0001314102false00013141022024-10-282024-10-28
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): October 28, 2024 |
EyePoint Pharmaceuticals, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
000-51122 |
26-2774444 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
480 Pleasant Street |
|
Watertown, Massachusetts |
|
02472 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (617) 926-5000 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.001 |
|
EYPT |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On October 28, 2024, EyePoint Pharmaceuticals, Inc. (the “Company”) posted an updated investor presentation (the "Presentation") on its website at www.eyepointpharma.com which included estimated cash and investments on hand as of September 30, 2024 and certain other corporate updates. The amounts included in the presentation were calculated prior to the completion of a review by the Company’s independent registered public accounting firm and are therefore subject to change upon completion of the Company’s quarterly report for the period ended September 30, 2024. Additional information and disclosures would be required for a more complete understanding of the Company’s financial position and results of operations as of September 30, 2024.
Item 8.01 Other Events.
On October 28, 2024, the Company issued a press release announcing positive interim data for the ongoing Phase 2 VERONA clinical trial evaluating DURAVYU as a six-month maintenance therapy for patients with diabetic macular edema (“DME”). A copy of the press release is attached hereto as Exhibit 99.1 and incorporated by reference herein.
On October 28, 2024, the Company posted the Presentation on its website at www.eyepointpharma.com. A copy of the Presentation is filed herewith as Exhibit 99.2 and is incorporated by reference herein.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
EYEPOINT PHARMACEUTICALS, INC. |
|
|
|
|
Date: |
October 28, 2024 |
By: |
/s/ George O. Elston |
|
|
|
George O. Elston
Executive Vice President and Chief Financial Officer |
Exhibit 99.1
EyePoint Pharmaceuticals Announces Positive Interim 16-Week Data for Ongoing Phase 2 VERONA Clinical Trial of DURAVYUTM for Diabetic Macular Edema
- DURAVYU 2.7mg demonstrated an early and sustained improvement in BCVA with a gain of +8.9 letters compared to baseline -
– DURAVYU 2.7mg demonstrated an early and sustained anatomical improvement mirroring BCVA results with a 68 micron reduction in CST -
– Favorable safety profile continues with no DURAVYU-related ocular or systemic SAEs to date –
– Full topline data anticipated in Q1 2025 –
WATERTOWN, Mass., October 28, 2024 (GLOBE NEWSWIRE) – EyePoint Pharmaceuticals, Inc. (NASDAQ: EYPT), a company committed to developing and commercializing innovative therapeutics to improve the lives of patients with serious retinal diseases, today announced positive interim 16-week data for the ongoing Phase 2 VERONA clinical trial evaluating DURAVYU, an investigational sustained delivery therapy delivering patent-protected vorolanib, a selective tyrosine kinase inhibitor formulated in proprietary bioerodible Durasert E™, for patients with diabetic macular edema (DME). DURAVYU 2.7mg demonstrated an early, sustained, and clinically meaningful improvement in best-corrected visual acuity (BCVA) and anatomical control versus the aflibercept control arm. A favorable safety and tolerability profile continued for both DURAVYU arms. The 2.7mg dose is also being evaluated in the Phase 3 pivotal trials for wet AMD. The Company expects to report the full topline results in the first quarter of 2025, once all patients complete the trial.
“We are encouraged and excited by these highly positive interim results demonstrating clinically meaningful functional and concomitant structural improvement with a continued favorable safety profile of DURAVYU,” said Jay S. Duker, M.D., President and Chief Executive Officer of EyePoint. “DME is a prevalent disease with a significant need for more durable treatments. The interim data from the VERONA trial demonstrates that after a single DURAVYU 2.7mg treatment there was a meaningful, early and maintained improvement in BCVA paired with strong anatomical improvement in retinal thickness, demonstrating the potential for DURAVYU in DME as a sustained delivery therapy. One of the most notable aspects of these data is that both DURAVYU doses showed an immediate benefit over aflibercept control in both BCVA and CST. We believe these compelling interim results support DURAVYU’s potential to advance to non-inferiority pivotal trials in DME. With pivotal wet AMD clinical trials underway and promising DME interim data, DURAVYU has the potential to be the first sustained release therapy to market in two significant indications.”
Phase 2 VERONA interim 16-week results as of October 1, 2024 data cut-off include:
•All patients (n=27) have completed the week 16 visit.
•DURAVYU 2.7mg demonstrated an early and sustained improvement in both BCVA and CST (central subfield thickness) as measured by optical coherence tomography (OCT).
oBCVA improved +8.9 letters versus +3.2 letters for aflibercept control compared to baseline.
oCST improved 68.1 microns versus 30.5 microns for aflibercept control compared to baseline.
•Visual and anatomical gains were observed at Week 4 demonstrating the immediate bioavailability of DURAVYU.
•Positive trend in BCVA and anatomy continued for patients that have reached the Week 24 visit.
•Continued positive safety and tolerability profile with no DURAVYU-related ocular or systemic serious adverse events. Additionally, there were no cases of:
oRetinal vasculitis (occlusive or non-occlusive)
oInsert migration to the anterior chamber
oIntraocular inflammation (IOI)
•82% of eyes in the DURAVYU 2.7mg arm were supplement-free versus 50% in the aflibercept control arm at 16 weeks.
“DME is a sight-threatening complication of diabetes that can lead to severe visual loss and eventual blindness,” said Charles Wykoff, M.D., Ph.D., Director of Research, Retina Consultants of Texas and Co-Chair of EyePoint’s Scientific Advisory Board. “There remains a significant need for differentiated and longer-acting treatments, as the current standard of care involves frequent intravitreal injections that can be a burden and have been associated with under-treatment. The interim data from the Phase 2 VERONA trial suggests promising activity in patients with active DME versus aflibercept alone and a favorable safety profile. These results support the potential for DURAVYU to bring substantial value to patients through stable, durable disease control.”
“Reducing the treatment burden in patients with DME is a critical unmet need,” said Adam Gerstenblith, M.D., a principal investigator in the VERONA clinical trial and vitreoretinal surgeon at Mid Atlantic Retina Specialists. “As a clinician dedicated to advancing retinal care, I am encouraged by the interim clinical data demonstrating the potential for DURAVYU 2.7mg to extend treatment intervals while improving vision without sacrificing anatomy. The VERONA trial is an important step in the pursuit of treatment options for patients that are safe and durable, and I am pleased to be participating in research that has the potential to shift the treatment paradigm in DME and ultimately improve patient outcomes.”
VERONA is a randomized, controlled, single-masked, open label Phase 2 trial of DURAVYU in DME patients previously treated with a standard-of-care anti-VEGF therapy. The trial enrolled 27 patients assigned to one of two intravitreal doses of DURAVYU (1.3mg or 2.7mg) or aflibercept control. The primary efficacy endpoint of the VERONA trial is time to first supplemental aflibercept injection up to 24 weeks based on established supplement criteria. Key secondary endpoints include safety, mean change in best corrected visual acuity (BCVA), mean change in central subfield thickness (CST) as measured by optical coherence tomography (OCT) and change in diabetic retinopathy severity scale (DRSS) over time. More information about the trial is available at clinicaltrials.gov (identifier: NCT06099184).
About Diabetic Macular Edema
Diabetic macular edema (DME) is the leading cause of vision loss in people with type 1 and type 2 diabetes. DME results when damaged blood vessels leak fluid into the macula, the central portion of the retina responsible for the sharp vision needed for routine tasks such as driving or reading. This resulting retinal swelling can cause blurred vision and may lead to severe vision loss or even blindness. DME is a common form of sight-threatening retinopathy in people with diabetes, with approximately 28 million people afflicted worldwide. As the prevalence of diabetes continues to grow, an increased number of people will be affected by diabetic eye diseases such as DME. The current standard of care for patients experiencing DME include intravitreal injections of short-acting anti-VEGF biologics, corticosteroids, or laser photocoagulation which can become a burden on patients, caregivers, and physicians due to the longevity of the disease.
About DURAVYU™
DURAVYUTM, f/k/a EYP-1901, is being developed as a potential paradigm-altering treatment for patients suffering from VEGF-mediated retinal diseases. DURAVYU delivers vorolanib, a potent, selective and patent-protected tyrosine kinase inhibitor (TKI) as a solid bioerodible insert using EyePoint’s proprietary sustained-release Durasert E™ technology. Vorolanib brings a new mechanistic approach to the treatment of VEGF-mediated retinal diseases as a pan-VEGF receptor inhibitor, inhibiting all VEGF receptors. Further, in an in-vivo model of retinal detachment, vorolanib demonstrated neuroprotection and may have antifibrotic benefits as it also blocks PDGF. DURAVYU is shipped and stored at ambient temperature and is administered with a standard intravitreal injection in the physician's office. DURAVYU is immediately bioavailable with zero-order kinetics release for up to nine months.
Positive data from both the Phase 1 DAVIO and Phase 2 DAVIO 2 clinical trials of DURAVYU in wet AMD demonstrated clinically meaningful efficacy data with stable visual acuity and CST and a favorable safety profile. Further, data from DAVIO 2 demonstrated an impressive treatment burden reduction of approximately 88% at eight months, six months after treatment with DURAVYU, with over 80% of patients supplement-free or receiving only one supplemental anti-VEGF injection through up to eight months, six months after treatment with DURAVYU. The data from the DAVIO 2 clinical trial supported the advancement of the wet AMD program and the initiation of the Phase 3 LUGANO trial, with the LUCIA pivotal trial to follow by year end 2024.
DURAVYU is also currently being studied in the Phase 2 VERONA trial for diabetic macular edema (DME). Full topline data is expected in the first quarter of 2025.
About EyePoint Pharmaceuticals
EyePoint (Nasdaq: EYPT) is a clinical-stage biopharmaceutical company committed to developing and commercializing innovative therapeutics to help improve the lives of patients with serious retinal diseases. The Company's pipeline leverages its proprietary bioerodible Durasert E™ technology for sustained intraocular drug delivery. The Company’s lead product candidate, DURAVYU™ (f/k/a EYP-1901), is an investigational sustained delivery treatment for VEGF-mediated retinal diseases combining vorolanib, a selective and patent-protected tyrosine kinase inhibitor with bioerodible Durasert E™. DURAVYU is presently in Phase 3 global, pivotal clinical trials as a sustained delivery treatment for wet age-related macular degeneration (wet AMD), the leading cause of vision loss among people 50 years of age and older in the United States, and in a Phase 2 clinical trial in diabetic macular edema (DME). EyePoint expects full topline data from the Phase 2 clinical trial in DME in Q1 2025 and topline data from both Phase 3 pivotal trials in wet AMD in 2026.
Pipeline programs include EYP-2301, a TIE-2 agonist, razuprotafib, formulated in Durasert E™ to potentially improve outcomes in serious retinal diseases. The proven Durasert® drug delivery technology has been safely administered to thousands of patient eyes across four U.S. FDA approved products. EyePoint Pharmaceuticals is headquartered in Watertown, Massachusetts.
Vorolanib is licensed to EyePoint exclusively by Equinox Sciences, a Betta Pharmaceuticals affiliate, for the localized treatment of all ophthalmic diseases outside of China, Macao, Hong Kong and Taiwan.
DURAVYU™ has been conditionally accepted by the FDA as the proprietary name for EYP-1901. DURAVYU is an investigational product; it has not been approved by the FDA. FDA approval and the timeline for potential approval is uncertain.
Forward Looking Statements
EYEPOINT PHARMACEUTICALS SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION ACT OF 1995: To the extent any statements made in this press release deal with information that is not historical, these are forward-looking statements under the Private Securities

Litigation Reform Act of 1995. Such statements include, but are not limited to, statements regarding our expectations regarding the timing and clinical development and potential of DURAVYU in DME and wet AMD, including our expectations regarding the announcement of full topline data from the VERONA trial in the first quarter of 2025 and initiation of the LUGANO trial and the LUCIA trial; the belief that the interim results from the VERONA trial support DURAVYU’s potential to advance to non-inferiority pivotal trials; our beliefs and expectations regarding the anticipated full results from the VERONA trial; the potential for DURAVYU 2.7mg to extend treatment intervals while improving vision without sacrificing anatomy; the potential for DURAVYU to provide an immediate benefit over aflibercept control in both BCVA and CST; our optimism that that DURAVYU has the potential to shift the treatment paradigm in DME and improve patient outcomes; our expectations regarding clinical development of our other product candidates, including EYP-2301; our business strategies and objectives; and other statements identified by words such as “will,” “potential,” “could,” “can,” “believe,” “intends,” “continue,” “plans,” “expects,” “anticipates,” “estimates,” “may,” other words of similar meaning or the use of future dates. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause EyePoint’s actual results to be materially different than those expressed in or implied by EyePoint’s forward-looking statements. For EyePoint, these risks and uncertainties include the timing, progress and results of the company’s clinical development activities; uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; the company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the risk that results of clinical trials may not be predictive of future results, and interim and preliminary data are subject to further analysis and may change as more data becomes available; unexpected safety or efficacy data observed during clinical trials; uncertainties related to the regulatory authorization or approval process, and available development and regulatory pathways for approval of the company’s product candidates; changes in the regulatory environment; changes in expected or existing competition; the success of current and future license agreements; our dependence on contract research organizations, and other outside vendors and service providers; product liability; the impact of general business and economic conditions; protection of our intellectual property and avoiding intellectual property infringement; retention of key personnel; delays, interruptions or failures in the manufacture and supply of our product candidates; the availability of and the need for additional financing; the company’s ability to obtain additional funding to support its clinical development programs; uncertainties regarding the timing and results of the August 2022 subpoena from the U.S. Attorney’s Office for the District of Massachusetts; uncertainties regarding the FDA warning letter pertaining to the company’s Watertown, MA manufacturing facility; and other factors described in our filings with the Securities and Exchange Commission. We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. EyePoint undertakes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events, or otherwise.
Investors:
Christina Tartaglia
Precision AQ (formerly Stern IR)
Direct: 212-698-8700
christina.tartaglia@sternir.com
Media Contact:
Amy Phillips
Green Room Communications
Direct: 412-327-9499
aphillips@greenroompr.com

Investor Presentation October 2024 ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Exhibit 99.2

Legal Disclaimers ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Various statements made in this presentation are forward-looking, within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, and are inherently subject to risks, uncertainties and potentially inaccurate assumptions. All statements that address activities, events or developments that we intend, expect, plan or believe may occur in the future, are forward-looking statements , including but not limited to statements regarding; our expectations regarding the timing and clinical development of DURAVYU™ in Wet AMD and DME, our expectations regarding the enrollment, dosing and data readouts for the LUGANO trial and the LUCIA trial; our optimism that that DURAVYU has the potential to change the current treatment paradigm and revolutionize real-world outcomes for patients suffering from serious retinal diseases; our belief that DURAVYU has the potential to maintain a majority of patients with active disease with no supplemental anti-VEGF therapy for six months or longer; and our expectations regarding the timing and clinical development of our other product candidates, including EYP-2301. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause EyePoint’s actual results to be materially different than those expressed in or implied by EyePoint’s forward-looking statements. For EyePoint, these risks and uncertainties include the timing, progress and results of the company’s clinical development activities; uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; the company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the risk that results of clinical trials may not be predictive of future results, and interim and preliminary data are subject to further analysis and may change as more data becomes available; unexpected safety or efficacy data observed during clinical trials; uncertainties related to the regulatory authorization or approval process, and available development and regulatory pathways for approval of the company’s product candidates; changes in the regulatory environment; changes in expected or existing competition; the success of current and future license agreements; our dependence on contract research organizations, and other outside vendors and service providers; product liability; the impact of general business and economic conditions; protection of our intellectual property and avoiding intellectual property infringement; retention of key personnel; delays, interruptions or failures in the manufacture and supply of our product candidates; the availability of and the need for additional financing; the company’s ability to obtain additional funding to support its clinical development programs; uncertainties regarding the timing and results of the August 2022 subpoena from the U.S. Attorney’s Office for the District of Massachusetts; uncertainties regarding the FDA warning letter pertaining to the company’s Watertown, MA manufacturing facility; and other factors described in our filings with the Securities and Exchange Commission (SEC). More detailed information on these and additional factors that could affect our actual results are described in our filings with the SEC, including our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, as revised or supplemented by its Quarterly Reports on Form 10-Q and other documents filed with the SEC. All forward-looking statements in this news release. We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. EyePoint undertakes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events, or otherwise. This presentation may also contain estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we compete are necessarily subject to a high degree of uncertainty and risk. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 3 Committed to Developing Innovative Therapeutics to Improve the Lives of Patients with Serious Retinal Diseases
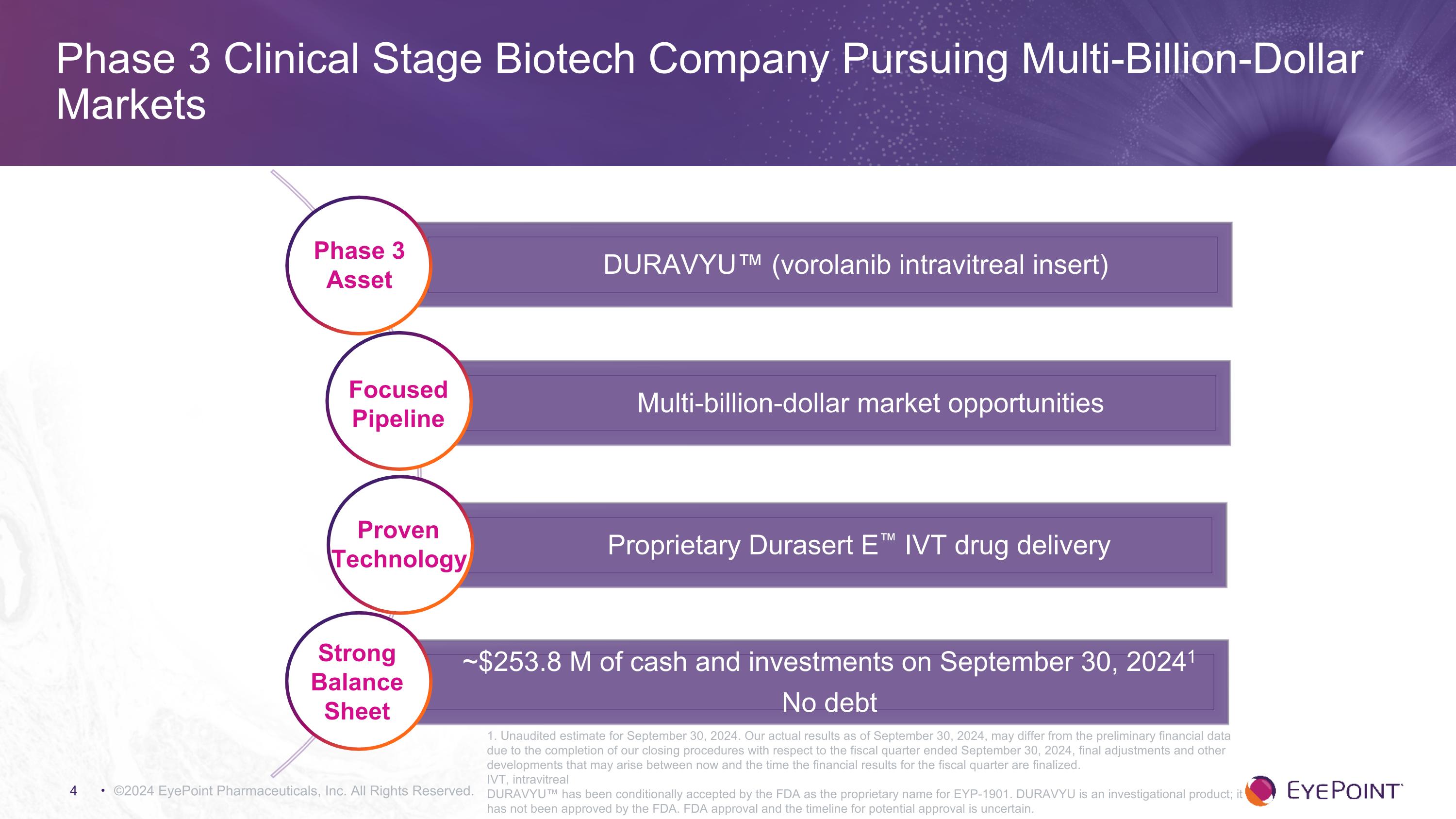
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 3 Clinical Stage Biotech Company Pursuing Multi-Billion-Dollar Markets DURAVYU™ (vorolanib intravitreal insert) Multi-billion-dollar market opportunities Proprietary Durasert E™ IVT drug delivery ~$253.8 M of cash and investments on September 30, 20241 No debt Phase 3 Asset Focused Pipeline Strong Balance Sheet Proven Technology 1. Unaudited estimate for September 30, 2024. Our actual results as of September 30, 2024, may differ from the preliminary financial data due to the completion of our closing procedures with respect to the fiscal quarter ended September 30, 2024, final adjustments and other developments that may arise between now and the time the financial results for the fiscal quarter are finalized. IVT, intravitreal DURAVYU™ has been conditionally accepted by the FDA as the proprietary name for EYP-1901. DURAVYU is an investigational product; it has not been approved by the FDA. FDA approval and the timeline for potential approval is uncertain.
