false0001564824NONE00015648242024-10-102024-10-10
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): October 10, 2024 |
Allakos Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-38582 |
45-4798831 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
825 Industrial Road, Suite 500 |
|
San Carlos, California |
|
94070 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 650 597-5002 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.001 |
|
ALLK |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On October 10, 2024, Allakos Inc. (the “Company”) released an updated corporate presentation. A copy of the presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K.
All of the information in this Item 7.01 and Item 9.01 of this Form 8-K, including the attached Exhibit 99.1, is intended to be furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing made by the Company under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Allakos Inc. |
|
|
|
|
Date: |
October 10, 2024 |
By: |
/s/ H. Baird Radford, III |
|
|
|
H. Baird Radford, III
Chief Financial Officer |

Corporate Presentation� October 2024 Developing Therapeutic Antibodies� Targeting Allergic, Inflammatory and Proliferative Disease Exhibit 99.1

Disclaimer This presentation contains forward-looking statements. All statements other than statements of historical fact contained in this presentation, including statements regarding the financial position of Allakos Inc. (“Allakos,” the “Company,” “we” or “our”); estimated lirentelimab closeout, severance and other costs; the timing of payment of restructuring expenditures; estimated ending 2024 cash, cash equivalents and investments; estimated cash runway; business strategy; plans and objectives for future operations; our expectations regarding the potential benefits, activity, effectiveness and safety of our product candidates; our expectations with regard to the initiation, design, timing and results of our clinical studies, preclinical studies and research and development programs, including the timing and availability of data from such studies; our preclinical, clinical and regulatory development plans for our product candidates; and our anticipated milestones are forward-looking statements. Allakos has based these forward-looking statements on its estimates and assumptions and its current expectations and projections about future events. