Filed
Pursuant to Rule 424(b)(5)
Registration
No. 333-278380
PROSPECTUS
SUPPLEMENT
(To
Prospectus dated May 10, 2024)
DARÉ
BIOSCIENCE, INC.

Up
to $18,089,630
Common
Stock
On
March 31, 2023, we entered into a sales agreement, or the Sales Agreement, with Stifel, Nicolaus & Company, Incorporated, or Stifel,
and Cantor Fitzgerald & Co., or Cantor, relating to the issuance and sale of shares of our common stock, $0.0001 par value per share,
including up to $18,089,630 of shares of common stock offered by this prospectus supplement and the accompanying prospectus. On April
30, 2024, we mutually agreed with Cantor to terminate the Sales Agreement with respect to Cantor. In accordance with the terms of the
Sales Agreement, we may offer and sell shares of our common stock from time to time through or to Stifel acting as our Sales Agent.
Our
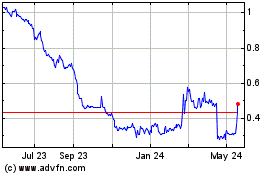
common stock is listed on the Nasdaq Capital Market under the symbol “DARE.” The last reported sale price of our common stock
on May 3, 2024 was $0.3112 per share. On March 28, 2024, the date we filed our Annual Report on Form 10-K for the fiscal year ended December
31, 2023, our prospectus became subject to the offering limits in General Instruction I.B.6 of Form S-3. As of the date hereof, the aggregate
market value of our common stock held by non-affiliates pursuant to General Instruction I.B.6 of Form S-3 is $54,830,751, which was calculated
based on 99,873,864 shares of our common stock outstanding held by non-affiliates and a price of $0.5490 per share, the closing price
of our common stock on March 27, 2024. As of the date of this prospectus supplement, we have offered and sold $187,287 of shares of our
common stock pursuant to General Instruction I.B.6 of Form S-3 during the 12 calendar months prior to, and including, the date of this
prospectus supplement. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell securities in public primary offerings
on Form S-3 with a value exceeding more than one-third of our public float (as defined by General Instruction I.B.6) in any 12 calendar
month period so long as our public float remains below $75 million.
Sales
of our common stock, if any, under this prospectus supplement and the accompanying prospectus may be made by any method deemed to be
an “at the market offering” as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended, or the
Securities Act, including sales made directly on the Nasdaq Capital Market or any other trading market for our common stock. The Sales
Agent will use commercially reasonable efforts to sell on our behalf the shares of our common stock requested by us to be sold, consistent
with their normal trading and sales practices, on mutually agreed terms set forth in the Sales Agreement. There is no arrangement for
funds to be received in any escrow, trust or similar arrangement.
The
compensation to the Sales Agent for sales of our common stock pursuant to the Sales Agreement will be 3.0% of the gross proceeds of the
sales price per share or such lower amount as we and the Sales Agent may agree. See “Plan of Distribution” beginning on page
S-11 for additional information regarding the compensation to be paid to the Sales Agent. In connection with the sale of our common
stock on our behalf, the Sales Agent will be deemed to be an “underwriter” within the meaning of the Securities Act and the
compensation of the Sales Agent will be deemed to be underwriting commissions or discounts. We have also agreed to provide indemnification
and contribution to the Sales Agent with respect to certain liabilities, including liabilities under the Securities Act or the Securities
Exchange Act of 1934, as amended, or the Exchange Act.
Investing
in our common stock involves a high degree of risk. Before deciding whether to purchase shares of our common stock, you should carefully
consider the risks and uncertainties that we have described under the caption “Risk Factors” beginning on page S-6 of this
prospectus supplement, as well as the information under similar captions in the documents incorporated by reference in this prospectus
supplement.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal
offense.
Stifel
The
date of this prospectus supplement is May 10, 2024
TABLE
OF CONTENTS
PROSPECTUS
SUPPLEMENT
PROSPECTUS
ABOUT
THIS PROSPECTUS SUPPLEMENT
This
document is in two parts. The first part is the prospectus supplement, including the documents incorporated by reference, which describes
the specific terms of this offering. The second part, the accompanying prospectus, including the documents incorporated by reference,
provides more general information. Before you invest, you should carefully read this prospectus supplement, the accompanying prospectus,
all information incorporated by reference herein and therein, as well as the additional information described under “Where You
Can Find Additional Information” on page S-13 of this prospectus supplement. These documents contain information you should consider
when making your investment decision. This prospectus supplement may add, update or change information contained in the accompanying
prospectus. To the extent there is a conflict between the information contained in this prospectus supplement, on the one hand, and the
information contained in the accompanying prospectus or any document incorporated by reference therein filed prior to the date of this
prospectus supplement, on the other hand, you should rely on the information in this prospectus supplement. If any statement in one of
these documents is inconsistent with a statement in another document having a later date—for example, a document filed after the
date of this prospectus supplement and incorporated by reference in this prospectus supplement and the accompanying prospectus—the
statement in the document having the later date modifies or supersedes the earlier statement.
You
should rely only on the information contained or incorporated by reference in this prospectus supplement, the accompanying prospectus
and in any free writing prospectus we may provide to you in connection with this offering. We have not, and the Sales Agent has not,
authorized any other person to provide you with any information that is different. If anyone provides you with different or inconsistent
information, you should not rely on it. We are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions
where offers and sales are permitted. The distribution of this prospectus supplement and the accompanying prospectus and the offering
of the common stock in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of
this prospectus supplement and the accompanying prospectus must inform themselves about, and observe any restrictions relating to, the
offering of the common stock and the distribution of this prospectus supplement and the accompanying prospectus outside the United States.
This prospectus supplement and the accompanying prospectus do not constitute, and may not be used in connection with, an offer to sell,
or a solicitation of an offer to buy, any securities offered by this prospectus supplement and the accompanying prospectus by any person
in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
We
further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document
that is incorporated by reference in this prospectus supplement and the accompanying prospectus were made solely for the benefit of the
parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should
not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate
only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing
the current state of our affairs.
Unless
the context otherwise requires, “Daré,” “Daré Bioscience,” “the Company,” “we,”
“us,” “our” and similar terms refer to Daré Bioscience, Inc. and its subsidiaries. When we refer to “you”
we mean potential holders of the securities we may offer under this prospectus supplement and the accompanying prospectus.
PROSPECTUS
SUPPLEMENT SUMMARY
This
summary highlights selected information about us, this offering and information appearing elsewhere in this prospectus supplement and
in the documents we incorporate by reference. This summary is not complete and does not contain all the information you should consider
before investing in our common stock pursuant to this prospectus supplement and the accompanying prospectus. Before making an investment
decision, to fully understand this offering and its consequences to you, you should carefully read this entire prospectus supplement
and the accompanying prospectus, including the financial statements and related notes and the other information that we incorporated
by reference herein, including our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K that we
file from time to time.
About
Daré Bioscience
We
are a biopharmaceutical company committed to advancing innovative products for women’s health. We are driven by a mission to identify,
develop and bring to market a diverse portfolio of differentiated therapies that prioritize women’s health and well-being, expand
treatment options, and improve outcomes, primarily in the areas of contraception, vaginal health, reproductive health, menopause, sexual
health and fertility. Our business strategy is to in-license or otherwise acquire the rights to differentiated product candidates in
our areas of focus, some of which have existing clinical proof-of-concept data, to take those candidates through mid to late-stage clinical
development or regulatory approval, and to establish and leverage strategic collaborations to achieve commercialization. We and our wholly
owned subsidiaries operate in one business segment.
The
first FDA-approved product to emerge from our portfolio of women’s health product candidates is XACIATO (clindamycin phosphate)
vaginal gel 2%, or XACIATO (pronounced zah-she-AH-toe). XACIATO was approved by the FDA in December 2021 as a single-dose prescription
medication for the treatment of bacterial vaginosis in females 12 years of age and older. In March 2022, we entered into an agreement
with an affiliate of Organon & Co., Organon International GmbH, or Organon, which became fully effective in June 2022, whereby Organon
licensed exclusive worldwide rights to develop, manufacture and commercialize XACIATO. Accordingly, our potential future revenue from
the commercialization of XACIATO will consist of royalties based on net sales and milestone payments from Organon. Organon commenced
U.S. marketing of XACIATO in the fourth quarter of 2023.
Our
product pipeline includes diverse programs that target unmet needs in women’s health in the areas of contraception, vaginal health,
reproductive health, menopause, sexual health and fertility, and aim to expand treatment options, enhance outcomes and improve ease of
use for women. We are primarily focused on progressing the development of our existing portfolio of product candidates. However, we also
explore opportunities to expand our portfolio by leveraging assets to which we hold rights or obtaining rights to new assets, with continued
focus solely on women’s health.
Our
current portfolio includes five product candidates in advanced clinical development (Phase 2-ready to Phase 3):
| ● | Ovaprene®,
a hormone-free, monthly intravaginal contraceptive; |
| ● | Sildenafil
Cream, 3.6%, a proprietary cream formulation of sildenafil for topical administration
to the female genitalia on demand for the treatment of female sexual arousal disorder (FSAD); |
| ● | DARE-HRT1,
an intravaginal ring designed to deliver both bio-identical estradiol and progesterone together,
continuously over a 28-day period, for the treatment of moderate-to-severe vasomotor symptoms,
as part of menopausal hormone therapy; |
| ● | DARE-VVA1,
a proprietary formulation of tamoxifen for intravaginal administration being developed as
a hormone-free alternative to estrogen-based therapies for the treatment of moderate-to-severe
dyspareunia, or pain during sexual intercourse, a symptom of vulvar and vaginal atrophy associated
with menopause; and |
| ● | DARE-CIN,
a proprietary, fixed-dose formulation of lopinavir and ritonavir in a soft gel vaginal insert
for the treatment of cervical intraepithelial neoplasia (CIN) and other human papillomavirus
(HPV)-related pathologies. |
Our
portfolio also includes five product candidates in Phase 1 clinical development or that we believe are Phase 1-ready:
| ● | DARE-PDM1,
a proprietary hydrogel formulation of diclofenac, a nonsteroidal anti-inflammatory drug,
for vaginal administration as a treatment for primary dysmenorrhea; |
| |
● |
DARE-204 and DARE-214, injectable formulations
of etonogestrel designed to provide contraception over 6-month and 12-month periods, respectively; |
| |
● |
DARE-FRT1, an intravaginal ring designed to deliver bio-identical
progesterone continuously for up to 14 days for luteal phase support as part of an in vitro fertilization treatment plan; and |
| |
● |
DARE-PTB1, an intravaginal ring designed to deliver bio-identical
progesterone continuously for up to 14 days for the prevention of preterm birth. |
In
addition, our portfolio includes five preclinical stage programs:
| |
● |
DARE-LARC1, a contraceptive implant delivering levonorgestrel
with a woman-centered design that has the potential to be a long-acting, yet convenient and user-controlled contraceptive option; |
| |
● |
DARE-LBT, a novel hydrogel formulation for vaginal delivery
of live biotherapeutics to support vaginal health; |
| |
● |
DARE-GML, an intravaginally-delivered potential multi-target
antimicrobial agent formulated with glycerol monolaurate (GML), which has shown broad antimicrobial activity, killing bacteria and viruses; |
| |
● |
DARE-RH1, a novel approach to non-hormonal contraception
for both men and women by targeting the CatSper ion channel; and |
| |
● |
DARE-PTB2, a novel approach for the prevention and treatment
of idiopathic preterm birth through inhibition of a stress response protein. |
The
product candidates and potential product candidates in our portfolio will require review and approval from the FDA, or a comparable foreign
regulatory authority, prior to being marketed or sold. See ITEM 1. “BUSINESS,” in Part I of our Annual Report on Form 10-K
for the year ended December 31, 2023 for additional information regarding our product and product candidates.
Our
primary operations have consisted of research and development activities to advance our portfolio of product candidates through late-stage
clinical development and/or regulatory approval. We expect our research and development expenses will continue to represent the majority
of our operating expenses for at least the next twelve months. Until we secure additional capital to fund our operating needs, we will
focus our resources primarily on advancement of Ovaprene and Sildenafil Cream. In addition, we expect to incur significant research and
development expenses for the DARE-LARC1 program for the next several years, but we also expect such expenses will be supported by non-dilutive
funding provided under a grant agreement we entered into in June 2021.
We
will need to raise substantial additional capital to continue to fund our operations and execute our current business strategy. We are
also subject to a number of other risks common to biopharmaceutical companies, including, but not limited to, dependence on key employees,
reliance on third-party collaborators, service providers and suppliers, being able to develop commercially viable products in a timely
and cost-effective manner, dependence on intellectual property we own or in-license and the need to protect that intellectual property
and maintain those license agreements, uncertainty of market acceptance of products, uncertainty of third-party payor coverage, pricing
and reimbursement for products, rapid technology change, intense competition, compliance with government regulations, product liability
claims, and exposure to cybersecurity threats and incidents.
The
process of developing and obtaining regulatory approvals for prescription drug and drug/device products in the United States and in foreign
jurisdictions is inherently uncertain and requires the expenditure of substantial financial resources without any guarantee of success.
To the extent we receive regulatory approvals to market and sell our product candidates, the commercialization of any product and compliance
with subsequently applicable laws and regulations requires the expenditure of further substantial financial resources without any guarantee
of commercial success. The amount of post-approval financial resources required for commercialization and the potential revenue we may
receive from sales of any product will vary significantly depending on many factors, including whether, and the extent to which, we establish
our own sales and marketing capabilities and/or enter into and maintain commercial collaborations with third parties with established
commercialization infrastructure.
Additional
Information
For
additional information related to our business and operations, please refer to the annual and quarterly reports incorporated herein by
reference, as described under the caption “Incorporation of Documents by Reference” on page S-13 of this prospectus supplement.
Corporate
Information
We
are incorporated under the laws of the State of Delaware. Our principal executive offices are located at 3655 Nobel Drive, Suite 260,
San Diego, California 92122, and our telephone number at that address is (858) 926-7655. We maintain a website at www.darebioscience.com,
to which we regularly post copies of our press releases as well as additional information about us. The information contained on, or
that can be accessed through, our website is not a part of this prospectus supplement. We have included our website address in this prospectus
supplement solely as an inactive textual reference.
Daré
Bioscience® is a registered trademark of Daré Bioscience, Inc. Ovaprene® is a registered trademark licensed to Daré
Bioscience, Inc. All brand names or trademarks appearing in this prospectus supplement are the property of their respective holders.
Use or display by us of other parties’ trademarks, trade dress, or products in this prospectus supplement is not intended to, and
does not, imply a relationship with, or endorsements or sponsorship of, us by the trademark or trade dress owners.
The
Offering
| Issuer |
|
Daré
Bioscience, Inc. |
| |
|
|
| Common
stock offered by us |
|
Shares
of our common stock having an aggregate offering price of not more than $18,089,630. |
| |
|
|
| Common
stock to be outstanding after this offering |
|
Up
to 158,102,563 shares, assuming sales of 58,128,631 shares of our common stock in this offering at an assumed offering price of $0.3112
per share, which was the last reported sale price of our common stock on the Nasdaq Capital Market on May 3, 2024. The actual number
of shares issued will vary depending on the sales prices at which our common stock is sold under this offering. |
| |
|
|
| Plan
of Distribution |
|
“At
the market offering” that may be made from time to time on the Nasdaq Capital Market or any other existing trading market for
our common stock through or to our sales agent, Stifel. See “Plan of Distribution” on page S-11 of this prospectus supplement. |
| |
|
|
| Use
of proceeds |
|
We
intend to use the net proceeds, if any, for general corporate purposes, which may include, among other things, increasing working
capital and funding operating expenses and capital expenditures to advance the development of and help bring to market our product
candidates. See “Use of Proceeds” on page S-9 of this prospectus supplement. |
| |
|
|
| Risk
factors |
|
Investment
in our common stock involves a high degree of risk. You should read the “Risk Factors” section of this prospectus supplement
on page S-6, as well as the other information included or incorporated by reference in this prospectus supplement and the accompanying
prospectus, for a discussion of risks and uncertainties you should carefully consider before deciding to purchase shares of our common
stock. |
| |
|
|
| Nasdaq
Capital Market symbol |
|
DARE |
The
number of our shares of common stock to be outstanding after this offering is based on 99,973,932 shares of our common stock outstanding
as of December 31, 2023 and excludes:
| ● | 15,006,500
shares of our common stock issuable upon exercise of warrants outstanding as of December
31, 2023, having a weighted-average exercise price of $0.63 per share; |
| ● | 9,463,556
shares of our common stock issuable upon exercise of options outstanding as of December 31,
2023, having a weighted-average exercise price of $1.46 per share; |
| ● | 6,725,579
shares of our common stock reserved and available as of December 31, 2023 for future issuance
under our 2022 Stock Incentive Plan; and |
| ● | 1,118,968
shares of our common stock sold under the Sales Agreement after December 31, 2023 and on
or before May 3, 2024. |
To
the extent that outstanding options or warrants have been or may be exercised, new equity awards were or are issued, shares of our common
stock are sold under our employee stock purchase plan, or we otherwise issued or sell equity or convertible debt securities, the issuance
of these securities could result in further dilution to our stockholders.
