As filed with the Securities and Exchange Commission on April 7, 2023
Registration No. 333-269076
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1/A
Amendment No. 2
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
| 
|
| COSTAS, INC. |
| (Exact name of registrant as specified in its charter) |
| Nevada | | 3843 | | 88-0411500 |
| (State or other jurisdiction of | | (Primary Standard Industrial | | (I.R.S. Employer |
| incorporation or organization) | | Classification Code Number) | | Identification Number) |
# 424 E Central Blvd, Suite 308,
Orlando, FL, 32801
321-465-9899
(Address, including zip code, and telephone number, including area code, of registrants principal executive offices)
With a copy to:
William (Bill) Macdonald Esq.
641 Lexington Avenue, 13th Floor
New York, NY 10022
Tel: (212) 271-4272
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Approximate Date of Commencement of Proposed Sale to the Public: As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, please check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act Prospectus number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company.
| ☐ Large accelerated filer | ☐ Accelerated filer | ☐ Non-accelerated filer | ☒ Smaller reporting company |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said section 8(a), may determine.
| The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities, nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. |
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED April ____, 2023 |

319,890,000 common shares underlying previously issued convertible promissory notes
750,000 common shares previously issued pursuant to asset acquisition
8,333,333 common shares issuable pursuant to purchase agreement
The date of this Prospectus is April__, 2023.
Costas, Inc. (“Costas”, “we”, “us”, “our” and “our company”) is registering 328,973,333 shares of common stock underlying previously issued convertible promissory notes, an asset purchase agreement and a purchase agreement for the future issuance of common shares as drawn down by our company, which may be resold from time to time held by nine selling security holders (the “ Selling Security Holders ”). This aggregate of 328,973,333 shares of common stock consists of: 319,890,000 shares of common stock underlying previously issued convertible promissory notes issued by our company to certain Selling Security Holders; 750,000 shares issued to one Selling Security Holder for the acquisition of certain assets; and 8,333,333 shares issuable upon drawdowns under the Purchase Agreement with another Selling Security Holder, World Amber Corporation. The convertible promissory notes were acquired by the Selling Security Holders directly from us in private offerings that were exempt from registration requirements of the Securities Act of 1933. A registration statement under the Exchange Act relating to these securities will have been filed with the Securities and Exchange Commission once declared effective. Our Selling Security Holders may not offer or sell their shares of our common stock until this registration statement is declared effective. We have been advised by the Selling Security Holders that they may offer to sell all or a portion of their shares of common stock being offered in this prospectus from time to time. Please see “Plan of Distribution” at page 26 for a detailed explanation of how the securities may be sold. The Selling Security Holders may sell all or a portion of their shares through public or private transactions at the fixed price of $0.00975 per share until such time as the shares are listed on a national securities exchange or quoted on the OTCQB or OTCQX operated by the OTC Markets Inc. We will not receive any of the proceeds from the sale of shares by the Selling Security Holders. None of the Selling Security Holders are affiliates of our company.
Our common stock is quoted under the trading symbol “CSSI” on the Pink tier of the OTC quotation service operated by OTC Markets Inc.
The Selling Security Holders may sell all or a portion of their shares through public or private transactions at the fixed price of $0.00975 per share until such time as the shares are listed on a national securities exchange or quoted on the OTCQB or OTCQX operated by the OTC Markets Inc., and thereafter at prevailing market prices or at privately negotiated prices. We have arbitrarily set the $0.00975 price per share set out in this registration statement solely for the purpose of determining the amount of the registration fee pursuant to Rule 457(c), based on the average of the high and low prices of the common stock as reported on the OTC Markets on December 19, 2022. The price does not reflect net worth, total asset value, or any other objective accounting measure of the value of our securities.
The Selling Security Holders are underwriters, within the meaning of section 2(a)(11) of the Securities Act. Any broker-dealers or agents that participate in the sale of the common stock or interests therein may also be deemed to be an “underwriter” within the meaning of section 2(a)(11) of the Securities Act. Any discounts, commissions, concessions or profit earned on any resale of the shares may be underwriting discounts and commissions under the Securities Act. We will not receive any proceeds from the sale of shares of our common stock by the Selling Security Holders. We will incur all costs associated with this Prospectus.
Our sole director and officer is James Brooks: Chief Executive and Financial Officer, Secretary, President, Chairman of the Board and Director.
Our common stock is presently not traded on any national securities exchange or the NASDAQ stock market. We do not intend to apply for listing on any national securities exchange or the NASDAQ stock market. The purchasers in this offering may be receiving an illiquid security.
An investment in our securities is speculative. See the section entitled “Risk Factors” beginning on page 8 of this Prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this Prospectus. Any representation to the contrary is a criminal offense.
The information in this Prospectus is not complete and may be changed. The Selling Security Holders may not sell these securities until this registration statement is declared effective by the Securities and Exchange Commission. This Prospectus shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall the Selling Security Holders sell any of these securities in any state where such an offer or solicitation would be unlawful before registration or qualification under such state’s securities laws.
You should rely only on the information contained in this Prospectus. We have not authorized anyone to provide you with information different from that contained in this Prospectus. The Selling Security Holders are offering to sell, and seeking offers to buy, their common shares. The information contained in this Prospectus is accurate only as of the date of this Prospectus, regardless of the time of delivery of this Prospectus or of any sale of our common shares.
Dealer Prospectus Delivery Obligation
Until __________________ (90th day after the later of (1) the effective date of the registration statement; or (2) the first date on which the securities are offered publicly), all dealers that effect in transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
The date of this Prospectus is April ____, 2023.
Table of Contents
PROSPECTUS SUMMARY
We qualify all the forward-looking statements contained in this Prospectus by the following cautionary statements.
This Prospectus, and any supplement to this Prospectus include “forward-looking statements”. To the extent that the information presented in this Prospectus discusses financial projections, information or expectations about our business plans, results of operations, products or markets, or otherwise makes statements about future events, such statements are forward-looking. Such forward-looking statements can be identified by the use of words such as “intends”, “anticipates”, “believes”, “estimates”, “projects”, “forecasts”, “expects”, “plans” and “proposes”. Although we believe that the expectations reflected in these forward-looking statements are based on reasonable assumptions, there are a number of risks and uncertainties that could cause actual results to differ materially from such forward-looking statements. These include, among others, the cautionary statements in the “Risk Factors” section beginning on page 9 of this Prospectus and the “Management’s Discussion and Analysis of Financial Position and Results of Operations” section elsewhere in this Prospectus.
The company currently conducts business using its acquired trade names Standard Dental Labs Inc. and Prime Dental Lab LLC, operating its first acquired dental lab assets, and seeking additional lab operations to expand its holdings with the purpose of consolidating several smaller regional lab operations into a single owned and operated lab facility in various locations across the United States. Our initial operational focus is in Florida.
In May 2022 the company acquired the trade name Standard Dental Labs Inc. (“SDL”), as well as certain other assets including a fully developed branding package and business plan including a model for acquisition and consolidation of small to medium sized dental lab operations on a market-by-market basis across North America, from a company controlled by our majority shareholder, and sole officer and director, Mr. James Brooks.
On August 15, 2022, the company subsequently acquired its first operating assets , including a customer base and manufacturing equipment from its first target dental lab, Prime Dental Lab LLC, based in Florida, including the tradename Prime Dental Lab, LLC. (“PDL”) and commenced operations as PDL on September 1, 2022. The company’s existing dental lab operation includes the manufacture of dental prosthetics, including crowns, bridges and other oral prosthetics for sale directly to dental offices. In order to facilitate ongoing lab operations and seamlessly service its acquired customer base the company entered into a service contract with Mr. John Kim, whereunder Mr. Kim will provide ongoing labor, quality control and delivery services during a period of up to two years while the company seeks additional lab assets and operations in order to consolidate several independent lab operations into its first large regional lab facility in Florida. To this end the company has plans to acquire several additional independent lab operations from owners who may be looking to exit the market, by either retiring from the industry, or who may want to sell their businesses.
Acquiring additional lab operations will be the focus of our operational and growth strategy. Ultimately, the fixed assets and key employees of acquired lab operations will be consolidated into one regionally central lab on a market-by-market basis, and from where we can service various client lists with technicians working side by side, sharing resources and overhead. Economies of scale are expected to improve profitability, while the acquisition of lab operations will add incremental revenue to the company's income statement. All North American markets with populations over 1 million are targets. The company is currently headquartered in Orlando, Florida.
While the company’s management, specifically James Brooks, is primarily focused on growth and further acquisitions, day-to-day manufacturing is supervised by contracted dental lab managers and technicians. Once the company has acquired at least 3 labs, it expects to consolidate those operations into one, central facility where the company can benefit from economies of scale. Our goal is that this facility should be able to accommodate 75 people, which would accommodate 15 to 20 independent labs. There are numerous risks associated with our proposed acquisition program, as noted herein under “Risk Factors”, specifically including that such program is currently dependent upon the actions of our sole director and officer James Brooks, as the loss of Mr. Brooks would seriously and negatively impact such activities.
The creation of a central dental lab facility, and the amalgamation of small dental labs into this facility, could greatly enhance the profitability of the company by reducing the day-to-day overhead, and by giving technicians access to shared equipment they might not have otherwise had access to in a smaller facility, that makes the technicians more productive.
We have a limited operating history in the acquisition and operation of dental labs and have a history of net losses, which we anticipate will continue for the immediate future.
Actual results may vary from those expected. Undue reliance should not be placed on any forward-looking statements, which are appropriate only for the date made. We do not plan to subsequently revise these forward-looking statements to reflect current circumstances after the date of such statements or to reflect the occurrence of anticipated or unanticipated events.
Corporate Background
Costas, Inc. was incorporated in the State of Nevada on December 10, 1998. Costas was a development stage company that had a primary business plan to acquire, improve, and re-market undeveloped real estate in Las Vegas, Nevada and its surrounding communities.
The company had pursued a number of different industries since its inception in 1998, including real estate speculation, financial technologies in 2014 through 2018, and in 2019, the company attempted to acquire a surgical materials supplier in Mexico (such acquisition was not completed).
In 2017, James Brooks commenced an action against the company and was awarded a judgment in the 8th Circuit against the company for breach of contract, and non-payment. A portion of that judgment was later converted into shares, and a controlling interest in Costas, Inc. Specifically, on September 20, 2017, the court filed an order effective September 18, 2017, whereby Mr. Brooks, as a creditor of the company was granted a judgment against the company in the principal amount of $1,114,500. On October 21, 2020, Mr. Brooks filed a motion requesting the appointment of a Receiver over the company. On March 25, 2021, the Receiver filed a motion with the Court requesting approval to appoint Mr. Brooks as an officer and director of the company and to increase the authorized capital of the issuer and subsequently to issue sufficient common and preferred shares on terms to be finalized with Mr. Brooks, whereby Mr. Brooks became the controlling shareholder of the company. On February 9, 2022, an Order was entered by the Eighth Judicial District Court, Clark County, Nevada, Case No. A-17-749977D, terminating the receivership for the company.
Mr. Brooks is now guiding the company into becoming a Florida - based dental laboratory services company. Our current operations are conducted through our acquired tradenames, Standard Dental Labs Inc. and Prime Dental Lab LLC .
The Offering
The 328,973,333 shares of common stock offered by the Selling Security Holders will represent approximately 42.2% of our issued and outstanding common stock as of March 31 2023, assuming all of the notes are converted and all drawdowns under the Purchase Agreement are completed.
We have entered into a registration rights agreement pursuant to which we are obligated to register 8,333,333 of the shares being registered in this Prospectus. We are not currently a reporting company with the SEC. We are bearing all costs associated with registering the shares being offered.
| Common Stock Outstanding Prior to the Offering | | 445,728,363 shares (774,701,696 shares upon issuance of the shares in the offering) |
| | | | | |
| Common Stock to be Outstanding Following the Offering | | 774,701,696 shares |
| | | | | |
| Common Stock Offered | | 328,973,333 shares of common stock consisting of: |
| | | | | |
| | | | · | 319,890,000 shares of common stock underlying previously issued convertible promissory notes |
| | | | | |
| | | | · | 750,000 shares issued for the acquisition of certain assets |
| | | | | |
| | | | · | 8,333,333 shares issuable upon drawdowns under a Purchase Agreement. |
| | | | | |
| Offering Price | | The Selling Shareholders will sell common shares being offered at the fixed price of $0.00975 per share (estimated solely for the purpose of determining the amount of the registration fee pursuant to Rule 457(c) based on the average of the high and low prices of the common stock as reported on the OTC Markets on December 19, 2022). The Selling Security Holders may sell all or a portion of their shares through public or private transactions at the fixed price of $0.00975 per share until such time as the shares are listed on a national securities exchange or quoted on the OTCQB or OTCQX operated by the OTC Markets Inc. |
| | | | | |
| Aggregate Offering Price | | N/A |
| | | | | |
| Number of Selling Security Holders | | 19 |
| | | | | |
| Use of Proceeds | | We will not receive any of the proceeds of the shares offered by the Selling Security Holders. Our company will pay all the expenses of this offering estimated at approximately $50,000. |
| | | | | |
| Underwriters | | The Selling Security Holders are underwriters, within the meaning of section 2(a)(11) of the Securities Act. |
| | | | | |
| Plan of Distribution | | The Selling Security Holders named in this Prospectus are making this offering and may sell at market or privately negotiated prices. |
| World Amber Purchase Agreement On November 22, 2022, 2021, we entered into a purchase agreement (the “Purchase Agreement”), and a registration rights agreement (the “Registration Rights Agreement”) with World Amber pursuant to which World Amber committed to purchase up to $2,500,000 of our common stock. Under the terms and subject to the conditions of the Purchase Agreement, we have the obligation, to sell to World Amber, and World Amber is obligated to purchase up to $2,500,000 of shares of our common stock. Future sales of common stock under the Purchase Agreement, if any, will be subject to certain limitations, and may occur from time to time, over the 24-month period commencing on the date that a registration statement of which this prospectus forms a part, which we agreed to file with the Securities and Exchange Commission (the “SEC”) pursuant to the Registration Rights Agreement, is declared effective by the SEC and a final prospectus in connection therewith is filed and the other conditions set forth in the Purchase Agreement are satisfied (such date on which all of such conditions are satisfied, the “Commencement Date”). After the Commencement Date, for every month over the term of the Purchase Agreement, we have the right, in our sole discretion, to direct World Amber to purchase up to 346,667 shares of common stock per business day, at $0.30 per share (each, a “Regular Purchase”). In each case, World Amber’s maximum commitment in any single Regular Purchase may not exceed $104,000. Pursuant to the terms of the Purchase Agreement, in no event may we issue or sell to World Amber any the shares of our common stock under the Purchase Agreement which, when aggregated with all other shares of common stock then beneficially owned by the World Amber and its affiliates (as calculated pursuant to Section 13(d) of the Exchange Act and Rule 13d-3 promulgated thereunder), would result in the beneficial ownership by World Amber and its affiliates of more than 9.99% of the then issued and outstanding shares of common stock (the “Beneficial Ownership Limitation”). The Purchase Agreement and the Registration Rights Agreement contain customary representations, warranties, agreements, conditions and indemnification obligations of the parties. We have the right to terminate the Purchase Agreement at any time, at no cost or penalty. Issuances of our common stock in this aspect of the offering will not affect the rights or privileges of our existing stockholders, except that the economic and voting interests of each of our existing stockholders will be diluted as a result of any such issuance. Although the number of shares of common stock that our existing stockholders own will not decrease, the shares owned by our existing stockholders will represent a smaller percentage of our total outstanding shares after any such issuance to World Amber. |
This summary does not contain all the information that should be considered before making an investment in Costas, Inc.’s common stock. The entire prospectus should be read including the “Risk Factors” on page 9 and financial statements before deciding to invest in our common stock.
Financial Summary Information
All references to currency in this Prospectus are to U.S. Dollars, unless otherwise noted.
The following table sets forth selected financial information, which should be read in conjunction with the information set forth in the “Management’s Discussion and Analysis of Financial Position and Results of Operations” section and the accompanying financial statements and related notes included elsewhere in this Prospectus.
Income Statement Data
| | | Year Ended December 31, 2022 (audited) $ | | | Year Ended December 31, 2021 (audited) $ | |
| Revenue | | | 173,329 | | | | - | |
| Operating Expenses | | | 391,646 | | | | 76,727 | |
| Net Income (Loss) | | | (1,651,896 | ) | | | (10,254,127 | ) |
| Net Earnings (Loss) Per Share | | | (0.00 | ) | | | (0.21 | ) |
Balance Sheet Data
| | | As at December 31, 2022 (audited) | | | As at December 31, 2021 (audited) | |
| Working Capital (Deficit) | | | (1,685,506 | ) | | | (133,023 | ) |
| Total Assets | | | 188,013 | | | | - | |
| Total Liabilities | | | 1,698,629 | | | | (133,023 | ) |
RISK FACTORS
An investment in our common stock involves a high degree of risk. You should carefully consider the risks described below, together with all of the other information included in this report, before making an investment decision. If any of the following risks actually occurs, our business, financial condition or results of operations could suffer. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment. You should read the section entitled “special Note Regarding Forward Looking Statements” above for a discussion of what types of statements are forward-looking statements, as well as the significance of such statements in the context of this report.
You should carefully consider the risks described below. Together with all of the other information included in this report before making an investment decision with regard to our securities. The statements contained in, or incorporated into, this registration statement that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks occur, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Associated with Our Business
Our business operations are subject to a number of risks and uncertainties, including, but not limited to those set forth below:
Our company has no operating history and an evolving business model which raises doubt about our ability to achieve profitability or obtain financing.
Our company has no operating history. Moreover, our business model is still evolving, subject to change, and will rely on the ability to make additional acquisitions of dental labs on commercially viable terms. Our company's ability to continue as a going concern is dependent upon our ability to obtain adequate financing and to reach profitable levels of operations has and we no proven history of performance, earnings, or success. There can be no assurance that we will achieve profitability or obtain future financing.
Acquiring additional dental labs is a key aspect of our business and growth strategy.
In order to grow and achieve the level of operations and margins that we hope to achieve, a key component of our strategy is the acquisition and integration of additional dental lab operations. While we are confident in our ability to execute on this, there can be no assurances that we will be able to identify suitable dental labs for acquisition, or that we will be able to acquire such labs on commercially viable terms. In the event that we are unable to make such additional acquisitions, or successfully integrate any acquired labs into existing operations, our growth prospects will be severely limited.
Conflicts of interest between our company and our sole director and officer may result in a loss of business opportunity.
Our sole director and officer is not obligated to commit his full time and attention to our business and, accordingly, he may encounter a conflict of interest in allocating his time between our future operations and those of other businesses. In the course of his other business activities, he may become aware of investment and business opportunities which may be appropriate for presentation to us as well as other entities to which he owes a fiduciary duty. As a result, he may have conflicts of interest in determining to which entity a particular business opportunity should be presented. He may also in the future become affiliated with entities, engaged in business activities similar to those we intend to conduct.
In general, officers and directors of a corporation are required to present business opportunities to a corporation if:
| | · | the corporation could financially undertake the opportunity; |
| | · | the opportunity is within the corporation’s line of business; and |
| | · | it would be unfair to the corporation and its stockholders not to bring the opportunity to the attention of the corporation. |
We plan to adopt a code of ethics that obligates our directors, officers and employees to disclose potential conflicts of interest and prohibits those persons from engaging in such transactions without our consent. Despite our intentions, conflicts of interest may nevertheless arise which may deprive our company of a business opportunity, which may impede the successful development of our business and negatively impact the value of an investment in our company.
The speculative nature of our business plan may result in the loss of your investment.
Our operations are in the start-up or stage only and are unproven. We may not be successful in implementing our business plan to become profitable. There may be less demand for our services than we anticipate. There is no assurance that our business will succeed, and you may lose your entire investment.
General economic factors may negatively impact the market for our dental products.
The willingness of dental practices and their patients/consumers to spend money on our products may be dependent upon general economic conditions; and any material downturn may reduce the likelihood of such parties incurring costs toward what some consumers may consider a discretionary expense item.
A wide range of economic and logistical factors may negatively impact our operating results.
Our operating results will be affected by a wide variety of factors that could materially affect revenues and profitability, including the timing and cancellation of customer orders and projects, competitive pressures on pricing, availability of personnel, and market acceptance of our services. As a result, we may experience material fluctuations in future operating results on a quarterly and annual basis which could materially affect our business, financial condition and operating results.
If we fail to effectively and efficiently advertise, the growth of our business may be compromised.
The future growth and profitability of our business will be dependent in part on the effectiveness and efficiency of our advertising and promotional expenditures, including our ability to (i) create greater awareness of our services, (ii) determine the appropriate creative message and media mix for future advertising expenditures, and (iii) effectively manage advertising and promotional costs in order to maintain acceptable operating margins. There can be no assurance that we will experience benefits from advertising and promotional expenditures in the future. In addition, no assurance can be given that our planned advertising and promotional expenditures will result in increased revenues, will generate levels of service and name awareness or that we will be able to manage such advertising and promotional expenditures on a cost-effective basis.
Our success is dependent on our unproven ability to attract qualified personnel.
We depend on our ability to attract, retain and motivate our management team, consultants and advisors. There is strong competition for qualified technical and management personnel in the dental business sector, and it is expected that such competition will increase. Our planned growth will place increased demands on our existing resources and will likely require the addition of technical personnel and the development of additional expertise by existing personnel. There can be no assurance that our compensation packages will be sufficient to ensure the continued availability of qualified personnel who are necessary for the development of our business.
We have a limited operating history with losses, and we expect the losses to continue, which raises concerns about our ability to continue as a going concern.
We have generated minimal revenues since our inception and will, in all likelihood, continue to incur operating expenses with minimal revenues until we are able to successfully develop our business. Our business plan will require us to incur further expenses. We may not be able to ever become profitable. These circumstances raise concerns about our ability to continue as a going concern. We have a limited operating history and must be considered in the start-up stage.
There is an explanatory paragraph to their audit opinion issued in connection with the financial statements for the years ended December 31, 2022 and 2021, with respect to their doubt about our ability to continue as a going concern. As discussed in Note 2 to our financial statements for the years ended December 31, 2022 and 2021, we have incurred cumulative losses of $(16,531,420) and $(14,879,523) which raises substantial doubt about its ability to continue as a going concern. Our management has been able, thus far, to finance the operations through equity financing and cash on hand. There is no assurance that our company will be able to continue to finance our company on this basis.
Without additional financing to develop our business plan, our business may fail.
Because we have generated only minimal revenue from our business and cannot anticipate when we will be able to generate meaningful revenue from our business, we will need to raise additional funds to conduct and grow our business. We do not currently have sufficient financial resources to completely fund the development of our business plan. We anticipate that we will need to raise further financing. With the exception of our Purchase Agreement with World Amber, we do not currently have any other arrangements for financing, and we can provide no assurance to investors that we will be able to find such financing if required. The most likely source of future funds presently available to us is through the sale of equity capital. Any sale of share capital will result in dilution to existing security-holders.
We may not be able to obtain all of the licenses necessary to operate our business, which would cause our business to fail.
Our operations require licenses and permits from various governmental authorities related to the operation of our acquired dental laboratory facilities. We believe that we will be able to obtain all necessary licenses and permits under applicable laws and regulations for our operations and believe we will be able to comply in all material respects with the terms of such licenses and permits. However, such licenses and permits are subject to change in various circumstances. There can be no guarantee that we will be able to obtain or maintain all necessary licenses and permits.
If we are unable to recruit or retain qualified personnel, it could have a material adverse effect on our operating results and stock price.
Our success depends in large part on the continued services of our sole executive officer and third-party relationships. We currently do not have key person insurance on these individuals. The loss of these people, especially without advance notice, could have a material adverse impact on our results of operations and our stock price. It is also very important that we be able to attract and retain highly skilled personnel, including technical personnel, to accommodate our acquisition and expansion plans and to replace personnel who leave. Competition for qualified personnel can be intense, and there are a limited number of people with the requisite knowledge and experience. Under these conditions, we could be unable to recruit, train, and retain employees. If we cannot attract and retain qualified personnel, it could have a material adverse impact on our operating results and stock price.
If we fail to effectively manage our growth our future business results could be harmed and our managerial and operational resources may be strained.
