settle or agree to settle any Action (including without limitation any suit, action, claim, proceeding or investigation
relating to this Agreement or Merger and the other the transactions contemplated hereby with adverse parties other than Parent or Merger
Sub) or insurance claim, other than compromises, settlements or agreements that involve only monetary payments not in excess of $25,000
individually or $100,000 in the aggregate, in any case without the imposition of material equitable relief on, or the admission of wrongdoing
by, the Company or any of its subsidiaries;
(xii) make
(other than in the ordinary course of business consistent with past practices), change or revoke any material income Tax election or
adopt or change any material method of Tax accounting (except as required by GAAP), (B) enter into any “closing agreement”
as described in Section 7121 of the Code (or any comparable or similar provisions of applicable Law), settle or compromise any material
liability with respect to Taxes, (C) amend any material Tax Return, or (D) consent to any extension or waiver of the limitations
period applicable to any claim or assessment with respect of Taxes (other than any extension pursuant to an extension to file any Tax
Return), in each case, to the extent such action would reasonably be expected to materially and adversely affect Parent, the Company,
or any of their respective subsidiaries in a taxable period (or portion thereof) ending after the Closing;
(xiii) make
or commit to any capital expenditures of greater than $100,000 in the aggregate (other than those set forth in the capital expenditure
budget delivered to Parent prior to the date hereof);
(xiv) (A) enter
into or terminate any Company Material Contract (other than an Acceptable Confidentiality Agreement to the extent permitted by Section 5.3),
(B) materially modify, amend, waive any right under or renew any Company Material Contract, (C) enter into or extend the term
or scope of any Contract that purports to restrict the Company, or any of its subsidiaries or Affiliates or any successor thereto, from
engaging or competing in any line of business or in any geographic area, or (D) enter into any Contract that would be breached by,
or require the consent of any third party in order to continue in full force following, consummation of the Merger and the other transactions
contemplated hereby;
(xv) make
any investment (by contribution to capital, property transfers, purchase of securities or otherwise) in, or loan or advance (other than
travel and similar advances to its employees in the ordinary course of business consistent with the Company’s past practice) to,
any Person;
(xvi) hire
or offer employment or engagement to, promote or terminate the employment or engagement of any director or officer, or any employee,
independent contractor or consultant with total annual compensation in excess of $100,000;
(xvii) merge
or consolidate the Company with any Person or adopt a plan of complete or partial liquidation or resolutions providing for a complete
or partial liquidation, dissolution, restructuring, recapitalization or other reorganization of the Company or any of its material subsidiaries;
(xviii) cancel,
dedicate to the public, disclaim, forfeit, reissue, reexamine, abandon without filing a substantially identical counterpart in the same
jurisdiction with the same priority, or allow to lapse (except with respect to issued Patents expiring in accordance with their terms)
any material Company Intellectual Property;
(xix) fail
to maintain in effect material insurance policies covering the Company and its subsidiaries and their respective properties, assets and
businesses;
(xx) (A) purchase
any marketable securities except in the ordinary course of business, or; (B) change in material manner the investment guidelines
with respect to the Company’s investment portfolio;
(xxi) forgive
any loans to any employees, officers or directors of the Company or its subsidiaries, or any of their respective Affiliates, except in
the ordinary course of business in connection with relocation activities to any employees of the Company or its subsidiaries;
(xxii) (i) sell,
transfer, assign, lease, license, covenant not to enforce, or otherwise dispose of (whether by merger, stock or asset sale or otherwise)
to any Person (including any Affiliate) any rights to any Company Intellectual Property material to the Company or its subsidiaries,
taken as a whole, other than licensing non-exclusive rights or entering in to customary nondisclosure or material transfer agreements
in the ordinary course of business consistent with past practice, (ii) cancel, dedicate to the public, disclaim, forfeit, reissue,
reexamine or abandon without filing a substantially identical counterpart in the same jurisdiction with the same priority or allow to
lapse (except with respect to Patents, Copyrights or Trademarks expiring in accordance with their terms) any Company Registered Intellectual
Property, which the Company or any of its subsidiary controls the prosecution or maintenance thereof, (iii) fail to make any filing,
pay any fee, or take any other action necessary to prosecute and maintain in full force and effect any Company Registered Intellectual
Property, (iv) make any change in Company Intellectual Property material to the business of the Company and its subsidiaries, taken
as a whole, that does or would reasonably be expected to impair such Company Intellectual Property or the Company’s or its subsidiaries
rights with respect thereto, (v) disclose to any Person (other than representatives of Parent and Merger Sub) any Trade Secrets,
know-how or confidential or proprietary information, except, in the case of confidential or proprietary information, in the ordinary
course of business to a Person that is subject to confidentiality obligations or (vi) fail to take or maintain reasonable measures
to protect the confidentiality and value of Trade Secrets included in any of the Owned Company Intellectual Property material to the
business of the Company and its subsidiaries, taken as a whole;
(xxiii) enter
into a definitive agreement providing for a Company Licensing Deal (other than Company Licensing Deal that is a Company Superior Proposal);
or
(xxiv) authorize
any of, or commit, resolve, propose or agree in writing or otherwise to take any of, the foregoing actions.
5.2 Conduct
of Parent Business.
(a) Parent
covenants and agrees as to itself and its subsidiaries that, during the Pre-Closing Period, except (i) as required or
specifically permitted by any other provision of this Agreement (or as expressly set forth in Section 5.2(a) of the
Parent Disclosure Letter), (ii) as required by applicable Law or (iii) with the Company’s prior written consent
(such consent not to be unreasonably withheld, conditioned or delayed), Parent and its subsidiaries shall conduct their respective
businesses in all material respects in the ordinary course of business consistent with past practice and, to the extent consistent
therewith, use their commercially reasonable efforts to (A) keep in effect casualty, product liability, workers’
compensation, property damage, business interruption and other insurance policies in coverage amounts substantially similar to those
in effect on the date of this Agreement, (B) preserve Parent’s business organization and maintain its existing relations
and goodwill with suppliers, distributors, creditors, lessors, consultants, regulators and business partners, and (C) preserve
and protect the material Parent Intellectual Property.
(b) Negative
Covenants Pending Closing. Except as required or specifically permitted by this Agreement (or as expressly set forth in Section 5.2(b) of
the Parent Disclosure Letter) or as required by applicable Law, from the date of this Agreement until the earlier of the Effective Time
and termination of this Agreement in accordance with Section 8.1, unless the Company otherwise consents in advance in writing
(such consent not to be unreasonably withheld, conditioned, or delayed), neither Parent nor any of its subsidiaries shall or may:
(i) amend
the Parent Charter Documents or the organizational or governing documents of any of Parent’s subsidiaries;
(ii) (A) issue,
deliver, sell, grant, dispose of, pledge or otherwise encumber any shares of capital stock of any class or any other Equity Interest
of Parent or any of its direct or indirect subsidiaries (the “Parent Securities”), or any rights, warrants, options,
calls, commitments or any other agreements of any character to purchase or acquire any Parent Securities, or any securities or rights
convertible into, exchangeable or exercisable for, or evidencing the right to subscribe for, any Parent Securities, in each case to or
in favor of a Person other than Parent or a wholly owned subsidiary of Parent, provided that Parent may issue shares of Parent Ordinary
Shares solely upon the exercise of Parent Options or the vesting or settlement of Parent restricted stock units that are outstanding
on the date of this Agreement in accordance with their terms as of the date of this Agreement or in accordance with the terms of any
Contract in effect as of the date of this Agreement; (B) redeem, purchase or otherwise acquire any outstanding Parent Securities,
or any rights, warrants, options, calls, commitments, convertible securities or any other agreements of any character to acquire any
Parent Securities, except in connection with the exercise of Parent Options that are outstanding on the date of this Agreement and in
accordance with their terms as of the date of this Agreement; (C) adjust, split, combine, subdivide or reclassify any Parent Securities;
(D) enter into, amend or waive any of the rights under any Contract with respect to the sale or repurchase of any Parent Securities;
or (E) except as expressly required by the terms of this Agreement, amend (including by reducing an exercise price or extending
a term) or waive any of its rights under any agreement evidencing any outstanding Parent Options;
(iii) directly
or indirectly acquire or agree to acquire in any transaction any Equity Interest in, or business of, any firm, corporation, partnership,
company, limited liability company, trust, joint venture, association or other entity or division thereof or the purchase (including
by license, collaboration or joint development agreement) directly or indirectly of any properties or assets (other than purchases of
supplies and inventory in the ordinary course of business consistent with Parent’s past practice);
(iv) sell,
pledge, dispose of, transfer, lease, license or mortgage or otherwise encumber or subject to any Lien (including pursuant to a sale leaseback
transaction or an asset securitization transaction) (other than a Company Permitted Lien) any properties, rights or assets (including
securities of Parent and its subsidiaries but excluding the Parent Intellectual Property), except dispositions of obsolete assets or
expired inventory;
(v) incur,
create, assume or otherwise become liable for any Indebtedness for borrowed money (including the issuance of any debt security and the
assumption or guarantee of obligations of any Person) (or enter into a “keep well” or similar agreement) in excess of $1,000,000
or issue or sell any debt securities or options, warrants, calls or other rights to acquire any debt securities of the Company, except
trade credit or trade payables in the ordinary course of business;
(vi) declare,
set aside, make or pay any dividend or other distribution, whether payable in cash, stock, property or otherwise, in respect of the Parent
Ordinary Shares, Parent ADSs or Equity Interests of any non-wholly owned subsidiary of Parent;
(vii) other
than as required by applicable Law or in accordance with the terms of any Contract or Parent Plan set forth in Section 5.2(b)(vii) of
the Parent Disclosure Letter, (A) increase the compensation or benefits (including severance benefits) of any current or former
employees, officers, directors or other service providers of Parent or its subsidiaries; (B) make any new equity or equity-based
awards to any current or former employees, officers, directors or other service providers of the Company or its subsidiaries; (C) take
any action to accelerate the vesting or payment, or prefund or in any other way secure the payment of, compensation or benefits under
any Parent Plan; (D) enter into, negotiate, establish, amend, extend or terminate any Parent Plan (including any arrangement that
would be a Parent Plan if in effect on the date hereof) or any Collective Bargaining Agreement; or (E) change any actuarial or other
assumptions used to calculate funding obligations with respect to any Parent Plan or to change the manner in which contributions to such
plans are made or the basis on which such contributions are determined, except insofar as may be required by GAAP, applicable Law or
regulatory guidelines;
(viii) communicate
in a writing that is intended for broad dissemination to the Company’s (or any of its subsidiary’s) employees regarding compensation,
benefits or other treatment they will receive following the Merger, unless any such communication is expressly permitted by this Agreement
(in which case, Parent shall provide the Company with prior notice of, and the opportunity to review and comment upon, any such communications);
(ix) make
any material changes in financial accounting methods, principles or practices (or change an annual accounting period), except insofar
as may be required by GAAP, applicable Law or regulatory guidelines;
(x) write
up, write down or write off the book value of any material assets, except to the extent required by GAAP;
(xi) release,
compromise, assign, settle or agree to settle any Action (including without limitation any suit, action, claim, proceeding or investigation
relating to this Agreement or Merger and the other the transactions contemplated hereby with adverse parties other than the Company)
or insurance claim, other than compromises, settlements or agreements that involve only monetary payments not in excess of $25,000 individually
or $100,000 in the aggregate, in any case without the imposition of material equitable relief on, or the admission of wrongdoing by,
Parent or any of its subsidiaries;
(xii) make
(other than in the ordinary course of business consistent with past practices), change or revoke any material income Tax election or
adopt or change any material method of Tax accounting (except as required by GAAP), (B) enter into any “closing agreement”
as described in Section 7121 of the Code (or any comparable or similar provisions of applicable Law), settle or compromise any material
liability with respect to Taxes, (C) amend any material Tax Return, or (D) consent to any extension or waiver of the limitations
period applicable to any claim or assessment with respect of Taxes (other than any extension pursuant to an extension to file any Tax
Return), in each case, to the extent such action would reasonably be expected to materially and adversely affect Parent, the Company,
or any of their respective subsidiaries in a taxable period (or portion thereof) ending after the Closing make or commit to any capital
expenditures (other than those set forth in the capital expenditure budget delivered to Parent prior to the date hereof);
(xiii) make
or commit to any capital expenditures of greater than $100,000 in the aggregate (other than those set forth in the capital expenditure
budget delivered to the Company prior to the date hereof);
(xiv) (A) enter
into or terminate any Parent Material Contract (other than an Acceptable Confidentiality Agreement containing a standstill agreement
to the extent permitted by Section 5.4), (B) materially modify, amend, waive any right under or renew any Parent Material
Contract, (C) enter into or extend the term or scope of any Contract that purports to restrict Parent, or any of its subsidiaries
or Affiliates or any successor thereto, from engaging or competing in any line of business or in any geographic area, or (D) enter
into any Contract that would be breached by, or require the consent of any third party in order to continue in full force following,
consummation of the Merger and the other transactions contemplated hereby;
(xv) make
any investment (by contribution to capital, property transfers, purchase of securities or otherwise) in, or loan or advance (other than
travel and similar advances to its employees in the ordinary course of business consistent with Parent’s past practice) to, any
Person;
(xvi) hire
or offer employment or engagement to, promote or terminate the employment or engagement of any director or officer, or any employee,
independent contractor or consultant with total annual compensation in excess of $100,000;
(xvii) merge
or consolidate Parent with any Person or adopt a plan of complete or partial liquidation or resolutions providing for a complete or partial
liquidation, dissolution, restructuring, recapitalization or other reorganization of Parent or any of its material subsidiaries;
(xviii) cancel,
dedicate to the public, disclaim, forfeit, reissue, reexamine, abandon without filing a substantially identical counterpart in the same
jurisdiction with the same priority, or allow to lapse (except with respect to issued Patents expiring in accordance with their terms)
any material Parent Intellectual Property;
(xix) fail
to maintain in effect material insurance policies covering Parent and its subsidiaries and their respective properties, assets and businesses;
(xx) (A) purchase
any marketable securities except in the ordinary course of business, or; (B) change in material manner the investment guidelines
with respect to the Parent’s investment portfolio;
(xxi) forgive
any loans to any employees, officers or directors of Parent or its subsidiaries, or any of their respective Affiliates, except in the
ordinary course of business in connection with relocation activities to any employees of Parent or its subsidiaries;
(xxii) (i) sell,
transfer, assign, lease, license, covenant not to enforce, or otherwise dispose of (whether by merger, stock or asset sale or otherwise)
to any Person (including any Affiliate) any rights to any Parent Intellectual Property material to Parent or its subsidiaries, taken
as a whole, other than licensing non-exclusive rights or entering in to customary nondisclosure or material transfer agreements in the
ordinary course of business consistent with past practice, (ii) cancel, dedicate to the public, disclaim, forfeit, reissue, reexamine
or abandon without filing a substantially identical counterpart in the same jurisdiction with the same priority or allow to lapse (except
with respect to Patents, Copyrights or Trademarks expiring in accordance with their terms) any Parent Registered Intellectual Property,
which the Company or any of its subsidiaries controls the prosecution or maintenance thereof, (iii) fail to make any filing, pay
any fee, or take any other action necessary to prosecute and maintain in full force and effect any Parent Registered Intellectual Property,
(iv) make any change in Parent Intellectual Property material to the business of Parent and its subsidiaries, taken as a whole,
that does or would reasonably be expected to impair such Parent Intellectual Property or Parent’s or its subsidiaries rights with
respect thereto, (v) disclose to any Person (other than representatives of the Company) any Trade Secrets, know-how or confidential
or proprietary information, except, in the case of confidential or proprietary information, in the ordinary course of business to a Person
that is subject to confidentiality obligations or (vi) fail to take or maintain reasonable measures to protect the confidentiality
and value of Trade Secrets included in any of the Owned Parent Intellectual Property material to the business of Parent and its subsidiaries,
taken as a whole;
(xxiii) enter
into a definitive agreement providing for a Parent Licensing Deal (other than a Parent Licensing Deal that is a Parent Superior Proposal);
or
(xxiv) authorize
any of, or commit, resolve, propose or agree in writing or otherwise to take any of, the foregoing actions.
5.3 No
Solicitation by the Company.
(a) From
the date of this Agreement until the earlier of the Effective Time and the valid termination of this Agreement in accordance with Section 8.1,
except as expressly permitted by Section 5.3(b) or Section 5.3(d), (i) the Company shall cease, and
shall cause its officers and directors and shall direct the other Company Representatives to cease, and cause to be terminated, all existing
discussions, negotiations and communications with any Persons with respect to any Company Acquisition Proposal (other than the transactions
contemplated hereby); (ii) the Company shall not, and shall not authorize or permit any officers, directors, investment bankers,
attorneys, accountants and other advisors, agents and representatives (collectively, “Company Representatives”) to,
directly or indirectly through another Person, (A) initiate, seek, solicit or knowingly encourage (including by way of furnishing
any non-public information relating to the Company or any of its subsidiaries), or knowingly induce or take any other action which would
reasonably be expected to lead to the making, submission or announcement of any Company Acquisition Proposal, (B) engage in negotiations
or discussions with, or provide any non-public information or non-public data to, any Person (other than Parent or any of its Affiliates
or any Parent Representatives) relating to any Company Acquisition Proposal or grant any waiver or release under any standstill or other
agreement (except that if the Company Board (or any committee thereof) determines in good faith that the failure to grant any waiver
or release would reasonably be expected to be inconsistent with the Company directors’ fiduciary duties under applicable law, the
Company may waive any such standstill provision in order to permit a third party to make a Company Acquisition Proposal), (C) enter
into any agreement, including any letter agreement, memorandum of understanding, agreement in principal, merger agreement or similar
agreement relating to any Company Acquisition Proposal, or (D) otherwise resolve to do any of the foregoing; (iii) the Company
shall not provide and shall, within twenty-four (24) hours of the date hereof, terminate access of any third party to any data room (virtual
or actual) containing any of the Company’s confidential information; and (iv) within two (2) Business Days after the
date hereof, the Company shall request the return or destruction of all confidential, non-public information provided to third parties
that have entered into confidentiality agreements relating to a possible Company Acquisition Proposal with the Company or any of its
subsidiaries. Notwithstanding the foregoing, nothing contained in this Section 5.3 or in Section 6.4 or any other
provision of this Agreement shall prohibit the Company or the Company Board (or any committee thereof) from (A) taking and disclosing
to the Company’s stockholders the fact that a Company Acquisition Proposal has been made, its position with respect to any tender
or exchange offer by a third party pursuant to Rules 14d-9 and 14e-2 promulgated under the Exchange Act or making any statement
contemplated by Item 1012(a) of Regulation MA or any “stop, look and listen” statement or (B) taking any of the
actions set forth in Section 5.3(a) with respect to a Company Licensing Deal.
(b) Notwithstanding
the foregoing, at any time prior to obtaining the Company Stockholder Approval, if the Company receives a written Company Acquisition
Proposal from a third party and the receipt of such Company Acquisition Proposal was not initiated, sought, solicited, knowingly encouraged
or knowingly induced in violation of Section 5.3(a), then the Company may (i) contact the Person who has made such Company
Acquisition Proposal in order to clarify the terms of such Company Acquisition Proposal so that the Company Board may inform itself about
such Company Acquisition Proposal, (ii) furnish information concerning its business, properties or assets to any Person pursuant
to an Acceptable Confidentiality Agreement and (iii) negotiate and participate in discussions and negotiations with such Person
concerning a Company Acquisition Proposal, in the case of clauses (ii) and (iii), only if the Company Board first determines in
good faith, after consultation with its financial advisor and outside legal counsel, that such Company Acquisition Proposal constitutes
or is reasonably likely to constitute or lead to a Company Superior Proposal. The Company (A) shall promptly (and in any case within
twenty-four (24) hours) provide Parent notice (1) of the receipt of any Company Acquisition Proposal, which notice shall include
a complete, unredacted copy of such Company Acquisition Proposal, and (2) of any inquiries, proposals or offers received by, any
requests for non-public information from, or any discussions or negotiations sought to be initiated or continued with, the Company or
any Company Representatives concerning a Company Acquisition Proposal that constitutes or is reasonably likely to constitute or lead
to a Company Acquisition Proposal, and disclose the identity of the other party (or parties) and the material terms of such inquiry,
offer, proposal or request and, in the case of written materials, provide copies of such materials, (B) shall promptly (and in any
case within twenty-four (24) hours) make available to Parent copies of all written materials provided by the Company or the Company’s
Representatives to such party but not previously made available to Parent and (C) shall keep Parent informed on a reasonably prompt
basis (and, in any case, within twenty-four (24) hours of any significant development) of the status and material details (including
amendments and proposed amendments) of any such Company Acquisition Proposal or other inquiry, offer, proposal or request.
(c) Except
as permitted by Section 5.3(d) or Section 5.3(e), neither the Company Board nor any committee thereof shall
(i) withdraw, qualify or modify, or publicly propose to withdraw, qualify or modify, the Company Recommendation, in each case in
a manner adverse to Parent or Merger Sub, (ii) approve or recommend any Company Acquisition Proposal, (iii) enter into any
agreement with respect to any Company Acquisition Proposal (other than an Acceptable Confidentiality Agreement pursuant to Section 5.3(b))
or (iv) if any Company Acquisition Proposal is publicly announced, fail to reaffirm or re-publish the Company Recommendation within
ten (10) Business Days of being requested by Parent to do so (provided that (A) Parent may make such request on no more
than two (2) occasions in response to the same facts, events, circumstances or set of circumstances arising in connection with a
Company Acquisition Proposal, (B) Parent may not make any such request at any time following the Company’s delivery of a notice
pursuant to clause (B) of Section 5.3(d) or clause (ii) of Section 5.3(e) and (C) if
Parent has made any such request and prior to the expiration of ten (10) Business Days, the Company delivers a notice pursuant to
clause (B) of Section 5.3(d) or clause (ii) of Section 5.3(e), the ten (10) Business Day
period set forth in this clause (iv) shall be tolled on a daily basis during the period beginning on the date of delivery of such
notice and ending on the date on which the Company Board shall have determined not to effect a Company Adverse Recommendation Change
pursuant to Section 5.3(d) or Section 5.3(e), as applicable) (any action described in this sentence being
referred to as a “Company Adverse Recommendation Change”).
(d) If,
at any time prior to the receipt of the Company Stockholder Approval, the Company Board receives a Company Acquisition Proposal that
the Company Board determines in good faith, after consultation with its financial advisor and outside legal counsel, constitutes a
Company Superior Proposal, the Company Board may (i) effect a Company Adverse Recommendation Change or (ii) authorize the
Company to terminate this Agreement pursuant to Section 8.1(c)(iii) in order to enter into a definitive agreement
providing for a Company Superior Proposal if (A) the Company Board determines in good faith, after consultation with its
financial advisor and outside legal counsel, that the failure to take such action would reasonably be expected to be inconsistent
with the Company’s directors’ fiduciary duties under applicable Law; (B) the Company has notified Parent in writing
that it intends to effect a Company Adverse Recommendation Change or terminate this Agreement; (C) if applicable, the Company
has provided Parent a copy of the proposed definitive agreements between the Company and the Person making such Company Superior
Proposal; (D) for a period of four (4) days following the notice delivered pursuant to clause (B) of this Section 5.3(d),
the Company shall have discussed and negotiated in good faith and made Company Representatives available to discuss and negotiate in
good faith (in each case to the extent Parent desires to negotiate) with Parent Representatives any proposed modifications to the
terms and conditions of this Agreement so that the failure to take such action would no longer reasonably be expected to be
inconsistent with the Company’s directors’ fiduciary duties under applicable Law (it being understood and agreed that
any amendment to any material term or condition of any Company Superior Proposal shall require a new notice and a new two
(2) day negotiation period); and (E) no earlier than the end of such negotiation period, the Company Board shall have
determined in good faith, after considering the terms of any proposed amendment or modification to this Agreement (and all
financial, legal and regulatory terms and conditions of such Company Acquisition Proposal and the expected timing of consummation
and the relative risk of consummation of the applicable proposal), that (x) the Company Acquisition Proposal that is the
subject of the notice described in clause (B) above still constitutes a Company Superior Proposal and (y) the failure to
take such action would still reasonably be expected to be inconsistent with the Company’s directors’ fiduciary duties
under applicable Law.
(e) Other
than in connection with a Company Superior Proposal (which shall be subject to Section 5.3(d) and shall not be subject
to this Section 5.3(e)), prior to obtaining the Company Stockholder Approval, the Company Board may take any action prohibited
by clause (i) of Section 5.3(c), but only in response to a Company Intervening Event and only if (i) the Company
Board determines in good faith, after consultation with its financial advisor and outside legal counsel, that the failure to take such
action would reasonably be expected to be inconsistent with the Company’s directors’ fiduciary duties under applicable Law;
(ii) the Company has notified Parent in writing that it intends to effect a Company Adverse Recommendation Change due to the occurrence
of a Company Intervening Event (which notice shall specify the Company Intervening Event in reasonable detail); (iii) for a period
of four (4) days following the notice delivered pursuant to clause (ii) of this Section 5.3(e), the Company shall
have discussed and negotiated in good faith and made Company Representatives available to discuss and negotiate in good faith (in each
case to the extent Parent desires to negotiate), with Parent Representatives any proposed modifications to the terms and conditions of
this Agreement so that the failure to take such action would no longer reasonably be expected to be inconsistent with the Company’s
directors’ fiduciary duties under applicable Law (it being understood and agreed that any material change to the facts and circumstances
relating to the Company Intervening Event shall require a new notice and a new two (2) day negotiation period); and (iv) no
earlier than the end of the negotiation period, the Company Board shall have determined in good faith, after consultation with its financial
advisor and outside legal counsel, after considering the terms of any proposed amendment or modification to this Agreement, that the
failure to take such action would still reasonably be expected to be inconsistent with the Company’s directors’ fiduciary
duties under applicable Law.
5.4 No
Solicitation by Parent.
(a) During
the Pre-Closing Period, except as expressly permitted by Section 5.4(b) or Section 5.4(d), (i) Parent
shall cease, and shall cause its officers and directors and shall direct the other Parent Representatives to cease, and cause to be terminated
all existing discussions, negotiations and communications with any Persons or entities with respect to any Parent Acquisition Proposal
(other than the transactions contemplated hereby); (ii) Parent shall not, and shall not authorize or permit any officers, directors,
investment bankers, attorneys, accountants and other advisors, agents and representatives (collectively, the “Parent Representatives”)
to, directly or indirectly through another Person, (A) initiate, seek, solicit or knowingly encourage (including by way of furnishing
any non-public information relating to Parent or any of its subsidiaries), or knowingly induce or take any other action which would reasonably
be expected to lead to the making, submission or announcement of any Parent Acquisition Proposal, (B) engage in negotiations or
discussions with, or provide any non-public information or non-public data to, any Person (other than the Company or any of its Affiliates
or any Company Representatives) relating to any Parent Acquisition Proposal or grant any waiver or release under any standstill or other
agreement (except that if the Parent Board (or any committee thereof) determines in good faith that the failure to grant any waiver or
release would reasonably be expected to be inconsistent with the Parent directors’ fiduciary duties under applicable law, Parent
may waive, any such standstill provision in order to permit a third party to make a Parent Acquisition Proposal), (C) enter into
any agreement, including any letter agreement, memorandum of understanding, agreement in principal, merger agreement or similar agreement
relating to any Parent Acquisition Proposal, or (D) otherwise resolve to do any of the foregoing; (iii) Parent shall not provide
and shall, within twenty four (24) hours of the date hereof, terminate access of any third party to any data room (virtual or actual)
containing any of Parent’s confidential information; and (iv) within two (2) Business Days after the date hereof, Parent
shall request the return or destruction of all confidential, non-public information provided to third parties that have entered into
confidentiality agreements relating to a possible Parent Acquisition Proposal with Parent or any of its subsidiaries. Notwithstanding
the foregoing, nothing contained in this Section 5.4 or in Section 6.4 or any other provision of this Agreement
shall prohibit Parent or the Parent Board (or any committee thereof) from (A) taking and disclosing to Parent Shareholders the fact
that any Parent Acquisition Proposal has been made, its position with respect to any tender or exchange offer by a third party pursuant
to Rules 14d-9 and 14e-2 promulgated under the Exchange Act or making any statement contemplated by Item 1012(a) of Regulation
MA or any “stop, look and listen” statement or (B) taking any of the actions set forth in Section 5.4(a) with
respect to a Parent Licensing Deal.
(b) Notwithstanding
the foregoing, at any time prior to obtaining the Parent Shareholder Approval, if Parent receives a written Parent Acquisition
Proposal from a third party and the receipt of such Parent Acquisition Proposal was not initiated, sought, solicited, knowingly
encouraged or knowingly induced in violation of Section 5.4(a), then Parent may (i) contact the Person who has made
such Parent Acquisition Proposal in order to clarify the terms of such Parent Acquisition Proposal so that the Parent Board may
inform itself about such Parent Acquisition Proposal, (ii) furnish information concerning its business, properties or assets to
any Person pursuant to an Acceptable Confidentiality Agreement and (iii) negotiate and participate in discussions and
negotiations with such Person concerning a Parent Acquisition Proposal, in the case of clauses (ii) and (iii), only if the
Parent Board first determines in good faith, after consultation with its financial advisor and outside legal counsel, that such
Parent Acquisition Proposal constitutes or is reasonably likely to constitute or to lead to a Parent Superior Proposal. Parent
(A) shall promptly (and in any case within twenty-four (24) hours) provide the Company notice (1) of the receipt of any
Parent Acquisition Proposal, which notice shall include a complete, unredacted copy of such Parent Acquisition Proposal, and
(2) of any inquiries, proposals or offers received by, any requests for non-public information from, or any discussions or
negotiations sought to be initiated or continued with, Parent or any Parent Representatives concerning a Parent Acquisition Proposal
that constitutes or is reasonably likely to constitute or lead to a Parent Acquisition Proposal, and disclose the identity of the
other party (or parties) and the material terms of such inquiry, offer, proposal or request and, in the case of written materials,
provide copies of such materials, (B) shall promptly (and in any case within twenty-four (24) hours) make available to the
Company copies of all written materials provided by Parent or Parent’s Representatives to such party but not previously made
available to the Company and (C) shall keep the Company informed on a reasonably prompt basis (and, in any case, within
twenty-four (24) hours of any significant development) of the status and material details (including amendments and proposed
amendments) of any such Parent Acquisition Proposal or other inquiry, offer, proposal or request.
(c) Except
as permitted by Section 5.4(d) or Section 5.4(e), neither the Parent Board nor any committee thereof shall
(i) withdraw, qualify or modify, or publicly propose to withdraw, qualify or modify, the Parent Recommendation, in each case in
a manner adverse to the Company, (ii) approve or recommend any Parent Acquisition Proposal, (iii) enter into any agreement
with respect to any Parent Acquisition Proposal (other than an Acceptable Confidentiality Agreement pursuant to Section 5.4(b))
or (iv) if any Parent Acquisition Proposal is publicly announced, fail to reaffirm or re-publish the Parent Recommendation within
ten (10) Business Days of being requested by the Company to do so (provided that (A) the Company may make such request on no
more than two (2) occasions in response to the same facts, events, circumstances or set of circumstances arising in connection with
a Parent Acquisition Proposal, (B) the Company may not make any such request at any time following Parent’s delivery of a
notice pursuant to clause (B) of Section 5.4(d) or clause (ii) of Section 5.4(e) and (C) if
the Company has made any such request and prior to the expiration of ten (10) Business Days Parent delivers a notice pursuant to
clause (B) of Section 5.4(d) or clause (ii) of Section 5.4(e), the ten (10) Business Day
period set forth in this clause (iv) shall be tolled on a daily basis during the period beginning on the date of delivery of such
notice and ending on the date on which the Parent Board shall have determined not to effect a Parent Adverse Recommendation Change pursuant
to Section 5.4(d) or 5.4(e), as applicable) (any action described in this sentence being referred to as a “Parent
Adverse Recommendation Change”).
(d) If,
at any time prior to the receipt of Parent Shareholder Approval, the Parent Board receives a Parent Acquisition Proposal that the Parent
Board determines in good faith, after consultation with its financial advisor and outside legal counsel, constitutes a Parent Superior
Proposal, the Parent Board may (i) effect a Parent Adverse Recommendation Change or (ii) authorize Parent to terminate this
Agreement pursuant to Section 8.1(b)(iii) in order to enter into a definitive agreement providing for a Parent Superior
Proposal, if the Parent Board determines in good faith, after consultation with its financial advisor and outside legal counsel, that
the failure to take such action would reasonably be expected to be inconsistent with Parent’s directors’ fiduciary duties
under applicable Law; (B) Parent has notified the Company in writing that it intends to effect a Parent Adverse Recommendation Change
or terminate this Agreement; (C) if applicable, Parent has provided the Company a copy of the proposed definitive agreements between
Parent and the Person making such Parent Superior Proposal; (D) for a period of four (4) days following the notice delivered
pursuant to clause (B) of this Section 5.4(d), Parent shall have discussed and negotiated in good faith and made Parent
Representatives available to discuss and negotiate in good faith (in each case to the extent the Company desires to negotiate) with Company
Representatives any proposed modifications to the terms and conditions of this Agreement so that the failure to take such action would
no longer be reasonably be expected to inconsistent with Parent’s directors’ fiduciary duties under applicable Law (it being
understood and agreed that any amendment to any material term or condition of any Parent Superior Proposal shall require a new notice
and a new two (2) day negotiation period; and (e) no earlier than the end of such negotiation period, the Parent Board shall
have determined in good faith, after consultation with its financial advisor and outside legal counsel, after considering the terms of
any proposed amendment or modification to this Agreement (and all financial, legal and regulatory terms and conditions of such Parent
Acquisition Proposal and the expected timing of consummation and the relative risk of consummation of the applicable proposal), that
(x) the Parent Acquisition Proposal that is the subject of the notice described in clause (B) above still constitutes a Parent
Superior Proposal and (y) the failure to take such action would still reasonably be expected to be inconsistent with Parent’s
directors’ fiduciary duties under applicable Law.