RISK
FACTORS
Investing
in our common stock involves a high degree of risk and uncertainty. In addition to the other information included or incorporated by
reference in this prospectus supplement and the accompanying prospectus, you should carefully consider the risks described below, before
making an investment decision with respect to our common stock. We expect to update these Risk Factors from time to time in the periodic
and current reports that we file with the SEC after the date of this prospectus supplement. These updated Risk Factors will be incorporated
by reference in this prospectus supplement and the accompanying prospectus. Please refer to these subsequent reports for additional information
relating to the risks associated with investing in our common stock. If any of such risks and uncertainties actually occurs, our business,
financial condition, and results of operations could be severely harmed. This could cause the trading price of our common stock to decline,
and you could lose all or part of your investment.
Risks
Related to this Offering
Resales
of our common stock in the public market by our stockholders during this offering may cause the market price of our common stock to fall.
We
may issue shares of our common stock from time to time in connection with this offering. The issuance from time to time of these new
shares, or our ability to issue new shares of common stock in this offering, could result in resales of our common stock by our current
stockholders concerned about the potential ownership dilution of their holdings. In turn, these resales could have the effect of depressing
the market price for our common stock.
There
may be future sales or other dilution of our equity, which may adversely affect the market price of our common stock.
We
are generally not restricted from issuing additional common stock, including any securities that are convertible into or exchangeable
for, or that represent the right to receive, common stock. The market price of our common stock could decline as a result of sales of
common stock or securities that are convertible into or exchangeable for, or that represent the right to receive, common stock after
this offering or the perception that such sales could occur.
Our
management will have broad discretion over the use of the net proceeds from this offering, you may not agree with how we use the proceeds
and any proceeds invested may not be invested successfully.
We
have not designated any portion of the net proceeds from this offering, if any, to be used for any particular purpose. Accordingly, our
management will have broad discretion as to the use of the net proceeds from this offering and could use them for purposes other than
those contemplated at the time of commencement of this offering. Accordingly, you will be relying on the judgment of our management with
regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether
the proceeds are being used appropriately. It is possible that, pending their use, we may invest the net proceeds in a way that does
not yield a favorable, or any, return for our company.
The
common stock offered hereby will be sold in “at the market offerings” and investors who buy shares at different times will
likely pay different prices.
Investors
who purchase shares in this offering at different times will likely pay different prices, and accordingly may experience different levels
of dilution and different outcomes in their investment results. We will have discretion, subject to market demand, to vary the timing,
prices and number of shares sold in this offering. Investors may experience a decline in the value of the shares they purchase in this
offering as a result of sales made at prices lower than the prices they paid.
The
actual number of shares we will issue under the Sales Agreement, at any one time or in total, is uncertain.
Subject
to certain limitations in the Sales Agreement and compliance with applicable law, we have the discretion to deliver a placement notice
to the Sales Agent at any time throughout the term of the Sales Agreement. The number of shares that are sold by a Sales Agent after
delivering a placement notice will fluctuate based on the market price of our common stock during the sales period and limits we set
with such Sales Agent. Because the price per share of each share sold will fluctuate based on the market price of our common stock during
the sales period, it is not possible at this stage to predict the number of shares that will be ultimately issued.
You
may experience immediate and substantial dilution in the book value per share of the common stock you purchase.
Because
the prices per share at which shares of our common stock are sold in this offering may be substantially higher than the book value per
share of our common stock, you may suffer immediate and substantial dilution in the net tangible book value of the common stock you purchase
in this offering. The shares sold in this offering, if any, will be sold from time to time at various prices. After giving effect to
the sale of our common stock in the maximum aggregate offering amount of $18,089,630 at an assumed offering price of $0.3112 per share,
which was the last reported sale price of our common stock on the Nasdaq Capital Market on May 3, 2024, and after deducting estimated
offering commissions and expenses payable by us, our net tangible book value as of December 31, 2023 would have been approximately $12.37
million, or $0.08 per share of common stock. This represents an immediate dilution of $0.23 in net tangible book value per share to purchasers
of our common stock in this offering and an immediate accretion in as-adjusted net tangible book value of approximately $0.13 per share
to our existing stockholders. See “Dilution” below for a more detailed discussion of the dilution you may incur in connection
with this offering.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus supplement, the accompanying prospectus and our SEC filings that are incorporated by reference into this prospectus supplement
and the accompanying prospectus contain or incorporate by reference forward-looking statements within the meaning of Section 27A of the
Securities Act and Section 21E of the Exchange Act. All statements, other than statements of historical fact, included or incorporated
by reference in this prospectus supplement and the accompanying prospectus regarding development and potential regulatory review or approval
and commercialization of our products, our future financial position, future operations, strategy, prospects, collaborations, competition,
intellectual property, objectives of management, and compliance with Nasdaq Capital Market listing standards are forward-looking statements.
Forward-looking statements may include, but are not limited to, any statements concerning:
| ● | the
anticipated timing, structure and results of clinical and nonclinical development of our
product candidates; |
| ● | the
anticipated pathway, timing and outcome of the regulatory review process for our product
candidates; |
| ● | the
plans, strategies and objectives of management for future operations, partnerships and other
collaborations; |
| ● | proposed
new products, services or developments; |
| ● | our
future funding requirements, the sufficiency of our cash resources and our need for additional
capital resources; |
| ● | our
future financial performance; |
| ● | future
economic and industry conditions; |
| ● | our
ability to protect our intellectual property and operate our business without infringing
upon the intellectual property rights of others; and |
| ● | our
intended use of the net proceeds from offerings of our securities under this prospectus supplement. |
The
words believe,” “may,” “will,” “estimate,” “continue,” “anticipate,”
“design,” “intend,” “expect,” “could,” “plan,” “potential,” “predict,”
“seek,” “should,” “would,” “contemplate,” project,” “target,” “tend
to,” or the negative version of these words and similar expressions are intended to identify forward-looking statements, although
not all forward-looking statements contain these identifying words. Forward-looking statements reflect our current views with respect
to future events, are based on assumptions and are subject to risks and uncertainties. We cannot guarantee that we actually will achieve
the plans, intentions or expectations expressed in our forward-looking statements and you should not place undue reliance on these statements.
There are a number of important factors that could cause our actual results to differ materially from those indicated or implied by forward-looking
statements. These important factors include those discussed under the heading “Risk Factors” contained or incorporated in
this prospectus supplement and the accompanying prospectus and any free writing prospectus we may authorize for use in connection with
a specific offering. These factors and the other cautionary statements made in this prospectus supplement and the accompanying prospectus
should be read as being applicable to all related forward-looking statements whenever they appear in this prospectus supplement and the
accompanying prospectus. Except as required by law, we do not assume any obligation to update any forward-looking statement. We disclaim
any intention or obligation to update or revise any forward-looking statement, whether as a result of new information, future events
or otherwise.
USE
OF PROCEEDS
We
may issue and sell shares of our common stock having aggregate sales proceeds of up to $18,089,630 from time to time. The amount of proceeds
from this offering will depend upon the number of shares of our common stock sold and the market price at which they are sold, if any,
and is not determinable at this time. We intend to use the net proceeds from this offering, if any, for general corporate purposes, which
include, but are not limited to, increasing working capital and funding operating expenses to advance the development of and help bring
to market our product candidates. We may also use a portion of the net proceeds to acquire or invest in complementary businesses, product
candidates or technologies, although we have no current commitments or agreements with respect to any such acquisitions or investments
as of the date of this prospectus supplement. We have not determined the amount of net proceeds to be used specifically for the purposes
described above. We will have broad discretion in the use of the net proceeds from this offering, if any. Pending use of the net proceeds,
we intend to invest the proceeds in interest-bearing, marketable securities.
DILUTION
If
you invest in our common stock, your interest will be diluted immediately to the extent of the difference between the public offering
price per share and the adjusted net tangible book value per share of our common stock after this offering.
The
historical net tangible book value of our common stock as of December 31, 2023, was approximately negative $5.05 million, or approximately
negative $0.05 per share. Net tangible book value per share represents the amount of our total tangible assets, excluding goodwill and
intangible assets, less total liabilities, divided by the total number of shares of our common stock outstanding. Dilution per share
to new investors represents the difference between the amount per share paid by purchasers for each share of common stock in this offering
and the net tangible book value per share of our common stock immediately following the completion of this offering.
After
giving effect to the sale of shares of our common stock in the aggregate amount of $18.09 million at an assumed offering price of $0.3112
per share, the last reported sale price of our common stock on May 3, 2024 on the Nasdaq Capital Market, and after deducting estimated
commissions and estimated offering expenses, our pro forma as-adjusted net tangible book value as of December 31, 2023 would have been
approximately $12.37 million, or approximately $0.08 per share. This represents an immediate dilution of $0.23 in pro forma net tangible
book value per share to purchasers of our common stock in this offering and an immediate accretion in pro forma as-adjusted net tangible
book value of approximately $0.13 per share to our existing stockholders, as illustrated by the following table:
| Assumed offering price per share of common stock | |
| | |
| $ |
0.31 |
|
| Historical net tangible book value per share as of December 31, 2023 | |
$ | (0.05 | ) |
|
|
|
|
| Increase in pro forma net tangible book value per share attributable to this offering | |
$ | 0.13 | |
|
|
|
|
| Pro forma as adjusted net tangible book value per share after giving effect to this offering | |
| | |
| $ |
0.08 |
|
| Dilution per share to new investors | |
| | |
| $ |
0.23 |
|
The
table above assumes, for illustrative purposes only, an aggregate of 58,128,631 shares of our common stock are sold at a price of $0.3112
per share, for aggregate gross proceeds of $18,089,630. The shares, if any, sold in this offering will be sold from time to time at various
prices. An increase of $0.031 per share in the price at which the shares are sold from the assumed offering price of $0.3112 per share
shown in the table above, assuming all of our common stock in the aggregate amount of $18,089,630 is sold at that price, would result
in an increase of $0.003 to our pro forma as-adjusted net tangible book value per share after the offering and result in an immediate
dilution of $0.26 in pro forma as-adjusted net tangible book value per share to purchasers of our common stock in this offering, after
deducting commissions and estimated aggregate offering expenses payable by us. A decrease of $0.031 per share in the price at which the
shares are sold from the assumed offering price of $0.3112 per share shown in the table above, assuming all of our common stock in the
aggregate amount of $18,089,630 is sold at that price, would result in a decrease of $0.003 to our pro forma as-adjusted net tangible
book value per share after the offering and result in an immediate dilution of $0.20 in pro forma as-adjusted net tangible book value
per share to purchasers of our common stock in this offering, after deducting commissions and estimated aggregate offering expenses payable
by us. This information is supplied for illustrative purposes only.
The
information in the table above is based on 99,973,932 shares of our common stock outstanding as of December 31, 2023 and excludes:
| ● | 15,006,500
shares of our common stock issuable upon exercise of warrants outstanding as of December
31, 2023, having a weighted-average exercise price of $0.63 per share; |
| ● | 9,463,556
shares of our common stock issuable upon exercise of options outstanding as of December 31,
2023, having a weighted-average exercise price of $1.46 per share; |
| ● | 6,725,579
shares of our common stock reserved and available as of December 31, 2023 for future issuance
under our 2022 Stock Incentive Plan; and |
| ● | 1,118,968
shares of our common stock sold under the Sales Agreement after December 31, 2023 and on
or before May 3, 2024. |
To
the extent that outstanding options or warrants have been or may be exercised, new equity awards were or are issued, shares of our common
stock are sold under our employee stock purchase plan, or we otherwise issued or sell equity or convertible debt securities, the issuance
of these securities could result in further dilution to our stockholders.
PLAN
OF DISTRIBUTION
We
have entered into the Sales Agreement with Stifel, Nicolaus & Company, Incorporated as the Sales Agent, under which we may issue
and sell shares of our common stock, including shares of common stock having an aggregate gross sales price of up to $18,089,630 from
time to time through or to the Sales Agent pursuant to this prospectus supplement and the accompanying prospectus. The Sales Agreement
has been filed as an exhibit to a current report on Form 8-K filed under the Exchange Act and incorporated by reference in this prospectus
supplement and the accompanying prospectus.
Upon
delivery of a placement notice and subject to the terms and conditions of the Sales Agreement, the Sales Agent may sell our common stock
by any method permitted by law deemed to be an “at the market offering” as defined in Rule 415 of the Securities Act, including
sales made directly on the Nasdaq Capital Market, on any other existing trading market for our common stock, or to or through a market
maker. We may instruct the Sales Agent not to sell common stock if the sales cannot be effected at or above the price designated by us
from time to time. We or the Sales Agent may suspend the offering of common stock upon notice and subject to other conditions.
We
will pay the Sales Agent commissions, in cash, for its services in acting as sales agent in the sale of our common stock. The Sales Agent
will be entitled to compensation at a fixed commission rate of 3.0% of the gross sales price per share sold or such lower amount as we
and the Sales Agent may agree. Because there is no minimum offering amount required as a condition to close this offering, the actual
total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. We also agreed to pay the fees
and associated expenses of the Sales Agent’s outside legal counsel for filings with the FINRA Corporate Financing Department in
an amount not to exceed $15,000 and the reasonable fees and disbursements of the Sales Agent’s outside legal counsel in an amount
not to exceed $75,000 arising out of the execution of the Sales Agreement and our delivery of the initial placement notice under the
Sales Agreement. We estimate that the total initial expenses of the offering payable by us, excluding commissions payable to the Sales
Agent under the Sales Agreement, will be approximately $125,000. We have also agreed to pay the ongoing fees and associated expenses
of the Sales Agent’s outside legal counsel in an amount not to exceed $15,000 per quarter arising out of each quarterly representation
date under the Sales Agreement.
The
remaining sales proceeds, after deducting any expenses payable by us and any transaction fees imposed by any governmental, regulatory
or self-regulatory organization in connection with this offering, will equal our net proceeds for the sale of such shares of our common
stock.
If
acting as a sales agent, the Sales Agent will provide written confirmation to us no later than the opening of the trading day on the
Nasdaq Capital Market after each trading day on which common stock is sold through it as sales agent under the Sales Agreement. Each
confirmation will include the number or amount of shares sold through it as sales agent on that day, the volume-weighted average price
of the shares sold and the net proceeds to us from such sales.
Settlement
for sales of common stock will occur on the second trading day following the date on which any sales are made, or on some other date
that is agreed upon by us and the Sales Agent in connection with a particular transaction, in return for payment of the net proceeds
to us. Sales of our common stock as contemplated in this prospectus supplement will be settled through the facilities of The Depository
Trust Company or by such other means as we and the Sales Agent may agree upon. There is no arrangement for funds to be received in an
escrow, trust or similar arrangement.
To
the extent any sales are made, we will report at least quarterly the number of shares of our common stock sold through the Sales Agent
under the Sales Agreement, the net proceeds to us and the compensation paid by us to the Sales Agent in connection with the sales of
our common stock.
The
Sales Agent will use their commercially reasonable efforts, consistent with their sales and trading practices, to solicit offers to purchase
shares of our common stock under the terms and subject to the conditions set forth in the Sales Agreement. In connection with the sale
of the common stock on our behalf, the Sales Agent will be deemed to be an “underwriter” within the meaning of the Securities
Act and the compensation of the Sales Agent will be deemed to be underwriting commissions or discounts. We have agreed to provide indemnification
and contribution to the Sales Agent against certain civil liabilities, including liabilities under the Securities Act.
This
offering of our common stock pursuant to the Sales Agreement will terminate upon the termination of the Sales Agreement as permitted
therein. We and the Sales Agent may each terminate the Sales Agreement at any time upon five days’ prior notice.
The
Sales Agent and/or its affiliates have provided, and may in the future provide, various investment banking, commercial banking and other
financial services for us and our affiliates, for which services they may in the future receive customary fees. The Sales Agent will
not engage in any transactions that stabilize our common stock. To the extent required by Regulation M, the Sales Agent will not engage
in any market making activities involving our common stock while the offering is ongoing under this prospectus supplement and the accompanying
prospectus.
This
prospectus supplement and the accompanying prospectus in electronic format may be made available on a website maintained by the Sales
Agent and the Sales Agent may distribute this prospectus supplement and the accompanying prospectus electronically.
LEGAL
MATTERS
Mintz,
Levin, Cohn, Ferris, Glovsky and Popeo, P.C., San Diego, California, will pass upon certain legal matters in connection with the offering
and validity of the securities offered by this prospectus supplement. Stifel is being represented in connection with this offering by
Duane Morris LLP, New York, New York.