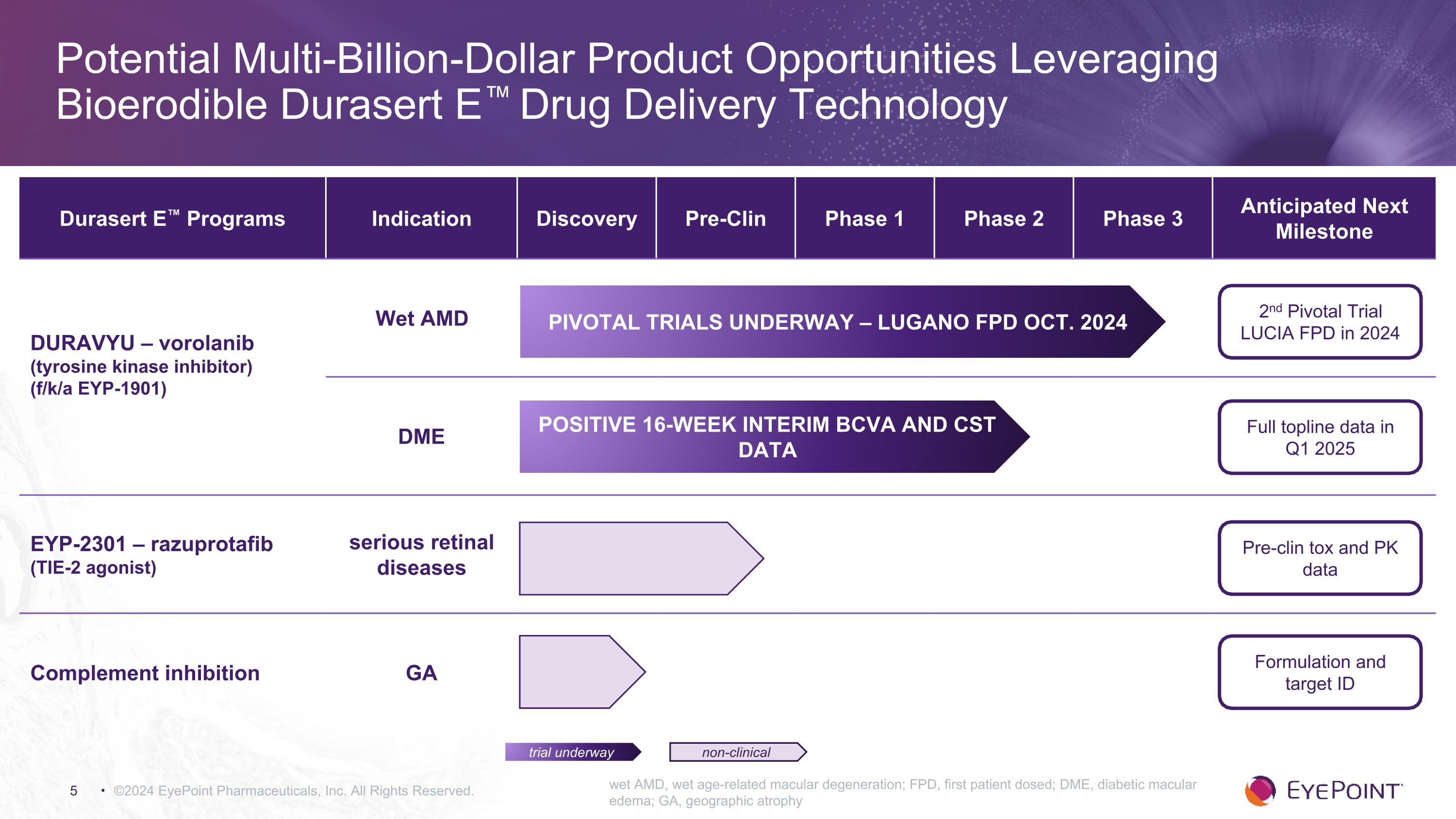
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. wet AMD, wet age-related macular degeneration; FPD, first patient dosed; DME, diabetic macular edema; GA, geographic atrophy Potential Multi-Billion-Dollar Product Opportunities Leveraging Bioerodible Durasert E™ Drug Delivery Technology Durasert E™ Programs Indication Discovery Pre-Clin Phase 1 Phase 2 Phase 3 Anticipated Next Milestone DURAVYU – vorolanib (tyrosine kinase inhibitor) (f/k/a EYP-1901) Wet AMD DME EYP-2301 – razuprotafib (TIE-2 agonist) serious retinal diseases Complement inhibition GA 2nd Pivotal Trial LUCIA FPD in 2024 Full topline data in Q1 2025 Pre-clin tox and PK data Formulation and target ID trial underway non-clinical PIVOTAL TRIALS UNDERWAY – LUGANO FPD OCT. 2024 Positive 16-WEEK interim BCVA and CST data

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. IVT, intravitreal Bioerodible DURASERT E™ TECHNOLOGY Sustained-Release Drug Delivery with favorable safety profile Delivered via a standard in-office IVT injection Continuous dosing Zero-order kinetics drug release Durasert E™: bioerodible Drug formulated within a bioerodible matrix as a solid insert Designed to deplete drug load before matrix fully erodes Favorable safety profile across multiple indications ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 6
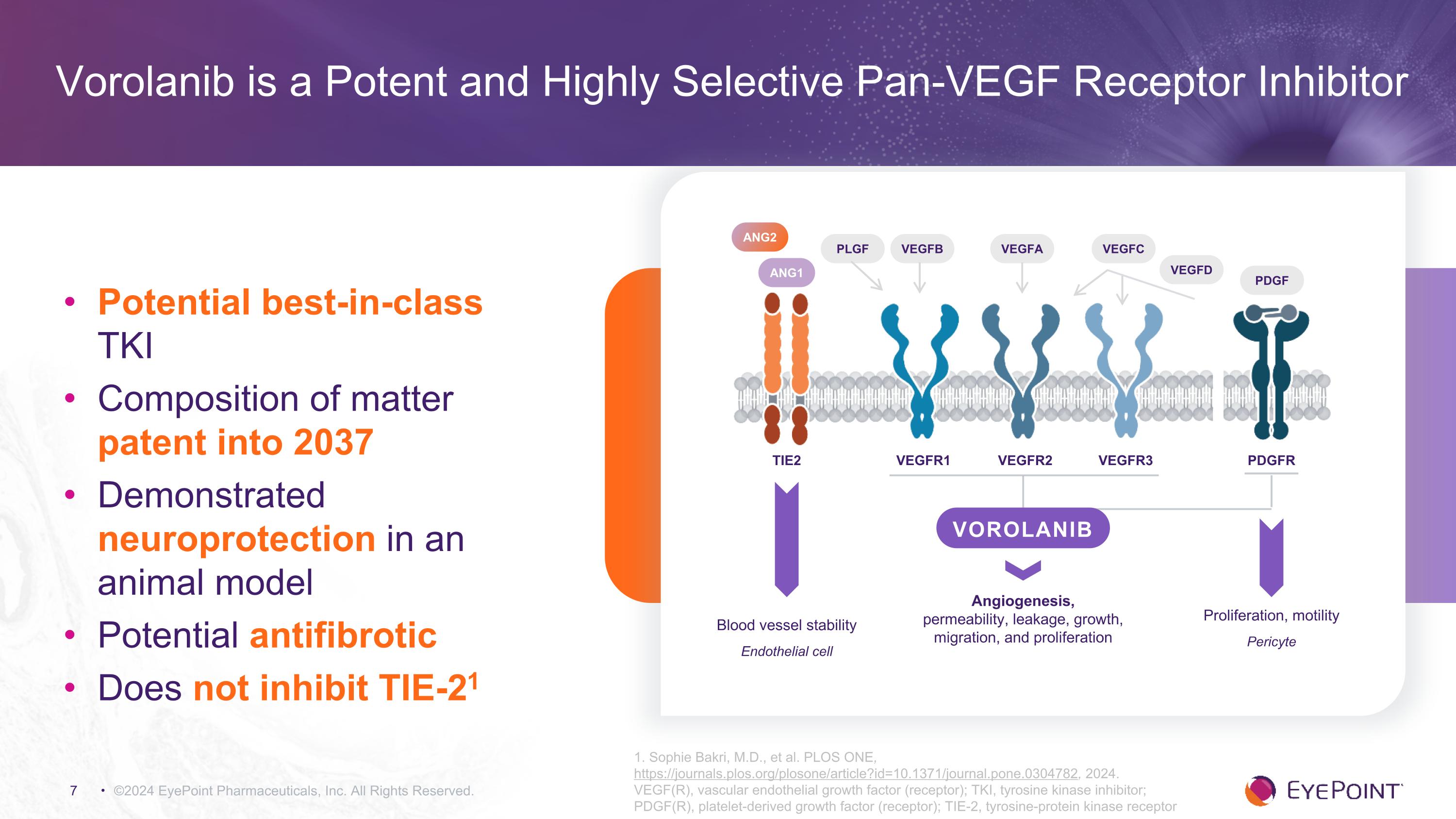
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 1. Sophie Bakri, M.D., et al. PLOS ONE, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0304782, 2024. VEGF(R), vascular endothelial growth factor (receptor); TKI, tyrosine kinase inhibitor; PDGF(R), platelet-derived growth factor (receptor); TIE-2, tyrosine-protein kinase receptor Vorolanib is a Potent and Highly Selective Pan-VEGF Receptor Inhibitor Potential best-in-class TKI Composition of matter patent into 2037 Demonstrated neuroprotection in an animal model Potential antifibrotic Does not inhibit TIE-21 VEGFB VEGFA VEGFC VEGFD VEGFR1 VEGFR2 VEGFR3 TIE2 PDGFR ANG1 ANG2 PDGF Angiogenesis, �permeability, leakage, growth, migration, and proliferation Blood vessel stability Endothelial cell Proliferation, motility Pericyte VOROLANIB PLGF
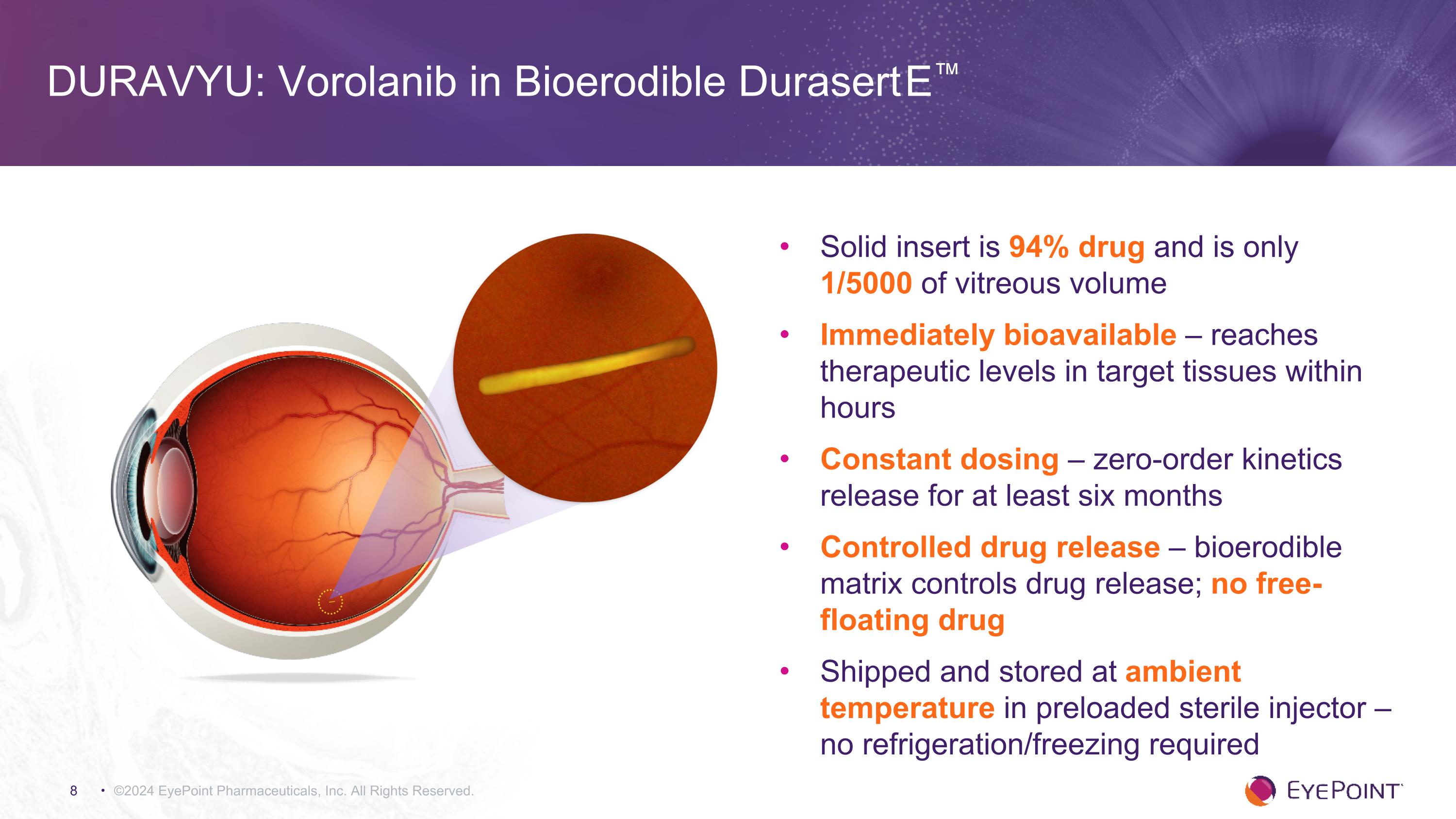
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Solid insert is 94% drug and is only 1/5000 of vitreous volume Immediately bioavailable – reaches therapeutic levels in target tissues within hours Constant dosing – zero-order kinetics release for at least six months Controlled drug release – bioerodible matrix controls drug release; no free-floating drug Shipped and stored at ambient temperature in preloaded sterile injector – no refrigeration/freezing required DURAVYU: Vorolanib in Bioerodible Durasert E™
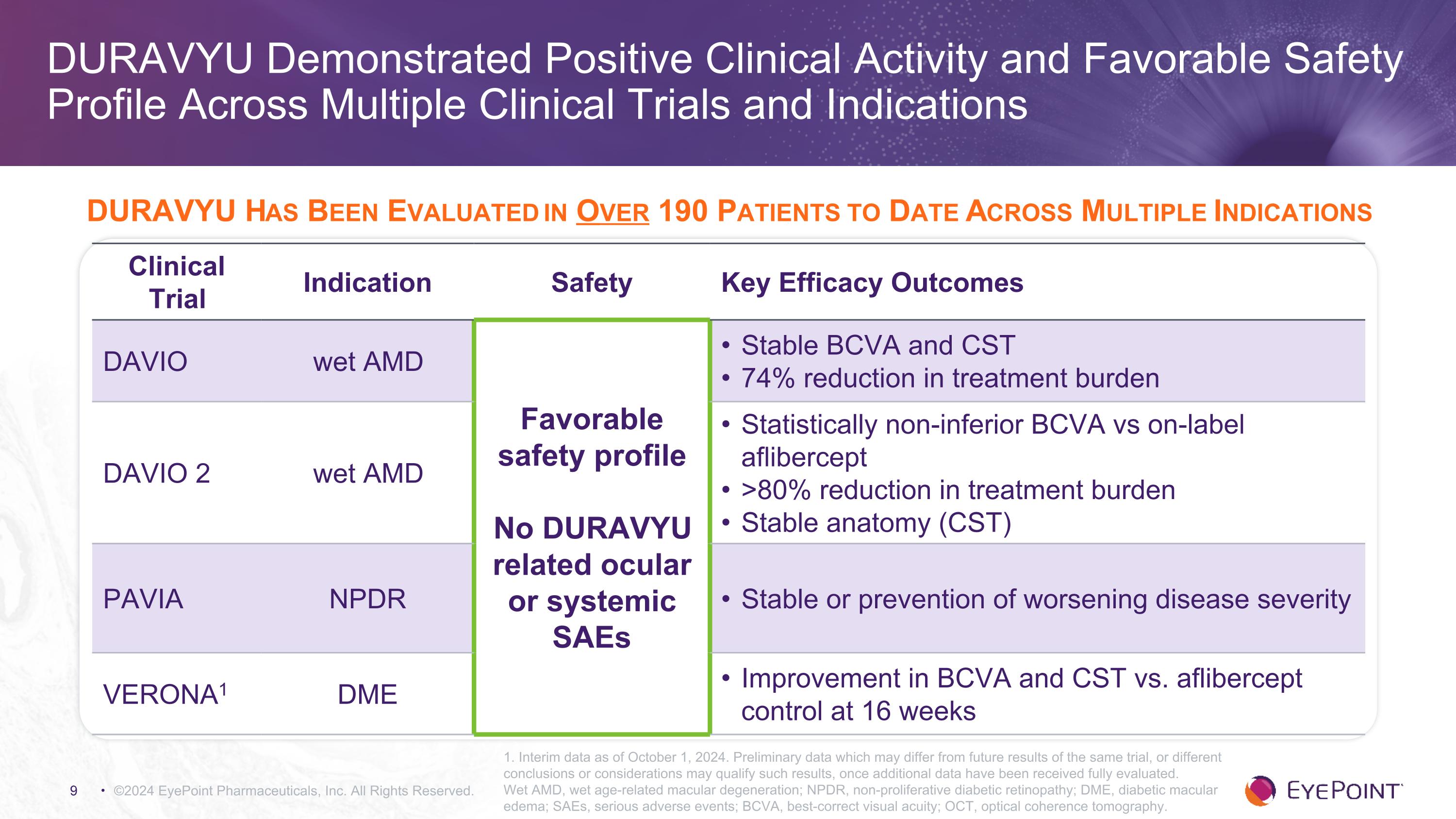
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU Demonstrated Positive Clinical Activity and Favorable Safety Profile Across Multiple Clinical Trials and Indications 1. Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received fully evaluated. Wet AMD, wet age-related macular degeneration; NPDR, non-proliferative diabetic retinopathy; DME, diabetic macular edema; SAEs, serious adverse events; BCVA, best-correct visual acuity; OCT, optical coherence tomography. Clinical Trial Indication Safety Key Efficacy Outcomes DAVIO wet AMD Favorable safety profile No DURAVYU related ocular or systemic SAEs Stable BCVA and CST 74% reduction in treatment burden DAVIO 2 wet AMD Statistically non-inferior BCVA vs on-label aflibercept >80% reduction in treatment burden Stable anatomy (CST) PAVIA NPDR Stable or prevention of worsening disease severity VERONA1 DME Improvement in BCVA and CST vs. aflibercept control at 16 weeks DURAVYU Has Been Evaluated in Over 190 Patients to Date Across Multiple Indications

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 2 VERONA Clinical Trial in DME – 16-Week Interim Results all patients Have completed the week 16 visit DME, diabetic macular edema Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received fully evaluated.
