Filed pursuant to Rule 424(b)(3)
Registration No. 333-277016
PROSPECTUS SUPPLEMENT NO. 3
(to Prospectus dated April 3, 2024)

10,242,092
Ordinary Shares Represented by 10,242,092 American Depositary Shares Issuable Upon
Exercise
of the Pre-Funded Warrants, Common Warrants, Series D Warrants and Series E Warrants
This
prospectus supplement updates, amends and supplements the prospectus contained in our Post-Effective Amendment No. 1 to Form S-1, Post-Effective
Amendment No. 2 to Form S-1 and Post-Effective Amendment No. 2 to Form S-1, effective as of April 3, 2024 (as supplemented or amended
from time to time, the “Prospectus”) (Registration No. 333-277016). Capitalized terms used in this prospectus supplement and
not otherwise defined herein have the meanings specified in the Prospectus.
This
prospectus supplement is being filed to update, amend and supplement the information included in the Prospectus with the information contained
in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2024 filed with the Securities and Exchange Commission (the “SEC”)
on August 8, 2024, which is set forth below.
This
prospectus supplement is not complete without the Prospectus. This prospectus supplement should be read in conjunction with the Prospectus,
which is to be delivered with this prospectus supplement, and is qualified by reference thereto, except to the extent that the information
in this prospectus supplement updates or supersedes the information contained in the Prospectus. Please keep this prospectus supplement
with your Prospectus for future reference.
Our
ADSs are listed on the Nasdaq Capital Market under the symbol “QNRX”. On August 8, 2024, the closing price for our ADSs on
the Nasdaq Capital Market was $0.5240 per ADS.
Investing
in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully the
risks and uncertainties under the heading “Risk Factors” beginning on page 5 of the Prospectus.
Neither
the SEC nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of
the Prospectus or this prospectus supplement. Any representation to the contrary is a criminal offense.
The date of this prospectus
supplement is August 8, 2024.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
☒
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended June 30, 2024
OR
☐
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ___________ to __________
Commission file number: 001-37846
QUOIN PHARMACEUTICALS LTD.
(Exact name of registrant as specified in its
charter)
| |
|
|
| State of Israel |
|
92-2593104 |
| (State or other jurisdiction of |
|
(I.R.S. Employer |
| incorporation or organization) |
|
Identification No.) |
| |
|
|
| 42127 Pleasant Forest Court |
|
|
| Ashburn, VA |
|
20148-7349 |
| (Address of principal executive offices) |
|
(Zip Code) |
(703) 980-4182
(Registrant’s telephone number, including
area code)
N/A
(Former name, former address and former fiscal
year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
| |
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| American Depositary Shares, each representing one (1) Ordinary Shares, no par value per share |
|
QNRX |
|
The Nasdaq Stock Market LLC |
| Ordinary Shares, no par value per share* |
|
|
|
N/A |
| * |
Not for trading, but only in connection with the registration of the American Depositary Shares (“ADSs”) pursuant to requirements of the Securities and Exchange Commission. |
| |
|
Indicate by check mark whether the registrant (1) has filed
all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for
such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for
the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant
has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405
of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes
☒ No ☐
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,”
and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
☐ |
Accelerated filer |
☐ |
| Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
| Emerging growth company |
☐ |
|
|
| |
|
|
|
If an emerging growth company, indicate by check mark if the
registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards
provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant
is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐
No ☒
As of August 7, 2024, the registrant had 3,979,970
ordinary shares, no par value per share, outstanding, and 3,979,970 ADSs outstanding (assuming all ordinary shares are represented by
ADSs), with each ADS representing one (1) ordinary share.
Table of Contents
QUOIN PHARMACEUTICALS LTD.
INDEX
| |
Page |
| CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS |
1 |
| |
|
| PART I-FINANCIAL INFORMATION |
3 |
| |
|
| Item 1. Financial Statements. |
3 |
| |
|
| Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
18 |
| |
|
| Item 3. Quantitative and Qualitative Disclosures About Market Risk. |
29 |
| |
|
| Item 4. Controls and Procedures. |
29 |
| |
|
| PART II-OTHER INFORMATION |
30 |
| |
|
| Item 1. Legal Proceedings. |
30 |
| |
|
| Item 1A. Risk Factors. |
30 |
| |
|
| Item 2. Unregistered Sales of Equity Securities and Use of Proceeds. |
31 |
| |
|
| Item 3. Defaults Upon Senior Securities. |
31 |
| |
|
| Item 4. Mine Safety Disclosures. |
31 |
| |
|
| Item 5. Other Information. |
31 |
| |
|
| Item 6. Exhibits. |
32 |
| |
|
| SIGNATURES |
33 |
Table of Contents
GENERAL INFORMATION
Unless otherwise indicated or the context otherwise
requires, all references in this Quarterly Report on Form 10-Q (the “Quarterly Report”) to the terms “Quoin,”
“Quoin Ltd.,” the “Company,” “us,” “we”, “our” and the “Registrant”
refer to Quoin Pharmaceuticals Ltd., an Israeli company, and its consolidated subsidiaries. In this Quarterly Report, the U.S. Securities
and Exchange Commission is referred to as the “SEC”, the Securities Act of 1933, as amended, is referred to as the “Securities
Act” and the Securities Exchange Act of 1934, as amended, is referred to as the “Exchange Act.”
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
AND SUMMARY OF RISK FACTORS
Certain information included in this Quarterly
Report may be deemed to be “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act
of 1995 and other securities laws. Forward-looking statements are often characterized by the use of forward-looking terminology such as
“may,” “will,” “expect,” “anticipate,” “estimate,” “continue,”
“believe,” “should,” “intend,” “project” or other similar words, but are not the only
way these statements are identified.
These forward-looking statements may include,
but are not limited to, statements relating to our objectives, plans and strategies, statements that contain projections of results of
operations or of financial condition, expected capital needs and expenses, statements relating to the research, development, completion
and use of our products, and all statements (other than statements of historical facts) that address activities, events or developments
that we intend, expect, project, believe or anticipate will or may occur in the future.
Forward-looking statements are not guarantees
of future performance and are subject to risks and uncertainties. We have based these forward-looking statements on assumptions and assessments
made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments
and other factors they believe to be appropriate.
Important factors that could cause actual results,
developments and business decisions to differ materially from those anticipated in these forward-looking statements include, among other
things:
| |
● |
our limited operating history and the difficulties encountered by a small developing company; |
| |
● |
our history of losses and need for additional capital to fund our operations and our inability to obtain additional capital on acceptable terms, or at all; |
| |
● |
our lack of revenue generated from product sales since inception, and potential inability to be profitable; |
| |
● |
uncertainties of cash flows and inability to meet working capital needs; |
| |
● |
our ability to obtain regulatory approvals; |
| |
● |
our ability to generate favorable pre-clinical and clinical trial results; |
| |
● |
our ability to identify and develop potential product candidates; |
| |
● |
additional costs or delays associated with unsuccessful clinical trials; |
| |
● |
the inability to predict the timing of revenue from sales of a future product; |
| |
● |
the extensive regulatory requirements and future developmental and regulatory challenges we will still face even if we obtain approval for a product candidate; |
| |
● |
our ability to obtain or maintain orphan drug designation or data exclusivity for our product candidates; |
| |
● |
our ability to obtain Orphan Disease and Rare Pediatric Disease designations for our product candidates; |
1
Table of Contents
| |
● |
the potential oversight of programs or product candidates that may be more profitable or more successful; |
| |
● |
our manufacturing processes may not be validated and our methodology may not be accepted by the scientific community; |
| |
● |
the ability to conduct clinical trials, because of difficulties enrolling patients or other reasons; |
| |
● |
the requirements of being a publicly traded company may strain our resources; |
| |
● |
potential adverse effects resulting from failure to maintain effective internal controls; |
| |
● |
our ability to comply with the applicable continued listing requirements of Nasdaq; |
| |
● |
the potential negative impact on our securities price and trading volume if securities or industry analysts do not publish reports about us or if they adversely change their recommendations about our business; |
| |
● |
failure to meet the continued listing requirements of the Nasdaq Capital Market could result in a delisting of our ADSs; |
| |
● |
the potential volatility of the market price for our ADSs; |
| |
● |
the potential dilution of our shareholders’ potential ownership due to future issuances of share capital; |
| |
● |
the requirement for holders of ADSs to act through the depositary to exercise their rights; |
| |
● |
the potential limitations on ADS holders with respect to the transfer of their ADSs; |
| |
● |
the risks of securities class action litigation; and |
| |
● |
other risks and uncertainties, including those listed under described in Part I – Item 1A. Risk Factors in our Annual Report on Form 10-K for the year ended December 31, 2023 (“Form 10-K”), as well as our subsequent reports filed with the SEC |
You are urged to carefully review and consider
the various disclosures made throughout this Quarterly Report which are designed to advise interested parties of the risks and factors
that may affect our business, financial condition, results of operations and prospects.
You should not put undue reliance on any forward-looking
statements. Although the forward-looking statements in this Quarterly Report are based on our beliefs, assumptions and expectations, taking
into account all information currently available to us, we cannot guarantee future transactions, results, performance, achievements or
outcomes. No assurance can be made that the expectations reflected in our forward-looking statements will be attained, or that deviations
from them will not be material and adverse. We undertake no obligation to publicly update or revise any forward-looking statements, whether
as a result of new information, future events or otherwise, except as required by law.
2
Table of Contents
PART I – FINANCIAL INFORMATION
Item 1. Financial Statements.
QUOIN PHARMACEUTICALS LTD.
Condensed Consolidated Balance Sheets
|
|
|
|
|
|
|
|
| |
|
June 30, |
|
December 31, |
| |
|
2024 |
|
2023 |
| |
|
(Unaudited) |
|
|
| ASSETS |
|
|
|
|
|
|
| Current assets: |
|
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
2,845,126 |
|
$ |
2,401,198 |
| Investments |
|
|
9,725,463 |
|
|
8,293,663 |
| Prepaid expenses and other current assets |
|
|
650,696 |
|
|
591,034 |
| Total current assets |
|
|
13,221,285 |
|
|
11,285,895 |
| |
|
|
|
|
|
|
| Prepaid expenses - long term |
|
|
300,000 |
|
|
300,000 |
| Intangible assets, net |
|
|
533,334 |
|
|
583,334 |
| Total assets |
|
$ |
14,054,619 |
|
$ |
12,169,229 |
| |
|
|
|
|
|
|
| LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
|
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
| Accounts payable |
|
$ |
819,674 |
|
$ |
526,523 |
| Accrued expenses |
|
|
1,386,625 |
|
|
1,308,706 |
| Accrued interest and financing expense |
|
|
1,146,251 |
|
|
1,146,251 |
| Due to officers - short term |
|
|
600,000 |
|
|
600,000 |
| Total current liabilities |
|
|
3,952,550 |
|
|
3,581,480 |
| |
|
|
|
|
|
|
| Due to officers - long term |
|
|
2,623,733 |
|
|
2,923,733 |
| Total liabilities |
|
$ |
6,576,283 |
|
$ |
6,505,213 |
| |
|
|
|
|
|
|
| Commitments and contingencies |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| Shareholders’ equity: |
|
|
|
|
|
|
| Ordinary shares, no par value per share, 100,000,000 ordinary shares authorized at |
|
$ |
— |
|
$ |
— |
| June 30, 2024 and December 31, 2023, respectively - 3,979,970 (3,979,970 ADS's) ordinary shares issued and outstanding at June 30, 2024 and 987,220 (987,220 ADS's) at December 31, 2023 |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| Additional paid in capital |
|
|
57,982,969 |
|
|
51,867,336 |
| Accumulated deficit |
|
|
(50,504,633) |
|
|
(46,203,320) |
| Total shareholders' equity |
|
|
7,478,336 |
|
|
5,664,016 |
| |
|
|
|
|
|
|
| Total liabilities and shareholders' equity |
|
$ |
14,054,619 |
|
$ |
12,169,229 |
The accompanying footnotes are an integral part of these unaudited
condensed consolidated financial statements
3
Table of Contents
QUOIN PHARMACEUTICALS LTD.
