0001474167false--12-31Q32024COSM0.001300000000864978649700100000000001487000148700000060000003533741P5YP5Y927003715010815321123700366900611500897165720908089716500P4Y7M21D315238648425764201.0000014741672024-01-012024-09-300001474167us-gaap:SubsequentEventMember2024-11-060001474167us-gaap:SubsequentEventMember2024-11-022024-11-060001474167abcd:UkMember2023-01-012023-09-300001474167abcd:UkMember2023-07-012023-09-300001474167abcd:UkMember2024-07-012024-09-300001474167abcd:UkMember2024-01-012024-09-300001474167abcd:UnitedStatesMember2023-01-012023-09-300001474167abcd:UnitedStatesMember2023-07-012023-09-300001474167abcd:UnitedStatesMember2024-07-012024-09-300001474167abcd:UnitedStatesMember2024-01-012024-09-300001474167abcd:GreeceMember2023-01-012023-09-300001474167abcd:GreeceMember2023-07-012023-09-300001474167abcd:GreeceMember2024-07-012024-09-300001474167abcd:BulgariaMember2023-01-012023-09-300001474167abcd:BulgariaMember2023-07-012023-09-300001474167abcd:BulgariaMember2024-07-012024-09-300001474167abcd:BulgariaMember2024-01-012024-09-300001474167abcd:CyprusMember2023-01-012023-09-300001474167abcd:CyprusMember2023-07-012023-09-300001474167abcd:CyprusMember2024-07-012024-09-300001474167abcd:CyprusMember2024-01-012024-09-300001474167abcd:CroatiaMember2023-01-012023-09-300001474167abcd:CroatiaMember2023-07-012023-09-300001474167abcd:CroatiaMember2024-07-012024-09-300001474167abcd:CroatiaMember2024-01-012024-09-300001474167abcd:IrelandMember2024-01-012024-09-300001474167abcd:IrelandMember2024-07-012024-09-300001474167abcd:IrelandMember2023-07-012023-09-300001474167abcd:IrelandMember2023-01-012023-09-300001474167abcd:WarrantExchangeMember2024-09-260001474167abcd:WarrantsMember2024-09-012024-09-260001474167abcd:WarrantExchangeMember2024-09-012024-09-260001474167abcd:WarrantsMember2023-12-012023-12-290001474167abcd:BoardOfDirectorsMember2023-08-210001474167abcd:TwoZeroTwentyThreePlanMember2023-08-210001474167abcd:TwoZeroTwentyTwoPlanMember2022-09-1900014741672023-04-012023-04-030001474167abcd:BoardOfDirectorsMember2024-01-012024-09-300001474167abcd:BoardOfDirectorsMember2024-07-012024-09-300001474167abcd:WarrantExchangeMember2024-01-012024-09-300001474167abcd:WarrantExchangeMember2023-01-012023-12-310001474167abcd:WarrantsMember2024-09-300001474167abcd:WarrantsMember2024-01-012024-09-300001474167abcd:WarrantsMember2023-01-012023-12-310001474167abcd:WarrantsMember2022-12-310001474167abcd:WarrantsMember2023-12-310001474167abcd:FourThirdPartyConsultantsMember2023-11-012023-11-210001474167abcd:PhaseOneMember2022-06-012022-06-260001474167abcd:PhaseTwoMember2022-06-012022-06-260001474167abcd:NationalMedicinesAgencyMemberus-gaap:GeneralAndAdministrativeExpenseMember2024-07-012024-09-300001474167abcd:NationalMedicinesAgencyMemberus-gaap:GeneralAndAdministrativeExpenseMember2023-01-012023-12-310001474167abcd:NationalMedicinesAgencyMemberus-gaap:GeneralAndAdministrativeExpenseMember2024-01-012024-09-300001474167abcd:NationalMedicinesAgencyMemberus-gaap:GeneralAndAdministrativeExpenseMember2023-01-012023-09-300001474167abcd:NationalMedicinesAgencyMemberus-gaap:GeneralAndAdministrativeExpenseMember2023-07-012023-09-3000014741672021-07-012021-07-310001474167us-gaap:InterestExpenseMember2024-01-012024-09-300001474167us-gaap:InterestExpenseMember2023-01-012023-09-300001474167us-gaap:InterestExpenseMember2023-07-012023-09-300001474167us-gaap:InterestExpenseMember2024-07-012024-09-300001474167us-gaap:GeneralAndAdministrativeExpenseMember2023-01-012023-09-300001474167us-gaap:GeneralAndAdministrativeExpenseMember2023-07-012023-09-300001474167us-gaap:GeneralAndAdministrativeExpenseMember2024-07-012024-09-300001474167us-gaap:GeneralAndAdministrativeExpenseMember2024-01-012024-09-300001474167abcd:FinanceLeaseMember2024-09-300001474167abcd:OperatingLeaseMember2024-09-300001474167abcd:SeniorPromissoryNotesMemberabcd:UnaffiliatedThirdPartyMember2024-01-012024-09-300001474167abcd:TFFMemberabcd:PrincipalBalanceTwoMemberabcd:SynthesisFacilityAgreementMember2018-10-170001474167abcd:TFFMemberabcd:PrincipalBalanceOneMemberabcd:SynthesisFacilityAgreementMember2018-10-170001474167abcd:TFFMemberabcd:PrincipalBalanceOneMemberabcd:SynthesisFacilityAgreementMember2020-12-300001474167abcd:JanuaryTwentyThreeTwentyTwentyFourMemberabcd:PromissoryNotesMember2023-12-310001474167abcd:DebtAgreementJulyTwentyNineMember2023-12-310001474167abcd:DebtAgreementJulyTwentyNineMember2024-09-300001474167abcd:DebtAgreementJuneOneMember2023-12-310001474167abcd:UnitedKingdomGovernmentMemberabcd:CovidNinteenMember2024-09-300001474167abcd:UnitedKingdomGovernmentMemberabcd:CovidNinteenMember2023-12-310001474167abcd:CovidNinteenMember2024-09-300001474167abcd:CovidNinteenMember2023-12-310001474167abcd:DebtAgreementJulyTwentyNineMember2020-06-012020-06-230001474167abcd:CovidNinteenMember2020-05-012020-05-120001474167abcd:CovidNinteenMember2024-01-012024-09-300001474167abcd:JulyFourteenTwoThousandTwentyThreeMemberabcd:PromissoryNotesMember2024-01-012024-09-300001474167abcd:DebtAgreementJuneOneMember2024-01-012024-09-300001474167abcd:DebtAgreementJuneMember2021-07-012021-07-300001474167abcd:DebtExchangeAgreementMember2020-11-012020-11-190001474167abcd:DebtAgreementJuneMember2024-01-012024-09-300001474167abcd:DebtExchangeAgreementMember2024-01-012024-09-300001474167abcd:JanuaryTwentyThreeTwentyTwentyFourMemberabcd:PromissoryNotesMember2024-01-012024-09-300001474167abcd:JuneTwentyThreeTwoThousandTwentyMemberabcd:NationalBankOfGreeceSAMember2024-01-012024-09-3000014741672022-01-012022-12-310001474167abcd:JuneTwentyThreeTwoThousandTwentyMemberabcd:NationalBankOfGreeceSAMember2020-06-012020-06-230001474167abcd:DebtAgreementJulyTwentyNineMember2024-01-012024-09-300001474167abcd:UnitedKingdomGovernmentMemberabcd:CovidNinteenMember2020-05-012020-05-120001474167abcd:UnitedKingdomGovernmentMemberabcd:CovidNinteenMember2024-01-012024-09-300001474167abcd:FullAndFinalSettlementAgreementMember2024-07-012024-09-300001474167abcd:JulyFourteenTwoThousandTwentyThreeMemberabcd:PromissoryNotesMember2023-12-310001474167abcd:JanuaryTwentyThreeTwentyTwentyFourMemberabcd:PromissoryNotesMember2024-09-300001474167abcd:DebtAgreementJuneOneMember2022-06-090001474167abcd:DebtAgreementJuneMember2023-12-310001474167abcd:DebtAgreementJuneMember2024-09-3000014741672021-07-300001474167abcd:DebtExchangeAgreementMember2020-11-190001474167abcd:JuneTwentyThreeTwoThousandTwentyMemberabcd:NationalBankOfGreeceSAMember2023-12-310001474167abcd:JuneTwentyThreeTwoThousandTwentyMemberabcd:NationalBankOfGreeceSAMember2024-09-300001474167abcd:FullAndFinalSettlementAgreementMember2024-09-300001474167abcd:FullAndFinalSettlementAgreementMember2023-12-3100014741672022-03-030001474167abcd:TFFMemberabcd:SynthesisFacilityAgreementMember2018-10-170001474167abcd:DebtExchangeAgreementMember2023-12-310001474167abcd:DebtExchangeAgreementMember2024-09-300001474167abcd:FullAndFinalSettlementAgreementMember2024-01-012024-09-300001474167abcd:COVIDLoansMember2024-09-300001474167abcd:ThirdPartyMember2024-09-300001474167abcd:TradeFacilityAgreementsMember2024-09-300001474167abcd:LoanFacilityAgreementMember2023-12-310001474167abcd:LoanFacilityAgreementMember2024-09-300001474167abcd:COVIDLoansMember2023-12-310001474167abcd:ThirdPartyMember2023-12-310001474167abcd:TradeFacilityAgreementsMember2023-12-310001474167abcd:COVIDLoansMember2024-01-012024-09-300001474167abcd:ThirdPartyMember2024-01-012024-09-300001474167abcd:TradeFacilityAgreementsMember2024-01-012024-09-300001474167abcd:LoanFacilityAgreementMember2023-01-012023-12-310001474167abcd:LoanFacilityAgreementMember2024-01-012024-09-300001474167abcd:COVIDLoansMember2023-01-012023-12-310001474167abcd:ThirdPartyMember2023-01-012023-12-310001474167abcd:TradeFacilityAgreementsMember2023-01-012023-12-310001474167abcd:LinesOfCreditMember2023-01-012023-09-300001474167abcd:LinesOfCreditMember2023-07-012023-09-300001474167abcd:LinesOfCreditMember2024-07-012024-09-300001474167abcd:LinesOfCreditMember2024-01-012024-09-300001474167abcd:EFGMemberus-gaap:LineOfCreditMember2023-01-012023-12-310001474167abcd:EFGMemberus-gaap:LineOfCreditMember2024-01-012024-09-300001474167abcd:PancretaOfGreeceMemberus-gaap:LineOfCreditMember2024-01-012024-09-300001474167abcd:PancretaOfGreeceMemberus-gaap:LineOfCreditMember2023-01-012023-12-310001474167abcd:AlphaBankOfGreeceMemberus-gaap:LineOfCreditMember2024-01-012024-09-300001474167abcd:AlphaBankOfGreeceMemberus-gaap:LineOfCreditMember2023-01-012023-12-310001474167abcd:NationalBankOfGreeceTwoMemberus-gaap:LineOfCreditMember2024-01-012024-09-300001474167abcd:NationalBankOfGreeceTwoMemberus-gaap:LineOfCreditMember2023-01-012023-12-310001474167abcd:NationalBankOfGreeceOneMemberus-gaap:LineOfCreditMember2024-01-012024-09-300001474167abcd:NationalBankOfGreeceOneMemberus-gaap:LineOfCreditMember2023-01-012023-12-310001474167abcd:EFGMemberus-gaap:LineOfCreditMember2024-09-300001474167abcd:EFGMemberus-gaap:LineOfCreditMember2023-12-310001474167abcd:PancretaOfGreeceMemberus-gaap:LineOfCreditMember2024-09-300001474167abcd:PancretaOfGreeceMemberus-gaap:LineOfCreditMember2023-12-310001474167abcd:AlphaBankOfGreeceMemberus-gaap:LineOfCreditMember2024-09-300001474167abcd:AlphaBankOfGreeceMemberus-gaap:LineOfCreditMember2023-12-310001474167abcd:NationalBankOfGreeceTwoMemberus-gaap:LineOfCreditMember2024-09-300001474167abcd:NationalBankOfGreeceTwoMemberus-gaap:LineOfCreditMember2023-12-310001474167abcd:NationalBankOfGreeceOneMemberus-gaap:LineOfCreditMember2024-09-300001474167abcd:NationalBankOfGreeceOneMemberus-gaap:LineOfCreditMember2023-12-310001474167abcd:EGFMember2024-09-300001474167abcd:EGFMember2023-12-310001474167abcd:PancretaMember2024-09-300001474167abcd:PancretaMember2023-12-310001474167abcd:AlphaMember2024-09-300001474167abcd:AlphaMember2023-12-310001474167abcd:NationalMember2024-09-300001474167abcd:NationalMember2023-12-310001474167abcd:DocPharmaSaMember2022-05-012022-05-170001474167abcd:DocPharmaSaMember2023-12-012023-12-290001474167abcd:DocPharmaSaMember2023-06-012023-06-280001474167abcd:InventoriesRelatedAgreementMemberabcd:DocPharmaSaMember2023-01-012023-09-300001474167abcd:InventoriesRelatedAgreementMemberabcd:DocPharmaSaMember2023-07-012023-09-300001474167abcd:InventoriesRelatedAgreementMemberabcd:DocPharmaSaMember2024-07-012024-09-300001474167abcd:InventoriesRelatedAgreementMemberabcd:DocPharmaSaMember2024-01-012024-09-300001474167abcd:DocPharmaSaMember2023-07-012023-09-300001474167abcd:DocPharmaSaMember2024-07-012024-09-300001474167abcd:MariaKozariMember2024-01-012024-09-300001474167abcd:MariaKozariMember2023-01-012023-09-300001474167abcd:MariaKozariMember2023-07-012023-09-300001474167abcd:MariaKozariMember2024-07-012024-09-300001474167abcd:MariaKozariMember2023-12-310001474167abcd:MariaKozariMember2024-09-300001474167abcd:KanarogloySiaEpeMember2023-12-310001474167abcd:KanarogloySiaEpeMember2024-09-300001474167abcd:DocPharmaSaMember2024-09-300001474167abcd:DocPharmaSaMember2023-12-310001474167abcd:BasothoInvestmentLimitedMember2023-12-310001474167abcd:BasothoInvestmentLimitedMember2024-09-300001474167abcd:BasothoInvestmentLimitedMember2024-01-012024-09-300001474167abcd:BasothoInvestmentLimitedMember2023-01-012023-12-310001474167abcd:PanagiotisKozarisMember2023-12-310001474167abcd:PanagiotisKozarisMember2024-09-300001474167abcd:PanagiotisKozarisMember2023-07-012023-09-300001474167abcd:PanagiotisKozarisMember2024-07-012024-09-300001474167abcd:GrigoriosSiokasMember2024-01-012024-09-300001474167abcd:DimitriosGoulielmosMember2024-01-012024-09-300001474167abcd:GrigoriosSiokasMember2023-12-310001474167abcd:GrigoriosSiokasMember2024-09-300001474167abcd:DimitriosGoulielmosMember2023-12-310001474167abcd:DimitriosGoulielmosMember2024-09-300001474167abcd:DocPharmaSaMember2023-01-012023-09-300001474167abcd:DocPharmaSaMember2024-01-012024-09-300001474167abcd:GeorgeTerzisMember2023-12-310001474167abcd:GeorgeTerzisMember2024-09-300001474167abcd:CanaHoldingsLaboratoriesHoldingLimitedMember2023-02-012023-02-280001474167abcd:LoansPayableRelatedPartyMember2024-09-300001474167abcd:LoansPayableRelatedPartyMember2023-01-012023-12-310001474167abcd:LoansPayableRelatedPartyMember2024-01-012024-09-300001474167abcd:LoansPayableRelatedPartyMember2023-12-310001474167abcd:LoansPayableRelatedPartyMember2022-12-310001474167abcd:NotesPayableRelatedPartyMember2024-09-300001474167abcd:NotesPayableRelatedPartyMember2023-01-012023-12-310001474167abcd:NotesPayableRelatedPartyMember2024-01-012024-09-300001474167abcd:NotesPayableRelatedPartyMember2023-12-310001474167abcd:NotesPayableRelatedPartyMember2022-12-310001474167abcd:WarrantExchangeMember2023-12-012023-12-290001474167abcd:WarrantInducementLetterMember2023-12-012023-12-290001474167abcd:WarrantInducementLetterMember2024-09-012024-09-2600014741672023-12-012023-12-2900014741672024-09-012024-09-260001474167us-gaap:SeriesAPreferredStockMember2024-09-300001474167us-gaap:SeriesAPreferredStockMember2023-12-310001474167abcd:TopMemberabcd:WarrantExchangeMember2023-12-290001474167abcd:TopMemberabcd:WarrantExchangeMember2024-09-260001474167abcd:BottomMemberabcd:WarrantExchangeMember2023-12-290001474167abcd:BottomMemberabcd:WarrantExchangeMember2024-09-260001474167abcd:WarrantInducementLetterMember2024-09-260001474167abcd:IssuanceofcommonstockMember2023-01-012023-09-300001474167abcd:TreasuryStocksOneMember2023-01-012023-01-240001474167abcd:TreasuryStocksOneMember2023-01-012023-12-310001474167abcd:TreasuryStocksOneMember2024-01-012024-09-300001474167abcd:WarrantExchangeMember2023-12-290001474167abcd:WarrantInducementLetterMember2023-12-290001474167abcd:WarrantExchangeMember2024-09-3000014741672023-12-2900014741672024-09-260001474167abcd:TreasuryStocksOneMember2024-09-300001474167abcd:TreasuryStocksOneMember2023-12-310001474167us-gaap:SeriesAPreferredStockMember2024-01-012024-09-300001474167us-gaap:SeriesAPreferredStockMember2022-01-012022-12-310001474167abcd:PlacementAgentMember2024-01-012024-09-300001474167abcd:UnitedKingdomMember2024-01-012024-09-3000014741672021-10-012021-10-300001474167us-gaap:CustomerListsMember2024-09-300001474167us-gaap:CustomerListsMember2023-12-310001474167abcd:TradeNameMarkMember2024-09-300001474167abcd:TradeNameMarkMember2023-12-310001474167abcd:SoftwareMember2024-09-300001474167us-gaap:LicenseMember2024-09-300001474167abcd:SoftwareMember2023-12-310001474167us-gaap:LicenseMember2023-12-310001474167us-gaap:BuildingImprovementsMember2023-12-310001474167us-gaap:LandMember2023-12-310001474167us-gaap:ComputerSoftwareIntangibleAssetMember2024-09-300001474167us-gaap:ComputerSoftwareIntangibleAssetMember2023-12-310001474167us-gaap:FurnitureAndFixturesMember2024-09-300001474167us-gaap:FurnitureAndFixturesMember2023-12-310001474167us-gaap:VehiclesMember2024-09-300001474167us-gaap:VehiclesMember2023-12-310001474167us-gaap:BuildingImprovementsMember2024-09-300001474167us-gaap:LandMember2024-09-300001474167us-gaap:LeaseholdImprovementsMember2024-09-300001474167us-gaap:LeaseholdImprovementsMember2023-12-310001474167abcd:DistributionAndEquityAcquisitionAgreementMemberus-gaap:SalesMemberabcd:MarathonGlobalIncMember2024-01-012024-09-300001474167abcd:DistributionAndEquityAcquisitionAgreementMemberabcd:SalesOneMemberabcd:MarathonGlobalIncMember2024-01-012024-09-300001474167abcd:CosmoFarmacyLPMember2024-09-300001474167abcd:CosmoFarmacyLPMember2024-01-012024-09-300001474167abcd:DistributionAndEquityAcquisitionAgreementMemberabcd:MarathonGlobalIncMember2024-01-012024-09-300001474167abcd:ShareExchangeAgreementMemberabcd:IccMember2023-12-310001474167abcd:ShareExchangeAgreementMemberabcd:IccMember2024-09-3000014741672018-12-190001474167abcd:NationalBankOfGreeceMember2024-01-012024-09-300001474167abcd:CANAPharmaceuticalLaboratoriesSACanaMember2023-02-012023-02-280001474167abcd:UnitedKingdomsOfEnglandMember2024-01-012024-09-300001474167abcd:GreeceMember2024-01-012024-09-300001474167abcd:ICCInternationalCannabisCorpMember2024-09-3000014741672023-01-060001474167abcd:PancretaBankMember2024-09-300001474167abcd:NationalBankOfGreeceMember2024-09-300001474167abcd:GreeceMember2023-06-150001474167abcd:CANAPharmaceuticalLaboratoriesSACanaMember2023-07-012023-09-300001474167abcd:GreeceMember2023-06-012023-06-150001474167abcd:ZipDoctorIncMember2023-03-012023-03-170001474167abcd:CloudscreenMember2024-01-012024-01-230001474167abcd:CloudscreenMember2023-04-022023-04-3000014741672023-01-012023-12-310001474167abcd:LeaseholdImprovementsAndTechnicalWorksMember2024-01-012024-09-300001474167us-gaap:ComputerEquipmentMemberabcd:TopMember2024-01-012024-09-300001474167us-gaap:ComputerEquipmentMemberabcd:BottomMember2024-01-012024-09-300001474167us-gaap:FurnitureAndFixturesMemberabcd:TopMember2024-01-012024-09-300001474167us-gaap:FurnitureAndFixturesMemberabcd:BottomMember2024-01-012024-09-300001474167abcd:MachineryMember2024-01-012024-09-300001474167abcd:BuildingsMemberabcd:TopMember2024-01-012024-09-300001474167us-gaap:VehiclesMember2024-01-012024-09-300001474167abcd:BuildingsMemberabcd:BottomMember2024-01-012024-09-300001474167abcd:CANAPharmaceuticalLaboratoriesSACanaMember2024-09-300001474167abcd:CANAPharmaceuticalLaboratoriesSACanaMember2024-01-012024-09-300001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-09-300001474167us-gaap:RetainedEarningsMember2024-09-300001474167abcd:SubscriptionReceivableMember2024-09-300001474167us-gaap:AdditionalPaidInCapitalMember2024-09-300001474167abcd:TreasurysStocksMember2024-09-300001474167abcd:CommonStockShareMember2024-09-300001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-07-012024-09-300001474167us-gaap:RetainedEarningsMember2024-07-012024-09-300001474167us-gaap:AdditionalPaidInCapitalMember2024-07-012024-09-300001474167abcd:CommonStockShareMember2024-07-012024-09-3000014741672024-06-300001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-06-300001474167us-gaap:RetainedEarningsMember2024-06-300001474167abcd:SubscriptionReceivableMember2024-06-300001474167us-gaap:AdditionalPaidInCapitalMember2024-06-300001474167abcd:TreasurysStocksMember2024-06-300001474167abcd:CommonStockShareMember2024-06-3000014741672024-04-012024-06-300001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-04-012024-06-300001474167us-gaap:RetainedEarningsMember2024-04-012024-06-300001474167us-gaap:AdditionalPaidInCapitalMember2024-04-012024-06-3000014741672024-03-310001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-03-310001474167us-gaap:RetainedEarningsMember2024-03-310001474167abcd:SubscriptionReceivableMember2024-03-310001474167us-gaap:AdditionalPaidInCapitalMember2024-03-310001474167abcd:TreasurysStocksMember2024-03-310001474167abcd:CommonStockShareMember2024-03-3100014741672024-01-012024-03-310001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-01-012024-03-310001474167us-gaap:RetainedEarningsMember2024-01-012024-03-310001474167abcd:TreasurysStocksMember2024-01-012024-03-310001474167abcd:SubscriptionReceivableMember2024-01-012024-03-310001474167us-gaap:AdditionalPaidInCapitalMember2024-01-012024-03-310001474167abcd:CommonStockShareMember2024-01-012024-03-310001474167us-gaap:PreferredStockMember2024-01-012024-03-310001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310001474167us-gaap:RetainedEarningsMember2023-12-310001474167abcd:SubscriptionReceivableMember2023-12-310001474167us-gaap:AdditionalPaidInCapitalMember2023-12-310001474167abcd:TreasurysStocksMember2023-12-310001474167abcd:CommonStockShareMember2023-12-310001474167us-gaap:PreferredStockMember2023-12-3100014741672023-09-300001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-09-300001474167us-gaap:RetainedEarningsMember2023-09-300001474167abcd:SubscriptionReceivableMember2023-09-300001474167us-gaap:AdditionalPaidInCapitalMember2023-09-300001474167abcd:TreasurysStocksMember2023-09-300001474167abcd:CommonStockShareMember2023-09-300001474167us-gaap:PreferredStockMember2023-09-300001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-07-012023-09-300001474167us-gaap:RetainedEarningsMember2023-07-012023-09-300001474167abcd:TreasurysStocksMember2023-07-012023-09-300001474167abcd:SubscriptionReceivableMember2023-07-012023-09-300001474167us-gaap:AdditionalPaidInCapitalMember2023-07-012023-09-300001474167abcd:CommonStockShareMember2023-07-012023-09-300001474167us-gaap:PreferredStockMember2023-07-012023-09-3000014741672023-06-300001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-06-300001474167us-gaap:RetainedEarningsMember2023-06-300001474167abcd:SubscriptionReceivableMember2023-06-300001474167us-gaap:AdditionalPaidInCapitalMember2023-06-300001474167abcd:TreasurysStocksMember2023-06-300001474167abcd:CommonStockShareMember2023-06-300001474167us-gaap:PreferredStockMember2023-06-3000014741672023-04-012023-06-300001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-04-012023-06-300001474167us-gaap:RetainedEarningsMember2023-04-012023-06-300001474167abcd:TreasurysStocksMember2023-04-012023-06-300001474167abcd:SubscriptionReceivableMember2023-04-012023-06-300001474167us-gaap:AdditionalPaidInCapitalMember2023-04-012023-06-300001474167abcd:CommonStockShareMember2023-04-012023-06-300001474167us-gaap:PreferredStockMember2023-04-012023-06-3000014741672023-03-310001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-310001474167us-gaap:RetainedEarningsMember2023-03-310001474167abcd:SubscriptionReceivableMember2023-03-310001474167us-gaap:AdditionalPaidInCapitalMember2023-03-310001474167abcd:TreasurysStocksMember2023-03-310001474167abcd:CommonStockShareMember2023-03-310001474167us-gaap:PreferredStockMember2023-03-310001474167us-gaap:RetainedEarningsMember2023-01-012023-03-310001474167us-gaap:AdditionalPaidInCapitalMember2023-01-012023-03-310001474167abcd:CommonStockShareMember2023-01-012023-03-310001474167abcd:SubscriptionReceivableMember2023-01-012023-03-3100014741672023-01-012023-03-310001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-03-3100014741672022-12-310001474167us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310001474167us-gaap:RetainedEarningsMember2022-12-310001474167abcd:SubscriptionReceivableMember2022-12-310001474167us-gaap:AdditionalPaidInCapitalMember2022-12-310001474167abcd:TreasurysStocksMember2022-12-310001474167abcd:CommonStockShareMember2022-12-310001474167us-gaap:PreferredStockMember2022-12-3100014741672023-01-012023-09-3000014741672023-07-012023-09-3000014741672024-07-012024-09-3000014741672023-12-3100014741672024-09-3000014741672024-11-14iso4217:USDxbrli:sharesiso4217:USDxbrli:sharesxbrli:pureiso4217:CADiso4217:EUR
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
☒ | QUARTERLY REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2024
☐ | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT |
For the transition period from __________ to __________
Commission file number: 000-54436
COSMOS HEALTH INC. |
(Exact name of registrant as specified in its charter) |
Nevada | | 27-0611758 |
(State or other jurisdiction of Company or organization) | | (I.R.S. Employer Identification No.) |
| | |
5 Agiou Georgiou Str, Pilea, Thessaloniki, Greece | | 55438 |
(Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number: (312) 536-3102
Securities registered under Section 12(b) of the Exchange Act:
Title of each class | | Name of each exchange on which registered |
Common Stock, par value $0.001 | | The Nasdaq Capital Market |
Securities registered under Section 12(g) of the Exchange Act:
Title of each class | | Name of each exchange on which registered |
Check whether the Issuer (1) filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act during the past 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | Accelerated filer | ☐ |
Non-accelerated Filer | ☐ | Smaller reporting company | ☒ |
(Do not check if a smaller reporting company) | Emerging growth company | ☐ |
If an emerging growth company, indicate by checkmark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Applicable only to Corporate Issuers:
Indicate the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date: 23,346,049 as of November 14, 2024.
TABLE OF CONTENTS
COSMOS HEALTH INC. |
CONDENSED CONSOLIDATED BALANCE SHEETS |
| | | | | | |
| | September 30, 2024 | | | December 31, 2023 | |
| | (Unaudited) | | | | |
ASSETS |
| | | | | | |
CURRENT ASSETS: | | | | | | |
Cash and cash equivalents | | $ | 3,314,845 | | | $ | 3,833,195 | |
Accounts receivable, net | | | 17,484,240 | | | | 19,759,254 | |
Accounts receivable - related party | | | 1,285,743 | | | | 1,099,098 | |
Marketable securities | | | 22,808 | | | | 20,075 | |
Inventory | | | 4,885,015 | | | | 4,789,054 | |
Loans receivable | | | 491,897 | | | | 411,858 | |
Loans receivable - related party | | | 445,800 | | | | 442,480 | |
Prepaid expenses and other current assets | | | 1,914,881 | | | | 1,811,911 | |
Prepaid expenses and other current assets - related party | | | 6,393,642 | | | | 4,440,855 | |
| | | | | | | | |
TOTAL CURRENT ASSETS | | | 36,238,871 | | | | 36,607,780 | |
| | | | | | | | |
Property and equipment, net | | | 10,575,928 | | | | 10,455,499 | |
Goodwill and intangible assets, net | | | 7,746,761 | | | | 7,684,183 | |
Loans receivable - long term portion | | | 3,225,879 | | | | 3,509,200 | |
Loans receivable - related party - long term | | | 3,234,604 | | | | 3,539,840 | |
Operating lease right-of-use asset | | | 710,711 | | | | 1,131,552 | |
Financing lease right-of-use asset | | | 22,343 | | | | 28,790 | |
Advances for building's acquisition | | | 2,000,020 | | | | 2,000,020 | |
Other assets | | | 764,865 | | | | 1,057,947 | |
| | | | | | | | |
TOTAL ASSETS | | $ | 64,519,982 | | | $ | 66,014,811 | |
| | | | | | | | |
LIABILITIES, MEZZANINE EQUITY AND STOCKHOLDERS' EQUITY |
| | | | | | | | |
CURRENT LIABILITIES: | | | | | | | | |
Accounts payable and accrued expenses | | $ | 11,605,255 | | | | 11,911,978 | |
Accounts payable and accrued expenses - related party | | | 1,027,327 | | | | 231,564 | |
Accrued interest | | | 185,561 | | | | 166,348 | |
Lines of credit | | | 5,989,425 | | | | 6,630,273 | |
Notes payable | | | 1,622,349 | | | | 1,570,886 | |
Notes payable - related party | | | 11,368 | | | | 11,283 | |
Loans payable - related party | | | 21,158 | | | | 13,257 | |
Operating lease liability, current portion | | | 241,422 | | | | 285,563 | |
Financing lease liability, current portion | | | 18,888 | | | | 27,222 | |
Other current liabilities | | | 4,488,465 | | | | 3,474,096 | |
| | | | | | | | |
TOTAL CURRENT LIABILITIES | | | 25,211,218 | | | | 24,322,470 | |
| | | | | | | | |
Share settled debt obligation | | | - | | | | - | |
Notes payable - long term portion | | | 2,645,623 | | | | 3,035,341 | |
Operating lease liability, net of current portion | | | 468,157 | | | | 844,866 | |
Financing lease liability, net of current portion | | | 5,845 | | | | 5,261 | |
Other liabilities | | | 1,212,540 | | | | 1,763,845 | |
TOTAL LIABILITIES | | | 29,543,383 | | | | 29,971,783 | |
| | | | | | | | |
Commitments and Contingencies (see Note 14) | | | - | | | | - | |
| | | | | | | | |
STOCKHOLDERS' EQUITY: | | | | | | | | |
Common stock, $0.001 par value; 300,000,000 shares authorized; 23,346,023 and 15,982,472 shares issued and 23,259,526 and 15,895,975 outstanding as of September 30, 2024 and December 31, 2023, respectively | | | 23,346 | | | | 15,983 | |
Additional paid-in capital | | | 140,797,456 | | | | 129,008,301 | |
Subscription receivable | | | (20 | ) | | | (20 | ) |
Treasury stock, at cost, 86,497 shares as of September 30, 2024 and December 31, 2023 | | | (917,159 | ) | | | (917,159 | ) |
Accumulated deficit | | | (104,479,192 | ) | | | (91,644,233 | ) |
Accumulated other comprehensive loss | | | (447,832 | ) | | | (419,844 | ) |
| | | | | | | | |
TOTAL STOCKHOLDERS' EQUITY | | | 34,976,599 | | | | 36,043,028 | |
| | | | | | | | |
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY | | $ | 64,519,982 | | | $ | 66,014,811 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
COSMOS HEALTH INC. |
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS |
| | | |
| | Three Months Ended September 30, | | | Nine Months Ended September 30, | |
| | 2024 | | | 2023 | | | 2024 | | | 2023 | |
| | | | | | | | | | | | |
REVENUE | | $ | 12,411,048 | | | $ | 12,823,797 | | | $ | 40,202,238 | | | $ | 37,537,003 | |
| | | | | | | | | | | | | | | | |
COST OF GOODS SOLD | | | 11,204,186 | | | | 11,609,039 | | | | 36,894,502 | | | | 34,418,334 | |
| | | | | | | | | | | | | | | | |
GROSS PROFIT | | | 1,206,862 | | | | 1,214,758 | | | | 3,307,736 | | | | 3,118,669 | |
| | | | | | | | | | | | | | | | |
OPERATING EXPENSES | | | | | | | | | | | | | | | | |
General and administrative expenses | | | 1,782,957 | | | | 2,573,414 | | | | 4,591,620 | | | | 6,662,579 | |
Salaries and wages | | | 1,317,782 | | | | 1,252,680 | | | | 4,030,823 | | | | 3,279,803 | |
Sales and marketing expenses | | | 41,848 | | | | 157,435 | | | | 326,291 | | | | 942,759 | |
Depreciation and amortization expense | | | 304,139 | | | | 248,530 | | | | 937,000 | | | | 478,466 | |
TOTAL OPERATING EXPENSES | | | 3,446,726 | | | | 4,232,059 | | | | 9,885,734 | | | | 11,363,607 | |
| | | | | | | | | | | | | | | | |
LOSS FROM OPERATIONS | | | (2,239,864 | ) | | | (3,017,301 | ) | | | (6,577,998 | ) | | | (8,244,938 | ) |
| | | | | | | | | | | | | | | | |
OTHER INCOME (EXPENSE) | | | | | | | | | | | | | | | | |
Other income (expense), net | | | (1,921 | ) | | | 14,404 | | | | 160,598 | | | | (14,330 | ) |
Interest expense | | | (181,429 | ) | | | (151,274 | ) | | | (692,547 | ) | | | (529,782 | ) |
Interest income | | | 101,236 | | | | 110,596 | | | | 309,031 | | | | 555,281 | |
Gain on equity investments, net | | | 428 | | | | (1,093 | ) | | | 2,518 | | | | 2,876 | |
Gain on extinguishment of debt | | | - | | | | 706 | | | | - | | | | 1,911,476 | |
Change in fair value of derivative liability | | | - | | | | - | | | | - | | | | 3,384 | |
Bargain purchase gain | | | - | | | | - | | | | - | | | | 1,633,842 | |
Foreign currency transaction, net | | | 139,016 | | | | (371,115 | ) | | | 158,463 | | | | (108,406 | ) |
TOTAL OTHER INCOME (EXPENSE), NET | | | 57,330 | | | | (397,776 | ) | | | (61,937 | ) | | | 3,454,341 | |
| | | | | | | | | | | | | | | | |
LOSS BEFORE INCOME TAXES | | | (2,182,534 | ) | | | (3,415,077 | ) | | | (6,639,935 | ) | | | (4,790,597 | ) |
| | | | | | | | | | | | | | | | |
INCOME TAX EXPENSE | | | - | | | | 65,873 | | | | - | | | | - | |
| | | | | | | | | | | | | | | | |
NET LOSS | | $ | (2,182,534 | ) | | $ | (3,349,204 | ) | | $ | (6,639,935 | ) | | $ | (4,790,597 | ) |
| | | | | | | | | | | | | | | | |
Deemed dividend on issuance of warrants | | | (6,185,231 | ) | | | - | | | | (6,185,231 | ) | | | | |
Deemed dividend on downround of warrants | | | - | | | | (15,053 | ) | | | - | | | | (15,053 | ) |
Deemed dividend on warrant exchange/modification | | | (9,793 | ) | | | - | | | | (9,793 | ) | | | | |
| | | | | | | | | | | | | | | | |
NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS | | $ | (8,377,558 | ) | | $ | (3,364,257 | ) | | $ | (12,834,959 | ) | | $ | (4,805,650 | ) |
| | | | | | | | | | | | | | | | |
OTHER COMPREHENSIVE INCOME (LOSS) | | | | | | | | | | | | | | | | |
Foreign currency translation adjustment, net | | | 747,879 | | | | (890,645 | ) | | | (27,988 | ) | | | (470,994 | ) |
| | | | | | | | | | | | | | | | |
TOTAL COMPREHENSIVE LOSS | | $ | (7,629,679 | ) | | $ | (4,254,902 | ) | | $ | (12,862,947 | ) | | $ | (5,276,644 | ) |
| | | | | | | | | | | | | | | | |
BASIC NET LOSS PER SHARE | | $ | (0.45 | ) | | $ | (0.27 | ) | | $ | (0.72 | ) | | $ | (0.42 | ) |
DILUTED NET LOSS PER SHARE | | $ | (0.45 | ) | | $ | (0.27 | ) | | $ | (0.72 | ) | | $ | (0.42 | ) |
| | | | | | | | | | | | | | | | |
WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING | | | | | | | | | | | | | | | | |
Basic | | | 18,418,287 | | | | 12,585,479 | | | | 17,724,305 | | | | 11,346,071 | |
Diluted | | | 18,418,287 | | | | 12,585,479 | | | | 17,724,305 | | | | 11,346,071 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
COSMOS HEALTH INC. |
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY AND MEZZANINE EQUITY |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Preferred Stock | | | Common Stock | | | Additional | | | | | | Treasury Stock | | | | | | Accumulated Other | | | Total | |
| | No. of Shares | | | Value | | | No. of Shares | | | Value | | | Paid-in Capital | | | Subscription Receivable | | | No. of Shares | | | Value | | | Accumulated Deficit | | | Comprehensive Loss | | | Stockholders' Equity | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at January 1, 2023 | | | - | | | $ | 372,414 | | | | 10,605,412 | | | $ | 10,606 | | | $ | 112,205,952 | | | $ | (4,750,108 | ) | | | 15,497 | | | $ | (816,707 | ) | | $ | (66,232,813 | ) | | $ | (1,132,635 | ) | | $ | 39,284,295 | |
Foreign currency translation adjustment, net | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 336,463 | | | | 336,463 | |
Proceeds from sale of common stock | | | | | | | | | | | | | | | | | | | | | | | 4,750,000 | | | | | | | | | | | | | | | | | | | | 4,750,000 | |
Shares issued in lieu of cash | | | | | | | | | | | 15,258 | | | | 15 | | | | 96,873 | | | | | | | | | | | | | | | | | | | | | | | | 96,888 | |
Net loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (459,863 | ) | | | | | | | (459,863 | ) |
Balance at March 31, 2023 | | | - | | | $ | 372,414 | | | | 10,620,670 | | | $ | 10,621 | | | $ | 112,302,825 | | | $ | (108 | ) | | | 15,497 | | | $ | (816,707 | ) | | $ | (66,692,676 | ) | | $ | (796,172 | ) | | $ | 44,007,783 | |
Foreign currency translation adjustment, net | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 83,188 | | | | 83,188 | |
Shares issued for purchase of customer base | | | - | | | | - | | | | 99,710 | | | | 100 | | | | 315,981 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 316,081 | |
Shares issued for purchase of Cana | | | - | | | | - | | | | 46,377 | | | | 46 | | | | 138,621 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 138,667 | |
Stock-based compensation | | | - | | | | - | | | | 185,000 | | | | 185 | | | | 104,684 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 104,869 | |
Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (981,530 | ) | | | - | | | | (981,530 | ) |
Balance at June 30, 2023 | | | - | | | $ | 372,414 | | | | 10,951,757 | | | | 10,952 | | | | 112,862,111 | | | | (108 | ) | | | 15,497 | | | | (816,707 | ) | | | (67,674,206 | ) | | | (712,984 | ) | | | 43,669,058 | |
Foreign currency translation adjustment, net | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (890,645 | ) | | | (890,645 | ) |
Proceeds from sale of common stock, net of financing fees of $442,870 | | | - | | | | - | | | | 2,116,936 | | | | 2,117 | | | | 4,804,921 | | | | (49,892 | ) | | | - | | | | - | | | | - | | | | - | | | | 4,757,146 | |
Repurchase of treasury stock | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 71,000 | | | | (100,251 | ) | | | | | | | | | | | (100,251 | ) |
Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | 109,636 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 109,636 | |
Deemed dividend | | | - | | | | - | | | | - | | | | - | | | | 15,053 | | | | - | | | | - | | | | - | | | | (15,053 | ) | | | - | | | | - | |
Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (3,349,204 | ) | | | - | | | | (3,349,204 | ) |
Balance at September 30, 2023 | | | - | | | | 372,414 | | | | 13,068,693 | | | | 13,069 | | | | 117,791,721 | | | | (50,000 | ) | | | 86,497 | | | | (916,958 | ) | | | (71,038,463 | ) | | | (1,603,629 | ) | | | 44,195,740 | |
| | Preferred Stock | | | Common Stock | | | Additional | | | | | | Treasury Stock | | | | | | Other | | | Total | |
| | No. of Shares | | | Value | | | No. of Shares | | | Value | | | Paid-in Capital | | | Subscription Receivable | | | No. of Shares | | | Value | | | Accumulated Deficit | | | Comprehensive Loss | | | Stockholders' Equity | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at January 1, 2024 | | | - | | | $ | - | | | | 15,982,472 | | | $ | 15,983 | | | $ | 129,008,301 | | | $ | (20 | ) | | | 86,497 | | | $ | (917,159 | ) | | $ | (91,644,233 | ) | | $ | (419,844 | ) | | $ | 36,043,028 | |
Foreign currency translation adjustment, net | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (599,276 | ) | | | (599,276 | ) |
Proceeds from sale of common stock, net of financing fees of $19,467 | | | - | | | | - | | | | 901,488 | | | | 901 | | | | 628,525 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 629,426 | |
Shares issued in lieu of cash | | | - | | | | - | | | | - | | | | - | | | | 108,297 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 108,297 | |
Shares issued pursuant to warrant exchange agreement | | | - | | | | - | | | | 950,063 | | | | 950 | | | | (950 | ) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | 231,897 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 231,897 | |
Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (1,866,690 | ) | | | - | | | | (1,866,690 | ) |
Balance at March 31, 2024 | | | - | | | $ | - | | | | 17,834,023 | | | $ | 17,834 | | | $ | 129,976,070 | | | $ | (20 | ) | | | 86,497 | | | $ | (917,159 | ) | | $ | (93,510,923 | ) | | $ | (1,019,120 | ) | | $ | 34,546,682 | |
Foreign currency translation adjustment, net | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (176,591 | ) | | | (176,591 | ) |
Shares issued in lieu of cash | | | - | | | | - | | | | - | | | | - | | | | 108,444 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 108,444 | |
Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | 231,750 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 231,750 | |
Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (2,590,711 | ) | | | - | | | | (2,590,711 | ) |
Balance at June 30, 2024 | | | - | | | | - | | | | 17,834,023 | | | | 17,834 | | | | 130,316,264 | | | | (20 | ) | | | 86,497 | | | | (917,159 | ) | | | (96,101,634 | ) | | | (1,195,711 | ) | | | 32,119,574 | |
Foreign currency translation adjustment, net | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 747,879 | | | | 747,879 | |
Shares issued in lieu of cash | | | - | | | | - | | | | 2,500,000 | | | | 2,500 | | | | 155,561 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 158,061 | |
Stock-based compensation | | | - | | | | - | | | | 680,000 | | | | 680 | | | | 264,070 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 264,750 | |
Proceeds from exercise of warrants, net of financing fees of $372,109 | | | - | | | | - | | | | 2,332,000 | | | | 2,332 | | | | 3,866,537 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 3,868,869 | |
Deemed dividend on warrant inducement | | | - | | | | - | | | | - | | | | - | | | | 6,195,024 | | | | - | | | | - | | | | - | | | | (6,195,024 | ) | | | - | | | | - | |
Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (2,182,534 | ) | | | - | | | | (2,182,534 | ) |
Balance at September 30, 2024 | | | - | | | | - | | | | 23,346,023 | | | | 23,346 | | | | 140,797,456 | | | | (20 | ) | | | 86,497 | | | | (917,159 | ) | | | (104,479,192 | ) | | | (447,832 | ) | | | 34,976,599 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
COSMOS HEALTH INC. |
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS |
| | | | | | |
| | Nine Months Ended September 30, | |
| | 2024 | | | 2023 | |
| | | | | | |
CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | |
Net Loss | | $ | (6,639,935 | ) | | $ | (4,790,597 | ) |
Adjustments to Reconcile Net Loss to Net Cash Used In Operating Activities: | | | | | | | | |
Depreciation and amortization expense | | | 914,493 | | | | 375,918 | |
Amortization of right-of-use assets | | | 22,507 | | | | 102,549 | |
Bad debt expense | | | (31,287 | ) | | | 836,300 | |
Provision for extraordinary tax charges | | | - | | | | 579,387 | |
Shares issued in lieu of cash | | | - | | | | 96,888 | |
Lease expense | | | 235,659 | | | | 178,893 | |
Interest on finance leases | | | 1,765 | | | | 20,629 | |
Stock-based compensation | | | 1,103,200 | | | | 214,505 | |
Deferred income taxes | | | - | | | | (1,923 | ) |
Gain on extinguishment of debt | | | - | | | | (1,911,476 | ) |
Bargain purchase gain | | | - | | | | (1,633,842 | ) |
Change in fair value of the derivative liability | | | - | | | | (3,384 | ) |
Gain on net change in fair value of equity investments | | | (2,518 | ) | | | (2,876 | ) |
Other income | | | - | | | | (928 | ) |
Changes in assets and liabilities: | | | | | | | | |
Accounts receivable | | | 2,392,104 | | | | (1,960,236 | ) |
Accounts receivable - related party | | | (176,512 | ) | | | (416,814 | ) |
Inventory | | | (46,301 | ) | | | (2,299,829 | ) |
Prepaid expenses and other assets | | | (283,932 | ) | | | (1,856,642 | ) |
Prepaid expenses and other current assets - related party | | | (1,873,513 | ) | | | (2,312,324 | ) |
Loan receivable - related party | | | - | | | | - | |
Accounts payable and accrued expenses | | | (437,391 | ) | | | 206,746 | |
Accounts payable and accrued expenses - related party | | | 795,471 | | | | (112,233 | ) |
Accrued interest | | | 17,531 | | | | (194,361 | ) |
Lease liabilities | | | (234,834 | ) | | | (179,081 | ) |
Taxes payable | | | - | | | | 307,357 | |
Other current liabilities | | | 910,885 | | | | (783,174 | ) |
Other liabilities | | | (550,606 | ) | | | (1,047,178 | ) |
NET CASH USED IN OPERATING ACTIVITIES | | | (3,883,215 | ) | | | (16,587,726 | ) |
| | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | |
Proceeds from loan receivable | | | 550,565 | | | | 609,455 | |
Cash paid for the acquisition of Cana | | | - | | | | (5,230,593 | ) |
Loan receivable - related party | | | - | | | | (168,469 | ) |
Sale of intangible assets | | | 1,999 | | | | - | |
Advances for building's acquisition | | | - | | | | (1,665,000 | ) |
Purchase of intangible assets | | | - | | | | (2,678,167 | ) |
Purchase of property and equipment | | | (345,593 | ) | | | (1,266,490 | ) |
NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES | | | 206,971 | | | | (10,399,264 | ) |
| | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | |
Payment of convertible note payable | | | - | | | | (100,000 | ) |
Payment of note payable | | | (814,267 | ) | | | (1,494,867 | ) |
Proceeds from note payable | | | 434,797 | | | | 1,059,300 | |
Payment of related party loan | | | (7,609 | ) | | | - | |
Proceeds from related party loan | | | 15,218 | | | | - | |
Payment of lines of credit | | | (19,504,594 | ) | | | (14,569,517 | ) |
Proceeds from lines of credit | | | 18,831,043 | | | | 14,218,787 | |
Proceeds from issuance of Series A Preferred Stock | | | - | | | | - | |
Proceeds from the issuance of common stock | | | 649,039 | | | | 9,950,037 | |
Proceeds from the exercise of warrants | | | 4,240,977 | | | | - | |
Payments of finance lease liability | | | (26,408 | ) | | | (118,847 | ) |
Payments for purchase of treasury stock | | | - | | | | (100,251 | ) |
Payments of financing fees | | | (391,575 | ) | | | (442,892 | ) |
NET CASH PROVIDED BY FINANCING ACTIVITIES | | | 3,426,621 | | | | 8,401,750 | |
| | | | | | | | |
Effect of exchange rate changes on cash | | | (268,727 | ) | | | 196,161 | |
| | | | | | | | |
NET CHANGE IN CASH | | | (518,350 | ) | | | (18,389,079 | ) |
| | | | | | | | |
CASH AT BEGINNING OF YEAR | | | 3,833,195 | | | | 20,749,683 | |
CASH AT END OF YEAR | | $ | 3,314,845 | | | $ | 2,360,604 | |
| | | | | | | | |
Supplemental Disclosure of Cash Flow Information | | | | | | | | |
| | | | | | | | |
Cash paid during the year: | | | | | | | | |
Interest | | $ | 198,194 | | | $ | 317,449 | |
Income tax | | $ | - | | | $ | - | |
| | | | | | | | |
Supplemental Disclosure of Non-Cash Investing and Financing Activities | | | | | | | | |
| | | | | | | | |
Common shares issued for acquisition of customer base | | $ | - | | | $ | 316,081 | |
Common shares issued for acquisition of Cana | | $ | - | | | $ | 138,667 | |
Closing of acquisition of Cloudscreen | | $ | 637,080 | | | $ | - | |
Deemed dividend upon warrant exchange | | $ | 6,195,024 | | | $ | - | |
Common stock issued to employees | | $ | 372,303 | | | $ | - | |
Common stock issued to consultants | | $ | 727,570 | | | $ | - | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
NOTE 1 – BASIS OF PRESENTATION
The terms “COSM,” “we,” the “Company,” the “Group” and “us” as used in this report refer to Cosmos Health Inc. The accompanying unaudited condensed consolidated balance sheet as of September 30, 2024 and unaudited condensed consolidated statements of operations and comprehensive loss for the three and nine months ended September 30, 2024 have been prepared in accordance with generally accepted accounting principles for interim financial information and with the instructions to Form 10-Q and Article 8 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by U.S. generally accepted accounting principles for complete financial statements. In the opinion of the management of COSM, all adjustments (consisting of normal recurring accruals) considered necessary for a fair presentation have been included. Operating results for the three and nine months ended September 30, 2024, are not necessarily indicative of the results that may be expected for the year ending December 31, 2024, or any other period. These unaudited condensed consolidated financial statements and notes should be read in conjunction with the financial statements for the year ended December 31, 2023, included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 (“Form 10-K”). The accompanying condensed consolidated balance sheet as of December 31, 2023 has been derived from the audited financial statements filed in our Form 10-K and is included for comparison purposes on the accompanying balance sheet.
Going Concern
The Company’s unaudited condensed consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”), which contemplates the continuation of the Company as a going concern. For the nine months ended September 30, 2024, the Company had revenue of $40,202,238, net loss of $6,639,935 and net cash used in operations of $3,883,215. Additionally, as of September 30, 2024, the Company had positive working capital of $11,027,653, an accumulated deficit of $104,479,192, and stockholders’ equity of $34,976,599. It is the management’s opinion that these conditions raise substantial doubt about the Company’s ability to continue as a going concern for a period of twelve months from the date of this filing.
The Company’s revenues are not able to sustain its operations, and concerns exist regarding the Company’s ability to meet its obligations as they become due. The Company is subject to a number of risks to those of smaller commercial companies, including dependence on key individuals and products, the difficulties inherent in the development of a commercial market, the need to obtain additional capital, competition from larger companies, and other pharmaceutical and health care companies.
Management evaluated the above conditions which raise substantial doubt about the Company’s ability to continue as a going concern to determine if it can meet its obligations for the subsequent twelve months from the date of this filing. Management considered its ability to access future capital, curtail expenses if needed, expand product lines, and acquire new products.
Management’s plans include expansion of brand name products to the market, expanding the current product portfolio, and evaluating acquisition targets to expand distribution. Furthermore, the Company intends to vertically integrate the supply chain distribution network. During the period up to the issuance of this report the Company has signed multiple distribution agreements for its SPL products in Europe and Asia and a variety of contract manufacturing agreements though its subsidiary, Cana Laboratories Holdings (Cyprus) Limited. Finally, the Company plans to further access the capital markets in order to raise additional funds through equity offerings. More specifically, up to the issuance of its consolidated financial statements for the nine months ended September 30, 2024, the Company has sold 901,488 shares of common stock for net proceeds of $629,426 through two Prospectus Supplements to its Registration Statement on Form S-3 (No. 333-267550) filed with the SEC on February 29 and March 7, 2024. Management will also consider postponing the repayment of its outstanding Trade Facility ($1,588,163 balance as of September 30, 2024), intends to make substantial efforts to receive additional debt financing through its subsidiary, Cosmofarm SA, and plans to raise additional equity funds through utilizing its outstanding warrants. Following such efforts, on September 26, 2024, the Company entered into a Warrant Inducement Letter with an investor pursuant to which the Company issued 9,748,252 new warrants and reduced the exercise price of 4,874,126 warrant shares from $1.45 to $0.8701 to induce exercise and receive gross cash proceeds of $4,240,977. On July 29, 2024 the Company’s subsidiary Cosmofarm SA entered into an agreement with a third-party lender in the principal amount of €400,000 ($432,760). Moreover, the Company’s management is considering postponing certain repayments of suppliers and creditors. However, management cannot provide any assurances that the Company will be successful in accomplishing any of its plans. The ability of the Company to continue as a going concern is dependent upon its ability to successfully accomplish the plans described herein and eventually secure other sources of financing and attain profitable operations.
Considering the above, management is of the view that substantial doubt exists about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments to reflect the possible future effect on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from the outcome of this uncertainty.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
NOTE 2 – ORGANIZATION AND NATURE OF BUSINESS
Cosmos Health Inc. and its subsidiaries (Nasdaq: COSM), (“us”, “we”, the “Group”, or the “Company”) are an international healthcare group headquartered in Thessaloniki, Greece. The group is engaged in the nutraceuticals sector through its own proprietary lines of products “Sky Premium Life” and “Mediterranation”. The Company is operating in the pharmaceutical sector as well, through the provision of a broad line of branded generics and OTC medications. In addition, the group is involved in the healthcare distribution sector through its subsidiaries in Greece and the UK, serving retail pharmacies and wholesale distributors. The Company is strategically focusing on the research and development (“R&D”) of novel patented nutraceuticals (Intellectual Property) and specialized root extracts as well as on the R&D of proprietary complex generics and innovative OTC products. The Company has developed a global distribution platform and is currently expanding throughout Europe, Asia and North America. The Company has offices and distribution centers in Thessaloniki and Athens, Greece and Harlow, UK.
The Company was incorporated in the State of Nevada under the name Prime Estates and Developments, Inc. on July 21, 2009. On November 14, 2013, we changed our name to Cosmos Holdings Inc., and on November 29, 2022, we changed our name to Cosmos Health Inc. Through its acquisition of Amplerissimo Ltd, on September 27, 2013, the Company changed its principal activities into trading of products, providing representation, and provision of consulting services to various sectors. On August 1, 2014, the Company formed SkyPharm S.A., a Greek Company (“SkyPharm”), a subsidiary that used to focus on the trading, sourcing and export of nutraceutical and pharmaceutical products. In February 2017, the Company acquired Decahedron Ltd., a UK Company (“Decahedron”) which is a fully licensed second-generation wholesaler specializing in imports and exports of generics and OTC pharmaceutical products within the EEA and distributor of Sky Premium Life nutraceutical products in the UK. On December 19, 2018, the Company acquired Cosmofarm, a pharmaceutical wholesaler specializing in the distribution and export of pharmaceutical products through its extensive pharmacies network. On April 3, 2023, the Company completed the acquisition of ZipDoctor Inc. (“ZipDoctor”), a telehealth company, a direct-to-consumer subscription-based telemedicine platform. On June 30, 2023, the Company acquired Cana Laboratories Holdings (Cyprus) Limited (“Cana”), which wholly owned an operating subsidiary, Pharmaceutical Laboratories Cana S.A. (“Cana SA”), a Greek pharmaceutical company that manufactures, sells, distributes, and markets original branded products researched and developed by leading global pharmaceutical and healthcare companies.
Acquisition Accounting
Cloudscreen
On January 23, 2024, the Company completed the acquisition of Cloudscreen, a cutting-edge Artificial Intelligence (AI) powered platform. The acquisition is pursuant to the purchase agreement announced on October 11, 2023. Cloudscreen is a multimodal platform specialized in drug repurposing, a process that involves uncovering new target proteins or indications for existing drugs for use in treating different diseases. The total purchase price amounted to $637,080 and consisted of 280,000 shares of common stock with a fair value of $319,200 and an amount of $317,880 to be settled in cash during 2024 based on the Promissory Note signed on October 10, 2023. The Company accounted for the acquisition as an asset acquisition in accordance with Accounting Standards Codification (“ASC”) Topic 805, Business Combinations, (“ASC 805”) and recorded $637,080 as an intangible asset related to the technology platform acquired.
ZipDoctor
On April 3, 2023, the Company completed the acquisition of ZipDoctor Inc. (“ZipDoctor”), a telehealth company for a total sum of $150,000 in cash and $8,788 in fees. The Company accounted for the acquisition as an asset acquisition in accordance with Accounting Standards Codification (“ASC”) Topic 805, Business Combinations, (“ASC 805”) and recorded $158,788 as an intangible asset related to the technology platform acquired.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Bikas
On June 15, 2023, Cosmos Health Inc. entered into an Assignment and Assumption Agreement (the “Agreement”) with Ioannis Bikas O.E., a Greek Company (“Bikas”). Bikas is owner of a pharmaceutical distribution network in Greece and agreed to sell to the Company their distribution network and customer base. The purchase price of the network was €100,000 ($109,330) in cash, and €300,000 ($316,081) in the Company’s common stock. The Company issued 99,710 shares of common stock related to the acquisition of the customer base, based on the fair value of the stock on the acquisition date. The Company accounted for the acquisition as an asset acquisition in accordance with ASC 805 and recorded $425,411 as an intangible asset related to the customer base acquired.
Buildings Acquisitions
On April 24, 2023, the Company purchased a building for a total sum of $1,054,872 in cash. The Company accounted for the acquisition as an asset acquisition in accordance with ASC 805 and recorded the cost of the building as “Property, plant and equipment” on the consolidated balance sheets.
On January 6, 2023, the Company agreed to purchase land and building located in Montreal, Canada from a third-party vendor. The total purchase price amounts to $3,950,000 and the closing date of the agreement based on the amendment signed on July 19, 2023, is December 31, 2023. As of September 30, 2024, the Company has made no additional prepayments concerning this building. The closing date of the agreement has been extended to December 31, 2024.
