Quoin Pharmaceuticals to Sponsor the Foundation for Ichthyosis & Related Skin Types (FIRST) 2024 National Conference
26 June 2024 - 6:05AM

Quoin Pharmaceuticals Ltd. (NASDAQ: QNRX) (the “Company” or
“Quoin”), a clinical stage specialty pharmaceutical company focused
on rare and orphan diseases, today announces that it will be a
Bronze Sponsor for the Foundation for Ichthyosis & Related Skin
Types (FIRST) 2024 national conference in Albuquerque, New Mexico
from June 28-30, 2024.
The three-day premier event is held every two
years with the aim of connecting individuals seeking information
about ichthyosis and other skin disorders. It brings together
members of the Foundation and their families to meet and network
with other affected individuals, and consult with leading medical
experts.
Quoin COO, Denise Carter, said, “Quoin is very
proud to be a sponsor of the 2024 FIRST national conference and
honored to be a member of this network supporting individuals and
families living with rare diseases. As we have learned from our
interactions with this community, “Rare diseases are only rare if
you don’t live with one™”. Raising awareness for these diseases,
supporting the patients and families, and funding development for
products to treat these underserved, unmet needs are all key
priorities for Quoin. That is why we are fully dedicated to
delivering a safe and effective treatment for Netherton Syndrome as
expeditiously as possible.”
Quoin is a Bronze Sponsor of the FIRST 2024
national conference, and will sponsor the conference’s celebration
dinner, which will be held on June 29, 2024.
Quoin is conducting two ongoing clinical trials
evaluating QRX003, a topical lotion, for the treatment of Netherton
Syndrome. For more information about the trials, please visit:
https://www.nethertonsyndromeclinicaltrials.com/.
About The Foundation for Ichthyosis
& Related Skin Types, Inc.® (FIRST)
The Foundation for Ichthyosis & Related Skin
Types, Inc.® (FIRST) is the only national non-profit foundation
located in the United States dedicated to assisting families
affected by ichthyosis. FIRST provides information on its website
www.firstskinfoundation.org, printed publications, and a
newsletter, Ichthyosis Focus. FIRST hosts a biennial national
conference, and patient support forums which provide families the
opportunity to create connections with each other and consult with
leading medical experts.
About Quoin Pharmaceuticals
Ltd.Quoin Pharmaceuticals Ltd. is a clinical stage
specialty pharmaceutical company focused on developing and
commercializing therapeutic products that treat rare and orphan
diseases. We are committed to addressing unmet medical needs for
patients, their families, communities and care teams. Quoin’s
innovative pipeline comprises four products in development that
collectively have the potential to target a broad number of rare
and orphan indications, including Netherton Syndrome, Peeling Skin
Syndrome, Palmoplantar Keratoderma, Scleroderma, Epidermolysis
Bullosa and others. For more information, visit:
www.quoinpharma.com or LinkedIn for updates.
Cautionary Note
Regarding Forward Looking Statements
The Company cautions that
statements in this press release that are not a description of
historical facts are forward-looking statements within the meaning
of the Private Securities Litigation Reform Act of 1995.
Forward-looking statements may be identified by the use of words
referencing future events or circumstances such as “expect,”
“intend,” “plan,” “anticipate,” “believe,” and “will,” among
others. All statements that reflect the Company’s expectations,
assumptions, projections, beliefs, or opinions about the future,
other than statements of historical fact, are forward-looking
statements, including, without limitation, statements relating to
the conference’s aim of connecting individuals seeking information
about ichthyosis and other skin disorders, the Company delivering a
safe and effective treatment for Netherton Syndrome as
expeditiously as possible, and the Company’s four products in
development collectively having the potential to target a broad
number of rare and orphan indications, including Netherton
Syndrome, Peeling Skin Syndrome, Palmoplantar Keratoderma,
Scleroderma, Epidermolysis. Because such statements are subject to
risks and uncertainties, actual results may differ materially from
those expressed or implied by such forward-looking statements.
These forward-looking statements are based upon the Company’s
current expectations and involve assumptions that may never
materialize or may prove to be incorrect. Actual results and the
timing of events could differ materially from those anticipated in
such forward-looking statements as a result of various risks and
uncertainties including, but not limited to, the Company ability to
deliver a safe and effective treatment for Netherton Syndrome, the
preclinical and clinical studies of the Company’s product
candidates may not be successful and other factors discussed in the
Company’s Annual Report on Form 10-K for the year ended December
31, 2023 that the Company filed with the SEC and the Company’s
subsequent filings with the SEC on Forms 10-Q and 8-K. One should
not place undue reliance on these forward-looking statements, which
speak only as of the date on which they were made. The Company
undertakes no obligation to update such statements to reflect
events that occur or circumstances that exist after the date on
which they were made, except as may be required by law.
For further information, contact:Investor
RelationsPCG AdvisoryJeff Ramsonjramson@pcgadvisory.com(646)
863-6341
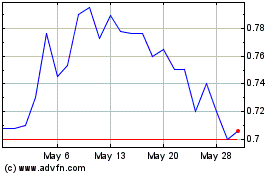
Quoin Pharmaceuticals (NASDAQ:QNRX)
Historical Stock Chart
From Jun 2024 to Jul 2024
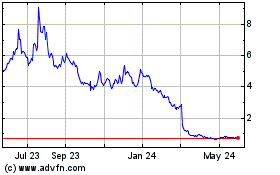
Quoin Pharmaceuticals (NASDAQ:QNRX)
Historical Stock Chart
From Jul 2023 to Jul 2024