Condensed Consolidated Statements of Operations (Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three months ended June 30, |
|
Six months ended June 30, |
| |
|
2024 |
|
2023 |
|
2024 |
|
2023 |
| Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
| General and administrative |
|
$ |
1,617,769 |
|
$ |
1,634,960 |
|
$ |
3,233,221 |
|
$ |
3,318,777 |
| Research and development |
|
|
519,349 |
|
|
625,104 |
|
|
1,362,181 |
|
|
1,716,837 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
2,137,118 |
|
|
2,260,064 |
|
|
4,595,402 |
|
|
5,035,614 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Other (income) and expenses |
|
|
|
|
|
|
|
|
|
|
|
|
| Unrealized loss |
|
|
2,177 |
|
|
34,472 |
|
|
8,686 |
|
|
14,045 |
| Realized and accrued interest income |
|
|
(165,262) |
|
|
(187,589) |
|
|
(302,775) |
|
|
(339,643) |
| Total other income |
|
|
(163,085) |
|
|
(153,117) |
|
|
(294,089) |
|
|
(325,598) |
| Net loss |
|
$ |
(1,974,033) |
|
$ |
(2,106,947) |
|
$ |
(4,301,313) |
|
$ |
(4,710,016) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Loss per ADS |
|
|
|
|
|
|
|
|
|
|
|
|
| Loss per ADS |
|
|
|
|
|
|
|
|
|
|
|
|
| Basic |
|
$ |
(0.39) |
|
$ |
(2.13) |
|
$ |
(1.20) |
|
$ |
(5.79) |
| Fully-diluted |
|
$ |
(0.39) |
|
$ |
(2.13) |
|
$ |
(1.20) |
|
$ |
(5.79) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted average number of ADS's outstanding |
|
|
|
|
|
|
|
|
|
|
|
|
| Basic |
|
|
5,049,720 |
|
|
987,220 |
|
|
3,576,506 |
|
|
813,187 |
| Fully-diluted |
|
|
5,049,720 |
|
|
987,220 |
|
|
3,576,506 |
|
|
813,187 |
The accompanying footnotes are an integral part of these unaudited
condensed consolidated financial statements
4
Table of Contents
QUOIN PHARMACEUTICALS LTD.
Condensed Consolidated Statements of Shareholders’ Equity
(Unaudited)
Three and six months ended June 30, 2023
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
No |
|
|
|
|
Additional |
|
|
|
|
|
|
| |
|
Ordinary |
|
|
|
Par |
|
Treasury |
|
Paid in |
|
Accumulated |
|
|
|
| |
|
Shares |
|
ADS's |
|
Value |
|
Stock |
|
Capital |
|
Deficit |
|
Total |
| Balance at January 1, 2023 |
|
403,887 |
|
403,887 |
|
— |
|
$ |
(2,932,000) |
|
$ |
47,855,521 |
|
$ |
(37,516,747) |
|
$ |
7,406,774 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
— |
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(2,603,069) |
|
|
(2,603,069) |
| Issuance of ADS and Pre - Funded Warrants, net |
|
583,333 |
|
583,333 |
|
— |
|
|
— |
|
|
5,849,266 |
|
|
— |
|
|
5,849,266 |
| Stock based compensation |
|
— |
|
— |
|
— |
|
|
— |
|
|
261,472 |
|
|
— |
|
|
261,472 |
| Balance at March 31, 2023 |
|
987,220 |
|
987,220 |
|
— |
|
$ |
(2,932,000) |
|
$ |
53,966,259 |
|
$ |
(40,119,816) |
|
$ |
10,914,443 |
| Net loss |
|
— |
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(2,106,947) |
|
|
(2,106,947) |
| Stock based compensation |
|
— |
|
— |
|
— |
|
|
— |
|
|
264,376 |
|
|
— |
|
|
264,376 |
| Balance at June 30, 2023 |
|
987,220 |
|
987,220 |
|
— |
|
$ |
(2,932,000) |
|
$ |
54,230,635 |
|
$ |
(42,226,763) |
|
$ |
9,071,872 |
Three and six months ended June 30, 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
No |
|
|
|
|
Additional |
|
|
|
|
|
|
| |
|
Ordinary |
|
|
|
Par |
|
Treasury |
|
Paid in |
|
Accumulated |
|
|
|
| |
|
Shares |
|
ADS's |
|
Value |
|
Stock |
|
Capital |
|
Deficit |
|
Total |
| Balance at January 1, 2024 |
|
987,220 |
|
987,220 |
|
— |
|
$ |
— |
|
$ |
51,867,336 |
|
$ |
(46,203,320) |
|
|
5,664,016 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
— |
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(2,327,280) |
|
|
(2,327,280) |
| Issuance of ADS's, pre-funded warrants and warrants, net |
|
2,808,750 |
|
2,808,750 |
|
— |
|
|
— |
|
|
5,482,472 |
|
|
— |
|
|
5,482,472 |
| Stock based compensation |
|
— |
|
— |
|
— |
|
|
— |
|
|
306,314 |
|
|
— |
|
|
306,314 |
| Balance at March 31, 2024 |
|
3,795,970 |
|
3,795,970 |
|
— |
|
$ |
— |
|
$ |
57,656,122 |
|
$ |
(48,530,600) |
|
$ |
9,125,522 |
| Net loss |
|
— |
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(1,974,033) |
|
|
(1,974,033) |
| Exercise of pre-funded warrants |
|
184,000 |
|
184,000 |
|
— |
|
|
— |
|
|
18 |
|
|
— |
|
|
18 |
| Reversal of accrued offering expenses |
|
— |
|
— |
|
— |
|
|
— |
|
|
13,707 |
|
|
— |
|
|
13,707 |
| Stock based compensation |
|
— |
|
— |
|
— |
|
|
— |
|
|
313,122 |
|
|
— |
|
|
313,122 |
| Balance at June 30, 2024 |
|
3,979,970 |
|
3,979,970 |
|
— |
|
$ |
— |
|
$ |
57,982,969 |
|
$ |
(50,504,633) |
|
$ |
7,478,336 |
The accompanying footnotes are an integral part of these unaudited
condensed consolidated financial statements
5
Table of Contents
QUOIN PHARMACEUTICALS LTD.
Condensed Consolidated Statements of Cash Flows (unaudited)
|
|
|
|
|
|
|
|
| |
|
Six Months Ended June 30, |
| |
|
2024 |
|
2023 |
| Cash flows used in operating activities: |
|
|
|
|
|
|
| Net loss |
|
$ |
(4,301,313) |
|
$ |
(4,710,016) |
| Stock based compensation |
|
|
619,436 |
|
|
525,848 |
| Amortization of intangibles |
|
|
50,000 |
|
|
52,022 |
| Unrealized gain and accrued interest on investments |
|
|
(121,026) |
|
|
(230,755) |
| Changes in assets and liabilities: |
|
|
|
|
|
|
| Increase in accounts payable and accrued expenses |
|
|
371,070 |
|
|
761,283 |
| Decrease (increase) in prepaid expenses & other assets |
|
|
(59,662) |
|
|
282,358 |
| Net cash used in operating activities |
|
$ |
(3,441,495) |
|
$ |
(3,319,260) |
| |
|
|
|
|
|
|
| Cash flows used in investing activities: |
|
|
|
|
|
|
| Purchase of investments |
|
$ |
(9,649,774) |
|
$ |
(13,491,505) |
| Proceeds from maturity of investments |
|
|
8,339,000 |
|
|
13,035,000 |
| Payment for license acquisition |
|
|
— |
|
|
— |
| Net cash used in investing activities |
|
$ |
(1,310,774) |
|
$ |
(456,505) |
| |
|
|
|
|
|
|
| Cash flows provided by financing activities: |
|
|
|
|
|
|
| Payment of amounts due to officers |
|
$ |
(300,000) |
|
|
(175,000) |
| Proceeds from sale of equity securities, net |
|
|
5,496,197 |
|
|
5,849,266 |
| Net cash provided by financing activities |
|
$ |
5,196,197 |
|
$ |
5,674,266 |
| |
|
|
|
|
|
|
| Net change in cash and cash equivalents: |
|
|
443,928 |
|
|
1,898,501 |
| Cash and cash equivalents - beginning of period |
|
|
2,401,198 |
|
|
2,860,628 |
| Cash and cash equivalents - end of period |
|
$ |
2,845,126 |
|
$ |
4,759,129 |
| |
|
|
|
|
|
|
| Supplemental information - Non cash items: |
|
|
|
|
|
|
| Offering expenses associated with warrant modification |
|
$ |
209,225 |
|
$ |
238,231 |
The accompanying footnotes are an integral part of these unaudited
condensed consolidated financial statements
6
Table of Contents
NOTE 1 – ORGANIZATION AND BUSINESS
Quoin Pharmaceuticals Ltd. (“Quoin Ltd.,”
or the “Company”), formerly known as Cellect Biotechnology Ltd. (“Cellect”), is the holding company for Quoin
Pharmaceuticals, Inc., a Delaware corporation (“Quoin Inc.”). Quoin Inc. was incorporated in Delaware on March 5, 2018. On
October 28, 2021, Cellect completed the business combination with Quoin Inc., with Quoin Inc. surviving as a wholly-owned subsidiary of
Cellect (the “Merger”). Immediately after completion of the Merger, Cellect changed its name to “Quoin Pharmaceuticals
Ltd.”
The Company is a clinical stage specialty pharmaceutical
company dedicated to the development and commercialization of therapeutic products that treat rare and orphan diseases for which there
are currently no approved treatments or cures. The Company’s initial focus is on the development of products, using proprietary
owned and in-licensed drug delivery technologies, that could help address rare skin diseases. The Company’s first lead product,
QRX003, is a topical lotion comprised of a broad-spectrum serine protease inhibitor, formulated with the proprietary in-licensed Invisicare®
technology, is under development as a potential treatment for Netherton Syndrome (“NS”), a rare hereditary genetic disease.
QRX003 is currently being tested in two clinical studies in the United States (“U.S.”) under an open Investigational New Drug
(“IND”) application with the Food and Drug Administration (“FDA”). Dosing of patients commenced in December 2022
for the first study and in March 2023 for the second study. The Company is also developing QRX004 as a potential treatment for Recessive
Dystrophic Epidermolysis Bullosa (“RDEB”). In addition, the Company has entered into Research Agreements with the Queensland
University of Technology (“QUT”), which include an option for global licenses to QRX007 for the potential treatment of NS
and QRX008 for the potential treatment of scleroderma , and with University College Cork (“UCC”) for the development of novel
topical formulations of rapamycin (sirolimus) as potential treatments for a number of rare and orphan diseases for which there are currently
no approved therapies or cures. To date, no products have been commercialized and no revenue has been generated.
NOTE 2 - LIQUIDITY RISKS AND OTHER UNCERTAINTIES
The unaudited condensed consolidated financial
statements have been prepared in conformity with generally accepted accounting principles in the United States (“U.S. GAAP”),
which contemplates continuation of the Company as a going concern. The Company has incurred net losses every year since inception and
has an accumulated deficit of approximately $50.5 million at June 30, 2024. The Company has a limited operating history and has historically
funded its operations through debt and equity financings. The Company incurred net losses of approximately $4.3 million, and negative
cash flows from operations of $3.4 million for the six months ended June 30, 2024. At June 30, 2024, the Company had cash and cash equivalents
balances totaling $2.8 million and investments of $9.7 million. The Company has determined that it has sufficient cash and liquidity to
effect its business plan for at least one year from the issuance of these unaudited condensed consolidated financial statements.
Additional financing will still be required to
complete the research and development of the Company’s therapeutic targets and its other operating requirements until it achieves
commercial profitability, if ever. Such financing may not be available at acceptable terms, if at all. If the Company is unable to obtain
additional funding when it becomes necessary, the development of its product candidates will be impacted and the Company would likely
be forced to delay, reduce, or terminate some or all of its development programs, all of which could have a material adverse effect on
the Company’s business, results of operations and financial condition.
Other risks and uncertainties:
The Company is subject to risks common to development
stage biopharmaceutical companies including, but not limited to, new technological innovations, dependence on key personnel, protection
of proprietary technology, compliance with government regulations, product liability, pre-clinical and clinical trial outcome risks, regulatory
approval risks, uncertainty of market acceptance and additional financing requirements.