(e) Other
than in connection with a Parent Superior Proposal (which shall be subject to Section 5.4(d) and shall not be subject
to this Section 5.4(e)), prior to obtaining the Parent Shareholder Approval, the Parent Board may take any action prohibited
by clause (i) of Section 5.4(c), but only in response to a Parent Intervening Event and only if (i) the Parent
Board determines in good faith, after consultation with its financial advisor and outside legal counsel, that the failure to take such
action would reasonably be expected to be inconsistent with the Parent directors’ fiduciary duties under applicable Law; (ii) Parent
has notified the Company in writing that it intends to effect a Parent Adverse Recommendation Change due to the occurrence of a Parent
Intervening Event (which notice shall specify the Parent Intervening Event in reasonable detail); (iii) for a period of four (4) days
following the notice delivered pursuant to clause (ii) of this Section 5.4(e), Parent shall have discussed and negotiated
in good faith, and shall have made Parent Representatives available to discuss and negotiate in good faith (in each case to the extent
the Company desires to negotiate), with Company Representatives any proposed modifications to the terms and conditions of this Agreement
so that the failure to take such action would no longer reasonably be expected to be inconsistent with the Parent directors’ fiduciary
duties under applicable Law (it being understood and agreed that any material change to the facts and circumstances relating to the Parent
Intervening Event shall require a new notice and a new two (2) day negotiation period; and (iv) no earlier than the end of
the negotiation period, the Parent Board shall have determined in good faith, after consultation with its financial advisor and outside
legal counsel, after considering the terms of any proposed amendment or modification to this Agreement, that the failure to take such
action would still reasonably be expected to be inconsistent with the Parent directors’ fiduciary duties under applicable Law.
Section 6
ADDITIONAL COVENANTS AND AGREEMENTS
6.1 Registration
Statement; Proxy Statement/Prospectus.
(a) As
promptly as practicable, and in any event within forty-five (45) days following the execution of this Agreement, (i) Parent and
the Company shall jointly prepare and cause to be filed with the SEC the Proxy Statement/Prospectus in preliminary form, which shall
contain the Company Recommendation (unless a Company Adverse Recommendation Change has occurred) and the Parent Recommendation (unless
a Parent Adverse Recommendation Change has occurred), and (ii) Parent shall prepare and cause to be filed with the SEC the Form S-4,
which shall include the Proxy Statement/Prospectus. To the extent necessary, (i) Parent shall cause the depositary of Parent ADSs
to prepare and file with the SEC, no later than the date prescribed by the rules and regulations under the Securities Act, a registration
statement, or a post-effective amendment thereto, as applicable, on Form F-6 or 8-K, as applicable, with respect to the Parent ADSs
deliverable in connection with the Merger and (ii) Parent shall use its commercially reasonable efforts to have such filing declared
effective under the Securities Act as promptly as practicable after such filing and to keep such filing effective as long as necessary
to consummate the transactions contemplated by this Agreement, including the Merger. Parent shall use its commercially reasonable efforts,
and the Company shall reasonably cooperate with Parent in such efforts (including by providing all information reasonably requested by
Parent in connection with the preparation of the Form S-4) to have the Form S-4 declared effective under the Securities Act
as promptly as practicable after such filing and to keep the Form S-4 effective as long as necessary to consummate the transactions
contemplated by this Agreement, including the Merger. The Company shall establish a record date for the Company Stockholders Meeting
and Parent shall establish a record date for the Parent Shareholders Meeting (which shall, to the extent practicable and consistent with
applicable Law or the rules of the relevant securities exchange, be the same date as the record date for the Company Stockholders
Meeting) and each of the Company and Parent shall commence a broker search in connection therewith, as promptly as practicable following
the date of this Agreement and mail the Proxy Statement/Prospectus to holders of the Company Common Stock and Parent Shareholders, as
applicable, as promptly as practicable after the Form S-4 is declared effective under the Securities Act (and in any event within
ten (10) days of the date the Form S-4 is declared effective by the SEC). Parent shall also use commercially reasonable efforts
to take any action required to be taken under any applicable state securities Laws and other applicable Laws in connection with the issuance
of Parent ADSs pursuant to this Agreement, and each party shall furnish all information concerning the Company and Parent, as applicable,
as may be reasonably requested by the other party in connection with any such action and the preparation, filing and distribution of
the Proxy Statement/Prospectus. For the avoidance of doubt, the obligations of each party in this Section 6.1(a) shall
include provision by such party of (x) all such information about itself, its directors and its Affiliates as may be reasonably
requested by the other party for inclusion in the Proxy Statement/Prospectus or Form S-4 and (y) reasonable access to, and
using commercially reasonable efforts to provide reasonable assistance from, the other party’s representatives in connection therewith.
No filing of, or amendment or supplement to, or correspondence to the SEC or its staff with respect to, the Form S-4, shall be made
by Parent, or with respect to the Proxy Statement/Prospectus shall be made by the Company, or in either case any of their respective
subsidiaries, without providing the other party a reasonable opportunity to review and comment thereon. Parent shall advise the Company,
promptly after it receives notice of the time when the Form S-4 has become effective or any supplement or amendment has been filed,
the issuance of any stop order, the suspension of the qualification of the Parent ADSs issuable in connection with the Merger for offering
or sale in any jurisdiction, or any request by the SEC for amendment of the Form S-4 or comments thereon and responses thereto or
requests by the SEC for additional information. The Company shall advise Parent, promptly after it receives notice of any request by
the SEC for the amendment of the Proxy Statement/Prospectus or comments thereon and responses thereto or requests by the SEC for additional
information. If at any time prior to the Effective Time the Company or Parent discover that any information relating to the Company or
Parent, or any of their respective Affiliates, officers or directors, which should be set forth in an amendment or supplement to either
the Form S-4 or the Proxy Statement/Prospectus, so that any of such documents would not include any misstatement of a material fact
or omit to state any material fact necessary to make the statements therein, in the light of the circumstances under which they were
made, not misleading, the party which discovers such information shall promptly notify the other parties hereto and an appropriate amendment
or supplement describing such information shall be promptly filed with the SEC, after the other party has had a reasonable opportunity
to review and comment thereon, and, to the extent required by applicable Law, disseminated to holders of the Company Common Stock.
(b) Whether
or not the Merger is consummated, Parent and the Company shall share equally all expenses incurred in connection with all filings and
other fees paid to the SEC (other than attorneys’ fees, accountants’ fees, investment bankers’ fees and related expenses).
6.2 Meetings
of Stockholders.
(a) The
Company shall, following the date on which the Form S-4 is declared effective by the SEC, duly call, give notice of, convene and
hold a meeting of its stockholders (the “Company Stockholders Meeting”) for the purpose of seeking the Company Stockholder
Approval and, unless the Company Board shall have effected a Company Adverse Recommendation Change in accordance with Sections 5.3(d) or
5.3(e), use its commercially reasonable efforts to solicit adoption of this Agreement by its stockholders. The Company shall,
after consultation with Parent, schedule the Company Stockholders Meeting to be held within thirty (30) days of the initial mailing of
the Proxy Statement/Prospectus; provided, however, that the Company may postpone, recess or adjourn the Company Stockholders
Meeting (i) with the consent of Parent, (ii) to ensure that any required supplement or amendment to the Proxy Statement/Prospectus
is provided to the Company’s stockholders with a reasonable amount of time in advance of the Company Stockholder Meeting, (iii) if
there are not sufficient affirmative votes in person or by proxy at such meeting to constitute a quorum or to obtain the Company’s
Stockholder Approval, to allow reasonable additional time for solicitation of proxies for purposes of obtaining a quorum or the Company
Stockholder Approval, as applicable, and (iv) as may be required by applicable Law.
(b) Parent
shall, following the date on which the Form S-4 is declared effective by the SEC, duly call, give notice of, convene and hold a
general meeting of the Parent Shareholders (the “Parent Shareholders Meeting”) for the purpose of seeking the
Parent Shareholder Approval and, unless the Parent Board shall have effected a Parent Adverse Recommendation Change in accordance
with Sections 5.4(d) or 5.4(e), use commercially reasonable efforts to solicit approval of the issuance and
delivery of Parent ADSs (and all Parent Ordinary Shares represented thereby) as provided in Section 2. Parent shall
provide the Company with a reasonable opportunity to review and comment upon the circular containing the notice of the Parent
Shareholders Meeting and shall consider any comments from Company thereon in good faith prior to the publication of such circular.
Subject to applicable Law or the rules of any relevant securities exchange, Parent shall schedule the Parent Shareholders
Meeting to be held substantially contemporaneously with (and in no event later than) the Company Stockholders Meeting; provided, however,
that Parent may postpone, recess or adjourn the Parent Shareholders Meeting (i) with the consent of the Company, (ii) to
ensure that any required supplement or amendment to the Proxy Statement/Prospectus is provided to the Parent Shareholders within a
reasonable amount of time in advance of the Parent Shareholders Meeting, (iii) if there are not sufficient affirmative votes in
person or by proxy at such meeting to constitute a quorum or to obtain the Parent Shareholder Approval, to allow reasonable
additional time for solicitation of proxies for purposes of obtaining a quorum or the Parent Shareholder Approval, as applicable and
(iv) as may be required by applicable Law.
(c) Parent
shall take all action necessary to cause Merger Sub to perform its obligations under this Agreement and to consummate the Merger and
other transactions contemplated by this Agreement on the terms and conditions set forth in this Agreement. Immediately following the
date of this Agreement, Parent shall provide or make available to the Company a copy of Parent’s approval of this Agreement as
the sole stockholder of Merger Sub.
6.3 Access
to Information.
(a) Prior
to the Effective Time, Parent shall be entitled, through its employees and representatives, to have such access to the assets, properties,
books, records, Contracts, business and operations of the Company as is reasonably necessary or appropriate in connection with Parent’s
investigation of the Company and its subsidiaries with respect to the transactions contemplated hereby and the execution, performance
or consummation (including with regard to the structure of the Merger and integration planning) of such transactions. Any such investigation
and examination shall be conducted at reasonable times during business hours upon reasonable advance notice and under reasonable circumstances
so as to minimize disruption to or impairment of the Company’s business and the Company shall cooperate fully therewith. In order
that Parent may have full opportunity to make such investigation, the Company shall furnish the Parent Representatives during such period
with all such information and copies of such documents concerning the affairs of the Company as such Parent Representatives may reasonably
request and cause its officers, employees, consultants, agents, accountants and attorneys to reasonably cooperate with such Parent Representatives
in connection with such investigation.
(b) Prior
to the Effective Time, the Company shall be entitled, through its employees and representatives, to have such access to the assets, properties,
books, records, Contracts, business and operations of Parent as is reasonably necessary or appropriate in connection with the Company’s
investigation of Parent and its subsidiaries with respect to the transactions contemplated hereby and the execution, performance or consummation
of such transactions. Any such investigation and examination shall be conducted at reasonable times during business hours upon reasonable
advance notice and under reasonable circumstances so as to minimize disruption to or impairment of Parent’s business and Parent
shall cooperate fully therewith. No investigation by Parent or the Company (whether conducted prior to or after the date of this Agreement)
shall diminish or obviate any of the representations, warranties and covenants or agreements of the Company or Parent contained in this
Agreement. In order that the Company may have full opportunity to make such investigation, Parent shall furnish the Company Representatives
during such period with all such information and copies of such documents concerning the affairs of Parent as such Company Representatives
may reasonably request and cause its officers, employees, consultants, agents, accountants and attorneys to reasonably cooperate with
such Parent Representatives in connection with such investigation.
(c) This
Section 6.3 shall not require a party hereunder to permit any inspection or other access, or to disclose any information,
that in its reasonable, good faith judgment (after consultation with outside counsel) would reasonably be expected to: (i) result
in such disclosure: (a) resulting in the disclosure of any Trade Secrets of third parties; (b) violating any Law to which such
party is subject or cause any privilege (including attorney-client privilege) which such party or any of its subsidiaries would be entitled
to assert to be undermined with respect to such information; (c) violating any obligation of the party with respect to confidentiality,
non-disclosure or privacy; (d) materially interfering with the conduct of the party’s business; or (e) of the party’s
board of directors or its committee’s materials that relate to a Company Acquisition Proposal or Parent Acquisition Proposal, provided,
that the parties shall use their reasonable best efforts to make appropriate substitute disclosure arrangements of such information under
circumstances in which restrictions in clauses (i)(a) through (e) apply; or (ii) be included in the minutes of the meeting
of the party’s board of directors or its committees and relates to the discussion by the party’s board of directors or any
applicable committee of the transactions contemplated herein or any similar transaction between the party and any other person (including
any presentations or other materials prepared by or for the party’s board of directors, whether in connection with a special meeting
or otherwise relating to such subject matter); or (iii) if the Company and its subsidiaries, on the one hand, and Parent or any
of its subsidiaries, on the other hand, are adverse parties in an Action, such information being reasonably pertinent thereto.
(d) No
investigation pursuant to this Section 6.3 or information provided, made available or delivered to any party pursuant to
this Agreement shall affect any of the representations, warranties, covenants, rights or remedies, or the conditions to the obligations
of, the parties hereunder. All information shared pursuant to this Section 6.3 shall be held confidential in accordance with
the terms of the Confidentiality Agreement.
6.4 Public
Disclosure. So long as this Agreement is in effect, neither Parent, nor the Company, nor any of their respective Affiliates,
shall disseminate any press release or other public announcement concerning this Agreement, the Merger or the other transactions
contemplated by this Agreement, except as may be required by Law or the rules of any listing authority or any securities
exchange, without the prior consent of each of the other parties hereto, which consent shall not be unreasonably withheld,
conditioned or delayed. The parties have agreed to the text of the joint press release announcing the execution of this Agreement.
Notwithstanding the foregoing, without prior consent of the other parties, each party (a) may communicate information that is
not confidential information of any other party to financial analysts, investors and media representatives in a manner consistent
with its past practice in compliance with applicable Law and (b) may disseminate the information included in a press release or
other document previously approved for external distribution by the other parties. Notwithstanding any other provision of this
Agreement, (i) no party shall be required to consult with the other party in connection with any such press release or public
announcement if (A) the Company Board has effected any Company Adverse Recommendation Change or shall have resolved to do so or
(B) the Parent Board has effected a Parent Adverse Recommendation Change or shall have resolved to do so and (ii) the
requirements of this Section 6.4 shall not apply to any disclosure by the Company or Parent of any information
concerning this Agreement, the Merger or the other transactions contemplated hereby in connection with a determination by
(A) the Company in accordance with Section 5.3(b) that a Company Acquisition Proposal constitutes, or may
constitute, a Company Superior Proposal, (B) Parent in accordance with Section 5.4(b) that a Parent
Acquisition Proposal constitutes, or may constitute, a Parent Superior Proposal, or (C) any dispute between the parties
regarding this Agreement, the Merger or the transactions contemplated by this Agreement.
6.5 Regulatory
Filings; Commercially Reasonable Efforts.
(a) Subject
to the terms and conditions of this Agreement, each party shall use its commercially reasonable efforts to take, or cause to be taken,
all actions and to do, or cause to be done, all things necessary, proper or advisable under applicable Laws to consummate the Merger
and the other transactions contemplated by this Agreement. Notwithstanding anything in this Agreement to the contrary, Parent and the
Company each agree to make any filings required by, or desirable under, applicable Antitrust Laws with respect to the Merger as promptly
as reasonably practicable following the date of this Agreement (and Parent may “pull and refile” any such form or filing,
if in its reasonable good faith judgment following consultation with the Company, such step is consistent with expeditiously obtaining
a required approval), and (ii) to respond as promptly as practicable to any request for additional information and documentary material
issued by a Governmental Authority pursuant to any Antitrust Law.
(b) Parent
and the Company shall consult and cooperate with one another, and consider in good faith the views of one another, in connection with,
and provide to the other in advance (to the extent legally permissible), any analyses, presentations, memoranda, briefs, arguments, opinions
and proposals made or submitted by or on behalf of any party hereto in connection with proceedings under or relating to the Antitrust
Laws. Without limiting the foregoing, the parties hereto agree (i) to give each other reasonable advance notice of all meetings
or substantive communications with any Governmental Authority relating to the transactions contemplated hereby under any Antitrust Laws,
(ii) to give each other an opportunity to participate in each of such meetings, (iii) to the extent practicable, to give each
other reasonable advance notice of all substantive oral communications with any Governmental Authority relating to the transactions contemplated
hereby under any Antitrust Laws, (iv) if any Governmental Authority initiates a substantive oral communication regarding the transactions
contemplated hereby under any Antitrust Laws, to promptly notify the other party of the substance of such communication, (v) to
provide each other with a reasonable advance opportunity to review and comment upon all written communications (including any analyses,
presentations, memoranda, briefs, arguments, opinions and proposals) with a Governmental Authority regarding the transactions contemplated
hereby under any Antitrust Laws and (vi) to provide each other with copies of all written communications from any Governmental Authority
relating to the transactions contemplated hereby under any Antitrust Laws. Any such disclosures or provision of copies by one party to
the other may be made on an outside counsel basis if appropriate.
(c) Notwithstanding
anything in this Agreement to the contrary, and subject to the prior good faith cooperation of the other parties and their subsidiaries,
each party shall, and shall cause each of its subsidiaries and Affiliates to, take reasonable actions necessary to obtain any consents,
clearances or approvals required under or in connection with the Antitrust Laws to enable all waiting periods under applicable Antitrust
Laws to expire, and to avoid or eliminate impediments under applicable Antitrust Laws asserted by any Governmental Authority, in each
case, to cause the Merger to occur prior to the Termination Date, including but not limited to promptly complying with or modifying any
requests for additional information by any Governmental Authority; provided, however, that, notwithstanding anything to the contrary
contained in this Agreement, neither party shall be required to sell, divest or otherwise dispose of, hold separate, enter into any license
or similar agreement with respect to, restrict the ownership or operation of, or agree to sell, divest or otherwise dispose of, hold
separate, enter into any license or similar agreement with respect to, or restrict the ownership or operation of, any assets or businesses
of the Company, Parent or any of their respective Affiliates or subsidiaries.
(d) Each
party shall bear its own expenses and costs incurred by such party in connection with any filings and submissions pursuant to Antitrust
Laws, except that Parent and the Company shall each pay one-half of the fees related to any filing made pursuant to Section 6.5(a).
(e) In
the event that any administrative or judicial Action is instituted (or threatened to be instituted) by a Governmental Authority challenging
the Merger, each of Parent, Merger Sub and the Company shall cooperate in all respects with each other and shall use its commercially
reasonable efforts to contest and resist any such action or proceeding and to have vacated, lifted, reversed or overturned any decree,
judgment, injunction or other order, whether temporary, preliminary or permanent, that is in effect and that prohibits, prevents or restricts
consummation of the Merger.
(f) Prior
to the Effective Time, each party shall use commercially reasonable efforts to obtain any consents, approvals or waivers of third parties
with respect to any Contracts to which it is a party as may be necessary for the consummation of the transactions contemplated by this
Agreement or required by the terms of any Contract as a result of the execution, performance or consummation of the transactions contemplated
by this Agreement.
6.6 Notification
of Certain Matters. Unless prohibited by applicable Law, each party shall give prompt notice to the other parties upon receiving
Knowledge of any event, effect, occurrence, fact, circumstance, condition or change that would reasonably be expected to give rise to
a failure of a condition precedent in Section 7; provided, however, that the failure to make any such notification (in and
of itself) shall not be taken into account in determining whether the conditions set forth in Section 7 have been satisfied
or give rise to any right of termination to any party hereto under Section 8.
6.7 Transaction
Litigation. The Company and Parent shall each notify the other party in writing as promptly as practicable after it has notice
of any Actions or governmental investigations or proceedings instituted or threatened against the Company or Parent, as applicable,
or any of their respective directors or officers (in their capacity as such), including by any stockholder of the Company or
Shareholder of Parent, as applicable, before any court or Governmental Authority, relating to this Agreement or the transactions
contemplated hereby (“Transaction Litigation”). Each of Parent and the Company shall have the right to
participate in (but not control) the defense of any such actions, suits, claims, investigations or proceedings, and each of Parent
and the Company shall consult with the other party regarding the defense of any such actions, suits, claims, investigations or
proceedings. Neither Parent nor the Company may settle or compromise any Transaction Litigation without the prior written consent of
the other party (not to be unreasonably withheld, conditioned or delayed).
6.8 Resignations.
Prior to the Effective Time, the Company and Parent shall use commercially reasonable efforts to each cause any director of the Company
or Parent, as applicable, or any of their respective subsidiaries, in each case, other than those directors chosen in accordance with
Section 1.6, to execute and deliver a letter effectuating his or her resignation as a director of such entity effective as of the
Effective Time; provided that, each such resigning director of Parent shall be paid by Parent his or her accrued director fees simultaneously with such
resignation.
6.9 Director
and Officer Liability.
(a) For
not less than six (6) years from and after the Effective Time, to the fullest extent permitted under applicable Law, the Surviving
Corporation shall maintain in effect the provisions of the certificate of incorporation, bylaws or similar governing documents of the
Company and its subsidiaries as in effect immediately prior to the Effective Time which provide for exculpation, indemnification or advancement
of expenses of current or former directors or officers of the Company or any of its subsidiaries and each individual who is serving or
has served at the request or for the benefit of the Company or any of its subsidiaries as a director, officer, employee, agent or fiduciary
of another Person (each Person entitled to indemnification under such governing documents, an “Indemnified Party”)
with respect to any matters existing or occurring at or prior to the Effective Time. For not less than six (6) years from and after
the Effective Time, to the fullest extent permitted under applicable Law, the Surviving Corporation shall cause any such provisions not
to be amended, repealed or otherwise modified in any manner that would adversely affect the rights of any Indemnified Party.
(b) For
not less than six (6) years from and after the Effective Time, each of Parent and the Surviving Corporation shall, to the fullest
extent permitted under applicable Law (including as it may be amended after the date of this Agreement to increase the extent to which
a corporation may provide indemnification), indemnify and hold harmless any Indemnified Party who was or is a party or is threatened
to be made a party to any actual or threatened Action or investigation in respect of acts or omissions occurring at or prior to the Effective
Time by reason of the fact that such Person is or was a director or officer of the Company, or is or was a director or officer of the
Company serving at the request of the Company as a director, officer, employee or agent of, or in a fiduciary capacity with respect
to, another corporation, partnership, joint venture, trust or other enterprise, against any resulting claims, losses, liabilities, damages,
fines, judgments, settlements and reasonable fees and expenses, including reasonable attorneys’ fees and expenses, and other costs,
arising therefrom. To the fullest extent permitted under applicable Law, each of Parent and the Surviving Corporation shall promptly
advance any reasonable expenses as incurred by any such Indemnified Party in connection with any such Action; provided, that any
Person to whom expenses are advanced provides an undertaking to repay such advances if it is ultimately determined by a final, non-appealable
judgment of a court of competent jurisdiction that such Person is not entitled to indemnification. Each of Parent and the Surviving Corporation
shall reasonably cooperate with each Indemnified Party in the defense of any Action.
(c) Prior
to the Effective Time, Parent shall (or shall cause the Surviving Corporation to), in each case following reasonable consultation with
the Company, obtain directors’ and officers’ liability and fiduciary liability insurance coverage providing substantially
similar protection to the Company’s directors and officers as the current insurance carried by the Company. This could include
a go-forward D&O insurance policy with Parent that includes prior acts coverage for such persons, tail policies, or some combination
of same, from current or new insurers, and involving separate or shared limits. The Company and Parent shall cooperate in this effort.
Company shall, at its option, be able to use a broker of its choice to facilitate the insurance placement described herein. Only on the
express written consent of the Company, which may be withheld in its sole discretion, may the insurance placement described herein be
placed for limits less than those currently insuring the Company’s directors and officers.
(d) In
the event that Parent, the Surviving Corporation or any of their respective successors or assigns (i) consolidates with or merges
with or into any other Person and shall not be the continuing or surviving corporation or entity in such consolidation or merger or (ii) transfers
all or substantially all of its properties and assets to any Person, then, and in either such case, proper provision shall be made so
that the successors and assigns of Parent or the Surviving Corporation, as the case may be, shall assume or succeed to all of the obligations
set forth in this Section 6.9.
(e) The
rights of each Indemnified Party under this Section 6.9 shall be in addition to, and not in limitation of, any other rights
any such Indemnified Party may have under the certificate of incorporation or bylaws or other organizational documents of the Company
or any of its subsidiaries or the Surviving Corporation, any other indemnification or other agreement or arrangement, the DGCL or otherwise.
All rights to exculpation, indemnification and advancement of expenses now existing in favor of any Indemnified Party as provided in
the certificate of incorporation, bylaws or other governing documents of the Company and its subsidiaries or in any agreement or in any
agreement to which the Company or any of its subsidiaries is a party shall survive the Merger in full force and effect and be assumed
by the Surviving Corporation and shall not be amended, repealed or otherwise modified in any manner that would adversely affect any right
thereunder of any such Indemnified Party.
(f) The
provisions of this Section 6.9 shall survive the Merger and are expressly intended to be for the benefit of, and shall be
enforceable by, each of the Indemnified Parties, each of whom is a third party beneficiary of this Section 6.9. Parent shall
pay all reasonable out of pocket expenses, including reasonable attorneys’ fees, that may be incurred by any Indemnified Party
in enforcing the indemnity and other obligations provided in this Section 6.9 if it is ultimately determined by a final,
non-appealable judgment of a court of competent jurisdiction that such Indemnified Party is entitled to indemnification hereunder.
6.10 Stock
Exchange De-Listing and Deregistration. Prior to the Effective Time, the Company shall cooperate with Parent and use
commercially reasonable efforts to take, or cause to be taken, all actions, and do or cause to be done all things, reasonably
necessary, proper or advisable on its part under applicable Laws and the rules and policies of OTC Markets Group applicable
with respect to its OTC Pink Market to cause the delisting of the Company Common Stock from the OTC Pink Market as promptly as
practicable after the Effective Time, and in any event no more than two (2) days after the Closing Date, and deregistration of
the Company Common Stock under the Exchange Act as promptly as practicable after such delisting. The Company shall not cause the
Company Common Stock to be delisted from OTC Pink Market prior to the Effective Time. If the Surviving Corporation is required to
file any quarterly or annual report by a filing deadline that is imposed by the Exchange Act and which falls on a date within the
ten (10) days following the Closing Date, the Company shall deliver to Parent at least five (5) Business Days prior to the
Closing a substantially final draft of any such annual or quarterly report reasonably likely to be required to be filed during such
period.
6.11 Stock
Exchange Listing. Parent shall use its commercially reasonable efforts to cause the Parent ADSs to be issued in connection with the
Merger to be approved and such other Parent Ordinary Shares to be reserved for issuance in the Merger to be authorized for listing on
Nasdaq, subject to official notice of issuance, prior to the Effective Time.
6.12 Section 16
Matters. Prior to the Effective Time, the Company shall take all such steps as may be required and permitted to cause any dispositions
of Company Common Stock (including derivative securities with respect to such Company Common Stock) by each director or officer of the
Company to be exempt under Rule 16b-3 promulgated under the Exchange Act.
6.13 Company’s
Auditors. From the date hereof until the Effective Time, the Company shall use its commercially reasonable efforts to cause the Company’s
auditors to complete their audit for the year ending December 31, 2023 as soon as reasonably practicable and, at the reasonable
request of Parent, to perform a review of the consolidated interim financial statements of the Company for any period beginning thereafter.
6.14 Takeover
Law. If any Takeover Law is or may become applicable to the Merger or any of the other transactions contemplated by this Agreement,
each of Parent and the Company and their respective boards of directors shall grant such approvals and take such actions as are necessary
so that such transactions may be consummated as promptly as practicable on the terms contemplated by this Agreement and otherwise act
to eliminate or minimize the effects of such statute or regulation on such transactions.
6.15 Integration
Planning. After the date hereof and prior to the Effective Time, Parent and the Company shall establish a mechanism, subject to applicable
Law, reasonably acceptable to both parties by which the parties shall confer on a regular and continued basis regarding the general status
of the ongoing operations of the Company and its subsidiaries, on the one hand, and Parent and its subsidiaries, on the other hand, and
integration planning matters and communicate and consult with specific persons to be identified by each party to the other with respect
to the foregoing.
6.16 PIPE
Investment. Prior to the Closing, Parent and the Company shall each use their respective commercially reasonable efforts to negotiate
with one or more third parties with respect to the purchase by such third parties of Parent Ordinary Shares and/or Parent ADSs simultaneously
with the Closing (the “PIPE Investment”). In connection with the PIPE Investment, prior to and conditioned on the
occurrence of the Closing, Parent shall enter into one or more subscription agreements in form and substance mutually acceptable in good
faith to Parent and the Company (each, a “Subscription Agreement”) among such third party investors and Parent. The
PIPE Investment shall result in aggregate net proceeds to Parent (net of all transaction expenses incurred by the parties pursuant to
or in connection with the transactions contemplated by this Agreement, including the Merger and the PIPE Investment) of at least $10,000,000
(the “Minimum Amount”) and shall be consummated immediately prior to the Effective Time subject to the condition that
the Closing occurs. Each of the Subscription Agreements, when executed by Parent, shall have been duly authorized, executed and delivered
by Parent, as applicable and constitute the valid and binding obligation of Parent, enforceable against Parent, and, to the Knowledge
of Parent, the other parties thereto, in accordance with its terms, subject to the Bankruptcy and Equity Exceptions. True and complete
original or signed copies of each of the Subscription Agreements shall be delivered to the Company prior to the Effective Time, and there
will have been no conditions to closing of the transactions contemplated therein other than the conditions (if any) specifically stated
therein.
6.17 Redomiciliation.
Parent covenants and agrees that as promptly as practicable following the Closing, Parent shall take all actions reasonably necessary
to seek to cause Parent and its subsidiaries to be redomiciled from the United Kingdom to the United States by way of initiation of a
court-sanctioned scheme of arrangement under the United Kingdom’s Companies Act 2006 or such other means as the Parent Board shall
deem appropriate and advisable in compliance with applicable Law and the applicable listing requirements of Nasdaq (the “Redomiciliation”).
The Redomiciliation shall be subject to obtaining the approval of Parent Shareholders and applicable Governmental Authorities (including
the Courts of England and Wales), including approval by Parent Shareholders at its annual general meeting or, if Parent deems appropriate,
at such shareholder and court meetings which as shall be convened to address in connection with the implementation of the Redomiciliation.
6.18 Determination
of Exchange Ratio.
(a) No
later than five (5) business days prior to the Closing Date (the “Determination Date”), the Company will deliver
to Parent a schedule (the “Company Net Cash Schedule”) setting forth, in reasonable detail, the Company’s good
faith, estimated calculation of (i) Net Cash of the Company and its consolidated subsidiaries as of the close of business on the
last business day prior to the anticipated Closing Date (the “Cash Determination Time”) and (ii) any potential
Company Licensing Deal Revenue, as determined in good faith by the Company, in each case, prepared and certified by the Company’s
Chief Financial Officer. The Company shall make available to Parent, as reasonably requested by Parent, the work papers and back-up materials
used or useful in preparing the Company Net Cash Schedule and the calculation of potential Company Licensing Deal Revenue and, if reasonably
requested by Parent, the Company’s accountants and counsel at reasonable times and upon reasonable notice.
(b) No
later than the Determination Date, Parent will deliver to the Company a schedule (the “Parent Net Cash Schedule”,
and together with the Company Net Cash Schedule, each, a “Net Cash Schedule”) setting forth, in reasonable detail,
Parent’s good faith, estimated calculation of (i) Net Cash of Parent and its consolidated subsidiaries as of the Cash Determination
Time and (ii) any potential Parent Licensing Deal Revenue, prepared and certified by Parent’s Chief Financial Officer. Parent
shall make available to the Company, as reasonably requested by the Company, the work papers and back-up materials used or useful in
preparing the Parent Net Cash Schedule and the calculation of potential Parent Licensing Deal Revenue and, if reasonably requested by
the Company, Parent’s accountants and counsel at reasonable times and upon reasonable notice.
(c) No
later than three (3) days after the date on which Parent or the Company, as applicable, delivers its applicable Net Cash Schedule
to the other party (the last day of such period, the “Response Date”), the other party (in such capacity, the “Reviewing
Party”) shall have the right to dispute any part of the applicable Net Cash Schedule by delivering a written notice (a “Dispute
Notice”) to that effect to the other party (in such capacity, the “Preparing Party”). Any Dispute Notice
shall identify in reasonable detail and to the extent known the nature and amounts of any proposed revisions to the calculations set
forth in the applicable Net Cash Schedule and will be accompanied by reasonably detailed materials supporting the basis for such revisions.
(d) If,
on or prior to the applicable Response Date, the Reviewing Party notifies the Preparing Party in writing that it has no objections to
the applicable Net Cash Schedule or, if on the Response Date, Parent fails to deliver a Dispute Notice as provided in Section 6.18(c) then
the amounts set forth in the applicable Net Cash Schedule shall be deemed to have been finally determined for purposes of this Agreement
and to represent the Preparing Party’s Net Cash at the Cash Determination Time (for purposes of calculating the Exchange Ratio,
which for the avoidance of doubt shall exclude any amounts in respect of potential Company Licensing Deal Revenue or Parent Licensing
Deal Revenue, as applicable) and the amount of potential Parent Licensing Deal Revenue or Company Licensing Deal Revenue, as applicable
(for purposes of calculating the maximum number of Additional Company Merger Shares that may be issued pursuant to Section 2.6).
(e) If
Representatives of the Company and Parent are unable to negotiate an agreed-upon determination of the Preparing Party’s Net Cash
as of the Cash Determination Time pursuant to Section 6.18(d) or the maximum number of Additional Company Merger Shares
that may be issued pursuant to Section 2.6 within three (3) days after delivery of the Dispute Notice (or such other
period as the Company and Parent may mutually agree upon), then any remaining disagreements as to the calculation of the Preparing Party’s
Net Cash or such maximum number of Additional Company Merger Shares that may be issued pursuant to Section 2.6 shall be referred
to an independent auditor of recognized national standing jointly selected by the Company and Parent. If the parties are unable to select
an independent auditor within five (5) days, then either the Company or Parent may thereafter request that the Boston, Massachusetts
Office of the American Arbitration Association (“AAA”) make such selection (either the independent auditor jointly
selected by both parties or such independent auditor selected by the AAA, the “Accounting Firm”). The Company and
Parent shall promptly deliver to the Accounting Firm the work papers and back-up materials used in preparing the Preparing Party’s
Net Cash Schedule and the Dispute Notice, and the Company or Parent shall use commercially reasonable efforts to cause the Accounting
Firm to make its determination within five (5) business days of accepting its selection. The Company and Parent shall be afforded
the opportunity to present to the Accounting Firm any material related to the unresolved disputes and to discuss the issues with the
Accounting Firm; provided, however, that no such presentation or discussion shall occur without the presence of a Representative
of each of the Company and Parent. The determination of the Accounting Firm shall be limited to the disagreements submitted to the Accounting
Firm. The determination of the amount of the Preparing Party’s Net Cash or the maximum number of Additional Company Merger Shares
that may be issued pursuant to Section 2.6 made by the Accounting Firm shall be made in writing delivered to each of the
Company and Parent, shall be final and binding on the Company and Parent and shall (absent manifest error) be deemed to have been finally
determined for purposes of this Agreement and to represent the Preparing Party’s Net Cash at the Cash Determination Time for purposes
of this Agreement and/or the maximum number of Additional Company Merger Shares that may be issued pursuant to Section 2.6.
