UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
DC 20549
FORM
10-KSB
|
x
|
ANNUAL
REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
|
|
|
|
|
For
the fiscal year ended December 31, 2007
|
|
o
|
TRANSITION
REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
Commission
File Number 001-33426
NEURO-HITECH,
INC.
(Exact
name of Small Business Issuer as Specified in its Charter)
|
Delaware
|
|
20-4121393
|
|
(State
or Other Jurisdiction of
|
|
(I.R.S.
Employer
|
|
Incorporation
or Organization)
|
|
Identification
No.)
|
One
Penn Plaza, Suite 1503, New York, NY 10019
(Address
of Principal Executive Offices)
(212)
594-1215
(Issuer’s
Telephone Number, Including Area Code)
Securities
registered under Section 12(b) of the Exchange Act: None
Securities
registered under Section 12(g) of the Exchange Act:
|
Title
of each class
|
|
|
|
Common
Stock, $0.001 par value per
share
|
Check
whether the issuer is not required to file reports pursuant to Section 13 or
15(d) of the Exchange Act.
o
Check
whether the issuer: (1) filed all reports required to be filed by Section 13
or
15(d) of the Exchange Act during the past 12 months (or for such shorter period
that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days.
Yes
þ
No
o
Check
if
there is no disclosure of delinquent filers in response to Item 405 of
Regulation S-B contained in this form, and no disclosure will be contained,
to
the best of registrant’s knowledge, in definitive proxy or information
statements incorporated by reference in Part III of Form 10-KSB or any amendment
to this Form 10-KSB.
o
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Exchange Act).
o
Yes
þ
No
State
issuer’s revenues for its most recent fiscal year. $458,870
The
aggregate market value of the voting and non-voting common equity held by
non-affiliates of the registrant as of
March
17,
2008
based upon the closing price of the common stock as reported then on the NASDAQ
Capital Market as of such date, was approximately $4,735,733.
The
number of shares outstanding of each of the issuer’s classes of common equity,
as of March 17, 2008 is:
DOCUMENTS
INCORPORATED BY REFERENCE
(1)
Portions
of the registrant’s Proxy Statement relating to its 2008 Annual Stockholders’
Meeting, to be filed subsequently—Part III.
Transitional
Small Business Disclosure Format (check
one):
o
Yes
þ
No
NEURO-HITECH,
INC.
FORM
10-KSB
FOR
THE FISCAL YEAR ENDED DECEMBER 31, 2007
|
|
|
Page
|
|
PART
I
|
|
|
|
Item
1. Description of Business
|
|
1
|
|
Item
2. Description of Property
|
|
21
|
|
Item
3. Legal Proceedings
|
|
21
|
|
Item
4. Submission of Matters to a Vote of Security Holders
|
|
21
|
|
|
|
|
|
PART
II
|
|
|
|
|
|
|
|
Item
5. Market for Common Equity, Related Stockholder Matters and Small
Business Issuer Purchases of Equity Securities
|
|
22
|
|
Item
6. Management’s Discussion and Analysis or Plan of
Operations
|
|
23
|
|
Item
7. Financial Statements
|
|
27
|
|
Item
8. Changes in and Disagreements with Accountants on Accounting and
Financial Disclosures
|
|
27
|
|
Item
8A(T). Controls and Procedures
|
|
27
|
|
Item
8B. Other Information
|
|
|
|
|
|
|
|
PART
III
|
|
|
|
|
|
|
|
Item
9. Directors, Executive Officers,
Promoters, Control Persons and Corporate Governance; Compliance with
Section 16(a) of the Exchange Act
|
|
29
|
|
Item
10. Executive Compensation
|
|
29
|
|
Item
11. Security Ownership of Certain Beneficial
Owners and Management and Related Stockholder Matters
|
|
29
|
|
Item
12. Certain Relationships and Related Transactions, and Director
Independence
|
|
29
|
|
Item
13. Exhibits
|
|
30
|
|
Item
14. Principal Accountant Fees and
Services
|
|
34
|
PART
I
|
Item
1.
|
Description
of Business
|
Description
of the Company
Neuro-Hitech,
Inc. (the “Company” or “Neuro-Hitech”) is an early stage pharmaceutical company
focused on developing innovative drugs for the treatment of degenerative
neurological diseases. The Company’s most advanced product candidate, Huperzine
A, recently completed a Phase II clinical trial in the U.S. which tested
Huperzine A for efficacy and safety in the treatment of mild to moderate
Alzheimer’s disease. Huperzine A is a cholinesterase inhibitor that the Company
believes may be effective in the treatment of Alzheimer’s disease and Mild
Cognitive Impairment (“MCI”), although, to date, its efforts have been focused
upon Huperzine A’s effectiveness in Alzheimer’s disease. Through a collaboration
with the Alzheimer’s Disease Cooperative Study (“ADCS”), the Company has
completed two Phase I studies and a Phase II clinical trial. ADCS was formed
in
1991 as a cooperative agreement between the National Institute of Aging and
the
University of California San Diego, with the goal of advancing the research
of
drugs for treating patients with Alzheimer’s disease, the National Institutes of
Health (“NIH”) and Georgetown University Medical Center
(“Georgetown”).
The
Phase
II clinical trial compared the safety, tolerability and efficacy of either
200
or 400 micrograms of Huperzine A on cognitive function, activities of daily
living and behavior. Results showed that there was no statistical difference
in
the mean change AD Assessment Scale-Cognitive (ADAS-Cog) scores, the primary
endpoint, after 16 weeks treatment with Huperzine A 200 micrograms bid compared
to placebo (p=0.81). However, data demonstrated that the higher dose tested,
400
micrograms bid, showed cognitive enhancement on the ADAS-Cog versus placebo.
The
maximum cognitive improvement was observed at week 11 of treatment (p=0.001).
Over 16 weeks Huperzine A (400 micrograms bid) improved cognition compared
to
placebo (p=0.03) and there was a trend to cognitive improvement over placebo
at
week 16 (p=0.069). In this clinical trial, there was an unexpected improvement
in cognition in the placebo group at week 16 versus baseline. On other secondary
endpoints, including clinical global impression of change (ADCS-CGIC) and the
Neuropsychiatric Inventory (NPI) there was no statistical difference between
placebo and either 200 or 400 micrograms bid after four months treatment.
However, there was a trend to improvement on activities of daily living
(ADCS-ADL) with 400 micrograms bid (p=0.077). Huperzine A was safe and well
tolerated. Overall the incidence of adverse events during the study was similar
between both doses of Huperzine A and placebo. Following completion of the
double-blind part of this clinical trial, subjects were invited to receive
Huperzine A treatment in an open-label fashion for up to one year; 82% of
subjects accepted this invitation.
After
receiving the results of the Phase II clinical trial of Huperzine A, the Company
conducted a detailed review of those results. Based on its review, the Company
believes that there is substantial support within the results that Huperzine
A
can become a safe and effective treatment for Alzheimer’s disease. In order to
validate the current information on the compound and review the Company’s
analysis of the results, in March 2008 the Company entered into an
agreement with Numoda Corporation (“Numoda”) pursuant to which Numoda will
review the results and will provide a report to the Company on its findings.
In
view
of the results of the Phase II clinical trial, the Company is currently
reviewing its options for moving forward with its business. Currently, the
Company expects to focus upon the development of collaborative, joint and
strategic alliances and licensing arrangements with one or more pharmaceutical
companies for the further development of Huperzine A and also expects to
consider merger and/or acquisition opportunities. The Company will further
evaluate these opportunities based upon the results of Numoda’s review and
report, and the Company also expects that Numoda will assist the Company in
interpreting and presenting the results of the Phase II clinical trial to
potential partners, licensees and merger or acquisition candidates.
The
Company has also studied the transdermal delivery of Huperzine A. The Company
believes that Huperzine A can effectively be delivered transdermally because
of
its low dosage requirement and low molecular weight. The Company believes that
a
transdermal patch could be a better way to deliver Huperzine A
because the patch may provide the drug for transdermal delivery for up to
between three and five days while avoiding the gastrointestinal tract. Although
the Company initially expected to begin Phase I clinical trials in the first
quarter of 2008, the Company has elected to postpone any decision or
expenditures related to such a trial until after Numoda has delivered its report
and the Company has considered its options.
Worldwide
research thus far suggests that, in addition to Alzheimer’s Disease, Huperzine A
may be effective in treating other dementias and myasthenia gravis. Also,
research suggests that it has potential neuroprotective properties that may
render it useful as a protection against neurotoxins, and it has an anti-oxidant
effect.
In
addition to Huperzine A, the Company has worked on two pre-clinical development
programs: one for second generation anti-amyloid compounds or disease modifying
drugs for Alzheimer’s disease and, secondly, development of a series of
compounds targeted to treat and prevent epilepsy. The Company’s efforts however,
have principally focused on its primary product.
The
Company has imported and sold inventories of natural Huperzine to vitamin and
supplement suppliers to generate revenues. However, the majority of the
Company’s operations to date have been funded through the Company’s private
placement of equity securities.
History
The
Company was originally formed on February 1, 2005, as Northern Way Resources,
Inc., a Nevada corporation, for the purpose of acquiring exploration and early
stage natural resource properties. On January 24, 2006, the Company entered
into
an Agreement and Plan of Reorganization (the “Merger Agreement”) by and among
the Company, Marco Hi-Tech JV Ltd., a privately held New York corporation
(“Marco”), and Marco Acquisition I, Inc., a newly formed wholly-owned Delaware
subsidiary of the Company (“Acquisition Sub”). Upon closing of the transactions
contemplated under the Merger Agreement (the “Merger”), Acquisition Sub was
merged with and into Marco, and Marco became a wholly-owned subsidiary of the
Company. The Merger was consummated on that date and in connection with that
Merger, the Company changed its name to Neuro-Hitech Pharmaceuticals, Inc.
The
Company subsequently changed its name to Neuro-Hitech, Inc. on August 11,
2006.
Pursuant
to the Merger Agreement, at closing, shareholders of Marco received 0.5830332
shares of the Company’s Common Stock for each issued and outstanding share of
Marco’s common stock, par value $.01 per share. As a result, at closing of the
Merger, the Company issued 6,164,006 shares of its Common Stock to the former
stockholders of Marco, which represented approximately 80% of the Company’s
outstanding Common Stock following the Merger, in exchange for 100% of the
outstanding capital stock of Marco.
All
references to the “Company” for periods prior to the closing of the Merger refer
to Marco, and references to the “Company” for periods subsequent to the closing
of the Merger refer to Neuro-Hitech and its subsidiaries.
Marco
was
incorporated in the State of New York on December 11, 1996. Through 2005, Marco
was focused primarily on licensing proprietary Huperzine A technology from
independent third-party developers and investigators, including the Mayo
Foundation for Medical Education and Research in Rochester, Minnesota (the
“Mayo
Foundation”), and conducting analytical work and clinical trials of Huperzine A,
and until such time operated with no full-time employees and minimal internal
resources. In addition, from time to time, Marco imported and sold inventories
of natural Huperzine and other dietary supplement ingredients to vitamin and
supplement suppliers to generate revenues. In 2005, Marco determined to raise
additional capital to pursue additional approvals and undertake necessary
studies for the development and commercialization of Huperzine A, including
securing rights to third-party transdermal patch technology.
Upon
the
Merger, the Company abandoned the line of business pursued by Northern Way
Resources prior to the Merger.
On
November 29, 2006, the Company completed an acquisition by merger of Q-RNA,
Inc. (“Q-RNA”), a New York-based biotechnology company focused on diseases such
as Alzheimer’s, epilepsy and Parkinson’s disease (the “Q-RNA Merger”), pursuant
to the Agreement and Plan of Merger (the “Q-RNA Merger Agreement”) with QA
Acquisition Corp., a Delaware corporation, QA Merger LLC, a Delaware limited
liability company, Q-RNA and Dr. David Dantzker, as the “Representative” of the
Q-RNA security holders.
The
Merger consideration paid to the Q-RNA securityholders pursuant to the Q-RNA
Merger Agreement consisted of an aggregate of: (i) 1,800,000 shares of the
Company’s common stock, (ii) warrants to purchase 600,356 shares of the
Company’s common stock at an exercise price of $13 per share, and (iii) warrants
to purchase 600,356 shares of the Company’s common stock at an exercise price of
$18 per share. The Company also assumed Q-RNA options outstanding which upon
exercise will be exercisable for 199,286 shares of the Company’s common
stock.
The
acquisition of Q-RNA provided the Company with a pipeline of compounds, many
of
which have been discovered and developed internally. Among the compounds that
Q-RNA believed were ready to move to optimization and pre-clinical development
were NHT0012, which is one of a number of second generation disease modifying
drugs for Alzheimer’s disease that inhibit A-beta and Tau oligomerization and
NHT1107, which is one of a large pharmaceutical library of drugs designed for
the treatment of epilepsy that offer both anti-ictogenci (ability to treat
epilepsy) and anti-epileptogenic (ability to prevent epilepsy)
properties.
Description
of the Business
Alzheimer’s
disease
and
MCI
Alzheimer’s
disease, the leading cause of dementia, is characterized by the progressive
loss
of memory, thinking (cognitive function) and the ability to perform the
activities of daily living (global function). There is currently no cure.
According to the Alzheimer’s Association and the American Health Assistance
Foundation:
|
·
|
Alzheimer’s
disease currently affects approximately 5 million people in the U.S.,
including as many as 13% of people age 65 and older and nearly 50%
of
those age 85 and older.
|
|
·
|
Worldwide,
Alzheimer’s disease affects 18 million people, and that number is
expected to reach 34 million by
2025.
|
|
·
|
There
are at least 350,000 new diagnoses of Alzheimer’s disease, and at least
65,000 Alzheimer’s disease deaths, per year in the U.S.
|
|
·
|
Following
initial diagnosis, patients live 4 – 6 years
on
average, but may live up
to 20 years with the disease.
|
|
·
|
Total
annual expenditures on Alzheimer’s disease in the U.S. exceed $100 billion
annually, and the average lifetime cost per Alzheimer’s disease patient is
$174,000.
|
The
precise physical changes in the brain that produce Alzheimer’s disease are
complex and not completely understood, but it is generally believed that the
misfolding of two proteins, A-beta and Tau, are central to the process. The
two
best-validated early drug targets for Alzheimer’s disease are cholinesterase and
the N-methyl-D-aspartate receptor (NMDA-receptor). There are only four
commonly-used drugs that the FDA has approved for the treatment of the symptoms
of Alzheimer’s disease. Although the precise mechanism of action of these four
drugs is unknown, three of these drugs are believed to inhibit cholinesterase,
and one is believed to inhibit the NMDA-receptor. These four drugs and their
respective marketers, FDA approval dates and mechanisms of action are set forth
in the following table.
|
Drug
(Trade Name/Generic)
|
|
Marketed
by
|
|
FDA
Approval Date
|
|
Postulated
Mechanism
|
|
Aricept
®
(donepezil)
|
|
Pfizer
Inc./Eisai Co., Ltd.
|
|
November 25, 1996
|
|
Cholinesterase
inhibition
|
|
Exelon
®
(rivastigmine)
|
|
Novartis
AG
|
|
April 21, 2000
|
|
Cholinesterase
inhibition
|
|
Razadyne
®
(galantamine)
|
|
Johnson
& Johnson
|
|
February 28, 2001
|
|
Cholinesterase
inhibition
|
|
Namenda
®
(memantine)
|
|
Forest
Laboratories, Inc.
|
|
October 16, 2003
|
|
NMDA-receptor
inhibition
|
According
to Merrill Lynch equity research, the worldwide market for Alzheimer’s disease
drugs in 2005 was $3 billion, with the largest selling cholinesterase
inhibitor, Aricept, generating $1.7 billion of those sales.
The
market performance of the existing Alzheimer’s disease therapeutics is
particularly noteworthy given that their clinical performance to date has been
modest. Specifically, as stated in their FDA-approved labeling, none of the
drugs approved by the FDA to treat Alzheimer’s disease has been proven to
prevent or change the underlying process of brain deterioration
(neurodegeneration) in patients with Alzheimer’s disease. Rather, these drugs
have been shown only to slow the worsening of the symptoms of Alzheimer’s
disease—primarily loss of cognitive and global function. Furthermore, in the
studies submitted in support of applications for FDA approval of these drugs,
none of these drugs was shown significantly to improve both cognitive and global
function over a six-month period in the patients studied. Thus, the Company
believes that there is room for improvement in this large and growing
pharmaceutical market.
In
addition to the market for Alzheimer’s disease, compounds such as Huperzine A
may provide potential benefits to patients diagnosed with MCI by slowing down
the advent of Alzheimer’s disease or other forms of dementia. According to the
Mayo Clinic, MCI afflicts up to 20% of the non-demented population over 65.
MCI is a relatively new classification of memory disorder that is
characterized by noticeable memory loss, but otherwise normal behavior.
According to the Mayo Clinic, MCI converts to Alzheimer’s disease at a rate of
10 to 15% a year.
Huperzine
A
The
Company’s decision to begin clinical development of Huperzine for Alzheimer’s
disease and to investigate potential clinical development of huperzine for
other
neurological indications was based in part on the following data:
Huperzine
A is a compound that is used in China as a prescription drug for treating
Alzheimer’s disease and other forms of dementia. Clinical trials conducted
outside the U.S of Huperzine A have been successful, and one Chinese study
using
the same clinical end points as an FDA-approved study for a leading Alzheimer’s
disease treatment show Huperzine A to be safe and effective. Both pre-clinical
and human clinical studies conducted both in China and in the U.S. suggest
that
Huperzine A:
|
·
|
may
have significantly longer inhibitory action at lower doses than the
other
approved drugs for early and middle stage Alzheimer’s
disease;
|
|
·
|
may
prove to reduce the unpleasant side effects resulting from use of
other
approved drugs for early and middle stage Alzheimer’s
disease;
|
|
·
|
may
be effective not only in increasing the brain’s acetylcholine levels, but
also levels of other important neurotransmitters such as dopamine
and
noradrenaline;
|
|
·
|
may
have high oral bioavailability and good penetration through the
blood-brain barrier; and
|
|
·
|
may
exhibit neuroprotective properties, and may significantly decrease
neuronal cell death due to glutamate-induced
excitotoxicity.
|
Although
not being pursued by the Company at this time, Chinese clinical studies have
indicated that Huperzine A may also have potential in the treatment of
myasthenia gravis, a progressive autoimmune disease resulting in neuromuscular
failure, which, untreated can lead to blindness and death from respiratory
failure. In a 1986 study by Y.S. Cheng et al., it was shown that Huperzine
A
controlled the clinical manifestations of the disease in 99% of the 128 patients
treated. Additional research at the Walter Reed Institute of Research indicates
that Huperzine A may also have application as a nerve gas antidote. Under the
Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended
to
treat a rare disease or condition, which is generally a disease or condition
that affects fewer than 200,000 individuals in the U.S. The Company believes
that Huperzine can qualify for orphan drug designation for treating myasthenia
gravis.
Future
Compounds
The
acquisition of Q-RNA provided the Company with a pipeline of compounds, many
of
which have been discovered and developed by Dr. Donald F. Weaver, an inventor
and expert in neuroscience and chemistry. The Company believes these compounds
provide it with an additional research and development pipeline. The following
compounds are ready to move to optimization and pre-clinical development:
NHT0012
is one of a number of second generation disease modifying drugs for Alzheimer’s
disease that inhibit A-beta and Tau oligomerization.
In
vitro
studies
suggest that these compounds are likely to offer a better pharmacological
profile than first generation drugs by having:
|
·
|
Enhanced
anti-aggregation activity
|
|
|
|
|
·
|
Better
blood-brain barrier (BBB) penetration
|
|
|
|
|
·
|
Milder
side effect profile
|
Currently
marketed medications only help to control symptoms and do not slow or reverse
the progression of the disease. NHT0012 belongs to an emerging class of
compounds focused on reducing the progression of Alzheimer’s disease while also
improving its debilitating symptoms. It is believed that NHT0012 will prevent
the formation and break down existing neurotoxic amyloid beta aggregates,
allowing amyloid peptides to clear from the brain rather than accumulate and
form amyloid plaques, a hallmark pathology of Alzheimer’s disease.
NHT1107
is one of a large pharmaceutical library of drugs designed for the treatment
of
epilepsy that offer both anti-ictogenic (ability to treat epilepsy) and
anti-epileptogenic (ability to prevent epilepsy) properties. Many of these
compounds have proven to be effective in animal model (
in
vivo
)
systems
conducted at the National Institutes of Health.
Existing
medications for epilepsy only control symptoms (i.e. seizures) and do not slow
the progression of the disorder. NHT1107 is a prototypic agent in a
pioneering class of new antiepileptogenic agents that actually prevent the
onset
of epilepsy after a brain injury. It is believed that NHT1107 prevents
excessive brain excitation that arises from abnormal activities of
glutamate and Gamma-Aminobutyric Acid within the injured brain and that
culminate in causing epilepsy.
Licenses
and Patents
PARTEQ
As
part
of the acquisition of Q-RNA, the Company assumed exclusive license agreements
and an option to purchase an exclusive license with PARTEQ Research and
Development Innovations (“PARTEQ”), the technology licensing arm of Queens
University, Kingston, Ontario, Canada. The exclusive license agreement grants
the Company exclusive worldwide licenses to all innovations and developments
made under the Sponsored Research Agreement, as defined therein.
PARTEQ
- Alzheimer’s Research
The
Company holds an exclusive license for patent applications directed to compounds
(including bi-aromatic and aromatic anionic (e.g., bi-indole and single indole
compounds) and methods for treating protein folding disorders (including
Alzheimer’s disease) from PARTEQ.
The
exclusive license agreement grants the Company an exclusive worldwide license
to
all innovations and developments, including the patent applications and
additional filings pursuant to the Exclusive Patent License Agreement with
PARTEQ (the “PARTEQ Licensing Agreement”) for Alzheimer’s research. The Company
paid a one-time license fee of C$25,000 when it entered into the PARTEQ
Licensing Agreement in 2005 and will be required to make quarterly royalty
payments of 3% of net sales of the licensed products, with a minimum annual
royalty of C$10,000 for 2007, C$20,000 for 2008, C$30,000 for 2009 and C$40,000
for 2010 and each subsequent calendar year. Until such time as the Company
has a
licensed product, the Company will not have to make quarterly payments. It
does
not anticipate having a licensed product in the near term. The Company is also
obligated to make the following milestone payments: C$100,000 upon completion
of
a Phase I trial of a licensed product, C$250,000 upon completion of a Phase
II
trial of a licensed product, and C$1,000,000 upon the first FDA approval (as
such term is defined in the PARTEQ Licensing Agreement). The Company also has
the right to sub-license with the payment of 20% of all non-royalty sublicensing
consideration.
Under
the
terms of the PARTEQ Licensing Agreement, which was amended in 2006, the Company
is obligated to pay fixed annual fees of C$256,802 for the Alzheimer’s research.
In October 2007, the Company renewed its licensing agreements with PARTEQ.
The
renewal extends the Company’s right to license any innovations and developments
thereunder for an additional year. Under the terms of the renewal, the Company
will be obligated to make additional payments of C$282,944, which payments
are
payable quarterly.
PARTEQ
- Epilepsy Research
The
Company holds an option to acquire an exclusive worldwide license to all
innovations and developments for certain compounds (including pyrimidine,
heterocyclic, beta-alanine, uracil, diuracil, beta amino acid analogs and
related compounds) and patents/patent applications related thereto from PARTEQ
for Epilepsy research, including the patent applications and additional filings,
pursuant to the Exclusive Patent License Option Agreement with PARTEQ (the
“PARTEQ Licensing Option Agreement”) for Epilepsy Research. The Company made a
non-refundable, non-creditable option payment of C$10,000 when it entered into
the PARTEQ Licensing Option Agreement in 2006. If the Company exercises its
option, the Company will make a non-refundable, non-creditable license payment
of C$17,500 at the time of such exercise. If the Company exercises its option,
it will be required to make quarterly royalty payments of 3% of net sales of
the
licensed products, with a minimum annual royalty of C$10,000 through the second
anniversary of the license, C$20,000 through the third anniversary of the
license, C$30,000 through the fourth anniversary of the license and C$40,000
through the fifth anniversary of the license and each subsequent anniversary.
The Company does not anticipate having a licensed product in the near term
and
thus will not be required to make these quarterly payments. If the Company
exercises its option, the Company is also obligated to make the following
milestone payments: C$100,000 upon completion of a Phase I trial of a licensed
product, C$250,000 upon completion of a Phase II trial of a licensed product,
and C$1,000,000 upon the first FDA approval (as such term is defined therein).
If the Company exercises its option, the Company also has the right to
sub-license with the payment of 20% of all non-royalty sublicensing
consideration.
Under
the
terms of the PARTEQ Licensing Option Agreement, the Company is required to
pay
fixed annual fees of C$150,800 for the epilepsy research.
For
the
year ended December 31, 2007, the payments made by the Company to PARTEQ under
these agreements have been approximately $457,115 and are reflected in the
Research and Development caption of the Statement of Operations. Following
the
results of the Phase II clinical trial of Huperzine A, the Company is evaluating
its alternatives for the further pursuit of the development of the PARTEQ
compounds.
Strategy
In
view
of the results of the Phase II clinical trial, the Company is currently
reviewing its options for moving forward with its business. Currently, the
Company expects to focus upon the development of collaborative, joint and
strategic alliances and licensing arrangements with one or more pharmaceutical
companies for the further development of Huperzine A and also expects to
consider merger and/or acquisition opportunities. The Company will further
evaluate these opportunities based upon the results of Numoda’s review and
report, and the Company also expects that Numoda will assist the Company in
interpreting and presenting the results of the Phase II clinical trial to
potential partners, licensees and merger or acquisition candidates.
Huperzine
A For
Alzheimer’s
disease
(Oral)
The
Company’s primary focus has been completion of the Phase II clinical trial for
Huperzine A in conjunction with Georgetown and the ADCS. Based upon the results
of this trial, the Company believes that additional testing is necessary. The
cost of this additional testing will be significant, and there are no assurances
that the Company can obtain the funds necessary to pay this expense or that
this
testing will provide favorable results.
Georgetown
(Phase II Clinical Trial)
In
December 2003, the Company entered into a clinical research agreement, which
was
amended in November 2005 and October 2007, with Georgetown pursuant to which
Georgetown provided the Company with Phase II research. The costs associated
with this agreement totaled $5,336,842 and were partially funded by the National
Institutes of Health. The Company’s portion of the total cost is $4,036,842, and
paid in installments upon the achievement of certain milestones.
For
the
year ended December 31, 2007, the payments made by the Company to Georgetown
under the terms of the clinical research agreement were approximately $1,183,167
and the total payments made by the Company to Georgetown since inception of
the
agreement were approximately $3,110,667. These costs are reflected in the
Research and Development caption of the Statement of Operations.