The Company’s products require approval
or clearance from the FDA prior to commencing commercial sales in the United States. There can be no assurance that the Company’s
products will receive all of the required approvals or clearances. Approvals or clearances are also required in foreign jurisdictions
in which the Company may license or sell its products.
There can be no assurance that the Company’s
products, if approved, will be accepted in the marketplace, nor can there be any assurance that any future products can be developed or
manufactured at an acceptable cost and with appropriate performance characteristics, or that such products will be successfully marketed.
7
Table of Contents
The Company is also dependent on several third
party suppliers, in some cases a single source supplier including the contract research organization managing both of the Company’s
current clinical studies, the supplier of the active pharmaceutical ingredient (API), as well as the contract manufacturer of the drug
product for clinical development.
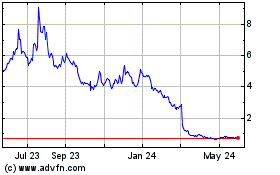
On April 29, 2024, the Company received a letter
from the Listing Qualifications staff (the “Staff”) of The Nasdaq Stock Market, LLC (“Nasdaq”) notifying the Company
that the closing bid price per ADS of the Company was below the required minimum of $1.00 for a period of 31 consecutive business days
and that the Company did not meet the minimum bid price requirements set forth in Nasdaq Rule 5550(a)(2). The notice has no immediate
effect on the listing or trading of the Company’s ADSs and the ADSs will continue to trade on The Nasdaq Capital Market under the
symbol “QNRX.”
Pursuant to Nasdaq Rule 5810(c)(3)(A), the Company
has a period of one hundred eighty (180) calendar days, or until October 28, 2024 (the “Compliance Period”), to regain compliance
with Nasdaq’s minimum bid price requirement. Compliance may be achieved without further action if the closing bid price of the Company’s
ADS is at or above $1.00 for a minimum of ten consecutive business days at any time during the Compliance Period, in which case Nasdaq
will notify the Company if it determines it is in compliance and the matter will be closed; however Nasdaq may require the closing bid
price to equal or to exceed the $1.00 minimum bid price requirement for more than 10 consecutive business days before determining that
a company complies. In the event the Company does not regain compliance by October 28, 2024, the Company may be eligible for an additional
180 calendar day grace period. To qualify, the Company will be required to meet the continued listing requirement for market value of
publicly held ADSs and all other initial listing standards for The Nasdaq Capital Market, with the exception of the minimum bid price
requirement, and will need to provide written notice of its intention to cure the deficiency during the second compliance period. The
Company intends to actively monitor the bid price of its ADS and will consider available options to regain compliance with the Nasdaq
listing requirements.
NOTE 3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation:
The accompanying unaudited condensed consolidated
financial statements have been prepared in accordance with U.S. GAAP for interim financial information. Accordingly, they do not include
all of the information and footnotes required by U.S. GAAP for complete financial statements. In the opinion of management, such statements
include all adjustments (consisting only of normal recurring items) which are considered necessary for a fair presentation of the unaudited
condensed consolidated financial statements of the Company as of June 30, 2024 and for the three and six months then ended. The results
of operations for the three and six months ended June 30, 2024 are not necessarily indicative of the operating results for the year or
any other period. These unaudited condensed consolidated financial statements should be read in conjunction with the audited financial
statements and related disclosures as of December 31, 2023 and for the year then ended which are included in the Company’s Annual
Report on Form 10- K, filed with the SEC on March 14, 2024. The Company operates in one segment.
Effective July 18, 2023, the ratio of American
Depositary Shares (“ADSs”) evidencing ordinary shares changed from 1 ADS representing five thousand (5,000) ordinary shares
to 1 ADS representing sixty thousand (60,000) ordinary shares, which resulted in a 1 for 12 reverse split of the issued and outstanding
ADSs. Effective November 8, 2023, the Company completed a 1 for 60,000 reverse split of the ordinary shares which resulted in the ratio
of ADSs evidencing ordinary shares to be changed from 1 ADS representing sixty thousand (60,000) ordinary shares to 1 ADS representing
one (1) ordinary share. Except as specifically provided, ADSs and related option and warrant information presented in these unaudited
condensed consolidated financial statements and accompanying footnotes has been retroactively adjusted to reflect the number of ordinary
shares and ADSs resulting from the aforementioned ordinary share reverse split and ADS ratio changes.
8
Table of Contents
Use of estimates:
The preparation
of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts
in the financial statements and accompanying notes. Actual results could materially differ from those estimates. Management considers
many factors in developing the estimates and assumptions that are used in the preparation of these unaudited condensed consolidated financial
statements including: expected business and operational changes, sensitivity and volatility associated with the assumptions used in developing
estimates, and whether historical trends are expected to be representative of future trends. The estimation process often may yield a
range of potentially reasonable estimates of the ultimate future outcomes and management must select an amount that falls within that
range of reasonable estimates. Estimates are used in the following areas, among others: settlement of debt or other obligations, stock-based
compensation, research and development expense recognition, intangible asset estimated useful lives and impairment assessments, allowances
of deferred tax assets, and cash flow assumptions regarding going concern considerations.
Cash and cash equivalents:
The Company considers all highly liquid investments
and short-term debt instruments with original maturities of three months or less to be cash equivalents. The Company, from time to time
during the periods presented, has had bank account balances in excess of federally insured limits where substantially all cash is held
in the United States. The Company has not experienced losses in such accounts. The Company believes that it is not subject to unusual
credit risk beyond the normal credit risk associated with commercial banking relationships.
Warrants:
The Company classifies as equity any contracts
that (i) require physical settlement or net-share settlement or (ii) provide the Company with a choice of net-cash settlement or settlement
in its own shares (physical settlement or net-share settlement) provided that such contracts are indexed to the Company’s own stock.
The Company classifies as assets or liabilities any contracts that (i) require net-cash settlement (including a requirement to net cash
settle the contract if an event occurs and if that event is outside the Company’s control) or (ii) give the counterparty a choice
of net-cash settlement or settlement in shares (physical settlement or net-share settlement).
The Company assesses classification of its warrants
and other free-standing derivatives at each reporting date to determine whether a change in classification between assets, liabilities
and equity is required. The Company evaluated its warrants outstanding to assess their proper classification using the applicable criteria
enumerated under U.S. GAAP and determined that such warrants meet the criteria for equity classification in the accompanying unaudited
condensed consolidated balance sheets as of June 30, 2024 and December 31, 2023, respectively.
Investments:
Investments
as of June 30, 2024 and December 31, 2023 consist of U.S. Treasury Bills and Notes, which are classified as trading securities, totaling
$9.7 million and $8.3 million, respectively. The Company determines the appropriate balance sheet classification of its investments at
the time of purchase and evaluates the classification at each balance sheet date. All of the Company’s U.S. Treasury Bills and Notes
held on June 30, 2024 have maturities within one year from the balance sheet date. As of June 30, 2024, the carrying value of the Company’s
U.S. Treasury Bills and Notes approximates their fair value due to their short-term maturities.
Long-lived assets:
Long-lived assets are comprised of acquired technology
and licensed rights to use technology, which are considered platform technology with alternative future uses beyond the current products
in development. Such intangible assets are being amortized on a straight-line basis over their expected useful life of 10 years.
The Company assesses the impairment for long-lived
assets whenever events or circumstances indicate the carrying value may not be recoverable. Factors we consider that could trigger an
impairment review include the following:
| |
● |
Significant changes in the manner of the Company’s use of the acquired assets or the strategy for its overall business, |
9
Table of Contents
| |
● |
Significant underperformance relative to expected historical or projected development milestones, |
| |
● |
Significant negative regulatory or economic trends, and |
| |
● |
Significant technological changes which could render the platform technology obsolete. |
The Company recognizes impairment when the sum
of the expected undiscounted future cash flows is less than the carrying amount of the asset. Impairment losses, if any, are measured
as the excess of the carrying amount of the asset over its estimated fair value. During the three and six months ended June 30, 2024 and
2023, there were no impairment indicators which required an impairment loss measurement.
Research and development:
Research and development costs are expensed as
incurred. Research and development expenses include personnel costs associated with research and development activities, including third-party
contractors to perform research, conduct clinical trials and manufacture drug supplies and materials. The Company accrues for costs incurred
by external service providers, including contract research organizations and clinical investigators, based on its estimates of service
performed and costs incurred. These estimates include the level of services performed by third parties, patient enrollment in clinical
trials when applicable, administrative costs incurred by third parties, and other indicators of the services completed. Based on the timing
of amounts invoiced by service providers, the Company may also record payments made to those providers as prepaid expenses that will be
recognized as expenses in future periods as the related services are rendered.
Stock based compensation:
The Company recognizes compensation costs resulting
from the issuance of stock-based awards to employees, non-employees and directors as an expense in the consolidated statements of operations
over the requisite service period based on a measurement of fair value for each stock-based award. The fair value of each option grant
is estimated as of the date of grant using the Black-Scholes option-pricing model, net of actual forfeitures. The fair value is amortized
as compensation cost on a straight-line basis over the requisite service period of the awards, which is generally the vesting period.
Since the Company has a limited history of trading
as a public company, the Company’s expected stock volatility is based on a weighting of its historical volatility along with a group
of a publicly traded set of peer companies. The Company utilizes the simplified method to estimate the expected term. The risk-free interest
rate was determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately
equal to the expected term of the award. The expected dividend yield was assumed to be zero as the Company has not paid and dividends
since its inception and does not anticipate paying dividends in the foreseeable future.
Fair value of financial instruments:
The Company considers its cash and cash equivalents,
investments, accounts payable, accrued expenses to meet the definition of financial instruments. The carrying amounts of these financial
instruments approximated their fair values due to the short maturities.
The Company measures fair value as required by
ASC Topic 820, Fair Value Measurements and Disclosures (“ASC Topic 820”). ASC Topic 820 defines fair value, establishes
a framework and gives guidance regarding the methods used for measuring fair value, and expands disclosures about fair value measurements.
ASC Topic 820 clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer
a liability in an orderly transaction between market participants.
10
Table of Contents
Earnings (loss) per share:
The Company reports loss per share in accordance
with ASC 260-10, Earnings Per Share, which provides for calculation of “basic” and “diluted” earnings per
share. Basic earnings per share includes no dilution and is computed by dividing net income or loss available to common shareholders by
the weighted average common shares outstanding for the period. Diluted earnings per share reflect the potential dilution of securities
that could share in the earnings of an entity. The calculation of diluted net earnings (loss) per share gives effect to ordinary shares
equivalents; however, other than unexercised prefunded warrants as described below, potential common shares are excluded if their effect
is anti-dilutive.
For the three and six months ended June 30, 2024,
the number of shares excluded from the diluted net earnings (loss) per share included outstanding warrants to purchase 8,988,346 ADS and
outstanding stock options to purchase 278,011 ADS, and for the three and six months ended June 30, 2023, the number of shares excluded
from the diluted net earnings (loss) per share included outstanding warrants to purchase 864,068 ADS and outstanding stock options to
purchase 25,595 ADS, respectively, as their inclusion in the denominator would be anti-dilutive. For the three and six months ended June
30, 2024 basic and diluted net earnings (loss) per share included 1,069,750 ADS issuable with respect to unexercised prefunded warrants
(See Note 13).
NOTE 4 – ACCRUED INTEREST AND FINANCING EXPENSE
On October 2, 2020, Quoin Inc. issued promissory
notes (the “2020 Notes”) to certain investors (“2020 Noteholders”). The 2020 Notes were mandatorily convertible
into 432 ADSs, subject to adjustment and were converted in 2021. The ADSs issued to the 2020 Noteholders did not include accrued interest.
Two of the five 2020 Noteholders received their amount due during the year ended December 31, 2022 and the Company’s estimate of
the liability to the remaining three 2020 Noteholders was estimated to be $1,146,000 as of June 30, 2024 and December 31, 2023.
There was no interest expense recognized in both
the three and six month periods ended June 30, 2024 and 2023.