The parties shall delay the Closing until the resolution of the matters described in this Section 6.18(e). The fees and expenses
of the Accounting Firm shall be allocated between the Company and Parent in the same proportion that the disputed amount of the Preparing
Party’s Net Cash that was unsuccessfully disputed by the Reviewing Party (as finally determined by the Accounting Firm) bears to
the total disputed amount of the Preparing Party’s Net Cash amount. If this Section 6.18(e) applies as to the
determination of the Preparing Party’s Net Cash at the Cash Determination Time described in Section 6.18(a), upon resolution
of the matter in accordance with this Section 6.18(e), the Parties shall not be required to determine the Preparing Party’s
Net Cash or the maximum number of Additional Company Merger Shares again even though the Closing Date may occur later than the original
Determination Date, except that the Reviewing Party may request a redetermination of the Preparing Party’s Net Cash or maximum
number of Additional Company Merger Shares if the Closing Date is more than thirty (30) days after the original Determination Date.
Section 7
CONDITIONS PRECEDENT TO THE OBLIGATION OF PARTIES
TO CONSUMMATE THE MERGER
7.1 Conditions
to Obligations of Each Party to Effect the Merger. The respective obligations of each party to this Agreement to effect the Merger
shall be subject to the satisfaction (or waiver, if permitted by applicable Law) at or prior to the Closing of the following conditions:
(a) Stockholder
Approval. Each of the Company Stockholder Approval and the Parent Shareholder Approval shall have been obtained.
(b) Registration
Statement. The Form S-4 shall have become effective in accordance with the provisions of the Securities Act, and no stop order
suspending the effectiveness of the Form S-4 shall have been issued by the SEC and remain in effect.
(c) Statutes;
Court Orders. No order, injunction, judgment, decree or ruling (whether temporary, preliminary or permanent) enacted, promulgated,
issued or entered by any Governmental Authority of competent authority (collectively, “Restraints”) or Laws shall
be in effect enjoining, restraining, preventing or prohibiting consummation of the Merger or making consummation of the Merger illegal.
(d) Nasdaq
Listing. The Parent ADSs to be issued in the Merger shall have been approved for listing on Nasdaq, subject to official notice of
issuance.
7.2 Additional
Conditions to the Obligations of Parent and Merger Sub. The obligations of Parent and Merger Sub to consummate and effect the Merger
shall be further subject to satisfaction (or waiver, if permitted by applicable Law) at or prior to the Closing of the following additional
conditions:
(a) Representations,
Warranties and Covenants. (i) Each of the representations and warranties of the Company contained in Section 3.1
(Organization, Standing and Corporate Power), Section 3.2 (Corporate Authorization), Section 3.4(a) (No
Conflict), Section 3.25 (Broker and Finder’s Fees) and Section 3.26 (Opinion of the Financial
Advisor) shall be true and correct in all material respects as of the date of this Agreement and as of the Closing Date as if made
as of such date (except for those representations and warranties which address matters as of an earlier date, which shall have been so
true and correct as of such earlier date), (ii) the representations and warranties of the Company contained in Section 3.9(a) (Absence
of Certain Changes) shall be true and correct in all respects as of the date of this Agreement and as of the Closing Date as if made
as of such date (except for those representations and warranties which address matters as of an earlier date, which shall have been so
true and correct as of such earlier date), (iii) the representations and warranties of the Company contained in Section 3.5(a) (Capitalization)
shall be true and correct other than in de minimis respects as of the date of this Agreement and as of the Closing Date as if
made on such date (except for those representations and warranties which address matters as of an earlier date, which shall have been
so true and correct as of such earlier date) and (iv) each of the other representations and warranties of the Company contained
in Section 3 of this Agreement shall be true and correct (without giving effect to any exception or qualification contained
therein relating to materiality or a Company Material Adverse Effect), except where the failure of such other representations and warranties
to be true and correct, individually or in the aggregate, has not had, or would not be reasonably expected to have, a Company Material
Adverse Effect, as of the date of this Agreement and as of the Closing Date, as if made as of such date (except for those representations
and warranties which address matters as of an earlier date, which shall have been so true and correct as of such earlier date).
(b) Performance
of Obligations of the Company. The Company shall have performed in all material respects the covenants and obligations required to
be performed by it under this Agreement at or prior to the Closing.
(c) No
Company Material Adverse Effect. Since the date of this Agreement, there shall not have occurred any effect, event, occurrence, development
or change that has had or would reasonably be expected to have individually or in the aggregate, a Company Material Adverse Effect.
(d) Closing
Certificate. The Company shall have furnished Parent with a certificate dated as of the Closing Date signed on its behalf by its
Chief Executive Officer or Chief Financial Officer to the effect that the conditions set forth in Sections 7.2(a), (b) and
(c) have been satisfied.
(e) PIPE
Investments. The PIPE Investment shall have been consummated simultaneously with, and conditioned only upon, the occurrence of the
Closing, and shall result in net proceeds to Parent of at least the Minimum Amount.
(f) Company
Net Cash. The Net Cash of the Company (as set forth in the Company Net Cash Schedule, as finally determined pursuant to Section 6.18)
(the “Company Net Cash”) shall be equal to or greater than negative $13,500,000.
(g) Additional
Conditions to the Obligations of Parent and Merger Sub. Each of the conditions set forth on Section 7.2(g) of the Company
Disclosure Letter shall have been satisfied.
(h) FIRPTA
Certificate. Parent shall have received from the Company a properly executed certification in accordance Treasury Regulations Sections
1.897-2(h)(1) and 1.1445-2(c), dated not more than thirty (30) days prior to the Closing Date, to the effect that the equity of
the Company does not constitute “United States real property interests” under Section 897(c) of the Code along
with evidence that the Company has complied with any notice requirement pursuant to Treasury Regulations Section 1.897-2(h)(2).
7.3 Additional
Conditions to the Obligations of the Company. The obligations of the Company to consummate and effect the Merger shall be further
subject to satisfaction (or waiver, if permitted by applicable Law) at or prior to the Closing of the following additional conditions:
(a) Representations,
Warranties and Covenants. (i) Each of the representations and warranties of Parent and Merger Sub contained in Section 4.1
(Organization, Standing and Corporate Power), Section 4.2 (Corporate Authorization), Section 4.4(a) (No
Conflict), Section 4.25 (Broker and Finder’s Fees) and Section 4.26 (Opinion of the Financial
Advisor) shall be true and correct in all material respects as of the date of this Agreement and as of the Closing Date as if made
as of such date (except for those representations and warranties which address matters as of an earlier date, which shall have been so
true and correct as of such earlier date), (ii) the representations and warranties of Parent and Merger Sub contained in Section 4.9(a) (Absence
of Certain Changes) shall be true and correct in all respects as of the date of this Agreement and as of the Closing Date as if made
as of such date (except for those representations and warranties which address matters as of an earlier date, which shall have been so
true and correct as of such earlier date), (iii) the representations and warranties of Parent contained in Section 4.5(a) (Capitalization)
shall be true and correct other than in de minimis respects as of the date of this Agreement and as of the Closing Date as if
made on such date (except for those representations and warranties which address matters as of an earlier date, which shall have been
so true and correct as of such earlier date), and (iv) each of the other representations and warranties of Parent and Merger Sub
contained in Section 4 of this Agreement shall be true and correct (without giving effect to any exception or qualification
contained therein relating to materiality or a Parent Material Adverse Effect), except where the failure of such other representations
and warranties to be true and correct, individually or in the aggregate, has not had, or would not be reasonably expected to have, a
Parent Material Adverse Effect, as of the date of this Agreement and as of the Closing Date, as if made as of such date (except for those
representations and warranties which address matters as of an earlier date which shall have been so true and correct as of such earlier
date).
(b) Performance
of Obligations of Parent and Merger Sub. Each of Parent and Merger Sub shall have performed in all material respects the covenants
and obligations required to be performed by it under this Agreement at or prior to the Closing.
(c) No
Parent Material Adverse Effect. Since the date of this Agreement, there shall not have occurred any effect, event, occurrence, development
or change that has had or would reasonably be expected to have, individually or in the aggregate, a Parent Material Adverse Effect.
(d) Closing
Certificate. Parent shall have furnished the Company with a certificate dated as of the Closing Date signed on its behalf by its
Chief Executive Officer or Chief Financial Officer to the effect that the conditions set forth in Sections 7.3(a), (b) and
(c) have been satisfied.
(e) PIPE
Investments. The PIPE Investment shall have been consummated simultaneously with, and conditioned only upon, the occurrence of the
Closing, and shall result in net proceeds to Parent of at least the Minimum Amount.
(f) Parent
Net Cash. The Net Cash of the Parent (as set forth in the Parent Net Cash Schedule, as finally determined pursuant to Section 6.18)
(the “Parent Net Cash”) shall be equal to or greater than negative $13,500,000.
(g) Director
Nominees. The Company director nominees and mutually chosen director nominee shall have been appointed to the Parent Board in accordance
with Section 1.6, effective as of the Closing, and the Resignations contemplated by Section 1.6 shall have been received
by Parent.
7.4 Frustration
of Closing Conditions. No party may rely on the failure of any condition set forth in this Section 7 to be satisfied
if such failure was caused by such party’s failure to act in compliance with the provisions of this Agreement.
Section 8
TERMINATION, AMENDMENT AND WAIVER
8.1 Termination.
This Agreement may be terminated and the transactions contemplated hereby may be abandoned, except as otherwise provided below, at any
time before the Effective Time, whether before or after the Company Stockholder Approval or the Parent Shareholder Approval is obtained,
as follows:
(a) By
mutual written consent of Parent and the Company;
(b) By
Parent:
(i) if
there has been a breach of, or inaccuracy in, any representation, warranty, covenant or agreement of the Company set forth in this Agreement,
which breach or inaccuracy would result in a failure of a condition set forth in Sections 7.2(a) or 7.2(b) to
be satisfied at the Closing (and such breach or inaccuracy has not been cured such that such condition would be capable of satisfaction
at the Closing within thirty (30) days after the receipt of notice thereof or such breach or inaccuracy is not reasonably capable of
being so cured within such thirty (30)-day period); provided, that Parent shall not have the right to terminate this Agreement
pursuant to this Section 8.1(b)(i) if Parent is then in material breach of any representation, warranty, covenant
or obligation hereunder;
(ii) if,
at any time prior to the receipt of the Company Stockholder Approval, the Company Board shall have effected a Company Adverse Recommendation
Change (provided, that any written notice pursuant to Sections 5.3(d) or 5.3(e) of the Company’s
intention to make a Company Adverse Recommendation Change in advance of a Company Adverse Recommendation Change shall not result in Parent
having any termination rights pursuant to this Section 8.1(b)(ii) unless such written notice otherwise constitutes a
Company Adverse Recommendation Change); or
(iii) at
any time prior to obtaining the Parent Shareholder Approval, in order to enter into a definitive agreement providing for a Parent Superior
Proposal in accordance with Section 5.4(d); provided, that Parent (A) shall have complied with all of the terms
and conditions set forth in Section 5.4, (B) shall have paid the Termination Fee to the Company substantially concurrently
with or prior to (and as a condition to) such termination in accordance and (C) substantially concurrently enters into such definitive
agreement with respect to such Parent Superior Proposal; or
(c) By
the Company:
(i) if
there has been a breach of, or inaccuracy in, any representation, warranty, covenant or agreement of Parent or Merger Sub set forth in
this Agreement, which breach or inaccuracy would result in a failure of a condition set forth in Sections 7.3(a) or 7.3(b) to
be satisfied at the Closing (and such breach or inaccuracy has not been cured such that such condition would be capable of satisfaction
at the Closing within thirty (30) days after the receipt of notice thereof or such breach or inaccuracy is not reasonably capable of
being so cured within such thirty (30)-day period); provided, that the Company shall not have the right to terminate this
Agreement pursuant to this Section 8.1(c)(i) if the Company is then in material breach of any representation, warranty,
covenant or obligation hereunder;
(ii) if,
at any time prior to the receipt of the Parent Shareholder Approval, the Parent Board shall have effected a Parent Adverse Recommendation
Change; or
(iii) at
any time prior to obtaining the Company Stockholder Approval, in order to enter into a definitive agreement providing for a Company
Superior Proposal in accordance with Section 5.3(d); provided, that the Company (A) shall have complied with
all of the terms and conditions set forth in Section 5.3, (B) shall have paid the Termination Fee to Parent
substantially concurrently with or prior to (and as a condition to) such termination in accordance and (C) substantially
concurrently enters into such definitive agreement with respect to such Company Superior Proposal; or
(d) By
either Parent or the Company:
(i) if
(A) a Restraint prohibiting the Merger shall be in effect and have become final and non-appealable or (B) the Effective Time
has not occurred by 5:00 p.m. Eastern time on September 4, 2024 (the “Termination Date”), unless extended
by mutual written agreement of Parent and the Company; provided, however, that the right to terminate this Agreement under
this Section 8.1(d) shall not be available to any party if the failure by such party to perform any of its obligations
under this Agreement has been the principal cause of the failure of any condition set forth in this Section 8.1(d) to
be satisfied;
(ii) if
the Company Stockholders Meeting (as it may be adjourned or postponed in accordance with this Agreement) shall have concluded and the
Company Stockholder Approval shall not have been obtained at such meeting; provided, however, that the right to terminate
this Agreement under this Section 8.1(d)(ii) shall not be available to the Company if the failure by the Company to
perform any of its obligations under this Agreement has been the principal cause of the failure to obtain the Company Stockholder Approval
and such action or failure to act constitutes a breach of this Agreement by such party; or
(iii) if
the Parent Shareholders Meeting (as it may be adjourned or postponed in accordance with this Agreement) shall have concluded and the
Parent Shareholder Approval shall not have been obtained at such meeting; provided, however, that the right to terminate this Agreement
under this Section 8.1(d)(iii) shall not be available to Parent if the failure by Parent or Merger Sub to perform any
of its obligations under this Agreement has been the principal cause of the failure to obtain the Parent Shareholder Approval and such
action or failure to act constitutes a breach of this Agreement by such party.
8.2 Effect
of Termination; Termination Fee.
(a) Effect
of Termination. In the event of termination of this Agreement as provided in Section 8.1 hereof, this Agreement shall
forthwith become null and void and be of no further force or effect, and there shall be no liability on the part of Parent, Merger Sub
or the Company (or any of their respective directors, officers, employees, stockholders, agents or representatives), except as set forth
in the last sentence of Section 6.3, Section 8 and Section 9, each of which shall remain in full
force and effect and survive any termination of this Agreement; provided, however, that nothing herein shall relieve any
party from liability for fraud with respect to any of its representations and warranties set forth herein or Intentional Breach of this
Agreement.
(b) Company
Termination Fee; Expense Reimbursement.
(i) The
Company shall deliver to Parent the sum of the Termination Fee plus the Parent Expense Reimbursement:
(A) as
promptly as possible (but in any event within two (2) Business Days) after the valid termination of this Agreement in accordance
with Section 8.1 if (x) Parent shall have terminated this Agreement pursuant to Section 8.1(b)(ii) (Company
Adverse Recommendation Change) or (y) the Company shall have terminated this Agreement pursuant to Section 8.1(c)(iii) (Company
Superior Proposal); or
(B) upon
consummation of such Company Acquisition Proposal if (x) this Agreement is terminated pursuant to Section 8.1(d)(i)(B) (Termination
Date), Section 8.1(b)(i) (Company Breach) or Section 8.1(d)(ii) (Company Stockholder
Approval), (y) prior to the time of termination and after the date of this Agreement, a Company Acquisition Proposal shall have
been publicly announced or made to the Company Board and not withdrawn and (z) within twelve (12) months after the date on which
this Agreement shall have been terminated the Company enters into a definitive agreement providing for a Company Acquisition Proposal
(which such Company Acquisition Proposal is later consummated) or a Company Acquisition Proposal is consummated.
(ii) All
amounts due hereunder shall be payable by wire transfer in immediately available funds to such account as Parent may designate in writing
to the Company. If the Company fails to promptly deliver any amounts required under this Section 8.2(b) and Parent commences
a suit to collect such amounts, the Company shall indemnify Parent for its fees and expenses (including attorneys’ fees and expenses)
incurred in connection with such suit and shall pay interest on the amount required to have been delivered at the prime rate in the Wall
Street Journal in effect on the date the amount was deliverable pursuant to this Section 8.2(b). The delivery by the Company
of the Termination Fee to Parent pursuant to this Section 8.2(b), including, if applicable, any fees and expenses incurred
as a result of the Company’s failure to timely deliver, if paid, shall be the sole and exclusive remedy of Parent in the event
of termination of this Agreement under circumstances requiring the delivery of the Termination Fee pursuant to this Section 8.2(b).
(c) Parent
Termination Fee; Expense Reimbursement.
(i) Parent
shall deliver to the Company the sum of the Termination Fee plus the Company Expense Reimbursement:
(A) As
promptly as possible (but in any event within two (2) Business Days) after the valid termination of this Agreement in accordance
with Section 8.1 if (x) the Company shall have terminated this Agreement pursuant to Section 8.1(c)(ii) (Parent
Adverse Recommendation Change) or (y) Parent shall have terminated this Agreement pursuant to Section 8.1(b)(iii) (Parent
Superior Proposal); or
(B) upon
consummation of such Parent Acquisition Proposal, if (x) this Agreement is terminated pursuant to Section 8.1(d)(i)(B) (Termination
Date), Section 8.1(c)(i) (Parent Breach) or Section 8.1(d)(iii) (Parent Shareholder
Approval), (y) prior to the time of termination and after the date of this Agreement, a Parent Acquisition Proposal shall have
been publicly announced or made to the Parent Board and not withdrawn and (z) within twelve (12) months after the date on which
this Agreement shall have been terminated Parent enters into a definitive agreement providing for a Parent Acquisition Proposal
(which such Parent Acquisition Proposal is later consummated) or a Parent Acquisition Proposal is consummated.
(ii) All
amounts due hereunder shall be payable by wire transfer in immediately available funds to such account or accounts as the Company may
designate in writing to Parent. If Parent fails to promptly deliver any amounts required under this Section 8.2(c) and
the Company commences a suit to collect such amounts, Parent shall indemnify the Company for its fees and expenses (including attorneys’
fees and expenses) incurred in connection with such suit and shall pay interest on the amount required to have been delivered at the
prime rate in the Wall Street Journal in effect on the date the amount was deliverable pursuant to this Section 8.2(c). The
payment by Parent of the Termination Fee to the Company pursuant to this Section 8.2(c), including, if applicable, any fees
and expenses incurred as a result of Parent’s failure to timely deliver, if paid, shall be the sole and exclusive remedy of the
Company in the event of termination of this Agreement under circumstances requiring the delivery of the Termination Fee pursuant to this
Section 8.2(c).
(d) The
parties acknowledge that the agreements contained in this Section 8.2 are an integral part of the transactions contemplated
hereby, that without these agreements the parties would not enter into this Agreement and that any amounts payable pursuant to this Section 8.2
do not constitute a penalty.
8.3 Fees
and Expenses. Subject to Section 6.1(b) and except as otherwise set forth in this Agreement, all costs and expenses
incurred in connection with this Agreement and the transactions contemplated hereby shall be paid by the party incurring such expenses.
8.4 Notice
of Termination. The party desiring to terminate this Agreement pursuant to Section 8.1 (other than under Section 8.1(a) (Mutual
Consent)) shall give written notice of such termination to the other party or parties specifying the provision or provisions of Section 8.1
pursuant to which such termination is purportedly effected.
8.5 Amendment.
Subject to applicable Law and as otherwise provided in this Agreement, this Agreement may be amended, modified and supplemented in any
and all respects, whether before or after any vote of the stockholders of the Company or the Parent Shareholders contemplated hereby,
only by written agreement of the parties hereto, but after the Company Stockholder Approval or the Parent Shareholder Approval, as applicable,
no amendment shall be made which by Law requires further approval by such stockholders without obtaining such further approval.
8.6 Waiver.
At any time prior to the Effective Time, each party hereto may (a) extend the time for the performance of any of the obligations
or other acts of any other party hereto or (b) waive compliance with any of the agreements of any other party or any conditions
to its own obligations, in each case only to the extent such obligations, agreements and conditions are intended for its benefit; provided,
that any such extension or waiver shall be binding upon a party only if such extension or waiver is set forth in a writing executed by
such party.
Section 9
MISCELLANEOUS
9.1 No
Survival. None of the representations, warranties, covenants or agreements in this Agreement or any instrument delivered pursuant
to this Agreement shall survive the Effective Time, other than those covenants or agreements of the parties which by their terms apply,
or are to be performed in whole or in part, after the Effective Time.
9.2 Notices.
Any notice or other communication required or permitted hereunder shall be in writing and shall be deemed given when delivered in Person,
by overnight courier or upon email transmission (provided, that no “bounce back” or similar message of non-delivery
is received with respect thereto), or two (2) Business Days after being sent by registered or certified mail (postage prepaid, return
receipt requested):
(a) if
to Parent or Merger Sub, to:
c/o Akari Therapeutics, Plc
22 Boston Wharf Road FL 7
Boston, MA 02210
Attn: Rachelle Jacques
Telephone: +1 929.274.7510
Email: rachelle.jacques@akaritx.com
with a copy to:
Goodwin Procter LLP
One Commerce Square
2005 Market Street, 32nd Floor
Philadelphia, PA 19103
Attention: Rachael Bushey, Jennifer Porter and Laura Gulick
Email: RBushey@goodwinlaw.com, JPorter@goodwinlaw.com and
LGulick@goodwinlaw.com
(b) if
to the Company, to:
Peak
Bio, Inc.
4900 Hopyard Road, Suite 100
Pleasanton, CA 94588
Attn: Stephen LaMond
Telephone: +1 650.477.4043
Email: steve.lamond@peak-bio.com
with a copy to:
DLA Piper LLP (US)
51 John F. Kennedy Parkway, Suite 120
Short Hills, NJ 07078
Attention: Andrew P. Gilbert and Scott A. Cowan
Email: andrew.gilbert@us.dlapiper.com; scott.cowan@us.dlapiper.com
and
DLA
Piper LLP (US)
303 Colorado Street, Suite 3000
Austin, TX 78701
Attention:
Jeffrey Scharfstein
Email: jeffrey.scharstein@us.dlapiper.com
Any party may by notice given in accordance with
this Section 9.2 to the other parties designate another address or Person for receipt of notices hereunder.
9.3 Entire
Agreement. This Agreement (including the Company Disclosure Letter, the Parent Disclosure Letter, Annexes and Exhibits hereto and
the documents and instruments referenced herein) contain the entire agreement among the parties with respect to the Merger and related
transactions, and supersede all prior agreements, written or oral, among the parties with respect thereto, other than the Confidentiality
Agreement, which shall survive and remain in full force and effect (other than the “standstill” provisions which shall expire
concurrently with the execution and delivery of this Agreement).
9.4 Governing
Law. This Agreement and all actions arising under or in connection therewith shall be governed by and construed in accordance with
the Laws of the State of Delaware, regardless of the Laws that might otherwise govern under applicable principles of conflicts of law
thereof.
9.5 Binding
Effect; No Assignment; No Third-Party Beneficiaries.
(a) This
Agreement shall not be assigned by any of the parties hereto (whether by operation of law or otherwise) without the prior written consent
of the other parties, except that (i) Merger Sub may assign, in its sole discretion and without the consent of any other party,
any or all of its rights, interests and obligations hereunder to Parent, and (ii) each of Parent and Merger Sub may assign, in their
respective sole discretion and without the consent of any other party, any or all of its rights, interests and obligations hereunder
to one or more direct or indirect wholly-owned subsidiaries of Parent (each, together with Parent, an “Assignee”);
provided that no such assignment shall release Parent or Merger Sub of its obligations hereunder. Any such Assignee may thereafter assign,
in its sole discretion and without the consent of any other party, any or all of its rights, interests and obligations hereunder to one
or more additional Assignees. Subject to the preceding sentence, but without relieving any party hereto of any obligation hereunder,
this Agreement shall be binding upon, inure to the benefit of and be enforceable by the parties and their respective successors and assigns.
(b) Other
than (i) Section 6.9 and (ii) from and after the Effective Time, the rights of holders of shares of Company Common
Stock to receive the Per Share Merger Consideration (and, if applicable, any Additional Per Share Merger Consideration pursuant to Section 2.6),
the holders of Company Options and Company Warrants to receive Adjusted Options and Adjusted Warrants, respectively, and other applicable
payments pursuant to Section 2 (which shall be enforceable by such Persons), nothing in this Agreement, express or implied,
is intended to or shall confer upon any Person other than Parent, Merger Sub and the Company and their respective successors and permitted
assigns any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
9.6 Counterparts
and Signature. This Agreement may be executed in two (2) or more counterparts (including by an electronic signature, electronic
scan or electronic transmission in portable document format (.pdf) including (but not limited to) DocuSign, delivered by electronic mail),
each of which shall be deemed an original but all of which together shall be considered one and the same agreement and shall become effective
when counterparts have been signed by each of the parties hereto and delivered to the other parties hereto, it being understood that
all parties hereto need not sign the same counterpart.
9.7 Severability.
If any provision of this Agreement is held invalid or unenforceable by any court of competent jurisdiction, the other provisions of this
Agreement shall remain in full force and effect. Any provision of this Agreement held invalid or unenforceable only in part or degree
shall remain in full force and effect to the extent not held invalid or unenforceable. The parties further agree to replace such invalid
or unenforceable provision of this Agreement with a valid and enforceable provision that shall achieve, to the extent possible, the economic,
business and other purposes of such invalid or unenforceable provision.
9.8 Submission
to Jurisdiction; Waiver. Each of the Company, Parent and Merger Sub irrevocably agrees that any legal action or proceeding with respect
to this Agreement or the transactions contemplated hereby or for recognition and enforcement of any judgment in respect hereof brought
by any other party hereto or its successors or assigns shall be brought and determined exclusively in the Delaware Court of Chancery
and any state appellate court therefrom within the State of Delaware (or, if and only if the Delaware Court of Chancery declines to accept
jurisdiction over a particular matter, any state or federal court within the State of Delaware) and each of the Company, Parent and Merger
Sub hereby irrevocably submits with regard to any action or proceeding for itself and in respect to its property, generally and unconditionally,
to the exclusive jurisdiction of the aforesaid courts and agrees that it shall not bring any action relating to this Agreement or the
transactions contemplated hereby in any court other than the aforesaid courts. Each of the Company, Parent and Merger Sub hereby irrevocably
waives, and agrees not to assert, by way of motion, as a defense, counterclaim or otherwise, in any action or proceeding with respect
to this Agreement, (a) any claim that it is not personally subject to the jurisdiction of the above-named courts for any reason
other than the failure to lawfully serve process, (b) that it or its property is exempt or immune from jurisdiction of any such
court or from any legal process commenced in such courts (whether through service of notice, attachment prior to judgment, attachment
in aid of execution of judgment, execution of judgment or otherwise), and (c) to the fullest extent permitted by applicable Law,
that (i) the suit, action or proceeding in any such court is brought in an inconvenient forum, (ii) the venue of such suit,
action or proceeding is improper or (iii) this Agreement, or the subject matter hereof, may not be enforced in or by such courts.
Each party agrees that notice or the service of process in any action or proceeding arising out of or relating to this Agreement or the
transactions contemplated hereof shall be properly served or delivered if delivered in the manner contemplated by Section 9.2
or in any other manner permitted by applicable Law.
9.9 Enforcement.
The parties recognize and agree that if for any reason any of the provisions of this Agreement are not performed in accordance with their
specific terms or are otherwise breached, immediate and irreparable harm or injury would be caused for which money damages would not
be an adequate remedy. Accordingly, each party agrees that, in addition to other remedies, any other party shall be entitled to an injunction
or injunctions to prevent breaches or restraining any violation or threatened violation of the provisions of this Agreement. In the event
that any action shall be brought in equity to enforce specifically the terms and provisions of this Agreement in the Delaware courts
and, in action for specific performance, no party shall allege, and each party hereby waives the defense, that there is an adequate remedy
at Law and waives any requirement for the securing or posting of any bond in connection with such remedy, this being in addition to any
other remedy to which they are entitled at law or in equity (subject to the limitations set forth in this Agreement).
9.10 No
Waiver; Remedies Cumulative. No failure or delay on the part of any party hereto in the exercise of any right hereunder shall impair
such right or be construed to be a waiver of, or acquiescence in, any breach of any representation, warranty or agreement herein, nor
shall any single or partial exercise of any such right preclude other or further exercise thereof or of any other right. All rights and
remedies existing under this Agreement are cumulative to, and not exclusive to, and not exclusive of, any rights or remedies otherwise
available.
9.11 Waiver
of Jury Trial. EACH OF PARENT, THE COMPANY AND MERGER SUB HEREBY IRREVOCABLY WAIVES THE RIGHT TO A TRIAL BY JURY IN ANY ACTION, PROCEEDING
OR COUNTERCLAIM (WHETHER BASED IN CONTRACT, TORT OR OTHERWISE) ARISING OUT OF, UNDER OR IN CONNECTION WITH THIS AGREEMENT OR ANY RELATED
DOCUMENT, OR ANY COURSE OF CONDUCT, COURSE OF DEALINGS, STATEMENT OR ACTION RELATED HERETO OR THERETO. Each party to this Agreement certifies
and acknowledges that (a) no Representative of any other party has represented, expressly or otherwise, that such other party would
not seek to enforce the foregoing waiver in the event of a legal action, (b) such party has considered the implications of this
waiver, (c) such party makes this waiver voluntarily, and (d) such party has been induced to enter into this Agreement by,
among other things, the mutual waivers and certifications in this Section 9.11.
Section 10
DEFINITIONS
10.1 Certain
Definitions. As used herein, the following terms have the following meanings:
“Acceptable Confidentiality
Agreement” means any agreement with the Company or Parent, as applicable, that is either (a) in effect as of the execution
and delivery of this Agreement or (b) executed, delivered and effective after the execution and delivery of this Agreement, in either
case containing provisions that require any counterparty thereto (and any of its Affiliates and Representatives) that receive material
non-public information of, or with respect to, the Company or Parent, as applicable, to keep such information confidential; provided,
however, that, in the case of clause (b), (i) the provisions contained therein are not materially less favorable in the aggregate
to the Company or Parent, as applicable, than the terms of the Confidentiality Agreement (it being agreed that such agreement need not
contain any “standstill” or similar provisions that prohibit the making of any Company Acquisition Proposal or Parent Acquisition
Proposal, as applicable) and (ii) such agreement does not contain any provision that prohibits the Company or Parent, as applicable,
from satisfying its obligations hereunder.
“Additional Company
Merger Shares” means a number of Parent Ordinary Shares, if any, equal to the positive difference between (a) the number
of Company Merger Shares resulting from a recalculation of the Company Adjustment Amount to include the amount of any Company Licensing
Deal Revenue (with respect to the calculation of Company Net Cash) and/or Parent Licensing Deal Revenue (with respect to the calculation
of Parent Net Cash) that is actually received in cash by Parent or the Surviving Corporation within 120 days following the Closing Date
and (b) the number of Company Merger Shares used for purposes of calculating the Exchange Ratio at the Closing. For the avoidance
of doubt, if the number of Company Merger Shares resulting from the calculation described in clause (a) of this definition is less
than the number of Company Merger Shares described in clause (b) of this definition, the number of Additional Company Merger Shares
shall be equal to zero (0).
“Affiliate”
means, with respect to any Person, any other Person, directly or indirectly, controlling, controlled by, or under common control with,
such Person. For purposes of this definition, the term “control” (including the correlative terms “controlling,”
“controlled by” and “under common control with”) means the possession, directly or indirectly, of the power to
direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by Contract
or otherwise.
“Antitrust Laws”
means the HSR Act or any other applicable U.S. or foreign competition, antitrust, merger control or investment Laws.
“Business Day”
means any day other than Saturday or Sunday or a day on which commercial banks are authorized or required by Law to be closed in New
York, New York.
“Company Acquisition
Proposal” means any proposal or offer, whether or not in writing, from any Person, Persons or group (other than Parent, Merger
Sub or any of their respective Affiliates) relating to any transaction or series of related transactions involving (a) any direct
or indirect acquisition or purchase from the Company or its subsidiaries of (i) 20% or more (based on the fair market value thereof,
as determined by the Company Board (or any committee thereof) in good faith) of assets (including capital stock of the Company’s
subsidiaries), or by means of any merger, consolidation, business combination, recapitalization, liquidation, dissolution, binding share
exchange or similar transaction to which the Company or its subsidiaries is a party, of the Company and its subsidiaries, taken as a
whole or (ii) 20% or more of the outstanding shares of Company Common Stock, (b) any tender offer or exchange offer that, if
consummated, would result in any Person, Persons or group owning, directly or indirectly, 20% or more of the outstanding shares of Company
Common Stock or (c) any merger, consolidation, business combination, recapitalization, liquidation, dissolution, binding share exchange
or similar transaction to which the Company or its subsidiaries is a party pursuant to which (i) any Person, Persons or group (or
the stockholders of any such Person(s)) would own, directly or indirectly, 20% or more of the voting securities of the Company or of
the surviving entity in a merger involving the Company or the resulting direct or indirect parent of the Company or such surviving entity,
or (ii) the owners of outstanding shares of Company Common Stock immediately prior to such transaction would own less than 80% of
the voting securities of the Company or of the surviving entity in a merger involving the Company or the resulting direct or indirect
parent of the Company or such surviving entity, other than, in each case, the Merger and the PIPE Investments; provided, for the avoidance
of doubt, a Company Licensing Deal shall not constitute a Company Acquisition Proposal.
“Company Charter”
means the Second Amended and Restated Certificate Incorporation of the Company, as amended on or prior to the date hereof.
“Company Equity Plan”
means the Company’s 2022 Long-Term Incentive Plan, as amended from time to time.
