0001622996falseNo--12-31FY20220.0012500000000001000000000.001600420006004200060042000P2YP2Y1one supplier00016229962022-01-012022-12-310001622996us-gaap:CostOfGoodsTotalMemberus-gaap:CustomerConcentrationRiskMember2022-01-012022-12-310001622996us-gaap:AccountsReceivableMemberacbm:CustomerSecondMember2021-01-012021-12-310001622996us-gaap:AccountsReceivableMemberacbm:CustomerFirstMember2021-01-012021-12-310001622996us-gaap:RevenueFromContractWithCustomerMemberacbm:CustomerFirstMember2021-01-012021-12-310001622996us-gaap:RevenueFromContractWithCustomerMemberacbm:CustomerSecondMember2021-01-012021-12-310001622996us-gaap:AccountsReceivableMemberacbm:TwoCustomerMember2021-01-012021-12-310001622996us-gaap:RevenueFromContractWithCustomerMemberacbm:TwoCustomerMember2021-01-012021-12-310001622996us-gaap:AccountsReceivableMemberacbm:CustomerSecondMember2022-01-012022-12-310001622996us-gaap:AccountsReceivableMemberacbm:CustomerFirstMember2022-01-012022-12-310001622996us-gaap:RevenueFromContractWithCustomerMemberacbm:CustomerFirstMember2022-01-012022-12-310001622996us-gaap:RevenueFromContractWithCustomerMemberacbm:CustomerSecondMember2022-01-012022-12-310001622996us-gaap:AccountsReceivableMemberacbm:TwoCustomerMember2022-01-012022-12-310001622996us-gaap:RevenueFromContractWithCustomerMemberacbm:TwoCustomerMember2022-01-012022-12-310001622996acbm:HongKongsMemberacbm:LeaseAgreementMember2021-11-012021-11-030001622996acbm:HongKongsMemberacbm:LeaseAgreementMember2019-12-012019-12-270001622996acbm:HongKongsMemberacbm:LeaseAgreementMember2022-01-012022-12-310001622996acbm:HongKongsMemberacbm:LeaseAgreementMember2022-12-310001622996srt:ChiefExecutiveOfficerMember2021-01-012021-12-310001622996srt:ChiefExecutiveOfficerMember2022-01-012022-12-310001622996acbm:CEOMember2021-01-012021-12-310001622996acbm:CEOMember2022-01-012022-12-310001622996acbm:TwoThousandTwentyEquityIncentivePlanMember2022-01-012022-12-310001622996acbm:TwoThousandTwentyEquityIncentivePlanMember2021-12-310001622996acbm:MayTwentyFiveTwentyTwentyOneMember2022-01-012022-12-310001622996acbm:AugustTwentyThreeTwentyTwentyOneMember2022-01-012022-12-310001622996acbm:MayTwentyFiveTwentyTwentyOneMember2021-12-310001622996acbm:AugustTwentyThreeTwentyTwentyOneMember2021-12-310001622996us-gaap:RetainedEarningsMember2022-12-310001622996acbm:DeferredStockCompensationMember2022-12-310001622996us-gaap:AdditionalPaidInCapitalMember2022-12-310001622996us-gaap:PreferredStockMember2022-12-310001622996us-gaap:CommonStockMember2022-12-310001622996us-gaap:RetainedEarningsMember2022-01-012022-12-310001622996acbm:DeferredStockCompensationMember2022-01-012022-12-310001622996us-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310001622996us-gaap:PreferredStockMember2022-01-012022-12-310001622996us-gaap:CommonStockMember2022-01-012022-12-310001622996us-gaap:RetainedEarningsMember2021-12-310001622996acbm:DeferredStockCompensationMember2021-12-310001622996us-gaap:AdditionalPaidInCapitalMember2021-12-310001622996us-gaap:PreferredStockMember2021-12-310001622996us-gaap:CommonStockMember2021-12-310001622996us-gaap:RetainedEarningsMember2021-01-012021-12-310001622996acbm:DeferredStockCompensationMember2021-01-012021-12-310001622996us-gaap:AdditionalPaidInCapitalMember2021-01-012021-12-310001622996us-gaap:PreferredStockMember2021-01-012021-12-310001622996us-gaap:CommonStockMember2021-01-012021-12-3100016229962020-12-310001622996us-gaap:RetainedEarningsMember2020-12-310001622996acbm:DeferredStockCompensationMember2020-12-310001622996us-gaap:AdditionalPaidInCapitalMember2020-12-310001622996us-gaap:PreferredStockMember2020-12-310001622996us-gaap:CommonStockMember2020-12-3100016229962021-01-012021-12-3100016229962021-12-3100016229962022-12-3100016229962023-07-1700016229962022-06-30iso4217:USDxbrli:sharesiso4217:USDxbrli:sharesxbrli:pureiso4217:HKD
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the year ended December 31, 2022
or
☐ | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 000-55643
ACRO BIOMEDICAL CO., LTD. |
(Exact name of registrant as specified in its charter) |
Nevada | | 47-1950356 |
(State or other jurisdiction of Incorporation or organization) | | (I.R.S. Employer Identification No.) |
12175 Visionary Way, Suite 1160; Fishers, Indiana 46038
(Address of principal executive offices)
Registrant’s telephone number, including area code: (317) 286-6788
Securities registered under Section 12(b) of the Exchange Act: None
Securities registered under Section 12(g) of the Exchange Act: Common Stock, par value $0.001 per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act.
Indicate by check mark whether the registrant (1) filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers in response to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendments to this From 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | Accelerated filer | ☐ |
Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| Emerging Growth Company | ☐ |
If an emerging growth company, indicate by a check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter: $127,678,500.
As of July 17, 2023, the registrant had 60,042,000 shares of common stock outstanding.
TABLE OF CONTENTS
As used in this report, the terms “we,” “us,” “our,” and words of like import, and the “Company” refers to Acro Biomedical Co., Ltd., unless the context indicates otherwise.
FORWARD LOOKING STATEMENTS
This report on Form 10-K contain “forward-looking statements,” within the meaning of the Private Securities Litigation Reform Act of 1995, all of which are subject to risks and uncertainties. Forward-looking statements can be identified by the use of words such as “expects,” “plans,” “will,” “forecasts,” “projects,” “intends,” “estimates,” and other words of similar meaning. One can identify them by the fact that they do not relate strictly to historical or current facts. These statements are likely to address our growth strategy, financial results and product and development programs. One must carefully consider any such statement and should understand that many factors could cause actual results to differ from our forward looking statements. These factors may include inaccurate assumptions and a broad variety of other risks and uncertainties, including some that are known and some that are not. No forward looking statement can be guaranteed and actual future results may vary materially.
These risks and uncertainties, many of which are beyond our control, include, and are not limited to:
| • | Our ability to develop any nutritional products based on cordyceps sinensis, of which our initial product is proposed to be a cordyceps-infused chicken feed, although as of the date of this annual report, we have not developed any such product, the engagement of consultants who worked on the proposed product having expired in May 2023 or will expire in August 2023 and whether we will continue on this product development; |
| | |
| • | If we are able to develop a product, our ability to any successful marketing program, which would require the establishment of a marketing organization, which we do not presently have, since all of our sales are made by our chief executive officer; |
| | |
| • | The extent to which there is a market for products such as our proposed products in the United States, and our ability to address any market which may develop; |
| | |
| • | If we are successful in developing a product and establishing a marketing organization, our ability to satisfy chicken farmers and distributors of chicken feed products that our cordyceps-infused chicken feed is safe for the health of the chickens and the health of the people who eat the chicken and the eggs and can be purchased at a reasonable cost; |
| | |
| • | Our ability to negotiate an agreement with animal feed distributors to carry our products in sufficient quantity to enable us to operate profitably; |
| | |
| • | Our ability to obtain the significant funding that we expect to require to develop and implement our proposed business plan; |
| | |
| • | Our ability to obtain any necessary regulatory approval necessary for us to market and sell our products and to comply with applicable regulatory requirements; |
| | |
| • | Our ability to generate revenue from the sale of our existing and any new products; |
| | |
| • | The effect of the COVID-19 pandemic on the market for our products, notwithstanding the elimination of China’s zero-COVID policy; |
| | |
| • | Our ability to generate a gross profit sufficient to cover our operating expenses; |
| | |
| • | Our ability to develop a customer base so that we are not dependent upon a small number of customers for all of our revenues, two of which accounted for 100% of revenue for 2022 and 91.7% of revenue for 2021; |
| | |
| • | Our ability to obtain products from suppliers on reasonable terms and in a timely manner; |
| | |
| • | Our ability to obtain the necessary financing for us to develop and market our products; |
| • | Our ability to obtain any necessary regulatory approval necessary for us to market and sell our products and to comply with applicable regulatory requirements; |
| | |
| • | Our ability to identify, hire and retain qualified executive, administrative, research and development, marketing and other personnel; |
| | |
| • | To the extent that we manufacture products, our ability to establish and maintain manufacturing facilities that comply with all applicable government regulations for any products which we may develop or manufacture; |
| | |
| • | Our ability to develop and maintain third-party manufacturing facilities for our product; |
| | |
| • | The ability and willingness of any third-party manufacturer that we engage for any products we may develop to meet our and our customers quality standards and delivery requirement; |
| | |
| • | Our ability to establish effective marketing and distribution arrangements; |
| | |
| • | Our ability and the ability of our suppliers to comply with government regulations relating to the manufacture, sale and marketing of our products; |
| | |
| • | Our ability to successfully develop a marketable product and market the product to a larger customer base that at present; |
| | |
| • | Any liability we may sustain as a result of adverse effects resulting from the use of the products we sell and any product recalls; |
| | |
| • | Any liability we may sustain as a result of either impurities or other problems relating to products we sell or adverse reactions to our products; |
| | |
| • | Our ability to protect any intellectual property we may develop; |
| | |
| • | Any infringement or claim of infringement which may be made if we develop our own products and our ability to defend and have the resources to defend any infringement action which may be brought against us; |
| | |
| • | The effects on our reputation or financial condition of any product recall, whether required or voluntary; |
| | |
| • | The effects of fluctuation of our sales on our operating results and on our ability to order products to meet the changing needs of the market; |
| | |
| • | The effects of any litigation which may arise concerning the use of our products; |
| | |
| • | The costs associated with defending and resolving potential legal claims, even if such claims are without merit; |
| | |
| • | The effects on our financial condition, operating results and reputation of any adverse reactions which users of our products may sustain; |
| | |
| • | Any liability we may sustain as a result of any impurities in any products we may sell; |
| | |
| • | The development of a market for our common stock, which is currently traded on the OTC Market Group’s Pink Limited Information tier, which includes companies that are delinquent in their filings with the SEC; |
| | |
| • | If a market in our common stock develops, actions by third parties to either sell or purchase our common stock in quantities that would have a significant effect on our stock price; |
| | |
| • | Risks generally associated with products that are considered nutritional supplements; |
| | |
| • | Current and future economic and political conditions including inflation and supply side issues, which have been acerbated by the Russian invasion of Ukraine; |
| | |
| • | The impact of changes in accounting rules on our financial statements; |
| | |
| • | Other assumptions described in this report; and |
| | |
| • | Other matters that are not within our control. |
Information regarding market and industry statistics contained in this report is included based on information available to us that we believe is accurate. It is generally based on industry and other publications that are not produced for purposes of securities offerings or economic analysis. We have not reviewed or included data from all sources. Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications and the additional uncertainties accompanying any estimates of future market size, revenue and market acceptance of products and services. We do not assume any obligation to update any forward-looking statement. As a result, you should not place undue reliance on these forward-looking statements.
The forward-looking statements in this annual report speak only as of the date of this annual report and you should not place undue reliance on any forward-looking statements. Forward-looking statements are subject to certain events, risks, and uncertainties that may be outside of our control. When considering forward-looking statements, you should carefully review the risks, uncertainties and other cautionary statements in this annual report as they identify certain important factors that could cause actual results to differ materially from those expressed in or implied by the forward-looking statements. These factors include, among others, the risks described under in this annual report, including those described under “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” as well as in other reports and documents we file with the SEC. We undertake no obligation to revise or publicly release the results of any revision to these forward-looking statements, except as required by law. Given these risks and uncertainties, you are cautioned not to place undue reliance on such forward-looking statements.
Information regarding market and industry statistics contained in this report is included based on information available to us that we believe is accurate. It is generally based on industry and other publications that are not produced for the purposes of securities offerings or economic analysis. We have not reviewed or included data from all sources. Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications and the additional uncertainties accompanying any estimates of future market size, revenue and market acceptance of products and services. We do not assume any obligation to update any forward-looking statement. As a result, you should not place undue reliance on these forward-looking statements.
Item 1. Business
Since January 30, 2017, following a change of control, we have been engaged in the business of developing and marketing nutritional products that promote wellness and a healthy lifestyle. Our business to date has involved the purchase of products from three suppliers in the Republic of China (Taiwan), one of which accounted for all of our purchases in the year ended December 31, 2022. We did not purchase any inventory in the year ended December 31, 2021. We sell products in bulk to companies who may use our products as ingredients in their products or sell the products they purchase from us to their own customers. All of our sales for the years ended December 31, 2022 and 2021 were made to two and three customer, respectively, two of which accounted for all of our sales for the year ended December 31, 2022 and 91.7% of our sales for the year ended December 31, 2021. Both of these customers generated less revenue in 2022 than they did in 2021. During the past several years, there have been a number of quarters in which we had no sales, including the second and third quarters of 2022 and the third quarter of 2021.
Substantially all of our sales to date have been sales of cordyceps related products. Cordyceps is a fungus that is used in traditional Chinese medicine. Cordyceps sinensis has been described as a medicine in old Chinese medical books and Tibetan medicine. It is a rare combination of a caterpillar and a fungus and found at altitudes above 4500m in Sikkim. We may also seek to market other products which we see as complimentary to our present products; however, we have not entered into negotiations with respect to the distribution of other products and we cannot assure you that we will be able to market any other products. In the quarter ended June 30, 2018, we sold metallothionein MT-3 elizer. We do not presently sell metallothionein MT-3 elizer, and we do not consider it part of our business.
We believe that our decrease in sales in 2022 from 2021 resulted substantially from COVID related issues that affected demand of our products. We cannot assure you that these factors will not continue to affect our ability to generate revenues in the future and, to the extent that any of these factors affects our ability to generate revenue, we may not be able to continue in business or that to the extent that COVID-because less of a factor, that our sales will increase,
Our Organization
We are a Nevada corporation incorporated on September 24, 2014 under the name Killer Waves Hawaii, Inc. On January 30, 2017, we changed our corporate name to Acro Biomedical Co., Ltd. Our address is 12175 Visionary Way, Suite 1160, Fishers, Indiana 46038, telephone (317) 286-6788. Our corporate website is http://acrobiomedicalco.com. Information on or derived from our website or any other website is not part of this annual report.
Our Business
To date, all of our sales have been made by our chief executive officer, who is our only employee. Although it has been part of our business plan to develop a marketing program for our existing products, we have not taken any active steps to implement such a program and, until either our revenue increases or we raise substantial funding for our operations it is not likely that we will be able to develop a marketing program and we will continue to be dependent upon our chief executive officer.
We currently intend to focus on marketing our products to companies in the Hong Kong and Republic of China markets. To date all of our sales have been sales of products which we purchased from our suppliers. In order to market products which would be based our or our customer’s specifications, we would need to provide our potential customers with evidence that we have the capacity to develop and manufacture the products that meet both the customer’s quality specifications and delivery requirements and comply with all government requirements. Since we anticipate that the products we sell will be marketed as over the counter health supplements, both the manufacturing facility, the product and the marketing materials would have to comply with all applicable government regulations in the country in which the products are sold.
We do not presently have either a marketing staff or any manufacturing facilities. In May and August 2021, we engaged consultants to work with us in various aspects of product development and marketing for a proposed product – cordyceps-based chicken feed – pursuant to consulting agreements. Because of our lack of funds, we compensated our consultants through the issuance of stock, primarily pursuant to our 2020 equity incentive plan. We issued 6,776,000 shares of common stock on May 25, 2021 and 5,506,000 shares of common stock on August 23, 2021 to consultants as stock grants pursuant to agreements with the consultants. The agreements provide for the consultants to perform services described in the contracts, which include research and development and marketing services for the two-year period commencing May 25, 2021 and August 23, 2021, respectively. The shares were valued at $31,424,800, based on the market price of the common stock on the respective dates of the agreements and are being amortized over the two-year terms of the consulting agreement. During 2022 and 2021, we recorded stock-based compensation of $15,712,400 and $7,651,417, respectively and at December 31, 2022 and 2021, we had deferred stock compensation of $8,060,983 and $23,773,383, respectively, which will be recognized over the balance of the terms of the agreement. The consulting agreements that were entered into in May 2021 have expired and they were not renewed. The consulting agreements that were entered into in August 2021 will expire in August 2023, and they will not be renewed or extended. However, we may engage one or more of the consultants to perform specific services.
The only product that was in development was our proposed cordyceps-infused chicken feed. As of the date of this annual report, we have not developed a marketable product and we cannot assure you that we can develop a product or, if we do develop a product, that we can or will generate any revenue from the product. We never produced a product for test marketing. The Company’s effort were devoted to work on the ratio of cordyceps to chicken feed in seeking to formulate a sample that could be used for test marketing.
We may continue the development work on this product, be we cannot give any assurance that we will continue the development work, or, if we continue the work, we will successfully develop a product or that the product can or will be successfully marketed. If we decide to continue this effort, we may hire one or more of the consultants; however, as of the date of this annual report, we have not made a decision with respect to engaging such individuals.
At present, we do not market products for retail sale. We may seek to develop our own proprietary products or we may have a supplier provide us with their products on a private label basis or we may sell a manufacturer’s brand. We cannot assure you that we can develop products, obtain products on a private-label basis or sell any products at retail. To the extent that we are selling products to consumers, whether directly through the Internet, or through retail outlets, we will need to comply with all applicable government regulations in each country in which we sell the products. In this connection, we are considering marketing products in the United States. If we sell products in the United States, we plan to sell products through distributors who sell other non-prescription products and who will be responsible for compliance with the applicable regulations of the United States Food and Drug Administration (the “FDA”). We also plan to market our products using the internet through a website that we plan to set up. As of the date of this annual report, we do not have any distribution agreements and we have not established a website. We cannot assure you that we will be successful in establishing any marketing program or establishing a website in the United States or elsewhere or that any website we establish will generate significant, if any, business.
Our initial product development work relates to the development of a cordyceps-infused chicken feed and the inspection, analysis and comparison of the nutritional components of eggs that are laid by chickens that are fed cordyceps-infused chicken feed. We had been formulating a marketing plan for cordyceps-based chicken feed. In order to be successful, we would need to satisfy chicken farmers that the use of cordyceps-infused check feed is safe, that there is improved nutrition in the chickens and the eggs and that the cost of the feed is reasonable and that there is a market for eggs laid by chickens that were feed with cordyceps-infused chicken feed. We cannot assure you that we will be successful in developing a marketable product or that we will generate any significant revenue from this product. We were not successful in developing a marketable product.