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “target,” “should,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. The forward-looking statements included in this presentation speak only as of the date of this presentation and are subject to a number of risks, uncertainties, and assumptions, including, but not limited to: the Company’s stages of clinical drug development; the Company’s ability to timely initiate and complete clinical trials for AK006; the Company’s ability to obtain required regulatory approvals for its clinical trials; uncertainties related to the enrollment of patients in its clinical trials; the Company’s ability to demonstrate sufficient safety and efficacy of its product candidates in its clinical trials; uncertainties related to the success of clinical trials, regardless of the outcomes of preclinical testing and prior clinical trials; the Company’s ability to advance additional product candidates beyond AK006; the Company’s ability to obtain additional capital to finance its operations; general economic and market conditions; and other risks described in the “Risk Factors” section included in our periodic filings that we have made and will make with the Securities and Exchange Commission (“SEC”). In addition, Allakos operates in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for Allakos’ management to predict all risks, nor can Allakos assess the impact of all factors on its business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements that Allakos may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation are inherently uncertain and may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Accordingly, you should not rely upon forward-looking statements as predictions of future events. Allakos does not undertake any obligation to update or revise any forward-looking statements, to conform these statements to actual results or to make changes in Allakos’ expectations, except as required by law. Accuracy of Data: This presentation contains statistical data based on independent industry publications or other publicly available