The
Company expects to make additional payments in 2008 to Georgetown of $926,175
related to an open label extension which is expected to be completed in the
second half of 2008.
Org
Syn Laboratory (Synthetic Huperzine)
On
February 1, 2006, the Company entered into an exclusive development agreement
(the “Development Agreement”) with Org Syn Laboratory, Inc. (“Org Syn”) for the
development by Org Syn of synthetic Huperzine A. Under the terms of the
Development Agreement, Org Syn received an aggregate of $175,894 upon the
execution of the Development Agreement.
For
the
year ended December 31, 2007, the payments made by the Company to Org Syn were
approximately $11,375 and are reflected in the Research and Development caption
of the Statement of Operations. Following the results of the Phase II clinical
trial of Huperzine A, the Company is evaluating its alternatives for the further
pursuit of the development of synthetic Huperzine A.
Huperzine
A For
Alzheimer’s
disease
(Transdermal)
The
Company’s strategy has been to make Huperzine A available in both oral and
transdermal form. The Company believes that Huperzine A can effectively be
delivered transdermally because of its low dosage requirement and low molecular
weight.
A
marketing study funded by the Company has shown that patient compliance is
a
crucial factor in determining patient and doctor choice in choosing a medication
for Alzheimer’s disease. Often it is the responsibility of a caregiver to remind
the patient to take the orally given medications. Recognizing this, the Company
hopes to develop and license a multi-day transdermal patch, which, if
successfully realized, will be able to be applied to the patient for more than
one day. The Company believes that some advantages of a transdermal delivery
may
include:
|
·
|
avoidance
of first-pass metabolism; better control of drug and metabolite plasma
levels leading to improved therapy with reduced side
effects;
|
|
·
|
avoidance
of non-compliance resulting, for example, from patients forgetting
to take
the medication; and
|
|
·
|
improved
quality of life for caregiver who only needs to replace the patch
up to
once per every three to five days.
|
XEL
Herbaceuticals, Inc. (Transdermal)
On
March
15, 2006, the Company entered into a development agreement with Xel
Herbaceuticals, Inc. (“XEL”) for the development of the Huperzine A Transdermal
Delivery System (“Delivery Product”). Under the terms of the agreement, the
Company paid XEL a $250,000 fee upon the execution of the agreement and paid
XEL
$92,500 per month during the development of the Delivery Product including
the
first six months of 2007. The Delivery Product was completed in July 2007.
Between August 2007 and January 2008, the Company paid XEL an additional $92,500
per month to fund the further development of the Product. Following the receipt
of the results of the Phase II clinical trial of Huperzine A, the Company
implemented certain cost reductions that have delayed expenditures relating
to
the study of the transdermal delivery system of Huperzine A.
The
Company and XEL may seek domestic and foreign patent protection for the Delivery
Product.
If
the
Company elects to exercise its right to license the Delivery Product in the
U.S.
and Canada (“North America”) and to develop the Delivery Product on its own, the
Company will pay XEL an initial license fee of $400,000 and up to an aggregate
of $2.4 million in additional payments upon the achievement of certain
milestones, including completion of a prototype, initial submission to the
Food
and Drug Administration (“FDA”), completion of phases of clinical studies and
completion of the FDA submission and FDA approval. Similarly, if the Company
elects to exercise its option to license the Delivery Product worldwide
excluding China, Taiwan, Hong Kong, Macau and Singapore (“Worldwide”), and
develop the Delivery Product on its own, the Company will pay XEL an additional
initial license fee of $400,000 and up to an aggregate of $2.4 million in
additional payments upon the achievement of comparable milestones. If XEL fails
to obtain a U.S. or international patent, the corresponding license fee and
milestone payments will be reduced by 50% until such time as XEL obtains such
patent, at which time the unpaid 50% of all such milestone payments previously
not made will be due. If the Company elects to exercise the licensing rights
described above, the additional payments that the Company has made to XEL to
further develop the Product would be applied toward the additional payments
payable to XEL upon the achievement of certain milestones.
The
Company will also be obligated to pay XEL royalty payments of between 7% and
10%
of net sales, with such royalty payments subject to reduction upon the
expiration of the patent or the launch of a generic product in the applicable
territory. If a patent has not been issued in either the U.S. or Canada, the
royalty payments will be subject to reduced rates of between 3% and 5% of net
sales. Royalty payments for sales in the Worldwide territory will be subject
to
good faith negotiations between the parties.
If
the
Company elects to exercise its right to license the Delivery Product in North
America and to develop the Delivery Product with a third party, the Company
will
pay XEL 50% of any initial signing fees and milestone fees (excluding any
research and development fees) paid by such third party. Similarly, in the
event
that the Company decides to exercise its option to license the Product Worldwide
and to develop the Product with a third party, the Company will pay XEL 50%
of
any initial signing fees and milestone fees (excluding any research and
development fees) paid by such third party. If XEL fails to obtain a U.S. or
international patent, the percentage of the corresponding fees will be reduced
to 25%. The Company will pay XEL 20% of any royalty payments received by the
Company from third-party sublicensees, or if the Product is not protected by
at
least one patent, 10% of any royalty received by the Company from
sublicensees.
If
the
Company elects not to exercise its right to license the Delivery Product and
XEL
elects to further develop the Delivery Product without the Company, XEL will
be
obligated to pay the Company 30% of any net profits realized up to a maximum
of
two times the amount paid by the Company to XEL during development, excluding
the initial $250,000 signing fee. Upon such election, XEL will be entitled
to
full ownership of the intellectual property of the Delivery Product. If the
Company elects to exercise its rights to license the Delivery Product in North
America, but not Worldwide, XEL will have certain rights to obtain intellectual
property protection in any country outside North America upon payment to the
Company for such rights, such fees to be negotiated in good faith by the
parties.
For
the
year ended December 31, 2007, the total payments made by the Company to XEL
under this agreement were approximately $1,111,250
and
are
reflected in the Research and Development caption of the Statement of
Operations.
Commercialization
of Huperzine A
The
Company believes that there is substantial support within the results that
Huperzine A can become a safe and effective treatment for Alzheimer’s disease.
The Company presently believes the estimated additional costs to bring Huperzine
A to market as an oral dose drug after completing clinical trials will be
substantial and no assurances as to future costs can be made or that the Company
will be able to fund them.
Currently,
the Company expects to focus upon the development of collaborative, joint and
strategic alliances and licensing arrangements with one or more pharmaceutical
companies for the further development of Huperzine A and also expects to
consider merger and/or acquisition opportunities. The Company anticipates that
it would be necessary to obtain collaborative partners who would be primarily
responsible for the sale and distribution of Huperzine A products after
obtaining FDA approval for Huperzine A.
Although
the Company’s primary focus continues to be the ultimate commercialization of
Huperzine A, the Company believes that a scientific and clinical rationale
exists for exploring the potential of both preclinical compounds discovered
by
Dr. Donald Weaver.
NHT0012
for
Alzheimer’s
disease
Protein
misfolding and aggregation are critical steps in the pathogenesis of a number
of
neurodegenerative diseases. In many cases,
self-assembly of two or more proteins is implicated in these diseases, including
Alzheimer’ s disease, in which aggregation of amyloid-β (Aβ), tau and
α-synuclein may all contribute to neurotoxicity. Inhibiting aberrant folding
and
assembly of one or more
of these
species is thus of great therapeutic interest. A novel class of bi-aromatic
compounds has been identified by researchers engaged by Neuro-Hitech that
pot
ently
inhibit the aggregation of Aβ, tau and α-synuclein in Thioflavin T (ThT) and
Thioflavin S (ThS) dye-binding fluorescence assays. The aggregation of both
major physiological isoforms of Aβ, i.e. Aβ40 and Aβ42, was inhibited and
compounds also caused disassembly of pre-formed aggregates. Further experiments
confirmed the anti-amyloidogenic activity of the compounds against Aβ40;
circular dichroism studies showed the compounds inhibited the random coil to
β-sheet conversion, while Aβ40 binding was studied in 1H NMR experiments.
Furthermore, compounds rescued SH-SY5Y neuroblastoma cells from Aβ40
toxicity
in a cell viability model. Ongoing pharmacokinetic testing for the compounds
has
been positive, with evidence of blood-brain barrier permeability, half- lives
of
several hours and minimal to no toxicity at doses as high as 300
mg/kg.
NHT1107
- Anti-ictogenci and anti-epileptogenic
Current
drugs for the treatment of epilepsy are little more than “symptomatic” agents,
suppressing the symptoms of epilepsy, while failing to deal with the causative
process underlying the susceptibility to seizures. The Neuro-Hitech approach
to
designing drugs to prevent epilepsy differentiates between “ictogenesis” and
“epileptogenesis”. No current anticonvulsant drug influences the natural history
of epilepsy, thus there is a crucial need for prototype “preventative”
antiepileptogenic drugs. Since epileptogenesis arises from altered
excitatory/inhibitory neurotransmitter activity, drug design exploiting such
neurotransmitters represents a therapeutic approach to epileptogenesis.
Neuro-Hitech’s approach focuses on
b
-alanine, an unexploited neuromodulator that affords multiple opportunities
for
drug design.
b
-alanine’s unique structure is intermediate between
a
- and
g
-amino
acids and thus
b
-alanine
can bind to glutamate, glycine and
g
-amino
butyric acid neurotransmitter receptors in brain.
b
-Alanine
analogs have “proGABAergic” and “antiglutamatergic” activities and thus
represent powerful platforms for drug design. Neuro-Hitech’s lead compound in
this therapeutic domain, NHT1107, prevents the onset of epilepsy in the
spontaneous recurrent seizure rat model of epilepsy by 82%, compared to
controls. NHT1107 is non-toxic in rodents at doses ranging from 100-300 mg/kg.
NHT1107 is thus a structurally unique analogue of
b
-alanine
with pioneering anti-epileptogenic activity. Preclinical development of this
compound is currently underway in collaboration with the Antiepileptic Drug
Development Program at NIH.
In
addition to developing a pioneering anti-epileptogenic drug, Neuro-Hitech’s
antiepilepsy program is also focusing on the design and development of improved
anticonvulsant drugs with fewer side-effects. Other analogues of
b
-alanine
are being optimized for their anti-ictogenic properties to permit the
development of an efficacious anticonvulsant agent with reduced cognitive and
memory-related side-effects.
Manufacturing
and Raw Materials
The
Company does not have, and does not intend to establish, manufacturing
facilities to produce its product candidates in the near or mid-term. The
Company plans to control capital expenditures by using contract manufacturers
to
produce product candidates. It is the Company’s belief that there are a
sufficient number of high quality GLP (Good Laboratory Practice) and GMP (Good
Manufacturing Practice) contract manufacturers available, and the Company has
had discussions and in some instances established relationships to fulfill
its
production needs for research and clinical use.
The
manufacturer of Neuro-Hitech’s product candidates or any future product, whether
done by third-party contractors as planned or internally, will be subject to
rigorous regulations, including the need to comply with the FDA’s current GMP
standards. As part of obtaining FDA approval for each product, each of the
manufacturing facilities must be inspected, approved by and registered with
the
FDA. In addition to obtaining FDA approval of the prospective manufacturer’s
quality control and manufacturing procedures, domestic and foreign manufacturing
facilities are subject to periodic inspection by the FDA and/or foreign
regulatory authorities.
The
Company currently obtains natural Huperzine from a limited number of suppliers
in China. If the Company is unable to develop synthetic Huperzine, the Company
may be wholly dependent upon its limited suppliers to provide the Company
natural Huperzine. Although the Company believes it has a good working
relationship with its suppliers, the Company is aware of the increased risk
and
challenges posed when relying on limited suppliers.
The
Company attempts to manage these risks by active inventory management. A
material shortage, contamination and/or recall could adversely affect the
manufacturing of the Company’s products.
Government
Regulation
The
research and development, manufacture and marketing of pharmaceuticals are
subject to regulation by U.S., Canadian and foreign governmental authorities
and
agencies. Such national agencies and other federal, state, provincial and local
entities regulate the testing, manufacturing, safety and promotion of the
Company’s products. Regulations applicable to the Company’s products may change
as the currently limited number of approved controlled-release products
increases and regulators acquire additional experience in this
area.
United
States Regulation
New
Drug Application
The
Company will be required by the FDA to comply with New Drug Application (“NDA”)
procedures for its products prior to commencement of marketing by the Company
or
the Company’s licensees. New drug compounds and new formulations for existing
drug compounds are subject to NDA procedures. These procedures include (a)
preclinical laboratory and animal toxicology tests; (b) scaling and testing
of
production batches; (c) submission of an investigational new drug application
(“IND”), and subsequent approval is required before any human clinical trials
can commence; (d) adequate and well controlled replicate human clinical trials
to establish the safety and efficacy of the drug for its intended indication;
(e) the submission of an NDA to the FDA; and (f) FDA approval of an NDA prior
to
any commercial sale or shipment of the product, including pre-approval and
post-approval inspections of its manufacturing and testing facilities. If all
of
these data in the product application is owned by the applicant, the FDA will
issue its approval without regard to patent rights that might be infringed
or
exclusivity periods that would affect the FDA’s ability to grant an approval if
the application relied upon data which the applicant did not own. The Company
currently intends to generate all data necessary to support FDA approval of
the
applications the Company files.
Preclinical
laboratory and animal toxicology tests may have to be performed to assess the
safety and potential efficacy of the product. The results of these preclinical
tests, together with information regarding the methods of manufacture of the
products and quality control testing, are then submitted to the FDA as part
of
an IND requesting authorization to initiate human clinical trials. Once the
IND
notice period has expired, clinical trials may be initiated, unless a hold
on
clinical trials has been issued by the FDA.
Clinical
trials involve the administration of a pharmaceutical product to individuals
under the supervision of qualified medical investigators that are experienced
in
conducting studies under “Good Clinical Practice” guidelines. Clinical studies
are conducted in accordance with protocols that detail the objectives of a
study, the parameters to be used to monitor safety and the efficacy criteria
to
be evaluated. Each protocol is submitted to the FDA and to an Institutional
Review Board prior to the commencement of each clinical trial. Clinical studies
are typically conducted in three sequential phases, which may overlap. In Phase
I, the initial introduction of the product into human subjects, the compound
is
tested for absorption, safety, dosage, tolerance, metabolic interaction,
distribution, and excretion. Phase II involves studies in a limited patient
population with the disease to be treated to (1) determine the efficacy of
the
product for specific targeted indications, (2) determine optimal dosage and
(3)
identify possible adverse effects and safety risks. In the event Phase II
evaluations demonstrate that a pharmaceutical product is effective and has
an
acceptable safety profile, Phase III clinical trials are undertaken to further
evaluate clinical efficacy of the product and to further test its safety within
an expanded patient population at geographically dispersed clinical study sites.
Periodic reports on the clinical investigations are required.
The
Company, or the FDA, may suspend clinical trials at any time if it is believed
that clinical subjects may be exposed to unacceptable health risks. The results
of the product development, analytical laboratory studies and clinical studies
are submitted to the FDA as part of an NDA for approval of the marketing and
commercialization of a pharmaceutical product.
In
certain companies where the objective is to develop a generic version of an
approved product already on the market in controlled-release dosages, an
Abbreviated New Drug Application (“ANDA”) may be filed in lieu of filing an NDA.
Under the ANDA procedure, the FDA waives the requirement to submit complete
reports of preclinical and clinical studies of safety and efficacy and instead
requires the submission of bio-equivalency data, that is, demonstration that
the
generic drug produces the same effect in the body as its brand-name counterpart
and has the same pharmacokinetic profile, or change in blood concentration
over
time. The advantages of an ANDA over an NDA include reduced research and
development costs associated with bringing a product to market, and generally
a
shorter review and approval time at the FDA. This procedure is not available
to
the Company’s planned products but might be available to the Company’s
competitors if the Company receives FDA approval for one or more of its
products.
Exclusivity
Issues
Under
U.S. law, the expiration of a patent on a drug compound does not create a right
to make, use or sell that compound. There may be additional patents relating
to
a person’s proposed manufacture, use or sale of a product that could potentially
prohibit such person’s proposed commercialization of a drug
compound.
The
Food
Drug and Cosmetic Act (“FDC”) contains non-patent market exclusivity provisions
that offer protection to pioneer drug products and are independent of any patent
coverage that might also apply. Five years of exclusivity are granted to the
first approval of a “new chemical entity.” Three years of exclusivity may apply
to products which are not new chemical entities, but for which new clinical
investigations are essential to the approval. For example, a new indication
for
use, or a new dosage strength of a previously approved product, may be entitled
to exclusivity, but only with respect to that indication or dosage strength.
Exclusivity only offers protection against a competitor entering the market
via
the ANDA route, and does not operate against a competitor that generates all
of
its own data and submits a full NDA.
If
applicable regulatory criteria are not satisfied, the FDA may deny approval
of
an NDA or an ANDA or may require additional testing. Product approvals may
be
withdrawn if compliance with regulatory standards is not maintained or if
problems occur after the product reaches the market. The FDA may require further
testing and surveillance programs to monitor the pharmaceutical product that
has
been commercialized. Noncompliance with applicable requirements can result
in
additional penalties, including product seizures, injunction actions and
criminal prosecutions.
Additional
Regulatory Considerations
Sales
of
the Company’s products by licensees outside the U.S. and Canada are subject to
regulatory requirements governing the testing, registration and marketing of
pharmaceuticals, which vary widely from country to country.
In
addition to the regulatory approval process, pharmaceutical companies are
subject to regulations under provincial, state and federal law, including
requirements regarding occupational safety, laboratory practices, environmental
protection and hazardous substance control, and may be subject to other present
and future local, provincial, state, federal and foreign regulations, including
possible future regulations of the pharmaceutical industry. The Company believes
that it is in compliance in all material respects with such regulations as
are
currently in effect.
Competition
The
Company intends to develop and market (through collaborative, joint and
strategic alliances or licensing arrangements with one or more pharmaceutical
companies) proprietary pharmaceutical products based on Huperzine A and NHT0012.
The Company’s competition consists of those companies which develop drugs to
treat Alzheimer’s disease, MCI and other forms of dementia, and companies that
develop Alzheimer’s disease and MCI drugs and drug delivery systems for theses
drugs.
Additionally,
the Company may seek to develop and market (through collaborative, joint and
strategic alliances or licensing arrangements with one or more pharmaceutical
companies) proprietary pharmaceutical products based on NHT1107. Currently,
there are 12 drugs on the market for the symptomatic treatment of epilepsy.
All of the drugs currently on the market are anti-seizure drugs that must
be taken after epilepsy has developed. These anti-seizure drugs suppress the
occurrence of seizures in an individual with established epilepsy. The Company
seeks to develop anti-epileptogenic agent that may prevent the onset of epilepsy
after a brain injury. Currently, there are no anti-epileptogenic drugs on the
market.
An
increasing number of pharmaceutical companies are interested in the development
and commercialization of products that treat these diseases. The Company also
expects that competition in the field of drug delivery will significantly
increase in the future since smaller specialized research and development
companies are beginning to concentrate on this aspect of the business. For
each
area, some of the major pharmaceutical companies have invested and are
continuing to invest significant resources in the development of these products,
and some have invested funds in specialized drug delivery
companies.
Other
companies may develop new drug formulations and products for Alzheimer’s
disease, Epilepsy or MCI, or may improve existing drug formulations and products
more efficiently than the Company can. In addition, almost all of the Company’s
competitors have vastly greater resources than the Company does. While the
Company’s product development capabilities and exclusive patent licenses may
help it maintain a market position in the field of drug delivery, there can
be
no assurance that others will not be able to develop these capabilities, or
alternative technologies outside the scope of the Company’s patents, if any, or
that even if patent protection is obtained, these patents will not be
successfully challenged in the future.
Scientific
and Clinical Advisory Board
The
Company maintains a Scientific and Clinical Advisory Board comprised of
scientists and physicians with experience relevant to the Company and its
product candidates. Members of the Company’s Scientific and Clinical Advisory
Board have agreed to consult and advise the Company in their respective areas
of
expertise. The Company has placed special emphasis on identifying members of
its
Scientific and Clinical Advisory Board with expertise in the treatment of the
clinical indications targeted by the Company’s programs. The Company’s
Scientific and Clinical Advisory Board consists of the following
members:
Paul
Aisen, M.D.
Dr.
Aisen
is a professor in the Department of Neurosciences at the University of
California, San Diego, and the National Institute of Aging-appointed director
of
the ADCS. Dr. Aisen was one of the first to conduct an Alzheimer’s disease
clinical trial in the U.S. and was an investigator in the pivotal FDA
registration studies for Namenda®. Dr. Aisen received his M.D. from Columbia
University, College of Physicians and Surgeons.
Robert
M. Moriarty, M.D.
Dr.
Robert M. Moriarty is a consultant of Org Syn Laboratory, Inc. Dr. Moriarty
received his Ph.D. degree from Princeton University. After completing
postdoctoral work at University of Munich and Harvard University, he worked
as a
research chemist at Merck from 1955-57. He founded Steroids Limited in 1981,
which became SynQuest Limited and was acquired by United Therapeutics in 2000.
Dr. Moriarty has authored over 200 articles on various topics in synthetic
and
mechanistic organic synthesis. Dr. Moriarty is a recipient of several
prestigious awards and grants.
Dr.
Dinesh Patel, M.D.
Dr.
Patel is the Chairman of the Board and Co-founder of Xel Herbaceuticals Inc.
Dr.
Patel has served fifteen years as Co-founder, Chairman of the Board of
Directors, President & CEO, of TheraTech, Inc., a Salt Lake City based
company that has been a pioneer in the development and manufacture of innovative
drug delivery products. Under Dr. Patel’s guidance, TheraTech established
strategic alliances with major pharmaceutical companies, including Eli Lilly,
Pfizer, Procter & Gamble, Roche, SmithKline Beecham, and Wyeth-Ayerst. Dr.
Patel directed the construction of a state-of-the-art manufacturing facility
and
oversaw the company’s R&D efforts through the manufacture of its currently
marketed transdermal products (transdermal testosterone and estrogen
hormone-replacement therapy patches). Dr. Patel has been the recipient of
numerous awards.
Donald
F. Weaver, M.D.
Dr.
Weaver is currently Canada Research Chair in Neuroscience/Chemistry, Professor
of the Department of Medicine (Neurology), Professor of the Department of
Chemistry and Professor of the School of Biomedical Engineering at Dalhousie
University in Halifax, Nova Scotia, Canada. With experience that spans academic
and commercial interests, he has published hundreds of articles, is a prolific
inventor with over 70 patents to his name and is the recipient of many awards
and honors. Dr. Weaver co-founded three biotechnology companies, including
Neurochem, Inc., and is a fellow of the Canadian Institute of Chemistry (FCIC),
a fellow of the Royal College of Physicians and Surgeons of Canada (FRCIP)
and a
board-certified neurologist.
Employees
As
of
March 17, 2008, the Company had two full-time employees and two employees that
work on a part-time basis.
Corporate
Information
The
Company’s corporate headquarters are located at One Penn Plaza, New York, NY
10019. The Company’s telephone number is (212) 594-1215, and its fax number is
(212) 798-8183.
Executive
Officers and Significant Employees of the Registrant
The
following sets forth certain information with regard to the executive officers
and certain significant employees of the Company as of March 31, 2008 (ages
are
as of December 31, 2007):
|
Name
|
|
Age
|
|
Position
|
|
Gary
T. Shearman
|
|
54
|
|
President,
Chief Executive Officer and Director
|
|
David
Barrett
|
|
32
|
|
Chief
Financial Officer
|
|
L.
William McIntosh
|
|
62
|
|
Chief
Operating Officer
|
|
William
Wong
|
|
60
|
|
Chief
Scientific Officer
|
Dr.
Gary T. Shearman
has
been
serving as Chief Executive Officer and Director of the Company since August
2007. Immediately prior to joining the Company, Dr. Shearman was serving as
a
consultant. Immediately prior to that, between September 2001 and April 2007,
Dr. Shearman had been serving as Chairman and Managing Director of HBM Advisors
USA, LLC, a provider of advisory services for HBM Partners (Cayman) Ltd., the
investment manager for all HBM investment vehicles including HBM BioVentures
and
HBM BioCapital, holding companies in the human medicine, biotechnology and
medical technology industries. Between May 1992 and January 2000, Dr. Shearman
was employed by Rhône-Poulenc Rorer where he served in a variety of positions,
most recently as Deputy Head of Global Research and Drug Development and Senior
Vice President, Global Development. Prior to that, Dr. Shearman held positions
with Merck & Co., Inc. and Sandoz Ltd.
David
Barrett
has been
serving as Chief Financial Officer since April 2006. Between January 2005 and
April 2006, Mr. Barrett had been serving as Chief Financial Officer of Overture
Financial Services, LLC, a company specializing in construction and management
of investment platforms for financial intermediaries. Between September 2003
and
January 2005, Mr. Barrett served in a variety of capacities, most recently
as
Chief Financial Officer of Overture Asset Managers, LLC, an asset management
holding company that partners, acquires and manages investment managers with
complementary investment products. Between June 1999 and September 2003, Mr.
Barrett was employed by Deloitte & Touche where he served in a variety of
capacities, most recently as Senior Consultant in merger and acquisition
services.
L.
William McIntosh
has been
serving as Chief Operating Officer since November 2006. Mr. McIntosh also served
as a director of the Company between November 2006 and August 2007. Prior to
joining the Company, Mr. McIntosh spent 30 years within the pharmaceutical
and
biotechnology industries in a variety capacities including marketing, sales,
business development, product development and general management. Immediately
prior to joining the Company, he served as a director and Chief Executive
Officer of Q-RNA between August 2004 and the closing of the Merger. Prior to
that, Mr. McIntosh served as Principal and Managing Director of Novatures
Consulting Group between June 2003 and July 2004. Between August 2001 and May
2003, Mr. McIntosh served as President and CBO of FASgen, Inc. Mr. McIntosh
has
also served in senior positions at Merck & Co., Inc., Medco Containment
Services, Boehringer Mannheim Pharmaceuticals Corporation, Zynaxis, Inc.,
Smith-Kline Beecham Pharmaceuticals, VIMRx Pharmaceuticals, Inc. and Nexell
Therapeutics, Inc. Mr. McIntosh received both his B.S. and M.B.A. from Lehigh
University in Pennsylvania.
William
Wong
has been
serving as Chief Scientific Officer since November 2006. Immediately prior
to
joining the Company, Dr. Wong worked in a variety of management capacities
at
pharmaceutical development, biotechnology and medical device companies during
his twenty-five year career, most recently at Q-RNA, where he served as Vice
President of Product Development, between August 2002 and November 2006. Prior
to that, he served as Principal at Novatures Consulting Group. He has served
on
the senior management teams at Lynx Therapeutics, IntraCel Corporation, E.I.