The activities in which our consultants were engaged included research relating to logistics for delivery and cost and pricing considerations, working with the chicken farmers who are participating in our project including preparing, maintaining and distributing the cordyceps egg packages for use by the farmers, conducting market research as to the buyer’s reactions and insights to the use of this product and market dynamics, designing packaging, developing seasonal product packaging, and seeking to communicate the benefits of the product as part of the package design, engaging in research as to competitor’s software, marketing, pricing and sales programs, as well as conducting marketing and research using the Internet, identifying, qualifying and contacting potential customers. The consultants also performed preliminary work for the possible international sales, including analyzing appropriate exhibitions and trade shows, assist in the proposed labeling of product to meet regulatory requirements, take steps to insure that ingredients are processed to meet the Taiwan Food and Drug Authority for health supplement certification registration, analyze products to gain assurance that that certified ingredients do not contain contaminants such as heavy metals, microbiological contaminants, pesticides, herbicides and mycotoxins or antibiotics. The consultants are also reviewing the possibility of patent protection.
We anticipate that for any products we sell pursuant to an agreement with our customer we will incur liability in the event that the product does not comply with the customer’s specifications or in the event of any product recall. We intend to provide in our agreement with the customer that the customer will be responsible for compliance with all laws applicable to the marketing, sale and labelling of the product. If we sell any products at retail, whether through the Internet or through retail outlets, it will be our responsibility to comply with all government regulations relating to manufacture, marketing, sale and labeling of the products. We will also incur liability in the event of any product recall. The cost of any liability which we may incur may be significant and, if we are found to be liable for any product noncompliance or product recall, we may not be able to continue in business.
Our business plan is in the preliminary stages, and we will require significant funding to implement our business plan, with no assurance that we can or will be successful in developing and implementing our business plan. If we are not able to implement our business plan, our business may be materially impaired.
Source of Supply
During the year ended December 31, 2022, we purchased inventory of Cordycepin and cordyceps powder, from one supplier, O’dimension Biotech Ltd., a Taiwan-based company. We did not purchase any inventory during 2021. The cordyceps that we used in our development efforts for our proposed our chicken feed product, which is the same product that we sell for human consumption, was taken from our inventory and the cordyceps used in the development project was not significant.
We do not plan to establish manufacturing facilities. In the past, we engaged in initial discussions with potential contract manufacturers in Taiwan but, as of the date of this annual report, we are not engaged in any negotiations with respect to a potential manufacturer, since we do not have any products for to be manufactured nor the funds to commence manufacturing operations.
Marketing and Sales
All of our marketing and sales activities to date have been conducted by our chief executive officer, Pao-Chi Chu, who is our only employee and who provides his services on a part-time basis. All sales to date were made by Mr. Chu.
During 2022, we had two customers, Vista Global Biotech Limited, a Hong Kong-based company, and Golden Biomedium Co., Ltd., a Taiwan-based company, accounted for all of our revenue in 2022 and 91.7% of our revenue in 2021. The following table sets forth information as to revenue from these customers:
| | Year Ended December 31, 2022 | | | Year Ended December 31, 2021 | |
| | Revenue | | | Percentage | | | Revenue | | | Percentage | |
Vista Global Biotech Limited | | $ | 360,000 | | | | 54.7 | % | | $ | 700,000 | | | | 58.5 | % |
Golden Biomedium Co., Ltd | | | 298,500 | | | | 45.3 | % | | | 398,000 | | | | 33.2 | % |
We do not have any long-term contracts with any customer. We ship products pursuant to purchase orders placed by the customers.
Effects of COVID-19; Inflation and Supply Chain Issues
Since our products are purchased by customers in Taiwan and Hong Kong either as one ingredient of a product to be sold to their customers in China or for resale to their customers, our business was effected by the COVID-19 pandemic as it effects manufacturers in Taiwan and Hong Kong and their customers in China, and our business may continue to be impacted by the sales lost during the pandemic.
The World Health Organization ended the global emergency status for COVID-19 on May 5, 2023. As restrictions that had been imposed, particularly China’s zero COVID policy, to address the pandemic have been lifted, we Company cannot give assurance that its sales will increase as a result of the reduction of such restrictions, and the Company’s revenues decreased from 2021 to 2022. Although China has lifted its COVID-19 restrictions, there continues to be a risk of the deaths and lockdowns from COVID-19.
As the world has begun to open following closures as a result of the pandemic, two other factors are facing businesses and consumers, which may be considered to be related to the effects of the COVID-19 pandemic. These are inflation and supply chain issues, which have been exacerbated by the recent Russian invasion of Ukraine. The Company cannot estimate the effect of these factors on its business. To the extent that these factors result in increased prices, the Company may not be able to pass along the increases to its customers. We do not believe that there is a shortages of cordyceps. Further, to the extent customers use the Company’s products as an ingredient in their own products, the inability of a potential customer to obtain other raw materials as well as cost increases for such products, could affect the timing and the amount of purchases from the Company. The Company cannot give assurance that its business will not be impaired by the effects of inflation and supply chain issues. Our revenue decreased in 2022 from 2021 by $539,000, or approximately 45%, primarily because of a decline in sales to two customers who accounted for 100% or our revenue in 2022 and 91.7% of revenue in 2021. Both the cost of our inventory and the prices we charge for our products were affected by inflation. Although we believe that the decline in revenue was COVID-related, we cannot assure you that our sales will increase as COVID-related factors become less important
Government Regulations
In the event that we seek to market and sell our products in the United States, we will be subject to various laws and regulations. The United States Federal Food, Drug, and Cosmetic Act defines a dietary ingredient as a vitamin, mineral, herb or other botanical, amino acid, dietary substance for use by man to supplement the diet by increasing the total dietary intake, or a concentrate, metabolite, constituent, extract, or combination of the preceding substances. Unlike drugs, supplements are not intended to treat, diagnose, prevent, or cure diseases, which means that supplements cannot make claims as to health benefits. Claims like these can only legitimately be made for drugs, not dietary supplements. Dietary supplements include such ingredients as vitamins, minerals, herbs, amino acids, and enzymes. Dietary supplements are marketed in forms such as tablets, capsules, softgels, gelcaps, powders, and liquids. Cordyceps is considered a dietary supplement.
The United States Food and Drug Administration regulates both finished dietary supplement products and dietary ingredients. The FDA regulates dietary supplements under a different set of regulations than those covering “conventional” foods and drug products. Under the Dietary Supplement Health and Education Act of 1994, manufacturers and distributors of dietary supplements and dietary ingredients are prohibited from marketing products that are adulterated or misbranded. That means that these firms are responsible for evaluating the safety and labeling of their products before marketing to ensure that they meet all the requirements of Dietary Supplement Health and Education Act and FDA regulations. The FDA is responsible for taking action against any adulterated or misbranded dietary supplement product after it reaches the market.
The Dietary Supplement and Nonprescription Drug Consumer Protection Act requires manufacturers, packers or distributors whose name appears on the product label of a dietary supplement to include contact information on the product label for consumers to use in reporting adverse events associated with the product’s use and to notify the FDA of any serious adverse event report within 15 business days of receiving such report. However, the reporting of an event is not an admission that the product caused the adverse event.
If we engage in business in the United States, we will be subject to a variety of other regulations, including those relating to health, safety, bioterrorism, environmental, cybersecurity, taxes, labor and employment, import and export, and environmental. These regulations may require significant financial and operational resources to ensure compliance, and we cannot assure you we will be able to be in compliance.
We do not presently sell products for retail sale to consumers although we may, to the extent that we implement our proposed business plan, develop products which are designed and packaged for consumer use. Our customers presently purchase our products in bulk and may use our products as ingredients in their products. Countries into which our products are sold have regulations relating to the marketing, labeling and claims for dietary supplements. Since we do not presently sell products in form for use by consumers, our customers must comply with applicable government regulations. Our present and recent former customers are located in Taiwan or Hong Kong, which have laws concerning the ingredients in products sold for consumption, including the purity of the ingredients. If products which include our products as ingredients are sold in Hong Kong or any other country, the products may be subject to the food and supplement regulations of the country. We do not make any of the products we sell. To the extent a claim arises either as a result of the use by a consumer of products which contain our ingredients or a government agency raises questions about the purity of ingredients purchased from us, we may incur liability for any adverse reactions to the products purchased by consumers or failures of our products to conform to the stated purity of our products and we cannot assure you that we will be able to claim over against our supplier. If we sell products that are designed and packaged for use by consumers, we may be subject to laws relating to such products, including the purity and labeling of the products and any other regulations that may be applicable.
If we sell products for consumer use in any country, we will be subject to the laws of that country. Each country has laws relating to products that are marketed as dietary supplements, including laws relating to the products and which describe the extent that products subject to the applicable laws, including the purity of the ingredients and marketing and labeling of products. We will need to comply with all applicable regulations and we may not be permitted to sell products in a country unless we have received prior approval from the applicable government agency.
We sell our products to distributors and it is the responsibility of our customers to comply with applicable regulations in the countries in which they sell products, including Taiwan, China and Hong Kong.
To the extent that we either manufacture our product or have our product manufactured by a third party, we intend to use a third party for inspection, verification, testing and certification services.
Research and Development
We incurred research and development expenses of approximately $10.1 million in 2022 and $5.3 million in 2021, which reflects the amortization of equity compensation provided to our consultants whose services related to the development of our proposed chicken feed product. We will continue to incur research and development expenses in 2023 for the balance of the consultant’s agreements which either expired in May 2023 or will expire in August 2023.
Intellectual Property Rights
We do not have any patent or other intellectual property rights with respect to any products.
Competition
A number of companies market and sell cordyceps products in the United States, including Real Mushrooms, Bulk Supplements, Terrasoul SuperFoods; Mental Refreshment Nutrition, NOW Foods, Aloha Medicinals, Natures Elements and Swanson Premium, and many companies market cordyceps in Asia. These products include cordyceps extract as well as products that include cordyceps along with other ingredients. Many, if not all, of these companies are better known and better capitalized than we are, and we cannot assure you that we will be able to compete successfully with these and other existing suppliers of cordyceps. There are a few companies that offer cordyceps in chicken feed. We believe that the market for this product is relatively small since cordyceps is extremely expensive and is usually used for human consumption. While cordyceps is considered a high price product, there are varying degrees of product, with the higher quality selling at higher prices. Cheaper products with lower quality that the products we sell are and have always been available in China. Although we believe there is a market for high quality product, we cannot give any assurance that our product or products which use our product as an ingredient are not priced at a level above that which a Chinese consumer will pay.
Employees
We have one employee, our chief executive officer and chief financial officer, Pao-Chi Chu, who works for us on a part-time basis.
ITEM 1A. RISK FACTORS
An investment in our common stock involves a high degree of risk. You should carefully consider the risks described below together with all of the other information included in this report before making an investment decision with regard to our securities. The statements contained in this report include forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. The risks set forth below are not the only risks facing us. Additional risks and uncertainties may exist that could also adversely affect our business, prospects or operations. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or a significant part of your investment.
Risks Concerning our Business
We have not generated significant revenues, we are operating at a loss, and in recent years we did not have any sales during several quarters, and we cannot assure you that we can or will ever operate profitably.
For the year ended December 31, 2022, we incurred a loss of approximately $15.9 million on revenues of $658,500, and for the year ended December 31, 2021, we incurred a loss of approximately $7.7 million on revenues of approximately 1.2 million. During 2022 we did not generate any revenue in the second and third quarters and in 2021 we did not generate any revenue in the third quarter. Our gross margin was 21.3% for the year ended December 31, 2022 and was 21.7% for the year ended December 31, 2021. We will not be able to operate profitably until and unless we are able to generate sufficient revenue so that our gross profit can cover our operating expenses. We cannot assure you that we be able to operate at a profit. We do not have any full-time employees and our chief executive officer, who provides his services on a part-time basis, has not received any salary. If we increase our operations and engage in selling, marketing and research and development activities, we will incur significant selling, general and administrative expenses. Unless we can significantly increase our revenue and gross profit or raise funds from other sources, including the sale or our equity securities, we may not be able to operate profitably. The lack of an active trading market in our common stock combined with our lack of sales can materially impair our ability to raise money through the sale of equity securities. We cannot assure you that we can or will ever operate profitably.
We require significant funding for us to conduct our business.
At December 31, 2022, we had cash of less than $6,000 and accounts receivable of $638,500. In order for us to continue in business, we will require significant additional capital either in the form of debt or equity. Because of the absence of any active trading market in our stock, our financial condition, our modest level of sales to date, as well as our lack of any history of significant operations, our low stock price and the absence of an active market for our stock, we may be unable to raise funds through the sale of equity securities.
We incurred significant research and development expenses in 2022 and 2021 and such research and development did not result in a marketable product.
A key element in the loss for both 2022 and 2021 is stock-based compensation relating to research and development of approximately $10.1 million in 2022 and approximately $5.3 million in 2021, reflecting the amortized portion of the value of common stock issued in 2021 to consultants for research and development. In addition, we incurred selling, general and administrative services largely relating to our proposed chicken feed product of $5,632,400 in 2022 and $2,366,242 in 2021, representing the amortization of common stock issued to consultants for such selling, general and administrative services. At December 31, 2022, we had deferred stock compensation of $8,060,983, which will be recognized over the balance of the agreements, which expired in May 2023 and will expire August 2023.The amortization of this stock-based compensation will continue to impact the results of our operations in 2023, although we have not developed a product from which we may derive revenue and we may never develop such a product.
Our financial statements include a going concern paragraph.
Our financial statements for the year ended December 31, 2022 include a going concern paragraph. We had minimal cash at December 31, 2022, we had limited gross profit and we incurred a loss from operations for the year ended December 31, 2022 and past few years. These factors, among others, raise substantial doubt about our ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. Although we propose to fund our operations through sales of cordyceps related products, we have not generated sales in three quarters during 2022 and 2021, and we do not have any new product which will be able to be marketed in the near future if at all. Because of the lack of sales and the absence of any active trading market for our common stock, our financial condition and our lack of an operating history, we will have difficulty raising funds in the equity market on reasonable, if any, terms, and we have had to rely on advances from a minority stockholder and our officer. If we cannot generate revenue from our products, we may not be able to continue in business.
If we are not able to increase revenue and our customer base, we may not be able to operate profitably.
Through December 31, 2022, our revenue was derived from a small number of customers. During 2022 and 2021 two customers accounted for 100% and 91.7% of our revenue. During 2021, we also has sales to one other customer. Our customers are based on in Taiwan and Hong Kong. Our two largest customers for 2021 were not customers in prior years. We do not have any long-term agreement with any customers and they may cease purchasing from us for any reason. Unless we are successful in generating revenue from a larger customer base, our ability to operate will be impaired. Further, we believe that the nature of the market is such that we have little ability to improve our gross margin.
Our revenue has been imparted by recent government efforts to politically stabilize Hong Kong.
Prior to 2019, our revenue was derived from Hong Kong –based customers, one of which was a customer in 2021. We believe that one factor was the political instability in Hong Kong, which had affected our customers’ ability to sell products into the People’s Republic of China and their purchases from us in the past. Although we believe that the political climate in Hong Kong has improved, we cannot be certain that this will continue to be the case.
Outbreaks of communicable diseases, natural disasters or other events have materially and adversely affected, and in the future, may materially and adversely affect our business, results of operations and financial condition.
Our business could be adversely affected by the effects of communicable diseases and epidemics. In recent years, there have been breakouts of epidemics in China and globally. In early 2020, there was a worldwide outbreak of a novel strain of coronavirus, later named COVID-19. The outbreak of COVID-19 severely impacted China and the rest of the world. In response to intensifying efforts to contain the spread of the coronavirus, in 2020, the Chinese government took a number of actions, which included extending the Chinese New Year holiday, temporary closure of corporate offices, retail outlets and manufacturing facilities, quarantining individuals in China who had COVID-19, asking citizens to remain at home and to avoid gathering in public, and other actions. Until recently, China imposed a zero-COVID policy which resulted in lockdown in major cities and in provinces. These actions resulted in a significant decline in the market for cordyceps products from our customers. The termination of the zero-COVID policy may result in increased deaths and hospitalizations. The extent to which COVID-19 and government actions to address it may affect our results of operations, financial condition and cash flows will depend on the future development of the outbreak. Our business could also be disrupted by outbreaks of H1N1 flu, avian flu or another epidemic. We are also vulnerable to natural disasters and other calamities that may affect our supplier and may affect us when we establish our own manufacturing facilities.
If we sell products or commence operations in the United States, we would be subject to government regulations in the United States.
If we sell products or commence operations in the United States, we would be subject to FDA regulations under the Dietary Supplement Health and Education Act, which generally provides a regulatory framework to help ensure safe, quality dietary supplements and the dissemination of accurate information about our products. The FDA does not generally regulate active ingredients in dietary supplements in the same manner as it regulates drugs unless the product makes claims, such as claims that a product may heal, mitigate, cure or prevent an illness, disease or malady, that may result in the product being subject to the restrictions and regulations imposed on drugs. If we commence operations in the United States, we would also be subject to government regulations that apply to business in general, including those relating to health, safety, bioterrorism, taxes, labor and employment, import and export, and the environment. At present, we do not have any business activities in the United States that require compliance with these regulations. However, at such time as we commence business in the United States, we may incur significant costs to comply with such regulations, and we cannot assure you we will be able to be in compliance. Other countries in which we may operate may have similar regulations, and, to the extent that we conduct business or sell products in these countries, we will be subject to those regulations.
Since we sell our products to customers in Taiwan and Hong Kong, we may be subject Taiwan and Hong Kong laws and regulations relating to our products.
We do not sell products for retail sale to consumers. Our customers purchase our products in bulk and use our products as ingredients in their products or they sell the products to customers. Countries into which our products are sold have regulations relating to the marketing, labeling and claims for dietary supplements. Since we do not sell products in form for use by consumers, our customers must comply with applicable government regulations. Our present customers are located in Taiwan and Hong Kong, which have laws concerning the ingredients in products sold for consumption, including the purity of the ingredients. If products which include our products as ingredients are sold in Taiwan, Hong Kong or any other country, including the PRC, the products may be subject to the food and supplement regulations of that country. We do not make any of the products we sell. To the extent a claim arises either as a result of the use by a consumer of products which contain our ingredients or a government agency raises questions about the purity of ingredients purchased from us, we may incur liability for any adverse reactions to the products purchased by consumers or failures of our products to conform to the stated purity of our products and we cannot assure you that we will be able to claim over against our supplier. Although we do not sell products in Taiwan, Hong Kong or any other country, we may be subject to liability or penalties in the event that our products do not have the purity which we claim We may, in the future, sell products that are designed and packaged for use by consumers, in which event we will be subject to laws relating to such products, including the purity and labeling of the products and any other regulations that may be applicable in the country in which the products are sold.
If we develop a chicken feed product, which would be used by chicken farmers to feed their chicken, we may be subject to government regulations.
We have been working on the development of a cordyceps-based ingredient for chicken feed which would be included as part of the chicken’s diet. The cordyceps in the chicken feed product is the same as cordyceps sold for human consumption and is treated as Chinese traditional medicine. To the extent that any of our products requires government approval, it is the responsibility of the manufacture to satisfy the government agency as to its compliance, and our product would need to be manufactured in a government-approved manufacturing facility.