EXPERTS
Haskell
& White LLP, an independent registered public accounting firm, has audited our consolidated financial statements included in our
Annual Report on Form 10-K for the year ended December 31, 2023, as set forth in its report, which is incorporated by reference in this
prospectus supplement and the registration statement. Our financial statements are incorporated by reference in reliance on Haskell &
White LLP’s report, given on the authority of said firm as experts in accounting and auditing.
Mayer
Hoffman McCann P.C., an independent registered public accounting firm, has audited our consolidated financial statements included in
our Annual Report on Form 10-K for the year ended December 31, 2022, as set forth in its report, which is incorporated by reference in
this prospectus supplement and the registration statement. Our financial statements are incorporated by reference in reliance on Mayer
Hoffman McCann P.C.’s report, given on the authority of said firm as experts in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
We
are subject to the reporting requirements of the Exchange Act and file annual, quarterly and current reports, proxy statements and other
information with the Securities and Exchange Commission, or the SEC. The SEC maintains an internet site that contains reports, proxy
and information statements and other information regarding issuers, such as our company, that file documents electronically with the
SEC. Our SEC filings are available to the public at the SEC’s website address at http://www.sec.gov. The information on
the SEC’s website is not part of this prospectus supplement, and any references to the SEC’s website or any other website
are inactive textual references only.
We
also maintain a website at www.darebioscience.com, through which you can access our SEC filings. The information set forth on
our website is not part of this prospectus supplement. We have included our website address in this prospectus supplement solely as an
inactive textual reference.
INCORPORATION
OF DOCUMENTS BY REFERENCE
The
SEC allows us to “incorporate by reference” into this prospectus supplement the information that we file with the SEC, which
means that we can disclose important information to you by referring you to those publicly available documents. The information that
we incorporate by reference is an important part of this prospectus supplement. This prospectus supplement incorporates by reference
the documents listed below:
| ● | our
Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC
on March 28, 2023 (the “Annual Report”), including all material incorporated
by reference therein; |
| | | |
| ● | our
Current Reports on Form 8-K and on Form 8-K/A filed with the SEC on January 19, 2024, January 26, 2024, and April 30, 2024 (except for any information furnished under Items 2.02 or 7.01
of Form 8-K and all exhibits related to such items); and |
| ● | the
description of our common stock contained in our Registration Statement on Form 8-A filed
on April 4, 2014, including any amendments thereto or reports filed for the purpose of updating
such description, including the description of our common stock in Exhibit 4.5 of the Annual
Report. |
The
SEC file number for each of the documents listed above is 001-36395.
In
addition, all documents filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 and 15(d) of the Exchange Act on and after the
date of this prospectus supplement and prior to the termination of this offering shall be deemed to be incorporated by reference into
this prospectus supplement and to be a part hereof from the date of filing such reports and other documents (excluding any information
in such documents furnished under Item 2.02 or Item 7.01 of any Current Report on Form 8-K and any exhibit furnished with such reports
that is related to those items). We also specifically incorporate by reference into this prospectus supplement all documents filed by
us with the SEC pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of the initial registration statement
of which this prospectus supplement is a part and prior to effectiveness of the registration statement (excluding any information in
such documents that is furnished under Item 2.02 or Item 7.01 of any Current Report on Form 8-K and any exhibit furnished with such reports
that is related to those items).
Any
statement contained in this prospectus supplement or in a document incorporated or deemed to be incorporated by reference into this prospectus
supplement will be deemed to be modified or superseded for purposes of this prospectus supplement to the extent that a statement contained
in this prospectus supplement or any other subsequently filed document that is deemed to be incorporated by reference into this prospectus
supplement modifies or supersedes the statement. Any statement so modified or superseded will not be deemed, except as so modified or
superseded, to constitute a part of this prospectus supplement.
We
will provide to each person, including any beneficial owner, to whom this prospectus supplement is delivered, upon written or oral request
and at no cost to the requester, a copy of any or all reports or documents that are incorporated by reference into this prospectus supplement,
but not delivered with the prospectus supplement. Such written or oral requests should be directed to:
Daré
Bioscience, Inc.
3655
Nobel Drive, Suite 260
San
Diego, CA 92122
Attn:
Chief Financial Officer
Telephone:
(858) 926-7655
You
may also access these documents on our website, www.darebioscience.com. The information contained on, or that can be accessed
through, our website is not a part of this prospectus supplement. We have included our website address in this prospectus supplement
solely as an inactive textual reference.
PROSPECTUS
DARÉ
BIOSCIENCE, INC.

$200,000,000
COMMON
STOCK
PREFERRED
STOCK
DEBT
SECURITIES
WARRANTS
RIGHTS
PURCHASE
CONTRACTS
UNITS
This
prospectus will allow us to issue, from time to time at prices and on terms to be determined at or prior to the time of the offering,
up to $200,000,000 of any combination of the securities described in this prospectus, either individually or in units. We may also offer
common stock or preferred stock upon conversion of or exchange for the debt securities; common stock upon conversion of or exchange for
the preferred stock; common stock, preferred stock or debt securities upon the exercise of warrants or rights; or any combination of
our equity securities upon the performance of purchase contracts.
This
prospectus describes the general terms of these securities and the general manner in which these securities will be offered. We will
provide you with the specific terms of any offering in one or more supplements to this prospectus. The prospectus supplements will also
describe the specific manner in which these securities will be offered and may also supplement, update or amend information contained
in this document. You should read this prospectus and any prospectus supplement, as well as any documents incorporated by reference into
this prospectus or any prospectus supplement, carefully before you invest.
Our
securities may be sold directly by us to you, through agents designated from time to time or to or through underwriters or dealers. For
additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus
and in the applicable prospectus supplement. If any underwriters or agents are involved in the sale of our securities with respect to
which this prospectus is being delivered, the names of such underwriters or agents and any applicable fees, commissions or discounts
and over-allotment options will be set forth in a prospectus supplement. The price to the public of such securities and the net proceeds
that we expect to receive from such sale will also be set forth in a prospectus supplement.
Our
common stock is listed on The Nasdaq Capital Market under the symbol “DARE.” On March 27, 2024, the last reported sale price
of our common stock was $0.55. On March 28, 2024, the date we filed our Annual Report on Form 10-K for the fiscal year ended December
31, 2023, our prospectus became subject to the offering limits in General Instruction I.B.6 of Form S-3. As of the date hereof, the aggregate
market value of our common stock held by non-affiliates pursuant to General Instruction I.B.6 of Form S-3 is $57,034,284, which was calculated
based on 99,362,864 shares of our common stock outstanding held by non-affiliates and a price of $0.57 per share, the closing price of
our common stock on February 29, 2024. As of the date of this prospectus, we have not offered or sold any securities pursuant to General
Instruction I.B.6 of Form S-3 during the 12 calendar months prior to, and including, the date of this prospectus. Pursuant to General
Instruction I.B.6 of Form S-3, in no event will we sell securities in public primary offerings on Form S-3 with a value exceeding more
than one-third of our public float (as defined by General Instruction I.B.6) in any 12 calendar month period so long as our public float
remains below $75 million. The applicable prospectus supplement will contain information, where applicable, as to any other listing,
if any, on any securities market or other securities exchange of the securities covered by the prospectus supplement. Prospective purchasers
of our securities are urged to obtain current information as to the market prices of our securities, where applicable.
Investing
in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully
the risks that we have described on page 8 of this prospectus under the caption “Risk Factors,” as well as in the documents
incorporated by reference in this prospectus.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The
date of this prospectus is May 10, 2024
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
This
prospectus is part of a registration statement that we filed with the Securities and Exchange Commission, or SEC, utilizing a “shelf”
registration process. Under this shelf registration process, we may offer shares of our common stock and preferred stock, various series
of debt securities and/or warrants, rights or purchase contracts to purchase any of such securities, either individually or in units,
in one or more offerings, with a total value of up to $200,000,000. This prospectus provides you with a general description of the securities
we may offer. Each time we offer securities under this prospectus, we will provide a prospectus supplement that will contain specific
information about the type or series of securities offered and the terms of that offering.
This
prospectus does not contain all of the information included in the registration statement of which this prospectus forms a part. For
a more complete understanding of the offering of the securities, you should refer to the registration statement, including its exhibits.
This prospectus, together with any prospectus supplement or free writing prospectus that we subsequently authorize for use in connection
with this offering and the documents incorporated by reference into this prospectus, includes all material information relating to the
offering of securities under this prospectus. You should carefully read this prospectus, any prospectus supplement or free writing prospectus
that we subsequently authorize for use in connection with this offering, the information and documents incorporated herein by reference
and the additional information under the headings “Where You Can Find More Information” and “Incorporation of Documents
by Reference” before making an investment decision.
You
should rely only on the information we have provided or incorporated by reference in this prospectus, or in any prospectus supplement
or free writing prospectus that we subsequently authorize for use in connection with this offering. We have not authorized anyone to
provide you with information different from that contained or incorporated by reference in this prospectus. If anyone provides you with
different or inconsistent information, you should not rely on it. You should assume that the information in this prospectus, or any related
prospectus supplement or free writing prospectus, is accurate only as of the date on the front of the document and that any information
we have incorporated herein by reference is accurate only as of the date of the document incorporated by reference, regardless of the
time of delivery of this prospectus, or such prospectus supplement or free writing prospectus, or any sale of a security.
We
are not offering to sell or seeking offers to purchase these securities in any jurisdiction where the offer or sale is not permitted.
We have not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where
action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this
prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities hereunder and the distribution
of this prospectus outside the United States.
The
representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated
by reference in this prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the
purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant
to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations,
warranties and covenants should not be relied on as accurately representing the current state of our affairs.
Unless
otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate, including
our general expectations and market position, market opportunity and market size, is based on information from various sources, including
peer reviewed journals, formal presentations at medical and scientific society meetings and third-parties commissioned by us or our licensors
to provide market research and analysis, and is subject to a number of assumptions and limitations. Although we are responsible for all
of the disclosure contained in this prospectus and we believe the information from industry publications and other third-party sources
included in this prospectus is reliable, such information is inherently imprecise. Information that is based on estimates, forecasts,
projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ
materially from events and circumstances that are assumed in this information. The industry in which we operate is subject to a high
degree of uncertainty and risk due to a variety of factors.
To
the extent there are inconsistencies between this prospectus, any related prospectus supplement or free writing prospectus, and any documents
incorporated by reference, the document with the most recent date will control.
Unless
the context otherwise requires, “Daré,” “Daré Bioscience,” “the Company,” “we,”
“us,” “our” and similar terms refer to Daré Bioscience, Inc. and its subsidiaries. When we refer to “you”
we mean potential holders of the securities we may offer under this prospectus.
PROSPECTUS
SUMMARY
This
summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information you should
consider before investing in our securities. We urge you to read this entire prospectus, including our consolidated financial statements,
notes to our consolidated financial statements and other information incorporated herein by reference to our other filings with the SEC,
or included in any applicable prospectus supplement.
About
Daré Bioscience
We
are a biopharmaceutical company committed to advancing innovative products for women’s health. We are driven by a mission to identify,
develop and bring to market a diverse portfolio of differentiated therapies that prioritize women’s health and well-being, expand
treatment options, and improve outcomes, primarily in the areas of contraception, vaginal health, reproductive health, menopause, sexual
health and fertility. Our business strategy is to in-license or otherwise acquire the rights to differentiated product candidates in
our areas of focus, some of which have existing clinical proof-of-concept data, to take those candidates through mid to late-stage clinical
development or regulatory approval, and to establish and leverage strategic collaborations to achieve commercialization. We and our wholly
owned subsidiaries operate in one business segment.
The
first FDA-approved product to emerge from our portfolio of women’s health product candidates is XACIATO (clindamycin phosphate)
vaginal gel 2%, or XACIATO (pronounced zah-she-AH-toe). XACIATO was approved by the FDA in December 2021 as a single-dose prescription
medication for the treatment of bacterial vaginosis in females 12 years of age and older. In March 2022, we entered into an agreement
with an affiliate of Organon & Co., Organon International GmbH, or Organon, which became fully effective in June 2022, whereby Organon
licensed exclusive worldwide rights to develop, manufacture and commercialize XACIATO. Accordingly, our potential future revenue from
the commercialization of XACIATO will consist of royalties based on net sales and milestone payments from Organon. Organon commenced
U.S. marketing of XACIATO in the fourth quarter of 2023.
Our
product pipeline includes diverse programs that target unmet needs in women’s health in the areas of contraception, vaginal health,
reproductive health, menopause, sexual health and fertility, and aim to expand treatment options, enhance outcomes and improve ease of
use for women. We are primarily focused on progressing the development of our existing portfolio of product candidates. However, we also
explore opportunities to expand our portfolio by leveraging assets to which we hold rights or obtaining rights to new assets, with continued
focus solely on women’s health.
Our
current portfolio includes five product candidates in advanced clinical development (Phase 2-ready to Phase 3):
| ● | Ovaprene®,
a hormone-free, monthly intravaginal contraceptive; |
| ● | Sildenafil
Cream, 3.6%, a proprietary cream formulation of sildenafil for topical administration
to the female genitalia on demand for the treatment of female sexual arousal disorder (FSAD); |
| ● | DARE-HRT1,
an intravaginal ring designed to deliver both bio-identical estradiol and progesterone together,
continuously over a 28-day period, for the treatment of moderate-to-severe vasomotor symptoms,
as part of menopausal hormone therapy; |
| ● | DARE-VVA1,
a proprietary formulation of tamoxifen for intravaginal administration being developed as
a hormone-free alternative to estrogen-based therapies for the treatment of moderate-to-severe
dyspareunia, or pain during sexual intercourse, a symptom of vulvar and vaginal atrophy associated
with menopause; and |
| ● | DARE-CIN,
a proprietary, fixed-dose formulation of lopinavir and ritonavir in a soft gel vaginal insert
for the treatment of cervical intraepithelial neoplasia (CIN) and other human papillomavirus
(HPV)-related pathologies. |
Our
portfolio also includes five product candidates in Phase 1 clinical development or that we believe are Phase 1-ready:
| ● | DARE-PDM1,
a proprietary hydrogel formulation of diclofenac, a nonsteroidal anti-inflammatory drug,
for vaginal administration as a treatment for primary dysmenorrhea; |
| ● | DARE-204
and DARE-214, injectable formulations of etonogestrel designed to provide contraception
over 6-month and 12-month periods, respectively; |
| ● | DARE-FRT1,
an intravaginal ring designed to deliver bio-identical progesterone continuously for up to
14 days for luteal phase support as part of an in vitro fertilization treatment plan; and |
| ● | DARE-PTB1,
an intravaginal ring designed to deliver bio-identical progesterone continuously for up to
14 days for the prevention of preterm birth. |
In
addition, our portfolio includes five preclinical stage programs:
| ● | DARE-LARC1,
a contraceptive implant delivering levonorgestrel with a woman-centered design that has the
potential to be a long-acting, yet convenient and user-controlled contraceptive option; |
| ● | DARE-LBT,
a novel hydrogel formulation for vaginal delivery of live biotherapeutics to support vaginal
health; |
| ● | DARE-GML,
an intravaginally-delivered potential multi-target antimicrobial agent formulated with glycerol
monolaurate (GML), which has shown broad antimicrobial activity, killing bacteria and viruses;
|
| ● | DARE-RH1,
a novel approach to non-hormonal contraception for both men and women by targeting the CatSper
ion channel; and |
| ● | DARE-PTB2,
a novel approach for the prevention and treatment of idiopathic preterm birth through inhibition
of a stress response protein. |
The
product candidates and potential product candidates in our portfolio will require review and approval from the FDA, or a comparable foreign
regulatory authority, prior to being marketed or sold. See ITEM 1. “BUSINESS,” in Part I of our Annual Report on Form 10-K
for the year ended December 31, 2023 for additional information regarding our product and product candidates.
Our
primary operations have consisted of research and development activities to advance our portfolio of product candidates through late-stage
clinical development and/or regulatory approval. We expect our research and development expenses will continue to represent the majority
of our operating expenses for at least the next twelve months. Until we secure additional capital to fund our operating needs, we will
focus our resources primarily on advancement of Ovaprene and Sildenafil Cream. In addition, we expect to incur significant research and
development expenses for the DARE-LARC1 program for the next several years, but we also expect such expenses will be supported by non-dilutive
funding provided under a grant agreement we entered into in June 2021.