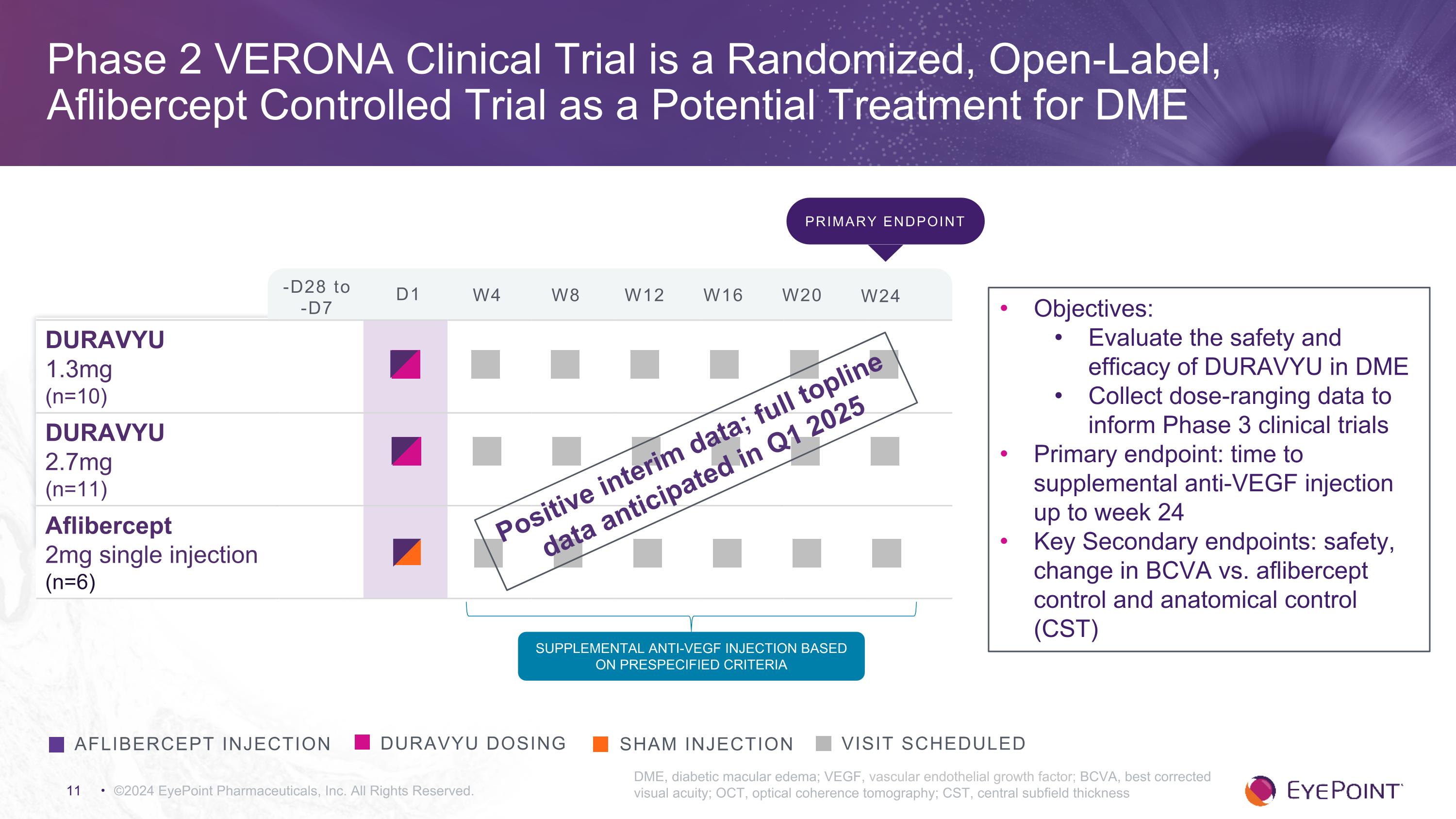
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DME, diabetic macular edema; VEGF, vascular endothelial growth factor; BCVA, best corrected visual acuity; OCT, optical coherence tomography; CST, central subfield thickness DURAVYU dosing Visit Scheduled aflibercept injection Sham injection DURAVYU 1.3mg (n=10) DURAVYU 2.7mg (n=11) Aflibercept 2mg single injection (n=6) Supplemental Anti-VEGF injection based on prespecified criteria Objectives: Evaluate the safety and efficacy of DURAVYU in DME Collect dose-ranging data to inform Phase 3 clinical trials Primary endpoint: time to supplemental anti-VEGF injection up to week 24 Key Secondary endpoints: safety, change in BCVA vs. aflibercept control and anatomical control (CST) Primary endpoint -D28 to -D7 D1 W4 W8 W12 W16 W20 W24 Positive interim data; full topline data anticipated in Q1 2025 Phase 2 VERONA Clinical Trial is a Randomized, Open-Label, Aflibercept Controlled Trial as a Potential Treatment for DME
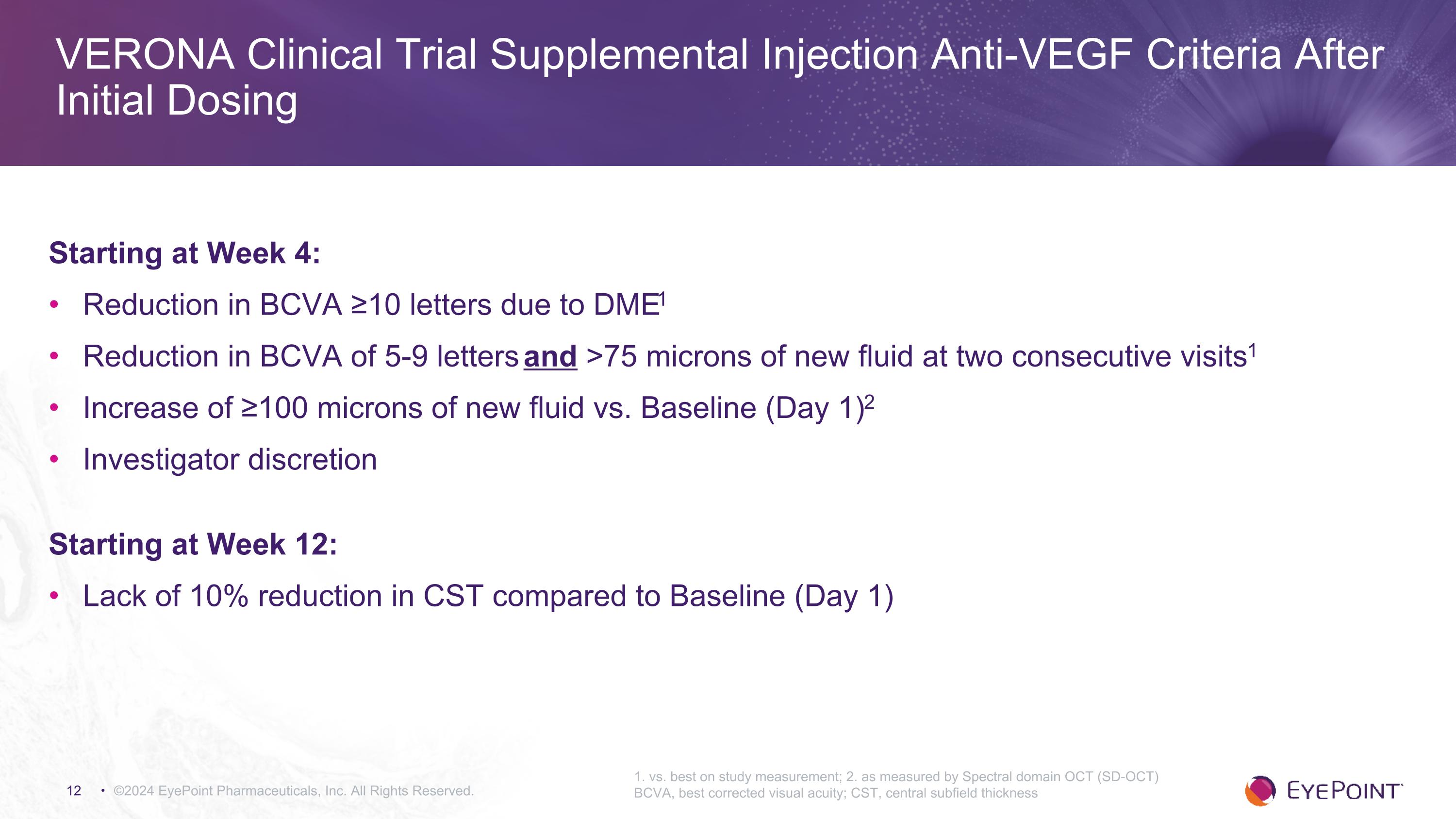
VERONA Clinical Trial Supplemental Injection Anti-VEGF Criteria After Initial Dosing ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 1. vs. best on study measurement; 2. as measured by Spectral domain OCT (SD-OCT) BCVA, best corrected visual acuity; CST, central subfield thickness Starting at Week 4: Reduction in BCVA ≥10 letters due to DME1 Reduction in BCVA of 5-9 letters and >75 microns of new fluid at two consecutive visits1 Increase of ≥100 microns of new fluid vs. Baseline (Day 1)2 Investigator discretion Starting at Week 12: Lack of 10% reduction in CST compared to Baseline (Day 1)
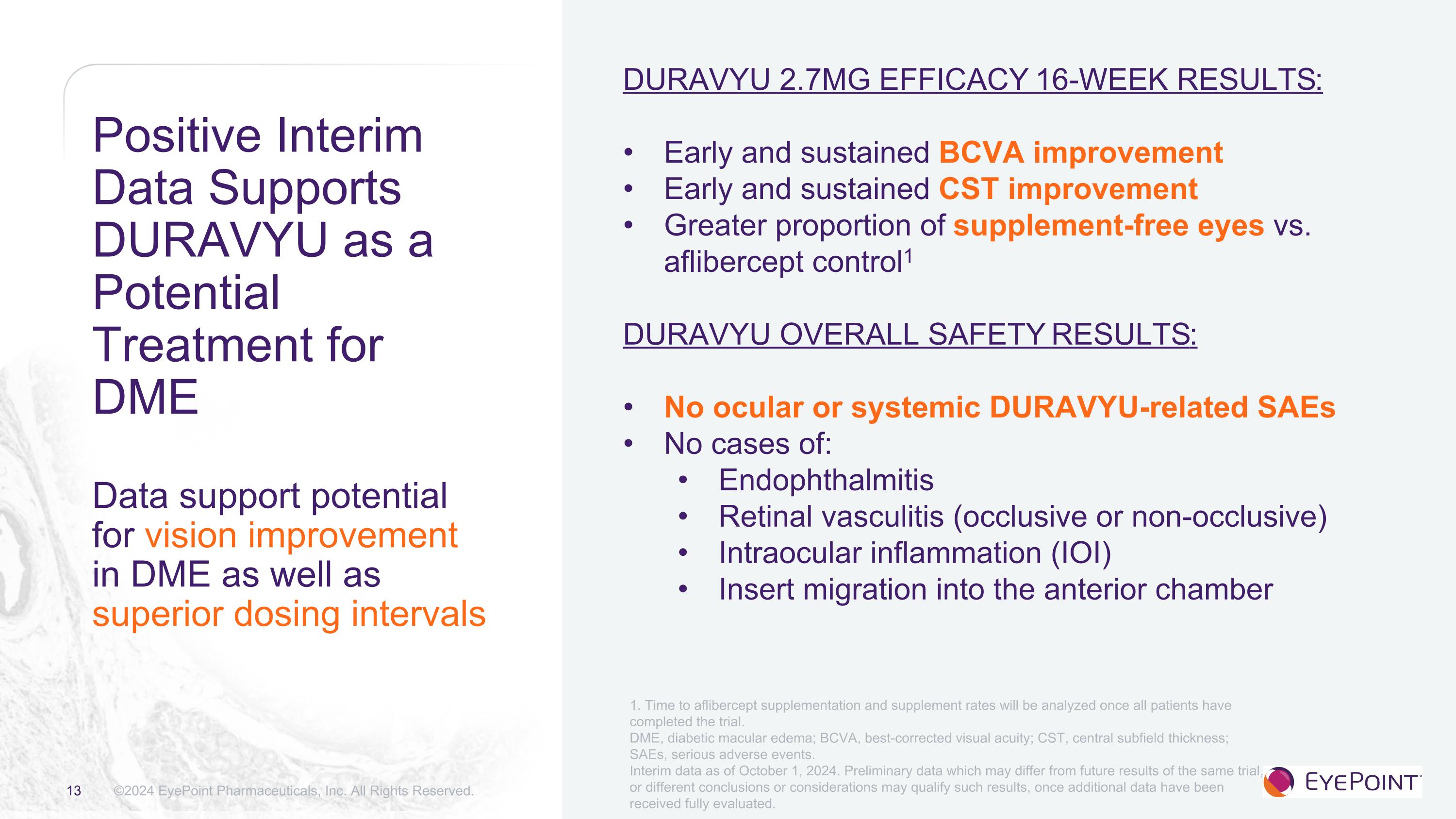
Positive Interim Data Supports DURAVYU as a Potential Treatment for DME��Data support potential for vision improvement in DME as well as superior dosing intervals ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU 2.7MG EFFICACY 16-Week Results: Early and sustained BCVA improvement Early and sustained CST improvement Greater proportion of supplement-free eyes vs. aflibercept control1 DURAVYU OVERALL SAFETY Results: No ocular or systemic DURAVYU-related SAEs No cases of: Endophthalmitis Retinal vasculitis (occlusive or non-occlusive) Intraocular inflammation (IOI) Insert migration into the anterior chamber 1. Time to aflibercept supplementation and supplement rates will be analyzed once all patients have completed the trial. DME, diabetic macular edema; BCVA, best-corrected visual acuity; CST, central subfield thickness; SAEs, serious adverse events. Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received fully evaluated.
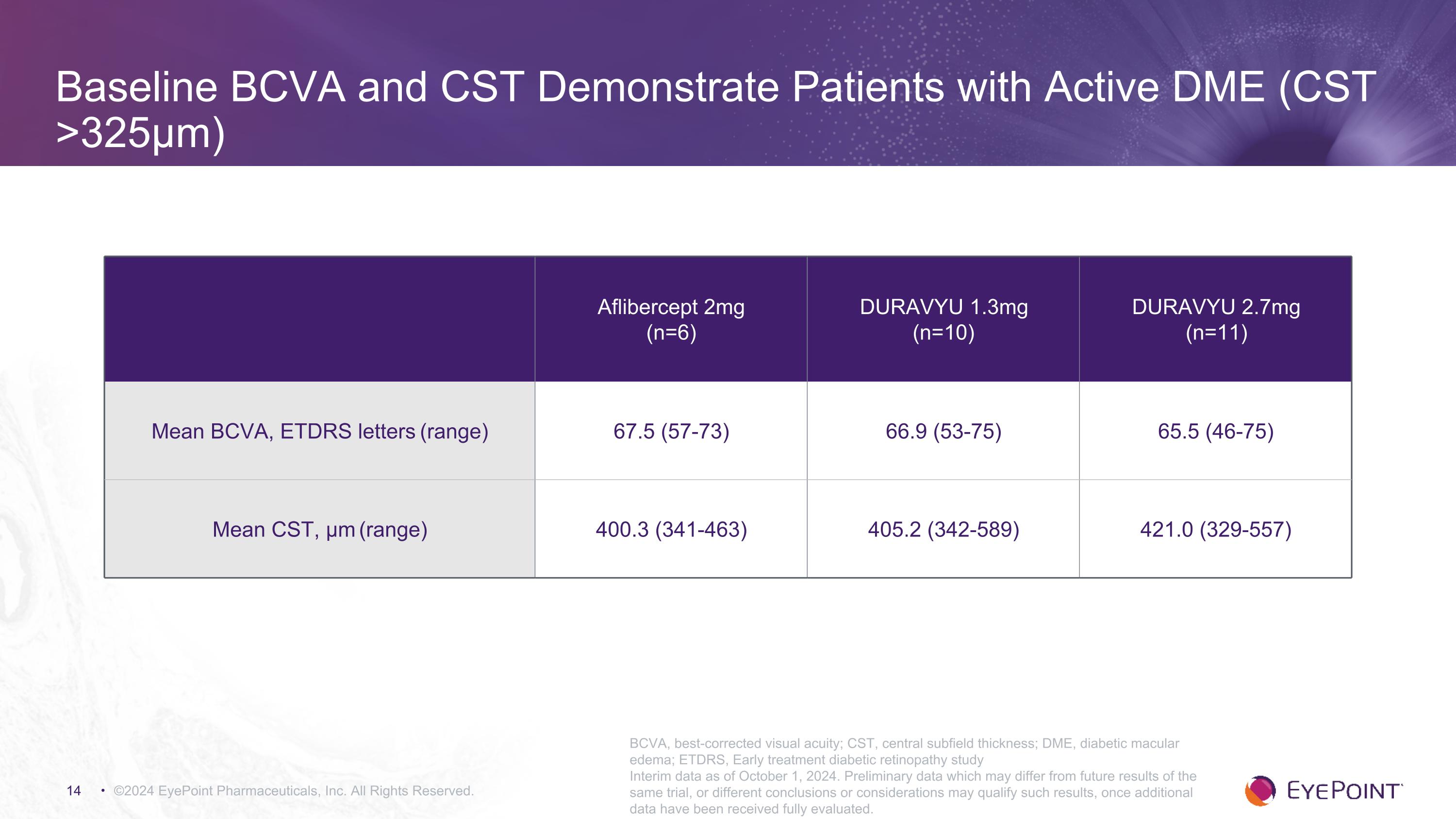
Baseline BCVA and CST Demonstrate Patients with Active DME (CST >325μm) ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Aflibercept 2mg (n=6) DURAVYU 1.3mg (n=10) DURAVYU 2.7mg (n=11) Mean BCVA, ETDRS letters (range) 67.5 (57-73) 66.9 (53-75) 65.5 (46-75) Mean CST, μm (range) 400.3 (341-463) 405.2 (342-589) 421.0 (329-557) BCVA, best-corrected visual acuity; CST, central subfield thickness; DME, diabetic macular edema; ETDRS, Early treatment diabetic retinopathy study Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received fully evaluated.