Cana
On June 30, 2023, the Company acquired Cana Laboratories Holdings (Cyprus) Limited (“Cana”), which wholly owned an operating subsidiary, Pharmaceutical Laboratories Cana S.A. (“Cana SA”) for €800,000 ($873,600) in cash and 46,377 shares of common stock, with fair value of $138,667 as of the date of acquisition. Moreover, on February 28, 2023, the Company had signed a Secured Promissory Note with Cana, whereby Cana borrowed the sum of €4,100,000 ($4,457,520), included in the total consideration of $5,469,787. The Company accounted for the acquisition as a business acquisition in accordance with ASC 805. The fair value of Cana assets acquired, and liabilities assumed was based upon management’s estimates assisted by an independent third-party valuation firm. The fixed assets of Cana (which included land, building & machinery) were valued as of December 31, 2022 and the Company believes that nothing has materially changed between such date and the acquisition date (June 30, 2023). The following table summarizes the preliminary allocation of purchase price of the acquisition:
Consideration | | | |
Cash | | $ | 5,331,120 | |
Fair value of common stock issued | | | 138,667 | |
Fair value of total consideration transferred | | $ | 5,469,787 | |
| | | | |
Recognized amounts of identifiable assets acquired | | | | |
Financial assets | | $ | 1,796,911 | |
Inventory | | | 297,340 | |
Property, plant and equipment | | | 7,488,818 | |
Identifiable intangible assets | | | 562,200 | |
Financial liabilities | | | (3,235,233 | ) |
Total identifiable net assets | | $ | 6,910,036 | |
| | | | |
Bargain purchase gain | | $ | 1,440,249 | |
Revenue for the 9 - month period ended September 30, 2024 | | $ | 549,567 | |
Loss for the 9 - month period ended September 30, 2024 | | $ | (1,674,785 | ) |
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
During the prior year period, Cana had minimal operations as it was in financial difficulties and seeking for an investor.
Basis of Financial Statement Presentation
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with U.S. GAAP.
Principles of Consolidation
Our consolidated accounts include our accounts and the accounts of our wholly owned subsidiaries, SkyPharm S.A., Decahedron Ltd., Cosmofarm S.A., Cana Laboratories Holdings (Cyprus) Limited and ZipDoctor Inc. The Group’s financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”). The consolidated financial statements reflect the consolidation of all entities in which the Company has control, as determined by the ability to direct the activities that significantly affect the entities’ economic performance. All significant intercompany balances and transactions have been eliminated.
Transactions in and Translations of Foreign Currency
The functional currency for the Greek subsidiaries of the Company (CANA Laboratories, Cosmofarm S.A. and SkyPharm SA) is Euro (€) and for the UK subsidiary (Decahedron Ltd) is GBP (£). ZipDoctor Inc. is a U.S. based entity. As a result, the financial statements of the subsidiaries (except for ZipDoctor Inc.) have been translated from the local currency into U.S. dollars using (i) year-end exchange rates for balance sheet accounts, and (ii) average exchange rates for the reporting period for all income statements accounts. Foreign currency translations gains and losses are reported as a separate component of the condensed consolidated statements of changes in stockholders’ equity and mezzanine equity.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
The Effects of War in the Ukraine
On February 24, 2022, Russian forces launched significant military action against Ukraine. There continues to be sustained conflict and disruption in the region, which is expected to endure for the foreseeable future. We do not conduct any commercial transactions with either Ukraine or Russia and the Company and, as such, is not aware of any specific event or circumstance that would require an update to its estimates or judgments or a revision of the carrying value of its assets or liabilities as of the date of issuance of this Quarterly Report on Form 10-Q. Such political issues and conflicts could have a material adverse effect on our results of operations and financial condition if they escalate in areas in which we do business. In addition, changes in and adverse actions by governments in foreign markets in which we do business could have a material adverse effect on our results of operations and financial condition.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Credit Losses
In June 2016, the FASB issued ASU 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses of Financial Instruments, which amends the requirement on the measurement and recognition of expected credit losses for financial assets held. Furthermore, amendments ASU 2019-10 and ASU 2019-11 provided additional clarification for implementing ASU 2016-13. ASU 2016-13 is effective for the Company beginning January 1, 2023, with early adoption permitted. The Company adopted the standard on January 1, 2023, and the standard did not have a material impact on the Company’s consolidated financial statements and related disclosures. The Company is exposed to credit losses primarily through sales to its customers and the loans that it has provided. The Company assesses each customer’s/ borrower’s ability to pay, and a credit loss estimate by conducting a credit review which includes consideration of established credit rating, or an internal assessment of the customer’s creditworthiness based on an analysis of their payment history when a credit rating is not available. The Company monitors credit exposure through active review of customer balances. The Company’s expected loss methodology for accounts receivable is developed through consideration of factors including, but not limited to, historical collection experience, current customer credit ratings, current customer financial condition, current and future economic and market conditions, and age of the receivables. More specifically, the Company assesses a number of customers with significant long outstanding balances on an individual basis, applying different credit loss percentages to them, and subsequently summarizes the ones not included in the individual analysis, groups them based on their rating (decided based on the factors described above) and applies specific credit loss percentages to each group. The Company has elected to follow the simplified ECL approach. The charges related to credit losses are included in “General and administrative expenses” and are recorded in the period that the outstanding receivables are determined to be doubtful. Account balances are written-off against the allowance when they are deemed uncollectible.
Cash and Cash Equivalents
For purposes of the statement of cash flows, the Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents.
The Company maintains bank accounts in the United States denominated in U.S. Dollars, in Greece denominated in Euros, U.S. Dollars and Great Britain Pounds (British Pounds Sterling), and in Bulgaria denominated in Euros. The Company also maintains bank accounts in the United Kingdom, denominated in Euros and Great Britain Pounds (British Pounds Sterling).
Accounts Receivable, net
Accounts receivable are stated at their net realizable value. The allowance for doubtful accounts against gross accounts receivable, prepaid expenses and other current assets and other assets reflects the best estimate of probable losses inherent in the receivables’ portfolio determined on the basis of historical experience, specific allowances for known troubled accounts and other currently available information. As of September 30, 2024 and December 31, 2023, the Company’s allowance for doubtful accounts was $19,905,776 and $19,686,091, respectively. Below is the summary of changes in the allowance for doubtful accounts:
| | September 30, 2024 | |
| | | |
Balance as of January 1st, 2024 | | $ | 19,686,091 | |
Provisions for credit losses | | | - | |
Write-offs | | | 250,971 | |
Foreign exchange adjustments | | | | |
Other adjustments | | | (31,286 | ) |
Balance as of September 30, 2024 | | $ | 19,905,776 | |
Tax Receivables
The Company pays Value Added Tax (“VAT”) or similar taxes (“input VAT”), income taxes, and other taxes within the normal course of its business in most of the countries in which it operates related to the procurement of merchandise and/or services it acquires and/or on sales and taxable income. The Company also collects VAT or similar taxes on behalf of the government (“output VAT”) for merchandise and/or services it sells. If the output VAT exceeds the input VAT, this creates a VAT payable to the government. If the input VAT exceeds the output VAT, this creates a VAT receivable from the government. The VAT tax return is filed on a monthly basis offsetting the payables against the receivables. In observance of EU regulations for intra-EU cross-border sales, our subsidiaries in Greece, SkyPharm and Cosmofarm, do not charge VAT for sales to wholesale drug distributors registered in other European Union member states. As of September 30, 2024 and December 31, 2023, the Company had a VAT net receivable balance of $322,576 and $187,512 respectively, recorded in the consolidated balance sheet as prepaid expenses and other current assets and accounts payable and accrued expenses, respectively.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Inventory
Inventory is stated at the lower-of-cost or net realizable value using the weighted average method. Inventory consists primarily of finished goods and packaging materials, i.e., packaged pharmaceutical products and the wrappers and containers they are sold in. A periodic inventory system is maintained by 100% count. Inventory is replaced periodically to maintain the optimum stock on hand available for immediate shipment.
The Company writes down inventories to net realizable value based on physical condition, expiration date, and current market conditions, as well as forecasted demand. The Company’s inventories are not highly susceptible to obsolescence. Many of the Company’s inventory items are eligible for return to our suppliers when pre-agreed product requirements, including, but not limited to, physical condition and expiration date, are not met. No significant judgments have been applied in estimating the selling price of our inventory.
Property and Equipment, net
Property and equipment are stated at cost, less accumulated depreciation. Depreciation is calculated on a straight-line basis over the useful lives (except for leasehold improvements which are depreciated over the lesser of the lease term or the useful life) of the assets as follows:
| | Estimated Useful Life | |
Leasehold improvements and technical works | | | Lesser of lease term or 25 years | |
Buildings | | | 25-30 years | |
Vehicles | | | 6 years | |
Machinery | | | 20 years | |
Furniture, fixtures and equipment | | | 5–10 years | |
Computers and software | | | 3-5 years | |
Depreciation expense was $89,694 and $124,910 for the three months ended September 30, 2024 and 2023, respectively and $306,126 and $237,479 for the nine months ended September 30, 2024 and 2023, respectively.
Property and Equipment additions
Property and Equipment additions are recognized as assets when it is probable that future economic benefits associated with the asset will flow to the entity and the cost of the asset can be measured reliably. Additions are initially measured at cost, which includes all costs directly attributable to bringing the asset to its working condition and location for its intended use. This may include purchase price, freight, installation, and any directly attributable professional fees. They are capitalized if their cost exceeds a certain threshold. The threshold is determined based on materiality considerations. Costs below the threshold are typically expensed as incurred. After initial recognition, additions are measured at cost less accumulated depreciation and any accumulated impairment losses. Depreciation is calculated systematically over the estimated useful life of the asset. They are tested for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. If the carrying amount exceeds the recoverable amount, an impairment loss is recognized, and the carrying amount of the asset is adjusted accordingly. Borrowing costs directly attributable to the acquisition, construction, or production of qualifying assets, including Property and Equipment additions, are capitalized as part of the cost of those assets.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Goodwill and Intangibles, net
The Company periodically reviews the carrying value of intangible assets not subject to amortization, including goodwill, to determine whether impairment may exist. Goodwill and certain intangible assets are assessed annually, or when certain triggering events occur, for impairment using fair value measurement techniques. These events could include a significant change in the business climate, legal factors, a decline in operating performance, competition, sale or disposition of a significant portion of the business, or other factors. First, under step 0, we determine whether it is more likely than not that the fair value of the reporting unit is less than the carrying amount. Following, if step 0 fails, goodwill impairment is determined using a two-step process. The first step of the goodwill impairment test is used to identify potential impairment by comparing the fair value of a reporting unit with its carrying amount, including goodwill. The Company uses level 3 inputs and a discounted cash flow methodology to estimate the fair value of a reporting unit. A discounted cash flow analysis requires one to make various judgmental assumptions including assumptions about future cash flows, growth rates, and discount rates. The assumptions about future cash flows and growth rates are based on the Company’s budget and long-term plans. Discount rate assumptions are based on an assessment of the risk inherent in the respective reporting units. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is considered not impaired and the second step of the impairment test is unnecessary. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test is performed to measure the amount of impairment loss, if any. The second step of the goodwill impairment test compares the implied fair value of the reporting unit’s goodwill with the carrying amount of that goodwill. If the carrying amount of the reporting unit’s goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess. The implied fair value of goodwill is determined in the same manner as the amount of goodwill recognized in a business combination. That is, the fair value of the reporting unit is allocated to all of the assets and liabilities of that unit (including any unrecognized intangible assets) as if the reporting unit had been acquired in a business combination and the fair value of the reporting unit was the purchase price paid to acquire the reporting unit.
On December 19, 2018, as a result of the acquisition of Cosmofarm, the Company recorded $49,697 of goodwill.
Intangible assets with definite useful lives are recorded on the basis of cost and are amortized on a straight-line basis over their estimated useful lives. The Company uses a useful life of 5 years for an import/export license and a useful life of 10 years for the pharmaceutical and nutraceutical products licenses included in Note 4 as “Licenses”. A useful life of 10 years is also used for the platforms included in Note 4 as “Software” and the customer bases. The Company evaluates the remaining useful life of intangible assets annually to determine whether events and circumstances warrant a revision to the remaining amortization period. If the estimate of the intangible asset’s remaining useful life is changed, the remaining carrying amount of the intangible asset will be amortized prospectively over that revised remaining useful life. As of September 30, 2024 and December 31, 2023, no revision to the remaining amortization period of the intangible assets was made.
Amortization expense was $196,183 and $88,168 for the three months ended September 30, 2024 and 2023, respectively and $579,556 and $138,438 for the nine months ended September 30, 2024 and 2023, respectively.
Impairment of Long-Lived Assets
In accordance with ASC 360-10, Long-lived Assets, property and equipment and intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the assets. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Equity Method Investment
For those investments in common stock or in-substance common stock in which the Company has the ability to exercise significant influence over the operating and financial policies of the investee, the investment is accounted for under the equity method. The Company records its share in the earnings of the investee and is included in “Equity earnings of affiliate” in the consolidated statement of operations. The Company assesses its investment for other-than-temporary impairment when events or changes in circumstances indicate that the carrying amount of the investment might not be recoverable and recognizes an impairment loss to adjust the investment to its then current fair value.
Investments in Equity Securities
Investments in equity securities are accounted for at fair value with changes in fair value recognized in net income (loss). Equity securities are classified as short-term or long-term based on the nature of the securities and their availability to meet current operating requirements. Equity securities that are readily available for sale in current operations are reported as a component of current assets on the accompanying consolidated balance sheets. Equity securities that are not considered available for use in current operations would be reported as a component of long-term assets on the accompanying consolidated balance sheets. For equity securities with no readily determinable fair value, the Company elects a measurement alternative to fair value. Under this alternative, the Company measures the investments at cost, less any impairment, and adjusted for changes resulting from observable price changes in transactions for identical or similar investments of the investee. The election to use the measurement alternative is made for each eligible investment.
As of September 30, 2024, investments consisted of 16,666 shares which traded at a closing price of $0.75 per share or value of $12,416 of National Bank of Greece. Additionally, the Company has $7,665 in equity securities of Pancreta Bank, which are revalued annually.
Fair Value Measurement
The Company applies ASC 820, Fair Value Measurements and Disclosures, (“ASC 820”), for assets and liabilities measured at fair value on a recurring basis. ASC 820 establishes a common definition for fair value to be applied to existing generally accepted accounting principles that require the use of fair value measurements establishes a framework for measuring fair value and expands disclosure about such fair value measurements.
ASC 820 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Additionally, ASC 820 requires the use of valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs. These inputs are prioritized below:
Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: Inputs other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
Level 3: Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use.
In addition, ASC 825-10-25, Fair Value Option, (“ASC 825-10-25”), expands opportunities to use fair value measurements in financial reporting and permits entities to choose to measure many financial instruments and certain other items at fair value. The Company did not elect the fair value options for any of its qualifying financial instruments.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Our financials also included the following financial instruments as of September 30, 2024 and December 31, 2023: cash, accounts receivable, inventory, prepaid expenses, loans receivable, accounts payable, notes payable and lines of credit. Except for the loans receivable which carry fixed interest rates, the carrying value of the remaining instruments, approximates fair value due to their short-term nature.
Customer Advances
The Company receives prepayments from certain customers for pharmaceutical products prior to those customers taking possession of the Company’s products. The Company records these receipts as current liabilities until it has met all the criteria for recognition of revenue including passing control of the products to its customer, at such point, the Company will reduce the customer advances balance and credit the Company’s revenues.
Revenue Recognition
In accordance with ASC Topic 606, Revenue from Contracts with Customers (“ASC 606”), the Company uses a five-step model for recognizing revenue by applying the following steps:
| 1) | Identification of the Contract: The Company identifies a contract with a customer when it enters into an agreement that creates enforceable rights and obligations. |
| 2) | Identification of Performance Obligations: The Company identifies distinct performance obligations within each contract, which represent promises to transfer goods or services to the customer. |
| 3) | Determination of Transaction Price: The Company determines the transaction price, which represents the amount of consideration to which it expects to be entitled in exchange for transferring promised goods or services to the customer, excluding any amounts collected on behalf of third parties. |
| 4) | Allocation of Transaction Price: The Company allocates the transaction price to each distinct performance obligation based on its standalone selling price. If the standalone selling price is not observable, the Company estimates it using an appropriate method. |
| 5) | Recognition of Revenue: Revenue is recognized when (or as) the Company satisfies a performance obligation by transferring a promised good or service to the customer. This typically occurs at a point in time or over time, depending on the nature of the performance obligation. |
Wholesale revenue and sales of own branded nutraceutical and pharmaceutical products
The Company has contracts or signed partnership forms (usual in the wholesale sector of the pharma industry) with its customers, stipulating enforceable rights and obligations. The Company is responsible for transferring the goods to the customer’s location, which represents its sole performance obligation. Thus, the transaction price, which is predetermined in most of the products sold, is exclusively allocated to this performance obligation. Revenue is recognized at a single point in time, which is upon issuance of the corresponding sales invoice. The Company has assessed the impact of the items invoiced but not delivered to the customer’s location as of December 31, 2023 and September 30, 2024, and deemed that it had no material effect.
Pharma manufacturing
The Company has active contracts with its customers, stipulating enforceable rights and obligations. The Company is responsible for the manufacturing and the packaging of specific products assigned by its customers, which represents its performance obligations to which the Company allocates the transaction price determined. The customers are responsible for providing the raw materials to the Company. Revenue is recognized over a period of time, which is during the production and packaging period of the respective products. As of September 30, 2024, there were no products or batches of products for which the production or packaging phase was in progress.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Medihelm SA
Commencing from January 1, 2023, and pursuant to the agreement with Medihelm, the exclusive distributor of the Company’s own proprietary line of nutraceuticals, the Company considers the transaction price to be variable and records an estimate of the transaction price, subject to the constraint for variable consideration. The Company is basing the change in transaction price with the exclusive distributor through assessment of significant overdue receivables from the exclusive distributor, which the Company reassesses each reporting period. Through this assessment, the Company applied the “expected value” model under ASC 606-10-32-5 and had applied specific constraints to revenue due from the customer at the end of each reporting period. Following the application of the “expected value” model, the Company had deferred an amount of $397,000 and recorded it against the sales to Medihelm for the twelve months ended December 31, 2023. However, the Company assessed once more the trading relationship with Medihelm SA at year end and since no significant receipts had taken place up to the issuance of the report, the Company recorded an allowance for the total receivable amount not received up to the issuance date. More specifically a cumulative reserve of $12,655,615 was applied, leaving a receivable of $532,704 due from Medihelm SA, as of December 31, 2023. The Company does not consider that new sales to Medihelm SA or sales to any other customer include a variable component as of September 30, 2024 and has limited such sales to the minimum required.
Stock-based Compensation
The Company records stock-based compensation in accordance with ASC 718, Stock Compensation (“ASC 718”) and Staff Accounting Bulletin No. 107 (“SAB 107”) regarding its interpretation of ASC 718. ASC 718 requires the fair value of all stock-based employee compensation awarded to employees to be recorded as an expense over the related requisite service period. The Company values any employee or non-employee stock-based compensation at fair value using the Black-Scholes Option Pricing Model.
The Company accounts for non-employee share-based awards in accordance with the measurement and recognition criteria of ASU 2018-07, “Compensation-Stock Compensation-Improvements to Nonemployee Share-Based Payment Accounting.”
Income Taxes
The Company accounts for income taxes under the asset and liability method, as required by the accounting standard for income taxes ASC 740. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis, as well as net operating loss carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
The Company is liable for income taxes in Greece and the United Kingdom The corporate income tax rate is 22% in Greece and 25% in the United Kingdom. Losses may also be subject to limitation under certain rules regarding change of ownership.
We regularly review deferred tax assets to assess their potential realization and establish a valuation allowance for portions of such assets to reduce the carrying value if we do not consider it to be more likely than not that the deferred tax assets will be realized. Our review includes evaluating both positive (e.g., sources of taxable income) and negative (e.g., recent historical losses) evidence that could impact the realizability of our deferred tax assets. At September 30, 2024, we believe our United Kingdom and Greece deferred tax assets will not be realized, as such, we did not record a reversal on the full valuation approach we followed during the year ended December 31, 2023.
Leases
The Company accounts for leases in accordance with ASC 842. For all leases, the Company recognizes a right-of-use (ROU) asset and a lease liability on the balance sheet. The ROU asset represents the Company's right to use the underlying asset for the lease term, and the lease liability represents the obligation to make lease payments arising from the lease, both measured at the present value of future lease payments. Lease payments are recognized as an operating expense on a straight-line basis over the lease term. The interest on the lease liability and the amortization of the ROU asset are recognized separately in the income statement. Initial direct costs incurred by the Company in negotiating and securing leases are capitalized and amortized over the lease term on a straight-line basis. The assets and liabilities from operating and finance leases are recognized at the commencement date based on the present value of remaining lease payments over the lease term using the Company’s secured incremental borrowing rates or implicit rates, when readily determinable. Short-term leases, which have an initial term of 12 months or less, are not recorded on the balance sheet. The Company’s operating leases do not provide an implicit rate that can readily be determined. Therefore, we use a discount rate based on our incremental borrowing rate, which is determined using the average interest rate of our long-term debt on the date of inception.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Retirement and Termination Benefits
Under Greek labor law, employees are entitled to lump-sum compensation in the event of termination or retirement. The amount depends on the employee’s work experience and remuneration as of the day of termination or retirement. If an employee remains with the company until full-benefit retirement, the employee is entitled to a lump-sum equal to 40% of the compensation to be received if the employee were to be dismissed on the same day. The Company periodically reviews the uncertainties and judgments related to the application of the relevant labor law regulations to determine retirement and termination benefits obligations of its Greek subsidiaries. The Company has evaluated the impact of these regulations and has identified a potential retirement and termination benefits liability. The amount of the liability as of September 30, 2024 and December 31, 2023, was $395,698 and $408,665, respectively, and has been recorded as a long-term liability within the consolidated balance sheets.
Basic and Diluted Net Loss per Common Share
Basic income per share is calculated by dividing the income available to common stockholders by the weighted average number of shares of common stock outstanding during the period. Diluted income per share is calculated by dividing income available to common stockholders by the weighted average number of common shares outstanding for the period and, when dilutive, potential shares from stock options and warrants to purchase common stock, using the treasury stock method. In accordance with ASC 260, Earnings Per Share, the following table reconciles basic shares outstanding to fully diluted shares outstanding.
| | September 30, 2024 | | | September 30, 2023 | |
Weighted average number of common shares outstanding Basic | | | 17,724,305 | | | | 11,346,071 | |
Potentially dilutive common stock equivalents | | | - | | | | - | |
Weighted average number of common and equivalent shares outstanding – Diluted | | | 17,724,305 | | | | 11,346,071 | |
The following table summarizes potential common shares that were excluded as their effect is anti-dilutive:
| | September 30, 2024 | | | September 30, 2023 | |
Warrants | | | 13,432,507 | | | | 6,124,412 | |
Total | | | 13,432,507 | | | | 6,124,412 | |
Common stock equivalents are included in the diluted income per share calculation only when option exercise prices are lower than the average market price of the common shares for the period presented.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
In March 2022, the FASB issued ASU 2022-02, Troubled Debt Restructurings and Vintage Disclosures. This ASU eliminates the accounting guidance for troubled debt restructurings by creditors that have adopted ASU 2016-13, Measurement of Credit Losses on Financial Instruments, which was adopted on January 1, 2020. The adoption of ASU 2016-13 did not have a material impact on the Company’s consolidated financial statements. ASU 2022-02 also enhances the disclosure requirements for certain loan refinancing and restructurings by creditors when a borrower is experiencing financial difficulty. In addition, the ASU amends the guidance on vintage disclosures to require entities to disclose current period gross write-offs by year of origination for financing receivables and net investments in leases within the scope of ASC 326-20. The ASU is effective for annual periods beginning after December 15, 2022, including interim periods within those fiscal years. Adoption of the ASU would be applied prospectively. Early adoption is also permitted, including adoption in an interim period. This ASU was adopted on January 1, 2023, which resulted in no cumulative-effect adjustment to retained earnings.
Recent Accounting Pronouncements
In March 2024, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2024-01, Compensation—Stock Compensation (Topic 718): Scope Application of Profits Interest and Similar Awards. This guidance is intended to improve generally accepted accounting principles (GAAP) by adding an illustrative example to demonstrate how an entity should apply the scope guidance in paragraph 718- 10-15-3 to determine whether profits interest and similar awards (“profits interest awards”) should be accounted for in accordance with Topic 718, Compensation—Stock Compensation. The amendments in this Update are effective for annual periods beginning after December 15, 2024, and interim periods within those annual periods. The amendments in this Update should be applied either (1) retrospectively to all prior periods presented in the financial statements or (2) prospectively to profits interest and similar awards granted or modified on or after the date at which the entity first applies the amendments. The Company is currently evaluating this guidance to determine the impact it may have on its consolidated financial statements disclosures.
In March 2024, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2024-02, Codification Improvements—Amendments to Remove References to the Concepts Statements. This guidance is intended to remove references to various FASB Concepts Statements. The Board has a standing project on its agenda to address suggestions received from stakeholders on the Accounting Standards Codification and other incremental improvements to generally accepted accounting principles (GAAP). This effort facilitates Codification updates for technical corrections such as conforming amendments, clarifications to guidance, simplifications to wording or the structure of guidance, and other minor improvements. The resulting amendments are referred to as Codification improvements. The amendments in this Update are not intended to result in significant accounting change for most entities. The Company is currently evaluating this guidance to determine the impact it may have on its consolidated financial statements disclosures.
In December 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. This guidance is intended to enhance the transparency and decision-usefulness of income tax disclosures. The amendments in ASU 2023-09 address investor requests for enhanced income tax information primarily through changes to disclosure regarding rate reconciliation and income taxes paid both in the U.S. and in foreign jurisdictions. ASU 2023-09 is effective for fiscal years beginning after December 15, 2024, on a prospective basis, with the option to apply the standard retrospectively. Early adoption is permitted. The Company is currently evaluating this guidance to determine the impact it may have on its consolidated financial statements disclosures.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting: Improvements to Reportable Segment Disclosures. This guidance expands public entities’ segment disclosures primarily by requiring disclosure of significant segment expenses that are regularly provided to the chief operating decision maker and included within each reported measure of segment profit or loss, an amount and description of its composition for other segment items, and interim disclosures of a reportable segment’s profit or loss and assets. The guidance is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. The amendments are required to be applied retrospectively to all prior periods presented in an entity’s financial statements. The Company is currently evaluating this guidance to determine the impact it may have on its consolidated financial statements related disclosures.
Management does not believe that any recently issued, but not effective, accounting standards, if currently adopted, would have a material effect on the Company’s consolidated financial statements.
NOTE 3 – EQUITY METHOD INVESTMENTS
Distribution and Equity Agreement
On March 19, 2018, the Company entered into a Distribution and Equity Acquisition Agreement with Marathon Global Inc. (“Marathon”), a company incorporated in the Province of Ontario, Canada. Marathon was formed to be a global supplier of cannabis, cannabidiol (CBD) and/or any cannabis extract products, extracts, ancillaries and derivatives (collectively, the “Products”). The Company was appointed the exclusive distributor of the Products initially throughout Europe and on a non-exclusive basis wherever else lawfully permitted. The Company has no present intention to distribute any Products under this Agreement in the United States or otherwise participate in cannabis operations in the United States. The Company intended to await further clarification from the U.S. government on cannabis regulation prior to determining whether to enter the domestic market.
The above transaction closed on May 22, 2018 after the due diligence period, following which the Company received: (a) a 33 1/3% equity interest or 5 million shares in Marathon as partial consideration for the Company’s distribution services; and (b) received cash of CAD $2,000,000, subject to repayment in common shares of the Company if it failed to meet certain performance milestones. The Company was entitled to receive an additional CAD $2,750,000 upon the Company’s receipt of gross sales of CAD $6,500,000 and an additional CAD $2,750,000 upon receipt of gross sales of CAD $13,000,000. The Company was also given the right to nominate one director to the Marathon board of directors. Since Marathon was a newly formed entity with no assets and no activity, the Company attributed no value to the 5 million shares in Marathon which was received as consideration for the distribution services.
The Distribution and Equity Acquisition Agreement was to remain in effect indefinitely unless Marathon fails to provide Market Competitive (as defined) product pricing and Marathon has not become profitable within five (5) years of the agreement. On March 20, 2023, the Company sent a termination notice, to Marathon, which became effective on April 19, 2023 as a result of Marathon’s failure to satisfy these conditions. The Company had accounted for its obligation to issue a variable number of the Company’s Common Shares as Share-settled debt obligation in accordance with ASC Topic 480, Distinguishing Liabilities from Equity (“ASC 480”), which was measured at fair value or the settlement amount of $1,554,590 (CAD $2 million). Due to termination of the Equity Agreement, the Company recorded a gain on extinguishment of debt of $1,554,590 due to the write-off of the share settled debt obligation, for the nine months ended September 30, 2023.
CosmoFarmacy LP
In September 2019, the Company entered into an agreement with an unaffiliated third party to incorporate CosmoFarmacy L.P. for the purpose of providing strategic management consulting services and the retail trade of pharmaceutical products, and OTC to pharmacies. CosmoFarmacy was incorporated with a 30-year term through May 31, 2049. The unaffiliated third party is the general partner (the “GP”) of the limited partnership and is responsible for management and decision-making associated with CosmoFarmacy. The initial share capital was set to EUR 150,000 ($163,080) which was later increased to EUR 500,000 ($543,600). The GP contributed the pharmacy license (the “License”) valued at EUR 350,000 (30-year term) to operate the business of CosmoFarmacy in exchange for a 70% equity ownership. The Company is a limited partner and contributed cash of EUR 150,000 ($163,080) for the remaining 30% equity ownership. CosmoFarmacy is not publicly traded and the Company’s investment has been recorded using the equity method of accounting. The value of the investment as of September 30, 2024 and December 31, 2023, was $167,175 and $165,930, respectively, and is included in “Other assets” on the Company’s consolidated balance sheets.
NOTE 4 – PROPERTY AND EQUIPMENT, NET
Property and equipment, net consists of the following at September 30, 2024 and December 31, 2023:
| | September 30, 2024 | | | December 31, 2023 | |
Land | | $ | 3,577,662 | | | $ | 3,551,020 | |
Buildings and improvements | | | 4,868,378 | | | | 4,787,963 | |
Leasehold improvements | | | 3,667 | | | | 3,639 | |
Vehicles | | | 285,609 | | | | 285,388 | |
Furniture, fixtures and equipment | | | 3,014,683 | | | | 2,707,442 | |
Computers and software | | | 203,215 | | | | 168,173 | |
| | | 11,953,214 | | | | 11,503,625 | |
Less: Accumulated depreciation and amortization | | | (1,377,286 | ) | | | (1,048,126 | ) |
Total | | $ | 10,575,928 | | | $ | 10,455,499 | |
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
NOTE 5 – INTANGIBLE ASSETS
Goodwill and intangible, net assets consist of the following at September 30, 2024 and December 31, 2023:
| | September 30, 2024 | | | December 31, 2023 | |
License | | $ | 6,976,209 | | | $ | 6,876,169 | |
Trade name / mark | | | 390,188 | | | | 392,197 | |
Customer base | | | 602,204 | | | | 602,204 | |
Software | | | 795,867 | | | | 158,787 | |
| | | 8,764,468 | | | | 8,029,357 | |
Less: Accumulated amortization | | | | | | | | |
License | | | (805,555 | ) | | | (235,925 | ) |
Trade name / mark | | | (36,997 | ) | | | (36,997 | ) |
Customer base | | | (157,333 | ) | | | (110,160 | ) |
Software | | | (67,520 | ) | | | (11,789 | ) |
Subtotal | | | 7,697,064 | | | | 7,634,486 | |
Goodwill | | | 49,697 | | | | 49,697 | |
Total | | $ | 7,746,761 | | | $ | 7,684,183 | |
At September 30, 2024, the estimated aggregate amortization expense for intangible assets subject to amortization for each of the five succeeding fiscal years is as follows:
Year | | Amount | |
2024 | | $ | 208,530 | |
2025 | | | 833,637 | |
2026 | | | 834,846 | |
2027 | | | 834,846 | |
2028 | | | 783,423 | |
Thereafter | | | 3,846,582 | |
Total | | $ | 7,341,864 | |
NOTE 6 – LOAN RECEIVABLE
On October 30, 2021, the Company entered into an agreement for a ten-year loan with Medihelm SA to memorialize €4,284,521 ($4,849,221) in prepayments the Company had made. The prepayments to Medihelm SA had been made in accordance with the parallel export business, through which Medihelm supplied and would supply SkyPharm SA with branded pharmaceuticals. This business is no longer in place for the Company and thus the Company entered into this agreement with Medihelm SA in order for the outstanding amount to be settled. Interest is calculated at a rate of 5.5% per annum on a 360-day basis. Under the terms of the agreement, the Company is to receive 120 equal payments over the term of the loan. During the year ended December 31, 2023, the Company received €352,438 ($389,867) in principal payments such that as of December 31, 2023, the Company had a short-term receivable balance of $411,858 and a long-term receivable balance of $3,509,200 under this loan. The Company also received €223,914 ($249,552) in principal payments and €107,144 ($119,411) in interest payments during the nine-month period ended September 30, 2024. The Note is considered fully recoverable.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
NOTE 7 – INCOME TAXES
The Company is incorporated in the United States of America and is subject to United States federal taxation. No provisions for income taxes have been made as the Company had no U.S. taxable income for the nine months ended September 30, 2024, and 2023.
The Company’s Greek subsidiaries are governed by the income tax laws of Greece. The corporate tax rate in Greece is 22% on income reported in the statutory financial statements after appropriate tax adjustments.
The Company’s United Kingdom subsidiaries are governed by the income tax laws of the United Kingdom. The corporate tax rate in the United Kingdom is 25% on income reported in the statutory financial statements after appropriate tax adjustments.
As of September 30, 2024, and 2023, the Company’s effective tax rate differs from the U.S. federal statutory tax rate primarily due to a valuation allowance recorded against net deferred tax assets in in the United States and the United Kingdom.
We regularly review deferred tax assets to assess their potential realization and establish a valuation allowance for portions of such assets to reduce the carrying value if we do not consider it to be more likely than not that the deferred tax assets will be realized. Our review includes evaluating both positive (e.g., sources of taxable income) and negative (e.g., recent historical losses) evidence that could impact the realizability of our deferred tax assets. As of September 30, 2024, and December 31, 2023, the Company has maintained a valuation allowance against all net deferred tax assets in the United States, Greece, and the UK.
For the three months ended September 30, 2024, and 2023, the Company has recorded tax benefit in any jurisdiction where it is subject to income tax, in the amount of $0 and $65,873, and respectively, on the Condensed Consolidated Statements of Operations and Comprehensive Loss. No tax loss or benefit was recorded for the equivalent nine month periods.
NOTE 8 – CAPITAL STRUCTURE
Preferred Stock
The Company is authorized to issue 100 million shares of preferred stock, of which 6,000,000 are designated as Series A convertible preferred stock. The preferred stock has a liquidation preference over the common stock and is non-voting. As of September 30, 2024 and December 31, 2023, no preferred shares were issued and outstanding.
Major Rights & Preferences of Series A Preferred Stock
On and effective October 4, 2021, the Company amended and restated its articles of incorporation (the “Amended and Restated Articles”) and filed a certificate of designation (the “COD”) for its Series A Preferred Stock (the “Series A Preferred Stock”) with the State of Nevada. The Amended and Restated Articles allow the Company’s Board of Directors the authority to authorize the issuance of preferred stock from time to time in one or more classes or series by resolution. On February 23, 2022, the Company filed Correction No. 1 to the COD. On July 28, 2022, the Company filed an Amendment to the COD with the State of Nevada to allow a holder to waive application of the Beneficial Ownership Limitation with respect to the conversion of Series A Preferred Stock.
With respect to payment of dividends and distribution of assets upon liquidation, dissolution, or winding up of the Company, whether voluntary or involuntary, all shares of the Series A Preferred Stock will rank: (i) senior to all of the Company’s Common Stock and any other equity securities that the Company may issue in the future, (ii) equal to any other equity securities that the Company may issue in the future, the terms of which specifically provide that such equity securities are on parity or senior to the Series A Preferred Stock (“Parity Securities”), (iii) junior to all other equity securities the Company issues, the terms of which specifically provide that such equity securities rank senior to the Series A Preferred Stock, and (iv) junior to all of the Company’s existing and future indebtedness; without the prior written consent of the Majority Holders.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
In the event of any voluntary or involuntary liquidation, dissolution or winding up of the Company (a “Liquidation”), the Holders of shares of Series A Preferred Stock shall be first entitled to receive out of the assets of the Company available for distribution to its shareholders.
Each Holder shall not be entitled to vote with holders of outstanding shares of Common Stock, voting together as a single class, with respect to any and all matters presented to the stockholders of the Company for their action or consideration, except as provided by law or as set forth in the COD. The holders of Series A Preferred Stock are entitled to receive dividends paid and distributions made to the holders of Common Stock to the same extent as if the holders of Series A Preferred Stock had converted such shares into shares of Common Stock.
The Series A Preferred Stock was initially convertible into the Company’s Common Stock as determined by dividing the number of shares of Series A Preferred Stock to be converted by the lower of (i) $75.00 or (ii) 80% of the average volume weighted average price for the Company’s Common Stock for the five trading days immediately following the effectiveness of the registration statement concerning the shares (the “Conversion Price”). On June 14, 2022, the Conversion Price was reset to $15.54 per share.
Each holder is entitled to receive dividends in shares of Series A Preferred Stock or cash determined based on the stated value of each Series A Preferred Stock at the dividend rate of 8.0% per year. For the year ended December 31, 2022, the Company recorded $372,414 as a deemed dividend in accordance with the Series A Preferred Stock cumulative dividend. As of December 31, 2022, the cumulative dividend has been recorded as mezzanine equity. Following, Mr. Siokas waiver of the right to receive the dividends on February 26, 2024, and the unanimous written consent of the Company’s Board of Directors on February 29, 2024, through which was resolved that the Company shall remove all accrued and unpaid dividends payable to the previous holders of Series A Preferred stock, the Company eliminated the total deemed dividend of $372,414 through retained earnings. Thus, the balance of mezzanine equity as of September 30, 2024, and December 31, 2023 is $0.
The Series A Shares rank senior to all of the Company’s Common Stock and any other equity securities that the Company may issue in the future with respect to payment of dividends and distribution of assets upon liquidation, dissolution or winding up. While the Series A Shares are outstanding, the Company may not amend, alter or change adversely the powers, preferences or rights given to the Series A Shares, create, or authorize the creation of, any additional class or series of capital stock of the Company (or any security convertible into or exercisable for any class or series of capital stock of the Company), including any class or series of capital stock of the Company that ranks superior to or in parity with the Series A Shares, alter, amend, modify, or repeal its Articles of Incorporation or other charter documents in any manner that adversely affects any rights of the holders of Series A Shares, increase or decrease the number of authorized shares of Series A Shares, any agreement, commitment or transaction that would result in a Change of Control, any sale or disposition of any material assets outside of the ordinary course of business of the Company, any material change in the principal business of the Company, including the entry into any new line of business or exit of any current line of business, and circumvent a right or preference of the Series A Shares. Any holder of the Series A Shares shall have the right by written election to the Company to convert all or any portion of the outstanding Series A Shares. Immediately upon effectiveness of a registration statement registering for resale all of the Registrable Securities (as defined in the Registration Rights Agreement), all outstanding Series A Shares shall automatically convert into Common Stock, subject to certain beneficial ownership limitations.
Treasury stock
As of September 30, 2024 and December 31, 2023, the Company held 86,497 and 86,497, respectively, shares of our common stock at a cost of $917,159 and $917,159, respectively. Shares of our common stock that are repurchased are classified as treasury stock pending future use and reduce the number of shares outstanding used in calculating earnings per share. Cosmos may repurchase shares from time to time through open market purchases in accordance with applicable securities laws and other restrictions. The Company repurchased no shares of our common stock during the nine months ended September 30, 2024. The Company repurchased 71,000 shares of our common stock for $100,452 during the year ended December 31, 2023. The Company repurchased no shares of our common stock during the nine months ended September 30, 2024.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
On January 24, 2023 the Company announced that its Board of Directors has approved a share repurchase program with authorization to purchase up to $3 million of its common stock. Cosmos may repurchase shares from time to time through open market purchases in accordance with applicable securities laws and other restrictions.
Common Stock
The Company is authorized to issue 300 million shares of common stock. As of September 30, 2024 and December 31, 2023, the Company had 17,834,023 and 15,982,472 shares of our common stock issued, respectively, and 21,346,023 and 15,895,975 shares outstanding, respectively.
Issuance of Common Stock
During the nine-month period ended September 30, 2023 the Company issued 15,258 shares to a consultant for services rendered. The shares were valued and expensed on the date of issuance and are separately presented in the condensed consolidated statement of changes in stockholders’ equity and mezzanine as “Shares issued in lieu of cash”.
During the nine months ended September 30, 2024, the Company raised additional equity funds through two Prospectus Supplements to its Registration Statement on Form S-3 (No. 333-267550) filed with the SEC on February 29 and March 7, 2024. More specifically, the Company sold 901,488 shares of common stock for gross proceeds of $648,893. Placement agent’s fees and other commissions amounted to $19,467 and thus the total net proceeds for the period were $629,426.
On December 29, 2023, the Company had entered into a warrant exchange agreement (the “Warrant Exchange”) with an investor to reduce the exercise price of 2,437,063 warrants from $2.75 per share to $1.45 per shares as an inducement to exercise. The Company issued 1,487,000 shares of common stock, held 950,063 shares in escrow until the investor’s beneficial ownership limitation allows for the transfer of the escrow shares, and received gross cash proceeds of 3,533,741. The 950,063 shares were issued within the nine-month period ended September 30, 2024 but were already valued in the year ended December 31, 2023.
On September 26, 2024, the Company entered into a Warrant Inducement Letter (the “Letter”) with an investor pursuant to which the Company issued 9,748,252 new warrants (the “New Warrants”) and reduced the exercise price of 4,874,126 warrant shares from $1.45 to $0.8701 to induce exercise and receive gross cash proceeds of $4,240,977 (the “Original Warrants”). The Company issued 2,332,000 shares of common stock, held 2,532,126 shares in escrow until the investor’s beneficial ownership limitation allows for the transfer of the escrow shares.
Exercise of Warrants
During the nine months ended September 30, 2024, the Company issued 2,332,000 shares of common stock upon the exercise of 2,332,000 warrants. The Company received gross proceeds of $4,240,977 upon exercise. The net proceeds after deducting legal, agent and escrow fees of $372,109 amounted to $3,868,868. The warrants were exercised following the Warrant Inducement letter the Company signed on September 26, 2024, through which their exercise price was reduced from $1.45 to $0.8701.
Warrant Classification
The Company determines the classification of its warrants upon issuance by identifying the instrument issued to determine if it is debt or equity classified. The Company determined its warrants meet the scope exception in ASC 815-10 and are equity classified because, (a) the warrant is indexed to the Company’s own stock, (b) require settlement in equity shares, and (c) the Company has enough authorized and unissued shares.
NOTE 9 – RELATED PARTY TRANSACTIONS
Doc Pharma S.A.
Doc Pharma S.A is considered a related party to the Company due to the fact that the CEO of Doc Pharma is the wife of Grigorios Siokas, the Company’s CEO and principal shareholder, who also served as a principal of Doc Pharma S.A. in the past.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Prepaid expenses and other current assets – related party
As of September 30, 2024 and December 31, 2023, the Company had a prepaid balance of $6,393,642 and $4,347,184, respectively, to Doc Pharma related to purchases of inventory and pharmaceutical and nutraceutical licenses to be purchased.
Accounts payable and accrued expenses - related party
As of September 30, 2024 and December 31, 2023, the Company had an accounts payable balance to Doc Pharma of $72,968 and $34,217, respectively.
Accounts receivable - related party
The Company had a receivable balance of $2,448,517 and $2,386,721 from Doc Pharma S.A as of September 30, 2024, and December 31, 2023, respectively.
Sales and Purchases
During the three months ended September 30, 2024 and 2023, the Company purchased a total of $85,073 and $456,257 of products from Doc Pharma S.A., respectively. During the three months ended September 30, 2024, and 2023, the Company had $40,370 and $61,163 revenue from Doc Pharma, respectively.
During the nine months ended September 30, 2024 and 2023, the Company purchased a total of $510,711 and $1,057,621 of products from Doc Pharma S.A., respectively. During the nine months ended September 30, 2024 and 2023, the Company had $581,862 and $43,107 revenue from Doc Pharma, respectively.
Other Agreements
On October 10, 2020, the Company entered into a contract manufacturer outsourcing (“CMO”) agreement with Doc Pharma whereby Doc Pharma is responsible for the development and manufacturing of pharmaceutical products and nutritional supplements according to the Company’s specifications based on strict pharmaceutical standards and good manufacturing practice (“GMP”) protocols as the National Organization for Medicines requires. The Company has the exclusive ownership rights for trading and distribution of its own branded nutritional supplements named “Sky Premium Life®”. The duration of the agreement is for five years, however, either party may terminate the agreement at any time giving six-month advance notice. Doc Pharma is exclusively responsible for supplying the raw materials and packaging required to manufacture the final product. However, they are not responsible for potential delays that may arise, concerning their import. Doc Pharma is also obligated to store the raw and packaging materials. The delivery of raw and packaging materials should be purchased at least 30 and 25 days, respectively, before the delivery date of the final product. The Manufacturer solely delivers the finished product to the Company. There is a minimum order quantity (“MoQ”) of 1,000 pieces per product code. Both parties have agreed that the Company will deposit 60% of the total cost upon agreement and assignment and 40% of the total cost including VAT charge upon the delivery date. The prices are indicative and are subject to amendments if the cost of the raw material or the production cost change.
For the three months ended September 30, 2024 and 2023, the Company has purchased €34,350 ($38,096) and €418,577 ($455,586) respectively, in inventory related to this agreement.
For the nine months ended September 30, 2024 and 2023, the Company has purchased €161,108 ($175,123) and €967,785 ($1,048,557) respectively, in inventory related to this agreement.
On May 17, 2021, Doc Pharma and the Company entered into a Research and Development (“R&D”) agreement whereby Doc Pharma will be responsible for the research, development, design, registration, copy rights and licenses of 250 nutritional supplements for the final products called Sky Premium Life®. These products will be sold in Greece and abroad. The total cost of this project will be €1,425,000 plus VAT and will be done over three phases as follows: Design & Development (€725,000); Control and Product Manufacturing (€250,000) and Clinical Study and Research (€450,000). SkyPharm has bought a total of as of 81 licenses at value of €554,500 ($593,204) which is 38.91% of the total cost, as of December 31, 2022. During the year ended December 31, 2023, 24 additional licenses were purchased at value of €475,014 ($525,461). During the three and nine months ended September 30, 2024, no additional licenses were purchased. The agreement will terminate on December 31, 2025.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Purchase of branded pharmaceuticals
On June 28, 2023, the Company approved the purchase of five proprietary and innovative branded pharmaceuticals with significant market presence and material profit contribution from Zakalia Ltd., the parent company of Doc Pharma, for €1,800,000 ($1,965,600). The transaction was settled on a non-cash basis through the reduction of an equivalent amount of prepaid expense balances the Company held with Doc Pharma. The purchased branded pharmaceuticals are presented in “Goodwill and intangible assets, net” on the accompanying consolidated balance sheets. On December 29, 2023, the Company approved the purchase of 19 additional licenses from DocPharma, of a total value of €3,200,000 ($3,539,840). This transaction was also settled on a non-cash basis through the reduction of an equivalent amount of prepaid expense balances the Company held with Doc Pharma.
Loans receivable - related party
The balance of prepaid expenses due Doc Pharma as of December 31, 2022, had increased to €7,103,706 ($7,599,545), which was mainly attributable to the prepayments SkyPharm S.A. made in accordance with the CMO agreement and the extensive orders and sales of the SPL products the Company expects to achieve within 2023, mainly through its Amazon channels in the UK, Singapore, Canada and other countries. However, as the benefit from a significant portion of the prepaid balance would not have been realized within a 12-month period, the Company opted to secure a portion of the outstanding prepaid balance through a loan agreement. SkyPharm S.A. (the “Lender”) entered into a loan agreement with Doc Pharma (the “Borrower”) for €4,000,000 ($4,279,200), all of which was financed through the outstanding prepaid balance. The duration of the loan is for a 10-year period up to December 31, 2032 (the “Maturity Date”). The loan bears a fixed interest rate of 5.5% payable on a monthly basis and will be repayable in 120 equal instalments of €33,333.33 ($37,150). The loan may be prepaid anytime during its duration in full or partially based on the Company’s product requirements and other factors, without Doc Pharma incurring any prepayment penalty.
As of September 30, 2024 and December 31, 2023, the loan had a current portion of €400,000 ($445,800) and €400,000 ($442,480), and a non-current portion of €2,900,000 ($3,232,050), and €3,200,000 ($3,539,840), respectively, which is classified as "Loans receivable – related party" on the accompanying consolidated balance sheets. During the nine months ended September 30, 2024, the Company received €300,000 ($334,350) in principal repayments, and €121,550 ($135,467) of interest repayments. Additionally, during the nine months ended September 30, 2024, the Company recorded €143,000 ($155,440) as interest income relating to this loan.
Cana Laboratories Holding Limited
Cana was considered a related party as the Company had signed a binding letter of intent and an SPA for the acquisition of Cana. The acquisition was completed on June 30, 2023 according to the SPA signed on May 31, 2023. Thus, all balances between the Company and Cana were eliminated upon consolidation as of December 31, 2023. The Secured Promissory Note discussed below was included in consideration transferred upon acquisition.
Loans receivable - Related Party - Long Term
On February 28, 2023 (Issue Date), the Company signed a Secured Promissory Note with Cana Laboratories Holdings (Cyprus) Limited (the “Holder”), whereby the Holder borrowed the sum of €4,100,000 ($4,457,520) from the Company. Interest on the Principal Amount under this Note shall accrue at a rate equal to Five Percent (5%) plus 1 month LIBOR per annum (5.47% as of December 31, 2023). The maturity date (“Maturity Date”) of this Note shall be five (5) years from the Issue Date. The Principal Amount, as well as all accrued interest shall be due and payable on the Maturity Date. Following the completion of Cana’s acquisition on June 30, 2023 the balance of the Note was eliminated on a consolidated level.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Panagiotis Kozaris
Panagiotis Kozaris is considered a related party due to the fact that he is a former General operational manager and current employee of Cosmofarm S.A.
Prepaid Expenses and Other Current Assets - Related Party
From time to time the Company purchases back shares that Panagiotis Kozaris owns and records them as treasury shares. The Company pays Panagiotis Kozaris in advance for the shares owned and obtains the shares upon execution of a cumulative stock-purchase agreement (“SPA”). During the three months ended September 30, 2024 and 2023, the Company paid Panagiotis Kozaris an additional sum of $0 and $51,159 respectively for shares owned, however, no SPA for these funds has been executed as of September 30, 2024. The Company intends to execute a cumulative SPA for these amounts during 2024. The total balances owed of $194,215 and $194,215 are included in “Prepaid expenses and other current assets - related party”, on the accompanying consolidated balance sheets as of September 30, 2024 and December 31, 2023, respectively.
Basotho Investment Limited
Basotho Investment Limited is considered a related party once Panagiotis Kozaris (former general operational manager and current employee of Cosmofarm SA) is one of its directors.
General and administrative expenses
On November 21, 2023, the Company issued 120,000 shares of common stock to Basotho Investment Limited for services rendered. The fair value of these shares for the period ended December 31, 2023 was $10,300, which was recorded as general and administrative expense. The fair value of the shares vested for the nine-month period ended September 30, 2024 was $92,700, which was recorded as general and administrative expense.
Maria Kozari
Maria Kozari is considered a related party to the Company due to the fact that she is the daughter of Panagiotis Kozaris, a former Operational General Manager and current employee of Cosmofarm S.A.
Accounts Receivable - Related Party
During 2021, the Company, through its subsidiary, Cosmofarm SA, commenced a partnership with a pharmacy called “Pharmacy & More”, owned by Maria Kozari. The transactions with the respective pharmacy were in Cosmofarm’s normal course of business, however, a more flexible credit policy was allowed as the pharmacy was new and needed to be established in the market. During the three and nine months ended September 30, 2024 and 2023 the Company’s net sales to Pharmacy & More amounted to $113,161 and $122,969 and $310,126 and $359,760 respectively. As of September 30, 2024 and December 31, 2023 the Company’s outstanding receivable balance due from the pharmacy amounted to $1,123,835 (€1,203,739) and $1,142,402 (€1,032,726), respectively, and are included in “Accounts receivable - related party”, on the accompanying consolidated balance sheets.
The Company plans to acquire Pharmacy & More within fiscal year 2024. Upon acquisition, the Company intends to offset the outstanding receivable balance with the corresponding purchase price and additionally plans to make Pharmacy & More the first shop-in-shop of its own branded line of nutraceutical products, Sky Premium Life® (SPL).
Other Related Parties
The Company has the following balances as of September 30, 2024: a) a balance of $731,000 relating to unpaid salaries and bonuses due to Grigorios Siokas, the CEO of the Company and $188,000 due to George Terzis, the CFO of the Company, classified as "Accounts payable and accrued expenses - related party" in the Company’s condensed consolidated balance sheets, b) a net payable balance of $29,832 due to Konstantinos Gaston Kanaroglou, former manager and current employee of the Company’s wholly owned subsidiary Cana, classified as " Accounts payable and accrued expenses - related party" in the Company’s condensed consolidated balance sheets.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Additionally, the Company had the following balances as of December 31, 2023: a) a balance of $98,000 relating to unpaid salaries and bonuses due to George Terzis, the CFO of the Company, classified as "Accounts payable and accrued expenses - related party" in the Company’s consolidated balance sheets, b) a net payable balance of $85,332 due to Konstantinos Gaston Kanaroglou, former manager and current employee of the Company’s wholly owned subsidiary Cana, classified as " Accounts payable and accrued expenses - related party" in the Company’s consolidated balance sheets.
Notes Payable – Related Party
A summary of the Company’s related party notes payable as of September 30, 2024 and December 31, 2023 is presented below:
| | September 30, 2024 | | | December 31, 2023 | |
| | | | | | |
Beginning Balance | | $ | 11,283 | | | $ | 10,912 | |
Payments | | | - | | | | - | |
Foreign currency translation | | | 85 | | | | 371 | |
Ending Balance | | $ | 11,368 | | | $ | 11,283 | |
Dimitrios Goulielmos
Dimitris Goulielmos was the Company’s former CEO and a Director of the Company.
On November 21, 2014, the Company entered into an agreement with Dimitrios Goulielmos, as amended on November 4, 2016. Pursuant to the amendment, this loan has no maturity date and is non-interest bearing. As of September 30, 2024 and December 31, 2023, the Company had a principal balance of €10,200 ($11,368) and €10,200 ($11,283), respectively.
The above balances are adjusted for the foreign currency rate as of the balance sheet date. For the nine months ended September 30, 2024, the Company recorded a foreign currency translation loss of $85.
Loans Payable – Related Party
A summary of the Company’s related party loans payable as of September 30, 2024 and December 31, 2023 is presented below:
| | September 30, 2024 | | | December 31, 2023 | |
| | | | | | |
Beginning balance | | $ | 13,257 | | | $ | 12,821 | |
Proceeds | | | 18,344 | | | | - | |
Payments | | | (8,918 | ) | | | - | |
Foreign currency translation | | | (1,525 | ) | | | 436 | |
Ending balance | | $ | 21,158 | | | $ | 13,257 | |
Grigorios Siokas
From time to time, Grigorios Siokas loans the Company funds in the form of non-interest bearing, no-term loans. As of September 30, 2024, the Company had an outstanding principal balance under these loans of $21,158 in loans payable to Grigorios Siokas. As of December 31, 2023, the Company had an outstanding principal balance of $13,257 related to this payable.
The above balances are adjusted for the foreign currency rate as of the balance sheet date. For the nine months ended September 30, 2024, the Company recorded a gain of $1,525.
Except as set forth above, we have not entered into any material transactions with any director, executive officer, and promoter, beneficial owner of five percent or more of our common stock, or family members of such persons.
NOTE 10 – LINES OF CREDIT
A summary of the Company’s lines of credit as of September 30, 2024 and December 31, 2023, is presented below:
| | September 30, 2024 | | | December 31, 2023 | |
National | | $ | 3,011,102 | | | $ | 3,918,523 | |
Alpha | | | 1,030,943 | | | | 1,130,140 | |
Pancreta | | | 1,560,803 | | | | 1,122,210 | |
EFG | | | 386,577 | | | | 459,400 | |
Ending balance | | $ | 5,989,425 | | | $ | 6,630,273 | |
The Company has three lines of credit with the National Bank of Greece, which are renewed annually. The three lines have interest rates of 6.00% (the "National Bank LOC"), 3.6% (the "COSME 2 Facility"), and 3.6% plus the six-month Euribor rate and any contributions currently in force by law on certain lines of credit (the "COSME 1 Facility").
The maximum borrowing allowed for the 6% line of credit was $3,315,638 and $3,290,945 as of September 30, 2024 and December 31, 2023, respectively. The outstanding balance of the facility was $2,102,765 and $2,829,828, as of September 30, 2024 and December 31, 2023, respectively.
The cumulative maximum borrowing allowed for the COSME 1 Facility and COSME 2 Facility (collectively, the "Facilities") was $1,114,500 and $1,106,200 as of September 30, 2024 and December 31, 2023, respectively. The outstanding balance of the Facilities was $943,466 and $1,099,255 as of September 30, 2024 and December 31, 2023, respectively.
The Company maintains a line of credit with Alpha Bank of Greece ("Alpha LOC"), which is renewed annually and has a current interest rate of 6.00%. The maximum borrowing allowed was $1,114,500 and $1,106,200 as of September 30, 2024 and December 31, 2023, respectively. The outstanding balance of the Alpha LOC was $1,030,944 and $1,130,141, as of September 30, 2024 and December 31, 2023, respectively.
The Company holds a line of credit with Pancreta Bank ("Pancreta LOC"), which is renewed annually and has a current interest rate of 4.10%. The maximum borrowing allowed as of September 30, 2024 and December 31, 2023 was $1,549,155 and $1,537,618, respectively. The outstanding balance of the Pancreta LOC as of September 30, 2024 and December 31, 2023 was $1,560,802 and $1,122,210, respectively.
The Company maintains a line of credit with EGF ("EGF LOC"), which is renewed annually and has a current interest rate of 4.49% plus 3-month Euribor. The maximum borrowing allowed as of September 30, 2024 and December 31, 2023 was $445,800 and $459,400, respectively. The outstanding balance of the EGF LOC as of September 30, 2024 and December 31, 2023 was $386,577 and $459,400, respectively.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Under the aforementioned line of credit agreements, the Company is required to maintain certain financial ratios and covenants. As of September 30, 2024, and December 31, 2023, the Company was in compliance with these ratios and covenants.
All lines of credit are guaranteed by customer receivable checks, which are a type of factoring in which postponed customer checks are assigned by the Company to the bank, in order to be financed at an agreed upon rate.
Interest expense on the Company’s outstanding lines of credit balances for the three and nine months ended September 30, 2024 and 2023, was $89,868 and $37,536, and 275,246 and $204,654, respectively.
