UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report: January 7, 2025
Commission File Number: 001-40377
Valneva SE
(Translation of registrant's name into English)
6 rue Alain Bombard
44800 Saint-Herblain, France
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1):
Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7):
Note: Regulation S-T Rule 101(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized (the registrant's "home country"), or under the rules of the home country exchange on which the registrant's securities are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the registrant's security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
On January 6, 2025, the Registrant issued a press release, a copy of which is attached hereto as Exhibit 99.1 and incorporated herein by reference. The information contained in this Form 6-K, including Exhibit 99.1, is hereby incorporated by reference into the registrant's Registration Statement on Form F-3 (File No. 333-266839).
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | | | | | | | | | | |
| | | | | Valneva SE (Registrant) | |
| | | | | | |
| Date: January 7, 2025 | | | | | /s/ Thomas Lingelbach Thomas Lingelbach Chief Executive Officer and President | |
Valneva to Meet with Investors during the J.P. Morgan Healthcare Conference
January 6, 2025
Saint Herblain (France), January 6, 2025 – Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company, today announces that members of its management team will meet one-on-one with existing shareholders and hold meetings with other institutional specialist investors during the 43rd Annual J.P. Morgan Healthcare Conference, January 13-15, 2025 in San Francisco.
Valneva’s CEO Thomas Lingelbach and CFO Peter Bühler will discuss the Company’s commercial portfolio of vaccines, which is expected to generate €160 – €170 million in revenues in 2024, and highlight upcoming catalysts from its clinical development pipeline, including the pivotal data readout for its Lyme disease vaccine later this year.
To schedule a 1on1 investor meeting with Valneva, institutional investors and analysts can contact Valneva’s investor relations department at investors@valneva.com.
About Valneva SE
We are a specialty vaccine company that develops, manufactures, and commercializes prophylactic vaccines for infectious diseases addressing unmet medical needs. We take a highly specialized and targeted approach, applying our deep expertise across multiple vaccine modalities, focused on providing either first-, best- or only-in-class vaccine solutions.
We have a strong track record, having advanced multiple vaccines from early R&D to approvals, and currently market three proprietary travel vaccines, including the world’s first and only chikungunya vaccine, as well as certain third-party vaccines.
Revenues from our growing commercial business help fuel the continued advancement of our vaccine pipeline. This includes the only Lyme disease vaccine candidate in advanced clinical development, which is partnered with Pfizer, the world’s most clinically advanced tetravalent Shigella vaccine candidate, as well as vaccine candidates against the Zika virus and other global public health threats. More information is available at www.valneva.com
Media and Investor Relations Contacts
Laetitia Bachelot-Fontaine
VP Global Communications & European Investor Relations
M +33 (0)6 4516 7099
laetitia.bachelot-fontaine@valneva.com
Joshua Drumm, Ph.D.
VP Global Investor Relations
M +001 917 815 4520
joshua.drumm@valneva.com
Forward-Looking Statements
This press release contains certain forward-looking statements relating to the business of Valneva, including with respect to business partnerships and the progress, timing, results and completion of technology transfer and regulatory approvals in additional markets. In addition, even if the actual results or development of Valneva are consistent with the forward-looking statements contained in this press release, those results or developments of Valneva may not be sustained in the future. In some cases, you can identify forward-looking statements by words such as “could,” “should,” “may,” “expects,” “anticipates,” “believes,” “intends,” “estimates,” “aims,” “targets,” or similar words. These forward-looking statements are based largely on the current expectations of Valneva as of the date of this press release and are subject to a number of known and unknown risks and uncertainties and other factors that may cause actual results, performance or achievements to be materially different from any future results, performance or achievement expressed or implied by these forward-looking statements. In particular, the expectations of Valneva could be affected by, among other things, uncertainties and delays involved in the development and manufacture of vaccines, unexpected clinical trial results, unexpected regulatory actions or delays, competition in general, currency fluctuations, the impact of the global and European credit crisis, and the ability to obtain or maintain patent or other proprietary intellectual property protection. Success in preclinical studies or earlier clinical trials may not be indicative of results in future clinical trials. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements made in this press release will in fact be realized. Valneva is providing this information as of the date of this press release and disclaims any intention or obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
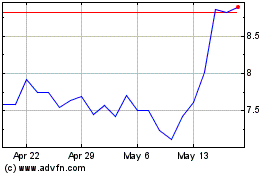
Valneva (NASDAQ:VALN)
Historical Stock Chart
From Dec 2024 to Jan 2025
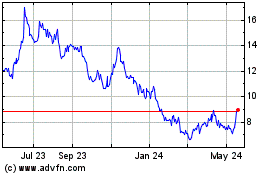
Valneva (NASDAQ:VALN)
Historical Stock Chart
From Jan 2024 to Jan 2025