As we proceed with our business plan, we expect to experience significant and rapid growth in the scope and complexity of our business. We will need to add staff to market our services, manage operations, handle sales and marketing efforts and perform finance and accounting functions. We will be required to hire a broad range of additional personnel in order to successfully advance our operations. This growth is likely to place a strain on our management and operational resources. The failure to develop and implement effective systems, or to hire and retain sufficient personnel for the performance of all of the functions necessary to effectively service and manage our potential business, or the failure to manage growth effectively, could have a materially adverse effect on our business and financial condition.
Coronavirus (COVID-19) Pandemic
On March 11, 2020 the World Health Organization declared the novel strain of coronavirus (“COVID-19”) a global pandemic and recommended containment and mitigation measures worldwide. The global outbreak of COVID-19 continues to rapidly evolve, and the extent to which COVID-19 may impact our business and the dental products and services market will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the ultimate geographic spread of the disease, the duration of the outbreak, travel restrictions and social distancing in the United States, Canada and other countries, business closures or business disruptions, and the effectiveness of actions taken in the United States and other countries to contain and treat the disease. We are continuing to vigilantly monitor the situation with our primary focus on the health and safety of our employees and contractors.
Risks Associated with the Shares of Our Company
Because we do not intend to pay any dividends on our shares, investors seeking dividend income or liquidity should not purchase our shares.
We have not declared or paid any dividends on our shares since inception, and do not anticipate paying any such dividends for the foreseeable future. We presently do not anticipate that we will pay dividends on any of our common stock in the foreseeable future. If payment of dividends does occur at some point in the future, it would be contingent upon our revenues and earnings, if any, capital requirements, and general financial condition. The payment of any common stock dividends will be within the discretion of our Board of Directors. We presently intend to retain all earnings to implement our business plan; accordingly, we do not anticipate the declaration of any dividends for common stock in the foreseeable future.
Investors seeking dividend income or liquidity should not invest in our shares.
Because we can issue additional shares, purchasers of our shares may incur immediate dilution and may experience further dilution.
We are authorized to issue up to 2,000,000,000 shares. The board of directors of our company has the authority to cause us to issue additional shares, and to determine the rights, preferences and privileges of such shares, without consent of any of our stockholders. Consequently, our stockholders may experience more dilution in their ownership of our company in the future.
Risks Associated with our Market and Governing Documents
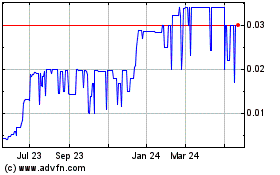
Trading on the OTC markets may be volatile and sporadic, which could depress the market price of our common stock and make it difficult for our stockholders to resell their shares.
Our common stock is quoted on the OTC Pink tier operated by OTC Markets Group Inc. Trading in stock quoted on the OTC markets is often thin and characterized by wide fluctuations in trading prices, due to many factors that may have little to do with our operations or business prospects. This volatility could depress the market price of our common stock for reasons unrelated to operating performance. Moreover, the OTC Pink tier not a stock exchange, and trading of securities on the OTC is often more sporadic than the trading of securities listed on a quotation system like Nasdaq or a stock exchange like the NYSE. Accordingly, shareholders may have difficulty reselling any of the shares.
Our stock is a penny stock. Trading of our stock may be restricted by the Securities and Exchange Commission’s penny stock regulations which may limit a stockholder’s ability to buy and sell our stock.
Our stock is a penny stock. The Securities and Exchange Commission has adopted Rule 15g-9 which generally defines “penny stock” to be any equity security that has a market price (as defined) less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exceptions. Our securities are covered by the penny stock rules, which impose additional sales practice requirements on broker-dealers who sell to persons other than established customers and “accredited investors”. The term “accredited investor” refers generally to institutions with assets in excess of $5,000,000 or individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 jointly with their spouse. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document in a form prepared by the Securities and Exchange Commission which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information, must be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing before or with the customer’s confirmation. In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from these rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for the stock that is subject to these penny stock rules. Consequently, these penny stock rules may affect the ability of broker-dealers to trade our securities. We believe that the penny stock rules discourage investor interest in and limit the marketability of our common stock.
The Financial Industry Regulatory Authority, or FINRA, has adopted sales practice requirements which may also limit a stockholder’s ability to buy and sell our stock.
In addition to the “penny stock” rules described above, FINRA has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low-priced securities will not be suitable for at least some customers. FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares.
Any change to government regulation/administrative practices may have a negative impact on our ability to operate and our profitability.
The laws, regulations, policies or current administrative practices of any government body, organization or regulatory agency in the United States, Canada, or any other jurisdiction, may be changed, applied or interpreted in a manner which will fundamentally alter the ability of our company to carry on our business.
The actions, policies or regulations, or changes thereto, of any government body or regulatory agency, or other special interest groups, may have a detrimental effect on us. Any or all of these situations may have a negative impact on our ability to operate and/or our profitably.
Because we can issue additional shares, purchasers of our shares may incur immediate dilution and may experience further dilution.
We are authorized to issue up to 2,000,000,000 shares. The board of directors of our company has the authority to cause us to issue additional shares, and to determine the rights, preferences and privileges of such shares, without consent of any of our stockholders. Consequently, our stockholders may experience more dilution in their ownership of our company in the future.
Our by-laws contain provisions indemnifying our officers and directors against all costs, charges and expenses incurred by them.
Our by-laws contain provisions with respect to the indemnification of our officers and directors against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, actually and reasonably incurred by him, including an amount paid to settle an action or satisfy a judgment in a civil, criminal or administrative action or proceeding to which he is made a party by reason of his being or having been one of our directors or officers.
Investors’ interests in our company will be diluted and investors may suffer dilution in their net book value per share if we issue additional shares or raise funds through the sale of equity securities.
Our constating documents authorize the issuance of 2,000,000,000 shares of common stock with a par value of $0.001. In the event that we are required to issue any additional shares or enter into private placements to raise financing through the sale of equity securities, investors” interests in our company will be diluted and investors may suffer dilution in their net book value per share depending on the price at which such securities are sold. If we issue any such additional shares, such issuances also will cause a reduction in the proportionate ownership and voting power of all other shareholders. Further, any such issuance may result in a change in our control.
We are authorized to issue up to 2,000,000,000 shares. The board of directors of our company have the authority to cause us to issue additional shares, and to determine the rights, preferences and privileges of such shares, without consent of any of our stockholders. Consequently, our stockholders may experience more dilution in their ownership of our company in the future.
Our by-laws do not contain anti-takeover provisions, which could result in a change of our management and directors if there is a take-over of our company.
We do not currently have a shareholder rights plan or any anti-takeover provisions in our By-laws. Without any anti-takeover provisions, there is no deterrent for a take-over of our company, which may result in a change in our management and directors.
Risks related to unknown trends, risks and uncertainties.
We have sought to identify what we believe to be the most significant risks to our business, but we cannot predict whether, or to what extent, any of such risks may be realized. There are numerous possible scenarios with sufficient potential impact to risk the future of the company as an independent business operating in the dental lab industry. For example, significant reputational impact as a result of a major issue resulting in injury to patients, possibly compounded by apparently negligent management behavior; extreme adverse press or social media coverage, would lead to a catastrophic share price fall, very significant loss of consumer confidence and inability to retain and recruit quality people. Investors should carefully consider all of such risk factors before making an investment decision with respect to our common shares.
USE OF PROCEEDS
This Prospectus relates to our common stock shares that will be offered on a continuous basis by the Selling Security Holders beginning immediately after the registration statement’s effective date, which is included in this Prospectus, and may continue for a period in excess of ninety (90) days from this effective date. We are completing this registration statement to allow the Selling Security Holders to sell their shares. The Selling Security Holders will receive all proceeds from this offering and, if all of the shares being offered by this Prospectus are sold at $0.00975 per share, those proceeds would be $3,207,490.00 (estimated based on, solely for the purpose of determining the amount of the registration fee pursuant to Rule 457(c), the average of the high and low prices of the common stock as reported on the OTC on December 19, 2022). The Selling Security Holders may actually sell all or a portion of their shares through public or private transactions at prevailing market prices or at privately negotiated prices.
We, the issuer, will not receive any of the proceeds from the common stock sale by the Selling Security Holders in this offering. Our company will pay all expenses of this offering estimated at $50,000. See Part II, Item 13.
This prospectus also relates to shares of our common stock that may be offered and sold from time to time by World Amber. We may receive up to $2,500,000 aggregate gross proceeds under the Purchase Agreement from any sales we make to World Amber pursuant to the Purchase Agreement after the date of this prospectus. However, we may not be registering for sale or offering for resale under the registration statement of which this prospectus is a part all of the shares issuable pursuant to the Purchase Agreement.
In any event, we will receive no proceeds from the sale of any shares of common stock by World Amber pursuant to this prospectus. As we are unable to predict the timing or amount of potential issuances of all of the shares offered hereby, we have not allocated any proceeds of such issuances to any particular purpose. Accordingly, all such proceeds actually received under the Purchase Agreement are expected to be used for general working capital and general corporate purposes. Regardless of the actual proceeds raised, we intend to apply available proceeds in the following approximate order of priority: general corporate maintenance, compensation of essential employees and/or consultants, professional fees and expenses related to our public reporting requirements, and expenses related to the evaluation and acquisition of additional dental labs. In the event the proceeds actually received under the Purchase Agreement are insufficient for our planned purposes, we intend to limit or defer our planned acquisition activities, until such time as we have sufficient working capital.
Pending other uses, we intend to invest any proceeds from the offering in short-term investments or hold them as cash. We cannot predict whether the proceeds invested will yield a favorable return. Our management will have broad discretion in the use of the net proceeds from this offering, and investors will be relying on the judgment of our management regarding the application of the net proceeds.
DETERMINATION OF OFFERING PRICE
The Selling Security Holders will sell their shares at prevailing market prices or privately negotiated prices. The number of securities that may be actually sold by a Selling Shareholder will be determined by each Selling Shareholder. The Selling Security Holders are under no obligation to sell all or any portion of the securities offered, nor are the Selling Security Holders obligated to sell such shares immediately under this Prospectus. A security holder may sell securities at any price depending on privately negotiated factors, such as a “shareholders” own cash requirements, or objective criteria of value such as the market value of our assets.
We have arbitrarily established the offering price of the common stock and it should not be considered to bear any relationship to our assets, book value or net worth and should not be considered to be an indication of our value. No valuation or appraisal has been prepared for our business.
Among the factors considered by our management were:
| | · | the market price for our common stock on the OTC; |
| | · | the potential of our acquisition program; |
| | · | our capital structure; |
| | · | the background of our management; and |
| | · | our cash requirements relative to our business operations. |
WORLD AMBER TRANSACTION
General
On November 22, 2022 (the “Execution Date”), we entered into a Purchase Agreement and a Registration Rights Agreement with World Amber. Pursuant to the terms of the Purchase Agreement, World Amber has agreed to purchase from us up to $2,500,000 of our common stock from time to time during the term of the Purchase Agreement, subject to certain limitations.
We do not have the right to commence any sales to World Amber under the Purchase Agreement until the Commencement Date (defined below) has occurred. Thereafter, we may, from time to time, in any one month period, direct World Amber to purchase shares of our common stock in amounts up to 346,667 shares, and subject to a maximum commitment by World Amber of $104,000 per Regular Purchase. The purchase price per share sold will be $0.30 per share of common stock. World Amber may not assign or transfer its rights and obligations under the Purchase Agreement.
Pursuant to the terms of the Purchase Agreement, in no event may we issue or sell to World Amber any shares of our common stock under the Purchase Agreement which, when aggregated with all other shares of Common Stock then beneficially owned by World Amber and its affiliates (as calculated pursuant to Section 13(d) of the Exchange Act and Rule 13d-3 promulgated thereunder), would result in the beneficial ownership by World Amber and its affiliates of more than 9.99% of the then issued and outstanding shares of common stock.
Pursuant to the Registration Rights Agreement, the company is required to register the shares of common stock that may be issued to World Amber under the Purchase Agreement. We have filed the registration statement with the SEC that includes this prospectus to register for resale under the Securities Act, up to 8,333,333 shares of common stock, representing 18% of our issued and outstanding shares of common stock on December 19, 2022 and assuming all shares under the Purchase Agreement are issued
Purchase of Shares Under the Purchase Agreement
Under the terms and subject to the conditions of the Purchase Agreement, the Company has the obligation, to sell to World Amber, and World Amber is obligated to purchase up to $2,500,000 of shares of common stock. Such sales of common stock by the Company, if any, will be subject to certain limitations, and may occur from time to time, over the 24-month period commencing on the Commencement Date when the registration statement covering the resale of shares of common stock that have been and may be issued under the Purchase Agreement, which the Company agreed to file with the SEC pursuant to the Registration Rights Agreement, is declared effective by the SEC and a final prospectus in connection therewith is filed and the other conditions set forth in the Purchase Agreement are satisfied, all of which are outside the control of World Amber.
Under the Purchase Agreement, in any one-month period over the term of the Purchase Agreement, the Company has the right to present World Amber with a purchase notice (each, a "Purchase Notice") directing World Amber to complete a Regular Purchase up to 346,667 shares of common stock per month In each case, World Amber's maximum commitment in any single Regular Purchase may not exceed $104,000. The Purchase Agreement provides for a purchase price per Purchase Share (the "Purchase Price") equal to $0.30 per Share.
Events of Default
Events of default under the Purchase Agreement include the following:
| | · | the effectiveness of a registration statement registering the resale of the common stock issued or issuable to World Amber lapses for any reason (including, without limitation, the issuance of a stop order or similar order) or such registration statement (or the prospectus forming a part thereof) is unavailable to World Amber for resale of any or all of the Securities to be issued to World Amber, and such lapse or unavailability continues for a period of ten (10) consecutive Business Days or for more than an aggregate of thirty (30) Business Days in any 365-day period, but excluding a lapse or unavailability where (i) the Company terminates a registration statement after World Amber has confirmed in writing that all of the Securities covered thereby have been resold or (ii) the Company supersedes one registration statement with another registration statement, including (without limitation) by terminating a prior registration statement when it is effectively replaced with a new registration statement covering Securities (provided in the case of this clause (ii) that all of the Securities covered by the superseded (or terminated) registration statement that have not theretofore been resold are included in the superseding (or new) registration statement); |
| | · | the suspension of the common stock from trading on the OTCQB or any other market on which the common stock trades for a period of one (1) Business Day, provided that the Company may not direct World Amber to purchase any shares of common stock during any such suspension; |
| | · | the delisting of the common stock from the OTCQB, provided, however, that the common stock is not immediately thereafter trading on The Nasdaq Capital Market, the New York Stock Exchange, The Nasdaq Global Market, The Nasdaq Global Select Market, the NYSE American, the NYSE Arca, the OTC Bulletin Board, the OTCQX operated by the OTC Markets Group, Inc. (or nationally recognized successor to any of the foregoing); |
| | · | the failure for any reason by the Company’s Transfer Agent to issue Purchase Shares to World Amber within two (2) Business Days after the applicable Purchase Date, on which World Amber is entitled to receive such Purchase Shares; |
| | · | the Company breaches any representation, warranty, covenant or other term or condition under any Transaction Document if such breach has or could have a Material Adverse Effect (as defined in the Purchase Agreement) and except, in the case of a breach of a covenant which is reasonably curable, only if such breach continues for a period of at least five (5) Business Days; |
| | · | if any Person commences a proceeding against the Company pursuant to or within the meaning of any Bankruptcy Law; |
| | · | if the Company is at any time insolvent, or pursuant to or within the meaning of any Bankruptcy Law, the Company (i) commences a voluntary case, (ii) consents to the entry of an order for relief against it in an involuntary case, (iii) consents to the appointment of a custodian of it or for all or substantially all of its property, or (iv) makes a general assignment for the benefit of its creditors or is generally unable to pay its debts as the same become due; |
| | · | a court of competent jurisdiction enters an order or decree under any Bankruptcy Law that (i) is for relief against the Company in an involuntary case, (ii) appoints a Custodian of the Company or for all or substantially all of its property, or (iii) orders the liquidation of the Company or any Subsidiary; or |
| | · | if at any time the Company is not eligible to transfer its common stock electronically as DWAC Shares. |
World Amber does have the right to terminate the Purchase Agreement upon any of the events of default set forth above. During an event of default, all of which are outside of World Amber’s control, we may not direct World Amber to purchase any shares of our common stock under the Purchase Agreement.
Termination Rights of the Company
We have the unconditional right, at any time, for any reason and without any payment or liability to World Amber, to give notice to World Amber to terminate the Purchase Agreement.
No Short-Selling or Hedging by World Amber
World Amber has agreed that neither it nor any of its affiliates shall engage in any direct or indirect short-selling or hedging of our common stock during any time prior to the termination of the Purchase Agreement.
Effect of Performance of the Purchase Agreement on Our Stockholders
All 8,333,333 shares registered in this offering which have been and may be issued or sold by us to World Amber under the Purchase Agreement are expected to be freely tradable. It is anticipated that shares registered in this offering may be sold over a period of up to 24-months commencing on the date that the registration statement including this prospectus becomes effective. The sale by World Amber of a significant number of shares registered in this offering at any given time could cause the market price of our common stock to decline and to be highly volatile. Sales of our common stock to World Amber, if any, will depend upon market conditions and other factors to be determined by us. We may ultimately decide to sell to World Amber all, some or none of the additional shares of our common stock that may be available for us to sell pursuant to the Purchase Agreement. If and when we do sell shares to World Amber, after World Amber has acquired the shares, World Amber may resell all, some or none of those shares at any time or from time to time in its discretion. Therefore, sales to World Amber by us under the Purchase Agreement may result in substantial dilution to the interests of other holders of our common stock. In addition, if we sell a substantial number of shares to World Amber under the Purchase Agreement, or if investors expect that we will do so, the actual sales of shares or the mere existence of our arrangement with World Amber may make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect such sales. However, we have the right to control the timing and amount of any additional sales of our shares to World Amber and the Purchase Agreement may be terminated by us at any time at our discretion without any cost to us.
The Purchase Agreement prohibits us from issuing or selling to World Amber under the Purchase Agreement any shares of our common stock if those shares, when aggregated with all other shares of our common stock then beneficially owned by World Amber and its affiliates, would exceed the Beneficial Ownership Limitation.
DILUTION
The Company intends to sell 8,333,333 shares of common stock, to World Amber, at a purchase price of $0.30 per share for the duration of the Offering (the “Offering”) for a period of two years from the effective date of this prospectus, unless extended by our Board of Directors for up to an additional ninety (90) days. Once a “Put Notice” is presented to World Amber, shares will be issued to World Amber. The Company is registering these shares on behalf of the selling shareholder. The following table sets forth the number of shares of Common Stock being registered for sale, the total consideration paid and the price per share. The table assumes all 8,333,333 shares of Common Stock will be sold.
| | | Shares Issued | | | Total Consideration | | | | |
| | | Number of Shares | | | Percent | | | Amount | | | Percent | | | Price Per Share | |
| Purchasers of Shares | | | 8,333,333 | | | | 100 | % | | $ | 2,500,000 | | | | 100 | % | | $ | 0.30 | |
| Total | | | 8,333,333 | | | | 100 | % | | $ | 2,500,000 | | | | 100 | % | | | | |
The following table sets forth the difference between the offering price of the shares of our Common Stock being sold by the Company to World Amber, and registered to World Amber, the net tangible book value per share, and the net tangible book value per share after giving effect to the Offering by us, assuming that 100%, 75%, 50%, 25% and 10% of the offered shares are sold. Net tangible book value per share represents the amount of total tangible assets less total liabilities at December 31, 2022, divided by the number of shares outstanding as of December 31, 2022. Totals may vary due to rounding. Note - the table below does not reflect offering costs estimated to be $50,000 which will be borne by the Company and not deducted from gross proceeds.
| | 100% of offered shares are sold | 75% of offered shares are sold | 50% of offered shares are sold | 25% of offered shares are sold | 10% of offered shares are sold |
| Offering Price | $0.30 per share | $0.30 per share | $0.30 per share | $0.30 per share | $0.30 per share |
| Net tangible book value at December 31, 2022 (1) | ($0.003) per share | ($0.003) per share | ($0.003) per share | ($0.003) per share | ($0.003) per share |
| Net tangible book value after giving effect to the Offering proceeds | $0.002 per share | $0.001 per share | $(0.001) per share | $(0.002) per share | $(0.003) per share |
| Increase in net tangible book value per share attributable to cash payments made by new investors | $0.005 per share | $0.004 per share | $0.002 per share | $0.001 per share | $0.00 per share |
| Per Share Dilution to New Investors | $0.298 per share | $0.299 per share | $0.301 per share | $0.302 per share | $0.303 per share |
| Percent Dilution to New Investors | 99% | 100% | 100% | 101% | 101% |
| (1) | Based on 445,728,363 shares issued and outstanding as of December 31, 2022 |
SELLING SECURITY HOLDERS
We are registering an aggregate of 328,973,333 shares of common stock held by the Selling Security Holders. The Selling Security Holders have the option to sell the 328,973,333 shares of our common stock at prevailing market prices or privately negotiated prices.
This Prospectus covers the offering of up to an aggregate of 328,973,333 shares of common stock, consisting of: 319,890,000 shares of common stock underlying previously issued convertible promissory notes issued by our company to certain Selling Security Holders; 750,000 shares issued pursuant to one Selling Security Holder for the acquisition of certain assets; and 8,333,333 shares issuable upon drawdowns under the Purchase Agreement with another Selling Security Holder, World Amber Corporation. The aggregate of 328,973,333 common shares issued or issuable to the nine Selling Security Holders are restricted under applicable federal and state security laws and are being registered to give them the opportunity to sell their shares.
They are offering for sale a total of 328,973,333 shares of common stock of our company. This comprises approximately 33% percent of the total issued and outstanding shares assuming all convertible notes are converted, and the Purchase Agreement is fully draw down. To the best of our knowledge, the Selling Security Holders have sole voting and investment power and rights over all their shares and are the beneficial owners. They have given all information regarding share ownership. The shares being offered are being registered to permit public secondary trading and the Selling Security Holders may offer all or part of their respective shares from time to time but is under no obligation to immediately sell them pursuant to this Prospectus. Thus, our company cannot guarantee that any shares will be sold after this registration statement is declared effective.
The offering of 319,890,000 shares of our issued and outstanding common stock by the Selling Security Holders were originally issued or are issuable pursuant to private placements, transactions or agreements as described herein.
Our common stock is quoted on the OTC Pink tier under the trading symbol “CSSI”.
The Selling Security Holders will have the option to sell their shares at an initial offering price of $0.00975 per share (estimated solely for the purpose of determining the amount of the registration fee pursuant to Rule 457(c) based on the average of the high and low prices of the common stock as reported on the OTC Markets on December 19, 2022) or at prevailing market prices or privately negotiated prices.
All of these shares were issued in reliance upon an exemption from registration pursuant to Section 4(2), Regulation S, or Regulation D under the Securities Act of 1933 (the “Securities Act ”). Our reliance upon Rule 903 of Regulation S was based on the fact that the sales of the securities were completed in an “offshore transaction”, as defined in Rule 902(h) of Regulation S. We did not engage in any directed selling efforts, as defined in Regulation S, in the United States in connection with the sale of the securities.
