As filed with the Securities and Exchange
Commission on April 8, 2014
Registration No. 333-______
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES
ACT OF 1933
STEVIA CORP.
(Exact name of registrant as specified in
its charter)
|
Nevada
|
|
700
|
|
98-0537233
|
|
(State or jurisdiction of
|
|
(Primary Standard Industrial
|
|
(I.R.S. Employer
|
|
incorporation or organization)
|
|
Classification Code Number)
|
|
Identification No.)
|
7117 US 31S
Indianapolis, IN 46227
(888) 250-2566
(Address and telephone number of principal
executive offices and principal place of business)
CSC Services of Nevada, Inc.
2215-B Renaissance Drive
Las Vegas, NV 89119
(702) 740-4244
(Name, address
and telephone number of agent for service)
Copies to:
Mark C. Lee
Saxon Peters
GREENBERG TRAURIG, LLP
1201 K Street, Suite 1100
Sacramento, California 95814
Telephone: (916) 442-1111
Facsimile: (916) 448-1709
Approximate date of proposed sale to
the public:
From time to time after the effective date
of this registration statement.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under
the Securities Act of 1933, check the following box.
þ
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check
the following box and list the Securities Act registration statement number of the earlier effective registration statement for
the same offering.
¨
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list
the Securities Act registration statement number of the earlier effective registration statement for the same offering.
¨
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list
the Securities Act registration statement number of the earlier effective registration statement for the same offering.
¨
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions
of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2
of the Exchange Act. (Check one):
|
Large accelerated filer
¨
|
Accelerated filer
¨
|
Non-accelerated filer
¨
|
Smaller reporting company
þ
|
|
|
|
(Do not check if a smaller reporting company)
|
|
|
|
CALCULATION OF REGISTRATION FEE
|
|
|
Amount of shares
|
|
|
Proposed maximum
|
|
|
Proposed maximum
|
|
|
Amount of
|
|
|
Title of each class of
|
|
to be
|
|
|
offering price
|
|
|
aggregate
|
|
|
Registration
|
|
|
securities to be registered
|
|
Registered
|
|
|
per share
|
|
|
offering price
|
|
|
Fee
|
|
|
Common Stock Underlying the Principal of Convertible Notes
|
|
|
10,674,182
|
(1)
|
|
$
|
0.266
|
(2)
|
|
$
|
2,839,332.41
|
|
|
$
|
365.71
|
|
|
Common Stock Underlying the Interest of Convertible Notes
|
|
|
508,905
|
(3)
|
|
$
|
0.266
|
(2)
|
|
$
|
135,368.73
|
|
|
$
|
17.44
|
|
|
Common Stock Underlying the Principal of Convertible Notes
|
|
|
12,809,018
|
(4)
|
|
$
|
0.266
|
(2)
|
|
$
|
3,407,198.79
|
|
|
$
|
438.85
|
|
|
Common Stock Underlying the Interest of Convertible Notes
|
|
|
610,687
|
(5)
|
|
$
|
0.266
|
(2)
|
|
$
|
162,442.74
|
|
|
$
|
20.92
|
|
|
Common Stock Underlying Warrants
|
|
|
20,296,139
|
(6)
|
|
$
|
0.053365
|
|
|
$
|
1,083,103.46
|
|
|
$
|
139.50
|
|
|
Common Stock Underlying a Convertible Note
|
|
|
3,000,000
|
(7)
|
|
$
|
0.266
|
(2)
|
|
$
|
798,000.00
|
|
|
$
|
102.78
|
|
|
Total
|
|
|
47,898,931
|
|
|
|
|
|
|
$
|
8,425,446.13
|
|
|
$
|
1085.20
|
|
|
|
(1)
|
Represents shares of common stock issuable by the registrant upon the conversion of the principal amount of the registrant’s Senior Convertible Note issued March 3, 2014 (the “Initial Nomis Bay Note”).
|
|
|
(2)
|
Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) under the Securities Act of 1933, as amended, based on the average high and low prices of the common stock as reported on the OTCQB on March 26, 2014.
|
|
|
|
|
|
|
(3)
|
Represents shares of common stock issuable by the registrant upon the conversion of interest accrued under
the Initial Nomis Bay Note.
|
|
|
|
|
|
|
(4)
|
Represents shares of common stock issuable by the registrant upon the conversion of the principal amount of the registrant’s Senior Convertible Note to be issued to Nomis Bay Ltd. (the “Secondary Nomis Bay Note”) after the effectiveness of this registration statement upon the terms and conditions set forth in the Securities Purchase Agreement dated March 3, 2014.
|
|
|
|
|
|
|
(5)
|
Represents shares of common stock issuable by the registrant upon the conversion of interest to be accrued
under the Secondary Nomis Bay Note.
|
|
|
(6)
|
Represents the number of shares of common stock offered for resale following the exercise of certain warrants to purchase common stock.
|
|
|
(7)
|
Represents shares of common stock issuable by the registrant upon the conversion of the registrant’s
promissory note issued July 10, 2013
(the “JMJ Note Shares”)
|
In the event of stock splits, stock dividends,
or similar transactions involving the Registrant’s common stock, the number of Shares registered shall, unless otherwise
expressly provided, automatically be deemed to cover the additional securities to be offered or issued pursuant to Rule 416 promulgated
under the Securities Act of 1933, as amended (the “Securities Act”); provided however that any additional shares received
due to subsequent equity issuances by the Registrant at a lower price per share than the current exercise price of any applicable
warrants would not be covered by this registration statement and would require separate registration or an exemption prior to sale.
We hereby amend this registration statement
on such date or dates as may be necessary to delay its effective date until we shall file a further amendment which specifically
states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act
or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant
to said Section 8(a), may determine.
SUBJECT TO COMPLETION, DATED APRIL 8,
2014
PROSPECTUS
47,898,931 Shares of Common Stock
STEVIA CORP.
Common Stock
This prospectus relates
to the resale of 47,898,931 shares of common stock, by the selling stockholders named in this prospectus. The shares of common
stock subject to this prospectus include:
|
|
(i)
|
24,602,792 shares of common stock issuable upon conversion of the principal amount of the registrant’s
Senior Convertible Note issued March 3, 2014 (the “Nomis Bay Note Shares”). Based upon current market price of the
Company’s common stock, the amount of Nomis Bay Note Shares being registered may be in excess of the number of shares into
which the Senior Convertible Note may currently be converted, however the parties have agreed upon the aggregate number of shares
to be registered to account for market fluctuations;
|
|
|
(ii)
|
23,026,318 shares of common stock issuable following the exercise of certain warrants issued in
accordance with a Warrant Exercise Reset Offer Letter Agreement entered into on May 3, 2013 as adjusted pursuant to its terms for
certain dilutive issuances, less 5,290,665 shares of common stock previously registered pursuant to the registrant’s Registration
Statement on Form S-1/A filed December 30, 2013 (the “Anson Reset Shares”);
|
|
|
(iii)
|
2,560,486 shares of common stock issuable following the exercise of certain warrants issued to
a selling stockholder in accordance with a Securities Purchase Agreement entered into on August 1, 2012, as adjusted pursuant to
the anti-dilution provision contained therein and the Common Stock Purchase Warrant issued February 20, 2014, less 683,202 shares
of common stock previously registered pursuant to the registrant’s Registration Statement on Form S-1/A filed December 30,
2013, plus an additional 683,202 shares of common stock issuable following the exercise of the warrant issued February 20, 2014
with an exercise price of $0.053365 per share (the “Cranshire Warrant Shares”); and
|
|
|
(iv)
|
3,000,000 shares of common stock issuable upon the conversion of the registrant’s $400,000
Promissory Note issued July 10, 2013 (the “JMJ Note Shares”);
|
We will not receive
any proceeds from the resale of any of the shares offered hereby. We may receive gross proceeds of up to $1,083,103.46 if all of
the warrants set forth above (the “Warrants”) are exercised for cash. The proceeds will be used for working capital
or general corporate purposes. We will bear all costs associated with this registration.
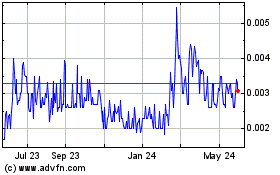
Our common stock is
quoted on the OTCQB under the symbol “STEV.” On March 26, 2014, the closing bid price of our common stock was
$0.28 per share.
INVESTING IN OUR
COMMON STOCK INVOLVES A HIGH DEGREE OF RISK. SEE “RISK FACTORS” BEGINNING ON PAGE 2 OF THIS PROSPECTUS.
NEITHER THE SECURITIES
AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS
PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The information in
this Prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with
the Securities and Exchange Commission becomes effective. This Prospectus is not an offer to sell these securities and we are not
soliciting an offer to buy these securities in any state where the offer or sale is not permitted or would be unlawful prior to
registration or qualification under the securities laws of any such state.
TABLE OF CONTENTS
|
|
Page
|
|
|
|
|
PART I - INFORMATION REQUIRED IN PROSPECTUS
|
|
|
|
|
|
PROSPECTUS SUMMARY
|
1
|
|
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
|
7
|
|
RISK FACTORS
|
7
|
|
RISKS RELATED TO OUR BUSINESS AND INDUSTRY
|
7
|
|
RISKS RELATED TO DOING BUSINESS IN VIETNAM AND OTHER DEVELOPING COUNTRIES
|
12
|
|
RISKS RELATED TO AN INVESTMENT IN OUR SECURITIES
|
14
|
|
USE OF PROCEEDS
|
15
|
|
DETERMINATION OF OFFERING PRICE
|
15
|
|
SELLING SECURITY HOLDER
|
15
|
|
PLAN OF DISTRIBUTION
|
17
|
|
DESCRIPTION OF SECURITIES TO BE REGISTERED
|
18
|
|
INTERESTS OF NAMED EXPERTS AND COUNSEL
|
20
|
|
INFORMATION WITH RESPECT TO THE REGISTRANT
|
20
|
|
PROPERTIES
|
32
|
|
LEGAL PROCEEDINGS
|
32
|
|
MARKET FOR COMMON EQUITY AND RELATED SHAREHOLDER MATTERS
|
32
|
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
33
|
|
DIRECTORS AND EXECUTIVE OFFICERS
|
37
|
|
EXECUTIVE COMPENSATION
|
38
|
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
|
39
|
|
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS AND DIRECTOR INDEPENDENCE
|
40
|
|
DISCLOSURE OF COMMISSION POSITION OF INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
|
41
|
|
WHERE YOU CAN FIND MORE INFORMATION
|
41
|
|
FINANCIAL STATEMENTS
|
F-1
|
|
|
|
|
PART II - INFORMATION NOT REQUIRED IN PROSPECTUS
|
42
|
|
|
|
|
OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
|
42
|
|
INDEMNIFICATION OF DIRECTORS AND OFFICERS
|
42
|
|
RECENT SALES OF UNREGISTERED SECURITIES
|
44
|
|
EXHIBIT INDEX
|
49
|
|
UNDERTAKINGS
|
50
|
|
SIGNATURES
|
53
|
You should rely only on the information
contained in this Prospectus. We have not authorized anyone to provide you with different information. We are not making an offer
of these securities in any state where the offer is not permitted.
PROSPECTUS SUMMARY
You should read the following summary
together with the more detailed information and the financial statements appearing elsewhere in this Prospectus. This Prospectus
contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those
anticipated in these forward-looking statements as a result of certain factors, including those set forth under “Risk Factors”
and elsewhere in this Prospectus. Unless the context indicates or suggests otherwise, references to “we,”
“our,” “us,” the “Company,” “Stevia” or the “Registrant” refer to Stevia
Corp., a Nevada corporation and its subsidiaries.
Overview
Stevia Corp. was incorporated on May 21,
2007 in the State of Nevada. On June 23, 2011, we closed a voluntary share exchange transaction with Stevia Ventures International
Ltd., a business company incorporated in the British Virgin Islands, pursuant to which we acquired the rights to purchase certain
strains of stevia leaf growing in Vietnam, including certain assignable exclusive purchase contracts and an assignable supply agreement
related to the stevia leaf.
We are a farm management company primarily
focused on crop agronomics from plant breeding to good agricultural practices to development of crop derived products, which can
be used for human consumption as well as for aquaculture and agriculture applications.
We have established a field test research
center in Vietnam on 10 Ha (25 acres) of leased land which is designed to support our commercial field trials that are on-going
in Vietnam and began our first commercial trial harvests in March 2012. We confirmed elite plant varieties, developed propagation
techniques, conducted field trials across several provinces, documented local operating procedures and post-harvest techniques,
and began commercial harvests in February 2013.
In July 2012 we formed a joint venture
with Tech-New Bio-Technology, a technology company in Hong Kong and acquired intellectual property covering several formulations
utilizing stevia extracts together with probiotics and enzymes which have applications for agriculture, aquaculture and post harvest
processing. We do not operate an extraction facility, but Tech-New Bio-Technology’s affiliate company in China has technologies
and the facility for the extraction and refinement of high purity stevia; we entered into a multi-year supply contract in March
2012 where they are committed to purchase all of our stevia leaf production for the first two years and we also have the ability
to use the resulting stevia extract to formulate our products. While we believe that our joint venture with Tech-New Bio-Technology
will increase the visibility of our intended services and products, there is no guarantee that such visibility will occur.
Our formulated products consist of ecological
fertilizers that address soil acidification, compaction and fertility decline caused by chemical fertilizer overuse; foliar fertilizers
that help plants resist infection and disease; feed formulations for livestock, fish and shrimp that enhance digestion and help
strengthen immunity; microbiological preparations that address pollution in marine environments that negatively impact aquaculture
activities; and natural preparations which aid in the preservation of crops after harvest and during processing. We also provide
private label pure stevia extracts which are suitable for food and beverage applications.
In August of 2012 we began to use our formulated
products as feed and fertilizer inputs under our farm management model and currently service several commercial operations providing
feed supplements for shrimp and fish production and fertilizer inputs for farmland as well as using it on our own trial farms.
In September 2012 we began providing samples
of stevia extract to food and beverage companies and we are working closely with local parties in several South East Asian countries
to provide technical information in support of recipe development. Although we believe that this product line will have growth
potential, there is no guarantee that a high volume of stevia will be utilized by our customers. We expect companies will take
another year to plan product launches.
In January 2014 we entered into a Farm
Management and Technology Agreement with ebbu LLC to provide farm management consultancy and technical expertise related to growing
the cannabis plant and extracting its cannabinoids. The cannabis plant produces many chemical compounds called cannabinoids and
there are more than 85 different cannabinioids that have been identified and isolated from the cannabis plant that exhibit varied
effects and many of these are being studied for their psychoactive and medical properties. Cannabis plants that have been bred
to produce high levels of tetrahydrocannabinol (“THC”), a psychoactive constituent, are commonly referred to as marijuana.
Current federal and most state regulations prevent us from participating directly in the marijuana industry and we cannot guarantee
that our services or technology will provide value if the laws do not evolve in favor of marijuana production and the commercial
sale of marijuana derived products.
In February 2014 we registered a wholly
owned subsidiary, Real Hemp LLC, as part of our strategy to enter the US hemp industry to import, manufacture and license products
containing hemp seeds, oil, protein, milk, fiber and cannabidiol. Hemp is the common term for cannabis plants that produce very
low levels of THC and are grown for industrial purposes and foodstuff products. Cannabidiol (“CBD”) is one of the active
cannabinoids in the cannabis plant and is a major constituent of hemp, accounting for up to 40% of the plants extract, and is considered
to have a wider scope of medical applications than THC. Hemp products, including CBD extracts, can be legally imported and traded
in the United States. We are currently exploring product opportunities and expect to launch our first products during 2014. There
is no guarantee that we will launch products containing hemp or that such products will be successful.
All of our formulated products used for
agriculture and aquaculture are approved for use in our areas of operation and the largest obstacle we will face will be farmer
confidence to use new products. We believe that we can overcome this obstacle by building a successful and demonstrable track record
working with the current operations of Tech-New Bio-Technology and its affiliates. All of the ingredients in the products are natural
compounds and are approved by the major developed countries if we choose to expand to other markets in the future. A list of the
major developed countries that have approved the use of stevia as a food additive can be found on page 29.
The stevia industry is segmented into several
business processes, which can broadly be categorized as i) plant breeding and propagation, ii) farming, iii) extraction and refining,
iv) product formulation, v) distribution and retail. As we achieve vertical integration along the supply chain we will continue
to focus on acquisitions and intellectual property development to support further downstream integration into the agriculture and
aquaculture sectors. We believe that over the long-run this will position the Company to become an industry leader, producing a
number of value-added stevia-enhanced products.
Our Business
We are a farm management company primarily
focused on stevia agronomics from plant breeding to good agricultural practices to development of stevia derived products which
can be used for human consumption as well as for aquaculture and agriculture applications. We plan to invest in research and development
and intellectual property acquisition and provide farm management services to contract growers and other industry growers integrating
our stevia focused research and development and intellectual property acquisitions.
Our farm management services include training
the farmers on the correct protocols and methodologies and providing ongoing technical assistance during the crop cycle as well
as providing inputs such as the seedlings, fertilizers and additives they are required to use.
We employ our services under three business
models which we classify as 1) contract farming model, 2) revenue share model and 3) product supply model.
Under the contract farming and revenue
share models we do not charge for the services and inputs, but rather our services provide us with a competitive advantage to secure
growers who are willing to dedicate their land and resources to grow crops with an expectation of high yielding, high quality crops
and guaranteed purchase prices. Under these models we will generate our revenue from the crops that are grown and we only enter
into production agreements with growers when there is already a committed buyer for the end crop. Under the contract farming model
we will purchase the crop from the grower at a fixed price and sell to our own customer. Under the revenue share model,
the grower already has their own buyer and we will share the revenue.
Under the product supply model we will
market our products in combination with technical services to buyers and charge a fee. We believe that this model will contribute
a small part of our overall revenue initially until we establish a proven track record and solid reputation for our services and
products under the first two models. We do not expect to focus on providing strictly farm management or technical services
for a fee and it is difficult to estimate what we would charge for such services.
We continue to focus on research and
development to further evolve and develop new protocols, methodologies and intellectual properties and believe that this will be
key to maintain our competitive advantage.
We utilize the contract farming model
to produce stevia leaf for our trial harvests and use the stevia extracts to produce our proprietary formulated products, which
we are applying under the revenue share model to an aquaculture operation beginning August 2012 and a chili operation beginning
October 2012.
Our mission is to maximize stockholder
value by consistently developing and acquiring the latest intellectual property and expanding our suite of formulated products
and their applications and leveraging our farm management business model to maximize market penetration and revenue margins.
To achieve these goals we intend to develop
a suite of intellectual property relating to stevia and its extracts that will enhance the value of our farm management operations.
Through our relationships with Tech-New Bio-Technology, Growers Synergy and local institutes, we are exploring the market for commercial
applications of stevia which will be vertically integrated into our services and production. We have engaged Growers Synergy, a
regional farm management services provider, to provide farm management operations and back-office and regional logistical support
for our Vietnam and Indonesia operations for a period of two years. George Blankenbaker, our president, director and stockholder
is the managing director of Growers Synergy. Growers Fresh Pte Ltd (“Growers Fresh) owns a 51% interest in Growers Synergy
and Mr. Blankenbaker controls a 49% interest in Growers Fresh.
Our current burn rate is approximately
$150,000 per month and we currently have approximately $350,000 in cash on hand. We are dependent on additional capital to continue
to operate. Failure to complete a financing will have an adverse effect on our ability to operate and execute our business plan.
We believe that $3 million of funding is sufficient for us to break-even and achieve self-sufficiency on a cash flow basis. Based
on the current burn rate, the Company does not currently have sufficient capital to operate and we are doing so on a very limited
budget, relying primarily on our goodwill with Growers Synergy and our other vendors, and during this period we will need to raise
additional capital and generate revenue. As a result, our accounts payable are expected to grow. However, there are no assurances
that Growers Synergy or our other vendors will continue to extend credit to the Company, and if they cease extending credit to
us, and we are unable to raise capital or generate sufficient revenue, we will have to liquidate or sell certain assets.
Our target markets are initially Vietnam,
Indonesia and China where we have contracted with growers and have established our own nurseries and test fields. In China we are
producing our proprietary formulated products and applying them to aquaculture projects under our revenue share model. Although
our priority is Asia, our services are not limited to specific countries and we plan to pursue viable opportunities in other markets.
Our operations to-date have primarily consisted
of securing purchase and supply contracts, office space and a research center, developing relationships with potential partners,
and developing products derived from the stevia plant. We have earned limited revenues since inception. For the nine month period
ended December 31, 2013 we incurred a net loss of $2,017,484 and for the period from inception (April 11, 2011) to March 31, 2013,
we have incurred a net loss of $4,359,415. Our assets total $3,641,781 and $2,194,251 as of as of December 31, 2013 and March 31,
2013, respectively. Further, our auditors have issued a going concern opinion in their audit report dated July 15, 2013. This means
that there is substantial doubt that we can continue as an on-going business for the next twelve months unless we obtain additional
capital.
Recent Developments
The table below sets forth shares of our
common stock that have been recently issued in exchange for certain services and rights.
|
Date
|
|
Issuance of Shares for Services and/or Rights
|
|
February 26, 2014
|
|
We issued an aggregate of 28,300,000
shares of our common stock to various service providers in exchange for services rendered, including 20,000,000 shares to Blankenbaker
Ventures (Asia) Pte. Ltd. on behalf of George Blankenbaker, our president, director and stockholder and 3,000,000 shares to Growers
Synergy Pte Ltd., a corporation organized under the laws of Singapore (“Growers Synergy”). Thomas Ong, a director of
the Company is a director of Growers Synergy and is also a 25% shareholder of Agriventure Pte Ltd., which is a 49% shareholder
of Growers Synergy. Growers Fresh Pte Ltd (“Growers Fresh) owns a 51% interest in Growers Synergy and Mr. Blankenbaker controls
a 49% interest in Growers Fresh.
|
|
February 26, 2014
|
|
We issued 16,744,682 shares of our common stock to Blankenbaker Ventures (Asia) Pte. Ltd. on behalf of George Blankenbaker, our president, director and stockholder, in exchange for the cancellation of approximately $893,579.93 of working capital advances provided by Mr. Blankenbaker and his affiliated companies.
|
|
|
|
|
Corporate
Information
Our principal executive offices are located
at 7117 US 31 S., Indianapolis, IN, 46227. Our telephone number is 888-250-2566. We maintain a corporate website at http://www.steviacorp.us.
Stock Transfer Agent
Our stock transfer agent is Securities
Transfer Corporation, and is located at 2591 Dallas Parkway, Suite 102, Frisco, Texas 75034. The agent’s telephone
number is 469-633-0101.
The Offering
|
Issuer
|
|
Stevia Corp.
|
|
|
|
|
|
Securities Offered for Resale
|
|
47,898,931 shares of Common Stock underling convertible notes and warrants to purchase Common Stock
|
|
|
|
|
|
Common Stock Outstanding Before the Offering
|
|
145,972,713 shares
|
|
|
|
|
|
Common Stock to be Outstanding After the Offering assuming all of the Securities are Resold
|
|
193,871,644 shares
|
|
|
|
|
|
Use of Proceeds
|
|
We will not receive any proceeds from the resale of the shares of common stock underlying the Warrants. We may receive proceeds in the event the Warrants are exercised for cash. Such proceeds from the offering will be used for working capital and general corporate purposes. See “Use of Proceeds.”
|
|
|
|
|
|
Trading
|
|
Our common stock is quoted on the OTCQB under the symbol “STEV.”
|
|
|
|
|
|
Risk Factors
|
|
You should carefully consider the information set forth in the section entitled “Risk Factors” beginning on page 2 of this prospectus in deciding whether or not to invest in our common stock.
|
Nomis Bay Note Shares
This offering, with respect to the Nomis Bay Note Shares, relates
to the resale of shares of common stock of the Company underlying a Senior Convertible Note, issued March 3, 2014 (the “Nomis
Bay Note”). On March 3, 2014, pursuant to a Securities Purchase Agreement (the “Purchase Agreement”) with Nomis
Bay Ltd., a Bermuda company (“Nomis Bay”), we issued the Nomis Bay Note with an initial principal amount of $500,000
for a purchase price of $340,000. The Nomis Bay Note matures on December 27, 2014 (subject to extension as provided
in the Nomis Bay Note) and, in addition to the 32% original issue discount, accrues interest at the rate of 8% per annum. The Purchase
Agreement also provides that, upon the terms and subject to the conditions set forth therein, the Company may require Nomis Bay
to purchase from the Company on or prior to the 10
th
trading day after the effective date of the registration statement
registering the shares issuable upon conversion of the Initial Convertible Note, an additional senior convertible note with an
initial principal amount of $600,000 (the “Additional Convertible Note” and together with the Nomis Bay Note, the “Convertible
Notes”) for a purchase price of $600,000. If issued, the Additional Convertible Note will mature on the date that is the
10-month anniversary of the date of issuance of the Additional Convertible Note (subject to extension as provided in the Initial
Convertible Note) and will accrue interest at the rate of 8% per annum. The Nomis Bay Note is convertible at any time, in whole
or in part, at Nomis Bay’s option into shares of the Company’s common stock, par value $0.001 per share (the “Common
Stock”), at a conversion price equal to the lesser of (i) the product of (x) the arithmetic average of the lowest three (3)
volume weighted average prices of the Common Stock during the 10 consecutive trading days ending and including the trading day
immediately preceding the applicable conversion date and (y) 40% (the “Variable Conversion Price”), and (ii) $0.30
(as adjusted for stock splits, stock dividends, stock combinations or other similar transactions). If issued, the Additional Convertible
Note will be convertible at any time, in whole or in part, at Nomis Bay’s option into shares of Common Stock at a conversion
price that will be equal to the lesser of (i) the Variable Conversion Price and (ii) $0.30 (as adjusted for stock splits, stock
dividends, stock combinations or other similar transactions). At no time will Nomis Bay be entitled to convert any portion of the
Convertible Notes to the extent that after such conversion, Nomis Bay (together with its affiliates) would beneficially own more
than 4.99% of the outstanding shares of Common Stock as of such date. The shares of common stock underlying the Nomis Bay Note
were issued in reliance upon an exemption from the registration requirements of the Securities Act and/or Rule 506 of Regulation
D promulgated thereunder.
Anson Reset Shares
This offering, with respect to the Anson
Reset Shares, relates to the resale of shares of common stock of the Company underlying three warrants in the amounts of 1,877,333,
1,066,666 and 2,346,666, with exercise prices of $0.20, $0.25 and $0.25 per share (the “Anson Warrants”). Pursuant
to the terms of a warrant exercise reset offer letter between the Company and the investor, the Anson Warrants were issued in consideration
for the investor’s agreement to immediately cash exercise an existing warrant to purchase 853,333 shares of common stock
of the Company at an exercise price of $0.20 per share for aggregate consideration to the Company of $170,666 originally issued
pursuant to the terms of the Securities Purchase Agreement, dated August 1, 2012 (the “Purchase Agreement”). Each Anson
Warrant has a five year term and was issued on May 3, 2013. Each Anson Warrant provides for adjustment of the exercise price and
share amount in the event of certain dilutive issuances by the Company. On February 20, 2014, the Company issued a notice to the
holder that the aggregate number of Anson Warrants had been adjusted to 23,026,318 and the exercise price of each had been adjusted
to $0.053365 as a result of certain other offerings of the Company. 5,290,665 of the shares underlying the Anson Warrants were
previously registered by the Company pursuant to the Registration Statement on Form S-1/A filed December 30, 2013. The warrants
and the shares of common stock underlying the warrants were issued in reliance upon an exemption from the registration requirements
of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder.
Cranshire Warrant Shares
This offering, with respect to the Cranshire
Warrant Shares, relates to the resale of the shares of common stock underlying a warrant in the amount of 2,560,486 with an exercise
price of $0.053365. The warrant has a five (5) year term and was issued pursuant to the terms of the Purchase Agreement. The warrant
was originally issued in the amount of 213,334 shares at an exercise price of $0.6405. Pursuant to the anti-dilution adjustment
provision contained therein, on February 20, 2014, the Company issued a notice to the holder that the total share amount had been
increased to 2,560,486 and the exercise price had been reduced to $0.053365 as a result of certain other offerings of the Company.
683,202 of the shares underlying the Cranshire Warrants were previously registered by the Company pursuant to the Registration
Statement on Form S-1/A filed December 30, 2013. Additionally, on February 20, 2014 in consideration for the investor’s agreement
to immediately cash exercise a portion of the Cranshire Warrants for aggregate consideration to the Company of $36,459, the Company
agreed to issue the investor an additional warrant to purchase 683,202 shares of common stock. The warrants and the shares of common
stock underlying the warrants were issued in reliance upon an exemption from the registration requirements of the Securities Act
and/or Rule 506 of Regulation D promulgated thereunder.
JMJ Note Shares
This offering, with respect to the JMJ Note Shares, relates
to the resale of 3,000,000 shares of common stock of the Company underlying a $400,000 Promissory Note issued July 10, 2013 (the
“JMJ Note”). The JMJ Note is subject to a one-time interest charge equal to 12% of the principal sum and is due and
payable July 10, 2014. The JMJ Note provides that it may be converted into common stock of the Company at any time at the election
of the holder, at a conversion price equal to the lesser of $0.26 or 65% of the lowest trade price in the 25 trading days previous
to the conversion. The JMJ Note and the shares of common stock underlying the JMJ Note were issued in reliance upon an exemption
from the registration requirements of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder.
SUMMARY OF FINANCIAL INFORMATION
The following selected financial information
is derived from the Company’s Financial Statements appearing elsewhere in this Prospectus and should be read in conjunction
with the Company’s Financial Statements, including the notes thereto, appearing elsewhere in this Prospectus.
Summary of Statements of Operations
For the Three Months Ended December 31, 2013:
|
Total revenue
|
|
$
|
388,746
|
|
|
|
|
|
|
|
|
Net income
|
|
|
(831,410
|
)
|
|
|
|
|
|
|
|
Net income per common share (basic and diluted)
|
|
$
|
(0.01
|
)
|
|
|
|
|
|
|
|
Weighted average common shares
|
|
|
79,632,959
|
|
For the Nine Months Ended December 31, 2013:
|
Total revenue
|
|
$
|
1,893,865
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
(2,017,484
|
)
|
|
|
|
|
|
|
|
Net loss per common share (basic and diluted)
|
|
$
|
(0.03
|
)
|
|
|
|
|
|
|
|
Weighted average common shares
|
|
|
72,842,975
|
|
For the Fiscal Year Ended March 31, 2013:
|
Total revenue
|
|
$
|
2,168,093
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
(2,035,864
|
)
|
|
|
|
|
|
|
|
Net loss per common share (basic and diluted)
|
|
$
|
(0.03
|
)
|
|
|
|
|
|
|
|
Weighted average common shares
|
|
|
62,092,487
|
|
Statement of Financial Position
|
|
|
December 31, 2013
|
|
|
|
|
|
|
|
Cash
|
|
$
|
85,366
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
$
|
164,988
|
|
|
|
|
|
|
|
|
Seeds
|
|
$
|
1,807,000
|
|
|
|
|
|
|
|
|
Prepayments and other current assets
|
|
$
|
74,946
|
|
|
|
|
|
|
|
|
Total current assets
|
|
$
|
2,132,300
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
3,641,781
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
$
|
2,591,869
|
|
|
|
|
|
|
|
|
Stockholders’ equity
|
|
$
|
798,339
|
|
|
|
|
|
|
|
|
Non-controlling interest
|
|
$
|
(345,946
|
)
|
|
|
|
|
|
|
|
Equity
|
|
$
|
452,393
|
|
|
|
|
|
|
|
|
Total liabilities and equity
|
|
$
|
3,641,781
|
|
|
|
|
March 31,
2013
|
|
|
|
|
|
|
|
Cash
|
|
$
|
424,475
|
|
|
|
|
|
|
|
|
Accounts Receivable
|
|
|
158,008
|
|
|
|
|
|
|
|
|
Prepayments and other current assets
|
|
|
33,096
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
615,579
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
2,194,251
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
$
|
1,457,531
|
|
|
|
|
|
|
|
|
Stockholders’ equity
|
|
$
|
464,765
|
|
|
|
|
|
|
|
|
Non-controlling interest
|
|
$
|
(214,158
|
)
|
|
|
|
|
|
|
|
Equity
|
|
$
|
250,607
|
|
|
|
|
|
|
|
|
Total liabilities and equity
|
|
$
|
2,194,251
|
|
DISCLOSURE REGARDING FORWARD-LOOKING
STATEMENTS
Except for statements of historical
facts, this Prospectus contains forward-looking statements involving risks and uncertainties. The words “anticipate,”
“believe,” “estimate,” “expect,” “future,” “intend,” “plan”
or the negative of these terms and similar expressions or variations thereof are intended to forward looking statements. Such statements
reflect the current view of the Registrant with respect to future events and are subject to risks, uncertainties, assumptions and
other factors (including the risks contained in the section of this registration statement on Form S-1 entitled “Risk Factors”)
relating to the Registrant’s industry, the Registrant’s operations and results of operations and any businesses that
may be acquired by the Registrant. Should one or more of these risks or uncertainties materialize, or should the underlying assumptions
prove incorrect, actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned.
Although the Registrant believes that
the expectations reflected in the forward looking statements are reasonable, the Registrant cannot guarantee future results, levels
of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States,
the Registrant does not intend to update any of the forward-looking statements to conform these statements to actual results. The
following discussion should be read in conjunction with the Registrant’s financial statements and the related notes included
in this registration statement on Form S-1.
RISK FACTORS
You should carefully consider the risks
described below together with all of the other information included in our public filings before making an investment decision
with regard to our securities. If any of the following events described in these risk factors actually occurs, our
business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could
decline, and you may lose all or part of your investment.
RISKS RELATING TO OUR BUSINESS AND INDUSTRY
We have a limited operating history on which to evaluate
our business or base an investment decision.
Our business prospects are difficult to
predict because of our limited operating history, early stage of development and unproven business strategy. Stevia is still a
relatively new product in the sweetener marketplace and it has historically not been commercially grown in Vietnam or many of our
other target locations. Both the continued growth of the stevia market in general, and our ability to introduce commercial development
of stevia to new regions, face numerous risks and uncertainties. In particular, we have not proven that we can produce stevia in
a manner that enables us to be profitable and meet manufacturer requirements, develop intellectual property to enhance stevia production,
develop and maintain relationships with key growers and strategic partners to extract value from our intellectual property, raise
sufficient capital in the public and/or private markets, or respond effectively to competitive pressures. If we are unable to accomplish
these goals, our business is unlikely to succeed and you should consider our prospects in light of these risks, challenges and
uncertainties.
We have incurred significant losses
and our auditors have expressed uncertainty about our ability to continue as a going concern.
Our auditors have expressed uncertainty
as to our ability to continue as a going concern as of our fiscal year ended March 31, 2013. As of December 31, 2013, we had an
accumulated deficit of $6,376,899. We anticipate that our existing cash and cash equivalents will not be sufficient to fund our
longer term business needs and we will need to generate additional revenue or receive additional investment in the Company to continue
operations. Such financing may not be available in sufficient amounts, or on terms acceptable to us and may dilute existing
stockholders.
If we fail to raise additional capital,
our ability to implement our business model and strategy could be compromised.
We have limited capital resources and operations.
On March 3, 2014, we raised $340,000 (subject to certain fees) through the issuance of a convertible promissory note (the “Initial
Convertible Note”), pursuant to a Securities Purchase Agreement (the “Purchase Agreement”) with Nomis Bay Ltd.,
a Bermuda company (“Nomis Bay”). The Purchase Agreement provides that, upon the terms and subject to the
conditions set forth therein, we may require Nomis Bay to purchase from the Company on or prior to the 10
th
trading
day after the effective date of the registration statement registering the shares issuable upon conversion of the Initial Convertible
Note, an additional senior convertible note with an initial principal amount of $600,000 (the “Additional Convertible Note”)
for a purchase price of $600,000. There is no guarantee that we will be able to meet the conditions for the funding of the Additional
Convertible Note and investors should not expect us to be able to do so.
To date, our operations have been funded
entirely from the proceeds from debt and equity financings. We expect to require substantial additional capital in the near future
to develop our intellectual property base and to establish the targeted levels of commercial production of stevia. We may not be
able to obtain additional financing on terms acceptable to us, or at all. Even if we obtain financing for our near term operations,
we expect that we will require additional capital beyond the near term. If we are unable to raise capital when needed, our business,
financial condition and results of operations would be materially adversely affected, and we could be forced to reduce or discontinue
our operations.
We face intense competition which could
prohibit us from developing a customer base and generating revenue.
The industries within which we compete,
including the sweetener industry and the fertilizer and feed industries, are highly competitive with companies that have greater
capital resources, facilities and diversity of product lines. Additionally, if demand for stevia continues to grow, we expect many
new competitors to enter the market as there are no significant barriers to stevia production. More established agricultural companies
with much greater financial resources which do not currently compete with us may be able to easily adapt their existing operations
to production of stevia. Due to this competition, there is no assurance that we will not encounter difficulties in obtaining revenues
and market share or in the positioning of our services or that competition in the industry will not lead to reduced prices for
the stevia leaf. Our competitors may also introduce new non-stevia based low-calorie sweeteners or be successful in developing
a fermentation-derived stevia ingredient or other alternative production method which could also increase competition and decrease
demand for stevia-based products.
Inability to protect our proprietary
rights could damage our competitive position.
Our business will be heavily dependent
upon the intellectual property we develop or acquire. Any infringement or misappropriation of our intellectual property could damage
its value and limit our ability to compete. We will rely on patents, copyrights, trademarks, trade secrets, confidentiality provisions
and licensing arrangements to establish and protect our intellectual property. We may have to engage in litigation to protect the
rights to our intellectual property, which could result in significant litigation costs and require a significant amount of our
time. In addition, our ability to enforce and protect our intellectual property rights may be limited in certain countries outside
the United States, which could make it easier for competitors to capture market position in such countries by utilizing technologies
that are similar to those developed or licensed by us.
Competitors may also harm our sales by
designing products that mirror the capabilities of our products or technology without infringing our intellectual property rights.
If we do not obtain sufficient protection for our intellectual property, or if we are unable to effectively enforce our intellectual
property rights, our competitiveness could be impaired, which would limit our growth and future revenue.
A successful claim of infringement against
us could result in a substantial damage award and materially harm our financial condition. Even if a claim against us is unsuccessful,
we would likely have to devote significant time and resources to defending against it.
We may also find it necessary to bring
infringement or other actions against third parties to seek to protect our intellectual property rights. Litigation of this nature,
even if successful, is often expensive and disruptive of a company’s management’s attention, and in any event may not
lead to a successful result relative to the resources dedicated to any such litigation.
We may be unable to effectively develop
an intellectual property portfolio or may fail to keep pace with advances in technology.
We have a limited operating history in
the agriculture industry and there is no certainty that we will be able to effectively develop a viable portfolio of intellectual
property. The success of our farm management services, which are the core of our business, depends upon our ability to create such
intellectual property.
Even if we are able to develop, manufacture
and obtain any regulatory approvals and clearances necessary for our technologies and methods, the success of such services will
depend upon market acceptance. Levels of market acceptance for our services could be affected by several factors, including:
|
|
·
|
the availability of alternative services from our competitors;
|
|
|
·
|
the price and reliability of the our services relative to that of our competitors; and
|
|
|
·
|
the timing of our market entry.
|
Additionally, our intellectual property
must keep pace with advances by our competitors. Failure to do so could cause our position in the industry to erode rapidly.
Confidentiality agreements with employees
and others may not adequately prevent disclosure of our trade secrets and other proprietary information.
Our success depends upon the skills, knowledge
and experience of our technical personnel, our consultants and advisors as well as our licensors and contractors. Because we operate
in a highly competitive field, we will rely significantly on trade secrets to protect our proprietary technology and processes.
However, trade secrets are difficult to protect. We enter into confidentiality and intellectual property assignment agreements
with our corporate partners, employees, consultants, outside scientific collaborators, developers and other advisors. These agreements
generally require that the receiving party keep confidential and not disclose to third parties confidential information developed
by us during the course of the receiving party’s relationship with us. These agreements also generally provide that inventions
conceived by the receiving party in the course of rendering services to us will be our exclusive property. However, these agreements
may be breached and may not effectively assign intellectual property rights to us. Our trade secrets also could be independently
discovered by competitors, in which case we would not be able to prevent use of such trade secrets by our competitors. The enforcement
of a claim alleging that a party illegally obtained and was using our trade secrets could be difficult, expensive and time consuming
and the outcome would be unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets.
The failure to obtain or maintain meaningful trade secret protection could adversely affect our competitive position.
We will produce products for consumption
by consumers that may expose us to litigation based on consumer claims and product liability.
The stevia produced at our farms will be
integrated into stevia-based products which will be consumed by the general public. Additionally, we may manufacture and sell private
label stevia-based food products. Even though we intend to grow and sell products that are safe, we have potential product risk
from the consuming public. We could be party to litigation based on consumer claims, product liability or otherwise that could
result in significant liability for us and adversely affect our financial condition and operations.
If our services do not gain acceptance
among stevia growers, we may not be able to recover the cost of our intellectual property development.
Our business model relies on the assumption
that we will be able to develop methods and protocols, secure valuable plant strains and develop other intellectual property for
stevia farming that will be attractive to both stevia growers and manufacturers. We spent $383,360 for this purpose as of March
31, 2013 and issued 3,000,000 shares to acquire intellectual property related to stevia and we estimate spending approximately
fifteen percent of our operating expense budget to continue developing and improving this intellectual property portfolio. If we
are unable to secure such intellectual property or if our methods and protocols do not gain acceptance among growers or manufacturers,
our intellectual property will have limited value. A number of factors may affect the market acceptance of our products and services,
including, among others, the perception by growers of the effectiveness of our intellectual property, the perception among manufacturers
of the quality of stevia produced using our intellectual property, our ability to fund marketing efforts, and the effectiveness
of such marketing efforts. If such products and services do not gain acceptance by growers and/or manufacturers, we may not be
able to fund future operations, including the expansion of our own farming projects and development and/or acquisition of additional
intellectual property, which inability would have a material adverse effect on our business, financial condition and operating
results.
Any failure to adequately establish
a network of growers and manufacturers will impede our growth.
We expect to be substantially dependent
on manufacturers to purchase the stevia produced both at our own farms and at those of our customers. We have entered into a supply
agreement with a manufacturer and two purchase agreements with growers and are in the process of establishing a network of growers
to produce stevia using the methods and protocols we are developing. The relationship with this manufacturer and its perception
of the stevia produced using our farm management services will determine its willingness to enter into purchase contracts with
us and our customers on attractive terms. Our ability to secure such contracts will influence our attractiveness to growers who
are potentially interested in partnering with us. Achieving significant growth in revenue will depend, in large part, on our success
in establishing this production network. If we are unable to develop an efficient production network, it will make our growth more
difficult and our business could suffer.
If we are unable to deliver a consistent,
high quality stevia leaf at sufficient volumes, our relationship with our manufacturers may suffer and our operating results will
be adversely affected.
Manufacturers will expect us to be able
to consistently deliver stevia at sufficient volumes, while meeting their established quality standards. If we are unable to consistently
deliver such volumes either from our own farms, or those of our grower partners, our relationship with these manufacturers could
be adversely affected which could have a negative impact on our operating results.
Laws and regulations affecting the
cannabis and marijuana industries are constantly changing, which could detrimentally affect our contemplated business, and we cannot
predict the impact that future regulations may have on us.
Local, state and federal cannabis and
marijuana laws and regulations are constantly changing and they are subject to evolving interpretations, which could require us
to incur substantial costs associated with compliance or to alter one or more of our contemplated service offerings. In addition,
violations of these laws, or allegations of such violations, could disrupt our contemplated business and result in a material adverse
effect on our revenues, profitability, and financial condition. We cannot predict the nature of any future laws, regulations, interpretations
or applications, nor can we determine what effect additional governmental regulations or administrative policies and procedures,
when and if promulgated, could have on our contemplated business. Any change in law or interpretation could have a material
adverse effect on our contemplated business, financial condition, and results of operations.
Marijuana remains illegal under federal law.
Marijuana remains illegal under federal law. It is
a schedule-I controlled substance. Even in those jurisdictions in which the use of medical marijuana has been legalized
at the state level, its prescription is a violation of federal law. Federal law criminalizing the use of marijuana trumps
state laws that legalize its use for medicinal purposes, although the current President’s administration has expressed a
reluctance to enforce federal law in this regard in jurisdictions where it conflicts with state law. However, a change in the federal
attitude towards enforcement could occur at any time and could cripple the industry.
It is possible that our contemplated activities
could be deemed to be facilitating the selling or distribution of marijuana in violation of the federal Controlled Substances Act,
or to constitute aiding or abetting, or being an accessory to, a violation of that Act. Federal authorities have not focused their
resources on such tangential or secondary violations of the Act, nor have they threatened to do so. However, if the federal government
were to change its practices, or were to expend its resources attacking providers of services or equipment that could be usable
by participants in the marijuana industry, such action could have a materially adverse effect on our contemplated business, financial
condition, and results of operations.
Hemp remains illegal to grow under federal
law.
Hemp
remains illegal to grow in the United States under federal law due to its relation to marijuana. However, it may be legally imported
and sold in the United States. In certain states, the cultivation of hemp is legal, however federal law criminalizing such cultivation
trumps state laws in this regard. The
current President’s administration has expressed a reluctance to enforce federal
law in this regard in jurisdictions where it conflicts with state law. However, a change in the federal attitude towards enforcement
could occur at any time and could cripple the industry.
It is possible that our contemplated activities
could be deemed to be facilitating hemp cultivation in violation of the federal Controlled Substances Act, or to constitute aiding
or abetting, or being an accessory to, a violation of that Act. Federal authorities have not focused their resources on such tangential
or secondary violations of the Act, nor have they threatened to do so. However, if the federal government were to change its practices,
or were to expend its resources attacking providers of services or equipment that could be usable by participants in the hemp cultivation
industry, such action could have a materially adverse effect on our contemplated business, financial condition, and results of
operations.
Changes in consumer preferences or negative
publicity or rumors may reduce demand for our products.
Recent data suggests consumers are adopting
stevia as a sweetener in many products. However, stevia is a relatively new ingredient in consumer products and many consumers
are not familiar with it. Therefore, any negative reports or rumors regarding either the taste or perceived health effects of stevia,
whether true or not, could have a severe impact on the demand for stevia-based products. Manufacturers may decide to rely on alternative
sweeteners which have a more established history with consumers. Primarily operating at the grower level, we will have little opportunity
to influence these perceptions and there can be no assurance that the increased adoption of stevia in consumer food and beverage
products will continue. Additionally, new sweeteners with similar characteristics to stevia may emerge which could be cheaper to
produce or be perceived to have other qualities superior to stevia. Any of these factors could adversely affect our ability to
produce revenues and our business, financial condition and results of operations would suffer.
Failure to effectively manage growth
of internal operations and business may strain our financial resources.
We intend to significantly expand the scope
of our farming operations and our research and development activities in the near term. Our growth rate may place a significant
strain on our financial resources for a number of reasons, including, but not limited to, the following:
|
|
·
|
The need for continued development of our financial and information management systems;
|
|
|
·
|
The need to manage strategic relationships and agreements with manufacturers, growers and partners; and
|
|
|
·
|
Difficulties in hiring and retaining skilled management, technical and other personnel necessary to support and manage our business.
|
Additionally, our strategy envisions a
period of rapid growth that may impose a significant burden on our administrative and operational resources. Our ability to effectively
manage growth will require us to substantially expand the capabilities of our administrative and operational resources and to attract,
train, manage and retain qualified management and other personnel. Our failure to successfully manage growth could result in our
sales not increasing commensurately with capital investments. Our inability to successfully manage growth could materially adversely
affect our business.
Adverse weather conditions, natural
disasters, crop disease, pests and other natural conditions can impose significant costs and losses on our business.
Weather-related events could significantly
affect our results of operations. We do not currently maintain insurance to cover weather-related losses and if we do obtain such
insurance it likely will not cover all weather-related events and, even when an event is covered, our retention or deductible may
be significant. Cooler temperatures in the regions where we operate could negatively affect us, while not affecting our competitors
in other regions.
Our crops, and those of our grower partners,
could also be affected by drought, temperature extremes, hurricanes, windstorms and floods. In addition, such crops could be vulnerable
to crop disease and to pests, which may vary in severity and effect, depending on the stage of agricultural production at the time
of infection or infestation, the type of treatment applied and climatic conditions. Unfavorable growing conditions caused by these
factors can reduce both crop size and crop quality. In extreme cases, entire harvests may be lost. These factors may result in
lower production and, in the case of farms we own or manage, increased costs due to expenditures for additional agricultural techniques
or agrichemicals, the repair of infrastructure, and the replanting of damaged or destroyed crops. We may also experience shipping
interruptions, port damage and changes in shipping routes as a result of weather-related disruptions.
Competitors and industry participants may
be affected differently by weather-related events based on the location of their production and supply. If adverse conditions are
widespread in the industry, it may restrict supplies and lead to an increase in prices for stevia leaf, but our typical fixed-price
supply contracts may prevent us from recovering these higher costs.
Our operations
and products are regulated in the areas of food safety and protection of human health and the environment.
Our operations and products are subject
to inspections by environmental, food safety, health and customs authorities and to numerous governmental regulations, including
those relating to the use and disposal of agrichemicals, the documentation of food shipments, the traceability of food products,
and labeling of our products for consumers, all of which involve compliance costs. Changes in regulations or laws may require,
operational modifications or capital improvements at various locations. If violations occur, regulators can impose fines, penalties
and other sanctions. The costs of these modifications and improvements and of any fines or penalties could be substantial. We can
be adversely affected by actions of regulators or if consumers lose confidence in the safety and quality of stevia, even if our
products are not implicated.
If we are unable to continually innovate
and increase efficiencies, our ability to attract new customers may be adversely affected.
In the area of innovation, we must be able
to develop new processes, plant strains, and other technologies that appeal to stevia growers. This depends, in part, on the technological
and creative skills of our personnel and on our ability to protect our intellectual property rights. We may not be successful in
the development, introduction, marketing and sourcing of new technologies or innovations, that satisfy customer needs, achieve
market acceptance or generate satisfactory financial returns.
Global economic conditions may adversely
affect our industry, business and result of operations.
Disruptions in the global credit and financial
market could result in diminished liquidity and credit availability, a decline in consumer confidence, a decline in economic growth,
an increased unemployment rate, and uncertainty about economic stability. These economic uncertainties can affect businesses such
as ours in a number of ways, making it difficult to accurately forecast and plan our future business activities. Such conditions
can lead consumers to postpone spending, which can cause manufacturers to cancel, decrease or delay orders with us. We are unable
to predict the likelihood of the occurrence, duration or severity of such disruptions in the credit and financial markets and adverse
global economic conditions and such economic conditions could materially and adversely affect our business and results of operations.
Our business depends substantially on
the continuing efforts of our executive officers and our business may be severely disrupted if we lose their services.
Our future success depends substantially
on the continued services of our executive officers, especially our President and director, Mr. George Blankenbaker. We do not
maintain key man life insurance on any of our executive officers and directors. If one or more of our executive officers are unable
or unwilling to continue in their present positions, we may not be able to replace them readily, if at all. Therefore, our business
may be severely disrupted, and we may incur additional expenses to recruit and retain new officers. In addition, if any of our
executives joins a competitor or forms a competing company, we may lose some of our customers.
Our engagement of Growers Synergy Pte
Ltd. may represent a potential conflict of interest.
We have engaged Growers Synergy Pte Ltd,
a regional farm management services provider, to provide farm management operations and back-office and regional logistical support
for our Vietnam and Indonesia operations for a period of two years. During the fiscal year ended March 31, 2012, Growers Synergy
received $180,000 for consulting services rendered to the Company and during the fiscal year ended March 31, 2013, Growers Synergy
received $240,000 for consulting services rendered to the Company. George Blankenbaker, our president, director and stockholder
is the managing director of Growers Synergy. Growers Fresh Pte Ltd (“Growers Fresh) owns a 51% interest in Growers Synergy
and Mr. Blankenbaker controls a 49% interest in Growers Fresh. As a result, there is a potential conflict of interest on Mr. Blankenbaker’s
role in the Company and Growers Synergy and such potential conflict could materially affect the terms of any engagement entered
into by the Company and Growers Synergy. Such terms, if not negotiated at arms length may not be in the best interest of the Company
and our stockholders.
Litigation may adversely affect our
business, financial condition and results of operations.
From time to time in the normal course
of our business operations, we may become subject to litigation that may result in liability material to our financial statements
as a whole or may negatively affect our operating results if changes to our business operation are required. The cost to defend
such litigation may be significant and may require a diversion of our resources. There also may be adverse publicity associated
with litigation that could negatively affect customer perception of our business, regardless of whether the allegations are valid
or whether we are ultimately found liable. As a result, litigation may adversely affect our business, financial condition and results
of operations.
We may be required to incur significant
costs and require significant management resources to evaluate our internal control over financial reporting as required under
Section 404 of the Sarbanes-Oxley Act, and any failure to comply or any adverse result from such evaluation may have an adverse
effect on our stock price.
As a smaller reporting company as defined
in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, we are required to evaluate our internal control over financial
reporting under Section 404 of the Sarbanes-Oxley Act of 2002 (“Section 404”). Section 404 requires us to include an
internal control report with our Annual Report on Form 10-K. This report must include management’s assessment of the effectiveness
of our internal control over financial reporting as of the end of the fiscal year. This report must also include disclosure of
any material weaknesses in internal control over financial reporting that we have identified. Failure to comply, or any adverse
results from such evaluation could result in a loss of investor confidence in our financial reports and have an adverse effect
on the trading price of our equity securities. As of December 31, 2013, the management of the Company assessed the effectiveness
of the Company’s internal control over financial reporting based on the criteria for effective internal control over financial
reporting established in
Internal Control - Integrated Framework
issued by the Committee of Sponsoring Organizations of
the Treadway Commission (“COSO”) and SEC guidance on conducting such assessments. Management concluded, as of the quarter
ended December 31, 2013, that its internal controls and procedures were not effective to detect the inappropriate application of
U.S. GAAP rules. Management realized there were deficiencies in the design or operation of our internal control that adversely
affected our internal controls which management considers to be material weaknesses including those described below:
|
|
·
|
We have not achieved the optimal level of segregation of duties relative to key financial reporting functions.
|
|
|
·
|
We do not have an audit committee or an independent audit committee financial expert. While not being legally obligated to have an audit committee or independent audit committee financial expert, it is the management’s view that to have an audit committee, comprised of independent board members, and an independent audit committee financial expert is an important entity-level control over our financial statements.
|
Achieving continued compliance with Section
404 may require us to incur significant costs and expend significant time and management resources. No assurance can be given that
we will be able to fully comply with Section 404 or that we and our independent registered public accounting firm would be able
to conclude that our internal control over financial reporting is effective at fiscal year end. As a result, investors could lose
confidence in our reported financial information, which could have an adverse effect on the trading price of our securities, as
well as subject us to civil or criminal investigations and penalties. In addition, our independent registered public accounting
firm may not agree with our management’s assessment or conclude that our internal control over financial reporting is operating
effectively.
RISKS RELATED TO DOING BUSINESS IN VIETNAM
AND OTHER DEVELOPING COUNTRIES
Our international operations will be
subject to the laws of the jurisdictions in which we operate.
A significant portion of our initial business
operations will occur in Vietnam. We will be generally subject to laws and regulations applicable to foreign investment
in Vietnam. The Vietnamese legal system is based, at least in part, on written statutes. However, since these
laws and regulations are relatively new and the Vietnamese legal system continues to rapidly evolve, the interpretations of many
laws, regulations and rules are not always uniform and enforcement of these laws, regulations and rules involves uncertainties.
In April 2012, we announced plans to begin
field tests in Indonesia. Similar to Vietnam, the modern Indonesia legal system was formed relatively recently and is continuing
to evolve. As we continue our expansion into Indonesia and other developing countries, we will face similar risks and uncertainties
regarding the legal system as we currently face in Vietnam.
We cannot predict the effect of future
developments in the legal systems of developing countries, including the promulgation of new laws, changes to existing laws or
the interpretation or enforcement thereof, the preemption of local regulations by national laws, or the overturn of local government’s
decisions by the superior government. These uncertainties may limit legal protections available to us.
Our international operations involve
the use of foreign currencies, which subjects us to exchange rate fluctuations and other currency risks.
The revenues and expenses of our international
operations are generally denominated in local currencies, which subjects us to exchange rate fluctuations between such local currencies
and the U.S. dollar. These exchange rate fluctuations will subject us to currency translation risk with respect to the reported
results of our international operations, as well as to other risks sometimes associated with international operations. In
the future, we could experience fluctuations in financial results from our operations outside of the United States, and there can
be no assurance we will be able, contractually or otherwise, to reduce the currency risks associated with our international operations.
We may be adversely affected by economic
and political conditions in the countries where we operate.
We operate in Vietnam and other countries
throughout the world. Economic and political changes in these countries, such as inflation rates, recession, foreign ownership
restrictions, restrictions on transfer of funds into or out of a country and similar factors may adversely affect results of operations.
While it is our understanding that the
economy in Vietnam has grown significantly in the past 20 years, the growth has been uneven, both geographically and among various
economic sectors. The government of Vietnam has implemented various measures to encourage or control economic growth
and guide the allocation of resources. Some of these measures benefit the overall Vietnamese economy, but may also have
a negative effect on us. For example, our financial condition and results of operations may be adversely affected by
government control over capital investments or changes in tax regulations that are applicable to us.
The Vietnamese economy has been transitioning
from a planned economy to a more market-oriented economy. Although in recent years the Vietnamese government has implemented
measures emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets
and the establishment of sound corporate governance in business enterprises, a substantial portion of the productive assets in
Vietnam are still owned by the Vietnamese government. The continued control of these assets and other aspects of the
national economy by Vietnam government could materially and adversely affect our business. The Vietnamese government
also exercises significant control over Vietnamese economic growth through the allocation of resources, controlling payment of
foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries
or companies. Efforts by the Vietnamese government to slow the pace of growth of the Vietnamese economy could negatively
affect our business.
Our insurance coverage may be inadequate
to cover all significant risk exposures.
We will be exposed to liabilities that
are unique to the products we provide. While we intend to maintain insurance for certain risks, the amount of our insurance
coverage may not be adequate to cover all claims or liabilities, and we may be forced to bear substantial costs resulting from
risks and uncertainties of our business. It is also not possible to obtain insurance to protect against all operational
risks and liabilities. The failure to obtain adequate insurance coverage on terms favorable to us, or at all, could
have a material adverse effect on our business, financial condition and results of operations. In addition, because
the insurance industry in Vietnam and other developing countries are still in their early stages of development, business interruption
insurance available in such countries relating to our intended services and products offers limited coverage compared to that offered
in many other developed countries. We do not have any business interruption insurance. Any business disruption
or natural disaster could result in substantial costs and diversion of resources.
It will be extremely difficult to acquire
jurisdiction and enforce liabilities against our officers, directors and assets outside the United States.
Substantially all of our assets are currently
located outside of the United States and a significant number of our officers and directors may reside outside of the United States
as well. As a result, it may not be possible for United States investors to enforce their legal rights, to effect service
of process upon our directors or officers or to enforce judgments of United States courts predicated upon civil liabilities and
criminal penalties of our directors and officers under Federal securities laws. Moreover, we have been advised that Vietnam in
particular does not have treaties providing for the reciprocal recognition and enforcement of judgments of courts with the United
States. Further, it is unclear if extradition treaties now in effect between the United States and Vietnam would permit effective
enforcement of criminal penalties of the Federal securities laws.