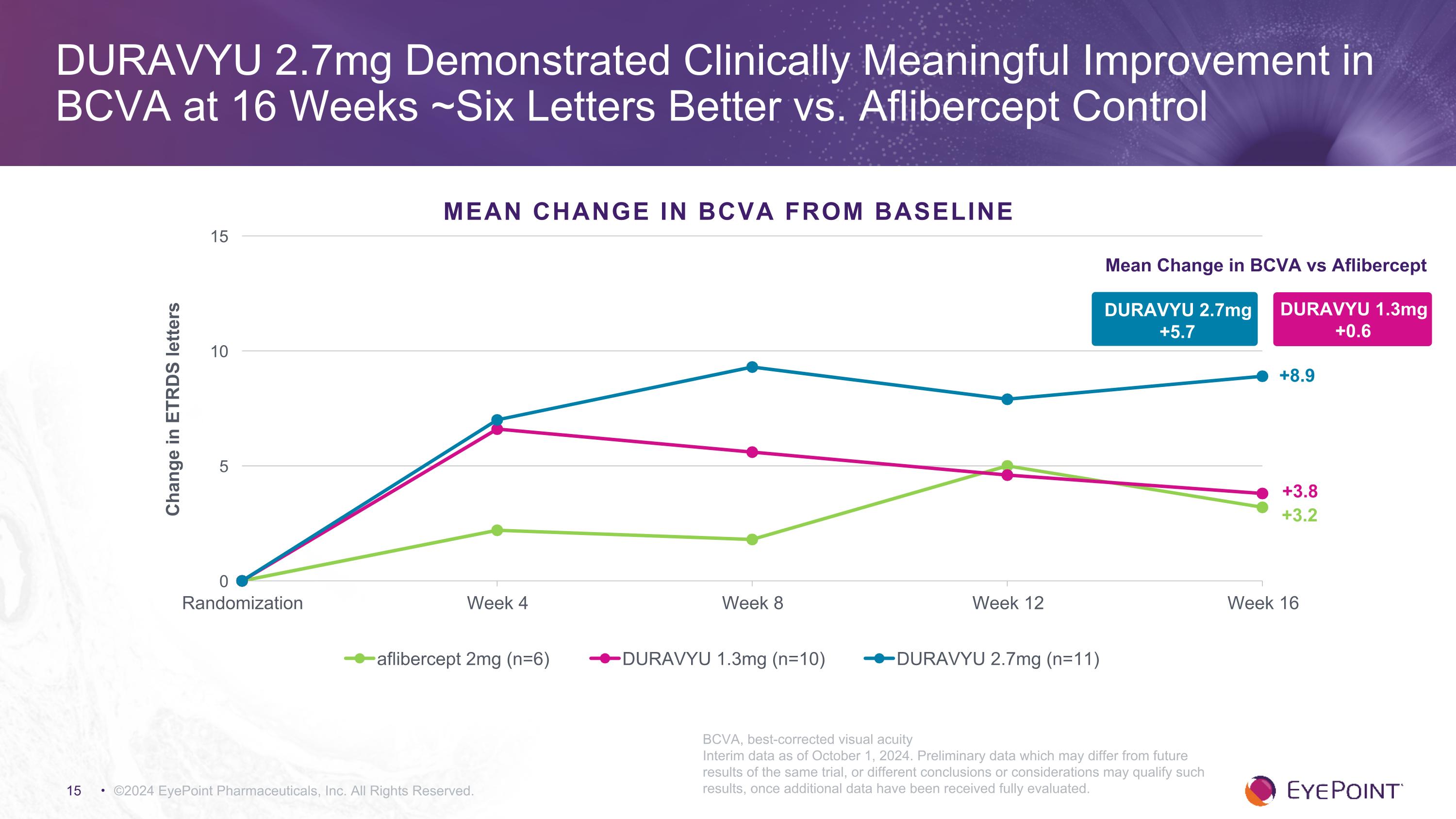
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU 2.7mg Demonstrated Clinically Meaningful Improvement in BCVA at 16 Weeks ~Six Letters Better vs. Aflibercept Control DURAVYU 2.7mg +5.7 Mean Change in BCVA vs Aflibercept DURAVYU 1.3mg +0.6 +3.8 +8.9 +3.2 MEAN CHANGE IN BCVA FROM BASELINE BCVA, best-corrected visual acuity Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received fully evaluated.
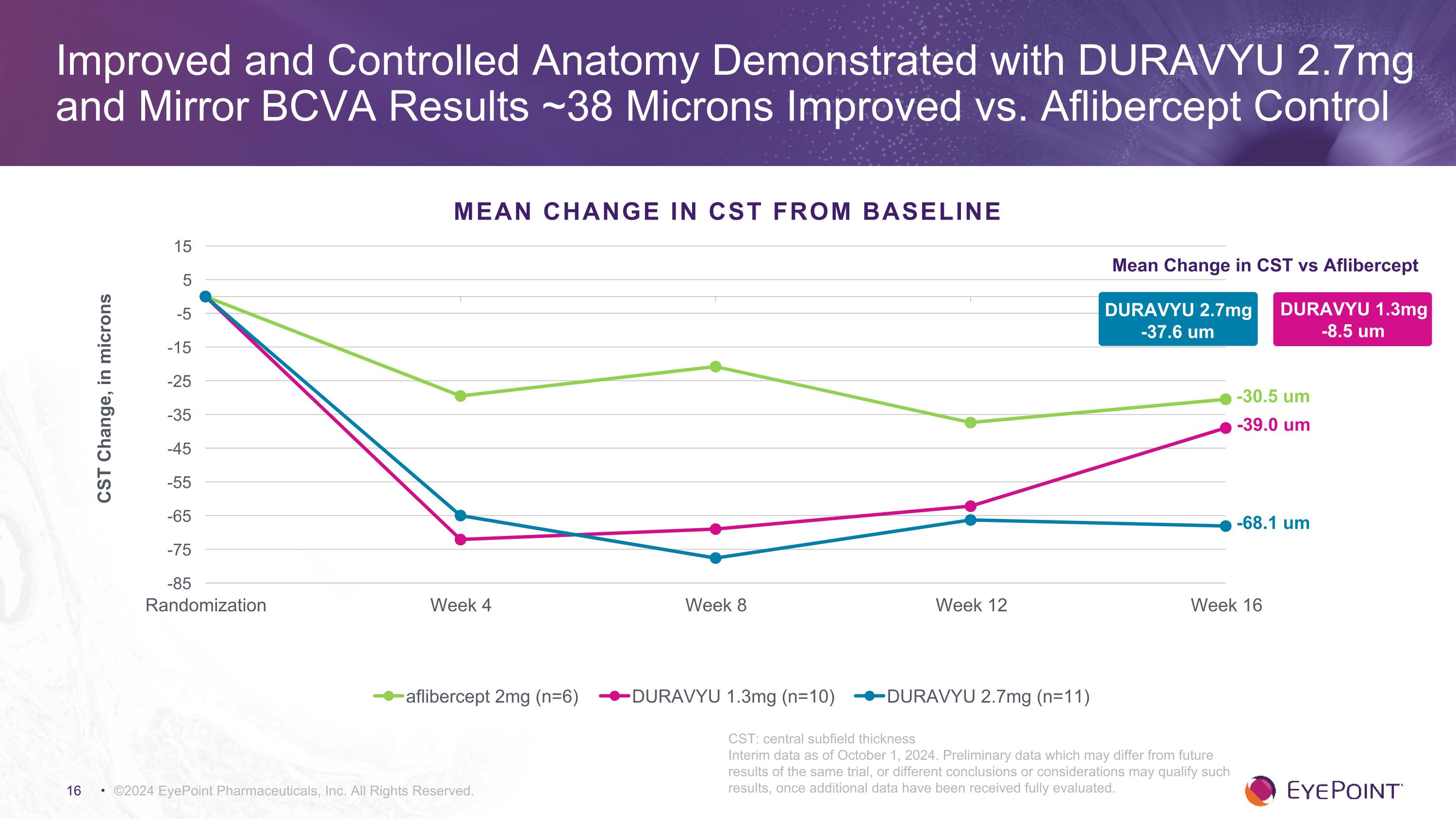
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Improved and Controlled Anatomy Demonstrated with DURAVYU 2.7mg and Mirror BCVA Results ~38 Microns Improved vs. Aflibercept Control Mean Change in CST vs Aflibercept DURAVYU 1.3mg -8.5 um -39.0 um -68.1 um -30.5 um MEAN CHANGE IN CST FROM BASELINE CST: central subfield thickness Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received fully evaluated. DURAVYU 2.7mg -37.6 um
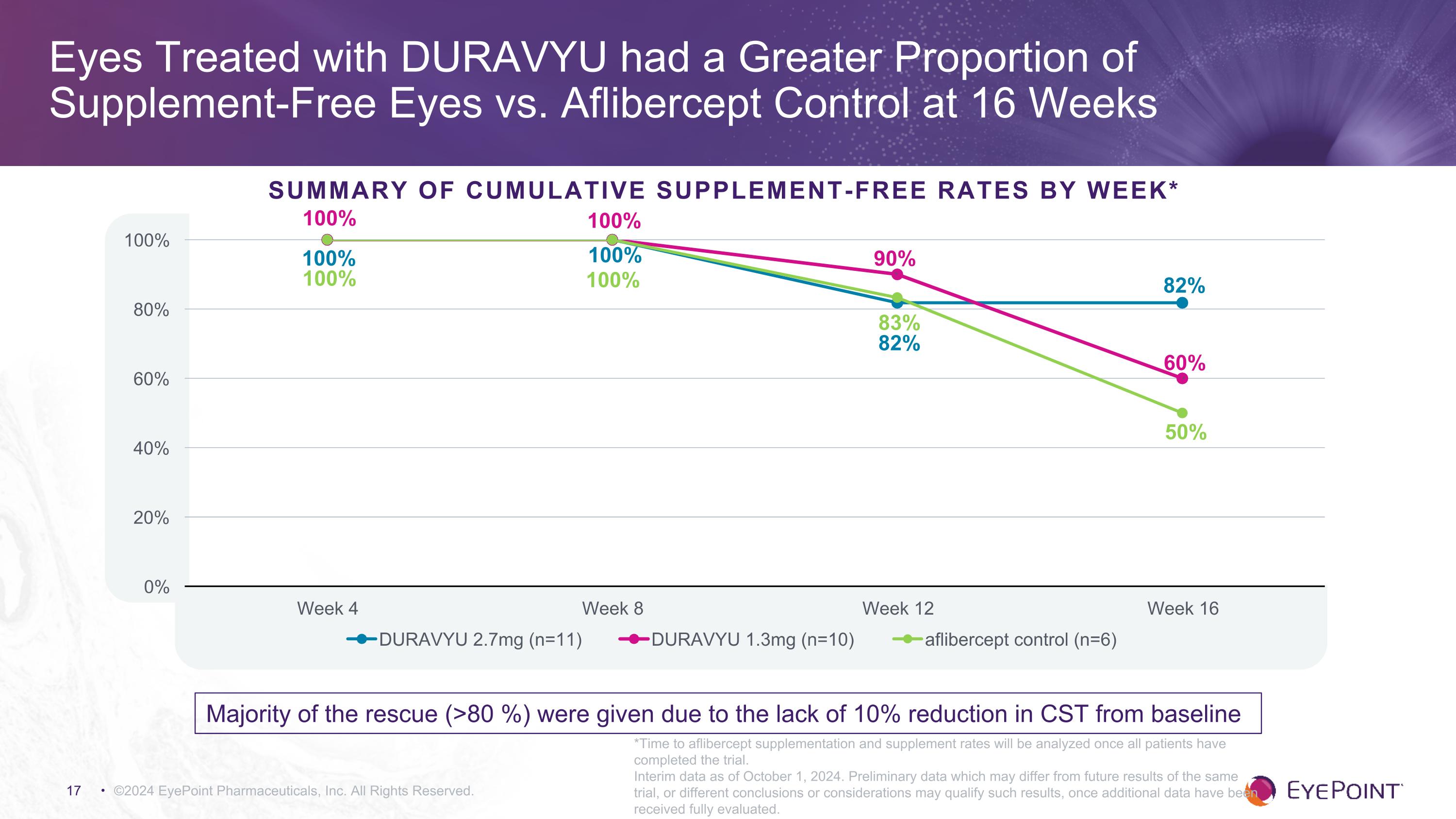
Eyes Treated with DURAVYU had a Greater Proportion of �Supplement-Free Eyes vs. Aflibercept Control at 16 Weeks ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Summary of Cumulative Supplement-Free Rates by Week* 100% 100% 100% *Time to aflibercept supplementation and supplement rates will be analyzed once all patients have completed the trial. Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received fully evaluated. Majority of the rescue (>80 %) were given due to the lack of 10% reduction in CST from baseline

Positive Interim Data for Ongoing Phase 2 VERONA Clinical Trial –��Data support potential for vision improvement in DME as well as superior dosing intervals ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU 2.7mg demonstrated an early and sustained improvement in both BCVA and CST 2.7mg is also being evaluated in the Phase 3 pivotal trials for wet AMD Eyes treated with DURAVYU 2.7mg improved nearly six letters more than aflibercept control Eyes treated with DURAVYU 2.7mg showed improved anatomy of ~38 microns better than aflibercept control DURAVYU drug release profile demonstrates immediate bioavailability DURAVYU had a greater proportion of supplement-free eyes vs. aflibercept control (82% v 50%)1 Continued favorable safety profile for DURAVYU to date (n = >190 patients) 1. Time to aflibercept supplementation and supplement rates will be analyzed once all patients have completed the trial. DME, diabetic macular edema; BCVA, best-corrected visual acuity; CST, central subfield thickness; SAEs, serious adverse events. Interim data as of October 1, 2024. Preliminary data which may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received fully evaluated.

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 2 DAVIO 2 Positive Results in�wet AMD A NON-INFERIORITY TRIAL VERSUS AN AFLIBERCEPT CONTROL
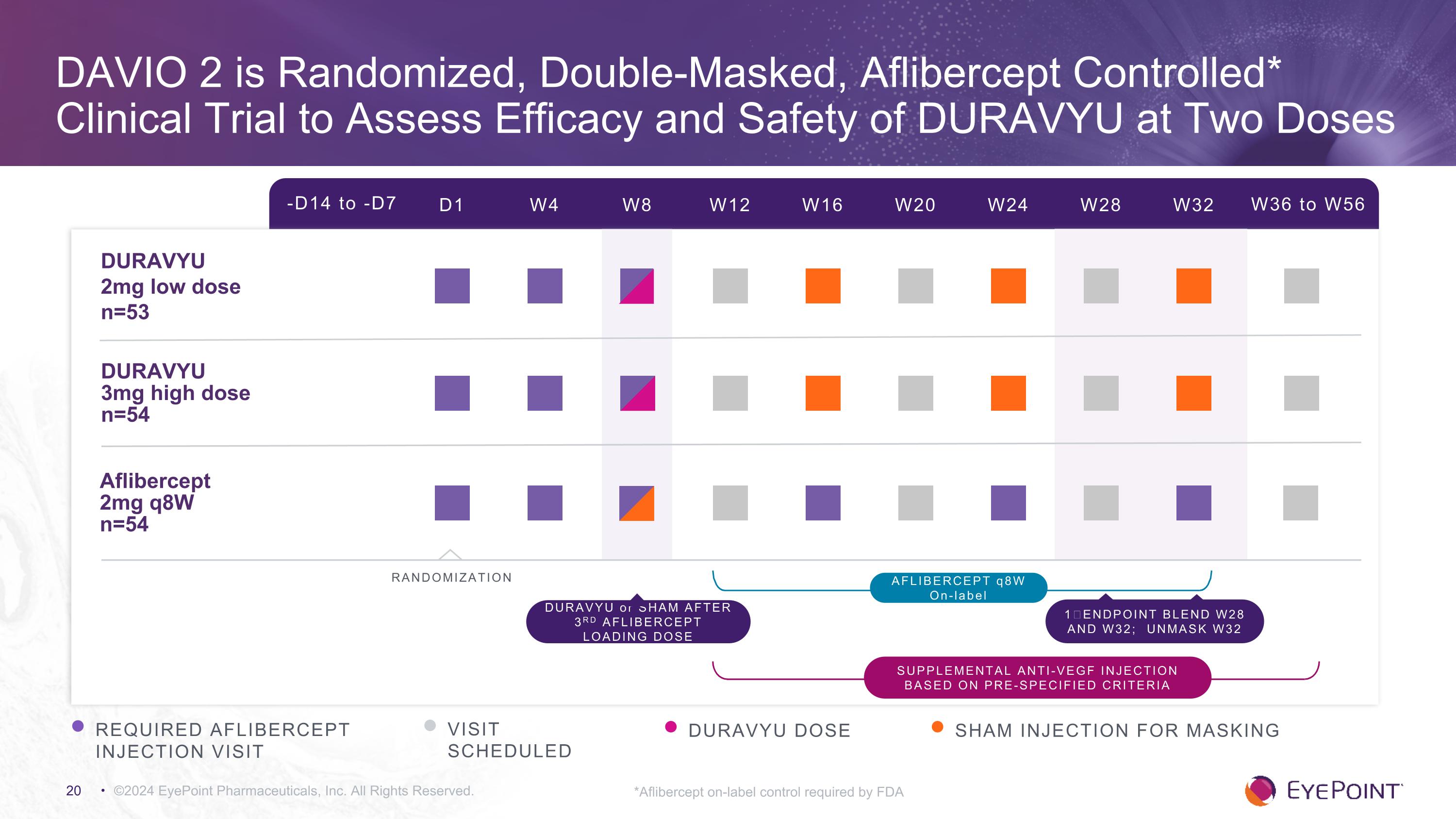
