As filed with the Securities and Exchange Commission on September 16, 2015
Registration No. 333-205666
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM F-3
REGISTRATION STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
NOVOGEN LIMITED ACN 063 259 754
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| New South Wales, Australia |
|
2834 |
|
Not Applicable |
| (State or other jurisdiction of
incorporation or organization) |
|
(Primary Standard Industrial
Classification Code Number) |
|
(I.R.S. Employer
Identification Number) |
Level 1, 16-20 Edgeworth David Avenue
Hornsby, New South Wales 2077
Australia
(61-2)
9472-4104
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Novogen North America, Inc.
110 Loomis Court
Ithaca,
NY 14850-8929
(607) 539-4002
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
|
|
| Michael Ryan, Esq.
Addisons Level 12, 60
Carrington Street Sydney, New South Wales 2000
Australia |
|
John F.F. Watkins, Esq.
Reitler Kailas & Rosenblatt LLC
885 Third Avenue 20th Floor New York, NY 10022 |
Approximate date of commencement of proposed sale to the public: From time to time after effectiveness of this registration statement (except as
otherwise described herein).
If only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans,
please check the following box. ¨
If any of the securities being registered on this Form are
to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and
list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act
registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a registration statement pursuant to General Instruction I.C. or a post-effective amendment thereto that shall become effective upon filing
with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ¨
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.C. filed to register additional securities or
additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ¨
CALCULATION OF REGISTRATION FEE
|
|
|
|
|
|
|
|
|
| |
| Title of each class of
Securities to be Registered |
|
Amount
to be
Registered(1) |
|
Proposed
Maximum Offering
Price
Per Share |
|
Proposed
Maximum Aggregate
Offering Price |
|
Amount of
Registration Fee |
| Ordinary Shares |
|
77,625,000(1) |
|
$0.161(2) |
|
$12,497,625(2) |
|
$1,452.22 |
| |
| (1) |
Consists of ordinary shares issuable upon exercise of options issued by Novogen Limited to the selling shareholders described in the prospectus. These ordinary shares may be represented by American Depository Shares, or
ADSs. The ADSs issuable upon deposit of the ordinary shares registered hereby have been registered under a separate registration statement on Form F-6 (Registration No. 333-128681). Each ADS represents twenty five ordinary shares.
|
| (2) |
Pursuant to Rule 457(c) of the Securities Act of 1933, the maximum aggregate offering price of shares being registered for resale by the selling shareholder was calculated on the basis of high and low sales prices of
the Novogen Limited ordinary shares represented by ADSs as reported on Nasdaq Capital Market on June 30, 2015. |
| (3) |
Previously paid based on 77,625,000 ordinary shares originally contemplated for registration as reflected in the original Form F-3 Registration Statement filed on
July 15, 2015. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its
effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until
the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to such Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. These securities may
not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where
the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED SEPTEMBER 16, 2015
Preliminary Prospectus
Up to 3,105,000 American Depositary Shares representing Ordinary Shares

Novogen Limited
This prospectus relates to the proposed resale by the selling shareholders named in the section “Selling Shareholders”
(collectively with any of their respective transferees or other successors-in-interest, the “Selling Shareholders”) of up to 3,105,000 American Depositary Shares, or ADSs”, representing 77,625,000 ordinary shares
(“ordinary shares”) of Novogen Limited (the “Company”). Each ADS represents twenty-five ordinary shares.
The ordinary shares underlying the ADSs are being registered pursuant to the requirements of a placement confirmation letter dated
April 20, 2015, between our Company and the Selling Shareholders to permit the Selling Shareholder to sell ADSs representing the ordinary shares from time to time in the public market (the “Placement Letter”).
We are not selling any securities under this prospectus and will not receive any of the proceeds from the sale of ADSs.
The ADSs covered by this prospectus may be offered or sold from time to time directly to purchasers or through agents, underwriters, brokers
or dealers at prevailing market or privately negotiated prices and on other terms to be determined at the time of sale. See “Plan Of Distribution.”
The ADSs are traded on the Nasdaq Capital Market, or Nasdaq, and our ordinary shares are listed on the Australian Stock Exchange, or ASX,
under the symbol “NRT”. On September 11, 2015, the closing price of the ADSs on Nasdaq was $2.52 per share and the closing price of our ordinary shares on the ASX was A$0.15 per share.
An investment in these securities involves risks. Please carefully consider the “Risk Factors”
beginning on page 3 of this prospectus, in Item 3.D of our Annual Report on Form 20-F for the fiscal year ended June 30, 2014 and other documents incorporated by reference in this prospectus, and in any applicable prospectus supplement,
for a discussion of the factors you should consider carefully before deciding to purchase these securities.
NEITHER THE SECURITIES
AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is September 16, 2015.
You should rely only on the information contained in or incorporated by reference into this
prospectus and any applicable prospectus supplement. Neither we nor the Selling Shareholders have authorized anyone to provide you with information different from that contained in this prospectus. The Selling Shareholders are not offering to sell
or soliciting offers to buy any security other than the ADSs and ordinary shares offered by this prospectus. In addition, the Selling Shareholders are not offering to sell or soliciting offers to buy any securities to or from any person in any
jurisdiction where it is unlawful to make this offer to or solicit an offer from a person in that jurisdiction. The information contained in this prospectus is accurate as of the date on the front of this prospectus only, regardless of the time of
delivery of this prospectus or of any sale of our securities. Our business, financial condition, results of operations and prospects may have changed since that date.
This prospectus is part of a registration statement on Form F-3 that we filed with the U.S. Securities and Exchange Commission, or the SEC.
This prospectus does not contain all of the information set forth in the registration statement, certain parts of which are omitted in accordance with the rules and regulations of the SEC. Accordingly, you should refer to the registration statement
and its exhibits for further information. Copies of the registration statement and its exhibits are on file with the SEC.
TABLE OF CONTENTS
IMPORTANT INFORMATION ABOUT THIS PROSPECTUS
Before you invest in our securities, you should carefully read this prospectus and any prospectus supplement together with the additional
information described in the sections entitled “Risk Factors,” “Where You Can Find Additional Information About Us” and “Incorporation of Certain Information by Reference” in this prospectus.
In this prospectus, unless otherwise indicated or the context otherwise requires:
| |
• |
|
the terms “we,” “us”, “our,” “the Company,” “our Company,” “Novogen,” or “NVGN” refer to Novogen Limited, an Australian company and its consolidated
subsidiaries; |
| |
• |
|
“Our shares,” “ordinary shares” and similar expressions refer to our Ordinary Shares; |
| |
• |
|
“ADSs” refers to American depositary shares, each of which represents twenty-five ordinary shares, and “ADRs” refers to the American depositary receipts that may evidence the ADSs; |
| |
• |
|
“US$,” “dollars” or “U.S. dollars” refers to the legal currency of the United States, unless otherwise indicated. |
i
This prospectus is part of a registration statement on Form F-3 that we filed with the SEC
utilizing a shelf registration process permitted under the Securities Act of 1933, as amended, or the Securities Act. By using a shelf registration statement, we or any selling security holder may sell any of our securities from time to time and in
one or more offerings. Each time we or any selling security holder sell securities, we may provide a supplement to this prospectus that contains specific information about the securities being offered and the specific terms of that offering. The
supplement may also add, update or change information contained in this prospectus. If there is any inconsistency between the information in this prospectus and any prospectus supplement, you should rely on the prospectus supplement.
SPECIAL CAUTIONARY NOTICE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements and matters discussed in this prospectus, the documents incorporated by reference, any related prospectus and any related
free writing prospectus constitute “forward-looking statements” within the meaning of, and intended to qualify for safe harbor from liability established by the Securities Act, the Securities Exchange Act of 1934, as amended, or the
Exchange Act, and the Private Securities Litigation Act of 1995. Forward-looking statements are statements that are not historical facts and may contain estimates, assumptions, projections, belief, expectations, future plans and strategies,
anticipated events and/or trends. Statements related to our future financial condition, results of operations and expected market growth are examples of forward-looking statements. Such forward-looking statements involve known and unknown risks,
uncertainties and other important factors that could cause our actual results, performance, or future results to differ materially from historical results or any future results, performance or achievements expressed, suggested or implied by such
forward-looking statements. In some instances, you can identify these forward-looking statements by words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,”
“plan,” “potential,” “will,” “should,” “would,” or similar expressions, including their negatives. These forward-looking statements include, without limitation, statements relating to our
expectations and beliefs regarding:
| |
• |
|
fluctuations in the market price of our securities; |
| |
• |
|
the possibility that our securities could be delisted from Nasdaq or the ASX; |
| |
• |
|
potential dilution to the holders of our securities as a result of future issuances of our securities; |
| |
• |
|
fluctuations in our results of operations; |
| |
• |
|
the accuracy of our financial forecasts in our drug development activity as well as in our medical device activity and the uncertainty regarding the adequacy of our liquidity to pursue our complete business objectives;
|
| |
• |
|
the timing and cost of the in-licensing, partnering and acquisition of new product opportunities; |
| |
• |
|
the timing of expenses associated with product development and manufacturing of the proprietary drug candidates; |
| |
• |
|
the costs involved in prosecuting and enforcing patent claims and other intellectual property rights; and |
| |
• |
|
other risks and uncertainties described in this prospectus. |
The risks included in this
section are not exhaustive. You should carefully consider the section entitled “Risk Factors” in this prospectus and reports filed with or furnished to the SEC, which include additional factors that could impact our business and financial
performance, before making any investment decision with respect to our securities. If any of these trends, risks or uncertainties actually occurs or continues, our business, financial condition and results of operations could be adversely affected,
the trading prices of our securities could decline and you could lose all or part of your investment.
Forward-looking statements
contained in this prospectus and documents incorporated by reference into this prospectus are based on our current plans, estimates and projections. Therefore, you should not place any reliance on any forward-looking statement as a prediction of
future results. Forward-looking statements made in this prospectus and the documents incorporated by reference are made as of the date of the respective documents. We undertake no obligation, and specifically decline any obligation, except as
required by law, to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
ii
PROSPECTUS SUMMARY
This summary provides a brief overview of the key aspects of Novogen Limited and certain material terms of the securities that may be
offered that are known as of the date of this prospectus. For a more complete understanding of the terms of a particular sale of offered securities, and before making your investment decision, you should carefully read:
| |
• |
|
this prospectus, including the materials incorporated herein by reference and the risk factors commencing on page 2 of this prospectus, which explains the general terms of the securities that may be offered;
|
| |
• |
|
the accompanying prospectus supplement for such issuance, which explains the specific terms of the securities being offered and which may update or change information in this prospectus; and |
| |
• |
|
the documents referred to in “Where You Can Find Additional Information” for information about us, including our financial statements. |
Our Company
We are
a pharmaceutical drug development company, with two main drug technology platforms: super-benzopyrans (SBPs) and anti-tropomyosins (ATMs). SBP compounds have the potential to kill the hierarchy of cancer cells that constitute a tumor, potentially
including the tumor-initiator cells. The ATM compounds target the microfilament cytoskeleton of a cancer cell. In vitro studies have shown that cancer cells treated with both the ATM compounds and standard of care anti-microtubule drugs, undergo
enhanced cancer cell death compared with the effect achieved with the respective monotherapy controls. Researchers have also observed this enhanced anti-cancer effect in in vivo studies (see below), ovarian cancer, colorectal cancer, prostate
cancer, neural cancers (glioblastoma, neuroblastoma in children), gastric cancer, pancreatic cancer, sarcomas and melanoma are the potential clinical indications being pursued, with the ultimate objective of employing both technologies as a unified
approach to therapy.
In November 2013, we formed a joint-venture company, CanTx Inc., with Yale University. The purpose of the joint
venture is to pool the resources of both parties in order to develop drugs for the treatment of ovarian cancer. Under a license agreement from Novogen, CanTx can access our patent portfolio of SBP drugs in order to identify a lead candidate compound
which leads to the development of a product for the treatment of intra-abdominal cancers, with a particular focus on ovarian cancer. A license agreement between Yale and CanTx provides CanTx access to certain Yale cell culture technology, animal
models, facilities, and resources. Arising from these studies, CanTx has identified a candidate drug product, Cantrixil. Cantrixil modulates the JNK and PKC pathways in cancer cells and induces caspase-mediated cell death. Cantrixil has been
designed to be injected into the peritoneal cavity with the aim of treating cancer initiating cells, the cells primarily responsible for cancer recurrence post chemotherapy. On April 20, 2015, we announced the results of pre-clinical studies
showing that Cantrixil was active in a stringent rodent model of human ovarian cancer. Pending successful completion of our formal toxicology program, an initial first in human Phase I clinical study is planned to be conducted in Australia
assessing the safety and tolerability of Cantrixil in patients with abdominal cancers with a bias toward patients with late-stage ovarian cancer in patients that no longer respond to standard of care therapy during 2016. We are planning to extend
this trial into US trial sites once we have received IND status for Cantrixil from the FDA. The FDA recently granted Cantrixil Orphan Drug status.
1
The Company’s second drug technology platform is called the anti-tropomyosin (ATM)
drug platform. The ATM’s are a class of small molecules designed to target a protein component of the actin microfilaments, tropomyosin Tpm3.1 which, as confirmed by in vitro studies, is essential for tumor cell survival. Inhibition of
Tpm3.1 function impacts the structural integrity of the cancer cell cytoskeleton causing the cancer cell to die. In addition to being an effective anti-cancer agent as a solo agent, these ATM compounds are also novel because they enhance the
anti-cancer activity of one of the most widely used classes of chemotherapy drugs - the anti-microtubule drugs.
In November 2014 we
announced that we had identified our lead ATM drug candidate, Anisina. Anisina was identified using Novogen’s proprietary VAL-ID - Versatile Approach to Library-based Iterative Design – medicinal chemistry program. This
strategy is based around the design, synthesis and evaluation of targeted small-molecule libraries and has proven to be a rapid and robust method of identifying lead compounds. Using a series of cell based triage screens, Anisina was selected
based on; i) its ability to bind to and inhibit the function of the target protein, Tpm3.1, ii) its effectiveness against a panel of both adult and pediatric tumor cell lines and iii) its ability to enhance the sensitivity of adult and pediatric
cancer cell lines to the standard of care microtubule targeting agents such as the taxanes and vinca alkaloids.
Preclinical in
vivo studies are underway to validate the effectiveness of Anisina in animal models of adult and paediatric (neuroblastoma) cancers both on its own and in combination with standard of care microtubule inhibitors. Importantly, a proof of
concept study done as part of the Children’s Oncology Drug Alliance (CODA) has confirmed that Anisina is not only effective on its own in reducing tumor growth in, but also enhances the sensitivity of the tumor to microtubule targeting
compounds in an animal model of neuroblastoma. The data from this study was presented at the Eighth Annual Cancer Molecular Therapeutics Research Association (CMTRA) meeting in the USA on July 13th of this year.
We are now focused on optimising the Anisina formulation and mode of delivery. Recent studies in a rodent model of cancer (announced
on the 24th of June 2015) have demonstrated that Anisina delivered in a cyclodextrin formulation is effective in reducing tumor growth, and was well tolerated when dosed orally (daily) or
intravenously (twice weekly). These studies clear the way to now initiate IND enabling studies with the view of taking Anisina through to the clinic as an IV delivered drug in combination with taxanes or vinca alkaloids. Pending the outcome
of our Anisina toxicology program, our first in man studies, are predicted to start during 2016.