We need to develop additional sources of supply.
Our revenue through December 31, 2022 has been derived from the sale of products purchased from three suppliers, one of which accounted for all of our purchases in the year ended December 31, 2022. We did not purchase any inventory in 2021. We do not have any long-term agreements with any suppliers, and, accordingly, our suppliers have no contractual obligation to sell us products at a price which would enable us to generate an acceptable gross margin, if at all. We will need to develop additional sources of supply for both raw materials and any finished products which we may sell. Although we believe that alternative sources of supply of both raw materials and finished products are available, any difficulty or delay in identifying and entering into supply arrangements with suppliers could impair both our gross margins and our ability to operate profitably. Further, any shortage of raw materials or interruption of supply could also result in higher prices for those materials which we may be unable to pass on to our customers. We cannot assure you that, if we develop our business, our suppliers will provide us with the quality of raw materials we need or the quantities we request or at a price we consider to be reasonable. Because we do not control the actual production of these raw materials, we are also subject to delays caused by interruption in production of materials based on conditions outside of our control, including weather, transportation interruptions, strikes, terrorism, natural disasters, or other catastrophic events.
We need to develop and maintain marketing and distribution channels.
We presently do not have any marketing or distribution arrangements. All of our sales through December 31, 2022 were made by our chief executive officer who is not a full-time employee. Unless we are able to hire qualified sales and marketing personnel and develop distribution channels to market and sell any products which we sell, we will not be able to generate sufficient revenue to enable us to operate profitably. We cannot assure you as to our ability to develop and maintain effective marketing and distribution channels or to operate profitably.
We do not have product liability insurance to protect us against any claims we may sustain.
We do not have any product liability insurance. Regardless of whether we manufacture products, we could face significant liabilities due to claims that the use of products we sell caused adverse reactions, regardless of whether we have the product manufactured for us or we purchase the product from a suppliers. We could be exposed to liability based on claims that, among others: our products contain contaminants; we provide consumers with inadequate instructions about product use; or we provide inadequate warning about side effects or interactions of our products with other substances. Even if we were to prevail in any such claims, the cost of litigation and settlement could be significant and could exceed any product liability coverage we may have. Although we intend to require any contract manufacturers to maintain product liability insurance, we cannot assure you that they will have adequate, if any, product liability insurance coverage. Since we do not have supply agreements with our present suppliers, we would have no contractual recourse against the suppliers in the event of any users should suffer adverse events following the use of products sold by us. In addition, as we are presently test marketing our chicken feed product with a small number of chicken farmers, we cannot assure you that we will develop a marketable product and we may be subject to claims from the chicken farmers if they believe that their chicken fell ill from our product.
The market for our products is very competitive, and we may not be able to compete successfully.
The cordyceps market is highly competitive and a number of products are readily available. Most, if not all, of our competitors are substantially larger and have greater financial resources and name recognition than we do. Further, new products which may be developed or sold may increase the competitiveness of the market. We anticipate that we will be dependent, at least initially, primarily on cordyceps products. Many of our competitors offer a range of products and are not dependent on a market for cordyceps products, which can protect them in the event that the market for cordyceps products declines. Furthermore, cordyceps is a relatively expensive product, and our customer’s products compete with cheaper products which may not have the same degree of purity as our products, which may affect the market for our products. If we develop a chicken feed products, we would compete with a number of major companies that manufacture chicken feed products, including products which are intended to improve the quality of the eggs and/or the heath of the chickens. We cannot assure you that, if we develop a chicken feed product, we will be able to generate any significant revenue from the product.
We have not conducted any study of the potential market for cordyceps-based in the United States and we cannot assure you that there is a significant market for these products in the United States.
Although we have a general familiarity with the market for cordyceps products in Asia, our business plan contemplates the sale of these products in the United States and possibly countries where there is a large Asian population. We have not conducted any study as to the market for cordyceps products in the United States and we cannot assure you that there is any significant market. Unless there is a significant market in the United States, we may not be able to operate profitably. We cannot assure you that there is a sufficient market in the United States to enable us to compete effectively or operate profitably or that, if a market exists for products of the type we sell, that we will be able to market our products successfully.
The market for cordyceps products or any chicken feed product we may develop may be affected by recalls or successful litigation arising from claimed adverse reactions to products.
Any recall or lawsuits arising out of adverse reactions or perceived adverse reactions to cordyceps products or any chicken feed product we may develop and market or unfavorable comments in the press or social media could impair the market for our products, even if the recall, adverse reaction or unfavorable comments related to products manufactured and sold by other companies.
The market for our products may be dependent on public tastes, which can rapidly change.
The market for any type of supplements, including supplements used in animal fees, is subject to change in public tastes, which changes may be based on the factors described in the preceding Risk Factor or other changes in taste not relating to any specific incident or problem. Since our business plan is presently limited to cordyceps products, we will be impacted more severely by changes in tastes than we would if we offered a range of different dietary supplements. We cannot assure you that we will be able to develop, offer and sell any products other than cordyceps-based products or that any market that may exist will continue.
We may not be successful in any research and development activities in which we may engage.
We have been engaged in research and development with result to our proposed chicken feed products. The consultants who performed such services as well are related selling, general and administrative services plan performed the services pursuant to agreements which either terminated in May 2023 or will terminate in August 2023. These services did not result in a marketable product We may hire one or more of these consultants to continue the research and development activities, however, as of the date of this annual report, we have not hired any of the consultant. Since our consultant are working from their own locations and not from our office and we have only one employee, our chief executive officer who is not a full-time employee, we cannot provide the same degree of supervision as we would use if we had employees engaging in research and development activities on our premises. We cannot assure you that we will pursuant any product development activities or that, if we do, that we will be successful in developing any product or that any product we may develop will be marketable in the United States or any other country or that we will not require regulatory approval for the sale of any such product in the United States or any other country in which we seek to market the product. If regulatory approval is required, compliance with such regulations may be very expensive and we cannot assure you that we will be able to obtain such approval. As a result, we may incur significant expenses in seeking to develop a product with no assurance that we can or will develop a marketable product that complies with applicable law.
We are dependent upon our chief executive officer.
We are dependent upon Pao-Chi Chu, our chief executive and financial officer, sole director and principal stockholder, who is our only employee and who works for us on a part-time basis. The loss of Mr. Chu would materially impair our ability to conduct our business. We do not have an employment agreement with Mr. Chu and we do not maintain key person life insurance on his life.
If we are unable to attract, train and retain technical and financial personnel, our business may be materially and adversely affected.
Our future success depends, to a significant extent, on our ability to attract, train and retain key management, marketing, sales, technical, product development and financial personnel. As of December 31, 2022 we had not taken steps to hire any such personnel. Recruiting and retaining capable personnel, particularly those with expertise in the natural supplement business are vital to our success. There is substantial competition for qualified personnel, and we cannot assure you we will be able to attract or retain our technical and financial personnel. If we are unable to attract and retain qualified employees, our business may be materially and adversely affected. Our financial condition, including the absence of sales in three of the eight quarters of 2022 and 2021, and the absence of any significant market in our common stock may make it difficult for us to attract qualified personnel.
Our chief executive officer may have a conflict of interest.
Pao-Chi Chu, our chief executive officer, chief financial officer and principal stockholder, has served as the chairman of Mucho Biotech Co., Ltd., Mucho Furich Co., Ltd., and Mucho Biomedical Co., Ltd., companies engaged in applications of cordyceps since 2006. These companies are controlled by Mr. Chu. As a result, he may have a conflict of interest in allocating his time and available resources among us and the other companies in related fields which he controls. We cannot assure you that Mr. Chu will be able to allocate sufficient time and resources to our business to enable us to develop our business plan.
We may not be able to protect any intellectual property which we may develop.
We do not have any patents or other proprietary intellectual property. While we may seek patents for any intellectual property which we may develop, we cannot assure you that we will develop any patentable product or that we will be able to obtain patents or that, if we do obtain patents, other companies will not be able to design around our patents and develop competitive or superior products. We cannot assure you that we will be able to enforce any patent rights which we may obtain. Patent litigation is very expensive, and, if we do not have the financial resources to enforce through litigation any patents we may obtain, we may not be able to retain the value of the patents. We believe that much of our intellectual property will be in the nature of trade secrets. Although we will seek to protect our intellectual property rights through nondisclosure agreements, including non-disclosure agreement with our employees and consultants and other companies with which we may conduct business, we cannot assure you that the other parties to the non-disclosure agreements will comply with their obligations, and we may not be aware of any breach until the intellectual property has been disclosed to a third party. We may not be able to enforce our rights under the non-disclosure agreements.
Our business and our ability to maintain our gross margin may be affected by inflation and the global supply chain issues.
After years of relatively low inflation, during the past several years, countries throughout the world, including Asia, have been subject to inflation at a rate significantly higher than in recent years. The slowdown resulting from the COVID-19 pandemic and steps taken by governments to address the pandemic, including China’s zero COVID policy, created major supply chain disruptions. We expect that both the inflationary pressures and supply chain disruption that affect other industries will affect us. These factors may result in delays in receipt of products we order, and increased costs which we may not be able to pass on to consumers. Both our cost of inventory and the prices we charged increased as a result of inflation. The recent Russian invasion of Ukraine also exacerbated the inflationary and supply chain issues. We cannot assure you that our business will not be materially impaired by inflationary and supply chain disruption.
Risks Concerning our Common Stock
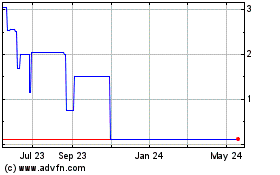
There is presently no active market for our common stock, which is currently quoted on the Pink Limited Information tier, which may make it difficult for you to sell your stock.
Although our common stock was quoted on the OTCQB marketplace, it is currently traded on the Pink Limited Information tier, which includes companies that are delinquent in their filings, under the symbol ACBM. There is no active trading market for our common stock, and the OTC Markets website shows that there are many days on which there is no trading volume or very limited trading volume. Accordingly, even if a market develops, as to which we can give no assurance, there can be no assurance as to the liquidity of our common stock, the ability of holders of our common stock to sell our common stock, or the prices at which holders may be able to sell our common stock. Further, if a market develops, it is likely that there will not be any significant float, with the result that the reported bid and asked prices may have little relationship to the price you would pay if you wanted to buy shares or the price you would receive if you wanted to sell shares. Further, we cannot determine whether or when our common stock will be traded on the OTCQB market.
Because our common stock is a penny stock, you may have difficulty selling our common stock in the secondary trading market.
If a market for our common stock develops, our common stock is, and will likely continue to be, a penny stock and therefore is subject to the rules adopted by the SEC regulating broker-dealer practices in connection with transactions in penny stocks. The SEC rules may have the effect of reducing trading activity in our common stock, making it more difficult for investors to purchase and sell their shares. The SEC’s rules require a broker or dealer proposing to effect a transaction in a penny stock to deliver the customer a risk disclosure document that provides certain information prescribed by the SEC, including, but not limited to, the nature and level of risks in the penny stock market. The broker or dealer must also disclose the aggregate amount of any compensation received or receivable by him in connection with such transaction prior to consummating the transaction. In addition, the SEC’s rules also require a broker or dealer to make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction before completion of the transaction. The existence of the SEC’s rules may result in a lower trading volume of our common stock and lower trading prices. Further, some broker-dealers will not process transactions in penny stocks.
Our lack of internal controls over financial reporting may affect the market for and price of our common stock.
Our disclosure controls and our internal controls over financial reporting are not effective. We do not have the financial resources or personnel to develop or implement systems that would provide us with the necessary information on a timely basis so as to be able to implement financial controls. Our continued poor financial condition together with the fact that we have one part-time employee, who is both our chief executive officer and chief financial officer, makes it difficult for us to implement a system of internal controls over financial reporting, and we cannot assure you that we will be able to develop and implement the necessary controls. The absence of internal controls over financial reporting may inhibit investors from purchasing our shares and may make it more difficult for us to raise debt or equity financing.
Our lack of a full-time chief financial officer affects our ability to develop financial controls, which could affect the market price for our common stock.
We do not have a full-time chief financial officer. At present, our chief executive officer, who does not have an accounting background, is also acting as our chief financial officer. We do not anticipate that we will be able to hire a qualified chief financial officer unless our financial condition improves significantly. The lack of an experienced chief financial officer, together with our lack of internal controls, may impair our ability to raise money through a debt or equity financing, the market for our common stock.
We do not have any independent directors.
We do not have any independent directors. Our sole director is Pao-Chi Chu, who is our chief executive officer, chief financial officer and principal stockholder. Because we have no independent director, we do not have any checks and balances on Mr. Chu, which may make it difficult for us to develop internal controls and to raise money in the financial markets.
Our stock price may be volatile and your investment in our common stock could suffer a decline in value.
As of the date of this report, there has been no active trading activity in our common stock. There can be no assurance that any significant market, or any market, will ever develop in our common stock. Because of the low public float and the lack of trading volume, any reported prices may not reflect the price at which you would be able to sell shares if you want to sell any shares you own or buy shares if you wish to buy shares. Further, stocks with a low public float may be more subject to manipulation than a stock that has a significant public float. The price may fluctuate significantly in response to a number of factors, many of which are beyond our control. These factors include, but are not limited to, the following, in addition to the risks described above and general market and economic conditions:
| · | our low stock price, which may result in a modest dollar purchase or sale of our common stock having a disproportionately large effect on the stock price; |
| · | the market’s perception as to our ability to generate revenue and positive cash flow or earnings; |
| · | changes in our or securities analysts’ estimate of our financial performance; |
| · | our ability or perceived ability to obtain necessary financing for our operations; |
| · | the perception of the future market for our products and whether we will be successful in developing and marketing our chicken feed product; |
| · | the anticipated or actual results of our operations; |
| · | changes in market valuations of other natural supplement companies; |
| · | any discrepancy between anticipated or projected results and actual results of our operations; |
| · | actions by third parties to either sell or purchase stock in quantities which would have a significant effect on our stock price; and |
| · | other factors not within our control. |
Raising funds by issuing equity or convertible debt securities could dilute the net tangible book value of the common stock and impose restrictions on our working capital.
If we were to raise additional capital by issuing equity securities, either alone or in connection with a non-equity financing, the net tangible book value of the then outstanding common stock could decline. If the additional equity securities were issued at a per share price less than the market price, which is customary in the private placement of equity securities, the holders of the outstanding shares would suffer a dilution, which could be significant. We may have difficulty in raising funds through the sale of debt securities because of both our financial position, the lack of any collateral on which a lender may place a value, and the absence of any revenue or operations. If we are able to raise funds from the sale of debt securities, the lenders may impose restrictions on our operations and may impair our working capital as we service any such debt obligations. Further, it is not uncommon for investors who provide private funding to companies with weak financial positions, to require the issuer to issue convertible securities which are convertible at a discount to the market price at the time the convertible security is converted. Such securities typically have a materially adverse effect on the market price for the issuer’s stock.
Because of our chief executive officer’s stock ownership, he has the power to elect all directors and to approve any action requiring stockholder approval.
Mr. Pao-Chi Chu, our chief executive officer, owns 30,000,000 shares of common stock, representing approximately 49.97% of our outstanding common stock. As a result, Mr. Chu has the power, without the vote of any other stockholders, to elect all of our directors and, with minimal support from other stockholders, take any action requiring stockholder approval, including any amendment to our certificate of incorporation, merger, sale of assets or other major corporate transaction.
We do not intend to pay any cash dividends in the foreseeable future. We have not paid any cash dividends on our common stock and do not intend to pay cash dividends on our common stock in the foreseeable future.
ITEM 2. PROPERTIES
On November 3, 2021, we entered into lease agreement to rent a storage facility in Hong Kong for a two-year term at HK$17,000 (approximately $2,190) per month and we paid HK$33,000 (approximately $4,230) as a security deposit.
ITEM 3. LEGAL PROCEEDINGS
None
ITEM 4. MINE SAFETY DISCLOSURES.
Not Applicable
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market Information
Our common stock has been quoted on the OTCQB market under the symbol ACBM and is presently quoted on the Pink Limited Information tier which includes companies that are delinquent in their SEC filing obligations. We are delinquent because we did not file this annual report when it was required to be filed. Any quotations for our common stock reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions
Stockholders of Record
As of July 17, 2023, we had 186 record holders of our common stock.
Transfer Agent
Cleartrust, LLC, 16540 Pointe Village Drive; Suite 210, Lutz, Florida 33558 is the transfer agent for our common stock.
Dividends
We have not paid any cash dividends to date and do not anticipate or contemplate paying dividends in the foreseeable future.
Securities Authorized for Issuance under Equity Compensation Agreements
We do not have any common stock available for grant or options under equity compensation plans as of December 31, 2022, since all shares approved for grant have been issued and no further shares are available.
Recent sales of unregistered securities.
None.
ITEM 6. SELECTED FINANCIAL DATA
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of financial condition and results of operations should be read in conjunction with our financial statements and related notes included elsewhere in this report. This discussion contains forward-looking statements that involve risks, uncertainties and assumptions. See “Note Regarding Forward-Looking Statements.” Our actual results could differ materially from those anticipated in the forward-looking statements as a result of certain factors discussed in “Risk Factors” and elsewhere in this report.
Overview
Since January 30, 2017, following a change of control, we have been engaged in the business of developing and marketing nutritional products that promote wellness and a healthy lifestyle. Our business to date has involved the purchase of products from three suppliers in Taiwan and the sale of these products to four unrelated customers, two of which accounted for all of our sales in the year ended December 31, 2022 and 91.7% of sales in the year ended December 31, 2021. We did not have any sales during the second and third quarters of 2022 or the third quarter of 2021. We sell products in bulk to companies who may use our products as ingredients in their products or sell the products they purchase from us to their own customers.
All of our sales to date have been sales of cordyceps related products except that, in the quarter ended June 30, 2018, we sold metallothionein MT-3 elizer, a product that we do not currently sell. Cordyceps is a fungus that is used in traditional Chinese medicine. Cordyceps sinensis has been described as a medicine in old Chinese medical books and Tibetan medicine. It is a rare combination of a caterpillar and a fungus and found at altitudes above 4500m in Sikkim. We may also seek to market other products which we see as complimentary to our present products; however, we have not entered into negotiations with respect to the distribution of other products, and we cannot assure you that we will be able to market any other products.
We believe that since a major market for cordyceps products is China and we believe our customers have significant customers in China, our business was impacted by COVID-19 and steps taken by the government of China, particularly its Zero COVID policy, which was relaxed in December 2022. We are continuing to feel the residual effect of China’s COVID policy and the loss of business during 2020 through 2022. Further, we also cannot assure you the political instability in Hong Kong will not affect our sales, since our customers in 2017 and 2018 were Hong Kong based customers who sold their products in the PRC and none of these customers has made purchases from us since the quarter ended December 31, 2018. We cannot assure you that these factors will not affect our ability to generate revenue in the future and, to the extent that any of these factors affect our ability to generate revenue, we may not be able to continue in business.