RISKS RELATED TO AN INVESTMENT IN OUR SECURITIES
The relative lack of public company
experience of our management team may put us at a competitive disadvantage.
Our management team lacks public company
experience and is generally unfamiliar with the requirements of the United States securities laws and U.S. Generally Accepted Accounting
Principles, which could impair our ability to comply with legal and regulatory requirements such as those imposed by Sarbanes-Oxley
Act of 2002. The individuals who now constitute our senior management team have never had responsibility for managing a publicly
traded company. Such responsibilities include complying with federal securities laws and making required disclosures on a timely
basis. Our senior management may not be able to implement programs and policies in an effective and timely manner that adequately
responds to such increased legal, regulatory compliance and reporting requirements. Our failure to comply with all applicable requirements
could lead to the imposition of fines and penalties and distract our management from attending to the growth of our business.
Our stock is categorized as a penny
stock. Trading of our stock may be restricted by the SEC’s penny stock regulations which may limit a stockholder’s
ability to buy and sell our stock.
Our stock is categorized as a “penny
stock”. The SEC has adopted Rule 15g-9 which generally defines “penny stock” to be any equity security that has
a market price (as defined) less than $4.00 per share or an exercise price of less than $5.00 per share, subject to certain exceptions.
Our securities are covered by the penny stock rules, which impose additional sales practice requirements on broker-dealers who
sell to persons other than established customers and accredited investors. The penny stock rules require a broker-dealer, prior
to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document in a
form prepared by the SEC which provides information about penny stocks and the nature and level of risks in the penny stock market.
The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of
the broker-dealer and its salesperson in the transaction and monthly account statements showing the market value of each penny
stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information,
must be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing
before or with the customer’s confirmation. In addition, the penny stock rules require that prior to a transaction in a penny
stock not otherwise exempt from these rules, the broker-dealer must make a special written determination that the penny stock is
a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure
requirements may have the effect of reducing the level of trading activity in the secondary market for the stock that is subject
to these penny stock rules. Consequently, these penny stock rules may affect the ability of broker-dealers to trade our securities.
We believe that the penny stock rules discourage investor interest in and limit the marketability of our common stock.
FINRA sales practice requirements may
also limit a stockholder’s ability to buy and sell our stock.
In addition to the “penny stock”
rules described above, the Financial Industry Regulatory Authority (“FINRA”) has adopted rules that require that in
recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable
for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers
must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives
and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low
priced securities will not be suitable for at least some customers. The FINRA requirements make it more difficult for broker-dealers
to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock and have an adverse
effect on the market for our shares.
We expect to experience volatility in
our stock price, which could negatively affect stockholders’ investments.
Although our common stock is quoted on
the OTCQB under the symbol “STEV”, there is a limited public market for our common stock. No assurance can
be given that an active market will develop or that a stockholder will ever be able to liquidate its shares of common stock without
considerable delay, if at all. Many brokerage firms may not be willing to effect transactions in the securities. Even
if a purchaser finds a broker willing to effect a transaction in these securities, the combination of brokerage commissions, state
transfer taxes, if any, and any other selling costs may exceed the selling price. Furthermore, our stock price may be
impacted by factors that are unrelated or disproportionate to our operating performance. These market fluctuations,
as well as general economic, political and market conditions, such as recessions, interest rates or international currency fluctuations
may adversely affect the market price and liquidity of our common stock.
In the past, securities class action litigation
has often been brought against a company following periods of volatility in the market price of its securities. Due to the
volatility of our common stock price, we may be the target of securities litigation in the future. Securities litigation
could result in substantial costs and divert management’s attention and resources.
Stockholders should also be aware that,
according to SEC Release No. 34-29093, the market for “penny stock”, such as our common stock, has suffered in recent
years from patterns of fraud and abuse. Such patterns include (1) control of the market for the security by one or a few
broker-dealers that are often related to the promoter or issuer; (2) manipulation of prices through prearranged matching of purchases
and sales and false and misleading press releases; (3) boiler room practices involving high-pressure sales tactics and unrealistic
price projections by inexperienced sales persons; (4) excessive and undisclosed bid-ask differential and markups by selling broker-dealers;
and (5) the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired
level, along with the resulting inevitable collapse of those prices and with consequent investor losses. Our management is
aware of the abuses that have occurred historically in the penny stock market. Although we do not expect to be in a position
to dictate the behavior of the market or of broker-dealers who participate in the market, management will strive within the confines
of practical limitations to prevent the described patterns from being established with respect to our securities. The occurrence
of these patterns or practices could increase the future volatility of our share price.
To date, we have not paid any cash dividends
and no cash dividends will be paid in the foreseeable future.
We do not anticipate paying cash dividends
on our common stock in the foreseeable future and we may not have sufficient funds legally available to pay dividends. Even if
the funds are legally available for distribution, we may nevertheless decide not to pay any dividends. We presently intend to retain
all earnings for our operations.to pay dividends. Even if the funds are legally available for distribution, we may nevertheless
decide not to pay any dividends. We presently intend to retain all earnings for our operations.
The elimination of monetary liability
against our directors, officers and employees under Nevada law and the existence of indemnification rights to our directors, officers
and employees may result in substantial expenditures by our company and may discourage lawsuits against our directors, officers
and employees.
Our Articles of Incorporation contain a
provision permitting us to eliminate the personal liability of our directors to our company and stockholders for damages for breach
of fiduciary duty as a director or officer to the extent provided by Nevada law. We may also have contractual indemnification obligations
under our employment agreements with our officers. The foregoing indemnification obligations could result in the Company incurring
substantial expenditures to cover the cost of settlement or damage awards against directors and officers, which we may be unable
to recoup. These provisions and resultant costs may also discourage our company from bringing a lawsuit against directors and officers
for breaches of their fiduciary duties, and may similarly discourage the filing of derivative litigation by our stockholders against
our directors and officers even though such actions, if successful, might otherwise benefit our company and stockholders.
If the Company’s outstanding warrants
and convertible notes are exercised and/or converted it will result in the dilution of our existing stockholders.
The outstanding warrants and convertible
notes being registered hereunder are exercisable for an aggregate of 47,898,931 shares of our common stock. Although the Company
has a call right or repayment right with respect to certain of the notes and warrants, the Company may not be financially capable
of exercising such call right or may otherwise choose not to do so, and therefore the Company may not control if and when the notes
and warrants are exercised and/or converted. The exercise of the notes and warrants would result in dilution to our existing stockholders
and could contribute to a reduction in the market price of the outstanding shares of our common stock.
USE OF PROCEEDS
The “Selling Security Holders”
set forth in “The Selling Security Holders Table” below may sell all of the common stock underlying the Warrants offered
by this Prospectus from time-to-time. We will not receive any proceeds from the sale of those shares of common stock. We may, however,
receive gross proceeds of up to $1,083,103.46 upon the cash exercise of the Warrants. Any such proceeds we receive will be used
for working capital and general corporate matters.
DETERMINATION OF OFFERING PRICE
There currently is a limited public market for our common stock.
Selling Security Holders will determine at what price they may sell the offered shares, and such sales may be made at prevailing
market prices or at privately negotiated prices. See “Plan of Distribution” below for more information.
SELLING SECURITY HOLDERS
Nomis Bay Note Shares
This offering, with respect to the Nomis Bay Note Shares, relates
to the resale of shares of common stock of the Company underlying a Senior Convertible Note, issued March 3, 2014 (the “Nomis
Bay Note”). On March 3, 2014, pursuant to a Securities Purchase Agreement (the “Purchase Agreement”) with Nomis
Bay Ltd., a Bermuda company (“Nomis Bay”), we issued the Nomis Bay Note with an initial principal amount of $500,000
for a purchase price of $340,000. The Nomis Bay Note matures on December 27, 2014 (subject to extension as provided
in the Nomis Bay Note) and, in addition to the 32% original issue discount, accrues interest at the rate of 8% per annum. The Purchase
Agreement also provides that, upon the terms and subject to the conditions set forth therein, the Company may require Nomis Bay
to purchase from the Company on or prior to the 10
th
trading day after the effective date of the registration statement
registering the shares issuable upon conversion of the Initial Convertible Note, an additional senior convertible note with an
initial principal amount of $600,000 (the “Additional Convertible Note” and together with the Nomis Bay Note, the “Convertible
Notes”) for a purchase price of $600,000. If issued, the Additional Convertible Note will mature on the date that is the
10-month anniversary of the date of issuance of the Additional Convertible Note (subject to extension as provided in the Initial
Convertible Note) and will accrue interest at the rate of 8% per annum. The Nomis Bay Note is convertible at any time, in whole
or in part, at Nomis Bay’s option into shares of the Company’s common stock, par value $0.001 per share (the “Common
Stock”), at a conversion price equal to the lesser of (i) the product of (x) the arithmetic average of the lowest three (3)
volume weighted average prices of the Common Stock during the 10 consecutive trading days ending and including the trading day
immediately preceding the applicable conversion date and (y) 40% (the “Variable Conversion Price”), and (ii) $0.30
(as adjusted for stock splits, stock dividends, stock combinations or other similar transactions). If issued, the Additional Convertible
Note will be convertible at any time, in whole or in part, at Nomis Bay’s option into shares of Common Stock at a conversion
price that will be equal to the lesser of (i) the Variable Conversion Price and (ii) $0.30 (as adjusted for stock splits, stock
dividends, stock combinations or other similar transactions). At no time will Nomis Bay be entitled to convert any portion of the
Convertible Notes to the extent that after such conversion, Nomis Bay (together with its affiliates) would beneficially own more
than 4.99% of the outstanding shares of Common Stock as of such date. The shares of common stock underlying the Nomis Bay Note
were issued in reliance upon an exemption from the registration requirements of the Securities Act and/or Rule 506 of Regulation
D promulgated thereunder.
Anson Reset Shares
This offering, with respect to the Anson
Reset Shares, relates to the resale of shares of common stock of the Company underlying three warrants in the amounts of 1,877,333,
1,066,666 and 2,346,666, with exercise prices of $0.20, $0.25 and $0.25 per share (the “Anson Warrants”). Pursuant
to the terms of a warrant exercise reset offer letter between the Company and the investor, the Anson Warrants were issued in consideration
for the investor’s agreement to immediately cash exercise an existing warrant to purchase 853,333 shares of common stock
of the Company at an exercise price of $0.20 per share for aggregate consideration to the Company of $170,666 originally issued
pursuant to the terms of the Securities Purchase Agreement, dated August 1, 2012 (the “Purchase Agreement”). Each Anson
Warrant has a five year term and was issued on May 3, 2013. Each Anson Warrant provides for adjustment of the exercise price and
share amount in the event of certain dilutive issuances by the Company. On February 20, 2014, the Company issued a notice to the
holder that the aggregate number of Anson Warrants had been adjusted to 23,026,318 and the exercise price of each had been adjusted
to $0.053365 as a result of certain other offerings of the Company. 5,290,665 of the shares underlying the Anson Warrants were
previously registered by the Company pursuant to the Registration Statement on Form S-1/A filed December 30, 2013. The warrants
and the shares of common stock underlying the warrants were issued in reliance upon an exemption from the registration requirements
of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder.
Cranshire Warrant Shares
This offering, with respect to the Cranshire
Warrant Shares, relates to the resale of the shares of common stock underlying a warrant in the amount of 2,560,486 with an exercise
price of $0.053365. The warrant has a five (5) year term and was issued pursuant to the terms of the Purchase Agreement. The warrant
was originally issued in the amount of 213,334 shares at an exercise price of $0.6405. Pursuant to the anti-dilution adjustment
provision contained therein, on February 20, 2014, the Company issued a notice to the holder that the total share amount had been
increased to 2,560,486 and the exercise price had been reduced to $0.053365 as a result of certain other offerings of the Company.
683,202 of the shares underlying the Cranshire Warrants were previously registered by the Company pursuant to the Registration
Statement on Form S-1/A filed December 30, 2013. Additionally, on February 20, 2014 in consideration for the investor’s agreement
to immediately cash exercise a portion of the Cranshire Warrants for aggregate consideration to the Company of $36,459, the Company
agreed to issue the investor an additional warrant to purchase 683,202 shares of common stock. The warrants and the shares of common
stock underlying the warrants were issued in reliance upon an exemption from the registration requirements of the Securities Act
and/or Rule 506 of Regulation D promulgated thereunder.
JMJ Note Shares
This offering, with respect to the JMJ
Note Shares, relates to the resale of 3,000,000 shares of common stock of the Company underlying a $400,000 Promissory Note issued
July 10, 2013. The JMJ Note is subject to a one-time interest charge equal to 12% of the principal sum and is due and payable July
10, 2014. The JMJ Note provides that it may be converted into common stock of the Company at any time at the election of the holder,
at a conversion price equal to the lesser of $0.26 or 65% of the lowest trade price in the 25 trading days previous to the conversion.
The JMJ Note and the shares of common stock underlying the JMJ Note were issued in reliance upon an exemption from the registration
requirements of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder.
The Selling Security Holders Table
The following table sets forth the names
of the Selling Security Holders, the number of shares of common stock beneficially owned by each Selling Security Holder as of
the date hereof and the number of shares of common stock being offered by each Selling Security Holder. The shares being
offered hereby are being registered to permit public secondary trading, and the Selling Security Holders may offer all or part
of the shares for resale from time to time. However, the Selling Security Holders are under no obligation to sell all or any portion
of such shares nor are the Selling Security Holders obligated to sell any shares immediately upon effectiveness of this prospectus.
All information with respect to share ownership has been furnished by the Selling Security Holders. The “Amount
Beneficially Owned After Offering” column assumes the sale of all shares offered.
|
|
|
Shares
|
|
|
|
|
|
Amount
|
|
|
Percent
|
|
|
|
|
Beneficially
|
|
|
|
|
|
Beneficially
|
|
|
Beneficially
|
|
|
|
|
Owned Prior To
|
|
|
Shares to
|
|
|
Owned After
|
|
|
Owned
|
|
|
Name
|
|
Offering(1)
|
|
|
be Offered
|
|
|
Offering(1)(5)
|
|
|
After Offering(6)
|
|
|
Nomis Bay Ltd.(2)
|
|
|
6,361,323
|
|
|
|
24,602,792
|
|
|
|
0
|
|
|
|
0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anson Investments Master Fund LP(3)
|
|
|
17,735,653
|
|
|
|
17,735,653
|
|
|
|
0
|
|
|
|
0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cranshire Capital Master Fund, Ltd.(4)
|
|
|
2,560,486
|
|
|
|
2,560,486
|
|
|
|
0
|
|
|
|
0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
JMJ Financial
|
|
|
0
|
|
|
|
3,000,000
|
|
|
|
0
|
|
|
|
0
|
%
|
|
|
(1)
|
Beneficial ownership has been determined in accordance
with Rule 13d-3 under the Exchange Act. Pursuant to the rules of the SEC, shares of common stock which an individual or group
has a right to acquire within 60 days pursuant to the exercise of options or warrants are deemed to be outstanding for the purpose
of computing the percentage ownership of such individual or group, but are not deemed to be beneficially owned and outstanding
for the purpose of computing the percentage ownership of any other person shown in the table.
|
|
|
(2)
|
Includes 6,361,323 shares of common stock issuable to
Nomis Bay Ltd. upon the conversion of the principal amount of the registrant’s Senior Convertible Note issued March 3, 2014,
based upon a current conversion price of $0.0786. EOM Management Ltd. (“EOM”) is the investment manager of Nomis Bay
Ltd. and has voting control and investment discretion over securities held by Nomis Bay Ltd. Chaja Carlebach (“Ms. Carlebach”)
owns all of the outstanding equity of EOM and has voting control over EOM. As a result, each of Ms. Carlebach and EOM may be deemed
to have beneficial ownership (as determined under Section 13(d) of the Securities Exchange Act of 1934, as amended) of the securities
held by Nomis Bay Ltd. We have been advised that neither EOM nor Nomis Bay Ltd. is a member of the Financial Industry Regulatory
Authority (“FINRA”) or an independent broker-dealer, and that neither EOM, Nomis Bay Ltd. or any of their respective
affiliates is an affiliate or an associated person of any FINRA member or independent broker-dealer.
|
|
|
(3)
|
Moez
Kassam has voting and dispositive control over the shares beneficially owned by Anson Investments Master Fund LP.
|
|
|
(4)
|
Cranshire
Capital Advisors, LLC (“CCA”) is the investment manager of Cranshire Capital Master Fund, Ltd. (“Cranshire Master
Fund”) and has voting control and investment discretion over securities held by Cranshire Master Fund. Mitchell P. Kopin
(“Mr. Kopin”), the president, the sole member and the sole member of the Board of Managers of CCA, has voting control
over CCA. As a result, each of Mr. Kopin and CCA may be deemed to have beneficial ownership (as determined under Section 13(d)
of the Securities Exchange Act of 1934, as amended) of the securities held by Cranshire Master Fund.
|
|
|
(5)
|
Includes
the shares of common stock issuable upon exercise of the Warrants.
|
|
|
(6)
|
Applicable
percentage ownership is based on 145,972,713 shares of our common stock outstanding as of March 26, 2014.
|
All expenses incurred with respect to the registration of the
common stock will be borne by us, but we will not be obligated to pay any underwriting fees, discounts, commission or other expenses
incurred by the Selling Security Holders in connection with the sale of the Shares.
Neither the Selling Security Holders nor any of their associates
or affiliates has held any position, office, or other material relationship with us in the past three years.
PLAN OF DISTRIBUTION
This prospectus relates to the resale of
47,898,931 shares of common stock, by the Selling Security Holders listed under “Selling Security Holders” on page
15, issuable upon the exercise of the outstanding convertible notes and warrants.
The Selling Security Holders and any of
their respective pledges, donees, assignees and other successors-in-interest may, from time to time, sell any or all of their shares
of our common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. The
Selling Security Holders may use any one or more of the following methods when selling shares:
|
|
·
|
ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
·
|
block
trades in which the broker-dealer will attempt to sell the shares as agent, but may position and resell a portion of the block
as principal to facilitate the transaction;
|
|
|
·
|
purchases
by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
|
·
|
an
exchange distribution in accordance with the rules of the applicable exchange;
|
|
|
·
|
privately
negotiated transactions;
|
|
|
·
|
broker-dealers
may agree with a Selling Security Holder to sell a specified number of such shares at a stipulated price per share;
|
|
|
·
|
through
the writing of options on the shares;
|
|
|
·
|
a combination of any such methods of sale; and
|
any
other method permitted pursuant to applicable law.
The Selling Security Holders or their respective
pledgees, donees, transferees or other successors in interest, may also sell the shares directly to market makers acting as principals
and/or broker-dealers acting as agents for themselves or their customers. Such broker-dealers may receive compensation
in the form of discounts, concessions or commissions from the Selling Security Holders and/or the purchasers of shares for whom
such broker-dealers may act as agents or to whom they sell as principal or both, which compensation as to a particular broker-dealer
might be in excess of customary commissions. Market makers and block purchasers purchasing the shares will do so for their own
account and at their own risk. It is possible that the Selling Security Holders will attempt to sell shares of common
stock in block transactions to market makers or other purchasers at a price per share which may be below the then market price. The
Selling Security Holders cannot assure that all or any of the shares offered in this prospectus will be issued to, or sold by,
the Selling Security Holders. In addition, the Selling Security Holders and any brokers, dealers or agents, upon effecting
the sale of any of the shares offered in this prospectus may be deemed “underwriters” as that term is defined under
the Securities Act or the Exchange Act, or the rules and regulations under such acts. In such event, any commissions
received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting
commissions or discounts under the Securities Act.
Discounts, concessions, commissions and
similar selling expenses, if any, attributable to the sale of shares will be borne by a Selling Security Holder. The Selling Security
Holders may agree to indemnify any agent, dealer or broker-dealer that participates in transactions involving sales of the shares
if liabilities are imposed on that person under the Securities Act.
The Selling Security Holders may from time
to time pledge or grant a security interest in some or all of the shares of common stock owned by them, and, if they default in
the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of common stock from
time to time under this prospectus after we have filed an amendment to this prospectus under Rule 424(b)(3) or any other applicable
provision of the Securities Act amending the list of Selling Security Holders to include the pledgee, transferee or other successors
in interest as Selling Security Holder under this prospectus.
The Selling Security Holders also may transfer
the shares of common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will
be the selling beneficial owners for purposes of this prospectus and may sell the shares of common stock from time to time under
this prospectus after we have filed an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities
Act amending the list of Selling Security Holders to include the pledgee, transferee or other successors in interest as a Selling
Security Holder under this prospectus.
The Selling Security Holders acquired or
will acquire the securities offered hereby in the ordinary course of business and have advised us that they have not entered into
any agreements, understandings or arrangements with any underwriters or broker-dealers regarding the sale of their shares of common
stock, nor is there an underwriter or coordinating broker acting in connection with a proposed sale of shares of common stock by
any Selling Security Holder. We will file a supplement to this prospectus if a Selling Security Holder enters into a
material arrangement with a broker-dealer for sale of common stock being registered. If the Selling Security Holders
use this prospectus for any sale of the shares of common stock, it will be subject to the prospectus delivery requirements of the
Securities Act.
Pursuant to a requirement by the Financial
Industry Regulatory Authority, or FINRA, the maximum commission or discount to be received by any FINRA member or independent broker/dealer
may not be greater than eight percent (8%) of the gross proceeds received by us for the sale of any securities being registered
pursuant to SEC Rule 415 under the Securities Act.
The anti-manipulation rules of Regulation
M under the Exchange Act, may apply to sales of our common stock and activities of the Selling Security Holders. The
Selling Security Holders will act independently of us in making decisions with respect to the timing, manner and size of each sale.
We will pay all expenses incident to the
registration, offering and sale of the shares of our common stock to the public hereunder other than commissions, fees and discounts
of underwriters, brokers, dealers and agents. We estimate that the expenses of the offering to be borne by us will
be approximately $16,145.53. We will not receive any proceeds from the resale of any of the shares of our common stock
in this Offering, other than $1,083,103.46 of potential proceeds upon the exercise of the Warrants for cash.
DESCRIPTION OF SECURITIES TO BE REGISTERED
General
The following summary includes a description
of material provisions of our capital stock.
Authorized and Outstanding Securities
The Company is authorized to issue 250,000,000
shares of common stock, par value $0.001 per share. As of March 26, 2014, there were issued and outstanding 145,972,713
shares of our common stock.
Common Stock
The holders of our common stock are entitled
to one vote for each share on all matters to be voted on by the stockholders. Holders of common stock do not have cumulative voting
rights. Holders of common stock are entitled to share ratably in dividends, if any, as may be declared from time to time by the
board of directors in its discretion from funds legally available therefore. In the event of a liquidation, dissolution or winding
up of the Company, the holders of common stock are entitled to share pro rata all assets remaining after payment in full of all
liabilities. Holders of common stock have no preemptive rights to purchase the Company’s common stock. There are no conversion
or redemption rights or sinking fund provisions with respect to the common stock.
Dividends
Dividends, if any, will be contingent upon
our revenues and earnings, if any, capital requirements and financial conditions. The payment of dividends, if any, will be within
the discretion of our board of directors. We intend to retain earnings, if any, for use in its business operations and accordingly,
the board of directors does not anticipate declaring any dividends in the foreseeable future.
Convertible Notes and Warrants
Nomis Bay Note Shares
This offering, with respect to the Nomis Bay Note Shares, relates
to the resale of shares of common stock of the Company underlying a Senior Convertible Note, issued March 3, 2014 (the “Nomis
Bay Note”). On March 3, 2014, pursuant to a Securities Purchase Agreement (the “Purchase Agreement”) with Nomis
Bay Ltd., a Bermuda company (“Nomis Bay”), we issued the Nomis Bay Note with an initial principal amount of $500,000
for a purchase price of $340,000. The Nomis Bay Note matures on December 27, 2014 (subject to extension as provided
in the Nomis Bay Note) and, in addition to the 32% original issue discount, accrues interest at the rate of 8% per annum. The Purchase
Agreement also provides that, upon the terms and subject to the conditions set forth therein, the Company may require Nomis Bay
to purchase from the Company on or prior to the 10
th
trading day after the effective date of the registration statement
registering the shares issuable upon conversion of the Initial Convertible Note, an additional senior convertible note with an
initial principal amount of $600,000 (the “Additional Convertible Note” and together with the Nomis Bay Note, the “Convertible
Notes”) for a purchase price of $600,000. If issued, the Additional Convertible Note will mature on the date that is the
10-month anniversary of the date of issuance of the Additional Convertible Note (subject to extension as provided in the Initial
Convertible Note) and will accrue interest at the rate of 8% per annum. The Nomis Bay Note is convertible at any time, in whole
or in part, at Nomis Bay’s option into shares of the Company’s common stock, par value $0.001 per share (the “Common
Stock”), at a conversion price equal to the lesser of (i) the product of (x) the arithmetic average of the lowest three (3)
volume weighted average prices of the Common Stock during the 10 consecutive trading days ending and including the trading day
immediately preceding the applicable conversion date and (y) 40% (the “Variable Conversion Price”), and (ii) $0.30
(as adjusted for stock splits, stock dividends, stock combinations or other similar transactions). If issued, the Additional Convertible
Note will be convertible at any time, in whole or in part, at Nomis Bay’s option into shares of Common Stock at a conversion
price that will be equal to the lesser of (i) the Variable Conversion Price and (ii) $0.30 (as adjusted for stock splits, stock
dividends, stock combinations or other similar transactions). At no time will Nomis Bay be entitled to convert any portion of the
Convertible Notes to the extent that after such conversion, Nomis Bay (together with its affiliates) would beneficially own more
than 4.99% of the outstanding shares of Common Stock as of such date. The shares of common stock underlying the Nomis Bay Note
were issued in reliance upon an exemption from the registration requirements of the Securities Act and/or Rule 506 of Regulation
D promulgated thereunder.
Anson Reset Shares
This offering, with respect to the Anson
Reset Shares, relates to the resale of shares of common stock of the Company underlying three warrants in the amounts of 1,877,333,
1,066,666 and 2,346,666, with exercise prices of $0.20, $0.25 and $0.25 per share (the “Anson Warrants”). Pursuant
to the terms of a warrant exercise reset offer letter between the Company and the investor, the Anson Warrants were issued in consideration
for the investor’s agreement to immediately cash exercise an existing warrant to purchase 853,333 shares of common stock
of the Company at an exercise price of $0.20 per share for aggregate consideration to the Company of $170,666 originally issued
pursuant to the terms of the Securities Purchase Agreement, dated August 1, 2012 (the “Purchase Agreement”). Each Anson
Warrant has a five year term and was issued on May 3, 2013. Each Anson Warrant provides for adjustment of the exercise price and
share amount in the event of certain dilutive issuances by the Company. On February 20, 2014, the Company issued a notice to the
holder that the aggregate number of Anson Warrants had been adjusted to 23,026,318 and the exercise price of each had been adjusted
to $0.053365 as a result of certain other offerings of the Company. 5,290,665 of the shares underlying the Anson Warrants were
previously registered by the Company pursuant to the Registration Statement on Form S-1/A filed December 30, 2013. The warrants
and the shares of common stock underlying the warrants were issued in reliance upon an exemption from the registration requirements
of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder.
Cranshire Warrant Shares
This offering, with respect to the Cranshire
Warrant Shares, relates to the resale of the shares of common stock underlying a warrant in the amount of 2,560,486 with an exercise
price of $0.053365. The warrant has a five (5) year term and was issued pursuant to the terms of the Purchase Agreement. The warrant
was originally issued in the amount of 213,334 shares at an exercise price of $0.6405. Pursuant to the anti-dilution adjustment
provision contained therein, on February 20, 2014, the Company issued a notice to the holder that the total share amount had been
increased to 2,560,486 and the exercise price had been reduced to $0.053365 as a result of certain other offerings of the Company.
683,202 of the shares underlying the Cranshire Warrants were previously registered by the Company pursuant to the Registration
Statement on Form S-1/A filed December 30, 2013. Additionally, on February 20, 2014 in consideration for the investor’s agreement
to immediately cash exercise a portion of the Cranshire Warrants for aggregate consideration to the Company of $36,459, the Company
agreed to issue the investor an additional warrant to purchase 683,202 shares of common stock. The warrants and the shares of common
stock underlying the warrants were issued in reliance upon an exemption from the registration requirements of the Securities Act
and/or Rule 506 of Regulation D promulgated thereunder.
JMJ Note Shares
This offering, with respect to the JMJ
Note Shares, relates to the resale of 3,000,000 shares of common stock of the Company underlying a $400,000 Promissory Note issued
July 10, 2013. The JMJ Note is subject to a one-time interest charge equal to 12% of the principal sum and is due and payable July
10, 2014. The JMJ Note provides that it may be converted into common stock of the Company at any time at the election of the holder,
at a conversion price equal to the lesser of $0.26 or 65% of the lowest trade price in the 25 trading days previous to the conversion.
The JMJ Note and the shares of common stock underlying the JMJ Note were issued in reliance upon an exemption from the registration
requirements of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder.
Registration Rights
Nomis Bay Registration Rights Agreement
In connection with the execution of the
Purchase Agreement, the Company and Nomis Bay also entered into a registration rights agreement (the “Registration Rights
Agreement”) dated March 3, 2014 (the “Closing Date”). Pursuant to the Registration Rights Agreement, the Company
has agreed to file this registration statement (“Registration Statement”) with the SEC to register the resale of 24,602,792
shares of Common Stock into which the Convertible Notes may be converted, on or prior to the 45
th
calendar day after
the Closing Date and have it declared effective at the earlier of (i) the 120
th
calendar day after the Closing Date
and (ii) the fifth business day after the date the Company is notified by the SEC that such Registration Statement will not be
reviewed or will not be subject to further review.
If at any time all of the shares of Common
Stock underlying the Convertible Notes are not covered by the initial Registration Statement, the Company has agreed to file with
the SEC one or more additional Registration Statements so as to cover all of the shares of Common Stock underlying the Convertible
Notes not covered by such initial Registration Statement, in each case, as soon as practicable, but in no event later than the
applicable filing deadline for such additional Registration Statements as provided in the Registration Rights Agreement.
Piggyback Registration Rights
The JMJ Note contains a provision whereby
the Company agreed to include on its next registration statement all shares issuable upon conversion of such debentures and notes.
Such conversion shares are included on this registration statement. All fees and expenses incident to the performance of or compliance
with such registration rights shall be borne by the Company.
INTERESTS OF NAMED EXPERTS AND COUNSEL
No expert or counsel named in this prospectus
as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being
registered or upon other legal matters in connection with the registration or offering of the common stock was employed on a contingency
basis, or had, or is to receive, in connection with the offering, a substantial interest, direct or indirect, in the registrant
or any of its parents or subsidiaries. Nor was any such person connected with the registrant or any of its parents or subsidiaries
as a promoter, managing or principal underwriter, voting trustee, director, officer, or employee.
The consolidated financial statements included
in this prospectus and in the registration statement have been audited by Li and Company, PC, and are included in reliance upon
such report given upon the authority of said firm as experts in auditing and accounting.
The validity of the issuance of the common
stock hereby will be passed upon for us by Greenberg Traurig, LLP.
INFORMATION WITH RESPECT TO THE REGISTRANT
Background
We are a farm management company primarily
focused on agronomics from plant breeding to good agricultural practices and the development of stevia derived products which can
be used for human consumption as well as for aquaculture and agriculture applications.
We were incorporated on May 21, 2007 in
the State of Nevada under the name Interpro Management Corp. On March 4, 2011, we changed our name to Stevia Corp. and effectuated
a 35 for 1 forward stock split of all of our issued and outstanding shares of common stock. Effective November 15, 2013,we filed
a Certificate of Amendment to the Company’s Articles of Incorporation to increase the total number of authorized shares of
Common Stock from one hundred million (100,000,000) shares of Common Stock to two hundred fifty million (250,000,000) shares of
Common Stock, each with a par value of $0.001.
On June 23, 2011, we closed a voluntary
share exchange transaction (the “Share Exchange Transaction”) with Stevia Ventures International Ltd., a business company
incorporated in the British Virgin Islands, pursuant to which we acquired certain rights relating to stevia production, including
certain exclusive purchase contracts and a supply agreement related to stevia. In connection with the Share Exchange Transaction,
on June 23, 2011, Mohanad Shurrab, a stockholder of the Company, surrendered 33,000,000 shares of the Company’s common stock
to the Company for cancellation.
On March 19, 2012, we formed a wholly-owned
subsidiary, Stevia Asia Limited, a company incorporated under the companies ordinance of Hong Kong (“Stevia Asia”)
that will allow the Company to expand its China operations. Hero Tact Limited, a wholly-owned subsidiary of Stevia Asia, was incorporated
under the companies ordinance of Hong Kong and renamed Stevia Technew Limited on April 28, 2012.
On July 5, 2012, Stevia Asia entered into
a Cooperative Agreement with Technew Technology Limited (“Technew Technology”), a company incorporated under the companies
ordinance of Hong Kong, and Zhang Ji, a Chinese citizen (together with Technew Technology, the “Partners”) pursuant
to which Stevia Asia and Partners have agreed to engage in a joint venture to be owned 70% by Stevia Asia and 30% by Technew Technology,
through the entity Stevia Technew Limited (the “Joint Venture”). The Partners will be responsible for managing the
Joint Venture and Stevia Asia has agreed to contribute $200,000 per month, up to a total of $2,000,000 in financing to be applied
on a project by project basis and subject to those projects remaining on target to generate positive EBITDA (earnings before interest,
tax, depreciation and amortization) of at least 1.5 times the investment in any particular project and subject to Stevia Asia’s
financial capabilities in terms of completing a financing or series of financings that provides the Company with the ability to
contribute at least $200,000 in any given month. Completion of a financing or series of financings depends on the size of any private
placements with investors that the Company may complete. Although Stevia Asia or Technew Technology may believe that a project
is on target to generate positive EBITDA of at least 1.5 times the investment, there is no guarantee that any particular project
will generate revenue. Stevia Asia contributed $200,000 to the Joint Venture in August 2012 which was applied to a specific aquaculture
project that is ongoing but has not and will not contribute additional funds until it completes a financing or series of financings
that provides the Company the ability to contribute at least $200,000 in any given month. The aquaculture project is focused on
producing prawns and fish using the Company’s formulated products. The Joint Venture will participate in the revenue of specific
ponds based on the pro-rata capital contribution allowing the flexibility to expand its participation as and when it has the ability
to contribute additional funds. The Joint Venture also participated in an agriculture project in Vietnam where 102.5 acres of chili
were cultivated using the Company’s formulated products. Part of the harvest occurred during the last quarter of the 2013
fiscal year generating revenue of $2,167,812.54 for the Company. The delay of additional capital is permissible pursuant to the
joint venture agreement and will only impact the number and size of specific projects and the companies continue to explore potential
stevia commercial applications but failure to complete a financing or series of financings sufficient to make additional contributions
will have an adverse effect on our ability to execute our business plan. The Cooperative Agreement shall automatically terminate
upon either Stevia Asia or Technew ceasing to be a stockholder in the Joint Venture, or may be terminated by either Stevia Asia
or Technew upon a material breach by the other party which is not cured within 30 days of notice of such breach.
On October 1, 2013, we formed SC Brands
Pte. Ltd., a Singapore corporation and a subsidiary in which we own a 70% equity interest. SC Brands will allow us to develop consumer
brand products.
On February 24, 2014, we formed Real Hemp
LLC, a wholly owned Indiana limited liability company that will focus on application of our proprietary processes to the commercial
farming of the cannabis plant.
On February 26, 2014, we entered into a
farm management and technology agreement with ebbu LLC to advise on scaling commercial extraction of identified cannabinoids from
the cannabis plant.
The following diagram illustrates our corporate structure:

Overview
Our focus is on implementing quality agribusiness
solutions to our partners, contract growers and customers to maximize the production of agri-products and the economic development
of stevia and its extracts.
Our mission is to maximize shareholder
value by consistently developing and acquiring the latest intellectual property and expanding our suite of formulated products
and their applications and leveraging our farm management business model to maximize market penetration and revenue margins.
To achieve these goals we intend to develop
a suite of intellectual property relating to stevia and its extracts that will enhance the value of our farm management operations.
Through our relationships with Tech-New Bio-Technology, Growers Synergy and local institutes, we are exploring the market for commercial
applications of stevia which will be vertically integrated into our services and production.
Our target markets are initially Vietnam,
Indonesia and China. In Vietnam and Indonesia we have contracted with growers and have established our own nurseries and test fields.
In China we are producing our proprietary formulated products and applying them to aquaculture projects under our revenue share
model. Although our priority is Asia, our services are not limited to specific countries and we plan to pursue viable opportunities
in other markets.
The Industry and Our Opportunity
Stevia as a Food Additive
We believe that health issues created by
the modern diet are causing consumers to look for more natural products and simpler ingredient lines on the foods and beverages
they purchase and causing governments to put pressure on the food industry to offer products with reduced calories.
In evaluating potential sweetener alternatives,
manufacturers focus on taste, pricing, and a sustainable and scalable supply. We believe stevia fulfills these four criteria and
has the added advantage of contributing no calories to food and beverage with a near zero glycemic index, making it safe for diabetics.
Originating from Paraguay, stevia leaf
has been valued for centuries because of its sweetening and herbal properties and has been used as an approved sweetener in Japan
and Korea for decades. Extracts from stevia contain a mixture of different molecules that vary depending upon climate and growing
conditions and it was historically impossible to come up with clear and consistent specifications of the product needed to make
it a reliable ingredient as well as conduct clinical trials required by the FDA for the approval process. This issue was only overcome
in recent years by identifying the steviol glycoside molecules with the best taste profiles and by developing innovative and unique
process technologies to separate and purify stevia extract to pharmaceutical levels of purity on a reliable and consistent basis:
and, importantly, to do so in commercially viable volumes.
In 2008, Rebaudioside A, a steviol glycoside,
was granted GRAS (Generally Recognized as Safe) status by the U.S. Food and Drug Administration following applications by Cargill
and Merisant. Since then, approval by legislators across the world has opened the door to new formulations and reformulations of
foods and beverages with zero or reduced calorie content. In 2009, stevia was incorporated into leading soft drinks brands manufactured
by Coca-Cola and PepsiCo and has since been incorporated into many categories of food and beverages.
The stevia industry is segmented into several
business processes, which can broadly be categorized as i) plant breeding and propagation, ii) farming, iii) extraction and refining,
iv) product formulation, v) distribution and retail.
A significant portion of the cost of Rebaudioside
A is a result of the leaf cost and we believe there remains considerable opportunity to build value in the supply chain by focusing
on stevia agronomics. The stevia genus includes more than 100 species and each species contains unique sweet compounds. However,
only two of these species contain steviol glycosides and of these two the variety with the sweetest compounds is stevia rebaudiana
bertoni. There is relatively little technical knowledge of this species and almost all commercial growing of stevia has occurred
in China because of the traditional Japanese and Korean markets. Now with the global market demand for high TSG (total steviol
glycoside) and high Reb-A (Rebaudioside A) producing plants, there is an increased demand for agronomic and farm management expertise
to establish new plantations and rapidly scale leaf production.
The primary competitors within this market
segment include: PureCircle, which has extensive operations in China as well as subsidiaries in South America (Paraguay) and Africa
(Kenya); Stevia One, an independent grower established in Peru; S&W Seed Company, who signed a supply agreement with PureCircle
in July of 2010 to grow stevia in North America under its subsidiary, Stevia California; and GLG Life Tech Corporation, a China-centric
company which has chosen to continue to focus on building and expanding its supply chain within China.
Stevia as a Commercial Product for Agriculture
Use
Stevia is classified as a medicinal herb
in China where more than 80% of the world’s supply of stevia is grown and stevia has been used as a medicinal herb as well
as a sweetener for centuries in its native country of Paraguay. Japan is the largest consumer of stevia extract and stevia has
accounted for more than 40% of Japan’s entire sweetener market consumption since 1992. Research articles studying the efficacy
of stevia as a feed supplement and fertilizer have been published by several universities in Japan, China and South Korea for more
than ten years. There are also several small local companies in Japan, South Korea and China that produce feed and fertilizer products
that are formulated using stevia extracts and they have been supplying these products to their local markets for several years.
We believe that the feed and fertilizer markets provide additional growth opportunities for stevia.
In July 2012, we obtained the rights to
product formulations that add stevia extracts to an existing probiotic and enzyme product line produced by our technology partner,
Tech-New Bio-Technology. We then obtained government approval in Vietnam to use the stevia product formulations for agricultural
use such as fertilizer and animal feed supplement.
The first commercial application was started
in August of 2012 for the production of approximately 2.5 acres of shrimp in China under the revenue share model with the intention
to expand as we confirm available funding. In October 2012, a commercial application was started for the production of 102.5 acres
of chili in Vietnam which was harvested during the first and second quarter of 2013. We are also using the formulations for the
commercial stevia trial fields in Vietnam.
Our product line includes aquaculture feed
for shrimp and fish, feed for livestock, granular fertilizers and foliar spray, each of which, we believe, holds the potential
to open new revenue opportunities to us.
By vertically integrating down the supply
chain, we believe we significantly enhance our revenue potential. An average hectare of stevia will produce approximately 6 tons
of dry leaf per year. We have entered into a five year supply contract with an option to renew for an additional four years with
a leaf buyer. Under the agreement, we will set a fixed price for the leaf each year based on the yearly average market prices for
the quality of leaf provided. We are growing elite strains and we believe prices for high quality leaf will continue to be more
stable than lower quality leaf, and as such, we believe our leaf prices will be more stable and predictable. We expect to generate
approximately $2,000 for each ton of dry leaf, so each hectare will potentially produce $12,000 of dry stevia leaf. On average,
dry stevia leaf produces approximately 10% of net usable extract by weight and the average price for the extract is approximately
$100,000 per ton, so each hectare can potentially produce $60,000 of extract (6 tons x 10% x $100,000) which is five times the
value of the dry stevia leaf and when we use the extracts to create our proprietary formulations, we can increase our revenue potential
further.
Alternative Applications of Our Technology
– Commercial Farming of Cannabis
We believe our expertise growing stevia
to produce specific types of extracts and our technologies for post-harvest processing, extraction and product formulation may
be applicable to the cannabis industry. In February 2014 we entered into a farm management and technology agreement with ebbu LLC
to provide farm management consultancy and technical expertise related to growing the cannabis plant and extracting its cannabinoids.
The cannabis plant produces many chemical compounds called cannabinoids and there are more than 85 different cannabinioids that
have been identified and isolated from the cannabis plant that exhibit varied effects and many of these are being studied for their
psychoactive and medical properties. Cannabis plants that have been bred to produce high levels of tetrahydrocannabinol (“THC”),
a psychoactive constituent, are commonly referred to as marijuana. In 2004 the United Nations estimated that global consumption
of marijuana indicated that approximately 4% of the adult world population (162 million people) used marijuana annually, and that
approximately 0.6% (22.5 million) people used marijuana daily. Current federal and most state regulations prevent us from participating
directly in the marijuana industry and we cannot guarantee that our services or technology will provide value if the laws do not
evolve in favor of marijuana production and the commercial sale of marijuana derived products, but it is our goal to position the
Company to take advantage of the shifting regulatory landscape where possible.
Cannabis plants that produce very low levels
of THC and are grown for industrial purposes and foodstuff products are commonly referred to as hemp. Hemp products such as seeds,
oil, protein, milk, fiber and cannabidiol (“CBD”) can be legally imported and traded in the United States, but it is
not legal for a U.S. company to grow hemp because of its relationship to marijuana. Seventy percent of the world’s
hemp production is currently produced in China. In February 2014 we registered a wholly owned subsidiary, Real Hemp LLC, to import,
manufacture, license and sell hemp products in the U.S. The 2012 retail value of North American hemp food, vitamin and body care
products was estimated to be in the range of $156 to $171 million by the Hemp Industries Association (HIA). When clothing, auto
parts, building materials and other non-food or body care products are included, the HIA estimates that the total retail value
of U.S. hemp products is about $500 million. Food and fiber uses for industrial hemp are growing rapidly and have increased
over 300 percent, to an estimated 25,000 products, in the past few years. Much of that growth is coming from the increased
sales of hemp food products. CBD is one of the active cannabinoids in the cannabis plant and is a major constituent of hemp, accounting
for up to 40% of the plants extract, and is considered to have a wider scope of medical applications than THC. Similar to our goals
in the marijuana industry, we intend to position the Company to take advantage of regulatory changes in the hemp industry.
Products and Services
Our farm management services include training
the farmers on the correct protocols and methodologies and providing ongoing technical assistance during the crop cycle as well
as providing inputs such as the seedlings, fertilizers and additives they are required to use. We apply our services under three
business models which we classify as 1) contract farming model, 2) revenue share model and 3) product supply model.
Under the contract farming and revenue
share models we do not charge for the services and inputs, but rather our services provide us with a competitive advantage to secure
growers who are willing to dedicate their land and resources to grow crops with an expectation of high yielding, high quality crops
and guaranteed purchase prices. Under these models we will generate our revenue from the crops that are grown and we only enter
into production agreements with growers when there is already a committed buyer for the end crop. Under the contract farming model
we will purchase the crop from the grower at a fixed price and sell to our own customer. Under the revenue share model, the grower
already has their own buyer and we will share the revenue.
Under the product supply model we will
market our products in combination with technical services to buyers and charge a fee. We believe that this model will contribute
a small part of our overall revenue initially until we establish a proven track record and solid reputation for our services and
products under the first two models. We do not expect to focus on providing strictly farm management or technical services for
a fee and it is difficult to estimate what we would charge for such services.
To support our farm management services
we established a research center in Vietnam on 25 acres of leased land. We confirmed elite plant varieties, developed propagation
techniques, conducted field trials across several provinces, documented local operating procedures and post-harvest techniques,
and began trial harvests in March 2012.
We continue to focus on research and development
to further evolve and develop new protocols, methodologies and intellectual properties and believe that this will be key to maintain
our competitive advantage.
We utilize the contract farming model to
produce stevia leaf for our trial harvests and use the stevia extracts to produce our proprietary formulated products, which we
are applying under the revenue share model to an aquaculture operation beginning August 2012 and a chili operation beginning October
2012.
In September 2012 we began providing samples
of stevia extract to food and beverage companies and we are working closely with local parties in several South East Asian countries
to provide technical information in support of recipe development. Although we believe that this product line will have growth
potential, there is no guarantee that a high volume of stevia will be utilized by our customers. We expect companies will take
another year to plan product launches.
Growth Cycle -
The stevia plant
is a perennial but the growing cycle varies greatly depending on the particular strain and location. Stevia is sensitive
to frost and in China where most stevia is grown today, it is common to only have one or two harvests. Closer to the equator
it is possible to harvest year round with some dormancy during the winter months. It is also possible to manipulate
the harvest cycle and in developing countries where manual labor is the preferred method, a short cycle of as little as 45 to 60
days between harvests is preferred. However, in more developed countries where mechanization is the focus, a longer growing
cycle is preferred and cycles of more than 120 days have been achieved.
Yield -
Expected annual dry leaf
yields of plant varieties commonly sourced from China is three to six tons per hectare (“Ha”). Field trial
data indicates that six tons or more per Ha can be achieved working with elite strains. By continuing to build our inventory of
elite strains and refine our farm management practices and technologies, we plan to improve yield and plant performance and
exploit the economic value of our intellectual property.
Harvest -
Stevia is a very labor
intensive plant and traditionally has been harvested by hand. As larger commercial operations have begun to focus on stevia,
a considerable amount of research is being put into the mechanization of planting, harvesting and leaf removal. While we
will need to maximize mechanization in the United States to be economical, in many Asian locations there is both an abundance
of low cost labor and an expectation that stevia will provide an economic stimulus and employ many of
the farmers in poor rural areas. So the adoption of mechanization will need to consider both economic
and social factors.
Location -
Currently over 80% of
stevia is grown in China and almost all of the high Reb-A variety stevia leaf is being produced in China. China is the center of
commercial stevia growing for historical reasons due to its proximity to Japan and Korea, which have historically been the major
markets for stevia. Due to its climate, we believe China is likely not the most geographically optimal location to grow stevia,
as stevia is sensitive to frost and China typically produces only one or two crops per year, requiring leaf processors to purchase
and store sufficient leaf for an entire year of production.
We believe that diversifying the supply chain of stevia leaf
would provide several advantages:
|
|
·
|
Incorporating Southern Hemisphere production provides two major growing seasons;
|
|
|
·
|
Incorporation Equatorial production provides for year round production;
|
|
|
·
|
Enables better control of leaf quality where major propagation of stevia varieties is controlled;
|
|
|
·
|
Provides protection against country-specific political, regulatory, disease, and natural disaster risk; and
|
|
|
·
|
Provides operations closer to end markets.
|
We believe infrastructure is a major criteria
for field site selection and can be especially challenging in developing countries. In addition, we believe a viable site
must have the proper weather and soil that is suitable for plant growth as well as being in a location that satisfies logistical
business considerations, such as being easily accessible and in close proximity to a capable labor pool. It is our belief
that access to water can often be a challenge and greatly limits the areas where an irrigation model can be applied. We
believe Vietnam has excellent road infrastructure and our fields are easily accessible by passenger car or lorry and most
potential growing areas are located within hours of a major port city. Indonesia has an abundance of low cost labor and land
available for acquisition that is suitable for new varieties of stevia that we are breeding and/or acquiring to grow in the equatorial
zone.
Land Use and Capital Requirements -
As we expand our operations, there are two primary business models available to manage farm operations. The plantation model will
involve us controlling the land and assets through lease or purchase arrangements and hiring the necessary workers which will require
higher upfront capital cost but enable rigorous control over operations with potentially higher revenue per acre. The contract
farm model involves entering into agreements with existing farmers to utilize our agriculture inputs and protocols in order to
produce specified crops under contract at negotiated prices. The contract farm model requires lower upfront capital and enables
us to more quickly scale over larger areas in those instances where we are able to efficiently manage operations and implement
supervisory control. If successfully implemented, we believe the contract farming model provides the fastest ramp to positive cash
flow while also conserving capital.
We are managing our trial harvest stevia
farms under the contract farming model and plan to continue using this model.
Under the revenue share model the grower
owns, leases or contracts the land and we provide our farm management services and products as part of the agriculture inputs and
then we share the revenue. This does not require us to have any obligations or liability for land and enables us to expand rapidly
and maximize revenue by leveraging existing operations with minimal capital commitment.
We intend to scale the use of our formulated
products using both the contract farm model and revenue share model which we started implementing in August of 2012. We will initially
work on projects with our joint venture partner.
Labor and Research and Development -
Our initial research and development funding was used to establish our research center and engage specialists who have secured
elite plant varieties, culled the original planted varieties, developed propagation techniques, conducted field trials, documented
local operating procedures and developed post-harvest techniques. We target spending approximately fifteen percent of our operating
expense budget towards research and development to continue improving and develop new intellectual properties.
Financial
- The value of the stevia
leaf fluctuates based on supply and demand and the quality of the leaf. Wide seasonal variances on the open
market are common and can make long-term planning difficult. Because we have entered into a long-term supply contract with
a leaf buyer and we are growing elite strains, we believe our prices will be more stable and predictable and we will be able
to plan our growth and commit to large contract growers. In addition, buyers of leaf pay a substantial premium
for high quality leaf. This places strong economic value on our intellectual property, including our elite stevia strains,
and our farm management solutions.
Current contracted selling price for leaf
that meets the minimum standards is set at a fixed price. Leaf exceeding the minimum standards will receive a premium for which
the benchmarks and price tiers will be reviewed each year based on comparative market leaf quality and supply and demand.
Historically, leaf that produced 13% TSG
and 70% Reb-A was purchased at a premium. Elite strains can potentially deliver TSG well above 12% and Reb-A above 80% providing
significant economic advantage. Minimum standards require a TSG of 12% or more, Reb-A to be at least 60% of TSG, maximum of 5%
impurities and a maximum moisture content of 10%. During the refining process, the net yields of usable extract will be slightly
lower.
Our Key Contracts and Relationships
Growers Synergy
Effective November 1, 2011, we engaged
Growers Synergy Pte Ltd, a regional farm management services provider (“Growers Synergy”), to provide farm management
operations and back-office and regional logistical support for our Vietnam and Indonesia operations for a period of two years at
a cost of $20,000 per month and the agreement was renewed on November 1, 2013 for an additional year. In addition, Growers Synergy
will enter into an agreement to purchase from us all the non-stevia crops produced at the farms for which they are providing management
services.
We believe that the relationship with Growers
Synergy will provide us with a strategic advantage and potential synergistic partnership by providing us with guaranteed off-take
agreements for agriculture crops other than stevia, which will be produced as part of inter-cropping practices to maintain optimal
soil conditions for stevia farming. Growers Synergy will work with us and our technology partner, Tech-New Bio-Technology, to combine
the agronomy protocol with the farming models. Models and their related protocols have been commercially field tested during the
first two years working with the provincial and national programs and establishing 100 Ha of field trials.
A local farm management service, such as
Growers Synergy, is critical to assist us in training local teams with the documented protocol sufficient to scale to 1,000 Ha
to create a turnkey project. Our goal is to be vested with fully documented protocols, local teams of trained staff capable of
supporting the scale up to 1,000 Ha and farmer communities that are capable of growing stevia and other crops. To help us achieve
this Growers Synergy will provide the necessary resources and assign staff to fill certain managerial and support staff positions.
Tech-New Bio-Technology
In March 2012, we entered into both a Supply
Agreement and Cooperative Agreement with Guangzhou Health China Technology Development Company Limited, operating under the trade
name Tech-New Bio-Technology (“TechNew”). TechNew is a developer and manufacturer of hi-tech biotechnology products
which offers a series of specialized ecological fertilizers, microbiological preparations and management systems for the agriculture
and aquaculture industry as well as technologies for the extraction and refinement of high purity stevia. Under the terms of the
Supply Agreement, we are able to sell dry stevia plant product exclusively to TechNew including all leaf and stem for a term of
five years with an option to renew for a further four years with the price to be negotiated by the parties on a yearly basis to
reflect changes in the specifications and market price. During the first two years TechNew is obligated to purchase all of our
production with quantity to be negotiated from the third year onwards. Under the terms of the Cooperative Agreement, we agreed
to explore potential technology partnerships with TechNew, with the intent to formalize a joint venture to pursue promising technologies
and businesses. These include the inclusion of stevia extracts in its current product formulations for use in agriculture and aquaculture
applications including fertilizers and feed.
Through our cooperative agreement with
TechNew, we will also explore a potential relationship to integrate extraction and refining technology to produce high purity Reb-A
and other steviol glycosides for the consumer market. We believe that vertically integrating our technologies for both commercial
and consumer products may provide advantages of a diversified market, but we do not intend to enter the consumer market with a
finished stevia product. It is our goal to develop core strengths in farm management and developing technologies for production
and post harvest processes, and we believe that the consumer market for stevia is extremely competitive.
We supplied leaf to TechNew from our trial
harvests and all of the leaf we have supplied has been used to produce products formulated with stevia extract. It is our intention
to apply as much leaf as possible towards producing the higher value added products rather than sell the leaf as a commodity under
the supply contract.
TechNew Technology Limited
On July 5, 2012, our wholly-owned subsidiary,
Stevia Asia entered into a Cooperative Agreement with Technew Technology Limited (“Technew Technology”), a company
incorporated under the companies ordinance of Hong Kong, and Zhang Ji, a Chinese citizen (together with Technew Technology, the
“Partners”) pursuant to which Stevia Asia and Partners have agreed to engage in a joint venture to be owned 70% by
Stevia Asia and 30% by Technew Technology (the “Joint Venture”), through the entity Stevia Technew Limited. The Joint
Venture will allow us to further explore potential stevia commercial applications, which we would integrate into our farm management
services and our own stevia production.
Independent Grower Relationships
We plan to develop a network of partner
growers who we can market our production methods and technologies to and who will also help supply us with the stevia product necessary
to fulfill our supply obligations. To date we have entered into initial purchase agreements for stevia under the contract farming
model where we provide the seedlings, fertilizer additives, protocols and technical supervision with an obligation to purchase
the stevia leaf at a fixed price per ton and the grower is responsible for the land, labor and all other inputs. The agreements
are reviewed annually to negotiate price and quantity for the subsequent renewal year to reflect changes in specifications, market
prices and demand. We have also entered into revenue share agreements with growers where we provide our proprietary feed or fertilizer
additives and farm management services in return for a share of the revenue. These agreements are reviewed each growing/harvest
cycle with renewal terms to be negotiated and confirmed for each subsequent cycle.
Our Farm Management Services and Intellectual
Property
Our objective is to provide a full spectrum
of farm management services to manage our contract farms, service industry growers and provide for optimal production. To achieve
this objective, our focus is on intellectual property development and continued development and improvement of cultivar varieties
for intended growing sites, propagation protocol, cultivation technology including an intercropping system and regional adaptability
test, and post-harvest and refinery processes.
We are also continuing to develop and improve
local SOP (standard operating procedures) manuals specific to each growing location and plant variety, which document the proper
use of all inputs including a proprietary crop production system that we believe is more efficient and cost effective than traditional
methods. We believe these customized operating manuals will result in advanced propagation and growing techniques that can improve
the quality and efficiency of a variety of crops.
We are also developing a wide portfolio
of highly efficient and environmentally friendly crop nutrition products. These products are performance minerals, plant phyto-chemicals,
functional nutrients and microbial formulations. All products are derived from natural sources and can be used as sustainable agriculture
solutions and/or for organic farming. While it is our intent to develop the foregoing highly efficient and environmentally friendly
crop nutrition products, there is no guarantee we will be successful in developing such a portfolio of products.
We are still developing protocols regarding
stevia production and we plan to provide a wide spectrum of agricultural consulting and solutions for stevia growers, including:
TechNew Suite of Products -
through
our technology partner, TechNew, we are able to contract manufacture the extraction and refinement of high purity stevia and we
acquired their formulas for using stevia extract in feed and fertilizer applications. We have also entered into a joint venture
with Technew Technology to further explore potential stevia commercial applications, which we would integrate into our farm management
services and our own stevia production.
Elite Germplasm -
high performance
mother stock suitable for varied regions and environment.
Advanced Propagation Techniques
-
methods that are efficient, more cost effective, and produce a higher quality plant.
To date we have not filed patents or registered
trademarks and we do not license any of our technologies. We previously had a license arrangement with Agro-Genesis, however, such
license was cancelled when we partnered with TechNew.
Our Competitive Advantage
We believe our intellectual property suite
that we are developing and our ability to serve across a wide spectrum of agricultural consulting and solutions will provide us
with a competitive advantage against our competitors.
We also believe our intellectual property,
particularly our fertilizers and feed additives and other input products used in our protocols, have the potential to create a
dedicated customer base because the protocols once implemented on a farm call for continual use of our fertilizers and feed additives
and other products as a mandatory production input. We believe this long-term customer relationship can enable us to create a substantial
barrier to entry to potential new competitors, while at the same time providing networking benefits that could further propagate
our business.
Our ability to fully develop our suite
of products and apply them to a customer base is dependent on our ability to raise sufficient capital to fund our business operations.
Market Trends
The original products launched that used
stevia were zero calorie beverages. Subsequent product launches included a blend of sugar and stevia that advertised reduced calories.
Stevia is now used across 38 categories of food and beverages with most of the applications involving a blend of sugar and stevia
for a reduced calorie product using all natural sweeteners.