The following table provides information as of March 31, 2023, regarding the beneficial ownership of our common stock by each of the Selling Security Holders, including:
| | · | the identity of the beneficial holder that owns the shares being offered |
| | | |
| | · | the number of shares owned by each prior to this offering; |
| | | |
| | · | the number of shares being offered by each; |
| | | |
| | · | the number of shares that will be owned by each upon completion of the offering, assuming that all the shares being offered are sold; and |
| | | |
| | · | the percentage of shares owned by each. |
Additional information regarding the holders of the 319,890,000 common shares underlying the convertible promissory notes, the 750,000 common shares issued pursuant to the asset acquisition with Prime Dental Lab LLC and the 8,333,333 common shares issuable pursuant to the Purchase Agreement with World Amber is included the following table.
| Name of Selling Security Holder | | Shares underlying Convertible Promissory Notes Owned Prior to this offering | | | Percent % | | | Maximum Number Shares being offered | | | Beneficial Ownership after offering | | | Percentage of Owned upon completion of the offering | |
| Ilya Aharon | | | 10,000,000 | | | | 1.3 | | | | 10,000,000 | | | | 0 | | | | 0 | |
| Yohanan Aharon | | | 20,000,000 | | | | 2.6 | | | | 20,000,000 | | | | 0 | | | | 0 | |
| Rosa Shimonov | | | 20,000,000 | | | | 2.6 | | | | 20,000,000 | | | | 0 | | | | 0 | |
| Aaron Abraham | | | 12,000,000 | | | | 1.5 | | | | 12,000,000 | | | | 0 | | | | 0 | |
| Shannon Sekhri | | | 20,900,000 | | | | 2.7 | | | | 20,900,000 | | | | 0 | | | | 0 | |
| Nirmal Sekhri | | | 119,240,000 | | | | 15.4 | | | | 119,240,000 | | | | 0 | | | | 0 | |
| Kenneth Brown | | | 10,000,000 | | | | 1.3 | | | | 10,000,000 | | | | 0 | | | | 0 | |
| Maddalena Popowich | | | 20,000,000 | | | | 2.6 | | | | 20,000,000 | | | | 0 | | | | 0 | |
| David Popowich | | | 5,000,000 | | | | 0.6 | | | | 5,000,000 | | | | 0 | | | | 0 | |
| George Alvarez Professional Corp | | | 20,000,000 | | | | 2.6 | | | | 20,000,000 | | | | 0 | | | | 0 | |
| Faisal Karmali | | | 25,000,000 | | | | 3.2 | | | | 25,000,000 | | | | 0 | | | | 0 | |
| Daniel & Debra Burrows | | | 2,500,000 | | | | 0.3 | | | | 2,500,000 | | | | 0 | | | | 0 | |
| Alek Dvoskin | | | 3,750,000 | | | | 0.5 | | | | 3,750,000 | | | | 0 | | | | 0 | |
| Vanessa Iorio | | | 3,250,000 | | | | 0.4 | | | | 3,250,000 | | | | 0 | | | | 0 | |
| Scott and Jennifer Sucharzewski | | | 2,500,000 | | | | 0.3 | | | | 2,500,000 | | | | 0 | | | | 0 | |
| Brian Koprowski and Vanessa D Belmares | | | 500,000 | | | | 0.1 | | | | 500,000 | | | | 0 | | | | 0 | |
| Luke Jacob Pascale | | | 12,500,000 | | | | 1.6 | | | | 12,500,000 | | | | | | | | | |
| Arcanumus Capital (Nirmal Sekhri) | | | 9,000,000 | | | | 1.2 | | | | 9,000,000 | | | | 0 | | | | 0 | |
| William Diaz | | | 3,750,000 | | | | 0.5 | | | | 3,750,000 | | | | 0 | | | | 0 | |
| | | | 319,890,000 | | | | 41 | % | | | 319,890,000 | | | | 0 | | | | 0 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | Shares previously issued pursuant to asset acquisition | | | | | | | | | | | | | | | | | |
| John Jonpil Kim | | | 750,000 | | | | 0.1 | % | | | 750,000 | | | | 0 | | | | 0 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | Shares issuable underlying Purchase Agreement | | | | | | | | | | | | | | | | | |
| World Amber Corporation(4) | | | 8,333,333 | | | | 1.1 | % | | | 8,333,333 | | | | 0 | | | | 0 | |
| Total | | | 328,973,333 | | | | 42.5 | % | | | 328,973,333 | | | | 0 | | | | 0 | |
NOTES:
| (1) | The number and percentage of shares beneficially owned is determined to the best of our knowledge in accordance with the Rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares as to which the selling security holder has sole or shared voting or investment power and also any shares which the selling security holder has the right to acquire within 60 days of the date of this Prospectus. |
| | |
| (2) | The percentages are based on a diluted basis assuming all 319,890,000 shares are issued upon conversion of the notes, 8,333,333 shares are issued under the Purchase Agreement and based on 445,728,363 shares of our common stock issued and outstanding as at March 31, 2023 for an aggregate of 774,701,696 issued and outstanding shares post-conversion. |
| | |
| (3) | Yohanan Aharon, the Chief Executive Officer of World Amber Corporation, is deemed to be beneficial owner of all of the shares of common stock owned by World Amber Corporation. Mr. Aharon has voting and investment power over the shares being offered under the prospectus filed with the SEC in connection with the transactions contemplated under the Purchase Agreement. World Amber Corporation is not a licensed broker dealer or an affiliate of a licensed broker dealer. |
Except as otherwise noted in the above lists, the named party beneficially owns and has sole voting and investment power over all the shares or rights to the shares. The numbers in this table assume that none of the Selling Security Holders will sell shares not being offered in this Prospectus or will purchase additional shares and assumes that all the shares being registered will be sold. There has been no material relationship between any of the Selling Security Holders and the company and its affiliates during the prior three years.
PLAN OF DISTRIBUTION
We are registering an aggregate of 328,973,333 shares of common stock, issuable pursuant to: the Purchase Agreement with World Amber, shares of common stock previously issued to Prime Dental Lab LLC, and shares of common stock underlying previously issued convertible promissory notes by our company, by Selling Security Holders. The Selling Security Holders have the option to sell the 328,973,333 shares of our common stock at prevailing market prices or privately negotiated prices.
The shares may be sold in a lawful manner using any one or more of the following methods: private transaction; ordinary brokerage transactions; transactions in which the broker-dealer solicits purchasers; broker-dealer as principal purchasers and resale by the broker-dealer for its own account; block trades in which the broker-dealer will attempt to sell the shares as an agent, but may position and resell a portion of the block as principal to facilitate the transaction; broker-dealer agreements with the selling shareholder to sell a specified number of such shares at a stipulated price per share; exchange distribution following the rules of the applicable exchange; short sales that are not violations of the laws and regulations of any state of the United States; through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; or through a combination of any such methods or other lawful means.
The Selling Security Holders are underwriters, within the meaning of section 2(a)(11) of the Securities Act. Any broker-dealers or agents that participate in the sale of the common stock or interests therein may also be deemed to be an “underwriter” within the meaning of section 2(a)(11) of the Securities Act. Any discounts, commissions, concessions or profit earned on any resale of the shares may be underwriting discounts and commissions under the Securities Act. The Selling Security Holders, who are “underwriters” within the meaning of section 2(a)(11) of the Securities Act, are subject to the prospectus delivery requirements of the Securities Act.
The brokers or dealers may receive commissions or discounts from the Selling Security Holders, if any of the broker-dealer acts as an agent for the purchaser of said shares, from the purchaser in the amount to be negotiated which are not expected to exceed those customary in the types of transactions involved. Broker-dealers may agree with the Selling Security Holders to sell a specified number of the shares of common stock at a stipulated price per share. In connection with such re-sales, the broker-dealer may pay to or receive from the purchasers of the shares, commissions as described above. Any broker or dealer participating in any distribution of the shares may be required to deliver a copy of this Prospectus, including any prospectus supplement, to any individual who purchases any shares from or through such broker-dealer.
Our common stock is quoted on the Pink tier of the OTC markets.
Trading in stocks quoted on the OTC is often thin and is characterized by wide fluctuations in trading prices due to many factors that may have little to do with a company’s operations or business prospects. The OTC should not be confused with the NASDAQ market. OTC companies are subject to far less restrictions and regulations than companies whose securities are traded on the NASDAQ market. Moreover, the OTC is not a stock exchange, and the trading of securities on the OTC is often more sporadic than the trading of securities listed on a quotation system like the NASDAQ Small Cap or a stock exchange. In the absence of an active trading market investors may have difficulty buying and selling or obtaining market quotations for our common stock and its market visibility may be limited, which may have a negative effect on the market price of our common stock.
We are bearing all costs relating to the registration of our common stock. The Selling Security Holders, however, will pay any commissions or other fees payable to brokers or dealers in connection with any sale of the shares of our common stock.
The Selling Security Holders must comply with the requirements of the Securities Act and the Exchange Act in the offer and sale of our common stock. In particular, during such times as the Selling Security Holders may be deemed to be engaged in a distribution of any securities, and therefore be considered to be an underwriter, they must comply with applicable laws and may, among other things:
| | · | furnish each broker or dealer through which our common stock may be offered such copies of this Prospectus, as amended from time to time, as may be required by such broker or dealer; |
| | | |
| | · | not engage in any stabilization activities in connection with our securities; and |
| | | |
| | · | not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities other than as permitted under the Exchange Act. |
Regulation M
During such time as the Selling Security Holders may be engaged in a distribution of any of the securities being registered by this Prospectus, the Selling Security Holders are required to comply with Regulation M under the Exchange Act. In general, Regulation M precludes any selling security holder, any affiliated purchaser and any broker-dealer or other person who participates in a distribution from bidding for or purchasing or attempting to induce any person to bid for or purchase, any security that is the subject of the distribution until the entire distribution is complete.
Regulation M defines a “distribution” as an offering of securities that is distinguished from ordinary trading activities by the magnitude of the offering and the presence of special selling efforts and selling methods. Regulation M also defines a “distribution participant as an underwriter, prospective underwriter, broker, dealer, or other person who has agreed to participate or who is participating in a distribution”.
Regulation M prohibits, with certain exceptions, participants in a distribution from bidding for or purchasing, for an account in which the participant has a beneficial interest, any of the securities that are the subject of the distribution. Regulation M also governs bids and purchases made in order to stabilize the price of a security in connection with a distribution of the security. We have informed the Selling Security Holders that the anti- manipulation provisions of Regulation M may apply to the sales of their shares offered by this Prospectus, and we have also advised the Selling Security Holders of the requirements for delivery of this Prospectus in connection with any sales of the shares offered by this Prospectus.
With regard to short sales, the Selling Security Holders cannot cover their short sales with securities from this offering. In addition, if a short sale is deemed to be a stabilizing activity, then the Selling Security Holders will not be permitted to engage in such an activity. All of these limitations may affect the marketability of our common stock.
The Selling Security Holders may also elect to sell their common shares in accordance with Rule 144 under the Securities Act, rather than pursuant to this Prospectus. After the sale of the shares offered by this Prospectus the Selling Security Holders will have no common shares. The sale of these shares could have an adverse impact on the price of our shares or on any trading market that is developed.
We have not registered or qualified offers and sales of shares of common stock under the laws of any country, other than the United States. To comply with certain states” securities laws, if applicable, the Selling Security Holders will offer and sell their shares of common stock in such jurisdictions only through registered or licensed brokers or dealers. In addition, in certain states the Selling Security Holders may not offer or sell shares of common stock unless we have registered or qualified such shares for sale in such states or we have complied with an available exemption from registration or qualification.
All expenses of this registration statement, estimated to be approximately $50,000 including but not limited to legal, accounting, printing and mailing fees will, be paid by our company. However, any selling costs or brokerage commissions incurred by each Selling Security Holder relating to the sale of their shares will be paid by them. See “Use of Proceeds” on page 16.
Penny Stock Rules
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00 (other than securities registered on certain national securities exchanges, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system).
The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document prepared by the SEC which:
| | · | contains a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading; |
| | | |
| | · | contains a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to violations of such duties or other requirements of federal securities laws; |
| | | |
| | · | contains a brief, clear, narrative description of a dealer market, including “bid” and “ask” prices for penny stocks and the significance of the spread between the bid and ask prices; |
| | | |
| | · | contains the toll-free telephone number for inquiries on disciplinary actions; |
| | | |
| | · | defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and |
| | | |
| | · | contains such other information, and is in such form (including language, type size, and format) as the SEC shall require by rule or regulation. |
Prior to effecting any transaction in a penny stock, a broker-dealer must also provide a customer with:
| | · | the bid and ask prices for the penny stock; |
| | | |
| | · | the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; |
| | | |
| | · | the amount and a description of any compensation that the broker-dealer and its associated salesperson will receive in connection with the transaction; and |
| | | |
| | · | a monthly account statement indicating the market value of each penny stock held in the customer’s account. |
In addition, the penny stock rules require that prior to effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement, (ii) a written agreement to transactions involving penny stocks, and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our securities, and therefore our stockholders may have difficulty selling their shares.
Blue Sky Restrictions on Resale
When a Selling Security Holder wants to sell shares of our common stock under this Prospectus in the United States, the Selling Security Holder will need to comply with state securities laws, also known as “blue sky laws”, with regard to secondary sales. All states offer a variety of exemptions from registration of secondary sales. Many states, for example, have an exemption for secondary trading of securities registered under section 12(g) of the Exchange Act or for securities of issuers that publish continuous disclosure of financial and non-financial information in a recognized securities manual, such as Standard & Poor’s. The broker for a selling security holder will be able to advise the stockholder as to which states have an exemption for secondary sales of our common stock.
Any person who purchases shares of our common stock from a Selling Security Holder pursuant to this Prospectus, and who subsequently wants to resell such shares will also have to comply with blue sky laws regarding secondary sales.
When this Registration Statement becomes effective, and a Selling Security Holder indicates in which state(s) he desires to sell his shares, we will be able to identify whether he will need to register or may rely on an exemption from registration.
DESCRIPTION OF SECURITIES TO BE REGISTERED
Our authorized capital stock consists of 2,000,000,000 shares of common stock, $0.001 par value, and no authorized shares of preferred stock.
Common Stock
As of March 31, 2023, we had 445,728,363 outstanding shares of our common stock, and no outstanding options or warrants.
Holders of our common stock have no preemptive rights to purchase additional shares of common stock or other subscription rights. Our common stock carries no conversion rights and is not subject to redemption or to any sinking fund provisions. All shares of our common stock are entitled to share equally in dividends from sources legally available, when, as and if declared by our Board of Directors, and upon our liquidation or dissolution, whether voluntary or involuntary, to share equally in our assets available for distribution to our stockholders.
Our Board of Directors is authorized to issue additional shares of our common stock not to exceed the amount authorized by our Articles of Incorporation, on such terms and conditions and for such consideration as our Board may deem appropriate without further security holder action.
Voting Rights
Each holder of our common stock is entitled to one vote per share on all matters on which such stockholders are entitled to vote. Since the shares of our common stock do not have cumulative voting rights, the holders of more than 50% of the shares voting for the election of directors can elect all the directors if they choose to do so and, in such event, the holders of the remaining shares will not be able to elect any person to our Board of Directors.
Dividend Policy
Holders of our common stock are entitled to dividends if declared by the Board of Directors out of funds legally available for payment of dividends. From our inception to March 31, 2023, we did not declare any dividends.
We do not intend to issue any cash dividends in the future. We intend to retain earnings, if any, to finance the development and expansion of our business. However, it is possible that our management may decide to declare a cash or stock dividend in the future. Our future dividend policy will be subject to the discretion of our Board of Directors and will be contingent upon future earnings, if any, our financial condition, our capital requirements, general business conditions and other factors.
Anti-takeover Effects of Our Articles of Incorporation and By-laws
Our amended and restated articles of incorporation and bylaws contain certain provisions that may have anti-takeover effects, making it more difficult for or preventing a third party from acquiring control of our company or changing its board of directors and management. According to our bylaws and articles of incorporation, neither the holders of our company’s common stock have cumulative voting rights in the election of our directors. The combination of an ownership by a few stockholders of a significant portion of our company’s issued and outstanding common stock and lack of cumulative voting makes it more difficult for other stockholders to replace our company’s board of directors or for a third party to obtain control of our company by replacing its board of directors.
Anti-takeover Effects of Nevada Law
Business Combinations
The “business combination” provisions of Sections 78.411 to 78.444, inclusive, of the Nevada Revised Statutes, or NRS, prohibit a Nevada corporation with at least 200 stockholders from engaging in various “combination” transactions with any interested stockholder: for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the transaction is approved by the board of directors prior to the date the interested stockholder obtained such status; or after the expiration of the three-year period, unless:
| · | the transaction is approved by the board of directors or a majority of the voting power held by disinterested stockholders, or |
| | |
| · | if the consideration to be paid by the interested stockholder is at least equal to the highest of: (a) the highest price per share paid by the interested stockholder within the three years immediately preceding the date of the announcement of the combination or in the transaction in which it became an interested stockholder, whichever is higher, (b) the market value per share of common stock on the date of announcement of the combination and the date the interested stockholder acquired the shares, whichever is higher, or (c) for holders of preferred stock, the highest liquidation value of the preferred stock, if it is higher. |
A “combination” is defined to include mergers or consolidations or any sale, lease exchange, mortgage, pledge, transfer or other disposition, in one transaction or a series of transactions, with an “interested stockholder” having: (a) an aggregate market value equal to 5% or more of the aggregate market value of the assets of the corporation, (b) an aggregate market value equal to 5% or more of the aggregate market value of all outstanding shares of the corporation, or (c) 10% or more of the earning power or net income of the corporation.
In general, an “interested stockholder” is a person who, together with affiliates and associates, owns (or within three years, did own) 10% or more of a corporation’s voting stock. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire our company even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
Control Share Acquisitions
The “control share” provisions of Sections 78.378 to 78.3793, inclusive, of the NRS, which apply only to Nevada corporations with at least 200 stockholders, including at least 100 stockholders of record who are Nevada residents, and which conduct business directly or indirectly in Nevada, prohibit an acquirer, under certain circumstances, from voting its shares of a target corporation’s stock after crossing certain ownership threshold percentages, unless the acquirer obtains approval of the target corporation’s disinterested stockholders. The statute specifies three thresholds: one-fifth or more but less than one-third, one-third but less than a majority, and a majority or more, of the outstanding voting power. Once an acquirer crosses one of the above thresholds, those shares in an offer or acquisition and acquired within 90 days thereof become “control shares” and such control shares are deprived of the right to vote until disinterested stockholders restore the right. These provisions also provide that if control shares are accorded full voting rights and the acquiring person has acquired a majority or more of all voting power, all other stockholders who do not vote in favor of authorizing voting rights to the control shares are entitled to demand payment for the fair value of their shares in accordance with statutory procedures established for dissenters” rights.
Transfer Agent and Registrar
Our independent stock transfer agent is Transfer Online, 512 SE Salmon Street, Portland, Oregon, 97214-3444 (Telephone: 503-227-2950; Facsimile: 503-227-6874).
INTERESTS OF NAMED EXPERTS AND COUNSEL
No expert or counsel named in this Prospectus as having prepared or certified any part thereof or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of our common stock was employed on a contingency basis or had or is to receive, in connection with the offering, a substantial interest, directly or indirectly, in us. Additionally, no such expert or counsel was connected with us as a promoter, managing or principal underwriter, voting trustee, director, officer or employee.
Experts
The audited financial statements of Costas, Inc. for the two most recent fiscal years ended December 31, 2022 and 2021 have been included in this Prospectus in reliance upon Olayinka Oyebola & Co, an independent registered public accounting firm, as experts in accounting and auditing.
Legal Matters
William Macdonald, Esq. has rendered a legal opinion regarding the validity of the shares of common stock offered by the Selling Security Holders. It is included at exhibit 5.1 to the registration statement of which this Prospectus is a part.
INFORMATION WITH RESPECT TO OUR COMPANY
Forward-Looking Statements
This Prospectus contains forward-looking statements. To the extent that any statements made in this report contain information that is not historical, these statements are essentially forward-looking. Forward-looking statements can be identified by the use of words such as “expects”, “plans”, “may”, “anticipates”, “believes”, “should”, “intends”, “estimates” and other words of similar meaning. These statements are subject to risks and uncertainties that cannot be predicted or quantified and, consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, our ability to raise additional capital to finance our activities; the effectiveness, profitability and marketability of our products; legal and regulatory risks; the future trading of our common stock; our ability to operate as a public company; our ability to protect our intellectual property; general economic and business conditions; the volatility of our operating results and financial condition; our ability to attract or retain qualified personnel; and other risks detailed from time to time in our filings with the SEC, or otherwise.
Information regarding market and industry statistics contained in this report is included based on information available to us that we believe is accurate. It is generally based on industry and other publications that are not produced for the purposes of securities offerings or economic analysis. Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications outlined above and the additional uncertainties accompanying any estimates of future market size, revenue and market acceptance of products and services. We do not undertake any obligation to publicly update any forward-looking statements.
DESCRIPTION OF BUSINESS
Overview of Business over the Last Five Years
Costas, Inc. was incorporated in the State of Nevada on December 10, 1998. Costas was a development stage company that had a primary business plan to acquire, improve, and re-market undeveloped real estate in Las Vegas, Nevada and its surrounding communities.
On September 20, 2017, the court filed an order effective September 18, 2017, whereby Mr. James Brooks, a creditor of the company was granted a judgment against the company in the principal amount of $1,114,500. On October 21, 2020, Mr. Brooks filed a motion requesting the appointment of a Receiver over the company. By order filed on November 7, 2020, the Eighth Judicial District Court for Clark County, Nevada appointed Fredrick P. Waid as Receiver for the company in Case No. A-17-749977-B notice of entry of that order was filed on November 9, 2020. On March 25, 2021, the Receiver filed a motion with the Court requesting approval to appoint Mr. Brooks as an officer and director of the company and to increase the authorized capital of the issuer and subsequently to issue sufficient common and preferred shares on terms to be finalized with Mr. Brooks, whereby Mr. Brooks became the controlling shareholder of the company.
On December 30, 2021, Fred Waid resigned as an officer and director of the company, and appointed James Brooks as the company’s sole officer and director. On February 9, 2022, an Order was entered by the Eighth Judicial District Court, Clark County, Nevada, Case No. A-17-749977D at the request of the Appointed Receiver of the Company, Frederick Waid, terminating the receivership for the company.
On May 6, 2022, the company entered into an asset purchase agreement with Standard Dental Labs Inc. (“SDL”), a Wyoming corporation controlled by the company’s CEO, James Brooks, in order to acquire certain assets including: (i) a ready to implement business model and platform for the identification and acquisition of small to medium sized dental labs in the United States, and (ii) a fully developed branding package created around SDL, including logo, website, presentation materials and corporate name. Under the terms of the acquisition agreement, assets valued at $75,900 were acquired through the issuance of a total of 31,661,760 shares of the company’s restricted common stock to SDL. With the conclusion of this acquisition, the company intends to operate in the dental lab industry, paving the way for future acquisitions and consolidations in the industry. The assets acquired from SDL will allow the company to immediately facilitate the acquisition of small to medium sized dental labs, of which there are thousands in the United States. The assets acquired from SDL did not constitute an operating business, but rather an immediately actionable plan and branding concept from which the company identified its first operating dental lab targets. Immediately following the acquisition of the aforementioned assets by the company, including the tradename “Standard Dental Labs Inc.”, SDL changed its name to “Fastbend Holdings Inc.” in order to continue its ongoing operations in the field of consulting services, including the creation of business plans for identifying and purchasing operating assets in various industry sectors.
On August 15, 2022, the company announced the completion of a definitive agreement to acquire the assets of Prime Dental Lab, LLC. (“Prime Dental" or "PDL"), an Orlando-based dental lab in operation since 2012. The Purchased Assets consisted of: all client contracts for existing PDL clients; certain physical assets of PDL including all dental lab equipment, furniture, computers and other office equipment; the assumption of certain contracts, equipment leases and office leases (if any); certain employees and management of PDL as determined by the company to be retained and/or contracted by the company; and specifically the right to continue to use the name “Prime Dental Lab LLC” along with certain other rights, trademarks, intellectual property and intangible assets of the Seller. The Purchase Price consisted of 750,000 unregistered, restricted Common Shares (the “Consideration Shares”) of the company (the “Share Consideration) plus additional cash consideration in the amount of $140,000.00 (the “Cash Consideration”) payable in two (2) equal instalments of seventy thousand ($70,000.00) dollars (each a “Cash Instalment”). The first Cash Instalment shall be paid by the company to PDL no later than fifteen (15) calendar days after receipt by the effective date of this Registration Statement (the “First Cash Instalment”). The second Cash Instalment of seventy thousand ($70,000.00) dollars shall be paid by the Acquiror to PDL on the date that is no later than ninety (90) calendar days subsequent to the payment of the First Cash Instalment (the “Second Cash Instalment”).
The Share Consideration is subject to a lock-up agreement for a term of twenty-four months from the issuance date, whereunder PDL shall be entitled to a release of 12.5% of the total Consideration Shares each quarter (93,750 shares) provided certain minimum quarterly revenue targets are achieved. Further, the company had agreed to include such Consideration Shares in any registration statement filed, and in the event that the company decides to approve and complete a share consolidation or share rollback within twelve (12) months after the date of the issuance of the Consideration Shares, such shares shall be protected from such share consolidation action (on a one-time basis).