We recently announced that pre-clinical
in vitro studies conducted on our other lead SBP drug candidate, TRXE-009, affects the viability of cancer cells by increasing rates of cell death (via caspase-mediated apoptosis) and reducing proliferation. This was observed in cancer cells
representative of glioblastoma, melanoma and prostate cancer. We have also demonstrated tumor growth inhibition in several animal models (flank models of melanoma, prostate and GBM) (see below). These findings combine with other recently announced
pre-clinical in vitro studies showing that TRXE-009 is highly cytotoxic to chemo-resistant pediatric brain cancers including Diffuse Intrinsic Pontine Glioma (DIPG). Through our collaboration with a local pediatric medical oncologist, the
Company has confirmed that Trilexium is highly active against patient derived explants of Diffuse Intrinsic Pontine Glioma (DIPG) in pre-clinical studies. DIPG is an aggressive but rare brain tumor primarily affecting children. Novogen has
identified a drug formulation that in animals is able to deliver Trilexium to brain tissue and researchers will soon commence a proof-of-concept pre-clinical study in an orthotropic model of DIPG. Once this proof-of-concept study is completed, the
Company plans to commence the required safety evaluation program. By targeting DIPG, the Company anticipates that this strategy may provide a fast-to-market opportunity and is also planning to file for an Orphan Drug Designation status with the FDA.
At this stage, first-in-human trials are targeted to commence by 2017.
Novogen has also recognised the potential depth of both the SBP
and ATM technology platforms which secures exceptional future growth opportunities, provides back-ups for our existing lead compounds and opens up new avenues for future discovery with a focus on Oncology. Our actions have also strengthened our
existing patent protection. In light of Novogen’s commitment to progressing at least two of its lead drug candidates into the clinic and focus on oncology-centric drug discovery, we have decided to pursue a patent protection approach for our
degenerative and regenerative medicine program known as Jacob’s Hope. This program will now take a lower profile while we focus on the nearer term core opportunities.
2
THE OFFERING AND PLACEMENT OF ORDINARY SHARES AND OPTIONS
We are registering for resale 77,625,000 ordinary shares, comprised of ordinary shares issuable upon exercise of (i) 51,750,000
options at the initial exercise price of A$0.30 per ordinary share that expire on December 30, 2015 (“Short-term Options”) and (ii) 25,875,000 options at the initial exercise price of A$0.40 per ordinary share that expire
on June 30, 2020 (“Long-term Options”; we refer in this Prospectus to the Short-term Options and the Long-terms Options collectively as the “Placement Options”), issued by our Company in a private placement
(the “Placement”) pursuant to a placement confirmation letter dated April 20, 2015 (the “Placement Letter”) between our Company and certain investors named in the section “Selling Stockholders”
(collectively, the “Selling Shareholders”), which may be represented by ADSs. In the Placement, our Company issued and sold to the Selling Shareholders an aggregate of 51,750,000 ordinary shares and 77,625,000 Placement Options. The
securities were issued without registration under the U.S. Securities Act of 1933, as amended (the “Securities Act”), pursuant to the exemption from registration under the Securities Act for transactions not involving any public
offering. We received gross proceeds of approximately A$15.5 million from the Placement, which we are using for, among other purposes, further development of pharmaceutical drug products and general corporate purposes to support further development
of our Company.
RISK FACTORS
Before you invest in the ADSs you should understand the high degree of risk involved. You should carefully consider the risks described below
and other information in this report, including our financial statements and related notes included elsewhere in this report, before you decide to purchase the ADSs. If any of the following risks actually occur, our business, financial condition and
operating results could be adversely affected. As a result, the trading price of the ADSs could decline and you could lose part or all of your investment.
3
Risks Related to Our Business
We are currently exploring the development of anti-cancer drugs based on two unproven drug technology platforms – no assurance can be given that
either of these platforms will prove successful.
We are committed to the identification of lead candidate anti-cancer drugs from
the two drug technology platforms, super-benzopyrans (SBPs) and anti-tropomyosins (ATMs). Although early pre-clinical studies have confirmed the utility of either drug technology platform in the generation of compounds with novel and potent
cytotoxicity against various human cancer cell lines in vitro, there are significant risks and uncertainties in translating those early laboratory results into drugs that will have meaningful clinical application and meet the stringent
requirements of regulatory authorities such as the United States Food and Drug Administration, that review Investigational New Drug (IND) Applications to enable the conduct of first-in-man clinical trials. The Company plans to obtain the appropriate
approvals to enable the trials.
Factors that may have a negative impact on early drug candidate selection include:
| |
• |
|
unacceptably high toxicity; |
| |
• |
|
Poor efficacy in in vivo models; |
| |
• |
|
unacceptably short drug half-life; |
| |
• |
|
inability to deliver the drug in a practical manner; and |
| |
• |
|
insurmountable difficulties in large-scale manufacture. |
Our ability to continue as a going concern is
dependent on a continuing positive news flow from our pre-clinical R&D programs, and our ability to raise capital to support those programs.
As a result of the placement and the rights offer completed by the Company on June 2, 2015, we have raised approximately A$33.2 million
which, together with the Company’s existing cash resources, will enable the Company to proceed with its proposed R&D programs over the next 2 years. However, in the longer run, we will need substantial additional funds to maintain our
planned level of R&D.
The factors that will determine the actual amount of additional capital required may include the
following:
| |
• |
|
the rate of success and the length of time it takes to identify lead candidate compounds in both the super-benzopyran (SBP) and anti-tropomyosin (ATM) drug technologies; |
| |
• |
|
the length of time and amount of work required to bring any lead candidate compounds through their pre-clinical programs |
| |
• |
|
the need to employ additional staff or contractors to meet the needs of the R&D programs. |
If we are unable to obtain additional funds on favorable terms or at all, we may be required to cease or reduce our operations. Also, if we
raise more funds by selling additional securities, the ownership interests of holders of our securities will be diluted.
We are at an early stage
of drug development and are in the process of applying for patents over composition and matter and use for both of our drug technology platforms. There is no certainty that patent protection will be granted.
Our patent portfolio is in a development stage. It comprises a certain number of provisional patents that have been lodged and others that
are in the process of being lodged. The patents usually have worldwide coverage, with a particular focus on USA, EU, Asia and Australia. While our patent strategy is closely supervised by experienced patent attorneys and every effort made to ensure
the likely success of achieving approval of patent claims in all major territories, there is no guarantee that any or all territories will grant such claims.
4
Negative global economic conditions may pose challenges to our business strategy, the success of which
relies on our continued ability to access capital from the markets or collaborators.
Negative conditions in the global economy,
including credit markets and the financial services industry, have generally made equity and debt financing more difficult to obtain, and may negatively impact our ability to complete financing transactions. The duration and severity of these
conditions is uncertain, as is the extent to which they may adversely affect our business and the business of current and prospective vendors and collaborators. If negative global economic conditions persist or worsen, we may not be able to secure
additional funding to sustain our operations or to find suitable collaborators to advance our internal programs, even if positive results are achieved from research and development efforts.
We have a history of incurring losses and expect to continue to incur losses.
We have not yet generated any income from our activities. We have had a history of incurring losses and are likely to continue to incur
operating losses in the near future, until such time as any possible commercial breakthrough occurs.
Final approval by regulatory authorities of
our drug candidates for commercial use may be delayed, limited or prevented, any of which would adversely affect our ability to generate operating revenues.
We will not generate any operating revenue until we, or our subsidiaries, successfully commercialize one or more of our drug candidates.
Currently, our drug candidates are at an early stage of development, and each will need to successfully proceed through a number of steps in order to obtain regulatory approval before potential commercialization.
For example, any of the following factors may serve to delay, limit or prevent final approval by regulatory authorities of our drug
candidates for commercial use:
| |
• |
|
we have identified two lead drug-candidates, Cantrixil and Anisina that are progressing through a safety evaluation program, and are in the process of identifying a number of other lead candidate compounds. All are in
the early stages of development, and we will need to conduct significant pre-clinical and clinical testing to demonstrate the safety and the efficacy of these drug candidates before applications for marketing can be filed with the FDA, or with the
regulatory authorities of other countries; |
| |
• |
|
data obtained from pre-clinical and clinical studies can be interpreted in different ways, which could delay, limit or prevent regulatory approval; |
| |
• |
|
development and testing of product formulation, including identification of suitable excipients, or chemical additives intended to facilitate delivery of our drug candidates; |
| |
• |
|
it may take us many years to complete the testing of our drug candidates, and failure can occur at any stage of this process; and |
| |
• |
|
negative or inconclusive results or adverse medical events during a clinical trial could cause us to delay or terminate our development efforts. |
The successful development of any of these drug candidates is uncertain and, accordingly, we may never commercialize any of these drug
candidates or generate revenue.
Even if we receive regulatory approval to commercialize our drug candidates, the ability to generate revenues from
any resulting products will be subject to a variety of risks, many of which are outside of our control.
Even if our drug
candidates obtain regulatory approval, resulting products may not gain market acceptance among physicians, patients, healthcare payers or the medical community. We believe that the degree of market acceptance and our ability to generate revenues
from such products will depend on a number of factors, including, but not limited to:
| |
• |
|
timing of market introduction of our drugs and competitive drugs; |
| |
• |
|
actual and perceived efficacy and safety of our drug candidates; |
| |
• |
|
prevalence and severity of any side effects; |
| |
• |
|
potential or perceived advantages or disadvantages over alternative treatments; |
| |
• |
|
strength of sales, marketing and distribution support; |
5
| |
• |
|
price of future products, both in absolute terms and relative to alternative treatments; |
| |
• |
|
the effect of current and future healthcare laws on our drug candidates; and |
| |
• |
|
availability of coverage and reimbursement from government and other third-party payers. |
If
any of our drugs are approved and fail to achieve market acceptance, we may not be able to generate significant revenue to achieve or sustain profitability.
We may not be able to establish the contractual arrangements necessary to develop, market and distribute the product candidates.
A key part of our business plan is to establish contractual relationships with strategic partners. We must successfully contract with third
parties to package, market and distribute our product candidates.
Potential partners may be discouraged by our limited operating
history.
There is no assurance that we will be able to negotiate commercially acceptable licensing or other agreements for the future
exploitation of our drug product candidates including continued clinical development, manufacture or marketing. If we are not able to successfully contract for these services, or if arrangements for these services are terminated, we may have to
delay the commercialization program which will adversely affect our ability to generate operating revenues.
Our commercial opportunity will be
reduced or eliminated if competitors develop and market products that are more effective, have fewer side effects or are less expensive than our drug candidates.
The development of drug candidates is highly competitive. A number of other companies have products or drug candidates in various stages of
pre-clinical or clinical development that are intended for the same therapeutic indications for which our drug candidates are being developed. Some of these potential competing drugs are further advanced in development than our drug candidates and
may be commercialized sooner. Even if we are successful in developing effective drugs, our compounds may not compete successfully with products produced by our competitors.
Our competitors include pharmaceutical companies and biotechnology companies, as well as universities and public and private research
institutions. In addition, companies active in different but related fields represent substantial competition. Many of our competitors developing oncology drugs have significantly greater capital resources, larger number of research and development
staff and facilities and greater experience in drug development, regulation, manufacturing and marketing. These organizations also compete with us and our service providers to recruit qualified personnel and to attract partners for joint ventures
and to license technologies. As a result, our competitors may be able to more easily develop technologies and products that would render our technologies or our drug candidates obsolete or non-competitive.
We rely on third parties to conduct our pre-clinical studies. If those parties do not successfully carry out their contractual duties or meet expected
deadlines, our drug candidates may not advance in a timely manner or at all.
In the course of discovery, pre-clinical
testing and clinical trials, we rely on third parties, including laboratories, investigators, contract research organizations, or CROs (Clinical Research Organisation) and manufacturers, to perform critical services. For example, we rely on third
parties to conduct all of our pre-clinical studies. These third parties may not be available when we need them or, if they are available, may not comply with all regulatory and contractual requirements or may not otherwise perform their services in
a timely or acceptable manner, and we may need to enter into new arrangements with alternative third parties and the studies may be extended, delayed or terminated. These independent third parties may also have relationships with other commercial
entities, some of which may compete with us. As a result of our dependence on third parties, we may face delays or failures outside of our direct control. These risks also apply to the development activities of collaborators, and we do not control
their research and development, clinical trial or regulatory activities.
We have no direct control over the cost of manufacturing our drug
candidates. Increases in the cost of manufacturing our drug candidates would increase the costs of conducting clinical trials and could adversely affect future profitability.
We do not intend to manufacture the drug product candidates in-house, and we will rely on third parties for drug supplies both for clinical
trials and for commercial quantities in the future. We have taken the strategic decision not to manufacture active pharmaceutical ingredients (‘API’) for the drug candidates, as these can be more economically supplied by third parties with
particular expertise in this area. We plan to manufacture product and test it to FDA requirements. We have identified contract facilities that are registered with the FDA, have a track record of large scale API manufacture, and have already invested
in capital and equipment. We have no direct control over the cost of manufacturing our product candidates. If the cost of manufacturing increases, or if the cost of the materials used increases, these costs will be passed on, making the cost of
conducting clinical trials more expensive. Increases in manufacturing costs could adversely affect our future profitability if we were unable to pass all of the increased costs along to our customers.
6
We may face a risk of product liability claims and may not be able to obtain adequate insurance.
Our business exposes us to the risk of product liability claims. This risk is inherent in the manufacturing, testing and
marketing of human therapeutic products. We have product liability insurance. The coverage is subject to deductibles and coverage limitations. We are in the process of identifying lead candidate compounds. When identified, and INDs are obtained,
they will be taken into the clinic. We may not be able to obtain or maintain adequate protection against potential liabilities, or claims may exceed the insurance limits. If we cannot or do not sufficiently insure against potential product liability
claims, we may be exposed to significant liabilities, which may materially and adversely affect our business development and commercialization efforts.
If we are unable to adequately protect our intellectual property, third parties may be able to use our technology, which could adversely affect our
ability to compete in the market.
Our commercial success will depend in part on our ability and the ability of our licensors to
obtain and maintain patent protection on our drug products and technologies and successfully defend these patents and technologies against third-party challenges. As part of our business strategy, our policy is to actively file patent applications
in the US and internationally to cover methods of use, new chemical compounds, pharmaceutical compositions and dosing of the compounds and composition and improvements in each of these. Because of the extensive time required for development, testing
and regulatory review of a potential product, it is possible that before we commercialize any of our products, any related patent may expire or remain in force for only a short period following commercialization, thus reducing any advantage of the
patent.
The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual
questions. No consistent policy regarding the breadth of claims allowed in biotechnology patents has emerged to date. Accordingly, the patents we use may not be sufficiently broad to prevent others from practicing our technologies or from developing
competing products. Furthermore, others may independently develop similar or alternative technologies or design around our patented technologies. The patents we use may be challenged or invalidated or may fail to provide us with any competitive
advantage.
Generally, patent applications in the US are maintained in secrecy for a period of at least 18 months. Since publication of
discoveries in the scientific or patent literature often lag behind actual discoveries, we are not certain that we were the first to make the inventions covered by each of our pending patent applications or that we were the first to file those
patent applications. We cannot predict the breadth of claims allowed in biotechnology and pharmaceutical patents, or their enforceability. Third parties or competitors may challenge or circumvent our patents or patent applications, if issued. If our
competitors prepare and file patent applications in the US that claim compounds or technology also claimed by us, we may be required to challenge competing patent rights, which could result in substantial cost, even if the eventual outcome is
favorable to us. While we have the right to defend patent rights related to the licensed drug candidates and technologies, we are not obligated to do so. In the event that we decide to defend our licensed patent rights, we will be obligated to
cover all of the expenses associated with that effort.