Our Properties
Our primary focus is on providing farm
management services to our contract growers. We have acquired two grower supply contracts and three nursery fields in Vietnam.
More than twenty fields have been established in five provinces in the northern half of Vietnam with total propagation exceeding
100 Ha (250 acres).
The provincial locations include Vinh Phuc,
Tuyen Quang, Thanh Hoa, Ha Tinh and Lai Chau.
On December 14, 2011 we entered into a
land lease agreement with Stevia Ventures Corporation, one of our Suppliers, and Vinh Phuc Province People’s Committee Tam
Dao Agriculture & Industry Co., Ltd (“Vinh Phuc”) whereby Stevia Ventures Corporation leased 10 Ha (25 acres) of
land over 5 years and we developed a research facility that will also serve as a propagation center for farms located in the surrounding
provinces and particularly those serving the provincial and national sponsored projects.
To better service multiple farms located
across the many provinces stretching from north central Vietnam to the Chinese border, we will utilize the greenhouse facilities
of our local grower partners in a decentralized model that more efficiently addresses the logistical challenges presented by the
contract farming model. It is assumed that the commercial fields will be scaled by stem cutting and we will provide the seedlings
to the growers as one of the inputs.
In addition to our Vietnam operations,
in April 2012, we established a 1 Ha (2.5 acres) initial field trial in Indonesia which utilizes our intercropping model.
We lease office space with Leverage Investments,
LLC, an entity owned and controlled by our President, for $500 per month on a month-to-month basis since July 1, 2011.
Regulation
Stevia extracts may be used in a wide variety
of consumer products including soft drinks, vegetable products, tabletop sweeteners, confectioneries, fruit products and processed
seafood products, in a wide range of countries, including almost all major markets, and as a dietary supplement in others.
Clinical studies have supported the safety and stability of stevia’s various high purity compounds used in food and beverages.
There is no documented health threat.
Cargill and Merisant each submitted applications
to the United Stated Food and Drug Administration (FDA) in 2008 for GRAS approval. On December 17, 2008 the stevia extract, Rebaudioside
A (Reb-A), received GRAS approval.
In December 2008, Australia and New Zealand
approved highly purified forms of stevia extracts as safe for use in food and beverages. Previously, such extracts had only been
permitted for use as a dietary supplement in these countries.
Stevia extracts have been sanctioned by
the Ministry of Health of China to be used as a food additive, and are listed in the Sanitation Standard of Food Additives.
In July 2010 the FDA issued GRAS clearance
for PureCircle’s high purity SG95 stevia product which opened up opportunities for many more applications as well as more
cost effective solutions.
In November 2011, the European Union cleared
stevia for use as a food additive in its twenty seven member states.
Further regulatory clearances were secured
for Reb-A in nearly all of the developed countries and South East Asian countries confirming the growing regulatory support for
high purity stevia.
Our proprietary fertilizer and feed additive
products are approved for use in China and South East Asia and we have started using them in commercial operations. All of the
ingredients in the products are natural compounds and are approved by the major developed countries, but registration of the products
will be required in each country before importation is allowed.
Foreign Currency Exchange Rate
The Company expects that international
revenues will account for a majority of our total revenues. Our international operations expose the Company to foreign currency
fluctuations. Revenues and related expenses generated from our international subsidiaries will generally be denominated in the
functional currencies of the local countries. For example, revenues derived from the People’s Republic of China (“PRC”)
will be denominated in Renminbi, or RMB.
Our statements of income of our international
operations are translated into United States dollars at the average exchange rates in each applicable period. To the extent the
United States dollar strengthens against foreign currencies, the translation of foreign currency denominated transactions will
result in reduced revenues, operating expenses and net income for our business. Similarly, our revenues, operating expenses and
net income will increase if the United States dollar weakens against foreign currencies.
We are also exposed to foreign exchange
rate fluctuations as we convert the financial statements of our foreign subsidiaries and our investments in equity interests into
United States dollars in consolidation. If there is a change in foreign currency exchange rates, the conversion of the foreign
subsidiaries’ financial statements into United States dollars will lead to a translation gain or loss which is recorded as
a component of accumulated other comprehensive income which is part of stockholders’ equity. In addition, we may have certain
assets and liabilities that are denominated in currencies other than the relevant entity’s functional currency. Changes in
the functional currency value of these assets and liabilities create fluctuations that will lead to a transaction gain or loss.
China
– The Company expects
to derive revenue from China. Pursuant to the Foreign Currency Administration Rules promulgated in 1996 and amended in 2008 and
various regulations issued by the State Administration of Foreign Exchange (“SAFE”), and other relevant PRC government
authorities, RMB is freely convertible only to the extent of current account items, such as trade-related receipts and payments,
interest and dividends. Capital account items, such as direct equity investments, loans and repatriation of investments, require
the prior approval from the SAFE or its local counterpart for conversion of RMB into a foreign currency, such as U.S. dollars,
and remittance of the foreign currency outside the PRC.
Payments for transactions that take place
within the PRC must be made in RMB. Unless otherwise approved, PRC companies must repatriate foreign currency payments received
from abroad. Foreign-invested enterprises may retain foreign exchange in accounts with designated foreign exchange banks subject
to a cap set by the SAFE or its local counterpart. Unless otherwise approved, domestic enterprises must convert all of their foreign
currency receipts into RMB. The value of the RMB against the U.S. dollar and other currencies is affected by, among other things,
changes in China’s political and economic conditions. Since July 2005, the RMB has no longer been pegged to the U.S. dollar.
The RMB may appreciate or depreciate significantly in value against the U.S. dollar in the medium to long term. Moreover, it is
possible that in the future, PRC authorities may lift restrictions on fluctuations in the RMB exchange rate and lessen intervention
in the foreign exchange market.
Because some of our revenue is expected
to come from China, appreciation or depreciation in the value of the RMB relative to the U.S. dollar would affect our financial
results reported in U.S. dollar terms without giving effect to any underlying change in our business or results of operations.
As a result, we face exposure to adverse movements in currency exchange rates as the financial results of our Chinese derived revenue
are translated from local currency into U.S. dollar upon consolidation. Our operations are subject to risks typical of international
business, including, but not limited to, differing economic conditions, changes in political climate, differing tax structures,
other regulations and restrictions, and foreign exchange rate volatility.
International Laws
A significant portion of our initial business
operations will occur in Vietnam. We will be generally subject to laws and regulations applicable to foreign investment
in Vietnam. Similarly, as we expand into Indonesia and other markets, we will be subject to the laws and regulations
of such jurisdictions. The Vietnam legal system is based, at least in part, on written statutes. However, since these
laws and regulations are relatively new and the Vietnamese legal system continues to rapidly evolve, the interpretations of many
laws, regulations and rules are not always uniform and enforcement of these laws, regulations and rules involves uncertainties. Similar
to Vietnam, the modern Indonesia legal system was formed relatively recently and is continuing to evolve.
|
Country
|
|
Type of Approval
|
|
|
|
|
|
North America
|
|
|
|
USA
|
|
Food additive
|
|
Canada
|
|
Food additive
|
|
Mexico
|
|
Food additive
|
|
Latin America
|
|
|
|
Argentina
|
|
Food additive
|
|
Brazil
|
|
Food additive
|
|
Chile
|
|
Food additive
|
|
Colombia
|
|
Food additive
|
|
Ecuador
|
|
Food additive
|
|
Paraguay
|
|
Food additive
|
|
Peru
|
|
Food additive
|
|
Uruguay
|
|
Food additive
|
|
Venezuela
|
|
Food additive
|
|
Asia Pacific
|
|
|
|
Australia
|
|
Food additive
|
|
Brunei
|
|
Food additive
|
|
China
|
|
Food additive
|
|
Hong Kong
|
|
Food additive
|
|
Indonesia
|
|
Food additive
|
|
Japan
|
|
Food additive
|
|
Malaysia
|
|
Food additive
|
|
New Zealand
|
|
Food additive
|
|
Singapore
|
|
Food additive
|
|
South Korea
|
|
Food additive
|
|
Taiwan
|
|
Food additive
|
|
Thailand
|
|
Food additive
|
|
Vietnam
|
|
Food additive
|
|
Europe
|
|
|
|
Austria
|
|
Food additive
|
|
Belgium
|
|
Food additive
|
|
Bulgaria
|
|
Food additive
|
|
Cyprus
|
|
Food additive
|
|
Czech Republic
|
|
Food additive
|
|
Denmark
|
|
Food additive
|
|
Estonia
|
|
Food additive
|
|
Finland
|
|
Food additive
|
|
France
|
|
Food additive
|
|
Germany
|
|
Food additive
|
|
Hungary
|
|
Food additive
|
|
Ireland
|
|
Food additive
|
|
Italy
|
|
Food additive
|
|
Latvia
|
|
Food additive
|
|
Lithuania
|
|
Food additive
|
|
Luxembourg
|
|
Food additive
|
|
Malta
|
|
Food additive
|
|
The Netherlands
|
|
Food additive
|
|
Poland
|
|
Food additive
|
|
Portugal
|
|
Food additive
|
|
Romania
|
|
Food additive
|
|
Slovakia
|
|
Food additive
|
|
Slovenia
|
|
Food additive
|
|
Spain
|
|
Food additive
|
|
Sweden
|
|
Food additive
|
|
Switzerland
|
|
Food additive
|
|
Russia
|
|
Food additive
|
|
United Kingdom
|
|
Food additive
|
We cannot predict the effect of future
developments in the legal systems of developing countries, including the promulgation of new laws, changes to existing laws or
the interpretation or enforcement thereof, the preemption of local regulations by national laws, or the overturn of local government’s
decisions by the superior government. These uncertainties may limit legal protections available to us.
Marketing
We believe it is important to educate the
local governments and farmer communities on the merits of stevia becoming a new commercial crop and its potential as a new economic
stimulus for rural farmers. Our President, Mr. George Blankenbaker, and our local partner have been conducting talks and training
sessions for more than three years in Vietnam and have fostered local support at many levels. To support the farmer’s transition
to stevia farming and provide an opportunity to showcase the stevia opportunity to farmers’ communities, the Vietnam government
provided financial support at both the provincial and national level to plant 20 Ha (50 acres) and 50 Ha (125 acres) respectively,
both of which were completed in 2012. The fields were small plots located in several villages and served as demonstration fields
and stepping stones to gain wide support from growers in several villages.
We have entered into formal cooperative
agreements with several local institutes, including the National Institute of Medicinal Materials in Hanoi and the Agricultural
Science Institute of Northern Central Vietnam. The terms of these agreements generally provide that we will provide stevia seedlings
and other products and services, at prices and in quantities as will be mutually agreed by the parties, at the clients’ nurseries
and provide the clients with off-take agreements for crops produced using our systems. As part of our services, we provide technical
assistance to assure the clients adhere to our established growing protocols. We also agree to work cooperatively with the clients
on research projects relating to stevia development, the cost of such projects to be shared between the parties as may be mutually
agreed. These agreements provide local technical assistance for our grower partners and also provide additional credibility when
our grower partners present the stevia opportunity to the local farmers’ communities.
We are also in contact with non-governmental
organizations (NGO) that are seeking programs to bring to the communities that they serve which are generally located in poor rural
areas in need of economically sound projects. If the stevia model proves to be viable for these locations, the NGOs have indicated
that they will be interested in introducing and funding stevia farming programs. However, many of these poor rural areas are located
in areas of poor soil quality, that lack adequate access to water or that suffer from other environmental constraints which limit
the opportunities for this approach.
We also hope to generate many local testimonials
from our field trials and the farmers in Vietnam are very fluid and willing to adopt new crops if the new crops are proven to be
more economically viable than their current crops.
In connection with commercial opportunities
for stevia derived products, we intend to develop a mark that can be applied to a buyer’s brand which would signify premium
quality stevia-derived products.
Currently our marketing efforts are focused
on educating our growers on our new proprietary formulations. These efforts are more administrative in nature and we do not currently
anticipate a need for a large marketing budget to support current operations.
Product Alternatives
As a full service stevia farm management
service provider we will face competition from both non-stevia sweetener products and from other service providers within the stevia
industry.
Food Additive Product Alternatives
- We believe stevia is the leader among natural zero calorie sweeteners at this time and it takes years to develop and bring to
market new sweeteners of which few end up possessing all the qualities needed to be adopted mainstream. At this time we are not
aware of any proven and viable alternative which possesses all of the positive qualities of stevia. As discussed above, the other
sweeteners currently on the market lack many of the qualities that make stevia attractive to consumers and manufacturers, including
the zero calorie/near zero glycemic index combination.
Therefore, we believe that the most likely
threat to stevia growers will come from alternative “natural” methods to produce stevia extracts that obviate the need
to farm stevia, such as fermentation-derived stevia.
A fermentation-derived stevia ingredient
can be produced in a lab where low cost plant materials are converted into sweet steviol glycosides through controlled fermentation
methods that duplicate the natural biochemical pathways that are involved in the natural production of the sweet components of
the stevia leaf and would still meet the requirements to be classified as a “natural” ingredient and when done at volume
could potentially be produced more economically than the farming method and without impurities.
Major known companies that are progressing
down this track include Evolva Holding SA of Switzerland who has acquired San Francisco based Abunda Nutrition, Inc., Blue California
of Rancho Santa Margarita, California and Stevia First Corporation of Yuba City, California.
There are four areas on which we will focus
to reduce the risk and/or impact of alternative methods of stevia ingredient production.
|
|
1.
|
Increase farming efficiencies
. The more efficient and scaled farming becomes, the higher the economic hurdle will be for other methods of production. We believe that our intellectual property and continued research and development activities will allow our farms and those of our customers to increase efficiencies, decrease cost of production and produce better quality leaf.
|
|
|
2.
|
Intellectual Property Protections.
We have a strong focus on developing protectable intellectual property which we believe should create barriers to entry and protect our methodologies. Additionally, where applicable we will continue to consider the acquisition of potentially synergistic intellectual property.
|
|
|
3.
|
Crop Diversification.
Our farm management infrastructure and the majority of our intellectual property is applicable to most crops providing us with the flexibility to diversify our crops and the customer base for our farm management solutions.
|
|
|
4.
|
Product Diversification
. We will explore additional markets and uses for stevia and seek to acquire technology to diversify its applications.
|
Commercial Product Alternatives
Small regional companies in Japan, China,
and South Korea have been producing commercial stevia products for several years, focusing on their local markets. We believe with
the awareness of stevia on a global scale, this will provide an opportunity to develop a large commercial market. Once the market
reaches critical mass, large companies will likely enter the market.
We intend to protect our market by positioning
ourselves as both the primary provider of raw extract to companies as well as establishing our own vertical markets utilizing our
farm management core competency to contract farm using our commercial stevia products.
Employees
George Blankenbaker, our President and
a director, is our sole employee.
Our relationship with our farm management
partner, Growers Synergy, currently provides the staffing necessary to operate our farms and our technology partner, TechNew, provides
the staffing for our technical operations.
We chose to outsource the operations management
during our development phase to minimize expenses and provide a team of qualified experienced staff to lead us through the development
phase until we are ready to commercialize. As we begin commercialization and revenue generation, we intend to begin to hire full
time staff.
Summary Plan of Operation
The following table provides our current
and intended plan of operation, including our goals, estimated costs and timelines in connection with our aim of expanding our
operations to provide farm management services and products in China and South East Asia. There can be no assurance that our plan
of operation and goals will be accomplished on the timelines set forth below, at the costs noted, or at all.
|
Goals
|
|
Status
|
|
Requirements
|
|
Timeline
|
|
Estimated
Budget
|
|
|
Build elite
strains of Stevia
|
|
Completed
(Continue Improving)
|
|
-
|
|
Ongoing
|
|
|
|
*
|
|
Develop Intellectual Property
|
|
Completed (Continue Improving)
|
|
-
|
|
Ongoing
|
|
|
|
*
|
|
Develop Farming Protocols
|
|
Completed (Continue Improving)
|
|
-
|
|
Ongoing
|
|
|
|
*
|
|
Develop Operating Manuals
|
|
Completed (Continue Improving)
|
|
-
|
|
Ongoing
|
|
|
|
*
|
|
Conduct Product Testing
|
|
Completed (Continue Improving)
|
|
Continue R&D
|
|
Ongoing
|
|
$
|
10,000/month
|
|
|
Trial harvests (multiple
countries)
|
|
Completed (Continue Improving)
|
|
Continue R&D
|
|
Ongoing
|
|
$
|
100,000/year
|
|
|
Feed Product approvals
|
|
Approved in South East
Asia & China
|
|
Register in other countries
|
|
by 2015
|
|
|
negligible
|
|
|
Fertilizer Product approvals
|
|
Approved in South East
Asia & China
|
|
Register in other countries
|
|
by 2015
|
|
|
negligible
|
|
|
Hire Additional Full Time
Staff
|
|
Staff are Identified
|
|
Adequate working capital
|
|
2nd Quarter 2014 (est)
|
|
$
|
50,000/month
|
|
|
Increase revenue
by expanding farm management operations under joint venture with Technew
|
|
Begun in August 2012
|
|
Adequate working capital
|
|
1st Quarter 2015 (est)
|
|
$
|
3,000,000
|
**
|
* These goals have initially been completed but improvements
are ongoing and the costs will depend on opportunities to make such improvements as they arise.
** This is what the Company is targeting through a combination
of revenue generation and external financing.
PROPERTIES
Our international corporate office is located
at 14 Chin Bee Road, Singapore 619824. We also maintain an office in Vietnam at No. 602, CC2A, Thanh Ha‘s building, Bac Linh
Dam, Hoang Mai district, Hanoi, Vietnam and in Hong Kong, at 19/F Kam Chung Comm Bldg 19-21, Hennessy Rd, Hong Kong and in the
United States, at 7117 US 31 South, Indianapolis, IN 46227.
We have also developed a research facility
on 10 Ha (25 Acres) of land leased by Stevia Ventures Corporation and have prepaid the first year lease payment of $30,000 and
the six month lease payment of $15,000 as security deposit.
LEGAL PROCEEDINGS
None.
MARKET FOR COMMON EQUITY AND RELATED
SHAREHOLDER MATTERS
Market Information
Our common stock is quoted on the OTCQB
under the symbol STEV. The closing bid price for our stock as of March 26, 2014, was $0.28.
The following is the range of high and
low bid prices for our common stock for the periods indicated. The quotations reflect inter-dealer prices, without retail mark-up,
mark-down or commissions and may not represent actual transactions.
|
Interim Period From April 1, 2013 to March 26, 2014
|
|
High
|
|
|
Low
|
|
|
First Quarter (April 1, 2013 to June 30, 2013)
|
|
$
|
.349
|
|
|
$
|
.20
|
|
|
Second Quarter (July 1, 2013 to September 30, 2013)
|
|
$
|
.2595
|
|
|
$
|
.1234
|
|
|
Third Quarter (October 1, 2013 to December 31, 2013)
|
|
$
|
.164
|
|
|
$
|
.097
|
|
|
Fourth Quarter (January 1, 2014 to March 26, 2014)
|
|
$
|
.28
|
|
|
$
|
.083
|
|
|
Fiscal Year Ended March 31, 2013
|
|
High
|
|
|
Low
|
|
|
First Quarter (June 30, 2012)
|
|
$
|
1.69
|
|
|
$
|
.75
|
|
|
Second Quarter (September 30, 2012)
|
|
$
|
.83
|
|
|
$
|
.26
|
|
|
Third Quarter (December 31, 2012)
|
|
$
|
.341
|
|
|
$
|
.101
|
|
|
Fourth Quarter (March 31, 2013)
|
|
$
|
.41
|
|
|
$
|
.146
|
|
|
Fiscal Year Ended March 31, 2012
|
|
High
|
|
|
Low
|
|
|
First Quarter (June 30, 2011)
|
|
$
|
1.60
|
|
|
$
|
.25
|
|
|
Second Quarter (September 30, 2011)
|
|
$
|
1.00
|
|
|
$
|
.85
|
|
|
Third Quarter (December 31, 2011)
|
|
$
|
1.05
|
|
|
$
|
.56
|
|
|
Fourth Quarter (March 31, 2012)
|
|
$
|
2.75
|
|
|
$
|
.667
|
|
Stockholders
As of March 26, 2014, there were 145,972,713
shares of common stock issued and outstanding held by 19 stockholders of record (including street name holders).
Dividends
We have not paid dividends to date and
do not anticipate paying any dividends in the foreseeable future. Our Board of Directors intends to follow a policy of retaining
earnings, if any, to finance our growth. The declaration and payment of dividends in the future will be determined by our Board
of Directors in light of conditions then existing, including our earnings, financial condition, capital requirements and other
factors.
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis
of the results of operations and financial condition for the fiscal years ended March 31, 2012 and 2013, and the three and nine
months ended December 31, 2013, should be read in conjunction with the financial statements and related notes and the other financial
information that are included elsewhere in this Prospectus. This discussion includes forward-looking statements based upon current
expectations that involve risks and uncertainties, such as our plans, objectives, expectations and intentions. Actual results and
the timing of events could differ materially from those anticipated in these forward-looking statements as a result of a number
of factors, including those set forth under the Risk Factors, Cautionary Notice Regarding Forward-Looking Statements and Business
sections in this registration statement on Form S-1. We use words such as “anticipate,” “estimate,” “plan,”
“project,” “continuing,” “ongoing,” “expect,” “believe,” “intend,”
“may,” “will,” “should,” “could,” and similar expressions to identify forward-looking
statements.
The following discussion should be read
in conjunction with our consolidated financial statements and notes thereto included elsewhere in this Registration Statement on
Form S-1. Forward looking statements are statements not based on historical information and which relate to future operations,
strategies, financial results or other developments. Forward-looking statements are based upon estimates, forecasts, and assumptions
that are inherently subject to significant business, economic and competitive uncertainties and contingencies, many of which are
beyond our control and many of which, with respect to future business decisions, are subject to change. These uncertainties and
contingencies can affect actual results and could cause actual results to differ materially from those expressed in any forward-looking
statements made by us, or on our behalf. We disclaim any obligation to update forward-looking statements.
Overview
We were incorporated on May 21, 2007 in
the State of Nevada under the name Interpro Management Corp. On March 4, 2011, we changed our name to Stevia Corp. and effectuated
a 35 for 1 forward stock split of all of our issued and outstanding shares of common stock. Effective November 15, 2013,we filed
a Certificate of Amendment to the Company’s Articles of Incorporation to increase the total number of authorized shares of
Common Stock from one hundred million (100,000,000) shares of Common Stock to two hundred fifty million (250,000,000) shares of
Common Stock, each with a par value of $0.001.
We generated revenues during the 2013 fiscal
year. We expect our primary sources of revenue will be (i) providing farm management services, which will provide protocols and
other services to agriculture, aquaculture, and livestock operators, (ii) the sale of inputs such as fertilizer and feed additives
to agriculture, aquaculture and livestock operators, (iii) the sale of crops and seafood produced under contract farming, (iv)
the sale of products derived from the stevia plant and other agriculture crops, (v) providing extraction and refining technology
services related to stevia and other medicinal herbs and (v) the sale of branded consumer products made from natural ingredients.
During 2012, we completed our first commercial
trials of stevia production in Vietnam. In connection with such production we have entered into supply agreements for the off-take
of the stevia we produce and entered into an agreement with Growers Synergy Pte Ltd to assist in the management of our Asia day-to-day
operations. We have also developed commercial applications of stevia derived products and have developed and acquired certain proprietary
technology relating to stevia development which we can integrate into our own stevia production and our farm management services.
In connection with our intellectual property development efforts we have engaged TechNew Technology Limited (“TechNew), as
our technology partner in Vietnam and on July 5, 2012 we entered into a Cooperative Agreement (the “Cooperative Agreement”)
through our subsidiary Stevia Asia Limited (“Stevia Asia”), with Technew and Zhang Ji, a Chinese citizen (together
with Technew, the “Partners”) pursuant to which Stevia Asia and Partners have agreed to engage in a joint venture to
develop certain intellectual property related to stevia development, such joint venture to be owned 70% by Stevia Asia and 30%
by Technew (the “Joint Venture”). Pursuant to the Cooperative Agreement Stevia Asia agreed to contribute $200,000 per
month, up to a total of $2,000,000 in financing, subject to the performance of the Joint Venture and Stevia Asia’s financial
capabilities.
We have also continued to establish research
and production relationships with local institutions and companies in Vietnam. In April, 2012 we announced plans to begin field
trials in Indonesia.
On March 19, 2012, we formed a wholly-owned
subsidiary, Stevia Asia Limited, a company incorporated under the companies ordinance of Hong Kong (“Stevia Asia”)
that will allow the Company to expand its China operations. Hero Tact Limited, a wholly-owned subsidiary of Stevia Asia, was incorporated
under the companies ordinance of Hong Kong and renamed Stevia Technew Limited on April 28, 2012.
On October 1, 2013, we formed SC Brands
Pte. Ltd., a Singapore corporation and a subsidiary in which we own a 70% equity interest. SC Brands will allow us to develop branded
consumer products.
On February 24, 2014, we formed Real Hemp
LLC, a wholly owned Indiana limited liability company that will focus on developing hemp products to be sold in the US.
Results of Operations
The following discussion of the financial
condition, results of operations, cash flows, and changes in our financial position should be read in conjunction with our audited
consolidated financial statements and notes included in our Annual Report on Form 10-K for the fiscal year ended March 31, 2013,
filed July 15, 2013. Such financial statements have been prepared in conformity with U.S. GAAP and are stated in United States
dollars.
Comparison of Three Month Periods
Ended December 31, 2013 and December 31, 2012
For the three month period ended December
31, 2013 we incurred a net loss of $831,410, compared to a net loss of $364,601 for the three month period ended December 31, 2012.
The increase was mainly attributed to a $561,077 loss attributed to debt settlement during the three month period ended December
31, 2013.
General and administration expenses
and professional fees for the three month period ended December 31, 2013 amounted to $133,641 and $178,135 respectively, compared
to $69,302 and $123,356 during the three month period ended December 31, 2012. Research and development fees for the three month
period ended December 31, 2013 were $71,930 compared to $0 during the three month period ended December 31, 2012. Directors fees,
officer salary and compensation and other salary and compensation were $7,813, $0 and $378 respectively, compared to $93,750, $0
and $59,105 during the three month period ended December 31, 2012.
Comparison of Nine Month Periods
Ended December 31, 2013 and December 31, 2012
For the nine month period ended December
31, 2013 we incurred a net loss of $2,017,484, compared to a net loss of $1,342,473 for the nine month period ended December 31,
2012. The increase was mainly attributed to an increase in officer salary and compensation expense from $0 to $600,000, an increase
in other income/expenses from a gain of $247,341 to a loss of $786,508, and offset by a an increase in revenues from $0 to $758,809.
General and administration expenses
and professional fees for the nine month period ended December 31, 2013 amounted to $387,040 and $487,867 respectively, compared
to $204,616 and $281,250 during the nine month period ended December 31, 2012. Research and development fees for the nine month
period ended December 31, 2013 were $262,810 compared to $118,669 during the nine month period ended December 31, 2012. Directors
fees, officer salary and compensation and other salary and compensation were $195,313, $600,000 and $66,556 respectively, compared
to $281,250, $0 and $110,982 during the nine month period ended December 31, 2012.
Results of Operations for the Fiscal
Years Ended March 31, 2012 and 2013
For the fiscal year ended March 31,
2013, we incurred a net loss of $2,035,864 and for the fiscal year ended March 31, 2012, we incurred a net loss of $2,323,551.
Revenues
Our revenues during the fiscal year
ended March 31, 2013 totaled $2,168,093, compared to $1,300 in the fiscal year ended March 31, 2012. This increase in
revenue was the result of the Company successfully completing its first commercial crop harvest in Vietnam.
Cost of Revenues
Cost of revenues during the fiscal
year ended March 31, 2013 totaled $2,617,381, compared to $711,246 during the fiscal year ended March 31, 2012. The
largest components of our cost of revenues are farm produce, which was $1,789,034 and farm management services – related
parties, which was $712,550.
Gross Margin
Gross margin for the fiscal year ended
March 31, 2013 was negative $449,288, compared to a negative $709,946 for the fiscal year ended March 31, 2012. The
decrease was attributable to the significant increase in revenues during the fiscal year ended March 31, 2013.
General and Administrative Expenses,
Salary and Compensation and Directors’ and Professional Fees
General and administration expenses
for the fiscal year ended March 31, 2013, amounted to $412,409 compared to $113,742 in the fiscal year ended March 31, 2012. Research
and development fees for the fiscal year ended March 31, 2013 were $177,169 compared to $206,191 for the fiscal year ended March
31, 2012. Salary and compensation expenses amounted to $190,549, directors’ fees amounted to $375,000 and professional fees
amounted to $454,958 in the fiscal year ended March 31, 2013 compared to $0, $187,500 and $255,959 for the fiscal year ended March
31, 2012.
Liquidity and Capital Resources
As at December 31, 2013 we have $2,132,300
in current assets, and $2,591,869 in current liabilities. As at December 31, 2013 we have $85,366 in cash. As at December 31, 2013,
our total assets were $3,641,781 and our total liabilities were $3,189,388. Our net working capital deficit as at December 31,
2013 was $459,569.
During the nine month period ended December
31, 2013, we used cash of $895,651 in operating activities and used cash of $16,475 in investing activities, respectively. During
the nine month period ended December 31, 2013, we funded our operations from revenue from operations and the proceeds of private
sales of convertible notes and the exercise proceeds of warrants. During the nine month period ended December 31, 2013, we raised
$431,500 through the issuance of convertible notes and $152,012 through the proceeds of warrant exercises.
As of December 31, 2013, convertible promissory
notes, net of discount, in the aggregate amount of $1,071,569 remained outstanding.
In July, 2012 outstanding convertible promissory
notes in the principal amount of $500,000 were converted into an aggregate of 634,193 shares of our common stock.
On August 1, 2012, we entered into a Securities
Purchase Agreement with certain accredited investors (the “Financing Stockholders”) to raise $500,000 in a private
placement financing (the “Offering”). On August 6, 2012, after the satisfaction of certain closing conditions, the
Offering closed and the Company issued to the Financing Stockholders: (i) an aggregate of 1,066,667 shares of the Company's common
stock at a price per share of $0.46875 and (ii) warrants to purchase an equal number of shares of the Company's common stock at
an exercise price of $0.6405 with a term of five (5) years, for gross proceeds of $500,000. Garden State Securities, Inc. (“GSS”)
served as the placement agent for such equity financing. Per the engagement agreement signed between GSS and the Company on June
18, 2012, in consideration for services rendered as the placement agent, the Company agreed to: (i) pay GSS cash commissions equal
to $40,000, or 8.0% of the gross proceeds received in the equity financing, and (ii) issue to GSS or its designee, a warrant to
purchase up to 85,333 shares of the Company's common stock representing 8% of the Shares sold in the Offering) with an exercise
price of $0.6405 per share and a term of five (5) years. Pursuant to the anti-dilution adjustment provision included in the Offering,
the total share amount under the Cranshire Warrant has been increased to 2,036,381 and the exercise price has been reduced to $0.0671
as a result of certain other offerings of the Company. We may receive gross proceeds of up to $136,640.40 upon the cash exercise
of the Cranshire Warrants. Any such proceeds we receive will be used for working capital and general corporate matters.
On May 3, 2013, in consideration for the
immediate cash exercise of outstanding warrants to purchase 853,333 shares of common stock of the Company at a price per share
of $0.20, the Company issued the Anson Warrants which are included in this registration statement. The warrant to purchase 1,877,333
shares of common stock is subject to a right of repurchase by the Company upon the satisfaction of certain conditions, at a price
of $0.001 per warrant share. The warrant to purchase 2,346,666 shares is only exercisable upon the investor’s exercise in
full of the warrant to purchase 1,877,333 shares. We will not receive any proceeds from the sale of those shares of common stock.
We may, however, receive gross proceeds of up to $1,228,799.60 upon the cash exercise of the Anson Warrants. Any such proceeds
we receive will be used for working capital and general corporate matters.
On July 16, 2013, the Company entered into
a $400,000 Promissory Note (the “June 2013 Note”) with an accredited investor (the “Investor”) whereby
the Investor agreed to loan to the Company up to $400,000 pursuant to the terms of the June 2013 Note. The June 2013 Note provides
for the first $100,000 to be advanced upon closing and additional amounts will be advanced at the Investor’s sole discretion.
Each advance is subject to a 10% original issue discount, such that the total amount which may actually be received by the Company
pursuant to the June 2013 Note is only $360,000. The maturity date for each advance made under the June 2013 Note is one year from
the date of such advance. If the Company repays the June 2013 Note on or before 90 days from the effective date, the interest rate
shall be 0%, otherwise a one-time interest charge of l2% shall be applied to the principal sum. The June 2013 Notes are convertible
into common stock of the Company on a cashless basis at any time, at a conversion price equal to the lesser of $0.26 or 65% of
the lowest trade price in the 25 trading days prior to the conversion. If the conversion shares are not deliverable by DWAC an
additional 10% discount will apply, and if the shares are ineligible for deposit into the DTC system and only eligible for Xclearing
deposit an additional 5% discount will apply. So long as the June 2013 Note is outstanding, upon any issuance by the Company or
any of its subsidiaries of any security with any term more favorable to the holder of such security or with a term in favor of
the holder of such security that was not similarly provided to the Investor in the June 2013 Note, then the Company shall notify
the Investor of such additional or more favorable term and such term, at the Investor's option, shall become a part of the transaction
documents with the Company.
On August 22, 2013, we issued a convertible
promissory note to Asher Enterprises, Inc. in the principal amount of $153,500 (the “Asher Note”), pursuant to the
terms of a Securities Purchase Agreement. The Note matures on May 26, 2014, incurs interest at the rate of 8% per annum, and is
convertible into shares of our common stock at a 35% discount to the average of the lowest three trading prices for our common
stock during the 30 day trading period prior to the conversion date.
On September 26, 2013, we issued a convertible
note in the principal amount of $27,778 with a 10% original issuance discount and a one-time interest charge of 12%. The note is
due one (1) year from the date of issuance with the conversion price at 65% of the lowest trade price for the 25 trade day period
before the conversion date.
On October 15, 2013, we issued a Convertible
Debenture in the principal amount of $58,000 (the “Debenture”), to Black Mountain Equities, Inc. (“Black Mountain”).
On March 31, 2014, the Debenture was converted in full into 1,119,299 shares of common stock. The Debenture provides that on the
next registration statement the Company files, the Company will include the shares issuable upon conversion of the Debenture. Black
Mountain also received a warrant to purchase 1,000,000 shares of our common stock with an exercise price of $0.25 per share, subject
to adjustment, and a term of five years.
On November 21, 2013, we issued a convertible
note in the principal amount of $53,000, convertible at 65% of the three lowest bids for 30 trading days before the conversion
date with interest at 8% per annum, due on August 25, 2014.
On December 9, 2013, we issued a convertible
note in the principal amount of $55,556 with a 10% original issuance discount and 12% one time interest. The note is due one (1)
year from the date of issuance with the conversion price at 65% of the lowest trade price for the 25 trade day period before the
conversion date.
Subsequent to the three months ended December
31, 2013, on February 7, 2014, we issued a Convertible Debenture to an investor in the principal amount of $80,000. The Convertible
Debenture matures on February 6, 2015, incurs interest at the rate of 8% per annum, and is convertible into shares of our common
stock at a conversion price of $0.10 per share.
Subsequent to the three months ended December
31, 2013, on February 13, 2014, an investor exercised warrants to purchase 1,877,333 shares of the Company’s common stock
with an exercise price of $0.0585 per share, yielding aggregate gross proceeds to the Company of $109,824 in cash.
Subsequent to the three months ended December
31, 2013, on February 20, 2014, an investor exercised warrants to purchase 683,202 shares of the Company’s common stock with
an exercise price of $0.053365 per share, yielding aggregate gross proceeds to the Company of $36,459 in cash.
Subsequent to the three months ended December
31, 2013, on February 24, 2014, an investor exercised warrants to purchase 1,413,326 shares of the Company’s common stock
with an exercise price of $0.053365 per share, yielding aggregate gross proceeds to the Company of $75,422 in cash.
Subsequent to the three months ended December
31, 2013, on February 26, 2014, the Company converted $893,579 of working capital advances, provided to the Company by George Blankenbaker
and his affiliated companies, into an aggregate of 16,744,682 shares of common stock.
Subsequent to the three months ended December
31, 2013, on February 28, 2014, an investor exercised warrants to purchase 250,000 shares of the Company’s common stock with
an exercise price of $0.053365 per share, yielding aggregate gross proceeds to the Company of $13,341 in cash.
Subsequent to the three months ended December
31, 2013, on March 3, 2014, an investor exercised warrants to purchase 1,750,006 shares of the Company’s common stock with
an exercise price of $0.053365 per share, yielding aggregate gross proceeds to the Company of $93,389 in cash.
Subsequent to the three months ended December
31, 2013, on March 3, 2014, the Company entered into a securities purchase agreement (the “
Purchase Agreement
”)
with Nomis Bay Ltd., a Bermuda company (“
Nomis Bay
”). The Purchase Agreement provides that, upon
the terms and subject to the conditions set forth therein, (i) Nomis Bay shall purchase from the Company on the Closing Date a
senior convertible note with an initial principal amount of $500,000 (the “
Initial Convertible Note
”) for a
purchase price of $340,000 (a 32% original issue discount) and (ii) the Company shall have the right to require Nomis Bay to purchase
from the Company on or prior to the 10
th
trading day after the effective date of this registration statement an additional
senior convertible note with an initial principal amount of $600,000 (the “
Additional Convertible Note
” and,
together with the Initial Convertible Note, the “
Convertible Notes
”) for a purchase price of $600,000. The Initial
Convertible Note matures on December 27, 2014 (subject to extension as provided in the Initial Convertible Note) and, in addition
to the 32% original issue discount, accrues interest at the rate of 8% per annum. If issued, the Additional Convertible Note will
mature on the date that is the 10-month anniversary of the date of issuance of the Additional Convertible Note (subject to extension
as provided in the Initial Convertible Note) and will accrue interest at the rate of 8% per annum. The Initial Convertible Note
is convertible at any time, in whole or in part, at Nomis Bay’s option into shares of the Company’s common stock, par
value $0.001 per share (the “
Common Stock
”), at a conversion price equal to the lesser of (i) the product of
(x) the arithmetic average of the lowest three (3) volume weighted average prices of the Common Stock during the 10 consecutive
trading days ending and including the trading day immediately preceding the applicable conversion date and (y) 40% (the “
Variable
Conversion Price
”), and (ii) $0.30 (as adjusted for stock splits, stock dividends, stock combinations or other similar
transactions). If issued, the Additional Convertible Note will be convertible at any time, in whole or in part, at Nomis Bay’s
option into shares of Common Stock at a conversion price that will be equal to the lesser of (i) the Variable Conversion Price
and (ii) $0.30 (as adjusted for stock splits, stock dividends, stock combinations or other similar transactions). At no time will
Nomis Bay be entitled to convert any portion of the Convertible Notes to the extent that after such conversion, Nomis Bay (together
with its affiliates) would beneficially own more than 4.99% of the outstanding shares of Common Stock as of such date. The Company
has the right at any time to redeem all, but not less than all, of the total outstanding amount then remaining under the Initial
Convertible Note and/or the Additional Convertible Note in cash at a price equal to 140% of the total amount of such Convertible
Note then outstanding.
We do not expect that our revenues from
operations will be wholly sufficient to fund our operating plan, so we are currently seeking further financing and we believe that,
along with our revenues, will provide sufficient working capital to fund our operations for at least the next six months. Changes
in our operating plans, increased expenses, acquisitions, or other events, may cause us to seek additional equity or debt financing
in the future.
Our current cash requirements are significant
due to the planned development and expansion of our business. The successful implementation of our business plan is dependent upon
our ability to develop valuable intellectual property relating to stevia through our research programs, as well as our ability
to develop and manage our own crop and aquaculture production operations. These planned research and agricultural development activities
require significant cash expenditures. We do not expect to generate the necessary cash from our operations during the next 6 to
12 months to expand our business as desired. As such, in order to fund our operations during the next 6 to 12 months, we anticipate
that we will have to raise additional capital through debt and/or equity financings, which may result in substantial dilution to
our existing stockholders. There are no assurances that we will be able to raise the required working capital on terms favorable,
or that such working capital will be available on any terms when needed. In addition, the terms of the Securities Purchase Agreement
contain certain restrictions on our ability to engage in financing transactions. Specifically, for a period of two years after
the effective date of the Securities Purchase Agreement, the Securities Purchase Agreement contains restrictions on certain types
of financing transactions. The Securities Purchase Agreement contains carveouts to such financing restrictions for certain exempted
transactions including (i) issuances pursuant to a stock option plan, (ii) securities issued upon the conversion of outstanding
securities, (iii) securities issued pursuant to acquisitions or other strategic transactions, (iv) up to $500,000 in stock and
warrants on the same terms as set forth in the Securities Purchase Agreement, and (v) up to $3,000,000 of the Company’s securities.
Contractual Obligations and Off-Balance
Sheet Arrangements
As of March 31, 2013, the end of our latest
fiscal year, we did not have any long-term debt or purchase obligations.
We have not entered into any other financial
guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative
contracts that are indexed to our shares and classified as stockholder’s equity or that are not reflected in our consolidated
financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated
entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated
entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or research and development
services with us.
Critical Accounting Estimates
The preparation of financial statements
in conformity with generally accepted accounting principles of the United States (GAAP) requires management to make estimates and
assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the
date of the financial statements and the reported amounts of revenues and expenses during the year. The more significant areas
requiring the use of estimates include asset impairment, stock-based compensation, and future income tax amounts. Management bases
its estimates on historical experience and on other assumptions considered to be reasonable under the circumstances. However, actual
results may differ from the estimates.
The discussion and analysis of our financial
condition and results of operations are based upon our financial statements, which have been prepared in accordance with GAAP.
We believe certain critical accounting policies affect our more significant judgments and estimates used in the preparation of
the financial statements. A description of our critical accounting policies is set forth in our Annual Report on Form 10-K for
the fiscal year ended March 31, 2013, filed on July 16, 2013. As of, and for the three months ended December 31, 2013, there have
been no material changes or updates to our critical accounting policies.
DIRECTORS AND EXECUTIVE OFFICERS
The following table sets forth the names and ages of our current
directors and executive officers, the principal offices and positions held by each person:
|
Person
|
|
Age
|
|
Position
|
|
George Blankenbaker
|
|
48
|
|
Director, President, Secretary and Treasurer
|
|
Dr. Pablo Erat
|
|
42
|
|
Director
|
|
Thomas Ong
|
|
42
|
|
Director
|
The information below with respect to our directors includes
such director’s experience, qualifications, attributes, and skills that led us to the conclusion that they should serve as
a director.
George Blankenbaker - President, Secretary, Treasurer and
Director
Mr. Blankenbaker became our President,
Secretary, Treasurer and Director in June, 2011. Since November 2008, Mr. Blankenbaker has been leading the development of high
Reb-A stevia farming in Vietnam. Mr. Blankenbaker was raised on a farm and became involved in large scale commercial agriculture
in 2002 when he began working with the Agri-Food Veterinary Authority of Singapore (AVA) to provide strategically important food
supplies to Singapore and has extensive experience managing agriculture projects in South East Asia. Mr. Blankenbaker received
a Bachelors of Science in Business Finance from Indiana University in 1988, where he also studied Asian Political Science. Mr.
Blankenbaker’s recent activities and experience in Vietnam have laid the groundwork for the Company’s current business
strategy, and his in-depth knowledge of such matters are invaluable to our Board of Directors.
Dr. Pablo Erat - Director
Dr. Erat was elected to our board of directors
on October 4, 2011. Since January 2009, Dr. Erat has served as CEO of Pal & Partners AG, a Swiss-based group domiciled in Zug
with offices in Zurich and Mumbai and with a focus on the Indian agriculture industry. Prior to joining Pal & Partners AG,
in 2008 Dr. Erat served as a consultant to corporations and start-up companies in various industries to assist in the development
and implementation of innovative strategies. In April 2001, he co-founded Executive Insight, a strategy consulting firm and in
January 2003, he co-founded DocsLogic, a company specialized on the development of knowledge applications, where he remained through
2007. Dr. Erat is also Assistant Professor at the ETH Zurich and regularly delivers speeches and workshops on strategic management
principles for educational and business communities. Dr. Erat received a Doctorate from the University of St. Gallen in Switzerland
in June 2003. Dr. Erat’s extensive knowledge and experience working for and advising early stage companies as well as his
experience in the agriculture industry will be extremely relevant to the Board of Directors.
Thomas Ong - Director
Since November 1, 2011, Mr. Ong has served
as our Director of Operations, Asia. Since November 6, 2009, Mr. Ong also serves as a Director of the Singapore registered farm
management firm Growers Synergy Pte Ltd, an agriculture consultancy and farm management company producing and trading crops for
the domestic and export markets. He is a member of the SPRING Start-up Enterprise Development Scheme (SPRING SEEDS) Investment
Panel, a wholly owned subsidiary of SPRING Singapore, a statutory board under the Singapore Ministry of Trade and Industry, that
provides equity-based co-financing options for Singapore-based early-stage companies. Prior to focusing on the food supply sector,
Mr. Ong was a director of A.D. Venture Limited, a Singapore-registered fund investment and management company with operating arms
in Hong Kong and the People's Republic of China (PRC). Previously, Mr. Ong served 5 years with the Ministry of the Environment
and subsequently joined the National Environment Agency (NEA) and worked with the Economic Development Board (EDB), International
Enterprise Singapore (IE Singapore), Workforce Development Agency (WDA) and related industry groups to promote high value environmental
services to the domestic and international markets. Mr. Ong received his Bachelor of Business Administration from the National
University of Singapore in 1995 and his Master of Science in Information Studies from Nanyang Technological University in 2000.
Mr. Ong's familiarity with our operations specifically and Asian farm management generally will be of great value to our Board
of Directors.
Involvement In Certain Legal Proceedings
No director, executive officer, significant
employee or control person of the Company has been involved in any legal proceeding listed in Item 401(f) of Regulation S-K in
the past 10 years.
Term of Office
Our directors are appointed for a one-year
term to hold office until the next annual general meeting of our stockholders or until removed from office in accordance with our
bylaws. Our officers are appointed by our Board of Directors and hold office until removed by the Board, absent an employment agreement.
EXECUTIVE COMPENSATION
Executive Compensation
The summary compensation table below shows
certain compensation information for services rendered in all capacities to us by our principal executive officer and principal
financial officer and by each other executive officer whose total annual salary and bonus exceeded $100,000 during the fiscal periods
ended March 31, 2012 and March 31, 2013. Other than as set forth below, no executive officer’s total annual compensation
exceeded $100,000 during our last fiscal period.
Summary Compensation Table
|
Name and Principal Position (a)
|
|
Year
(b)
|
|
|
Salary
($)
(c)
|
|
|
Bonus
($)
(d)
|
|
|
Stock
Awards
($)
(e)
|
|
|
Option
Awards
($)
(f)
|
|
|
Non
Equity
Incentive
Plan
Compensation
($)
(g)
|
|
|
Non-qualified
Deferred
Compensation
Earnings
($)
(h)
|
|
|
All Other
Compensation
($)
(i)
|
|
|
Total
($)
(j)
|
|
|
George Blankenbaker
|
|
|
2013
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
President,
Secretary, Treasurer, Director (Principal Executive Officer and Principal Financial Officer)
|
|
|
2012
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
750,000
|
|
|
$
|
750,000
|
|
On June 23, 2011, as a result of the Share
Exchange Agreement, the sole stockholder of Stevia Ventures International Ltd. (“Stevia Ventures”) received 12,000,000
shares of our common stock in exchange for 100% of the issued and outstanding common stock of Stevia Ventures. Mr. Blankenbaker,
our President and director, was the sole stockholder and officer of Stevia Ventures. Accordingly, he was a recipient of 12,000,000
shares of our common stock issued in connection with the Share Exchange Transaction, 6,000,000 of which were to be held in escrow
pending the achievement by the Company of certain business milestones (the “Escrow Shares”). On December 23, 2011,
3,000,000 of the 6,000,000 Escrow Shares were earned and released to Mr. Blankenbaker upon achievement of certain business objectives
by the Company. Those shares were valued at $0.25 per share or $750,000 on the date of release and recorded as compensation. The
remaining 3,000,000 Escrow Shares were earned and released from escrow on July 12, 2013 upon achievement of certain business objectives
by the Company. Those shares were valued at $0.20 per share or $600,000 on the date of release and recorded as compensation.
Other than as set forth above, none of
our executive officers received, nor do we have any arrangements to pay out, any bonus, stock awards, option awards, non-equity
incentive plan compensation, or non-qualified deferred compensation.
Director Compensation
On October 14, 2011 we issued 1,500,000 shares to each of Rodney
L. Cook and Pablo Erat, as newly appointed members of our Board of Directors, as compensation for future services. These shares
shall vest with respect to 750,000 shares of restricted stock for each director on each of the first two anniversaries of the date
of grant, subject to the director’s continuous service to the Company. These shares were valued at $0.25 per share, or an
aggregate of $750,000, on the date of grant and are being amortized over the vesting period of two (2) years or $93,750 per quarter.
We recorded $187,500 in directors’ fees for the period
from April 11, 2011 (inception) through March 31, 2012 and $375,000 in directors’ fees for the fiscal year ended March 31,
2013.
We have no standard arrangement to compensate directors for
their services in their capacity as directors. Except as set forth above, directors are not paid for meetings attended. All travel
and lodging expenses associated with corporate matters are reimbursed by us, if and when incurred.
Employment Agreements
None of our executive officers currently
have employment agreements with us and the manner and amount of compensation for the above-referenced new officer and director
has not yet been determined.
Potential Payments Upon Termination
or Change-in-Control
We currently have no employment agreements
with any of our executive officers, nor any compensatory plans or arrangements resulting from the resignation, retirement or any
other termination of any of our executive officers, from a change-in-control, or from a change in any executive officer’s
responsibilities following a change-in-control. As a result, we have omitted this table.
Compensation Committee Interlocks and
Insider Participation
No interlocking relationship exists between
our Board of Directors and the Board of Directors or compensation committee of any other company, nor has any interlocking relationship
existed in the past.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL
OWNERS AND MANAGEMENT
The following table sets forth certain
information as of March 26, 2014 with respect to the beneficial ownership of our common stock for (i) each director and officer,
(ii) all of our directors and officers as a group, and (iii) each person known to us to own beneficially 5% or more of the outstanding
shares of our common stock. To our knowledge, except as indicated in any footnotes to this table or pursuant to applicable community
property laws, the persons named in the table have sole voting and investment power with respect to the shares of common stock
indicated.
Name and Address of Beneficial
Owner (1)
|
|
Amount and Nature of
Beneficial Ownership
|
|
|
Percentage of Class
(2)
|
|
|
George Blankenbaker(3)
|
|
|
52,244,682
|
|
|
|
35.79
|
%
|
|
President, Secretary, Treasurer,
|
|
|
|
|
|
|
|
|
|
and Director
|
|
|
|
|
|
|
|
|
|
6451 Buck Creek Pkwy
|
|
|
|
|
|
|
|
|
|
Indianapolis, IN 46227
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thomas Ong(4)
|
|
|
5,000,000
|
|
|
|
3.42
|
%
|
|
Director
|
|
|
|
|
|
|
|
|
|
7117 US 31S
|
|
|
|
|
|
|
|
|
|
Indianapolis, IN 46227
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pablo Erat
|
|
|
1,500,000
|
|
|
|
1.03
|
%
|
|
Director
|
|
|
|
|
|
|
|
|
|
Ludretikonerstrasse 53
|
|
|
|
|
|
|
|
|
|
880 Thalwil
|
|
|
|
|
|
|
|
|
|
Switzerland
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All Officers and Directors as a Group
|
|
|
55,244,982
|
|
|
|
40.24
|
%
|
|
|
|
|
|
|
|
|
|
|
(1) Beneficial ownership has been determined
in accordance with Rule 13d-3 under the Exchange Act. Pursuant to the rules of the SEC, shares of common stock which an individual
or group has a right to acquire within 60 days pursuant to the exercise of options or warrants are deemed to be outstanding for
the purpose of computing the percentage ownership of such individual or group, but are not deemed to be beneficially owned and
outstanding for the purpose of computing the percentage ownership of any other person shown in the table.
(2) Based on 145,972,713 shares of our common stock outstanding
as of March 26, 2014.
(3) Mr. Blankenbaker is the beneficial
owner of 52,244,982 shares of common stock. Mr. Blankenbaker owns 12,000,000 shares of common stock directly. 3,500,000 shares
of common stock are owned by Growers Synergy Pte Ltd. (“Growers Synergy”). Mr. Blankenbaker is the managing director
of Growers Synergy. Growers Fresh Pte Ltd (“Growers Fresh) owns a 51% interest in Growers Synergy and the Reporting
Person controls a 49% interest in Growers Fresh. Mr. Blankenbaker may be deemed to be the indirect beneficial owner of the
shares held by Growers Synergy under Rule 13d-3(a) promulgated under the Securities Exchange Act of 1934 (the “Exchange Act”).
However, pursuant to Rule 13d-4 promulgated under the Exchange Act, Mr. Blankenbaker disclaims that he is a beneficial owner of
such shares, except to the extent of his pecuniary interest herein. 36,744,682 shares of common stock are owned by Blankenbaker
Ventures (Asia) Pte. Ltd. (“BV Asia”). Mr. Blankenbaker owns a 65% controlling interest in BV Asia.
(4) Mr. Ong is the beneficial owner of
5,000,000 shares of common stock. Mr. Ong owns 1,500,000 shares of common stock directly and 3,500,000 shares of common stock are
owned by Growers Synergy. Mr. Ong, a director of the Company, is a director of Growers Synergy and is also a 25% shareholder of
Agriventure Pte Ltd., which is a 49% shareholder of Growers Synergy. Mr. Ong may be deemed to be the indirect beneficial owner
of the shares held by Growers Synergy under Rule 13d-3(a) promulgated under the Securities Exchange Act of 1934 (the “Exchange
Act”). However, pursuant to Rule 13d-4 promulgated under the Exchange Act, Mr. Ong disclaims that he is a beneficial owner
of such shares, except to the extent of his pecuniary interest herein.
CERTAIN RELATIONSHIPS AND RELATED PARTY
TRANSACTIONS
AND DIRECTOR INDEPENDENCE
Certain Relationships and Related Party Transactions
On June 23, 2011, as a result of the Share
Exchange Agreement, the sole stockholder of Stevia Ventures International Ltd. (“Stevia Ventures”) received 12,000,000
shares of our common stock in exchange for 100% of the issued and outstanding common stock of Stevia Ventures. Mr. Blankenbaker,
our President and director, was the sole stockholder and officer of Stevia Ventures. Accordingly, he was a recipient of 12,000,000
shares of our common stock issued in connection with the Share Exchange Transaction, 6,000,000 of which were to be held in escrow
pending the achievement by the Company of certain business milestones (the “Escrow Shares”). On December 23, 2011,
3,000,000 of the 6,000,000 Escrow Shares were earned and released to Mr. Blankenbaker upon achievement of certain business objectives
by the Company. Those shares were valued at $0.25 per share or $750,000 on the date of release and recorded as compensation. The
remaining 3,000,000 Escrow Shares were earned and released from escrow on July 12, 2013 upon achievement of certain business objectives
by the Company. Those shares were valued at $0.20 per share or $600,000 on the date of release and recorded as compensation.
On November 1, 2011, the Company entered
into a Management and Off-Take Agreement (the "Management Agreement") with Growers Synergy Pte Ltd. ("Growers Synergy"),
a Singapore corporation. Mr. Ong, a director of the Company, is a director of Growers Synergy and is also a 25% shareholder of
Agriventure Pte Ltd., which is a 49% shareholder of Growers Synergy. Mr. Blankenbaker is the managing director of Growers Synergy.
Growers Fresh Pte Ltd (“Growers Fresh) owns a 51% interest in Growers Synergy and Mr. Blankenbaker controls a 49% interest
in Growers Fresh. Under the terms of the Management Agreement, the Company engaged Growers Synergy to supervise the Company's farm
management operations, recommend quality farm management programs for stevia cultivation, assist in the hiring of employees and
provide training to help the Company meet its commercialization targets, develop successful models to propagate future agribusiness
services, and provide back-office and regional logistical support for the development of proprietary stevia farm systems in Vietnam,
Indonesia and potentially other countries. Growers Synergy will provide services for a term of two (2) years from the date of signing,
at $20,000 per month. The Management Agreement may be terminated by the Company upon 30 day notice. In connection with the Management
Agreement, the parties agreed to enter into an off-take agreement whereby Growers Synergy agreed to purchase all of the non-stevia
crops produced at the Company's Growers Synergy supervised farms. On July 5, 2012, the Company issued 500,000 shares of its common
stock to Growers Synergy as consideration for services rendered by Growers Synergy to the Company. On February 26, 2014, the Company
issued 3,000,000 shares of its common stock to Growers Synergy as consideration for services rendered by Growers Synergy to the
Company.
On February 26, 2014, we issued 20,000,000
shares to Blankenbaker Ventures (Asia) Pte. Ltd., on behalf of George Blankenbaker, our president, director and stockholder in
exchange for services to be rendered by Mr. Blankenbaker. 4,000,000 of the shares were fully vested at the time of grant and the
remainder vest in four equal installments on each anniversary of February 26, 2014. Mr. Blankenbaker owns a 65% controlling interest
in BV Asia.
On February 26, 2014, we issued 16,744,682
shares of our common stock to Blankenbaker Ventures (Asia) Pte. Ltd., on behalf of George Blankenbaker, our president, director
and stockholder, in exchange for the cancellation of approximately $893,579.93 of working capital advances received from Mr. Blankenbaker
and his affiliated companies.
Review, Approval or Ratification of Transactions with Related
Persons
Although we have adopted a Code of Ethics,
we still rely on our Board to review related party transactions on an ongoing basis to prevent conflicts of interest. Our Board
reviews a transaction in light of the affiliations of the director, officer or employee and the affiliation’s of such person’s
immediate family. Transactions are presented to our Board for approval before they are entered into or, if this is not possible,
for ratification after the transaction has occurred. If our Board finds that a conflict of interest exists, then it will determine
the appropriate remedial action, if any. Our Board approves or ratifies a transaction if it determines that the transaction is
consistent with the best interests of the Company.
Director Independence
During the year ended March 31, 2013, we
had two independent directors on our Board, Dr. Erat and Mr. Cook. Mr. Blankenbaker is not independent. We evaluate independence
by the standards for director independence established by applicable laws, rules, and listing standards including, without limitation,
the standards for independent directors established by The New York Stock Exchange, Inc., the NASDAQ National Market, and the SEC.
Subject to some exceptions, these standards
generally provide that a director will not be independent if (a) the director is, or in the past three years has been, an employee
of ours; (b) a member of the director’s immediate family is, or in the past three years has been, an executive officer of
ours; (c) the director or a member of the director’s immediate family has received more than $120,000 per year in direct
compensation from us other than for service as a director (or for a family member, as a non-executive employee); (d) the director
or a member of the director’s immediate family is, or in the past three years has been, employed in a professional capacity
by our independent public accountants, or has worked for such firm in any capacity on our audit; (e) the director or a member of
the director’s immediate family is, or in the past three years has been, employed as an executive officer of a company where
one of our executive officers serves on the compensation committee; or (f) the director or a member of the director’s immediate
family is an executive officer of a company that makes payments to, or receives payments from, us in an amount which, in any twelve-month
period during the past three years, exceeds the greater of $1,000,000 or 2% of that other company’s consolidated gross revenues.
