Zymeworks Announces Participation in Upcoming Investor Conferences
30 January 2025 - 10:00PM
UK Regulatory
Zymeworks Announces Participation in Upcoming Investor Conferences
VANCOUVER, British Columbia, Jan. 30, 2025 (GLOBE
NEWSWIRE) -- Zymeworks Inc. (Nasdaq: ZYME), a clinical-stage
biotechnology company developing a diverse pipeline of novel,
multifunctional biotherapeutics to improve the standard of care for
difficult-to-treat diseases, including cancer, inflammation, and
autoimmune disease, today announced that management will
participate in the following upcoming investor conferences:
- Oppenheimer 35th Annual Healthcare Life Sciences
Conference: Zymeworks’ management will participate in virtual
one-on-one meetings and a fireside chat on February 11 at 11:20 am
Eastern Time (ET).
- Citi's 2025 Virtual Oncology Leadership Summit: Zymeworks’
management will participate in a virtual fireside chat on February
20 at 2:00 pm ET.
- B. Riley Securities Precision Oncology & Radiopharma
Conference: Zymeworks’ management will participate in one-on-one
meetings and a panel discussion on February 28 in New York,
NY.
About Zymeworks Inc.
Zymeworks is a global clinical-stage
biotechnology company committed to the discovery, development, and
commercialization of novel, multifunctional biotherapeutics.
Zymeworks’ mission is to make a meaningful difference in the lives
of people impacted by difficult-to-treat conditions such as cancer,
inflammation, and autoimmune disease. The Company’s complementary
therapeutic platforms and fully integrated drug development engine
provide the flexibility and compatibility to precisely engineer and
develop highly differentiated antibody-based therapeutic
candidates. Zymeworks engineered and developed
zanidatamab, a HER2-targeted bispecific antibody using the
Company’s proprietary Azymetric™
technology. Zymeworks has entered into separate
agreements with BeiGene, Ltd. (BeiGene) and Jazz
Pharmaceuticals Ireland Limited (Jazz Pharmaceuticals),
granting each exclusive rights to develop and commercialize
zanidatamab in different territories. The U.S. FDA
granted accelerated approval of
Ziihera® (zanidatamab-hrii) 50mg/mL for injection
for intravenous use for the treatment of adults with
previously-treated, unresectable or metastatic HER2-positive (IHC
3+) second-line biliary tract cancer (BTC).
Ziihera® is the first and only dual HER2-targeted
bispecific antibody approved for HER2-positive BTC in the U.S.
Zanidatamab is currently under regulatory review in the EU and
China for second-line BTC and is being evaluated in multiple global
clinical trials as a potential best-in-class treatment for patients
with multiple HER2-expressing cancers. Zymeworks is rapidly
advancing a robust pipeline of wholly-owned product candidates,
leveraging its expertise in both antibody-drug conjugates and
multispecific antibody therapeutics targeting novel pathways in
areas of significant unmet medical need. Phase 1 studies for ZW171
and ZW191 are now actively recruiting with investigational new drug
applications for ZW220 and ZW251 planned for 2025. In addition to
Zymeworks’ pipeline, its therapeutic platforms have been further
leveraged through strategic partnerships with global
biopharmaceutical companies. For information about Zymeworks,
visit www.zymeworks.com and
follow @ZymeworksInc on X.
Contacts:
Investor Inquiries:
Shrinal Inamdar
Senior Director, Investor Relations
(604) 678-1388
ir@zymeworks.com
Media Inquiries:
Diana Papove
Senior Director, Corporate Communications
(604) 678-1388
media@zymeworks.com


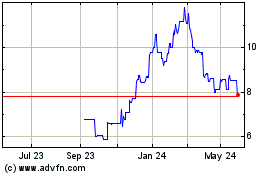
Zymeworks BC (TG:ZA8)
Historical Stock Chart
From Feb 2025 to Mar 2025
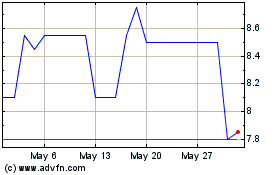
Zymeworks BC (TG:ZA8)
Historical Stock Chart
From Mar 2024 to Mar 2025