“Company Expense Reimbursement”
means the aggregate amount of all reasonable, documented, out-of-pocket legal fees and expenses incurred or paid by or on behalf of the
Company and its Affiliates in connection with the transactions contemplated by this Agreement or related to the authorization, preparation,
negotiation, execution and performance of this Agreement and the termination thereof, provided that, in no event shall the “Company
Expense Reimbursement” exceed $1,500,000.
“Company Foreign Plan”
means (i) any Company Plan that is maintained, sponsored or contributed (or required to be contributed) to primarily for the benefit
of any current or former employee, officer, director or other service provider of the Company or any of its subsidiaries or with respect
to which the Company or any of its subsidiaries has or could have any liability, contingent or otherwise, who are or were providing services
outside the United States and (ii) any plan that would be a Company Plan except for the fact that it is subject to any Law other
than U.S. federal, state or local Law.
“Company Intervening
Event” means a material event or circumstance not known to the Company Board on the date of this Agreement, which event or
circumstance becomes known to the Company Board prior to the Effective Time; provided, however, that in no event shall
the following constitute a Company Intervening Event: (a) a Company Acquisition Proposal, (b) any material event or circumstance
that was known or reasonably foreseeable to the Company Board as of the date hereof (or if known or reasonably foreseeable, the consequences
of which were not reasonably foreseeable) or (c) changes in the Company Common Stock price, in and of itself.
“Company Licensing
Deal” means any acquisition or license (other than any non-exclusive and non-material license granted by the Company in the
ordinary course of business consistent with past practice) of, or joint venture, partnership, revenue or profit-sharing arrangement,
collaboration or other similar transaction with respect to any Company product or asset, including, but not limited to, any product or
asset (a) in the antibody-drug conjugate (ADC) platform or (b) related to PHP-303.
“Company Licensing
Deal Revenue” means the amount of any upfront cash payment proposed to be paid to the Company in respect of a Company Licensing
Deal pursuant to a bona fide term sheet entered into between the Company and an unaffiliated third party, negotiated on arms’
length terms and without assigning value to any assets or product lines of Parent, which term sheet remains in effect as of the Closing
Date and which is reasonably likely to be paid within 120 days following the Closing Date.
“Company Material
Adverse Effect” means any effect, event, occurrence, development or change that has a material adverse effect on the
financial condition, assets, liabilities, business or results of operations of the Company and its subsidiaries, taken as a whole; provided, however,
that a Company Material Adverse Effect shall not be deemed to include effects, events, occurrences, developments or changes arising
out of, relating to or resulting from: (A) changes or prospective changes generally affecting the economy, financial or
securities markets or political, legislative or regulatory conditions, except and only to the extent such changes adversely affect
the Company in a disproportionate manner relative to other participants in the Company’s industry; (B) changes or
prospective changes in the Company’s industry, except and only to the extent such changes adversely affect the Company in a
disproportionate manner relative to other participants in the Company’s industry; (C) any change or prospective change in
Law or the interpretation thereof, except and only to the extent such changes adversely affect the Company in a disproportionate
manner relative to other participants in the Company’s industry; (D) any change or prospective change in applicable
accounting regulations or principles, including GAAP, or the interpretation thereof; (E) acts of war, armed hostility,
terrorism, volcanic eruptions, tsunamis, pandemics, earthquakes, floods, storms, hurricanes, tornadoes or other natural disasters,
except and only to the extent such acts adversely affect the Company in a disproportionate manner relative to other participants in
the Company’s industry; (F) the public announcement by Parent of its proposal to acquire the Company or the execution and
delivery of this Agreement (except to the extent such effect, event, occurrence, development or change was the result of a breach of Section 3.4)
or the announcement of the Merger, including the impact thereof on contractual or other relationships with suppliers, distributors,
partners, employees, lenders, investors or Governmental Authorities, or any Transaction Litigation; (G) any failure by the
Company to meet any internal or published industry analyst projections or forecasts or estimates of revenues or earnings (it being
understood and agreed that the facts and circumstances giving rise to such failure may be deemed to constitute, and may be taken
into account in determining whether there has been, a Company Material Adverse Effect); (H) any change or prospective change in
the price or trading volume of the Company Common Stock on the OTC Pink Market (it being understood and agreed that the facts and
circumstances giving rise to such change may be deemed to constitute, and may be taken into account in determining whether there has
been, a Company Material Adverse Effect); (I) actions or omissions required by this Agreement, or the failure to take any
action prohibited by this Agreement; (J) changes or prospective changes in the Company’s credit ratings (it being
understood and agreed that the facts and circumstances giving rise to such change may be deemed to constitute, and may be taken into
account in determining whether there has been, a Company Material Adverse Effect); (K) (i) any delay in obtaining or
making, or failure to obtain or make, any regulatory approval, clearance or application with respect to any of the Company’s
Products or (ii) any results, outcomes or data, adverse events, side effects or safety observations arising from, or any delay
in the timing or conduct of, any nonclinical, preclinical or clinical studies, trials or tests related to any of the Company’s
Products or (L) changes or prospective changes in interest rates or foreign exchange rates.
“Company Permitted
Liens” means any (i) statutory Liens for Taxes, business improvement district charges, water and sewer charges, assessments
and other lienable services and other governmental charges and impositions not yet due or payable or that are being contested in good
faith through appropriate proceedings, and in each case, for which adequate reserves have been established, in accordance with GAAP,
on the consolidated financial statements included in the most recent Company SEC Documents, (ii) statutory Liens arising out of
operation of Law, including carriers’, warehousemen’s, mechanics’, materialmen’s, repairmen’s or other
similar Liens incurred in the ordinary course of business, (iii) pledges or deposits in connection with workers’ compensation,
unemployment insurance and other social security legislation, (iv) with respect to Company Leased Real Property, (1) all matters,
whether or not of record, that arise out of the actions of Parent or its agents, representatives or contractors, (2) all easements,
covenants, rights-of-way, restrictions and other encumbrances affecting any Company Leased Real Property, (3) all Liens and other
matters disclosed, or in any title commitment, report, listing or policy, or in any survey or survey update relating to the Company Leased
Real Property, in each case to the extent publicly available or made available by the Company to Parent (including those relating to
physical condition or variations in location or dimension), and (4) any and all Laws affecting the Company Leased Real Property
(including any Laws relating to zoning, building and the use, occupancy, subdivision or improvement of the Company Leased Real Property);
provided that such matters described in clauses (1) through (4) do not prohibit or materially impair the current use and operation
of the Company Leased Real Property subject thereto in the business of the Company, (v) statutory landlords’ Liens and Liens
granted to landlords under any lease or sublease, (vi) licenses, options or other covenants of, or other contractual obligations
with respect to, any Intellectual Property incurred in the ordinary course of business (vii) any Liens created pursuant to or in
connection with this Agreement or disclosed in the Company Disclosure Letter, (viii) Liens approved in writing by Parent and (ix) Liens
that, individually or in the aggregate, do not materially impair the current use and operation of the assets to which they relate.
“Company Plan”
means each Employee Benefit Plan that is sponsored, maintained, or contributed (or required to be contributed) to by the Company or any
of its subsidiaries for the benefit of one or more current or former employees, officers, directors or other service providers of the
Company or any of its subsidiaries and with respect to which the Company or any of its subsidiaries has any liability, contingent or
otherwise.
“Company Superior
Proposal” means (i) a Company Acquisition Proposal (except that all percentages in the definition of Company Acquisition
Proposal shall be deemed to be 50%) made by any Person on terms that the Company Board (or any committee thereof) determines in good
faith, after consultation with the Company’s outside financial advisors and outside legal counsel, and considering such factors
as the Company Board (or any committee thereof) considers to be appropriate (including conditionality, timing, likelihood of consummation
of such proposal and consideration per share), that is reasonably likely to be consummated in accordance with its terms, and, if consummated,
would result in a transaction that is more favorable to stockholders of the Company than the Merger (including taking into account any
applicable Termination Fee of the Company) or (ii) a Company Licensing Deal if and only if the Company has complied with Section 5.3
(ignoring clause (B) of the last sentence of Section 5.3(a)) with respect to such Company Licensing Deal as if a
Company Licensing Deal were included within the definition of Company Acquisition Proposal.
“Company Warrant”
means each warrant to purchase capital stock of the Company.
“Confidentiality Agreement”
means the Confidentiality Agreement, dated June 7, 2023 (as it may be amended from time to time), between Parent and the Company.
“Contract”
means, with respect to any Person, any of the agreements, contracts, leases (whether for real or personal property), notes, bonds, mortgages,
indentures, deeds of trust, loans, evidences of Indebtedness, letters of credit, settlement agreements, franchise agreements, undertakings,
employment agreements, license agreements or instruments to which such Person or its subsidiaries is a party, whether oral or written.
“Copyrights”
means works of authorship (whether or not copyrightable, including all Software, whether in source code or object code format) and all
copyrights (whether or not registered), including all registrations thereof and applications therefor, and all renewals, extensions,
restorations and reversions of the foregoing.
“Deposit Agreement”
means the Deposit Agreement, dated as of December 7, 2012, among Parent (as successor-in-interest to Celsus Therapeutics plc), Deutsche
Bank Trust Company Americas, as depositary, and all holders from time to time of Parent ADSs, as amended.
“Employee Benefit
Plan” means any (A) employee benefit plan within the meaning of Section 3(3) of ERISA, whether or not subject
to ERISA; (B) stock option plan, stock purchase plan, equity-based plan, retention plan, profit sharing plan, bonus or incentive
plan, program, agreement or arrangement, deferred compensation arrangement or agreement, severance pay plan, program or agreement, compensation
plan, program, agreement or arrangement, change in control plan, program, agreement or arrangement, supplemental income arrangement,
vacation plan, and any other employee benefit plan, agreement or arrangement, not described in (A) above; and (C) plan or arrangement
providing compensation to employee and non-employee directors.
“Environmental Claim”
means any and all written complaints, summons, citations, directives, orders, decrees, claims, Liens, litigation, investigations, notices
of violation, judgments, administrative, regulatory or judicial actions, suits, demands or proceedings, or notices of noncompliance or
violation by any Governmental Authority or Person involving or alleging potential liability of a party to this Agreement or one of its
subsidiaries arising out of or resulting from any violation of any Environmental Law or the presence or Release of Hazardous Material
at, from, or otherwise relating to: (i) any of the Company’s or its subsidiaries’ facilities or any other properties
or facilities currently or formerly owned, leased, operated or otherwise used by Company or any of its subsidiary; (ii) nearby properties
or businesses; or (iii) any facilities that received Hazardous Material generated by the Company or any of its subsidiaries.
“Environmental Laws”
means all applicable federal, state, local or foreign Laws, statutes, regulations, ordinances, decrees, directives, judgments, common
law, or other enforceable requirements of Governmental Authorities, relating to pollution or protection of human health and safety (including
workplace health and safety) or the environment, including, without limitation, Laws relating to Releases or threatened Release of Hazardous
Materials, the protection of human health as a result of exposure to Hazardous Materials, the storage, transport or disposal of solid
and hazardous waste, discharges of substances to surface water or groundwater, air emissions, recordkeeping, notification, disclosure
and reporting requirements respecting Hazardous Materials, and all Laws relating to endangered or threatened species of fish, wildlife
and plants and the management or use of natural resources.
“Environmental Liability”
means all liabilities, monetary obligations, losses, damages of any kind including without limitation punitive damages, consequential
damages, treble damages, and natural resource damages, costs and expenses (including all fees, disbursements and expenses of counsel,
experts and consultants, costs of investigations and feasibility studies, compliance costs, abatement and cleanup costs), fines, penalties,
sanctions and interest incurred as a result of any claim or demand by any Governmental Authority or any third party or requirement of
Environmental Law, and which relate to any environmental condition, violation or alleged violation of Environmental Laws or Releases
of Hazardous Materials at, from, or otherwise relating to (i) any of the Company’s or its subsidiaries’ facilities or
any other properties or facilities currently or formerly owned, leased, operated or otherwise used by Company, any of its subsidiaries
or the Company’s current business; (ii) nearby properties or businesses; or (iii) any facilities that received Hazardous
Material generated by the Company or any of its subsidiaries.
“Environmental Permits”
means any permit, registration, license or other authorization required or issued under any applicable Environmental Law.
“Equity Interest”
means any share, capital stock, partnership, limited liability company, membership, member, joint venture or similar interest, and any
option, restricted stock, restricted stock unit, phantom equity interest, stock appreciation right, warrant, right or security (including
debt securities) convertible, exchangeable or exercisable thereto or therefor.
“ERISA”
means the Employee Retirement Income Security Act of 1974, as amended.
“ERISA Affiliate”
of any entity means any entity, trade or business that is, or at any applicable time was, a member of a group described in Section 414(b),
(c), (m) or (o) of the Code or Section 4001(b)(1) of ERISA that includes such entity.
“Exchange Act”
means the Securities Exchange Act of 1934, as amended.
“Exchange Ratio”
means, subject to Section 2.1(d), the following ratio (rounded to four decimal places): the quotient obtained by dividing
(a) the Company Merger Shares by (b) the Company Outstanding Shares, in which:
| ● | “Company
Adjustment Amount” means the sum of (i) 0.50 plus (ii) the
Company Adjustment Factor minus (iii) the Parent Adjustment Factor. |
| ● | “Company
Adjustment Factor” means: |
| (i) | if Company Net Cash is greater than zero,
the quotient obtained by dividing (a) Company Net Cash by (b) 100,000,000. |
| (i) | if Company Net Cash is equal to or greater
than the Net Cash Target but less than or equal to zero, zero; and |
| (iii) | if Company Net Cash is less than the Net
Cash Target, the quotient obtained by dividing (a) the amount by which the Net Cash
Target exceeds Company Net Cash (expressed as a positive number) by (b) negative 100,000,000. |
| ● | “Company
Merger Shares” means product determined by multiplying (a) the quotient obtained
by dividing (i) the Parent Outstanding Shares by (ii) the Parent Adjustment Amount,
by (b) the Company Adjustment Amount. |
| ● | “Company
Outstanding Shares” means the total number of shares of Company Common Stock outstanding
immediately prior to the Effective Time expressed on a fully-diluted and as-converted to
Company Common Stock basis, calculated in accordance with the treasury method, and assuming,
without limitation or duplication, the issuance of shares of Company Common Stock in respect
of all Company Options and any other options, warrants or other rights to receive shares
of Company Common Stock (but specifically excluding all Company Warrants or Company Options
having an exercise price that exceeds the implied value of the Exchange Ratio). |
| ● | “Parent
Outstanding Shares” means, subject to Section 2.1(d), (i) the
total number of Parent Ordinary Shares (including all Parent Ordinary Shares represented
by Parent ADSs) outstanding immediately prior to the Effective Time expressed on a fully-diluted
and as-converted to Parent Ordinary Shares basis, calculated in accordance with the treasury
method, and assuming, without limitation or duplication, the issuance of Parent Ordinary
Shares in respect of all Parent Options, Parent RSUs, and other rights to receive such shares
that will be outstanding immediately after the Effective Time (but specifically excluding
any shares issued or to be issued pursuant to the PIPE Investment and all Parent Warrants
or Parent Options having an exercise price that exceeds the implied value of the Post-Closing
Parent Shares as determined on the basis of the Exchange Ratio), plus (ii) Parent Broker
Shares. |
| ● | “Parent
Adjustment Amount” means the sum of (i) 0.50 plus (ii) the
Parent Adjustment Factor minus the Company Adjustment Factor. |
| ● | “Parent
Adjustment Factor” means: |
| (i) | if Parent Net Cash is greater than zero,
the quotient obtained by dividing (a) Parent Net Cash by (b) 100,000,000. |
| (i) | if Parent Net Cash is equal to or greater
than the Net Cash Target but less than or equal to zero, zero; and |
| (iii) | if Parent Net Cash is less than the Net
Cash Target, the quotient obtained by dividing (a) the amount by which the Net Cash
Target exceeds Parent Net Cash (expressed as a positive number) by (b) negative 100,000,000. |
| ● | “Net
Cash Target” means negative six million dollars (-$6,000,000). |
“FCPA” means
the U.S. Foreign Corrupt Practices Act of 1977, as amended.
“GAAP” means
generally accepted accounting principles in the United States.
“Global
Trade Control Laws” means, to the extent applicable, the U.S. Export Administration Regulations; the U.S. International Traffic
in Arms Regulations; the economic sanctions rules and regulations implemented under statutory authority and/or President’s
Executive Orders and administered by the U.S. Treasury Department’s Office of Foreign Assets Control; U.S. Customs Regulations;
European Union (E.U.) Council Regulations on export controls, including Nos. 428/2009, 267/2012; other E.U. Council sanctions regulations,
as implemented in E.U. Member States; United Nations sanctions policies; all relevant regulations and legislative instruments made under
any of the above; other relevant economic sanctions, export and import control laws, and other laws, regulations, legislation, orders
and requirements imposed by a relevant Governmental Authority applicable to the Company or Parent.
“Government
Official” means (i) any elected or appointed government official (e.g., a legislator or a member of a ministry
of health); (ii) any employee or Person acting for or on behalf of a government, a government department or agency, an institution
or entity owned or controlled by a government (e.g., a healthcare professional employed by a government-owned or -controlled hospital,
or a Person serving on a healthcare committee that advises a government), or an enterprise or instrumentality performing a governmental
function; (iii) any candidate for public office, or officer, employee, or Person acting for or on behalf of a political party or
candidate for public office; (iv) an employee or Person acting for or on behalf of a public international organization (e.g., the
United Nations, the Red Cross, or the World Bank); (v) any member of a military or a royal or ruling family; or (vi) any Person
otherwise categorized as a government official under Law.
“Governmental Authority”
means any arbitrator, court, nation, government, any state or other political subdivision thereof and any entity exercising executive,
legislative, judicial regulatory or administrative functions of, or pertaining to or on behalf of, government.
“Hazardous
Materials” means any materials, chemicals, pollutants, contaminants, wastes, toxic or hazardous substances, including without
limitation petroleum and petroleum products or compounds, gasoline, diesel fuel, solvents, asbestos and asbestos-containing materials,
polychlorinated biphenyls, lead and lead-based paints and materials, radon, radioactive materials, pesticides, urea formaldehyde, and
mold, (i) that can cause harm to living organisms, human welfare, or the environment, (ii) that are regulated, or for which
liability can be imposed, pursuant to Environmental Laws, or (iii) the presence, handling, or management of which requires registration,
authorization, investigation or remediation under Environmental Laws, including by example “hazardous substances” and “hazardous
wastes” as defined in the Comprehensive Environmental Response, Compensation, and Liability
Act of 1980, as amended, and Resource Conservation and Recovery Act, respectively.
“Healthcare
Laws” means, to the extent related to the conduct of the Company’s and its subsidiaries or Parent and its
subsidiaries businesses, as applicable, as of the date of this Agreement, means (a) all federal and state fraud and abuse Laws,
including, the federal Anti-Kickback Statute (42 U.S.C. § 1320a-7b(b)), the Stark Law (42 U.S.C. § 1395nn), the civil
False Claims Act (31 U.S.C. § 3729 et seq.), Sections 1320a-7 and 1320a-7a of Title 42 of the United States Code and the
regulations promulgated pursuant to such statutes; (b) the administrative simplification provisions of the Health Insurance
Portability and Accountability Act of 1996 (18 U.S.C. §§669, 1035, 1347 and 1518; 42 U.S.C. §1320d et seq.) and
the regulations promulgated thereunder; (c) Titles XVIII (42 U.S.C. §1395 et seq.) and XIX (42 U.S.C. §1396 et seq.)
of the Social Security Act and the regulations promulgated thereunder; (d) the Medicare Prescription Drug, Improvement,
and Modernization Act of 2003 (42 U.S.C. §1395w-101 et seq.) and the regulations promulgated thereunder; (e) the so-called
federal “Sunshine Law” or Open Payments (42 U.S.C. § 1320a-7h) and state or local Laws regulating or requiring
reporting of interactions between pharmaceutical manufacturers and members of the healthcare industry and regulations promulgated
thereunder; (f) Laws governing government pricing or price reporting programs and regulations promulgated thereunder, including
the Medicaid Drug Rebate Program (42 U.S.C. § 1396r-8) and any state supplemental rebate program, the Public Health Service Act
(42 U.S.C. § 256b), the VA Federal Supply Schedule (38 U.S.C. § 8126) or any state pharmaceutical assistance program or
U.S. Department of Veterans Affairs agreement, and any successor government programs; (g) the Federal Food, Drug and Cosmetic
Act, 21 U.S.C. § 321 et seq., and all regulations, agency guidance or similar legal requirement promulgated thereunder; and
(h) any and all other federal, state, local or foreign health care Law applicable to the Company and its subsidiaries or Parent
and its subsidiaries, as applicable, or affecting their respective businesses.
“HSR Act”
means the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended.
“Indebtedness”
means, with respect to any Person, (i) indebtedness, notes payable, bonds, debentures or other obligations of such Person for borrowed
money, whether current, short-term or long-term, secured or unsecured; (ii) lease obligations under leases which are classified
as capital leases of such Person under GAAP (excluding any operating leases of such Person under GAAP); (iii) indebtedness created
or arising under any conditional sale or other title retention agreement with respect to property acquired by such Person; (iv) obligations
of such Person for the deferred purchase price of property or services (other than trade payables and obligations of such Person to creditors
incurred in the ordinary course of business); (v) obligations of such Person pursuant to or evidenced by hedging, swap, factoring,
interest rate, currency or commodity derivatives arrangements or other similar instruments; (vi) off-balance sheet financing of
such Person including synthetic leases and project financing; (vii) indebtedness of another Person referred to in clauses (i) through
(vi) above guaranteed, directly or indirectly, jointly or severally, in any manner by such Person; (viii) indebtedness referred
to in clauses (i) through (vii) above secured by (or for which the holder of such indebtedness has an existing right, contingent
or otherwise, to be secured by) any Lien on property or assets owned by such Person; and (ix) reimbursement obligations of such
Person with respect to letters of credit (other than (A) letters of credit issued for the benefit of suppliers to support accounts
payable to suppliers incurred in the ordinary course of business consistent with past practices, (B) standby letters of credit relating
to workers’ compensation insurance and surety bonds, and (C) surety bonds and customs bonds), bankers’ acceptance or
similar facilities issued for the account of such Person.
“Intellectual Property”
means, in any jurisdiction throughout the world, all rights, title, and interests in and to all intellectual property rights of every
kind and nature however denominated, intangible industrial property rights, and all related priority rights protected, created or arising
under the Laws of the United States or any other jurisdiction or under any international convention, including: (i) all Patents,
(ii) Trade Secrets, (iii) Copyrights, (iv) Software, (v) Trademarks (vi) registered domain names and social
media designations, (vii) all tangible embodiments of the foregoing (in whatever form or medium) and any rights equivalent to any
of the foregoing anywhere in the world, (viii) all royalties, fees, income, payments, and other proceeds now or hereafter due or
payable with respect to any of the foregoing, (ix) any and all registrations, applications, recordings, licenses, common-law rights,
statutory rights, administrative rights, and contractual rights relating to any of the foregoing, and (x) all claims and causes
of action, with respect to any of the foregoing, whether accruing before, on or after the date of this Agreement, including all rights
to and claims for damages, restitution and injunctive relief for infringement, dilution, misappropriation, violation, misuse, breach
or default, with the right but not the obligation to sue for such legal and equitable relief, and to collect, or otherwise recover, any
such damages, including costs and attorneys’ fees.
“Intentional
Breach” means the taking of a deliberate act or a deliberate failure to act, in either case which act or failure to act constitutes
in and of itself a material breach of the covenants or agreements set forth in this Agreement, even if breaching was not the conscious
object of the act.
“IP Governmental Authority”
means the United States Patent and Trademark Office, the United States Copyright Office, or any foreign equivalent thereof, or any foreign
Governmental Authority that performs the same or similar functions to either of the United States Patent and Trademark Office or the
United States Copyright Office with respect to the registration of Trademarks or Copyrights or the issuance of Patents.
“Knowledge of Parent”
means the actual knowledge, after reasonable inquiry, of the individuals listed on Section 10.1(b) of the Parent Disclosure
Letter. With respect to matters involving Intellectual Property, Knowledge does not require any Person to have conducted or have obtained
any freedom to operate opinions of any patent or any trademark or other intellectual property clearance searches or reviews, and if not
conducted, obtained, or reviewed, no knowledge of any patents, trademarks or other intellectual property of any Person that would have
been revealed by such opinions, searches, or reviews will be imputed to the Parent.
“Knowledge of the
Company” means the actual knowledge, after reasonable inquiry, of the individuals listed on Section 10.1(c) of
the Company Disclosure Letter. With respect to matters involving Intellectual Property, Knowledge does not require any Person to have
conducted or have obtained any freedom to operate opinions of any patent or any trademark or other intellectual property clearance searches
or reviews, and if not conducted, obtained, or reviewed, no knowledge of any patents, trademarks or other intellectual property of any
Person that would have been revealed by such opinions, searches, or reviews will be imputed to the Company.
“Law” means
any federal, state, local, national or supranational or foreign law (including common law), statute, ordinance, rule, regulation, order,
code ruling, decree, arbitration award, agency requirement, license, permit, standard, binding guideline or policy, or other enforceable
requirements of any Governmental Authority.
“Lien” means,
with respect to any property or asset (including any security), any lien, mortgage, pledge, encumbrance, security interest or deed of
trust.
“Net Cash”
means, without duplication, as of the Cash Determination Time and determined in accordance with the Net Cash Accounting Principles,
with respect to the applicable party (and rounded down to the nearest $100,000):
(I) the
sum of (without duplication):
(i) such
party’s cash and cash equivalents, plus
(ii) the
aggregate amount of any Specified Transaction Costs that have been paid by such party prior to the Closing, plus
(iii) the
aggregate amount of any severance payments made to any current or former officer, director, employee, consultant or independent contractor
of such party during the Pre-Closing Period in connection with or in anticipation of the transactions contemplated by this Agreement
(in an amount not to exceed $1,500,000 in the case of the Company and $3,000,000 in the case of Parent), plus
(iv) the aggregate
amount of all prepaid expenses for D&O insurance of such party that will be utilized by Parent and/or the Surviving Company on and
following the Closing;
minus (II) the
sum of (without duplication):
(a) (1) all
accounts payable and accrued expenses (other than accrued expenses which are such party’s Transaction Costs or Specified Employee
Costs) and (2) other current and long-term liabilities or other obligations for borrowed money (excluding, for the avoidance of
doubt, in the case of the foregoing clauses (1) and (2), (x) convertible notes or promissory notes that actually convert into
Company equity at or prior to the Closing and are extinguished in full in connection with such conversion) and (y) any amounts of
the type described in clauses (b) and (c) of this paragraph (II); plus
(b) any
and all liabilities of such party to any current or former officer, director, employee, consultant or independent contractor of such
party in respect of:
(1) any
sale, change of control, “stay around”, success, retention payments or other similar payments that are or could become due
as a result of the consummation of the Transactions, but excluding any severance or related termination costs (which shall be addressed
by clause (2) of this paragraph (II)(b));
(2) that
constitute severance or related termination costs that are or could become due as a result of the termination of such current or former
officer, director, employee, consultant or independent contractor of such party at or following the Closing (whether paid prior to the
Closing or unpaid as of the Closing) in excess of, together with any amounts added to such party’s Net Cash in accordance with
(I)(iii) of the definition of Net Cash, $1,500,000 in the case of the Company and $3,000,000 in the case of Parent;
(3) pursuant
to any Employee Benefit Plan maintained by such party, including deferred compensation, accrued but unpaid bonuses and accrued but unpaid
vacation or paid time off;
(4) any
claims for unpaid salary, bonuses, vacation pay and expense reimbursement obligations or other compensatory amounts, whether accrued
or unaccrued, related to the performance of services at any time prior to the Closing; or
(5) the
employer portion of any payroll taxes associated with any of the payments set forth in the foregoing clauses (1) – (4)
(collectively, the “Specified
Employee Costs”); plus
(c) all
of such party’s unpaid Transaction Costs (excluding any Specified Transaction Costs); plus
(d) to
the extent not included in the calculation of clause (a) of this paragraph (II), all payables or obligations, whether absolute,
contingent or otherwise, related to such party’s lease obligations (net of any rights of such party to receive payments relating
to the property subject to such lease obligation pursuant to an arrangement reasonably acceptable in form and substance (including the
creditworthiness of the counterparty thereto) to such party, such acceptance not to be unreasonably withheld, conditioned or delayed);
plus
(e) to
the extent not included in the calculation of clause (a) of this paragraph (II), all payables or obligations, whether absolute,
contingent or otherwise, related to such party’s (1) research and development obligations and (2) utilization of laboratory
space.
For the avoidance of doubt,
notwithstanding anything to the contrary in the definition of “Net Cash”, Net Cash shall exclude the proceeds of the PIPE
Investment pursuant to any Subscription Agreement and shall not be reduced by (w) any party’s unpaid Specified Transaction
Costs, (x) any payables and obligations related to convertible notes or promissory notes that will convert into Company equity at
or prior to the Closing, (y) any non-cash warrant liability or derivative liability or (z) any fees or expenses incurred by
the Company or Parent in connection with acquiring a D&O “tail” insurance policy, as may be acquired consistent with
Section 6.9(c) of the Agreement, or other expenses of the Company or Parent associated with obtaining or maintaining directors’
and officers’ insurance policies related to the period following the Closing in the ordinary course of business.
“Net Cash Accounting
Principles” means (a) to the extent consistent with GAAP, the accounting policies, principles, practices and methodologies
used to calculate a given item in such party’s latest financial statements that are audited or reviewed, and (b) if the policies,
principles, practices and methodologies described in clause (a) are not consistent with GAAP, GAAP.
“OFAC” means
the Office of Foreign Assets Control.
“Owned Company Intellectual
Property” means any and all Company Intellectual Property owned or purported to be owned by the Company or its subsidiaries.
“Owned Parent Intellectual
Property” means any and all Parent Intellectual Property owned or purported to be owned by Parent or its subsidiaries.
“Parent Acquisition
Proposal” means any proposal or offer, whether or not in writing, from any Person, Persons or group (other than Company or
any of its respective Affiliates) relating to any transaction or series of related transactions involving (a) any direct or indirect
acquisition or purchase from Parent or its subsidiaries, in a single transaction or a series of transactions, of (i) 20% or more
(based on the fair market value thereof, as determined by the Parent Board (or any committee thereof) in good faith) of assets (including
capital stock of the Parent’s subsidiaries), or by means of any merger, consolidation, business combination, recapitalization,
liquidation, dissolution, binding share exchange or similar transaction to which Parent or its subsidiaries is a party, of Parent and
its subsidiaries, taken as a whole or (ii) 20% or more of the outstanding Parent ADSs, (b) any tender offer or exchange offer
that, if consummated, would result in any Person, Persons or group owning, directly or indirectly, 20% or more of the outstanding Parent
ADSs or (c) any merger, consolidation, business combination, recapitalization, liquidation, dissolution, binding share exchange
or similar transaction to which Parent or its subsidiaries is a party pursuant to which (i) any Person, Persons or group (or the
stockholders of any such Person(s)) would own, directly or indirectly, 20% or more of the voting securities of Parent or of the surviving
entity in a merger involving Parent or the resulting direct or indirect parent of Parent or such surviving entity, or (ii) the owners
of outstanding Parent ADSs immediately prior to such transaction would own less than 80% of the voting securities of Parent or of the
surviving entity in a merger involving Parent or the resulting direct or indirect parent of Parent or such surviving entity, other than,
in each case, the Merger and the PIPE Investments; provided, for the avoidance of doubt, a Parent Licensing Deal shall not constitute
a Parent Acquisition Proposal.
“Parent
Broker Shares” means the number of Parent Ordinary Shares represented by 121,500 Parent ADSs.
“Parent Expense Reimbursement”
means the aggregate amount of all reasonable, documented, out-of-pocket legal fees and expenses incurred or paid by or on behalf of Parent
and its Affiliates in connection with the transactions contemplated by this Agreement or related to the authorization, preparation, negotiation,
execution and performance of this Agreement and the termination thereof, provided that, in no event shall “Parent Expense Reimbursement”
exceed $1,500,000.
“Parent Financial
Advisor” means Locust Walk Securities LLC.
“Parent Foreign Plan”
means (i) any Parent Plan that is maintained, sponsored or contributed to (or required to be contributed to) primarily for the benefit
of any current or former employee, officer, director or other service provider of Parent or any of its subsidiaries or with respect to
which Parent or any of its subsidiaries has or could have any liability, contingent or otherwise, who are or were providing services
outside the United States and (ii) any plan that would be a Parent Plan except for the fact that it is subject to any Law other
than U.S. federal, state or local Law.
“Parent Intervening
Event” means a material event or circumstance not known to the Parent Board on the date of this Agreement, which event or circumstance
becomes known to the Parent Board prior to the Effective Time; provided, however, that in no event shall the following
constitute a Parent Intervening Event: (a) a Parent Acquisition Proposal, (b) any material event or circumstance that was known
or reasonably foreseeable to the Parent Board as of the date hereof (or if known or reasonably foreseeable, the consequences of which
were not reasonably foreseeable), or (c) changes in the stock price of the Parent ADSs, in and of itself.
“Parent Licensing
Deal” means any acquisition or license (other than any non-exclusive and non-material license granted by Parent in the ordinary
course of business consistent with past practice) of, or joint venture, partnership, revenue or profit-sharing arrangement, collaboration
or other similar transaction with respect to PAS-600 nomacopan for the treatment of Geographic Atrophy (GA),
“Parent Licensing
Deal Revenue” means the amount of any upfront cash payment proposed to be paid to Parent in respect of a Parent Licensing Deal
pursuant to a bona fide term sheet entered into between Parent and an unaffiliated third party, negotiated on arms’ length
terms and without assigning value to any assets or product lines of the Company, which term sheet remains in effect as of the Closing
Date and which is reasonably likely to be paid within 120 days following the Closing Date.
