0000835662
false
0000835662
2022-01-01
2022-06-30
0000835662
2021-12-31
0000835662
2020-12-31
0000835662
2022-06-30
0000835662
us-gaap:ProductMember
2021-01-01
2021-12-31
0000835662
us-gaap:ProductMember
2020-01-01
2020-12-31
0000835662
us-gaap:AdvertisingMember
2021-01-01
2021-12-31
0000835662
us-gaap:AdvertisingMember
2020-01-01
2020-12-31
0000835662
AIXN:RoomRevenueMember
2021-01-01
2021-12-31
0000835662
AIXN:RoomRevenueMember
2020-01-01
2020-12-31
0000835662
AIXN:FoodAndBeverageRevenuesMember
2021-01-01
2021-12-31
0000835662
AIXN:FoodAndBeverageRevenuesMember
2020-01-01
2020-12-31
0000835662
AIXN:OtherMember
2021-01-01
2021-12-31
0000835662
AIXN:OtherMember
2020-01-01
2020-12-31
0000835662
2021-01-01
2021-12-31
0000835662
2020-01-01
2020-12-31
0000835662
us-gaap:ProductMember
2022-04-01
2022-06-30
0000835662
us-gaap:ProductMember
2021-04-01
2021-06-30
0000835662
us-gaap:ProductMember
2022-01-01
2022-06-30
0000835662
us-gaap:ProductMember
2021-01-01
2021-06-30
0000835662
us-gaap:AdvertisingMember
2022-04-01
2022-06-30
0000835662
us-gaap:AdvertisingMember
2021-04-01
2021-06-30
0000835662
us-gaap:AdvertisingMember
2022-01-01
2022-06-30
0000835662
us-gaap:AdvertisingMember
2021-01-01
2021-06-30
0000835662
AIXN:RoomRevenueMember
2022-04-01
2022-06-30
0000835662
AIXN:RoomRevenueMember
2021-04-01
2021-06-30
0000835662
AIXN:RoomRevenueMember
2022-01-01
2022-06-30
0000835662
AIXN:RoomRevenueMember
2021-01-01
2021-06-30
0000835662
AIXN:FoodAndBeverageRevenuesMember
2022-04-01
2022-06-30
0000835662
AIXN:FoodAndBeverageRevenuesMember
2021-04-01
2021-06-30
0000835662
AIXN:FoodAndBeverageRevenuesMember
2022-01-01
2022-06-30
0000835662
AIXN:FoodAndBeverageRevenuesMember
2021-01-01
2021-06-30
0000835662
AIXN:OtherMember
2022-04-01
2022-06-30
0000835662
AIXN:OtherMember
2021-04-01
2021-06-30
0000835662
AIXN:OtherMember
2022-01-01
2022-06-30
0000835662
AIXN:OtherMember
2021-01-01
2021-06-30
0000835662
2022-04-01
2022-06-30
0000835662
2021-04-01
2021-06-30
0000835662
2021-01-01
2021-06-30
0000835662
us-gaap:CommonStockMember
2019-12-31
0000835662
us-gaap:AdditionalPaidInCapitalMember
2019-12-31
0000835662
AIXN:StatutoryReservesMember
2019-12-31
0000835662
us-gaap:RetainedEarningsMember
2019-12-31
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2019-12-31
0000835662
2019-12-31
0000835662
us-gaap:CommonStockMember
2020-12-31
0000835662
us-gaap:AdditionalPaidInCapitalMember
2020-12-31
0000835662
AIXN:StatutoryReservesMember
2020-12-31
0000835662
us-gaap:RetainedEarningsMember
2020-12-31
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2020-12-31
0000835662
us-gaap:CommonStockMember
2021-12-31
0000835662
us-gaap:AdditionalPaidInCapitalMember
2021-12-31
0000835662
AIXN:StatutoryReservesMember
2021-12-31
0000835662
us-gaap:RetainedEarningsMember
2021-12-31
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-12-31
0000835662
us-gaap:CommonStockMember
2022-03-31
0000835662
us-gaap:AdditionalPaidInCapitalMember
2022-03-31
0000835662
AIXN:StatutoryReservesMember
2022-03-31
0000835662
us-gaap:RetainedEarningsMember
2022-03-31
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-03-31
0000835662
2022-03-31
0000835662
us-gaap:CommonStockMember
2021-03-31
0000835662
us-gaap:AdditionalPaidInCapitalMember
2021-03-31
0000835662
AIXN:StatutoryReservesMember
2021-03-31
0000835662
us-gaap:RetainedEarningsMember
2021-03-31
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-03-31
0000835662
2021-03-31
0000835662
us-gaap:CommonStockMember
2020-01-01
2020-12-31
0000835662
us-gaap:AdditionalPaidInCapitalMember
2020-01-01
2020-12-31
0000835662
AIXN:StatutoryReservesMember
2020-01-01
2020-12-31
0000835662
us-gaap:RetainedEarningsMember
2020-01-01
2020-12-31
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2020-01-01
2020-12-31
0000835662
us-gaap:CommonStockMember
2021-01-01
2021-12-31
0000835662
us-gaap:AdditionalPaidInCapitalMember
2021-01-01
2021-12-31
0000835662
AIXN:StatutoryReservesMember
2021-01-01
2021-12-31
0000835662
us-gaap:RetainedEarningsMember
2021-01-01
2021-12-31
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-01-01
2021-12-31
0000835662
us-gaap:CommonStockMember
2022-01-01
2022-03-31
0000835662
us-gaap:AdditionalPaidInCapitalMember
2022-01-01
2022-03-31
0000835662
AIXN:StatutoryReservesMember
2022-01-01
2022-03-31
0000835662
us-gaap:RetainedEarningsMember
2022-01-01
2022-03-31
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-01-01
2022-03-31
0000835662
2022-01-01
2022-03-31
0000835662
us-gaap:CommonStockMember
2022-04-01
2022-06-30
0000835662
us-gaap:AdditionalPaidInCapitalMember
2022-04-01
2022-06-30
0000835662
AIXN:StatutoryReservesMember
2022-04-01
2022-06-30
0000835662
us-gaap:RetainedEarningsMember
2022-04-01
2022-06-30
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-04-01
2022-06-30
0000835662
us-gaap:CommonStockMember
2021-01-01
2021-03-31
0000835662
us-gaap:AdditionalPaidInCapitalMember
2021-01-01
2021-03-31
0000835662
AIXN:StatutoryReservesMember
2021-01-01
2021-03-31
0000835662
us-gaap:RetainedEarningsMember
2021-01-01
2021-03-31
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-01-01
2021-03-31
0000835662
2021-01-01
2021-03-31
0000835662
us-gaap:CommonStockMember
2021-04-01
2021-06-30
0000835662
us-gaap:AdditionalPaidInCapitalMember
2021-04-01
2021-06-30
0000835662
AIXN:StatutoryReservesMember
2021-04-01
2021-06-30
0000835662
us-gaap:RetainedEarningsMember
2021-04-01
2021-06-30
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-04-01
2021-06-30
0000835662
us-gaap:CommonStockMember
2022-06-30
0000835662
us-gaap:AdditionalPaidInCapitalMember
2022-06-30
0000835662
AIXN:StatutoryReservesMember
2022-06-30
0000835662
us-gaap:RetainedEarningsMember
2022-06-30
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-06-30
0000835662
us-gaap:CommonStockMember
2021-06-30
0000835662
us-gaap:AdditionalPaidInCapitalMember
2021-06-30
0000835662
AIXN:StatutoryReservesMember
2021-06-30
0000835662
us-gaap:RetainedEarningsMember
2021-06-30
0000835662
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-06-30
0000835662
2021-06-30
0000835662
AIXN:ChinaConcentricMember
2017-02-02
0000835662
AIXN:ChinaConcentricMember
2017-02-02
0000835662
AIXN:ChinaConcentricMember
2017-02-01
2017-02-02
0000835662
AIXN:QuanzhongLinMember
2017-12-11
2017-12-12
0000835662
AIXN:EquityTransferAgreementMember
AIXN:AixinShangyanHotelManagementMember
2021-05-25
0000835662
AIXN:AixinShangyanHotelManagementMember
2021-05-24
2021-05-25
0000835662
AIXN:AixintangPharmacisesMember
2021-05-24
2021-05-25
0000835662
AIXN:EquityTransferAgreementMember
AIXN:AixinShangyanHotelManagementMember
2021-05-25
0000835662
AIXN:ChengduAixintangPharmacyCoLtdMember
AIXN:PharmaciesPurchaseAgreementMember
2021-06-01
2021-06-02
0000835662
AIXN:AixintangPharmacisesMember
2021-06-01
2021-06-02
0000835662
AIXN:PriorToMayOneTwpThousandEighteenMember
2021-01-01
2021-12-31
0000835662
srt:HotelMember
2021-01-01
2021-12-31
0000835662
us-gaap:HealthCareMember
2021-01-01
2021-12-31
0000835662
AIXN:SinceAprilOneTwoThousandNineteenMember
2022-01-01
2022-06-30
0000835662
us-gaap:HealthCareMember
2022-01-01
2022-06-30
0000835662
AIXN:OfficeFurnitureMember
2021-01-01
2021-12-31
0000835662
AIXN:ElectronicEquipmentMember
srt:MinimumMember
2021-01-01
2021-12-31
0000835662
AIXN:ElectronicEquipmentMember
srt:MaximumMember
2021-01-01
2021-12-31
0000835662
AIXN:MachineryMember
2021-01-01
2021-12-31
0000835662
us-gaap:LeaseholdImprovementsMember
2021-01-01
2021-12-31
0000835662
us-gaap:VehiclesMember
2021-01-01
2021-12-31
0000835662
AIXN:OfficeFurnitureMember
2022-01-01
2022-06-30
0000835662
AIXN:ElectronicEquipmentMember
srt:MinimumMember
2022-01-01
2022-06-30
0000835662
AIXN:ElectronicEquipmentMember
srt:MaximumMember
2022-01-01
2022-06-30
0000835662
AIXN:MachineryMember
2022-01-01
2022-06-30
0000835662
us-gaap:LeaseholdImprovementsMember
2022-01-01
2022-06-30
0000835662
us-gaap:VehiclesMember
2022-01-01
2022-06-30
0000835662
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
AIXN:CustomerAMember
2021-01-01
2021-12-31
0000835662
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
AIXN:CustomerBMember
2021-01-01
2021-12-31
0000835662
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
AIXN:CustomerAMember
2020-01-01
2020-12-31
0000835662
us-gaap:CostOfSalesMember
us-gaap:SupplierConcentrationRiskMember
AIXN:SupplierCMember
2021-12-31
0000835662
us-gaap:CostOfSalesMember
us-gaap:SupplierConcentrationRiskMember
AIXN:SupplierCMember
2021-01-01
2021-12-31
0000835662
us-gaap:CostOfSalesMember
us-gaap:SupplierConcentrationRiskMember
AIXN:SupplierAMember
2020-12-31
0000835662
us-gaap:CostOfSalesMember
us-gaap:SupplierConcentrationRiskMember
AIXN:SupplierAMember
2020-01-01
2020-12-31
0000835662
us-gaap:CostOfSalesMember
AIXN:SupplierDMember
us-gaap:SupplierConcentrationRiskMember
2020-12-31
0000835662
us-gaap:CostOfSalesMember
us-gaap:SupplierConcentrationRiskMember
AIXN:SupplierDMember
2020-01-01
2020-12-31
0000835662
us-gaap:CostOfSalesMember
AIXN:SupplierEMember
us-gaap:SupplierConcentrationRiskMember
2020-12-31
0000835662
us-gaap:CostOfSalesMember
us-gaap:SupplierConcentrationRiskMember
AIXN:SupplierEMember
2020-01-01
2020-12-31
0000835662
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
AIXN:CustomerAMember
2021-04-01
2021-06-30
0000835662
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
AIXN:CustomerBMember
2021-04-01
2021-06-30
0000835662
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
AIXN:CustomerAMember
2021-01-01
2021-06-30
0000835662
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
AIXN:CustomerBMember
2021-01-01
2021-06-30
0000835662
us-gaap:CostOfSalesMember
us-gaap:SupplierConcentrationRiskMember
AIXN:SupplierCMember
2022-04-01
2022-06-30
0000835662
us-gaap:CostOfSalesMember
us-gaap:SupplierConcentrationRiskMember
AIXN:SupplierCMember
2022-01-01
2022-06-30
0000835662
us-gaap:CostOfSalesMember
AIXN:SupplierAMember
us-gaap:SupplierConcentrationRiskMember
2021-04-01
2021-06-30
0000835662
us-gaap:CostOfSalesMember
AIXN:SupplierDMember
us-gaap:SupplierConcentrationRiskMember
2021-01-01
2021-06-30
0000835662
AIXN:AdvertisingAndProductMember
2021-01-01
2021-12-31
0000835662
AIXN:AdvertisingAndProductMember
2020-01-01
2020-12-31
0000835662
AIXN:PharmaciesMember
2021-01-01
2021-12-31
0000835662
AIXN:PharmaciesMember
2020-01-01
2020-12-31
0000835662
srt:HotelMember
2020-01-01
2020-12-31
0000835662
AIXN:AdvertisingAndProductMember
2021-12-31
0000835662
AIXN:AdvertisingAndProductMember
2020-12-31
0000835662
AIXN:PharmaciesMember
2021-12-31
0000835662
AIXN:PharmaciesMember
2020-12-31
0000835662
srt:HotelMember
2021-12-31
0000835662
srt:HotelMember
2020-12-31
0000835662
AIXN:AdvertisingAndProductMember
2022-04-01
2022-06-30
0000835662
AIXN:AdvertisingAndProductMember
2021-04-01
2021-06-30
0000835662
AIXN:PharmaciesMember
2022-04-01
2022-06-30
0000835662
AIXN:PharmaciesMember
2021-04-01
2021-06-30
0000835662
srt:HotelMember
2022-04-01
2022-06-30
0000835662
srt:HotelMember
2021-04-01
2021-06-30
0000835662
AIXN:AdvertisingAndProductMember
2022-01-01
2022-06-30
0000835662
AIXN:AdvertisingAndProductMember
2021-01-01
2021-06-30
0000835662
AIXN:PharmaciesMember
2022-01-01
2022-06-30
0000835662
AIXN:PharmaciesMember
2021-01-01
2021-06-30
0000835662
srt:HotelMember
2022-01-01
2022-06-30
0000835662
srt:HotelMember
2021-01-01
2021-06-30
0000835662
AIXN:AdvertisingAndProductMember
2022-06-30
0000835662
AIXN:PharmaciesMember
2022-06-30
0000835662
srt:HotelMember
2022-06-30
0000835662
AIXN:VehicleMember
2021-12-31
0000835662
AIXN:VehicleMember
2020-12-31
0000835662
AIXN:OfficeFurnitureMember
2021-12-31
0000835662
AIXN:OfficeFurnitureMember
2020-12-31
0000835662
AIXN:ElectronicEquipmentMember
2021-12-31
0000835662
AIXN:ElectronicEquipmentMember
2020-12-31
0000835662
AIXN:MachineryMember
2021-12-31
0000835662
AIXN:MachineryMember
2020-12-31
0000835662
us-gaap:LeaseholdImprovementsMember
2021-12-31
0000835662
us-gaap:LeaseholdImprovementsMember
2020-12-31
0000835662
us-gaap:PropertyPlantAndEquipmentOtherTypesMember
2021-12-31
0000835662
us-gaap:PropertyPlantAndEquipmentOtherTypesMember
2020-12-31
0000835662
AIXN:VehicleMember
2022-06-30
0000835662
AIXN:OfficeFurnitureMember
2022-06-30
0000835662
AIXN:ElectronicEquipmentMember
2022-06-30
0000835662
AIXN:MachineryMember
2022-06-30
0000835662
us-gaap:LeaseholdImprovementsMember
2022-06-30
0000835662
us-gaap:PropertyPlantAndEquipmentOtherTypesMember
2022-06-30
0000835662
AIXN:ValueAddedMember
2021-12-31
0000835662
AIXN:ValueAddedMember
2020-12-31
0000835662
AIXN:IncomeMember
2021-12-31
0000835662
AIXN:IncomeMember
2020-12-31
0000835662
AIXN:CityConstructionMember
2021-12-31
0000835662
AIXN:CityConstructionMember
2020-12-31
0000835662
AIXN:EducationMember
2021-12-31
0000835662
AIXN:EducationMember
2020-12-31
0000835662
AIXN:OtherMember
2021-12-31
0000835662
AIXN:OtherMember
2020-12-31
0000835662
AIXN:ValueAddedMember
2022-06-30
0000835662
AIXN:IncomeMember
2022-06-30
0000835662
AIXN:CityConstructionMember
2022-06-30
0000835662
AIXN:EducationMember
2022-06-30
0000835662
AIXN:OtherMember
2022-06-30
0000835662
2020-06-08
0000835662
2020-06-08
2020-06-08
0000835662
2019-03-31
0000835662
srt:MinimumMember
2021-12-31
0000835662
srt:MaximumMember
2021-12-31
0000835662
AIXN:AixinShangyanHotelManagementMember
2021-12-31
0000835662
AIXN:AixintangPharmacisesMember
srt:MinimumMember
2021-12-31
0000835662
AIXN:AixintangPharmacisesMember
srt:MaximumMember
2021-12-31
0000835662
srt:MinimumMember
2022-06-30
0000835662
srt:MaximumMember
2022-06-30
0000835662
AIXN:AixinShangyanHotelManagementMember
2022-06-30
0000835662
AIXN:AixintangPharmacisesMember
srt:MinimumMember
2022-06-30
0000835662
AIXN:AixintangPharmacisesMember
srt:MaximumMember
2022-06-30
0000835662
AIXN:ChengduWenJiangAixinNanjiangPharmacyCoLtdMember
2021-12-31
0000835662
AIXN:ChengduWenJiangAixinNanjiangPharmacyCoLtdMember
2020-12-31
0000835662
AIXN:QionglaiWeidePharmacyMember
2021-12-31
0000835662
AIXN:QionglaiWeidePharmacyMember
2020-12-31
0000835662
AIXN:SichuanAixinInvestmentCoLtdMember
2021-12-31
0000835662
AIXN:SichuanAixinInvestmentCoLtdMember
2020-12-31
0000835662
AIXN:ChengduXinduCundetangPharmacyCoLimitedMember
2021-12-31
0000835662
AIXN:ChengduXinduCundetangPharmacyCoLimitedMember
2020-12-31
0000835662
AIXN:ChengduLishengHuirenTangPharmacyCoLimitedMember
2021-12-31
0000835662
AIXN:ChengduLishengHuirenTangPharmacyCoLimitedMember
2020-12-31
0000835662
AIXN:QuanzhongLinMember
2021-12-31
0000835662
AIXN:QuanzhongLinMember
2020-12-31
0000835662
AIXN:YirongShenMember
2021-12-31
0000835662
AIXN:YirongShenMember
2020-12-31
0000835662
AIXN:BranchManagerMember
2021-12-31
0000835662
AIXN:BranchManagerMember
2020-12-31
0000835662
AIXN:ChengduAixinECommerceCompanyLtdMember
2021-12-31
0000835662
AIXN:ChengduAixinECommerceCompanyLtdMember
2020-12-31
0000835662
AIXN:ChengduAixinInternationalTravelserviceCoLtdMember
2021-12-31
0000835662
AIXN:ChengduAixinInternationalTravelserviceCoLtdMember
2020-12-31
0000835662
AIXN:ChengduBeibangPharmacyMember
2021-12-31
0000835662
AIXN:ChengduBeibangPharmacyMember
2020-12-31
0000835662
AIXN:AixinLifeBeautyMember
2021-12-31
0000835662
AIXN:AixinLifeBeautyMember
2020-12-31
0000835662
AIXN:ChengduWenJiangAixinNanjiangPharmacyCoLtdMember
2022-06-30
0000835662
AIXN:SichuanAixinInvestmentCoLtdMember
2022-06-30
0000835662
AIXN:ChengduFuxiangTangPharmacyCoLtdMember
2022-06-30
0000835662
AIXN:ChengduFuxiangTangPharmacyCoLtdMember
2021-12-31
0000835662
AIXN:ChengduWenjiangDistrictHenengHupuPharmacyCoLtdMember
2022-06-30
0000835662
AIXN:ChengduWenjiangDistrictHenengHupuPharmacyCoLtdMember
2021-12-31
0000835662
AIXN:ChengduLishengHuirenTangPharmacyCoLimitedMember
2022-06-30
0000835662
AIXN:QuanzhongLinMember
2022-06-30
0000835662
AIXN:YirongShenMember
2022-06-30
0000835662
AIXN:BranchManagerMember
2022-06-30
0000835662
AIXN:ChengduAixinECommerceCompanyLtdMember
2022-06-30
0000835662
AIXN:ChengduAixinInternationalTravelserviceCoLtdMember
2022-06-30
0000835662
AIXN:AixinLifeBeautyMember
2022-06-30
0000835662
2014-05-31
0000835662
2014-05-01
2014-05-31
0000835662
AIXN:DecemberThirtyOneTwoThousandTwentyTwoMember
2021-12-31
0000835662
AIXN:DecemberThirtyOneTwoThousandTwentyThreeMember
2021-12-31
0000835662
AIXN:RenewedMember
2014-05-01
2014-05-31
0000835662
AIXN:DecemberThirtyOneTwoThousandTwentyTwoMember
2022-06-30
0000835662
us-gaap:ForeignCountryMember
2021-01-01
2021-12-31
0000835662
us-gaap:ForeignCountryMember
AIXN:AixinShangyanHotelAndAixintangPharmaciesMember
2021-12-31
0000835662
us-gaap:ForeignCountryMember
2022-01-01
2022-06-30
0000835662
2010-12-31
0000835662
2020-08-16
2020-08-17
0000835662
AIXN:QuanzhongLinMember
2020-06-30
0000835662
AIXN:EmployeesAndContractorsMember
AIXN:TwoThousandAndNinettenEquityIncentivePlanMember
2019-10-21
2019-10-22
0000835662
AIXN:EmployeesAndContractorsMember
AIXN:TwoThousandAndNinettenEquityIncentivePlanMember
2019-10-22
0000835662
AIXN:EmployeesAndContractorsMember
AIXN:TwoThousandAndNinettenEquityIncentivePlanMember
2019-10-23
2019-10-24
0000835662
AIXN:EmployeesAndContractorsMember
AIXN:TwoThousandAndNinettenEquityIncentivePlanMember
2019-10-24
0000835662
AIXN:EmployeesAndContractorsMember
AIXN:TwoThousandAndNinettenEquityIncentivePlanMember
2021-01-01
2021-12-31
0000835662
AIXN:QuanzhongLinMember
2021-01-01
2021-12-31
0000835662
AIXN:AixinShangyanHotelAndAixintangPharmaciesMember
2021-01-01
2021-12-31
0000835662
AIXN:EmployeesAndContractorsMember
AIXN:TwoThousandAndNinettenEquityIncentivePlanMember
2022-01-01
2022-06-30
0000835662
srt:MinimumMember
2021-01-01
2021-12-31
0000835662
srt:MaximumMember
2021-01-01
2021-12-31
0000835662
srt:MinimumMember
2022-01-01
2022-06-30
0000835662
srt:MaximumMember
2022-01-01
2022-06-30
0000835662
us-gaap:IndemnificationGuaranteeMember
2020-12-01
2020-12-31
0000835662
2020-12-01
2020-12-31
0000835662
AIXN:AixintangPharmacisesMember
2021-06-02
0000835662
AIXN:AixinShangyanHotelManagementMember
2021-01-01
2021-12-31
0000835662
AIXN:AixinShangyanHotelManagementMember
2020-01-01
2020-12-31
0000835662
AIXN:AixinShangyanHotelManagementMember
2021-04-01
2021-06-30
0000835662
AIXN:AixinShangyanHotelManagementMember
2021-01-01
2021-06-30
0000835662
AIXN:EquityTransferAgreementMember
AIXN:YunnanShengshengyuanTechnologyMember
us-gaap:SubsequentEventMember
2022-07-19
0000835662
AIXN:EquityTransferAgreementMember
AIXN:YunChenMember
us-gaap:SubsequentEventMember
2022-07-19
0000835662
AIXN:YunnanShengshengyuanTechnologyMember
us-gaap:SubsequentEventMember
2022-07-01
2022-07-19
0000835662
AIXN:YunnanShengshengyuanTechnologyMember
us-gaap:SubsequentEventMember
AIXN:SupplementalAgreementMember
2022-09-01
2022-09-30
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
iso4217:CNY
As
filed with the Securities and Exchange Commission on _________, 2022
Registration
No. 333-
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE SECURITIES ACT OF 1933
AIXIN LIFE INTERNATIONAL, INC.
(Exact
name of registrant as specified in its charter)
| colorado |
|
5149 |
|
84-1085935 |
(State
or other jurisdiction of
incorporation
or organization) |
|
(Primary
Standard Industrial
Classification
Code Number) |
|
(I.R.S.
Employer
Identification
Number) |
Hongxing
International Business Building 2, 14th FL,
No. 69 Qingyun South Ave.,
Jinjiang District
Chengdu
City, Sichuan Province, China
+(86)
313-6732526
(Address, including zip code, and telephone number,
including
area code, of registrant’s principal executive offices)
Corporation
Service Company
1900
W. Littleton Boulevard
Littleton,
Colorado 80120
Tel:
(303) 832 7579
(Name,
address, including zip code, and telephone number,
including
area code, of agent for service of process)
Copies
To:
Vincent
J. McGill, Esq.
Ellenoff
Grossman & Schole LLP
1345
Avenue of the Americas
New
York, NY 10105
Telephone:
(516) 220-6569 |
|
Fang
Liu, Esq.
VCL Law LLP
1945 Old Gallows Road, Suite 630
Vienna, VA 22182
Telephone: (703) 919-7285 |
Approximate
date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement is declared effective.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933 check the following box: ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large
accelerated filer ☐ |
Accelerated
filer ☐ |
| Non-accelerated
filer ☒ |
Smaller
reporting company ☒ |
| |
Emerging
growth company ☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
The
registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the
registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act or until the Registration Statement shall become effective on such date as the
Securities and Exchange Commission acting pursuant to said Section 8(a), may determine.
The
information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement
filed with the U.S. Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is
not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT
TO COMPLETION, DATED , 2022
PRELIMINARY
PROSPECTUS
Aixin
Life International, Inc.

$50,000,000
Shares
of Common Stock
This
is a public offering of common stock, par value $0.00001 per share (“common stock”), by Aixin Life International, Inc., a
Colorado company. Throughout this prospectus, unless the context requires otherwise, all references to “Aixin” or “Aixin
Colorado” refer to Aixin Life International, Inc., a holding company and references to “we,” “us,” “our,”
the “Registrant,” the “Company” or “our company” are to Aixin and/or its consolidated subsidiaries.
We are offering [●] shares of Common Stock on a firm commitment basis. We currently expect the public offering price to
be $[●] per share.
The
offering is being made on a “firm commitment” basis by Network 1 Financial Securities, Inc. See “Underwriting.”
Our
shares of common stock offered in this prospectus are shares of Aixin Colorado which has no material operations of its own and conducts
substantially all of its operations through operating companies established in the People’s Republic of China, or the PRC, which
are wholly owned through an intermediary company established in the British Virgin Islands. We are not a Chinese operating company. We
are a holding company and do not directly own any substantive business operations in China. Therefore, you will not directly hold any
equity interests in our Chinese operating companies. Our holding company structure involves unique risks to investors. Chinese regulatory
authorities could disallow our operating structure, which would likely result in a material change in our operations and/or the value
of our common stock, including causing the value of our common stock to significantly decline or become worthless. For a detailed description
of risks related to our corporate structure, see “Risk Factors—Risks Relating to Our Holding Company Structure”
for detailed discussions.
Additionally,
we are subject to certain legal and operational risks associated with our business operations in China. PRC laws and regulations
governing our current business operations are sometimes vague and uncertain, and we face the risk that changes in the policies of
the PRC government could have a significant impact upon the business we may be able to conduct in the PRC and the profitability of
such business. These risks associated with our being based in or conducting substantially all of our operations through operating
companies established in China could cause the value of our securities to significantly decline or become worthless. Furthermore,
these risks may result in a material change in our business operations or a complete hinderance of our ability to offer or continue
to offer our securities to investors. Recently, the PRC government initiated a series of regulatory actions and statements to
regulate business operations in China with little advance notice, including cracking down on illegal activities in securities
markets, enhancing supervision over China-based companies listed overseas using a variable interest entity structure, adopting new
measures to extend the scope of cybersecurity reviews, and expanding efforts to enforce anti-monopoly laws and regulations. As
confirmed by our PRC counsel, Sichuan Junhe Law Firm (“Sichuan Junhe”) the business conducted by our subsidiaries until
now is not subject to cybersecurity review with the Cyberspace Administration of China, or CAC, given that: (i) we do not possess a
large amount of personal information in our business operations and (ii) data that we do possess and process in our business does
not have a bearing on national security and thus may not be classified as core or important data by the authorities. In addition, as
confirmed by our PRC counsel, Sichuan Junhe, we are not subject to merger control review by China’s anti-monopoly enforcement
agency due to the level of revenues reported by us and audited by our auditor KCCW Accountancy Corp, and the fact that we currently
do not expect to propose or implement any acquisition of control of, or decisive influence over, any company with revenues within
China of more than RMB400 million. Currently, the statements and regulatory actions related to cybersecurity and anti-monopoly
policies have had no impact on our daily business operations, the ability to accept foreign investments and list our securities on a
U.S. or other foreign exchange. As of the date of this prospectus, no effective laws or regulations in the PRC explicitly require us
to seek approval from the China Securities Regulatory Commission (the “CSRC”) or any other PRC governmental authorities
for our overseas listing, nor has our company or any of our subsidiaries received any inquiry, notice, warning or sanctions
regarding our overseas listing from the CSRC or any other PRC governmental authorities. However, since these statements and
regulatory actions are new, it is highly uncertain as to how soon legislative or administrative regulation making bodies will
respond to ongoing developments and what existing or new laws or regulations or detailed implementation policies and interpretations
will be promulgated or modified and the potential impact such new laws or modified laws and regulations will have on our daily
business operations, the ability to accept foreign investments and list our securities on a U.S. or other foreign exchange. See
“Risk Factors” beginning on page 15 for a discussion of these legal and operational risks and other information
that should be considered before making a decision to purchase our common stock.
As
a holding company, our ability to pay dividends to our shareholders and to service any debt we may incur at the holding company level
may depend upon dividends or interest paid by our operating companies in China (our “PRC Subsidiaries”). Current PRC regulations
permit our PRC Subsidiaries to pay dividends to us only out of their accumulated profits, if any, determined in accordance with Chinese
accounting standards and regulations. In addition, each of our PRC Subsidiaries is required to set aside at least 10% of its after-tax
profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of its registered capital. As of the date hereof,
we have had no material transactions that involved the transfer of cash or assets throughout our corporate structure. The PRC Subsidiaries
have not transferred cash or other assets to Aixin, including by way of dividends or interest payments. Aixin does not currently plan
or anticipate transferring cash or other assets from our operations in China to any non-Chinese entity. For a detailed description of
how cash is transferred through our corporate structure, see “Prospectus Summary - Transfers of Cash to and from our Subsidiaries.”
Pursuant
to the Holding Foreign Companies Accountable Act (“HFCAA”), the Public Company Accounting Oversight Board (United States)
(the “PCAOB”) issued a Determination Report on December 16, 2021 which found that the PCAOB is unable to inspect or investigate
completely registered public accounting firms headquartered in: (1) mainland China of the PRC and (2) Hong Kong, a Special Administrative
Region and dependency of the PRC. In addition, the PCAOB’s report identified the specific registered public accounting firms which
are subject to these determinations. Our registered public accounting firm, KCCW Accountancy Corp. is not headquartered in mainland
China or Hong Kong and was not identified in this report as a firm subject to the PCAOB’s determinations. KCCW Accountancy
Corp. is registered with the PCAOB and is subject to laws in the United States pursuant to which the PCAOB conducts regular inspections
to assess KCCW Accountancy Corp.’s compliance with applicable professional standards. KCCW Accountancy Corp. has been inspected
by the PCAOB on a regular basis, with the last inspection in 2022. On August 26, 2022, the China Securities Regulatory Commission,
or CSRC, the Ministry of Finance of the PRC, and the PCAOB signed a Statement of Protocol, or the Protocol, governing inspections and
investigations of audit firms based in China and Hong Kong. Pursuant to the Protocol, the PCAOB shall have independent discretion to
select any issuer audits for inspection or investigation and has the unfettered ability to transfer information to the SEC. Notwithstanding
the foregoing, if the PCAOB is not able to inspect and investigate completely our auditor’s work papers in China, you may be deprived
of the benefits of such inspection which could result in limitation or restriction to our access to the U.S. capital markets and trading
of our securities may be prohibited under the HFCAA and the Nasdaq may determine to delist our securities if the PCAOB determines that
it cannot inspect or investigate completely our auditor under the HFCAA. For more details, see “Risk Factors –
Risk Associated with Our Company -A recent joint statement by the SEC and the Public Company Accounting Oversight Board (United States),
or the “PCAOB,” proposed rule changes submitted by Nasdaq, and the newly enacted “Holding Foreign Companies Accountable
Act” all call for additional and more stringent criteria to be applied to emerging market companies upon assessing the qualification
of their auditors, especially the non-U.S. auditors who are not inspected by the PCAOB. These developments could add uncertainties to
our offering.”
We
are a reporting company under Section 12(g) of the Securities Exchange Act of 1934, as amended. Our common stock is currently quoted
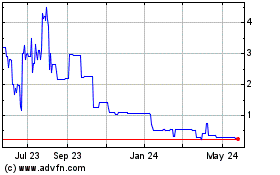
on the OTCQX Marketplace (the “OTCQX”) under the symbol “AIXN.” The last reported closing price
for our common stock on November 2, 2022, was $3.80 per share. There is a limited public trading market for
our common stock. We have applied to list our common stock on the Nasdaq Capital Market under the symbol “AIXN.” There can
be no assurance that our application will be approved. The closing of this offering is contingent upon the successful listing of our
common stock on the Nasdaq Capital Market.
Investing
in our securities involves a significant degree of risks. You should carefully consider the risk factors beginning on page 15 of
this prospectus and set forth in the documents incorporated by reference herein before making any decision to invest in our
securities.
Neither
the U.S. Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon
the accuracy or adequacy of this registration statement. Any representation to the contrary is a criminal offense.
| | |
Per
share | | |
Total | |
| Public
offering price | |
$ | | | |
$ | 50,000,000 | |
| Underwriting
discounts and commissions (1) | |
$ | | | |
$ | 4,000,000 | |
| Offering
proceeds to us, before expenses | |
$ | | | |
$ | 46,000,000 | |
| (1) |
Does
not include additional items of compensation payable to Network 1 Financial Securities, Inc., the underwriter, which includes warrants
to purchase 8% of the aggregate number of shares issued in this offering, with an exercise price equal to 110% of the
price per share sold in this offering. We have also agreed to reimburse the underwriter for certain accountable expenses incurred
by them. See “Underwriting.” |
We
have also granted a 45-day option to the underwriter to purchase up to additional [●] shares of common stock solely to cover
over-allotments, if any.
The
underwriter expects to deliver our shares of common stock to purchasers in this offering on or about [●], 2023.

The
date of this prospectus is , 2022
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
This
prospectus is part of a registration statement that we filed with the Securities and Exchange Commission (the “SEC”
or the “Commission”). You should rely only on the information contained in this prospectus or any supplement or amendment
hereto. Neither we, nor the underwriter have authorized any person to provide you with different information. Neither we, nor the underwriter
are offering to sell, or seeking an offer to buy, our common stock in any jurisdiction where such offer or sale is not permitted. You
should assume that the information contained in this prospectus and any supplement or amendment hereto is accurate only as of their respective
dates, regardless of the time of delivery of this prospectus or of any sale of our common stock. Our business, financial condition, results
of operations and prospects may have changed since that date. On October 27, 2020, we effected a 1-for-4 reverse split of our shares
of common stock. Concurrently with the reverse split we reduced the number of our authorized shares of common stock from 900,000,000
shares to 500,000,000 shares. All share and per share numbers contained herein have been adjusted to give effect to our reverse stock
split.
You
should read this prospectus, together with additional information described under “Where You Can Find More Information”,
beginning on page 78, before making an investment decision.
The
market data and certain other statistical information used throughout this prospectus is based on independent industry publications,
reports by market research firms or other independent sources that we believe to be reliable sources. Industry publications and third-party
research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although
they do not guarantee the accuracy or completeness of such information. We are responsible for all of the disclosure contained in this
prospectus, and we believe these industry publications and third-party research, surveys and studies are reliable. While we are not aware
of any misstatements regarding any third-party information presented in this prospectus, their estimates, in particular, as they relate
to projections, involve numerous assumptions, are subject to risks and uncertainties, and are subject to change based on various factors.
Some market and other data included herein, as well as the data of competitors as they relate to Aixin, is also based on our good faith
estimates.
Unless
the context otherwise requires, all references in this prospectus to:
| |
● |
“Aixin”
or “Aixin Colorado” refers to Aixin Life International, Inc., a Colorado corporation; |
| |
● |
“We,”
“us,” “our,” the “Registrant”, the “Company,” or “our
company” refer to Aixin and its consolidated subsidiaries; |
| |
● |
“Exchange
Act” refers to the Securities Exchange Act of 1934, as amended; |
| |
● |
“SEC”
or the “Commission” refers to the United States Securities and Exchange Commission; |
| |
● |
“Securities
Act” refers to the Securities Act of 1933, as amended; |
| |
● |
“China,”
“Chinese” or the “PRC” refers to the People’s Republic of China, excluding, for the purposes
of this prospectus only, Hong Kong, Macau and Taiwan; |
| |
● |
all
references to “RMB” or “Chinese Yuan” is to the legal currency of the People’s Republic
of China; and |
| |
● |
all
references to “U.S. dollars,” “dollars,” “USD” or “$”
are to the legal currency of the United States; |
The
Company’s reporting currency is the U.S. dollar. The functional currency of the parent company is the U.S. dollar and the functional
currency of the Company’s operating subsidiaries is the Chinese Renminbi (“RMB”). For the subsidiaries whose functional
currencies are the RMB, all assets and liabilities are translated at exchange rates at the balance sheet date, which are 0.1493 and 0.1569
as at June 30, 2022 and December 31, 2021, respectively. Revenue and expenses are translated at the average yearly exchange rates, which
are 0.1550 and 0.1448 for the two years ended December 31, 2021 and 2020, respectively. The equity is translated at historical exchange
rates. Any translation adjustments resulting are not included in determining net income but are included in foreign exchange adjustments
to other comprehensive loss, a component of equity.
PROSPECTUS
SUMMARY
This
summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider
in making your investment decision. Before investing in our securities, you should carefully read this entire prospectus, especially
the risks of investing in our securities as discussed under “Risk Factors” and the financial statements and notes
thereto herein. The following summary is qualified in its entirety by the detailed information appearing elsewhere in this prospectus.
Overview
Our
Business
We,
Aixin Life International, Inc. ((“Aixin or “Aixin Colorado”) together with our subsidiaries, referred to as the “Company”,
“we”, “us” and “our” unless specified otherwise)) are a Colorado holding company with no material
operations of our own. We conduct substantially all of our operations through our wholly-owned operating companies in the PRC.
Our
subsidiaries are (i) Aixin (BVI) International Group Co., Ltd. (“Aixin BVI”), 100% ownwd by Aixin Colorado, (ii) HK
AiXin International Group Co., Limited, a Hong Kong limited company (“AiXin HK”), 100% owned by Aixin BVI, and the
following entities, each of which was organized in the PRC, is wholly-owned by Aixin HK and which are collectively referred to
as our “PRC Subsidiaries” (iii) Chengdu AiXinZhonghong Biological Technology Co., Ltd., a Chinese limited company
(“AiXinZhonghong”), (iv) Yunnan Runcangsheng Technology Co., Ltd (“Yunnan Runcangsheng”), (v) Chengdu Aixintang
Pharmacy Co., Ltd. and its wholly-owned subsidiaries, each of which operates a pharmacy and (vi) Aixin Shangyan Hotel Management
Co., Ltd.
This
is an offering of common stock of Aixin Colorado, not shares of our operating companies in China. Therefore, you will not directly
hold any equity interests in our operating companies. Our holding company structure involves unique risks to investors. Chinese regulatory
authorities could disallow our operating structure, which would likely result in a material change in our operations and/or the value
of our common stock, including causing the value of our common stock to significantly decline or become worthless. Aixin Colorado
is listed on the OTCQX under the symbol “AIXN”.
We
are focused on providing health and wellness products to the growing middle class in China. We currently develop, manufacture, market
and sell premium-quality healthcare, nutritional products and wellness supplements, including herbs and greens, traditional Chinese remedies,
functional products, such as weight management tools, probiotics, foods and drinks. We also offer products purchased from third parties
and provide advertising and marketing services to clients which engage us to market and distribute their products. We offer our products
and those of clients for which we provide marketing services, through a diversified, omni-channel business model which generates revenues
through retail and wholesale product sales, through company-owned pharmacies, direct marketing and e-commerce. Our marketing approach
emphasizes proactively approaching customers such as by hosting marketing events for clients, which we believe is ideally suited to marketing
the products we offer because sales of healthcare, nutritional products and supplements are strengthened by ongoing personal contact
and support, coaching and education among the Company and our clients towards how to achieve a healthy and active lifestyle.
In
December 2017, when we acquired Aixin BVI, it indirectly owned all of the capital stock of AixinZhonghong, which began distributing
nutritional products in 2013.
In
June 2021, we acquired nine retail pharmacies in Chengdu. We utilize these pharmacies to distribute health and wellness products and
serve as learning centers for our clients. Since June 2021 we have added 4 additional pharmacies bringing the number of
pharmacies we currently operate to 13. We intend to seek to continue to expand our chain of pharmacies and to use these
outlets as part of our overall marketing strategy.
More
recently, in September 2022, we acquired Yunnan Runcangsheng based in Yunnan Province which engages in the research, development, manufacture
and wholesale distribution of health foods. Yunnan Runcangsheng operates a 13,000 square meter production facility, which houses R&D
centers, extraction facilities, preparation workshops and a warehouse. Yunnan Runcangsheng has more than 30 sub brands and operates planting
facilities where it grows some of the key ingredients used in its products. Many of the products it has developed are specifically targeted
to alleviate symptoms associated with the increasingly competitive and pressured lifestyle of the Chinese middle class and disorders
associated with changing eating habits and the presence of environmental toxins that are becoming more widespread through the use of
western style pesticides and fertilizers.
In
addition to our acquisitions in the health and nutritional sector, in July 2021, we acquired a hotel located in the Jinniu District,
Chengdu City. The hotel covers more than 8,000 square meters and has a large restaurant that can accommodate 600 people, 6 luxury dining
rooms, a 200 square meter music tea house, 13 private tea rooms, 108 guest rooms and other supporting facilities. We envision utilizing
the hotel to conduct marketing events and seminars for our customers, and training sessions for our personnel at which we introduce new
products and services intended to promote healthy living.
Business
Objectives
Key
elements of our business strategy are detailed below:
Leading
brand of nutritional supplements. Our primary goal is to develop recognized brand names with a reputation as a provider of quality
health and wellness products which deliver demonstrable benefits to our customers. Our objective is to provide members of the growing
Chinese middle class with the information necessary to maintain a healthy lifestyle and to offer a broad and deep mix of products whether
the customer is looking to treat a specific health-related issue, maintain their overall wellness or improve their performance. Our premium,
value-added offerings will include both proprietary branded products and other branded products provided by third parties to meet needs
not met by our in-house products.
We
believe that by offering a variety of branded exclusive products and a wide range of merchandise, and close customer support and services,
we will be able to differentiate our company from competitors and effectively compete against other food, drug and mass channel players,
specialty stores, independent vitamin, supplement and natural food shops and online retailers.
Product
development and innovation. With the acquisition of Yunnan Runcangsheng we now have the facilities and, more importantly, knowledgeable,
experienced personnel to develop high-quality, innovative health foods and nutritional supplements that will be offered by our
direct marketing team and available through our company-owned pharmacies, our website and, as demand grows other select marketplaces
and wholesale partners. To the extent desirable we will grow our own key ingredients. When we choose to use ingredients of third parties,
they will be rigorously tested before they are added to our products, undergoing multiple quality checks to ensure that they meet our
high standards for purity, composition and absence of contaminants.
We
believe our ability to innovate and respond to customer needs by designing new products gives us a significant competitive advantage.
At Yunnan Runcangsheng we directly employ scientists, nutritionists, formulators, and quality control experts who work at our research
and manufacturing facilities.
A
differentiated customer experience. One of the key differentiators to our marketing strategy is the continued development of
well-trained team members first used at AiXinZhonghong that work closely with customers. We will provide our team members with regular
training and education focused on the benefits of a healthy lifestyle, the advantages of our products and solution-based selling. With
their knowledge of the available products our team members can engage customers in conversation, access the customer’s purchasing
history through our data base, share product information and testimonials, and recommend solutions and help customers add complimentary
products and build wellness regimens. We operate in a highly personalized, aspirational sector and believe that health food and nutritional
supplement consumers often desire and seek out product expertise and knowledgeable customer service.
Our
loyalty programs used early on by AiXinZhonghong will allow us to develop and maintain a large and loyal customer base, provide targeted
offers and information, and connect with our customers on a regular basis. We routinely harness data generated by these programs to better
understand customers’ buying behaviors and needs, so we can deliver a stronger experience, bring like-minded consumers into the
channel and make well-informed decisions about our business.
As
we grow and develop our customer base, we can use our hotel as a site for educational seminars, conferences and marketing events featuring
well known experts in the wellness sector. These events can be used to increase customer loyalty and to educate our team members as to
the benefits of a healthy life style and our products so they can better serve our customers by guiding them to products that meet their
needs.,
Omni-channel
development. We believe our diversified, omni-channel model, which includes company-owned pharmacies, direct marketing by our
team members, individually and at large scale company sponsored marketing events, wholesale locations and e-commerce channels, can differentiate
us from many of our competitors, particularly those that rely exclusively on online marketing efforts. Our strategy is to give consumers
a seamless, integrated experience across digital, mobile and in-store channels and in every interaction with us and our products.
Through
our website and in conversations with our well-trained team members, customers can research our products and purchase them online or
at one of our local pharmacies or sales offices. We believe our physical store base provides a competitive advantage, allowing customers
to experience our products and get expert advice from our team members.
Vertical
Integration. We believe that our multi-channel distribution model, combined with our research, development and manufacturing
capabilities offers us a unique advantage over our competitors. The daily feedback received from interactions between our team members
and customers will be monitored to recognize what our customers want and rapidly develop products to meet their desires. Instead of simply
responding to trends in the market, we will seek to bring new topical products to the market before they are generally available.
Our
Corporate Structure
We,
Aixin Life International, Inc. (“Aixin” or “Aixin Colorado”), are a corporation organized under the laws of Colorado.
We are a holding company with no material operations of our own. We conduct all of our operations through our wholly owned subsidiaries
which are operating companies established in the PRC. Our operating companies in the PRC are wholly owned by HK AiXin International Group
Co., Limited (“AiXin HK”), our wholly owned subsidiary organized in Hong Kong which, in turn is wholly owned by Aixin (BVI)
International Group Co., Ltd. (“Aixin BVI”), an entity organized in the British Virgin Islands which is wholly owned by us.
Our
subsidiaries are (i) AiXin BVI), (ii) AiXin HK, and the following entities, each of which was organized in the PRC and which are collectively
referred to as our “PRC Subsidiaries” (iii) Chengdu AiXinZhonghong Biological Technology Co., Ltd., a Chinese limited
company (“AiXinZhonghong”), (iv) Yunnan Runcangsheng Technology Co., Ltd (“Yunnan Runcangsheng”), (v) Chengdu
Aixintang Pharmacy Co., Ltd. and its subsidiaries, each of which operates a pharmacy and (vi) Aixin Shangyan Hotel Management Co., Ltd.
Unless the context otherwise requires, all references in this prospectus to “Aixin” or “Aixin Colorado”)
refer to Aixin Life International, Inc., and references to “we,” “us,” “our,”
the “Registrant”, the “Company,” or “our company” refer to Aixin and/or its
consolidated subsidiaries.

Summary
of Risk Factors
Investing
in our common stock involves a high degree of risk. Below is a summary of material factors that make an investment in our common stock
speculative or risky. This summary does not address all of the risks that we face. Please refer to the information contained in and incorporated
by reference under the heading “Risk Factors” on page 15 of this prospectus.
Risks
Associated with Our Company
| ● |
The
health and wellness industry is highly competitive and we face fierce competition from numerous industry participants, including
many larger, well capitalized nationally and internationally recognized companies, see “Risk Factors — Risks Associated
with Our Company — We operate in a highly competitive industry and our failure to compete effectively could adversely affect
our market share, revenues and growth prospects. |
| |
|
| ● |
We
first entered the health and wellness business in 2018 when we acquired Aixin BVI. Consequently, we have a limited operating history
on which to evaluate our prospects, see “Risk Factors — Risks Associated with Our Company — We have a limited
operating history on which to judge our performance and assess our prospects for success.” |
| |
|
| ● |
We
recently completed a significant acquisition and intend to seek to expand through future acquisitions. Our future expansion plans
are subject to uncertainties and risks. For more details, see “Risk Factors – Risks Associated with Our Company
– Our recently completed acquisition is subject to uncertainties and risks” and – “Our future expansion
plans are subject to uncertainties and risks.” |
| |
|
| ● |
Our
success depends upon our ability to anticipate and timely react to changes in consumers demands, see “Risk Factors –
Risks Associated with Our Company - Failure to effectively anticipate consumer preferences could negatively impact the demand for
our products and our ability to generate revenues and the market price of our common stock.” |
| |
|
| ● |
We
may not succeed in our efforts to develop new products which could cause us to devote our resources to projects that do not succeed,
see “Risk Factors – Risks Associated with Our Company—Resources devoted to product innovation may not yield
new products that achieve commercial success.” |
| |
|
| ● |
Our
success depends on our ability to attract and retain customers through a coordinated omni channel distribution network which includes
pharmacies, direct marketing and e-commerce. If we do not succeed in these efforts we may fail to grow our customer base. For more
details, see “Risk Factors – Risks Associated with Our Company – If we do not successfully develop and maintain
a robust omni-channel experience for our customers, our business and results of operations could be materially and adversely affected.” |
| |
|
| ● |
If
we fail to succeed in developing and protecting our brand names we could fail in our efforts to grow our customer base, see “Risk
Factors – Risks Associated with Our Company —If we fail to develop and protect our brand names and reputation, we may
not attract and retain new customers which could adversely affect our revenues and financial performance.” |
| |
|
| ● |
We
are highly dependent upon consumer perception of our products and unfavorable publicity about us or our business may materially and
adversely affect our reputation, business, results of operations and financial condition. For more details, see “Risk Factors
– Risks Associated with Our Company –Unfavorable publicity about us or consumer perception of our products, the ingredients
they contain and any similar products distributed by other companies could cause fluctuations in our operating results and could
have a material adverse effect on our reputation, the demand for our products and our ability to generate revenues and the market
price of our common stock.” |
| |
|
●
|
We
face risks related to our ability to effectively source raw materials and any adverse change
in the supply or cost of raw materials may adversely affect our business. See “Risk
Factors – Risks Associated with Our Company -A significant disruption to our
timely receipt of raw materials and inventory could adversely impact sales and operations
or increase our transportation costs, which would decrease our profits. |
| |
|
| ● |
We
are highly dependent upon the services of Quanzhong Lin, our President, Chief Executive Officer and principal shareholder. The loss
of his services could have a Material adverse impact on our business. See, Risk Factors – Risks Associated with Our
Company - Our business depends on the continued contributions made by Mr. Quanzhong Lin, our key executive officer. The loss of his
services may result in a severe impediment to our business.” |
General
Risks Associated with Business Operations in China
| ● |
In
March 2020, the World Health Organization announced that infections caused by the coronavirus disease of 2019 (“COVID-19”)
had become pandemic. Economies throughout the world have been severely disrupted by the effects of the pandemic and the quarantines,
stay at home orders, business closures and the reluctance of individuals to leave their homes resulting from the outbreak of the
Covid-19 Pandemic. China adopted and continues to rely upon a “zero-tolerance” policy pursuant to which it has declared
a number of total and partial lockdowns in cities throughout China, including Chengdu. These lockdowns and any accompanying travel
restrictions have adversely impacted many industries in China, see “Risk Factors — General Risks Associated with
Business Operation in China – Recent developments related to the global outbreak of the novel strain of the coronavirus
known as “COVID-19 and the possibility of future outbreaks.” |
| |
|
| ● |
The
Chinese government may intervene or influence our operations at any time or may exert more control over offerings conducted overseas
and/or foreign investment in China-based issuers, which could result in material changes in our operations and/or the value of your
common stock. For more details, see “Risk Factors – General Risks Associated with Business Operation in China - The
PRC government has significant oversight and discretion over the conduct of a PRC company’s business operations or to exert
control over any offering of securities conducted overseas and/or foreign investment in China-based issuers, and may intervene with
or influence our operations, may limit or completely hinder our ability to offer or continue to offer securities to investors, and
may cause the value of such securities to significantly decline or become worthless, as the government deems appropriate to
further regulatory, political and societal goals.” |
| |
|
| ● |
On
December 24, 2021, the CSRC released the Administrative Provisions of the State Council Regarding the Overseas Issuance and Listing
of Securities by Domestic Enterprises (Draft for Comments) (the “Draft Administrative Provisions”) and the Measures for
the Overseas Issuance of Securities and Listing Record-Filings by Domestic Enterprises (Draft for Comments) (the “Draft Filing
Measures,” collectively with the Draft Administrative Provisions, the “Draft Rules Regarding Overseas Listing”).
The Draft Rules Regarding Overseas Listing lay out the filing regulation arrangement for both direct and indirect overseas listing,
and clarify the determination criteria for indirect overseas listing in overseas markets. Among other things, if a domestic enterprise
intends to indirectly offer and list securities in an overseas market, the record-filing obligation is with a major operating entity
incorporated in the PRC and such filing obligation shall be completed within three working days after the overseas listing application
is submitted. The required filing materials for an initial public offering and listing shall include but not limited to: regulatory
opinions, record-filing, approval and other documents issued by competent regulatory authorities of relevant industries (if applicable);
and security assessment opinion issued by relevant regulatory authorities (if applicable). The Draft Rules Regarding Overseas Listing,
if enacted, may subject us to additional compliance requirement in the future. Any failure of us to fully comply with new regulatory
requirements may significantly limit or completely hinder our ability to offer or continue to offer our common stock, cause
significant disruption to our business operations, and severely damage our reputation, which would materially and adversely affect
our financial condition and results of operations and cause our common stock to significantly decline in value or become worthless.
See “Risk Factors – General Risks Associated with Business Operations in China — The CSRC has released
for public consultation the draft rules for China-based companies seeking to conduct initial public offerings in foreign markets.
The Chinese government may exert more oversight and control over offerings that are conducted overseas and foreign investment in
China-based issuers, which could significantly limit or completely hinder our ability to offer or continue to offer our common
stock to investors and could cause the value of our common stock to significantly decline or become worthless.” |
| |
|
| ● |
You
may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in China against
us or our management named in the prospectus based on foreign laws. For more details, see “Risk Factors – General
Risks Associated with Business Operations in China - You may have difficulty enforcing judgments against us.” |
| ● |
Changes
in the policies, regulations, rules and the enforcement of laws of the PRC government may be quick with little advance notice and
could have a significant impact upon the business we may be able to conduct in the PRC and the profitability of such business. For
more details, see “Risk Factors - General Risks Associated with Business Operations in China - Changes in the policies,
regulations, rules and the enforcement of laws of the PRC government may be quick with little advance notice and could have a significant
impact upon the business we may be able to conduct in the PRC and the profitability of such business.” |
| |
|
| ● |
Inflation
could pose a risk to our business. For more details, see “Risk Factors - General Risks Associated with Business Operations
in China - Inflation could pose a risk to our business.” |
| |
|
| ● |
There
are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations. For more details, see “Risk
Factors – General Risks Associated with Business Operation in China - There are uncertainties regarding the interpretation
and enforcement of PRC laws, rules and regulations.”. |
| |
|
| ● |
PRC
regulations regarding acquisitions impose significant regulatory approval and review requirements, which could make it more difficult
for us to pursue growth through acquisitions. For more details, see “Risk Factors – General Risks Associated with
Business Operation in China - PRC regulations regarding acquisitions impose significant regulatory approval and review
requirements, which could make it more difficult for us to pursue growth through acquisitions.” |
| |
|
| ● |
While
the approval of the China Securities Regulatory Commission is not currently required for this offering, it may be required in the
future in connection with this offering under the M&A Rules and, if required, we cannot predict whether we will be able to obtain
such approval. For more details, see “Risk Factors – General Risks Associated with Business Operation in China
- While the approval of the China Securities Regulatory Commission is not currently required for this offering, it may
be required in the future in connection with this offering under the M&A Rules and, if required, we cannot predict whether we
will be able to obtain such approval.” |
| |
|
| ● |
Our
business may be subject to a variety of PRC laws and other obligations regarding cybersecurity and data protection. For more details,
see “Risk Factors – General Risks Associated with Business Operation in China - Our business may be subject to a variety
of PRC laws and other obligations regarding cybersecurity and data protection.” |
| |
|
| ● |
PRC
regulations relating to investments in offshore companies by PRC residents may subject our PRC-resident beneficial owners or our
PRC Subsidiaries to liability or penalties, limit our ability to inject capital into our PRC Subsidiaries or limit our PRC Subsidiaries’
ability to increase their registered capital or distribute profits. For more details, see “Risk Factors – General
Risks Associated with Business Operation in China - PRC regulations relating to investments in offshore companies by PRC residents
may subject our PRC-resident beneficial owners or our PRC Subsidiaries to liability or penalties, limit our ability to inject capital
into our PRC Subsidiaries or limit our PRC Subsidiaries’ ability to increase their registered capital or distribute profits.” |
| ● |
We
may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise Income Tax Law, and we may therefore be subject
to PRC income tax on our global income. For more details, see “Risk Factors – General Risks
Associated with Business Operation in China - We may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise
Income Tax Law, and we may therefore be subject to PRC income tax on our global income.” |
| |
|
| ● |
Restrictions
on currency exchange may limit our ability to utilize our PRC revenue effectively. For more details, see “Risk Factors –
General Risks Associated with Business Operation in China - Restrictions on currency exchange may limit our ability to utilize our
PRC revenue effectively.” |
| |
|
| ● |
Introduction
of new laws or regulations or changes to existing laws or regulations by the PRC government may adversely affect our business. For
more details, see “Risk Factors – General Risks Associated with Business Operation in China - Introduction of new
laws or changes to existing laws by the PRC government may adversely affect our business.” |
Risks
Relating to Our Holding Company Structure
| ● |
Substantial
uncertainties exist with respect to the interpretation and implementation of the newly enacted Foreign Investment Law and how it
may impact the viability of our current corporate structure, corporate governance and business operations. For more details, see
“Risk Factors – Risks Relating to Our Holding Company Structure - Substantial uncertainties exist with
respect to the interpretation and implementation of the newly enacted Foreign Investment Law and how it may impact the viability
of our current corporate structure, corporate governance and business operations.” |
| |
|
| ● |
We
may rely on dividends and other distributions on equity paid by our PRC Subsidiaries and loans between us and our PRC subsidiaries
to fund any cash and financing requirements we or any of our PRC Subsidiaries may have, and any limitation on the ability of our
PRC Subsidiaries to make payments too or transfer funds to us or our other PRC Subsidiaries could have a material and adverse effect
on our ability to conduct our business and the ability of our PRC Subsidiaries to conduct their respective businesses. For more details,
see “Risk Factors – Risks Relating to Our Holding Company Structure - We may rely on dividends and other distributions
on equity paid by our PRC Subsidiaries to fund any cash and financing requirements we may have, and any limitation on the ability
of our PRC subsidiaries to make payments to us could have a material and adverse effect on our ability to conduct our business.” |
| |
|
| ● |
PRC
regulation of loans to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion
may delay or prevent us from using the proceeds of this offering to make loans or additional capital contributions to our PRC Subsidiaries,
which could materially and adversely affect our liquidity and our ability to fund and expand our business. For more details, see
“Risk Factors – Risks Relating to Our Holding Company Structure - PRC regulation of loans to and direct investment
in PRC entities by offshore holding companies and governmental control of currency conversion may delay or prevent us from using
the proceeds of this offering to make loans or additional capital contributions to our PRC Subsidiaries, which could materially and
adversely affect our liquidity and our ability to fund and expand our business.” |
Risks
Relating to this Offering and Our Common Stock
| ● |
Prior
to this offering, we had a limited public market for our common stock and you may
not be able to resell our shares at or above the price you paid, or at all. For more details,
see “Risk Factors – Risks Relating to this Offering and Our Common Stock -
Prior to this offering, we had a limited public market for our common stock and
you may not be able to resell our shares at or above the price you paid, or at all.”
|
| |
|
| ● |
Future
sales of substantial amounts of our common stock by existing shareholders, including sales resulting from a foreclosure of shares
pledged by holders of a substantial number of shares of our common stock, could adversely affect the price of our common stock. For
more details, see “Risk Factors – Risks Relating to this Offering and Our Common Stock - The sale or availability
for sale of substantial amounts of our common stock could adversely affect its market price.” |
| ● |
The
market price of our shares is likely to be highly volatile and subject to wide fluctuations
in response to various factors. For more details, see “Risk Factors – Risks
Relating to this Offering and Our Common Stock - The market price of our shares is likely
to be highly volatile, which could result in substantial losses to inivestors.”
|
| |
|
| ● |
We
may never be able to pay dividends and are unlikely to do so. For more details, see “Risk Factors – Risks Relating
to this Offering and Our Common Stock - Because we do not expect to pay dividends in the foreseeable future after this offering,
you must rely on price appreciation of our common stock for return on your investment.” |
PRC
Limitation on Overseas Listing and Share Issuances
Neither
we nor our subsidiaries are currently required to obtain approval from Chinese authorities, including the China Securities Regulatory
Commission, or CSRC, or Cybersecurity Administration Committee, or CAC, to list on U.S. exchanges or issue securities to foreign investors,
however, if our subsidiaries or the holding company were required to obtain approval in the future and were denied permission from Chinese
authorities to list on U.S. exchanges, we would not be able to continue listing on any U.S. exchange, which would materially affect the
interests of investors in our securities. It is uncertain when and whether we will be required to obtain permission from the PRC government
to list on U.S. exchanges in the future, and even if such permission is obtained, whether it will be rescinded. Although we currently
are not required to obtain permission from any of the PRC central or local government agencies and have not been denied the right to
list on a U.S. exchange by any Chinese governmental authority, our operations could be adversely affected, directly or indirectly, by
existing or future laws and regulations denying us the right to list on any U. S. exchange. If we inadvertently conclude that such approvals
are not required when they are, or applicable laws, regulations, or interpretations change and we are required to obtain approval in
the future, our operations and the interests of our shareholders could be adversely affected. For more detailed information, see “Risk
Factors – General Risks Associated with Business Operations in China – While the approval of the China Securities Regulatory
Commission is not currently required for this offering, it may be required in the future in connection with this offering under the M&A
Rules and, if required, we cannot predict whether we will be able to obtain such approval.”