NOTE 5 - FAIR VALUE OF FINANCIAL INSTRUMENTS
The Company applies fair value accounting for
all assets and liabilities that are recognized or disclosed at fair value in the financial statements on a recurring basis. Fair value
is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between
market participants at the measurement date. When determining the fair value measurements for assets and liabilities the Company considers
the principal or most advantageous market in which it would transact and the market-based risk measurements or assumptions that market
participants would use in pricing the asset or liability, such as risks inherent in valuation techniques, transfer restrictions and credit
risk. For certain instruments, including cash and cash equivalents, accounts payable, and accrued expenses, it was estimated that the
carrying amount approximated fair value because of the short maturities of these instruments.
Fair value is estimated using various valuation
models, which utilize certain inputs and assumptions that market participants would use in pricing the asset or liability. The inputs
and assumptions used in valuation models are classified in the fair value hierarchy as follows:
Level 1: Quoted prices (unadjusted) in
active markets that are accessible at the measurement date for assets or liabilities. The fair value hierarchy gives the highest priority
to Level 1 inputs.
Level 2: Quoted market prices for similar
instruments in an active market; quoted prices for identical or similar assets and liabilities in markets that are not active; and model-derived
valuations inputs of which are observable and can be corroborated by market data.
Level 3: Unobservable inputs and assumptions
that are supported by little or no market activity and that are significant to the fair value of the asset and liability. The fair value
hierarchy gives the lowest priority to Level 3 inputs.
In determining the appropriate hierarchy levels,
the Company analyzes the assets and liabilities that are subject to fair value disclosure. Financial assets and liabilities are classified
in their entirety based on the lowest level of input that is significant to their fair value measurement.
11
Table of Contents
The following table presents the Company’s
assets and liabilities that are measured at fair value on a recurring basis by fair value hierarchy at June 30, 2024 and December 31,
2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| June 30, 2024 |
|
|
Level 1 |
|
|
Level 2 |
|
Level 3 |
|
Total |
| US Treasury Bills and Notes |
|
$ |
9,725,463 |
|
$ |
— |
|
$ |
— |
|
$ |
9,725,463 |
| Total US Treasury Bills amd Notes Asset |
|
$ |
9,725,463 |
|
$ |
— |
|
$ |
— |
|
$ |
9,725,463 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| December 31, 2023 |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
| US Treasury Bills and Notes |
|
$ |
8,293,663 |
|
$ |
— |
|
$ |
— |
|
$ |
8,293,663 |
| Total US Treasury Bills amd Notes Asset |
|
$ |
8,293,663 |
|
$ |
— |
|
$ |
— |
|
$ |
8,293,663 |
NOTE 6 – STOCK BASED COMPENSATION
In March 2022, the Board of Directors of
the Company approved the Amended and Restated Equity Incentive Plan (the “Amended Plan”), which was approved by the shareholders
at the Company’s Annual General Meeting of Shareholders held on April 12, 2022, which increased the number of ordinary shares reserved
for issuance under such equity incentive plan to 15% of the Company’s outstanding ordinary shares on a fully-diluted basis, or 2,147,412
ordinary shares represented by 2,147,412 ADSs as of June 30, 2024. Under the Amended Plan, the Company may grant options to its directors,
officers, employees, consultants, advisers and service providers. As of June 30, 2024, 1,869,401 shares remained available for issuance.
The following table summarizes stock-based activities
under the Amended Plan:
|
|
|
|
|
|
|
|
|
| |
|
|
|
Weighted |
|
Weighted |
| |
|
|
|
Average |
|
Average |
| |
|
ADS Underlying |
|
Exercise |
|
Contractual |
| |
|
Options |
|
Price |
|
Terms |
| Outstanding at December 31, 2023 |
|
278,011 |
|
$ |
25.34 |
|
9.68 |
| Granted |
|
— |
|
|
— |
|
|
| Forfeited/Cancelled |
|
— |
|
|
— |
|
|
| Outstanding at June 30, 2024 |
|
278,011 |
|
$ |
25.34 |
|
9.18 |
| |
|
|
|
|
|
|
|
| Exercisable options at June 30, 2024 |
|
14,228 |
|
$ |
210.00 |
|
7.83 |
The intrinsic value of outstanding options at
June 30, 2024 was $0.
Stock based compensation expense was approximately
$313,000 ($68,000 included in research and development expense and $245,000 included in general and administrative expenses) in the three
months ended June 30, 2024 and approximately $619,000 ($118,000 included in research and development expense and $501,000 included in
general and administrative expenses) in the six months ended June 30, 2024
Stock based compensation expense was approximately
264,000 ($34,000 included in research and development expense and $230,000 included in general and administrative expenses) in the three
months ended June 30, 2023 and approximately $526,000 ($69,000 included in research and development expense and $457,000 included in general
and administrative expenses) in the six months ended June 30, 2023
At June 30, 2024, the total unrecognized compensation
expense related to non-vested options was approximately $2.4 million and is expected to be recognized over the remaining weighted average
service period of approximately 3.25 years.
12
Table of Contents
NOTE 7 – PREPAID EXPENSES
Prepaid expenses are as follows:
|
|
|
|
|
|
|
|
| |
|
June 30, |
|
December 31, |
| |
|
2024 |
|
2023 |
| Prepaid R&D costs |
|
$ |
649,241 |
|
$ |
447,979 |
| Prepaid insurance |
|
|
159,746 |
|
|
401,972 |
| Prepaid expense |
|
|
33,605 |
|
|
8,500 |
| Deferred offering costs (note 13) |
|
|
108,104 |
|
|
32,583 |
| Total |
|
$ |
950,696 |
|
$ |
891,034 |
| Less: Short-term portion |
|
|
(650,696) |
|
|
(591,034) |
| Long-term portion |
|
$ |
300,000 |
|
$ |
300,000 |
NOTE 8 - ACCRUED EXPENSES
Accrued expenses are as follows:
|
|
|
|
|
|
|
|
| |
|
June 30, |
|
December 31, |
| |
|
2024 |
|
2023 |
| Research contract expenses (note 12) |
|
$ |
550,391 |
|
$ |
358,287 |
| Payroll (note 11) |
|
|
611,843 |
|
|
804,156 |
| Payroll taxes (note 11) |
|
|
90,781 |
|
|
93,989 |
| Professional fees |
|
|
97,622 |
|
|
50,534 |
| Other expenses |
|
|
35,988 |
|
|
1,740 |
| Total |
|
$ |
1,386,625 |
|
$ |
1,308,706 |
NOTE 9 – IN-LICENSED TECHNOLOGY
Skinvisible:
In October 2019, Quoin Inc. entered into the Exclusive
Licensing Agreement (as amended from time to time, the “License Agreement”) with Skinvisible Pharmaceuticals, Inc. (“Skinvisible”),
under which Skinvisible granted the Company an exclusive royalty-bearing license relating to the production and manufacture of prescription
drug products related to certain patents held by Skinvisible, including those related to QRX003 and QRX004. The Company made Skinvisible
a one-time non-refundable, non-creditable license fee of $1 million (the “License Fee”). In addition, the Company agreed to
pay Skinvisible a single digit royalty percentage of the Company’s net sales revenues for any licensed product covered by the patent
rights licensed under the License Agreement. The Company also agreed to pay Skinvisible 25% of any revenues the Company receives as royalties
in the event that the Company sublicenses any licensed products to a third party. The License Agreement also requires that the Company
make a $5 million payment to Skinvisible upon receiving approval in the U.S. or European Union, whichever occurs first, for the first
drug product developed using intellectual property licensed thereunder. There were no milestone or royalty obligations due at June 30,
2024 and December 31, 2023.
NOTE 10 - INTANGIBLE ASSETS
Intangible assets are as follows:
|
|
|
|
|
|
|
|
| |
|
June 30, |
|
December 31, |
| |
|
2024 |
|
2023 |
| Technology license – Skinvisible |
|
$ |
1,000,000 |
|
$ |
1,000,000 |
| Total cost |
|
|
1,000,000 |
|
|
1,000,000 |
| Accumulated amortization |
|
|
(466,666) |
|
|
(416,666) |
| Net book value |
|
$ |
533,334 |
|
$ |
583,334 |
13
Table of Contents
The Company recorded amortization expense of approximately
$25,000 and $26,000 in the three months ended June 30, 2024 and 2023, respectively. The Company recorded amortization expense of approximately
$50,000 and $52,000 in the six months ended June 30, 2024 and 2023, respectively. The annual amortization expense expected to be recorded
for existing intangible assets for the years 2024 through 2027, and thereafter, is approximately $50,000, $100,000, $100,000, $100,000
and $183,000, respectively.
NOTE 11 - RELATED PARTY TRANSACTIONS
Employment Agreements and Due to Officers/Founders:
Due to the limited funding of Quoin Inc. prior
to the consummation of the Merger, the compensation, including salary, office and car allowances and other benefits, due to Dr. Myers
and Ms. Carter under their respective employment agreements, as well as reimbursement of expenses and other amounts paid by Dr. Myers
and Ms. Carter to third parties on behalf of Quoin Inc., were not paid by Quoin Inc. to Dr. Myers and Ms. Carter, and were accrued as
indebtedness to Dr. Myers and Ms. Carter. Following the closing of the Merger, Quoin Inc. began making payments of $25,000 per month to
each of Dr. Myers and Ms. Carter to repay the above-described non-interest-bearing indebtedness. The Company repaid $75,000 and $75,000
of such indebtedness to Dr. Myers and $75,000 and $0 to Ms. Carter in the three months ended June 30, 2024 and 2023, respectively. The
Company repaid $150,000 and $150,000 of such indebtedness to Dr. Myers and $150,000 and $25,000 to Ms. Carter in the six months ended
June 30, 2024 and 2023, respectively.
As of June 30, 2024, approximately $1.8 million
and $1.4 million of such indebtedness was outstanding to Dr. Myers and Ms. Carter, respectively.
Amounts due to officers at June 30, 2024 and December
31, 2023 consisted of the following:
|
|
|
|
|
|
|
|
| |
|
March 31, |
|
December 31, |
| |
|
2024 |
|
2023 |
| Salaries and other compensation |
|
$ |
3,223,733 |
|
$ |
3,523,733 |
| Total |
|
$ |
3,223,733 |
|
$ |
3,523,733 |
| Less: Short-term portion |
|
|
(600,000) |
|
|
(600,000) |
| Long-term portion |
|
$ |
2,623,733 |
|
$ |
2,923,733 |
Interest Payable:
See Note 4 for interest payable on the 2020 Notes.
NOTE 12 – RESEARCH, CONSULTING AGREEMENTS AND
COMMITMENTS
Research agreements
In November 2020, Quoin Inc. entered into a Master
Service Agreement with Therapeutics Inc. for the management of the preclinical and clinical development of QRX003 for Netherton Syndrome.
The initial term of the agreement was three years with automatic one year extensions, and the agreement required the execution of individual
work orders. Quoin Inc. may terminate any work order for any reason with 90 days written notice subject to costs incurred through
termination and a defined termination fee, unless there is a material breach by Therapeutics Inc. A work order was entered into in June
2022 for the first QRX003 clinical study at an expected estimated cost of approximately $4.4 million. An additional work order was entered
into in December 2022 for a second QRX003 clinical study at an expected estimated cost of approximately $830,000. For the three and six
months ended June 30, 2024 and 2023, the Company incurred a research and development expense under these agreements of approximately $162,000
and $650,000 and 360,000 and $959,000 respectively.
14
Table of Contents
In November 2021, the Company entered into a research
agreement with Queensland University of Technology (QUT) for a pre-clinical research program for the development of a product to treat
Netherton Syndrome of approximately $250,000. In May 2022, the Company entered into a second research agreement with QUT for the development
of a product to treat Scleroderma of approximately $610,000. Each agreement remains in place until the completion of the research program,
which in each case was initially anticipated to be 18 months from execution. For the three and six months ended June 30, 2024 the
Company did not incur any costs related to these agreements. For the three and six months ended June 30, 2023, the Company incurred research
and development costs related to these agreements of approximately $138,000 and $276,000 respectively.
