Tandem Diabetes Care, Inc. (Nasdaq: TNDM) and Dexcom, Inc. today
announced that following the recent authorization of sale by Health
Canada, in-warranty t:slim X2™ insulin pump users can now access
full compatibility with both the Dexcom G7 and Dexcom G6 Continuous
Glucose Monitoring (CGM) Systems. The t:slim X2 insulin pump with
Control-IQ technology is the #1-rated automated insulin delivery
(AID) system1, and now the first and only insulin pump in Canada
that is integrated with both Dexcom G6 and Dexcom G7.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20241210731189/en/
t:slim X2 Insulin Pump with Dexcom G7 CGM
(Photo: Business Wire)
“The commercial availability of this new offering is a testament
to our decade-long partnership with Dexcom and shared commitment to
continued leadership in advancing AID systems,” said Mark Novara,
executive vice president and chief commercial officer of Tandem
Diabetes Care. “Now, our t:slim X2 users in Canada can have access
to a range of innovative tools that not only simplify diabetes
management but also enhance their quality of life.”
“Through our continued partnership with Tandem, we have seen the
proven benefits of automated insulin delivery. We’re proud that
people living in Canada can now access the latest AID offering with
Dexcom G7, our smallest and most accurate CGM2,” said André Côté,
executive vice president and general manager of Dexcom Canada. “Our
two organizations hold the same core value: foster technological
innovation that leads to a better life for those living with
diabetes. We couldn’t be more pleased to officially bring this
offering to Canadians.”
In December 2023, the t:slim X2 pump with Dexcom G7 integration
launched in the U.S., marking the first time an AID option was
compatible with Dexcom’s latest CGM technology. Now, t:slim X2
users in Canada can experience even more choice when it comes to
CGM compatibility, along with the option to spend more time in
closed loop with Dexcom G7’s 30-minute sensor warm-up time, faster
than any other CGM on the market.3 In addition, t:slim X2 users who
pair Dexcom G7 with an Apple smartwatch4 can see their glucose
numbers directly from their watch without having to access their
pump or smartphone4.
Tandem will email all in-warranty t:slim X2 users in Canada with
instructions on how to add the new compatibility feature free of
charge via remote software update. t:slim X2 pumps pre-loaded with
the updated software will begin shipping to new customers in early
January 2025.
To check coverage and start the process of getting a Tandem
insulin pump, please visit tandemdiabetes.ca.
To learn more about Dexcom G6 or Dexcom G7, please visit
dexcom.com or ask your healthcare provider.
1 dQ&A Canada Patient Voice H1 2024. 2 Welsh JB, et al. J
Diabetes Sci Technol. 2024;18(1):143-7 3 Dexcom G7 can complete
warm up within 30 minutes, whereas other CGM brands require up to
an hour or longer 4 Smart device sold separately. To view a list of
compatible devices, visit dexcom.com/compatibility. Compatible
smartphone is required to pair a new Dexcom G7 sensor with a
compatible Apple Watch. Dexcom G7 users must continuously have
their smartphone within 10 meters (33 feet) to utilize
Share/Follow.
About Tandem Diabetes Care, Inc.
Tandem Diabetes Care, a global insulin delivery and diabetes
technology company, manufactures and sells advanced automated
insulin delivery systems that reduce the burden of diabetes
management, while creating new possibilities for patients, their
loved ones, and healthcare providers. The Company’s pump portfolio
features the Tandem Mobi system and the t:slim X2 insulin pump,
both of which feature Control-IQ advanced hybrid closed-loop
technology. Tandem Diabetes Care is based in San Diego, California.
For more information, visit tandemdiabetes.com.
Follow Tandem Diabetes Care on X @tandemdiabetes; use #tslimX2
and #TandemDiabetes.
Follow Tandem Diabetes Care on Facebook at
facebook.com/TandemDiabetesCanada.
Follow Tandem Diabetes Care on LinkedIn at
linkedin.com/company/tandemdiabetes.
© 2024 Tandem Diabetes Care, Inc. All rights reserved. Tandem
Diabetes Care, the Tandem logo, Control-IQ, t:slim X2, and Tandem
Mobi are either registered trademarks or trademarks of Tandem
Diabetes Care, Inc. in the United States and/or other countries.
Dexcom, Dexcom G6, Dexcom G7, and any related logos and design
marks are either registered trademarks or trademarks of Dexcom,
Inc. in the United States and/or other countries. All other
third-party marks are the property of their respective owners.