At present, we have no full-time employees. Our only employee is our chief executive officer who works for us on a part-time basis. We face significant risks in implementing our business plan, as described under Item 1.Business – Our Business, including, but not limited to, our ability to raise the necessary financing either through the sale of debt or equity securities or through a loan facility, our ability to increase our customer base and supply chain, our ability to increase our gross margins, our ability to hire and retain qualified research and development, marketing and administrative personnel, our ability to develop products and to market in the United States and other western markets any products we may develop, our ability to comply with any government regulations relating to the manufacture, distribution and marketing any products we develop. We cannot assure you that we can or will develop any products or generate revenue or profits in the future.
In May and August 2021, we entered into two-year service agreements with consultants who performed research and development services as well as selling general and administrative services in connection with a proposed product – a cordyceps-infused chicken feed. The agreements with the consultant either expired in May 2023 or will expire in August 2023, and they will not be renewed. We may consider hiring one or more of the consultants as employees, but, as of the date of this annual report, we have not made a decision with respect to hiring any of the consultants. Our statement of operation reflects the amortization of common stock issued to consultants in connection with our proposed chicken feed product. We issued a total of 12,282,000 shares of common stock to consultants as stock grants pursuant to agreements with the consultants in May and August 2021. The agreements provide for the consultants to perform the services described in the contracts for the two-year period commencing the date of the agreements. The shares were valued at $31,424,800, based on the market price of the common stock on the respective dates of the agreements, and is being amortized over the two-year period of the agreement terms using the straight-line method. During the years ended December 31, 2022 and 2021, we recorded stock-based compensation of $15,712,400 and $7,651,417, respectively, and had deferred stock compensation of $8,060,983 and $23,773,383 as of December 31, 2022 and 2021 which will be recognized over the balance of the agreements. Our selling, general and administrative expenses do not include any compensation for our chief executive officer, who serves without compensation and is responsible for our purchases, sales and directing our research and development program. As a result, the results of our operations do not reflect costs that would normally be associated with a chief executive officer who performs such functions.
We require funds for our operations. At December 31, 2022, we had $5,852 in cash, $638,500 in accounts receivables, of which $498,500 remains outstanding to date. Although we may seek to raise funds in the equity market, we have no agreements or understandings with respect to any funding and we can give no assurance as to the availability or terms of any such financing. Because of our financial condition, the lack of sales in the six out of twelve quarters in 2022, 2021 and 2020, our reliance of sales primarily of one product, along with the absence of an active market for our stock and our stock being traded on the OTC Market Group’s Pink Limited Information Tier, and our market capitalization in relation to our financial performance, together with risk related to the COVID-19 pandemic and the political and legal situation in Hong Kong, it may be difficult for us to raise funds in the equity market, and, if we are able to raise funds our stockholders may suffer significant dilution.
To the extent that we implement our business plan, we anticipate that we will incur marketing and other expenses without any assurance that such expenses will generate any significant revenue or net income. Because of our cash position, we may use equity-based compensation for our employees and independent contractors. Because of our low cash position, we may rely on loans from stockholders or related parties, although we do not have any agreements or understandings at this time, and we may issue equity to attract employees and consultants to help us develop our business plan.
Effects of COVID-19
Since our products are purchased by customers in Taiwan and Hong Kong either as one ingredient of a product to be sold to their customers in China or for resale to their customers, our business was by the effects of the COVID-19 pandemic as it effects manufacturers in Taiwan and Hong Kong and their customers in China, and our business may continue to be impacted by the sales lost during the pandemic.
The World Health Organization ended the global emergency status for COVID-19 on May 5, 2023. As restrictions that had been imposed, particularly China’s zero COVID policy, to address the pandemic have been lifted, the Company cannot give assurance that its sales will increase as a result of the reduction of such restrictions, and the Company’s revenues decreased from 2021 to 2022. Although China as lifted COVID-19 restrictions, there continues to be a risk of the deaths and lockdowns from COVID-19. Further, we continue to feel the effects of the loss of business during the pandemic.
Inflation and Supply Chain Disruption
After years of relatively low inflation, during the past two years, countries throughout the world, including Asia, have been subject to inflation at a rate significantly higher than in recent years. The slowdown resulting from the COVID-19 pandemic and steps taken by governments to address the pandemic, including the recent lockdown in a number of Chinese provinces and cities, have created major supply chain disruptions. Although we did not purchase any inventory in 2021, expect that both the inflationary pressures and supply chain disruption that affect other industries will affect us. These factors may result in delays in receipt of products we order, and increased costs which we may not be able to pass on to consumers. Both our cost of inventory and the prices we charged increased as a result of inflation. The recent Russian invasion of Ukraine has also exacerbated the inflationary and supply chain issues. We cannot assure you that our business will not be materially impaired by inflationary and supply chain disruption.
Results of Operations
Years Ended December 31, 2022 and 2021
For the year ended December 31, 2022, we had revenue of $658,500, representing sales to two customers. Our revenue decreased in 2022 from 2021 by $539,000, or approximately 45%, primarily because of a decline in sales to two customers who accounted for 100% or our revenue in 2022 and 91.7% of revenue in 2021. We had no revenue for the second and third quarters of 2022. Our gross profit was $140,500, and our gross margin was 21.3%. We had operating expenses of $16,010,237, representing research and development expenses of $10,080,000 and selling, general and administrative expenses of $5,930,237, which included stock-based compensation of $15,712,400 paid to consultants working on research and development and marketing and related expense relating to our proposed chicken feed product. As a result of the foregoing, we had a net loss of $15,871,443, or $(0.26) per share (basic and diluted).
For the year ended December 31, 2021, we had revenue of $1,197,500, representing sales to three customers. We had no revenue for the third quarter of 2021. Our gross profit was $259,500, and our gross margin was 21.7%. We had operating expenses of $7,953,078, representing research and development expenses of $5,285,175 and selling, general and administrative expenses of $2,667,903, which included stock-based compensation of $7,651,417 paid to consultants working on research and development and marketing and related expense relating to our proposed chicken feed product. As a result of the foregoing, we had a net loss of $7,697,828, or $(0.14) per share (basic and diluted).
We imputed interest at the rate of 4% on the advances made to us by a stockholder and CEO in the amount of $1,706 and $4,250 for the years ended December 31, 2022 and 2021, respectively.
Because of our dependence on a few customers, our revenue in any quarter is dependent upon both the timing of orders from customers and the delivery of products from our suppliers.
Liquidity and Capital Resources
The following table sets forth information relating to our working capital at December 31, 2022 and 2021:
| | December 31, 2022 | | | December 31, 2021 | | | Change | |
Current assets | | $ | 657,537 | | | $ | 706,415 | | | $ | (48,878 | ) |
Current liabilities | | $ | 201,116 | | | $ | 91,651 | | | $ | 109,465 | |
Working capital | | $ | 456,421 | | | $ | 614,764 | | | $ | (158,343 | ) |
The following is a summary of the statements of cash flows for the years ended December 30, 2022 and 2021:
| | Years Ended December 31, | |
| | 2022 | | | 2021 | |
Cash (used in) provided by operating activities | | $ | (147,481 | ) | | $ | 299,335 | |
Cash provided by investing activities | | | - | | | | - | |
Cash provided by (used in) financing activities | | | 58,085 | | | | (222,210 | ) |
Cash and cash equivalents end of year | | | 5,852 | | | | 95,248 | |
Cash used in operating activities for the year ended December 31, 2022 reflected the net loss of $15,871,443, decreased primarily by stock-based compensation of $15,712,400.
Cash provided by operating activities for the year ended December 31, 2021 reflected the net loss of $7,697,828, decreased primarily by stock-based compensation of $7,651,417, decrease in inventories of $938,000, increased by an increase in accounts receivable of $598,000.
Cash provided by financing activities for the year ended December 31, 2022 of $58,085 represented advances from a minority stockholder.
Cash used in financing activities for the year ended December 31, 2021 of $222,210 represented repayments of loans to a minority stockholder of $241,851 and advances from the minority stockholder of $19,641.
Going Concern
Our financial statements have been prepared assuming that we will continue as a going concern, which contemplates the realization of assets and the liquidation of liabilities in the normal course of business. We had minimal cash as of December 31, 2022, had limited gross profit and incurred a loss from operations for the year ended December 31, 2022 and past few years. These factors, among others, raise substantial doubt about our ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Although we propose to fund operations through sales of our products and equity financing arrangements, because of the lack of sales and the absence of any active trading market for our common stock, our financial condition and our lack of an operating history, we may not be able to raise funds for capital expenditures, working capital or other cash requirements and will have to rely on advances from a minority stockholder and our officer. If we cannot generate revenue from our products, we may not be able to continue in business.
Critical Accounting Policies and Estimates
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements. The estimates and judgments will also affect the reported amounts for certain revenues and expenses during the reporting period. Actual results could differ from these good faith estimates and judgments.
Inventories
Inventories consist primarily of finished goods. Inventories are valued at the lower of cost or net realizable value. We determine cost on the basis of first-in, first-out methods. We periodically review inventories for obsolescence and any inventories identified as obsolete are written down or written off. Although we believe that the assumptions we use to estimate inventory write-downs are reasonable, future changes in these assumptions could provide a significantly different result. No inventory markdown was recorded for the years ended December 31, 2022 and 2021.
Leases
We determine if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, other current liabilities, and operating lease liabilities on our balance sheets if the lease term is more than one year. Finance leases are included in property and equipment, other current liabilities, and other long-term liabilities on our balance sheets.
ROU assets represent our right to use an underlying asset for the lease term, and lease liabilities represent our obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As most of our leases do not provide an implicit rate, we generally use our incremental borrowing rate based on the estimated rate of interest for collateralized borrowing over a similar term of the lease payments at commencement date. The operating lease ROU asset also includes any lease payments made and excludes lease incentives. Our lease terms may include options to extend or terminate the lease when it is reasonably certain that we will exercise that option.
Leases with a lease term of one year or less at inception are not recorded on the Company’s balance sheet and are expensed on a straight- line basis over the lease term in the statement of operations.
Net Income (Loss) Per Share of Common Stock
We adopted ASC Topic 260, “Earnings per Share” which requires presentation of basic earnings per share on the face of the statements of operations for all entities with complex capital structures and requires a reconciliation of the numerator and denominator of the basic earnings per share computation. Basic loss per share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per share is computed by dividing net income by the weighted average number of shares of common stock and potentially dilutive outstanding shares of common stock during the period to reflect the potential dilution that could occur from common shares issuable through contingent share arrangements, stock options and warrants unless the result would be antidilutive. There were no potentially dilutive shares of common stock outstanding for the years ended December 31, 2022 and 2021.
Concentrations of Credit Risk
Our financial instruments that are exposed to concentrations of credit risk primarily consist of its cash and cash equivalents and related party payables that we will likely incur in the near future. We place our cash and cash equivalents with financial institutions of high credit worthiness. At times, our cash and cash equivalents with a particular financial institution may exceed any applicable government insurance limits.
Stock-Based Compensation
We recognize compensation expense for stock-based compensation in accordance with ASC Topic 718. For employee and non-employee stock-based awards, we calculate the fair value of the award on the date of grant using the option-pricing model for stock options and the quoted price of its common stock for unrestricted shares; the expense is recognized over the service period for awards expected to vest. We consider many factors when estimating expected forfeitures, including types of awards, employee class and historical experience.
Deferred Income Taxes and Valuation Allowance
We account for income taxes under ASC 740 “Income Taxes.” Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize the deferred tax assets through future operations.
Related Parties
We follow ASC 850, “Related Party Disclosures,” for the identification of related parties and disclosure of related party transactions.
Revenue Recognition
We recognize revenue in accordance with Topic 606, which requires revenues are recognized when control of the promised goods or services are transferred to a customer, in an amount that reflects the consideration that we expect to receive in exchange for those goods or services. We apply the following five steps in order to determine the appropriate amount of revenue to be recognized as we fulfill our obligations under each of its agreements:
| · | identify the contract with a customer; |
| · | identify the performance obligations in the contract; |
| · | determine the transaction price; |
| · | allocate the transaction price to performance obligations in the contract; and |
| · | recognize revenue as the performance obligation is satisfied. |
We recognize revenue when products are delivered to customers in accordance with the written sales terms.
Recent Accounting Pronouncements
In June 2022, the FASB issued ASU 2022-03, ASC Subtopic “Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions”. These amendments clarify that a contractual restriction on the sale of an equity security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value. The amendments in this update are effective for public business entities for fiscal years, including interim periods within those fiscal years, beginning after December 15, 2023. Early adoption is permitted. We do not believe the adoption of this standard will have any material impact on our financial statements.
We have reviewed all other recently issued, but not yet effective, accounting pronouncements and do not believe the future adoption of any such pronouncements may be expected to have a material impact on our financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements start on Page F-1.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not Applicable
ITEM 9A. CONTROLS AND PROCEDURES
Management’s Conclusions Regarding Effectiveness of Disclosure Controls and Procedures
We conducted an evaluation of the effectiveness of our “disclosure controls and procedures” (“Disclosure Controls”), as defined by Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), as of December 31, 2022, the end of the period covered by this annual report on Form 10-K. The Disclosure Controls evaluation was done under the supervision and with the participation of management, including our chief executive officer and chief financial officer, who is the same person and our sole employee. There are inherent limitations to the effectiveness of any system of disclosure controls and procedures. Accordingly, even effective disclosure controls and procedures can only provide reasonable assurance of achieving their control objectives. Based upon this evaluation, our chief executive officer and chief financial officer concluded that, due to our limited internal audit function and our very limited staff, our disclosure controls were not effective as of December 31, 2022, such that the information required to be disclosed by us in reports filed under the Securities Exchange Act of 1934 is (i) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to the chief executive officer/chief financial officer, as appropriate to allow timely decisions regarding disclosure.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act. Our management is also required to assess and report on the effectiveness of our internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 (“Section 404”). Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2022. In making this assessment, we used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control - Integrated Framework. During our assessment of the effectiveness of internal control over financial reporting as of December 31, 2022, management identified material weaknesses related to (i) our internal audit functions (ii) inadequate levels of review of the financial statements and (iii) a lack of segregation of duties within accounting functions. Therefore, our internal controls over financial reporting were not effective as of December 31, 2022.
Management has determined that our internal controls contain material weaknesses due to the absence of segregation of duties, as well as lack of qualified accounting personnel and excessive reliance on third party consultants for accounting and a reliance on outside services for our accounting functions, financial reporting and related activities. The lack of any separation of duties, with the same person, who is our only employee who serves as both chief executive officer and chief financial officer, and who does not have an accounting background and serves on a part-time basis, makes it unlikely that we will be able to implement effective internal controls over financial reporting in the near future.
Due to our size and nature, segregation of all conflicting duties is not possible. However, to the extent possible, we plan to implement procedures to assure that the initiation of transactions, the custody of assets and the recording of transactions will be performed by separate individuals if and when we have sufficient income to enable us to hire such individuals, and we cannot give any assurance that we will be able to hire such personnel. Our financial condition makes it difficult for us to implement a system of internal controls over financial reporting.
Until we generate significantly greater revenues and employ accounting personnel, it is doubtful that we will be able to implement any system which provides us with any degree of internal controls over financial reporting. Due to the nature of this material weakness in our internal control over financial reporting, there is more than a remote likelihood that misstatements which could be material to our annual or interim financial statements could not be prevented or detected.
A material weakness (within the meaning of PCAOB Auditing Standard No. 5) is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. A significant deficiency is a deficiency, or a combination of deficiencies, in internal control over financial reporting that is less severe than a material weakness, yet important enough to merit attention by those responsible for oversight of our financial reporting.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies and procedures may deteriorate.
Changes in Internal Control over Financial Reporting.
During the year ended December 31, 2022, there was no change in our internal control over financial reporting (as such term is defined in Rule 13a-15(f) under the Exchange Act) that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following table presents information with respect to our officers, directors:
Name | | Age | | Position(s) |
Pao-Chi Chu | | 69 | | Chief executive officer, chief financial officer, president, secretary and director |
Mr. Chu has been our chief executive officer, chief financial officer, president, secretary and a director since January 30, 2017. Mr. Chu has served as the chairman of Mucho Biotech Co., Ltd., Mucho Furich Co., Ltd., and Mucho Biomedical Co., Ltd., companies engaged in applications of cordyceps since 2006 and which are controlled by Mr. Chu. Mr. Chu has more than ten years of experience in the biotech industry with a focus on initiating the integration of cordyceps technology development, which includes cordyceps strains management, cordyceps cultivation, food processing and health products development. Mr. Chu is a graduate of Fu Jen Catholic University in Taipei, Taiwan.
Code of Ethics
We have not yet adopted a code of ethics that applies to our principal executive officers, principal financial officer, principal accounting officer or controller, or persons performing similar functions, since we have been focusing our efforts on developing our business. We expect to adopt a code as we develop our business.
Committees of the Board of Directors
We do not have any committees of our board of directors.
Compliance with Section 16(a) of the Securities Exchange Act of 1934
Our chief executive officer, who is our only officer and director, is current is these filing.
ITEM 11: EXECUTIVE COMPENSATION
The following summary compensation table sets forth information concerning compensation for services rendered in all capacities during the years ended December 31, 2022 and 2021, earned by or paid to our executive officers.
Name and Principal Position | | Period | | Salary ($) | | | Bonus Awards ($) | | | Stock Awards ($) | | | Options/ Warrant Awards (1) ($) | | | Non-Equity Plan Compensation ($) | | | Nonqualified Deferred Earnings ($) | | | All Other Compensation ($) | | | Total ($) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Pao-Chi Chu | | 2022 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
CEO, CFO and President | | 2021 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
Employment Agreements
We have no employment agreements with Mr. Chu.
Pension Benefits
We currently have no plans that provide for payments or other benefits at, following, or in connection with retirement of our officer.
Outstanding Equity Awards at Fiscal Year-End
There are no outstanding equity awards at December 31, 2022
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table provides information as to shares of common stock beneficially owned as of June 20, 2023, by:
| · | Each director; |
| · | Each current officer named in the summary compensation table; |
| · | Each person owning of record or known by us, based on information provided to us by the persons named below, at least 5% of our common stock; and |
| · | All directors and officers as a group. |
For purposes of the following table, “beneficial ownership” means the sole or shared power to vote, or to direct the voting of, a security, or sole or shared investment power with respect to a security, or any combination thereof, and the right to acquire such power (for example, through the exercise of options, warrants or convertible securities) within 60 days of June 20, 2023. None of the named beneficial owners held any options, warrants or convertible securities at June 20, 2023.
Name and Address of Beneficial Owner | | Amount and Nature of Beneficial Ownership | | | % of Class | |
| | | | | | |
Pao-Chi Chu 2F, No. 356, Dunhua S. Road, Da’an Dist Taipei City 106, Taiwan, ROC | | | 30,000,000 | | | | 49.97 | % |
| | | | | | | | |
All officers and directors as a group (one individual) | | | 30,000,000 | | | | 49.97 | % |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Related Party Transactions
During the years ended December 31, 2022 and 2021, a minority stockholder advanced $55,000 and $0, paid expenses of $3,085 and $19,641 on our behalf, and we repaid advances of $0 and $241,851, respectively.
At December 31, 2022 and 2021, we owed $1,100 and $1,100 to our chief executive officer for non-interest-bearing advances made to us or on our behalf. These advances are due on demand.