DAVIO 2 is Randomized, Double-Masked, Aflibercept Controlled* Clinical Trial to Assess Efficacy and Safety of DURAVYU at Two Doses ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *Aflibercept on-label control required by FDA -D14 to -D7 D1 W4 W8 W12 W16 W24 W32 W36 to W56 W20 W28 DURAVYU �2mg low dose n=53 DURAVYU �3mg high dose n=54 Aflibercept 2mg q8W n=54 RANDOMIZATION REQUIRED AFLIBERCEPT INJECTION VISIT VISIT SCHEDULED DURAVYU DOSE AFLIBERCEPT q8W On-label DURAVYU or SHAM AFTER 3RD AFLIBERCEPT LOADING DOSE 1⁰ ENDPOINT BLEND W28 AND W32; UNMASK W32 SHAM INJECTION FOR MASKING Supplemental anti-VEGF injection based on pre-specified criteria
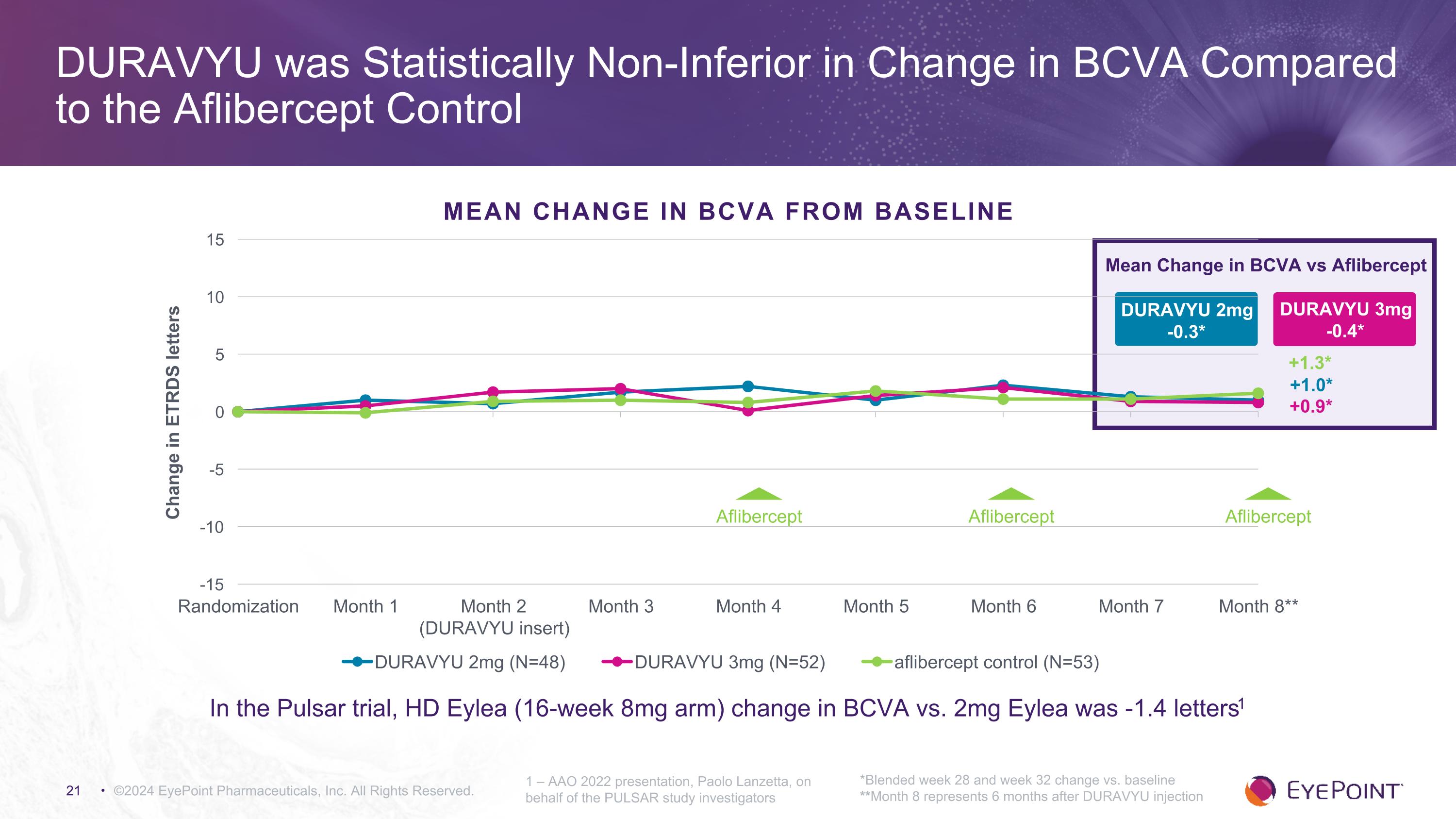
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU was Statistically Non-Inferior in Change in BCVA Compared to the Aflibercept Control *Blended week 28 and week 32 change vs. baseline **Month 8 represents 6 months after DURAVYU injection 1 – AAO 2022 presentation, Paolo Lanzetta, on behalf of the PULSAR study investigators DURAVYU 2mg -0.3* Mean Change in BCVA vs Aflibercept DURAVYU 3mg -0.4* +0.9* +1.0* +1.3* MEAN CHANGE IN BCVA FROM BASELINE In the Pulsar trial, HD Eylea (16-week 8mg arm) change in BCVA vs. 2mg Eylea was -1.4 letters1 Aflibercept Aflibercept Aflibercept
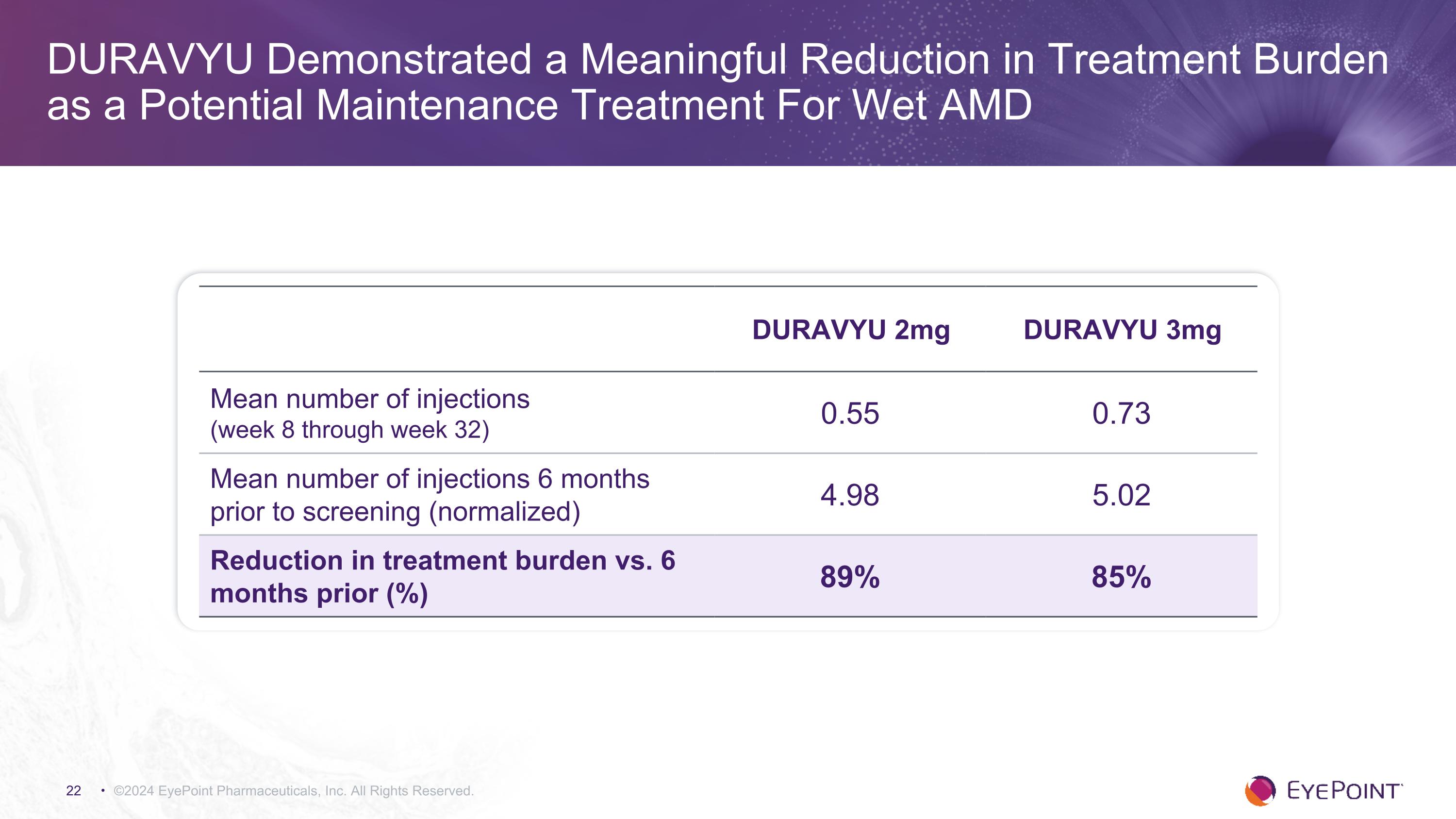
DURAVYU Demonstrated a Meaningful Reduction in Treatment Burden as a Potential Maintenance Treatment For Wet AMD ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU 2mg DURAVYU 3mg Mean number of injections (week 8 through week 32) 0.55 0.73 Mean number of injections 6 months prior to screening (normalized) 4.98 5.02 Reduction in treatment burden vs. 6 months prior (%) 89% 85%
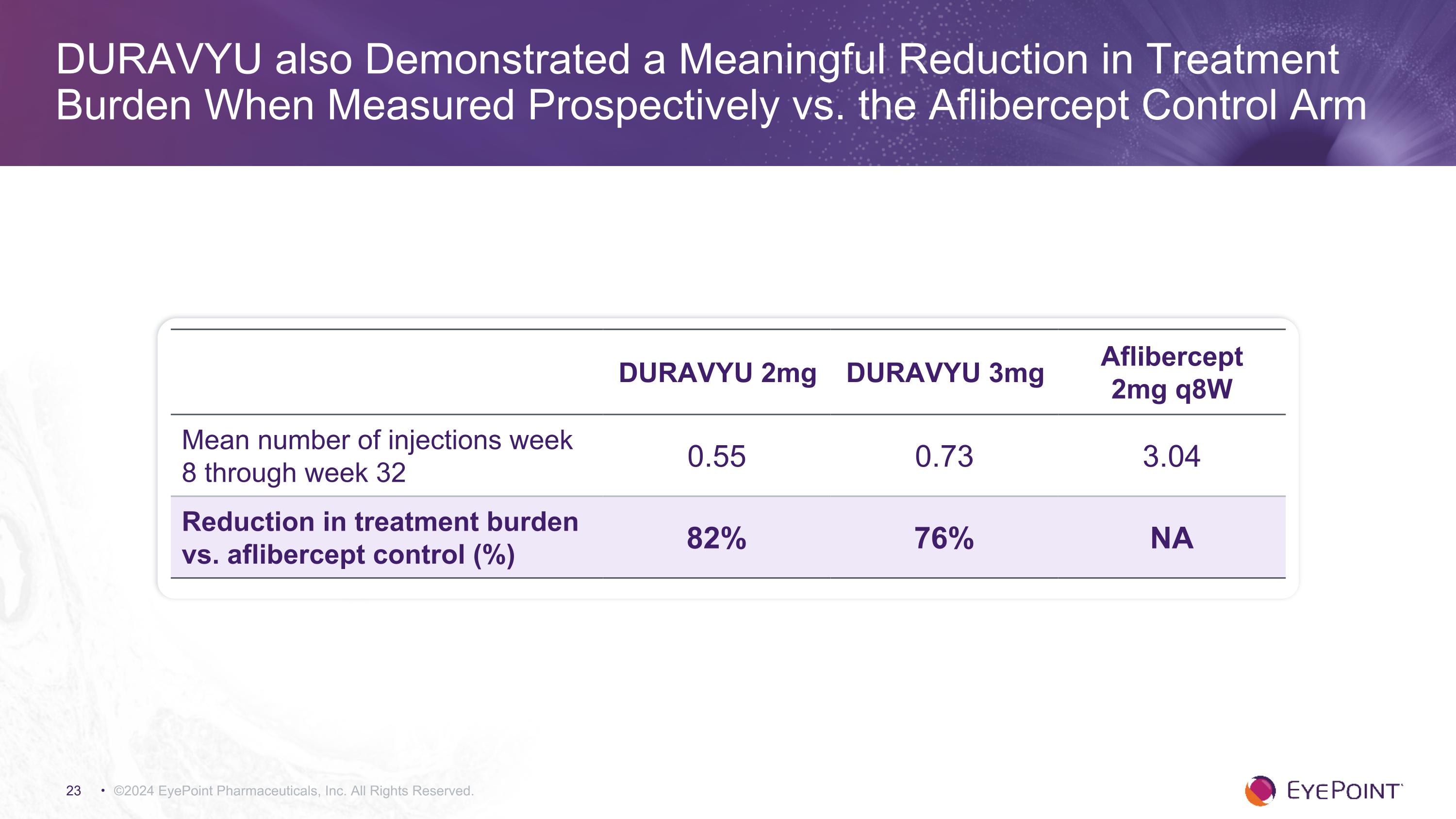
DURAVYU also Demonstrated a Meaningful Reduction in Treatment Burden When Measured Prospectively vs. the Aflibercept Control Arm ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU 2mg DURAVYU 3mg Aflibercept 2mg q8W Mean number of injections week 8 through week 32 0.55 0.73 3.04 Reduction in treatment burden vs. aflibercept control (%) 82% 76% NA
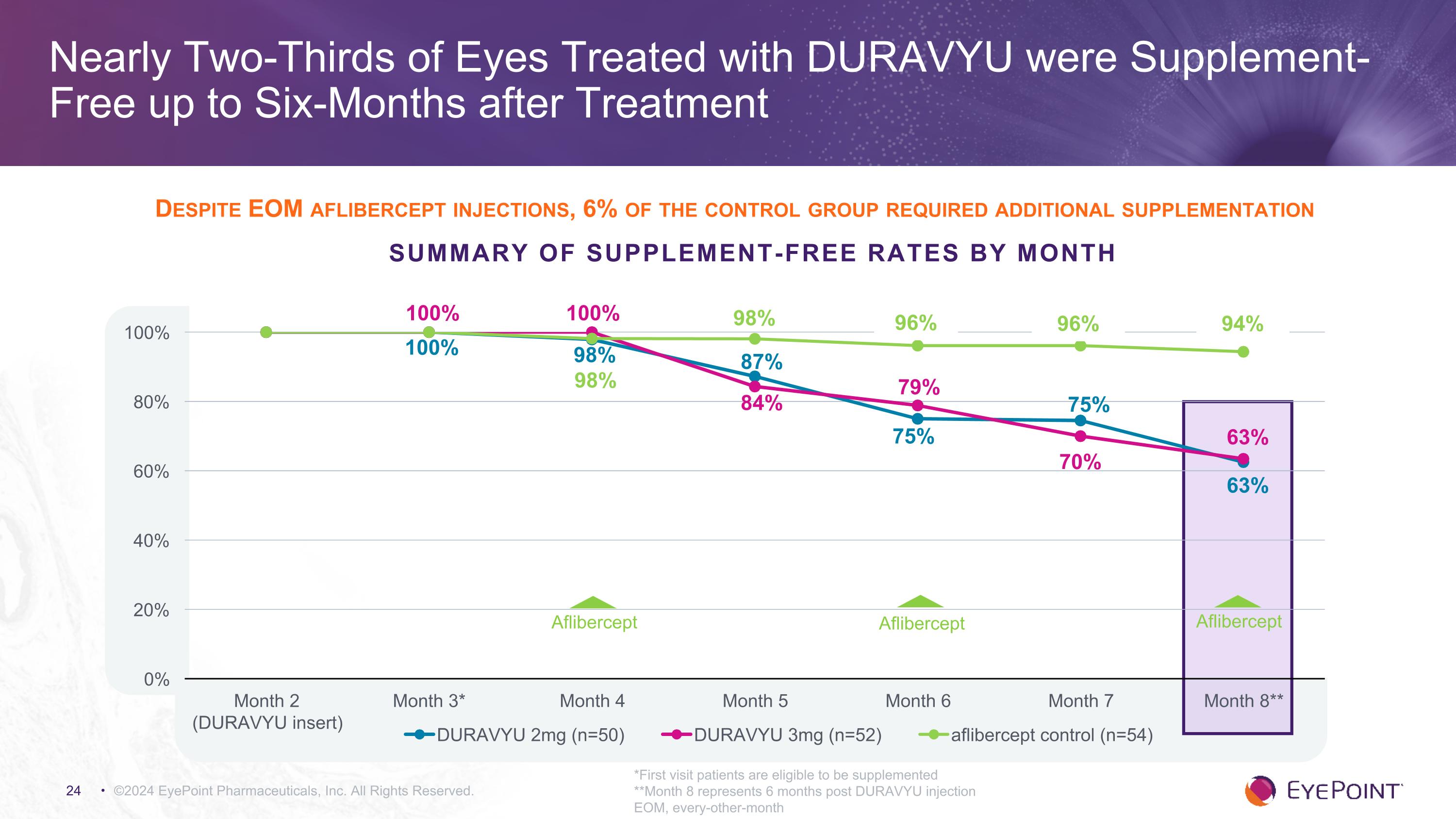
Nearly Two-Thirds of Eyes Treated with DURAVYU were Supplement-Free up to Six-Months after Treatment ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *First visit patients are eligible to be supplemented **Month 8 represents 6 months post DURAVYU injection EOM, every-other-month Summary of Supplement-Free Rates by Month 100% 100% Despite EOM aflibercept injections, 6% of the control group required additional supplementation Aflibercept Aflibercept Aflibercept 96% 96% 94% 98%
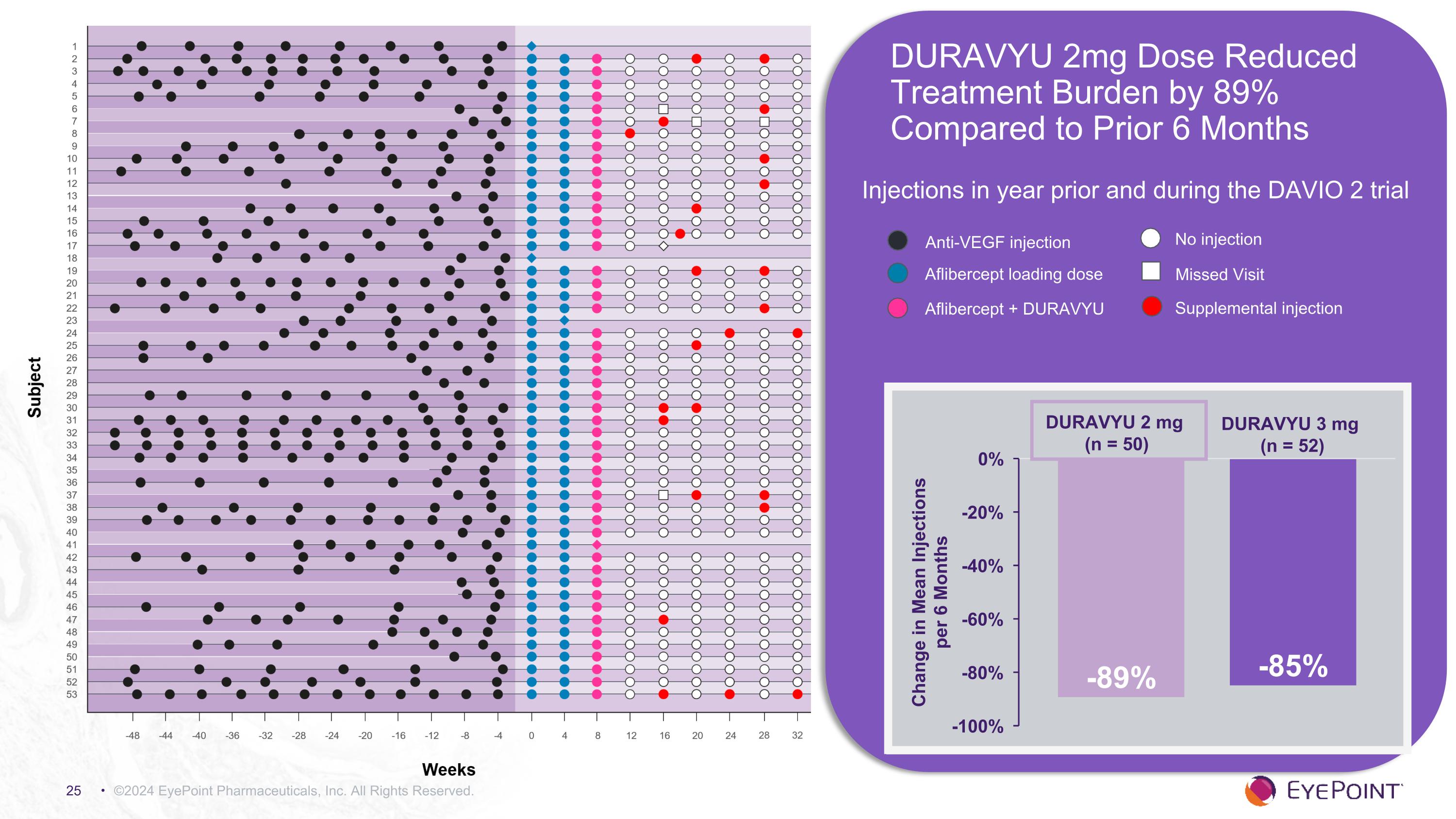
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU 2mg Dose Reduced Treatment Burden by 89% Compared to Prior 6 Months Injections in year prior and during the DAVIO 2 trial DURAVYU 3 mg (n = 52) DURAVYU 2 mg (n = 50) Anti-VEGF injection Aflibercept loading dose Aflibercept + DURAVYU No injection Missed Visit Supplemental injection
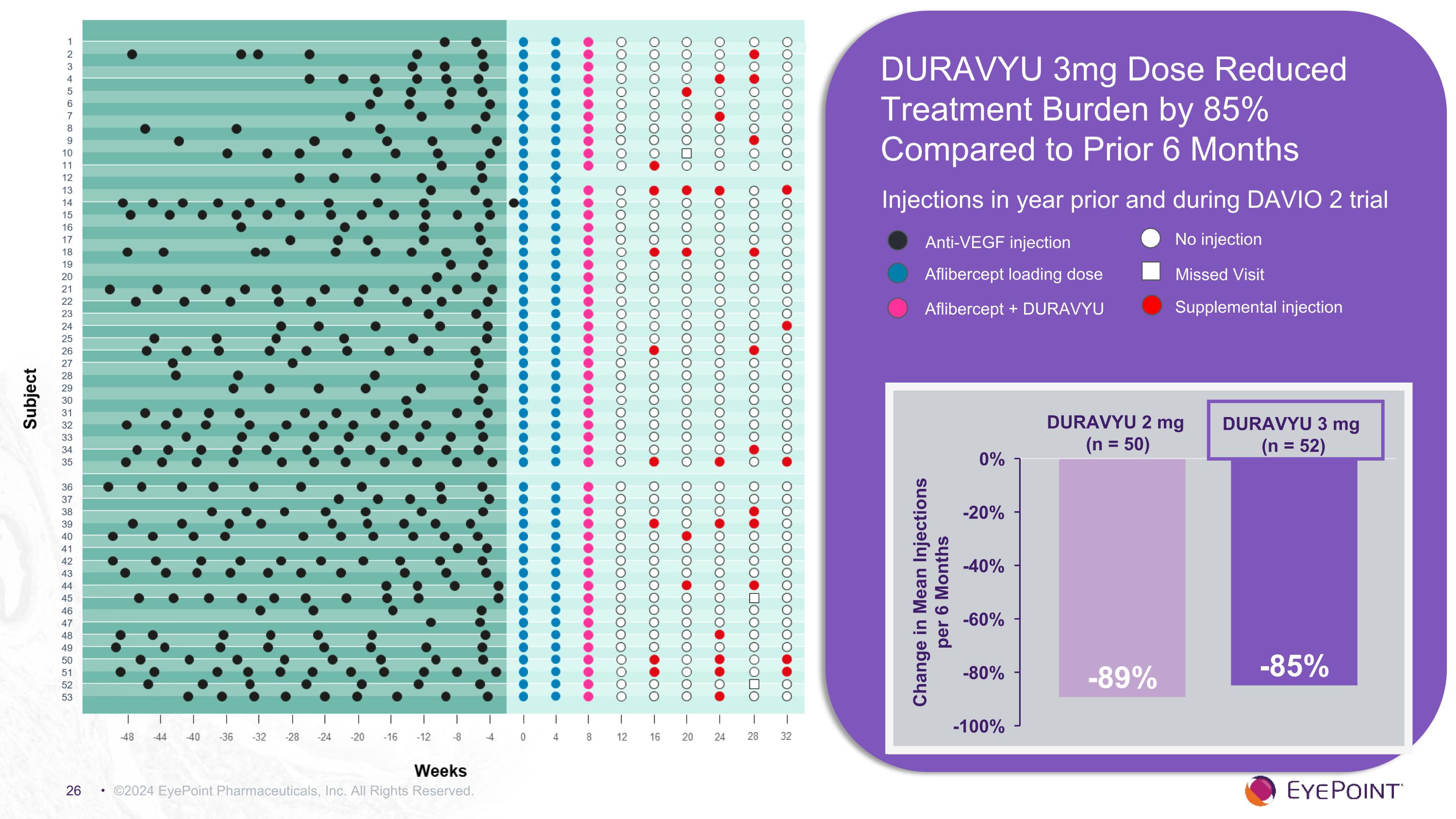