Forward-looking Statements
This press release contains “forward-looking statements” within
the meaning of Section 27A of the Securities Act of 1933, as
amended, and Section 21E of the Securities Exchange Act of 1934, as
amended. These forward-looking statements relate to, among other
things, the anticipated timing for the Canadian availability of the
t:slim X2 pump with Dexcom G7 integration as well as our ability to
provide the software update for current t:slim X2 pump users and
the t:slim X2 pumps pre-loaded with Dexcom G7 integration software.
These statements are subject to numerous risks and uncertainties,
including our ability to start commercial scale manufacturing of
the t:slim X2 pumps pre-loaded with Dexcom G7 integration software
for the Canadian market, our ability to operate and maintain a
system to facilitate online training for existing t:slim X2 pump
customers upgrading their existing devices, and the risk that we
may encounter other challenges that may delay the availability of
t:slim X2 pumps pre-loaded with Dexcom G7 integration software.
These and other risks are identified and described in greater
detail under the “Risk Factors” heading of our most recent Annual
Report on Form 10-K, Quarterly Reports on Form 10-Q, and other
documents filed with the Securities and Exchange Commission.
Readers are cautioned not to place undue reliance on these
forward-looking statements, which speak only as of the date of this
release. Actual results could differ materially from those
anticipated or projected in the forward-looking statements. Tandem
undertakes no obligation to update or review any forward-looking
statement in this press release because of new information, future
events, or other factors.
Responsible Use of Control-IQ Technology
Control-IQ technology does not prevent all highs and lows. Users
must still bolus for meals and actively manage their diabetes.
Visit tandemdiabetes.com/safetyinfo for additional important safety
information.
This product may not be right for you. Always read and follow
the label.
Important Safety Information: The t:slim X2 insulin pump with
Control-IQ technology (the System) consists of the t:slim X2
insulin pump, which contains Control-IQ technology, and a
compatible continuous glucose monitor (CGM, sold separately). The
t:slim X2 insulin pump is intended for the subcutaneous delivery of
insulin, at set and variable rates, for the management of diabetes
mellitus in people requiring insulin. The t:slim X2 insulin pump
can be used solely for continuous insulin delivery and as part of
the System. When used with a compatible CGM, the System can be used
to automatically increase, decrease, and suspend delivery of basal
insulin based on CGM sensor readings and predicted glucose values.
The System can also deliver correction boluses when the glucose
value is predicted to exceed a predefined threshold. The pump and
the System are indicated for use in individuals 6 years of age and
greater. The pump and the System are intended for single user use.
The pump and the System are indicated for use with NovoRapid,
Admelog, Trurapi, or Humalog U-100 insulin. The System is intended
for the management of Type 1 diabetes.
WARNING: Control-IQ technology should not be used by anyone
under the age of 6 years old. It should also not be used in users
who require less than 10 units of insulin per day or who weigh less
than 25 kilograms.
The System is not indicated for use in pregnant women, people on
dialysis, or critically ill users. Do not use the System if using
hydroxyurea.
Users of the pump and the System must: be willing and able to
use the insulin pump, CGM, and all other system components in
accordance with their respective instructions for use; test blood
glucose levels as recommended by their healthcare provider;
demonstrate adequate carb-counting skills; maintain sufficient
diabetes self-care skills; see healthcare provider(s) regularly;
and have adequate vision and/or hearing to recognize all functions
of the pump, including alerts, alarms, and reminders. The t:slim X2
pump must be removed before MRI, CT, or diathermy treatment. Visit
tandemdiabetes.com/safetyinfo for additional important safety
information.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20241210731189/en/
Tandem Diabetes Care Media Contact: 858-366-6900
media@tandemdiabetes.com
Tandem Diabetes Care Investor Contact: 858-366-6900
IR@tandemdiabetes.com
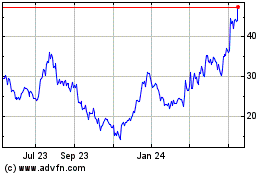
Tandem Diabetes Care (NASDAQ:TNDM)
Historical Stock Chart
From Dec 2024 to Jan 2025
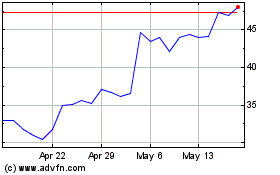
Tandem Diabetes Care (NASDAQ:TNDM)
Historical Stock Chart
From Jan 2024 to Jan 2025