We
will need to raise substantial additional capital to continue to fund our operations and execute our current business strategy. We are
also subject to a number of other risks common to biopharmaceutical companies, including, but not limited to, dependence on key employees,
reliance on third-party collaborators, service providers and suppliers, being able to develop commercially viable products in a timely
and cost-effective manner, dependence on intellectual property we own or in-license and the need to protect that intellectual property
and maintain those license agreements, uncertainty of market acceptance of products, uncertainty of third-party payor coverage, pricing
and reimbursement for products, rapid technology change, intense competition, compliance with government regulations, product liability
claims, and exposure to cybersecurity threats and incidents.
The
process of developing and obtaining regulatory approvals for prescription drug and drug/device products in the United States and in foreign
jurisdictions is inherently uncertain and requires the expenditure of substantial financial resources without any guarantee of success.
To the extent we receive regulatory approvals to market and sell our product candidates, the commercialization of any product and compliance
with subsequently applicable laws and regulations requires the expenditure of further substantial financial resources without any guarantee
of commercial success. The amount of post-approval financial resources required for commercialization and the potential revenue we may
receive from sales of any product will vary significantly depending on many factors, including whether, and the extent to which, we establish
our own sales and marketing capabilities and/or enter into and maintain commercial collaborations with third parties with established
commercialization infrastructure.
Summary
of Risk Factors
Investing
in our securities involves a high degree of risk. Before deciding whether to purchase any of our securities, you should carefully consider
the risks and uncertainties described in the section captioned “Risk Factors” in this prospectus and the applicable accompanying
prospectus supplement, and under similar headings in the documents incorporated by reference into this prospectus and the applicable
accompanying prospectus supplement. The occurrence of any of these risk factors could have a material adverse effect on our business,
financial condition, results of operations and growth prospects. In these circumstances, the market price of our common stock could decline,
and you may lose all or part of your investment in our common stock. These risk factors include, but are not limited to, the following:
| ● | We
will need to raise substantial additional capital to continue our operations, execute our
business strategy and remain a going concern, and we may not be able to raise adequate capital
on a timely basis, on favorable terms, or at all. Raising additional capital may cause substantial
dilution to our stockholders, restrict our operations or require us to relinquish rights
in our technologies or product candidates and their future revenue streams. |
| ● | We
have a limited operating history, have incurred significant losses since our inception and
expect to continue to incur losses for the foreseeable future, which, together with our limited
financial resources and substantial capital requirements, make it difficult to assess our
prospects. |
| ● | Our
business depends on obtaining the approval of regulatory authorities, and in particular,
FDA approval, to market products that we develop. All of our product candidates are investigational,
require the conduct and successful completion of clinical studies and nonclinical work, and
may never complete development or be submitted for or receive regulatory approval. The FDA’s
approval of XACIATO is not predictive of favorable development or marketing approval outcomes
for our product candidates. |
| ● | Clinical
development is a lengthy and expensive process with an inherently uncertain outcome. Failure
to successfully complete clinical trials and nonclinical activities and obtain regulatory
approval to market and sell our product candidates on our anticipated timelines at reasonable
costs to us, or at all, particularly Ovaprene and Sildenafil Cream, could have a material
adverse effect on our business, operating results and financial condition. |
| ● | The
regulatory approval processes of the FDA and comparable foreign authorities are expensive,
lengthy, time-consuming, and inherently unpredictable. If we are not able to obtain regulatory
approvals for our product candidates, our ability to generate product revenue will be materially
impaired. |
| ● | Drug
products and drug/device combination products are complex to manufacture and we face significant
challenges in scaling up manufacturing of our product candidates for larger clinical trials
and commercial production. Manufacturing and supply delays and disruptions could postpone
the initiation of or interrupt our clinical studies, extend the timeframe and cost of development
of our product candidates, delay potential regulatory approvals and adversely impact the
commercialization of any approved products. |
| ● | Strategic
collaborations are a key part of our strategy and our existing strategic collaborations are
important to our business. If we are unable to maintain existing strategic collaborations
or establish new ones, or if they are not successful, we may require substantial additional
capital to develop and commercialize our products and product candidates and our business
and prospects may be materially harmed. |
| ● | Unless
and until one of our product candidates receives regulatory approval, payments under our
license agreement with Organon based on net sales of XACIATO represent our only potential
source of ongoing revenue and the amount of those net sales is largely outside of our control.
|
| ● | We
have no manufacturing, sales, marketing or distribution infrastructure. We depend heavily
on, and expect to continue to rely on, the performance of third parties, including our strategic
collaborators, contract manufacturers and suppliers, CROs, medical institutions, and scientific,
medical, regulatory and other consultants and advisors, to develop our product candidates
and commercialize any approved products. Failure of these third parties to perform as expected
could result in substantial delays, increased costs or failures of our product development
programs, delayed or unsuccessful commercialization of any approved products, and the need
for significant additional capital. |
| ● | Due
in part to our limited financial and human resources, we may fail to effectively execute
our product development, regulatory submission and commercialization plans in accordance
with communicated timelines, or at all. |
| ● | The
loss or impairment of our rights under our license agreements for XACIATO or any of our product
candidates could prevent us from developing or commercializing them, which could have a material
adverse effect on our business prospects, operations and viability. |
| ● | The
commercial success of XACIATO will depend on Organon’s efforts and capabilities and
a variety of factors, many of which currently are unknown or uncertain, and if commercialization
of XACIATO is not successful, our reputation, business and prospects may suffer. |
| ● | XACIATO
and any future products will face intense competition, including from generic products, and
may fail to achieve the degree of market acceptance necessary for commercial success. Our
business, operating results and financial condition will suffer if we, or our commercial
collaborators, fail to compete effectively and fail to achieve market acceptance. |
| ● | Failure
to successfully obtain coverage and adequate reimbursement for XACIATO and any future products
from government health care programs and other third-party payors would diminish our ability,
or that of a commercial collaborator, to generate net product revenue or net sales. If out-of-pocket
costs for products we develop are deemed by women to be unaffordable, a commercial market
may never develop. |
| ● | We
have a relatively small number of employees, and if we fail to attract and retain key personnel
or effectively manage our personnel costs, our business may materially suffer. |
| ● | We
may not be successful in our efforts to identify and acquire or in-license additional product
candidates or technologies, which may limit our growth potential. |
| ● | Our
failure to adequately protect or enforce our intellectual property rights, and those of our
licensors, could materially harm our proprietary position in the marketplace or prevent or
impede the commercialization of XACIATO and any future products. |
| ● | Lack
of patent protection for the active ingredients in certain of our product candidates, including
Sildenafil Cream and DARE-HRT1, may limit the commercial opportunity for those products if
competitors are able to develop and commercialize safe and effective alternative formulations
or methods of delivery of the active ingredients. |
| ● | Volatility
in the financial markets, geopolitical conflicts and events, public health emergencies such
as the COVID-19 pandemic and other macroeconomic factors may negatively impact our business,
financial condition and results and our stock price, including by increasing the cost and
timelines for our clinical development programs or making it more difficult or costly to
raise additional capital when needed. |
| ● | Product
liability lawsuits against us could cause us to incur substantial liabilities and divert
management attention from our business. |
| ● | The
price of our common stock has been and may continue to be highly volatile and such volatility
may be related or unrelated to our performance and operating results. Volatility in our stock
price may subject us to increased risk of securities litigation, including class-action lawsuits,
which could be expensive and divert management attention. |
| ● | If
we fail to regain and maintain compliance with the continued listing requirements of the
Nasdaq Capital Market, our common stock could be delisted, which could, among other things,
limit demand for our common stock, substantially impair our ability to raise additional capital
and have an adverse effect on the market price of, and the efficiency of the trading market
for, our common stock. |
| ● | Future
dilution to our existing stockholders from sales and issuances of our common stock through
at-the-market, or ATM, offerings, other types of public or private offerings of equity or
equity-linked securities and upon the exercise of stock options, or the market’s expectation
that such sales could adversely affect our stock price, even if our business is doing well. |
| ● | We
have been subject to a cyber-related crime and our controls and security measures may not
be successful in preventing other cybersecurity incidents in the future. Cyber-attacks, security
breaches, loss of data and other disruptions to our information technology systems or those
of our strategic collaborators or third-party service providers could compromise sensitive
information related to our business, delay or prevent us from accessing critical information,
subject us to significant financial loss, or expose us to liability, any of which could adversely
affect our business and our reputation. |
Additional
Information
For
additional information related to our business and operations, please refer to the annual and quarterly reports incorporated herein by
reference, as described under the caption “Incorporation of Documents by Reference” on page 33 of this prospectus.
Corporate
Information
We
are incorporated under the laws of the State of Delaware. Our principal executive offices are located at 3655 Nobel Drive, Suite 260,
San Diego, California 92122, and our telephone number at that address is (858) 926-7655. We maintain a website at www.darebioscience.com,
to which we regularly post copies of our press releases as well as additional information about us. The information contained on, or
that can be accessed through, our website is not a part of this prospectus. We have included our website address in this prospectus solely
as an inactive textual reference.
Daré
Bioscience® is a registered trademark of Daré Bioscience, Inc. Ovaprene® is a registered trademark licensed to Daré
Bioscience, Inc. All brand names or trademarks appearing in this prospectus are the property of their respective holders. Use or display
by us of other parties’ trademarks, trade dress, or products in this prospectus is not intended to, and does not, imply a relationship
with, or endorsements or sponsorship of, us by the trademark or trade dress owners.
Offerings
Under This Prospectus
Under
this prospectus, we may offer shares of our common stock and preferred stock, various series of debt securities and/or warrants, rights
or purchase contracts to purchase any of such securities, either individually or in units, with a total value of up to $200,000,000,
from time to time at prices and on terms to be determined by market conditions at the time of the offering. This prospectus provides
you with a general description of the securities we may offer. Each time we offer a type or series of securities under this prospectus,
we will provide a prospectus supplement that will describe the specific amounts, prices and other important terms of the securities,
including, to the extent applicable:
| ● | designation
or classification; |
| ● | aggregate
principal amount or aggregate offering price; |
| ● | maturity,
if applicable; |
| ● | rates
and times of payment of interest or dividends, if any; |
| ● | redemption,
conversion or sinking fund terms, if any; |
| ● | voting
or other rights, if any; and |
| ● | conversion
or exercise prices, if any. |
The
prospectus supplement also may add, update or change information contained in this prospectus or in documents we have incorporated by
reference into this prospectus. However, no prospectus supplement will fundamentally change the terms that are set forth in this prospectus
or offer a security that is not registered and described in this prospectus at the time of its effectiveness.
We
may sell the securities directly to investors or to or through agents, underwriters or dealers. We, and our agents or underwriters, reserve
the right to accept or reject all or part of any proposed purchase of securities. If we offer securities through agents or underwriters,
we will include in the applicable prospectus supplement:
| ● | the
names of those agents or underwriters; |
| ● | applicable
fees, discounts and commissions to be paid to them; |
| ● | details
regarding over-allotment options, if any; and |
| ● | the
net proceeds to us. |
This
prospectus may not be used to consummate a sale of any securities unless it is accompanied by a prospectus supplement.
RISK
FACTORS
Investing
in our securities involves significant risk. The prospectus supplement applicable to each offering of our securities will contain a discussion
of risks related to that offering. Prior to making a decision about investing in our securities, you should carefully consider the specific
factors discussed under the heading “Risk Factors” in the applicable prospectus supplement and any related free writing prospectus,
together with all of the other information contained or incorporated by reference in the prospectus supplement or contained or incorporated
by reference in this prospectus. You should carefully consider the risks, uncertainties and assumptions discussed under the heading “Risk
Factors” in our most recent annual report on Form 10-K, as may be amended, supplemented or superseded from time to time by information
in quarterly reports on Form 10-Q and current reports on Form 8-K that we file with the SEC after the date of this prospectus, all of
which are incorporated herein by reference (other than current reports on Form 8-K, or portions thereof, furnished under Items 2.02 or
7.01 of Form 8-K). The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not
presently known to us or that we currently deem immaterial may also affect our operations. The occurrence of any of these risks might
cause you to lose all or part of your investment in the offered securities.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and the documents incorporated by reference in this prospectus contain or incorporate by reference forward-looking statements
that involve substantial risks and uncertainties. All statements, other than statements of historical facts, are forward-looking statements,
including statements regarding our strategy, future operations, future financial position, projected costs, prospects, plans and objectives
of management. Forward-looking statements, in some cases, can be identified by terms such as “believe,” “may,”
“will,” “estimate,” “continue,” “anticipate,” “design,” “intend,”
“expect,” “could,” “plan,” “potential,” “predict,” “seek,” “should,”
“would,” “contemplate,” project,” “target,” “tend to,” or the negative version
of these words and similar expressions.
Forward-looking
statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements
to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements,
including those factors discussed under the heading “Risk Factors” contained or incorporated in this prospectus and in any
related prospectus supplement or free writing prospectus we may authorize for use in connection with a specific offering. These factors
and the other cautionary statements contained or incorporated in this prospectus and in the applicable prospectus supplement and any
free writing prospectus we may authorize for use in connection with a specific offering should be read as being applicable to all related
forward-looking statements whenever they appear in this prospectus. Given these uncertainties, you should not place undue reliance on
any forward-looking statement. The following factors are among those that may cause such differences:
| ● | Inability
to raise additional capital, under favorable terms or at all, to fund our operating needs
and continue as a going concern; |
| ● | The
number and scope of product development programs we pursue; |
| ● | Clinical
trial outcomes and results of preclinical development; |
| ● | Failure
to complete development of our product candidates or submit and obtain United States Food
and Drug Administration, or FDA, or foreign regulatory authority approval for our product
candidates on projected timelines or budgets, or at all; |
| ● | Challenges
and delays in obtaining timely supplies of our product candidates, including their components
as well as the finished product, in the quantities needed in accordance with current good
manufacturing practices, our specifications and other applicable requirements; |
| ● | The
performance of third parties on which we rely to conduct nonclinical studies and clinical
trials of our product candidates; |
| ● | Our
failure, or a failure of a strategic collaborator, to successfully commercialize our product
candidates, if approved, or our failure to otherwise monetize our portfolio programs and
assets; |
| ● | The
timing and amount of future royalty and milestone payments to us, if any, under our out-license
agreements for commercialization of XACIATO™ (clindamycin phosphate) vaginal gel 2%,
or XACIATO, and Ovaprene®; |
| ● | Termination
by a collaborator of our respective out-license agreements for commercialization of XACIATO
and Ovaprene, or, in the case of Ovaprene, a decision by the collaborator not to make the
license grant fully effective following its review of the results of the ongoing pivotal
clinical trial of Ovaprene; |
| ● | The
performance of third parties on which we rely to commercialize, or assist us in commercializing,
XACIATO and any future product; |
| ● | Difficulties
with maintaining existing collaborations relating to the development and/or commercialization
of our product candidates, or establishing new ones on a timely basis or on acceptable terms,
or at all; |
| ● | The
terms and conditions of any future strategic collaborations relating to our product candidates; |
| ● | The
degree of market acceptance that XACIATO and any future product achieves; |
| ● | Coverage
and reimbursement levels for XACIATO and any future product by government health care programs,
private health insurance companies and other third-party payors; |
| ● | Our
loss of, or inability to attract, key personnel; |
| ● | A
change in the FDA’s prior determination that the Center for Devices and Radiological
Health would lead the review of a premarket approval application for potential marketing
approval of Ovaprene; |
| ● | A
change in regulatory requirements for our product candidates, including the development pathway
pursuant to Section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act, or the FDA’s
505(b)(2) pathway; |
| ● | Unfavorable
differences between preliminary, interim or topline clinical study data reported by us and
final study results; |
| ● | Communication
from the FDA or another regulatory authority, including a complete response letter, that
such agency does not accept or agree with our assumptions, estimates, calculations, conclusions
or analyses of clinical or nonclinical study data regarding a product candidate, or that
such agency interprets or weighs the importance of study data differently than we have in
a manner that negatively impacts the candidate’s prospects for regulatory approval
in a timely manner, or at all; |
| ● | Failure
to select product candidates that capitalize on the most scientifically, clinically or commercially
promising or profitable indications or therapeutic areas within women’s health including
due to our limited financial resources; |
| ● | Loss
or impairment of our in-licensed rights to develop and commercialize XACIATO and our product
candidates; |
| ● | Our
payment and other obligations under our in-license and acquisition agreements for XACIATO
and our product candidates; |
| ● | Developments
by our competitors that make XACIATO, or any potential product we develop, less competitive
or obsolete; |
| ● | Unfavorable
or unanticipated macroeconomic factors, geopolitical events or conflicts, public health emergencies,
or natural disasters; |
| ● | Weak
interest in women’s health relative to other healthcare sectors from the investment
community or from pharmaceutical companies and other potential development and commercialization
collaborators; |
| ● | Cyber-attacks,
security breaches or similar events compromising our technology systems and data, our financial
resources and other assets, or the technology systems and data of third parties on which
we rely; |
| ● | Difficulty
in introducing branded products in a market made up of generic products; |
| ● | Inability
to adequately protect or enforce our, or our licensor’s, intellectual property rights; |
| ● | Lack
of patent protection for the active ingredients in XACIATO and certain of our product candidates
that expose them to competition from other formulations using the same active ingredients;
|
| ● | Higher
risk of failure associated with product candidates in preclinical stages of development that
may lead investors to assign them little to no value and make these assets difficult to fund; |
| ● | Dependence
on grant funding to advance the development of several of our product candidates; |
| ● | Disputes
or other developments concerning our intellectual property rights; |
| ● | Actual
and anticipated fluctuations in our quarterly or annual operating results or results that
differ from investors’ expectations for such results; |
| ● | Price
and volume fluctuations in the stock market, and in our stock in particular, which could
cause investors to experience losses and subject us to securities class-action litigation; |
| ● | Failure
to maintain the listing of our common stock on the Nasdaq Capital Market or another nationally
recognized exchange; |
| ● | Development
of safety, efficacy or quality concerns related to our product or product candidates (or
third-party products or product candidates that share similar characteristics or drug substances),
whether or not scientifically justified, leading to delays in or discontinuation of product
development, product recalls or withdrawals, diminished sales, and/or other significant negative
consequences; |
| ● | Product
liability claims or governmental investigations; |
| ● | Changes
in government laws and regulations in the United States and other jurisdictions, including
laws and regulations governing the research, development, approval, clearance, manufacturing,
supply, distribution, pricing and/or marketing of our products, product candidates and related
intellectual property, health care information and data privacy and security laws, transparency
laws and fraud and abuse laws, and the enforcement thereof affecting our business; and |
| ● | Increased
costs as a result of operating as a public company, and substantial time devoted by our management
to compliance initiatives and corporate governance practices. |
You
are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date of this prospectus or the date
of the document incorporated by reference in this prospectus. We do not undertake any obligation to publicly update any forward-looking
statement to reflect events or circumstances after the date on which any statement is made or to reflect the occurrence of unanticipated
events, except as required by law.