On
December 24, 2021, the China Securities Regulatory Commission, or the CSRC, issued Provisions of the State Council on the Administration
of Overseas Securities Offering and Listing by Domestic Companies (Draft for Comments) (the “Administration Provisions”),
and the Administrative Measures for the Filing of Overseas Securities Offering and Listing by Domestic Companies (the “Measures”),
which were open for public comments by January 23, 2022. The Administration Provisions and Measures lay out specific
requirements for filing documents and include unified regulation management, strengthening regulatory coordination, and cross-border
regulatory cooperation. Domestic companies seeking to list abroad must carry out relevant security screening procedures if their businesses
involve activities requiring supervision such as foreign investment security and cyber security reviews. Companies deemed to endanger
national security are among those off-limits for overseas listings. The Administration Provisions and Measures have not yet come into
effect. However, it is uncertain when the Administration Provision and the Measures will take effect, whether they will take effect as
currently drafted or if we will be subject to such measures when they come into effect.
As
of the date of this prospectus, other than the response we recently received from the CSRC confirming that our offering under this prospectus
does not require the examination and approval of the CSRC in accordance with the existing PRC legislation and regulations, we
have not received any inquiry, notice, warning, sanctions or regulatory objection to this offering from the CSRC, CAC or any other PRC
governmental authorities, and we believe our PRC Subsidiaries have obtained all requisite permissions and approvals from PRC governmental
authorities to operate our business as currently conducted under relevant PRC laws and regulations.
Currently,
each of our PRC Subsidiaries holds and maintains a business license issued by the local market supervision and administration bureau
and has received all requisite permissions and approvals in order to conduct and operate its business. As of the date of this prospectus,
none of our PRC Subsidiaries has been denied a business license or punished by relevant governmental authorities due to its business
operations. In addition, we and our non-PRC subsidiaries have also received all requisite permissions and approvals in order to conduct
and operate our business.
Transfers
of Cash to and from our Subsidiaries
We
(Aixin Life) are a Colorado holding company with no material operations of our own. We conduct all of our operations through operating
companies established in the PRC. As a result, our ability to pay dividends to our shareholders and to service any debt we may
incur may depend upon dividends paid by our PRC Subsidiaries. If any of our subsidiaries incurs debt on its own, the instruments
governing such debt may restrict its ability to pay dividends to us or otherwise transfer funds to us or any of our other subsidiaries.
In addition, our PRC Subsidiaries are required to make appropriations to certain statutory reserve funds, which are not distributable
as cash dividends except in the event of a liquidation of the company at a time when it is solvent.
Current
PRC regulations permit our PRC Subsidiaries to pay dividends to us through Aixin HK, our intermediate holding subsidiary in Hong Kong,
only out of their accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. As of the
date hereof, none of our PRC Subsidiaries has distributed any dividends to Aixin HK.
The
PRC government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies outside the PRC.
Therefore, we may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency
for the payment of dividends from our profits, if any, or for any other purpose.
Cash
dividends, if any, on our common stock will be paid in U.S. dollars. If we are considered a PRC tax resident enterprise for tax purposes,
any dividends we pay to our overseas shareholders may be regarded as China-sourced income and as a result may be subject to PRC withholding
tax at a rate of up to 10.0%.
Pursuant
to the Arrangement between Mainland China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and Tax
Evasion on Income, or the Double Tax Avoidance Arrangement, the 10% withholding tax rate may be lowered to 5% if a Hong Kong resident
enterprise owns no less than 25% of a PRC project. However, the 5% withholding tax rate does not automatically apply and certain requirements
must be satisfied, including without limitation that (a) the Hong Kong project must be the beneficial owner of the relevant dividends;
and (b) the Hong Kong project must directly hold no less than 25% share ownership in the PRC project during the 12 consecutive months
preceding its receipt of the dividends. In current practice, a Hong Kong project must obtain a tax resident certificate from the Hong
Kong tax authority to apply for the 5% lower PRC withholding tax rate. As the Hong Kong tax authority will issue such a tax resident
certificate on a case-by-case basis, we cannot assure you that we will be able to obtain the tax resident certificate from the relevant
Hong Kong tax authority and enjoy the preferential withholding tax rate of 5% under the Double Taxation Arrangement with respect to dividends
to be paid by any of our PRC Subsidiaries to Aixin HK. As of the date of this prospectus, we have not applied for the tax resident certificate
from the relevant Hong Kong tax authority. Aixin HK intends to apply for the tax resident certificate at such time as one of our PRC
Subsidiaries plans to declare and pay a dividend to Aixin HK. See “Risk Factors – Risks Relating to Our Holding Company
Structure – We may rely on dividends and other distributions on equity paid by our PRC Subsidiaries to fund any cash and financing
requirements we may have, and any limitation on the ability of our PRC Subsidiaries to make payments to us could have a material and
adverse effect on our ability to conduct our business.”
As
of the date hereof, we have had no transactions that involved the transfer of cash or assets throughout our corporate structure. The
PRC Subsidiaries have not transferred cash or other assets to Aixin Life, including by way of dividends. Aixin Life does not currently
plan or anticipate transferring cash or other assets from our operations in China to any non-Chinese entity. As of the date hereof, no
transfers, dividends, or distributions have been made to our investors.
Holding
Foreign Companies Accountable Act
Trading
in our securities may be prohibited under the Holding Foreign Companies Accountable Act, or the HFCAA, if the PCAOB determines that it cannot inspect or investigate completely our auditor.
Pursuant
to the HFCAA, the PCAOB issued a Determination Report on December 16, 2021 which found that the PCAOB is unable to inspect or investigate
completely registered public accounting firms headquartered in: (1) mainland China of the People’s Republic of China because of
a position taken by one or more authorities in mainland China; and (2) Hong Kong, a Special Administrative Region and dependency of the
PRC, because of a position taken by one or more authorities in Hong Kong. In addition, the PCAOB’s report identified the specific
registered public accounting firms which are subject to these determinations.
The
PCAOB is currently unable to conduct inspections in China without the approval of Chinese government authorities. If it is later determined
that the PCAOB is unable to inspect or investigate our auditor completely, investors may be deprived of the benefits of such inspection.
Any audit reports not issued by auditors that are completely inspected by the PCAOB, or a lack of PCAOB inspections of audit work undertaken
in China that prevents the PCAOB from regularly evaluating our auditors’ audits and their quality control procedures, could result
in a lack of assurance that our financial statements and disclosures are adequate and accurate.
Our
auditor, KCCW Accountancy Corp., is an independent registered public accounting firm with the PCAOB, and as an auditor of publicly traded
companies in the U.S., is subject to laws in the U.S. pursuant to which the PCAOB conducts regular inspections to assess its compliance
with the applicable professional standards. KCCW Accountancy Corp. is based in the United States and has been inspected by the PCAOB
on a regular basis, with the last inspection in 2022. KCCW Accountancy Corp., is not headquartered in mainland China or Hong Kong
and was not identified as a firm subject to the determinations announced by the PCAOB on December 16, 2021. Should the PCAOB be unable
to fully conduct inspection of our auditor’s work papers in China, it will make it difficult to evaluate the effectiveness of our
auditor’s audit procedures or equity control procedures. Investors may consequently lose confidence in our reported financial information
and procedures or quality of the financial statements, which would adversely affect us and our securities.
Moreover,
if trading in our securities is prohibited under the HFCAA because the PCAOB determines that it cannot inspect or fully investigate our
auditor, an exchange may determine to delist our securities.
Furthermore,
on June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act (“AHFCAA”), which, if
enacted, would amend the HFCAA and require the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchanges
if its auditor is not subject to PCAOB inspections for two consecutive years instead of three. If the AHFCAA is enacted, and if we are
subject to it, it would decrease the number of “non-inspection years” from three years to two years, and thus, would reduce
the time before our securities may be prohibited from trading or delisted. On August 26, 2022, the CSRC, the Ministry of Finance of the
PRC, and the PCAOB signed a Statement of Protocol, governing inspections and investigations of audit firms based in China and Hong Kong.
Pursuant to the Protocol, the PCAOB shall have independent discretion to select any issuer audits for inspection or investigation and
has the unfettered ability to transfer information to the SEC. However, uncertainties still exist whether the framework will be fully
complied. Our auditor, KCCW Accountancy Corp. is not subject to the determinations announced by the PCAOB on December 16, 2021.
See “Risk Factors—Risks Associated with Our Company— A recent joint statement by the SEC and the Public Company
Accounting Oversight Board (United States), or the “PCAOB,” proposed rule changes submitted by Nasdaq, and the newly enacted
“Holding Foreign Companies Accountable Act” all call for additional and more stringent criteria to be applied to emerging
market companies upon assessing the qualification of their auditors, especially the non-U.S. auditors who are not inspected by the PCAOB.
These developments could add uncertainties to our offering.”
Corporate
Information
Aixin
Life International, Inc. was incorporated in the State of Colorado in 2001. We have a fiscal year-end of December 31. Our principal executive
offices are located at Hongxing International Business Building 2, 14th FL, No. 69 Qingyun South Ave., Jinjiang District,
Chengdu City, Sichuan Province, China and our telephone number is + (86) 313-6732526. We maintain a website at www.aixinjituan.com. The
information contained on our website is not, and should not be interpreted to be, a part of this prospectus.
THE
OFFERING
| Shares
of common stock offered by us: |
|
[●] shares of common stock, or [●]
shares if the underwriter exercises the over-allotment option in full. |
| |
|
|
| Number
of shares of common stock outstanding after this offering: (1) |
|
[●]
shares of common stock will be outstanding after
this offering is completed, [●] shares if the underwriter exercises the over-allotment option in full. |
| |
|
|
| Over-allotment
option: |
|
We
have granted the underwriter the right to purchase up to [●] additional shares of common stock from us at the public
offering price less the underwriting discount within 45 days from the date of this prospectus to cover over-allotments. |
| |
|
|
| Underwriter’s
warrants: |
|
We
will issue to Network 1 Financial Securities, Inc., upon closing of this offering, compensation warrants, or the Underwriter’s
Warrants, entitling the underwriter to purchase 10% of the aggregate number of shares of common stock issued in this offering, including
shares issued pursuant to the exercise of the over-allotment option, at an exercise price of $[●] per share. The Underwriter’s
Warrants will have a term of five years beginning on date of commencement of sales of the shares offered hereby and may be
exercised upon commencement of sales of the shares offered hereby. The Underwriter’s Warrants may be exercised
on a cashless basis. |
| |
|
|
| Use
of proceeds: |
|
Our
proceeds from this offering are expected to be approximately $[●], before payment of underwriter commissions and other
expenses. We intend to use the proceeds from this offering for the purchase and sale of raw materials and developing our own brands,
including working capital and general corporate purposes. See “Use of Proceeds” on 38 page. |
| |
|
|
| Proposed
Nasdaq Capital Market symbol: |
|
We
have applied to list our common stock on the Nasdaq Capital Market under the symbol “AIXN”. There can be no assurance
that our application will be approved. The closing of this offering is contingent upon the successful listing of our common stock
on the Nasdaq Capital Market. |
| |
|
|
| Lock-Up
Agreements: |
|
“See
“Plan of Distribution” for more information. |
| |
|
|
| Risk
factors: |
|
Investing
in our common stock is highly speculative and involves a significant degree of risk. As an investor you should be able to bear a
complete loss of your investment. You should carefully consider the information set forth in the “Risk Factors” section
beginning on page 15. |
| |
|
|
| OTCQX
Market Symbol: |
|
AIXN. |
| |
|
|
Transfer Agent: |
|
Securities Transfer Corporation. |
| |
(1) |
The
number of shares of our common stock to be outstanding after this offering is based on 49,999,891 shares outstanding as of October
[●], 2022 and gives no effect to shares which may be issued upon exercise of (i) the Underwriter’s over-allotment
option and (ii) the Underwriter’s Warrants. |
FORWARD-LOOKING
STATEMENTS
This
prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act and the Private Securities Litigation
Reform Act of 1995, as amended. These forward-looking statements are based on our management’s belief and assumptions and on information
currently available to our management. Although we believe that the expectations reflected in these forward-looking statements are reasonable,
these statements relate to future events or our future financial performance, and involve known and unknown risks, uncertainties and
other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any
future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
In
some cases, you can identify forward-looking statements by terminology such as “may,” “should,”
“expects,” “intends,” “plans,” “anticipates,” “believes,”
“estimates,” “predicts,” “potential,” “continue” or the negative
of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking
statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases, beyond our control
and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include,
among other things, those listed under “Risk Factors” and elsewhere in this prospectus. If one or more of these risks
or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from
those implied or projected by the forward-looking statements. No forward-looking statement is a guarantee of future performance. You
should read this prospectus and those documents which we have filed with the SEC as exhibits to the registration statement, of which
this prospectus is a part, completely and with the understanding that our actual future results may be materially different from any
future results expressed or implied by these forward-looking statements.
The
forward-looking statements in this prospectus represent our views as of the date of this prospectus. We anticipate that subsequent events
and developments may cause our views to change. However, while we may elect to update these forward-looking statements at some point
in the future, we have no current intention of doing so except to the extent required by applicable law. You should therefore not rely
on these forward-looking statements as representing our views as of any date subsequent to the date of this prospectus.
You
should also consider carefully the statements under “Risk Factors” and other sections of this prospectus, which address
additional facts that could cause our actual results to differ from those set forth in the forward-looking statements. We caution investors
not to place significant reliance on the forward-looking statements contained in this prospectus. We undertake no obligation to publicly
update or review any forward-looking statements, whether as a result of new information, future developments or otherwise, except as
otherwise required by law.
RISK
FACTORS
You
should carefully consider the risks described below and elsewhere in this prospectus, which could materially and adversely affect our
business, results of operations or financial condition. Our business faces significant risks and the risks described below may not be
the only risks we face. Additional risks not presently known to us or that we currently believe are immaterial may materially affect
our business, results of operations, or financial condition. If any of these risks occur, the trading price of our common stock could
decline, and you may lose all or part of your investment. You should consider our business and prospects in light of the challenges we
face, including the ones discussed in this section. In the event that any of the events described in the risk factors below occur, it
could have a material adverse effect on our operations and cash flow and cause the value of our securities to decline in value or become
worthless.
Risks
Associated with Our Company
We
operate in a highly competitive industry and our failure to compete effectively could adversely affect our market share, revenues and
growth prospects.
The
market for health and wellness products is large, highly fragmented and intensely competitive. Current and prospective participants include
specialty retailers, supermarkets, drugstores, mass merchants, multi-level marketing organizations, online merchants, mail-order companies
and a variety of other smaller participants. The market is also highly sensitive to the introduction of new products, which may rapidly
gain consumer acceptance. We compete for sales with heavily advertised brands manufactured by large pharmaceutical and food companies,
as well as other brands, some of which have greater market presence, name recognition and financial, marketing and other resources, including
some competitors that may spend more aggressively on advertising and promotional activities than we do. Further, if we fail to build
out our e-commerce platform or fail to provide our customers with a desired omni-channel experience, we may lose business to online retailers
with a more robust and engaging e-commerce platform.
Further,
the ability of consumers to compare prices on a real-time basis through the use of smartphones and digital technology puts additional
pressure on us to maintain competitive pricing. We compete in multiple product categories and sales channels, including pharmaceutical
stores, wholesale to specialty store formats, direct marketing and increasingly internet-based and direct-sell retailers and vendors.
Many factors affect the extent to which competition could affect our results, including as it relates to pricing, quality, assortment,
marketing, promotions and advertising, service, locations, capital expenditures, category share and reputation, any of which could have
a material effect on our results of operations.
We
have a limited operating history on which to judge our performance and assess our prospects for future success.
We
first entered the health and wellness business in December 2017 when we acquired Aixin BVI, which at that time owned all of the equity
of Aixin Zhonghong, then engaged in the distribution of health and wellness foods. Consequently, we have a limited operating history
on which to evaluate our prospects in the health and wellness industry. We may fail to continue our growth. You should not consider our
historical growth and expansion of our business through acquisitions as indicative of our ability to grow in the future.
Our
recently completed acquisition is subject to uncertainties and risks.
We
recently completed a significant acquisition. There is no assurance that we
will realize the benefits anticipated from our acquisition of Yunnan Runcangsheng. There is no assurance that the members of the
management of Yunnan Runcangsheng that choose to join our company can be successfully integrated into our management. The process
of combining the operations of Yunnan Runcangsheng with our existing operations could have a material adverse effect on us and
our financial condition.
Our
future expansion plans are subject to uncertainties and risks.
We
intend to seek to expand our operations through acquisitions. The implementation of such plan requires us to integrate any newly acquired
business and its management teams. If we fail to effectively and efficiently implement our plan for acquisitions, we may not be successful
in achieving profitable results. Even if we effectively and efficiently implement our future plans, there may be other unexpected events
or factors that prevent us from achieving success. Our management has limited experience in effecting a rapid expansion and in managing
larger operations. Our business, financial condition, results of operations and growth prospects may be materially and adversely affected
if our future expansion plans fail to achieve positive results.
Failure
to effectively anticipate consumer preferences could negatively impact the demand for our products and our ability to generate
revenues and the market price of our common stock.
Our
success also depends on our ability to anticipate and respond in a timely manner to changing consumer demand, consumer preferences, and
shopping patterns regarding health and wellness products. Consumer preferences cannot be predicted with certainty and are subject to
continual change and evolution. Additionally, our customers may have expectations about how they shop in stores or through e-Commerce
or more generally engage with businesses across different channels or media (through online and other digital or mobile channels or particular
forms of social media), which may change over time which may make it more difficult for us to adapt to rapid changes in consumer preferences.
Our
sales may decline significantly if we misjudge the market for our new products, which may result in significant inventory markdowns and
lower margins, missed opportunities for other products, or inventory write-downs, and could have a negative impact on our reputation
and profitability.
Resources
devoted to product innovation may not yield new products that achieve commercial success.
Our
ability to develop new and innovative products or identify and acquire new and innovative products from third-party vendors, depends
on, among other factors, our ability to understand evolving market trends and translate our insights into identifying, and then designing
and manufacturing or otherwise obtaining, commercially successful new products. If we are unable to do so, our customer relationships
and product sales could be harmed significantly. The health and wellness industry is characterized by rapid and frequent changes in demand
for products. Our failure to accurately predict these trends could harm our customer relationships and cause us to fail to grow our revenues.
The development of new and innovative products requires significant investment in research and development and testing of new ingredients,
formulas and possibly new production processes. The research and development process entails considerable uncertainty. Products may appear
promising in development but fail to reach market within the expected time frame, or at all. Further, products also may fail to achieve
commercial success. Finally, there is no guarantee that our development teams will be able to successfully respond to competitive products
that could render some of our offerings obsolete.
If
we do not successfully develop and maintain a robust omni-channel experience for our customers, our business and results of operations
could be materially and adversely affected.
Omni-channel
retailing where customers are accessed through various coordinated channels, is rapidly evolving, and we must keep pace with changing
customer expectations and new developments by our competitors. The growing middle class in China is increasingly seeking relevant information
regarding healthy lifestyles and products that promote a healthy life. In addition, customers are increasingly shopping for products
online instead of in traditional brick-and-mortar shopping centers and retail locations. As part of our omni-channel strategy, we anticipate
the need to continue making investments in technology and to provide ready means for our customers to access the information and products
they want in a comfortable environment. If we are unable to make, improve, or develop relevant channels to interact with customers in
a timely manner, our ability to compete and our business and results of operations could be materially and adversely affected. In addition,
if our e-commerce businesses or our other customer-facing technology systems do not function as designed or we are unable to effectively
blend our digital, online and in-store platforms, we may experience a loss of customer confidence, lost sales, or data security breaches,
any of which could materially and adversely affect our business and results of operations.
We
may incur material product liability claims, or experience product recalls, which could increase our costs and adversely affect our sales
and margin, reputation, revenues and operating income.
As
a retailer, distributor and manufacturer of products for human consumption, we are subject to product liability claims if the use of
our products is alleged to result in injury. The products that we sell consist of minerals, herbs, supplements and other ingredients
that are classified as foods or dietary supplements. The products that we sell could contain contaminated substances, and some of the
products we sell contain ingredients that do not have long histories of human consumption. Previously unknown adverse reactions resulting
from human consumption of these ingredients could occur.
In
addition, third-party manufacturers produce many of the products we sell. We rely on these manufacturers to ensure the integrity of their
ingredients and formulations. As a distributor of products manufactured by third parties, we may also be liable for various product liability
claims for products we do not manufacture. Our ultimate liability for these products that are manufactured by third parties depends on
a number of factors, including our contractual relationship with the vendor, the creditworthiness of the vendor and any insurance that
we have. Therefore, we may be unable to adequately protect ourselves against claims with respect to products manufactured by a third-party.
We
may be subject to product liability claims, including, among others, that our products include inadequate instructions for use or inadequate
warnings concerning possible side effects and interactions with other substances. Product liability claims could significantly damage
our reputation and consumer confidence in our products, regardless of the merits or outcomes of such claims. Our litigation expenses
could increase as well, which also could have a material adverse effect on our results of operations even if a product liability claim
is unsuccessful or is not fully pursued.
We
may be subject to product recalls, withdrawals or seizures if any of the products we sell are believed to be adulterated, cause injury
or illness or if we are alleged to have violated governmental regulations in the manufacturing, labeling, promotion, sale or distribution
of such products. A significant recall, withdrawal or seizure of any of the products may require significant management attention, would
likely result in substantial and unexpected costs and may materially and adversely affect our business, financial condition or results
of operations. Furthermore, a recall, withdrawal or seizure of any of the products that we sell may adversely affect consumer confidence
in our brands and thus decrease consumer demand for such products.
If
we fail to develop and protect our brand names and reputation, we may not attract and retain new customers which could adversely affect
our revenues and financial performance.
We
will invest significant resources to promote our brand names to obtain favorable public recognition for us and our products. If we do
not achieve the reputation we envision, we may not be able to attract and retain a significant customer base, which could in turn adversely
affect our revenues, profitability and the market price of our common stock.
Our
ability to adequately protect our trade names, trademarks and patents could have an impact on our brand images and ability to penetrate
new markets.
We
believe that our trade names and trademarks and patents are important assets and an essential element of our strategy. We have applied
for the registration of many of our trade names, trademarks and patents in China. Some of these applications have been granted
and some of these registrations are currently pending approval from the corresponding departments. There can be no assurance that we
will obtain such registrations or that the registrations we obtain will prevent the imitation of our products or infringement of our
intellectual property rights by others. In particular, the laws of the PRC may not protect proprietary rights to the same extent
as the laws of the U.S. Claims that others are infringing our intellectual property would be costly and would divert the attention of
management and key personnel. Our failure to successfully protect our trademarks could diminish the value and effectiveness of our past
and future marketing efforts and could cause customer confusion. This could in turn adversely affect our revenues, profitability and
the market price of our common stock.
Unfavorable
publicity about us or consumer perception of our products, the ingredients they contain and any similar products distributed by other
companies could cause fluctuations in our operating results and could have a material adverse effect on our reputation, the demand for
our products and our ability to generate revenues and the market price of our common stock.
We
are highly dependent upon consumer perception of the safety and quality of our products and the ingredients they contain, as well as
that of similar products distributed by other companies. Consumer perception of products and the ingredients they contain can be significantly
influenced by scientific research, national media attention and other publicity, including that generated via social media. A research
report or publicity related to our products and the ingredients they contain that is perceived by our consumers as less favorable or
that questions earlier favorable research or publicity could have a material adverse effect on our ability to generate revenues. Adverse
publicity in the form of published scientific research or otherwise, whether or not accurate, that associates consumption of our products
or the ingredients they contain or similar products distributed by other companies with illness or other adverse effects, that questions
the benefits of our or similar products, or that claims that such products are ineffective could have a material adverse effect on our
reputation, the demand for our products, our ability to generate revenues and the market price of our common stock.
We
depend on the services of key executives and other skilled professionals and our ability to attract, train and retain highly qualified
associates. Any failure to attract or retain such individuals could affect our business strategy and adversely impact our performance
and results of operations.
Our
senior executives are instrumental in setting our strategic direction, operating our business, identifying, recruiting and training personnel,
identifying opportunities, designing new products and arranging necessary financing. In addition, other key employees below the executive
level, with deep knowledge of our business, are critical to the execution and success of our strategy. We must attract, train and retain a growing number of qualified individuals.
Losing
the services of any of these groups of individuals could adversely affect our business and we may be unable to identify candidates of
sufficient experience and capabilities in a timely fashion or at all, which could negatively impact our business and operations. Further,
our ability to control labor and benefit costs is subject to numerous external factors, including regulatory changes, prevailing wage
rates, and healthcare and other insurance costs. We compete with other retail and non-retail businesses for these store and field associates
and invest significant resources in training and motivating them. There is no assurance that we will be able to attract or retain qualified
store and field associates in the future, which could have a material adverse effect on our business, financial condition and results
of operations.
Compliance
with new and existing laws and governmental regulations could increase our costs significantly and adversely affect our results of operations.
The
processing, formulation, safety, manufacturing, packaging, labeling, advertising and distribution of our products are subject to numerous
Chinese laws and regulations as well as laws and regulations imposed by provincial and local governments and agencies. Government regulations
may prevent or delay the introduction, or require the reformulation, of our products, which could result in lost revenues and increased
costs to us. Manufacturers and distributors of health and wellness products and dietary supplements and dietary ingredients are prohibited
from marketing products that are adulterated or misbranded, and the governing agencies may take enforcement action against any adulterated
or misbranded health and wellness product or dietary supplement on the market. If we violate applicable regulatory requirements, we may
be subject to enforcement actions against us, which could have a material adverse effect on our business, prospects, financial condition,
and results of operations. The government may not accept the evidence of safety for any new product or ingredient we may wish to market,
may determine that a particular product or ingredient presents an unacceptable health risk based on reported serious adverse events or
other information, and may determine that a particular claim or statement of efficacy or nutritional value that we use to support the
marketing of a product is not substantiated, or is an unauthorized version of a “health claim.” Any of these actions could
prevent us from marketing particular products or making certain claims or statements with respect to those products. We could also be
required to remove a particular product from the market. Any future recall or removal would result in costs to us, including lost revenues
from any products that we are required to remove from the market, any of which could be material. Any product recalls or removals could
also lead to an increased risk of litigation and liability, substantial costs, and reduced growth prospects.
Additional
or more stringent laws and regulations of health and wellness products have been considered from time to time. These developments could
require reformulation of some products to meet new standards, recalls or discontinuance of some products not able to be reformulated,
additional record-keeping requirements, increased documentation of the properties of some products, additional or different labeling,
additional scientific substantiation, or other new requirements. Any of these developments could increase our costs significantly. In
addition, regulators’ evolving interpretation of existing laws could have similar effects.
A
significant disruption to our timely receipt of raw materials and inventory could adversely impact sales and operations or increase our
transportation costs, which would decrease our profits.
Unexpected
delays in the deliveries of raw materials from our vendors or inventories or increases in transportation costs (including through increased
fuel costs) could significantly decrease our ability to make sales and earn profits. We must maintain sufficient levels of raw materials
and inventories to operate our business successfully. However, we also must guard against accumulating excess inventory. If we fail to
anticipate accurately either the market for the merchandise we offer or our customers’ purchasing habits, we may be forced to rely
on markdowns or promotional sales to dispose of excess or slow moving inventory, which could have a material adverse effect on our business,
financial condition, and results of operations.
In
addition, sales of adulterated products received from third parties could result in a product liability judgment or a widespread product
recall that may negatively impact our sales and profitability for a period of time depending on product availability, competition reaction
and consumer attitudes. Even if the product liability claim is unsuccessful or is not fully pursued, the negative publicity surrounding
any assertions could adversely impact our reputation with existing and potential customers and our brand image.
Natural
disasters (whether or not caused by climate change), unusually adverse weather conditions, pandemic outbreaks and terrorist acts in China
and the response of the Chinese governments to such occurrences, could impair our ability to purchase, receive or replenish inventory
or raw materials or cause customer traffic to decline, all of which could result in lost sales and otherwise adversely affect our financial
performance.
The
occurrence of one or more natural disasters, such as hurricanes, fires, floods and earthquakes (whether or not caused by climate change),
unusually adverse weather conditions, pandemic outbreaks (including the recent outbreak of the coronavirus, or COVID-19) or terrorist
acts in China and the response of the Chinese government to such occurrences, could adversely affect our operations and financial performance.
To the extent these events result in the closure of one or more of our pharmacies, manufacturing facility or our corporate headquarters,
or impact one or more of our key suppliers, our operations and financial performance could be materially adversely affected through lost
sales. Such events could also cause a disruption in travel making it difficult for consumers to order our products, such as has happened
as a result of recent lock-downs in Chengdu. In addition, these events could result in increases in fuel (or other energy) prices or
a fuel shortage, the temporary lack of an adequate work force in a market, the temporary or long-term disruption in the supply of products
from some suppliers, the temporary disruption in the transport of goods, the temporary reduction in the availability of products in our
stores, expiration of inventory and disruption to our information systems.
We
may be impacted by our ability to attract, develop and retain qualified associates and manage labor-related costs.
We
believe one of our competitive advantages is providing a positive, engaging and satisfying experience for each customer, which requires
us to have highly trained and engaged associates. Our success depends in part upon our ability to attract, develop and retain a sufficient
number of qualified associates to enable us to grow our customer base. The turnover rate in the health and wellness industry is generally
high, and qualified individuals of the requisite caliber and number needed may be in short supply. Competition for such qualified individuals
or changes in labor laws could require us to incur higher labor costs. Our inability to recruit a sufficient number of qualified individuals
may delay the expansion of our customer base and our growth. Significant increases in associate turnover rates or significant increases
in labor-related costs could have a material adverse effect on our results of operations, financial condition and cash flows.
Large
and similar sized competitors could steal our market share by offering lower prices.
We
endeavor to provide high quality service to our clients at the best possible price, however, large and similar sized competitors might
steal some of our market share by offering lower prices, causing us to lose some of our clients which could have a material adverse effect
on our results of operations, financial condition and cash flows.
Our
insurance may not be sufficient.
We
carry insurance that we consider adequate in regard to the nature of the covered risks and the costs of coverage. We are not fully insured
against all possible risks, nor are all such risks insurable.
Our
business depends on the continued contributions made by Mr. Quanzhong Lin, our key executive officer. The loss of his services may result
in a severe impediment to our business.
Our
success is dependent upon the continued contributions made by our CEO and President, Mr. Quanzhong Lin. The Company has no “Key
Man” insurance to cover the resulting losses in the event that any of our officer or directors should die or resign.
If
Mr. Lin cannot serve the Company or is no longer willing to do so, the Company may not be able to find alternatives in a timely manner
or at all. This would likely result in a severe damage to our business operations and would have an adverse material impact on our financial
position and operational results. To continue as a viable operation, the Company may have to recruit and train replacement personnel
at a higher cost.
Our
key executive does not devote his full business time to our operations.
Our
President and Chief Executive Officer, Mr. Quanzhong Lin, is involved in a number of businesses and does not devote all of his working
time to our business. Our positive reputation in Chengdu is derived from the standing of Mr. Lin in the Chengdu
business community. If Mr. Lin does not devote sufficient time to our business, our operations could suffer which would have an adverse
material impact on our financial position and operational results.
Some
of the other businesses engaged in by Mr. Lin could be deemed competitive with aspects of our business. Should such other businesses prove more successful than ours, Mr. Lin could choose to focus his attention on such businesses which could cause him to fail
to devote sufficient attention to our business and our operations could suffer and our financial conditions and results of operations
may be materially and adversely affected
Our
principal shareholder is not familiar with American business practices.
Mr.
Quanzhong Lin, our founder and principal shareholder, is a citizen of the PRC and an active entrepreneur in Chengdu. Mr. Lin is not familiar
with American business practices and is heavily influenced by the business culture in the PRC. There is a certain level of respect and
prestige associated with being the Chinese principal of a company which is publicly traded in the U.S. Mr. Lin’s motivation for
causing the business of AiXinZhongdong to become a part of a U.S. publicly-traded company may differ from those of American entrepreneurs
and his values may cause him to operate the business differently than would an American entrepreneur which could have a material adverse
effect on our results of operations, financial condition and cash flows
We
may be adversely impacted by certain compliance or legal matters.
We,
along with third parties with which we do business, are subject to complex compliance and litigation risks. The cost of defending against
claims that might be brought against us or the ultimate resolution of such claims, whether by settlement or adverse court decision, may
harm our business. Further, potential claimants may be encouraged to bring lawsuits based on a settlement from us or adverse court decisions
against us. We cannot currently assess the likely outcome of such suits, but if the outcome were negative, it could have a material adverse
effect on our reputation, results of operations, financial condition and cash flows.
In
addition, we may be impacted by litigation trends, including class action lawsuits involving consumers and shareholders, that could have
a material adverse effect on our reputation, the market price of our common stock, results of operations, financial condition and cash
flows.
Failure
to make adequate contributions to various employee benefits plans as required by PRC regulations may subject us to penalties.
Companies
operating in China are required to participate in various government sponsored employee benefit plans, including certain social insurance,
housing funds and other welfare-oriented payment obligations, and contribute to the plans in amounts equal to certain percentage of salaries,
including bonuses and allowances, of employees up to a maximum amount specified by the local government from time to time at locations
where they operate their businesses. The requirement of employee benefit plans has not been implemented consistently by the local governments
in China given the different levels of economic development in different locations. Employers that fail to make adequate social insurance
and housing provident fund contributions may be subject to late payment fees, fines and sanctions. If the relevant PRC authorities determine
that we need to make supplemental contributions or that we are subject to late payment fees, fines or other legal sanctions, such
as order of timely rectification, in relation to our failure to make social insurance and housing fund contributions in full for our
employees, our business and financial condition may be adversely affected.
Our
common stock may be delisted under the Holding Foreign Companies Accountable Act if the PCAOB is unable to inspect our auditors. The
delisting of our common stock or the threat of their being delisted, may materially and adversely affect the value of your investment.
The
HFCAA was enacted on December 18, 2020. The HFCAA states if the SEC determines that a company has filed audit reports issued
by a registered public accounting firm that has not been subject to inspection by the PCAOB for three consecutive years beginning in
2021, the SEC shall prohibit the securities of such company from being traded on a national securities exchange or in the over
the counter trading market in the U.S. On June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable
Act which, if enacted, would amend the HFCAA and require the SEC to prohibit an issuer’s securities from trading on
any U.S. stock exchange if its auditor is not subject to PCAOB inspections for two consecutive years instead of three.
On
March 24, 2021, the SEC adopted interim final rules relating to the implementation of certain disclosure and documentation requirements
of the HFCAA. A company will be required to comply with these rules if the SEC identifies it as having a “non-inspection”
year under a process to be subsequently established by the SEC. The SEC is assessing how to implement other requirements of the HFCAA,
including the listing and trading prohibition requirements described above. On September 22, 2021, the PCAOB adopted a final rule
implementing the HFCAA, which provides a framework for the PCAOB to use when determining whether the PCAOB is unable to inspect
or investigate completely registered public accounting firms located in a foreign jurisdiction because of a position taken by one or
more authorities in that jurisdiction. On December 2, 2021, the SEC issued amendments to finalize rules implementing the submission and
disclosure requirements in the HFCAA. The rules apply to registrants that the SEC identifies as having filed an annual report
with an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction and that PCAOB is unable
to inspect or investigate completely because of a position taken by an authority in foreign jurisdictions. On December 16, 2021, the
PCAOB issued a Determination Report which found that the PCAOB is unable to inspect or investigate completely registered public accounting
firms headquartered in: (i) China, and (ii) Hong Kong. On August 26, 2022, the CSRC, the Ministry of Finance of the PRC, and the PCAOB
signed a Statement of Protocol, or the Protocol, governing inspections and investigations of audit firms based in China and Hong Kong.
Pursuant to the Protocol, the PCAOB shall have independent discretion to select any issuer audits for inspection or investigation and
has the unfettered ability to transfer information to the SEC. Notwithstanding the foregoing, if the PCAOB is not able to inspect and
investigate completely our auditor’s work papers in China, you may be deprived of the benefits of such inspection which could result
in limitation or restriction to our access to the U.S. capital markets and trading of our securities may be prohibited under the HFCAA
and the Nasdaq may determine to delist our securities if the PCAOB determines that it cannot inspect or investigate completely our auditor
under the HFCAA.
Our auditor is not headquartered in China or Hong Kong and was not identified
in this report as a firm subject to the PCAOB’s determination.
The
SEC may propose additional rules or guidance that could impact us if our auditor is not subject to PCAOB inspection. For example, on
August 6, 2020, the President’s Working Group on Financial Markets, or the PWG, issued the Report on Protecting United States Investors
from Significant Risks from Chinese Companies to the then President of the United States. This report recommended the SEC implement five
recommendations to address companies from jurisdictions that do not provide the PCAOB with sufficient access to fulfil its statutory
mandate. Some of the concepts of these recommendations were implemented with the enactment of the HFCAA. However, some of the
recommendations were more stringent than the HFCAA. For example, if a company’s auditor was not subject to PCAOB inspection,
the report recommended that the transition period before a company would be delisted would end on January 1, 2022.
The
SEC has announced that the SEC staff is preparing a consolidated proposal for the rules regarding the implementation of the HFCAA
and to address the recommendations in the PWG report. The implications of possible additional regulation in addition to the requirements
of the HFCAA and what was recently adopted on December 2, 2021 are uncertain. While we understand that there has been dialogue
among the CSRC, the SEC and the PCAOB regarding the inspection of PCAOB-registered accounting firms in China, there can be no assurance
that we will be able to comply with requirements imposed by U.S. regulators. Such uncertainty could cause the market price of our common
stock to be materially and adversely affected, and our securities could be delisted and prohibited from being traded on the national
securities exchange earlier than would be required by the HFCAA. If our securities are unable to be listed on another securities
exchange by then, such a delisting would substantially impair your ability to sell or purchase our common stock when you wish to do so,
and the risk and uncertainty associated with a potential delisting would have a negative impact on the price of our common stock.
Further,
new laws and regulations or changes in laws and regulations in both the United States and China could affect our ability to list our
common stock on Nasdaq, which could materially impair the market for and market price of our common stock.
A
recent joint statement by the SEC and the Public Company Accounting Oversight Board (United States), or the “PCAOB,” proposed
rule changes submitted by Nasdaq, and the newly enacted “Holding Foreign Companies Accountable Act” all call for additional
and more stringent criteria to be applied to emerging market companies upon assessing the qualification of their auditors, especially
the non-U.S. auditors who are not inspected by the PCAOB. These developments could add uncertainties to our offering.
On
April 21, 2020, the SEC and the PCAOB released a joint statement highlighting the risks associated with investing in companies based
in or having substantial operations in emerging markets including China. The joint statement emphasized the risks associated with lack
of access for the PCAOB to inspect auditors and audit work papers in China and higher risks of fraud in emerging markets.
On
May 18, 2020, Nasdaq filed three proposals with the SEC to (i) apply a minimum offering size requirement for companies primarily operating
in a “Restrictive Market,” (ii) adopt a new requirement relating to the qualification of management or the board of directors
for Restrictive Market companies, and (iii) apply additional and more stringent criteria to an applicant or listed company based on the
qualifications of the company’s auditor. We are very likely to be deemed as a company primarily operating in a Restrictive Market
under such proposed rules of Nasdaq. Therefore, Nasdaq might apply the additional and more stringent criteria for our initial and continued
listing, which might cause delay or even denial of our listing application.
On
December 18, 2020, the HFCAA was signed and became law. This legislation requires certain issuers of securities to establish that they
are not owned or controlled by a foreign government. Specifically, an issuer must make this certification if the PCAOB is unable to audit
specified reports because the issuer has retained a foreign public accounting firm not subject to inspection by the PCAOB. Furthermore,
if the PCAOB is unable to inspect the issuer’s public accounting firm for three consecutive years, the issuer’s securities
are banned from trade on a national exchange or through other methods.
On
March 24, 2021, the SEC announced that it had adopted interim final amendments to implement congressionally mandated submission and disclosure
requirements of the HFCAA. The interim final amendments will apply to registrants that the SEC identifies as having filed an annual report
on Forms 10-K, 20-F, 40-F or N-CSR with an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction
and that the PCAOB has determined it is unable to inspect or investigate completely because of a position taken by an authority in that
jurisdiction. The SEC will implement a process for identifying such a registrant and any such identified registrant will be required
to submit documentation to the SEC establishing that it is not owned or controlled by a governmental entity in that foreign jurisdiction,
and will also require disclosure in the registrant’s annual report regarding the audit arrangements of, and governmental influence
on, such a registrant.
Furthermore,
on June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act (“AHFCAA”), which, if
enacted, would amend the HFCAA and require the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchanges
if its auditor is not subject to PCAOB inspections for two consecutive years instead of three. If the AHFCAA is enacted, and if we are
subject to it, it would decrease the number of “non-inspection years” from three years to two years, and thus, would reduce
the time before our securities may be prohibited from trading or delisted.
On
September 22, 2021, the PCAOB adopted rules to create a framework for the PCAOB to use when determining, as contemplated under the HFCAA,
whether it is unable to inspect or investigate completely registered public accounting firms located in a foreign jurisdiction because
of a position taken by one or more authorities in that jurisdiction.
On
December 2, 2021, the SEC issued amendments to finalize the interim final rules previously adopted in March 2021 to implement the submission
and disclosure requirements in the HFCAA. The rules apply to registrants that the SEC identifies as having filed an annual report with
an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction and that the PCAOB is unable
to inspect or investigate completely because of a position taken by an authority in a foreign jurisdiction.
On
December 16, 2021, the PCAOB issued a Determination Report which found that the PCAOB is unable to inspect or investigate completely
registered public accounting firms headquartered in: (1) mainland China of the People’s Republic of China, because of a position
taken by one or more authorities in mainland China; and (2) Hong Kong, a Special Administrative Region and dependency of the PRC, because
of a position taken by one or more authorities in Hong Kong. The PCAOB has made such designations as mandated under the HFCAA. Pursuant
to each annual determination by the PCAOB, the SEC will, on an annual basis, identify issuers that have used non-inspected audit firms
and thus are at risk of such suspensions in the future.
On August 26, 2022, the CSRC, the Ministry
of Finance of the PRC, and the PCAOB signed a Statement of Protocol, or the Protocol, governing inspections and investigations of audit
firms based in China and Hong Kong. Pursuant to the Protocol, the PCAOB shall have independent discretion to select any issuer audits
for inspection or investigation and has the unfettered ability to transfer information to the SEC. Notwithstanding the foregoing, if
the PCAOB is not able to inspect and investigate completely our auditor’s work papers in China, you may be deprived of the benefits
of such inspection which could result in limitation or restriction to our access to the U.S. capital markets and trading of our securities
may be prohibited under the HFCAA and the Nasdaq may determine to delist our securities if the PCAOB determines that it cannot inspect
or investigate completely our auditor under the HFCAA.
Our
auditor, KCCW Accountancy Corp. (“KCCW CPA”), the independent registered public accounting firm with the PCAOB, and
as an auditor of publicly traded companies in the U.S., is subject to laws in the U.S. pursuant to which the PCAOB conducts regular inspections
to assess its compliance with the applicable professional standards. Our auditor is based in the United States and has been inspected
by the PCAOB on a regular basis, with the last inspection in 2022. Our auditor is not headquartered in mainland China or Hong
Kong and was not identified as a firm subject to the determinations announced by the PCAOB on December 16, 2021. Should the PCAOB be
unable to fully conduct inspection of our auditor’s work papers in China, it will make it difficult to evaluate the effectiveness
of our auditor’s audit procedures or equity control procedures. Investors may consequently lose confidence in our reported financial
information and procedures or quality of the financial statements, which would adversely affect us and our securities. Moreover, if trading
in our securities is prohibited under the HFCAA in the future because the PCAOB determines that it cannot inspect or fully investigate
our auditor at such future time, an exchange may determine to delist our securities.
There
are uncertainties under the PRC Securities Law relating to the procedures and requisite timing for the U.S. securities regulatory agencies
to conduct investigations and collect evidence within the territory of the PRC.
On
December 28, 2019, the newly amended Securities Law of the PRC (the “PRC Securities Law”) was promulgated, which became effective
on March 1, 2020. According to Article 177 of the PRC Securities Law (“Article 177”), the securities regulatory authority
of the State Council may establish a regulatory cooperation mechanism with securities regulatory authorities of another country or region
for the implementation of cross-border supervision and administration. Article 177 further provides that overseas securities regulatory
authorities shall not engage in activities pertaining to investigations or evidence collection directly conducted within the territories
of the PRC, and that no Chinese entities or individuals shall provide documents and information in connection with securities business
activities to any organizations and/or persons aboard without the prior consent of the securities regulatory authority of the State Council
and the competent departments of the State Council. As of the date of this prospectus, we are not aware of any implementing rules or
regulations which have been published regarding application of Article 177.
As
advised by our PRC counsel, Sichuan Junhe, Article 177 is only applicable where the activities of overseas authorities constitute a direct
investigation or evidence collection by such authorities within the territory of the PRC. Our principal business operation is conducted
in the PRC. In the event that the U.S. securities regulatory agencies carry out an investigation on us such as an enforcement action
by the Department of Justice, the SEC or other authorities, such agencies’ activities will constitute conducting an investigation
or collecting evidence directly within the territory of the PRC and accordingly fall within the scope of Article 177. In that case, the
U.S. securities regulatory agencies may have to consider establishing cross-border cooperation with the securities regulatory authority
of the PRC by way of judicial assistance, diplomatic channels or establishing a regulatory cooperation mechanism with the securities
regulatory authority of the PRC. However, there is no assurance that the U.S. securities regulatory agencies will succeed in establishing
such cross-border cooperation in this particular case and/or establish such cooperation in a timely manner.
Furthermore,
as the date of this prospectus, there have not been implementing rules or regulations regarding the application of Article 177, it remains
unclear as to how it will be interpreted, implemented or applied by the Chinese Securities Regulatory Commission or other relevant government
authorities. As such, there are uncertainties as to the procedures and requisite timing for the U.S. securities regulatory agencies to
conduct investigations and collect evidence within the territory of the PRC. If the U.S. securities regulatory agencies are unable to
conduct such investigations, there exists a risk that they may determine to suspend or de-register our registration with the SEC and
may also delist our securities from Nasdaq or other applicable trading market within the US.
We
are exposed to liabilities relating to environmental protection and safety laws and regulations.
Our
operations are subject to comprehensive and frequently changing laws and regulations relating to environmental protection and health
and safety. The discharge of waste and pollutants from our manufacturing operations into the environment may give rise to liabilities
that may require us to incur costs to remedy such discharge. If we violate such laws or regulations, we may be required to implement
corrective actions and could be subject to civil or criminal fines or penalties or other sanctions.
However,
we cannot assure you that any environmental laws adopted in the future will not materially increase our operating costs and other expenses.
We cannot assure you that we will not have to make significant capital or operating expenditures in the future in order to comply with
existing or new laws and regulations or that we will comply with applicable environmental laws at all times. Such violations or liability
could have a material adverse effect on our business, financial condition and results of operations.
We
may require additional financing and our operations could be curtailed if we are unable to obtain required additional financing when
needed.
We
may need to obtain additional debt or equity financing to fund the immediate development of Yunnan Runcangsheng and future capital
expenditures. While we do not anticipate seeking additional financing in the immediate future, any additional equity may result in dilution
to the holders of our outstanding shares of capital stock. Additional debt financing may include conditions that would restrict our freedom
to operate our business, such as conditions that:
| |
● |
limit
our ability to pay dividends or require us to seek consent for the payment of dividends; |
| |
|
|
| |
● |
increase
our vulnerability to general adverse economic and industry conditions; |
| |
|
|
| |
● |
require
us to dedicate a portion of our cash flow from operations to payments on our debt, thereby reducing the availability of our cash
flow to fund capital expenditures, working capital and other general corporate purposes; and |
| |
|
|
| |
● |
limit
our flexibility in planning for, or reacting to, changes in our business and our industry. |
We
cannot guarantee that we will be able to obtain any additional financing on terms that are acceptable to us, or at all.
General
Risks Associated with Business Operations in China
Recent
developments related to the global outbreak of the novel strain of the coronavirus known as “COVID-19”and the possibility
of future outbreaks.
Our
business could be materially and adversely affected by the outbreak of a widespread epidemic or pandemic or other public health crisis,
including arising from the novel strain of the coronavirus known as “COVID-19”, particularly when such health epidemic or
pandemic has an adverse effect on China. The occurrence of such an outbreak or other adverse public health developments could affect
us in various ways, including disrupting our operations, supply chains and distribution systems and increasing our expenses, including
as a result of impacts associated with preventive and precautionary measures that we, governments and other businesses may take. Such
events could also significantly impact our industry and cause us to curtail some of our personal marketing outreach programs and to close
some or all of our stores, which would severely disrupt our operations and have a material adverse effect on our business, financial
condition and results of operations.
In
March 2020, the World Health Organization announced that infections caused by the coronavirus disease of 2019 (“COVID-19”)
had become pandemic. Economies throughout the world have been severely disrupted by the effects of the pandemic and the quarantines,
stay at home orders, business closures and the reluctance of individuals to leave their homes resulting from the outbreak of the Covid-19
Pandemic. China adopted and continues to rely upon a “zero-tolerance” policy pursuant to which it has declared a number of
total and partial lockdowns in cities throughout China, including Chengdu. These lockdowns and any accompanying travel restrictions have
adversely impacted many industries in China.
In
addition, certain illnesses may be transmitted through human or surface contact, and the risk of contracting such illnesses could cause
employees and customers to avoid gathering in public places, as was the case in many places during February and March 2020 due to concerns
about the coronavirus. This could not only adversely affect store traffic to our pharmacies, but also our ability to host large scale
marketing events and seminars, adequately staff and operate our manufacturing facility and the willingness of customers to meet with
members of our sales team. We could be adversely affected if the national or regional governments in China impose mandatory closures,
seek voluntary closures, imposes restrictions on operations of stores, or if suppliers are unable to provide us with timely delivery.
Even if such measures are not implemented and a communicable illness does not spread significantly, the perceived risk of infection or
health risk may adversely affect our business, financial condition and results of operations.
The
PRC government has significant oversight and discretion over the conduct of a PRC company’s business operations or to exert control
over any offering of securities conducted overseas and/or foreign investment in China-based issuers, and may intervene with or influence
our operations, may limit or completely hinder our ability to offer or continue to offer securities to investors, and may cause the value
of such securities to significantly decline or become worthless, as the government deems appropriate to further regulatory, political
and societal goals.
The
PRC government may intervene or influence our operations at any time, which could result in a material change in our operations and/or
the value of our common stock. For example, the PRC government has recently published new policies that significantly affected certain
industries such as the education and internet industries, and we cannot rule out the possibility that it will release regulations
or policies regarding any industry that could adversely affect the business, financial condition and results of operations of our company.
Furthermore, the PRC government has also recently indicated an intent to exert more oversight and control over securities offerings and
other capital markets activities that are conducted overseas and foreign investment in China-based companies. Any such action, once taken
by the PRC government, could significantly limit or completely hinder our ability to offer or continue to offer securities to investors
and cause the value of such securities to significantly decline or in extreme cases, become worthless.
Recently,
the PRC government initiated a series of regulatory actions and statements to regulate business operations in China with little advance
notice, including cracking down on illegal activities in the securities market, enhancing supervision over China-based companies listed
overseas using variable interest entity (“VIE”) structures, adopting new measures to extend the scope of cybersecurity
reviews, and expanding anti-monopoly enforcement efforts. As confirmed by our PRC counsel, Sichuan Junhe, we are not subject to cybersecurity
review with the Cyberspace Administration of China, or CAC, given that: (i) we do not possess a large amount of personal information
in our business operations; and (ii) data processed in our business does not have a bearing on national security and thus may not be
classified as core or important data by the authorities. See also “Risk Factors - General Risks Associated with Business Operations
in China - Our business may be subject to a variety of PRC laws and other obligations regarding cybersecurity and data protection.”
In addition, as confirmed by our PRC counsel, Sichuan Junhe, we are not subject to merger control review by China’s anti-monopoly
enforcement agency due to the level of our revenues and the fact that we currently do not expect to propose or implement any acquisition
of control of, or decisive influence over, any company with revenues within China of more than RMB400 million. Currently, these statements
and regulatory actions have had no impact on our daily business operations, the ability to accept foreign investments or list our securities
on a U.S. or other foreign exchange. Since these statements and regulatory actions are new, it is highly uncertain how soon legislative
or administrative regulation making bodies will respond and what existing or new laws or regulations or detailed implementations and
interpretations will be modified or promulgated, if any, and the potential impact such modified or new laws and regulations will have
on our daily business operation, the ability to accept foreign investments and list our securities on an U.S. or other foreign exchange.
The
CSRC has released for public consultation the draft rules for China-based companies seeking to conduct initial public offerings in foreign
markets. The Chinese government may exert more oversight and control over offerings that are conducted overseas and foreign investment
in China-based issuers, which could significantly limit or completely hinder our ability to offer or continue to offer our common stock
to investors and could cause the value of our common stock to significantly decline or become worthless.
On
December 24, 2021, the CSRC released the Administrative Provisions of the State Council Regarding the Overseas Issuance and Listing of
Securities by Domestic Enterprises (Draft for Comments) (the “Administrative Provisions”) and the Measures for the Overseas
Issuance of Securities and Listing Record-Filings by Domestic Enterprises (Draft for Comments) (the “Measures”). The Administrative
Provisions and Measures lay out the filing regulation arrangement for both direct and indirect overseas listing and clarify the determination
criteria for indirect overseas listing in overseas markets. The Admoinistrative Provisions and Measures, if enacted, may subject
us to additional compliance requirement in the future. Any failure of us to fully comply with new regulatory requirements may significantly
limit or completely hinder our ability to offer or continue to offer our common stock, cause significant disruption to our business
operations, and severely damage our reputation, which would materially and adversely affect our financial condition and results of operations
and cause our common stock shares to significantly decline in value or become worthless.
You
may have difficulty enforcing judgments against us.
We
are a Colorado company conducting our operations in China through wholly owned subsidiaries with direct equity ownership and most of
our assets are and will be located outside the United States. Almost all of our operations will be conducted in China. In addition, nearly
all of our officers and directors are nationals and residents of China and their assets are located outside the United
States. As a result, it may be difficult for you to effect service of process upon them or to bring actions against us or our management
in China. It may also be difficult for you to enforce in U.S. courts judgments based on violations of the civil liability provisions
of the U.S. federal securities laws against us and our officers and directors, since none of them is a resident in the United States.
In addition, there is uncertainty as to whether the courts of China would recognize or enforce judgments of U.S. courts.
Foreign
exchange fluctuations may affect our business.
We
accept payment for our products in RMB. Therefore, foreign exchange fluctuations may influence our business in unpredictable ways.
The
value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political
and economic conditions and the foreign exchange policy adopted by the PRC government. For instance, in August 2015, the People’s
Bank of China, or PBOC, changed the way it calculates the mid-point price of Renminbi against the U.S. dollar, requiring the market-makers
who submit for reference rates to consider the previous day’s closing spot rate, foreign-exchange demand and supply as well as
changes in major currency rates. In fiscal year 2019 and 2020, the value of the Renminbi depreciated by approximately 6.9% and 5.5% against
the U.S. dollar, respectively. In fiscal year 2021 and 2022 the value of the Renminbi appreciated by approximately 7.4% and 3.2% against
the U.S. dollar. It is difficult to predict how market forces or PRC or U.S. government policy, including any interest rate increases
by the Federal Reserve, may impact the exchange rate between the Renminbi and the U.S. dollar in the future. There remains significant
international pressure on the PRC government to adopt a more flexible currency policy, including from the U.S. government, which has
threatened to label China as a “currency manipulator,” which could result in greater fluctuation of the Renminbi against
the U.S. dollar.
A
substantial percentage of our revenues and costs are denominated in Renminbi, and a significant portion of our assets are also denominated
in Renminbi. We are a holding company and we rely on dividends, loans and other distributions on equity paid by our operating subsidiaries
in China. Any significant fluctuations in the value of the Renminbi may materially and adversely affect our liquidity and cash flows.
Appreciation of the U.S. dollar against the Renminbi would have a negative effect on the U.S. dollar amount we would receive. Conversely,
to the extent that we need to convert U.S. dollars into Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar
would have an adverse effect on the Renminbi amount we would receive.
Inflation
could pose a risk to our business.
Inflation
is an important factor that must be considered as we move forward. A change in the rate of inflation could influence the profits that
we generate from our business. When the rate of inflation rises, the operational costs of running our company would increase, such as
labor costs, raw materials and public utilities, affecting our ability to provide our services at competitive prices. An increase in
the rate of inflation would force our clients to search for other service providers, causing us to lose business and revenue.
Changes
in the policies, regulations, rules and the enforcement of laws of the PRC government may be quick with little advance notice and could
have a significant impact upon the business we may be able to conduct in the PRC and the profitability of such business.
The
PRC’s economy is in a transition from a planned economy to a market oriented economy subject to five-year and annual plans adopted
by the central government that set national economic development goals. Policies of the PRC government can have significant effects on
the economic conditions of the PRC. The PRC government has confirmed that economic development will follow the model of a market economy.
Under this direction, we believe that the PRC will continue to strengthen its economic and trading relationships with foreign countries
and business development in the PRC will follow market forces. While we believe that this trend will continue, we cannot assure you that
this will be the case. Changes in policies, regulations, rules and the enforcement of laws by the PRC government, which changes may be
quick with little advance notice, could adversely affect our interests by. Although the PRC government has been pursuing economic reform
policies for more than two decades, we cannot assure you that the government will continue to pursue such policies or that such policies
may not be significantly altered, especially in the event of a change in leadership, social or political disruption, or other circumstances
affecting the PRC’s political, economic and social environment.
There
are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations.
Most
of our operations are conducted in the PRC, and are governed by PRC laws, rules and regulations. Our PRC Subsidiaries are subject to
laws, rules and regulations applicable to foreign investment in China. The PRC legal system is a civil law system based on written statutes.
Unlike the common law system, prior court decisions may be cited for reference but have limited precedential value.
In
1979, the PRC government began to promulgate a comprehensive system of laws, rules and regulations governing economic matters in general. However, China has not developed a fully integrated legal system, and recently enacted laws, rules and regulations
may not sufficiently cover all aspects of economic activities in China or may be subject to significant degree of interpretation by PRC
regulatory agencies and courts. In particular, because these laws, rules and regulations are relatively new, and because of the limited
number of published decisions and the non-precedential nature of these decisions, and because the laws, rules and regulations often give
the relevant regulator significant discretion in how to enforce them, the interpretation and enforcement of these laws, rules and regulations
involve uncertainties and can be inconsistent and unpredictable. Therefore, it is possible that our existing operations may be found
not to be in full compliance with relevant laws and regulations. In addition, the PRC legal system is based in part on government policies
and internal rules, some of which are not published on a timely basis or at all, and which may have a retroactive effect. As a result,
we may not be aware of our violation of these policies and rules until after the occurrence of the violation.
Any
administrative and court proceedings in China may be protracted, resulting in substantial costs and diversion of resources and management
attention. Since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and
contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection
we enjoy than in more developed legal systems. These uncertainties may impede our ability to enforce the contracts we have entered into
and could materially and adversely affect our business, financial condition and results of operations.
PRC
regulations regarding acquisitions impose significant regulatory approval and review requirements, which could make it more difficult
for us to pursue growth through acquisitions.
Under
the PRC Anti-Monopoly Law, companies undertaking acquisitions relating to businesses in China must notify the anti-monopoly enforcement
agency in advance of any transaction where the parties’ revenues in the China market exceed certain thresholds and the buyer would
obtain control of, or decisive influence over, the other party. In addition, on August 8, 2006, six PRC regulatory agencies, including
the MOFCOM, the State-Owned Assets Supervision and Administration Commission, the State Administration of Taxation, the SAIC, the China
Securities Regulatory Commission, or the CSRC, and the State Administration of Foreign Exchange, or SAFE, jointly adopted Regulations
on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rules, which came into effect on September 8,
2006 and was amended on June 22, 2009. Under the M&A Rules, the approval of MOFCOM must be obtained in circumstances where overseas
companies established or controlled by PRC enterprises or residents acquire domestic companies affiliated with such PRC enterprises or
residents. Applicable PRC laws, rules and regulations also require certain merger and acquisition transactions to be subject to security
review.
While
the approval of the China Securities Regulatory Commission is not currently required for this offering, it may be required in the future
in connection with this offering under the M&A Rules and, if required, we cannot predict whether we will be able to obtain such approval.
The
application of the M&A Rules remains unclear. According to the results of the searches conducted by our PRC counsel, Sichuan
Junhe, on the official website of the CSRC and its administrative license processing hall
(https://neris.csrc.gov.cn/alappl/home/guideH), at present, only one administrative license related to overseas public offering and
listing is enacted, that is, “examination and approval of overseas public offering shares and listing (including additional
issuance) of joint-stock companies”. Such examination and approval license requirements are only applicable to issuers which
are formed as PRC joint-stock companies in China under PRC law. None of our operating PRC Subsidiaries is formed as a PRC
joint-stock company in China, and as such, we do not believe that we need CSRC approval. We do not believe that the current PRC
regulations and rules including China Securities Law require explicitly and directly that the overseas listing of foreign issuers
who indirectly hold the rights and interests of Chinese domestic enterprises be examined and approved by the CSRC. If CSRC approval
is required, it is uncertain whether it would be possible for us to obtain the approval. Any failure to obtain or delay in obtaining
CSRC approval for this offering would subject us to sanctions imposed by the CSRC and other PRC regulatory agencies.
While
the application of the M&A Rules remain unclear, we believe, based on the advice of our PRC legal counsel, Sichuan Junhe, based on
its understanding of the current PRC laws, regulations and rules, that the CSRC’s approval is not required for this offering.
We neither received nor were denied permission from CSRC or other PRC government agencies to list our securities on the NASDAQ and
issue our securities to foreign investors.
There
remains some uncertainty as to how the M&A Rules will be interpreted or implemented in the context of an overseas offering. We cannot
assure you that relevant PRC government agencies, including the CSRC, would reach the same conclusion as we do. If it is determined that
CSRC approval is required for this offering, we may face sanctions by the CSRC or other PRC regulatory agencies for failure to obtain
or delay in obtaining CSRC approval for this offering. These sanctions may include fines and penalties on our operations in China, limitations
on our operating privileges in China, delays in or restrictions on the repatriation of the proceeds from this offering into the PRC,
restrictions on or prohibition of the payments or remittance of dividends by our subsidiaries in China, or other actions that could have
a material and adverse effect on our business, financial condition, results of operations, reputation and prospects, as well as the trading
price of our securities. The CSRC or other PRC regulatory agencies may also take actions requiring us or making it advisable for us,
to halt this offering before the settlement and delivery of the securities that we are offering. Consequently, if you engage in market
trading or other activities in anticipation of and prior to the settlement and delivery of the securities we are offering, you would
be doing so at the risk that the settlement and delivery may not occur. In addition, if the CSRC or other regulatory agencies later promulgate
new rules or explanations requiring that we obtain their approvals for this offering, we may be unable to obtain a waiver of such approval
requirements.
As
of the date of this prospectus, we have not received any inquiry, notice, warning,
sanctions or regulatory objection to this offering from the CSRC or any other PRC governmental authorities, and our PRC Subsidiaries
have obtained all requisite permissions and approvals from PRC governmental authorities to operate our business as currently conducted
under relevant PRC laws and regulations.