DISCLOSURE OF COMMISSION POSITION OF
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Sections 78.7502 and 78.751 of the Nevada
Revised Statutes authorizes a court to award, or a corporation’s board of directors to grant indemnity to directors and officers
in terms sufficiently broad to permit indemnification, including reimbursement of expenses incurred, under certain circumstances
for liabilities arising under the Securities Act of 1933, as amended. In addition, the registrant’s Bylaws provide
that the registrant has the authority to indemnify the registrant’s directors and officers and may indemnify the registrant’s
employees and agents (other than officers and directors) against liabilities to the fullest extent permitted by Nevada law. The
registrant is also empowered under the registrant’s Bylaws to purchase insurance on behalf of any person whom the registrant
is required or permitted to indemnify.
Insofar as indemnification for liabilities
arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions,
or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in
the Securities Act and is therefore unenforceable.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement
on Form S-1, together with all amendments and exhibits, with the SEC. This Prospectus, which forms a part of that registration
statement, does not contain all information included in the registration statement. Certain information is omitted and you should
refer to the registration statement and its exhibits. With respect to references made in this Prospectus to any of our contracts
or other documents, the references are not necessarily complete and you should refer to the exhibits attached to the registration
statement for copies of the actual contracts or documents. You may read and copy any document that we file at the Commission’s
Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information
on the operation of the public reference rooms. Our filings and the registration statement can also be reviewed by accessing the
SEC’s website at http://www.sec.gov. We maintain a website at http://www.steviacorp.us
FINANCIAL STATEMENTS
Our interim unaudited consolidated financial
statements as of and for the three and nine months ended December 31, 2013 and 2012, and audited consolidated financial statements
as of and for the fiscal year ended March 31, 2013 and for the period from April 11, 2011 (inception) to March 31, 2012 are included
herewith.
Stevia Corp.
March 31, 2013 and 2012
Index to the Consolidated Financial Statements
|
Contents
|
Page(s)
|
|
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
|
|
|
Consolidated Balance Sheets at March 31, 2013 and 2012
|
F-3
|
|
|
|
|
Consolidated Statements of Operations for the Fiscal Year Ended March 31, 2013 and for the Period from April 11, 2011 (Inception) through March 31, 2012
|
F-4
|
|
|
|
|
Consolidated Statement of Equity (Deficit) for the Fiscal Year Ended March 31, 2013 and for the Period from April 11, 2011 (Inception) through March 31, 2012
|
F-5
|
|
|
|
|
Consolidated Statements of Cash Flows for the Fiscal Year Ended March 31, 2013 and for the Period from April 11, 2011 (Inception) through March 31, 2012
|
F-6
|
|
|
|
|
Notes to the Consolidated Financial Statements
|
F-7
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Stevia Corp.
Indianapolis, Indiana
We have audited the accompanying consolidated balance sheets of Stevia Corp. (the “Company”) as of March 31, 2013 and 2012, and the related consolidated statements of operations, equity (deficit) and cash flows for the fiscal year ended March 31, 2013 and for the period from April 11, 2011 (inception) through March 31, 2012. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purposes of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining on a test basis, evidence supporting the amount and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of March 31, 2013 and 2012, and the related consolidated statements of operations, equity (deficit) and cash flows for the fiscal year ended March 31, 2013 and for the period from April 11, 2011 (inception) through March 31, 2012 in conformity with accounting principles generally accepted in the United States of America.
The consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates continuity of operations, realization of assets, and liquidation of liabilities in the normal course of business. As discussed in Note 3 to the consolidated financial statements, the Company had an accumulated deficit at March 31, 2013, a net loss and net cash used in operating activities for the fiscal year then ended. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regards to these matters are also described in Note 3. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/Li and Company, PC
Li and Company, PC
Skillman, New Jersey
July 15, 2013
Stevia Corp.
Consolidated Balance Sheets
|
|
|
March 31, 2013
|
|
|
March 31, 2012
|
|
|
|
|
|
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
Cash
|
|
$
|
424,475
|
|
|
$
|
15,698
|
|
|
Accounts receivable
|
|
|
158,008
|
|
|
|
-
|
|
|
Prepayments and other current assets
|
|
|
33,096
|
|
|
|
168,874
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
615,579
|
|
|
|
184,572
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current assets:
|
|
|
|
|
|
|
|
|
|
Property and equipment
|
|
|
7,925
|
|
|
|
3,036
|
|
|
Accumulated depreciation
|
|
|
(1,234
|
)
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net
|
|
|
6,691
|
|
|
|
3,036
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquired technology
|
|
|
1,635,300
|
|
|
|
-
|
|
|
Accumulatd amortization
|
|
|
(81,765
|
)
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquired technology, net
|
|
|
1,553,535
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Website development costs
|
|
|
5,315
|
|
|
|
5,315
|
|
|
Accumulated amortization
|
|
|
(1,869
|
)
|
|
|
(801
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Website development costs, net
|
|
|
3,446
|
|
|
|
4,514
|
|
|
|
|
|
|
|
|
|
|
|
|
Security deposit
|
|
|
15,000
|
|
|
|
15,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
2,194,251
|
|
|
$
|
207,122
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and equity (deficit)
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
948,073
|
|
|
$
|
237,288
|
|
|
Accounts payable - president and CEO
|
|
|
89,193
|
|
|
|
20,220
|
|
|
Accrued expenses
|
|
|
19,700
|
|
|
|
5,400
|
|
|
Accrued interest
|
|
|
21,627
|
|
|
|
15,521
|
|
|
|
|
|
|
|
|
|
|
|
|
Advances from president and significant stockholder
|
|
|
21,238
|
|
|
|
19,138
|
|
|
Convertible notes payable - net of discount
|
|
|
357,700
|
|
|
|
700,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Current portion of derivative liability
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
1,457,531
|
|
|
|
997,567
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Current liabilities:
|
|
|
|
|
|
|
|
|
|
Derivative liability
|
|
|
486,113
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current liabilities
|
|
|
486,113
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
1,943,644
|
|
|
|
997,567
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity (Deficit)
|
|
|
|
|
|
|
|
|
|
Stevia Corp stockholders' equity (deficit):
|
|
|
|
|
|
|
|
|
|
Preferred stock at $0.001 par value: 1,000,000 shares authorized;
|
|
|
|
|
|
|
|
|
|
none issued or outstanding
|
|
|
-
|
|
|
|
-
|
|
|
Common stock at $0.001 par value: 100,000,000 shares authorized,
|
|
|
|
|
|
|
|
|
|
63,555,635 and 58,354,775 shares issued and outstanding, respectively
|
|
|
63,556
|
|
|
|
58,355
|
|
|
Additional paid-in capital
|
|
|
4,760,624
|
|
|
|
1,474,751
|
|
|
Accumulated deficit
|
|
|
(4,359,415
|
)
|
|
|
(2,323,551
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total Stevia Corp stockholders' equity (deficit)
|
|
|
464,765
|
|
|
|
(790,445
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Non-controlling interest in subsidiary
|
|
|
(214,158
|
)
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Equity (Deficit)
|
|
|
250,607
|
|
|
|
(790,445
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total Liabilities and Equity (Deficit)
|
|
$
|
2,194,251
|
|
|
$
|
207,122
|
|
See accompanying notes to the consolidated financial statements.
Stevia Corp.
Consolidated Statements of Operations
|
|
|
|
|
|
|
|
For the Period from
|
|
|
|
|
|
For the Fiscal Year
|
|
|
April 11, 2011
|
|
|
|
|
|
Ended
|
|
|
(inception) through
|
|
|
|
|
|
March 31, 2013
|
|
|
March 31, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues
|
|
|
$
|
2,168,093
|
|
|
$
|
1,300
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of revenues
|
|
|
|
|
|
|
|
|
|
Farm produce
|
|
|
1,789,034
|
|
|
|
-
|
|
|
Farm expenses
|
|
|
94,547
|
|
|
|
523,746
|
|
|
Farm field lease
|
|
|
21,250
|
|
|
|
7,500
|
|
|
Farm management services - related parties
|
|
|
712,550
|
|
|
|
180,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cost of revenues
|
|
|
2,617,381
|
|
|
|
711,246
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross margin
|
|
|
(449,288)
|
|
|
|
(709,946)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
Directors' fees
|
|
|
375,000
|
|
|
|
187,500
|
|
|
Professional fees
|
|
|
454,958
|
|
|
|
255,959
|
|
|
Research and development
|
|
|
177,169
|
|
|
|
206,191
|
|
|
Salary and compensation - officer
|
|
|
-
|
|
|
|
750,000
|
|
|
Salary and compensation - others
|
|
|
190,549
|
|
|
|
-
|
|
|
General and administrative expenses
|
|
|
412,409
|
|
|
|
113,742
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
1,610,085
|
|
|
|
1,513,392
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(2,059,373)
|
|
|
|
(2,223,338)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other (income) expense:
|
|
|
|
|
|
|
|
|
|
Change in fair value of derivative liability
|
|
|
74,308
|
|
|
|
-
|
|
|
Financing cost
|
|
|
28,625
|
|
|
|
70,500
|
|
|
Foreign currency transaction gain (loss)
|
|
|
1,316
|
|
|
|
-
|
|
|
Interest expense
|
|
|
86,400
|
|
|
|
29,757
|
|
|
Interest income
|
|
|
-
|
|
|
|
(44)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other (income) expense
|
|
|
190,649
|
|
|
|
100,213
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before income tax provision and non-controlling interest
|
|
|
(2,250,022)
|
|
|
|
(2,323,551)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax provision
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss before non-controlling interest
|
|
|
(2,250,022)
|
|
|
|
(2,323,551)
|
|
|
Net loss attributable to the non-controlling interest
|
|
|
(214,158)
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to Stevia Corp.
|
|
$
|
(2,035,864)
|
|
|
$
|
(2,323,551)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share
|
|
|
|
|
|
|
|
|
|
- Basic and diluted:
|
|
$
|
(0.03)
|
|
|
$
|
(0.05)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding
|
|
|
|
|
|
|
|
|
|
- basic and diluted
|
|
|
62,092,487
|
|
|
|
45,093,271
|
|
See accompanying notes to the consolidated financial statements.
Stevia Corp.
Consolidated Statement of Equity (Deficit)
For the Fiscal Year Ended March 31, 2013 and for the Period from April 11, 2011 (Inception) through March 31, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total STEV
|
|
|
|
|
|
|
|
|
|
|
Common Stock, $0.001 Par Value
|
|
|
Additional
|
|
|
Accumulated
|
|
|
Stockholders'
|
|
|
Non-controlling
|
|
|
Total
|
|
|
|
|
Number of Shares
|
|
|
Amount
|
|
|
paid-in Capital
|
|
|
Deficit
|
|
|
Equity (Deficit)
|
|
|
Interest
|
|
|
Equity (Deficit)
|
|
|
Balance, April 11, 2011 (inception)
|
|
|
6,000,000
|
|
|
$
|
6,000
|
|
|
$
|
(5,900
|
)
|
|
$
|
-
|
|
|
$
|
100
|
|
|
$
|
-
|
|
|
$
|
100
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares deemed issued in reverse acquisition
|
|
|
79,800,000
|
|
|
|
79,800
|
|
|
|
(198,088
|
)
|
|
|
|
|
|
|
(118,288
|
)
|
|
|
|
|
|
|
(118,288
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares cancelled in reverse acquisition
|
|
|
(33,000,000
|
)
|
|
|
(33,000
|
)
|
|
|
33,000
|
|
|
|
|
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for cash at $0.25 per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
on October 4, 2011
|
|
|
400,000
|
|
|
|
400
|
|
|
|
99,600
|
|
|
|
|
|
|
|
100,000
|
|
|
|
|
|
|
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for notes conversion
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at $0.25 per share on October 4, 2011
|
|
|
1,400,000
|
|
|
|
1,400
|
|
|
|
348,600
|
|
|
|
|
|
|
|
350,000
|
|
|
|
|
|
|
|
350,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for conversion of accrued interest
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at $0.25 per share on October 4, 2011
|
|
|
74,850
|
|
|
|
75
|
|
|
|
18,638
|
|
|
|
|
|
|
|
18,713
|
|
|
|
|
|
|
|
18,713
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares cancelled by significant stockholder
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
on October 4, 2011
|
|
|
(3,000,000
|
)
|
|
|
(3,000
|
)
|
|
|
3,000
|
|
|
|
|
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for future director
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
services on October 4, 2011
|
|
|
3,000,000
|
|
|
|
3,000
|
|
|
|
747,000
|
|
|
|
|
|
|
|
750,000
|
|
|
|
|
|
|
|
750,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for future director
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
services on October 4, 2011
|
|
|
|
|
|
|
|
|
|
|
(750,000
|
)
|
|
|
|
|
|
|
(750,000
|
)
|
|
|
|
|
|
|
(750,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for future director
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
services on October 4, 2011
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
earned during the period
|
|
|
|
|
|
|
|
|
|
|
187,500
|
|
|
|
|
|
|
|
187,500
|
|
|
|
|
|
|
|
187,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Make good shares released to officer for achieving
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
the first milestone on December 23, 2011
|
|
|
3,000,000
|
|
|
|
3,000
|
|
|
|
747,000
|
|
|
|
|
|
|
|
750,000
|
|
|
|
|
|
|
|
750,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for notes conversion
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at $0.25 per share on January 18, 2012
|
|
|
600,000
|
|
|
|
600
|
|
|
|
149,400
|
|
|
|
|
|
|
|
150,000
|
|
|
|
|
|
|
|
150,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for conversion of accrued interest
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at $0.25 per share on January 18, 2012
|
|
|
17,425
|
|
|
|
17
|
|
|
|
4,339
|
|
|
|
|
|
|
|
4,356
|
|
|
|
|
|
|
|
4,356
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for financing services upon agreement
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at $1.50 per share on January 26, 2012
|
|
|
35,000
|
|
|
|
35
|
|
|
|
52,465
|
|
|
|
|
|
|
|
52,500
|
|
|
|
|
|
|
|
52,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for consulting services
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at $1.39 per share on March 31, 2012
|
|
|
27,500
|
|
|
|
28
|
|
|
|
38,197
|
|
|
|
|
|
|
|
38,225
|
|
|
|
|
|
|
|
38,225
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2,323,551
|
)
|
|
|
(2,323,551
|
)
|
|
|
|
|
|
|
(2,323,551
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2012
|
|
|
58,354,775
|
|
|
|
58,355
|
|
|
|
1,474,751
|
|
|
|
(2,323,551
|
)
|
|
|
(790,445
|
)
|
|
|
-
|
|
|
|
(790,445
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted common shares issued for farm management services to a relatd party
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
valued at $0.79 per share discounted at 69%
on July 5, 2012
|
|
|
500,000
|
|
|
|
500
|
|
|
|
272,050
|
|
|
|
|
|
|
|
272,550
|
|
|
|
|
|
|
|
272,550
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted common shares issued for technology rights
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
valued at $0.79 per share discounted at 69%
on July 5, 2012
|
|
|
3,000,000
|
|
|
|
3,000
|
|
|
|
1,632,300
|
|
|
|
|
|
|
|
1,635,300
|
|
|
|
|
|
|
|
1,635,300
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for notes conversion
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at $0.832143 per share on July 6, 2012
|
|
|
600,858
|
|
|
|
601
|
|
|
|
499,399
|
|
|
|
|
|
|
|
500,000
|
|
|
|
|
|
|
|
500,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for conversion of accrued interest
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at $0.832143 per share on July 6, 2012
|
|
|
33,335
|
|
|
|
33
|
|
|
|
27,707
|
|
|
|
|
|
|
|
27,740
|
|
|
|
|
|
|
|
27,740
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares and warrants issued to two investors for cash
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at $0.46875 per unit on August 6, 2012
|
|
|
1,066,667
|
|
|
|
1,067
|
|
|
|
498,933
|
|
|
|
|
|
|
|
500,000
|
|
|
|
|
|
|
|
500,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued to investors in connection with the sale of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
equity units on August 6, 2012 classified as derivative liability
|
|
|
|
|
|
|
|
|
|
|
(381,300
|
)
|
|
|
|
|
|
|
(381,300
|
)
|
|
|
|
|
|
|
(381,300
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commissions and legal fees paid in connection with the sale of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
equity units on August 6, 2012
|
|
|
|
|
|
|
|
|
|
|
(52,500
|
)
|
|
|
|
|
|
|
(52,500
|
)
|
|
|
|
|
|
|
(52,500
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued to placement agent in connection with the sale of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
equity units on August 6, 2012 classified as derivative liability
|
|
|
|
|
|
|
|
|
|
|
(30,504
|
)
|
|
|
|
|
|
|
(30,504
|
)
|
|
|
|
|
|
|
(30,504
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of warrants in connection with
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
convertible note payable issued in February and March 2013
|
|
|
|
|
|
|
|
|
|
|
220,438
|
|
|
|
|
|
|
|
220,438
|
|
|
|
|
|
|
|
220,438
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beneficial conversion feature in connection with
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
convertible note payable issued in February and March 2013
|
|
|
|
|
|
|
|
|
|
|
224,350
|
|
|
|
|
|
|
|
224,350
|
|
|
|
|
|
|
|
224,350
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for future director
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
services on October 4, 2011
earned during the period
|
|
|
|
|
|
|
|
|
|
|
375,000
|
|
|
|
|
|
|
|
375,000
|
|
|
|
|
|
|
|
375,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2,035,864
|
)
|
|
|
(2,035,864
|
)
|
|
|
(214,158
|
)
|
|
|
(2,250,022
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2013
|
|
|
63,555,635
|
|
|
$
|
63,556
|
|
|
$
|
4,760,624
|
|
|
$
|
(4,359,415
|
)
|
|
$
|
464,765
|
|
|
$
|
(214,158
|
)
|
|
$
|
250,607
|
|
See accompanying notes to the consolidated financial statements.
Stevia Corp.
Consolidated Statements of Cash Flows
|
|
|
|
|
|
|
For the Period from
|
|
|
|
|
For the Fiscal Year
|
|
|
April 11, 2011
|
|
|
|
|
Ended
|
|
|
(inception) through
|
|
|
|
|
March 31, 2013
|
|
|
March 31, 2012
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
Adjustments to reconcile net loss to net cash used in operating activities
|
|
|
|
|
|
|
|
|
|
Depreciation expense
|
|
|
1,234
|
|
|
|
-
|
|
|
Amortization expense - acquired technology
|
|
|
81,765
|
|
|
|
-
|
|
|
Amortization expense - website development costs
|
|
|
1,068
|
|
|
|
801
|
|
|
Discount of convertible notes payable
|
|
|
(412,738
|
)
|
|
|
-
|
|
|
Change in fair value of derivative liability
|
|
|
74,308
|
|
|
|
-
|
|
|
Common shares issued for compensation
|
|
|
-
|
|
|
|
750,000
|
|
|
Common shares issued for director services earned during the period
|
|
|
375,000
|
|
|
|
187,500
|
|
|
Common shares issued for services-related party
|
|
|
272,550
|
|
|
|
-
|
|
|
Common shares issued for outside services
|
|
|
-
|
|
|
|
90,725
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(158,008
|
)
|
|
|
-
|
|
|
Prepaid expenses
|
|
|
135,778
|
|
|
|
(168,874
|
)
|
|
Accounts payable
|
|
|
710,785
|
|
|
|
141,530
|
|
|
Accounts payable - President and CEO
|
|
|
68,973
|
|
|
|
20,220
|
|
|
Accrued expenses
|
|
|
14,300
|
|
|
|
(1,290
|
)
|
|
Accrued interest
|
|
|
54,284
|
|
|
|
38,589
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(1,030,723
|
)
|
|
|
(1,279,350
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment
|
|
|
(4,889
|
)
|
|
|
(3,036
|
)
|
|
Website development costs
|
|
|
-
|
|
|
|
(5,315
|
)
|
|
Cash received from reverse acquisition
|
|
|
-
|
|
|
|
3,199
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(4,889
|
)
|
|
|
(5,152
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
Advances from president and stockholder
|
|
|
2,100
|
|
|
|
200
|
|
|
Proceeds from issuance of convertible notes
|
|
|
550,000
|
|
|
|
1,200,000
|
|
|
Proceeds from sale of common stock, net of costs
|
|
|
892,289
|
|
|
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
1,444,389
|
|
|
|
1,300,200
|
|
|
|
|
|
|
|
|
|
|
|
|
Net change in cash
|
|
|
408,777
|
|
|
|
15,698
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash at beginning of the fiscal year
|
|
|
15,698
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash at end of the fiscal year
|
|
$
|
424,475
|
|
|
$
|
15,698
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flows information:
|
|
|
|
|
|
|
|
|
|
Interest paid
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax paid
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash investing and financing activities:
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for conversion of convertible notes
|
|
$
|
500,000
|
|
|
$
|
500,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Converstion of accrued interest to convertible notes
|
|
$
|
20,438
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for conversion of accrued interest
|
|
$
|
27,740
|
|
|
$
|
23,068
|
|
See accompanying notes to the consolidated financial statements.
Stevia Corp.
March 31, 2013 and 2012
Notes to the Consolidated Financial Statements
Note 1 – Organization and Operations
Stevia Corp. (Formerly Interpro Management Corp.)
Interpro Management Corp (“Interpro”) was incorporated under the laws of the State of Nevada on May 21, 2007. Interpro focused on developing and offering web based software that was designed to be an online project management tool used to enhance an organization’s efficiency through planning and monitoring the daily operations of a business. The Company discontinued its web-based software business upon the acquisition of Stevia Ventures International Ltd. on June 23, 2011.
On March 4, 2011, Interpro amended its Articles of Incorporation, and changed its name to Stevia Corp. (“Stevia” or the “Company”) and effectuated a 35 for 1 (1:35) forward stock split of all of its issued and outstanding shares of common stock (the “Stock Split”).
All shares and per share amounts in the consolidated financial statements have been adjusted to give retroactive effect to the Stock Split.
Stevia Ventures International Ltd.
Stevia Ventures International Ltd. (“Ventures”) was incorporated on April 11, 2011 under the laws of the Territory of the British Virgin Islands (“BVI”). Ventures owns certain rights relating to stevia production, including certain assignable exclusive purchase contracts and an assignable supply agreement related to stevia.
Acquisition of Stevia Ventures International Ltd. Recognized as a Reverse Acquisition
On June 23, 2011 (the “Closing Date”), the Company closed a voluntary share exchange transaction with Ventures pursuant to a Share Exchange Agreement (the “Share Exchange Agreement”) by and among the Company, Ventures and George Blankenbaker, the stockholder of Ventures (the “Ventures Stockholder”).
Immediately prior to the Share Exchange Transaction on June 23, 2011, the Company had 79,800,000 common shares issued and outstanding. Simultaneously with the Closing of the Share Exchange Agreement, on the Closing Date, Mohanad Shurrab, a shareholder and, as of the Closing Date, the Company’s former Director, President, Treasurer and Secretary, surrendered 33,000,000 shares of the Company's common stock to the Company for cancellation.
As a result of the Share Exchange Agreement, the Company issued 12,000,000 common shares for the acquisition of 100% of the issued and outstanding shares of Ventures. Of the 12,000,000 common shares issued 6,000,000 shares were being held in escrow pending the achievement by the Company of certain post-Closing business milestones (the “Milestones”), pursuant to the terms of the Make Good Escrow Agreement, between the Company, Greenberg Traurig, LLP, as escrow agent and the Ventures’ Stockholder (the “Escrow Agreement”). Even though the shares issued only represented approximately 20.4% of the issued and outstanding common stock immediately after the consummation of the Share Exchange Agreement the stockholder of Ventures completely took over and controlled the board of directors and management of the Company upon acquisition.
As a result of the change in control to the then Ventures Stockholder, for financial statement reporting purposes, the merger between the Company and Ventures has been treated as a reverse acquisition with Ventures deemed the accounting acquirer and the Company deemed the accounting acquiree under the acquisition method of accounting in accordance with section 805-10-55 of the FASB Accounting Standards Codification. The reverse acquisition is deemed a capital transaction and the net assets of Ventures (the accounting acquirer) are carried forward to the Company (the legal acquirer and the reporting entity) at their carrying value before the acquisition. The acquisition process utilizes the capital structure of the Company and the assets and liabilities of Ventures which are recorded at their historical cost. The equity of the Company is the historical equity of Ventures retroactively restated to reflect the number of shares issued by the Company in the transaction.
Formation of Stevia Asia Limited
On March 19, 2012, the Company formed Stevia Asia Limited (“Stevia Asia”) under the laws of the Hong Kong Special Administrative Region (“HK SAR”) of the People’s Republic of China (“PRC”), a wholly-owned subsidiary.
Formation of Stevia Technew Limited (Formerly Hero Tact Limited)/Cooperative Agreement
On April 28, 2012, Stevia Asia formed Hero Tact Limited, a wholly-owned subsidiary, under the laws of HK SAR, which subsequently changed its name to Stevia Technew Limited (“Stevia Technew”). Stevia Technew intends to facilitate a joint venture relationship with the Company’s technology partner, Guangzhou Health China Technology Development Company Limited, operating under the trade name Tech-New Bio-Technology and Guangzhou’s affiliates Technew Technology Limited. Prior to July 5, 2012, the date of entry into the Cooperative Agreement, Stevia Technew was inactive and had no assets or liabilities.
On July 5, 2012, Stevia Asia entered into a Cooperative Agreement (the "Cooperative Agreement") with Technew Technology Limited ("Technew"), a company incorporated under the companies ordinance of Hong Kong and an associate of Guangzhou Health China Technology Development Company Limited, and Zhang Jia, a Chinese citizen (together with Technew, the "Partners") pursuant to which Stevia Asia and Partners have agreed to make Stevia Technew, a joint venture, of which Stevia Asia legally and beneficially owns 70% of the shares (representing 70% of the issued shares) and Technew legally and beneficially owns 30% of the shares (representing 30% of the issued shares). The Partners will be responsible for managing Stevia Technew and Stevia Asia has agreed to contribute $200,000 per month, up to a total of $2,000,000 in financing, subject to the performance of Stevia Technew and Stevia Asia's financial capabilities.
The Cooperative Agreement shall automatically terminate upon either Stevia Asia or Technew ceasing to be a shareholder in Stevia Technew, or may be terminated by either Stevia Asia or Technew upon a material breach by the other party which is not cured within 30 days of notice of such breach.
Note 2 – Summary of Significant Accounting Policies
Basis of Presentation
The Company’s consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Principles of Consolidation
The Company applies the guidance of Topic 810
“Consolidation”
of the FASB Accounting Standards Codification to determine whether and how to consolidate another entity. Pursuant to ASC Paragraph 810-10-15-10 all majority-owned subsidiaries—all entities in which a parent has a controlling financial interest—shall be consolidated except (1) when control does not rest with the parent, the majority owner; (2) if the parent is a broker-dealer within the scope of Topic 940 and control is likely to be temporary; (3) consolidation by an investment company within the scope of Topic 946 of a non-investment-company investee. Pursuant to ASC Paragraph 810-10-15-8 the usual condition for a controlling financial interest is ownership of a majority voting interest, and, therefore, as a general rule ownership by one reporting entity, directly or indirectly, of more than 50 percent of the outstanding voting shares of another entity is a condition pointing toward consolidation. The power to control may also exist with a lesser percentage of ownership, for example, by contract, lease, agreement with other stockholders, or by court decree. The Company consolidates all less-than-majority-owned subsidiaries, if any, in which the parent’s power to control exists.
The Company's consolidated subsidiaries and/or entities are as follows:
|
Name of consolidated
subsidiary or entity
|
|
State or other jurisdiction of
incorporation or organization
|
|
Date of incorporation or formation
(date of acquisition, if applicable)
|
|
Attributable interest
|
|
|
|
|
|
|
|
|
|
Stevia Ventures International Ltd.
|
|
The Territory of the British Virgin Islands
|
|
April 11, 2011
|
|
100%
|
|
|
|
|
|
|
|
|
|
Stevia Asia Limited
|
|
Hong Kong SAR
|
|
March 19, 2012
|
|
100%
|
|
|
|
|
|
|
|
|
|
Stevia Technew Limited
|
|
Hong Kong SAR
|
|
April 28, 2012
|
|
70%
|
The consolidated financial statements include all accounts of the Company and the consolidated subsidiaries and/or entities as of reporting period ending date(s) and for the reporting period(s) then ended.
All inter-company balances and transactions have been eliminated.
Reclassification
Certain amounts in the prior period financial statements have been reclassified to conform to the current period presentation. These reclassifications had no effect on reported losses.
Use of Estimates and Assumptions
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period.
The Company’s significant estimates and assumptions include the fair value of financial instruments; the carrying value, recoverability and impairment of long-lived assets, including the values assigned to and the estimated useful lives of website development costs; interest rate; revenue recognized or recognizable; sales returns and allowances; foreign currency exchange rate; income tax rate, income tax provision, deferred tax assets and valuation allowance of deferred tax assets; expected term of share options and similar instruments, expected volatility of the entity’s shares and the method used to estimate it, expected annual rate of quarterly dividends, and risk free rate(s); and the assumption that the Company will continue as a going concern. Those significant accounting estimates or assumptions bear the risk of change due to the fact that there are uncertainties attached to those estimates or assumptions, and certain estimates or assumptions are difficult to measure or value.
Management bases its estimates on historical experience and on various assumptions that are believed to be reasonable in relation to the financial statements taken as a whole under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources.
Management regularly evaluates the key factors and assumptions used to develop the estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such evaluations, if deemed appropriate, those estimates are adjusted accordingly.
Actual results could differ from those estimates.
Fair Value of Financial Instruments
The Company follows paragraph 820-10-35-37 of the FASB Accounting Standards Codification (“Paragraph 820-10-35-37”) to measure the fair value of its financial instruments and paragraph 825-10-50-10 of the FASB Accounting Standards Codification for disclosures about fair value of its financial instruments. Paragraph 820-10-35-37 establishes a framework for measuring fair value in accounting principles generally accepted in the United States of America (U.S. GAAP), and expands disclosures about fair value measurements. To increase consistency and comparability in fair value measurements and related disclosures, Paragraph 820-10-35-37 establishes a fair value hierarchy which prioritizes the inputs to valuation techniques used to measure fair value into three (3) broad levels. The three (3) levels of fair value hierarchy defined by Paragraph 820-10-35-37 are described below:
|
Level 1
|
|
Quoted market prices available in active markets for identical assets or liabilities as of the reporting date.
|
|
|
|
|
|
Level 2
|
|
Pricing inputs other than quoted prices in active markets included in Level 1, which are either directly or indirectly observable as of the reporting date.
|
|
|
|
|
|
Level 3
|
|
Pricing inputs that are generally observable inputs and not corroborated by market data.
|
Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable.
The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. If the inputs used to measure the financial assets and liabilities fall within more than one level described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
The carrying amounts of the Company’s financial assets and liabilities, such as cash, accounts receivable, prepayments and other current assets, accounts payable, accrued expenses, and accrued interest, approximate their fair values because of the short maturity of these instruments.
The Company’s convertible notes payable approximates the fair value of such instrument based upon management’s best estimate of interest rates that would be available to the Company for similar financial arrangements at March 31, 2013 and March 31, 2012.
The Company’s Level 3 financial liabilities consist of the derivative warrant issued in August 2012 for which there is no current market for these securities such that the determination of fair value requires significant judgment or estimation. The Company valued the automatic conditional conversion, re-pricing/down-round, change of control; default and follow-on offering provisions using a lattice model, with the assistance of a third party valuation specialist, for which management understands the methodologies. These models incorporate transaction details such as Company stock price, contractual terms, maturity, risk free rates, as well as assumptions about future financings, volatility, and holder behavior as of the date of issuance and each balance sheet date.
It is not, however, practical to determine the fair value of advances from president and significant stockholder, if any, due to their related party nature.
Fair Value of Financial Assets and Liabilities Measured on a Recurring Basis
Level 3 Financial Liabilities – Derivative Warrant Liabilities
The Company uses Level 3 of the fair value hierarchy to measure the fair value of the derivative liabilities and revalues its derivative warrant liability at every reporting period and recognizes gains or losses in the consolidated statements of operations and comprehensive income (loss) that are attributable to the change in the fair value of the derivative warrant liability.
Carrying Value, Recoverability and Impairment of Long-Lived Assets
The Company has adopted paragraph 360-10-35-17 of the FASB Accounting Standards Codification for its long-lived assets. The Company’s long-lived assets, which include property and equipment, acquired technology, and website development costs are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable.
The Company assesses the recoverability of its long-lived assets by comparing the projected undiscounted net cash flows associated with the related long-lived asset or group of long-lived assets over their remaining estimated useful lives against their respective carrying amounts. Impairment, if any, is based on the excess of the carrying amount over the fair value of those assets. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable. If long-lived assets are determined to be recoverable, but the newly determined remaining estimated useful lives are shorter than originally estimated, the net book values of the long-lived assets are depreciated over the newly determined remaining estimated useful lives.
The Company considers the following to be some examples of important indicators that may trigger an impairment review: (i) significant under-performance or losses of assets relative to expected historical or projected future operating results; (ii) significant changes in the manner or use of assets or in the Company’s overall strategy with respect to the manner or use of the acquired assets or changes in the Company’s overall business strategy; (iii) significant negative industry or economic trends; (iv) increased competitive pressures; (v) a significant decline in the Company’s stock price for a sustained period of time; and (vi) regulatory changes. The Company evaluates acquired assets for potential impairment indicators at least annually and more frequently upon the occurrence of such events.
The key assumptions used in management’s estimates of projected cash flow deal largely with forecasts of sales levels and gross margins. These forecasts are typically based on historical trends and take into account recent developments as well as management’s plans and intentions. Other factors, such as increased competition or a decrease in the desirability of the Company’s products or services, could lead to lower projected sales levels, which would adversely impact cash flows. A significant change in cash flows in the future could result in an impairment of long lived assets.
The impairment charges, if any, is included in operating expenses in the accompanying consolidated statements of operations.
Fiscal Year End
The Company elected March 31 as its fiscal year ending date.
Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less at the time of purchase to be cash equivalents.
Property and Equipment
Property and equipment is recorded at cost. Expenditures for major additions and betterments are capitalized. Maintenance and repairs are charged to operations as incurred. Depreciation of furniture and fixture is computed by the straight-line method (after taking into account their respective estimated residual values) over the assets estimated useful life of five (5) years. Upon sale or retirement of property and equipment, the related cost and accumulated depreciation are removed from the accounts and any gain or loss is reflected in the statements of operations.
Intangible Assets Other Than Goodwill
The Company has adopted paragraph 350-30-25-3 of the FASB Accounting Standards Codification for intangible assets other than goodwill. Under the requirements, the Company amortizes the acquisition costs of intangible assets other than goodwill inclusive of acquired technology and website development costs on a straight-line basis over their relevant estimated useful lives of fifteen (15) and five (5) years, respectively. Upon becoming fully amortized, the related cost and accumulated amortization are removed from the accounts.
Related Parties
The Company follows subtopic 850-10 of the FASB Accounting Standards Codification for the identification of related parties and disclosure of related party transactions.
Pursuant to Section 850-10-20 the related parties include a. affiliates of the Company; b. entities for which investments in their equity securities would be required, absent the election of the fair value option under the Fair Value Option Subsection of Section 825–10–15, to be accounted for by the equity method by the investing entity; c. trusts for the benefit of employees, such as pension and profit-sharing trusts that are managed by or under the trusteeship of management; d. principal owners of the Company; e. management of the Company; f. other parties with which the Company may deal if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests; and g. other parties that can significantly influence the management or operating policies of the transacting parties or that have an ownership interest in one of the transacting parties and can significantly influence the other to an extent that one or more of the transacting parties might be prevented from fully pursuing its own separate interests.
The financial statements shall include disclosures of material related party transactions, other than compensation arrangements, expense allowances, and other similar items in the ordinary course of business. However, disclosure of transactions that are eliminated in the preparation of consolidated or combined financial statements is not required in those statements. The disclosures shall include: a. the nature of the relationship(s) involved; b. a description of the transactions, including transactions to which no amounts or nominal amounts were ascribed, for each of the periods for which income statements are presented, and such other information deemed necessary to an understanding of the effects of the transactions on the financial statements; c. the dollar amounts of transactions for each of the periods for which income statements are presented and the effects of any change in the method of establishing the terms from that used in the preceding period; and d. amounts due from or to related parties as of the date of each balance sheet presented and, if not otherwise apparent, the terms and manner of settlement.
Derivative Instruments and Hedging Activities
The Company accounts for derivative instruments and hedging activities in accordance with paragraph 810-10-05-4 of the FASB Accounting Standards Codification (“Paragraph 810-10-05-4”). Paragraph 810-10-05-4 requires companies to recognize all derivative instruments as either assets or liabilities in the balance sheet at fair value. The accounting for changes in the fair value of a derivative instrument depends upon: (i) whether the derivative has been designated and qualifies as part of a hedging relationship, and (ii) the type of hedging relationship. For those derivative instruments that are designated and qualify as hedging instruments, a company must designate the hedging instrument based upon the exposure being hedged as either a fair value hedge, cash flow hedge or hedge of a net investment in a foreign operation.
From time to time, the Company may employ foreign currency forward contracts to convert unforeseeable foreign currency exchange rates to fixed foreign currency exchange rates. The Company does not use derivatives for speculation or trading purposes. Changes in the fair value of derivatives are recorded each period in current earnings or through other comprehensive income, depending on whether a derivative is designated as part of a hedge transaction and the type of hedge transaction. The ineffective portion of all hedges is recognized in current earnings. The Company has sales and purchase commitments denominated in foreign currencies. Foreign currency forward contracts are used to hedge against the risk of change in the fair value of these commitments attributable to fluctuations in exchange rates (“Fair Value Hedges”). Changes in the fair value of the derivative instrument are generally offset in the income statement by changes in the fair value of the item being hedged.
The Company did not employ foreign currency forward contracts to convert unforeseeable foreign currency exchange rates to fixed foreign currency exchange rates for the fiscal year ended March 31, 2013 or 2012.
Derivative Liability
The Company evaluates its convertible debt, options, warrants or other contracts, if any, to determine if those contracts or embedded components of those contracts qualify as derivatives to be separately accounted for in accordance with paragraph 810-10-05-4 and Section 815-40-25 of the FASB Accounting Standards Codification. The result of this accounting treatment is that the fair value of the embedded derivative is marked-to-market each balance sheet date and recorded as either an asset or a liability. In the event that the fair value is recorded as a liability, the change in fair value is recorded in the consolidated statement of operations and comprehensive income (loss) as other income or expense. Upon conversion, exercise or cancellation of a derivative instrument, the instrument is marked to fair value at the date of conversion, exercise or cancellation and then that the related fair value is reclassified to equity.
In circumstances where the embedded conversion option in a convertible instrument is required to be bifurcated and there are also other embedded derivative instruments in the convertible instrument that are required to be bifurcated, the bifurcated derivative instruments are accounted for as a single, compound derivative instrument.
The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period. Equity instruments that are initially classified as equity that become subject to reclassification are reclassified to liability at the fair value of the instrument on the reclassification date. Derivative instrument liabilities will be classified in the balance sheet as current or non-current based on whether or not net-cash settlement of the derivative instrument is expected within 12 months of the balance sheet date.
The Company adopted Section 815-40-15 of the FASB Accounting Standards Codification (“Section 815-40-15”)
to determine whether an instrument (or an embedded feature) is indexed to the Company’s own stock. Section 815-40-15 provides that an entity should use a two-step approach to evaluate whether an equity-linked financial instrument (or embedded feature) is indexed to its own stock, including evaluating the instrument’s contingent exercise and settlement provisions. The adoption of Section 815-40-15 has affected the accounting for (i) certain freestanding warrants that contain exercise price adjustment features and (ii) convertible bonds issued by foreign subsidiaries with a strike price denominated in a foreign currency.
The Company marks to market the fair value of the embedded derivative warrants at each balance sheet date and records the change in the fair value of the embedded derivative warrants as other income or expense in the consolidated statements of operations and comprehensive income (loss).
The Company utilizes the Lattice model that values the liability of the derivative warrants based on a probability weighted discounted cash flow model with the assistance of the third party valuation firm. The reason the Company picks the Lattice model is that in many cases there may be multiple embedded features or the features of the bifurcated derivatives may be so complex that a Black-Scholes valuation does not consider all of the terms of the instrument. Therefore, the fair value may not be appropriately captured by simple models. In other words, simple models such as Black-Scholes may not be appropriate in many situations given complex features and terms of conversion option (e.g., combined embedded derivatives). The Lattice model is based on future projections of the various potential outcomes. The features that were analyzed and incorporated into the model included the exercise and full reset features. Based on these features, there are two primary events that can occur; the Holder exercises the Warrants or the Warrants are held to expiration. The Lattice model analyzed the underlying economic factors that influenced which of these events would occur, when they were likely to occur, and the specific terms that would be in effect at the time (i.e. stock price, exercise price, volatility, etc.). Projections were then made on the underlying factors which led to potential scenarios. Probabilities were assigned to each scenario based on management projections. This led to a cash flow projection and a probability associated with that cash flow. A discounted weighted average cash flow over the various scenarios was completed to determine the value of the derivative warrants.
Beneficial Conversion Feature
When the Company issues an debt or equity security that is convertible into common stock at a discount from the fair value of the common stock at the date the debt or equity security counterparty is legally committed to purchase such a security (Commitment Date), a beneficial conversion charge is measured and recorded on the Commitment Date for the difference between the fair value of the Company's common stock and the effective conversion price of the debt or equity security. If the intrinsic value of the beneficial conversion feature is greater than the proceeds allocated to the debt or equity security, the amount of the discount assigned to the beneficial conversion feature is limited to the amount of the proceeds allocated to the debt or equity security.
Commitment and Contingencies
The Company follows subtopic 450-20 of the FASB Accounting Standards Codification to report accounting for contingencies. Certain conditions may exist as of the date the consolidated financial statements are issued, which may result in a loss to the Company but which will only be resolved when one or more future events occur or fail to occur. The Company assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought therein.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s consolidated financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, and an estimate of the range of possible losses, if determinable and material, would be disclosed.
Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the guarantees would be disclosed. Management does not believe, based upon information available at this time, that these matters will have a material adverse effect on the Company’s consolidated financial position, results of operations or cash flows. However, there is no assurance that such matters will not materially and adversely affect the Company’s business, financial position, and results of operations or cash flows.
Non-controlling Interest
The Company follows paragraph 810-10-65-1 of the FASB Accounting Standards Codification to report the non-controlling interest in Stevia Technew Limited, its majority owned subsidiary in the consolidated statements of balance sheets within the equity section, separately from the Company’s stockholders’ equity. Non-controlling interest represents the non-controlling interest holder’s proportionate share of the equity of the Company’s majority-owned subsidiary, Stevia Technew Limited. Non-controlling interest is adjusted for the non-controlling interest holder’s proportionate share of the earnings or losses and other comprehensive income (loss) and the non-controlling interest continues to be attributed its share of losses even if that attribution results in a deficit non-controlling interest balance.
Revenue Recognition
The Company follows paragraph 605-10-S99-1 of the FASB Accounting Standards Codification for revenue recognition. The Company recognizes revenue when it is realized or realizable and earned. The Company considers revenue realized or realizable and earned when all of the following criteria are met: (i) persuasive evidence of an arrangement exists, (ii) the product has been shipped or the services have been rendered to the customer, (iii) the sales price is fixed or determinable, and (iv) collectability is reasonably assured.
Research and Development
The Company follows paragraph 730-10-25-1 of the FASB Accounting Standards Codification (formerly Statement of Financial Accounting Standards No. 2
“Accounting for Research and Development Costs”
) and paragraph 730-20-25-11 of the FASB Accounting Standards Codification (formerly Statement of Financial Accounting Standards No. 68
“Research and Development Arrangements”
) for research and development costs. Research and development costs are charged to expense as incurred. Research and development costs consist primarily of remuneration for research and development staff, depreciation and maintenance expenses of research and development equipment, material and testing costs for research and development as well as research and development arrangements with unrelated third party research and development institutions.
Non-refundable Advance Payments for Goods or Services to be Used in Future Research and Development Activities
The research and development arrangements usually involve specific research and development projects. Often times, the Company makes non-refundable advances upon signing of these arrangements. The Company adopted paragraph 730-20-25-13 and 730-20-35-1 of the FASB Accounting Standards Codification (formerly Emerging Issues Task Force Issue No. 07-3
“Accounting for Nonrefundable Advance Payments for Goods or Services to be Used in Future Research and Development Activities”
) for those non-refundable advances. Non-refundable advance payments for goods or services that will be used or rendered for future research and development activities are deferred and capitalized. Such amounts are recognized as an expense as the related goods are delivered or the related services are performed. The management continues to evaluate whether the Company expect the goods to be delivered or services to be rendered. If the management does not expect the goods to be delivered or services to be rendered, the capitalized advance payment are charged to expense.
Stock-Based Compensation for Obtaining Employee Services
The Company accounts for its stock based compensation in which the Company obtains employee services in share-based payment transactions under the recognition and measurement principles of the fair value recognition provisions of section 718-10-30 of the FASB Accounting Standards Codification. Pursuant to paragraph 718-10-30-6 of the FASB Accounting Standards Codification, all transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. The measurement date used to determine the fair value of the equity instrument issued is the earlier of the date on which the performance is complete or the date on which it is probable that performance will occur. If shares of the Company are thinly traded the use of share prices established in the Company’s most recent private placement memorandum ("PPM”), or weekly or monthly price observations would generally be more appropriate than the use of daily price observations as such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market.
The fair value of non-derivative option award is estimated on the date of grant using a Black-Scholes option-pricing valuation model. The ranges of assumptions for inputs are as follows:
|
·
|
Expected term of share options and similar instruments: The expected life of options and similar instruments represents the period of time the option and/or warrant are expected to be outstanding. Pursuant to Paragraph 718-10-50-2(f)(2)(i) of the FASB Accounting Standards Codification the expected term of share options and similar instruments represents the period of time the options and similar instruments are expected to be outstanding taking into consideration of the contractual term of the instruments and employees’ expected exercise and post-vesting employment termination behavior into the fair value (or calculated value) of the instruments. Pursuant to paragraph 718-10-S99-1, it may be appropriate to use the
simplified method
,
i.e.,
expected term = ((vesting term + original contractual term) / 2)
, if (i) A company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term due to the limited period of time its equity shares have been publicly traded; (ii) A company significantly changes the terms of its share option grants or the types of employees that receive share option grants such that its historical exercise data may no longer provide a reasonable basis upon which to estimate expected term; or (iii) A company has or expects to have significant structural changes in its business such that its historical exercise data may no longer provide a reasonable basis upon which to estimate expected term. The Company uses the simplified method to calculate expected term of share options and similar instruments as the company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term.
|
|
·
|
Expected volatility of the entity’s shares and the method used to estimate it. Pursuant to ASC Paragraph 718-10-50-2(f)(2)(ii) a thinly-traded or nonpublic entity that uses the calculated value method shall disclose the reasons why it is not practicable for the Company to estimate the expected volatility of its share price, the appropriate industry sector index that it has selected, the reasons for selecting that particular index, and how it has calculated historical volatility using that index. The Company uses the average historical volatility of the comparable companies over the expected contractual life of the share options or similar instruments as its expected volatility. If shares of a company are thinly traded the use of weekly or monthly price observations would generally be more appropriate than the use of daily price observations as the volatility calculation using daily observations for such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market.
|
|
·
|
Expected annual rate of quarterly dividends. An entity that uses a method that employs different dividend rates during the contractual term shall disclose the range of expected dividends used and the weighted-average expected dividends. The expected dividend yield is based on the Company’s current dividend yield as the best estimate of projected dividend yield for periods within the expected term of the share options and similar instruments.
|
|
·
|
Risk-free rate(s). An entity that uses a method that employs different risk-free rates shall disclose the range of risk-free rates used. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods within the expected term of the share options and similar instruments.
|
The Company’s policy is to recognize compensation cost for awards with only service conditions and a graded vesting schedule on a straight-line basis over the requisite service period for the entire award.
E
quity Instruments Issued to Parties other than Employees for Acquiring Goods or Services
The Company accounts for equity instruments issued to parties other than employees for acquiring goods or services under guidance of Subtopic 505-50 of the FASB Accounting Standards Codification (“Subtopic 505-50”).
Pursuant to ASC Section 505-50-30, all transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. The measurement date used to determine the fair value of the equity instrument issued is the earlier of the date on which the performance is complete or the date on which it is probable that performance will occur. If shares of the Company are thinly traded the use of share prices established in the Company’s most recent private placement memorandum ("PPM”), or weekly or monthly price observations would generally be more appropriate than the use of daily price observations as such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market.
The fair value of non-derivative option or warrant award is estimated on the date of grant using a Black-Scholes option-pricing valuation model. The ranges of assumptions for inputs are as follows:
|
·
|
Expected term of share options and similar instruments: Pursuant to Paragraph 718-10-50-2 of the FASB Accounting Standards Codification the expected term of share options and similar instruments represents the period of time the options and similar instruments are expected to be outstanding taking into consideration of the contractual term of the instruments and holder’s expected exercise behavior into the fair value (or calculated value) of the instruments. The Company uses historical data to estimate holder’s expected exercise behavior. If the Company is a newly formed corporation or shares of the Company are thinly traded the contractual term of the share options and similar instruments is used as the expected term of share options and similar instruments as the Company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term.
|
|
·
|
Expected volatility of the entity’s shares and the method used to estimate it. An entity that uses a method that employs different volatilities during the contractual term shall disclose the range of expected volatilities used and the weighted-average expected volatility. A thinly-traded or nonpublic entity that uses the calculated value method shall disclose the reasons why it is not practicable for the Company to estimate the expected volatility of its share price, the appropriate industry sector index that it has selected, the reasons for selecting that particular index, and how it has calculated historical volatility using that index. The Company uses the average historical volatility of the comparable companies over the expected contractual life of the share options or similar instruments as its expected volatility. If shares of a company are thinly traded the use of weekly or monthly price observations would generally be more appropriate than the use of daily price observations as the volatility calculation using daily observations for such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market.
|
|
·
|
Expected annual rate of quarterly dividends. An entity that uses a method that employs different dividend rates during the contractual term shall disclose the range of expected dividends used and the weighted-average expected dividends. The expected dividend yield is based on the Company’s current dividend yield as the best estimate of projected dividend yield for periods within the expected contractual life of the option and similar instruments.
|
|
·
|
Risk-free rate(s). An entity that uses a method that employs different risk-free rates shall disclose the range of risk-free rates used. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods within the contractual life of the option and similar instruments.
|
Pursuant to Paragraphs 505-50-25-8, if fully vested, non-forfeitable equity instruments are issued at the date the grantor and grantee enter into an agreement for goods or services (no specific performance is required by the grantee to retain those equity instruments), then, because of the elimination of any obligation on the part of the counterparty to earn the equity instruments, a measurement date has been reached. A grantor shall recognize the equity instruments when they are issued (in most cases, when the agreement is entered into). Whether the corresponding cost is an immediate expense or a prepaid asset (or whether the debit should be characterized as contra-equity under the requirements of paragraph 505-50-45-1) depends on the specific facts and circumstances. Pursuant to ASC paragraph 505-50-45-1, a grantor may conclude that an asset (other than a note or a receivable) has been received in return for fully vested, non-forfeitable equity instruments that are issued at the date the grantor and grantee enter into an agreement for goods or services (and no specific performance is required by the grantee in order to retain those equity instruments). Such an asset shall not be displayed as contra-equity by the grantor of the equity instruments. The transferability (or lack thereof) of the equity instruments shall not affect the balance sheet display of the asset. This guidance is limited to transactions in which equity instruments are transferred to other than employees in exchange for goods or services. Section 505-50-30 provides guidance on the determination of the measurement date for transactions that are within the scope of this Subtopic.
Pursuant to Paragraphs 505-50-25-8 and 505-50-25-9, an entity may grant fully vested, non-forfeitable equity instruments that are exercisable by the grantee only after a specified period of time if the terms of the agreement provide for earlier exercisability if the grantee achieves specified performance conditions. Any measured cost of the transaction shall be recognized in the same period(s) and in the same manner as if the entity had paid cash for the goods or services or used cash rebates as a sales discount instead of paying with, or using, the equity instruments. A recognized asset, expense, or sales discount shall not be reversed if a stock option that the counterparty has the right to exercise expires unexercised.
Pursuant to ASC paragraph 505-50-30-S99-1, if the Company receives a right to receive future services in exchange for unvested, forfeitable equity instruments, those equity instruments are treated as unissued for accounting purposes until the future services are received (that is, the instruments are not considered issued until they vest). Consequently, there would be no recognition at the measurement date and no entry should be recorded.
Income Tax Provision
The Company accounts for income taxes under Section 740-10-30 of the FASB Accounting Standards Codification, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are based on the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the consolidated statements of income and comprehensive income (loss) in the period that includes the enactment date.
The Company adopted section 740-10-25 of the FASB Accounting Standards Codification (“Section 740-10-25”). Section 740-10-25 addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under Section 740-10-25, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than fifty (50) percent likelihood of being realized upon ultimate settlement. Section 740-10-25 also provides guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures.
The estimated future tax effects of temporary differences between the tax basis of assets and liabilities are reported in the accompanying consolidated balance sheets, as well as tax credit carry-backs and carry-forwards. The Company periodically reviews the recoverability of deferred tax assets recorded on its consolidated balance sheets and provides valuation allowances as management deems necessary.
Management makes judgments as to the interpretation of the tax laws that might be challenged upon an audit and cause changes to previous estimates of tax liability. In addition, the Company operates within multiple taxing jurisdictions and is subject to audit in these jurisdictions. In management’s opinion, adequate provisions for income taxes have been made for all years. If actual taxable income by tax jurisdiction varies from estimates, additional allowances or reversals of reserves may be necessary.
Uncertain Tax Positions
The Company did not take any uncertain tax positions and had no adjustments to its income tax liabilities or benefits pursuant to the provisions of Section 740-10-25 for the fiscal year ended March 31, 2013 or for the period from April 11, 2011 (Inception) through December 31, 2012.
Limitation on Utilization of NOLs due to Change in Control
Pursuant to the Internal Revenue Code Section 382 (“Section 382”), certain ownership changes may subject the NOL’s to annual limitations which could reduce or defer the NOL. Section 382 imposes limitations on a corporation’s ability to utilize NOLs if it experiences an “ownership change.” In general terms, an ownership change may result from transactions increasing the ownership of certain stockholders in the stock of a corporation by more than 50 percentage points over a three-year period. In the event of an ownership change, utilization of the NOLs would be subject to an annual limitation under Section 382 determined by multiplying the value of its stock at the time of the ownership change by the applicable long-term tax-exempt rate. Any unused annual limitation may be carried over to later years. The imposition of this limitation on its ability to use the NOLs to offset future taxable income could cause the Company to pay U.S. federal income taxes earlier than if such limitation were not in effect and could cause such NOLs to expire unused, reducing or eliminating the benefit of such NOLs.
Net Income (Loss) per Common Share
Net income (loss) per common share is computed pursuant to section 260-10-45 of the FASB Accounting Standards Codification. Basic net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock outstanding during the period. Diluted net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock and potentially outstanding shares of common stock during the period to reflect the potential dilution that could occur from common shares issuable through contingent shares issuance arrangement, stock options or warrants.
The following table shows the potentially outstanding dilutive common shares excluded from the diluted net income (loss) per common share calculation as they were anti-dilutive:
|
|
|
Potentially Outstanding Dilutive
Common Shares
|
|
|
|
For Fiscal Year Ended
March 31, 2013
|
|
For the Period
from April 11, 2011
(inception) through
March 31, 2012
|
|
|
|
|
|
|
|
|
Make Good Escrow Shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Make Good Escrow Agreement shares issued and held with the escrow agent in connection with the Share Exchange Agreement consummated on June 23, 2011 pending the achievement by the Company of certain post-Closing business milestones (the “Milestones”).
|
|
|
3,000,000
|
|
|
6,000,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sub-total Make Good Escrow Shares
|
|
|
3,000,000
|
|
|
6,000,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible Note Shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
On March 7, 2012, the Company issued a convertible note in the amount of $200,000 with interest at 10% per annum due one (1) year from the date of issuance with the conversion price to be the same as the next private placement price on a per share basis, provided that the Company completes a private placement with gross proceeds of at least $100,000. On August 6, 2012, the Company completed the very next private placement at $0.46875 per share with gross proceeds of at least $100,000. On March 15, 2013, the above note was cancelled and reissued with a new convertible note consisting of the prior principal amount and all the accrued unpaid interest for a total amount of $220,438 with interest at 12% per annum due on September 30, 2013 with the conversion price is $0.25 per share.
|
|
|
881,752
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
On May 30, 2012, the Company issued a convertible note in the amount of $200,000 with interest at 10% per annum due one (1) year from the date of issuance with the conversion price to be the same as the next private placement price on a per share basis, provided that the Company completes a private placement with gross proceeds of at least $100,000. On August 6, 2012, the Company completed the very next private placement at $0.46875 per share with gross proceeds of at least $100,000.
|
|
|
426,667
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
On February 26, 2013 , the Company issued two (2) convertible notes in the amount of $250,000 and $100,000, respectively, with interest at 12% per annum due on September 30, 2013 with the conversion price at $0.25 per share
|
|
|
1,400,000
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sub-total Convertible Note Shares
|
|
|
2,708,419
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant Shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
On August 6, 2012, the Company issued (i) warrants to purchase 1,066,667 shares, in the aggregate, of the Company’s common stock to the investors (the “investors warrants”) and (ii) warrants to purchase 85,333 shares of the Company's common stock to the placement agent (the "agent warrants") with an exercise price of $0.6405 per share subject to certain adjustments pursuant to Section 3(b) Subsequent Equity Sales of the SPA expiring five (5) years from the date of issuance. On February 26, 2013, the
new warrants issued
triggered a reset of the above warrants exercise price to $0.25 per share and the shares to be issued under the warrants were adjusted accordingly.
|
|
|
2,951,424
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
On February 26, 2013, the Company issued warrants to purchase 1,000,000 and 400,000 shares respectively, 1,400,000 shares in the aggregate, of the Company’s common stock to two notes holders in connection with the issuance of convertible notes.
|
|
|
1,400,000
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
On March 15, 2013, the Company issued a warrant to purchase 881,753 shares of the Company’s common stock to the note holder in connection with the issuance of the convertible note.
|
|
|
881,753
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sub-total Warrant Shares
|
|
|
5,233,177
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total potentially outstanding dilutive common shares
|
|
|
10,941,596
|
|
|
6,000,000
|
|
|
Cash Flows Reporting
The Company adopted paragraph 230-10-45-24 of the FASB Accounting Standards Codification for cash flows reporting, classifies cash receipts and payments according to whether they stem from operating, investing, or financing activities and provides definitions of each category, and uses the indirect or reconciliation method (“Indirect method”) as defined by paragraph 230-10-45-25 of the FASB Accounting Standards Codification to report net cash flow from operating activities by adjusting net income to reconcile it to net cash flow from operating activities by removing the effects of (a) all deferrals of past operating cash receipts and payments and all accruals of expected future operating cash receipts and payments and (b) all items that are included in net income that do not affect operating cash receipts and payments. The Company reports the reporting currency equivalent of foreign currency cash flows, using the current exchange rate at the time of the cash flows and the effect of exchange rate changes on cash held in foreign currencies is reported as a separate item in the reconciliation of beginning and ending balances of cash and cash equivalents and separately provides information about investing and financing activities not resulting in cash receipts or payments in the period pursuant to paragraph 830-230-45-1 of the FASB Accounting Standards Codification.