At December 31, 2022 and 2021, we owed $77,726 and $19,641 to a minority stockholder for non-interest-bearing advances made to us or on our behalf, respectively. These advances are due on demand.
We have imputed interest at the rate of 4% on the advances made to us or on our behalf. Imputed interest amounted to $1,706 and $4,250, during the years ended December 31, 2022 and 2021, respectively.
As of December 31, 2022 and 2021, the Company had amounts due to related parties of $78,826 and $20,741, respectively.
Director Independence
We have no independent directors.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The following table sets forth the fees billed by our independent accountants, Prager Metis, CPAs LLC for the years ended December 31, 2022 and 2021.
| | Year Ended December 31, | |
| | 2022 | | | 2021 | |
Audit fees | | $ | 40,500 | | | $ | 44,250 | |
Audit-related fees | | | - | | | | - | |
Tax fees | | | - | | | | - | |
All other fees | | | - | | | | - | |
Audit fees consist of fees related to professional services rendered in connection with the audit and review of our annual financial statements.
Our policy is to pre-approve all audit and permissible non-audit services performed by the independent accountants. These services may include audit services, audit-related services, tax services and other services. Under our audit committee’s policy, pre-approval is generally provided for particular services or categories of services, including planned services, project based services and routine consultations. In addition, the audit committee may also pre-approve particular services on a case-by-case basis. Our board approved all services that our independent accountants provided to us in the past two fiscal years.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
EXHIBIT
______________
(1) | Filed as an exhibit to the Company’s report on Form 8-K, which was filed with the SEC on February 1, 2017. |
(2) | Filed as an exhibit on to the Company’s registration statement on Form 10, which was filed on September 8, 2020. |
(3) | Filed as an exhibit to the Company’s report on Form 8-K, which was filed with the SEC on August 13, 2020. |
Item 16. FORM 10-K SUMMARY
Not applicable
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Date: July 21, 2023
| ACRO BIOMEDICAL CO., LTD. | |
| | | |
| By: | /s/ Pao-Chi Chu | |
| Name: | Pao-Chi Chu | |
| Title: | Chief Executive Officer | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | | Title | | Date |
| | | | |
/s/ Pao-Chi Chu | | Director, chief executive officer and | | July 21, 2023 |
Pao-Chi Chu | | chief financial officer (principal executive, financial and accounting officer) | | |
ACRO BIOMEDICAL CO., LTD.
Index to Financial Statements
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of
Acro Biomedical Co., Ltd.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Acro Biomedical Co., Ltd. ("the Company") as of December 31, 2022, and 2021, and the related statements of operations, changes in stockholders' equity and cash flows for the years ended December 31, 2022, and 2021, and related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022, and 2021 and the results of its operations and its cash flows for the years ended December 31, 2022, and 2021 in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company had limited cash as of December 31, 2022, had limited gross profit and incurred a loss from its operations for the year ended December 31, 2022, and past few years. These circumstances, among others, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are described in Note 3 to the financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters for the current audit period.
/s/ Prager Metis CPAs, LLC
We have served as the Company's auditor since 2018.
Hackensack, New Jersey
July 21, 2023
ACRO BIOMEDICAL CO., LTD.
Balance Sheets
| | December 31, | | | December 31, | |
| | 2022 | | | 2021 | |
| | | | | | |
ASSETS | | | | | | |
Current Assets | | | | | | |
Cash | | $ | 5,852 | | | $ | 95,248 | |
Accounts receivable | | | 638,500 | | | | 598,000 | |
Purchase deposit for inventory | | | 12,000 | | | | 12,000 | |
Prepaid expenses | | | 1,185 | | | | 1,167 | |
Total Current Assets | | | 657,537 | | | | 706,415 | |
| | | | | | | | |
Operating lease right of use asset | | | 25,719 | | | | 50,432 | |
Security deposit | | | 4,230 | | | | 4,230 | |
TOTAL ASSETS | | $ | 687,486 | | | $ | 761,077 | |
| | | | | | | | |
LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | | |
| | | | | | | | |
Current Liabilities | | | | | | | | |
Accounts payable and accrued expenses | | $ | 76,571 | | | $ | 26,197 | |
Deferred revenue | | | 20,000 | | | | 20,000 | |
Due to related parties | | | 78,826 | | | | 20,741 | |
Operating lease liabilities - current | | | 25,719 | | | | 24,713 | |
Total Current Liabilities | | | 201,116 | | | | 91,651 | |
| | | | | | | | |
Operating lease liabilities - noncurrent | | | - | | | | 25,719 | |
TOTAL LIABILITIES | | | 201,116 | | | | 117,370 | |
| | | | | | | | |
Stockholders' Equity | | | | | | | | |
Preferred stock: 25,000,000 authorized; $0.001 par value; no shares issued and outstanding | | | - | | | | - | |
Common stock: 100,000,000 authorized; $0.001 par value; 60,042,000 shares issued and outstanding as of December 31,2022 and 2021 | | | 60,042 | | | | 60,042 | |
Additional paid-in capital | | | 32,295,236 | | | | 32,293,530 | |
Deferred stock compensation | | | (8,060,983 | ) | | | (23,773,383 | ) |
Accumulated deficit | | | (23,807,925 | ) | | | (7,936,482 | ) |
Total Stockholders’ Equity | | | 486,370 | | | | 643,707 | |
| | | | | | | | |
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY | | $ | 687,486 | | | $ | 761,077 | |
The accompanying notes are an integral part of these financial statements.
ACRO BIOMEDICAL CO., LTD.
Statements of Operations
| | Year ended | |
| | December 31, | |
| | 2022 | | | 2021 | |
| | | | | | |
Revenues | | $ | 658,500 | | | $ | 1,197,500 | |
Cost of revenues | | | 518,000 | | | | 938,000 | |
Gross profit | | | 140,500 | | | | 259,500 | |
| | | | | | | | |
Operating expenses | | | | | | | | |
Selling, general and administrative | | | 5,930,237 | | | | 2,667,903 | |
Research and development | | | 10,080,000 | | | | 5,285,175 | |
Total operating expenses | | | 16,010,237 | | | | 7,953,078 | |
| | | | | | | | |
Loss from operations | | | (15,869,737 | ) | | | (7,693,578 | ) |
| | | | | | | | |
Other expense | | | | | | | | |
Interest expense - related party | | | 1,706 | | | | 4,250 | |
Total other expenses | | | 1,706 | | | | 4,250 | |
| | | | | | | | |
Loss before income tax credit | | | (15,871,443 | ) | | | (7,697,828 | ) |
Income taxes expenses (credit) | | | - | | | | - | |
Net loss | | $ | (15,871,443 | ) | | $ | (7,697,828 | ) |
| | | | | | | | |
Basic and diluted loss per share of common stock | | $ | (0.26 | ) | | $ | (0.14 | ) |
| | | | | | | | |
Weighted average number of shares of common stock outstanding | | | 60,042,000 | | | | 53,838,855 | |
The accompanying notes are an integral part of these financial statements.
ACRO BIOMEDICAL CO., LTD.
Statements of Changes in Stockholders’ Equity
| | | | | | | | | | | | | | Additional | | | Deferred | | | | | | | |
| | Preferred Stock | | | Common Stock | | | Paid in | | | stock | | | Accumulated | | | | |
| | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | compensation | | | Deficit | | | Total | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2020 | | | - | | | $ | - | | | | 47,760,000 | | | $ | 47,760 | | | $ | 876,762 | | | $ | - | | | $ | (238,654 | ) | | $ | 685,868 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Share issuance for service | | | - | | | | - | | | | 12,282,000 | | | | 12,282 | | | | 31,412,518 | | | | (23,773,383 | ) | | | - | | | | 7,651,417 | |
Imputed interest on related party loans | | | - | | | | - | | | | - | | | | - | | | | 4,250 | | | | - | | | | - | | | | 4,250 | |
Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (7,697,828 | ) | | | (7,697,828 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2021 | | | - | | | $ | - | | | | 60,042,000 | | | $ | 60,042 | | | $ | 32,293,530 | | | $ | (23,773,383 | ) | | $ | (7,936,482 | ) | | $ | 643,707 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Amortization of deferred stock compensation | | | - | | | | - | | | | - | | | | - | | | | - | | | | 15,712,400 | | | | - | | | | 15,712,400 | |
Imputed interest on related party loans | | | - | | | | - | | | | - | | | | - | | | | 1,706 | | | | - | | | | - | | | | 1,706 | |
Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (15,871,443 | ) | | | (15,871,443 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2022 | | | - | | | $ | - | | | | 60,042,000 | | | $ | 60,042 | | | $ | 32,295,236 | | | $ | (8,060,983 | ) | | $ | (23,807,925 | ) | | $ | 486,370 | |
The accompanying notes are an integral part of these financial statements.
ACRO BIOMEDICAL CO., LTD.
Statements of Cash Flows
| | Year ended | |
| | December 31, | |
| | 2022 | | | 2021 | |
| | | | | | |
CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | |
Net loss | | $ | (15,871,443 | ) | | $ | (7,697,828 | ) |
Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | | | | | | | | |
Imputed interest - related parties | | | 1,706 | | | | 4,250 | |
Amortization of deferred stock compensation | | | 15,712,400 | | | | 7,651,417 | |
Change of ROU and lease liabilities | | | - | | | | 200 | |
Changes in operating assets and liabilities: | | | | | | | | |
Accounts receivable | | | (40,500 | ) | | | (598,000 | ) |
Inventories | | | - | | | | 938,000 | |
Prepaid expenses | | | (18 | ) | | | (167 | ) |
Accounts payable and accrued expenses | | | 50,374 | | | | 1,463 | |
Net cash provided by (used in) operating activities | | | (147,481 | ) | | | 299,335 | |
| | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | | |
Advances from related parties | | | 58,085 | | | | 19,641 | |
Repayment to related parties | | | - | | | | (241,851 | ) |
Net cash provided by (used in) financing activities | | | 58,085 | | | | (222,210 | ) |
| | | | | | | | |
Net change in cash | | | (89,396 | ) | | | 77,125 | |
Cash at beginning of year | | | 95,248 | | | | 18,123 | |
Cash at end of year | | $ | 5,852 | | | $ | 95,248 | |
| | | | | | | | |
SUPPLEMENTAL CASH FLOW INFORMATION: | | | | | | | | |
Cash paid for income taxes | | $ | - | | | $ | - | |
Cash paid for interest | | $ | - | | | $ | - | |
| | | | | | | | |
NON CASH INVESTING AND FINANCING ACTIVITIES | | | | | | | | |
Increase in the right-of-use asset and lease liability | | $ | - | | | $ | 50,432 | |
The accompanying notes are an integral part of these financial statements.
ACRO BIOMEDICAL CO., LTD.
Notes to the Financial Statements
NOTE 1 -ORGANIZATION AND DESCRIPTION OF BUSINESS
Acro Biomedical Co., Ltd. (the “Company”) is a Nevada corporation incorporated on September 24, 2014 under the name Killer Waves Hawaii, Inc. On January 30, 2017, the Company’s corporate name was changed to Acro Biomedical Co., Ltd.
The Company’s business is the sale of cordyceps related products. Cordyceps is a fungus that is used in traditional Chinese medicine. During the second and third quarters of 2021, the Company engaged consultants to take the initial steps to develop and implement a research and development and marketing program. These consultants are working independently and report to the chief executive officer. As of December 31, 2022, the research and development efforts had not developed any products, the contracts with the consultants expired in May 2023 and will expire in August 2023, and the Company cannot give any assurance that the marketing and research development activities will generate any new product or new marketing opportunities or generate any revenue.
NOTE 2 -SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The financial statements have been prepared in accordance with Generally Accepted Accounting Principles (“GAAP”) of the United States.
Use of Estimates
The preparation of financial statements in conformity with GAAP in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements. The estimates and judgments will also affect the reported amounts for certain revenues and expenses during the reporting period. Actual results could differ from these good faith estimates and judgments.
Cash and Cash Equivalents
Cash and cash equivalents include cash in banks, money market funds, and certificates of term deposits with maturities of less than three months from inception, which are readily convertible to known amounts of cash and which, in the opinion of management, are subject to an insignificant risk of loss in value.
Accounts Receivable
Accounts receivable are recorded in accordance with ASC 310, “Receivables.” Accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts is the Company’s best estimate of the amount of probable credit losses in its existing accounts receivable. The Company does not currently have any amount recorded as an allowance for doubtful accounts. Based on management’s estimate, the Company has not deemed it necessary to reserve for doubtful accounts at this time.
Inventories
Inventories consist of finished goods. Inventories are valued at the lower of cost or net realizable value. The Company determines cost on the basis of first-in, first-out methods. The Company periodically reviews inventories for obsolescence and any inventories identified as obsolete are written down or written off. Although the Company believes that the assumptions it uses to estimate inventory write-downs are reasonable, future changes in these assumptions could provide a significantly different result. No inventory markdown was recorded for the years ended December 31, 2022 and 2021.
Leases
The Company determines if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, other current liabilities, and operating lease liabilities in the Company’ balance sheets if the lease term is more than one year. Finance leases are included in property and equipment, other current liabilities, and other long-term liabilities in the Company’s balance sheets.
ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As most of the Company’s leases do not provide an implicit rate, the Company generally uses its incremental borrowing rate based on the estimated rate of interest for collateralized borrowing over a similar term of the lease payments at commencement date. The operating lease ROU asset also includes any lease payments made and excludes lease incentives. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option.
Leases with a lease term of 12 months or less at inception are not recorded on the Company’s balance sheet and are expensed on a straight- line basis over the lease term in the statement of operations.
Net (Loss) Per Share of Common Stock
The Company has adopted ASC Topic 260, ”Earnings per Share” which requires presentation of basic earnings per share on the face of the statements of operations for all entities with complex capital structures and requires a reconciliation of the numerator and denominator of the basic earnings per share computation. In the accompanying financial statements, basic loss per share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per share is computed by dividing net income by the weighted average number of shares of common stock and potentially dilutive outstanding shares of common stock during the period to reflect the potential dilution that could occur from common stock issuable through contingent share arrangements, stock options and warrants unless the result would be antidilutive. There were no potentially dilutive shares of common stock outstanding for the years ended December 31, 2022 and 2021, respectively.
Concentrations of Credit Risk
The Company’s financial instruments that are exposed to concentrations of credit risk primarily consist of its cash and cash equivalents and related party payables that it will likely incur in the near future. The Company places its cash and cash equivalents with a United States financial institutions which the Company believes are of high creditworthiness. At times, its cash and cash equivalents with a particular financial institution may exceed any applicable government insurance limits.
Financial Instruments
The Company follows ASC 820, “Fair Value Measurements and Disclosures,” which defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy that distinguishes between (1) market participant assumptions developed based on market data obtained from independent sources (observable inputs) and (2) an entity’s own assumptions about market participant assumptions developed based on the best information available in the circumstances (unobservable inputs). The fair value hierarchy consists of three broad levels, which gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3). The three levels of the fair value hierarchy are described below:
Level 1
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3
Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
The carrying values of financial instruments, including, cash, accounts receivable, prepaid expenses, accounts payable and accrued expenses and due to related parties approximate their fair values due to the short-term maturities of these financial instruments.
Stock-Based Compensation
The Company recognizes compensation expense for stock-based compensation in accordance with ASC Topic 718. For employee and non-employee stock-based awards, the Company calculates the fair value of the award on the date of grant using the option-pricing model for stock options and the quoted price of its common stock for unrestricted shares; the expense is recognized over the service period for awards expected to vest. The Company considers many factors when estimating expected forfeitures, including types of awards, employee class, and historical experience.
Deferred Income Taxes and Valuation Allowance
The Company accounts for income taxes under ASC 740 “Income Taxes.” Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize the deferred tax assets through future operations.
Related Parties
The Company follows ASC 850, ”Related Party Disclosures,” for the identification of related parties and disclosure of related party transactions and balances.
Revenue Recognition
The Company recognizes revenue in accordance with Topic 606, which requires revenues are recognized when control of the promised goods or services are transferred to a customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services. The Company applies the following five steps in order to determine the appropriate amount of revenue to be recognized as it fulfills its obligations under each of its agreements:
| · | identify the contract with a customer; |
| · | identify the performance obligations in the contract; |
| · | determine the transaction price; |
| · | allocate the transaction price to performance obligations in the contract; and |
| · | recognize revenue as the performance obligation is satisfied. |
The Company recognizes revenue when products are delivered to customers in accordance with the written sales terms.
Deferred Revenue
The Company sells products pursuant to agreements for the delivery of products to customers. The Company receives cash in advance and records it as deferred revenue.
Deferred revenue was $20,000 at December 31, 2022 and 2021.
Recent Accounting Pronouncements
In June 2022, the FASB issued ASU 2022-03, ASC Subtopic “Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions”. These amendments clarify that a contractual restriction on the sale of an equity security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value. The amendments in this update are effective for public business entities for fiscal years, including interim periods within those fiscal years, beginning after December 15, 2023. Early adoption is permitted. The Company does not believe that the impact of the adoption of this standard will have a material effect on its financial statements.
The Company has reviewed all other recently issued, but not yet effective, accounting pronouncements and does not believe the future adoption of any such pronouncements may be expected to cause a material impact on the Company’s financial statements.
COVID 19
Since the Company’s products are purchased by customers in the Republic of China (Taiwan) and Hong Kong who sold products to their customers in the People’s Republic of China (the “PRC”), the Company’s business was impacted by the effects of the COVID-19 pandemic and the actions taken by the governments of the PRC, the Republic of China (Taiwan) and Hong Kong.
The World Health Organization ended the global emergency status for COVID-19 on May 5, 2023. As restrictions that had been imposed, particularly China’s zero COVID policy, to address the pandemic have been lifted, the Company cannot give assurance that its sales will increase as a result of the reduction of such restrictions, and the Company’s revenues decreased from 2021 to 2022. Although China has lifted COVID-19 restrictions, there continues to be a risk of the deaths and lockdowns from COVID-19.
As the world has begun to open following closures as a result of the pandemic, two other factors are facing businesses and consumers, which are considered to be related to the effects of the COVID-19 pandemic. These are inflation and supply chain issues, which have been exacerbated by the recent Russian invasion of Ukraine. The Company cannot estimate the effect of these factors on its business. To the extent that these factors result in increased prices, the Company may not be able to pass along the increases to its customers. Further, to the extent customers use the Company’s products as an ingredient in their own products, the inability of a potential customer to obtain other raw materials as well as cost increases for such products, could affect the timing and the amount of purchases from the Company. The Company cannot give assurance that its business will not be impaired by the effects of inflation and supply chain issues.
NOTE 3 – GOING CONCERN
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates the realization of assets and the liquidation of liabilities in the normal course of business. The Company had minimal cash as of December 31, 2022, had limited gross profit and incurred a loss from operations for the year ended December 31, 2022 and past few years. These factors, among others, raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
The Company proposes to fund operations through sales of its products and equity financing arrangements. However, because of the lack of sales and the absence of any active trading market for its common stock, its financial condition and its lack of an operating history, the Company may not be able to raise funds for capital expenditures, working capital and other cash requirements and will have to rely on advances from a minority stockholder and an officer. If the Company cannot generate revenue from its products, it may not be able to continue in its business.