NOTE 11 – NOTES PAYABLE
A summary of the Company’s third-party debt as of and for the nine months ended September 30, 2024, and the year ended December 31, 2023 is presented below:
September 30, 2024 | | Trade Facility | | | Third Party | | | COVID Loans | | | Total | |
Beginning balance, December 31, 2023 | | $ | 1,908,195 | | | $ | 2,511,148 | | | $ | 186,884 | | | $ | 4,606,227 | |
Proceeds | | | - | | | | 445,800 | | | | - | | | | 445,800 | |
Payments | | | (334,350 | ) | | | (481,254 | ) | | | (19,073 | ) | | | (834,677 | ) |
Conversion of debt | | | - | | | | - | | | | - | | | | - | |
Recapitalized upon debt modification | | | - | | | | - | | | | - | | | | - | |
Accretion of debt and debt discount | | | - | | | | - | | | | - | | | | - | |
Foreign currency translation | | | 14,318 | | | | 32,613 | | | | 3,691 | | | | 50,622 | |
Ending balance, September 30, 2024 | | | 1,588,163 | | | | 2,508,307 | | | | 171,502 | | | | 4,267,972 | |
Notes payable - long-term | | | (1,086,638 | ) | | | (1,416,802 | ) | | | (142,183 | ) | | | (2,645,623 | ) |
Notes payable - short-term | | $ | 501,525 | | | $ | 1,091,505 | | | $ | 29,319 | | | | 1,622,349 | |
December 31, 2023 | | Trade Facility | | | Third Party | | | COVID Loans | | | Total | |
Beginning balance, December 31, 2022 | | $ | 3,305,532 | | | $ | 1,505,078 | | | $ | 207,377 | | | $ | 5,017,987 | |
Proceeds | | | - | | | | 1,082,231 | | | | - | | | | 1,082,231 | |
Payments | | | (1,155,310 | ) | | | (415,557 | ) | | | (27,027 | ) | | | (1,597,894 | ) |
Oher additions | | | - | | | | 317,880 | | | | - | | | | 317,880 | |
Debt forgiveness | | | (306,637 | ) | | | - | | | | - | | | | (306,637 | ) |
Foreign currency translation | | | 64,610 | | | | 21,516 | | | | 6,534 | | | | 92,660 | |
Ending balance, December 31, 2023 | | | 1,908,195 | | | | 2,511,148 | | | | 186,884 | | | | 4,606,227 | |
Notes payable – long-term | | | (1,327,440 | ) | | | (1,549,768 | ) | | | (158,133 | ) | | | (3,035,341 | ) |
Notes payable - short-term | | $ | 580,755 | | | $ | 961,380 | | | $ | 28,751 | | | $ | 1,570,886 | |
Our outstanding debt as of September 30, 2024 is repayable as follows: |
| | September 30, 2024 | |
2025 | | $ | 1,622,349 | |
2026 | | | 1,717,738 | |
2027 | | | 420,633 | |
2028 | | | 353,961 | |
2029 and thereafter | | | 153,291 | |
Total debt | | | 4,267,972 | |
Less: notes payable - current portion | | | (1,622,349 | ) |
Notes payable - long term portion | | $ | 2,645,623 | |
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Trade Facility Agreements
On May 12, 2017, SkyPharm entered into a Trade Finance Facility Agreement (the “TFF”) with Synthesis Structured Commodity Trade Finance Limited (the “Lender”) as amended on November 16, 2017, and May 16, 2018.
On October 17, 2018, the Company entered into a further amended agreement with Synthesis whereby the current balance on the TFF as of October 1, 2018, which was €4,866,910 ($5,629,555) and related accrued interest of €453,094 ($524,094) would be split into two principal balances of Euro €2,000,000 ($2,316,000), (the "EURO Loan") and USD $4,000,000 (the "USD Loan"). Interest on both the EURO Loan and USD Loan commenced on October 1, 2018, at 6% per annum plus one-month Euribor (3.90% as of December 31, 2023), and 6% plus one-month LIBOR (fully paid as of December 31, 2023), respectively.
On December 30, 2020, the Company transferred the EURO Loan to a new third-party lender. The terms remained the same except interest accrues at 5.5% per annum plus one-month Euribor (3.87% as of December 31, 2023). The principal was scheduled to be repaid in a total of five quarterly installments beginning October 31, 2021 of €50,000 ($54,600) each with a final repayment of €1,800,000 ($1,965,600) Euro payable on October 31, 2022.
On March 3, 2022, the Company entered into a modification agreement to extend the maturity date to January 10, 2023 and payments under the USD Loan. During June 2022, the Company agreed with the Lender to postpone the repayment of an installment of $500,000 due on June 30, 2022 (based on the modification agreement signed on March 3, 2022) until January 2023. During September 2022, the Company entered into an agreement with the Lender to postpone the repayment of the outstanding balance on the USD Loan of $3,950,000, plus unpaid accrued interest until January 2023. The Company capitalized fees paid upon modification of €200,000 ($221,060) that are being amortized over the life of the loan. The Company incurred non-cash interest expense of $200,000 during the year ended December 31, 2022 concerning the above capitalized fees.
On December 22, 2022, SkyPharm signed an agreement for the extension of the payments and an increase in interest rate due under the EURO Loan that was extended to be repaid with a balloon payment now due on October 31, 2025. This extension was agreed upon in writing on December 22, 2022, with a retroactive modification date to October 31, 2022 (the original maturity date).
As of December 31, 2023 the Company had an outstanding principal balance of €1,725,000 ($1,908,195), of which $1,327,440 is classified as ''Notes payable - long term portion" on the consolidated balance sheets. As of December 31, 2023, the Company had accrued $161,274 in interest expense related to these agreements.
The Company repaid €300,000 ($334,350) of the EURO Loan during the nine months ended September 30, 2024. As of September 30, 2024, the Company had an outstanding principal balance of €1,425,000 ($1,588,163), of which $1,086,638 is classified as ''Notes payable - long term portion" on the consolidated balance sheets. For the three and nine months ended September 30, 2024, the Company had accrued $29,365 and $110,170, respectively, in interest expense related to these agreements.
June 23, 2020 Debt Agreement
On June 23, 2020, the Company’s subsidiary, Cosmofarm, entered into an agreement with the National Bank of Greece S.A. (the “Bank”) to borrow a maximum of €500,000 ($611,500). The note has a maturity date of sixty (60) months from the date of the first disbursement, which includes a grace period of nine months. The total amount of the initial proceeds was received in 3 equal monthly installments. The note is interest bearing from the date of receipt and is payable every three months at an interest rate of 3.06% plus 3-month Euribor (3.47% as of September 30, 2024). The outstanding balance was €117,647 ($131,118) and €205,882 ($227,747) as of September 30, 2024 and December 31, 2023, respectively, of which $0 and $97,606 was classified as “Notes payable - long-term portion”, on the accompanying condensed consolidated balance sheets. During the nine months ended September 30, 2024, the Company repaid €88,235 ($98,338) of the principal balance.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
June 24, 2020 Debt Agreement
On June 24, 2020, the Company’s subsidiary, Decahedron, received a loan £50,000 ($68,310) from the United Kingdom government. The loan has a ten-year maturity and bears interest at a rate of 2.5% per annum beginning 12-months after the initial disbursement, which was on July 10, 2020. The Company may prepay this loan without penalty at any time. As of December 31, 2023, the principal balance was £40,858 ($52,066). As of September 30, 2024, the principal balance was £38,320 ($51,345).
November 19, 2020 Debt Agreement
On November 19, 2020, the Company entered into an agreement with a third-party lender in the principal amount of €500,000 ($611,500). The note matures on November 18, 2025 and bears an annual interest rate, based on a 360-day year, of 3% plus 0.6% plus 6-month Euribor when Euribor is positive (3.35% as of September 30, 2024). The principal is to be repaid in 18 quarterly installments of €27,778 ($30,333). During the nine months ended September 30, 2024, the Company repaid €88,333 ($92,875) of the principal. As of September 30, 2024 and December 31, 2023, the Company has accrued interest of €5,434 ($6,057) and €11,191 ($12,379) related to this note and a principal balance of €138,889 ($154,792) and €222,222 ($245,822), of which $30,958 and $122,911 is classified as "Notes payable - long term portion" on the accompanying condensed consolidated balance sheets.
July 30, 2021 Debt Agreement
On July 30, 2021, the Company entered into an agreement with a third-party lender in the principal amount of €500,000 ($578,850). The note matures on August 5, 2026 and bears an annual interest rate that applies to 60% of the principal of the note that is based on a 365-day year, of 5.84% plus 3-month Euribor when Euribor is positive (3.47% as of September 30, 2024). Pursuant to the terms of the agreement, there is a nine-month grace period for principal repayment during which interest is accrued. The principal is to be repaid in 18 quarterly installments of €27,778 commencing three months from the end of the grace period. During the nine months ended September 30, 2024, the Company repaid €81,891 ($91,267) of the principal. As of September 30, 2024 and December 31, 2023, the Company had accrued interest of €15,328 ($17,083) and €10,905 ($12,063), respectively, and principal of €262,662 ($281,338) and €235,009 ($261,918), respectively, of which $134,929 and $227,065 is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets.
June 9, 2022 Debt Agreement
On June 9, 2022 the Company entered into an agreement with a third-party lender in the principal amount of €320,000 ($335,008), the “Note”. The Note matures on June 16, 2027 and bears an annual interest rate of 3.89% plus an additional rate of 0.60%, plus the 3-month Euribor (3.47% as of September 30, 2024). Pursuant to the agreement, there is a twelve-month grace period for principal repayment during which interest is accrued. The principal is to be repaid in 16 equal quarterly installments of €20,000 commencing on June 30, 2023. During the nine months ended September 30, 2024, the Company repaid €60,000 ($66,870) of the principal. As of September 30, 2024 and December 31, 2023, the Company has accrued interest of €4,673 ($5,208) and €11,043 ($12,215), respectively, and an outstanding balance of €200,000 ($222,900) and €260,000 ($287,612) of which $133,740 and $204,322, respectively, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets.
July 14, 2023 Debt Agreement
On July 14, 2023 the Company entered into an agreement with a third-party lender in the principal amount of €1,000,000 ($1,123,700), the “Note”. The Note matures on July 31, 2028 and bears an annual interest rate of 2.46% plus the 3-month Euribor (3.47% as of September 30, 2024). Pursuant to the agreement, there is a nine-month grace period for interest and principal repayment. The principal is to be repaid in 18 equal quarterly installments of €55,556 commencing on May 2, 2024. During the nine months ended September 30, 2024, the Company repaid €108,633 ($58,179) of the principal. As of September 30, 2024, and December 31, 2023, the Company has accrued interest of €7,845 ($8,743) and €19,820 ($21,925), respectively. As of September 30, 2024, and December 31, 2023 the Company an outstanding balance of €869,067 ($968,575) and €977,700 ($1,081,532), of which $720,908 and $897,165, respectively, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets.
July 29, 2024 Debt Agreement
On July 29, 2024 the Company entered into an agreement with a third-party lender in the principal amount of €400,000 ($432,760), the “Note”. The Note matures on July 31, 2029 and bears an annual interest rate of 2.58% plus the 3-month Euribor (3.47% as of September 30, 2024). Pursuant to the agreement, there is a six-month grace period for principal and interest repayment. The principal is to be repaid in 18 equal quarterly installments of €22,222 commencing on April 30, 2025. During the nine months ended September 30, 2024, the Company repaid no principal and had not accrued any interest. As of September 30, 2024, and December 31, 2023 the Company an outstanding balance of €400,000 ($445,800) and €0 ($0), of which $396,367 and $0, respectively, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
COVID-19 Loans
On May 12, 2020, the Company’s subsidiary, SkyPharm, was granted and on May 22, 2020 received a €300,000 ($366,900) loan from the Greek government. The loan will be repaid in 40 equal monthly installments beginning on July 29, 2022. As a condition to the loan, the Company was required to retain the same number of employees until October 31, 2020. As of December 31, 2023, the principal balance was $134,818. During the nine months ended September 30, 2024, the Company repaid €14,063 ($15,673) of the principal balance. The outstanding balance as of September 30, 2024 is €107,813 ($120,157) of which $99,260, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheet.
Cloudscreen Promissory Note
On January 23, 2024 the Company entered into an agreement with a third-party in the principal amount of €300,000 ($324,870), the “Promissory Note”. The Promissory Note matures on March 25, 2025 and is interest free. This Note is being given in connection with the Closing of the Asset Purchase, Sale and Transfer Agreement dated as of October 9, 2023 and as amended from time to time pursuant to which the Company agreed to purchase from the third-party a drug repurposing Artificial Intelligence “AI” powered platform known as “Cloudscreen®” (refer to Note 2, section “Acquisition accounting”). The principal is to be repaid in 15 equal monthly installments of €20,000 commencing on January 25, 2024. During the 9 months ended September 30, 2024, the Company repaid €10,000 ($10,830) of the principal and recorded a foreign currency loss of $16,155. As of September 30, 2024, and December 31, 2023 the Company had an outstanding balance of $323,205 and $317,880 of which $0 and $0, respectively, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets.
Distribution and Equity Agreement
As discussed in Note 3 above, the Company entered into a Distribution and Equity Acquisition Agreement with Marathon. The Company was appointed the exclusive distributor of the Products (as defined) initially throughout Europe and on a non-exclusive basis wherever else lawfully permitted. As consideration for its services, Company received: (a) a 33 1/3% equity interest or 5 million shares in Marathon as partial consideration for the Company’s distribution services; and (b) received cash of CAD $2,000,000, subject to repayment in Common Shares of the Company if it fails to meet certain performance milestones. The Company is entitled to receive an additional CAD $2,750,000 upon the Company’s receipt of gross sales of CAD $6,500,000 and an additional CAD $2,750,000 upon receipt of gross sales of CAD $13,000,000.
As discussed in Note 3, the Company attributed no value to the shares received in Marathon pursuant to (a) above. In relation to the CAD $2 million cash received noted in (b) above, the Company accounted for its obligation to issue a variable number of the Company’s Common Shares as Share-settled debt obligation in accordance with ASC 480 measured at fair value or the settlement amount of $1,554,590 (CAD $2 million). If settlement had occurred on December 31, 2022, the Company would have been required to issue 420,471 common shares to settle its debt obligation. The Company could be obligated to potentially issue an unlimited number of common shares to settle its Share-settled debt obligation.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
On March 20, 2023, the Company’s legal counsel provided notice to Marathon Global Inc, that Cosmos terminated the Equity agreement dated on March 19, 2018 pursuant to Section 3.2 and that termination is effective thirty days from the date of the letter.
None of the above loans were made by any related parties.
NOTE 12 – LEASES
The Company has various operating and finance lease agreements with terms up to 10 years, for various types of property and equipment (such as office space and vehicles) etc. Some leases include options to purchase, terminate or extend for one or more years. These options are included in the lease term when it is reasonably certain that the option will be exercised. Leases with an initial term of 12 months or less are not recorded on the balance sheet; we recognize lease expense for these leases on a straight-line basis over the lease term.
Operating Leases
The Company’s weighted-average remaining lease term relating to its operating leases is 4.08 years, with a weighted-average discount rate of 6.74%.
The following table presents information about the amount, timing and uncertainty of cash flows arising from the Company’s operating leases as of September 30, 2024:
Maturity of Operating Lease Liability | | | |
2024 | | | 82,319 | |
2025 | | | 243,175 | |
2026 | | | 179,151 | |
2027 and thereafter | | | 300,301 | |
Total undiscounted operating lease payments | | $ | 804,946 | |
Less: Imputed interest | | | (95,366 | ) |
Present value of operating lease liabilities | | $ | 709,580 | |
The Company incurred lease expense, due to amortization of operating lease right-of-use assets, of $76,229 and $50,690 and $235,659 and $158,407, which was included in “General and administrative expenses,” for the three and nine months ended September 30, 2024 and 2023, respectively.
Finance Leases
The Company’s weighted-average remaining lease term relating to its finance leases is 1.19 years, with a weighted-average discount rate of 6.74%.
The following table presents information about the amount, timing and uncertainty of cash flows arising from the Company’s finance leases as of September 30, 2024:
Maturity of Lease Liability | | | |
2024 | | | 9,039 | |
2025 | | | 12,892 | |
2026 | | | 3,712 | |
Total undiscounted finance lease payments | | $ | 25,643 | |
Less: Imputed interest | | | (910 | ) |
Present value of finance lease liabilities | | $ | 24,733 | |
The Company had financing cash flows used in finances leases of $9,765 and $41,094 and $27,118 and $118,847 for the three and nine months ended September 30, 2024 and 2023, respectively.
The Company incurred interest expense on its finance leases of $457 and $6,920 and interest expense of $1,765 and $20,629 which was included in “Interest expense”, for the three and nine months ended September 30, 2024 and 2023, respectively. The Company incurred amortization expense on its finance leases of $7,589 and $35,452 and amortization expense of $22,507 and $102,549 which was included in “Depreciation and amortization expense,” for the three and nine months ended September 30, 2024 and 2023, respectively.
NOTE 13 – OTHER LIABILITIES
The Company’s other liabilities include but are not limited to liabilities to local tax authorities, fines and payroll taxes, which comprise the largest portion of the balance as of September 30, 2024. The Company’s Greek subsidiaries have $1,812,919 in settled tax liabilities payable to the tax authorities in installments and $1,799,431 in payroll and other tax related current liabilities. Moreover, we have recorded a provision relating to the unaudited tax years of our subsidiary SkyPharm SA, of $644,779 and a provision for staff leaving compensation, based on the corresponding actuarial reports, of $411,732. Additionally, we have received prepayments from our customers of $451,575 and recorded accrued sales discounts of $407,725 included in “Other current liabilities” as of September 30, 2024. We classify the liabilities payable within the twelve months following the balance sheet date in “Other current liabilities” and the remaining balance is included in “Other Liabilities”.
NOTE 14 – COMMITMENTS AND CONTINGENCIES
Legal Matters
From time to time, the Company may be involved in litigation relating to claims arising out of the Company’s operations in the normal course of business. As of September 30, 2024, the following litigations were pending. None of the below is expected to have a material financial or operational impact.
On July 22, 2015, the National Medicines Agency approved the license of wholesale sale of pharmaceutical products under the name SkyPharm SA with set validity at five years and an expiration date of July 22, 2020. Subsequently, SkyPharm on June 15, 2020, legally and timely submitted the application for renewal of the wholesale license of pharmaceutical products to the National Medicines Agency. The National Medicines Agency did not respond, therefore the Company asked for an immediate decision on the renewal. Two months after the filing of the no. 3459 / 15.01.2021 letter and almost nine months after the no. 627615.06.2020 Company application for the renewal, the National Medicines Agency replied by rejecting the renewal request on March 9, 2021 (ref. 62769 / 20-25.02.2021). In addition, document No. 127351-16.12.2021 of EOF (Greek National Medicines Organization) to SkyPharm states that after an inspection of EOF at the premises of Doc Pharma, we did not have a wholesale license in violation of article 106 par. 1b and par. 1c of the ministerial decision D.YG3a / GP.32221 / 29-4-2019. The National Medicines Agency imposed a fine of €15,000 ($16,214) on SkyPharm for the above case, which was included in “General and administrative expenses” on the accompany statement of operations and comprehensive loss for the twelve-month ended December 31, 2023.
There has been a payment request by the Greek court, which relates to a fine arising from Cosmofarm’s tax audit for financial year 2014. The law with no. 483/16.12.2020 was used by the court against Cosmofarm (the “defendant”). The defendant appealed against the decision using the law with no.11541/09.03.2021. This appeal was dismissed after 120 days from its submission to the court. Additionally, there had been an obligation for payment of additional tax and fines related to this matter in the amount of €91,652 ($99,644), which the defendant has already settled. However, the defendant has claimed back the respective amount through appeal. As of September 30, 2024, the trial is still pending.
On January 25, 2023, a criminal case of dishonored checks against Cosmofarm’s customer Filippou, was heard at the Z’ Three-Member Misdemeanor Court of Athens, which was postponed to November 27, 2023, when the defendant was tried and found guilty.
On January 26, 2023, the appeal of the Company against Eleutheria Drakopoulou and decision 1389/2021of the Single-Member Court of First Instance of Athens was heard at the Athens Court of Appeal. The appeal was partially accepted. The Court ordered the return of the fee to the appellants, dismissed the action against the third defendant, Kozaris and accepted the action as regards the first and the second defendants (Kastrantas & Cosmofarm).
On October 23, 2023, a criminal case of dishonored checks against Cosmofarm’s customer Kafantaris was heard at the Sixth Single-Member Misdemeanor Court of Athens, which was postponed to January 26, 2024, when the defendant was convicted by decision no. 1599/2024.
In October 2023, the Company’s subsidiary, Cana Laboratories was approached by an attorney on behalf of two clients which were requesting an amount of €39,211 as compensation for the value of 34.70 square meters in relation to an urban sprawl with respect to which an Act of Imputation had been issued by the department of Urban Planning. Our legal counsel’s response was that CANA was not obliged to accept the compensatory value agreed and suggested exploring out of court settlement. As of today, the clients’ Attorney at law has not come back with any suggestions.
Our subsidiary, Cana Laboratories, has two pending lawsuits against Euaggelismos Hospital for a total sum of EUR 526,436 due to unpaid bills. The court date for one of the two lawsuits is set for December 11, 2024, and for the other one has not yet been set. The opinion of our legal advisor is that the collection of the total sum by the Company is almost certain.
Our subsidiary, Cana Laboratories, has an unasserted claim against Papanikolaou Hospital for a total sum of EUR 89,300 due to unpaid bills, which will be asserted through a lawsuit. The opinion of our legal advisor is that the collection of the sum by the Company is almost certain.
A lawsuit dated April 5, 2018 against the Company’s subsidiary Cana Laboratories by a former employee before the Athens court of instance was initially heard on October 12, 2018. The former employee was seeking that the termination of her employment contract to be considered null and void and was requesting compensation for late wages and moral damages. Following numerous appeals, Judgment No. 1192/2024 was issued on September 26, 2023, which as explicitly stated by our legal counsel, requires CANA to rehire the former employee with the threat of a penalty of €200 for each day of non-compliance. As informed by our legal counsel, in order for the penalty to be effective the former employee should file a new lawsuit against CANA and request to get rehired. In case CANA denies employment, then the penalty should be in effect. As of today, we have not received neither a lawsuit nor any request of employment by the former employee.
Advisory Agreements
On July 1, 2021, the Company entered into a two-year advisory agreement with a third party (the “Consultant”) for advisory and consulting services related to the Company’s intention to become listed on Nasdaq. Peter Goldstein, a then director of the Company is a principal of the Consultant. As consideration for services rendered, and successful Nasdaq listing, the Company paid $100,000. The $100,000 bonus was incurred and settled within 2022. Finally, the Consultant received a total of 10,000 shares of the Company’s common stock, 2,000 of such shares that have been previously issued pursuant to previous agreements and additional 15,258 shares that were issued on February 2, 2023, based on the amendment signed on February 1, 2023.
On November 21, 2023, the Company entered into certain consulting agreements with four third-party consultants for the provision of a variety of services such as digital marketing, advisory services relating to target acquisitions and M&As and other additional services as described in the respective agreements. The agreements have a duration from 10 to 18 months and the consultants will solely receive stock consideration for the services rendered. More precisely, they have been awarded a total of 970,000 shares of the Company’s common stock valued at a total of $999,100 based on the fair value of the Company’s common stock as of the agreements’ date. On September 17, 2024 the termination of two out of the four aforementioned consulting agreements was extended and the consultants received additional 440,000 shares as complementary compensation for the extended services to be provided. The additional stock consideration was valued at a total of $501,600 based on the fair value of the Company’s common stock as of the agreements’ date.
On July 1, 2024 the Company entered into a consulting agreement with a third-party consultant for the provision of a variety of services such as preparation of press releases and other publications, relationship management and other additional services as described in the respective agreement. The agreement has a duration of sixteen months and the consultant will solely receive stock consideration for the services rendered. More precisely, they have been awarded a total of 240,000 shares of the Company’s common stock valued at a total of $264,000 based on the fair value of the Company’s common stock as of the agreements’ date.
The corresponding consulting expense is accrued evenly over the term of the agreements. For the twelve-month period ended December 31, 2023 the Company has recorded $77,250 as stocked based compensation for the above agreements, classified as “General and administrative expenses” in the Company’s consolidated statements of operations and comprehensive loss. For the three and nine months ended September 30, 2024 and 2023 the Company has recorded $264,750 and $728,250 and $0 and $0 as stocked based compensation for the above agreements, classified as “General and administrative expenses” in the Company’s Condensed Consolidated Statements of Operations and Comprehensive Loss.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
Research and Development Agreements
The Company entered into a Research & Development agreement with Doc Pharma S.A. on May 17, 2021. Under this agreement, Doc Pharma is responsible for the research, development, design, registration, copy rights and licenses of 250 nutritional supplements for the final products called Sky Premium Life®. More specifically, Doc Pharma is responsible for the product development and the Company had added 105 of such products codes in its portfolio as of December 31, 2023. No additional ones were added within the nine-month period ended September 30, 2024. The licenses purchased by Doc Pharma SA are capitalized and included in “Goodwill and intangible assets, net” of the Company’s Consolidated Balance Sheets as of September 30, 2024. Thus, no relevant R&D expense had been charged to the Company’s Consolidated Statements of Operations and Comprehensive Loss.
On June 26, 2022, the Company signed a research and development (“R&D”) agreement with a third party, through which the Company assigns to the third party the development of new products and services in the field of health, focusing on the human intestinal microbiome. The project includes two phases. Phase 1 has a 20-month duration and its cost amounts to EUR 758,000 ($838,450) and phase 2, has a 22-month duration and a cost of EUR 820,000 ($907,084). The amount will be due and payable upon completion of the corresponding phases. The Company records the corresponding R&D expense based on the project’s progress, which is invoiced by the third party in the relevant period. For the nine-month period ended September 30, 2024, the Company has not incurred such costs.
NOTE 15 – STOCK OPTIONS AND WARRANTS
Omnibus Equity Incentive Plan
On September 19, 2022, the Company held a Board of Directors meeting, whereas, the Board of Directors had elected to adopt an Omnibus Equity Incentive Plan (the “2022 Plan”), that includes reserving 200,000 shares of common stock eligible for issuance under the 2022 Plan to be registered on a Form S-8 Registration Statement with the SEC. The 2022 Plan is designed to enable the flexibility to grant equity awards to the Company’s officers, employees, non-employee directors and consultants and to ensure that it can continue to grant equity awards to eligible recipients at levels determined to be appropriate by the Board and/or the Compensation Committee. According to the Proxy Statement filed with the SEC on October 20, 2022 the 2022 Plan received final approval by the Company’s stockholders at the Annual Meeting of Stockholders held on December 2, 2022.
On April 3, 2023, the Company approved incentive stock awards for the CFO, certain officers and directors and other employees of the Company. The awards are in the form of restricted stock and will vest in two parts: 50% on October 2, 2023 and 50% on October 2, 2024. A total of 185,000 shares were awarded and a corresponding share-based compensation expense of $109,636 and $326,525 was recorded for the three and nine months ended September 30, 2024, based on the amortization of fair value from the date of issuance of April 3, 2023, through September 30, 2024.
The equivalent share-based compensation expense for the three and nine months ended September 30, 2023 was $109,636 and $214,505, respectively.
On August 21, 2023, the Board adopted, subject to stockholder approval, the Cosmos Health Inc. 2023 Omnibus Equity Incentive Plan (the “2023 Plan”). The 2023 Plan is designed to enable the flexibility to grant equity awards to our officers, employees, non-employee directors and consultants and to ensure that we can continue to grant equity awards to eligible recipients at levels determined to be appropriate by the Board and/or the Compensation Committee. Subject to certain adjustments (as provided in Section 4.2 of the 2023 Plan) and exception (as provided in Section 5.6(b) of the 2023 Plan), the maximum number of shares reserved for issuance under the 2023 Plan (including incentive share options) is 2,500,000 shares. The 2023 Plan was approved by the Company’s stockholders at the Annual Meeting of Stockholders held on September 18, 2023.
On September 16, 2024 the Company’s Board of Directors approved incentive stock awards for the CEO, the CFO, certain officers and directors and other key employees of the Company pursuant to the 2023 Plan adopted on August 21, 2023. The awards are in the form of restricted stock and will vest in two parts: 50% on September 16, 2025 and 50% on September 16, 2026. A total of 2,500,000 shares were awarded and a corresponding share-based compensation expense of $48,425 was recorded for the three and nine months ended September 30, 2024, based on the amortization of fair value from the date of issuance of September 16, 2024, through September 30, 2024.
Warrant Anti-Dilution Adjustment and Deemed Dividend
The Company’s warrants outstanding contain certain anti-dilution adjustments if the Company issues shares of its common stock at a lower price per share than the applicable exercise price of the underlying warrant. If any such dilutive issuance occurs prior to the exercise of such warrant, the exercise price will be adjusted downward to a price equal to the common stock issuance, and the number of warrants that may be purchase upon exercise is increased proportionately so that the aggregate exercise price payable under the warrant shares shall be the same as the aggregate exercise price in effect immediately prior to such adjustment.
On December 29, 2023, the Company entered into a warrant exchange agreement (the “Warrant Exchange”) with an investor to reduce the exercise price of 2,437,063 warrants from $2.75 per share to $1.45 per shares as an inducement to exercise. The Company issued 1,487,000 shares of common stock, held 950,063 shares in escrow until the investor’s beneficial ownership limitation allows for the transfer of the escrow shares, and received gross cash proceeds of 3,533,741. The Company contingently granted 4,874,126 additional warrants to be issued upon shareholder approval, with an exercise price of $1.45 and a term of five years. For the year ended December 31, 2023, the Company recorded a deemed dividend of $7,642 for the inducement to exercise and $7,218,485 for the grant of new warrants. The Company valued (a) the fair value of the warrants immediately before the re-pricing in the amount of $3,603,183, (b) the fair value of the warrants immediately after the re-pricing in the amount of $3,610,825, and (c) recorded the difference as deemed dividend in the amount of $7,642. The warrants were valued using the Black-Scholes option pricing model using the following terms: a) fair value of common stock of $1.49, b) exercise price of $1.45 before re-pricing, c) exercise price of $2.75 after re-pricing, d) terms of 5.07 years and 5.02 years, e) dividend rate of 0%, and f) risk free interest rate of 3.83%. Regarding the valuation of the 4,874,126 new warrants (and the recognition of a deemed dividend of $7,218,485) the following terms were used: a) fair value of common stock of $1.49, b) exercise price of $1.45, d) term of 5 years, e) dividend rate of 0%, and f) risk free interest rate of 3.83%.
On September 26, 2024, the Company entered into a Warrant Inducement Letter (the “Letter”) with an investor pursuant to which the Company issued 9,748,252 new warrants (the “New Warrants”) and reduced the exercise price of 4,874,126 warrant shares from $1.45 to $0.8701 to induce exercise and receive gross cash proceeds of $4,240,977 (the “Original Warrants”). Of the 9,748,252 warrants 4,874,126 of them have a term of 5 years (“Series A Warrants”) and the remaining 4,874,126 have a term of 1.5 years (“Series B Warrants”). The Company issued 2,332,000 shares of common stock, held 2,532,126 shares in escrow until the investor’s beneficial ownership limitation allows for the transfer of the escrow shares. For the period ended September 30, 2024, the Company recorded a deemed dividend of $9,793 for the inducement to exercise and $6,185,231 for the grant of new warrants. The Company valued (a) the fair value of the warrants immediately before the re-pricing in the amount of $4,197,280, (b) the fair value of the warrants immediately after the re-pricing in the amount of $4,207,073, and (c) recorded the difference as deemed dividend in the amount of $9,793. The warrants were valued using the Black-Scholes option pricing model using the following terms: a) fair value of common stock of $0.8701, b) exercise price of $1.45 before re-pricing, c) exercise price of $0.8701 after re-pricing, d) term of 4.26 years, e) dividend rate of 0%, and f) risk free interest rate of 3.55%. Regarding the valuation of the 9,748,252 new warrants (and the recognition of a deemed dividend of $6,185,321) the following terms were used: a) fair value of common stock of $0.8701, b) exercise price of $0.95, d) terms of 5 years for the Series A Warrants and 1.5 years for the Series B Warrants, e) dividend rate of 0%, and f) risk free interest rate of 3.55% for the Series A Warrants and 3.57% for the Series B Warrants.
As of September 30, 2024, there were 13,432,506 warrants outstanding and 8,558,380 warrants exercisable with 13,419,172 warrants having expiration dates from October 2024 through October 2029 and 13,334 warrants with no expiration date.
A summary of the Company’s warrant activity for the nine months ended September 30, 2024 and the year ending December 31, 2023 is as follows:
| | | | | | | | Weighted | | | | |
| | | | | Weighted | | | Average | | | | |
| | | | | Average | | | Remaining | | | Aggregate | |
| | Number of | | | Exercise | | | Contractual | | | Intrinsic | |
Warrants | | Shares | | | Price | | | Term | | | Value | |
Balance Outstanding, January 1, 2023 | | | 4,194,236 | | | $ | 8.31 | | | | 5.04 | | | $ | 2,562,621 | |
Granted | | | 7,524,933 | | | | 1.65 | | | | 5.13 | | | | - | |
Forfeited | | | - | | | | - | | | | - | | | | - | |
Exercised | | | (3,152,386 | ) | | | - | | | | - | | | | - | |
Expired | | | (5,307 | ) | | | - | | | | - | | | | - | |
Balance Outstanding, December 31, 2023 | | | 8,561,476 | | | $ | 3.91 | | | | 4.64 | | | $ | 18,801 | |
Granted | | | 9,748,252 | | | | 0.95 | | | | 3.24 | | | | - | |
Forfeited | | | - | | | | - | | | | - | | | | - | |
Exercised | | | (4,874,126 | ) | | | - | | | | - | | | | - | |
Expired | | | (3,096 | ) | | | - | | | | - | | | | - | |
Balance Outstanding, September 30, 2024 | | | 13,432,506 | | | $ | 2.64 | | | | 3.28 | | | $ | 11,681 | |
| | | | | | | - | | | | - | | | | - | |
Exercisable, September 30, 2024 | | | 13,432,506 | | | $ | 2.64 | | | | 3.28 | | | $ | 11,681 | |
NOTE 16 – DISAGGREGATION OF REVENUE
ASC 606-10-50-5 requires that entities disclose disaggregated revenue information in categories (such as type of good or service, geography, market, type of contract, etc.). ASC 606-10-55-89 explains that the extent to which an entity’s revenue is disaggregated depends on the facts and circumstances that pertain to the entity’s contracts with customers and that some entities may need to use more than one type of category to meet the objective for disaggregating revenue.
COSMOS HEALTH INC.
Notes to Unaudited Condensed Consolidated Financial Statements
September 30, 2024
The Company disaggregates revenue by country to depict the nature and economic characteristics affecting revenue.
The following table presents our revenue disaggregated by country for the three months ended:
Country | | September 30, 2024 | | | September 30, 2023 | |
Croatia | | $ | 107 | | | | 14,159 | |
Cyprus | | | 16,519 | | | | 72,754 | |
Bulgaria | | | 25,507 | | | | - | |
Greece | | | 12,247,597 | | | | 12,544,643 | |
USA | | | - | | | | 210 | |
Ireland | | | - | | | | 1,417 | |
UK | | | 121,318 | | | | 190,614 | |
Total | | $ | 12,411,048 | | | $ | 12,823,797 | |
The following table presents our revenue disaggregated by country for the nine months ended:
Country | | September 30, 2024 | | | September 30, 2023 | |
Croatia | | $ | 19,370 | | | | 14,159 | |
Cyprus | | | 89,064 | | | | 141,402 | |
Bulgaria | | | 43,849 | | | | - | |
Greece | | | 39,385,730 | | | | 36,041,012 | |
USA | | | - | | | | 504 | |
Ireland | | | - | | | | 1,417 | |
UK | | | 664,225 | | | | 1,338,509 | |
Total | | $ | 40,202,238 | | | $ | 37,537,003 | |
NOTE 17 – SUBSEQUENT EVENTS
On November 6, 2024, the Company received a non-compliance letter from Nasdaq for its failure to maintain a minimum bid price of 1.00 per share for thirty consecutive business days in accordance with Nasdaq Listing Rule 5550(a)(2). The Company has one hundred eighty calendar days from November 6, 2024, to regain compliance by the closing bid price of the Company’s common stock being at least $1.00 per share for ten consecutive business days. In the event the Company cannot otherwise regain compliance with the listing rule, it intends to effect a reverse stock split to regain compliance.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Available Information
The following discussion should be read in conjunction with our interim Condensed Consolidated Financial Statements and the related notes and other financial information appearing elsewhere in this report as well as Management’s Discussion and Analysis of Financial Condition and Results of Operations included in our Form 10-K for the year ended December 31, 2023 (“Form 10-K”) and this Quarterly Report on Form 10-Q for the quarter ended September 30, 2024.
Forward-Looking Statements
Certain statements, other than purely historical information, including estimates, projections, statements relating to our business plans, objectives, and expected operating results, and the assumptions upon which those statements are based, are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These forward-looking statements generally are identified by the words “believes,” “project,” “expects,” “anticipates,” “estimates,” “intends,” “strategy,” “plan,” “may,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions.
We intend such forward-looking statements to be covered by the safe-harbor provisions for forward-looking statements and are including this statement for purposes of complying with those safe-harbor provisions. Forward-looking statements are based on current expectations and assumptions that are subject to risks and uncertainties which may cause actual results to differ materially from the forward-looking statements. Our ability to predict results or the actual effect of future plans or strategies is inherently uncertain.
Factors which could have a material adverse effect on our operations and future prospects on a consolidated basis include but are not limited to: changes in economic conditions, legislative/regulatory changes, availability of capital, interest rates, competition, and generally accepted accounting principles. These risks and uncertainties should also be considered in evaluating forward-looking statements and undue reliance should not be placed on such statements. We undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise. Further information concerning our business, including additional factors that could materially affect our financial results, is included herein and in our other filings with the SEC.
Overview
Summary
We are an international healthcare company with a proprietary line of nutraceuticals and distributor of branded and generic pharmaceuticals, nutraceuticals, OTC medications and medical devices. The Company uses a differentiated operating model based on a lean, nimble and decentralized structure, with an emphasis on acquisitions of established companies and our ability to maintain better pharmaceutical assets than others. This operating model and the execution of our corporate strategy are designed to enable the Company to achieve sustainable growth and create added value for our shareholders. In particular, we look to enhance our pharmaceutical and over-the-counter product lines by acquiring or licensing rights to additional products and regularly evaluate selective company acquisition opportunities. The Company, through its subsidiaries, is operating within the pharmaceutical industry and in order to compete successfully in the healthcare industry, must demonstrate that its products offer medical benefits as well as cost advantages. Currently, most of the products that the Company is trading, compete with other products already in the market in the same therapeutic category, and are subject to potential competition from new products that competitors may introduce in the future.
We continue to rapidly expand our distribution network worldwide and open new markets for our proprietary line of branded pharmaceuticals, nutraceuticals, and nutraceuticals through our distribution channels and e-commerce marketplace. We use our extensive network with direct access to Europe’s primary sales channels for pharmaceuticals and nutraceuticals, which includes over 160 pharmaceutical wholesale distributors in Europe’s largest markets, over 40,000 pharmacies in Europe and 1,500 pharmacies in Greece. We achieve stable supply of pharmaceuticals from DocPharma, a related party, which enhances our ability to scale our expansion. Additionally, following the successful completion of the acquisition of Cana on June 30, 2023, the Company expects to also utilize Cana’s facilities for the production of both pharmaceutical and nutraceutical products. We receive full priority in the production of nutraceuticals and volumes. Our full production in Greece ensures a decisive production-cost advantage while we secure additional discounts by leveraging our purchasing scale.
Our focus on investing in technology enhances yield cost savings and economies of scale the safety, distribution and warehousing efficiency and reliability, as a result of 0% error selection rate and acceleration order fulfillment.
Revenue sources
The Company operates in nutraceuticals industry, distribution of pharmaceuticals and healthcare distribution.
Branded Pharmaceuticals & Generics
We are engaged in the production, promotion, distribution and sale of licensed branded generics and OTC products throughout Europe by our subsidiaries in Greece and UK. Our capital efficient business model is based on infrastructure, efficiency and scale. We believe that there is a significant growth on opportunities through product additions and geographic expansion.
Healthcare Distribution
We conduct direct distribution and sales of pharmaceuticals, medical devices, branded generics and OTC products. Our automated and GDP licensed distribution facilities ensure all medications reach their destination daily on an efficient and secure way. Our network exceeds over 1,500 pharmacies in Greece. We have created an upgraded and high-end distribution center in Greece due to our Robotic systems and integrated automations (“ROWA” robotics).
Nutraceutical
We have created and developed our own proprietary branded nutraceutical products, named “Sky Premium Life®” which was launched in 2018 and “Mediterranation®” which was launched in 2022. Utilizing unique formulations, and specialized extraction processes which follow strict pharmaceutical standards, our proprietary lines of nutraceuticals aim for excellence. We have a full portfolio of fast-moving and specialty formulas with more than 105 product codes including vitamins, minerals and other herbal extracts. Our nutraceutical products are manufactured exclusively by Doc Pharma, a related party of the Company. Our nutraceutical products have penetrated several markets within 2022 and 2023 through digital channels such as Amazon and Tmall and through significant partnerships such as the one with Pharmalink for the distribution of our products in the United Arab Emirates (the “UAE”). We focus on nutraceutical products because we foresee it as a market with high grow opportunities due to its large market size and margin contribution as the demand for nutraceutical products is increasing globally.
Regulations and Licenses
Our subsidiary, Decahedron, was granted the license for the wholesale of medicinal products for human use in February 2021 pursuant to the regulation of 18 of The Human Medicines Regulations 2012 (SI 2012/1916). It fulfills the guidelines of the Wholesale Distribution Authorization (Human). Our subsidiary, Cosmofarm S.A., was granted the license for the wholesale of pharmaceutical products for human use on February 2019 pursuant to the EU directive of (2013/C 343/01). It fulfils the Guidelines of the Good Distribution Practices of medical products for human use. Finally, our subsidiary, Cana SA, is a holder of Good Manufacturing Practices license (GMP), which means that it is certified for fulfilling the minimum standards that a medicines manufacturer must meet in the production processes. All licenses were granted based on inspections and are valid unless current inspections occur which will revise their status.
Risks
Supply chain disruption is a growing concern for the European pharmaceutical industry as it increasingly looks to cut costs by relying on ‘emerging markets’, where standards can be lower in terms of compliance, ethics and health and safety.
Hikes in the price of medicine and their impact on the sustainability of the healthcare systems are garnering more and more attention. European regulators are willing to play their part in safeguarding continued access to safe and effective medicines. Regulators can speed up the approval of branded pharmaceuticals and biosimilars to boost competition and drive down prices.
Cuts in healthcare spending keep occurring since the financial crises of the late of 2000s. Europe’s slow recovery has been uneven, with austerity and economic uncertainty, especially in the EU’s poorer member states, such as Greece.
Distribution and Trade Agreements
On July 1, 2021, the Company’s subsidiary SkyPharm SA, entered into an exclusive distribution agreement with a company based in Germany, the “Distributor A”, whereas SkyPharm appointed Distributor A to be the responsible Partner for the distribution, promotion, trade marketing, logistics and sale of the nutraceuticals manufactured and supplied by SkyPharm (Sky Premium Life®), in the territories of Austria and Germany. Distributor A places purchase orders with SkyPharm at the company’s address and the purchase order is necessary to initiate any shipment.
On July 7, 2021, SkyPharm SA signed a trade agreement with a company specializing in e-commerce mall advice and operation, henceforward referred as “Distributor B”. Based on the agreement, SkyPharm will sell its own branded products Sky Premium Life ® to final consumers through the e-commerce store opened by Distributor B on Tmall International MALL and Distributor B will provide platform operation services to SkyPharm. The services provided by Distributor B will include mall construction, mall operation and network promotion, along with collection, settlement, customer service, logistics and distribution.
On November 25, 2021, SkyPharm SA signed a trade agreement with a wholesaler which operates in the storage, distribution, trading and promotion of pharmaceutical products) henceforward referred as “Distributor C”. Based on the agreement Distributor C is appointed as the exclusive representative for the promotion & distribution of our proprietary nutraceutical products Sky Premium Life®, in Greece.
During July 2021, the Company’s subsidiary Decahedron Ltd, created a distribution page on Amazon UK, through which it sells, advertises and promotes our own proprietary branded nutraceutical product line “Sky Premium Life®, directly to final consumers.
On September 22, 2022, the Company entered into a distribution agreement with a third party in order to become the distributor of Monkeypox Virus Real-Time PCR Detection Kits. Cosmos will have exclusive distribution rights for Greece and Cyprus, with the opportunity to distribute the test kits across Europe on a non-exclusive basis.
On June 27, 2024 the Company signed an exclusive distribution agreement (the "Agreement") with Pharmalink for its Sky Premium Life products in the UAE. As part of the Agreement, Pharmalink will be responsible for all key functions, including sales and marketing, regulatory affairs, logistics, supply, and distribution of Sky Premium Life products in the UAE. Cosmos Health has secured its first purchase order from Pharmalink for 130,000 units and anticipates receiving orders of more than 500,000 units in the first year and in excess of 3,000,000 units over the next five years.
Acquisitions and Co-Ventures
ZipDoctor
On September 28, 2022, the Company entered into a non-binding letter of intent (“LOI”) agreement to wholly acquire ZipDoctor Inc., a company that possesses a direct-to-consumer subscription-based telemedicine platform, that expects to provide its customers affordable, unlimited, 24/7 access to board certified physicians and licensed mental and behavioral health counselors and therapists. The current parent company of the acquiree will continue to manage all its aspects of the day-to-day operations, including product development, marketing, and operational support.
On March 17, 2023, the Company announced that it has entered into a definitive agreement to acquire ZipDoctor Inc. for a total sum of $150,000. The Sale and Purchase Agreement (“SPA”) was signed on March 17, 2023, and the transaction closed on April 3, 2023.
CANA
On May 31, 2023, the Company entered into a Stock Purchase Agreement with the owners of one hundred (100%) percent of the equity (the “Shares”) of Cana Laboratories Holdings (Cyprus) Limited (“Cana”), which wholly owned an operating subsidiary, Pharmaceutical Laboratories Cana S.A. (“Cana SA”). The purchase price for the shares for the two Sellers is €800,000 and 46,377 shares of Cosmos restricted common stock at an issuance price of $17.25 per share or $800,000. Moreover, on February 28, 2023, the Company signed a Secured Promissory Note with Cana, whereby Cana borrowed the sum of €4,100,000 ($4,457,520), included in the total cash consideration provided for the acquisition. The acquisition was successfully completed on June 30, 2023.
Cana SA is a Greek pharmaceutical company that manufactures, sells, distributes, and markets original branded products researched and developed by leading global pharmaceutical and healthcare companies. Cana stands out as it brings significant synergies and vertical integration. With a long-standing history spanning almost a century, Cana has earned the trust of industry giants like AstraZeneca, Merck, Unilever, and Procter & Gamble. Cana's Good Manufacturing Practice (GMP) license enables us to manufacture pharmaceuticals, including medicines, within the EU, which creates attractive opportunities for high-margin contract manufacturing agreements with major multinational clients.
Bikas
On June 15, 2023, Cosmos Health Inc. entered into an Assignment and Assumption Agreement (the “Agreement”) with Ioannis Bikas O.E., a Greek Company (“Bikas”). Bikas is owner of a pharmaceutical distribution network in Greece and agreed to sell the Company their distribution network and customer base. The purchase price of the network was €100,000 ($109,330) of cash, and €300,000 ($316,081) of the Company’s stock. The Company issued 99,710 shares of common stock related to the acquisition of the customer base, based on the fair value of the stock on the acquisition date. The Company accounted for the acquisition as an asset acquisition in accordance with ASC 805 and recorded $425,411 as an intangible asset related to the customer base acquired.
This acquisition positively impacted on our revenue (an increase of more than $10 million annually) and enhanced the Company's gross margins (due to economies of scale). Additionally, synergies with Cosmofarm's state-of-the-art facility, which employs robotic technologies for procurement, inventory management, and order execution, provide an elevated level of service to pharmacies, leading to increased orders. We are pleased to announce that we have now successfully integrated Bikas within the Cosmofarm platform.
Cloudscreen
On January 23, 2024, the Company completed the acquisition of Cloudscreen, a cutting-edge Artificial Intelligence (AI) powered platform. The acquisition is pursuant to the purchase agreement announced on October 11, 2023. Cloudscreen is a multimodal platform specialized in drug repurposing, a process that involves uncovering new target proteins or indications for existing drugs for use in treating different diseases. The total purchase price amounted to $637,080 and consisted of 280,000 shares of common stock with a fair value of $319,200 and an amount of $317,880 to be settled in cash during 2024 based on the Promissory Note signed on October 10, 2023. The Company accounted for the acquisition as an asset acquisition in accordance with Accounting Standards Codification (“ASC”) Topic 805, Business Combinations, (“ASC 805”) and recorded $637,080 as another asset related to the technology platform acquired. The total amount was reclassified to “Goodwill and intangible assets, net” in January 2024 with the closing of the agreement (refer to Notes 2 & 5).
Results of Operations
Three and Nine Months Ended September 30, 2024 and 2023
Revenue and net loss
The Company had revenue of $12,411,048 and $12,823,797 (a decrease of 3.22%) for the three months ended September 30, 2024 and 2023, respectively and $40,202,238 and $37,537,003 (an increase of 7.10%) for the nine months ended September 30, 2024 and 2023, respectively. Revenue increased overall, compared to the prior periods, and the increase in the nine-month period is mainly attributed to the wholesale revenue stream which was further increased with the enhancement of the overall customer base (acquisition of Bikas and other customer bases from smaller wholesalers). The revenue decrease in the three-month period relates to CANA’s decreased revenue for the period due to a few delays in the production process and Decahedron’s decreased sales of nutraceutical products. Management evaluates that both subsidiaries will rebound and increase their revenue in the fourth quarter of 2024. The Company had a net loss of $2,182,534 on revenue of $12,411,048 versus a net loss of $3,415,077 on revenue of $12,823,797 for the three months ended September 30, 2024 and 2023, respectively and net loss of $6,639,935 on revenue of $40,202,238 versus a net loss of $4,790,597 on revenue of $37,537,003 for the nine months ended September 30, 2024 and 2023, respectively. The increase in net loss for the nine-month period ended September 30, 2024 compared to the 2023 period of $1,849,338 was due to the extraordinary 2023 items such as the gain on debt extinguishment of $1,910,770 and the bargain purchase gain of $1,633,842. The decrease in net loss for the three-month period ended September 31, 2024 compared to the three-month period ended September 31, 2023 is mainly attributable to significantly higher general and administrative costs in the 2023 period, which include material provisions relating to allowances on trade and other receivables and other tax related provisions.
Cost of Goods Sold
The Company had costs of goods sold of $11,204,186 versus $11,609,039 (a decrease of 3.49%) for the three months ended September 30, 2024 and 2023, respectively and $36,894,502 versus $34,418,334 (an increase of 7.19%) for the nine months ended September 30, 2024 and 2023, respectively. The increase in the cost of goods sold in the nine month period is a consequence of the increased sales and the equivalent decrease in the three month period is also in accordance with the decreased revenue.
Our future revenue growth is expected to continue to be affected by various factors such as industry growth trends, including drug utilization, the introduction of new innovative brand therapies, the likely increase in the number of branded pharmaceutical products that will be available over the next few years’ price increases and price deflation, general economic conditions, including the effects of the current conflict in the Ukraine, the coronavirus in the United Kingdom and the member states of European Union, competition within the industry, customer consolidation, changes in pharmaceutical manufacturer pricing and distribution policies and practices, increased downward pressure on government and other third party reimbursement rates to our customers, and changes in government rules and regulations.
Gross Profit
The Company had gross profit of $1,206,862 versus $1,214,758 (a decrease of 0.65%) for the three months ended September 30, 2024 and 2023, respectively and $3,307,736 versus $3,118,669 (an increase of 6.06%) for the nine months ended September 30, 2024 and 2023, respectively. The increase in gross profit for the nine-month period is attributable to the inclusion of CANA’s pharma manufacturing stream which attributes high gross margins, whereas it was merely included for three months in the equivalent 2023 period (acquisition was dated on June 30, 2023). The gross profit was relatively stable between the three-month period ended September 30, 2024 and 2023.
Operating Expenses
The Company had general and administrative costs of $1,782,957 and $2,573,414, salaries and wage expenses of $1,317,782 and $1,252,680, sales and marketing expenses of $41,848 and $157,435 and depreciation and amortization expense of $304,139 and $248,530 for a loss from operations of $2,239,864 and a loss from operations of $3,017,301 for the three months ended September 30, 2024 and 2023, respectively. The operating expenses decreased by 18.56% for the three-month period ended September 30, 2024, mainly derived from the decrease in general and administrative costs. They decreased by 30.72% due to the significant provisions for bad debt allowances and other tax liabilities recorded in 2023. The salaries and wages increased by 5.2% which is mainly attributable to the addition of CANA and the corresponding payroll costs, once all employees remained with the Company following its acquisition on June 30, 2023. The increase in depreciation and amortization expense for the three-month period ended September 30, 2024 of $55,609 (22.38%) is in accordance increase in intangible assets (mainly attributable to the purchase of pharmaceutical licenses in December 2023).
For the nine months ended September 30, 2024 and 2023, the Company had general and administrative costs of $4,591,620 and $6,662,579, salaries and wage expenses of $4,030,823 and $3,279,803, sales and marketing expenses of $326,291 and $942,759 and depreciation and amortization expense of $937,000 and $478,466 for a loss from operations of $6,577,998 and a loss from operations of $8,244,938, respectively. The significant increase in salaries and wages of $751,020 (22.9%) is due to CANA’s inclusion in the group for the whole nine-month period ended September 30, 2024 whereas it was consolidated for three months in the comparative period. The 95.83% increase in depreciation and amortization expense for the nine-month period ended September 30, 2024 is attributable to increased depreciation expense charged for Cosmofarm’s and CANA’s wholly owned facilities (acquired in April and June 2023 respectively) as long as the amortization expense for the pharmaceutical licenses acquired in December 2023.
Other Income (Expense)
The Company had interest expense related to notes payable and lines of credit of $181,429 and $151,274 versus $692,547 and $529,782 for the three and nine months ended September 30, 2024 and 2023, respectively. The increase in interest expense of 19.93% and 30.72% for the three and nine months ended September 30, 2024 and 2023 respectively, contrary to the overall decrease in notes payable and lines of credit balances for the equivalent periods, is mainly attributable to the increased floating rates of the Company’s facilities in 2024 (Euribor, Libor and Euro Short Term rate).
Interest income amounted to $101,236 and $309,031 versus $110,596 and $555,281 for the three and nine months ended September 30, 2024 and 2023, respectively and the decrease is attributable to both the decreased outstanding balances of the Company’s Loans Receivable and Loans Receivable from related parties and the fact that the Company had interest income arising from treasury bills in the 2023 comparative periods.
The other income, net recorded in the 9-month period ended September 30, 2024, of $160,598 mostly relates to write-offs of liabilities of our dormant subsidiary Cana Laboratories Holdings (Cyprus) Limited (“Cana”) arising from the past which had no substance and thus written off. The corresponding other expense and other income amounts in the three-month periods ended September 30, 2024, and 2023 of $1,921 and $14,404, respectively, primarily relate to prior period income/(expenses) of the Greek subsidiaries.
Additionally, a gain on debt extinguishment relating to the write-off of a share settled debt obligation and the forgiveness of a notes payable balance for a total gain of $1,911,476 was recorded in the nine months ended September 30, 2023. Finally, the Company has recorded a bargain purchase gain of $1,633,842 for the nine months ended September 30, 2023. The bargain purchase gain recorded is solely arising from the gain recognized upon acquisition of Cana. No equivalent extraordinary items were included in the three and nine-month periods ended September 30, 2024.
Foreign currency translation adjustment, net and total comprehensive gain/loss
The Company had a foreign currency translation adjustment gain of $747,879 and a foreign currency translation adjustment loss of $27,988 versus losses of $890,645 and $470,994, attributable to the positive movement of the exchange rates during the three months ended September 30, 2024, respectively, and a net comprehensive loss of $7,629,679 and $12,862,947 versus a loss of $4,254,902 and $5,276,644 for the three and nine months ended September 30, 2024 and 2023, respectively. The increase in comprehensive loss in the three and nine months ended September 30, 2024 mostly derives from the deemed dividends of $9,793 for the inducement to exercise the warrants exercised on September 26, 2024 and $6,185,231 for the grant of new warrants (200% of the ones exercised). For more details regarding the warrant transaction and the deemed dividends recorded please refer to Note 15.
Liquidity and Capital Resources
As of September 30, 2024, the Company had working capital of $11,027,653 compared to $12,285,310 as of December 31, 2023.
The Company had cash and cash equivalents of $3,314,845 versus $3,833,195 as of September 30, 2024 and December 31, 2023, respectively. The Company had net cash used in operating activities of $3,883,215 and $16,587,726 for the nine months ended September 30, 2024 and 2023, respectively. The Company has devoted substantially all of its cash resources to expand through organic business growth and has incurred significant general and administrative expenses in order to enable the financing and growth of its business and operations. The decrease is attributable to the significant working capital outflows in 2023, when the company utilized the proceeds from 2022 equity offerings to repay its significant outstanding liabilities in addition to providing prepayments to certain suppliers.
The Company had net cash used provided by investing activities of $206,971 and net cash used in investing activities $10,399,264 during the nine months ended September 30, 2024, and 2023, respectively. For the nine months ended September 30, 2023, the net cash used in investing activities was mainly attributable to the outflow of consideration transferred through the Cana acquisition, the purchase of ZipDoctor Inc, the purchase of Bikas customer base, the purchase of a list of pharmaceutical licenses, the purchase of Cosmofarm’s warehouse facilities and the advances provided for the purchase of the facilities in Montreal, Canada from the parent company.
The Company had net cash provided by financing activities of $3,426,621 versus $8,401,750 during the nine months ended September 30, 2024, and 2023, respectively. For the nine months ended September 30, 2024, the Company received proceeds from lines of credit of $18,831,043 and payments of lines of credit of $19,504,594, for a net decrease on the lines of credit of $673,551. The significantly higher inflows arising from financing activities in 2023 was mainly attributable to the receipt of the $4,750,107 subscription receivable, due from December’s 2022 offering. However, the Company repaid $1,494,867 of the outstanding notes payable during the nine-month period ended September 30, 2023, versus $814,267 of debt repayments within the nine-month period ended September 30, 2024 whilst having proceeds from notes payable of $434,797 versus $1,059,300 for the nine month periods ended September 30, 2024 and 2023 respectively. During the nine-month period ended September 30, 2024, the Company raised additional equity funds through two Prospectus Supplements to its Registration Statement on Form S-3 (No. 333-267550) filed with the SEC on February 29 and March 7, 2024. More specifically, the Company sold 901,488 shares of common stock for gross proceeds of $649,039. Additionally, the Company received gross proceeds from the exercise of a number of its outstanding warrants pursuant to the warrant inducement transaction occurred on September 26, 2024 of $4,240,977.
We anticipate using cash in our bank account as of September 30, 2024, cash generated from debt or equity financing, from investing activities or from management loans to the extent that funds are available to do so to conduct our business in the upcoming year. Management is not obligated to provide these or any other funds. If we fail to meet these requirements, we may lose the qualification for quotation and our securities would no longer trade on Nasdaq Capital Market. Further, as a consequence we would fail to satisfy our reporting obligations with the Securities and Exchange Commission (“SEC”), and investors would then own stock in a company that does not provide the disclosure available in quarterly and annual reports filed with the SEC and investors may have increased difficulty in selling their stock as we will be non-reporting.
Going Concern
The Company’s unaudited condensed consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”), which contemplates the continuation of the Company as a going concern. For the nine months ended September 30, 2024, the Company had revenue of $40,202,238, net loss of $6,639,935 and net cash used in operations of $3,883,215. Additionally, as of September 30, 2024, the Company had positive working capital of $11,027,653, an accumulated deficit of $104,479,692, and stockholders’ equity of $34,976,599. It is the management’s opinion that these conditions raise substantial doubt about the Company’s ability to continue as a going concern for a period of twelve months from the date of this filing.
The Company’s revenues are not able to sustain its operations, and concerns exist regarding the Company’s ability to meet its obligations as they become due. The Company is subject to a number of risks to those of smaller commercial companies, including dependence on key individuals and products, the difficulties inherent in the development of a commercial market, the need to obtain additional capital, competition from larger companies, and other pharmaceutical and health care companies.
Management evaluated the above conditions which raise substantial doubt about the Company’s ability to continue as a going concern to determine if it can meet its obligations for the subsequent twelve months from the date of this filing. Management considered its ability to access future capital, curtail expenses if needed, expand product lines, and acquire new products.