On June 10, 2024, the Company signed a research
agreement with The School of Pharmacy at UCC. The scope of the agreement encompasses the development of novel topical formulations of
rapamycin (sirolimus) as potential treatments for a number of rare and orphan diseases for which there are currently no approved therapies
or cures. Under the terms of the agreement, based on the achievement of certain milestones, the Company will fund up to approximately
€567,000 ($608,000) plus VAT over an anticipated 2-1/2 year period to support UCC research program to investigate the development
of a number of topical rapamycin formulations for future development as potential treatments for several rare and orphan diseases. Following
completion of the research program, the Company will have the option to advance the clinical development of rapamycin formulations developed
by UCC. The Company incurred no costs related to this agreement in the three and six months ended June 30, 2024.
Performance milestones and Royalties
See Note 9 for asset and in-licensed technology
commitments.
NOTE 13 – SHAREHOLDERS’ EQUITY
Alumni Equity Line and Purchase Agreement
On January 25, 2024, the Company entered into
a purchase agreement (the “Alumni Purchase Agreement”) with Alumni Capital LP (“Alumni”). Pursuant to the Alumni
Purchase Agreement, the Company has the right to sell to Alumni up to $8,000,000 (the “Commitment Amount”) of newly issued
ordinary shares that are represented by ADS, subject to certain conditions and limitations, from time to time during the term of the Alumni
Purchase Agreement.
The Company does not have the right to commence
any sales of ordinary shares represented by ADSs to Alumni under the Alumni Purchase Agreement until the date, which the Company refers
to as the Commencement Date, that all of the conditions set forth in the Alumni Purchase Agreement have been satisfied, including that
the registration statement the Company agreed to file with the SEC pursuant to the Alumni Purchase Agreement is declared effective by
the SEC, and the Company’s shareholders have approved of the issuance of ADSs under the Alumni Purchase Agreement, which approval
was obtained on April 5, 2024.
From and after the Commencement Date, the Company
may, from time to time and at the Company’s sole discretion for a period of three months, which the Company at its sole discretion
may increase by an additional three months (such period, including any extension, the “Commitment Period”), on any business
day that the Company may select, direct Alumni to purchase ordinary shares represented by ADSs. The purchase price for the ordinary shares
represented by ADSs the Company may sell to Alumni will be based upon formulas set forth in the Alumni Purchase Agreement based on the
then current market price of the ADSs as computed under the Alumni Purchase Agreement and will depend on the type of purchase notice the
Company submits to Alumni from time to time. There is no upper limit on the price per share that Alumni could be obligated to pay for
the ADSs under the Alumni Purchase Agreement; provided, however at no time can the purchase price be below a floor price of $1.00 per
share (subject to adjustment). The Company agreed to issue purchase notices for an aggregate of at least $4,000,000 of the Commitment
Amount prior to the end of the Commitment Period.
As consideration for Alumni’s irrevocable
commitment to purchase ADSs under the Alumni Purchase Agreement, the Company agreed to issue to Alumni, at the times set forth in the
Alumni Purchase Agreement beginning with the trading day after the Commencement Date, a number of ADSs with a value at the time of issuance
not to exceed $240,000 in the aggregate (the “Commitment Securities”). The Company may pay cash in lieu of issuing all or
any portion of the Commitment Securities.
15
Table of Contents
Public Offering
On March 7, 2024, (the “2024 Closing Date”)
the Company completed an offering (the “2024 Offering”) of the following securities (i) 811,250 ordinary shares represented
by ADSs, (ii) 4,062,500 Series D warrants (the “Series D Warrants”) to purchase 4,062,500 ordinary shares represented by ADSs,
(iii) 4,062,500 Series E warrants (the “Series E Warrants” and together with the Series D Warrants, the “2024 Warrants”)
to purchase 4,062,500 ordinary shares represented by ADSs, and (iv) 3,251,250 pre-funded warrants (the “2024 Pre-Funded Warrants”)
to purchase 3,251,250 ordinary shares represented by ADSs for aggregate gross proceeds of approximately $6.5 million, resulting in net
proceeds of approximately $5.5 million, after deducting the placement agent’s fees and offering expenses paid by the Company. Each
ADS (or 2024 Pre-Funded Warrant to purchase one ADS in lieu thereof) was sold together with a Series D Warrant to purchase one ADS and
a Series E Warrant to purchase one ADS. The ADSs and accompanying 2024 Warrants were sold at a combined public offering price of $1.60
and the 2024 Pre-Funded Warrants and accompanying 2024 Warrants were sold at a combined public offering price of $1.5999, which is equal
to the combined purchase price per ADS and accompanying 2024 Warrants, minus the exercise price of each 2024 Pre-Funded Warrant of $0.0001.
The Series D and Series E warrants have an exercise price of $1.60 per share, were exercisable immediately following the 2024 Closing
Date and expire in two years and five years, respectively, from the closing of the 2024 Offering. As of June 30, 2024, 2,181,500 2024
Pre-Funded Warrants have been exercised and are included in issued and outstanding ADSs.
In connection with the 2024 Offering, the Company
entered into a Securities Purchase Agreement (the “2024 Purchase Agreement”) dated March 4, 2024, with certain institutional
investors signatory thereto, pursuant to which the Company agreed to issue and sell to such investors, certain of the ADSs, 2024 Pre-Funded
Warrants and 2024 Warrants sold in the 2024 Offering. Pursuant to the terms of the 2024 Purchase Agreement, the Company agreed, subject
to certain exceptions, (i) to not enter into variable rate financings for a period of 180 days following the closing of the 2024 Offering,
and (ii) to not enter into any equity financings for 90 days from closing of the 2024 Offering.
On March 7, 2024, the Company also entered into
privately negotiated agreements with the holders of certain existing outstanding warrants to purchase up to 638,834 ADSs, 207,499 of which
were warrants issued in the Company’s 2022 public offering and 431,335 of which were warrants issued in the Company’s 2023
public offering (collectively, the “Prior Warrants”) to, among other things, reduce the exercise price of such Prior Warrants
to $1.60 and to extend the current expiration date of the Prior Warrants until March 7, 2029. The incremental fair value of the modified
warrants was approximately $209,000, which was accounted for as an offering expense in connection with the 2024 Offering.
Warrants
The following table summarizes warrant activities
during the six months ended June 30, 2024:
|
|
|
|
|
|
|
|
| |
|
|
|
Weighted |
|
| |
|
ADSs |
|
Average |
|
| |
|
Underlying |
|
Exercise Price |
|
| |
|
Warrants |
|
Per ADS |
|
| Outstanding and exercisable at December 31, 2023 |
|
864,081 |
|
$ |
16.13 |
* |
| Granted Common Warrants |
|
8,125,000 |
|
|
1.60 |
|
| Granted Pre-Funded Warrants |
|
3,251,250 |
|
|
— |
|
| Exercised Pre-Funded Warrants |
|
(2,181,500) |
|
|
— |
|
| Terminated |
|
(735) |
|
|
1,650.00 |
|
| Outstanding and exercisable at June 30, 2024 |
|
10,058,096 |
|
$ |
1.87 |
|
As of June 30, 2024, outstanding common warrants
expire in 2026, 2027 and 2029, and the outstanding pre-funded warrants have an approximate intrinsic value of $620,000.
* Note that the
exercise price of certain existing outstanding warrants to purchase up to 638,834 ADSs was reduced from $12.00 and $13.20 to $1.60 on
March 7, 2024, see above.
16
Table of Contents
NOTE 14 – CONTINGENCIES
From time to time, the Company may become involved
in various legal matters arising in the ordinary course of business. Management is unaware of any matters requiring accrual for related
losses in the unaudited condensed consolidated financial statements.
NOTE 15 – LICENSE AGREEMENTS
As of June 30, 2024, the Company had nine commercial
license and supply agreements outstanding, whereby the Company will receive a royalty or other proceeds from the specified product revenues
from the licensor, if and when the underlying products are approved and commercialized or sold via compassionate use or early access programs.
No royalty revenues have been received through June 30, 2024 from any of these agreements.
17
Table of Contents
Item 2. Management’s Discussion and Analysis of Financial
Condition and Results of Operations
You should read the following discussion and
analysis of our financial condition and results of operations together with our unaudited condensed consolidated financial statements
and related notes thereto included in Part I-Item 1 of this Form 10-Q. This discussion and other parts of this report contain forward-looking
statements that involve risks and uncertainties, such as statements of our plans, objectives, expectations and intentions that are based
on the beliefs of our management, as well as assumptions made by, and information currently available to, our management. Our actual results
could differ materially from those discussed in these forward-looking statements. See “Cautionary Note Regarding Forward-Looking
Statements” in this Form 10-Q.
Overview
We are a clinical stage specialty pharmaceutical
company dedicated to the development and commercialization of therapeutic products that treat rare and orphan diseases for which there
are currently no approved treatments or cures. Our initial focus is on the development of products, using our proprietary owned and in-licensed
drug delivery technologies, that could help address rare skin diseases. Our first lead product is QRX003, a once daily, topical lotion
comprised of a broad-spectrum serine protease inhibitor, formulated with the proprietary in-licensed Invisicare® technology, is under
development as a potential treatment for Netherton Syndrome (“NS”), a rare hereditary genetic disease. QRX003 is currently
being tested in two clinical studies in the United States (“U.S.”) under an open Investigational New Drug (“IND”)
application with the Food and Drug Administration (“FDA”). We are also developing QRX004 as a potential treatment for Recessive
Dystrophic Epidermolysis Bullosa (“RDEB”). In addition, we entered into Research Agreements with the Queensland University
of Technology (“QUT”), under which we have obtained an option for global licenses to QRX007 for the potential treatment of
NS and QRX008 for the potential treatment of scleroderma, and with University College Cork (“UCC”) for the development of
novel topical formulations of Rapamycin (sirolimus) as potential treatments for a number of rare and orphan diseases for which there are
currently no approved therapies or cures.
Our objective is to develop and commercialize
proprietary therapeutic drug products. To this effect, we intend to develop and seek marketing approvals from the FDA and other worldwide
regulatory bodies for rare and orphan diseases. To achieve these objectives, we plan to:
| |
● |
complete the late-stage clinical testing of QRX003 and, if successful, file for marketing approval in the United States and other territories; |
| |
● |
prepare to commercialize QRX003 by establishing our own sales infrastructure in the U.S. and Europe and entering into distribution partnerships in other territories such as those currently established for Canada, Australia/New Zealand, the Middle East, China, Hong Kong, Taiwan, Latin America, Central and Eastern Europe, Turkey and Singapore; and |
| |
● |
pursue business development activities by seeking partnering, licensing, merger and acquisition opportunities or other transactions to further expand our pipeline and drug-development capabilities. |
We do not expect to generate revenue from product
sales unless and until we successfully complete development and obtain marketing approval for one or more of our product candidates, which
we expect will take a number of years and is subject to significant uncertainty. Accordingly, we will need to raise additional capital
prior to the commercialization of QRX003 or any other product candidate. Until such time, if ever, as we can generate substantial revenue
from product sales, we expect to finance our operating activities through a combination of equity offerings, debt financings, government
or other third-party funding, commercialization, marketing and distribution arrangements and other collaborations, strategic alliances
and licensing arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable
terms or at all. Our failure to raise capital or enter into such other arrangements as and when needed would have a negative impact on
our financial condition and our ability to continue our operations. See “Liquidity and Capital Resources”.
18
Table of Contents
ADS Ratio Change and Ordinary Share Reverse Split
Effective July 18, 2023, the ratio of ADSs evidencing
ordinary shares changed from 1 ADS representing five thousand (5,000) ordinary shares to 1 ADS representing sixty thousand (60,000) ordinary
shares, which resulted in a 1 for 12 reverse split of the issued and outstanding ADSs. Effective November 8, 2023, the Company completed
a 1 for 60,000 reverse split of the ordinary shares which resulted in the ratio of ADSs evidencing ordinary shares to be changed from
1 ADS representing sixty thousand (60,000) ordinary shares to 1 ADS representing one (1) ordinary share. Except as specifically provided,
ordinary share, ADS and related option and warrant information presented herein, including our unaudited condensed consolidated financial
statements and accompanying footnotes, has been retroactively adjusted to reflect the number of ordinary shares and ADSs resulting from
the aforementioned ordinary share reverse split and ADS ratio changes.