“Parent Material
Adverse Effect” means any effect, event, occurrence, development or change that has a material adverse effect on the
financial condition, assets, liabilities, business or results of operations of Parent and its subsidiaries, taken as a whole; provided, however,
that a Parent Material Adverse Effect shall not be deemed to include effects, events, occurrences, developments or changes arising
out of, relating to or resulting from: (A) changes or prospective changes generally affecting the economy, financial or
securities markets or political, legislative or regulatory conditions, except and only to the extent such changes adversely affect
Parent in a disproportionate manner relative to other participants in Parent’s industry; (B) changes or prospective
changes in Parent’s industry, except and only to the extent such changes adversely affect Parent in a disproportionate manner
relative to other participants in Parent’s industry; (C) any change or prospective change in Law or the interpretation
thereof, except and only to the extent such changes adversely affect Parent in a disproportionate manner relative to other
participants in Parent’s industry; (D) any change or prospective change in applicable accounting regulations or
principles, including GAAP, or the interpretation thereof; (E) acts of war, armed hostility, terrorism, volcanic eruptions,
tsunamis, pandemics, earthquakes, floods, storms, hurricanes, tornadoes or other natural disasters, except and only to the extent
such acts adversely affect Parent in a disproportionate manner relative to other participants in Parent’s industry;
(F) the public announcement by Parent of its proposal to acquire the Company or the execution and delivery of this Agreement
(except to the extent such effect, event, occurrence, development or change was the result of a breach of Section 4.4)
or the announcement of the Merger, including the impact thereof on contractual or other relationships with suppliers, distributors,
partners, employees, lenders, investors, Governmental Authorities, and any Transaction Litigation; (G) any failure by Parent to
meet any internal or published industry analyst projections or forecasts or estimates of revenues or earnings (it being understood
and agreed that the facts and circumstances giving rise to such failure may be deemed to constitute, and may be taken into account
in determining whether there has been, a Parent Material Adverse Effect); (H) any change or prospective change in the price or
trading volume of the Parent ADSs on Nasdaq (it being understood and agreed that the facts and circumstances giving rise to such
change may be deemed to constitute, and may be taken into account in determining whether there has been, a Parent Material Adverse
Effect); (I) actions or omissions or required by this Agreement, or the failure to take any action prohibited by this
Agreement; (J) changes or prospective changes in Parent’s credit ratings (it being understood and agreed that the facts
and circumstances giving rise to such change may be deemed to constitute, and may be taken into account in determining whether there
has been, a Parent Material Adverse Effect); (K) (i) any delay in obtaining or making, or failure to obtain or make, any
regulatory approval, clearance or application with respect to any of the Company’s Products or (ii) any results, outcomes
or data, adverse events, side effects or safety observations arising from, or any delay in the timing or conduct of, any
nonclinical, preclinical or clinical studies, trials or tests related to any of the Company’s Products or (L) changes or
prospective changes in interest rates or foreign exchange rates.
“Parent Permitted
Liens” means any (i) statutory Liens for Taxes, business improvement district charges, water and sewer charges, assessments
and other lienable services and other governmental charges and impositions not yet due or payable or that are being contested in good
faith through appropriate proceedings, and in each case, for which adequate reserves have been established, in accordance with GAAP,
on the consolidated financial statements included in the most recent Parent SEC Documents, (ii) statutory Liens arising out of operation
of Law, including carriers’, warehousemen’s, mechanics’, materialmen’s, repairmen’s or other similar Liens
incurred in the ordinary course of business, (iii) pledges or deposits in connection with workers’ compensation, unemployment
insurance and other social security legislation, (iv) with respect to real property leased by Parent (“Parent Leased Real
Property”), (1) all matters, whether or not of record, that arise out of the actions of the Company or its agents, representatives
or contractors, (2) all easements, covenants, rights-of-way, restrictions and other encumbrances affecting any Parent Leased Real
Property, (3) all Liens and other matters disclosed, or in any title commitment, report, listing or policy, or in any survey or
survey update relating to the Parent Leased Real Property, in each case to the extent publicly available or made available by Parent
to the Company (including those relating to physical condition or variations in location or dimension), and (4) any and all Laws
affecting the Parent Leased Real Property (including any Laws relating to zoning, building and the use, occupancy, subdivision or improvement
of the Parent Leased Real Property); provided that such matters described in clauses (1) through (4) do not prohibit or materially
impair the current use and operation of the Parent Leased Real Property subject thereto in the business of Parent, (v) statutory
landlords’ Liens and Liens granted to landlords under any lease or sublease, (vi) any Liens created pursuant to or in connection
with this Agreement or disclosed in the Parent Disclosure Letter, (vii) Liens approved in writing by the Company and (viii) Liens
that, individually or in the aggregate, do not materially impair the current use and operation of the assets to which they relate
“Parent Plan”
means each Employee Benefit Plan that is sponsored, maintained, or contributed (or required to be contributed) to by Parent or any of
its subsidiaries for the benefit of current or former employees, officers, directors or other service providers of Parent or any of its
subsidiaries or with respect to which Parent or any of its subsidiaries has any liability, contingent or otherwise.
“Parent
Superior Proposal” means (i) a Parent Acquisition Proposal (except that (percentages in the definition of Parent
Acquisition Proposal shall be deemed to be 50%) made by any Person on terms that the Parent Board (or any committee thereof) determines
in good faith, after consultation with Parent’s outside financial advisors and outside legal counsel, and considering such factors
as the Parent Board (or any committee thereof) considers to be appropriate (including conditionality, timing, likelihood of consummation
of such proposal and consideration per share), that is reasonably likely to be consummated in accordance with its terms, and, if consummated,
would result in a transaction that is more favorable to Parent Shareholders than the Merger (including taking into account any applicable
Termination Fee of Parent) or (ii) a Parent Licensing Deal if and only if Parent has complied with Section 5.4 (ignoring clause
(B) of the last sentence of Section 5.4(a)) with respect to such Parent Licensing Deal as if a Parent Licensing Deal were included
within the definition of Parent Acquisition Proposal.
“Parent Warrant”
means each warrant to purchase capital stock of Parent.
“Patents”
means patents, registrations, invention disclosures, and patent applications, including divisionals, provisionals, continuations,
continuations-in-part, renewals, supplementary protection certificates, extensions, reissues and reexaminations thereof, and all patents
that may issue on such applications.
“Permits”
means all approvals, authorizations, certificates, consents, licenses, orders and permits and other similar authorizations of all Governmental
Authorities and all other Persons.
“Person”
means any individual, corporation (including any non-profit corporation), general partnership, limited partnership, limited liability
partnership, joint venture, estate, trust, company (including any limited liability company or joint stock company), firm or other enterprise,
association, organization, entity or Governmental Authority.
“Products”
means any product that the Company or Parent or any of their respective subsidiaries, as applicable, has manufactured, distributed, marketed
or sold, or is manufacturing, distributing, marketing or selling and any products currently under preclinical or clinical development
by the Company or Parent, as applicable.
“Release”
means any release, spill, emission, discharge, leaking, pumping, injection, deposit, disposal, dispersal, leaching, migration, or other
movement or presence in, into or through the indoor or outdoor environment (including, without limitation, ambient air, surface water,
groundwater and surface or subsurface strata) or at or from any property.
“Representative”
means any officers, directors, investment bankers, attorneys, accountants and other advisors, agents and representatives of a
party.
“Restricted
Market” means any of the Crimea, so-called Donetsk People’s Republic and so-called Luhansk People’s Republic
regions of Ukraine, Russia, Cuba, Iran, Venezuela, North Korea and Syria.
“Restricted Party”
means any Person that is the target of sanctions, including (a) any Person listed in any sanctions-related list of designated Persons
maintained by OFAC or the U.S. Department of State, the United Nations Security Council, the European Union, Her Majesty’s Treasury
of the United Kingdom, the Federal Department of Finance of Switzerland or such similar Governmental Authority of any European Union
member state or (b) any Person located, organized or resident in a Restricted Market.
“Sarbanes-Oxley Act”
means the Sarbanes-Oxley Act of 2002, including its rules and regulations.
“SEC” means
the United States Securities and Exchange Commission.
“Securities Act”
means the Securities Act of 1933, as amended.
“Software”
means any (a) computer programs, including all software implementations of algorithms, models and methodologies, whether in source
code or object code, (b) technical databases and compilations, including all technical data and collections of data, whether machine
readable or otherwise, including program files, data files, computer-related data, field and technical data definitions and relationships,
data definition specifications, data models, program and system logic, interfaces, program modules, routines, sub-routines, algorithms,
program architecture, design concepts, system designs, program structure, sequence and organization, screen displays and report layouts,
(c) descriptions, flow charts and other work product used to design, plan, organize and develop any of the foregoing, screens, user
interfaces, report formats, firmware, development tools, templates, menus, buttons and icons and (d) all documentation including
user manuals and other training documentation related to any of the foregoing, and any improvements, updates, upgrades or derivative
works of any of the foregoing.
“Specified Transaction
Costs” means the aggregate amount of all legal fees, expenses and disbursements incurred by such party in respect of legal
counsel in connection with the negotiation, execution and performance of this Agreement, the Ancillary Agreements and the consummation
of the transactions contemplated hereby and thereby.
“subsidiary”
of any specified Person means any other Person of which such first Person owns (either directly or indirectly through one or more other
subsidiaries) a majority of the outstanding equity securities or securities carrying a majority of the voting power in the election of
the board of directors or other governing body of such Person, and with respect to which entity such first Person is not otherwise prohibited
contractually or by other legally binding authority from exercising control.
“Tax” (including,
with correlative meaning, the term “Taxes”) includes all federal, state, local and foreign income, profits, franchise, gross
receipts, environmental, customs duty, capital stock, severances, stamp, payroll, sales, employment, unemployment, disability, use, property,
withholding, excise, production, value-added, occupancy and other taxes, governmental charges, duties or assessments of any nature whatsoever,
together with all interest, penalties and additions imposed with respect to such amounts and any interest in respect of such penalties
and additions.
“Tax Return”
means all returns and reports (including elections, declarations, disclosures, schedules, estimates and information returns) required
to be supplied to a Tax authority relating to Taxes.
“Tax Sharing Agreements”
means all agreements binding a party or any of its subsidiaries that provide for the allocation, apportionment, sharing or assignment
of any Tax liability or benefit (excluding any indemnification agreement or arrangement pertaining to the sale or lease of assets or
subsidiaries and any commercially reasonable indemnity, sharing or similar agreements or arrangements where the inclusion of a Tax indemnification
or allocation provision is customary or incidental to an agreement the primary nature of which is not Tax sharing or indemnification).
“Termination Fee”
means an amount equal to $300,000.
“third party”
means any Person, including as defined in Section 13(d) of the Exchange Act, other than Parent or any of its Affiliates or
the Company and any of its Affiliates, and the representatives of such Person.
“Trade Secrets”
means trade secrets and any other confidential information, including ideas, research and development, know-how, formulations of products,
proprietary biologic and chemical materials, drawings, prototypes, models, designs, manufacturing, production and other processes and
techniques, schematics, engineering, production and other designs, business methods, customer lists and supplier lists.
“Trademarks”
means trademarks, service marks, corporate names, trade names, brand names, product names, logos, slogans, trade dress and other indicia
of source or origin, any applications and registrations for any of the foregoing and all renewals and extensions thereof, and all goodwill
associated therewith and symbolized thereby.
“Transaction
Costs” means, with respect to any party, the aggregate amount of all out-of-pocket fees and expenses, incurred by such party
and its subsidiaries relating to the negotiation, preparation or execution of this Agreement or any documents or agreements contemplated
hereby or the performance or consummation of the transactions contemplated hereby, which shall include (a) any fees and expenses
associated with obtaining necessary or appropriate waivers, consents or approvals of any Governmental Authority on behalf of such party
or its subsidiaries; (b) any fees or expenses associated with obtaining the release and termination of any Lien; (c) all brokers’
or finders’ fees; and (d) fees and expenses of counsel, advisors, consultants, investment bankers, accountants, auditors and
experts.
“Treasury Regulations”
means the regulations promulgated under the Code.
10.2 Other
Definitional and Interpretative Provisions. The words “hereof”, “herein” and “hereunder” and
words of like import used in this Agreement shall refer to this Agreement as a whole and not to any particular provision of this Agreement.
The captions herein are included for convenience of reference only and shall be ignored in the construction or interpretation hereof.
References to Sections, Annexes and Exhibits are to Sections, Annexes and Exhibits of this Agreement unless otherwise specified. Any
singular term in this Agreement shall be deemed to include the plural, and any plural term the singular. Whenever the words “include”,
“includes” or “including” are used in this Agreement, they shall be deemed to be followed by the words “without
limitation”, whether or not they are in fact followed by those words or words of like import. References to any statute shall be
deemed to refer to such statute as amended from time to time and to any rules or regulations promulgated thereunder. References
to “made available” (or similar words of import) in respect of information made available by the Company or Parent mean any
information made available to Parent or the Company, as applicable, and their respective Affiliates or Representatives, as applicable
(including any information made available prior to the date hereof in the virtual data room maintained by the Company or Parent, as applicable,
or in writing with respect to materials specifically references in the Company Disclosure Letter and the Parent Disclosure Letter). References
to any agreement or contract are to that agreement or contract as amended, modified or supplemented from time to time in accordance with
the terms hereof and thereof. References to any Person include the successors and permitted assigns of that Person. All references to
“dollars” or “$” are to United States dollars. This Agreement is the product of negotiation by the parties having
the assistance of counsel and other advisors and, accordingly, it is the intention of the parties that this Agreement not be construed
more strictly with regard to one party than with regard to the others.
[Remainder of Page Intentionally Left Blank]
IN WITNESS WHEREOF, the parties
have executed this Agreement and Plan of Merger under seal as of the date first stated above.
| |
AKARI THERAPEUTICS, PLC By: |
/s/ Rachelle Jacques |
| |
Name: Rachelle Jacques
Title: Chief Executive Officer |
| |
|
| |
PEGASUS MERGER SUB, INC. By: |
/s/ Rachelle Jacques |
| |
Name: Rachelle Jacques
Title: Chief Executive Officer |
| |
|
| |
PEAK BIO, INC. By: |
/s/ Hoyoung Huh |
| |
Name: Hoyoung Huh
Title: Authorized Signatory |
[Signature Page to Agreement and Plan of
Merger]
Exhibit A
Form of Company Voting Agreement
Exhibit B
Form of Parent Voting Agreement
Exhibit 10.1
VOTING
AND SUPPORT AGREEMENT
This Voting and Support Agreement
(this “Agreement”) is made and entered into as of [●], 2024 (the “Agreement Date”), by and
among Akari Therapeutics, Plc, a public company limited by shares incorporated in England and Wales (“Parent”), and
[Stockholder] (the “Stockholder”). Each of Parent and the Stockholder are sometimes referred to as a “Party”
and collectively as the “Parties”. Capitalized terms used but not defined herein have the meanings ascribed to such
terms in the Merger Agreement (as defined below).
RECITALS
A. Concurrently
with the execution and delivery of this Agreement, Parent, Peak Bio, Inc., a Delaware corporation (the “Company”)
and Pegasus Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of Parent, are entering into an Agreement and Plan
of Merger (as it may be amended, supplemented or otherwise modified from time to time, the “Merger Agreement”) in substantially
the form attached hereto as Exhibit B.
B. As
of the Agreement Date, the Stockholder is the record and/or “beneficial owner” (within the meaning of Rule 13d-3 under
the Exchange Act) of the Company Common Stock, described on Exhibit A (the “Owned Shares”, and together
with any additional Company Common Stock that the Stockholder may acquire record and/or beneficial ownership of (including through the
exercise of any Company Options or Company Warrants) after the Agreement Date and prior to the Expiration Time, the “Covered
Shares”).
C. As
an inducement to the willingness of the Parent to enter into the Merger Agreement, the Parent has required that the Stockholder enter
into this Agreement with respect to the Covered Shares, and the Stockholder desires to enter into this Agreement to induce the Parent
to enter into the Merger Agreement.
NOW, THEREFORE, in consideration
of the foregoing and the respective representations, warranties, covenants and agreements set forth below and for other good and valuable
consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto, intending to be legally bound, do hereby
agree as follows:
1. Agreement
to Vote the Covered Shares; Appointment of Proxy
1.1. Agreement
to Vote. Until the earliest to occur of (a) the Effective Time, (b) receipt of the Company Stockholder Approval, and (c) such
date and time as the Merger Agreement is validly terminated pursuant to Section 8 thereof (as applicable, the “Expiration
Time”), at every meeting of the Company’s stockholders, however called, including any adjournment or postponement thereof,
and in connection with any action proposed to be taken by written consent of the Company’s stockholders, at which the approval of
the Merger Agreement or the Merger is to be voted on (and at every adjournment or postponement thereof), the Stockholder shall vote (including
via proxy) on or before the fifth (5th) business day prior to any meeting of Company’s stockholders, all of the Stockholder’s
Covered Shares in accordance with the Company Recommendation. Until the Expiration Time, the Stockholder agrees that the Stockholder will
not in the Stockholder's capacity as a stockholder of the Company bring, commence, institute, maintain, prosecute or voluntarily aid any
Action, which (i) challenges the validity of or seeks to enjoin the operation of any provision of this Agreement or (ii) alleges
that the execution and delivery of this Agreement by the Stockholder, either alone or together with the other stockholder voting agreements
and proxies to be delivered in connection with the execution of the Merger Agreement, or the approval of the Merger Agreement by the Company
Board, breaches any fiduciary duty of the Company Board or any member thereof.
1.2. Forms
and Power of Attorney. The Stockholder agrees to duly complete forms of proxy in respect of all of his, her or its Covered Shares,
and any other required documents in connection therewith, and cause same to be validly delivered in accordance with (and indicating that
all Covered Shares are voted in accordance with) the Company Recommendation at the Company Stockholders Meeting and will not withdraw
the forms of proxy or other documentation except as expressly otherwise provided in this Agreement. The obligations of the Stockholder
specified in Section 1.1 and Section 1.2 herein shall apply whether or not the transactions contemplated by the Merger Agreement,
including the Merger, or any other action described above are recommended by the Company Board. The Stockholder irrevocably and by way
of security for its obligations hereunder appoints any director of the Company to be its attorney in its name and on its behalf to take
effect on the dispatch of the Proxy Statement/Prospectus and only then if such Stockholder has failed to comply with its obligations under
Section 1.1 or Section 1.2 of this Agreement, with full power and authority to sign, execute and deliver a form of proxy and/or
such other documents and to do all such acts and things as may be necessary for or incidental to the performance of their obligations
under this Agreement.
1.3. Quorum.
Until the Expiration Time, at every meeting of the Company’s stockholders (and at every adjournment or postponement thereof), the
Stockholder shall be represented in person or by proxy at such meeting (or cause the holders of record on any applicable record date to
be represented in person or by proxy at such meeting) in order for the Covered Shares to be counted as present for purposes of establishing
a quorum.
2. Representations
and Warranties of the Stockholder. The Stockholder hereby severally, and not jointly or jointly and severally, represents and warrants
as follows:
2.1. Incorporation;
Authorization. If the Stockholder is a corporation, other legal entity, or otherwise not a natural person, the Stockholder is duly
organized and validly existing under the laws of the jurisdiction of its incorporation, formation or organization. The Stockholder has
all necessary power, authority, capacity and right to enter into this Agreement and to carry out each of its obligations under this Agreement.
This Agreement has been duly executed and delivered by the Stockholder and, assuming due authorization, execution and delivery of this
Agreement by the other parties hereto, constitutes a legal, valid and binding obligation of the Stockholder, enforceable against the Stockholder
in accordance with its terms, except that such enforceability (a) may be limited by bankruptcy, insolvency, fraudulent transfer,
reorganization, moratorium and other similar Laws of general application affecting or relating to the enforcement of creditors’
rights generally and (b) is subject to general principles of equity, whether considered in a proceeding at Law or in equity.
2.2. Ownership
of Subject Securities. The Stockholder is, and, subject to any transfer permitted pursuant to Section 4.1, will be continuously
up until the Effective Time, the direct or indirect beneficial owner of the Owned Shares set out opposite such Stockholder's name in Exhibit A,
with good and marketable title thereto, free and clear of any and all mortgages, liens, charges, restrictions, security interests, adverse
claims, pledges, encumbrances and demands or rights of others of any nature or kind whatsoever. The Stockholder does not own or have any
interest in any securities of the Company other than the Owned Shares set forth opposite such Stockholder's name on Exhibit A
hereto. The Stockholder is not a party to, bound or affected by or subject to, any charter or by-law, contract, agreement provision, statute,
regulation, judgment, order, decree or law which would be violated, contravened, breached by, or under which any default would occur as
a result of, the execution and delivery of this Agreement or the consummation of any of the transactions provided for in this Agreement.
2.3. Consents.
No consents or approvals of, or filings with, any Governmental Authority are necessary for the execution and delivery of this Agreement
by the Stockholder and the consummation by the Stockholder of the transactions contemplated hereby in connection with (a) the execution
and delivery by the Stockholder and enforcement against the Stockholder of this Agreement, or (b) the consummation of any transactions
by the Stockholder provided for herein.
2.4. No
Conflicts. Neither the execution and delivery of this Agreement by the Stockholder nor compliance by the Stockholder with any provision
of this Agreement (a) conflicts with or violates any organizational documents of the Stockholder, (b) violates any order, injunction,
judgment, decree or ruling (whether temporary, preliminary or permanent) enacted, promulgated, issued or entered by any Governmental Authority
or any Law applicable to the Stockholder or (c) violates, breaches, results in the loss of any benefit under, conflicts with any
provisions of, or constitutes a default (or an event which, with the notice or lapse of time, or both, would constitute a default) under,
or results in the termination of or a right of termination or cancellation under any contract to which the Stockholder is bound.
2.5. Legal
Proceedings. There are no material complaints, claim, action, charge, suit, arbitration, mediation, investigation or proceeding pending
or threatened against the Stockholder, or any of the Owned Shares of the Stockholder, and there are no material outstanding judgments,
writs, injunctions, decrees or orders of any Governmental Authority against or binding on the Stockholder, or any of the Owned Shares
of the Stockholder, in each case, that would reasonably be expected to materially impair the ability of the Stockholder to perform its
obligations under this Agreement.
2.6. No
Commitment. None of the Owned Shares held by the Stockholder is the subject of any commitment, undertaking or agreement, the terms
of which would affect in any way the ability of the Stockholder to perform the Stockholder’s obligations with respect to such Owned
Shares as set out in this Agreement.
2.7. No
Finder’s Fees. No broker, investment banker, financial advisor, finder, agent or other Person is entitled to any broker’s,
finder’s, financial adviser’s or other similar fee or commission in connection with this Agreement based upon arrangements
made by or on behalf of the Stockholder in his or her capacity as such.
2.8. Reliance
by the Company. The Stockholder understands and acknowledges that the Company is entering into the Merger Agreement in reliance upon
the Stockholder's execution and delivery of this Agreement.
3. Representations
and Warranties of the Parent. Parent hereby represents and warrants to the Stockholder as follows:
3.1. Incorporation;
Authorization. Parent is a public company limited by shares duly organized and validly existing under the laws of England and Wales.
Parent has all necessary power, authority, capacity and right to enter into this Agreement and to carry out each of its obligations under
this Agreement. This Agreement has been duly executed and delivered by Parent and, assuming due authorization, execution and delivery
of this Agreement by the other parties hereto, constitutes a legal, valid and binding obligation of Parent, enforceable against Parent
in accordance with its terms, except that such enforceability (a) may be limited by bankruptcy, insolvency, fraudulent transfer,
reorganization, moratorium and other similar Laws of general application affecting or relating to the enforcement of creditors’
rights generally and (b) is subject to general principles of equity, whether considered in a proceeding at Law or in equity.
3.2. No
Conflicts. Neither the execution and delivery of this Agreement by Parent nor compliance by Parent with any provision of this Agreement
shall (a) conflict with or violate the certificate of incorporation, by-laws or other charter documents of Parent, (b) violate
any order, injunction, judgment, decree or ruling (whether temporary, preliminary or permanent) enacted, promulgated, issued or entered
by any Governmental Authority or any Law applicable to Parent or (c) violate, breach, result in the loss of any benefit under, conflict
with any provisions of, or constitute a default (or an event which, with the notice or lapse of time, or both, would constitute a default)
under, result in the termination of or a right of termination or cancellation under any contract to which Parent is bound.
3.3. Legal
Proceedings. There are no material complaints, claim, action, charge, suit, arbitration, mediation, investigation or proceeding pending
or threatened against Parent, or any securities of Parent, and there are no material outstanding judgments, writs, injunctions, decrees
or orders of any Governmental Authority against or binding on Parent, or any securities of Parent, in each case, that would reasonably
be expected to materially impair the ability of Parent to perform its obligations under this Agreement.
4. Miscellaneous.
4.1. No
Transfer of Covered Shares. Prior to the Expiration Time, the Stockholder agrees not to, directly or indirectly, (i) sell, transfer,
pledge, encumber, assign, hedge, swap, convert or otherwise dispose of (including by merger (including by conversion into securities or
other consideration), by tendering into any tender or exchange offer, by operation of law or otherwise), either voluntarily or involuntarily,
offer to transfer or consent to any transfer or enter into any contract, option or other agreement or understanding with respect to the
transfer of any or all of the interest in such Stockholder's Covered Shares, or (ii) take any action or agree or commit to take any
action that would make any representation or warranty of such Stockholder contained in this Agreement untrue or incorrect or have the
effect of preventing or materially delaying the Stockholder from performing its obligations under this Agreement; provided, however, that
nothing in this Section 4.1 shall prohibit a transfer of Covered Shares (w) with the prior written consent of Parent, (x) to
any member of the Stockholder's immediate family, or to a trust for the benefit of the Stockholder or any member of the Stockholder's
immediate family, or otherwise for estate planning purposes, (y) by will or under the laws of intestacy upon the death of the Stockholder,
(z) pursuant to a qualified domestic order, (aa) to any charitable organization or (bb) any Stockholder that is an entity may transfer
Covered Shares to any Affiliate of such Stockholder or to one or more partners or members of such Stockholder; provided, further, that
a transfer referred to in the foregoing clauses of this sentence shall be permitted only if the transferee agrees in a written document,
reasonably satisfactory in form and substance to Parent, to be bound by all of the terms of this Agreement.
4.2. Non-Solicitation.
From and after the date hereof until the Expiration Time, the Stockholder will not, and will not permit any entity under such Stockholder’s
control to, take any action the Company is prohibited from taking pursuant to Section 5.3 of the Merger Agreement.
4.3. Further
Assurances. From time to time, at Parent’s request and without further consideration, the Stockholder shall execute and deliver
such additional documents and take all such further action as may be reasonably necessary or reasonably requested to effect the actions
and consummate the transactions contemplated by this Agreement and the Merger Agreement.
4.4. Termination.
This Agreement shall automatically terminate without further action by any of the Parties hereto and shall have no further force or effect
as of the Expiration Time, provided that Section 4.7 shall continue until the earlier of the Effective Time and the valid termination
of the Merger Agreement.
4.5. Capacity
as a Stockholder. Notwithstanding anything in this Agreement to the contrary, the Stockholder signs this Agreement solely in the Stockholder's
capacity as a stockholder of the Company, and not in any other capacity (including, if applicable, in such Stockholder's capacity as a
director or officer of the Company) and this Agreement shall not limit or otherwise affect the actions or inactions of any Affiliate,
representative or designee of the Stockholder or any of its Affiliates in his or her capacity, if applicable, as an officer or director
of any other person. Nothing herein shall in any way restrict a Stockholder that is a director or officer of the Company in the taking
of any actions (or failure to act) in his or her capacity as a director or officer of the Company if such action (or failure to act) would
reasonably be expected to be inconsistent with the exercise of his or her fiduciary duties as a director or officer of the Company.
4.6. Certain
Adjustments. In the event of a stock split, stock dividend or distribution, or any change in the Company Common Stock by reason of
any split-up, reverse stock split, recapitalization, combination, reclassification, exchange of shares or the like, the terms “Company
Common Stock”, “Covered Shares”, and “Owned Shares” shall be deemed to refer to and include such shares
as well as all such stock dividends and distributions and any securities into which or for which any or all of such shares may be changed
or exchanged or which are received in such transaction.
4.7. Other
Miscellaneous Provisions. The following provisions of the Merger Agreement shall apply mutatis mutandis to this Agreement:
Section 8.5 (Amendment), Section 9.2 (Notices), Section 9.4 (Governing Law), Section 9.6 (Counterparts and Signature),
Section 9.9 (Enforcement) and Section 9.11 (Waiver of Jury Trial).
[Signature page follows]
IN WITNESS WHEREOF, the Parties have caused this
Agreement to be duly executed and delivered on the date and year first above written.
| |
AKARI
Therapeutics, PLC |
| |
|
| |
By: |
|
| |
|
Name: |
| |
|
Title: |
[Signature Page to the
Parent Voting Agreement]
Exhibit A
Owned Shares
Exhibit B
Merger Agreement
Attached.
Exhibit 10.2
VOTING
AND SUPPORT AGREEMENT
This Voting and Support Agreement
(this “Agreement”) is made and entered into as of [●], 2024 (the “Agreement Date”), by and
among Peak Bio, Inc. (the “Company”), a Delaware corporation and [SHAREHOLDER] (the “Shareholder”).
Each of the Company and the Shareholder are sometimes referred to as a “Party” and collectively as the “Parties”.
Capitalized terms used but not defined herein have the meanings ascribed to such terms in the Merger Agreement (as defined below).
RECITALS
A. Concurrently
with the execution and delivery of this Agreement, Akari Therapeutics, Plc, a public company limited by shares incorporated in England
and Wales (“Parent”), the Company and Pegasus Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary
of Parent, are entering into an Agreement and Plan of Merger (as it may be amended, supplemented or otherwise modified from time to time,
the “Merger Agreement”) in substantially the form attached hereto as Exhibit B.
B.
As of the Agreement Date, the Shareholder is the record and/or “beneficial owner”
(within the meaning of Rule 13d-3 under the Exchange Act) of the Parent Ordinary Shares or Parent ADSs, as applicable,
described on Exhibit A (the “Owned Shares”, and together with any additional Parent Ordinary Shares
or Parent ADSs that the Shareholder may acquire record and/or beneficial ownership of (including through the exercise of any Parent
Options or Parent Warrants or vesting of Parent RSUs) after the Agreement Date and prior to the Expiration Time, the
“Covered Shares”).
C.
As an inducement to the willingness of the Company to enter into the Merger Agreement,
the Company has required that the Shareholder enter into this Agreement with respect to the Covered Shares, and the Shareholder
desires to enter into this Agreement to induce the Company to enter into the Merger Agreement.
NOW, THEREFORE, in consideration
of the foregoing and the respective representations, warranties, covenants and agreements set forth below and for other good and valuable
consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto, intending to be legally bound, do hereby
agree as follows:
1.
Agreement to Vote the Covered Shares; Appointment of Proxy
1.1. Agreement
to Vote. Until the earliest to occur of (a) the Effective Time, (b) receipt of the Parent Shareholder Approval, and (c) such
date and time as the Merger Agreement is validly terminated pursuant to Section 8 thereof (as applicable, the “Expiration
Time”), at every meeting of the Parent Shareholders, however called, including any adjournment or postponement thereof, and
in connection with any action proposed to be taken by written consent of the Parent Shareholders, at or in which the approval of the authorization
of the Parent Board to issue and allot all Parent Ordinary Shares, which shall be represented by Parent ADSs, to be issued in connection
with the Merger (the “Issuance”) is to be voted on (and at every adjournment or postponement thereof), the Shareholder
shall (i) if a holder of Parent Ordinary Shares, vote (including via proxy) all of the Shareholder’s Covered Shares in accordance
with the Parent Recommendation, on or before 48 hours prior to any meeting of Parent Shareholders, and (ii) if a holder of Parent
ADSs, instruct the registered holder/depositary to vote all of the Shareholder’s Covered Shares in accordance with the Parent Recommendation
and in accordance with the voting procedures of the Parent ADSs applicable to any general meeting of Parent Shareholders on or before
the fifth (5th) business day prior to any meeting of Parent Shareholders or such other period as may be required by the depositary
for the Parent ADSs. Until the Expiration Time, the Shareholder agrees that the Shareholder will not in the Shareholder’s capacity
as a shareholder of Parent bring, commence, institute, maintain, prosecute or voluntarily aid any Action, which (i) challenges the
validity of or seeks to enjoin the operation of any provision of this Agreement or (ii) alleges that the execution and delivery of
this Agreement by the Shareholder, either alone or together with the other shareholder voting agreements and proxies to be delivered in
connection with the execution of the Merger Agreement, or the approval of the Merger Agreement by the Parent Board, breaches any fiduciary
duty of the Parent Board or any member thereof.
1.2. Forms
and Power of Attorney. The Shareholder agrees to duly complete forms of proxy and, in respect of Parent ADSs, the relevant voting
forms in accordance with the rules applicable to the Parent ADSs in respect of all of his, her or its Covered Shares, and any other
required documents in connection therewith, and cause same to be validly delivered in accordance with (and indicating that all Covered
Shares are voted in accordance with) the Parent Recommendation at the Parent Shareholders Meeting and will not withdraw the forms of proxy
or other documentation except as expressly otherwise provided in this Agreement. The obligations of the Shareholder specified in Section 1.1
and Section 1.2 herein shall apply whether or not the transactions contemplated by the Merger Agreement, including the Merger, or
any other action described above are recommended by the Parent Board. The Shareholder irrevocably and by way of security for its obligations
hereunder appoints any director of Parent to be its attorney in its name and on its behalf to take effect on the dispatch of the Proxy
Statement/Prospectus and only then if such Shareholder has failed to comply with its obligations under Section 1.1 or Section 1.2
of this Agreement, with full power and authority to sign, execute and deliver a form of proxy, form of instruction to the depositary of
the Parent ADSs and/or such other documents and to do all such acts and things as may be necessary for or incidental to the performance
of their obligations under this Agreement.
1.3. Quorum.
Until the Expiration Time, at every meeting of the Parent Shareholders (and at every adjournment or postponement thereof), the Shareholder
shall be represented in person or by proxy at such meeting (or cause the holders of record on any applicable record date to be represented
in person or by proxy at such meeting) in order for the Covered Shares to be counted as present for purposes of establishing a quorum.
2.
Representations and Warranties of the Shareholder. The Shareholder hereby
severally, and not jointly or jointly and severally, represents and warrants as follows:
2.1. Incorporation;
Authorization. If the Shareholder is a corporation, other legal entity, or otherwise not a natural person, the Shareholder is duly
organized and validly existing under the laws of the jurisdiction of its incorporation, formation or organization. The Shareholder has
all necessary power, authority, capacity and right to enter into this Agreement and to carry out each of its obligations under this Agreement.
This Agreement has been duly executed and delivered by the Shareholder and, assuming due authorization, execution and delivery of this
Agreement by the other parties hereto, constitutes a legal, valid and binding obligation of the Shareholder, enforceable against the Shareholder
in accordance with its terms, except that such enforceability (a) may be limited by bankruptcy, insolvency, fraudulent transfer,
reorganization, moratorium and other similar Laws of general application affecting or relating to the enforcement of creditors’
rights generally and (b) is subject to general principles of equity, whether considered in a proceeding at Law or in equity.