NOTE 4 - EQUITY
Common Stock
The Company issued a total of 6,776,000 and 5,506,000 shares of common stock to consultants as stock grants pursuant to agreements with the consultants in May 2021 and August 2021, respectively, of which 11,912,000 shares were issued pursuant to the 2020 equity incentive plan. (the “Plan”) and 370,000 were issued as restricted stock outside of the Plan. The agreements provide for the consultants to perform services described in the contracts for the two-year period commencing May 25, 2021 and August 23, 2021. The shares were valued at $19,311,600 and $12,113,200, based on the market price of the common stock on the respective dates of the agreements, which was $2.85 and $2.20 per share, respectively, and is being amortized over two-year period starting from the date of the agreement using the straight-line method. During the year ended December 31, 2022 and 2021, the Company recorded stock-based compensation of $15,712,400 and $7,651,417, respectively, and had deferred stock compensation of $8,060,983 and $23,773,383 as of December 31, 2022 and 2021 which will be recognized over the balance of the agreements.
During the year ended December 31, 2022, the Company did not issue any shares of common stock.
NOTE 5 - INCOME TAXES
The reconciliation of income tax expense at the U.S. statutory rate of 21% to the Company’s effective tax rate is as follows:
| | Year ended | |
| | December 31, | |
| | 2022 | | | 2021 | |
Income tax credit at statutory rate | | $ | (3,333,003 | ) | | $ | (1,616,544 | ) |
Income tax adjustment | | | | | | | | |
Imputed interest | | | 358 | | | | 893 | |
Change of valuation allowance | | | 3,332,645 | | | | 1,615,651 | |
Income tax expense | | $ | - | | | $ | - | |
Net deferred tax assets consist of the following components as of:
| | December 31, | | | December 31, | |
| | 2022 | | | 2021 | |
Operating loss carry forward | | $ | 5,014,941 | | | $ | 1,682,296 | |
Valuation allowance | | | (5,014,941 | ) | | | (1,682,296 | ) |
Deferred tax asset | | $ | - | | | $ | - | |
The Company has approximately $24 million net operating loss carryforwards that are available to reduce future taxable income. In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers projected future taxable income and tax planning strategies in making this assessment. Based on the assessment, management has established a full valuation allowance against all of the deferred tax assets for the year ended December 31, 2022 and 2021 because it is more likely than not that all of the deferred tax assets will not be realized.
NOTE 6 - RELATED PARTY TRANSACTIONS
At December 31, 2022 and 2021, the Company owed $1,100 and $1,100 to its CEO for non-interest-bearing advances made to or on behalf of the Company, respectively. These advances are due on demand.
During the years ended December 31, 2022 and 2021, a minority stockholder advanced $55,000 and $0 paid expenses of $3,085 and $19,641 on behalf of the Company, and the Company repaid $0 and $241,851, respectively.
At December 31, 2022 and 2021, the Company owed $77,726 and $19,641 to a stockholder who is not a 5% stockholder for non-interest-bearing advances made to or on behalf of the Company, respectively. These advances are due on demand.
The Company has imputed interest at the rate of 4% on the advances made to or on behalf of the Company. Imputed interest amounted to $1,706 and $4,250, during the years ended December 31, 2022 and 2021, respectively.
As of December 31, 2022 and 2021, the Company had amounts due to related parties of $78,826 and $20,741, respectively.
NOTE 7 – LEASES
On December 27, 2019, the Company entered into a lease agreement to rent a storage facility in Hong Kong for a two-year term at HK$16,500 (approximately $2,115) per month. A stockholder paid HK$33,000 (approximately $4,230) as a security deposit and HK$16,500 (approximately $2,115) as prepaid rent on behalf of the Company. The lease expired in December 2021.
On November 3, 2021, the Company entered into a lease agreement to rent a storage facility in Hong Kong for a two-year term at HK$17,000 (approximately $2,190) per month and HK$33,000 (approximately $4,230) as a security deposit. These payments were paid by the stockholder on behalf of the Company.
In accordance with ASC 842, the Company recognized operating lease ROU assets and lease liabilities as follows:
| | December 31, | | | December 31, | |
| | 2022 | | | 2021 | |
Operating lease ROU asset | | $ | 25,719 | | | $ | 50,432 | |
| | December 31, | | | December 31, | |
Operating lease liabilities | | 2022 | | | 2021 | |
Current portion | | $ | 25,719 | | | $ | 24,713 | |
Non-current portion | | | - | | | | 25,719 | |
Total | | $ | 25,719 | | | $ | 50,432 | |
Future minimum lease payments under operating leases at December 31, 2022 were as follows:
2023 | | $ | 26,280 | |
Thereafter | | | - | |
Total | | $ | 26,280 | |
The Company recognized total lease expense of $26,280 and $25,140 for the years ended December 31, 2022 and 2021, respectively.
NOTE 8 – CONCENTRATION
Revenue and accounts receivable
For the years ended December 31, 2022 and 2021, customer concentrations (more than 10%) were as follows:
| | Percentage of revenue | | | Percentage of Accounts receivable | |
| | Year ended December 31 | | | December 31, | | | December 31, | |
| | 2022 | | | 2021 | | | 2022 | | | 2021 | |
Customer A | | | 55 | % | | | 59 | % | | | 56 | % | | | 33 | % |
Customer B | | | 45 | % | | | 33 | % | | | 44 | % | | | 67 | % |
Total (as a group) | | | 100 | % | | | 92 | % | | | 100 | % | | | 100 | % |
Purchases
During the year ended December 31, 2022, all purchases were made from one supplier.
During the year ended December 31, 2021, the Company did not purchase inventory.
NOTE 9 - SUBSEQUENT EVENTS
The Company has evaluated subsequent events that have occurred after the date of the balance sheet through the date of issuance of these financial statements and determined that no subsequent event requires recognition or disclosure to the financial statements.
nullnull
v3.23.2
Cover - USD ($)
|
12 Months Ended |
|
|
Dec. 31, 2022 |
Jul. 17, 2023 |
Jun. 30, 2022 |
| Cover [Abstract] |
|
|
|
| Entity Registrant Name |
ACRO BIOMEDICAL CO., LTD.
|
|
|
| Entity Central Index Key |
0001622996
|
|
|
| Document Type |
10-K
|
|
|
| Amendment Flag |
false
|
|
|
| Entity Voluntary Filers |
No
|
|
|
| Current Fiscal Year End Date |
--12-31
|
|
|
| Entity Well Known Seasoned Issuer |
No
|
|
|
| Entity Small Business |
true
|
|
|
| Entity Shell Company |
false
|
|
|
| Entity Emerging Growth Company |
false
|
|
|
| Entity Current Reporting Status |
Yes
|
|
|
| Document Period End Date |
Dec. 31, 2022
|
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
|
| Document Fiscal Period Focus |
FY
|
|
|
| Document Fiscal Year Focus |
2022
|
|
|
| Entity Common Stock Shares Outstanding |
|
60,042,000
|
|
| Entity Public Float |
|
|
$ 127,678,500
|
| Document Annual Report |
true
|
|
|
| Document Transition Report |
false
|
|
|
| Entity File Number |
000-55643
|
|
|
| Entity Incorporation State Country Code |
NV
|
|
|
| Entity Tax Identification Number |
47-1950356
|
|
|
| Entity Interactive Data Current |
Yes
|
|
|
| Entity Address Address Line 1 |
12175 Visionary Way
|
|
|
| Entity Address Address Line 2 |
Suite 1160
|
|
|
| Entity Address City Or Town |
Fishers
|
|
|
| Entity Address State Or Province |
IN
|
|
|
| Entity Address Postal Zip Code |
46038
|
|
|
| City Area Code |
317
|
|
|
| Local Phone Number |
286-6788
|
|
|
| Security 12g Title |
Common Stock, par value $0.001 per share
|
|
|
| Auditor Name |
Prager Metis CPAs, LLC
|
|
|
| Auditor Location |
Hackensack, New Jersey
|
|
|
| Auditor Firm Id |
273
|
|
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPCAOB issued Audit Firm Identifier Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorFirmId |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:nonemptySequenceNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorLocation |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an annual report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentAnnualReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter.
| Name: |
dei_EntityPublicFloat |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
| Name: |
dei_EntityVoluntaryFilers |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Is used on Form Type: 10-K, 10-Q, 8-K, 20-F, 6-K, 10-K/A, 10-Q/A, 20-F/A, 6-K/A, N-CSR, N-Q, N-1A. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 405
| Name: |
dei_EntityWellKnownSeasonedIssuer |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(g) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection g
| Name: |
dei_Security12gTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Balance Sheets - USD ($)
|
Dec. 31, 2022 |
Dec. 31, 2021 |
| Current Assets |
|
|
| Cash |
$ 5,852
|
$ 95,248
|
| Accounts receivable |
638,500
|
598,000
|
| Purchase deposit for inventory |
12,000
|
12,000
|
| Prepaid expenses |
1,185
|
1,167
|
| Total Current Assets |
657,537
|
706,415
|
| Operating lease right of use asset |
25,719
|
50,432
|
| Security deposit |
4,230
|
4,230
|
| TOTAL ASSETS |
687,486
|
761,077
|
| Current Liabilities |
|
|
| Accounts payable and accrued expenses |
76,571
|
26,197
|
| Deferred revenue |
20,000
|
20,000
|
| Due to related parties |
78,826
|
20,741
|
| Operating lease liabilities - current |
25,719
|
24,713
|
| Total Current Liabilities |
201,116
|
91,651
|
| Operating lease liabilities - noncurrent |
0
|
25,719
|
| TOTAL LIABILITIES |
201,116
|
117,370
|
| Stockholders' Equity |
|
|
| Preferred stock: 25,000,000 authorized; $0.001 par value; no shares issued and outstanding |
0
|
0
|
| Common stock: 100,000,000 authorized; $0.001 par value; 60,042,000 shares issued and outstanding as of December 31,2022 and 2021 |
60,042
|
60,042
|
| Additional paid-in capital |
32,295,236
|
32,293,530
|
| Deferred stock compensation |
(8,060,983)
|
(23,773,383)
|
| Accumulated deficit |
(23,807,925)
|
(7,936,482)
|
| Total Stockholders' Equity |
486,370
|
643,707
|
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY |
$ 687,486
|
$ 761,077
|
| X |
- References
+ Details
| Name: |
acbm_DeferredStockCompensationEquity |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
acbm_PurchaseDepositForInventoryCurrent |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 310
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480833/946-310-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(5)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 310
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481058/954-310-45-1
| Name: |
us-gaap_AccountsReceivableNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 45
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-21
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 210
-Topic 946
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-20
| Name: |
us-gaap_Cash |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred income and obligation to transfer product and service to customer for which consideration has been received or is receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DeferredRevenue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesAndStockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
| Name: |
us-gaap_PrepaidExpenseCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of an asset, typically cash, provided to a counterparty to provide certain assurance of performance by the entity pursuant to the terms of a written or oral agreement, such as a lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_SecurityDeposit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
Balance Sheets (Parentheticals) - $ / shares
|
Dec. 31, 2022 |
Dec. 31, 2021 |
| Balance Sheets |
|
|
| Preferred stock, par value |
$ 0.001
|
$ 0.001
|
| Preferred stock, shares authorized |
25,000,000
|
25,000,000
|
| Preferred stock, shares issued |
0
|
0
|
| Preferred stock, shares outstanding |
0
|
0
|
| Common stock, shares authorized |
100,000,000
|
100,000,000
|
| Common stock, par value |
$ 0.001
|
$ 0.001
|
| Common stock, shares issued |
60,042,000
|
60,042,000
|
| Common stock, shares outstanding |
60,042,000
|
60,042,000
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) issued to shareholders (includes related preferred shares that were issued, repurchased, and remain in the treasury). May be all or portion of the number of preferred shares authorized. Excludes preferred shares that are classified as debt. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Statements of Operations - USD ($)
|
12 Months Ended |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Statements of Operations |
|
|
| Revenues |
$ 658,500
|
$ 1,197,500
|
| Cost of revenues |
518,000
|
938,000
|
| Gross profit |
140,500
|
259,500
|
| Operating expenses |
|
|
| Selling, general and administrative |
5,930,237
|
2,667,903
|
| Research and development |
10,080,000
|
5,285,175
|
| Total operating expenses |
16,010,237
|
7,953,078
|
| Loss from operations |
(15,869,737)
|
(7,693,578)
|
| Other expense |
|
|
| Interest expense - related party |
1,706
|
4,250
|
| Total other expenses |
1,706
|
4,250
|
| Loss before income tax credit |
(15,871,443)
|
(7,697,828)
|
| Income taxes expenses (credit) |
0
|
0
|
| Net loss |
$ (15,871,443)
|
$ (7,697,828)
|
| Basic and diluted loss per share of common stock |
$ (0.26)
|
$ (0.14)
|
| Weighted average number of shares of common stock outstanding |
60,042,000
|
53,838,855
|
| X |
- References
+ Details
| Name: |
acbm_EarningsPerSharesBasicAndDiluted |
| Namespace Prefix: |
acbm_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
acbm_WeightedAverageNumberOfSharesOfCommonStockOutstanding |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate cost of goods produced and sold and services rendered during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_CostOfRevenue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate revenue less cost of goods and services sold or operating expenses directly attributable to the revenue generation activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.1,2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GrossProfit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncomeStatementAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4,6)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total costs related to selling a firm's product and services, as well as all other general and administrative expenses. Direct selling expenses (for example, credit, warranty, and advertising) are expenses that can be directly linked to the sale of specific products. Indirect selling expenses are expenses that cannot be directly linked to the sale of specific products, for example telephone expenses, Internet, and postal charges. General and administrative expenses include salaries of non-sales personnel, rent, utilities, communication, etc. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_SellingGeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.2
Statements of Changes in Stockholders' Equity - USD ($)
|
Total |
Common Stock |
Preferred Stock |
Additional Paid-In Capital |
Deferred stock compensation |
Retained Earnings (Accumulated Deficit) |
| Balance, shares at Dec. 31, 2020 |
|
47,760,000
|
|
|
|
|
| Balance, amount at Dec. 31, 2020 |
$ 685,868
|
$ 47,760
|
$ 0
|
$ 876,762
|
$ 0
|
$ (238,654)
|
| Share issuance for service, shares |
|
12,282,000
|
|
|
|
|
| Share issuance for service, amount |
7,651,417
|
$ 12,282
|
0
|
31,412,518
|
(23,773,383)
|
0
|
| Imputed interest on related party loans |
4,250
|
0
|
0
|
4,250
|
0
|
0
|
| Net loss |
(7,697,828)
|
$ 0
|
0
|
0
|
0
|
(7,697,828)
|
| Balance, shares at Dec. 31, 2021 |
|
60,042,000
|
|
|
|
|
| Balance, amount at Dec. 31, 2021 |
643,707
|
$ 60,042
|
0
|
32,293,530
|
(23,773,383)
|
(7,936,482)
|
| Imputed interest on related party loans |
1,706
|
0
|
0
|
1,706
|
0
|
0
|
| Net loss |
(15,871,443)
|
0
|
0
|
0
|
0
|
(15,871,443)
|
| Amortization of deferred stock compensation |
15,712,400
|
$ 0
|
0
|
0
|
15,712,400
|
0
|
| Balance, shares at Dec. 31, 2022 |
|
60,042,000
|
|
|
|
|
| Balance, amount at Dec. 31, 2022 |
$ 486,370
|
$ 60,042
|
$ 0
|
$ 32,295,236
|
$ (8,060,983)
|
$ (23,807,925)
|
| X |
- References
+ Details
| Name: |
acbm_AmortizationOfDeferredStockCompensation |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
acbm_ShareIssuanceForServicesAmount |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
acbm_ShareIssuanceForServicesShares |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued as of the balance sheet date, including shares that had been issued and were previously outstanding but which are now held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
| Name: |
us-gaap_SharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
Statements of Cash Flows - USD ($)
|
12 Months Ended |
Dec. 31, 2022 |
Dec. 31, 2021 |
| CASH FLOWS FROM OPERATING ACTIVITIES |
|
|
| Net loss |
$ (15,871,443)
|
$ (7,697,828)
|
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
|
|
| Imputed interest - related parties |
1,706
|
4,250
|
| Amortization of deferred stock compensation |
15,712,400
|
7,651,417
|
| Change of ROU and lease liabilities |
0
|
200
|
| Changes in operating assets and liabilities: |
|
|
| Accounts receivable |
(40,500)
|
(598,000)
|
| Inventories |
0
|
938,000
|
| Prepaid expenses |
(18)
|
(167)
|
| Accounts payable and accrued expenses |
50,374
|
1,463
|
| Net cash provided by (used in) operating activities |
(147,481)
|
299,335
|
| CASH FLOWS FROM FINANCING ACTIVITIES |
|
|
| Advances from related parties |
58,085
|
19,641
|
| Repayment to related parties |
0
|
(241,851)
|
| Net cash provided by (used in) financing activities |
58,085
|
(222,210)
|
| Net change in cash |
(89,396)
|
77,125
|
| Cash at beginning of year |
95,248
|
18,123
|
| Cash at end of year |
5,852
|
95,248
|
| SUPPLEMENTAL CASH FLOW INFORMATION: |
|
|
| Cash paid for income taxes |
0
|
0
|
| Cash paid for interest |
0
|
0
|
| NON CASH INVESTING AND FINANCING ACTIVITIES |
|
|
| Increase in the right-of-use asset and lease liability |
$ 0
|
$ 50,432
|
| X |
- References
+ Details
| Name: |
acbm_ChangeOfRouAndLeaseLiabilities |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
acbm_OperatingLeaseLiabilities |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expense recognized in the current period for the periodic realization of capitalized fees that were paid to salespeople, distributors, brokers, and agents at the time of the conclusion of the sale. As a noncash expense, this element is added back to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_AmortizationOfDeferredSalesCommissions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; excluding effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseExcludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in amount due within one year (or one business cycle) from customers for the credit sale of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate value of all inventory held by the reporting entity, associated with underlying transactions that are classified as operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInInventories |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amount of outstanding money paid in advance for goods or services that bring economic benefits for future periods. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-2
| Name: |
us-gaap_InterestPaidNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NoncashInvestingAndFinancingItemsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
ORGANIZATION AND DESCRIPTION OF BUSINESS
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| ORGANIZATION AND DESCRIPTION OF BUSINESS |
|
| ORGANIZATION AND DESCRIPTION OF BUSINESS |
NOTE 1 -ORGANIZATION AND DESCRIPTION OF BUSINESS Acro Biomedical Co., Ltd. (the “Company”) is a Nevada corporation incorporated on September 24, 2014 under the name Killer Waves Hawaii, Inc. On January 30, 2017, the Company’s corporate name was changed to Acro Biomedical Co., Ltd. The Company’s business is the sale of cordyceps related products. Cordyceps is a fungus that is used in traditional Chinese medicine. During the second and third quarters of 2021, the Company engaged consultants to take the initial steps to develop and implement a research and development and marketing program. These consultants are working independently and report to the chief executive officer. As of December 31, 2022, the research and development efforts had not developed any products, the contracts with the consultants expired in May 2023 and will expire in August 2023, and the Company cannot give any assurance that the marketing and research development activities will generate any new product or new marketing opportunities or generate any revenue.