Management’s plans include expansion of brand name products to the market, expanding the current product portfolio, and evaluating acquisition targets to expand distribution. Furthermore, the Company intends to vertically integrate the supply chain distribution network. During the period up to the issuance of this report the Company has signed multiple distribution agreements for its SPL products in Europe and Asia and a variety of contract manufacturing agreements though its subsidiary, CANA. Finally, the Company plans to further access the capital markets in order to raise additional funds through equity offerings. More specifically, up to the issuance of its consolidated financial statements for the nine months ended September 30, 2024, the Company has sold 901,488 shares of common stock for net proceeds of $629,426 through two Prospectus Supplements to its Registration Statement on Form S-3 (No. 333-267550) filed with the SEC on February 29 and March 7, 2024. Management will also consider postponing the repayment of its outstanding Trade Facility ($1,588,163 balance as of September 30, 2024), intends to make substantial efforts to receive additional debt financing through its subsidiary, Cosmofarm SA, and plans to raise additional equity funds through utilizing its outstanding warrants. Following such efforts, on September 26, 2024, the Company entered into a Warrant Inducement Letter with an investor pursuant to which the Company issued 9,748,252 new warrants and reduced the exercise price of 4,874,126 warrant shares from $1.45 to $0.8701 to induce exercise and receive gross cash proceeds of $4,240,977 and on July 29, 2024 the Company’s subsidiary Cosmofarm SA entered into an agreement with a third-party lender in the principal amount of €400,000 ($432,760). The Company additionally plans to file with the U.S. SEC a registration statement on Form S-1 for an offering of up to $7.5 million by the end of November 2024. Moreover, the Company’s management is considering postponing certain repayments of suppliers and creditors. However, management cannot provide any assurances that the Company will be successful in accomplishing any of its plans. The ability of the Company to continue as a going concern is dependent upon its ability to successfully accomplish the plans described herein and eventually secure other sources of financing and attain profitable operations.
Considering the above, management is of the view that substantial doubt exists about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments to reflect the possible future effect on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from the outcome of this uncertainty.
Plan of Operation in the Next Twelve Months
Specifically, our plan of operations for the next twelve months is as follows:
We assess the foreseeable development of the Company as being positive. Over the medium term we expect to further expand our market share. However, during the course of further organizational optimization there may be associated extraordinary additional costs.
Our plan for our own branded nutraceuticals is to enlarge our portfolio up to 150 SKUs by the end of 2023, including more basic line formulas to cover more customer needs of any age, advanced formulations, formulas based on herbs and further clinical studies with R&D for further products. Our plan for geographic expansion in distributing and market penetration in the EU, Asia, USA and Canada is based on exclusive distributors, wholesalers, e-commerce, and development of franchising model, alliances and acquisitions of nutraceutical companies.
In addition, our plan for branded pharmaceuticals is geographic expansion across the world, especially in the EU and UK, as well as in other countries with fast registration and developed markets with liberalized OTC policies for online pharmacies and supermarkets. We also intend to enhance our exclusive distribution rights with a growing basis of cooperating partners whilst purchasing generic, biosimilar drugs and OTC licenses. We also intend to enhance our product expectance by registered copyrights and trademarks in all OTC drugs. In addition, we remain committed to strategic research and development across each business unit with a particular focus on assets with inherently lower risk profiles and clearly defined governmental regulatory pathways.
Our plan for our full line wholesale is to expand in the Greek territory, enlarge our customer portfolio and integrate of established sales network of pharmacies through the use of B2B and B2C e-commerce platforms and exclusive distributors. We are also aiming in increasing the exports of branded pharmaceuticals as we focus on higher profit margins categories (OTC and VMS), deliver 3PL (third-party logistics) services to pharma companies, put in force loyalty programs, provide added value services to pharmacies and emergency deliveries to VIP customers. The Company will evaluate and, where appropriate, execute on opportunities to expand its network of pharmacies and products in areas that it believes will offer above average growth characteristics and attractive margins.
The Company is growing its business through organic growth, market penetration, geographic expansion and acquisitions which would add value to its business and its shareholders. The Company is also committed to pursuing various forms of business development; this can include trading, alliances, joint ventures and dispositions. Moreover, it hopes to continue to build on its portfolio of pharmaceutical products and expand its OTC and nutraceutical product portfolio. Thus, the Company is developing a sound sales distribution network specializing in its own branded nutraceutical products.
The Company’s main objective is expanding the business operations of its subsidiaries by concentrating its efforts on becoming an international pharmaceutical Company. The Company views its business development activity as an enabler of its strategies, and it seeks to generate earnings growth and enhance shareholder value by pursuing a disciplined, strategic, and financial approach to evaluating business development opportunities. Under these principles the Company assesses businesses and assets as part of its regular, ongoing portfolio review process and continues to consider trading development activities for its businesses. The Company’s objective is the optimization of operating expenses across all entities without compromising the quality of the Company’s services and products.
Changes in the behavior and spending patterns of purchasers of pharmaceutical and healthcare products and services, including delaying medical procedures, rationing prescription medications, reducing the frequency of doctor visits, and foregoing healthcare insurance coverage, may impact the Company’s business.
The pharmaceutical sector offers a large growth potential within the European pharmaceutical market if service, price and quality are strictly directed towards the customer requirements. The Company will continue to encounter competition in the market by product, service, reliability, and a high level of quality. On the procurement side, the Company can access a wide range of supply possibilities. To minimize business risks, the Company diversifies its sources of supply all over Europe. It secures its high-quality demands through careful supplier qualification and selection, as well as active suppliers’ system management.
Strategic Plan
Our strategic plan, which strikes a balance between growth and sustainability, emphasizes synergies, vertical integration, operational efficiencies, R&D, brand expansion, and the global growth of our distribution network and facilities.
We intend to continue to pursue active ongoing acquisitions. In fact, many of our acquisitions entail exploring opportunities, with discounted assets through business combinations or joint ventures, all to enhance our distribution network. We will expand our R&D division which is a platform and incubator to develop new patented pharmaceuticals and proprietary innovative nutraceutical products. To foster organic growth, we will enhance our business development and marketing efforts, pursue global expansion via prominent retailers, pharmacies and e-commerce platforms, and recapture lost markets such as the infant and baby care categories. In addition, we will invest in the expansion of our production capacity and global network of facilities to boost sales of our brands, engage in contract manufacturing with large multinational pharmaceutical companies, produce pharma grade ethanol for hospitals, and expand into new large markets capitalizing on our comparative advantages. Last but not least, we aim to strategically invest in key personnel, from seasoned export managers to highly skilled scientists, to ensure we have the necessary expertise at our fingertips.
Organic Growth
Proprietary Portfolio of Branded Products: A bright spot so far in 2024 is the strong demand for our branded nutraceuticals, as we aspire to transform them into global brands. Our products have received very positive feedback at leading events like Arab Health in Dubai, Infarma in Barcelona, Vitafoods in Geneva, and Pharmacy Show in Birmingham.
Sky Premium Life®: We are selling Sky Premium Life products in an increasing number of countries through pharmacies, retail chains, and online platforms. Among our prominent retailers is Holland & Barrett, with over 1,600 stores in 18 countries across the world, it is not only Europe's largest health and wellbeing retailer but also one of the world's largest, generating about $1 billion in annual revenue. Additionally, our products are available online through platforms like eBay and Amazon in the UK, Canada, the US, Germany, France, Spain, and Singapore. We are investing in our infrastructure, expanding our production capacity to accommodate increasing volumes, accelerating our efforts to broaden our distribution network, and planning to penetrate new major markets. This is boosted by strategic collaborations like the one announced with C.A. PAPAELLINAS Group, a market leader with an extensive distribution network throughout Cyprus. PAPAELLINAS will represent and distribute Sky Premium Life, not only in Holland & Barrett stores but also in pharmacies throughout Cyprus.
Mediterranation®: Building upon the success of Sky Premium Life, we also launched Mediterranation, our premium food supplements brand. Inspired by the Mediterranean way of life, renowned for its healthy food, sunny climate, and longevity, Mediterranation utilizes organic herbs and plant extracts, such as dittany of Crete, oregano, mastic, and kritamos from the Mediterranean region. All of our products are manufactured under strict pharmaceutical standards and adhere to GMP protocols.
Bio-Bebe® and C-Sept®/C-Scrub: Among Cana's many valuable assets, Cosmos Health also obtained a proprietary portfolio of pharmaceutical, dermocosmetic, antiseptic, and food supplement branded products. These include, among others: Bio-Bebe, an organic infant care and nutrition brand, which we are in the process of relaunching. This presents us with a great opportunity to enter the lucrative global baby food market that, according to Fortune Business Insights, is worth $102.90 billion per year. C-Sept, an antiseptic brand, which we are expanding with the launch of the new C-Scrub Wash 4% CHG Biocide. We are well positioned to capitalize on the global antiseptic and disinfectant market that, according to Grand View Research, is worth $29 billion per year.
While the Company intends to pursue these milestones, there may be circumstances where for valid business reasons or due to factors beyond the control of the Company, a reallocation of efforts may be necessary or advisable.
The Company intends to spend the funds available to strengthen working capital, inventories, intangible assets, acquisitions, research and development, sales and marketing expenses. Due to the uncertain nature of the industry in which the Company operates, projects may be frequently reviewed and reassessed. Accordingly, while it is currently intended by management that the available funds will be expended as set forth above, actual expenditures may in fact differ from these amounts and allocations.
Off Balance Sheet Arrangements
As of September 30, 2024, there were no off-balance sheet arrangements.
Critical Accounting Policies
In December 2001, the SEC requested that all registrants list their most “critical accounting polices” under the Management’s Discussion and Analysis section. The SEC indicated that a “critical accounting policy” is one which is both important to the portrayal of a company’s financial condition and results, and requires management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain.
Revenue Recognition: The Company adopted Topic 606 Revenue from Contracts with Customers on January 1, 2018. As a result, it has changed its accounting policy for revenue recognition as detailed in Note 2.
Foreign Currency. Assets and liabilities of all foreign operations are translated at period-end rates of exchange, and the statements of operations are translated at the average rates of exchange for the period. Gains or losses resulting from translating foreign currency financial statements are accumulated in a separate component of stockholders’ equity until the entity is sold or substantially liquidated. Gains or losses from foreign currency transactions (transactions denominated in a currency other than the entity’s local currency) are included in net (loss) earnings.
Income Taxes. The Company accounts for income taxes under the asset and liability method, as required by the accounting standard for income taxes, ASC 740. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis, as well as net operating loss carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
The Company is liable for income taxes in Greece and the United Kingdom. The corporate income tax rate is 22% in Greece (tax losses are carried forward for five years effective January 1, 2013) and 25% in United Kingdom. Losses may also be subject to limitation under certain rules regarding change of ownership.
We regularly review deferred tax assets to assess their potential realization and establish a valuation allowance for portions of such assets to reduce the carrying value if we do not consider it to be more likely than not that the deferred tax assets will be realized. Our review includes evaluating both positive (e.g., sources of taxable income) and negative (e.g., recent historical losses) evidence that could impact the realizability of our deferred tax assets.
We recognize the impact of an uncertain tax position in our financial statements if, in management’s judgment, the position is not more-likely-than-not sustainable upon audit based on the position’s technical merits. This involves the identification of potential uncertain tax positions, the evaluation of applicable tax laws and an assessment of whether a liability for an uncertain tax position is necessary. We operate and are subject to audit in multiple taxing jurisdictions.
We record interest and penalties related to income taxes as a component of interest and other expense as incurred, respectively.
Potential benefits of income tax losses are not recognized in the accounts until realization is more likely than not. The Company has adopted ASC 740 “Accounting for Income Taxes” as of its inception. Pursuant to ASC 740, the Company is required to compute tax asset benefits for net operating losses carried forward. The potential benefits of net operating losses have not been recognized in this financial statement because the Company cannot be assured it is more likely than not it will utilize the net operating losses carried forward in future years.
The Company has net operating loss carry-forwards in our parent, Cosmos Health Inc., which are applicable to future taxable income in the United States (if any). Additionally, the Company has income tax liabilities in the United Kingdom. The income tax assets and liabilities are not able to be netted. We therefore reserve the income tax assets applicable to the United States but recognize the income tax liabilities in Greece and the United Kingdom. Losses may also be subject to limitation under certain rules regarding change of ownership.
Accounts Receivable and Allowance for Credit Losses
The Company follows ASC 310 to estimate the allowance for doubtful accounts. Pursuant to FASB ASC paragraph 310-10-35-9, losses from uncollectible receivables shall be accrued when both of the following conditions are met: (a) information available before the financial statements are issued or are available to be issued (as discussed in Section 855-10-25) indicates that it is probable that an asset has been impaired at the date of the financial statements, and (b) the amount of the loss can be reasonably estimated. Those conditions may be considered in relation to individual receivables or in relation to groups of similar types of receivables. If the conditions are met, accrual shall be made even though the receivables that are uncollectible may not be identifiable. The Company reviews individually each trade receivable for collectability and performs on-going credit evaluations of its customers and adjusts credit limits based upon payment history and the customer’s current credit worthiness, as determined by the review of their current credit information; and determines the allowance for doubtful accounts based on historical write-off experience, customer specific facts and general economic conditions that may affect a client’s ability to pay. Bad debt expense is included in general and administrative expenses, if any.
Inventory Reserves
Our merchandise inventories are made up of finished goods and are valued at the lower of cost or market using the weighted-average cost method. Average cost includes the direct purchase price, net of vendor allowances and cash discounts, of merchandise inventory. We record valuation reserves on an annual basis for merchandise damage and defective returns, merchandise items with slow-moving or obsolescence exposure and merchandise that has a carrying value that exceeds market value. These reserves are estimates of a reduction in value to reflect inventory valuation at the lower of cost or market. The reserve for merchandise returns is based upon the determination of the historical net realizable value of products sold from our returned goods inventory or returned to vendors for credit. Our reserve for merchandise returns includes amounts for returned product on-hand as well as for new merchandise on-hand that we estimate will ultimately become returned goods inventory after being sold based on historical return rates.
Item 3. Quantitative and Qualitative Disclosures about Market Risk.
Not applicable. A smaller reporting company is not required to provide the information required by this Item.
Item 4. Controls and Procedures.
Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act) that are designed to ensure that information required to be disclosed in the Company’s Securities Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and that such information is accumulated and communicated to the Company’s management, including its Principal Executive Officer and Principal Financial Officer, as appropriate, to allow timely decisions regarding required disclosures.
Evaluation of Disclosure Controls and Procedures
The Company’s management, with the participation of the Company’s Principal Executive Officer and Principal Financial Officer, has evaluated the effectiveness of the Company’s disclosure controls and procedures as of the end of the period covered by this report. Based upon that evaluation, the Principal Executive Officer and the Principal Financial Officer have concluded that, as of the end of the period covered by this report, the Company’s disclosure controls and procedures were ineffective due to material weaknesses stated below:
| · | The Company has a lack of proper segregation of duties. |
| | |
| · | The Company’s internal control structure lacks multiple levels of review and oversight and does not have appropriate IT General Controls (ITGCs) for the applications used in the Financial Reporting process, caused by lack of design of relevant controls and overall IT risk management. |
We are in the process of remediating all material weaknesses present in our internal controls and we plan to have completed the remediation by December 31, 2024.
- The Company has a lack of proper segregation of duties.
We are in the process of updating the organizational chart in order to reallocate roles among personnel and emphasize sharing the responsibilities of key business processes by distributing the discrete functions of these processes to multiple people and departments.
- The Company’s internal control structure lacks multiple levels of review and oversight and does not have appropriate IT General Controls (ITGCs) for the applications used in the Financial Reporting process, caused by lack of design of relevant controls and overall IT risk management.
We are in the process of developing multiple levels of review based on job responsibilities and level of personnel. For example, management reviews whether the bank reconciliations are being prepared on a timely basis by the preparer and whether there are discrepancies between the general ledger and the bank statements. Another example is the review of management accounting information by the CFO and his authorization for material transactions and adjustments. Furthermore, we are in the process of assessing a new financial reporting application that will be used by all the companies of the Group and would be able to support, process financially relevant information, provide financially relevant reporting and house financially relevant interfaces and application controls in order for us to more efficiently establish IT General Controls to a single and more reliable application.
Changes in Internal Controls Over Financial Reporting
There were no changes in our internal control over financial reporting that have materially affected or are reasonably likely to materially affect, our internal control over financial reporting.
Our Audit Committee is in the process of evaluating our existing controls and procedures, while communicating with the Management on quarterly basis.
Audit Committee
We have a separately designated standing audit committee, which is appointed by the Board of Directors of Cosmos Health Inc. On April 28, 2022, Dr. Anastasios Aslidis was elected to serve on the Board of Directors and was appointed as a chair of the Audit Committee. Our three independent directors, Anastasios Aslidis, John Hoidas and Demetrios Demetriades serve on the Audit Committee. The primary function of the committee is to assist the Board of Directors in overseeing (1) the financial reporting and accounting processes of the Company, and (2) the financial statements audits of the Company. The Committee also prepares a written report to be included in the annual proxy statement of the Company pursuant to the applicable rules and regulations of the “SEC”. In furtherance of these purposes, the Committee shall maintain direct communication among the Company’s independent auditors and the Board of Directors. The independent auditors and any other registered public accounting firm engaged in preparing or issuing an audit report or performing other audit review or attest services for the Company shall report directly to the Committee and are ultimately accountable to the Committee and the Board of Directors.
In discharging its oversight role, the Committee is authorized to investigate any matter brought to its attention with full access to all books, records, facilities and personnel of the Company. The Committee shall have the sole authority to retain at the Company’s expense outside legal, accounting or other advisors to advise the Committee and to receive appropriate funding, as determined by the Committee, from the Company for the payment of the compensation of such advisors and for the payment of ordinary administrative expenses of the Committee that are necessary to carry out its duties. The Committee may request any officer or employee of the Company or the Company’s outside counsel or independent auditors to attend a meeting of the Committee or to meet with any member of, or advisors to, the Committee. The Committee may also meet with the Company’s investment bankers or financial analysts who follow the Company.
The Committee shall meet no less frequently than four times per year, with additional meetings as circumstances warrant. The Committee shall also meet periodically with management, the internal auditors, if any, and the independent auditors in separate executive sessions. The Committee shall record the minutes of all such meetings and shall submit the minutes of its meetings to, or discuss the matters deliberated at each meeting with, the Board of Directors. The Company’s chief financial or accounting officer shall function as the management liaison officer to the Committee.
PART II - OTHER INFORMATION
Item 1. Legal Proceedings.
There have been no changes since the filing of the Company’s Form 10-K for the year ended December 31, 2023 and Form 10-Q for the quarter ending June 30, 2024.
Item 1A. Risk Factors
The Company is not required to provide the information called for in this item due to its status as a Smaller Reporting Company. You should refer to the other information set forth in this report, including the information set forth in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” as well as in our consolidated financial statements and the related notes. Our business prospects, financial condition or results of operations could be adversely affected by any of these risks.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
None. Previously reported on Form 8-K.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Other Information.
During the nine months ended September 30, 2024, no director or officer of the Company adopted or terminated a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408(a) of Regulation S-K.
Item 6. Exhibits.
(a) Exhibits.
_____________
* | This exhibit shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Securities Exchange Act of 1934, whether made before or after the date hereof and irrespective of any general incorporation language in any filings. |
| |
** | XBRL (Extensible Business Reporting Language) information is furnished and not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise is not subject to liability under these sections. |
SIGNATURES
In accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Cosmos Health Inc. | |
| | | |
Date: November 14, 2024 | By: | /s/ Grigorios Siokas | |
| | Grigorios Siokas | |
| | Chief Executive Officer | |
| | (Principal Executive Officer) | |
Date: November 14, 2024 | By: | /s/ Georgios Terzis | |
| | Georgios Terzis | |
| | Chief Financial Officer | |
| | (Principal Financial Officer, And Principal Accounting Officer) | |
EXHIBIT INDEX
___________
* | This exhibit shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Securities Exchange Act of 1934, whether made before or after the date hereof and irrespective of any general incorporation language in any filings. |
| |
** | XBRL (Extensible Business Reporting Language) information is furnished and not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise is not subject to liability under these sections. |
nullnullnullnull
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an quarterly report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-Q
-Number 240
-Section 308
-Subsection a
| Name: |
dei_DocumentQuarterlyReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
CONDENSED CONSOLIDATED BALANCE SHEETS - USD ($)
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| CURRENT ASSETS: |
|
|
| Cash and cash equivalents |
$ 3,314,845
|
$ 3,833,195
|
| Accounts receivable, net |
17,484,240
|
19,759,254
|
| Accounts receivable - related party |
1,285,743
|
1,099,098
|
| Marketable securities |
22,808
|
20,075
|
| Inventory |
4,885,015
|
4,789,054
|
| Loans receivable |
491,897
|
411,858
|
| Loans receivable - related party |
445,800
|
442,480
|
| Prepaid expenses and other current assets |
1,914,881
|
1,811,911
|
| Prepaid expenses and other current assets - related party |
6,393,642
|
4,440,855
|
| TOTAL CURRENT ASSETS |
36,238,871
|
36,607,780
|
| Property and equipment, net |
10,575,928
|
10,455,499
|
| Goodwill and intangible assets, net |
7,746,761
|
7,684,183
|
| Loans receivable - long term portion |
3,225,879
|
3,509,200
|
| Loans receivable - related party - long term |
3,234,604
|
3,539,840
|
| Operating lease right-of-use asset |
710,711
|
1,131,552
|
| Financing lease right-of-use asset |
22,343
|
28,790
|
| Advances for building's acquisition |
2,000,020
|
2,000,020
|
| Other assets |
764,865
|
1,057,947
|
| TOTAL ASSETS |
64,519,982
|
66,014,811
|
| CURRENT LIABILITIES: |
|
|
| Accounts payable and accrued expenses |
11,605,255
|
11,911,978
|
| Accounts payable and accrued expenses - related party |
1,027,327
|
231,564
|
| Accrued interest |
185,561
|
166,348
|
| Lines of credit |
5,989,425
|
6,630,273
|
| Notes payable |
1,622,349
|
1,570,886
|
| Notes payable - related party |
11,368
|
11,283
|
| Loans payable - related party |
21,158
|
13,257
|
| Operating lease liability, current portion |
241,422
|
285,563
|
| Financing lease liability, current portion |
18,888
|
27,222
|
| Other current liabilities |
4,488,465
|
3,474,096
|
| TOTAL CURRENT LIABILITIES |
25,211,218
|
24,322,470
|
| Share settled debt obligation |
0
|
0
|
| Notes payable - long term portion |
2,645,623
|
3,035,341
|
| Operating lease liability, net of current portion |
468,157
|
844,866
|
| Financing lease liability, net of current portion |
5,845
|
5,261
|
| Other liabilities |
1,212,540
|
1,763,845
|
| TOTAL LIABILITIES |
29,543,383
|
29,971,783
|
| Commitments and Contingencies (see Note 14) |
0
|
0
|
| STOCKHOLDERS' EQUITY: |
|
|
| Common stock, $0.001 par value; 300,000,000 shares authorized; 23,346,023 and 15,982,472 shares issued and 23,259,526 and 15,895,975 outstanding as of September 30, 2024 and December 31, 2023, respectively |
23,346
|
15,983
|
| Additional paid-in capital |
140,797,456
|
129,008,301
|
| Subscription receivable |
(20)
|
(20)
|
| Treasury stock, at cost, 86,497 shares as of September 30, 2024 and December 31, 2023 |
(917,159)
|
(917,159)
|
| Accumulated deficit |
(104,479,192)
|
(91,644,233)
|
| Accumulated other comprehensive loss |
(447,832)
|
(419,844)
|
| TOTAL STOCKHOLDERS' EQUITY |
34,976,599
|
36,043,028
|
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY |
$ 64,519,982
|
$ 66,014,811
|
| X |
- References
+ Details
| Name: |
abcd_AdvancesForBuildingsAcquisition |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_LoansReceivableNoncurrent |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities incurred to vendors for goods and services received, and accrued liabilities classified as other, payable within one year or the normal operating cycle, if longer.
| Name: |
us-gaap_AccountsPayableAndOtherAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business, classified as current. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481990/310-10-45-2
| Name: |
us-gaap_AccountsReceivableNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 12: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 30: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of subscription receivable from investors who have been allocated common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 310
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477802/946-310-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(5)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockShareSubscribedButUnissuedSubscriptionsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of short-term and long-term debt and lease obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(13))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_DebtAndCapitalLeaseObligations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from finance lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_FinanceLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from finance lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_FinanceLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after accumulated amortization, of right-of-use asset from finance lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_FinanceLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after impairment and amortization, of goodwill, indefinite-lived, and finite-lived intangible assets. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 10
-Name Accounting Standards Codification
-Section S45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480265/350-10-S45-1
| Name: |
us-gaap_IntangibleAssetsNetIncludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liability recognized for present obligation requiring transfer or otherwise providing economic benefit to others. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-5
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe carrying value as of the balance sheet date of the current portion of long-term obligations drawn from a line of credit, which is a bank's commitment to make loans up to a specific amount. Examples of items that might be included in the application of this element may consist of letters of credit, standby letters of credit, and revolving credit arrangements, under which borrowings can be made up to a maximum amount as of any point in time conditional on satisfaction of specified terms before, as of and after the date of drawdowns on the line. Includes short-term obligations that would normally be classified as current liabilities but for which (a) postbalance sheet date issuance of a long term obligation to refinance the short term obligation on a long term basis, or (b) the enterprise has entered into a financing agreement that clearly permits the enterprise to refinance the short-term obligation on a long term basis and the following conditions are met (1) the agreement does not expire within 1 year and is not cancelable by the lender except for violation of an objectively determinable provision, (2) no violation exists at the BS date, and (3) the lender has entered into the financing agreement is expected to be financially capable of honoring the agreement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(13))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_LinesOfCreditCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of notes payable (with maturities initially due after one year or beyond the operating cycle if longer), excluding current portion. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of investment in marketable security, classified as current. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_MarketableSecuritiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmortized cost, after allowance for credit loss, of financing receivable classified as current. Excludes net investment in lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481990/310-10-45-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_NotesAndLoansReceivableNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of the portions of long-term notes payable due within one year or the operating cycle if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_NotesPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of assets classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(10))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_OtherLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other, due after one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherLiabilitiesNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for other costs that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483032/340-10-45-1
| Name: |
us-gaap_OtherPrepaidExpenseCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478451/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount allocated to previously issued common shares repurchased by the issuing entity and held in treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481520/505-30-50-4
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481549/505-30-45-1
| Name: |
us-gaap_TreasuryStockCommonValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.3
CONDENSED CONSOLIDATED BALANCE SHEETS (Parenthetical) - $ / shares
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| CONDENSED CONSOLIDATED BALANCE SHEETS |
|
|
| Common stock, shares par value |
$ 0.001
|
$ 0.001
|
| Common stock, shares authorized |
300,000,000
|
300,000,000
|
| Common stock, shares issued |
23,346,023
|
15,982,472
|
| Common stock, shares outstanding |
23,259,526
|
15,895,975
|
| Treasury stock |
86,497
|
86,497
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of previously issued common shares repurchased by the issuing entity and held in treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481549/505-30-45-1
| Name: |
us-gaap_TreasuryStockCommonShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.3
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS |
|
|
|
|
| REVENUE |
$ 12,411,048
|
$ 12,823,797
|
$ 40,202,238
|
$ 37,537,003
|
| COST OF GOODS SOLD |
11,204,186
|
11,609,039
|
36,894,502
|
34,418,334
|
| GROSS PROFIT |
1,206,862
|
1,214,758
|
3,307,736
|
3,118,669
|
| OPERATING EXPENSES |
|
|
|
|
| General and administrative expenses |
1,782,957
|
2,573,414
|
4,591,620
|
6,662,579
|
| Salaries and wages |
1,317,782
|
1,252,680
|
4,030,823
|
3,279,803
|
| Sales and marketing expenses |
41,848
|
157,435
|
326,291
|
942,759
|
| Depreciation and amortization expense |
304,139
|
248,530
|
937,000
|
478,466
|
| TOTAL OPERATING EXPENSES |
3,446,726
|
4,232,059
|
9,885,734
|
11,363,607
|
| LOSS FROM OPERATIONS |
(2,239,864)
|
(3,017,301)
|
(6,577,998)
|
(8,244,938)
|
| OTHER INCOME (EXPENSE) |
|
|
|
|
| Other income (expense), net |
(1,921)
|
14,404
|
160,598
|
(14,330)
|
| Interest expense |
(181,429)
|
(151,274)
|
(692,547)
|
(529,782)
|
| Interest income |
101,236
|
110,596
|
309,031
|
555,281
|
| Gain on equity investments, net |
428
|
(1,093)
|
2,518
|
2,876
|
| Gain on extinguishment of debt |
0
|
706
|
0
|
1,911,476
|
| Change in fair value of derivative liability |
0
|
0
|
0
|
3,384
|
| Bargain purchase gain |
0
|
0
|
0
|
1,633,842
|
| Foreign currency transaction, net |
139,016
|
(371,115)
|
158,463
|
(108,406)
|
| TOTAL OTHER INCOME (EXPENSE), NET |
57,330
|
(397,776)
|
(61,937)
|
3,454,341
|
| LOSS BEFORE INCOME TAXES |
(2,182,534)
|
(3,415,077)
|
(6,639,935)
|
(4,790,597)
|
| INCOME TAX EXPENSE |
0
|
65,873
|
0
|
0
|
| NET LOSS |
(2,182,534)
|
(3,349,204)
|
(6,639,935)
|
(4,790,597)
|
| Deemed dividend on issuance of warrants |
(6,185,231)
|
0
|
(6,185,231)
|
|
| Deemed dividend on downround of warrants |
0
|
(15,053)
|
0
|
(15,053)
|
| Deemed dividend on warrant exchange/modification |
(9,793)
|
0
|
(9,793)
|
|
| NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS |
(8,377,558)
|
(3,364,257)
|
(12,834,959)
|
(4,805,650)
|
| Foreign currency translation adjustment, net |
747,879
|
(890,645)
|
(27,988)
|
(470,994)
|
| TOTAL COMPREHENSIVE LOSS |
$ (7,629,679)
|
$ (4,254,902)
|
$ (12,862,947)
|
$ (5,276,644)
|
| BASIC NET LOSS PER SHARE |
$ (0.45)
|
$ (0.27)
|
$ (0.72)
|
$ (0.42)
|
| DILUTED NET LOSS PER SHARE |
$ (0.45)
|
$ (0.27)
|
$ (0.72)
|
$ (0.42)
|
| WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING |
|
|
|
|
| Basic |
18,418,287
|
12,585,479
|
17,724,305
|
11,346,071
|
| Diluted |
18,418,287
|
12,585,479
|
17,724,305
|
11,346,071
|
| X |
- References
+ Details
| Name: |
abcd_BargainPurchaseGain |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_DeemedDividendOnDownroundOfWarrants |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_DeemedDividendOnWarrantExchangeModification |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs related to goods produced and sold and services rendered by an entity during the reporting period. This excludes costs incurred during the reporting period related to financial services rendered and other revenue generating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(2)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(2)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479941/924-10-S99-1
| Name: |
us-gaap_CostOfGoodsAndServicesSold |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe current period expense charged against earnings on long-lived, physical assets not used in production, and which are not intended for resale, to allocate or recognize the cost of such assets over their useful lives; or to record the reduction in book value of an intangible asset over the benefit period of such asset; or to reflect consumption during the period of an asset that is not used in production. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_DepreciationAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in the fair value of derivatives recognized in the income statement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 815
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4A
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480434/815-10-50-4A
| Name: |
us-gaap_DerivativeGainLossOnDerivativeNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of realized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-6
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481956/830-20-45-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481926/830-20-50-1
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossRealized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of realized and unrealized gain (loss) on investment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(9)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_GainLossOnInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionDifference between the fair value of payments made and the carrying amount of debt which is extinguished prior to maturity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481303/470-50-40-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481303/470-50-40-4
| Name: |
us-gaap_GainsLossesOnExtinguishmentOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate revenue less cost of goods and services sold or operating expenses directly attributable to the revenue generation activity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 9: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-31
| Name: |
us-gaap_GrossProfit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncomeStatementAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest expense classified as operating and nonoperating. Includes, but is not limited to, cost of borrowing accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-24
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest income earned from interest bearing assets classified as other.
| Name: |
us-gaap_InterestIncomeOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of dividend income on nonoperating securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_InvestmentIncomeDividend |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of tax, noncontrolling interests, dividends on preferred stock and participating securities; of income (loss) available to common shareholders. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SAB Topic 6.B)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-5
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
| Name: |
us-gaap_NetIncomeLossAvailableToCommonStockholdersBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-31
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (a)
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-10A
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
| Name: |
us-gaap_OtherComprehensiveIncomeLossForeignCurrencyTransactionAndTranslationAdjustmentNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of income (expense) related to nonoperating activities, classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherNonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-40
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477314/942-235-S99-1
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for salary and wage arising from service rendered by nonofficer employee. Excludes allocated cost, labor-related nonsalary expense, and direct and overhead labor cost included in cost of good and service sold. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_SalariesAndWages |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total amount of expenses directly related to the marketing or selling of products or services.
| Name: |
us-gaap_SellingAndMarketingExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY AND MEZZANINE EQUITY - USD ($)
|
Total |
Preferred Stock |
Common Stock |
Additional Paid-In Capital |
Subscription Receivable |
Retained Earnings (Accumulated Deficit) |
Accumulated other comprehensive loss |
Treasurys Stocks |
| Balance, shares at Dec. 31, 2022 |
|
|
10,605,412
|
|
|
|
|
15,497
|
| Balance, amount at Dec. 31, 2022 |
$ 39,284,295
|
$ 372,414
|
$ 10,606
|
$ 112,205,952
|
$ (4,750,108)
|
$ (66,232,813)
|
$ (1,132,635)
|
$ (816,707)
|
| Foreign currency translation adjustment, net |
336,463
|
|
|
|
|
|
336,463
|
|
| Proceeds from sale of common stock |
4,750,000
|
|
|
|
4,750,000
|
|
|
|
| Shares issued in lieu of cash, shares |
|
|
15,258
|
|
|
|
|
|
| Shares issued in lieu of cash, amount |
96,888
|
|
$ 15
|
96,873
|
|
|
|
|
| Net loss |
(459,863)
|
|
|
|
|
(459,863)
|
|
|
| Balance, shares at Mar. 31, 2023 |
|
|
10,620,670
|
|
|
|
|
15,497
|
| Balance, amount at Mar. 31, 2023 |
44,007,783
|
372,414
|
$ 10,621
|
112,302,825
|
(108)
|
(66,692,676)
|
(796,172)
|
$ (816,707)
|
| Balance, shares at Dec. 31, 2022 |
|
|
10,605,412
|
|
|
|
|
15,497
|
| Balance, amount at Dec. 31, 2022 |
39,284,295
|
372,414
|
$ 10,606
|
112,205,952
|
(4,750,108)
|
(66,232,813)
|
(1,132,635)
|
$ (816,707)
|
| Foreign currency translation adjustment, net |
(470,994)
|
|
|
|
|
|
|
|
| Net loss |
(4,790,597)
|
|
|
|
|
|
|
|
| Balance, shares at Sep. 30, 2023 |
|
|
13,068,693
|
|
|
|
|
86,497
|
| Balance, amount at Sep. 30, 2023 |
44,195,740
|
372,414
|
$ 13,069
|
117,791,721
|
(50,000)
|
(71,038,463)
|
(1,603,629)
|
$ (916,958)
|
| Balance, shares at Dec. 31, 2022 |
|
|
10,605,412
|
|
|
|
|
15,497
|
| Balance, amount at Dec. 31, 2022 |
39,284,295
|
372,414
|
$ 10,606
|
112,205,952
|
(4,750,108)
|
(66,232,813)
|
(1,132,635)
|
$ (816,707)
|
| Balance, shares at Dec. 31, 2023 |
|
|
15,982,472
|
|
|
|
|
86,497
|
| Balance, amount at Dec. 31, 2023 |
36,043,028
|
0
|
$ 15,983
|
129,008,301
|
(20)
|
(91,644,233)
|
(419,844)
|
$ (917,159)
|
| Balance, shares at Mar. 31, 2023 |
|
|
10,620,670
|
|
|
|
|
15,497
|
| Balance, amount at Mar. 31, 2023 |
44,007,783
|
372,414
|
$ 10,621
|
112,302,825
|
(108)
|
(66,692,676)
|
(796,172)
|
$ (816,707)
|
| Foreign currency translation adjustment, net |
83,188
|
0
|
0
|
0
|
0
|
0
|
83,188
|
0
|
| Net loss |
(981,530)
|
0
|
$ 0
|
0
|
0
|
(981,530)
|
0
|
0
|
| Shares issued for purchase of customer base, shares |
|
|
99,710
|
|
|
|
|
|
| Shares issued for purchase of customer base, amount |
316,081
|
0
|
$ 100
|
315,981
|
0
|
0
|
0
|
0
|
| Shares issued for purchase of Cana, shares |
|
|
46,377
|
|
|
|
|
|
| Shares issued for purchase of Cana, amount |
138,667
|
0
|
$ 46
|
138,621
|
0
|
0
|
0
|
0
|
| Stock-based compensation, shares |
|
|
185,000
|
|
|
|
|
|
| Stock-based compensation, amount |
104,869
|
0
|
$ 185
|
104,684
|
0
|
0
|
0
|
$ 0
|
| Balance, shares at Jun. 30, 2023 |
|
|
10,951,757
|
|
|
|
|
15,497
|
| Balance, amount at Jun. 30, 2023 |
43,669,058
|
372,414
|
$ 10,952
|
112,862,111
|
(108)
|
(67,674,206)
|
(712,984)
|
$ (816,707)
|
| Foreign currency translation adjustment, net |
(890,645)
|
0
|
0
|
0
|
0
|
0
|
(890,645)
|
0
|
| Net loss |
(3,349,204)
|
0
|
0
|
0
|
0
|
(3,349,204)
|
0
|
0
|
| Stock-based compensation, amount |
109,636
|
0
|
$ 0
|
109,636
|
0
|
0
|
0
|
0
|
| Proceeds from sale of common stock, net of financing fees of $442,870, shares |
|
|
2,116,936
|
|
|
|
|
|
| Proceeds from sale of common stock, net of financing fees of $442,870, amount |
4,757,146
|
0
|
$ 2,117
|
4,804,921
|
(49,892)
|
0
|
0
|
$ 0
|
| Repurchase of treasury stock, shares |
|
|
|
|
|
|
|
71,000
|
| Repurchase of treasury stock, amount |
100,251
|
0
|
0
|
0
|
0
|
|
|
$ 100,251
|
| Deemed dividend |
0
|
0
|
$ 0
|
15,053
|
0
|
(15,053)
|
0
|
$ 0
|
| Balance, shares at Sep. 30, 2023 |
|
|
13,068,693
|
|
|
|
|
86,497
|
| Balance, amount at Sep. 30, 2023 |
44,195,740
|
372,414
|
$ 13,069
|
117,791,721
|
(50,000)
|
(71,038,463)
|
(1,603,629)
|
$ (916,958)
|
| Balance, shares at Dec. 31, 2023 |
|
|
15,982,472
|
|
|
|
|
86,497
|
| Balance, amount at Dec. 31, 2023 |
36,043,028
|
0
|
$ 15,983
|
129,008,301
|
(20)
|
(91,644,233)
|
(419,844)
|
$ (917,159)
|
| Foreign currency translation adjustment, net |
(599,276)
|
0
|
0
|
0
|
0
|
0
|
(599,276)
|
0
|
| Shares issued in lieu of cash, amount |
108,297
|
0
|
0
|
108,297
|
0
|
0
|
0
|
0
|
| Net loss |
(1,866,690)
|
0
|
0
|
0
|
0
|
(1,866,690)
|
0
|
0
|
| Stock-based compensation, amount |
231,897
|
0
|
$ 0
|
231,897
|
0
|
0
|
0
|
0
|
| Proceeds from sale of common stock, net of financing fees of $19,467, shares |
|
|
901,488
|
|
|
|
|
|
| Proceeds from sale of common stock, net of financing fees of $19,467, amount |
629,426
|
0
|
$ 901
|
628,525
|
0
|
0
|
0
|
0
|
| Shares issued pursuant to warrant exchange agreement, shares |
|
|
950,063
|
|
|
|
|
|
| Shares issued pursuant to warrant exchange agreement, amount |
0
|
0
|
$ 950
|
(950)
|
0
|
0
|
0
|
$ 0
|
| Balance, shares at Mar. 31, 2024 |
|
|
17,834,023
|
|
|
|
|
86,497
|
| Balance, amount at Mar. 31, 2024 |
34,546,682
|
|
$ 17,834
|
129,976,070
|
(20)
|
(93,510,923)
|
(1,019,120)
|
$ (917,159)
|
| Balance, shares at Dec. 31, 2023 |
|
|
15,982,472
|
|
|
|
|
86,497
|
| Balance, amount at Dec. 31, 2023 |
36,043,028
|
$ 0
|
$ 15,983
|
129,008,301
|
(20)
|
(91,644,233)
|
(419,844)
|
$ (917,159)
|
| Foreign currency translation adjustment, net |
(27,988)
|
|
|
|
|
|
|
|
| Net loss |
(6,639,935)
|
|
|
|
|
|
|
|
| Balance, shares at Sep. 30, 2024 |
|
|
23,346,023
|
|
|
|
|
86,497
|
| Balance, amount at Sep. 30, 2024 |
34,976,599
|
|
$ 23,346
|
140,797,456
|
(20)
|
(104,479,192)
|
(447,832)
|
$ (917,159)
|
| Balance, shares at Mar. 31, 2024 |
|
|
17,834,023
|
|
|
|
|
86,497
|
| Balance, amount at Mar. 31, 2024 |
34,546,682
|
|
$ 17,834
|
129,976,070
|
(20)
|
(93,510,923)
|
(1,019,120)
|
$ (917,159)
|
| Foreign currency translation adjustment, net |
(176,591)
|
|
|
|
|
|
(176,591)
|
|
| Shares issued in lieu of cash, amount |
108,444
|
|
|
108,444
|
|
|
|
|
| Net loss |
(2,590,711)
|
|
|
|
|
(2,590,711)
|
|
|
| Stock-based compensation, amount |
231,750
|
|
|
231,750
|
|
|
|
|
| Balance, shares at Jun. 30, 2024 |
|
|
17,834,023
|
|
|
|
|
86,497
|
| Balance, amount at Jun. 30, 2024 |
32,119,574
|
|
$ 17,834
|
130,316,264
|
(20)
|
(96,101,634)
|
(1,195,711)
|
$ (917,159)
|
| Foreign currency translation adjustment, net |
747,879
|
|
|
|
|
|
747,879
|
|
| Shares issued in lieu of cash, shares |
|
|
2,500,000
|
|
|
|
|
|
| Shares issued in lieu of cash, amount |
158,061
|
|
$ 2,500
|
155,561
|
|
|
|
|
| Net loss |
(2,182,534)
|
|
|
|
|
(2,182,534)
|
|
|
| Stock-based compensation, shares |
|
|
680,000
|
|
|
|
|
|
| Stock-based compensation, amount |
264,750
|
|
$ 680
|
264,070
|
|
|
|
|
| Proceeds from exercise of warrants, net of financing fees of $372,109, shares |
|
|
2,332,000
|
|
|
|
|
|
| Proceeds from exercise of warrants, net of financing fees of $372,109, amount |
3,868,869
|
|
$ 2,332
|
3,866,537
|
|
|
|
|
| Deemed dividend on warrant inducement |
|
|
|
6,195,024
|
|
(6,195,024)
|
|
|
| Balance, shares at Sep. 30, 2024 |
|
|
23,346,023
|
|
|
|
|
86,497
|
| Balance, amount at Sep. 30, 2024 |
$ 34,976,599
|
|
$ 23,346
|
$ 140,797,456
|
$ (20)
|
$ (104,479,192)
|
$ (447,832)
|
$ (917,159)
|
| X |
- References
+ Details
| Name: |
abcd_DeemedDividend |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_DeemedDividendOnWarrantInducement |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ProceedsFromExerciseOfWarrantsNetOfFinancingFeesAmount |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ProceedsFromExerciseOfWarrantsNetOfFinancingFeesShares |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ProceedsFromSaleOfCommonStock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ProceedsFromSaleOfCommonStockNetOfFinancingFeesAmount |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ProceedsFromSaleOfCommonStockNetOfFinancingFeesOfAmount |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ProceedsFromSaleOfCommonStockNetOfFinancingFeesOfShares |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ProceedsFromSaleOfCommonStockNetOfFinancingFeesShares |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_SharesIssuedForPurchaseOfCanaAmount |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_SharesIssuedForPurchaseOfCanaShares |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_SharesIssuedForPurchaseOfCustomerBaseAmount |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_SharesIssuedForPurchaseOfCustomerBaseShares |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_SharesIssuedPursuantToWarrantExchangeAgreementAmount |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_SharesIssuedPursuantToWarrantExchangeAgreementShares |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (a)
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-10A
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
| Name: |
us-gaap_OtherComprehensiveIncomeLossForeignCurrencyTransactionAndTranslationAdjustmentNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued as of the balance sheet date, including shares that had been issued and were previously outstanding but which are now held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
| Name: |
us-gaap_SharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber, after forfeiture, of shares or units issued under share-based payment arrangement. Excludes shares or units issued under employee stock ownership plan (ESOP). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue, after forfeiture, of shares issued under share-based payment arrangement. Excludes employee stock ownership plan (ESOP). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_StockIssuedDuringPeriodValueShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares that have been repurchased during the period and have not been retired and are not held in treasury. Some state laws may govern the circumstances under which an entity may acquire its own stock and prescribe the accounting treatment therefore. This element is used when state law does not recognize treasury stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockRepurchasedDuringPeriodShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of stock that has been repurchased during the period and has not been retired and is not held in treasury. Some state laws may mandate the circumstances under which an entity may acquire its own stock and prescribe the accounting treatment therefore. This element is used when state law does not recognize treasury stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockRepurchasedDuringPeriodValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.3
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS - USD ($)
|
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
| CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
| Net Loss |
$ (6,639,935)
|
$ (4,790,597)
|
| Adjustments to Reconcile Net Loss to Net Cash Used In Operating Activities: |
|
|
| Depreciation and amortization expense |
914,493
|
375,918
|
| Amortization of right-of-use assets |
22,507
|
102,549
|
| Bad debt expense |
(31,287)
|
836,300
|
| Provision for extraordinary tax charges |
0
|
$ 579,387
|
| Shares issued in lieu of cash |
|
96,888
|
| Lease expense |
235,659
|
$ 178,893
|
| Interest on finance leases |
1,765
|
20,629
|
| Stock-based compensation |
1,103,200
|
214,505
|
| Deferred income taxes |
0
|
(1,923)
|
| Gain on extinguishment of debt |
0
|
(1,911,476)
|
| Bargain purchase gain |
0
|
(1,633,842)
|
| Change in fair value of the derivative liability |
0
|
(3,384)
|
| Gain on net change in fair value of equity investments |
(2,518)
|
(2,876)
|
| Other income |
0
|
(928)
|
| Changes in assets and liabilities: |
|
|
| Accounts receivable |
2,392,104
|
(1,960,236)
|
| Accounts receivable - related party |
(176,512)
|
(416,814)
|
| Inventory |
(46,301)
|
(2,299,829)
|
| Prepaid expenses and other assets |
(283,932)
|
(1,856,642)
|
| Prepaid expenses and other current assets - related party |
(1,873,513)
|
(2,312,324)
|
| Loan receivable - related party |
0
|
|
| Accounts payable and accrued expenses |
(437,391)
|
206,746
|
| Accounts payable and accrued expenses - related party |
795,471
|
(112,233)
|
| Accrued interest |
17,531
|
(194,361)
|
| Lease liabilities |
(234,834)
|
(179,081)
|
| Taxes payable |
0
|
307,357
|
| Other current liabilities |
910,885
|
(783,174)
|
| Other liabilities |
(550,606)
|
(1,047,178)
|
| NET CASH USED IN OPERATING ACTIVITIES |
(3,883,215)
|
(16,587,726)
|
| CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
| Proceeds from loan receivable |
550,565
|
609,455
|
| Cash paid for the acquisition of Cana |
0
|
(5,230,593)
|
| Loan receivable - related party |
0
|
(168,469)
|
| Sale of intangible assets |
1,999
|
0
|
| Advances for building's acquisition |
0
|
(1,665,000)
|
| Purchase of intangible assets |
0
|
(2,678,167)
|
| Purchase of property and equipment |
(345,593)
|
(1,266,490)
|
| NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES |
206,971
|
(10,399,264)
|
| CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
| Payment of convertible note payable |
0
|
(100,000)
|
| Payment of note payable |
(814,267)
|
(1,494,867)
|
| Proceeds from note payable |
434,797
|
1,059,300
|
| Payment of related party loan |
(7,609)
|
0
|
| Proceeds from related party loan |
15,218
|
0
|
| Payment of lines of credit |
(19,504,594)
|
(14,569,517)
|
| Proceeds from lines of credit |
18,831,043
|
14,218,787
|
| Proceeds from issuance of Series A Preferred Stock |
0
|
0
|
| Proceeds from the issuance of common stock |
649,039
|
9,950,037
|
| Proceeds from the exercise of warrants |
4,240,977
|
0
|
| Payments of finance lease liability |
(26,408)
|
(118,847)
|
| Payments for purchase of treasury stock |
0
|
(100,251)
|
| Payments of financing fees |
(391,575)
|
(442,892)
|
| NET CASH PROVIDED BY FINANCING ACTIVITIES |
3,426,621
|
8,401,750
|
| Effect of exchange rate changes on cash |
(268,727)
|
196,161
|
| NET CHANGE IN CASH |
(518,350)
|
(18,389,079)
|
| CASH AT BEGINNING OF YEAR |
3,833,195
|
20,749,683
|
| CASH AT END OF YEAR |
3,314,845
|
2,360,604
|
| Cash paid during the year: |
|
|
| Interest |
198,194
|
317,449
|
| Income tax |
0
|
0
|
| Supplemental Disclosure of Non-Cash Investing and Financing Activities |
|
|
| Common shares issued for acquisition of customer base |
|
316,081
|
| Common shares issued for acquisition of Cana |
|
138,667
|
| Closing of acquisition of Cloudscreen |
637,080
|
0
|
| Deemed dividend upon warrant exchange |
6,195,024
|
0
|
| Common stock issued to employees |
372,303
|
0
|
| Common stock issued to consultants |
$ 727,570
|
$ 0
|
| X |
- References
+ Details
| Name: |
abcd_CashPaidDuringTheYearNewAbstract |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_CashPaidForTheAcquisitionOfCana |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ChangeInFairValueOfTheDerivativeLiability |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ClosingOfAcquisitionOfCloudscreen |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_CommonSharesIssuedForAcquisitionOfCana |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_CommonSharesIssuedForAcquisitionOfCustomerBase |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_CommonStockIssuedToConsultants |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_DeemedDividendUponWarrantExchange |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_GainLossOnBargainPurchase |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_PaymentForAdvancesForBuildingsAcquisition |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_SharesIssuedForCash |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477401/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CashFlowNoncashInvestingAndFinancingActivitiesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense recognized in the current period that allocates the cost of tangible assets, intangible assets, or depleting assets to periods that benefit from use of the assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
| Name: |
us-gaap_DepreciationDepletionAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) from effect of exchange rate changes on cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; held in foreign currencies. Excludes amounts for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 230
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477401/830-230-45-1
| Name: |
us-gaap_EffectOfExchangeRateOnCashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest expense on finance lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_FinanceLeaseInterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow for principal payment on finance lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-5
| Name: |
us-gaap_FinanceLeasePrincipalPayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of realized and unrealized gain (loss) on investment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(9)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_GainLossOnInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionDifference between the fair value of payments made and the carrying amount of debt which is extinguished prior to maturity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481303/470-50-40-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481303/470-50-40-4
| Name: |
us-gaap_GainsLossesOnExtinguishmentOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amounts payable to vendors for goods and services received and the amount of obligations and expenses incurred but not paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in amount due within one year (or one business cycle) from customers for the credit sale of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period of all taxes owed but not paid, including income, property and other taxes. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedTaxesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in interest payable, which represents the amount owed to note holders, bond holders, and other parties for interest earned on loans or credit extended to the reporting entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInInterestPayableNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate value of all inventory held by the reporting entity, associated with underlying transactions that are classified as operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInInventories |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation for operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-SubTopic 20
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_IncreaseDecreaseInOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in current liabilities classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInOtherCurrentLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in operating liabilities classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInOtherOperatingLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-2
| Name: |
us-gaap_InterestPaidNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of rent expense incurred for leased assets, including but not limited to, furniture and equipment, that is not directly or indirectly associated with the manufacture, sale or creation of a product or product line.
| Name: |
us-gaap_LeaseAndRentalExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of periodic reduction over lease term of carrying amount of right-of-use asset from operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_OperatingLeaseRightOfUseAssetAmortizationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue and income classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column E)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column E)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column E)(Footnote 6)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(1)(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
| Name: |
us-gaap_OtherIncome |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow to reacquire common stock during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 15
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsForRepurchaseOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for loan and debt issuance costs. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsOfFinancingCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow to acquire asset without physical form usually arising from contractual or other legal rights, excluding goodwill. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds from issuance of capital stock which provides for a specific dividend that is paid to the shareholders before any dividends to common stockholders and which takes precedence over common stockholders in the event of liquidation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfPreferredStockAndPreferenceStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from contractual arrangement with the lender, including but not limited to, letter of credit, standby letter of credit and revolving credit arrangements. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(f))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromLinesOfCredit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from a borrowing supported by a written promise to pay an obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from disposal of asset without physical form usually arising from contractual or other legal rights, excluding goodwill. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 12
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromSaleOfIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the sale of loans receivables arising from the financing of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 12
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromSaleOfLoansReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from holders exercising their stock warrants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromWarrantExercises |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 11: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-3
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477314/942-235-S99-1
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 31: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4J
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4K
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-2
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (reversal of expense) for expected credit loss on accounts receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479319/326-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_ProvisionForDoubtfulAccounts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow from the repayment of a long-term debt instrument which can be exchanged for a specified amount of another security, typically the entity's common stock, at the option of the issuer or the holder. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfConvertibleDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow for payment of an obligation from a lender, including but not limited to, letter of credit, standby letter of credit and revolving credit arrangements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(f))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_RepaymentsOfLinesOfCredit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for a borrowing supported by a written promise to pay an obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate change in value for stock issued during the period as a result of employee stock purchase plan. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueEmployeeStockPurchasePlan |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.3
BASIS OF PRESENTATION
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| BASIS OF PRESENTATION |
|
| BASIS OF PRESENTATION |
NOTE 1 – BASIS OF PRESENTATION The terms “COSM,” “we,” the “Company,” the “Group” and “us” as used in this report refer to Cosmos Health Inc. The accompanying unaudited condensed consolidated balance sheet as of September 30, 2024 and unaudited condensed consolidated statements of operations and comprehensive loss for the three and nine months ended September 30, 2024 have been prepared in accordance with generally accepted accounting principles for interim financial information and with the instructions to Form 10-Q and Article 8 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by U.S. generally accepted accounting principles for complete financial statements. In the opinion of the management of COSM, all adjustments (consisting of normal recurring accruals) considered necessary for a fair presentation have been included. Operating results for the three and nine months ended September 30, 2024, are not necessarily indicative of the results that may be expected for the year ending December 31, 2024, or any other period. These unaudited condensed consolidated financial statements and notes should be read in conjunction with the financial statements for the year ended December 31, 2023, included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 (“Form 10-K”). The accompanying condensed consolidated balance sheet as of December 31, 2023 has been derived from the audited financial statements filed in our Form 10-K and is included for comparison purposes on the accompanying balance sheet. Going Concern The Company’s unaudited condensed consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”), which contemplates the continuation of the Company as a going concern. For the nine months ended September 30, 2024, the Company had revenue of $40,202,238, net loss of $6,639,935 and net cash used in operations of $3,883,215. Additionally, as of September 30, 2024, the Company had positive working capital of $11,027,653, an accumulated deficit of $104,479,192, and stockholders’ equity of $34,976,599. It is the management’s opinion that these conditions raise substantial doubt about the Company’s ability to continue as a going concern for a period of twelve months from the date of this filing. The Company’s revenues are not able to sustain its operations, and concerns exist regarding the Company’s ability to meet its obligations as they become due. The Company is subject to a number of risks to those of smaller commercial companies, including dependence on key individuals and products, the difficulties inherent in the development of a commercial market, the need to obtain additional capital, competition from larger companies, and other pharmaceutical and health care companies. Management evaluated the above conditions which raise substantial doubt about the Company’s ability to continue as a going concern to determine if it can meet its obligations for the subsequent twelve months from the date of this filing. Management considered its ability to access future capital, curtail expenses if needed, expand product lines, and acquire new products. Management’s plans include expansion of brand name products to the market, expanding the current product portfolio, and evaluating acquisition targets to expand distribution. Furthermore, the Company intends to vertically integrate the supply chain distribution network. During the period up to the issuance of this report the Company has signed multiple distribution agreements for its SPL products in Europe and Asia and a variety of contract manufacturing agreements though its subsidiary, Cana Laboratories Holdings (Cyprus) Limited. Finally, the Company plans to further access the capital markets in order to raise additional funds through equity offerings. More specifically, up to the issuance of its consolidated financial statements for the nine months ended September 30, 2024, the Company has sold 901,488 shares of common stock for net proceeds of $629,426 through two Prospectus Supplements to its Registration Statement on Form S-3 (No. 333-267550) filed with the SEC on February 29 and March 7, 2024. Management will also consider postponing the repayment of its outstanding Trade Facility ($1,588,163 balance as of September 30, 2024), intends to make substantial efforts to receive additional debt financing through its subsidiary, Cosmofarm SA, and plans to raise additional equity funds through utilizing its outstanding warrants. Following such efforts, on September 26, 2024, the Company entered into a Warrant Inducement Letter with an investor pursuant to which the Company issued 9,748,252 new warrants and reduced the exercise price of 4,874,126 warrant shares from $1.45 to $0.8701 to induce exercise and receive gross cash proceeds of $4,240,977. On July 29, 2024 the Company’s subsidiary Cosmofarm SA entered into an agreement with a third-party lender in the principal amount of €400,000 ($432,760). Moreover, the Company’s management is considering postponing certain repayments of suppliers and creditors. However, management cannot provide any assurances that the Company will be successful in accomplishing any of its plans. The ability of the Company to continue as a going concern is dependent upon its ability to successfully accomplish the plans described herein and eventually secure other sources of financing and attain profitable operations. Considering the above, management is of the view that substantial doubt exists about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments to reflect the possible future effect on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from the outcome of this uncertainty.