2.2. Ownership
of Subject Securities. The Shareholder is, and, subject to any transfer permitted pursuant to Section 4.1, will be continuously
up until the Effective Time, the direct or indirect beneficial owner of the Owned Shares set out opposite such Shareholder’s name
in Exhibit A, with good and marketable title thereto, free and clear of any and all mortgages, liens, charges, restrictions,
security interests, adverse claims, pledges, encumbrances and demands or rights of others of any nature or kind whatsoever. The Shareholder
does not own or have any interest in any securities of Parent other than the Owned Shares set forth opposite such Shareholder’s
name on Exhibit A hereto. The Shareholder is not a party to, bound or affected by or subject to, any charter or by-law, contract,
agreement provision, statute, regulation, judgment, order, decree or law which would be violated, contravened, breached by, or under which
any default would occur as a result of, the execution and delivery of this Agreement or the consummation of any of the transactions provided
for in this Agreement.
2.3. Consents.
No consents or approvals of, or filings with, any Governmental Authority are necessary for the execution and delivery of this Agreement
by the Shareholder and the consummation by the Shareholder of the transactions contemplated hereby in connection with (a) the execution
and delivery by the Shareholder and enforcement against the Shareholder of this Agreement, or (b) the consummation of any transactions
by the Shareholder provided for herein.
2.4. No
Conflicts. Neither the execution and delivery of this Agreement by the Shareholder nor compliance by the Shareholder with any provision
of this Agreement (a) conflicts with or violates any organizational documents of the Shareholder, (b) violates any order, injunction,
judgment, decree or ruling (whether temporary, preliminary or permanent) enacted, promulgated, issued or entered by any Governmental Authority
or any Law applicable to the Shareholder or (c) violates, breaches, results in the loss of any benefit under, conflicts with any
provisions of, or constitutes a default (or an event which, with the notice or lapse of time, or both, would constitute a default) under,
or results in the termination of or a right of termination or cancellation under any contract to which the Shareholder is bound.
2.5. Legal
Proceedings. There are no material complaints, claim, action, charge, suit, arbitration, mediation, investigation or proceeding pending
or threatened against the Shareholder, or any of the Owned Shares of the Shareholder, and there are no material outstanding judgments,
writs, injunctions, decrees or orders of any Governmental Authority against or binding on the Shareholder, or any of the Owned Shares
of the Shareholder, in each case, that would reasonably be expected to materially impair the ability of the Shareholder to perform its
obligations under this Agreement.
2.6. No
Commitment. None of the Owned Shares held by the Shareholder is the subject of any commitment, undertaking or agreement, the terms
of which would affect in any way the ability of the Shareholder to perform the Shareholder’s obligations with respect to such Owned
Shares as set out in this Agreement.
2.7. No
Finder’s Fees. No broker, investment banker, financial advisor, finder, agent or other Person is entitled to any broker’s,
finder’s, financial adviser’s or other similar fee or commission in connection with this Agreement based upon arrangements
made by or on behalf of the Shareholder in his or her capacity as such.
2.8. Reliance
by the Company. The Shareholder understands and acknowledges that the Company is entering into the Merger Agreement in reliance upon
the Shareholder’s execution and delivery of this Agreement.
3. Representations
and Warranties of the Company. The Company hereby represents and warrants to the Shareholder as follows:
3.1. Incorporation;
Authorization. The Company is a corporation duly organized and validly existing under the laws of the State of Delaware. The Company
has all necessary power, authority, capacity and right to enter into this Agreement and to carry out each of its obligations under this
Agreement. This Agreement has been duly executed and delivered by the Company and, assuming due authorization, execution and delivery
of this Agreement by the other parties hereto, constitutes a legal, valid and binding obligation of the Company, enforceable against the
Company in accordance with its terms, except that such enforceability (a) may be limited by bankruptcy, insolvency, fraudulent transfer,
reorganization, moratorium and other similar Laws of general application affecting or relating to the enforcement of creditors’
rights generally and (b) is subject to general principles of equity, whether considered in a proceeding at Law or in equity.
3.2. No
Conflicts. Neither the execution and delivery of this Agreement by the Company nor compliance by the Company with any provision of
this Agreement shall (a) conflict with or violate the certificate of incorporation, by-laws or other charter documents of the Company,
(b) violate any order, injunction, judgment, decree or ruling (whether temporary, preliminary or permanent) enacted, promulgated,
issued or entered by any Governmental Authority or any Law applicable to the Company or (c) violate, breach, result in the loss of
any benefit under, conflict with any provisions of, or constitute a default (or an event which, with the notice or lapse of time, or both,
would constitute a default) under, result in the termination of or a right of termination or cancellation under any contract to which
the Company is bound.
3.3. Legal
Proceedings. There are no material complaints, claim, action, charge, suit, arbitration, mediation, investigation or proceeding pending
or threatened against the Company, or any securities of the Company, and there are no material outstanding judgments, writs, injunctions,
decrees or orders of any Governmental Authority against or binding on the Company, or any securities of the Company, in each case, that
would reasonably be expected to materially impair the ability of the Company to perform its obligations under this Agreement.
4. Miscellaneous.
4.1. No
Transfer of Covered Shares. Prior to the Expiration Time, the Shareholder agrees not to, directly or indirectly, (i) sell, transfer,
pledge, encumber, assign, hedge, swap, convert or otherwise dispose of (including by merger (including by conversion into securities
or other consideration), by tendering into any tender or exchange offer, by operation of law or otherwise), either voluntarily or involuntarily,
offer to transfer or consent to any transfer or enter into any contract, option or other agreement or understanding with respect to the
transfer of any or all of the interest in such Shareholder’s Covered Shares, or (ii) take any action or agree or commit to
take any action that would make any representation or warranty of such Shareholder contained in this Agreement untrue or incorrect or
have the effect of preventing or materially delaying the Shareholder from performing its obligations under this Agreement; provided,
however, that nothing in this Section 4.1 shall prohibit a transfer of Covered Shares (w) with the prior written consent of
the Company, (x) to any member of the Shareholder’s immediate family, or to a trust for the benefit of the Shareholder or
any member of the Shareholder’s immediate family, or otherwise for estate planning purposes, (y) by will or under the laws
of intestacy upon the death of the Shareholder, (z) pursuant to a qualified domestic order, (aa) to any charitable organization
or (bb) any Shareholder that is an entity may transfer Covered Shares to any Affiliate of such Shareholder or to one or more partners
or members of such Shareholder; provided, further, that a transfer referred to in the foregoing clauses of this sentence shall be permitted
only if the transferee agrees in a written document, reasonably satisfactory in form and substance to the Company, to be bound by all
of the terms of this Agreement.
4.2. Non-Solicitation.
From and after the date hereof until the Expiration Time, the Shareholder will not, and will not permit any entity under such Shareholder’s
control to, take any action that Parent is prohibited from taking pursuant to Section 5.4 of the Merger Agreement.
4.3. Further
Assurances. From time to time, at the Company’s request and without further consideration, the Shareholder shall execute and
deliver such additional documents and take all such further action as may be reasonably necessary or reasonably requested to effect the
actions and consummate the transactions contemplated by this Agreement and the Merger Agreement.
4.4. Termination.
This Agreement shall automatically terminate without further action by any of the Parties hereto and shall have no further force or effect
as of the Expiration Time, provided that Section 4.7 shall continue until the earlier of the Effective Time and the valid termination
of the Merger Agreement.
4.5. Capacity
as a Shareholder. Notwithstanding anything in this Agreement to the contrary, the Shareholder signs this Agreement solely in the Shareholder’s
capacity as a shareholder of Parent, and not in any other capacity (including, if applicable, in such Shareholder’s capacity as
a director or officer of Parent) and this Agreement shall not limit or otherwise affect the actions or inactions of any Affiliate, representative
or designee of the Shareholder or any of its Affiliates in his or her capacity, if applicable, as an officer or director of any other
person. Nothing herein shall in any way restrict a Shareholder that is a director or officer of Parent in the taking of any actions (or
failure to act) in his or her capacity as a director or officer of Parent if such action (or failure to act) would reasonably be expected
to be inconsistent with the exercise of his or her fiduciary duties as a director or officer of Parent.
4.6. Certain
Adjustments. In the event of a stock split, stock dividend or distribution, or any change in the Parent Ordinary Shares or Parent
ADSs by reason of any split-up, reverse stock split, recapitalization, combination, reclassification, exchange of shares or the like,
the terms “Parent Ordinary Shares”, “Parent ADSs”, “Covered Shares”, and “Owned Shares”
shall be deemed to refer to and include such shares as well as all such stock dividends and distributions and any securities into which
or for which any or all of such shares may be changed or exchanged or which are received in such transaction.
4.7. Other
Miscellaneous Provisions. The following provisions of the Merger Agreement shall apply mutatis mutandis to this Agreement:
Section 8.5 (Amendment), Section 9.2 (Notices), Section 9.4 (Governing Law), Section 9.6 (Counterparts and Signature),
Section 9.9 (Enforcement) and Section 9.11 (Waiver of Jury Trial).
[Signature page follows]
IN WITNESS WHEREOF, the Parties have caused this
Agreement to be duly executed and delivered on the date and year first above written.
| |
PEAK BIO, INC. |
| |
|
| |
By: |
|
| |
|
Name: |
| |
|
Title: |
[Signature Page to the
Parent Voting Agreement]
Exhibit A
Owned Shares
| |
|
| Parent Ordinary Shares |
Parent ADSs |
| |
|
Exhibit B
Merger Agreement
Attached.
Exhibit 99.1
Akari Therapeutics
and Peak Bio Announce Definitive Agreement to Merge as Equals Creating an Expanded Pipeline
That Features a Novel Antibody Drug Conjugate (ADC) Toolkit
BOSTON,
MA and PLEASANTON, CA – March 5, 2024 (GLOBE NEWSWIRE) — Akari Therapeutics, Plc (Nasdaq: AKTX), a late-stage
biotechnology company developing advanced therapies for autoimmune and inflammatory diseases, and Peak Bio Inc. (OTC: PKBO), a
clinical-stage biopharmaceutical company focused on developing therapeutics in areas of inflammation and oncology announce a
definitive agreement to merge as equals in an all-stock transaction. The combined entity will operate as Akari Therapeutics, Plc,
which is expected to continue to be listed and trade on the Nasdaq Capital Market as AKTX.
Following closing,
the company will have an expanded pipeline that contains multiple compelling assets spanning early and late development stages.
An assessment of the pipeline is planned, including program prioritization, updated timelines, near-term value creation opportunities,
and other considerations. Key highlights of the merger include:
Peak’s innovative ADC toolkit and lead program
| · | The merged pipeline features a robust ADC toolkit with novel payload
and linker technologies. By combining chemotherapy with immunotherapy strategies, the merged company aims to develop cutting-edge solutions
for cancer patients. In addition, the program includes a novel pre-clinical ADC candidate targeting TROP-2. |
Multiple compelling assets spanning early and late stages
| · | Akari’s nomacopan is a bispecific recombinant inhibitor of complement
C5 and leukotriene B4 (LTB4) being evaluated in a Phase 3 clinical trial for pediatric hematopoietic stem cell transplant-related thrombotic
microangiopathy (HSCT-TMA). It has potential to be the first approved treatment for HSCT-TMA, a rare complication of stem cell transplantation
that has an 80% mortality rate among severe adult and pediatric patients. |
| · | Akari’s long-acting version of nomacopan (PASylated-nomacopan) is in
the final stages of pre-clinical development for geographic atrophy (GA). It has the potential to address significant unmet patient needs
including a longer dose interval between intravitreal injections and reduction of choroidal neovascularization (CNV) risk that is associated
with approved complement-only inhibitors currently used in GA treatment. |
| · | Peak Bio’s Phase 2-ready PHP-303 program is targeting alpha-1 antitrypsin
deficiency (AATD). The program was licensed from Bayer Healthcare and is a 5th generation neutrophil elastase inhibitor (NEI)
targeting the inflammatory aspects of AATD, a rare condition. |
Strategic focus
| · | The merged company is expected to emphasize business development and licensing
with broad potential impact on patients. |
Proven leadership
| · | Leadership has extensive strategic and operational experience. Hoyoung Huh,
M.D., Ph.D. is expected to serve as incoming Chairman of the Board of the combined entity. Dr. Huh is currently Chairman of the Board
of Directors at Pliant Therapeutics and co-founder of BridgeBio Pharma. He is former Chairman of Geron Corporation, CytomX Therapeutics,
Epizyme, and is a former partner at McKinsey & Company. |
The post-merger Board of Directors will
consist of three directors selected by each company and one independent director jointly selected.
Transaction Details
Under the terms
of the agreement, Peak stockholders will receive a number of Akari ordinary shares (represented by American Depositary Shares) for each
share of Peak stock they own, as determined on the basis of the exchange ratio described in the agreement. The exchange is expected to
result in implied equity ownership in the combined company of approximately 50% for Akari shareholders and approximately 50% percent
for Peak stockholders on a fully-diluted basis, subject to adjustment under certain circumstances, including based on each party’s
relative level of net cash at the closing of the proposed transaction.
Timing
and Approvals
The transaction
is expected to close late in the second quarter of this year subject to the satisfaction of customary closing conditions, including approval
by the shareholders of both companies.
Legal Advisors
Goodwin Procter
LLP is serving as legal advisor to Akari and DLA Piper LLP is serving as legal advisor to Peak Bio.
About Akari Therapeutics
Akari Therapeutics,
plc (Nasdaq: AKTX) is a biotechnology company developing advanced therapies for autoimmune and inflammatory diseases. Akari’s lead
asset, investigational nomacopan, is a bispecific recombinant inhibitor of complement C5 activation and leukotriene B4 (LTB4) activity.
Akari’s pipeline includes a Phase 3 clinical trial program investigating nomacopan for severe hematopoietic stem cell
transplant-related thrombotic microangiopathy (HSCT-TMA).
Akari has been
granted Orphan Drug, Fast Track and Rare Pediatric Disease designations from the FDA for nomacopan for the treatment of pediatric HSCT-TMA
and orphan drug designation from the European Commission for treatment in hematopoietic stem cell transplantation. Akari’s pipeline
also includes pre-clinical research of long-acting PAS-nomacopan in geographic
atrophy (GA).
For more information
about Akari, please visit akaritx.com.
About Peak Bio, Inc.
Peak Bio is a clinical-stage
biopharmaceutical company focused on developing therapeutics addressing significant unmet needs in the areas of oncology and inflammation.
The Peak Bio pipeline includes an antibody-drug-conjugate (ADC) platform that includes novel toxins and linkers coupled with important
cancer antibody targets and a Phase 2-ready neutrophil elastase inhibitor for alpha1 anti-trypsin deficiency disorder (AATD).
The Peak Bio’s
clinical asset includes a Phase 2-ready program. PHP-303 was licensed from Bayer Healthcare in which Peak Bio has conducted additional
clinical studies to advance the program. PHP-303 is a neutrophil elastase inhibitor (NEI) targeting alpha-1 antitrypsin deficiency (AATD)
that is in development with the potential for best-in-class properties including increased potency and selectivity, and oral versus infused
administration.
For more information
about Peak Bio, please visit peak-bio.com.
Cautionary Note Regarding Forward-Looking
Statements
This communication relates to the proposed
transaction pursuant to the terms of the Merger Agreement, by and among Akari, Pegasus Merger Sub, Inc., and Peak Bio. This communication
includes express or implied forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended,
and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), about the proposed transaction
between Peak Bio and Akari and the operations of the combined company that involve risks and uncertainties relating to future events
and the future performance of Akari and Peak Bio. Actual events or results may differ materially from these forward-looking statements.
Words such as “will,” “could,” “would,” “should,” “expect,” “plan,”
“anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,”
“potential,” “continue,” “future,” “opportunity” “will likely result,” “target,”
variations of such words, and similar expressions or negatives of these words are intended to identify such forward-looking statements,
although not all forward-looking statements contain these identifying words. Examples of such forward-looking statements include, but
are not limited to, express or implied statements regarding: the business combination and related matters, including, but not limited
to, satisfaction of closing conditions to the proposed transaction, prospective performance and opportunities with respect to Akari or
Peak Bio, post-closing operations and the outlook for the companies’ businesses; Akari’s, Peak Bio’s or the combined
company’s targets, plans, objectives or goals for future operations, including those related to Akari’s and Peak Bio’s
product candidates, research and development, product candidate introductions and product candidate approvals as well as cooperation
in relation thereto; projections of or targets for revenues, costs, income (or loss), earnings per share, capital expenditures, dividends,
capital structure, net financials and other financial measures; future economic performance, future actions and outcome of contingencies
such as legal proceedings; and the assumptions underlying or relating to such statements.
These statements are based on Akari’s
and Peak Bio’s current plans, estimates and projections. By their very nature, forward-looking statements involve inherent risks
and uncertainties, both general and specific. A number of important factors, including those described in this communication, could cause
actual results to differ materially from those contemplated in any forward-looking statements. Factors that may affect future results
and may cause these forward-looking statements to be inaccurate include, without limitation: uncertainties as to the timing for completion
of the proposed transaction; uncertainties as to Peak Bio’s and/or Akari’s ability to obtain the approval of Akari’s
shareholders or Peak Bio’s stockholders required to consummate the proposed transaction; the possibility that competing offers
will be made by third parties; the occurrence of events that may give rise to a right of one or both of Akari and Peak Bio to terminate
the merger agreement; the possibility that various closing conditions for the proposed transaction may not be satisfied or waived on
a timely basis or at all, including the possibility that a governmental entity may prohibit, delay, or refuse to grant approval, if required,
for the consummation of the proposed transaction (or only grant approval subject to adverse conditions or limitations); the difficulty
of predicting the timing or outcome of consents or regulatory approvals or actions, if any; the possibility that the proposed transaction
may not be completed in the time frame expected by Akari and Peak Bio, or at all; the risk that Akari and Peak Bio may not realize the
anticipated benefits of the proposed transaction in the time frame expected, or at all; the effects of the proposed transaction on relationships
with Akari’s or Peak Bio’s employees, business or collaboration partners or governmental entities; the ability to retain
and hire key personnel; potential adverse reactions or changes to business relationships resulting from the announcement or completion
of the proposed transaction; significant or unexpected costs, charges or expenses resulting from the proposed transaction; the potential
impact of unforeseen liabilities, future capital expenditures, revenues, costs, expenses, earnings, synergies, economic performance,
indebtedness, financial condition and losses on the future prospects, business and management strategies for the management, expansion
and growth of the combined business after the consummation of the proposed transaction; potential negative effects related to this announcement
or the consummation of the proposed transaction on the market price of Akari’s American Depositary Shares or Peak Bio’s common
stock and/or Akari’s or Peak Bio’s operating or financial results; uncertainties as to the long-term value of Akari’s
American Depositary Shares (and the ordinary shares represented thereby), including the dilution caused by Akari’s issuance of
additional American Depositary Shares (and the ordinary shares represented thereby) in connection with the proposed transaction; unknown
liabilities related to Akari or Peak Bio; the nature, cost and outcome of any litigation and other legal proceedings involving Akari,
Peak Bio or their respective directors, including any legal proceedings related to the proposed transaction; risks related to global
as well as local political and economic conditions, including interest rate and currency exchange rate fluctuations; potential delays
or failures related to research and/or development of Akari’s or Peak Bio’s programs or product candidates; risks related
to any loss of Akari’s or Peak Bio’s patents or other intellectual property rights; any interruptions of the supply chain
for raw materials or manufacturing for Akari or Peak Bio’s product candidates, the nature, timing, cost and possible success and
therapeutic applications of product candidates being developed by Akari, Peak Bio and/or their respective collaborators or licensees;
the extent to which the results from the research and development programs conducted by Akari, Peak Bio, and/or their respective collaborators
or licensees may be replicated in other studies and/or lead to advancement of product candidates to clinical trials, therapeutic applications,
or regulatory approval; uncertainty of the utilization, market acceptance, and commercial success of Akari’s or Peak Bio’s
product candidates, and the impact of studies (whether conducted by Akari, Peak Bio or others and whether mandated or voluntary) on any
of the foregoing; unexpected breaches or terminations with respect to Akari’s or Peak Bios’s material contracts or arrangements;
risks related to competition for Akari’s or Peak Bio’s product candidates; Akari’s or Peak Bio’s ability to successfully
develop or commercialize Akari’s or Peak Bio’s product candidates; Akari’s, Peak Bio’s, and their collaborators’
abilities to continue to conduct current and future developmental, preclinical and clinical programs; potential exposure to legal proceedings
and investigations; risks related to changes in governmental laws and related interpretation thereof, including on reimbursement, intellectual
property protection and regulatory controls on testing, approval, manufacturing, development or commercialization of any of Akari’s
or Peak Bio’s product candidates; unexpected increase in costs and expenses with respect to the potential transaction or Akari’s
or Peak Bio’s business or operations; and risks and uncertainties related to epidemics, pandemics or other public health crises
and their impact on Akari’s and Peak Bio’s respective businesses, operations, supply chain, patient enrollment and retention,
preclinical and clinical trials, strategy, goals and anticipated milestones. While the foregoing list of factors presented here is considered
representative, no list should be considered to be a complete statement of all potential risks and uncertainties. There can be no assurance
that the proposed transaction or any other transaction described above will in fact be consummated in the manner described or at all.
A more complete description of these and other material risks can be found in Akari’s and Peak Bios’s respective filings
with the U.S. Securities and Exchange Commission (the “SEC”), including each of their Annual Reports on Form 20-F and
10-K, respectively, for the year ended December 31, 2022, subsequent periodic reports, and other documents that may be filed from
time to time with the SEC. These risks, as well as other risks associated with the proposed transaction, will be more fully discussed
in the joint proxy statement/prospectus that will be included in the registration statement on Form S-4 that will be filed with
the SEC in connection with the proposed transaction, which joint proxy statement/prospectus will be mailed or otherwise disseminated
to Akari’s shareholders and Peak Bio’s stockholders when it becomes available.
Any forward-looking statements speak
only as of the date of this communication and are made based on the current beliefs and judgments of Akari’s and Peak Bio’s
management, and the reader is cautioned not to rely on any forward-looking statements made by Akari or Peak Bio. Unless required by law,
neither Akari nor Peak Bio is under no duty and undertakes no obligation to update or revise any forward-looking statement after the
distribution of this document, including without limitation any financial projection or guidance, whether as a result of new information,
future events or otherwise.
No Offer or Solicitation
This communication is not intended to and shall not constitute an offer to subscribe for, buy or sell or the solicitation
of an offer to subscribe for, buy or sell any securities, or a solicitation of any vote or approval, nor shall there be any sale of, or
offer to sell or buy, securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration
or qualification under the securities laws of any such jurisdiction. This communication is for informational purposes only. No offering
of securities shall be made, except by means of a prospectus meeting the requirements of Section 10 of the U.S. Securities Act of 1933,
as amended, and otherwise in accordance with applicable law.
Additional Information
and Where to Find It
In connection with the proposed transaction,
Akari and Peak Bio expect to file with the SEC a Registration Statement on Form S-4. The Registration Statement on Form S-4
will include a prospectus of Akari and a joint proxy statement of Akari and Peak Bio, and each party may also file other documents regarding
the proposed transaction with the SEC. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ CAREFULLY THE REGISTRATION STATEMENT ON FORM S-4,
JOINT PROXY STATEMENT/PROSPECTUS AND OTHER RELEVANT DOCUMENTS FILED OR WILL BE FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS
THERETO AND ANY DOCUMENTS INCORPORATED BY REFERENCE THEREIN, IN THEIR ENTIRETY IF AND WHEN THEY BECOME AVAILABLE BECAUSE THEY CONTAIN
OR WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION, RELATED MATTERS AND THE PARTIES TO THE PROPOSED TRANSACTION.
You may obtain a free copy of the Registration
Statement on Form S-4, joint proxy statement/prospectus and other relevant documents (if and when they become available) that are
or will be filed with the SEC for free at the SEC’s website at www.sec.gov. Copies of the documents filed with the SEC by Akari
will be available free of charge on Akari’s website at http://investor.akaritx.com/ or by contacting Akari’s Investor Relations
Department at http://investor.akaritx.com/investor-resources/contact-us. Copies of the documents filed with the SEC by Peak Bio will
be available free of charge on Peak Bio’s website at https://peak-bio.com/investors or by contacting Peak Bio’s Investor
Relations Department at https://peak-bio.com/contact.
Participants in the Solicitation
Akari, Peak Bio and their respective
directors and executive officers and other members of management and employees may be deemed to be participants in the solicitation of
proxies in respect of the proposed transaction. Information about the directors and executive officers of Akari, including a description
of their direct or indirect interests, by security holdings or otherwise, is set forth in Akari’s Annual Report on Form 20-F
for the year ended December 31, 2022 filed with the SEC on May 1, 2023, subsequent quarterly and current reports on Form 10-Q
and -K, respectively, and other documents that may be filed from time to time with the SEC. Information about the directors and executive
officers of Peak Bio, including a description of their direct or indirect interests, by security holdings or otherwise, is set forth
in Peak Bio’s proxy statement for its 2022 Special Meeting of Stockholders, which was filed with the SEC on October 19, 2022,
the Annual Report on Form 10-K for the year ended December 31, 2022 filed with the SEC on June 29, 2023, subsequent quarterly
and current reports on Form 10-Q and Form 8-K, respectively, and other documents that may be filed from time to time with the
SEC. Other information regarding the participants in the proxy solicitations and a description of their direct and indirect interests,
by security holdings or otherwise, will be contained in the joint proxy statement/prospectus included in the Registration Statement on
Form S-4 and other relevant materials to be filed with the SEC regarding the proposed transaction when such materials become available.
Security holders, potential investors and other readers should read the joint proxy statement/prospectus, included in the Registration
Statement on Form S-4 carefully when it becomes available before making any voting or investment decision. You may obtain free copies
of these documents from Akari or Peak Bio using the sources indicated above.
For more information
Investor Contact:
Mike Moyer
LifeSci Advisors
(617) 308-4306
mmoyer@lifesciadvisors.com
Media Contact:
Eliza Schleifstein
Schleifstein PR
(917) 763-8106
eliza@schleifsteinpr.com
Exhibit 99.2
Akari employee communication from Rachelle Jacques:
Team,
Today we issued a press release announcing that Akari and Peak Bio
have reached a definitive agreement for a merger of equals in an all-stock transaction. Please see the press release here.
This merger is an important step forward for our company. I’d
like to discuss this with you and answer your questions personally, so we’ll hold a town hall later today. I look forward to seeing
you there.
Best regards,
Rachelle
Forward-looking Statements
This communication relates to the proposed transaction pursuant to
the terms of the Agreement and Plan of Merger (the “Merger Agreement”), by and among Akari Therapeutics, Plc, a public company
limited by shares incorporated in England and Wales (“Akari”), Pegasus Merger Sub, Inc., a Delaware corporation and a wholly-owned
subsidiary of Akari and Peak Bio, Inc. (“Peak Bio”) and includes express or implied forward-looking statements within the
meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Exchange Act, about the proposed transaction
between Peak Bio and Akari and the operations of the combined company that involve risks and uncertainties relating to future events and
the future performance of Akari and Peak Bio. Actual events or results may differ materially from these forward-looking statements. Words
such as “will,” “could,” “would,” “should,” “expect,” “plan,”
“anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,”
“potential,” “continue,” “future,” “opportunity” “will likely result,” “target,”
variations of such words, and similar expressions or negatives of these words are intended to identify such forward-looking statements,
although not all forward-looking statements contain these identifying words. Examples of such forward-looking statements include, but
are not limited to, express or implied statements regarding: the Merger (as defined in the Merger Agreement) and related matters, including,
but not limited to, satisfaction of closing conditions to the proposed transaction, prospective performance and opportunities with respect
to Akari or Peak Bio, post-closing operations and the outlook for the companies’ businesses; Akari’s, Peak Bio’s or
the combined company’s targets, plans, objectives or goals for future operations, including those related to Akari’s and Peak
Bio’s product candidates, research and development, product candidate introductions and product candidate approvals as well as cooperation
in relation thereto; projections of or targets for revenues, costs, income (or loss), earnings per share, capital expenditures, dividends,
capital structure, net financials and other financial measures; future economic performance, future actions and outcome of contingencies
such as legal proceedings; and the assumptions underlying or relating to such statements.
These statements are based on Akari’s and Peak Bio’s current
plans, estimates and projections. By their very nature, forward-looking statements involve inherent risks and uncertainties, both general
and specific. A number of important factors, including those described in this communication, could cause actual results to differ materially
from those contemplated in any forward-looking statements. Factors that may affect future results and may cause these forward-looking
statements to be inaccurate include, without limitation: uncertainties as to the timing for completion of the proposed transaction; uncertainties
as to Peak Bio’s and/or Akari’s ability to obtain the approval of Akari’s shareholders or Peak Bio’s stockholders
required to consummate the proposed transaction; the possibility that competing offers will be made by third parties; the occurrence
of events that may give rise to a right of one or both of Akari and Peak Bio to terminate the Merger Agreement; the possibility that various
closing conditions for the proposed transaction may not be satisfied or waived on a timely basis or at all, including the possibility
that a governmental entity may prohibit, delay, or refuse to grant approval, if required, for the consummation of the proposed transaction
(or only grant approval subject to adverse conditions or limitations); the difficulty of predicting the timing or outcome of consents
or regulatory approvals or actions, if any; the possibility that the proposed transaction may not be completed in the time frame expected
by Akari and Peak Bio, or at all; the risk that Akari and Peak Bio may not realize the anticipated benefits of the proposed transaction
in the time frame expected, or at all; the effects of the proposed transaction on relationships with Akari’s or Peak Bio’s
employees, business or collaboration partners or governmental entities; the ability to retain and hire key personnel; potential adverse
reactions or changes to business relationships resulting from the announcement or completion of the proposed transaction; significant
or unexpected costs, charges or expenses resulting from the proposed transaction; the potential impact of unforeseen liabilities, future
capital expenditures, revenues, costs, expenses, earnings, synergies, economic performance, indebtedness, financial condition and losses
on the future prospects, business and management strategies for the management, expansion and growth of the combined business after the
consummation of the proposed transaction; potential negative effects related to this announcement or the consummation of the proposed
transaction on the market price of Akari’s American Depositary Shares or Peak Bio’s common stock and/or Akari’s or Peak
Bio’s operating or financial results; uncertainties as to the long-term value of Akari’s American Depositary Shares (and the
ordinary shares represented thereby), including the dilution caused by Akari’s issuance of additional American Depositary Shares
(and the ordinary shares represented thereby) in connection with the proposed transaction; unknown liabilities related to Akari or Peak
Bio; the nature, cost and outcome of any litigation and other legal proceedings involving Akari, Peak Bio or their respective directors,
including any legal proceedings related to the proposed transaction; risks related to global as well as local political and economic conditions,
including interest rate and currency exchange rate fluctuations; potential delays or failures related to research and/or development of
Akari’s or Peak Bio’s programs or product candidates; risks related to any loss of Akari’s or Peak Bio’s patents
or other intellectual property rights; any interruptions of the supply chain for raw materials or manufacturing for Akari or Peak Bio’s
product candidates, the nature, timing, cost and possible success and therapeutic applications of product candidates being developed by
Akari, Peak Bio and/or their respective collaborators or licensees; the extent to which the results from the research and development
programs conducted by Akari, Peak Bio, and/or their respective collaborators or licensees may be replicated in other studies and/or lead
to advancement of product candidates to clinical trials, therapeutic applications, or regulatory approval; uncertainty of the utilization,
market acceptance, and commercial success of Akari’s or Peak Bio’s product candidates, and the impact of studies (whether
conducted by Akari, Peak Bio or others and whether mandated or voluntary) on any of the foregoing; unexpected breaches or terminations
with respect to Akari’s or Peak Bios’s material contracts or arrangements; risks related to competition for Akari’s
or Peak Bio’s product candidates; Akari’s or Peak Bio’s ability to successfully develop or commercialize Akari’s
or Peak Bio’s product candidates; Akari’s, Peak Bio’s, and their collaborators’ abilities to continue to conduct
current and future developmental, preclinical and clinical programs; potential exposure to legal proceedings and investigations; risks
related to changes in governmental laws and related interpretation thereof, including on reimbursement, intellectual property protection
and regulatory controls on testing, approval, manufacturing, development or commercialization of any of Akari’s or Peak Bio’s
product candidates; unexpected increase in costs and expenses with respect to the potential transaction or Akari’s or Peak Bio’s
business or operations; and risks and uncertainties related to epidemics, pandemics or other public health crises and their impact on
Akari’s and Peak Bio’s respective businesses, operations, supply chain, patient enrollment and retention, preclinical and
clinical trials, strategy, goals and anticipated milestones. While the foregoing list of factors presented here is considered representative,
no list should be considered to be a complete statement of all potential risks and uncertainties. There can be no assurance that the proposed
transaction or any other transaction described above will in fact be consummated in the manner described or at all. A more complete description
of these and other material risks can be found in Akari’s and Peak Bios’s respective filings with the U.S. Securities and
Exchange Commission (the “SEC”), including each of their Annual Reports on Form 20-F and 10-K, respectively, for the year
ended December 31, 2022, subsequent periodic reports, and other documents that may be filed from time to time with the SEC. These risks,
as well as other risks associated with the proposed transaction, will be more fully discussed in the joint proxy statement/prospectus
that will be included in the registration statement on Form S-4 that will be filed with the SEC in connection with the proposed transaction,
which joint proxy statement/prospectus will be mailed or otherwise disseminated to Akari’s shareholders and Peak Bio’s stockholders
when it becomes available.
Any forward-looking statements speak only as of the date of this communication
and are made based on the current beliefs and judgments of Akari’s and Peak Bio’s management, and the reader is cautioned
not to rely on any forward-looking statements made by Akari or Peak Bio. Unless required by law, neither Akari nor Peak Bio is under no
duty and undertakes no obligation to update or revise any forward-looking statement after the distribution of this document, including
without limitation any financial projection or guidance, whether as a result of new information, future events or otherwise.
No Offer or Solicitation
This communication is not intended to and shall not constitute an offer
to subscribe for, buy or sell or the solicitation of an offer to subscribe for, buy or sell any securities, or a solicitation of any vote
or approval, nor shall there be any sale of, or offer to sell or buy, securities in any jurisdiction in which such offer, solicitation
or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. This communication
is for informational purposes only. No offering of securities shall be made, except by means of a prospectus meeting the requirements
of Section 10 of the U.S. Securities Act of 1933, as amended, and otherwise in accordance with applicable law.