|
| X |
- DefinitionThe entire disclosure for the nature of an entity's business, major products or services, principal markets including location, and the relative importance of its operations in each business and the basis for the determination, including but not limited to, assets, revenues, or earnings. For an entity that has not commenced principal operations, disclosures about the risks and uncertainties related to the activities in which the entity is currently engaged and an understanding of what those activities are being directed toward. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
| Name: |
us-gaap_NatureOfOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
|
| SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
NOTE 2 -SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES Basis of Presentation The financial statements have been prepared in accordance with Generally Accepted Accounting Principles (“GAAP”) of the United States. Use of Estimates The preparation of financial statements in conformity with GAAP in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements. The estimates and judgments will also affect the reported amounts for certain revenues and expenses during the reporting period. Actual results could differ from these good faith estimates and judgments. Cash and Cash Equivalents Cash and cash equivalents include cash in banks, money market funds, and certificates of term deposits with maturities of less than three months from inception, which are readily convertible to known amounts of cash and which, in the opinion of management, are subject to an insignificant risk of loss in value. Accounts Receivable Accounts receivable are recorded in accordance with ASC 310, “Receivables.” Accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts is the Company’s best estimate of the amount of probable credit losses in its existing accounts receivable. The Company does not currently have any amount recorded as an allowance for doubtful accounts. Based on management’s estimate, the Company has not deemed it necessary to reserve for doubtful accounts at this time. Inventories Inventories consist of finished goods. Inventories are valued at the lower of cost or net realizable value. The Company determines cost on the basis of first-in, first-out methods. The Company periodically reviews inventories for obsolescence and any inventories identified as obsolete are written down or written off. Although the Company believes that the assumptions it uses to estimate inventory write-downs are reasonable, future changes in these assumptions could provide a significantly different result. No inventory markdown was recorded for the years ended December 31, 2022 and 2021. Leases The Company determines if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, other current liabilities, and operating lease liabilities in the Company’ balance sheets if the lease term is more than one year. Finance leases are included in property and equipment, other current liabilities, and other long-term liabilities in the Company’s balance sheets. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As most of the Company’s leases do not provide an implicit rate, the Company generally uses its incremental borrowing rate based on the estimated rate of interest for collateralized borrowing over a similar term of the lease payments at commencement date. The operating lease ROU asset also includes any lease payments made and excludes lease incentives. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option. Leases with a lease term of 12 months or less at inception are not recorded on the Company’s balance sheet and are expensed on a straight- line basis over the lease term in the statement of operations. Net (Loss) Per Share of Common Stock The Company has adopted ASC Topic 260, ”Earnings per Share” which requires presentation of basic earnings per share on the face of the statements of operations for all entities with complex capital structures and requires a reconciliation of the numerator and denominator of the basic earnings per share computation. In the accompanying financial statements, basic loss per share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per share is computed by dividing net income by the weighted average number of shares of common stock and potentially dilutive outstanding shares of common stock during the period to reflect the potential dilution that could occur from common stock issuable through contingent share arrangements, stock options and warrants unless the result would be antidilutive. There were no potentially dilutive shares of common stock outstanding for the years ended December 31, 2022 and 2021, respectively. Concentrations of Credit Risk The Company’s financial instruments that are exposed to concentrations of credit risk primarily consist of its cash and cash equivalents and related party payables that it will likely incur in the near future. The Company places its cash and cash equivalents with a United States financial institutions which the Company believes are of high creditworthiness. At times, its cash and cash equivalents with a particular financial institution may exceed any applicable government insurance limits. Financial Instruments The Company follows ASC 820, “Fair Value Measurements and Disclosures,” which defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy that distinguishes between (1) market participant assumptions developed based on market data obtained from independent sources (observable inputs) and (2) an entity’s own assumptions about market participant assumptions developed based on the best information available in the circumstances (unobservable inputs). The fair value hierarchy consists of three broad levels, which gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3). The three levels of the fair value hierarchy are described below: Level 1 Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities. Level 2 Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data. Level 3 Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities. The carrying values of financial instruments, including, cash, accounts receivable, prepaid expenses, accounts payable and accrued expenses and due to related parties approximate their fair values due to the short-term maturities of these financial instruments. Stock-Based Compensation The Company recognizes compensation expense for stock-based compensation in accordance with ASC Topic 718. For employee and non-employee stock-based awards, the Company calculates the fair value of the award on the date of grant using the option-pricing model for stock options and the quoted price of its common stock for unrestricted shares; the expense is recognized over the service period for awards expected to vest. The Company considers many factors when estimating expected forfeitures, including types of awards, employee class, and historical experience. Deferred Income Taxes and Valuation Allowance The Company accounts for income taxes under ASC 740 “Income Taxes.” Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize the deferred tax assets through future operations. Related Parties The Company follows ASC 850, ”Related Party Disclosures,” for the identification of related parties and disclosure of related party transactions and balances. Revenue Recognition The Company recognizes revenue in accordance with Topic 606, which requires revenues are recognized when control of the promised goods or services are transferred to a customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services. The Company applies the following five steps in order to determine the appropriate amount of revenue to be recognized as it fulfills its obligations under each of its agreements: | · | identify the contract with a customer; | | · | identify the performance obligations in the contract; | | · | determine the transaction price; | | · | allocate the transaction price to performance obligations in the contract; and | | · | recognize revenue as the performance obligation is satisfied. |
The Company recognizes revenue when products are delivered to customers in accordance with the written sales terms. Deferred Revenue The Company sells products pursuant to agreements for the delivery of products to customers. The Company receives cash in advance and records it as deferred revenue. Deferred revenue was $20,000 at December 31, 2022 and 2021. Recent Accounting Pronouncements In June 2022, the FASB issued ASU 2022-03, ASC Subtopic “Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions”. These amendments clarify that a contractual restriction on the sale of an equity security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value. The amendments in this update are effective for public business entities for fiscal years, including interim periods within those fiscal years, beginning after December 15, 2023. Early adoption is permitted. The Company does not believe that the impact of the adoption of this standard will have a material effect on its financial statements. The Company has reviewed all other recently issued, but not yet effective, accounting pronouncements and does not believe the future adoption of any such pronouncements may be expected to cause a material impact on the Company’s financial statements. COVID 19 Since the Company’s products are purchased by customers in the Republic of China (Taiwan) and Hong Kong who sold products to their customers in the People’s Republic of China (the “PRC”), the Company’s business was impacted by the effects of the COVID-19 pandemic and the actions taken by the governments of the PRC, the Republic of China (Taiwan) and Hong Kong. The World Health Organization ended the global emergency status for COVID-19 on May 5, 2023. As restrictions that had been imposed, particularly China’s zero COVID policy, to address the pandemic have been lifted, the Company cannot give assurance that its sales will increase as a result of the reduction of such restrictions, and the Company’s revenues decreased from 2021 to 2022. Although China has lifted COVID-19 restrictions, there continues to be a risk of the deaths and lockdowns from COVID-19. As the world has begun to open following closures as a result of the pandemic, two other factors are facing businesses and consumers, which are considered to be related to the effects of the COVID-19 pandemic. These are inflation and supply chain issues, which have been exacerbated by the recent Russian invasion of Ukraine. The Company cannot estimate the effect of these factors on its business. To the extent that these factors result in increased prices, the Company may not be able to pass along the increases to its customers. Further, to the extent customers use the Company’s products as an ingredient in their own products, the inability of a potential customer to obtain other raw materials as well as cost increases for such products, could affect the timing and the amount of purchases from the Company. The Company cannot give assurance that its business will not be impaired by the effects of inflation and supply chain issues.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
GOING CONCERN
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| GOING CONCERN |
|
| GOING CONCERN |
NOTE 3 – GOING CONCERN The accompanying financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates the realization of assets and the liquidation of liabilities in the normal course of business. The Company had minimal cash as of December 31, 2022, had limited gross profit and incurred a loss from operations for the year ended December 31, 2022 and past few years. These factors, among others, raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. The Company proposes to fund operations through sales of its products and equity financing arrangements. However, because of the lack of sales and the absence of any active trading market for its common stock, its financial condition and its lack of an operating history, the Company may not be able to raise funds for capital expenditures, working capital and other cash requirements and will have to rely on advances from a minority stockholder and an officer. If the Company cannot generate revenue from its products, it may not be able to continue in its business.
|
| X |
- References
+ Details
| Name: |
us-gaap_RisksAndUncertaintiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure when substantial doubt is raised about the ability to continue as a going concern. Includes, but is not limited to, principal conditions or events that raised substantial doubt about the ability to continue as a going concern, management's evaluation of the significance of those conditions or events in relation to the ability to meet its obligations, and management's plans that alleviated or are intended to mitigate the conditions or events that raise substantial doubt about the ability to continue as a going concern. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-SubTopic 40
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205-40/tableOfContent
| Name: |
us-gaap_SubstantialDoubtAboutGoingConcernTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
EQUITY
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| EQUITY |
|
| EQUITY |
NOTE 4 - EQUITY Common Stock The Company issued a total of 6,776,000 and 5,506,000 shares of common stock to consultants as stock grants pursuant to agreements with the consultants in May 2021 and August 2021, respectively, of which 11,912,000 shares were issued pursuant to the 2020 equity incentive plan. (the “Plan”) and 370,000 were issued as restricted stock outside of the Plan. The agreements provide for the consultants to perform services described in the contracts for the two-year period commencing May 25, 2021 and August 23, 2021. The shares were valued at $19,311,600 and $12,113,200, based on the market price of the common stock on the respective dates of the agreements, which was $2.85 and $2.20 per share, respectively, and is being amortized over two-year period starting from the date of the agreement using the straight-line method. During the year ended December 31, 2022 and 2021, the Company recorded stock-based compensation of $15,712,400 and $7,651,417, respectively, and had deferred stock compensation of $8,060,983 and $23,773,383 as of December 31, 2022 and 2021 which will be recognized over the balance of the agreements. During the year ended December 31, 2022, the Company did not issue any shares of common stock.
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
INCOME TAX
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| INCOME TAX |
|
| INCOME TAX |
NOTE 5 - INCOME TAXES The reconciliation of income tax expense at the U.S. statutory rate of 21% to the Company’s effective tax rate is as follows: | | Year ended | | | | December 31, | | | | 2022 | | | 2021 | | Income tax credit at statutory rate | | $ | (3,333,003 | ) | | $ | (1,616,544 | ) | Income tax adjustment | | | | | | | | | Imputed interest | | | 358 | | | | 893 | | Change of valuation allowance | | | 3,332,645 | | | | 1,615,651 | | Income tax expense | | $ | - | | | $ | - | |
Net deferred tax assets consist of the following components as of: | | December 31, | | | December 31, | | | | 2022 | | | 2021 | | Operating loss carry forward | | $ | 5,014,941 | | | $ | 1,682,296 | | Valuation allowance | | | (5,014,941 | ) | | | (1,682,296 | ) | Deferred tax asset | | $ | - | | | $ | - | |
The Company has approximately $24 million net operating loss carryforwards that are available to reduce future taxable income. In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers projected future taxable income and tax planning strategies in making this assessment. Based on the assessment, management has established a full valuation allowance against all of the deferred tax assets for the year ended December 31, 2022 and 2021 because it is more likely than not that all of the deferred tax assets will not be realized.
|
| X |
- DefinitionThe entire disclosure for income taxes. Disclosures may include net deferred tax liability or asset recognized in an enterprise's statement of financial position, net change during the year in the total valuation allowance, approximate tax effect of each type of temporary difference and carryforward that gives rise to a significant portion of deferred tax liabilities and deferred tax assets, utilization of a tax carryback, and tax uncertainties information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//740/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-14
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482526/740-270-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
RELATED PARTY TRANSACTIONS
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| RELATED PARTY TRANSACTIONS |
|
| RELATED PARTY TRANSACTIONS |
NOTE 6 - RELATED PARTY TRANSACTIONS At December 31, 2022 and 2021, the Company owed $1,100 and $1,100 to its CEO for non-interest-bearing advances made to or on behalf of the Company, respectively. These advances are due on demand. During the years ended December 31, 2022 and 2021, a minority stockholder advanced $55,000 and $0 paid expenses of $3,085 and $19,641 on behalf of the Company, and the Company repaid $0 and $241,851, respectively. At December 31, 2022 and 2021, the Company owed $77,726 and $19,641 to a stockholder who is not a 5% stockholder for non-interest-bearing advances made to or on behalf of the Company, respectively. These advances are due on demand. The Company has imputed interest at the rate of 4% on the advances made to or on behalf of the Company. Imputed interest amounted to $1,706 and $4,250, during the years ended December 31, 2022 and 2021, respectively. As of December 31, 2022 and 2021, the Company had amounts due to related parties of $78,826 and $20,741, respectively.
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
LEASES
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| LEASES |
|
| LEASES |
NOTE 7 – LEASES On December 27, 2019, the Company entered into a lease agreement to rent a storage facility in Hong Kong for a two-year term at HK$16,500 (approximately $2,115) per month. A stockholder paid HK$33,000 (approximately $4,230) as a security deposit and HK$16,500 (approximately $2,115) as prepaid rent on behalf of the Company. The lease expired in December 2021. On November 3, 2021, the Company entered into a lease agreement to rent a storage facility in Hong Kong for a two-year term at HK$17,000 (approximately $2,190) per month and HK$33,000 (approximately $4,230) as a security deposit. These payments were paid by the stockholder on behalf of the Company. In accordance with ASC 842, the Company recognized operating lease ROU assets and lease liabilities as follows: | | December 31, | | | December 31, | | | | 2022 | | | 2021 | | Operating lease ROU asset | | $ | 25,719 | | | $ | 50,432 | |
| | December 31, | | | December 31, | | Operating lease liabilities | | 2022 | | | 2021 | | Current portion | | $ | 25,719 | | | $ | 24,713 | | Non-current portion | | | - | | | | 25,719 | | Total | | $ | 25,719 | | | $ | 50,432 | |
Future minimum lease payments under operating leases at December 31, 2022 were as follows: 2023 | | $ | 26,280 | | Thereafter | | | - | | Total | | $ | 26,280 | |
The Company recognized total lease expense of $26,280 and $25,140 for the years ended December 31, 2022 and 2021, respectively.
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for operating leases of lessee. Includes, but is not limited to, description of operating lease and maturity analysis of operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//842-20/tableOfContent
| Name: |
us-gaap_LesseeOperatingLeasesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
CONCENTRATION
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| CONCENTRATION |
|
| CONCENTRATION |
NOTE 8 – CONCENTRATION Revenue and accounts receivable For the years ended December 31, 2022 and 2021, customer concentrations (more than 10%) were as follows: | | Percentage of revenue | | | Percentage of Accounts receivable | | | | Year ended December 31 | | | December 31, | | | December 31, | | | | 2022 | | | 2021 | | | 2022 | | | 2021 | | Customer A | | | 55 | % | | | 59 | % | | | 56 | % | | | 33 | % | Customer B | | | 45 | % | | | 33 | % | | | 44 | % | | | 67 | % | Total (as a group) | | | 100 | % | | | 92 | % | | | 100 | % | | | 100 | % |
Purchases During the year ended December 31, 2022, all purchases were made from one supplier. During the year ended December 31, 2021, the Company did not purchase inventory.
|
| X |
- References
+ Details
| Name: |
acbm_ConcentrationDisclosureTextBlock |
| Namespace Prefix: |
acbm_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_ConcentrationRisksTypesNoConcentrationPercentageAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
SUBSEQUENT EVENTS
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| SUBSEQUENT EVENTS |
|
| SUBSEQUENT EVENTS |
NOTE 9 - SUBSEQUENT EVENTS The Company has evaluated subsequent events that have occurred after the date of the balance sheet through the date of issuance of these financial statements and determined that no subsequent event requires recognition or disclosure to the financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Policies)
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
|
| Basis of Presentation |
The financial statements have been prepared in accordance with Generally Accepted Accounting Principles (“GAAP”) of the United States.
|
| Use of Estimates |
The preparation of financial statements in conformity with GAAP in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements. The estimates and judgments will also affect the reported amounts for certain revenues and expenses during the reporting period. Actual results could differ from these good faith estimates and judgments.
|
| Cash and Cash Equivalents |
Cash and cash equivalents include cash in banks, money market funds, and certificates of term deposits with maturities of less than three months from inception, which are readily convertible to known amounts of cash and which, in the opinion of management, are subject to an insignificant risk of loss in value.
|
| Accounts Receivable |
Accounts receivable are recorded in accordance with ASC 310, “Receivables.” Accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts is the Company’s best estimate of the amount of probable credit losses in its existing accounts receivable. The Company does not currently have any amount recorded as an allowance for doubtful accounts. Based on management’s estimate, the Company has not deemed it necessary to reserve for doubtful accounts at this time.
|
| Inventories |
Inventories consist of finished goods. Inventories are valued at the lower of cost or net realizable value. The Company determines cost on the basis of first-in, first-out methods. The Company periodically reviews inventories for obsolescence and any inventories identified as obsolete are written down or written off. Although the Company believes that the assumptions it uses to estimate inventory write-downs are reasonable, future changes in these assumptions could provide a significantly different result. No inventory markdown was recorded for the years ended December 31, 2022 and 2021.
|
| Leases |
The Company determines if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, other current liabilities, and operating lease liabilities in the Company’ balance sheets if the lease term is more than one year. Finance leases are included in property and equipment, other current liabilities, and other long-term liabilities in the Company’s balance sheets. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As most of the Company’s leases do not provide an implicit rate, the Company generally uses its incremental borrowing rate based on the estimated rate of interest for collateralized borrowing over a similar term of the lease payments at commencement date. The operating lease ROU asset also includes any lease payments made and excludes lease incentives. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option. Leases with a lease term of 12 months or less at inception are not recorded on the Company’s balance sheet and are expensed on a straight- line basis over the lease term in the statement of operations.
|
| Net Income (Loss) Per Share of Common Stock |
The Company has adopted ASC Topic 260, ”Earnings per Share” which requires presentation of basic earnings per share on the face of the statements of operations for all entities with complex capital structures and requires a reconciliation of the numerator and denominator of the basic earnings per share computation. In the accompanying financial statements, basic loss per share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per share is computed by dividing net income by the weighted average number of shares of common stock and potentially dilutive outstanding shares of common stock during the period to reflect the potential dilution that could occur from common stock issuable through contingent share arrangements, stock options and warrants unless the result would be antidilutive. There were no potentially dilutive shares of common stock outstanding for the years ended December 31, 2022 and 2021, respectively.
|
| Concentrations of Credit Risk |
The Company’s financial instruments that are exposed to concentrations of credit risk primarily consist of its cash and cash equivalents and related party payables that it will likely incur in the near future. The Company places its cash and cash equivalents with a United States financial institutions which the Company believes are of high creditworthiness. At times, its cash and cash equivalents with a particular financial institution may exceed any applicable government insurance limits.