DuPont Co. and Becton-Dickinson Corp. Dr. Wong received his Ph.D. from the
University of Rochester School of Medicine in the Department of Microbiology
and
Immunology.
The
Company is committed to legal and ethical conduct in fulfilling its
responsibilities. The Board expects all directors, as well as officers and
employees, to act ethically at all times. Additionally, the Board expects the
Chief Executive Officer, the Chief Financial Officer, and all senior financial
and accounting officials to adhere to the Company’s Code of Ethics which was
adopted on February 23, 2006. The Code of Ethics incorporates the Company’s
expectations of its executive officers that enable the Company to provide
accurate and timely disclosure in its filings with the SEC and other public
communications. In addition, they incorporate the Company’s guidelines
pertaining to topics such as complying with applicable laws, rules, and
regulations; reporting of code violations; and maintaining accountability for
adherence to the code.
The
full
text of the Code of Ethics is published on the investor relations portion of
our
website at
www.neurohitech.com
.
The
Company intends to disclose any amendments to provisions of its Code of Ethics,
or waivers of such provisions granted to executive officers and directors,
on
this website within four business days following the date of any such amendment
or waiver.
Risk
Factors
Investing
in the Company’s common stock involves a high degree of risk. Prospective
investors should carefully consider the risks described below, together with
all
of the other information included or referred to in this Annual Report on Form
10-KSB, before purchasing shares of the Company’s common stock. There are
numerous and varied risks, known and unknown, that may prevent the Company
from
achieving its goals. The risks described below are not the only ones the Company
will face. If any of these risks actually occur, the Company’s business,
financial condition or results of operation may be materially adversely
affected. In such case, the trading price of the Company’s common stock could
decline and investors in the Company’s common stock could lose all or part of
their investment.
Risks
Related to the Company and the Company’s Business
The
Company has limited cash available, and the Company may not have sufficient
cash
to continue its business operations.
As
of
March 30, 2008 the Company had approximately $4.3 million available in cash
and
cash equivalents. The Company expects to continue to incur losses in future
months as the Company engages in further expenditures as it pursues its business
plan.
The
Company has relied almost entirely on external financing to fund its operations
to date. Such financing has historically come from the sale of common stock
to
third parties. The Company will need to raise additional capital in the future
to fund its operations and there is no guarantee that financing from external
sources will be available if needed or on favorable terms. The sale of the
Company’s common stock to raise capital may cause dilution to its existing
stockholders. If additional financing is not available when required or is
not
available on acceptable terms, the Company may be unable to fund its operations
and expansion, successfully develop its products, take advantage of business
opportunities or respond to competitive market pressures, any of which could
make it more difficult for the Company to continue operations. Any financing
on
unfavorable terms or a reduction in the Company’s operations may result in a
lower stock price.
Following
receipt of the results of its Phase II clinical trial of Huperzine A, the
Company implemented cost reductions. Presently, the Company expects that its
available cash, cash equivalents and interest income earned are sufficient
to
meet its operating expenses and capital requirements for a period of at least
twelve months, however, if the Company fails to raise additional capital it
may
not have sufficient cash to meet future operating expenses and capital
requirements beyond twelve months. Even with additional capital, the Company
may
not be able to execute its current business plan nor fund its operations long
enough to achieve positive cash flow. Furthermore, the Company may be forced
to
implement more significant reductions of its expenses and cash expenditures,
which would impair the Company’s ability to execute its business
operations.
The
failure to complete development of Huperzine A, obtain government approvals,
including required FDA approvals, or to comply with ongoing governmental
regulations, could delay or limit introduction of proposed products and result
in failure to achieve revenues or maintain the Company’s ongoing
business.
The
Company’s research and development activities, and the manufacture and marketing
of its intended products, are subject to extensive regulation for safety,
efficacy and quality by numerous government authorities in the U.S. and abroad.
Before receiving FDA clearance to market the Company’s proposed products, the
Company will have to demonstrate that its products are safe and effective on
the
patient population and for the diseases that are to be treated. Clinical trials,
manufacturing and marketing of drugs are subject to the rigorous testing and
approval process of the FDA and equivalent foreign regulatory authorities.
The
FDA and other federal, state and foreign statutes and regulations govern and
influence the testing, manufacture, labeling, advertising, distribution and
promotion of drugs and medical devices. As a result, clinical trials and
regulatory approval can take a number of years or longer to accomplish and
require the expenditure of substantial financial, managerial and other
resources.
In
order
to be commercially viable, the Company must successfully research, develop,
obtain regulatory approval for, manufacture, introduce, market and distribute
its technologies. For each drug the Company must successfully meet a number
of
critical developmental milestones, including:
|
·
|
demonstrate
benefit from each specific drug
technology,
|
|
·
|
demonstrate
through pre-clinical and clinical trials that the drug and patient
specific therapy is safe and effective,
and
|
|
·
|
demonstrate
that its drug offers a material benefit to patients, especially in
cases
where the patent exclusivity on some of its competitors drugs, like
Pfizer’s Aricept®, is expiring.
|
|
|
|
|
·
|
establish
a viable Good Manufacturing Process capable of potential scale
up.
|
The
time
frame necessary to achieve these developmental milestones may be long and
uncertain, and the Company may not successfully complete these milestones for
any of its intended products in development.
Although
the Company completed the Phase II clinical trial of Huperzine A, Huperzine
A is
subject to additional developmental risks which include the
following:
|
·
|
the
uncertainties arising from the interpretation of the Phase II
results;
|
|
|
|
|
·
|
the
uncertainties arising from the rapidly growing scientific aspects
of drug
therapies and potential treatments;
|
|
·
|
uncertainties
arising as a result of the broad array of potential treatments related
to
neurological disease; and
|
|
·
|
anticipated
expense and time believed to be associated with the development and
regulatory approval of treatments for neurological
disease.
|
In
order
to conduct clinical trials that are necessary to obtain approval by the FDA
to
market a product, it is necessary to receive clearance from the FDA to conduct
such clinical trials. The FDA can halt clinical trials at any time for safety
reasons or because the Company or its clinical investigators do not follow
the
FDA’s requirements for conducting clinical trials. If the Company is unable to
receive clearance to conduct clinical trials or the trials are halted by the
FDA, the Company would not be able to achieve any revenue from such product,
as
it is illegal to sell any drug or medical device for human consumption without
FDA approval.
Data
obtained from clinical trials is susceptible to varying interpretations, which
could delay, limit or prevent regulatory clearances.
Data
already obtained, or in the future obtained, from pre-clinical studies and
clinical trials do not necessarily predict the results that will be obtained
from later pre-clinical studies and clinical trials. Moreover, pre-clinical
and
clinical data is susceptible to varying interpretations, which could delay,
limit or prevent regulatory approval. The Company is also not able to assure
that the results of the tests already conducted will be consistent with prior
observations or support the Company’s applications for regulatory approval. A
number of companies in the pharmaceutical industry have suffered significant
setbacks in advanced clinical trials, even after promising results in earlier
trials. The failure to adequately demonstrate the safety and effectiveness
of an
intended product under development could delay or prevent regulatory clearance
of a potential drug, resulting in delays to commercialization, and could
materially harm the Company’s business. The Company’s clinical trials may not
demonstrate sufficient levels of safety and efficacy necessary to obtain the
requisite regulatory approvals for the Company’s drugs, and thus its proposed
drugs may not be approved for marketing. Even after approval, further studies
could result in withdrawal of FDA and other regulatory approvals and voluntary
or involuntary withdrawal of products from the market.
The
Company may encounter delays or rejections based upon additional government
regulation from future legislation or administrative action or changes in FDA
policy during the period of development, clinical trials and FDA regulatory
review. The Company may encounter similar delays in foreign countries. Sales
of
the Company’s products outside the U.S. would be subject to foreign regulatory
approvals that vary from country to country. The time required to obtain
approvals from foreign countries may be shorter or longer than that required
for
FDA approval, and requirements for foreign licensing may differ from FDA
requirements. The Company may be unable to obtain requisite approvals from
the
FDA and foreign regulatory authorities, and even if obtained, such approvals
may
not be on a timely basis, or they may not cover the uses that the Company
requests.
The
Company’s product development efforts may not result in commercial
products.
Successful
product development in the biotechnology industry is highly uncertain, and
very
few research and development projects produce a commercial product. Products
that appear promising in the early phases of development, such as in early
human
clinical trials, may fail to reach the market for a number of reasons, such
as:
|
·
|
the
product did not demonstrate acceptable clinical trial results even
though
it demonstrated positive preclinical trial
results;
|
|
·
|
the
product was not effective in treating a specified condition or
illness;
|
|
·
|
the
product had harmful side effects on
humans;
|
|
·
|
the
necessary regulatory bodies, such as the FDA, did not approve the
Company’s product for an intended
use;
|
|
·
|
the
product was not economical for the Company to
commercialize;
|
|
·
|
other
companies or people have or may have proprietary rights over the
Company’s
product, such as patent rights, and will not let the Company sell
it on
reasonable terms, or at all; or
|
|
·
|
the
product is not cost effective in light of existing
therapeutics.
|
As
a
result, there can be no assurance that any of the Company’s products currently
in development will ever be successfully commercialized.
The
Company will require significant additional funding and may have difficulty
raising needed capital in the future because of its limited operating history
and business risks associated with the Company’s drug
technology.
The
Company’s business currently does not generate significant revenue from the
Company’s proposed products and its limited revenue may not be sufficient to
meet its future capital requirements. The Company does not know when, or if,
this will change. The Company has expended and will continue to expend
substantial funds in the research, development and clinical and pre-clinical
testing of its drug technology. The Company will require additional funds to
conduct research and development, establish and conduct clinical and
pre-clinical trials, establish commercial scale manufacturing arrangements,
provide for the marketing and distribution of its products and/or support its
obligations under collaborative, joint and strategic alliances or licensing
arrangements. Additional funds may not be available on acceptable terms, if
at
all. The Company has already implemented cost reductions following receipt
of
the results of its Phase II clinical trial of Huperzine A and delayed any
expenditures relating to the study of the transdermal delivery system of
Huperzine A. If adequate funds are unavailable from any available source, the
Company may have to further delay, reduce the scope of or eliminate one or
more
of its research or development programs or product launches or marketing efforts
which may materially harm the Company’s business, financial condition and
results of operations.
The
Company’s long term capital requirements are expected to depend on many factors,
including:
|
·
|
the
number of potential products and technologies in
development;
|
|
·
|
continued
progress and cost of the Company’s research and development
programs;
|
|
·
|
progress
with pre-clinical studies and clinical
trials;
|
|
·
|
the
time and costs involved in obtaining regulatory
clearance;
|
|
·
|
costs
involved in preparing, filing, prosecuting, maintaining and enforcing
patent claims;
|
|
·
|
costs
of developing sales, marketing and distribution channels and the
Company’s
ability to sell its drugs;
|
|
·
|
costs
involved in establishing manufacturing capabilities for clinical
trial and
commercial quantities of the Company’s
drugs;
|
|
·
|
competing
technological and market
developments;
|
|
·
|
market
acceptance or the Company’s
products;
|
|
·
|
costs
for recruiting and retaining management, employees and consultants;
and
|
|
·
|
costs
for training physicians.
|
The
Company may consume available resources more rapidly than currently anticipated,
resulting in the need for additional funding. The Company may seek to raise
any
necessary additional funds through the exercising of warrants, equity or debt
financings, collaborative arrangements with corporate partners or other sources,
which may be dilutive to existing stockholders or otherwise have a material
effect on the Company’s current or future business prospects. In addition, in
the event that additional funds are obtained through arrangements with
collaborative partners or other sources, the Company may have to relinquish
economic and/or proprietary rights to some of the Company’s technologies or
products under development that the Company would otherwise seek to develop
or
commercialize by itself. If adequate funds are not available, the Company may
be
required to significantly reduce or refocus its development efforts with regard
to its drug technology, compounds and drugs.
If
the Company fails to negotiate or maintain successful collaborative arrangements
with third parties, the Company’s development and commercialization activities
may be delayed or reduced.
In
the
past, the Company has entered into, and expects to enter into in the future,
collaborative arrangements with third parties, such as universities,
governmental agencies, charitable foundations, manufacturers, contract research
organizations and corporate partners, who provide the Company with funding
and/or who perform research, development, regulatory compliance, manufacturing
or commercialization activities relating to some or all of the Company’s product
candidates. If the Company fails to secure or maintain successful collaborative
arrangements, its development and commercialization activities may be delayed
or
reduced.
Under
certain circumstances, partners, universities and other collaborators, may
acquire certain rights in newly developed intellectual property developed in
conjunction with the Company.
These
collaborative agreements can be terminated under certain conditions by the
Company’s partners. The Company’s partners may also under some circumstances
independently pursue competing products, delivery approaches or technologies.
Even if the Company’s partners continue their contributions to the Company’s
collaborative arrangements, they may nevertheless determine not to actively
pursue the development or commercialization of any resulting products. Also,
the
Company’s partners may fail to perform their obligations under the collaborative
arrangements or may be slow in performing their obligations. In addition, the
Company’s partners may experience financial difficulties at any time that could
prevent them from having available funds to contribute to these collaborations.
In these circumstances, the Company’s ability to develop and market potential
products could be severely limited.
Because
the Company has accumulated deficits in the research and development of
Huperzine A since inception, there is no guarantee that the Company will ever
become profitable even if one or more of the Company’s drugs are approved for
commercialization.
Since
inception the Company has recorded operating losses. As of December 31, 2007,
the Company had a stockholders’ equity of approximately $5,263,649 and an
accumulated deficit of approximately $(32,830,653). In addition, the Company
expects to incur increasing operating losses over the next several years as
the
Company continues to incur increasing costs for research and development and
clinical trials, compliance with governmental regulations and in other
development activities. The Company’s ability to generate revenue and achieve
profitability depends upon its ability, alone or with others, to complete the
development of its proposed products, obtain the required regulatory approvals
and manufacture, market and sell its proposed products. Development is costly
and requires significant investment. In addition, the Company may choose to
license rights to particular drugs. The license fees for such drugs may increase
the Company’s costs.
The
Company has not generated any revenue from the commercial sale of its proposed
products in development or any drugs and does not expect to receive such revenue
in the near future. The Company’s primary activity to date has been research and
development. Revenues to date have been primarily from sales of
inventory of imported Huperzine, which may be continued by the Company. The
Company may however, elect to reduce or eliminate entirely these sales
efforts as the Company refocuses its efforts on drug development and
approval.
The
Company cannot be certain as to when or whether to anticipate commercializing
and marketing its proposed products in development, and do not expect to
generate sufficient revenues from proposed product sales to cover its expenses
or achieve profitability in the near future.
Acceptance
of the Company’s products in the marketplace is uncertain and failure to achieve
market acceptance will prevent or delay its ability to generate
revenues.
The
Company’s future financial performance will depend, at least in part, upon the
introduction and acceptance of the Company’s proposed Huperzine A products by
physicians, patients, payors and the broader medical community. Even if approved
for marketing by the necessary regulatory authorities, the Company’s products
may not achieve market acceptance. The degree of market acceptance will depend
upon a number of factors, including:
|
·
|
the
receipt of regulatory clearance of marketing claims for the uses
that the
Company is developing;
|
|
·
|
the
establishment and demonstration of the advantages, safety and efficacy
of
Huperzine A;
|
|
·
|
pricing
and reimbursement policies of government and third party payors such
as
insurance companies, health maintenance organizations and other health
plan administrators;
|
|
·
|
the
Company’s ability to attract corporate partners, including pharmaceutical
companies, to assist in commercializing the Company’s intended products;
and
|
|
·
|
the
Company’s ability to market its
products.
|
The
Company may face costly and time consuming litigation from third parties which
claim that the Company’s products infringe on their intellectual property
rights, particularly because there is substantial uncertainty about the validity
and breadth of medical patents.
There
is
significant litigation in the biotechnology field regarding patents and other
intellectual property rights. Biotechnology companies of roughly the Company’s
size and financial position have gone out of business after fighting and losing
an infringement battle. The Company may be exposed to future litigation by
third
parties based on claims that the Company’s technologies, products or activities
infringe the intellectual property rights of others or that the Company has
misappropriated the trade secrets of others. This risk is exacerbated by the
fact that the validity and breadth of claims covered in medical technology
patents and the breadth and scope of trade secret protection involve complex
legal and factual questions for which important legal principles are unresolved.
In particular, there are many patents relating to specific genes, nucleic acids,
polypeptides or the uses thereof to treat Alzheimer’s disease and other central
nervous system diseases. Some of these may encompass genes or polypeptides
that
the Company utilizes in its drug development activities. Any litigation or
claims against the Company, whether or not valid, could result in substantial
costs, could place a significant strain on the Company’s financial and
managerial resources and could harm the Company’s reputation. Most of the
Company’s license agreements would likely require that the Company pay the costs
associated with defending this type of litigation. In addition, intellectual
property litigation or claims could force the Company to do one or more of
the
following:
|
·
|
cease
selling, incorporating or using any of the Company’s Huperzine A products
and/or products that incorporate the challenged intellectual property,
which would adversely affect the Company’s future
revenue;
|
|
·
|
pay
significant damages and the patentee could prevent the Company from
using
the patented genes or polypeptides for the identification or development
of drug compounds;
|
|
·
|
Obtain
a license from the holder of the infringed intellectual property
right,
which license may be costly or may not be available on reasonable
terms,
if at all; or
|
|
·
|
redesign
the Company’s products, which would be costly and time
consuming.
|
As
of
March 17, 2008, the Company has not engaged in discussions, received any
communications, nor does the Company have any reason to believe that any third
party is challenging or has the proper legal authority to challenge the
Company’s intellectual property rights or those of the actual patent holders, or
the Company’s licenses.
If
the Company is unable to adequately protect or enforce its rights to
intellectual property or secure rights to third party patents, the Company
may
lose valuable rights, experience reduced market share, assuming any, or incur
costly litigation to protect such rights.
The
Company’s ability to obtain licenses to patents, apply for new patents on a
Huperzine A product, maintain trade secret protection and operate without
infringing the proprietary rights of others will be important to its
commercializing any products under development. Therefore, any disruption in
access to the technology could substantially delay the development of Huperzine
A or other products.
The
patent positions of biotechnology and pharmaceutical companies, including ours,
which also involve licensing agreements, are frequently uncertain and involve
complex legal and factual questions. In addition, the coverage claimed in a
patent application can be significantly reduced before the patent is issued.
Consequently, the Company’s patent applications and any issued and licensed
patents may not provide protection against competitive technologies or may
be
held invalid if challenged or circumvented. The Company’s competitors may also
independently develop drug technologies or products similar to the Company’s or
design around or otherwise circumvent patents issued or licensed to the Company.
In addition, the laws of some foreign countries may not protect the Company’s
proprietary rights to the same extent as U.S. law.
The
Company also relies upon trade secrets, technical know how and continuing
technological innovation to develop and maintain the Company’s competitive
position. The Company generally will seek to require its employees, consultants,
advisors and collaborators to execute appropriate confidentiality and assignment
of inventions agreements. These agreements typically provide that all materials
and confidential information developed or made known to the individual during
the course of the individual’s relationship with the Company is to be kept
confidential and not disclosed to third parties except in specific
circumstances, and that all inventions arising out of the individual’s
relationship with the Company shall be the its exclusive property. These
agreements may be breached, or unavailable, and in some instances, the Company
may not have an appropriate remedy available for breach of the agreements.
Furthermore, the Company’s competitors may independently develop substantially
equivalent proprietary information and techniques, reverse engineer the
Company’s information and techniques, or otherwise gain access to its
proprietary technology. The Company may be unable to meaningfully protect its
rights in trade secrets, technical know how and other non patented
technology.
Although
the Company’s trade secrets and technical know how are important, the Company’s
continued access to the patents and ability to develop, and apply for, new
patents is a significant factor in the development and commercialization of
Huperzine A and other products. Aside from the general body of scientific
knowledge from other drug processes and technology, these patents and processes,
to the best of the Company’s knowledge and based upon its current scientific
data, are the only intellectual property necessary to develop its proposed
drugs. The Company does not believe that it is or will be knowingly violating
any other patents in developing Huperzine A or its other products.
The
Company may have to resort to litigation to protect its rights for certain
intellectual property, or to determine their scope, validity or
enforceability.
Enforcing
or defending the Company’s rights is expensive, could cause diversion of its
resources and may not prove successful. Any failure to enforce or protect the
Company’s rights could cause it to lose the ability to exclude others from using
Huperzine A or to develop or sell competing products.
The
Company may rely on third party contract research organizations, service
providers and suppliers to support development and clinical testing of its
products.
Failure
of any of these contractors to provide the required services in a timely manner
or on reasonable commercial terms could materially delay the development and
approval of the Company’s products, increase its expenses and materially harm
its business, financial condition and results of operations.
Key
components of the Company’s drug technologies may be provided by sole or limited
numbers of suppliers, and supply shortages or loss of these suppliers could
result in interruptions in supply or increased costs.
Certain
components used in the Company’s research and development activities such as
naturally occurring or synthetic Huperzine are currently purchased from a single
or a limited number of sources primarily located in China in the case of
naturally occurring supplies. The reliance on a sole or limited number of
suppliers could result in:
|
·
|
potential
delays associated with research and development and clinical and
preclinical trials due to an inability to timely obtain a single
or
limited source component;
|
|
·
|
potential
inability to timely obtain an adequate supply;
and
|
|
·
|
potential
of reduced control over pricing, quality and timely
delivery.
|
The
Company does not have long-term agreements with any of its suppliers, and
therefore the supply of a particular component could be terminated without
penalty to the supplier. Any interruption in the supply of components could
cause the Company to seek alternative sources of supply or manufacture these
components internally. If the supply of any components is interrupted,
components from alternative suppliers may not be available in sufficient volumes
within required timeframes, if at all, to meet the Company’s needs. This could
delay the Company’s ability to complete clinical trials, obtain approval for
commercialization or commence marketing, or cause the Company to lose sales,
incur additional costs, delay new product introductions or harm the Company’s
reputation. Further, components from a new supplier may not be identical to
those provided by the original supplier. Such differences if they exist could
affect product formulations or the safety and effect of the Company’s products
that are being developed and delay regulatory approvals.
Due
to the Company’s limited marketing, sales and distribution experience, the
Company may be unsuccessful in its efforts to sell its products, enter into
relationships with third parties or develop a direct sales
organization.
The
Company has yet to establish marketing, sales or distribution capabilities
for
its proposed products. Until such time as the Company’s products are further
along in the regulatory process, the Company will not devote meaningful time
and
resources to this effort. At the appropriate time, the Company intends to enter
into agreements with third parties to sell its products or the Company may
develop its own sales and marketing force. The Company may be unable to
establish or maintain third party relationships on a commercially reasonable
basis, if at all. In addition, these third parties may have similar or more
established relationships with the Company’s competitors.
If
the
Company does not enter into relationships with third parties for the sales
and
marketing of its products, the Company will need to develop its own sales and
marketing capabilities. The Company has limited experience in developing,
training or managing a sales force. If the Company chooses to establish a direct
sales force, the Company may incur substantial additional expenses in
developing, training and managing such an organization. The Company may be
unable to build a sales force on a cost effective basis or at all. Any such
direct marketing and sales efforts may prove to be unsuccessful. In addition,
the Company will compete with many other companies that currently have extensive
marketing and sales operations. The Company’s marketing and sales efforts may be
unable to compete against these other companies. The Company may be unable
to
establish a sufficient sales and marketing organization on a timely basis,
if at
all.
The
Company may be unable to engage qualified distributors. Even if engaged, these
distributors may:
|
·
|
fail
to satisfy financial or contractual obligations to the
Company;
|
|
·
|
fail
to adequately market the Company’s
products;
|
|
·
|
cease
operations with little or no notice;
or
|
|
·
|
offer,
design, manufacture or promote competing
products.
|
If
the
Company fails to develop sales, marketing and distribution channels, the Company
would experience delays in product sales and incur increased costs, which would
harm the Company’s financial results. If the Company is unable to convince
physicians as to the benefits of its intended products, it may incur delays
or
additional expense in its attempt to establish market acceptance.
Broad
use
of the Company’s drug technology may require physicians to be informed regarding
its intended products and the intended benefits. The time and cost of such
an
educational process may be substantial. Inability to successfully carry out
this
physician education process may adversely affect market acceptance of the
Company’s products. The Company may be unable to timely educate physicians
regarding its intended products in sufficient numbers to achieve the Company’s
marketing plans or to achieve product acceptance. Any delay in physician
education may materially delay or reduce demand for the Company’s products. In
addition, the Company may expend significant funds towards physician education
before any acceptance or demand for the Company’s products is created, if at
all.
The
market for the Company’s products is rapidly changing and competitive, and new
drug mechanisms, drug technologies, new therapeutics, new drugs and new
treatments which may be developed by others could impair the Company’s ability
to maintain and grow its business and remain
competitive.
The
pharmaceutical and biotechnology industries are subject to rapid and substantial
technological change. Developments by others may render the Company’s
technologies and intended products noncompetitive or obsolete, or the Company
may be unable to keep pace with technological developments or other market
factors.
Technological
competition from pharmaceutical and biotechnology companies, universities,
governmental entities and others diversifying into the field is intense and
is
expected to increase. Many of these entities have significantly greater research
and development capabilities and budgets than the Company do, as well as
substantially more marketing, manufacturing, financial and managerial resources.
These entities represent significant competition for the Company. Acquisitions
of, or investments in, competing pharmaceutical or biotechnology companies
by
large corporations could increase such competitors’ financial, marketing,
manufacturing and other resources.
The
Company’s resources are limited and the Company may experience management,
operational or technical challenges inherent in such activities and novel
technologies.
Competitors
have developed or are in the process of developing technologies that are, or
in
the future may be, the basis for competition.
Some
of
these technologies may have an entirely different approach or means of
accomplishing similar therapeutic effects. The Company’s competitors may develop
drug technologies and drugs that are safer, more effective or less costly than
its intended products and, therefore, present a serious competitive threat
to
the Company.
The
Company has no manufacturing capabilities. If third-party manufacturers of
the
Company’s product candidates fail to devote sufficient time and resources to the
Company’s concerns, or if their performance is substandard, its clinical trials
and product introductions may be delayed.
Currently,
the Company has no internal manufacturing capabilities for any of its product
candidates. The Company cannot be sure that the Company will be able to: (i)
acquire or build facilities that will meet quality, quantity and timing
requirements; or (ii) enter into manufacturing contracts with others on
acceptable terms. Failure to accomplish these tasks would impede the Company’s
efforts to bring its product candidates to market, which would adversely affect
its business. Moreover, if the Company decides to manufacture one or more
product candidates, the Company would incur substantial start-up expenses and
would need to expand the Company’s facilities and hire additional
personnel.