Additional Information and Where to Find It
In connection with the proposed transaction, Akari and Peak Bio expect
to file with the SEC a Registration Statement on Form S-4. The Registration Statement on Form S-4 will include a prospectus of Akari and
a joint proxy statement of Akari and Peak Bio, and each party may also file other documents regarding the proposed transaction with the
SEC. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ CAREFULLY THE REGISTRATION STATEMENT ON FORM S-4, JOINT PROXY STATEMENT/PROSPECTUS
AND OTHER RELEVANT DOCUMENTS FILED OR WILL BE FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS THERETO AND ANY DOCUMENTS INCORPORATED
BY REFERENCE THEREIN, IN THEIR ENTIRETY IF AND WHEN THEY BECOME AVAILABLE BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT
THE PROPOSED TRANSACTION, RELATED MATTERS AND THE PARTIES TO THE PROPOSED TRANSACTION.
You may obtain a free copy of the Registration Statement on Form S-4,
joint proxy statement/prospectus and other relevant documents (if and when they become available) that are or will be filed with the SEC
for free at the SEC’s website at www.sec.gov. Copies of the documents filed with the SEC by Akari will be available free of charge
on Akari’s website at http://investor.akaritx.com/ or by contacting Akari’s Investor Relations Department at http://investor.akaritx.com/investor-resources/contact-us.
Copies of the documents filed with the SEC by Peak Bio will be available free of charge on Peak Bio’s website at https://peak-bio.com/investors
or by contacting Peak Bio’s Investor Relations Department at https://peak-bio.com/contact.
Participants in the Solicitation
Akari, Peak Bio and their respective directors and executive officers
and other members of management and employees may be deemed to be participants in the solicitation of proxies in respect of the proposed
transaction. Information about the directors and executive officers of Akari, including a description of their direct or indirect interests,
by security holdings or otherwise, is set forth in Akari’s Annual Report on Form 20-F for the year ended December 31, 2022 filed
with the SEC on May 1, 2023, subsequent quarterly and current reports on Form 10-Q and -K, respectively, and other documents that may
be filed from time to time with the SEC. Information about the directors and executive officers of Peak Bio, including a description
of their direct or indirect interests, by security holdings or otherwise, is set forth in Peak Bio’s proxy statement for its 2022
Special Meeting of Stockholders, which was filed with the SEC on October 19, 2022, the Annual Report on Form 10-K for the year ended
December 31, 2022 filed with the SEC on June 29, 2023, subsequent quarterly and current reports on Form 10-Q and Form 8-K, respectively,
and other documents that may be filed from time to time with the SEC. Other information regarding the participants in the proxy solicitations
and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the joint proxy statement/prospectus
included in the Registration Statement on Form S-4 and other relevant materials to be filed with the SEC regarding the proposed transaction
when such materials become available. Security holders, potential investors and other readers should read the joint proxy statement/prospectus,
included in the Registration Statement on Form S-4 carefully when it becomes available before making any voting or investment decision.
You may obtain free copies of these documents from Akari or Peak Bio using the sources indicated above.
Exhibit 99.3
Akari investor email (those who have inquired in the
past) from Rachelle Jacques:
[Name],
I wanted to reach out personally and share the news we issued this
morning. Akari and Peak Bio have reached a definitive agreement for a merger of equals in an all-stock transaction. Please see the press
release here.
What is most exciting about this merged company, which will
operate as Akari and continue to trade on Nasdaq as AKTX after closing, is our expanded pipeline that will feature an antibody drug conjugate
(ADC) toolkit with novel toxin and linker technologies. The ADC toolkit is expected to be the merged company’s lead asset and the program includes a novel pre-clinical ADC candidate targeting TROP-2. You may be aware that ADCs are one of the most exciting segments in oncology
and are the subjects of significant deal activity.
The pipeline also spans multiple stages of development,
including Akari’s Phase 3 program investigating nomacopan in pediatric HSCT-TMA, Peak Bio’s Phase 2-ready neutrophil elastase
inhibitor targeting alpha-1 antitrypsin deficiency, and Akari’s long-acting version of nomacopan (PAS-nomacopan) that is in the
final stages of pre-clinical development for geographic atrophy. Each of these programs has the potential to raise standards of care and
address unmet patient needs. We believe we have significant business development and licensing opportunities with broad potential to impact
patients and we intend to make these opportunities a strategic emphasis going forward.
This is an important step for our company, and I want to take this opportunity to thank you for your [investment/interest] in Akari and
your continued support.
Best regards,
Rachelle
Forward-looking Statements
This communication relates to the proposed transaction pursuant to
the terms of the Agreement and Plan of Merger (the “Merger Agreement”), by and among Akari Therapeutics, Plc, a public company
limited by shares incorporated in England and Wales (“Akari”), Pegasus Merger Sub, Inc., a Delaware corporation and a wholly-owned
subsidiary of Akari and Peak Bio, Inc. (“Peak Bio”) and includes express or implied forward-looking statements within the
meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Exchange Act, about the proposed transaction
between Peak Bio and Akari and the operations of the combined company that involve risks and uncertainties relating to future events and
the future performance of Akari and Peak Bio. Actual events or results may differ materially from these forward-looking statements. Words
such as “will,” “could,” “would,” “should,” “expect,” “plan,”
“anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,”
“potential,” “continue,” “future,” “opportunity” “will likely result,” “target,”
variations of such words, and similar expressions or negatives of these words are intended to identify such forward-looking statements,
although not all forward-looking statements contain these identifying words. Examples of such forward-looking statements include, but
are not limited to, express or implied statements regarding: the Merger (as defined in the Merger Agreement) and related matters, including,
but not limited to, satisfaction of closing conditions to the proposed transaction, prospective performance and opportunities with respect
to Akari or Peak Bio, post-closing operations and the outlook for the companies’ businesses; Akari’s, Peak Bio’s or
the combined company’s targets, plans, objectives or goals for future operations, including those related to Akari’s and Peak
Bio’s product candidates, research and development, product candidate introductions and product candidate approvals as well as cooperation
in relation thereto; projections of or targets for revenues, costs, income (or loss), earnings per share, capital expenditures, dividends,
capital structure, net financials and other financial measures; future economic performance, future actions and outcome of contingencies
such as legal proceedings; and the assumptions underlying or relating to such statements.
These statements are based on Akari’s and Peak Bio’s current
plans, estimates and projections. By their very nature, forward-looking statements involve inherent risks and uncertainties, both general
and specific. A number of important factors, including those described in this communication, could cause actual results to differ materially
from those contemplated in any forward-looking statements. Factors that may affect future results and may cause these forward-looking
statements to be inaccurate include, without limitation: uncertainties as to the timing for completion of the proposed transaction; uncertainties
as to Peak Bio’s and/or Akari’s ability to obtain the approval of Akari’s shareholders or Peak Bio’s stockholders
required to consummate the proposed transaction; the possibility that competing offers will be made by third parties; the occurrence
of events that may give rise to a right of one or both of Akari and Peak Bio to terminate the Merger Agreement; the possibility that various
closing conditions for the proposed transaction may not be satisfied or waived on a timely basis or at all, including the possibility
that a governmental entity may prohibit, delay, or refuse to grant approval, if required, for the consummation of the proposed transaction
(or only grant approval subject to adverse conditions or limitations); the difficulty of predicting the timing or outcome of consents
or regulatory approvals or actions, if any; the possibility that the proposed transaction may not be completed in the time frame expected
by Akari and Peak Bio, or at all; the risk that Akari and Peak Bio may not realize the anticipated benefits of the proposed transaction
in the time frame expected, or at all; the effects of the proposed transaction on relationships with Akari’s or Peak Bio’s
employees, business or collaboration partners or governmental entities; the ability to retain and hire key personnel; potential adverse
reactions or changes to business relationships resulting from the announcement or completion of the proposed transaction; significant
or unexpected costs, charges or expenses resulting from the proposed transaction; the potential impact of unforeseen liabilities, future
capital expenditures, revenues, costs, expenses, earnings, synergies, economic performance, indebtedness, financial condition and losses
on the future prospects, business and management strategies for the management, expansion and growth of the combined business after the
consummation of the proposed transaction; potential negative effects related to this announcement or the consummation of the proposed
transaction on the market price of Akari’s American Depositary Shares or Peak Bio’s common stock and/or Akari’s or Peak
Bio’s operating or financial results; uncertainties as to the long-term value of Akari’s American Depositary Shares (and the
ordinary shares represented thereby), including the dilution caused by Akari’s issuance of additional American Depositary Shares
(and the ordinary shares represented thereby) in connection with the proposed transaction; unknown liabilities related to Akari or Peak
Bio; the nature, cost and outcome of any litigation and other legal proceedings involving Akari, Peak Bio or their respective directors,
including any legal proceedings related to the proposed transaction; risks related to global as well as local political and economic conditions,
including interest rate and currency exchange rate fluctuations; potential delays or failures related to research and/or development of
Akari’s or Peak Bio’s programs or product candidates; risks related to any loss of Akari’s or Peak Bio’s patents
or other intellectual property rights; any interruptions of the supply chain for raw materials or manufacturing for Akari or Peak Bio’s
product candidates, the nature, timing, cost and possible success and therapeutic applications of product candidates being developed by
Akari, Peak Bio and/or their respective collaborators or licensees; the extent to which the results from the research and development
programs conducted by Akari, Peak Bio, and/or their respective collaborators or licensees may be replicated in other studies and/or lead
to advancement of product candidates to clinical trials, therapeutic applications, or regulatory approval; uncertainty of the utilization,
market acceptance, and commercial success of Akari’s or Peak Bio’s product candidates, and the impact of studies (whether
conducted by Akari, Peak Bio or others and whether mandated or voluntary) on any of the foregoing; unexpected breaches or terminations
with respect to Akari’s or Peak Bios’s material contracts or arrangements; risks related to competition for Akari’s
or Peak Bio’s product candidates; Akari’s or Peak Bio’s ability to successfully develop or commercialize Akari’s
or Peak Bio’s product candidates; Akari’s, Peak Bio’s, and their collaborators’ abilities to continue to conduct
current and future developmental, preclinical and clinical programs; potential exposure to legal proceedings and investigations; risks
related to changes in governmental laws and related interpretation thereof, including on reimbursement, intellectual property protection
and regulatory controls on testing, approval, manufacturing, development or commercialization of any of Akari’s or Peak Bio’s
product candidates; unexpected increase in costs and expenses with respect to the potential transaction or Akari’s or Peak Bio’s
business or operations; and risks and uncertainties related to epidemics, pandemics or other public health crises and their impact on
Akari’s and Peak Bio’s respective businesses, operations, supply chain, patient enrollment and retention, preclinical and
clinical trials, strategy, goals and anticipated milestones. While the foregoing list of factors presented here is considered representative,
no list should be considered to be a complete statement of all potential risks and uncertainties. There can be no assurance that the proposed
transaction or any other transaction described above will in fact be consummated in the manner described or at all. A more complete description
of these and other material risks can be found in Akari’s and Peak Bios’s respective filings with the U.S. Securities and
Exchange Commission (the “SEC”), including each of their Annual Reports on Form 20-F and 10-K, respectively, for the year
ended December 31, 2022, subsequent periodic reports, and other documents that may be filed from time to time with the SEC. These risks,
as well as other risks associated with the proposed transaction, will be more fully discussed in the joint proxy statement/prospectus
that will be included in the registration statement on Form S-4 that will be filed with the SEC in connection with the proposed transaction,
which joint proxy statement/prospectus will be mailed or otherwise disseminated to Akari’s shareholders and Peak Bio’s stockholders
when it becomes available.
Any forward-looking statements speak only as of the date of this communication
and are made based on the current beliefs and judgments of Akari’s and Peak Bio’s management, and the reader is cautioned
not to rely on any forward-looking statements made by Akari or Peak Bio. Unless required by law, neither Akari nor Peak Bio is under no
duty and undertakes no obligation to update or revise any forward-looking statement after the distribution of this document, including
without limitation any financial projection or guidance, whether as a result of new information, future events or otherwise.
No Offer or Solicitation
This communication is not intended to and shall not constitute an offer
to subscribe for, buy or sell or the solicitation of an offer to subscribe for, buy or sell any securities, or a solicitation of any vote
or approval, nor shall there be any sale of, or offer to sell or buy, securities in any jurisdiction in which such offer, solicitation
or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. This communication
is for informational purposes only. No offering of securities shall be made, except by means of a prospectus meeting the requirements
of Section 10 of the U.S. Securities Act of 1933, as amended, and otherwise in accordance with applicable law.
Additional Information and Where to Find It
In connection with the proposed transaction, Akari and Peak Bio expect
to file with the SEC a Registration Statement on Form S-4. The Registration Statement on Form S-4 will include a prospectus of Akari and
a joint proxy statement of Akari and Peak Bio, and each party may also file other documents regarding the proposed transaction with the
SEC. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ CAREFULLY THE REGISTRATION STATEMENT ON FORM S-4, JOINT PROXY STATEMENT/PROSPECTUS
AND OTHER RELEVANT DOCUMENTS FILED OR WILL BE FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS THERETO AND ANY DOCUMENTS INCORPORATED
BY REFERENCE THEREIN, IN THEIR ENTIRETY IF AND WHEN THEY BECOME AVAILABLE BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT
THE PROPOSED TRANSACTION, RELATED MATTERS AND THE PARTIES TO THE PROPOSED TRANSACTION.
You may obtain a free copy of the Registration Statement on Form S-4,
joint proxy statement/prospectus and other relevant documents (if and when they become available) that are or will be filed with the SEC
for free at the SEC’s website at www.sec.gov. Copies of the documents filed with the SEC by Akari will be available free of charge
on Akari’s website at http://investor.akaritx.com/ or by contacting Akari’s Investor Relations Department at http://investor.akaritx.com/investor-resources/contact-us.
Copies of the documents filed with the SEC by Peak Bio will be available free of charge on Peak Bio’s website at https://peak-bio.com/investors
or by contacting Peak Bio’s Investor Relations Department at https://peak-bio.com/contact.
Participants in the Solicitation
Akari, Peak Bio and their respective directors and executive officers
and other members of management and employees may be deemed to be participants in the solicitation of proxies in respect of the proposed
transaction. Information about the directors and executive officers of Akari, including a description of their direct or indirect interests,
by security holdings or otherwise, is set forth in Akari’s Annual Report on Form 20-F for the year ended December 31, 2022 filed
with the SEC on May 1, 2023, subsequent quarterly and current reports on Form 10-Q and -K, respectively, and other documents that may
be filed from time to time with the SEC. Information about the directors and executive officers of Peak Bio, including a description
of their direct or indirect interests, by security holdings or otherwise, is set forth in Peak Bio’s proxy statement for its 2022
Special Meeting of Stockholders, which was filed with the SEC on October 19, 2022, the Annual Report on Form 10-K for the year ended
December 31, 2022 filed with the SEC on June 29, 2023, subsequent quarterly and current reports on Form 10-Q and Form 8-K, respectively,
and other documents that may be filed from time to time with the SEC. Other information regarding the participants in the proxy solicitations
and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the joint proxy statement/prospectus
included in the Registration Statement on Form S-4 and other relevant materials to be filed with the SEC regarding the proposed transaction
when such materials become available. Security holders, potential investors and other readers should read the joint proxy statement/prospectus,
included in the Registration Statement on Form S-4 carefully when it becomes available before making any voting or investment decision.
You may obtain free copies of these documents from Akari or Peak Bio using the sources indicated above.
Exhibit 99.4

Akari Therapeutics Company Presentation March 2024

2 This communication relates to the proposed transaction pursuant to the terms of the Agreement and Plan of Merger (the “Merger Ag reement”), by and among Akari Therapeutics, Plc, a public company limited by shares incorporated in England and Wales (“ Akari ”),, Pegasus Merger Sub, Inc., a Delaware corporation and a wholly - owned subsidiary of Akari and Peak Bio, Inc. (“Peak Bio”) and includes express or implied forward - looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Exchange Act, about the proposed transaction between Peak Bio and Akari and the operations of the combined company that involve risks and uncertainties relating to future events and the future perf or mance of Akari and Peak Bio. Actual events or results may differ materially from these forward - looking statements. Words such as “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipat e,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “future,” “opportunity” “will likely resu lt, ” “target,” variations of such words, and similar expressions or negatives of these words are intended to identify such forward - looking statements, although not all f orward - looking statements contain these identifying words. Examples of such forward - looking statements include, but are not limi ted to, express or implied statements regarding: the Merger (as defined in the Merger Agreement) and related matters, including, but not limited to , satisfaction of closing conditions to the proposed transaction, prospective performance and opportunities with respect to Akari or Peak Bio, post - closing operations and the outlook for the companies’ businesses; Akari’s , Peak Bio’s or the combined company’s targets, plans, objectives or goals for future operations, including those related to Akari’s and Peak Bio’s product candidates, research and development, product candidate introductions and product candidate approvals as well as cooperation in relation thereto; projections of or targets fo r revenues, costs, income (or loss), earnings per share, capital expenditures, dividends, capital structure, net financials a nd other financial measures; future economic performance, future actions and outcome of contingencies such as legal proceedings; and the assumptions under lyi ng or relating to such statements. These statements are based on Akari’s and Peak Bio’s current plans, estimates and projections. By their very nature, forward - looking statements involve inherent risk s and uncertainties, both general and specific. A number of important factors, including those described in this communication, could cause actual results to differ materially from those contemplated in any forward - looking statements. F actors that may affect future results and may cause these forward - looking statements to be inaccurate include, without limitatio n: uncertainties as to the timing for completion of the proposed transaction; uncertainties as to Peak Bio’s and/or Akari’s ability to obtain the approval of Akari’s shareholders or Peak Bio’s stockholders required to consummate the proposed transaction; the possibility that competing offer s will be made by third parties; the occurrence of events that may give rise to a right of one or both of Akari and Peak Bio to terminate the Merger Agreement; the possibility that various closing conditions for the proposed transaction ma y not be satisfied or waived on a timely basis or at all, including the possibility that a governmental entity may prohibit, delay, or refuse to grant approval, if required, f or the consummation of the proposed transaction (or only grant approval subject to adverse conditions or limitations); the diffi cul ty of predicting the timing or outcome of consents or regulatory approvals or actions, if any; the possibility that the proposed transaction may not be comp let ed in the time frame expected by Akari and Peak Bio, or at all; the risk that Akari and Peak Bio may not realize the anticipated benefits of the proposed transaction in the time frame expected, or at all; the effects of the proposed transaction on relationships with Akari’s or Peak Bio’s employees, business or collaboration partners or governmental entities; the ability to retain and hire key pers on nel; potential adverse reactions or changes to business relationships resulting from the announcement or completion of the proposed transaction; sig nif icant or unexpected costs, charges or expenses resulting from the proposed transaction; the potential impact of unforeseen li abi lities, future capital expenditures, revenues, costs, expenses, earnings, synergies, economic performance, indebtedness, financial condition an d losses on the future prospects, business and management strategies for the management, expansion and growth of the combined bu siness after the consummation of the proposed transaction; potential negative effects related to this announcement or the consummati on of the proposed transaction on the market price of Akari’s American Depositary Shares or Peak Bio’s common stock and/or Akari’s or Peak Bio’s operating or financial results; uncertainties as to the long - term value of Akari’s American Depositary Shares (and the ordinary shares represented thereby), including the dilution caused by Akari’s issuance of additional American Depositary Shares (and the ordinary shares represented thereby) in connection with the proposed transaction; unknown liabilities related to Akari or Peak Bio; the nature, cost and outcome of any litigation and other legal proceedings involving Akari , Peak Bio or their respective directors, including any legal proceedings related to the proposed transaction; risks related to global as well as local political and economic conditions, including in ter est rate and currency exchange rate fluctuations; potential delays or failures related to research and/or development of Akari’s or Peak Bio’s programs or product candidates; risks related to any loss of Akari’s or Peak Bio’s patents or other intellectual property rights; any interruptions of the supply chain for raw materials or manuf ac turing for Akari or Peak Bio’s product candidates, the nature, timing, cost and possible success and therapeutic applications of product candidates being developed by Akari , Peak Bio and/or their respective collaborators or licensees; the extent to which the results from the research and developm ent programs conducted by Akari , Peak Bio, and/or their respective collaborators or licensees may be replicated in other studies and/or lead to advancement of product candidates to clinical tr ial s, therapeutic applications, or regulatory approval; uncertainty of the utilization, market acceptance, and commercial succes s o f Akari’s or Peak Bio’s product candidates, and the impact of studies (whether conducted by Akari , Peak Bio or others and whether mandated or voluntary) on any of the foregoing; unexpected breaches or terminations with res pec t to Akari’s or Peak Bios’s material contracts or arrangements; risks related to competition for Akari’s or Peak Bio’s product candidates; Akari’s or Peak Bio’s ability to successfully develop or commercialize Akari’s or Peak Bio’s product candidates; Akari’s , Peak Bio’s, and their collaborators’ abilities to continue to conduct current and future developmental, preclinical and clinical programs; potential exposure to legal proceedings and investigations; risks related t o c hanges in governmental laws and related interpretation thereof, including on reimbursement, intellectual property protection and regulatory controls on testing, approval, manufacturing, development or commercialization of any of Akari’s or Peak Bio’s product candidates; unexpected increase in costs and expenses with respect to the potential transaction or Akari’s or Peak Bio’s business or operations; and risks and uncertainties related to epidemics, pandemics or other public health crises and their impact on Akari’s and Peak Bio’s respective businesses, operations, supply chain, patient enrollment and retention, preclinical and clinical tr ia ls, strategy, goals and anticipated milestones. While the foregoing list of factors presented here is considered representative, no list should be considered to be a complet e s tatement of all potential risks and uncertainties. There can be no assurance that the proposed transaction or any other trans act ion described above will in fact be consummated in the manner described or at all. A more complete description of these and other material risks can be found in Akari’s and Peak Bios’s respective filings with the U.S. Securities and Exchange Commission (the “SEC”), including each of their Annual Reports on Form 20 - F and 10 - K, respectively, for the year ended December 31, 2022, subsequent periodic reports, and other documents that may be filed from time to time with the SEC. These risks, as well as other risks associated with the proposed tr ansaction, will be more fully discussed in the joint proxy statement/prospectus that will be included in the registration statement on Form S - 4 that will be filed with the SEC in connection with the proposed transaction, which joint proxy statement/prospectus will be m ail ed or otherwise disseminated to Akari’s shareholders and Peak Bio’s stockholders when it becomes available. Any forward - looking statements speak only as of the date of this communication and are made based on the current beliefs and jud gments of Akari’s and Peak Bio’s management, and the reader is cautioned not to rely on any forward - looking statements made by Akari or Peak Bio. Unless required by law, neither Akari nor Peak Bio is under no duty and undertakes no obligation to update or revise any forward - looking statement after the distribu tion of this document, including without limitation any financial projection or guidance, whether as a result of new information, future events or otherwise. Forward - Looking Statements

3 Additional Disclaimers No Offer or Solicitation This communication is not intended to and shall not constitute an offer to subscribe for, buy or sell or the solicitation of an offer to subscribe for, buy or sell any securities, or a solicitation of any vote or approval, nor shall there be any sale of , or offer to sell or buy, securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to reg istration or qualification under the securities laws of any such jurisdiction. This communication is for informational purposes only. No offering of securities shall be made, except by means of a prospectus meeting the requirements of Section 1 0 o f the U.S. Securities Act of 1933, as amended, and otherwise in accordance with applicable law. Additional Information and Where to Find It In connection with the proposed transaction, Akari and Peak Bio expect to file with the SEC a Registration Statement on Form S - 4. The Registration Statement on Form S - 4 will incl ude a prospectus of Akari and a joint proxy statement of Akari and Peak Bio, and each party may also file other documents regarding the proposed transaction with the SEC. INVESTORS AND SEC UR ITY HOLDERS ARE URGED TO READ CAREFULLY THE REGISTRATION STATEMENT ON FORM S - 4, JOINT PROXY STATEMENT/PROSPECTUS AND OTHER RELEVANT DOCUMENTS FILED OR WILL BE FILED WITH THE SEC, AS WELL AS ANY AMEN DMENTS OR SUPPLEMENTS THERETO AND ANY DOCUMENTS INCORPORATED BY REFERENCE THEREIN, IN THEIR ENTIRETY IF AND WHEN THEY BECOME AVAILABLE BECAUSE THEY CONTAIN OR WILL CONTAIN I MPO RTANT INFORMATION ABOUT THE PROPOSED TRANSACTION, RELATED MATTERS AND THE PARTIES TO THE PROPOSED TRANSACTION. You may obtain a free copy of the Registration Statement on Form S - 4, joint proxy statement/prospectus and other relevant docume nts (if and when they become available) that are or will be filed with the SEC for free at the SEC’s website at www.sec.gov. Copies of the documents filed with the SEC by Akari will be available free of charge on Akari’s website at http://investor.akaritx.com/ or by contacting Akari’s Investor Relations Department at http://investor.akaritx.com/investor - resources/contact - us. Copies of the documents filed with the SEC by Peak Bio will be availa ble free of charge on Peak Bio’s website at https://peak - bio.com/investors or by contacting Peak Bio’s Investor Relations Department at https://peak - bio.com/contact. Participants in the Solicitation Akari , Peak Bio and their respective directors and executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in respect of the proposed transaction. Information about the directors and executive officers of Akari , including a description of their direct or indirect interests, by security holdings or otherwise, is set forth in Akari’s Annual Report on Form 20 - F for the year ended December 31, 2022 filed with the SEC on May 1, 2023, subsequent quarterly and current reports on Form 10 - Q and - K, respectively, and other documents tha t may be filed from time to time with the SEC. Information about the directors and executive officers of Peak Bio, including a description of their direct or indirect interests, by security holdings or otherwise, is set forth in P eak Bio’s proxy statement for its 2022 Special Meeting of Stockholders, which was filed with the SEC on October 19, 2022, the Annual Report on Form 10 - K for the year ended December 31, 2022 filed with the SEC on June 29, 2023, subsequent quarterly an d current reports on Form 10 - Q and Form 8 - K, respectively, and other documents that may be filed from time to time with the SEC. Other information regarding the participants in the proxy solicitations and a description of their di rect and indirect interests, by security holdings or otherwise, will be contained in the joint proxy statement/prospectus included in the Registration Statement on Form S - 4 and other relevant materials to be filed with the SEC regarding the proposed transaction when such materials become available. Security holders, potential investors and other readers should read the joint proxy statement/prospectus, included in the Registration Statement on Form S - 4 carefully when it becomes a vailable before making any voting or investment decision. You may obtain free copies of these documents from Akari or Peak Bio using the sources indicated above.