As of the date of this
prospectus, none of our PRC Subsidiaries has been denied or punished by relevant governmental authorities due to its business qualifications
Our
business may be subject to a variety of PRC laws and other obligations regarding cybersecurity and data protection.
Our
business may be subject to PRC laws relating to the collection, use, sharing, retention, security, and transfer of confidential and private
information, such as personal information and other data. These laws continue to develop, and the PRC government may adopt other rules
and regulations in the future. Non-compliance could result in penalties or other significant legal liabilities.
Pursuant
to the PRC Cybersecurity Law, which was promulgated by the Standing Committee of the National People’s Congress on November 7,
2016 and took effect on June 1, 2017, personal information and important data collected and generated by a critical information infrastructure
operator in the course of its operations in China must be stored in China, and if a critical information infrastructure operator purchases
internet products and services that affects or may affect national security, it should be subject to cybersecurity review by the Cyberspace
Administration of China (“CAC”). Due to the lack of further interpretations, the exact scope of “critical information
infrastructure operator” remains unclear.
On
December 28, 2021, twelve Chinese government agencies jointly promulgated the Measures for Cybersecurity Review, which became effective
on February 15, 2022, set forth the cybersecurity review mechanism for critical information infrastructure operators, and provided that
critical information infrastructure operators who intend to purchase internet products and services that affect or may affect national
security shall be subject to a cybersecurity review. On June 10, 2021, the Standing Committee of the National People’s Congress
promulgated the PRC Data Security Law, which took effect in September 2021. The Data Security Law provides for a security review procedure
for data activities that may affect national security. Moreover, the State Internet Information Office issued the Measures of Cybersecurity
Review (Revised Draft for Comments, not yet effective) on July 10, 2021, which requires operators with personal information of more than
1 million users who want to list abroad to file a cybersecurity review with the CAC. Furthermore, the General Office of the Central Committee
of the Communist Party of China and the General Office of the State Council jointly issued the Opinions on Severe and Lawful Crackdown
on Illegal Securities Activities, which was available to the public on July 6, 2021. These opinions emphasized the need to strengthen
the administration over illegal securities activities and the supervision of overseas listings by China-based companies. These opinions
proposed to take effective measures, such as promoting the construction of relevant regulatory systems, to deal with the risks and incidents
facing China-based overseas-listed companies and the demand for cybersecurity and data privacy protection. As these laws, opinions and
the draft measures were recently issued, official guidance and interpretation of these remain unclear in several respects at this time,
and the PRC government authorities have wide discretion in the interpretation and enforcement of these laws, opinions and draft measures.
Therefore, it is uncertain whether the future regulatory changes would impose additional restrictions on our business
The
Data Security Law also sets forth the data security protection obligations for entities and individuals handling personal data, including
that no entity or individual may acquire such data by stealing or other illegal means, and the collection and use of such data should
not exceed the necessary limits The costs of compliance with, and other burdens imposed by, PRC Cybersecurity Law and any other cybersecurity
and related laws may limit the utility of our internet sales channel of distribution and could have an adverse impact on our business.
Further, if the enacted version of the Measures for Cybersecurity Review mandates clearance of cybersecurity review and other specific
actions to be completed by companies like us, we face uncertainties as to whether such clearance can be timely obtained, or at all.
As
confirmed by our PRC counsel, we are not be subject to the cybersecurity review by the CAC for this offering, given that: (i) we do not
possess a large amount of personal information in our business operations; and (iii) data processed in our business does not have a bearing
on national security and thus may not be classified as core or important data by the authorities. However, there remains uncertainty
as to how the Draft Measures will be interpreted or implemented and whether the PRC regulatory agencies, including the CAC, may adopt
new laws, regulations, rules, or detailed implementation and interpretation related to the Draft Measures. If any such new laws, regulations,
rules, or implementation and interpretation comes into effect, we will take all reasonable measures and actions to comply and to minimize
the adverse effect of such laws on us.
We
cannot assure you that PRC regulatory agencies, including the CAC, would take the same view as we do, and there is no assurance that
we can fully or timely comply with such laws. In the event that we are subject to any mandatory cybersecurity review and other specific
actions required by the CAC, we face uncertainty as to whether any clearance or other required actions can be timely completed, or at
all. Given such uncertainty, we may be further required to shut down our website, or face other penalties, which could materially and
adversely affect our business, financial condition, and results of operations.
PRC
regulations relating to investments in offshore companies by PRC residents may subject our PRC-resident beneficial owners or our PRC
Subsidiaries to liability or penalties, limit our ability to inject capital into our PRC Subsidiaries or limit our PRC Subsidiaries’
ability to increase their registered capital or distribute profits.
SAFE
promulgated the Circular on Relevant Issues Concerning Foreign Exchange Control on Domestic Residents’ Offshore Investment and
Financing and Roundtrip Investment through Special Purpose Vehicles, or SAFE Circular 37, on July 4, 2014, which replaced the former
circular commonly known as “SAFE Circular 75.” SAFE Circular 37 requires PRC residents to register with local branches of
SAFE in connection with their direct establishment or indirect control of an offshore entity, for the purpose of overseas investment
and financing, with such PRC residents’ legally owned assets or equity interests in domestic enterprises or offshore assets or
interests, referred to in SAFE Circular 37 as a “special purpose vehicle.” SAFE Circular 37 further requires amendment to
the registration in the event of any significant changes with respect to the special purpose vehicle, such as changes in capital
contributed by PRC individuals, share transfers or exchanges, mergers, divisions or other material events. In the event that a
PRC shareholder holding interests in a special purpose vehicle fails to fulfill the required SAFE registration, the PRC Subsidiaries
of that special purpose vehicle may be prohibited from making profit distributions to the offshore parent and from carrying out subsequent
cross-border foreign exchange activities, and the special purpose vehicle may be restricted in its ability to contribute additional capital
into its PRC Subsidiaries. Moreover, failure to comply with the various SAFE registration requirements could result in liability under
PRC law for evasion of foreign exchange controls.
As
we have little control over the registration procedures, we cannot assure you of the outcome of such registration, and we cannot assure
you that any of our shareholders who are PRC residents will submit the required registration or amend or update their registration
as required under Circular 37 and the Notice of the State Administration of Foreign Exchange on Further Simplifying and Improving the
Foreign Exchange Administration Policies for Direct Investment [Hui Fa (2015) No.13] issued by SAFE with effect from June 1, 2015, or
SAFE Notice 13, in a timely manner or at all. In addition, we may not be aware of the identities of all of our beneficial owners who
are PRC residents. We do not have control over our beneficial owners and cannot assure you that all of our PRC-resident beneficial owners
will comply with SAFE Circular 37 and subsequent implementation rules. The failure of our current and future beneficial owners
who are PRC residents to register or amend their SAFE registrations in a timely manner pursuant to SAFE Circular 37 and subsequent implementation
rules, may subject the beneficial owners or our PRC Subsidiaries to fines and
legal sanctions. On February 13, 2015, SAFE promulgated a Notice on Further Simplifying and Improving Foreign Exchange Administration
Policy on Direct Investment, or SAFE Notice 13, which became effective on June 1, 2015. Pursuant to SAFE Notice 13, entities and individuals
are required to apply for foreign exchange registration of foreign direct investment and overseas direct investment, including those
required under the SAFE Circular 37, with designated domestic banks, instead of SAFE. The designated domestic banks will directly review
the applications and conduct the registration.
Furthermore,
since it is unclear how the new SAFE regulations, and any future regulation concerning offshore or cross-border transactions, will
be interpreted, amended and implemented by the relevant PRC government authorities, we cannot predict how these regulations will affect
our business operations or future strategy. Failure to register or comply with relevant requirements may also limit our ability to contribute
additional capital to our PRC Subsidiaries and limit our PRC Subsidiaries’ ability to distribute dividends to our company. These
risks may have a material adverse effect on our business, financial condition and results of operations.
We
may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise Income Tax Law, and we may therefore be subject
to PRC income tax on our global income.
Under
the PRC Enterprise Income Tax Law and its implementing rules, both of which came into effect on January 1, 2008, enterprises established
under the laws of jurisdictions outside of China with “de facto management bodies” located in China may be considered PRC
tax resident enterprises for tax purposes and may be subject to the PRC enterprise income tax at the rate of 25% on their global income.
“De facto management body” refers to a managing body that exercises substantive and overall management and control over the
production and business, personnel, accounting books and assets of an enterprise. The State Administration of Taxation issued the Notice
Regarding the Determination of Chinese-Controlled Offshore-Incorporated Enterprises as PRC Tax Resident Enterprises on the basis of de
facto management bodies, or Circular 82, on April 22, 2009. Circular 82 provides certain specific criteria for determining whether the
“de facto management body” of a Chinese-controlled offshore-incorporated enterprise is located in China. Although Circular
82 only applies to offshore enterprises controlled by PRC enterprises, not those controlled by foreign enterprises or individuals, the
determining criteria set forth in Circular 82 may reflect the State Administration of Taxation’s general position on how the “de
facto management body” test should be applied in determining the tax resident status of offshore enterprises, regardless of whether
they are controlled by PRC enterprises. If we, Aixin Colorado and Aixin BVI, were to be considered a PRC resident enterprise, we would
be subject to PRC enterprise income tax at the rate of 25% on our global income. In such case, our profitability and cash flow may be
materially reduced as a result of our global income being taxed under the Enterprise Income Tax Law. We believe that none of our entities
outside of China is a PRC resident enterprise for PRC tax purposes. However, the tax resident status of an enterprise is subject to determination
by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body.”
Restrictions
on currency exchange may limit our ability to utilize our PRC revenue effectively.
We
(Aixin Colorado) are a Colorado holding company with no material operations of our own. We conduct substantially all of our operations
through the operating companies established in the PRC. We are a holding company and do not directly own any substantive business operations
in China. Substantially all of our revenue is denominated in Renminbi. The Renminbi is currently convertible under the “current
account,” which includes dividends, trade and service-related foreign exchange transactions, but requires approval from or registration
with appropriate government authorities or designated banks under the “capital account,” which includes foreign direct investment
and loans, including loans we may secure from our onshore subsidiaries. Currently, one of our PRC Subsidiaries, may purchase foreign
currency for settlement of “current account transactions,” including payment of dividends to us, without the approval of
SAFE by complying with certain procedural requirements. However, the relevant PRC governmental authorities or the local bank may limit
or eliminate our ability to purchase foreign currencies in the future for current account transactions.
Since
2016, PRC governmental authorities have imposed more stringent restrictions on outbound capital flows, including heightened scrutiny
over “irrational” overseas investments for certain industries, as well as over four kinds of “abnormal” offshore
investments, which are:
●
investments through enterprises established for only a few months without substantive operation;
●
investments with amounts far exceeding the registered capital of the onshore parent and not supported by its business performance shown
on financial statements;
●
investments in targets which are unrelated to an onshore parent’s main business; and
●
investments with abnormal sources of Renminbi funding suspected to be involved in illegal transfer of assets or illegal operation of
underground banking.
On
January 26, 2017, SAFE promulgated the Circular on Further Improving Reform of Foreign Exchange Administration and Optimizing Genuineness
and Compliance Verification, which tightened the authenticity and compliance verification of cross-border transactions and cross-border
capital flow, including requiring banks to verify board resolutions, tax filing forms and audited financial statements before wiring
foreign invested enterprises’ foreign exchange dividend distribution of over US$50,000. In addition, the Outbound Investment Sensitive
Industry Catalogue (2018) lists certain sensitive industries that are subject to NDRC pre-approval requirements prior to remitting investment
funds offshore, which subjects us to increased approval requirements and restrictions should we have overseas investments. Since a significant
amount of our PRC revenue is denominated in Renminbi, any existing and future restrictions on currency exchange may limit our ability
to utilize revenue generated in Renminbi to fund our business activities outside of the PRC, make investments, service any debt we may
incur outside of China or pay dividends in foreign currencies to our shareholders.
Fluctuations
in foreign exchange rates will impact our reported results.
The
functional currency utilized by our PRC Subsidiaries is the RMB. Our financial results are reported in U.S. Dollars. Therefore, foreign
exchange fluctuations will impact the reporting of our financial results and significant fluctuations in the value of the RMB relative
to the U.S. Dollar may materially and adversely affect our financial results as reported in our filings with the SEC. Such variations
in our perceived operating results may increase the volatility in the market for our common stock.
The
disclosures in our reports and other filings with the SEC and our other public pronouncements are not subject to the scrutiny of any
regulatory bodies in the PRC.
We
are subject to the regulations of the SEC and our reports and other filings with the SEC are subject to SEC review in accordance with
the rules and regulations promulgated by the SEC under the Securities Act and the Exchange Act. Our SEC reports and other disclosure
and public pronouncements are not subject to the review or scrutiny of any PRC regulatory authority. For example, the disclosure in our
SEC reports and other filings are not subject to the review by China Securities Regulatory Commission, a PRC regulator that is responsible
for oversight of the capital markets in China. Accordingly, you should review our SEC reports, filings and our other public pronouncements
with the understanding that no local regulator has done any review of us, our SEC reports, other filings or any of our other public pronouncements.
Introduction
of new laws or changes to existing laws by the PRC government may adversely affect our business.
The
PRC legal system is a codified legal system made up of written laws, regulations, circulars, administrative directives and internal guidelines.
Unlike common law jurisdictions like the U.S., decided cases (which may be taken as reference) do not form part of the legal structure
of the PRC and thus have no binding effect on subsequent cases with similar issues and fact patterns. Furthermore, in line with its transformation
from a centrally-planned economy to a more free market-oriented economy, the PRC government is still in the process of developing a comprehensive
set of laws and regulations. As the legal system in the PRC is still evolving, laws and regulations or the interpretation of the same
may be subject to further changes. For example, the PRC government may impose more stringent environmental regulations which would affect
our ability to comply with, or our costs to comply with, such regulations. Such changes, if implemented, may adversely affect our business
operations and may reduce our profitability.
Risks
Relating to Our Holding Company Structure
Substantial
uncertainties exist with respect to the interpretation and implementation of the newly enacted Foreign Investment Law and how it may
impact the viability of our current corporate structure, corporate governance and business operations.
On
March 15, 2019, the PRC National People’s Congress approved the Foreign Investment Law, which came into effect on January 1, 2020
and replaced existing laws regulating foreign investment in the PRC and become the legal foundation for foreign investment in the PRC.
Meanwhile, the Implementation Regulation of the Foreign Investment Law and the Measures for Reporting of Information on Foreign Investment
came into effect as of January 1, 2020, which clarified and elaborated the relevant provisions of the Foreign Investment Law.
The
Foreign Investment Law sets out the basic regulatory framework for foreign investments and proposes to implement a system of pre-entry
national treatment with a restricted list for foreign investments, pursuant to which (i) foreign entities and individuals are prohibited
from investing in the areas that are not open to foreign investments, (ii) foreign investments in the restricted industries must satisfy
certain requirements under the law, and (iii) foreign investments in business sectors outside of the restricted list will be treated
equally with domestic investments. The Foreign Investment Law also sets forth necessary mechanisms to facilitate, protect and manage
foreign investments and proposes to establish a foreign investment information reporting system, through which foreign investors or foreign-invested
enterprises are required to submit initial report, report of changes, report of deregistration and annual report relating to their investments
to the Ministry of Commerce, or MOFCOM, or its local branches.
Although
our operating structure is legal and permissible under the current Chinese law and regulations, including the Foreign Investment Law,
Chinese regulatory authorities could disallow our operating structure, which would likely result in a material change in our operations
and/or could cause the value of our common stock to significantly decline or become worthless.
We
may rely on dividends and other distributions on equity paid by our PRC Subsidiaries to fund any cash and financing requirements we may
have, and any limitation on the ability of our PRC Subsidiaries to make payments to us could have a material and adverse effect on our
ability to conduct our business.
We
are a Colorado holding company and we rely principally on dividends and other distributions on equity from our PRC Subsidiaries for our
cash requirements, including the funds necessary to pay dividends and other cash distributions to our shareholders, and to service any
debt we may incur outside of China. If our PRC Subsidiaries incur debt, the instruments governing the debt may restrict their ability
to pay dividends or make other distributions to us. Under PRC laws and regulations, our PRC Subsidiaries may pay dividends only out of
their respective accumulated profits as determined in accordance with PRC accounting standards and regulations. In addition each of our
PRC Subsidiaries are required to set aside at least 10% of its accumulated after-tax profits each year, if any, to fund a certain statutory
reserve fund, until the aggregate amount of such fund reaches 50% of its registered capital. Such reserve funds cannot be distributed
to us as dividends. At its discretion, each of our PRC subsidiaries may allocate a portion of its after-tax profits based on PRC accounting
standards to an enterprise expansion fund, or a staff welfare and bonus fund.
The
PRC government may continue to strengthen its capital controls, and more restrictions and substantial vetting processes may be put forward
by SAFE for cross-border transactions falling under both the current account and the capital account. Any limitation on the ability of
our PRC Subsidiaries to pay dividends or make other kinds of payments to us could materially and adversely limit our ability to grow,
make investments or acquisitions that could be beneficial to our business, pay dividends, or otherwise fund and conduct our business.
In
addition, the Enterprise Income Tax Law and its implementation rules provide that a withholding tax rate of up to 10% will be applicable
to dividends payable by Chinese companies to non-PRC-resident enterprises unless otherwise exempted or reduced according to treaties
or arrangements between the PRC central government and governments of other countries or regions where the non-PRC-resident enterprises
are incorporated.
PRC
regulation of loans to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion
may delay or prevent us from using the proceeds of this offering to make loans or additional capital contributions to our PRC subsidiaries,
which could materially and adversely affect our liquidity and our ability to fund and expand our business.
We
are an offshore holding company conducting our operations in China through our PRC Subsidiaries. We may make loans or provide guarantees
for the benefit of our PRC Subsidiaries subject to the approval of or registration with governmental authorities and limitations of the
amount, or we may make additional capital contributions to our PRC Subsidiaries. Any loans to our PRC Subsidiaries, which are treated
as foreign-invested enterprises under PRC law, are subject to foreign exchange loan registrations. In addition, a foreign-invested enterprise,
or FIE, shall use its capital pursuant to the principle of authenticity and self-use within its business scope. The capital of an FIE
shall not be used for the following purposes: (i) directly or indirectly for payments beyond the business scope of the enterprise or
payments prohibited by relevant laws and regulations; (ii) directly or indirectly for investments in securities or investments other
than in banks’ principal-secured products unless otherwise provided by relevant laws and regulations; (iii) the granting of loans
to non-affiliated enterprises, except where it is expressly permitted in the business license; and (iv) paying the expenses related to
the purchase of real estate that is not for self-use (except for foreign-invested real estate enterprises).
In
light of the requirements imposed by PRC regulations on loans to and direct investments in PRC entities by offshore holding companies,
we cannot assure you that we will be able to complete the necessary government registrations or obtain the necessary government approvals
on a timely basis, if at all, with respect to future loans by us to our PRC Subsidiaries or with respect to future capital contributions
by us to our PRC Subsidiaries. If we fail to complete such registrations or obtain such approvals, our ability to use the proceeds from
this offering and to capitalize or otherwise fund our PRC operations may be negatively affected, which could materially and adversely
affect our liquidity and our ability to fund and expand our business.
Risks
Related to this Offering and our Common Stock
Prior
to this offering, we had a limited public market for our common stock and you may not be able to resell our shares at or above the price
you paid or at all.
Prior
to this offering, there was a limited public market for our common stock in the OTCQX. On many days during the twelve months ended September
30, 2022, no shares were traded. We cannot assure you that an active public market for our common stock will develop or that the market
price of our shares will not decline below the public offering price. The public offering price of our shares may not be indicative of
prices that will prevail in the trading market following the offering.
An
active trading market for our common stock may not develop and the trading price for our common stock may fluctuate significantly.
We
have applied to have our common stock approved for listing on the Nasdaq. Prior to the completion of this offering, there has been no
public market for our common stock, and we cannot assure you that a liquid public market for our common stock will develop
even if they are listed on NASDAQ. The public offering price for our common stock offered hereby was determined by negotiation between
us and the underwriters based upon several factors, and we can provide no assurance that the trading price of our common stock
after this offering will not decline below the initial public offering price. As a result, investors in our securities may experience
a significant decrease in the value of their common stock.
Nasdaq
may apply additional and more stringent criteria for our initial and continued listing because we plan to have a small public offering
and insiders will hold a large portion of our listed securities.
Nasdaq
Listing Rule 5101 provides Nasdaq with broad discretionary authority over the initial and continued listing of securities in Nasdaq and
Nasdaq may use such discretion to deny initial listing, apply additional or more stringent criteria for the initial or continued listing
of particular securities, or suspend or delist particular securities based on any event, condition, or circumstance that exists or occurs
that makes initial or continued listing of the securities on Nasdaq inadvisable or unwarranted in the opinion of Nasdaq, even though
the securities meet all enumerated criteria for initial or continued listing on Nasdaq. In addition, Nasdaq has used its discretion to
deny initial or continued listing or to apply additional and more stringent criteria in the instances, including but not limited to:
(i) where the company engaged an auditor that has not been subject to an inspection by PCAOB, an auditor that PCAOB cannot inspect, or
an auditor that has not demonstrated sufficient resources, geographic reach, or experience to adequately perform the company’s
audit; (ii) where the company planned a small public offering, which would result in insiders holding a large portion of the company’s
listed securities and Nasdaq was concerned that the offering size was insufficient to establish the company’s initial valuation,
and there would not be sufficient liquidity to support a public market for the company; and (iii) where the company did not demonstrate
sufficient nexus to the U.S. capital market, including having no U.S. shareholders, operations, or members of the board of directors
or management. Nasdaq might apply the additional and more stringent criteria for our initial and continued listing, which might cause
delay or even denial of our listing application.
The
market price of securities of certain small cap companies, including companies whose operations are based in China, have been highly
volatile and have raised the concerns of market regulators and if our common stock were to have such high volatility could cause NASDAQ
to temporarily halt trading in or delist our securities.
The
market prices of certain small cap companies, including companies whose operations are based in China, recently have been highly volatile.
Such volatility in the market price of our common stock Nasdaq under the authority of Listing Rule 5101 to halt trading in our common
stock or apply additional or more stringent criteria for the continued listing of our common stock, assuming initial listing is granted,
even though our common stock then meets all enumerated criteria for initial or continued listing on Nasdaq.
If
a limited number of participants in this offering purchase a significant percentage of the offering, the effective public float may be
smaller than anticipated and the trading price of our common stock may be volatile which could subject us to securities litigation and
make it more difficult for you to sell your shares and could cause NASDAQ to temporarily halt trading in or delist our common stock.
The
market prices of certain small cap companies, including companies whose operations are based in China, recently have been highly volatile.
As a company conducting a public offering, we are subject to the risk that a small number of investors will purchase a high percentage
of the offering. We have not imposed any obligations on the underwriters as to the maximum number of shares they may place with individual
investors. If, in the course of marketing the offering, the underwriters were to determine that demand for our shares was concentrated
in a limited number of investors and such investors determined to hold their shares after the offering rather than trade them in the
market, other shareholders could find the trading and price of our shares affected (positively or negatively) by the limited availability
of our shares. If this were to happen, investors could find our shares to be more volatile than they might otherwise anticipate. Companies
that experience such volatility in their share price may be more likely to be the subject of securities litigation. In addition, if a
large portion of our public float were to be held by a few investors, smaller investors may find it more difficult to sell their shares.
Further, such volatility in the market price of our common stock could cause Nasdaq under the authority of Listing Rule 5101 to halt
trading in our common stock or apply additional or more stringent criteria for the continued listing of our common stock, assuming initial
listing is granted, even though our common stock then meets all enumerated criteria for initial or continued listing on Nasdaq
The
trading price of our common stock has been volatile.
The
trading price for our common stock has varied during the twelve-month period ended September 30, 2022, between a high of $6.99 in January
and February of this year and a low of $3.22 in March and April of this year. Our stock price is likely to continue to be volatile and
subject to significant price and volume fluctuations in response to market and other factors. In addition, a substantial decline in the
price of our securities that persists for a significant period of time could cause our securities to be delisted from the NASDAQ Capital
Market, further reducing market liquidity. If an active market for our securities does not develop or cannot be sustained, the liquidity
of an investor’s investment may be limited, and the price of our securities may decline. If an active market does not exist, investors
may lose their entire investment. As a result of any of these factors, the market price of our securities at any given point in time
may not accurately reflect our long-term value.
The
market price of our shares is likely to be highly volatile, which could result in substantial losses to investors.
The
trading price of our common stock may be volatile and could fluctuate widely due to factors beyond our control. This may happen because
of the broad market and industry factors, like the performance and fluctuation of the market prices of other companies with business
operations located mainly in China that have listed their securities in the United States. The securities of some companies with operations
in China and securities listed in the United States have experienced significant volatility, including price declines in connection with
their initial public offerings. The trading performance of these Chinese companies’ securities after their offerings may affect
the attitudes of investors toward Chinese companies listed in the United States, including those of investors based in China, in general
and consequently may impact the trading performance of our common stock, regardless of our actual operating performance.
In
addition to market and industry factors, the trading price and volume of our common stock may be highly volatile due to factors specific
to our operations, including the following:
| ● |
variations
in our actual and perceived operating results; |
| |
|
| ● |
news
regarding gains or losses of customers or partners by us or our competitors; |
| |
|
| ● |
news
regarding gains or losses of key personnel by us or our competitors; |
| |
|
| ● |
announcements
of competitive developments, acquisitions or strategic alliances in our industry by us or our competitors; |
| |
|
| ● |
changes
in earnings estimates or buy/sell recommendations by financial analysts; |
| |
|
| ● |
potential
litigation; |
| |
|
| ● |
the
imposition of fines or penalties related to our activities in the PRC and failure to comply with applicable rules and regulations;
|
| |
|
| ● |
general
market conditions or other developments affecting us or our industry; and |
| |
|
| ● |
the
operating and stock price performance of other companies, other industries and other events or factors beyond our control. |
In addition, the stock market has experienced
extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of particular
companies. These fluctuations may be even more pronounced in the trading market for our shares shortly following this offering. If the
market price of our common stock after this offering does not exceed the offering price, you may not realize any return on your investment
in us and may lose some or all of your investment. Securities class action litigation has often been instituted against companies following
periods of volatility in the overall market and in the market price of a company’s securities. This litigation, if instituted against
us, could result in very substantial costs, divert our management’s attention and resources, and harm our business, operating results
and financial condition. In addition, recent fluctuations in the financial and capital markets have resulted in volatility in securities
prices.
The
sale or availability for sale of substantial amounts of our common stock could adversely affect its market price.
Sales
of substantial amounts of our common stock in the public market after the completion of this offering, or the perception that these sales
could occur, could adversely affect the market price of our common stock and could materially impair our ability to raise capital through
equity offerings in the future. The common stock sold in this offering will be freely tradable without restriction or further registration
under the Securities Act, and shares held by our existing shareholders may also be sold in the public market in the future subject to
the restrictions in Rule 144 under the Securities Act and the applicable lock-up agreements. Following the consummation of our initial
public offering, there will be [●] shares of common stock outstanding immediately after this offering or [●]
shares assuming the full exercise of the underwriters’ over-allotment option. In connection with this offering, we and each of
our directors and officers named in the section “Management,” have agreed not to sell any common stock for
six months from the date of this prospectus without the prior written consent of the underwriter, subject to certain exceptions. However,
the underwriters may release these securities from these restrictions at any time, subject to applicable regulations of the Financial
Industry Regulatory Authority, Inc. (“FINRA”). We cannot predict what effect, if any, market sales of securities held by
our significant shareholders or any other shareholder or the availability of these securities for future sale will have on the market
price of our common stock. See “Underwriting” and “Shares Eligible for Future Sale” for
a more detailed description of the restrictions on selling our securities after this offering.
If
securities or industry analysts do not publish research or reports about our business, or if they adversely change their recommendations
regarding our common stock, the market price for our common stock and trading volume could decline.
The
trading market for our common stock will be influenced by research or reports that industry or securities analysts publish about
our business. If one or more analysts who cover us downgrade our common stock, the market price for our common stock would
likely decline. If one or more of these analysts cease to cover us or fail to regularly publish reports on us, we could lose visibility
in the financial markets, which in turn could cause the market price or trading volume for our common stock to decline.
Because
we do not expect to pay dividends in the foreseeable future after this offering, you must rely on price appreciation of our common
stock for return on your investment.
To
date, we have not paid dividends on our common stock. We currently intend to retain all of our available funds and any future earnings
after this offering to fund the development and growth of our business. As a result, we do not expect to pay any cash dividends in the
foreseeable future. Therefore, you should not rely on an investment in our common stock as a source for dividend income.
Our
board of directors has complete discretion as to whether to distribute dividends. The timing, amount and form of future dividends,
if any, will depend on, among other things, our future results of operations and cash flow, our capital requirements and surplus, the
amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors
deemed relevant by our board of directors. Accordingly, the return on your investment in our common stock will likely depend entirely
upon any future price appreciation of our common stock. There is no guarantee that our common stock will appreciate in value after this
offering or even maintain the price at which you purchased our common stock and you may even lose your entire investment.
You
must rely on the judgment of our management as to the use of the net proceeds from this offering, and such use may not produce income
or increase our share price.
We
plan to use the net proceeds of this offering primarily for expanding our manufacturing facilities, pursuing business development opportunities
and working capital and other general corporate purposes. See “Use of Proceeds.” However, our management will have
considerable discretion in the application of the net proceeds received by us. You will not have the opportunity, as part of your investment
decision, to assess whether proceeds are being used appropriately. The net proceeds may be used for corporate purposes that do not improve
our efforts to achieve or maintain profitability or increase our share price. The net proceeds from this offering may be placed in investments
that do not produce income or that lose value.
Our
CEO has substantial influence over our company. His interests may not be aligned with the interests of our other shareholders, and he
could prevent or cause a change of control or other transactions.
As
of the date of this prospectus, Quanzhong Lin, our Chairman of the Board of Directors and Chief Executive Officer, beneficially owns
an aggregate of 58% of our outstanding common stock. Upon completion of this offering, Mr. Lin will beneficially own approximately [●]% of our outstanding common stock.
Accordingly,
Mr. Lin could have significant influence in determining the outcome of any corporate transaction or other matter submitted to the shareholders
for approval, including mergers, consolidations, the appointment of directors and other significant corporate actions. Mr. Lin will also
have the power to prevent or cause a change in control. Without the consent of Mr. Lin, we may be prevented from entering into transactions
that could be beneficial to us or our minority shareholders. In addition, Mr. Lin could violate his fiduciary duties by diverting business
opportunities from us to himself or others. The interests of Mr. Lin may differ from the interests of our other shareholders. The concentration
in the ownership of our common stock shares may cause a material decline in the value of our common stock. For more information regarding
Mr. Lin, see “Principal Shareholders.”
USE
OF PROCEEDS
After
deducting the underwriting commissions and estimated offering expenses payable by us, we expect to receive net proceeds of [●]
from this offering. We anticipate that the proceeds will be applied as follows:
| Planned Actions | |
Amount | |
| Working capital and general corporate purposes | |
$ | | |
| Fund existing businesses operation (product design and manufacture) | |
| | |
| Expansion of manufacturing facility | |
| - | |
| Expand e-commerce network | |
| | |
| Branding and marketing | |
| | |
| Offering expenses | |
| | |
| Underwriting commissions and expenses | |
| | |
| | |
| | |
| TOTAL | |
$ | 50,000,000 | |
The
amount and timing of these expenditures will vary depending on a number of factors, including the amount of cash generated by our operations
and the rate of growth, if any, of our business.
Although
we may use a portion of the proceeds for the acquisition of, or investment in, companies, technologies, products or assets that complement
our business, we have no present understandings, commitments or agreements to enter into any acquisitions or make any investments. We
cannot assure you that we will make any acquisitions or investments in the future.
CAPITALIZATION
The
following table sets forth our cash and cash equivalents, accounts payable, loans and advances from third parties and capitalization
as of June 30, 2022:
| |
● |
on
an actual basis; and |
| |
|
|
| |
● |
on a pro forma to give effect to the acquisition of Yunnan
Runcangsheng and the items described in the Notes to Unaudited Pro Forma Consolidated Financial Statements included
in the Financial Statements beginning on page F-1; and |
| |
|
|
| |
● |
on a pro forma, as adjusted basis, to reflect the acquisition
of Yunnan Runcangsheng and the items described in the Notes to Unaudited Pro Forma Consolidated Financial Statements
included in the Financial Statements beginning on page F-1 and the completion of this offering. |
You
should read this table in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
and the financial statements and related notes included elsewhere in this prospectus.
| | |
| | |
June 30, 2022 | |
| | |
Actual | | |
Pro
Forma | | |
Pro Forma As
Adjusted | |
| | |
| | |
(unaudited) | | |
(unaudited) | |
| Cash and cash equivalents | |
$ | 8,129,696 | | |
$ | 8,836,449 | | |
| | |
| Accounts Payable | |
$ | 472,857 | | |
$ | 4,922,505 | | |
| | |
| Loans from third parties | |
$ | 89,578 | | |
$ | 89,578 | | |
| | |
| Advance from related parties, actual, prof forma and pro forma as adjusted | |
$ | 2,431,138 | | |
$ | 2,392,984 | | |
| | |
| | |
| | | |
| | | |
| | |
| Common Stock, actual, prof forma and pro forma as adjusted | |
$ | 500 | | |
$ | 500 | | |
| | |
| Additional Paid in Capital, actual, prof forma and pro forma as adjusted | |
| 14,272,563 | | |
| 14,272,563 | | |
| | |
| Statutory Reserve | |
| 151,988 | | |
| 151,988 | | |
| | |
| Accumulated Deficit | |
| (10,360,963 | ) | |
| (10,381,762 | ) | |
| | |
| Accumulated other comprehensive income (loss) | |
| 697,739 | | |
| 694,210 | | |
| | |
| Total Equity | |
$ | 4,761,827 | | |
$ | 4,737,499 | | |
| | |
DILUTION
If
you invest in our common stock in this offering, you will experience immediate and substantial dilution to the extent of the difference
between the public offering price per share you will pay in this offering and the pro forma as adjusted net tangible book value per share
of our common stock after this offering.
Our
net tangible book value as of September 30, 2022 was ($ ), or $ per share of common stock. Our pro forma net tangible book value per
share as of September 30, 2022, was $ million, or $ per share of
common stock. Net tangible book value represents total tangible assets less total liabilities. Tangible assets represent total assets
excluding goodwill and other intangible assets. Net tangible book value per share represents net tangible book value divided by the aggregate
number of shares of common stock outstanding immediately prior to this offering.
After
giving effect to the pro forma adjustments described in the Notes to Unaudited Pro Forma Consolidated Financial Statements included in
the Financial Statements beginning on page F-1, our pro forma net tangible book value as of September 30, 2022 would have been approximately
$ or approximately $ per share of common stock. After giving
effect to these pro forma adjustments, including the sale of shares
of our common stock in this offering (assuming the underwriters do not exercise the option to purchase additional shares of our common
stock) at an assumed initial public offering price of $ per share of common stock and after
deducting the underwriting discounts and commissions and estimated offering expenses payable by us, and the application of such net proceeds
as described under “Use of Proceeds,” our pro forma as adjusted net tangible book value as of September 30, 2022 would have
been approximately $ million, or approximately $ per
share of common stock. This represents an immediate increase in pro forma as adjusted net tangible book value of $ per
share of common stock to our existing stockholders and an immediate dilution (i.e., the difference between the offering price and the
pro forma as adjusted net tangible book value after this offering) to investors participating in this offering of $ per
share of common stock. The following table illustrates this per share dilution to the new investors purchasing shares of common stock
in this offering:
| | |
Offering(1) | | |
Full Over- allotment Post-offering(2) | |
| Assumed offering price per share | |
$ | | | |
| | |
| Net tangible book value per share as of September 30, 2022 | |
$ | | | |
| | |
Decrease in net tangible book value per share attributable to the
pro forma adjustments described above | |
| | | |
| | |
Pro forma net tangible book value per share before completion of
this offering | |
| | | |
| | |
Increase in net tangible book value per share attributable to
this offering | |
$ | | | |
| | |
Pro forma as adjusted net tangible book value per common stock
after the offering | |
$ | | | |
| | |
Dilution in pro forma as adjusted net tangible book value per share
to investors in this offering | |
$ | | | |
| | |
| (1) |
Assumes
gross proceeds from offering of shares. |
| (2) |
Assumes
gross proceeds from offering of shares, if over-allotment
option is exercised in full. |
A
$1.00 increase (decrease) in the public offering price of $ per share would increase (decrease) the pro forma as adjusted net tangible
book value by $ , the pro forma as adjusted net tangible book value per share after this offering by $ per share and the dilution in
pro forma as adjusted net tangible book value per share to investors in this offering by $ per share, assuming that the number of shares
offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriter commissions and
estimated offering expenses payable by us.
MARKET
FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Our
common stock is currently quoted on the OTCQX under the symbol “AIXN.” From February 6, 2019, until January 22, 2021, our
common stock traded on the OTCQB
Trading
in stocks quoted on the OTCQX and OTCQB is often thin and is characterized by wide fluctuations in trading prices due to many factors
that may have little to do with a company’s operations or business prospects. We cannot assure you that there will be a market
for our common stock in the future.
The
following table sets forth the high and low trading prices of one share of our common stock for each fiscal quarter over the past two
fiscal years, and from January 1, 2022 to the date of this prospectus. The quotations provided are for the over-the-counter market, which
reflect interdealer prices without retail mark-up, mark-down or commissions, and may not represent actual transactions. Our common stock
trades on a limited, sporadic and volatile basis. These high and low bid prices per share of common stock have been adjusted to give
effect to the 1 for 4 reverse stock split of our common stock effected on October 27, 2020.
| Year ended December 31, 2022 | |
High | | |
Low | |
| First Quarter | |
$ | 6.98 | | |
$ | 3.22 | |
| Second Quarter | |
$ | 5.47 | | |
$ | 4.002 | |
| Third Quarter | |
$ | 4.00 | | |
$ | 3.32 | |
| Fourth Quarter (through November 2, 2022) | |
$ | 3.80 | | |
$ | 3.35 | |
| Year
ended December 31, 2021 |
|
High
|
|
|
Low
|
|
| First
Quarter |
|
$ |
4.80 |
|
|
$ |
6.13 |
|
| Second
Quarter |
|
$ |
7.00 |
|
|
$ |
7.00 |
|
| Third
Quarter |
|
$ |
4.00 |
|
|
$ |
6.79 |
|
| Fourth
Quarter |
|
$ |
3.05 |
|
|
$ |
6.45 |
|
| Year
ended December 31, 2020 |
|
High
|
|
|
Low
|
|
| First
Quarter |
|
$ |
2.40 |
|
|
$ |
8.20 |
|
| Second
Quarter |
|
$ |
2.49 |
|
|
$ |
4.38 |
|
| Third
Quarter |
|
$ |
3.01 |
|
|
$ |
4.00 |
|
| Fourth
Quarter |
|
$ |
3.05 |
|
|
$ |
6.45 |
|
Holders
of Our Common Stock
49,999,891shares
of common stock were issued and outstanding as of November 2, 2022. They were held by a total of 650 shareholders of
record. The holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders.
Holders of the common stock have no pre-emptive rights and no right to convert their common stock into any other securities. There are
no redemption or sinking fund provisions applicable to the common stock.
Transfer
Agent
The
transfer agent for the common stock is Securities Transfer Corporation. The transfer agent’s address is 2901 N. Dallas Parkway,
Suite 380, Plano Texas 75093, and its telephone number is +1 (469) 633-0101.
Dividend
Policy
No
cash dividends were paid on our shares of common stock since we acquired Aixin BVI. We do not foresee declaring any cash dividends on
our common stock in the foreseeable future.
Securities
Authorized for Issuance under Equity Compensation Plans
In
2019 we adopted the 2019 Equity Incentive Plan (the “2019 Plan”), which authorizes the issuance of shares of common stock
for grants of stock options, stock appreciation rights, restricted stock, stock units, bonus stock, dividend equivalents, other stock
related awards and performance awards that may be settled in cash, stock, or other property. The 2019 Plan authorizes the issuance of
up to 1,250,000 shares. Options to purchase an aggregate of 187,500 shares have been granted pursuant to the Plan and remain outstanding.
We
adopted the 2019 Plan to provide a means by which employees, directors, and consultants of our Company and those of our subsidiaries
and other designated affiliates, which we refer to together as our affiliates, may be given an opportunity to purchase our common stock,
to assist in retaining the services of such persons, to secure and retain the services of persons capable of filling such positions,
and to provide incentives for such persons to exert maximum efforts for our success and the success of our affiliates. The material features
of the 2019 Plan are outlined below. This summary is qualified in its entirety by reference to the complete text of the 2019 Plan which
has been filed with the SEC.
SELECTED
HISTORICAL FINANCIAL AND OPERATING DATA
The
following tables set forth our summary historical financial data for the periods presented and as adjusted to give effect to the acquisition
of Yunnan Runcangsheng and the adjustments described in the Notes to Unaudited Pro Forma Consolidated Financial Statements included in
the Financial Statements beginning on page F-1. The following summary financial data for the years ended December 31, 2021 and 2020
are derived from our audited financial statements appearing elsewhere in this prospectus; the summary financial data for the six
months ended June 30, 2022, and the pro forma financial statements as of June 30, 2022, are unaudited.
This
summary financial data should be read together with the historical financial statements and related notes to those statements, as well
as “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” which are included elsewhere
in this prospectus.
Balance
Sheet Data:
| | |
Aixin December 31,2020 | | |
Aixin December 31,2021 | | |
Aixin June 30,2022 | | |
Ruicangsheng June 30, 2022 | | |
June 30, 2022 Pro Forma adjustments | | |
June 30, 2022 Pro forma consolidated | |
| ASSETS | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Current Assets | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 7,676,689 | | |
$ | 8,556,642 | | |
$ | 8,129,696 | | |
$ | 706,753 | | |
$ | - | | |
$ | 8,836,449 | |
| Restricted cash | |
| - | | |
| 44,211 | | |
| 61,588 | | |
| - | | |
| - | | |
| 61,588 | |
| Accounts receivable, net | |
| - | | |
| 45,923 | | |
| 60,548 | | |
| 239,681 | | |
| (51,712 | ) | |
| 248,517 | |
| Accounts receivable, related party | |
| - | | |
| - | | |
| - | | |
| 220,746 | | |
| - | | |
| 220,746 | |
| Other receivables and prepaid expense | |
| 32,323 | | |
| 143,281 | | |
| 46,702 | | |
| 15,024 | | |
| - | | |
| 61,726 | |
| Advance to suppliers | |
| 155,686 | | |
| 162,969 | | |
| 143,421 | | |
| 112,684 | | |
| - | | |
| 256,105 | |
| Advance to related parties | |
| 15,739 | | |
| 19,055 | | |
| 82,374 | | |
| - | | |
| - | | |
| 82,374 | |
| Inventory | |
| 45,535 | | |
| 233,454 | | |
| 291,918 | | |
| 394,745 | | |
| - | | |
| 686,663 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total Current Assets | |
| 7,925,972 | | |
| 9,205,535 | | |
| 8,816,247 | | |
| 1,689,633 | | |
| (51,712 | ) | |
| 10,454,168 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Non-Current Assets | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Goodwill | |
| - | | |
| - | | |
| - | | |
| - | | |
| 3,812,093 | | |
| 3,812,093 | |
| Property and equipment, net | |
| 67,817 | | |
| 290,148 | | |
| 221,958 | | |
| 1,809,711 | | |
| - | | |
| 2,031,669 | |
| Intangible assets, net | |
| - | | |
| 1,940 | | |
| 594 | | |
| - | | |
| - | | |
| 594 | |
| Operating lease Right-of-use assets | |
| 100,029 | | |
| 2,049,775 | | |
| 1,444,683 | | |
| 3,382 | | |
| - | | |
| 1,448,065 | |
| Other non-current assets | |
| - | | |
| 112,948 | | |
| 106,529 | | |
| - | | |
| - | | |
| 106,529 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total Non-Current Assets | |
| 167,846 | | |
| 2,454,811 | | |
| 1,773,764 | | |
| 1,813,093 | | |
| 3,812,093 | | |
| 7,398,950 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total Assets | |
$ | 8,093,818 | | |
$ | 11,660,346 | | |
$ | 10,590,011 | | |
$ | 3,502,726 | | |
$ | 3,760,381 | | |
$ | 17,853,118 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total Current Liabilities | |
| 1,172,486 | | |
| 4,452,145 | | |
| 5,213,393 | | |
| 3,440,251 | | |
| 3,847,184 | | |
| 12,500,828 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total Non-Current Liabilities | |
| 29,250 | | |
| 1,138,710 | | |
| 614,791 | | |
| - | | |
| - | | |
| 614,791 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total Liabilities | |
| 1,201,736 | | |
| 5,590,855 | | |
| 5,828,184 | | |
| 3,440,251 | | |
| 3,847,184 | | |
| 13,115,619 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stockholders’ Equity | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total Stockholders’ Equity | |
| 6,892,082 | | |
| 6,069,491 | | |
| 4,761,827 | | |
| 62,475 | | |
| (86,803 | ) | |
| 4,737,499 | |
Statements
of Operations Data:
| | |
Aixin December 31,2020 | | |
Aixin June 30,2022 | | |
Runcangsheng June 30, 2022 | | |
PRO FORMA ADJUSTMENTS | | |
PRO FORMA CONSOLIDATED | |
| | |
| | |
| | |
| | |
| | |
| |
| Revenue | |
| | | |
| | | |
| | | |
| | | |
| | |
| Products | |
$ | 742,624 | | |
$ | 385,628 | | |
$ | 295,375 | | |
$ | (63,681 | ) | |
$ | 617,322 | |
| Advertising | |
| 1,944,811 | | |
| - | | |
| - | | |
| - | | |
| - | |
| Room revenue | |
| 114,086 | | |
| 94,716 | | |
| - | | |
| - | | |
| 94,716 | |
| Food and beverage revenue | |
| 201,755 | | |
| 292,519 | | |
| - | | |
| - | | |
| 292,519 | |
| Others | |
| 62,957 | | |
| 66,702 | | |
| - | | |
| - | | |
| 66,702 | |
| Total revenues, net | |
| 3,066,233 | | |
| 839,565 | | |
| 295,375 | | |
| (63,681 | ) | |
| 1,071,259 | |
| Total operating costs and expenses | |
| 3,093,171 | | |
| 2,350,957 | | |
| 334,935 | | |
| (63,681 | ) | |
| 2,622,211 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss from operations | |
| (26,938 | ) | |
| (1,511,392 | ) | |
| (39,560 | ) | |
| - | | |
| (1,550,952 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total non-operating income, net | |
| 34,023 | | |
| 32,007 | | |
| 18,761 | | |
| - | | |
| 50,768 | |
| Income (loss) before income tax | |
| 7,085 | | |
| (1,479,385 | ) | |
| (20,799 | ) | |
| - | | |
| (1,500,184 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Income tax expense | |
| 274,321 | | |
| 965 | | |
| - | | |
| - | | |
| 965 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| (267,236 | ) | |
| (1,480,350 | ) | |
| (20,799 | ) | |
| - | | |
| (1,501,149 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss per share | |
$ | (0.005 | ) | |
$ | - | | |
$ | - | | |
| | | |
$ | (0.030 | ) |
Please see the Notes to Unaudited
Pro Forma Consolidated Financial Statements for a discussion of the adjustments made in combining the historical balance sheet data and
statements of operations data of Aixin and Yunnan Runcangsheng.
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF
FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
Forward
Looking Statements
The
following discussion should be read in conjunction with our consolidated audited financial statements and related notes thereto and other
financial information appearing elsewhere in this prospectus. In addition to historical information, the following discussion contains
forward-looking statements that involve risks, uncertainties and assumptions. Where possible, we have tried to identify these forward-looking
statements by using words such as “anticipate,” “believe,” “intends,” or similar expressions. As
a result of many factors, including those factors set forth in the “Risk Factors” section of this prospectus, our actual
results could differ materially from the results described in or implied by the forward-looking statements contained in this prospectus.
This
prospectus contains statements that we believe are, or may be considered to be, “forward-looking statements”. All
statements other than statements of historical fact included in this prospectus regarding the prospects of our industry or our prospects,
plans, financial position or business strategy, may constitute forward-looking statements. In addition, forward-looking statements generally
can be identified by the use of forward-looking words such as “may,” “will,” “expect,”
“intend,” “estimate,” “foresee,” “project,” “anticipate,”
“believe,” “plans,” “forecasts,” “continue” or “could”
or the negatives of these terms or variations of them or similar terms. Furthermore, such forward-looking statements may be included
in various filings that we make with the Securities and Exchange Commission or press releases or oral statements made by or with the
approval of one of our authorized executive officers. Although we believe that the expectations reflected in these forward-looking statements
are reasonable, we cannot assure you that these expectations will prove to be correct. These forward-looking statements are subject to
certain known and unknown risks and uncertainties, as well as assumptions that could cause actual results to differ materially from those
reflected in these forward-looking statements. Readers are cautioned not to place undue reliance on any forward-looking statements contained
herein, which reflect management’s opinions only as of the date hereof. Except as required by law, we undertake no obligation to
revise or publicly release the results of any revision to any forward-looking statements. You are advised, however, to consult any additional
disclosures we make in our reports to the SEC. All subsequent written and oral forward-looking statements attributable to us or persons
acting on our behalf are expressly qualified in their entirety by the cautionary statements contained in this prospectus.
You
should read the matters described in “Risk Factors” and the other cautionary statements made in this prospectus, and
incorporated by reference herein, as being applicable to all related forward-looking statements wherever they appear in this prospectus.
We cannot assure you that the forward-looking statements in this prospectus will prove to be accurate and therefore prospective investors
are encouraged not to place undue reliance on forward-looking statements.
Corporate
History
Aixin
Life International, Inc. (“Aixin” or “Aixin Colorado”) was incorporated under the laws of the State of Colorado
on December 30, 1987. In February 2, 2017, Mr. Quanzhong Lin (Mr. Lin) purchased 7,380,352 shares of our common stock, 65.0% of its outstanding
shares for $300,000. In December 2017, we issued 56,838,151 shares of common stock to Mr. Lin, the sole stockholder of AiXin (BVI) International
Group Co., Ltd. a British Virgin Islands corporation (“AiXin BVI”), for all of the outstanding shares of AiXin BVI. As a
result, AiXin BVI became our wholly-owned subsidiary, and through Aixin BVI we now own all of the outstanding shares of HK AiXin International
Group Co., Limited, a Hong Kong limited company (“AiXin HK”), which in turn owns all of the outstanding shares of Chengdu
AiXinZhonghong Biological Technology Co., Ltd., a Chinese limited company (“AiXinZhonghong”), which markets and sells premium-quality
nutritional products in China. AiXinZhonghong began distributing nutritional products in 2013.
For
accounting purposes, the acquisition of AiXin BVI was accounted for as a reverse acquisition and treated as a recapitalization of the
Company effected by a share exchange, with AiXin BVI as the accounting acquirer. Since neither AiXin BVI nor AiXin HK had operations
prior to the date on which we acquired AiXin BVI, the historical consolidated financial statements of AiXinZhonghong are now the historical
consolidated financial statements of the Company. The assets and liabilities of AiXinZhonghong were brought forward at their book value
and no goodwill was recognized.
In
September 2019 we completed the acquisition of nine pharmacies located in Chengdu, each of which is owned by a separate subsidiary. Since
that time, the number of our pharmacies has increased to 13. The staff at our pharmacies is being trained to reach out to customers and
make them aware of the benefits of our health and wellness products. In addition, the pharmacies serve as convenient locations
where repeat customers can buy our products.
More
recently, in September 2022, we acquired Yunnan Runcangsheng based in Yunnan Province which engages in the research, development, manufacture
and wholesale distribution of health foods. Yunnan Runcangsheng operates a 13,000 square meter production facility, which houses R&D
centers, extraction facilities, preparation workshops and a warehouse. Yunnan Runcangsheng has more than 30 sub brands and operates planting
facilities where it grows some of the key ingredients used in its products. Many of the products it has developed are specifically targeted
to alleviate symptoms associated with the increasingly competitive and pressured lifestyle of the Chinese middle class.
In
addition to our acquisitions in the health and nutritional sector, in July 2021, we acquired a hotel located in the Jinniu District,
Chengdu City. We envision utilizing the hotel to conduct marketing events and seminars for our customers, and training sessions for our
personnel at which we introduce new products and services intended to promote healthy living.
We
intend to look for additional opportunities to profit from the growing healthcare market in China. Though currently we are not party
to any agreements, we will explore, among other opportunities, expanding our product line through internal research and acquiring complementary
products from third parties, acquiring additional pharmacies and other retail outlets and operating nursing homes and possibly clinics
which provide medical care to clients.
Our
Business
We
are focused on providing health and wellness products to the growing middle class in China. We currently develop, manufacture, market
and sell premium-quality healthcare, nutritional products and wellness supplements, including herbs and greens, traditional Chinese remedies,
functional products, such as weight management tools, probiotics, foods and drinks. We also offer products purchased from third parties
and provide advertising and marketing services to clients which engage us to market and distribute their products. We offer our products
and those of clients for which we provide marketing services, through a diversified, omni-channel business model which generates revenues
through retail and wholesale product sales, through company-owned pharmacies, direct marketing and e-commerce. Our marketing approach
emphasizes proactively approaching customers such as by hosting marketing events for clients, which we believe is ideally suited to marketing
the products we offer because sales of healthcare, nutritional products and supplements are strengthened by ongoing personal contact
and support, coaching and education among the Company and our clients towards how to achieve a healthy and active lifestyle.
We
believe the competitive strengths that will enable us to grow in the health and wellness market include our ability to design and manufacture
products that are responsive to consumers’ needs as the life style of China’s middle class evolves, our coordinated omni-channel
distribution network where we enable consumers to obtain the information they need to improve their lifestyle on our website, at our
pharmacies and through individual meetings with our team members.
Our ability to operate profitably and generate
positive cash flow will be determined by our ability to attract a large and loyal customer base and provide the information and products
they need cost effectively. Our revenue will largely be determined by our ability to achieve and maintain a strong brand name and company
image, the volume of products we sell and the prices we can charge for such products, which will require that we compete effectively.
Our costs will largely be determined by the cost of raw materials and acquired inventory, the labor used to design and manufacture products,
and the costs incurred to deliver these products to the consumer.
In March 2020, the World
Health Organization announced that infections caused by the coronavirus disease of 2019 (“COVID-19”) had become pandemic
and national, provincial and local authorities, including those whose jurisdictions include Chengdu, where our offices, hotel and pharmacies
are located, adopted various regulations and orders, including “shelter in place” rules, restrictions on travel, mandates
on the number of people that may gather in one location and closing non-essential businesses. Many of these measures were relaxed due
to the decrease in the prevalence of Covid-19 in China. However, COVID-19 cases in China have periodically
risen in many cities of China. As China continues to enforce its zero tolerance policy, Chengdu has recently been subject to shelter
in place rules, lockdowns, restrictions on travel and other measures which have negatively impacted our business operations. In particular,
lockdowns, limitations on travel and limits on the number of people that may gather in one location negatively impact our marketing efforts.
Moreover, the perception that Covid-19 and other infectious diseases are on the rise, may make some potential customers reluctant to
attend large gatherings or meet with members of our sales team which could limit our sales growth. We have implemented procedures
to promote employee and customer safety. These measures will not significantly increase our operating costs. However, we cannot predict
with certainty what measures may be taken by our suppliers and customers and the impact these measures may have on our financial results
for 2022.
Our
Current Strategy
We
intend to build a reputation as a provider of premium health and wellness products that seeks to improve our customers health and well
being. Our objective is to offer a broad and deep mix of products for consumers interested in living well, whether they are looking to
treat a health-related issue or simply maintain their overall wellness, Our premium, value-added offerings include both proprietary products
developed and manufactured by us as well as products acquired from or sold on behalf of third parties. We believe our range of products
and ability to develop new products, combined with the customer support and service we offer, differentiate us and allow us to effectively
compete against food, drug and mass channel players, specialty stores, independent vitamin, supplement and natural food shops and online
retailers.
We
believe that the innovative capability we now have as a result of our acquisition of Yunnan Runcangsheng is a significant competitive
advantage. The vertical integration this gives us allows us to monitor the changing buying patterns of our customers to quickly and efficiently
design new products they desire and drives increased efficiencies in manufacturing and supply chain.
Our
retail strategy is to deliver a compelling experience at every customer touch point. We recognize that the growing Chinese middle class
is searching for product expertise and knowledgeable customer service in choosing health and wellness products. We differentiate ourselves
from competitors by educating our team members as to the benefits of our products with regular training that focuses on solution-based
selling. With this training and information regarding a customer’s purchasing habits available in our data bases, members of our
sales team can engage customers in conversation, share product information and testimonials, recommend solutions and help customers add
complimentary products and build wellness regimens.
Our
loyalty programs will allow us to develop and maintain a large and loyal customer base, provide targeted offers and information, and
connect with our customers on a regular basis. We harness data generated by these programs to better understand customers’ buying
behaviors and needs, so we can deliver a stronger experience, bring like-minded consumers into the channel and make well-informed decisions
about the business.
We
believe our diversified, omni-channel model, which includes company-owned pharmacies, direct marketing, large scale marketing events,
and e-commerce channels, differentiates us from online-only competitors. Our strategy is to give consumers a seamless, integrated experience
across all distribution channels. We believe our sales offices and pharmacies is a competitive advantage which enhances our online presence
and platform, allowing customers to experience our products and get expert advice from a coach. Our omni-channel model can enhance the
customer experience and increase the lifetime value of our customers and we are implementing strategies over the next 12-18 months to
blend our digital, online and in-store platforms.
Our
business is dependent on consumer demand for our products and services. We believe that the significant growing middle class and changing
lifestyles in China offer an opportunity to provide consumers with products that will enable them to maintain their health and well being
and deal with the ailments that accompany their changing lifestyles. Many of these customers are highly sensitive to the cost of our
products and lack the knowledge to choose the optimal products. We believe that the combination of information and products we will provide
will best serve to meet our customers’ needs.
Critical
Accounting Policies and Estimates
The
discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which
have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of our
financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues
and expenses, and related disclosure of contingent liabilities. On an on-going basis, management evaluates past judgments and estimates,
including those related to bad debts, accrued liabilities, convertible promissory notes and contingencies. Management bases its estimates
on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of
which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other
sources. Actual results may differ from these estimates under different assumptions or conditions. We describe below those critical accounting
policies that, as a result of judgments, uncertainties, uniqueness and complexities of the underlying accounting standards and operation
involved could result in material changes to our financial position or results of operations under different conditions or using different
assumptions.
We
acquired Yunnan Runcangsheng on September 30, 2022. The historical financial information of our Company reported below do not
include the historical operating results of Yunnan Runcangsheng which will only be included in our financial results from and
after October 1, 2022.
The
following table sets forth the results of our operations for the periods indicated as a percentage of net revenue, certain columns may
not add due to rounding:
| | |
Six
Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| | |
$ | | |
%
of Revenue | | |
$ | | |
%
of Revenue | |
| Revenue | |
$ | 839,565 | | |
| 100 | % | |
$ | 1,550,926 | | |
| 100 | % |
| Operating
costs and expenses | |
| 2,350,957 | | |
| 280 | % | |
| 860,252 | | |
| 55 | % |
| Income
(loss) from operations | |
| (1,511,392 | ) | |
| (180 | )% | |
| 690,674 | | |
| 45 | % |
| Non-operating
income (expenses), net | |
| 32,007 | | |
| 4 | % | |
| (4,235 | ) | |
| (0.3 | )% |
| Income
(loss) before income tax | |
| (1,479,385 | ) | |
| (176 | )% | |
| 686,439 | | |
| 44 | % |
| Income
tax expense | |
| 965 | | |
| - | % | |
| 218,052 | | |
| 14 | % |
| Net
income (loss) | |
$ | (1,480,350 | ) | |
| (176 | )% | |
$ | 468,387 | | |
| 30 | % |
The
following table shows our operations by business segment for the six months ended June 30, 2022 and 2021.
| | |
For
the Six Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| Net
revenue | |
| | | |
| | |
| Advertising
and products | |
$ | 32,689 | | |
$ | 1,550,926 | |
| Pharmacies | |
| 352,939 | | |
| - | |
| Hotel | |
| 453,937 | | |
| - | |
| Total
revenues, net | |
$ | 839,565 | | |
$ | 1,550,926 | |
| | |
| | | |
| | |
| Operating
costs and expenses | |
| | | |
| | |
| Advertising
and products | |
| | | |
| | |
| Cost
of goods sold | |
$ | 8,418 | | |
$ | 160,680 | |
| Operating
expenses | |
| 660,261 | | |
| 699,572 | |
| Pharmacies | |
| | | |
| | |
| Cost
of goods sold | |
| 270,104 | | |
| - | |
| Operating
expenses | |
| 323,151 | | |
| - | |
| Hotel | |
| | | |
| | |
| Hotel
operating costs | |
| 929,719 | | |
| - | |
| Operating
expenses | |
| 159,304 | | |
| - | |
| Total
operating costs and expenses | |
$ | 2,350,957 | | |
$ | 860,252 | |
| | |
| | | |
| | |
| (Loss)
income from operations | |
| | | |
| | |
| Advertising
and products | |
$ | (635,990 | ) | |
$ | 690,674 | |
| Pharmacies | |
| (240,316 | ) | |
| - | |
| Hotel | |
| (635,086 | ) | |
| - | |
| (Loss)
income from operations | |
$ | (1,511,392 | ) | |
$ | 690,674 | |
Revenue
Revenue
was $839,565 in the six months ending June 30, 2022, compared to $1,550,926 in the same period of 2021, a decrease of $711,361 or 46%.
The decrease in revenue was mainly due to decreases in direct sales of our nutritional products and advertising revenues as due to COVID-19
restrictions we were not able to host the types of events at which we market nutritional products, which were partly offset by revenues
from our pharmacies and hotel which we did not own in the first half year of 2021. For the six months ended of June 30, 2022, we had
$0 advertising revenue and $385,628 product revenues (of which $32,689 were from direct sales and $352,939 represented sales at our pharmacies),
and hotel revenue of $453,937. For the six months ended June 30, 2021, we had $1,297,681 of advertising revenue and $253,245 of product
revenue from our direct sales activities and no revenues from our pharmacies and hotel as the acquisitions were not completed until the
third quarter of 2021.
Operation
Costs and Expenses
Cost
of Goods Sold
Cost
of goods sold was $278,522 for the six months ended June 30, 2022, compared to $160,680
for the six months ended June 30, 2021, an increase of $117,842 or 73%. The increase in
our cost of goods sold is attributable to pharmacy products sales partly offset by decrease in direct product
sales. The cost of goods sold for our direct product sales as a percentage of sales was 26% in 2022, compared to 63% for 2021.
The cost of goods sold for products sold through our pharmacies as a percentage of pharmacy product sales was 77% in 2022, and
no comparable costs were incurred in the six months ended June 30, 2021 as the acquisition was completed in the third quarter of 2021.
Hotel
Operating Costs
Hotel
operating costs were $929,719 for the six months ended June 30, 2022. There were no comparable costs in the six months ended June 30,
2021 as the acquisition was completed in the third quarter of 2021.