The
Company currently expects to utilize third-party manufacturers to produce the
drug compounds used in clinical trials and for the potential commercialization
of future products. If the Company is unable to obtain or retain third-party
manufacturers, the Company will not be able to commercialize its products.
The
Company’s reliance on contract manufacturers also will expose the Company to the
following risks:
|
·
|
contract
manufacturers may encounter difficulties in achieving volume production,
quality control and quality assurance and also may experience shortages
in
qualified personnel. As a result, the Company’s contract manufacturers
might not be able to meet its clinical schedules or adequately manufacture
the Company’s products in commercial quantities when
required;
|
|
·
|
switching
manufacturers may be difficult because the number of potential
manufacturers is limited. It may be difficult or impossible for the
Company to find a replacement manufacturer quickly on acceptable
terms, or
at all;
|
|
·
|
the
Company’s contract manufacturers may not perform as agreed or may not
remain in the contract manufacturing business for the time required
to
successfully produce, store or distribute the Company’s products;
and
|
|
·
|
if
the Company’s primary contract manufacturer should be unable to
manufacture any of its product candidates for any reason, or should
fail
to receive FDA approval or Drug Enforcement Administration approval,
commercialization of the Company’s product candidates could be delayed
which would negatively impact its
business.
|
Third-party
manufacturers also must comply with the FDA, the Drug Enforcement Administration
and other regulatory requirements for their facilities. The Company does not
have control over third-party manufacturers’ compliance with the regulations and
standards established by these agencies. In addition, manufacture of product
candidates on a limited basis for investigational use in animal studies or
human
clinical trials does not guarantee that large-scale, commercial production
is
viable. Small changes in methods of manufacture can affect the safety, efficacy,
controlled release or other characteristics of a product. Changes in methods
of
manufacture, including commercial scale-up, can, among other things, require
the
performance of new clinical studies.
If
the Company is unable to hire and retain additional qualified personnel, the
Company’s business may be harmed.
The
Company is small and if unable to continue to attract, retain and motivate
highly qualified management and scientific personnel and develop and maintain
important relationships with leading academic institutions and scientists,
may
not be able to achieve its research and development objectives. Competition
for
personnel and academic collaborations is intense.
Although
the Company has outsourced and intends to continue to outsource its development
programs, the Company also may need to hire additional qualified personnel
with
expertise in preclinical testing, clinical research and testing, government
regulation, formulation and manufacturing and sales and marketing. The Company
competes for qualified individuals with numerous biopharmaceutical companies,
universities and other research institutions and other emerging entrepreneurial
companies. Competition for such individuals, particularly in the New York City
area, where the Company is located, is intense and the Company cannot be certain
that the Company’s search for such personnel will be successful. Attracting and
retaining qualified personnel will be critical to the Company’s success. Skilled
employees in the Company’s industry are in great demand. The Company is
competing for employees against companies located in the New York metropolitan
area that are more established than the Company is and has the ability to pay
more cash compensation than the Company does. The Company will require
experienced scientific personnel in many fields in which there are a limited
number of qualified personnel and will have to compete with other technology
companies and academic institutions for such personnel. As a result, depending
upon the success and the timing of clinical tests, the Company may continue
to
experience difficulty in hiring and retaining highly skilled employees,
particularly scientists. If the Company is unable to hire and retain skilled
scientists, its business, financial condition, operating results and future
prospects could be materially adversely affected.
If
users of the Company’s products are unable to obtain adequate reimbursement from
third party payors, or if new restrictive legislation is adopted, market
acceptance of the Company’s products may be limited and the Company may not
achieve anticipated revenues.
The
continuing efforts of government and insurance companies, health maintenance
organizations and other payors of healthcare costs to contain or reduce costs
of
health care may affect the Company’s future revenues and profitability, and the
future revenues and profitability of the Company’s potential customers,
suppliers and collaborative partners and the availability of capital. For
example, in certain foreign markets, pricing or profitability of prescription
pharmaceuticals is subject to government control. In the U.S., given recent
federal and state government initiatives directed at lowering the total cost
of
health care, the U.S. Congress and state legislatures will likely continue
to
focus on health care reform, the cost of prescription pharmaceuticals and on
the
reform of the Medicare and Medicaid systems. While the Company cannot predict
whether any such legislative or regulatory proposals will be adopted, the
announcement or adoption of such proposals could materially harm the Company’s
business, financial condition and results of operations.
The
Company’s ability to commercialize its products will depend in part on the
extent to which appropriate reimbursement levels for the cost of its products
and related treatment are obtained by governmental authorities, private health
insurers and other organizations, such as HMOs. Third party payors are
increasingly challenging the prices charged for medical drugs and services.
Also, the trend toward managed health care in the U.S. and the concurrent growth
of organizations such as HMOs, which could control or significantly influence
the purchase of health care services and drugs, as well as legislative proposals
to reform health care or reduce government insurance programs, may all result
in
lower prices for or rejection of the Company’s drugs. The cost containment
measures that health care payors and providers are instituting and the effect
of
any health care reform could materially harm the Company’s ability to operate
profitably.
The
Company’s limited operating history makes evaluating its common stock more
difficult, and therefore, investors have limited information upon which to
rely.
An
investor can only evaluate the Company’s business based on a limited operating
history. The Company’s operations are expected to change dramatically as the
Company evolves from primarily a “virtual” technology holding company with no
full-time employees to a capitalized company with larger internal operations
and
costs. This limited history may not be adequate to enable an investor to fully
assess the Company’s ability to develop Huperzine A and proposed drugs, obtain
FDA approval, and achieve market acceptance of the Company’s proposed products
and respond to competition, or conduct such affairs as are presently
contemplated.
The
Company’s compliance with the reporting requirements of federal securities laws
and SEC rules concerning internal controls may be time consuming, difficult
and
expensive.
The
Company is a public reporting company and, accordingly, subject to the
information and reporting requirements of the Exchange Act and other federal
securities laws, including compliance with the Sarbanes-Oxley Act. It may be
time consuming, difficult and costly for the Company to develop and implement
the internal controls and reporting procedures required by the Sarbanes-Oxley
Act. The costs of preparing and filing annual and quarterly reports, proxy
statements and other information with the SEC and furnishing audited reports
to
stockholders will cause the Company’s expenses to be higher than they would be
if the Company had remained privately-held. The Company may need to hire
additional financial reporting, internal controls and other finance personnel
in
order to develop and implement appropriate internal controls and reporting
procedures. If the Company is unable to comply with the internal controls
requirements of the Sarbanes-Oxley Act, the Company may not be able to obtain
the independent accountant certifications required by the Sarbanes-Oxley Act.
Additionally, the Company will incur substantial expenses in connection with
the
preparation of a registration statement and related documents to register
certain shares of the Company’s common stock which it is obligated to
register.
Risks
Relating to the Company’s Common Stock
The
market price of the Company’s common stock has been, and is likely to continue
to be, highly volatile and subject to wide
fluctuations.
The
market price of the Company’s common stock has been, and is likely to continue
to be, highly volatile and could be subject to wide fluctuations in response
to
a number of factors, some of which are beyond the Company’s control,
including:
|
·
|
announcements
relating to the interpretation of the results from the Company’s Phase II
clinical trial;
|
|
·
|
announcements
or developments related to the products of the Company’s
competitors;
|
|
·
|
quarterly
variations in the Company’s operating
expenses;
|
|
·
|
issuances
or sales of capital stock by the Company;
and
|
|
·
|
sales
of the common stock by the Company’s founders or other selling
stockholders.
|
Applicable
SEC rules governing the trading of “penny stocks” may limit the trading and
liquidity of the Company’s common stock in the future, which could affect its
trading price.
The
Company’s common stock is currently traded on the Pink Sheets under
the symbol “NHPI.”
The
Company’s common stock may be considered a “penny stock” and subject to SEC
rules and regulations which impose limitations upon the manner in which such
shares may be publicly traded. These regulations require the delivery, prior
to
any transaction involving a penny stock, of a disclosure schedule explaining
the
penny stock market and the associated risks. Under these regulations, certain
brokers who recommend such securities to persons other than established
customers or certain accredited investors must make a special written
suitability determination regarding such a purchaser and receive such
purchaser’s written agreement to a transaction prior to sale. These regulations
have the effect of limiting the trading activity of the common stock and
reducing the liquidity of an investment in the common stock.
Because
the Company became public by means of a reverse merger, it may not be able
to
attract the attention of major brokerage firms.
There
may
be risks associated with the Company becoming public through a “reverse merger.”
Securities analysts of major brokerage firms may not provide coverage of the
Company because there is no incentive to brokerage firms to recommend the
purchase of the Company’s common stock.
The
common stock is controlled by insiders.
Alan
Kestenbaum, Reuben Seltzer and certain affiliated parties beneficially own
a
large percentage of the Company’s outstanding shares of common stock. Such
concentrated control of the Company may adversely affect the price of the common
stock. The Company’s principal security holders may be able to control matters
requiring approval by security holders, including the election of directors.
Such concentrated control may also make it difficult for stockholders to receive
a premium for their shares of common stock in the event of a merger with a
third
party or different transaction that requires stockholder approval. In addition,
certain provisions of Delaware law could have the effect of making it more
difficult or more expensive for a third party to acquire, or of discouraging
a
third party from attempting to acquire, control of the Company. Accordingly,
under certain circumstances, investors may have no effective voice in the
management of the Company.
The
Company does not expect to pay dividends for the foreseeable
future.
The
Company currently intends to retain any future earnings to support the
development and expansion of its business and does not anticipate paying cash
dividends in the foreseeable future. Any payment of future dividends will be
at
the discretion of the board of directors after taking into account various
factors, including but not limited to the Company’s financial condition,
operating results, cash needs, growth plans and the terms of any credit
agreements that the Company may be a party to at the time.
|
Item
2.
|
Description
of Property.
|
The
Company leases office space at One Penn Plaza, Suite 1503, New York, New York
10019.
|
Item
3.
|
Legal
Proceedings.
|
The
Company is not a party to any pending legal proceedings.
|
Item4.
|
Submission
of Matters to a Vote of Security Holders.
|
The
Company did not submit any matter to a vote of security holders through the
solicitation of proxies or otherwise during the fourth quarter of the fiscal
year ended December 31, 2007.
PART
II
|
Item
5.
|
Market
for Common Equity, Related Stockholder Matters and Small Business
Issuer
Purchases of Equity
Securities
|
The
Company’s common stock is currently traded on the Pink Sheets under
the symbol “NHPI.”
The
Company’s common stock was first traded on the OTCBB market on February 2, 2006
until the common stock began trading on the NASDAQ Capital Market on April
24,
2007. The Company
’
s common
stock was
delisted from the NASDAQ Capital Market on April 2, 2008. The following table
sets forth the high and low sales prices for the period during which the common
stock has been traded on the NASDAQ Capital Market and the range of the high
and
low bid prices for the period during which the common stock was traded on the
OTCBB market. The information provided for our trading on the OTCBB market,
as
reported by the National Quotation Bureau, represents interdealer quotations,
without retail markup, markdown or commission and may not be reflective of
actual transactions.
|
|
|
Bid Price Per Share
|
|
|
|
|
High
|
|
Low
|
|
|
February
2006 - March 2006
|
|
$
|
10.73
|
|
$
|
5.49
|
|
|
April
2006 - June 2006
|
|
$
|
9.15
|
|
$
|
5.00
|
|
|
July
2006 - September 2006
|
|
$
|
8.00
|
|
$
|
5.25
|
|
|
October
2006 - December 2006
|
|
$
|
7.50
|
|
$
|
5.00
|
|
|
January
2007 - March 2007
|
|
$
|
7.25
|
|
$
|
4.75
|
|
|
April
2007 – June 2007
|
|
$
|
8.15
|
|
$
|
5.82
|
|
|
July
2007 – September 2007
|
|
$
|
6.47
|
|
$
|
4.25
|
|
|
October
2007 – December 2007
|
|
$
|
5.50
|
|
$
|
3.01
|
|
|
January
2008 – March 2008
|
|
$
|
4.40
|
|
$
|
0.40
|
|
Stockholders
As
of
March 17, 2008, the Company believes there were approximately 123
holders
of record of its common stock. The Company believes that a greater number of
holders of its common stock are “street name” or beneficial holders, whose
shares are held of record by banks, brokers and other financial
institutions.
Dividends
The
Company has never declared or paid any cash dividends on its common stock.
The
Company currently intends to retain all available funds and any future earnings
to fund the development and growth of its business and does not anticipate
declaring or paying any cash dividends on its common stock in the foreseeable
future.
Recent
Sales of Unregistered Securities
The
Company did not sell any unregistered equity securities during the year ended
December 31, 2007 that were not previously reported on a Quarterly Report on
Form 10-QSB or a Current Report on Form 8-K.
|
Item
6.
|
Management’s
Discussion and Analysis or Plan of Operation
|
FORWARD-LOOKING
STATEMENTS
This
Annual Report on Form 10-KSB contains forward-looking statements (as defined
in
Section 27A of the Securities Act and Section 21E of the Exchange Act). To
the
extent that any statements made in this Report contain information that is
not
historical, these statements are essentially forward-looking. Forward-looking
statements can be identified by the use of words such as “expects,” “plans”
“will,” “may,” “anticipates,” “believes,” “should,” “intends,” “estimates,”
“projects” and other words of similar meaning. These statements are subject to
risks and uncertainties that cannot be predicted or quantified and consequently,
actual results may differ materially from those expressed or implied by such
forward-looking statements. Such risks and uncertainties include those outlined
in “Risk Factors” found within our Annual Report on Form 10-KSB and include,
without limitation, the Company’s early stage, limited history, limited
revenues, limited cash and ability to raise capital to finance the growth of
the
Company’s operations, the ability of the Company to develop its products and
obtain necessary governmental approvals, the Company’s ability to protect its
proprietary information, the Company’s ability to attract or retain qualified
personnel, including scientific and technical personnel and other risks detailed
from time to time in the Company’s filings with the SEC, or
otherwise.
All
references to the “Company” for periods prior to the closing of the Merger refer
to Marco, and references to the “Company” for periods subsequent to the closing
of the Merger refer to Neuro-Hitech and its subsidiaries.
THE
FOLLOWING DISCUSSION SHOULD BE READ TOGETHER WITH THE INFORMATION CONTAINED
IN
THE FINANCIAL STATEMENTS AND RELATED NOTES INCLUDED ELSEWHERE IN THIS ANNUAL
REPORT ON FORM 10-KSB.
The
Company is an early stage pharmaceutical company focused on developing
innovative drugs for the treatment of degenerative neurological diseases. The
Company’s most advanced product candidate, Huperzine A, recently completed a
Phase II clinical trial in the U.S. which tested Huperzine A for efficacy and
safety in the treatment of mild to moderate Alzheimer’s disease.
The
Phase
II clinical trial compared the safety, tolerability and efficacy of either
200
or 400 micrograms of Huperzine A on cognitive function, activities of daily
living and behavior. Results showed that there was no statistical difference
in
the mean change AD Assessment Scale-Cognitive (ADAS-Cog) scores, the primary
endpoint, after 16 weeks treatment with Huperzine A 200 micrograms bid compared
to placebo (p=0.81). However, data demonstrated that the higher dose tested, 400
micrograms bid, showed cognitive enhancement on the ADAS-Cog versus placebo.
The
maximum cognitive improvement was observed at week 11 of treatment (p=0.001).
Over 16 weeks Huperzine A (400 micrograms bid) improved cognition compared
to
placebo (p=0.03) and there was a trend to cognitive improvement over placebo
at
week 16 (p=0.069). In this clinical trial, there was an unexpected improvement
in cognition in the placebo group at week 16 versus baseline. On other secondary
endpoints, including clinical global impression of change (ADCS-CGIC) and the
Neuropsychiatric Inventory (NPI) there was no statistical difference between
placebo and either 200 or 400 micrograms bid after four months treatment.
However, there was a trend to improvement on activities of daily living
(ADCS-ADL) with 400 micrograms bid (p=0.077). Huperzine A was safe and well
tolerated. Overall the incidence of adverse events during the study was similar
between both doses of Huperzine A and placebo. Following completion of the
double-blind part of this clinical trial, subjects were invited to receive
Huperzine A treatment in an open-label fashion for up to one year: 82% of
subjects accepted this invitation.
After
receiving the results of the Phase II clinical trial of Huperzine A, the Company
conducted a detailed review of those results. Based on its review, the Company
believes that there is substantial support within the results that Huperzine
A
can become a safe and effective treatment for Alzheimer’s disease. In order to
validate the current information on the compound and review the Company’s
analysis of the results, in March 2008 the Company entered into an
agreement with Numoda Corporation (“Numoda”) pursuant to which Numoda will
review the results and will provide a report to the Company on its findings.
In
view
of the results of the Phase II clinical trial, the Company is currently
reviewing its options for moving forward with its business. Currently, the
Company expects to focus upon the development of collaborative, joint and
strategic alliances and licensing arrangements with one or more pharmaceutical
companies for the further development of Huperzine A and also expects to
consider merger and/or acquisition opportunities. The Company will further
evaluate these opportunities based upon the results of Numoda’s review and
report, and the Company also expects that Numoda will assist the Company in
interpreting and presenting the results of the Phase II clinical trial to
potential partners, licensees and merger or acquisition candidates.
The
Company has also studied the transdermal delivery of Huperzine A. The Company
believes that Huperzine A can effectively be delivered transdermally because
of
its low dosage requirement and low molecular weight. The Company believes that
a
transdermal patch could be a better way to deliver Huperzine A because the
patch
may provide the drug for transdermal delivery for up to between three and five
days while avoiding the gastrointestinal tract. Although the Company initially
expected to begin Phase I clinical trials in the first quarter of 2008, the
Company has elected to postpone any decision or expenditures related to such
a
trial until after Numoda has delivered its report and the Company has considered
its options.
Worldwide
research thus far suggests that, in addition to Alzheimer’s Disease, Huperzine A
may be effective in treating other dementias and myasthenia gravis. Also,
research suggests that it has potential neuroprotective properties that may
render it useful as a protection against neurotoxins, and it has an anti-oxidant
effect.
In
addition to Huperzine A, the Company has worked on two pre-clinical development
programs: one for second generation anti-amyloid compounds or disease modifying
drugs for Alzheimer’s disease and, secondly, development of a series of
compounds targeted to treat and prevent epilepsy. The Company’s efforts however,
have principally focused on its primary product.
The
Company has imported and sold inventories of natural Huperzine to vitamin and
supplement suppliers to generate revenues. However, the majority of the
Company’s operations to date have been funded through Company’s private
placement of equity securities.
History
The
Company was originally formed on February 1, 2005, as Northern Way Resources,
Inc., a Nevada corporation, for the purpose of acquiring exploration and early
stage natural resource properties. On January 24, 2006, the Company entered
into
an Agreement and Plan of Reorganization (the “Merger Agreement”) by and among
the Company, Marco Hi-Tech JV Ltd., a privately held New York corporation
(“Marco”), and Marco Acquisition I, Inc., a newly formed wholly-owned Delaware
subsidiary of ours (“Acquisition Sub”). Upon closing of the transactions
contemplated under the Merger Agreement (the “Merger”), Acquisition Sub was
merged with and into Marco, and Marco became a wholly-owned subsidiary of the
Company. The Merger was consummated on January 24, 2006, and in connection
with
that Merger, the Company changed its name to Neuro-Hitech Pharmaceuticals,
Inc.
The Company subsequently changed its name to Neuro-Hitech, Inc. on August 11,
2006.
Marco
was
incorporated in the State of New York on December 11, 1996. Through 2005, Marco
conducted analytical work and clinical trials of Huperzine A and was focused
primarily on licensing proprietary Huperzine A technology from independent
third-party developers and investigators, including the Mayo Foundation, and
until such time operated with no full-time employees and minimal internal
resources. In addition, from time to time, Marco has imported and sold
inventories of natural Huperzine and other dietary supplement ingredients to
vitamin and supplement suppliers to generate revenues. In 2005, Marco decided
to
raise additional capital to pursue additional approvals and undertake necessary
studies for the development and commercialization of Huperzine A, including
funding development and securing rights to third-party transdermal patch
technology.
Upon
the
Merger, the Company abandoned the line of business pursued by Northern Way
Resources prior to the Merger.
On
November 29, 2006, the Company completed its acquisition by merger of
Q-RNA, Inc. (“Q-RNA”), a New York-based biotechnology company focused on
diseases such as Alzheimer’s, epilepsy and Parkinson’s disease, pursuant to the
Agreement and Plan of Merger with QA Acquisition Corp., a Delaware corporation,
QA Merger LLC, a Delaware limited liability company, Q-RNA and Dr. David
Dantzker, as the “Representative” of the Q-RNA securityholders.
Plan
of Operation
Based
upon the completion of a Phase II clinical trial of Huperzine A, the Company
is
currently reviewing its options for moving forward with its business. Currently,
the Company expects to focus upon the development of collaborative, joint and
strategic alliances and licensing arrangements with one or more pharmaceutical
companies for the further development of Huperzine A and also expects to
consider merger and/or acquisition opportunities. The Company will further
evaluate these opportunities based upon the results of Numoda’s review and
report, and the Company also expects that Numoda will assist the Company in
interpreting and presenting the results of the Phase II clinical trial to
potential partners, licensees and merger or acquisition candidates.
The
Company presently believes the estimated additional costs to bring Huperzine
A
to market as an oral dose drug after completing clinical trials will be
substantial and no assurances as to future costs can be made or that the Company
will be able to fund them. Currently, the Company expects to focus upon the
development of collaborative, joint and strategic alliances and licensing
arrangements with one or more pharmaceutical companies for the further
development of Huperzine A and also expects to consider merger and/or
acquisition opportunities. The Company anticipates that it would be necessary
to
obtain collaborative partners who would be primarily responsible for the sale
and distribution of Huperzine A products after obtaining FDA approval for
Huperzine A.
The
principal use of the Company’s cash and cash equivalents is to review the
results of its Phase II clinical trial of Huperzine A and pursuing the
development of collaborative, joint and strategic alliances and licensing
arrangements with one or more pharmaceutical companies and/or acquisition or
merger candidates.
The
Company has generated limited revenue from operations to date, and expects
to
continue generating limited operating revenue for several years. Substantially
all of the Company’s operations to date have been funded through the sale of its
securities, and the Company expects this to continue to be the case for the
foreseeable future.
Following
the receipt of the results of the Phase II clinical trial of Huperzine A, to
improve its liquidity and help ensure that it has sufficient cash and cash
equivalents for the next 12 months, the Company implemented certain cost
reductions in the first quarter of 2008 and delayed the implementation of
certain research programs, including the previously planned Phase I trial for
the transdermal patch. The Company anticipates, based on current plans and
assumptions relating to operations, including the implementation of its cost
reductions, that its cash and cash equivalents are sufficient to satisfy its
contemplated cash requirements to implement its business plan for the next
twelve months. In the event that the Company’s cash and cash equivalents prove
to be insufficient to fund the implementation of its business plan (due to
a
change in the Company’s plans or a material inaccuracy in its assumptions, or as
a result of unanticipated expenses, technical difficulties or other
unanticipated problems), the Company will be required to seek additional
financing sooner than anticipated in order to proceed with such implementation.
Additional funds may not be available on acceptable terms, if at all. If
adequate funds are unavailable the Company may have to delay, reduce the scope
of or eliminate one or more of its research or development programs, product
launches or marketing efforts which may materially harm the Company’s financial
condition and operations.
Results
of Operations
The
following discussion provides a comparison of the Company’s results from
operations for the year ended December 31, 2007 to the year ended December
31,
2006.
The
Company had revenues from operations of $458,870 for the year ended December
31,
2007, a 50.8% increase from the $304,240 in revenue achieved from the year
ended
December 31, 2006. The increase in revenue was a result of an increase in
product sales to the Company’s single customer of natural Huperzine.
Cost
of
goods sold as a percentage of the Company’s revenue was 47% for the year ended
December 31, 2007, compared with 51% for the year ended December 31, 2006.
The
Company’s cost of goods sold remained relatively constant as a percentage of the
Company’s revenue as the Company continues to purchase its natural Huperzine
from the same supplier as the prior year on substantially similar
terms.
The
Company’s total selling, general and administrative expenses increased from
$1,765,486 for the year ended December 31, 2006 to $2,561,402 for the year
ended
December 31, 2007. This increase was the result of the Company’s expansion of
its executive management and salaries and benefits in response to the
acquisition of Q-RNA. Additionally, a portion of the increase in the selling,
general and administrative expenses from the prior year is attributable to
the
increased costs associated with being a public company.
Share
based compensation for the year ended December 31, 2007 was $2,427,904, an
increase from the $327,835 for the year ended December 31, 2006. The increase
in
share based compensation was attributable to options granted during the year
ended December 31, 2007 to Messrs. Barrett, McIntosh, Shearman and Wong and
options paid to directors in lieu of director fees pursuant to the
Non-Management Director Deferral Program.
The
Company’s research and development costs increased from $2,674,714 for the year
ended December 31, 2006 to $3,523,954 for the year ended December 31, 2007,
as a
result of the expansion of the Company’s clinical development portfolio for
Huperzine A and preclinical research in compounds acquired from Q-RNA. These
costs exclude $16,805,787 assigned by the Company in the year ended December
31,
2006, as in-process research and development in accordance with FASB Statement
No. 4,
“Applicability
of FASB
Statement No. 2 to Business Combinations Accounted for by the Purchase
Method.
”
The Company expects operating expenses in 2008 to decrease as it looks
to
pass on future expenditures to collaborative licensing partners with one or
more
pharmaceutical companies and/or acquisition or merger candidates.
In
December 2003, the Company entered into a clinical research agreement, which
was
amended in November 2005, with Georgetown pursuant to which Georgetown provided
the Company with Phase II research on Huperzine A. The costs associated with
this agreement totaled $3,146,667 and were partially funded by the National
Institutes of Health. The Company’s portion of the total cost was $1,846,667 and
payable in installments upon the achievement of certain milestones. On
December 8, 2006, the Company announced the expansion in the size of the Phase
II clinical trial by 60 participants, an increase of 40%. The Company’s
portion of the total cost increased by another $2,190,175 and is payable in
installments. For the years ended December 31, 2007 and 2006, the payments
made
by the Company to Georgetown under the terms of the clinical research agreement
were approximately $1,183,167 and $952,500, respectively, and the total
payments made by the Company to Georgetown since inception of the agreement
were
approximately $3,110,667. These costs are reflected in the Research and
Development caption of the Statement of Operations.
The
Company expects to make additional payments in 2008 to Georgetown of $926,175
related to an open label extension which extension is expected to be completed
in the second half of 2008.