4 DEFINITIVE AGREEMENT FOR MERGER OF EQUALS WITH PEAK BIO

Akari/Peak Bio Definitive Agreement/Merger of Equals 5 Akari Therapeutics and Peak Bio Announce Definitive Agreement to Merge as Equals Creating an Expanded Pipeline That Features a Novel Antibody Drug Conjugate (ADC) Toolkit Following closing, the combined company will have an expanded pipeline that contains multiple compelling assets spanning early and late development stages Key highlights of the merger include: o Peak Bio’s innovative antibody drug conjugate (ADC) toolkit with novel toxin and linker technology, expected to be the merged company’s lead asset: program includes a novel pre - clinical ADC candidate targeting TROP - 2 o Akari’s nomacopan, a bispecific recombinant inhibitor of complement C5 and leukotriene B4 (LTB4), in Phase 3 for pediatric hematopoietic stem cell transplant - related thrombotic microangiopathy (HSCT - TMA) o Akari’s long - acting version of nomacopan (PASylated - nomacopan) in the final stages of pre - clinical development for geographic atrophy (GA) with potential to address significant unmet needs o Peak Bio’s Phase 2 - ready neutrophil elastase inhibitor (NEI) targeting alpha - 1 antitrypsin deficiency (AATD); program licensed from Bayer Healthcare and is a 5th generation neutrophil elastase inhibitor (NEI) targeting the inflammatory aspects of AATD, a rare condition o Strategic emphasis on business development and licensing with broad potential impact on patients o Proven leadership with extensive strategic and operational experience

6 AKARI OVERVIEW
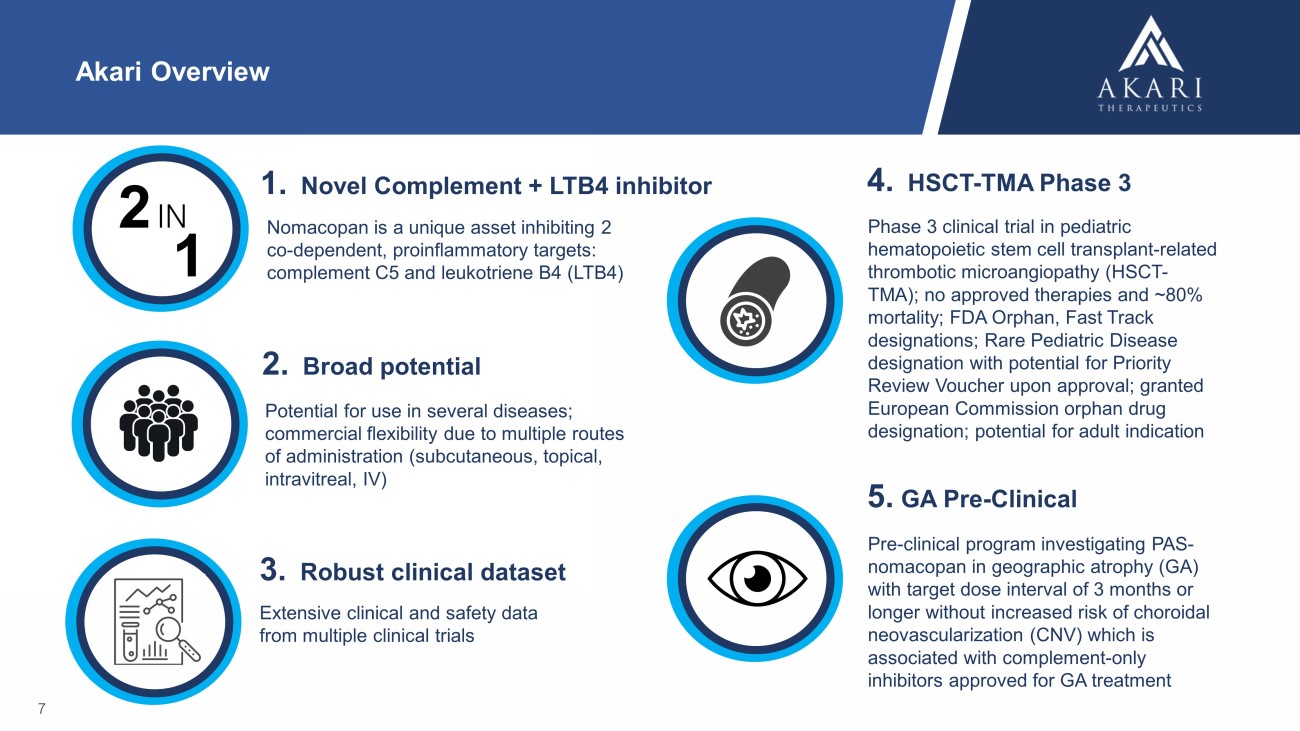
Akari Overview Nomacopan is a unique asset inhibiting 2 co - dependent, proinflammatory targets: complement C5 and leukotriene B4 (LTB4) 7 Potential for use in several diseases; commercial flexibility due to multiple routes of administration (subcutaneous, topical, intravitreal, IV) 1. Novel Complement + LTB4 inhibitor 2. Broad potential Extensive clinical and safety data from multiple clinical trials 3. Robust clinical dataset 2 IN 1 Phase 3 clinical trial in pediatric hematopoietic stem cell transplant - related thrombotic microangiopathy (HSCT - TMA); no approved therapies and ~80% mortality; FDA Orphan, Fast Track designations; Rare Pediatric Disease designation with potential for Priority Review Voucher upon approval; granted European Commission orphan drug designation; potential for adult indication 4. HSCT - TMA Phase 3 5. GA Pre - Clinical Pre - clinical program investigating PAS - nomacopan in geographic atrophy (GA) with target dose interval of 3 months or longer without increased risk of choroidal neovascularization (CNV) which is associated with complement - only inhibitors approved for GA treatment
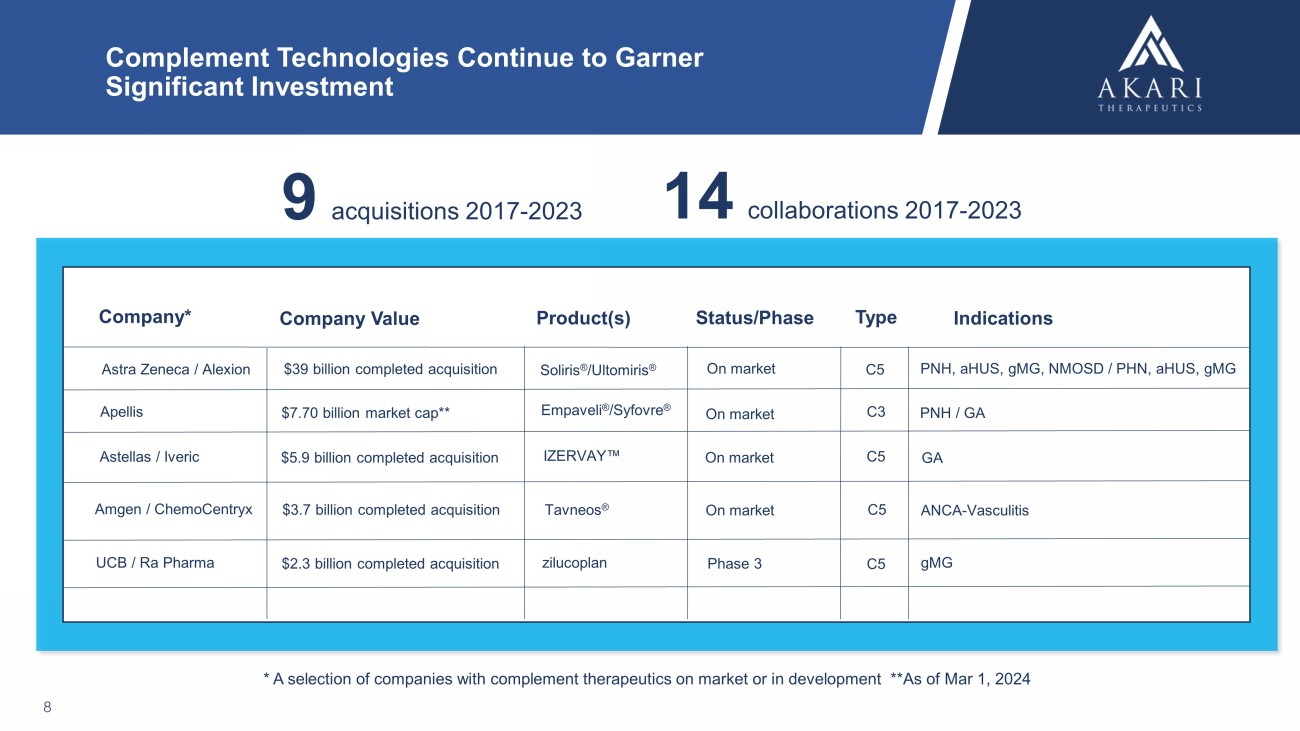
Complement Technologies Continue to Garner Significant Investment 8 Astra Zeneca / Alexion Company* Company Value Status/Phase Indications $39 billion completed acquisition Soliris ® / Ultomiris ® PNH, aHUS , gMG , NMOSD / PHN, aHUS , gMG Tavneos ® Amgen / ChemoCentryx ANCA - Vasculitis $7.70 billion market cap** Empaveli ® / Syfovre ® PNH / GA * A selection of companies with complement therapeutics on market or in development **As of Mar 1, 2024 Product(s) Type $3.7 billion completed acquisition C5 On market Apellis C3 On market Astellas / Iveric $5.9 billion completed acquisition C5 GA On market IZERVAY Œ C5 On market UCB / Ra Pharma $2.3 billion completed acquisition zilucoplan Phase 3 C5 gMG 9 acquisitions 2017 - 2023 14 collaborations 2017 - 2023
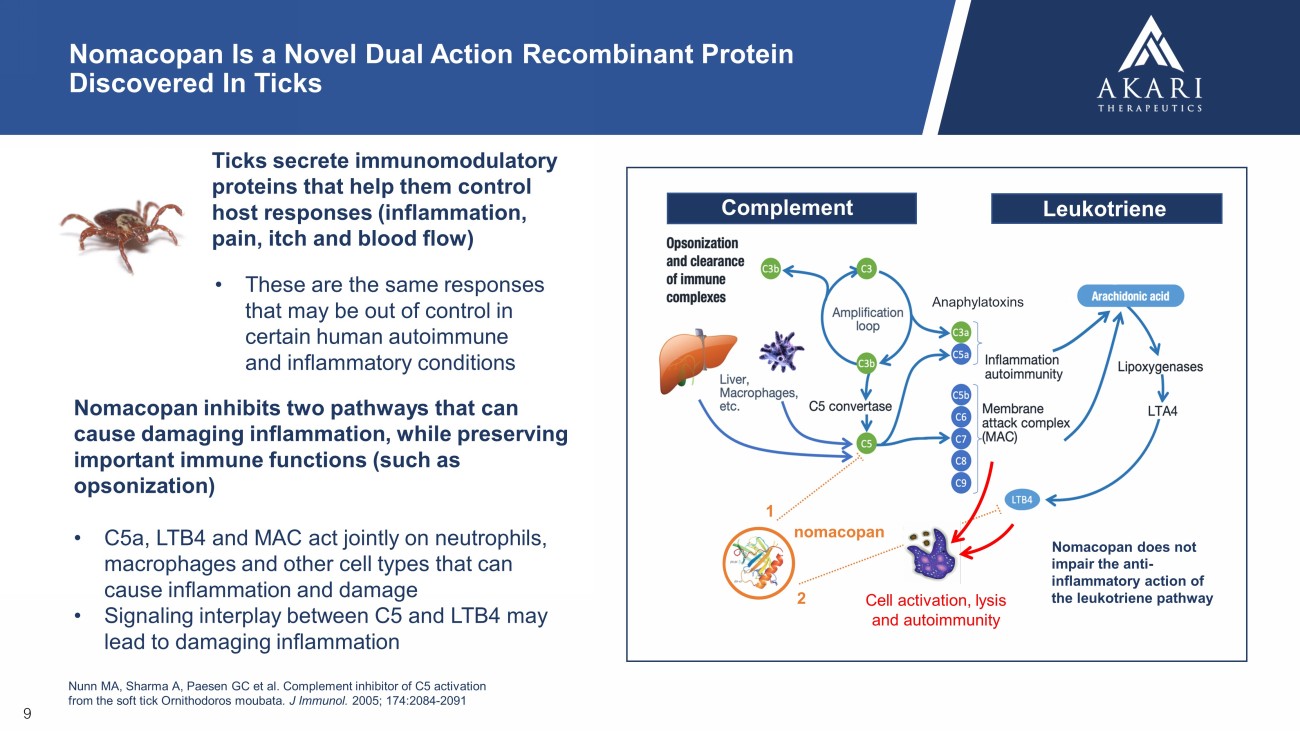
Nomacopan inhibits two pathways that can cause damaging inflammation, while preserving important immune functions (such as opsonization) • C5a, LTB4 and MAC act jointly on neutrophils, macrophages and other cell types that can cause inflammation and damage • Signaling interplay between C5 and LTB4 may lead to damaging inflammation Nunn MA, Sharma A, Paesen GC et al. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata . J Immunol. 2005; 174:2084 - 2091 Cell activation, lysis and autoimmunity nomacopan 1 2 Complement Leukotriene Anaphylatoxins Nomacopan does not impair the anti - inflammatory action of the leukotriene pathway 9 Nomacopan Is a Novel Dual Action Recombinant Protein Discovered In Ticks T icks secrete immunomodulatory proteins that help them control host responses (inflammation, pain, itch and blood flow) • These are the same responses that may be out of control in certain human autoimmune and inflammatory conditions
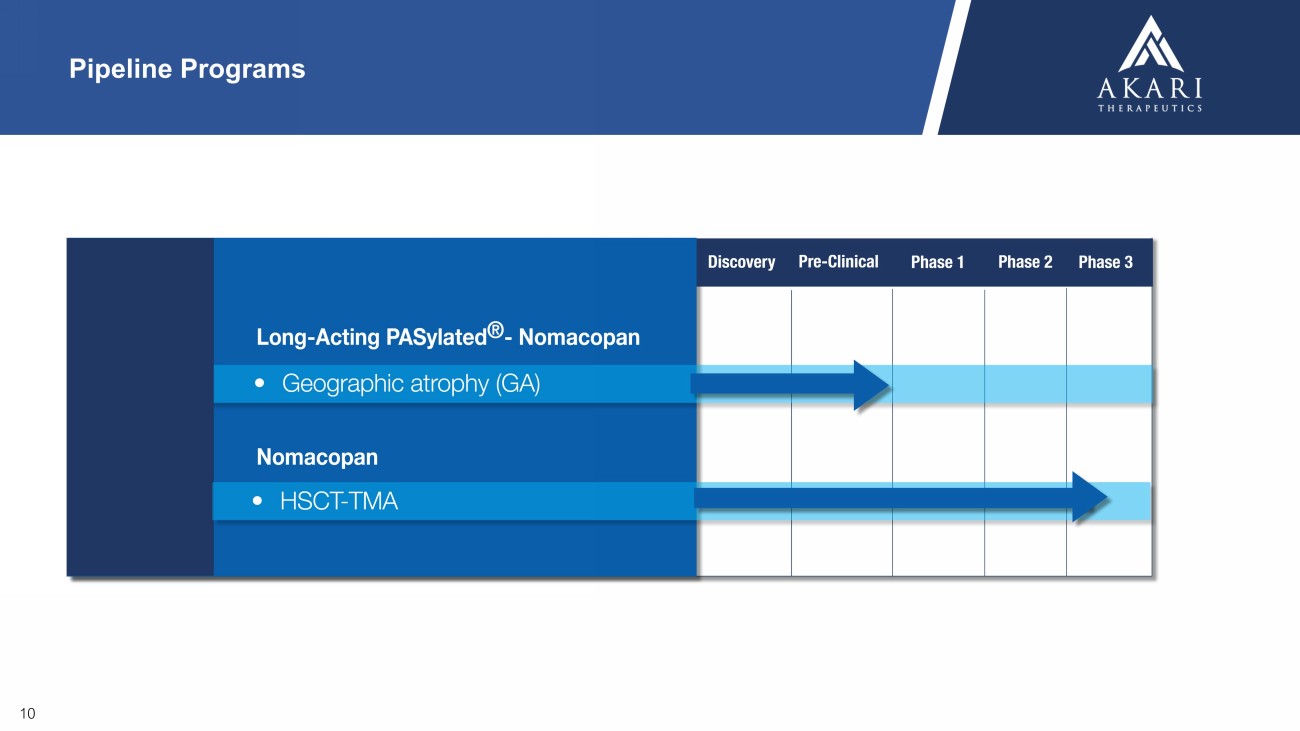
10 Pipeline Programs
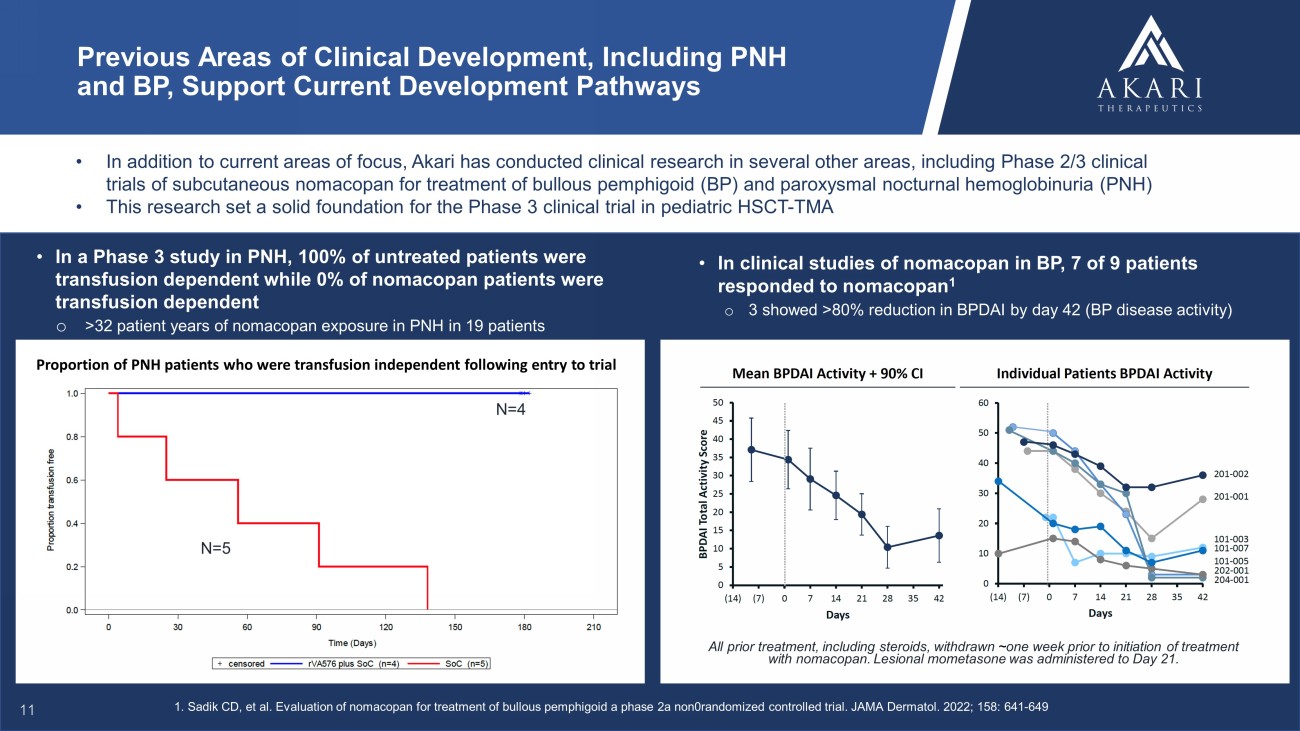
• In addition to current areas of focus, Akari has conducted clinical research in several other areas, including Phase 2/3 clin ica l trials of subcutaneous nomacopan for treatment of bullous pemphigoid (BP) and paroxysmal nocturnal hemoglobinuria (PNH) • This research set a solid foundation for the Phase 3 clinical trial in pediatric HSCT - TMA Previous Areas of Clinical Development, Including PNH and BP, Support Current Development Pathways • In clinical studies of nomacopan in BP, 7 of 9 patients responded to nomacopan 1 o 3 showed >80% reduction in BPDAI by day 42 (BP disease activity) All prior treatment, including steroids, withdrawn ~ one week prior to initiation of treatment with nomacopan. Lesional mometasone was administered to Day 21. • In a Phase 3 study in PNH, 100% of untreated patients were transfusion dependent while 0% of nomacopan patients were transfusion dependent o >32 patient years of nomacopan exposure in PNH in 19 patients Proportion of PNH patients who were transfusion independent following entry to trial 1. Sadik CD, et al. Evaluation of nomacopan for treatment of bullous pemphigoid a phase 2a non0randomized controlled trial. JAMA Dermatol. 2022; 158: 641 - 649 N=4 N=5 11

12 AKARI DEVELOPMENT PROGRAMS

13 NOMACOPAN IN HSCT - TMA
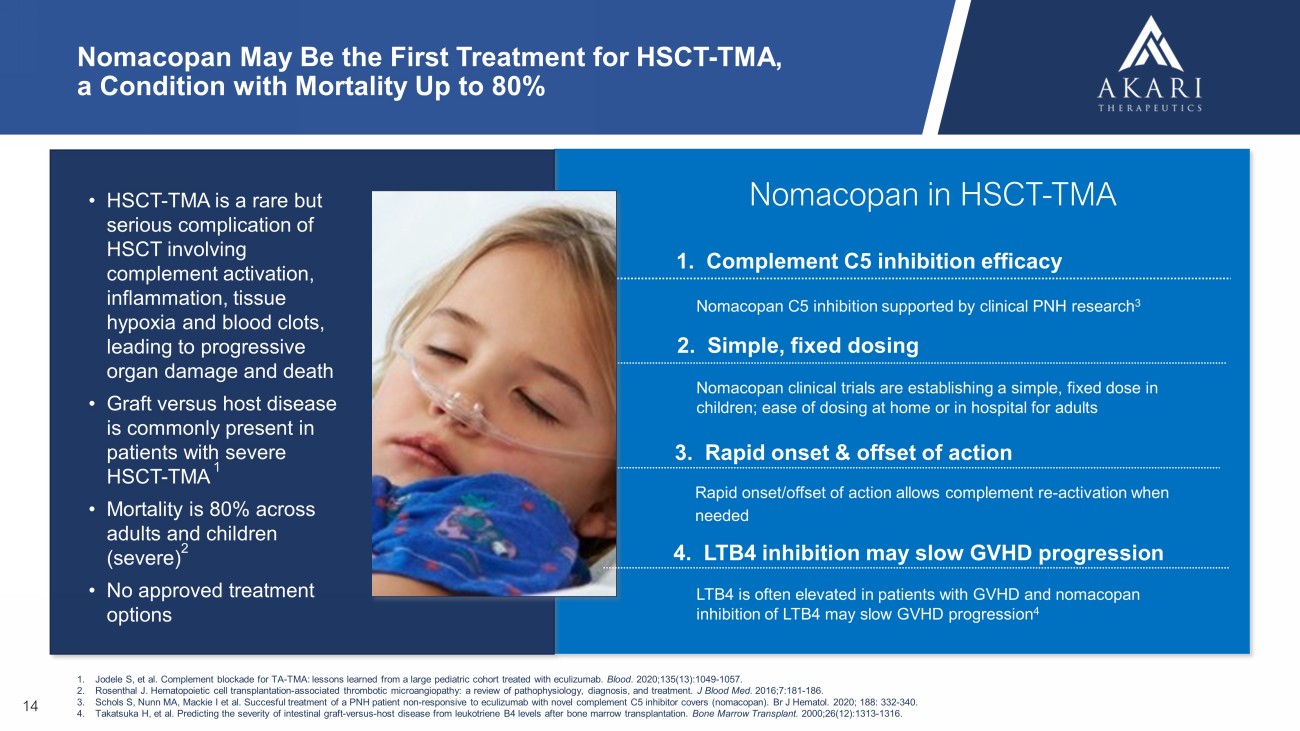
14 • HSCT - TMA is a rare but serious complication of HSCT involving complement activation, inflammation, tissue hypoxia and blood clots, leading to progressive organ damage and death • Graft versus host disease is commonly present in patients with severe HSCT - TMA 1 • Mortality is 80% across adults and children (severe) 2 • No approved treatment options Nomacopan May Be the First Treatment for HSCT - TMA, a Condition with Mortality Up to 80% 1. Complement C5 inhibition efficacy Nomacopan clinical trials are establishing a simple, fixed dose in children; ease of dosing at home or in hospital for adults LTB4 is often elevated in patients with GVHD and nomacopan inhibition of LTB4 may slow GVHD progression 4 1. Jodele S, et al. Complement blockade for TA - TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood . 2020;135(13):1049 - 1057. 2. Rosenthal J. Hematopoietic cell transplantation - associated thrombotic microangiopathy: a review of pathophysiology, diagnosis, a nd treatment. J Blood Med. 2016;7:181 - 186. 3. Schols S, Nunn MA, Mackie I et al. Succesful treatment of a PNH patient non - responsive to eculizumab with novel complement C5 inhibitor covers (nomacopan). Br J Hematol . 2020; 188: 332 - 340. 4. Takatsuka H, et al. Predicting the severity of intestinal graft - versus - host disease from leukotriene B4 levels after bone marrow transpla ntation. Bone Marrow Transplant. 2000;26(12):1313 - 1316. Nomacopan C5 inhibition supported by clinical PNH research 3 Nomacopan in HSCT - TMA 2. Simple, fixed dosing 3. Rapid onset & offset of action 4. LTB4 inhibition may slow GVHD progression Rapid onset/offset of action allows complement re - activation when needed
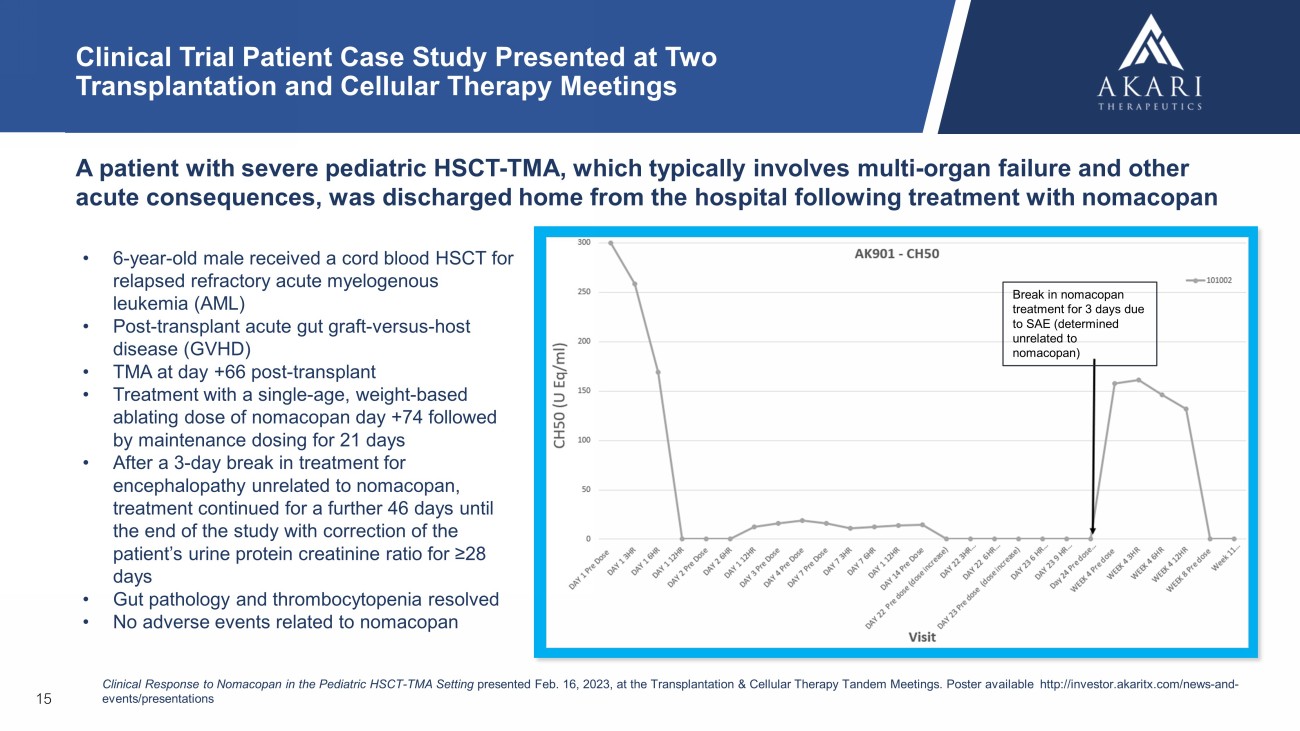
15 Clinical Trial Patient Case Study Presented at Two Transplantation and Cellular Therapy Meetings A patient with severe pediatric HSCT - TMA, which typically involves multi - organ failure and other acute consequences, was discharged home from the hospital following treatment with nomacopan • 6 - year - old male received a cord blood HSCT for relapsed refractory acute myelogenous leukemia (AML) • Post - transplant acute gut graft - versus - host disease (GVHD) • TMA at d ay +66 post - transplant • T reatment with a single - age, weight - based ablating dose of nomacopan day +74 followed by maintenance dosing for 21 days • After a 3 - day break in treatment for encephalopathy unrelated to nomacopan, treatment continued for a further 46 days until the end of the study with correction of the patient’s urine protein creatinine ratio for ≥28 days • Gut pathology and thrombocytopenia resolved • No adverse events related to nomacopan Clinical Response to Nomacopan in the Pediatric HSCT - TMA Setting presented Feb. 16, 2023, at the Transplantation & Cellular Therapy Tandem Meetings. P oster available http:// investor.akaritx.com /news - and - events/presentations Break in nomacopan treatment for 3 days due to SAE (determined unrelated to nomacopan)

16 GEOGRAPHIC ATROPHY (GA)

17 • Geographic atrophy (GA) manifests as a chronic progressive degeneration of the macula, which occurs during late - stage dry age - related macular degeneration (dAMD) and can lead to irreversible vision loss • Approximately 5 million people worldwide are affected, 1,2 with nearly 1 million in the U.S. 3 • The first treatments for GA have been approved by the FDA in 2023 References 1. Wong WL, et al. Global prevalence of age - related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta - analysis. Lancet Glob Health. 2014;2(2):e106 - e116. 2. Rudnicka AR, et al. Age and gender variations in age - related macular degeneration prevalence in populations of European ancestry: a meta - analysis. Ophthalmology. 2012;119(3):571 - 580. 3. Friedman DS, et al. Prevalence of age - related macular degeneration in the United States [published correction appears in Arch Ophthalmol . 2011 Sep;129(9):1188]. Arch Ophthalmol . 2004;122(4):564 - 572. Geographic Atrophy (GA)

KOL Insights on GA Treatment Landscape and Unmet Needs 18 “ ” Despite FDA approvals of the first treatments for GA, there are still significant unmet needs. It’s important that we reduce the frequency of therapy, which must be administered through intravitreal injection into the eye. In addition, treating geographic atrophy while preventing choroidal neovascularization from developing is another important unmet need. Elias Reichel, M.D. Professor of Ophthalmology Tufts University School of Medicine A key opinion leader event hosted by Akari discussed GA diagnosis, treatment, and significant unmet needs https:// lifescievents.com /event/ akari - event/

1. Complement C5 inhibition to slow GA progression Frequent needle injections into the back of the eye, a source of fear, discomfort and disruption for patients 3 ; potential for 4 or fewer injections with PAS - nomacopan each year LTB4 inhibition may prevent VEGF - A overexpression, a key driver of sight - threatening CNV, 4 a safety risk (treated with VEGF inhibitors) associated with complement - only inhibitors approved for GA treatment Efficacy of complement C3 and C5 inhibition slowing progression of GA lesions is well understood 1,2 PAS - nomacopan in GA 2. Fewer needle injections into the eye 3. LTB4 inhibition may reduce risk of CNV PAS - Nomacopan May Provide 3 Key Benefits: Complement Inhibition, Fewer Doses & LTB4 Inhibition to Address CNV Risk 19 References 1. Liao DS, et al., Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age - related macular degeneration - a randomised phase 2 trial. Opthalmology 2019; 127: 586 - 195. 2. Jaffe GJ, et al., C5 inhibitor avacincaptad peg for geographic atrophy due to age - related macular degeneration - a randomised pivotal phase 2/3 trial. Ophthalmology 2021; 128: 576 - 586. 3. McClard CK, et al. Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII): Development of a p ati ent - reported measure to assess treatment burden of repeat intravitreal injections. BMJ Open Ophthalmol . 2021;6(1):e000669. 4. Sasaki F, et al., Leukotriene B4 promotes neovascularisation and macrophage recruitment in murine wet - type AMD models. JCI Insight 2018; 3: e96902.
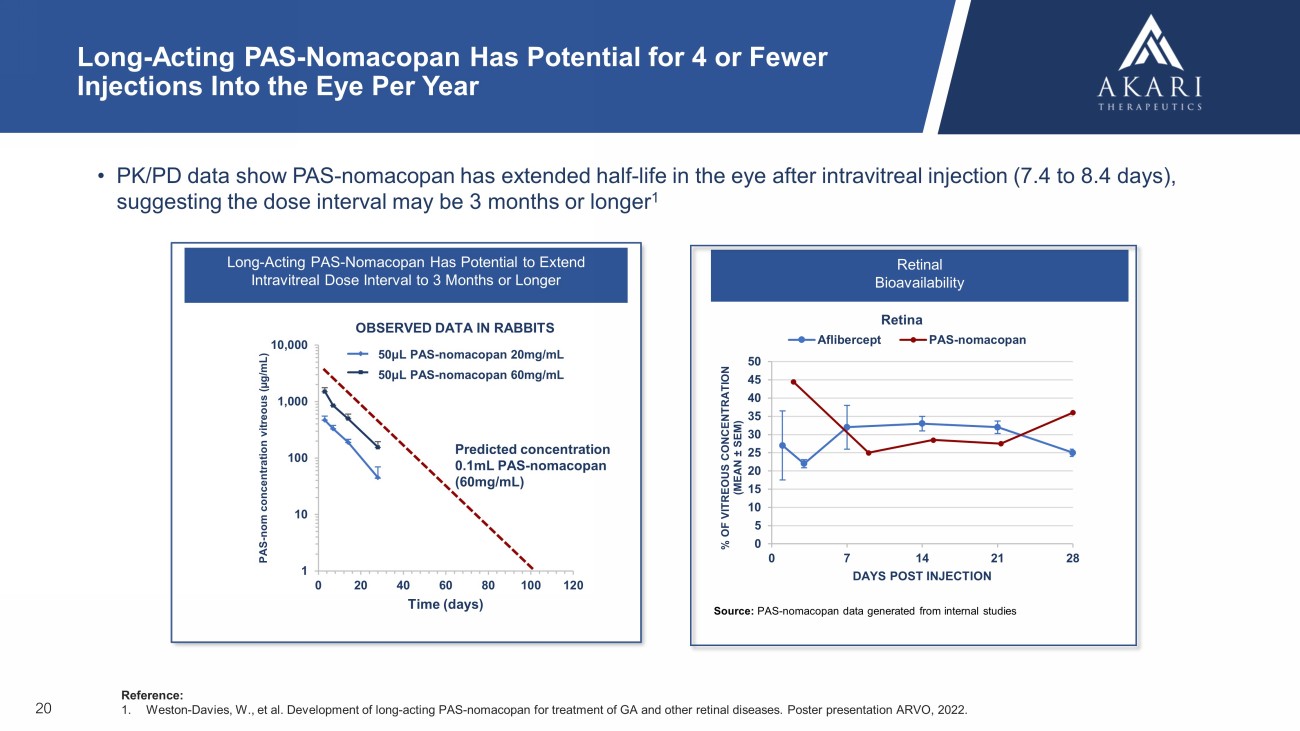
Long - Acting PAS - Nomacopan Has Potential to Extend Intravitreal Dose Interval to 3 Months or Longer 1 10 100 1,000 10,000 0 20 40 60 80 100 120 PAS - nom concentration vitreous ( µg/mL) Time (days) 0.05mL PAS600-nomacopan (20 mg/mL) 0.05mL PAS600-nomacopan (60 mg/mL) Long - Acting PAS - Nomacopan Has Potential for 4 or Fewer Injections Into the Eye Per Year • PK/PD data show PAS - nomacopan has extended half - life in the eye after intravitreal injection (7.4 to 8.4 days), suggesting the dose interval may be 3 months or longer 1 Reference: 1. Weston - Davies, W., et al. Development of long - acting PAS - nomacopan for treatment of GA and other retinal diseases. Poster presen tation ARVO, 2022. 20 OBSERVED DATA IN RABBITS Predicted concentration 0.1mL PAS - nomacopan (60mg/mL) 50μL PAS - nomacopan 20mg/mL 50μL PAS - nomacopan 60mg/mL Source: PAS - nomacopan data generated from internal studies Retinal Bioavailability Retina 0 5 10 15 20 25 30 35 40 45 50 0 7 14 21 28 % OF VITREOUS CONCENTRATION (MEAN ± SEM) DAYS POST INJECTION Aflibercept PAS-nomacopan
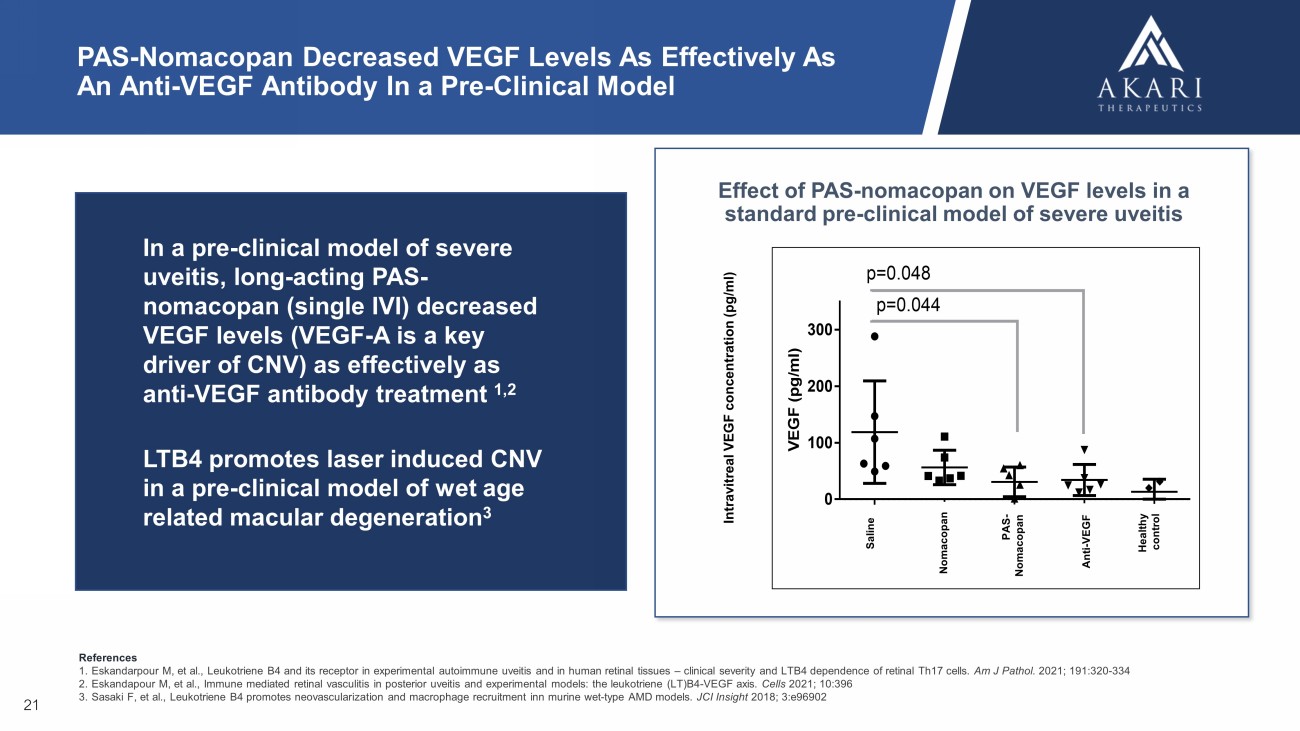
PAS - Nomacopan Decreased VEGF Levels As Effectively As An Anti - VEGF Antibody In a Pre - Clinical Model V E G F ( p g / m l ) S a l i n e n o m a P A S - n o m a a n t i - V E G F H e a l t h y c t 0 100 200 300 p=0.044 p=0.048 Effect of PAS - nomacopan on VEGF levels in a standard pre - clinical model of severe uveitis 21 In a pre - clinical model of severe uveitis, long - acting PAS - nomacopan (single IVI) decreased VEGF levels (VEGF - A is a key driver of CNV) as effectively as anti - VEGF antibody treatment 1,2 LTB4 promotes laser induced CNV in a pre - clinical model of wet age related macular degeneration 3 Intravitreal VEGF concentration ( pg /ml) Nomacopan Saline Anti - VEGF Healthy control PAS - Nomacopan References 1. Eskandarpour M, et al., Leukotriene B4 and its receptor in experimental autoimmune uveitis and in human retinal tissues – clinical severity and LTB4 dependence of retinal Th17 cells. Am J Pathol . 2021; 191:320 - 334 2. Eskandapour M, et al., Immune mediated retinal vasculitis in posterior uveitis and experimental models: the leukotriene (LT)B4 - VEGF axis. Cells 2021; 10:396 3. Sasaki F, et al., Leukotriene B4 promotes neovascularization and macrophage recruitment inn murine wet - type AMD models. JCI Insight 2018; 3:e96902

22 THANK YOU
Akari Therapeutics (NASDAQ:AKTX)
Historical Stock Chart
From Nov 2024 to Dec 2024
Akari Therapeutics (NASDAQ:AKTX)
Historical Stock Chart
From Dec 2023 to Dec 2024