|
| X |
- References
+ Details
| Name: |
abcd_BASISOFPRESENTATIONAbstract |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the business description and basis of presentation concepts. Business description describes the nature and type of organization including but not limited to organizational structure as may be applicable to holding companies, parent and subsidiary relationships, business divisions, business units, business segments, affiliates and information about significant ownership of the reporting entity. Basis of presentation describes the underlying basis used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/275/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/205/tableOfContent
| Name: |
us-gaap_BusinessDescriptionAndBasisOfPresentationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
ORGANIZATION AND NATURE OF BUSINESS
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| ORGANIZATION AND NATURE OF BUSINESS |
|
| ORGANIZATION AND NATURE OF BUSINESS |
NOTE 2 – ORGANIZATION AND NATURE OF BUSINESS Cosmos Health Inc. and its subsidiaries (Nasdaq: COSM), (“us”, “we”, the “Group”, or the “Company”) are an international healthcare group headquartered in Thessaloniki, Greece. The group is engaged in the nutraceuticals sector through its own proprietary lines of products “Sky Premium Life” and “Mediterranation”. The Company is operating in the pharmaceutical sector as well, through the provision of a broad line of branded generics and OTC medications. In addition, the group is involved in the healthcare distribution sector through its subsidiaries in Greece and the UK, serving retail pharmacies and wholesale distributors. The Company is strategically focusing on the research and development (“R&D”) of novel patented nutraceuticals (Intellectual Property) and specialized root extracts as well as on the R&D of proprietary complex generics and innovative OTC products. The Company has developed a global distribution platform and is currently expanding throughout Europe, Asia and North America. The Company has offices and distribution centers in Thessaloniki and Athens, Greece and Harlow, UK. The Company was incorporated in the State of Nevada under the name Prime Estates and Developments, Inc. on July 21, 2009. On November 14, 2013, we changed our name to Cosmos Holdings Inc., and on November 29, 2022, we changed our name to Cosmos Health Inc. Through its acquisition of Amplerissimo Ltd, on September 27, 2013, the Company changed its principal activities into trading of products, providing representation, and provision of consulting services to various sectors. On August 1, 2014, the Company formed SkyPharm S.A., a Greek Company (“SkyPharm”), a subsidiary that used to focus on the trading, sourcing and export of nutraceutical and pharmaceutical products. In February 2017, the Company acquired Decahedron Ltd., a UK Company (“Decahedron”) which is a fully licensed second-generation wholesaler specializing in imports and exports of generics and OTC pharmaceutical products within the EEA and distributor of Sky Premium Life nutraceutical products in the UK. On December 19, 2018, the Company acquired Cosmofarm, a pharmaceutical wholesaler specializing in the distribution and export of pharmaceutical products through its extensive pharmacies network. On April 3, 2023, the Company completed the acquisition of ZipDoctor Inc. (“ZipDoctor”), a telehealth company, a direct-to-consumer subscription-based telemedicine platform. On June 30, 2023, the Company acquired Cana Laboratories Holdings (Cyprus) Limited (“Cana”), which wholly owned an operating subsidiary, Pharmaceutical Laboratories Cana S.A. (“Cana SA”), a Greek pharmaceutical company that manufactures, sells, distributes, and markets original branded products researched and developed by leading global pharmaceutical and healthcare companies. Acquisition Accounting Cloudscreen On January 23, 2024, the Company completed the acquisition of Cloudscreen, a cutting-edge Artificial Intelligence (AI) powered platform. The acquisition is pursuant to the purchase agreement announced on October 11, 2023. Cloudscreen is a multimodal platform specialized in drug repurposing, a process that involves uncovering new target proteins or indications for existing drugs for use in treating different diseases. The total purchase price amounted to $637,080 and consisted of 280,000 shares of common stock with a fair value of $319,200 and an amount of $317,880 to be settled in cash during 2024 based on the Promissory Note signed on October 10, 2023. The Company accounted for the acquisition as an asset acquisition in accordance with Accounting Standards Codification (“ASC”) Topic 805, Business Combinations, (“ASC 805”) and recorded $637,080 as an intangible asset related to the technology platform acquired. ZipDoctor On April 3, 2023, the Company completed the acquisition of ZipDoctor Inc. (“ZipDoctor”), a telehealth company for a total sum of $150,000 in cash and $8,788 in fees. The Company accounted for the acquisition as an asset acquisition in accordance with Accounting Standards Codification (“ASC”) Topic 805, Business Combinations, (“ASC 805”) and recorded $158,788 as an intangible asset related to the technology platform acquired. Bikas On June 15, 2023, Cosmos Health Inc. entered into an Assignment and Assumption Agreement (the “Agreement”) with Ioannis Bikas O.E., a Greek Company (“Bikas”). Bikas is owner of a pharmaceutical distribution network in Greece and agreed to sell to the Company their distribution network and customer base. The purchase price of the network was €100,000 ($109,330) in cash, and €300,000 ($316,081) in the Company’s common stock. The Company issued 99,710 shares of common stock related to the acquisition of the customer base, based on the fair value of the stock on the acquisition date. The Company accounted for the acquisition as an asset acquisition in accordance with ASC 805 and recorded $425,411 as an intangible asset related to the customer base acquired. Buildings Acquisitions On April 24, 2023, the Company purchased a building for a total sum of $1,054,872 in cash. The Company accounted for the acquisition as an asset acquisition in accordance with ASC 805 and recorded the cost of the building as “Property, plant and equipment” on the consolidated balance sheets. On January 6, 2023, the Company agreed to purchase land and building located in Montreal, Canada from a third-party vendor. The total purchase price amounts to $3,950,000 and the closing date of the agreement based on the amendment signed on July 19, 2023, is December 31, 2023. As of September 30, 2024, the Company has made no additional prepayments concerning this building. The closing date of the agreement has been extended to December 31, 2024. Cana On June 30, 2023, the Company acquired Cana Laboratories Holdings (Cyprus) Limited (“Cana”), which wholly owned an operating subsidiary, Pharmaceutical Laboratories Cana S.A. (“Cana SA”) for €800,000 ($873,600) in cash and 46,377 shares of common stock, with fair value of $138,667 as of the date of acquisition. Moreover, on February 28, 2023, the Company had signed a Secured Promissory Note with Cana, whereby Cana borrowed the sum of €4,100,000 ($4,457,520), included in the total consideration of $5,469,787. The Company accounted for the acquisition as a business acquisition in accordance with ASC 805. The fair value of Cana assets acquired, and liabilities assumed was based upon management’s estimates assisted by an independent third-party valuation firm. The fixed assets of Cana (which included land, building & machinery) were valued as of December 31, 2022 and the Company believes that nothing has materially changed between such date and the acquisition date (June 30, 2023). The following table summarizes the preliminary allocation of purchase price of the acquisition: Consideration | | | | Cash | | $ | 5,331,120 | | Fair value of common stock issued | | | 138,667 | | Fair value of total consideration transferred | | $ | 5,469,787 | | | | | | | Recognized amounts of identifiable assets acquired | | | | | Financial assets | | $ | 1,796,911 | | Inventory | | | 297,340 | | Property, plant and equipment | | | 7,488,818 | | Identifiable intangible assets | | | 562,200 | | Financial liabilities | | | (3,235,233 | ) | Total identifiable net assets | | $ | 6,910,036 | | | | | | | Bargain purchase gain | | $ | 1,440,249 | |
Revenue for the 9 - month period ended September 30, 2024 | | $ | 549,567 | | Loss for the 9 - month period ended September 30, 2024 | | $ | (1,674,785 | ) |
During the prior year period, Cana had minimal operations as it was in financial difficulties and seeking for an investor. Basis of Financial Statement Presentation The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with U.S. GAAP. Principles of Consolidation Our consolidated accounts include our accounts and the accounts of our wholly owned subsidiaries, SkyPharm S.A., Decahedron Ltd., Cosmofarm S.A., Cana Laboratories Holdings (Cyprus) Limited and ZipDoctor Inc. The Group’s financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”). The consolidated financial statements reflect the consolidation of all entities in which the Company has control, as determined by the ability to direct the activities that significantly affect the entities’ economic performance. All significant intercompany balances and transactions have been eliminated. Transactions in and Translations of Foreign Currency The functional currency for the Greek subsidiaries of the Company (CANA Laboratories, Cosmofarm S.A. and SkyPharm SA) is Euro (€) and for the UK subsidiary (Decahedron Ltd) is GBP (£). ZipDoctor Inc. is a U.S. based entity. As a result, the financial statements of the subsidiaries (except for ZipDoctor Inc.) have been translated from the local currency into U.S. dollars using (i) year-end exchange rates for balance sheet accounts, and (ii) average exchange rates for the reporting period for all income statements accounts. Foreign currency translations gains and losses are reported as a separate component of the condensed consolidated statements of changes in stockholders’ equity and mezzanine equity. Use of Estimates The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. The Effects of War in the Ukraine On February 24, 2022, Russian forces launched significant military action against Ukraine. There continues to be sustained conflict and disruption in the region, which is expected to endure for the foreseeable future. We do not conduct any commercial transactions with either Ukraine or Russia and the Company and, as such, is not aware of any specific event or circumstance that would require an update to its estimates or judgments or a revision of the carrying value of its assets or liabilities as of the date of issuance of this Quarterly Report on Form 10-Q. Such political issues and conflicts could have a material adverse effect on our results of operations and financial condition if they escalate in areas in which we do business. In addition, changes in and adverse actions by governments in foreign markets in which we do business could have a material adverse effect on our results of operations and financial condition. Credit Losses In June 2016, the FASB issued ASU 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses of Financial Instruments, which amends the requirement on the measurement and recognition of expected credit losses for financial assets held. Furthermore, amendments ASU 2019-10 and ASU 2019-11 provided additional clarification for implementing ASU 2016-13. ASU 2016-13 is effective for the Company beginning January 1, 2023, with early adoption permitted. The Company adopted the standard on January 1, 2023, and the standard did not have a material impact on the Company’s consolidated financial statements and related disclosures. The Company is exposed to credit losses primarily through sales to its customers and the loans that it has provided. The Company assesses each customer’s/ borrower’s ability to pay, and a credit loss estimate by conducting a credit review which includes consideration of established credit rating, or an internal assessment of the customer’s creditworthiness based on an analysis of their payment history when a credit rating is not available. The Company monitors credit exposure through active review of customer balances. The Company’s expected loss methodology for accounts receivable is developed through consideration of factors including, but not limited to, historical collection experience, current customer credit ratings, current customer financial condition, current and future economic and market conditions, and age of the receivables. More specifically, the Company assesses a number of customers with significant long outstanding balances on an individual basis, applying different credit loss percentages to them, and subsequently summarizes the ones not included in the individual analysis, groups them based on their rating (decided based on the factors described above) and applies specific credit loss percentages to each group. The Company has elected to follow the simplified ECL approach. The charges related to credit losses are included in “General and administrative expenses” and are recorded in the period that the outstanding receivables are determined to be doubtful. Account balances are written-off against the allowance when they are deemed uncollectible. Cash and Cash Equivalents For purposes of the statement of cash flows, the Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. The Company maintains bank accounts in the United States denominated in U.S. Dollars, in Greece denominated in Euros, U.S. Dollars and Great Britain Pounds (British Pounds Sterling), and in Bulgaria denominated in Euros. The Company also maintains bank accounts in the United Kingdom, denominated in Euros and Great Britain Pounds (British Pounds Sterling). Accounts Receivable, net Accounts receivable are stated at their net realizable value. The allowance for doubtful accounts against gross accounts receivable, prepaid expenses and other current assets and other assets reflects the best estimate of probable losses inherent in the receivables’ portfolio determined on the basis of historical experience, specific allowances for known troubled accounts and other currently available information. As of September 30, 2024 and December 31, 2023, the Company’s allowance for doubtful accounts was $19,905,776 and $19,686,091, respectively. Below is the summary of changes in the allowance for doubtful accounts: | | September 30, 2024 | | | | | | Balance as of January 1st, 2024 | | $ | 19,686,091 | | Provisions for credit losses | | | - | | Write-offs | | | 250,971 | | Foreign exchange adjustments | | | | | Other adjustments | | | (31,286 | ) | Balance as of September 30, 2024 | | $ | 19,905,776 | |
Tax Receivables The Company pays Value Added Tax (“VAT”) or similar taxes (“input VAT”), income taxes, and other taxes within the normal course of its business in most of the countries in which it operates related to the procurement of merchandise and/or services it acquires and/or on sales and taxable income. The Company also collects VAT or similar taxes on behalf of the government (“output VAT”) for merchandise and/or services it sells. If the output VAT exceeds the input VAT, this creates a VAT payable to the government. If the input VAT exceeds the output VAT, this creates a VAT receivable from the government. The VAT tax return is filed on a monthly basis offsetting the payables against the receivables. In observance of EU regulations for intra-EU cross-border sales, our subsidiaries in Greece, SkyPharm and Cosmofarm, do not charge VAT for sales to wholesale drug distributors registered in other European Union member states. As of September 30, 2024 and December 31, 2023, the Company had a VAT net receivable balance of $322,576 and $187,512 respectively, recorded in the consolidated balance sheet as prepaid expenses and other current assets and accounts payable and accrued expenses, respectively. Inventory Inventory is stated at the lower-of-cost or net realizable value using the weighted average method. Inventory consists primarily of finished goods and packaging materials, i.e., packaged pharmaceutical products and the wrappers and containers they are sold in. A periodic inventory system is maintained by 100% count. Inventory is replaced periodically to maintain the optimum stock on hand available for immediate shipment. The Company writes down inventories to net realizable value based on physical condition, expiration date, and current market conditions, as well as forecasted demand. The Company’s inventories are not highly susceptible to obsolescence. Many of the Company’s inventory items are eligible for return to our suppliers when pre-agreed product requirements, including, but not limited to, physical condition and expiration date, are not met. No significant judgments have been applied in estimating the selling price of our inventory. Property and Equipment, net Property and equipment are stated at cost, less accumulated depreciation. Depreciation is calculated on a straight-line basis over the useful lives (except for leasehold improvements which are depreciated over the lesser of the lease term or the useful life) of the assets as follows: | | Estimated Useful Life | | Leasehold improvements and technical works | | | Lesser of lease term or 25 years | | Buildings | | | 25-30 years | | Vehicles | | | 6 years | | Machinery | | | 20 years | | Furniture, fixtures and equipment | | | 5–10 years | | Computers and software | | | 3-5 years | |
Depreciation expense was $89,694 and $124,910 for the three months ended September 30, 2024 and 2023, respectively and $306,126 and $237,479 for the nine months ended September 30, 2024 and 2023, respectively. Property and Equipment additions Property and Equipment additions are recognized as assets when it is probable that future economic benefits associated with the asset will flow to the entity and the cost of the asset can be measured reliably. Additions are initially measured at cost, which includes all costs directly attributable to bringing the asset to its working condition and location for its intended use. This may include purchase price, freight, installation, and any directly attributable professional fees. They are capitalized if their cost exceeds a certain threshold. The threshold is determined based on materiality considerations. Costs below the threshold are typically expensed as incurred. After initial recognition, additions are measured at cost less accumulated depreciation and any accumulated impairment losses. Depreciation is calculated systematically over the estimated useful life of the asset. They are tested for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. If the carrying amount exceeds the recoverable amount, an impairment loss is recognized, and the carrying amount of the asset is adjusted accordingly. Borrowing costs directly attributable to the acquisition, construction, or production of qualifying assets, including Property and Equipment additions, are capitalized as part of the cost of those assets. Goodwill and Intangibles, net The Company periodically reviews the carrying value of intangible assets not subject to amortization, including goodwill, to determine whether impairment may exist. Goodwill and certain intangible assets are assessed annually, or when certain triggering events occur, for impairment using fair value measurement techniques. These events could include a significant change in the business climate, legal factors, a decline in operating performance, competition, sale or disposition of a significant portion of the business, or other factors. First, under step 0, we determine whether it is more likely than not that the fair value of the reporting unit is less than the carrying amount. Following, if step 0 fails, goodwill impairment is determined using a two-step process. The first step of the goodwill impairment test is used to identify potential impairment by comparing the fair value of a reporting unit with its carrying amount, including goodwill. The Company uses level 3 inputs and a discounted cash flow methodology to estimate the fair value of a reporting unit. A discounted cash flow analysis requires one to make various judgmental assumptions including assumptions about future cash flows, growth rates, and discount rates. The assumptions about future cash flows and growth rates are based on the Company’s budget and long-term plans. Discount rate assumptions are based on an assessment of the risk inherent in the respective reporting units. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is considered not impaired and the second step of the impairment test is unnecessary. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test is performed to measure the amount of impairment loss, if any. The second step of the goodwill impairment test compares the implied fair value of the reporting unit’s goodwill with the carrying amount of that goodwill. If the carrying amount of the reporting unit’s goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess. The implied fair value of goodwill is determined in the same manner as the amount of goodwill recognized in a business combination. That is, the fair value of the reporting unit is allocated to all of the assets and liabilities of that unit (including any unrecognized intangible assets) as if the reporting unit had been acquired in a business combination and the fair value of the reporting unit was the purchase price paid to acquire the reporting unit. On December 19, 2018, as a result of the acquisition of Cosmofarm, the Company recorded $49,697 of goodwill. Intangible assets with definite useful lives are recorded on the basis of cost and are amortized on a straight-line basis over their estimated useful lives. The Company uses a useful life of 5 years for an import/export license and a useful life of 10 years for the pharmaceutical and nutraceutical products licenses included in Note 4 as “Licenses”. A useful life of 10 years is also used for the platforms included in Note 4 as “Software” and the customer bases. The Company evaluates the remaining useful life of intangible assets annually to determine whether events and circumstances warrant a revision to the remaining amortization period. If the estimate of the intangible asset’s remaining useful life is changed, the remaining carrying amount of the intangible asset will be amortized prospectively over that revised remaining useful life. As of September 30, 2024 and December 31, 2023, no revision to the remaining amortization period of the intangible assets was made. Amortization expense was $196,183 and $88,168 for the three months ended September 30, 2024 and 2023, respectively and $579,556 and $138,438 for the nine months ended September 30, 2024 and 2023, respectively. Impairment of Long-Lived Assets In accordance with ASC 360-10, Long-lived Assets, property and equipment and intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the assets. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable. Equity Method Investment For those investments in common stock or in-substance common stock in which the Company has the ability to exercise significant influence over the operating and financial policies of the investee, the investment is accounted for under the equity method. The Company records its share in the earnings of the investee and is included in “Equity earnings of affiliate” in the consolidated statement of operations. The Company assesses its investment for other-than-temporary impairment when events or changes in circumstances indicate that the carrying amount of the investment might not be recoverable and recognizes an impairment loss to adjust the investment to its then current fair value. Investments in Equity Securities Investments in equity securities are accounted for at fair value with changes in fair value recognized in net income (loss). Equity securities are classified as short-term or long-term based on the nature of the securities and their availability to meet current operating requirements. Equity securities that are readily available for sale in current operations are reported as a component of current assets on the accompanying consolidated balance sheets. Equity securities that are not considered available for use in current operations would be reported as a component of long-term assets on the accompanying consolidated balance sheets. For equity securities with no readily determinable fair value, the Company elects a measurement alternative to fair value. Under this alternative, the Company measures the investments at cost, less any impairment, and adjusted for changes resulting from observable price changes in transactions for identical or similar investments of the investee. The election to use the measurement alternative is made for each eligible investment. As of September 30, 2024, investments consisted of 16,666 shares which traded at a closing price of $0.75 per share or value of $12,416 of National Bank of Greece. Additionally, the Company has $7,665 in equity securities of Pancreta Bank, which are revalued annually. Fair Value Measurement The Company applies ASC 820, Fair Value Measurements and Disclosures, (“ASC 820”), for assets and liabilities measured at fair value on a recurring basis. ASC 820 establishes a common definition for fair value to be applied to existing generally accepted accounting principles that require the use of fair value measurements establishes a framework for measuring fair value and expands disclosure about such fair value measurements. ASC 820 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Additionally, ASC 820 requires the use of valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs. These inputs are prioritized below: Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities. Level 2: Inputs other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. Level 3: Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use. In addition, ASC 825-10-25, Fair Value Option, (“ASC 825-10-25”), expands opportunities to use fair value measurements in financial reporting and permits entities to choose to measure many financial instruments and certain other items at fair value. The Company did not elect the fair value options for any of its qualifying financial instruments. Our financials also included the following financial instruments as of September 30, 2024 and December 31, 2023: cash, accounts receivable, inventory, prepaid expenses, loans receivable, accounts payable, notes payable and lines of credit. Except for the loans receivable which carry fixed interest rates, the carrying value of the remaining instruments, approximates fair value due to their short-term nature. Customer Advances The Company receives prepayments from certain customers for pharmaceutical products prior to those customers taking possession of the Company’s products. The Company records these receipts as current liabilities until it has met all the criteria for recognition of revenue including passing control of the products to its customer, at such point, the Company will reduce the customer advances balance and credit the Company’s revenues. Revenue Recognition In accordance with ASC Topic 606, Revenue from Contracts with Customers (“ASC 606”), the Company uses a five-step model for recognizing revenue by applying the following steps: | 1) | Identification of the Contract: The Company identifies a contract with a customer when it enters into an agreement that creates enforceable rights and obligations. | | 2) | Identification of Performance Obligations: The Company identifies distinct performance obligations within each contract, which represent promises to transfer goods or services to the customer. | | 3) | Determination of Transaction Price: The Company determines the transaction price, which represents the amount of consideration to which it expects to be entitled in exchange for transferring promised goods or services to the customer, excluding any amounts collected on behalf of third parties. | | 4) | Allocation of Transaction Price: The Company allocates the transaction price to each distinct performance obligation based on its standalone selling price. If the standalone selling price is not observable, the Company estimates it using an appropriate method. | | 5) | Recognition of Revenue: Revenue is recognized when (or as) the Company satisfies a performance obligation by transferring a promised good or service to the customer. This typically occurs at a point in time or over time, depending on the nature of the performance obligation. |
Wholesale revenue and sales of own branded nutraceutical and pharmaceutical products The Company has contracts or signed partnership forms (usual in the wholesale sector of the pharma industry) with its customers, stipulating enforceable rights and obligations. The Company is responsible for transferring the goods to the customer’s location, which represents its sole performance obligation. Thus, the transaction price, which is predetermined in most of the products sold, is exclusively allocated to this performance obligation. Revenue is recognized at a single point in time, which is upon issuance of the corresponding sales invoice. The Company has assessed the impact of the items invoiced but not delivered to the customer’s location as of December 31, 2023 and September 30, 2024, and deemed that it had no material effect. Pharma manufacturing The Company has active contracts with its customers, stipulating enforceable rights and obligations. The Company is responsible for the manufacturing and the packaging of specific products assigned by its customers, which represents its performance obligations to which the Company allocates the transaction price determined. The customers are responsible for providing the raw materials to the Company. Revenue is recognized over a period of time, which is during the production and packaging period of the respective products. As of September 30, 2024, there were no products or batches of products for which the production or packaging phase was in progress. Medihelm SA Commencing from January 1, 2023, and pursuant to the agreement with Medihelm, the exclusive distributor of the Company’s own proprietary line of nutraceuticals, the Company considers the transaction price to be variable and records an estimate of the transaction price, subject to the constraint for variable consideration. The Company is basing the change in transaction price with the exclusive distributor through assessment of significant overdue receivables from the exclusive distributor, which the Company reassesses each reporting period. Through this assessment, the Company applied the “expected value” model under ASC 606-10-32-5 and had applied specific constraints to revenue due from the customer at the end of each reporting period. Following the application of the “expected value” model, the Company had deferred an amount of $397,000 and recorded it against the sales to Medihelm for the twelve months ended December 31, 2023. However, the Company assessed once more the trading relationship with Medihelm SA at year end and since no significant receipts had taken place up to the issuance of the report, the Company recorded an allowance for the total receivable amount not received up to the issuance date. More specifically a cumulative reserve of $12,655,615 was applied, leaving a receivable of $532,704 due from Medihelm SA, as of December 31, 2023. The Company does not consider that new sales to Medihelm SA or sales to any other customer include a variable component as of September 30, 2024 and has limited such sales to the minimum required. Stock-based Compensation The Company records stock-based compensation in accordance with ASC 718, Stock Compensation (“ASC 718”) and Staff Accounting Bulletin No. 107 (“SAB 107”) regarding its interpretation of ASC 718. ASC 718 requires the fair value of all stock-based employee compensation awarded to employees to be recorded as an expense over the related requisite service period. The Company values any employee or non-employee stock-based compensation at fair value using the Black-Scholes Option Pricing Model. The Company accounts for non-employee share-based awards in accordance with the measurement and recognition criteria of ASU 2018-07, “Compensation-Stock Compensation-Improvements to Nonemployee Share-Based Payment Accounting.” Income Taxes The Company accounts for income taxes under the asset and liability method, as required by the accounting standard for income taxes ASC 740. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis, as well as net operating loss carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company is liable for income taxes in Greece and the United Kingdom The corporate income tax rate is 22% in Greece and 25% in the United Kingdom. Losses may also be subject to limitation under certain rules regarding change of ownership. We regularly review deferred tax assets to assess their potential realization and establish a valuation allowance for portions of such assets to reduce the carrying value if we do not consider it to be more likely than not that the deferred tax assets will be realized. Our review includes evaluating both positive (e.g., sources of taxable income) and negative (e.g., recent historical losses) evidence that could impact the realizability of our deferred tax assets. At September 30, 2024, we believe our United Kingdom and Greece deferred tax assets will not be realized, as such, we did not record a reversal on the full valuation approach we followed during the year ended December 31, 2023. Leases The Company accounts for leases in accordance with ASC 842. For all leases, the Company recognizes a right-of-use (ROU) asset and a lease liability on the balance sheet. The ROU asset represents the Company's right to use the underlying asset for the lease term, and the lease liability represents the obligation to make lease payments arising from the lease, both measured at the present value of future lease payments. Lease payments are recognized as an operating expense on a straight-line basis over the lease term. The interest on the lease liability and the amortization of the ROU asset are recognized separately in the income statement. Initial direct costs incurred by the Company in negotiating and securing leases are capitalized and amortized over the lease term on a straight-line basis. The assets and liabilities from operating and finance leases are recognized at the commencement date based on the present value of remaining lease payments over the lease term using the Company’s secured incremental borrowing rates or implicit rates, when readily determinable. Short-term leases, which have an initial term of 12 months or less, are not recorded on the balance sheet. The Company’s operating leases do not provide an implicit rate that can readily be determined. Therefore, we use a discount rate based on our incremental borrowing rate, which is determined using the average interest rate of our long-term debt on the date of inception. Retirement and Termination Benefits Under Greek labor law, employees are entitled to lump-sum compensation in the event of termination or retirement. The amount depends on the employee’s work experience and remuneration as of the day of termination or retirement. If an employee remains with the company until full-benefit retirement, the employee is entitled to a lump-sum equal to 40% of the compensation to be received if the employee were to be dismissed on the same day. The Company periodically reviews the uncertainties and judgments related to the application of the relevant labor law regulations to determine retirement and termination benefits obligations of its Greek subsidiaries. The Company has evaluated the impact of these regulations and has identified a potential retirement and termination benefits liability. The amount of the liability as of September 30, 2024 and December 31, 2023, was $395,698 and $408,665, respectively, and has been recorded as a long-term liability within the consolidated balance sheets. Basic and Diluted Net Loss per Common Share Basic income per share is calculated by dividing the income available to common stockholders by the weighted average number of shares of common stock outstanding during the period. Diluted income per share is calculated by dividing income available to common stockholders by the weighted average number of common shares outstanding for the period and, when dilutive, potential shares from stock options and warrants to purchase common stock, using the treasury stock method. In accordance with ASC 260, Earnings Per Share, the following table reconciles basic shares outstanding to fully diluted shares outstanding. | | September 30, 2024 | | | September 30, 2023 | | Weighted average number of common shares outstanding Basic | | | 17,724,305 | | | | 11,346,071 | | Potentially dilutive common stock equivalents | | | - | | | | - | | Weighted average number of common and equivalent shares outstanding – Diluted | | | 17,724,305 | | | | 11,346,071 | |
The following table summarizes potential common shares that were excluded as their effect is anti-dilutive: | | September 30, 2024 | | | September 30, 2023 | | Warrants | | | 13,432,507 | | | | 6,124,412 | | Total | | | 13,432,507 | | | | 6,124,412 | |
Common stock equivalents are included in the diluted income per share calculation only when option exercise prices are lower than the average market price of the common shares for the period presented. In March 2022, the FASB issued ASU 2022-02, Troubled Debt Restructurings and Vintage Disclosures. This ASU eliminates the accounting guidance for troubled debt restructurings by creditors that have adopted ASU 2016-13, Measurement of Credit Losses on Financial Instruments, which was adopted on January 1, 2020. The adoption of ASU 2016-13 did not have a material impact on the Company’s consolidated financial statements. ASU 2022-02 also enhances the disclosure requirements for certain loan refinancing and restructurings by creditors when a borrower is experiencing financial difficulty. In addition, the ASU amends the guidance on vintage disclosures to require entities to disclose current period gross write-offs by year of origination for financing receivables and net investments in leases within the scope of ASC 326-20. The ASU is effective for annual periods beginning after December 15, 2022, including interim periods within those fiscal years. Adoption of the ASU would be applied prospectively. Early adoption is also permitted, including adoption in an interim period. This ASU was adopted on January 1, 2023, which resulted in no cumulative-effect adjustment to retained earnings. Recent Accounting Pronouncements In March 2024, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2024-01, Compensation—Stock Compensation (Topic 718): Scope Application of Profits Interest and Similar Awards. This guidance is intended to improve generally accepted accounting principles (GAAP) by adding an illustrative example to demonstrate how an entity should apply the scope guidance in paragraph 718- 10-15-3 to determine whether profits interest and similar awards (“profits interest awards”) should be accounted for in accordance with Topic 718, Compensation—Stock Compensation. The amendments in this Update are effective for annual periods beginning after December 15, 2024, and interim periods within those annual periods. The amendments in this Update should be applied either (1) retrospectively to all prior periods presented in the financial statements or (2) prospectively to profits interest and similar awards granted or modified on or after the date at which the entity first applies the amendments. The Company is currently evaluating this guidance to determine the impact it may have on its consolidated financial statements disclosures. In March 2024, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2024-02, Codification Improvements—Amendments to Remove References to the Concepts Statements. This guidance is intended to remove references to various FASB Concepts Statements. The Board has a standing project on its agenda to address suggestions received from stakeholders on the Accounting Standards Codification and other incremental improvements to generally accepted accounting principles (GAAP). This effort facilitates Codification updates for technical corrections such as conforming amendments, clarifications to guidance, simplifications to wording or the structure of guidance, and other minor improvements. The resulting amendments are referred to as Codification improvements. The amendments in this Update are not intended to result in significant accounting change for most entities. The Company is currently evaluating this guidance to determine the impact it may have on its consolidated financial statements disclosures. In December 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. This guidance is intended to enhance the transparency and decision-usefulness of income tax disclosures. The amendments in ASU 2023-09 address investor requests for enhanced income tax information primarily through changes to disclosure regarding rate reconciliation and income taxes paid both in the U.S. and in foreign jurisdictions. ASU 2023-09 is effective for fiscal years beginning after December 15, 2024, on a prospective basis, with the option to apply the standard retrospectively. Early adoption is permitted. The Company is currently evaluating this guidance to determine the impact it may have on its consolidated financial statements disclosures. In November 2023, the FASB issued ASU 2023-07, Segment Reporting: Improvements to Reportable Segment Disclosures. This guidance expands public entities’ segment disclosures primarily by requiring disclosure of significant segment expenses that are regularly provided to the chief operating decision maker and included within each reported measure of segment profit or loss, an amount and description of its composition for other segment items, and interim disclosures of a reportable segment’s profit or loss and assets. The guidance is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. The amendments are required to be applied retrospectively to all prior periods presented in an entity’s financial statements. The Company is currently evaluating this guidance to determine the impact it may have on its consolidated financial statements related disclosures. Management does not believe that any recently issued, but not effective, accounting standards, if currently adopted, would have a material effect on the Company’s consolidated financial statements.
|
| X |
- References
+ Details
| Name: |
abcd_OrganizationAndNatureOfTheBusinessAbstract |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the general note to the financial statements for the reporting entity which may include, descriptions of the basis of presentation, business description, significant accounting policies, consolidations, reclassifications, new pronouncements not yet adopted and changes in accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/275/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 250
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/250/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationBasisOfPresentationBusinessDescriptionAndAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
EQUITY METHOD INVESTMENTS
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| EQUITY METHOD INVESTMENTS |
|
| EQUITY METHOD INVESTMENTS |
NOTE 3 – EQUITY METHOD INVESTMENTS Distribution and Equity Agreement On March 19, 2018, the Company entered into a Distribution and Equity Acquisition Agreement with Marathon Global Inc. (“Marathon”), a company incorporated in the Province of Ontario, Canada. Marathon was formed to be a global supplier of cannabis, cannabidiol (CBD) and/or any cannabis extract products, extracts, ancillaries and derivatives (collectively, the “Products”). The Company was appointed the exclusive distributor of the Products initially throughout Europe and on a non-exclusive basis wherever else lawfully permitted. The Company has no present intention to distribute any Products under this Agreement in the United States or otherwise participate in cannabis operations in the United States. The Company intended to await further clarification from the U.S. government on cannabis regulation prior to determining whether to enter the domestic market. The above transaction closed on May 22, 2018 after the due diligence period, following which the Company received: (a) a 33 1/3% equity interest or 5 million shares in Marathon as partial consideration for the Company’s distribution services; and (b) received cash of CAD $2,000,000, subject to repayment in common shares of the Company if it failed to meet certain performance milestones. The Company was entitled to receive an additional CAD $2,750,000 upon the Company’s receipt of gross sales of CAD $6,500,000 and an additional CAD $2,750,000 upon receipt of gross sales of CAD $13,000,000. The Company was also given the right to nominate one director to the Marathon board of directors. Since Marathon was a newly formed entity with no assets and no activity, the Company attributed no value to the 5 million shares in Marathon which was received as consideration for the distribution services. The Distribution and Equity Acquisition Agreement was to remain in effect indefinitely unless Marathon fails to provide Market Competitive (as defined) product pricing and Marathon has not become profitable within five (5) years of the agreement. On March 20, 2023, the Company sent a termination notice, to Marathon, which became effective on April 19, 2023 as a result of Marathon’s failure to satisfy these conditions. The Company had accounted for its obligation to issue a variable number of the Company’s Common Shares as Share-settled debt obligation in accordance with ASC Topic 480, Distinguishing Liabilities from Equity (“ASC 480”), which was measured at fair value or the settlement amount of $1,554,590 (CAD $2 million). Due to termination of the Equity Agreement, the Company recorded a gain on extinguishment of debt of $1,554,590 due to the write-off of the share settled debt obligation, for the nine months ended September 30, 2023. CosmoFarmacy LP In September 2019, the Company entered into an agreement with an unaffiliated third party to incorporate CosmoFarmacy L.P. for the purpose of providing strategic management consulting services and the retail trade of pharmaceutical products, and OTC to pharmacies. CosmoFarmacy was incorporated with a 30-year term through May 31, 2049. The unaffiliated third party is the general partner (the “GP”) of the limited partnership and is responsible for management and decision-making associated with CosmoFarmacy. The initial share capital was set to EUR 150,000 ($163,080) which was later increased to EUR 500,000 ($543,600). The GP contributed the pharmacy license (the “License”) valued at EUR 350,000 (30-year term) to operate the business of CosmoFarmacy in exchange for a 70% equity ownership. The Company is a limited partner and contributed cash of EUR 150,000 ($163,080) for the remaining 30% equity ownership. CosmoFarmacy is not publicly traded and the Company’s investment has been recorded using the equity method of accounting. The value of the investment as of September 30, 2024 and December 31, 2023, was $167,175 and $165,930, respectively, and is included in “Other assets” on the Company’s consolidated balance sheets.
|
| X |
- DefinitionThe entire disclosure for equity method investments and joint ventures. Equity method investments are investments that give the investor the ability to exercise significant influence over the operating and financial policies of an investee. Joint ventures are entities owned and operated by a small group of businesses as a separate and specific business or project for the mutual benefit of the members of the group. Reference 1: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478156/740-323-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 323
-Publisher FASB
-URI https://asc.fasb.org/323/tableOfContent
| Name: |
us-gaap_EquityMethodInvestmentsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_InvestmentsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
PROPERTY AND EQUIPMENT, NET
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| PROPERTY AND EQUIPMENT, NET |
|
| PROPERTY AND EQUIPMENT, NET |
NOTE 4 – PROPERTY AND EQUIPMENT, NET Property and equipment, net consists of the following at September 30, 2024 and December 31, 2023: | | September 30, 2024 | | | December 31, 2023 | | Land | | $ | 3,577,662 | | | $ | 3,551,020 | | Buildings and improvements | | | 4,868,378 | | | | 4,787,963 | | Leasehold improvements | | | 3,667 | | | | 3,639 | | Vehicles | | | 285,609 | | | | 285,388 | | Furniture, fixtures and equipment | | | 3,014,683 | | | | 2,707,442 | | Computers and software | | | 203,215 | | | | 168,173 | | | | | 11,953,214 | | | | 11,503,625 | | Less: Accumulated depreciation and amortization | | | (1,377,286 | ) | | | (1,048,126 | ) | Total | | $ | 10,575,928 | | | $ | 10,455,499 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/360/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-7
| Name: |
us-gaap_PropertyPlantAndEquipmentDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
INTANGIBLE ASSETS
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| INTANGIBLE ASSETS |
|
| INTANGIBLE ASSETS |
NOTE 5 – INTANGIBLE ASSETS Goodwill and intangible, net assets consist of the following at September 30, 2024 and December 31, 2023: | | September 30, 2024 | | | December 31, 2023 | | License | | $ | 6,976,209 | | | $ | 6,876,169 | | Trade name / mark | | | 390,188 | | | | 392,197 | | Customer base | | | 602,204 | | | | 602,204 | | Software | | | 795,867 | | | | 158,787 | | | | | 8,764,468 | | | | 8,029,357 | | Less: Accumulated amortization | | | | | | | | | License | | | (805,555 | ) | | | (235,925 | ) | Trade name / mark | | | (36,997 | ) | | | (36,997 | ) | Customer base | | | (157,333 | ) | | | (110,160 | ) | Software | | | (67,520 | ) | | | (11,789 | ) | Subtotal | | | 7,697,064 | | | | 7,634,486 | | Goodwill | | | 49,697 | | | | 49,697 | | Total | | $ | 7,746,761 | | | $ | 7,684,183 | |
At September 30, 2024, the estimated aggregate amortization expense for intangible assets subject to amortization for each of the five succeeding fiscal years is as follows: Year | | Amount | | 2024 | | $ | 208,530 | | 2025 | | | 833,637 | | 2026 | | | 834,846 | | 2027 | | | 834,846 | | 2028 | | | 783,423 | | Thereafter | | | 3,846,582 | | Total | | $ | 7,341,864 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for goodwill and intangible assets. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/350-30/tableOfContent
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/350-20/tableOfContent
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
LOAN RECEIVABLE
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| LOAN RECEIVABLE |
|
| LOAN RECEIVABLE |
NOTE 6 – LOAN RECEIVABLE On October 30, 2021, the Company entered into an agreement for a ten-year loan with Medihelm SA to memorialize €4,284,521 ($4,849,221) in prepayments the Company had made. The prepayments to Medihelm SA had been made in accordance with the parallel export business, through which Medihelm supplied and would supply SkyPharm SA with branded pharmaceuticals. This business is no longer in place for the Company and thus the Company entered into this agreement with Medihelm SA in order for the outstanding amount to be settled. Interest is calculated at a rate of 5.5% per annum on a 360-day basis. Under the terms of the agreement, the Company is to receive 120 equal payments over the term of the loan. During the year ended December 31, 2023, the Company received €352,438 ($389,867) in principal payments such that as of December 31, 2023, the Company had a short-term receivable balance of $411,858 and a long-term receivable balance of $3,509,200 under this loan. The Company also received €223,914 ($249,552) in principal payments and €107,144 ($119,411) in interest payments during the nine-month period ended September 30, 2024. The Note is considered fully recoverable.
|
| X |
- References
+ Details
| Name: |
abcd_LoanReceivableabstract |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for claims held for amounts due to entity, excluding financing receivables. Examples include, but are not limited to, trade accounts receivables, notes receivables, loans receivables. Includes disclosure for allowance for credit losses. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/310-10/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482925/835-30-45-2
| Name: |
us-gaap_LoansNotesTradeAndOtherReceivablesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
INCOME TAXES
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| INCOME TAXES |
|
| INCOME TAXES |
NOTE 7 – INCOME TAXES The Company is incorporated in the United States of America and is subject to United States federal taxation. No provisions for income taxes have been made as the Company had no U.S. taxable income for the nine months ended September 30, 2024, and 2023. The Company’s Greek subsidiaries are governed by the income tax laws of Greece. The corporate tax rate in Greece is 22% on income reported in the statutory financial statements after appropriate tax adjustments. The Company’s United Kingdom subsidiaries are governed by the income tax laws of the United Kingdom. The corporate tax rate in the United Kingdom is 25% on income reported in the statutory financial statements after appropriate tax adjustments. As of September 30, 2024, and 2023, the Company’s effective tax rate differs from the U.S. federal statutory tax rate primarily due to a valuation allowance recorded against net deferred tax assets in in the United States and the United Kingdom. We regularly review deferred tax assets to assess their potential realization and establish a valuation allowance for portions of such assets to reduce the carrying value if we do not consider it to be more likely than not that the deferred tax assets will be realized. Our review includes evaluating both positive (e.g., sources of taxable income) and negative (e.g., recent historical losses) evidence that could impact the realizability of our deferred tax assets. As of September 30, 2024, and December 31, 2023, the Company has maintained a valuation allowance against all net deferred tax assets in the United States, Greece, and the UK. For the three months ended September 30, 2024, and 2023, the Company has recorded tax benefit in any jurisdiction where it is subject to income tax, in the amount of $0 and $65,873, and respectively, on the Condensed Consolidated Statements of Operations and Comprehensive Loss. No tax loss or benefit was recorded for the equivalent nine month periods.
|
| X |
- DefinitionThe entire disclosure for income tax. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 231
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482663/740-10-55-231
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12C
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12B
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477891/740-270-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479360/740-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/740/tableOfContent
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-14
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-21
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-17
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479360/740-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
CAPITAL STRUCTURE
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| CAPITAL STRUCTURE |
|
| CAPITAL STRUCTURE |
NOTE 8 – CAPITAL STRUCTURE Preferred Stock The Company is authorized to issue 100 million shares of preferred stock, of which 6,000,000 are designated as Series A convertible preferred stock. The preferred stock has a liquidation preference over the common stock and is non-voting. As of September 30, 2024 and December 31, 2023, no preferred shares were issued and outstanding. Major Rights & Preferences of Series A Preferred Stock On and effective October 4, 2021, the Company amended and restated its articles of incorporation (the “Amended and Restated Articles”) and filed a certificate of designation (the “COD”) for its Series A Preferred Stock (the “Series A Preferred Stock”) with the State of Nevada. The Amended and Restated Articles allow the Company’s Board of Directors the authority to authorize the issuance of preferred stock from time to time in one or more classes or series by resolution. On February 23, 2022, the Company filed Correction No. 1 to the COD. On July 28, 2022, the Company filed an Amendment to the COD with the State of Nevada to allow a holder to waive application of the Beneficial Ownership Limitation with respect to the conversion of Series A Preferred Stock. With respect to payment of dividends and distribution of assets upon liquidation, dissolution, or winding up of the Company, whether voluntary or involuntary, all shares of the Series A Preferred Stock will rank: (i) senior to all of the Company’s Common Stock and any other equity securities that the Company may issue in the future, (ii) equal to any other equity securities that the Company may issue in the future, the terms of which specifically provide that such equity securities are on parity or senior to the Series A Preferred Stock (“Parity Securities”), (iii) junior to all other equity securities the Company issues, the terms of which specifically provide that such equity securities rank senior to the Series A Preferred Stock, and (iv) junior to all of the Company’s existing and future indebtedness; without the prior written consent of the Majority Holders. In the event of any voluntary or involuntary liquidation, dissolution or winding up of the Company (a “Liquidation”), the Holders of shares of Series A Preferred Stock shall be first entitled to receive out of the assets of the Company available for distribution to its shareholders. Each Holder shall not be entitled to vote with holders of outstanding shares of Common Stock, voting together as a single class, with respect to any and all matters presented to the stockholders of the Company for their action or consideration, except as provided by law or as set forth in the COD. The holders of Series A Preferred Stock are entitled to receive dividends paid and distributions made to the holders of Common Stock to the same extent as if the holders of Series A Preferred Stock had converted such shares into shares of Common Stock. The Series A Preferred Stock was initially convertible into the Company’s Common Stock as determined by dividing the number of shares of Series A Preferred Stock to be converted by the lower of (i) $75.00 or (ii) 80% of the average volume weighted average price for the Company’s Common Stock for the five trading days immediately following the effectiveness of the registration statement concerning the shares (the “Conversion Price”). On June 14, 2022, the Conversion Price was reset to $15.54 per share. Each holder is entitled to receive dividends in shares of Series A Preferred Stock or cash determined based on the stated value of each Series A Preferred Stock at the dividend rate of 8.0% per year. For the year ended December 31, 2022, the Company recorded $372,414 as a deemed dividend in accordance with the Series A Preferred Stock cumulative dividend. As of December 31, 2022, the cumulative dividend has been recorded as mezzanine equity. Following, Mr. Siokas waiver of the right to receive the dividends on February 26, 2024, and the unanimous written consent of the Company’s Board of Directors on February 29, 2024, through which was resolved that the Company shall remove all accrued and unpaid dividends payable to the previous holders of Series A Preferred stock, the Company eliminated the total deemed dividend of $372,414 through retained earnings. Thus, the balance of mezzanine equity as of September 30, 2024, and December 31, 2023 is $0. The Series A Shares rank senior to all of the Company’s Common Stock and any other equity securities that the Company may issue in the future with respect to payment of dividends and distribution of assets upon liquidation, dissolution or winding up. While the Series A Shares are outstanding, the Company may not amend, alter or change adversely the powers, preferences or rights given to the Series A Shares, create, or authorize the creation of, any additional class or series of capital stock of the Company (or any security convertible into or exercisable for any class or series of capital stock of the Company), including any class or series of capital stock of the Company that ranks superior to or in parity with the Series A Shares, alter, amend, modify, or repeal its Articles of Incorporation or other charter documents in any manner that adversely affects any rights of the holders of Series A Shares, increase or decrease the number of authorized shares of Series A Shares, any agreement, commitment or transaction that would result in a Change of Control, any sale or disposition of any material assets outside of the ordinary course of business of the Company, any material change in the principal business of the Company, including the entry into any new line of business or exit of any current line of business, and circumvent a right or preference of the Series A Shares. Any holder of the Series A Shares shall have the right by written election to the Company to convert all or any portion of the outstanding Series A Shares. Immediately upon effectiveness of a registration statement registering for resale all of the Registrable Securities (as defined in the Registration Rights Agreement), all outstanding Series A Shares shall automatically convert into Common Stock, subject to certain beneficial ownership limitations. Treasury stock As of September 30, 2024 and December 31, 2023, the Company held 86,497 and 86,497, respectively, shares of our common stock at a cost of $917,159 and $917,159, respectively. Shares of our common stock that are repurchased are classified as treasury stock pending future use and reduce the number of shares outstanding used in calculating earnings per share. Cosmos may repurchase shares from time to time through open market purchases in accordance with applicable securities laws and other restrictions. The Company repurchased no shares of our common stock during the nine months ended September 30, 2024. The Company repurchased 71,000 shares of our common stock for $100,452 during the year ended December 31, 2023. The Company repurchased no shares of our common stock during the nine months ended September 30, 2024. On January 24, 2023 the Company announced that its Board of Directors has approved a share repurchase program with authorization to purchase up to $3 million of its common stock. Cosmos may repurchase shares from time to time through open market purchases in accordance with applicable securities laws and other restrictions. Common Stock The Company is authorized to issue 300 million shares of common stock. As of September 30, 2024 and December 31, 2023, the Company had 17,834,023 and 15,982,472 shares of our common stock issued, respectively, and 21,346,023 and 15,895,975 shares outstanding, respectively. Issuance of Common Stock During the nine-month period ended September 30, 2023 the Company issued 15,258 shares to a consultant for services rendered. The shares were valued and expensed on the date of issuance and are separately presented in the condensed consolidated statement of changes in stockholders’ equity and mezzanine as “Shares issued in lieu of cash”. During the nine months ended September 30, 2024, the Company raised additional equity funds through two Prospectus Supplements to its Registration Statement on Form S-3 (No. 333-267550) filed with the SEC on February 29 and March 7, 2024. More specifically, the Company sold 901,488 shares of common stock for gross proceeds of $648,893. Placement agent’s fees and other commissions amounted to $19,467 and thus the total net proceeds for the period were $629,426. On December 29, 2023, the Company had entered into a warrant exchange agreement (the “Warrant Exchange”) with an investor to reduce the exercise price of 2,437,063 warrants from $2.75 per share to $1.45 per shares as an inducement to exercise. The Company issued 1,487,000 shares of common stock, held 950,063 shares in escrow until the investor’s beneficial ownership limitation allows for the transfer of the escrow shares, and received gross cash proceeds of 3,533,741. The 950,063 shares were issued within the nine-month period ended September 30, 2024 but were already valued in the year ended December 31, 2023. On September 26, 2024, the Company entered into a Warrant Inducement Letter (the “Letter”) with an investor pursuant to which the Company issued 9,748,252 new warrants (the “New Warrants”) and reduced the exercise price of 4,874,126 warrant shares from $1.45 to $0.8701 to induce exercise and receive gross cash proceeds of $4,240,977 (the “Original Warrants”). The Company issued 2,332,000 shares of common stock, held 2,532,126 shares in escrow until the investor’s beneficial ownership limitation allows for the transfer of the escrow shares. Exercise of Warrants During the nine months ended September 30, 2024, the Company issued 2,332,000 shares of common stock upon the exercise of 2,332,000 warrants. The Company received gross proceeds of $4,240,977 upon exercise. The net proceeds after deducting legal, agent and escrow fees of $372,109 amounted to $3,868,868. The warrants were exercised following the Warrant Inducement letter the Company signed on September 26, 2024, through which their exercise price was reduced from $1.45 to $0.8701. Warrant Classification The Company determines the classification of its warrants upon issuance by identifying the instrument issued to determine if it is debt or equity classified. The Company determined its warrants meet the scope exception in ASC 815-10 and are equity classified because, (a) the warrant is indexed to the Company’s own stock, (b) require settlement in equity shares, and (c) the Company has enough authorized and unissued shares.
|
| X |
- References
+ Details
| Name: |
abcd_CapitalStructureTextBlock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
RELATED PARTY TRANSACTIONS
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| RELATED PARTY TRANSACTIONS |
|
| RELATED PARTY TRANSACTIONS |
NOTE 9 – RELATED PARTY TRANSACTIONS Doc Pharma S.A. Doc Pharma S.A is considered a related party to the Company due to the fact that the CEO of Doc Pharma is the wife of Grigorios Siokas, the Company’s CEO and principal shareholder, who also served as a principal of Doc Pharma S.A. in the past. Prepaid expenses and other current assets – related party As of September 30, 2024 and December 31, 2023, the Company had a prepaid balance of $6,393,642 and $4,347,184, respectively, to Doc Pharma related to purchases of inventory and pharmaceutical and nutraceutical licenses to be purchased. Accounts payable and accrued expenses - related party As of September 30, 2024 and December 31, 2023, the Company had an accounts payable balance to Doc Pharma of $72,968 and $34,217, respectively. Accounts receivable - related party The Company had a receivable balance of $2,448,517 and $2,386,721 from Doc Pharma S.A as of September 30, 2024, and December 31, 2023, respectively. Sales and Purchases During the three months ended September 30, 2024 and 2023, the Company purchased a total of $85,073 and $456,257 of products from Doc Pharma S.A., respectively. During the three months ended September 30, 2024, and 2023, the Company had $40,370 and $61,163 revenue from Doc Pharma, respectively. During the nine months ended September 30, 2024 and 2023, the Company purchased a total of $510,711 and $1,057,621 of products from Doc Pharma S.A., respectively. During the nine months ended September 30, 2024 and 2023, the Company had $581,862 and $43,107 revenue from Doc Pharma, respectively. Other Agreements On October 10, 2020, the Company entered into a contract manufacturer outsourcing (“CMO”) agreement with Doc Pharma whereby Doc Pharma is responsible for the development and manufacturing of pharmaceutical products and nutritional supplements according to the Company’s specifications based on strict pharmaceutical standards and good manufacturing practice (“GMP”) protocols as the National Organization for Medicines requires. The Company has the exclusive ownership rights for trading and distribution of its own branded nutritional supplements named “Sky Premium Life®”. The duration of the agreement is for five years, however, either party may terminate the agreement at any time giving six-month advance notice. Doc Pharma is exclusively responsible for supplying the raw materials and packaging required to manufacture the final product. However, they are not responsible for potential delays that may arise, concerning their import. Doc Pharma is also obligated to store the raw and packaging materials. The delivery of raw and packaging materials should be purchased at least 30 and 25 days, respectively, before the delivery date of the final product. The Manufacturer solely delivers the finished product to the Company. There is a minimum order quantity (“MoQ”) of 1,000 pieces per product code. Both parties have agreed that the Company will deposit 60% of the total cost upon agreement and assignment and 40% of the total cost including VAT charge upon the delivery date. The prices are indicative and are subject to amendments if the cost of the raw material or the production cost change. For the three months ended September 30, 2024 and 2023, the Company has purchased €34,350 ($38,096) and €418,577 ($455,586) respectively, in inventory related to this agreement. For the nine months ended September 30, 2024 and 2023, the Company has purchased €161,108 ($175,123) and €967,785 ($1,048,557) respectively, in inventory related to this agreement. On May 17, 2021, Doc Pharma and the Company entered into a Research and Development (“R&D”) agreement whereby Doc Pharma will be responsible for the research, development, design, registration, copy rights and licenses of 250 nutritional supplements for the final products called Sky Premium Life®. These products will be sold in Greece and abroad. The total cost of this project will be €1,425,000 plus VAT and will be done over three phases as follows: Design & Development (€725,000); Control and Product Manufacturing (€250,000) and Clinical Study and Research (€450,000). SkyPharm has bought a total of as of 81 licenses at value of €554,500 ($593,204) which is 38.91% of the total cost, as of December 31, 2022. During the year ended December 31, 2023, 24 additional licenses were purchased at value of €475,014 ($525,461). During the three and nine months ended September 30, 2024, no additional licenses were purchased. The agreement will terminate on December 31, 2025. Purchase of branded pharmaceuticals On June 28, 2023, the Company approved the purchase of five proprietary and innovative branded pharmaceuticals with significant market presence and material profit contribution from Zakalia Ltd., the parent company of Doc Pharma, for €1,800,000 ($1,965,600). The transaction was settled on a non-cash basis through the reduction of an equivalent amount of prepaid expense balances the Company held with Doc Pharma. The purchased branded pharmaceuticals are presented in “Goodwill and intangible assets, net” on the accompanying consolidated balance sheets. On December 29, 2023, the Company approved the purchase of 19 additional licenses from DocPharma, of a total value of €3,200,000 ($3,539,840). This transaction was also settled on a non-cash basis through the reduction of an equivalent amount of prepaid expense balances the Company held with Doc Pharma. Loans receivable - related party The balance of prepaid expenses due Doc Pharma as of December 31, 2022, had increased to €7,103,706 ($7,599,545), which was mainly attributable to the prepayments SkyPharm S.A. made in accordance with the CMO agreement and the extensive orders and sales of the SPL products the Company expects to achieve within 2023, mainly through its Amazon channels in the UK, Singapore, Canada and other countries. However, as the benefit from a significant portion of the prepaid balance would not have been realized within a 12-month period, the Company opted to secure a portion of the outstanding prepaid balance through a loan agreement. SkyPharm S.A. (the “Lender”) entered into a loan agreement with Doc Pharma (the “Borrower”) for €4,000,000 ($4,279,200), all of which was financed through the outstanding prepaid balance. The duration of the loan is for a 10-year period up to December 31, 2032 (the “Maturity Date”). The loan bears a fixed interest rate of 5.5% payable on a monthly basis and will be repayable in 120 equal instalments of €33,333.33 ($37,150). The loan may be prepaid anytime during its duration in full or partially based on the Company’s product requirements and other factors, without Doc Pharma incurring any prepayment penalty. As of September 30, 2024 and December 31, 2023, the loan had a current portion of €400,000 ($445,800) and €400,000 ($442,480), and a non-current portion of €2,900,000 ($3,232,050), and €3,200,000 ($3,539,840), respectively, which is classified as "Loans receivable – related party" on the accompanying consolidated balance sheets. During the nine months ended September 30, 2024, the Company received €300,000 ($334,350) in principal repayments, and €121,550 ($135,467) of interest repayments. Additionally, during the nine months ended September 30, 2024, the Company recorded €143,000 ($155,440) as interest income relating to this loan. Cana Laboratories Holding Limited Cana was considered a related party as the Company had signed a binding letter of intent and an SPA for the acquisition of Cana. The acquisition was completed on June 30, 2023 according to the SPA signed on May 31, 2023. Thus, all balances between the Company and Cana were eliminated upon consolidation as of December 31, 2023. The Secured Promissory Note discussed below was included in consideration transferred upon acquisition. Loans receivable - Related Party - Long Term On February 28, 2023 (Issue Date), the Company signed a Secured Promissory Note with Cana Laboratories Holdings (Cyprus) Limited (the “Holder”), whereby the Holder borrowed the sum of €4,100,000 ($4,457,520) from the Company. Interest on the Principal Amount under this Note shall accrue at a rate equal to Five Percent (5%) plus 1 month LIBOR per annum (5.47% as of December 31, 2023). The maturity date (“Maturity Date”) of this Note shall be five (5) years from the Issue Date. The Principal Amount, as well as all accrued interest shall be due and payable on the Maturity Date. Following the completion of Cana’s acquisition on June 30, 2023 the balance of the Note was eliminated on a consolidated level. Panagiotis Kozaris Panagiotis Kozaris is considered a related party due to the fact that he is a former General operational manager and current employee of Cosmofarm S.A. Prepaid Expenses and Other Current Assets - Related Party From time to time the Company purchases back shares that Panagiotis Kozaris owns and records them as treasury shares. The Company pays Panagiotis Kozaris in advance for the shares owned and obtains the shares upon execution of a cumulative stock-purchase agreement (“SPA”). During the three months ended September 30, 2024 and 2023, the Company paid Panagiotis Kozaris an additional sum of $0 and $51,159 respectively for shares owned, however, no SPA for these funds has been executed as of September 30, 2024. The Company intends to execute a cumulative SPA for these amounts during 2024. The total balances owed of $194,215 and $194,215 are included in “Prepaid expenses and other current assets - related party”, on the accompanying consolidated balance sheets as of September 30, 2024 and December 31, 2023, respectively. Basotho Investment Limited Basotho Investment Limited is considered a related party once Panagiotis Kozaris (former general operational manager and current employee of Cosmofarm SA) is one of its directors. General and administrative expenses On November 21, 2023, the Company issued 120,000 shares of common stock to Basotho Investment Limited for services rendered. The fair value of these shares for the period ended December 31, 2023 was $10,300, which was recorded as general and administrative expense. The fair value of the shares vested for the nine-month period ended September 30, 2024 was $92,700, which was recorded as general and administrative expense. Maria Kozari Maria Kozari is considered a related party to the Company due to the fact that she is the daughter of Panagiotis Kozaris, a former Operational General Manager and current employee of Cosmofarm S.A. Accounts Receivable - Related Party During 2021, the Company, through its subsidiary, Cosmofarm SA, commenced a partnership with a pharmacy called “Pharmacy & More”, owned by Maria Kozari. The transactions with the respective pharmacy were in Cosmofarm’s normal course of business, however, a more flexible credit policy was allowed as the pharmacy was new and needed to be established in the market. During the three and nine months ended September 30, 2024 and 2023 the Company’s net sales to Pharmacy & More amounted to $113,161 and $122,969 and $310,126 and $359,760 respectively. As of September 30, 2024 and December 31, 2023 the Company’s outstanding receivable balance due from the pharmacy amounted to $1,123,835 (€1,203,739) and $1,142,402 (€1,032,726), respectively, and are included in “Accounts receivable - related party”, on the accompanying consolidated balance sheets. The Company plans to acquire Pharmacy & More within fiscal year 2024. Upon acquisition, the Company intends to offset the outstanding receivable balance with the corresponding purchase price and additionally plans to make Pharmacy & More the first shop-in-shop of its own branded line of nutraceutical products, Sky Premium Life® (SPL). Other Related Parties The Company has the following balances as of September 30, 2024: a) a balance of $731,000 relating to unpaid salaries and bonuses due to Grigorios Siokas, the CEO of the Company and $188,000 due to George Terzis, the CFO of the Company, classified as "Accounts payable and accrued expenses - related party" in the Company’s condensed consolidated balance sheets, b) a net payable balance of $29,832 due to Konstantinos Gaston Kanaroglou, former manager and current employee of the Company’s wholly owned subsidiary Cana, classified as " Accounts payable and accrued expenses - related party" in the Company’s condensed consolidated balance sheets. Additionally, the Company had the following balances as of December 31, 2023: a) a balance of $98,000 relating to unpaid salaries and bonuses due to George Terzis, the CFO of the Company, classified as "Accounts payable and accrued expenses - related party" in the Company’s consolidated balance sheets, b) a net payable balance of $85,332 due to Konstantinos Gaston Kanaroglou, former manager and current employee of the Company’s wholly owned subsidiary Cana, classified as " Accounts payable and accrued expenses - related party" in the Company’s consolidated balance sheets. Notes Payable – Related Party A summary of the Company’s related party notes payable as of September 30, 2024 and December 31, 2023 is presented below: | | September 30, 2024 | | | December 31, 2023 | | | | | | | | | Beginning Balance | | $ | 11,283 | | | $ | 10,912 | | Payments | | | - | | | | - | | Foreign currency translation | | | 85 | | | | 371 | | Ending Balance | | $ | 11,368 | | | $ | 11,283 | |
Dimitrios Goulielmos Dimitris Goulielmos was the Company’s former CEO and a Director of the Company. On November 21, 2014, the Company entered into an agreement with Dimitrios Goulielmos, as amended on November 4, 2016. Pursuant to the amendment, this loan has no maturity date and is non-interest bearing. As of September 30, 2024 and December 31, 2023, the Company had a principal balance of €10,200 ($11,368) and €10,200 ($11,283), respectively. The above balances are adjusted for the foreign currency rate as of the balance sheet date. For the nine months ended September 30, 2024, the Company recorded a foreign currency translation loss of $85. Loans Payable – Related Party A summary of the Company’s related party loans payable as of September 30, 2024 and December 31, 2023 is presented below: | | September 30, 2024 | | | December 31, 2023 | | | | | | | | | Beginning balance | | $ | 13,257 | | | $ | 12,821 | | Proceeds | | | 18,344 | | | | - | | Payments | | | (8,918 | ) | | | - | | Foreign currency translation | | | (1,525 | ) | | | 436 | | Ending balance | | $ | 21,158 | | | $ | 13,257 | |
Grigorios Siokas From time to time, Grigorios Siokas loans the Company funds in the form of non-interest bearing, no-term loans. As of September 30, 2024, the Company had an outstanding principal balance under these loans of $21,158 in loans payable to Grigorios Siokas. As of December 31, 2023, the Company had an outstanding principal balance of $13,257 related to this payable. The above balances are adjusted for the foreign currency rate as of the balance sheet date. For the nine months ended September 30, 2024, the Company recorded a gain of $1,525. Except as set forth above, we have not entered into any material transactions with any director, executive officer, and promoter, beneficial owner of five percent or more of our common stock, or family members of such persons.