The company allocated the acquired assets on the company’s balance sheets as of the date of closing as Property and Equipment and Intangible Assets at fair market value. Assets acquired were as follows:
| 750,000 shares of common stock | | $ | 10,500 | |
| Cash consideration – other current liability | | | 140,000 | |
| Total consideration purchase cost | | $ | 150,500 | |
| | | | | |
| Allocation: | | | | |
| Property and equipment | | $ | 78,060 | |
| Customer relationships | | | 72,440 | |
| Total purchased assets | | $ | 150,500 | |
Upon acquisition of the customers and operational assets of PDL, the company immediately commenced revenue generating operations effective September 1, 2022, and reported gross revenues of $173,329 in the four -month period ended December 31, 2022, and associated costs of goods sold totaling $101,054 for gross profit of $72,275.
Subsequent to December 31, 2022 the Company and John Kim executed an addendum to the definitive agreement with PDL (the “PDL Amendment”) whereunder the Cash Consideration as part of Purchase Price was revised so that the first instalment shall be released immediately upon execution of the PDL Amendment, or March 24, 2023, and the second Cash Instalment shall be payable on the date that is no later than seven (7) calendar days subsequent to the receipt of proceeds from the first payment under the Purchase Agreement with World Amber.
The company is now operating in the dental lab industry under its acquired tradenames, Standard Dental Labs Inc. and Prime Dental Lab LLC, and is currently manufacturing dental prosthetics for dentists and dental clinics via its first operational lab facility. Our existing dental lab supplies dentists and dental clinics with dental prosthetics such as crowns, bridges, and implants, including other prosthetics.
Our Current Business
Our current business activities include discovering, acquiring, operating and developing dental labs throughout the United States. The company plans to acquire independent labs looking to exit the market, or whom may be interested in retirement. Acquiring labs will be the focus of our growth strategy and the operations of the acquired labs will be the cornerstone of our revenue model. Labs will be consolidated into one regionally central lab, and continue to operate, adding incremental revenue to the company's income statement. All North American markets with populations over 1 million are targets.
As noted above, in May of 2022, Costas, Inc. acquired certain assets of Standard Dental Labs Inc. (SDL), a privately owned Wyoming corporation controlled by our CEO, James Brooks, which is in the business of providing consulting services, including the creation of business plans for identifying and purchasing operating assets in various industry sectors. Immediately following the acquisition of the aforementioned assets by the company, including the tradename “Standard Dental Labs Inc.”, SDL changed its name to “Fastbend Holdings Inc.” in order to continue its ongoing operations. The company commenced operating under the dba “Standard Dental Labs Inc.”
In August of 2022, Costas , Inc. purchased all of the assets, including the revenue, of Prime Dental Lab LLC, a Florida dental laboratory and engaged Mr. John Kim as a subcontractor to provide labor and manufacturing services as well as certain management oversight for the ongoing operation of the dental lab until such time as additional lab assets are acquired in order to facilitate consolidation of operations into one owned and operated lab facility in Florida. The company continues to service clients under its acquired tradename, Prime Dental Lab LLC.
The consolidation of dental and medical clinics has been an increasing trend over the past two decades, but only recently have investors focused on dental labs. We see this as an opportunity to apply similar strategies to what has been learned from other consolidations. For example, over the past 3 years Invisible Dental Service Operators (IDSOs) have been actively investing in dental practices, and have invested billions of dollars, with the objective of bringing liquidity to the owners (typically dentists)(from: https://www.dentaleconomics.com/practice/multi-practice-growth/article/14233624/why-dental-practice-consolidation-is-accelerating). Although there are a few differences in the models, the company’s business plan shares many components with that of the IDSOs. Historical examples of consolidation strategies could include the pharmaceutical industry, which has many examples of M&A growth successes, and also the waste management industry, which has been led by Waste Management Company, a $60B company which has used the model since the 1970s. Our growth model will build on the strategy of companies that have successfully gone through an industry consolidation.
Our company’s view is that there is no faster way to grow than through acquisition. This industry, in particular, has not yet made its way into the public markets. Consolidation of this industry, currently represented by 7,000+ privately owned businesses, will be the company’s focus. Based on his past experience, we believe that our current director and officer, James Brooks, understands how to design strong companies, and how to build and scale operations to become regionally competitive. In the process, we will be positioning ourselves as leaders in setting new standards for the dental lab industry.
Management of the Company has completed a review of industry metrics and outlooks relying on various sources of industry data in order to prepare its assessment of the key market factors affecting the dental lab industry, and acquisition opportunities, including but not limited to the National Association of Dental Laboratories' (NADL) 2019 Business Survey (1) and other related NADL reports, Inside Dental Technology (“IDL”) October 2019 Volume 10, Issue 10, “Effects of Consolidation” (2) and other IDL reports, Grandview R esearch (“Grandview”) Dental Laboratories Market Size, Share & Trends Analysis Report By Product (Oral Care, Restorative), By Equipment Type (Systems & Parts, Dental Lasers), By Region, And Segment Forecasts, 2022 – 2030 (3) , Ibis World (“Ibis World”) research reports including “ Dental Laboratories in the US - Number of Businesses 2004–2029” (4) and other related reports and the US Bureau of Labor Statistics (5) as well as other independently conducted research locally in our initial target market, Florida. Upon a review of the market data, in management’s opinion, despite growth in demand from dental clinics, small and medium sized labs, which we believe represent more than three-quarters of the overall industry, and are controlled by independent owners, may not be able to exit or retire due to lack of interest and expansion in the independent dental lab market.It is management’s assessment from a review of the industry data including the IDL and Grandview reports that a majority of these dental labs nationally operate with annual sales of near $1 million US in gross receipts considering there are a total of roughly 9,200 labs in the United States with 2021 reported cumulative gross sales of $9.7Bn. Further management believes that these owners will be faced with barriers to exit from the market, potentially creating a substantial acquisition opportunity for a liquid, publicly traded company that can identify target lab operations in large US cities to acquire and consolidate. Currently the dental lab industry is highly fragmented according to an October 2022 Research and Markets study (6) and “….is anticipated to witness significant mergers & acquisitions….”. Our first target market, the State of Florida, has more than 400 privately owned labs. The NADL indicates its members are 55 or older, including 13.7% who are 65 or older, and who are, or are near, retirement and may already be interested in selling their businesses. We have identified several suitable single owner lab operations and believe that these business owners may be interested in selling their labs, or their book of business to our company on terms that we propose to include both cash consideration and equity in the company. In this way, we will be able to preserve working capital for operations and expansion by using our equity as the acquisition consideration. The company is currently at various stages of negotiations/discussions with five independently owned dental labs in the Orlando area. We cannot provide any assurances that any negotiations will result in a successful acquisition or that commercially reasonable terms can be agreed to; and if consummated cannot provide any assurances that any such acquisition will result in the hoped for synergies for our company.
| | (1) | https://nadlonlinestore.payscapecommerce.com/2019-Costs-of-Doing-Business-Survey-Full-Survey-Including-Executive-Summary-p237290665 |
| | | |
| | (2) | https://www.aegisdentalnetwork.com/idt/2019/10/the-effect-of-consolidation |
| | | |
| | (3) | https://www.grandviewresearch.com/industry-analysis/dental-laboratory-market |
| | | |
| | (4) | https://www.ibisworld.com/industry-statistics/number-of-businesses/dental-laboratories-united-states/ |
| | | |
| | (5) | https://www.bls.gov/ |
| | | |
| | (6) | https://www.researchandmarkets.com/reports/5406425/dental-laboratories-market-size-share-and-trends?utm_source=GNOM&utm_medium=PressRelease&utm_code=4h3l3w&utm_campaign=1626216++Global+%2443.5+Billion+Dental+Laboratories+(Restorative%2c+Implant%2c+Oral+Care)+Markets+to+2028%3a+Market+is+Highly+Fragmented+and+is+Anticipated+to+Witness+a+Significant+Merger+and+Acquisition+Activity&utm_exec=chdo54prd |
In addition to the company’s expansion program, it operates day-to-day as a full-service dental lab. With more than 50 dental clinics as clients, Prime Dental Lab produces roughly 500 dental prosthetics each month. While presently the company has engaged the labor and manufacturing services of Mr. John Kim and his employees as a contract manufacturer, our goal is to open a large and modern facility in Orlando, Florida to accommodate 75 full-time employees, including dental lab technicians. We have not undertaken and specific steps at this time for this facility, and would not anticipate doing so until we have acquired at least 3 dental labs. We sell our dental lab products and market these products to our clients using our acquired tradename Prime Dental Lab LLC and compensate Mr. Kim for contract services provided. Mr. Kim in turn reimburses the company for the use of our acquired dental lab equipment.
The 500 orders that are delivered each month are completed by the contracted dental lab techs and invoiced by Prime Dental Labs. With 60+ techs working in the new facility, we expect tech productivity to factor on par or better than current levels due to improved support technologies and equipment in our new facility.
Products and services
Dental labs supply dentists and dental clinics with dental prosthetics such as crowns, bridges, and implants. Although they manufacture many other prosthetics, these represent a large majority of what is supplied, and would be called the “bread and butter” of the industry.
The type of dental labs the company has and will continue to acquire typically serve 20 to 25 dental clinics each and employ 4 to 6 dental lab technicians who prepare dental prosthetics for dentists. The dentists are normally responsible for the final fitting with the patient, but most of the work is done by the lab.
Over the past 5 years, 3D printing, and modelling technology has become more mainstream. As most of this technology has been unaffordable for small business owners, advanced technology is uncommon in these smaller operations. While the dental labs we have and will be acquiring will have to be profitable with their existing equipment, going forward we plan to purchase the latest and most advanced equipment available in the sector, and integrate into in its operations following the consolidation of acquisition targets. This becomes possible through economies of scale. While our operations will not be dependent on the purchase of such equipment, we intend to capitalize on the fact that the more labs acquired, the more competitive the company will become and this should contribute to an overall higher purchasing power.
The Market
The transition from skilled labor to computer driven technology in the dental lab industry, although in its infancy, is well underway. The changeover of skilled artisans to 3D printers and milling equipment is causing a shift in the perception for would-be investors. No one can predict how this technology might evolve, how long it will take, or how it may impact the industry in the long term.
The uncertain future of dental labs means there is less interest from investors in the field. The erosion of the one, two, and three person businesses is certain. (Forbes, Nov. 2019). The result: A generation of lab owners that may not be able to sell their business for retirement or raise the necessary capital to retool to this advancing technology.
The company has identified this exciting opportunity and has begun the acquisition of dental lab operations from individual owners that are interested in selling their business. The company will establish offices across America, where acquisition targets are concentrated, where these smaller lab operations will be consolidated into one owned and operated lab facility allowing the company to take advantage of economies of scale with an immediate revenue stream.
Competition
The biggest competition to the private dental lab owner in the United States is large, Chinese factory labs operating in southern China. We estimate that a large percentage of dental prosthetics are now sourced through these offshore manufacturers. This is detrimental to the local industry, and there is very little an individual lab owner can do to protect himself from the market erosion caused by this competitor.
However, this may represent an opportunity for the company to lobby state government to enact legislation requiring the materials be “certified” prior to being used to supply the industry with the raw materials required to manufacture dental products.
Certified materials could only become certified through local inspectors which could be funded through a state or national association. So far in the US, the only such association is The National Association of Dental Laboratories, located in Tallahassee, Florida.
As a start-up company in the dental lab industry, we will be at a significant disadvantage to other more established and better capitalized companies that we are in competition with for the acquisition of additional dental labs. However as a smaller company we believe that we will have some advantages in our ability to structure flexible acquisition terms that are tied to a lab’s performance and will include equity in our company, and hence liquidity, for a vendor. We believe that larger more established companies will not be as flexible as we have the potential to be, in regards to acquisition terms with potential lab owners, particularly with our ability to offer equity. As the company is able to acquire additional labs and enhance its revenue, when appropriate the company will seek to uplist to the OTCQB Market or a more senior exchange to further enhance liquidity benefits for shareholders and vendors of dental lab assets.
Compliance with Government Regulation
In a 2009, American Dental Association member survey, nearly 65 percent of dentists responded that they believe dental technicians and laboratories are regulated in their state. This is not the case. In fact, only four states in the United States require either certification or continuing education.
However, 12 states require basic minimum standards be met for laboratories, which include state registration. The most up-to-date regulations were last set in 2019, in Washington State, but most haven't updated their requirements for 25 years or longer. We feel that there is an opportunity to contribute to the industry by proposing new, more current legislation that addresses the importation of materials from foreign countries. New legislation that requires certification of materials could greatly enhance the safety of the materials for patients, and also contribute to the success of US based dental labs.
Other than the payment of a modest registration fee every two years to maintain our dental lab registration with the State of Florida , ongoing continuing education of 18 hours biennially by one certified lab technician in the lab in accordance with Florida Administrative Code rule Chapter 64B-5, we do not require any governmental approvals or authorizations for the operation of our existing dental lab in Florida. Further, we are not aware of any pending or probable regulations that would have an impact upon our operations.
| Dental Laboratory Minimum Standards By State |
| | Florida | Illinois | Kentucky | MN | Missouri | NC | Ohio | Oklahoma | PA | S.Carolina | Texas | Virginia | Washton |
| Year of Initial Enactment | 1987 | 1993 | 1977 | 2012 | 1995 | 1962 | 2008 | 1992 | 1987 | 1942 | 1985 | 2012 | 2019 |
| Laboratory Registration Fee | $200/2yrs. | N/A | $150/yr. | $50/yr. | N/A | N/A | N/A | $300/yr. | $25/yr. | $150/2yrs. | $135/yr. | N/A | $250 |
| Laboratory Registration | Yes | No | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | No | Yes |
| Technician Registration Fee | No | No | No | No | No | No | No | No | No | $100 Initial $150 Renewal | No | No | No |
| Certificate to Perform Dental Technology | No | No | No | No | No | No | No | No | No | Yes - δ | No | No | No |
| CDT or Equivalent Required | No | No -  | Yes | No | No | No | No | No | No | Yes | Yes | No | Yes (2025) |
| State Laws and Rules Exam Required | No | No | No | No | No | No | No | No | No | Yes | Yes | No | No |
| Out-Of-State Laboratories Required To Register | No | No | Yes | No | No | No | No | Yes | No - δ | Yes | Yes | No | Yes |
| Dentists Required to Use Registered Laboratory | No | No | Yes | Yes** | No | No | No | Yes | No | Yes | Yes | No | Yes |
| Materials Disclosure | Yes | Yes | Yes | Yes | Yes*** | Yes | Yes | No | No | Yes | No | Yes | Yes |
| Point of Origin Disclosure | Yes | Yes | Yes | Yes | Yes*** | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| CE for One Technician in Each Laboratory | Yes | No | Yes | No | No | No | No | No | No | Yes | Yes | No | Yes |
| |
| NOTES: |
|  - Dentists receive CE credit for attending NBC approved CE courses. - Dentists receive CE credit for attending NBC approved CE courses.
|
| * - $20 on renewal |
| ** - Only for lab work done in MN |
| *** - Required |
| δ - Out-Of-State labs required to register if employing sales representative or agents within the state. |
|  - The certificate to perform dental technological work in SC requires each of the following: - The certificate to perform dental technological work in SC requires each of the following:
|
| HS or GED; 2 year DT degree, or 3 year OJT; CDT or pass SC state board exam; pass SC laws and rules exam. |
| |
| - Updated March 2020 by NADL |
-Source: National Association of Dental Laboratories
The company’s current lab operations in Florida are not subject to OSHA regulations or regulations governing the handling or disposing of medical waste. There are currently no specific OSHA standards for dentistry, however the labs follow best practices including:
(a) having a lab space that is clean and orderly and in good repair, with regard to normal fabrication procedures;
(b) All waste materials properly disposed of at the end of each day;
(c) Maintaining on the laboratory premises a copy of the laboratory registration so it is readily available for inspection by Department personnel;
(d) Maintaining on the laboratory premises, for each separate appliance and for a period of four years, a work order from a licensed dentist authorizing construction or repair of the specified artificial oral appliance;
AND
(e) Maintaining on the laboratory premises a written policy and procedure document on sanitation. Said policy shall include, but not necessarily be limited to:
1. Intake and disinfection procedure for each appliance, impression, bite, or other material posing a possible contamination risk received by the laboratory.
In the State of Florida the prosthetics and other oral appliances manufactured and repaired by the lab do not for the most part meet the definition of medical waste according to the Florida Department of Health which includes, “ Any solid or liquid waste which may present a threat of infection to humans, including nonliquid tissue, body parts, blood, blood products, and body fluids from humans and other primates; laboratory and veterinary wastes which contain human disease-causing agents; and discarded sharps. ” Most prosthetics and appliances coming to the lab from a dental office have been disinfected prior to intake. However, the company’s lab operations follow the recommended procedures for waste including properly labeled and identified waste bags, proper seals on waste containers and proper disposal of waste in accordance with the State of Florida department of health chapter 64E-16 of the Florida Administrative Code.
Further the company’s dental lab operations do not deal directly with any personal medical records and there are no requirements for the company’s labs to meet federal HPAA compliance standards. Products manufactured at our dental labs are sold directly to dental offices and are based on product specifications provided by such dental offices.
Significant Acquisitions
In May of 2022, Costas, Inc. acquired certain assets of Standard Dental Labs Inc. (SDL), a privately owned Wyoming corporation controlled by our CEO, James Brooks, which is in the business of providing consulting services, including the creation of business plans for identifying and purchasing operating assets in various industry sectors. Immediately following the acquisition of the aforementioned assets by the company, including the tradename “Standard Dental Labs Inc.”, SDL changed its name to “Fastbend Holdings Inc.” in order to continue its ongoing operations. The company commenced operating under the dba “Standard Dental Labs Inc.”
The business model acquired from SDL includes metrics and data in order to allow the company to quickly identify and purchase privately owned dental lab operations in the United States, allowing for these operations to be consolidated regionally, and to operate them efficiently applying economies of scale . To that end, in August of 2022, Costas signed an agreement to purchase all of the assets, including the revenue, of Prime Dental Lab LLC, a Florida dental laboratory, and contracted Prime Dental Lab to continue to operate the company’s assets.
On August 15, 2022, the company announced the completion of the definitive agreement to acquire the assets of Prime Dental Lab LLC, an Orlando-based dental lab in operation since 2012. The Purchased Assets pursuant to the agreement consisted of: all client contracts for existing PDL clients; certain physical assets of PDL including all dental lab equipment, furniture, computers and other office equipment; the assumption of certain contracts, equipment leases and office leases (if any); certain employees and management of PDL as determined by the company to be retained and/or contracted by the company; and specifically the right to continue to use the name “Prime Dental Lab LLC” along with certain other rights, trademarks, intellectual property and intangible assets of the seller. The assets acquired more specifically consisted of the lab equipment, the client base and the corporate brand. The Purchase Price consisted of 750,000 unregistered, restricted Common Shares (the “Consideration Shares”) of the company (the “Share Consideration) plus additional cash consideration in the amount of $140,000.00 (the “Cash Consideration”).
Subsequent to December 31, 2022 the Company and John Kim executed an addendum to the definitive agreement with PDL (the “PDL Amendment”) whereunder the Cash Consideration as part of Purchase Price was revised so that the first instalment shall be released immediately upon execution of the PDL Amendment, or March 24, 2023, and the second Cash Instalment shall be payable on the date that is no later than seven (7) calendar days subsequent to the receipt of proceeds from the first payment under the Purchase Agreement with World Amber.
In order to facilitate ongoing lab operations and seamlessly service its acquired customer base the company entered into a service contract with Mr. John Kim on August 31, 2022, whereunder Mr. Kim will provide ongoing labor, quality control and delivery services and management oversight during a period of up to two years while the company seeks additional lab assets and operations in order to consolidate several independent lab operations into its first large regional lab facility in Florida. The company operates using its acquired tradename Prime Dental Labs LLC for purposes of continuity in invoicing its acquired client base. The company did not acquire the property owned by Mr. John Kim where the manufacturing of Prime Dental Lab appliances and prosthetics takes place. The Company and Mr. Kim have agreed that the cost of manufacturing goods paid by the company to Mr. Kim shall include overhead costs including applied costs on a per item bases for the use of the space where the lab operations take place. There is no separate agreement for the rental or lease of the space where the prosthetics are manufactured.
Offices
The mailing address of our company is # 424 E Central Blvd, Suite 308, Orlando, FL, 32801. This space is leased by our President, James Brooks, for a term of one year from January 2023 through 2024, is approximately 1,500 square feet and has a cost of approximately $3,185 per month, plus utilities and insurances , which amounts are reimbursed to Mr. Brooks. The mailing address is a shared office and residential space. Our main telephone number is 321-465-9899. Our initial lab location is at 1008 N Pine Hills Rd, Orlando, FL 32808. We do not own or lease our lab location, as the location is owned by Mr. John Kim with whom we have contracted to provide ongoing labor, quality control and delivery services and management services during a period of up to two years while the company seeks additional lab assets and operations in order to consolidate several independent lab operations into its first large regional lab facility in Florida. Mr. Kim is reimbursed for the use of this lab space through a usage fee included in the costs of goods sold charged to the company by Mr. Kim for each dental appliance. In addition, Mr. Kim remits to the company a usage fee monthly for access to the lab equipment acquired by the company and used in the manufacturing process. Our current locations provide adequate space for our purposes at this stage of our development.
Employees
We currently have employees consisting of our sole director and officer, James Brooks and five contractors in the dental lab operations under our contract service agreement with Mr. John Kim. We primarily also use the services of sub-contractors and consultants for certain aspects of our business operations.
We do expect material changes in the number of employees over the next 12-month period, as will be required as we expand with our acquisitions of additional dental lab operations. Also, we do and will continue to outsource contract employment as needed.
Summary
The continuation of our business is dependent upon obtaining further financing, a successful program of development, and, finally, achieving a profitable level of operations. The issuance of additional equity securities by us could result in a significant dilution in the equity interests of our current stockholders. Obtaining commercial loans, assuming those loans would be available, will increase our liabilities and future cash commitments.
There are no assurances that we will be able to obtain further funds required for our continued operations. As noted herein, we are pursuing various financing alternatives to meet our immediate and long-term financial requirements. There can be no assurance that additional financing will be available to us when needed or, if available, that it can be obtained on commercially reasonable terms. If we are not able to obtain the additional financing on a timely basis, we will be unable to conduct our operations as planned, and we will not be able to meet our other obligations as they become due. In such event, we will be forced to scale down or perhaps even cease our operations. There is significant uncertainty as to whether we can obtain additional financing.
DESCRIPTION OF PROPERTIES
Executive Offices
Our mailing address is # 424 E Central Blvd, Suite 308, Orlando, FL, 32801. This space is leased at $ 3,185 per month, plus the cost of utilities and insurances. Our main telephone number is 407-789-1923. Our initial lab location is at 1008 N Pine Hills Rd, Orlando, FL 32808. These locations provide adequate space for our purposes at this stage of our development.
Laboratories
Our lab facilities operations can be described in 6 steps as follows:
Step 1
Day to day business at Standard Dental Labs starts with materials management. In order to keep up with demand, a review of inventories is conducted each day, and supplies are topped up. Raw materials are ordered from several different dental supply companies, and 80% of the raw materials are ordered from Henry Shein (NASDAQ: HSIC), one of the world’s largest medical and dental supply companies. We are not dependent on Henry Shein, or any other supplier, as there are numerus dental supply companies, and raw materials are readily available. We have elected to use Henry Shein as our main supplier due to the raw material certification process.
Meta Dental Supply, and Atlantic Dental Supply provide on demand inventories, and supply roughly 10% each to Standard Dental Labs.
Step 2
Customer orders are currently being placed by phone, but the company is developing software to streamline this process, and to provide transparency for dentist and patients.
Each order requires a courier to pick up dental impressions created by the dentist of the patient’s mouth, and a prescription with notes which outline exactly what is prescribed by the doctor, including details like shading the enamel (ceramic) prosthetic to match.
Dental impressions can also be transferred electronically to the company by using an intra-oral scanner, but fewer than 10% of dental clinics possess that technology today due to limitations in the technology that have not yet become economical for dentists.
Step 3
Once received by the lab, and depending on the job, one of 3 possible technician types is assigned the work. First, a model is made by a model work technician from the impression, and the type of model depends on the prescription. The end product could be a ceramic implant, crown or bridge, or it could be a metal product made of silver or gold. These processes require different skill sets, and therefore different technicians.