On
February 1, 2006, the Company entered into an exclusive development agreement
with Org Syn for the development by Org Syn of synthetic Huperzine A, in
accordance with the terms of the Agreement. Org Syn received an aggregate of
$175,894 upon the execution of the Agreement. For the years ended December
31,
2007 and 2006, the payments made by the Company to Org Syn were approximately
$11,375 and $419,500, respectively, and are reflected in the Research and
Development caption of the Statement of Operations. Org Syn may receive up
to an
additional $75,000 upon the achievement of certain milestones for services
rendered under the Agreement.
On
March
15, 2006, the Company entered into a development agreement with XEL for the
development of the Huperzine A Transdermal Delivery System (“Product”). Under
the terms of the agreement, the Company paid XEL a $250,000 fee upon the
execution of the agreement and paid an additional $92,500 per month during
the
development of the Product,
including
the first six months of 2007. The Delivery Product was completed in July
2007
. Between August 2007 and December 2007, the Company paid XEL an
additional $92,500 per month to fund the further development of the Product.
For
the years ended December 31, 2007 and 2006, the payments made by the Company
to
XEL were approximately $1,111,250 and $1,081,000, respectively, and are
reflected in the Research and Development caption of the Statement of
Operations. The Company and XEL intend to seek domestic and foreign patent
protection for the Product.
As
part
of the acquisition of Q-RNA, the Company assumed exclusive license agreements
with PARTEQ. Under the terms of the exclusive PARTEQ Licensing Agreement the
Company made an initial one-time license fee of C$25,000 and since January
1,
2008 has been obligated to pay fixed annual fees of C$283,000 for the
Alzheimer’s research in equal quarterly installments. The Company may also be
required to make quarterly royalty payments of 3% of net sales of the licensed
products, with a minimum annual royalty of C$10,000 for 2007, C$20,000 for
2008,
C$30,000 for 2009 and C$40,000 for 2010 and each subsequent calendar year.
Until
such time as the Company has a licensed product, the Company will not have
to
make any quarterly payments. The Company is also obligated to make the following
milestone payments: C$100,000 upon completion of a Phase I trial of a licensed
product, C$250,000 upon completion of a Phase II trial of a licensed product,
and C$1,000,000 upon the first FDA approval (as such term is defined in the
PARTEQ Licensing Agreement). The Company does not currently anticipate having
a
licensed product in the near term. The Company also has the right to sub-license
with the payment of 20% of all non-royalty sublicensing consideration.
Under
the
terms of the Exclusive Patent License Option Agreement with PARTEQ (the
“Epilepsy Agreement”) grants the Company a three-year option to acquire an
exclusive worldwide license to all innovations and developments, including
the
patent applications and additional filings, related to specified patents for
research on Epilepsy. The Company made a non-refundable, non-creditable option
payment of $10,000 when it entered into the Epilepsy Agreement in 2006.
The
Company made an additional payment of $45,000 to PARTEQ in the year ended
December 31, 2007 for expenses that should have been paid by Q-RNA in the year
ended December 31, 2005.
The Company also began to pay fixed annual fees
of C$150,800 in quarterly installments of C$37,700 beginning on March 1,
2008. If the Company exercises its option, the Company will make a
non-refundable, non-creditable license payment of C$17,500 at the time of such
exercise. If the Company exercises its option, it will be required to make
quarterly royalty payments of 3% of net sales of the licensed products (as
such
term is defined in Epilepsy Agreement), with a minimum annual royalty of
C$10,000 through the second anniversary of the license, C$20,000 through the
third anniversary of the license, C$30,000 through the fourth anniversary of
the
license and C$40,000 through the fifth anniversary of the license and each
subsequent anniversary. If the Company exercises its option, it is also
obligated to make the following milestone payments: C$100,000 upon completion
of
a Phase I trial of a licensed product, C$250,000 upon completion of a Phase
II
trial of a licensed product, and C$1,000,000 upon the first FDA approval (as
such term is defined therein). If the Company exercises its option, the Company
also has the right to sub-license with the payment of 20% of all non-royalty
sublicensing consideration. For the years ended December 31, 2007 and 2006,
the
payments made by the Company to PARTEQ under these agreements have been
approximately $457,115 and $48,600, respectively.
The
Company holds a license for two composition of matter patents and four process
patents for Racemic Huperzine A, Huperzine A and their analogues and derivatives
from the Mayo Foundation. The Company has a license to the four process patents
pursuant to a Technology License Contract with the Mayo Foundation (the “Mayo
Licensing Agreement”). For the year ended December 31, 2007 and 2006, the total
research and development costs incurred by the Company under this Agreement
have been approximately $18,888 and $205,000, respectively. The total costs
accrued and incurred since inception of the agreement were approximately
$350,000 and are reflected in the Research and Development caption of the
Statement of Operations.
Although
the Company has accrued certain costs relating to the Mayo Licensing Agreement,
because of uncertainty between the Company and Mayo over the interpretation
of the agreement, it is uncertain when or whether those expenses actually
will
be paid.
Liquidity
and Capital Resources
The
Company has generated limited revenue from operations to date, and expects
to
continue generating limited operating revenue from the sale of natural
Huperzine. Substantially all of the Company’s operations to date have been
funded through the sale of its securities, and the Company expects this to
continue in the foreseeable future.
Historically,
the principal uses of the Company’s cash and cash equivalents have been
concluding the Phase II clinical trials, developing alternative delivery
technologies, improving on the synthetic processes, and continuing to fund
pre-clinical compounds associated with the agreements with PARTEQ. Although
the
Company has developed plans related to its operations, management continues
to
retain significant flexibility for the uses of Company funds. In addition to
meeting its working capital needs, the Company may also use its cash and cash
equivalents to acquire additional products or technologies. Currently, the
principal uses of the Company’s cash and cash equivalents are investigating the
results of the Phase II clinical trials.
During
the year ended December 31, 2007, the Company experienced an increase in
selling, general and administrative expenses compared to the year ended
December 31, 2006. The increases were largely attributable to increases in
salaries and employee benefit expenses resulting from the Company’s expansion of
its executive management team following the acquisition of Q-RNA and the
employment of a new Chief Executive Officer. To improve its liquidity and ensure
that it has sufficient cash and cash equivalents for the next 12 months, the
Company implemented certain cost reductions in the first quarter of 2008 and
delayed the implementation of certain research programs, including the
previously planned Phase I trial for the transdermal patch.
The
Company expects to make additional payments by June 30, 2008 to Georgetown
of
$926,175 related to an open label extension which extension will expire in
June
2008. The Company also expects to continue making payments to PARTEQ of
C$108,000 each quarter as a result of the renewal of the Alzheimer’s Agreement
and Epilepsy Agreement with PARTEQ. The Company may also incur additional
expenses if it pursues certain contractual rights or activities that are at
its
discretion, including exercising its option to license the Product in North
America from XEL, pursuant to which it would pay XEL an initial license fee
of
$400,000 and up to an aggregate of $2.8 million in additional payments upon
the
achievement of certain milestones.
The
Company may also incur additional expenses in connection with its agreement
with
Numoda Corporation (“Numoda”). Following Numoda’s delivery of its analysis of
the Phase II clinical trials, the Company will pay Numoda $100,000. The Company
may be obligated to pay Numoda up to $400,000 of additional payments (a
“Deferred Payment”) if the Company enters into an agreement for the sale or
license of the Company’s products, or an agreement to merge or sell the Company
(each a “Transaction”). If the aggregate consideration paid to the Company in
such a Transaction is $1,000,000 or less, the Deferred Payment will be $200,000.
If the aggregate consideration paid to the Company in a Transaction is more
than
$1,000,000 but less than $5,000,000, the Deferred Payment will be $250,000.
If
the aggregate consideration paid to the Company in a Transaction is more than
$5,000,001 but less than $10,000,000, the Deferred Payment will be $300,000.
If
the aggregate consideration paid to the Company in the Transaction is more
than
$10,000,000, the Deferred Payment will be $400,000. Additionally, the Company
will be obligated to pay Numoda a fee equal to 3.5% of the aggregate
consideration paid to the Company in a Transaction, provided that the
Transaction is completed at any time during the term of the agreement, or prior
to March 3, 2011, and Numoda has either introduced such party to the Company
or
materially assisted the Company in facilitating such a Transaction.
To
fund
the implementation of its business plan, the Company has historically engaged
in
equity financing through existing investors and potential new investors. If
the
Company does not enter into a Transaction that provides it substantial
liquidity, it may engage in additional financing efforts. Additional funds
may
not be available or not available on acceptable terms, if at all. Given the
anticipated cash expenditures, the potential cash requirements and the lack
of
sufficient cash to fully fund those expenses, the Company is continually
analyzing alternative ways in which it can preserve its cash and cash
equivalents, including the potential delay, reduction in scope of or elimination
of some of its research or development programs. If the Company is unable to
raise additional financing and is forced to take such measures, they may
materially harm the Company’s prospects, financial condition and future
operations.
During
2007, the Company issued 2,016,930 shares of its Common Stock in private
placements of the Company’s securities, raising gross proceeds of $7,379,005.
Costs and expenses related to these private offerings, including, placement
agent, legal, accounting and printing fees, totaled in the aggregate,
$376,548 and were charged to additional paid-in capital.
In
addition to the aforementioned private placements, the Company received
approximately $46,000 from the exercise of options to purchase 12,059 shares
of
common stock.
During
the year ended December 31, 2007, the Company issued 20,000 shares of restricted
stock for services rendered. The Company recognized an expense of $121,000
relating to the issuance of those shares and are reflected in the Selling,
General and Administrative Expenses caption of the Statement of
Operations.
During
the third quarter of 2007, 100,729 shares of common stock were issued to
investors that purchased common stock and warrants from the Company in the
private offerings that took place in the first quarter of 2006 and between
November 2006 and March 2007. The shares were issued in accordance with the
provisions of registration rights agreements between the Company and the
purchasers of securities in those offerings. The aforementioned registration
rights agreements required the Company to pay an amount equal to 1% payable
in
the Company’s common stock, up to a maximum 6%, of the aggregate dollar amount
of securities purchased by such investors if the Company did not file a resale
registration statement or have such registration statement declared effective
by
the dates set forth in each agreement. The Company issued the maximum 6% of
shares and is under no obligation to issue any additional shares pursuant to
the
terms of the registration rights agreements described above.
Critical
Accounting Policies
Our
financial statements are prepared in accordance with accounting principles
generally accepted in the United States of America. Preparing financial
statements in accordance with generally accepted accounting principles requires
management to make estimates and assumptions which affect the reported amounts
of assets and liabilities and the disclosure of contingent assets and
liabilities at the balance sheet dates, and the recognition of revenues and
expenses for the reporting periods. These estimates and assumptions are affected
by management’s application of accounting policies.
Research
and Development Costs
All
research and development costs are expensed as incurred and include costs
paid
to sponsored third parties to perform research and conduct clinical
trials.
Share-Based
Compensation
Effective
January 1, 2006, the Company adopted Statement of Financial Accounting
Standards No. 123(R), (“SFAS No. 123(R)”), “Share-Based Payment,” which requires
the Company to record as an expense in its financial statements the fair
value
of all stock-based compensation awards. The Company currently utilizes the
Black-Scholes option pricing model to measure the fair value of stock options
granted to employees using the “modified prospective” method. Under the
“modified prospective” method, compensation cost is recognized in the financial
statements beginning with the effective date, based on the requirements of
SFAS
No. 123(R) for all share-based payments granted after that date, and based
on
the requirements of SFAS No. 123(R) for all unvested awards granted prior
to the
effective date of SFAS No. 123(R).
Deferred
Compensation
In
accordance with EITF Abstract No. 96-18, “Accounting for Equity Instruments That
Are Issued to Other Than Employees for Acquiring, or in Conjunction with
Selling, Goods or Services,” the Company initially measures the fair value of
any equity granted to consultants on the date of grant and subsequently
remeasures such grants in accordance with promulgated accounting principles
using the Black-Scholes pricing model. Amounts are initially recorded as
Deferred Compensation in the Stockholders’ Equity section of the balance sheet
and are subsequently charged to the appropriate expense over the period to
which
the service relates.
|
Item
7.
|
Financial
Statements
|
The
Company’s Consolidated Financial Statements and the Report of the
Independent Registered Public Accounting Firm appears at the end of
this annual report.
|
Item
8.
|
Changes
in and Disagreements With Accountants on Accounting and Financial
Disclosure
|
N/A
|
Item
8A.
|
Controls
and Procedures
|
Evaluation
of Disclosure Controls and Procedures
Based
on
management’s evaluation (with the participation of our Chief Executive Officer
(“CFO”) and Chief Financial Officer (“CFO”)), as of the end of the period
covered by this report, our CEO and CFO have concluded that our disclosure
controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under
the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), are
effective to provide reasonable assurance that information required to be
disclosed by us in reports that we file or submit under the Exchange Act is
recorded, processed, summarized, and reported within the time periods specified
in SEC rules and forms, and is accumulated and communicated to management,
including our principal executive officer and principal financial officer,
as
appropriate to allow timely decisions regarding required disclosure.
Changes
in Internal Control Over Financial Reporting
There
were no changes to our internal control over financial reporting (as defined
in
Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during
the period covered by this report that have materially affected, or are
reasonably likely to materially affect, our internal control over financial
reporting.
Management
Report on Internal Control Over Financial Reporting
Our
management is responsible for establishing and maintaining adequate internal
control over financial reporting (as defined in Rules 13a-15(f) and
15d-15(f) under the Exchange Act) to provide reasonable assurance regarding
the
reliability of our financial reporting and the preparation of financial
statements for external purposes in accordance with U.S. generally accepted
accounting principles.
Management
assessed our internal control over financial reporting as of December 31,
2007, the end of our fiscal year. Management based its assessment on criteria
established in Internal Control—Integrated Framework issued by the Committee of
Sponsoring Organizations of the Treadway Commission. Management’s assessment
included evaluation of elements such as the design and operating effectiveness
of key financial reporting controls, process documentation, accounting policies,
and our overall control environment.
Based
on
our assessment, management has concluded that our internal control over
financial reporting was effective as of the end of the fiscal year to provide
reasonable assurance regarding the reliability of financial reporting and the
preparation of financial statements for external reporting purposes in
accordance with U.S. generally accepted accounting principles. We reviewed
the results of management’s assessment with the Audit Committee of our Board of
Directors.
This
report does not include an attestation report of our registered public
accounting firm regarding our internal controls over financial reporting. The
disclosure contained under this Item 8A was not subject to attestation by our
independent registered public accounting firm pursuant to temporary rules
of the SEC that permit us to provide only the disclosure under this Item 8A
in
this Report.
Inherent
Limitations on Effectiveness of Controls
Our
management, including the CEO and CFO, does not expect that our disclosure
controls or our internal control over financial reporting will prevent or detect
all errors and all fraud. A control system, no matter how well designed and
operated, can provide only reasonable, not absolute, assurance that the control
system’s objectives will be met. The design of a control system must reflect the
fact that there are resource constraints, and the benefits of controls must
be
considered relative to their costs. Further, because of the inherent limitations
in all control systems, no evaluation of controls can provide absolute assurance
that misstatements due to error or fraud will not occur or that all control
issues and instances of fraud, if any, have been detected. These inherent
limitations include the realities that judgments in decision-making can be
faulty and that breakdowns can occur because of simple error or mistake.
Controls can also be circumvented by the individual acts of some persons, by
collusion of two or more people, or by management override of the controls.
The
design of any system of controls is based in part on certain assumptions about
the likelihood of future events, and there can be no assurance that any design
will succeed in achieving its stated goals under all potential future
conditions. Projections of any evaluation of controls effectiveness to future
periods are subject to risks. Over time, controls may become inadequate because
of changes in conditions or deterioration in the degree of compliance with
policies or procedures.
|
Item
8B.
|
Other
Information
|
None.
PART
III
Certain
information required by Part III is omitted from this report in that the Company
will file a definitive proxy statement pursuant to Regulation 14A with respect
to its 2008 Annual Meeting (the “Proxy Statement”) no later than 120 days after
the end of the fiscal year covered by this report, and certain information
included therein is incorporated herein by reference. Only those sections of
the
Proxy Statement which specifically address the items set forth herein are
incorporated by reference. In addition, the Company has adopted a Code of Ethics
which can be reviewed and printed from its website,
www.neurohitech.com.
|
Item
9.
|
Directors,
Executive Officers, Promoters, Control Persons and Corporate Governance;
Compliance with Section 16(a) of the Exchange Act
|
The
information required by this Item is hereby incorporated herein by reference
to
the Proxy Statement. The information under the heading “Executive Officers of
the Registrant” in Part I, Item 1 of this Form 10-KSB is also incorporated by
reference in this section.
|
Item
10.
|
Executive
Compensation
|
The
information required by this Item is hereby incorporated herein by reference
to
the Proxy Statement.
|
Item
11.
|
Security
Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters
|
The
information required by this Item is hereby incorporated herein by reference
to
the Proxy Statement.
|
Item
12.
|
Certain
Relationships and Related Transactions, and Director
Independence
|
The
information required by this Item is hereby incorporated herein by reference
to
the Proxy Statement.
|
Exhibit
|
|
|
|
Incorporated
by Reference
|
|
Filed
|
|
Number
|
|
Exhibit
Description
|
|
Form
|
|
Exhibit
|
|
Filing
Date
|
|
Herewith
|
|
2.1
|
|
Agreement
and Plan of Merger, dated January 17, 2006, between Northern Way
Resources, Inc., a Nevada corporation and Northern Way Resources,
Inc., a
Delaware corporation
|
|
8-K
|
|
2.1
|
|
1/23/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.2
|
|
Certificate
of Ownership and Merger merging Northern Way Resources, Inc., a Nevada
corporation into Northern Way Resources, Inc., a Delaware
corporation
|
|
8-K
|
|
2.2
|
|
1/23/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.3
|
|
Articles
of Merger merging Northern Way Resources, Inc., a Nevada corporation
into
Northern Way Resources, Inc., a Delaware corporation
|
|
8-K
|
|
2.3
|
|
1/23/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.4
|
|
Agreement
of Merger and Plan of Reorganization, dated as of January 24, 2006,
by and
among Neurotech Pharmaceuticals, Inc., Marco Hi-Tech JV Ltd., and
Marco
Acquisition I, Inc.
|
|
8-K
|
|
2.1
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.5
|
|
Agreement
and Plan of Merger, dated as of November 16, 2006, by and among
Neuro-Hitech, Inc., QA Acquisition Corp., QA Merger LLC, Q-RNA, Inc.,
and
Dr. David Dantzker, as the Representative of the Q-RNA, Inc. security
holders.
|
|
8-K
|
|
2.1
|
|
12/5/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1
|
|
Certificate
of Incorporation of Neurotech Pharmaceuticals, Inc.
|
|
8-K
|
|
3.1
|
|
1/23/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2
|
|
Certificate
of Merger of Marco Acquisition I, Inc. with and into Marco Hi-Tech
JV
Ltd.
|
|
8-K
|
|
3.5
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.3
|
|
Certificate
of Merger of Marco Acquisition I, Inc. with and into Marco Hi-Tech
JV
Ltd.
|
|
8-K
|
|
3.6
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.4
|
|
Certificate
of Amendment of Certificate of Incorporation of Neurotech Pharmaceuticals,
Inc., changing name to Neuro-Hitech Pharmaceuticals, Inc.
|
|
8-K
|
|
3.7
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.5
|
|
Certificate
of Ownership and Merger effective August 11, 2006
|
|
8-K
|
|
3.1
|
|
8/11/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.6
|
|
By-laws
of the Company
|
|
8-K
|
|
3.2
|
|
1/23/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.1
|
|
Form
of Common Stock Purchase Warrant Certificate
|
|
8-K
|
|
4.1
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.2
|
|
Warrant
to purchase common stock of Marco-Hitech JV Ltd. issued to Brown
Brothers
Harriman & Co.
|
|
8-K
|
|
4.2
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.3
|
|
Warrant
to purchase common stock of Marco-Hitech JV Ltd. issued to Barry
Honig
|
|
8-K
|
|
4.3
|
|
1/30/06
|
|
|
|
4.4
|
|
Form
of Marco Hi-Tech JV Ltd. Registration Rights Agreement
|
|
8-K
|
|
10.4
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.5
|
|
Registration
Rights Agreement, dated as of November 29, 2006, by and among
Neuro-Hitech, Inc. and David Dantzker as the Representative of the
Q-RNA,
Inc. security holders
|
|
8-K
|
|
4.1
|
|
12/5/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.6
|
|
Registration
Rights Agreement, dated as of November 29, 2006, by and among
Neuro-Hitech, Inc. and individuals and entities that are parties
to the
Securities Purchase Agreement dated as of November 16,
2006
|
|
8-K
|
|
4.2
|
|
12/5/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.7
|
|
Form
of $13 Warrant issued pursuant to the Merger.
|
|
8-K
|
|
4.3
|
|
12/5/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.8
|
|
Form
of $18Warrant issued pursuant to the Merger.
|
|
8-K
|
|
4.4
|
|
12/5/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.9
|
|
Form
of Warrant issued in connection with private offering
|
|
8-K
|
|
4.5
|
|
12/5/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.10
|
|
Stock
and Warrant Purchase Agreement, dated as of November 29, 2007, by
and
among Neuro-Hitech, Inc. and the investors identified therein
|
|
8-K
|
|
4.1
|
|
12/19/07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.11
|
|
Registration
Rights Agreement, dated as of November 29, 2007, by and among
Neuro-Hitech, Inc. and the investors identified therein
|
|
8-K
|
|
4.2
|
|
12/19/07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.12
|
|
Form
of Warrant issued in connection with private offering
|
|
8-K
|
|
4.3
|
|
12/19/07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1
|
|
Neurotech
Pharmaceuticals, Inc. 2006 Incentive Stock Plan
|
|
8-K
|
|
10.1
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.2
|
|
Neurotech
Pharmaceuticals, Inc. 2006 Non-Employee Directors Stock Option
Plan
|
|
8-K
|
|
10.2
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.3
|
|
Form
of Private Placement Subscription Agreement
|
|
8-K
|
|
10.3
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.4
|
|
Securities
Purchase Agreement, dated January 5, 2006, by and between Marco Hi-Tech
JV
Ltd. and the investors signatory thereto
|
|
8-K
|
|
10.5
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.5
|
|
Director
and Officer Indemnification Agreement dated January 24, 2006, between
Neurotech Pharmaceuticals, Inc. and Reuben Seltzer
|
|
8-K
|
|
10.6
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.6
|
|
Director
and Officer Indemnification Agreement dated January 24, 2006, between
Neurotech Pharmaceuticals, Inc. and Alan Kestenbaum
|
|
8-K
|
|
10.7
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.7
|
|
Director
and Officer Indemnification Agreement dated January 24, 2006, between
Neurotech Pharmaceuticals, Inc. and John Abernathy
|
|
8-K
|
|
10.8
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.8
|
|
Director
and Officer Indemnification Agreement dated January 24, 2006, between
Neurotech Pharmaceuticals, Inc. and Mark Auerbach
|
|
8-K
|
|
10.9
|
|
1/30/06
|
|
|
|
10.9
|
|
Technology
License Contract, dated as of June 1, 1997, by and between Mayo
Foundation
for Medical Education and Research and Marco Hi-Tech JV
Ltd.
|
|
8-K
|
|
10.12
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.10
|
|
Clinical
Research Agreement, dated March 1, 2002, by and between Georgetown
University and Marco Hi-Tech JV Ltd.
|
|
8-K
|
|
10.13
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.11
|
|
Offer
Letter, dated January 6, 2006, to John Abernathy from Marco Hi-Tech
JV
Ltd.
|
|
8-K
|
|
10.14
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.12
|
|
Offer
Letter, dated January 5, 2006, to Mark Auerbach from Marco Hi-Tech
JV
Ltd.
|
|
8-K
|
|
10.15
|
|
1/30/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.13
|
|
Development
Agreement dated February 1, 2006, between the Company and Org Syn
Laboratory, Inc
|
|
10-QSB
|
|
10.1
|
|
5/15/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.14
|
|
Development
Agreement dated March 15, 2006, between the Company and Xel
Herbaceuticals, Inc
|
|
10-QSB
|
|
10.2
|
|
5/15/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.15
|
|
Securities
Purchase Agreement, dated as of November 16, 2006, by and among
Neuro-Hitech, Inc. and the investors identified therein.
|
|
8-K
|
|
2.2
|
|
12/5/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.16
|
|
Amendment
No. 1 to 2006 Incentive Stock Plan
|
|
8-K
|
|
4.6
|
|
12/5/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.17
|
|
Amendment
No. 2 to 2006 Incentive Stock Plan
|
|
8-K
|
|
4.7
|
|
12/5/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.18
|
|
Consultant
Agreement, dated as of November 29, 2006, by and between Neuro-Hitech,
Inc., and D.F. Weaver Medical, Inc., Donald F. Weaver, Principal
Consultant.
|
|
8-K
|
|
10.1
|
|
12/5/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.19
|
|
2002
Q-RNA, Inc. Stock Incentive Plan
|
|
S-8
|
|
10.1
|
|
12/13/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.20
|
|
2006
Stock Incentive Plan
|
|
DEF
14A
|
|
|
|
6/05/07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.21
|
|
Non-Management
Directors Deferral Program
|
|
10-QSB
|
|
10.2
|
|
8/08/07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.22
|
|
Officers
Deferral Program
|
|
10-QSB
|
|
10.3
|
|
8/08/07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.23
|
|
Employment
Agreement, dated August 22, 2007, between Neuro-Hitech, Inc. and
Gary
Shearman
|
|
8-K
|
|
10.1
|
|
8/29/07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.24
|
|
Director
and Officer Indemnification Agreement, dated August 22, 2007, between
Neuro-Hitech, Inc. and Gary Shearman
|
|
8-K
|
|
10.2
|
|
8/29/07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.25
|
|
Employment
Agreement, dated December 7, 2007, between Neuro-Hitech, Inc. and
David
Barrett
|
|
8-K
|
|
10.1
|
|
12/11/07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
14.1
|
|
Code
of Ethics
|
|
10-KSB
|
|
14.1
|
|
3/31/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
16.1
|
|
Letter
from Dale, Matheson, Carr-Hilton Labonte, dated as of January 27,
2006
|
|
8-K
|
|
16.1
|
|
2/6/06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
21.1
|
|
Subsidiaries
|
|
10-KSB
|
|
21.1
|
|
4/13/07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23.01
|
|
Consent
of Independent Registered Public Accounting Firm
|
|
|
|
|
|
|
|
X
|
|
31.1
|
|
Certification
of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley
Act of 2002
|
|
|
|
|
|
|
|
X
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.2
|
|
Certification
of the Chief Financial Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002
|
|
|
|
|
|
|
|
X
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.1
|
|
Certification
of the Chief Executive Officer and Chief Financial Officer Pursuant
to 18
U.S.C. 1350, as adopted Pursuant to Section 906 of the Sarbanes-Oxley
Act
of 2002
|
|
|
|
|
|
|
|
X
|
|
Item
14.
|
Principal
Accountant Fees and
Services
|
The
information required by this Item is hereby incorporated herein by reference
to
the Proxy Statement.