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
LINES OF CREDIT
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| LINES OF CREDIT |
|
| LINES OF CREDIT |
NOTE 10 – LINES OF CREDIT A summary of the Company’s lines of credit as of September 30, 2024 and December 31, 2023, is presented below: | | September 30, 2024 | | | December 31, 2023 | | National | | $ | 3,011,102 | | | $ | 3,918,523 | | Alpha | | | 1,030,943 | | | | 1,130,140 | | Pancreta | | | 1,560,803 | | | | 1,122,210 | | EFG | | | 386,577 | | | | 459,400 | | Ending balance | | $ | 5,989,425 | | | $ | 6,630,273 | |
The Company has three lines of credit with the National Bank of Greece, which are renewed annually. The three lines have interest rates of 6.00% (the "National Bank LOC"), 3.6% (the "COSME 2 Facility"), and 3.6% plus the six-month Euribor rate and any contributions currently in force by law on certain lines of credit (the "COSME 1 Facility"). The maximum borrowing allowed for the 6% line of credit was $3,315,638 and $3,290,945 as of September 30, 2024 and December 31, 2023, respectively. The outstanding balance of the facility was $2,102,765 and $2,829,828, as of September 30, 2024 and December 31, 2023, respectively. The cumulative maximum borrowing allowed for the COSME 1 Facility and COSME 2 Facility (collectively, the "Facilities") was $1,114,500 and $1,106,200 as of September 30, 2024 and December 31, 2023, respectively. The outstanding balance of the Facilities was $943,466 and $1,099,255 as of September 30, 2024 and December 31, 2023, respectively. The Company maintains a line of credit with Alpha Bank of Greece ("Alpha LOC"), which is renewed annually and has a current interest rate of 6.00%. The maximum borrowing allowed was $1,114,500 and $1,106,200 as of September 30, 2024 and December 31, 2023, respectively. The outstanding balance of the Alpha LOC was $1,030,944 and $1,130,141, as of September 30, 2024 and December 31, 2023, respectively. The Company holds a line of credit with Pancreta Bank ("Pancreta LOC"), which is renewed annually and has a current interest rate of 4.10%. The maximum borrowing allowed as of September 30, 2024 and December 31, 2023 was $1,549,155 and $1,537,618, respectively. The outstanding balance of the Pancreta LOC as of September 30, 2024 and December 31, 2023 was $1,560,802 and $1,122,210, respectively. The Company maintains a line of credit with EGF ("EGF LOC"), which is renewed annually and has a current interest rate of 4.49% plus 3-month Euribor. The maximum borrowing allowed as of September 30, 2024 and December 31, 2023 was $445,800 and $459,400, respectively. The outstanding balance of the EGF LOC as of September 30, 2024 and December 31, 2023 was $386,577 and $459,400, respectively. Under the aforementioned line of credit agreements, the Company is required to maintain certain financial ratios and covenants. As of September 30, 2024, and December 31, 2023, the Company was in compliance with these ratios and covenants. All lines of credit are guaranteed by customer receivable checks, which are a type of factoring in which postponed customer checks are assigned by the Company to the bank, in order to be financed at an agreed upon rate. Interest expense on the Company’s outstanding lines of credit balances for the three and nine months ended September 30, 2024 and 2023, was $89,868 and $37,536, and 275,246 and $204,654, respectively.
|
| X |
- References
+ Details
| Name: |
abcd_LineOfCreditFacilitiesDisclosureTextBlock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LineOfCreditFacilityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
NOTES PAYABLE
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| NOTES PAYABLE |
|
| NOTES PAYABLE |
NOTE 11 – NOTES PAYABLE A summary of the Company’s third-party debt as of and for the nine months ended September 30, 2024, and the year ended December 31, 2023 is presented below: September 30, 2024 | | Trade Facility | | | Third Party | | | COVID Loans | | | Total | | Beginning balance, December 31, 2023 | | $ | 1,908,195 | | | $ | 2,511,148 | | | $ | 186,884 | | | $ | 4,606,227 | | Proceeds | | | - | | | | 445,800 | | | | - | | | | 445,800 | | Payments | | | (334,350 | ) | | | (481,254 | ) | | | (19,073 | ) | | | (834,677 | ) | Conversion of debt | | | - | | | | - | | | | - | | | | - | | Recapitalized upon debt modification | | | - | | | | - | | | | - | | | | - | | Accretion of debt and debt discount | | | - | | | | - | | | | - | | | | - | | Foreign currency translation | | | 14,318 | | | | 32,613 | | | | 3,691 | | | | 50,622 | | Ending balance, September 30, 2024 | | | 1,588,163 | | | | 2,508,307 | | | | 171,502 | | | | 4,267,972 | | Notes payable - long-term | | | (1,086,638 | ) | | | (1,416,802 | ) | | | (142,183 | ) | | | (2,645,623 | ) | Notes payable - short-term | | $ | 501,525 | | | $ | 1,091,505 | | | $ | 29,319 | | | | 1,622,349 | |
December 31, 2023 | | Trade Facility | | | Third Party | | | COVID Loans | | | Total | | Beginning balance, December 31, 2022 | | $ | 3,305,532 | | | $ | 1,505,078 | | | $ | 207,377 | | | $ | 5,017,987 | | Proceeds | | | - | | | | 1,082,231 | | | | - | | | | 1,082,231 | | Payments | | | (1,155,310 | ) | | | (415,557 | ) | | | (27,027 | ) | | | (1,597,894 | ) | Oher additions | | | - | | | | 317,880 | | | | - | | | | 317,880 | | Debt forgiveness | | | (306,637 | ) | | | - | | | | - | | | | (306,637 | ) | Foreign currency translation | | | 64,610 | | | | 21,516 | | | | 6,534 | | | | 92,660 | | Ending balance, December 31, 2023 | | | 1,908,195 | | | | 2,511,148 | | | | 186,884 | | | | 4,606,227 | | Notes payable – long-term | | | (1,327,440 | ) | | | (1,549,768 | ) | | | (158,133 | ) | | | (3,035,341 | ) | Notes payable - short-term | | $ | 580,755 | | | $ | 961,380 | | | $ | 28,751 | | | $ | 1,570,886 | |
Our outstanding debt as of September 30, 2024 is repayable as follows: | | | September 30, 2024 | | 2025 | | $ | 1,622,349 | | 2026 | | | 1,717,738 | | 2027 | | | 420,633 | | 2028 | | | 353,961 | | 2029 and thereafter | | | 153,291 | | Total debt | | | 4,267,972 | | Less: notes payable - current portion | | | (1,622,349 | ) | Notes payable - long term portion | | $ | 2,645,623 | |
Trade Facility Agreements On May 12, 2017, SkyPharm entered into a Trade Finance Facility Agreement (the “TFF”) with Synthesis Structured Commodity Trade Finance Limited (the “Lender”) as amended on November 16, 2017, and May 16, 2018. On October 17, 2018, the Company entered into a further amended agreement with Synthesis whereby the current balance on the TFF as of October 1, 2018, which was €4,866,910 ($5,629,555) and related accrued interest of €453,094 ($524,094) would be split into two principal balances of Euro €2,000,000 ($2,316,000), (the "EURO Loan") and USD $4,000,000 (the "USD Loan"). Interest on both the EURO Loan and USD Loan commenced on October 1, 2018, at 6% per annum plus one-month Euribor (3.90% as of December 31, 2023), and 6% plus one-month LIBOR (fully paid as of December 31, 2023), respectively. On December 30, 2020, the Company transferred the EURO Loan to a new third-party lender. The terms remained the same except interest accrues at 5.5% per annum plus one-month Euribor (3.87% as of December 31, 2023). The principal was scheduled to be repaid in a total of five quarterly installments beginning October 31, 2021 of €50,000 ($54,600) each with a final repayment of €1,800,000 ($1,965,600) Euro payable on October 31, 2022. On March 3, 2022, the Company entered into a modification agreement to extend the maturity date to January 10, 2023 and payments under the USD Loan. During June 2022, the Company agreed with the Lender to postpone the repayment of an installment of $500,000 due on June 30, 2022 (based on the modification agreement signed on March 3, 2022) until January 2023. During September 2022, the Company entered into an agreement with the Lender to postpone the repayment of the outstanding balance on the USD Loan of $3,950,000, plus unpaid accrued interest until January 2023. The Company capitalized fees paid upon modification of €200,000 ($221,060) that are being amortized over the life of the loan. The Company incurred non-cash interest expense of $200,000 during the year ended December 31, 2022 concerning the above capitalized fees. On December 22, 2022, SkyPharm signed an agreement for the extension of the payments and an increase in interest rate due under the EURO Loan that was extended to be repaid with a balloon payment now due on October 31, 2025. This extension was agreed upon in writing on December 22, 2022, with a retroactive modification date to October 31, 2022 (the original maturity date). As of December 31, 2023 the Company had an outstanding principal balance of €1,725,000 ($1,908,195), of which $1,327,440 is classified as ''Notes payable - long term portion" on the consolidated balance sheets. As of December 31, 2023, the Company had accrued $161,274 in interest expense related to these agreements. The Company repaid €300,000 ($334,350) of the EURO Loan during the nine months ended September 30, 2024. As of September 30, 2024, the Company had an outstanding principal balance of €1,425,000 ($1,588,163), of which $1,086,638 is classified as ''Notes payable - long term portion" on the consolidated balance sheets. For the three and nine months ended September 30, 2024, the Company had accrued $29,365 and $110,170, respectively, in interest expense related to these agreements. June 23, 2020 Debt Agreement On June 23, 2020, the Company’s subsidiary, Cosmofarm, entered into an agreement with the National Bank of Greece S.A. (the “Bank”) to borrow a maximum of €500,000 ($611,500). The note has a maturity date of sixty (60) months from the date of the first disbursement, which includes a grace period of nine months. The total amount of the initial proceeds was received in 3 equal monthly installments. The note is interest bearing from the date of receipt and is payable every three months at an interest rate of 3.06% plus 3-month Euribor (3.47% as of September 30, 2024). The outstanding balance was €117,647 ($131,118) and €205,882 ($227,747) as of September 30, 2024 and December 31, 2023, respectively, of which $0 and $97,606 was classified as “Notes payable - long-term portion”, on the accompanying condensed consolidated balance sheets. During the nine months ended September 30, 2024, the Company repaid €88,235 ($98,338) of the principal balance. June 24, 2020 Debt Agreement On June 24, 2020, the Company’s subsidiary, Decahedron, received a loan £50,000 ($68,310) from the United Kingdom government. The loan has a ten-year maturity and bears interest at a rate of 2.5% per annum beginning 12-months after the initial disbursement, which was on July 10, 2020. The Company may prepay this loan without penalty at any time. As of December 31, 2023, the principal balance was £40,858 ($52,066). As of September 30, 2024, the principal balance was £38,320 ($51,345). November 19, 2020 Debt Agreement On November 19, 2020, the Company entered into an agreement with a third-party lender in the principal amount of €500,000 ($611,500). The note matures on November 18, 2025 and bears an annual interest rate, based on a 360-day year, of 3% plus 0.6% plus 6-month Euribor when Euribor is positive (3.35% as of September 30, 2024). The principal is to be repaid in 18 quarterly installments of €27,778 ($30,333). During the nine months ended September 30, 2024, the Company repaid €88,333 ($92,875) of the principal. As of September 30, 2024 and December 31, 2023, the Company has accrued interest of €5,434 ($6,057) and €11,191 ($12,379) related to this note and a principal balance of €138,889 ($154,792) and €222,222 ($245,822), of which $30,958 and $122,911 is classified as "Notes payable - long term portion" on the accompanying condensed consolidated balance sheets. July 30, 2021 Debt Agreement On July 30, 2021, the Company entered into an agreement with a third-party lender in the principal amount of €500,000 ($578,850). The note matures on August 5, 2026 and bears an annual interest rate that applies to 60% of the principal of the note that is based on a 365-day year, of 5.84% plus 3-month Euribor when Euribor is positive (3.47% as of September 30, 2024). Pursuant to the terms of the agreement, there is a nine-month grace period for principal repayment during which interest is accrued. The principal is to be repaid in 18 quarterly installments of €27,778 commencing three months from the end of the grace period. During the nine months ended September 30, 2024, the Company repaid €81,891 ($91,267) of the principal. As of September 30, 2024 and December 31, 2023, the Company had accrued interest of €15,328 ($17,083) and €10,905 ($12,063), respectively, and principal of €262,662 ($281,338) and €235,009 ($261,918), respectively, of which $134,929 and $227,065 is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets. June 9, 2022 Debt Agreement On June 9, 2022 the Company entered into an agreement with a third-party lender in the principal amount of €320,000 ($335,008), the “Note”. The Note matures on June 16, 2027 and bears an annual interest rate of 3.89% plus an additional rate of 0.60%, plus the 3-month Euribor (3.47% as of September 30, 2024). Pursuant to the agreement, there is a twelve-month grace period for principal repayment during which interest is accrued. The principal is to be repaid in 16 equal quarterly installments of €20,000 commencing on June 30, 2023. During the nine months ended September 30, 2024, the Company repaid €60,000 ($66,870) of the principal. As of September 30, 2024 and December 31, 2023, the Company has accrued interest of €4,673 ($5,208) and €11,043 ($12,215), respectively, and an outstanding balance of €200,000 ($222,900) and €260,000 ($287,612) of which $133,740 and $204,322, respectively, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets. July 14, 2023 Debt Agreement On July 14, 2023 the Company entered into an agreement with a third-party lender in the principal amount of €1,000,000 ($1,123,700), the “Note”. The Note matures on July 31, 2028 and bears an annual interest rate of 2.46% plus the 3-month Euribor (3.47% as of September 30, 2024). Pursuant to the agreement, there is a nine-month grace period for interest and principal repayment. The principal is to be repaid in 18 equal quarterly installments of €55,556 commencing on May 2, 2024. During the nine months ended September 30, 2024, the Company repaid €108,633 ($58,179) of the principal. As of September 30, 2024, and December 31, 2023, the Company has accrued interest of €7,845 ($8,743) and €19,820 ($21,925), respectively. As of September 30, 2024, and December 31, 2023 the Company an outstanding balance of €869,067 ($968,575) and €977,700 ($1,081,532), of which $720,908 and $897,165, respectively, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets. July 29, 2024 Debt Agreement On July 29, 2024 the Company entered into an agreement with a third-party lender in the principal amount of €400,000 ($432,760), the “Note”. The Note matures on July 31, 2029 and bears an annual interest rate of 2.58% plus the 3-month Euribor (3.47% as of September 30, 2024). Pursuant to the agreement, there is a six-month grace period for principal and interest repayment. The principal is to be repaid in 18 equal quarterly installments of €22,222 commencing on April 30, 2025. During the nine months ended September 30, 2024, the Company repaid no principal and had not accrued any interest. As of September 30, 2024, and December 31, 2023 the Company an outstanding balance of €400,000 ($445,800) and €0 ($0), of which $396,367 and $0, respectively, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets. COVID-19 Loans On May 12, 2020, the Company’s subsidiary, SkyPharm, was granted and on May 22, 2020 received a €300,000 ($366,900) loan from the Greek government. The loan will be repaid in 40 equal monthly installments beginning on July 29, 2022. As a condition to the loan, the Company was required to retain the same number of employees until October 31, 2020. As of December 31, 2023, the principal balance was $134,818. During the nine months ended September 30, 2024, the Company repaid €14,063 ($15,673) of the principal balance. The outstanding balance as of September 30, 2024 is €107,813 ($120,157) of which $99,260, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheet. Cloudscreen Promissory Note On January 23, 2024 the Company entered into an agreement with a third-party in the principal amount of €300,000 ($324,870), the “Promissory Note”. The Promissory Note matures on March 25, 2025 and is interest free. This Note is being given in connection with the Closing of the Asset Purchase, Sale and Transfer Agreement dated as of October 9, 2023 and as amended from time to time pursuant to which the Company agreed to purchase from the third-party a drug repurposing Artificial Intelligence “AI” powered platform known as “Cloudscreen®” (refer to Note 2, section “Acquisition accounting”). The principal is to be repaid in 15 equal monthly installments of €20,000 commencing on January 25, 2024. During the 9 months ended September 30, 2024, the Company repaid €10,000 ($10,830) of the principal and recorded a foreign currency loss of $16,155. As of September 30, 2024, and December 31, 2023 the Company had an outstanding balance of $323,205 and $317,880 of which $0 and $0, respectively, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets. Distribution and Equity Agreement As discussed in Note 3 above, the Company entered into a Distribution and Equity Acquisition Agreement with Marathon. The Company was appointed the exclusive distributor of the Products (as defined) initially throughout Europe and on a non-exclusive basis wherever else lawfully permitted. As consideration for its services, Company received: (a) a 33 1/3% equity interest or 5 million shares in Marathon as partial consideration for the Company’s distribution services; and (b) received cash of CAD $2,000,000, subject to repayment in Common Shares of the Company if it fails to meet certain performance milestones. The Company is entitled to receive an additional CAD $2,750,000 upon the Company’s receipt of gross sales of CAD $6,500,000 and an additional CAD $2,750,000 upon receipt of gross sales of CAD $13,000,000. As discussed in Note 3, the Company attributed no value to the shares received in Marathon pursuant to (a) above. In relation to the CAD $2 million cash received noted in (b) above, the Company accounted for its obligation to issue a variable number of the Company’s Common Shares as Share-settled debt obligation in accordance with ASC 480 measured at fair value or the settlement amount of $1,554,590 (CAD $2 million). If settlement had occurred on December 31, 2022, the Company would have been required to issue 420,471 common shares to settle its debt obligation. The Company could be obligated to potentially issue an unlimited number of common shares to settle its Share-settled debt obligation. On March 20, 2023, the Company’s legal counsel provided notice to Marathon Global Inc, that Cosmos terminated the Equity agreement dated on March 19, 2018 pursuant to Section 3.2 and that termination is effective thirty days from the date of the letter. None of the above loans were made by any related parties.
|
| X |
- References
+ Details
| Name: |
us-gaap_DebtDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for information about short-term and long-term debt arrangements, which includes amounts of borrowings under each line of credit, note payable, commercial paper issue, bonds indenture, debenture issue, own-share lending arrangements and any other contractual agreement to repay funds, and about the underlying arrangements, rationale for a classification as long-term, including repayment terms, interest rates, collateral provided, restrictions on use of assets and activities, whether or not in compliance with debt covenants, and other matters important to users of the financial statements, such as the effects of refinancing and noncompliance with debt covenants. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/470/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482925/835-30-45-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1C
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1C
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1C
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1I
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1I
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1I
| Name: |
us-gaap_DebtDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
LEASES
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| LEASES |
|
| LEASES |
NOTE 12 – LEASES The Company has various operating and finance lease agreements with terms up to 10 years, for various types of property and equipment (such as office space and vehicles) etc. Some leases include options to purchase, terminate or extend for one or more years. These options are included in the lease term when it is reasonably certain that the option will be exercised. Leases with an initial term of 12 months or less are not recorded on the balance sheet; we recognize lease expense for these leases on a straight-line basis over the lease term. Operating Leases The Company’s weighted-average remaining lease term relating to its operating leases is 4.08 years, with a weighted-average discount rate of 6.74%. The following table presents information about the amount, timing and uncertainty of cash flows arising from the Company’s operating leases as of September 30, 2024: Maturity of Operating Lease Liability | | | | 2024 | | | 82,319 | | 2025 | | | 243,175 | | 2026 | | | 179,151 | | 2027 and thereafter | | | 300,301 | | Total undiscounted operating lease payments | | $ | 804,946 | | Less: Imputed interest | | | (95,366 | ) | Present value of operating lease liabilities | | $ | 709,580 | |
The Company incurred lease expense, due to amortization of operating lease right-of-use assets, of $76,229 and $50,690 and $235,659 and $158,407, which was included in “General and administrative expenses,” for the three and nine months ended September 30, 2024 and 2023, respectively. Finance Leases The Company’s weighted-average remaining lease term relating to its finance leases is 1.19 years, with a weighted-average discount rate of 6.74%. The following table presents information about the amount, timing and uncertainty of cash flows arising from the Company’s finance leases as of September 30, 2024: Maturity of Lease Liability | | | | 2024 | | | 9,039 | | 2025 | | | 12,892 | | 2026 | | | 3,712 | | Total undiscounted finance lease payments | | $ | 25,643 | | Less: Imputed interest | | | (910 | ) | Present value of finance lease liabilities | | $ | 24,733 | |
The Company had financing cash flows used in finances leases of $9,765 and $41,094 and $27,118 and $118,847 for the three and nine months ended September 30, 2024 and 2023, respectively. The Company incurred interest expense on its finance leases of $457 and $6,920 and interest expense of $1,765 and $20,629 which was included in “Interest expense”, for the three and nine months ended September 30, 2024 and 2023, respectively. The Company incurred amortization expense on its finance leases of $7,589 and $35,452 and amortization expense of $22,507 and $102,549 which was included in “Depreciation and amortization expense,” for the three and nine months ended September 30, 2024 and 2023, respectively.
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for lessee entity's leasing arrangements including, but not limited to, all of the following: (a.) The basis on which contingent rental payments are determined, (b.) The existence and terms of renewal or purchase options and escalation clauses, (c.) Restrictions imposed by lease agreements, such as those concerning dividends, additional debt, and further leasing. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/840/tableOfContent
| Name: |
us-gaap_LeasesOfLesseeDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
OTHER LIABILITIES
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| OTHER LIABILITIES |
|
| OTHER LIABILITIES |
NOTE 13 – OTHER LIABILITIES The Company’s other liabilities include but are not limited to liabilities to local tax authorities, fines and payroll taxes, which comprise the largest portion of the balance as of September 30, 2024. The Company’s Greek subsidiaries have $1,812,919 in settled tax liabilities payable to the tax authorities in installments and $1,799,431 in payroll and other tax related current liabilities. Moreover, we have recorded a provision relating to the unaudited tax years of our subsidiary SkyPharm SA, of $644,779 and a provision for staff leaving compensation, based on the corresponding actuarial reports, of $411,732. Additionally, we have received prepayments from our customers of $451,575 and recorded accrued sales discounts of $407,725 included in “Other current liabilities” as of September 30, 2024. We classify the liabilities payable within the twelve months following the balance sheet date in “Other current liabilities” and the remaining balance is included in “Other Liabilities”.
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherLiabilitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for other liabilities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 405
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/405/tableOfContent
| Name: |
us-gaap_OtherLiabilitiesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
COMMITMENTS AND CONTINGENCIES
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| COMMITMENTS AND CONTINGENCIES |
|
| COMMITMENTS AND CONTINGENCIES |
NOTE 14 – COMMITMENTS AND CONTINGENCIES Legal Matters From time to time, the Company may be involved in litigation relating to claims arising out of the Company’s operations in the normal course of business. As of September 30, 2024, the following litigations were pending. None of the below is expected to have a material financial or operational impact. On July 22, 2015, the National Medicines Agency approved the license of wholesale sale of pharmaceutical products under the name SkyPharm SA with set validity at five years and an expiration date of July 22, 2020. Subsequently, SkyPharm on June 15, 2020, legally and timely submitted the application for renewal of the wholesale license of pharmaceutical products to the National Medicines Agency. The National Medicines Agency did not respond, therefore the Company asked for an immediate decision on the renewal. Two months after the filing of the no. 3459 / 15.01.2021 letter and almost nine months after the no. 627615.06.2020 Company application for the renewal, the National Medicines Agency replied by rejecting the renewal request on March 9, 2021 (ref. 62769 / 20-25.02.2021). In addition, document No. 127351-16.12.2021 of EOF (Greek National Medicines Organization) to SkyPharm states that after an inspection of EOF at the premises of Doc Pharma, we did not have a wholesale license in violation of article 106 par. 1b and par. 1c of the ministerial decision D.YG3a / GP.32221 / 29-4-2019. The National Medicines Agency imposed a fine of €15,000 ($16,214) on SkyPharm for the above case, which was included in “General and administrative expenses” on the accompany statement of operations and comprehensive loss for the twelve-month ended December 31, 2023. There has been a payment request by the Greek court, which relates to a fine arising from Cosmofarm’s tax audit for financial year 2014. The law with no. 483/16.12.2020 was used by the court against Cosmofarm (the “defendant”). The defendant appealed against the decision using the law with no.11541/09.03.2021. This appeal was dismissed after 120 days from its submission to the court. Additionally, there had been an obligation for payment of additional tax and fines related to this matter in the amount of €91,652 ($99,644), which the defendant has already settled. However, the defendant has claimed back the respective amount through appeal. As of September 30, 2024, the trial is still pending. On January 25, 2023, a criminal case of dishonored checks against Cosmofarm’s customer Filippou, was heard at the Z’ Three-Member Misdemeanor Court of Athens, which was postponed to November 27, 2023, when the defendant was tried and found guilty. On January 26, 2023, the appeal of the Company against Eleutheria Drakopoulou and decision 1389/2021of the Single-Member Court of First Instance of Athens was heard at the Athens Court of Appeal. The appeal was partially accepted. The Court ordered the return of the fee to the appellants, dismissed the action against the third defendant, Kozaris and accepted the action as regards the first and the second defendants (Kastrantas & Cosmofarm). On October 23, 2023, a criminal case of dishonored checks against Cosmofarm’s customer Kafantaris was heard at the Sixth Single-Member Misdemeanor Court of Athens, which was postponed to January 26, 2024, when the defendant was convicted by decision no. 1599/2024. In October 2023, the Company’s subsidiary, Cana Laboratories was approached by an attorney on behalf of two clients which were requesting an amount of €39,211 as compensation for the value of 34.70 square meters in relation to an urban sprawl with respect to which an Act of Imputation had been issued by the department of Urban Planning. Our legal counsel’s response was that CANA was not obliged to accept the compensatory value agreed and suggested exploring out of court settlement. As of today, the clients’ Attorney at law has not come back with any suggestions. Our subsidiary, Cana Laboratories, has two pending lawsuits against Euaggelismos Hospital for a total sum of EUR 526,436 due to unpaid bills. The court date for one of the two lawsuits is set for December 11, 2024, and for the other one has not yet been set. The opinion of our legal advisor is that the collection of the total sum by the Company is almost certain. Our subsidiary, Cana Laboratories, has an unasserted claim against Papanikolaou Hospital for a total sum of EUR 89,300 due to unpaid bills, which will be asserted through a lawsuit. The opinion of our legal advisor is that the collection of the sum by the Company is almost certain. A lawsuit dated April 5, 2018 against the Company’s subsidiary Cana Laboratories by a former employee before the Athens court of instance was initially heard on October 12, 2018. The former employee was seeking that the termination of her employment contract to be considered null and void and was requesting compensation for late wages and moral damages. Following numerous appeals, Judgment No. 1192/2024 was issued on September 26, 2023, which as explicitly stated by our legal counsel, requires CANA to rehire the former employee with the threat of a penalty of €200 for each day of non-compliance. As informed by our legal counsel, in order for the penalty to be effective the former employee should file a new lawsuit against CANA and request to get rehired. In case CANA denies employment, then the penalty should be in effect. As of today, we have not received neither a lawsuit nor any request of employment by the former employee. Advisory Agreements On July 1, 2021, the Company entered into a two-year advisory agreement with a third party (the “Consultant”) for advisory and consulting services related to the Company’s intention to become listed on Nasdaq. Peter Goldstein, a then director of the Company is a principal of the Consultant. As consideration for services rendered, and successful Nasdaq listing, the Company paid $100,000. The $100,000 bonus was incurred and settled within 2022. Finally, the Consultant received a total of 10,000 shares of the Company’s common stock, 2,000 of such shares that have been previously issued pursuant to previous agreements and additional 15,258 shares that were issued on February 2, 2023, based on the amendment signed on February 1, 2023. On November 21, 2023, the Company entered into certain consulting agreements with four third-party consultants for the provision of a variety of services such as digital marketing, advisory services relating to target acquisitions and M&As and other additional services as described in the respective agreements. The agreements have a duration from 10 to 18 months and the consultants will solely receive stock consideration for the services rendered. More precisely, they have been awarded a total of 970,000 shares of the Company’s common stock valued at a total of $999,100 based on the fair value of the Company’s common stock as of the agreements’ date. On September 17, 2024 the termination of two out of the four aforementioned consulting agreements was extended and the consultants received additional 440,000 shares as complementary compensation for the extended services to be provided. The additional stock consideration was valued at a total of $501,600 based on the fair value of the Company’s common stock as of the agreements’ date. On July 1, 2024 the Company entered into a consulting agreement with a third-party consultant for the provision of a variety of services such as preparation of press releases and other publications, relationship management and other additional services as described in the respective agreement. The agreement has a duration of sixteen months and the consultant will solely receive stock consideration for the services rendered. More precisely, they have been awarded a total of 240,000 shares of the Company’s common stock valued at a total of $264,000 based on the fair value of the Company’s common stock as of the agreements’ date. The corresponding consulting expense is accrued evenly over the term of the agreements. For the twelve-month period ended December 31, 2023 the Company has recorded $77,250 as stocked based compensation for the above agreements, classified as “General and administrative expenses” in the Company’s consolidated statements of operations and comprehensive loss. For the three and nine months ended September 30, 2024 and 2023 the Company has recorded $264,750 and $728,250 and $0 and $0 as stocked based compensation for the above agreements, classified as “General and administrative expenses” in the Company’s Condensed Consolidated Statements of Operations and Comprehensive Loss. Research and Development Agreements The Company entered into a Research & Development agreement with Doc Pharma S.A. on May 17, 2021. Under this agreement, Doc Pharma is responsible for the research, development, design, registration, copy rights and licenses of 250 nutritional supplements for the final products called Sky Premium Life®. More specifically, Doc Pharma is responsible for the product development and the Company had added 105 of such products codes in its portfolio as of December 31, 2023. No additional ones were added within the nine-month period ended September 30, 2024. The licenses purchased by Doc Pharma SA are capitalized and included in “Goodwill and intangible assets, net” of the Company’s Consolidated Balance Sheets as of September 30, 2024. Thus, no relevant R&D expense had been charged to the Company’s Consolidated Statements of Operations and Comprehensive Loss. On June 26, 2022, the Company signed a research and development (“R&D”) agreement with a third party, through which the Company assigns to the third party the development of new products and services in the field of health, focusing on the human intestinal microbiome. The project includes two phases. Phase 1 has a 20-month duration and its cost amounts to EUR 758,000 ($838,450) and phase 2, has a 22-month duration and a cost of EUR 820,000 ($907,084). The amount will be due and payable upon completion of the corresponding phases. The Company records the corresponding R&D expense based on the project’s progress, which is invoiced by the third party in the relevant period. For the nine-month period ended September 30, 2024, the Company has not incurred such costs.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 405
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/405-30/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/450/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478522/954-440-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
STOCK OPTIONS AND WARRANTS
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| STOCK OPTIONS AND WARRANTS |
|
| STOCK OPTIONS AND WARRANTS |
NOTE 15 – STOCK OPTIONS AND WARRANTS Omnibus Equity Incentive Plan On September 19, 2022, the Company held a Board of Directors meeting, whereas, the Board of Directors had elected to adopt an Omnibus Equity Incentive Plan (the “2022 Plan”), that includes reserving 200,000 shares of common stock eligible for issuance under the 2022 Plan to be registered on a Form S-8 Registration Statement with the SEC. The 2022 Plan is designed to enable the flexibility to grant equity awards to the Company’s officers, employees, non-employee directors and consultants and to ensure that it can continue to grant equity awards to eligible recipients at levels determined to be appropriate by the Board and/or the Compensation Committee. According to the Proxy Statement filed with the SEC on October 20, 2022 the 2022 Plan received final approval by the Company’s stockholders at the Annual Meeting of Stockholders held on December 2, 2022. On April 3, 2023, the Company approved incentive stock awards for the CFO, certain officers and directors and other employees of the Company. The awards are in the form of restricted stock and will vest in two parts: 50% on October 2, 2023 and 50% on October 2, 2024. A total of 185,000 shares were awarded and a corresponding share-based compensation expense of $109,636 and $326,525 was recorded for the three and nine months ended September 30, 2024, based on the amortization of fair value from the date of issuance of April 3, 2023, through September 30, 2024. The equivalent share-based compensation expense for the three and nine months ended September 30, 2023 was $109,636 and $214,505, respectively. On August 21, 2023, the Board adopted, subject to stockholder approval, the Cosmos Health Inc. 2023 Omnibus Equity Incentive Plan (the “2023 Plan”). The 2023 Plan is designed to enable the flexibility to grant equity awards to our officers, employees, non-employee directors and consultants and to ensure that we can continue to grant equity awards to eligible recipients at levels determined to be appropriate by the Board and/or the Compensation Committee. Subject to certain adjustments (as provided in Section 4.2 of the 2023 Plan) and exception (as provided in Section 5.6(b) of the 2023 Plan), the maximum number of shares reserved for issuance under the 2023 Plan (including incentive share options) is 2,500,000 shares. The 2023 Plan was approved by the Company’s stockholders at the Annual Meeting of Stockholders held on September 18, 2023. On September 16, 2024 the Company’s Board of Directors approved incentive stock awards for the CEO, the CFO, certain officers and directors and other key employees of the Company pursuant to the 2023 Plan adopted on August 21, 2023. The awards are in the form of restricted stock and will vest in two parts: 50% on September 16, 2025 and 50% on September 16, 2026. A total of 2,500,000 shares were awarded and a corresponding share-based compensation expense of $48,425 was recorded for the three and nine months ended September 30, 2024, based on the amortization of fair value from the date of issuance of September 16, 2024, through September 30, 2024. Warrant Anti-Dilution Adjustment and Deemed Dividend The Company’s warrants outstanding contain certain anti-dilution adjustments if the Company issues shares of its common stock at a lower price per share than the applicable exercise price of the underlying warrant. If any such dilutive issuance occurs prior to the exercise of such warrant, the exercise price will be adjusted downward to a price equal to the common stock issuance, and the number of warrants that may be purchase upon exercise is increased proportionately so that the aggregate exercise price payable under the warrant shares shall be the same as the aggregate exercise price in effect immediately prior to such adjustment. On December 29, 2023, the Company entered into a warrant exchange agreement (the “Warrant Exchange”) with an investor to reduce the exercise price of 2,437,063 warrants from $2.75 per share to $1.45 per shares as an inducement to exercise. The Company issued 1,487,000 shares of common stock, held 950,063 shares in escrow until the investor’s beneficial ownership limitation allows for the transfer of the escrow shares, and received gross cash proceeds of 3,533,741. The Company contingently granted 4,874,126 additional warrants to be issued upon shareholder approval, with an exercise price of $1.45 and a term of five years. For the year ended December 31, 2023, the Company recorded a deemed dividend of $7,642 for the inducement to exercise and $7,218,485 for the grant of new warrants. The Company valued (a) the fair value of the warrants immediately before the re-pricing in the amount of $3,603,183, (b) the fair value of the warrants immediately after the re-pricing in the amount of $3,610,825, and (c) recorded the difference as deemed dividend in the amount of $7,642. The warrants were valued using the Black-Scholes option pricing model using the following terms: a) fair value of common stock of $1.49, b) exercise price of $1.45 before re-pricing, c) exercise price of $2.75 after re-pricing, d) terms of 5.07 years and 5.02 years, e) dividend rate of 0%, and f) risk free interest rate of 3.83%. Regarding the valuation of the 4,874,126 new warrants (and the recognition of a deemed dividend of $7,218,485) the following terms were used: a) fair value of common stock of $1.49, b) exercise price of $1.45, d) term of 5 years, e) dividend rate of 0%, and f) risk free interest rate of 3.83%. On September 26, 2024, the Company entered into a Warrant Inducement Letter (the “Letter”) with an investor pursuant to which the Company issued 9,748,252 new warrants (the “New Warrants”) and reduced the exercise price of 4,874,126 warrant shares from $1.45 to $0.8701 to induce exercise and receive gross cash proceeds of $4,240,977 (the “Original Warrants”). Of the 9,748,252 warrants 4,874,126 of them have a term of 5 years (“Series A Warrants”) and the remaining 4,874,126 have a term of 1.5 years (“Series B Warrants”). The Company issued 2,332,000 shares of common stock, held 2,532,126 shares in escrow until the investor’s beneficial ownership limitation allows for the transfer of the escrow shares. For the period ended September 30, 2024, the Company recorded a deemed dividend of $9,793 for the inducement to exercise and $6,185,231 for the grant of new warrants. The Company valued (a) the fair value of the warrants immediately before the re-pricing in the amount of $4,197,280, (b) the fair value of the warrants immediately after the re-pricing in the amount of $4,207,073, and (c) recorded the difference as deemed dividend in the amount of $9,793. The warrants were valued using the Black-Scholes option pricing model using the following terms: a) fair value of common stock of $0.8701, b) exercise price of $1.45 before re-pricing, c) exercise price of $0.8701 after re-pricing, d) term of 4.26 years, e) dividend rate of 0%, and f) risk free interest rate of 3.55%. Regarding the valuation of the 9,748,252 new warrants (and the recognition of a deemed dividend of $6,185,321) the following terms were used: a) fair value of common stock of $0.8701, b) exercise price of $0.95, d) terms of 5 years for the Series A Warrants and 1.5 years for the Series B Warrants, e) dividend rate of 0%, and f) risk free interest rate of 3.55% for the Series A Warrants and 3.57% for the Series B Warrants. As of September 30, 2024, there were 13,432,506 warrants outstanding and 8,558,380 warrants exercisable with 13,419,172 warrants having expiration dates from October 2024 through October 2029 and 13,334 warrants with no expiration date. A summary of the Company’s warrant activity for the nine months ended September 30, 2024 and the year ending December 31, 2023 is as follows: | | | | | | | | Weighted | | | | | | | | | | Weighted | | | Average | | | | | | | | | | Average | | | Remaining | | | Aggregate | | | | Number of | | | Exercise | | | Contractual | | | Intrinsic | | Warrants | | Shares | | | Price | | | Term | | | Value | | Balance Outstanding, January 1, 2023 | | | 4,194,236 | | | $ | 8.31 | | | | 5.04 | | | $ | 2,562,621 | | Granted | | | 7,524,933 | | | | 1.65 | | | | 5.13 | | | | - | | Forfeited | | | - | | | | - | | | | - | | | | - | | Exercised | | | (3,152,386 | ) | | | - | | | | - | | | | - | | Expired | | | (5,307 | ) | | | - | | | | - | | | | - | | Balance Outstanding, December 31, 2023 | | | 8,561,476 | | | $ | 3.91 | | | | 4.64 | | | $ | 18,801 | | Granted | | | 9,748,252 | | | | 0.95 | | | | 3.24 | | | | - | | Forfeited | | | - | | | | - | | | | - | | | | - | | Exercised | | | (4,874,126 | ) | | | - | | | | - | | | | - | | Expired | | | (3,096 | ) | | | - | | | | - | | | | - | | Balance Outstanding, September 30, 2024 | | | 13,432,506 | | | $ | 2.64 | | | | 3.28 | | | $ | 11,681 | | | | | | | | | - | | | | - | | | | - | | Exercisable, September 30, 2024 | | | 13,432,506 | | | $ | 2.64 | | | | 3.28 | | | $ | 11,681 | |
|
| X |
- References
+ Details
| Name: |
abcd_StockOptionsAndWarrantsAbstract |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_StockOptionsAndWarrantsDisclouserTextBlock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
DISAGGREGATION OF REVENUE
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| DISAGGREGATION OF REVENUE |
|
| DISAGGREGATION OF REVENUE |
NOTE 16 – DISAGGREGATION OF REVENUE ASC 606-10-50-5 requires that entities disclose disaggregated revenue information in categories (such as type of good or service, geography, market, type of contract, etc.). ASC 606-10-55-89 explains that the extent to which an entity’s revenue is disaggregated depends on the facts and circumstances that pertain to the entity’s contracts with customers and that some entities may need to use more than one type of category to meet the objective for disaggregating revenue. The Company disaggregates revenue by country to depict the nature and economic characteristics affecting revenue. The following table presents our revenue disaggregated by country for the three months ended: Country | | September 30, 2024 | | | September 30, 2023 | | Croatia | | $ | 107 | | | | 14,159 | | Cyprus | | | 16,519 | | | | 72,754 | | Bulgaria | | | 25,507 | | | | - | | Greece | | | 12,247,597 | | | | 12,544,643 | | USA | | | - | | | | 210 | | Ireland | | | - | | | | 1,417 | | UK | | | 121,318 | | | | 190,614 | | Total | | $ | 12,411,048 | | | $ | 12,823,797 | |
The following table presents our revenue disaggregated by country for the nine months ended: Country | | September 30, 2024 | | | September 30, 2023 | | Croatia | | $ | 19,370 | | | | 14,159 | | Cyprus | | | 89,064 | | | | 141,402 | | Bulgaria | | | 43,849 | | | | - | | Greece | | | 39,385,730 | | | | 36,041,012 | | USA | | | - | | | | 504 | | Ireland | | | - | | | | 1,417 | | UK | | | 664,225 | | | | 1,338,509 | | Total | | $ | 40,202,238 | | | $ | 37,537,003 | |
|
| X |
- References
+ Details
| Name: |
abcd_DisaggregationOfRevenueDisclosureTextBlock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_RevenueFromContractWithCustomerAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
SUBSEQUENT EVENTS
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| SUBSEQUENT EVENTS |
|
| SUBSEQUENT EVENTS |
NOTE 17 – SUBSEQUENT EVENTS On November 6, 2024, the Company received a non-compliance letter from Nasdaq for its failure to maintain a minimum bid price of 1.00 per share for thirty consecutive business days in accordance with Nasdaq Listing Rule 5550(a)(2). The Company has one hundred eighty calendar days from November 6, 2024, to regain compliance by the closing bid price of the Company’s common stock being at least $1.00 per share for ten consecutive business days. In the event the Company cannot otherwise regain compliance with the listing rule, it intends to effect a reverse stock split to regain compliance.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
ORGANIZATION AND NATURE OF BUSINESS (Policies)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| ORGANIZATION AND NATURE OF BUSINESS |
|
| Acquisition Accounting |
Cloudscreen On January 23, 2024, the Company completed the acquisition of Cloudscreen, a cutting-edge Artificial Intelligence (AI) powered platform. The acquisition is pursuant to the purchase agreement announced on October 11, 2023. Cloudscreen is a multimodal platform specialized in drug repurposing, a process that involves uncovering new target proteins or indications for existing drugs for use in treating different diseases. The total purchase price amounted to $637,080 and consisted of 280,000 shares of common stock with a fair value of $319,200 and an amount of $317,880 to be settled in cash during 2024 based on the Promissory Note signed on October 10, 2023. The Company accounted for the acquisition as an asset acquisition in accordance with Accounting Standards Codification (“ASC”) Topic 805, Business Combinations, (“ASC 805”) and recorded $637,080 as an intangible asset related to the technology platform acquired. ZipDoctor On April 3, 2023, the Company completed the acquisition of ZipDoctor Inc. (“ZipDoctor”), a telehealth company for a total sum of $150,000 in cash and $8,788 in fees. The Company accounted for the acquisition as an asset acquisition in accordance with Accounting Standards Codification (“ASC”) Topic 805, Business Combinations, (“ASC 805”) and recorded $158,788 as an intangible asset related to the technology platform acquired. Bikas On June 15, 2023, Cosmos Health Inc. entered into an Assignment and Assumption Agreement (the “Agreement”) with Ioannis Bikas O.E., a Greek Company (“Bikas”). Bikas is owner of a pharmaceutical distribution network in Greece and agreed to sell to the Company their distribution network and customer base. The purchase price of the network was €100,000 ($109,330) in cash, and €300,000 ($316,081) in the Company’s common stock. The Company issued 99,710 shares of common stock related to the acquisition of the customer base, based on the fair value of the stock on the acquisition date. The Company accounted for the acquisition as an asset acquisition in accordance with ASC 805 and recorded $425,411 as an intangible asset related to the customer base acquired. Buildings Acquisitions On April 24, 2023, the Company purchased a building for a total sum of $1,054,872 in cash. The Company accounted for the acquisition as an asset acquisition in accordance with ASC 805 and recorded the cost of the building as “Property, plant and equipment” on the consolidated balance sheets. On January 6, 2023, the Company agreed to purchase land and building located in Montreal, Canada from a third-party vendor. The total purchase price amounts to $3,950,000 and the closing date of the agreement based on the amendment signed on July 19, 2023, is December 31, 2023. As of September 30, 2024, the Company has made no additional prepayments concerning this building. The closing date of the agreement has been extended to December 31, 2024. Cana On June 30, 2023, the Company acquired Cana Laboratories Holdings (Cyprus) Limited (“Cana”), which wholly owned an operating subsidiary, Pharmaceutical Laboratories Cana S.A. (“Cana SA”) for €800,000 ($873,600) in cash and 46,377 shares of common stock, with fair value of $138,667 as of the date of acquisition. Moreover, on February 28, 2023, the Company had signed a Secured Promissory Note with Cana, whereby Cana borrowed the sum of €4,100,000 ($4,457,520), included in the total consideration of $5,469,787. The Company accounted for the acquisition as a business acquisition in accordance with ASC 805. The fair value of Cana assets acquired, and liabilities assumed was based upon management’s estimates assisted by an independent third-party valuation firm. The fixed assets of Cana (which included land, building & machinery) were valued as of December 31, 2022 and the Company believes that nothing has materially changed between such date and the acquisition date (June 30, 2023). The following table summarizes the preliminary allocation of purchase price of the acquisition: Consideration | | | | Cash | | $ | 5,331,120 | | Fair value of common stock issued | | | 138,667 | | Fair value of total consideration transferred | | $ | 5,469,787 | | | | | | | Recognized amounts of identifiable assets acquired | | | | | Financial assets | | $ | 1,796,911 | | Inventory | | | 297,340 | | Property, plant and equipment | | | 7,488,818 | | Identifiable intangible assets | | | 562,200 | | Financial liabilities | | | (3,235,233 | ) | Total identifiable net assets | | $ | 6,910,036 | | | | | | | Bargain purchase gain | | $ | 1,440,249 | |
Revenue for the 9 - month period ended September 30, 2024 | | $ | 549,567 | | Loss for the 9 - month period ended September 30, 2024 | | $ | (1,674,785 | ) |
During the prior year period, Cana had minimal operations as it was in financial difficulties and seeking for an investor.
|
| Basis of Financial Statement Presentation |
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with U.S. GAAP.
|
| Principles of Consolidation |
Our consolidated accounts include our accounts and the accounts of our wholly owned subsidiaries, SkyPharm S.A., Decahedron Ltd., Cosmofarm S.A., Cana Laboratories Holdings (Cyprus) Limited and ZipDoctor Inc. The Group’s financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”). The consolidated financial statements reflect the consolidation of all entities in which the Company has control, as determined by the ability to direct the activities that significantly affect the entities’ economic performance. All significant intercompany balances and transactions have been eliminated.
|
| Transactions in and Translations of Foreign Currency |
The functional currency for the Greek subsidiaries of the Company (CANA Laboratories, Cosmofarm S.A. and SkyPharm SA) is Euro (€) and for the UK subsidiary (Decahedron Ltd) is GBP (£). ZipDoctor Inc. is a U.S. based entity. As a result, the financial statements of the subsidiaries (except for ZipDoctor Inc.) have been translated from the local currency into U.S. dollars using (i) year-end exchange rates for balance sheet accounts, and (ii) average exchange rates for the reporting period for all income statements accounts. Foreign currency translations gains and losses are reported as a separate component of the condensed consolidated statements of changes in stockholders’ equity and mezzanine equity.
|
| Use of Estimates |
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
|
| The Effects of War in the Ukraine |
On February 24, 2022, Russian forces launched significant military action against Ukraine. There continues to be sustained conflict and disruption in the region, which is expected to endure for the foreseeable future. We do not conduct any commercial transactions with either Ukraine or Russia and the Company and, as such, is not aware of any specific event or circumstance that would require an update to its estimates or judgments or a revision of the carrying value of its assets or liabilities as of the date of issuance of this Quarterly Report on Form 10-Q. Such political issues and conflicts could have a material adverse effect on our results of operations and financial condition if they escalate in areas in which we do business. In addition, changes in and adverse actions by governments in foreign markets in which we do business could have a material adverse effect on our results of operations and financial condition.
|
| Credit Losses |
In June 2016, the FASB issued ASU 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses of Financial Instruments, which amends the requirement on the measurement and recognition of expected credit losses for financial assets held. Furthermore, amendments ASU 2019-10 and ASU 2019-11 provided additional clarification for implementing ASU 2016-13. ASU 2016-13 is effective for the Company beginning January 1, 2023, with early adoption permitted. The Company adopted the standard on January 1, 2023, and the standard did not have a material impact on the Company’s consolidated financial statements and related disclosures. The Company is exposed to credit losses primarily through sales to its customers and the loans that it has provided. The Company assesses each customer’s/ borrower’s ability to pay, and a credit loss estimate by conducting a credit review which includes consideration of established credit rating, or an internal assessment of the customer’s creditworthiness based on an analysis of their payment history when a credit rating is not available. The Company monitors credit exposure through active review of customer balances. The Company’s expected loss methodology for accounts receivable is developed through consideration of factors including, but not limited to, historical collection experience, current customer credit ratings, current customer financial condition, current and future economic and market conditions, and age of the receivables. More specifically, the Company assesses a number of customers with significant long outstanding balances on an individual basis, applying different credit loss percentages to them, and subsequently summarizes the ones not included in the individual analysis, groups them based on their rating (decided based on the factors described above) and applies specific credit loss percentages to each group. The Company has elected to follow the simplified ECL approach. The charges related to credit losses are included in “General and administrative expenses” and are recorded in the period that the outstanding receivables are determined to be doubtful. Account balances are written-off against the allowance when they are deemed uncollectible.
|
| Cash and Cash Equivalents |
For purposes of the statement of cash flows, the Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. The Company maintains bank accounts in the United States denominated in U.S. Dollars, in Greece denominated in Euros, U.S. Dollars and Great Britain Pounds (British Pounds Sterling), and in Bulgaria denominated in Euros. The Company also maintains bank accounts in the United Kingdom, denominated in Euros and Great Britain Pounds (British Pounds Sterling).
|
| Accounts Receivable, net |
Accounts receivable are stated at their net realizable value. The allowance for doubtful accounts against gross accounts receivable, prepaid expenses and other current assets and other assets reflects the best estimate of probable losses inherent in the receivables’ portfolio determined on the basis of historical experience, specific allowances for known troubled accounts and other currently available information. As of September 30, 2024 and December 31, 2023, the Company’s allowance for doubtful accounts was $19,905,776 and $19,686,091, respectively. Below is the summary of changes in the allowance for doubtful accounts: | | September 30, 2024 | | | | | | Balance as of January 1st, 2024 | | $ | 19,686,091 | | Provisions for credit losses | | | - | | Write-offs | | | 250,971 | | Foreign exchange adjustments | | | | | Other adjustments | | | (31,286 | ) | Balance as of September 30, 2024 | | $ | 19,905,776 | |
|
| Tax Receivables |
The Company pays Value Added Tax (“VAT”) or similar taxes (“input VAT”), income taxes, and other taxes within the normal course of its business in most of the countries in which it operates related to the procurement of merchandise and/or services it acquires and/or on sales and taxable income. The Company also collects VAT or similar taxes on behalf of the government (“output VAT”) for merchandise and/or services it sells. If the output VAT exceeds the input VAT, this creates a VAT payable to the government. If the input VAT exceeds the output VAT, this creates a VAT receivable from the government. The VAT tax return is filed on a monthly basis offsetting the payables against the receivables. In observance of EU regulations for intra-EU cross-border sales, our subsidiaries in Greece, SkyPharm and Cosmofarm, do not charge VAT for sales to wholesale drug distributors registered in other European Union member states. As of September 30, 2024 and December 31, 2023, the Company had a VAT net receivable balance of $322,576 and $187,512 respectively, recorded in the consolidated balance sheet as prepaid expenses and other current assets and accounts payable and accrued expenses, respectively.
|
| Inventory |
Inventory is stated at the lower-of-cost or net realizable value using the weighted average method. Inventory consists primarily of finished goods and packaging materials, i.e., packaged pharmaceutical products and the wrappers and containers they are sold in. A periodic inventory system is maintained by 100% count. Inventory is replaced periodically to maintain the optimum stock on hand available for immediate shipment. The Company writes down inventories to net realizable value based on physical condition, expiration date, and current market conditions, as well as forecasted demand. The Company’s inventories are not highly susceptible to obsolescence. Many of the Company’s inventory items are eligible for return to our suppliers when pre-agreed product requirements, including, but not limited to, physical condition and expiration date, are not met. No significant judgments have been applied in estimating the selling price of our inventory.
|
| Property and Equipment, net |
Property and equipment are stated at cost, less accumulated depreciation. Depreciation is calculated on a straight-line basis over the useful lives (except for leasehold improvements which are depreciated over the lesser of the lease term or the useful life) of the assets as follows: | | Estimated Useful Life | | Leasehold improvements and technical works | | | Lesser of lease term or 25 years | | Buildings | | | 25-30 years | | Vehicles | | | 6 years | | Machinery | | | 20 years | | Furniture, fixtures and equipment | | | 5–10 years | | Computers and software | | | 3-5 years | |
Depreciation expense was $89,694 and $124,910 for the three months ended September 30, 2024 and 2023, respectively and $306,126 and $237,479 for the nine months ended September 30, 2024 and 2023, respectively. Property and Equipment additions Property and Equipment additions are recognized as assets when it is probable that future economic benefits associated with the asset will flow to the entity and the cost of the asset can be measured reliably. Additions are initially measured at cost, which includes all costs directly attributable to bringing the asset to its working condition and location for its intended use. This may include purchase price, freight, installation, and any directly attributable professional fees. They are capitalized if their cost exceeds a certain threshold. The threshold is determined based on materiality considerations. Costs below the threshold are typically expensed as incurred. After initial recognition, additions are measured at cost less accumulated depreciation and any accumulated impairment losses. Depreciation is calculated systematically over the estimated useful life of the asset. They are tested for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. If the carrying amount exceeds the recoverable amount, an impairment loss is recognized, and the carrying amount of the asset is adjusted accordingly. Borrowing costs directly attributable to the acquisition, construction, or production of qualifying assets, including Property and Equipment additions, are capitalized as part of the cost of those assets.
|
| Goodwill and Intangibles, net |
The Company periodically reviews the carrying value of intangible assets not subject to amortization, including goodwill, to determine whether impairment may exist. Goodwill and certain intangible assets are assessed annually, or when certain triggering events occur, for impairment using fair value measurement techniques. These events could include a significant change in the business climate, legal factors, a decline in operating performance, competition, sale or disposition of a significant portion of the business, or other factors. First, under step 0, we determine whether it is more likely than not that the fair value of the reporting unit is less than the carrying amount. Following, if step 0 fails, goodwill impairment is determined using a two-step process. The first step of the goodwill impairment test is used to identify potential impairment by comparing the fair value of a reporting unit with its carrying amount, including goodwill. The Company uses level 3 inputs and a discounted cash flow methodology to estimate the fair value of a reporting unit. A discounted cash flow analysis requires one to make various judgmental assumptions including assumptions about future cash flows, growth rates, and discount rates. The assumptions about future cash flows and growth rates are based on the Company’s budget and long-term plans. Discount rate assumptions are based on an assessment of the risk inherent in the respective reporting units. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is considered not impaired and the second step of the impairment test is unnecessary. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test is performed to measure the amount of impairment loss, if any. The second step of the goodwill impairment test compares the implied fair value of the reporting unit’s goodwill with the carrying amount of that goodwill. If the carrying amount of the reporting unit’s goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess. The implied fair value of goodwill is determined in the same manner as the amount of goodwill recognized in a business combination. That is, the fair value of the reporting unit is allocated to all of the assets and liabilities of that unit (including any unrecognized intangible assets) as if the reporting unit had been acquired in a business combination and the fair value of the reporting unit was the purchase price paid to acquire the reporting unit. On December 19, 2018, as a result of the acquisition of Cosmofarm, the Company recorded $49,697 of goodwill. Intangible assets with definite useful lives are recorded on the basis of cost and are amortized on a straight-line basis over their estimated useful lives. The Company uses a useful life of 5 years for an import/export license and a useful life of 10 years for the pharmaceutical and nutraceutical products licenses included in Note 4 as “Licenses”. A useful life of 10 years is also used for the platforms included in Note 4 as “Software” and the customer bases. The Company evaluates the remaining useful life of intangible assets annually to determine whether events and circumstances warrant a revision to the remaining amortization period. If the estimate of the intangible asset’s remaining useful life is changed, the remaining carrying amount of the intangible asset will be amortized prospectively over that revised remaining useful life. As of September 30, 2024 and December 31, 2023, no revision to the remaining amortization period of the intangible assets was made. Amortization expense was $196,183 and $88,168 for the three months ended September 30, 2024 and 2023, respectively and $579,556 and $138,438 for the nine months ended September 30, 2024 and 2023, respectively.
|
| Impairment of Long-Lived Assets |
In accordance with ASC 360-10, Long-lived Assets, property and equipment and intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the assets. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable.
|
| Equity Method Investment |
For those investments in common stock or in-substance common stock in which the Company has the ability to exercise significant influence over the operating and financial policies of the investee, the investment is accounted for under the equity method. The Company records its share in the earnings of the investee and is included in “Equity earnings of affiliate” in the consolidated statement of operations. The Company assesses its investment for other-than-temporary impairment when events or changes in circumstances indicate that the carrying amount of the investment might not be recoverable and recognizes an impairment loss to adjust the investment to its then current fair value.
|
| Investments in Equity Securities |
Investments in equity securities are accounted for at fair value with changes in fair value recognized in net income (loss). Equity securities are classified as short-term or long-term based on the nature of the securities and their availability to meet current operating requirements. Equity securities that are readily available for sale in current operations are reported as a component of current assets on the accompanying consolidated balance sheets. Equity securities that are not considered available for use in current operations would be reported as a component of long-term assets on the accompanying consolidated balance sheets. For equity securities with no readily determinable fair value, the Company elects a measurement alternative to fair value. Under this alternative, the Company measures the investments at cost, less any impairment, and adjusted for changes resulting from observable price changes in transactions for identical or similar investments of the investee. The election to use the measurement alternative is made for each eligible investment. As of September 30, 2024, investments consisted of 16,666 shares which traded at a closing price of $0.75 per share or value of $12,416 of National Bank of Greece. Additionally, the Company has $7,665 in equity securities of Pancreta Bank, which are revalued annually.