Operating
Expenses
Operating
costs and expenses were $1,142,716 for the six months ended June 30, 2022, compared to $699,572 for the same period of 2021, an increase
of $443,144 or 63%. The increase in operating expenses was mainly due to the inclusion of the operating expenses of our pharmacies and
hotel.
Income
(loss) from Operations
Loss
from operations was $1,511,392 in the six months ended June 30, 2022, compared to income of $690,674 in the same period of 2021, a decrease
in results of $2,202,066 or 319%. The decrease in our income from operations for 2022 was due to the loss incurred from our direct
sales activities and the inclusion of the losses incurred by our pharmacies and hotel. All of our operations and in particular our direct
marketing activities were materially adversely impacted by travel and work restrictions and limits on the number of people that might
gather in one place imposed on a temporary basis in China and Chengdu to limit the spread of COVID-19.
Non-operating
Income (Expense)
Non-operating
income was $32,007 for the six months ended June 30, 2022, compared to non-operating expense of $4,235 for the six months ended June
30, 2021. For the six months ended June 30, 2022, we had interest income of $2,612 and other income of $29,655, partly offset by other
expenses of $260. For the six months ended June 30, 2021, we had interest income of $2,489 and other income of $161, partly offset by
other expenses of $6,885.
Income
tax expense
Income
tax expense was $965 and $218,052 for the six months ended June 30, 2022 and 2021, respectively, a decrease of $217,087 or 100% for the
six months ended June 30, 2022 compared with the same period of 2021.
Net
Income (Loss)
Our
net loss for the six months ended June 30, 2022 was $1,480,350, compared to net income of $468,387 in the same period of 2021, a decrease
of $1,948,737 or 416%. The decrease in the six months ended June 30, 2022 was mainly due to decreased sales and increased operating costs
and expense as explained above.
Yunnan
Runcangsheng
Yunnan
Runcangsheng commenced operations in April 2020 and remains in the development stage. During the six months ended June 30, 2022 and 2021,
Yunnan Runcangsheng generate revenues of $295,375 and $62,391, respectively, and incurred losses from operations of $39,560 and
$25,739, respectively. Of the revenues generated in the six months ended June 30, 2022, $63,681 was the result of purchases
made by Aixin. We do not anticipate the elimination of significant expenses as a result of our acquisition of Yunnan Runcangsheng and
our ability to operate Yunnan Runcangsheng profitably is dependent upon our ability to increase its revenues.
Results
of Operations for the years ended December 31, 2021 and 2020
The
following table sets forth the results of our operations for the periods indicated as a percentage of net revenue, certain columns may
not add due to rounding (In reviewing the tables below, please note that the operations of our pharmacies and our hotel are reflected
in our financial results from August 2022, the dates as of which the acquisitions were completed):
| | |
Years
Ended December 31, | |
| | |
2021 | | |
2020 | |
| | |
$ | | |
%
of Revenue | | |
$ | | |
%
of Revenue | |
| Revenue | |
$ | 3,066,233 | | |
| 100 | % | |
$ | 2,451,055 | | |
| 100 | % |
| Operating
costs and expenses | |
| 3,093,171 | | |
| 101 | % | |
| 1,656,595 | | |
| 68 | % |
| Income
(Loss) from operations | |
| (26,938 | ) | |
| (1 | )% | |
| 794,460 | | |
| 32 | % |
| Non-operating
income (expenses), net | |
| 34,023 | | |
| 1 | % | |
| 563,178 | | |
| 23 | % |
| Income
tax expense | |
| 274,321 | | |
| 9 | % | |
| 340,127 | | |
| 14 | % |
| Net
income(loss) | |
$ | (267,236 | ) | |
| (9 | )% | |
$ | 1,017,511 | | |
| 42 | % |
The
following table shows our operations by business segment for the years ended December 31, 2021 and 2020.
| | |
2021 | | |
2020 | |
| Net
revenue | |
| | | |
| | |
| Advertising
and products | |
$ | 2,406,988 | | |
$ | 2,451,055 | |
| Pharmacies | |
| 280,447 | | |
| - | |
| Hotel | |
| 378,798 | | |
| - | |
| Total
revenues, net | |
$ | 3,066,233 | | |
$ | 2,451,055 | |
| | |
| | | |
| | |
| Operating
costs and expenses | |
| | | |
| | |
| Advertising
and products | |
| | | |
| | |
| Cost
of goods sold | |
$ | 316,750 | | |
$ | 224,675 | |
| Operating
expenses | |
| 1,344,543 | | |
| 1,431,920 | |
| Pharmacies | |
| | | |
| | |
| Cost
of goods sold | |
| 218,735 | | |
| - | |
| Operating
expenses | |
| 252,513 | | |
| - | |
| Hotel | |
| | | |
| | |
| Hotel
operating costs | |
| 744,594 | | |
| - | |
| Operating
expenses | |
| 216,036 | | |
| - | |
| | |
| - | | |
| - | |
| Total
operating costs and expenses | |
$ | 3,093,171 | | |
$ | 1,656,595 | |
| | |
| | | |
| | |
| Income
(loss) from operations | |
| | | |
| | |
| Advertising
and products | |
$ | 745,695 | | |
$ | 794,460 | |
| Pharmacies | |
| (190,801 | ) | |
| - | |
| Hotel | |
| (581,832 | ) | |
| - | |
| Income
(loss) from operations | |
$ | (26,938 | ) | |
$ | 794,460 | |
Revenue
Revenue
was $3,066,233 in the year ending December 31, 2021, compared to $2,451,055 in the same period of 2020, an increase of $615,178 or 25%.
The increase in revenue was mainly due to increased advertising revenue and the inclusion of revenue from our hotel and pharmacies, partly
offset by a decline in product revenues of the Advertising and products segment. For 2021, we had advertising and product revenues of
$2,406,988, pharmacies revenue of $280,447, and hotel revenue of $378,798. For 2020, we had $2,451,055 in advertising and products revenue
and no revenues from the hotel and pharmacies as the acquisitions were not completed until 2021.
Operation
Costs and Expenses
Cost
of Goods Sold
Cost
of goods sold was $535,485 in the year ended December 31, 2021, compared to $224,675 for
2020, an increase of $310,810 or 138%. The increase in our cost of goods sold is attributable
to the increase in product sales due to the acquisition of the pharmacies as well as an
increase in cost of goods sold from our traditional products. The cost of goods sold for our nutritional products as a percentage of
sales was 69% in 2021, compared to 39% for 2020. The cost of goods sold as a percentage of nutritional
product sales was higher in 2021 than 2020 due to increased sales volume of lower profit margin products in 2021.
Hotel
Operating Costs
Hotel
Operating costs were $744,594 for the year ended December 31, 2021. There were no comparable costs in 2020 as the acquisition was completed
in 2021.
Operating
Expenses
Operating
costs and expenses were $2,557,688 for the year ended December 31 2021, compared to $1,431,920 for 2020, an increase of $1,125,766. The
increase in operating expenses was mainly due to the inclusion of the operating expenses of the hotel and pharmacies since their respective
dates of acquisitions.
Income
(loss) from Operations
Loss
from operations was $(26,938) in the year ended December 31 2021, compared to income of $794,460 in 2020, a decrease of $821,398 or 103%.
The decrease in our income from operations for 2021 was mainly due to the inclusion of the losses occurred by our pharmacies and hotel,
along with a slight decrease in our income from advertising services and product sales.
Non-operating
Income
Non-operating
income was $34,023 for the year ended December 31, 2021, compared to $563,178 for 2020. For 2021, we had interest income of $4,113 and
other income of $63,064 and other expenses of $33,154. For 2020, we had interest income of $537,580 and other income $28,924 and other
expense $3,326. The interest income in 2020 was primarily due to the interest income from a loan to third party in 2020, which was repaid
in full during the year ended December 31, 2020.
Income
tax expense
Income
tax expense was $274,321 and $340,127 for the years ended December 31, 2021 and 2020, a decrease of $65,806 or 19% for 2021 compared
with 2020.
Net
Income (Loss)
Our
net income (loss) for the years ended December 31, 2021 and 2020 was a loss of $(267,236) and income of $1,017,511, respectively, a decrease
in net income of $1,284,747 or 126% for 2021 compared
with 2020. The decrease in net income in 2021 was mainly due to a decrease in interest income and
operating losses incurred by our hotel and pharmacies.
Yunnan
Runcangsheng
During
the years ended December 31, 2021 and 2020, Yunnan Runcangsheng generate revenues of $783,149 and $7,304 respectively, and incurred losses
from operations of $25,243 and $28,232, respectively. The substantial increase in revenues in 2021 as compared to 2020, reflects the
fact that Yunnan Runcangsheng only commenced operations in April 2020, and began distributing products in late 2020.
Summary
of cash flows
The
following is a summary of cash provided by or used in each of the indicated types of activities during the years ended December 31, 2021
and 2020, respectively.
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Net
cash (used in) provided by operating activities | |
$ | (57,804 | ) | |
$ | 1,613,207 | |
| Net
cash (used in) provided by investing activities | |
$ | (4,431,513 | ) | |
$ | 4,085,236 | |
| Net
cash (used in) provided by financing activities | |
$ | 5,221,864 | | |
$ | 1,546,854 | |
Net
cash provided by operating activities
For
the year ended December 31, 2021, net cash used in operating activities was $57,804.
This reflects our net loss of $267,236, adjusted by non-cash related expenses including depreciation and amortization expense of $96,106,
change in deferred tax of $18,570, operating lease expense of $411,607 and stock-based compensation of $371,540, and then decreased by
changes in working capital of $651,251. The cash outflow from changes in working capital mainly resulted from unearned revenue of $122,897,
payments of taxes payable of $57,467, payments of lease liabilities of $473,508 and payments of accrued liabilities of $142,027, partly
offset by cash inflow from other receivables and prepaid expenses $94,992 and inventory of $69,738.
For
the year ended December 31, 2020, net cash provided by operating activities was $1,613,207. This was primarily due to our net income
of $1,017,511, adjusted by non-cash related expenses including depreciation of $43,462, provision for bad debt of $13,624, and stock-based
compensation of $371,540, and then increased by favorable changes in working capital of $15,340. The favorable changes in working capital
mainly resulted from a decrease in advance to suppliers of $136,479, a decrease in other receivables and prepaid expenses of $20,003,
a decrease in inventory of $6,866, and an increase in taxes payable of $169,734, offset by a decrease in accrued liabilities and other
payables of $166,012.
Net
cash (used in) provided by investing activities
For
the year ended December 31, 2021, net cash used in investing activities was $4,431,513, mainly for the acquisition of a hotel and pharmacies.
For
the year ended December 31, 2020, net cash provided by investing activities was $4,085,236, which was mainly due to the return of prepayments
for acquisitions of $4,087,409, partly offset by purchases of property and equipment of $2,173.
Net
cash (used in) provided by financing activities
For
the year ended December 31, 2021, net cash provided by financing activities reflected a capital contribution of $4,386,070 and the proceeds
from advances from related parties of $1,204,442, partially offset by the repayment of loans from third parties of $368,648.
For
the year ended in December 31, 2020, net cash provided by financing activities were advances from related parties of $1,546,854.
During
the six months ended June 30, 2022, we used $771,496 in operations. As of June 30, 2022, cash and cash equivalents were $8,129,696 (excluding
$61,588 of restricted cash), compared to $8,556,642 (excluding $44,211 of restricted cash) as of December 31, 2021. At June 30, 2022,
we had working capital of $3,602,854 compared to $4,753,390 at December 31, 2021.
The
following is a summary of cash provided by or used in each of the indicated types of activities during the six Months ended June 30,
2022 and 2021, respectively.
| | |
June
30, 2022 | | |
June
30, 2021 | |
| Net
cash (used in) provided by operating activities | |
$ | (771,496 | ) | |
$ | 439,801 | |
| Net
cash (used in) provided by investing activities | |
$ | - | | |
$ | (4,494,966 | ) |
| Net
cash (used in) provided by financing activities | |
$ | 779,015 | | |
$ | (444,754 | ) |
Net
cash provided by (used in) operating activities
For
the six months ended June 30, 2022, net cash used in operating activities was $771,496.
This reflects our net loss of $1,480,350, adjusted by non-cash related expenses including depreciation and amortization expense of $57,219,
the change in deferred tax of $965, bad debt expense of $47,857, operating lease expense of $449,445 and stock-based compensation of
$185,770, and then decreased by changes in working capital of $32,402. The cash outflow from changes in working capital mainly resulted
from an increase in accounts receivable of $65,284, payments of lease liabilities of $418,711, a change in inventory of $72,168, unearned
revenue of $10,099 and taxes payable of $7,034, which was partly offset by cash inflow from accrued liability and other payables of $346,883,
other receivable and prepaid expense of $92,619, accounts payable of $89,349 and advances to suppliers of $12,043.
For
the six months ended June 30, 2021, net cash provided by operating activities was $439,801. This was primarily due to our net income
of $468,387, adjusted by non-cash related expenses including depreciation of $10,870, operating lease expense of $56,253 and stock-based
compensation of $185,770, and then decreased by changes in working capital of $281,479. The cash outflow from changes in working capital
mainly resulted from inventory purchase of $88,863; payment of advances to suppliers of $113,556; payment of lease liability of $56,253
and tax payments of $47,058, which was partly offset by cash inflow from accrued liability and other payables of $22,326 and unearned
revenue of $2,787.
Net
cash provided by (used in) investing activities
For
the six months ended June 30, 2022 and 2021, net cash used in investing activities was $0 and $4,494,966, respectively. For the six months
ended June 30, 2021, net cash used in investing activities was $4,494,966, mainly for the prepayment for the acquisition of a hotel and
pharmacies from our major shareholder.
Net
cash provided by (used in) financing activities
For
the six months ended June 30, 2022, net cash provided by financing activities were increased advances from related parties of $779,015.
For
the six months ended June 30, 2021, net cash used in financing activities were net repayments to advances from related parties of $444,754.
Yunnan
Runcangsheng
Because
it only commenced operations in April 2020 and is investing in equipment and fixtures necessary for its research and manufacturing operations,
and acquiring raw materials and inventory, Yunnan Runcangsheng used net cash of $312,901 in operations, all of which was supported
by proceeds from a government grant of approximately $1,011,421 received during the six months ended June 30, 2022 as compared to
$115,382 used in operations in the six months ended June 30, 2021. For the year ended December 31, 2021, Yunnan Runcangsheng generated
net cash from operations of $1,735,611, largely as a result of an increase in accrued liabilities and other payables of $1,808,593, partially
offset by reductions in accounts receivable of $419,382, receivables due from a related party of $229,174 and an increase in inventory
of $219,109. Yunnan Runcangsheng’s cash needs to date were funded primarily through proceeds from its shareholders and a
government grant of $1,011,421. We anticipate that Yunnan Runcangsheng will continue to need cash for the immediate future to continue
its investment in plant and equipment and as working capital to increase its sales.
Liquidity
We
are subject to all of the substantial risks inherent in the development of a new business enterprise within an extremely competitive
industry. Due to the absence of a long standing operating history and the emerging nature of the markets in which we compete, we anticipate
operating losses until we can successfully implement our business strategy. Our revenue model is new and evolving, and we cannot be certain
that it will be successful. The potential profitability of our business model is unproven. We may never ever achieve profitable operations.
Our future operating results depend on many factors, including demand for our services, the level of competition, and the ability of
our officers to manage our business and growth. As a result of the emerging nature of the market in which we compete, we may incur operating
losses until such time as we can develop a substantial and stable revenue base. Additional development expenses may delay or negatively
impact the ability of the Company to generate profits. Accordingly, we cannot assure you that our business model will be successful or
that we can sustain revenue growth, achieve or sustain profitability, or continue as a going concern.
We
expect to finance our operations primarily through cash flow from operations and the proceeds of this offering until such time as our
cash is used to support operations or increase our business through acquisitions. At this time, we are not party to any agreement with
respect to the acquisition of new products, business or operations outside of the ordinary course of business.
On
September 30, 2022, we entered into a Supplementary Agreement to Equity Transfer Agreement with the former owners of Yunnan Runcangsheng
whereby we deemed our acquisition of Yunnan Runcangsheng to be effective. We subsequently entered into a second anticipate
Supplementary Agreement with the former owners of Yunnan Runcangsheng wherein we agreed to reduce the purchase price for Yunnan
Runcangsheng from RMB 45,082,600 to RMB 31,557,820 and confirmed that to date we had paid RMB4,850,000 of the amounts due the Sellers.
Amounts paid subsequent to June 30, 2022, for the purchase of Yunnan Runcangsheng, including the amount to be paid in November, represent
a substantial portion of the $8,129,696 in cash and cash equivalents on hand as of June
30, 2022. During the six months ended June 30, 2022, Yunnan Runcangsheng incurred a net
loss of $20,799 and used $312,901 to support its operations. We anticipate that Yunnan Runcangsheng will continue to generate
negative cash flow for the immediate future. During the six months ended June 30, 2022, we used $771,496 in our historical operations.
Should we continue to incur operating losses and incur negative cash flow on a combined basis, we may have to seek to raise capital.
We may also have to raise additional financing as our working capital requirements are expected to increase in line with the growth
of our business as a result of our acquisition of Yunnan Runcangsheng. In the past we have funded
our operations through the proceeds from private placements of equity securities completed in China and advances from our principal
shareholder. Should we require capital to fund our business, we intend to finance our business by raising additional capital or, when
available, borrowing additional funds. Additional issuances of equity or convertible debt securities will result in dilution to our current
shareholders and could cause the price of our common stock to decrease. Further, such securities might have rights, preferences or privileges
senior to our common stock. Additional financing may not be available upon acceptable terms, or at all. If adequate funds are not available
or are not available on acceptable terms, we may not be able to take advantage of prospective new business endeavors or opportunities,
which could significantly and materially restrict our business operations.
Our
ability to obtain funds through the issuance of debt or equity is dependent upon the state of the financial markets at such time as we
may seek to raise funds and the willingness of participants in the capital markets to fund business whose operations are in China. The
state of the capital market markets may be adversely impacted by various risks and uncertainties, including, but not limited to future
and current impacts of global events such as COVID-19 and the war in the Ukraine, increases in inflation and other risks set forth herein.
During
the year ended of December 31, 2021, we used $57,804 in operations. As of December 31, 2021, cash and cash equivalents were $8,556,642
(excluding $44,211 of restricted cash), compared to $7,676,689 as of December 31, 2020. At December 31, 2021, we had working capital
of $4,753,390 compared to $6,753,486 at December 31, 2020.
Impact
of Inflation
Our
results of operations may be affected by inflation, particularly rising prices for labor, raw materials, products and other operating
costs, if we cannot pass such increases along to our customers in the form of higher prices for our products and services. Generally,
our inventory turns multiple times per year and we anticipate that we will be able to increase prices on products to reflect increases
in the cost of inventory.
Foreign
Currency Translation Risk
Our
operations are located in China, which may give rise to significant foreign currency risks from fluctuations and the degree of volatility
in foreign exchange rates between the U.S. dollar and the Chinese Renminbi (“RMB”). All of our sales are in RMB. In the past
years, RMB continued to appreciate against the U.S. dollar. As of December 31, 2021, the market foreign exchange rate had
decreased to RMB 6.37 to one U.S. dollar. Our financial statements are translated into U.S. dollars using the closing rate method.
The balance sheet items are translated into U.S. dollars using the exchange rates at the respective balance sheet dates. The capital
and various reserves are translated at historical exchange rates prevailing at the time of the transactions while income and expenses
items are translated at the average exchange rate for the period. All translation adjustments are included in accumulated other comprehensive
income in the statement of equity. The foreign currency translation gain for the years ended December 31, 2021 and 2020
was $0.1 million and $0.4 million, respectively.
Off-Balance
Sheet Arrangements
We
have no off-balance sheet arrangements (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) as of June 30, 2022 that
have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues
or expenses, results of operations, liquidity, capital expenditures or capital resources.
Contingencies
Our
operations are conducted in the PRC and are subject to specific considerations and significant risks not typically associated with companies
in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments
in China and foreign currency exchange rates. Our results may be adversely affected by changes in PRC government policies with respect
to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad and rates and methods of taxation, among
other things.
Our
sales, purchases and expense transactions in China are denominated in RMB and all of our assets and liabilities in China are also denominated
in RMB. The RMB is not freely convertible into foreign currencies under the current PRC law. In China, foreign exchange transactions
are required by law to be transacted only by authorized financial institutions. Remittances in currencies other than RMB may require
certain supporting documentation in order to affect the remittance.
Significant
Accounting Policies
Our
management’s discussion and analysis of our financial condition and results of operations are based on our consolidated financial
statements, which were prepared in accordance with accounting principles generally accepted in the United States of America (“US
GAAP”). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts
of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as
the reported net sales and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and assumptions. We
base our estimates on historical experience and various other factors that we believe are reasonable under the circumstances, the results
of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other
sources. Actual results may differ from these estimates under different assumptions or conditions.
While
our significant accounting policies are more fully described in Note 2 to our consolidated financial statements, we believe the following
accounting policies are the most critical to assist you in fully understanding and evaluating this management discussion and analysis.
Basis
of Presentation
The
accompanying financial statements are prepared in conformity with U.S. Generally Accepted Accounting Principles (“US GAAP”).
The functional currency of Aixin is Chinese Renminbi (“RMB”). The accompanying financial statements are translated
from RMB and presented in U.S. dollars (“USD”).
Use
of Estimates
In
preparing financial statements in conformity with US GAAP, management makes estimates and assumptions that affect the reported amounts
of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the
reported amounts of revenues and expenses during the reporting period.
Significant
estimates, required by management, include the recoverability of long-lived assets, allowance for doubtful accounts, and the reserve
for obsolete and slow-moving inventories. Actual results could differ from those estimates.
Accounts
Receivable
We
maintain an allowance for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and
analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment
patterns to evaluate the adequacy of these reserves. As of December 31, 2021 and 2020, the bad debt allowance was $213,787
and $148,520, respectively.
Revenue
Recognition
ASU
No. 2014-09, Revenue from Contracts with Customers (“Topic 606”), became effective for us on January 1, 2018.
Our revenue recognition disclosure reflects updated accounting policies that are affected by this new standard. We applied the “modified
retrospective” transition method for open contracts for the implementation of Topic 606. As revenues are and have been primarily
from the delivery of products and the performance of services, and we have no significant post-delivery obligations, this did not result
in a material recognition of revenue on the accompanying consolidated financial statements for the cumulative impact of applying this
new standard. We made no adjustments to previously-reported total revenues, as those periods continue to be presented in accordance with
our historical accounting practices under Topic 605, Revenue Recognition.
Revenue
from sale of goods under Topic 606 is recognized in a manner that reasonably reflects the delivery of our products and services
to customers in return for expected consideration and includes the following elements:
| |
● |
executed
contract(s) with customers that we believe are legally enforceable; |
| |
|
|
| |
● |
identification
of performance obligation in the respective contract; |
| |
|
|
| |
● |
determination
of the transaction price for each performance obligation in the respective contract; |
| |
|
|
| |
● |
allocation
of the transaction price to each performance obligation; and |
| |
|
|
| |
● |
recognition
of revenue only when we satisfy each performance obligation. |
Our
revenue recognition policies for our operating segments are as follows:
Advertising
and Products
Advertising
Revenue
Commencing
in the third quarter of 2019 we began to provide advertising services to our clients. Advertising contracts are signed to establish the
price and advertising services to be provided. Pursuant to the advertising contracts, we provided advertising and marketing services
to clients through exhibition events, conferences, and person-to-person marketing. We perform a credit assessment of each customer to
assess the collectability of the contract price prior to entering into contracts.
Most
of the advertisement contracts designated that we perform advertising services for the client through exhibition events, conferences,
and person-to-person marketing during the contracted period, regardless of the number of such events. As such, we determined that the
performance obligation is satisfied over time during the contracted period and revenue is recognized accordingly. Such advertising revenue
amounted to $1,944,811 and $1,863,785 for the years ended December 31, 2021 and 2020, respectively.
A
smaller proportion of our advertising revenue is generated from services to clients through exhibition events, conferences, and person-to-person
marketing, and our compensation is based on the number of products sold. Such advertising revenue amounted to $0 and $6,558 for
the years ended December 31, 2021 and 2020, respectively.
All
of the advertising revenue is subject to the PRC VAT of 6%. This VAT may be offset by VAT paid by us for raw materials and other materials
purchased in China.
Products
Revenue
Our
revenue from sales of products is recognized when goods are delivered to the customer and no other obligation exists. We do not provide
unconditional return or other concessions to customers. Our sales policy allows for the return of unopened products for cash after deducting
certain service and transaction fees. As an alternative to returning a product, customers may request an exchange for products with the
same value.
Product
sales revenue represents the invoiced value of goods, net of value-added taxes (“VAT”). All of our products sold in China
are subject to the PRC VAT of 17% of the gross sales price prior to May 1, 2018, 16% since May 1, 2018 and 13% since April 1, 2019. This
VAT may be offset by VAT paid by for raw materials and other materials purchased in China. We record VAT payables and VAT receivables
net of payments in the financial statements. The VAT tax return is filed offsetting the payables against the receivables. Sales and purchases
are recorded net of VAT collected and paid as we act as an agent for the government.
Hotel
Hotel
revenues are primarily derived from the rental of rooms, food and beverage sales and other ancillary goods and services, including but
not limited to souvenir, parking and conference reservations. Each of these products and services represents a distinct performance obligation
and, in exchange for these services, we receive fixed amounts based on published rates or negotiated contracts. Payment is due in full
at the time when the services are rendered or the goods are provided. Room rental revenue is recognized on a daily basis when rooms are
occupied. Food and beverage revenue and other goods and services revenue are recognized when they have been delivered or rendered to
the guests as the respective performance obligations
are satisfied. All of the hotel’s goods sold in China are subject to the PRC VAT of 6%. This VAT may be offset by VAT paid by on
raw materials and other materials purchased in China.
Pharmacies
Our
retail drugstores recognize revenue at the time the customer takes possession of the merchandise. For pharmacy sales, each prescription
claim is its own arrangement with the customer and is a performance obligation. We generally receive payment from pharmacy customers
we satisfy our performance obligations. We record a receivable when we have an unconditional right to receive payment and only the passage
of time is required before payment is due. Sales revenue represents the invoiced value of goods, net of VAT. All of the products sold
in our pharmacies are exempt from VAT as the pharmacies qualify for a small business exemption.
Foreign
Currency Translation and Comprehensive Income (Loss)
The
functional currency of our business operations is RMB. For financial reporting purposes, RMB is translated into USD as the reporting
currency. Assets and liabilities are translated at the exchange rate in effect at the balance sheet dates. Revenues and expenses are
translated at the average rate of exchange prevailing during the reporting period.
Translation
adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’
equity as “Accumulated other comprehensive income”. Gains and losses resulting from foreign currency transactions are included
in income. There was no significant fluctuation in the exchange rate for the conversion of RMB to USD after the balance sheet date.
We
use FASB ASC Topic 220, “Comprehensive Income”. Comprehensive income (loss) is comprised of net income (loss) and all changes
to the statements of stockholders’ equity, except those due to investments by stockholders, changes in paid-in capital and distributions
to stockholders. Comprehensive loss for years ended December 31, 2021 and 2020 consisted of net loss and foreign currency translation
adjustments.
BUSINESS
Overview
We
(Aixin Life International, Inc.) are a Colorado holding company with no material operations of our own. We conduct substantially all
of our operations through our operating companies established in the PRC. From the time we acquired
Aixin BVI in December 2017, our focus has been providing health and wellness products to the growing middle class in China. We
currently develop, manufacture, market and sell premium-quality healthcare, nutritional products and wellness supplements, including
herbs and greens, traditional Chinese remedies, functional products, such as weight management products, probiotics, foods and drinks.
We also provide advertising and marketing services to clients which engage us to market and distribute their products. We offer our products
and those of clients for which we provide marketing services, through a diversified, omni-channel business model which generates revenues
through retail and wholesale product sales, through company-owned pharmacies, direct marketing and e-commerce.
In
December 2017, when we acquired Aixin BVI, it indirectly owned all of the capital stock of AixinZhonghong. AixinZhinghong began distributing
nutritional products in 2013. AixinZhonghong offers nutritional products to its customers through a direct marketing team, at large scale
entertainment style events which it organizes for the benefit of its clients and more recently, through e-commerce. In 2019, AixinZhonghong
began to provide marketing and distribution services to other manufacturers and suppliers of healthcare products. AixinZhonghong has
a proactive approach reaching out to customers to provide them with information as to how to live a healthy lifestyle. We believe this
approach is ideally suited to marketing health and wellness products as sales of these products are strengthened by ongoing personal
contact and support, coaching and education of clients, as to the benefits of a healthy and active lifestyle.
In
June 2021, we acquired nine retail pharmacies in Chengdu. We utilize these pharmacies to distribute our health and wellness products
and serve as learning centers for our clients along with their traditional business. Since June 2021 we have added four additional pharmacies
and now operate thirteen pharmacies. We intend to seek to continue to expand our chain of pharmacies and to use these outlets as part
of our overall marketing strategy. As part of our marketing efforts, we will educate the employees at our pharmacies as to the benefits
of our health and wellness products so they can closely work with our clients.
More
recently, in September 2022, we acquired Yunnan Runcangsheng which engages in the research,
development, manufacture and wholesale distribution of health and wellness products. Yunnan Runcangsheng operates a 13,000 square meter
production facility, including, R&D centers, extraction facilities, preparation workshops and a warehouse. Yunnan Runcangsheng has
more than 30 brand names under which it distributes products and maintains and operates planting facilities where it grows some of the
key ingredients used in its products. Many of the products it has developed are specifically targeted to alleviate ailments associated
with the increasingly competitive and pressured lifestyle of Chinese people, such as hypertension and obesity which result from a more
pressured yet sedentary lifestyle, symptoms associated with changing eating habits and the presence of environmental toxins that are
becoming more widespread through the use of western style pesticides and fertilizers. When we entered into the agreement to acquire Yunnan
Runcangsheng we began to distribute its products through our distribution channels and continue to do so.
In
addition to our acquisitions in the health and nutritional sector, in July 2021, we acquired Chengdu Aixin Shangyan Hotel Management
Co., Ltd (“Aixin Shangyan Hotel”) which operates
the Shangyan Hotel located in the Jinniu District, Chengdu City. While we have continued the hotel’s pre-acquisition business,
we intend to use the hotel to host events at which we educate our team members and customers as to the benefits of a healthy lifestyle,
introduce new products and offer our complete product line for sale.
We
intend to look for additional opportunities to profit from the growing healthcare market in China. Though currently we are not party
to any agreements, we will explore, among other opportunities, expanding our product line through internal research and acquiring complementary
products from third parties, acquiring additional pharmacies and other retail outlets and operating nursing homes and possibly clinics
which provide medical care to clients.
Business
Objectives
Key
elements of our strategy are detailed below:
Leading
brand of nutritional supplements. Our primary goal is to develop recognized brand names with a reputation as a provider of quality
health and wellness products which deliver demonstrable benefits to our customers. Our objective is to provide members of the growing
Chinese middle class with the information necessary to maintain a healthy lifestyle and to offer them a broad and deep mix of products
whether the customer is looking to treat a specific health-related issue, maintain their overall wellness or improve their performance.
Our premium, value-added offerings will include both proprietary branded products and other branded products provided by third parties
to meet needs not met by our in-house products.
We
believe that by offering a variety of branded exclusive products and a wide range of merchandise, and close customer support and services,
we will be able to differentiate our company from competitors and effectively compete against other food, drug and mass channel players,
specialty stores, independent vitamin, supplement and natural food shops and online retailers. We further believe that if we succeed
in developing and maintaining a positive reputation, we will be able to offer additional products and services to our clients.
Product
development and innovation. With the acquisition of Yunnan Runcangsheng we now have the facilities and, more importantly, knowledgeable,
experienced personnel, to develop high-quality, innovative health foods and nutritional supplement products that will be offered by our
direct marketing team and available through our company-owned pharmacies, our website and, as demand grows other select marketplaces
and wholesale partners. To the extent desirable we will grow some of the key ingredients in our products. When we choose to use ingredients
of third parties, they will be rigorously tested before they are added to our products, undergoing multiple quality checks to ensure
that they meet our high standards for purity, composition and absence of contaminants.
We
believe our capability to innovate and respond to customer needs by designing new products gives us a significant competitive advantage.
At Yunnan Runcangsheng we directly employ scientists, nutritionists, formulators, and quality control experts who work at our research
and manufacturing facilities.
A
differentiated customer experience. One of the key differentiators to our marketing strategy is the continued development of
well-trained team members first used at AiXinZhonghong that work closely with customers.
We will provide our team members with regular training and education focused on the benefits of a healthy lifestyle, the advantages of
our products and solution-based selling. We will also maintain and make available to our team members data bases of our customers purchasing
patterns. With their knowledge of the available products our team members can engage customers in conversation, access the customer’s
purchasing history through our data base, share product information and testimonials, and recommend solutions and help customers add
complimentary products and build wellness regimens. We operate in a highly personalized, aspirational sector and believe that health
food and nutritional supplement consumer often desires and seeks out product expertise and knowledgeable customer service.
At
AiXinZhonghong we developed loyalty programs to reward customers and incentivize them to
introduce our products to their friends. A number of AiXinZhonghong’s customers in
fact acted as independent marketers, buying products from us and distributing them to others at a profit. We intend to continue to use
loyalty programs to develop and maintain a large and loyal customer base, provide targeted offers and information, and connect with our
customers on a regular basis. We will harness data generated by these programs to better understand customers’ buying behaviors
and needs, so we can deliver a more rewarding experience, encourage our customers to introduce our products to their friends and make
well-informed decisions about the direction of our business.
As
we grow and develop our customer base, we can use our hotel as a site for educational seminars, conferences and marketing events featuring
well known experts in the wellness sector. These events can be used to increase customer loyalty and to educate our team members as to
the benefits of a healthy life style and our products so they can better serve our customers by guiding them to products that meet their
needs.
Omni-channel
development. We believe our diversified, omni-channel model, which includes company-owned offices open to clients, pharmacies,
direct marketing by our team members, individually and at large scale company sponsored marketing events, wholesale locations and e-commerce
channels, can differentiate us from many of our competitors, particularly those that rely exclusively on online marketing efforts. Our
strategy is to give consumers a seamless, integrated experience across digital, mobile and in-store channels and in every interaction
with us and our products.
Through
our website and in conversations with our well-trained team members, customers can research our products and purchase them online or
at one of our local pharmacies or sales offices. We believe our physical store base provides a competitive advantage, allowing customers
to experience our products and get expert advice from our team members.
Our
omni-channel model can enhance the customer experience and increase the value of a customer to us and we will implement strategies to
blend our digital, online and in-store platforms, always stressing the availability and benefits of a conversation with one of our team
members. These initiatives will include increased cross-channel marketing, online and in-store subscription services, giving customers
the option of picking up online purchases at our stores, delivering products purchased via e-commerce directly from stores, and providing
educational content, information and advice online, at our pharmacies o through a personal visit by one of our team members.
Vertical
Integration. We believe that our multi-channel distribution model, combined with our research, development and manufacturing
capabilities offers us a unique advantage over our competitors. The daily feedback received from interactions between our team members
and customers will be monitored to recognize what our customers want and rapidly develop products to meet their desires. Instead of simply
responding to trends in the market, we will seek to bring new topical products to the market before they are generally available.
Products
Our
pharmacies offer a wide variety of personal healthcare products and traditional Chinese remedies in addition to our health and wellness
products. They began to carry the products developed by Yunnan Runcanscheng prior to completion of that acquisition. The primary products
currently distributed by us, including those of Yunnan Runcangsheng include rhino king, colostrum powder derived from goat’s
milk, tong li ke, gammaoling granules, amoxicillin capsules, loratadine tablets, and fuyang granules.
Marketing
Our
target customer is the growing population of middle- and upper-class Chinese who are willing to devote increasing amounts to healthcare
products as their incomes grow. We believe that as this group grows, becomes more affluent and adopts a more western sedentary life-style
and diet, it will experience obesity, hypertension, heart disease, back pain and some of the negative symptoms common in developed countries.
Our marketing efforts include proactively approaching these customers through person-to-person marketing and by hosting educational and
informative events, which we believe is ideally suited to marketing our products and those of our clients for which we perform advertising
services because sales of health and wellness products and supplements are strengthened by ongoing personal contact and support, coaching
and education towards how to achieve a healthy and active lifestyle.
We
currently market our products through our pharmacies, at large scale marketing events which feature popular local entertainers, at our
offices which customers are encouraged to use for social gatherings, at wholesale and online. Key to our marketing efforts are the members
of our marketing team who make themselves available to individual customers and work with such customers to develop a healthy well balanced
dietary regimen. As part of our educational efforts, we distribute informational videos to our clients on-line and make multiple posts
on social media daily. Off-line, in addition to distributing products through our pharmacies, we utilize person-to-person marketing to
promote and sell products. These personal marketing efforts include hosting educational events and allowing customers to utilize our
facilities for social gatherings.
In
addition to our marketing efforts, through our loyalty program we encourage our customers to distribute our products to others. Our program
for increasing the number of clients interested in reselling the products we offer and for developing new clients who demonstrate the
ability to sell our products to others, is to provide them with compensation, in the form of quantity purchase discounts and other incentives,
such as free meals, travel or vacations.
We
offer our clients a customer satisfaction guarantee. Our sales policy allows for the return of unopened products for cash after deducting
certain service and transaction fees. As an alternative to returning a product, customers can exchange a product for another of the same
value.
Manufacturing
As
a result of the acquisition of Yunnan Runcangsheng, we now have a 13,000 sq. mtr. manufacturing center inclusive of office space,
R&D centers, extraction workshops, preparation workshops, traditional Chinese medicine decoction pieces workshops and storage areas.
The activities include research and development, extraction and intensive processing of traditional Chinese medicines and processing
to incorporate active ingredients into tablets, capsules, granules and pills according to market demand and product attributes. The facility
was designed and constructed and is operated in compliance with applicable national health food and pharmaceutical standards (GMP), and
has achieved ISO quality system certification and HACCP food safety system certification.
Raw
Materials
The
raw materials used in products we manufacture are generally readily available, subject to fluctuations in price due to supply and demand
issues. We do not look to enter into long term contracts with any of our suppliers as the materials we need shifts as demand for our
products changes.
Intellectual
Property
We believe that we need to develop well
recognized brand names and, when possible, obtain patents covering products we develop, their formulae or the processes by which
they are manufactured. Runcangsheng has received patents on certain manufacturing processes and has used multiple brand names for
its products. Given the early stage of Runcangsheng’s development and the limited historical operations of AiXinZhonghong, our
products and brand names have yet to achieve the widespread recognition we envision. Further, we have yet to determine whether the
patents Runcangsheng has received will prevent others from marketing competitive products or using manufacturing processes similar
to those we have developed. See, “Risk Factors – Risks Associated with Our Company -, Our ability to adequately
protect our trade names, trademarks and patents could have an impact on our brand images and ability to penetrate new
markets.”
Competition
The
category of nutritional products is very competitive and there are various channels through which such products are marketed to consumers,
including direct selling, through the internet, through specialty retailers, pharmacies and discount channels of food, drug and mass
merchandisers. We seek to differentiate ourselves by being familiar with our clients and providing a personalized sales experience and
focusing on after-sale services where sales employees focus on the consultative sales process through product education and the frequent
contact and support that many sales employees have with the clients. From a competitive standpoint, there are many providers and sales
outlets of nutritional products in China. We believe that none have effectively combined the product, personal coaching, education and
the product access provided by our sales employees and, further, that these efforts are compounded by the peer pressure our clients generate
through our organized group sales presentations.
Employees
As
of November 1, 2022, we had approximately 159 employees and there was no labor union established by our employees. The following table
sets out a breakdown of the number of employees by function as of November 1, 2022:
| Function | |
Number
of employees | |
| Administration | |
| 25 | |
| Finance | |
| 15 | |
| Manufacturing | |
| 42 | |
| Marketing
(sales) | |
| 70 | |
| R&D | |
| 4 | |
| Procurement | |
| 3 | |
We
believe that we maintain a good working relationship with our employees, and to date we have not experienced any significant labor disputes.
Government
Regulations
We
are subject to numerous regulations applicable to businesses generally, including regulations relating to working conditions, employee
compensation and the maintenance of welfare plans, environmental regulation and truth in advertising. In addition, the manufacture and
distribution of nutritional products is subject to many laws, governmental regulations, administrative determinations and guidance. Such
laws, regulations and other constraints exist at the national, provincial and local levels, including regulations pertaining to: (1)
the formulation, manufacturing, packaging, labeling, distribution, importation, sale and storage of products; (2) product claims and
advertising, including direct claims and advertising by us, as well as claims and advertising by manufacturers and distributors of the
products we offer, for which we may be held responsible; and (3) taxes. As a distributor, we are subject to only a portion of these laws
and regulations. We believe that we are fully compliant with those applicable to our activities.
Prior
to commencing manufacture or distribution of a product, the manufacturer or distributor may be required to obtain an approval, license
or certification from the national, provincial or local government in China. Although we attempt to determine whether all regulatory
requirements have been met, we cannot monitor the manufacture of products we acquire for distribution from third parties and cannot be
certain that all applicable regulations are satisfied. Moreover, even if we were to determine that a manufacturer or distributor had
the requisite license or certification at the beginning of a relationship, we might not become aware if it were to forfeit any regulatory
approvals or fail to adhere to applicable requirements.
Dietary
supplements are subject to regulation by the China Food and Drug Administration. China has highly restrictive nutritional supplement
product regulations. Products marketed as “health foods” are subject to extensive laboratory and clinical analysis by government
authorities, and the product registration process in China generally takes one to two years but, may be substantially longer. We market
both “health foods” and “general foods.” For products we distribute on behalf of or acquired from others, we
are not in a position to obtain any required license, though we may be held liable if we were to distribute a product which had not been
properly tested and registered with the authorities. There is some risk associated with the common practice in China of marketing a product
as a “general food” while seeking “health food” classification. If government officials feel the categorization
of a product distributed by us is inconsistent with product claims, ingredients or function, this could end or limit our ability to market
such products.
As
the middle class has grown, the number of manufacturers and distributors of nutritional supplements in China has dramatically increased.
Many of these enterprises have often ignored applicable laws and distributed adulterated or inferior products. We believe this has created
a marketing opportunity which we have tried to exploit as a trusted source of products on which our clients can rely. To the extent our
reputation results from reviewing and testing products prior to distributing them, and then distributing only products determined to
be safe, it is incumbent upon us to ensure that the manufacturers and distributors upon which we rely are trustworthy. A failure by any
of these third parties could cause substantial damage to our reputation, business and financial results.
Direct
selling and multi-level marketing are two forms of marketing regulated by various national, provincial and local government agencies
in China. These laws and regulations are generally intended to prevent fraudulent or deceptive schemes, including “pyramid”
schemes, which compensate participants primarily for recruiting additional participants without significant emphasis on product sales
to consumers.
Under
PRC regulations, “direct selling” refers to a type of business mode in which a company recruits door-to-door salesmen to
sell products directly to ultimate consumers outside the companies’ fixed places of business. Businesses engaged in “direct
selling” are required to obtain a license from the PRC government. A direct selling company is required to provide vocational training
for, and conduct an examination of, any sales promoter it recruits, and obtain a certificate for each sales promoter after the sales
promoter has passed the examination. A direct selling company is also required, when commencing operations, to deposit RMB 20 million
($2.9 million) in a special account with a designated bank, which deposit is adjusted on a monthly basis to equal 15% of the operator’s
sales from direct selling products up to RMB 0.1 billion ($14.5 million).
We
do not engage in direct selling activities subject to regulations prevalent in China since we do not employ sales personnel engaged in
door-to-door sales outside our place of business.
Under
PRC regulations, “multi-level marketing” refers to marketing, promotional and sales activities whereby organizers or operators
take in new members and compensate each member based upon the number of new members introduced by such member, directly or indirectly,
or based upon the level of sales generated by the members introduced by such member. The regulations also prohibit an organizer from
requiring new members to deposit a sum of money as a condition to membership or requiring that members recruit additional members to
establish a multi-level relationship. PRC regulations distinguish direct selling from multi-level marketing in that all direct sellers
are normally trained by the direct selling company and any direct seller is not allowed to develop new followers or form multiple levels.
We
are not in the direct selling category or multi-level marketing category since (i) we do not pay salaries or commissions to our member
distributors, who decide as a matter of personal preference whether to introduce our products to relatives or friends based on their
own personal experience of usage and/or trust of our company’s products; (ii) we do not require individuals to deposit a sum of
money to become a member; and (iii) we do not pay members to recruit individuals to join in or to form a multi-level relationship.
Nevertheless,
the laws and regulations governing direct selling and multi-level marketing may be modified or reinterpreted from time to time, which
may cause us to change our business model. Regulations are subject to discretionary interpretation by regulators and governmental authorities.
There is often ambiguity and uncertainty with respect to the implication of direct selling and anti-pyramiding laws and regulations.
Properties
We
currently operate 13 retail pharmacies in Chengdu. The pharmacies are operated subject to short term leases at market rate
rents. In addition to the pharmacies, we maintain our administrative offices in Chengdu.
Yunnan
Runcangsheng operates a 13,000 square meter production facility, including, R&D centers, extraction facilities, preparation workshops
and a warehouse. Yunnan Runcangsheng has more than 30 brand names under which it distributes products and maintains and operates planting
facilities where it grows some of the key ingredients used in its products. The manufacturing center is leased through February 2023
at an annual rent of RMB 36,000 (approximately USD $5,000).
Shangyan
Hotel Company owns and operates a hotel located in the Jinniu District, Chengdu City. The hotel covers more than 8,000 square meters
and has a large restaurant that can accommodate 600 people, 6 luxury dining rooms, a 200 square meter music tea house, 13 private tea
rooms, 108 guest rooms and other supporting facilities. The hotel is conveniently located, connected to the express ring line of Chengdu
and the Chendgdu bus system, within a 30-minute drive to Shuangliu International Airport. The hotel is equipped with all modern facilities,
including central air conditioning and each guest room features luxury bedding, high-speed internet, a safe for valuables and minibar.
To accommodate businesses, the banquet hall is equipped with advanced audio-visual equipment and dedicated high-speed wireless Internet
to facilitate large group presentations. The staff includes a professional banquet team to ensure the success of any private function
or business gathering. A full range of catering services, including Chinese-style boutique Sichuan cuisine are provided in a stylish
environment. The hotel is leased pursuant to an agreement which expires at the end of 2023 at an annual rent
of approximately RMB4,600,000 (USD $650,000).
Legal
Proceedings
From
time to time, we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We
are not presently a party to any legal proceedings that in the opinion of our management, if determined adversely to us, would individually
or taken together have a material adverse effect on our business, operating results, financial condition, or cash flows.
DIRECTORS
AND EXECUTIVE OFFICERS
The
name, address, age and titles of our executive officers and director are as follows:
The
following table sets forth the names and ages of all directors and executive officers as of the end of the last fiscal year and on the
date of this report:
| Name |
|
Age |
|
Position |
| Quanzhong
Lin |
|
43 |
|
Director,
Chairman, President and Chief Executive Officer |
| Yao-Te
Wang (1) (2) (3) |
|
44 |
|
Director |
| Chang-Ping
Lin (1) (2) (3) |
|
44 |
|
Director |
| Christopher
Lee (1) (2) (3) |
|
50 |
|
Director |
| Guolu
Li |
|
55 |
|
Chief
Financial Officer |
| (1) |
Member
of the Audit Committee |
| (2) |
Member
of the Compensation Committee |
| (3) |
Member of the Nominating and Corporate Governance Committee |
Quanzhong
Lin has served as a director, President and Chief Executive Officer of our company since February 2, 2017. Mr. Lin is a highly active
entrepreneur in China, and currently serves as Chairman of AiXin Company Group, a diversified company which he founded in 2008. In addition
to Ai Xin Company Group, Mr. Lin has founded a number of companies located in Chengdu City, Sichuan Province, China, engaged in various
types of business, including pharmacies, retail outlets, hotel management services and global tourism.
In
2009, Mr. Lin founded QingBaiJiangJinWanXiang Daily Necessities store, predecessor to AixinZhonghong Biotechnology Co., Ltd. From 2010
to 2013, Mr. Lin opened branches in Xindu and Xinjin district, officially entering the Chengdu market.
In
September 2013, Mr. Lin founded Chengdu Aixin E-Commerce Company Ltd., which in the following twelve months opened branches in cities
and counties including Huayuan and Wenjiang district, and Mianyang and Jianyang city. In April, 2015, Aixin E-commerce Co., Ltd. changed
its name to Chengdu AixinZhonghong Biotechnology Co., Ltd., whose shares became listed on the Shanghai Stock Exchange (Ticker Symbol:
207448) in October 2015; and during 2015, AixinZhonghong opened branches in Dujiangyan City, and Chongzhou City. What happened to this
company?
Yao-Te
Wang has served as a director of our company since December 12, 2017. Mr. Wang has been the Chief Executive Officer of Ivy Service
Group (China), which is a transnational consultant company in China, since 2015. From January 2016 to June 2016, Mr. Wang participated
in the overall operation planning for Chongqing Cultural Assets and Equity Exchange. From June 2015 to January 2016, Mr. Wang helped
with the overall brand strategy development for Swire Group, who merged the biggest baking brand in Southwest China within more than
150 million RMB. From September 2014 to February 2015, Mr. Wang was the chairman special assistant for JECUI Health Science Company.
From July 2012 to August 2014, Mr. Wang was the Chief Executive Officer of Ivy Service Group (Taipei). From August 2007 to June 2012,
Mr. Wang was an instructor of National Defense University (Taipei), taught International Politics and Economic Analysis.
Chang-Ping
Lin was elected a director of our company on January 8, 2020. Mr. Lin has more than twenty years of experience in the financial services
industry in which he has held numerous management level positions. He currently serves as the Chief Executive Officer of the Taiwan Financial
Development Association and operates Bo-Si International Holdings Ltd., a private consulting firm he formed in January 2018. From January
2016 to January 2018 Mr. Lin served as Marketing Director - Taiwan, for Globalink Securities. From January 2012 to January 2015 Mr. Lin
was affiliated with KGI Securities, last serving as Deputy Manager, Product Management Development. Mr. Lin received an MS Degree in
Public Administration and Policy from Jinan University and a BS from Ling Tung University, Department of Business Administration. Mr.
Lin is an Associate Professor instructing Professional Level and Technical Personnel at Feng Chia University. He has also lectured in
the Data Science Department at Providence University and the Finance Department of Da Yeh University.
Christopher
Lee was appointed as a Member of the Board of Directors of the Company on February 5, 2021. Mr. Lee has served as Chief Financial
Officer of Semileds Corporation since September 2015. Mr. Lee joined Semileds Corporation in September 2014 and from November 2014 until
his appointment as Chief Financial Officer, Mr. Lee was the interim Chief Financial Officer of Semileds Corporation. Semileds develops,
manufactures and sells high performance light emitting diodes and is currently listed on The Nasdaq Stock Market. Mr. Lee has over 20
years of experience in accounting and finance, including US GAAP, PCAOB standards and SEC rules and regulations. Mr. Lee was a partner
of KEDP CPA Group from August 2009 to June 2011 and a self-employed accountant from July 2011 to August 2014. Mr. Lee holds a BS degree
in accounting from Ohio State University and a MS degree in business taxation from Golden Gate University and is licensed as a Certified
Public Accountant (CPA) in the United States.
Guolu
Li became our Chief Financial Officer on December 12, 2017. Mr. Li is a CPA who has served as managing director and Senior Accountant
at Chengdu Bixin, an accounting firm, since August 2016. From October 2013 to July 2016, Mr. Li was Deputy Financial Director of Chengdu
Geeya Science and Technology Co. (Shenzhen Stock Exchange). From August 2010 to May 2013, he was Senior Auditor at Sichuan HengKun CPA
Co., Ltd. From December 2007 to July 2010, he was Manager of the Audit Department at Sichuan Zhonglian, an accounting firm. Mr. Li received
a Bachelor degree in Engineer Management from China University of Petroleum (Beijing), in July 1989.
There
are no family relationships among any of our officers and directors.
Directors
hold office until the next annual meeting of shareholders and until their successors have been duly elected and qualified. Officers are
elected by the board of directors and hold office until the earliest of their death, resignation or removal from office.
Board
Committees
Our
board of directors has established standing committees in connection with the discharge of its responsibilities. These committees include
an Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee. Our board of directors has adopted
written charters for each of these committees. Upon completion of this offering, copies of the charters will be available on our website.
Our board of directors may establish other committees as it deems necessary or appropriate from time to time.
Audit
Committee Our Audit Committee consists of Messrs. Lee, Lin and Wang, each of whom is independent. The Audit Committee assists the
Board of Directors oversight of (i) the integrity of financial statements, (ii) our compliance with legal and regulatory requirements,
(iii) the independent auditor’s qualifications and independence, and (iv) the performance of our internal audit function and independent
auditor and prepares the report that the SEC requires to be included in our annual proxy statement. The audit committee operates under
a written charter. Mr. Lee is the Chairman of our audit committee.
The
Board of Directors determined that Mr. Lee possesses accounting or related financial management experience that qualifies him as financially
sophisticated within the meaning of Rule 4350(d)(2)(A) of the Nasdaq Marketplace Rules and that he is an “audit committee financial
expert” as defined by the rules and regulations of the SEC.
According
to its charter, the Audit Committee shall consist of at least three members, each of whom shall be a non-employee director who has been
determined by the Board to meet the independence requirements of NASDAQ, and also Rule 10A-3(b)(1) of the SEC, subject to the exemptions
provided in Rule 10A-3(c). We do not have a website containing a copy of the Audit Committee Charter. The Audit Committee Charter describes
the primary functions of the Audit Committee, including the following:
| |
● |
Oversee
the Company’s accounting and financial reporting processes; |
| |
|
|
| |
● |
Oversee
audits of the Company’s financial statements; |
| |
|
|
| |
● |
Discuss
policies with respect to risk assessment and risk management, and discuss the Company’s major financial risk exposures and
the steps management has taken to monitor and control such exposures; |
| |
|
|
| |
● |
Review
and discuss with management the Company’s audited financial statements and review with management and the Company’s independent
registered public accounting firm the Company’s financial statements prior to the filing with the SEC of any report containing
such financial statements. |
| |
|
|
| |
● |
Recommend
to the board that the Company’s audited financial statements be included in its annual report on Form 10-K for the last fiscal
year; |
| |
|
|
| |
● |
Meet
separately, periodically, with management, with the Company’s internal auditors (or other personnel responsible for the internal
audit function) and with the Company’s independent registered public accounting firm; |
| |
● |
Be
directly responsible for the appointment, compensation, retention and oversight of the work of any independent registered public
accounting firm engaged to prepare or issue an audit report for the Company; |
| |
|
|
| |
● |
Take,
or recommend that the board take, appropriate action to oversee and ensure the independence of the Company’s independent registered
public accounting firm; and |
| |
|
|
| |
● |
Review
major changes to the Company’s auditing and accounting principles and practices as suggested by the Company’s independent
registered public accounting firm, internal auditors or management. |
Nominating
and Corporate Governance Committee
The
purpose of the Nominating and Corporate Governance Committee is to assist the Board of Directors in identifying qualified individuals
to become members of our Board of Directors, in determining the composition of the Board of Directors and in monitoring the process to
assess Board effectiveness. Each of Messrs. Lee, Lin and Wang are members of the Nominating and Corporate Governance Committee. Mr. Wang
serves as Chairman of the Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee operates under
a written charter.
| |
● |
Our
Nominating and Corporate Governance Committee has, among the others, the following authority and responsibilities: |
| |
|
|
| |
● |
To
determine and recommend to the Board, the criteria to be considered in selecting nominees for the director; |
| |
|
|
| |
● |
To
identify and screen candidate consistent with such criteria and consider any candidates recommended by our stockholders pursuant
to the procedures described in our proxy statement or in accordance with applicable laws, rules and regulations and provisions of
our charter documents. |
| |
|
|
| |
● |
To
select and approve the nominees for director to be submitted to a stockholder vote at the annual meeting of stockholders. |
Compensation
Committee
The
Compensation Committee is responsible for overseeing and, as appropriate, making recommendations to the Board of Directors regarding
the annual salaries and other compensation of our executive officers and general employees and other policies, and for providing assistance
and recommendations with respect to our compensation policies and practices. Each of Messrs. Lee, Lin and Wang are members of the Compensation
Committee. The Compensation Committee operates under a written charter. Mr. Lin is the Chairman of Compensation Committee.
Our
Compensation Committee has, among the others, the following responsibilities and authority.
| |
● |
The
compensation committee may, in its sole discretion, retain or obtain the advice of a compensation consultant, legal counsel or other
adviser. |
| |
|
|
| |
● |
The
compensation committee shall be directly responsible for the appointment, compensation and oversight of the work of any compensation
consultant, legal counsel and other adviser retained by the compensation committee or said group. |
| |
|
|
| |
● |
The
Company must provide for appropriate funding, as determined by the compensation committee, for payment of reasonable compensation
to a compensation consultant, legal counsel or any other adviser retained by the compensation committee or said group. |
| |
● |
The
compensation committee select, or receive advice from, a compensation consultant, legal counsel or other adviser to the compensation
committee or said group, other than in-house legal counsel, only after conducting an independence assessment with respect to the
adviser as provided for in the Exchange Act. |
Board
Leadership Structure and Role in Risk Oversight
Mr.
Quanzhong Lin holds the positions of chief executive officer and chairman of the board of the Company. The board believes that Mr. Lin’s
services as both chief executive officer and chairman of the board is in the best interest of the Company and its shareholders. Mr. Lin
is a well-recognized successful entrepreneur in Chengdu. He possesses detailed and in-depth knowledge of the issues, opportunities and
challenges facing the Company in its business and is thus best positioned to develop agendas that ensure that the Board’s time
and attention are focused on the most critical matters relating to the business of the Company. His combined role enables decisive leadership,
ensures clear accountability, and enhances the Company’s ability to communicate its message and strategy clearly and consistently
to the Company’s shareholders, employees and customers.
The
board has not designated a lead director. Given the limited number of directors comprising the Board, the independent directors call
and plan their executive sessions collaboratively and, between meetings of the Board, communicate with management and one another directly.
Under these circumstances, the directors believe designating a lead director to take on responsibility for functions in which they all
currently participate might detract from rather than enhance performance of their responsibilities as directors.
Management
is responsible for assessing and managing risk, subject to oversight by the board of directors. The board oversees our risk management
policies and risk appetite, including operational risks and risks relating to our business strategy and transactions. Various committees
of the board assist the board in this oversight responsibility in their respective areas of expertise.
Code
of Ethics
We
have adopted a Code of Ethical Business Conduct that applies to, among other persons, members of our board of directors, our Company’s
officers including our Chief Executive Officer, employees, consultants and advisors. As adopted, our Code of Business Conduct and Ethics
sets forth written standards that are designed to deter wrongdoing and to promote:
| |
1. |
honest
and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional
relationships; |
| |
|
|
| |
2. |
full,
fair, accurate, timely, and understandable disclosure in reports and documents that we file with, or submit to, the SEC and in other
public communications made by us; |
| |
|
|
| |
3. |
compliance
with applicable governmental laws, rules and regulations; |
| |
|
|
| |
4. |
the
prompt internal reporting of violations of the Code of Ethical Business Conduct to an appropriate person or persons identified in
the Code of Ethical Business Conduct; and |
| |
|
|
| |
5. |
accountability
for adherence to the Code of Ethical Business Conduct. |
Our
Code of Code of Ethical Business Conduct requires, among other things, that all of our company’s senior officers commit to timely,
accurate and consistent disclosure of information; that they maintain confidential information; and that they act with honesty and integrity.
In
addition, our Code of Ethical Business Conduct emphasizes that all employees, and particularly senior officers, have a responsibility
for maintaining financial integrity within our company, consistent with generally accepted accounting principles, and federal and state
securities laws. Any senior officer, who becomes aware of any incidents involving financial or accounting manipulation or other irregularities,
whether by witnessing the incident or being told of it, must report it to our Company. Any failure to report such inappropriate or irregular
conduct of others is to be treated as a severe disciplinary matter. It is against our Company policy to retaliate against any individual
who reports in good faith the violation or potential violation of our company’s Code of Ethical Business Conduct by another.
A
copy of the Code of Ethics and Business Conduct was filed as an Exhibit to our report on Form 8-K filed on September 25, 2020, and is
available on the SEC’s website, www.sec.gov.
Family
Relationships
There
are no family relationships between any director or executive officer of the Company.
Independence
The
Board has determined that each of Christopher Lee, Yao-Te Wang and Chang-Ping Lin satisfies the definition of “independent director”
in accordance with Rule 5605(a)(2) of the Marketplace Rules of The Nasdaq Stock Market, Inc. and Section 10(A)(m)(3) of the Securities
Exchange Act of 1934, as amended.
EXECUTIVE
COMPENSATION
The
following tables set forth certain information about compensation paid, earned or accrued for services by our Executive Officer for the
fiscal years ended December 31, 2021 and 2020:
Summary
Compensation Table
| Name
and Principal Position |
|
Year |
|
|
Salary
($) |
|
|
Bonus
($) |
|
|
Total
($) |
|
| Quanzhong
Lin, President(1) |
|
2021 |
|
|
$ |
24,000 |
|
|
$ |
|
|
|
$ |
24,000
|
|
| Guolu
Li, Chief Financial Officer(2) |
|
2021 |
|
|
$ |
18,750 |
|
|
$ |
|
|
|
$ |
18,750 |
|
(1)
Amounts attributed to Mr. Lin represent amounts paid as President and CEO of AiXinZhonghong.
(2)
Amounts attributed to Mr. Li represent amounts paid as CFO of AiXinZhonghong.
Neither
Mr. Lin nor Mr. Li has an employment agreement with the Company.
Narrative
Disclosure to Summary Compensation Table
There
are no annuity, pension or retirement benefits proposed to be paid to the officer or director or employees in the event of retirement
at normal retirement date pursuant to any presently existing plan provided or contributed to by the Company or any of its subsidiaries,
if any.
Stock
Option Plan
In
2019 we adopted the 2019 Equity Incentive Plan (the “2019 Plan”), which authorizes the issuance of shares of common stock
for grants of stock options, stock appreciation rights, restricted stock, stock units, bonus stock, dividend equivalents, other stock
related awards and performance awards that may be settled in cash, stock, or other property. The 2019 Plan authorizes the issuance of
up to 5,000,000 shares
We
adopted the 2019 Plan to provide a means by which employees, directors, and consultants of our Company and those of our subsidiaries
and other designated affiliates, which we refer to together as our affiliates, may be given an opportunity to purchase our common stock,
to assist in retaining the services of such persons, to secure and retain the services of persons capable of filling such positions,
and to provide incentives for such persons to exert maximum efforts for our success and the success of our affiliates. The material features
of the 2019 Plan are outlined below. This summary is qualified in its entirety by reference to the complete text of the 2019 Plan which
has been filed with the SEC.
Grants
of Plan-Based Awards
None
of our executive officers was granted any options or equity awards during 2021 or 2020 or held any options or other equity awards
as of the date hereof.
In
October 22, 2019, we issued an aggregate of 587,500 shares to employees and contractors under the 2019 Equity Incentive Plan.
Outstanding
Equity Awards
There
are no outstanding equity awards granted pursuant to the 2019 Equity Incentive Plan.