|
| Financial Instruments |
The Company follows ASC 820, “Fair Value Measurements and Disclosures,” which defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy that distinguishes between (1) market participant assumptions developed based on market data obtained from independent sources (observable inputs) and (2) an entity’s own assumptions about market participant assumptions developed based on the best information available in the circumstances (unobservable inputs). The fair value hierarchy consists of three broad levels, which gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3). The three levels of the fair value hierarchy are described below: Level 1 Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities. Level 2 Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data. Level 3 Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities. The carrying values of financial instruments, including, cash, accounts receivable, prepaid expenses, accounts payable and accrued expenses and due to related parties approximate their fair values due to the short-term maturities of these financial instruments.
|
| Stock-Based Compensation |
The Company recognizes compensation expense for stock-based compensation in accordance with ASC Topic 718. For employee and non-employee stock-based awards, the Company calculates the fair value of the award on the date of grant using the option-pricing model for stock options and the quoted price of its common stock for unrestricted shares; the expense is recognized over the service period for awards expected to vest. The Company considers many factors when estimating expected forfeitures, including types of awards, employee class, and historical experience.
|
| Deferred Income Taxes and Valuation Allowance |
The Company accounts for income taxes under ASC 740 “Income Taxes.” Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize the deferred tax assets through future operations.
|
| Related Parties |
The Company follows ASC 850, ”Related Party Disclosures,” for the identification of related parties and disclosure of related party transactions and balances.
|
| Revenue Recognition |
The Company recognizes revenue in accordance with Topic 606, which requires revenues are recognized when control of the promised goods or services are transferred to a customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services. The Company applies the following five steps in order to determine the appropriate amount of revenue to be recognized as it fulfills its obligations under each of its agreements: | · | identify the contract with a customer; | | · | identify the performance obligations in the contract; | | · | determine the transaction price; | | · | allocate the transaction price to performance obligations in the contract; and | | · | recognize revenue as the performance obligation is satisfied. |
The Company recognizes revenue when products are delivered to customers in accordance with the written sales terms.
|
| Deferred Revenue |
The Company sells products pursuant to agreements for the delivery of products to customers. The Company receives cash in advance and records it as deferred revenue. Deferred revenue was $20,000 at December 31, 2022 and 2021.
|
| Recent Accounting Pronouncements |
In June 2022, the FASB issued ASU 2022-03, ASC Subtopic “Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions”. These amendments clarify that a contractual restriction on the sale of an equity security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value. The amendments in this update are effective for public business entities for fiscal years, including interim periods within those fiscal years, beginning after December 15, 2023. Early adoption is permitted. The Company does not believe that the impact of the adoption of this standard will have a material effect on its financial statements. The Company has reviewed all other recently issued, but not yet effective, accounting pronouncements and does not believe the future adoption of any such pronouncements may be expected to cause a material impact on the Company’s financial statements.
|
| COVID-19 |
Since the Company’s products are purchased by customers in the Republic of China (Taiwan) and Hong Kong who sold products to their customers in the People’s Republic of China (the “PRC”), the Company’s business was impacted by the effects of the COVID-19 pandemic and the actions taken by the governments of the PRC, the Republic of China (Taiwan) and Hong Kong. The World Health Organization ended the global emergency status for COVID-19 on May 5, 2023. As restrictions that had been imposed, particularly China’s zero COVID policy, to address the pandemic have been lifted, the Company cannot give assurance that its sales will increase as a result of the reduction of such restrictions, and the Company’s revenues decreased from 2021 to 2022. Although China has lifted COVID-19 restrictions, there continues to be a risk of the deaths and lockdowns from COVID-19. As the world has begun to open following closures as a result of the pandemic, two other factors are facing businesses and consumers, which are considered to be related to the effects of the COVID-19 pandemic. These are inflation and supply chain issues, which have been exacerbated by the recent Russian invasion of Ukraine. The Company cannot estimate the effect of these factors on its business. To the extent that these factors result in increased prices, the Company may not be able to pass along the increases to its customers. Further, to the extent customers use the Company’s products as an ingredient in their own products, the inability of a potential customer to obtain other raw materials as well as cost increases for such products, could affect the timing and the amount of purchases from the Company. The Company cannot give assurance that its business will not be impaired by the effects of inflation and supply chain issues.
|
| X |
- References
+ Details
| Name: |
acbm_AccountsReceivablePolicyTextBlock |
| Namespace Prefix: |
acbm_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
acbm_ConcentrationsofCreditRiskPolicyTextBLOCK |
| Namespace Prefix: |
acbm_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for COVID-19.
| Name: |
acbm_CoronavirusDisease2019PolicyTextBlock |
| Namespace Prefix: |
acbm_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
acbm_DeferredRevenuePolicyTextBlock |
| Namespace Prefix: |
acbm_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for related parties.
| Name: |
acbm_RelatedPartiesPolicyTextBlock |
| Namespace Prefix: |
acbm_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the basis of presentation and significant accounting policies concepts. Basis of presentation describes the underlying basis used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Accounting policies describe all significant accounting policies of the reporting entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_BasisOfPresentationAndSignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for salaries, bonuses, incentive awards, postretirement and postemployment benefits granted to employees, including equity-based arrangements; discloses methodologies for measurement, and the bases for recognizing related assets and liabilities and recognizing and reporting compensation expense. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (b),(f(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_CompensationRelatedCostsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for credit loss on financial instrument measured at amortized cost basis, net investment in lease, off-balance sheet credit exposure, and available-for-sale debt security. Includes, but is not limited to, methodology used to estimate allowance for credit loss, how writeoff of uncollectible amount is recognized, and determination of past due status and nonaccrual status. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-14
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 30
-Paragraph 5A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479391/326-20-30-5A
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-3C
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 35
-Paragraph 8A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479366/326-20-35-8A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-3D
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479344/326-20-45-5
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 30
-Paragraph 1B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479175/326-30-30-1B
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 3C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-3C
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 3A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-3A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 3D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-3D
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 35
-Paragraph 13A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479148/326-30-35-13A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 30
-Paragraph 4A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479391/326-20-30-4A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 35
-Paragraph 7A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479148/326-30-35-7A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-21
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-7
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-17
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 6.M.Q4)
-SubTopic 20
-Topic 326
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483530/326-20-S99-1
| Name: |
us-gaap_CreditLossFinancialInstrumentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of inventory accounting policy for inventory classes, including, but not limited to, basis for determining inventory amounts, methods by which amounts are added and removed from inventory classes, loss recognition on impairment of inventories, and situations in which inventories are stated above cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483489/210-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 912
-SubTopic 330
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482105/912-330-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//330/tableOfContent
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-4
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (a)
-SubTopic 10
-Topic 270
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482989/270-10-45-6
| Name: |
us-gaap_InventoryPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangement entered into by lessee. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-1
| Name: |
us-gaap_LesseeLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for establishing and maintaining the valuation allowance related to real estate owned.
| Name: |
us-gaap_RealEstateOwnedValuationAllowancePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for revenue. Includes revenue from contract with customer and from other sources. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (e)
-SubTopic 10
-Topic 235
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
| Name: |
us-gaap_RevenueRecognitionPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
INCOME TAX (Tables)
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| INCOME TAX |
|
| Schedule of reconciliation of income tax expense benefit |
| | Year ended | | | | December 31, | | | | 2022 | | | 2021 | | Income tax credit at statutory rate | | $ | (3,333,003 | ) | | $ | (1,616,544 | ) | Income tax adjustment | | | | | | | | | Imputed interest | | | 358 | | | | 893 | | Change of valuation allowance | | | 3,332,645 | | | | 1,615,651 | | Income tax expense | | $ | - | | | $ | - | |
|
| Schedule of net deferred tax assets |
| | December 31, | | | December 31, | | | | 2022 | | | 2021 | | Operating loss carry forward | | $ | 5,014,941 | | | $ | 1,682,296 | | Valuation allowance | | | (5,014,941 | ) | | | (1,682,296 | ) | Deferred tax asset | | $ | - | | | $ | - | |
|
| X |
- DefinitionTabular disclosure of the components of income tax expense attributable to continuing operations for each year presented including, but not limited to: current tax expense (benefit), deferred tax expense (benefit), investment tax credits, government grants, the benefits of operating loss carryforwards, tax expense that results from allocating certain tax benefits either directly to contributed capital or to reduce goodwill or other noncurrent intangible assets of an acquired entity, adjustments of a deferred tax liability or asset for enacted changes in tax laws or rates or a change in the tax status of the entity, and adjustments of the beginning-of-the-year balances of a valuation allowance because of a change in circumstances that causes a change in judgment about the realizability of the related deferred tax asset in future years. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 9
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
| Name: |
us-gaap_ScheduleOfComponentsOfIncomeTaxExpenseBenefitTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of net deferred tax asset or liability recognized in an entity's statement of financial position, including the following: the total of all deferred tax liabilities, the total of all deferred tax assets, the total valuation allowance recognized for deferred tax assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
LEASES (Tables)
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| LEASES |
|
| Schedule of operating lease ROU assets and lease liabilites |
| | December 31, | | | December 31, | | | | 2022 | | | 2021 | | Operating lease ROU asset | | $ | 25,719 | | | $ | 50,432 | |
| | December 31, | | | December 31, | | Operating lease liabilities | | 2022 | | | 2021 | | Current portion | | $ | 25,719 | | | $ | 24,713 | | Non-current portion | | | - | | | | 25,719 | | Total | | $ | 25,719 | | | $ | 50,432 | |
|
| Schedule of future minimum lease payments |
2023 | | $ | 26,280 | | Thereafter | | | - | | Total | | $ | 26,280 | |
|
| X |
- References
+ Details
| Name: |
acbm_OperationLeaseRightOfUseAssetsAndLiabilitiesTableTextBlock |
| Namespace Prefix: |
acbm_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of future minimum payments required in the aggregate and for each of the five succeeding fiscal years for operating leases having initial or remaining noncancelable lease terms in excess of one year and the total minimum rentals to be received in the future under noncancelable subleases as of the balance sheet date. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481501/840-20-50-2
| Name: |
us-gaap_ScheduleOfFutureMinimumRentalPaymentsForOperatingLeasesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
CONCENTRATION (Tables)
|
12 Months Ended |
Dec. 31, 2022 |
|---|
| CONCENTRATION |
|
| Schedule of concentrations risk percentage |
| | Percentage of revenue | | | Percentage of Accounts receivable | | | | Year ended December 31 | | | December 31, | | | December 31, | | | | 2022 | | | 2021 | | | 2022 | | | 2021 | | Customer A | | | 55 | % | | | 59 | % | | | 56 | % | | | 33 | % | Customer B | | | 45 | % | | | 33 | % | | | 44 | % | | | 67 | % | Total (as a group) | | | 100 | % | | | 92 | % | | | 100 | % | | | 100 | % |
|
| X |
- References
+ Details
| Name: |
us-gaap_ConcentrationRisksTypesNoConcentrationPercentageAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the nature of a concentration, a benchmark to which it is compared, and the percentage that the risk is to the benchmark. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-21
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-18
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-20
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-16
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-21
| Name: |
us-gaap_SchedulesOfConcentrationOfRiskByRiskFactorTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- References
+ Details
| Name: |
acbm_DiscriptionOfContracts |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of deferred income and obligation to transfer product and service to customer for which consideration has been received or is receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DeferredRevenue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
EQUITY (Detail Narrative) - USD ($)
|
12 Months Ended |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Deferred stock compensation |
$ (8,060,983)
|
$ (23,773,383)
|
| Stock based compensation expenses |
$ 15,712,400
|
$ 7,651,417
|
| 2020 Equity Incentive Plan [Member] |
|
|
| Common stock share stock grants |
|
11,912,000
|
| Agreements description |
The agreements provide for the consultants to perform services described in the contracts for the two-year period commencing May 25, 2021 and August 23, 2021
|
|
| August 23, 2021 [Member] |
|
|
| Common stock share stock grants, value |
|
$ 12,113,200
|
| Common stock shares price per share |
|
$ 2.20
|
| Common stock share stock grants, share |
5,506,000
|
|
| May 25, 2021 [Member] |
|
|
| Common stock shares price per share |
|
$ 2.85
|
| Common stock share stock grants, share |
6,776,000
|
|
| Common stock share stock grants, value |
|
$ 19,311,600
|
| X |
- References
+ Details
| Name: |
acbm_AgreementsDescription |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
acbm_CommonStockShareStockGrantsShare |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
acbm_CommonStockShareStockGrantsShares |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
acbm_CommonStockShareStockGrantsValue |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
acbm_CommonStockShareStockGrantsvalue |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
acbm_CommonStockSharesPricePerShare |
| Namespace Prefix: |
acbm_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionValue of stock issued under share-based plans to employees or officers which is the unearned portion, accounted for under the fair value method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.30)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DeferredCompensationEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued as of the balance sheet date, including shares that had been issued and were previously outstanding but which are now held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
| Name: |
us-gaap_SharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=acbm_TwoThousandTwentyEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=acbm_AugustTwentyThreeTwentyTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=acbm_MayTwentyFiveTwentyTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
INCOME TAX (Details) - USD ($)
|
12 Months Ended |
Dec. 31, 2022 |
Dec. 31, 2021 |
| INCOME TAX |
|
|
| Income tax expense (credit) at statutory rate |
$ (3,333,003)
|
$ (1,616,544)
|
| Imputed interest |
358
|
893
|
| Change of valuation allowance |
3,332,645
|
1,615,651
|
| Income tax expense (credit) |
$ 0
|
$ 0
|
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (210.5-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in valuation and qualifying accounts and reserves from adjustment. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 4
-Subparagraph (SX 210.12-09)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-4
| Name: |
us-gaap_ValuationAllowancesAndReservesAdjustments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.2
INCOME TAX (Details 1) - USD ($)
|
Dec. 31, 2022 |
Dec. 31, 2021 |
| INCOME TAX |
|
|
| Operating loss carry forward |
$ 5,014,941
|
$ 1,682,296
|
| Valuation allowance |
(5,014,941)
|
(1,682,296)
|
| Deferred tax asset |
$ 0
|
$ 0
|
| X |
- DefinitionAmount after allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible operating loss carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsOperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
| X |
- DefinitionAmount of operating loss carryforward, before tax effects, available to reduce future taxable income under enacted tax laws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_OperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
RELATED PARTY TRANSACTIONS (Details Narrative) - USD ($)
|
12 Months Ended |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Due to related parties |
$ 3,085
|
$ 19,641
|
| Non interest-bearing |
5.00%
|
|
| Imputed interest - related parties |
$ 1,706
|
4,250
|
| Percentage of inputed interest |
4.00%
|
|
| Amounts due to related parties |
$ 78,826
|
20,741
|
| Advances from related parties |
55,000
|
0
|
| Advances from related parties |
58,085
|
19,641
|
| Repayment to related parties |
0
|
241,851
|
| CEO [Member] |
|
|
| Due to related parties |
1,100
|
1,100
|
| Chief Executive Officer [Member] |
|
|
| Advances from related parties |
$ 77,726
|
$ 19,641
|
| X |
- References
+ Details
| Name: |
acbm_NonInterestBearingPercentage |
| Namespace Prefix: |
acbm_ |
| Data Type: |
num:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of required minimum rental payments for leases having an initial or remaining non-cancelable letter-terms in excess of one year. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Subparagraph (Note 3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481418/840-10-55-40
Reference 2: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481501/840-20-50-2
| Name: |
us-gaap_OperatingLeasesFutureMinimumPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of required minimum rental payments for operating leases having an initial or remaining non-cancelable lease term in excess of one year due in the third fiscal year following the latest fiscal year. Excludes interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Subparagraph (Note 3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481418/840-10-55-40
Reference 2: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481501/840-20-50-2
| Name: |
us-gaap_OperatingLeasesFutureMinimumPaymentsDueInThreeYears |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of required minimum rental payments for operating leases having an initial or remaining non-cancelable lease term in excess of one year due after the fifth fiscal year following the latest fiscal year. Excludes interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Subparagraph (Note 3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481418/840-10-55-40
Reference 2: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481501/840-20-50-2
| Name: |
us-gaap_OperatingLeasesFutureMinimumPaymentsDueThereafter |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
LEASES (Details Narrative)
|
|
1 Months Ended |
12 Months Ended |
|
Nov. 03, 2021 |
Dec. 27, 2019 |
Dec. 31, 2022
USD ($)
|
Dec. 31, 2022
HKD ($)
|
Dec. 31, 2021
USD ($)
|
Dec. 31, 2022
HKD ($)
|
| Security deposit |
|
|
$ 4,230
|
|
$ 4,230
|
|
| Prepaid rent |
|
|
|
|
|
$ 2,190
|
| Rent expenses |
|
|
2,115
|
|
|
|
| Total lease expenses |
|
|
$ 26,280
|
|
$ 25,140
|
|
| Hong Kong [Member] | Lease Agreement [Member] |
|
|
|
|
|
|
| Security deposit |
|
|
|
|
|
33,000
|
| Prepaid rent |
|
|
|
|
|
$ 16,500
|
| Rent expenses |
|
|
|
$ 16,500
|
|
|
| Lease term |
2 years
|
2 years
|
|
|
|
|
| X |
- References
+ Details
| Name: |
acbm_LeaseTerm |
| Namespace Prefix: |
acbm_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of rent expense incurred for leased assets, including but not limited to, furniture and equipment, that is not directly or indirectly associated with the manufacture, sale or creation of a product or product line.
| Name: |
us-gaap_LeaseAndRentalExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of operating lease expense. Excludes sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-4
| Name: |
us-gaap_OperatingLeaseExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for rent that provides economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
| Name: |
us-gaap_PrepaidRent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of an asset, typically cash, provided to a counterparty to provide certain assurance of performance by the entity pursuant to the terms of a written or oral agreement, such as a lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_SecurityDeposit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=acbm_HongKongsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=acbm_LeaseAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionFor an entity that discloses a concentration risk in relation to quantitative amount, which serves as the "benchmark" (or denominator) in the equation, this concept represents the concentration percentage derived from the division. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 21
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-21
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-20
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-18
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-20
| Name: |
us-gaap_ConcentrationRiskPercentage1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_RevenueFromContractWithCustomerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByTypeAxis=acbm_CustomerFirstMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByTypeAxis=acbm_CustomerSecondMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByTypeAxis=acbm_TwoCustomerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_AccountsReceivableMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- References
+ Details
| Name: |
acbm_ConcentrationCreditRisk |
| Namespace Prefix: |
acbm_ |
| Data Type: |
num:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of risks that arise due to the volume of business transacted with a particular customer. At a minimum, the description informs financial statement users of the general nature of the risk, but excludes "Information about Major Customers" that may be disclosed elsewhere (for instance, segment disclosures). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-20
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-16
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-18
| Name: |
us-gaap_ConcentrationRiskCustomer |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_RevenueFromContractWithCustomerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByTypeAxis=acbm_TwoCustomerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_CostOfGoodsTotalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByTypeAxis=us-gaap_CustomerConcentrationRiskMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Arco Biomedical (CE) (USOTC:ACBM)
Historical Stock Chart
From Jan 2025 to Feb 2025
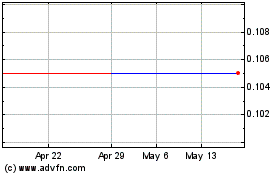
Arco Biomedical (CE) (USOTC:ACBM)
Historical Stock Chart
From Feb 2024 to Feb 2025