New data presented at ATTD demonstrates the
system's ability to help individuals with type 1 diabetes exceed
international targets on outcome measures
DUBLIN and FLORENCE,
Italy, March 9, 2024 /PRNewswire/ -- Medtronic
plc. (NYSE: MDT), a global leader in healthcare technology,
today shared a robust set of new clinical and real-world evidence
on the MiniMed™ 780G system from around the world including the
largest set of data from early users in the United States. The data was presented at
the 17th International Conference on Advanced
Technologies and Treatments for Diabetes (ATTD) in Florence, Italy. These results build on the
3-year data published in Diabetes Technology &
Therapeutics showing over 100,000 real-world users achieving a
Time in Range (TIR) of 78% with the use of recommended optimal
settings, outperforming international targets of 70% TIR.
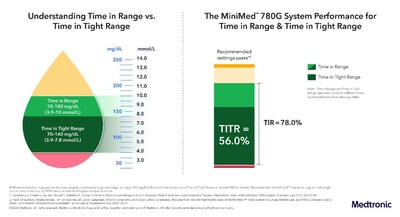
New data sought to evaluate the MiniMed™ 780G system's
ability to help users achieve Time in Tight Range (TITR) goals, a
new and emerging supplementary metric being discussed amongst
experts, which more closely mirrors the glucose levels of
individuals without diabetes. Also referred to as normoglycemia or
euglycemia, it is defined as the percentage of time a person spends
in the glucose range of 70-140 mg/dL. TITR lowers the upper
threshold of Time in Range from 180 mg/dL to 140 mg/dL. Results
showed users (n=13,461) achieved a TITR of greater than 56% with
the use of recommended optimal settings (100 mg/dL set target with
an active insulin time of 2 hours). This data adds to a growing
body of evidence that a TITR goal of 50% or greater is a reasonably
achievable goal with the right therapeutic option.
"Since the landmark DCCT study, numerous retrospective studies
have demonstrated the association between increased Time in Range
and a reduction of diabetic complications.1-12 There's
no doubt elevated glucose is harmful and the average blood sugars
of those living with type 1 diabetes are higher than we should
accept as a clinical community," said Robert Vigersky, MD, Chief
Medical Officer, Medtronic Diabetes. "The preponderance of data
across randomized controlled trials and real-world studies show
that the MiniMed™ 780G system is maximizing Time in Range far
surpassing international targets and is taking it a step beyond by
getting people closer to euglycemia.13,14 In the absence
of a cure, our goal is to relentlessly innovate therapies to help
people maximize their health without adding burden, which our
newest AID system has proven to do."
MiniMed™ 780G System Early Success in the U.S.
In an oral presentation, Dr. James Thrasher, MD, Founder, Arkansas Diabetes
and Endocrinology Center, shared data on early real-world users
with type 1 diabetes of the MiniMed™ 780G system in the U.S.
(n=7,499). Results showed users achieved over 80% TIR when
employing the recommended optimal settings, exceeding international
glycemic targets (ADA guidelines recommend 70% time in range
between 70-180 mg/dL), with closed loop exits occurring less than
once per week on average. The enhancements introduced in this
latest system have resulted in high satisfaction and improved
quality of life benefits.15,16 Indeed, the latest
dQ&A U.S. Pump Patient Survey (n=1,997), found that among pump
users, the MiniMed™ 780G system scored first in overall pump
satisfaction.*,17 The survey also showed that among
people with type 1 diabetes using CGM, the Guardian™ 4 sensor
mirrored competitor sensors in overall
satisfaction.*,18
"The results demonstrate that when the MiniMed™ 780G system is
optimized with recommended optimal settings, it helps people with
diabetes far exceed the ADA recommended goal of 70% Time in
Range,*" said Dr. Thrasher. "The advent of AID systems has been
nothing short of transformative in the practice of endocrinology
and is really pushing all of us to introduce its protective
benefits on overall health as early and often as possible. This
data reinforces that the determinant of choice for AID systems
should be first and foremost the power of the algorithm."
About Time in Tight Range
The development of
continuous glucose monitoring enabled the development of Time in
Range (TIR), a metric used today to determine whether an individual
with type 1 diabetes is meeting blood sugar management goals. Since
2019, the goal of diabetes management has been to maintain the
highest TIR for as long as possible while also minimizing
hypoglycemia. The introduction of automated insulin delivery (AID)
systems has transformed diabetes care by enabling a wider range of
individuals to safely achieve blood-sugar goals with less burden
and effort. AID systems are helping people achieve more ambitious
goals with glucose management, prompting the emergence of a new
supplementary metric that mirrors blood sugar levels of individuals
without diabetes (normoglycemia or euglycemia). The MiniMed™ 780G
system is demonstrating that a Time in Tight Range above 50 percent
is achievable and serves as a powerful tool for those seeking more
time in euglycemia.