Compensation
of Directors
| | |
DIRECTOR
COMPENSATION | |
| Name | |
Fees
Earned
or Paid In
Cash
($) | | |
Stock
Awards
($) | | |
Option
Awards
($) | | |
Non-Equity
Incentive Plan
Compensation
($) | | |
Non-Qualified
Deferred
Compensation
Earnings ($) | | |
All
Other
Compensation
($) | | |
Total
($) | |
| Yao-Te
Wang | |
$ | 16,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 0 | | |
$ | 16,000 | |
| Quanzhong
Lin | |
$ | 24,000 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 0 | | |
$ | 24,000 | |
| Yuhua
Zhu | |
| 0 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 0 | | |
| 0 | |
| Chang-Ping
Lin | |
| 0 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 0 | | |
| 0 | |
| Christopher
Lee | |
| 9,600 | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 9,600 | |
Pension,
Retirement or Similar Benefit Plans
There
are no arrangements or plans in which we provide pension, retirement or similar benefits for directors or executive officers. We have
no material bonus or profit sharing plans pursuant to which cash or non-cash compensation is or may be paid to our directors or executive
officers, except that stock options may be granted at the discretion of the board of directors or a committee thereof.
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS
Transactions
with Related Persons
The
following includes a summary of transactions since January 1, 2021, or any currently proposed transaction, in which we were or are to
be a participant and the amount involved exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets
at year-end for the last two completed fiscal years, and in which any related person had or will have a direct or indirect material interest
(other than compensation described under “Executive Compensation”). We believe the terms obtained or consideration that we
paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts
that would be paid or received, as applicable, in arm’s-length transactions.
Advance
to/from related parties
At
December 31, 2021, the Company had advances from a shareholders and affiliates of shareholders of $1,947,154. At December 31, 2021, the
Company had advances to affiliates of our major shareholder of $19,055. The advances are payable on demand, and bear no interest.
Acquisitions
from a Major Shareholder
In
September 2021, we completed the acquisition of nine pharmacies located in Chengdu by acquiring the
entities which owned the pharmacies for an aggregate purchase price of RMB 34,635,845, or approximately US$5.31 million (“Transfer
Price”). Our Chairman, Quanzhong Lin, was the principal shareholder of the entity which owned the pharmacies we acquired.
In
July we completed the acquisition of Aixin Shangyan Hotel. Shangyan
Hotel Company which owns and operates a hotel located in the Jinniu District, Chengdu City. The hotel covers more than 8,000 square
meters and has a large restaurant that can accommodate 600 people, 6 luxury dining rooms, a 200 square meter music tea house, 13 private
tea rooms, 108 guest rooms and other supporting facilities. We acquired the hotel through an acquisition
of the outstanding equity of Aixin Shangyan Hotel of which Mr. Lin was the principal shareholder, for a purchase price
of RMB 7,598,887, or approximately $1.16 million (“Transfer Price”).
In
connection with the acquisitions of the pharmacies and hotel we made payments to Mr Lin in the aggregate amount of $4.50 million.
Forgiveness
of Loan
On
December 31, 2021, Mr. Lin forgave a loan previously made to the Company in the amount of $6,912,513. This was treated as a contribution
to capital.
Office
Lease from a Major Shareholder
In
May 2014, we entered a lease with Mr. Lin for use of an office. We renewed the lease until May 28, 2023, with monthly rent of
RMB 5,000 ($766), payable quarterly.
Other
than the foregoing, none of the directors or executive officers of the Company, nor any person who owned of record or was known to own
beneficially more than 5% of the Company’s outstanding shares of its Common Stock, nor any associate or affiliate of such persons
or companies, had any material interest, direct or indirect, in any transaction that occurred since January 1, 2019, or in any proposed
transaction, which has materially affected or will affect the Company.
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The
following table sets forth, as of November 1, 2022, certain information concerning the beneficial ownership of our common stock
by (i) each stockholder known by us to own beneficially five percent or more of our outstanding common stock; (ii) each director; (iii)
each named executive officer; and (iv) all of our executive officers and directors as a group, and their percentage ownership and voting
power. The column entitled “Percentage of Shares Beneficially Owned—Before Offering” is based on 49,999,891 shares
of our common stock outstanding.
The
information presented below regarding beneficial ownership of our voting securities has been presented in accordance with the rules of
the Securities and Exchange Commission and is not necessarily indicative of ownership for any other purpose. Under these rules, a person
is deemed to be a “beneficial owner” of a security if that person has or shares the power to vote or direct the voting of
the security or the power to dispose or direct the disposition of the security. A person is deemed to own beneficially any security which
such person has the right to acquire sole or shared voting or investment power within sixty (60) days through the conversion or exercise
of any convertible security, warrant, option, or other right. More than one (1) person may be deemed to be a beneficial owner of the
same securities. The percentage of beneficial ownership by any person as of a particular date is calculated by dividing the number of
shares beneficially owned by such person, which includes the number of shares as to which such person has the right to acquire voting
or investment power within sixty (60) days, by the sum of the number of shares outstanding as of such date including the number of such
shares which such person has the right to acquire. Consequently, the denominator used for calculating such percentage may be different
for each beneficial owner. Except as otherwise indicated below and under applicable community property laws, we believe that the beneficial
owners of our common stock listed below have sole voting and investment power with respect to the shares shown.
| Name
of Shareholder (1) | |
Amount
and Nature of Beneficial Ownership | | |
Percent
of Common Stock Before the Offering | | |
Percent
of Common Stock After the Offering | |
| Directors
and Executive Officers: | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
Quanzhong
Lin, Chairman and CEO
9 An Rong Lu Jingniu, Bldg 4 Unit 163
Chengdu, Sichuan Province, China | |
| 29,069,353 | | |
| 58.14 | % | |
| | % |
| | |
| | | |
| | | |
| | |
Yao-Te
Wang, Director
704 No.9, Lane 14, Shijian St.
Tainan City, Taiwan, R.O.C. | |
| 3,768,673 | | |
| 7.54 | % | |
| | % |
| | |
| | | |
| | | |
| | |
| All
directors and executive officers as a group (4 persons) | |
| 32,838,026 | | |
| 65.68 | % | |
| | % |
(1)
The address of each beneficial owner is c/o Aixin Life International, Inc., X Hongxing International Business Building 2, 14th
FL, No. 69 Qingyun South Ave., Jinjiang District, Chengdu City, Sichuan Province, China
DESCRIPTION
OF CAPITAL STOCK
We
have authorized capital stock consisting of 500,000,000 shares of common stock, $0.00001 par value per share and 20,000,000 shares of
preferred stock, $0.001 par value per share.
As
of the date of this prospectus, we have 49,999,891 shares of our common stock outstanding.
The
following description of our capital stock is a summary only and is subject to and qualified in its entirety by reference to the applicable
provisions of the Colorado Business Corporations Act (“Colorado Act”), and our charter and Bylaws, copies of which have been
filed as exhibits to the registration statement of which this prospectus is part. You should refer to, and read this summary together
with, our Articles of Incorporation and Bylaws, each as amended and restated to date, to review all of the terms of our capital stock.
Our Articles of Incorporation and amendments thereto are incorporated by reference as exhibits to the registration statement of which
this prospectus is a part.
Common
Stock
Holders
of Shares of Common Stock have the right to cast one vote for each share of Common Stock in their name on the books of our company, whether
represented in person or by proxy, on all matters submitted to a vote of holders of Common Stock, including election of directors. There
is no right to cumulative voting in election of directors. Except where a greater requirement is provided by statute, by our articles
of incorporation, or by our bylaws, the presence, in person or by proxy duly authorized, of one or more holders of a majority of the
outstanding Shares of our Common Stock constitutes a quorum for the transaction of business. The vote by the holders of a majority of
outstanding Shares is required to effect certain fundamental corporate changes such as liquidation, merger, or amendment of our articles
of incorporation.
There
are no restrictions in our articles of incorporation or bylaws that prevent us from declaring dividends. The Colorado Act does, however,
prohibit us from declaring dividends where, after giving effect to the distribution of the dividend (1) we would not be able to pay our
debts as they become due in the usual course of business or (2) our total assets would be less than the sum of our total liabilities
plus the amount that would be needed to satisfy the rights of stockholders who have preferential rights superior to those receiving the
distribution.
Holders
of Shares of our Common Stock are not entitled to preemptive or subscription or conversion rights, and no redemption or sinking fund
provisions are applicable to our Common Stock. All outstanding Shares of Common Stock sold in the offering are, or when issued pursuant
to the terms of any convertible securities or warrants will be, fully paid and non-assessable.
Each
share of our common stock is entitled to equal dividends and distributions per share with respect to the common stock when, as and if
declared by our Board of Directors. No holder of any shares of our common stock has a preemptive right to subscribe for any of our securities,
nor are any shares of our common stock subject to redemption or convertible into other securities. Upon liquidation, dissolution or winding-up
of the Company, and after payment to our creditors and preferred stockholders, if any, our assets will be divided pro rata on a share-for-share
basis among the holders of our common stock. Each share of our common stock is entitled to one vote on all stockholder matters. Shares
of our common stock do not possess any cumulative voting rights.
The
presence of the persons entitled to vote a majority of the outstanding voting shares on a matter before the stockholders constitute the
quorum necessary for the consideration of the matter at a stockholders’ meeting.
Except
as otherwise required by law, the Articles of Incorporation, or any certificate of designations, (i) at all meetings of stockholders
for the election of directors, a plurality of votes cast are sufficient to elect such directors; (ii) any other action taken by stockholders
are be valid and binding upon the Company if the number of votes cast in favor of the action exceeds the number of votes cast in opposition
to the action, at a meeting at which a quorum is present, except that adoption, amendment or repeal of the Bylaws by stockholders requires
the vote of a majority of the shares entitled to vote; and (iii) broker non-votes and abstentions are considered for purposes of establishing
a quorum but not considered as votes cast for or against a proposal or director nominee. Each stockholder has one vote for every share
of stock having voting rights registered in his or her name, except as otherwise provided in any preferred stock designation setting
forth the right of preferred stock stockholders.
The
common stock does not have cumulative voting rights, which means that the holders of 51% of the common stock voting for election of directors
can elect 100% of our directors if they choose to do so.
Preferred
Stock
Our
Articles of Incorporation gives our Board of Directors authority to issue shares of “blank check” preferred stock from time
to time in one or more series, pursuant to resolutions adopted by the Board in accordance with the Colorado Act each having the voting
powers, if any, designations, powers, preferences, and the relative, participating, optional, or other rights, if any, and the qualifications,
limitations, or restrictions thereof, of any unissued series of preferred stock, to fix the number of shares constituting such series,
and to increase or decrease the number of shares of any such series, but not below the number of shares thereof then outstanding, without
the delay attendant to obtaining stockholder approval for such issuance.
We
may issue preferred stock to effect a business combination, to raise capital or for other reasons. In addition, preferred stock could
be utilized as a method of discouraging, delaying or preventing a change in control of our company.
Our
board of directors has no intention at the present time to create any additional series of preferred stock. The issuance of any new series
of preferred stock could, depending on the terms of such series, impede the completion of a merger, tender offer or other takeover attempt.
Any
future issuance of preferred stock may have the effect of delaying, deferring or preventing a change in control of us without further
action by the shareholders and may adversely affect the voting and/or other rights of the holders of common stock or any other securities
we may issue in the future. The issuance of shares of preferred stock, or the issuance of rights to purchase such shares, could be used
to discourage an unsolicited acquisition proposal. For instance, the issuance of a series of preferred stock might impede a business
combination by including class voting rights that would enable the holders to block such a transaction or facilitate a business combination
by including voting rights that would provide a required percentage vote of the stockholders. In addition, under certain circumstances,
the issuance of preferred stock could adversely affect the voting power of the holders of the common stock. Although our board of directors
is required to make any determination to issue such stock based on its judgment as to the best interests of our stockholders, the board
of directors could act in a manner that would discourage an acquisition attempt or other transaction that some, or a majority, of the
stockholders might believe to be in their best interests or in which stockholders might receive a premium for their stock over the then
market price of such stock. Our board of directors does not at present intend to seek stockholder approval prior to any issuance of currently
authorized preferred stock, unless otherwise required by law.
SHARES
ELIGIBLE FOR FUTURE SALE
Prior
to this offering, only a limited public market for our common stock existed on the OTCQX. Future sales of substantial amounts of our
common stock in the public market, including shares issued upon exercise of outstanding warrants, or the anticipation of such sales,
could adversely affect prevailing market prices of our common stock from time to time and could impair our ability to raise equity capital
in the future.
Upon
the closing of this offering, we will have [●] shares of our common stock issued and outstanding. In addition, we
will have [●] shares of common stock issuable upon the exercise of the Underwriter Warrants.
Lock-Up
For
further details on the lock-up agreements, see the section entitled “Underwriting – Lock Up Agreements.”
Rule
144
In
general, under Rule 144 of the Securities Act, as in effect on the date of this prospectus, any person who is not our affiliate at any
time during the preceding three months, and who has beneficially owned their shares for at least six months, including the holding period
of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares of our common stock provided
current public information about us is available, and, after owning such shares for at least one year, including the holding period of
any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares of our common stock without
restriction.
A
person who is our affiliate or who was our affiliate at any time during the preceding three months, and who has beneficially owned restricted
securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to
sell within any three-month period a number of shares that does not exceed the greater of:
| |
● |
1%
of the number of shares of our common stock then outstanding, which will equal approximately [●] shares immediately
after this offering; or |
| |
|
|
| |
● |
the
average weekly trading volume of our common stock during the four calendar weeks preceding the filing of a Notice of Proposed Sale
of Securities pursuant to Rule 144 with respect to the sale. |
Sales
under Rule 144 by our affiliates are also subject to manner of sale provisions and notice requirements and to the availability of current
public information about us.
UNDERWRITING
In
connection with this offering, we will enter into an underwriting agreement with Network 1 Financial Securities, Inc., which we sometimes
refer to herein as the Underwriter. The Underwriter may retain other brokers or dealers to act as sub-agents on its behalf in connection
with this offering and may pay any sub-agent a solicitation fee with respect to any securities placed by it. The Underwriter has agreed
to purchase, and we have agreed to sell to the Underwriter, the number of shares indicated below:
| Name |
|
Number
of shares |
|
| Network
1 Financial Securities, Inc. |
|
|
|
|
| Total |
|
|
|
|
The
underwriting agreement provides that the Underwriter is obligated to purchase all shares in the offering if any are purchased, other
than those shares covered by the over-allotment option described below.
We
have agreed to indemnify the Underwriter and certain of its controlling persons against certain liabilities, including liabilities under
the Securities Act, and to contribute to payments that the Underwriter may be required to make in respect of those liabilities.
We
have granted to the Underwriter a 45-day option to purchase up to additional [●] shares from us at the initial public offering
price less the underwriting discounts and commissions. The option may be exercised in whole or in part, and may be exercised more than
once, during the 45-day option period. The Underwriter may exercise this option solely for the purpose of covering over-allotments, if
any, made in connection with the offering contemplated by this prospectus.
Fees
and Expenses
The
Underwriter has advised us that it proposes to offer the shares to the public at the public offering price set forth on the cover
page of this prospectus and to certain dealers at that price less a concession not in excess of $[●] per share. After
this offering, the public offering price and concession to dealers may be reduced by the Underwriter. No such reduction shall change
the amount of proceeds to be received by us as set forth on the cover page of this prospectus. The securities are offered by the
Underwriter as stated herein, subject to receipt and acceptance by it and subject to its right to reject any order in whole or in
part. The Underwriter has informed us that it does not intend to confirm sales to any accounts over which it exercises discretionary
authority.
We
have agreed to pay the Underwriter a cash fee equal to eight percent (8%) of the aggregate gross proceeds raised in this offering. The
following table shows the price per share and total public offering price, underwriting discounts and commissions, and proceeds before
expenses to us. These amounts are shown assuming both no exercise and full exercise of the Underwriter’s over-allotment option.
| | |
Total | |
| | |
Per
Share | | |
No
Exercise | | |
Full
Exercise | |
| Public
offering price | |
$ | | | |
| | | |
| | |
| Underwriting
discounts and commissions to be paid by us: | |
$ | | | |
| | | |
| | |
| Proceeds,
before expenses, to us | |
$ | | | |
| | | |
| | |
We
will also pay to the Underwriter by deduction from the net proceeds of the offering, a non-accountable expense allowance equal to two
percent (2%) of the gross proceeds received by us from the sale of the shares.
We
have agreed to reimburse the Underwriter up to a maximum of $125,000 for out-of-pocket accountable expenses. We have paid expense deposits
of $xx,000 to the Underwriter for its anticipated out-of-pocket expenses; any expense deposits will be returned to us to the extent the
Underwriter’s out-of-pocket accountable expenses are not actually incurred in accordance with FINRA Rule 5110(g)(5)(A).
We
have agreed to pay expenses relating to the offering, including, without limitation: the Company’s legal and accounting fees and
disbursements; the costs of preparing, printing, mailing and delivering the Registration Statement, the preliminary and final prospectus
contained therein and amendments thereto, post-effective amendments and supplements thereto, the underwriting agreement and related documents
(all in such quantities as the Underwriter may reasonably require); preparing and printing stock certificates and warrant certificates;
the costs of any “due diligence” meetings; all reasonable and documented fees and expenses for conducting a net road show
presentation; all filing fees (including SEC filing fees) and communication expenses relating to the registration of the shares to be
sold in the Offering, FINRA filing fees; the reasonable and documented fees and disbursements of the Underwriter’s counsel up to
an amount of $60,000 (which maximum shall apply solely to such fees and disbursements of counsel and not to other fees and expenses);
background checks of the Company’s officers and directors up to a maximum of $15,000; preparation of bound volumes and mementos
in such quantities as the Underwriter may reasonably request up to an amount of $2,500; transfer taxes, if any, payable upon the transfer
of securities from the Company to the Underwriter; and the fees and expenses of the transfer agent, clearing firm and registrar for the
shares; provided that the actual accountable expenses of the Underwriter shall not exceed $125,000.
We
estimate that the total expenses of the offering payable by us, excluding the total underwriting discount and commissions will be approximately
$ , including a maximum aggregate reimbursement of $150,000 of the Underwriter’s
accountable expenses.
Underwriter
Warrants
In
addition, we have agreed to grant the underwriter non-redeemable warrants to purchase an amount equal to eight percent (8%) of the shares
of common stock sold in the offering at the commencement of sales of the shares offered hereby, which warrants will be exercisable
upon issuance, have a five (5) year term beginning
on the date on which sales of the shares offered hereby commence, and a cashless exercise feature. Such warrants are exercisable
at a price of 110% of the public offering price of the shares of common stock offered pursuant to this offering. We will register the
shares underlying the Underwriter Warrants and will file all necessary undertakings in connection therewith. The Underwriter Warrants
may not be sold, transferred, assigned, pledged or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call
transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days immediately
following the commencement of the offering contemplated by this prospectus (in accordance with FINRA Rule 5110), except that they may
be assigned, in whole or in part, to any member participating in the offering and the officers or partners thereof, and that all securities
so transferred remain subject to the lock-up restriction for the remainder of the time period. The Underwriter Warrants may be exercised
as to all or a lesser number of shares, will provide for cashless exercise and will contain provisions for one demand registration of
the sale of the underlying shares of common stock at the Company’s expense, an additional demand registration at the warrant holders’
expense, and unlimited “piggyback” registration rights for a period of five years after the effective date of the registration
statement at the Company’s expense. The Underwriter’s Warrants shall further provide for adjustment in the number and price
of such warrants (and the shares of common stock underlying such warrants) in the event of recapitalization, merger or other structural
transaction to prevent dilution. The underwriter will have the option to exercise their warrants at any time, provided that such shares
are not transferred during the lock-up period; the 180-day lock period will remain on these underlying shares.
Electronic
Offer, Sale and Distribution of Common Stock
A
prospectus in electronic format may be made available on the websites maintained by the underwriter. In addition, the common stock may
be sold by the underwriter to securities dealers who resell the common stock to online brokerage account holders. Other than the prospectus
in electronic format, the information on the underwriter’s website and any information contained in any other website maintained
by the underwriter is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved
and/or endorsed by us or the underwriter in its capacity as underwriter and should not be relied upon by investors.
Lock-up
Agreements
We
have agreed not to, for a period of 180 days beginning on the date of the effectiveness of the registration statement of which this prospectus
forms a part, offer, issue, sell, contract to sell, encumber, grant any option for the sale of, or otherwise dispose of, except in this
offering, any of the shares of our common stock or securities that are substantially similar to our shares, including but not limited
to any options or warrants to purchase our shares, or any securities that are convertible into or exchangeable for, or that represent
the right to receive, our shares or any such substantially similar securities (other than pursuant to employee stock option plans existing
on, or upon the conversion or exchange of convertible or exchangeable securities outstanding as of, the date such lock-up agreement was
executed), without the prior written consent of the underwriter.
Furthermore,
each of our directors, officers and holders
of 5% or more of the outstanding shares of our common stock have agreed or are otherwise contractually restricted for a period of
twelve months after the date of this prospectus, without the prior written consent of the Underwriter not to directly or indirectly:
| |
● |
issue
(in the case of us), offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract
to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of any shares of our common stock
or other capital stock or any securities convertible into or exercisable or exchangeable for our common stock or other capital stock; |
| |
|
|
| |
● |
in
the case of us, file or cause the filing of any registration statement under the Securities Act with respect to any shares of our
common stock or other capital stock or any securities convertible into or exercisable or exchangeable for our common stock or other
capital stock, other than registration statements on Form S-8 filed with the SEC after the closing date of this offering; or |
| |
|
|
| |
● |
enter
into any swap or other agreement, arrangement, hedge or transaction that transfers to another, in whole or in part, directly or indirectly,
any of the economic consequences of ownership of our common stock or other capital stock or any securities convertible into or exercisable
or exchangeable for our common stock or other capital stock, |
whether
any transaction described in any of the foregoing bullet points is to be settled by delivery of our common stock or other capital stock,
other securities, in cash or otherwise, or publicly announce an intention to do any of the foregoing.
There
are no existing agreements between the Underwriter and any person who will execute a lock-up agreement in connection with this offering
providing consent to the sale of shares prior to the expiration of the lock-up period. The lock up does not apply to the issuance of
shares upon the exercise of rights to acquire shares of common stock pursuant to any existing stock option or the conversion of any of
our preferred convertible stock.
Right of First Refusal
Until twelve (12) months from the commencement
of sales of the Offering, the Underwriter shall have a right of first refusal to act as lead managing underwriter for any public
underwriting, or private placement, of debt or equity (excluding (i) shares issued under any compensation or stock option plan approved
by our stockholders, (ii) shares issued in payment of the consideration for an acquisition or as part of strategic partnerships and transactions;
and (iii) conventional banking arrangements and commercial debt financing) of the Company or any subsidiary or successor of the company,
with the Underwriter receiving the right to underwrite or place a number of the securities to be sold therein having an aggregate purchase
price therein equal to a minimum of the aggregate purchase price of the shares in the Offering. If the Underwriter fails to accept in
writing any such proposal for such public or private sale within ten (10) days after receipt of a written notice from the Company containing
such proposal, then the Underwriter will have no claim or right with respect to any such sale contained in any such notice. If such proposal
is modified in any material respect, the Company will adopt the same procedures as with respect to the original proposed public or private
sale, and the Underwriter shall have the right of first refusal with respect to such revised proposal.
Determination
of Offering Price
Upon
the declaration of effectiveness of the registration statement of which this prospectus is a part, we will enter into an underwriting
agreement with the Underwriter. The terms of the underwriting agreement provide that the obligations of the Underwriter are subject to
certain conditions precedent, including the absence of any material adverse change in our business and the receipt of certain certificates,
opinions and letters from us, our counsel and our auditors.
We
have applied to list our common stock on the Nasdaq Capital Market under the symbol “AIXN”.
Prior
to this offering, there has not been an active public market for our shares. The initial public offering price of the shares offered
hereby was determined by negotiations between us and the Underwriter and will not necessarily reflect the market price of our common
stock following this offering. The principal factors that were considered in determining the initial public offering price included:
| |
● |
the
information presented in this prospectus and otherwise available to the Underwriter; |
| |
● |
the
history of, and prospects for, the industry in which we will compete; |
| |
● |
the
ability of our management; |
| |
● |
the
prospects for our future earnings; |
| |
● |
the
present state of our development, results of operations and our current financial condition |
| |
● |
the
general condition of the securities markets at the time of this offering; and |
| |
● |
the
recent market prices of, and the demand for, publicly traded common stock of generally comparable companies. |
We
cannot assure you that the initial public offering price will correspond to the price at which our common stock will trade in the public
market subsequent to this offering or that an active trading market for our common stock will develop and continue after this offering.
Stabilization
In
connection with this offering the Underwriter may engage in stabilizing transactions, over-allotment transactions, syndicate covering
transactions, penalty bids and passive market making in accordance with Regulation M under the Securities Exchange Act of 1934 (the “Exchange
Act”).
| |
● |
Stabilizing
transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. |
| |
● |
Over-allotment
transactions involve sales by the Underwriter of the common stock in excess of the number of shares the Underwriter is obligated
to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short
position. In a covered short position, the number of shares over-allotted by the Underwriter is not greater than the number of shares
that it may purchase pursuant to the over-allotment option. In a naked short position, the number of shares involved is greater than
the number of shares in the over-allotment option. The Underwriter may close out any covered short position by either exercising
its over-allotment option and/or purchasing shares in the open market. |
| |
● |
Syndicate
covering transactions involve purchases of shares in the open market after the distribution has been completed in order to cover
syndicate short positions. In determining the source of shares to close out the short position, the Underwriter will consider, among
other things, the price of our common stock available for purchase in the open market as compared to the price at which they may
purchase shares through the over-allotment option. If the Underwriter sells more shares than could be covered by the over-allotment
option, a naked short position, the position can only be closed out by buying shares in the open market. A naked short position is
more likely to be created if the Underwriter is concerned that there could be downward pressure on the price of the shares in the
open market after pricing that could adversely affect investors who purchase in the offering. |
| |
● |
Penalty
bids permit the Underwriter to reclaim a selling concession from a syndicate member when the common stock originally sold by the
syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
| |
● |
In
passive market making, market makers in the shares including the Underwriter or prospective underwriters may, subject to limitations,
make bids for or purchases of our common stock until the time, if any, at which a stabilizing bid is made. |
These
stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price
of our common stock or preventing or retarding a decline in the market price of the shares. As a result the price of our common stock
may be higher than the price that might otherwise exist in the open market. These transactions may be effected on the Nasdaq Capital
Market or otherwise and, if commenced, may be discontinued at any time.
A
prospectus in electronic format may be made available on the web sites maintained by one or more of the Underwriter, or selling group
members, if any, participating in this offering and the Underwriter may distribute prospectuses electronically. The Underwriter may agree
to allocate a number of shares to selling group members for sale to their online brokerage account holders. Internet distributions will
be allocated by the Underwriter and selling group members that will make internet distributions on the same basis as other allocations.
The
Underwriter and their respective affiliates are full-service financial institutions engaged in various activities, which may include
securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment,
hedging, financing and brokerage activities. The Underwriter has, from time to time, performed, and may in the future perform, various
financial advisory and investment banking services for us, for which it received or will receive customary fees and expenses.
In
addition, in the ordinary course of the business activities, the Underwriter and their affiliates may make or hold a broad array of investments
and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for
their own account and for the accounts of their customers. These investments and securities activities may involve securities and/or
instruments of ours or our affiliates. The Underwriter and their affiliates may also make investment recommendations and/or publish or
express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that
they acquire, long and/or short positions in such securities and instruments.
Offer
Restrictions outside the United States
Other
than in the United States, no action has been taken by us or the Underwriter that would permit a public offering of the securities offered
by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be
offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with
the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result
in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are
advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus.
This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in
any jurisdiction in which such an offer or a solicitation is unlawful.
Australia.
This prospectus is not a product disclosure statement, prospectus or other type of disclosure document for the purposes of Corporations
Act 2001 (Commonwealth of Australia) (the “Act”) and does not purport to include the information required of a product disclosure
statement, prospectus or other disclosure document under Chapter 6D.2 of the Act. No product disclosure statement, prospectus, disclosure
document, offering material or advertisement in relation to the offer of the shares has been or will be lodged with the Australian Securities
and Investments Commission or the Australian Securities Exchange.
Accordingly,
(1) the offer of the shares under this prospectus may only be made to persons in Australia: (i) to whom it is lawful to offer the shares
without disclosure to investors under Chapter 6D.2 of the Act under one or more exemptions set out in Section 708 of the Act, and (ii)
who are “wholesale clients” as that term is defined in section 761G of the Act, (2) this prospectus may only be made available
in Australia to persons as set forth in clause (1) above, and (3) by accepting this offer, the offeree represents that the offeree is
such a person as set forth in clause (1) above, and the offeree agrees not to sell or offer for sale any of the shares sold to the offeree
within 12 months after their issue except as otherwise permitted under the Act.
Canada.
The shares may not be offered, sold or distributed, directly or indirectly, in any province or territory of Canada other than the
provinces of Ontario and Quebec or to or for the benefit of any resident of any province or territory of Canada other than the provinces
of Ontario and Quebec, and only on a basis that is pursuant to an exemption from the requirement to file a prospectus in the relevant
province, and only through a dealer duly registered under the applicable securities laws of such province or in accordance with an exemption
from the applicable registered dealer requirements.
Cayman
Islands. This prospectus does not constitute a public offer of the shares, whether by way of sale or subscription, in the Cayman
Islands. The Underwriter has represented and agreed that it has not offered or sold, and will not offer or sell, directly or indirectly,
any shares to any member of the public in the Cayman Islands.
European
Economic Area. In relation to each Member State of the European Economic Area that has implemented the Prospectus Directive, or a
Relevant Member State, from and including the date on which the Prospectus Directive is implemented in that Relevant Member State, or
the Relevant Implementation Date, an offer of the shares to the public may not be made in that Relevant Member State prior to the publication
of a prospectus in relation to the shares that has been approved by the competent authority in that Relevant Member State or, where appropriate,
approved in another Relevant Member State and the competent authority in that Relevant Member State has been notified, all in accordance
with the Prospectus Directive, except that, with effect from and including the Relevant Implementation Date, an offer of the shares to
the public may be made in that Relevant Member State at any time,
| |
● |
to
legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose
corporate purpose is solely to invest in securities; |
| |
|
|
| |
● |
to
any legal entity that has two or more of (1) an average of at least 250 employees during the last financial year, (2) a total balance
sheet of more than €43,000,000, and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or
consolidated accounts; |
| |
|
|
| |
● |
to
fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive); or |
| |
|
|
| |
● |
in
any other circumstances that do not require the publication by the company of a prospectus pursuant to Article 3 of the Prospectus
Directive; |
provided
that no such offer of shares shall result in a requirement for the publication by us of a prospectus pursuant to Article 3 of the Prospectus
Directive.
For
purposes of the above provision, the expression “an offer of shares to the public” in relation to any shares in any Relevant
Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares
to be offered so as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in that Member State
by any measure implementing the Prospectus Directive in that Member State, and the expression “Prospectus Directive” means
Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
Hong
Kong. The shares may not be offered or sold by means of this document or any other document other than (i) in circumstances that
do not constitute an offer or invitation to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong) or the
Securities and Futures Ordinance (Cap.571, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of
the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances that
do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong),
and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose
of issue (in each case whether in Hong Kong or elsewhere), that is directed at, or the contents of which are likely to be accessed or
read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares which are
or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning
of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Malaysia
The
shares have not been and may not be approved by the securities commission Malaysia, or SC, and this document has not been and will not
be registered as a prospectus with the SC under the Malaysian capital markets and services act of 2007, or CMSA. Accordingly, no securities
or offer for subscription or purchase of securities or invitation to subscribe for or purchase securities are being made to any person
in or from within Malaysia under this document except to persons falling within any of paragraphs 2(g)(i) to (xi) of schedule 5 of the
CMSA and distributed only by a holder of a capital markets services license who carries on the business of dealing in securities and
subject to the issuer having lodged this prospectus with the SC within seven days from the date of the distribution of this prospectus
in Malaysia. The distribution in Malaysia of this document is subject to Malaysian laws. No action has been taken in Malaysia under its
securities laws in respect of this document. This document does not constitute and may not be used for the purpose of a public offering
or an issue, offer for subscription or purchase, invitation to subscribe for or purchase any securities requiring the approval of the
SC or the registration of a prospectus with the SC under the CMSA.
People’s
Republic of China. This prospectus may not be circulated or distributed in the PRC and the shares may not be offered or sold and
will not be offered or sold to any person for re-offering or resale directly or indirectly to any resident of the PRC except pursuant
to applicable laws and regulations of the PRC. For the purpose of this paragraph, PRC does not include Taiwan and the special administrative
regions of Hong Kong and Macau.
Singapore
The
securities which are the subject of this prospectus may not be offered or sold, nor may any document or other material in connection
with such securities be distributed, either directly or indirectly, (i) to persons in Singapore other than under circumstances in which
such offer or sale does not constitute an offer or sale of such securities to the public in Singapore or (ii) to the public or any member
of the public in Singapore other than pursuant to, and in accordance with the conditions of, an exemption invoked under division 5a or
part iv of the companies act, chapter 50 of Singapore and to persons to whom the securities may be offered or sold under such exemption.
United
Kingdom. An offer of the shares may not be made to the public in the United Kingdom within the meaning of Section 102B of the Financial
Services and Markets Act 2000, as amended, or the FSMA, except to legal entities that are authorized or regulated to operate in the financial
markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities or otherwise in circumstances
that do not require the publication by the company of a prospectus pursuant to the Prospectus Rules of the Financial Services Authority,
or the FSA.
An
invitation or inducement to engage in investment activity (within the meaning of Section 21 of FSMA) may only be communicated to persons
who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets
Act 2000 (Financial Promotion) Order 2005 or in circumstances in which Section 21 of FSMA does not apply to the company.
All
applicable provisions of the FSMA with respect to anything done by the underwriter in relation to the shares must be complied with in,
from or otherwise involving the United Kingdom.
LEGAL
MATTERS
The
validity of the shares of our common stock offered hereby has been passed upon for us by Ellenoff Grossman & Schole LLP, New York,
New York. VCL Law LLP, is acting as counsel to the Underwriter in connection with the securities offered hereby. Certain legal matters
relating to the offering as to PRC law will be passed upon for us by Sichuan Junhe Law Firm and for the Underwriter by Allbright Law
Offices.
EXPERTS
KCCW
Accountancy Corp., independent registered public accounting firm, has audited our financial statements as of and for the years ended
December 31, 2021 and 2020 as set forth in their report.
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement on Form S-1 under the Securities Act, with respect to the shares of common stock being
offered by this prospectus. This prospectus does not contain all of the information in the registration statement and its exhibits. For
further information with respect to us and the common stock offered by this prospectus, we refer you to the registration statement and
its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily
complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement.
Each of these statements is qualified in all respects by this reference.
You
can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at www.sec.gov. You may
also read and copy any document we file with the SEC at its public reference facilities at 100 F Street NE, Washington, D.C. 20549. You
may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street NE,
Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
You may also request a copy of these filings, at no cost, by writing us at Aixin Life International Inc., Hongxing International Business
Building 2, 14th FL, No. 69 Qingyun South Ave., Jinjiang District, Chengdu City, Sichuan Province, China
We
are subject to the information reporting requirements of the Exchange Act, and file reports, proxy statements and other information with
the SEC. These reports, proxy statements and other information are available for inspection and copying at the public reference room
and web site of the SEC referred to above. We also maintain a website at www.addentax.com, at which, following the closing of this offering,
you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished
to, the SEC. The information contained in, or that can be accessed through, our website incorporated by reference in, and is not part
of, this prospectus.
FINANCIAL
STATEMENTS
| Index
to Consolidated Financial Statements |
|
Page |
| Audited
Financial Statements of Aixin Life International, Inc. |
|
|
| Report of Independent Registered Public Accounting Firm (PCAOB ID: 2851) |
|
F-2 |
| Consolidated Balance Sheets as of December 31, 2021 and 2020 |
|
F-4 |
| Consolidated Statements of Operations and Comprehensive Income (Loss) for the years ended December 31, 2021 and 2020 |
|
F-5 |
| Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2021 and 2020 |
|
F-6 |
| Consolidated Statements of Cash Flows for the years ended December 31, 2021 and 2020 |
|
F-7 |
| Notes to Consolidated Financial Statements for the years ended December 31, 2021 and 2020 |
|
F-8
– F-25 |
| |
|
|
Unaudited
Financial Statements of Aixin Life International, Inc.
|
|
|
| Consolidated
Balance sheets as of June 30, 2022 and December 31, 2021 |
|
F-26 |
| Consolidated
Statements of Operations and Comprehensive Income (Loss) for the six months ended June 30, 2022 and
2021 |
|
F-27 |
| Consolidated Statements of Stockholders Equity for the six months ended June 30, 2022 and 2021 |
|
F-28 |
| Consolidated Statements of Cash Flows for the six months ended June 30, 2022 and 2021 |
|
F-29 |
| Notes
to Consolidated Financial Statements for the six months ended June 30, 2022 and 2021 |
|
F-30 – F-47 |
| |
|
|
| Audited Financial Statements of Yunnan Runcangsheng Technology Company Limited |
| Report of Independent Registered Public Accounting Firm (PCAOB ID: 2851) |
|
F-48 |
| Balance Sheets as of December 31, 2021 and 2020 |
|
F-49 |
| Statements of Operations and Comprehensive Loss for the years ended December 31, 2021 and 2020 |
|
F-50 |
| Statements of Equity for the years ended December 31, 2021 and 2020 |
|
F-51 |
| Statements of Cash Flows for the years ended December 31, 2021 and 2020 |
|
F-52 |
| Notes to Financial Statements for the years ended December 31, 2021 and 2020 |
|
F-53 – F-63 |
| |
|
|
| Unaudited Financial Statements of Yunnan Runcangsheng Technology Company Limited |
| Balance
Sheets as of June 30, 2022 and December 31, 2021 |
|
F-64 |
| Statements of Operations and Comprehensive Loss for the six months ended June 30, 2022 and 2021 |
|
F-65 |
| Statements of Stockholders Equity for the six months ended June 30, 2022 and 2021 |
|
F-66 |
| Statements of Cash Flows for the six months ended June 30, 2022 and 2021 |
|
F-67 |
Notes to Financial Statements for the six months ended June 30, 2022 and 2021
|
|
F-68 – F-78 |
| |
|
|
| Unaudited Pro Forma Combined Financial Statements as of June 30, 2022, and for the six months ended June 30, 2022 |
|
|
| Unaudited Pro Forma Consolidated Balance Sheets as of June 30, 2022 |
|
F-79 |
| Unaudited Pro Forma Consolidated Statements of Operations and Comprehensive Loss the six months ended June 30, 2022 |
|
F-80 |
| Unaudited Pro Forma Consolidated Statements of Operations and Comprehensive Loss the year ended December 31, 2021 |
|
F-81 |
| Notes to Unaudited Pro Forma Consolidated Financial Statements |
|
F-82 |

REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Board of Directors and Stockholders of
AiXin
Life International, Inc.
Opinion
on the Financial Statements
We
have audited the accompanying consolidated balance sheets of AiXin Life International, Inc. (the “Company”) as of December
31, 2021 and 2020, the related consolidated statements of income and comprehensive income (loss), stockholders’
equity, and cash flows for the years then ended, and the related notes (collectively referred to as the “consolidated financial
statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position
of the Company at December 31, 2021 and 2020, and the results of its operations and its cash flows for the years ended
December 31, 2021 and 2020, in conformity with the U.S. generally accepted accounting principles in the United States of
America.
Basis
for Opinion
These
consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion
on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public
Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company
in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission
and the PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part
of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing
an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our
audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether
due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence
regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles
used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that our audits provide a reasonable basis for our opinion.
Critical
Audit Matter
The
critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was communicated
or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial
statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters
does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit
matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Revenue
Recognition – Identifying and evaluating the timing and amount of revenue recognition
Description
of the Matter
As
described in Note 2 of the consolidated financial statements, the Company provides advertising services to its customers, and revenue
related to these services were recognized over the applicable service period. The Company also purchases products from the same customer.
The principal considerations for our determination that performing procedures relating to revenue recognition, specifically the identification
and evaluation of the timing and amount of revenue recognition, is a critical audit matter, involved judgment exercised by management
in identifying and evaluating the timing and amount of advertising revenue recognition. Auditor judgement is involved in performing our
audit procedures to evaluate whether the timing and amount of revenue recognition on advertising was appropriately stated.
How
We Addressed the Matter in Our Audit
Our
audit procedures over determining the time period over which the advertising revenue is recognized involved, among others, evaluation
of management’s assessment in regard to the timing and amount of advertising revenue recognition. We selected customer agreements
and performed the following procedures:
| |
● |
Obtained
and read the customer agreements or contracts for each selected agreement. |
| |
● |
Evaluated
and tested management’s identification of significant terms for completeness, including the identification of performance obligations. |
| |
● |
From
the terms in the customer agreement, evaluated the appropriateness of management’s application of their accounting policies,
in their determination of revenue recognition conclusions. |
For
the payment made to customer, we evaluated the application of the Company’s accounting policies in the context of applicable accounting
standards. In addition, we tested the mathematical accuracy of management’s calculations of revenue and the associated timing and
amount of revenue recognized in the consolidated financial statements.
| /s/
KCCW Accountancy Corp. |
|
We
have served as the Company’s auditor since 2019.
Diamond
Bar, California
April
15, 2022

AIXIN
LIFE INTERNATIONAL, INC.
CONSOLIDATED
BALANCE SHEETS
The
accompanying notes are an integral part of these financial statements
AIXIN
LIFE INTERNATIONAL, INC.
CONSOLIDATED
STATEMENTS OF INCOME AND COMPREHENSIVE INCOME (LOSS)
The
accompanying notes are an integral part of these financial statements
AIXIN
LIFE INTERNATIONAL, INC.
CONSOLIDATED
STATEMENTS OF STOCKHOLDERS’ EQUITY
The
accompanying notes are an integral part of these financial statements
AIXIN
LIFE INTERNATIONAL, INC.
CONSOLIDATED
STATEMENTS OF CASH FLOWS
The
accompanying notes are an integral part of these financial statements
AIXIN
LIFE INTERNATIONAL, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
1.
ORGANIZATION AND DESCRIPTION OF BUSINESS
Aixin
Life International, Inc. (the “Company” or “Aixin Life” or “we”) was incorporated under the laws
of the State of Colorado on December 30, 1987 under the name Mercari Communications Group, Ltd (“Mercari”). On February 2,
2017, Mr. Quanzhong Lin (Mr. Lin) purchased 7,380,352 shares of the Company’s common stock, 65.0% of its outstanding shares from
China Concentric Capital Group for $300,000, pursuant to a Stock Purchase Agreement dated December 21, 2016, which resulted in a change
in control of our company.
On
December 12, 2017, the Company issued 56,838,151 shares of common stock to Mr. Lin, the sole stockholder of AiXin (BVI) International
Group Co., Ltd. a British Virgin Islands corporation (“AiXin BVI”), for his shares of AiXin BVI, pursuant to a Share Exchange
Agreement.
As
a result of the Share Exchange, AiXin BVI became the Company’s wholly-owned subsidiary, and the Company now owns all of the outstanding
shares of HK AiXin International Group Co., Limited, a Hong Kong limited company (“AiXin HK”), which in turn owns all of
the outstanding shares of Chengdu AiXinZhonghong Biological Technology Co., Ltd., a Chinese limited company (“AiXinZhonghong”),
which markets and sells premium-quality nutritional products in China.
AiXin
BVI was incorporated on September 21, 2017 as a holding company and AiXin HK was established in Hong Kong on February 25, 2016 as an
intermediate holding company. AiXinZhonghong was established in the People’s Republic of China (“PRC”) on March 4,
2013, and on May 27, 2017, the local government of the PRC issued a certificate of approval regarding the foreign ownership of AiXinZhonghong
by AiXin HK. Neither AiXin BVI nor AiXin HK had operations prior to December 12, 2017.
For
accounting purposes, the acquisition of AiXin BVI was accounted for as a reverse acquisition and treated as a recapitalization of the
Company effected by a share exchange, with AiXin BVI as the accounting acquirer. Since neither AiXin BVI nor AiXin HK had operations
prior to December 12, 2017, the historical consolidated financial statements of AiXinZhonghong are now the historical consolidated financial
statements of the Company. The assets and liabilities of AiXinZhonghong were brought forward at their book value and no goodwill was
recognized.
Effective
February 1, 2018, pursuant to Articles of Amendment to the Company’s Articles of Incorporation filed with the Secretary of State
of Colorado, the Company changed its name to AiXin Life International., Inc (“Aixin Life”).
The
Company, through its indirectly owned AiXinZhonghong subsidiary, mainly develops and distributes consumer products by offering a line
of nutritional products. The Company sells the products through exhibition events, conferences, and person-to-person marketing. Beginning
in 2019, the Company began to provide advertising services to clients who engaged the Company to help distribute their products. The
Company’s business mainly focuses on a proactive approach to its customers such as hosting events for clients, which it believes
is ideally suited to marketing its products because sales of nutrition products are strengthened by ongoing personal contact and support,
coaching and education of its clients, as to the benefits of a healthy and active lifestyle.
On
May 25, 2021, AiXin HK entered into an Equity Transfer Agreement with Chengdu Aixin Shangyan Hotel Management Co., Ltd (“Aixin
Shangyan Hotel”), and its two shareholders Quanzhong Lin and Yirong Shen (“Transferor”). Pursuant to the agreement
(the “Hotel Purchase Agreement”), Aixin Life agreed to purchase 100% ownership of Aixin Shangyan Hotel from Mr. Lin and Ms.
Shen. Eighty percent of the equity of Aixin Shangyan Hotel was owned by Mr. Lin, and the remaining balance was owned by Ms. Shen. Under
the terms of the Hotel Purchase Agreement, Aixin Life agreed to purchase all of the outstanding equity of Aixin Shangyan Hotel for a
purchase price of RMB 7,598,887, or approximately $1.16 million (the “Transfer Price”). The Transfer Price will be reduced
by an amount equal to any amounts paid or distributed by Aixin Shangyan Hotel to the Transferor after December 31, 2020 and will be increased
by an amount equal to any amounts contributed to Aixin Shangyan Hotel by the Transferor after December 31, 2020. The acquisition was
completed in July 2021.
On
June 2, 2021, AiXin HK entered into an Equity Transfer Agreement with Chengdu Aixintang Pharmacy Co., Ltd. and certain affiliated entities,
each of which operates a pharmacy (together, “Aixintang Pharmacies”) and its three shareholders, Quanzhong Lin, Ting Li and
Xiao Ling Li (“Transferor”). Mr. Lin owned in excess of 95% of the outstanding equity the Aixintang Pharmacies. The remaining
equity interest was owned by Ting Li and Xiao Ling Li. Pursuant to the agreement (the “Pharmacies Purchase Agreement”), AiXin
HK agreed to purchase all of the outstanding equity of Aixintang Pharmacies for an aggregate purchase price of RMB 34,635,845, or approximately
US$5.31 million (the “Transfer Price”). The Transfer Price will be reduced by an amount equal to any amounts paid or distributed
by any of the Aixintang Pharmacies to the Transferor after December 31, 2020 and increased by an amount contributed to any of the Aixintang
Pharmacies by the Transferor after such date. The acquisition was completed in September 2021.
2.
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis
of Presentation
The
accompanying consolidated financial statements are prepared in conformity with U.S. Generally Accepted Accounting Principles (“US
GAAP”). The functional currency of AiXinZhonghong, Aixin Shangyan Hotel and Aixintang Pharmacies is Chinese Renminbi (“RMB”).
The accompanying consolidated financial statements are translated from RMB and presented in U.S. dollars (“USD”).
The
consolidated financial statements include the accounts of the Company and its current wholly owned subsidiaries, AiXin HK, AiXinZhonghong,
Aixin Shangyan Hotel and Aixintang Pharmacies. Intercompany transactions and accounts were eliminated in consolidation.
Unaudited
Interim Financial Information
Reclassification
Certain
prior period amounts have been reclassified to conform to the current period presentation and had no effect on previously reported consolidated
net income (loss) or accumulated deficit.
Covid
– 19
On
March 11, 2020, the World Health Organization announced that infections caused by the corona virus disease of 2019 (“COVID-19”)
had become pandemic. The Government of China has adopted various regulations and orders, including mandatory quarantines, limits on the
number of people that may gather in one location, closing non-essential businesses and travel bans to limit the spread of the disease.
Many of these measures have been relaxed due to the decrease in the prevalence of Covid-19 in China. However, since February 2022 to
date, COVID-19 cases have increased again in many cities of China. There has been only a slight increase in the number of cases in Sichuan
Province, the Province in which the Company is located, and the Company does not expect that the increase will impact the Company’s
operations.
Financial
impacts related to COVID-19, including the Company’s actions and costs incurred in response to the pandemic, were not material
to the Company’s financial position, results of operations or cash flows for the year ended December 31, 2021. The Company has
implemented procedures to promote employee and customer safety. These measures will not significantly increase its operating costs. However,
the Company cannot predict with certainty what measures may be taken by its suppliers and customers and the impact these measures may
have on its future financial position, results of operations or cash flows.
Use
of Estimates
In
preparing consolidated financial statements in conformity with US GAAP, management makes estimates and assumptions that affect the reported
amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the consolidated financial statements,
as well as the reported amounts of revenues and expenses during the reporting period.
Significant
estimates, required by management, include the recoverability of long-lived assets, allowance for doubtful accounts, and the reserve
for obsolete and slow-moving inventories. Actual results could differ from those estimates.
Cash
and Cash Equivalents
For
financial statement purposes, the Company considers all highly liquid investments with an original maturity of three months or less to
be cash and cash equivalents.
Restricted
Cash
The
restricted cash was for the temporary frozen of bank accounts held by Aixintang Pharmacy Co., Ltd. (“Aixintang Pharmacy”)
and its branches by the court for a complaint against the Aixintang Pharmacy while Aixintang Pharmacy is the process of appeal (see Note
17 – litigation).
Accounts
Receivable
The
Company’s policy is to maintain an allowance for potential credit losses on accounts receivable. Management reviews the composition
of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends
and changes in customer payment patterns to evaluate the adequacy of these reserves. As of December 31, 2021 and 2020, the bad debt allowance
was $213,787 and $148,520, respectively.
Inventories
Inventories
mainly consists of health supplements, drugs, pharmaceutical and nutritional products, food and beverage, hotel supplies and consumables.
Inventories are valued at the lower of average cost or market, cost being determined on a moving weighted average method at the end of
the month. Management compares the cost of inventories with the net realizable value and an allowance is made for writing down inventories
to market value, if lower. The Company recorded no inventory impairment for the years ended December 31, 2021 and 2020.
In
July 2015, the FASB issued Accounting Standards Update (“ASU”) 2015-11, “Inventory (Topic 330) - Simplifying the Measurement
of Inventory,” which requires that inventory within the scope of the guidance be measured at the lower of cost and net realizable
value. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of
completion, disposal, and transportation.
Property
and Equipment
Property
and equipment are stated at cost, less accumulated depreciation, and impairment losses, if any. Major repairs and betterments that significantly
extend original useful lives or improve productivity are capitalized and depreciated over the period benefited. Maintenance and repairs
are expensed as incurred. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation
are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided
using the straight-line method for substantially all assets with 5% salvage value and estimated lives as follows:
SCHEDULE
OF PROPERTY AND EQUIPMENT ESTIMATED LIVES
| Office furniture | |
5 years |
| Electronic equipment | |
2-3 years |
| Machinery | |
3 years |
| Leasehold improvements | |
3 years |
| Vehicles | |
5 years |
Impairment
of Long-Lived Assets
Long-lived
assets, which include property and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances
indicate that the carrying amount of an asset may not be recoverable, but at least annually.
Recoverability
of long-lived assets to be held and used is measured by comparing the carrying amount of an asset to the estimated undiscounted future
cash flows expected to be generated by it. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an
impairment charge is recognized by the amount by which the carrying amount of the asset exceeds its fair value. Fair value is generally
determined using the asset’s expected future discounted cash flows or market value, if readily determinable. Based on its review,
the Company believes that, as of December 31, 2021 and 2020, there were no significant impairments of its long-lived assets.
Income
Taxes
Income
taxes are accounted for using an asset and liability method. Under this method, deferred income taxes are recognized for the tax consequences
in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end
based on enacted tax laws and statutory tax rates, applicable to the periods in which the differences are expected to affect taxable
income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
The
Company follows Accounting Standards Codification (“ASC”) Topic 740, which prescribes a more-likely-than-not threshold for
financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740 also provides
guidance on recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities,
accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods, and income tax disclosures.
Under
ASC Topic 740, when tax returns are filed, it is likely that some positions taken would be sustained upon examination by the taxing authorities,
while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately
sustained. The benefit of a tax position is recognized in the consolidated financial statements in the period during which, based on
all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including
the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax
positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than
50% likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions
taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying
balance sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination. Interest
associated with unrecognized tax benefits is classified as interest expense and penalties are classified in selling, general and administrative
expenses in the statement of income.
At
December 31, 2021 and 2020, the Company did not take any uncertain positions that would necessitate recording a tax related liability.
Revenue
Recognition
ASU
No. 2014-09, Revenue from Contracts with Customers (“Topic 606”), became effective for the Company on January
1, 2018. The Company’s revenue recognition disclosure reflects its updated accounting policies that are affected by this new standard.
The Company applied the “modified retrospective” transition method for open contracts for the implementation of Topic
606. As revenues are and have been primarily from the delivery of products and the performance of services, and the Company has no
significant post-delivery obligations, this did not result in a material recognition of revenue on the Company’s accompanying consolidated
financial statements for the cumulative impact of applying this new standard. The Company made no adjustments to its previously-reported
total revenues, as those periods continue to be presented in accordance with its historical accounting practices under Topic 605,
Revenue Recognition.
Revenue
from sale of goods under Topic 606 is recognized in a manner that reasonably reflects the delivery of the Company’s products
and services to customers in return for expected consideration and includes the following elements:
| |
● |
executed
contract(s) with customers that the Company believes is legally enforceable; |
| |
● |
identification
of performance obligation in the respective contract; |
| |
● |
determination
of the transaction price for each performance obligation in the respective contract; |
| |
● |
allocation
of the transaction price to each performance obligation; and |
| |
● |
recognition
of revenue only when the Company satisfies each performance obligation. |
The
Company’s revenue recognition policies for its various operating segments are as follows:
Advertising
and Products
Advertising
Revenue
Commencing
in the third quarter of 2019, AiXin Zhonghong began to provide advertising services to its clients. Advertising contracts are signed
to establish the price and advertising services to be provided. Pursuant to the advertising contracts, the Company provides advertising
and marketing services to its clients through exhibition events, conferences, and person-to-person marketing. The Company performs a
credit assessment of the customer to assess the collectability of the contract price prior to entering into contracts.
Most
of the advertisement contracts designated that the Company perform such advertising services for its clients through exhibition events,
conferences, and person-to-person marketing during the contracted period, regardless of the number of such events. As such, the Company
determined that the performance obligation is satisfied over time during the contracted period and revenue is recognized accordingly.
Such advertising revenue amounted to $1,944,811 and $1,863,785 for the years ended December 31, 2021 and 2020, respectively.
A
smaller proportion of the Company’s advertising revenue is generated from services to its clients through exhibition events, conferences,
and person-to-person marketing, and charges based on the number of promotional products sold. Such advertising revenue amounted to $0
and $6,558
for the years ended
December 31, 2021 and 2020, respectively.
All
of the advertising revenue is subject to the PRC VAT of 6%. This VAT may be offset by VAT paid by the Company on raw materials and other
materials purchased in China.
Products
Revenue
The
Company’s revenue from sale of products is recognized when goods are delivered to the customer and no other obligation exists.
The Company does not provide unconditional return or other concessions to the customer. The Company’s sales policy allows for the
return of unopened products for cash after deducting certain service and transaction fees. As an alternative to the product return option,
the customers have options of asking for an exchange for products with the same value.
Sales
revenue of AiXin Zhonghong represents the invoiced value of goods, net of value-added taxes (“VAT”). All of the Company’s
products sold in China are subject to the PRC VAT of 13% since April 1, 2019. This VAT may be offset by VAT paid by the Company on raw
materials and other materials purchased in China. The Company records VAT payable and VAT receivable net of payments in the financial
statements. The VAT tax return is filed offsetting the payables against the receivables. Sales and purchases are recorded net of VAT
collected and paid as the Company acts as an agent for the government.
Hotel
Hotel
revenues are primarily derived from the rental of rooms, food and beverage sales and other ancillary goods and services, including but
not limited to souvenir, parking and conference reservation. Each of these products and services represents a distinct performance obligation
and, in exchange for these services, the Company receives fixed amounts based on published rates or negotiated contracts. Payment is
due in full at the time when the services are rendered or the goods are provided. Room rental revenue is recognized on a daily basis
when rooms are occupied. Food and beverage revenue and other goods and services revenue are recognized when they have been delivered
or rendered to the guests as the respective performance obligations are satisfied. All of the hotel’s goods sold in China are subject
to the PRC VAT of 6%. This VAT may be offset by VAT paid by the Company on raw materials and other materials purchased in China.
Pharmacies
The
Company’s retail drugstores (Aixintang Pharmacies) recognize revenue at the time the customer takes possession of the merchandise.
For pharmacy sales, each prescription claim is its own arrangement with the customer and is a performance obligation. Aixintang Pharmacies
generally receives payments from customers as it satisfies its performance obligations. The Company records a receivable when it has
an unconditional right to receive payment and only the passage of time is required before payment is due. Sales revenue represents the
invoiced value of goods, net of VAT. All of Aixintang Pharmacies’ products sold in China are eligible for the PRC VAT of 0% as
it qualifies as a small businesses.
Unearned
Revenue
The
Company’s unearned revenue primarily consists of advances received from customers for the rental of hotel rooms prior to the delivery
of service. The room rental services are delivered (normally within one year) based upon contract terms and customer demand.
Concentration
of Credit Risk
The
operations of the Company are in the PRC. Accordingly, the Company’s business, financial condition, and results of operations may
be influenced by the political, economic, and legal environments in the PRC, and by the general state of the PRC economy.
The
Company has cash on hand and demand deposits in accounts maintained with state-owned banks within the PRC. Cash in state-owned banks
is covered by insurance up to RMB 500,000 ($72,500) per bank. The Company has not experienced any losses in such accounts and believes
they are not exposed to any risks on its cash in these bank accounts.
During
the year ended December 31, 2021, the Company had two major customers that accounted for over 10% of its total revenue.
SCHEDULE
OF CONCENTRATION OF RISK BY RISK FACTORS
| Customer | |
Net
sales for the
year ended
December 31, 2021 | | |
%
of total revenue | |
| A* | |
$ | 1,286,792 | | |
| 42 | % |
| B | |
| 658,018 | | |
| 21 | % |
During
the year ended December 31, 2020, the Company had one major customer that accounted for over 10% of its total revenue.
| Customer | |
Net
sales for the
year ended
December 31, 2020 | | |
%
of total revenue | |
| A* | |
$ | 1,863,785 | | |
| 76 | % |
During
the year ended December 31, 2021, the Company had one major supplier that accounted for over 10% of its total purchases.
| Supplier | |
Net
purchases for the year ended
December 31, 2021 | | |
%
of total purchase | |
| C | |
$ | 233,187 | | |
| 40 | % |
During
the year ended December 31, 2020, the Company had three major suppliers that accounted for over 10% of its total purchases.
| Supplier | |
Net
purchase for the year ended
December 31, 2020 | | |
%
of total purchase | |
| A* | |
$ | 110,037 | | |
| 55 | % |
| D | |
| 27,225 | | |
| 14 | % |
| E | |
| 20,000 | | |
| 10 | % |
| * |
Represented
advertising revenues from this customer during the years ended December 31, 2021 and 2020. The Company also purchased inventory from
this customer during the years ended December 31, 2021 and 2020. |
Leases
The
Company adopted FASB Accounting Standards Codification, Topic 842, Leases (“ASC 842”) using the modified retrospective approach,
electing the practical expedient that allows the Company not to restate prior to the adoption of the standard on January 1, 2019.
The
Company applied the following practical expedients in the transition to the new standard allowed under ASC 842:
| Practical
Expedient |
|
Description |
| Reassessment
of expired or existing contracts |
|
The
Company elected not to reassess, at the application date, whether any expired or existing contracts contained leases, the lease classification
for any expired or existing leases, and the accounting for initial direct costs for any existing leases. |
| Use
of hindsight |
|
The
Company elected to use hindsight in determining the lease term (that is, when considering options to extend or terminate the lease
and to purchase the underlying asset) and in assessing impairment of right-to-use assets. |
| Reassessment
of existing or expired land easements |
|
The
Company elected not to evaluate existing or expired land easements that were not previously accounted for as leases under ASC 840,
as allowed under the transition practical expedient. Going forward, new or modified land easements will be evaluated under ASU No.
2016-02. |
| Separation
of lease and non-lease components |
|
Lease
agreements that contain both lease and non-lease components are generally accounted for separately. |
| Short-term
lease recognition exemption |
|
The
Company also elected the short-term lease recognition exemption and will not recognize ROU assets or lease liabilities for leases
with a term less than 12 months. |
The
Company determines if an arrangement is a lease at inception under FASB ASC Topic 842, Right of Use Assets (“ROU”) and lease
liabilities are recognized at commencement date based on the present value of remaining lease payments over the lease term. For this
purpose, the Company considers only payments that are fixed and determinable at the time of commencement. As most of its leases do not
provide an implicit rate, it uses its incremental borrowing rate based on the information available at commencement date in determining
the present value of lease payments. The Company’s incremental borrowing rate is a hypothetical rate based on its understanding
of what its credit rating would be. The ROU assets include adjustments for prepayments and accrued lease payments. The ROU asset also
includes any lease payments made prior to commencement and is recorded net of any lease incentives received. The Company’s lease
terms may include options to extend or terminate the lease when it is reasonably certain that it will exercise such options.
ROU
assets are reviewed for impairment when indicators of impairment are present. ROU assets from operating and finance leases are subject
to the impairment guidance in ASC 360, Property, Plant, and Equipment, as ROU assets are long-lived nonfinancial assets.
ROU
assets are tested for impairment individually or as part of an asset group if the cash flows related to the ROU asset are not independent
from the cash flows of other assets and liabilities. An asset group is the unit of accounting for long-lived assets to be held and used,
which represents the lowest level for which identifiable cash flows are largely independent of the cash flows of other groups of assets
and liabilities. The Company recognized no impairment of ROU assets as of December 31, 2021 and 2020. Operating leases are included in
operating lease ROU and operating lease liabilities (current and non-current), on the consolidated balance sheets.
Statement
of Cash Flows
In
accordance with ASC Topic 230, “Statement of Cash Flows,” cash flows from the Company’s operations are calculated
based on the local currencies using the average translation rates. As a result, amounts related to assets and liabilities reported on
the consolidated statements of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance
sheets.
Fair
Value of Financial Instruments
The
carrying amounts of certain of the Company’s financial instruments, including cash and equivalents, accrued liabilities and accounts
payable, approximate their fair value due to their short maturities. FASB ASC Topic 825, “Financial Instruments,” requires
disclosure of the fair value of financial instruments held by the Company. The carrying amounts reported in the consolidated balance
sheets for current liabilities each qualify as financial instruments and are a reasonable estimate of their fair value because of the
short period of time between the origination of such instruments and their expected realization and the current market rate of interest.