information, as well as other information based on Allakos’ internal sources. We have not independently verified the accuracy or completeness of the data contained in these industry publications and other publicly available information. Accordingly, Allakos makes no representations as to the accuracy or completeness of that data. Additional Information: The Company has filed and will file Current Reports on Form 8-K, Quarterly Reports on Form 10-Q, and Annual Reports on Form 10-K, and other documents with the SEC. You should read these documents for more complete information about the Company. You may get these documents for free by visiting EDGAR on the SEC website at www.sec.gov. This presentation concerns products that are under clinical investigation, and which have not yet been approved for marketing by the U.S. Food and Drug Administration. It is currently limited by federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated. Allakos and the 3-circles design are federally registered trademarks owned by Allakos Inc. Any unauthorized use is expressly prohibited

Allakos Opportunity Novel Target Milestones Early Q1’25 – Report topline Phase 1 data of AK006 in patients with CSU AK006 (anti-Siglec-6 mAb) selectively inhibits multiple modes of mast cell activation Inhibits IgE-dependent and IgE-independent mast cell activation pathways, including IgE, KIT and MRGPRX2 Depletes mast cells by ADCP in the presence of activated macrophages Upcoming Data Catalysts and Expected Milestones AK006 has the potential to treat a broad range of mast cell driven diseases IV and SC formulations of AK006 achieved high Siglec-6 occupancy on skin mast cells SC Formulation of AK006 has high bioavailability and was well-tolerated AK006 is being tested in Chronic Spontaneous Urticaria (CSU) Significant Need for New Agents
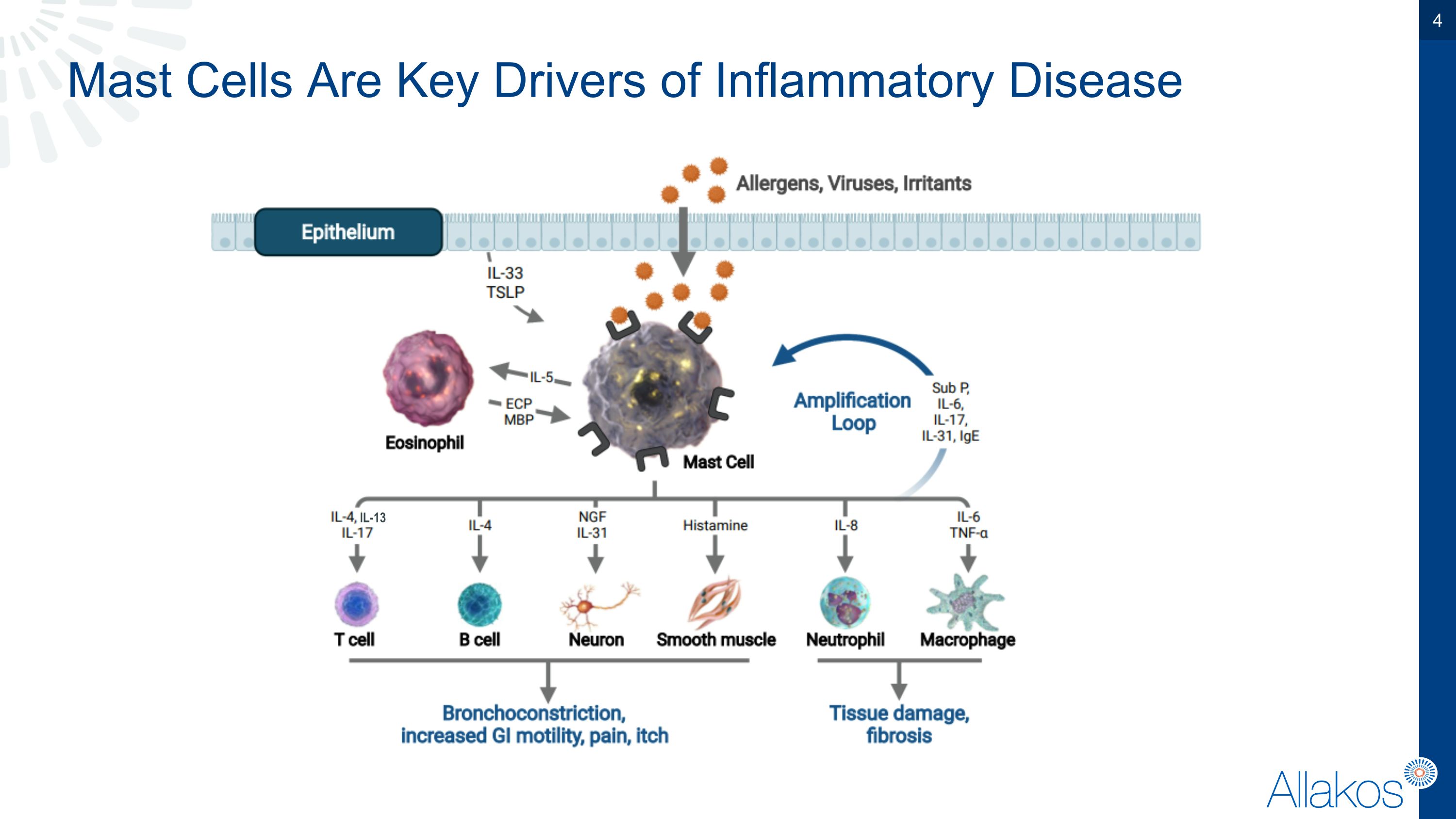
Mast Cells Are Key Drivers of Inflammatory Disease IL-13
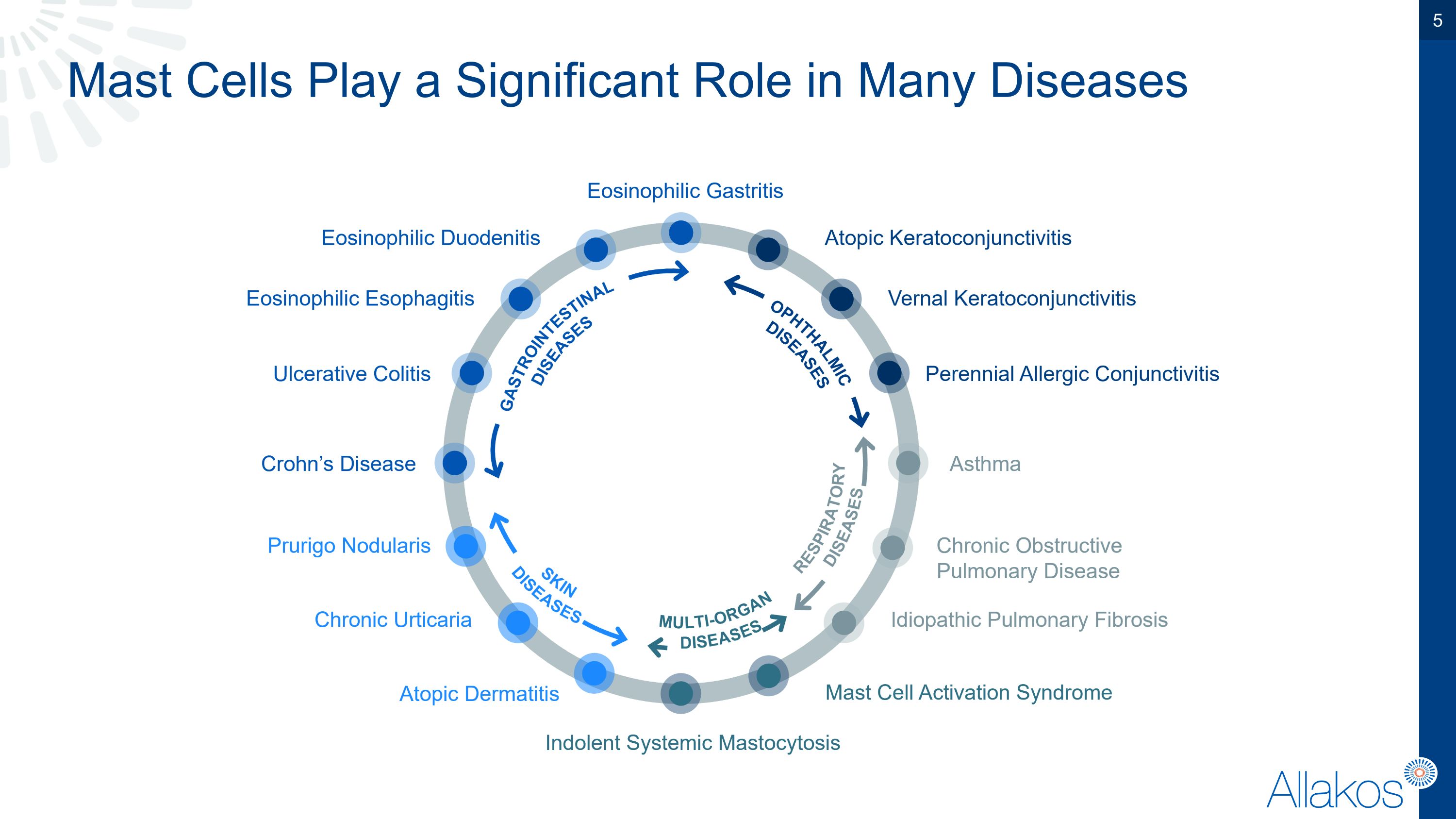
Mast Cells Play a Significant Role in Many Diseases
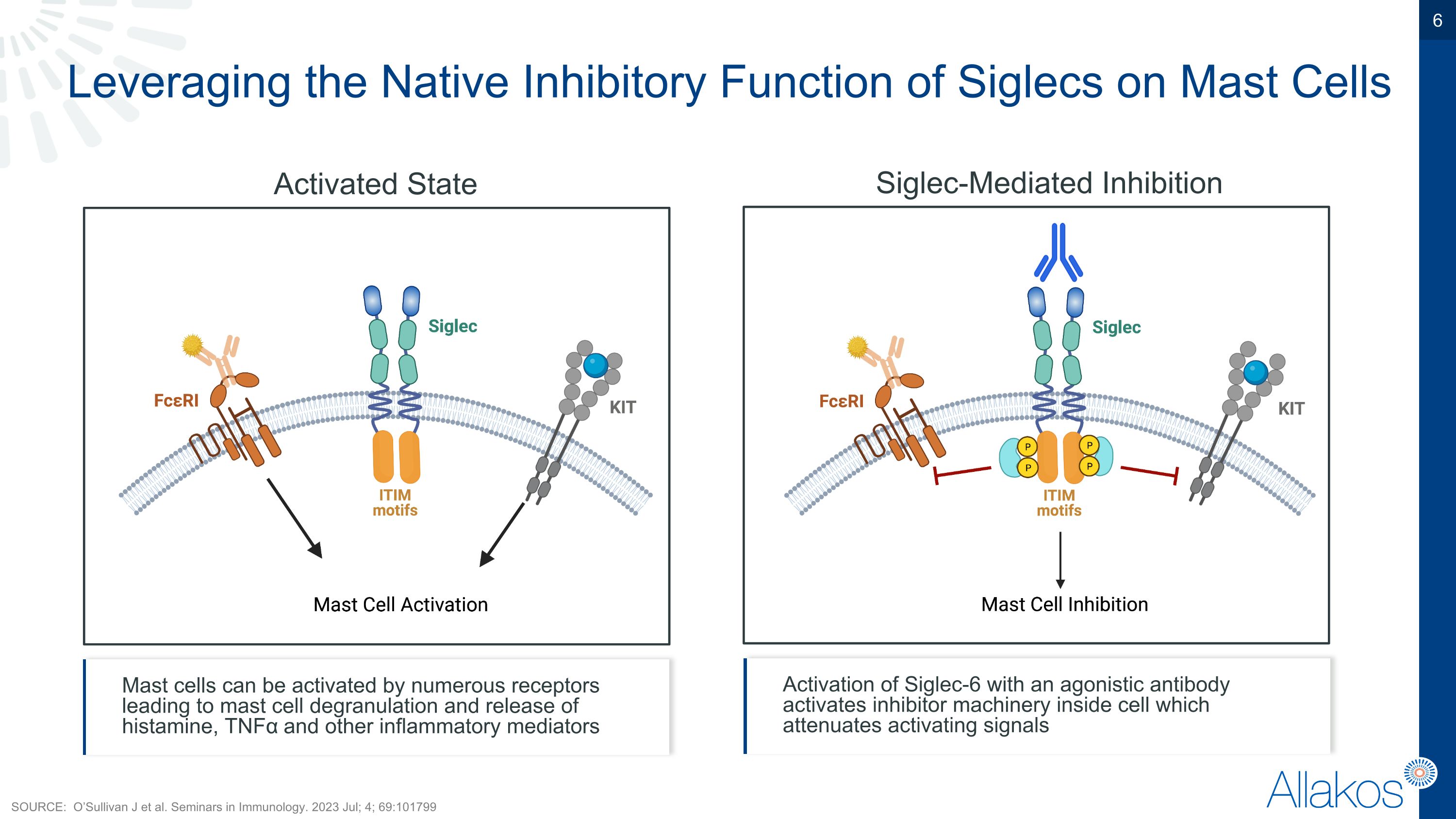