We also rely on trade secrets to protect technology where we believe patent
protection is not appropriate or obtainable. Trade secrets are difficult to protect. While we require our employees, collaborators and consultants to enter into confidentiality agreements, this may not be sufficient to protect our trade secrets or
other proprietary information adequately. In addition, we share ownership and publication rights to data relating to some of our drug candidates and technologies with our research collaborators and scientific advisors. If we cannot maintain the
confidentiality of this information, our ability to protect our proprietary information will be at risk.
7
Litigation or third-party claims of intellectual property infringement could require us to spend
substantial time, money and other resources defending such claims and adversely affect our ability to develop and commercialize our products.
Third parties may assert that we are using their proprietary technology without authorization. In addition, third parties may have or obtain
patents in the future and claim that our products infringe their patents. If we are required to defend against patent suits brought by third parties, or if we sue third parties to protect our patent rights, we may be required to pay substantial
litigation costs, and our management’s attention may be diverted from operating our business. In addition, any legal action against our licensors or us that seeks damages or an injunction of our commercial activities relating to the affected
products could subject us to monetary liability and require our licensors or us to obtain a license to continue to use the affected technologies. We cannot predict whether our licensors or we would prevail in any of these types of actions or that
any required license would be made available on commercially acceptable terms, if at all. In addition, any legal action against us that seeks damages or an injunction relating to the affected activities could subject us to monetary liability and/or
require us to discontinue the affected technologies or obtain a license to continue use thereof.
In addition, there can be no assurance
that our patents or patent applications or those licensed to us will not become involved in opposition or revocation proceedings instituted by third parties. If such proceedings were initiated against one or more of our patents, or those licensed to
us, the defense of such rights could involve substantial costs and the outcome could not be predicted.
Competitors or potential
competitors may have filed applications for, may have been granted patents for, or may obtain additional patents and proprietary rights that may relate to compounds or technologies competitive with ours. If patents are granted to other parties that
contain claims having a scope that is interpreted to cover any of our products (including the manufacture thereof), there can be no assurance that we will be able to obtain licenses to such patents at reasonable cost, if at all, or be able to
develop or obtain alternative technology.
Risks Related to the ADSs
The trading price of our ordinary shares and ADSs is highly volatile. Your investment could decline in value and we may incur significant costs from
class action litigation and our securities may be delisted from Nasdaq.
The trading price of our ordinary shares and ADSs is
highly volatile in response to various factors, many of which are beyond our control, including:
| |
• |
|
announcements of technological innovations by us and our competitors; |
| |
• |
|
new products introduced or announced by us or our competitors; |
| |
• |
|
changes in financial estimates by securities analysts; |
| |
• |
|
actual or anticipated variations in operating results; |
| |
• |
|
expiration or termination of licenses, research contracts or other collaboration agreements; |
| |
• |
|
conditions or trends in the regulatory climate in the biotechnology, pharmaceutical and genomics industries; |
| |
• |
|
changes in the market values of similar companies; |
| |
• |
|
the liquidity of any market for our securities; and |
| |
• |
|
additional sales by us of our shares. |
In addition, equity markets in general and the market
for biotechnology and life sciences companies in particular, have experienced substantial price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of the companies traded in those markets. Further
changes in economic conditions in Australia, the United States, Europe, or globally, could impact our ability to raise funding. Adverse economic changes are outside our control and may result in material adverse effects on our business or results of
operations. These broad market and industry factors may materially affect the market price of our ordinary shares and ADSs regardless of our development and operating performance. In the past, following periods of volatility in the market price of a
company’s securities, securities class action litigation has often been instituted against that company. Such litigation, if instituted against us, could cause us to incur substantial costs and divert management’s attention and resources.
The market price of the ADSs remains below US$5.00 per share. As such, under stock exchange rules, our stockholders will not be able to
use such ADSs as collateral for borrowing in margin accounts. This inability to use ADSs as collateral may depress demand as certain institutional investors are restricted from investing in securities priced below US$5.00 and may lead to sales of
such ADSs creating downward pressure on and increased volatility in the market price of our ordinary shares and ADSs.
8
In addition, under Nasdaq rules, companies listed on the Nasdaq Capital Market are required to
maintain a share price of at least US$1.00 per share and if the share price declines below US$1.00 for a period of 30 consecutive business days, then the listed company would have 180 days to regain compliance with the US$1.00 per share minimum. In
the event that our share price for an ADS declines below US$1.00, we may be required to take action, such as a reverse stock split, in order to comply with the Nasdaq rules that may be in effect at the time.
The ADSs are traded in small volumes, limiting your ability to sell your ADSs that represent ordinary shares at a desirable price, if at all.
The trading volume of the ADSs has historically been low. Even if the trading volume of the ADSs increases, we can give no
assurance that it will be maintained or will result in a desirable stock price. As a result of this low trading volume, it may be difficult to identify buyers to whom you can sell your ADSs in desirable volume and you may be unable to sell your ADSs
at an established market price, at a price that is favorable to you, or at all. A low volume market also limits your ability to sell large blocks of the ADSs at a desirable or stable price at any one time. You should be prepared to own the ADSs
indefinitely.
Our stock price can be volatile, which increases the risk of litigation and may result in a significant decline in the value of your
investment.
The trading price of the ADSs representing our ordinary shares is likely to be highly volatile and subject to wide
fluctuations in price in response to various factors, many of which are beyond our control. These factors include:
| |
• |
|
developments concerning our drug candidates or medical devices; |
| |
• |
|
announcements of technological innovations by us or our competitors; |
| |
• |
|
introductions or announcements of new products by us or our competitors; |
| |
• |
|
developments in the markets of the field of activities and changes in customer attributes; |
| |
• |
|
announcements by us of significant acquisitions, in/out license transactions, strategic partnerships, joint ventures or capital commitments; |
| |
• |
|
changes in financial estimates by securities analysts; |
| |
• |
|
actual or anticipated variations in interim operating results and near-term working capital as well as failure to raise required funds for the continued development and operations of the Company; |
| |
• |
|
expiration or termination of licenses, patents, research contracts or other collaboration agreements; |
| |
• |
|
conditions or trends in the regulatory climate and the biotechnology and pharmaceutical industries; |
| |
• |
|
failure to obtain orphan drug designation status for the relevant drug candidates in the relevant regions; |
| |
• |
|
increase in costs and lengthy timing of the clinical trials according to regulatory requirements; |
| |
• |
|
failure to increase awareness to our non-medicinal non-invasive therapy and its benefits; |
| |
• |
|
changes in reimbursement policy by governments or insurers in markets we operate or may operate in the future; |
| |
• |
|
any changes in the regulatory environment relating to our products may impact our ability to market and sell our products; |
| |
• |
|
failure to obtain or renew the required licenses for the marketing and sale of our products (once developed) in the main markets in which those products are to be sold; |
| |
• |
|
changes in the market valuations of similar companies; and |
| |
• |
|
additions or departures of key personnel. |
In addition, equity markets in general, and the
market for biotechnology and life sciences companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of companies traded in those markets. These
broad market and industry factors may materially affect the market price of the ADSs, regardless of our development and operating performance. In the past, following periods of volatility in the market price of a company’s securities,
securities class-action litigation has often been instituted against that company. Such litigation, if instituted against us, could cause us to incur substantial costs to defend such claims and divert management’s attention and resources even
if we prevail in the litigation, all of which could seriously harm our business.
9
Future issuances or sales of ADSs could depress the market for the ADSs.
Future issuances of a substantial number of ADSs, or the perception by the market that those issuances could occur, could cause the market
price of our ordinary shares or the ADSs to decline or could make it more difficult for us to raise funds through the sale of equity in the future. Also, if we make one or more significant acquisitions in which the consideration includes ordinary
shares or other securities, your portion of shareholders’ equity in us may be significantly diluted.
ADS holders are not shareholders and do
not have shareholder rights.
The Bank of New York Mellon, as depositary, delivers the ADSs. ADS holders will not be treated as
shareholders and do not have the rights of shareholders. The depositary will be the holder of the shares underlying the ADSs. Holders of ADSs will have ADS holder rights. A deposit agreement among us, the depositary and ADS holders, and the
beneficial owners of ADSs, sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs. Our shareholders have shareholder rights prescribed by Australian law. Australian
law and our Constitution, govern such shareholder rights. ADS holders do not have the same voting rights as our shareholders. Shareholders are entitled to our notices of general meetings and to attend and vote at our general meetings of
shareholders. At a general meeting, every shareholder present (in person or by proxy, attorney or representative) and entitled to vote has one vote on a show of hands. Every shareholder present (in person or by proxy, attorney or representative) and
entitled to vote has one vote per fully paid ordinary share on a poll. This is subject to any other rights or restrictions which may be attached to any shares. ADS holders may instruct the depositary to vote the ordinary shares underlying their
ADSs. If we do not ask the depositary to ask for their instructions, ADS holders will not receive our notices of general meeting and may not find out about it in time to give voting instructions. ADS holders will not be entitled to attend and vote
at a general meeting unless they surrender ADSs and withdraw the ordinary shares. ADS holders may not know about the meeting enough in advance to withdraw the ordinary shares. If we ask for ADS holders’ instructions, the depositary will notify
ADS holders of the upcoming vote and arrange to deliver our voting materials and form of notice to them. The depositary will try, as far as is practical, subject to the provisions of the deposit agreement, to vote the shares as ADS holders instruct.
The depositary will not vote or attempt to exercise the right to vote other than in accordance with the instructions of ADS holders. We cannot assure ADS holders that they will receive the voting materials in time to ensure that they can instruct
the depositary to vote their shares.
ADS holders do not have the same rights to receive dividends or other distributions as our
shareholders. Subject to any special rights or restrictions attached to a share, the directors may determine that a dividend will be payable on a share and fix the amount, the time for payment and the method for payment (although we have never
declared or paid any cash dividends on our ordinary stock and we do not anticipate paying any cash dividends in the foreseeable future). Dividends and other distributions payable to our shareholders with respect to our ordinary shares generally will
be payable directly to them. Any dividends or distributions payable with respect to ordinary shares represented by ADSs will be paid to the depositary, which has agreed to pay to ADS holders the cash dividends or other distributions it or the
custodian receives on shares or other deposited securities, after deducting its fees and expenses. ADS holders will receive these distributions in proportion to the number of shares their ADSs represent. In addition, there may be certain
circumstances in which the depositary may not pay to the ADS holders amounts distributed by us as a dividend or distribution.
If we fail to
maintain an effective system of internal controls over financial reporting, we may not be able to accurately report our financial results or prevent fraud.
We are subject to reporting obligations under U.S. securities laws. The SEC, as required by Section 404 of the Sarbanes-Oxley Act of
2002, or the Sarbanes-Oxley Act, adopted rules requiring most public companies to include a management report on such company’s internal controls over financial reporting in its annual report, which contains management’s assessment of the
effectiveness of the Company’s internal controls over financial reporting. Our management may conclude that our internal controls over our financial reporting are not effective.
If we fail to maintain the adequacy of our internal controls, we may not be able to conclude that we have effective internal control over
financial reporting. Moreover, effective internal control over financial reporting is necessary for us to produce reliable financial reports and is important to help prevent fraud. As a result, our failure to maintain effective internal control over
financial reporting could result in the loss of investor confidence in the reliability of our financial statements, which in turn could harm our business and negatively impact the trading price of the ADSs. Furthermore, we have incurred and expect
to continue to incur considerable costs and devote significant management time and efforts and other resources to comply with Section 404 of the Sarbanes-Oxley Act.
10
Compliance with rules and regulations applicable to companies publicly listed in the United States is
costly and complex and any failure by us to comply with these requirements on an ongoing basis could negatively affect investor confidence in us and cause the market price of the ADSs to decrease.
In addition to Section 404, the Sarbanes-Oxley Act also mandates, among other things, that companies adopt corporate governance
measures, imposes comprehensive reporting and disclosure requirements, sets strict independence and financial expertise standards for audit committee members, and imposes civil and criminal penalties for companies, their chief executive officers,
chief financial officers and directors for securities law violations. Our current and future compliance efforts will continue to require significant management attention. In addition, our board members, chief executive officer and chief financial
officer could face an increased risk of personal liability in connection with the performance of their duties. As a result, we may have difficulty attracting and retaining qualified board members and executive officers to fill critical positions
within our Company. Any failure by us to comply with these requirements on an ongoing basis could negatively affect investor confidence in us, cause the market price of the ADSs to decrease or even result in the delisting of the ADSs from NASDAQ.
Because we are not organized under U.S. law, we are subject to certain less detailed disclosure requirements under U.S. federal securities
laws.
We are a “foreign private issuer,” as defined in the SEC’s regulations, and consequently we are not
subject to all of the same disclosure requirements applicable to domestic (U.S.) companies. We prepare annual financial statements in accordance with International Financial Reporting Standards. We are exempt from the SEC’s proxy rules, and our
annual reports contain less detailed disclosure than reports of domestic issuers regarding such matters as management, executive compensation and outstanding options, beneficial ownership of our securities and certain related party transactions.
Also, our officers, directors and beneficial owners of more than 10% of our equity securities are exempt from the reporting requirements and short-swing profit recovery provisions of Section 16 of the Exchange Act. We are also generally exempt
from most of the governance rules applicable to companies listed on NASDAQ. These limits on available information about our Company and exemptions from many governance rules applicable to U.S. domestic issuers may adversely affect the market prices
for our securities.
You may experience difficulties in effecting service of legal process, enforcing foreign judgments or
bringing original actions in Australia based on United States or other foreign laws against us or our management named in the annual report.
We are incorporated in Australia and conduct most of our operations in Australia. Most of our assets are located in Australia. In addition,
many of our directors and senior executive officers reside within Australia and some or all of the assets of those persons are located outside of the United States. As a result, it may not be possible to effect service of process within the United
States or elsewhere outside Australia upon our directors and senior executive officers, including with respect to matters arising under U.S. federal securities laws or applicable state securities laws. Even if you are successful in bringing an
action of this kind, the laws of Australia may render you unable to enforce a judgment against our assets or the assets of our directors and officers.
Australia has no statutory reciprocal enforcement arrangements in place with the US. Therefore, to enforce a US judgment in Australia, the
common law prerequisites to the recognition and enforcement of an overseas’ judgment must be met.
The common law prerequisites are
that the foreign Court must have exercised a jurisdiction which the Australian Courts recognise, the judgment is final and conclusive, the parties to the judgment and in the application to register the judgment are identical and the judgment is for
a fixed, or readily calculable debt.
The debtor can apply to have the enforcement of a judgment refused on grounds such as for public
policy reasons, because judgment was obtained by fraud, because the foreign Court failed to act in accordance with natural justice or to apply the appropriate law, or the party seeking the enforcement is stopped from doing so.
11
You may not be able to participate in rights offerings and may experience dilution of your holdings as a
result.
We may from time to time distribute rights to our shareholders, including rights to acquire our securities. Under the
deposit agreement for the ADSs, the depositary will not offer those rights to ADS holders unless both the rights and the underlying securities to be distributed to ADS holders are either registered under the Securities Act or are exempt from
registration under the Securities Act with respect to all holders of ADSs. We are under no obligation to file a registration statement with respect to any such rights or underlying securities or to endeavor to cause such a registration statement to
be declared effective. In addition, we may not be able to take advantage of any exemptions from registration under the Securities Act. Accordingly, holders of ADSs may be unable to participate in our rights offerings and may experience dilution in
their holdings as a result.