Fair
Value Measurements and Disclosures
ASC
Topic 820, “Fair Value Measurements and Disclosures,” defines fair value, and establishes a three-level valuation hierarchy
for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The three levels are defined
as follow:
| |
● |
Level
1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| |
● |
Level
2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that
are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| |
● |
Level
3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
As
of December 31, 2021 and 2020, the Company did not identify any assets and liabilities that are required to be presented on the balance
sheet at fair value.
Foreign
Currency Translation and Comprehensive Income (Loss)
The
functional currency of the Company is RMB. For financial reporting purposes, RMB is translated into USD as the reporting currency. Assets
and liabilities are translated at the exchange rate in effect at the balance sheet dates. Revenues and expenses are translated at the
average rate of exchange prevailing during the reporting period.
Translation
adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’
equity as “Accumulated other comprehensive income”. Gains and losses resulting from foreign currency transactions are included
in income. There was no significant fluctuation in the exchange rate for the conversion of RMB to USD after the balance sheet date.
The
Company uses FASB ASC Topic 220, “Comprehensive Income”. Comprehensive loss is comprised of net loss and all changes to the
statements of stockholders’ equity, except those due to investments by stockholders, changes in paid-in capital and distributions
to stockholders. Comprehensive income (loss) for the year ended December 31, 2021 and 2020 consisted of net income (loss) and foreign
currency translation adjustments.
Earnings
per Share
Basic
income (loss) per share is computed on the basis of the weighted average number of common shares outstanding during the period.
Dilution
is computed by applying the treasury stock method for options and warrants. Under this method, options and warrants are assumed to be
exercised at the beginning of the period (or at the time of issuance, if later), and as if funds obtained thereby were used to purchase
common stock at the average market price during the period.
As
of December 31, 2021 and 2020, the Company did not have any potentially dilutive instruments.
Stock-Based
Compensation
The
Company periodically grants stock options, warrants and awards to employees and non-employees in non-capital raising transactions as
compensation for services rendered. The Company accounts for stock option, stock warrant and stock award grants to employees based on
the authoritative guidance provided by the FASB where the value of the award is measured on the date of grant and recognized over the
vesting period. The Company accounts for stock option, stock warrant and stock award grants to non-employees in accordance with the authoritative
guidance of the FASB where the value of the stock compensation is determined based upon the measurement date at either a) the date at
which a performance commitment is reached, or b) at the date at which the necessary performance to earn the equity instruments is complete.
Stock-based compensation charges generally are amortized over the vesting period on a straight-line basis. In certain circumstances where
there are no future performance requirements by the employees and non-employees, option, warrant and award grants are immediately vested
and the total stock-based compensation charge is recorded in the period of the measurement date.
Segment
Reporting
ASC
Topic 280, “Segment Reporting,” requires use of the “management approach” model for segment reporting. The management
approach model is based on the way a company’s chief operating decision maker organizes segments within the Company for making
operating decisions assessing performance and allocating resources. Reportable segments are based on products and services, geography,
legal structure, management structure, or any other manner in which management disaggregates a company.
The
Company manages its business as three operating segments, advertising and products, pharmacies, and hotels, all of which are located
in the PRC. All of its revenues are derived in the PRC. All long-lived assets are located in PRC.
The
following table shows the Company’s operations by business segment for the year ended December 31, 2021 and 2020. Revenues and
expenses for the Pharmacies and Hotel segments commenced as of the respective dates of the completion of their acquisitions:
SCHEDULE
OF INFORMATION SEGMENTS
| | |
2021 | | |
2020 | |
| Net revenue | |
| | | |
| | |
| Advertising
and products | |
$ | 2,406,988 | | |
$ | 2,451,055 | |
| Pharmacies | |
| 280,447 | | |
| - | |
| Hotel | |
| 378,798 | | |
| - | |
| Total revenues, net | |
$ | 3,066,233 | | |
$ | 2,451,055 | |
| | |
| | | |
| | |
| Operating costs and expenses | |
| | | |
| | |
| Advertising and products | |
| | | |
| | |
| Cost of goods sold | |
$ | 316,750 | | |
$ | 224,675 | |
| Operating expenses | |
| 1,344,543 | | |
| 1,431,920 | |
| Pharmacies | |
| | | |
| | |
| Cost of goods sold | |
| 218,735 | | |
| - | |
| Operating expenses | |
| 252,513 | | |
| - | |
| Hotel | |
| | | |
| | |
| Hotel operating costs | |
| 744,594 | | |
| - | |
| Operating
expenses | |
| 216,036 | | |
| - | |
| Total operating costs
and expenses | |
$ | 3,093,171 | | |
$ | 1,656,595 | |
| | |
| | | |
| | |
| Income (loss) from operations | |
| | | |
| | |
| Advertising and products | |
$ | 745,695 | | |
$ | 794,460 | |
| Pharmacies | |
| (190,801 | ) | |
| - | |
| Hotel | |
| (581,832 | ) | |
| - | |
| Income (loss) from operations | |
$ | (26,938 | ) | |
$ | 794,460 | |
| Segment
assets | |
As
of
December 31, 2021 | | |
As
of
December 31, 2020 | |
| Advertising
and products | |
$ | 8,914,211 | | |
$ | 8,093,818 | |
| Pharmacies | |
| 931,706 | | |
| - | |
| Hotel | |
| 1,814,429 | | |
| - | |
| Total assets | |
$ | 11,660,346 | | |
$ | 8,093,818 | |
New
Accounting Pronouncements
In
June 2016, the FASB issued ASU No. 2016-13, Financial Instruments-Credit Losses (Topic 326), which requires entities to measure all expected
credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and
supportable forecasts. This replaces the existing incurred loss model and is applicable to the measurement of credit losses on financial
assets measured at amortized cost. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning
after December 15, 2022. Early application will be permitted for all entities for fiscal years, and interim periods within those fiscal
years, beginning after December 15, 2018. The Company is currently evaluating the impact that the standard will have on its consolidated
financial statements.
In
January 2017, the FASB issued ASU No. 2017-04, Simplifying the Test for Goodwill Impairment. The guidance removes Step 2 of the goodwill
impairment test, which requires a hypothetical purchase price allocation. A goodwill impairment will now be the amount by which a reporting
unit’s carrying value exceeds its FV, not to exceed the carrying amount of goodwill. The guidance should be adopted on a prospective
basis. As a smaller reporting company, the standard will be effective for the Company for interim and annual reporting periods beginning
after December 15, 2022, with early adoption permitted. The Company is currently evaluating the impact of adopting this standard on the
Company’s consolidated financial statements presentation or disclosures.
In
August 2020, the FASB issued ASU 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470- 20) and Derivatives and Hedging
- Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s
Own Equity (“ASU 2020-06”), which simplifies the accounting for certain financial instruments with characteristics of liabilities
and equity. This ASU (1) simplifies the accounting for convertible debt instruments and convertible preferred stock by removing the existing
guidance in ASC 470-20, Debt: Debt with Conversion and Other Options, that requires entities to account for beneficial conversion features
and cash conversion features in equity, separately from the host convertible debt or preferred stock; (2) revises the scope exception
from derivative accounting in ASC 815-40 for freestanding financial instruments and embedded features that are both indexed to the issuer’s
own stock and classified in stockholders’ equity, by removing certain criteria required for equity classification; and (3) revises
the guidance in ASC 260, Earnings Per Share, to require entities to calculate diluted earnings per share (EPS) for convertible instruments
by using the if-converted method. In addition, entities must presume share settlement for purposes of calculating diluted EPS when an
instrument may be settled in cash or shares. For SEC filers, excluding smaller reporting companies, ASU 2020-06 is effective for fiscal
years beginning after December 15, 2021 including interim periods within those fiscal years. Early adoption is permitted, but no earlier
than fiscal years beginning after December 15, 2020. For all other entities, ASU 2020-06 is effective for fiscal years beginning after
December 15, 2023, including interim periods within those fiscal years. Entities should adopt the guidance as of the beginning of the
fiscal year of adoption and cannot adopt the guidance in an interim reporting period. The adoption of ASU 2020-06 is not expected to
have any impact on the Company’s consolidated financial statements presentation or disclosures.
In
May 2021, the FASB issued ASU 2021-04, Earnings Per Share (Topic 260), Debt — Modifications and Extinguishments (Subtopic 470-50),
Compensation — Stock Compensation (Topic 718), and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic
815-40): Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options (“ASU
2021-04”). ASU 2021-04 provides guidance as to how an issuer should account for a modification of the terms or conditions or an
exchange of a freestanding equity-classified written call option (i.e., a warrant) that remains classified after modification or exchange
as an exchange of the original instrument for a new instrument. An issuer should measure the effect of a modification or exchange as
the difference between the fair value of the modified or exchanged warrant and the fair value of that warrant immediately before modification
or exchange and then apply a recognition model that comprises four categories of transactions and the corresponding accounting treatment
for each category (equity issuance, debt origination, debt modification, and modifications unrelated to equity issuance and debt origination
or modification). ASU 2021-04 is effective for all entities for fiscal years beginning after December 15, 2021, including interim periods
within those fiscal years. An entity should apply the guidance provided in ASU 2021-04 prospectively to modifications or exchanges occurring
on or after the effective date. Early adoption is permitted for all entities, including adoption in an interim period. If an entity elects
to early adopt ASU 2021-04 in an interim period, the guidance should be applied as of the beginning of the fiscal year that includes
that interim period. The adoption of ASU 2021-04 is not expected to have any impact on the Company’s consolidated financial statements
presentation or disclosures.
The
Company’s management does not believe that any other recently issued, but not yet effective, authoritative guidance, if currently
adopted, would have a material impact on the Company’s financial statement presentation or disclosures.
3.
OTHER RECEIVABLES AND PREPAID EXPENSES
Other
receivables and prepaid expenses consisted of the following at December 31, 2021 and 2020:
SCHEDULE OF OTHER
RECEIVABLES AND PREPAID EXPENSES
| | |
December 31,
2021 | | |
December 31,
2020 | |
| Deposits | |
$ | 68,433 | | |
$ | 6,624 | |
| Prepaid expenses | |
| 50,221 | | |
| 12,884 | |
| Employees’ social insurance | |
| 13,839 | | |
| 7,562 | |
| Others | |
| 10,788 | | |
| 5,253 | |
| Total | |
$ | 143,281 | | |
$ | 32,323 | |
4.
ADVANCES TO SUPPLIERS
The
Company had advances to suppliers of $162,969 and $155,686 as of December 31, 2021 and 2020, respectively. Advances to suppliers primarily
include prepayments for products expected to be delivered subsequent to balance sheet dates.
5.
INVENTORIES
Inventories
consisted of the following at December 31, 2021 and 2020:
SCHEDULE
OF INVENTORIES
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Finished goods – health supplements | |
$ | 6,201 | | |
$ | 45,535 | |
| Drugs, pharmaceutical and nutritional products | |
| 122,966 | | |
| - | |
| Food and beverage, hotel
supplies and consumables | |
| 104,287 | | |
| - | |
| Total | |
$ | 233,454 | | |
$ | 45,535 | |
6.
PROPERTY AND EQUIPMENT, NET
Property
and equipment consisted of the following at December 31, 2021 and 2020:
SCHEDULE OF PROPERTY AND EQUIPMENT
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Vehicles | |
$ | 295,502 | | |
$ | 288,604 | |
| Office furniture | |
| 64,263 | | |
| 48,464 | |
| Electronic equipment | |
| 22,304 | | |
| 14,852 | |
| Machinery | |
| 106,080 | | |
| - | |
| Leasehold improvements | |
| 256,548 | | |
| - | |
| Other | |
| 6,374 | | |
| - | |
| Total | |
| 751,071 | | |
| 351,920 | |
| Less: Accumulated depreciation | |
| (460,923 | ) | |
| (284,103 | ) |
| Property and equipment,
net | |
$ | 290,148 | | |
$ | 67,817 | |
Depreciation
expense for the years ended December 31, 2021 and 2020 was $95,026 and $43,462, respectively.
7.
INTANGIBLE ASSET, NET
Intangible
asset consisted of the following at December 31, 2021 and 2020:
SCHEDULE
OF INTANGIBLE ASSET
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Software | |
$ | 7,896 | | |
$ | - | |
| Less: Accumulated amortization | |
| (5,956 | ) | |
| - | |
| Intangible asset, net | |
$ | 1,940 | | |
$ | - | |
Amortization
expense for the years ended December 31, 2021 and 2020 was $1,080 and $0.
8.
TAXES PAYABLE
Taxes
payable consisted of the following at December 31, 2021 and 2020:
SCHEDULE
OF TAX PAYABLE
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Value-added | |
$ | 97,917 | | |
$ | 32,318 | |
| Income | |
| 109,396 | | |
| 235,300 | |
| City construction | |
| 7,018 | | |
| 2,422 | |
| Education | |
| 5,064 | | |
| 1,781 | |
| Other | |
| 13,242 | | |
| 11,674 | |
| Taxes payable | |
$ | 232,637 | | |
$ | 283,495 | |
9.
ACCRUED LIABILITIES AND OTHER PAYABLES
Accrued
liabilities and other payables consisted of the following at December 31, 2021 and 2020:
SCHEDULE OF ACCRUED LIABILITIES AND OTHER PAYABLES
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Accrued employees’ social
insurance | |
$ | 327,735 | | |
$ | 364,870 | |
| Accrued payroll and commission | |
| 179,183 | | |
| 105,844 | |
| Accrued rent expense | |
| 29,000 | | |
| - | |
| Construction payable | |
| 111,807 | | |
| - | |
| Accrued professional fees | |
| 50,840 | | |
| 16,927 | |
| Deposit | |
| 12,239 | | |
| - | |
| Other payables | |
| 41,596 | | |
| 26,598 | |
| Total | |
$ | 752,400 | | |
$ | 514,239 | |
10.
LOAN FROM THIRD PARTIES
As
of December 31, 2021 and 2020, the Company had advances from former shareholders and unrelated third parties of Aixin Shangyan Hotel
in an aggregate amount of $94,153 and $0, respectively. There was no written agreement, and these loans are payable on demand and bear
no interest.
11. LOAN
TO THIRD PARTY
On June
8, 2020, the Company entered into an unsecured loan agreement with a third party, pursuant to which the Company agreed to lend RMB 50,300,000,
equivalent to $7,408,389, to the third party. This loan bears interest of RMB 74,000, equivalent to $10,718, per day, and will mature
on July 28, 2020. As of December 31, 2020, the Company has received the repayment of principal and interest in full amount, and recorded
interest income of $535,906 during the year ended December 31, 2020.
12.
LEASE
Concurrent
with the completion of the sale of its rights to a portion of a building completed in 2019, the Company entered into an agreement to
lease a portion of the building back from the buyer over a lease term of 2 years. The Company accounted for this lease as an operating
lease right-of-use asset and a corresponding operating lease liability in accordance with the Lease Standard. As a result, $207,049 (RMB
1,389,731) was recorded as operating lease right-of-use asset and lease liability on March 31, 2019 when the lease commenced based on
a 4.75% discount factor. The lease agreement expired on March 31, 2021. Commencing in April, 2021, the Company continues to lease the
office on a monthly basis.
The
Company also has operating leases for other sales locations under various operating lease arrangements. The leases have remaining lease
terms of approximately 0.5 to 5 years.
Aixin
Shangyan Hotel leases its hotel premises under an operating lease arrangement. The lease has a remaining lease term of approximately
2 years.
Aixintang
Pharmacies lease retail pharmacy stores under operating lease arrangements, with remaining lease terms of 2 to 5 years.
Balance
sheet information related to the Company’s leases is presented below:
SCHEDULE OF OPERATING LEASE LIABILITIES
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Operating Leases | |
| | | |
| | |
| Operating lease right-of-use assets | |
$ | 2,049,775 | | |
$ | 100,029 | |
| | |
| | | |
| | |
| Operating lease liabilities – current | |
$ | 848,230 | | |
$ | 70,780 | |
| Operating lease liability
– non-current | |
| 1,138,710 | | |
| 29,250 | |
| Total operating lease
liabilities | |
$ | 1,986,940 | | |
$ | 100,030 | |
The
following provides details of the Company’s lease expenses:
SCHEDULE OF OPERATING LEASE EXPENSES
| | |
2021 | | |
2020 | |
| | |
Years
Ended December 31, | |
| | |
2021 | | |
2020 | |
| Operating lease expenses | |
$ | 411,607 | | |
$ | 151,730 | |
Other
information related to leases is presented below:
SCHEDULE OF OTHER INFORMATION RELATED LEASES
| | |
Years
Ended December 31, | |
| | |
2021 | | |
2020 | |
| Cash Paid For Amounts Included
In Measurement of Liabilities: | |
| | | |
| | |
| Operating cash flows from operating
leases | |
$ | 473,508 | | |
| 151,730 | |
| | |
| | | |
| | |
| Weighted Average Remaining Lease Term: | |
| | | |
| | |
| Operating leases | |
| 2.32
years | | |
| 1.37
years | |
| | |
| | | |
| | |
| Weighted Average Discount Rate: | |
| | | |
| | |
| Operating leases | |
| 4.75 | % | |
| 4.75 | % |
Maturities
of lease liabilities were as follows:
SCHEDULE OF MATURITIES OF LEASE LIABILITIES
| | |
| | |
| For the year ending December 31: | |
| |
| 2022 (excluding the six months ended June 30, 2022) | |
$ | | |
| 2022 | |
$ | 912,635 | |
| 2023 | |
| 959,650 | |
| 2024 | |
| 142,251 | |
| 2025 | |
| 52,176 | |
| 2026 | |
| 24,280 | |
| Total lease payments | |
| 2,090,992 | |
| Less: imputed interest | |
| (104,052 | ) |
| Total lease liabilities | |
| 1,986,940 | |
| Less: current portion | |
| (848,230 | ) |
| Lease liabilities –
non-current portion | |
$ | 1,138,710 | |
13.
RELATED PARTY TRANSACTIONS
Advance
to related parties
SCHEDULE OF RELATED PARTY TRANSACTIONS
Advance
to related parties consisted of the following as of the periods indicated:
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Chengdu WenJiang Aixin Nanjiang
Pharmacy Co., Ltd. | |
$ | 4,583 | | |
$ | - | |
| Qionglai Weide Pharmacy | |
| - | | |
| 10,421 | |
| Sichuan Aixin Investment Co., Ltd | |
| 4,237 | | |
| - | |
| Chengdu Xindu Cundetang Pharmacy Co., Ltd. | |
| - | | |
| 5,318 | |
| Chengdu Lisheng Huiren
Tang Pharmacy Co., Ltd. | |
| 10,235 | | |
| - | |
| Total | |
$ | 19,055 | | |
$ | 15,739 | |
Advance
from related parties
Advance
from related parties consisted of the following as of the periods indicated:
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Quanzhong Lin | |
$ | 1,822,705 | | |
$ | 258,862 | |
| Yirong Shen | |
| 97,292 | | |
| - | |
| Branch manager | |
| 1,667 | | |
| - | |
| Chengdu Aixin E-Commerce Company Ltd. | |
| 15,378 | | |
| 3,240 | |
| Chengdu Aixin International travel service
Co, Ltd | |
| 2,388 | | |
| - | |
| Chengdu Beibang Pharmacy | |
| - | | |
| 2,748 | |
| Aixin Life Beauty | |
| 7,724 | | |
| - | |
| Total | |
$ | 1,947,154 | | |
$ | 264,850 | |
All
the related party entities are controlled by Mr. Quanzhong Lin (the Chairman, President and major shareholder of Aixin Life). These advances
to and from related parties were for working capital purpose, payable on demand, and bear no interest. Yirong Shen was a major shareholder
of Aixin Shangyan Hotel prior to the closing of Hotel Purchase Agreement, and she serves as the supervisor of Aixin Shangyan Hotel.
Office
lease from a Major Shareholder
In
May 2014, the Company entered a lease with its major shareholder for an office. The lease term was for three years expiring in May 2017
with an option to renew. The monthly rent was RMB 5,000 ($774), the Company was required to prepay each year’s annual rent at 15th
of May of each year. The Company renewed the lease until May 28, 2023 with monthly rent of RMB 5,000 ($774), payable quarterly. The future
annual minimum lease payment at December 31, 2021 is $9,300 and $3,875 for each of the years ended December 31, 2022 and 2023, respectively.
14.
INCOME TAXES
The
Company was incorporated in the United States of America (“USA”) and has operations in one tax jurisdiction, i.e. the PRC.
The Company generated substantially all of its sales from its operations in the PRC for the years ended December 31, 2021 and 2020, and
recorded an income tax provision for each of such periods.
China
has a tax rate of 25% for all enterprises (including foreign-invested enterprises).
The
components of the provision for income taxes for the years ended December 31, 2021 and 2020 consisted of the following:
SCHEDULE OF COMPONENTS OF THE PROVISION FOR INCOME TAXES
| | |
2021 | | |
2020 | |
| | |
For
the Year Ended December 31, | |
| | |
2021 | | |
2020 | |
| Current: | |
| | |
| |
| China | |
$ | 292,891 | | |
$ | 340,127 | |
| Total
current | |
| 292,891 | | |
| 340,127 | |
| Deferred: | |
| | | |
| | |
| China | |
| (18,570 | ) | |
| - | |
| Total
deferred | |
| (18,570 | ) | |
| - | |
| Total income tax expense | |
$ | 274,321 | | |
$ | 340,127 | |
Deferred
tax assets as of December 31, 2021 and 2020 consisted of the following:
SCHEDULE OF DEFERRED TAX ASSETS
| | |
December
31, 2021 | | |
December
31, 2010 | |
| Deferred tax assets: | |
| | | |
| | |
| Accumulated amortization | |
$ | 18,795 | | |
$ | - | |
The
following table reconciles the statutory rates to the Company’s effective tax rate for years ended December 31, 2021 and 2020:
SCHEDULE OF EFFECTIVE INCOME TAX RATE
| | |
2021 | | |
2020 | |
| Statutory U.S. federal income tax rate | |
| 21.0 | % | |
| 21.0 | % |
| Foreign tax rate differential | |
| 216.5 | % | |
| 5.1 | % |
| Change in valuation allowances | |
| 3,634.4 | % | |
| 5.7 | % |
| Other (1) | (1) |
| - | % | |
| (6.7 | )% |
| Effective combined tax rate | |
| 3,871.9 | % | |
| 25.1 | % |
| (1) | | Primarily consists
of utilization of net operating losses in China |
As of December 31, 2021, the Company had $1,753,139
of net operating loss carry-forwards available to offset future taxable income in China from the acquisition of Aixin Shangyan Hotel
and Aixintang Pharmacies. Some of the net operating loss carry-forwards, if not utilized, will begin to expire in 2022.
Uncertain
Tax Positions
Interest
associated with unrecognized tax benefits are classified as income tax, and penalties are classified in selling, general and administrative
expenses in the statements of operations. For the years ended December 31, 2021 and 2020, the Company had no unrecognized tax benefits
and related interest and penalties expenses. Currently, the Company is not subject to examination by major tax jurisdictions.
15.
STOCKHOLDERS’ EQUITY
On
August 17, 2020, by unanimous written consent in lieu of a meeting, the Board adopted resolutions authorizing a one (1)-for-four (4)
reverse stock. The reverse stock split became effective on October 27, 2020. According to the Articles of Amendment, the Company is authorized
to issue 20,000,000 shares of blank check preferred stock at $0.001 par value and 500,000,000 shares of common stock at $.00001 par value
per share. All share and earnings per share information has been retroactively adjusted to reflect the reverse stock split.
As
of December 31, 2021 and 2020, the Company had 49,999,891 common shares issued and outstanding.
In
June 2020, 35,049,685 shares owned by Quanzhong Lin (the Chairman, President and major shareholder of Aixin Life) were cancelled.
Stock
Awards Issued for Services
On
October 22, 2019, the Company granted and issued 37,500 shares to its employees and contractors under its 2019 Equity Incentive Plan.
The stock awards were valued at $337,500 based on the post-split closing price of $9 on the grant date.
On
October 24, 2019, the Company granted and issued 550,000 shares to its employees and contractors under its 2019 Equity Incentive Plan.
The stock awards were valued at $1,520,200 based on the post-split closing price of $2.764 on the grant date.
The
stock awards will vest over five (5)
years from the grant date, and the grantee will forfeit a portion of the shares granted (“Shares Granted”) if the grantee
is no longer employed by or contracted with the Company. Specifically, the
grantee will forfeit 80% of Shares Granted if no longer employed by or contracted with the Company on the date that is one year from
the grant date, forfeit 60% of Shares Granted if no longer employed by or contracted with the Company on the date that is two years from
the grant date, forfeit 40% of Shares Granted if no longer employed by or contracted with the Company on the date that is three years
from the grant date, and forfeit 20% of Shares Granted if no longer employed by or contracted with the Company on the date that is four
years from the grant date. Effective on the 5th year from the grant date, none of the shares will be subject to forfeiture.
For
the years ended December 31, 2021 and 2020, stock-based compensation expenses were $371,540. As of December 31, 2021, unrecognized compensation
expenses related to these stock awards are $1,044,207. These expenses are expected to be recognized over 3 years.
Forgiveness
of shareholder’s loan
As
of December 31, 2021, the Company’s major shareholder Mr. Lin forgave his loan to the Company for $6,912,513. The Company recorded
this forgiveness of shareholder loan as additional paid-in capital.
Acquisition
of Subsidiaries
As
of December 31, 2021, the Company completed the acquisitions of Aixin Shangyan Hotel and Aixintang Pharmacies (see Note 1). The
acquisitions were accounted for as acquisitions of entities under common control. In connection with the acquisitions, the Company
made payments to Mr. Lin in the aggregate amount of $4.50
million, or RMB 29
million. The difference between the consideration given and the net assets received was recognized in equity, resulting in a
decrease of additional paid-in capital of $4,313,025.
16.
STATUTORY RESERVES
Pursuant
to the PRC corporate law, the Company is now only required to maintain one statutory reserve by appropriating from its after-tax profit
before declaration or payment of dividends. The statutory reserve represents restricted retained earnings.
Surplus
reserve fund
The
Company is required to transfer 10% of its net income, as determined under PRC accounting rules and regulations, to a statutory surplus
reserve fund until such reserve balance reaches 50% of the Company’s registered capital. During the years ended December 31, 2021
and 2020, the Company make $0 and $140,267 contribution to statutory reserve fund.
The
surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any,
and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion
to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance
after such issue is not less than 25% of the registered capital.
Common
welfare fund
Common
welfare fund is a voluntary fund to which the Company can elect to transfer 5% to 10% of its net income, as determined under PRC accounting
rules and regulations. The Company did not make any contribution to this fund during the years ended December 31, 2021 and 2020.
This
fund can only be utilized on capital items for the collective benefit of the Company’s employees, such as construction of dormitories,
cafeteria facilities, and other staff welfare facilities. This fund is non-distributable other than upon liquidation.
17.
OPERATING CONTINGENCIES
The
Company’s operations in the PRC are subject to specific considerations and significant risks not typically associated with companies
in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments
and foreign currency exchange. The Company’s results may be adversely affected by changes in governmental policies with respect
to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among
other things.
The
Company’s sales, purchases and expenses are denominated in RMB and all of the Company’s assets and liabilities are also denominated
in RMB. The RMB is not freely convertible into foreign currencies under the current law. In China, foreign exchange transactions are
required by law to be transacted only by authorized financial institutions. Remittances in currencies other than RMB may require certain
supporting documentation to affect the remittance.
Litigation
The
Company is, from time to time, involved in litigation incidental to the conduct of its business regarding merchandise sold, employment
matters, and litigation regarding intellectual property rights.
In
December 2020, Jian Yiao (the “Plaintiff”) filed a complaint against Chengdu Aixintang Pharmacy Co., Ltd. (“Aixintang
Pharmacy”, or the “Defendant”) in Zhangjiagang People’s Court in Jiangsu Province. The complaint alleges that
Jian Yiao is entitled to $392,305 (RMB 2,500,000) from Aixintang Pharmacy for not fulfilling the contractual obligation of a purchase
agreement entered in March 2020 (the “Purchase Agreement”). Aixintang Pharmacy claimed that the Purchase Agreement was falsely
entered by an employee through forged documents, and that Aixintang Pharmacy did not enter the Purchase Agreement. The Court determined
that Aixintang Pharmacy breached the Purchase Agreement by not delivering the products ordered and ordered Aixintang Pharmacy to pay
$392,305 (RMB 2,500,000) to the Plaintiff. In December 2020, Aixintang Pharmacy filed a motion in the Jiangsu Suzhou Intermediate
People’s Court against the determination reached from the first trial.
In
February 2021, the judge in the Jiangsu Suzhou Intermediate People’s Court denied the Defendant’s motion and upheld the judgment
from the first trial. In March 2021, Aixintang Pharmacy filed another motion to the Jiangsu High People’s Court on the basis that
the Purchase Agreement was forged. In February 2022, Aixintang Pharmacy filed an appeal in Jiangsu High People’s Court against
the judgment reached by Jiangsu Suzhou Intermediate People’s Court in February 2021. To date, this legal proceeding remains
pending.
In
November 2021, the Company and Mr. Quanzhong Lin agreed that Mr. Lin shall assume any losses arising from this legal proceeding. As such,
the Company did not accrue contingent losses from this legal proceeding as of December 31, 2021.
The
Company believes that current pending litigation will not have a material adverse effect on its consolidated financial position, results
of operations or cash flows.
18.
ACQUISITION OF SUBSIDIARIES
In
July and September, 2021, the Company completed the required governmental procedures and obtained the documents necessary to consider
the acquisitions of Aixin Shangyan Hotel and Aixintang Pharmacies completed.
Pursuant
to the Hotel Purchase Agreement dated May 25, 2021, AiXin HK purchased all of the outstanding equity of Aixin Shangyan Hotel from Mr.
Lin and the other shareholder for a purchase price of RMB 7,598,887,
or $1.16
million. The Transfer
Price will be reduced by an amount equal to any amounts paid or distributed by Aixin Shangyan Hotel to the Transferor after December
31, 2020 and will be increased by an amount equal to any amounts contributed to Aixin Shangyan Hotel by the Transferor after December
31, 2020.
Pursuant
to the Pharmacies Purchase Agreement entered on June 2, 2021, AiXin HK purchased 100%
ownership of Aixintang
Pharmacies from Mr. Lin and the other two shareholders for a purchase price of RMB 34,635,845
or $5.31
million. The purchase
price will be reduced by an amount equal to any amounts paid or distributed by any of the Aixintang Pharmacies to Mr. Lin or the other
two shareholders after December 31, 2020, and increased by an amount equal to any monies they contributed to any of the Aixintang Pharmacies
after such date.
The
acquisitions will be accounted for as acquisitions of entities under common control under ASC 805-50-15-6, and the assets and liabilities
acquired will be measured and recorded at the carrying amount under ASC 805-50-30-5. The following condensed unaudited pro forma consolidated
results of operations for the Company, Aixin Shangyan Hotel and Aixintang Pharmacies for the years ended December 31, 2021 and 2020 present
the results of operations of the Company, Aixin Shangyan Hotel and Aixintang Pharmacies as if the acquisitions occurred on January 1,
2021 and 2020, respectively. The pro forma results are not necessarily indicative of the actual results that would have occurred had
the acquisitions been completed as of the beginning of the periods presented, nor are they necessarily indicative of future consolidated
results.
SCHEDULE
OF BUSINESS ACQUISITION PRO FORMA
| | |
2021 | | |
2020 | |
| Revenue | |
$ | 4,241,991 | | |
$ | 4,789,633 | |
| Operating costs and
expenses | |
| 5,244,272 | | |
| 4,949,947 | |
| Loss from operations | |
| (1,002,281 | ) | |
| (160,314 | ) |
| Other income (expense) | |
| 100,026 | | |
| 643,458 | |
| Income tax expense | |
| 274,321 | | |
| 340,155 | |
| Net income (loss) | |
$ | (1,176,576 | ) | |
$ | 142,989 | |
19.
SUBSEQUENT EVENT
The
Company follows the guidance in FASB ASC 855-10 for the disclosure of subsequent events. The Company evaluated subsequent events through
the date the financial statements were issued and determined the Company has no material subsequent events.
AIXIN
LIFE INTERNATIONAL, INC.
CONSOLIDATED
BALANCE SHEETS
AIXIN
LIFE INTERNATIONAL, INC.
CONSOLIDATED
STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME (LOSS)
(UNAUDITED)
AIXIN
LIFE INTERNATIONAL, INC.
CONSOLIDATED
STATEMENTS OF STOCKHOLDERS’ EQUITY
(UNAUDITED)
AIXIN
LIFE INTERNATIONAL, INC.
CONSOLIDATED
STATEMENTS OF CASH FLOWS
(UNAUDITED)
AIXIN
LIFE INTERNATIONAL, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
1.
ORGANIZATION AND DESCRIPTION OF BUSINESS
Aixin
Life International, Inc. (the “Company” or “Aixin Life” or “we”) was incorporated under the laws
of the State of Colorado on December 30, 1987 under the name Mercari Communications Group, Ltd (“Mercari”). On February 2,
2017, Mr. Quanzhong Lin (Mr. Lin) purchased 7,380,352 shares of the Company’s common stock, 65.0% of its outstanding shares from
China Concentric Capital Group for $300,000, pursuant to a Stock Purchase Agreement dated December 21, 2016, which resulted in a change
in control of our company.
On
December 12, 2017, the Company issued 56,838,151 shares of common stock to Mr. Lin, the sole stockholder of AiXin (BVI) International
Group Co., Ltd. a British Virgin Islands corporation (“AiXin BVI”), for his shares of AiXin BVI, pursuant to a Share Exchange
Agreement.
As
a result of the Share Exchange, AiXin BVI became the Company’s wholly-owned subsidiary, and the Company now owns all of the outstanding
shares of HK AiXin International Group Co., Limited, a Hong Kong limited company (“AiXin HK”), which in turn owns all of
the outstanding shares of Chengdu AiXinZhonghong Biological Technology Co., Ltd., a Chinese limited company (“AiXinZhonghong”),
which markets and sells premium-quality nutritional products in China.
AiXin
BVI was incorporated on September 21, 2017 as a holding company and AiXin HK was established in Hong Kong on February 25, 2016 as an
intermediate holding company. AiXinZhonghong was established in the People’s Republic of China (“PRC”) on March 4,
2013, and on May 27, 2017, the local government of the PRC issued a certificate of approval regarding the foreign ownership of AiXinZhonghong
by AiXin HK. Neither AiXin BVI nor AiXin HK had operations prior to December 12, 2017.
For
accounting purposes, the acquisition of AiXin BVI was accounted for as a reverse acquisition and treated as a recapitalization of the
Company effected by a share exchange, with AiXin BVI as the accounting acquirer. Since neither AiXin BVI nor AiXin HK had operations
prior to December 12, 2017, the historical consolidated financial statements of AiXinZhonghong are now the historical consolidated financial
statements of the Company. The assets and liabilities of AiXinZhonghong were brought forward at their book value and no goodwill was
recognized.
Effective
February 1, 2018, the Company changed its name to AiXin Life International, Inc. (“Aixin Life”).
The
Company, through its indirectly owned AiXinZhonghong subsidiary, develops and distributes consumer products by offering a line of nutritional
products. The Company sells the products through exhibition events, conferences, and person-to-person marketing. Beginning in 2019, the
Company began to provide advertising services to clients who engaged the Company to help distribute their products. The Company’s
business mainly focuses on a proactive approach to its customers such as hosting events for clients, which it believes is ideally suited
to marketing its products because sales of nutrition products are strengthened by ongoing personal contact and support, coaching and
education of its clients, as to the benefits of a healthy and active lifestyle.
On
May 25, 2021, AiXin HK entered into an Equity Transfer Agreement (the “Hotel Purchase Agreement”) with Chengdu Aixin Shangyan
Hotel Management Co., Ltd (“Aixin Shangyan Hotel”), and its two shareholders Quanzhong Lin and Yirong Shen (“Transferor”).
Pursuant to the Hotel Purchase Agreement, Aixin Life purchased 100% ownership of Aixin Shangyan Hotel from Transferor. Eighty percent
of the equity of Aixin Shangyan Hotel was owned by Mr. Lin, and the remaining balance was owned by Ms. Shen. Under the terms of the Hotel
Purchase Agreement, Aixin Life purchased all of the outstanding equity of Aixin Shangyan Hotel for a purchase price of RMB 7,598,887,
or approximately $1.16 million (the “Transfer Price”). The Transfer Price will be reduced by an amount equal to any amounts
paid or distributed by Aixin Shangyan Hotel to the Transferor after December 31, 2020 and will be increased by an amount equal to any
amounts contributed to Aixin Shangyan Hotel by the Transferor after December 31, 2020. The acquisition was completed in July 2021.
On
June 2, 2021, AiXin HK entered into an Equity Transfer Agreement (the “Pharmacies Purchase Agreement”) with Chengdu Aixintang
Pharmacy Co., Ltd. and certain affiliated entities, each of which operates a pharmacy (together, “Aixintang Pharmacies”)
and its three shareholders, Quanzhong Lin, Ting Li and Xiao Ling Li (“Transferor”). Mr. Lin owned in excess of 95% of the
outstanding equity the Aixintang Pharmacies. The remaining equity interest was owned by Ting Li and Xiao Ling Li. Pursuant to the Pharmacies
Purchase Agreement, AiXin HK purchased all of the outstanding equity of Aixintang Pharmacies for an aggregate purchase price of RMB 34,635,845,
or approximately US$5.31 million (the “Transfer Price”). The Transfer Price will be reduced by an amount equal to any amounts
paid or distributed by any of the Aixintang Pharmacies to the Transferor after December 31, 2020 and increased by an amount contributed
to any of the Aixintang Pharmacies by the Transferor after such date. The acquisition was completed in September 2021.
2.
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis
of Presentation
The
accompanying consolidated financial statements are prepared in conformity with U.S. Generally Accepted Accounting Principles (“US
GAAP”). The functional currency of AiXinZhonghong, Aixin Shangyan Hotel and Aixintang Pharmacies is Chinese Renminbi (“RMB”).
The accompanying consolidated financial statements are translated from RMB and presented in U.S. dollars (“USD”).
The
consolidated financial statements include the accounts of the Company and its current wholly owned subsidiaries, AiXin HK, AiXinZhonghong,
Aixin Shangyan Hotel and Aixintang Pharmacies. Intercompany transactions and accounts were eliminated in consolidation.
Unaudited
Interim Financial Information
These
unaudited interim financial statements have been prepared in accordance with GAAP for interim financial reporting and the rules and regulations
of the Securities and Exchange Commission that permit reduced disclosure for interim periods. Therefore, certain information and footnote
disclosures normally included in financial statements prepared in accordance with GAAP have been condensed or omitted. In the opinion
of management, all adjustments of a normal recurring nature necessary for a fair presentation of the financial position, results of operations
and cash flows for the periods presented have been made. The results of operations for the interim periods presented are not necessarily
indicative of the results to be expected for the year ending December 31, 2022.
The
balance sheets and certain comparative information as of December 31, 2021 are derived from the audited financial statements and related
notes for the year ended December 31, 2021, included in the Company’s 2021 Annual Report on Form 10-K. These unaudited interim
financial statements should be read in conjunction with the annual consolidated financial statements and the accompanying notes contained
in our Annual Report on Form 10-K for the year ended December 31, 2021.
Covid
– 19; The Invasion of Ukraine
On
March 11, 2020, the World Health Organization announced that infections caused by the corona virus disease of 2019 (“COVID-19”)
had become pandemic. In furtherance of its zero tolerance policy, the Government of China has adopted various regulations and orders,
including mandatory quarantines, limits on the number of people that may gather in one location, closing non-essential businesses and
travel bans to limit the spread of the disease. Many of these measures have been relaxed from time to time in various localities. However,
beginning in the second half of 2021 and continuing to date, the rate of COVID-19 cases has fluctuated in China and has increased in
many provinces and cities including in Sichuan Province, where the Company is located. As a result of such increases there have been
periodic short-term lockdowns and restrictions on travel in Sichuan Province. All of the Company’s operations, in particular its
direct sales business and hotel, have been adversely impacted by the travel and work restrictions imposed on a temporary basis in China
and Chengdu to limit the spread of COVID-19.
In
response to COVID-19, the Company has implemented procedures to promote employee and customer safety. These measures will not significantly
increase its operating costs. However, the Company cannot predict with certainty what measures may be taken by its suppliers and customers
and the impact these measures may have on its future financial position, results of operations or cash flows.
The
invasion of Ukraine by the Russian Federation had an immediate impact on the global economy resulting in higher prices for oil and other
commodities. The United States, United Kingdom, European Union and other countries responded to Russia’s invasion of Ukraine by
imposing various economic sanctions and bans. Russia has responded with its own retaliatory measures. These measures have disrupted financial
and economic markets. The global impact of these measures is continually evolving and cannot be predicted with certainty and there is
no assurance that Russia’s invasion of Ukraine and responses thereto will not further disrupt the global economy and financial
markets.
While
the invasion of Ukraine and responses thereto have not interrupted the Company’s operations, these or future developments resulting
from the invasion of Ukraine could make it difficult to access debt and equity capital on attractive terms, if at all, and impact the
Company’s ability to fund business activities, including proposed acquisitions.
Use
of Estimates
In
preparing consolidated financial statements in conformity with US GAAP, management makes estimates and assumptions that affect the reported
amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the consolidated financial statements,
as well as the reported amounts of revenues and expenses during the reporting period.
Significant
estimates, required by management, include the recoverability of long-lived assets, allowance for doubtful accounts, and the reserve
for obsolete and slow-moving inventories. Actual results could differ from those estimates.
Reclassification
Certain
prior period amounts have been reclassified to conform to the current period presentation and had no effect on previously reported consolidated
net income (loss) or accumulated deficit.
Cash
and Cash Equivalents
For
financial statement purposes, the Company considers all highly liquid investments with an original maturity of three months or less to
be cash and cash equivalents.
Restricted
Cash
The
restricted cash was for the temporary frozen of bank accounts held by Aixintang Pharmacy Co., Ltd. (“Aixintang Pharmacy”)
and its branches by the court for a complaint against the Aixintang Pharmacy while Aixintang Pharmacy is in the process of appeal (see
Note 16 – litigation).
Accounts
Receivable
The
Company’s policy is to maintain an allowance for potential credit losses on accounts receivable. Management reviews the composition
of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends
and changes in customer payment patterns to evaluate the adequacy of these reserves. As of June 30, 2022 and December 31, 2021, the bad
debt allowance was $249,711 and $213,787, respectively.
Inventories
Inventories
mainly consists of health supplements, drugs, pharmaceutical and nutritional products, food and beverage, hotel supplies and
consumables. Inventories are valued at the lower of average cost or market, cost being determined on a moving weighted average
method at the end of the month. Management compares the cost of inventories with the net realizable value and an allowance is made
for writing down inventories to market value, if lower. The Company recorded no
inventory impairment for the three and six months ended June 30, 2022 and 2021.
In
July 2015, the FASB issued Accounting Standards Update (“ASU”) 2015-11, “Inventory (Topic 330) - Simplifying the Measurement
of Inventory,” which requires that inventory within the scope of the guidance be measured at the lower of cost and net realizable
value. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of
completion, disposal, and transportation.
Property
and Equipment
Property
and equipment are stated at cost, less accumulated depreciation, and impairment losses, if any. Major repairs and betterments that significantly
extend original useful lives or improve productivity are capitalized and depreciated over the period benefited. Maintenance and repairs
are expensed as incurred. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation
are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided
using the straight-line method for substantially all assets with 5% salvage value and estimated lives as follows:
SCHEDULE OF PROPERTY AND EQUIPMENT ESTIMATED LIVES
| Office furniture | |
| 5 years | |
| Electronic equipment | |
| 2-3 years | |
| Machinery | |
| 3 years | |
| Leasehold improvements | |
| 3 years | |
| Vehicles | |
| 5 years | |
Impairment
of Long-Lived Assets
Long-lived
assets, which include property and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances
indicate that the carrying amount of an asset may not be recoverable, but at least annually.
Recoverability
of long-lived assets to be held and used is measured by comparing the carrying amount of an asset to the estimated undiscounted future
cash flows expected to be generated by it. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an
impairment charge is recognized by the amount by which the carrying amount of the asset exceeds its fair value. Fair value is generally
determined using the asset’s expected future discounted cash flows or market value, if readily determinable. Based on its review,
the Company believes that, as of June 30, 2022 and December 31, 2021, there were no significant impairments of its long-lived assets.
Income
Taxes
Income
taxes are accounted for using an asset and liability method. Under this method, deferred income taxes are recognized for the tax consequences
in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end
based on enacted tax laws and statutory tax rates, applicable to the periods in which the differences are expected to affect taxable
income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
The
Company follows Accounting Standards Codification (“ASC”) Topic 740, which prescribes a more-likely-than-not threshold for
financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740 also provides
guidance on recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities,
accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods, and income tax disclosures.
Under
ASC Topic 740, when tax returns are filed, it is likely that some positions taken would be sustained upon examination by the taxing authorities,
while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately
sustained. The benefit of a tax position is recognized in the consolidated financial statements in the period during which, based on
all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including
the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax
positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than
50% likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions
taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying
balance sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination. Interest
associated with unrecognized tax benefits is classified as interest expense and penalties are classified in selling, general and administrative
expenses in the statement of income.
At
June 30, 2022 and December 31, 2021, the Company did not take any uncertain positions that would necessitate recording a tax related
liability.
Revenue
Recognition
ASU
No. 2014-09, Revenue from Contracts with Customers (“Topic 606”), became effective for the Company on January
1, 2018. The Company’s revenue recognition disclosure reflects its updated accounting policies that are affected by this new standard.
The Company applied the “modified retrospective” transition method for open contracts for the implementation of Topic
606. As revenues are and have been primarily from the delivery of products and the performance of services, and the Company has no
significant post-delivery obligations, this did not result in a material recognition of revenue on the Company’s accompanying consolidated
financial statements for the cumulative impact of applying this new standard. The Company made no adjustments to its previously-reported
total revenues, as those periods continue to be presented in accordance with its historical accounting practices under Topic 605,
Revenue Recognition.
Revenue
from sale of goods under Topic 606 is recognized in a manner that reasonably reflects the delivery of the Company’s products
and services to customers in return for expected consideration and includes the following elements:
| |
● |
executed
contract(s) with customers that the Company believes is legally enforceable; |
| |
|
|
| |
● |
identification
of performance obligation in the respective contract; |
| |
|
|
| |
● |
determination
of the transaction price for each performance obligation in the respective contract; |
| |
|
|
| |
● |
allocation
of the transaction price to each performance obligation; and |
| |
|
|
| |
● |
recognition
of revenue only when the Company satisfies each performance obligation. |
The
Company’s revenue recognition policies for its various operating segments are as follows:
Advertising
and Products
Advertising
Revenue
Commencing
in the third quarter of 2019, AiXin Zhonghong began to provide advertising services to its clients. Advertising contracts are signed
to establish the price and advertising services to be provided. Pursuant to the advertising contracts, the Company provides advertising
and marketing services to its clients through exhibition events, conferences, and person-to-person marketing. The Company performs a
credit assessment of the customer to assess the collectability of the contract price prior to entering into contracts.
Most
of the advertisement contracts designated that the Company perform such advertising services for its clients through exhibition events,
conferences, and person-to-person marketing during the contracted period, regardless of the number of such events. As such, the Company
determined that the performance obligation is satisfied over time during the contracted period and revenue is recognized accordingly.
Such advertising revenue amounted to $0 and $802,817 for the three months ended June 30, 2022 and 2021, respectively. Such advertising
revenue amounted to $0 and
$1,297,681 for
the six months ended June 30, 2022 and 2021, respectively.
All
of the advertising revenue is subject to the PRC VAT of 6%. This VAT may be offset by VAT paid by the Company on raw materials and other
materials purchased in China.
Products
Revenue
The
Company’s revenue from sale of products is recognized when goods are delivered to the customer and no other obligation exists.
The Company does not provide unconditional return or other concessions to the customer. The Company’s sales policy allows for the
return of unopened products for cash after deducting certain service and transaction fees. As an alternative to the product return option,
the customers have options of asking for an exchange for products with the same value.
Sales
revenue of AiXin Zhonghong represents the invoiced value of goods, net of value-added taxes (“VAT”). All of the Company’s
products sold in China are subject to the PRC VAT of 13% since April 1, 2019. This VAT may be offset by VAT paid by the Company on raw
materials and other materials purchased in China. The Company records VAT payable and VAT receivable net of payments in the financial
statements. The VAT tax return is filed offsetting the payables against the receivables. Sales and purchases are recorded net of VAT
collected and paid as the Company acts as an agent for the government.
Hotel
Hotel
revenues are primarily derived from the rental of rooms, food and beverage sales and other ancillary goods and services, including but
not limited to souvenir, parking and conference reservation. Each of these products and services represents a distinct performance obligation
and, in exchange for these services, the Company receives fixed amounts based on published rates or negotiated contracts. Payment is
due in full at the time when the services are rendered or the goods are provided. Room rental revenue is recognized on a daily basis
when rooms are occupied. Food and beverage revenue and other goods and services revenue are recognized when they have been delivered
or rendered to the guests as the respective performance obligations are satisfied. All of the hotel’s goods sold in China are subject
to the PRC VAT of 6%. This VAT may be offset by VAT paid by the Company on raw materials and other materials purchased in China.
Pharmacies
The
Company’s retail drugstores (Aixintang Pharmacies) recognize revenue at the time the customer takes possession of the merchandise.
For pharmacy sales, each prescription claim is its own arrangement with the customer and is a performance obligation. Aixintang Pharmacies
generally receives payments from customers as it satisfies its performance obligations. The Company records a receivable when it has
an unconditional right to receive payment and only the passage of time is required before payment is due. Sales revenue represents the
invoiced value of goods, net of VAT. All of Aixintang Pharmacies’ products sold in China are eligible for the PRC VAT of 0% as
it qualifies as a small business.
Unearned
Revenue
The
Company’s unearned revenue primarily consists of advances received from customers for the rental of hotel rooms prior to the delivery
of service. The room rental services are delivered (normally within one year) based upon contract terms and customer demand.
Concentration
of Credit Risk
The
operations of the Company are in the PRC. Accordingly, the Company’s business, financial condition, and results of operations may
be influenced by the political, economic, and legal environments in the PRC, and by the general state of the PRC economy.
The
Company has cash on hand and demand deposits in accounts maintained with state-owned banks within the PRC. Cash in state-owned banks
is covered by insurance up to RMB 500,000 ($72,500) per bank. The Company has not experienced any losses in such accounts and believes
they are not exposed to any risks on its cash in these bank accounts.
During
the three and six months ended June 30, 2022, the Company had no customer that accounted for over 10% of its total
revenue.
During
the three months ended June 30, 2021, the Company had two major customers accounted for over 10% of its total
revenue.
SCHEDULE OF CONCENTRATION OF RISK BY RISK FACTORS
| Customer | |
Net sales for the three months
ended
June 30, 2021 | | |
% of total revenue | |
| A(1) | |
$ | 511,204 | | |
| 60 | % |
| B | |
| 291,613 | | |
| 34 | % |
During the six months ended June
30, 2021, the Company had two major customers accounted for over 10% of its total revenue.
| Customer | |
Net sales for the six
months ended
June 30, 2021 | | |
% of total revenue | |
| A(1) | |
$ | 1,006,068 | | |
| 65 | % |
| B | |
| 291,613 | | |
| 19 | % |
During
the three months ended June 30, 2022, the Company had one major supplier that accounted for over 10% of its total purchases.
| Supplier | |
Net purchase for the
three months ended
June 30, 2022 | | |
% of total purchase | |
| C(2) | |
$ | 63,681 | | |
| 23 | % |
During
the six months ended June 30, 2022, the Company had one major supplier that accounted for over 10% of its total purchases.
| Supplier | |
Net purchase for the
six months ended
June 30, 2022 | | |
% of total purchase | |
| C(2) | |
$ | 63,681 | | |
| 12 | % |
During
the three months ended June 30, 2021, the Company had one major supplier accounted for over 10% of its total purchase.
| Supplier | |
Net purchase for the
three months ended
June 30, 2021 | | |
% of total purchase | |
| A(1) | |
$ | 3,940 | | |
| 91 | % |
During
the six months ended June 30, 2021, the Company had one major supplier accounted for over 10% of its total purchase.
| Supplier | |
Net purchase for the
six months ended
June 30, 2021 | | |
% of total purchase | |
| D | |
$ | 232,516 | | |
| 97 | % |
| (1) |
Represented
advertising revenues from this customer during the three and six months ended June 30, 2021. The Company also purchased inventory
from this customer in the three and six months ended June 30, 2021. |
| (2) |
The Company purchased inventory from this supplier,
Yunnan Runcangsheng, in the three and six months ended June 30, 2022. In July 2022, the Company agreed to acquire all of the
outstanding interest of Yunnan Runcangsheng (see Note 18). |
Leases
The
Company determines if an arrangement is a lease at inception under FASB ASC Topic 842, Right of Use Assets (“ROU”) and lease
liabilities are recognized at commencement date based on the present value of remaining lease payments over the lease term. For this
purpose, the Company considers only payments that are fixed and determinable at the time of commencement. As most of its leases do not
provide an implicit rate, it uses its incremental borrowing rate based on the information available at commencement date in determining
the present value of lease payments. The Company’s incremental borrowing rate is a hypothetical rate based on its understanding
of what its credit rating would be. The ROU assets include adjustments for prepayments and accrued lease payments. The ROU asset also
includes any lease payments made prior to commencement and is recorded net of any lease incentives received. The Company’s lease
terms may include options to extend or terminate the lease when it is reasonably certain that it will exercise such options.
ROU
assets are reviewed for impairment when indicators of impairment are present. ROU assets from operating and finance leases are subject
to the impairment guidance in ASC 360, Property, Plant, and Equipment, as ROU assets are long-lived nonfinancial assets.
ROU
assets are tested for impairment individually or as part of an asset group if the cash flows related to the ROU asset are not independent
from the cash flows of other assets and liabilities. An asset group is the unit of accounting for long-lived assets to be held and used,
which represents the lowest level for which identifiable cash flows are largely independent of the cash flows of other groups of assets
and liabilities. The Company recognized no impairment of ROU assets as of June 30, 2022 and December 31, 2021. Operating leases are included
in operating lease ROU and operating lease liabilities (current and non-current), on the consolidated balance sheets.
Statement
of Cash Flows
In
accordance with ASC Topic 230, “Statement of Cash Flows,” cash flows from the Company’s operations are calculated
based on the local currencies using the average translation rates. As a result, amounts related to assets and liabilities reported on
the consolidated statements of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance
sheets.
Fair
Value of Financial Instruments
The
carrying amounts of certain of the Company’s financial instruments, including cash and equivalents, accrued liabilities and accounts
payable, approximate their fair value due to their short maturities. FASB ASC Topic 825, “Financial Instruments,” requires
disclosure of the fair value of financial instruments held by the Company. The carrying amounts reported in the consolidated balance
sheets for current liabilities each qualify as financial instruments and are a reasonable estimate of their fair value because of the
short period of time between the origination of such instruments and their expected realization and the current market rate of interest.
Fair
Value Measurements and Disclosures
ASC
Topic 820, “Fair Value Measurements and Disclosures,” defines fair value, and establishes a three-level valuation hierarchy
for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The three levels are defined
as follow:
| |
● |
Level
1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| |
● |
Level
2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that
are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| |
● |
Level
3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
As
of June 30, 2022 and December 31, 2021, the Company did not identify any assets and liabilities that are required to be presented on
the balance sheet at fair value.
Foreign
Currency Translation and Comprehensive Income (Loss)
The
functional currency of the Company is RMB. For financial reporting purposes, RMB is translated into USD as the reporting currency. Assets
and liabilities are translated at the exchange rate in effect at the balance sheet dates. Revenues and expenses are translated at the
average rate of exchange prevailing during the reporting period.
Translation
adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’
equity as “Accumulated other comprehensive income”. Gains and losses resulting from foreign currency transactions are included
in income. There was no significant fluctuation in the exchange rate for the conversion of RMB to USD after the balance sheet date.
The
Company uses FASB ASC Topic 220, “Comprehensive Income”. Comprehensive loss is comprised of net loss and all changes to
the statements of stockholders’ equity, except those due to investments by stockholders, changes in paid-in capital and
distributions to stockholders. Comprehensive income (loss) for the three and six months ended June 30, 2022 and 2021
consisted of net income (loss) and foreign currency translation adjustments.
Earnings
per Share
Basic
income (loss) per share is computed on the basis of the weighted average number of common shares outstanding during the period.
Dilution
is computed by applying the treasury stock method for options and warrants. Under this method, options and warrants are assumed to be
exercised at the beginning of the period (or at the time of issuance, if later), and as if funds obtained thereby were used to purchase
common stock at the average market price during the period.
As
of June 30, 2022 and December 31, 2021, the Company did not have any potentially dilutive instruments.
Stock-Based
Compensation
The
Company periodically grants stock options, warrants and awards to employees and non-employees in non-capital raising transactions as
compensation for services rendered. The Company accounts for stock option, stock warrant and stock award grants to employees based on
the authoritative guidance provided by the FASB where the value of the award is measured on the date of grant and recognized over the
vesting period. The Company accounts for stock option, stock warrant and stock award grants to non-employees in accordance with the authoritative
guidance of the FASB where the value of the stock compensation is determined based upon the measurement date at either a) the date at
which a performance commitment is reached, or b) at the date at which the necessary performance to earn the equity instruments is complete.
Stock-based compensation charges generally are amortized over the vesting period on a straight-line basis. In certain circumstances where
there are no future performance requirements by the employees and non-employees, option, warrant and award grants are immediately vested
and the total stock-based compensation charge is recorded in the period of the measurement date.
Segment
Reporting
ASC
Topic 280, “Segment Reporting,” requires use of the “management approach” model for segment reporting. The management
approach model is based on the way a company’s chief operating decision maker organizes segments within the Company for making
operating decisions assessing performance and allocating resources. Reportable segments are based on products and services, geography,
legal structure, management structure, or any other manner in which management disaggregates a company.
The
Company manages its business as three operating segments, advertising and products, pharmacies, and hotels, all of which are located
in the PRC. All of its revenues are derived in the PRC. All long-lived assets are located in PRC.
The
following table shows the Company’s operations by business segment for the three months ended June 30, 2022 and 2021. Revenues
and expenses for the Pharmacies and Hotel segments commenced as of the respective dates of the completion of their acquisitions:
SCHEDULE OF INFORMATION SEGMENTS
| | |
2022 | | |
2021 | |
| | |
For the Three Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| Net revenue | |
| | | |
| | |
| Advertising and products | |
$ | 16,591 | | |
$ | 852,768 | |
| Pharmacies | |
| 194,045 | | |
| - | |
| Hotel | |
| 210,251 | | |
| - | |
| Total revenues, net | |
$ | 420,887 | | |
$ | 852,768 | |
| | |
| | | |
| | |
| Operating costs and expenses | |
| | | |
| | |
| Advertising and products | |
| | | |
| | |
| Cost of goods sold | |
$ | 3,735 | | |
$ | 25,021 | |
| Operating expenses | |
| 362,220 | | |
| 359,243 | |
| Pharmacies | |
| | | |
| | |
| Cost of goods sold | |
| 150,263 | | |
| - | |
| Operating expenses | |
| 153,842 | | |
| - | |
| Hotel | |
| | | |
| | |
| Hotel operating costs | |
| 418,100 | | |
| - | |
| Operating expenses | |
| 71,965 | | |
| - | |
| Total operating costs and expenses | |
$ | 1,160,125 | | |
$ | 384,264 | |
| | |
| | | |
| | |
| (Loss) income from operations | |
| | | |
| | |
| Advertising and products | |
$ | (349,364 | ) | |
$ | 468,504 | |
| Pharmacies | |
| (110,060 | ) | |
| - | |
| Hotel | |
| (279,814 | ) | |
| - | |
| (Loss) income from operations | |
$ | (739,238 | ) | |
$ | 468,504 | |
The
following table shows the Company’s operations by business segment for the six months ended June 30, 2022 and 2021. Revenues and
expenses for the Pharmacies and Hotel segments commenced as of the respective dates of the completion of their acquisitions:
| | |
2022 | | |
2021 | |
| | |
For the Six Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| Net revenue | |
| | | |
| | |
| Advertising and products | |
$ | 32,689 | | |
$ | 1,550,926 | |
| Pharmacies | |
| 352,939 | | |
| - | |
| Hotel | |
| 453,937 | | |
| - | |
| Total revenues, net | |
$ | 839,565 | | |
$ | 1,550,926 | |
| | |
| | | |
| | |
| Operating costs and expenses | |
| | | |
| | |
| Advertising and products | |
| | | |
| | |
| Cost of goods sold | |
$ | 8,418 | | |
$ | 160,680 | |
| Operating expenses | |
| 660,261 | | |
| 699,572 | |
| Pharmacies | |
| | | |
| | |
| Cost of goods sold | |
| 270,104 | | |
| - | |
| Operating expenses | |
| 323,151 | | |
| - | |
| Hotel | |
| | | |
| | |
| Hotel operating costs | |
| 929,719 | | |
| - | |
| Operating expenses | |
| 159,304 | | |
| - | |
| Total operating costs and expenses | |
$ | 2,350,957 | | |
$ | 860,252 | |
| | |
| | | |
| | |
| (Loss) income from operations | |
| | | |
| | |
| Advertising and products | |
$ | (635,990 | ) | |
$ | 690,674 | |
| Pharmacies | |
| (240,316 | ) | |
| - | |
| Hotel | |
| (635,086 | ) | |
| - | |
| (Loss) income from operations | |
$ | (1,511,392 | ) | |
$ | 690,674 | |
| Segment assets | |
As of
June 30, 2022 | | |
As of
December 31, 2021 | |
| Advertising and products | |
$ | 8,459,975 | | |
$ | 8,914,211 | |
| Pharmacies | |
| 767,817 | | |
| 931,706 | |
| Hotel | |
| 1,362,219 | | |
| 1,814,429 | |
| Total assets | |
$ | 10,590,011 | | |
$ | 11,660,346 | |
New
Accounting Pronouncements
In
June 2016, the FASB issued ASU No. 2016-13, Financial Instruments-Credit Losses (Topic 326), which requires entities to measure all expected
credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and
supportable forecasts. This replaces the existing incurred loss model and is applicable to the measurement of credit losses on financial
assets measured at amortized cost. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning
after December 15, 2022. Early application will be permitted for all entities for fiscal years, and interim periods within those fiscal
years, beginning after December 15, 2018. The Company is currently evaluating the impact that the standard will have on its consolidated
financial statements.
In
January 2017, the FASB issued ASU No. 2017-04, Simplifying the Test for Goodwill Impairment. The guidance removes Step 2 of the goodwill
impairment test, which requires a hypothetical purchase price allocation. A goodwill impairment will now be the amount by which a reporting
unit’s carrying value exceeds its FV, not to exceed the carrying amount of goodwill. The guidance should be adopted on a prospective
basis. As a smaller reporting company, the standard will be effective for the Company for interim and annual reporting periods beginning
after December 15, 2022, with early adoption permitted. The Company is currently evaluating the impact of adopting this standard on the
Company’s consolidated financial statements presentation or disclosures.
