Eton Pharma Gets FDA Complete Response Letter for Dehydrated Alcohol Injection
28 June 2023 - 9:42PM
Dow Jones News
By Robb M. Stewart
Eton Pharmaceuticals said Wednesday the U.S. Food and Drug
Administration issued a complete response letter in response to its
new drug application for dehydrated alcohol injection for the
treatment of methanol poisoning that raised certain issued.
In premarket trading, the pharmaceutical company's shares
dropped 26% after ending Tuesday at $4.04, up 43% so far this
year.
Eton said the issues raised in the complete response letter
relate primarily to chemistry manufacturing and controls, and that
it believes all issues in the letter are addressable. The company
said it will develop a comprehensive action plan to address the
FDA's concerns.
"While we are disappointed with the FDA's decision, our
commercial business remains strong, and we are pleased that our
momentum in product revenue growth has continued," Chief Executive
Sean Brynjelsen said. "We expect to once again report record
product revenue in the second quarter of 2023."
Write to Robb M. Stewart at robb.stewart@wsj.com
(END) Dow Jones Newswires
June 28, 2023 07:27 ET (11:27 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
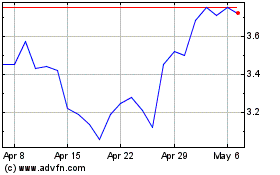
Eton Pharmaceuticals (NASDAQ:ETON)
Historical Stock Chart
From Jun 2024 to Jul 2024
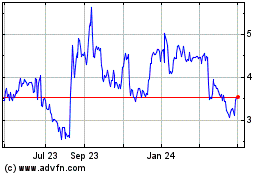
Eton Pharmaceuticals (NASDAQ:ETON)
Historical Stock Chart
From Jul 2023 to Jul 2024