GLUCOTRACK ANNOUNCES THE APPOINTMENT OF ANDY BALO TO BOARD OF DIRECTORS
20 June 2024 - 10:30PM

Glucotrack, Inc. (Nasdaq: GCTK) (“Glucotrack” or the “Company”), a
medical technology company focused on the design, development, and
commercialization of novel technologies for people with diabetes,
today announced that it has appointed Andy Balo to its Board of
Directors, effective immediately.
Mr. Balo brings decades of regulatory, clinical
and quality experience in the medical technology industry. In 2002
he joined Dexcom as part of the original executive team where he
remained for the next 22 years playing a critical role in shaping
the company’s future. During his tenure, he was responsible for
numerous glucose monitoring regulatory submissions and clinical
trials worldwide and coordinated quality activities across multiple
manufacturing facilities. In March 2024, Mr. Balo retired from
Dexcom as Executive Vice President of Clinical, Global Access, and
Medical Affairs. Prior to joining Dexcom, Mr. Balo held several
leadership positions at St. Jude Medical, including Corporate Vice
President of Regulatory, Clinical, and Quality, and also served in
executive roles at Baxter, Pacesetter and Endocardial
Solutions.
Mr. Balo is widely regarded as an industry
expert in regulatory and clinical strategies. He has served on
several FDA panels as an industry representative, spanning
cardiovascular, neurological, and gastrointestinal technologies. He
has been instrumental in bringing several breakthrough medical
devices to market, including continuous glucose monitors,
tissue-based and mechanical heart valves, 3-D electrophysiology
mapping devices, pacemakers, and has obtained approval for over 100
PMAs, PMA supplements and 510ks.
Mr. Balo holds a Bachelor of Science degree in
microbiology and chemistry from the University of Maryland and
completed graduate studies at UCLA.
“We are honored to have Andy join our Board of
Directors,” said Paul Goode, PhD, CEO of Glucotrack. “He is
nationally recognized as a thought leader in the regulatory and
clinical landscape, and his experience in diabetes and
cardiovascular technologies will be invaluable as we move into
human clinical studies for our unique technology. Additionally,
Andy is an authentic, driven and passionate leader who will help
guide the company through our regulatory, clinical and
commercialization milestones.”
“I am excited to be joining the Board of such an
innovative and nimble medical technology company,” said Mr. Balo.
“Glucotrack’s continuous blood glucose monitor (CBGM) is a very
compelling product that bridges diabetes and cardiovascular
technology, leveraging established techniques and tools to create a
truly differentiated glucose monitoring product. I look forward to
working with the Board and the leadership team to bring this
groundbreaking technology to people living with diabetes every
day.”
For more information about Glucotrack, visit
glucotrack.com. Information on the Company’s website does not
constitute a part of and is not incorporated by reference into this
press release.
# # #
About Glucotrack, Inc.
Glucotrack, Inc. (NASDAQ: GCTK) is focused on
the design, development, and commercialization of novel
technologies for people with diabetes. The Company is currently
developing a long-term implantable continuous blood glucose
monitoring system for people living with diabetes.
Glucotrack’s CBGM is a long-term, implantable
system that continually measures blood glucose levels with a sensor
longevity of 2+ years, no on-body wearable component and with
minimal calibration. For more information, please
visit http://www.glucotrack.com.
Forward-Looking Statements
This news release contains forward-looking
statements within the meaning of the Private Securities Litigation
Reform Act of 1995. Statements contained in this news release that
are not statements of historical fact may be deemed to be
forward-looking statements. Without limiting the generality of the
foregoing, words such as “believe”, “expect”, “plan” and “will” are
intended to identify forward-looking statements. Such
forward-looking statements are based on the beliefs of management,
as well as assumptions made by, and information currently available
to, management. These statements relate only to events as of the
date on which the statements are made, and Glucotrack undertakes no
obligation to publicly update any forward-looking statements,
whether as a result of new information, future events or otherwise,
except as required by law. All of the forward-looking statements
made in this press release are qualified by these cautionary
statements, and there can be no assurance that the actual results
anticipated by Glucotrack will be realized or, even if
substantially realized, that they will have the expected
consequences to or effects on us or our business or operations.
Readers are cautioned that certain important factors may affect
Glucotrack’s actual results and could cause such results to differ
materially from any forward-looking statements that may be made in
this news release. Factors that may affect Glucotrack’s results
include, but are not limited to, the ability of Glucotrack to raise
additional capital to finance its operations (whether through
public or private equity offerings, debt financings, strategic
collaborations or otherwise); risks relating to the receipt (and
timing) of regulatory approvals (including U.S. Food and Drug
Administration approval); risks relating to enrollment of patients
in, and the conduct of, clinical trials; risks relating to
Glucotrack’s future distribution agreements; risks relating to its
ability to hire and retain qualified personnel, including sales and
distribution personnel; and the additional risk factors described
in Glucotrack’s filings with the U.S. Securities and Exchange
Commission (the “SEC”), including its Annual Report on Form 10-K
for the year ended December 31, 2023 as filed with the SEC on March
28, 2024.
Contacts:
Investor Relations:investors@glucotrack.com
Media:GlucotrackPR@icrinc.com
GlucoTrack (NASDAQ:GCTK)
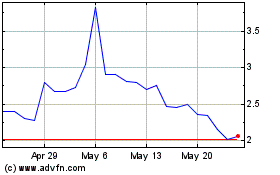
Historical Stock Chart
From May 2024 to Jun 2024
GlucoTrack (NASDAQ:GCTK)
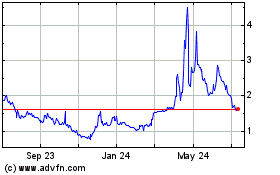
Historical Stock Chart
From Jun 2023 to Jun 2024