In
August 2020, the FASB issued ASU 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470- 20) and Derivatives and Hedging
- Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s
Own Equity (“ASU 2020-06”), which simplifies the accounting for certain financial instruments with characteristics of liabilities
and equity. This ASU (1) simplifies the accounting for convertible debt instruments and convertible preferred stock by removing the existing
guidance in ASC 470-20, Debt: Debt with Conversion and Other Options, that requires entities to account for beneficial conversion features
and cash conversion features in equity, separately from the host convertible debt or preferred stock; (2) revises the scope exception
from derivative accounting in ASC 815-40 for freestanding financial instruments and embedded features that are both indexed to the issuer’s
own stock and classified in stockholders’ equity, by removing certain criteria required for equity classification; and (3) revises
the guidance in ASC 260, Earnings Per Share, to require entities to calculate diluted earnings per share (EPS) for convertible instruments
by using the if-converted method. In addition, entities must presume share settlement for purposes of calculating diluted EPS when an
instrument may be settled in cash or shares. For SEC filers, excluding smaller reporting companies, ASU 2020-06 is effective for fiscal
years beginning after December 15, 2021 including interim periods within those fiscal years. Early adoption is permitted, but no earlier
than fiscal years beginning after December 15, 2020. For all other entities, ASU 2020-06 is effective for fiscal years beginning after
December 15, 2023, including interim periods within those fiscal years. Entities should adopt the guidance as of the beginning of the
fiscal year of adoption and cannot adopt the guidance in an interim reporting period. The adoption of ASU 2020-06 is not expected to
have any impact on the Company’s consolidated financial statements presentation or disclosures.
The
Company’s management does not believe that any other recently issued, but not yet effective, authoritative guidance, if currently
adopted, would have a material impact on the Company’s financial statement presentation or disclosures.
3.
OTHER RECEIVABLES AND PREPAID EXPENSES
Other
receivables and prepaid expenses consisted of the following at June 30, 2022 and December 31, 2021:
SCHEDULE
OF OTHER RECEIVABLES AND PREPAID EXPENSES
| | |
June 30, 2022 | | |
December 31, 2021 | |
| Deposits | |
$ | 16,153 | | |
$ | 68,433 | |
| Prepaid expenses | |
| 3,489 | | |
| 50,221 | |
| Employees’ social insurance | |
| 10,768 | | |
| 13,839 | |
| Others | |
| 16,292 | | |
| 10,788 | |
| Total | |
$ | 46,702 | | |
$ | 143,281 | |
4.
ADVANCES TO SUPPLIERS
The
Company had advances to suppliers of $143,421 and $162,969 as of June 30, 2022 and December 31, 2021, respectively. Advances to suppliers
primarily include prepayments for products expected to be delivered subsequent to balance sheet dates.
5.
INVENTORIES
Inventories
consisted of the following at June 30, 2022 and December 31, 2021:
SCHEDULE
OF INVENTORIES
| | |
June 30, 2022 | | |
December 31, 2021 | |
| Finished goods – health supplements | |
$ | 67,997 | | |
$ | 6,201 | |
| Drugs, pharmaceutical and nutritional products | |
| 132,684 | | |
| 122,966 | |
| Food and beverage, hotel supplies and consumables | |
| 91,237 | | |
| 104,287 | |
| Total | |
$ | 291,918 | | |
$ | 233,454 | |
6.
PROPERTY AND EQUIPMENT, NET
Property
and equipment consisted of the following at June 30, 2022 and December 31, 2021:
SCHEDULE
OF PROPERTY AND EQUIPMENT
| | |
June 30, 2022 | | |
December 31, 2021 | |
| Vehicles | |
$ | 281,146 | | |
$ | 295,502 | |
| Office furniture | |
| 61,140 | | |
| 64,263 | |
| Electronic equipment | |
| 21,220 | | |
| 22,304 | |
| Machinery | |
| 94,273 | | |
| 106,080 | |
| Leasehold improvements | |
| 244,081 | | |
| 256,548 | |
| Other | |
| 12,716 | | |
| 6,374 | |
| Total | |
| 714,576 | | |
| 751,071 | |
| Less: Accumulated depreciation | |
| (492,618 | ) | |
| (460,923 | ) |
| Property and equipment, net | |
$ | 221,958 | | |
$ | 290,148 | |
Depreciation
expense for the three months ended June 30, 2022 and 2021 was $26,947 and $3,645, respectively.
Depreciation
expense for the six months ended June 30, 2022 and 2021 was $55,925 and $10,870, respectively.
7.
INTANGIBLE ASSET, NET
Intangible
asset consisted of the following at June 30, 2022 and December 31, 2021:
SCHEDULE
OF INTANGIBLE ASSET
| | |
June 30, 2022 | | |
December 31, 2021 | |
| Software | |
$ | 7,513 | | |
$ | 7,896 | |
| Less: Accumulated amortization | |
| (6,919 | ) | |
| (5,956 | ) |
| Intangible asset, net | |
$ | 594 | | |
$ | 1,940 | |
Amortization
expense for the three months ended June 30, 2022 and 2021 was $633 and $0.
Amortization
expense for the six months ended June 30, 2022 and 2021 was $1,294 and $0.
8.
TAXES PAYABLE
Taxes
payable consisted of the following at June 30, 2022 and December 31, 2021:
SCHEDULE
OF TAX PAYABLE
| | |
June 30, 2022 | | |
December 31, 2021 | |
| Value-added | |
$ | 87,150 | | |
$ | 97,917 | |
| Income | |
| 104,094 | | |
| 109,396 | |
| City construction | |
| 6,788 | | |
| 7,018 | |
| Education | |
| 4,898 | | |
| 5,064 | |
| Other | |
| 11,831 | | |
| 13,242 | |
| Taxes payable | |
$ | 214,761 | | |
$ | 232,637 | |
9.
ACCRUED LIABILITIES AND OTHER PAYABLES
Accrued
liabilities and other payables consisted of the following at June 30, 2022 and December 31, 2021:
SCHEDULE OF ACCRUED LIABILITIES AND OTHER PAYABLES
| | |
June 30, 2022 | | |
December 31, 2021 | |
| Accrued employees’ social insurance | |
$ | 297,335 | | |
$ | 327,735 | |
| Accrued payroll and commission | |
| 302,118 | | |
| 179,183 | |
| Accrued rent expense | |
| 109,957 | | |
| 29,000 | |
| Construction payable | |
| 106,095 | | |
| 111,807 | |
| Accrued professional fees | |
| 175,107 | | |
| 50,840 | |
| Deposit | |
| 11,644 | | |
| 12,239 | |
| Other payables | |
| 49,656 | | |
| 41,596 | |
| Total | |
$ | 1,051,912 | | |
$ | 752,400 | |
10.
LOAN FROM THIRD PARTIES
As
of June 30, 2022 and December 31, 2021, the Company had advances from former shareholders and unrelated third parties of Aixin Shangyan
Hotel in an aggregate amount of $89,578 and $94,153, respectively. There was no written agreement, and these loans are payable on demand
and bear no interest.
11.
LEASE
Concurrent
with the completion of the sale of its rights to a portion of a building completed in 2019, the Company entered into an agreement to
lease a portion of the building back from the buyer over a lease term of 2 years. The Company accounted for this lease as an operating
lease right-of-use asset and a corresponding operating lease liability in accordance with the Lease Standard. As a result, $207,049 (RMB
1,389,731) was recorded as operating lease right-of-use asset and lease liability on March 31, 2019 when the lease commenced based on
a 4.75% discount factor. The lease agreement expired on March 31, 2021. Commencing in April, 2021, the Company continues to lease the
office on a monthly basis.
The
Company also has operating leases for other sales locations under various operating lease arrangements. The leases have remaining lease
terms of approximately 0.17
to 3.95
years.
Aixin
Shangyan Hotel leases its hotel premises under an operating lease arrangement. The lease has a remaining lease term of approximately 1.5 years.
Aixintang
Pharmacies lease retail pharmacy stores under operating lease arrangements, with remaining lease terms of 0.87 to 4.17 years.
Balance
sheet information related to the Company’s leases is presented below:
SCHEDULE OF OPERATING LEASE LIABILITIES
| | |
June 30, 2022 | | |
December 31, 2021 | |
| Operating Leases | |
| | | |
| | |
| Operating lease right-of-use assets | |
$ | 1,444,683 | | |
$ | 2,049,775 | |
| | |
| | | |
| | |
| Operating lease liabilities – current | |
$ | 799,839 | | |
$ | 848,230 | |
| Operating lease liability – non-current | |
| 614,791 | | |
| 1,138,710 | |
| Total operating lease liabilities | |
$ | 1,414,630 | | |
$ | 1,986,940 | |
The
following provides details of the Company’s lease expenses:
SCHEDULE OF OPERATING LEASE EXPENSES
| | |
2022 | | |
2021 | |
| | |
Three Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| Operating lease expenses | |
$ | 220,203 | | |
$ | 14,953 | |
| | |
2022 | | |
2021 | |
| | |
Six Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| Operating lease expenses | |
$ | 449,445 | | |
$ | 56,253 | |
Other
information related to leases is presented below:
SCHEDULE OF OTHER INFORMATION RELATED LEASES
| | |
Six Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| Cash Paid For Amounts Included In Measurement of Liabilities: | |
| | | |
| | |
| Operating cash flows from operating leases | |
$ | 418,711 | | |
| 56,253 | |
| | |
| | | |
| | |
| Weighted Average Remaining Lease Term: | |
| | | |
| | |
| Operating leases | |
| 1.91 years | | |
| 2.66 years | |
| | |
| | | |
| | |
| Weighted Average Discount Rate: | |
| | | |
| | |
| Operating leases | |
| 4.75 | % | |
| 4.75 | % |
Maturities
of lease liabilities were as follows:
SCHEDULE
OF MATURITIES OF LEASE LIABILITIES
| For the year ending December 31: | |
| | |
| 2022 (excluding the six months ended June 30, 2022) | |
$ | 418,055 | |
| 2023 | |
| 847,673 | |
| 2024 | |
| 131,146 | |
| 2025 | |
| 54,778 | |
| 2026 | |
| 23,100 | |
| Total lease payments | |
| 1,474,752 | |
| Less: imputed interest | |
| (60,122 | ) |
| Total lease liabilities | |
| 1,414,630 | |
| Less: current portion | |
| (799,839 | ) |
| Lease liabilities – non-current portion | |
$ | 614,791 | |
12.
RELATED PARTY TRANSACTIONS
Advance
to related parties
Advance
to related parties consisted of the following as of the periods indicated:
SCHEDULE
OF RELATED PARTY TRANSACTIONS
| | |
June 30, 2022 | | |
December 31, 2021 | |
| Chengdu WenJiang Aixin Nanjiang Pharmacy Co., Ltd. | |
$ | 8,875 | | |
$ | 4,583 | |
| Sichuan Aixin Investment Co., Ltd. | |
| 150 | | |
| 4,237 | |
| Chengdu Fuxiang Tang Pharmacy Co., Ltd. | |
| 27,303 | | |
| - | |
| Chengdu Wenjiang district Heneng hupu Pharmacy Co., Ltd | |
| 34,068 | | |
| - | |
| Chengdu Lisheng Huiren Tang Pharmacy Co., Ltd. | |
| 11,978 | | |
| 10,235 | |
| Total | |
$ | 82,374 | | |
$ | 19,055 | |
Advance
from related parties
Advance
from related parties consisted of the following as of the periods indicated:
| | |
June 30, 2022 | | |
December 31, 2021 | |
| Quanzhong Lin | |
$ | 2,317,265 | | |
$ | 1,822,705 | |
| Yirong Shen | |
| 92,564 | | |
| 97,292 | |
| Branch manager | |
| - | | |
| 1,667 | |
| Chengdu Aixin E-Commerce Company Ltd. | |
| 14,631 | | |
| 15,378 | |
| Chengdu Aixin International travel service Co, Ltd | |
| 6,678 | | |
| 2,388 | |
| Aixin Life Beauty | |
| - | | |
| 7,724 | |
| Total | |
$ | 2,431,138 | | |
$ | 1,947,154 | |
All
the related party entities are controlled by Mr. Quanzhong Lin (the Chairman, President and major shareholder of Aixin Life). These advances
to and from related parties were for working capital purpose, payable on demand, and bear no interest. Yirong Shen was a major shareholder
of Aixin Shangyan Hotel prior to the closing of Hotel Purchase Agreement, and she serves as the supervisor of Aixin Shangyan Hotel.
Office
lease from a Major Shareholder
In
May 2014, the Company entered a lease with its major shareholder for an office. The lease term was for three years expiring in May 2017
with an option to renew. The monthly rent was RMB 5,000
($789),
the Company was required to prepay each year’s annual rent at 15th of May of each year. The Company renewed the lease until May
28, 2023 with monthly
rent of RMB 5,000
($789),
payable quarterly. The future annual minimum lease payment at June 30, 2022 is $8,211
for the year ended June
30, 2023.
13.
INCOME TAXES
The
Company was incorporated in the United States of America (“USA”) and has operations in one tax jurisdiction, i.e. the
PRC. The Company generated substantially all of its sales from its operations in the PRC for the three and six months
ended June 30, 2022 and 2021, and recorded income tax provision for the periods.
China
has a tax rate of 25% for all enterprises (including foreign-invested enterprises).
Uncertain
Tax Positions
Interest
associated with unrecognized tax benefits are classified as income tax, and penalties are classified in selling, general and
administrative expenses in the statements of operations. For the three and six months ended June 30, 2022 and 2021, the
Company had no unrecognized tax benefits and related interest and penalties expenses. Currently, the Company is not
subject to examination by major tax jurisdictions.
14.
STOCKHOLDERS’ EQUITY
On
August 17, 2020, by unanimous written consent in lieu of a meeting, the Board adopted resolutions authorizing a one (1)-for-four (4)
reverse stock. The reverse stock split became effective on October 27, 2020. According to the Articles of Amendment, the Company is authorized
to issue 20,000,000 shares of blank check preferred stock at $0.001 par value and 500,000,000 shares of common stock at $.00001 par value
per share. All share and earnings per share information has been retroactively adjusted to reflect the reverse stock split.
As
of June 30, 2022 and December 31, 2021, the Company had 49,999,891 common shares issued and outstanding.
In
June 2020, 35,049,685 shares owned by Quanzhong Lin (the Chairman, President and major shareholder of Aixin Life) were cancelled.
Stock
Awards Issued for Services
On
October 22, 2019, the Company granted and issued 37,500 shares to its employees and contractors under its 2019 Equity Incentive Plan.
The stock awards were valued at $337,500 based on the post-split closing price of $9 on the grant date.
On
October 24, 2019, the Company granted and issued 550,000 shares to its employees and contractors under its 2019 Equity Incentive Plan.
The stock awards were valued at $1,520,200 based on the post-split closing price of $2.764 on the grant date.
The
stock awards will vest over five (5)
years from the grant date, and the grantee will forfeit a portion of the shares granted (“Shares Granted”) if the grantee
is no longer employed by or contracted with the Company. Specifically, the
grantee will forfeit 80% of Shares Granted if no longer employed by or contracted with the Company on the date that is one year from
the grant date, forfeit 60% of Shares Granted if no longer employed by or contracted with the Company on the date that is two years from
the grant date, forfeit 40% of Shares Granted if no longer employed by or contracted with the Company on the date that is three years
from the grant date, and forfeit 20% of Shares Granted if no longer employed by or contracted with the Company on the date that is four
years from the grant date. Effective on the 5th year from the grant date, none of the shares will be subject to forfeiture.
For the three months ended June 30,
2022 and 2021, stock-based compensation expenses were $92,885
each. For the six months ended
June 30, 2022 and 2021, stock-based compensation expenses were $185,770 each.
As of June 30, 2022, unrecognized compensation expenses related to these stock awards are $858,437.
These expenses are expected to be recognized over 3 years.
Forgiveness
of shareholder’s loan
As
of December 31, 2021, the Company’s major shareholder Mr. Lin forgave his loan to the Company for $6,912,513. The Company recorded
this forgiveness of shareholder loan as additional paid-in capital.
Acquisition
of Subsidiaries
As
of December 31, 2021, the Company completed the acquisitions of Aixin Shangyan Hotel and Aixintang Pharmacies (see Note 1). The acquisitions
were accounted for as acquisitions of entities under common control. In connection with the acquisitions, the Company made payments to
Mr. Lin in the aggregate amount of $4.50 million, or RMB 29 million. The difference between the consideration given and the net assets
received was recognized in equity, resulting in a decrease of additional paid-in capital of $4,313,025.
15.
STATUTORY RESERVES
Pursuant
to the PRC corporate law, the Company is now only required to maintain one statutory reserve by appropriating from its after-tax profit
before declaration or payment of dividends. The statutory reserve represents restricted retained earnings.
Surplus
reserve fund
The
Company is required to transfer 10%
of its net income, as determined under PRC accounting rules and regulations, to a statutory surplus reserve fund until such reserve
balance reaches 50% of the
Company’s registered capital. During the three and six months ended
June 30, 2022 and 2021, the Company make $0
and $0
contribution to statutory reserve fund.
The
surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any,
and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion
to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance
after such issue is not less than 25% of the registered capital.
Common
welfare fund
Common
welfare fund is a voluntary fund to which the Company can elect to transfer 5%
to 10%
of its net income, as determined under PRC accounting rules and regulations. The Company did not make any contribution to this fund
during the three and six months ended June 30, 2022 and 2021.
This
fund can only be utilized on capital items for the collective benefit of the Company’s employees, such as construction of dormitories,
cafeteria facilities, and other staff welfare facilities. This fund is non-distributable other than upon liquidation.
16.
OPERATING CONTINGENCIES
The
Company’s operations in the PRC are subject to specific considerations and significant risks not typically associated with companies
in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments
and foreign currency exchange. The Company’s results may be adversely affected by changes in governmental policies with respect
to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among
other things.
The
Company’s sales, purchases and expenses are denominated in RMB and all of the Company’s assets and liabilities are also denominated
in RMB. The RMB is not freely convertible into foreign currencies under the current law. In China, foreign exchange transactions are
required by law to be transacted only by authorized financial institutions. Remittances in currencies other than RMB may require certain
supporting documentation to affect the remittance.
Litigation
The
Company is, from time to time, involved in litigation incidental to the conduct of its business regarding merchandise sold, employment
matters, and litigation regarding intellectual property rights.
In
December 2020, Jian Yiao (the “Plaintiff”) filed a complaint against Chengdu Aixintang Pharmacy Co., Ltd. (“Aixintang
Pharmacy”, or the “Defendant”) in Zhangjiagang People’s Court in Jiangsu Province. The complaint alleges that
Jian Yiao is entitled to $392,305 (RMB 2,500,000) from Aixintang Pharmacy for not fulfilling the contractual obligation of a purchase
agreement entered in March 2020 (the “Purchase Agreement”). Aixintang Pharmacy claimed that the Purchase Agreement was falsely
entered by an employee through forged documents, and that Aixintang Pharmacy did not enter the Purchase Agreement. The Court determined
that Aixintang Pharmacy breached the Purchase Agreement by not delivering the products ordered and ordered Aixintang Pharmacy to pay
$392,305 (RMB 2,500,000) to the Plaintiff. In December 2020, Aixintang Pharmacy filed a motion in the Jiangsu Suzhou Intermediate People’s
Court against the determination reached from the first trial.
In
February 2021, the judge in the Jiangsu Suzhou Intermediate People’s Court denied the Defendant’s motion and upheld the judgment
from the first trial. In March 2021, Aixintang Pharmacy filed another motion to the Jiangsu High People’s Court on the basis that
the Purchase Agreement was forged. In February 2022, Aixintang Pharmacy filed an appeal in Jiangsu High People’s Court against
the judgment reached by Jiangsu Suzhou Intermediate People’s Court in February 2021. To date, this legal proceeding remains pending.
In
November 2021, the Company and Mr. Quanzhong Lin agreed that Mr. Lin shall assume any losses arising from this legal proceeding. As such,
the Company did not accrue contingent losses from this legal proceeding as of June 30, 2022.
The
Company believes that current pending litigation will not have a material adverse effect on its consolidated financial position, results
of operations or cash flows.
17.
ACQUISITION OF SUBSIDIARIES
In
July and September, 2021, the Company completed the required governmental procedures and obtained the documents necessary to consider
the acquisitions of Aixin Shangyan Hotel and Aixintang Pharmacies completed.
Pursuant
to the Hotel Purchase Agreement, AiXin HK purchased all of the outstanding equity of Aixin Shangyan Hotel from Mr. Lin and the other
shareholder for a purchase price of RMB 7,598,887, or $1.16 million. The Transfer Price will be reduced by an amount equal to any amounts
paid or distributed by Aixin Shangyan Hotel to the Transferor after December 31, 2020 and will be increased by an amount equal to any
amounts contributed to Aixin Shangyan Hotel by the Transferor after December 31, 2020.
Pursuant
to the Pharmacies Purchase Agreement, AiXin HK purchased 100% ownership of Aixintang Pharmacies from Mr. Lin and the other two shareholders
for a purchase price of RMB 34,635,845 or $5.31 million. The purchase price will be reduced by an amount equal to any amounts paid or
distributed by any of the Aixintang Pharmacies to Mr. Lin or the other two shareholders after December 31, 2020, and increased by an
amount equal to any monies they contributed to any of the Aixintang Pharmacies after such date.
The
acquisitions will be accounted for as acquisitions of entities under common control under ASC 805-50-15-6, and the assets and
liabilities acquired will be measured and recorded at the carrying amount under ASC 805-50-30-5. The following condensed unaudited
pro forma consolidated results of operations for the Company, Aixin Shangyan Hotel and Aixintang Pharmacies for the three and
six months ended June 30, 2021 present the results of operations of the Company, Aixin Shangyan Hotel and Aixintang
Pharmacies as if the acquisitions occurred on January 1, 2021, respectively. The pro forma results are not necessarily indicative of
the actual results that would have occurred had the acquisitions been completed as of the beginning of the periods presented, nor
are they necessarily indicative of future consolidated results.
SCHEDULE
OF BUSINESS ACQUISITION PRO FORMA
| | |
For the
Three Months Ended
June 30, 2021 | |
| Revenue | |
$ | 1,406,874 | |
| Operating costs and expenses | |
| 1,278,574 | |
| Income from operations | |
| 128,300 | |
| Other income | |
| 28,637 | |
| Income tax expense | |
| 218,052 | |
| Net loss | |
$ | (61,115 | ) |
| | |
For the
Six Months Ended
June 30, 2021 | |
| Revenue | |
$ | 2,601,982 | |
| Operating costs and expenses | |
| 2,716,243 | |
| Loss from operations | |
| (114,261 | ) |
| Other income | |
| 51,174 | |
| Income tax expense | |
| 218,052 | |
| Net loss | |
$ | (281,139 | ) |
18.
SUBSEQUENT EVENT
On July 19, 2022, the Company entered into an Equity
Transfer Agreement (the “Transfer Agreement”) with Yunnan Shengshengyuan Technology Co., Ltd (“Shengshengyuan”)
and Yun Chen (collectively “the Sellers”), who own 95% and 5% equity interest of Yunnan Runcangsheng Technology Co., Ltd (“Yunnan
Runcangsheng”), respectively.
Under the terms of the Transfer Agreement, the Company
agreed to purchase all of the outstanding equity interest of Yunnan Runcangsheng for an aggregate purchase price of RMB 45,082,600 or
US$6.68 million based on an exchange rate of RMB / US$ 6.75 yuan per dollar on July 19, 2022. In addition to transferring their respective
equity interest in Yunnan Runcangsheng by the Sellers, both Sellers agree to forgive any loans Yunnan Runcangsheng due to them.
If the audited net worth
of Yunnan Runcangsheng as of December 31, 2021 is less than 97% of the initial net worth (net worth prior to the audit that were used
to determine the equity transfer price), then the equity transfer price shall be reduced by an amount equal to the initial net worth minus
the audited net worth.
Effective
on September 30, 2022, both parties entered a supplemental agreement to reduce the purchase price to RMB31,557,820 (US$4.42 million).
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Board of Directors and Stockholder of
Yunnan
Runcangsheng Technology Company Limited
Opinion
on the Financial Statements
We
have audited the accompanying balance sheets of Yunnan Runcangsheng Technology Company Limited (the “Company”) as of December
31, 2021 and 2020, the related statements of operations and comprehensive loss, stockholders’ equity, and cash flows for the years
then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements
present fairly, in all material respects, the financial position of the Company at December 31, 2021 and 2020, and the results of its
operations and its cash flows for the years ended December 31, 2021 and 2020, in conformity with the U.S. generally accepted accounting
principles in the United States of America.
Basis
for Opinion
These
financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s
financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board
(United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal
securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company
is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits,
we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion
on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our
audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error
or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding
the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant
estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits
provide a reasonable basis for our opinion.
Critical
Audit Matters
Critical
audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be
communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and
(2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/
KCCW Accountancy Corp.
We
have served as the Company’s auditor since 2022.
Diamond
Bar, California
September
29, 2022
YUNNAN
RUNCANGSHENG TECHNOLOGY COMPANY LIMITED
BALANCE
SHEETS
| | |
December
31, | | |
December
31, | |
| | |
2021 | | |
2020 | |
| ASSETS | |
| | | |
| | |
| CURRENT
ASSETS | |
| | | |
| | |
| Cash
and cash equivalents | |
$ | 52,052 | | |
$ | 2,773 | |
| Accounts
receivable, net | |
| 254,271 | | |
| 8,432 | |
| Accounts
receivable - related party | |
| 231,983 | | |
| - | |
| Advance
to suppliers | |
| 30,232 | | |
| 8,278 | |
| Other
receivables and prepaid expenses | |
| 2,942 | | |
| 30,648 | |
| Inventory,
net | |
| 222,373 | | |
| 564 | |
| Total
current assets | |
| 793,853 | | |
| 50,695 | |
| | |
| | | |
| | |
| NON-CURRENT
ASSETS | |
| | | |
| | |
| Property
and equipment, net | |
| 2,072,984 | | |
| 154,104 | |
| Construction
in progress | |
| - | | |
| 84,385 | |
| Deferred
tax asset | |
| - | | |
| 5,642 | |
| Operating
lease right-of-use assets | |
| 6,219 | | |
| 11,285 | |
| Total
non-current assets | |
| 2,079,203 | | |
| 255,416 | |
| | |
| | | |
| | |
| TOTAL
ASSETS | |
$ | 2,873,056 | | |
$ | 306,111 | |
| | |
| | | |
| | |
| LIABILITIES
AND STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| CURRENT
LIABILITIES | |
| | | |
| | |
| Accounts
payable | |
$ | 258,103 | | |
$ | 4,599 | |
| Accounts
payable - related party | |
| 212,230 | | |
| - | |
| Accrued
liabilities and other payables | |
| 1,842,045 | | |
| 11,023 | |
| Advance
from customers | |
| 35,106 | | |
| - | |
| Taxes
payable | |
| 19,492 | | |
| - | |
| Operating
lease liabilities - current | |
| 16,880 | | |
| 10,723 | |
| Due
to related parties | |
| 402,397 | | |
| 159,432 | |
| Total
current liabilities | |
| 2,786,253 | | |
| 185,777 | |
| | |
| | | |
| | |
| NON-CURRENT
LIABILITIES | |
| | | |
| | |
| Operating
lease liabilities - non-current | |
| - | | |
| 5,455 | |
| Total
non-current liabilities | |
| - | | |
| 5,455 | |
| | |
| | | |
| | |
| TOTAL
LIABILITIES | |
| 2,786,253 | | |
| 191,232 | |
| | |
| | | |
| | |
| STOCKHOLDERS’
DEFICIT | |
| | | |
| | |
| Paid-in
capital | |
| 133,678 | | |
| 128,321 | |
| Accumulated
deficit | |
| (58,662 | ) | |
| (22,897 | ) |
| Accumulated
other comprehensive income | |
| 11,787 | | |
| 9,455 | |
| Total
stockholders’ equity | |
| 86,803 | | |
| 114,879 | |
| | |
| | | |
| | |
| TOTAL
LIABILITIES AND STOCKHOLDERS’ EQUITY | |
$ | 2,873,056 | | |
$ | 306,111 | |
YUNNAN
RUNCANGSHENG TECHNOLOGY COMPANY LIMITED
STATEMENTS
OF OPERATIONS AND COMPREHENSIVE LOSS
| | |
Year
Ended December 31, | |
| | |
2021 | | |
2020 | |
| | |
| | |
| |
| Revenues | |
$ | 783,149 | | |
$ | 7,304 | |
| Cost
of goods sold | |
| 445,796 | | |
| 4,551 | |
| | |
| | | |
| | |
| Gross
profit | |
| 337,353 | | |
| 2,753 | |
| | |
| | | |
| | |
| Operating
expense | |
| | | |
| | |
| Selling | |
| 61,283 | | |
| - | |
| Research
and development | |
| 53,696 | | |
| 4,129 | |
| General
and administrative | |
| 247,617 | | |
| 26,856 | |
| Total
operating expenses | |
| 362,596 | | |
| 30,985 | |
| | |
| | | |
| | |
| Loss
from operations | |
| (25,243 | ) | |
| (28,232 | ) |
| | |
| | | |
| | |
| Non-operating
income | |
| | | |
| | |
| Interest
income | |
| 56 | | |
| 6 | |
| Other
income | |
| 4 | | |
| - | |
| | |
| | | |
| | |
| Total
non-operating income | |
| 60 | | |
| 6 | |
| | |
| | | |
| | |
| Loss
before income tax | |
| (25,183 | ) | |
| (28,226 | ) |
| | |
| | | |
| | |
| Income
tax expense (benefit) | |
| 10,582 | | |
| (5,329 | ) |
| | |
| | | |
| | |
| Net
loss | |
$ | (35,765 | ) | |
$ | (22,897 | ) |
| | |
| | | |
| | |
| Other
comprehensive item | |
| | | |
| | |
| Foreign
currency translation gain | |
| 2,332 | | |
| 9,455 | |
| Comprehensive
loss | |
$ | (33,433 | ) | |
$ | (13,442 | ) |
YUNNAN
RUNCANGSHENG TECHNOLOGY COMPANY LIMITED
STATEMENTS OF STOCKHOLDERS’ EQUITY
| | |
| | |
| | |
Accumulated
other | | |
| |
| | |
Paid
in | | |
Accumulated | | |
comprehensive | | |
| |
| | |
capital | | |
deficit | | |
income | | |
Total | |
| Balance
at April 17, 2020 (inception) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| Capital
contribution | |
| 128,321 | | |
| - | | |
| - | | |
| 128,321 | |
| Net
loss | |
| - | | |
| (22,897 | ) | |
| - | | |
| (22,897 | ) |
| Foreign
currency translation | |
| | | |
| - | | |
| 9,455 | | |
| 9,455 | |
| Balance
at December 31, 2020 | |
| 128,321 | | |
| (22,897 | ) | |
| 9,455 | | |
| 114,879 | |
| Capital
contribution | |
| 5,357 | | |
| - | | |
| - | | |
| 5,357 | |
| Net
loss | |
| - | | |
| (35,765 | ) | |
| - | | |
| (35,765 | ) |
| Foreign
currency translation | |
| - | | |
| - | | |
| 2,332 | | |
| 2,332 | |
| Balance
at December 31, 2021 | |
$ | 133,678 | | |
$ | (58,662 | ) | |
$ | 11,787 | | |
$ | 86,803 | |
YUNNAN
RUNCANGSHENG TECHNOLOGY, INC
STATEMENTS OF CASH FLOWS
| | |
Year
Ended December 31, | |
| | |
2021 | | |
2020 | |
| | |
| | |
| |
| CASH
FLOWS FROM OPERATING ACTIVITIES | |
| | | |
| | |
| Net
loss | |
$ | (35,765 | ) | |
$ | (22,897 | ) |
| Adjustments
to reconcile net loss to net cash provided by (used in) operating activities: |
| Depreciation | |
| 121,936 | | |
| 60 | |
| Provision
for allowance for doubtful accounts | |
| 176,715 | | |
| - | |
| Operating
lease expense | |
| 5,584 | | |
| 4,622 | |
| Deferred
tax | |
| 5,704 | | |
| (5,329 | ) |
| Changes
in operating assets and liabilities: | |
| | | |
| | |
| Accounts
receivable | |
| (419,382 | ) | |
| (7,964 | ) |
| Accounts
receivable - related party | |
| (229,174 | ) | |
| - | |
| Advance
to suppliers | |
| (21,496 | ) | |
| (7,819 | ) |
| Other
receivable and prepaid expenses | |
| 28,081 | | |
| (28,948 | ) |
| Inventory | |
| (219,109 | ) | |
| (533 | ) |
| Accounts
payable | |
| 250,327 | | |
| 4,344 | |
| Accounts
payable - related party | |
| 209,660 | | |
| - | |
| Accrued
liabilities and other payables | |
| 1,808,593 | | |
| 10,412 | |
| Advance
from customers | |
| 34,681 | | |
| - | |
| Taxes
payable | |
| 19,256 | | |
| - | |
| Net
cash provided by (used in) operating activities | |
| 1,735,611 | | |
| (54,052 | ) |
| | |
| | | |
| | |
| CASH
FLOWS FROM INVESTING ACTIVITIES | |
| | | |
| | |
| Construction
in progress | |
| - | | |
| (79,706 | ) |
| Purchase
of equipment | |
| (1,928,683 | ) | |
| (145,619 | ) |
| Net
cash used in investing activities | |
| (1,928,683 | ) | |
| (225,325 | ) |
| | |
| | | |
| | |
| CASH
FLOWS FROM FINANCING ACTIVITIES | |
| | | |
| | |
| Proceeds
from related parties | |
| 236,324 | | |
| 150,592 | |
| Capital
contribution | |
| 5,364 | | |
| 131,406 | |
| Net
cash provided by financing activities | |
| 241,688 | | |
| 281,998 | |
| | |
| | | |
| | |
| Effect
of exchange rate changes on cash and cash equivalents | |
| 663 | | |
| 152 | |
| | |
| | | |
| | |
| Net
increase in cash and cash equivalents | |
| 49,279 | | |
| 2,773 | |
| | |
| | | |
| | |
| Cash
and cash equivalents - beginning of year | |
| 2,773 | | |
| - | |
| Cash
and cash equivalents - end of year | |
$ | 52,052 | | |
$ | 2,773 | |
| | |
| | | |
| | |
| Supplemental
Cash Flow Disclosures | |
| | | |
| | |
| Cash
paid for interest | |
$ | - | | |
$ | - | |
| Cash
paid for income taxes | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Supplemental
Non-Cash Investing and Financing Activities | |
| | | |
| | |
| Transfer
of construction in progress to property and equipment | |
$ | 853,211 | | |
$ | - | |
YUNNAN
RUNCANGSHENG TECHNOLOGY COMPANY LIMITED
NOTES
TO FINANCIAL STATEMENTS
1.
ORGANIZATION AND DESCRIPTION OF BUSINESS
Yunnan
Runcangsheng Technology Company Limited (the “Company” or “Runcangsheng”), was incorporated on April 17, 2020
in Kunming City, Yunnan Province in China. The Company is engaged in biotechnology research and consultation, and the manufacturing and
sales of health supplement products.
2.
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis
of Presentation
The
accompanying financial statements are
prepared in conformity with U.S. Generally Accepted Accounting Principles (“US GAAP”). The functional currency of the
Company is Chinese Renminbi (“RMB”). The accompanying financial statements are
translated from RMB and presented in U.S. dollars (“USD”).
Covid
– 19
On
March 11, 2020, the World Health Organization announced that infections caused by the corona virus disease of 2019 (“COVID-19”)
had become pandemic. The Government of China has adopted various regulations and orders, including mandatory quarantines, limits on the
number of people that may gather in one location, closing non-essential businesses, and travel bans to limit the spread of the disease.
Many of these measures have been relaxed due to the decrease in the prevalence of Covid-19 in China. To date, the ongoing operations
of the Company have not been materially adversely affected by the measures taken to limit the spread of the disease in China.
Financial
impacts related to COVID-19, including the Company’s actions and costs incurred in response to the pandemic, were not material
to the Company’s financial position, results of operations or cash flows for the years ended December 31, 2021 and 2020. The Company
has implemented procedures to promote employee and customer safety. These measures will not significantly increase its operating costs.
Use
of Estimates
In
preparing financial statements in
conformity with US GAAP, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures
of contingent assets and liabilities at the dates of the financial statements, as well as
the reported amounts of revenues and expenses during the reporting period.
Significant
estimates, required by management, include the recoverability of long-lived assets, allowance for doubtful accounts, and the reserve
for obsolete and slow-moving inventories. Actual results could differ from those estimates.
Cash
and Cash Equivalents
For
financial statement purposes, the Company considers all highly liquid investments with an original maturity of three months or less to
be cash and cash equivalents.
Accounts
Receivable and Allowance for Doubtful Accounts
Accounts
receivable mainly consist of amounts due from customers. The Company’s policy is to maintain an allowance for potential credit
losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer
concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy
of these reserves. As of December 31, 2021 and 2020, allowance for doubtful accounts was $178,881 and
$0, respectively.
Advance
to Suppliers
The
Company makes advance payment to certain vendors for purchase of raw material, tools and equipment for production. The advances are interest-free
and unsecured. As of December 31, 2021 and 2020, the Company had advance to suppliers of $30,232 and $8,278 respectively.
Inventory
Inventories
are valued at the lower of cost or net realizable value using the weighted average cost method. Management compares the cost of inventories
with the net realizable value and an allowance is made for writing down inventories to market value, if lower. The Company recorded no
inventory impairment for the years ended December
31, 2021 and 2020.
Property
and Equipment
Property
and equipment are stated at cost, less accumulated depreciation, and impairment losses, if any. Major repairs and betterments that
significantly extend original useful lives or improve productivity are capitalized and depreciated over the period benefited.
Maintenance and repairs are expensed as incurred. When property and equipment are retired or otherwise disposed of, the related cost
and accumulated depreciation are removed from the respective accounts, and any gain or loss is included in operations. Depreciation
of property and equipment is provided using the straight-line method for substantially all assets with 5% salvage value and
estimated lives as follows:
| Office
equipment and furniture |
1~5
years |
| Machinery
and equipment |
10
years |
| Leasehold
improvement |
5
years |
| Vehicle |
5
years |
Impairment
of Long-Lived Assets
Long-lived
assets, which include property and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances
indicate that the carrying amount of an asset may not be recoverable, but at least annually.
Recoverability
of long-lived assets to be held and used is measured by comparing the carrying amount of an asset to the estimated undiscounted future
cash flows expected to be generated by it. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an
impairment charge is recognized by the amount by which the carrying amount of the asset exceeds its fair value. Fair value is generally
determined using the asset’s expected future discounted cash flows or market value, if readily determinable. Based on its review,
the Company believes that, as of December 31, 2021 and 2020, there was no significant impairment of its long-lived assets.
Income
Taxes
Income
taxes are accounted for using an asset and liability method. Under this method, deferred income taxes are recognized for the tax consequences
in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end
based on enacted tax laws and statutory tax rates, applicable to the periods in which the differences are expected to affect taxable
income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
The
Company follows Accounting Standards Codification (“ASC”) Topic 740, which prescribes a more-likely-than-not threshold for
financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740 also provides
guidance on recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities,
accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods, and income tax disclosures.
Under
ASC Topic 740, when tax returns are filed, it is likely that some positions taken would be sustained upon examination by the taxing authorities,
while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately
sustained. The benefit of a tax position is recognized in the financial statements in the period during which, based on all available
evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution
of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that
meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50% likely of
being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken
that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying balance
sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination. Interest associated
with unrecognized tax benefits is classified as interest expense and penalties are classified in selling, general and administrative
expenses in the statement of operations.
At
December 31, 2021 and 2020, the Company did not take any uncertain positions that would necessitate recording a tax related liability.
Revenue
Recognition
In
accordance with ASC 606, revenue is recognized upon the transfer of control of promised goods or services to customers in an amount that
reflects the consideration the Company expects to receive in exchange for those products or services.
Revenue
from sale of goods under Topic 606 is recognized in a manner that reasonably reflects the delivery of the Company’s
products and services to customers in return for expected consideration and includes the following elements:
| |
● |
executed
contract(s) with customers that the Company believes is legally enforceable; |
| |
|
|
| |
● |
identification
of performance obligation in the respective contract; |
| |
|
|
| |
● |
determination
of the transaction price for each performance obligation in the respective contract; |
| |
|
|
| |
● |
allocation
of the transaction price to each performance obligation; and |
| |
|
|
| |
● |
recognition
of revenue only when the Company satisfies each performance obligation. |
The
Company recognizes revenue at the time the products are shipped as it satisfies its performance obligations. The Company records a receivable
for the sales when it has an unconditional right to receive payment and only the passage of time is required before payment is due.
Sales
revenue represents the invoiced value of goods, net of value-added taxes (“VAT”). All of the Company’s products sold
in China are subject to the PRC VAT of 13%. The VAT may be offset by VAT paid by the Company on inventories and products purchased in
China. The Company records VAT payable and VAT receivable net of payments in the financial statements. The VAT tax return is filed offsetting
the payables against the receivables. Sales and purchases are recorded net of VAT collected and paid as the Company acts as an agent
for the government.
Cost
of Goods Sold
Cost
of goods sold consists primarily of the cost of products manufactured during the reporting period, mainly including direct labor, raw
material and overhead costs. Reserve for inventory allowance due to lower of cost or market is also recorded in cost of goods sold.
Research
and Development Costs
Research
and development costs are expensed as incurred and included in general and administrative expenses. These costs primarily consist of
cost of materials used, salaries paid to the Company’s development team, and fees paid to third parties. Research and development
expenses for the years ended December 31, 2021 and 2020 were $53,696 and $4,129, respectively.
Concentration
of Credit Risk
The
operations of the Company are in the PRC. Accordingly, the Company’s business, financial condition, and results of operations may
be influenced by the political, economic, and legal environments in the PRC, and by the general state of the PRC economy.
The
Company has cash on hand and demand deposits in accounts maintained with state-owned banks within the PRC. Cash in state-owned banks
is covered by insurance up to RMB 500,000 ($72,500) per bank. The Company has not experienced any losses in such accounts and believes
they are not exposed to any risks on its cash in these bank accounts.
Leases
ASC
Topic 842, “Leases,” requires recognition of leases on the balance sheets as right-of-use (“ROU”)
assets and lease liabilities. ROU assets represent the Company’s right to use underlying assets for the lease terms and lease liabilities
represent the Company’s obligation to make lease payments arising from the leases. Operating lease ROU assets and operating lease
liabilities are recognized based on the present value and future minimum lease payments over the lease term at commencement date. The
Company’s future minimum based payments used to determine the Company’s lease liabilities mainly include minimum based rent
payments. As most of the Company’s leases do not provide an implicit rate, the Company uses its estimated incremental borrowing
rate based on the information available at commencement date in determining the present value of lease payments.
Operating
lease cost is recognized as a single lease cost on a straight-line basis over the lease term and is recorded in selling, general and
administrative expenses. Variable lease payments for common area maintenance, property taxes and other operating expenses are recognized
as expense in the period when the changes in facts and circumstances on which the variable lease payments are based occur.
Related
Parties
Parties
are considered to be related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are
controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management,
members of the immediate families of principal owners of the Company and its management and other parties with which the Company may
deal with if one party controls or can significantly influence the management or operating policies of the other to an extent that one
of the transacting parties might be prevented from fully pursuing its own separate interests. The Company discloses all significant related
party transactions.
Statement
of Cash Flows
In
accordance with ASC Topic 230, “Statement of Cash Flows,” cash flows from the Company’s operations
are calculated based on the local currencies using the average translation rates. As a result, amounts related to assets and liabilities
reported on the statements of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheets.
Fair
Value of Financial Instruments
The
carrying amounts of certain of the Company’s financial instruments, including cash and equivalents, accrued liabilities and accounts
payable, approximate their fair value due to their short maturities. FASB ASC Topic 825, “Financial Instruments,” requires
disclosure of the fair value of financial instruments held by the Company. The carrying amounts reported in the balance sheets for current
liabilities each qualify as financial instruments and are a reasonable estimate of their fair value because of the short period of time
between the origination of such instruments and their expected realization and the current market rate of interest.
Fair
Value Measurements and Disclosures
ASC
Topic 820, “Fair Value Measurements and Disclosures,” defines fair value, and establishes a three-level
valuation hierarchy for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The three
levels are defined as follow:
| |
● |
Level
1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| |
● |
Level
2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that
are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| |
● |
Level
3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
As
of December 31, 2021 and 2020, the Company did not identify any assets and liabilities that are required to be presented on the balance
sheet at fair value.
Foreign
Currency Translation and Comprehensive Income (Loss)
The
functional currency of the Company is RMB. For financial reporting purposes, RMB is translated into USD as the reporting currency. Assets
and liabilities are translated at the exchange rate in effect at the balance sheet dates. Revenues and expenses are translated at the
average rate of exchange prevailing during the reporting period.
Translation
adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’
equity as “Accumulated other comprehensive income.” Gains and losses resulting from foreign currency transactions are included
in income. There was no significant fluctuation in the exchange rate for the conversion of RMB to USD after the balance sheet date.
The
Company uses FASB ASC Topic 220, “Comprehensive Income”. Comprehensive income (loss) is comprised of net income
(loss) and all changes to the statements of stockholders’ equity, except those due to investments by stockholders, changes in paid-in
capital and distributions to stockholders. Comprehensive income (loss) for the years ended December
31, 2021 and 2020 consisted of net income (loss) and foreign currency translation adjustments.
Earnings
per Share
The
Company is a limited Company (“LC”) formed under the laws of the PRC. Like limited liability company in the US, limited company
in the PRC do not issue shares to the owners. The owners however, are called shareholders. Ownership interest is determined in proportion
to capital contributed. Accordingly, earnings per share data is not presented.
Segment
Reporting
ASC
Topic 280, “Segment Reporting,” requires use of the “management approach” model for segment
reporting. The management approach model is based on the way a company’s chief operating decision maker organizes segments within
the Company for making operating decisions assessing performance and allocating resources. Reportable segments are based on products
and services, geography, legal structure, management structure, or any other manner in which management disaggregates a company.
Management
determined the Company’s operations constitute a single reportable segment in accordance with ASC 280. The Company operates exclusively
in one business and industry segment: health supplemental products manufacture and sale.
New
Accounting Pronouncements
In
June 2016, the FASB issued ASU No. 2016-13, Financial Instruments-Credit Losses (Topic 326), which requires entities to measure all expected
credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and
supportable forecasts. This replaces the existing incurred loss model and is applicable to the measurement of credit losses on financial
assets measured at amortized cost. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning
after December 15, 2022. Early application is permitted for all entities for fiscal years, and interim periods within those fiscal years,
beginning after December 15, 2018. The Company is currently evaluating the impact that the standard will have on its financial statements.
In
March 2020, the FASB issued ASU 2020-04, Reference Rate Reform (Topic 848) (“ASU 2020-04”). ASU 2020-04 contains practical
expedients for reference rate reform related activities that impact debt, leases, derivatives and other contracts. The guidance in ASU
2020-04 is optional and may be elected over time as reference rate reform activities occur. The Company continues to evaluate the impact
of the guidance and may apply the elections as applicable as changes in the market occur.
3.
OTHER RECEIVABLES AND PREPAID EXPENSES
The
Company had other receivables and prepaid expenses of $2,942 and $30,648 as of December 31, 2021 and 2020, respectively, which mainly
consisted of prepaid VAT.
4.
ACCOUNTS RECEIVABLES, NET
Accounts
receivable, net consisted of the following:
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Accounts
receivable – third parties | |
$ | 433,152 | | |
$ | 8,432 | |
| Less:
allowance for doubtful accounts | |
| (178,881 | ) | |
| - | |
| Accounts
receivable, net | |
$ | 254,271 | | |
$ | 8,432 | |
The
following table presents the activities in the allowance for doubtful accounts for the years ended December 31, 2021 and 2020.
| | |
2021 | | |
2020 | |
| Balance,
beginning of period | |
$ | - | | |
$ | - | |
| Provision
for doubtful debts | |
| 178,881 | | |
| - | |
| Balance,
end of period | |
$ | 178,881 | | |
$ | - | |
Provision
for allowance were recognized $178,881 and $0 during the years ended December31, 2021 and 2020, respectively.
5.
INVENTORY
Inventory
consisted of the following at December 31,
2021 and 2020:
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Raw
materials | |
$ | 44,399 | | |
$ | 564 | |
| Finished
goods | |
| 177,974 | | |
| - | |
| Total | |
$ | 222,373 | | |
$ | 564 | |
6.
PROPERTY AND EQUIPMENT
Property
and equipment consisted of the following at December 31, 2021 and 2020:
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Office
equipment and furniture | |
$ | 25,079 | | |
$ | - | |
| Leasehold
improvement | |
| 977,644 | | |
| - | |
| Machinery
and equipment | |
| 1,078,399 | | |
| 55,675 | |
| Vehicle | |
| 112,050 | | |
| 98,492 | |
| Other | |
| 3,307 | | |
| - | |
| Total | |
| 2,196,479 | | |
| 154,167 | |
| Less:
Accumulated depreciation | |
| (123,495 | ) | |
| (63 | ) |
| Property
and equipment, net | |
$ | 2,072,984 | | |
$ | 154,104 | |
Depreciation
expense for the years ended December 31, 2021 and 2020 was $121,936 and $60, respectively.
7.
CONSTRUCTION IN PROGRESS
As
of December 31, 2021 and December 31, 2020, the Company had construction in progress of $0 and $84,385, respectively, which was for constructing
the Biological Resources GMP Purification workshop. The construction was completed in 2021 and was transferred to the Company’s
fixed assets.
8.
TAXES PAYABLE
Taxes
payable mainly consisted of VAT and corporate income tax payable of $19,492 and $0 as of December 31, 2021 and 2020.
9.
ACCRUED LIABILITIES AND OTHER PAYABLES
Accrued
liabilities and other payables consisted of the following at December
31, 2021 and 2020:
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Accrued
payroll | |
$ | 18,245 | | |
$ | 1,679 | |
| Payable
for construction and improvement | |
| 1,642,570 | | |
| - | |
| Payable
for equipment purchase | |
| 180,857 | | |
| 9,344 | |
| Other | |
| 373 | | |
| - | |
| Total | |
$ | 1,842,045 | | |
$ | 11,023 | |
The
payable for construction and improvement was mainly for the payment of workshop construction and biological resources purification workshop
project. Total construction costs for workshop construction were $1.84 million. All construction has been completed in 2021.
10.
LEASE
The
Company leases its office under an operating lease arrangement with a related party, with the lease term of 3 years from March 1, 2020
through February 28, 2023. The monthly rent is RMB 3,000 ($470), payable every six month in advance.
Balance
sheet information related to the Company’s leases is presented below:
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Operating
Leases | |
| | | |
| | |
| Operating
lease right-of-use assets | |
$ | 6,219 | | |
$ | 11,285 | |
| | |
| | | |
| | |
| Operating
lease liabilities - current | |
$ | 16,880 | | |
$ | 10,723 | |
| Operating
lease liabilities – non-current | |
| - | | |
| 5,455 | |
| Total
operating lease liabilities | |
$ | 16,880 | | |
$ | 16,178 | |
The
following provides details of the Company’s lease expenses:
| | |
Years
Ended December 31, | |
| | |
2021 | | |
2020 | |
| Operating
lease expenses | |
$ | 5,584 | | |
$ | 4,622 | |
Other
information related to leases is presented below:
| | |
Years
Ended December 31, | |
| | |
2021 | | |
2020 | |
| Cash
Paid For Amounts Included In Measurement of Liabilities: | |
| | |
| |
| Operating
cash flows from operating leases | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Weighted
Average Remaining Lease Term: | |
| | | |
| | |
| Operating
leases | |
| 1.16
years | | |
| 2.16
years | |
| | |
| | | |
| | |
| Weighted
Average Discount Rate: | |
| | | |
| | |
| Operating
leases | |
| 4.75 | % | |
| 4.75 | % |
Maturities
of lease liabilities were as follows:
| For
the year ending December 31, | |
| |
| 2022 | |
$ | 16,945 | |
| Less:
imputed interest | |
| 65 | |
| Total
lease liabilities | |
$ | 16,880 | |
11.
RELATED PARTY TRANSACTIONS
Accounts
receivable – related party
As
of December 31, 2021 and 2020,
the Company had accounts receivable due from related party in the amount of $231,983 and $0, respectively.
As
of December 31, 2021, accounts receivable of $231,983 was due from Yunnan Shengshengyuan Technology Co., Ltd. (“Shengshengyuan”),
the 95% shareholder of the Company.
Accounts
payable– related party
As
of December 31, 2021 and 2020,
the Company had accounts payable due to related party in the amount of $212,230 and $0, respectively.
As
of December 31, 2021,
the Company had accounts payable of $212,230 due to Luquan Shengcaofeng Biotechnology Co., Ltd. (“Shengcaofeng”), an entity
controlled by the ultimate major shareholder of the Company.
Due
to related parties
| | |
December
31, 2021 | | |
December
31, 2020 | |
| Yunnan
Shengshengyuan Technology Inc (95% shareholder of the Company) | |
$ | 61,191 | | |
$ | 59,787 | |
| Huiliang
Jiao (ultimate major shareholder of the Company) | |
| 325,516 | | |
| 84,315 | |
| Xiaoyan
Zhou (spouse of the ultimate major shareholder) | |
| 15,690 | | |
| 15,330 | |
| Total | |
$ | 402,397 | | |
$ | 159,432 | |
Due
to related parties represented the advance from the related parties for the Company’s operating needs. Due to related parties were
unsecured, bear no interest and payable upon demand.
12.
INCOME TAXES
The
Company is governed by the Income Tax Laws of the PRC and various local tax laws. Effective January 1, 2008, China adopted a uniform
tax rate of 25% for all enterprises (including foreign-invested enterprises).
Provision
for income tax expense (benefit) consists of the following:
| | |
For
the Year Ended December
31, | |
| | |
2021 | | |
2020 | |
| Current | |
| | |
| |
| China | |
$ | 4,877 | | |
$ | - | |
| Deferred | |
| | | |
| | |
| China | |
| 5,705 | | |
| (5,329 | ) |
| Total
provision for income tax expense (benefit) | |
$ | 10,582 | | |
$ | (5,329 | ) |
The
following table reconciles the PRC statutory rate to the Company’s effective tax rate for years ended December 31, 2021 and 2020:
| | |
2021 | | |
2020 | |
| Income
tax (benefit) at PRC statutory rate | |
| (25.0 | )% | |
| (25.0 | )% |
| Nondeductible
items | |
| 263.4 | % | |
| 6.1 | % |
| Excess
research and development deduction (1) | |
| (51.9 | )% | |
| - | % |
| Tax
credit (2) | |
| (144.5 | )% | |
| - | % |
| Effective
income tax rate | |
| 42.0 | % | |
| (18.9 | )% |
| (1) | During
the year ended December 31, 2021, the Company qualifies for excess deduction related to research
and development costs incurred. |
| (2) | During
the year ended December 31, 2021, the Company is qualified for tax credit for Small and Low-profit
Enterprises as a result of the COVID-19 tax relief. |
13.
STATUTORY RESERVES
Pursuant
to the PRC corporate law, the Company is now only required to maintain one statutory reserve by appropriating from its after-tax profit
before declaration or payment of dividends. The statutory reserve represents restricted retained earnings.
Surplus
reserve fund
The
Company is now required to transfer 10% of its net income, as determined under PRC accounting rules and regulations, to a statutory surplus
reserve fund until such reserve balance reaches 50% of the Company’s registered capital. During the years ended December 31, 2021
and 2020, the Company made $0 to its statutory reserve fund.
The
surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any,
and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion
to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance
after such issue is not less than 25% of the registered capital.
Common
welfare fund
Common
welfare fund is a voluntary fund to which the Company can elect to transfer 5% to 10% of its net income, as determined under PRC accounting
rules and regulations. The Company did not make any contribution to this fund during the years ended December 31, 2021 and 2020.
This
fund can only be utilized on capital items for the collective benefit of the Company’s employees, such as construction of dormitories,
cafeteria facilities, and other staff welfare facilities. This fund is non-distributable other than upon liquidation.
14.
OPERATING CONTINGENCIES
The
Company’s operations in the PRC are subject to specific considerations and significant risks not typically associated with companies
in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments
and foreign currency exchange. The Company’s results may be adversely affected by changes in governmental policies with respect
to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among
other things.
The
Company’s sales, purchases and expenses are denominated in RMB and all of the Company’s assets and liabilities are also denominated
in RMB. The RMB is not freely convertible into foreign currencies under the current law. In China, foreign exchange transactions are
required by law to be transacted only by authorized financial institutions. Remittances in currencies other than RMB may require certain
supporting documentation to affect the remittance.
The
Company is, from time to time, involved in litigation incidental to the conduct of its business regarding merchandise sold, employment
matters, and litigation regarding intellectual property rights.
The
Company believes that current pending litigation will not have a material adverse effect on its financial position, results of operations
or cash flows.
15.
CONCENTRATIONS
Customers
During
the year ended December 31, 2021, the Company had two major customers that accounted for over 10% of its total revenue.
| Customer | |
Net
sales for the
year ended
December 31, 2021 | | |
%
of total revenue | | |
Accounts
receivable as of December
31, 2021 | |
| A* | |
$ | 229,174 | | |
| 29 | % | |
$ | 231,983 | |
| B | |
| 108,795 | | |
| 14 | % | |
| 113,810 | |
*
The Company had sales to and accounts receivable due from Shengshengyuan, a related party (see Note 11).
During
the year ended December 31, 2020, the Company had one major customer that accounted for over 10% of its total revenue.
| Customer | |
Net
sales for the
year ended
December 31, 2020 | | |
%
of total revenue | | |
Accounts
receivable as of December
31, 2020 | |
| C | |
$ | 6,407 | | |
| 88 | % | |
$ | 7,665 | |
Suppliers
During
the year ended December 31, 2021, the Company had two major suppliers that accounted for over 10% of its total purchase.
| Supplier | |
Net
purchase for the
year ended
December 31, 2021 | | |
%
of total purchase | | |
Accounts
payable as
of December
31, 2021 | |
| D* | |
$ | 226,332 | | |
| 57 | % | |
$ | 212,230 | |
| E | |
| 85,568 | | |
| 22 | % | |
| 171,729 | |
*
The Company had purchase from and accounts payable to Shengcaofeng, a related party (see Note 11).
During
the year ended December 31, 2020, the Company had one major supplier that accounted for over 10% of its total purchase.
| Supplier | |
Net
purchase for the
year ended
December 31, 2021 | | |
%
of total purchase | | |
Accounts
payable as
of December
31, 2021 | |
| F | |
$ | 4,344 | | |
| 100 | % | |
$ | 4,599 | |
16.
SUBSEQUENT EVENTS
On
December 1, 2021, the Company and Luquan Yizu Miaozu Autonomous County People’s Government (“the People’s Government”)
entered into a cooperation agreement for a cooperation term of 10 years. According to the agreement, the People’s Government will
contribute RMB 8,000,000 ($1,194,400) as a one-time payment to the Company for supporting the Company on deep processing of Chinese herbs
in exchange for the Company’s proportionate equity interest. The Company can retain the contributed amount at the end of the cooperation
term if it passes the performance assessment by People’s Government; otherwise, the Company should return the full proceeds they
received plus 20% penalty. During the six months ended June 30, 2022, the Company received $978,647 from the People’s Government.
Based on management’s assessment, the Company does not expect to receive the remaining funds, and determined that they will return
the $978,647 to the People Government.
On
July 19, 2022, Hong Kong Aixin International Group Co., Limited (“HK Aixin”), a wholly owned subsidiary of Aixin Life International,
Inc. (“Aixin Life”), entered into an Equity Transfer Agreement (the “Transfer Agreement”) with Yunnan Shengshengyuan
Technology Co., Ltd and Yun Chen (collectively “the Sellers”), who own 95% and 5% equity interest of Runcangsheng, respectively.
Under
the terms of the Transfer Agreement, HK Aixin agreed to purchase all of the outstanding equity interest of Runcangsheng for an aggregate
purchase price of RMB 45,082,600 or US$6.68 million based on an exchange rate of RMB / US$ 6.75 yuan per dollar on July 19, 2022. In
addition to transferring their respective equity interest in Runcangsheng by the Sellers, both Sellers agree to forgive any loans Runcangsheng
due to them.
If
the audited net worth of the Company as of December 31, 2021 is less than 97% of the initial net worth (net worth prior to the audit
that were used to determine the equity transfer price), then the above purchase price shall be reduced by an amount equal to the initial
net worth minus the audited net worth.
Management
has evaluated subsequent events through the date which the financial statements were available to be issued. All subsequent events requiring
recognition as of December 31, 2021 have been incorporated into these financial statements and there are no subsequent events that require
disclosure in accordance with FASB ASC Topic 855, “Subsequent Events.”