Subsequent Events
The Company follows the guidance in Section 855-10-50 of the FASB Accounting Standards Codification for the disclosure of subsequent events. The Company will evaluate subsequent events through the date when the financial statements are issued. Pursuant to ASU 2010-09 of the FASB Accounting Standards Codification, the Company as an SEC filer considers its financial statements issued when they are widely distributed to users, such as through filing them on EDGAR.
Recently Issued Accounting Pronouncements
In January 2013, the FASB issued ASU No. 2013-01, "
Balance Sheet (Topic 210): Clarifying the Scope of Disclosures about Offsetting Assets and Liabilities
". This ASU clarifies that the scope of ASU No. 2011-11, "
Balance Sheet (Topic 210): Disclosures about Offsetting Assets and Liabilities.
" applies only to derivatives, repurchase agreements and reverse purchase agreements, and securities borrowing and securities lending transactions that are either offset in accordance with specific criteria contained in FASB Accounting Standards Codification or subject to a master netting arrangement or similar agreement. The amendments in this ASU are effective for fiscal years, and interim periods within those years, beginning on or after January 1, 2013.
In February 2013, the FASB issued ASU No. 2013-02, "
Comprehensive Income (Topic 220): Reporting of Amounts Reclassified Out of Accumulated Other Comprehensive Income.
" The ASU
adds new disclosure requirements for items reclassified out of accumulated other comprehensive income by component and their corresponding effect on net income. The ASU is effective for public entities for fiscal years beginning after December 15, 2013.
In February 2013, the Financial Accounting Standards Board, or FASB, issued ASU No. 2013-04, "
Liabilities (Topic 405): Obligations Resulting from Joint and Several Liability Arrangements for which the Total Amount of the Obligation Is Fixed at the Reporting Date
." This ASU addresses the recognition, measurement, and disclosure of certain obligations resulting from joint and several arrangements including debt arrangements, other contractual obligations, and settled litigation and judicial rulings. The ASU is effective for public entities for fiscal years, and interim periods within those years, beginning after December 15, 2013.
In March 2013, the FASB issued ASU No. 2013-05, "
Foreign Currency Matters (Topic 830): Parent's Accounting for the Cumulative Translation Adjustment upon Derecognition of Certain Subsidiaries or Groups of Assets within a Foreign Entity or of an Investment in a Foreign Entity
." This ASU addresses the accounting for the cumulative translation adjustment when a parent either sells a part or all of its investment in a foreign entity or no longer holds a controlling financial interest in a subsidiary or group of assets that is a nonprofit activity or a business within a foreign entity. The guidance outlines the events when cumulative translation adjustments should be released into net income and is intended by FASB to eliminate some disparity in current accounting practice. This ASU is effective prospectively for fiscal years, and interim periods within those years, beginning after December 15, 2013.
In March 2013, the FASB issued ASU 2013-07,
“Presentation of Financial Statements (Topic 205): Liquidation Basis of Accounting.”
The amendments require an entity to prepare its financial statements using the liquidation basis of accounting when liquidation is imminent. Liquidation is imminent when the likelihood is remote that the entity will return from liquidation and either (a) a plan for liquidation is approved by the person or persons with the authority to make such a plan effective and the likelihood is remote that the execution of the plan will be blocked by other parties or (b) a plan for liquidation is being imposed by other forces (for example, involuntary bankruptcy). If a plan for liquidation was specified in the entity’s governing documents from the entity’s inception (for example, limited-life entities), the entity should apply the liquidation basis of accounting only if the approved plan for liquidation differs from the plan for liquidation that was specified at the entity’s inception. The amendments require financial statements prepared using the liquidation basis of accounting to present relevant information about an entity’s expected resources in liquidation by measuring and presenting assets at the amount of the expected cash proceeds from liquidation. The entity should include in its presentation of assets any items it had not previously recognized under U.S. GAAP but that it expects to either sell in liquidation or use in settling liabilities (for example, trademarks). The amendments are effective for entities that determine liquidation is imminent during annual reporting periods beginning after December 15, 2013, and interim reporting periods therein. Entities should apply the requirements prospectively from the day that liquidation becomes imminent. Early adoption is permitted.
Management does not believe that any other recently issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on the accompanying financial statements.
Note 3 – Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates continuity of operations, realization of assets, and liquidation of liabilities in the normal course of business.
As reflected in the accompanying consolidated financial statements, the Company had an accumulated deficit at March 31, 2013, a net loss and net cash used in operating activities for the fiscal year then ended. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
While the Company is attempting to generate sufficient revenues, the Company’s cash position may not be sufficient enough to support the Company’s daily operations. Management intends to raise additional funds by way of a public or private offering. Management believes that the actions presently being taken to further implement its business plan and generate sufficient revenues provide the opportunity for the Company to continue as a going concern. While the Company believes in the viability of its strategy to generate sufficient revenues and in its ability to raise additional funds, there can be no assurances to that effect. The ability of the Company to continue as a going concern is dependent upon the Company’s ability to further implement its business plan and generate sufficient revenues.
The consolidated financial statements do not include any adjustments related to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
Note 4 – Prepaid Expenses
Prepaid expenses consisted of the following:
|
|
|
March 31, 2013
|
|
|
March 31, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
Prepaid research and development
|
|
$
|
25,546
|
|
|
$
|
128,445
|
|
|
|
|
|
|
|
|
|
|
|
|
Prepaid rent
|
|
|
6,667
|
|
|
|
21,250
|
|
|
|
|
|
|
|
|
|
|
|
|
Retainer
|
|
|
451
|
|
|
|
15,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
|
|
|
432
|
|
|
|
4,179
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
33,096
|
|
|
$
|
168,874
|
|
Note 5 – Property and Equipment
Property and equipment, stated at cost, less accumulated depreciation consisted of the following:
|
|
Estimated Useful Life (Years)
|
|
March 31, 2013
|
|
|
March 31, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment
|
5
|
|
$
|
7,925
|
|
|
$
|
3,036
|
|
|
|
|
|
|
|
|
|
|
|
Less accumulated depreciation
|
|
|
|
(1,234
|
)
|
|
|
(-
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
6,691
|
|
|
$
|
3,036
|
|
Depreciation Expense
The Company acquired furniture and fixture near the end of February 2012 and started to depreciate as of April 1, 2012. Depreciation expense for the fiscal year ended March 31, 2013 was $1,234.
Note 6 – Acquired Technology
On July 5, 2012, the Company acquired the rights to certain technology from Technew Technology Limited in exchange for 3,000,000 restricted shares of the Company's common stock. These restricted shares were valued at $0.79 per share discounted at 69% taking into consideration its restricted nature and lack of liquidity and consistent trading in the market for a total value of $1,635,300, which was recorded as acquired technology and amortized on a straight-line basis over the acquired technology's estimated useful life of fifteen (15) years.
|
|
Estimated Useful Life (Years)
|
|
March 31, 2013
|
|
|
March 31, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Technology right
|
15
|
|
$
|
1,635,300
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
Less accumulated amortization
|
|
|
|
(81,765
|
)
|
|
|
(-
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,553,535
|
|
|
$
|
-
|
|
Amortization Expense
Amortization expense for the fiscal year ended March 31, 2013 was $81,765.
Note 7 – Website Development Costs
Website development costs, stated at cost, less accumulated amortization consisted of the following:
|
|
Estimated Useful Life (Years)
|
|
March 31, 2013
|
|
|
March 31, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Website development costs
|
5
|
|
$
|
5,315
|
|
|
$
|
5,315
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated amortization
|
|
|
|
(1,869
|
)
|
|
|
(801
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
3,446
|
|
|
$
|
4,514
|
|
Amortization expense
Amortization expense was $1,068 and $801 for the fiscal year ended March 31, 2013 and for the period from April 11, 2011 (inception) through December 31, 2012, respectively.
Note 8 – Related Party Transactions
Related parties
Related parties with whom the Company had transactions are:
|
Related Parties
|
|
Relationship
|
|
|
|
|
|
George Blankenbaker
|
|
President and significant stockholder of the Company
|
|
|
|
|
|
Leverage Investments LLC
|
|
An entity owned and controlled by the president and significant stockholder of the Company
|
|
|
|
|
|
Technew Technology Limited
|
|
Non-controlling interest holder
|
|
|
|
|
|
Growers Synergy Pte Ltd.
|
|
An entity owned and controlled by the president and significant stockholder of the Company
|
|
|
|
|
|
Guangzhou Health Technology Development Company Limited
|
|
An entity owned and controlled by Non-controlling interest holder
|
Advances from
Stockholder
From time to time, stockholder of the Company advances funds to the Company for working capital purpose. Those advances are unsecured, non-interest bearing and due on demand.
Lease of Certain Office Space from
Leverage Investments, LLC
The Company leases certain office space with Leverage Investments, LLC for $500 per month on a month-to-month basis since July 1, 2011. For the fiscal year ended March 31, 2013 and for the period from April 11, 2011 (inception) through December 31, 2012, the Company recorded $6,000 and $4,500 in rent expenses due Leverage Investment LLC, respectively.
Farm Management and Off-Take Agreement with Growers Synergy Pte Ltd.
For the period from July 1, 2011 through October 31, 2011, the Company engaged Growers Synergy Pte Ltd. to provide farm management services on a month-to-month basis, at $20,000 per month.
On November 1, 2011, the Company entered into a Management and Off-Take Agreement (the “Management Agreement”) with Growers Synergy Pte Ltd. (“GSPL”), a Singapore corporation owned and controlled by the president and major stockholder of the Company. Under the terms of the Management Agreement, the Company will engage GSPL to supervise the Company’s farm management operations, recommend quality farm management programs for stevia cultivation, assist in the hiring of employees and provide training to help the Company meet its commercialization targets, develop successful models to propagate future agribusiness services, and provide back-office and regional logistical support for the development of proprietary stevia farm systems in Vietnam, Indonesia and potentially other countries. GSPL will provide services for a term of two (2) years from the date of signing, at $20,000 per month. The Management Agreement may be terminated by the Company upon 30 day notice. In connection with the Management Agreement, the parties agreed to enter into an off-take agreement whereby GSPL agreed to purchase all of the non-stevia crops produced at the Company’s GSPL supervised farms.
Farm management services provided by Growers Synergy Pte Ltd. is as follows:
|
|
|
For the Fiscal Year
Ended
March 31, 2013
|
|
|
For the Period from
April 11, 2011 (inception)
through
March 31, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
Farm management services – related parties
|
|
$
|
240,000
|
|
|
$
|
180,000
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
240,000
|
|
|
$
|
180,000
|
|
Future minimum payments required under this agreement were as follows:
|
Fiscal Year Ending March 31:
|
|
|
|
|
|
|
|
|
|
|
|
2014
|
|
$
|
140,000
|
|
|
|
|
|
|
|
|
|
$
|
140,000
|
|
Cash Commitment in Connection with the Operations of Stevia Technew
On July 5, 2012, Stevia Asia, entered into a Cooperative Agreement (the "Cooperative Agreement") with Technew Technology Limited ("Technew"), a company incorporated under the companies ordinance of Hong Kong and an associate of Guangzhou Health China Technology Development Company Limited, and Zhang Jia, a Chinese citizen (together with Technew, the "Partners") pursuant to which Stevia Asia and Partners have agreed to make Stevia Technew, a joint venture, of which Stevia Asia legally and beneficially owns 70% shares (representing 70% of the issued shares) and Technew legally and beneficially owns 30% shares (representing 30% of the of the issued shares). The Partners will be responsible for managing Stevia Technew and Stevia Asia has agreed to provide $200,000 per month, up to a total of $2,000,000 in financing, subject to the performance of Stevia Technew and Stevia Asia's financial capabilities.
For the fiscal year ended March 31, 2013, Stevia Asia provided Stevia Technew $230,000, all of which has been paid to Guangzhou Health and expended and recorded as farm management services - related parties.
Note 9 – Convertible Notes Payable
On February 14, 2011, the Company issued a convertible note in the amount of $250,000 with interest at 10% per annum due one (1) year from the date of issuance. On October 4, 2011, the note holder converted the entire principal of $250,000 and accrued interest through the date of conversion of $15,890.41 at $0.25 per share to 1,000,000 shares and 63,561 shares of the Company’s common stock, respectively.
On June 23, 2011, the Company issued a convertible note in the amount of $100,000 with interest at 10% per annum due one (1) year from the date of issuance. On October 4, 2011, the note holder converted the entire principal of $100,000 and accrued interest through the date of conversion of $2,821.92 at $0.25 per share to 400,000 shares and 11,288 shares of the Company’s common stock.
On October 4, 2011, the Company issued a convertible note in the amount of $150,000 with interest at 10% per annum due one (1) year from the date of issuance. On January 18, 2012, the note holder converted the entire principal of $150,000 and accrued interest through the date of conversion of $4,356 at $0.25 per share to 617,425 shares of the Company’s common stock.
(i) February 26, 2013 issuance of convertible notes with warrants
On February 26, 2013, the Company entered into two (2) 12% convertible notes payable of $350,000 in aggregate (“Convertible Notes”) with two investors (the “Payees”) maturing on September 30, 2013. The Payees have the option to convert the outstanding notes and interest due into the Company’s common shares at $0.25 per share at any time prior to September 30, 2013. In connection with the issuance of the Convertible Notes, the Company granted to the Payees a warrant to purchase 1,400,000 common shares exercisable at $0.25 per share expiring three (3) years from the date of issuance.
The Company estimated the relative fair value of these warrants on the date of grant, using the Black-Scholes option-pricing model with the following weighted-average assumptions:
|
Expected option life (year)
|
|
|
3.00
|
|
|
|
|
|
|
|
|
Expected volatility
|
|
|
74.53
|
%
|
|
|
|
|
|
|
|
Risk-free interest rate
|
|
|
0.37
|
%
|
|
|
|
|
|
|
|
Dividend yield
|
|
|
0.00
|
%
|
|
|
|
|
|
The relative fair value of these warrants granted, estimated on the date of grant, was $110,425 in aggregate, which was recorded as a discount to the convertible notes payable. After allocating the $110,425 portion of the proceeds to the warrants as a discount to the Convertible Note, an additional $113,925 was allocated to a beneficial conversion feature by crediting additional $113,925 to additional paid-in capital and debiting the same amount to the beneficial conversion feature. The Company amortizes the discount and beneficial conversion feature over the term of the Convertible Notes. The amortization of the discount and beneficial conversion feature amounted to $32,050 for the fiscal year ended March 31, 2013.
(ii) March 15, 2013 issuance of convertible note with warrant
On March 15, 2013, the Company cancelled a prior convertible note and entered into a 12% convertible note payable of $220,438, which is the total amount of the prior note principal and accrued interest, with the existing investor (the “Payee”), maturing on September 30, 2013. The Payee has the option to convert the outstanding note into the Company’s common shares at $0.25 per share at any time prior to payment in full of the principal balance of the Convertible Note. In connection with the issuance of the Convertible Note, the Company granted the Payee a warrant to purchase 881,753 common shares exercisable at $0.25 per share expiring three (3) years from the date of issuance.
The Company estimated the relative fair value of these warrants on the date of grant using the Black-Scholes option-pricing model with the following weighted-average assumptions:
|
Expected option life (year)
|
|
|
3.00
|
|
|
|
|
|
|
|
|
Expected volatility
|
|
|
75.11
|
%
|
|
|
|
|
|
|
|
Risk-free interest rate
|
|
|
0.40
|
%
|
|
|
|
|
|
|
|
Dividend yield
|
|
|
0.00
|
%
|
|
|
|
|
|
The relative fair value of these warrants was $98,095, which was recorded as a discount to the convertible note payable. After allocating the $98,095, portion of the proceeds to the warrants as a discount to the Convertible Note, the effective conversion price of the convertible notes payable was lower than the market price at the date of issuance and per calculation the remaining balance of the face amount was allocated to a beneficial conversion feature by crediting $122,343 to additional paid-in capital and debiting the same amount to the beneficial conversion feature. The Company amortizes the discount and beneficial conversion feature over the term of the Convertible Note and began to amortize the amount from April 1, 2013.
Convertible notes payable consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
March 31, 2013
|
|
|
March 31, 2012
|
|
|
|
|
|
|
|
|
|
|
On November 16, 2011, the Company issued a convertible note in the amount of $250,000 with interest at 10% per annum due one (1) year from the date of issuance with the conversion price to be the same as the private placement price on a per share basis provided the Company completes a private placement with gross proceeds of at least $100,000. On July 6, 2012, the note holder converted the entire principal of $250,000 and accrued interest through the date of conversion of $15,959 to 319,607 shares of the Company’s common stock at $0.83 per share.
|
$
|
-
|
|
$
|
250,000
|
|
|
|
|
|
|
|
|
|
|
On January 16, 2012, the Company issued a convertible note in the amount of $250,000 with interest at 10% per annum due one (1) year from the date of issuance with the conversion price to be the same as the private placement price on a per share basis provided the Company completes a private placement with gross proceeds of at least $100,000. On July 6, 2012, the note holder converted the entire principal of $250,000 and accrued interest through the date of conversion of $11,781 to 314,586 shares of the Company’s common stock at $0.83 per share.
|
|
-
|
|
|
250,000
|
|
|
On March 7, 2012, the Company issued a convertible note in the amount of $200,000 with interest at 10% per annum due one (1) year from the date of issuance with the conversion price to be the same as the next private placement price on a per share basis provided that the Company completes a private placement with gross proceeds of at least $100,000. On August 6, 2012, the Company completed the very next private placement at $0.46875 per share with gross proceeds of at least $100,000. On March 15, 2013, the above note was cancelled and reissued with a new convertible note consisting of the prior principal amount and all the accrued unpaid interest for a total amount of $220,438 with interest at 12% per annum due on September 30, 2013 with the conversion price $0.25 per share
|
|
220,438
|
|
|
200,000
|
|
|
|
|
|
|
|
|
|
|
On May 30, 2012, the Company issued a convertible note in the amount of $200,000 with interest at 10% per annum due one (1) year from the date of issuance with the conversion price to be the same as the next private placement price on a per share basis provided that the Company completes a private placement with gross proceeds of at least $100,000. On August 6, 2012, the Company completed the very next private placement at $0.46875 per share with gross proceeds of at least $100,000.
|
|
200,000
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
On February 26, 2013, the Company issued two (2) convertible notes in the amount of $250,000 and $100,000, respectively, for an aggregate of $350,000 with interest at 12% per annum, due on September 30, 2013, with the conversion price at $0.25 per share. In connection with the issuance of the convertible notes, the Company issued to both notes holders a warrant to purchase 1,000,000 shares and 400,000 shares, respectively, in the aggregate of 1,400,000 shares of the Company’s common stock.
|
|
350,000
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
Sub-total: convertible notes payable
|
|
770,438
|
|
|
700,000
|
|
|
|
|
|
|
|
|
|
|
Discount representing (i) the relative fair value of the warrants issued and (ii) the beneficial conversion features
|
|
(444,788
|
)
|
|
-
|
|
|
|
|
|
|
|
|
|
|
Accumulated amortization of discount on convertible notes payable
|
|
32,050
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
Remaining discount
|
|
(412,738
|
)
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
$
|
357,770
|
|
$
|
700,000
|
|
Note 10 – Derivative Instruments and the Fair Value of Financial Instruments
(i)
Warrants Issued on August 6, 2012
Description of Warrants and Fair Value on Date of Grant
On August 6, 2012, the Company issued (i) warrants to purchase 1,066,667 shares of the Company’s common stock to the investors (the “investors warrants”) and (ii) warrants to purchase 85,333 shares of the Company's common stock to the placement agent (the "agent warrants") with an exercise price of $0.6405 per share, subject to certain adjustments, pursuant to Section 3(b) Subsequent Equity Sales of the SPA, expiring five (5) years from the date of issuance. The Company issued the warrants on February 26 and March 15, 2013 with an exercise price of $0.25 per share. Pursuant to Section 3(b) Subsequent Equity Sales of the SPA those warrants’ exercise price was reset to $0.25 per share and the number of warrant shares was increased to 2,732,801 and 218,623, respectively, for a total of 2,951,424.
Derivative Analysis
The exercise price of the August 6, 2012 warrants and the number of shares issuable upon exercise is subject to reset adjustment in the event of stock splits, stock dividends, recapitalization, most favored nation status and similar corporate events. Pursuant to Section 3(b) Subsequent Equity Sales of the SPA, if the Company issues any common stock or securities other than the excepted issuances, to any person or entity at a purchase or exercise price per share less than the share purchase price of the August 6, 2012 Unit Offering without the consent of the subscriber holding purchased shares, warrants or warrant shares of the August 6, 2012 Unit Offering, then the subscriber shall have the right to apply the lowest such purchase price or exercise price of the offering or sale of such new securities to the purchase price of the purchased shares then held by the subscriber (and, if necessary, the Company will issue additional shares), the reset adjustments are also referred to as full reset adjustments.
Because these warrants have full reset adjustments tied to future issuances of equity securities by the Company, they are subject to derivative liability treatment under Section 815-40-15 of the FASB Accounting Standard Codification (“Section 815-40-15”) (
formerly FASB Emerging Issues Task Force (“EITF”) Issue No. 07-5: Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity's Own Stock (“EITF 07-5”)
). Section 815-40-15 became effective on January 1, 2009 and the Warrants issued in the August 6, 2012 Unit Offering have been measured at fair value using a Lattice model at each reporting period with gains and losses from the change in fair value of derivative liabilities recognized on the consolidated statement of income and comprehensive income.
Valuation of Derivative Liability
(a)
Valuation Methodology
The Company’s August 6, 2012 warrants do not trade in an active securities market, as such, the Company developed a Lattice model that values the derivative liability of the warrants based on a probability weighted discounted cash flow model. This model is based on future projections of the various potential outcomes. The features that were analyzed and incorporated into the model included the exercise feature and the full ratchet reset.
Based on these features, there are two primary events that can occur; the Holder exercises the Warrants or the Warrants are held to expiration. The model analyzed the underlying economic factors that influenced which of these events would occur, when they were likely to occur, and the specific terms that would be in effect at the time (i.e. stock price, exercise price, volatility, etc.). Projections were then made on these underlying factors which led to a set of potential scenarios. As the result of the large Warrant overhang we accounted for the dilution affects, volatility and market cap to adjust the projections.
Probabilities were assigned to each of these scenarios based on management projections. This led to a cash flow projection and a probability associated with that cash flow. A discounted weighted average cash flow over the various scenarios was completed to determine the value of the derivative warrant liability.
(b)
Valuation Assumptions
The Company’s 2012 derivative warrants were valued at each period ending date with the following assumptions:
|
·
|
The stock price would fluctuate with the Company projected volatility.
|
|
·
|
The stock price would fluctuate with an annual volatility. The projected volatility curve was based on historical volatilities of the Company for the valuation periods.
|
|
·
|
The Holder would exercise the warrant as they become exercisable (effective registration is projected 4 months from issuance and the earliest exercise is projected 180 days from issuance) at target prices of 2 times the higher of the projected reset price or stock price.
|
|
·
|
The Holder would exercise the warrant at maturity if the stock price was above the project reset prices.
|
|
·
|
A 100% probability of a reset event and a projected financing each quarter for 3 years at prices approximating 93% of market
|
|
·
|
The 2,732,801 Investor Warrants with an exercise price of $0.25 per share (1,066,667 Investor Warrants with an exercise price of $0.6405 per share prior to the occurrence of the February 26, 2013 reset event) is projected to reset to $0.095 at maturity; and the 218,623 Placement Agent Warrants with an exercise price of $0.25 per share (85,333 Placement Agent Warrants with an exercise price of $0.6405 per share prior to the occurrence of the February 26, 2013 reset event) is projected to reset to $0.095 at maturity.
|
|
·
|
No warrants have been exercised or expired.
|
|
·
|
The projected volatility curve for the valuation dates was:
|
|
|
|
1 Year
|
|
|
2 Tear
|
|
|
3 Year
|
|
|
4 Year
|
|
|
5 Year
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
August 6, 2012
|
|
129%
|
|
|
178%
|
|
|
218%
|
|
|
252%
|
|
|
281%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2012
|
|
127%
|
|
|
173%
|
|
|
211%
|
|
|
244%
|
|
|
272%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2013
|
|
122%
|
|
|
167%
|
|
|
205%
|
|
|
236%
|
|
|
264%
|
|
(c)
Fair Value of Derivative Warrants
The table below provides a summary of the fair value of the derivative warrant liability and the changes in the fair value of the derivative warrants to purchase 2,951,424 shares of the Company’s common stock, including net transfers in and/or out, of derivative warrants measured at fair value on a recurring basis using significant unobservable inputs (Level 3).
|
|
|
Fair Value Measurement Using Level 3 Inputs
|
|
|
|
Derivative warrants
|
|
|
|
|
|
|
|
Assets (Liability)
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
Balance, August 6, 2012
|
|
$
|
(411,805
|
)
|
|
$
|
(411,805
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total gains or losses (realized/unrealized) included in:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
231,521
|
|
|
|
231,521
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income (loss)
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases, issuances and settlements
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Transfers in and/or out of Level 3
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, August 6, 2012
|
|
|
(180,284
|
)
|
|
|
(180,284
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total gains or losses (realized/unrealized) included in:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
(305,829
|
)
|
|
|
(305,829
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income (loss)
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases, issuances and settlements
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Transfers in and/or out of Level 3
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2013
|
|
$
|
(486,113
|
)
|
|
$
|
(486,113
|
)
|
(d)
Warrants Outstanding
As of March 31, 2013 no warrants have been exercised and warrants to purchase 2,951,424shares of Company common stock remain outstanding.
The table below summarizes the Company’s derivative warrant activity:
|
|
|
2012 Warrant Activities
|
|
APIC
|
|
(Gain) Loss
|
|
|
|
|
Derivative
Shares
|
|
Non-derivative
Shares
|
|
Total Warrant
Shares
|
|
Fair Value of
Derivative
Warrants
|
|
Reclassification
of Derivative
Liability
|
|
Change in
Fair Value of
Derivative
Liability
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative warrant at August 6, 2012
|
|
|
1,152,000
|
|
|
-
|
|
|
1,152,000
|
|
|
(411,805
|
)
|
|
-
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mark to market
|
|
|
|
|
|
|
|
|
|
|
|
231,521
|
|
|
|
|
|
(231,521
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative warrant at September 30, 2012
|
|
|
1,152,000
|
|
|
-
|
|
|
1,152,000
|
|
|
(180,284
|
)
|
|
|
|
|
(231,521
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mark to market
|
|
|
|
|
|
|
|
|
|
|
|
(73,723
|
)
|
|
|
|
|
(73,723
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative warrant at December 31, 2012
|
|
|
1,152,000
|
|
|
-
|
|
|
1,152,000
|
|
|
(106,561
|
)
|
|
|
|
|
(305,244
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reset of warrant shares
|
|
|
1,799,424
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mark to market
|
|
|
|
|
|
|
|
|
|
|
|
(379,552
|
)
|
|
|
|
|
379,552
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative warrant at March 31, 2013
|
|
|
2,951,424
|
|
|
-
|
|
|
2,951,424
|
|
|
(486,113
|
)
|
|
|
|
|
74,308
|
|
(ii) Warrant Activities
The table below summarizes the Company’s warrant activities through March 31, 2013:
Summary of the Company’s Warrant Activities
The table below summarizes the Company’s warrant activities:
|
|
|
Number of
|
|
|
Exercise Price
|
|
|
Weighted Average
|
|
|
Fair Value at Date
|
|
|
Aggregate
|
|
|
|
|
Warrant Shares
|
|
|
Range
Per Share
|
|
|
Exercise Price
|
|
|
of Issuance
|
|
|
Intrinsic
Value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2012
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
5,233,177
|
|
|
|
0.25
|
|
|
|
0.25
|
|
|
|
620,350
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Canceled
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expired
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2013
|
|
|
5,233,177
|
|
|
$
|
0.25
|
|
|
$
|
0.25
|
|
|
$
|
620,350
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Earned and exercisable, March 31, 2013
|
|
|
5,233,177
|
|
|
$
|
0.25
|
|
|
$
|
0.25
|
|
|
$
|
620,350
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unvested, March 31, 2013
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
The following table summarizes information concerning outstanding and exercisable warrants as of
March 31, 2013
:
|
|
|
Warrants Outstanding
|
|
Warrants Exercisable
|
|
|
Range of Exercise Prices
|
|
Number
Outstanding
|
|
Average Remaining
Contractual Life
(in years)
|
|
Weighted Average Exercise Price
|
|
Number
Exercisable
|
|
Average Remaining Contractual Life
(in years)
|
|
Weighted Average Exercise Price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$0.25
|
|
|
5,233,177
|
|
|
3.73
|
|
$
|
0.25
|
|
|
5,233,177
|
|
|
3.73
|
|
$
|
0.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$0.25
|
|
|
5,233,177
|
|
|
3.73
|
|
$
|
0.25
|
|
|
5,233,177
|
|
|
3.73
|
|
$
|
0.25
|
|
Note 14 – Commitments and Contingencies
Supply Agreement – between Stevia Ventures International Ltd. and Asia Stevia Investment Development Company Ltd.
On April 12, 2011, Stevia Ventures International Ltd, a subsidiary of the Company entered into a Supply Agreement (the “Supply Agreement”) with Asia Stevia Investment Development Company Ltd (“ASID”), a foreign-invested limited liability company incorporated in Vietnam.
(I)
Scope of Services
Under the terms of the Agreement, the Company engaged ASID to plant the Stevia Seedlings and supply the Products only to the Company to the exclusion of other customers and the Company is desirous to purchase the same, on the terms and conditions as set out in this Agreement produce Products and the Company purchase the Products from ASID.
(ii)
Term
This Agreement shall come into force on the date of signing and, subject to earlier termination pursuant to certain clauses specified in the Agreement, shall continue in force for a period of three (3) years ("Term") and thereafter automatically renew on its anniversary each year for an additional period of one (1) year ("Extended Term").
(iii)
Purchase Price
ASID and the Company shall review and agree, on or before September 30th of each year, on the quantity of the Products to be supplied by ASID to the Company in the forthcoming year and ASID shall provide the Company with prior written notice at any time during the year following the revision if it has reason to believe that it would be unable to fulfill its forecast volumes under this clause.
Supply Agreement – between Stevia Ventures International Ltd. And Stevia Ventures Corporation
On April 12, 2011, Stevia Ventures International Ltd, a subsidiary of the Company also entered into a Supply Agreement (the “Supply Agreement”) with Stevia Ventures Corporation (“SVC”), a foreign-invested limited liability company incorporated in Vietnam.
(i)
Scope of Services
Under the terms of the Agreement, the Company engaged SVC to plant the Stevia Seedlings and supply the Products only to the Company to the exclusion of other customers and the Company is desirous to purchase the same, on the terms and conditions as set out in this Agreement produce Products and the Company purchase the Products from SVC.
(ii)
Term
This Agreement shall come into force on the date of signing and, subject to earlier termination pursuant to certain clauses specified in the Agreement, shall continue in force for a period of three (3) years ("Term") and thereafter automatically renew on its anniversary each year for an additional period of one (1) year ("Extended Term").
(iii)
Purchase Price
SVC and the Company shall review and agree, on or before September 30
th
,
of each Year on the quantity of the Products to be supplied by SVC to the Company in the forthcoming year and SVC shall provide the Company with prior written notice at any time during the year following the revision if it has reason to believe that it would be unable to fulfill its forecast volumes under this clause.
Consulting Agreement – Dorian Banks
Entry into Consulting Agreement
On July 1, 2011 the Company entered into a consulting agreement (the “Consulting Agreement”) with Dorian Banks (“Banks”).
(i)
Scope of Services
Under the terms of the Consulting Agreement, the Company engaged the Consultant to provide advice in general business development, strategy, assistance with new business and land acquisition, introductions, and assistance with Public Relations (“PR”) and Investor Relations (“IR”).
(ii)
Term
The term of this Agreement shall be six (6) months, commencing on July 1, 2011 and continue until December 31, 2011. This Agreement may be terminated by either the Company or the Consultant at any time prior to the end of the Consulting Period by giving thirty (30) days written notice of termination. Such notice may be given at any time for any reason, with or without cause. The Company will pay Consultant for all Service performed by Consultant through the date of termination.
(iii)
Compensation
The Company shall pay the Consultant a fee of $3,000 per month.
Extension of the Consulting Agreement
On December 30, 2011, the Consulting Agreement was extended with the same terms and conditions to expire on March 31, 2013.
Expiration of the Consulting Agreement
The Consulting Agreement expired on March 31, 2013 with no further extension.
Summary of the Consulting Fees
For the fiscal year ended March 31, 2013 and for the period from April 11, 2011 (inception) through March 31, 2012, the Company recorded $41,250 and $27,000 in consulting fees under the Consulting Agreement, respectively.
Financing Consulting Agreement – David Clifton
Entry into Financial Consulting Agreement
On July 1, 2011 the Company entered into a consulting agreement (the “Consulting Agreement”) with David Clifton ( “Clifton”).
(i)
Scope of Services
Under the terms of the Consulting Agreement, the Company engaged Clifton to introduce interested investors to the Company, advise the Company on available financing options and provide periodic updates on the stevia sector and provide insights and strategies for the Company to undertake.
(ii)
Term
The term of this Agreement shall be six (6) months, commencing on July 1, 2011 and continuing until December 31, 2011. This Agreement may be terminated by either the Company or Clifton at any time prior to the end of the consulting period by giving thirty (30) days written notice of termination. Such notice may be given at any time for any reason, with or without cause. The Company will pay Clifton for all service performed by him through the date of termination.
On December 31, 2011, the financial consulting agreement expired.
(iii)
Compensation
The Company shall pay Clifton a fee of $3,000 per month.
Summary of the Consulting Fees
For the period from April 11, 2011 (inception) through December 31, 2011, the Company recorded $18,000 in financing cost under this Financing Consulting Agreement.
Entry into Engagement Agreement – Garden State Securities Inc.
On June 18, 2012, the Company entered into an engagement agreement (the “Agreement”) with Garden State Securities Inc. (“GSS”) for GSS to act as a selling/placement agent for the Company.
(i)
Scope of Services
Under the terms of the Agreement, the Company engaged GSS to review the business and operations of the Company and its historical and projected financial condition, advise the Company on a “best efforts” Private Placement offering of debt or equity securities to fulfill the Company’s business plan, and contacts for the Company possible financing sources.
(ii)
Term
GSS shall act as the Company’s exclusive placement agent for the period of the later of; (i) 60 days from the execution of the term sheet; or (ii) the final termination date of the securities financing (the “Exclusive Period”). GSS shall act as the Company’s non-exclusive placement agent after the Exclusive Period until terminated.
(iii) Compensation
The Company agrees to pay to GSS at each full or incremental closing of any equity financing, convertible debt financing, debt conversion or any instrument convertible into the Company’s common stock (the “Securities Financing”) during the Exclusive Period; (i) a cash transaction fee in the amount of 8% of the amount received by the Company under the Securities Financing; and (ii) warrants (the “Warrants”) with “piggy back” registration rights, equal to 8% of the stock issued in the Securities Financing at an exercise price equal to the investors’ warrant exercise price of the Securities Financing or the price of the Securities Financing if no warrants are issued to investors. The Company will also pay, at closing, the expense of GSS’s legal counsel pursuant to the Securities Financing and/or Shelf equal to $25,000 for Securities Financing and/or Shelf resulting in equal to or greater than $500,000 of gross proceeds to the Company, and $18,000 for a Securities Financing and/or Shelf resulting in less than $500,000 of gross proceeds to the Company. In addition, the Company shall cause, at its cost and expense, the “Blue sky filing” and Form D in due and proper form and substance and in a timely manner.
Note 11 – Equity
Shares Authorized
Upon formation the total number of shares of common stock which the Company is authorized to issue is One Hundred Million (100,000,000) shares, par value $0.001 per share.
Common Stock
Reverse Acquisition Transaction
Immediately prior to the Share Exchange Transaction on June 23, 2011, the Company had 79,800,000 common shares issued and outstanding. Simultaneously with the Closing of the Share Exchange Agreement, on the Closing Date, Mohanad Shurrab, a shareholder and, as of the Closing Date, the Company’s former Director, President, Treasurer and Secretary, surrendered 33,000,000 shares of the Company’s common stock to the Company for cancellation.
As a result of the Share Exchange Agreement, the Company issued 12,000,000 common shares for the acquisition of 100% of the issued and outstanding shares of Stevia Ventures International Ltd. Of the 12,000,000 common shares issued in connection with the Share Exchange Agreement, 6,000,000 of such shares are being held in escrow (“Escrow Shares”) pending the achievement by the Company of certain post-Closing business milestones (the “Milestones”), pursuant to the terms of the Make Good Escrow Agreement, between the Company, Greenberg Traurig, LLP, as escrow agent and the Ventures’ Stockholder (the “Escrow Agreement”).
Make Good Agreement Shares
(i)
Duration of Escrow Agreement
The Make Good Escrow Agreement shall terminate on the sooner of (i) the distribution of all escrow shares, or (ii) December 31, 2013.
(ii)
Disbursement of Make Good Shares
Upon achievement of any Milestone on or before the date associated with such Milestone on Exhibit A, the Company shall promptly provide written notice to the Escrow Agent and the Selling Shareholder of such achievement (each a “COMPLETION NOTICE”). Upon the passage of any Milestone date set forth on Exhibit A for which the Company has not achieved the associated Milestone, the Company shall promptly provide written notice to the Escrow Agent and the Selling Shareholder of such failure to achieve the milestone (each a “NON-COMPLETION NOTICE”).
(iii)
Exhibit A – Schedule of Milestones
|
Milestones
|
|
|
Completion Date
|
|
|
Number of
Escrow
Shares
|
|
|
|
|
|
|
|
|
|
|
|
I.
(1)Enter into exclusive international license agreement for all Agro Genesis intellectual property and products as it applies to stevia
(2)Enter into cooperative agreements to work with Vietnam Institutes (a) Medical Plant Institute in Hanoi; (b) Agricultural Science Institute of Northern Central Vietnam
(3)Enter into farm management agreements with local growers including the Provincial and National projects;
(4)Take over management of three existing nurseries
|
|
|
Within 180 days
of the Closing Date
|
|
|
3,000,000 shares
only if and when ALL four (4) milestones reached (*)
|
|
|
|
|
|
|
|
|
|
|
|
II. Achieve 100 Ha field trials and first test shipment of dry leaf
|
|
|
Within two (2) years
of the Closing Date
|
|
|
1,500,000 shares (**)
|
|
|
|
|
|
|
|
|
|
|
|
III. Test shipment of dry leaf to achieve minimum specs for contracted base price (currently $2.00 per kilogram)
|
|
|
Within two (2) years
of the Closing Date
|
|
|
1,500,000 shares (**)
|
|
|
*
|
On December 23, 2011, 3,000,000 out of the 6,000,000 Escrow Shares have been earned and released to Ventures stockholder upon achievement of the First Milestone within 180 days of June 23, 2011, the Closing Date associated with the First Milestone. These shares were valued at $0.25 per share or $750,000 on the date of release and recorded as compensation.
|
|
**
|
On June 23, 2013, the remaining 3,000,000 Escrow Shares have been earned to Ventures stockholder upon achievement of the Second and the Third Milestones within two (2) years of June 23, 2011, the Closing Date associated with the Milestones. These shares were valued at $0.20 per share or $600,000 on June 23, 2013 and recorded as compensation.
|
Common Shares Surrendered for Cancellation
On October 4, 2011, a significant stockholder of the Company, Mohanad Shurrab, surrendered another 3,000,000 shares of the Company’s common stock to the Company for cancellation. The Company recorded this transaction by debiting common stock at par of $3,000 and crediting additional paid-in capital of the same.
Common Shares Issued for Cash
On October 4, 2011 the Company sold 400,000 shares of its common stock to one investor at $0.25 per share or $100,000.
Common Shares Issued for Obtaining Employee and Director Services
On October 14, 2011 the Company issued 1,500,000 shares each to two (2) newly appointed members of the board of directors or 3,000,000 shares of its common stock in aggregate as compensation for future services. These shares shall vest with respect to 750,000 shares of restricted stock on each of the first two anniversaries of the date of grant, subject to the director’s continuous service to the Company as directors. These shares were valued at $0.25 per share or $750,000 on the date of grant and are being amortized over the vesting period of two (2) years or $93,750 per quarter. The Company recorded $187,500 in directors’ fees for the period from April 11, 2011 (inception) through March 31, 2012. The Company recorded $375,000 in directors’ fees for the fiscal year ended March 31, 2013.
Common Shares Issued
to Parties other than Employees for Acquiring Goods or Services
Equity Purchase Agreement and Related Registration Rights Agreement
(i)
Equity Purchase Agreement
On January 26, 2012, the Company entered into an equity purchase agreement (“Equity Purchase Agreement”) with Southridge Partners II, LP, a Delaware limited partnership (The “Investor”). Upon the terms and subject to the conditions contained in the agreement, the Company shall issue and sell to the Investor, and the Investor shall purchase, up to Twenty Million Dollars ($20,000,000) of its common stock, par value $0.001 per share.
At any time and from time to time during the Commitment Period, the period commencing on the effective date, and ending on the earlier of (i) the date on which investor shall have purchased put shares pursuant to this agreement for an aggregate purchase price of the maximum commitment amount, or (ii) the date occurring thirty six (36) months from the date of commencement of the commitment period. the Company may exercise a put by the delivery of a put notice, the number of put shares that investor shall purchase pursuant to such put shall be determined by dividing the investment amount specified in the put notice by the purchase price with respect to such put notice. However, that the investment amount identified in the applicable put notice shall not be greater than the maximum put amount and, when taken together with any prior put notices, shall not exceed the maximum commitment The purchase price shall mean 93% of the market price on such date on which the purchase price is calculated in accordance with the terms and conditions of this Agreement.
(ii)
Registration Rights Agreement
In connection with the Equity Purchase Agreement, on January 26, 2012, the Company entered into a registration rights agreement (“Registration Rights Agreement”) with Southridge Partners II, LP, a Delaware limited partnership (the “Investor”). To induce the investor to execute and deliver the equity purchase agreement which the Company has agreed to issue and sell to the investor shares (the “put shares”) of its common stock, par value $0.001 per share (the “common stock”) from time to time for an aggregate investment price of up to twenty million dollars ($20,000,000) (the “registrable securities”), the Company has agreed to provide certain registration rights under the securities act of 1933, as amended, and the rules and regulations thereunder, or any similar successor statute (collectively, “securities act”), and applicable state securities laws with respect to the registrable securities.
(iii)
Common Shares Issued Upon Signing
As a condition for the execution of this agreement by the investor, the company issued to the investor 35,000 shares of restricted common stock (the “restricted shares”) upon the signing of this agreement. The restricted shares shall have no registration rights. These shares were valued at $1.50 per share or $52,500 on the date of issuance and recorded as financing cost.
Marketing Service Agreement – Empire Relations Group, Inc.
On March 14, 2012 the Company entered into a consulting agreement (the “Consulting Agreement”) with Empire Relations Group, Inc. (“Empire”).
(i)
Scope of Services
Under the terms of the Consulting Agreement, the Company engaged Empire to introduce interested investors to the Company, advise the Company on available financing options, provide periodic updates on the stevia sector and provide insights and strategies for the Company to undertake.
(ii)
Term
The term of this agreement were consummated from the date hereof, and automatically terminated on May 30, 20 12.
(iii)
Compensation
For the term of this agreement, the consultant shall be paid an upfront, non-refundable, non-cancellable retainer fee of 27,500 restricted shares. For the purposes of this agreement, these shares shall be considered to be fully earned by March 31, 2012. These shares were valued at $1.39 per share or $38,225 on March 31, 2012, the date when they were earned.
Common Shares Issued in Connection with Entry into Technology Acquisition Agreement
On July 5, 2012, the Company entered into a Technology Acquisition Agreement (the "Technology Acquisition Agreement") with Technew Technology Limited (“Technew”), pursuant to which the Company acquired the rights to certain technology from Technew in exchange for 3,000,000 restricted shares of the Company's common stock. These restricted shares were valued at $0.79 per share discounted at 69% taking into consideration its restricted nature and lack of liquidity and consistent trading in the market or $1,635,300, which was recorded as acquired technology and amortized on a straight-line basis over the acquired technology's estimated useful life of fifteen (15) years.
Common Shares Issued to a Related Party
On July 5, 2012, the Company issued 500,000 restricted shares of its common shares to Growers Synergy Pte Ltd., a corporation organized under the laws of the Republic of Singapore ("Singapore"), owned and controlled by George Blankenbaker, the president, director and a significant stockholder of the Company ("Growers Synergy"), as consideration for services rendered by Growers Synergy to the Company. These restricted shares were valued at $0.79 per share discounted at 69% taking into consideration of its restricted nature and lack of liquidity and consistent trading in the market or $272,550 and included in the farm management services - related party in the consolidated statements of operations.
Sale of Equity Unit Inclusive of Common Stock and Warrants
Entry into Securities Purchase Agreement
On August 1, 2012, the Company entered into a Securities Purchase Agreement (the "SPA") with two (2) accredited institutional investors (the "Purchasers") to raise $500,000 in a private placement financing. On August 6, 2012, after the satisfaction of certain closing conditions, the Offering closed and the Company issued to the Purchasers: (i) an aggregate of 1,066,667
shares of the Company's common stock at $0.46875 per share and (ii) warrants to purchase 1,066,667 shares of the Company's common stock at an exercise price of $0.6405 expiring five (5) years from the date of issuance for a gross proceeds of $500,000.
At closing, the Company reimbursed the investor for legal fees of $12,500 and paid Garden State Securities, Inc,(“GSS”), who served as placement agent for the Company in the offering, (i) cash commissions equal to 8.0% of the gross proceeds received in the equity financing or $40,000, and (ii) a warrant to purchase 85,333 shares of the Company's common stock representing 8% of the Shares sold in the Offering with an exercise price of $0.6405 per share expiring five (5) years from the date of issuance (the "agent warrants") to GSS or its designee.
The units were sold at $0.46875 per unit consisting one common share and the warrant to purchase one (1) common share for gross proceeds of $500,000. In connection with the August 6, 2012 equity unit offering the Company paid (i) GSS cash commissions equal to 8.0% of the gross proceeds received in the equity financing, or $40,000 and (ii) $12,500 in legal fees and resulted in a net proceeds of $447,500.
The Company intended to use the net proceeds from the Offering to advance the Company's ability to execute its growth strategy and to aid in the commercial development of the recently announced launch of the Company's majority-owned subsidiary, Stevia Technew Limited.
Entry into Registration Rights Agreement
In connection with the equity financing on August 1, 2012, the Company also entered into a registration rights agreement with the Purchasers (the "rights agreement"). The Rights Agreement requires the Company to file a registration statement (the "Registration Statement") with the Securities and Exchange Commission (the "SEC") within thirty (30) days of the Company's entrance into the rights agreement (the "filing date") for the resale by the Purchasers of all of the Shares and all of the shares of common stock issuable upon exercise of the Warrants (the "registrable securities"). The Company filed the Registration Statement with the Securities and Exchange Commission (the "SEC") within thirty (30) days of the Company's entrance into the rights agreement.
The registration statement must be declared effective by the SEC within one hundred and twenty (120) days of the closing date of the Offering subject to certain adjustments. If the registration statement is not filed prior to the filing date, the Company will be required to pay certain liquidated damages, not to exceed in the aggregate 6% of the purchase price paid by the Purchasers pursuant to the SPA. The Registration Statement was declared effective by the SEC within one hundred and twenty (120) days of the closing date of the Offering subject to certain adjustments.
Warrants
Issuances of Warrants in Connection with Entry into Securities Purchase Agreement
On August 6, 2012, the Company issued (i) warrants to purchase 1,066,667 shares, in the aggregate, of the Company’s common stock to the investors with an exercise price of $0.6405 per share subject to certain adjustments per Section 3(b) Subsequent Equity Sales of the SPA expiring five (5) years from the date of issuance in connection with the sale of common shares. The exercise price and number of warrant shares were reset to $0.25 per share and 2,732,801 shares, respectively, due to the occurrence of the February 26, 2013 reset event.
Issuance of Warrants to the Placement Agent as Compensation
Garden State Securities, Inc. (the "GSS") served as the placement agent of the Company for the equity financing on August 1, 2012. Per the engagement agreement signed between GSS and the Company, in consideration for services rendered as the placement agent, the Company agreed to: (i) pay GSS cash commissions equal to 8.0% of the gross proceeds received in the equity financing, or $40,000, and (ii) issue to GSS or its designee, a warrant to purchase 85,333 shares of the Company's common stock representing 8% of the warrants sold in the Offering) with an exercise price of $0.6405 per share subject to certain adjustments per Section 3(b) Subsequent Equity Sales of the SPA expiring five (5) years from the date of issuance (the "agent warrants"). The agent warrants also provide for the same registration rights and obligations as set forth in the Rights Agreement with respect to the Warrants and Warrant Shares. The exercise price and number of warrant shares were reset to $0.25 per share and 2,732,801 shares, respectively, due to the occurrence of the February 26, 2013 reset event.
Note 12 – Non-controlling Interest
Non-controlling interest consisted of the following:
|
|
|
Contributed
and
additional
paid-in
capital
|
|
|
Earnings
and losses
|
|
|
Other
Comprehensive
Income
|
|
|
Total
non-controlling
interest
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at March 31, 2012
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current period earnings and losses
|
|
|
-
|
|
|
|
(214,082
|
)
|
|
|
-
|
|
|
|
(214,082
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at March 31, 2013
|
|
$
|
-
|
|
|
$
|
(214,082
|
)
|
|
$
|
-
|
|
|
$
|
(214,082
|
)
|
Note 13 – Research and Development
Agribusiness Development Agreement – Agro Genesis Pte Ltd.
Entry into Agribusiness Development Agreement
On July 16, 2011, the Company entered into an Agribusiness Development Agreement (the “Agribusiness Development Agreement”) with Agro Genesis Pte Ltd. (“AGPL”), a corporation organized under the laws of the Republic of Singapore expiring two (2) years from the date of signing.
Under the terms of the Agreement, the Company engaged AGPL to be the Company’s technology provider consultant for stevia propagation and cultivation in Vietnam, and potentially other countries for a period of two (2) years. AGPL will be tasked with developing stevia propagation and cultivation technology in Vietnam, recommend quality agronomic programs for stevia cultivation, harvest and post harvest, alert findings on stevia propagation and cultivation that may impact profitability and develop a successful model in Vietnam that can be replicated elsewhere (the “Project”). The Project will be on-site at stevia fields in Vietnam and will have a term of at least two (2) years. For its services, AGPL could receive a fee of up to 275,000 Singapore dollars, plus related expenses estimated at $274,000 as specified in Appendix A to the Agribusiness Development Agreement. Additionally, the Company will be AGPL’s exclusive distributor
for AGPL’s g'farm system (a novel crop production system) for stevia growing resulting from the Project. AGPL will receive a commission of no less than 2% of the price paid for crops other than stevia, from cropping systems that utilize the g'farm system resulting from the Project. All technology-related patents resulting from the Project will be jointly owned by AGPL and the Company, with the Company holding a right of first offer for the use and distribution rights to registered patents resulting from the Project.
Addendum to Agribusiness Development Agreement
On August 26, 2011, in accordance with Appendix A , 3(a), the Company and AGPL have mutually agreed to add to the current Project budget $100,000 per annum for one, on-site resident AGPL expert for 2 (two) years effective September 1, 2011, or $200,000 in aggregate for the term of the contract as specified in Appendix C. In-country accommodation for the resident expert will be born separately by the Company and is excluded from the above amount. The expert, Dr. Cho, Young-Cheol, Director, Life Sciences has been appointed and commenced on September 1, 2011.
Termination of Agribusiness Development Agreement
On March 31, 2012, the Company and AGPL mutually agreed to terminate the Agribusiness Development Agreement, effective immediately.
Lease of Agricultural Land
On December 14, 2011, the Company and Stevia Ventures Corporation (“Stevia Ventures”) entered into a Land Lease Agreement with Vinh Phuc Province People's Committee Tam Dao Agriculture & Industry Co., Ltd. pursuant to which Stevia Ventures has leased l0 hectares of land (the “Leased Property”) for a term expiring five (5) years from the date of signing.
The Company has begun development of a research facility on the Leased Property and has prepaid (i) the first year lease payment of $30,000 and (ii) the six month lease payment of $15,000 as security deposit, or $45,000 in aggregate upon signing of the agreement.
Future minimum payments required under this agreement at March 31, 2013 were as follows:
|
Fiscal Year Ending March 31:
|
|
|
|
|
|
|
|
|
|
|
|
2014
|
|
$
|
30,000
|
|
|
|
|
|
|
|
|
2015
|
|
|
30,000
|
|
|
|
|
|
|
|
|
2016
|
|
|
30,000
|
|
|
|
|
|
|
|
|
|
$
|
90,000
|
|
Supply and Cooperative Agreement – Guangzhou Health Technology Development Company Limited
Entry into Supply Agreement
On February 21, 2012, the Company entered into a Supply Agreement (the "Supply Agreement") with Guangzhou Health China Technology Development Company Limited, a foreign-invested limited liability company incorporated in the People's Republic of China (the "Guangzhou Health").
Under the terms of the Supply Agreement, the Company will sell dry stevia plant materials, including stems and leaves ("Product") exclusively to Guangzhou Health. For the first two years of the agreement, Guangzhou Health will purchase all Product produced by the Company. Starting with the third year of the agreement, the Company and Guangzhou Health will review and agree on the quantity of Product to be supplied in the forthcoming year, and Guangzhou Health will be obliged to purchase up to 130 percent of that amount. The specifications and price of Product will also be revised annually according to the mutual agreement of the parties. The term of the Supply Agreement is five years with an option to renew for an additional four years.
Entry into Cooperative Agreement
On February 21, 2012, the Company also entered into Cooperative Agreement (the “Cooperative Agreement”) with Guangzhou Health Technology Development Company Limited.
Under the terms of the Cooperative Agreement, the parties agree to explore potential technology partnerships with the intent of formalizing a joint venture to pursue the most promising technologies and businesses. The parties also agree to conduct trials to test the efficacy of certain technologies as applied specifically to the Company's business model as well as the marketability of harvests produced utilizing such technologies. Guangzhou Health will share all available information of its business structure and technologies with the Company, subject to the confidentiality provisions of the Cooperative Agreement. Guangzhou Health will also permit the Company to enter its premises and grow-out sites for purposes of inspection and will, as reasonably requested by the Company, supply without cost, random samples of products and harvests for testing.
Note 14 – Income Tax Provision
United States Income Tax
Stevia Corp is the parent Company which incorporated in the State of Nevada and is subjected to United States of America tax law.
Hong Kong SAR Income Tax
Stevia Asia Limited, a wholly-owned subsidiary of the Company, is registered in the Hong Kong Special Administrative Region (“HK SAR”) of the People’s Republic of China (“PRC”) and is subject to HK SAR tax law. Armco HK’s statutory income tax rate is 16.5% and there were no significant differences between income reported for financial reporting purposes and income reported for income tax purposes for the year ended March 31, 2013.
Stevia Technew Limited, a majority owned subsidiary of the Company, is registered in the Hong Kong Special Administrative Region (“HK SAR”) of the People’s Republic of China (“PRC”) and is subject to HK SAR tax law. Armco HK’s statutory income tax rate is 16.5% and there were no significant differences between income reported for financial reporting purposes and income reported for income tax purposes for the year ended March 31, 2013.
Deferred Tax Assets
At March 31, 2013, the Company has available for federal income tax purposes net operating loss (“NOL”) carry-forwards of $3,857,505 that may be used to offset future taxable income through the fiscal year ending March 31, 2033. No tax benefit has been reported with respect to these net operating loss carry-forwards in the accompanying financial statements since the Company believes that the realization of its net deferred tax asset of approximately $1,317,552 was not considered more likely than not and accordingly, the potential tax benefits of the net loss carry-forwards are fully offset by the full valuation allowance.
Deferred tax assets consist primarily of the tax effect of NOL carry-forwards. The Company has provided a full valuation allowance on the deferred tax assets because of the uncertainty regarding its realizability. The valuation allowance increased approximately $521,545 and $790,007 for the fiscal year ended March 31, 2013 and for the period from April 11, 2011 (inception) through March 31, 2012, respectively.
Components of deferred tax assets are as follows:
|
|
|
March 31, 2013
|
|
|
March 31, 2012
|
|
|
Net deferred tax assets – Non-current:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expected income tax benefit from NOL carry-forwards
|
|
|
1,311,552
|
|
|
|
790,007
|
|
|
|
|
|
|
|
|
|
|
|
|
Less valuation allowance
|
|
|
(1,311,552
|
)
|
|
|
(790,007
|
)
|
|
|
|
|
|
|
|
|
|
Deferred tax assets, net of valuation allowance
|
|
$
|
-
|
|
|
$
|
-
|
|
Limitation on Utilization of NOLs due to Change in Control
The Company had ownership changes as defined by the Internal Revenue Code Section 382 (“Section 382”), which may subject the NOL’s to annual limitations which could reduce or defer the NOL. Section 382 imposes limitations on a corporation’s ability to utilize NOLs if it experiences an “ownership change.” In general terms, an ownership change may result from transactions increasing the ownership of certain stockholders in the stock of a corporation by more than 50 percentage points over a three-year period. In the event of an ownership change, utilization of the NOLs would be subject to an annual limitation under Section 382 determined by multiplying the value of its stock at the time of the ownership change by the applicable long-term tax-exempt rate. Any unused annual limitation may be carried over to later years. The imposition of this limitation on its ability to use the NOLs to offset future taxable income could cause the Company to pay U.S. federal income taxes earlier than if such limitation were not in effect and could cause such NOLs to expire unused, reducing or eliminating the benefit of such NOLs.
Income Tax Provision in the Consolidated Statement of Operations
A reconciliation of the federal statutory income tax rate and the effective income tax rate as a percentage of income before income taxes is as follows:
|
|
|
For the Fiscal Year
Ended
March 31, 2013
|
|
|
For the Period from
April 11, 2011 (inception)
through
March 31, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal statutory income tax rate
|
|
|
34.0
|
%
|
|
|
34.0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
Change in valuation allowance on net operating loss carry-forwards
|
|
|
(34.0
|
)
|
|
|
(34.0
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Effective income tax rate
|
|
|
0.0
|
%
|
|
|
0.0
|
%
|
Note 15 – Concentrations and Credit Risk
Credit Risk
Financial instruments that potentially subject the Company to significant concentration of credit risk consist primarily of cash and cash equivalents.
As of March 31, 2013, substantially all of the Company’s cash and cash equivalents were held by major financial institutions, and the balance at certain accounts exceeded the maximum amount insured by the Federal Deposits Insurance Corporation (“FDIC”). However, the Company has not experienced losses on these accounts and management believes that the Company is not exposed to significant risks on such accounts.
Customers and Credit Concentrations
One (1) customer accounted for all of the sales for the fiscal year ended March 31, 2013 and the accounts receivable balance at March 31, 2013. A reduction in sales from or loss of such customer would have a material adverse effect on the Company’s results of operations and financial condition.