You may be subject to limitations on transfer of your ADSs.
Your ADSs are transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to
time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any
time if we or the depositary deem it advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason.
CAPITALIZATION AND INDEBTEDNESS
The table below sets forth our capitalization as of July 31, 2015.
|
|
|
|
|
| |
|
As of
July 31,
2015 |
|
| |
|
(In A$, except
number of shares) |
|
| Ordinary Shares issued and outstanding (1) |
|
|
424,117,465 |
|
| Borrowings |
|
$ |
0 |
|
| Contributed Equity |
|
$ |
190,555,804 |
|
| Reserves |
|
$ |
2,566,053 |
|
| Accumulated losses |
|
($ |
148,696,769 |
) |
| Non-controlling interest |
|
|
(318,573 |
) |
| Total shareholders’ equity |
|
$ |
44,105,615 |
|
| (1) |
Excludes 178,924,987 ordinary shares issuable upon the exercise of 178,924,987 options, having exercise prices ranging from A$0.1250 to A$0.40 per ordinary share and having a weighted average exercise price of
A$0.3278 per ordinary share. |
12
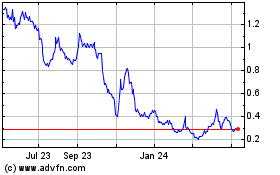
MARKET FOR OUR ORDINARY SHARES
Australian Securities Exchange
Our
ordinary shares are traded on the ASX. The following table sets forth, for the periods indicated, the high and low market quotations for our ordinary shares, as quoted on the ASX.
|
|
|
|
|
|
|
|
|
| |
|
Per Ordinary Share
(A$) |
|
| |
|
High |
|
|
Low |
|
| Fiscal Year Ended June 30, |
|
|
|
|
|
|
|
|
| 2010 |
|
|
0.89 |
|
|
|
0.17 |
|
| 2011 |
|
|
0.46 |
|
|
|
0.10 |
|
| 2012 |
|
|
0.25 |
|
|
|
0.08 |
|
| 2013 |
|
|
0.47 |
|
|
|
0.06 |
|
| 2014 |
|
|
0.40 |
|
|
|
0.15 |
|
| 2015 |
|
|
0.45 |
|
|
|
0.08 |
|
|
|
|
| Fiscal Year Ended June 30, 2013: |
|
|
|
|
|
|
|
|
| First Quarter |
|
|
0.10 |
|
|
|
0.07 |
|
| Second Quarter |
|
|
0.20 |
|
|
|
0.06 |
|
| Third Quarter |
|
|
0.47 |
|
|
|
0.07 |
|
| Fourth Quarter |
|
|
0.37 |
|
|
|
0.16 |
|
|
|
|
| Fiscal Year Ended June 30, 2014: |
|
|
|
|
|
|
|
|
| First Quarter |
|
|
0.21 |
|
|
|
0.16 |
|
| Second Quarter |
|
|
0.40 |
|
|
|
0.17 |
|
| Third Quarter |
|
|
0.23 |
|
|
|
0.18 |
|
| Fourth Quarter |
|
|
0.18 |
|
|
|
0.15 |
|
|
|
|
| Fiscal Year Ended June 30, 2015: |
|
|
|
|
|
|
|
|
| First Quarter |
|
|
0.16 |
|
|
|
0.12 |
|
| Second Quarter |
|
|
0.16 |
|
|
|
0.08 |
|
| Third Quarter |
|
|
0.24 |
|
|
|
0.10 |
|
| Fourth Quarter |
|
|
0.45 |
|
|
|
0.20 |
|
|
|
|
| Fiscal Year Ended June 30, 2016: |
|
|
|
|
|
|
|
|
| First Quarter (through to September 11, 2015) |
|
|
0.27 |
|
|
|
0.13 |
|
|
|
|
| Month Ended: |
|
|
|
|
|
|
|
|
| July 2014 |
|
|
0.16 |
|
|
|
0.12 |
|
| August 2014 |
|
|
0.15 |
|
|
|
0.13 |
|
| September 2014 |
|
|
0.16 |
|
|
|
0.13 |
|
| October 2014 |
|
|
0.14 |
|
|
|
0.12 |
|
| November 2014 |
|
|
0.12 |
|
|
|
0.08 |
|
| December 2014 |
|
|
0.16 |
|
|
|
0.08 |
|
| January 2015 |
|
|
0.11 |
|
|
|
0.09 |
|
| February 2015 |
|
|
0.17 |
|
|
|
0.12 |
|
| March 2015 |
|
|
0.24 |
|
|
|
0.13 |
|
| April 2015 |
|
|
0.45 |
|
|
|
0.21 |
|
| May 2015 |
|
|
0.38 |
|
|
|
0.28 |
|
| June 2015 |
|
|
0.30 |
|
|
|
0.23 |
|
| July 2015 |
|
|
0.27 |
|
|
|
0.20 |
|
| August 2015 |
|
|
0.21 |
|
|
|
0.15 |
|
| September 2015 (through to September 11, 2015) |
|
|
0.15 |
|
|
|
0.13 |
|
13
NASDAQ Capital Market
The ADSs are traded on the NASDAQ Capital Market under the symbol “NVGN.” The following table sets forth, for the periods
indicated, the high ask and low bid prices of the ADSs on the NASDAQ Capital Market:
|
|
|
|
|
|
|
|
|
| |
|
Per ADS (US$)* |
|
| |
|
High |
|
|
Low |
|
| Fiscal Year Ended June 30, |
|
|
|
|
|
|
|
|
| 2010 |
|
|
26.20 |
|
|
|
2.85 |
|
| 2011 |
|
|
14.85 |
|
|
|
2.05 |
|
| 2012 |
|
|
8.25 |
|
|
|
1.95 |
|
| 2013 |
|
|
10.49 |
|
|
|
0.76 |
|
| 2014 |
|
|
6.84 |
|
|
|
3.42 |
|
| 2015 |
|
|
5.20 |
|
|
|
2.50 |
|
|
|
|
| Fiscal Year Ended June 30, 2013: |
|
|
|
|
|
|
|
|
| First Quarter |
|
|
2.44 |
|
|
|
1.81 |
|
| Second Quarter |
|
|
6.14 |
|
|
|
0.76 |
|
| Third Quarter |
|
|
10.49 |
|
|
|
1.75 |
|
| Fourth Quarter |
|
|
6.92 |
|
|
|
3.57 |
|
|
|
|
| Fiscal Year Ended June 30, 2014: |
|
|
|
|
|
|
|
|
| First Quarter |
|
|
5.18 |
|
|
|
3.50 |
|
| Second Quarter |
|
|
6.84 |
|
|
|
3.73 |
|
| Third Quarter |
|
|
5.70 |
|
|
|
3.85 |
|
| Fourth Quarter |
|
|
4.16 |
|
|
|
3.42 |
|
|
|
|
| Fiscal Year Ended June 30, 2015: |
|
|
|
|
|
|
|
|
| First Quarter |
|
|
3.62 |
|
|
|
2.74 |
|
| Second Quarter |
|
|
5.38 |
|
|
|
1.64 |
|
| Third Quarter |
|
|
4.33 |
|
|
|
2.09 |
|
| Fourth Quarter |
|
|
9.50 |
|
|
|
3.41 |
|
|
|
|
| Fiscal Year Ended June 30, 2016: |
|
|
|
|
|
|
|
|
| First Quarter (through to September 11, 2015) |
|
|
5.20 |
|
|
|
2.50 |
|
|
|
|
| Month Ended: |
|
|
|
|
|
|
|
|
| July 2014 |
|
|
3.23 |
|
|
|
3.09 |
|
| August 2014 |
|
|
3.60 |
|
|
|
2.72 |
|
| September 2014 |
|
|
3.46 |
|
|
|
2.70 |
|
| October 2014 |
|
|
3.05 |
|
|
|
2.76 |
|
| November 2014 |
|
|
2.98 |
|
|
|
1.65 |
|
| December 2014 |
|
|
5.38 |
|
|
|
1.64 |
|
| January 2015 |
|
|
2.65 |
|
|
|
2.09 |
|
| February 2015 |
|
|
4.07 |
|
|
|
2.52 |
|
| March 2015 |
|
|
4.33 |
|
|
|
2.50 |
|
| April 2015 |
|
|
9.50 |
|
|
|
4.05 |
|
| May 2015 |
|
|
7.66 |
|
|
|
5.50 |
|
| June 2015 |
|
|
5.82 |
|
|
|
3.86 |
|
| July 2015 |
|
|
5.20 |
|
|
|
3.41 |
|
| August 2015 |
|
|
3.49 |
|
|
|
2.50 |
|
| September 2015 (Through to September 11, 2015) |
|
|
2.58 |
|
|
|
2.70 |
|
| * |
Note that we effected a change to the ADS ratio on January 3, 2012. The ratio changed from each ADS representing 5 ordinary shares to each ADS representing 25 ordinary shares. All of the ADR prices presented above
have been adjusted to be comparative to the current ratio. |
14
USE OF PROCEEDS
We will not receive proceeds from any sales by the Selling Shareholders of the ADSs. The Selling Shareholders will receive the proceeds from
such sale of shares.
DESCRIPTION OF ORDINARY SHARES AND AMERICAN DEPOSITARY SHARES
ORDINARY SHARES
The following is a
summary description of our ordinary shares under our Constitution.
Rights Attached to Ordinary Shares
Holders of ordinary shares are entitled to attend and vote at general meetings of the Company. They are entitled to participate equally in the
payment of dividends and share distributions and, in the event of our liquidation, in the distribution of assets after satisfaction of liabilities to creditors. No preferred shares are currently issued. All outstanding ordinary shares are validly
issued and fully paid.
We are not required to issue stock certificates.
Transfer of Shares
Fully-paid
ordinary shares may be freely transferred under our Constitution unless the transfer is restricted or prohibited by another instrument or applicable securities laws.
Dividend and Liquidation Rights
We may declare a dividend to be paid to the holders of ordinary shares according to their rights and interests in our profits. In the event of
our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of ordinary shares in proportion to the number of shares held.
This right may be affected by the grant of preferential dividend or distribution rights, to the holders of a class of shares with
preferential rights that may be authorized in the future. Under the Australian Companies Law, the declaration of a dividend does not require the approval of the shareholders of the Company, unless the Company’s Constitution requires otherwise.
Our Constitution provides that the Board of Directors may declare and distribute dividends without the approval of the shareholders.
Voting
Rights
Our ordinary shares do not have cumulative voting rights in the election of directors. As a result, the holders of
ordinary shares that represent more than 50% of the voting power represented at a shareholders meeting at which a quorum is present have the power to elect all of our directors.
Holders of ordinary shares have one vote each on all matters submitted to a vote of shareholders on a show of hands and one vote for each
ordinary share held on all matters submitted to a vote of shareholders on a poll. Shareholders may vote in person or by proxy. These voting rights may be affected by the grant of any special voting rights to the holders of a class of shares with
preferential rights that may be authorized in the future.
Under the Australian Companies Law, unless otherwise provided in the
Constitution or by applicable law, all resolutions of the shareholders require a simple majority.
Voting by Proxy and in Other Manners
Our Constitution enables a shareholder to appoint a proxy, who need not be a shareholder, to vote at any shareholders meeting. We require that
the appointment of a proxy be in writing signed by the person making the appointment or by an attorney authorized for this purpose, and if the person making the appointment is a corporation, by a person or persons authorized to bind the corporation.
In the document appointing a proxy, each shareholder may specify how the proxy should vote on any matter presented at a shareholders meeting. The document appointing the proxy shall be deposited in our offices or at such other address as shall be
specified in the notice of the meeting not less than 48 hours before the time of the meeting at which the person specified in the appointment is due to vote.
15
Anti-Takeover Provisions under the Australian Corporations Law
Whilst the takeover of listed companies is regulated under the Corporations Act, there are no specific anti-takeover provisions in the
Corporations Act.
However, we are able by shareholder resolution (special resolution requiring at least a 75% of votes of voting
shareholders at the meeting personally or by proxy) to require a shareholder resolution (by simple majority) to be passed as a condition precedent to completion of a proportional takeover offer of the Company’s shares.
Rights of Shareholders
Under
our Constitution, our directors have the right to determine whether and to what extent and under what conditions our accounting records and other documents and records are open to the inspection of our shareholders. No shareholder has any right of
inspection except as provided by law, as authorized by our directors or as authorized by our shareholders at a general meeting. Pursuant to the Australia Corporations Act 2001, on application by a member of a company, an Australian court may make an
order (i) authorizing the applicant to inspect books of the Company; or (ii) authorizing another person (whether a member or not) to inspect books of the Company on the applicant’s behalf. The court may only make the order if it is
satisfied that the applicant is acting in good faith and that the inspection is to be made for a proper purpose. A person authorized to inspect books may make copies of the books unless the Court orders otherwise.
Enforceability of Civil Liabilities
We are incorporated in Australia. Two of our directors reside in the United States. The other four directors and all our officers and the
Australian experts named in this registration statement reside outside the United States. Service of process upon them may be difficult to effect within the US. Furthermore, because most of our assets, and those of our non-US directors and officers
and the Australian experts named herein, are located outside the United States, any judgment obtained in the United States against us or any of these persons may not be collectible within the United States.
We have been informed by our legal counsel in Australia, Addisons, that there is doubt as to the enforceability in Australia of civil
liabilities under the Securities Act or the Exchange Act. There is no statutory recognition in Australia of judgments obtained in the United States, although the courts of Australia will generally recognize and enforce a non-penal judgment of a
foreign court of competent jurisdiction without retrial on the merits.
Subject to particular time limitations, executory judgments of a
US court for monetary damages in civil matters may be enforced by an Australian court, provided that:
| |
• |
|
the judgment was obtained not more than 12 years ago and was after due process before a court of competent jurisdiction, that recognizes and enforces similar judgments of Australian courts, and the court had authority
according to the rules of private international law currently prevailing in Australia; |
| |
• |
|
adequate service of process was effected and the defendant had a reasonable opportunity to be heard; |
| |
• |
|
the judgment is not contrary to the law, public policy, security or sovereignty of Australia and its enforcement is not contrary to the laws governing enforcement of judgments; |
| |
• |
|
the judgment was not obtained by fraud and does not conflict with any other valid judgment in the same matter between the same parties; |
| |
• |
|
if the judgment is against a party is it for a fixed or readily calculable sum |
| |
• |
|
the judgment is final and conclusive and no longer appealable; and |
| |
• |
|
an action between the same parties in the same matter is not pending in any Australian court at the time the lawsuit is instituted in the foreign court the parties to the judgment and in the application to register the
judgment are identical. |
Foreign judgments enforced by Australian courts generally will be payable in Australian dollars.
The usual practice in an action before an Australian court to recover an amount in a non-Australian currency is for the Australian court to render judgment for the equivalent amount in Australian currency at the rate of exchange in force on the date
of the judgment. Current Australian exchange control regulations also permit a judgment debtor to make payment in foreign currency. Pending collection, judgment creditors must bear the risk of unfavorable exchange rates.
16
AMERICAN DEPOSITARY SHARES
The Bank of New York Mellon, as depositary, will register and deliver American Depositary Shares, also referred to as ADSs. Each ADS will
represent twenty-five ordinary shares (or a right to receive twenty-five ordinary shares) deposited with a custodian for the depositary. Each ADS will also represent any other securities, cash or other property which may be held by the depositary in
Australia. The depositary’s office at which the ADSs will be administered is located at 101 Barclay Street, New York, New York 10286. The Bank of New York Mellon’s principal executive office is located at One Wall Street, New York, New
York 10286.