With a model made from an impression, a wax-up technician can manually create a metal crown or bridge. When an impression is digitally scanned, it can be used to create a ceramic model from a digital milling machine, which can be finished by a ceramic technician. Ceramic technicians normally require a digital milling machine to create the base prosthetic, but could produce up to 4 prosthetics per hour if supported by the right equipment.
A tech working manually, ceramic or metal, can only produce one prosthetic per hour.
The company is planning several upgrades that will include more advanced 3D printing and milling technologies, and is encouraging dental clinics to adopt intra-oral scanning technology to streamline the transfer of information, and improve turnaround times for patients.
Step 4
Once completed, the technician packages the finished product and updates the file with billing details. An invoice is created, attached to the package, and then moved to a staging area to await delivery.
Step 5
The next step is delivery. Several times per day, and more than 500 times per month, deliveries are performed by our in-house couriers. The final product, including the invoice, is delivered to the dental clinics. On delivery, the courier uses an itemized manifest which details each invoice in the delivery. The receiver signs for the delivery which completes the process.
Step 6
Finally, at the end of each billing cycle (normally monthly), a billing statement is sent to each client with a request for payment, which closes the job.
LEGAL PROCEEDINGS
We know of no material, existing or pending legal proceedings against us, nor are we involved as a plaintiff in any material proceeding or pending litigation. There are no proceedings in which any of our directors, officers or affiliates, or any registered or beneficial shareholder, is an adverse party or has a material interest adverse to our company.
MARKET PRICE OF AND DIVIDENDS ON OUR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Our common shares are quoted on the OTC markets under the symbol “CSSI”. The following quotations, obtained from OTC Markets Inc., reflect the high and low bids for our common shares based on inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions.
The high and low bid prices of our common stock for the periods indicated below are as follows:
| Quarter Ended (1) | | High | | | Low | |
| March 31, 2023 | | | 0.0076 | | | | 0.0045 | |
| December 31, 2022 | | | 0.015 | | | | 0.0031 | |
| September 30, 2022 | | | 0.015 | | | | 0.0055 | |
| June 30, 2022 | | | 0.0355 | | | | 0.121 | |
| March 31, 2022 | | | 0.04 | | | | 0.0102 | |
| December 31, 2021 | | | 0.055 | | | | 0.0141 | |
| September 30, 2021 | | | 0.0788 | | | | 0.0038 | |
| June 30, 2021 | | | 0.0393 | | | | 0.015 | |
| March 31, 2021 | | | 0.074 | | | | 0.0125 | |
| December 31, 2020 | | | 0.0375 | | | | 0.0065 | |
| September 30, 2020 | | | 0.0257 | | | | 0.003 | |
| June 30, 2020 | | | 0.0296 | | | | 0.01 | |
| March 31, 2020 | | | 0.0839 | | | | 0.02 | |
NOTES:
| (1) | The quotations above reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions. |
On March 31, 2023, the last closing price for one share of our common stock as reported by the OTC was $0.0072. This closing price reflects an inter-dealer price, without retail mark-up, mark-down or commission, and may not represent an actual transaction.
Our common shares are issued in registered form. Our independent stock transfer agent is Transfer Online, 512 SE Salmon Street, Portland, Oregon, 97214-3444 (Telephone: 503-227-2950; Facsimile: 503-227-6874) is the transfer agent for our common shares.
Dividend Policy
We have not paid any cash dividends on our common stock and have no present intention of paying any dividends on the shares of our common stock. Our current policy is to retain earnings, if any, for use in our operations and in the development of our business. Our future dividend policy will be determined from time to time by our board of directors.
Equity Compensation Plan Information
We have no long-term incentive plans.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
We did not purchase any of our shares of common stock or other securities during our fiscal years ended December 31, 2022 or 2021.
FINANCIAL STATEMENTS
This Prospectus includes the following financial statements:
| | · | Audited financial statements of our company for fiscal years ended December 31, 2022 and 2021 |
| | · | Audited financial statements of Prime Dental Labs LLC for the fiscal years ended December 31, 2021 and 2020 |
Our financial statements are prepared in accordance with United States generally accepted accounting principles and are stated in United States Dollars ($). The financial statements appear beginning on page F-1.
COSTAS, INC.
TABLE OF CONTENTS FOR AUDITED
FINANCIAL STATEMENTS
Year ended December 31, 2022
Report of Independent Public Accounting Firm
To the shareholders and the board of directors of Costas Inc
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Costas Inc (the "Company") as of December 31, 2022, and 2021 and the related statements of operations, changes in shareholders' equity and cash flows, for the years ended December 31, 2022, and 2021, and the related notes collectively referred to as the "financial statements”. In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022, and 2021 and the results of its operations and its cash flows for the years ended December 31, 2022, in conformity with U.S. generally accepted accounting principles.
Going Concern
The accompanying financial statements have been prepared assuming the company will continue as a going concern as disclosed in Note 2 to the financial statement, the Company incurred a net loss of $(1,651,897) for the year ended December 31, 2022 and working capital deficiency of $(1,685,506) and accumulated deficit of $(16,531,420) The continuation of the Company as a going concern through December 31, 2021, is dependent upon improving the profitability and the continuing financial support from its stockholders. Management believes the existing shareholders or external financing will provide additional cash to meet the Company’s obligations as they become due.
These factors raise substantial doubt about the company’s ability to continue as a going concern. These financial statements do not include any adjustments that might result from the outcome of the uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB. We conducted our audits in accordance with the standards of the PCAOB.
Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
OLAYINKA OYEBOLA & CO.
(Chartered Accountants)
We have served as the Company's auditor since August 2022.
March 27th, 2023.
Lagos Nigeria
Costas, Inc.
Balance Sheets
| | | December 31, 2022 | | | December 31, 2021 | |
| | | | | | | |
| Assets | | | | | | |
| | | | | | | |
| Current assets: | | | | | | |
| Cash and cash equivalents | | $ | 13,123 | | | $ | - | |
| Total current assets | | | 13,123 | | | | - | |
| | | | | | | | | |
| Property and equipment, net | | | 70,786 | | | | - | |
| Intangible assets | | | 104,103 | | | | - | |
| Total assets | | $ | 188,012 | | | $ | - | |
| | | | | | | | | |
| Liabilities and Stockholders’ Equity (Deficit) | | | | | | | | |
| | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable and accrued liabilities | | $ | 204,741 | | | $ | 73,755 | |
| Accounts payable – related party | | | 89,915 | | | | 17,054 | |
| Advances Payable – related party | | | - | | | | 16,532 | |
| Convertible notes | | | 92,246 | | | | - | |
| Convertible note – shareholder, net | | | 1,171,727 | | | | 25,682 | |
| Other current liability | | | 140,000 | | | | - | |
| Total current liabilities | | | 1,698,629 | | | | 133,023 | |
| | | | | | | | | |
| Total liabilities | | | 1,698,629 | | | | 133,023 | |
| | | | | | | | | |
| Commitments and contingencies | | | | | | | | |
| | | | | | | | | |
| Stockholders’ (deficit): | | | | | | | | |
| Common stock, 2,000,000,000 shares authorized, $0.001 par value, 445,728,363 and 413,314,603 shares issued and outstanding at December 31, 2022 and December 31, 2021, respectively | | | 445,728 | | | | 413,315 | |
| Additional Paid in Capital | | | 14,545,075 | | | | 14,333,185 | |
| Accumulated deficit | | | (16,531,420 | ) | | | (14,879,523 | ) |
| Stockholders’ (deficit) | | | (1,510,617 | ) | | | (133,023 | ) |
| Total Liabilities and Stockholders’ Deficit | | $ | 188,012 | | | $ | - | |
The accompanying notes are an integral part of these audited financial statements.
Costas, Inc.
Statements of Operations
| | | For the Years Ended | |
| | | December 31, | |
| | | 2022 | | | 2021 | |
| | | | | | | |
| Revenue | | $ | 173,329 | | | $ | - | |
| Costs of goods sold | | | 101,054 | | | | - | |
| Gross profit | | | 72,275 | | | | - | |
| | | | | | | | | |
| Operating expenses: | | | | | | | | |
| Selling and marketing expenses | | | 74,393 | | | | - | |
| General and administrative expenses | | | 132,416 | | | | 20,053 | |
| Professional Fees | | | 177,564 | | | | 56,674 | |
| Depreciation | | | 7,274 | | | | - | |
| Total Operating Expenses | | | 391,647 | | | | 76,727 | |
| | | | | | | | | |
| Loss from Operations | | | (319,372 | ) | | | (76,727 | ) |
| | | | | | | | | |
| Other Income (Expense) | | | | | | | | |
| Gain (Loss) on Settlement of Debt | | | 6,884 | | | | (10,025,000 | ) |
| Interest Expense | | | (1,339,409 | ) | | | (152,400 | ) |
| Total Other (expense) | | | (1,332,525 | ) | | | (10,177,400 | ) |
| | | | | | | | | |
| Net (Loss) | | $ | (1,651,897 | ) | | $ | (10,254,127 | ) |
| | | | | | | | | |
| Net loss per share attributable to common shareholders: | | | | | | | | |
| Basic and diluted | | $ | (0.00 | ) | | $ | (0.21 | ) |
| | | | | | | | | |
| Weighted average shares outstanding | | | | | | | | |
| Basic and diluted | | | 434,296,490 | | | | 49,177,617 | |
The accompanying notes are an integral part of these audited financial statements.
Costas, Inc.
Statements of Stockholders’ Equity
| | | Common Stock | | | Additional Paid-in | | | Accumulated | | | Stockholders’ | |
| | | Shares | | | Amount ($) | | | Capital ($) | | | (Deficit) ($) | | | (Deficit) ($) | |
| | | | | | | | | | | | | | | | |
| Balance, December 31, 2020 | | | 43,314,603 | | | | 43,315 | | | | 3,191,458 | | | | (4,625,396 | ) | | | (1,390,623 | ) |
| Beneficial conversion feature associated with convertible notes | | | - | | | | - | | | | 1,241,727 | | | | - | | | | 1,241,727 | |
| Shares issued for debt | | | 370,000,000 | | | | 370,000 | | | | 9,900,000 | | | | - | | | | 10,270,000 | |
| Net loss for the year | | | - | | | | - | | | | - | | | | (10,254,127 | ) | | | (10,254,127 | ) |
| Balance, December 31, 2021 | | | 413,314,603 | | | | 413,315 | | | | 14,333,185 | | | | (14,879,523 | ) | | | (133,023 | ) |
| Beneficial conversion feature associated with Convertible notes | | | - | | | | - | | | | 232,140 | | | | - | | | | 232,140 | |
| Shares issued under acquisition agreements | | | 32,413,760 | | | | 32,413 | | | | 9,750 | | | | - | | | | 42,163 | |
| Net loss for the year | | | - | | | | - | | | | - | | | | (1,651,897 | ) | | | (1,651,897 | ) |
| Balance, December 31, 2022 | | | 445,728,363 | | | | 445,728 | | | | 14,575,075 | | | | (16,531,420 | ) | | | (1,510,617 | ) |
The accompanying notes are an integral part of these audited financial statements.
Costas, Inc.
Statements of Cash Flows
| | | For the Years ended December 31, | |
| | | 2022 | | | 2021 | |
| | | | | | | |
| Cash flows from operating activities: | | | | | | |
| Net loss | | $ | (1,651,897 | ) | | $ | (10,254,127 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation | | | 7,274 | | | | - | |
| (Gain) Loss on extinguishment of debt | | | (6,884 | ) | | | 10,025,000 | |
| Amortization of debt discount | | | 1,238,291 | | | | - | |
| Non-cash interest | | | - | | | | 95,682 | |
| Changes in certain assets and liabilities: | | | | | | | | |
| Accounts payable – related party | | | 72,861 | | | | 15,329 | |
| Accounts payable and other liabilities | | | 137,870 | | | | 101,584 | |
| Net cash used in operating activities | | | (202,485 | ) | | | (16,532 | ) |
| | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | |
| Net cash provided by investing activities | | | - | | | | - | |
| | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
| Proceeds from convertible notes | | | 232,140 | | | | | |
| Advances payable – related party | | | - | | | | 16,532 | |
| Repayments to related party | | | (16,532 | ) | | | - | |
| Net cash provided by financing activities | | | 215,608 | | | | 16,532 | |
| | | | | | | | | |
| Net decrease in cash and cash equivalents | | | 13,123 | | | | - | |
| | | | | | | | | |
| Cash and cash equivalents, beginning of year | | | - | | | | - | |
| | | | | | | | | |
| Cash and cash equivalents, end of year | | $ | 13,123 | | | $ | - | |
| | | | | | | | | |
| Supplemental Disclosure of Cash Flows Information: | | | | | | | | |
| Shares issued to settle shareholder loans | | $ | - | | | $ | 245,000 | |
| Accounts payable classified to convertible note | | $ | - | | | $ | 302,227 | |
| Shareholder loan classified to convertible note | | $ | - | | | $ | 869,500 | |
| Beneficial conversion feature associated with convertible notes | | $ | 232,140 | | | $ | 1,241,727 | |
| Property and equipment acquired under asset purchase agreement | | $ | 78,060 | | | $ | - | |
| Intangible assets acquired under acquisition agreement | | $ | 31,664 | | | $ | - | |
| Other current liability acquired under asset purchase agreement | | $ | 140,000 | | | $ | - | |
| Common stock issued under asset purchase agreement | | $ | 10,500 | | | $ | - | |
The accompanying notes are an integral part of these audited financial statements.
Costas, Inc.
Notes to Financial Statements
For Years Ended December 31, 2022 and 2021
NOTE 1 - NATURE OF OPERATIONS
Historical Information
The Company was originally organized as Costas, Inc. under the corporate laws of the State of Nevada on December 10, 1998. On July 1,2010, the Company purchased the technology assets of eJob Resource, Inc. The purchase included eJob Resources’ online job search and posting site to provide a virtual bridge between the Indian and U.S. technology job markets; all job search technology, which aggregates job posting from many sites, and make them available via XML, API.
On July 17, 2014, the Company amended its Articles of Incorporation by approving a 25 for 1 reverse split.
On January 21, 2015, the Company entered into an agreement with Mr. James Brooks to provide certain services to the Company in exchange for a salary of $10,000 per month and 2,550,000 common shares of the Company.
In January 2016 the Company purchased 48% of AuthentaTradeLtd, a Seychelles based corporation, with operations in Cyprus whose function was building a digital currency exchange platform. The remaining 52% was purchased in January 2018.
On September 20, 2017, Mr. James Brooks, a creditor of the Company was granted a Judgment against the Issuer in the principal amount of $1,114,500 with respect to unpaid salary and non-issuance of common shares as required under the original 2015 agreement.
In November 2018, the Company changed its business model to participate in on-line gaming, which operations ceased during the three months period ended March 31, 2019.
On May 20, 2019, the Company announced the completion of the acquisition of Nano Creaciones Sapi de C.V., a Mexican company. The Company issued a total of 25,000,000 shares as consideration for the acquisition. The Company has not received sufficient support from the vendors to confirm ownership of this Mexican entity, and therefore has not included its operations in these condensed financial statements.
On October 21, 2020, Mr. James Brooks, the creditor of the Company holding a judgment in the principal amount of $1,114,500 filed a motion requesting the appointment of a Receiver over the Company. By order filed on November 7, 2020, the Eighth Judicial District Court for Clark County, Nevada appointed Frederick P. Waid as Receiver for the Company in Case No. A-17-749977-B. Notice of entry of that order was filed on November 9, 2020. Mr. Waid became the sole officer and director of the Company. The Receiver was not provided any historical accounting documents from former management as part of the proceedings. As a result of the aforementioned actions, the Court approved an amended opening balance sheet for the Company as of December 31, 2019 which reflects the debt owing to Mr. Brooks, previously omitted, including accrued interest as well as any other approved amounts while eliminating any outstanding debts not approved during the receivership.
On March 25, 2021, the Receiver filed a motion with the Court requesting approval to appoint Mr. Brooks as an officer and director of the Company and to increase the authorized capital of the Company and subsequently to issue sufficient common and preferred shares on terms to be finalized between the Receiver and Mr. Brooks, to settle a total of $175,000 of outstanding debt. Further, subsequent to the March 25, 2021, order, the Receiver sought and received approval from the Court to eliminate certain unsupported assets, outstanding payables and convertible loans on the financial statements of the Company as at December 31, 2019. The Receiver further placed an administrative hold on a total of 26,500,000 shares issued in 2019 for the acquisition of Nano Creaciones Sapi de C.V., a Mexican company, and as consideration for services purported to be rendered, due to the fact that there was no verifiable support for the completion of the acquisition or the provision of services.
Costas, Inc.
Notes to Financial Statements
For Years Ended December 31, 2022 and 2021
NOTE 1 - NATURE OF OPERATIONS (Continued)
Current Information (cont’d)
On January 26, 2022, with an effective date of December 23, 2021, three hundred million (300,000,000) shares of the Company’s common stock were issued to Mr. James Brooks pursuant to a Court Order entered in the Eighth District Judicial Court, Clark County, Nevada, Case No. A-17-749977-B, resulting in a change of control of the Company. The issuance of 300,000,000 shares to Mr. Brooks was issued in partial settlement of debt owed to Mr. Brooks. On December 30, 2021, Mr. Brooks was named the sole officer and director of the Company. On February 9, 2022, an Order was entered in the Eighth Judicial District Judicial Court, Clark County, Nevada, Case No. A-17-749977D by the Appointed Receiver of the Company, Frederick Waid, terminating the receivership for the Company. Concurrently, the Company changed its operating address to 424 E Central Blvd, Suite 308, Orlando, Florida 32801.
On May 6, 2022, the Company entered into a formal acquisition agreement with Standard Dental Labs Inc. (“SDL”), a Wyoming corporation controlled by the Company’s CEO, James Brooks, in order to acquire certain assets including: (i) a ready to implement business model and platform for the identification and acquisition of small to medium sized dental labs in the United States, and (ii) a fully developed branding package created under SDL, including logo, website, presentation materials and corporate name. Under the terms of the acquisition agreement, assets valued at $75,900 was acquired through the issuance of a total of 31,663,760 shares of the Company’s unregistered, restricted common stock to SDL. With the conclusion of this acquisition, the Company intends to operate in the dental lab industry, paving the way for future acquisitions and consolidations in the industry. The assets acquired from SDL will allow the Company to immediately facilitate the acquisition of small to medium sized dental labs, of which there are thousands in the United States.
On August 15, 2022 the Company completed of a definitive agreement to acquire the assets of Prime Dental Lab, LLC. ("Prime Dental"), an Orlando-based dental lab in operation since 2012. Total consideration was paid to the shareholders of Prime Dental in a combination of cash and registered shares for the assets, which includes all equipment, customer relationships, and associated revenue. The Company commenced operations in the dental lab business effective September 1, 2022.
On August 17, 2022 the Company’s board of directors and majority shareholder increased the Company’s authorized share capital from 1.25bn to 2bn shares of common stock.
NOTE 2 – GOING CONCERN
The Company’s financial statements are prepared in accordance with generally accepted accounting principles applicable to a going concern. This contemplates the realization of assets and the liquidation of liabilities in the normal course of business. The Company has recently acquired assets including branding and a detailed business plan to facilitate the acquisition of small to medium sized dental labs, as well as its first dental lab operation. Presently the Company does not have a source of revenue sufficient to cover all of its operating costs. While we have recently commenced revenue generating operations, the Company’s sole officer and director is currently providing capital for operational shortfalls as needed by the Company, and we continue to raise proceeds from the sale of convertible notes having received gross proceeds of $232,140 through December 31, 2022, there remains substantial doubt about our ability to continue as a going concern. As at December 31, 2022, the Company has $13,123 cash on hand, and substantial debt. As we continue to implement our business plan, the Company may continue to be dependent upon financing from our sole officer and director, and the raising of additional capital through placement of our common stock or debt financing. There can be no assurance that the Company will be successful in either situation in order to continue as a going concern.
The ability of the Company to continue as a going concern is dependent on attaining profitable operations. There are no assurances that the Company will be able to meet its obligations, raise funds or conclude additional acquisitions of identified businesses. Further upon acquisition of any target businesses there is no guarantee these operations will reach profitability. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts, or amount and classification of liabilities that might cause results from this uncertainty.
Costas, Inc.
Notes to Financial Statements
For Years Ended December 31, 2022 and 2021
NOTE 2 – GOING CONCERN (continued)
COVID-19 and other factors
While the COVID-19 pandemic has subsided, COVID-19 could continue to have an adverse impact on the Company going forward. COVID-19 caused significant disruptions to the global financial markets, which could impact the Company’s ability to obtain materials necessary for its dental lab operations, result in staffing shortfalls or create difficulty raising capital to fund future acquisitions as the global economy continues to recover. Additional factors which may impact the Company’s ongoing operations include inflation, the recent war in the Ukraine, climate change and others. These events may have serious adverse impact on domestic and foreign economies which may impact the Company’s operations as a result of a variety of factors including the potential for reduced consumer spending. The Company is unable to predict the ongoing impact of these factors on the Company’s financial operations.
NOTE 3 - USE OF ESTIMATES IN THE PREPARATION OF FINANCIAL STATEMENTS
The preparation of financial statements in conformity with Generally Accepted Accounting Principles (GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent assets and liabilities, at the date of these financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Fiscal Year End
The Company has selected December 31 as its fiscal year end.
Basis of Presentation
The accompanying unaudited condensed financial statements have been prepared in accordance with generally accepted accounting principles (US GAAP). In the opinion of management, all adjustments considered necessary for a fair presentation have been included. All such adjustments are of a normal recurring nature.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less to be cash equivalents.
Beneficial Conversion Feature
For conventional convertible debt where the rate of conversion is below market value, the Company records any “beneficial conversion feature” (“BCF”) intrinsic value as additional paid in capital and related debt discount. When the Company records a BCF, the relative fair value of the BCF is recorded as a debt discount against the face amount of the respective debt instrument. The discount is amortized over the life of the debt. If a conversion of the underlying debt occurs, a proportionate share of the unamortized amounts is immediately expensed.
Property and Equipment
Property and equipment are recorded at cost. Depreciation on property and equipment are determined using the straight-line method over the one to eight year estimated useful lives of the assets.
Intangible Assets
During the year ended December 31, 2022, the Company has acquired (i) a ready to implement business model and platform for the identification and acquisition of small to medium sized dental labs in the United States, and (ii) a fully developed branding package including logo, website, presentation materials and corporate name. A total of $31,663 has been capitalized in respect to these assets. The Company has also recognized assets for customer relationships in the amount of $72,440 in respect to a recent asset purchase agreement with Prime Dental Labs, whereunder we acquired assets to commence operation of our first dental lab. The Company will review these intangible assets for impairment at a minimum of once per year or whenever events or changes in circumstances suggest a need for evaluation. There is no impairment expense for the intangible assets in the period ended December 31, 2022.
Costas, Inc.
Notes to Financial Statements
For Years Ended December 31, 2022 and 2021
NOTE 4 – SUMMARY OF ACCOUNTING POLICIES (continued)
Impairment
Our long-lived assets are subject to an impairment test if there is an indicator of impairment. The carrying value and ultimate realization of these assets is dependent upon our estimates of future earnings and benefits that we expect to generate from their use. If our expectations of future results and cash flows are significantly diminished, other long-lived assets may be impaired and the resulting charge to operations may be material. When we determine that the carrying value of intangibles or other long-lived assets may not be recoverable based upon the existence of one or more indicators of impairment, we use the projected undiscounted cash flow method or realizable value to determine whether an impairment exists, and then measure the impairment using discounted cash flows.
Revenue Recognition
The Company applies ASC 606 — Revenue from Contracts with Customers. Under ASC 606, the Company recognizes revenue from the sales of products by applying the following steps: (1) identify the contract with a customer; (2) identify the performance obligations in the contract; (3) determine the transaction price; (4) allocate the transaction price to each performance obligation in the contract; and (5) recognize revenue when each performance obligation is satisfied.
The Company recognizes revenue when the earnings process is complete and persuasive evidence of an arrangement exists. This generally occurs when a purchased product has been delivered to a customer from our lab facility, at which time both title and the risks and rewards of ownership are transferred to and accepted by the customer, and the selling price has been collected.
Inventory
Inventories, if maintained, consist of work-in-progress inventory or replacement parts on hand in order to complete customer orders.