NEURO-HITECH,
INC. AND SUBSIDIARIES
INDEX
TO CONSOLIDATED FINANCIAL STATEMENTS
|
|
|
Page
|
|
Report
of Independent Registered Public Accounting Firm
|
|
F-1
|
|
|
|
|
|
Consolidated
Balance Sheet as of December 31, 2007
|
|
F-2
|
|
|
|
|
|
Consolidated
Statements of Operations for the Years Ended December 31, 2007 and
2006
|
|
F-3
|
|
|
|
|
|
Consolidated
Statements of Changes in Stockholders’ [Deficit] Equity
|
|
|
|
for
the Years Ended December 31, 2007 and 2006
|
|
F-4
|
|
|
|
|
|
Consolidated
Statements of Cash Flows for the Years Ended December 31, 2007
and 2006
|
|
F-5
|
|
|
|
|
|
Notes
to the Consolidated Financial Statements
|
|
F-7
|
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the
Board of Directors
Neuro-Hitech,
Inc.
New
York,
New York
We
have
audited the consolidated balance sheet of Neuro-Hitech, Inc. and Subsidiaries
as
of December 31, 2007 and the related consolidated statements of operations,
changes in stockholders' equity and cash flows for each of the two years
in the
period ended December 31, 2007. These financial statements are the
responsibility of the Company's management. Our responsibility is to express
an
opinion on these financial statements based on our audits.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that
we plan
and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement. An audit includes examining,
on a
test basis, evidence supporting the amounts and disclosures in the financial
statements. An audit also includes assessing the accounting principles used
and
significant estimates made by management, as well as evaluating the overall
financial statement presentation. We believe that our audits provide a
reasonable basis for our opinion.
In
our
opinion, the consolidated financial statements referred to above present
fairly,
in all material respects, the financial position of Neuro-Hitech, Inc. and
Subsidiaries as of December 31, 2007 and the results of their operations
and
their cash flows for each of the two years in the period ended December 31,
2007, in conformity with U.S. generally accepted accounting
principles.
|
|
/s/
Moore Stephens, P.C.
|
|
|
MOORE
STEPHENS, P.C.
|
|
|
Certified
Public Accountants
|
New
York,
New York
March
28,
2008
Neuro-Hitech,
Inc. and Subsidiaries
Consolidated
Balance Sheet
|
|
|
As
of
December 31, 2007
|
|
|
ASSETS:
|
|
|
|
|
Current
Assets:
|
|
|
|
|
Cash
and Cash Equivalents
|
|
$
|
6,137,592
|
|
|
Accounts
Receivable
|
|
|
63,300
|
|
|
Inventory
|
|
|
33,821
|
|
|
Prepaid
Expenses
|
|
|
11,861
|
|
|
Total
Current Assets
|
|
|
6,246,574
|
|
|
|
|
|
|
|
|
Property
and Equipment, net
|
|
|
4,248
|
|
|
|
|
|
|
|
|
Other
Assets:
|
|
|
|
|
|
Security
Deposit
|
|
|
13,226
|
|
|
|
|
|
|
|
|
Total
Assets
|
|
$
|
6,264,048
|
|
|
|
|
|
|
|
|
LIABILITIES
AND STOCKHOLDERS' EQUITY:
|
|
|
|
|
|
|
|
|
|
|
|
Current
Liabilities:
|
|
|
|
|
|
Accounts
Payable and Accrued Expenses
|
|
$
|
1,000,399
|
|
|
Total
Current Liabilities
|
|
|
1,000,399
|
|
|
|
|
|
|
|
|
Stockholders'
Equity:
|
|
|
|
|
|
Common
Stock $.001 Par Value, Authorized: 44,999,900, Issued and Outstanding:
14,004,853
|
|
|
14,005
|
|
|
Common
Stock - Class A $.001 Par Value, Authorized: 100, Issued and Outstanding:
0
|
|
|
-
|
|
|
Additional
Paid-in Capital
|
|
|
39,270,951
|
|
|
Deferred
Compensation
|
|
|
(1,190,654
|
)
|
|
Accumulated
Deficit
|
|
|
(32,830,653
|
)
|
|
|
|
|
|
|
|
Total
Stockholders' Equity
|
|
|
5,263,649
|
|
|
|
|
|
|
|
|
Total
Liabilities and Stockholders' Equity
|
|
$
|
6,264,048
|
|
The
accompanying notes are an integral part of these consolidated financial
statements
Neuro-Hitech,
Inc. and Subsidiaries
Consolidated
Statements of Operations
|
|
|
For
the
Years Ended December
31,
|
|
|
|
|
2007
|
|
2006
|
|
|
|
|
|
|
|
|
|
Sales
|
|
$
|
458,870
|
|
$
|
304,240
|
|
|
Cost
of Goods Sold
|
|
|
215,854
|
|
|
155,014
|
|
|
Gross
Profit
|
|
|
243,016
|
|
|
149,226
|
|
|
|
|
|
|
|
|
|
|
|
Operating
Expenses:
|
|
|
|
|
|
|
|
|
Selling,
General and Administrative Expenses
|
|
|
2,561,402
|
|
|
1,765,486
|
|
|
Research
and Development Costs
|
|
|
3,523,954
|
|
|
19,480,501
|
|
|
Share-Based
Compensation
|
|
|
2,427,904
|
|
|
327,835
|
|
|
Amortization
of Deferred Compensation
|
|
|
242,447
|
|
|
130,905
|
|
|
Registration
Payment Arrangement
|
|
|
490,550
|
|
|
-
|
|
|
Total
Operating Expenses
|
|
|
9,246,257
|
|
|
21,704,727
|
|
|
|
|
|
|
|
|
|
|
|
(Loss)
from Operations
|
|
|
(9,003,241
|
)
|
|
(21,555,501
|
)
|
|
|
|
|
|
|
|
|
|
|
Other
Income:
|
|
|
|
|
|
|
|
|
Interest
and Dividend Income
|
|
|
206,804
|
|
|
147,730
|
|
|
Total
Other Income
|
|
|
206,804
|
|
|
147,730
|
|
|
|
|
|
|
|
|
|
|
|
(Loss)
Before Provision for IncomeTaxes
|
|
|
(8,796,437
|
)
|
|
(21,407,771
|
)
|
|
|
|
|
|
|
|
|
|
|
Provision
for Income Taxes
|
|
|
-
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
Net
(Loss)
|
|
$
|
(8,796,437
|
)
|
$
|
(21,407,771
|
)
|
|
|
|
|
|
|
|
|
|
|
Basic
and Diluted (Loss) per Weighted Average Common Shares
Outstanding
|
|
$
|
(0.71
|
)
|
$
|
(2.25
|
)
|
|
|
|
|
|
|
|
|
|
|
Basic
and Diluted Weighted Average - Common Shares
Outstanding
|
|
|
12,351,746
|
|
|
9,528,650
|
|
The
accompanying notes are an integral part of these consolidated financial
statements
Neuro-Hitech,
Inc. and Subsidiaries
Consolidated
Statement of Changes in Stockholders' [Deficit] Equity
|
|
|
Convertible
Preferred
Stock Series A
|
|
Class
A -
Common
Stock
|
|
Common
Stock
|
|
Additional
Paid- In
|
|
Deferred
|
|
Accumulated
|
|
|
|
|
|
|
Shares
|
|
Amount
|
|
Shares
|
|
Amount
|
|
Shares
|
|
Amount
|
|
Capital
|
|
Compensation
|
|
Deficit
|
|
Totals
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance
as of January 1, 2006
|
|
|
12,005
|
|
$
|
12,005
|
|
|
-
|
|
|
-
|
|
|
8,654,112
|
|
$
|
86,541
|
|
$
|
2,478,373
|
|
|
-
|
|
$
|
(2,626,445
|
)
|
$
|
(49,526
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Recapitalization
as of January 18, 2006
|
|
|
(12,005
|
)
|
|
(12,005
|
)
|
|
100
|
|
$
|
-
|
|
|
(1,626,860
|
)
|
|
(79,514
|
)
|
|
91,519
|
|
|
|
|
|
|
|
|
-
|
|
|
Private
Placement of Common Stock, net of issuance costs of
$353,127
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,026,204
|
|
|
3,026
|
|
|
8,152,378
|
|
|
|
|
|
|
|
|
8,155,404
|
|
|
Common
Stock and Warrants Issued in Connection with Q-RNA merger, net
of issuance
costs of $271,394
|
|
|
|
|
|
|
|
|
-
|
|
|
-
|
|
|
1,800,000
|
|
|
1,800
|
|
|
16,532,593
|
|
|
|
|
|
|
|
|
16,534,393
|
|
|
Exercise
of Stock Options
|
|
|
|
|
|
|
|
|
-
|
|
|
-
|
|
|
1,679
|
|
|
2
|
|
|
217
|
|
|
|
|
|
|
|
|
219
|
|
|
Share-Based
Compensation Expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
327,835
|
|
|
|
|
|
|
|
|
327,835
|
|
|
Recognition
of Non-Qualified Stock Options in accordance with Consulting
Agreement
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,309,052
|
|
$
|
(1,309,052
|
)
|
|
|
|
|
-
|
|
|
Amortization
of Deferred Compensation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
130,905
|
|
|
|
|
|
130,905
|
|
|
Net
(Loss)
|
|
|
-
|
|
|
|
|
|
-
|
|
|
|
|
|
-
|
|
|
|
|
|
|
|
|
-
|
|
|
(21,407,771
|
)
|
|
(21,407,771
|
)
|
|
Balance
as of December 31, 2006
|
|
|
-
|
|
$
|
-
|
|
|
100
|
|
$
|
-
|
|
|
11,855,135
|
|
$
|
11,855
|
|
$
|
28,891,967
|
|
$
|
(1,178,147
|
)
|
$
|
(24,034,216
|
)
|
$
|
3,691,459
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Private
Placement of Common Stock, net of issuance costs of
$376,548
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,016,930
|
|
|
2,017
|
|
|
7,000,440
|
|
|
|
|
|
|
|
|
7,002,457
|
|
|
Repurchase
of Class A Common Stock
|
|
|
|
|
|
|
|
|
(100
|
)
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-
|
|
|
Exercise
of Stock Options
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12,059
|
|
|
12
|
|
|
45,812
|
|
|
|
|
|
|
|
|
45,824
|
|
|
Share-Based
Compensation Expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,427,904
|
|
|
|
|
|
|
|
|
2,427,904
|
|
|
Recognition
of Warrants granted in connection with services to be
rendered
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
254,954
|
|
|
(254,954
|
)
|
|
|
|
|
|
|
|
Common
Stock issued in connection with services rendered
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20,000
|
|
|
20
|
|
|
120,980
|
|
|
|
|
|
|
|
|
|
|
|
Remeasurement
of Non-Qualified Stock Options in accordance with Consulting
Agreement
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
38,445
|
|
|
|
|
|
|
|
|
|
|
|
Amortization
of Deferred Compensation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
242,447
|
|
|
|
|
|
242,447
|
|
|
Penalty
shares issued in connection with Registration Rights
Agreement
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100,729
|
|
|
101
|
|
|
490,449
|
|
|
|
|
|
|
|
|
490,550
|
|
|
Net
(Loss)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(8,796,437
|
)
|
|
(8,796,437
|
)
|
|
Balance
as of December 31, 2007
|
|
|
-
|
|
$
|
-
|
|
|
|
|
$
|
-
|
|
|
14,004,853
|
|
$
|
14,005
|
|
$
|
39,270,951
|
|
$
|
(1,190,654
|
)
|
$
|
(32,830,653
|
)
|
$
|
5,263,649
|
|
The
accompanying notes are an integral part of these consolidated financial
statements
Neuro-Hitech,
Inc. and Subsidiaries
Consolidated
Statements of Cash Flows
|
|
|
For
the Years Ended
|
|
|
|
|
December
31,
|
|
|
|
|
2007
|
|
2006
|
|
|
|
|
|
|
|
|
|
Cash
flows used in operating activities:
|
|
|
|
|
|
|
Net
(Loss)
|
|
$
|
(8,796,437
|
)
|
$
|
(21,407,771
|
)
|
|
|
|
|
|
|
|
|
|
|
Adjustments
to Reconcile Net (Loss) to Net Cash (Used In) Operating
Activities:
|
|
|
|
|
|
|
|
|
Acquired
Research and Development Costs
|
|
|
-
|
|
|
16,805,787
|
|
|
Share-Based
Compensation Expense
|
|
|
2,427,904
|
|
|
327,835
|
|
|
Amortization
of Deferred Compensation
|
|
|
242,447
|
|
|
130,905
|
|
|
Registration
Payment Arrangement
|
|
|
490,550
|
|
|
-
|
|
|
Depreciation
Expense
|
|
|
2,998
|
|
|
1,749
|
|
|
Other
Share-Based Selling, General and Administrative Expenses
|
|
|
225,808
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
Change
in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
(Increase)
Decrease in Assets:
|
|
|
|
|
|
|
|
|
Accounts
Receivable
|
|
|
(37,500
|
)
|
|
26,325
|
|
|
Inventory
|
|
|
(2,530
|
)
|
|
(31,293
|
)
|
|
Prepaid
Expenses
|
|
|
(1,166
|
)
|
|
(10,695
|
)
|
|
Deferred
Charges
|
|
|
93,750
|
|
|
(93,750
|
)
|
|
Security
Deposit
|
|
|
-
|
|
|
(13,226
|
)
|
|
Increase
(Decrease) in Liabilities:
|
|
|
|
|
|
|
|
|
Accounts
Payable and Accrued Expenses
|
|
|
(261,707
|
)
|
|
1,045,793
|
|
|
Due
To Affiliate
|
|
|
-
|
|
|
(42,304
|
)
|
|
Net
cash (Used In) Operating activities
|
|
|
(5,615,883
|
)
|
|
(3,260,645
|
)
|
|
|
|
|
|
|
|
|
|
|
Cash
flows from investing activities:
|
|
|
|
|
|
|
|
|
Business
Acquisition and Related Costs
|
|
|
-
|
|
|
(271,394
|
)
|
|
Investment
in Property and Equipment
|
|
|
-
|
|
|
(8,995
|
)
|
|
Net
cash (Used In) Investing activities
|
|
|
-
|
|
|
(280,389
|
)
|
|
|
|
|
|
|
|
|
|
|
Cash
flows from financing activities:
|
|
|
|
|
|
|
|
|
Net
Proceeds from Private Placement Offering of Common Stock
|
|
|
7,002,456
|
|
|
8,155,404
|
|
|
Proceeds
from the Exercise of Options
|
|
|
45,824
|
|
|
219
|
|
|
Net
cash provided by Financing activities
|
|
|
7,048,280
|
|
|
8,155,623
|
|
|
|
|
|
|
|
|
|
|
|
Net
increase in cash and cash equivalents
|
|
|
1,432,397
|
|
|
4,614,589
|
|
|
|
|
|
|
|
|
|
|
|
Cash
and cash equivalents, beginning of years
|
|
|
4,705,195
|
|
|
90,606
|
|
|
|
|
|
|
|
|
|
|
|
Cash
and cash equivalents, end of years
|
|
$
|
6,137,592
|
|
$
|
4,705,195
|
|
|
|
|
|
|
|
|
|
|
|
Cash
Paid For:
|
|
|
|
|
|
|
|
|
Income
Taxes
|
|
$
|
-
|
|
$
|
-
|
|
|
Interest
|
|
$
|
-
|
|
$
|
-
|
|
The
accompanying notes are an integral part of these consolidated financial
statements
Supplemental
Disclosure of Non-Cash Investing and Financing
Activities:
The
Company entered into a service agreement and issued 100,000 warrants measured
at
fair value as of the date the service agreement was ratified by the Company's
Board of Directors. The fair value of these warrants approximated $255,000
and
was recognized as Deferred Compensation in the Stockholders' Equity section
of
the Balance Sheet. During 2007, the Company ratably recognized approximately
$112,000 of expense for the amortization of Deferred Compensation in accordance
with the service term of the agreement.
In
connection with the Q-RNA merger, the Company entered into a consulting
agreement and issued 500,000 non-qualified stock options valued at their
grant
date fair value as of the date of the merger closing. The total fair value
of
these options approximated $1,309,000 and was recognized as Deferred
Compensation in the Stockholders' Equity section of the Balance Sheet.
During
2007, in accordance with vesting terms of the options, the Company remeasured
and recognized approximately $170,000 of expense for the amortization of
Deferred Compensation.
In
connection with the reverse merger into Northern Way Resources - a non-operating
public shell - shareholders of the former privately held company - Marco
Hi-Tech
J.V. LTD - converted their shares of preferred stock into common stock
of the
publicly traded Company for approximately $12,000.
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
[1]
Nature of Operations
Neuro-Hitech,
Inc. (the “Company” or “Neuro-Hitech”) is an early stage pharmaceutical company
focused on developing innovative drugs for the treatment of degenerative
neurological diseases. The Company’s most advanced product candidate, Huperzine
A, recently completed a Phase II clinical trial in the U.S. which tested
Huperzine A for efficacy and safety in the treatment of mild to moderate
Alzheimer’s disease. The Company has also studied the transdermal delivery of
Huperzine A. The Company believes that Huperzine A can effectively be delivered
transdermally because of its low dosage requirement and low molecular weight.
After
receiving the results of the Phase II clinical trial of Huperzine A, the Company
conducted a detailed review of those results. Based on its review, the Company
believes that there is substantial support within the results that Huperzine
A
can become a safe and effective treatment for Alzheimer’s disease. In order to
validate the current information on the compound and review the Company’s
analysis of the results, in March 2008 the Company entered into an
agreement with Numoda Corporation (“Numoda”) pursuant to which Numoda will
review the results and will provide a report to the Company on its findings.
In
view
of the results of the Phase II clinical trial, the Company is currently
reviewing its options for moving forward with its business. Currently, the
Company expects to focus upon the development of collaborative, joint and
strategic alliances and licensing arrangements with one or more pharmaceutical
companies for the further development of Huperzine A and also expects to
consider merger and/or acquisition opportunities. The Company will further
evaluate these opportunities based upon the results of Numoda’s review and
report, and the Company also expects that Numoda will assist the Company in
interpreting and presenting the results of the Phase II clinical trial to
potential partners, licensees and merger or acquisition candidates.
In
addition to Huperzine A, the Company has worked on two pre-clinical development
programs: one for second generation anti-amyloid compounds or disease modifying
drugs for Alzheimer’s disease and, secondly, development of a series of
compounds targeted to treat and prevent epilepsy. The Company’s efforts however,
have principally focused on its primary product.
The
Company has imported and sold inventories of natural Huperzine to vitamin and
supplement suppliers to generate revenues. However, the majority of the
Company’s operations to date have been funded through Company’s private
placement of equity securities.
Liquidity
The
accompanying consolidated financial statements have been prepared on a
going-concern basis, which contemplates the continuation of operations,
realization of assets, and liquidation of liabilities in the ordinary course
of
business. For the year ending December 31, 2007, the Company generated a net
loss of approximately $8.8 million. As of December 31, 2007, the Company has
funded its working capital requirements primarily through the sale of equity
to
founders, institutional and individual investors. Management intends to fund
future operations through entrance into the commercial marketplace as well
as
additional equity or debt offerings.
There
can
be no assurance that the Company will be successful in obtaining financing
at
the level needed for long-term operations or on terms acceptable to the Company.
In addition, there can be no assurance, assuming the Company is successful
in
commercializing its product, realizing revenues and obtaining new equity or
debt
offerings that the Company will achieve profitability or positive operating
cash
flow. The Company is incurring significant losses, which give rise to questions
about its ability to continue as a going concern. The accompanying financial
statements do not include any adjustments that might result from the outcome
of
this uncertainty.
[2]
Summary of Significant Accounting Policies
Principles
of Consolidation
The
consolidated financial statements include the accounts of the Company and its
wholly owned subsidiaries. All inter-company balances and transactions have
been
eliminated. Approximately $459,000 and $6,264,000 of consolidated revenue and
assets, after eliminations, respectively, are based upon the accounts of the
parent and $0 and $0 of consolidated revenue and assets, after eliminations
respectively, of the subsidiaries.
Use
of
Estimates
The
preparation of financial statements in conformity with accounting principles
generally accepted in the United States of America requires management to make
estimates and assumptions that effect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities at the date
of
the financial statements and the reported amounts of revenue and expenses during
the reporting periods. Actual results could differ from those
estimates.
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
Cash
and
Cash Equivalents
Cash
and
cash equivalents consist of highly liquid financial instruments with an original
maturity of three months or less from the date acquired.
Concentrations
of Credit Risk
Financial
statement items which potentially subject the Company to concentrations of
credit risk are cash and cash equivalents and trade accounts receivable arising
from the Company’s normal business activities. To mitigate cash risks, the
Company places its cash with a high credit quality financial institution. At
December 31, 2007, the Company had approximately $6,000,000 in this financial
institution that is subject to normal credit risk beyond federally insured
amounts.
Accounts
Receivable
Accounts
receivable are recorded at invoiced amounts and do not bear interest. The
Company has established guidelines relative to credit ratings and maturities
that seek to maintain stability and liquidity. The Company routinely assesses
the financial strength of its customers and maintains allowances for doubtful
accounts for estimate of the amount of probable credit losses of accounts
receivable balances outstanding. Account balances are charged against the
allowance after all means of collection have been pursued and likelihood of
collection is remote. The Company does not have any off-balance sheet credit
exposure related to its customers. Based on management’s assessment of credit
losses as of December 31, 2007, no allowance for doubtful accounts has
been deemed necessary.
Inventory
Inventory
is stated at the lower of average cost or market.
Property
and Equipment
Furniture,
fixtures and equipment are carried at cost. Depreciation is recorded on the
straight-line method over three years, which approximates their useful
life. Depreciation expense in 2007 and 2006 was $2,998 and $1,749,
respectively.
Routine
maintenance and repair costs are charged to expense as incurred and renewals
and
improvements that extend the useful life of the assets are capitalized. Upon
sale or retirement, the cost and related accumulated depreciation are eliminated
from the respective accounts and any resulting gain or loss is reported in
the
statement of operations. During 2007 and 2006, there were no sales or
retirements of property and equipment.
Earnings
Per Share
The
Company has adopted the provisions of SFAS No. 128. Basic earnings per share
is
computed by dividing income available to common stockholders by the weighted
average number of common shares outstanding during the period. SFAS No. 128
also
requires a dual presentation of basic and diluted earnings per share on the
face
of the statement of operations for all companies with complex capital
structures. Diluted earnings per share reflects the amount of earnings for
the
period available to each share of common stock outstanding during the reporting
period, while giving effect to all dilutive potential common shares that were
outstanding during the period, such as common shares that could result from
the
potential exercise or conversion of securities into common stock.
The
computation of diluted earnings per share does not assume conversion, exercise,
or contingent issuance of securities that would have an antidilutive effect
on
per share amounts [i.e., increasing earnings per share or reducing loss per
share]. The dilutive effect of outstanding options and warrants and their
equivalents are reflected in dilutive earnings per share by the application
of
the treasury stock method which recognizes the use of proceeds that could be
obtained upon exercise of options and warrants in computing diluted earnings
per
share. It assumes that any proceeds would be used to purchase common stock
at
the average market price during the period. Options and warrants will have
a
dilutive effect only when the average market price of the common stock during
the period exceeds the exercise price of the options or warrants.
In
January 2006, the Company merged into a non-operating public shell, analogous
to
a reverse acquisition as more fully described in Note 5 - Capital Stock.
The
shares issued in connection with this transaction are presented as outstanding
for all periods presented.
Basic
earnings (loss) per share reflect the amount of earnings (loss) for the period
attributable to each share of common stock outstanding during the reporting
period. For the year ended December 31, 2007 and 2006, the Company recorded
losses and as a result, the average number of common shares used in the
calculation of basic and diluted loss per share have not been adjusted for
the
effects of potential common shares from unexercised stock options and
warrants.
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
The
basic
and diluted loss per common share outstanding on the Consolidated Statement
of
Operations excludes common shares represented by warrants and options from
the
computation of diluted net loss per share, as they have an anti-dilutive effect
but may dilute earnings per share in the future.
Share-Based
Compensation
Effective
January 1, 2006, the Company adopted SFAS No. 123(R), “Share-Based
Payment,” which requires the Company to record as an expense in its financial
statements the fair value of all stock-based compensation awards. The Company
currently utilizes a standard option pricing model (i.e., Black-Scholes) to
measure the fair value of stock options granted to employees using the “modified
prospective” method. Under the “modified prospective” method, compensation cost
is recognized in the financial statements beginning with the effective date,
based on the requirements of SFAS No. 123(R) for all share-based payments
granted after that date, and based on the requirements of SFAS No. 123(R) for
all unvested awards granted prior to the effective date of SFAS No.
123(R).
Deferred
Compensation
In
accordance with EITF Abstract No. 96-18, “Accounting for Equity Instruments That
Are Issued to Other Than Employees for Acquiring, or in Conjunction with
Selling, Goods or Services,” the Company uses the Black-Scholes
pricing model to value options granted in connection with services
rendered, which are recorded as Deferred Compensation in the Stockholders’
Equity section of the balance sheet and are subsequently remeasured and charged
to the appropriate expense for which the service relates.
Income
Taxes
The
Company follows the provisions of the Statement of Financial Accounting
Standards No. 109, Accounting for Income Taxes (“SFAS 109”). Income taxes are
provided on taxable income at the statutory rates applicable to such income.
Deferred taxes arise from the temporary differences in the basis of assets
and
liabilities for income tax and financial reporting purposes. Deferred tax assets
are reduced by a valuation allowance if it is more likely than not that some
portion or all of the deferred tax asset will not be realized.
Fair
Value of Financial Instruments
The
carrying amounts of financial instruments including cash and cash equivalents,
accounts receivable, other assets, accounts payable and accrued expenses,
approximate fair value due to the relatively short maturity of these
instruments.
Revenue
Recognition
Revenues
from product sales are recognized when products are shipped to the customer
and
risk of loss and transfer of title have carried over to the
customer.
Research
and Development Costs
All
research and development costs are expensed as incurred and include costs paid
to sponsored third parties to perform research and conduct clinical
trials.
Advertising
Expense
The
cost
of advertising is expensed as incurred. During 2007 and 2006, the Company
incurred approximately $0 in advertising costs.
[3]
Property and Equipment
|
|
|
December
31,
2007
|
|
|
Office
Equipment
|
|
$
|
8,995
|
|
|
Less:
Accumulated depreciation
|
|
|
4,747
|
|
|
Net
Office Equipment
|
|
$
|
4,248
|
|
[4]
Operating Lease
The
Company leases office space under a three year lease expiring June 30, 2009.
