NewAmsterdam Pharma Reports Inducement Grants Under Nasdaq Listing Rule 5635(c)(4)
08 February 2025 - 8:01AM

NewAmsterdam Pharma Company N.V. (Nasdaq: NAMS or “NewAmsterdam” or
the “Company”), a late-stage, clinical biopharmaceutical company
developing oral, non-statin medicines for patients at risk of
cardiovascular disease (“CVD”) with elevated low-density
lipoprotein cholesterol (“LDL-C”), for whom existing therapies are
not sufficiently effective or well-tolerated, today announced that
the Compensation Committee of NewAmsterdam’s Board of Directors
approved the grant of inducement share options covering an
aggregate of 138,000 of NewAmsterdam’s ordinary shares to four (4)
non-executive new hires. The share options were granted as an
inducement material to the employees’ acceptance of employment with
NewAmsterdam pursuant to the NewAmsterdam Pharma Company N.V. 2024
Inducement Plan (the “2024 Inducement Plan”) and in accordance with
Nasdaq Listing Rule 5635(c)(4).
The share options have an exercise price per share
equal to $21.36, which represents the closing market price on the
Nasdaq Stock Market of the Company’s ordinary shares on, February
3, 2025, the grant date. The shares subject to the options will
vest over four years, with 25% of the shares vesting on the
one-year anniversary of the applicable vesting commencement date
and the balance of the shares vesting in a series of 36 equal
monthly installments thereafter, subject to each employee’s
continued service with NewAmsterdam on such vesting dates. The
options are subject to the terms and conditions of the 2024
Inducement Plan and the terms and conditions of an option award
agreement covering the grant.
About NewAmsterdamNewAmsterdam
Pharma (Nasdaq: NAMS) is a late-stage biopharmaceutical company
whose mission is to improve patient care in populations with
metabolic diseases where currently approved therapies have not been
adequate or well tolerated. We seek to fill a significant unmet
need for a safe, well-tolerated and convenient LDL-lowering
therapy. In multiple phase 3 studies, NewAmsterdam is investigating
obicetrapib, an oral, low-dose and once-daily CETP inhibitor, alone
or as a fixed-dose combination with ezetimibe, as LDL-C lowering
therapies to be used as an adjunct to statin therapy for patients
at risk of CVD with elevated LDL-C, for whom existing therapies are
not sufficiently effective or well tolerated.
Company ContactMatthew PhilippeP:
1-917-882-7512matthew.philippe@newamsterdampharma.com
NewAmsterdam Pharma Comp... (NASDAQ:NAMSW)
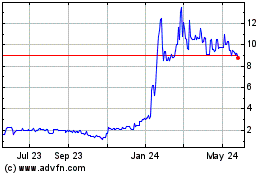
Historical Stock Chart
From Jan 2025 to Feb 2025
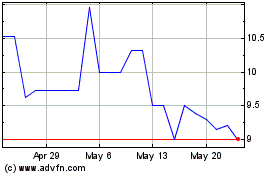
NewAmsterdam Pharma Comp... (NASDAQ:NAMSW)
Historical Stock Chart
From Feb 2024 to Feb 2025