Vendors and Accounts Payable Concentrations
Vendor purchase concentrations and accounts payable concentration are as follows:
|
|
|
Accounts Payable at
|
|
Net Purchases
|
|
|
|
|
March 31, 2013
|
|
|
March 31, 2012
|
|
|
March 31, 2013
|
|
|
March 31, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Growers Synergy Pte. Ltd. – related party
|
|
|
50.1
|
%
|
|
|
16.4
|
%
|
|
|
26.4
|
%
|
|
|
13.5
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stevia Ventures Corporation
|
|
|
16. 9
|
%
|
|
|
54.1
|
%
|
|
|
55.7
|
%
|
|
|
14.4
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
67.0
|
%
|
|
|
70.5
|
%
|
|
|
82.1
|
%
|
|
|
27.9
|
%
|
Note 16 – Subsequent Events
The Company has evaluated all events that occurred after the balance sheet date through the date when the financial statements were issued to determine if they must be reported. The Management of the Company determined that there were certain reportable subsequent events to be disclosed as follows:
On April 30, 2013, the Company issued 1,000,000 shares of its common stock to Mountain Sky International Limited, a Hong Kong corporation (“Mountain Sky”), in partial consideration for consulting services rendered by Mountain Sky. 500,000 of the shares vested at the time of grant, and 500,000 will vest on the one year anniversary of the date of grant. The 500,000 shares vested on April 30, 2013 were valued at $0.20 per share or $100,000 and recorded as consulting fee.
On May 3, 2013, the Company entered into a Warrant Exercise Reset Offer Letter Agreement (the "Reset Letter") with an investor (the "Investor") whereby the Company and the Investor agreed that the Investor would immediately exercise his warrant to purchase 853,333 shares of common stock of the Company at an exercise price of $0.20 per share for the aggregate of $170,667. In consideration for the Investor's immediate exercise, the Company agreed to issue to the Investor three new warrants in the amounts of 1,877,333, 1,066,666 and 2,346,666, with exercise prices of $0.20, $0.25 and $0.25 per share, respectively (the "Series A Warrants", "Series B Warrants" and "Series C Warrants", respectively, and collectively the "New Warrants"). The Series A Warrants are subject to the Company's call right, and the Series C Warrants are only exercisable upon the Investor's exercise in full of the Series A Warrants. In connection with the Reset Letter, the Company agreed to use its best efforts to file a registration statement (the "Registration Statement") with the Securities and Exchange Commission (the "SEC") within ten (10) business days. The Company will use its best efforts to have the Registration Statement declared effective by the SEC within thirty (30) days. The Company filed a registration statement (the "Registration Statement") with the Securities and Exchange Commission (the "SEC") within ten (10) business days which was declared effective by the SEC within thirty (30) days.
Garden State Securities, Inc. (the "Placement Agent") served as the placement agent of the Company for the Offering. In consideration for services rendered as the Placement Agent, the Company agreed to: (i) pay to the Placement Agent cash commissions equal to $13,600, (ii) warrants equal to eight percent (8%) of the aggregate number of shares exercised by the Investor, and (iii) upon exercise of the New Warrants by the Company, the Placement Agent will receive additional warrants equal to eight percent (8%) of the number of shares issued upon exercise of the New Warrants (collectively, the "Agent Warrants").
On June 23, 2013, the remaining 3,000,000 Escrow Shares have been earned by Ventures stockholder upon achievement of the Second and the Third Milestones within two (2) years of June 23, 2011, the Closing Date associated with the Milestones. These shares were valued at $0.20 per share or $600,000 on June 23, 2013 and recorded as compensation.
December 31, 2013 and 2012
Index to the Consolidated Financial Statements
|
Contents
|
|
Page(s)
|
|
|
|
|
|
Consolidated Balance Sheets at December 31, 2013 (Unaudited) and March 31, 2013
|
|
5
|
|
|
|
|
|
Consolidated Statements of Operations for the Nine Months and Three Months Ended December 31, 2013 and 2012 (Unaudited)
|
|
6
|
|
|
|
|
|
Consolidated Statement of Equity (Deficit) for the Interim Period Ended December 31, 2013 (Unaudited) and for the Fiscal Year Ended March 31, 2013
|
|
7
|
|
|
|
|
|
Consolidated Statements of Cash Flows for the Nine Months Ended December 31, 2013 and 2012 (Unaudited)
|
|
8
|
|
|
|
|
|
Notes to the Consolidated Financial Statements (Unaudited)
|
|
9
|
Stevia Corp.
Consolidated Balance Sheets
|
|
|
|
December 31, 2013
|
|
|
March 31, 2013
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
|
$
|
85,366
|
|
|
$
|
424,475
|
|
|
Accounts receivable
|
|
|
164,988
|
|
|
|
158,008
|
|
|
Seeds
|
|
|
|
1,807,000
|
|
|
|
-
|
|
|
Prepayments and other current assets
|
|
|
74,946
|
|
|
|
33,096
|
|
|
Total current assets
|
|
|
2,132,300
|
|
|
|
615,579
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current assets:
|
|
|
|
|
|
|
|
|
|
Property and equipment
|
|
|
24,400
|
|
|
|
7,925
|
|
|
Accumulated depreciation
|
|
|
(4,334)
|
|
|
|
(1,234)
|
|
|
Property and equipment, net
|
|
|
20,066
|
|
|
|
6,691
|
|
|
Acquired technology
|
|
|
1,635,300
|
|
|
|
1,635,300
|
|
|
Accumulatd amortization
|
|
|
(163,530)
|
|
|
|
(81,765)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquired technology, net
|
|
|
1,471,770
|
|
|
|
1,553,535
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Website development costs
|
|
|
5,315
|
|
|
|
5,315
|
|
|
Accumulated amortization
|
|
|
(2,670)
|
|
|
|
(1,869)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Website development costs, net
|
|
|
2,645
|
|
|
|
3,446
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Security deposit
|
|
|
15,000
|
|
|
|
15,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
3,641,781
|
|
|
$
|
2,194,251
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
1,092,237
|
|
|
$
|
948,073
|
|
|
Accounts payable - president and CEO
|
|
|
262,576
|
|
|
|
89,193
|
|
|
Accrued expenses
|
|
|
9,600
|
|
|
|
19,700
|
|
|
Accrued interest
|
|
|
145,144
|
|
|
|
21,627
|
|
|
Advances from president and significant stockholder
|
|
|
10,743
|
|
|
|
21,238
|
|
|
Convertible notes payable - net of discount
|
|
|
1,071,569
|
|
|
|
357,700
|
|
|
Current portion of derivative liability
|
|
|
-
|
|
|
|
-
|
|
|
Total current liabilities
|
|
|
2,591,869
|
|
|
|
1,457,531
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Current liabilities:
|
|
|
|
|
|
|
|
|
|
Derivative note liabilities
|
|
|
227,772
|
|
|
|
-
|
|
|
Derivative warrant liabilities
|
|
|
369,747
|
|
|
|
486,113
|
|
|
Total non-current liabilities
|
|
|
597,519
|
|
|
|
486,113
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
3,189,388
|
|
|
|
1,943,644
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
|
Stevia Corp stockholders' equity:
|
|
|
|
|
|
|
|
|
|
Preferred stock par value $0.001: 1,000,000 shares authorized;
|
|
|
|
|
|
|
|
|
|
none issued or outstanding
|
|
|
-
|
|
|
|
-
|
|
|
Common stock par value $0.001: 250,000,000 shares authorized,
|
|
|
|
|
|
|
|
|
|
82,695,634 and 63,555,635 shares issued and outstanding, respectively
|
|
|
82,695
|
|
|
|
63,556
|
|
|
Additional paid-in capital
|
|
|
6,813,321
|
|
|
|
4,760,624
|
|
|
Common stock to be issued
|
|
|
279,222
|
|
|
|
-
|
|
|
Accumulated deficit
|
|
|
(6,376,899)
|
|
|
|
(4,359,415)
|
|
|
Total Stevia Corp stockholders' equity
|
|
|
798,339
|
|
|
|
464,765
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-controlling interest in subsidiary
|
|
|
|
|
|
|
|
|
|
Noncontrolling interest - retained earnings in consolidated subsidiaries
|
|
|
(345,946)
|
|
|
|
(214,158)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-controlling interest in subsidiary
|
|
|
(345,946)
|
|
|
|
(214,158)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Equity
|
|
|
452,393
|
|
|
|
250,607
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Liabilities and Equity
|
|
$
|
3,641,781
|
|
|
$
|
2,194,251
|
|
See accompanying notes to the consolidated financial statements.
Stevia Corp.
Consolidated Statements of Operations
|
|
|
For the Nine Months
|
|
|
For the Three Months
|
|
|
For the Nine Months
|
|
|
For the Three Months
|
|
|
|
|
Ended
|
|
|
Ended
|
|
|
Ended
|
|
|
Ended
|
|
|
|
|
December 31, 2013
|
|
|
December 31, 2013
|
|
|
December 31, 2012
|
|
|
December 31, 2012
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues
|
|
$
|
1,893,865
|
|
|
$
|
388,746
|
|
|
$
|
120,939
|
|
|
$
|
8,142
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Farm produce
|
|
|
758,809
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Farm expenses
|
|
|
318,234
|
|
|
|
4,925
|
|
|
|
66,743
|
|
|
|
66,743
|
|
|
Farm field lease
|
|
|
-
|
|
|
|
-
|
|
|
|
21,250
|
|
|
|
6,250
|
|
|
Farm management services - related parties
|
|
|
180,000
|
|
|
|
60,000
|
|
|
|
652,550
|
|
|
|
60,000
|
|
|
Total cost of revenues
|
|
|
1,257,043
|
|
|
|
64,925
|
|
|
|
740,543
|
|
|
|
132,993
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross margin
|
|
|
636,822
|
|
|
|
323,821
|
|
|
|
(619,604
|
)
|
|
|
(124,851
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Directors' fees
|
|
|
195,313
|
|
|
|
7,813
|
|
|
|
281,250
|
|
|
|
93,750
|
|
|
Professional fees
|
|
|
487,867
|
|
|
|
178,135
|
|
|
|
352,031
|
|
|
|
123,356
|
|
|
Research and development
|
|
|
262,810
|
|
|
|
71,930
|
|
|
|
118,669
|
|
|
|
-
|
|
|
Salary and compensation - officer
|
|
|
600,000
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Salary and compensation - others
|
|
|
66,556
|
|
|
|
378
|
|
|
|
110,982
|
|
|
|
59,105
|
|
|
General and administrative expenses
|
|
|
387,040
|
|
|
|
133,641
|
|
|
|
204,616
|
|
|
|
69,302
|
|
|
Total operating expenses
|
|
|
1,999,586
|
|
|
|
391,897
|
|
|
|
1,067,548
|
|
|
|
345,513
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(1,362,764
|
)
|
|
|
(68,076
|
)
|
|
|
(1,687,152
|
)
|
|
|
(470,364
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other (income) expense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of derivative liability
|
|
|
(675,949
|
)
|
|
|
(40,105
|
)
|
|
|
(305,244
|
)
|
|
|
(73,723
|
)
|
|
Debt discount
|
|
|
626,446
|
|
|
|
63,263
|
|
|
|
-
|
|
|
|
-
|
|
|
Debt settlement loss
|
|
|
561,077
|
|
|
|
561,077
|
|
|
|
-
|
|
|
|
-
|
|
|
Excess of fair value of warrants over notes, net of OID
|
|
|
38,075
|
|
|
|
38,075
|
|
|
|
-
|
|
|
|
-
|
|
|
Financing cost
|
|
|
30,000
|
|
|
|
8,000
|
|
|
|
16,125
|
|
|
|
2,807
|
|
|
Foreign currency transaction gain (loss)
|
|
|
-
|
|
|
|
-
|
|
|
|
1,316
|
|
|
|
1
|
|
|
Interest expense
|
|
|
123,516
|
|
|
|
75,613
|
|
|
|
40,462
|
|
|
|
10,130
|
|
|
Other (income) expense
|
|
|
83,343
|
|
|
|
83,343
|
|
|
|
-
|
|
|
|
-
|
|
|
Total other (income) expense
|
|
|
786,508
|
|
|
|
789,266
|
|
|
|
(247,341
|
)
|
|
|
(60,785
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before income tax provision and non-controlling interest
|
|
|
(2,149,272
|
)
|
|
|
(857,342
|
)
|
|
|
(1,439,811
|
)
|
|
|
(409,579
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax provision
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss before non-controlling interest
|
|
|
(2,149,272
|
)
|
|
|
(857,342
|
)
|
|
|
(1,439,811
|
)
|
|
|
(409,579
|
)
|
|
Net loss attributable to the non-controlling interest
|
|
|
(131,788
|
)
|
|
|
(25,932
|
)
|
|
|
(97,338
|
)
|
|
|
(44,978
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) attributable to Stevia Corp.
|
|
$
|
(2,017,484
|
)
|
|
$
|
(831,410
|
)
|
|
$
|
(1,342,473
|
)
|
|
$
|
(364,601
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per common share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- Basic and diluted:
|
|
$
|
(0.03
|
)
|
|
$
|
(0.01
|
)
|
|
$
|
(0.02
|
)
|
|
$
|
(0.01
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- basic and diluted
|
|
|
72,842,975
|
|
|
|
79,632,959
|
|
|
|
61,613,572
|
|
|
|
63,225,253
|
|
See accompanying notes to the consolidated financial statements.
Stevia Corp.
Consolidated Statement of Equity (Deficit)
For the Interim Period Ended December 31, 2013 (
Unaudited
) and for the Fiscal Year Ended March 31, 2013
|
|
|
Common Stock Par Value $0.001
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
|
|
|
|
Additional
|
|
|
Common stock
|
|
|
Accumulated
|
|
|
Stockholders'
|
|
|
Non-controlling
|
|
|
Total
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
paid-in Capital
|
|
|
to be Issued
|
|
|
Deficit
|
|
|
Equity (Deficit)
|
|
|
Interest
|
|
|
Equity (Deficit)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2012
|
|
|
58,354,775
|
|
|
$
|
58,355
|
|
|
$
|
1,474,751
|
|
|
$
|
-
|
|
|
$
|
(2,323,551
|
)
|
|
$
|
(790,445
|
)
|
|
$
|
-
|
|
|
$
|
(790,445
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted common shares issued for farm management services to
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
a related party valued at $0.79 per share discounted at 69%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
on July 5, 2012
|
|
|
500,000
|
|
|
|
500
|
|
|
|
272,050
|
|
|
|
|
|
|
|
|
|
|
|
272,550
|
|
|
|
|
|
|
|
272,550
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted common shares issued for technology rights
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
valued at $0.79 per share discounted at 69%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
on July 5, 2012
|
|
|
3,000,000
|
|
|
|
3,000
|
|
|
|
1,632,300
|
|
|
|
|
|
|
|
|
|
|
|
1,635,300
|
|
|
|
|
|
|
|
1,635,300
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for notes conversion
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at $0.832143 per share on July 6, 2012
|
|
|
600,858
|
|
|
|
601
|
|
|
|
499,399
|
|
|
|
|
|
|
|
|
|
|
|
500,000
|
|
|
|
|
|
|
|
500,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for conversion of accrued interest
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at $0.832143 per share on July 6, 2012
|
|
|
33,335
|
|
|
|
33
|
|
|
|
27,707
|
|
|
|
|
|
|
|
|
|
|
|
27,740
|
|
|
|
|
|
|
|
27,740
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares and warrants issued to two investors for cash
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at $0.46875 per unit on August 6, 2012
|
|
|
1,066,667
|
|
|
|
1,067
|
|
|
|
498,933
|
|
|
|
|
|
|
|
|
|
|
|
500,000
|
|
|
|
|
|
|
|
500,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued to investors in connection with the sale of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
equity units on August 6, 2012 classified as derivative liability
|
|
|
|
|
|
|
|
|
|
|
(381,300
|
)
|
|
|
|
|
|
|
|
|
|
|
(381,300
|
)
|
|
|
|
|
|
|
(381,300
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commissions and legal fees paid in connection with the sale of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
equity units on August 6, 2012
|
|
|
|
|
|
|
|
|
|
|
(52,500
|
)
|
|
|
|
|
|
|
|
|
|
|
(52,500
|
)
|
|
|
|
|
|
|
(52,500
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued to placement agent in connection with the sale of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
equity units on August 6, 2012 classified as derivative liability
|
|
|
|
|
|
|
|
|
|
|
(30,504
|
)
|
|
|
|
|
|
|
|
|
|
|
(30,504
|
)
|
|
|
|
|
|
|
(30,504
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of warrants in connection with
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
convertible note payable issued in February and March 2013
|
|
|
|
|
|
|
|
|
|
|
220,438
|
|
|
|
|
|
|
|
|
|
|
|
220,438
|
|
|
|
|
|
|
|
220,438
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beneficial conversion feature in connection with
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
convertible note payable issued in February and March 2013
|
|
|
|
|
|
|
|
|
|
|
224,350
|
|
|
|
|
|
|
|
|
|
|
|
224,350
|
|
|
|
|
|
|
|
224,350
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for future director
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
services on October 4, 2011
earned during the period
|
|
|
|
|
|
|
|
|
|
|
375,000
|
|
|
|
|
|
|
|
|
|
|
|
375,000
|
|
|
|
|
|
|
|
375,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2,035,864
|
)
|
|
|
(2,035,864
|
)
|
|
|
(214,158
|
)
|
|
|
(2,250,022
|
)
|
|
Balance, March 31, 2013
|
|
|
63,555,635
|
|
|
|
63,556
|
|
|
|
4,760,624
|
|
|
|
-
|
|
|
|
(4,359,415
|
)
|
|
|
464,765
|
|
|
|
(214,158
|
)
|
|
|
250,607
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for consulting services
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
valued at $0.20 per share on April 30, 2013
|
|
|
500,000
|
|
|
|
500
|
|
|
|
99,500
|
|
|
|
|
|
|
|
|
|
|
|
100,000
|
|
|
|
|
|
|
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of warrant with exercise price adjusted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
to $0.20 per share on May 6, 2013
|
|
|
853,333
|
|
|
|
853
|
|
|
|
169,813
|
|
|
|
|
|
|
|
|
|
|
|
170,666
|
|
|
|
|
|
|
|
170,666
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commissions and legal fees paid in connection with the exercise of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
warrants on May 6, 2013
|
|
|
|
|
|
|
|
|
|
|
(18,653
|
)
|
|
|
|
|
|
|
|
|
|
|
(18,653
|
)
|
|
|
|
|
|
|
(18,653
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reclassification of derivative liability to additional paid-in capital
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
associated with the exercise of warrants
|
|
|
|
|
|
|
|
|
|
|
595,852
|
|
|
|
|
|
|
|
|
|
|
|
595,852
|
|
|
|
|
|
|
|
595,852
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued to investors in connection with warrants
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
exercised on May 6, 2013 classified as derivative liability
|
|
|
|
|
|
|
|
|
|
|
(833,106
|
)
|
|
|
|
|
|
|
|
|
|
|
(833,106
|
)
|
|
|
|
|
|
|
(833,106
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Make good shares released to officer for achieving
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
the second and third milestones on June 21, 2013
|
|
|
3,000,000
|
|
|
|
3,000
|
|
|
|
597,000
|
|
|
|
|
|
|
|
|
|
|
|
600,000
|
|
|
|
|
|
|
|
600,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for future director
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
services on October 4, 2011
earned during the period endng June 30,2013
|
|
|
|
|
|
|
|
|
|
|
93,750
|
|
|
|
|
|
|
|
|
|
|
|
93,750
|
|
|
|
|
|
|
|
93,750
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reclassification to derivative liability for warrants
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
that became derivatives
|
|
|
|
|
|
|
|
|
|
|
(167,949
|
)
|
|
|
|
|
|
|
|
|
|
|
(167,949
|
)
|
|
|
|
|
|
|
(167,949
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for future director
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
services on October 4, 2011
earned during the period endng September 30, 2013
|
|
|
|
|
|
|
|
|
|
|
93,750
|
|
|
|
|
|
|
|
|
|
|
|
93,750
|
|
|
|
|
|
|
|
93,750
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anti-dilution shares issued in accordance with the Security Purchase
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Agreement dated August 1, 2012 on October 1, 2013
|
|
|
286,666
|
|
|
|
286
|
|
|
|
(286
|
)
|
|
|
|
|
|
|
|
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for future director service
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
on December 4, 2013
|
|
|
1,500,000
|
|
|
|
1,500
|
|
|
|
186,000
|
|
|
|
|
|
|
|
|
|
|
|
187,500
|
|
|
|
|
|
|
|
187,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for future director service
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
on December 4, 2013
|
|
|
|
|
|
|
|
|
|
|
(187,500
|
)
|
|
|
|
|
|
|
|
|
|
|
(187,500
|
)
|
|
|
|
|
|
|
(187,500
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued per debt settlement agreement
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
for past due accounts payable and related settlement costs
|
|
|
13,000,000
|
|
|
|
13,000
|
|
|
|
1,416,715
|
|
|
|
279,222
|
|
|
|
|
|
|
|
1,708,937
|
|
|
|
|
|
|
|
1,708,937
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for future director service on December 4, 2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
earned during the period endng December 31, 2013
|
|
|
|
|
|
|
|
|
|
|
7,811
|
|
|
|
|
|
|
|
|
|
|
|
7,811
|
|
|
|
|
|
|
|
7,811
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2,017,484
|
)
|
|
|
(2,017,484
|
)
|
|
|
(131,788
|
)
|
|
|
(2,149,272
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2013
|
|
|
82,695,634
|
|
|
$
|
82,695
|
|
|
$
|
6,813,321
|
|
|
$
|
279,222
|
|
|
$
|
(6,376,899
|
)
|
|
$
|
798,339
|
|
|
$
|
(345,946
|
)
|
|
$
|
452,393
|
|
See accompanying notes to the consolidated financial statements.
Stevia Corp.
Consolidated Statements of Cash Flows
|
|
|
For the Nine months
|
|
|
For the Nine Months
|
|
|
|
|
Ended
|
|
|
Ended
|
|
|
|
|
December 31, 2013
|
|
|
December 31, 2012
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
Net loss before non-controlling interest
|
|
$
|
(2,149,272
|
)
|
|
$
|
(1,439,811
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating activities
|
|
|
|
|
|
|
|
|
|
Depreciation expense
|
|
|
3,100
|
|
|
|
762
|
|
|
Amortization expense - acquired technology
|
|
|
81,765
|
|
|
|
54,510
|
|
|
Amortization expense - website development costs
|
|
|
801
|
|
|
|
801
|
|
|
Amortization of discount of convertible notes payable
|
|
|
626,446
|
|
|
|
-
|
|
|
Debt settlement loss
|
|
|
561,077
|
|
|
|
-
|
|
|
Excess of fair value of warrants over notes, net of OID
|
|
|
38,075
|
|
|
|
-
|
|
|
Change in fair value of derivative liability
|
|
|
(675,949
|
)
|
|
|
(305,244
|
)
|
|
Common shares issued for director services earned during the period
|
|
|
195,312
|
|
|
|
281,250
|
|
|
Common shares issued for services-related party
|
|
|
600,000
|
|
|
|
272,550
|
|
|
Common shares issued for outside services
|
|
|
205,860
|
|
|
|
-
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(6,980
|
)
|
|
|
(120,659
|
)
|
|
Seeds
|
|
|
(765,000
|
)
|
|
|
-
|
|
|
Prepayments and other current assets
|
|
|
(41,850
|
)
|
|
|
142,858
|
|
|
Accounts payable
|
|
|
144,164
|
|
|
|
412,110
|
|
|
Accounts payable - president and CEO
|
|
|
173,383
|
|
|
|
-
|
|
|
Accrued expenses
|
|
|
(10,100
|
)
|
|
|
6,045
|
|
|
Accrued interest
|
|
|
123,517
|
|
|
|
40,397
|
|
|
Net cash used in operating activities
|
|
|
(895,651
|
)
|
|
|
(654,431
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment
|
|
|
(16,475
|
)
|
|
|
(4,889
|
)
|
|
Net cash used in investing activities
|
|
|
(16,475
|
)
|
|
|
(4,889
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
Advances from (repayments to) president and significant stockholder
|
|
|
(10,495
|
)
|
|
|
2,100
|
|
|
Proceeds from issuance of convertible notes, net of costs
|
|
|
431,500
|
|
|
|
200,000
|
|
|
Proceeds from sale of common stock, net of costs
|
|
|
-
|
|
|
|
447,500
|
|
|
Proceeds from exercise of warrants, net of costs
|
|
|
152,012
|
|
|
|
-
|
|
|
Net cash provided by financing activities
|
|
|
573,017
|
|
|
|
649,600
|
|
|
|
|
|
|
|
|
|
|
|
|
Net change in cash
|
|
|
(339,109
|
)
|
|
|
(9,720
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash at beginning of the period
|
|
|
424,475
|
|
|
|
15,698
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash at end of the period
|
|
$
|
85,366
|
|
|
$
|
5,978
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flows information:
|
|
|
|
|
|
|
|
|
|
Interest paid
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Income tax paid
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash investing and financing activities:
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for past due payables
|
|
$
|
1,042,000
|
|
|
$
|
-
|
|
|
Issuance of common stock for conversion of convertible notes
|
|
$
|
-
|
|
|
$
|
500,000
|
|
|
Issuance of common stock for conversion of accrued interest
|
|
$
|
-
|
|
|
$
|
27,739
|
|
See accompanying notes to the consolidated financial statements.
Stevia Corp.
December 31, 2013 and 2012
Notes to the Consolidated Financial Statements
(Unaudited)
Note 1 – Organization and Operations
Stevia Corp. (Formerly Interpro Management Corp.)
Interpro Management Corp (“Interpro”) was incorporated under the laws of the State of Nevada on May 21, 2007. Interpro focused on developing and offering web based software that was designed to be an online project management tool used to enhance an organization’s efficiency through planning and monitoring the daily operations of a business.
On March 4, 2011, Interpro amended its Articles of Incorporation, and changed its name to Stevia Corp. (“Stevia” or the “Company”) to reflect its intended acquisition of Stevia Ventures International Ltd.
The Company discontinued its web-based software business upon the acquisition of Stevia Ventures International Ltd. on June 23, 2011.
Stevia Ventures International Ltd.
Stevia Ventures International Ltd. (“Ventures”) was incorporated on April 11, 2011 under the laws of the Territory of the British Virgin Islands (“BVI”). Ventures owns certain rights relating to stevia production, including certain assignable exclusive purchase contracts and an assignable supply agreement related to stevia
.
Acquisition of Stevia Ventures International Ltd. Recognized as a Reverse Acquisition
On June 23, 2011 (the “Closing Date”), the Company closed a voluntary share exchange transaction with Ventures pursuant to a Share Exchange Agreement (the “Share Exchange Agreement”) by and among the Company, Ventures and George Blankenbaker, the stockholder of Ventures (the “Ventures Stockholder”).
Immediately prior to the consummation of the Share Exchange Agreement on June 23, 2011, the Company had 79,800,000 common shares issued and outstanding. Simultaneously with the closing of the Share Exchange Agreement, on the Closing Date, Mohanad Shurrab, a shareholder and, as of the Closing Date, the Company’s former Director, President, Treasurer and Secretary, surrendered 33,000,000 shares of the Company's common stock to the Company for cancellation.
As a result of the Share Exchange Agreement, the Company issued 12,000,000 common shares for the acquisition of 100% of the issued and outstanding shares of Ventures. Of the 12,000,000 common shares issued 6,000,000 shares were being held in escrow pending the achievement by the Company of certain post-Closing business milestones (the “Milestones”), pursuant to the terms of the Make Good Escrow Agreement, between the Company, Greenberg Traurig, LLP, as escrow agent and the Ventures’ Stockholder (the “Escrow Agreement”). Even though the shares issued only represented approximately 20.4% of the issued and outstanding common stock, immediately after the consummation of the Share Exchange Agreement, the stockholder of Ventures completely took over and controlled the board of directors and management of the Company upon acquisition.
As a result of the change in control to the then Ventures Stockholder, for financial statement reporting purposes, the merger between the Company and Ventures has been treated as a reverse acquisition with Ventures deemed the accounting acquirer and the Company deemed the accounting acquiree under the acquisition method of accounting in accordance with section 805-10-55 of the FASB Accounting Standards Codification. The reverse acquisition is deemed a capital transaction and the net assets of Ventures (the accounting acquirer) are carried forward to the Company (the legal acquirer and the reporting entity) at their carrying value before the acquisition. The acquisition process utilizes the capital structure of the Company and the assets and liabilities of Ventures which are recorded at their historical cost. The equity of the Company is the historical equity of Ventures retroactively restated to reflect the number of shares issued by the Company in the transaction.
Formation of Stevia Asia Limited
On March 19, 2012, the Company formed Stevia Asia Limited (“Stevia Asia”) under the laws of the Hong Kong Special Administrative Region (“HK SAR”) of the People’s Republic of China (“PRC”), as a wholly-owned subsidiary.
Formation of Stevia Technew Limited (Formerly Hero Tact Limited)/Cooperative Agreement
On April 28, 2012, Stevia Asia formed Hero Tact Limited, as a wholly-owned subsidiary, under the laws of HK SAR, which subsequently changed its name to Stevia Technew Limited (“Stevia Technew”). Stevia Technew intends to facilitate a joint venture relationship with the Company’s technology partner, Guangzhou Health China Technology Development Company Limited, operating under the trade name Tech-New Bio-Technology and Guangzhou’s affiliates Technew Technology Limited. Prior to July 5, 2012, the date of entry into the Cooperative Agreement, Stevia Technew was inactive and had no assets or liabilities.
On July 5, 2012, Stevia Asia entered into a Cooperative Agreement (the "Cooperative Agreement") with Technew Technology Limited ("Technew"), a company incorporated under the companies ordinance of Hong Kong and an associate of Guangzhou Health China Technology Development Company Limited, and Zhang Jia, a Chinese citizen (together with Technew, the "Partners") pursuant to which Stevia Asia and Partners have agreed to make Stevia Technew, a joint venture, of which Stevia Asia legally and beneficially owns 70% of the issued shares and Technew legally and beneficially owns 30% of the issued shares. The Partners will be responsible for managing Stevia Technew and Stevia Asia has agreed to contribute $200,000 per month, up to a total of $2,000,000 in financing, subject to the performance of Stevia Technew and Stevia Asia's financial capabilities. On March 1, 2013, the partners agreed to terminate the Cooperative Agreement specific to the investment in an agricultural project and no further obligation by either party related to the payment of $200,000.
The Cooperative Agreement shall automatically terminate upon either Stevia Asia or Technew ceasing to be a shareholder in Stevia Technew, or may be terminated by either Stevia Asia or Technew upon a material breach by the other party which is not cured within 30 days of notice of such breach.
Formation of SC Brands Pte Ltd
On October 1, 2013, the Company formed SC Brands Pte Ltd (“SC Brands”) under the laws of Singapore, with the Company owning 70% of the shares and 30% owned by a Singapore strategic partner that will provide the working capital funds via fixed convertible notes to the Company. As of December 31, 2013 SC Brands was inactive.
Note 2 – Summary of Significant Accounting Policies
The Management of the Company is responsible for the selection and use of appropriate accounting policies and the appropriateness of accounting policies and their application. Critical accounting policies and practices are those that are both most important to the portrayal of the Company’s financial condition and results and require management’s most difficult, subjective, or complex judgments, often as a result of the need to make estimates about the effects of matters that are inherently uncertain. The Company’s significant and critical accounting policies and practices are disclosed below as required by generally accepted accounting principles.
Basis of Presentation – Unaudited Interim Financial Information
The unaudited interim consolidated financial statements and related notes have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information, and with the rules and regulations of the United States Securities and Exchange Commission (“SEC”) to Form 10-Q and Article 8 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by U.S. GAAP for complete financial statements. The unaudited interim consolidated financial statements furnished reflect all adjustments (consisting of normal recurring accruals) which are, in the opinion of management, necessary to a fair statement of the results for the interim periods presented. Interim results are not necessarily indicative of the results for the full fiscal year. These consolidated financial statements should be read in conjunction with the consolidated financial statements of the Company for the fiscal year ended March 31, 2013 and notes thereto contained in the Company’s Annual Report on Form 10-K as filed with the SEC on July 16, 2013.
Fiscal Year End
The Company elected March 31st as its fiscal year end date upon its formation.
Use of Estimates and Assumptions and Critical Accounting Estimates and Assumptions
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date(s) of the financial statements and the reported amounts of revenues and expenses during the reporting period(s).
Critical accounting estimates are estimates for which (a) the nature of the estimate is material due to the levels of subjectivity and judgment necessary to account for highly uncertain matters or the susceptibility of such matters to change and (b) the impact of the estimate on financial condition or operating performance is material. The Company’s critical accounting estimates and assumptions affecting the financial statements were:
|
|
(i)
|
Assumption as a going concern
:
Management assumes that the Company will continue as a going concern, which contemplates continuity of operations, realization of assets, and liquidation of liabilities in the normal course of business
.
|
|
|
(ii)
|
Allowance for doubtful accounts
:
Management’s
estimate of the allowance for doubtful accounts is based on historical sales, historical loss levels, and an analysis of the collectability of individual accounts;
and general economic conditions that may affect a client’s ability to pay
. The Company evaluated the key factors and assumptions used to develop the allowance in determining that it is reasonable in relation to the financial statements taken as a whole.
|
|
|
(iii)
|
Inventory Obsolescence and Markdowns
:
The Company’s estimate of potentially excess and slow-moving inventories is based on evaluation of inventory levels and aging, review of inventory turns and historical sales experiences. The Company’s estimate of reserve for inventory shrinkage is based on the historical results of physical inventory cycle counts.
|
|
|
(iv)
|
Fair value of long-lived assets
:
Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable. If long-lived assets are determined to be recoverable, but the newly determined remaining estimated useful lives are shorter than originally estimated, the net book values of the long-lived assets are depreciated over the newly determined remaining estimated useful lives. The Company considers the following to be some examples of important indicators that may trigger an impairment review: (i) significant under-performance or losses of assets relative to expected historical or projected future operating results; (ii) significant changes in the manner or use of assets or in the Company’s overall strategy with respect to the manner or use of the acquired assets or changes in the Company’s overall business strategy; (iii) significant negative industry or economic trends; (iv) increased competitive pressures; (v) a significant decline in the Company’s stock price for a sustained period of time; and (vi) regulatory changes. The Company evaluates acquired assets for potential impairment indicators at least annually and more frequently upon the occurrence of such events.
|
|
|
(v)
|
Valuation allowance for deferred tax assets
:
Management assumes that the realization of the Company’s net deferred tax assets resulting from its net operating loss (“NOL”) carry–forwards for Federal income tax purposes that may be offset against future taxable income was not considered more likely than not and accordingly, the potential tax benefits of the net loss carry-forwards are offset by a full valuation allowance. Management made this assumption based on (a) the Company has incurred recurring losses, (b) general economic conditions, and (c) its ability to raise additional funds to support its daily operations by way of a public or private offering, among other factors.
|
|
|
(vi)
|
Estimates and assumptions used in valuation of equity instruments
: Management estimates
expected term of share options and similar instruments, expected volatility of the Company’s common shares and the method used to estimate it, expected annual rate of quarterly dividends, and risk free rate(s) to value share options and similar instruments.
|
These significant accounting estimates or assumptions bear the risk of change due to the fact that there are uncertainties attached to these estimates or assumptions, and certain estimates or assumptions are difficult to measure or value.
Management bases its estimates on historical experience and on various assumptions that are believed to be reasonable in relation to the financial statements taken as a whole under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources.
Management regularly evaluates the key factors and assumptions used to develop the estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such evaluations, if deemed appropriate, those estimates are adjusted accordingly.
Actual results could differ from those estimates.
Principles of Consolidation
The Company applies the guidance of Topic 810
“Consolidation”
of the FASB Accounting Standards Codification to determine whether and how to consolidate another entity. Pursuant to ASC Paragraph 810-10-15-10 all majority-owned subsidiaries—all entities in which a parent has a controlling financial interest—shall be consolidated except (1) when control does not rest with the parent, the majority owner; (2) if the parent is a broker-dealer within the scope of Topic 940 and control is likely to be temporary; (3) consolidation by an investment company within the scope of Topic 946 of a non-investment-company investee. Pursuant to ASC Paragraph 810-10-15-8 the usual condition for a controlling financial interest is ownership of a majority voting interest, and, therefore, as a general rule ownership by one reporting entity, directly or indirectly, of more than 50 percent of the outstanding voting shares of another entity is a condition pointing toward consolidation. The power to control may also exist with a lesser percentage of ownership, for example, by contract, lease, agreement with other stockholders, or by court decree. The Company consolidates all less-than-majority-owned subsidiaries, if any, in which the parent’s power to control exists.
The Company's consolidated subsidiaries and/or entities are as follows:
|
Name of consolidated
subsidiary or entity
|
|
State or other jurisdiction
of incorporation or organization
|
|
Date of incorporation or formation
(date of acquisition, if applicable)
|
|
Attributable
interest
|
|
|
|
|
|
|
|
|
|
|
|
Stevia Ventures International Ltd.
|
|
The Territory of the British Virgin Islands
|
|
April 11, 2011
|
|
|
100
|
%
|
|
|
|
|
|
|
|
|
|
|
|
Stevia Asia Limited
|
|
Hong Kong SAR
|
|
March 19, 2012
|
|
|
100
|
%
|
|
|
|
|
|
|
|
|
|
|
|
Stevia Technew Limited
|
|
Hong Kong SAR
|
|
April 28, 2012
|
|
|
70
|
%
|
|
|
|
|
|
|
|
|
|
|
|
SC Brands Pte Ltd
|
|
Singapore
|
|
October 1, 2013
|
|
|
70
|
%
|
The consolidated financial statements include all accounts of the Company and the consolidated subsidiaries and/or entities as of reporting period ending date(s) and for the reporting period(s) then ended.
All inter-company balances and transactions have been eliminated.
Reclassification
Certain amounts in the prior period financial statements have been reclassified to conform to the current period presentation. These reclassifications had no effect on reported losses.
Fair Value of Financial Instruments
The Company follows paragraph 820-10-35-37 of the FASB Accounting Standards Codification (“Paragraph 820-10-35-37”) to measure the fair value of its financial instruments and paragraph 825-10-50-10 of the FASB Accounting Standards Codification for disclosures about fair value of its financial instruments. Paragraph 820-10-35-37 establishes a framework for measuring fair value in accounting principles generally accepted in the United States of America (U.S. GAAP), and expands disclosures about fair value measurements. To increase consistency and comparability in fair value measurements and related disclosures, Paragraph 820-10-35-37 establishes a fair value hierarchy which prioritizes the inputs to valuation techniques used to measure fair value into three (3) broad levels. The three (3) levels of fair value hierarchy defined by Paragraph 820-10-35-37 are described below:
|
Level 1
|
|
Quoted market prices available in active markets for identical assets or liabilities as of the reporting date.
|
|
|
|
|
|
Level 2
|
|
Pricing inputs other than quoted prices in active markets included in Level 1, which are either directly or indirectly observable as of the reporting date.
|
|
|
|
|
|
Level 3
|
|
Pricing inputs that are generally observable inputs and not corroborated by market data.
|
Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable.
The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. If the inputs used to measure the financial assets and liabilities fall within more than one level described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
The carrying amounts of the Company’s financial assets and liabilities, such as cash, accounts receivable, prepayments and other current assets, accounts payable,accrued expenses, and accrued interest, approximate their fair values because of the short maturity of these instruments.
The Company’s convertible notes payable approximates the fair value of such instrument based upon management’s best estimate of interest rates that would be available to the Company for similar financial arrangements at December 31, 2013 and March 31, 2013.
The Company’s Level 3 financial liabilities consist of the derivative warrant issued in August 2012 for which there is no current market for these securities such that the determination of fair value requires significant judgment or estimation and the derivative liability on the conversion feature. The Company valued the automatic conditional conversion, re-pricing/down-round, change of control; default and follow-on offering provisions using a lattice model, with the assistance of a third party valuation specialist, for which management understands the methodologies. These models incorporate transaction details such as Company stock price, contractual terms, maturity, risk free rates, as well as assumptions about future financings, volatility, and holder behavior as of the date of issuance and each balance sheet date.
Fair Value of Financial Assets and Liabilities Measured on a Recurring Basis
Level 3 Financial Liabilities – Derivative Warrant Liabilities and Derivative Liability on Conversion Feature
The Company uses Level 3 of the fair value hierarchy to measure the fair value of the derivative liabilities and revalues its derivative warrant liability and derivative liability on the conversion feature at every reporting period and recognizes gains or losses in the consolidated statements of operations that are attributable to the change in the fair value of the derivative liabilities.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are recorded at the invoiced amount, net of an allowance for doubtful accounts. The Company follows paragraph 310-10-50-9 of the FASB Accounting Standards Codification to estimate the allowance for doubtful accounts. The Company performs on-going credit evaluations of its customers and adjusts credit limits based upon payment history and the customer’s current credit worthiness, as determined by the review of their current credit information; and determines the allowance for doubtful accounts based on historical write-off experience, customer specific facts and economic conditions.
Pursuant to paragraph 310-10-50-2 of the FASB Accounting Standards Codification account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company has adopted paragraph 310-10-50-6 of the FASB Accounting Standards Codification and determine when receivables are past due or delinquent based on how recently payments have been received.
Outstanding account balances are reviewed individually for collectability. The allowance for doubtful accounts is the Company’s best estimate of the amount of probablecredit losses in the Company’s existing accounts receivable. Bad debt expense is included in general and administrative expenses, if any.
There was no allowance for doubtful accounts as December 31, 2013 or March 31, 2013.
The Company does not have any off-balance-sheet credit exposure to its customers.
Carrying Value, Recoverability and Impairment of Long-Lived Assets
The Company has adopted paragraph 360-10-35-17 of the FASB Accounting Standards Codification for its long-lived assets. The Company’s long-lived assets, which include property and equipment, acquired technology, and website development costs are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable.
The Company assesses the recoverability of its long-lived assets by comparing the projected undiscounted net cash flows associated with the related long-lived asset or group of long-lived assets over their remaining estimated useful livesagainst their respective carrying amounts. Impairment, if any, is based on the excess of the carrying amount over the fair value of those assets. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable.If long-lived assets are determined to be recoverable, but the newly determined remaining estimated useful lives are shorter than originally estimated, the net book values of the long-lived assets are depreciated over the newly determined remaining estimated useful lives.
The Company considers the following to be some examples of important indicators that may trigger an impairment review: (i) significant under-performance or losses of assets relative to expected historical or projected future operating results; (ii) significant changes in the manner or use of assets or in the Company’s overall strategy with respect to the manner or use of the acquired assets or changes in the Company’s overall business strategy; (iii) significant negative industry or economic trends; (iv) increased competitive pressures; (v) a significant decline in the Company’s stock price for a sustained period of time; and (vi) regulatory changes. The Company evaluates acquired assets for potential impairment indicators at least annually and more frequently upon the occurrence of such events.
The key assumptions used in management’s estimates of projected cash flow deal largely with forecasts of sales levels and gross margins. These forecasts are typically based on historical trends and take into account recent developments as well as management’s plans and intentions. Other factors, such as increased competition or a decrease in the desirability of the Company’s products or services, could lead to lower projected sales levels, which would adversely impact cash flows. A significant change in cash flows in the future could result in an impairment of long lived assets.
The impairment charges, if any, is included in operating expenses in the accompanying consolidated statements of operations.
Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less at the time of purchase to be cash equivalents.
Inventories
Inventory Valuation
The Company values inventory, consisting of finished goods, at the lower of cost or market. Cost is determined on the first-in and first-out (“FIFO”) method. The Company reduces inventory for the diminution of value, resulting from product obsolescence, damage or other issues affecting marketability, equal to the difference between the cost of the inventory and its estimated market value. Factors utilized in the determination of estimated market value include (i) estimates of future demand, and (ii) competitive pricing pressures.
Inventory Obsolescence and Markdowns
The Company evaluates its current level of inventory considering historical sales and other factors and, based on this evaluation, classify inventory markdowns in the income statement as a component of cost of goods sold pursuant to Paragraph 420-10-S99 of the FASB Accounting Standards Codification to adjust inventory to net realizable value. These markdowns are estimates, which could vary significantly from actual requirements if future economic conditions, customer demand or competition differ from expectations.
There was no inventory obsolescence for the interim period ended December 31, 2013 or 2012.
There was no lower of cost or market adjustments for the interim period ended December 31, 2013 or 2012.
Property and Equipment
Property and equipment is recorded at cost. Expenditures for major additions and betterments are capitalized. Maintenance and repairs are charged to operations as incurred. Depreciation of furniture and fixture is computed by the straight-line method (after taking into account their respective estimated residual values) over the assets estimated useful life of five (5) years. Upon sale or retirement of property and equipment, the related cost and accumulated depreciation are removed from the accounts and any gain or loss is reflected in the statements of operations.
Intangible Assets Other Than Goodwill
The Company has adopted paragraph 350-30-25-3 of the FASB Accounting Standards Codification for intangible assets other than goodwill. Under the requirements, the Company amortizes the acquisition costs of intangible assets other than goodwillon a straight-line basis over the estimated useful lives of the respective assets as follows:
|
|
|
Estimated Useful Life (Years)
|
|
|
|
|
|
|
|
|
Acquired technology
|
|
|
15
|
|
|
Website development costs
|
|
|
5
|
|
Upon becoming fully amortized, the related cost and accumulated amortization are removed from the accounts.
Related Parties
The Company follows subtopic 850-10 of the FASB Accounting Standards Codification for the identification of related parties and disclosure of related party transactions.
Pursuant to Section 850-10-20 the related parties include a. affiliates of the Company; b. entities for which investments in their equity securities would be required, absent the election of the fair value option under the Fair Value Option Subsection of Section 825–10–15, to be accounted for by the equity method by the investing entity; c. trusts for the benefit of employees, such as pension and profit-sharing trusts that are managed by or under the trusteeship of management; d. principal owners of the Company; e. management of the Company; f. other parties with which the Company may deal if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests; and g. other parties that can significantly influence the management or operating policies of the transacting parties or that have an ownership interest in one of the transacting parties and can significantly influence the other to an extent that one or more of the transacting parties might be prevented from fully pursuing its own separate interests.
The financial statements shall include disclosures of material related party transactions, other than compensation arrangements, expense allowances, and other similar items in the ordinary course of business. However, disclosure of transactions that are eliminated in the preparation of consolidated or combined financial statements is not required in those statements. The disclosures shall include: a. the nature of the relationship(s) involved; b. a description of the transactions, including transactions to which no amounts or nominal amounts were ascribed, for each of the periods for which income statements are presented, and such other information deemed necessary to an understanding of the effects of the transactions on the financial statements; c. the dollar amounts of transactions for each of the periods for which income statements are presented and the effects of any change in the method of establishing the terms from that used in the preceding period; and d. amounts due from or to related parties as of the date of each balance sheet presented and, if not otherwise apparent, the terms and manner of settlement.
Extinguishment Accounting
On July 25, 2013, the Supreme Court of the State of New York, County of New York (the "Court"), entered an order (the "Order") approving the settlement (the "Settlement Agreement") between the Company and Hanover Holdings I, LLC, a New York limited liability company ("Hanover"), Hanover commenced the action against the Company on July 12, 2013 to recover $1,042,000 of past-due accounts payable of the Company, plus fees and costs (the "Claim"). The Settlement Agreement became effective and binding upon the Company and Hanover upon execution of the Order by the Court on July 25, 2013.
The Settlement Agreement provides that the Initial Settlement Shares will be subject to adjustment on the trading day immediately following the Calculation Period to reflect the intention of the parties that the total number of shares of Common Stock to be issued to Hanover pursuant to the Settlement Agreement be based upon a specified discount to the trading volume weighted average price (the "VWAP") of the Common Stock for a specified period of time subsequent to the Court's entry of the Order.
The Company considered the settlement of debt with common shares as an extinguishment of debt and applied extinguishment accounting accordingly. The Company compared the trade accounts payable and related settlement costs with the fair value of common shares issued. Because the fair value of common shares issued was $561,077 greater than trade accounts payable and related settlement costs, the Company applied extinguishment accounting, resulting in a loss on extinguishment of debt of $561,077, for the reporting period ended December 31, 2013.
Derivative Instruments and Hedging Activities
The Company accounts for derivative instruments and hedging activities in accordance with paragraph 810-10-05-4 of the FASB Accounting Standards Codification (“Paragraph 810-10-05-4”). Paragraph 810-10-05-4 requires companies to recognize all derivative instruments as either assets or liabilities in the balance sheet at fair value. The accounting for changes in the fair value of a derivative instrument depends upon: (i) whether the derivative has been designated and qualifies as part of a hedging relationship, and (ii) the type of hedging relationship. For those derivative instruments that are designated and qualify as hedging instruments, a company must designate the hedging instrument based upon the exposure being hedged as either a fair value hedge, cash flow hedge or hedge of a net investment in a foreign operation.
Derivative Liability
The Company evaluates its convertible debt, options, warrants or other contracts, if any, to determine if those contracts or embedded components of those contracts qualify as derivatives to be separately accounted for in accordance with paragraph 810-10-05-4 and Section 815-40-25 of the FASB Accounting Standards Codification. The result of this accounting treatment is that the fair value of the embedded derivative is marked-to-market each balance sheet date and recorded as either an asset or a liability. In the event that the fair value is recorded as a liability, the change in fair value is recorded in the consolidated statement of operations and comprehensive income (loss) as other income or expense. Upon conversion, exercise or cancellation of a derivative instrument, the instrument is marked to fair value at the date of conversion, exercise or cancellation and then that the related fair value is reclassified to equity.
In circumstances where the embedded conversion option in a convertible instrument is required to be bifurcated and there are also other embedded derivative instruments in the convertible instrument that are required to be bifurcated, the bifurcated derivative instruments are accounted for as a single, compound derivative instrument.
The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period. Equity instruments that are initially classified as equity that become subject to reclassification are reclassified to liability at the fair value of the instrument on the reclassification date. Derivative instrument liabilities will be classified in the balance sheet as current or non-current based on whether or not net-cash settlement of the derivative instrument is expected within 12 months of the balance sheet date.
The Company adopted Section 815-40-15 of the FASB Accounting Standards Codification (“Section 815-40-15”)to determine whether an instrument (or an embedded feature) is indexed to the Company’s own stock. Section 815-40-15 provides that an entity should use a two-step approach to evaluate whether an equity-linked financial instrument (or embedded feature) is indexed to its own stock, including evaluating the instrument’s contingent exercise and settlement provisions. The adoption of Section 815-40-15 has affected the accounting for (i) certain freestanding warrants that contain exercise price adjustment features and (ii) convertible bonds issued by foreign subsidiaries with a strike price denominated in a foreign currency.
The Company marks to market the fair value of the embedded derivative warrants at each balance sheet date and records the change in the fair value of the embedded derivative warrants as other income or expense in the consolidated statements of operations and comprehensive income (loss).
The Company utilizes the Lattice model that values the liability of the derivative warrants based on a probability weighted discounted cash flow model with the assistance of the third party valuation firm. The reason the Company picks the Lattice model is that in many cases there may be multiple embedded features or the features of the bifurcated derivatives may be so complex that a Black-Scholes valuation does not consider all of the terms of the instrument. Therefore, the fair value may not be appropriately captured by simple models. In other words, simple models such as Black-Scholes may not be appropriate in many situations given complex features and terms of conversion option (e.g., combined embedded derivatives). The Lattice model is based on future projections of the various potential outcomes. The features that were analyzed and incorporated into the model included the exercise and full reset features. Based on these features, there are two primary events that can occur; the Holder exercises the Warrants or the Warrants are held to expiration. The Lattice model analyzed the underlying economic factors that influenced which of these events would occur, when they were likely to occur, and the specific terms that would be in effect at the time (i.e. stock price, exercise price, volatility, etc.). Projections were then made on the underlying factors which led to potential scenarios. Probabilities were assigned to each scenario based on management projections. This led to a cash flow projection and a probability associated with that cash flow. A discounted weighted average cash flow over the various scenarios was completed to determine the value of the derivative warrants.
Beneficial Conversion Feature
When the Company issues an debt or equity security that is convertible into common stock at a discount from the fair value of the common stock at the date the debt or equity security counterparty is legally committed to purchase such a security (Commitment Date), a beneficial conversion charge is measured and recorded on the Commitment Date for the difference between the fair value of the Company's common stock and the effective conversion price of the debt or equity security. If the intrinsic value of the beneficial conversion feature is greater than the proceeds allocated to the debt or equity security, the amount of the discount assigned to the beneficial conversion feature is limited to the amount of the proceeds allocated to the debt or equity security.
Commitment and Contingencies
The Company follows subtopic 450-20 of the FASB Accounting Standards Codification to report accounting for contingencies. Certain conditions may exist as of the date the consolidated financial statements are issued, which may result in a loss to the Company but which will only be resolved when one or more future events occur or fail to occur. The Company assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or un-asserted claims that may result in such proceedings, the Company evaluates the perceived merits of any legal proceedings or un-asserted claims as well as the perceived merits of the amount of relief sought or expected to be sought therein.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s consolidated financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, and an estimate of the range of possible losses, if determinable and material, would be disclosed.
Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the guarantees would be disclosed. Management does not believe, based upon information available at this time, that these matters will have a material adverse effect on the Company’s consolidated financial position, results of operations or cash flows. However, there is no assurance that such matters will not materially and adversely affect the Company’s business, financial position, and results of operations or cash flows.
Non-controlling Interest
The Company follows paragraph 810-10-65-1 of the FASB Accounting Standards Codification to report the non-controlling interests in its majority owned subsidiaries in the consolidated statements of balance sheets within the equity section, separately from the Company’s stockholders’ equity. Non-controlling interests represents the non-controlling interest holder’s proportionate share of the equity of the Company’s majority-owned subsidiaries. Non-controlling interest is adjusted for the non-controlling interest holder’s proportionate share of the earnings or losses and other comprehensive income (loss) and the non-controlling interest continues to be attributed its share of losses even if that attribution results in a deficit non-controlling interest balance.
Revenue Recognition
The Company follows paragraph 605-10-S99-1 of the FASB Accounting Standards Codification for revenue recognition. The Company recognizes revenue when it is realized or realizable and earned. The Company considers revenue realized or realizable and earned when all of the following criteria are met: (i) persuasive evidence of an arrangement exists, (ii) the product has been shipped or the services have been rendered to the customer, (iii) the sales price is fixed or determinable, and (iv)collectability is reasonably assured.
Shipping and Handling Costs
The Company accounts for shipping and handling fees in accordance with paragraph 605-45-45-19 of the FASB Accounting Standards Codification. While amounts charged to customers for shipping products are included in revenues, the related costs are classified in cost of goods sold as incurred.
Research and Development
The Company follows paragraph 730-10-25-1 of the FASB Accounting Standards Codification (formerly Statement of Financial Accounting Standards No. 2
“Accounting for Research and Development Costs”
) and paragraph 730-20-25-11 of the FASB Accounting Standards Codification (formerly Statement of Financial Accounting Standards No. 68
“Research and Development Arrangements”
) for research and development costs. Research and development costs are charged to expense as incurred.Research and development costs consist primarily of remuneration for research and development staff, depreciation and maintenance expenses of research and development equipment, material and testing costs for research and development as well as research and development arrangements with unrelated third party research and development institutions.
Non-refundable Advance Payments for Goods or Services to be Used in Future Research and Development Activities
The research and development arrangements usually involve specific research and development projects. Often times, the Company makes non-refundable advances upon signing of these arrangements. The Company adopted paragraph 730-20-25-13 and 730-20-35-1 of the FASB Accounting Standards Codification (formerly Emerging Issues Task Force Issue No. 07-3
“Accounting for Nonrefundable Advance Payments for Goods or Services to be Used in Future Research and Development Activities”
) for those non-refundable advances. Non-refundable advance payments for goods or services that will be used or rendered for future research and development activities are deferred and capitalized. Such amounts are recognized as an expense as the related goods are delivered or the related services are performed. The management continues to evaluate whether the Company expect the goods to be delivered or services to be rendered. If the management does not expect the goods to be delivered or services to be rendered, the capitalized advance payment are charged to expense.
Stock-Based Compensation for Obtaining Employee Services
The Company accounts for its stock based compensation in which the Company obtains employee services in share-based payment transactions under the recognition and measurement principles of the fair value recognition provisions of section 718-10-30 of the FASB Accounting Standards Codification. Pursuant to paragraph 718-10-30-6 of the FASB Accounting Standards Codification, all transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. The measurement date used to determine the fair value of the equity instrument issued is the earlier of the date on which the performance is complete or the date on which it is probable that performance will occur. If shares of the Company are thinly traded the use of share prices established in the Company’s most recent private placement memorandum ("PPM”), or weekly or monthly price observations would generally be more appropriate than the use of daily price observations as such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market.
The fair value of non-derivative option award is estimated on the date of grant using a Black-Scholes option-pricing valuation model. The ranges of assumptions for inputs are as follows:
|
·
|
Expected term of share options and similar instruments: The expected life of options and similar instruments represents the period of time the option and/or warrant are expected to be outstanding. Pursuant to Paragraph 718-10-50-2(f)(2)(i) of the FASB Accounting Standards Codification the expected term of share options and similar instruments represents the period of time the options and similar instruments are expected to be outstanding taking into consideration of the contractual term of the instruments and employees’ expected exercise and post-vesting employment termination behavior into the fair value (or calculated value) of the instruments. Pursuant to paragraph 718-10-S99-1, it may be appropriate to use the
simplified method
,
i.e.,
expected term = ((vesting term + original contractual term) / 2)
, if (i) A company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term due to the limited period of time its equity shares have been publicly traded; (ii) A company significantly changes the terms of its share option grants or the types of employees that receive share option grants such that its historical exercise data may no longer provide a reasonable basis upon which to estimate expected term; or (iii) A company has or expects to have significant structural changes in its business such that its historical exercise data may no longer provide a reasonable basis upon which to estimate expected term. The Company uses the simplified method to calculate expected term of share options and similar instruments as the company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term.
|
|
·
|
Expected volatility of the entity’s shares and the method used to estimate it. Pursuant to ASC Paragraph 718-10-50-2(f)(2)(ii) a thinly-traded or nonpublic entity that uses the calculated value method shall disclose the reasons why it is not practicable for the Company to estimate the expected volatility of its share price, the appropriate industry sector index that it has selected, the reasons for selecting that particular index, and how it has calculated historical volatility using that index. The Company uses the average historical volatility of the comparable companies over the expected contractual life of the share options or similar instruments as its expected volatility. If shares of a company are thinly traded the use of weekly or monthly price observations would generally be more appropriate than the use of daily price observations as the volatility calculation using daily observations for such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market.
|
|
·
|
Expected annual rate of quarterly dividends. An entity that uses a method that employs different dividend rates during the contractual term shall disclose the range of expected dividends used and the weighted-average expected dividends. The expected dividend yield is based on the Company’s current dividend yield as the best estimate of projected dividend yield for periods within the expected term of the share options and similar instruments.
|
|
·
|
Risk-free rate(s). An entity that uses a method that employs different risk-free rates shall disclose the range of risk-free rates used. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods within the expected term of the share options and similar instruments.
|
The Company’s policy is to recognize compensation cost for awards with only service conditions and a graded vesting schedule on a straight-line basis over the requisite service period for the entire award.
E
quity Instruments Issued to Parties other than Employees for Acquiring Goods or Services
The Company accounts for equity instruments issued to parties other than employees for acquiring goods or services under guidance of Subtopic 505-50 of the FASB Accounting Standards Codification (“Subtopic 505-50”).