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Injections in year prior and during DAVIO 2 trial DURAVYU 3 mg (n = 52) DURAVYU 2 mg (n = 50) Anti-VEGF injection Aflibercept loading dose Aflibercept + DURAVYU No injection Missed Visit Supplemental injection DURAVYU 3mg Dose Reduced Treatment Burden by 85% Compared to Prior 6 Months
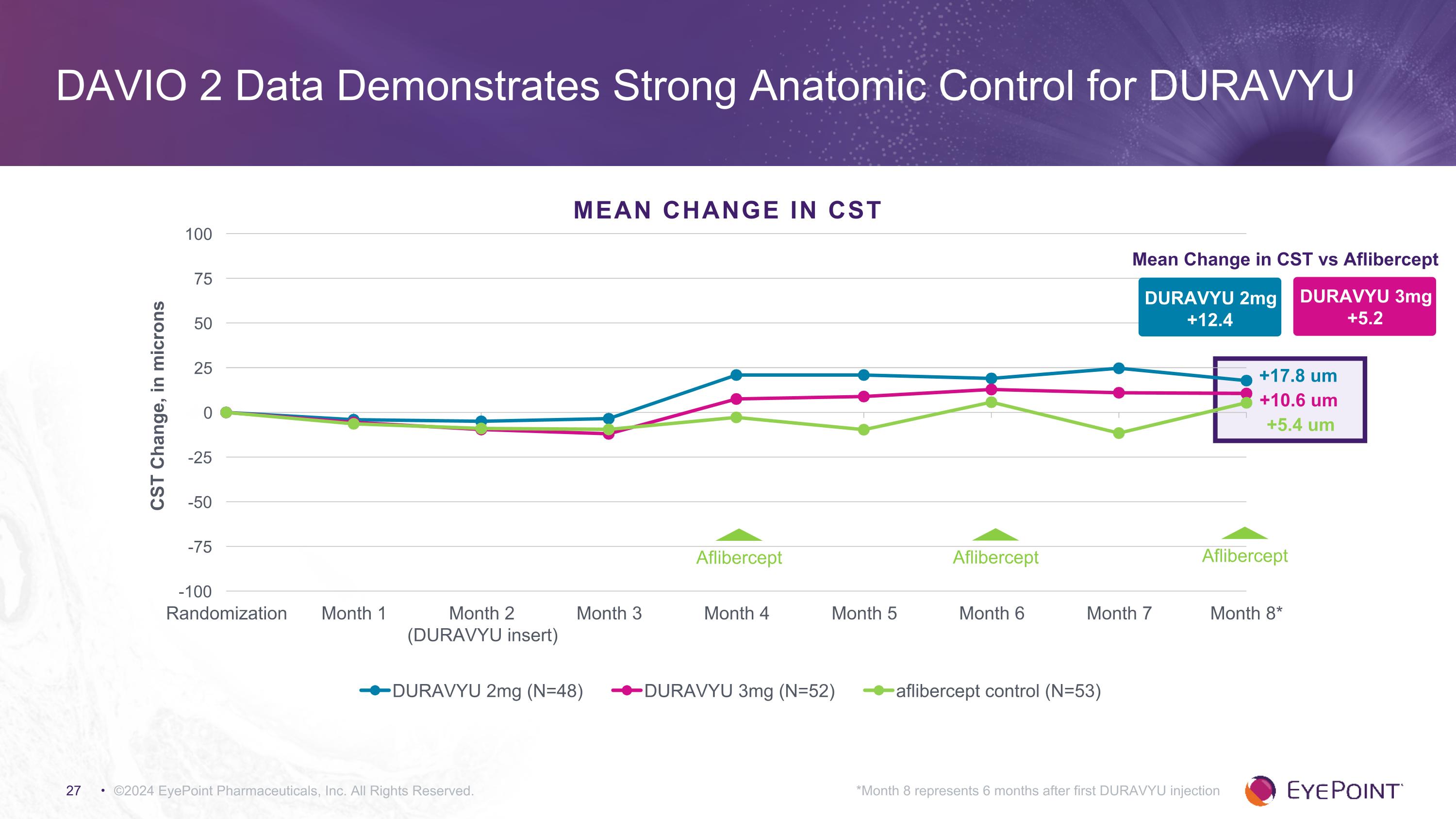
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *Month 8 represents 6 months after first DURAVYU injection DAVIO 2 Data Demonstrates Strong Anatomic Control for DURAVYU DURAVYU 2mg +12.4 Mean Change in CST vs Aflibercept DURAVYU 3mg +5.2 +10.6 um +17.8 um +5.4 um MEAN CHANGE in CST Aflibercept Aflibercept Aflibercept
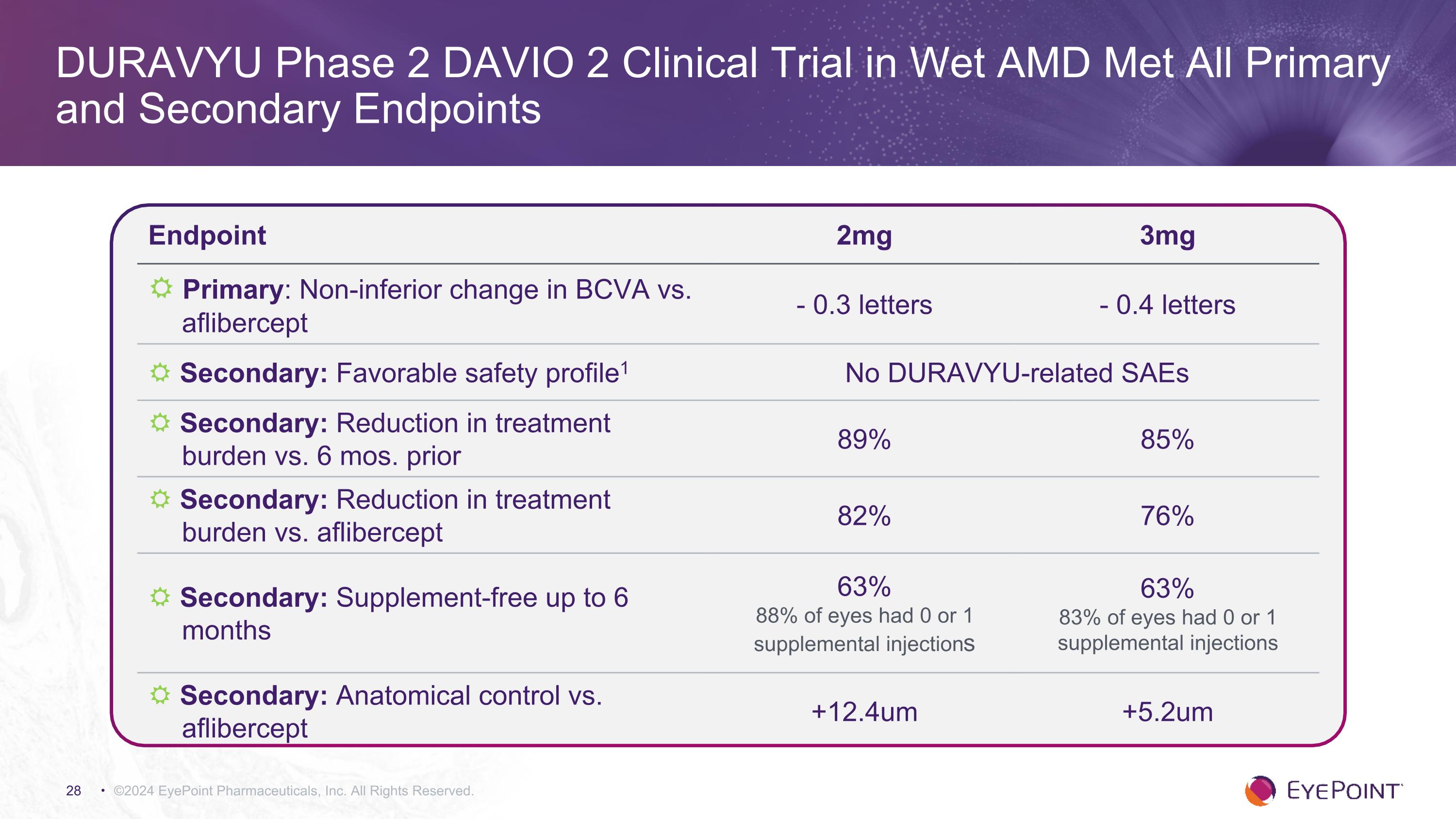
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU Phase 2 DAVIO 2 Clinical Trial in Wet AMD Met All Primary and Secondary Endpoints Endpoint 2mg 3mg R Primary: Non-inferior change in BCVA vs. aflibercept - 0.3 letters - 0.4 letters R Secondary: Favorable safety profile1 No DURAVYU-related SAEs R Secondary: Reduction in treatment burden vs. 6 mos. prior 89% 85% R Secondary: Reduction in treatment burden vs. aflibercept 82% 76% R Secondary: Supplement-free up to 6 months 63% 88% of eyes had 0 or 1 supplemental injections 63% 83% of eyes had 0 or 1 supplemental injections R Secondary: Anatomical control vs. aflibercept +12.4um +5.2um

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 2 DAVIO 2 Clinical Trial in Wet AMD��Sub-Group Analysis of Patients Anti-VEGF Supplement-Free Up to 6 Months
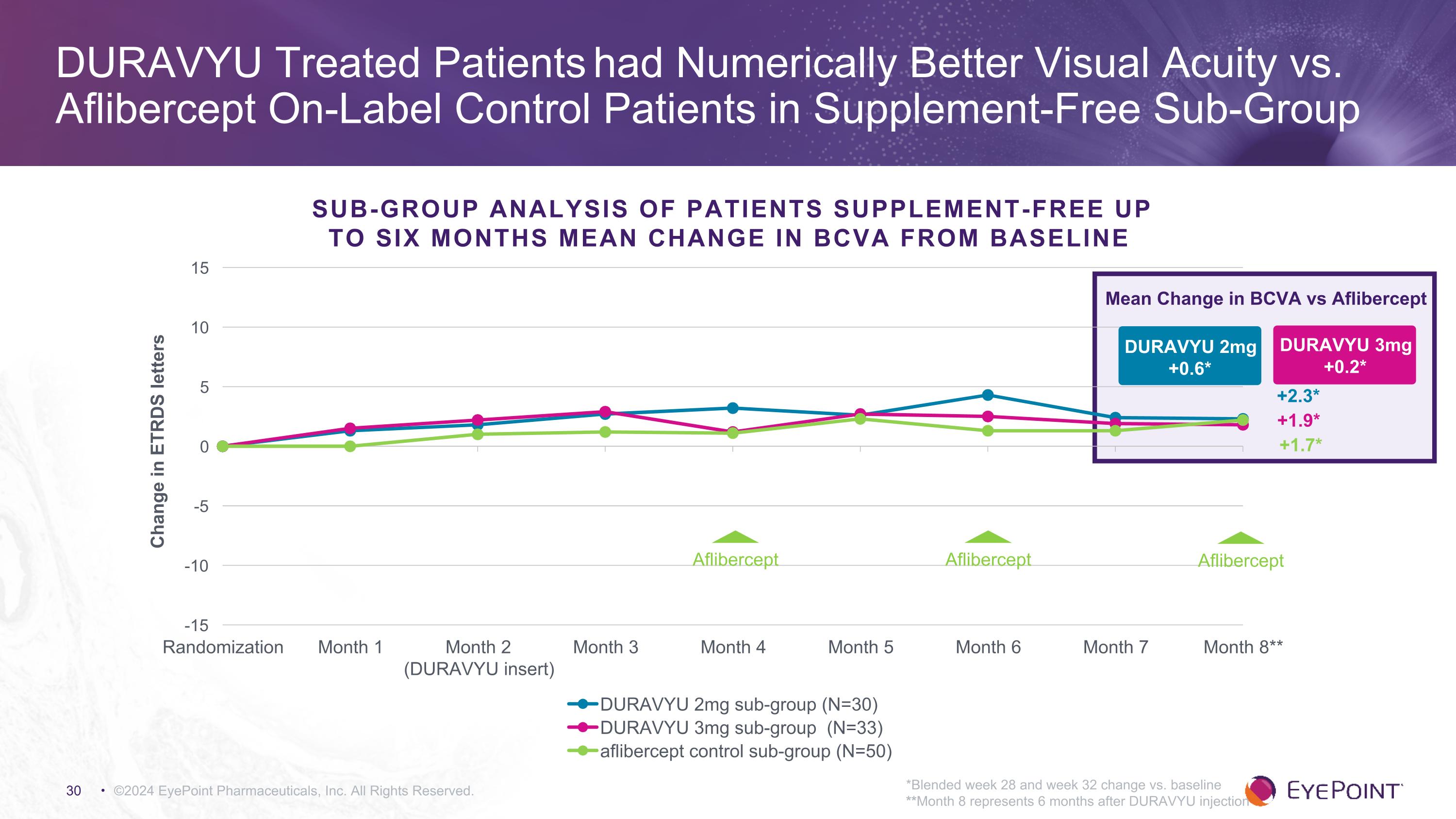
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *Blended week 28 and week 32 change vs. baseline **Month 8 represents 6 months after DURAVYU injection DURAVYU Treated Patients had Numerically Better Visual Acuity vs. Aflibercept On-Label Control Patients in Supplement-Free Sub-Group DURAVYU 2mg +0.6* Mean Change in BCVA vs Aflibercept DURAVYU 3mg +0.2* +1.9* +2.3* +1.7* Sub-Group Analysis of Patients Supplement-Free Up to Six Months MEAN CHANGE IN BCVA FROM BASELINE Aflibercept Aflibercept Aflibercept
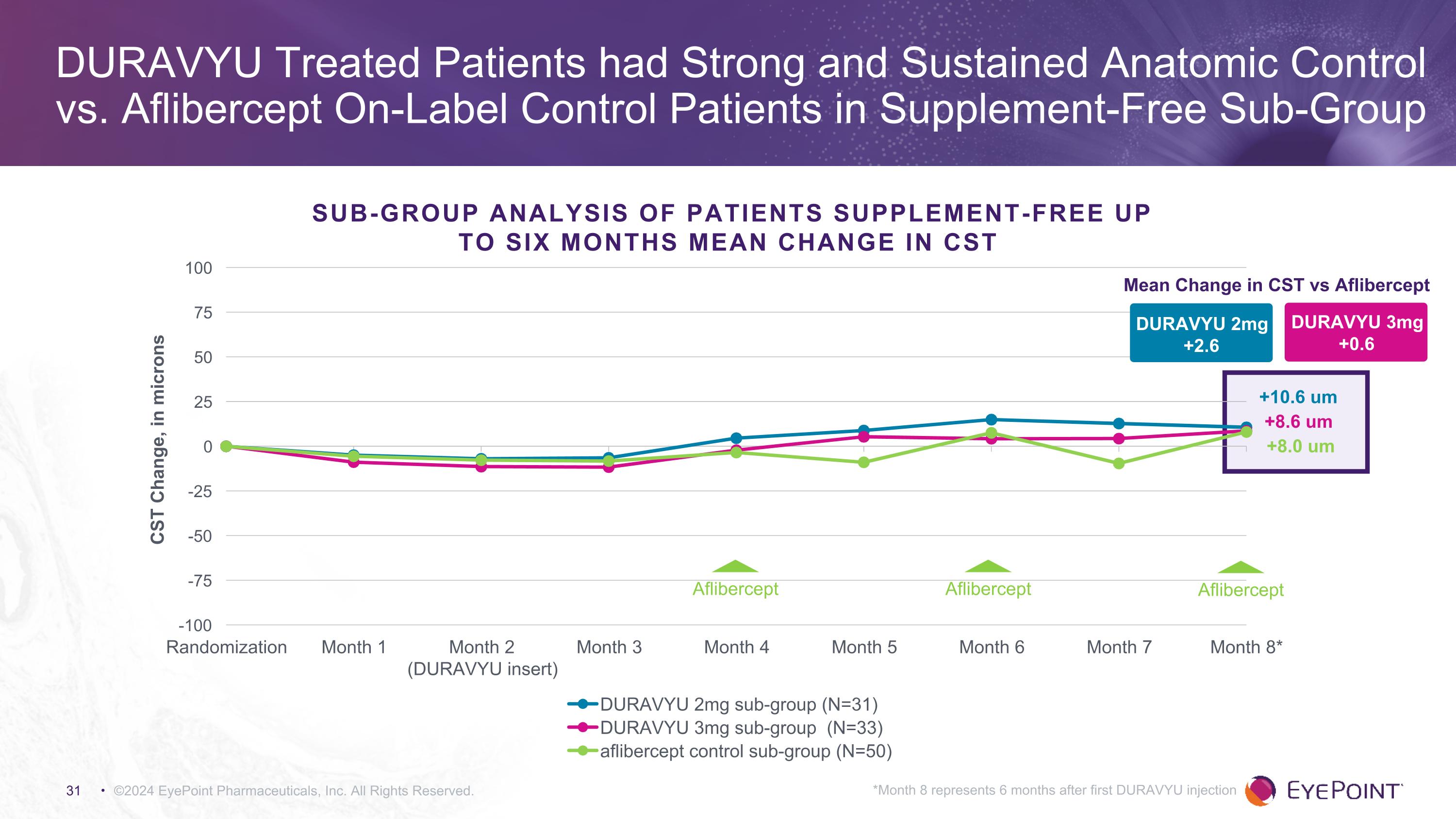
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *Month 8 represents 6 months after first DURAVYU injection DURAVYU Treated Patients had Strong and Sustained Anatomic Control vs. Aflibercept On-Label Control Patients in Supplement-Free Sub-Group DURAVYU 2mg +2.6 Mean Change in CST vs Aflibercept DURAVYU 3mg +0.6 +8.6 um +10.6 um +8.0 um Sub-Group Analysis of Patients Supplement-Free Up to Six Months MEAN CHANGE in CST Aflibercept Aflibercept Aflibercept

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 2 DAVIO 2 Clinical Trial 12-Month Results in wet AMD A NON-INFERIORITY TRIAL VERSUS AN AFLIBERCEPT CONTROL
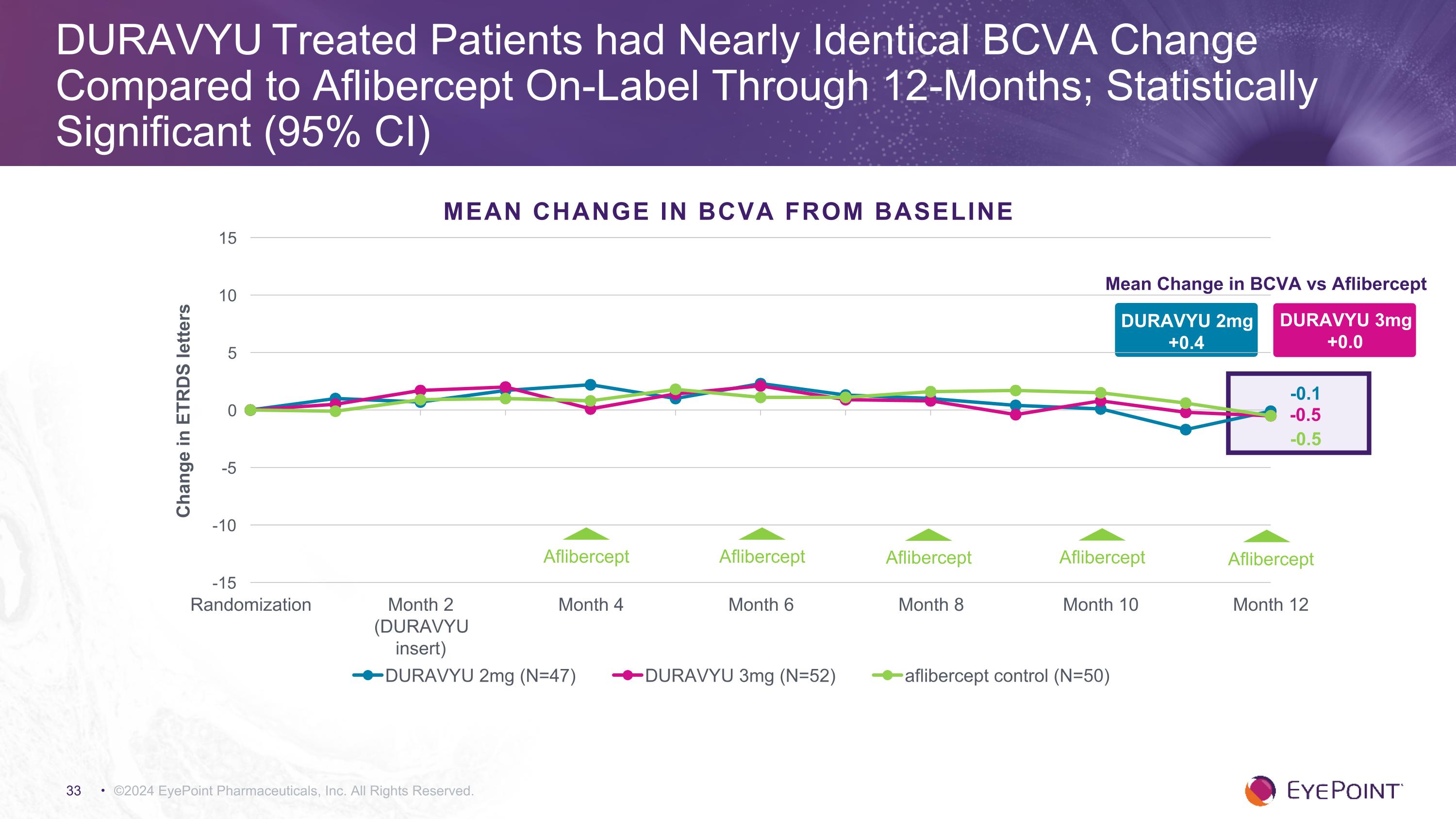
