Regeneron Gets FDA Priority Review of Odronextamab in Follicular, Diffuse Large B-cell Lymphoma
29 September 2023 - 11:11PM
Dow Jones News
By Colin Kellaher
Regeneron Pharmaceuticals has won U.S. Food and Drug
Administration priority review of its application seeking approval
of odronextamab for certain patients with the two most common
subtypes of non-Hodgkin lymphoma.
The Tarrytown, N.Y., biotechnology company said the application
covers odronextamab for adults with relapsed/refractory follicular
lymphoma or diffuse large B-cell lymphoma who have progressed after
at least two prior systemic therapies.
The FDA grants priority review to medicines that have the
potential to provide significant improvements in the treatment of a
serious disease, and the designation shortens the review period.
Regeneron said the agency set a target action date of March 31,
2024, for its application.
Regeneron said an FDA green light would make odronextamab the
first and only bispecific antibody approved in both follicular
lymphoma and diffuse large B-cell lymphoma.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
September 29, 2023 08:56 ET (12:56 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
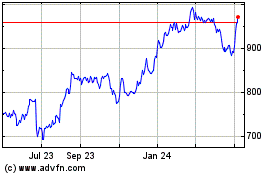
Regeneron Pharmaceuticals (NASDAQ:REGN)
Historical Stock Chart
From Apr 2024 to May 2024
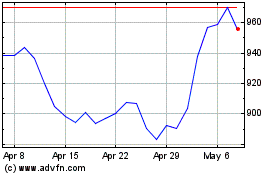
Regeneron Pharmaceuticals (NASDAQ:REGN)
Historical Stock Chart
From May 2023 to May 2024