Warranty
We do not record warranty liabilities at the time of sale for the estimated costs that may be incurred under the terms of the applicable limited warranty as all component parts are covered by our respective industry suppliers. Our products are custom created for the individual client, and therefore we have no formal return policy or money back guarantee, however, if a product is determined to be defective, we will deliver a replacement unit to meet expected customer service terms. We assess the need for warranty and return liabilities at each report date.
Cost of Sales
Cost of sales includes actual product cost, labor, and allocated overheard, which is applied on a per unit basis.
Accounts Receivable
Accounts receivable is trade related. The Company’s management has established an allowance for bad debt based upon accounts receivable that are more than one year past due. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the necessary for reserves for bad debt. Reserves, if required, are recorded on management’s best estimate of collection. At December 31, 2022 there were no outstanding accounts receivable.
Basic and Diluted Loss Per Share
The Company computed basic and diluted loss per share amounts pursuant to the ASC 260 “Earnings per Share.” There are no potentially dilutive shares outstanding and, accordingly, dilutive per share amounts have not been presented in the accompanying statements of operations.
Potential common stock consists of the incremental common stock issuable upon the exercise of convertible notes (using the if-converted method). The table below reflects the potentially dilutive securities at each reporting period, which have not been included in the computation of diluted net loss per share due to their anti-dilutive effect:
Costas, Inc.
Notes to Financial Statements
For Years Ended December 31, 2022 and 2021
NOTE 4 – SUMMARY OF ACCOUNTING POLICIES (continued)
Basic and Diluted Loss Per Share (cont’d)
| | | December 31, 2022 | | | December 31, 2021 | |
| Convertible notes (principal balance) at $0.001 per share | | | 1,403,867,310 | | | | 1,171,727,310 | |
Income Taxes
Income taxes are recognized in accordance with ASC 740, “Income Taxes”, whereby deferred income tax liabilities or assets at the end of each period are determined using the tax rate expected to be in effect when the taxes are actually paid or recovered. A valuation allowance is recognized on deferred tax assets when it is more likely than not that some or all of these deferred tax assets will not be realized.
Recent Accounting Pronouncements
In August 2020, the FASB issued ASU 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. The new guidance, among other things, simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments, and amends existing earnings-per-share (“EPS”) guidance by requiring that an entity use the if-converted method when calculating diluted EPS for convertible instruments. ASU 2020-06 is effective for public business entities that meet the definition of an SEC filer, excluding entities eligible to be smaller reporting companies as defined by the SEC, for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. The Company plans to adopt the new guidance effective January 1, 2024.
NOTE 5 – ASSET PURCHASE AGREEMENTS
(1) On May 6, 2022, the Company entered into a formal acquisition agreement with Standard Dental Labs Inc. (“SDL”), a Wyoming corporation controlled by the Company’s CEO, James Brooks, to acquire certain assets including: (i) a ready to implement business model and platform for the identification and acquisition of small to medium sized dental labs in the United States, and (ii) a fully developed branding package created under SDL, including logo, website, presentation materials and corporate name. Under the terms of the acquisition agreement, assets valued at $75,900 were acquired through the issuance of a total of 31,663,760 shares of the Company’s unregistered, restricted common stock to SDL. The transaction occurred under common control and as a result, the issued shares were valued at par value, or $0.001 per share, and a total of $31,663 was recorded as intangible assets on the Company’s balance sheet.
(2) On August 15, 2022 the Company (the “Acquiror”) announced the completion of a definitive agreement to acquire the assets of Prime Dental Lab, LLC. ("PDL"), an Orlando-based dental lab in operation since 2012. The Purchased Assets consisted of: all client contracts for existing PDL clients; certain physical assets of PDL including all dental lab equipment, furniture, computers and other office equipment; the assumption of certain contracts, equipment leases and office leases (if any); certain employees and management of PDL as determined by the Acquiror to be retained and/or contracted by the Acquiror; and specifically the right to continue to use the name “Prime Dental Lab LLC” along with certain other rights, trademarks, intellectual property and intangible assets of the Seller. The Purchase Price consisted of 750,000 unregistered, restricted Common Shares (the “Consideration Shares”) of the Acquiror (the “Share Consideration) plus additional cash consideration in the amount of $140,000.00 (the “Cash Consideration”) payable in two (2) equal instalments of seventy thousand ($70,000.00) dollars (each a “Cash Instalment”). The first Cash Instalment shall be paid by the Acquiror to PDL no later than fifteen (15) calendar days after receipt by the Acquiror of a Notice of Effect from the Securities and Exchange Commission of a Form S-1 Registration Statement (the “First Cash Instalment”). The second Cash Instalment of seventy thousand ($70,000.00) dollars shall be paid by the Acquiror to PDL on the date that is no later than ninety (90) calendar days subsequent to the payment of the First Cash Instalment (the “Second Cash Instalment”).
Costas, Inc.
Notes to Financial Statements
For Years Ended December 31, 2022 and 2021
NOTE 5 – ASSET PURCHASE AGREEMENTS (continued)
The Share Consideration is subject to a lock-up agreement for a term of twenty-four months from the issuance date, whereunder PDL shall be entitled to a release of 12.5% of the total Consideration Shares each quarter (93,750 shares) provided certain minimum quarterly revenue targets are achieved. Further, the Company has agreed to include such Consideration Shares in any registration statement filed, and in the event that the Company decides to approve and complete a share consolidation or share rollback within twelve (12) months after the date of the issuance of the Consideration Shares, such shares shall be protected from such share consolidation action (on a one-time basis).
The Company allocated the acquired assets on the Company’s balance sheets as of the date of closing as Property and Equipment and Intangible Assets at fair market value. Assets acquired were as follows:
| 750,000 shares of common stock | | $ | 10,500 | |
| Cash consideration – other current liability | | | 140,000 | |
| Total consideration purchase cost | | $ | 150,500 | |
| | | | | |
| Allocation: | | | | |
| Property and equipment | | $ | 78,060 | |
| Customer relationships | | | 72,440 | |
| Total purchased assets | | $ | 150,500 | |
The purchase accounting for the acquisition of assets from PDL includes an analysis of all available information as at the acquisition date. Management may continue to evaluate information about circumstances that existed as of the acquisition date and recognize measurement period adjustments prospectively. The measurement period is not to exceed 12 months from the respective date of acquisition.
NOTE 6 – SUBCONTRACTOR AGREEMENT
Concurrent with the closing of the acquisition of certain assets from Prime Dental Lab, LLC (“PDL”) (see Note 5(2)) on August 31, 2022, the Company entered into a subcontractor agreement with Mr. John Kim for the provision of certain services including labor and manufacturing expertise and management oversight services with respect to the production of appliances, orthotics and prosthetics in order to service the Company’s acquired client list. Mr. Kim shall have available for his exclusive use certain acquired assets in order to facilitate the provision of finished products and shall charge the company a fixed cost per product manufactured including an overhead rate for the use of the laboratory space owned by Mr. Kim where the Company’s manufacturing equipment is located. In addition, Mr. Kim shall remit to the Company a flat usage fee monthly for access to the Company’s equipment and shall receive a monthly management fee in consideration of his services. Invoicing to the customer is managed by the Company under its dba Prime Dental Lab LLC and all obligations for performance rest with the Company under the terms of the Company’s agreements with the customers as set out in the respective purchase orders for the manufactured goods.
NOTE 7 – JUDGMENT PAYABLE AND CONVERTIBLE NOTE
During fiscal 2017, Mr. James Brooks (“Brooks”), a creditor of the Company, obtained a judgment in the principal amount of $1,114,500. Previously, on January 21, 2015, the Company entered into an agreement with Mr. Brooks whereunder he would provide certain services to the Company in exchange for a salary of $10,000 per month and 2,550,000 common shares of the Company. Under the terms of this contract, Mr. Brooks was owed $120,000 in salary and 2,550,000 shares, which consideration was not provided by the Company in accordance with the contract terms. On January 23, 2017 Mr. Brooks filed a complaint in respect to amounts payable and applicable damages. The Company failed to respond to the action, and on August 2, 2017, Mr. Brooks filed a motion for entry of default judgment. On September 6, 2017, the court determined the unpaid 2,550,000 common shares had a market value of $994,500 at the time they were originally deliverable to Mr. Brooks. In addition to the value of the unpaid shares, unpaid salary of $120,000 resulted in a judgment of $1,114,500. Concurrently, the court granted post-judgment interest pursuant to Nevada Revised Statute 17.130 which provides that when there is no express contract in writing, interest must be allowed at a rate equal to the prime rate at the largest bank in Nevada, as ascertained by the Commissioner of Financial Institution on January 1 or July 1 as the case may be, immediately preceding the date of the transaction, plus 2 percent. The rate must be adjusted accordingly on each January 1 and July 1 thereafter until the judgment is satisfied.
Costas, Inc.
Notes to Financial Statements
For Years Ended December 31, 2022 and 2021
NOTE 7 – JUDGMENT PAYABLE AND CONVERTIBLE NOTE (continued)
As a result, interest applied on the judgment over the applicable periods was as follows:
| January 1, 2021 | 5.25% | July 1, 2020 | 5.25% | January 1, 2020 | 6.75% |
On March 25, 2021, the Court approved the first proposed settlement of a portion of Brooks’ debt, in the amount of One Hundred Seventy-Five Thousand Dollars ($175,000) (the “Settlement Debt”) to be paid via the issuance of certain common shares of the Company. On December 6, 2021, Brooks entered into certain Debt Assignment and Purchase Agreements with several third parties in the accumulated amount of $70,000. (See Note 9). On December 23, 2021, the Company entered into an 8% Convertible Promissory Note with Brooks, our then sole officer and director, in the amount of $1,171,727. Concurrently, three hundred million (300,000,000) shares of the Company’s common stock were issued to Brooks pursuant to a Court Order entered in the Eighth District Judicial Court, Clark County, Nevada, Case No. A-17-749977-B. The Company valued the 300,000,000 shares at the closing price of the Company’s stock as traded on the OTCMarkets on the date of issuance and recorded a loss on the extinguishment of debt of $10,025,000.
The convertible promissory note bears interest rate at 8% per annum for a period of 12 months. The holder has the right to convert any or all of the outstanding principal into shares of the Company’s common stock at a conversion price of $0.001 per share. The beneficial conversion feature associated with the note and realized on issuance date totaled $1,171,727, which amount is being amortized over the term of the note, or 12 months. Prior to maturity, Mr. Brooks agreed to extend the repayment date of the note to December 31, 2023.
Interest payable included in accounts payable and the principal outstanding balance of the debt as at each period-end are as follows:
| | | Interest Payable | | | Shareholder Loan | | | Convertible Note | | | Total | |
| Balance, December 31, 2020 | | | 247,564 | | | | 1,114,500 | | | | - | | | | 1,362,064 | |
| Interest expense on shareholder loan | | | 54,663 | | | | - | | | | - | | | | 54,663 | |
| Interest expense on convertible note | | | 2,054 | | | | - | | | | - | | | | 2,054 | |
| Debt assignment and purchase agreements | | | - | | | | (70,000 | ) | | | - | | | | (70,000 | ) |
| Issuance of 300,000,000 shares | | | | | | | (175,000 | ) | | | | | | | (175,000 | ) |
| Debt reclassification | | | (302,227 | ) | | | (869,500 | ) | | | 1,171,727 | | | | - | |
| Subtotal | | | 2,054 | | | | - | | | | 1,171,727 | | | | 1,173,781 | |
| Unamortized debt discount | | | - | | | | - | | | | (1,146,045 | ) | | | (1,146,045 | ) |
| Balance December 31, 2021 | | | 2,054 | | | | - | | | | 25,682 | | | | 27,736 | |
| Interest expense on convertible note | | | 93,738 | | | | - | | | | - | | | | 93,738 | |
| (Repayments to interest expense) | | | (5,877 | ) | | | - | | | | - | | | | (5,877 | ) |
| Amortized Debt Discount | | | - | | | | - | | | | 1,146,045 | | | | 1,146,045 | |
| Balance, December 31, 2022 | | $ | 89,915 | | | $ | - | | | $ | 1,171,727 | | | $ | 1,261,642 | |
NOTE 8 – CONVERTIBLE NOTES
During the fiscal year ended December 31, 2022, the company issued convertible promissory notes in the principal amount of $232,140 to several investors bearing interest at 8% per annum for a period of 12 months. The holders have the right to convert any or all of the outstanding principal into shares of the Company’s common stock at a conversion price of $0.001 per share. The beneficial conversion feature associated with the note and realized on issuance date totaled $232,140, which amount is being amortized over the term of the note, or 12 months.
Costas, Inc.
Notes to Financial Statements
For Years Ended December 31, 2022 and 2021
NOTE 8 – CONVERTIBLE NOTES (continued)
| | | Interest Payable | | | Convertible Note | | | Total | |
| Balance, December 31, 2021 | | $ | - | | | $ | - | | | $ | - | |
| Proceeds | | | - | | | | 232,140 | | | | 232,140 | |
| Unamortized debt discount | | | - | | | | (232,140 | ) | | | (232,140 | ) |
| Subtotal | | | - | | | | - | | | | - | |
| Interest expense on convertible note | | | 7,380 | | | | - | | | | 7,380 | |
| Amortized Debt Discount | | | - | | | | 92,246 | | | | 92,246 | |
| Balance, December 31, 2022 | | $ | 7,380 | | | $ | 92,246 | | | $ | 99,626 | |
NOTE 9 – DEBT ASSIGNMENTS AND PURCHASE AGREEMENT
On December 6, 2021, Mr. James Brooks, a creditor of the Company entered into certain Debt Assignment and Purchase Agreements with third parties in the accumulated amount of $70,000. Subsequent to the Debt Assignment and Purchase Agreements, these third parties entered into non-interest-bearing convertible notes with the Company whereunder the debt may be converted into shares of the Company’s common stock at par value or, $0.001 per share. The total discount recognized as a result of the beneficial conversion feature realized on the date of the notes was $70,000, which amount was fully amortized on issuance date, and recorded as interest expenses.
On December 13 and December 14, 2021, the Company received Notices of Conversion from the aforementioned third parties, and debt in the amount of $70,000 was converted into 70,000,000 shares of common stock.
NOTE 10 – RELATED PARTY TRANSACTIONS
Fred Waid, former sole officer and director
During the year ended December 31, 2020, Intermountain Fiduciary Services Inc., a company of which Fred Waid, the former Receiver and our former sole officer and director, is also director and officer (“IFSI”), advanced a total of $1,725 for legal and professional which remained unpaid at December 31, 2020.
During the fiscal year ended December 31, 2021, IFSI incurred an additional $13,275 in legal and professional fees, respectively. The Company made cash payment in the amount of $15,000 to Intermountain and no additional invoices were received during the year ended December 31, 2022. At December 31, 2022 and December 31, 2021, $0 and $15,000, respectively, is reflected on the balance sheet as accounts payable – related party.
James Brooks, sole officer and director, controlling shareholder
On December 30, 2021, Mr. James Brooks was appointed the Company’s sole officer and director in place of Mr. Fred Waid. Immediately prior Mr. Brooks became the Company’s controlling shareholder upon issuance of 300,000,000 shares of common stock for certain debt in the amount of $175,000. Concurrently the Company and Mr. Brooks entered into a convertible note with respect to amounts payable totaling an accumulated $1,171,727 (Note 7 above).
During the year ended December 31, 2021, Mr., Brooks and companies controlled by him advanced a total of $16,532 for operating expenses.
During the year ended December 31, 2022, Mr. Brooks and companies controlled by him advanced a further $162,220 for operating expenses and the Company made cash payments to reduce the total balance payable in the amount of $178,752. As at December 31, 2022, the amount owing to Mr. Brooks for advances for operations is $0 (December 31, 2021 - $16,532) which amount is reflected on the balance sheets as advances payable – related party.
Costas, Inc.
Notes to Financial Statements
For Years Ended December 31, 2022 and 2021
NOTE 10 – RELATED PARTY TRANSACTIONS (continued)
James Brooks, sole officer and director, controlling shareholder (continued)
During the year ended December 31, 2022 the Company also recorded interest expenses of $93,738 (2021 - $2,054) for Mr. Brooks on his convertible note (see Note 7 above). Mr. Brooks received payments against interest of $5,887 in the year ended December 31, 2022 (2021 - $0) leaving a balance owing of $89,915 which is reflected on the balance sheets as accounts payable – related party.
During the year ended December 31, 2022, the Company acquired certain assets by way of issuance of 31,663,760 common shares of the Company’s restricted, unregistered common stock to a company controlled by Mr. Brooks. (see Note 5 (1)).
During the year ended December 31, 2022 the Company paid $30,000 to Mr. Brooks in respect to a monthly expense allowance of $2,500.
NOTE 11 – COMMON STOCK
The Company has authorized a total of 2,000,000,000 shares of common stock, $0.001 par value.
During the year ended December 31, 2021, 70,000,000 shares were issued upon conversion of certain convertible notes. (see Note 9).
During the year ended December 31, 2021, 300,000,000 shares were issued pursuant to a court order in settlement of $175,000 payable to our sole officer and director, Mr. James Brooks. (see Note 7)
On May 6, 2022, 31,663,760 shares were issued in respect to an asset purchase agreement. (see Note 5 (1)).
On August 31, 2022, 750,000 shares were issued in respect to an asset purchase agreement. (see Note 5(2)).
At December 31, 2022 and December 31, 2021, there was a total of 445,728,363 and 413,314,603 shares issued and outstanding, respectively.
NOTE 12 – PURCHASE AGREEMENT WORLD AMBER CORP.
On November 22, 2022, we entered into a purchase agreement (the “Purchase Agreement”), and a registration rights agreement (the “Registration Rights Agreement”) with World Amber Corp. (“World Amber”), a Nevada corporation, pursuant to which World Amber committed to purchase up to $2,500,000 of our common stock. Under the terms and subject to the conditions of the Purchase Agreement, we have the obligation, to sell to World Amber, and World Amber is obligated to purchase up to $2,500,000 of shares of our common stock. Future sales of common stock under the Purchase Agreement, if any, will be subject to certain limitations, and may occur from time to time, over the 24-month period commencing on the date that a registration statement is filed with the Securities and Exchange Commission (the “SEC”) pursuant to the Registration Rights Agreement, and is declared effective by the SEC and a final prospectus in connection therewith is filed and the other conditions set forth in the Purchase Agreement are satisfied (such date on which all of such conditions are satisfied, the “Commencement Date”).
After the Commencement Date, for every month over the term of the Purchase Agreement, the Company has the right, in its sole discretion, to direct World Amber to purchase up to 346,667 shares of common stock per business day, at $0.30 per share (each, a “Regular Purchase”). In each case, World Amber’s maximum commitment in any single Regular Purchase may not exceed $104,000.
Costas, Inc.
Notes to Financial Statements
For Years Ended December 31, 2022 and 2021
NOTE 12 – PURCHASE AGREEMENT WORLD AMBER CORP. (continued)
Pursuant to the terms of the Purchase Agreement, in no event may the Company issue or sell to World Amber any shares of our common stock under the Purchase Agreement which, when aggregated with all other shares of common stock then beneficially owned by the World Amber and its affiliates, would result in the beneficial ownership by World Amber and its affiliates of more than 9.99% of the then issued and outstanding shares of common stock (the “Beneficial Ownership Limitation”).
The Purchase Agreement and the Registration Rights Agreement contain customary representations, warranties, agreements, conditions and indemnification obligations of the parties. The Company has the right to terminate the Purchase Agreement at any time, at no cost or penalty.
The Company filed its initial registration statement on December 30, 2022 and has not yet received Notice of Effect.
NOTE 13 - SUBSEQUENT EVENTS
Subsequent to December 31, 2022, the Company entered into certain 8% interest convertible note agreements (the “CPNs” with various individual investors for total gross proceeds of $50,000. Under the terms of the agreements holders of the CPNs may convert the principal balance of the notes to unregistered, restricted shares of the Company’s common stock at $0.001 at any time with three (3) days written notice.
Subsequent to December 31, 2022, the Company entered into certain zero interest convertible note agreements (the “CPNs” with various individual investors for total gross proceeds of $136,000. Under the terms of the agreements holders of the CPNs may convert the principal balance of the notes to unregistered, restricted shares of the Company’s common stock at $0.004 at any time with three (3) days written notice.
Subsequent to December 31, 2022 the Company and John Kim executed an addendum to the definitive agreement with PDL (Ref: Note 5(2)) (the “PDL Amendment”) whereunder the Cash Consideration as part of Purchase Price was revised so that the first instalment shall be released immediately upon execution of the PDL Amendment, or March 24, 2023, and the second Cash Instalment shall be payable on the date that is no later than seven (7) calendar days subsequent to the receipt of proceeds from the first payment under the Purchase Agreement with World Amber (Ref: Note 12).
The Company has evaluated subsequent events from the balance sheet date through the date that the financial statements were issued and determined that there are no additional subsequent events to disclose.
Prime Dental Lab, LLC
Audited Financial Statements for the years ended
December 31, 2021, and 2020
PRIME DENTAL LAB LLC
TABLE OF CONTENTS FOR AUDITED
FINANCIAL STATEMENTS

Report of Independent Registered Public Accounting Firm
To the Members of Prime Dental Lab, LLC.
We have audited the accompanying balance sheets of Prime Dental Lab, LLC (the “Company”) as of December 31, 2021, and 2020, the related statements of operations and cash flows, for each of the two years in the period ended December 31, 2021, and 2020, and the related notes collectively referred to as the "financial statements”.
In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021, and 2020, and the results of its operations and its cash flows for the year ended December 31, 2021, and 2020, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB. We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.

OLAYINKA OYEBOLA & CO.
(Chartered Accountants)
We have served as the Company's auditor since August 2022.
December 29th, 2022.
Prime Dental Lab, LLC.
(Stated in U.S. Dollars)
| | | December 31, 2021 | | | December 31, 2020 | |
| | | | | | | |
| ASSETS | | | | | | |
| | | | | | | |
| Current Assets | | | | | | |
| Cash and cash equivalents | | $ | 32,736 | | | $ | 45,131 | |
| Accounts receivable | | | 46,469 | | | | 45,480 | |
| Other current assets | | | - | | | | 8,710 | |
| Total Current Assets | | | 79,205 | | | | 99,321 | |
| | | | | | | | | |
| Property and equipment, net | | | 12,758 | | | 17,950 | |
| Total Assets | | $ | 91,963 | | | $ | 117,271 | |
| | | | | | | | | |
| LIABILITIES AND MEMBERS’ EQUITY (DEFICIT) | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable and accrued expenses | | | 6,451 | | | | 33,974 | |
| Loan payable | | | 152,500 | | | | 153,900 | |
| Total Current Liabilities | | | 158,951 | | | | 187,874 | |
| | | | | | | | | |
| Total Liabilities | | | 158,951 | | | | 187,874 | |
| | | | | | | | | |
| Members’ Equity (Deficit) | | | | | | | | |
| Members Capital | | | 100 | | | | 100 | |
| Accumulated Deficit | | | (67,088 | ) | | | (70,703 | ) |
| Total Members’ Equity (Deficit) | | | (66,988 | ) | | | (70,603 | ) |
| Total Liabilities and Stockholders’ Equity (Deficit) | | $ | 91,963 | | | $ | 117,271 | |
The accompanying notes are an integral part of these financial statements
Prime Dental Lab, LLC.
Statements of Operations and Comprehensive Loss
(Stated in U.S. Dollars)
| | | For the year ended December 31, | |
| | | 2021 | | | 2020 | |
| Revenues | | $ | 627,894 | | | $ | 464,359 | |
| Cost of revenues | | | 438,161 | | | | 339,220 | |
| Gross profit | | | 189,733 | | | | 125,139 | |
| | | | | | | | | |
| Operating expenses | | | | | | | | |
| General and Administrative | | | 221,838 | | | | 249,323 | |
| Total operating expenses | | | 221,838 | | | | 249,323 | |
| | | | | | | | | |
| Loss from Operations | | | (32,105 | ) | | | (124,184 | ) |
| | | | | | | | | |
| Other Income | | | | | | | | |
| PPP forgiveness | | | 35,720 | | | | 29,790 | |
| Total Other Income | | | 35,720 | | | | 29,790 | |
| | | | | | | | | |
| Provisions for income taxes | | | - | | | | - | |
| | | | | | | | | |
| Net income (loss) | | $ | 3,615 | | | $ | (94,394 | ) |
The accompanying notes are an integral part of these financial statements
Prime Dental Lab, LLC.