You may hold ADSs either (A) directly (i) by having an American Depositary Receipt, also referred to as an ADR,
which is a certificate evidencing a specific number of ADSs, registered in your name, or (ii) by having ADSs registered in your name in the Direct Registration System, or (B) indirectly by holding a security entitlement in ADSs through
your broker or other financial institution. If you hold ADSs directly, you are a registered ADS holder, also referred to as an ADS holder. This description assumes you are an ADS holder. If you hold the ADSs indirectly, you must rely on the
procedures of your broker or other financial institution to assert the rights of ADS holders described in this section. You should consult with your broker or financial institution to find out what those procedures are.
The Direct Registration System, also referred to as DRS, is a system administered by The Depository Trust Company, also referred to as DTC,
under which the depositary may register the ownership of uncertificated ADSs, which ownership is confirmed by statements sent by the depositary to the registered holders of uncertificated ADSs.
As an ADS holder, we will not treat you as one of our shareholders and you will not have shareholder rights. Australian law governs
shareholder rights. The depositary will be the holder of the shares underlying your ADSs. As a registered holder of ADSs, you will have ADS holder rights. A deposit agreement among us, the depositary, ADS holders and all other persons beneficially
owning ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs.
The following is a summary of the material provisions of the deposit agreement. For more complete information, you should read the entire
deposit agreement and the form of ADR. Directions on how to obtain copies of those documents are provided on page 29.
Dividends and Other
Distributions
How will you receive dividends and other distributions on the shares?
The depositary has agreed to pay to ADS holders the cash dividends or other distributions it or the custodian receives on ordinary shares or
other deposited securities, after deducting its fees and expenses. You will receive these distributions in proportion to the number of ordinary shares your ADSs represent.
|
|
|
| Cash. |
|
The depositary will convert any cash dividend or other cash distribution we pay on the ordinary shares into U.S. dollars, if it can do so on a reasonable basis and can transfer the U.S. dollars to the United States. If that is not
possible or if any government approval is needed and cannot be obtained, the deposit agreement allows the depositary to distribute the foreign currency only to those ADS holders to whom it is possible to do so. It will hold the foreign currency it
cannot convert for the account of the ADS holders who have not been paid. It will not invest the foreign currency and it will not be liable for any interest. |
|
|
|
|
Before making a distribution, any withholding taxes, or other governmental charges that must be paid will be deducted. The depositary will distribute only whole U.S. dollars and cents and will round fractional cents to the nearest
whole cent. If the exchange rates fluctuate during a time when the depositary cannot convert the foreign currency, you may lose some or all of the value of the distribution. |
17
|
|
|
| Shares. |
|
The depositary may distribute additional ADSs representing any ordinary shares we distribute as a dividend or free distribution. The depositary will only distribute whole ADSs. It will sell ordinary shares which would require it to
deliver a fraction of an ADS (or ADSs representing those shares) and distribute the net proceeds in the same way as it does with cash. If the depositary does not distribute additional ADSs, the outstanding ADSs will also represent the new ordinary
shares. |
|
| Rights to Purchase Additional |
| Shares. |
|
If we offer holders of our securities any rights to subscribe for additional shares or any other rights, the depositary may make these rights
available to ADS holders. If the depositary decides it is not legal and practical to make the rights available but that it is practical to sell the rights, the depositary will use reasonable efforts to sell the rights and distribute the proceeds in
the same way as it does with cash. The depositary will allow rights that are not distributed or sold to lapse. In that case, you will receive no value for them.
If the depositary makes rights available to ADS holders, it will exercise the rights and purchase the shares on your behalf. The depositary will then deposit
the shares and deliver ADSs to the persons entitled to them. It will only exercise rights if you pay it the exercise price and any other charges the rights require you to pay.
U.S. securities laws may restrict transfers and cancellation of the ADSs represented by ordinary shares purchased upon exercise of rights. For example, you may
not be able to trade these ADSs freely in the United States. In this case, the depositary may deliver restricted depositary shares that have the same terms as the ADSs described in this section except for changes needed to put the necessary
restrictions in place. |
|
|
| Other |
|
|
| Distributions. |
|
The depositary will send to ADS holders anything else we distribute on deposited securities by any means it thinks is legal, fair and
practical. If it cannot make the distribution in that way, the depositary has a choice. It may decide to sell what we distributed and distribute the net proceeds, in the same way as it does with cash. Or, it may decide to hold what we distributed,
in which case ADSs will also represent the newly distributed property. However, the depositary is not required to distribute any securities (other than ADSs) to ADS holders unless it receives satisfactory evidence from us that it is legal to make
that distribution. The depositary is not responsible if it decides that it is unlawful
or impractical to make a distribution available to any ADS holders. We have no obligation to register ADSs, shares, rights or other securities under the Securities Act. We also have no obligation to take any other action to permit the
distribution of ADSs, shares, rights or anything else to ADS holders. This means that you may not receive the distributions we make on our shares or any value for them if it is illegal or impractical for us to make them available to
you. |
Deposit, Withdrawal and Cancellation
How are ADSs issued?
The
depositary will deliver ADSs if you or your broker deposits shares or evidence of rights to receive ordinary shares with the custodian. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or
fees, the depositary will register the appropriate number of ADSs in the names you request and will deliver the ADSs to or upon the order of the person or persons that made the deposit.
How can ADS holders withdraw the deposited securities?
You may surrender your ADSs at the depositary’s office. Upon payment of its fees and expenses and of any taxes or charges, such as stamp
taxes or stock transfer taxes or fees, the depositary will deliver the ordinary shares and any other deposited securities underlying the ADSs to the ADS holder or a person the ADS holder designates at the office of the custodian. Or, at your
request, risk and expense, the depositary will deliver the deposited securities at its office, if feasible.
18
How do ADS holders interchange between certificated ADSs and uncertificated ADSs?
You may surrender your ADR to the depositary for the purpose of exchanging your ADR for uncertificated ADSs. The depositary will cancel that
ADR and will send to the ADS holder a statement confirming that the ADS holder is the registered holder of uncertificated ADSs. Alternatively, upon receipt by the depositary of a proper instruction from a registered holder of uncertificated ADSs
requesting the exchange of uncertificated ADSs for certificated ADSs, the depositary will execute and deliver to the ADS holder an ADR evidencing those ADSs.
Voting Rights
How do you vote?
ADS holders may instruct the depositary how to vote the number of deposited shares their ADSs represent. Otherwise, you won’t
be able to exercise your right to vote unless you withdraw the shares. However, you may not know about the meeting enough in advance to withdraw the shares.
The depositary will notify ADS holders of shareholders’ meetings and arrange to deliver our voting materials to them if we ask it to.
Those materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary to vote. For instructions to be valid, they must reach the depositary by a date set by the depositary.
The depositary will try, as far as practical, subject to the laws of Australia and of our Constitution or similar documents, to vote or to
have its agents vote the deposited ordinary shares or other deposited securities as instructed by ADS holders. The depositary will only vote or attempt to vote as instructed.
We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your shares.
In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that you may not be able to exercise your right to vote and there may be
nothing you can do if your ordinary shares are not voted as you requested.
Fees and Expenses
|
|
|
| Persons depositing or
withdrawing shares or ADS holders must pay: |
|
For: |
| $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) |
|
Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property
Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement
terminates |
|
|
| $.02 (or less) per ADS |
|
Any cash distribution to ADS holders |
|
|
| A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs |
|
Distribution of securities distributed to holders of deposited securities which are distributed by the depositary to ADS holders |
|
|
| $.02 (or less) per ADS per calendar year |
|
Depositary services |
|
|
| Registration or transfer fees |
|
Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares |
|
|
| Expenses of the depositary |
|
Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement)
Converting foreign currency to U.S. dollars |
|
|
| Taxes and other governmental charges the depositary or the custodian has to pay on any ADSs or shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes |
|
As necessary |
|
|
| Any charges incurred by the depositary or its agents for servicing the deposited securities |
|
As necessary |
19
The depositary collects its fees for delivery and surrender of ADSs directly from investors
depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed. The depositary may
collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may generally refuse to provide
fee-attracting services until its fees for those services are paid.
From time to time, the depositary may make payments to us to
reimburse and/or share revenue from the fees collected from ADS holders, or waive fees and expenses for services provided, generally relating to costs and expenses arising out of establishment and maintenance of the ADS program. In performing its
duties under the deposit agreement, the depositary may use brokers, dealers, foreign currency dealers or other service providers owned by or that are affiliates of the depositary and that may earn or share fees, spreads or commissions.
The depositary may convert foreign currency itself or through any of its affiliates and, in those cases, acts as principal for its own
account and not as an agent, fiduciary or broker on behalf of any other person and earns revenue, including, without limitation, fees and spreads that I will retain for its own account. The spread is the difference between the exchange rate assigned
to the currency conversion made under the deposit agreement and the rate that the depositary or its affiliate receives in an offsetting foreign currency trade. The depositary makes no representation that the exchange rate used or obtained in any
currency conversion under the deposit agreement will be the most favorable rate that could be obtained at the time or as to the method by which that rate will be determined, subject to its obligations under the deposit agreement.
Payment of Taxes
You will be
responsible for any taxes or other governmental charges payable on your ADSs or on the deposited securities represented by any of your ADSs. The depositary may refuse to register any transfer of your ADSs or allow you to withdraw the deposited
securities represented by your ADSs until such taxes or other charges are paid. It may apply payments owed to you or sell deposited securities represented by your American Depositary Shares to pay any taxes owed and you will remain liable for any
deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number of ADSs to reflect the sale and pay to ADS holders any proceeds, or send to ADS holders any property, remaining after it has paid the taxes.
Reclassifications, Recapitalizations and Mergers
|
|
|
| If we: |
|
Then: |
| • Reclassify, split up or consolidate any of the
deposited securities
• Distribute securities on the shares that are not distributed to
you
• Recapitalize, reorganize, merge, liquidate, sell all or substantially
all of our assets, or take any similar action |
|
The cash, shares or other securities received by the depositary will become deposited securities. Each ADS will automatically represent
its equal share of the new deposited securities. The depositary may distribute new
ADSs representing the new deposited securities or ask you to surrender your outstanding ADRs in exchange for new ADRs identifying the new deposited securities. |
20
Amendment and Termination
How may the deposit agreement be amended?
We may agree with the depositary to amend the deposit agreement and the ADRs without your consent for any reason. If an amendment adds or
increases fees or charges, except for taxes and other governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery charges or similar items, or prejudices a substantial right of ADS holders, it will not become
effective for outstanding ADSs until 30 days after the depositary notifies ADS holders of the amendment. At the time an amendment becomes effective, you are considered, by continuing to hold your ADSs, to agree to the amendment and to be bound by
the ADRs and the deposit agreement as amended.
How may the deposit agreement be terminated?
The depositary will terminate the deposit agreement at our direction or if the depositary resigns or is removed by mailing notice of
termination to the ADS holders then outstanding at least 30 days prior to the date fixed in such notice for such termination.
After
termination, the depositary and its agents will do the following under the deposit agreement but nothing else: collect distributions on the deposited securities, sell rights and other property, and deliver shares and other deposited securities upon
cancellation of ADSs. One year after termination, the depositary may sell any remaining deposited securities by public or private sale. After that, the depositary will hold the money it received on the sale, as well as any other cash it is holding
under the deposit agreement for the pro rata benefit of the ADS holders that have not surrendered their ADSs. It will not invest the money and has no liability for interest. The depositary’s only obligations will be to account for the
money and other cash. After termination our only obligations will be to indemnify the depositary and to pay fees and expenses of the depositary that we agreed to pay.
Limitations on Obligations and Liability
Limits on
our Obligations and the Obligations of the Depositary; Limits on Liability to Holders of ADSs
The deposit agreement expressly
limits our obligations and the obligations of the depositary. It also limits our liability and the liability of the depositary. We and the depositary:
| • |
|
are only obligated to take the actions specifically set forth in the deposit agreement without negligence or bad faith; |
| • |
|
are not liable if we are or it is prevented or delayed by law or circumstances beyond our or its control from performing our or its obligations under the deposit agreement; |
| • |
|
are not liable if we or it exercises discretion permitted under the deposit agreement; |
| • |
|
are not liable for the inability of any holder of ADSs to benefit from any distribution on deposited securities that is not made available to holders of ADSs under the terms of the deposit agreement, or for any special,
consequential or punitive damages for any breach of the terms of the deposit agreement; |
| • |
|
have no obligation to become involved in a lawsuit or other proceeding related to the ADSs or the deposit agreement on your behalf or on behalf of any other person; and |
| • |
|
may rely upon any documents we believe or it believes in good faith to be genuine and to have been signed or presented by the proper person. |
In the deposit agreement, we and the depositary agree to indemnify each other under certain circumstances.
Requirements for Depositary Actions
Before the depositary will deliver or register a transfer of ADSs, make a distribution on ADSs, or permit withdrawal of shares, the depositary
may require:
| |
• |
|
payment of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for the transfer of any shares or other deposited securities; |
21
| |
• |
|
satisfactory proof of the identity and genuineness of any signature or other information it deems necessary; and |
| |
• |
|
compliance with regulations it may establish, from time to time, consistent with the deposit agreement, including presentation of transfer documents. |
The depositary may refuse to deliver ADSs or register transfers of ADSs when the transfer books of the depositary or our transfer books are
closed or at any time if the depositary or we think it advisable to do so.
Your Right to Receive the Shares Underlying your ADSs
ADS holders have the right to cancel their ADSs and withdraw the underlying shares at any time except:
| |
• |
|
when temporary delays arise because: (i) the depositary has closed its transfer books or we have closed our transfer books; (ii) the transfer of shares is blocked to permit voting at a shareholders’
meeting; or (iii) we are paying a dividend on our shares; |
| |
• |
|
when you owe money to pay fees, taxes and similar charges; or |
| |
• |
|
when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of shares or other deposited securities. |
This right of withdrawal may not be limited by any other provision of the deposit agreement.
Pre-release of ADSs
The deposit
agreement permits the depositary to deliver ADSs before deposit of the underlying ordinary shares. This is called a pre-release of the ADSs. The depositary may also deliver shares upon cancellation of pre-released ADSs (even if the ADSs are canceled
before the pre-release transaction has been closed out). A pre-release is closed out as soon as the underlying shares are delivered to the depositary. The depositary may receive ADSs instead of ordinary shares to close out a pre-release. The
depositary may pre-release ADSs only under the following conditions: (1) before or at the time of the pre-release, the person to whom the pre-release is being made represents to the depositary in writing that it or its customer owns the
ordinary shares or ADSs to be deposited; (2) the pre-release is fully collateralized with cash or other collateral that the depositary considers appropriate; and (3) the depositary must be able to close out the pre-release on not more than
five business days’ notice. In addition, the depositary will limit the number of ADSs that may be outstanding at any time as a result of pre-release, although the depositary may disregard the limit from time to time if it thinks it is
appropriate to do so.
Shareholder communications; inspection of register of holders of ADSs
The depositary will make available for your inspection at its office all communications that it receives from us as a holder of deposited
securities that we make generally available to holders of deposited securities. The depositary will send you copies of those communications if we ask it to. You have a right to inspect the register of holders of ADSs, but not for the purpose of
contacting those holders about a matter unrelated to our business or the ADSs.