Pursuant to ASC Section 505-50-30, all transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. The measurement date used to determine the fair value of the equity instrument issued is the earlier of the date on which the performance is complete or the date on which it is probable that performance will occur. If shares of the Company are thinly traded the use of share prices established in the Company’s most recent private placement memorandum ("PPM”), or weekly or monthly price observations would generally be more appropriate than the use of daily price observations as such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market.
The fair value of non-derivative option or warrant award is estimated on the date of grant using a Black-Scholes option-pricing valuation model. The ranges of assumptions for inputs are as follows:
|
·
|
Expected term of share options and similar instruments: Pursuant to Paragraph 718-10-50-2 of the FASB Accounting Standards Codification the expected term of share options and similar instruments represents the period of time the options and similar instruments are expected to be outstanding taking into consideration of the contractual term of the instruments and holder’s expected exercise behavior into the fair value (or calculated value) of the instruments. The Company uses historical data to estimate holder’s expected exercise behavior. If the Company is a newly formed corporation or shares of the Company are thinly traded the contractual term of the share options and similar instruments is used as the expected term of share options and similar instruments as the Company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term.
|
|
·
|
Expected volatility of the entity’s shares and the method used to estimate it. An entity that uses a method that employs different volatilities during the contractual term shall disclose the range of expected volatilities used and the weighted-average expected volatility. A thinly-traded or nonpublic entity that uses the calculated value method shall disclose the reasons why it is not practicable for the Company to estimate the expected volatility of its share price, the appropriate industry sector index that it has selected, the reasons for selecting that particular index, and how it has calculated historical volatility using that index.The Company uses the average historical volatility of the comparable companies over the expected contractual life of the share options or similar instruments as its expected volatility. If shares of a company are thinly traded the use of weekly or monthly price observations would generally be more appropriate than the use of daily price observations as the volatility calculation using daily observations for such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market.
|
|
·
|
Expected annual rate of quarterly dividends. An entity that uses a method that employs different dividend rates during the contractual term shall disclose the range of expected dividends used and the weighted-average expected dividends. The expected dividend yield is based on the Company’s current dividend yield as the best estimate of projected dividend yield for periods within the expected contractual life of the option and similar instruments.
|
|
·
|
Risk-free rate(s). An entity that uses a method that employs different risk-free rates shall disclose the range of risk-free rates used. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods within the contractual life of the option and similar instruments.
|
Pursuant to Paragraphs 505-50-25-8, if fully vested, non-forfeitable equity instruments are issued at the date the grantor and grantee enter into an agreement for goods or services (no specific performance is required by the grantee to retain those equity instruments), then,
because of the elimination of any obligation on the part of the counterparty to earn the equity instruments, a measurement date has been reached.
A grantor shall recognize the equity instruments when they are issued (in most cases, when the agreement is entered into). Whether the corresponding cost is an immediate expense or a prepaid asset (or whether the debit should be characterized as contra-equity under the requirements of paragraph
505-50-45-1) depends on the specific facts and circumstances. Pursuant to ASC paragraph 505-50-45-1, a grantor may conclude that an asset (other than a note or a receivable) has been received in return for fully vested, non-forfeitable equity instruments that are issued at the date the grantor and grantee enter into an agreement for goods or services (and no specific performance is required by the grantee in order to retain those equity instruments).
Such an asset shall not be displayed as contra-equity by the grantor of the equity instruments. The transferability (or lack thereof) of the equity instruments shall not affect the balance sheet display of the asset. This guidance is limited to transactions in which equity instruments are transferred to other than employees in exchange for goods or services. Section
505-50-30 provides guidance on the determination of the measurement date for transactions that are within the scope of this Subtopic.
Pursuant to Paragraphs 505-50-25-8 and 505-50-25-9,an entity may grant fully vested, non-forfeitable equity instruments that are exercisable by the grantee only after a specified period of time if the terms of the agreement provide for earlier exercisability if the grantee achieves specified performance conditions.
Any measured cost of the transaction shall be recognized in the same period(s) and in the same manner as if the entity had paid cash for the goods or services or used cash rebates as a sales discount instead of paying with, or using, the equity instruments. A recognized asset, expense, or sales discount shall not be reversed if a stock option that the counterparty has the right to exercise expires unexercised.
Pursuant to ASC paragraph 505-50-30-S99-1, if the Company receives a right to receive future services in exchange for unvested, forfeitable equity instruments, those equity instruments are treated as unissued for accounting purposes until the future services are received (that is, the instruments are not considered issued until they vest). Consequently, there would be no recognition at the measurement date and no entry should be recorded.
Income Tax Provision
The Company accounts for income taxes under Section 740-10-30 of the FASB Accounting Standards Codification, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are based on the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse.Deferred tax assets are reduced by a valuation allowance to the extent management concludes it ismore likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the consolidated statements of income and comprehensive income (loss) in the period that includes the enactment date.
The Company adopted section 740-10-25 of the FASB Accounting Standards Codification (“Section 740-10-25”). Section 740-10-25 addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under Section 740-10-25, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than fifty (50) percent likelihood of being realized upon ultimate settlement. Section 740-10-25 also provides guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures.
The estimated future tax effects of temporary differences between the tax basis of assets and liabilities are reported in the accompanying consolidated balance sheets, as well as tax credit carry-backs and carry-forwards. The Company periodically reviews the recoverability of deferred tax assets recorded on its consolidated balance sheets and provides valuation allowances as management deems necessary.
Management makes judgments as to the interpretation of the tax laws that might be challenged upon an audit and cause changes to previous estimates of tax liability. In addition, the Company operates within multiple taxing jurisdictions and is subject to audit in these jurisdictions. In management’s opinion, adequate provisions for income taxes have been made for all years. If actual taxable income by tax jurisdiction varies from estimates, additional allowances or reversals of reserves may be necessary.
Uncertain Tax Positions
The Company did not take any uncertain tax positions and had no adjustments to its income tax liabilities or benefits pursuant to the provisions of Section 740-10-25 for the reporting period ended December 31, 2013 or 2012.
Limitation on Utilization of NOLs due to Change in Control
Pursuant to the Internal Revenue Code Section 382 (“Section 382”), certain ownership changes may subject the NOL’s to annual limitations which could reduce or defer the NOL. Section 382 imposes limitations on a corporation’s ability to utilize NOLs if it experiences an “ownership change.” In general terms, an ownership change may result from transactions increasing the ownership of certain stockholders in the stock of a corporation by more than 50 percentage points over a three-year period. In the event of an ownership change, utilization of the NOLs would be subject to an annual limitation under Section 382 determined by multiplying the value of its stock at the time of the ownership change by the applicable long-term tax-exempt rate. Any unused annual limitation may be carried over to later years. The imposition of this limitation on its ability to use the NOLs to offset future taxable income could cause the Company to pay U.S. federal income taxes earlier than if such limitation were not in effect and could cause such NOLs to expire unused, reducing or eliminating the benefit of such NOLs.
Net Income (Loss)per Common Share
Net income (loss) per common share is computed pursuant to section 260-10-45 of the FASB Accounting Standards Codification. Basic net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock outstanding during the period. Diluted net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock and potentially outstanding shares of common stock during the period to reflect the potential dilution that could occur from common shares issuable through contingent shares issuance arrangement, stock options or warrants.
The following table shows the potentially outstanding dilutive common shares excluded from the diluted net income (loss) per common share calculation as they were anti-dilutive:
|
|
|
Potentially Outstanding Dilutive Common Shares
|
|
|
|
|
For Interim
PeriodEnded
December 31, 2013
|
|
For Interim
Period Ended
December 31, 2012
|
|
Make Good Escrow Shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Make Good Escrow Agreement shares issued and held with the escrow agent in connection with the Share Exchange Agreement consummated on June 23, 2011 pending the achievement by the Company of certain post-Closing business milestones (the “Milestones”).
|
|
|
-
|
|
3,000,000
|
|
|
|
|
|
|
|
|
Sub-total Make Good Escrow Shares
|
|
|
-
|
|
3,000,000
|
|
Convertible Note Shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
On March 7, 2012, the Company issued a convertible note in the principal amount of $200,000 with interest at 10% per annum due one (1) year from the date of issuance with the conversion price to be the same as the next private placement price on a per share basis, provided that the Company completes a private placement with gross proceeds of at least $100,000. On August 6, 2012, the Company completed the very next private placement at $0.46875 per share with gross proceeds of at least $100,000. On March 15, 2013, the above note was cancelled and reissued with a new convertible note consisting of the prior principal amount and the entire accrued unpaid interest for the total amount of $220,438 with interest at 12% per annum convertible at $0.25 per share due on September 30, 2013. The note is currently past due with no penalty and the Company continues to accrue the interest at 10% per annum.
|
|
|
881,752
|
|
426,667
|
|
|
|
|
|
|
|
|
On May 30, 2012, the Company issued a convertible note in the principal amount of $200,000 with interest at 10% per annum due one (1) year from the date of issuance with the unpaid principal and any accrued and unpaid interest thereon convertible, as of the Conversion Date, at the lower of (a) the price per share at which shares of capital stock issued in the Financing are sold in the Financing, or (b) the closing price of the Company's securities if traded on a securities exchange, or if actively traded over-the-counter, the average closing bid price for the securi1ies, in each case over the thirty (30) day period prior to the Conversion Date; provided however, that if no active trading market for the securities exists at the time of the conversion, such amount shall be the fair market value of a share of the Company's common stock as determined in good faith by Company's Board of Directors. A "Financing" means the closing of the sale of shares of capital stock of the Company in the first equity financing transaction after the date first set forth above, in which the Company receives gross proceeds of at least $100,000, excluding conversion of this Note. The note is currently past due with no penalty and the Company continues to accrue the interest at 10% per annum.
|
|
|
1,739,130
|
|
426,667
|
|
|
|
|
|
|
|
|
On February 26, 2013, the Company issued two (2) convertible notes in the principal amount of $250,000 and $100,000, respectively, convertible at $0.25 per share, with interest at 12% per annum due on September 30, 2013. The note is currently past due with no penalty and the Company continues to accrue the interest at 12% per annum.
|
|
|
1,400,000
|
|
-
|
|
|
|
|
|
|
|
|
On July 16, 2013, the Company issued a convertible note in the principal amount of $111,111 with a 10% Original Issuance Discount ("OID") and a one-time interest charge of 12% after 90 days. The note is due one (1) year from the date of issuance with the conversion price at 65% of the lowest trade price for the 25 trade day period before the conversion date.
|
|
|
1,762,228
|
|
-
|
|
|
|
|
|
|
|
|
On August 27, 2013, the Company issued a convertible note in the principal amount of $153,500, convertible at 65% of the three lowest bids for 30 trading days before the conversion date with interest at 8% per annum, due on May 26, 2014.
|
|
|
2,353,573
|
|
-
|
|
|
|
|
|
|
|
|
On September 26, 2013, the Company issued a convertible note in the principal amount of $27,778 with a 10% Original Issuance Discount ("OID") and a one-time interest charge of 12%. The note is due one (1) year from the date of issuance with the conversion price at 65% of the lowest trade price for the 25 trade day period before the conversion date.
|
|
|
440,571
|
|
-
|
|
|
|
|
|
|
|
|
On October 15, 2013, the Company issued a convertible note in the principal amount of $58,000 with an $8,000 Original Issuance Discount ("OID") and with interest at 10% per annum, convertible at $0.20 per share, due on May 1, 2014.
|
|
|
290,000
|
|
-
|
|
|
|
|
|
|
|
|
On November 21, 2013, the Company issued a convertible note in the principal amount of $53,000, convertible at 65% of the three lowest bids for 30 trading days before the conversion date with interest at 8% per annum, due on August 25, 2014.
|
|
|
812,634
|
|
-
|
|
|
|
|
|
|
|
|
On December 9, 2013, the Company issued a convertible note in the principal amount of $55,556 with a 10% Original Issuance Discount ("OID") and 12% one time interest. The note is due one (1) year from the date of issuance with the conversion price at 65% of the lowest trade price for the 25 trade day period before the conversion date .
|
|
|
881,142
|
|
-
|
|
|
|
|
|
|
|
|
Sub-total Convertible Note Shares
|
|
|
10,561,070
|
|
853,334
|
|
Warrant Shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
On August 6, 2012, the Company issued (i) warrants to purchase 1,066,667 shares, in the aggregate, of the Company’s common stock to investors (the “investor warrants”) and (ii) warrants to purchase 85,333 shares of the Company's common stock to the placement agent (the "agent warrants") with an exercise price of $0.6405 per share, subject to certain adjustments pursuant to Section 3(b) Subsequent Equity Sales of the SPA, expiring five (5) years from the date of issuance. On February 26, 2013, warrantsissued subsequent to these warrantstriggered a reset of these warrants exercise price to $0.25 per share and the shares to be issued under the warrants were adjusted to 2,951,424 shares accordingly. On May 8, 2013, the Company completed a private placement at $0.20 per share with gross proceeds more than $100,000; this event triggered the reset of the conversion price of the convertible note to $0.20 per share and the shares to be issued under the warrants were adjusted to 3,689,280 shares accordingly. On May 8, 2013, investors exercised the warrants to purchase 2,732,799 shares (853,333 original shares) at $0.20 per share.
|
|
|
956,481
|
|
1,152,000
|
|
|
|
|
|
|
|
|
On February 26, 2013, the Company issued warrants to purchase 1,000,000 and 400,000 shares respectively, or 1,400,000 shares in the aggregate, of the Company’s common stock to two (2) note holders in connection with the issuance of convertible notes.
|
|
|
1,400,000
|
|
-
|
|
|
|
|
|
|
|
|
On March 15, 2013, the Company issued a warrant to purchase 881,753 shares of the Company’s common stock to the note holder in connection with the issuance of the convertible note.
|
|
|
881,753
|
|
-
|
|
|
|
|
|
|
|
|
On May 6, 2013, the Company issued three (3) series of warrants:
Series A warrants include (i) warrants to purchase 1,877,333 shares of the Company’s common stock to the investor and (ii) warrants to purchase 150,187 shares of the Company's common stock to the placement agent (the "agent warrants") with an exercise price of $0.20 per share expiring five (5) years from the date of issuance.
|
|
|
2,027,520
|
|
-
|
|
|
|
|
|
|
|
|
Series B warrants include (i) warrants to purchase 1,066,666 shares of the Company’s common stock to the investor and (ii) warrants to purchase 85,333 shares of the Company's common stock to the placement agent (the "agent warrants") with an exercise price of $0.25 per share expiring five (5) years from the date of issuance.
|
|
|
1,151,999
|
|
-
|
|
|
|
|
|
|
|
|
Series C warrants include (i) warrants to purchase 2,346,666 shares of the Company’s common stock to the investor and (ii) warrants to purchase 187,733 shares of the Company's common stock to the placement agent (the "agent warrants") with an exercise price of $0.25 per share expiring five (5) years from the date of issuance. The warrants are exercisable under the condition of Series A warrants are exercised.
|
|
|
2,534,399
|
|
-
|
|
|
|
|
|
|
|
|
On October 15, 2013, the Company issued warrants to purchase 1,000,000 shares of the Company’s common stock to a note holder with an exercise price of $0.25 per share in connection with the issuance of convertible note.
|
|
|
1,000,000
|
|
-
|
|
|
|
|
|
|
|
|
Sub-total Warrant Shares
|
|
|
9,952,152
|
|
1,152,000
|
|
|
|
|
|
|
|
|
Total potentially outstanding dilutive common shares
|
|
|
20,513,222
|
|
5,005,334
|
Cash Flows Reporting
The Company adopted paragraph 230-10-45-24 of the FASB Accounting Standards Codification for cash flows reporting, classifies cash receipts and payments according to whether they stem from operating, investing, or financing activities and provides definitions of each category, and uses the indirect or reconciliation method (“Indirect method”) as defined by paragraph 230-10-45-25 of the FASB Accounting Standards Codification to report net cash flow from operating activities by adjusting net income to reconcile it to net cash flow from operating activities by removing the effects of (a) all deferrals of past operating cash receipts and payments and all accruals of expected future operating cash receipts and payments and (b) all items that are included in net income that do not affect operating cash receipts and payments. The Company reports the reporting currency equivalent of foreign currency cash flows, using the current exchange rate at the time of the cash flows and the effect of exchange rate changes on cash held in foreign currencies is reported as a separate item in the reconciliation of beginning and ending balances of cash and cash equivalents and separately provides information about investing and financing activities not resulting in cash receipts or payments in the period pursuant to paragraph 830-230-45-1 of the FASB Accounting Standards Codification.
Subsequent Events
The Company follows the guidance in Section 855-10-50 of the FASB Accounting Standards Codification for the disclosure of subsequent events. The Company will evaluate subsequent events through the date when the financial statements are issued. Pursuant to ASU 2010-09 of the FASB Accounting Standards Codification, the Company as an SEC filer considers its financial statements issued when they are widely distributed to users, such as through filing them on EDGAR.
Recently Issued Accounting Pronouncements
In February 2013, the FASB issued ASU No. 2013-02, "
Comprehensive Income (Topic 220): Reporting of Amounts Reclassified Out of Accumulated Other Comprehensive Income.
" The ASUadds new disclosure requirements for items reclassified out of accumulated other comprehensive income by component and their corresponding effect on net income. The ASU is effective for public entities for fiscal years beginning after December 15, 2013.
In February 2013, the Financial Accounting Standards Board, or FASB, issued ASU No. 2013-04, "
Liabilities (Topic 405): Obligations Resulting from Joint and Several Liability Arrangements for which the Total Amount of the Obligation Is Fixed at the Reporting Date
." This ASU addresses the recognition, measurement, and disclosure of certain obligations resulting from joint and several arrangements including debt arrangements, other contractual obligations, and settled litigation and judicial rulings. The ASU is effective for public entities for fiscal years, and interim periods within those years, beginning after December 15, 2013.
In March 2013, the FASB issued ASU No. 2013-05, "
Foreign Currency Matters (Topic 830): Parent's Accounting for the Cumulative Translation Adjustment upon Derecognition of Certain Subsidiaries or Groups of Assets within a Foreign Entity or of an Investment in a Foreign Entity
." This ASU addresses the accounting for the cumulative translation adjustment when a parent either sells a part or all of its investment in a foreign entity or no longer holds a controlling financial interest in a subsidiary or group of assets that is a nonprofit activity or a business within a foreign entity. The guidance outlines the events when cumulative translation adjustments should be released into net income and is intended by FASB to eliminate some disparity in current accounting practice. This ASU is effective prospectively for fiscal years, and interim periods within those years, beginning after December 15, 2013.
In March 2013, the FASB issued ASU 2013-07,
“Presentation of Financial Statements (Topic 205): Liquidation Basis of Accounting.”
The amendments require an entity to prepare its financial statements using the liquidation basis of accounting when liquidation is imminent. Liquidation is imminent when the likelihood is remote that the entity will return from liquidation and either (a) a plan for liquidation is approved by the person or persons with the authority to make such a plan effective and the likelihood is remote that the execution of the plan will be blocked by other parties or (b) a plan for liquidation is being imposed by other forces (for example, involuntary bankruptcy). If a plan for liquidation was specified in the entity’s governing documents from the entity’s inception (for example, limited-life entities), the entity should apply the liquidation basis of accounting only if the approved plan for liquidation differs from the plan for liquidation that was specified at the entity’s inception. The amendments require financial statements prepared using the liquidation basis of accounting to present relevant information about an entity’s expected resources in liquidation by measuring and presenting assets at the amount of the expected cash proceeds from liquidation. The entity should include in its presentation of assets any items it had not previously recognized under U.S. GAAP but that it expects to either sell in liquidation or use in settling liabilities (for example, trademarks). The amendments are effective for entities that determine liquidation is imminent during annual reporting periods beginning after December 15, 2013, and interim reporting periods therein. Entities should apply the requirements prospectively from the day that liquidation becomes imminent. Early adoption is permitted.
Management does not believe that any other recently issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on the accompanying financial statements.
Note 3 – Going Concern
The consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates continuity of operations, realization of assets, and liquidation of liabilities in the normal course of business.
As reflected in the consolidated financial statements, the Company had an accumulated deficit at December 31, 2013, a net loss and net cash used in operating activities for the reporting period then ended. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
The Company is attempting to generate sufficient revenue; however, the Company’s cash position may not be sufficient to support its daily operations.While the Company believes in the viability of its strategy to generate sufficient revenue and in its ability to raise additional funds, there can be no assurances to that effect. The ability of the Company to continue as a going concern is dependent upon its ability to further implement its business plan and generate sufficient revenue and its ability to raise additional funds.
The consolidated financial statements do not include any adjustments related to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
Note 4 – Inventory - Seeds
Inventory – seeds consisted of the following:
|
|
|
December 31, 2013
|
|
|
March 31, 2013
|
|
|
|
|
|
|
|
|
|
|
|
|
Seeds (*)
|
|
$
|
1,807,000
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,807,000
|
|
|
$
|
-
|
|
* The company acquired certain seeds in the amount of $1,807,000 in aggregate which was used for preparation of the fall planting for this spring harvest which will start from the second half of February, 2014 and last through April, 2014, $1,042,000 of which was in default. The vendor sold its accounts receivable of $1,042,000 to a third party, which sued the Company and settled the accounts payable and related legal costs and fees with the Company for the issuance of 15,538,882 common shares in aggregate.
Slow-Moving or Obsolescence Markdowns
The Company recorded no inventory obsolescence adjustments for the reporting period ended December 31, 2013 or 2012.
Note 5 – Property and Equipment
(i)
Depreciation Expense
Depreciation expense was $3,100 and $762 for the interim period ended December 31, 2013 and 2012, respectively.
Note 6 – Acquired Technology
On July 5, 2012, the Company acquired the rights to certain technology from Technew Technology Limited in exchange for 3,000,000 restricted shares of the Company's common stock. These restricted shares were valued at $0.79 per share, discounted at 69% taking into consideration its restricted nature and lack of liquidity and consistent trading in the market, for a total value of $1,635,300, which was recorded as acquired technology and is being amortized on a straight-line basis over the acquired technology's estimated useful life of fifteen (15) years.
(i)
Amortization Expense
Amortization expense was $81,765 and $54,510 for the interim period ended December 31, 2013 and 2012, respectively.
Note 7 – Website Development Costs
(i)
Amortization expense
Amortization expense was $801 each for the interim period ended December 31, 2013 and 2012, respectively.
Note 8 – Related Party Transactions
Related parties
Related parties with whom the Company had transactions are:
|
Related Parties
|
|
Relationship
|
|
|
|
|
|
George Blankenbaker
|
|
President and significant stockholder of the Company
|
|
|
|
|
|
Leverage Investments LLC
|
|
An entity owned and controlled by the president and significant stockholder of the Company
|
|
|
|
|
|
Technew Technology Limited
|
|
Non-controlling interest holder
|
|
Growers Synergy Pte Ltd.
|
|
An entity owned and controlled by the president and significant stockholder of the Company
|
|
|
|
|
|
Guangzhou Health Technology Development Company Limited
|
|
An entity owned and controlled by Non-controlling interest holder
|
Advances from
Stockholder
From time to time, stockholder of the Company advances funds to the Company for working capital purpose. Those advances are unsecured, non-interest bearing and due on demand.
Lease of Certain Office Space from
Leverage Investments, LLC
The Company leases certain office space with Leverage Investments, LLC for $500 per month on a month-to-month basis since July 1, 2011. For the interim periods ended December 31, 2013 and 2012, the Company recorded $4,500 each in rent expense, respectively.
Farm Management and Off-Take Agreement with Growers Synergy Pte Ltd.
On November 1, 2011, the Company entered into a Management and Off-Take Agreement (the “Management Agreement”) with Growers Synergy Pte Ltd. (“GSPL”), a Singapore corporation. Under the terms of the Management Agreement, the Company will engage GSPL to supervise the Company’s farm management operations, recommend quality farm management programs for stevia cultivation, assist in the hiring of employees and provide training to help the Company meet its commercialization targets, develop successful models to propagate future agribusiness services, and provide back-office and regional logistical support for the development of proprietary stevia farm systems in Vietnam, Indonesia and potentially other countries. GSPL will provide services at $20,000 per month for a term of two (2) years from the date of signing expiring on November 1, 2013. The Management Agreement may be terminated by the Company upon 30 day notice. In connection with the Management Agreement, the parties agreed to enter into an off-take agreement whereby GSPL agreed to purchase all of the non-stevia crops produced at the Company’s GSPL supervised farms.
On October 31, 2013 ("Effective Date"), the Company extended the Management and Off-Take Agreement (the “Management Agreement”) with GSPL with the same terms and conditions for a period of two (2) years ("Term") from the Effective Date expiring October 31, 2015 and shall automatically be extended for subsequent period of one (1) year expiring October 31, 2016 ("Extended Term") unless earlier terminated in writing.
Farm management services provided by Growers Synergy Pte Ltd. were as follows:
|
|
|
For the interim
period ended
December 31, 2013
|
|
|
For the interim
period ended
December 31, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
Farm management services – related parties
|
|
$
|
180,000
|
|
|
$
|
180,000
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
180,000
|
|
|
$
|
180,000
|
|
Future minimum payments required under this agreement were as follows:
|
Fiscal Year Ending March 31:
|
|
|
|
|
|
|
|
|
|
|
|
2014 (remainder of the fiscal year)
|
|
$
|
240,000
|
|
|
2015
|
|
|
240,000
|
|
|
2016
|
|
|
240,000
|
|
|
2017
|
|
|
140,000
|
|
|
|
|
|
|
|
|
|
$
|
860,000
|
|
Cash Commitment in Connection with the Operations of Stevia Technew
For the fiscal year ended March 31, 2013, Stevia Asia provided Stevia Technew $200,000, all of which has been paid to Guangzhou Health and expended and recorded as farm management services - related parties. On March 1, 2013, the partners agreed to terminate the Cooperative Agreement specific to the investment in an agricultural project and no further obligation by either party related to the payment of $200,000.
Note 9 – Convertible Notes Payable
(i) February 26, 2013 issuance of convertible notes with warrants
On February 26, 2013, the Company entered into two (2) 12% convertible notes payable of $350,000 in aggregate (“Convertible Notes”) with two investors (the “Payees”) maturing on September 30, 2013. The Payees have the option to convert the outstanding notes and interest due into the Company’s common shares at $0.25 per share at any time prior to September 30, 2013. In connection with the issuance of the Convertible Notes, the Company granted to the Payees a warrant to purchase 1,400,000 common shares exercisable at $0.25 per share expiring three (3) years from the date of issuance. The notes were currently past due with no penalty and the Company continues to accrue the interest at 12% per annum.
The Company estimated the relative fair value of these warrants on the date of grant, using the Black-Scholes option-pricing model with the following weighted-average assumptions:
|
Expected option life (year)
|
|
|
3.00
|
|
|
Expected volatility
|
|
|
74.53
|
%
|
|
Risk-free interest rate
|
|
|
0.37
|
%
|
|
Dividend yield
|
|
|
0.00
|
%
|
The relative fair value of these warrants granted, estimated on the date of grant, was $110,425, which was recorded as a discount to the convertible notes payable. After allocating the $110,425 portion of the proceeds to the warrants as a discount to the Convertible Notes, an additional $113,925 was allocated to a beneficial conversion feature by crediting $113,925 to additional paid-in capital and debiting the same amount to the beneficial conversion feature. The Company amortizes the discount and beneficial conversion feature over the term of the Convertible Notes. The amortization of the discount and beneficial conversion feature were fully amortized as of September 30, 2013.
(ii) March 15, 2013 issuance of convertible note with warrant
On March 15, 2013, the Company cancelled a prior convertible note and entered into a 12% convertible note payable of $220,438, which is the total amount of the prior note principal and accrued interest, with the existing investor (the “Payee”), maturing on September 30, 2013. The Payee has the option to convert the outstanding note into the Company’s common shares at $0.25 per share at any time prior to payment in full of the principal balance of the convertible note. In connection with the issuance of the convertible note, the Company granted the Payee a warrant to purchase 881,753 common shares exercisable at $0.25 per share expiring three (3) years from the date of issuance. The note is currently past due with no penalty and the Company continues to accrue the interest at 12% per annum.
The Company estimated the relative fair value of these warrants on the date of grant using the Black-Scholes option-pricing model with the following weighted-average assumptions:
|
Expected option life (year)
|
|
|
3.00
|
|
|
Expected volatility
|
|
|
75.11
|
%
|
|
Risk-free interest rate
|
|
|
0.40
|
%
|
|
Dividend yield
|
|
|
0.00
|
%
|
The relative fair value of these warrants was $98,095, which was recorded as a discount to the convertible note payable. After allocating the $98,095, portion of the proceeds to the warrants as a discount to the convertible note, the effective conversion price of the convertible notes payable was lower than the market price at the date of issuance and per calculation the remaining balance of the face amount was allocated to a beneficial conversion feature by crediting $122,343 to additional paid-in capital and debiting the same amount to the beneficial conversion feature. The Company amortizes the discount and beneficial conversion feature over the term of the convertible note and the amounts were fully amortized as of September 30, 2013.
(iii) October 15, 2013 issuance of convertible note with derivative warrant
General Terms
On October 15, 2013, the Company issued a convertible note in the principal amount of $58,000 convertible at $0.20 per share, with an $8,000 Original Issue Discount ("OID") and interest at 10% per annum maturing on May 1, 2014. The Debenture is secured by 1,250,000 restricted common shares of the Company. The restricted shares will be issued in the name of Black Mountain Equities, Inc. upon closing.
Events of Defaults
An “Event of Default”, wherever used herein, means any one of the following events: (i) An “Event of Default”, wherever used herein, means any one of the following events, (ii) A Conversion Failure; (iii) The Company or any subsidiary of the Company shall commence, or there shall be commenced against the Company or any subsidiary of the Company under any applicable bankruptcy or insolvency laws; (iv) (a) The Company or any subsidiary of the Company shall default in any of its obligations under any other indebtness in an amount exceeding $100,000, whether such indebtedness now exists or shall hereafter be created and (b) The Common Stock is suspended or delisted for trading on the Over the Counter Bulletin Board market (the “Primary Market”), (c) The Company loses its ability to deliver shares via “DWAC/FAST” electronic transfer, (d) The Company loses its status as “DTC Eligible.”, (e) The Company shall become late or delinquent in its filing requirements as a fully-reporting issuer registered with the Securities & Exchange Commission.
Piggyback Registration Rights
The Company shall include on the next registration statement the Company files with SEC (or on the subsequent registration statement if such registration statement is withdrawn) all shares issuable upon conversion of this Note. Failure to do so will result in liquidated damages of 25% of the outstanding principal balance of this Note, but not less than $25,000, being immediately due and payable to the Holder at its election in the form of cash payment or addition to the balance of this Note.
Warrants
In connection with the issuance of the convertible note, the Company granted the note holder a warrant to purchase 1,000,000 common shares with an exercise price of $0.25 per share, subject to certain adjustments pursuant to Section 3(b) Subsequent Equity Sales and Section 3(c) Subsequent Rights Offerings of the warrant ("full price and share reset provisions") expiring five (5) years from the date of issuance.
Pursuant to Section 3 (b) Subsequent Equity Sales if the Company or any Subsidiary thereof, as applicable, at any time while this Warrant is outstanding, shall sell or grant any option to purchase, or sell or grant any right to re-price, or otherwise dispose of or issue (or announce any offer, sale, grant or any option to purchase or other disposition) any Common Stock or Common Stock Equivalents entitling any Person to acquire shares of Common Stock, at an effective price per share less than the then Exercise Price (such lower price, the “Base Share Price” and such issuances collectively, a “Dilutive Issuance”) (if the holder of the Common Stock or Common Stock Equivalents so issued shall at any time, whether by operation of purchase price adjustments, reset provisions, floating conversion, exercise or exchange prices or otherwise, or due to warrants, options or rights per share which are issued in connection with such issuance, be entitled to receive shares of Common Stock at an effective price per share which is less than the Exercise Price, such issuance shall be deemed to have occurred for less than the Exercise Price on such date of the Dilutive Issuance), then the Exercise Price shall be reduced and only reduced to equal the Base Share Price and the number of Warrant Shares issuable hereunder shall be increased such that the aggregate Exercise Price payable hereunder, after taking into account the decrease in the Exercise Price, shall be equal to the aggregate Exercise Price prior to such adjustment.
Pursuant to Section 3 (c) Subsequent Rights Offerings if the Company, at any time while the Warrant is outstanding, shall issue rights, options or warrants to all holders of Common Stock (and not to Holders) entitling them to subscribe for or purchase shares of Common Stock at a price per share less than the VWAP at the record date, then the Exercise Price shall be multiplied by a fraction, of which the denominator shall be the number of shares of the Common Stock outstanding on the date of issuance of such rights or warrants plus the number of additional shares of Common Stock offered for subscription or purchase, and of which the numerator shall be the number of shares of the Common Stock outstanding on the date of issuance of such rights or warrants plus the number of shares which the aggregate offering price of the total number of shares so offered (assuming receipt by the Company in full of all consideration payable upon exercise of such rights, options or warrants) would purchase at such VWAP.
The fair value of note derivative liability and the warrant liability were $11,428 and $76,647, respectively, or $88,075 in aggregate; $50,000 of which was recorded as a discount to the convertible note and the $38,075 remaining balance was recorded as other expense. The Company amortizes OID and the discount to the note over the term of the convertible note and marks to market the warrant value as of each quarter end.
Convertible notes payable consisted of the following:
|
|
|
December 31, 2013
|
|
|
March 31, 2013
|
|
|
On May 30, 2012, the Company issued a convertible note in the principal amount of $200,000 with interest at 10% per annum due one (1) year from the date of issuance with the unpaid principal of this note and any accrued and unpaid interest thereon, as of the Conversion Date, at the lower of (a) the price per share at which shares of capital stock issued in the Financing are sold in the Financing, or (b) the closing price of the Company's securities if traded on a securities exchange, or if actively traded over-the-counter, the average closing bid price for the securi1ies, in each case over the thirty (30) day period prior to the Conversion Date; provided however, that if no active trading market for the securities exists at the time of the conversion, such amount shall be the fair market value of a share of the Company's common stock as determined in good faith by Company's Board of Directors. A "Financing" means the closing of the sale of shares of capital stock of the Company in the first equity financing transaction after the date first set forth above, in which the Company receives gross proceeds of at least $100,000, excluding conversion of this Note. The note is currently past due with no penalty and the Company continues to accrue the interest at 10% per annum.
|
|
|
200,000
|
|
|
|
200,000
|
|
|
|
|
|
|
|
|
|
|
|
|
February 26, 2013 convertible notes
|
|
|
350,000
|
|
|
|
350,000
|
|
|
|
|
|
|
|
|
|
|
|
|
March 15, 2013 convertible note
|
|
|
220,438
|
|
|
|
220,438
|
|
|
|
|
|
|
|
|
|
|
|
|
On July 16, 2013, the Company issued a convertible note in the principal amount of $111,111 with a 10% Original Issuance Discount ("OID") and a one-time interest charge of 12% after 90 days. The note is due one (1) year from the date of issuance with the conversion price at 65% of the lowest trade price for the 25 trade day period before the conversion date .
|
|
|
111,111
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
On August 27, 2013, the Company issued a convertible notes in the principal amount of $153,500 convertible at 65% of the three lowest bids for 30 trading days before the conversion date with interest at 8% per annum due on May 26, 2014.
|
|
|
153,500
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
On September 26, 2013, the Company issued a convertible note in the principal amount of $27,778 with a 10% Original Issuance Discount ("OID") and a one-time interest charge of 12%. The note is due one (1) year from the date of issuance with the conversion price at 65% of the lowest trade price for the 25 trade day period before the conversion date .
|
|
|
27,778
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
On October 15, 2013, the Company issued a convertible note in the principal amount of $58,000 convertible at $0.20 per share, with an $8,000 Original Issue Discount ("OID") and interest at 10% per annum maturing on May 1, 2014. The Debenture is secured by 1,250,000 restricted common shares of the Company. In connection with the issuance of the convertible note, the Company granted the note holder a warrant to purchase 1,000,000 common shares with an exercise price of $0.25 per share, subject to certain adjustments pursuant to Section 3(b) Subsequent Equity Sales and Section 3(c) Subsequent Rights Offerings of the warrant ("full price and share reset provisions") expiring five (5) years from the date of issuance.
|
|
|
58,000
|
|
|
|
-
|
|
|
On November 21, 2013, the Company issued a convertible note in the principal amount of $53,000, convertible at 65% of the three lowest bids for 30 trading days before the conversion date, with interest at 8% per annum, due on August 25, 2014.
|
|
|
53,000
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
On December 9, 2013, the Company issued a convertible note in the principal amount of $55,556 with a 10% Original Issuance Discount ("OID") and a one-time interest charge of 12%. The note is due one (1) year from the date of issuance with the conversion price at 65% of the lowest trade price for the 25 trade day period before the conversion date .
|
|
|
55,556
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Sub-total: convertible notes payable
|
|
|
1,229,383
|
|
|
|
770,438
|
|
|
|
|
|
|
|
|
|
|
|
|
Discount representing (i) the relative fair value of the warrants issued, (ii) the beneficial conversion features and (iii) the derivative liability on conversion features
|
|
|
(816,310
|
)
|
|
|
(444,788
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated amortization of discount on convertible notes payable
|
|
|
658,496
|
|
|
|
32,050
|
|
|
|
|
|
|
|
|
|
|
|
|
Remaining discount
|
|
|
(157,814
|
)
|
|
|
(412,738
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,071,569
|
|
|
$
|
357,770
|
|
Note 10 – Derivative Instruments and the Fair Value of Financial Instruments
(i) Warrants Issued
Description of Warrants and Fair Value on Date of Grant
On August 6, 2012, the Company issued (i) warrants to purchase 1,066,667 shares of the Company’s common stock to the investors (the “investors warrants”) and (ii) warrants to purchase 85,333 shares of the Company's common stock to the placement agent (the "agent warrants")with an exercise price of $0.6405 per share, subject to certain adjustments, pursuant to Section 3(b) Subsequent Equity Sales of the SPA, expiring five (5) years from the date of issuance.
On February 26, 2013 and March 15, 2013 the Company issued warrants with an exercise price of $0.25 per share. Pursuant to Section 3(b), the previously issued warrants’ exercise price was reset to $0.25 per share and the number of warrant shares was increased to 2,732,801 and 218,623, respectively, for a total of 2,951,424.
On May 6, 2013, the Company issued warrants with an exercise price of $0.25 per share. Pursuant to Section 3(b), the previously issued warrants' exercise price was reset again to $0.20 per share and the number of warrant shares was increased to 3,416,001 and 273,279, respectively, for a total of 3,689,280. On May 6, 2013, investors exercised warrants to purchase 2,732,799 (out of 3,416,001) shares of the Company’s common stock at $0.20 per share.
On May 6, 2013, the Company issued (i) warrants to purchase 1,877,333 (Series A), 1,066,667 (Series B) and 2,346,666 (Series C), in aggregate 5,290,665 shares of the Company’s common stock to the investors (the “investor warrants”) and (ii) warrants to purchase 150,187 (Series A), 85,333 (Series B) and 187,733 (Series C) shares of the Company's common stock to the placement agent (the "agent warrants")with an exercise price of $0.20 (Series A) per share, $0.25 (Series B) per share and $0.25 (Series C) per share subject to certain adjustments, pursuant to Section 3(b), expiring five (5) years from the date of issuance.
On October 15, 2013, the Company issued a warrant to purchase 1,000,000 common shares with an exercise price at $0.25 per share with full ratchet reset features expiring five (5) years from the date of issuance in connection with the issuance of a convertible note.
Derivative Analysis
Because these warrants have full reset adjustments tied to future issuances of equity securities by the Company, they are subject to derivative liability treatment under Section 815-40-15 of the FASB Accounting Standard Codification (“Section 815-40-15”).
Valuation of Derivative Liability
(a)
Valuation Methodology
The Company’s August 6, 2012 and May 6, 2013 warrants do not trade in an active securities market, as such, the Company developed a Lattice model that values the derivative liability of the warrants based on a probability weighted discounted cash flow model. This model is based on future projections of the various potential outcomes. The features that were analyzed and incorporated into the model included the exercise feature and the full ratchet reset.
Based on these features, there are two primary events that can occur; the Holder exercises the Warrants or the Warrants are held to expiration. The model analyzed the underlying economic factors that influenced which of these events would occur, when they were likely to occur, and the specific terms that would be in effect at the time (i.e. stock price, exercise price, volatility, etc.). Projections were then made on these underlying factors which led to a set of potential scenarios. As the result of the large Warrant overhang we accounted for the dilution affects, volatility and market cap to adjust the projections.
Probabilities were assigned to each of these scenarios based on management projections. This led to a cash flow projection and a probability associated with that cash flow. A discounted weighted average cash flow over the various scenarios was completed to determine the value of the derivative warrant liability.
(b)
Valuation Assumptions
The Company’s 2013 derivative warrants were valued at each period ending date with the following assumptions:
|
·
|
The stock price would fluctuate with the Company projected volatility.
|
|
·
|
The stock price would fluctuate with an annual volatility. The projected volatility curve was based on historical volatilities of the Company for the valuation periods.
|
|
·
|
The Holder would exercise the warrant as they become exercisable (effective registration is projected 4 months from issuance and the earliest exercise is projected 180 days from issuance) at target prices of 2 times the higher of the projected reset price or stock price.
|
|
·
|
The Holder would exercise the warrant at maturity if the stock price was above the project reset prices.
|
|
·
|
A 100% probability of a reset event and a projected financing each quarter for 3 years at prices approximating 93% of market
|
|
·
|
The Warrants with an exercise price of $0.25 exercise price is projected to reset to $0.047 at maturity; the Warrants with an exercise price of $0.20 per share is projected to reset to $0.043at maturity
|
|
·
|
The Company had no reset event during this quarter period ending 12/31/2013. Prior reset events occurred on 2/26/2013 to $0.25 and 5/6/2013 to $0.20.
|
|
·
|
No warrants have expired. Warrants with full reset feature issued during this quarter period ending 12/31/2013
|
|
·
|
The projected volatility curve for the valuation dates was:
|
|
|
|
|
1 Year
|
|
|
2 Year
|
|
|
3 Year
|
|
|
4 Year
|
|
|
5 Year
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
August 6, 2012
|
|
|
129%
|
|
|
178%
|
|
|
218%
|
|
|
252%
|
|
|
281%
|
|
|
September 30, 2012
|
|
|
127%
|
|
|
173%
|
|
|
211%
|
|
|
244%
|
|
|
272%
|
|
|
March 31, 2013
|
|
|
122%
|
|
|
167%
|
|
|
205%
|
|
|
236%
|
|
|
264%
|
|
|
December 31, 2013
|
|
|
111%
|
|
|
168%
|
|
|
202%
|
|
|
233%
|
|
|
261%
|
|
(c)
Fair Value of Derivative Warrants
The table below provides a summary of the fair value of the derivative warrant liability and the changes in the fair value of the derivative warrants to purchase 2,951,424 (reset to 6,247,146 on May 1, 2013) shares of the Company’s common stock, including net transfers in and/or out, of derivative warrants measured at fair value on a recurring basis using significant unobservable inputs (Level 3).
|
|
Derivative warrants
Assets (Liability)
|
|
Total
|
|
|
|
|
|
|
Balance, September 30, 2012
|
|
$
|
(180,284
|
)
|
|
|
$
|
(180,284
|
)
|
|
Total gains or losses (realized/unrealized) included in:
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
(305,829
|
)
|
|
|
|
(305,829
|
)
|
|
Other comprehensive income (loss)
|
|
|
-
|
|
|
|
|
-
|
|
|
Purchases, issuances and settlements
|
|
|
-
|
|
|
|
|
-
|
|
|
Transfers in and/or out of Level 3
|
|
|
-
|
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2013
|
|
|
(486,113
|
)
|
|
|
|
(486,113
|
)
|
|
Total gains or losses (realized/unrealized) included in:
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
675,949
|
|
|
|
|
675,949
|
|
|
Other comprehensive income (loss)
|
|
|
-
|
|
|
|
|
-
|
|
|
Purchases, issuances and settlements
|
|
|
(787,355
|
)
|
|
|
|
(787,355
|
)
|
|
Transfers in and/or out of Level 3
|
|
|
-
|
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2013
|
|
$
|
(597,519
|
)
|
|
|
$
|
(597,519
|
)
|
(d)
Warrants Outstanding
As of December 31, 2013, 853,333 warrants (2,732,799 warrants after the exercise price being reset to $0.20 per share) have been exercised and warrants to purchase 9,952,152 shares of Company common stock remain outstanding.
The table below summarizes the Company’s derivative warrant activity:
|
|
|
Warrant Activities
|
|
APIC
|
|
(Gain) Loss
|
|
|
|
|
Derivative
Shares
|
|
Non-derivative
Shares
|
|
Total Warrant
Shares
|
|
Fair Value of
Derivative
Warrants
|
|
Reclassification
of Derivative
Liability
|
|
Change in
Fair Value of
Derivative
Liability
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative warrant at August 6, 2012
|
|
|
1,152,000
|
|
|
-
|
|
|
1,152,000
|
|
|
(411,805
|
)
|
|
-
|
|
|
-
|
|
|
Mark to market
|
|
|
|
|
|
|
|
|
|
|
|
231,521
|
|
|
|
|
|
(231,521
|
)
|
|
Derivative warrant at September 30, 2012
|
|
|
1,152,000
|
|
|
-
|
|
|
1,152,000
|
|
|
(180,284
|
)
|
|
|
|
|
|
|
|
Mark to market
|
|
|
|
|
|
|
|
|
|
|
|
(73,723
|
)
|
|
|
|
|
(73,723
|
)
|
|
Derivative warrant at December 31, 2012
|
|
|
1,152,000
|
|
|
-
|
|
|
1,152,000
|
|
|
(106,561
|
)
|
|
|
|
|
|
|
|
Reset of warrant shares
|
|
|
1,799,424
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mark to market
|
|
|
|
|
|
|
|
|
|
|
|
(379,552
|
)
|
|
|
|
|
379,552
|
|
|
Derivative warrant at March 31, 2013
|
|
|
2,951,424
|
|
|
-
|
|
|
2,951,424
|
|
|
(486,113
|
)
|
|
|
|
|
|
|
|
Exercise of warrants on May 6, 2013
|
|
|
(2,732,799
|
)
|
|
-
|
|
|
(2,732,799
|
)
|
|
-
|
|
|
-
|
|
|
-
|
|
|
Issuance of warrants on May 6, 2013
|
|
|
5,713,918
|
|
|
-
|
|
|
5,713,918
|
|
|
(106,360
|
)
|
|
-
|
|
|
-
|
|
|
Reset of warrant shares
|
|
|
737,856
|
|
|
|
|
|
737,856
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of warrants on Oct 15, 2013
|
|
|
1,000,000
|
|
|
|
|
|
1,000,000
|
|
|
(76,647
|
)
|
|
|
|
|
|
|
|
Mark to market
|
|
|
|
|
|
|
|
|
|
|
|
299,373
|
|
|
|
|
|
(299,373)
|
|
|
Derivative warrant at December 31, 2013
|
|
|
7,670,399
|
|
|
-
|
|
|
7,670,399
|
|
|
(369,747
|
)
|
|
|
|
|
|
|
(ii) Warrant Activities
The table below summarizes the Company’s warrant activities through December 31, 2013:
Summary of the Company’s Warrant Activities
The table below summarizes the Company’s warrant activities:
|
|
|
Number of
Warrant Shares
|
|
|
Exercise Price
Range
Per Share
|
|
|
Weighted
Average
Exercise Price
|
|
|
Fair Value
at Date
of Issuance
|
|
|
Aggregate
Intrinsic
Value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2013
|
|
|
5,233,177
|
|
|
$
|
0.20
|
|
|
$
|
0.20
|
|
|
$
|
620,325
|
|
|
$
|
-
|
|
|
Issuance of warrant shares Pursuant to Section 3(b) Subsequent Equity Sales
|
|
|
737,856
|
|
|
|
0.20
|
|
|
|
0.20
|
|
|
|
|
|
|
|
-
|
|
|
Granted
|
|
|
6,713,918
|
|
|
|
0.20 - 0.25
|
|
|
|
0.23
|
|
|
|
183,007
|
|
|
|
-
|
|
|
Canceled
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
Exercised
|
|
|
(2,732,799
|
)
|
|
|
0.20
|
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
Expired
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
Balance, December 31, 2013
|
|
|
9,952,152
|
|
|
$
|
0.20 - 0.25
|
|
|
$
|
0.23
|
|
|
$
|
803,332
|
|
|
$
|
-
|
|
|
Earned and exercisable, December 31, 2013
|
|
|
9,952,152
|
|
|
$
|
0.20 - 0.25
|
|
|
$
|
0.23
|
|
|
$
|
803,332
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unvested, December 31, 2013
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
The following table summarizes information concerning outstanding and exercisable warrants as of
December 31, 2013
:
|
|
|
Warrants Outstanding
|
|
Warrants Exercisable
|
|
|
Range of Exercise Prices
|
|
Number
Outstanding
|
|
Average
Remaining
Contractual
Life (in years)
|
|
Weighted
Average
Exercise Price
|
|
Number
Exercisable
|
|
Average
Remaining
Contractual
Life (in years)
|
|
Weighted
Average
Exercise Price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$0.20 - 0.25
|
|
9,952,152
|
|
4.36
|
|
$
|
0.23
|
|
9,952,152
|
|
4.36
|
|
$
|
0.23
|
|
|
$0.20 - 0.25
|
|
9,952,152
|
|
4.36
|
|
$
|
0.23
|
|
9,952,152
|
|
4.36
|
|
$
|
0.23
|
|
Note 11 – Commitments and Contingencies
Supply Agreement – between Stevia Ventures International Ltd. and Asia Stevia Investment Development Company Ltd.
On April 12, 2011, Stevia Ventures International Ltd., a subsidiary of the Company entered into a Supply Agreement (the “Supply Agreement”) with Asia Stevia Investment Development Company Ltd. (“ASID”), a foreign-invested limited liability company incorporated in Vietnam.
(i)
Scope of Services
Under the terms of the Agreement, the Company engaged ASID to plant the Stevia Seedlings and supply the Products only to the Company to the exclusion of other customers and the Company is desirous to purchase the same, on the terms and conditions as set out in this Agreement produce Products and the Company purchase the Products from ASID.
(ii)
Term
This Agreement shall come into force on the date of signing and, subject to earlier termination pursuant to certain clauses specified in the Agreement, shall continue in force for a period of three (3) years ("Term") expiring on April 1, 2014 and thereafter automatically renew on its anniversary for an additional period of one (1) year expiring on April 1, 2015 ("Extended Term").
(iii)
Purchase Price
ASID and the Company shall review and agree, on or before September 30th of each year, on the quantity of the Products to be supplied by ASID to the Company in the forthcoming year and ASID shall provide the Company with prior written notice at any time during the year following the revision if it has reason to believe that it would be unable to fulfill its forecast volumes under this clause.
Supply Agreement – between Stevia Ventures International Ltd. And Stevia Ventures Corporation
On April 12, 2011, Stevia Ventures International Ltd., a subsidiary of the Company also entered into a Supply Agreement (the “Supply Agreement”) with Stevia Ventures Corporation (“SVC”), a foreign-invested limited liability company incorporated in Vietnam.
(i)
Scope of Services
Under the terms of the Agreement, the Company engaged SVC to plant the Stevia Seedlings and supply the Products only to the Company to the exclusion of other customers and the Company is desirous to purchase the same, on the terms and conditions as set out in this Agreement produce Products and the Company purchase the Products from SVC.
(ii)
Term
This Agreement shall come into force on the date of signing and, subject to earlier termination pursuant to certain clauses specified in the Agreement, shall continue in force for a period of three (3) years expiring April 1, 2014 ("Term") and thereafter automatically renew on its anniversary for an additional period of one (1) year expiring April 1, 2015 ("Extended Term").
(iii)
Purchase Price
SVC and the Company shall review and agree, on or before September 30
th
, of each Year on the quantity of the Products to be supplied by SVC to the Company in the forthcoming year and SVC shall provide the Company with prior written notice at any time during the year following the revision if it has reason to believe that it would be unable to fulfill its forecast volumes under this clause.
Engagement Agreement – Garden State Securities Inc.
On June 18, 2012, the Company entered into an engagement agreement (the “Agreement”) with Garden State Securities Inc. (“GSS”) for GSS to act as a selling/placement agent for the Company.
(i)
Scope of Services
Under the terms of the Agreement, the Company engaged GSS to review the business and operations of the Company and its historical and projected financial condition, advise the Company on a “best efforts” Private Placement offering of debt or equity securities to fulfill the Company’s business plan, and contacts for the Company possible financing sources.
(ii)
Term
GSS shall act as the Company’s exclusive placement agent for the period of the later of; (i) 60 days from the execution of the term sheet; or (ii) the final termination date of the securities financing (the “Exclusive Period”). GSS shall act as the Company’s non-exclusive placement agent after the Exclusive Period until terminated.
(iii)
Compensation
The Company agrees to pay to GSS at each full or incremental closing of any equity financing, convertible debt financing, debt conversion or any instrument convertible into the Company’s common stock (the “Securities Financing”) during the Exclusive Period;(i) a cash transaction fee in the amount of 8% of the amount received by the Company under the Securities Financing; and (ii) warrants (the “Warrants”) with “piggy back” registration rights, equal to 8% of the stock issued in the Securities Financing at an exercise price equal to the investors’ warrant exercise price of the Securities Financing or the price of the Securities Financing if no warrants are issued to investors. The Company will also pay, at closing, the expense of GSS’s legal counsel pursuant to the Securities Financing and/or Shelf equal to $25,000 for Securities Financing and/or Shelf resulting in equal to or greater than $500,000 of gross proceeds to the Company, and $18,000 for a Securities Financing and/or Shelf resulting in less than $500,000 of gross proceeds to the Company. In addition, the Company shall cause, at its cost and expense, the “Blue sky filing” and Form D in due and proper form and substance and in a timely manner.
Consulting Agreement –
Mountain Sky International Limited
On April 18, 2013, the Company entered into a consulting agreement (the “Consulting Agreement”) with Mountain Sky International Limited (“Mountain Sky”) to perform consulting certain services for the Company. In consideration of the mutual covenants and promises contained herein and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged by the parties hereto, the parties agree as follows.
(i)
Scope
of Services
The Consultant agrees to perform certain consulting, advisory and related services to the Company.
(ii)
Term
This Agreement shall commence on April 18, 2013 (the "Commencement Date") and shall continue until April 30, 2015 unless terminated. This Agreement may be terminated by either the Company or the Consultant at any time prior to the end of the Consulting Period by giving thirty (30) days written notice of termination. Such notice may be given at any time for any reason, with or without cause. The Company will pay Consultant for all Services performed by Consultant through the date of termination.
(iii)
Compensation
The Company issued 1,000,000 shares of its common stock to Mountain Sky International Limited, a Hong Kong corporation (“Mountain Sky”), in partial consideration for consulting services to be rendered by Mountain Sky. 500,000 of the 1,000,000 shares vested at the time of grant, and 500,000 will vest on the one (1) year anniversary of the date of grant. The 500,000 shares vested on April 30, 2013 were valued at $0.20 per share or $100,000 and recorded as consulting fee.
Note 12 – Equity
Shares Authorized
Upon formation the total number of shares of common stock which the Company is authorized to issue is One Hundred Million (100,000,000) shares, par value $0.001 per share.
On November 15, 2013, the Company approved an amendment to the Articles of Incorporations to increase the authorized number of shares to Two hundred and fifty million (250,000,000) shares, par value $0.001 per share.
Common Stock
Reverse Acquisition Transaction
Immediately prior to the Share Exchange Agreement on June 23, 2011, the Company had 79,800,000 common shares issued and outstanding. Simultaneously with the Closing of the Share Exchange Agreement, on the Closing Date, Mohanad Shurrab, a shareholder and, as of the Closing Date, the Company’s former Director, President, Treasurer and Secretary, surrendered 33,000,000 shares of the Company’s common stock to the Company for cancellation.
As a result of the Share Exchange Agreement, the Company issued 12,000,000 common shares for the acquisition of 100% of the issued and outstanding shares of Stevia Ventures International Ltd. Of the 12,000,000 common shares issued in connection with the Share Exchange Agreement, 6,000,000 of such shares were being held in escrow (“Escrow Shares”) pending the achievement by the Company of certain post-Closing business milestones (the “Milestones”), pursuant to the terms of the Make Good Escrow Agreement, between the Company, Greenberg Traurig, LLP, as escrow agent and the Ventures’ Stockholder (the “Escrow Agreement”).
|
|
*
|
On December 23, 2011, 3,000,000 out of the 6,000,000 Escrow Shares have been earned by and released to Ventures stockholder upon achievement of the First Milestone within 180 days of June 23, 2011, the Closing Date associated with the First Milestone. These shares were valued at $0.25 per share or $750,000 on the date of release and recorded as salary and compensation - officer.
|
|
|
**
|
On June 23, 2013, the remaining 3,000,000 Escrow Shares have been earned by and released to Ventures stockholder upon achievement of the Second and the Third Milestones within two (2) years of June 23, 2011, the Closing Date associated with the Milestones. These shares were valued at $0.20 per share or $600,000 on June 23, 2013 and recorded as salary and compensation - officer.
|
Common Shares Issued for Obtaining Employee and Director Services
October 14, 2011 Issuance of Common Shares for Director Services
On October 14, 2011 the Company issued 1,500,000 shares each to two (2) newly appointed members of the board of directors or 3,000,000 shares of its common stock in aggregate as compensation for future services. These shares shall vest with respect to 750,000 shares of restricted stock on each of the first two anniversaries of the date of grant, subject to the director’s continuous service to the Company as directors. These shares were valued at $0.25 per share or $750,000 on the date of grant and are being amortized over the vesting period of two (2) years or $93,750 per quarter.
The Company recorded $375,000 and $187,500 in directors’ fees for the fiscal year ended March 31, 2013 and for the period from April 11, 2011 (inception) through March 31, 2012, respectively.
The Company recorded the remaining balance of $187,500 for the interim period ended December 31, 2013.
December 4, 2013 Issuance of Common Shares for Director Services
On December 4, 2013 the Company issued 1,500,000 shares to a newly appointed member of the board of directors as compensation for future services. These shares shall vest with respect to 750,000 shares of restricted stock on each of the first two anniversaries of the date of grant, subject to the director’s continuous service to the Company as a director. These shares were valued at $0.125 per share or $187,500 on the date of grant and are being amortized over the vesting period of two (2) years or $7,811 per month.
The Company recorded $7,811 in director’s fees for the interim period ended December 31, 2013.
Common Shares Issued
to Parties other than Employees for Acquiring Goods or Services
Common Shares Issued to a Related Party
On July 5, 2012, the Company issued 500,000 restricted shares of its common shares to Growers Synergy Pte Ltd., a corporation organized under the laws of the Republic of Singapore ("Singapore"), owned and controlled by George Blankenbaker, the president, director and a significant stockholder of the Company ("Growers Synergy"), as consideration for services rendered by Growers Synergy to the Company. These restricted shares were valued at $0.79 per share discounted at 69% taking into consideration of its restricted nature and lack of liquidity and consistent trading in the market or $272,550 and included in the farm management services - related party.