Statements of Cash Flows
(Stated in U.S. Dollars)
| | | For the year ended December 31, | |
| | | 2021 | | | 2020 | |
| | | | | | | |
| CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | |
| Net income (loss) | | $ | 3,615 | | | $ | (94,394 | ) |
| Adjustments to reconcile net (loss) to net cash used in operating activities: | | | | | | | | |
| Depreciation | | | 14,102 | | | | 4,187 | |
| PPP forgiveness | | | (35,720 | ) | | | (29,790 | ) |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | (989 | ) | | | (11,870 | ) |
| Other current assets | | | 8,710 | | | | - | |
| Accounts payable and accrued expenses | | | (27,523 | ) | | | (1,858 | ) |
| Net Cash used in operating activities | | | (37,805 | ) | | | (133,725 | ) |
| | | | | | | | | |
| CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | | | |
| Purchase property and equipment | | | (8,910 | ) | | | (8,837 | ) |
| Net Cash used in financing activities | | | (8,910 | ) | | | (8,837 | ) |
| | | | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | |
| Proceeds from loan payable | | | 40,720 | | | | 183,690 | |
| Payment to loan payable | | | (6,400 | ) | | | - | |
| Net Cash provided by financing activities | | | 34,320 | | | | 183,690 | |
| | | | | | | | | |
| INCREASE (DECREASE) IN CASH | | | (12,395 | ) | | | 41,128 | |
| CASH AT BEGINNING OF YEAR | | | 45,131 | | | | 4,003 | |
| CASH AT END OF YEAR | | $ | 32,736 | | | $ | 45,131 | |
| | | | | | | | | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION | | | | | | | | |
| Interest Paid | | $ | - | | | $ | - | |
| Taxes Paid | | $ | - | | | $ | - | |
The accompanying notes are an integral part of these financial statements
Prime Dental Lab, LLC.
Notes to the Financial Statements
December 31, 2021 and 2020
NOTE 1. DESCRIPTION OF BUSINESS
Prime Dental Lab, LLC. (the “Company”) is an Orlando-based dental lab in operation since 2012.
The business purpose of the company is currently manufacturing dental prosthetics for dentists and dental clinics at its operational lab facility. The Company’s founder and sole director and officer is Jongpil (John) Kim.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Fiscal year
The Company has selected December 31 as its fiscal year end.
Basis of Presentation
The accompanying financial statements have been prepared by the Company in accordance with accounting principles generally accepted in the United States (“GAAP”), and pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”).
Use of Estimates
The preparation of these consolidated financial statements in conformity with United States generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to long-lived assets and deferred income tax asset valuation allowances. The Company bases its estimates and assumptions on current facts, historical experience and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between the estimates and the actual results, future results of operations will be affected.
Cash and Cash Equivalents
For financial accounting purposes, cash and cash equivalents are considered to be all highly liquid investments with a maturity of three (3) months or less at the time of purchase.
Revenue Recognition
The Company applies ASC 606 — Revenue from Contracts with Customers. Under ASC 606, the Company recognizes revenue from the sales of products by applying the following steps: (1) identify the contract with a customer; (2) identify the performance obligations in the contract; (3) determine the transaction price; (4) allocate the transaction price to each performance obligation in the contract; and (5) recognize revenue when each performance obligation is satisfied.
The Company recognizes revenue when the earnings process is complete and persuasive evidence of an arrangement exists. This generally occurs when a purchased product has been delivered to a customer from our lab facility, at which time both title and the risks and rewards of ownership are transferred to and accepted by the customer, and the selling price has been collected.
Prime Dental Lab, LLC.
Notes to the Financial Statements
December 31, 2021 and 2020
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Inventory
Inventories, if maintained, consist of work-in-progress inventory or replacement parts on hand in order to complete customer orders.
Warranty
We do not record warranty liabilities at the time of sale for the estimated costs that may be incurred under the terms of the applicable limited warranty as all component parts are covered by our respective industry suppliers. Our products are custom created for the individual client, and therefore we have no formal return policy or money back guarantee, however, if a product is determined to be defective, we will deliver a replacement unit to meet expected customer service terms. We assess the need for warranty and return liabilities at each report date.
Cost of Sales
Cost of sales includes actual product cost, labor, and allocated overheard, which is applied on a per unit basis.
Accounts receivable
Accounts receivable are recognized and carried at original invoiced amount less an estimated allowance for uncollectible accounts.
The Company determines the adequacy of reserves for doubtful accounts based on general and individual account analysis and historical collection trend. The Company establishes general and specific allowance when there is objective evidence that the Company may not be able to collect amounts due. The allowance is based on management’s best estimate of specific losses on individual exposures, as well as a provision on historical trends of collections. The provision is recorded against accounts receivable balances, with a corresponding charge recorded in the consolidated statements of income and comprehensive income. Actual amounts received may differ from management’s estimate of credit worthiness and the economic environment. Delinquent account balances are written-off against the allowance for doubtful accounts after management has determined that the likelihood of collection is not probable. As of December 31, 2021 and 2020, allowance for uncollectible balances amounted to $0.
Fair Value of Financial Instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. A three-level fair value hierarchy prioritizes the inputs used to measure fair value. The hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
| | ● | Level 1 — inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| | ● | Level 2 — inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, quoted market prices for identical or similar assets in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| | ● | Level 3 — inputs to the valuation methodology are unobservable. |
Unless otherwise disclosed, the fair value of the Company’s financial instruments, including cash, accounts receivable, and prepaid expenses, short-term borrowings, accounts payable, due to related parties, and other payables and other current liabilities, approximate the fair value of the respective assets and liabilities as of December 31, 2021 based upon the short-term nature of the assets and liabilities.
Prime Dental Lab, LLC.
Notes to the Financial Statements
December 31, 2021 and 2020
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Property and Equipment
At December 31, 2021 and 2020, property and equipment consists of laboratory building improvements, lab equipment and computers, stated at cost. The Company depreciates the cost of property and equipment using the straight-line method for financial reporting purposes at rates based on the following estimated useful lives:
| | | Years | |
| Lab equipment | | 3-15 | |
| Computers | | | 3 | |
| Building Improvements | | | 5 | |
Expenditures for maintenance and repairs are expensed as incurred.
Income Taxes
The Company has adopted ASC Topic 740 – Income Taxes, which requires the use of the asset and liability method of accounting for income taxes. Under the asset and liability method of ASC Topic 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
Recent accounting pronouncements
Certain new accounting pronouncements that have been issued are not expected to have a material effect on the Company’s financial statements.
Covid-19 Pandemic
COVID-19 has caused significant disruptions to the global financial markets over the past several years, which impacted our ability to raise additional capital. Management is actively monitoring the situation but given the daily evolution of the COVID-19 outbreak, the Company is not able to fully estimate the effects of the COVID-19 outbreak on its planned operations or financial condition in the next 12 months. While significant uncertainty remains as the global pandemic appears to be on the decline, the Company believes it is possible that the COVID-19 outbreak will continue to have a negative impact on its ability to raise additional financing and may result in delays in fully implementing our plan of operations.
NOTE 3: PROPERTY AND EQUIPMENT
Property and equipment, net consists of the following:
| | | December 31, 2021 | | | December 31, 2020 | |
| Office equipment | | $ | 13,288 | | | $ | 4,378 | |
| Leasehold improvements | | | 102,175 | | | | 102,175 | |
| Lab equipment | | | 55,837 | | | | 55,837 | |
| Total | | | 171,300 | | | | 162,390 | |
| Less: accumulated depreciation and amortization | | | (158,542 | ) | | | (144,440 | ) |
| Total property and equipment, net | | $ | 12,758 | | | $ | 17,950 | |
Depreciation expense amounted to $14,102 and $4,187 for the years ended December 31, 2021 and 2020, respectively.
Prime Dental Lab, LLC.
Notes to the Financial Statements
December 31, 2021 and 2020
NOTE 4: LOANS PAYABLE
Loans payable consists of the following:
| | | Paycheck Protection Program (“PPP loan”) | | | SBA loan | |
| December 31, 2019 | | $ | - | | | $ | - | |
| Proceeds from PPP | | | 29,790 | | | | - | |
| Proceeds from SBA | | | - | | | | 153,900 | |
| PPP forgiveness | | | (29,790 | ) | | | - | |
| Balance, December 31, 2020 | | | - | | | | 153,900 | |
| Proceeds from PPP | | | 35,720 | | | | | |
| Proceeds from SBA | | | - | | | | 5,000 | |
| Payment to SBA | | | - | | | | (6,400 | ) |
| PPP forgiveness | | | (35,720 | ) | | | | |
| Balance, December 31, 2021 | | $ | - | | | $ | 152,500 | |
NOTE 5: RELATED PARTY TRANSACTIONS
| Jongpil (John) Kim, Sole officer and director | | December 31, 2021 | | | December 31, 2020 | |
| Cost of revenue, services provided | | $ | 238,990 | | | $ | 206,980 | |
| General and Administrative, services provided | | | 59,746 | | | | 102,175 | |
| Total | | $ | 298,736 | | | $ | 258,725 | |
| Yung Kim (Spouse of Jongpil (John) Kim) | | December 31, 2021 | | | December 31, 2020 | |
| General and Administrative, services provided | | $ | 81,855 | | | $ | 91,410 | |
NOTE 6: SUBSEQUENT EVENTS
On August 15, 2022 the assets of Prime Dental Lab, LLC. was acquired by Costas, Inc. (“Acquiror”) The Purchased Assets consisted of: all client contracts for existing PDL clients; certain physical assets of PDL including all dental lab equipment, furniture, computers and other office equipment; the assumption of certain contracts, equipment leases and office leases (if any); certain employees and management of PDL as determined by the Acquiror to be retained and/or contracted by the Acquiror; and specifically the right to continue to use the name “Prime Dental Lab LLC” along with certain other rights, trademarks, intellectual property and intangible assets of the Seller. The Purchase Price consisted of 750,000 unregistered, restricted Common Shares (the “Consideration Shares”) of the Acquiror (the “Share Consideration) plus additional cash consideration in the amount of $140,000.00 (the “Cash Consideration”) payable in two (2) equal instalments of seventy thousand ($70,000.00) dollars (each a “Cash Instalment”). The first Cash Instalment shall be paid by the Acquiror to PDL no later than fifteen (15) calendar days after receipt by the Acquiror of a Notice of Effect from the Securities and Exchange Commission of a Form S-1 Registration Statement (the “First Cash Instalment”). The second Cash Instalment of seventy thousand ($70,000.00) dollars shall be paid by the Acquiror to PDL on the date that is no later than ninety (90) calendar days subsequent to the payment of the First Cash Instalment (the “Second Cash Instalment”). The Share Consideration is subject to a lock-up agreement for a term of twenty-four months from the issuance date, whereunder PDL shall be entitled to a release of 12.5% of the total Consideration Shares each quarter (93,750 shares) provided certain minimum quarterly revenue targets are achieved. Further, the Company has agreed to include such Consideration Shares in any registration statement filed, and in the event that the Company decides to approve and complete a share consolidation or share rollback within twelve (12) months after the date of the issuance of the Consideration Shares, such shares shall be protected from such share consolidation action (on a one-time basis).
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Forward-Looking Statements
This registration statement contains forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements relate to future events or our future financial performance. In some cases, you can identify forward-looking statements by terminology such as “may”, ‘should”, “expects”, “plans”, “anticipates”, “believes”, “estimates”, “predicts”, “potential” or “continue” or the negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks in the section entitled “Risk Factors” that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to actual results.
Our financial statements are stated in United States Dollars ($) except as otherwise indicated and are prepared in accordance with United States Generally Accepted Accounting Principles. The following discussion should be read in conjunction with our unaudited interim consolidated financial statements and the related notes that appear elsewhere in this registration statement. The following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed below and elsewhere in this registration statement, particularly in the section entitled “Risk Factors” of this registration statement.
In this registration statement, unless otherwise specified, all dollar amounts are expressed in United States dollars and all references to “common shares” refer to the common shares in our capital stock.
Plan of Operation
During the next twelve-month period (beginning January 1, 2023), we intend to identify and secure sources of equity and/or debt financing for additional acquisitions.
We anticipate that we will incur the following operating expenses during this period:
Estimated Funding Required During the 12 Months beginning January 1, 2023
| Expense | | Amount ($) | |
| Improvements to planned corporate facility | | | 300,000 | |
| Executive salaries, including new hires | | | 540,000 | |
| Administrative support | | | 150,000 | |
| Professional fees | | | 240,000 | |
| General and administrative expense including rent, transfer agent, travel, office and sundry | | | 300,000 | |
| Advertising, marketing, and website costs | | | 80,000 | |
| Total operating expense | | | 1,610,000 | |
| Cash required for lab acquisitions | | | 250,000 | |
| Total | | | 1,860,000 | |
As at the date of this prospectus, we do not have sufficient cash on hand to finance our entire potential and estimated $1,860,000 cash obligation to the proposed spending for the 12 months beginning January 1, 2023. Based on our current cash position of $3,188 we anticipate that we will require approximately $1,800,000 in additional cash to execute our business plan. In the event that we are unable raise sufficient cash we intend to reduce our planned expenditures to accommodate our means with a view toward prioritizing revenue generating activity and fulfilling our public reporting obligations. As at the date of this registration statement we have no financing arrangements in place.
Results of Operations for our Years Ended December 31, 2022 and 2021
Our net income (loss) and comprehensive income (loss) for our year ended December 31, 2022, for our year ended December 31, 2021, and the changes between those periods for the respective items are summarized as follows:
| | | Year Ended December 31, 2022 ($) | | | Year Ended December 31, 2021 ($) | | | Change Between Year Ended December 31, 2022 and Year Ended December 31, 2021 ($) | |
| Revenue | | | 173,329 | | | | - | | | | 173,329 | |
| Cost of Goods Sold | | | 101,054 | | | | - | | | | 101,054 | |
| Gross Profit | | | 72,275 | | | | - | | | | 72,275 | |
| Expenses | | | | | | | | | | | | |
| Selling and marketing expenses | | | 74,393 | | | | - | | | | 74,393 | |
| General and administrative expenses | | | 132,416 | | | | 20,053 | | | | 112,363 | |
| Professional Fees | | | 177,564 | | | | 56,674 | | | | 120,890 | |
| Depreciation | | | 7,274 | | | | - | | | | 7,274 | |
| Total Operating expenses | | | 391,647 | | | | 76,727 | | | | 314,920 | |
| Loss from operations | | | (319,372 | ) | | | (76,727 | ) | | | (242,645 | ) |
| | | Year Ended December 31, 2022 ($) | | | Year Ended December 31, 2021 ($) | | | Change Between Year Ended December 31, 2022 and Year Ended December 31, 2021 ($) | |
| Other (income) expense | | | | | | | | | |
| Interest expenses | | | (1,339,409 | ) | | | (152,400 | ) | | | (1,187,009 | ) |
| Gain (Loss) on extinguishment of debt | | | 6,884 | | | | (10,025,000 | ) | | | 10,018,116 | |
| Net (income) loss | | | (1,651,897 | ) | | | (10,254,127 | ) | | | 8,602,230 | |
During the years ended December 31, 2022 and 2021, respectively the Company reported $173,329 and $0 in gross revenue, $101,054 and $0 in costs of goods sold and $72,275 and $0 as gross profit. In the years ended December 31, 2022 and 2021 the Company reported net operating losses of $319,371 and $76,727, respectively. Losses from operations in the year ended December 31, 2022 consist of professional fees of $177,564, depreciation of $7,274, Selling and marketing expenses of $74,393 and general and administrative expenses, including amounts paid to the transfer agent, rent, consultants and filing fees of $132,416. Losses from operations in the year ended December 31, 2021 consist of professional fees of $56,674 and general and administrative expenses, including amounts paid to the transfer agent, rent, consultants and filing fees of $20,053. The increases to both professional fees and administrative costs period over period related to increased operations as the Company incurred costs with respect to the acquisition of the assets of Prime Dental Lab, in addition in the year ended December 31, 2022 the Company reported selling and marketing expenses of $74,393 and depreciation of $7,274 with no comparative expenses in the year ended December 31, 2021.
During the comparative years ended December 31, 2022 and 2021 the Company incurred interest expenses of $1,339,409 and $152,400 respectively as a result of interest accruing on a judgment payable in favor of our sole officer and director, Mr. James Brooks and an underlying convertible promissory note in the principal amount of $1,171,727, as well as certain other convertible notes issued during the year ended December 31, 2022. Further in the year ended December 31, 2021 the Company issued 300,000,000 shares to Mr. Brooks in order to extinguish debt in the amount of $175,000 which shares were valued at the closing price of the Company’s stock as traded on the OTC Markets on the date of issuance and recorded as a loss on the extinguishment of debt of $10,025,000, with no similar expense in the year ended December 31, 2022. The Company recorded a gain of $6,884 in the year ended December 31, 2022 as a result of a settlement of certain accounts payable at a negotiated discount.
Liquidity and Financial Condition
| Working Capital | | At | | | At | |
| | | December 31 | | | December 31 | |
| | | 2022 | | | 2021 | |
| Current assets | | $ | 13,123 | | | | - | |
| Current liabilities | | | 1,698,629 | | | | 133,023 | |
| | | | | | | | | |
| Working capital surplus/(deficit) | | $ | (1,685,506 | ) | | | (133,023 | ) |
| Cash Flows | | Year Ended | |
| | | December 31, 2022 | | | December 31, 2021 | |
| | | | | |
| Cash flows (used in) operating activities | | $ | (202,485 | ) | | $ | (16,532 | |
| Cash flows from investing activities | | | - | | | | - | |
| Cash flows from financing activities | | | 215,608 | | | | 16,532 | |
| Net increase (decrease) in cash during year | | $ | 13,123 | | | $ | - | |
During the year ended December 31, 2021, net cash used in operating activities totaled $16,532, which consists of our net loss of $10,254,127, offset by noncash adjustments of $10,025,000 as a result of a loss on extinguishment of debt, and $95,682 in non-cash interest expenses. Changes in operating assets included an increase to accounts payable and accrued liabilities of $103,638 and in increase to accounts payable, related party of $13,275. During the year ended December 31, 2022 cash used in operating expenses was $202,485, and consisted of our net loss of $1,651,896 offset by depreciation of $7,274, a gain on extinguishment of debt of $6,884, amortization of discounts on convertible debt of $1,238,291, an increase to accounts payable – related parties of $72,861 and an increase to accounts payable and other liabilities of $137,870.
Cash Provided by Investing Activities
There was no cash used in investing activities in the years ended December 31, 2022, and 2021.
Cash Provided by Financing Activities
Cash provided by financing activities totaled $215,608 for the year ended December 31, 2022, including proceeds from convertible notes payable of $232,140 and repayments to advances from related parties of $16,532.
Cash provided by financing activities totaled $16,532 in the year ended December 31, 2021 as a result of advances from related parties.
Off-Balance Sheet Arrangements
We have no significant off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to stockholders.
Critical Accounting Policies
Our financial statements and accompanying notes are prepared in accordance with generally accepted accounting principles used in the United States of America. Preparing financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses. These estimates and assumptions are affected by management's application of accounting policies. We believe that understanding the basis and nature of the estimates and assumptions involved with the following aspects of our financial statements is critical to an understanding of our financials.
Going Concern
We have suffered recurring losses from operations. The continuation of our Company as a going concern is dependent upon our Company attaining and maintaining profitable operations and/or raising additional capital. The financial statements do not include any adjustment relating to the recovery and classification of recorded asset amounts or the amount and classification of liabilities that might be necessary should our Company discontinue operations.
The continuation of our business is dependent upon us raising additional financial support and/or attaining and maintaining profitable levels of internally generated revenue. The issuance of additional equity securities by us could result in a significant dilution in the equity interests of our current stockholders. Obtaining commercial loans, assuming those loans would be available, will increase our liabilities and future cash commitments.
Recently Issued Accounting Standards
In August 2020, the FASB issued ASU 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470- 20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. The new guidance, among other things, simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments, and amends existing earnings-per-share (“EPS”) guidance by requiring that an entity use the if converted method when calculating diluted EPS for convertible instruments. ASU 2020-06 is effective for public business entities that meet the definition of an SEC filer, excluding entities eligible to be smaller reporting companies as defined by the SEC, for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. The Company plans to adopt the new guidance effective January 1, 2024.
Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports filed under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms, and that such information is accumulated and communicated to our management, including our president (also our principal executive officer) and our secretary, treasurer and chief financial officer (also our principal financial and accounting officer) to allow for timely decisions regarding required disclosure.
As of September 30, 2022, the end of the third quarter covered by the comparative information of this prospectus, we carried out an evaluation, under the supervision and with the participation of our president (also our principal executive officer) and our secretary, treasurer and chief financial officer (also our principal financial and accounting officer), of the effectiveness of the design and operation of our disclosure controls and procedures. Based on the foregoing, our president (also our principal executive officer) and who is also our secretary, treasurer and chief financial officer (also our principal financial and accounting officer) concluded that our disclosure controls and procedures were effective in providing reasonable assurance in the reliability of our financial reports as of the end of the period.
Inherent limitations on effectiveness of controls
Internal control over financial reporting has inherent limitations which include but is not limited to the use of independent professionals for advice and guidance, interpretation of existing and/or changing rules and principles, segregation of management duties, scale of organization, and personnel factors. Internal control over financial reporting is a process which involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements on a timely basis, however these inherent limitations are known features of the financial reporting process and it is possible to design into the process safeguards to reduce, though not eliminate, this risk. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal controls over financial reporting that occurred during the year ended December 31, 2022, that have materially or are reasonably likely to materially affect, our internal controls over financial reporting.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
We have not had any changes in or disagreements with our independent public accountants since our inception.
DIRECTORS AND EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Directors and Executive Officers
All directors of our company hold office until the next annual meeting of the security holders or until their successors have been elected and qualified. The officers of our company are appointed by our board of directors and hold office until their death, resignation or removal from office. Our directors and executive officers, their ages, positions held, and duration as such, are as follows:
| Name | Position Held with our Company | Age | Date First Elected Or Appointed |
| James Brooks | President, Secretary and Treasurer, Director | 52 | December 30, 2021 |
Our directors will serve in that capacity until our next annual shareholder meeting or until his successor is elected or appointed and qualified. Officers hold their positions at the will of our Board of Directors.
There are no arrangements, agreements or understandings between non-management security holders and management under which non-management security holders may directly or indirectly participate in or influence the management of our affairs.
James Brooks, President, Secretary, Treasurer and Director
Mr. Brooks is the company’s sole officer and director and was appointed as president and director on December 30, 2021.
James Brooks first began working with public companies in 1998, and has founded and exited from two substantial companies in the transportation logistics sector since. The first, Urban Dispatch, where Mr. Brooks served as the CEO, COO and a board member was a Canadian logistics company, and employed more than 250 people, operating in 4 provinces in Western Canada. Following his time with Urban Dispatch, Mr. Brooks founded Oveldi Worldwide Shipping Company, based in Hong Kong, where he served as CEO, COO and a board member until divestiture in 2012.
From 2014 through 2019, Mr. Brooks served as an officer and director of Oveldi Strategic Capital Ltd. where he also provided services as a business consultant. Oveldi Strategic Capital assisted small businesses in a variety of industries with corporate structure, governance, business planning, exit strategies, strategic partnerships and overall corporate objectives and direction. Such clients included companies in moving and storage, transportation logistics, jewelry distribution, real estate development, clothing manufacturing, and others.
During the Covid-19 pandemic commencing in early 2019, Mr. Brooks repositioned his focus and chose to dedicate his business time exclusively to his next controlled corporation, Fastbend Holdings Inc. (formerly Standard Dental Labs), where he is the CEO, President, and a director. Fastbend Holdings is a consulting firm and project incubation facilitator, drawing on the experience gained from his former venture Oveldi Strategic Capital. Upon acquiring a controlling interest in Costas Inc. in December 2021 and becoming the sole officer and director, Mr. Brooks again reallocated his time resources to spend 90% of his time developing the ongoing business of Costas, with the remaining 10% allocated to oversight of his controlled private corporation, Fastbend Holdings.
Given his background and experience building operations from the ground up, Mr. Brooks has a clear vision of how to identify and acquire target companies for Costas, and how to execute the company’s business plan. Mr. Brooks has been responsible for advancing the company’s objectives, including the financing the efforts made to date for Costas, Inc., as its largest creditor, shareholder and CEO.