Recent Developments
Clinical Development
Quoin’s lead asset, QRX003, is currently
in late-stage clinical development in the U.S. under an open IND application with the FDA. Five clinical sites in the U.S. have been opened
for our initial study, patients are actively being screened and recruited into the study and dosing commenced in December 2022. This study
originally was designed as a randomized, double blinded assessment of two different doses of QRX003 versus a placebo vehicle in 18 adult
NS patients. The test materials are applied once daily, over a twelve-week period, to pre-selected areas of the patient’s body.
Based on discussions with the FDA, a number of different clinical endpoints are being assessed in the study, including but not limited
to, an Investigators Global Assessment (IGA), Patient’s Global Assessment (PaGA) and Pruritis.
In November 2022, we submitted a protocol for
our second clinical study in NS patients to the FDA under our currently open IND (the “Open Label Study”). This study was
cleared by the FDA to initiate in December 2022. This study originally was designed to be conducted in ten adult NS patients who are currently
receiving, and will continue to do so throughout the study, off-label systemic therapy, primarily systemic biologic therapy. This is an
open-label study with no placebo control. Both of our NS clinical studies are running concurrently and utilize the same clinical trial
sites and investigators.
On October 24, 2023, we released positive initial
clinical results obtained from the first six evaluable subjects in our open-label study. As a result of this positive initial data and
the absence of any safety concerns from both studies, on November 8, 2023 we submitted a number of protocol amendments to the FDA, under
our open IND, with a view to optimizing both studies and potentially leading to even better clinical outcomes and a more rapid regulatory
approval. These protocol amendments included eliminating the lower dose from the double-blinded study, modifying the dosing frequency
from once-daily to twice-daily and increasing the number of subjects from 18 to 30. For the open-label study, the number of subjects was
increased from 10 to 20 and dosing was modified from once-daily to twice-daily. On December 13, 2023, we announced that we were cleared
by the FDA to implement these protocol amendments. In February 2024 we submitted a further protocol amendment to the FDA requesting permission
to lower the age of eligibility for participation in both studies to 14 years and older from 18 years and older. On March 4, 2024 we announced
that we were cleared to implement this protocol amendment. All protocol amendments have now been implemented and going forward participants
in both studies will be dosed twice-daily with those enrolled in the blinded study receiving either a 4% dose of QRX003 or a placebo,
while subjects in the open-label will receive a 4% dose of QRX003 only.
On June 27, 2024 we announced that we will expand
our ongoing Netherton Syndrome clinical studies to include international sites. The first international site will be opened at a research
hospital in Saudi Arabia. This hospital is currently treating a number of Netherton patients who will now become eligible for recruitment
into our studies. An experienced Clinical Research Organization has been engaged to manage the study locally. The Saudi site will operate
under the auspices of our open Investigational New Drug (IND) application with the US Food and Drug Administration. Plans to open additional
international clinical sites are at an advanced stage.
19
Table of Contents
Research Agreement with University College
Cork
On June 10, 2024 we signed a research agreement
with The School of Pharmacy at UCC. The scope of the agreement encompasses the development of novel topical formulations of rapamycin
(sirolimus) as potential treatments for a number of rare and orphan diseases for which there are currently no approved therapies or cures.
UCC will apply its proprietary dissolvable microneedle delivery technology along with other formulation approaches to optimize the local
delivery of rapamycin and potentially enhance its therapeutic effectiveness as a potential treatment for several pre-identified clinical
targets.
Under the terms of the agreement, we will fund
a research program at UCC over an anticipated 2-1/2 year period to investigate the development of a number of topical rapamycin formulations
for future development as potential treatments for several rare and orphan diseases, where it is believed that the drug’s mechanism
of action may provide for clinical efficacy in these settings. Following completion of the research program, we will have the option to
advance the clinical development of rapamycin formulations developed by UCC. The terms of the agreement do not require us to pay any upfront
license or milestone fees or any royalties based on future product sales.
Public Offering
On March 7, 2024, (the “2024 Closing Date”)
we completed an offering (the “2024 Offering”) of the following securities (i) 811,250 ordinary shares represented by ADSs,
(ii) 4,062,500 Series D warrants (the “Series D Warrants”) to purchase 4,062,500 ordinary shares represented by ADSs, (iii)
4,062,500 Series E warrants (the “Series E Warrants” and together with the Series D Warrants, the “2024 Warrants”)
to purchase 4,062,500 ordinary shares represented by ADSs, and (iv) 3,251,250 pre-funded warrants (the “2024 Pre-Funded Warrants”)
to purchase 3,251,250 ordinary shares represented by ADSs for aggregate gross proceeds of approximately $6.5 million, resulting in net
proceeds of approximately $5.5 million, after deducting the placement agent’s fees and offering expenses paid by us. Each ADS (or
2024 Pre-Funded Warrant to purchase one ADS in lieu thereof) was sold together with a Series D Warrant to purchase one ADS and a Series
E Warrant to purchase one ADS. The ADSs and accompanying 2024 Warrants were sold at a combined public offering price of $1.60 and the
2024 Pre-Funded Warrants and accompanying 2024 Warrants were sold at a combined public offering price of $1.5999, which is equal to the
combined purchase price per ADS and accompanying 2024 Warrants, minus the exercise price of each 2024 Pre-Funded Warrant of $0.0001. The
Series D Warrants and the Series E Warrants have an exercise price of $1.60 per share, were exercisable immediately following the closing
of the 2024 Offering and expire in two years and five years, respectively, from the closing of the 2024 Offering. As of June 30, 2024
2,181,500 2024 Pre-Funded Warrants have been exercised and are included in issued and outstanding ADSs.
In connection with the 2024 Offering, we entered
into a Securities Purchase Agreement (the “2024 Purchase Agreement”) dated March 4, 2024, with certain institutional investors
signatory thereto, pursuant to which we agreed to issue and sell to such investors, certain of the ADSs, 2024 Pre-Funded Warrants and
2024 Warrants sold in the 2024 Offering. Pursuant to the terms of the 2024 Purchase Agreement, we agreed, subject to certain exceptions,
(i) to not enter into variable rate financings for a period of 180 days following the closing of the 2024 Offering, and (ii) to not enter
into any equity financings for 90 days from the closing of the 2024 Offering.
On March 7, 2024, we also entered into privately
negotiated agreements with the holders of certain existing outstanding warrants to purchase up to 638,834 ADSs (the “Prior Warrants”)
to, among other things, reduce the exercise price of such Prior Warrants to $1.60 and to extend the current expiration date of the Prior
Warrants until March 7, 2029. The incremental fair value of the modified warrants was approximately $209,000, which was accounted for
as an offering expense in connection with the 2024 Offering.
20
Table of Contents
Alumni Equity Line and Purchase Agreement
On January 25, 2024, we entered into a Purchase
Agreement (the “Alumni Purchase Agreement”) with Alumni Capital LP (“Alumni”). Pursuant to the Alumni Purchase
Agreement, we have the right to sell to Alumni up to $8,000,000 (the “Commitment Amount”) of newly issued ordinary shares
that are represented by ADS (the “Purchase Notice Securities”), subject to certain conditions and limitations, from time to
time during the term of the Alumni Purchase Agreement.
We do not have the right to commence any sales
of ordinary shares represented by ADSs to Alumni under the Alumni Purchase Agreement until the date, which we refer to as the Commencement
Date, that all of the conditions set forth in the Alumni Purchase Agreement have been satisfied, including that the registration statement
we agreed to file with the SEC pursuant to the Alumni Purchase Agreement is declared effective by the SEC, and our shareholders have approved
of the issuance of ADSs under the Alumni Purchase Agreement, which approval was obtained on April 5, 2024.
From and after the Commencement Date, we may,
from time to time and at our sole discretion for a period of three months, which we at our sole discretion may increase by an additional
three months (such period, including any extension, the “Commitment Period”), on any business day that we select, direct Alumni
to purchase ordinary shares represented by ADSs. The purchase price for the ordinary shares represented by ADSs we may sell to Alumni
will be based upon formulas set forth in the Alumni Purchase Agreement based on the then current market price of the ADSs as computed
under the Alumni Purchase Agreement and will depend on the type of purchase notice we submit to Alumni from time to time. There is no
upper limit on the price per share that Alumni could be obligated to pay for the ADSs under the Alumni Purchase Agreement; provided, however
at no time can the purchase price be below a floor price of $1.00 per share (subject to adjustment). We agreed to issue purchase notices
for an aggregate of at least $4,000,000 of the Commitment Amount prior to the end of the Commitment Period.
As consideration for Alumni’s irrevocable
commitment to purchase ADSs under the Alumni Purchase Agreement, we agreed to issue to Alumni, at the times set forth in the Alumni Purchase
Agreement beginning with the trading day after the Commencement Date, a number of ADSs with a value at the time of issuance not to exceed
$240,000 in the aggregate (the “Commitment Securities”). The ADSs to be issued will be valued at the average of the closing
prices of the ADSs on Nasdaq for the five trading days immediately prior to the date such ADSs are issued. We may pay cash in lieu of
issuing all or any portion of the Commitment Securities.
In connection with the 2024 Offering, we agreed
not to sell any ADS to Alumni under the Alumni Purchase agreement for a period of 180 days from the 2024 Closing Date.
Nasdaq Listing
On April 29, 2024, we received a letter from the
Listing Qualifications staff (the “Staff”) of The Nasdaq Stock Market, LLC (“Nasdaq”) notifying us that the closing
bid price per ADS of the Company was below the required minimum of $1.00 for a period of 31 consecutive business days and that we did
not meet the minimum bid price requirements set forth in Nasdaq Rule 5550(a)(2). The notice has no immediate effect on the listing or
trading of our ADSs and the ADSs will continue to trade on The Nasdaq Capital Market under the symbol “QNRX.”
Pursuant to Nasdaq Rule 5810(c)(3)(A), we have
a period of one hundred eighty (180) calendar days, or until October 28, 2024 (the “Compliance Period”), to regain compliance
with Nasdaq’s minimum bid price requirement. Compliance may be achieved without further action if the closing bid price of our ADSs
is at or above $1.00 for a minimum of ten consecutive business days at any time during the Compliance Period, in which case Nasdaq will
notify us if it determines we are in compliance and the matter will be closed; however Nasdaq may require the closing bid price to equal
or to exceed the $1.00 minimum bid price requirement for more than 10 consecutive business days before determining that a company complies.
In the event we do not regain compliance by October 28, 2024, we may be eligible for an additional 180 calendar day grace period. To qualify,
we will be required to meet the continued listing requirement for market value of publicly held ADSs and all other initial listing standards
for The Nasdaq Capital Market, with the exception of the minimum bid price requirement, and will need to provide written notice of its
intention to cure the deficiency during the second compliance period. We intend to actively monitor the bid price of our ADS and will
consider available options to regain compliance with the Nasdaq listing requirements.
21
Table of Contents
Components of Our Results of Operations
Operating Expenses
Our current operating expenses consist of two
components - research and development expenses, and general and administrative expenses.
Research and Development Expenses
Research and development costs are expensed as
incurred. Research and development expenses include personnel costs associated with research and development activities, including third-party
contractors to perform research, conduct clinical trials and manufacture drug supplies and materials. We utilize outside consultants and
third parties to conduct the majority of our research and development, under the supervision of our management team.
Future research and development expenses may include:
| |
● |
employee-related expenses, such as salaries, bonuses and benefits, consultant-related expenses, share-based compensation, overhead related expenses and travel related expenses for our research and development personnel; |
| |
● |
expenses incurred under agreements with CROs, as well as consultants that support the implementation of the clinical studies described above; |
| |
● |
manufacturing and packaging costs in connection with conducting clinical trials and for stability and other studies required to support the NDA filing as well as manufacturing drug product for commercial launch; |
| |
● |
formulation, research and development expenses related to QRX003; and other product candidates we may choose to develop; and |
| |
● |
costs for sponsored research. |
Research and development activities will continue
to be central to our business plan. Product candidates in later stages of clinical development generally have higher development costs
than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials.
We expect our research and development expenses to be significant over the next several years as personnel and compensation costs increase
and we conduct late-stage clinical studies and prepare to seek regulatory approval for QRX003 and any other future product candidate.