SELLING SHAREHOLDERS
The ADSs being offered by the Selling Shareholders represent ordinary shares that are issuable to the Selling Shareholders upon exercise of
the Placement Options. For additional information regarding the issuance of the ordinary shares, see “The Offering and Placement of Ordinary Shares and Options” above. We are registering, in accordance with the Placement Letter, the
ordinary shares issuable upon exercise of the Placement Options in order to permit the Selling Shareholders to offer ordinary shares in the form of ADSs for resale from time to time.
The Short-term Options are exercisable at an initial exercise price of A$0.30 per ordinary share, and the Long-term Options are exercisable
at an initial exercise price of A$0.40 per ordinary share. The shares issuable upon exercise of the Placement Options will rank pari passu with our then issued and fully paid ordinary shares.
22
The Placement Letter contains customary representations and warranties and indemnification
provisions. We have agreed with each Selling Shareholder that we will not for a period of ninety (90) days from the day of such Selling Shareholder’s Placement Letter issue any additional ordinary shares or options to purchase ordinary
shares, other than pursuant to the rights offering we completed on June 2, 2015, which is discussed below (the “Rights Offering”).
The foregoing descriptions of the Placement Letter and the Placement Options do not purport to be a complete description of the terms of the
documents, and are qualified in their entirety by the terms of the definitive documents or forms thereof which have been filed with the SEC.
Except for the purchase of the Placement Options and the ordinary shares issued pursuant to the Placement Letter and their rights under the
Placement Letter, the previous dealings with Hudson Bay Master Fund Limited (“HBMF”) discussed below, the previous private placement by our Company of ordinary shares and options pursuant to placement confirmation letters dated
December 16 and 18, 2014 (“December PIPE”) discussed below, and the Rights Offering, which is also discussed below, none of the Selling Shareholders has had any material relationship with us within the past three years.
On July 3, 2013 we entered into a Convertible Securities Agreement with HBMF pursuant to which HBMF agreed to invest, at the
Company’s option, up to an aggregate amount of A$5,000,000 in return for us issuing HBMF up to five convertible securities having an aggregate face value of up to A$5,500,000 (the “Convertible Securities Agreement”). The
Convertible Securities Agreement was amended on November 15, 2013 to among other things increase the total amount which HBMF agreed to invest to A$8,000,000. Under the Convertible Securities Agreement, we also issued to HBMF an option to
purchase 4,000,000 ordinary shares (the “2013 HBMF Option”), at an option exercise price of A$0.237 per ordinary share and having an expiration date of July 4, 2016. On July 4, 2013, we also issued 822,369 ordinary shares
to HBMF in satisfaction of our obligation to pay a commencement fee under the Convertible Securities Agreement. Between July 3, 2013 and February 10, 2015, we issued four convertible promissory notes to HBMF having an aggregate face value
of A$6,050,000. HBMF or its nominees have converted all of these convertible promissory notes and were issued a further 47,173,141 ordinary shares. On January 16, 2015, we terminated the Convertible Securities Agreement, therefore no further
Notes will be issued under the Convertible Securities Agreement.
In the December PIPE, we issued 46,900,800 ordinary shares at a
purchase price of A$0.125 per share and 50,652,864 options, with an initial exercise price of A$0.15 per option (the “December PIPE Options”). The table below names the Selling Shareholders that participated in the December PIPE and
the number of ordinary shares and December PIPE Options that each of them purchased:
|
|
|
|
|
|
|
|
|
| Selling Shareholder |
|
Shares issued |
|
|
Options issued |
|
| Anson Investments Master Fund LP |
|
|
19,000,800 |
|
|
|
19,000,800 |
|
| Hudson Bay Master Fund Ltd. |
|
|
14,950,000 |
|
|
|
14,950,000 |
|
| Iroquois Master Fund Ltd. |
|
|
12,950,000 |
|
|
|
12,950,000 |
|
As consideration for the issue of ordinary shares, we received gross proceeds of approximately A$5,850,000.
As of June 30, 2015, we have received approximately A$6,800,000 from the proceeds of the exercise of the December PIPE Options.
On April 21, 2015, we also announced a non-renounceable rights issue offering pursuant to which shareholders of our Company located in
Australia and New Zealand who held shares as of May 1, 2015 (the “Record Date”) could purchase one ordinary share for every six ordinary shares held as of the Record Date at a purchase price of A$0.30 per share. Participants
also received, for no additional consideration, for every ordinary share purchased in the Rights Offering one option, with an initial exercise price of A0.30 per option and an expiration date of December 4, 2015, and one-half of an option, with
an initial exercise price of A$0.40 per option and an expiration date of June 4, 2020 (collectively, the “Rights Offering Options”). In the Rights Offering, we issued 58,971,207 ordinary shares and 88,456,810 Rights Offering
Options. As consideration for the issue of ordinary shares in the Rights Offering, we received gross proceeds of approximately A$17,691,000. The table below names the Selling Shareholders that participated in the Rights Offering and the number of
ordinary shares and Rights Offering Options that each of them purchased:
|
|
|
|
|
|
|
|
|
| Selling Shareholder |
|
Shares issued |
|
|
Options issued |
|
| Anson Investments Master Fund LP |
|
|
5,000,166 |
|
|
|
7,500,249 |
|
| Hudson Bay Master Fund Ltd. |
|
|
800,000 |
|
|
|
1,200,000 |
|
| Iroquois Master Fund Ltd. |
|
|
4,319,822 |
|
|
|
6,479,733 |
|
| Empery Asset Master, LTD |
|
|
1,299,086 |
|
|
|
1,948,629 |
|
| Empery Tax Efficient, LP |
|
|
1,123,618 |
|
|
|
1,685,427 |
|
| Empery Tax Efficient II, LP |
|
|
1,577,269 |
|
|
|
2,365,917 |
|
23
The table below names the Selling Shareholders and sets forth certain other information
regarding the beneficial ownership of our ordinary shares by the Selling Shareholders. The second column lists the number of securities, including ordinary shares and December PIPE Options, Rights Offering Options and the Placement Options
(collectively, “Options”), held by each Selling Shareholder, prior to the offering, as of September 16, 2015, assuming the full exercise of the Options held by such Selling Shareholder. The third column lists the ordinary
shares being offered by this prospectus, assuming the full exercise of the Options held by such Selling Shareholder. The fourth column shows the ordinary shares beneficially owned by each Selling Shareholder upon completion of the offering being
made by this prospectus, assuming the full exercise of the Options held by such Selling Shareholder. Because a Selling Shareholder may offer all or less than all of our ordinary shares covered by this prospectus, we cannot estimate the amount of our
ordinary shares that will be held by each Selling Shareholder upon completion of the offering, and the fourth column therefore assumes the sale of all such ordinary shares. For information on the procedure for sales by the Selling Shareholders,
please read the disclosure set forth under the heading “Plan of Distribution.”
The following ordinary shares may be sold
by a Selling Shareholder or by transferees, donees, pledgees or other successors-in-interest received after the date of this prospectus. Information concerning the Selling Shareholders may change from time to time. This prospectus may be
supplemented or amended from time to time as appropriate to update the information set forth below and to identify any additional selling shareholder who may offer ordinary shares hereunder.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Shares Beneficially Owned
Prior to Offering (1) |
|
|
Shares
Being
Offered
Hereby |
|
|
Shares Beneficially Owned
After Offering (1) |
|
| Selling Shareholder |
|
Number |
|
|
Percent |
|
|
|
Number |
|
|
Percent |
|
| Anson Investments Master Fund LP |
|
|
24,000,976 |
(2) |
|
|
5.66 |
% |
|
|
16,500,000 |
|
|
|
12,411,612 |
|
|
|
2.88 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Hudson Bay Master Fund Ltd. |
|
|
17,700,000 |
(3) |
|
|
4.17 |
% |
|
|
16,500,000 |
|
|
|
1,200,000 |
|
|
|
0.28 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Iroquois Master Fund Ltd. |
|
|
21,579,733 |
(4) |
|
|
5.09 |
% |
|
|
15,000,000 |
|
|
|
6,579,733 |
|
|
|
1.76 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Iroquois Capital Investment Group LLC |
|
|
1,500,000 |
|
|
|
0.35 |
% |
|
|
1,500,000 |
|
|
|
0 |
|
|
|
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sabby Healthcare Master Fund Ltd |
|
|
5,945,730 |
|
|
|
1.40 |
% |
|
|
5,500,001 |
|
|
|
445,729 |
|
|
|
0.11 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sabby Volatility Warrant Master Fund Ltd |
|
|
5,945,730 |
|
|
|
1.40 |
% |
|
|
5,500,001 |
|
|
|
445,729 |
|
|
|
0.11 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Empery Asset Master Ltd |
|
|
5,521,089 |
(5) |
|
|
1.30 |
% |
|
|
3,572,460 |
|
|
|
1,948,629 |
|
|
|
0.46 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Empery Tax Efficient LP |
|
|
4,775,391 |
(6) |
|
|
1.13 |
% |
|
|
3,089,964 |
|
|
|
1,685,427 |
|
|
|
0.40 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Empery Tax Efficient II LP |
|
|
6,703,494 |
(7) |
|
|
1.58 |
% |
|
|
4,337,576 |
|
|
|
2,365,918 |
|
|
|
0.56 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Pinz Capital International LP |
|
|
6,124,998 |
|
|
|
1.44 |
% |
|
|
6,124,998 |
|
|
|
0 |
|
|
|
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
|
|
99,797,141 |
|
|
|
23.77 |
% |
|
|
77,625,000 |
|
|
|
27,082,777 |
|
|
|
6.39 |
% |
24
| (1) |
Beneficial ownership is determined in accordance with the rules of the SEC. Percentage ownership is based on 424,117,465 ordinary shares outstanding as of September 16, 2015. |
| (2) |
Comprised of (i) 16,500,000 ordinary shares issuable upon exercise of the Placement Options and (ii) 7,500,249 ordinary shares issuable upon exercise of Rights Offering Options and (iii) 1,000 ordinary
shares acquired in the open market. |
| (3) |
Comprised of (i) 16,500,000 ordinary shares issuable upon exercise of the Placement Options and (ii) 1,200,000 ordinary shares issuable upon exercise of Rights Offering Options. |
| (4) |
Comprised of (i) 15,000,000 ordinary share issuable upon exercise of the Placement Options and (ii) 100,000 ordinary shares acquired in the Right Offering and 6,479,733 ordinary shares issuable upon exercise
of Rights Offering Options. |
| (5) |
Comprised of (i) 3,572,460 ordinary shares issuable upon exercise of the Placement Options and (ii) 1,948,629 ordinary shares issuable upon exercise of Rights Offering Options. |
| (6) |
Comprised of (i) 3,089,964 ordinary shares issuable upon exercise of the Placement Options and (ii) 1,685,427 ordinary shares issuable upon exercise of Rights Offering Options. |
| (7) |
Comprised of (i) 4,337,577 ordinary shares issuable upon exercise of the Placement Options and (ii) 2,365,918 ordinary shares issuable upon exercise of Rights Offering Options. |
PLAN OF DISTRIBUTION
We are registering the ordinary shares that are issuable to all the Selling Shareholders upon exercise of the Options, to permit the Selling
Shareholders to sell these ordinary shares in the form of ADSs from time to time after the date of this prospectus. We will not receive any of the proceeds from the sale by the Selling Shareholders of the ADSs. We will bear all fees and expenses
incident to our obligation to register the ordinary shares.
Each Selling Shareholder may sell all or a portion of the ADSs beneficially
owned by it and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If the ADSs are sold through underwriters or broker-dealers, the Selling Shareholder will be responsible for underwriting
discounts or commissions or agent’s commissions. The ADSs may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices.
These sales may be effected in transactions, which may involve crosses or block transactions, pursuant to one or more of the following methods:
| |
• |
|
on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale; |
| |
• |
|
in the over-the-counter market; |
| |
• |
|
in transactions otherwise than on these exchanges or systems or in the over-the-counter market; |
| |
• |
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| |
• |
|
block trades in which the broker-dealer will attempt to sell the ADSs as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
25
| |
• |
|
purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| |
• |
|
an exchange distribution in accordance with the rules of the applicable exchange; |
| |
• |
|
privately negotiated transactions; |
| |
• |
|
sales pursuant to Rule 144; |
| |
• |
|
broker-dealers may agree with the Selling Shareholder to sell a specified number of such ADSs at a stipulated price per ADS; |
| |
• |
|
a combination of any such methods of sale; and |
| |
• |
|
any other method permitted pursuant to applicable law. |
If a Selling Shareholder effects such
transactions by selling ADSs to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from such Selling Shareholder or commissions
from purchasers of the ADSs for whom they may act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of
transactions involved). In connection with sales of the ADSs or otherwise, a Selling Shareholder may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of the ADSs in the course of hedging in positions they
assume. A Selling Shareholder may also lend or pledge ADSs to broker-dealers that in turn may sell such ADSs.
A Selling Shareholder may
pledge or grant a security interest in some or all of the ordinary shares or ADSs owned by it and, if it defaults in the performance of its secured obligations, the pledgees or secured parties may offer and sell the ADSs from time to time pursuant
to this prospectus or any amendment to this prospectus under Rule 424(b)(3) under, or another applicable provision of, the Securities Act, amending, if necessary, the list of Selling Shareholders to include the pledgee, transferee or other
successor-in-interest as a Selling Shareholder under this prospectus. A Selling Shareholder also may transfer the ADSs in other circumstances in which case the transferees, donees, pledgees or other successors-in-interest may be the selling
beneficial owners for purposes of this prospectus and may sell such ADSs from time to time under this prospectus after an amendment or supplement has been filed under Rule 424(b)(3) under, or another applicable provision of, the Securities Act,
amending, if necessary, the list of Selling Shareholders to include the transferees, donees, pledgees or other successors-in-interest as a Selling Shareholder under this prospectus.
The Selling Shareholders and any broker-dealer participating in the distribution of the ADSs may be deemed to be “underwriters”
within the meaning of the Securities Act, and any commission paid, or any discounts or concessions allowed or fees paid to, any such broker-dealer and any profit on the resale of the ADSs sold by them may be deemed to be underwriting commissions or
discounts. In the event any underwriter, dealer or agent who is a member of the Financial Industry Regulatory Authority, or FINRA, participates in the distribution of any securities offered pursuant to this prospectus and any applicable prospectus
supplement, the maximum underwriters’ compensation to be received by such FINRA member will not be greater than eight percent of the gross proceeds from the sale of the ADSs. At the time a particular offering of the ADSs is made, a
prospectus supplement, if required, will be distributed which will set forth the aggregate amount of ADSs being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other
terms constituting compensation from the Selling Shareholder and any discounts, commissions or concessions allowed or reallowed or paid to broker-dealers.
Under the securities laws of some states, the ADSs may be sold in such states only through registered or licensed brokers or dealers. In
addition, in some states the ADSs may not be sold unless such ADSs have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
The Selling Shareholders and any other person participating in such distribution will be subject to applicable provisions of the Exchange
Act, and the rules and regulations thereunder, including, without limitation, Regulation M under the Exchange Act, which may limit the timing of purchases and sales of any of the ADSs by the Selling Shareholders and any other participating person.
(Regulation M does not, however, limit the ability of the Selling Shareholders to convert the Options into ordinary shares.) Regulation M may also restrict the ability of any person engaged in the distribution of the ADSs to engage in market-making
activities with respect to the ADSs. All of the foregoing may affect the marketability of the ADSs and the ability of any person or entity to engage in market-making activities with respect to the ADSs.