|
| Fair Value Measurement |
The Company applies ASC 820, Fair Value Measurements and Disclosures, (“ASC 820”), for assets and liabilities measured at fair value on a recurring basis. ASC 820 establishes a common definition for fair value to be applied to existing generally accepted accounting principles that require the use of fair value measurements establishes a framework for measuring fair value and expands disclosure about such fair value measurements. ASC 820 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Additionally, ASC 820 requires the use of valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs. These inputs are prioritized below: Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities. Level 2: Inputs other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. Level 3: Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use. In addition, ASC 825-10-25, Fair Value Option, (“ASC 825-10-25”), expands opportunities to use fair value measurements in financial reporting and permits entities to choose to measure many financial instruments and certain other items at fair value. The Company did not elect the fair value options for any of its qualifying financial instruments. Our financials also included the following financial instruments as of September 30, 2024 and December 31, 2023: cash, accounts receivable, inventory, prepaid expenses, loans receivable, accounts payable, notes payable and lines of credit. Except for the loans receivable which carry fixed interest rates, the carrying value of the remaining instruments, approximates fair value due to their short-term nature.
|
| Customer Advances |
The Company receives prepayments from certain customers for pharmaceutical products prior to those customers taking possession of the Company’s products. The Company records these receipts as current liabilities until it has met all the criteria for recognition of revenue including passing control of the products to its customer, at such point, the Company will reduce the customer advances balance and credit the Company’s revenues.
|
| Revenue Recognition |
In accordance with ASC Topic 606, Revenue from Contracts with Customers (“ASC 606”), the Company uses a five-step model for recognizing revenue by applying the following steps: | 1) | Identification of the Contract: The Company identifies a contract with a customer when it enters into an agreement that creates enforceable rights and obligations. | | 2) | Identification of Performance Obligations: The Company identifies distinct performance obligations within each contract, which represent promises to transfer goods or services to the customer. | | 3) | Determination of Transaction Price: The Company determines the transaction price, which represents the amount of consideration to which it expects to be entitled in exchange for transferring promised goods or services to the customer, excluding any amounts collected on behalf of third parties. | | 4) | Allocation of Transaction Price: The Company allocates the transaction price to each distinct performance obligation based on its standalone selling price. If the standalone selling price is not observable, the Company estimates it using an appropriate method. | | 5) | Recognition of Revenue: Revenue is recognized when (or as) the Company satisfies a performance obligation by transferring a promised good or service to the customer. This typically occurs at a point in time or over time, depending on the nature of the performance obligation. |
Wholesale revenue and sales of own branded nutraceutical and pharmaceutical products The Company has contracts or signed partnership forms (usual in the wholesale sector of the pharma industry) with its customers, stipulating enforceable rights and obligations. The Company is responsible for transferring the goods to the customer’s location, which represents its sole performance obligation. Thus, the transaction price, which is predetermined in most of the products sold, is exclusively allocated to this performance obligation. Revenue is recognized at a single point in time, which is upon issuance of the corresponding sales invoice. The Company has assessed the impact of the items invoiced but not delivered to the customer’s location as of December 31, 2023 and September 30, 2024, and deemed that it had no material effect. Pharma manufacturing The Company has active contracts with its customers, stipulating enforceable rights and obligations. The Company is responsible for the manufacturing and the packaging of specific products assigned by its customers, which represents its performance obligations to which the Company allocates the transaction price determined. The customers are responsible for providing the raw materials to the Company. Revenue is recognized over a period of time, which is during the production and packaging period of the respective products. As of September 30, 2024, there were no products or batches of products for which the production or packaging phase was in progress. Medihelm SA Commencing from January 1, 2023, and pursuant to the agreement with Medihelm, the exclusive distributor of the Company’s own proprietary line of nutraceuticals, the Company considers the transaction price to be variable and records an estimate of the transaction price, subject to the constraint for variable consideration. The Company is basing the change in transaction price with the exclusive distributor through assessment of significant overdue receivables from the exclusive distributor, which the Company reassesses each reporting period. Through this assessment, the Company applied the “expected value” model under ASC 606-10-32-5 and had applied specific constraints to revenue due from the customer at the end of each reporting period. Following the application of the “expected value” model, the Company had deferred an amount of $397,000 and recorded it against the sales to Medihelm for the twelve months ended December 31, 2023. However, the Company assessed once more the trading relationship with Medihelm SA at year end and since no significant receipts had taken place up to the issuance of the report, the Company recorded an allowance for the total receivable amount not received up to the issuance date. More specifically a cumulative reserve of $12,655,615 was applied, leaving a receivable of $532,704 due from Medihelm SA, as of December 31, 2023. The Company does not consider that new sales to Medihelm SA or sales to any other customer include a variable component as of September 30, 2024 and has limited such sales to the minimum required.
|
| Stock-based Compensation |
The Company records stock-based compensation in accordance with ASC 718, Stock Compensation (“ASC 718”) and Staff Accounting Bulletin No. 107 (“SAB 107”) regarding its interpretation of ASC 718. ASC 718 requires the fair value of all stock-based employee compensation awarded to employees to be recorded as an expense over the related requisite service period. The Company values any employee or non-employee stock-based compensation at fair value using the Black-Scholes Option Pricing Model. The Company accounts for non-employee share-based awards in accordance with the measurement and recognition criteria of ASU 2018-07, “Compensation-Stock Compensation-Improvements to Nonemployee Share-Based Payment Accounting.”
|
| Income Taxes |
The Company accounts for income taxes under the asset and liability method, as required by the accounting standard for income taxes ASC 740. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis, as well as net operating loss carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company is liable for income taxes in Greece and the United Kingdom The corporate income tax rate is 22% in Greece and 25% in the United Kingdom. Losses may also be subject to limitation under certain rules regarding change of ownership. We regularly review deferred tax assets to assess their potential realization and establish a valuation allowance for portions of such assets to reduce the carrying value if we do not consider it to be more likely than not that the deferred tax assets will be realized. Our review includes evaluating both positive (e.g., sources of taxable income) and negative (e.g., recent historical losses) evidence that could impact the realizability of our deferred tax assets. At September 30, 2024, we believe our United Kingdom and Greece deferred tax assets will not be realized, as such, we did not record a reversal on the full valuation approach we followed during the year ended December 31, 2023.
|
| Leases |
The Company accounts for leases in accordance with ASC 842. For all leases, the Company recognizes a right-of-use (ROU) asset and a lease liability on the balance sheet. The ROU asset represents the Company's right to use the underlying asset for the lease term, and the lease liability represents the obligation to make lease payments arising from the lease, both measured at the present value of future lease payments. Lease payments are recognized as an operating expense on a straight-line basis over the lease term. The interest on the lease liability and the amortization of the ROU asset are recognized separately in the income statement. Initial direct costs incurred by the Company in negotiating and securing leases are capitalized and amortized over the lease term on a straight-line basis. The assets and liabilities from operating and finance leases are recognized at the commencement date based on the present value of remaining lease payments over the lease term using the Company’s secured incremental borrowing rates or implicit rates, when readily determinable. Short-term leases, which have an initial term of 12 months or less, are not recorded on the balance sheet. The Company’s operating leases do not provide an implicit rate that can readily be determined. Therefore, we use a discount rate based on our incremental borrowing rate, which is determined using the average interest rate of our long-term debt on the date of inception.
|
| Retirement and Termination Benefits |
Under Greek labor law, employees are entitled to lump-sum compensation in the event of termination or retirement. The amount depends on the employee’s work experience and remuneration as of the day of termination or retirement. If an employee remains with the company until full-benefit retirement, the employee is entitled to a lump-sum equal to 40% of the compensation to be received if the employee were to be dismissed on the same day. The Company periodically reviews the uncertainties and judgments related to the application of the relevant labor law regulations to determine retirement and termination benefits obligations of its Greek subsidiaries. The Company has evaluated the impact of these regulations and has identified a potential retirement and termination benefits liability. The amount of the liability as of September 30, 2024 and December 31, 2023, was $395,698 and $408,665, respectively, and has been recorded as a long-term liability within the consolidated balance sheets.
|
| Basic and Diluted Net Loss per Common Share |
Basic income per share is calculated by dividing the income available to common stockholders by the weighted average number of shares of common stock outstanding during the period. Diluted income per share is calculated by dividing income available to common stockholders by the weighted average number of common shares outstanding for the period and, when dilutive, potential shares from stock options and warrants to purchase common stock, using the treasury stock method. In accordance with ASC 260, Earnings Per Share, the following table reconciles basic shares outstanding to fully diluted shares outstanding. | | September 30, 2024 | | | September 30, 2023 | | Weighted average number of common shares outstanding Basic | | | 17,724,305 | | | | 11,346,071 | | Potentially dilutive common stock equivalents | | | - | | | | - | | Weighted average number of common and equivalent shares outstanding – Diluted | | | 17,724,305 | | | | 11,346,071 | |
The following table summarizes potential common shares that were excluded as their effect is anti-dilutive: | | September 30, 2024 | | | September 30, 2023 | | Warrants | | | 13,432,507 | | | | 6,124,412 | | Total | | | 13,432,507 | | | | 6,124,412 | |
Common stock equivalents are included in the diluted income per share calculation only when option exercise prices are lower than the average market price of the common shares for the period presented. In March 2022, the FASB issued ASU 2022-02, Troubled Debt Restructurings and Vintage Disclosures. This ASU eliminates the accounting guidance for troubled debt restructurings by creditors that have adopted ASU 2016-13, Measurement of Credit Losses on Financial Instruments, which was adopted on January 1, 2020. The adoption of ASU 2016-13 did not have a material impact on the Company’s consolidated financial statements. ASU 2022-02 also enhances the disclosure requirements for certain loan refinancing and restructurings by creditors when a borrower is experiencing financial difficulty. In addition, the ASU amends the guidance on vintage disclosures to require entities to disclose current period gross write-offs by year of origination for financing receivables and net investments in leases within the scope of ASC 326-20. The ASU is effective for annual periods beginning after December 15, 2022, including interim periods within those fiscal years. Adoption of the ASU would be applied prospectively. Early adoption is also permitted, including adoption in an interim period. This ASU was adopted on January 1, 2023, which resulted in no cumulative-effect adjustment to retained earnings.
|
| Recent Accounting Pronouncements |
In March 2024, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2024-01, Compensation—Stock Compensation (Topic 718): Scope Application of Profits Interest and Similar Awards. This guidance is intended to improve generally accepted accounting principles (GAAP) by adding an illustrative example to demonstrate how an entity should apply the scope guidance in paragraph 718- 10-15-3 to determine whether profits interest and similar awards (“profits interest awards”) should be accounted for in accordance with Topic 718, Compensation—Stock Compensation. The amendments in this Update are effective for annual periods beginning after December 15, 2024, and interim periods within those annual periods. The amendments in this Update should be applied either (1) retrospectively to all prior periods presented in the financial statements or (2) prospectively to profits interest and similar awards granted or modified on or after the date at which the entity first applies the amendments. The Company is currently evaluating this guidance to determine the impact it may have on its consolidated financial statements disclosures. In March 2024, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2024-02, Codification Improvements—Amendments to Remove References to the Concepts Statements. This guidance is intended to remove references to various FASB Concepts Statements. The Board has a standing project on its agenda to address suggestions received from stakeholders on the Accounting Standards Codification and other incremental improvements to generally accepted accounting principles (GAAP). This effort facilitates Codification updates for technical corrections such as conforming amendments, clarifications to guidance, simplifications to wording or the structure of guidance, and other minor improvements. The resulting amendments are referred to as Codification improvements. The amendments in this Update are not intended to result in significant accounting change for most entities. The Company is currently evaluating this guidance to determine the impact it may have on its consolidated financial statements disclosures. In December 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. This guidance is intended to enhance the transparency and decision-usefulness of income tax disclosures. The amendments in ASU 2023-09 address investor requests for enhanced income tax information primarily through changes to disclosure regarding rate reconciliation and income taxes paid both in the U.S. and in foreign jurisdictions. ASU 2023-09 is effective for fiscal years beginning after December 15, 2024, on a prospective basis, with the option to apply the standard retrospectively. Early adoption is permitted. The Company is currently evaluating this guidance to determine the impact it may have on its consolidated financial statements disclosures. In November 2023, the FASB issued ASU 2023-07, Segment Reporting: Improvements to Reportable Segment Disclosures. This guidance expands public entities’ segment disclosures primarily by requiring disclosure of significant segment expenses that are regularly provided to the chief operating decision maker and included within each reported measure of segment profit or loss, an amount and description of its composition for other segment items, and interim disclosures of a reportable segment’s profit or loss and assets. The guidance is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. The amendments are required to be applied retrospectively to all prior periods presented in an entity’s financial statements. The Company is currently evaluating this guidance to determine the impact it may have on its consolidated financial statements related disclosures. Management does not believe that any recently issued, but not effective, accounting standards, if currently adopted, would have a material effect on the Company’s consolidated financial statements.
|
| X |
- References
+ Details
| Name: |
abcd_AcquisitionAccountingPolicyTextBlock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_CreditLossesPolicyTextBlock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_CustomerAdvancesPolicyTextBlock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_EffectsOfWarInTheUkrainePolicyTextBlock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_OrganizationAndNatureOfTheBusinessAbstract |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_TaxReceivablesPolicyTextBlock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for salaries, bonuses, incentive awards, postretirement and postemployment benefits granted to employees, including equity-based arrangements; discloses methodologies for measurement, and the bases for recognizing related assets and liabilities and recognizing and reporting compensation expense. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_CompensationRelatedCostsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy regarding (1) the principles it follows in consolidating or combining the separate financial statements, including the principles followed in determining the inclusion or exclusion of subsidiaries or other entities in the consolidated or combined financial statements and (2) its treatment of interests (for example, common stock, a partnership interest or other means of exerting influence) in other entities, for example consolidation or use of the equity or cost methods of accounting. The accounting policy may also address the accounting treatment for intercompany accounts and transactions, noncontrolling interest, and the income statement treatment in consolidation for issuances of stock by a subsidiary. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1
| Name: |
us-gaap_ConsolidationPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for equity method of accounting for investments and other interests. Investment includes, but is not limited to, unconsolidated subsidiary, corporate joint venture, noncontrolling interest in real estate venture, limited partnership, and limited liability company. Information includes, but is not limited to, ownership percentage, reason equity method is or is not considered appropriate, and accounting policy election for distribution received. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 21D
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-21D
| Name: |
us-gaap_EquityMethodInvestmentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for fair value measurements of financial and non-financial assets, liabilities and instruments classified in shareholders' equity. Disclosures include, but are not limited to, how an entity that manages a group of financial assets and liabilities on the basis of its net exposure measures the fair value of those assets and liabilities.
| Name: |
us-gaap_FairValueMeasurementPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for (1) transactions denominated in a currency other than the reporting enterprise's functional currency, (2) translating foreign currency financial statements that are incorporated into the financial statements of the reporting enterprise by consolidation, combination, or the equity method of accounting, and (3) remeasurement of the financial statements of a foreign reporting enterprise in a hyperinflationary economy. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/830/tableOfContent
| Name: |
us-gaap_ForeignCurrencyTransactionsAndTranslationsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for goodwill and intangible assets. This accounting policy also may address how an entity assesses and measures impairment of goodwill and intangible assets. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/350-30/tableOfContent
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/350-20/tableOfContent
| Name: |
us-gaap_GoodwillAndIntangibleAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for recognizing and measuring the impairment of long-lived assets. An entity also may disclose its accounting policy for long-lived assets to be sold. This policy excludes goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.CC)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480091/360-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 05
-Paragraph 4
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482338/360-10-05-4
| Name: |
us-gaap_ImpairmentOrDisposalOfLongLivedAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-20
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-19
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482525/740-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-17
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-9
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482525/740-10-45-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-1
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of inventory accounting policy for inventory classes, including, but not limited to, basis for determining inventory amounts, methods by which amounts are added and removed from inventory classes, loss recognition on impairment of inventories, and situations in which inventories are stated above cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483080/330-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483489/210-10-50-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-4
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 912
-SubTopic 330
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478411/912-330-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/330/tableOfContent
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483080/330-10-50-4
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (a)
-SubTopic 10
-Topic 270
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482989/270-10-45-6
| Name: |
us-gaap_InventoryPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for investment in financial asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 12
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-12
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 19
-Subparagraph (2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-19
| Name: |
us-gaap_InvestmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangements entered into by lessor. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479773/842-30-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 3A
-Subparagraph (a)
-SubTopic 30
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479773/842-30-50-3A
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 3A
-Subparagraph (b)
-SubTopic 30
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479773/842-30-50-3A
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-SubTopic 30
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479773/842-30-50-14
| Name: |
us-gaap_LessorLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for postemployment benefits. Postemployment benefits are benefits provided to former or inactive employees, their beneficiaries, and covered dependents after employment but before retirement, except for: a) benefits provided through a pension or postretirement benefit plan, b) individual deferred compensation arrangements, c) special or contractual termination benefits, and d) stock compensation plans. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 712
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/712/tableOfContent
| Name: |
us-gaap_PostemploymentBenefitPlansPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for recognizing unearned income or deferred revenue related to transactions involving the sale of a product or performance of services.
| Name: |
us-gaap_RevenueRecognitionDeferredRevenue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for accounts receivable. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-6
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481569/310-20-50-1
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-15
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-11B
| Name: |
us-gaap_TradeAndOtherAccountsReceivablePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
ORGANIZATION AND NATURE OF BUSINESS (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| ORGANIZATION AND NATURE OF BUSINESS |
|
| Schedule of exchange rate |
Consideration | | | | Cash | | $ | 5,331,120 | | Fair value of common stock issued | | | 138,667 | | Fair value of total consideration transferred | | $ | 5,469,787 | | | | | | | Recognized amounts of identifiable assets acquired | | | | | Financial assets | | $ | 1,796,911 | | Inventory | | | 297,340 | | Property, plant and equipment | | | 7,488,818 | | Identifiable intangible assets | | | 562,200 | | Financial liabilities | | | (3,235,233 | ) | Total identifiable net assets | | $ | 6,910,036 | | | | | | | Bargain purchase gain | | $ | 1,440,249 | |
Revenue for the 9 - month period ended September 30, 2024 | | $ | 549,567 | | Loss for the 9 - month period ended September 30, 2024 | | $ | (1,674,785 | ) |
|
| Schedule of Accounts Receivable & Allowance for Doubtful Accounts |
| | September 30, 2024 | | | | | | Balance as of January 1st, 2024 | | $ | 19,686,091 | | Provisions for credit losses | | | - | | Write-offs | | | 250,971 | | Foreign exchange adjustments | | | | | Other adjustments | | | (31,286 | ) | Balance as of September 30, 2024 | | $ | 19,905,776 | |
|
| Schedule of Property and Equipment, net |
| | Estimated Useful Life | | Leasehold improvements and technical works | | | Lesser of lease term or 25 years | | Buildings | | | 25-30 years | | Vehicles | | | 6 years | | Machinery | | | 20 years | | Furniture, fixtures and equipment | | | 5–10 years | | Computers and software | | | 3-5 years | |
|
| Schedule of Basic and Diluted Net Loss per Common Share |
| | September 30, 2024 | | | September 30, 2023 | | Weighted average number of common shares outstanding Basic | | | 17,724,305 | | | | 11,346,071 | | Potentially dilutive common stock equivalents | | | - | | | | - | | Weighted average number of common and equivalent shares outstanding – Diluted | | | 17,724,305 | | | | 11,346,071 | |
|
| Schedule of potential shares of common stock |
| | September 30, 2024 | | | September 30, 2023 | | Warrants | | | 13,432,507 | | | | 6,124,412 | | Total | | | 13,432,507 | | | | 6,124,412 | |
|
| X |
- References
+ Details
| Name: |
abcd_OrganizationAndNatureOfTheBusinessAbstract |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ScheduleOfForeignCurrencyTranslationAndOtherComprehensiveLossTableTextBlock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of allowance for credit loss on accounts receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479319/326-20-50-13
| Name: |
us-gaap_AccountsReceivableAllowanceForCreditLossTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) in the future that were not included in the computation of diluted EPS because to do so would increase EPS amounts or decrease loss per share amounts for the period presented, by antidilutive securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of public utility physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation expense and method used, including composite depreciation, and accumulated depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 980
-SubTopic 20
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481834/980-20-45-1
| Name: |
us-gaap_ScheduleOfPublicUtilityPropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the weighted average number of shares used in calculating basic net earnings per share (or unit) and diluted earnings per share (or unit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfWeightedAverageNumberOfSharesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
PROPERTY AND EQUIPMENT, NET (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| PROPERTY AND EQUIPMENT, NET |
|
| Schedule of Property and Equipment, net |
| | September 30, 2024 | | | December 31, 2023 | | Land | | $ | 3,577,662 | | | $ | 3,551,020 | | Buildings and improvements | | | 4,868,378 | | | | 4,787,963 | | Leasehold improvements | | | 3,667 | | | | 3,639 | | Vehicles | | | 285,609 | | | | 285,388 | | Furniture, fixtures and equipment | | | 3,014,683 | | | | 2,707,442 | | Computers and software | | | 203,215 | | | | 168,173 | | | | | 11,953,214 | | | | 11,503,625 | | Less: Accumulated depreciation and amortization | | | (1,377,286 | ) | | | (1,048,126 | ) | Total | | $ | 10,575,928 | | | $ | 10,455,499 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
INTANGIBLE ASSETS (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| INTANGIBLE ASSETS |
|
| Schedule of Intangible Assets |
| | September 30, 2024 | | | December 31, 2023 | | License | | $ | 6,976,209 | | | $ | 6,876,169 | | Trade name / mark | | | 390,188 | | | | 392,197 | | Customer base | | | 602,204 | | | | 602,204 | | Software | | | 795,867 | | | | 158,787 | | | | | 8,764,468 | | | | 8,029,357 | | Less: Accumulated amortization | | | | | | | | | License | | | (805,555 | ) | | | (235,925 | ) | Trade name / mark | | | (36,997 | ) | | | (36,997 | ) | Customer base | | | (157,333 | ) | | | (110,160 | ) | Software | | | (67,520 | ) | | | (11,789 | ) | Subtotal | | | 7,697,064 | | | | 7,634,486 | | Goodwill | | | 49,697 | | | | 49,697 | | Total | | $ | 7,746,761 | | | $ | 7,684,183 | |
|
| Schedule of amortization expense for intangible assets |
Year | | Amount | | 2024 | | $ | 208,530 | | 2025 | | | 833,637 | | 2026 | | | 834,846 | | 2027 | | | 834,846 | | 2028 | | | 783,423 | | Thereafter | | | 3,846,582 | | Total | | $ | 7,341,864 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of goodwill and intangible assets, which may be broken down by segment or major class. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/350-30/tableOfContent
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/350-20/tableOfContent
| Name: |
us-gaap_ScheduleOfIntangibleAssetsAndGoodwillTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the amount of amortization expense expected to be recorded in succeeding fiscal years for finite-lived intangible assets. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
| Name: |
us-gaap_ScheduleofFiniteLivedIntangibleAssetsFutureAmortizationExpenseTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
RELATED PARTY TRANSACTIONS (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| RELATED PARTY TRANSACTIONS |
|
| Schedule of Related Party Notes Payable activity |
| | September 30, 2024 | | | December 31, 2023 | | | | | | | | | Beginning Balance | | $ | 11,283 | | | $ | 10,912 | | Payments | | | - | | | | - | | Foreign currency translation | | | 85 | | | | 371 | | Ending Balance | | $ | 11,368 | | | $ | 11,283 | |
|
| Schedule of Related Party Loans Payable |
| | September 30, 2024 | | | December 31, 2023 | | | | | | | | | Beginning balance | | $ | 13,257 | | | $ | 12,821 | | Proceeds | | | 18,344 | | | | - | | Payments | | | (8,918 | ) | | | - | | Foreign currency translation | | | (1,525 | ) | | | 436 | | Ending balance | | $ | 21,158 | | | $ | 13,257 | |
|
| X |
- References
+ Details
| Name: |
abcd_ScheduleOfRelatedPartyLoansPayableTableTextBlock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of related party transactions. Examples of related party transactions include, but are not limited to, transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners and (d) affiliates.
| Name: |
us-gaap_ScheduleOfRelatedPartyTransactionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
LINES OF CREDIT (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| LINES OF CREDIT |
|
| Schedule of Lines of Credit |
| | September 30, 2024 | | | December 31, 2023 | | National | | $ | 3,011,102 | | | $ | 3,918,523 | | Alpha | | | 1,030,943 | | | | 1,130,140 | | Pancreta | | | 1,560,803 | | | | 1,122,210 | | EFG | | | 386,577 | | | | 459,400 | | Ending balance | | $ | 5,989,425 | | | $ | 6,630,273 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_LineOfCreditFacilityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of short-term or long-term contractual arrangements with lenders, including letters of credit, standby letters of credit, and revolving credit arrangements, under which borrowings can be made up to maximum amount as of any point in time conditional on satisfaction of specified terms before, as of and after the date of drawdowns on the line. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ScheduleOfLineOfCreditFacilitiesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
NOTES PAYABLE (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| NOTES PAYABLE |
|
| Summary of roll forward of the third party Debt |
September 30, 2024 | | Trade Facility | | | Third Party | | | COVID Loans | | | Total | | Beginning balance, December 31, 2023 | | $ | 1,908,195 | | | $ | 2,511,148 | | | $ | 186,884 | | | $ | 4,606,227 | | Proceeds | | | - | | | | 445,800 | | | | - | | | | 445,800 | | Payments | | | (334,350 | ) | | | (481,254 | ) | | | (19,073 | ) | | | (834,677 | ) | Conversion of debt | | | - | | | | - | | | | - | | | | - | | Recapitalized upon debt modification | | | - | | | | - | | | | - | | | | - | | Accretion of debt and debt discount | | | - | | | | - | | | | - | | | | - | | Foreign currency translation | | | 14,318 | | | | 32,613 | | | | 3,691 | | | | 50,622 | | Ending balance, September 30, 2024 | | | 1,588,163 | | | | 2,508,307 | | | | 171,502 | | | | 4,267,972 | | Notes payable - long-term | | | (1,086,638 | ) | | | (1,416,802 | ) | | | (142,183 | ) | | | (2,645,623 | ) | Notes payable - short-term | | $ | 501,525 | | | $ | 1,091,505 | | | $ | 29,319 | | | | 1,622,349 | |
December 31, 2023 | | Trade Facility | | | Third Party | | | COVID Loans | | | Total | | Beginning balance, December 31, 2022 | | $ | 3,305,532 | | | $ | 1,505,078 | | | $ | 207,377 | | | $ | 5,017,987 | | Proceeds | | | - | | | | 1,082,231 | | | | - | | | | 1,082,231 | | Payments | | | (1,155,310 | ) | | | (415,557 | ) | | | (27,027 | ) | | | (1,597,894 | ) | Oher additions | | | - | | | | 317,880 | | | | - | | | | 317,880 | | Debt forgiveness | | | (306,637 | ) | | | - | | | | - | | | | (306,637 | ) | Foreign currency translation | | | 64,610 | | | | 21,516 | | | | 6,534 | | | | 92,660 | | Ending balance, December 31, 2023 | | | 1,908,195 | | | | 2,511,148 | | | | 186,884 | | | | 4,606,227 | | Notes payable – long-term | | | (1,327,440 | ) | | | (1,549,768 | ) | | | (158,133 | ) | | | (3,035,341 | ) | Notes payable - short-term | | $ | 580,755 | | | $ | 961,380 | | | $ | 28,751 | | | $ | 1,570,886 | |
|
| Summary of Outstanding Debt |
Our outstanding debt as of September 30, 2024 is repayable as follows: | | | September 30, 2024 | | 2025 | | $ | 1,622,349 | | 2026 | | | 1,717,738 | | 2027 | | | 420,633 | | 2028 | | | 353,961 | | 2029 and thereafter | | | 153,291 | | Total debt | | | 4,267,972 | | Less: notes payable - current portion | | | (1,622,349 | ) | Notes payable - long term portion | | $ | 2,645,623 | |
|
| X |
- References
+ Details
| Name: |
abcd_ScheduleOfOutstandingDebtTableTextBlock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DebtDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of information pertaining to short-term and long-debt instruments or arrangements, including but not limited to identification of terms, features, collateral requirements and other information necessary to a fair presentation.
| Name: |
us-gaap_ScheduleOfDebtTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
LEASES (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| LEASES |
|
| Schedule of maturity of Operating Lease liability |
Maturity of Operating Lease Liability | | | | 2024 | | | 82,319 | | 2025 | | | 243,175 | | 2026 | | | 179,151 | | 2027 and thereafter | | | 300,301 | | Total undiscounted operating lease payments | | $ | 804,946 | | Less: Imputed interest | | | (95,366 | ) | Present value of operating lease liabilities | | $ | 709,580 | |
|
| Schedule of maturity of finance lease liability |
Maturity of Lease Liability | | | | 2024 | | | 9,039 | | 2025 | | | 12,892 | | 2026 | | | 3,712 | | Total undiscounted finance lease payments | | $ | 25,643 | | Less: Imputed interest | | | (910 | ) | Present value of finance lease liabilities | | $ | 24,733 | |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of finance lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to finance lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_FinanceLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
STOCK OPTIONS AND WARRANTS (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| STOCK OPTIONS AND WARRANTS |
|
| Schedule of option activity |
| | | | | | | | Weighted | | | | | | | | | | Weighted | | | Average | | | | | | | | | | Average | | | Remaining | | | Aggregate | | | | Number of | | | Exercise | | | Contractual | | | Intrinsic | | Warrants | | Shares | | | Price | | | Term | | | Value | | Balance Outstanding, January 1, 2023 | | | 4,194,236 | | | $ | 8.31 | | | | 5.04 | | | $ | 2,562,621 | | Granted | | | 7,524,933 | | | | 1.65 | | | | 5.13 | | | | - | | Forfeited | | | - | | | | - | | | | - | | | | - | | Exercised | | | (3,152,386 | ) | | | - | | | | - | | | | - | | Expired | | | (5,307 | ) | | | - | | | | - | | | | - | | Balance Outstanding, December 31, 2023 | | | 8,561,476 | | | $ | 3.91 | | | | 4.64 | | | $ | 18,801 | | Granted | | | 9,748,252 | | | | 0.95 | | | | 3.24 | | | | - | | Forfeited | | | - | | | | - | | | | - | | | | - | | Exercised | | | (4,874,126 | ) | | | - | | | | - | | | | - | | Expired | | | (3,096 | ) | | | - | | | | - | | | | - | | Balance Outstanding, September 30, 2024 | | | 13,432,506 | | | $ | 2.64 | | | | 3.28 | | | $ | 11,681 | | | | | | | | | - | | | | - | | | | - | | Exercisable, September 30, 2024 | | | 13,432,506 | | | $ | 2.64 | | | | 3.28 | | | $ | 11,681 | |
|
| X |
- References
+ Details
| Name: |
abcd_StockOptionsAndWarrantsAbstract |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
DISAGGREGATION OF REVENUE (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| DISAGGREGATION OF REVENUE |
|
| Schedule of Revenue Disaggregation |
Country | | September 30, 2024 | | | September 30, 2023 | | Croatia | | $ | 107 | | | | 14,159 | | Cyprus | | | 16,519 | | | | 72,754 | | Bulgaria | | | 25,507 | | | | - | | Greece | | | 12,247,597 | | | | 12,544,643 | | USA | | | - | | | | 210 | | Ireland | | | - | | | | 1,417 | | UK | | | 121,318 | | | | 190,614 | | Total | | $ | 12,411,048 | | | $ | 12,823,797 | |
Country | | September 30, 2024 | | | September 30, 2023 | | Croatia | | $ | 19,370 | | | | 14,159 | | Cyprus | | | 89,064 | | | | 141,402 | | Bulgaria | | | 43,849 | | | | - | | Greece | | | 39,385,730 | | | | 36,041,012 | | USA | | | - | | | | 504 | | Ireland | | | - | | | | 1,417 | | UK | | | 664,225 | | | | 1,338,509 | | Total | | $ | 40,202,238 | | | $ | 37,537,003 | |
|
| X |
- DefinitionTabular disclosure of disaggregation of revenue into categories depicting how nature, amount, timing, and uncertainty of revenue and cash flows are affected by economic factor. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-5
| Name: |
us-gaap_DisaggregationOfRevenueTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_RevenueFromContractWithCustomerAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
BASIS OF PRESENTATION (Details Narrative) - USD ($)
|
9 Months Ended |
|
|
|
|
|
|
Sep. 30, 2024 |
Sep. 30, 2023 |
Jun. 30, 2024 |
Mar. 31, 2024 |
Dec. 31, 2023 |
Jun. 30, 2023 |
Mar. 31, 2023 |
Dec. 31, 2022 |
| Net loss |
$ (6,639,935)
|
$ (4,790,597)
|
|
|
|
|
|
|
| Amortization of right-of-use assets |
22,507
|
102,549
|
|
|
|
|
|
|
| Accumulated deficit |
$ (104,479,192)
|
|
|
|
$ (91,644,233)
|
|
|
|
| Description of the warrants and reduced the exercise price |
which the Company issued 9,748,252 new warrants and reduced the exercise price of 4,874,126 warrant shares from $1.45 to $0.8701 to induce exercise and receive gross cash proceeds of $4,240,977
|
|
|
|
|
|
|
|
| Stockholders' equity |
$ 34,976,599
|
$ 44,195,740
|
$ 32,119,574
|
$ 34,546,682
|
$ 36,043,028
|
$ 43,669,058
|
$ 44,007,783
|
$ 39,284,295
|
| CANA Pharmaceutical Laboratories, S.A. ("Cana") [Member] |
|
|
|
|
|
|
|
|
| Net cash used in operations |
3,883,215
|
|
|
|
|
|
|
|
| Working capital |
11,027,653
|
|
|
|
|
|
|
|
| Amortization of right-of-use assets |
1,588,163
|
|
|
|
|
|
|
|
| Cash proceed |
$ 629,426
|
|
|
|
|
|
|
|
| Proceeds from sale of common stock |
901,488
|
|
|
|
|
|
|
|
| X |
- References
+ Details
| Name: |
abcd_DescriptionOfTheWarrantsAndReducedTheExercisePrice |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ProceedsFromSaleOfCommonStockNetOfFinancingFeesOf19467Shares |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_WorkingCapital |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe increase (decrease) in cash associated with the entity's continuing operating, investing, and financing activities. While for technical reasons this element has no balance attribute, the default assumption is a debit balance consistent with its label. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInContinuingOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of periodic reduction over lease term of carrying amount of right-of-use asset from operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_OperatingLeaseRightOfUseAssetAmortizationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 11: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-3
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477314/942-235-S99-1
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 31: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4J
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4K
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-2
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash restricted as to withdrawal or usage. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(1)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_RestrictedCash |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=abcd_CANAPharmaceuticalLaboratoriesSACanaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
ORGANIZATION AND NATURE OF BUSINESS (Details) - USD ($)
|
3 Months Ended |
9 Months Ended |
|
Sep. 30, 2024 |
Jun. 30, 2024 |
Mar. 31, 2024 |
Sep. 30, 2023 |
Jun. 30, 2023 |
Mar. 31, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Dec. 31, 2023 |
| Financial assets |
$ 64,519,982
|
|
|
|
|
|
$ 64,519,982
|
|
$ 66,014,811
|
| Net loss |
(2,182,534)
|
$ (2,590,711)
|
$ (1,866,690)
|
$ (3,349,204)
|
$ (981,530)
|
$ (459,863)
|
(6,639,935)
|
$ (4,790,597)
|
|
| CANA Pharmaceutical Laboratories, S.A. ("Cana") [Member] |
|
|
|
|
|
|
|
|
|
| Cash |
|
|
|
|
|
|
5,331,120
|
|
|
| Fair value of common stock issued |
|
|
|
$ 138,667
|
|
|
138,667
|
|
|
| Fair value of total consideration transferred |
|
|
|
|
|
|
5,469,787
|
|
|
| Financial assets |
1,796,911
|
|
|
|
|
|
1,796,911
|
|
|
| Inventory |
297,340
|
|
|
|
|
|
297,340
|
|
|
| Property, plant and equipment |
7,488,818
|
|
|
|
|
|
7,488,818
|
|
|
| Identifiable intangible assets |
562,200
|
|
|
|
|
|
562,200
|
|
|
| Financial liabilities |
(3,235,233)
|
|
|
|
|
|
(3,235,233)
|
|
|
| Total identifiable net assets |
6,910,036
|
|
|
|
|
|
6,910,036
|
|
|
| Bargain purchase gain |
$ 1,440,249
|
|
|
|
|
|
1,440,249
|
|
|
| Revenue |
|
|
|
|
|
|
549,567
|
|
|
| Net loss |
|
|
|
|
|
|
$ (1,674,785)
|
|
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 12: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 30: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of consideration transferred, consisting of acquisition-date fair value of assets transferred by the acquirer, liabilities incurred by the acquirer, and equity interest issued by the acquirer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 30
-Paragraph 8
-SubTopic 30
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479637/805-30-30-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 30
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479581/805-30-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 30
-Paragraph 7
-SubTopic 30
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479637/805-30-30-7
| Name: |
us-gaap_BusinessCombinationConsiderationTransferred1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity interests of the acquirer, including instruments or interests issued or issuable in consideration for the business combination. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 30
-Paragraph 8
-SubTopic 30
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479637/805-30-30-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 30
-Paragraph 7
-SubTopic 30
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479637/805-30-30-7
| Name: |
us-gaap_BusinessCombinationConsiderationTransferredEquityInterestsIssuedAndIssuable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of financial liabilities assumed (as defined) which have been recognized as of the acquisition date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 10
-Section 55
-Paragraph 37
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479303/805-10-55-37
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedFinancialLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of inventory recognized as of the acquisition date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 10
-Section 55
-Paragraph 37
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479303/805-10-55-37
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedInventory |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount recognized as of the acquisition date for the identifiable assets acquired in excess of (less than) the aggregate liabilities assumed. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 10
-Section 55
-Paragraph 37
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479303/805-10-55-37
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of assets expected to be realized or consumed after one year or the normal operating cycle, if longer, acquired at the acquisition date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedNoncurrentAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of property, plant, and equipment recognized as of the acquisition date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 10
-Section 55
-Paragraph 37
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479303/805-10-55-37
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount recognized for assets, including goodwill, in excess of (less than) the aggregate liabilities assumed. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredGoodwillAndLiabilitiesAssumedNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of business during the period. The cash portion only of the acquisition price. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479581/805-30-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireBusinessesGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, including tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value-added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 4: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479941/924-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-5
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-40
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerIncludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=abcd_CANAPharmaceuticalLaboratoriesSACanaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- References
+ Details
| Name: |
abcd_ChangesInAllowanceForDoubtfulAccountsBeginningBalance |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ChangesInAllowanceForDoubtfulAccountsEndingBalance |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_OrganizationAndNatureOfTheBusinessAbstract |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of recurring noncash expense charged against earnings in the period to allocate the cost of assets over their estimated remaining economic lives. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_AdjustmentForAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of loans and leases that have been written off from both loan receivables and allowance reserve for credit loss. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11B
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-11B
| Name: |
us-gaap_AllowanceForLoanAndLeaseLossesWriteOffs |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense related to credit loss from transactions other than loan and lease transactions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(11))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_ProvisionForOtherCreditLosses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAdjustments to temporary equity resulting from foreign currency translation adjustments.
| Name: |
us-gaap_TemporaryEquityForeignCurrencyTranslationAdjustments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.3
| X |
- References
+ Details
| Name: |
abcd_PropertyPlantAndEquipmentUsefulLifes |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_PropertyPlantsAndEquipmentEstimatedUsefulLives |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_PropertyPlantsAndEquipmentUsefulLife |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_VehiclesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=abcd_MachineryMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=abcd_LeaseholdImprovementsAndTechnicalWorksMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=abcd_BuildingsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_BottomMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_TopMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_FurnitureAndFixturesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_ComputerEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
ORGANIZATION AND NATURE OF BUSINESS (Details 3) - shares
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| ORGANIZATION AND NATURE OF BUSINESS |
|
|
|
|
| Weighted average number of common shares outstanding Basic |
18,418,287
|
12,585,479
|
17,724,305
|
11,346,071
|
| Weighted average number of common and equivalent shares outstanding - Diluted |
|
|
17,724,305
|
11,346,071
|
| X |
- References
+ Details
| Name: |
abcd_OrganizationAndNatureOfTheBusinessAbstract |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_WeightedAverageNumberOfCommonAndEquivalentSharesOutstandingDiluted |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
ORGANIZATION AND NATURE OF BUSINESS (Details 4) - shares
|
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
| ORGANIZATION AND NATURE OF BUSINESS |
|
|
| Common Stock Warrants |
13,432,507
|
6,124,412
|
| Convertible Debt |
13,432,507
|
6,124,412
|
| X |
- References
+ Details
| Name: |
abcd_OrganizationAndNatureOfTheBusinessAbstract |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares issued in exchange for the original debt being converted in a noncash (or part noncash) transaction. "Part noncash" refers to that portion of the transaction not resulting in cash receipts or payments in the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-5
| Name: |
us-gaap_DebtConversionConvertedInstrumentSharesIssued1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAdditional shares included in the calculation of diluted EPS as a result of the potentially dilutive effect of call options and warrants using the treasury stock method. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-22
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 26
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-26
| Name: |
us-gaap_IncrementalCommonSharesAttributableToCallOptionsAndWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
ORGANIZATION AND NATURE OF BUSINESS (Details Narrative) - USD ($)
|
1 Months Ended |
3 Months Ended |
9 Months Ended |
12 Months Ended |
|
|
Jan. 23, 2024 |
Jun. 15, 2023 |
Apr. 30, 2023 |
Mar. 17, 2023 |
Feb. 28, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Jun. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Dec. 31, 2023 |
Jan. 06, 2023 |
Dec. 19, 2018 |
| VAT net receivable and payable balance |
|
|
|
|
|
$ 322,576
|
|
|
$ 322,576
|
|
$ 187,512
|
|
|
| Long-term liability |
|
|
|
|
|
395,698
|
|
|
395,698
|
|
408,665
|
|
|
| Allowance for doubtful accounts |
|
|
|
|
|
|
|
|
19,905,776
|
|
19,686,091
|
|
|
| Deferred amount |
|
|
|
|
|
|
|
|
|
|
397,000
|
|
|
| Foreign accounts |
|
|
|
|
|
12,655,615
|
|
|
12,655,615
|
|
532,704
|
|
|
| Depreciation expense |
|
|
|
|
|
89,694
|
|
$ 124,910
|
306,126
|
$ 237,479
|
|
|
|
| Amortization expense |
|
|
|
|
|
196,183
|
|
$ 88,168
|
579,556
|
$ 138,438
|
|
|
|
| Equity method investment aggregate cost |
|
|
|
|
|
|
|
|
|
|
|
$ 3,950,000
|
|
| Goodwill |
|
|
|
|
|
49,697
|
|
|
49,697
|
|
$ 49,697
|
|
$ 49,697
|
| ICC International Cannabis Corp [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Equity method investment aggregate cost |
|
|
|
|
|
0
|
|
|
$ 0
|
|
|
|
|
| National Bank of Greece [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares of common stock issued |
|
|
|
|
|
|
|
|
16,666
|
|
|
|
|
| Equity method investment aggregate cost |
|
|
|
|
|
12,416
|
|
|
$ 12,416
|
|
|
|
|
| Closing price |
|
|
|
|
|
|
|
|
$ 0.75
|
|
|
|
|
| Pancreta Bank [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Equity method investment aggregate cost |
|
|
|
|
|
$ 7,665
|
|
|
$ 7,665
|
|
|
|
|
| Greece [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash |
|
$ 109,330
|
|
|
|
|
|
|
|
|
|
|
|
| Intangible assets |
|
425,411
|
|
|
|
|
|
|
|
|
|
|
|
| Issuable stock amount |
|
$ 316,081
|
|
|
|
|
|
|
|
|
|
|
|
| Income tax rate |
|
|
|
|
|
|
|
|
22.00%
|
|
|
|
|
| Shares issued |
|
99,710
|
|
|
|
|
|
|
|
|
|
|
|
| United Kingdom Of England [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Income tax rate |
|
|
|
|
|
|
|
|
25.00%
|
|
|
|
|
| CANA Pharmaceutical Laboratories, S.A. ("Cana") [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash |
|
|
|
|
|
|
$ 873,600
|
|
|
|
|
|
|
| Issuable stock amount |
|
|
|
|
|
|
$ 138,667
|
|
$ 138,667
|
|
|
|
|
| Shares issued |
|
|
|
|
|
|
46,377
|
|
|
|
|
|
|
| Asset acquision |
|
|
|
|
$ 5,469,787
|
|
|
|
|
|
|
|
|
| Secured Promissory Note |
|
|
|
|
$ 4,457,520
|
|
|
|
|
|
|
|
|
| Zip Doctor Inc [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash |
|
|
|
$ 150,000
|
|
|
|
|
|
|
|
|
|
| Payment in fees |
|
|
|
$ 8,788
|
|
|
|
|
|
|
|
|
|
| Cloudscreen [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash |
$ 317,880
|
|
$ 1,054,872
|
|
|
|
|
|
|
|
|
|
|
| Total purchase price amount |
$ 637,080
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares of common stock issued |
280,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares of common fair value |
$ 319,200
|
|
|
|
|
|
|
|
|
|
|
|
|
| Asset acquisition |
$ 637,080
|
|
|
|
|
|
|
|
|
|
|
|
|
| X |
- References
+ Details
| Name: |
abcd_AllowanceForDoubtfulAccounts |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_BusinessCombinationConsiderationTransferredEquityInterestsIssuedAndIssuableShares |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ClosingPrice |
| Namespace Prefix: |
abcd_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ForeignAccounts |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_LongTermLiabilities |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_PaymentInFees |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe adjustment needed to reconcile previously recorded amounts to the actual aggregate amount paid, whether in cash or other consideration, to acquire all of the shares purchased under an Accelerated Share Repurchase arrangement.
| Name: |
us-gaap_AcceleratedShareRepurchasesAdjustmentToRecordedAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense charged against earnings to allocate the cost of intangible assets (nonphysical assets not used in production) in a systematic and rational manner to the periods expected to benefit from such assets. As a noncash expense, this element is added back to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482686/350-30-45-2
| Name: |
us-gaap_AmortizationOfIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of tangible and intangible assets included as part of consideration transferred in asset acquisition, classified as other. Excludes cash. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 805
-SubTopic 50
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480060/805-50-25-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 805
-SubTopic 50
-Name Accounting Standards Codification
-Section 30
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480027/805-50-30-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 805
-SubTopic 50
-Name Accounting Standards Codification
-Section 30
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480027/805-50-30-2
| Name: |
us-gaap_AssetAcquisitionConsiderationTransferredOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionPurchase price of expected asset acquisition prior to consideration being transferred. Excludes business acquisition. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 805
-SubTopic 50
-Name Accounting Standards Codification
-Section 15
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480123/805-50-15-3
| Name: |
us-gaap_AssetAcquisitionPriceOfAcquisitionExpected |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity interests of the acquirer, including instruments or interests issued or issuable in consideration for the business combination. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 30
-Paragraph 8
-SubTopic 30
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479637/805-30-30-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 30
-Paragraph 7
-SubTopic 30
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479637/805-30-30-7
| Name: |
us-gaap_BusinessCombinationConsiderationTransferredEquityInterestsIssuedAndIssuable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of intangible assets, excluding goodwill, acquired at the acquisition date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 20
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479907/805-20-50-1
| Name: |
us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedIntangibleAssetsOtherThanGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe total fair value of shares issued during the period under a deferred compensation arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DeferredCompensationArrangementWithIndividualFairValueOfSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of deferred cost, excluding capitalized cost related to contract with customer; classified as noncurrent. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_DeferredCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThis element represents the aggregate cost of investments accounted for under the equity method of accounting. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(12))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_EquityMethodInvestmentAggregateCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after accumulated impairment loss, of asset representing future economic benefit arising from other asset acquired in business combination or from joint venture formation or both, that is not individually identified and separately recognized. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482548/350-20-55-24
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482598/350-20-45-1
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482573/350-20-50-1
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482573/350-20-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Goodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash outflow for purchases of and capital improvements on property, plant and equipment (capital expenditures), software, and other intangible assets. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 805
-SubTopic 50
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480060/805-50-25-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 805
-SubTopic 50
-Name Accounting Standards Codification
-Section 30
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480027/805-50-30-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 805
-SubTopic 50
-Name Accounting Standards Codification
-Section 30
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480027/805-50-30-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireProductiveAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from borrowings supported by a written promise to pay an obligation that is collateralized (backed by pledge, mortgage or other lien in the entity's assets). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromSecuredNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued during the period pursuant to acquisitions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesAcquisitions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying amount as of the balance sheet date of value added taxes due either from customers arising from sales on credit terms, or as previously overpaid to tax authorities. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(3)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ValueAddedTaxReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=abcd_CANAPharmaceuticalLaboratoriesSACanaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=abcd_ZipDoctorIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=abcd_CloudscreenMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
EQUITY METHOD INVESTMENTS (Details Narrative)
|
3 Months Ended |
9 Months Ended |
|
|
Sep. 30, 2024
USD ($)
|
Sep. 30, 2023
USD ($)
|
Sep. 30, 2024
USD ($)
|
Sep. 30, 2024
CAD ($)
|
Sep. 30, 2024
EUR (€)
|
Sep. 30, 2023
USD ($)
|
Dec. 31, 2023
USD ($)
|
| Gain on extinguishment of debt |
$ 0
|
$ 706
|
$ 0
|
|
|
$ 1,911,476
|
|
| Cosmo Farmacy LP [Member] |
|
|
|
|
|
|
|
| Initial share capital | € |
|
|
|
|
€ 150,000
|
|
|
| Initial share capital increased | € |
|
|
|
|
500,000
|
|
|
| Pharmacy license value | € |
|
|
|
|
€ 350,000
|
|
|
| Maturity period of license |
|
|
30 years
|
30 years
|
30 years
|
|
|
| Ownership equity |
70.00%
|
|
70.00%
|
|
|
|
|
| Cash contributed to limited partner | € |
|
|
|
|
€ 150,000
|
|
|
| Equity ownership remaining |
30.00%
|
|
30.00%
|
|
|
|
|
| Marathon Global Inc [Member] | Distribution and Equity Acquisition Agreement [Member] |
|
|
|
|
|
|
|
| Upfront cash received |
|
|
|
$ 2,000,000
|
|
|
|
| Fair value of settlement account |
|
|
$ 1,554,590
|
|
|
|
|
| Gain on extinguishment of debt |
|
|
$ 1,554,590
|
|
|
|
|
| Agreement term |
|
|
five (5) years
|
five (5) years
|
five (5) years
|
|
|
| Cash received upon gross sales |
|
|
|
$ 2,750,000
|
|
|
|
| Marathon Global Inc [Member] | Distribution and Equity Acquisition Agreement [Member] | Gross Sales One [Member] |
|
|
|
|
|
|
|
| Cash received upon gross sales |
|
|
|
2,750,000
|
|
|
|
| Gross sales |
|
|
|
13,000,000
|
|
|
|
| Marathon Global Inc [Member] | Distribution and Equity Acquisition Agreement [Member] | Gross Sales [Member] |
|
|
|
|
|
|
|
| Cash received upon gross sales |
|
|
|
2,750,000
|
|
|
|
| Gross sales |
|
|
|
$ 6,500,000
|
|
|
|
| Share Exchange Agreement [Member] | ICC [Member] |
|
|
|
|
|
|
|
| Investement |
$ 167,175
|
|
$ 167,175
|
|
|
|
$ 165,930
|
| X |
- References
+ Details
| Name: |
abcd_AgreementTerm |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_CashContributedToLimitedPartner |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_CashReceivedUponGrossSales |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_EquityMethodInvestmentOwnershipRemainingPercentage |
| Namespace Prefix: |
abcd_ |
| Data Type: |
num:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_EquityMethodInvestmentsOwnershipPercentage |
| Namespace Prefix: |
abcd_ |
| Data Type: |
num:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_GrossSales |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_InitialShareCapital |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_InitialShareCapitalIncreased |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_MaturityPeriodOfLicense |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_PharmacyLicenseValue |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_SettlementAmountDebtObligation |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_UpfrontCashReceived |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionDifference between the fair value of payments made and the carrying amount of debt which is extinguished prior to maturity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481303/470-50-40-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481303/470-50-40-4
| Name: |
us-gaap_GainsLossesOnExtinguishmentOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all investments. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 944
-SubTopic 80
-Name Accounting Standards Codification
-Section 55
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480078/944-80-55-14
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 944
-SubTopic 80
-Name Accounting Standards Codification
-Section 55
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480078/944-80-55-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(1)(h))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(1)(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_Investments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=abcd_DistributionAndEquityAcquisitionAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=abcd_SalesOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_SalesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_ShareExchangeAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
PROPERTY PLANT AND EQUIPMENT (Details) - USD ($)
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Property plant and equipment |
$ 11,953,214
|
$ 11,503,625
|
| Less: Accumulated depreciation |
(1,377,286)
|
(1,048,126)
|
| Total |
10,575,928
|
10,455,499
|
| Land [Member] |
|
|
| Property plant and equipment |
3,577,662
|
3,551,020
|
| Computers and software [Member] |
|
|
| Property plant and equipment |
203,215
|
168,173
|
| Vehicles [Member] |
|
|
| Property plant and equipment |
285,609
|
285,388
|
| Leasehold Improvements [Member] |
|
|
| Property plant and equipment |
3,667
|
3,639
|
| Furniture, fixtures and equipment [Member] |
|
|
| Property plant and equipment |
3,014,683
|
2,707,442
|
| Building And Improvements [Member] |
|
|
| Property plant and equipment |
$ 4,868,378
|
$ 4,787,963
|
| X |
- DefinitionAmount of accumulated depreciation, depletion and amortization for physical assets used in the normal conduct of business to produce goods and services. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(14))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(13))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478451/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=us-gaap_LandMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_ComputerSoftwareIntangibleAssetMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_VehiclesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_LeaseholdImprovementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_FurnitureAndFixturesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_BuildingImprovementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
INTANGIBLE ASSETS NET (Details) - USD ($)
|
Sep. 30, 2024 |
Dec. 31, 2023 |
Dec. 19, 2018 |
| Goodwill and intangible assets, gross |
$ 8,764,468
|
$ 8,029,357
|
|
| Subtotal |
7,697,064
|
7,634,486
|
|
| Goodwill |
49,697
|
49,697
|
$ 49,697
|
| Total |
7,746,761
|
7,684,183
|
|
| Customer Base [Member] |
|
|
|
| Goodwill and intangible assets, gross |
602,204
|
602,204
|
|
| Less: Accumulated Amortization |
157,333
|
110,160
|
|
| Trade name / mark [Member] |
|
|
|
| Goodwill and intangible assets, gross |
390,188
|
392,197
|
|
| Less: Accumulated Amortization |
(36,997)
|
(36,997)
|
|
| License [Member] |
|
|
|
| Goodwill and intangible assets, gross |
6,976,209
|
6,876,169
|
|
| Less: Accumulated Amortization |
805,555
|
235,925
|
|
| Software [Member] |
|
|
|
| Goodwill and intangible assets, gross |
795,867
|
158,787
|
|
| Less: Accumulated Amortization |
$ 67,520
|
$ 11,789
|
|
| X |
- DefinitionAccumulated amount of amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 10
-Name Accounting Standards Codification
-Section S45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480265/350-10-S45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAccumulatedAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after accumulated impairment loss, of asset representing future economic benefit arising from other asset acquired in business combination or from joint venture formation or both, that is not individually identified and separately recognized. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482548/350-20-55-24
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482598/350-20-45-1
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482573/350-20-50-1
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482573/350-20-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Goodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts of all intangible assets, excluding goodwill, as of the balance sheet date, net of accumulated amortization and impairment charges. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482686/350-30-45-1
| Name: |
us-gaap_IntangibleAssetsNetExcludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after impairment and amortization, of goodwill, indefinite-lived, and finite-lived intangible assets. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 10
-Name Accounting Standards Codification
-Section S45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480265/350-10-S45-1
| Name: |
us-gaap_IntangibleAssetsNetIncludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before accumulated amortization of finite-lived intangible assets classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherFiniteLivedIntangibleAssetsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_CustomerListsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IndefiniteLivedIntangibleAssetsByMajorClassAxis=abcd_TradeNameMarkMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=us-gaap_LicenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=abcd_SoftwareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
INTANGIBLE ASSETS NET (Details 1)
|
Sep. 30, 2024
USD ($)
|
| INTANGIBLE ASSETS |
|
| 2024 |
$ 208,530
|
| 2025 |
833,637
|
| 2026 |
834,846
|
| 2027 |
834,846
|
| 2028 |
783,423
|
| Thereafter |
3,846,582
|
| Total amortization |
$ 7,341,864
|
| X |
- DefinitionAmount of amortization for asset, excluding financial asset and goodwill, lacking physical substance with finite life expected to be recognized after fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseAfterYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483154/926-20-50-5
| Name: |
us-gaap_FiniteLivedIntangibleAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
LOAN RECEIVABLE (Details Narrative) - USD ($)
|
1 Months Ended |
9 Months Ended |
12 Months Ended |
Oct. 30, 2021 |
Sep. 30, 2024 |
Dec. 31, 2023 |
| LOAN RECEIVABLE |
|
|
|
| Prepayments of loan |
$ 4,849,221
|
|
|
| Interest rate |
5.50%
|
|
|
| Principal payments |
|
|
$ 389,867
|
| Short-term receivable |
|
$ 249,552
|
411,858
|
| Interest payments |
|
$ 119,411
|
|
| Long term receivables |
|
|
$ 3,509,200
|
| Loan receivables term |
360 days
|
|
|
| Decription of loan payment |
the Company is to receive 120 equal payments over the term of the loan
|
|
|
| X |
- References
+ Details
| Name: |
abcd_DecriptionOfLoanPayment |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_InterestPayments |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_LoanReceivableabstract |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ThresholdPeriodPastDueForWriteoffsOfFinancingReceivable |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the required periodic payments including both interest and principal payments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 470
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477734/942-470-50-3
| Name: |
us-gaap_DebtInstrumentPeriodicPayment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying amount as of the balance sheet date of interest earned but not received. Also called accrued interest or accrued interest receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 310
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477802/946-310-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(3)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InterestReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying amounts due as of the balance sheet date of the sum of amounts receivable other than from customers. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(3)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_NontradeReceivables |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash outflow for loan origination associated cost which is usually collected through escrow. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsOfLoanCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage increase in the stated interest rate on a short-term debt instrument.