The duration, costs and timing of clinical trials
of QRX003 and any other future product candidate will depend on a variety of factors that include, but are not limited to:
| |
● |
the number of trials required for approval; |
| |
● |
the per patient trial costs; |
| |
● |
the number of patients that participate in the trials; |
| |
● |
the number of sites included in the trials; |
| |
● |
the countries in which the trial is conducted; |
| |
● |
the length of time required to enroll eligible patients; |
| |
● |
the number of doses that patients receive; |
22
Table of Contents
| |
● |
the drop-out or discontinuation rates of patients; |
| |
● |
the potential additional safety monitoring or other studies requested by regulatory agencies; |
| |
● |
the duration of patient follow-up; |
| |
● |
the timing and receipt of regulatory approvals; and |
| |
● |
the efficacy and safety profile of our product candidates. |
General and Administrative Expenses
General and administrative expenses consist primarily
of compensation and employee related expenses including non-cash stock-based compensation, professional fees and other corporate expenses.
We anticipate that our general and administrative
expenses will increase in the future to support our continued research and development activities. These increases will likely include
compensation and employee-related expenses including stock-based compensation, increased costs related to the potential hiring of personnel,
travel costs and fees to outside consultants, lawyers and accountants.
Other Expenses (income)
Other expenses (income) consist primarily of interest
income and unrealized loss (gain) on investments.
Results of Operations – Three months
ended June 30, 2024 compared to the three months ended June 30, 2023
The following table sets forth our results of
operations for the three months ended June 30, 2024, compared to the three months ended June 30, 2023:
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three months ended June 30, |
|
|
|
| |
|
2024 |
|
2023 |
|
Change |
| Operating Expenses |
|
|
|
|
|
|
|
|
|
| General and administrative |
|
$ |
1,617,769 |
|
$ |
1,634,960 |
|
$ |
(17,191) |
| Research and development |
|
|
519,349 |
|
|
625,104 |
|
|
(105,755) |
| Total operating expenses |
|
|
2,137,118 |
|
|
2,260,064 |
|
|
(122,946) |
| Other (income) and expenses |
|
|
|
|
|
|
|
|
|
| Unrealized loss |
|
|
2,177 |
|
|
34,472 |
|
|
(32,295) |
| Realized and accrued interest income |
|
|
(165,262) |
|
|
(187,589) |
|
|
22,327 |
| Total other income |
|
|
(163,085) |
|
|
(153,117) |
|
|
(9,968) |
| Net loss |
|
$ |
(1,974,033) |
|
$ |
(2,106,947) |
|
$ |
132,914 |
23
Table of Contents
General and Administrative Expenses
General and administrative expenses were approximately
$1,618,000 and $1,635,000, in the three months ended June 30, 2024 and 2023, respectively, representing a decrease of $17,000, or approximately
1.0%. The decrease is primarily due to the change in allocation of compensation expense between general and administrative expense to
research and development expense of $120,000, as management devoted more time and effort to research and development activity during the
period, and a decrease in insurance costs of $32,000, offset by increases in public company costs of $46,000, legal and professional fees
of $21,000, travel related expenses of $34,000, and an increase in non-cash stock-based compensation expense of $15,000.
Research and Development Expenses
Research and development expenses were approximately $519,000 and $625,000,
in the three months ended June 30, 2024 and 2023, respectively, representing a decrease of $106,000, or approximately 17.0%. The decrease
was primarily due to $138,000 in decreased expenditures on our development programs, including work related to the clinical studies for
the development of QRX003 and our research collaborations with Queensland University of Technology. Offsetting this decrease was an increase
of $34,000 of non-cash stock-based compensation. “Components of Our Results of Operations - Research and Development Expenses”
above.
We amortize licensed or acquired intellectual property over its expected
useful life, included in research and development expenses set out above. Amortization of intangible assets was approximately $25,000
and $26,000 in each of the three month periods ended June 30, 2024 and 2023.
Other Expenses:
We had approximately $2,000 in unrealized loss
and $165,000 in interest income in the three months ended June 30, 2024 from our cash and cash equivalents and investments in marketable
debt securities. We earned $188,000 in interest income and incurred approximately $34,000 in unrealized loss in the three months ended
June 30, 2023 from our cash and cash equivalents and investments in marketable debt securities.
Results of Operations – Six months ended
June 30, 2024 compared to the six months ended June 30, 2023
The following table sets forth our results of
operations for the six months ended June 30, 2024, compared to the six months ended June 30, 2023:
|
|
|
|
|
|
|
|
|
|
|
| |
|
Six months ended June 30, |
|
|
|
| |
|
2024 |
|
2023 |
|
Change |
| Operating Expenses |
|
|
|
|
|
|
|
|
|
| General and administrative |
|
$ |
3,233,221 |
|
$ |
3,318,777 |
|
$ |
(85,556) |
| Research and development |
|
|
1,362,181 |
|
|
1,716,837 |
|
|
(354,656) |
| Total operating expenses |
|
|
4,595,402 |
|
|
5,035,614 |
|
|
(440,212) |
| Other (income) and expenses |
|
|
|
|
|
|
|
|
|
| Unrealized loss |
|
|
8,686 |
|
|
14,045 |
|
|
(5,359) |
| Realized and accrued interest income |
|
|
(302,775) |
|
|
(339,643) |
|
|
36,868 |
| Total other income |
|
|
(294,089) |
|
|
(325,598) |
|
|
31,509 |
| Net loss |
|
$ |
(4,301,313) |
|
$ |
(4,710,016) |
|
$ |
408,703 |
24
Table of Contents
General and Administrative Expenses
General and administrative expenses were approximately
$3,233,000 and $3,319,000, in the six months ended June 30, 2024 and 2023, respectively, representing a decrease of $86,000, or approximately
2.6%. The decrease was primarily due to a decrease of $92,000 in legal and professional fees, a reduction of insurance costs of $65,000
and a reduction of travel related expenses of $15,000, offset by increases in payroll and benefits of $43,000, and an increase in non-cash
stock-based compensation expense of $44,000.
Research and Development Expenses
Research and development expenses were approximately
$1,362,000 and $1,717,000, in the six months ended June 30, 2024 and 2023, respectively, representing a decrease of $355,000, or approximately
20.7%. The decrease was primarily due to $401,000 in decreased expenditures on our development programs, including work related to the
clinical studies for the development of QRX003 and our research collaborations with Queensland University of Technology. Offsetting this
decrease was an increase of $49,000 of non-cash stock-based compensation expense allocated to research and development expenses. See “Components
of Our Results of Operations - Research and Development Expenses” above.
We amortize licensed or acquired intellectual
property over its expected useful life, included in research and development expenses set out above. Amortization of intangible assets
was approximately $50,000 and $52,000 in each of the six month periods ended June 30, 2024 and 2023.
Other Expenses:
We had approximately $9,000 in unrealized loss and $303,000 in realized
gain and interest income in the six months ended June 30, 2024 from our cash and cash equivalents and investments in marketable debt securities.
We earned $339,000 in interest income and incurred approximately $14,000 in unrealized loss in the six months ended June 30, 2023 from
our cash and cash equivalents and investments in marketable debt securities.
Liquidity and Capital Resources
We have incurred net losses every year since inception and had an accumulated
deficit of approximately $50.5 million at June 30, 2024. We have a limited operating history and have historically funded our operations
through debt and equity financings. We incurred net losses of approximately $4.3 million and negative cash flows from operations of $3.4
million for the six months ended June 30, 2024. At June 30, 2024, we had cash and cash equivalent balances totaling $2.8 million and investments
of $9.7 million. We have determined that we have sufficient resources to effect our business plan for at least one year from the issuance
of the unaudited consolidated financial statements included in this report; however, we are subject to risks common to development stage
biopharmaceutical companies including, but not limited to, unanticipated clinical trial costs and the ability to estimate such occurrences,
if any, on our cash, liquidity, additional financing requirements, and availability. Accordingly, we may need to raise additional funds
sooner than planned. We do not expect to generate revenue from product sales unless and until we successfully complete development and
obtain marketing approval for one or more of our product candidates, which we expect will take a number of years and is subject to significant
uncertainty. Additional financing will be required to complete the research and development of our therapeutic targets and our other operating
requirements, which may not be available at acceptable terms, if at all. If we are unable to obtain additional funding when it becomes
necessary, the development of our product candidates will be impacted and we would likely be forced to delay, reduce, or terminate some
or all of our development programs, all of which could have a material adverse effect on our business, results of operations and financial
condition.
Our net losses may fluctuate significantly from quarter-to-quarter
and year-to-year, depending on the timing of planned clinical trials and our expenditures on other research and development activities.
25
Table of Contents
Future Funding Requirements
We will need to obtain further funding through
public or private offerings of our capital stock, debt financing, collaboration and licensing arrangements or other sources, the requirements
for which will depend on many factors, including:
| |
● |
the scope, timing, rate of progress and costs of our drug development efforts, preclinical development activities, the timing of laboratory testing and clinical trials for our product candidates; |
| |
● |
the number and scope of clinical programs we decide to pursue; |
| |
● |
the cost, timing and outcome of preparing for and undergoing regulatory review of our product candidates; |
| |
● |
the scope and costs of development and commercial manufacturing activities; |
| |
● |
the cost and timing associated with commercializing our product candidates, if they receive marketing approval; |
| |
● |
the extent to which we acquire or in-license other product candidates and technologies; |
| |
● |
the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; |
| |
● |
our ability to establish and maintain collaborations on favorable terms, if at all; |
| |
● |
our efforts to enhance operational systems and our ability to attract, hire and retain qualified personnel, including personnel to support the development of our product candidates and, ultimately, the sale of our products, following FDA approval; |
| |
● |
our implementation of operational, financial and management systems; and |
| |
● |
the costs associated with being a public company. |
Adequate additional funding may not be available
to us on acceptable terms, or at all. If we are unable to raise capital in sufficient amounts or on terms acceptable to us, we may have
to significantly delay, scale back or discontinue the development or commercialization of QRX003, any future product candidate, or potentially
discontinue operations.
To the extent that we raise additional capital
through the sale of our equity or convertible debt securities, and pursuant to the exercise of the warrants issued to investors in 2022,
2023 and 2024 public offerings, the ownership interest of our equity holders will be diluted, and the terms of these securities may include
liquidation or other preferences that adversely affect the rights of our equity holders. Debt financing and preferred equity financing,
if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring
additional debt, making capital expenditures or declaring dividends.
26
Table of Contents
If we raise additional funds through collaborations,
strategic alliances or marketing, distribution or licensing arrangements with third parties, we may be required to relinquish valuable
rights to our technologies, future revenue streams, research programs or proposed products, or to grant licenses on terms that may not
be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay,
limit, reduce or terminate our drug development or future commercialization efforts or grant rights to develop and market any future product
that we would otherwise prefer to develop and market ourselves.
Summary Statement of Cash Flows
As of June 30, 2024, we had approximately $12,600,000 in cash and cash
equivalents and investments in marketable securities. The table below presents our cash flows for the six month periods ended June 30,
2024 and 2023:
|
|
|
|
|
|
|
|
| |
|
Six months ended June 30, |
| |
|
2024 |
|
2023 |
| Net cash used in operating activities |
|
$ |
(3,441,495) |
|
$ |
(3,319,260) |
| Net cash used in investing activities |
|
|
(1,310,774) |
|
|
(456,505) |
| Net cash provided by financing activities |
|
|
5,196,197 |
|
|
5,674,266 |
| Net change in cash and cash equivalents |
|
$ |
443,928 |
|
$ |
1,898,501 |
Operating Activities
Net cash used in operating activities was approximately
$3,442,000 and $3,319,000 in the six months ended June 30, 2024 and 2023, respectively. The increase in 2024 was mostly due to a smaller
increase in accounts payable and accrued expenses and an increase of prepaid expenses, offset by a decrease in net loss adjusted for non-cash
income and expenses.
Investing Activities
Net cash used in investing activities was approximately
$1,311,000 and $457,000 in the six months ended June 30, 2024 and 2023, respectively. The cash used in investing activities for the six
months ended June 30, 2024 consisted of net purchases of short maturity US Treasury Bills and Notes from the proceeds of the 2024 Offering,
the cash used in investing activities in the six months ended June 30, 2023 consisted of net purchases of short maturity US Treasury Bills
and Notes from the proceeds of our public offering in February 2023 (the “2023 Offering”).