We will pay all expenses of the registration of the ordinary
shares pursuant to the Placement Letters, including, without limitation, SEC filing fees and expenses of compliance with state securities or “blue sky” laws; provided, however, that the Selling Shareholders will pay all underwriting
discounts and selling commissions, if any. See “Offering Expenses” on page 26. We will indemnify the Selling Shareholders against liabilities, including some liabilities under the Securities Act, in accordance with the Placement Letters,
or the Selling Shareholders will be entitled to contribution. We may be indemnified by the Selling Shareholders against civil liabilities, including liabilities under the Securities Act, that may arise from any written information furnished to us by
the Selling Shareholders specifically for use in this prospectus, in accordance with the related registration rights agreement, or we may be entitled to contribution.
26
Once sold under the registration statement of which this prospectus forms a part, the ordinary
shares and ADSs representing those ordinary shares will be freely tradable in the hands of persons other than our affiliates.
There can
be no assurance that the Selling Shareholders will sell any or all of the ordinary shares in the form of ADSs registered pursuant to the registration statement of which this prospectus forms a part.
ENFORCEMENT OF CIVIL LIABILITIES
We are incorporated in Australia. Most of our current operations are conducted in Australia, and most of our assets are located in the
Australia. A majority of our directors and officers are nationals or residents of jurisdictions other than the United States and a substantial portion of their assets are located outside of the United States. As a result, it may be difficult for a
shareholder to effect service of process within the United States upon us or such persons, or to enforce against us or them judgments obtained in United States courts, including judgments predicated upon the civil liability provisions of the
securities laws of the United States or any state in the United States.
We have appointed Corporation Trust Company as our agent to
receive service of process with respect to any action brought against us in the United States District Court for the Southern District of New York under the federal securities laws of the United States or of any state in the United States or any
action brought against us in the Supreme Court of the State of New York in the County of New York under the securities laws of the State of New York.
Addisons, our counsel as to Australian law, have advised us that there is uncertainty as to whether the courts of Australia would:
| |
• |
|
recognize or enforce judgments of United States courts obtained against us or our directors or officers predicated upon the civil liability provisions of the securities laws of the United States or any state within the
United States; or |
| |
• |
|
entertain original actions brought in each respective jurisdiction against us or our directors or officers predicated solely upon the securities laws of the United States or any state within the United States.
|
Addisons has further advised us that a final and conclusive judgment in the federal or state courts of the United States
under which a sum of money is payable, other than a sum payable in respect of taxes, fines, penalties or similar charges, may be subject to enforcement proceedings as debt in the courts of Australia under the common law. Civil liability provisions
of the U.S. federal and state securities law permit punitive damages against us; however, according to Addisons, Australian courts would not recognize or enforce judgments against us to the extent the judgment is punitive or penal. It is uncertain
as to whether a judgment obtained from the U.S. courts under civil liability provisions of the securities laws would be determined by Australian courts as penal or punitive in nature.
OFFERING EXPENSES
The following is a statement of expenses in connection with the distribution of the securities registered. All amounts shown are estimates except the SEC
registration fee. The estimates do not include expenses related to offerings of particular securities. Each prospectus supplement describing an offering of securities will reflect the estimated expenses related to the offering of securities under
that prospectus supplement.
|
|
|
|
|
|
|
| SEC registration fee |
|
|
|
$ |
1,452.22 |
|
| EDGAR and printing fees |
|
A |
|
$ |
1,628 |
|
| Legal fees and expenses |
|
A |
|
$ |
55,129 |
|
| Accounting fees and expenses |
|
A |
|
$ |
8,480 |
|
| Depositary fees and expenses |
|
|
|
$ |
2,000 |
|
| Miscellaneous |
|
|
|
|
|
|
| Total |
|
|
|
$ |
68,689.22 |
|
|
|
|
|
|
|
|
27
LEGAL MATTERS
Certain legal matters with respect to Australian law, including the validity of the ordinary shares, will be passed upon for us by Addisons.
EXPERTS
The audited consolidated financial statements incorporated by reference in this prospectus and elsewhere in the registration statement have
been so incorporated by reference in reliance upon the report of Grant Thornton Audit Pty Limited, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” the information we file with it, which means that we can disclose important
information to you by referring you to those documents. Each document incorporated by reference is current only as of the date of such document, and the information incorporated by reference is considered to be part of this prospectus. The
information we file later with the SEC will automatically update and supersede this information. As such, in the case of a conflict or inconsistency between information contained in this prospectus and information incorporated by reference into this
prospectus, you should rely on the information contained in the document that was filed later.
We hereby incorporate by reference the
following:
| |
• |
|
Our Annual Report on Form 20-F for the fiscal year ended June 30, 2014 filed with the SEC on October 31, 2014 (File No. 000-29962); |
| |
• |
|
Current report on Form 6-K filed on October 31, 2014; |
| |
• |
|
Current report on Form 6-K filed on November 3, 2014; |
| |
• |
|
Current report on Form 6-K filed on November 6, 2014; |
| |
• |
|
Current report on Form 6-K filed on November 7, 2014; |
| |
• |
|
Current report on Form 6-K filed on November 19, 2014; |
| |
• |
|
Current report on Form 6-K filed on November 20, 2014; |
| |
• |
|
Current report on Form 6-K filed on December 4, 2014; |
| |
• |
|
Current report on Form 6-K filed on December 16, 2014; |
| |
• |
|
Current report on Form 6-K filed on December 17, 2014; |
| |
• |
|
Current report on Form 6-K filed on December 19, 2014; |
| |
• |
|
Current report on Form 6-K filed on January 7, 2015; |
| |
• |
|
Current report on Form 6-K filed on January 15, 2015; |
| |
• |
|
Current report on Form 6-K filed on January 16, 2015; |
| |
• |
|
Current report on Form 6-K filed on January 29, 2015; |
| |
• |
|
Registration Statement on Form F-3 filed on January 30, 2015 |
| |
• |
|
Current report on Form 6-K filed on January 30, 2015; |
| |
• |
|
Current report on Form 6-K filed on February 2, 2015; |
| |
• |
|
Current report on Form 6-K filed on February 11, 2015; |
| |
• |
|
Current report on Form 6-K filed on March 4, 2015; |
| |
• |
|
Current report on Form 6-K filed on March 6, 2015; |
| |
• |
|
Current report on Form 6-K filed on March 11, 2015; |
| |
• |
|
Amendment to Registration Statement on Form F-3/A filed on March 17, 2015; |
| |
• |
|
Release of Notice of Effectiveness on Form EFFECT filed on March 17, 2015 |
| |
• |
|
Current report on Form 6-K filed on March 18, 2015; |
| |
• |
|
Release of Prospectus on Form 424B1 on March 19, 2015; |
| |
• |
|
Current report on Form 6-K filed on March 30, 2015; |
| |
• |
|
Current report on Form 6-K filed on April 9, 2015 |
| |
• |
|
Current report on Form 6-K filed on April 17, 2015 |
| |
• |
|
Current report on Form 6-K filed on April 20, 2015 |
28
| |
• |
|
Current report on Form 6-K filed on April 21, 2015 |
| |
• |
|
Current report on Form 6-K filed on April 27, 2015 |
| |
• |
|
Current report on Form 6-K filed on April 28, 2015 |
| |
• |
|
Current report on Form 6-K filed on April 29, 2015 |
| |
• |
|
Current report on Form 6-K filed on May 8, 2015 |
| |
• |
|
Current report on Form 6-K filed on May 14, 2015 |
| |
• |
|
Current report on Form 6-K filed on May 18, 2015 |
| |
• |
|
Current report on Form 6-K filed on May 26, 2015 |
| |
• |
|
Current report on Form 6-K filed on May 27, 2015 |
| |
• |
|
Current report on Form 6-K filed on May 28, 2015 |
| |
• |
|
Current report on Form 6-K filed on June 2, 2015 |
| |
• |
|
Current report on Form 6-K filed on June 3, 2015 |
| |
• |
|
Current report on Form 6-K filed on June 4, 2015 |
| |
• |
|
Current report on Form 6-K filed on June 12, 2015 |
| |
• |
|
Current report on Form 6-K filed on June 24, 2015 |
| |
• |
|
Current report on Form 6-K filed on July 1, 2015 |
| |
• |
|
Current report on Form 6-K filed on July 2, 2015 |
| |
• |
|
Current report on Form 6-K filed on July 13, 2015 |
| |
• |
|
Current report on Form 6-K filed on July 16, 2015 |
| |
• |
|
Current report on Form 6-K filed on July 22, 2015 |
| |
• |
|
Current report on Form 6-K filed on July 22, 2015 |
| |
• |
|
Current report on Form 6-K filed on July 30, 2015 |
| |
• |
|
Current report on Form 6-K filed on August 5, 2015 |
| |
• |
|
Current report on Form 6-K filed on August 31, 2015 |
| |
• |
|
Current report on Form 6-K filed on September 10, 2015 |
| |
• |
|
With respect to each offering of securities under this prospectus, all other subsequent annual reports on Form 20-F and any report on Form 6-K indicating that it is being incorporated by reference and that we file with
the SEC on or after the date on which the registration statement is first filed with the SEC and until the termination or completion of the offering under this prospectus. |
All Annual Reports on Form 20-F and all Reports of Foreign Issuer on Form 6-K, which are identified by us as being incorporated herein by
reference, filed subsequent to the date of the registration statement on Form F-3, of which this prospectus forms a part, including documents filed prior to the effectiveness of such registration statement, but before the termination of the
offering by this prospectus, shall be deemed to be incorporated by reference into this prospectus and deemed to be a part hereof from the date of the filing of such documents.
Any statement contained in a document incorporated by reference herein shall be deemed to be modified or superseded for all purposes to the
extent that a statement contained in this prospectus or in any other subsequently filed document which is also incorporated or deemed to be incorporated by reference, modifies or supersedes such statement. Any statement so modified or superseded
shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
We will provide at no cost to each
person, including any beneficial owner, to whom a prospectus is delivered, a copy of any or all information that has been incorporated by reference herein but has not been previously delivered upon written or oral request to:
Iain Ross
Acting Chief Executive
Officer
Novogen Limited
Level 1, 16-20 Edgeworth David Avenue
Hornsby, New South Wales 2007
Australia
+61 (02) 9472 4111
WHERE YOU CAN FIND ADDITIONAL
INFORMATION ABOUT US
As required by the Securities Act, we filed a registration statement on Form F-3 relating to the securities
offered by this prospectus with the SEC. This prospectus is a part of that registration statement, which includes additional information. You should refer to the registration statement and its exhibits for additional information. Whenever we make
reference in this prospectus to any of our contracts, agreements or other documents, the references are not necessarily complete and you should refer to the exhibits attached to the registration statement for copies of the actual contract,
agreements or other document.
29
We are subject to the informational requirements of the Exchange Act applicable to foreign
private issuers. We, as a “foreign private issuer,” are exempt from the rules under the Exchange Act prescribing certain disclosure and procedural requirements for proxy solicitations, and our officers, directors and principal shareholders
are exempt from the reporting and “short-swing” profit recovery provisions contained in Section 16 of the Exchange Act, with respect to their purchases and sales of shares. In addition, we are not required to file annual, quarterly
and current reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. However, we anticipate filing with the SEC, within four months after the end of each
fiscal year, an Annual Report on Form 20-F containing financial statements audited by an independent accounting firm. We also file with the SEC Reports of Foreign Private Issuer on Form 6-K.
You may read and copy any document we file or furnish with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington,
D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities. The SEC maintains an Internet site that contains reports, proxy and information statements and other information regarding
issuers that file electronically with the SEC. You can review our SEC filings and the registration statement by accessing the SEC’s Internet site at http://www.sec.gov.
You can review our ASX filings and the registration statement by accessing the ASX’s Internet site at http://www.asx.com.au.
We also maintain a website at http://www.novogen.com, but information contained on our website does not constitute a part of this
prospectus and is not incorporated by reference into this prospectus.
30
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 8. INDEMNIFICATION OF DIRECTORS AND OFFICERS
Our Constitution provides that, subject to the Australian Corporations Act, every director, secretary, manager or officer of our Company or
any person employed by our Company as auditor or agent shall be indemnified out of our funds against all liability incurred by such person as a director or officer in defending proceedings, whether civil or criminal, in which judgment is given in
the persons favor or in which the person is acquitted in connection with any application under the Australian Corporations Act in which relief is granted to the person by a Court.
Under our Constitution no director or other officer shall be liable for any acts, receipts, neglect or defaults of any other director or
officer for joining in any receipt or other act for conformity or for any loss, expense or damage whatever which arises in the execution of the duties of his or her office unless the same arises through his or her own negligence, default, breach of
duty or breach of trust.
In addition, our Constitution provides that every person who is or was a director or other officer, Auditor or
agent of the Company is indemnified (to the maximum extent permitted by law) by the Company from and against all costs losses and expenses which such person may properly incur or become liable to pay in connection with the performance by him or her
of his or her duties as director or such other officer in accordance with the Constitution and the law except where any such costs, loss or expense results from the negligence, default, breach of duty or breach of trust of such director or other
officer in relation to the Company, and it is the duty of the directors to pay any cost, loss or expense the subject of this indemnity from out of the funds of the Company.
We maintain a directors’ and officers’ liability insurance policy.
ITEM 9. EXHIBITS
|
|
|
Exhibit
Number |
|
Description of Document |
|
|
| 4.1 |
|
Placement Confirmation Letter dated April 20, 2015+ |
|
|
| 4.2 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Anson Investments Master Fund LP (“Anson”)+ |
|
|
| 4.3 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Anson+ |
|
|
| 4.4 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Anson+ |
|
|
| 4.5 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Hudson Bay Master Fund Ltd. (“HBMF”)+ |
|
|
| 4.6 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to HMBF+ |
|
|
| 4.7 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to HMBF+ |
|
|
| 4.8 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Iroquois Master Fund Ltd. (“Iroquois MF”)+ |
|
|
| 4.9 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Iroquois MF+ |
II - 1
|
|
|
|
|
| 4.10 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Iroquois MF+ |
|
|
| 4.11 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Iroquois Capital Investment Group LLC (“Iroquois CIG”)+ |
|
|
| 4.12 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Iroquois CIG+ |
|
|
| 4.13 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Iroquois CIG+ |
|
|
| 4.14 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Sabby Healthcare Master Fund Ltd. (“Sabby HMF”)+ |
|
|
| 4.15 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Sabby HMF+ |
|
|
| 4.16 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Sabby HMF+ |
|
|
| 4.17 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Sabby Volatility Warrant Master Fund Ltd (“Sabby VWMF”)+ |
|
|
| 4.18 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Sabby VWMF+ |
|
|
| 4.19 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Sabby VWMF+ |
|
|
| 4.20 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Empery Asset Master Ltd (“Empery AM”)+ |
|
|
| 4.21 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Empery AM+ |
|
|
| 4.22 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Empery AM+ |
|
|
| 4.23 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Empery Tax Efficient, LP (“Empery TE”)+ |
|
|
| 4.24 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Empery TE+ |
|
|
| 4.25 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Empery TE+ |
|
|
| 4.26 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Pinz Capital International (“Empery TE2”)+ |
|
|
| 4.27 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Empery TE2+ |
|
|
| 4.28 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Empery TE2+ |
|
|
| 4.29 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Pinz Capital International LP (“Pinz”)+ |
|
|
| 4.30 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Pinz+ |
|
|
| 4.31 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Pinz+ |
|
|
| 4.32 |
|
Engagement Letter Novogen HC Wainwright+ |
|
|
| 4.33 |
|
Amendment to Engagement letter Novogen HC Wainwright – December 2014+ |
II - 2
|
|
|
|
|
| 4.34 |
|
Amendment to Engagement letter Novogen HC Wainwright – April 2015+ |
|
|
| 5.1 |
|
Opinion of Addisons |
|
|
| 23.1 |
|
Consent of Grant Thornton Audit Pty Limited, Independent Registered Public Accounting Firm |
|
|
| 23.2 |
|
Consent of Addisons |
|
|
| 24.1 |
|
Power of Attorney (filed herewith as part of the signature page) |
ITEM 10. UNDERTAKINGS
(a) The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the
most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of
securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with
the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the
effective registration statement.
