- The VENTANA Kappa and Lambda Dual ISH mRNA Probe Cocktail
assay is the first clinically approved in-situ hybridisation (ISH)
test with the sensitivity to assess the full spectrum of B-cell
lymphoma subtypes.1,2
- The test helps differentiate a B-cell cancer from a normal,
reactive immune response, offering diagnostic certainty for
healthcare providers and their patients.
- B-cell lymphoma accounts for approximately 85 percent of
non-Hodgkin lymphoma (NHL) cases, which is one of the most common
cancers in the US.3
TUCSON,
Ariz., Jan. 13, 2025 /PRNewswire/ -- Roche (SIX:
RO, ROG; OTCQX: RHHBY) announced today that it has received 510(k)
clearance from the United States Food and Drug Administration (FDA)
for its highly-sensitive in-situ hybridisation (ISH) test, the
VENTANA® Kappa and Lambda Dual ISH mRNA Probe Cocktail.4
The test is designed to help pathologists differentiate a B-cell
malignancy from a normal, reactive response to an infection, thus
facilitating faster access to treatment.4 This
announcement follows the assay's CE Mark approval in June 2024.
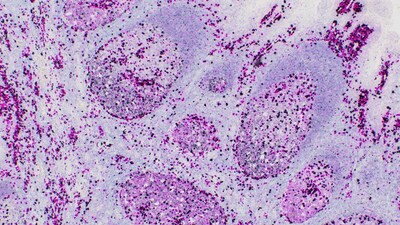
B-cell lymphoma is a type of cancer that typically develops in
the lymphatic system. It accounts for approximately 85 percent of
non-Hodgkin lymphoma (NHL) cases.3 NHL is one of the
most common forms of cancer in the US, accounting for about 4% of
all cancer cases,5 and causing more than 80,000 deaths
each year.5 In the early stages of NHL, patients may
experience symptoms like swelling of the lymph nodes, fever,
fatigue, loss of appetite or a red rash.
"Accurately differentiating lymphoma from an infection is
critical in ensuring accurate and timely diagnosis, especially as
the symptoms can appear similar," said Jill
German, Head of Pathology Lab at Roche Diagnostics. "With
this new test, clinicians can have confidence in their diagnosis,
while the test reduces the need for multiple samples and time
consuming follow up tests, giving patients certainty sooner, and
enabling faster access to the right treatment."
With increased sensitivity, the new VENTANA Kappa and Lambda
Dual ISH mRNA Probe Cocktail enables assessment across the more
than 60 B-cell lymphoma subtypes and
plasma cell neoplasms on a single tissue slide. The test can assess
small biopsies and formalin-fixed tissue, reducing the need for a
fresh tissue sample, which may not be available especially if
lymphoma was not originally suspected. These test properties
preserve tissue, may result in fewer additional patient biopsies
and make interpretation quicker and easier for the pathologist,
helping facilitate a faster diagnosis and access to treatment for
patients.
This first-of-its-kind assay is a significant addition to
Roche's industry-leading hematopathology portfolio, which includes
more than 65 biomarkers.
About the VENTANA Kappa and Lambda Dual ISH mRNA Probe
Cocktail
The VENTANA Kappa and Lambda Dual ISH mRNA Probe Cocktail Assay is
a qualitative assay that is used to detect the expression of kappa
and lambda immunoglobulin light chains in formalin-fixed paraffin
embedded (FFPE) human hematolymphoid specimens by in situ
hybridization (ISH).
The assay is intended as an aid in the diagnosis of mature
B-cell lymphomas and plasma cell neoplasms. The VENTANA Kappa and
Lambda Dual ISH mRNA Probe Cocktail is indicated for use when a
biopsy of lymph node or bone marrow (core biopsy and clot section)
indicates inconclusive results. It enables the assessment of both
markers in the context of one another on a single slide as an aid
in differentiating between a reactive process or B-cell lymphoma
and plasma cell neoplasms.
This is not a standalone test, and results should be evaluated
by a qualified pathologist within the context of the patient's
clinical history and other diagnostic tests. This product is
intended for in vitro diagnostic (IVD) use.4
About Roche
Founded in 1896 in Basel,
Switzerland, as one of the first industrial manufacturers of
branded medicines, Roche has grown into the world's largest
biotechnology company and the global leader in in-vitro
diagnostics. The company pursues scientific excellence to discover
and develop medicines and diagnostics for improving and saving the
lives of people around the world. We are a pioneer in personalised
healthcare and want to further transform how healthcare is
delivered to have an even greater impact. To provide the best care
for each person we partner with many stakeholders and combine our
strengths in Diagnostics and Pharma with data insights from the
clinical practice.
For over 125 years, sustainability has been an integral part of
Roche's business. As a science-driven company, our greatest
contribution to society is developing innovative medicines and
diagnostics that help people live healthier lives. Roche is
committed to the Science Based Targets initiative and the
Sustainable Markets Initiative to achieve net zero by 2045.
Genentech, in the United
States, is a wholly owned member of the Roche Group. Roche
is the majority shareholder in Chugai Pharmaceutical, Japan.
For more information, please visit www.roche.com.
All trademarks used or mentioned in this release are protected
by law.
References
1Rimsza LM, et al. Kappa and lambda light chain mRNA
in situ hybridization compared to flow cytometry and
immunohistochemistry in B cell lymphomas. Diagn Pathol.
2014;9:144.
2F. Hoffmann-La Roche Ltd. Conjoint Market Research.
[Survey; Cited 2024 April 4].
Data on File.
3American Cancer Society. Types of B-cell Lymphoma.
[Internet; Cited 3 December 2024].
Available
at: https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/b-cell-lymphoma.html#:~:text=B%2Dcell%20lymphomas%20make%20up,cell%20lymphomas%20are%20listed%20below.
4F. Hoffmann-La Roche Ltd. VENTANA Kappa and
Lambda Dual ISH mRNA Probe Cocktail. [Method Sheet; cited 2025
January 10]. Data on file.
5American Cancer Society. Key Statistics for Non-Hodgkin
Lymphoma [Internet; Cited 3 December
2024]. Available at:
https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html#:~:text=Non%2DHodgkin%20lymphoma%20(NHL),11%2C780%20males%20and%208%2C360%20females).
Roche Media Relations
Jo Lynn
Garing, +1 317-363-7286
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/roche-receives-fda-clearance-for-new-highly-sensitive-test-to-aid-clinicians-in-diagnosing-b-cell-lymphoma-302348583.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/roche-receives-fda-clearance-for-new-highly-sensitive-test-to-aid-clinicians-in-diagnosing-b-cell-lymphoma-302348583.html
SOURCE Roche