Common Shares Issued in Connection with Consulting Agreement
On April 18, 2013, the Company issued 1,000,000 shares of its common stock to Mountain Sky International Limited, a Hong Kong corporation (“Mountain Sky”), in partial consideration for consulting services rendered by Mountain Sky. 500,000 of the 1,000,000 shares vested at the time of grant, and 500,000 will vest on the one (1) year anniversary of the date of grant. The 500,000 shares vested on April 30, 2013 were valued at $0.20 per share or $100,000 and recorded as consulting fee.
Sale of Equity Units Including Common Stock and Warrants
Entry into Securities Purchase Agreement
On August 1, 2012, the Company entered into a Securities Purchase Agreement (the "SPA") with two (2) accredited institutional investors (the "Purchasers") to raise $500,000 in a private placement financing. On August 6, 2012, after the satisfaction of certain closing conditions, the Offering closed and the Company issued to the Purchasers: (i) an aggregate of 1,066,667shares of the Company's common stock at $0.46875 per share and (ii) warrants to purchase 1,066,667 shares of the Company's common stock at an exercise price of $0.6405 expiring five (5) years from the date of issuance for a gross proceeds of $500,000.
At closing, the Company reimbursed the investor for legal fees of $12,500 and paid Garden State Securities, Inc,(“GSS”), who served as placement agent for the Company in the offering, (i) cash commissions equal to 8.0% of the gross proceeds received in the equity financing or $40,000, and (ii) a warrant to purchase 85,333 shares of the Company's common stock representing 8% of the Shares sold in the Offering with an exercise price of $0.6405 per share expiring five (5) years from the date of issuance (the "agent warrants") to GSS or its designee.
The units were sold at $0.46875 per unit consisting one common share and the warrant to purchase one (1) common share for gross proceeds of $500,000. In connection with the August 6, 2012 equity unit offering the Company paid (i) GSS cash commissions equal to 8.0% of the gross proceeds received in the equity financing, or $40,000 and (ii) $12,500 in legal fees and resulted in a net proceeds of $447,500.
Per the terms of the SPA, from the date until one (1) year anniversary of the closing date, if the Company issues or sells any shares of the Company’s common stock at a price that is less than the per share purchase price, than immediately without any obligation of or notice to the Purchasers, the per share purchase price paid shall be reduced to be the discounted per share purchase price and the number of shares issuable under this agreement shall be deemed increased to the subscription amount paid by such Purchaser. On October 1, 2013,
The Company issued 286,666 common shares to the investor according to this anti-dilutive term.
Exercise of Warrants with Issuance of New Warrants per the Warrant Reset Offer
On May 3, 2013, the Company entered into a Warrant Exercise Reset Offer Letter Agreement (the "Reset Letter") with an investor (the "Investor") whereby the Company and the Investor agreed that the Investor would immediately exercise his warrant to purchase 853,333 shares of common stock of the Company at an exercise price of $0.20 per share for cash in the aggregate of $170,667. In consideration for the Investor's immediate exercise, the Company agreed to issue to the Investor three (3) new warrants in the amounts of 1,877,333, 1,066,666 and 2,346,666, with exercise prices of $0.20, $0.25 and $0.25 per share,
respectively (the "Series A Warrants", "Series B Warrants" and "Series C Warrants", respectively, and collectively the "New Warrants"). The Series A Warrants are subject to the Company's call right, and the Series C Warrants are only exercisable upon the Investor's exercise in full of the Series A Warrants. In connection with the Reset Letter, the Company agreed to use its best efforts to file a registration statement (the "Registration Statement") with the United States Securities and Exchange Commission (the "SEC") within ten (10) business days. The Company will use its best efforts to have the Registration Statement declared effective by the SEC within thirty (30) days. The Company filed a registration statement (the "Registration Statement") with the Securities and Exchange Commission (the "SEC") within ten (10) business days which was declared effective by the SEC within thirty (30) days.
Issuance of Common Stock per the Settlement Agreement
On July 25, 2013, the Supreme Court of the State of New York, County of New York (the "Court"), entered an order (the "Order") approving the settlement (the "Settlement Agreement") between the Company and Hanover Holdings I, LLC, a New York limited liability company ("Hanover"), Hanover commenced the action against the Company on July 12, 2013 to recover $1,042,000 of past-due accounts payable of the Company, plus fees and costs (the "Claim"). The Settlement Agreement became effective and binding upon the Company and Hanover upon execution of the Order by the Court on July 25, 2013.
On July 26, 2013, the Company issued and delivered to Hanover 7,500,000 shares (the "Initial Settlement Shares") of the Company's common stock, $0.001 par value (the "Common Stock"), pursuant to the terms of the Settlement Agreement approved by the Order.
The Settlement Agreement provides that the Initial Settlement Shares will be subject to adjustment on the trading day immediately following the Calculation Period to reflect the intention of the parties that the total number of shares of Common Stock to be issued to Hanover pursuant to the Settlement Agreement be based upon a specified discount to the trading volume weighted average price (the "VWAP") of the Common Stock for a specified period of time subsequent to the Court's entry of the Order. Specifically, the total number of shares of Common Stock to be issued to Hanover pursuant to the Settlement Agreement shall be equal to the sum of: (i) the quotient obtained by dividing (A) $1,042,000 (representing the total amount of the Claim), by (B) 65% of the average of the lowest 40 VWAPs of the Common Stock over the 120-consecutive trading day period (subject to extension under the Settlement Agreement) immediately following the date of issuance of the Initial Settlement Shares (or such shorter trading-day period as may be determined by Hanover in its sole discretion by delivery of written notice to the Company) (the "Calculation Period"); (ii) the quotient obtained by dividing (A) $22,500, representing (1) $25,000 of Hanover's legal fees and expenses incurred in connection with the Action that the Company has agreed to pay less (2) $2,500 heretofore paid by the Company, by (B) 100% of the VWAP of the Common Stock over the Calculation Period; and (iii) the quotient obtained by dividing (A) agent fees of $83,360, by (B) 100% of the VWAP of the Common Stock over the Calculation Period, rounded up to the nearest whole share (the "VWAP Shares"). As a result, the Company ultimately may be required to issue to Hanover substantially more shares of Common Stock than the number of Initial Settlement Shares issued (subject to the limitations described below). The Settlement Agreement further provides that if, at any time and from time to time during the Calculation Period, Hanover reasonably believes that the total number of Settlement Shares previously issued to Hanover shall be less than the total number of VWAP Shares to be issued to Hanover or its designee in connection with the Settlement Agreement, Hanover may, in its sole discretion, deliver one or more written notices to the Company, at any time and from time to time during the Calculation Period, requesting that a specified number of additional shares of Common Stock promptly be issued and delivered to Hanover or its designee (subject to the limitations described below), and the Company will upon such request reserve and issue the number of additional shares of Common Stock requested to be so issued and delivered in the notice (all of such additional shares of Common Stock, "Additional Settlement Shares"). At the end of the Calculation Period, (i) if the number of VWAP Shares exceeds the number of Initial Settlement Shares and Additional Settlement Shares issued, then the Company will issue to Hanover or its designee additional shares of Common Stock equal to the difference between the number of VWAP Shares and the number of Initial Settlement Shares and Additional Settlement Shares, and (ii) if the number of VWAP Shares is less than the number of Initial Settlement Shares and Additional Settlement Shares issued, then Hanover or its designee will return to the Company for cancellation that number of shares of Common Stock equal to the difference between the number of VWAP Shares and the number of Initial Settlement Shares and Additional Settlement Shares. Hanover may sell the shares of Common Stock issued to it or its designee in connection with the Settlement Agreement at any time without restriction, even during the Calculation Period.
The Settlement Agreement provides that in no event shall the number of shares of Common Stock issued to Hanover or its designee in connection with the Settlement Agreement, when aggregated with all other shares of Common Stock then beneficially owned by Hanover and its affiliates (as calculated pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), and the rules and regulations thereunder), result in the beneficial ownership by Hanover and its affiliates (as calculated pursuant to Section 13(d) of the Exchange Act and the rules and regulations thereunder) at any time of more than 9.99% of the Common Stock.
On September 30, 2013, the Company issued and delivered to Hanover 2,000,000 Additional Settlement Shares pursuant to the terms of the Settlement Agreement approved by the Order. Since the issuance of the Initial Settlement Shares and Additional Settlement Shares described above, Hanover demonstrated to the Company's satisfaction that it was entitled to receive another 3,500,000 Additional Settlement Shares, based on the adjustment formula described above, and that the issuance of such Additional Settlement Shares to Hanover would not result in Hanover exceeding the beneficial ownership limitation set forth above. On December 13, 2013, the Company issued and delivered to Hanover another 3,500,000 Additional Settlement Shares and on January 22, 2014, the Company issued and delivered to Hanover the final 2,538,882 Additional Settlement Shares pursuant to the terms of the Settlement Agreement approved by the Order.
The Company considered the settlement of debt with common shares as an extinguishment of debt and applied extinguishment accounting accordingly. The Company compared the trade accounts payable and related settlement costs with the fair value of common shares issued. Because the fair value of common shares issued was $561,077 greater than trade accounts payable and related settlement costs, the Company applied extinguishment accounting, resulting in a loss on extinguishment of debt of $561,077, for the reporting period ended December 31, 2013.
Warrants
Issuances of Warrants in Connection with Securities Purchase Agreement
On August 6, 2012, the Company issued (i) warrants to purchase 1,066,667 shares of the Company’s common stock to the investors with an exercise price of $0.6405 per share subject to certain adjustments per Section 3(b) Subsequent Equity Sales of the SPA expiring five (5) years from the date of issuance in connection with the sale of common shares. The exercise price and number of warrant shares were reset to $0.25 per share and 2,732,801 shares, respectively, due to the occurrence of the February 26, 2013 reset event.
Issuance of Warrants to the Placement Agent as Compensation
Garden State Securities, Inc. (the "GSS") served as the placement agent of the Company for the equity financing on August 1, 2012. Per the engagement agreement signed between GSS and the Company, in consideration for services rendered as the placement agent, the Company agreed to: (i) pay GSS cash commissions equal to 8.0% of the gross proceeds received in the equity financing, or $40,000, and (ii) issue to GSS or its designee, a warrant to purchase 85,333 shares of the Company's common stock representing 8% of the warrants sold in the Offering) with an exercise price of $0.6405 per share subject to certain adjustments per Section 3(b) Subsequent Equity Sales of the SPA expiring five (5) years from the date of issuance (the "agent warrants"). The agent warrants also provide for the same registration rights and obligations as set forth in the Rights Agreement with respect to the Warrants and Warrant Shares. The exercise price and number of warrant shares were reset to $0.25 per share and 2,732,801 shares, respectively, due to the occurrence of the February 26, 2013 reset event.
Garden State Securities, Inc. (the "Placement Agent") served as the placement agent of the Company for the Warrant Reset Offering on May 6, 2013. In consideration for services rendered as the Placement Agent, the Company agreed to: (i) pay to the Placement Agent cash commissions equal to $13,653, (ii) warrants equal to eight percent (8%) of the aggregate number of shares exercised by the Investor, and (iii) upon exercise of the New Warrants by the Company, the Placement Agent will receive additional warrants equal to eight percent (8%) of the number of shares issued upon exercise of the New Warrants (collectively, the "Agent Warrants").
Note 13 – Research and Development
Lease of Agricultural Land
On December 14, 2011, the Company and Stevia Ventures Corporation (“Stevia Ventures”) entered into a Land Lease Agreement with Vinh Phuc Province People's Committee Tam Dao Agriculture & Industry Co., Ltd. pursuant to which Stevia Ventures has leased l0 hectares of land (the “Leased Property”) for a term expiring five (5) years from the date of signing expiring December 14, 2016.
The Company has begun development of a research facility on the Leased Property and has prepaid (i) the first year lease payment of $30,000 and (ii) the six month lease payment of $15,000 as security deposit, or $45,000 in aggregate upon signing of the agreement.
Future minimum payments required under this agreement at December 31, 2013 were as follows:
|
Fiscal Year Ending March 31:
|
|
|
|
|
|
|
|
|
|
|
|
2014 (remainder of the year)
|
|
$
|
7,500
|
|
|
2015
|
|
|
30,000
|
|
|
2016
|
|
|
30,000
|
|
|
|
|
|
|
|
|
|
$
|
67,500
|
|
Supply and Cooperative Agreement – Guangzhou Health Technology Development Company Limited
Entry into Supply Agreement
On February 21, 2012, the Company entered into a Supply Agreement (the "Supply Agreement") with Guangzhou Health China Technology Development Company Limited, a foreign-invested limited liability company incorporated in the People's Republic of China (the "Guangzhou Health").
Under the terms of the Supply Agreement, the Company will sell dry stevia plant materials, including stems and leaves ("Product") exclusively to Guangzhou Health. For the first two years of the agreement, Guangzhou Health will purchase all Product produced by the Company. Starting with the third year of the agreement, the Company and Guangzhou Health will review and agree on the quantity of Product to be supplied in the forthcoming year, and Guangzhou Health will be obliged to purchase up to 130 percent of that amount. The specifications and price of Product will also be revised annually according to the mutual agreement of the parties. The term of the Supply Agreement is five years with an option to renew for an additional four years.
Entry into Cooperative Agreement
On February 21, 2012, the Company also entered into Cooperative Agreement (the “Cooperative Agreement”) with Guangzhou Health Technology Development Company Limited.
Under the terms of the Cooperative Agreement, the parties agree to explore potential technology partnerships with the intent of formalizing a joint venture to pursue the most promising technologies and businesses. The parties also agree to conduct trials to test the efficacy of certain technologies as applied specifically to the Company's business model as well as the marketability of harvests produced utilizing such technologies. Guangzhou Health will share all available information of its business structure and technologies with the Company, subject to the confidentiality provisions of the Cooperative Agreement. Guangzhou Health will also permit the Company to enter its premises and grow-out sites for purposes of inspection and will, as reasonably requested by the Company, supply without cost, random samples of products and harvests for testing.
Note 14 – Concentrations and Credit Risk
Credit Risk
Financial instruments that potentially subject the Company to significant concentration of credit risk consist primarily of cash and cash equivalents.
As of December 31, 2013, substantially all of the Company’s cash and cash equivalents were held by major financial institutions, and the balance at certain accounts exceeded the maximum amount insured by the Federal Deposits Insurance Corporation (“FDIC”). However, the Company has not experienced losses on these accounts and management believes that the Company is not exposed to significant risks on such accounts.
Customers and Credit Concentrations
One (1) customer accounted for all of the sales for the interim period ended December 31, 2013 and the accounts receivable at December 31, 2013. A reduction in sales from or loss of such customer would have a material adverse effect on the Company’s results of operations and financial condition.
Vendors and Accounts Payable Concentrations
Vendor purchase concentrations and accounts payable concentration are as follows:
|
|
Accounts Payable at
|
|
|
Net Purchases
|
|
|
|
December 31, 2013
|
|
|
March 31, 2013
|
|
|
For the Reporting
Period Ended
December 31, 2013
|
|
|
For the Reporting
Period Ended
December 31, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Growers Synergy Pte. Ltd. – related party
|
|
45.0
|
%
|
|
|
50.1
|
%
|
|
|
6.0
|
%
|
|
|
49.8
|
%
|
|
Stevia Ventures Corporation
|
|
19.3
|
%
|
|
|
16. 9
|
%
|
|
|
26.6
|
%
|
|
|
10.3
|
%
|
|
SG Agro Tech Pte Ltd
|
|
-
|
%
|
|
|
-
|
%
|
|
|
52.2
|
%
|
|
|
-
|
%
|
|
|
|
64.3
|
%
|
|
|
67.0
|
%
|
|
|
84.8
|
%
|
|
|
60.1
|
%
|
Note 15 – Subsequent Events
The Company has evaluated all events that occurred after the balance sheet date through the date when the financial statements were issued to determine if they must be reported. The Management of the Company determined that there were certain reportable subsequent event(s) to be disclosed as follows:
On January 21, 2014 and February 4, 2014, a convertible note holder converted part of the accrued interest through the date of conversion of $23,400 and $26,325, respectively, at $0.0585 per share to 400,000 and 450, 000 shares of the Company’s common stock, respectively.
On February 7, 2014, the Company issued a convertible note in the principal amount of $80,000 convertible at $0.10 per share with interest at 8%, per annum, maturing one year from the date of issuance on February 7, 2015. In connection with the issuance of the convertible note,the Company issued the note holder a warrant to purchase 1,000,000 common shares at an exercise price of $0.10 per share with full reset features, expiring five (5) years from the date of issuance.
On February 13, 2014, one investor exercised warrants to purchase 1,877,333 shares of the Company’s common stock with an exercise price of $0.0585 per share for $109,824 in cash.
Entry into Service Agreement
On January 27, 2014, the Company entered into a Farm Management and Technology Agreement (the “Agreement”) with Ebbu LLC ("Ebbu"), that is developing proprietary marijuana farming and extraction technologies. Under the terms of the Agreement, Ebbu wishes to engage the Company to provide technical support for farm management operations and extraction for both cash and assignable warrants. The scope of work and exact compensation is still under negotiation and will be completed by the end of next week.
PART II - INFORMATION NOT REQUIRED IN
PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs
and expenses payable by us in connection with the issuance and distribution of the securities being registered hereunder. No expenses
will be borne by the Selling Security Holders. All of the amounts shown are estimates, except for the SEC registration fee.
|
SEC registration fee
|
|
$
|
1,145.53
|
|
|
Accounting fees and expenses
|
|
$
|
5,000.00
|
|
|
Legal fees and expenses
|
|
$
|
10,000.000
|
|
|
Total
|
|
$
|
16,145.53
|
|
Item 14. Indemnification of Directors and Officers
Nevada Law
Section 78.7502 of the Nevada Revised Statutes
permits a corporation to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending
or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the
right of the corporation, by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or
is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership,
joint venture, trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid
in settlement actually and reasonably incurred by him in connection with the action, suit or proceeding if he:
|
|
(a)
|
is
not liable pursuant to Nevada Revised Statute 78.138, or
|
|
|
(b)
|
acted
in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, and,
with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful.
|
In addition, Section 78.7502 permits a
corporation to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed
action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that he is or was
a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director,
officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including
amounts paid in settlement and attorneys’ fees actually and reasonably incurred by him in connection with the defense or
settlement of the action or suit if he:
|
|
(a)
|
is
not liable pursuant to Nevada Revised Statute 78.138; or
|
|
|
(b)
|
acted
in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation.
|
To the extent that a director, officer,
employee or agent of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding
referred to above, or in defense of any claim, issue or matter, the corporation is required to indemnify him against expenses,
including attorneys’ fees, actually and reasonably incurred by him in connection with the defense.
Section 78.751 of the Nevada Revised Statutes provides that
such indemnification may also include payment by the Company of expenses incurred in defending a civil or criminal action or proceeding
in advance of the final disposition of such action or proceeding upon receipt of an undertaking by the person indemnified to repay
such payment if he shall be ultimately found not to be entitled to indemnification under Section 78.751. Indemnification may be
provided even though the person to be indemnified is no longer a director, officer, employee or agent of the Company or such other
entities.
Section 78.752 of the Nevada Revised Statutes
allows a corporation to purchase and maintain insurance or make other financial arrangements on behalf of any person who is or
was a director, officer, employee or agent of the corporation or is or was serving at the request of the corporation as a director,
officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise for any liability asserted
against him and liability and expenses incurred by him in his capacity as a director, officer, employee or agent, or arising out
of his status as such, whether or not the corporation has the authority to indemnify him against such liability and expenses.
Other financial arrangements made by the
corporation pursuant to Section 78.752 may include the following:
|
|
(a)
|
the
creation of a trust fund;
|
|
|
(b)
|
the
establishment of a program of self-insurance;
|
|
|
(c)
|
the
securing of its obligation of indemnification by granting a security interest or other lien on any assets of the corporation;
and
|
|
|
(d)
|
the
establishment of a letter of credit, guaranty or surety
|
No financial arrangement made pursuant
to Section 78.752 may provide protection for a person adjudged by a court of competent jurisdiction, after exhaustion of all appeals,
to be liable for intentional misconduct, fraud or a knowing violation of law, except with respect to the advancement of expenses
or indemnification ordered by a court.
Any discretionary indemnification pursuant
to NRS 78.7502, unless ordered by a court or advanced pursuant to an undertaking to repay the amount if it is determined by a court
that the indemnified party is not entitled to be indemnified by the corporation, may be made by the corporation only as authorized
in the specific case upon a determination that indemnification of the director, officer, employee or agent is proper in the circumstances.
The determination must be made:
|
|
(b)
|
by
the board of directors by majority vote of a quorum consisting of directors who were not parties to the action, suit or proceeding;
|
|
|
(c)
|
if
a majority vote of a quorum consisting of directors who were not parties to the action, suit or proceeding so orders, by independent
legal counsel in a written opinion, or
|
|
|
(d)
|
if
a quorum consisting of directors who were not parties to the action, suit or proceeding cannot be obtained, by independent legal
counsel in a written opinion.
|
Charter Provisions and Other Arrangements
of the Registrant
Pursuant to the provisions of Nevada Revised
Statutes, the Registrant has adopted the following indemnification provisions in its Bylaws for its directors and officers:
The Company shall indemnify, to the maximum
extent permitted by the law, any person who was or is a party or is threatened to be made a party to any threatened, pending or
completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right
of the Company, by reason of the fact that such person is or was a director, officer, employee, or agent of the Company, or is
or was serving at the request of the Company as a director, officer, employee, or agent of another company, partnership, joint
venture, trust, or other enterprise, against expenses, including attorneys’ fees, judgments, fines, and amounts paid in settlement
actually and reasonably incurred by such person in connection with such action, suit, or proceeding if such person acted in good
faith and in a manner which such person reasonably believed to be in or not opposed to the best interests of the Company, and,
with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. The
termination of any action, suit or proceeding by judgment, order, settlement, conviction, or upon a plea of
no lo contendere
or its equivalent, shall not, of itself, create a presumption that the person did not act in good faith and in a manner which such
person reasonably believed to be in or not opposed to the best interests of the Company, and that, with respect to any criminal
action or proceeding, such person had reasonable cause to believe that his conduct was unlawful.
The Company shall indemnify, to the maximum
extent permitted by the law, any person who was or is a party or is threatened to be made a party to any threatened, pending or
completed action or suit by or in the right of the Company to procure a judgment in its favor by reason of the fact that such person
is or was a director, officer, employee, or agent of the Company, or is or was serving at the request of the Company as a director,
officer, employee or agent of another company, partnership, joint venture, trust, or other enterprise against expenses, including
attorneys’ fees, actually and reasonably incurred by such person in connection with the defense or settlement of such action
or suit if such person acted in good faith and in a manner which such person reasonably believed to be in or not opposed to the
best interests of the Company, but no indemnification shall be made in respect of any claim, issue or matter as to which such person
has been adjudged to be liable for negligence or misconduct in the performance of such person’s duty to the Company unless
and only to the extent that the court in which such action or suit was brought determines upon application that, despite the adjudication
of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such
expenses as the court deems proper.
To the extent that a director, officer,
employee or agent of the Company has been successful on the merits or otherwise in defense of any action, suit or proceeding referred
to in the prior two paragraphs, or in defense of any claim, issue or matter therein, such person shall be indemnified by the Company
against expenses, including attorneys’ fees, actually and reasonably incurred by such person in connection with such defense. Any
indemnification under the prior two paragraphs, unless ordered by a court, shall be made by the Company only as authorized in the
specific case upon a determination that indemnification of the director, officer, employee or agent is proper in the circumstances
because such person has met the applicable standard of conduct set forth in the prior two paragraphs. Such determination
shall be made:
|
|
(ii)
|
by
the board of directors by majority vote of a quorum consisting of directors who were not parties to such act, suit or proceeding;
|
|
|
(iii)
|
if
such a quorum of disinterested directors so orders, by independent legal counsel in a written opinion; or
|
|
|
(iv)
|
if
such a quorum of disinterested directors cannot be obtained, by independent legal counsel in a written opinion.
|
Expenses incurred in defending a civil
or criminal action, suit or proceeding may be paid by the Company in advance of the final disposition of such action, suit or proceeding
as authorized by the board of directors unless it is ultimately determined that such director, officer, employee or agent is not
entitled to be indemnified by the Company as authorized in the Bylaws or as provided by law.
The indemnification provided by the Bylaws:
|
|
(i)
|
does
not exclude any other rights to which a person seeking indemnification may be entitled under any bylaw, agreement, vote of stockholders,
or disinterested directors or otherwise, both as to action in such person’s official capacity and as to action in another
capacity while holding such office; and
|
|
|
(ii)
|
shall
continue as to a person who has ceased to be a director, officer, employee or agent and shall inure to the benefit of the heirs,
executors, and administrators of such a person.
|
The Company may purchase and maintain insurance
on behalf of any person who is or was a director, officer, employee or agent of the Company, or is or as serving at the request
of the Company as a director, officer, employee, or agent of another company, partnership, joint venture, trust, or other enterprise
against any liability asserted against such person and incurred by such person in any such capacity, or arising out of such person’s
status as such, whether or not the Company would have the power to indemnify such person against such liability under the provisions
of the Bylaws.
Item 15. Recent Sales of Unregistered Securities
Promissory Notes
On February 14, 2011 we issued a convertible
promissory note in the principal amount of $250,000 to Vantage Associates SA (“Vantage”) and on June 23, 2011, we issued
an additional convertible promissory note to Vantage in the principal amount of $100,000 (the “Original Notes”). The
Original Notes were convertible into shares of the Company’s common stock upon the closing by the Company of an equity financing
yielding aggregate gross proceeds of at least $100,000. The Original Notes convert at the price per share of the securities issued
in such financing. The Original Notes were issued in reliance upon exemption from registration under the Securities Act pursuant
to Regulation S thereof. The Original Notes were converted into common stock on October 4, 2011 and are no longer outstanding.
Share Exchange Transaction
In connection with the Share Exchange Transaction,
on June 23, 2011 we issued a total of 12,000,000 shares of our common stock in exchange for 100% of the issued and outstanding
common stock of BVI. The common stock was issued in reliance upon exemption from registration under the Securities Act pursuant
to Rule 506 of Regulation D thereof, and comparable exemptions under state securities laws. The common stock was issued to “accredited
investors,” as such term is defined in Rule 501(a) under the Securities Act, based upon representations made by such investor.
Sale of Common Stock and Additional
Promissory Notes
On October 6, 2011, we raised $100,000
through the sale of 400,000 shares of our common stock at a price of $0.25 per share (the “October Shares”).
On October 6, 2011, we raised $150,000
from the proceeds of a convertible note (the “October Note”). The October Note was based upon the Company’s standard
form of promissory note, accrues interest at the rate of ten percent per annum, simple interest and the principal balance of the
October Note and any accrued interest thereon is convertible into our common stock at a $0.25 per share conversion price. The October
Note was converted into common stock on January 18, 2012 and is no longer outstanding.
On November 16, 2011, we raised $250,000
from the proceeds of a convertible note (the “November Note”). The November Note was based upon the Company’s
standard form of promissory note, accrues interest at the rate of ten percent per annum, simple interest and the principal balance
of the November Note and any accrued interest thereon is convertible into our common stock at the lower of (a) the price per share
at which shares of capital stock are sold in our next equity financing, or (b) the closing price of our securities if traded on
a securities exchange, or if actively traded over-the-counter, the average closing bid price for the securities, in each case over
the thirty (30) day period prior to the date of conversion; provided however, that if no active trading market for the securities
exists at the time of the conversion, such conversion price shall be the fair market value of a share of our common stock as determined
in good faith by our board of directors. The November Note was converted into common stock on July 6, 2012 and is no longer outstanding.
On January 16, 2012, March 7, 2012 and
May 30, 2012, we raised $250,000, $200,000 and $200,000 respectively from the proceeds of convertible notes (the “Subsequent
Notes”). The Subsequent Notes were based upon the Company’s standard form of promissory note, accrue interest at the
rate of ten percent per annum, simple interest and the principal balance of the Subsequent Notes and any accrued interest thereon
is convertible into our common stock at the lower of (a) the price per share at which shares of capital stock are sold in our next
equity financing, or (b) the closing price of our securities if traded on a securities exchange, or if actively traded over-the-counter,
the average closing bid price for the securities, in each case over the thirty (30) day period prior to the date of conversion;
provided however, that if no active trading market for the securities exists at the time of the conversion, such conversion price
shall be the fair market value of a share of our common stock as determined in good faith by our board of directors. The Note issued
on January 16, 2012 was converted into common stock on July 16, 2012 and is no longer outstanding. The other Subsequent Notes remain
outstanding.
On January 26, 2012, we entered into an Equity Purchase Agreement
(the “Southridge Agreement”) with Southridge Partners II, LP, a Delaware limited partnership (“Southridge”).
Upon execution of the Southridge Agreement, we issued 35,000 shares of our common stock to Southridge as a commitment fee (the
“Southridge Shares”).
On March 19, 2012, we issued 27,500 shares
of our common stock to Empire Relations Group (“Empire”) as consideration for consulting services rendered by Empire
to the Company (the “Empire Shares”).
On July 5, 2012, we entered into a Technology
Acquisition Agreement (the “Technology Agreement”) with Technew, pursuant to which we acquired the rights to certain
technology from Technew in exchange for 3,000,000 shares of our common stock (the “Technew Shares”).
On July 5, 2012, we issued 500,000 shares
of our common stock (the “Growers Synergy Shares”) to Growers Synergy Pte Ltd., a corporation organized under the laws
of Singapore (“Growers Synergy”), as consideration for services rendered by Growers Synergy to the Company. George
Blankenbaker, our president, director and stockholder is the managing director of Growers Synergy. Growers Fresh Pte Ltd (“Growers
Fresh) owns a 51% interest in Growers Synergy and Mr. Blankenbaker controls a 49% interest in Growers Fresh. owned and controlled
by the president and major stockholder of the Company.
On February 26, 2013 we raised an aggregate of $350,000, from
the proceeds of convertible notes. Additionally, on March 15, 2013, we issued a convertible promissory note in the aggregate principal
amount of $220,438.36 in exchange for the cancellation of an outstanding promissory note in the principal amount of $200,000 issued
March 7, 2012 (collectively, the "2013 Notes").
The 2013 Notes were each based upon our standard form of promissory
note, accrue interest at the rate of twelve percent per annum simple interest, and the principal balance of each 2013 Note and
any accrued interest thereon is convertible into our common stock at a price per share of $0.25.
In connection with the issuance of the Notes, we issued to each
investor a warrant to purchase such number of shares of common stock as is equal to four times the principal amount of their 2013
Note (the “2013 Warrants”). The 2013 Warrants have a three year term and an exercise price of $0.25 per share.
The issuance of the October Shares, the
Notes, the Technew Shares and the Growers Synergy Shares were conducted in reliance upon Regulation S of the Securities Act of
1933, as amended and the rules and regulations promulgated thereunder (the “Securities Act”), to investors who are
“accredited investors,” as such term is defined in Rule 501(a) under the Securities Act, in offshore transactions (as
defined in Rule 902 under Regulation S of the Securities Act), based upon representations made by such investors.
The issuance of the Southridge Shares and
the Empire Shares were conducted in reliance upon Regulation D of the Securities Act to investors who are “accredited investors,”
as such term is defined in Rule 501(a) under the Securities Act, based upon representations made by such investors.
The issuances of the 2013 Notes and the 2013 Warrants were conducted
in reliance upon Regulation D and Regulation S of the Securities Act of 1933, as amended and the rules and regulations promulgated
thereunder (the "Securities Act"), to investors who are "accredited investors," as such term is defined in
Rule 501(a) under the Securities Act or in offshore transactions (as defined in Rule 902 under Regulation S of the Securities Act),
based upon representations made by such investors.
2012 Financing
On August 6, 2012, we raised $500,000 in
a private placement financing (the “Offering”) through the sale of (i) an aggregate of 1,066,667 shares of common stock
at a price per share of $0.46875 and (ii) warrants to purchase an equal number of shares of the Company’s common stock at
an exercise price of $0.6405 with a term of 5 years (the “Financing Securities”). The Company intends to use the net
proceeds from this offering to advance the Company’s ability to execute its growth strategy and to aid in the commercial
development of the recently announced launch of the Company’s majority-owned subsidiary, Stevia Technew Limited.
Garden State Securities, Inc. (the “Placement
Agent”) served as the placement agent of the Company for the Offering. In consideration for services rendered as the Placement
Agent, the Company agreed to: (i) pay to the Placement Agent cash commissions equal to $40,000, or 8.0% of the gross proceeds received
in the Offering, and (ii) issue to the Placement Agent, or its designee, a Warrant to purchase up to 85,333 shares of the Company’s
common stock (representing 8% of the Shares sold in the Offering) with an exercise price of$0.6405 per share and a term of 5 years
(the “GSS Securities”).
The issuance of the Financing Securities
and the GSS Securities were conducted in reliance upon Regulation D of the Securities Act to investors who are “accredited
investors,” as such term is defined in Rule 501(a) under the Securities Act, based upon representations made by such investors.
Reset Warrants
On May 3, 2013, the Company entered into
a Warrant Exercise Reset Offer Letter Agreement (the “Reset Letter”) with an accredited investor (the “Investor”)
whereby the Company and the Investor agreed that the Investor would immediately cash exercise its warrant to purchase 853,333 shares
of common stock of the Company at an exercise price of $0.20 per share. In consideration for the Investor’s immediate exercise,
the Company agreed to issue to the Investor three new warrants in the amounts of 1,877,333, 1,066,666 and 2,346,666, with exercise
prices of $0.20, $0.25 and $0.25 per share, respectively (the “Series A Warrants”, “Series B Warrants”
and “Series C Warrants”, respectively, and collectively the “New Warrants”). With the exception of the
different exercise prices, the Warrants all contain the same terms, except that the Series A Warrants are subject to the Company’s
call right, and the Series C Warrants are only exercisable upon the Investor’s exercise in full of the Series A Warrants,
pursuant to the terms of a Warrant Exercise Reset Offer Letter Agreement.
In connection with the Reset Letter, the
Company agreed to use its best efforts to file a registration statement (the “Registration Statement”) with the Securities
and Exchange Commission (the “SEC”) within ten (10) business days. The Company will use its best efforts to have
the Registration Statement declared effective by the SEC within thirty (30) days.
Garden State Securities, Inc. (the “Placement
Agent”) served as the placement agent of the Company for the Offering. In consideration for services rendered as the Placement
Agent, the Company agreed to: (i) pay to the Placement Agent cash commissions equal to $13,600, (ii) warrants equal to
eight percent (8%) of the aggregate number of shares exercised by the Investor, and (iii) upon exercise of the New Warrants by
the Company, the Placement Agent will receive additional warrants equal to eight percent (8%) of the number of shares issued upon
exercise of the New Warrants (collectively, the “Agent Warrants”).
The New Warrants and the Agent Warrants
(including the shares of the Company’s common stock underlying the New Warrants and the Agent Warrants) were offered and
sold in reliance on the exemption from registration afforded by Section 4(2) and Regulation D (Rule 506) under the Securities Act
and corresponding provisions of state securities laws.
Stock Issuance for Services
On April 30, 2013, the Company issued 1,000,000
shares of our common stock (the “Mountain Sky Shares”) to Mountain Sky International Limited, a Hong Kong corporation
(“Mountain Sky”), in partial consideration for consulting services rendered by Mountain Sky. 500,000 of the shares
vest at the time of grant, and 500,000 vest on the one year anniversary of the date of grant.
June 2013 Note
On July 16, 2013, the Company entered into
a $400,000 Promissory Note (the “June 2013 Note”) with an accredited investor (the “Investor”) whereby
the Investor agreed to loan to the Company up to $400,000 pursuant to the terms of the June 2013 Note. The June 2013 Note provides
for the first $100,000 to be advanced upon closing and additional amounts will be advanced at the Investor’s sole discretion.
Each advance is subject to a 10% original issue discount, such that the total amount which may actually be received by the Company
pursuant to the June 2013 Note is only $360,000. The maturity date for each advance made under the June 2013 Note is one year from
the date of such advance. If the Company repays the June 2013 Note on or before 90 days from the effective date, the interest rate
shall be 0%, otherwise a one-time interest charge of l2% shall be applied to the principal sum.
The June 2013 Notes are convertible into
common stock of the Company on a cashless basis at any time, at a conversion price equal to the lesser of $0.26 or 65% of the lowest
trade price in the 25 trading days prior to the conversion. If the conversion shares are not deliverable by DWAC an additional
10% discount will apply, and if the shares are ineligible for deposit into the DTC system and only eligible for Xclearing deposit
an additional 5% discount will apply. Unless otherwise agreed in writing by both parties, at no time will the Investor convert
any amount of the June 2013 Note into common stock that would result in the Investor owning more than 4.99% of the common stock
outstanding. The Company will reserve at least 3,000,000 shares of Common Stock for issuance upon conversion of the Preferred Stock.
The Company agreed to include all shares
issuable upon conversion of the June 2013 Note in its next registration statement filed with the Securities and Exchange Commission.
So long as the June 2013 Note is outstanding,
upon any issuance by the Company or any of its subsidiaries of any security with any term more favorable to the holder of such
security or with a term in favor of the holder of such security that was not similarly provided to the Investor in the June 2013
Note, then the Company shall notify the Investor of such additional or more favorable term and such term, at the Investor's option,
shall become a part of the transaction documents with the Company.
Garden State Securities, Inc. (the “Placement
Agent”) served as the placement agent of the Company for the June 2013 Note. In consideration for services rendered as the
Placement Agent, the Company and Investor agreed that eight percent (8%) of any amounts advanced under the June 2013 Note would
be wired directly to the Placement Agent by the Investor as payment of the Placement Agent’s fee.
Settlement Shares
On July 25, 2013, we issued 7,500,000 shares
of our common stock to Hanover Holdings I, LLC, a New York limited liability company (“Hanover”), in full and final
settlement, subject to adjustment, of past-due accounts payable of the Company, which Hanover had purchased from a farming supplies
vendor of the Company, plus fees and costs (the “Hanover Settlement”).
On September 30, 2013, pursuant to the
adjustment provision included in the Hanover Settlement, an additional 2,000,000 shares of our common stock were issued to Hanover
based upon the volume weighted average price of our common stock.
The issuance of shares of our common stock
to Hanover was exempt from the registration requirements of the Securities Act pursuant to Section 3(a)(10) thereof, as an issuance
of securities in exchange for bona fide outstanding claims, where the terms and conditions of such issuance are approved by a court
after a hearing upon the fairness of such terms and conditions at which all persons to whom it is proposed to issue securities
in such exchange shall have the right to appear.
Asher Note
On August 22, 2013, we issued the Asher
Note, pursuant to the terms of a Securities Purchase Agreement. The Note matures on May 26, 2014, incurs interest at the rate of
8% per annum, and is convertible into shares of our common stock at a 35% discount to the average of the lowest three trading prices
for our common stock during the 30 day trading period prior to the conversion date. The Asher Note was offered and sold in reliance
on the exemption from registration afforded by Section 4(2) and Regulation D (Rule 506) under the Securities Act and corresponding
provisions of state securities laws.
Black Mountain Shares
On October 15, 2013, we issued a Convertible
Debenture in the principal amount of $58,000 (the “Debenture”), to Black Mountain Equities, Inc. (“Black Mountain”).
The Debenture matures on May 1, 2014, incurs a one-time interest charge of 10%, and is convertible into shares of our common stock
at a conversion price of $0.20 per share. The Debenture is secured by 1,250,000 shares of our common stock. The Debenture provides
that on the next registration statement the Company files, the Company will include the shares issuable upon conversion of the
Debenture. Black Mountain also received a warrant to purchase 1,000,000 shares of our common stock, with an exercise price of $0.25
per share and a term of five years. The Debenture was offered and sold in reliance on the exemption from registration afforded
by Section 4(2) and Regulation D (Rule 506) under the Securities Act and corresponding provisions of state securities laws.
Note Issuances
On November 21, 2013, we issued a convertible
note in the principal amount of $53,000, convertible at 65% of the three lowest bids for 30 trading days before the conversion
date with interest at 8% per annum, due on August 25, 2014 and on December 9, 2013, we issued a convertible note in the principal
amount of $55,556 with a 10% original issuance discount and 12% one time interest. The convertible notes were offered and sold
in reliance on the exemption from registration afforded by Section 4(2) and Regulation D (Rule 506) under the Securities Act and
corresponding provisions of state securities laws.
Foster Debenture and Warrants
Effective February
7, 2014, we issued a Convertible Debenture to an investor in the principal amount of $80,000. The Convertible Debenture
matures on February 6, 2015, incurs interest at the rate of 8% per annum, and is convertible into shares of our common stock at
a conversion price of $0.10 per share. The Convertible Debenture requires that we reserve at least 2,000,000 shares
for issuance upon its conversion and provides the holder with participation rights in future financings and piggyback registration
rights on the next registration statement the Company files. In connection with the issuance of the Convertible Debenture,
the investor also received a Common Stock Purchase Warrant to purchase 1,000,000 shares of our common stock, with an
exercise price of $0.10 per share, subject to adjustment, and a term of five years. The Convertible Debenture and Common
Stock Purchase Warrant were offered and sold in reliance on the exemption from registration afforded by Section 4(2) and Regulation
D (Rule 506) under the Securities Act and corresponding provisions of state securities laws.
Supplemental Warrant
On February 20, 2014, in consideration
for Cranshire Capital Master Fund, Ltd.’s (“Cranshire”) immediate cash exercise of an outstanding warrant to
purchase common stock of the Company, we agreed to issue Cranshire an additional warrant to purchase 683,202 shares of common stock
(the “Supplemental Warrant”). The issuance of the Supplemental Warrant was conducted in reliance upon Regulation D
of the Securities Act to investors who are “accredited investors,” as such term is defined in Rule 501(a) under the
Securities Act.
Restricted Stock Awards
On February 26, 2014, we issued an aggregate of 28,300,000 shares
of common stock pursuant to restricted stock award agreements to employees and consultants of the Company for services rendered
(the “Restricted Shares”). 20,000,000 of the Restricted Shares were issued to Blankenbaker Ventures (Asia) Pte. Ltd.
on behalf of George Blankenbaker, the Company’s President and director; 4,000,000 of such shares vest at the time of issuance
and the remainder vest over the following four years in equal annual installments. 3,000,000 of the shares were issued to Growers
Synergy Pte Ltd., a corporation organized under the laws of Singapore (“Growers Synergy”), all of which were fully
vested at the time of issuance. Mr. Blankenbaker is the managing director of Growers Synergy and Growers Fresh Pte Ltd (“Growers
Fresh) owns a 51% interest in Growers Synergy and Mr. Blankenbaker controls a 49% interest in Growers Fresh. Thomas Ong, a director
of the Company is a director of Growers Synergy and
is also a 25% shareholder of Agriventure
Pte Ltd., which is a 49% shareholder of Growers Synergy
. The issuance of the Restricted Stock was conducted in reliance
upon Regulation D of the Securities Act to investors who are “accredited investors,” as such term is defined in Rule
501(a) under the Securities Act and Regulation S of the Securities Act, in offshore transactions (as defined in Rule 902 under
Regulation S of the Securities Act).
Accounts Payable Conversion
On February 26, 2014, the Company agreed
to convert an aggregate of approximately $893,579.93 of advances for working capital received from George Blankenbaker, the Company’s
President and director, and entities affiliated with Mr. Blankenbaker, into an aggregate of 16,744,682 shares of common stock at
a deemed fair market value of $0.053365 per share. The issuance was conducted in reliance upon Regulation D of the Securities Act
to investors who are “accredited investors,” as such term is defined in Rule 501(a) under the Securities Act.
Nomis Bay Transaction
On March 3, 2014, pursuant to a Securities
Purchase Agreement (the “
Purchase Agreement
”) with Nomis Bay Ltd., a Bermuda company (“
Nomis Bay
”),
we issued a senior convertible note with an initial principal amount of $500,000 (the “
Initial Convertible Note
”)
for a purchase price of $340,000. The Purchase Agreement provides that, upon the terms and subject to the conditions
set forth therein, we shall have the right to require Nomis Bay to purchase from the Company on or prior to the 10
th
trading day after the effective date of a registration statement registering the shares issuable upon conversion of the Initial
Convertible Note, an additional senior convertible note with an initial principal amount of $600,000 (the “
Additional
Convertible Note
” and, together with the Initial Convertible Note, the “
Convertible Notes
”) for a
purchase price of $600,000.
The Initial Convertible Note matures on
December 27, 2014 (subject to extension as provided in the Initial Convertible Note) and, in addition to the 32% original issue
discount, accrues interest at the rate of 8% per annum. If issued, the Additional Convertible Note will mature on the date that
is the 10-month anniversary of the date of issuance of the Additional Convertible Note (subject to extension as provided in the
Initial Convertible Note) and will accrue interest at the rate of 8% per annum. The Initial Convertible Note is convertible at
any time, in whole or in part, at Nomis Bay’s option into shares of the Company’s common stock, par value $0.001 per
share (the “
Common Stock
”), at a conversion price equal to the lesser of (i) the product of (x) the arithmetic
average of the lowest three (3) volume weighted average prices of the Common Stock during the 10 consecutive trading days ending
and including the trading day immediately preceding the applicable conversion date and (y) 40% (the “
Variable Conversion
Price
”), and (ii) $0.30 (as adjusted for stock splits, stock dividends, stock combinations or other similar transactions).
If issued, the Additional Convertible Note will be convertible at any time, in whole or in part, at Nomis Bay’s option into
shares of Common Stock at a conversion price that will be equal to the lesser of (i) the Variable Conversion Price and (ii) $0.30
(as adjusted for stock splits, stock dividends, stock combinations or other similar transactions). At no time will Nomis Bay be
entitled to convert any portion of the Convertible Notes to the extent that after such conversion, Nomis Bay (together with its
affiliates) would beneficially own more than 4.99% of the outstanding shares of Common Stock as of such date.
The issuances were conducted in reliance
upon Regulation D of the Securities Act to investors who are “accredited investors,” as such term is defined in Rule
501(a) under the Securities Act.
Warrant Exercises
On August 1, 2012, the Company issued to Cranshire a warrant
to purchase an aggregate of 213,334 shares of the Company’s common stock at an exercise price of $0.6405 with a term of 5
years (the “
Cranshire Warrant
”). On May 1, 2013, the Company issued to Anson Investments Master Fund LP (“
Anson
”),
three warrants to purchase 1,877,333, 1,066,666 and 2,346,666 shares respectively, at exercise prices of $0.20, $0.25 and $0.25
respectively (the “
Anson Warrants
” and together with the Cranshire Warrants, the “
Investor Warrants
”).
The Investor Warrants each contained certain adjustment provisions in the event the Company undertook subsequent stock issuances
at a price per share less than the exercise price of the Investor Warrants. As of February 20, 2014, as a result of dilutive issuances,
the Investor Warrants were each adjusted to an exercise price of $0.053365. As of February 20, 2014, the Cranshire Warrant is exercisable
for an aggregate of 2,560,486 shares and the Anson Warrants are exercisable for an aggregate of 7,035,820, 4,997,029 and 10,993,469
shares respectively. As of February 28, 2014 Cranshire had exercised an aggregate of 683,202 shares pursuant to the Cranshire Warrant
and Anson had exercised an aggregate of 3,540,659 shares pursuant to the Anson Warrants. The issuances were conducted in reliance
upon Regulation D of the Securities Act to investors who are “accredited investors,” as such term is defined in Rule
501(a) under the Securities Act.
Item 16. Exhibit Index
The following exhibits are included as part of this registration
statement by reference:
|
Number
|
|
Description
|
|
|
|
|
|
2.1
|
|
Share Exchange Agreement, dated June 23, 2011 (incorporated by reference to Exhibit 2.1 of the Registrant’s Current Report on Form 8-K filed on June 29, 2011)
|
|
|
|
|
|
3.1
|
|
Articles of Incorporation of the Registrant, dated May 18, 2007, including all amendments to date (Incorporated by reference to the Quarterly Report on Form 10-Q filed on November 20, 2013)
|
|
|
|
|
|
3.2
|
|
Amended and Restated Bylaws of the Registrant, as amended, dated March 18, 2011 (incorporated by reference to Exhibit 3.2 of the Registrant’s Current Report on Form 8-K filed on March 22, 2011)
|
|
|
|
|
|
4.1
|
|
Specimen Common Stock Certificate (incorporated by reference to Exhibit 4.1 of the Registrant’s Registration Statement on Form S-1 filed on July 16, 2008)
|
|
|
|
|
|
5.1
|
|
Opinion of Greenberg Traurig, LLP*
|
|
|
|
|
|
10.1
|
|
Supply Agreement with Asia Stevia Investment Development Company Ltd, dated April 12, 2011 (incorporated by reference to the registrant’s Form 8-K filed on June 29, 2011)
|
|
|
|
|
|
10.2
|
|
Supply Agreement with Stevia Ventures Corporation, dated April 12, 2011 (incorporated by reference to the registrant’s Form 8-K filed on June 29, 2011)
|
|
|
|
|
|
10.3
|
|
Convertible Promissory Note, with Vantage Associates SA, dated February 14, 2011 (incorporated by reference to the registrant’s Form 8-K filed on June 29, 2011)
|
|
|
|
|
|
10.4
|
|
Convertible Promissory Note, with Vantage Associates SA, dated June 23, 2011 (incorporated by reference to the registrant’s Form 8-K filed on June 29, 2011)
|
|
|
|
|
|
10.5
|
|
Form of Convertible Promissory Note (incorporated by reference to the registrant’s Form 10-Q filed on November 21, 2011)
|
|
|
|
|
|
10.6
|
|
Stock Purchase Agreement (incorporated by reference to the registrant’s Form 10-Q filed on November 21, 2011)
|
|
|
|
|
|
10.7
|
|
Management and Off-Take Agreement with Growers Synergy Pte Ltd., effective November 1, 2011 (incorporated by reference to the registrant’s Form 8-K filed on October 31, 2011)
|
|
10.8
|
|
The Minutes for Land Transferring Agreement for New Crop Plants Variety, dated December 14, 2011 (incorporated by reference to the registrant’s Form 10-Q filed on February 17, 2012)
|
|
|
|
|
|
10.9
|
|
Supply Agreement with Guangzhou Health China Technology Development Company Limited, dated February 21, 2012 (incorporated by reference to the registrant’s Form 8-K filed on February 27, 2012)
|
|
|
|
|
|
10.10
|
|
Cooperative Agreement (incorporated by reference to the registrant’s Current Report on Form 8-K filed on July 11, 2012)
|
|
|
|
|
|
10.11
|
|
Technology Acquisition Agreement (incorporated by reference to the registrant’s Current Report on Form 8-K filed on July 11, 2012)
|
|
|
|
|
|
10.12
|
|
Securities Purchase Agreement (incorporated by reference to the Current Report on Form 8-K filed on August 7, 2012)
|
|
|
|
|
|
10.13
|
|
Registration Rights Agreement (incorporated by reference to the Current Report on Form 8-K filed on August 7, 2012)
|
|
|
|
|
|
10.14
|
|
Form of Warrant (incorporated by reference to the Current Report on Form 8-K filed on August 7, 2012)
|
|
|
|
|
|
10.15
|
|
Reset Letter with Anson Investments Master Fund LP, dated May 1, 2013 (incorporated by reference to the Current Report on Form 8-K filed on May 6, 2013)
|
|
|
|
|
|
10.16
|
|
Form of Warrant (incorporated by reference to the Current Report on Form 8-K filed on May 6, 2013)
|
|
|
|
|
|
10.17
|
|
Stipulation of Settlement with Hanover Holdings I, LLC, dated July 16, 2013 (incorporated by reference to the Current Report on Form 8-K filed on July 29, 2013)
|
|
|
|
|
|
10.18
|
|
$400,000 Promissory Note, dated July 16, 2013(incorporated by reference to the Quarterly Report on Form 10-Q filed August 19, 2013)
|
|
|
|
|
|
10.19
|
|
Form of Senior Convertible Note (incorporated by reference to the Current Report on Form 8-K filed March 4, 2014)
|
|
|
|
|
|
10.20
|
|
Securities Purchase Agreement, dated as of March 3, 2014, by and between Nomis Bay Ltd. and Stevia Corp. (incorporated by reference to the Current Report on Form 8-K filed March 4, 2014)
|
|
|
|
|
|
10.21
|
|
Registration Rights Agreement, dated as of March 3, 2014, by and between Nomis Bay Ltd. and Stevia Corp. (incorporated by reference to the Current Report on Form 8-K filed March 4, 2014)
|
|
|
|
|
|
10.22
|
|
Note Purchase Agreement, dated as of April 2, 2014, by and between Stevia Corp. and YOPCP, LLC (incorporated
by reference to the Current Report on Form 8-K filed on April 3, 2014)
|
|
|
|
|
|
10.23
|
|
Form of Senior Secured Convertible Promissory Note (incorporated by reference to the Current Report on
Form 8-K filed on April 3, 2014)
|
|
|
|
|
|
10.24
|
|
Security Agreement, dated as of April 2, 2014, by and between Stevia Corp. and YOPCP, LLC (incorporated
by reference to the Current Report on Form 8-K filed on April 3, 2014)
|
|
|
|
|
|
21
|
|
List of Subsidiaries
*
|
|
|
|
|
|
23.1
|
|
Consent of Li and Company, PC*
|
|
|
|
|
|
23.2
|
|
Consent of Greenberg Traurig, LLP (filed as part of Exhibit 5.1)*
|
|
|
|
|
|
24
|
|
Power of Attorney (included on the signature page of this Registration Statement)*
|
|
|
|
|
|
101
|
|
Interactive Data File*
|
*Filed Herewith
Item 17. Undertakings
The undersigned registrant hereby undertakes
to:
(a) Rule 415 Offering
:
1. To file, during
any period in which offers or sales are being made, a post-effective amendment to this registration statement:
i. To include any
prospectus required by section 10(a)(3) of the Securities Act of 1933;
ii. To reflect in
the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the
registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar
value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated
maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) (§ 230.424
of this chapter) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate
offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
iii. To include any
material information with respect to the plan of distribution not previously disclosed in the registration statement or any material
change to such information in the registration statement;
Provided, however, that:
(A) Paragraphs (a)(1)(i)
and (a)(1)(ii) of this section do not apply if the registration statement is on Form S-8 (§ 239.16b of this chapter), and
the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or
furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 (15
U.S.C. 78m or 78o(d)) that are incorporated by reference in the registration statement; and
(B) Paragraphs (a)(1)(i),
(a)(1)(ii) and (a)(1)(iii) of this section do not apply if the registration statement is on Form S-3 (§ 239.13 of this chapter)
or Form F-3 (§ 239.33 of this chapter) and the information required to be included in a post-effective amendment by those
paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section
15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained
in a form of prospectus filed pursuant to Rule 424(b) (§ 230.424(b) of this chapter) that is part of the registration statement.
(C) Provided further,
however, that paragraphs (a)(1)(i) and (a)(1)(ii) do not apply if the registration statement is for an offering of asset-backed
securities on Form S-1 (§ 239.11 of this chapter) or Form S-3 (§ 239.13 of this chapter), and the information required
to be included in a post-effective amendment is provided pursuant to Item 1100(c) of Regulation AB (§ 229.1100(c)).
2. That, for the purpose
of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the
initial bona fide offering thereof.
3. To remove from
registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination
of the offering.
4. If the registrant
is a foreign private issuer, to file a post-effective amendment to the registration statement to include any financial statements
required by “Item 8.A. of Form 20-F (17 CFR 249.220f)” at the start of any delayed offering or throughout a continuous
offering. Financial statements and information otherwise required by Section 10(a)(3) of the Act need not be furnished, provided
that the registrant includes in the prospectus, by means of a post-effective amendment, financial statements required pursuant
to this paragraph (a)(4) and other information necessary to ensure that all other information in the prospectus is at least as
current as the date of those financial statements. Notwithstanding the foregoing, with respect to registration statements on Form
F-3 (§239.33 of this chapter), a post-effective amendment need not be filed to include financial statements and information
required by Section 10(a)(3) of the Act or §210.3-19 of this chapter if such financial statements and information are contained
in periodic reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the
Securities Exchange Act of 1934 that are incorporated by reference in the Form F-3.
5. That, for the purpose
of determining liability under the Securities Act of 1933 to any purchaser:
i. If the registrant
is relying on Rule 430B (§230.430B of this chapter):
(A) Each
prospectus filed by the registrant pursuant to Rule 424(b)(3) (§230.424(b)(3) of this chapter) shall be deemed to be part
of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement;
and
(B) Each
prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) (§230.424(b)(2), (b)(5), or (b)(7) of this chapter)
as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii),
or (x) (§230.415(a)(1)(i), (vii), or (x) of this chapter) for the purpose of providing the information required by section
10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier
of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in
the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is
at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the
securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or
prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into
the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract
of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus
that was part of the registration statement or made in any such document immediately prior to such effective date; or
ii. If the registrant
is subject to Rule 430C (§230.430C of this chapter), each prospectus filed pursuant to Rule 424(b) as part of a registration
statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in
reliance on Rule 430A (§230.430A of this chapter), shall be deemed to be part of and included in the registration statement
as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus
that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration
statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior
to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of
the registration statement or made in any such document immediately prior to such date of first use.
6. That, for the purpose
of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the
securities:
The undersigned registrant
undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless
of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser
by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered
to offer or sell such securities to such purchaser:
i. Any preliminary
prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424
of this chapter);
ii. Any free writing
prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned
registrant;
iii. The portion of
any other free writing prospectus relating to the offering containing material information about the undersigned registrant or
its securities provided by or on behalf of the undersigned registrant; and
iv. Any other communication
that is an offer in the offering made by the undersigned registrant to the purchaser.
Insofar as indemnification for liabilities
arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant
to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission
such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim
for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director,
officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such
director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the
opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the
question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final
adjudication of such issue.
SIGNATURES
Pursuant to the requirements
of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned,
thereunto duly authorized in the City of Indianapolis, State of Indiana, on April 8, 2014.
|
|
STEVIA CORP.
|
|
|
a Nevada corporation
|
|
|
|
|
Dated: April 8, 2014
|
/s/ George Blankenbaker
|
|
|
By: George Blankenbaker
|
|
|
Its: President, Secretary, Treasurer and Director
|
|
|
(Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer)
|
SIGNATURES AND POWER OF ATTORNEY
Known All Persons
By These Present
, that each person whose signature appears below appoints Mr. George Blankenbaker as his true and lawful attorney-in-fact
and agent, with full power of substitution, for him and in his name, place and stead, to sign any amendment (including post-effective
amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection
therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority
to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents
and purposes as he may do in person, hereby ratifying and confirming all that said attorney-in-fact and agent or any of them, or
of his/her substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements
of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the
dates indicated:
|
Dated: April 8, 2014
|
/s/ George Blankenbaker
|
|
|
George Blankenbaker
|
|
|
President, Secretary, Treasurer and Director (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer)
|
|
|
|
|
|
|
|
Dated: April 8, 2014
|
/s/ Pablo Erat
|
|
|
Pablo Erat
|
|
|
Director
|
|
|
|
|
|
|
|
Dated: April 8, 2014
|
/s/ Thomas Ong
|
|
|
Thomas Ong
|
|
|
Director
|
Stevia (PK) (USOTC:STEV)
Historical Stock Chart
From Feb 2025 to Mar 2025
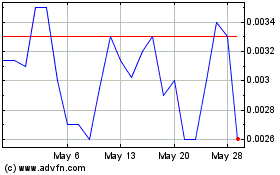
Stevia (PK) (USOTC:STEV)
Historical Stock Chart
From Mar 2024 to Mar 2025