YUNNAN
RUNCANGSHENG TECHNOLOGY COMPANY LIMITED
BALANCE
SHEETS
| | |
June
30, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| | |
(Unaudited) | | |
| |
| ASSETS | |
| | | |
| | |
| CURRENT
ASSETS | |
| | | |
| | |
| Cash
and cash equivalents | |
$ | 706,753 | | |
$ | 52,052 | |
| Accounts
receivable, net | |
| 239,681 | | |
| 254,271 | |
| Accounts
receivable - related party | |
| 220,746 | | |
| 231,983 | |
| Advance
to suppliers | |
| 112,684 | | |
| 30,232 | |
| Other
receivables and prepaid expenses | |
| 15,024 | | |
| 2,942 | |
| Inventory,
net | |
| 394,745 | | |
| 222,373 | |
| Total
current assets | |
| 1,689,633 | | |
| 793,853 | |
| | |
| | | |
| | |
| NON-CURRENT
ASSETS | |
| | | |
| | |
| Property
and equipment, net | |
| 1,809,711 | | |
| 2,072,984 | |
| Operating
lease right-of-use assets, net | |
| 3,382 | | |
| 6,219 | |
| Total
non-current assets | |
| 1,813,093 | | |
| 2,079,203 | |
| | |
| | | |
| | |
| TOTAL
ASSETS | |
$ | 3,502,726 | | |
$ | 2,873,056 | |
| | |
| | | |
| | |
| LIABILITIES
AND STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| CURRENT
LIABILITIES | |
| | | |
| | |
| Accounts
payable | |
$ | 200,067 | | |
$ | 258,103 | |
| Accounts
payable - related party | |
| 170,895 | | |
| 212,230 | |
| Accrued
liabilities and other payables | |
| 1,620,489 | | |
| 1,842,045 | |
| Advance
from customers | |
| 55,727 | | |
| 35,106 | |
| Interest
payable | |
| 11,127 | | |
| - | |
| Government
grant payable | |
| 978,647 | | |
| - | |
| Taxes
payable | |
| 22,932 | | |
| 19,492 | |
| Operating
lease liabilities - current | |
| 16,124 | | |
| 16,880 | |
| Due
to related parties | |
| 364,243 | | |
| 402,397 | |
| Total
current liabilities | |
| 3,440,251 | | |
| 2,786,253 | |
| | |
| | | |
| | |
| TOTAL
LIABILITIES | |
| 3,440,251 | | |
| 2,786,253 | |
| | |
| | | |
| | |
| STOCKHOLDERS’
EQUITY | |
| | | |
| | |
| Paid-in
capital | |
| 133,678 | | |
| 133,678 | |
| Accumulated
deficit | |
| (79,461 | ) | |
| (58,662 | ) |
| Accumulated
other comprehensive income | |
| 8,258 | | |
| 11,787 | |
| Total
stockholders’ equity | |
| 62,475 | | |
| 86,803 | |
| | |
| | | |
| | |
| TOTAL
LIABILITIES AND STOCKHOLDERS’ EQUITY | |
$ | 3,502,726 | | |
$ | 2,873,056 | |
YUNNAN
RUNCANGSHENG TECHNOLOGY COMPANY LIMITED
STATEMENTS
OF OPERATIONS AND COMPREHENSIVE LOSS
(UNAUDITED)
| | |
Six
Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Revenues | |
$ | 295,375 | | |
$ | 62,391 | |
| Cost
of goods sold | |
| 125,850 | | |
| 42,974 | |
| | |
| | | |
| | |
| Gross
profit | |
| 169,525 | | |
| 19,417 | |
| | |
| | | |
| | |
| Operating
expense | |
| | | |
| | |
| Selling | |
| 8,788 | | |
| 4,613 | |
| Research
and development | |
| 45,092 | | |
| 15,903 | |
| General
and administrative | |
| 155,205 | | |
| 24,640 | |
| Total
operating expenses | |
| 209,085 | | |
| 45,156 | |
| | |
| | | |
| | |
| Loss
from operations | |
| (39,560 | ) | |
| (25,739 | ) |
| | |
| | | |
| | |
| Non-operating
income | |
| | | |
| | |
| Interest
income | |
| 551 | | |
| 16 | |
| Other
income | |
| 18,210 | | |
| 2 | |
| Total
non-operating income | |
| 18,761 | | |
| 18 | |
| | |
| | | |
| | |
| Loss
before income tax | |
| (20,799 | ) | |
| (25,721 | ) |
| | |
| | | |
| | |
| Income
tax expense | |
| - | | |
| - | |
| | |
| | | |
| | |
| Net
loss | |
$ | (20,799 | ) | |
$ | (25,721 | ) |
| | |
| | | |
| | |
| Other
comprehensive item | |
| | | |
| | |
| Foreign
currency translation | |
| (3,529 | ) | |
| 1,152 | |
| Comprehensive
loss | |
$ | (24,328 | ) | |
$ | (24,569 | ) |
YUNNAN
RUNCANGSHENG TECHNOLOGY COMPANY LIMITED
STATEMENTS
OF STOCKHOLDERS’ EQUITY
(UNAUDITED)
| | |
| | |
| | |
Accumulated
other | | |
| |
| | |
Paid
in | | |
Accumulated | | |
comprehensive | | |
| |
| | |
capital | | |
deficit | | |
income | | |
Total | |
| Balance
at December 31, 2021 | |
$ | 133,678 | | |
$ | (58,662 | ) | |
$ | 11,787 | | |
$ | 86,803 | |
| Net
loss | |
| - | | |
| (20,799 | ) | |
| - | | |
| (20,799 | ) |
| Foreign
currency translation | |
| - | | |
| - | | |
| (3,529 | ) | |
| (3,529 | ) |
| Balance
at June 30, 2022 | |
$ | 133,678 | | |
$ | (79,461 | ) | |
$ | 8,258 | | |
$ | 62,475 | |
| | |
| | |
| | |
Accumulated
other | | |
| |
| | |
Paid
in | | |
Accumulated | | |
comprehensive | | |
| |
| | |
capital | | |
deficit | | |
income | | |
Total | |
| Balance
at December 31, 2020 | |
$ | 128,321 | | |
$ | (22,897 | ) | |
$ | 9,455 | | |
$ | 114,879 | |
| Net
loss | |
| - | | |
| (25,721 | ) | |
| - | | |
| (25,721 | ) |
| Foreign
currency translation | |
| - | | |
| - | | |
| 1,152 | | |
| 1,152 | |
| Balance
at June 30, 2021 | |
$ | 128,321 | | |
$ | (48,618 | ) | |
$ | 10,607 | | |
$ | 90,310 | |
YUNNAN
RUNCANGSHENG TECHNOLOGY, INC
STATEMENTS
OF CASH FLOWS
(UNAUDITED)
| | |
Six
Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| CASH
FLOWS FROM OPERATING ACTIVITIES | |
| | | |
| | |
| Net
loss | |
$ | (20,799 | ) | |
$ | (25,721 | ) |
| Adjustments
to reconcile net loss to net cash provided by (used in) operating activities: | |
| | | |
| | |
| Depreciation | |
| 168,129 | | |
| 12,470 | |
| Provision
for allowance for doubtful accounts | |
| 71,990 | | |
| - | |
| Operating
lease expense | |
| 2,686 | | |
| 2,816 | |
| Changes
in operating assets and liabilities: | |
| | | |
| | |
| Accounts
receivable | |
| (69,641 | ) | |
| 8,138 | |
| Accounts
receivable - related party | |
| - | | |
| (20,274 | ) |
| Advance
to suppliers | |
| (86,726 | ) | |
| (20,149 | ) |
| Other
receivable and prepaid expenses | |
| (12,448 | ) | |
| (49,409 | ) |
| Inventory | |
| (189,277 | ) | |
| (275,140 | ) |
| Accounts
payable | |
| (47,059 | ) | |
| 59,744 | |
| Accounts
payable - related party | |
| (32,094 | ) | |
| 184,694 | |
| Accrued
liabilities and other payables | |
| (136,762 | ) | |
| 4,850 | |
| Advance
from customers | |
| 23,069 | | |
| 2,601 | |
| Interest
payable | |
| 11,500 | | |
| - | |
| Taxes
payable | |
| 4,531 | | |
| (2 | ) |
| Net
cash used in operating activities | |
| (312,901 | ) | |
| (115,382 | ) |
| | |
| | | |
| | |
| CASH
FLOWS FROM INVESTING ACTIVITIES | |
| | | |
| | |
| Construction
in progress | |
| - | | |
| (42,550 | ) |
| Purchase
of equipment | |
| - | | |
| (5,473 | ) |
| Net
cash used in investing activities | |
| - | | |
| (48,023 | ) |
| | |
| | | |
| | |
| CASH
FLOWS FROM FINANCING ACTIVITIES | |
| | | |
| | |
| Repayment
to / proceeds from related parties | |
| (19,287 | ) | |
| 164,865 | |
| Proceeds
from government grant payable | |
| 1,011,421 | | |
| - | |
| Net
cash provided by financing activities | |
| 992,134 | | |
| 164,865 | |
| | |
| | | |
| | |
| Effect
of exchange rate changes on cash and cash equivalents | |
| (24,532 | ) | |
| 32 | |
| | |
| | | |
| | |
| Net
increase in cash and cash equivalents | |
| 654,701 | | |
| 1,492 | |
| | |
| | | |
| | |
| Cash
and cash equivalents - beginning of period | |
| 52,052 | | |
| 2,773 | |
| Cash
and cash equivalents - end of period | |
$ | 706,753 | | |
$ | 4,265 | |
| | |
| - | | |
| - | |
| Supplemental
Cash Flow Disclosures | |
| | | |
| | |
| Cash
paid for interest | |
$ | - | | |
$ | - | |
| Cash
paid for income taxes | |
$ | 4,855 | | |
$ | - | |
YUNNAN
RUNCANGSHENG TECHNOLOGY COMPANY LIMITED
NOTES
TO FINANCIAL STATEMENTS
(UNAUDITED)
1.
ORGANIZATION AND DESCRIPTION OF BUSINESS
Yunnan
Runcangsheng Technology Company Limited (the “Company” or “Runcangsheng”), was incorporated on April 17, 2020
in Kunming City, Yunnan Province in China. The Company is engaged in biotechnology research and consultation, and the manufacturing and
sales of health supplement products.
2.
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis
of Presentation
The
accompanying financial statements are
prepared in conformity with U.S. Generally Accepted Accounting Principles (“US GAAP”). The functional currency of the
Company is Chinese Renminbi (“RMB”). The accompanying financial statements are
translated from RMB and presented in U.S. dollars (“USD”).
Covid
- 19
On
March 11, 2020, the World Health Organization announced that infections caused by the corona virus disease of 2019 (“COVID-19”)
had become pandemic. The Government of China has adopted various regulations and orders, including mandatory quarantines, limits on the
number of people that may gather in one location, closing non-essential businesses, and travel bans to limit the spread of the disease.
Many of these measures have been relaxed due to the decrease in the prevalence of Covid-19 in China. To date, the ongoing operations
of the Company have not been materially adversely affected by the measures taken to limit the spread of the disease in China.
Financial
impacts related to COVID-19, including the Company’s actions and costs incurred in response to the pandemic, were not material
to the Company’s financial position, results of operations or cash flows for the six months ended June 30, 2022 and 2021. The Company
has implemented procedures to promote employee and customer safety. These measures will not significantly increase its operating costs.
Use
of Estimates
In
preparing financial statements in
conformity with US GAAP, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures
of contingent assets and liabilities at the dates of the financial statements, as well as
the reported amounts of revenues and expenses during the reporting period.
Significant
estimates, required by management, include the recoverability of long-lived assets, allowance for doubtful accounts, and the reserve
for obsolete and slow-moving inventories. Actual results could differ from those estimates.
Cash
and Cash Equivalents
For
financial statement purposes, the Company considers all highly liquid investments with an original maturity of three months or less to
be cash and cash equivalents.
Accounts
Receivable and Allowance for Doubtful Accounts
Accounts
receivable mainly consist of amounts due from customers. The Company’s policy is to maintain an allowance for potential credit
losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer
concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy
of these reserves. As of June 30, 2022 and December 31, 2021, allowance for doubtful accounts was
$239,874 and $178,881, respectively.
Advance
to Suppliers
The
Company makes advance payment to certain vendors for purchase of raw material, tools and equipment for production. The advances are interest-free
and unsecured. As of June 30, 2022 and December 31, 2021, the Company had advance to suppliers of $112,684 and $30,232 respectively.
Inventory
Inventories
are valued at the lower of cost or net realizable value using the weighted average cost method. Management compares the cost of inventories
with the net realizable value and an allowance is made for writing down inventories to market value, if lower. The Company recorded no
inventory impairment for the six months ended June
30, 2022 and 2021.
Property
and Equipment
Property
and equipment are stated at cost, less accumulated depreciation, and impairment losses, if any. Major repairs and betterments that
significantly extend original useful lives or improve productivity are capitalized and depreciated over the period benefited.
Maintenance and repairs are expensed as incurred. When property and equipment are retired or otherwise disposed of, the related cost
and accumulated depreciation are removed from the respective accounts, and any gain or loss is included in operations. Depreciation
of property and equipment is provided using the straight-line method for substantially all assets with 5% salvage value and
estimated lives as follows:
| Office
equipment and furniture |
|
1~5
years |
| Machinery
and equipment |
|
10
years |
| Leasehold
improvement |
|
5
years |
| Vehicle |
|
5
years |
Impairment
of Long-Lived Assets
Long-lived
assets, which include property and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances
indicate that the carrying amount of an asset may not be recoverable, but at least annually.
Recoverability
of long-lived assets to be held and used is measured by comparing the carrying amount of an asset to the estimated undiscounted future
cash flows expected to be generated by it. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an
impairment charge is recognized by the amount by which the carrying amount of the asset exceeds its fair value. Fair value is generally
determined using the asset’s expected future discounted cash flows or market value, if readily determinable. Based on its review,
the Company believes that, as of June 30, 2022 and December 31, 2021, there was no significant impairment of its long-lived assets.
Income
Taxes
Income
taxes are accounted for using an asset and liability method. Under this method, deferred income taxes are recognized for the tax consequences
in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end
based on enacted tax laws and statutory tax rates, applicable to the periods in which the differences are expected to affect taxable
income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
The
Company follows Accounting Standards Codification (“ASC”) Topic 740, which prescribes a more-likely-than-not threshold for
financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740 also provides
guidance on recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities,
accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods, and income tax disclosures.
Under
ASC Topic 740, when tax returns are filed, it is likely that some positions taken would be sustained upon examination by the taxing authorities,
while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately
sustained. The benefit of a tax position is recognized in the financial statements in the period during which, based on all available
evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution
of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that
meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50% likely of
being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken
that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying balance
sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination. Interest associated
with unrecognized tax benefits is classified as interest expense and penalties are classified in selling, general and administrative
expenses in the statement of operations.
At
June 30, 2022 and December 31, 2021, the Company did not take any uncertain positions that would necessitate recording a tax related
liability.
Revenue
Recognition
In
accordance with ASC 606, revenue is recognized upon the transfer of control of promised goods or services to customers in an amount that
reflects the consideration the Company expects to receive in exchange for those products or services.
Revenue
from sale of goods under Topic 606 is recognized in a manner that reasonably reflects the delivery of the Company’s
products and services to customers in return for expected consideration and includes the following elements:
| |
● |
executed
contract(s) with customers that the Company believes is legally enforceable; |
| |
|
|
| |
● |
identification
of performance obligation in the respective contract; |
| |
|
|
| |
● |
determination
of the transaction price for each performance obligation in the respective contract; |
| |
|
|
| |
● |
allocation
of the transaction price to each performance obligation; and |
| |
|
|
| |
● |
recognition
of revenue only when the Company satisfies each performance obligation. |
The
Company recognizes revenue at the time the products are shipped as it satisfies its performance obligations. The Company records a receivable
for the sales when it has an unconditional right to receive payment and only the passage of time is required before payment is due.
Sales
revenue represents the invoiced value of goods, net of value-added taxes (“VAT”). All of the Company’s products sold
in China are subject to the PRC VAT of 13%. The VAT may be offset by VAT paid by the Company on inventories and products purchased in
China. The Company records VAT payable and VAT receivable net of payments in the financial statements. The VAT tax return is filed offsetting
the payables against the receivables. Sales and purchases are recorded net of VAT collected and paid as the Company acts as an agent
for the government.
Cost
of Goods Sold
Cost
of goods sold consists primarily of the cost of products manufactured during the reporting period, mainly including direct labor, raw
material and overhead costs. Reserve for inventory allowance due to lower of cost or market is also recorded in cost of goods sold.
Research
and Development Costs
Research
and development costs are expensed as incurred and included in general and administrative expenses. These costs primarily consist of
cost of materials used, salaries paid to the Company’s development team, and fees paid to third parties. Research and development
expenses for the six months ended June 30, 2022 and 2021 were $45,092 and $15,903, respectively.
Concentration
of Credit Risk
The
operations of the Company are in the PRC. Accordingly, the Company’s business, financial condition, and results of operations may
be influenced by the political, economic, and legal environments in the PRC, and by the general state of the PRC economy.
The
Company has cash on hand and demand deposits in accounts maintained with state-owned banks within the PRC. Cash in state-owned banks
is covered by insurance up to RMB 500,000 ($74,650) per bank. The Company has not experienced any losses in such accounts and believes
they are not exposed to any risks on its cash in these bank accounts.
Leases
ASC
Topic 842 “Leases,” requires recognition of leases on the balance sheets as right-of-use (“ROU”) assets
and lease liabilities. ROU assets represent the Company’s right to use underlying assets for the lease terms and lease liabilities
represent the Company’s obligation to make lease payments arising from the leases. Operating lease ROU assets and operating lease
liabilities are recognized based on the present value and future minimum lease payments over the lease term at commencement date. The
Company’s future minimum based payments used to determine the Company’s lease liabilities mainly include minimum based rent
payments. As most of the Company’s leases do not provide an implicit rate, the Company uses its estimated incremental borrowing
rate based on the information available at commencement date in determining the present value of lease payments.
Operating
lease cost is recognized as a single lease cost on a straight-line basis over the lease term and is recorded in selling, general and
administrative expenses. Variable lease payments for common area maintenance, property taxes and other operating expenses are recognized
as expense in the period when the changes in facts and circumstances on which the variable lease payments are based occur.
Related
Parties
Parties
are considered to be related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are
controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management,
members of the immediate families of principal owners of the Company and its management and other parties with which the Company may
deal with if one party controls or can significantly influence the management or operating policies of the other to an extent that one
of the transacting parties might be prevented from fully pursuing its own separate interests. The Company discloses all significant related
party transactions.
Statement
of Cash Flows
In
accordance with ASC Topic 230, “Statement of Cash Flows,” cash flows from the Company’s operations are calculated
based on the local currencies using the average translation rates. As a result, amounts related to assets and liabilities reported on
the statements of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheets.
Fair
Value of Financial Instruments
The
carrying amounts of certain of the Company’s financial instruments, including cash and equivalents, accrued liabilities and accounts
payable, approximate their fair value due to their short maturities. FASB ASC Topic 825, “Financial Instruments,” requires
disclosure of the fair value of financial instruments held by the Company. The carrying amounts reported in the balance sheets for current
liabilities each qualify as financial instruments and are a reasonable estimate of their fair value because of the short period of time
between the origination of such instruments and their expected realization and the current market rate of interest.
Fair
Value Measurements and Disclosures
ASC
Topic 820, “Fair Value Measurements and Disclosures,”defines fair value, and establishes a three-level
valuation hierarchy for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The three
levels are defined as follow:
| |
● |
Level
1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| |
● |
Level
2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that
are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| |
● |
Level
3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
As
of June 30, 2022 and December 31, 2021, the Company did not identify any assets and liabilities that are required to be presented on
the balance sheet at fair value.
Foreign
Currency Translation and Comprehensive Income (Loss)
The
functional currency of the Company is RMB. For financial reporting purposes, RMB is translated into USD as the reporting currency. Assets
and liabilities are translated at the exchange rate in effect at the balance sheet dates. Revenues and expenses are translated at the
average rate of exchange prevailing during the reporting period.
Translation
adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’
equity as “Accumulated other comprehensive income.” Gains and losses resulting from foreign currency transactions are included
in income. There was no significant fluctuation in the exchange rate for the conversion of RMB to USD after the balance sheet date.
The
Company uses FASB ASC Topic 220, “Comprehensive Income”. Comprehensive income (loss) is comprised of net income
(loss) and all changes to the statements of stockholders’ equity, except those due to investments by stockholders, changes in paid-in
capital and distributions to stockholders. Comprehensive income (loss) for the six months ended June
30, 2022 and 2021 consisted of net income (loss) and foreign currency translation adjustments.
Earnings
per Share
The
Company is a limited Company (“LC”) formed under the laws of the PRC. Like limited liability company in the US, limited company
in the PRC do not issue shares to the owners. The owners however, are called shareholders. Ownership interest is determined in proportion
to capital contributed. Accordingly, earnings per share data is not presented.
Segment
Reporting
ASC
Topic 280, “Segment Reporting,” requires use of the “management approach” model for segment
reporting. The management approach model is based on the way a company’s chief operating decision maker organizes segments within
the Company for making operating decisions assessing performance and allocating resources. Reportable segments are based on products
and services, geography, legal structure, management structure, or any other manner in which management disaggregates a company.
Management
determined the Company’s operations constitute a single reportable segment in accordance with ASC 280. The Company operates exclusively
in one business and industry segment: health supplemental products manufacture and sale.
New
Accounting Pronouncements
In
June 2016, the FASB issued ASU No. 2016-13, Financial Instruments-Credit Losses (Topic 326), which requires entities to measure all expected
credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and
supportable forecasts. This replaces the existing incurred loss model and is applicable to the measurement of credit losses on financial
assets measured at amortized cost. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning
after December 15, 2022. Early application is permitted for all entities for fiscal years, and interim periods within those fiscal years,
beginning after December 15, 2018. The Company is currently evaluating the impact that the standard will have on its financial statements.
In
March 2020, the FASB issued ASU 2020-04, Reference Rate Reform (Topic 848) (“ASU 2020-04”). ASU 2020-04 contains practical
expedients for reference rate reform related activities that impact debt, leases, derivatives and other contracts. The guidance in ASU
2020-04 is optional and may be elected over time as reference rate reform activities occur. The Company continues to evaluate the impact
of the guidance and may apply the elections as applicable as changes in the market occur.
3.
OTHER RECEIVABLES AND PREPAID EXPENSES
The
Company had other receivables and prepaid expenses of $15,024 and $2,942 as of June 30, 2022 and December 31, 2021, respectively, which
mainly consisted of prepaid service fees.
4.
ACCOUNTS RECEIVABLES, NET
Accounts
receivable, net consisted of the following:
| | |
June
30, 2022 | | |
December
31, 2021 | |
| Accounts
receivable - third parties | |
$ | 479,555 | | |
$ | 433,152 | |
| Less:
allowance for doubtful accounts | |
| (239,874 | ) | |
| (178,881 | ) |
| Accounts
receivable, net | |
$ | 239,681 | | |
$ | 254,271 | |
The
following table presents the activities in the allowance for doubtful accounts for the six months ended June 30, 2022 and 2021.
| | |
Six
Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| Balance,
beginning of period | |
$ | 178,881 | | |
$ | - | |
| Provision
for doubtful debts | |
| 71,990 | | |
| - | |
| Effect
of foreign currency translation | |
| (10,997 | ) | |
| - | |
| Balance,
end of period | |
$ | 239,874 | | |
$ | - | |
Provision
for allowance were recognized $71,990 and $0 during the six months ended June 30, 2022 and 2021, respectively.
5.
INVENTORY
Inventory
consisted of the following at June 30, 2022 and December
31, 2021:
| | |
June
30, 2022 | | |
December
31, 2021 | |
| Raw
materials | |
$ | 119,588 | | |
$ | 44,399 | |
| Work
in process | |
| 4,261 | | |
| - | |
| Finished
goods | |
| 270,896 | | |
| 177,974 | |
| Total | |
$ | 394,745 | | |
$ | 222,373 | |
6.
PROPERTY AND EQUIPMENT
Property
and equipment consisted of the following at June 30, 2022 and December 31, 2021:
| | |
June
30, 2022 | | |
December
31, 2021 | |
| Office
equipment and furniture | |
$ | 23,864 | | |
$ | 25,079 | |
| Leasehold
improvement | |
| 928,890 | | |
| 977,644 | |
| Machinery
and equipment | |
| 1,026,163 | | |
| 1,078,399 | |
| Vehicle | |
| 106,622 | | |
| 112,050 | |
| Other | |
| 4,366 | | |
| 3,307 | |
| Total | |
| 2,089,905 | | |
| 2,196,479 | |
| Less:
Accumulated depreciation | |
| (280,194 | ) | |
| (123,495 | ) |
| Property
and equipment, net | |
$ | 1,809,711 | | |
$ | 2,072,984 | |
Depreciation
expense for the six months ended June 30, 2022 and 2021 was $168,129 and $12,470, respectively.
7.
CONSTRUCTION IN PROGRESS
As
of June 30, 2022 and December 31, 2021, the Company had construction in progress of $0. During the year ended December 31, 2021, the
Company completed the construction of the Biological Resources GMP Purification workshop, and was transferred to the Company’s
fixed assets.
8.
TAXES PAYABLE
Taxes
payable mainly consisted of VAT and corporate income tax payable of $22,932 and $19,492 as of June 30, 2022 and December 31, 2021.
9.
ACCRUED LIABILITIES AND OTHER PAYABLES
Accrued
liabilities and other payables consisted of the following at June 30, 2022 and December
31, 2021:
| | |
June
30, 2022 | | |
December
31, 2021 | |
| Accrued
payroll | |
$ | 9,681 | | |
$ | 18,245 | |
| Payable
for construction and improvement | |
| 1,447,960 | | |
| 1,642,570 | |
| Payable
for equipment purchase | |
| 162,473 | | |
| 180,857 | |
| Other | |
| 375 | | |
| 373 | |
| Total | |
$ | 1,620,489 | | |
$ | 1,842,045 | |
The
payable for construction and improvement was mainly for the payment of workshop construction and biological resources purification workshop
project. Total construction costs for workshop construction were $1.75 million. All construction has been completed in 2021.
10.
GOVERNMENT GRANT PAYABLE
On
December 1, 2021, the Company and Luquan Yizu Miaozu Autonomous County People’s Government (“the People’s Government”)
entered into a cooperation agreement for a cooperation term of 10 years. According to the agreement, the People’s Government will
contribute RMB 8,000,000 ($1,194,400) as a one-time payment to the Company for supporting the Company on deep processing of Chinese herbs
in exchange for the Company’s proportionate equity interest. The Company can retain the contributed amount at the end of the cooperation
term if it passes the performance assessment by People’s Government; otherwise, the Company should return the full proceeds they
received plus 20% penalty. As of June 30, 2022, the Company received $978,647 from the People’s Government, and recorded as government
grant payable. Based on management’s assessment, the Company does not expect to receive the remaining funds, and determined that
they will return the $978,647 to the People Government.
11.
LEASE
The
Company leases its office under an operating lease arrangement with a related party, with the lease term of 3 years from March 1, 2020
through February 28, 2023. The monthly rent is RMB 3,000 ($464), payable every six month in advance.
Balance
sheet information related to the Company’s leases is presented below:
| | |
June
30, 2022 | | |
December
31, 2021 | |
| Operating
Leases | |
| | | |
| | |
| Operating
lease right-of-use assets | |
$ | 3,382 | | |
$ | 6,219 | |
| | |
| | | |
| | |
| Operating
lease liabilities - current | |
$ | 16,124 | | |
$ | 16,880 | |
The
following provides details of the Company’s lease expenses:
| | |
Six
Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| Operating
lease expenses | |
$ | 2,686 | | |
$ | 2,816 | |
Other
information related to leases is presented below:
| | |
Six
Months Ended June 30, | |
| | |
2022 | | |
2021 | |
| Cash
Paid For Amounts Included In Measurement of Liabilities: | |
| | |
| |
| Operating
cash flows from operating leases | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Weighted
Average Remaining Lease Term: | |
| | | |
| | |
| Operating
leases | |
| 0.67
years | | |
| 1.67
years | |
| | |
| | | |
| | |
| Weighted
Average Discount Rate: | |
| | | |
| | |
| Operating
leases | |
| 4.75 | % | |
| 4.75 | % |
Maturities
of lease liabilities were as follows:
| For
the year ending December 31, | |
| |
| 2022 | |
$ | 16,124 | |
| Less:
imputed interest | |
| - | |
| Total
lease liabilities | |
$ | 16,124 | |
12.
RELATED PARTY TRANSACTIONS
Accounts
receivable - related party
As
of June 30, 2022 and December 31, 2021,
the Company had accounts receivable from related party in the amount of $220,746 and $231,983, respectively, which was from sales of
products to Yunnan Shengshengyuan Technology Co., Ltd. (“Shengshengyuan”), the 95% shareholder of the Company. During the
six months ended June 30, 2022 and 2021, the Company had sales of $0 and $17,942 to Shengshengyuan, respectively.
Accounts
payable- related party
As
of June 30, 2022 and December 31, 2021,
the Company had accounts payable to related party in the amount of $170,895 and $212,230, respectively, which was from purchase of raw
materials from Luquan Shengcaofeng Biotechnology Co., Ltd. (“Shengcaofeng”), an entity controlled by the ultimate
major shareholder of the Company. During the six months ended June 30, 2022 and 2021, the Company
made purchase of $0 and $199,776 from Shengcaofeng, respectively.
Due
to related parties
| | |
June
30, 2022 | | |
December
31, 2021 | |
| Yunnan
Shengshengyuan Technology Inc (95% shareholder of the Company) | |
$ | 52,255 | | |
$ | 61,191 | |
| Huiliang
Jiao (ultimate major shareholder of the Company) | |
| 299,596 | | |
| 325,516 | |
| Xiaoyan
Zhou (spouse of the ultimate major shareholder) | |
| 12,392 | | |
| 15,690 | |
| Total | |
$ | 364,243 | | |
$ | 402,397 | |
Due
to related parties represented the advance from the related parties for the Company’s operating needs. Due to related parties were
unsecured, bear no interest and payable upon demand.
Consulting
Fee
During
the six months ended June 30, 2022 and 2021, the Company paid consulting fee of $3,879 and $0 to Shengshengyuan, respectively.
13.
INCOME TAXES
The
Company is governed by the Income Tax Laws of the PRC and various local tax laws. Effective January 1, 2008, China adopted a uniform
tax rate of 25% for all enterprises (including foreign-invested enterprises).
The
following table reconciles the PRC statutory rate to the Company’s effective tax rate for six months ended June 30, 2022 and 2021:
| |
|
2022 |
|
|
2021 |
|
| Income
tax (benefit) at PRC statutory rate |
|
|
(25.0 |
)% |
|
|
(25.0 |
)% |
| Change
in valuation allowance |
|
|
25.0 |
% |
|
|
25.0 |
% |
| Effective
income tax rate |
|
|
- |
% |
|
|
- |
% |
14.
STATUTORY RESERVES
Pursuant
to the PRC corporate law, the Company is now only required to maintain one statutory reserve by appropriating from its after-tax profit
before declaration or payment of dividends. The statutory reserve represents restricted retained earnings.
Surplus
reserve fund
The
Company is now required to transfer 10% of its net income, as determined under PRC accounting rules and regulations, to a statutory surplus
reserve fund until such reserve balance reaches 50% of the Company’s registered capital. During the six months ended June 30, 2022
and 2021, the Company made $0 to its statutory reserve fund.
The
surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any,
and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion
to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance
after such issue is not less than 25% of the registered capital.
Common
welfare fund
Common
welfare fund is a voluntary fund to which the Company can elect to transfer 5% to 10% of its net income, as determined under PRC accounting
rules and regulations. The Company did not make any contribution to this fund during the six months ended June 30, 2022 and 2021.
This
fund can only be utilized on capital items for the collective benefit of the Company’s employees, such as construction of dormitories,
cafeteria facilities, and other staff welfare facilities. This fund is non-distributable other than upon liquidation.
15.
OPERATING CONTINGENCIES
The
Company’s operations in the PRC are subject to specific considerations and significant risks not typically associated with companies
in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments
and foreign currency exchange. The Company’s results may be adversely affected by changes in governmental policies with respect
to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among
other things.
The
Company’s sales, purchases and expenses are denominated in RMB and all of the Company’s assets and liabilities are also denominated
in RMB. The RMB is not freely convertible into foreign currencies under the current law. In China, foreign exchange transactions are
required by law to be transacted only by authorized financial institutions. Remittances in currencies other than RMB may require certain
supporting documentation to affect the remittance.
The
Company is, from time to time, involved in litigation incidental to the conduct of its business regarding merchandise sold, employment
matters, and litigation regarding intellectual property rights.
The
Company believes that current pending litigation will not have a material adverse effect on its financial position, results of operations
or cash flows.
16.
CONCENTRATIONS
Customers
During
the six months ended June 30, 2022, the Company had two major customers that accounted for over 10% of its total revenue.
| Customer | |
Net
sales for the
six months ended
June 30, 2022 | | |
%
of total revenue | | |
Accounts
receivable as of June
30, 2022 | |
| A | |
$ | 84,793 | | |
| 29 | % | |
$ | 80,982 | |
| B | |
| 60,497 | | |
| 20 | % | |
| 130,485 | |
During
the six months ended June 30, 2021, the Company had three major customers that accounted for over 10% of its total revenue.
| Customer | |
Net
sales for the
six months ended
June 30, 2021 | | |
%
of total revenue | | |
Accounts
receivable as of June
30, 2021 | |
| C | |
$ | 20,522 | | |
| 33 | % | |
$ | - | |
| D* | |
| 17,942 | | |
| 29 | % | |
| 20,314 | |
| E | |
| 13,348 | | |
| 21 | % | |
| - | |
*
The Company had sales to and accounts receivable due from Shengshengyuan, a related party (see Note 12).
Suppliers
During
the six months ended June 30, 2022, the Company had three major suppliers that accounted for over 10% of its total purchase.
| Supplier | |
Net
purchase for the
six months ended
June 30, 2022 | | |
%
of total purchase | | |
Accounts
payable as
of June
30, 2022 | |
| F | |
$ | 74,467 | | |
| 33 | % | |
$ | 115,450 | |
| G | |
| 69,659 | | |
| 31 | % | |
| - | |
| H | |
| 27,821 | | |
| 12 | % | |
| 1,860 | |
During
the six months ended June 30, 2021, the Company had two major suppliers that accounted for over 10% of its total purchase.
| Supplier | |
Net
purchase for the
six months ended
June 30, 2021 | | |
%
of total purchase | | |
Accounts
payable as
of June
30, 2021 | |
| I* | |
$ | 199,777 | | |
| 68 | % | |
| 185,052 | |
| J | |
| 54,488 | | |
| 19 | % | |
| 54,594 | |
*
The Company had purchase from and accounts payable to Shengcaofeng, a related party (see Note 12).
17.
SUBSEQUENT EVENTS
On
July 19, 2022, Hong Kong Aixin International Group Co., Limited (“HK Aixin”), a wholly owned subsidiary of Aixin Life International,
Inc. (“Aixin Life”), entered into an Equity Transfer Agreement (the “Transfer Agreement”) with Yunnan Shengshengyuan
Technology Co., Ltd and Yun Chen (collectively “the Sellers”), who own 95% and 5% equity interest of Runcangsheng, respectively.
Under
the terms of the Transfer Agreement, HK Aixin agreed to purchase all of the outstanding equity interest of Runcangsheng for an aggregate
purchase price of RMB 45,082,600 or US$6.68 million based on an exchange rate of RMB / US$ 6.75 yuan per dollar on July 19, 2022. In
addition to transferring their respective equity interest in Runcangsheng by the Sellers, both Sellers agree to forgive any loans Runcangsheng
due to them.
If
the audited net worth of the Company as of December 31, 2021 is less than 97% of the initial net worth (net worth prior to the audit
that were used to determine the equity transfer price), then the above purchase price shall be reduced by an amount equal to the initial
net worth minus the audited net worth.
Management
has evaluated subsequent events through the date which the financial statements were available to be issued. All subsequent events requiring
recognition as of June 30, 2022 have been incorporated into these financial statements and there are no subsequent events that require
disclosure in accordance with FASB ASC Topic 855, “Subsequent Events.”
AIXIN
LIFE INTERNATIONAL, INC.
UNAUDITED
PRO FORMA CONSOLIDATED BALANCE SHEETS
AS
OF JUNE 30, 2022
| | |
AIXIN LIFE INTERNATIONAL, INC. | | |
YUNNAN RUNCANGSHENG TECHNOLOGY COMPANY LIMITED | | |
PRO FORMA ADJUSTMENTS | |
| |
PRO FORMA CONSOLIDATED | |
| | |
| | |
| | |
| |
| |
| |
| ASSETS | |
| | | |
| | | |
| | |
| |
| | |
| Current Assets | |
| | | |
| | | |
| | |
| |
| | |
| Cash and cash equivalents | |
$ | 8,129,696 | | |
$ | 706,753 | | |
$ | - | |
| |
$ | 8,836,449 | |
| Restricted cash | |
| 61,588 | | |
| - | | |
| - | |
| |
| 61,588 | |
| Accounts receivable, net | |
| 60,548 | | |
| 239,681 | | |
| (51,712 | ) |
{d} | |
| 248,517 | |
| Accounts receivable, related party | |
| - | | |
| 220,746 | | |
| - | |
| |
| 220,746 | |
| Other receivables and prepaid expense | |
| 46,702 | | |
| 15,024 | | |
| - | |
| |
| 61,726 | |
| Advance to suppliers | |
| 143,421 | | |
| 112,684 | | |
| - | |
| |
| 256,105 | |
| Advance to related parties | |
| 82,374 | | |
| - | | |
| - | |
| |
| 82,374 | |
| Inventory | |
| 291,918 | | |
| 394,745 | | |
| - | |
| |
| 686,663 | |
| Total Current Assets | |
| 8,816,247 | | |
| 1,689,633 | | |
| (51,712 | ) |
| |
| 10,454,168 | |
| Non-Current Assets | |
| | | |
| | | |
| | |
| |
| | |
| Investment | |
| - | | |
| - | | |
| 4,301,293 | |
{b} | |
| - | |
| | |
| | | |
| | | |
| (4,301,293 | ) |
{b} | |
| | |
| Goodwill | |
| - | | |
| - | | |
| 3,812,093 | |
{c} | |
| 3,812,093 | |
| Property and equipment, net | |
| 221,958 | | |
| 1,809,711 | | |
| - | |
| |
| 2,031,669 | |
| Intangible assets, net | |
| 594 | | |
| - | | |
| - | |
| |
| 594 | |
| Deferred tax asset | |
| 16,951 | | |
| - | | |
| - | |
| |
| 16,951 | |
| Security deposits | |
| 89,578 | | |
| - | | |
| - | |
| |
| 89,578 | |
| Operating lease right-of-use assets | |
| 1,444,683 | | |
| 3,382 | | |
| - | |
| |
| 1,448,065 | |
| Total Non-Current Assets | |
| 1,773,764 | | |
| 1,813,093 | | |
| 3,812,093 | |
| |
| 7,398,950 | |
| Total Assets | |
$ | 10,590,011 | | |
$ | 3,502,726 | | |
$ | 3,760,381 | |
| |
$ | 17,853,118 | |
| | |
| | | |
| | | |
| | |
| |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |
| | | |
| | | |
| | |
| |
| | |
| Current Liabilities | |
| | | |
| | | |
| | |
| |
| | |
| Accounts payable | |
$ | 472,857 | | |
$ | 200,067 | | |
$ | 4,301,293 | |
{b} | |
$ | 4,922,505 | |
| | |
| | | |
| | | |
| (51,712 | ) |
{d} | |
| | |
| Accounts payable-related party | |
| - | | |
| 170,895 | | |
| - | |
| |
| 170,895 | |
| Unearned revenue | |
| 153,308 | | |
| 55,727 | | |
| - | |
| |
| 209,035 | |
| Taxes payable | |
| 214,761 | | |
| 22,932 | | |
| | |
| |
| 237,693 | |
| Accrued liabilities and other payables | |
| 1,051,912 | | |
| 1,620,489 | | |
| - | |
| |
| 2,672,401 | |
| Interest payable | |
| - | | |
| 11,127 | | |
| - | |
| |
| 11,127 | |
| Loan from third parties | |
| 89,578 | | |
| - | | |
| - | |
| |
| 89,578 | |
| Government grant payable | |
| - | | |
| 978,647 | | |
| - | |
| |
| 978,647 | |
| Operating lease liabilities - current | |
| 799,839 | | |
| 16,124 | | |
| - | |
| |
| 815,963 | |
| Advance from related parties | |
| 2,431,138 | | |
| 364,243 | | |
| (402,397 | ) |
{a} | |
| 2,392,984 | |
| Total Current Liabilities | |
| 5,213,393 | | |
| 3,440,251 | | |
| 3,847,184 | |
| |
| 12,500,828 | |
| Non-Current Liabilities | |
| | | |
| | | |
| | |
| |
| | |
| Operating lease liabilities - noncurrent | |
| 614,791 | | |
| - | | |
| - | |
| |
| 614,791 | |
| Total Non-Current Liabilities | |
| 614,791 | | |
| - | | |
| - | |
| |
| 614,791 | |
| Total Liabilities | |
| 5,828,184 | | |
| 3,440,251 | | |
| 3,847,184 | |
| |
| 13,115,619 | |
| Stockholders’ Equity | |
| | | |
| | | |
| | |
| |
| | |
| Common stock | |
| 500 | | |
| - | | |
| - | |
| |
| 500 | |
| Additional paid in capital | |
| 14,272,563 | | |
| 133,678 | | |
| (133,678 | ) |
{c} | |
| 14,272,563 | |
| Statutory reserve | |
| 151,988 | | |
| - | | |
| - | |
| |
| 151,988 | |
| Accumulated deficit | |
| (10,360,963 | ) | |
| (79,461 | ) | |
| 58,662 | |
{c} | |
| (10,381,762 | ) |
| Accumulated other comprehensive income (loss) | |
| 697,739 | | |
| 8,258 | | |
| (11,787 | ) |
{c} | |
| 694,210 | |
| Total Stockholders’ Equity | |
| 4,761,827 | | |
| 62,475 | | |
| (86,803 | ) |
| |
| 4,737,499 | |
| Total Liabilities and Stockholders’ Equity | |
$ | 10,590,011 | | |
$ | 3,502,726 | | |
$ | 3,760,381 | |
| |
$ | 17,853,118 | |
See
accompanying notes to pro forma consolidated financial statements
AIXIN
LIFE INTERNATIONAL, INC.
UNAUDITED
PRO FORMA CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
FOR
THE SIX MONTHS ENDED JUNE 30, 2022
| | |
AIXIN LIFE INTERNATIONAL, INC. | | |
YUNNAN RUNCANGSHENG TECHNOLOGY COMPANY LIMITED | | |
PRO FORMA ADJUSTMENTS | |
| |
PRO FORMA CONSOLIDATED | |
| | |
| | |
| | |
| |
| |
| |
| Revenue | |
| | | |
| | | |
| | |
| |
| | |
| Products | |
$ | 385,628 | | |
$ | 295,375 | | |
$ | (63,681 | ) |
{d} | |
$ | 617,322 | |
| Room revenue | |
| 94,716 | | |
| - | | |
| - | |
| |
| 94,716 | |
| Food and beverage revenue | |
| 292,519 | | |
| - | | |
| - | |
| |
| 292,519 | |
| Others | |
| 66,702 | | |
| - | | |
| - | |
| |
| 66,702 | |
| Total revenues, net | |
| 839,565 | | |
| 295,375 | | |
| (63,681 | ) |
| |
| 1,071,259 | |
| | |
| | | |
| | | |
| | |
| |
| | |
| Operating costs and expenses: | |
| | | |
| | | |
| | |
| |
| | |
| Cost of goods sold | |
| 278,522 | | |
| 125,850 | | |
| (63,681 | ) |
{d} | |
| 340,691 | |
| Hotel operating costs | |
| 929,719 | | |
| - | | |
| - | |
| |
| 929,719 | |
| Selling expenses | |
| 389,618 | | |
| 8,788 | | |
| - | |
| |
| 398,406 | |
| General and administrative expenses | |
| 519,471 | | |
| 200,297 | | |
| - | |
| |
| 719,768 | |
| Provision for bad debts | |
| 47,857 | | |
| - | | |
| - | |
| |
| 47,857 | |
| Stock-based compensation | |
| 185,770 | | |
| - | | |
| - | |
| |
| 185,770 | |
| Total operating costs and expenses | |
| 2,350,957 | | |
| 334,935 | | |
| (63,681 | ) |
| |
| 2,622,211 | |
| | |
| | | |
| | | |
| | |
| |
| | |
| Loss from operations | |
| (1,511,392 | ) | |
| (39,560 | ) | |
| - | |
| |
| (1,550,952 | ) |
| | |
| | | |
| | | |
| | |
| |
| | |
| Non-operating income (expenses) | |
| | | |
| | | |
| | |
| |
| | |
| Interest income | |
| 2,612 | | |
| 551 | | |
| - | |
| |
| 3,163 | |
| Other income | |
| 29,655 | | |
| 18,210 | | |
| - | |
| |
| 47,865 | |
| Other expenses | |
| (260 | ) | |
| - | | |
| - | |
| |
| (260 | ) |
| Total non-operating income, net | |
| 32,007 | | |
| 18,761 | | |
| - | |
| |
| 50,768 | |
| | |
| | | |
| | | |
| | |
| |
| | |
| Loss before income tax | |
| (1,479,385 | ) | |
| (20,799 | ) | |
| - | |
| |
| (1,500,184 | ) |
| | |
| | | |
| | | |
| | |
| |
| | |
| Income tax expense | |
| 965 | | |
| - | | |
| - | |
| |
| 965 | |
| | |
| | | |
| | | |
| | |
| |
| | |
| Net loss | |
| (1,480,350 | ) | |
| (20,799 | ) | |
| - | |
| |
| (1,501,149 | ) |
| | |
| | | |
| | | |
| | |
| |
| | |
| Other comprehensive items | |
| | | |
| | | |
| | |
| |
| | |
| Foreign currency translation loss | |
| (13,084 | ) | |
| (3,529 | ) | |
| - | |
| |
| (16,613 | ) |
| | |
| | | |
| | | |
| | |
| |
| | |
| Comprehensive loss | |
$ | (1,493,434 | ) | |
$ | (24,328 | ) | |
| - | |
| |
$ | (1,517,762 | ) |
| | |
| | | |
| | | |
| | |
| |
| | |
| Loss per share | |
$ | (0.030 | ) | |
$ | - | | |
| - | |
| |
$ | (0.030 | ) |
| | |
| | | |
| | | |
| | |
| |
| | |
| Weighted average shares outstanding | |
| 49,999,891 | | |
| - | | |
| - | |
| |
| 49,999,891 | |
See
accompanying notes to pro forma consolidated financial statements
AIXIN
LIFE INTERNATIONAL, INC.
UNAUDITED
PRO FORMA CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
FOR
THE YEAR ENDED DECEMBER 31, 2021
| | |
AIXIN LIFE INTERNATIONAL, INC. | | |
YUNNAN RUNCANGSHENG TECHNOLOGY COMPANY LIMITED | | |
PRO FORMA ADJUSTMENTS | | |
PRO FORMA CONSOLIDATED | |
| | |
| | |
| | |
| | |
| |
| Revenue | |
| | | |
| | | |
| | | |
| | |
| Products | |
$ | 742,624 | | |
$ | 783,149 | | |
| | | |
$ | 1,525,773 | |
| Advertising | |
| 1,944,811 | | |
| - | | |
| | | |
| 1,944,811 | |
| Room revenue | |
| 114,086 | | |
| - | | |
| | | |
| 114,086 | |
| Food and beverage revenue | |
| 201,755 | | |
| - | | |
| | | |
| 201,755 | |
| Others | |
| 62,957 | | |
| - | | |
| | | |
| 62,957 | |
| Total revenues, net | |
| 3,066,233 | | |
| 783,149 | | |
| | | |
| 3,849,382 | |
| | |
| | | |
| | | |
| | | |
| | |
| Operating costs and expenses: | |
| | | |
| | | |
| | | |
| | |
| Cost of goods sold | |
| 535,485 | | |
| 445,796 | | |
| | | |
| 981,281 | |
| Hotel operating costs | |
| 744,594 | | |
| - | | |
| | | |
| 744,594 | |
| Selling expenses | |
| 470,798 | | |
| 61,283 | | |
| | | |
| 532,081 | |
| General and administrative expenses | |
| 1,033,830 | | |
| 301,313 | | |
| | | |
| 1,335,143 | |
| Reversal of for bad debts | |
| (63,076 | ) | |
| - | | |
| | | |
| (63,076 | ) |
| Stock-based compensation | |
| 371,540 | | |
| - | | |
| | | |
| 371,540 | |
| Total operating costs and expenses | |
| 3,093,171 | | |
| 808,392 | | |
| | | |
| 3,901,563 | |
| | |
| | | |
| | | |
| | | |
| | |
| Loss from operations | |
| (26,938 | ) | |
| (25,243 | ) | |
| | | |
| (52,181 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Non-operating income (expenses) | |
| | | |
| | | |
| | | |
| | |
| Interest income | |
| 4,113 | | |
| 56 | | |
| | | |
| 4,169 | |
| Other income | |
| 63,064 | | |
| 4 | | |
| | | |
| 63,068 | |
| Other expenses | |
| (33,154 | ) | |
| - | | |
| | | |
| (33,154 | ) |
| Total non-operating income, net | |
| 34,023 | | |
| 60 | | |
| | | |
| 34,083 | |
| | |
| | | |
| | | |
| | | |
| | |
| Income (loss) before income tax | |
| 7,085 | | |
| (25,183 | ) | |
| | | |
| (18,098 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Income tax expense | |
| 274,321 | | |
| 10,582 | | |
| | | |
| 284,903 | |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| (267,236 | ) | |
| (35,765 | ) | |
| | | |
| (303,001 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Other comprehensive items | |
| | | |
| | | |
| | | |
| | |
| Foreign currency translation income | |
| 122,283 | | |
| 2,332 | | |
| | | |
| 124,615 | |
| | |
| | | |
| | | |
| | | |
| | |
| Comprehensive loss | |
$ | (144,953 | ) | |
$ | (33,433 | ) | |
| | | |
$ | (178,386 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Loss per share | |
$ | (0.005 | ) | |
$ | - | | |
| | | |
$ | (0.006 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted average shares outstanding | |
| 49,999,891 | | |
| - | | |
| | | |
| 49,999,891 | |
See
accompanying notes to pro forma consolidated financial statements
AIXIN
LIFE INTERNATIONAL, INC.
NOTES
TO UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
NOTE
1 - INTRODUCTION
On
July 19, 2022, HK Aixin International Group Co., Limited (“HK Aixin”), a wholly owned subsidiary of Aixin Life International,
Inc (“Aixin Life” or the “Company”) entered into an Equity Transfer Agreement (the “Transfer Agreement”)
with Yunnan Shengshengyuan Technology Co., Ltd, and Yun Chen (the “Sellers”), the shareholders of Yunnan Runcangsheng Technology
Company Ltd. (“Yunnan Runcangsheng”). Yunnan Shengshengyuan owns in excess of 95% of the outstanding equity the Yunnan Runcangsheng.
The remaining equity interest is owned by Yun Chen. Pursuant to the Transfer Agreement, HK Aixin agreed to purchase all of the outstanding
equity of Yunnan Runcangsheng for an aggregate purchase price of RMB 45,082,600, or approximately US$6.3 million (“Purchase Price”).
In contemplation of the transaction, Yunnan Runcangsheng will prepare a balance sheet based upon its books and records as of December
31, 2021. Consummation of the acquisition is subject to the completion of an audit of the financial statements of Yunnan Runcangsheng
showing that the net worth of Yunnan Runcangsheng as of December 31, 2021, is no less than RMB 1,530,000. In addition, if the net worth
of Yunnan Runcangsheng as of December 31, 2021, as shown by the audit is less than 97% of the net worth on the balance sheet as of that
date initially prepared by Yunnan Runcangsheng, the purchase price shall be reduced by the amount of the deficiency (“Adjusted
Purchase Price”). In addition to transferring their respective equity interest in Yunnan Runcangsheng by the Sellers, both Sellers
agree to forgive any loans Yunnan Runcangsheng due to them. The Transfer Agreement also provides that Aixin Life will be the beneficiary
of any profit generated by Yunnan Runcangsheng commencing January 1, 2022, and bear any loss it incurs from and after such date.
NOTE
2 - BASIS OF PRESENTATION
The
unaudited pro forma consolidated balance sheet as of June 30, 2022 combines the historical balance sheets of Aixin Life and Yunnan Runcangsheng
as if the Transactions had occurred on June 30, 2022. The unaudited pro forma consolidated statements of operations and comprehensive
loss for the six months ended June 30, 2022 and for the year ended December 31, 2021 combine the historical consolidated statements of
operations and comprehensive loss of Aixin Life and Yunnan Runcangsheng, and have been prepared as if the Transactions had closed on
January 1, 2022 and 2021, respectively. The unaudited pro forma consolidated financial statements have also been adjusted to give effect
to pro forma events that are directly attributable to the Transactions, factually supportable and expected to have a continuing impact
on the combined results.
The
preliminary unaudited pro forma information is presented solely for informational purposes and is not necessarily indicative of the consolidated
results of operations or financial position that might have been achieved for the periods or dates indicated, nor is it necessarily indicative
of the future results of the consolidated company.
NOTE
3 – PRO FORMA ADJUSTMENTS
The
following adjustments were made in the preparation of the unaudited pro forma consolidated balance sheet and unaudited pro forma consolidated
statements of operations and comprehensive loss:
{a}
Forgiveness of $402,397 outstanding balance of due to related parties at December 31, 2021 by the Sellers.
{b}
Represents the Adjusted Purchase Price to be paid by Aixin Life. The Adjusted Purchase Price is
based on the Purchase Price of $4,418,095, decreased by $116,802 resulting from the audited net worth of Yunnan Runcangsheng as
of December 31, 2021, being less than 97% of the net worth on the balance sheet as of that date initially prepared by Yunnan Runcangsheng.
{c}
Represents the goodwill arising from the Adjusted Purchase Price exceeded the net assets of Yunnan
Runcangsheng.
{d}
To eliminate the accounts payable to and purchase from Runcangsheng as of and during the six months ended June 30, 2022.
AIXIN
LIFE IINTERNATIONAL, INC.

$50,000,000
Shares
of Common Stock
PROSPECTUS
You
should rely only on the information contained in this prospectus. No dealer, salesperson or other person is authorized to give information
that is not contained in this prospectus. This prospectus is not an offer to sell nor is it seeking an offer to buy these securities
in any jurisdiction where the offer or sale is not permitted. The information contained in this prospectus is correct only as of the
date of this prospectus, regardless of the time of the delivery of this prospectus or the sale of these securities.
Until
, 2023, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver
a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriter with respect to
their unsold subscriptions.
The
date of this prospectus is , 2022
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
ITEM
13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The
following table sets forth the various expenses, all of which will be borne by us, in connection with the sale and distribution of the
securities being registered, other than the underwriter commissions. All amounts shown are estimates except for the Securities and Exchange
Commission registration fee and the FINRA filing fee.
| Description |
|
Amount |
|
| |
|
|
|
| Filing
Fee - Securities and Exchange Commission |
|
$ |
6,843.42. |
|
| FINRA
Filing Fee |
|
|
7,500 |
* |
| NASDAQ
Application and Listing Fee |
|
|
75,000 |
|
| Attorney’s
fees and expenses |
|
|
325,000 |
* |
| Accountant’s
fees and expenses |
|
|
125,000 |
* |
| Transfer
agent’s and registrar fees and expenses |
|
|
5,000 |
* |
| Printing
and engraving expenses |
|
|
7,500 |
* |
| Miscellaneous
expenses |
|
|
3,500 |
* |
| |
|
|
|
|
| Total |
|
$ |
555,353.42 |
* |
*
Estimated expenses.
ITEM
14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
The
Colorado Business Corporations Act provides that a director or officer is not individually liable to the corporation or its stockholders
or creditors for any damages as a result of any act or failure to act in his capacity as a director or officer unless it is proven that
his act or failure to act constituted a breach of his fiduciary duties as a director or officer and his breach of those duties involved
intentional misconduct, fraud or a knowing violation of law. The Articles of Incorporation or an amendment thereto may, however, provide
for greater individual liability.
This
provision is intended to afford directors and officers protection against and to limit their potential liability for monetary damages
resulting from suits alleging a breach of the duty of care by a director or officer. As a consequence of this provision, stockholders
of our company will be unable to recover monetary damages against directors or officers for action taken by them that may constitute
negligence or gross negligence in performance of their duties unless such conduct meets the requirements of Colorado law to impose such
liability. The provision, however, does not alter the applicable standards governing a director’s or officer’s fiduciary
duty and does not eliminate or limit the right of our company or any stockholder to obtain an injunction or any other type of non-monetary
relief in the event of a breach of fiduciary duty.
The
Colorado Business Corporations Act also provides that under certain circumstances, a corporation may indemnify any person for amounts
incurred in connection with a pending, threatened or completed action, suit or proceeding in which he is, or is threatened to be made,
a party by reason of his being a director, officer, employee or agent of the corporation or serving at the request of the corporation
as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, if such person
(a) is not liable for a breach of fiduciary duty involving intentional misconduct, fraud or a knowing violation of law or such greater
standard imposed by the corporation’s articles of incorporation; or (b) acted in good faith and in a manner which he reasonably
believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had
no reasonable cause to believe his conduct was unlawful. Additionally, a corporation may indemnify a director, officer, employee or agent
with respect to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its
favor, if such person (a) is not liable for a breach of fiduciary duty involving intentional misconduct, fraud or a knowing violation
of law or such greater standard imposed by the corporation’s articles of incorporation; or (b) acted in good faith and in a manner
which he reasonably believed to be in or not opposed to the best interests of the corporation, however, indemnification may not be made
for any claim, issue or matter as to which such a person has been adjudged by a court to be liable to the corporation or for amounts
paid in settlement to the corporation, unless the court determines that the person is fairly and reasonably entitled to indemnity for
such expenses as the court deems proper. To the extent that a director, officer, employee or agent of a corporation has been successful
on the merits or otherwise in defense of any action, suit or proceeding referred to above, or in defense of any claim, issue or matter
therein, the corporation shall indemnify him against expenses, including attorneys’ fees, actually and reasonably incurred by him
in connection with the defense.
The
Company’s By-laws provide in substance that every director and officer of the Company shall be entitled to indemnification against
expense actually and necessarily incurred in any action suit or proceeding, in which he or she may be named as a party by reason of being
or having been a director or officer of the Company, except to the extent that such officer is finally adjudicated to be liable for negligence
or misconduct.
Neither
our Bylaws nor our Articles of Incorporation include any specific indemnification provisions for our officers or directors against liability
under the Securities Act. Additionally, insofar as indemnification for liabilities arising under the Securities Act may be permitted
to directors, officers and controlling persons of the Company pursuant to the foregoing provisions, or otherwise, the Company has been
advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the
Securities Act and is, therefore, unenforceable.
ITEM
15. RECENT SALES OF UNREGISTERED SECURITIES.
Not
Applicable
ITEM
16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)
Exhibits.
Pursuant
to Item 601 of Regulation S-K:
A
list of exhibits filed with this registration statement on Form S-1 is set forth on the Exhibit Index and is incorporated herein by reference.
ITEM
17. UNDERTAKINGS.
The
undersigned registrant hereby undertakes:
(1)
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement to:
(i)
Include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii)
Reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities
offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range
may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume
and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration
Fee” table in the effective registration statement; and
(iii)
Include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any
material change to such information in the registration statement.
(2)
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed
to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof.
(3)
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering.
(4)
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser each prospectus filed by the registrant
pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part
of and included in the registration statement; and each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7)
as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x)
for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and
included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the
date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability
purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the
registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of
such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made
in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated
by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a
time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement
or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date
(5)
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling
persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of
the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred
or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is
asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless
in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the
question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication
of such issue.
EXHIBIT
INDEX
Exhibit
Number |
|
Description
of Document |
| 1.1** |
|
Form
of Underwriting Agreement |
| 3.1 |
|
Articles of Incorporation (incorporated by reference to the Company’s Annual Report on Form 10-KSB for the fiscal year ended May 31, 2006 as filed with the SEC on March 7, 2007). |
| 3.2 |
|
Articles of Amendment to Articles of Incorporation (incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on June 3, 2008). |
| 3.3 |
|
Articles of Amendment to Articles of Incorporation (incorporated by reference to Exhibit 3.3 the Company’s Quarterly Report on Form 10-Q for the quarterly period ended November 30, 2017 as filed with the SEC on January 16, 2018). |
3.4 |
|
Articles of Amendment to Articles of Incorporation (incorporated by reference to Appendix A to the 14C Information Schedule filed with the SEC on August 24, 2020). |
| 3.5 |
|
Bylaws of the Registrant (incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on June 3, 2008). |
| 4.1** |
|
Registrant’s
Specimen Certificate for Common Stock. |
| 4.2** |
|
Form
of Underwriter’s Warrant. |
| 5.1* |
|
Opinion of Ellenoff Grossman & Scholes LLP regarding the validity of the common stock being registered. |
| 10.1 |
|
2019 Incentive Stock Plan (incorporated by reference to Exhibit 10.1 to the Company’s Registration Statement on Form S-8 filed on January 10, 2019). |
| 10.2 |
|
English Translation of Equity Transfer Agreement with Respect to Shangyan Hotel Company (Incorporated by reference to Report on Form 8-K dated May 25, 2021). |
| 10.3 |
|
English Translation of Equity Transfer Agreement with respect to Chengdu Aixin Pharmacy Co., Ltd. and affiliated entities (Incorporated by reference to Report on Form 8-K dated June 2, 2021). |
| 10.4 |
|
English Translation of Equity Transfer Agreement among the Company, Chen Yun and Yunnan Sheng Shengyuan Technology Co., Ltd. (Incorporated by reference to Report on Form 8-K dated July 7, 2022)
|
| 10.5 |
|
English Translation of Supplementary Agreement to Equity Transfer Agreement among the Company, Yunnan Sheng Shengyan Technology Co., Ltd. And Chen Yun. (Incorporated by reference to Report on Form 8-K/A filed October 10, 2022). |
| 10.6 |
|
English Translation of Supplementary 2 Agreement to Equity Transfer Agreement among the Company, Yunnan Sheng Shengyan Technology Co., Ltd. And Chen Yun. (Incorporated by reference to Report on Form 8-K/A filed October 27, 2022). |
| 10.7** |
|
English
translation of Yunnan Runcangsheng Plant Lease Agreement |
| 21.1* |
|
List of Subsidiaries |
| 23.1* |
|
Consent of KCCW Accountancy Corp. |
| 23.2* |
|
Consent of Ellenoff Grossman & Schole LLP (included in Exhibit 5.1) |
| 23.3* |
|
Consent of Sichuan Junhe Law Firm |
| 24.1* |
|
Powers of Attorney (included on signature page to Registration Statement on Form F-1) |
| 99.1 |
|
Code of Business Conduct and Ethics (Incorporated by reference to Report on Form 8-K filed September 25, 2020) |
| 99.2 |
|
Form of Audit Committee Charter (Incorporated by reference to Report on Form 8-K filed September 25, 2020) |
| 99.3 |
|
Form of Compensation Committee Charter (Incorporated by reference to Report on Form 8-K filed September 25, 2020) |
| 99.4 |
|
Form of Nominating and Corporate Governance Committee Charter (Incorporated by reference to Report on Form 8-K filed September 25, 2020) |
| 107* |
|
Calculation
of Filing Fee table |
| * |
Filed herewith |
| ** |
To
be filed by amendment |
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement on Form S-1 to be signed
on its behalf by the undersigned, thereunto duly authorized, in the City of Chengdu, China, on November 4, 2022.
| |
AIXIN
LIFE INNTERNATIONAL, INC. |
| |
|
| |
/s/
Quanzhong Lin |
| |
Quanzhong
Lin |
| |
CEO,
President, Secretary and Director |
| |
(Principal
Executive Officer) |
POWER
OF ATTORNEY
KNOW
ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Quanzhong Lin and Yao-Te Wang,
and each acting individually, his true and lawful attorney-in-fact, with full power of substitution and re-substitution for
him and in his name, place and stead, in any and all capacities to sign any and all amendments including post-effective amendments to
this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities
and Exchange Commission, hereby ratifying and confirming all that said attorney-in-fact or his substitute, each acting alone, may lawfully
do or cause to be done by virtue thereof.
Pursuant
to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in
the capacities and on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/
Quanzhong Lin |
|
CEO,
President, Secretary and Director |
|
November
4, 2022 |
Quanzhong
Lin
|
|
(Principal
Executive Officer)
|
|
|
| /s/
Guolo Li |
|
CFO
and Treasurer |
|
November
4, 2022 |
| Guolo
Li |
|
(Principal
Financial and Accounting Officer) |
|
|
| |
|
|
|
|
| /s/
Yao-Te Wang |
|
Independent
Director |
|
November
4, 2022 |
| Yao-Te
Wang |
|
|
|
|
| |
|
|
|
|
| Christopher
Lee |
|
Independent
Director |
|
November
4, 2022 |
| Christopher
Lee |
|
|
|
|
| |
|
|
|
|
| Chang-Ping
Ling |
|
Independent
Director |
|
November
4, 2022 |
| Chang-Ping
Ling |
|
|
|
|
AiXin Life (QB) (USOTC:AIXN)
Historical Stock Chart
From Oct 2024 to Nov 2024
AiXin Life (QB) (USOTC:AIXN)
Historical Stock Chart
From Nov 2023 to Nov 2024