About the Medtronic Diabetes
(www.medtronicdiabetes.com)
Medtronic Diabetes is on a mission to alleviate the burden of
diabetes by empowering individuals to live life on their terms,
with the most advanced diabetes technology and always-on support
when and how they need it. We've pioneered first-of-its-kind
innovations for over 40 years and are committed to designing the
future of diabetes management through next-generation sensors
(CGM), intelligent dosing systems, and the power of data science
and AI while always putting the customer experience at the
forefront.
About Medtronic
Bold thinking. Bolder actions. We are
Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global
healthcare technology company that boldly attacks the most
challenging health problems facing humanity by searching out and
finding solutions. Our Mission — to alleviate pain, restore health,
and extend life — unites a global team of 95,000+ passionate people
across more than 150 countries. Our technologies and therapies
treat 70 health conditions and include cardiac devices, surgical
robotics, insulin pumps, surgical tools, patient monitoring
systems, and more. Powered by our diverse knowledge, insatiable
curiosity, and desire to help all those who need it, we deliver
innovative technologies that transform the lives of two people
every second, every hour, every day. Expect more from us as we
empower insight-driven care, experiences that put people first, and
better outcomes for our world. In everything we do, we are
engineering the extraordinary. For more information on Medtronic
(NYSE:MDT), visit www.Medtronic.com and follow Medtronic on
LinkedIn.
Any forward-looking statements are subject to risks and
uncertainties such as those described in Medtronic's periodic
reports on file with the Securities and Exchange Commission. Actual
results may differ materially from anticipated results.
*Adults, T1 and parents of children with T1 diabetes < 18
years were surveyed; Individual results may vary.
Sources
- Yapanis M, James S, Craig ME, et al. Complications of Diabetes
and metrics of glycemic management derived from continuous glucose
monitoring. J Clin Endocrinol Metab 2022;107(6):e2221–e2236
- Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation
of time in range as an outcome measure for diabetes clinical
trials. Diabetes Care 2019;42(3):400–405
- Lu J, Ma X, Zhou J, et al. Association of time in range, as
assessed by continuous glucose monitoring, with diabetic
retinopathy in type 2 diabetes. Diabetes Care
2018;41(11):2370–2376
- Raj R, Mishra R, Jha N, et al. Time in range, as measured by
continuous glucose monitor, as a predictor of microvascular
complications in type 2 diabetes: A systematic review. BMJ Open
Diab Res Care 2022;10(1):e002573
- Lu J, Ma X, Shen Y, et al. Time in range is associated with
carotid intima-media thickness in type 2 diabetes. Diabetes Technol
Ther 2020;22(2):72–78
- Yoo JH, Choi MS, Ahn J, et al. Association between continuous
glucose monitoring-derived time in range, other core metrics, and
albuminuria in type 2 diabetes. Diabetes Technol Ther
2020;22(10):768–776
- Yang J, Yang X, Zhao D, et al. Association of time in
range, as assessed by continuous glucose monitoring, with painful
diabetic polyneuropathy. J Diabetes Invest 2021;12(5):828–836
- Hirsch IB, Sherr JL, Hood KK. Connecting the dots:
Validation of time in range metrics with microvascular outcomes.
Diabetes Care 2019;42(3):345–348
- Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and
peripheral neuropathy in type 2 diabetes mellitus and chronic
kidney disease. BMJ Open diabetes Res Care 2020;8(1):e000991
- El Malahi A, Van Elsen M, Charleer S, et al. Relationship
between time in range, glycemic variability, HbA1c, and
complications in adults with type 1 diabetes mellitus. J Clin
Endocrinol Metab 2022;107(2):e570–e581
- Beck RW. The association of time in range and diabetic
complications: The evidence is strong. Diabetes Technol Ther
2023;25(6):375–377
- Zhu DD, Wu X, Cheng XX, et al. Time in range as a useful marker
for evaluating retinal functional changes in diabetic retinopathy
patients. Int J Ophthalmol 2023;16(6):915–920
- CGM & Time in Range. American Diabetes Association.
Available at:
https://diabetes.org/tools-support/devices-technology/cgm-time-in-range.
Accessed June 19, 2023.
- American Diabetes Association (2019). Standards of medical care
in diabetes—2019. Diabetes Care, 42(Suppl 1): S61-S70.
- MiniMed™ 780G system SSED
- Medtronic data on file: MiniMed™780G users survey conducted in
April – May 202in UK, Sweden,
Italy, Netherlands and Belgium. N 789
- dQ&A US Diabetes Patient Panel Report; Customer Overall
Satisfaction, n=146; Q4 2023: P.52 (November
2023)
- dQ&A US Diabetes Patient Panel Report; Customer Overall
Satisfaction, n=207; Q4 2023: P.85 (November
2023)
|
Contacts:
|
|
|
Ashley
Patterson
|
Ryan
Weispfenning
|
|
Public
Relations
|
Investor
Relations
|
|
+1-818-576-3025
|
+1-763-505-4626
|
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/new-real-world-data-shows-minimed-780g-system-sustains-strong-global-performance-exceeding-international-targets-for-diabetes-management-302084607.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/new-real-world-data-shows-minimed-780g-system-sustains-strong-global-performance-exceeding-international-targets-for-diabetes-management-302084607.html
SOURCE Medtronic plc