Leveraging the Native Inhibitory Function of Siglecs on Mast Cells Activated State Siglec-Mediated Inhibition Mast cells can be activated by numerous receptors leading to mast cell degranulation and release of histamine, TNFα and other inflammatory mediators Activation of Siglec-6 with an agonistic antibody activates inhibitor machinery inside cell which attenuates activating signals SOURCE: O’Sullivan J et al. Seminars in Immunology. 2023 Jul; 4; 69:101799
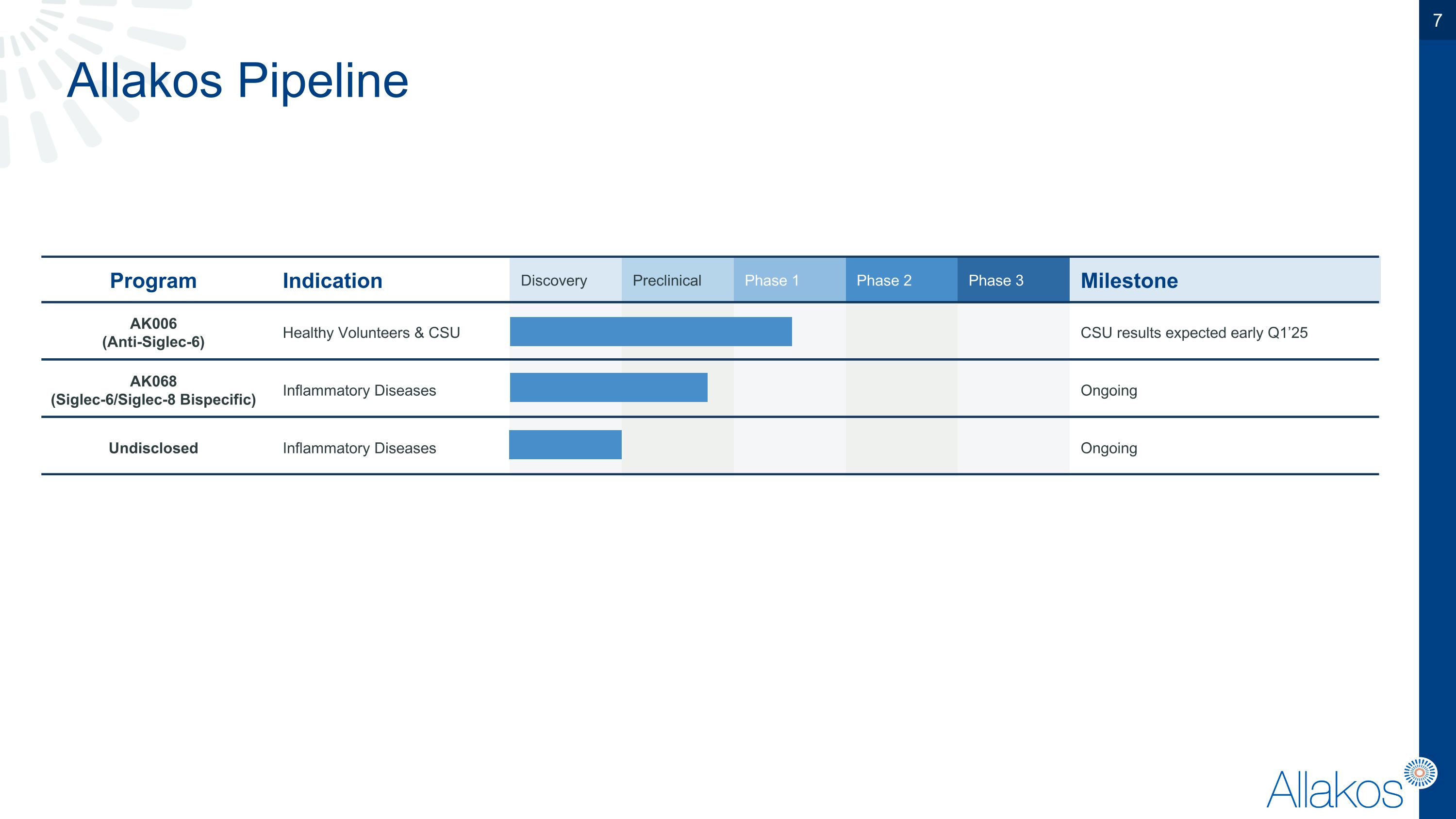
Program Indication Discovery Preclinical Phase 1 Phase 2 Phase 3 Milestone AK006 �(Anti-Siglec-6) Healthy Volunteers & CSU CSU results expected early Q1’25 AK068 (Siglec-6/Siglec-8 Bispecific) Inflammatory Diseases Ongoing Undisclosed Inflammatory Diseases Ongoing Allakos Pipeline

AK006: Siglec-6 mAb that Selectively and Potently Inhibits Mast Cells
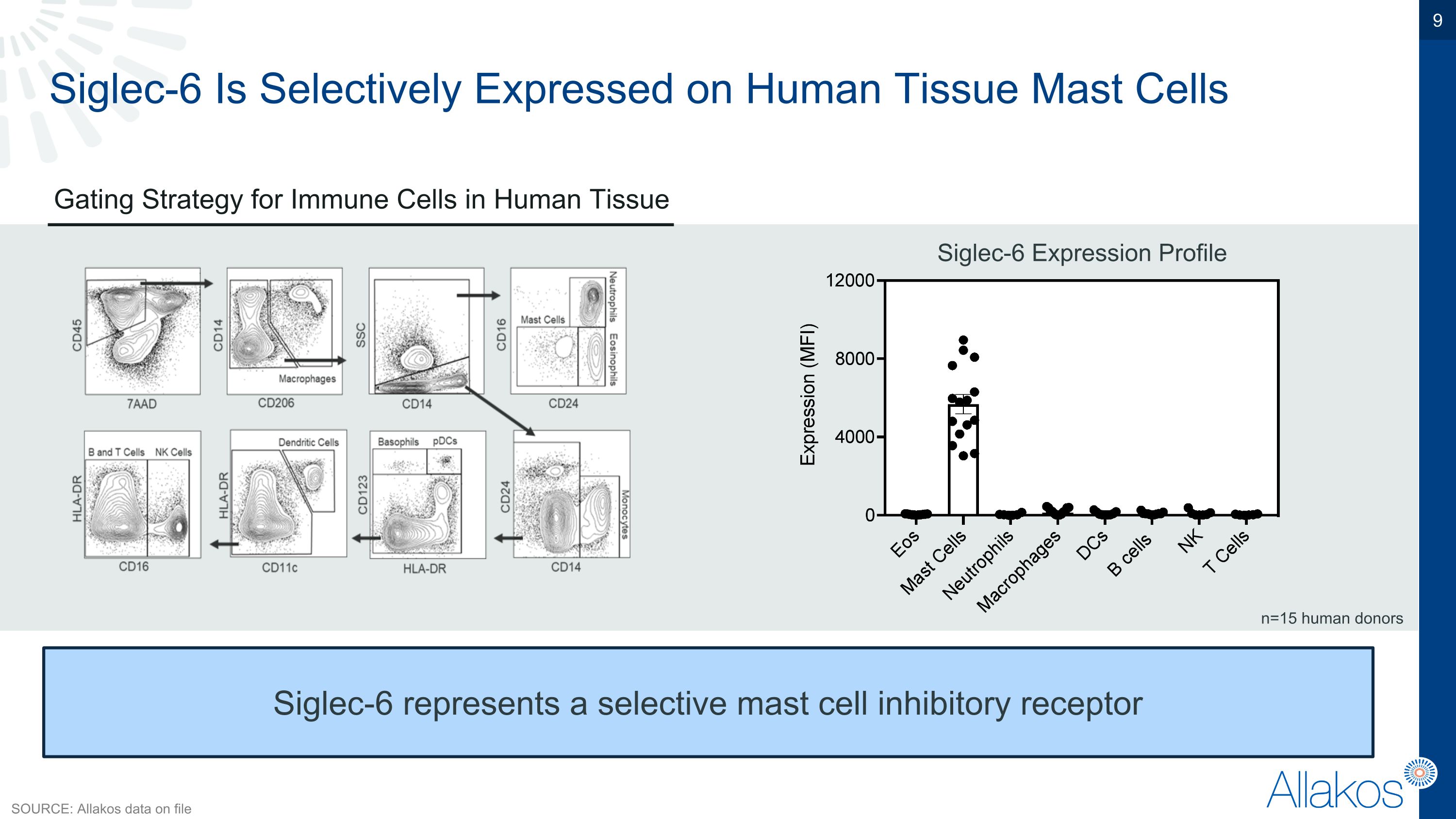
Siglec-6 Is Selectively Expressed on Human Tissue Mast Cells Gating Strategy for Immune Cells in Human Tissue Siglec-6 Expression Profile Siglec-6 represents a selective mast cell inhibitory receptor n=15 human donors SOURCE: Allakos data on file
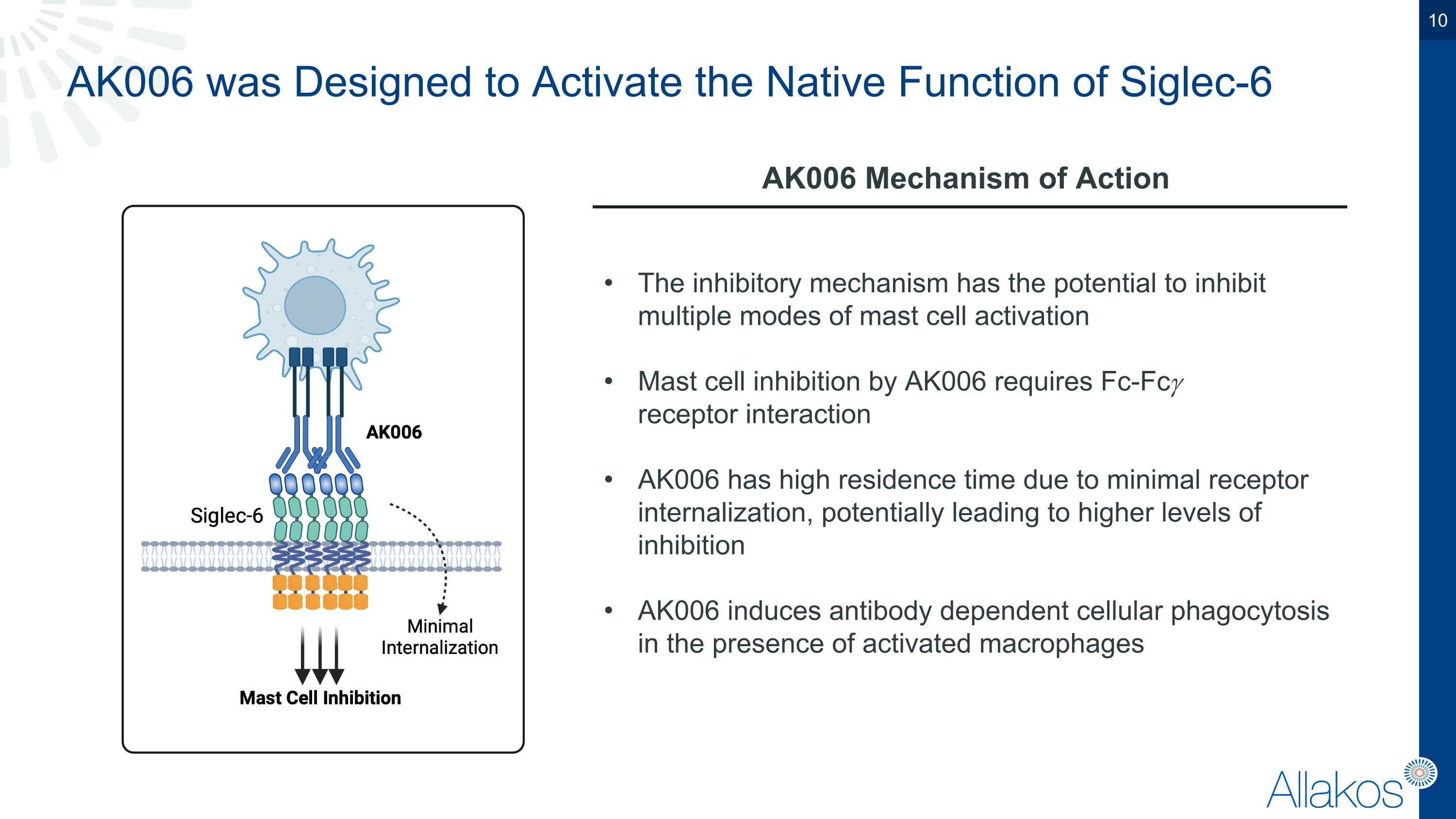
AK006 was Designed to Activate the Native Function of Siglec-6 AK006 Mechanism of Action The inhibitory mechanism has the potential to inhibit multiple modes of mast cell activation Mast cell inhibition by AK006 requires Fc-Fc𝛾 �receptor interaction AK006 has high residence time due to minimal receptor internalization, potentially leading to higher levels of inhibition AK006 induces antibody dependent cellular phagocytosis in the presence of activated macrophages
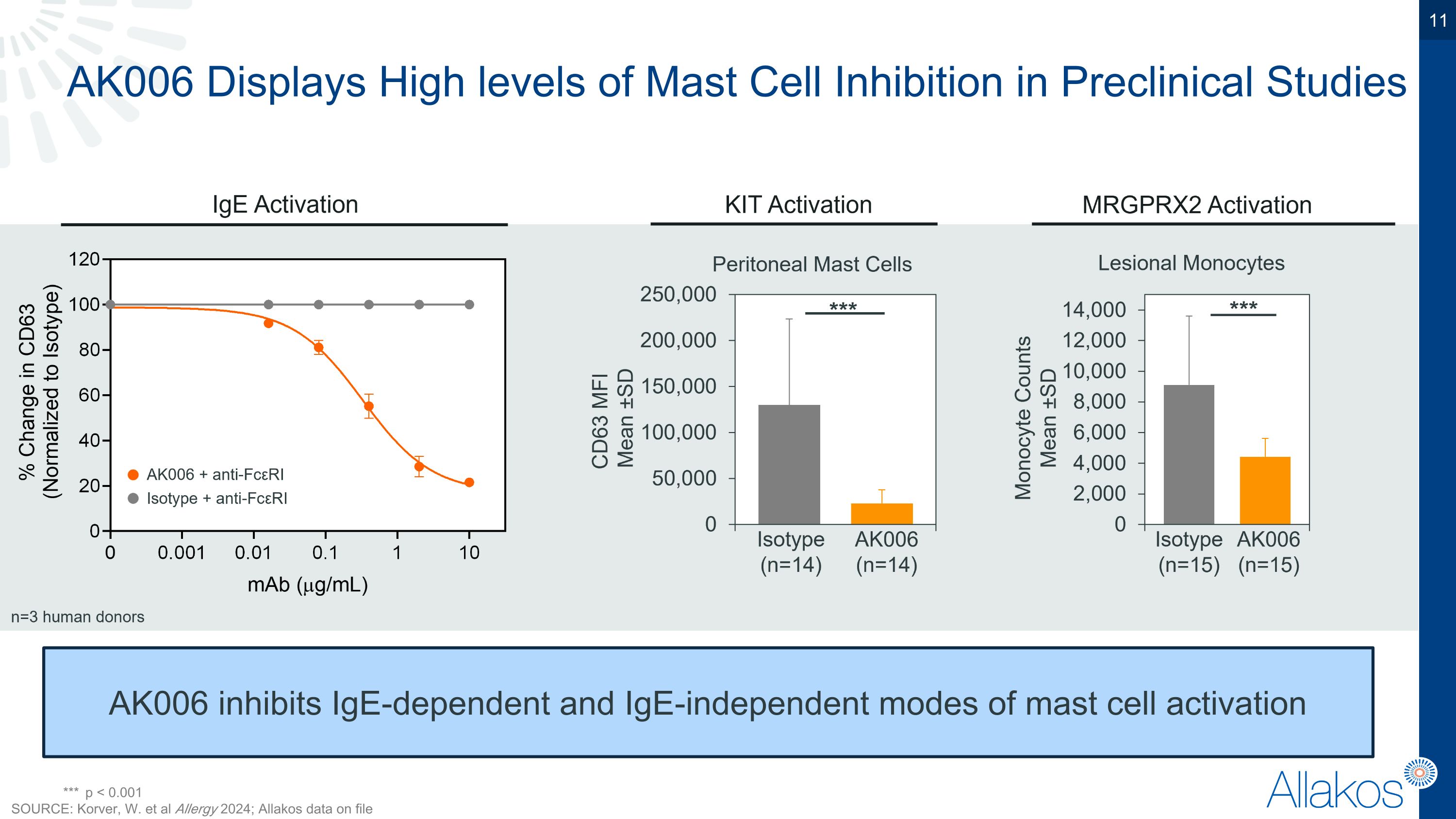
AK006 Displays High levels of Mast Cell Inhibition in Preclinical Studies AK006 inhibits IgE-dependent and IgE-independent modes of mast cell activation *** p < 0.001 SOURCE: Korver, W. et al Allergy 2024; Allakos data on file
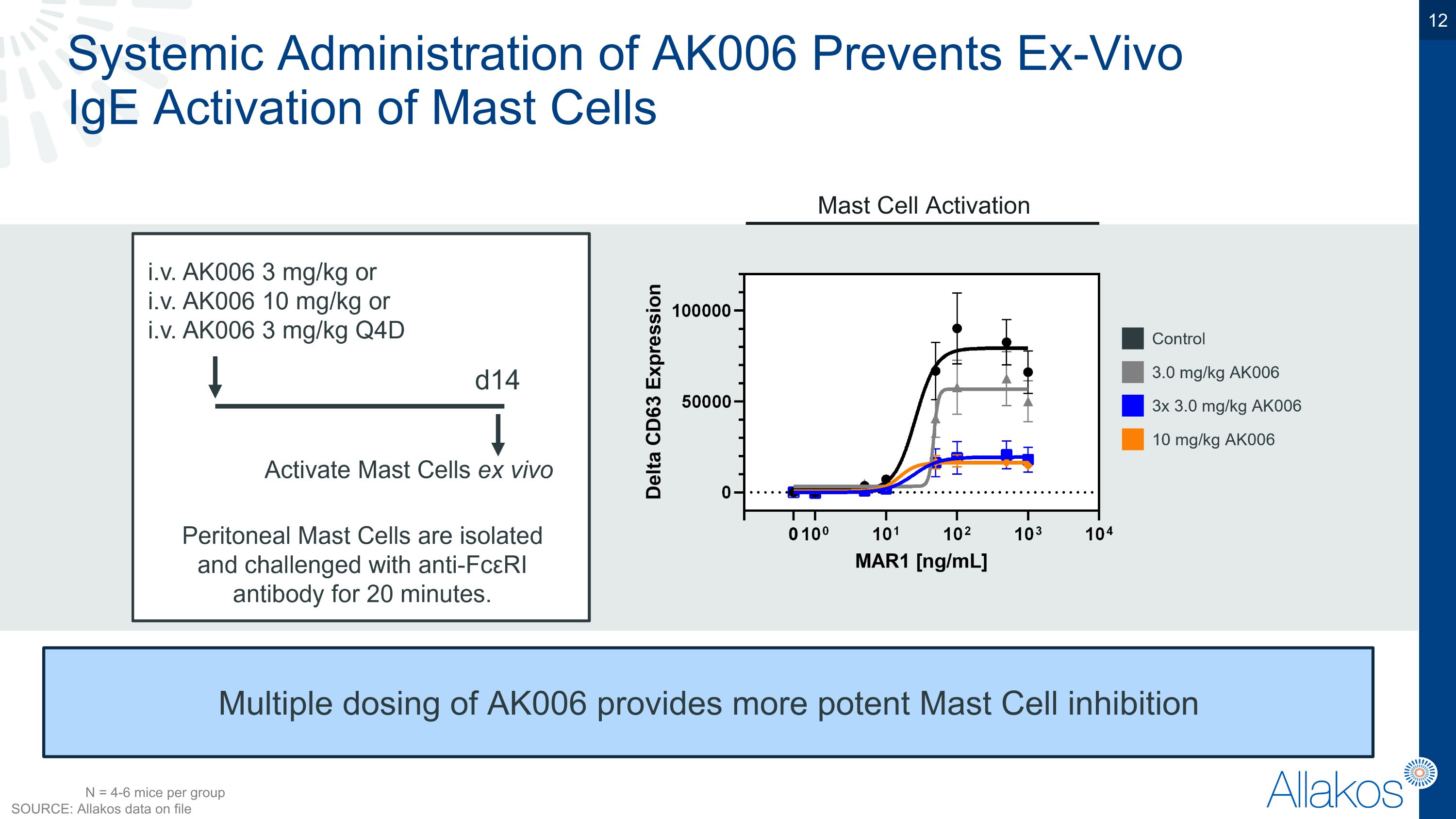
Systemic Administration of AK006 Prevents Ex-Vivo �IgE Activation of Mast Cells Multiple dosing of AK006 provides more potent Mast Cell inhibition Mast Cell Activation N = 4-6 mice per group SOURCE: Allakos data on file
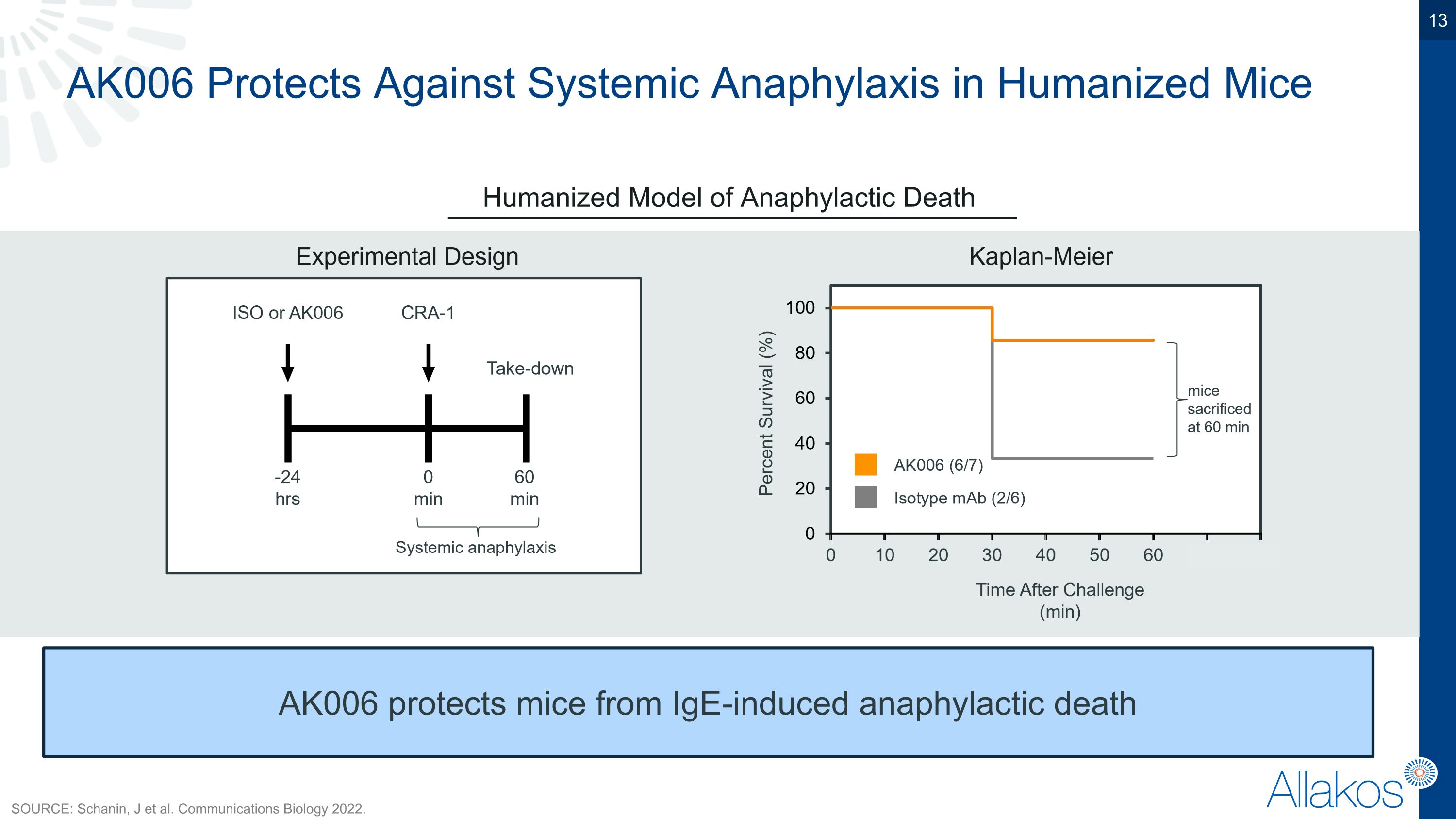
AK006 Protects Against Systemic Anaphylaxis in Humanized Mice Humanized Model of Anaphylactic Death AK006 protects mice from IgE-induced anaphylactic death SOURCE: Schanin, J et al. Communications Biology 2022.