Mr. Brooks currently devotes approximately 90% of his professional time to the business and intends to continue to devote this amount of time in the future, or more as required.
Our company believes that all of our directors” respective educational background, operational and business experience give them the qualifications and skills necessary to serve as directors and officers, respectively, of our company. Our board of directors consists solely of Mr. Brooks.
Significant Employees
Other than the foregoing named officers and directors, we have no employees whose services are materially significant to our business and operations who is employed by Costas. We have certain contractors providing services under the terms of a subcontractor agreement entered into August 31, 2022 with Mr. John Kim for services including labor and manufacturing expertise as well as management services with respect to the servicing of the Company’s client list for dental prosthetics and orthotics, who we consider significant to our current operations.
Jongpil (John) Kim immigrated from Seoul, South Korea to Boston, Massachusetts in 2006, where he studied for 3 years to become a dental lab technician at Mass Dental Technique, a private school in Boston. In 2008, Mr. Kim received permanent and green card residency and started his first full-time job at Reliable Dental Lab in Andover, MA, and worked there until 2011. In 2011, Mr. Kim moved to Orlando, FL and after one year of practical clinical experience in Florida at Mount Dora Modern Dentistry, opened his own lab, Prime Dental Lab LLC in 2012. In 2014 Mr. Kim received US Citizenship. Mr. Kim established a solid client base and operated Prime Dental until the sale of its material assets in 2021.
Family Relationships
There are no family relationships between any of our directors and officers.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors or executive officers has, during the past ten years:
1. been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offences);
2. had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time;
3. been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity;
4. been found by a court of competent jurisdiction in a civil action or by the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated;
5. been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
6. been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
Compliance with Section 16(a) of the Securities Exchange Act of 1934
When we have a class of securities registered under the Securities Exchange Act of 1934, Section 16(a) of the Securities Exchange Act of 1934 requires our executive officers and directors and persons who own more than 10% of our common stock to file with the Securities and Exchange Commission initial statements of beneficial ownership, reports of changes in ownership and annual reports concerning their ownership of our common stock and other equity securities, on Forms 3, 4 and 5 respectively. Executive officers, directors and greater than 10% shareholders are required by the SEC regulations to furnish us with copies of all Section 16(a) reports that they file.
Code of Ethics
We have not adopted a code of ethics that applies to our officers, directors and employees. When we do adopt a code of ethics, we will disclose it in a Current Report on Form 8-K.
Audit Committee and Audit Committee Financial Expert
Our board of directors (currently consisting of one member) also acts as the audit committee and has determined that it does not have a member that qualifies as an “audit committee financial expert” as defined in Item 407(d)(5)(ii) of Regulation S-K, and is “independent” as the term is used in Item 7(d)(3)(iv) of Schedule 14A under the Securities Exchange Act of 1934, as amended.
We believe that our board of directors is capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting. We believe that retaining an independent director who would qualify as an “audit committee financial expert” would be overly costly and burdensome and is not warranted in our circumstances given the early stages of our development and the fact that we have not generated any material revenues to date. In addition, we currently do not have nominating, compensation or audit committees or committees performing similar functions nor do we have a written nominating, compensation or audit committee charter. Our directors do not believe that it is necessary to have such committees because they believe the functions of such committees can be adequately performed by the members of our board of directors.
EXECUTIVE COMPENSATION
The particulars of the compensation paid to the following persons:
(a) our principal executive officers;
(b) each of our two most highly compensated executive officers who were serving as executive officers at the end of the years ended December 31, 2022, and 2021; and
c) up to two additional individuals for whom disclosure would have been provided under (b) but for the fact that the individual was not serving as our executive officer at the end of the years ended December 31, 2022 and 2021, who we will collectively refer to as the named executive officers of our Company, are set out in the following summary compensation table, except that no disclosure is provided for any named executive officer, other than our principal executive officers, whose total compensation did not exceed $100,000 for the respective fiscal year:
SUMMARY COMPENSATION TABLE
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation | Non-Qualified Deferred Compensation Earnings ($) | All Other Compensation | Total ($) |
| James Brooks(1) President and Director | 2022 | Nil | Nil | Nil | Nil | Nil | Nil | 30,000 | 30,000 |
| 2021 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| Fred Waid(1) Former President and Director | 2021 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
Notes:
| (1) | On December 30, 2021, Mr. Brooks was appointed as our President and as a director, replacing Mr. Waid who held those positions due to the company’s receivership proceedings as appointed by the court. |
Employment/Consulting Agreements
Other than as set out herein we have not entered into any employment or consulting agreements with any of our current officers, directors or employees. Our Chief Executive Officer receives a monthly allowance of $2,500 to offset certain expenses for vehicle, travel and other costs.
Grants of Plan-Based Awards Table
None
Outstanding Equity Awards at Fiscal Year End
Not applicable.
Option Exercises
During our fiscal years ended December 31, 2022 and 2021 there were no options exercised by our named officers.
Compensation of Directors
Except as otherwise disclosed, we do not have any agreements for compensating our directors for their services in their capacity as directors, although such directors are expected in the future to receive stock options to purchase shares of our common stock as awarded by our board of directors.
Pension, Retirement or Similar Benefit Plans
There are no arrangements or plans in which we provide pension, retirement or similar benefits for directors or executive officers. We have no material bonus or profit-sharing plans pursuant to which cash or non-cash compensation is or may be paid to our directors or executive officers, except that stock options may be granted at the discretion of the board of directors or a committee thereof.
Indebtedness of Directors, Senior Officers, Executive Officers and Other Management
None of our directors or executive officers or any associate or affiliate of our Company during the last two fiscal years is or has been indebted to our company by way of guarantee, support agreement, letter of credit or other similar agreement or understanding currently outstanding.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of March 31, 2023, certain information with respect to the beneficial ownership of our common shares by each shareholder known by us to be the beneficial owner of more than 5% of our common shares, as well as by each of our current directors and executive officers as a group. Each person has sole voting and investment power with respect to the shares of common stock, except as otherwise indicated. Beneficial ownership consists of a direct interest in the shares of common stock, except as otherwise indicated.
| Name and Address of Beneficial Owner | Amount and Nature of Beneficial Ownership | Percentage of Class(1) |
| James Brooks 12 S Osceola Ave. Orlando, Florida, 32801 | 331,663,760(2) | 74.4% |
| Directors and Executive Officers as a Group | 331,663,760 | 74.4% |
NOTES
| (1) | Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights. As a result, the percentage of outstanding shares of any person as shown in this table does not necessarily reflect the person’s actual ownership or voting power with respect to the number of shares of common stock actually outstanding on January 31, 2023. As of January 31, 2023, there were 445,728,363 shares of our company’s common stock issued and outstanding. |
| (2) | Of the shares held by Mr. Brooks, 31,663,760 are held in the name of Fastbend Holdings Inc., formerly Standard Dental Labs Inc., over which Mr. Brooks has voting and investment authority, and is the 63% shareholder. |
Changes in Control
We are unaware of any contract or other arrangement the operation of which may at a subsequent date result in a change in control of our company.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Except as disclosed herein, no director, executive officer, shareholder holding at least 5% of shares of our common stock, or any family member thereof, had any material interest, direct or indirect, in any transaction, or proposed transaction since the years ended December 31, 2022 and 2021, in which the amount involved in the transaction exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at the year-end for the last two completed fiscal years.
Fred Waid, former sole officer and director
During the year ended December 31, 2020, Intermountain Fiduciary Services Inc., a company of which Fred Waid, the former Receiver and our former sole officer and director, is also director and officer (“IFSI”), advanced a total of $1,725 for legal and professional which remained unpaid at December 31, 2020.
During the fiscal year ended December 31, 2021, IFSI incurred an additional $13,275 in legal and professional fees, respectively. The Company made cash payment in the amount of $15,000 to Intermountain and no additional invoices were received during the year ended December 31, 2022. At December 31, 2022 and December 31, 2021, $0 and $15,000, respectively, is reflected on the balance sheet as accounts payable – related party.
James Brooks, sole officer and director, controlling shareholder
On December 30, 2021, Mr. James Brooks was appointed the Company’s sole officer and director in place of Mr. Fred Waid. Immediately prior Mr. Brooks became the Company’s controlling shareholder upon issuance of 300,000,000 shares of common stock for certain debt in the amount of $175,000. On December 23, 2021, the Company entered into an 8% Convertible Promissory Note with Brooks in the amount of $1,171,727. The convertible promissory note bears interest rate at 8% per annum for a period of 12 months. The holder has the right to convert any or all of the outstanding principal into shares of the Company’s common stock at a conversion price of $0.001 per share.
Prior to maturity, Mr. Brooks agreed to extend the repayment date of the note to December 31, 2023.
During the year ended December 31, 2021, Mr. Brooks and companies controlled by him advanced a total of $16,532 for operating expenses.
On November 7, 2022, Mr. Brooks entered into a lease agreement with MAA Parkside for a term of one year commencing January 2023 for approximately 1,500 square feet which is a shared use office and residential space at a cost of $3,185 per month, plus insurance and utilities, which is reimbursed by the Company.
During the year ended December 31, 2022, Mr. Brooks and companies controlled by him advanced a further $162,220 for operating expenses and the Company made cash payments to reduce the total balance payable in the amount of $178,752. As at December 31, 2022, the amount owing to Mr. Brooks for advances for operations is $0 (December 31, 2021 - $16,532) which amount is reflected on the balance sheets as advances payable – related party.
During the year ended December 31, 2022 the Company also recorded interest expenses of $93,738 (2021 - $2,054) for Mr. Brooks on his convertible note. Mr. Brooks received payments against interest of $5,887 in the year ended December 31, 2022 (2021 - $0) leaving a balance owing of $89,915 which is reflected on the balance sheets as accounts payable – related party.
During the year ended December 31, 2022, the Company acquired certain assets by way of issuance of 31,663,760 common shares of the Company’s restricted, unregistered common stock to Fastbend Holdings, Inc. (formerly Standard Dental Labs Inc.) a company controlled by Mr. Brooks.
During the year ended December 31, 2022 the Company paid $30,000 to Mr. Brooks in respect to a monthly expense allowance of $2,500.
Director Independence
We currently act with one director, consisting of James Brooks. We have determined that James Brooks is not an “independent director” as defined in NASDAQ Marketplace Rule 4200(a)(15).
Currently our audit committee consists of all of our sole member of our board of directors. We currently do not have nominating, compensation committees or committees performing similar functions. There has not been any defined policy or procedure requirements for shareholders to submit recommendations or nomination for directors.
Our board of directors has determined that it does not have a member of its audit committee who qualifies as an “audit committee financial expert” as defined in as defined in Item 407(d)(5)(ii) of Regulation S-K.
From inception to present date, we believe that the members of our audit committee and the board of directors have been and are collectively capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting.
We do not have a standing compensation or nominating committee. We believe that our directors are capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting. Our directors do not believe that it is necessary to have an audit committee because we believe that the functions of an audit committee can be adequately performed by the board of directors. In addition, we believe that retaining additional independent directors who would qualify as an “audit committee financial expert” would be overly costly and burdensome and is not warranted in our circumstances given the early stages of our development.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION OF SECURITIES ACT LIABILITIES
Our bylaws provide that we indemnify our directors and officers to the fullest extent not prohibited by Nevada law.
The general effect of the foregoing is to indemnify a control person, officer or director from liability, thereby making us responsible for any expenses or damages incurred by such control person, officer or director in any action brought against them based on their conduct in such capacity, provided they did not engage in fraud or criminal activity.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or control persons pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
We are incorporated under the laws of the State of Nevada. Section 78.138 of the Nevada Revised Statutes (“ NRS ”) provides that neither a director nor an officer of a Nevada corporation can be held personally liable to the corporation, its stockholders or its creditors unless the director or officer committed both a breach of fiduciary duty and such breach was accompanied by intentional misconduct, fraud, or knowing violation of law. Nevada does not exclude breaches of the duty of loyalty or instances where the director has received an improper personal benefit.
A Nevada corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right of the corporation, by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys” fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with the action, suit or proceeding, if he is not liable under NRS 78.138 (see above), acted in “good faith” and in a manner he reasonably believed to be in and not opposed to the best interests of the corporation, and with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. However, with respect to actions by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which such court shall deem proper. A director or officer who is successful, on the merits or otherwise, in defense of any proceeding subject to the Nevada corporate statutes” indemnification provisions must be indemnified by the corporation for reasonable expenses incurred in connection therewith, including attorneys” fees.
Our company’s bylaws provide that the corporation shall, to the maximum extent and in the manner permitted by the NRS, indemnify and hold harmless any and all persons whom it shall have power to indemnify under said provisions from and against any and all liabilities (including expenses) imposed upon or reasonably incurred by him or her in connection with any action, suit or other proceeding in which he or she may be involved or with which he or she may be threatened, or other matters referred to in or covered by said provisions both as to action in his or her official capacity and as to action in another capacity while holding such office, and shall continue as to a person who has ceased to be a director or officer of the corporation. Our company’s bylaws do not modify Nevada law in this respect.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and persons controlling us pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
We have no liability insurance.
DEALER PROSPECTUS DELIVERY OBLIGATION
Until a date, which is 90 days after the date of this Prospectus, all dealers that effect transactions in these securities whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealer’s obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II – INFORMATION NOT REQUIRED IN PROSPECTUS
OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
No expenses will be borne by the Selling Security Holders. Our estimated expenses in connection with the issuance and distribution of the securities being registered in this Prospectus are as follows:
| Commission filing fee | | | |
| Legal fees and expenses | | $ | 20,000 | |
| Accounting fees and expenses | | | 30,000 | |
| Total | | $ | 50,000 | |
INDEMNIFICATION OF DIRECTORS AND OFFICERS
Section 78.138 of the NRS provides that a director or officer will not be individually liable unless it is proven that (i) the director’s or officer’s acts or omissions constituted a breach of his or her fiduciary duties, and (ii) such breach involved intentional misconduct, fraud or a knowing violation of the law.
Section 78.7502 of NRS permits a company to indemnify its directors and officers against expenses, judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with a threatened, pending or completed action, suit or proceeding if the officer or director (i) is not liable pursuant to NRS 78.138 or (ii) acted in good faith and in a manner the officer or director reasonably believed to be in or not opposed to the best interests of the corporation and, if a criminal action or proceeding, had no reasonable cause to believe the conduct of the officer or director was unlawful.
Section 78.751 of NRS permits a Nevada company to indemnify its officers and directors against expenses incurred by them in defending a civil or criminal action, suit or proceeding as they are incurred and in advance of final disposition thereof, upon receipt of an undertaking by or on behalf of the officer or director to repay the amount if it is ultimately determined by a court of competent jurisdiction that such officer or director is not entitled to be indemnified by the company. Section 78.751 of NRS further permits the company to grant its directors and officers additional rights of indemnification under its articles of incorporation or bylaws or otherwise.
Section 78.752 of NRS provides that a Nevada company may purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director, officer, employee or agent of the company, or is or was serving at the request of the company as a director, officer, employee or agent of another company, partnership, joint venture, trust or other enterprise, for any liability asserted against him and liability and expenses incurred by him in his capacity as a director, officer, employee or agent, or arising out of his status as such, whether or not the company has the authority to indemnify him against such liability and expenses.
Our Articles of Incorporation provide that no director or officer of our company will be personally liable to our company or any of its stockholders for damages for breach of fiduciary duty as a director or officer; provided, however, that the foregoing provision shall not eliminate or limit the liability of a director or officer (i) for acts or omissions which involve intentional misconduct, fraud or knowing violation of law, or (ii) the unlawful payment of dividends. In addition, our bylaws permit for the indemnification and insurance provisions in Chapter 78 of the NRS.
Insofar as indemnification by us for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling our company pursuant to provisions of our articles of incorporation and bylaws, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification by such director, officer or controlling person of us in the successful defense of any action, suit or proceeding is asserted by such director, officer or controlling person in connection with the securities being offered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
At the present time, there is no pending litigation or proceeding involving a director, officer, employee or other agent of ours in which indemnification would be required or permitted. We are not aware of any threatened litigation or proceeding, which may result in a claim for such indemnification.
Further, in the normal course of business, we may have in our contracts indemnification clauses, written as either mutual where each party will indemnify, defend, and hold each other harmless against losses arising from a breach of representations or covenants, or out of intellectual property infringement or other claims made against certain parties; or single where we have agreed to hold certain parties harmless against losses etc.
Our Bylaws
Our bylaws provide that we will indemnify our directors and officers to the fullest extent not prohibited by Nevada law.
The general effect of the foregoing is to indemnify a control person, officer or director from liability, thereby making us responsible for any expenses or damages incurred by such control person, officer or director in any action brought against them based on their conduct in such capacity, provided they did not engage in fraud or criminal activity.
RECENT SALES OF UNREGISTERED SECURITIES
During February and March 2023, the company issued non-interest bearing convertible promissory notes in the principal amount of $151,000 to several investors for a period of 12 months. The holders have the right to convert any or all of the outstanding principal into shares of the Company’s common stock at a conversion price of $0.004 per share. These notes were issued to seven (7) non-U.S. persons (as that term is defined in Regulation S of the Securities Act of 1933, as amended) in an offshore transaction relying on Regulation of the Securities Act of 1933, as amended).
During January 2023, the company issued convertible promissory notes in the principal amount of $50,000 to several investors bearing interest at 8% per annum for a period of 12 months. The holders have the right to convert any or all of the outstanding principal into shares of the Company’s common stock at a conversion price of $0.001 per share. These notes were issued to three (3) non-U.S. persons (as that term is defined in Regulation S of the Securities Act of 1933, as amended) in an offshore transaction relying on Regulation of the Securities Act of 1933, as amended).
During the year ended December 31, 2022, the company issued convertible promissory notes in the principal amount of $232,140 to several investors bearing interest at 8% per annum for a period of 12 months. The holders have the right to convert any or all of the outstanding principal into shares of the Company’s common stock at a conversion price of $0.001 per share. These notes were issued to eight (8) non-U.S. persons (as that term is defined in Regulation S of the Securities Act of 1933, as amended) in an offshore transaction relying on Regulation of the Securities Act of 1933, as amended).
On September 1, 2022 the Company issued 750,000 shares to Mr. Jongpil Kim in consideration for the purchase of certain assets under an asset purchase agreement. We issued the share to Mr. Jongpil Kim, an accredited investor (as that term is defined in Regulation D of the Securities Act of 1933) relying on Rule 506 of Regulation D and/or Section 4(a)(2) of the Securities Act of 1933.
On May 6, 2022, 31,663,760 shares were issued in respect to a certain asset purchase agreement. We issued the shares to an accredited investor (as that term is defined in Regulation D of the Securities Act of 1933) relying on Rule 506 of Regulation D and/or Section 4(a)(2) of the Securities Act of 1933.
During the year ended December 31, 2021, 70,000,000 shares were issued upon conversion of certain convertible notes. These shares were issued to three (3) non-U.S. persons (as that term is defined in Regulation S of the Securities Act of 1933, as amended) in an offshore transaction relying on Regulation of the Securities Act of 1933, as amended)
During the year ended December 31, 2021, 300,000,000 shares were issued pursuant to a court order in settlement of $175,000 payable to our current sole officer and director, Mr. James Brooks, our sole officer and director, and valued at the fair market value on the date of issue or $10,200,000.
On December 23, 2021, the Company entered into an 8% Convertible Promissory Note with Mr. James Brooks, our sole officer and director, in the amount of $1,171,727. The convertible promissory note bears interest rate at 8% per annum for a period of 12 months. The holder has the right to convert any or all of the outstanding principal into shares of the Company’s common stock at a conversion price of $0.001 per share.
EXHIBITS
| Exhibit Number | | Exhibit Description |
| 3.1 | | Articles of Incorporation of Costas, Inc. (incorporated by reference to our Registration Statement on Form 10-SB filed January 14, 2003 as Exhibit 3(a)) |
| | | |
| 3.2 | | Certificate of Amendment and Restated Articles filed with the Nevada Secretary of State on July 11, 2010.+ |
| | | |
| 3.3 | | Certificate of Amendment to Articles of Incorporation filed with the Nevada Secretary of State on July 17, 2014.+ |
| | | |
| 3.4 | | Bylaws (incorporated by reference to Exhibit 3(b) to our Registration Statement on Form 10-SB filed January 14, 2003). |
| | | |
| 3.5 | | Certificate of Correction Amending Reverse Split filed with the State of Nevada on November 6, 2014.+ |
| | | |
| 3.6 | | Certificate of Dissolution as filed with the State of Nevada on April 15, 2019.+ |
| | | |
| 3.7 | | Certificate of Correction and Reinstatement as filed with the State of Nevada on July 3, 2019.+ |
| | | |
| 3.8 | | Certificate of Amendment amending name filed with the State of Nevada on February 24, 2020.+ |
| | | |
| 3.9 | | Certificate of Correction regarding name amendment filled with the State of Nevada on June 1, 2021.+ |
| | | |
| 3.10 | | Certificate of Amendment increasing authorized share capital filed with the State of Nevada on January 27, 2022.+ |
| | | |
| 3.11 | | Certificate of Amendment increasing authorized share capital filed with the State of Nevada on August 17,2022.+ |
| | | |
| 5.1 | | Legal Opinion of William (Bill) Macdonald Esq.+ |
| | | |
| 10.1 | | Asset Purchase Agreement between Costas, Inc. and Standard Dental Labs Inc. dated May 6, 2022+ |
| | | |
| 10.2 | | Asset Purchase Agreement between Costas, Inc. and Prime Dental Lab LLC dated August 15, 2022+ |
| | | |
| 10.3 | | Purchase Agreement between Costas, Inc. and World Amber Corporation dated November 22, 2022+ |
| | | |
| 10.4 | | Registration Rights Agreement between Costas, Inc. and World Amber Corporation dated November 22, 2022+ |
| | | |
| 10.5 | | Form of Convertible Promissory Note# |
| | | |
| 10.6* | | Addendum No. 1 to Asset Purchase Agreement between Costas, Inc. and Prime Dental Lab LLC dated March 24, 2023 |
| | | |
| 10.7* | | Subcontractor agreement between Costas Inc. and Mr. John Kim dated August 31, 2022 |
| | | |
| 10.8* | | Lease Agreement between James Brooks and MAA Parkside |
| | | |
| 23.1 | | Consent of Olayinka Oyebola & Co.* |
| | | |
| 23.2 | | Consent of Olayinka Oyebola & Co.* |
| | | |
| 23.3 | | Consent of William (Bill) Macdonald Esq. (incorporated in Exhibit 5.1) |
| | | |
| 107 | | Filing fees table* |
*Filed herewith
+Incorporated by reference to our Registration Statement on Form S-1 filed on December 30, 2022.
# Incorporated by reference to Amendment No. 1 to our Registration Statement on Form S-1 filed on February 10, 2023.
UNDERTAKINGS
The registrant hereby undertakes:
1. To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
| | (i) | To include any prospectus required by section 10(a)(3) of the Securities Act; |
| | | |
| | (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post- effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| | | |
| | (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
| | | |
2. That for the purpose of determining liability under the Securities Act, each post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof;
3. To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering; and
4. That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the registrant undertakes that in a primary offering of securities of the registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
| | (i) | Any preliminary prospectus or prospectus of the registrant relating to the offering required to be filed pursuant to Rule 424; |
| | | |
| | (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the registrant or used or referred to by the registrant; |
| | | |
| | (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the registrant or its securities provided by or on behalf of the registrant; and |
| | | |
| | (iv) | Any other communication that is an offer in the offering made by the registrant to the purchaser. |
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
Signatures
Pursuant to the requirements of the Securities Act, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Orlando, Florida on April 7, 2023.
| | COSTAS, INC. | |
| | | | |
| | By: | //Signed// James Brooks | |
| | | James Brooks President and Director | |
In accordance with the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities and on the dates stated.
| SIGNATURES | | TITLE | | DATE |
| | | | | |
| //Signed// James Brooks | | Director and President | | April 7, 2023 |
| James Brooks | | (Principal Executive and Accounting Officer) | | |
Costas (PK) (USOTC:CSSI)
Historical Stock Chart
From Apr 2024 to May 2024
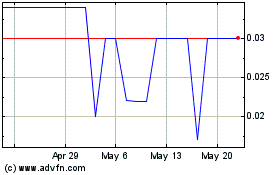
Costas (PK) (USOTC:CSSI)
Historical Stock Chart
From May 2023 to May 2024