The
Company is required to pay utilities, insurance and other costs related to
the
leased facilities.
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
|
2008
|
|
|
55,616
|
|
|
2009
|
|
|
28,131
|
|
|
Totals
|
|
$
|
83,747
|
|
The
Company’s lease on its office space has an escalation clause requiring increases
over the lease term. The effect of the escalation clause which requires
straight-line recognition over the lease term has not been recorded in
accordance with SFAS No. 13 “Accounting for Leases” as it has been deemed
immaterial.
[5]
Common Stock
Recapitalization
On
January 18, 2006, Northern Way Resources, Inc., a Nevada corporation
(“Northern-NV”) was merged with and into Northern Way Resources Inc., a Delaware
corporation (“Northern-DE”) for the sole purpose of changing its state of
incorporation from Nevada to Delaware pursuant to an Agreement and Plan of
Merger dated January 12, 2006 (“Reincorporation Merger Agreement”), which was
approved through an action by written consent of a majority of the stockholders
on the same date (“Reincorporation Merger”). Under the terms of the
Reincorporation Merger, each share of Northern-NV was exchanged for one share
of
Northern-DE. In connection with the Reincorporation Merger, Northern-DE changed
its name to Neurotech Pharmaceuticals, Inc. (“Neurotech”).
On
January 24, 2006 Neurotech entered into an Agreement of Merger and Plan of
Reorganization by and among Neurotech, Marco Hi-Tech J.V. Ltd., a privately
held
New York corporation, and Marco Acquisition I, Inc., (“Acquisition Sub”) a newly
formed wholly-owned Delaware subsidiary of Neurotech. Upon closing of the merger
transactions contemplated under the Merger Agreement, Acquisition Sub was merged
with and into Marco, and Marco became a wholly-owned subsidiary of
Neurotech.
On
January 25, 2006, Neurotech filed a Certificate of Amendment to its Certificate
of Incorporation in the State of Delaware in order to change its name to
Neuro-Hitech Pharmaceuticals, Inc.
For
accounting purposes, the acquisition was treated as an issuance of shares for
cash by Marco with Marco as the acquirer. The accounting is identical to that
resulting from a reverse acquisition except that no goodwill or other intangible
assets are recorded. Pro forma information is not presented as of the date
of
this transaction as Neurotech was considered a public shell and
accordingly, the transaction was not considered a business
combination.
Thereafter,
on August 11, 2006, Neuro-Hitech Pharmaceuticals, Inc. amended its Certificate
of Incorporation to change its name to “Neuro-Hitech, Inc.”
Business
Combination
On
November 29, 2006, the Company completed an acquisition by merger of Q-RNA,
Inc. a privately held New York-based biotechnology company. In connection
with
the acquisition, the Company issued (i) 1,800,000 shares of the Company’s common
stock, (ii) warrants to purchase 600,356 shares of the Company’s common stock at
an exercise price of $13 per share, and (iii) warrants to purchase 600,356
shares of the Company’s common stock at an exercise price of $18 per share. The
Company also assumed Q-RNA options outstanding which upon exercise will
be
exercisable into 199,286 shares of the Company’s common stock. The common shares
and warrants were valued at the adjusted close price of the NHPI stock
on the
closing date of the agreement which was $5.60 per share. The issuance costs
related to the Q-RNA Merger totaled approximately $271,000.
Upon
acquisition of Q-RNA, the Company adopted Interpretation No. 4 (FIN),
“
Applicability
of
FASB Statement No. 2 to Business Combinations Accounted for by the Purchase
Method.
”
In
accordance with this interpretation, a substantial portion of the costs
of the
Q-RNA acquisition, $16,805,787 million, was assigned to in-process research
and
development and expensed upon acquisition as management deemed these assets
to
have no alternative future use.
The
unaudited pro forma information below assumes that Q-RNA was acquired in
fiscal
2006 with the impact of charges for in-process research and development
costs being excluded.
The
unaudited proforma information is presented for informational purposes
only and
is not indicative of the results of future operations or results that would
have
been achieved had the acquisition taken place at the beginning of fiscal
year.
|
|
|
For the Year
Ended December
31,
|
|
|
|
|
2006
|
|
|
Total
Revenue
|
|
$
|
304,200
|
|
|
|
|
|
|
|
|
Net
[Loss]
|
|
$
|
(5,402,784
|
)
|
|
|
|
|
|
|
|
Basic
and Diluted [Loss] Per Common Share
|
|
$
|
(0.57
|
)
|
The
acquisition of Q-RNA provided the Company with intellectual property
and a
pipeline of preclinical compounds.
Private
Placement Offerings
Immediately
after the closing of the Merger on January 24, 2006, there were 7,027,252 shares
of Neuro-Hitech Common Stock issued and outstanding and 100 shares of
Neuro-Hitech Class A Common Stock issued and outstanding.
Prior
to
the closing of the Merger, the Company’s predecessor, Marco completed a private
offering in which Marco received total gross proceeds of $996,006, which
after the closing of the merger with the Company, were converted into 664,004
shares of the Company’s common stock. Subsequent to the closing of the Merger,
Neuro-Hitech completed a private offering of 1,750,000 shares of its common
stock and warrants to purchase 437,500 shares of its common stock for $4,375,000
million in cash. The common stock was sold in the offering at $2.50 per share
and the exercise price of the warrants is $5.00 per share. The warrants
expire on January 24, 2009.
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
On
November 29, 2006 Neuro-Hitech closed on the sale in a private offering of
612,200 shares of its common stock and warrants to purchase 306,100 shares
of
its common stock for $3,137,525 in cash. The common stock was sold in the
offering at $5.125 per share and the exercise price of the warrants is $7.00
per
share. The warrants expire on November 29, 2011.
Between
January 2007 and March 2007, the Company received total gross proceeds of
$2,379,005 from the private placement with accredited investors of an aggregate
of 464,196 shares of the Company’s common stock and warrants to purchase 232,098
shares of the Company’s common stock. The common stock was sold in the offering
at $5.125 per share and the exercise price of the warrants was $7.00 per share.
The warrants expire on November 29, 2011.
In
December 2007, the Company received total gross proceeds of $5,000,000 from
the
private placement with accredited investors receiving an aggregate of 1,250,000
shares of the Company’s common stock and warrants to purchase 625,000 shares of
the Company’s common stock. The common stock was sold in the offering at $4.00
per share and the exercise price of the warrants was $7.00 per share. The
warrants expire on December 14, 2012.
In
connection with these private placement offerings, placement agent, legal,
accounting, printing and other costs in the aggregate amount of $376,548 and
$353,127, were charged to Additional Paid-In Capital in the year ended December
31, 2007 and December 31, 2006, respectively.
Registration
Payment Arrangement
During
2007, 100,729 shares of common stock were issued to investors that purchased
common stock and warrants from the Company in the private offerings that the
Company conducted in 2006 and 2007. The shares were issued in accordance with
the provisions of a Registration Rights Agreement dated as of January 5, 2006
between the Company and the purchasers of securities in the 2006 Private
Offering and a Registration Rights Agreement dated November 29, 2006 between
the
Company and the purchasers of securities in the 2007 Private Offering. The
aforementioned registration rights agreements, required the Company to pay
an
amount equal to 1% payable in the Company’s common stock, up to a maximum 6%, of
the aggregate dollar amount of securities purchased by such investors if the
Company did not file a resale registration statement or have such registration
statement declared effective by the dates set forth in each agreement. The
shares of common stock issued to these investors had an aggregate value of
approximately $491,000 and were expensed as a Registration Payment Arrangement
which is reflected in the Selling, General and Administrative Expense caption
of
the 2007 Statement of Operations. The Company issued the maximum 6% of shares
and is under no obligation to issue any additional shares pursuant to the terms
of the registration rights agreements previously described.
Option
Exercises
In
addition to the aforementioned private placements, the Company received
approximately $46,000 and $200 from the exercise of options to purchase
12,059 and 1,679 shares during 2007 and 2006,
respectively.
Class
A
Common Stock
On
April
24, 2007, immediately prior to listing of the Company’s Common Stock on the
NASDAQ Capital Market, the Chief Executive Officer and Executive Vice President
of the Company surrendered all of the Class A Common Stock they owned. The
shares were repurchased for $2 and as of December 31, 2007, no Class A Common
Stock remains outstanding.
[6]
Research and License Agreements
Georgetown
University
In
December 2003, the Company entered into a clinical research agreement, which
was
amended in November 2005 and October 2007, with Georgetown pursuant to which
Georgetown provided the Company with Phase II research. The costs associated
with this agreement totaled $5,336,842 and were partially funded by the National
Institutes of Health. The Company’s portion of the total cost is $4,036,842, and
paid in installments upon the achievement of certain
milestones.
For
the
years ended December 31, 2007 and 2006, the payments made by the Company to
Georgetown under the terms of the clinical research agreement were approximately
$1,183,167 and $952,500, respectively, and the total payments made by the
Company to Georgetown since inception of the agreement were approximately
$3,110,667. These costs are reflected in the Research and Development caption
of
the Statement of Operations.
The
Company expects to make additional payments in 2008 to Georgetown of $926,175
related to an open label extension which extension will expire in June
2008.
Org
Syn
Laboratory, Inc.
On
February 1, 2006, the Company entered into an exclusive development agreement
with Org Syn for the development of synthetic Huperzine A. Org Syn received
an
aggregate of $175,894 upon the execution of the Agreement
.
For
the
years ended December 31, 2007 and 2006, the payments made by the Company to
Org
Syn were approximately $11,375 and $419,500, respectively.
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
For
the
years ended December 31, 2007 and 2006, the payments made by the Company to
Org
Syn were approximately $45,000 and $419,500, respectively, and are reflected
in
the Research and Development caption of the Statement of
Operations.
Xel
Herbaceuticals, Inc.
On
March
15, 2006, the Company entered into a development agreement with Xel
Herbaceuticals, Inc. (“XEL”) for the development of the Huperzine A Transdermal
Delivery System (“Delivery Product”). Under the terms of the agreement, the
Company paid XEL a $250,000 fee upon the execution of the agreement and paid
XEL
$92,500 per month during the development of the Delivery Product, including
the
first six months of 2007. The Delivery Product was completed in July 2007.
The
$250,000 signing fee paid upon the execution of the agreement was amortized
ratably over the 16 month term of the agreement. Through December 31, 2007,
the
remaining $93,750 of unamortized signing fee was charged to the Statement of
Operations and is reflected in the Research and Development Costs caption.
Between August 2007 and December 31, 2007, the Company paid XEL an
additional $92,500 per month to fund the further development of the Product.
The
Company and XEL intend to seek domestic and foreign patent protection for the
Delivery Product.
If
the
Company elects to exercise its right to license the Delivery Product in the
U.S.
and Canada (“North America”) and to develop the Delivery Product on its own, the
Company will pay XEL an initial license fee of $400,000 and up to an aggregate
of $2.4 million in additional payments upon the achievement of certain
milestones, including completion of a prototype, initial submission to the
Food
and Drug Administration (“FDA”), completion of phases of clinical studies and
completion of the FDA submission and FDA approval. Similarly, if the Company
elects to exercise its option to license the Delivery Product worldwide
excluding China, Taiwan, Hong Kong, Macau and Singapore (“Worldwide”), and
develop the Delivery Product on its own, the Company will pay XEL an additional
initial license fee of $400,000 and up to an aggregate of $2.4 million in
additional payments upon the achievement of comparable milestones. If XEL fails
to obtain a U.S. or international patent, the corresponding license fee and
milestone payments will be reduced by 50% until such time as XEL obtains such
patent, at which time the unpaid 50% of all such milestone payments previously
not made will be due. If the Company elects to exercise the licensing rights
described above, the additional payments that the Company has made to XEL to
further develop the Product would be applied toward the additional payments
payable to XEL upon the achievement of certain milestones.
The
Company will also be obligated to pay XEL royalty payments of between 7% and
10%
of net sales, with such royalty payments subject to reduction upon the
expiration of the patent or the launch of a generic product in the applicable
territory. If a patent has not been issued in either the U.S. or Canada, the
royalty payments will be subject to reduced rates of between 3% and 5% of net
sales. Royalty payments for sales in the Worldwide territory will be subject
to
good faith negotiations between the parties.
If
the
Company elects to exercise its right to license the Delivery Product in North
America and to develop the Delivery Product with a third party, the Company
will
pay XEL 50% of any initial signing fees and milestone fees (excluding any
research and development fees) paid by such third party. Similarly, in the
event
that the Company decides to exercise its option to license the Product Worldwide
and to develop the Product with a third party, the Company will pay XEL 50%
of
any initial signing fees and milestone fees (excluding any research and
development fees) paid by such third party. If XEL fails to obtain a U.S. or
international patent, the percentage of the corresponding fees will be reduced
to 25%. The Company will pay XEL 20% of any royalty payments received by the
Company from third-party sublicensees, or if the Product is not protected by
at
least one patent, 10% of any royalty received by the Company from
sublicensees.
If
the
Company elects not to exercise its right to license the Delivery Product and
XEL
elects to further develop the Delivery Product without the Company, XEL will
be
obligated to pay the Company 30% of any net profits realized up to a maximum
of
two times the amount paid by the Company to XEL during development, excluding
the initial $250,000 signing fee. Upon such election, XEL will be entitled
to
full ownership of the intellectual property of the Delivery Product. If the
Company elects to exercise its rights to license the Delivery Product in North
America, but not Worldwide, XEL will have certain rights to obtain intellectual
property protection in any country outside North America upon payment to the
Company for such rights, such fees to be negotiated in good faith by the
parties.
For
the
years ended December 31, 2007 and 2006, the total payments made by the Company
to XEL under this agreement were approximately $1,111,250
and
$1,081,000, respectively, and are reflected in the Research and Development
caption of the Statement of Operations.
Dalhousie
License Agreements (PARTEQ)
As
part
of the acquisition of Q-RNA, the Company assumed exclusive License Agreements
with PARTEQ Research and Development Innovations (“PARTEQ”), the technology
licensing arm of Queens University, Kingston, Ontario, Canada.
The
Exclusive Patent License Agreement with PARTEQ (the “Alzheimer’s Agreement”)
grants the Company an exclusive worldwide license to all innovations and
developments, including the patent applications and additional filings, related
to specified patents related to research on Alzheimer’s disease. The Company
made a one-time license fee of C$25,000 when it entered into the Alzheimer’s
Agreement in 2005 and will be required to make quarterly royalty payments of
3%
of net sales of the licensed products (as such term is defined in Alzheimer’s
Agreement), with a minimum annual royalty of C$10,000 for 2007, C$20,000 for
2008, C$30,000 for 2009 and C$40,000 for 2010 and each subsequent calendar
year.
The Company is also obligated to make the following milestone payments:
C$100,000 upon completion of a Phase I trial of a licensed product, C$250,000
upon completion of a Phase II trial of a licensed product, and C$1,000,000
upon
the first FDA approval (as such term is defined in the Alzheimer’s Agreement).
The Company also has the right to sub-license with the payment of 20% of all
non-royalty sublicensing consideration.
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
Under
the
terms of the Alzheimer’s Agreement which was amended in early 2007, the Company
will pay fixed annual fees of C$282,944.
The
Exclusive Patent License Option Agreement with PARTEQ (the “Epilepsy Agreement”)
grants the Company an option to acquire an exclusive worldwide license to all
innovations and developments, including the patent applications and additional
filings, related to specified patents related to research on Epilepsy. The
Company made a non-refundable, non-creditable option payment of $10,000 when
it
entered into the Epilepsy Agreement in 2006.
The
Company made an additional payment of $45,000 to PARTEQ in the year ended
December 31, 2007 for expenses that should have been paid by Q-RNA in the year
ended December 31, 2005.
If the Company exercises its option, the
Company will make a non-refundable, non-creditable license payment of C$17,500
at the time of such exercise. If the Company exercises its option, it will
be
required to make quarterly royalty payments of 3% of net sales of the licensed
products (as such term is defined in Epilepsy Agreement), with a minimum annual
royalty of C$10,000 through the second anniversary of the license, C$20,000
through the third anniversary of the license, C$30,000 through the fourth
anniversary of the license and C$40,000 through the fifth anniversary of the
license and each subsequent anniversary. If the Company exercises its option,
the Company is also obligated to make the following milestone payments:
C$100,000 upon completion of a Phase I trial of a licensed product, C$250,000
upon completion of a Phase II trial of a licensed product, and C$1,000,000 upon
the first FDA approval (as such term is defined therein). If the Company
exercises its option, the Company also has the right to sub-license with the
payment of 20% of all non-royalty sublicensing consideration.
Under
the
terms of the Epilepsy Agreement which was amended in early 2007, the Company
will pay fixed annual fees of C$150,800.
For
the
years
ended
December 31, 2007 and 2006, the payments made by the Company to PARTEQ under
these agreements have been approximately $457,115 and $48,600,
respectively, and are reflected in the Research and Development caption of
the
Statement of Operations.
The
following chart estimates the fixed annual research and development costs of
the
Company, excluding any exercise of the option under the Epilepsy Agreement
and
any milestone payments to Numoda or XEL.
Mayo
Foundation
The
Company holds a license for two composition of matter patents and four process
patents for Racemic Huperzine A, Huperzine A and their analogues and derivatives
from the Mayo Foundation. The Company has a license to the four process patents
pursuant to a Technology License Contract with the Mayo Foundation (the “Mayo
Licensing Agreement”). For the years ended December 31, 2007 and 2006, the total
research and development costs incurred by the Company under this Agreement
have been approximately $18,888 and $205,000, respectively. The total costs
accrued and incurred since inception of the agreement were approximately
$350,000 and are reflected in the Research and Development caption of the
Statement of Operations
.
Although the Company has accrued certain costs
relating to the Mayo Licensing Agreement, because of uncertainty between the
Company and Mayo over the interpretation of the agreement, it is uncertain
when
or whether those expenses actually will be paid.
|
Year
|
|
Amount
|
|
|
2008
|
|
$
|
1,416,545
|
|
|
2009
|
|
|
20,000
|
|
|
2010
|
|
|
30,000
|
|
|
2011
|
|
|
40,000
|
|
|
Total
|
|
$
|
1,506,545
|
|
See
Note 13 for description of a subsequent event related to the License Agreement
with Numoda.
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
[7]
Employee, Director and Third Party Stock-Based Compensation
As
of
December 31, 2007 the Company had two stock-based compensation plans, which
are
described below. Effective January 1, 2006, the Company adopted the fair value
method of recording stock-based compensation in accordance with the modified
prospective method of SFAS No. 123(R), “Share-Based Payment.”
The
Company’s Incentive Plan provides for the issuance of options and other
equity-based awards for the Company’s common stock. Shares issued upon exercise
of equity-based awards are shares held in treasury that have been reserved
for
issuance under the plan. The Company’s Incentive Plan is administered by the
Company’s board of directors, or a committee appointed by the Board, which
determines recipients and types of awards to be granted, including the number
of
shares subject to the awards, the exercise price and the vesting schedule.
Options generally have an exercise price equal to the fair market value of
the
Company’s common stock as of the grant date.
Non-Employee
Stock Option Plan
On
January 24, 2006, Neuro-Hitech’s shareholders approved the Company’s 2006
Non-Employee Directors Stock Option Plan (the “Directors Plan”). Key features of
this Plan include:
|
·
|
Non-employee
directors of the Company and its subsidiaries are eligible to participate
in the Directors Plan. The term of the Directors Plan is ten years.
400,000 shares of common stock have been reserved for issuance under
the
Directors Plan.
|
|
·
|
Options
may only be issued as non-qualified stock
options.
|
|
·
|
Stockholder
approval is required in order to replace or reprice
options.
|
|
·
|
The
Directors Plan is administered by the a committee designated by the
board.
|
|
·
|
Options
shall be granted within ten years from the effective
date.
|
|
·
|
Upon
a “change in control” any unvested options shall vest and become
immediately exercisable.
|
The
fair
value used in calculating the stock option expense has been estimated using
Black-Scholes pricing model which takes into account as of the grant date,
the
exercise price, expected life of the option, contractual term, current price
of
the underlying stock, expected volatility, expected dividends on the stock
and
the risk-free interest rate based on expected term of the option. The risk-free
rate is estimated based on U.S. Treasury security rates for the applicable
terms.
The
following is the average of the data used for the aforementioned
assumptions:
|
Year
Ended December 31,
|
|
Risk-Free
Interest Rate
|
|
Expected
Term
|
|
Expected
Volatility
|
|
Expected
Dividends
|
|
|
2007
|
|
|
3.45
|
%
|
|
|
|
|
5
|
|
|
55.16
|
%
|
|
None
|
|
|
2006
|
|
|
4.45
|
%
|
|
|
|
|
5
|
|
|
48.60
|
%
|
|
None
|
|
A
summary
of the stock option activity under the Company’s Non-Employee Directors Stock
Option Plan during the year ended December 31, 2007 is presented below:
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
|
|
|
Options Outstanding as of 12/31/2007
|
|
Options Exercisable
|
|
|
|
|
Weighted Average
|
|
|
|
Weighted
|
|
|
|
|
Exercise Price Range
|
|
Number of
Shares
Outstanding
|
|
Remaining
Contractual Life
|
|
Exercise Price
Per Share
|
|
As of
12/31/2007
|
|
Average
Exercise Price
Per Share
|
|
Aggregate
Intrinsic
Value
|
|
|
$0
to $2.50 per share
|
|
|
350,000
|
|
|
2.00
|
|
$
|
2.50
|
|
|
233,334
|
|
$
|
2.50
|
|
$
|
396,668
|
|
|
|
|
Year Ended December 31, 2007
|
|
|
|
|
Number of
Options
|
|
Weighted
Average Exercise
Price Per Share
|
|
Aggregate
Intrinsic
Value
|
|
|
Outstanding
at December 31, 2006
|
|
|
350,000
|
|
|
2.50
|
|
$
|
595,000
|
|
|
Granted
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
Exercised
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
Expired
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
Outstanding
at December 31, 2007
|
|
|
350,000
|
|
$
|
2.50
|
|
$
|
595,000
|
|
Employee
Stock Option Plan
The
Company’s 2006 Incentive Stock Plan was initially approved by the Company’s
stockholders on January 24, 2006. Amendments to the 2006 Incentive Stock Plan
(as amended, the “Incentive Plan”) were approved by the Company’s stockholders
at a meeting of the Company’s stockholders on June 26, 2007. Key features of the
Incentive Plan include:
|
·
|
Company’s
officers, directors, key employees and consultants of the Company
and its
subsidiaries are eligible to participate in the Incentive Plan. The
term
of the Incentive Plan is ten years. 3,250,000 shares of common stock
are
reserved for issuance under the Incentive
Plan.
|
|
·
|
Both
incentive and nonqualified stock options may be granted under the
Incentive Plan, as well as Stock Appreciation Rights, Restricted
Stock,
Restricted Stock Units and Unrestricted
Stock.
|
|
·
|
The
Incentive Plan terminates on January 23,
2016.
|
|
·
|
The
Incentive Plan is administered by a committee designated by the
board.
|
On
January 24, 2006, the Company's Chief Executive Officer and Executive Vice
President were granted options to purchase 220,000 shares and 70,000 shares
of the Company’s common stock, respectively, each at an exercise price of $2.50
per share. One-third of the shares granted vested on the date of grant and
the
remaining shares vest in equal proportions on the first and second
anniversaries of the grant date.
In
connection with the acquisition of Q-RNA, the Company also entered into a
consulting relationship with Dr. Donald F. Weaver and signed an Employment
Agreement with William McIntosh, as Chief Operating Officer, and William Wong,
as Chief Scientific Officer.
Under
the
terms of the Consultant Agreement, the Company issued 500,000 non-qualified
stock options to Dr. Donald Weaver. These options have a contractual term of
15
years and are exercisable at $5.85 per share.
Vesting
of the options is based on two separate criteria. The first vesting criteria
is
for each successful year of completion of the sponsored research agreement
measured from July to June and commencing in 2006 the consultant will earn
the
right to exercise the purchase of 50,000 options which total 200,000 options
over a four year vesting term.
The
second vesting criteria allows for the ability to earn the right to purchase
the
other 300,000 options measured by achievement of specified milestones in
connection with the sponsored research agreement. There are four milestones
allowing for the right to purchase an additional 75,000 shares,
respectively.
Initial
recognition of the deferred compensation approximated $1.3 million valued using
the Black-Scholes option pricing model and was amortized by approximately
$131,000 through December 31, 2006 due to vesting of the first 50,000 shares
in
accordance with achievement of the first successful year of completion of the
sponsored research agreement from July 2005 through June 2006.
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
On
November 29, 2006, Mr. McIntosh signed an employment agreement and received
options to purchase 300,000 shares of the Company’s common stock, which option
becomes exercisable as to 18,750 shares per quarter beginning as of the date
of
this agreement. The option has an exercise price of $5.85 per share. Pursuant
to
an amendment to Mr. McIntosh’s employment agreement which extended the term
until May 28, 2008, in the event Mr. McIntosh remains employed by the Company
until May 28, 2008, in addition to the vesting set forth above, the option
shall
then be immediately vested as to an additional 37,500 shares.
On
November 29, 2006, Dr. Wong signed an employment agreement and received options
to purchase 150,000 shares of the Company’s common stock, which option became
exercisable as to 9,375 shares per quarter beginning as of the date of this
agreement. The option has an exercise price of $5.85 per share.
Pursuant
to an amendment to Dr. Wong’s employment agreement which extended the term until
May 28, 2008, in the event Dr. Wong remains employed by the Company until May
28, 2008, in addition to the vesting set forth above, the option shall then
be
immediately vested as to an additional 18,750 shares.
On
April
10, 2007, the Company issued options to purchase an aggregate of 100,000
shares
of its common stock to two of its non-employee directors, each at an exercise
price of $5.85 per share.
During
the Year ended December 31, 2007, David Barrett, the Company’s Chief
Financial Officer, was granted various options to purchase the Company’s
common stock. On April 20, 2007, Mr. Barrett was granted an option that was
immediately exercisable as to 60,000 shares at an exercise price of $5.85 per
share. On June 26, 2007, Mr. Barrett was granted an option to purchase 100,000
shares at an exercise price of $7.97
per
share, which shares were immediately vested as to 40,000 shares and will vest
as
to 20,000 shares on May 10, 2008 and an additional 40,000 shares on May 10,
2009. On December 6, 2007, Mr. Barrett was granted an option to purchase 25,000
shares at an exercise price of $3.88 per share, which shares will vest as to
8,333 shares on December 1, 2008, an additional 8,333 shares on December 1,
2009
and 8,334 shares on December 1, 2010.