(iii) To include any material information with respect to the plan of distribution not
previously disclosed in the registration statement or any material change to such information in the registration statement;
Provided,
however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) of this section do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the
Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934, as amended, that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule
424(b) that is part of the registration statement; and
(2) That, for the purpose of determining any liability under the Securities Act of
1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering.
(4) To file a post-effective amendment to the registration statement to include any financial statements
required by Item 8.A. of Form 20-F at the start of any delayed offering or throughout a continuous offering. Financial statements and information otherwise required by Section 10(a)(3) of the Act need not be
furnished, provided that the registrant includes in the prospectus, by means of a post-effective amendment, financial statements required pursuant to this paragraph (a)(4) and other information necessary to ensure that all other
information in the prospectus is at least as current as the date of those financial statements. Notwithstanding the foregoing, a post-effective amendment need not be filed to include financial statements and information required by
Section 10(a)(3) of the Act if such financial statements and information are contained in periodic reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of
1934 that are incorporated by reference in the Form F-3.
II - 3
(5) That, for the purpose of determining liability under the Securities Act of 1933 to any
purchaser:
(i) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the
registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(ii) Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in
reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in
the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for
liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus
relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is
part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of
sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date.
(b) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the
registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities
Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to
be the initial bona fide offering thereof.
(c) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to
directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public
policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling
person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its
counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final
adjudication of such issue.
II - 4
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant certifies that it has reasonable grounds to believe
that it meets all of the requirements of filing on Form F-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, in the City of Sydney, Australia, on the 16th day of September 2015.
|
|
|
|
|
| Novogen Limited |
|
|
| By: |
|
/s/ Iain Ross |
|
|
Name: |
|
Iain Ross |
|
|
Title: |
|
Acting Chief Executive Officer |
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints each of Iain Ross and Cristyn
Humphreys, severally, his true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him and his name, place and stead, in any and all capacities, to sign any or all amendments (including pre-effective and
post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto and other documents in connection therewith, including any Registration Statement filed pursuant to Rule 462(b) under the Securities Act of
1933, with the SEC, granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might
or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent or any of his substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons
in the capacities indicated as of September 16, 2015:
|
|
|
|
|
| Signatures |
|
|
|
Title |
|
|
|
| /s/ Ian Phillips |
|
|
|
Interim Chairman |
| Ian Phillips |
|
|
|
|
|
|
|
| /s/ Iain Ross |
|
|
|
Director |
| Iain Ross |
|
|
|
Acting Chief Executive Officer |
|
|
|
| /s/ Steven Coffey |
|
|
|
Non-Executive Director |
| Steven Coffey |
|
|
|
|
|
|
|
| /s/ John O’Connor |
|
|
|
Non-Executive Director |
| John O’Connor |
|
|
|
|
|
|
|
| /s/ Peter Gunning |
|
|
|
Non-Executive Director |
| Prof. Peter Gunning |
|
|
|
|
|
|
|
| /s/ Bryce Carmine |
|
|
|
Non-Executive Director |
| Bryce Carmine |
|
|
|
|
|
|
|
| /s/ Cristyn Humphreys |
|
|
|
Chief Financial Officer and Chief Accounting Officer |
| Cristyn Humphreys |
|
|
|
|
|
|
|
| NOVOGEN NORTH AMERICA, INC. |
|
| Authorized representative in the United States |
|
|
| By: |
|
/s/ Andrew Heaton |
| Name: |
|
Andrew Heaton |
| Title: |
|
President |
II - 5
EXHIBIT INDEX
|
|
|
Exhibit
Number |
|
Description of Document |
|
|
| 4.1 |
|
Placement Confirmation Letter dated April 20, 2015+ |
|
|
| 4.2 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Anson Investments Master Fund LP (“Anson”)+ |
|
|
| 4.3 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Anson+ |
|
|
| 4.4 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Anson+ |
|
|
| 4.5 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Hudson Bay Master Fund Ltd. (“HBMF”)+ |
|
|
| 4.6 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to HMBF+ |
|
|
| 4.7 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to HMBF+ |
|
|
| 4.8 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Iroquois Master Fund Ltd. (“Iroquois MF”)+ |
|
|
| 4.9 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Iroquois MF+ |
|
|
| 4.10 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Iroquois MF+ |
|
|
| 4.11 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Iroquois Capital Investment Group LLC (“Iroquois CIG”)+ |
|
|
| 4.12 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Iroquois CIG+ |
|
|
| 4.13 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Iroquois CIG+ |
|
|
| 4.14 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Sabby Healthcare Master Fund Ltd. (“Sabby HMF”)+ |
|
|
| 4.15 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Sabby HMF+ |
|
|
| 4.16 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Sabby HMF+ |
|
|
| 4.17 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Sabby Volatility Warrant Master Fund Ltd (“Sabby VWMF”)+ |
|
|
| 4.18 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Sabby VWMF+ |
|
|
| 4.19 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Sabby VWMF+ |
|
|
| 4.20 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Empery Asset Master Ltd (“Empery AM”)+ |
|
|
| 4.21 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Empery AM+ |
|
|
| 4.22 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Empery AM+ |
II - 6
|
|
|
| |
|
|
|
|
| 4.23 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Empery Tax Efficient, LP (“Empery TE”)+ |
|
|
| 4.24 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Empery TE+ |
|
|
| 4.25 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Empery TE+ |
|
|
| 4.26 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Pinz Capital International (“Empery TE2”)+ |
|
|
| 4.27 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Empery TE2+ |
|
|
| 4.28 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Empery TE2+ |
|
|
| 4.29 |
|
Placement Confirmation Letter signature page dated April 20, 2015 by and between Novogen Limited and Pinz Capital International LP (“Pinz”)+ |
|
|
| 4.30 |
|
Terms and Conditions of Options Expiring December 27, 2015 Granted to Pinz+ |
|
|
| 4.31 |
|
Terms and Conditions of Options Expiring June 27, 2020 Granted to Pinz+ |
|
|
| 4.32 |
|
Engagement Letter Novogen HC Wainwright+ |
|
|
| 4.33 |
|
Amendment to Engagement letter Novogen HC Wainwright – December 2014+ |
|
|
| 4.34 |
|
Amendment to Engagement letter Novogen HC Wainwright – April 2015+ |
|
|
| 5.1 |
|
Opinion of Addisons |
|
|
| 23.1 |
|
Consent of Grant Thornton Audit Pty Limited, Independent Registered Public Accounting Firm |
|
|
| 23.2 |
|
Consent of Addisons |
|
|
| 24.1 |
|
Power of Attorney (filed herewith as part of the signature page) |
II - 7
Exhibit 5.1

14 September 2015
Our
Ref: MVR: NOV001/4007
Novogen Limited
16-20 Edgeworth
David Avenue
Hornsby NSW 2077
Australia
Attention: Board of Directors
Dear Sirs
Amendment No. 1 to SEC Registration Statement on Form F-3 filed 15 July 2015
We have acted as counsel to Novogen Limited, a company organized under the laws of Australia (the Company). This opinion is being delivered in
connection with the Company’s filing of Amendment No. 1 to a Registration Statement on Form F-3 filed by the Company 15 July 2015 (the Amended Registration Statement) with the U.S. Securities and Exchange Commission (the
Commission), on or about the date of this letter, pursuant to the U.S. Securities Act of 1933, as amended (the Securities Act), relating to the registration under the Securities Act and the proposed issuance and sale from time to time
pursuant to Rule 415 under the Securities Act of ordinary shares of the Company, in the form of ordinary shares or American Depositary Shares (the Ordinary Shares).
For purposes of giving this opinion, we have examined the documents listed in Schedule 1 hereto and we have relied upon the assumptions set out in Schedule 2
hereto, which we have not independently verified.
We are legal practitioners admitted to practise in the State of New South Wales. Our examination of
matters of law in connection with the opinions issued herein has been limited to, and accordingly our opinions herein are limited to, the laws of New South Wales.
Based on the foregoing, we are of the opinion that with respect to each of the Ordinary Shares, when (i) the Board of Directors of the Company or a
committee thereof properly empowered (such Board of Directors or committee being hereinafter referred to as the “Board”) has taken all necessary corporate action to approve the issuance of and the terms of the offering of the
Ordinary Shares and related matters, and (ii) holding statements in respect of the Ordinary Shares have been duly delivered (a) for consideration approved by the Board or (b) upon consideration by way of conversion or exercise of any
other Security issued by the Company in accordance with the terms of such Security, or the instrument governing such Security, providing for such conversion or exercise as approved by the Board, the Ordinary Shares will be duly authorized, validly
issued, fully paid and nonassessable. By “nonassessable” we mean that the potential liability of holders of the Ordinary Shares is limited to the amount the holders paid for the shares and that the holders may not be charged or assessed
additional amounts by the Company or the Company’s creditors to pay for the Company’s debts.
The reference in the paragraph above to “all
necessary corporate action” includes (but is not limited to) the obtaining of any approvals from shareholders of the Company required under the Corporations Act 2001 of the Commonwealth of Australia or the Listing Rules of the Australian
Securities Exchange.
This opinion is limited to the matters referred to herein and shall not be construed as extending to any other matters or document
not referred to herein. In particular (but without limiting the generality of the
ABN 55 365 334 124
|
|
|
|
|
|
|
|
|
| Level 12, 60 Carrington Street |
|
GPO Box 1433 |
|
DX 262 |
|
Telephone +61 2 8915 1000 |
|
mail@addisonslawyers.com.au |
| Sydney NSW 2000 |
|
Sydney NSW 2001 |
|
Sydney |
|
Facsimile +61 2 8916 2000 |
|
addisonslawyers.com.au |
Liability limited by a scheme approved under Professional Standards Legislation
|
|
|
|
|
| Novogen Limited |
|
|
|
14 September 2015 |
| |
foregoing), no opinion is expressed as to what further documentation may need to be entered into, or what other requirements may need to be complied with, to permit an offering of the shares in
Australia or any other jurisdiction. This opinion is governed by and shall be construed in accordance with the laws of New South Wales.
We consent to the
filing of this opinion as an exhibit to the Amended Registration statement and to the use of our name as the attorneys who will pass upon Australian legal matters in connection with the issuance of Ordinary Shares under the Amended Registration
Statement and any prospectus supplements related thereto. By giving this consent, we do not admit that we are within the category of persons whose consent is required under Section 7 of the Securities Act or under the rules and regulations of
the Commission relating thereto. This opinion is rendered as of the date above and we disclaim any obligation to advise you of facts, circumstances, events or developments that may alter, affect or modify the opinion expressed herein.
Yours faithfully
/s/ Jeff Mansfield
Jeff Mansfield
Partner
Direct Line: +61 2 8915 1016
Direct Fax: +61 2 8916 2016
Email: jeff.mansfield@addisonslawyers.com.au
2
|
|
|
|
|
| Novogen Limited |
|
|
|
14 September 2015 |
| |
Schedule 1 - List of Documents Examined
| 1. |
Such constating documents and corporate records as deemed necessary; |
| 2. |
The Registration Statement; |
| 3. |
A search on 14 September 2015 of the public database maintained by the Australian Securities and Investments Commission; and |
| 4. |
Such other documents as we have considered necessary for the purposes of rendering this opinion. |
3
|
|
|
|
|
| Novogen Limited |
|
|
|
14 September 2015 |
| |
Schedule 2 - Assumptions
This opinion is given based upon the following assumptions:
| 1. |
Payment in full for the Ordinary Shares will be received by the Company. |
| 2. |
The Constitution of the Company reviewed by us is the Constitution of the Company in force at the date hereof. |
| 3. |
The copies of such constating documents and corporate records of the Company examined, and as deemed necessary to be examined, by us are complete and accurate and constitute a complete and accurate record of the
business transacted by the Company. |
4
Exhibit 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We have issued our report dated October 31, 2014 with respect to the consolidated financial statements of Novogen Limited included in the Annual Report on
Form 20-F for the year ended June 30, 2014, which is incorporated by reference in this Registration Statement.
We consent to the incorporation by
reference of the aforementioned report in this Registration Statement and to the use of our name as it appears under the caption “Experts”.
/s/
GRANT THORNTON
GRANT THORNTON AUDIT PTY LTD
Chartered
Accountants
Sydney NSW Australia
16 September 2015
Exhibit 23.2

14 September 2015
Our
ref: MVR:NOV001/4007
Novogen Limited
16-20 Edgeworth David
Avenue
Hornsby NSW 2077
Australia
Attention: Board of Directors
Dear Sirs
Consent of Addisons to be named in Amendment No. 1 to SEC Registration Statement on Form F-3 filed 15 July 2015
Reference is made to the document prepared by Novogen Limited (ACN 063 259 754) (Company) titled “Amendment No. 1 to F-3 Registration
Statement”, which is proposed to be lodged with the Securities and Exchange Commission on or about the date of this letter, in relation to the registration for resale of 77,625,000 ordinary shares issuable upon exercise of various short and
long term options (Amended Registration Statement).
Addisons was provided with a final draft of the Amended Registration Statement on
September 14, 2015 (Final Draft).
Addisons consents to being named and referred to in its capacity as the Australian legal counsel to the
Company in the form and context in which it is named in the Final Draft.
In all other respects, to the maximum extent permitted by law, Addisons
expressly disclaims and takes no liability for any part of the Amended Registration Statement.
Addisons has not authorised or caused the issue of the
Amended Registration Statement or any part of the Amended Registration Statement.
By signing this consent, I confirm that I am authorised to give this
consent on behalf of Addisons.
Yours faithfully
/s/ Jeff
Mansfield
Jeff Mansfield
Partner
Direct Line: +61 2 8915 1016
Direct Fax: +61 2 8916 2016
Email: jeff.mansfield@addisonslawyers.com.au
ABN 55 365 334 124
|
|
|
|
|
|
|
|
|
| Level 12, 60 Carrington Street |
|
GPO Box 1433 |
|
DX 262 |
|
Telephone +61 2 8915 1000 |
|
mail@addisonslawyers.com.au |
| Sydney NSW 2000 |
|
Sydney NSW 2001 |
|
Sydney |
|
Facsimile +61 2 8916 2000 |
|
addisonlawyers.com.au |
Liability limited by a scheme approved under Professional Standards Legislation
Kazia Therapeutics (NASDAQ:KZIA)
Historical Stock Chart
From Oct 2024 to Nov 2024
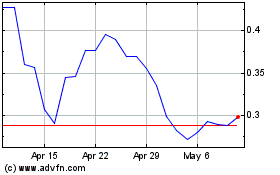
Kazia Therapeutics (NASDAQ:KZIA)
Historical Stock Chart
From Nov 2023 to Nov 2024