USE
OF PROCEEDS
We
will have broad discretion over the use of net proceeds from the sale of securities offered hereby. Unless otherwise indicated in the
applicable prospectus supplement and any free writing prospectus we may authorize for use in connection with a specific offering, we
intend to use the net proceeds from the sale of securities under this prospectus for general corporate purposes, including working capital,
operating expenses and capital expenditures. We may also use the net proceeds to repay any debts and/or acquire or invest in complementary
businesses, products or technologies, although we have no current commitments or agreements with respect to any such acquisitions or
investments as of the date of this prospectus.
We
have not determined the amount of net proceeds to be used specifically for the foregoing purposes. As a result, you will be relying on
the judgment of our management regarding the application of the proceeds of any sale of securities offered hereby. If a material part
of the net proceeds is to be used to repay indebtedness, we will set forth the interest rate and maturity of such indebtedness in a prospectus
supplement. Pending use of the net proceeds, we intend to invest the proceeds in interest-bearing, marketable securities.
PLAN
OF DISTRIBUTION
We
may offer securities under this prospectus from time to time pursuant to underwritten public offerings, negotiated transactions, block
trades or a combination of these methods. We may sell the securities (1) through underwriters or dealers, (2) through agents or (3) directly
to one or more purchasers, or through a combination of such methods. We may distribute the securities from time to time in one or more
transactions at:
| ● | a
fixed price or prices, which may be changed from time to time; |
| ● | market
prices prevailing at the time of sale; |
| ● | prices
related to the prevailing market prices; or |
| ● | negotiated
prices. |
We
may directly solicit offers to purchase the securities being offered by this prospectus. We may also designate agents to solicit offers
to purchase the securities from time to time, and may enter into arrangements for “at-the-market,” equity line or similar
transactions. We will name in a prospectus supplement any underwriter or agent involved in the offer or sale of the securities.
If
we utilize a dealer in the sale of the securities being offered by this prospectus, we will sell the securities to the dealer, as principal.
The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale.
If
we utilize an underwriter in the sale of the securities being offered by this prospectus, we will execute an underwriting agreement with
the underwriter at the time of sale, and we will provide the name of any underwriter in the prospectus supplement which the underwriter
will use to make resales of the securities to the public. In connection with the sale of the securities, we, or the purchasers of the
securities for whom the underwriter may act as agent, may compensate the underwriter in the form of underwriting discounts or commissions.
The underwriter may sell the securities to or through dealers, and the underwriter may compensate those dealers in the form of discounts,
concessions or commissions.
With
respect to underwritten public offerings, negotiated transactions and block trades, we will provide in the applicable prospectus supplement
information regarding any compensation we pay to underwriters, dealers or agents in connection with the offering of the securities, and
any discounts, concessions or commissions allowed by underwriters to participating dealers. Underwriters, dealers and agents participating
in the distribution of the securities may be deemed to be underwriters within the meaning of the Securities Act of 1933, as amended,
or the Securities Act, and any discounts and commissions received by them and any profit realized by them on resale of the securities
may be deemed to be underwriting discounts and commissions. We may enter into agreements to indemnify underwriters, dealers and agents
against civil liabilities, including liabilities under the Securities Act, or to contribute to payments they may be required to make
in respect thereof.
If
so indicated in the applicable prospectus supplement, we will authorize underwriters, dealers or other persons acting as our agents to
solicit offers by certain institutions to purchase securities from us pursuant to delayed delivery contracts providing for payment and
delivery on the date stated in each applicable prospectus supplement. Each contract will be for an amount not less than, and the aggregate
amount of securities sold pursuant to such contracts shall not be less nor more than, the respective amounts stated in each applicable
prospectus supplement. Institutions with whom the contracts, when authorized, may be made include commercial and savings banks, insurance
companies, pension funds, investment companies, educational and charitable institutions and other institutions, but shall in all cases
be subject to our approval. Delayed delivery contracts will not be subject to any conditions except that:
| ● | the
purchase by an institution of the securities covered under that contract shall not at the
time of delivery be prohibited under the laws of the jurisdiction to which that institution
is subject; and |
| ● | if
the securities are also being sold to underwriters acting as principals for their own account,
the underwriters shall have purchased such securities not sold for delayed delivery. The
underwriters and other persons acting as our agents will not have any responsibility in respect
of the validity or performance of delayed delivery contracts. |
One
or more firms, referred to as “remarketing firms,” may also offer or sell the securities, if a prospectus supplement so indicates,
in connection with a remarketing arrangement upon their purchase. Remarketing firms will act as principals for their own accounts or
as our agents. These remarketing firms will offer or sell the securities in accordance with the terms of the securities. Each prospectus
supplement will identify and describe any remarketing firm and the terms of its agreement, if any, with us and will describe the remarketing
firm’s compensation. Remarketing firms may be deemed to be underwriters in connection with the securities they remarket. Remarketing
firms may be entitled under agreements that may be entered into with us to indemnification by us against certain civil liabilities, including
liabilities under the Securities Act, and may be customers of, engage in transactions with or perform services for us in the ordinary
course of business.
Certain
underwriters may use this prospectus and any accompanying prospectus supplement for offers and sales related to market-making transactions
in the securities. These underwriters may act as principal or agent in these transactions, and the sales will be made at prices related
to prevailing market prices at the time of sale. Any underwriters involved in the sale of the securities may qualify as “underwriters”
within the meaning of Section 2(a)(11) of the Securities Act. In addition, the underwriters’ commissions, discounts or concessions
may qualify as underwriters’ compensation under the Securities Act and the rules of the Financial Industry Regulatory Authority,
Inc., or FINRA.
All
securities we may offer, other than shares of our common stock, may be new issues of securities with no established trading market. Any
agents or underwriters may make a market in these securities, but will not be obligated to do so and may discontinue any market making
at any time without notice. We can make no assurance as to the liquidity of or the existence, development or maintenance of trading markets
for any of the securities. There is currently no market for any of the securities we may offer hereby, other than our common stock which
is listed on the Nasdaq Capital Market. Underwriters may make a market in our common stock, but will not be obligated to do so and may
discontinue any market making at any time without notice. We have no current plans for listing of the preferred stock, debt securities,
warrants, rights or units on any securities exchange or quotation system; any such listing with respect to any particular preferred stock,
debt securities, warrants, rights or units will be described in the applicable prospectus supplement or other offering materials, as
the case may be.
In
order to facilitate the offering of the securities, certain persons participating in the offering may engage in transactions that stabilize,
maintain or otherwise affect the price of the securities. This may include over-allotments or short sales of the securities, which involve
the sale by persons participating in the offering of more securities than we sold to them. In these circumstances, these persons would
cover such over-allotments or short positions by making purchases in the open market or by exercising their over-allotment option. In
addition, these persons may stabilize or maintain the price of the securities by bidding for or purchasing the applicable security in
the open market or by imposing penalty bids, whereby selling concessions allowed to dealers participating in the offering may be reclaimed
if the securities sold by them are repurchased in connection with stabilization transactions. The effect of these transactions may be
to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail in the open market. These
transactions may be discontinued at any time.
The
underwriters, dealers and agents may engage in other transactions with us, or perform other services for us, in the ordinary course of
their business.
Under
Rule 15c6-1 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, trades in the secondary market generally are required
to settle in two business days, unless the parties to any such trade expressly agree otherwise or the securities are sold by us to an
underwriter in a firm commitment underwritten offering. The applicable prospectus supplement may provide that the original issue date
for your securities may be more than two scheduled business days after the trade date for your securities. Accordingly, in such a case,
if you wish to trade securities on any date prior to the second business day before the original issue date for your securities, you
will be required, by virtue of the fact that your securities initially are expected to settle in more than two scheduled business days
after the trade date for your securities, to make alternative settlement arrangements to prevent a failed settlement.
DESCRIPTION
OF COMMON STOCK
We
are authorized to issue 240,000,000 shares of common stock, par value $0.0001 per share. As of March 27, 2024, we had 100,581,900 shares
of common stock outstanding.
The
following summary of certain provisions of our common stock does not purport to be complete. You should refer to the section of this
prospectus entitled “Certain Provisions of Delaware Law and of Our Certificate of Incorporation and Bylaws” and our Restated
Certificate of Incorporation, as amended, referred to herein as our restated certificate of incorporation, and our Third Amended and
Restated By-laws, as amended, referred to herein as our restated bylaws, both of which are included as exhibits to the registration statement
of which this prospectus is a part. The summary below is also qualified by provisions of applicable law.
General
Voting
Rights
Holders
of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders, except that unless
otherwise required by law, holders of our common stock are not entitled to vote on any amendment to our restated certificate of incorporation
that relates solely to the terms of one or more outstanding series of preferred stock, if the holders of such affected series are entitled,
either separately or together as a class with the holders of one or more such other series, to vote thereon pursuant to our restated
certificate of incorporation. Holders of our common stock do not have cumulative voting rights.
An
election of directors will be decided by a plurality of the votes cast by the stockholders entitled to vote on the election at a duly
held stockholders’ meeting at which a quorum is present. All other questions will be decided by a majority of the votes cast by
stockholders entitled to vote thereon at a duly held meeting of stockholders at which a quorum is present, except when a different vote
is required by law, our restated certificate of incorporation or restated bylaws.
Dividends
Holders
of our common stock are entitled to receive proportionately any dividends as may be declared by our board of directors out of legally
available funds, subject to any preferential dividend or other rights of any series of preferred stock that we may designate and issue
in the future.
Liquidation
and Dissolution
In
the event of our liquidation, dissolution or winding up, the holders of our common stock will be entitled to share ratably in the net
assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction
of any liquidation preference of any then outstanding shares of preferred stock.
No
Preemptive Rights
Holders
of our common stock have no preemptive, conversion, subscription or other rights, and there are no redemption or sinking fund provisions
applicable to our common stock.
Rights
of Preferred Stock May be Senior to Rights of Common Stock
Our
board of directors has the authority, without further action by our stockholders, to issue up to 5,000,000 shares of preferred stock
in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges
could include dividend rights, conversion rights, voting rights, terms of redemption, and liquidation preferences, any or all of which
may be greater than the rights of the holders of our common stock.
Transfer
Agent and Registrar
The
transfer agent and registrar for our common stock is Equiniti Trust Company, LLC, with offices at 6201 15th Avenue, Brooklyn, NY 11219.
Stock
Exchange Listing
Our
common stock is listed on the Nasdaq Capital Market under the symbol “DARE.”
DESCRIPTION
OF PREFERRED STOCK
We
are authorized to issue up to 5,000,000 shares of preferred stock, par value $0.01 per share. As of the date of this prospectus, no shares
of our preferred stock were outstanding or designated. The following summary of certain provisions of our preferred stock does not purport
to be complete. You should refer to our restated certificate of incorporation and restated bylaws, both of which are included as exhibits
to the registration statement of which this prospectus is a part. The summary below is also qualified by provisions of applicable law.
General
Our
board of directors may, from time to time, direct the issuance of shares of preferred stock in one or more series and may, at the time
of issuance, determine the rights, preferences and limitations of each series, including voting rights, dividend rights, conversion rights,
redemption privileges and liquidation preferences. Satisfaction of any dividend preferences of outstanding shares of preferred stock
would reduce the amount of funds available for the payment of dividends on shares of our common stock. Holders of shares of preferred
stock may be entitled to receive a preference payment in the event of any liquidation, dissolution or winding-up of our company before
any payment is made to the holders of shares of our common stock. In some circumstances, the issuance of shares of preferred stock may
render more difficult or tend to discourage a merger, tender offer or proxy contest, the assumption of control by a holder of a large
block of our securities or the removal of incumbent management. Upon the affirmative vote of our board of directors, without stockholder
approval, unless and only to the extent stockholder approval is required by the listing standards of any securities exchange on which
our securities are listed, we may issue shares of preferred stock with voting and conversion rights which could adversely affect the
holders of shares of our common stock.
If
we offer a specific series of preferred stock under this prospectus, we will describe the terms of that series of preferred stock in
the prospectus supplement for such offering and will file a copy of the amended and restated certificate of incorporation or the certificate
of designations establishing the terms of the preferred stock with the SEC. To the extent required, this description will include:
| ● | the
title and stated value; |
| ● | the
number of shares offered, the liquidation preference, if any, per share and the purchase
price; |
| ● | the
dividend rate(s), period(s) and/or payment date(s), or method(s) of calculation for such
dividends; |
| ● | whether
dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends
will accumulate; |
| ● | the
procedures for any auction and remarketing, if any; |
| ● | the
provisions for a sinking fund, if any; |
| ● | the
provisions for redemption, if applicable; |
| ● | any
listing of the preferred stock on any securities exchange or market; |
| ● | whether
the preferred stock will be convertible into our common stock, and, if applicable, the conversion
price (or how it will be calculated) and conversion period; |
| ● | whether
the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange
price (or how it will be calculated) and exchange period; |
| ● | voting
rights, if any, of the preferred stock; |
| ● | a
discussion of any material and/or special U.S. federal income tax considerations applicable
to the preferred stock; |
| ● | the
relative ranking and preferences of the preferred stock as to dividend rights and rights
upon liquidation, dissolution or winding up of the affairs of our company; and |
| ● | any
material limitations on issuance of any class or series of preferred stock ranking pari passu
with or senior to the series of preferred stock as to dividend rights and rights upon liquidation,
dissolution or winding up of our company. |
Transfer
Agent and Registrar
The
transfer agent and registrar for our preferred stock will be set forth in the applicable prospectus supplement.
DESCRIPTION
OF DEBT SECURITIES
The
following description, together with the additional information we include in any applicable prospectus supplements, summarizes the material
terms and provisions of the debt securities that we may offer under this prospectus. While the terms we have summarized below will apply
generally to any future debt securities we may offer pursuant to this prospectus, we will describe the particular terms of any debt securities
that we may offer in more detail in the applicable prospectus supplement. If we so indicate in a prospectus supplement, the terms of
any debt securities offered under such prospectus supplement may differ from the terms we describe below, and to the extent the terms
set forth in a prospectus supplement differ from the terms described below, the terms set forth in the prospectus supplement shall control.
We
may sell from time to time, in one or more offerings under this prospectus, debt securities, which may be senior or subordinated. We
will issue any such senior debt securities under a senior indenture that we will enter into with a trustee to be named in the senior
indenture. We will issue any such subordinated debt securities under a subordinated indenture, which we will enter into with a trustee
to be named in the subordinated indenture. We have filed forms of these documents as exhibits to the registration statement, of which
this prospectus is a part. We use the term “indentures” to refer to either the senior indenture or the subordinated indenture,
as applicable. The indentures will be qualified under the Trust Indenture Act of 1939, as in effect on the date of the indenture. We
use the term “debenture trustee” to refer to either the trustee under the senior indenture or the trustee under the subordinated
indenture, as applicable.