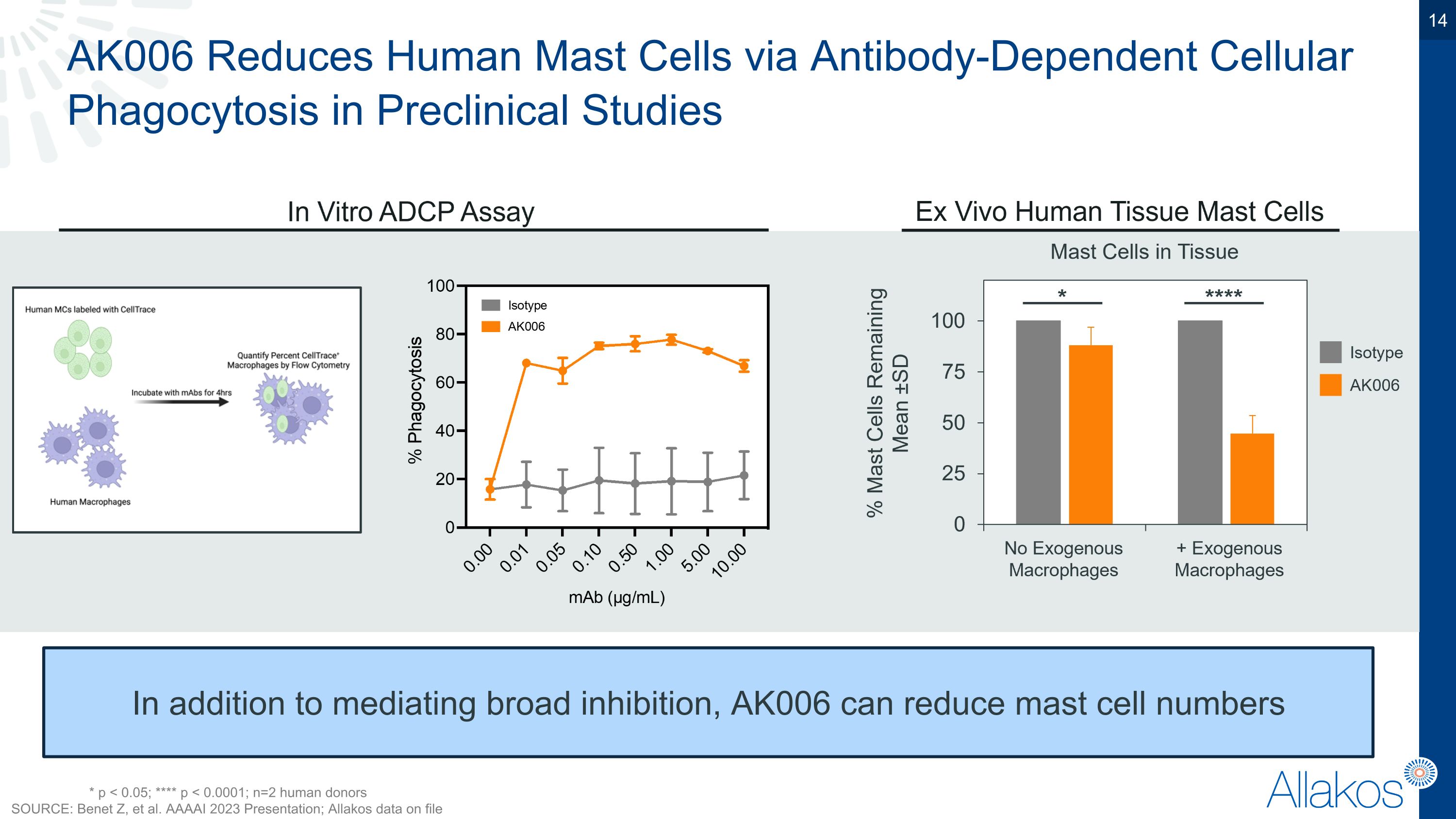
AK006 Reduces Human Mast Cells via Antibody-Dependent Cellular Phagocytosis in Preclinical Studies In addition to mediating broad inhibition, AK006 can reduce mast cell numbers * p < 0.05; **** p < 0.0001; n=2 human donors SOURCE: Benet Z, et al. AAAAI 2023 Presentation; Allakos data on file
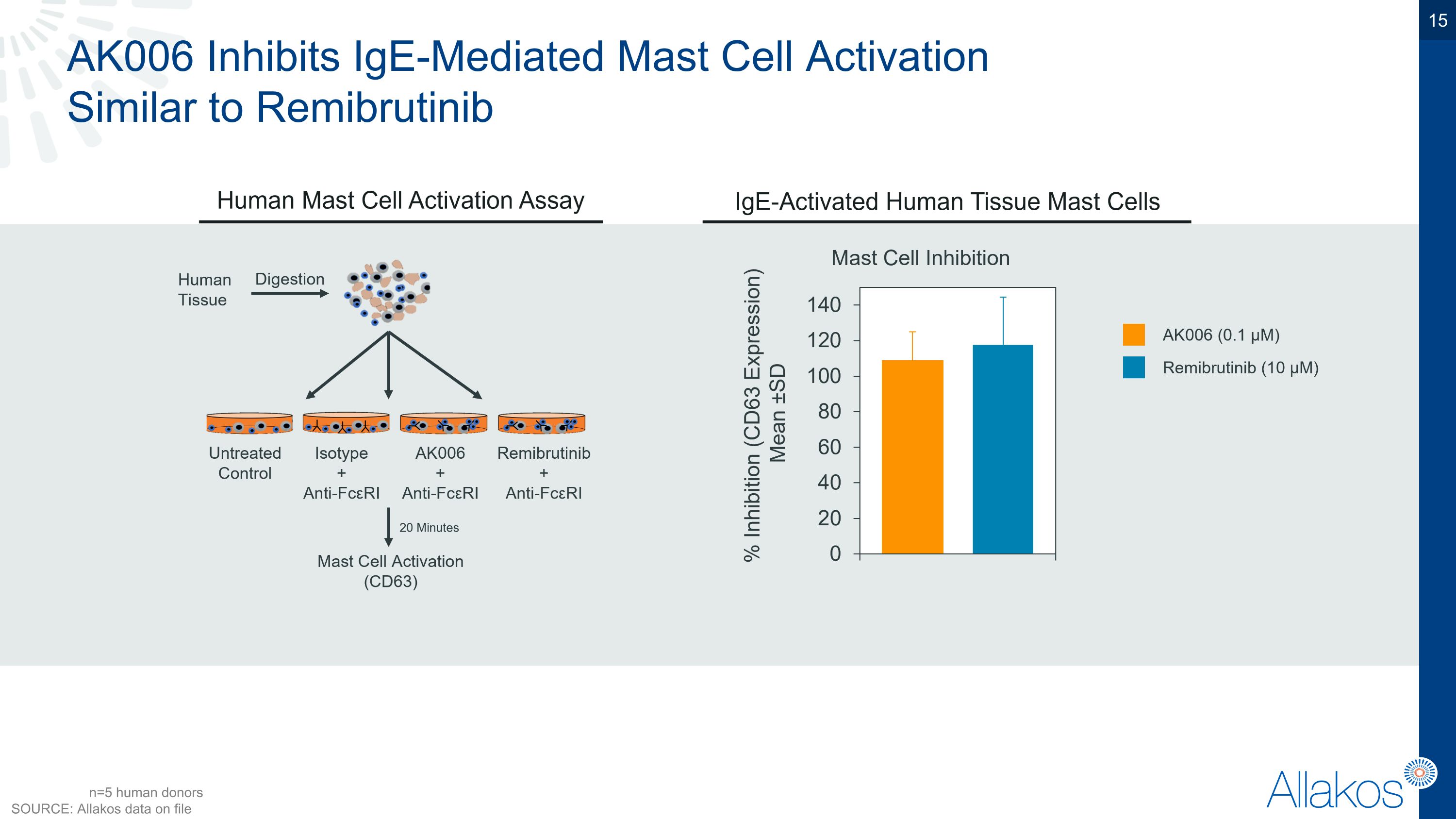
AK006 Inhibits IgE-Mediated Mast Cell Activation �Similar to Remibrutinib n=5 human donors SOURCE: Allakos data on file

AK006 in Phase 1 Clinical Study in Healthy Volunteers and Chronic Spontaneous Urticaria
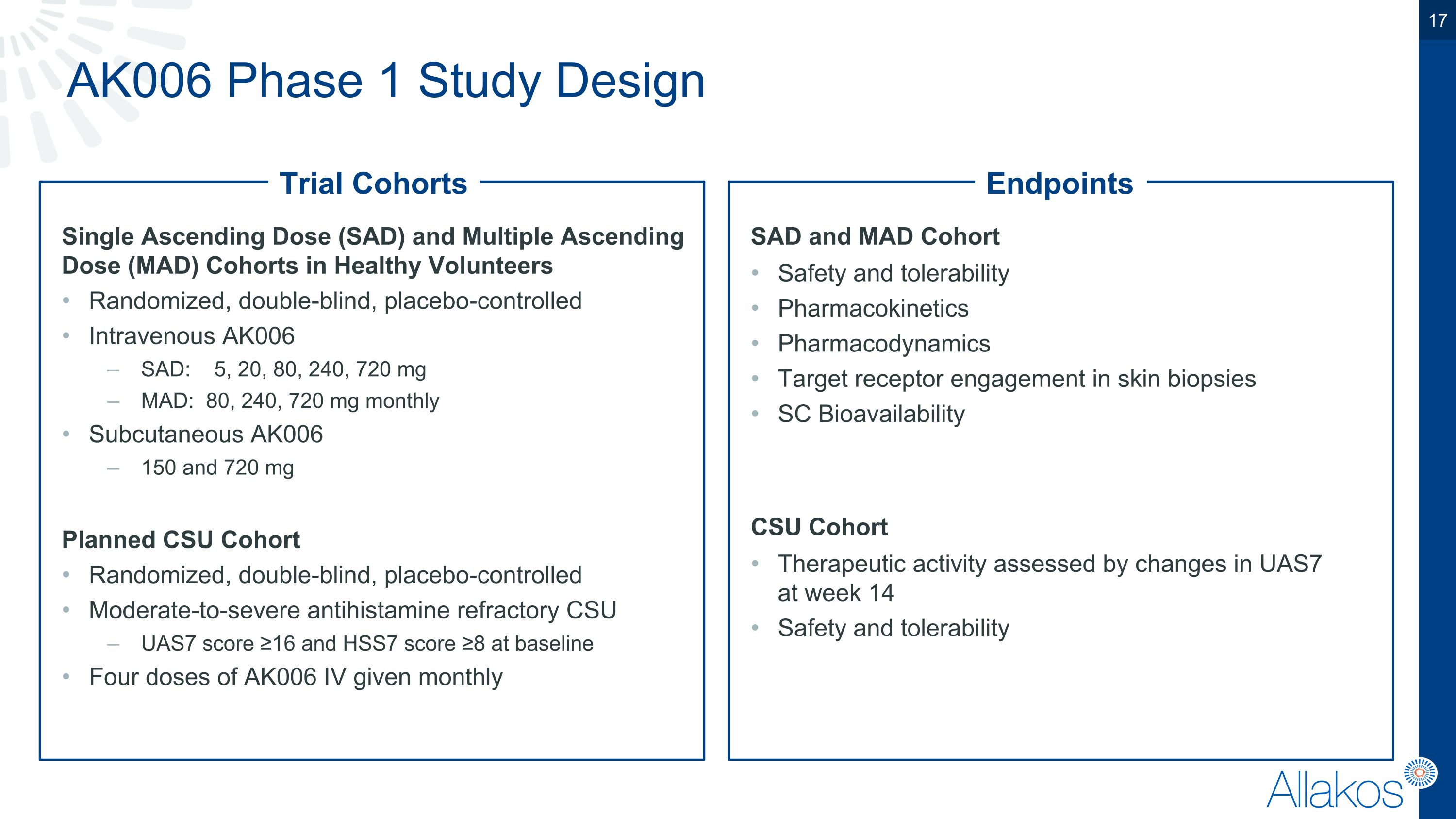
AK006 Phase 1 Study Design Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD) Cohorts in Healthy Volunteers Randomized, double-blind, placebo-controlled Intravenous AK006 SAD: 5, 20, 80, 240, 720 mg MAD: 80, 240, 720 mg monthly Subcutaneous AK006 150 and 720 mg Planned CSU Cohort Randomized, double-blind, placebo-controlled Moderate-to-severe antihistamine refractory CSU UAS7 score ≥16 and HSS7 score ≥8 at baseline Four doses of AK006 IV given monthly SAD and MAD Cohort Safety and tolerability Pharmacokinetics Pharmacodynamics Target receptor engagement in skin biopsies SC Bioavailability CSU Cohort Therapeutic activity assessed by changes in UAS7�at week 14 Safety and tolerability Trial Cohorts Endpoints
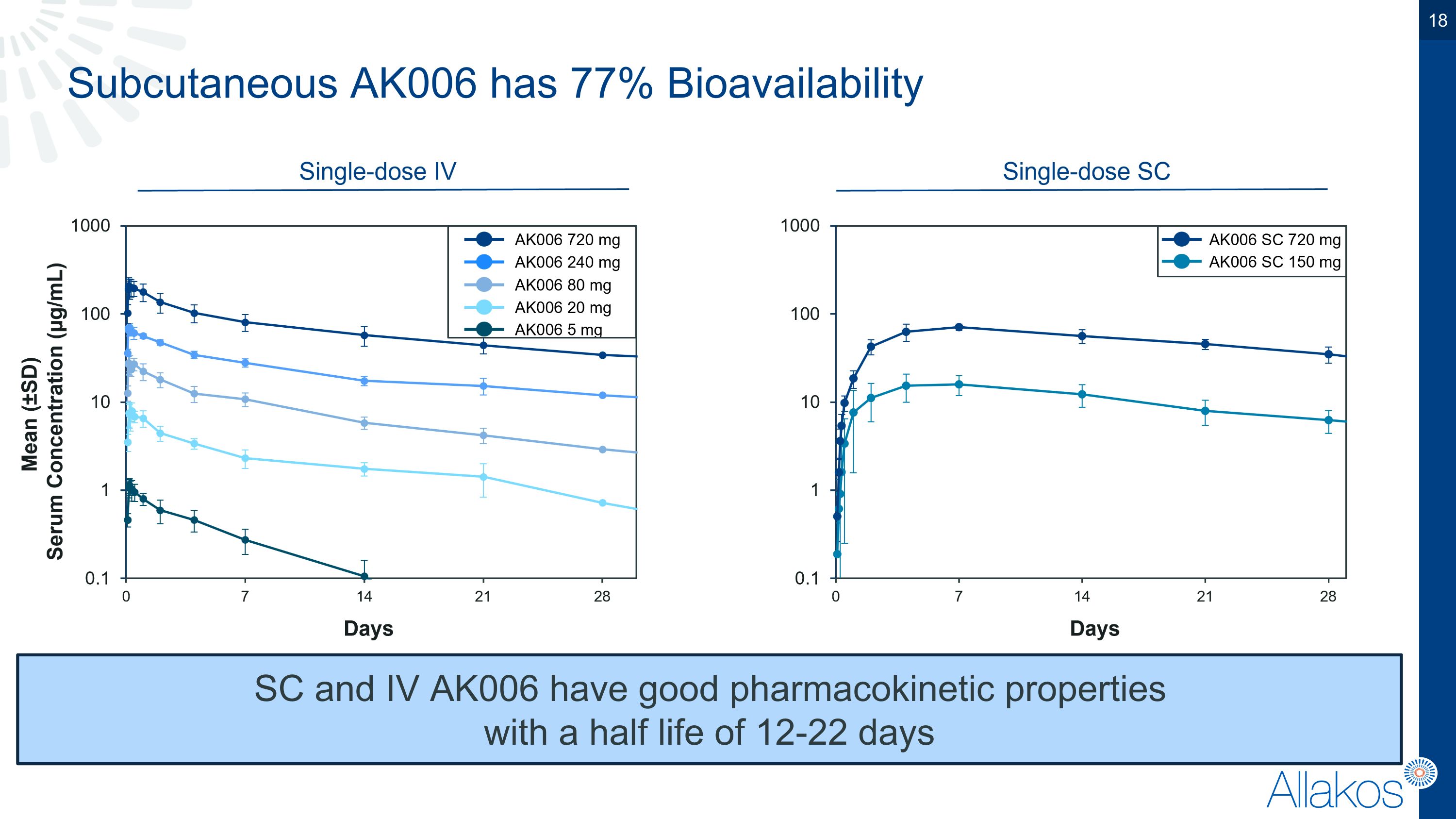
Subcutaneous AK006 has 77% Bioavailability SC and IV AK006 have good pharmacokinetic properties �with a half life of 12-22 days
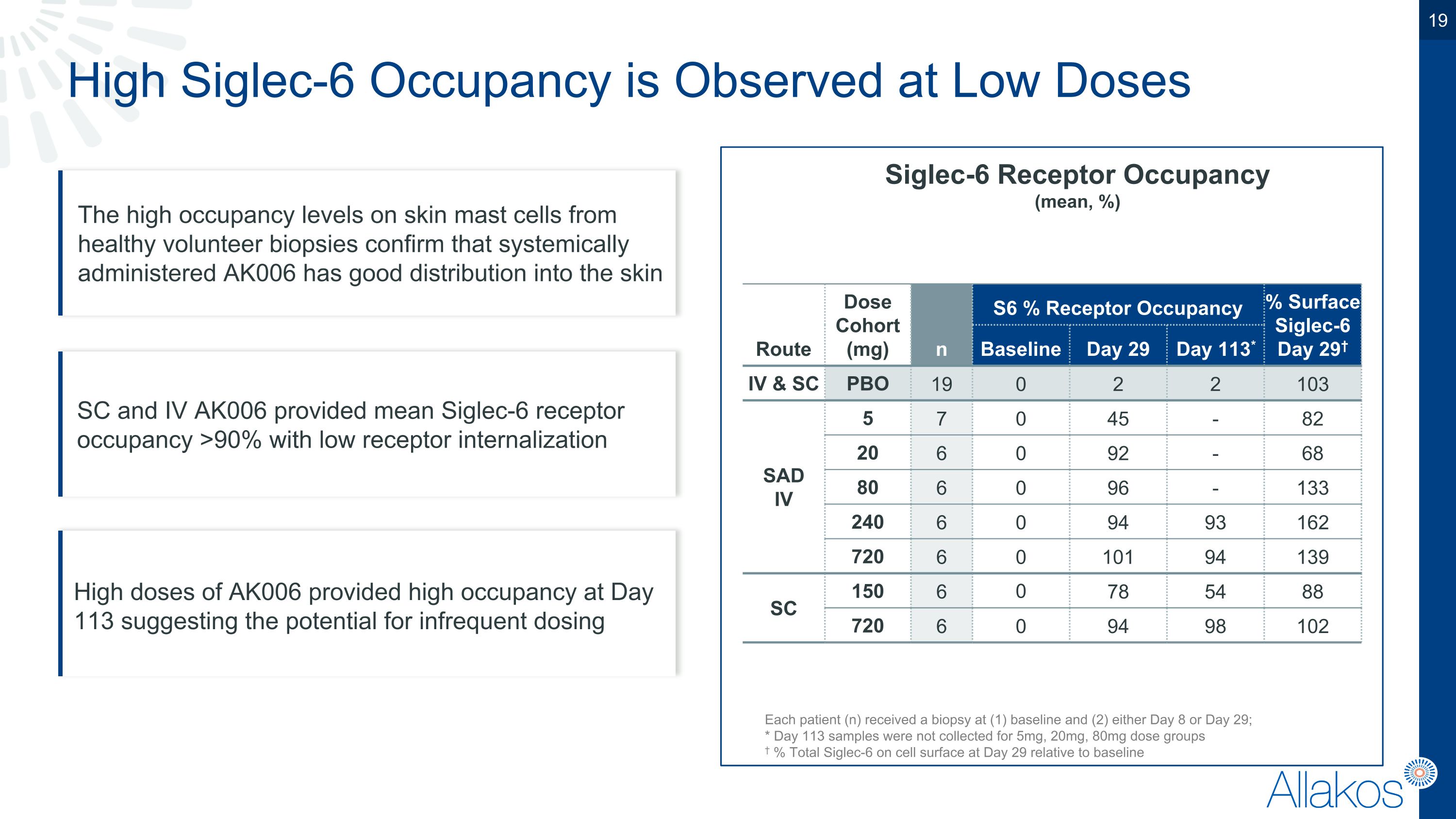
High Siglec-6 Occupancy is Observed at Low Doses Siglec-6 Receptor Occupancy�(mean, %) Route Dose Cohort (mg) n S6 % Receptor Occupancy % Surface Siglec-6 Day 29† Baseline Day 29 Day 113* IV & SC PBO 19 0 2 2 103 SAD IV 5 7 0 45 - 82 20 6 0 92 - 68 80 6 0 96 - 133 240 6 0 94 93 162 720 6 0 101 94 139 SC 150 6 0 78 54 88 720 6 0 94 98 102 Each patient (n) received a biopsy