Financing Activities
Net cash provided by financing activities was
approximately $5,196,000 for the six months ended June 30, 2024. The net cash provided increased due to the receipt of approximately $5.5
million in net proceeds from the 2024 Offering partially offset by repayments of amounts due to officers of $300,000. Net cash provided
by financing activities was approximately $5,674,000 for the six months ended June 30, 2023. The net cash provided decreased due to the
receipt of $5,849,000 in net proceeds from the 2023 Offering partially offset by repayments of amounts due to officers of $175,000.
Research and Development Commitments
In October 2019, Quoin Inc. entered into the Exclusive
Licensing Agreement (as amended from time to time, the “License Agreement”) with Skinvisible Pharmaceuticals, Inc. (“Skinvisible”),
under which Skinvisible granted us an exclusive royalty-bearing license relating to the production and manufacture of prescription drug
products related to certain patents held by Skinvisible, including those related to QRX003 and QRX004. We made Skinvisible a one-time
non-refundable, non-creditable license fee of $1 million (the “License Fee”). In addition, we agreed to pay Skinvisible a
single digit royalty percentage of our net sales revenues for any licensed product covered by the patent rights licensed under the License
Agreement. We also agreed to pay Skinvisible 25% of any revenues we receive as royalties in the event that we sublicense any licensed
products to a third party. The License Agreement also requires that we make a $5 million payment to Skinvisible upon receiving approval
in the U.S. or European Union, whichever occurs first, for the first drug product developed using intellectual property licensed thereunder.
27
Table of Contents
In November 2020, Quoin Inc. entered into a Master
Service Agreement with Therapeutics Inc. for the management of the preclinical and clinical development of QRX003 for Netherton Syndrome.
The initial term of the agreement was three years with automatic one year extensions, and the agreement required the execution of individual
work orders. Quoin Inc. may terminate any work order for any reason with 90 days written notice subject to costs incurred through termination
and a defined termination fee, unless there is a material breach by Therapeutics Inc. A work order was entered into in June 2022 for the
first QRX003 clinical study at an expected estimated cost of approximately $4.4 million through 2024. An additional work order was entered
into in December 2022 for a second QRX003 clinical study at an expected estimated cost of approximately $830,000. For the three and six
months ended June 30, 2024 and 2023, we incurred research and development expenses under these agreements of approximately $162,000 and
$650,000, and $360,000 and $959,000, respectively.
In November 2021, we entered into a research agreement
with Queensland University of Technology (“QUT”) for a pre-clinical research program for the development of a product to treat
Netherton Syndrome of approximately $250,000. In May 2022, we entered into a second research agreement with QUT for the development of
a product to treat Scleroderma of approximately $610,000. Each agreement remains in place until the completion of the research program.
For the three and six months ended June 30, 2024 we did not incur any costs related to these agreements. For the three and six months
ended June 30, 2023, we incurred research and development costs related to these agreements of approximately $138,000 and $276,000 respectively.
On June 10, 2024, we entered into a research agreement
with The School of Pharmacy at UCC. The scope of the agreement encompasses the development of novel topical formulations of Rapamycin
(sirolimus) as potential treatments for a number of rare and orphan diseases for which there are currently no approved therapies or cures.
Under the terms of the agreement, based on the achievement of certain milestones, we will fund up to approximately €567,000 ($608,000)
plus VAT over an anticipated 2-1/2 year period to support UCC research program to investigate the development of a number of topical rapamycin
formulations for future development as potential treatments for several rare and orphan diseases. Following completion of the research
program, we will have the option to advance the clinical development of rapamycin formulations developed by UCC. For the three and
six months ended June 30, 2024 we did not incur any costs related to this agreement.
Critical Accounting Estimates
There have been no material changes to our critical
accounting estimates from the information provided in Item 7, “Management’s Discussion and Analysis of Financial Condition
and Results of Operations,” included in our Annual Report on Form 10-K for the year ended December 31, 2023.
28
Table of Contents
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
We are a smaller reporting company as defined
in Rule 12b-2 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and are not required to provide the
information otherwise required under this Item 3.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures,
which are designed to provide reasonable assurance that information required to be disclosed in the reports we file or submit under the
Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC rules and forms, and that such
information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate
to allow timely decisions regarding required disclosure. Our management, with the participation of our Chief Executive Officer and Chief
Financial Officer, conducted an evaluation, as of the end of the period covered by this report, of the effectiveness of our disclosure
controls and procedures (as defined in Rules 13a-15e under the Exchange Act). Based on that evaluation, our Chief Executive Officer and
Chief Financial Officer have concluded that, as of the end of the period covered by this report, our disclosure controls and procedures
were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
During the quarter ended June 30, 2024, there
were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act)
that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
29
Table of Contents
PART II – OTHER INFORMATION
Item 1. Legal Proceedings.
From time to time, we may become involved in legal
or administrative proceedings or be subject to claims arising in the ordinary course of our business. We are currently not a party to
any material legal or administrative proceedings, and we are not aware of any pending or threatened material legal or administrative proceedings
against us.
Item 1A. Risk Factors.
Except as set forth below, there have been no
material changes in our risk factors from the risks previously reported in Part 1, Item 1A, “Risk Factors” of our Form 10-K.
You should carefully consider the factors discussed in Form 10-K, which could materially affect our business, financial condition or future
results. The risks described in our Form 10-K are not the only risks we face. Additional risks and uncertainties not currently known to
us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition and/or operating
results. We may disclose changes to such factors or disclose additional factors from time to time in our future filings with the SEC.
Our failure to meet the continued listing
requirements of The Nasdaq Capital Market could result in a delisting of our ADSs.
Our ADSs are listed on the Nasdaq Capital Market,
which imposes, among other requirements, a minimum bid requirement.
On April 29, 2024, we received a deficiency letter
from the Listing Qualifications Department of the Nasdaq notifying us that for the preceding 31 consecutive business days (March 14, 2024
through April 26, 2024), our ADSs did not maintain a minimum closing bid price of $1.00 (“Minimum Bid Price Requirement”)
per ADS as required by Nasdaq Listing Rule 5550(a)(2). In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we have a compliance period
of 180 calendar days, or until October 28, 2024, to regain compliance with Nasdaq Listing Rule 5550(a)(2). Compliance may be achieved
automatically and without further action if the closing bid price of our ADSs is at or above $1.00 for a minimum of ten consecutive business
days at any time during the 180-day compliance period, in which case, in its discretion, Nasdaq will notify the Company of its compliance
and the matter will be closed. If, however, we do not achieve compliance with the Minimum Bid Price Requirement by October 28, 2024, we
may be eligible for additional time to comply. In order to be eligible for such additional time, we will be required to meet the continued
listing requirement for market value of publicly held ADSs and all other initial listing standards for the Nasdaq Capital Market, with
the exception of the Minimum Bid Price Requirement, and must notify Nasdaq in writing of our intention to cure the deficiency during the
second compliance period. We intend to actively monitor the bid price of our ADSs and will consider available options to regain compliance
with the Nasdaq listing requirements.
If we cannot regain compliance with the Minimum
Bid Price Requirement or if we otherwise fail to meet any of Nasdaq’s listing standards, our ADSs will be subject to delisting.
If that were to occur, our ADSs would be subject to rules that impose additional sales practice requirements on broker-dealers who sell
our securities. The additional burdens imposed upon broker-dealers by these requirements could discourage broker-dealers from effecting
transactions in our ADSs. This would adversely affect the ability of investors to trade our ADSs and would adversely affect the value
of our ADSs. Delisting from Nasdaq would cause us to pursue eligibility for trading of our ADSs on other markets or exchanges, or on an
over-the-counter market. In such case, our stockholders’ ability to trade or obtain quotations of the market value of our ADSs would
be severely limited because of lower trading volumes and transaction delays. These factors could contribute to lower prices and larger
spreads in the bid and ask prices of these securities. There can be no assurance that our ADSs, if delisted from the Nasdaq, would be
listed on a national securities exchange, a national quotation service or the over-the-counter markets. Delisting from the Nasdaq could
also result in negative publicity, adversely affect the market liquidity of our ADSs, decrease securities analysts’ coverage of
us or diminish investor, supplier and employee confidence.
30
Table of Contents
The delisting of our ADSs from Nasdaq may make
it more difficult for us to raise capital on favorable terms in the future, or at all. Such a delisting would likely have a negative effect
on the price of our ADSs and would impair your ability to sell or purchase our ADSs when you wish to do so. Further, if our ADSs were
to be delisted from Nasdaq, our ADSs would cease to be recognized as a covered security, and we would be subject to additional regulation
in each state in which we offer our securities. Moreover, there is no assurance that any actions that we take to restore our compliance
with the Nasdaq Minimum Bid Price Requirement would stabilize the market price or improve the liquidity of our ADSs, prevent our ADSs
from falling below the Nasdaq minimum bid price required for continued listing again or prevent future non-compliance with other applicable
Nasdaq listing requirements, including maintaining minimum levels of stockholders’ equity or market values of our ADSs, our ADSs
could be delisted.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
None.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Other Information.
During the second quarter of 2024, none of our
directors or executive officers adopted or terminated any “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading
arrangement” (as each term is defined in Item 408(a) of Registration S-K).
31
Table of Contents
Item 6. Exhibits.
The following exhibits are included in this Form
10-Q or incorporated herein by reference:
|
|
|
| Exhibit No. |
|
Exhibit Description |
| 3.1 |
|
Amended and Restated Articles of Association of Quoin Pharmaceuticals Ltd., adopted on February 28, 2022 (incorporated by reference to Annex A included in Exhibit 99.1 to Form 6-K filed with the SEC on February 8, 2022). |
| 3.2 |
|
Amendment to the Amended and Restated Articles of Association of Quoin Pharmaceuticals Ltd., adopted on April 12, 2022 (incorporated by reference to Annex A included in Exhibit 99.1 to Form 6-K filed with the SEC on March 8, 2022). |
| 3.3 |
|
Amendment to the Amended and Restated Articles of Association of Quoin Pharmaceuticals Ltd., adopted on November 3, 2022 (incorporated by reference to Annex A included in Exhibit 99.1 to Form 6-K filed with the SEC on September 21, 2022). |
| 3.4 |
|
Amendment to the Amended and Restated Articles of Association of Quoin Pharmaceuticals Ltd., adopted on October 26, 2023 (incorporated by reference to Annex A included in the proxy statement filed with the SEC on September 12, 2023). |
| 31.1* |
|
Certification of Chief Executive Officer pursuant to Rules 13a-14(a) or 15d-14(a) under the Securities Exchange Act of 1934 |
| 31.2* |
|
Certification of Chief Financial Officer pursuant to Rules 13a-14(a) or 15d-14(a) under the Securities Exchange Act of 1934 |
| 32.1* |
|
Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350 |
| 32.2* |
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350 |
| 101* |
|
Information formatted in Extensible Business Reporting Language (XBRL): (i) Condensed Consolidated Balance Sheets, (ii) Condensed Consolidated Statements of Operations, (iii) Condensed Consolidated Statements of Shareholders’ Equity, (iv) Condensed Consolidated Statements of Cash Flows, and (v) Notes to Condensed Consolidated Financial Statements. |
| 104* |
|
Cover Page Interactive Data File (Embedded within the Inline XBRL document and included in Exhibit 101) |
*
Previously filed or furnished with the Company’s Form 10-Q filed on August 8, 2024.
32
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
| |
Quoin Pharmaceuticals Ltd. |
| |
|
|
| August 8, 2024 |
By: |
/s/ Gordon Dunn |
| |
|
Name: Gordon Dunn |
| |
|
Title: Chief Financial Officer |
33
Quoin Pharmaceuticals (NASDAQ:QNRX)
Historical Stock Chart
From Feb 2025 to Mar 2025
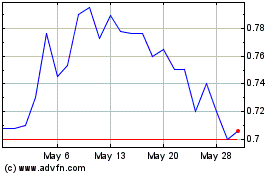
Quoin Pharmaceuticals (NASDAQ:QNRX)
Historical Stock Chart
From Mar 2024 to Mar 2025