The
following summaries of material provisions of the senior debt securities, the subordinated debt securities and the indentures are subject
to, and qualified in their entirety by reference to, all the provisions of the indenture applicable to a particular series of debt securities.
General
Each
indenture provides that debt securities may be issued from time to time in one or more series and may be denominated and payable in foreign
currencies or units based on or relating to foreign currencies. Neither indenture limits the amount of debt securities that may be issued
thereunder, and each indenture provides that the specific terms of any series of debt securities shall be set forth in, or determined
pursuant to, an authorizing resolution and/or a supplemental indenture, if any, relating to such series.
We
will describe in each prospectus supplement the following terms relating to a series of debt securities:
| ● | the
title or designation; |
| ● | the
aggregate principal amount and any limit on the amount that may be issued; |
| ● | the
currency or units based on or relating to currencies in which debt securities of such series
are denominated and the currency or units in which principal or interest or both will or
may be payable; |
| ● | whether
we will issue the series of debt securities in global form, the terms of any global securities
and who the depositary will be; |
| ● | the
maturity date and the date or dates on which principal will be payable; |
| ● | the
interest rate, which may be fixed or variable, or the method for determining the rate and
the date interest will begin to accrue, the date or dates interest will be payable and the
record dates for interest payment dates or the method for determining such dates; |
| ● | whether
or not the debt securities will be secured or unsecured, and the terms of any secured debt; |
| ● | the
terms of the subordination of any series of subordinated debt; |
| ● | the
place or places where payments will be payable; |
| ● | our
right, if any, to defer payment of interest and the maximum length of any such deferral period; |
| ● | the
date, if any, after which, and the price at which, we may, at our option, redeem the series
of debt securities pursuant to any optional redemption provisions; |
| ● | the
date, if any, on which, and the price at which we are obligated, pursuant to any mandatory
sinking fund provisions or otherwise, to redeem, or at the holder’s option to purchase,
the series of debt securities; |
| ● | whether
the indenture will restrict our ability to pay dividends, or will require us to maintain
any asset ratios or reserves; |
| ● | whether
we will be restricted from incurring any additional indebtedness; |
| ● | a
discussion of any material or special U.S. federal income tax considerations applicable to
a series of debt securities; |
| ● | the
denominations in which we will issue the series of debt securities, if other than denominations
of $1,000 and any integral multiple thereof; and |
| ● | any
other specific terms, preferences, rights or limitations of, or restrictions on, the debt
securities. |
We
may issue debt securities that provide for an amount less than their stated principal amount to be due and payable upon declaration of
acceleration of their maturity pursuant to the terms of the indenture. We will provide you with information on the federal income tax
considerations and other special considerations applicable to any of these debt securities in the applicable prospectus supplement.
Conversion
or Exchange Rights
We
will set forth in the prospectus supplement the terms, if any, on which a series of debt securities may be convertible into or exchangeable
for our common stock or our other securities. We will include provisions as to whether conversion or exchange is mandatory, at the option
of the holder or at our option. We may include provisions pursuant to which the number of shares of our common stock or our other securities
that the holders of the series of debt securities receive would be subject to adjustment.
Consolidation,
Merger or Sale; No Protection in Event of a Change of Control or Highly Leveraged Transaction
The
indentures do not contain any covenant that restricts our ability to merge or consolidate, or sell, convey, transfer or otherwise dispose
of all or substantially all of our assets. However, any successor to or acquirer of such assets must assume all of our obligations under
the indentures or the debt securities, as appropriate.
Unless
we state otherwise in the applicable prospectus supplement, the debt securities will not contain any provisions that may afford holders
of the debt securities protection in the event we have a change of control or in the event of a highly leveraged transaction (whether
or not such transaction results in a change of control), which could adversely affect holders of debt securities.
Events
of Default Under the Indenture
The
following are events of default under the indentures with respect to any series of debt securities that we may issue:
| ● | if
we fail to pay interest when due and our failure continues for 90 days and the time for payment
has not been extended or deferred; |
| ● | if
we fail to pay the principal, or premium, if any, when due and the time for payment has not
been extended or delayed; |
| ● | if
we fail to observe or perform any other covenant set forth in the debt securities of such
series or the applicable indentures, other than a covenant specifically relating to and for
the benefit of holders of another series of debt securities, and our failure continues for
90 days after we receive written notice from the debenture trustee or holders of not less
than a majority in aggregate principal amount of the outstanding debt securities of the applicable
series; and |
| ● | if
specified events of bankruptcy, insolvency or reorganization occur as to us. |
No
event of default with respect to a particular series of debt securities (except as to certain events of bankruptcy, insolvency or reorganization)
necessarily constitutes an event of default with respect to any other series of debt securities. The occurrence of an event of default
may constitute an event of default under any bank credit agreements we may have in existence from time to time. In addition, the occurrence
of certain events of default or an acceleration under the indenture may constitute an event of default under certain of our other indebtedness
outstanding from time to time.
If
an event of default with respect to debt securities of any series at the time outstanding occurs and is continuing, then the trustee
or the holders of not less than a majority in principal amount of the outstanding debt securities of that series may, by a notice in
writing to us (and to the debenture trustee if given by the holders), declare to be due and payable immediately the principal (or, if
the debt securities of that series are discount securities, that portion of the principal amount as may be specified in the terms of
that series) of and premium and accrued and unpaid interest, if any, on all debt securities of that series. Before a judgment or decree
for payment of the money due has been obtained with respect to debt securities of any series, the holders of a majority in principal
amount of the outstanding debt securities of that series (or, at a meeting of holders of such series at which a quorum is present, the
holders of a majority in principal amount of the debt securities of such series represented at such meeting) may rescind and annul the
acceleration if all events of default, other than the non-payment of accelerated principal, premium, if any, and interest, if any, with
respect to debt securities of that series, have been cured or waived as provided in the applicable indenture (including payments or deposits
in respect of principal, premium or interest that had become due other than as a result of such acceleration). We refer you to the prospectus
supplement relating to any series of debt securities that are discount securities for the particular provisions relating to acceleration
of a portion of the principal amount of such discount securities upon the occurrence of an event of default.
Subject
to the terms of the indentures, if an event of default under an indenture shall occur and be continuing, the debenture trustee will be
under no obligation to exercise any of its rights or powers under such indenture at the request or direction of any of the holders of
the applicable series of debt securities, unless such holders have offered the debenture trustee reasonable indemnity. The holders of
a majority in principal amount of the outstanding debt securities of any series will have the right to direct the time, method and place
of conducting any proceeding for any remedy available to the debenture trustee, or exercising any trust or power conferred on the debenture
trustee, with respect to the debt securities of that series, provided that:
| ● | the
direction so given by the holder is not in conflict with any law or the applicable indenture;
and |
| ● | subject
to its duties under the Trust Indenture Act, the debenture trustee need not take any action
that might involve it in personal liability or might be unduly prejudicial to the holders
not involved in the proceeding. |
A
holder of the debt securities of any series will only have the right to institute a proceeding under the indentures or to appoint a receiver
or trustee, or to seek other remedies if:
| ● | the
holder previously has given written notice to the debenture trustee of a continuing event
of default with respect to that series; |
| ● | the
holders of at least a majority in aggregate principal amount of the outstanding debt securities
of that series have made written request, and such holders have offered reasonable indemnity
to the debenture trustee to institute the proceeding as trustee; and |
| ● | the
debenture trustee does not institute the proceeding, and does not receive from the holders
of a majority in aggregate principal amount of the outstanding debt securities of that series
(or at a meeting of holders of such series at which a quorum is present, the holders of a
majority in principal amount of the debt securities of such series represented at such meeting)
other conflicting directions within 60 days after the notice, request and offer. |
These
limitations do not apply to a suit instituted by a holder of debt securities if we default in the payment of the principal, premium,
if any, or interest on, the debt securities.
We
will periodically file statements with the applicable debenture trustee regarding our compliance with specified covenants in the applicable
indenture.
Modification
of Indenture; Waiver
The
debenture trustee and we may change the applicable indenture without the consent of any holders with respect to specific matters, including:
| ● | to
fix any ambiguity, defect or inconsistency in the indenture; and |
| ● | to
change anything that does not materially adversely affect the interests of any holder of
debt securities of any series issued pursuant to such indenture. |
In
addition, under the indentures, the rights of holders of a series of debt securities may be changed by us and the debenture trustee with
the written consent of the holders of at least a majority in aggregate principal amount of the outstanding debt securities of each series
(or, at a meeting of holders of such series at which a quorum is present, the holders of a majority in principal amount of the debt securities
of such series represented at such meeting) that is affected. However, the debenture trustee and we may make the following changes only
with the consent of each holder of any outstanding debt securities affected:
| ● | extending
the fixed maturity of the series of debt securities; |
| ● | reducing
the principal amount, reducing the rate of or extending the time of payment of interest,
or any premium payable upon the redemption of any debt securities; |
| ● | reducing
the principal amount of discount securities payable upon acceleration of maturity; |
| ● | making
the principal of or premium or interest on any debt security payable in currency other than
that stated in the debt security; or |
| ● | reducing
the percentage of debt securities, the holders of which are required to consent to any amendment
or waiver. |
Except
for certain specified provisions, the holders of at least a majority in principal amount of the outstanding debt securities of any series
(or, at a meeting of holders of such series at which a quorum is present, the holders of a majority in principal amount of the debt securities
of such series represented at such meeting) may on behalf of the holders of all debt securities of that series waive our compliance with
provisions of the indenture. The holders of a majority in principal amount of the outstanding debt securities of any series may on behalf
of the holders of all the debt securities of such series waive any past default under the indenture with respect to that series and its
consequences, except a default in the payment of the principal of, premium or any interest on any debt security of that series or in
respect of a covenant or provision, which cannot be modified or amended without the consent of the holder of each outstanding debt security
of the series affected; provided, however, that the holders of a majority in principal amount of the outstanding debt securities of any
series may rescind an acceleration and its consequences, including any related payment default that resulted from the acceleration.
Discharge
Each
indenture provides that we can elect to be discharged from our obligations with respect to one or more series of debt securities, except
for obligations to:
| ● | the
transfer or exchange of debt securities of the series; |
| ● | replace
stolen, lost or mutilated debt securities of the series; |
| ● | maintain
paying agencies; |
| ● | hold
monies for payment in trust; |
| ● | compensate
and indemnify the trustee; and |
| ● | appoint
any successor trustee. |
In
order to exercise our rights to be discharged with respect to a series, we must deposit with the trustee money or government obligations
sufficient to pay all the principal of, the premium, if any, and interest on, the debt securities of the series on the dates payments
are due.
Form,
Exchange, and Transfer
We
will issue the debt securities of each series only in fully registered form without coupons and, unless we otherwise specify in the applicable
prospectus supplement, in denominations of $1,000 and any integral multiple thereof. The indentures provide that we may issue debt securities
of a series in temporary or permanent global form and as book-entry securities that will be deposited with, or on behalf of, The Depository
Trust Company or another depositary named by us and identified in a prospectus supplement with respect to that series.
At
the option of the holder, subject to the terms of the indentures and the limitations applicable to global securities described in the
applicable prospectus supplement, the holder of the debt securities of any series can exchange the debt securities for other debt securities
of the same series, in any authorized denomination and of like tenor and aggregate principal amount.
Subject
to the terms of the indentures and the limitations applicable to global securities set forth in the applicable prospectus supplement,
holders of the debt securities may present the debt securities for exchange or for registration of transfer, duly endorsed or with the
form of transfer endorsed thereon duly executed if so required by us or the security registrar, at the office of the security registrar
or at the office of any transfer agent designated by us for this purpose. Unless otherwise provided in the debt securities that the holder
presents for transfer or exchange or in the applicable indenture, we will make no service charge for any registration of transfer or
exchange, but we may require payment of any taxes or other governmental charges.
We
will name in the applicable prospectus supplement the security registrar, and any transfer agent in addition to the security registrar,
that we initially designate for any debt securities. We may at any time designate additional transfer agents or rescind the designation
of any transfer agent or approve a change in the office through which any transfer agent acts, except that we will be required to maintain
a transfer agent in each place of payment for the debt securities of each series.
If
we elect to redeem the debt securities of any series, we will not be required to:
| ● | issue,
register the transfer of, or exchange any debt securities of that series during a period
beginning at the opening of business 15 days before the day of mailing of a notice of redemption
of any debt securities that may be selected for redemption and ending at the close of business
on the day of the mailing; or |
| ● | register
the transfer of or exchange any debt securities so selected for redemption, in whole or in
part, except the unredeemed portion of any debt securities we are redeeming in part. |
Information
Concerning the Debenture Trustee
The
debenture trustee, other than during the occurrence and continuance of an event of default under the applicable indenture, undertakes
to perform only those duties as are specifically set forth in the applicable indenture. Upon an event of default under an indenture,
the debenture trustee under such indenture must use the same degree of care as a prudent person would exercise or use in the conduct
of his or her own affairs. Subject to this provision, the debenture trustee is under no obligation to exercise any of the powers given
it by the indentures at the request of any holder of debt securities unless it is offered reasonable security and indemnity against the
costs, expenses and liabilities that it might incur.
Payment
and Paying Agents
Unless
we otherwise indicate in the applicable prospectus supplement, we will make payment of the interest on any debt securities on any interest
payment date to the person in whose name the debt securities, or one or more predecessor securities, are registered at the close of business
on the regular record date for the interest.
We
will pay principal of and any premium and interest on the debt securities of a particular series at the office of the paying agents designated
by us, except that unless we otherwise indicate in the applicable prospectus supplement, will we make interest payments by check which
we will mail to the holder. Unless we otherwise indicate in a prospectus supplement, we will designate the corporate trust office of
the debenture trustee in the City of New York as our sole paying agent for payments with respect to debt securities of each series. We
will name in the applicable prospectus supplement any other paying agents that we initially designate for the debt securities of a particular
series. We will maintain a paying agent in each place of payment for the debt securities of a particular series.
All
money we pay to a paying agent or the debenture trustee for the payment of the principal of or any premium or interest on any debt securities
which remains unclaimed at the end of two years after such principal, premium or interest has become due and payable will be repaid to
us, and the holder of the security thereafter may look only to us for payment thereof.
Governing
Law
The
indentures and the debt securities will be governed by and construed in accordance with the laws of the State of New York, except to
the extent that the Trust Indenture Act is applicable.
Subordination
of Subordinated Debt Securities
Our
obligations pursuant to any subordinated debt securities will be unsecured and will be subordinate and junior in priority of payment
to certain of our other indebtedness to the extent described in a prospectus supplement. The subordinated indenture does not limit the
amount of senior indebtedness we may incur. It also does not limit us from issuing any other secured or unsecured debt.
DESCRIPTION
OF WARRANTS
General
We
may issue warrants to purchase shares of our common stock, preferred stock and/or debt securities in one or more series together with
other securities or separately, as described in the applicable prospectus supplement. Below is a description of certain general terms
and provisions of the warrants that we may offer. Particular terms of the warrants will be described in the warrant agreements and the
prospectus supplement relating to the warrants.
The
applicable prospectus supplement will contain, where applicable, the following terms of and other information relating to the warrants:
| ● | the
specific designation and aggregate number of, and the price at which we will issue, the warrants; |
| ● | the
currency or currency units in which the offering price, if any, and the exercise price are
payable; |
| ● | the
designation, amount and terms of the securities purchasable upon exercise of the warrants; |
| ● | if
applicable, the exercise price for shares of our common stock and the number of shares of
common stock to be received upon exercise of the warrants; |
| ● | if
applicable, the exercise price for shares of our preferred stock, the number of shares of
preferred stock to be received upon exercise, and a description of that series of our preferred
stock; |
| ● | if
applicable, the exercise price for our debt securities, the amount of debt securities to
be received upon exercise, and a description of that series of debt securities; |
| ● | the
date on which the right to exercise the warrants will begin and the date on which that right
will expire or, if you may not continuously exercise the warrants throughout that period,
the specific date or dates on which you may exercise the warrants; |
| ● | whether
the warrants will be issued in fully registered form or bearer form, in definitive or global
form or in any combination of these forms, although, in any case, the form of a warrant included
in a unit will correspond to the form of the unit and of any security included in that unit; |
| ● | any
applicable material U.S. federal income tax consequences; |
| ● | the
identity of the warrant agent for the warrants and of any other depositories, execution or
paying agents, transfer agents, registrars or other agents; |
| ● | the
proposed listing, if any, of the warrants or any securities purchasable upon exercise of
the warrants on any securities exchange; |
| ● | if
applicable, the date from and after which the warrants and the common stock, preferred stock
and/or debt securities will be separately transferable; |
| ● | if
applicable, the minimum or maximum amount of the warrants that may be exercised at any one
time; |
| ● | information
with respect to book-entry procedures, if any; |
| ● | the
anti-dilution provisions of the warrants, if any; |
| ● | any
redemption or call provisions; |
| ● | whether
the warrants may be sold separately or with other securities as parts of units; and |
| ● | any
additional terms of the warrants, including terms, procedures and limitations relating to
the exchange and exercise of the warrants. |
Transfer
Agent and Registrar
The
transfer agent and registrar for any warrants will be set forth in the applicable prospectus supplement.