at (1) baseline and (2) either Day 8 or Day 29; * Day 113 samples were not collected for 5mg, 20mg, 80mg dose groups † % Total Siglec-6 on cell surface at Day 29 relative to baseline The high occupancy levels on skin mast cells from healthy volunteer biopsies confirm that systemically administered AK006 has good distribution into the skin SC and IV AK006 provided mean Siglec-6 receptor occupancy >90% with low receptor internalization High doses of AK006 provided high occupancy at Day 113 suggesting the potential for infrequent dosing

AK006 was Well-Tolerated with a Favorable Safety Profile Single and multiple doses of IV AK006 and single doses of SC up to 720 mg were �well-tolerated with a favorable safety profile. In the safety profile to date: No treatment emergent SAEs in subjects on AK006 There were no treatment emergent adverse events leading to discontinuation of AK006 There were no dose limiting toxicities The most common adverse events (≥10%) occurring more frequently in subjects on AK006 were headache and dysmenorrhea, all of which were mild-to-moderate in severity

AK006 for Chronic Spontaneous Urticaria
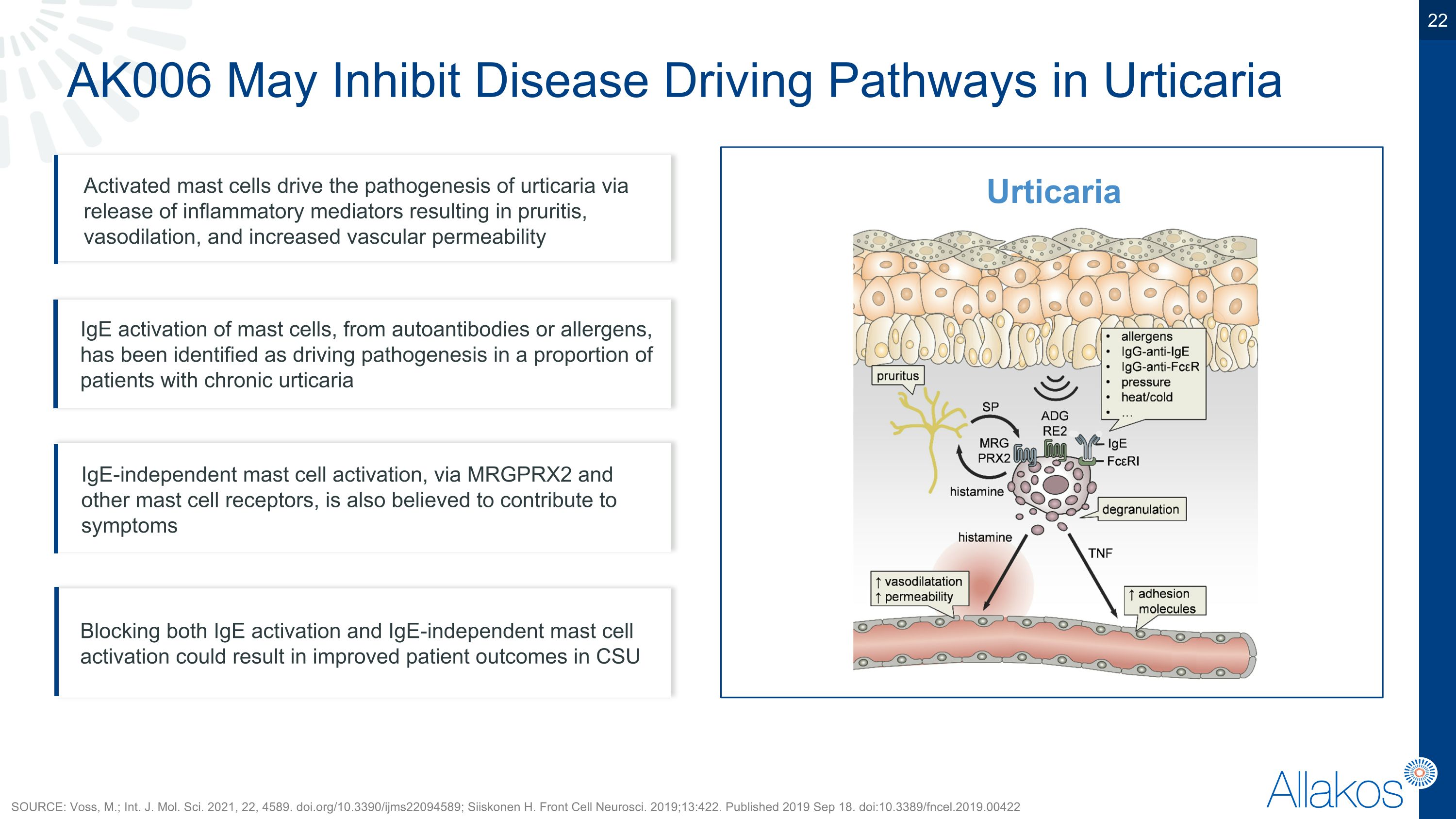
AK006 May Inhibit Disease Driving Pathways in Urticaria Activated mast cells drive the pathogenesis of urticaria via release of inflammatory mediators resulting in pruritis, vasodilation, and increased vascular permeability Blocking both IgE activation and IgE-independent mast cell activation could result in improved patient outcomes in CSU IgE activation of mast cells, from autoantibodies or allergens, has been identified as driving pathogenesis in a proportion of patients with chronic urticaria IgE-independent mast cell activation, via MRGPRX2 and other mast cell receptors, is also believed to contribute to symptoms SOURCE: Voss, M.; Int. J. Mol. Sci. 2021, 22, 4589. doi.org/10.3390/ijms22094589; Siiskonen H. Front Cell Neurosci. 2019;13:422. Published 2019 Sep 18. doi:10.3389/fncel.2019.00422 Urticaria
