Limbach Laboratory Network Group Selects Bruker´s MALDI Biotyper for Mass Spectrometry-Based Microbial Identification by Mol...
15 September 2009 - 9:00PM
Business Wire
Prior to the 61st Annual Meeting of the German Society for
Hygiene and Microbiology on September 20-23, 2009 in Goettingen,
Bruker Daltonics announces a new framework agreement with the
Limbach laboratory network, concerning microbial identification
based on MALDI-TOF mass spectrometry. According to the agreement,
Bruker Daltonics will be the exclusive provider of MALDI-TOF based
microbial identification solutions for the Limbach network of more
than 30 European clinical laboratories.
Since being introduced at the Labor Limbach headquarters in
Heidelberg in 2007, the MALDI Biotyper has been established
in a number of other laboratories in the Limbach association. The
MALDI Biotyper is currently in use in Limbach clinical
microbiology centers in Germany in Heidelberg, Koblenz, Hamburg,
Karlsruhe, Singen, Magdeburg and Nordhorn. After the signing of the
new framework agreement, Bruker has received additional orders for
MALDI Biotyper systems from the Limbach laboratories in
Ravensburg and in Freiburg, and an additional system was ordered
for Limbach site in Heidelberg. The Limbach laboratory network
intends to introduce this new molecular technology broadly as a
very fast routine method for microbial identification in additional
Limbach laboratories in Germany. Moreover, Bruker and Labor Limbach
intend to jointly introduce the MALDI Biotyper in Poland via
Limbach´s laboratories in Warsaw and Gdansk.
Dr. Anne-Marie Fahr, Head of Microbiology at Labor Limbach,
commented: “In contrast to previous introductions of other
technologies, in the case of the MALDI Biotyper we have seen
a very wide acceptance of this new approach nearly throughout our
entire network. Bruker is the leading supplier of MALDI-TOF mass
spectrometry with a global support and service network, and is thus
the reliable long-term partner that we were looking for in the
Limbach association.”
Dr. Wolfgang Pusch, Vice President for Clinical Research
Solutions & IVD at Bruker Daltonics, added: “This exclusive
framework agreement is a major step ahead into a new era of fast
molecular identification in clinical microbiology. The last two
years have shown the fundamental advantages of our MALDI
Biotyper approach for microbial identification, i.e. higher
specificity, faster time-to-result and lower consumables costs.
Moreover, the MALDI Biotyper has proven to be robust and
easy to use in demanding environments. We are very pleased that our
MALDI Biotyper fulfils the high expectations of leading
clinical laboratories like the Labor Limbach association, and we
look forward to presenting the IVD-CE marked MALDI Biotyper
at the upcoming 61st DGHM Annual Meeting.”
About the Bruker MALDI Biotyper
Bruker’s dedicated MALDI Biotyper solution enables
molecular identification, taxonomical classification or
dereplication of microorganisms like bacteria, yeasts and fungi.
Classification and identification of microorganisms is achieved
reliably and quickly using proteomic fingerprinting by
high-throughput MALDI-TOF mass spectrometry. Applications include
clinical routine microbial identification, environmental and
pharmaceutical analysis, taxonomical research, food and consumer
product processing and quality control, as well as marine
microbiology. Bruker’s robust MALDI Biotyper method requires
minimal sample preparation efforts and offers low consumables cost
per sample. The MALDI Biotyper is available in a
research-use-only version, as well as in an IVD-CE version
according to EU directive EC/98/79 in certain European countries.
For more information, please visit www.bdal.com/maldibiotyper.
ABOUT BRUKER DALTONICS
For more information about Bruker Daltonics and Bruker
Corporation (NASDAQ: BRKR), please visit www.bdal.com and
www.bruker.com.
CAUTIONARY STATEMENT
Any statements contained in this press release that do not
describe historical facts may constitute forward-looking statements
as that term is defined in the Private Securities Litigation Reform
Act of 1995. Any forward-looking statements contained herein are
based on current expectations, but are subject to a number of risks
and uncertainties. The factors that could cause actual future
results to differ materially from current expectations include, but
are not limited to, risks and uncertainties relating to adverse
changes in conditions in the global economy and volatility in the
capital markets, the integration of businesses we have acquired or
may acquire in the future, changing technologies, product
development and market acceptance of our products, the cost and
pricing of our products, manufacturing, competition, dependence on
collaborative partners and key suppliers, capital spending and
government funding policies, changes in governmental regulations,
intellectual property rights, litigation, and exposure to foreign
currency fluctuations. These and other factors are identified and
described in more detail in our filings with the SEC, including,
without limitation, our annual report on Form 10-K for the year
ended December 31, 2008, our most recent quarterly reports on Form
10-Q and our current reports on Form 8-K. We disclaim any intent or
obligation to update these forward-looking statements other than as
required by law.
Photos/Multimedia Gallery Available:
http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6048603&lang=en
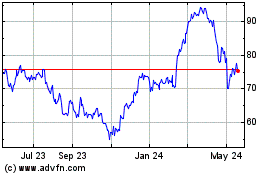
Bruker (NASDAQ:BRKR)
Historical Stock Chart
From Jun 2024 to Jul 2024
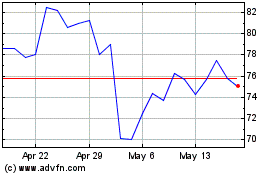
Bruker (NASDAQ:BRKR)
Historical Stock Chart
From Jul 2023 to Jul 2024