Outstanding
Warrants
December
2023 Warrants
In
connection with the royalty investment financing agreement we entered into in December 2023, we issued a warrant to purchase up to an
aggregate of 5,000,000 shares of our common stock. The warrant has a term of five years from the date of issuance and an exercise price
of $0.3467 per share, subject to customary adjustment for stock splits and similar transactions. A holder (together with its affiliates)
may not exercise any portion of the warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder
9.99%) of our outstanding common stock immediately after exercise. The warrant includes certain rights in favor of the holder upon a
“fundamental transaction” as described in the warrant, including the right of the holder to receive from us or the successor
entity an amount of cash equal to the Black-Scholes value (as described in the warrants) of the unexercised portion of the warrant on
the date of the consummation of such fundamental transaction.
The
warrant was allocated a value of $0.8 million using a Black-Scholes option pricing model based on the relative fair value method as they
were issued with common stock. The Black-Scholes model used the following assumptions: expected volatility: 85.91%; risk-free interest
rate: 4.05%; expected dividend yield: 0%; and expected term: 5 years. The warrant was deemed to be classified as equity and recorded
within additional paid in capital on the condensed consolidated balance sheets. As of December 31, 2023, no portion of the warrant has
been exercised.
September
2023 Warrants
In
connection with the registered direct offering completed in September 2023, we issued warrants to purchase up to an aggregate of 10,000,000
shares of our common stock. The warrants became exercisable on March 1, 2024, expire March 1, 2029 and have an exercise price of $0.77
per share, subject to customary adjustment for stock splits and similar transactions. A holder (together with its affiliates) may not
exercise any portion of a warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder 9.99%) of
our outstanding common stock immediately after exercise. The warrants include certain rights in favor of the holders upon a “fundamental
transaction” as described in the warrants, including the right of the holders to receive from us or the successor entity an amount
of cash equal to the Black-Scholes value (as described in the warrants) of the unexercised portion of the warrants on the date of the
consummation of such fundamental transaction.
The
warrants were allocated a value of $2.9 million using a Black-Scholes option pricing model based on the relative fair value method as
they were issued with common stock. The Black-Scholes model used the following assumptions: expected volatility: 87.77%; risk-free interest
rate: 4.29%; expected dividend yield: 0%; and expected term: 5.5 years. The warrants were deemed to be classified as equity and recorded
within additional paid in capital on the condensed consolidated balance sheets. As of December 31, 2023, none of the warrants have been
exercised.
Aquilo
Warrant
On
October 4, 2016, we issued Aquilo Partners, L.P. a warrant to purchase 6,500 shares of common stock at an exercise price of $10.00 per
share over a 10 year period.
DESCRIPTION
OF RIGHTS
General
We
may issue rights to our stockholders to purchase shares of our common stock, preferred stock or the other securities described in this
prospectus. We may offer rights separately or together with one or more additional rights, debt securities, preferred stock, common stock,
warrants or purchase contracts, or any combination of those securities in the form of units, as described in the applicable prospectus
supplement. Each series of rights will be issued under a separate rights agreement to be entered into between us and a bank or trust
company, as rights agent. The rights agent will act solely as our agent in connection with the certificates relating to the rights of
the series of certificates and will not assume any obligation or relationship of agency or trust for or with any holders of rights certificates
or beneficial owners of rights. The following description sets forth certain general terms and provisions of the rights to which any
prospectus supplement may relate. The particular terms of the rights to which any prospectus supplement may relate and the extent, if
any, to which the general provisions may apply to the rights so offered will be described in the applicable prospectus supplement. To
the extent that any particular terms of the rights, rights agreement or rights certificates described in a prospectus supplement differ
from any of the terms described below, then the terms described below will be deemed to have been superseded by that prospectus supplement.
We encourage you to read the applicable rights agreement and rights certificate for additional information before you decide whether
to purchase any of our rights. We will provide in a prospectus supplement the following terms of the rights being issued:
| ● | the
date of determining the stockholders entitled to the rights distribution; |
| ● | the
aggregate number of shares of common stock, preferred stock or other securities purchasable
upon exercise of the rights; |
| ● | the
exercise price; |
| ● | the
aggregate number of rights issued; |
| ● | whether
the rights are transferrable and the date, if any, on and after which the rights may be separately
transferred; |
| ● | the
date on which the right to exercise the rights will commence, and the date on which the right
to exercise the rights will expire; |
| ● | the
method by which holders of rights will be entitled to exercise; |
| ● | the
conditions to the completion of the offering, if any; |
| ● | the
withdrawal, termination and cancellation rights, if any; |
| ● | whether
there are any backstop or standby purchaser or purchasers and the terms of their commitment,
if any; |
| ● | whether
stockholders are entitled to oversubscription rights, if any; |
| ● | any
applicable material U.S. federal income tax considerations; and |
| ● | any
other terms of the rights, including terms, procedures and limitations relating to the distribution,
exchange and exercise of the rights, as applicable. |
Each
right will entitle the holder of rights to purchase for cash the principal amount of shares of common stock, preferred stock or other
securities at the exercise price provided in the applicable prospectus supplement. Rights may be exercised at any time up to the close
of business on the expiration date for the rights provided in the applicable prospectus supplement.
Holders
may exercise rights as described in the applicable prospectus supplement. Upon receipt of payment and the rights certificate properly
completed and duly executed at the corporate trust office of the rights agent or any other office indicated in the prospectus supplement,
we will, as soon as practicable, forward the shares of common stock, preferred stock or other securities, as applicable, purchasable
upon exercise of the rights. If less than all of the rights issued in any rights offering are exercised, we may offer any unsubscribed
securities directly to persons other than stockholders, to or through agents, underwriters or dealers or through a combination of such
methods, including pursuant to standby arrangements, as described in the applicable prospectus supplement.
Rights
Agent
The
rights agent for any rights we offer will be set forth in the applicable prospectus supplement.
DESCRIPTION
OF PURCHASE CONTRACTS
We
may issue purchase contracts, including contracts obligating holders to purchase from us, and for us to sell to holders, a specific or
variable number of our debt securities, shares of common stock, preferred stock, warrants or rights, or securities of an entity unaffiliated
with us, or any combination of the above, at a future date or dates. Alternatively, the purchase contracts may obligate us to purchase
from holders, and obligate holders to sell to us, a specific or variable number of our debt securities, shares of common stock, preferred
stock, warrants, rights or other property, or any combination of the above. The price of the securities or other property subject to
the purchase contracts may be fixed at the time the purchase contracts are issued or may be determined by reference to a specific formula
described in the purchase contracts. We may issue purchase contracts separately or as a part of units each consisting of a purchase contract
and one or more of our other securities described in this prospectus or securities of third parties, including U.S. Treasury securities,
securing the holder’s obligations under the purchase contract. The purchase contracts may require us to make periodic payments
to holders or vice versa and the payments may be unsecured or pre-funded on some basis. The purchase contracts may require holders to
secure the holder’s obligations in a manner specified in the applicable prospectus supplement.
The
applicable prospectus supplement will describe the terms of any purchase contracts in respect of which this prospectus is being delivered,
including, to the extent applicable, the following:
| ● | whether
the purchase contracts obligate the holder or us to purchase or sell, or both purchase and
sell, the securities subject to purchase under the purchase contract, and the nature and
amount of each of those securities, or the method of determining those amounts; |
| ● | whether
the purchase contracts are to be prepaid; |
| ● | whether
the purchase contracts are to be settled by delivery, or by reference or linkage to the value,
performance or level of the securities subject to purchase under the purchase contract; |
| ● | any
acceleration, cancellation, termination or other provisions relating to the settlement of
the purchase contracts; |
| ● | any
applicable material U.S. federal income tax considerations; and |
| ● | whether
the purchase contracts will be issued in fully registered or global form. |
The
preceding description sets forth certain general terms and provisions of the purchase contracts to which any prospectus supplement may
relate. The particular terms of the purchase contracts to which any prospectus supplement may relate and the extent, if any, to which
the general provisions may apply to the purchase contracts so offered will be described in the applicable prospectus supplement. To the
extent that any particular terms of the purchase contracts described in a prospectus supplement differ from any of the terms described
above, then the terms described above will be deemed to have been superseded by that prospectus supplement.
We
encourage you to read the applicable purchase contract for additional information before you decide whether to purchase any of our purchase
contracts.
DESCRIPTION
OF UNITS
The
following description, together with the additional information that we include in any applicable prospectus supplements summarizes the
material terms and provisions of the units that we may offer under this prospectus. While the terms we have summarized below will apply
generally to any units that we may offer under this prospectus, we will describe the particular terms of any series of units in more
detail in the applicable prospectus supplement. The terms of any units offered under a prospectus supplement may differ from the terms
described below.
We
will incorporate by reference from reports that we file with the SEC, the form of unit agreement that describes the terms of the series
of units we are offering, and any supplemental agreements, before the issuance of the related series of units. The following summaries
of material terms and provisions of the units are subject to, and qualified in their entirety by reference to, all the provisions of
the unit agreement and any supplemental agreements applicable to a particular series of units. We urge you to read the applicable prospectus
supplements related to the particular series of units that we may offer under this prospectus, as well as any related free writing prospectuses
and the complete unit agreement and any supplemental agreements that contain the terms of the units.
General
We
may issue units consisting of common stock, preferred stock, one or more debt securities, warrants, rights or purchase contacts for the
purchase of common stock, preferred stock and/or debt securities in one or more series, in any combination. Each unit will be issued
so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights
and obligations of a holder of each security included in the unit. The unit agreement under which a unit is issued may provide that the
securities included in the unit may not be held or transferred separately, at any time or at any time before a specified date.
We
will describe in the applicable prospectus supplement the terms of the series of units being offered, including:
| ● | the
designation and terms of the units and of the securities comprising the units, including
whether and under what circumstances those securities may be held or transferred separately; |
| ● | any
provisions of the governing unit agreement that differ from those described below; and |
| ● | any
provisions for the issuance, payment, settlement, transfer or exchange of the units or of
the securities comprising the units. |
The
provisions described in this section, as well as those set forth in any prospectus supplement or as described under “Description
of Common Stock,” “Description of Preferred Stock,” “Description of Debt Securities,” “Description
of Warrants,” “Description of Rights” and “Description of Purchase Contracts” will apply to each unit,
as applicable, and to any common stock, preferred stock, debt security, warrant, right or purchase contract included in each unit, as
applicable.
Unit
Agent
The
name and address of the unit agent for any units we offer will be set forth in the applicable prospectus supplement.
Issuance
in Series
We
may issue units in such amounts and in such numerous distinct series as we determine.
Enforceability
of Rights by Holders of Units
Each
unit agent will act solely as our agent under the applicable unit agreement and will not assume any obligation or relationship of agency
or trust with any holder of any unit. A single bank or trust company may act as unit agent for more than one series of units. A unit
agent will have no duty or responsibility in case of any default by us under the applicable unit agreement or unit, including any duty
or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a unit may, without the
consent of the related unit agent or the holder of any other unit, enforce by appropriate legal action its rights as holder under any
security included in the unit.
FORMS
OF SECURITIES
Each
debt security, warrant, purchase contract and unit will be represented either by a certificate issued in definitive form to a particular
investor or by one or more global securities representing the entire issuance of securities. Definitive securities name you or your nominee
as the owner of the security, and in order to transfer or exchange these securities or to receive payments other than interest or other
interim payments, you or your nominee must physically deliver the securities to the trustee, registrar, paying agent or other agent,
as applicable. Global securities name a depositary or its nominee as the owner of the debt securities, warrants, purchase contracts or
units represented by these global securities. The depositary maintains a computerized system that will reflect each investor’s
beneficial ownership of the securities through an account maintained by the investor with its broker/dealer, bank, trust company or other
representative, as we explain more fully below.
Global
Securities
We
may issue our debt securities, warrants, purchase contracts and units in the form of one or more fully registered global securities that
will be deposited with a depositary or its nominee identified in the applicable prospectus supplement and registered in the name of that
depositary or nominee. In those cases, one or more global securities will be issued in a denomination or aggregate denominations equal
to the portion of the aggregate principal or face amount of the securities to be represented by global securities. Unless and until it
is exchanged in whole for securities in definitive registered form, a global security may not be transferred except as a whole by and
among the depositary for the global security, the nominees of the depositary or any successors of the depositary or those nominees.
If
not described below, any specific terms of the depositary arrangement with respect to any securities to be represented by a global security
will be described in the prospectus supplement relating to those securities. We anticipate that the following provisions will apply to
all depositary arrangements.
Ownership
of beneficial interests in a global security will be limited to persons, called participants, that have accounts with the depositary
or persons that may hold interests through participants. Upon the issuance of a global security, the depositary will credit, on its book-entry
registration and transfer system, the participants’ accounts with the respective principal or face amounts of the securities beneficially
owned by the participants. Any dealers, underwriters or agents participating in the distribution of the securities will designate the
accounts to be credited. Ownership of beneficial interests in a global security will be shown on, and the transfer of ownership interests
will be effected only through, records maintained by the depositary, with respect to interests of participants, and on the records of
participants, with respect to interests of persons holding through participants. The laws of some states may require that some purchasers
of securities take physical delivery of these securities in definitive form. These laws may impair your ability to own, transfer or pledge
beneficial interests in global securities.
So
long as the depositary, or its nominee, is the registered owner of a global security, that depositary or its nominee, as the case may
be, will be considered the sole owner or holder of the securities represented by the global security for all purposes under the applicable
indenture, warrant agreement, purchase contract or unit agreement. Except as described below, owners of beneficial interests in a global
security will not be entitled to have the securities represented by the global security registered in their names, will not receive or
be entitled to receive physical delivery of the securities in definitive form and will not be considered the owners or holders of the
securities under the applicable indenture, warrant agreement, purchase contract or unit agreement. Accordingly, each person owning a
beneficial interest in a global security must rely on the procedures of the depositary for that global security and, if that person is
not a participant, on the procedures of the participant through which the person owns its interest, to exercise any rights of a holder
under the applicable indenture, warrant agreement, purchase contract or unit agreement. We understand that under existing industry practices,
if we request any action of holders or if an owner of a beneficial interest in a global security desires to give or take any action that
a holder is entitled to give or take under the applicable indenture, warrant agreement, purchase contract or unit agreement, the depositary
for the global security would authorize the participants holding the relevant beneficial interests to give or take that action, and the
participants would authorize beneficial owners owning through them to give or take that action or would otherwise act upon the instructions
of beneficial owners holding through them.
Principal,
premium, if any, and interest payments on debt securities, and any payments to holders with respect to warrants or units, represented
by a global security registered in the name of a depositary or its nominee will be made to the depositary or its nominee, as the case
may be, as the registered owner of the global security. None of us, or any trustee, warrant agent, unit agent or other agent of ours,
or any agent of any trustee, warrant agent or unit agent will have any responsibility or liability for any aspect of the records relating
to payments made on account of beneficial ownership interests in the global security or for maintaining, supervising or reviewing any
records relating to those beneficial ownership interests.
We
expect that the depositary for any of the securities represented by a global security, upon receipt of any payment to holders of principal,
premium, interest or other distribution of underlying securities or other property on that registered global security, will immediately
credit participants’ accounts in amounts proportionate to their respective beneficial interests in that global security as shown
on the records of the depositary. We also expect that payments by participants to owners of beneficial interests in a global security
held through participants will be governed by standing customer instructions and customary practices, as is now the case with the securities
held for the accounts of customers or registered in “street name,” and will be the responsibility of those participants.
If
the depositary for any of the securities represented by a global security is at any time unwilling or unable to continue as depositary
or ceases to be a clearing agency registered under the Exchange Act, and a successor depositary registered as a clearing agency under
the Exchange Act is not appointed by us within 90 days, we will issue securities in definitive form in exchange for the global security
that had been held by the depositary. Any securities issued in definitive form in exchange for a global security will be registered in
the name or names that the depositary gives to the relevant trustee, warrant agent, unit agent or other relevant agent of ours or theirs.
It is expected that the depositary’s instructions will be based upon directions received by the depositary from participants with
respect to ownership of beneficial interests in the global security that had been held by the depositary.
CERTAIN
PROVISIONS OF DELAWARE LAW AND OF OUR CERTIFICATE OF INCORPORATION AND BYLAWS
Anti-Takeover
Effect Provisions
Certain
provisions in our restated certificate of incorporation and our restated bylaws and applicable provisions under the Delaware General
Corporation Law, or the DGCL, may have an anti-takeover effect, including:
Classified
Board. We have a classified board of directors with three-year staggered terms, which may delay the ability of stockholders to change
the composition of a majority of our board of directors.