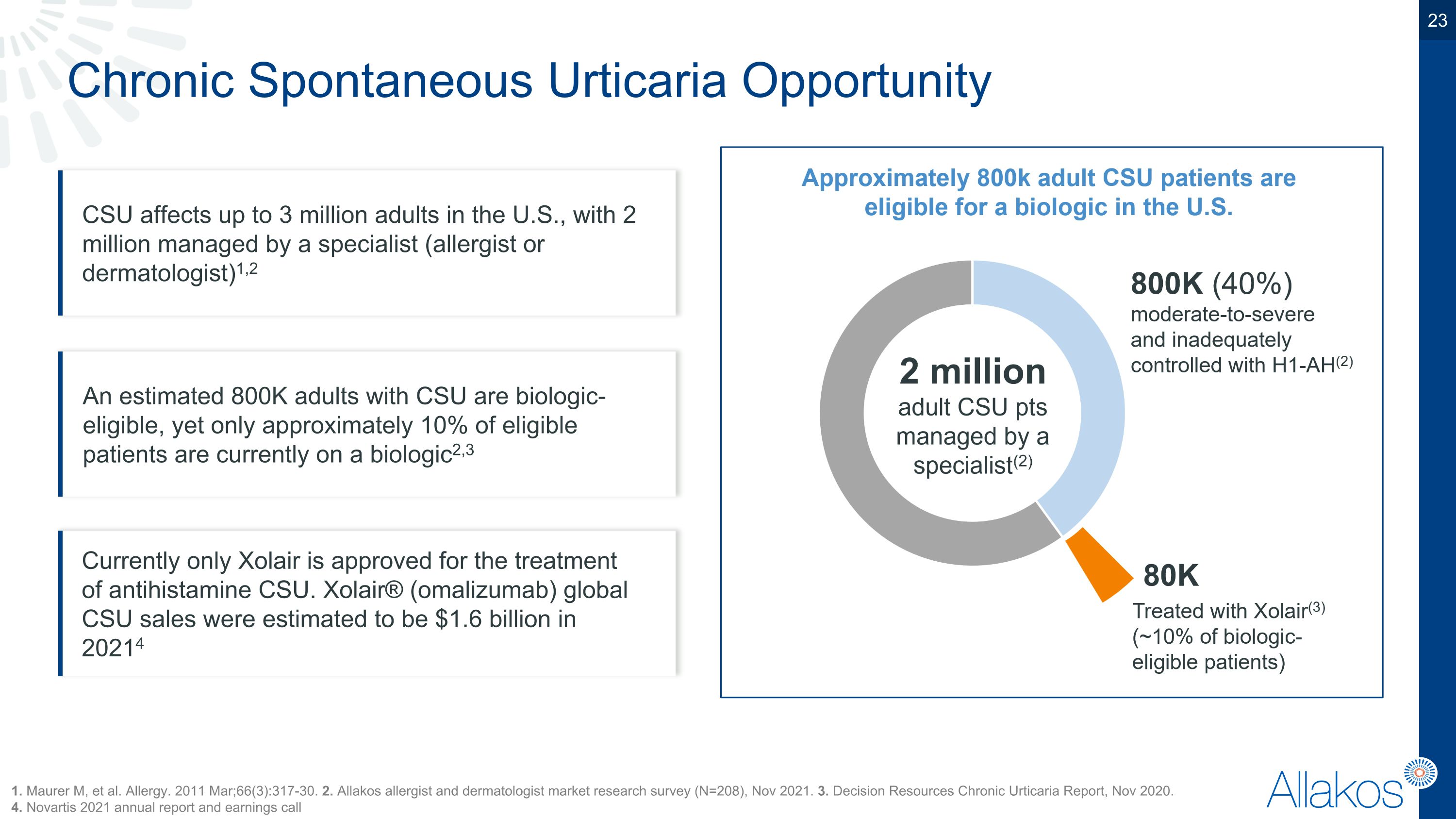
Chronic Spontaneous Urticaria Opportunity CSU affects up to 3 million adults in the U.S., with 2 million managed by a specialist (allergist or dermatologist)1,2 1. Maurer M, et al. Allergy. 2011 Mar;66(3):317-30. 2. Allakos allergist and dermatologist market research survey (N=208), Nov 2021. 3. Decision Resources Chronic Urticaria Report, Nov 2020. �4. Novartis 2021 annual report and earnings call An estimated 800K adults with CSU are biologic-eligible, yet only approximately 10% of eligible patients are currently on a biologic2,3 Currently only Xolair is approved for the treatment of antihistamine CSU. Xolair® (omalizumab) global CSU sales were estimated to be $1.6 billion in 20214
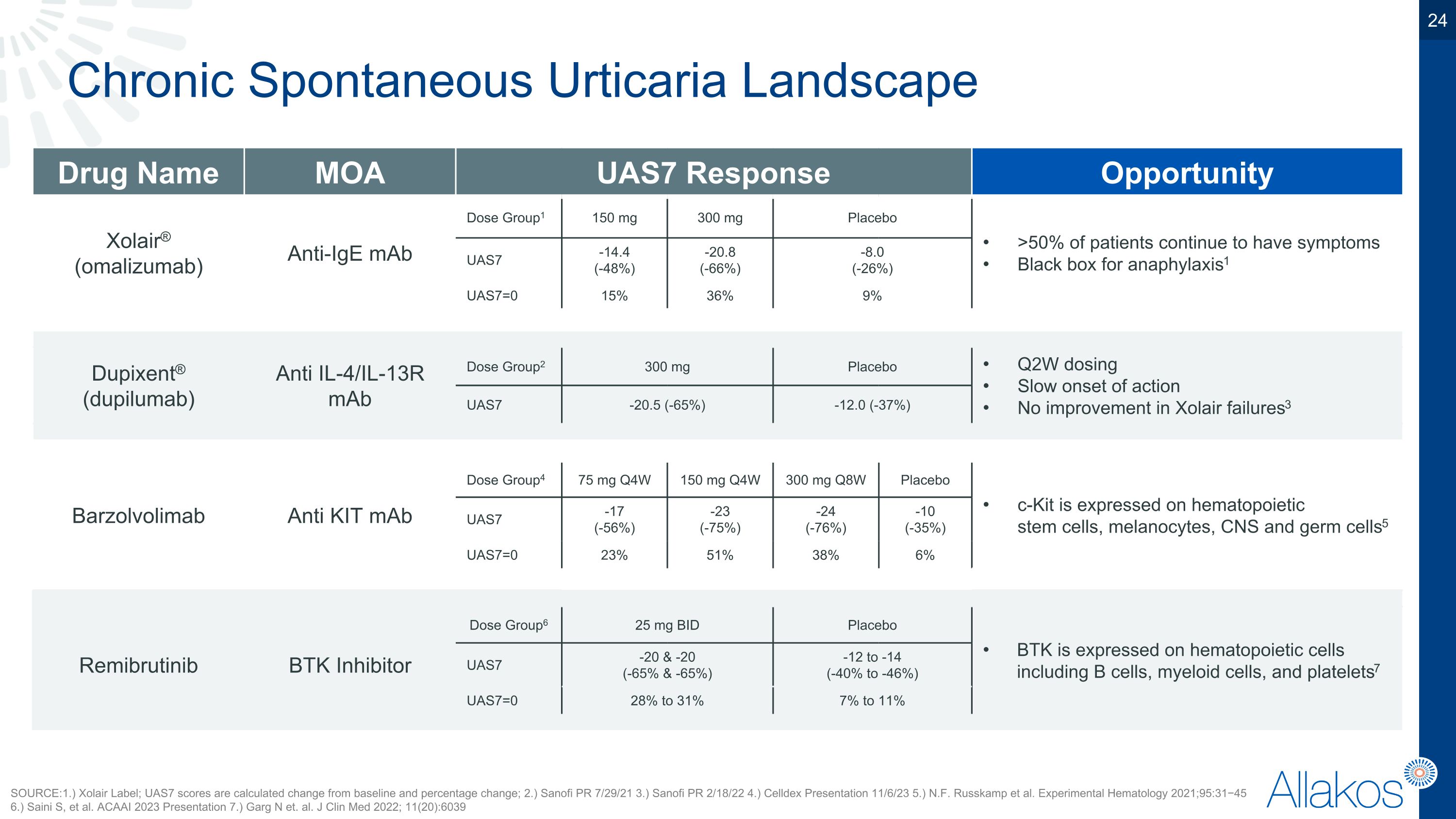
Chronic Spontaneous Urticaria Landscape Drug Name MOA UAS7 Response Opportunity Xolair® (omalizumab) Anti-IgE mAb Dose Group1 150 mg 300 mg Placebo Placebo >50% of patients continue to have symptoms Black box for anaphylaxis1 UAS7 -14.4 (-48%) -20.8�(-66%) -8.0 (-26%) -8.0 (-26%) UAS7=0 15% 36% 9% 9% Dupixent® (dupilumab) Anti IL-4/IL-13R mAb Dose Group2 300 mg 300 mg Placebo Placebo Q2W dosing Slow onset of action No improvement in Xolair failures3 UAS7 -20.5 (-65%) -20.5 (-65%) -12.0 (-37%) -12.0 (-37%) Barzolvolimab Anti KIT mAb Dose Group4 75 mg Q4W 150 mg Q4W 300 mg Q8W Placebo c-Kit is expressed on hematopoietic�stem cells, melanocytes, CNS and germ cells5 UAS7 -17�(-56%) -23�(-75%) -24�(-76%) -10�(-35%) UAS7=0 23% 51% 38% 6% Dose Group6 25 mg BID Placebo BTK is expressed on hematopoietic cells including B cells, myeloid cells, and platelets7 Remibrutinib BTK Inhibitor UAS7 -20 & -20�(-65% & -65%) -12 to -14�(-40% to -46%) Warnings and Precautions: hemorrhage, infections, cardiac arrhythmias & failure, hypertension, cytopenia, malignancies8 UAS7=0 28% to 31% 7% to 