| Name: |
us-gaap_ShortTermDebtInterestRateIncrease |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
| X |
- References
+ Details
| Name: |
abcd_CorporateTaxRate |
| Namespace Prefix: |
abcd_ |
| Data Type: |
num:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
CAPITAL STRUCTURE (Details Narrative) - USD ($)
|
1 Months Ended |
3 Months Ended |
9 Months Ended |
12 Months Ended |
Sep. 26, 2024 |
Dec. 29, 2023 |
Jan. 24, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Preferred stock, shares authorized |
|
|
|
100,000,000
|
|
100,000,000
|
|
100,000,000
|
|
| Shares issued to raised additional equity funds, shares |
|
|
|
|
|
901,488
|
|
|
|
| Preferred stock, shares outstanding |
|
|
|
|
|
|
|
0
|
|
| Shares issued to raised additional equity funds, value |
|
|
|
|
|
$ 648,893
|
|
|
|
| Preferred stock, shares issued |
|
|
|
|
|
|
|
0
|
|
| Deemed dividend |
|
|
|
$ 6,185,231
|
$ 0
|
$ 6,185,231
|
|
|
|
| Common stock, shares issued |
2,332,000
|
1,487,000
|
|
17,834,023
|
|
17,834,023
|
|
15,982,472
|
|
| Common stock, shares authorized |
|
|
|
300,000,000
|
|
300,000,000
|
|
300,000,000
|
|
| Common stock, shares issued |
|
|
|
23,346,023
|
|
23,346,023
|
|
15,982,472
|
|
| Common stock, shares purchased amount |
|
|
|
|
|
$ 0
|
$ 100,251
|
|
|
| Common stock shares value |
|
|
|
$ 23,346
|
|
$ 23,346
|
|
$ 15,983
|
|
| Common stock, shares outstanding |
|
|
|
21,346,023
|
|
21,346,023
|
|
15,895,975
|
|
| Gross cash proceeds from warrants exercised |
$ 4,240,977
|
$ 3,533,741
|
|
|
|
$ 4,240,977
|
$ 0
|
|
|
| Shares held in escrow |
2,532,126
|
950,063
|
|
|
|
|
|
|
|
| Warrant Inducement Letter [Member] |
|
|
|
|
|
|
|
|
|
| Common stock, shares issued |
|
1,487,000
|
|
|
|
|
|
|
|
| Pre-funded warrants issued |
9,748,252
|
2,437,063
|
|
|
|
|
|
|
|
| Gross cash proceeds from warrants exercised |
$ 4,240,977
|
$ 3,533,741
|
|
|
|
|
|
|
|
| Shares held in escrow |
|
950,063
|
|
|
|
|
|
|
|
| Term |
|
5 years
|
|
|
|
|
|
|
|
| Placement Agent [Member] |
|
|
|
|
|
|
|
|
|
| Fees and other commissions |
|
|
|
|
|
19,467
|
|
|
|
| Net proceeds |
|
|
|
|
|
$ 629,426
|
|
|
|
| Warrant Exchange [Member] |
|
|
|
|
|
|
|
|
|
| Common stock, shares issued |
2,332,000
|
1,487,000
|
|
2,332,000
|
|
2,332,000
|
|
|
|
| Pre-funded warrants issued |
4,874,126
|
2,437,063
|
|
2,332,000
|
|
2,332,000
|
|
|
|
| Gross cash proceeds from warrants exercised |
$ 4,240,977
|
$ 3,533,741
|
|
|
|
|
|
|
|
| Escrow fees |
|
|
|
$ 372,109
|
|
$ 372,109
|
|
|
|
| Shares held in escrow |
2,532,126
|
950,063
|
|
|
|
|
|
|
|
| Term |
5 years
|
5 years
|
|
|
|
|
|
|
|
| Warrant Exchange [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
|
| Warrants exercise price |
$ 1.45
|
$ 1.45
|
|
|
|
|
|
|
|
| Warrant Exchange [Member] | Minimum [Member] |
|
|
|
|
|
|
|
|
|
| Warrants exercise price |
$ 0.8701
|
$ 2.75
|
|
|
|
|
|
|
|
| Series A Preferred Stock [Member] |
|
|
|
|
|
|
|
|
|
| Deemed dividend |
|
|
|
|
|
$ 372,414
|
|
|
$ 372,414
|
| Description of convertible into Common Stock |
|
|
|
|
|
The Series A Preferred Stock was initially convertible into the Company’s Common Stock as determined by dividing the number of shares of Series A Preferred Stock to be converted by the lower of (i) $75.00 or (ii) 80% of the average volume weighted average price for the Company’s Common Stock for the five trading days immediately following the effectiveness of the registration statement concerning the shares (the “Conversion Price”). On June 14, 2022, the Conversion Price was reset to $15.54 per share
|
|
|
|
| Dividend rate, per year |
|
|
|
|
|
|
|
|
8.00%
|
| Total mezzanine equity |
|
|
|
$ 0
|
|
$ 0
|
|
$ 0
|
|
| Preferred stock, Liquidation Preference |
|
|
|
6,000,000
|
|
6,000,000
|
|
6,000,000
|
|
| Issuance of common stock [Member] |
|
|
|
|
|
|
|
|
|
| Common stock shares issued for services |
|
|
|
|
|
|
15,258
|
|
|
| Treasury Stocks One [Member] |
|
|
|
|
|
|
|
|
|
| Common stock, shares issued |
|
|
|
86,497
|
|
86,497
|
|
86,497
|
|
| Common stock, shares purchased |
|
|
|
|
|
0
|
|
71,000
|
|
| Common stock, shares purchased amount |
|
|
|
|
|
$ 0
|
|
$ 100,452
|
|
| Common stock, authorization to purchase |
|
|
$ 3,000,000
|
|
|
|
|
|
|
| Common stock shares value |
|
|
|
$ 917,159
|
|
$ 917,159
|
|
$ 917,159
|
|
| X |
- References
+ Details
| Name: |
abcd_AuthorizationToRepurchaseCommonStock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_CommonStocksSharesIssued |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_CommonStocksSharesOutstanding |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_DescriptionOfConvertibleIntoCommonStock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_EscrowFees |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_PreferredStockLiquidationPreference1 |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_SharesHeldInEscrow |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of warrants or rights which entitle the entity to receive future services in exchange for the unvested, forfeitable warrants or rights.
| Name: |
us-gaap_ClassOfWarrantOrRightUnissued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of dividend income on nonoperating securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_InvestmentIncomeDividend |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow to reacquire common stock during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 15
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsForRepurchaseOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe percentage rate used to calculate dividend payments on preferred stock. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-6
| Name: |
us-gaap_PreferredStockDividendRatePercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued for nonredeemable preferred shares and preferred shares redeemable solely at option of issuer. Includes, but is not limited to, preferred shares issued, repurchased, and held as treasury shares. Excludes preferred shares classified as debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash inflow from debt classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromOtherDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from holders exercising their stock warrants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromWarrantExercises |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPrimarily represents commissions incurred in the period based upon the sale by commissioned employees or third parties of the entity's goods or services, and fees for sales assistance or product enhancements performed by third parties (such as a distributor or value added reseller). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_SalesCommissionsAndFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued in lieu of cash for services contributed to the entity. Number of shares includes, but is not limited to, shares issued for services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued attributable to transactions classified as other.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of shares of stock issued attributable to transactions classified as other.
| Name: |
us-gaap_StockIssuedDuringPeriodValueOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares that have been repurchased during the period and have not been retired and are not held in treasury. Some state laws may govern the circumstances under which an entity may acquire its own stock and prescribe the accounting treatment therefore. This element is used when state law does not recognize treasury stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockRepurchasedDuringPeriodShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying amount, attributable to parent, of an entity's issued and outstanding stock which is not included within permanent equity. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Includes any type of security that is redeemable at a fixed or determinable price or on a fixed or determinable date or dates, is redeemable at the option of the holder, or has conditions for redemption which are not solely within the control of the issuer. Includes stock with a put option held by an ESOP and stock redeemable by a holder only in the event of a change in control of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.E.Q2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479830/718-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
| Name: |
us-gaap_TemporaryEquityCarryingAmountAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_WarrantInducementLetterMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=abcd_WarrantExchangeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_TopMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_BottomMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_SeriesAPreferredStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=abcd_IssuanceofcommonstockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=abcd_TreasuryStocksOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- DefinitionAmount, before tax, of realized and unrealized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 35
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482014/830-20-35-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481956/830-20-45-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481926/830-20-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481839/830-10-45-17
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionCash payments for and related to principal collection on loans related to operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (g)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_PaymentsForLoans |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.3
| X |
- DefinitionAmount before tax, after reclassification adjustments of gain (loss) on foreign currency translation adjustments, on foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature, attributable to parent entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_OtherComprehensiveIncomeForeignCurrencyTransactionAndTranslationAdjustmentBeforeTaxPortionAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionCash payments for and related to principal collection on loans related to operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (g)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_PaymentsForLoans |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionCash received from principal payments made on loans related to operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_ProceedsFromLoans |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.3
RELATED PARTY TRANSACTIONS (Details Narrative) - USD ($)
|
1 Months Ended |
3 Months Ended |
9 Months Ended |
12 Months Ended |
Dec. 29, 2023 |
Jun. 28, 2023 |
Feb. 28, 2023 |
May 17, 2022 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Dec. 31, 2023 |
| Interest income |
|
|
|
|
$ 101,236
|
$ 110,596
|
$ 309,031
|
$ 555,281
|
|
| Principal repayments |
|
|
|
|
|
|
$ 26,408
|
118,847
|
|
| Common stock share issued |
|
|
|
|
23,346,023
|
|
23,346,023
|
|
15,982,472
|
| Accounts receivable balance |
|
|
|
|
$ 17,484,240
|
|
$ 17,484,240
|
|
$ 19,759,254
|
| Prepaid expenses and other current assets - related party |
|
|
|
|
1,914,881
|
|
1,914,881
|
|
1,811,911
|
| Revenue |
|
|
|
|
$ 12,411,048
|
12,823,797
|
40,202,238
|
37,537,003
|
|
| Basotho Investment Limited [Member] |
|
|
|
|
|
|
|
|
|
| General and administrative expenses |
|
|
|
|
|
|
$ 92,700
|
|
$ 10,300
|
| Common stock share issued |
|
|
|
|
92,700
|
|
92,700
|
|
120,000
|
| Grigorios Siokas [Member] |
|
|
|
|
|
|
|
|
|
| Outstanding principal balance |
|
|
|
|
$ 21,158
|
|
$ 21,158
|
|
$ 13,257
|
| Foreign curreny translation |
|
|
|
|
|
|
1,525
|
|
|
| Cana Holdings Laboratories Holding Limited [Member] |
|
|
|
|
|
|
|
|
|
| Secured promissory note |
|
|
$ 4,457,520
|
|
|
|
|
|
|
| Description related to interest on principal |
|
|
Interest on the Principal Amount under this Note shall accrue at a rate equal to Five Percent (5%) plus 1 month LIBOR per annum (5.47% as of December 31, 2023)
|
|
|
|
|
|
|
| DOC Pharma S.A. [Member] |
|
|
|
|
|
|
|
|
|
| Interest income |
|
|
|
|
|
|
143,000
|
|
|
| Principal repayments |
|
|
|
|
|
|
334,350
|
135,467
|
|
| Interest repayments |
|
|
|
|
|
|
$ 155,440
|
|
|
| Interest rate |
|
|
|
|
|
|
5.50%
|
|
|
| Loan term |
|
|
|
|
|
|
10 years
|
|
|
| Maturity date |
|
|
|
|
|
|
Dec. 31, 2032
|
|
|
| Accounts payable and accrued expenses - related party |
|
|
|
|
72,968
|
|
$ 72,968
|
|
34,217
|
| Prepaid balance |
|
|
|
|
|
|
|
|
4,279,200
|
| Accounts receivable - related party |
|
|
|
|
2,448,517
|
|
2,448,517
|
|
2,386,721
|
| Prepaid expenses and other current assets - related party |
|
|
|
|
6,393,642
|
|
$ 6,393,642
|
|
4,347,184
|
| Agreement terminate |
|
|
|
|
|
|
Dec. 31, 2025
|
|
|
| Pieces per product |
|
|
|
|
|
|
1,000 pieces
|
|
|
| Purchased of additional licenses |
|
|
|
|
|
|
$ 525,461
|
|
|
| Loan current portion |
|
|
|
|
445,800
|
|
445,800
|
|
442,480
|
| Loan non-current portion |
|
|
|
|
3,232,050
|
|
3,232,050
|
|
3,539,840
|
| Revenue |
|
|
|
|
40,370
|
61,163
|
581,862
|
43,107
|
|
| Inventroy purchase |
|
|
|
|
85,073
|
456,257
|
510,711
|
1,057,621
|
|
| Purchase of branded pharmaceuticals |
$ 3,539,840
|
$ 1,965,600
|
|
|
|
|
|
|
|
| Description of research and development |
|
|
|
The total cost of this project will be €1,425,000 plus VAT and will be done over three phases as follows: Design & Development (€725,000); Control and Product Manufacturing (€250,000) and Clinical Study and Research (€450,000). SkyPharm has bought a total of as of 81 licenses at value of €554,500 ($593,204) which is 38.91% of the total cost, as of December 31, 2022. During the year ended December 31, 2023, 24 additional licenses were purchased at value of €475,014 ($525,461)
|
|
|
|
|
|
| Monthly basis instalments |
|
|
|
|
37,150
|
|
37,150
|
|
|
| DOC Pharma S.A. [Member] | Inventories Related Agreement [Member] |
|
|
|
|
|
|
|
|
|
| Inventroy purchase |
|
|
|
|
38,096
|
455,586
|
175,123
|
1,048,557
|
|
| Dimitrios Goulielmos [Member] |
|
|
|
|
|
|
|
|
|
| Outstanding principal balance |
|
|
|
|
11,368
|
|
11,368
|
|
11,283
|
| Foreign curreny translation |
|
|
|
|
|
|
85
|
|
|
| Panagiotis Kozaris [Member] |
|
|
|
|
|
|
|
|
|
| Shares owned |
|
|
|
|
0
|
51,159
|
|
|
|
| Prepaid expenses |
|
|
|
|
194,215
|
|
194,215
|
|
194,215
|
| Maria Kozari [Member] |
|
|
|
|
|
|
|
|
|
| Accounts receivable balance |
|
|
|
|
1,123,835
|
|
1,123,835
|
|
1,142,402
|
| Net sales |
|
|
|
|
113,161
|
$ 310,126
|
122,969
|
$ 359,760
|
|
| George Terzis [Member] |
|
|
|
|
|
|
|
|
|
| Unpaid salaries and bonuses |
|
|
|
|
731,000
|
|
731,000
|
|
98,000
|
| Unpaid salaries and bonuses dues |
|
|
|
|
188,000
|
|
188,000
|
|
|
| Kanarogloy & Sia Epe [Member] |
|
|
|
|
|
|
|
|
|
| Accounts payable and accrued expenses - related party |
|
|
|
|
$ 29,832
|
|
$ 29,832
|
|
$ 85,332
|
| X |
- References
+ Details
| Name: |
abcd_AgreementTerminate |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_DescriptionInterestOfSecuredPromissoryNote |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_InterestRepayments |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_LoanCurrentPortion |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_LoanNonCurrentPortion |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_NetSales |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_PaymentReceivedFromInstallment |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_PiecesPerProduct |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_PurchaseOfInventory |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_PurchaseOfPharmaceuticalsMedicine |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_SharesOwnedAdditionalPaymentUnderStockPurchaseAgreement |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_UnpaidSalariesAndBonusesDues |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business, classified as current. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481990/310-10-45-2
| Name: |
us-gaap_AccountsReceivableNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of the obligations incurred through that date and payable for employees' services provided. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(15)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_AccruedSalariesCurrentAndNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDate when the debt instrument is scheduled to be fully repaid, in YYYY-MM-DD format. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
| Name: |
us-gaap_DebtInstrumentMaturityDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod of time between issuance and maturity of debt instrument, in PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.
| Name: |
us-gaap_DebtInstrumentTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow for principal payment on finance lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-5
| Name: |
us-gaap_FinanceLeasePrincipalPayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before tax of foreign currency transaction realized and unrealized gain recognized in the income statement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481956/830-20-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481926/830-20-50-1
| Name: |
us-gaap_ForeignCurrencyTransactionGainBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest income earned from interest bearing assets classified as other.
| Name: |
us-gaap_InterestIncomeOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe effective interest rate during the reporting period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityInterestRateDuringPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of the nature of payments to managing member or general partner for management of the day-to-day business functions of the limited liability company (LLC) or limited partnership (LP), including the fee rate, basis of calculation, relevant accounting period, whether the fee is paid to an entity other than the managing member or general partner, or whether the fee is waived. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 850
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
| Name: |
us-gaap_ManagementFeeDescription |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIncluding the current and noncurrent portions, aggregate carrying amount of all types of notes payable, as of the balance sheet date, with initial maturities beyond one year or beyond the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_NotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of general and administrative expense classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
| Name: |
us-gaap_OtherGeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets.
| Name: |
us-gaap_PrepaidExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for interest that provides economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483032/340-10-45-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482955/340-10-05-5
| Name: |
us-gaap_PrepaidInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow from borrowings supported by a written promise to pay an obligation that is collateralized (backed by pledge, mortgage or other lien in the entity's assets). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromSecuredNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-40
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477314/942-235-S99-1
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount purchased during the period under an unrecorded unconditional purchase obligation (for example, under the take-or-pay or throughput contract). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
| Name: |
us-gaap_UnrecordedUnconditionalPurchaseObligationPurchases |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.3
LINES OF CREDIT (Details) - USD ($)
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Subtotal |
$ 5,989,425
|
$ 6,630,273
|
| National [Member] |
|
|
| Subtotal |
3,011,102
|
3,918,523
|
| Alpha [Member] |
|
|
| Subtotal |
1,030,943
|
1,130,140
|
| Pancreta [Member] |
|
|
| Subtotal |
1,560,803
|
1,122,210
|
| EGF [Member] |
|
|
| Subtotal |
$ 386,577
|
$ 459,400
|
| X |
- DefinitionThe carrying value as of the balance sheet date of the current and noncurrent portions of long-term obligations drawn from a line of credit, which is a bank's commitment to make loans up to a specific amount. Examples of items that might be included in the application of this element may consist of letters of credit, standby letters of credit, and revolving credit arrangements, under which borrowings can be made up to a maximum amount as of any point in time conditional on satisfaction of specified terms before, as of and after the date of drawdowns on the line. Includes short-term obligations that would normally be classified as current liabilities but for which (a) postbalance sheet date issuance of a long term obligation to refinance the short term obligation on a long term basis, or (b) the enterprise has entered into a financing agreement that clearly permits the enterprise to refinance the short-term obligation on a long term basis and the following conditions are met (1) the agreement does not expire within 1 year and is not cancelable by the lender except for violation of an objectively determinable provision, (2) no violation exists at the BS date, and (3) the lender has entered into the financing agreement is expected to be financially capable of honoring the agreement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_LineOfCredit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=abcd_NationalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=abcd_AlphaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=abcd_PancretaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=abcd_EGFMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
LINES OF CREDIT (Details Narrative) - USD ($)
|
3 Months Ended |
9 Months Ended |
12 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Dec. 31, 2023 |
| Line of credit description |
|
|
The Company has three lines of credit with the National Bank of Greece, which are renewed annually. The three lines have interest rates of 6.00% (the "National Bank LOC"), 3.6% (the "COSME 2 Facility"), and 3.6% plus the six-month Euribor rate and any contributions currently in force by law on certain lines of credit (the "COSME 1 Facility")
|
|
|
| Interest Expense |
$ 181,429
|
$ 151,274
|
$ 692,547
|
$ 529,782
|
|
| Line of Credit [Member] | National Bank of Greece Two [Member] |
|
|
|
|
|
| Borrowing |
1,114,500
|
|
1,114,500
|
|
$ 1,106,200
|
| Outstanding debt balance |
|
|
943,466
|
|
1,099,255
|
| Line of Credit [Member] | Alpha Bank of Greece [Member] |
|
|
|
|
|
| Borrowing |
1,114,500
|
|
1,114,500
|
|
1,106,200
|
| Outstanding debt balance |
|
|
1,030,944
|
|
1,130,141
|
| Line of Credit [Member] | Pancreta Bank of Greece [Member] |
|
|
|
|
|
| Borrowing |
1,549,155
|
|
1,549,155
|
|
1,537,618
|
| Outstanding debt balance |
|
|
1,560,802
|
|
1,122,210
|
| National Bank of Greece One [Member] | Line of Credit [Member] |
|
|
|
|
|
| Borrowing |
3,315,638
|
|
3,315,638
|
|
3,290,945
|
| Outstanding debt balance |
|
|
2,102,765
|
|
2,829,828
|
| EGF [Member] | Line of Credit [Member] |
|
|
|
|
|
| Borrowing |
445,800
|
|
445,800
|
|
459,400
|
| Outstanding debt balance |
|
|
386,577
|
|
$ 459,400
|
| Lines of credit [Member] |
|
|
|
|
|
| Interest Expense |
$ 89,868
|
$ 37,536
|
$ 275,246
|
$ 204,654
|
|
| X |
- DefinitionCarrying value as of the balance sheet date of the sum of the obligations to contract holders to provide to them an agreed upon rate of return pursuant to the terms of the underlying contract. These contracts represent lending by the contract holders to the entity in return for a guaranteed (primarily fixed) interest rate until maturity, unless called earlier if the contracts provide that option to the contract holders (usually institutions). There is little or no insurance risk for the entity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_BorrowingsUnderGuaranteedInvestmentAgreements |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of interest expense classified as operating and nonoperating. Includes, but is not limited to, cost of borrowing accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-24
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAverage amount borrowed under the credit facility during the period.
| Name: |
us-gaap_LineOfCreditFacilityAverageOutstandingAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of the terms of a credit facility arrangement. Terms typically include interest rate, collateral required, guarantees required, repayment requirements, and restrictions on use of assets and activities of the entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityDescription |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=us-gaap_LineOfCreditMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=abcd_NationalBankOfGreeceTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=abcd_AlphaBankOfGreeceMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=abcd_PancretaOfGreeceMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=abcd_LinesOfCreditMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
NOTES PAYABLE (Details) - USD ($)
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2024 |
Dec. 31, 2023 |
| Notes payable - long-term |
$ (2,645,623)
|
$ (3,035,341)
|
| Notes payable - short-term |
1,622,349
|
1,570,886
|
| Trade Facility [Member] |
|
|
| Beginning balance loans |
1,908,195
|
3,305,532
|
| Proceeds |
0
|
0
|
| Payments |
(334,350)
|
(1,155,310)
|
| Conversion of debt |
0
|
|
| Recapitalized upon debt modification |
0
|
|
| Accretion of debt and debt discount |
0
|
|
| Foreign currency translation |
14,318
|
(64,610)
|
| Subtotal |
1,588,163
|
1,908,195
|
| Notes payable - long-term |
(1,086,638)
|
(1,327,440)
|
| Oher additions |
|
0
|
| Notes payable- short -term |
501,525
|
580,755
|
| Debt forgiveness |
|
(306,637)
|
| COVID Loans [Member] |
|
|
| Beginning balance loans |
186,884
|
207,377
|
| Proceeds |
0
|
0
|
| Payments |
(19,073)
|
(27,027)
|
| Conversion of debt |
0
|
|
| Recapitalized upon debt modification |
0
|
|
| Accretion of debt and debt discount |
0
|
|
| Foreign currency translation |
3,691
|
6,534
|
| Subtotal |
171,502
|
186,884
|
| Notes payable - long-term |
(142,183)
|
(158,133)
|
| Notes payable - short-term |
29,319
|
28,751
|
| Oher additions |
|
0
|
| Debt forgiveness |
|
0
|
| Loan Facility [Member] |
|
|
| Beginning balance loans |
4,267,972
|
5,017,987
|
| Proceeds |
445,800
|
1,082,231
|
| Payments |
(834,677)
|
(1,597,894)
|
| Conversion of debt |
0
|
(306,637)
|
| Recapitalized upon debt modification |
0
|
317,880
|
| Foreign currency translation |
50,622
|
92,660
|
| Subtotal |
4,606,227
|
4,606,227
|
| Notes payable - long-term |
(2,645,623)
|
(3,035,341)
|
| Notes payable - short-term |
1,622,349
|
1,570,886
|
| Third Party [Member] |
|
|
| Beginning balance loans |
2,511,148
|
1,505,078
|
| Proceeds |
445,800
|
1,082,231
|
| Payments |
(481,254)
|
(415,557)
|
| Conversion of debt |
0
|
|
| Recapitalized upon debt modification |
0
|
|
| Accretion of debt and debt discount |
0
|
|
| Foreign currency translation |
32,613
|
21,516
|
| Subtotal |
2,508,307
|
2,511,148
|
| Notes payable - long-term |
(1,416,802)
|
(1,549,768)
|
| Notes payable - short-term |
$ 1,091,505
|
961,380
|
| Oher additions |
|
317,880
|
| Debt forgiveness |
|
$ 0
|
| X |
- References
+ Details
| Name: |
abcd_BeginningBalanceLoans |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ConversionOfDebt |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ForeignCurrencyTranslation |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_OherAdditionsOfDebt |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_PaymentsOfDebt |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ProceedFromDebt |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_RecapitalizedUponDebtModification |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_SubtotalOfDebts |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe sum of the periodic adjustments of the differences between securities' face values and purchase prices that are charged against earnings. This is called accretion if the security was purchased at a discount and amortization if it was purchased at premium. As a noncash item, this element is an adjustment to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_AccretionAmortizationOfDiscountsAndPremiumsInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGross amount of debt extinguished.
| Name: |
us-gaap_ExtinguishmentOfDebtAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of notes payable (with maturities initially due after one year or beyond the operating cycle if longer), excluding current portion. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionIncluding the current and noncurrent portions, aggregate carrying amount of all types of notes payable, as of the balance sheet date, with initial maturities beyond one year or beyond the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_NotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of the portions of long-term notes payable due within one year or the operating cycle if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_NotesPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_DeferredRevenueArrangementTypeAxis=abcd_TradeFacilityAgreementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DeferredRevenueArrangementTypeAxis=abcd_COVIDLoansMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=abcd_LoanFacilityAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=abcd_ThirdPartyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
NOTES PAYABLE (Details 1)
|
Sep. 30, 2024
USD ($)
|
| NOTES PAYABLE |
|
| 2025 |
$ 1,622,349
|
| 2026 |
1,717,738
|
| 2027 |
420,633
|
| 2028 |
353,961
|
| 2029 and thereafter |
153,291
|
| Total Debt |
4,267,972
|
| Less: notes payable - current portion |
1,622,349
|
| Notes payable - long term portion |
$ (2,645,623)
|
| X |
- References
+ Details
| Name: |
abcd_LessNotesPayableCurrentPortion |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_NotesPayableLongTermPortion |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_DebtDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt. Excludes lease obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482949/835-30-55-8
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69B
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69C
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1D
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-4
| Name: |
us-gaap_LongTermDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing after fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 470
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
| Name: |
us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalAfterYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 470
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-1
| Name: |
us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalInNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 470
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-1
| Name: |
us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalInYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 470
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-1
| Name: |
us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalInYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 470
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-1
| Name: |
us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalInYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.3
NOTES PAYABLE (Details Narrative)
|
|
1 Months Ended |
3 Months Ended |
9 Months Ended |
12 Months Ended |
|
|
|
|
|
|
May 12, 2020
USD ($)
|
Jul. 30, 2021
USD ($)
|
Nov. 19, 2020
USD ($)
|
Jun. 23, 2020
USD ($)
|
Sep. 30, 2024
USD ($)
|
Sep. 30, 2024
USD ($)
shares
|
Sep. 30, 2024
CAD ($)
shares
|
Dec. 31, 2022
USD ($)
|
Dec. 31, 2023
USD ($)
|
Jun. 09, 2022
USD ($)
|
Mar. 03, 2022
USD ($)
|
Dec. 30, 2020 |
Oct. 17, 2018
USD ($)
|
| Stock issued for debt obligation | shares |
|
|
|
|
|
420,471
|
420,471
|
|
|
|
|
|
|
| Outstanding principal loan balance |
|
$ 578,850
|
|
|
|
|
|
|
|
|
$ 3,950,000
|
|
|
| Note payable long term |
|
|
|
|
$ 2,645,623
|
$ 2,645,623
|
|
|
$ 3,035,341
|
|
|
|
|
| Interest rate description |
|
|
|
|
|
one-month Euribor (3.90% as of December 31, 2023), and 6% plus one-month LIBOR (fully paid as of December 31, 2023), respectively
|
one-month Euribor (3.90% as of December 31, 2023), and 6% plus one-month LIBOR (fully paid as of December 31, 2023), respectively
|
|
|
|
|
|
|
| Non cash interest expenses |
|
|
|
|
|
$ 221,060
|
|
$ 200,000
|
|
|
|
|
|
| Five quarterly installments | shares |
|
|
|
|
|
54,600
|
54,600
|
|
|
|
|
|
|
| Final repayment | shares |
|
|
|
|
|
1,965,600
|
1,965,600
|
|
|
|
|
|
|
| July 29, 2024 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest rate description |
|
|
|
|
|
The Note matures on July 31, 2029 and bears an annual interest rate of 2.58% plus the 3-month Euribor (3.47% as of September 30, 2024). Pursuant to the agreement, there is a six-month grace period for principal and interest repayment
|
The Note matures on July 31, 2029 and bears an annual interest rate of 2.58% plus the 3-month Euribor (3.47% as of September 30, 2024). Pursuant to the agreement, there is a six-month grace period for principal and interest repayment
|
|
|
|
|
|
|
| Repayments of debt |
|
|
|
|
|
$ 445,800
|
|
|
|
|
|
|
|
| Debt amount received from related party |
|
|
|
$ 611,500
|
|
|
|
|
|
|
|
|
|
| Notes payable long term |
|
|
|
|
396,367
|
396,367
|
|
|
0
|
|
|
|
|
| Full and Final Settlement Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment of loan |
|
|
|
|
|
334,350
|
|
|
|
|
|
|
|
| Outstanding principal loan balance |
|
|
|
|
1,588,163
|
1,588,163
|
|
|
1,908,195
|
|
|
|
|
| Note payable long term |
|
|
|
|
1,086,638
|
1,086,638
|
|
|
1,327,440
|
|
|
|
|
| Accrued interest expenses |
|
|
|
|
|
|
|
|
161,274
|
|
|
|
|
| Interest expenses |
|
|
|
|
29,365
|
110,170
|
|
|
|
|
|
|
|
| Debt Exchange Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal loan balance |
|
|
$ 611,500
|
|
154,792
|
154,792
|
|
|
245,822
|
|
|
|
|
| Accrued interest expenses |
|
|
|
|
6,057
|
6,057
|
|
|
12,379
|
|
|
|
|
| Repayments of debt |
|
|
|
|
|
92,875
|
|
|
|
|
|
|
|
| Agreement description |
|
|
The note matures on November 18, 2025 and bears an annual interest rate, based on a 360-day year, of 3% plus 0.6% plus 6-month Euribor when Euribor is positive (3.35% as of September 30, 2024). The principal is to be repaid in 18 quarterly installments of €27,778 ($30,333)
|
|
|
|
|
|
|
|
|
|
|
| Notes payable long term |
|
|
|
|
30,958
|
30,958
|
|
|
122,911
|
|
|
|
|
| July 30, 2021 Debt Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal loan balance |
|
|
|
|
281,338
|
281,338
|
|
|
261,918
|
|
|
|
|
| Note payable long term |
|
|
|
|
134,929
|
134,929
|
|
|
227,065
|
|
|
|
|
| Accrued interest expenses |
|
|
|
|
17,083
|
17,083
|
|
|
12,063
|
|
|
|
|
| Repayments of debt |
|
|
|
|
|
91,267
|
|
|
|
|
|
|
|
| Agreement description |
|
The note matures on August 5, 2026 and bears an annual interest rate that applies to 60% of the principal of the note that is based on a 365-day year, of 5.84% plus 3-month Euribor when Euribor is positive (3.47% as of September 30, 2024). Pursuant to the terms of the agreement, there is a nine-month grace period for principal repayment during which interest is accrued. The principal is to be repaid in 18 quarterly installments of €27,778 commencing three months from the end of the grace period
|
|
|
|
|
|
|
|
|
|
|
|
| Covid Ninteen [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loan received from related party |
$ 366,900
|
|
|
|
|
1,123,700
|
|
|
|
|
|
|
|
| Debt principal balance |
|
|
|
|
720,908
|
$ 720,908
|
|
|
897,165
|
|
|
|
|
| Covid Ninteen [Member] | United Kingdom Government [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest rate description |
The loan will be repaid in 40 equal monthly installments beginning on July 29, 2022. As a condition to the loan, the Company was required to retain the same number of employees until October 31, 2020. As of December 31, 2023, the principal balance was $134,818. During the nine months ended September 30, 2024, the Company repaid €14,063 ($15,673) of the principal balance. The outstanding balance as of September 30, 2024 is €107,813 ($120,157) of which $99,260, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheet
|
|
|
|
|
The loan has a ten-year maturity and bears interest at a rate of 2.5% per annum beginning 12-months after the initial disbursement, which was on July 10, 2020
|
The loan has a ten-year maturity and bears interest at a rate of 2.5% per annum beginning 12-months after the initial disbursement, which was on July 10, 2020
|
|
|
|
|
|
|
| Loan received from related party |
$ 366,900
|
|
|
|
|
$ 68,310
|
|
|
|
|
|
|
|
| Loan prinipal amount |
|
|
|
|
51,345
|
$ 51,345
|
|
|
52,066
|
|
|
|
|
| June 9, 2022 Debt Agreement One [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal loan balance |
|
|
|
|
|
|
|
|
|
$ 335,008
|
|
|
|
| Agreement description |
|
|
|
|
|
the Company entered into an agreement with a third-party lender in the principal amount of €320,000 ($335,008), the “Note”. The Note matures on June 16, 2027 and bears an annual interest rate of 3.89% plus an additional rate of 0.60%, plus the 3-month Euribor (3.47% as of September 30, 2024). Pursuant to the agreement, there is a twelve-month grace period for principal repayment during which interest is accrued. The principal is to be repaid in 16 equal quarterly installments of €20,000 commencing on June 30, 2023. During the nine months ended September 30, 2024, the Company repaid €60,000 ($66,870) of the principal. As of September 30, 2024 and December 31, 2023, the Company has accrued interest of €4,673 ($5,208) and €11,043 ($12,215), respectively, and an outstanding balance of €200,000 ($222,900) and €260,000 ($287,612) of which $133,740 and $204,322, respectively, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets
|
the Company entered into an agreement with a third-party lender in the principal amount of €320,000 ($335,008), the “Note”. The Note matures on June 16, 2027 and bears an annual interest rate of 3.89% plus an additional rate of 0.60%, plus the 3-month Euribor (3.47% as of September 30, 2024). Pursuant to the agreement, there is a twelve-month grace period for principal repayment during which interest is accrued. The principal is to be repaid in 16 equal quarterly installments of €20,000 commencing on June 30, 2023. During the nine months ended September 30, 2024, the Company repaid €60,000 ($66,870) of the principal. As of September 30, 2024 and December 31, 2023, the Company has accrued interest of €4,673 ($5,208) and €11,043 ($12,215), respectively, and an outstanding balance of €200,000 ($222,900) and €260,000 ($287,612) of which $133,740 and $204,322, respectively, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets
|
|
|
|
|
|
|
| Accured interest expense |
|
|
|
|
|
|
|
|
12,215
|
|
|
|
|
| Notes payable long term |
|
|
|
|
|
|
|
|
204,322
|
|
|
|
|
| Synthesis Facility Agreement [Member] | TFF [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal loan balance |
|
|
|
|
|
|
|
|
|
|
|
|
$ 5,629,555
|
| Accrued expenses |
|
|
|
|
|
|
|
|
|
|
|
|
524,094
|
| Synthesis Facility Agreement [Member] | TFF [Member] | Principal Balance One [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Debt instrument, accrue interest rate |
|
|
|
|
|
|
|
|
|
|
|
5.50%
|
|
| Debt intrument split, principal balance |
|
|
|
|
|
|
|
|
|
|
|
|
$ 2,316,000
|
| Synthesis Facility Agreement [Member] | TFF [Member] | Principal balance 2 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stated interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
6.00%
|
| Debt split, balance |
|
|
|
|
|
|
|
|
|
|
|
|
$ 4,000,000
|
| National Bank of Greece SA [Member] | June 23, 2020 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal loan balance |
|
|
|
|
131,118
|
$ 131,118
|
|
|
227,747
|
|
|
|
|
| Interest rate description |
|
|
|
The note is interest bearing from the date of receipt and is payable every three months at an interest rate of 3.06% plus 3-month Euribor (3.47% as of September 30, 2024)
|
|
|
|
|
|
|
|
|
|
| Repayments of debt |
|
|
|
|
|
98,338
|
|
|
|
|
|
|
|
| Debt amount received from related party |
|
|
|
$ 611,500
|
|
|
|
|
|
|
|
|
|
| Notes payable long term |
|
|
|
|
0
|
$ 0
|
|
|
97,606
|
|
|
|
|
| Senior Promissory Notes [Member] | Unaffiliated Third Party [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Description of loan repayment |
|
|
|
|
|
CAD $2 million cash received noted in (b) above, the Company accounted for its obligation to issue a variable number of the Company’s Common Shares as Share-settled debt obligation in accordance with ASC 480 measured at fair value or the settlement amount of $1,554,590 (CAD $2 million
|
CAD $2 million cash received noted in (b) above, the Company accounted for its obligation to issue a variable number of the Company’s Common Shares as Share-settled debt obligation in accordance with ASC 480 measured at fair value or the settlement amount of $1,554,590 (CAD $2 million
|
|
|
|
|
|
|
| Cloudscreen Promissory Note [Member] | January 23, 2024 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal loan balance |
|
|
|
|
$ 324,870
|
$ 324,870
|
|
|
|
|
|
|
|
| Repayments of debt |
|
|
|
|
|
$ 10,830
|
|
|
|
|
|
|
|
| Agreement description |
|
|
|
|
|
Note matures on March 25, 2025 and is interest free. This Note is being given in connection with the Closing of the Asset Purchase, Sale and Transfer Agreement dated as of October 9, 2023 and as amended from time to time pursuant to which the Company agreed to purchase from the third-party a drug repurposing Artificial Intelligence “AI” powered platform known as “Cloudscreen®” (refer to Note 2, section “Acquisition accounting”). The principal is to be repaid in 15 equal monthly installments of €20,000 commencing on January 25, 2024. During the 9 months ended September 30, 2024, the Company repaid €10,000 ($10,830) of the principal and recorded a foreign currency loss of $16,155. As of September 30, 2024, and December 31, 2023 the Company had an outstanding balance of $323,205 and $317,880 of which $0 and $0, respectively, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets
|
Note matures on March 25, 2025 and is interest free. This Note is being given in connection with the Closing of the Asset Purchase, Sale and Transfer Agreement dated as of October 9, 2023 and as amended from time to time pursuant to which the Company agreed to purchase from the third-party a drug repurposing Artificial Intelligence “AI” powered platform known as “Cloudscreen®” (refer to Note 2, section “Acquisition accounting”). The principal is to be repaid in 15 equal monthly installments of €20,000 commencing on January 25, 2024. During the 9 months ended September 30, 2024, the Company repaid €10,000 ($10,830) of the principal and recorded a foreign currency loss of $16,155. As of September 30, 2024, and December 31, 2023 the Company had an outstanding balance of $323,205 and $317,880 of which $0 and $0, respectively, is classified as “Notes payable - long term portion” on the accompanying condensed consolidated balance sheets
|
|
|
|
|
|
|
| Notes payable long term |
|
|
|
|
|
|
|
|
317,880
|
|
|
|
|
| Cloudscreen Promissory Note [Member] | July 14, 2023 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal loan balance |
|
|
|
|
|
|
|
|
1,081,532
|
|
|
|
|
| Agreement description |
|
|
|
|
|
the Company entered into an agreement with a third-party lender in the principal amount of €1,000,000 ($1,123,700), the “Note”. The Note matures on July 31, 2028 and bears an annual interest rate of 2.46% plus the 3-month Euribor (3.47% as of September 30, 2024). Pursuant to the agreement, there is a nine-month grace period for interest and principal repayment. The principal is to be repaid in 18 equal quarterly installments of €55,556 commencing on May 2, 2024. During the nine months ended September 30, 2024, the Company repaid €108,633 ($58,179) of the principal. As of September 30, 2024, and December 31, 2023, the Company has accrued interest of €7,845 ($8,743) and €19,820 ($21,925), respectively. As of September 30, 2024, and December 31, 2023 the Company an outstanding balance of €869,067 ($968,575) and €977,700 ($1,081,532), of which $720,908 and $897,165, respectively
|
the Company entered into an agreement with a third-party lender in the principal amount of €1,000,000 ($1,123,700), the “Note”. The Note matures on July 31, 2028 and bears an annual interest rate of 2.46% plus the 3-month Euribor (3.47% as of September 30, 2024). Pursuant to the agreement, there is a nine-month grace period for interest and principal repayment. The principal is to be repaid in 18 equal quarterly installments of €55,556 commencing on May 2, 2024. During the nine months ended September 30, 2024, the Company repaid €108,633 ($58,179) of the principal. As of September 30, 2024, and December 31, 2023, the Company has accrued interest of €7,845 ($8,743) and €19,820 ($21,925), respectively. As of September 30, 2024, and December 31, 2023 the Company an outstanding balance of €869,067 ($968,575) and €977,700 ($1,081,532), of which $720,908 and $897,165, respectively
|
|
|
|
|
|
|
| Notes payable long term |
|
|
|
|
|
|
|
|
$ 897,165
|
|
|
|
|
| Distribution and Equity Acquisition Agreement [Member] | Marathon Global Inc [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash received upon gross sales |
|
|
|
|
|
|
$ 2,750,000
|
|
|
|
|
|
|
| Upfront cash received |
|
|
|
|
|
|
$ 2,000,000
|
|
|
|
|
|
|
| Equity interest acquired description |
|
|
|
|
|
a 33 1/3% equity interest or 5 million shares in Marathon as partial consideration for the Company’s distribution services
|
a 33 1/3% equity interest or 5 million shares in Marathon as partial consideration for the Company’s distribution services
|
|
|
|
|
|
|
| Distribution and Equity Acquisition Agreement [Member] | Marathon Global Inc [Member] | Gross Sales One [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash received upon gross sales |
|
|
|
|
|
|
$ 2,750,000
|
|
|
|
|
|
|
| Distribution and Equity Acquisition Agreement [Member] | Marathon Global Inc [Member] | Gross Sales [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash received upon gross sales |
|
|
|
|
|
|
2,750,000
|
|
|
|
|
|
|
| Gross sales |
|
|
|
|
|
|
$ 13,000,000
|
|
|
|
|
|
|
| X |
- References
+ Details
| Name: |
abcd_AgreementDescription |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_CashReceivedUponGrossSales |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_DebtInstrumentAccrueInterestRate |
| Namespace Prefix: |
abcd_ |
| Data Type: |
num:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_DebtIntrumentSplitPrincipalBalance |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_DebtPrincipalBalance |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_DebtSplitBalance |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_DescriptionOfLoanRepayment |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_EquityInterestAcquiredpercentageDescription |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_FinalRepaymentShares |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_FiveQuarterlyInstallments |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_GrossSalesValue |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_NonCashInterestExpenses |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_PrincipalAmountLoan |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_RepaymentsOfDebtsExchange |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_RepaymentsOfDebtsTwo |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_StockIssuedForDebtObligation |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_TotalRepaymentsOfDebt |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_TradeFinanceFacilityAgreementInterestRateDescription |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_UpfrontCashReceived |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid nor invoiced, and liabilities classified as other.
| Name: |
us-gaap_AccruedLiabilitiesAndOtherLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of debt and lease obligation, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionContractual interest rate for funds borrowed, under the debt agreement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
| Name: |
us-gaap_DebtInstrumentInterestRateStatedPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of interest expense classified as other.
| Name: |
us-gaap_InterestExpenseOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of [accrued] interest payable on all forms of debt, including trade payables, that has been incurred and is unpaid. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InterestPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of interest payable on debt, including, but not limited to, trade payables. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(15)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(15)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_InterestPayableCurrentAndNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt classified as noncurrent. Excludes lease obligation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermDebtNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of notes payable (with maturities initially due after one year or beyond the operating cycle if longer), excluding current portion. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash outflow for short-term and long-term debt. Excludes payment of lease obligation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_DebtAgreementJulyTwentyNineMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_FullAndFinalSettlementAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_DebtExchangeAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_DebtAgreementJuneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_CovidNinteenMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AccountsNotesLoansAndFinancingReceivablesByLegalEntityOfCounterpartyTypeAxis=abcd_UnitedKingdomGovernmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_DebtAgreementJuneOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_SynthesisFacilityAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=abcd_TFFMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=abcd_PrincipalBalanceOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=abcd_PrincipalBalanceTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=abcd_JuneTwentyThreeTwoThousandTwentyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LongtermDebtTypeAxis=abcd_SeniorPromissoryNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LongtermDebtTypeAxis=abcd_PromissoryNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=abcd_JanuaryTwentyThreeTwentyTwentyFourMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=abcd_JulyFourteenTwoThousandTwentyThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=abcd_DistributionAndEquityAcquisitionAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=abcd_SalesOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_SalesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- References
+ Details
| Name: |
abcd_OperatingLeaseLiabilityLessImputedInterest |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease due after fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueAfterYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PropertySubjectToOrAvailableForOperatingLeaseAxis=abcd_OperatingLeaseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- References
+ Details
| Name: |
abcd_FinanceLeaseLiabilityPaymentsDueYearFiveImputedInterest |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_FinanceLeaseLiabilityPaymentsDueYearFiveTotalUndiscountedFinanceLeasePayments |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments for finance lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_FinanceLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for finance lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_FinanceLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for finance lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_FinanceLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for finance lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_FinanceLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=abcd_FinanceLeaseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
LEASES (Details Narrative) - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Operating lease, term of agreements |
|
|
The Company has various operating and finance lease agreements with terms up to 10 years
|
|
| Amortization of right-of-use assets |
|
|
$ 22,507
|
$ 102,549
|
| Operating lease cash flows used in finance lease |
$ 9,765
|
$ 27,118
|
$ 41,094
|
118,847
|
| Operating lease, weighted average discount rate |
|
|
6.74%
|
|
| Operating lease Weighted-average remaining lease term |
|
|
4 years 29 days
|
|
| Finance lease, weighted average remaining lease term |
|
|
1 year 2 months 8 days
|
|
| Finance lease, interest expense |
|
|
$ 1,765
|
20,629
|
| Finance lease, weighted average discount rate |
|
|
6.74%
|
|
| Finance lease, amortization expense |
7,589
|
22,507
|
$ 35,452
|
102,549
|
| Interest expense [Member] |
|
|
|
|
| Finance lease, interest expense |
457
|
1,765
|
6,920
|
20,629
|
| General And Administrative Expense [Member] |
|
|
|
|
| Amortization of right-of-use assets |
$ 76,229
|
$ 235,659
|
$ 50,690
|
$ 158,407
|
| X |
- References
+ Details
| Name: |
abcd_FinanceLeaseAmortizationExpense |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_FinanceLeaseWeightedAverageDiscountRate |
| Namespace Prefix: |
abcd_ |
| Data Type: |
num:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_OperatingLeaseCashFlowsUsedInFinanceLease |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_OperatingLeaseTermOfAgreements |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_OperatingLeaseWeightedAverageDiscountRate |
| Namespace Prefix: |
abcd_ |
| Data Type: |
num:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average period before the next renewal or extension for intangible assets with renewal or extension terms, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_AcquiredFiniteLivedIntangibleAssetWeightedAveragePeriodBeforeRenewalOrExtension |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average amortization period of finite-lived intangible assets acquired either individually or as part of a group of assets, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_AcquiredFiniteLivedIntangibleAssetsWeightedAverageUsefulLife |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest expense on finance lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_FinanceLeaseInterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of periodic reduction over lease term of carrying amount of right-of-use asset from operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_OperatingLeaseRightOfUseAssetAmortizationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_InterestExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- References
+ Details
| Name: |
abcd_ProvisionForStaffLeavingCompensation |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_TaxLiabilitiesPayable |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred through that date and payable for sales commissions. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 8
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-8
| Name: |
us-gaap_AccruedSalesCommissionCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherLiabilitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCash received from customers as progress payments on projects that have been partially completed. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 912
-SubTopic 310
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478345/912-310-45-11
| Name: |
us-gaap_ProceedsFromCustomersForProgressPayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.3
COMMITMENTS AND CONTINGENCIES (Details Narrativec) - USD ($)
|
1 Months Ended |
3 Months Ended |
9 Months Ended |
12 Months Ended |
Nov. 21, 2023 |
Jun. 26, 2022 |
Jul. 31, 2021 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Dec. 31, 2023 |
| Description for advisory agreement |
|
|
As consideration for services rendered, and successful Nasdaq listing, the Company paid $100,000. The $100,000 bonus was incurred and settled within 2022. Finally, the Consultant received a total of 10,000 shares of the Company’s common stock, 2,000 of such shares that have been previously issued pursuant to previous agreements and additional 15,258 shares that were issued on February 2, 2023, based on the amendment signed on February 1, 2023
|
|
|
|
|
|
| Stock based compensation |
|
|
|
$ 109,636
|
$ 109,636
|
$ 326,525
|
$ 214,505
|
|
| Payment of additional tax |
|
|
|
|
|
99,644
|
|
|
| Additional stock consideration |
|
|
|
|
|
$ 264,000
|
|
|
| Complementary compensation Shares |
|
|
|
|
|
440,000
|
|
|
| Four Third Party Consultants [Member] |
|
|
|
|
|
|
|
|
| Awarded shares |
970,000
|
|
|
|
|
|
|
|
| Awarded share value |
$ 999,100
|
|
|
|
|
|
|
|
| Phase 1 [Member] |
|
|
|
|
|
|
|
|
| Project cost |
|
$ 838,450
|
|
|
|
|
|
|
| Phase 2 [Member] |
|
|
|
|
|
|
|
|
| Project cost |
|
$ 907,084
|
|
|
|
|
|
|
| National Medicines Agency [Member] | General And Administrative Expense [Member] |
|
|
|
|
|
|
|
|
| Stock based compensation |
|
|
|
$ 264,750
|
$ 0
|
$ 728,250
|
$ 0
|
$ 77,250
|
| Imposed fine |
|
|
|
|
|
$ 16,214
|
|
|
| X |
- References
+ Details
| Name: |
abcd_AdditionalStockConsideration |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_AdvisoryDescription |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ComplementaryCompensationShares |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ImposedFine |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_PaymentTax |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ProjectCosts |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_StockIssuedDuringPeriodSharesAwarded |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe fair value of stock issued in noncash financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-3
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-5
| Name: |
us-gaap_StockIssued1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_LitigationCaseAxis=abcd_NationalMedicinesAgencyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
STOCK OPTIONS AND WARRANTS (Details) - Warrants [Member] - USD ($)
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2024 |
Dec. 31, 2023 |
| Number of Shares Outstanding, Beginning balance |
8,561,476
|
4,194,236
|
| Number of Shares Outstanding, Granted |
9,748,252
|
7,524,933
|
| Number of Shares Outstanding, Forfeited |
0
|
0
|
| Number of Shares Outstanding, Exercised |
(4,874,126)
|
(3,152,386)
|
| Number of Shares Outstanding, Expired |
(3,096)
|
|
| Number of Shares Outstanding, Ending balance |
13,432,506
|
8,561,476
|
| Number of Shares Outstanding, Exercisable |
13,432,506
|
|
| Weighted Average Exercise Price Outstanding, Beginning balance |
$ 3.91
|
$ 8.31
|
| Weighted Average Exercise Price, Granted |
0.95
|
1.65
|
| Weighted Average Exercise Price Outstanding, Ending balance |
2.64
|
$ 3.91
|
| Weighted Average Exercise Price, Exercisable |
$ 2.64
|
|
| Weighted Average Remaining Contractual Term Outstanding, Beginning |
4 years 7 months 21 days
|
5 years 14 days
|
| Weighted Average Remaining Contractual Term, Granted |
3 years 2 months 26 days
|
5 years 1 month 17 days
|
| Weighted Average Remaining Contractual Term Outstanding, Ending |
3 years 3 months 10 days
|
4 years 7 months 20 days
|
| Weighted Average Remaining Contractual Term Exercisable |
3 years 3 months 10 days
|
|
| Aggregate Intrinsic Value Outstanding, Beginning balance |
$ 18,801
|
$ 2,562,621
|
| Aggregate Intrinsic Value Outstanding, Ending balance |
11,681
|
$ 18,801
|
| Aggregate Intrinsic Value Outstanding, Exercisable |
$ 11,681
|
|
| X |
- References
+ Details
| Name: |
abcd_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExercisableInPeriod |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingExercisableIntrinsicValue |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisableInPeriodWeightedAverageExercisePrice92 |
| Namespace Prefix: |
abcd_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTermGranted |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_WeightedAverageRemainingContractualTermExercisable |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_WeightedAverageRemainingContractualTermOutstandingBeginning |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_WeightedAverageRemainingContractualTermOutstandingEnding |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor presentations that combine terminations, the number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan or that expired. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNet number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which the current fair value of the underlying stock exceeds the exercise price of options outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=abcd_WarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
STOCK OPTIONS AND WARRANTS (Details Narrative) - USD ($)
|
|
1 Months Ended |
3 Months Ended |
9 Months Ended |
12 Months Ended |
|
|
Apr. 03, 2023 |
Sep. 26, 2024 |
Dec. 29, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Dec. 31, 2023 |
Aug. 21, 2023 |
Sep. 19, 2022 |
| Share-based compensation expense |
|
|
|
$ 109,636
|
$ 109,636
|
$ 326,525
|
$ 214,505
|
|
|
|
| Discription of incentive stock awards |
|
|
|
|
|
The awards are in the form of restricted stock and will vest in two parts: 50% on October 2, 2023 and 50% on October 2, 2024
|
|
|
|
|
| Total shares of awarded |
185,000
|
|
|
|
|
|
|
|
|
|
| Common stock share issued |
|
|
|
23,346,023
|
|
23,346,023
|
|
15,982,472
|
|
|
| Gross cash proceeds from warrants exercised |
|
$ 4,240,977
|
$ 3,533,741
|
|
|
$ 4,240,977
|
$ 0
|
|
|
|
| Shares held in escrow |
|
2,532,126
|
950,063
|
|
|
|
|
|
|
|
| Board of Directors [Member] |
|
|
|
|
|
|
|
|
|
|
| Share-based compensation expense |
|
|
|
$ 48,425
|
|
$ 48,425
|
|
|
|
|
| Share reserv for issuance |
|
|
|
|
|
|
|
|
2,500,000
|
|
| 2022 Plan [Member] |
|
|
|
|
|
|
|
|
|
|
| Share reserv for issuance |
|
|
|
|
|
|
|
|
|
200,000
|
| 2023 Plan [Member] |
|
|
|
|
|
|
|
|
|
|
| Share reserv for issuance |
|
|
|
|
|
|
|
|
2,500,000
|
|
| Warrant Exchange [Member] |
|
|
|
|
|
|
|
|
|
|
| Number of Shares Outstanding, Exercised |
|
|
(4,874,126)
|
|
|
(9,748,252)
|
|
(3,152,386)
|
|
|
| Fair value of warrants immediately before the re-pricing |
|
$ 4,197,280
|
$ 3,603,183
|
|
|
|
|
|
|
|
| Fair value of warrants immediately after re-pricing |
|
4,207,073
|
3,610,825
|
|
|
|
|
|
|
|
| Deemed dividend |
|
$ 6,185,321
|
$ 7,218,485
|
|
|
|
|
$ 7,642
|
|
|
| Warrant with no expiration |
|
4,874,126
|
2,437,063
|
2,332,000
|
|
2,332,000
|
|
|
|
|
| Common stock share issued |
|
2,332,000
|
1,487,000
|
2,332,000
|
|
2,332,000
|
|
|
|
|
| Gross cash proceeds from warrants exercised |
|
$ 4,240,977
|
$ 3,533,741
|
|
|
|
|
|
|
|
| Shares held in escrow |
|
2,532,126
|
950,063
|
|
|
|
|
|
|
|
| Term |
|
5 years
|
5 years
|
|
|
|
|
|
|
|
| Warrant Exchange [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
|
|
| Warrants exercise price |
|
$ 0.8701
|
$ 1.45
|
|
|
|
|
|
|
|
| Warrant Exchange [Member] | Minimum [Member] |
|
|
|
|
|
|
|
|
|
|
| Warrants exercise price |
|
$ 1.45
|
$ 2.75
|
|
|
|
|
|
|
|
| Warrants [Member] |
|
|
|
|
|
|
|
|
|
|
| Number of Shares Outstanding, Exercised |
|
|
|
|
|
(4,874,126)
|
|
(3,152,386)
|
|
|
| Fair value of exercise prices before re-pricing |
|
|
fair value of common stock of $1.49, b) exercise price of $1.45 before re-pricing, c) exercise price of $2.75 after re-pricing
|
|
|
exercise price of $1.45 before re-pricing, c) exercise price of $0.8701 after re-pricing,
|
|
|
|
|
| Fair value of terms re-pricing |
|
|
terms of 5.07 years and 5.02 years
|
|
|
term of 4.26 years
|
|
|
|
|
| Deemed dividend |
|
|
$ 7,642
|
|
|
$ 9,793
|
|
$ 7,642
|
|
|
| Fair value of common stock |
|
|
|
$ 1.49
|
|
$ 1.49
|
|
|
|
|
| Divined rate |
|
0.00%
|
|
|
|
0.00%
|
|
|
|
|
| Risk free interest rate |
|
3.83%
|
|
|
|
3.55%
|
|
|
|
|
| Number of Warrants Exercisable |
|
|
|
8,558,380
|
|
8,558,380
|
|
|
|
|
| Warrant otstanding |
|
|
|
13,432,506
|
|
13,432,506
|
|
|
|
|
| Warrant expiration date |
|
|
|
|
|
expiration dates from October 2024 through October 2029 and 13,334 warrants with no expiration date
|
|
|
|
|
| Warrant with no expiration |
|
|
|
13,419,172
|
|
13,419,172
|
|
|
|
|
| X |
- References
+ Details
| Name: |
abcd_AwardedShares |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_DiscriptionOfIncentiveStockAwards |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ExercisePricesBeforeRePricingDescription |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_FairValueOfCommonStockPerShare |
| Namespace Prefix: |
abcd_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_FairValueOfWarrantsAfterRepricing |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_FairValueOfWarrantsBeforeRepricing |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionExercisableNumber |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_SharesHeldInEscrow |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_TermsRepricingDescription |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_WarrantExercisePriceOfWarrants |
| Namespace Prefix: |
abcd_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
abcd_WarrantExpirationDateDescription |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of warrants or rights which entitle the entity to receive future services in exchange for the unvested, forfeitable warrants or rights.
| Name: |
us-gaap_ClassOfWarrantOrRightUnissued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate number of common shares reserved for future issuance. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockCapitalSharesReservedForFutureIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of paid and unpaid cash, stock, and paid-in-kind (PIK) dividends declared, for example, but not limited to, common and preferred stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-SubTopic 405
-Topic 942
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477787/942-405-45-2
| Name: |
us-gaap_Dividends |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from holders exercising their stock warrants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromWarrantExercises |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_TwoZeroTwentyTwoPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_TwoZeroTwentyThreePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=abcd_WarrantExchangeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_TopMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=abcd_BottomMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=abcd_WarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
DISAGGREGATION OF REVENUE (Details) - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Revenue |
$ 12,411,048
|
$ 12,823,797
|
$ 40,202,238
|
$ 37,537,003
|
| Greece [Member] |
|
|
|
|
| Revenue |
12,247,597
|
12,544,643
|
39,385,730
|
36,041,012
|
| Croatia [Member] |
|
|
|
|
| Revenue |
107
|
14,159
|
19,370
|
14,159
|
| Cyprus [Member] |
|
|
|
|
| Revenue |
16,519
|
72,754
|
89,064
|
141,402
|
| Bulgaria [Member] |
|
|
|
|
| Revenue |
25,507
|
0
|
43,849
|
0
|
| Ireland [Member] |
|
|
|
|
| Revenue |
0
|
1,417
|
0
|
1,417
|
| UK [Member] |
|
|
|
|
| Revenue |
121,318
|
190,614
|
664,225
|
1,338,509
|
| United States [Member] |
|
|
|
|
| Revenue |
$ 0
|
$ 210
|
$ 0
|
$ 504
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-40
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477314/942-235-S99-1
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.3
| X |
- References
+ Details
| Name: |
abcd_BidingPriceDescripion |
| Namespace Prefix: |
abcd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_ClosingBidOfSharesOfCommonStock |
| Namespace Prefix: |
abcd_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
abcd_MinimumBidPricePerShare |
| Namespace Prefix: |
abcd_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Cosmos Health (NASDAQ:COSM)
Historical Stock Chart
From Dec 2024 to Jan 2025
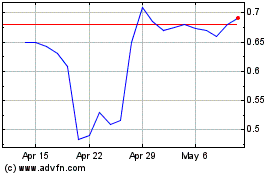
Cosmos Health (NASDAQ:COSM)
Historical Stock Chart
From Jan 2024 to Jan 2025