In
connection with his employment by the Company and in accordance with the terms
of his employment agreement, on August 22, 2007, Dr. Gary Shearman was granted
an option to purchase 800,000 shares of the Company’s common stock at an
exercise price of $5.35 per share. The option to purchase 240,000 shares vested
immediately on August 27, 2007 and the remaining 560,000 shares shall vest
in
equal one-third increments on the first, second and third anniversaries of
the
commencement of the term of his employment.
On
June
26, 2007, the Company issued options to purchase 9,541 shares to each of its
non-employee directors, or an aggregate of 38,164, each at an exercise price
of
$6.25 per share.
During
2007 and 2006, compensation costs on vested awards totaled approximately
$2,428,000 an $328,000, and are reflected in Share-Based Compensation
caption of the Statement of Operations.
During
the year ended December 31, 2007, the Company accrued an expense of $138,308
for
directors’ fees that would have been paid in that year. In lieu of the fees that
would otherwise have been paid, the Company issued options to purchase 53,880
shares of the Company’s common stock to the non-management directors that
elected to receive compensation for their service in the form of options
pursuant to the terms of the Non-Management Directors Deferral Program. The
number of shares underlying the options issued was determined by dividing
the
amount deferred by the Black-Scholes “value” of the last fair market value
option grant made in the preceding fiscal year, as set forth in the Company’s
audited financial statements for that preceding fiscal year. Similarly, the
Company accrued an expense of $21,244 for compensation that would have otherwise
been paid to one of its officers during the year ended December 31, 2007.
In
lieu of compensation that would have been paid in that year, the officer
received options to purchase 8,276 shares of the Company’s common stock pursuant
to the terms of the Officers Deferral Program. The number of shares underlying
the option issued was determined by dividing the amount deferred by the
Black-Scholes “value” of the last fair market value option grant made in the
preceding fiscal year, as set forth in the Company’s audited financial
statements for that preceding fiscal year.
|
|
|
Options Outstanding as of 12/31/2007
|
|
Options Exercisable
|
|
|
|
|
Weighted Average
|
|
|
|
Weighted
|
|
|
|
|
Exercise Price Range
|
|
Number of
Shares Under
Option
|
|
Remaining
Contractual Life
|
|
Exercise Price
Per Share
|
|
As of
12/31/2007
|
|
Average
Exercise Price
Per Share
|
|
Aggregate
Intrinsic
Value
|
|
|
$0
to $2.50 per share
|
|
|
290,000
|
|
|
8.00
|
|
$
|
2.50
|
|
|
193,334
|
|
$
|
2.50
|
|
$
|
328,668
|
|
|
$2.51
to $4.25 per share
|
|
|
56,078
|
|
|
9.96
|
|
$
|
4.06
|
|
|
31,078
|
|
$
|
4.20
|
|
|
-
|
|
|
$4.26
to $6.25 per share
|
|
|
2,079,242
|
|
|
9.01
|
|
$
|
5.68
|
|
|
673,118
|
|
$
|
5.68
|
|
|
-
|
|
|
|
|
|
2,425,319
|
|
|
|
|
|
|
|
|
897,529
|
|
|
|
|
|
328,668
|
|
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
|
|
|
Year Ended December 31, 2007
|
|
|
|
|
Number of
Options
|
|
Weighted
Average Exercise
Price Per Share
|
|
Aggregate
Intrinsic
Value
|
|
|
Outstanding
at December 31, 2006
|
|
|
1,240,000
|
|
$
|
5.07
|
|
|
-
|
|
|
Granted
|
|
|
1,185,319
|
|
$
|
5.46
|
|
|
-
|
|
|
Exercised
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
Expired
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
Outstanding
at December 31, 2007
|
|
|
2,425,319
|
|
$
|
5.26
|
|
|
-
|
|
As
of
December 31, 2007, there was $1,190,654 of total unrecognized compensation
cost
related to nonvested stock-based compensation in connection with a consulting
agreement assumed by the Company pursuant to the Q-RNA merger and a consulting
agreement the Company entered into in 2007 for services to be rendered.
Approximately $131,000 and $111,000 of the amounts recognized as Amortization
of
Deferred Compensation in the 2007 Statement of Operations are in accordance
with
vesting provisions of the consulting agreement entered into in 2006 and the
performance of services pursuant to the consulting agreement entered into
in
2007, respectively. Similarly, all of the amounts reflected in Amortization
of
Deferred Compensation in the 2006 Statement of Operations relate to the vesting
provisions of the consulting agreement entered into in 2006. For the years
ended
December 31, 2007 and 2006, approximately $242,000 and $131,000 was reflected
in
Amortization of Deferred Compensation in the Statement of Operations,
respectively.
Options
Assumed From Q-RNA Merger
As
part
of the Q-RNA merger, the Company assumed Q-RNA options outstanding which upon
exercise will be exercisable into 199,286 shares of the Company’s common stock.
Upon consummation of the Q-RNA Merger, the shares were immediately exercisable
into the Company’s common stock.
|
|
|
Options Outstanding as of 12/31/2007
|
|
Options Exercisable
|
|
|
|
|
Weighted Average
|
|
|
|
Weighted
|
|
|
|
|
Exercise Price Range
|
|
Number of
Shares Under
Option
|
|
Remaining
Contractual Life
|
|
Exercise Price
Per Share
|
|
As of
12/31/2007
|
|
Average
Exercise Price
Per Share
|
|
Aggregate
Intrinsic
Value
|
|
|
$0
to $3.80 per share
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
$3.81
to $9.49 per share
|
|
|
1,210
|
|
|
5.75
|
|
$
|
9.49
|
|
|
1,210
|
|
$
|
9.49
|
|
|
-
|
|
|
$9.50
to $12.66 per share
|
|
|
151,080
|
|
|
5.88
|
|
$
|
12.66
|
|
|
151,080
|
|
$
|
12.66
|
|
|
-
|
|
|
|
|
|
152,290
|
|
|
|
|
|
|
|
|
152,290
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, 2007
|
|
|
|
|
Number of
Options
|
|
Weighted
Average Exercise
Price Per Share
|
|
Aggregate
Intrinsic
Value
|
|
|
Assumed
Upon Acquisition
|
|
|
199,286
|
|
$
|
11.98
|
|
|
-
|
|
|
Exercised
|
|
|
1,679
|
|
|
.13
|
|
$
|
6,797
|
|
|
Expired
|
|
|
(2,049
|
)
|
|
11.44
|
|
|
-
|
|
|
Granted
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding
at December 31, 2006
|
|
|
195,558
|
|
|
12.09
|
|
|
-
|
|
|
Exercised
|
|
|
12,059
|
|
|
3.80
|
|
$
|
4,824
|
|
|
Expired
|
|
|
(31,209
|
)
|
|
12.66
|
|
|
-
|
|
|
Granted
|
|
|
-
|
|
|
|
|
|
|
|
|
Outstanding
at December 31, 2007
|
|
|
152,290
|
|
$
|
12.62
|
|
|
-
|
|
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
The
intrinsic value for the 12,059 and 1,679 Q-RNA options exercised
during the years ended December 31, 2007 and 2006 was $18,330 and $9,486,
respectively.
See
Note 5 for further description of the options assumed by the Company in
connection with the Q-RNA merger.
Warrants
to Non-Employees
Prior
to
the closing of the Merger with Northern Way Resources, Inc., the Company’s
predecessor, the Company granted warrants to purchase an aggregate of 100,000
shares of common stock at an exercise price of $2.50 per share.
In
January 2006, in connection with a private offering of its securities, the
Company granted warrants to purchase an aggregate of 437,500 shares of common
stock at an exercise price of $5.00 per share.
In
November 2006, in connection with a private placement offering, the Company
granted warrants to purchase an aggregate of 306,100 shares of common stock
at
an exercise price of $7.00 per share.
Between
January 1, 2007 and March 15, 2007, the Company granted warrants to purchase
an
aggregate of 232,098 shares of common stock at an exercise price of $7.00
per
share. The warrants expire on November 29, 2011.
During
the year ended December 31, 2007, the Company granted an aggregate of warrants
to purchase an aggregate of 202,581 shares of common stock, 17,670 of which
can
be purchased at an exercise price of $7.00 per share prior to the expiration
of
such warrants on May 18, 2012, 9,911 at an exercise price of $5.13 per share
prior to the expiration of such warrants on February 14, 2012, 75,000 at
an
exercise price of $7.97 per share prior to the expiration of such warrants
on
May 10, 2012 and 100,000 at an exercise price of $7.15 per share prior to
the
expiration of such warrants on February 14, 2011.
On
December 14, 2007, the Company granted warrants to purchase an aggregate
of
625,000 shares of common stock.
The
exercise price of these warrants is $7.00 per share unless the warrants are
exercised prior to the later of (i) April 30, 2008 or (ii) thirty days
after a registration statement registering the shares of common stock underlying
the warrants is declared effective by the Securities and Exchange Commission,
in
which case the exercise price will be $5.00 per share.
The
warrants expire on December 14, 2012.
On
December 14, 2007, the Company adjusted the number of shares issuable upon
the
exercise of warrants to purchase common stock issued in private offerings
between November 2006 and March 2007. These adjustments were made in accordance
with certain anti-dilution adjustment provisions in the issued warrants.
In
accordance therewith, the number of shares of common stock issuable upon
exercise of those warrants will result, if exercised, in the issuance of
an
additional 151,366 shares of the Company’s common stock.
A
summary
of the warrant activity during the year ended December 31, 2007 is presented
below:
|
|
|
Options Outstanding as of 12/31/2007
|
|
Options Exercisable
|
|
|
|
|
Weighted Average
|
|
|
|
Weighted
|
|
|
|
|
Exercise Price Range
|
|
Number of
Shares Under
Option
|
|
Remaining
Contractual Life
|
|
Exercise Price
Per Share
|
|
As of
12/31/2007
|
|
Average
Exercise Price
Per Share
|
|
Aggregate
Intrinsic
Value
|
|
|
$0
to $2.50 per share
|
|
|
100,000
|
|
|
2.00
|
|
$
|
2.50
|
|
|
100,000
|
|
$
|
2.50
|
|
$
|
170,000
|
|
|
$2.51
to $5.00 per share
|
|
|
437,500
|
|
|
3.10
|
|
$
|
5.00
|
|
|
437,500
|
|
$
|
5.00
|
|
|
-
|
|
|
$5.01
to $7.97 per share
|
|
|
1,517,145
|
|
|
4.35
|
|
$
|
7.05
|
|
|
1,517,145
|
|
$
|
7.05
|
|
|
-
|
|
|
*$7.98
to $13.00 per share
|
|
|
600,356
|
|
|
8.92
|
|
$
|
13.00
|
|
|
600,356
|
|
$
|
13.00
|
|
|
-
|
|
|
*$13.01
to $18.00 per share
|
|
|
600,356
|
|
|
8.92
|
|
$
|
18.00
|
|
|
600,356
|
|
$
|
18.00
|
|
|
-
|
|
|
|
|
|
3,225,357
|
|
|
|
|
|
|
|
|
3,225,357
|
|
|
|
|
$
|
170,000
|
|
|
|
|
Year Ended December 31, 2007
|
|
|
|
|
Number of
Warrants
|
|
Weighted
Average Exercise
Price Per Share
|
|
Aggregate
Intrinsic
Value
|
|
|
Outstanding
at December 31, 2006
|
|
|
2,044,312
|
|
$
|
11.34
|
|
|
-
|
|
|
Granted
|
|
|
1,211,045
|
|
$
|
7.06
|
|
|
-
|
|
|
Exercised
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
Expired
|
|
|
(40,000
|
)
|
$
|
0.98
|
|
|
-
|
|
|
Outstanding
at December 31, 2007
|
|
|
3,225,357
|
|
$
|
9.75
|
|
|
-
|
|
*See
Note 5 for further description of the 1,200,712 warrants issued in
connection with the acquisition of Q-RNA.
[8]
Employment Contracts and Consulting Agreements
Under
the
terms of the Q-RNA Merger Agreement, the Company entered into a consulting
agreement with Dr. Donald F. Weaver and employment agreements with William
McIntosh, as Chief Operating Officer, and William Wong, as Chief Scientific
Officer.
Under
the
terms of the Consultant Agreement, Dr. Weaver will serve as a member of the
Company’s Scientific Advisory Board and consult with the Company on matters
pertaining to the research and development of products owned or licensed by
the
Company. Dr. Weaver will also serve as the coordinator and administrator of
any
formal licensing, sponsored research or other agreements as executed by the
Company with Queens University, PARTEQ Innovations and Dalhousie
University.
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
In
accordance with the terms of his Consultant Agreement, Dr. Weaver was eligible
to receive $1,000 per day, pro rata as appropriate for services actually
performed. The term of the Agreement was for one year from November 29, 2006
until November 29, 2007. Dr. Weaver did not receive any compensation in the
years ended December 31, 2006 and December 31, 2007.
L.
William McIntosh became the Company’s Chief Operating Officer on November 29,
2006. Mr. McIntosh’s annualized base salary will be $240,000 per year. Under the
terms of his employment agreement, Mr. McIntosh shall devote, on average, four
days a week to the business affairs of the Company and be compensated
accordingly. The original term of the Agreement expired on November 29, 2007,
but was extended until May 28, 2008 pursuant to an amendment of the
agreement.
For
the
years ended December 31, 2007 and 2006, Mr. McIntosh was paid $192,000 and
$16,000, respectively.
William
Wong became the Company’s Chief Scientific Officer on November 29, 2006. Dr.
Wong’s annualized base salary will be $180,000 per year. Under the terms of his
employment agreement, Dr. Wong shall devote, on average, three days a week
to
the business affairs of the Company and be compensated accordingly. The original
term of the Agreement expired on November 29, 2007, but was extended until
May
28, 2008 pursuant to an amendment of the agreement.
For
the
years ended December 31, 2007 and 2006, Dr. Wong was paid $114,000 and $6,000,
respectively.
On
August
27, 2007, Dr. Gary Shearman became the Company’s Chief Executive Officer,
President and a member of the Company’s board of directors. Dr. Shearman’s base
salary will be $450,000 per year. In addition, Dr. Shearman will be eligible
to
receive bonus compensation of up to 50% of his base salary, in amounts to be
determined by the Compensation Committee of the Board following Dr. Shearman’s
achievement of certain performance objectives. The term of the agreement is
four
years from August 27, 2007, unless earlier terminated or extended and the fixed
and determinable salary to be paid under the agreement is $450,000 or $1,800,000
in the aggregate.
For
the
year ended December 31, 2007, Dr. Shearman was paid $159,750.
David
Barrett, the Company’s Chief Financial Officer, entered into an executive
employment agreement with the Company on December 7, 2007. Under the terms
of
Mr. Barrett’s employment agreement, Mr. Barrett will receive an annual base
salary of $250,000. The agreement was effective December 1, 2007 and expires
on
November 30, 2010. In addition to his base compensation, Mr. Barrett will
be
eligible to receive bonus compensation of up to 25% of his base salary, in
amounts to be determined by the Chief Executive Officer and approved by the
Compensation Committee of the Board following Mr. Barrett’s achievement of
certain performance objectives. For the year ended December 31, 2007, Mr.
Barrett was paid $20,833 pursuant to the terms of his employment agreement.
Mr.
Barrett was paid $191,167 in compensation prior to the effectiveness of his
employment agreement.
The
following table summarizes the annual fixed minimum employment and
consulting agreement obligations of the Company.
|
Year
|
|
Amount
|
|
|
2008
|
|
|
812,000
|
|
|
2009
|
|
|
700,000
|
|
|
2010
|
|
|
700,000
|
|
|
2011
|
|
|
519,417
|
|
|
2012
|
|
|
-
|
|
|
Thereafter
|
|
|
-
|
|
|
Total
|
|
$
|
2,731,417
|
|
See
Note 8 for a description of the option grants.
[9]
Related Party Transaction
During
2006, the Company recorded allocated salary, payroll taxes and other operational
expenses from a company affiliated through common ownership. In March 2006,
the
Company fully repaid the amount due to the affiliate totaling approximately
$45,000.
[10]
Income Taxes
For
2007
and 2006, the Company had no current or deferred income tax provision. Deferred
taxes are based upon differences between the financial statement and tax basis
of assets and liabilities and available net operating loss carryforwards
summarized as follows:
|
|
|
December
31,
|
|
|
|
|
2007
|
|
2006
|
|
|
Deferred
Tax Asset - Non-Current:
|
|
|
|
|
|
|
Net
Operating Loss Carryforwards
|
|
$
|
3,613,600
|
|
$
|
2,475,600
|
|
|
Valuation
Allowance
|
|
|
(
3,613,600
|
)
|
|
(
2,475,600
|
)
|
|
|
|
$
|
-
|
|
$
|
-
|
|
A
full
valuation allowance has been established due to the uncertainty about the
realization of the deferred tax asset. The net change during 2007 and 2006
in
the total valuation allowance was approximately $1,138,000 and $1,439,600,
respectively.
As
of
December 31, 2007, the Company has net operating loss carry-forwards of
approximately $9,034,000. The Company’s net operating loss carry-forwards as
of December 31, 2007 expire as set forth in the following table.
|
Year
of Expiration
|
|
Amount
|
|
|
2012
|
|
$
|
153,000
|
|
|
2021
|
|
|
21,000
|
|
|
2022
|
|
|
113,000
|
|
|
2023
|
|
|
634,000
|
|
|
2024
|
|
|
713,000
|
|
|
2025
|
|
|
955,000
|
|
|
2026
|
|
|
3,600,000
|
|
|
2027
|
|
|
2,845,000
|
|
|
|
|
$
|
9,034,000
|
|
|
For
the Years Ended December 31,
|
|
2007
|
|
2006
|
|
|
Federal
statutory tax rate
|
|
|
-35.0
|
%
|
|
-35.0
|
%
|
|
Changes
to valuation allowance
|
|
|
12.9
|
%
|
|
0.06
|
%
|
|
Effective
state tax rate
|
|
|
0.1
|
%
|
|
0.5
|
%
|
|
Permanent
differences
|
|
|
22.0
|
%
|
|
34.44
|
%
|
|
Effective
Income tax rate
|
|
|
0.00
|
%
|
|
0.00
|
%
|
The
utilization of the net operating losses were limited during 2007 based upon
provisions established in Section 382 of the Internal Revenue Code.
Total
net
operating losses incurred were approximately $6,200,000, however, limitation
of
these losses pursuant to applicable Internal Revenue Code provisions, based
upon
changes in ownership resulted in only $2,800,000 of these losses being available
to offset future taxable income.
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
[11] Supply Concentrations
The
number of available suppliers of natural Huperzine is limited. The Company
does
not have a long-term supply contract with its current suppliers, and purchases
natural Huperzine on a purchase order basis. If the Company is unable to obtain
sufficient quantities of natural Huperzine, when needed, or develop a synthetic
version of Huperzine A in the future, the Company’s ability to pursue its
business plan could be delayed or reduced, or the Company may be forced to
pay
higher prices for the natural Huperzine.
[12] Recent
Accounting Pronouncements
In
February 2007, the Financial Accounting Standards Board (FASB) issued SFAS
No.
159, “The Fair Value Option for Financial Assets and Financial Liabilities -
Including an Amendment of FASB Statement No. 115”. This statement permits
entities to choose to measure many financial instruments and certain other
items
at fair value. Most of the provisions of SFAS No. 159 apply only to entities
that elect the fair value option. However, the amendment to SFAS No. 115
“Accounting for Certain Investments in Debt and Equity Securities” applies to
all entities with available-for-sale and trading securities. SFAS No. 159 is
effective as of the beginning of an entity’s first fiscal year that begins after
November 15, 2007. Early adoption is permitted as of the beginning of a fiscal
year that begins on or before November 15, 2007, provided the entity also elects
to apply the provision of SFAS No. 157, “Fair Value Measurements”. The adoption
of this statement is not expected to have a material effect on the Company’s
financial statements.
In
December 2007, the Financial Accounting Standards Board (FASB) issued SFAS
No. 141 (revised 2007), "Business Combinations" (SFAS 141(R)), which
replaces SFAS No. 141, "Business Combinations." SFAS 141(R) retains the
underlying concepts of SFAS 141 in that all business combinations are still
required to be accounted for at fair value under the acquisition method of
accounting but SFAS 141(R) changed the method of applying the acquisition method
in a number of significant aspects. Acquisition costs will generally be expensed
as incurred; noncontrolling interests will be valued at fair value at the
acquisition date; in-process research and development will be recorded at fair
value as an indefinite-lived intangible asset at the acquisition date;
restructuring costs associated with a business combination will generally be
expensed subsequent to the acquisition date; and changes in deferred tax asset
valuation allowances and income tax uncertainties after the acquisition date
generally will affect income tax expense. SFAS 141(R) is effective on a
prospective basis for all business combinations for which the acquisition date
is on or after the beginning of the first annual period subsequent to
December 15, 2008, with the exception of the accounting for valuation
allowances on deferred taxes and acquired tax contingencies. SFAS 141(R) amends
SFAS 109 such that adjustments made to valuation allowances on deferred taxes
and acquired tax contingencies associated with acquisitions that closed prior
to
the effective date of SFAS 141(R) would also apply the provisions of SFAS
141(R). Early adoption is not permitted. We are currently evaluating the
effects, if any, that SFAS 141(R) may have on our financial statements and
believe it could have a significant impact if business combinations are
consummated. However, the effect is indeterminable as of December 31,
2007.
In
December 2007, the Financial Accounting Standards Board (FASB) issued Financial
Accounting Standards No. 160, "Noncontrolling Interests in Consolidated
Financial Statements—an amendment of ARB No. 51." This statement is
effective for fiscal years, and interim periods within those fiscal years,
beginning on or after December 15, 2008, with earlier adoption prohibited.
This statement requires the recognition of a noncontrolling interest (minority
interest) as equity in the consolidated financial statements and separate from
the parent's equity. The amount of net income attributable to the noncontrolling
interest will be included in consolidated net income on the face of the income
statement. It also amends certain of ARB No. 51's consolidation procedures
for consistency with the requirements of SFAS 141(R). This statement also
includes expanded disclosure requirements regarding the interests of the parent
and its noncontrolling interest. We are currently evaluating this new statement
and anticipate that the statement will not have a significant impact on the
reporting of our results of operations.
In
March
2008, the Financial Accounting Standards Board (FASB) issued FASB Statement
No.
161, Disclosures about Derivative Instruments and Hedging Activities. The new
standard is intended to improve financial reporting about derivative instruments
and hedging activities by requiring enhanced disclosures to enable investors
to
better understand their effects on an entity’s financial position, financial
performance, and cash flows. It is effective for financial statements issued
for
fiscal years and interim periods beginning after November 15, 2008, with early
application encouraged. The Company is currently evaluating the impact of
adopting SFAS. No. 161 on its financial statements.
[13]
Subsequent Events
On
February 1, 2008, the Company announced top-line results from the double-blind
part of a phase 2 clinical trial of Huperzine A in mild to moderate Alzheimer’s
disease (AD) patients.
On
March
3, 2008, the Company entered into an agreement with Numoda corporation
(“Numoda”) pursuant to which Numoda will review the results of the Company’s
recently completed Phase II clinical trial of Huperzine A. After completing
its
review and delivering a report of its findings to the Company, the Company
will
consider its options for moving forward and Numoda will assist the Company
in
interpreting and presenting the results to potential licensing partners,
purchasers and/or acquisition or merger candidates.
NEURO-HITECH,
INC. AND SUBSIDIARIES
NOTES
TO
THE CONSOLIDATED FINANCIAL STATEMENTS
(Continued)
The
terms
of its agreement with Numoda, the Company made an initial payment to Numoda
of
$100,000. The Company will pay Numoda an additional $100,000 upon the delivery
of their analysis. Deferred payments of up to $400,000 will be payable to Numoda
(a “Deferred Payment”) if the Company enters into an agreement for the sale or
license of the Company’s products, or an agreement to merge or sell the Company
(each a “Transaction”). If the aggregate consideration paid to the Company in
such a Transaction is $1,000,000 or less, the Deferred Payment will be $200,000.
If the aggregate consideration paid to the Company in a Transaction is more
than
$1,000,000 but less than $5,000,000, the Deferred Payment will be $250,000.
If
the aggregate consideration paid to the Company in a Transaction is more than
$5,000,000 but less than $10,000,000, the Deferred Payment will be $300,000.
If
the aggregate consideration paid to the Company in the Transaction is more
than
$10,000,000, the Deferred Payment will be $400,000. Additionally, the Company
will be obligated to pay Numoda a fee equal to 3.5% of the aggregate
consideration paid to the Company in a Transaction, provided that the
Transaction is completed at any time during the term of the agreement, or prior
to March 3, 2011, and Numoda has either introduced such party to the Company
or
materially assisted the Company in facilitating such a Transaction.
SIGNATURES
In
accordance with Section 13 or 15(d) of the Exchange Act, the registrant has
duly
caused this report to be signed on its behalf by the undersigned thereunto
duly
authorized.
|
|
NEURO-HITECH,
INC.
(Registrant)
|
|
|
|
|
Date:
March 31, 2008
|
|
|
|
/s/
Gary T. Shearman
|
|
|
Gary
T. Shearman
President,
Chief Executive Officer and
Principal
Executive Officer
|
In
accordance with the Exchange Act, this report has been signed below by the
following persons on behalf of the registrant and in the capacities and on
the
dates indicated.
|
SIGNATURE
|
|
TITLE
|
|
DATE
|
|
|
|
|
|
|
|
/s/
Gary T. Shearman
|
|
President,
Chief Executive Officer,
|
|
March
31, 2008
|
|
Gary
T. Shearman
|
|
Principal
Executive Officer and Director
|
|
|
|
|
|
|
|
|
|
/s/
David Barrett
|
|
Chief
Financial Officer and
|
|
March
28, 2008
|
|
David
Barrett
|
|
Principal
Accounting Officer
|
|
|
|
|
|
|
|
|
|
/s/
John Abernathy
|
|
Director
|
|
March
29, 2008
|
|
John
Abernathy
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Mark
Auerbach
|
|
Director
|
|
March
31, 2008
|
|
Mark
Auerbach
|
|
|
|
|
|
|
|
|
|
|
|
/s/
David
Dantzker
|
|
Director
|
|
March
31, 2008
|
|
David
Dantzker
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Director
|
|
March
__, 2008
|
|
Alan
Kestenbaum
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Director
|
|
March
__, 2008
|
|
Jay
Lombard
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Reuben Seltzer
|
|
Director
|
|
March
28, 2008
|
|
Reuben
Seltzer
|
|
|
|
|
Woodbrook (PK) (USOTC:WDBG)
Historical Stock Chart
From Nov 2024 to Dec 2024
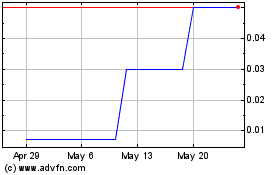
Woodbrook (PK) (USOTC:WDBG)
Historical Stock Chart
From Dec 2023 to Dec 2024