Number
of Directors. The number of directors on our board of directors is established by our board of directors, which may delay the ability
of stockholders to change the composition of a majority of our board of directors.
No
Cumulative Voting. Our stockholders cannot cumulate their votes in the election of directors, which limits the ability of minority
stockholders to elect director candidates.
Filling
of Vacancies. Our board of directors have the exclusive right to elect a director to fill any vacancy or newly created directorship.
Removing
Directors. A director may be removed only for cause and only by the affirmative vote of at least 75% of the votes which all the stockholders
would be entitled to cast in any annual election of directors or class of directors.
Prohibition
on Written Consent. Our stockholders are prohibited from acting by written consent, which forces stockholder action to be taken at
an annual or special meeting of our stockholders.
Calling
Special Meetings. Special meetings of our stockholders may be called only by our board of directors, the chairman of our board of
directors or our chief executive officer, which may delay the ability of our stockholders to force consideration of a proposal or to
take action, including the removal of directors.
Advance
Notice Procedures. Stockholders must comply with the advance notice procedures in our restated bylaws to nominate candidates to our
board of directors and to propose matters to be acted upon at a stockholders’ meeting, which may discourage or deter a potential
acquirer from soliciting proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of us;
Supermajority
Provisions. The affirmative vote of the holders of at least 75% of the votes which all the stockholders would be entitled to cast
in any annual election of directors or class of directors is required to amend or repeal, or to adopt any provision inconsistent with,
the provisions in our restated certificate of incorporation that relate to, among other matters, the classification of our board of directors,
the number of our directors, the removal of our directors, the filling of vacancies on our board of directors, the prohibition on our
stockholders to act by written consent, and the calling of special meetings of our stockholders.
Bylaw
Amendments. Our board of directors, by majority vote, may amend, alter or repeal our restated bylaws and may adopt new bylaws. Our
stockholders may not adopt, amend, alter or repeal our restated bylaws or adopt any provision inconsistent therewith, unless such action
is approved, in addition to any vote required by our restated certificate of incorporation, by the affirmative vote of holders of at
least 75% of the votes that all the stockholders would be entitled to cast in any annual election of directors or class of directors,
and the affirmative vote of holders of at least 75% of the votes that all the stockholders would be entitled to cast in any annual election
of directors or class of directors is required to amend or repeal, or to adopt any provision inconsistent with, the foregoing. These
provisions may inhibit the ability of an acquirer from amending our restated certificate of incorporation or our restated bylaws to facilitate
a hostile acquisition and may allow our board of directors to take additional actions to prevent a hostile acquisition.
Preferred
Stock. Our board of directors can determine to issue shares of preferred stock and to determine the price and other terms of those
shares, including preferences and voting rights, without stockholder approval, which could significantly dilute the ownership of a hostile
acquirer.
Additional
Authorized Shares of Capital Stock. The shares of authorized common stock and preferred stock available for issuance under our restated
certificate of incorporation could be issued at such times, under such circumstances, and with such terms as to impede a change in control.
DGCL
Section 203. We are subject to Section 203 of the DGCL, which, subject to certain exceptions, prohibits a Delaware corporation from
engaging in any “business combination” with any “interested stockholder” for three years following the date that
such stockholder became an interested stockholder, unless: (i) before such date, the board of directors of the corporation approved either
the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; (ii) on consummation
of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85%
of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number
of shares outstanding those shares owned (a) by persons who are directors and also officers and (b) by employee stock plans in which
employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a
tender or exchange offer; or (iii) on or after such date, the business combination is approved by the board of directors and authorized
at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2⁄3% of the
outstanding voting stock not owned by the interested stockholder.
The
term “business combination” generally includes mergers or consolidations resulting in a financial benefit to the interested
stockholder. The term “interested stockholder” generally means any person, other than the corporation and any direct or indirect
majority-owned subsidiary of the corporation, who, together with affiliates and associates, owns (or owned within three years prior to
the determination of interested stockholder status) 15% or more of the outstanding voting stock of the corporation. The provisions of
Section 203 of the DGCL may deter a hostile takeover or delay a change in control.
Choice
of Forum
Our
restated bylaws provide that, among other things, unless we consent in writing to the selection of an alternative forum, the courts located
within the State of Delaware will serve as the sole and exclusive forum for the adjudication of (i) any derivative action or proceeding
brought on behalf of us; (ii) any action or proceeding asserting a claim of breach of a fiduciary duty owed by any current or former
director, officer or other employee of ours, to us or our stockholders; (iii) any action or proceeding asserting a claim against us or
any current or former director, officer or other employee of ours, arising out of or pursuant to any provision of the DGCL, our restated
certificate of incorporation or our restated bylaws (as each may be amended from time to time); (iv) any action or proceeding to interpret,
apply, enforce or determine the validity of our restated certificate of incorporation or our restated bylaws (including any right, obligation,
or remedy thereunder); (v) any action or proceeding as to which the DGCL confers jurisdiction to the Court of Chancery of the State of
Delaware; and (vi) any action asserting a claim against us or any director, officer or other employee of ours, governed by the internal
affairs doctrine, in all cases to the fullest extent permitted by law and subject to the court’s having personal jurisdiction over
the indispensable parties named as defendants. This choice of forum provision will not apply to suits brought to enforce a duty or liability
created by the Securities Act, the Exchange Act, or any other claim for which the federal courts have exclusive jurisdiction. In addition,
our restated bylaws provide that, unless we consent in writing to the selection of an alternative forum, to the fullest extent permitted
by law, the federal district courts of the United States of America shall be the sole and exclusive forum for the resolution of any complaint
asserting a cause of action arising under the Securities Act. Our restated bylaws also provides that any person or entity purchasing
or otherwise acquiring or holding any interest in any of our securities will be deemed to have notice of and consented to this choice
of forum provision.
We
believe these choice of forum provisions may benefit us by providing increased consistency in the application of Delaware law and federal
securities laws by chancellors and judges, as applicable, particularly experienced in resolving corporate disputes, efficient administration
of cases on a more expedited schedule relative to other forums and protection against the burdens of multi-forum litigation. These provisions
may have the effect of discouraging lawsuits against us and/or our directors, officers and employees as they may limit a stockholder’s
ability to bring a claim in a judicial forum that the stockholder finds favorable for disputes with us or our directors, officers or
employees.
Limitation
of Liability and Indemnification
Section
102 of the DGCL permits a corporation to provide in its certificate of incorporation that a director or officer of the corporation shall
not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director or officer,
except for liability (i) for any breach of the director’s or officer’s duty of loyalty to the corporation or its stockholders,
(ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) for payments
of unlawful dividends or unlawful stock purchases or redemptions, (iv) for any transaction from which the director or officer derived
an improper personal benefit, or (v) of officers in any action by or in the right of the corporation.
Our
restated certificate of incorporation provides that no director of our corporation shall be personally liable to us or our stockholders
for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except
to the extent that the DGCL prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Our
restated certificate of incorporation provides that we will indemnify each individual who was or is a party or threatened to be made
a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative
(other than an action by or in the right of us), by reason of the fact that such individual is or was, or has agreed to become, our director
or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or
in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (all such individuals being referred
to as an Indemnitee), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including
attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action,
suit or proceeding and any appeal therefrom if such Indemnitee acted in good faith and in a manner the Indemnitee reasonably believed
to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, such Indemnitee had no reasonable
cause to believe the Indemnitee’s conduct was unlawful.
Our
restated certificate of incorporation also provides that we will indemnify any Indemnitee who was or is a party to an action or suit
by or in the right of us to procure a judgment in our favor by reason of the fact that the Indemnitee is or was, or has agreed to become,
our director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee
or, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, or by reason of any action
alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees) and, to the extent permitted
by law, amounts paid in settlement actually and reasonably incurred by Indemnitee in connection with such action, suit or proceeding
and any appeal therefrom, if the Indemnitee acted in good faith and in a manner which the Indemnitee reasonably believed to be in, or
not opposed to, our best interests, except that no indemnification shall be made in respect of any claim, issue or matter as to which
the Indemnitee shall have been adjudged to be liable to us, unless, and only to the extent, that the Court of Chancery of Delaware or
the court in which such action or suit was brought shall determine upon application that, despite the adjudication of such liability
but in view of all the circumstances of the case, the Indemnitee is fairly and reasonably entitled to indemnity for such expenses (including
attorneys’ fees) which the Court of Chancery of Delaware or such other court shall deem proper. Notwithstanding the foregoing,
to the extent that any Indemnitee has been successful, on the merits or otherwise, such Indemnitee will be indemnified by us against
all expenses (including attorneys’ fees) actually and reasonably incurred by or on behalf of the Indemnitee in connection therewith.
Without limiting the foregoing, if any action, suit or proceeding is disposed of, on the merits or otherwise (including a disposition
without prejudice), without (i) the disposition being adverse to the Indemnitee, (ii) an adjudication that the Indemnitee was liable
to us, (iii) a plea of guilty or nolo contendere by the Indemnitee, (iv) an adjudication that the Indemnitee did not act in good faith
and in a manner the Indemnitee reasonably believed to be in or not opposed to our best interests, and (v) with respect to any criminal
proceeding, an adjudication that Indemnitee had reasonable cause to believe his or her conduct was unlawful, the Indemnitee shall be
considered for the purposes hereof to have been wholly successful with respect thereto. If we do not assume the defense, expenses must
be advanced to an Indemnitee under certain circumstances.
Section
145 of the DGCL provides that a corporation has the power to indemnify a director, officer, employee, or agent of the corporation, or
a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in
related capacities against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably
incurred by the person in connection with an action, suit or proceeding to which such individual was or is a party or is threatened to
be made a party to any threatened, ending or completed action, suit or proceeding by reason of such position, if such individual acted
in good faith and in a manner the individual reasonably believed to be in or not opposed to the best interests of the corporation, and,
in any criminal action or proceeding, had no reasonable cause to believe such individual’s conduct was unlawful, except that, in
the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue
or matter as to which such individual shall have been adjudged to be liable to the corporation unless and only to the extent that the
Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances
of the case, such individual is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other
court shall deem proper.
We
have entered into indemnification agreements with our directors and certain officers, in addition to the indemnification provided in
our restated certificate of incorporation, and intend to enter into indemnification agreements with any new directors and executive officers
in the future. In general, these agreements provide that we will indemnify the director or officer to the fullest extent permitted by
law for claims arising in such individual’s capacity as a director or officer of our company or in connection with their service
at our request for another corporation or entity. The indemnification agreements also provide for procedures that will apply in the event
that the director or officer makes a claim for indemnification and establish certain presumptions that are favorable to the director
or officer, as applicable.
We
have purchased and intend to maintain insurance on behalf of any individual who is or was a director or officer against any loss arising
from any claim asserted against such individual and incurred by the individual in any such capacity, subject to certain exclusions.
The
foregoing discussion of our restated certificate of incorporation, restated bylaws, indemnification agreements and Delaware law is not
intended to be exhaustive and is qualified in its entirety by such documents or law.
Insofar
as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons
pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against
public policy as expressed in the Securities Act and is, therefore, unenforceable.
Dissenter’s
Rights of Appraisal and Payment
Under
the DGCL, with certain exceptions, our stockholders have appraisal rights in connection with a merger or consolidation of our company.
Under the DGCL, stockholders who properly request and perfect appraisal rights in connection with such merger or consolidation will have
the right to receive payment of the fair value of their shares as determined by the Delaware Court of Chancery.
LEGAL
MATTERS
Mintz,
Levin, Cohn, Ferris, Glovsky and Popeo, P.C., San Diego, California, will pass upon certain legal matters in connection with the offering
and validity of the securities offered by this prospectus.
EXPERTS
Haskell
& White LLP, an independent registered public accounting firm, has audited our consolidated financial statements included in our
Annual Report on Form 10-K for the year ended December 31, 2023, as set forth in its report, which is incorporated by reference in this
prospectus and the registration statement. Our financial statements are incorporated by reference in reliance on Haskell & White
LLP’s report, given on the authority of said firm as experts in accounting and auditing.
Mayer
Hoffman McCann P.C., an independent registered public accounting firm, has audited our consolidated financial statements included in
our Annual Report on Form 10-K for the year ended December 31, 2022, as set forth in its report (which report includes an explanatory
paragraph regarding the existence of substantial doubt about our ability to continue as a going concern), which is incorporated by reference
in this prospectus and the registration statement. Our financial statements are incorporated by reference in reliance on Mayer Hoffman
McCann P.C.’s report, given on the authority of said firm as experts in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
We
are subject to the reporting requirements of the Exchange Act and file annual, quarterly and current reports, proxy statements and other
information with the SEC. The SEC maintains an internet site that contains reports, proxy and information statements and other information
regarding issuers, such as our company, that file documents electronically with the SEC. Our SEC filings are available to the public
at the SEC’s website address at http://www.sec.gov. The information on the SEC’s website is not part of this prospectus,
and any references to the SEC’s website or any other website are inactive textual references only.
We
also maintain a website at www.darebioscience.com, through which you can access our SEC filings. The information set forth on
our website is not part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
This
prospectus is only part of a registration statement on Form S-3 that we filed with the SEC. This prospectus omits some information contained
in the registration statement in accordance with the SEC rules and regulations. You should review the information in and schedules and/or
exhibits to the registration statement for further information about us and the securities being offered pursuant to this prospectus.
Statements in this prospectus concerning any document we filed as an exhibit or schedule to the registration statement or that we otherwise
filed with the SEC are not intended to be comprehensive and are qualified by reference to these filings. You should review the complete
document to evaluate these statements.
INCORPORATION
OF DOCUMENTS BY REFERENCE
The
SEC allows us to “incorporate by reference” into this prospectus the information that we file with the SEC, which means that
we can disclose important information to you by referring you to those publicly available documents. The information that we incorporate
by reference is an important part of this prospectus. This prospectus incorporates by reference the documents listed below:
| ● | our
Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC
on March 28, 2023 (the “Annual Report”), including all material incorporated
by reference therein; |
| ● | our
Current Reports on Form 8-K and on Form 8-K/A filed with the SEC on January 19, 2024 and
January 26, 2024 (except for any information furnished under Items 2.02 or 7.01 of Form 8-K
and all exhibits related to such items); and |
| ● | the
description of our common stock contained in our Registration Statement on Form 8-A filed
on April 4, 2014, including any amendments thereto or reports filed for the purpose of updating
such description, including the description of our common stock in Exhibit 4.5 of the Annual
Report. |
The
SEC file number for each of the documents listed above is 001-36395.
In
addition, all documents filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 and 15(d) of the Exchange Act after the date of
this prospectus and prior to the termination of this offering shall be deemed to be incorporated by reference into this prospectus and
to be a part hereof from the date of filing such reports and other documents (excluding any information in such documents furnished under
Item 2.02 or Item 7.01 of any Current Report on Form 8-K and any exhibit furnished with such reports that is related to those items).
We also specifically incorporate by reference into this prospectus all documents filed by us with the SEC pursuant to Section 13(a),
13(c), 14 or 15(d) of the Exchange Act after the date of the initial registration statement of which this prospectus is a part and prior
to effectiveness of the registration statement (excluding any information in such documents that is furnished under Item 2.02 or Item
7.01 of any Current Report on Form 8-K and any exhibit furnished with such reports that is related to those items).
Any
statement contained in this prospectus or in a document incorporated or deemed to be incorporated by reference into this prospectus will
be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus or
any other subsequently filed document that is deemed to be incorporated by reference into this prospectus modifies or supersedes the
statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this
prospectus.
We
will provide to each person, including any beneficial owner, to whom this prospectus is delivered, upon written or oral request and at
no cost to the requester, a copy of any or all reports or documents that are incorporated by reference into this prospectus, but not
delivered with the prospectus. Such written or oral requests should be directed to:
Daré
Bioscience, Inc.
3655
Nobel Drive, Suite 260
San
Diego, CA 92122
Attn:
Chief Accounting Officer
Telephone:
(858) 926-7655
You
may also access these documents on our website, www.darebioscience.com. The information contained on, or that can be accessed
through, our website is not a part of this prospectus. We have included our website address in this prospectus solely as an inactive
textual reference.
DARÉ
BIOSCIENCE, INC.

Up
to $18,089,630
Common
Stock
PROSPECTUS
SUPPLEMENT
May
10, 2024
Dare Bioscience (NASDAQ:DARE)
Historical Stock Chart
From Apr 2024 to May 2024
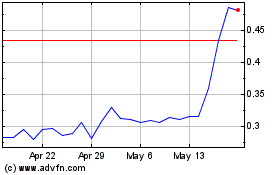
Dare Bioscience (NASDAQ:DARE)
Historical Stock Chart
From May 2023 to May 2024