Form 6-K - Report of foreign issuer [Rules 13a-16 and 15d-16]
01 June 2024 - 6:24AM
Edgar (US Regulatory)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
of the Securities Exchange Act of 1934
For the month of May 2024
Commission File Number: 001-39481
PainReform Ltd.
(Translation of registrant’s name into English)
65 Yigal Alon St., Tel Aviv 6744316
Israel
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
On May 31, 2024, PainReform Ltd. (the “Company”) issued a press release announcing the receipt of a letter from the Nasdaq Listing Qualifications. A copy of the press release is attached hereto as
Exhibit 99.1 and incorporated herein by reference.
The press release attached as Exhibit 99.1 to this Form 6-K is incorporated by reference into the Company’s Registration Statement on Form S-8 (Registration No. 333-257968 and 333-265902) and the
Company’s Registration Statement on Form F-3 (Registration No. 333-259318, 333-254982 and 333-276485).
|
99.1
|
Press Release dated May 31, 2024
|
Exhibit Index
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Date: May 31, 2024
|
PAINREFORM LTD.
|
|
|
|
|
|
By:
|
/s/ Ilan Hadar
|
|
|
|
Ilan Hadar
|
|
|
|
Chief Executive Officer
|
PainReform Announces Receipt of Nasdaq Minimum Bid Price Notification
Tel Aviv, Israel, May 31, 2024 — PainReform Ltd. (Nasdaq: PRFX) (“PainReform” or the “Company”), a clinical-stage specialty pharmaceutical company focused on the reformulation of established therapeutics, today announced that the Company
received a letter from the Nasdaq Listing Qualifications (the “Letter”), indicating that the Company is not in compliance with the minimum bid price requirement for continued listing set forth in Listing Rule 5550(a)(2), which requires listed
securities to maintain a minimum bid price of $1.00 per share.
Further, the Rules also provide the Company a compliance period of 180 calendar days to regain compliance. According to the Letter, the Company has from May 28, 2024, or until November 25, 2024, to
regain compliance with the minimum bid price requirement. The Company can regain compliance, if at any time during this 180-day period, the closing bid price of its ordinary shares is at least $1 for a minimum of ten consecutive business days, in
which case the Company will be provided with a written confirmation of compliance and this matter will be closed. In the event the Company does not regain compliance after the initial 180-day period, the Company may then be eligible for an
additional time if it meets the continued listing requirement for market value of publicly held shares and all other initial listing standards for The Nasdaq Capital Market, with the exception of the bid price requirement, and will need to provide
written notice of its intention to cure the deficiency during the second compliance period.
If the Company cannot demonstrate compliance by the end of the 180-day period (or during any subsequent second compliance period), the Nasdaq’s staff will notify the Company that its ordinary
shares are subject to delisting.
The Letter has no immediate effect on the Company’s Nasdaq listing or the trading of its ordinary shares, and during the grace period, as may be extended, PainReform’s ordinary shares will continue
to trade on the Nasdaq Capital Market under the symbol “PRFX”.
About PainReform Ltd.
PainReform is a clinical-stage specialty pharmaceutical company focused on the reformulation of established therapeutics. PRF-110, the Company's lead product, is based on the local anesthetic
ropivacaine, targeting the post-operative pain relief market. PRF-110 is an oil-based, viscous, clear solution that is deposited directly into the surgical wound bed prior to closure to provide localized and extended post-operative analgesia. The
Company's proprietary extended-release drug-delivery system is designed to provide an extended period of post-surgical pain relief without the need for repeated dose administration while reducing the potential need for the use of opiates. For more
information, please visit www.painreform.com.
Notice Regarding Forward-Looking Statements
This press release contains forward-looking statements about our expectations, beliefs and intentions including with respect to objectives, plans and strategies and expected
timing of results. Forward-looking statements can be identified by the use of forward-looking words such as "believe", "expect", "intend", "plan", "may", "should", "could", "might", "seek", "target", "will", "project", "forecast", "continue" or
"anticipate" or their negatives or variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical matters. These forward-looking statements are based on assumptions and assessments made
in light of management's experience and perception of historical trends, current conditions, expected future developments and other factors believed to be appropriate. Forward-looking statements in this press release are made as of the date of this
press release, and we undertake no duty to update or revise any such statements, whether as a result of new information, future events or otherwise. Forward-looking statements are not guarantees of future performance and are subject to risks and
uncertainties, many of which are outside of our control. Many factors could cause our actual activities or results to differ materially from the activities and results anticipated in forward- looking statements, including, but not limited to, the
following: our ability to continue as a going concern, our history of significant losses, our need to raise additional capital and our ability to obtain additional capital on acceptable terms, or at all; our dependence on the success of our
initial product candidate, PRF-110; the outcomes of preclinical studies, clinical trials and other research regarding PRF-110 and future product candidates; our limited experience managing clinical trials; our ability to retain key personnel and
recruit additional employees; our reliance on third parties for the conduct of clinical trials, product manufacturing and development; the impact of competition and new technologies; our ability to comply with regulatory requirements relating to
the development and marketing of our product candidates; our ability to establish and maintain strategic partnerships and other corporate collaborations; the implementation of our business model and strategic plans for our business and product
candidates; the scope of protection we are able to establish and maintain for intellectual property rights and our ability to operate our business without infringing the intellectual property rights of others; the overall global economic
environment; our ability to develop an active trading market for our ordinary shares and whether the market price of our ordinary shares is volatile; and statements as to the impact of the political and security situation in Israel on our business,
including due to the current war between Israel and Hamas. More detailed information about the risks and uncertainties affecting us is contained under the heading "Risk Factors" included in the Company's most recent Annual Report on Form 20-F and
in other filings that we have made and may make with the Securities and Exchange Commission in the future.
Contact:
Crescendo Communications, LLC
Tel: 212-671-1021
Email: prfx@crescendo-ir.com
Ilan Hadar
Chief Executive Officer
PainReform Ltd.
Tel: +972-54-5331725
Email: ihadar@painreform.com
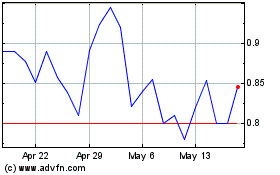
PainReform (NASDAQ:PRFX)
Historical Stock Chart
From May 2024 to Jun 2024
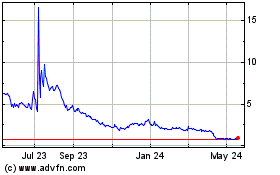
PainReform (NASDAQ:PRFX)
Historical Stock Chart
From Jun 2023 to Jun 2024