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU Treated Patients had Nearly Identical BCVA Change Compared to Aflibercept On-Label Through 12-Months; Statistically Significant (95% CI) DURAVYU 2mg +0.4 Mean Change in BCVA vs Aflibercept DURAVYU 3mg +0.0 -0.5 -0.1 -0.5 MEAN CHANGE IN BCVA FROM BASELINE Aflibercept Aflibercept Aflibercept Aflibercept Aflibercept
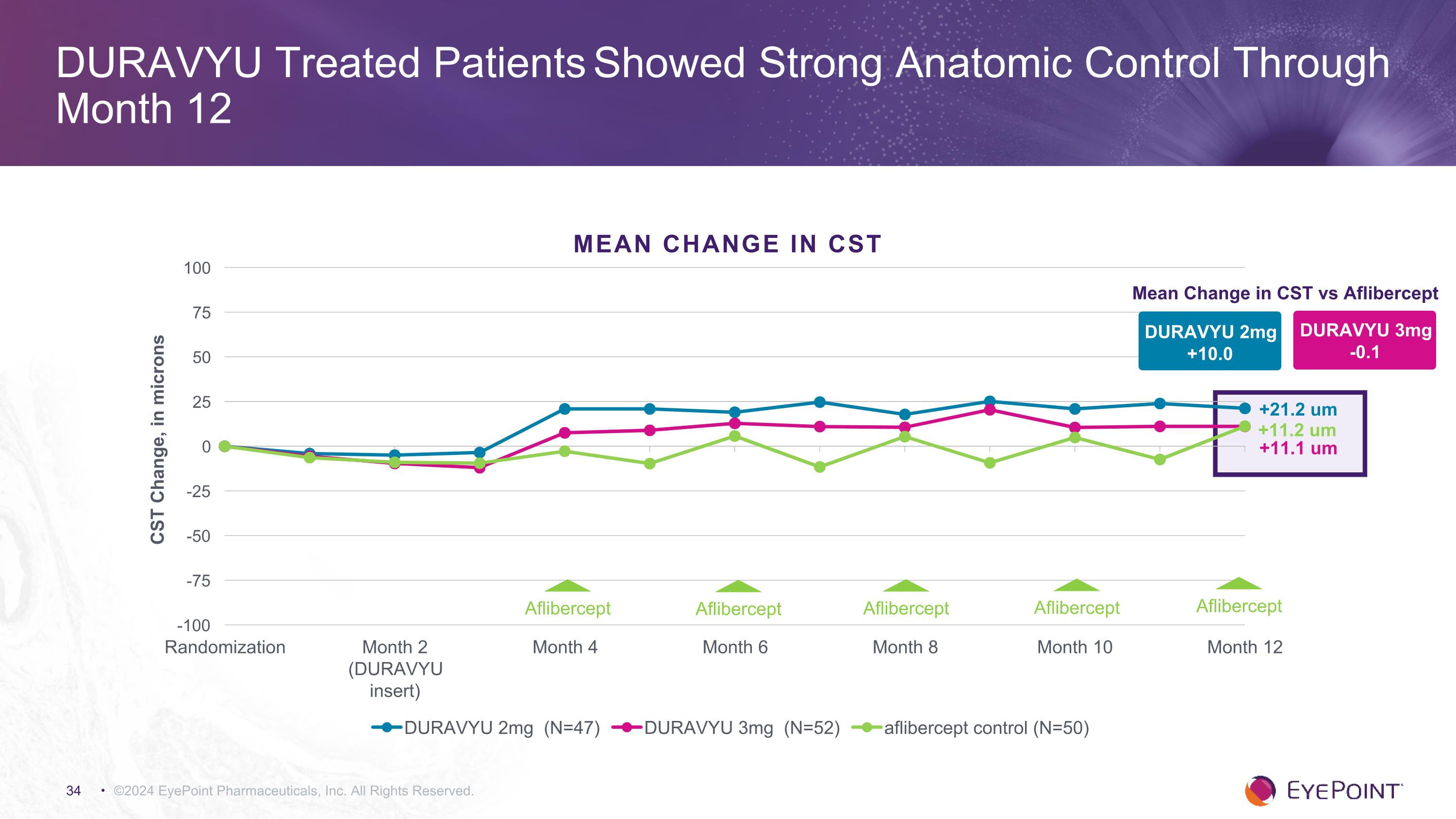
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU Treated Patients Showed Strong Anatomic Control Through Month 12 DURAVYU 2mg +10.0 Mean Change in CST vs Aflibercept DURAVYU 3mg -0.1 +11.1 um +21.2 um +11.2 um MEAN CHANGE in CST Aflibercept Aflibercept Aflibercept Aflibercept Aflibercept
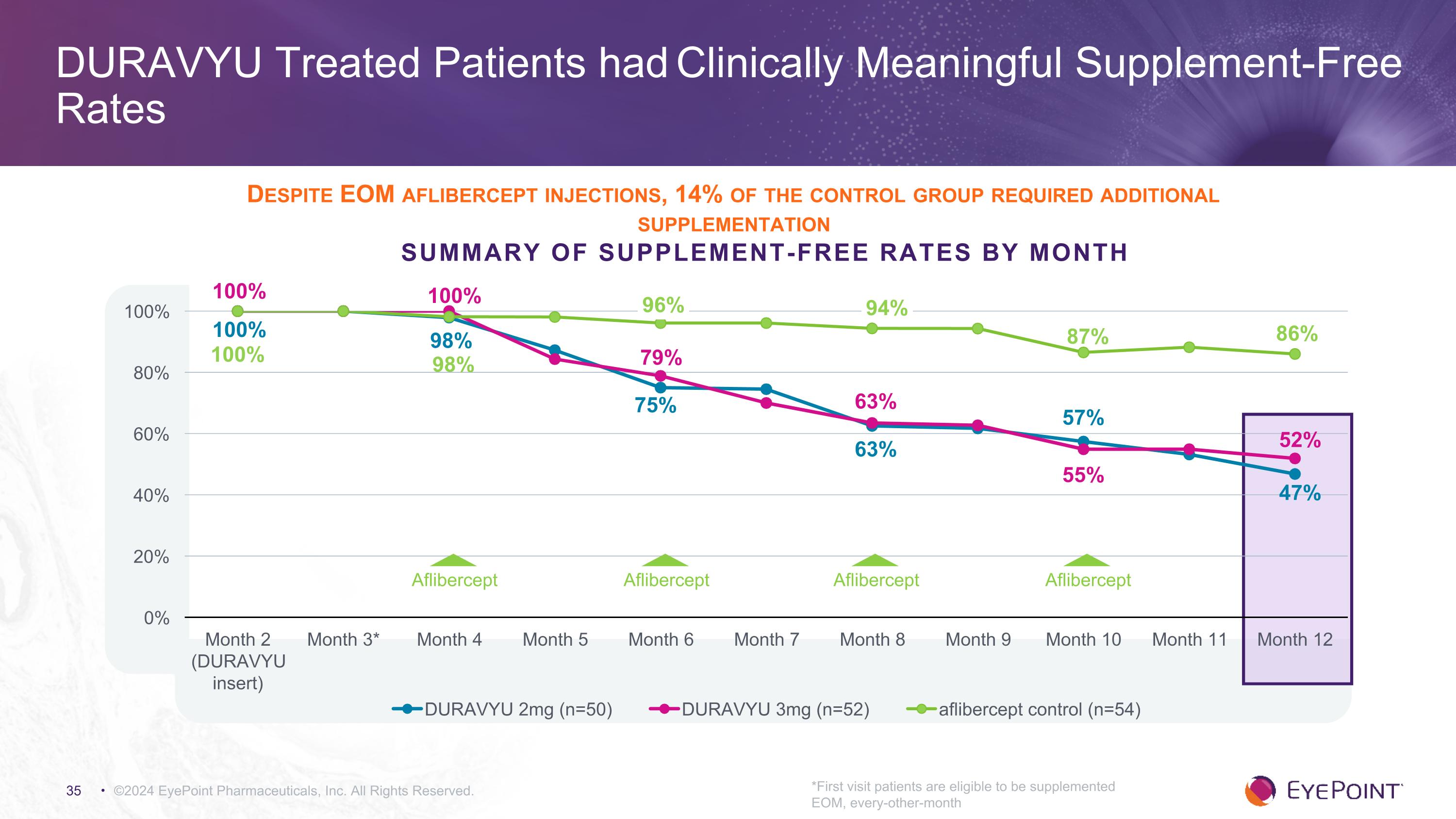
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *First visit patients are eligible to be supplemented EOM, every-other-month DURAVYU Treated Patients had Clinically Meaningful Supplement-Free Rates Summary of Supplement-Free Rates by Month 100% 100% 100% Aflibercept Aflibercept Aflibercept Aflibercept Despite EOM aflibercept injections, 14% of the control group required additional supplementation
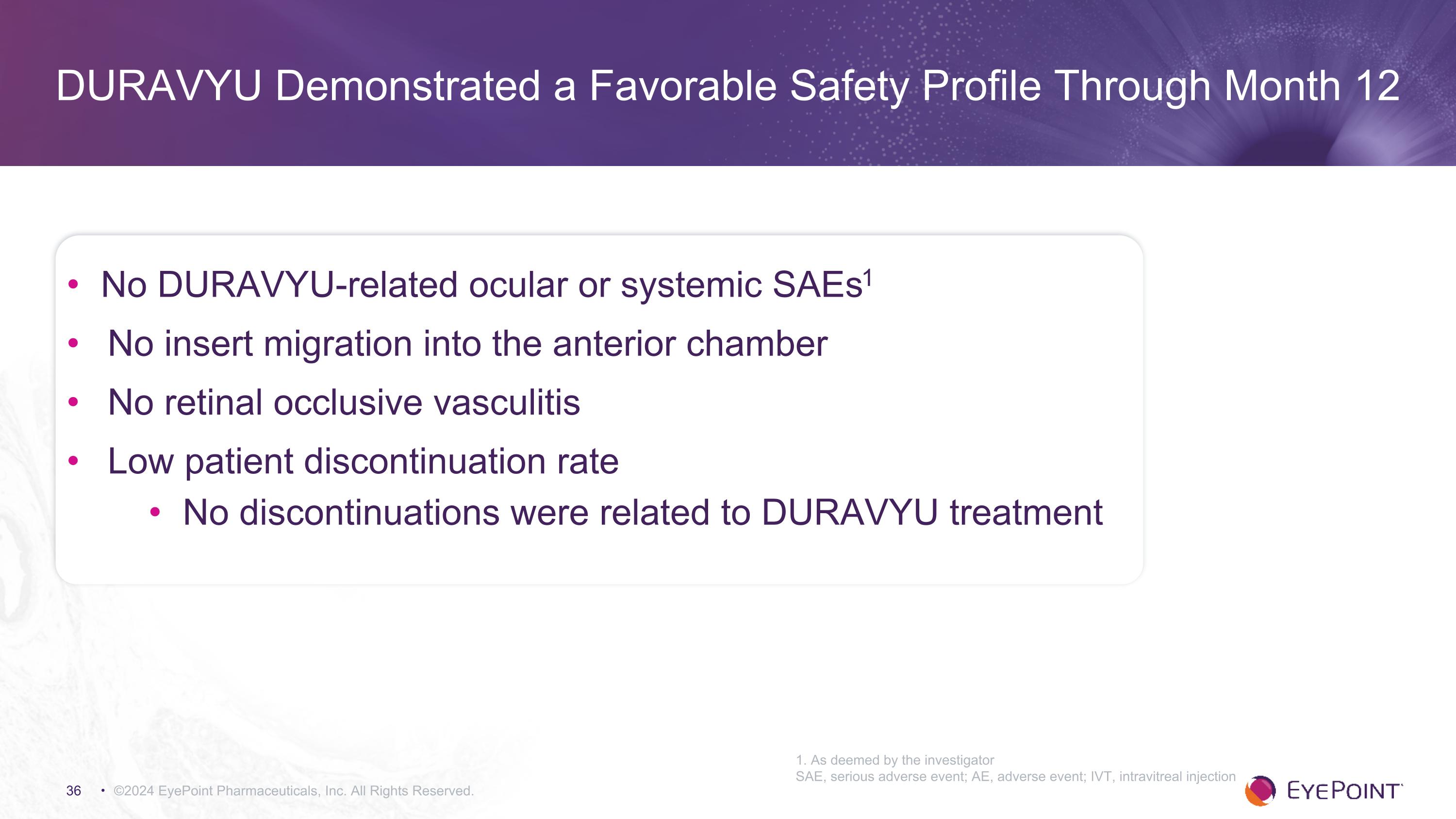
©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 1. As deemed by the investigator SAE, serious adverse event; AE, adverse event; IVT, intravitreal injection DURAVYU Demonstrated a Favorable Safety Profile Through Month 12 No DURAVYU-related ocular or systemic SAEs1 No insert migration into the anterior chamber No retinal occlusive vasculitis Low patient discontinuation rate No discontinuations were related to DURAVYU treatment

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 3 Pivotal Trials Design NON-INFERIORITY VERSUS AN AFLIBERCEPT CONTROL
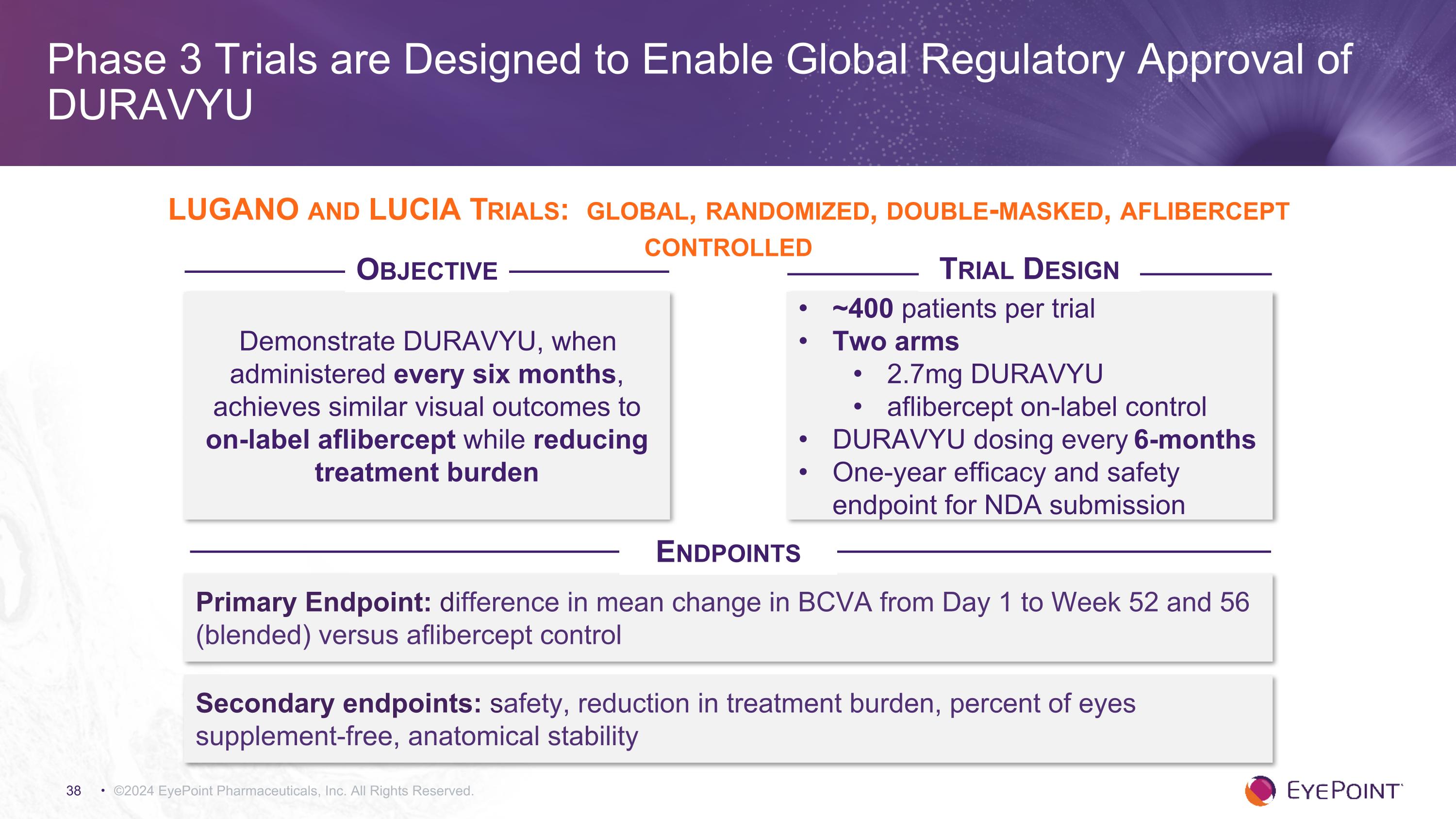
Phase 3 Trials are Designed to Enable Global Regulatory Approval of DURAVYU ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Demonstrate DURAVYU, when administered every six months, achieves similar visual outcomes to on-label aflibercept while reducing treatment burden ~400 patients per trial Two arms 2.7mg DURAVYU aflibercept on-label control DURAVYU dosing every 6-months One-year efficacy and safety endpoint for NDA submission Primary Endpoint: difference in mean change in BCVA from Day 1 to Week 52 and 56 (blended) versus aflibercept control Secondary endpoints: safety, reduction in treatment burden, percent of eyes supplement-free, anatomical stability LUGANO and LUCIA Trials: global, randomized, double-masked, aflibercept controlled Objective Trial Design Endpoints
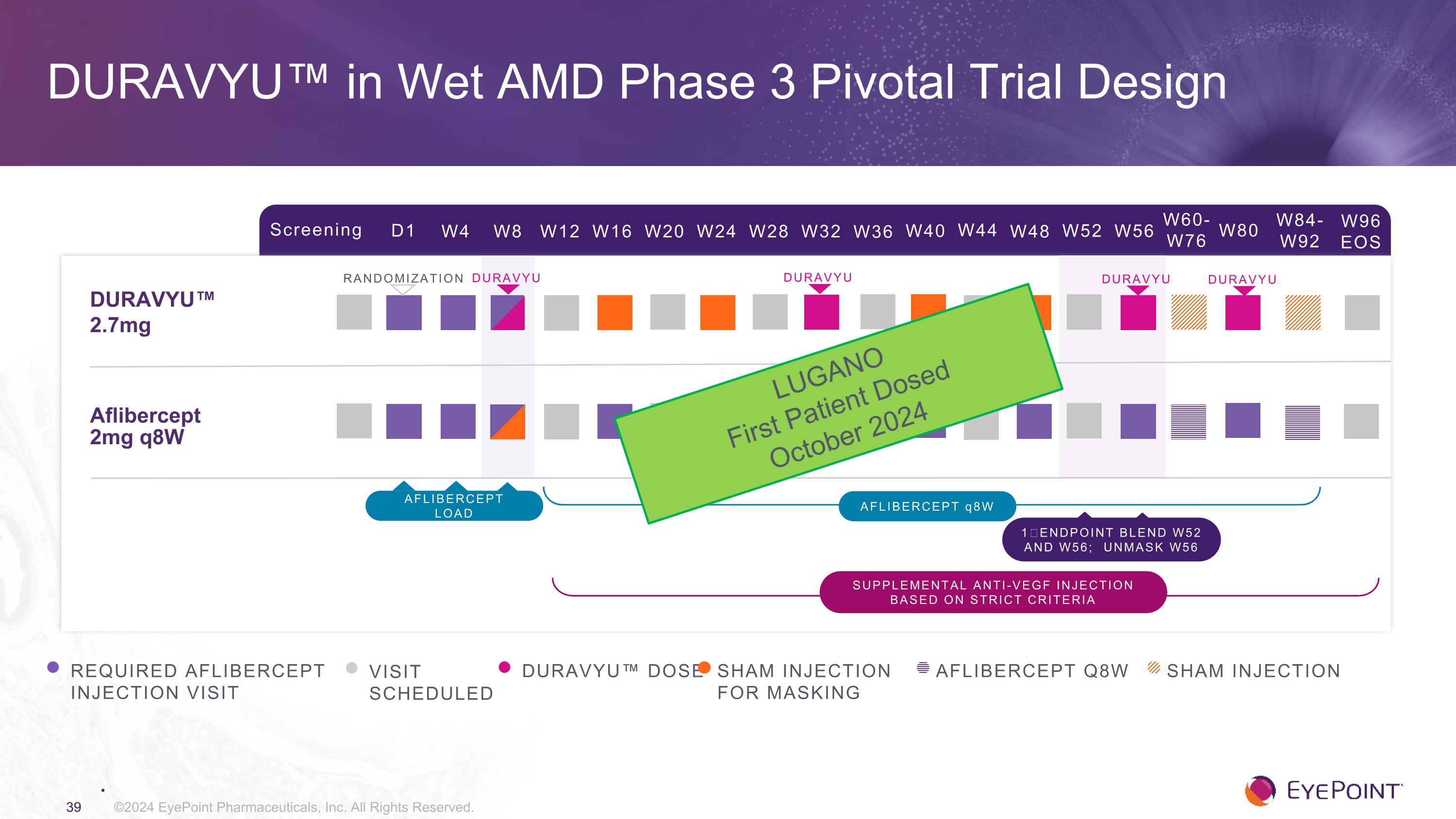
DURAVYU™ in Wet AMD Phase 3 Pivotal Trial Design ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Screening D1 W4 W8 W12 W16 W24 W32 W84-W92 W20 W28 DURAVYU™ �2.7mg Aflibercept 2mg q8W RANDOMIZATION REQUIRED AFLIBERCEPT INJECTION VISIT VISIT SCHEDULED DURAVYU™ DOSE AFLIBERCEPT q8W 1⁰ ENDPOINT BLEND W52 AND W56; UNMASK W56 SHAM INJECTION FOR MASKING W36 W40 W44 W48 W52 W56 W60-W76 W80 W96 EOS AFLIBERCEPT Q8W Sham injection Supplemental anti-VEGF injection based on strict criteria AFLIBERCEPT load DURAVYU DURAVYU DURAVYU DURAVYU LUGANO First Patient Dosed October 2024