11% SOURCE: 1.) Xolair Label; UAS7 scores are calculated change from baseline and percentage change; 2.) Sanofi PR 7/29/21 3.) Sanofi PR 2/18/22 4.) Celldex Presentation 11/6/23 5.) N.F. Russkamp et al. Experimental Hematology 2021;95:31−45 6.) Saini S, et al. ACAAI 2023 Presentation 7.) Garg N et. al. J Clin Med 2022; 11(20):6039

Financial Overview & Key Milestones

Data Catalysts and Expected Milestones Q2 2024: Report SAD and MAD safety, pharmacokinetics (PK), and pharmacodynamic (PD) results from the Phase 1 IV AK006 trial in healthy volunteers, including data to confirm Siglec-6 receptor occupancy in skin biopsy samples. Q2 2024: Initiate the randomized, double-blind, placebo-controlled Phase 1 trial of IV AK006 in patients with chronic spontaneous urticaria (CSU). Q3 2024: Report subcutaneous (SC) AK006 safety, PK, and PD results from the Phase 1 trial in healthy volunteers, including data to confirm Siglec-6 receptor occupancy in skin biopsy samples. Early Q1 2025: Report topline data from the Phase 1 trial of IV AK006 in patients with CSU.

Cash, Cash Equivalents and Investments in Marketable Securities as of December 31, 2023 $170.8 M – Estimated 2024 cash used in restructuring (lirentelimab closeout, severance and other costs) $30 M – Estimated 2024 cash used in ongoing business operations $55 to $60 M Estimated, Cash, Cash Equivalents and Investments in Marketable Securities at year end 2024 $81 to $86 M Common Shares Outstanding as of December 31, 2023 87.8 M Allakos expects that the restructuring activities will extend the cash runway into mid-2026 AK006 composition of matter�to expire in 2042 without extensions Planning subcutaneous AK006 for Phase 2 studies Balance Sheet and IP Protection
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Allakos (NASDAQ:ALLK)
Historical Stock Chart
From Jan 2025 to Feb 2025
Allakos (NASDAQ:ALLK)
Historical Stock Chart
From Feb 2024 to Feb 2025