Commercial Manufacturing Facility ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. FDA, Federal Drug Administration; EMA, European Medicines Agency Conveniently located in Northbridge, MA, near EyePoint headquarters Built to US FDA and EU EMA standards 40,000sf cGMP manufacturing facility Built to EYPT specifications with no capital investment required preserving cash New manufacturing site for clinical and commercial products

©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. EYP-2301: razuprotafib in Durasert E™ A sustained delivery tie-2 agonist for severe retinal diseases
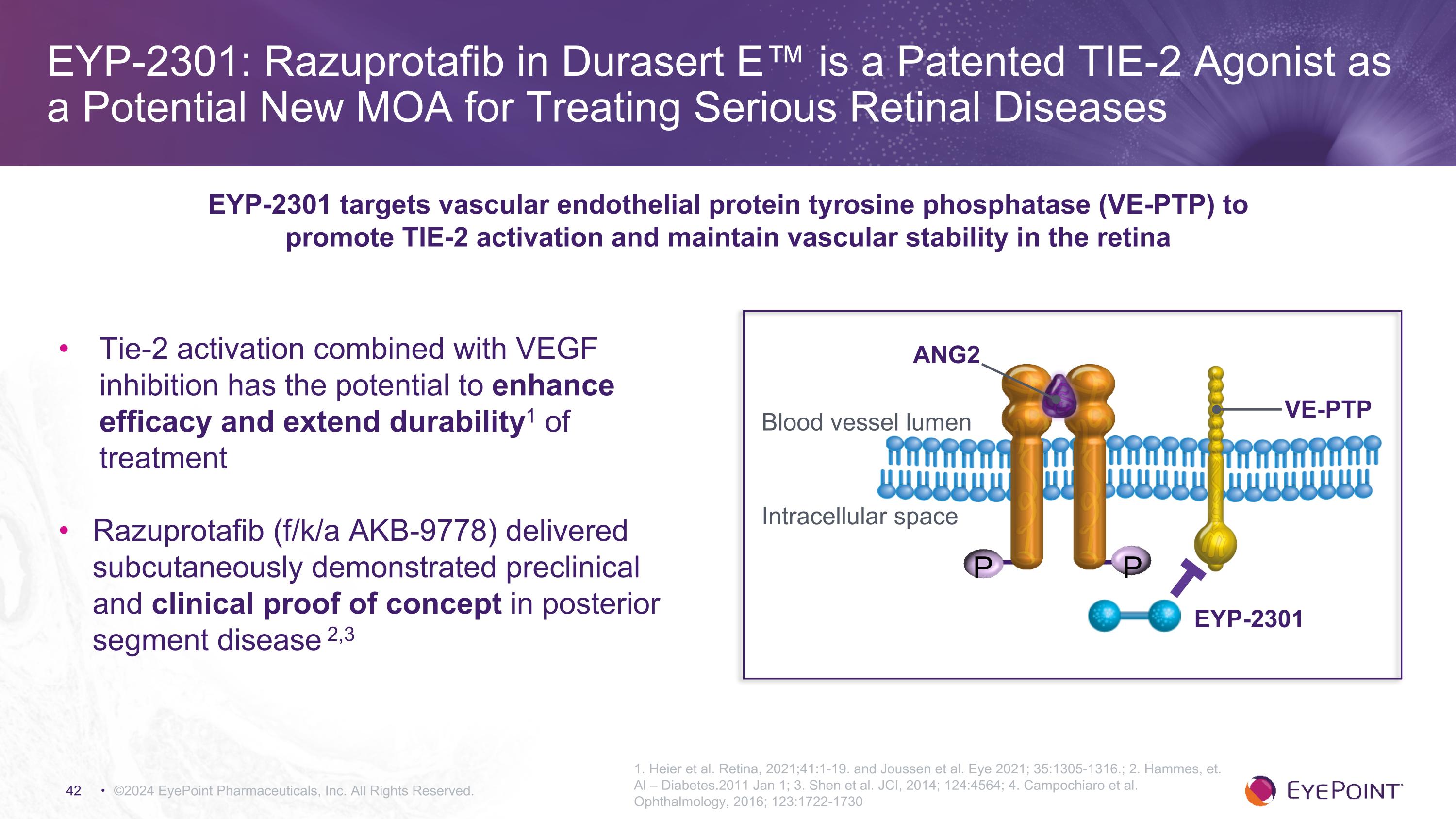
EYP-2301: Razuprotafib in Durasert E™ is a Patented TIE-2 Agonist as a Potential New MOA for Treating Serious Retinal Diseases ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 1. Heier et al. Retina, 2021;41:1-19. and Joussen et al. Eye 2021; 35:1305-1316.; 2. Hammes, et. Al – Diabetes.2011 Jan 1; 3. Shen et al. JCI, 2014; 124:4564; 4. Campochiaro et al. Ophthalmology, 2016; 123:1722-1730 EYP-2301 targets vascular endothelial protein tyrosine phosphatase (VE-PTP) to promote TIE-2 activation and maintain vascular stability in the retina P P EYP-2301 Blood vessel lumen Intracellular space VE-PTP ANG2 Tie-2 activation combined with VEGF inhibition has the potential to enhance efficacy and extend durability1 of treatment Razuprotafib (f/k/a AKB-9778) delivered subcutaneously demonstrated preclinical and clinical proof of concept in posterior segment disease 2,3
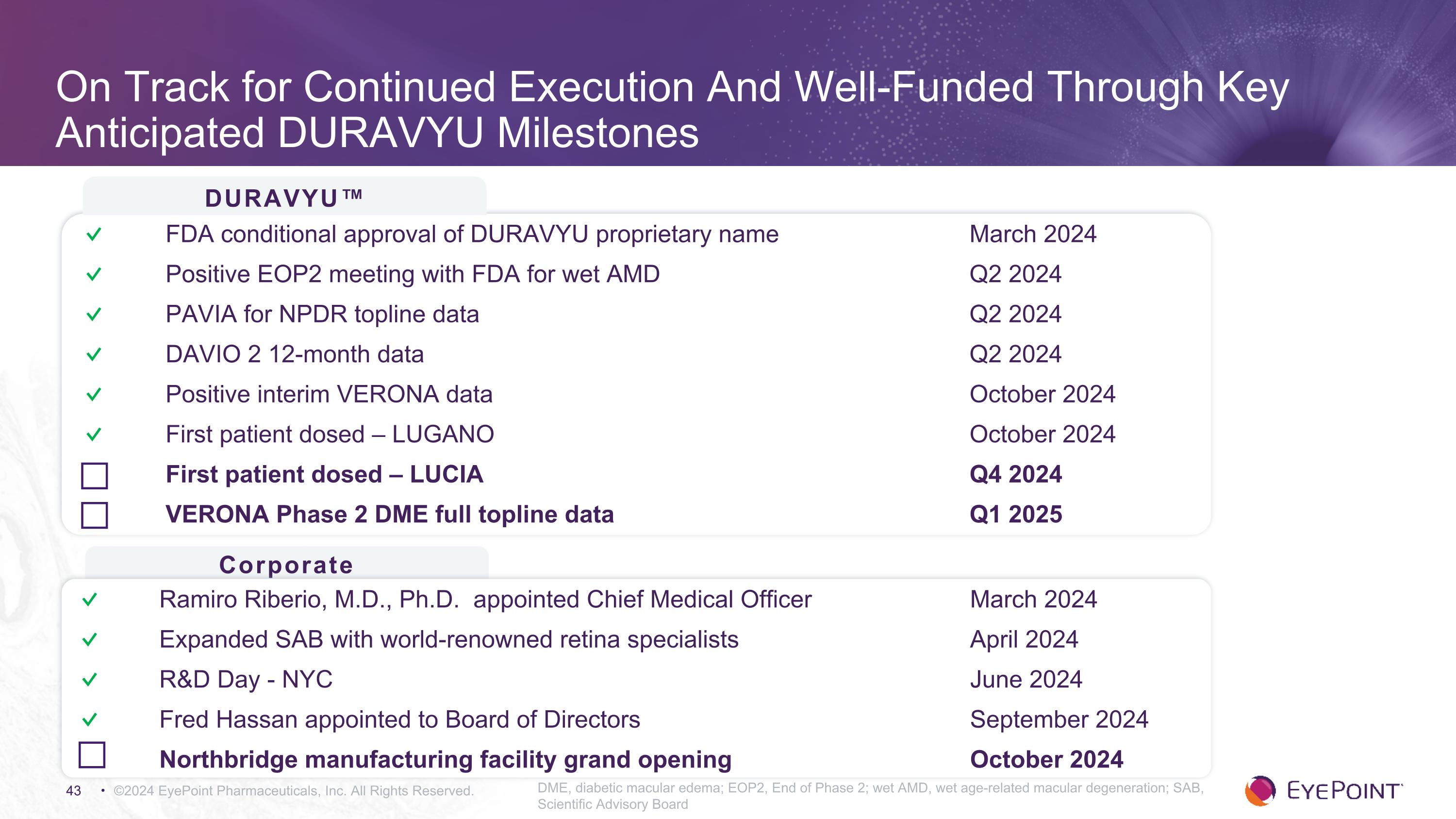
On Track for Continued Execution And Well-Funded Through Key Anticipated DURAVYU Milestones ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DME, diabetic macular edema; EOP2, End of Phase 2; wet AMD, wet age-related macular degeneration; SAB, Scientific Advisory Board DURAVYU™ Corporate ✓ FDA conditional approval of DURAVYU proprietary name March 2024 ✓ Positive EOP2 meeting with FDA for wet AMD Q2 2024 ✓ PAVIA for NPDR topline data Q2 2024 ✓ DAVIO 2 12-month data Q2 2024 ✓ Positive interim VERONA data October 2024 ✓ First patient dosed – LUGANO October 2024 First patient dosed – LUCIA Q4 2024 VERONA Phase 2 DME full topline data Q1 2025 ✓ Ramiro Riberio, M.D., Ph.D. appointed Chief Medical Officer March 2024 ✓ Expanded SAB with world-renowned retina specialists April 2024 ✓ R&D Day - NYC June 2024 ✓ Fred Hassan appointed to Board of Directors September 2024 Northbridge manufacturing facility grand opening October 2024

Investor Presentation October 2024 ©2024 EyePoint Pharmaceuticals, Inc. All Rights Reserved.
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
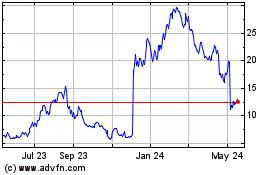
EyePoint Pharmaceuticals (NASDAQ:EYPT)
Historical Stock Chart
From Oct 2024 to Nov 2024
EyePoint Pharmaceuticals (NASDAQ:EYPT)
Historical Stock Chart
From Nov 2023 to Nov 2024