AIM announces Paul Goepfert, MD, as the principal investigator for the planned clinical study of Ampligen and FluMist as a vaccine for avian influenza
01 March 2025 - 12:45AM

AIM ImmunoTech Inc. (NYSE American:
AIM)
(“AIM” or the “Company”) today announced
that Paul Goepfert, MD, of the University of Alabama-Birmingham
(“UAB”), has agreed to act as the Principal Investigator for the
company’s planned clinical study in the combination of Ampligen and
AstraZeneca’s FluMist as an intranasal vaccine for influenza,
including avian influenza. Ampligen would serve as a vaccine
adjuvant.
This will be a follow-up study to a previous
clinical trial at UAB, which indicated that intranasal delivery of
Ampligen after the intranasal delivery of the FluMist seasonal
influenza vaccine not only increased the immune response to
seasonal variants in the vaccine by greater than four-fold, but
most importantly induced cross-reactive secretory Immunoglobulin A
against highly pathogenic avian influenza virus strains H5N1, H7N9
and H7N3.
Paul Goepfert, MD, Director for the Alabama
Vaccine Research Clinic, stated: “I’m excited for the opportunity
to follow-up on my previous work regarding the combination of
Ampligen and FluMist, especially with the rising threat of avian
influenza.”
AIM has engaged Amarex Clinical Research, its
Clinical Research Organization, with the preparation of an
Investigational New Drug application and the eventual management of
the planned clinical study. A key next step will be to identify
study funding through industry or governmental grants.
AIM CEO Thomas K. Equels states: “Our strong
belief in the potential of a second Ampligen and FluMist study in
humans stems directly from the pre-clinical and clinical work
performed with Ampligen and multiple influenza variants, including
in the original UAB study. We believe that the U.S. government —
which has made the growing threat of avian influenza a top priority
— should take a long and close look at this data when deciding how
best to prepare for a potential epidemic. Rather than spend perhaps
billions of dollars on the lengthy development of a new mRNA
vaccine, the government should instead consider the combination of
Ampligen and FluMist, which would be no more than $10 million in
development costs and far quicker to develop, since it already has
strong human and non-human primate data suggesting its potential
preventive efficacy against avian influenza. AIM believes that the
potential for a rapidly deployable vaccine that includes Ampligen
is clear.”
Read more about the Ampligen-involved avian
influenza pre-clinical and clinical work here.
About AIM ImmunoTech Inc.
AIM ImmunoTech Inc., an Ocala, Florida-based
company, is an immuno-pharma company focused on the research and
development of therapeutics to treat multiple types of cancers,
immune disorders and viral diseases, including COVID-19. The
Company’s lead product is a first-in-class investigational drug
called Ampligen® (rintatolimod), a dsRNA and highly selective TLR3
agonist immuno-modulator with broad spectrum activity in clinical
trials for globally important cancers, viral diseases and disorders
of the immune system.
For more information, please
visit aimimmuno.com and connect with the Company
on X, LinkedIn, and Facebook.
Cautionary Statement
This press release contains forward-looking
statements within the meaning of the Private Securities Litigation
Reform Act of 1995 (the “PSLRA”). Words such as “may,” “will,”
“expect,” “plan,” “anticipate,” “continue,” “believe,” “potential,”
“upcoming” and other variations thereon and similar expressions (as
well as other words or expressions referencing future events or
circumstances) are intended to identify forward-looking statements.
Many of these forward-looking statements involve a number of risks
and uncertainties. Data, pre-clinical success and clinical success
seen to date do not guarantee that Ampligen will be approved as a
therapy or vaccine adjuvant for any variant of influenza. The
Company urges investors to consider specifically the various risk
factors identified in its most recent Form 10-K, and any risk
factors or cautionary statements included in any subsequent Form
10-Q or Form 8-K, filed with the U.S. Securities and Exchange
Commission. You are cautioned not to place undue reliance on these
forward-looking statements, which speak only as of the date of this
press release. Among other things, for those statements, the
Company claims the protection of the safe harbor for
forward-looking statements contained in the PSLRA. The Company does
not undertake to update any of these forward-looking statements to
reflect events or circumstances that occur after the date
hereof.
A photo accompanying this announcement is available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/afb103f2-0128-42af-ba4b-5a3c720118aa
Investor Contact:
JTC Team, LLC
Jenene Thomas
908.824.0775
AIM@jtcir.com
AIM ImmunoTech (AMEX:AIM)
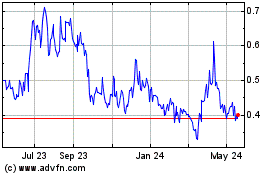
Historical Stock Chart
From Feb 2025 to Mar 2025
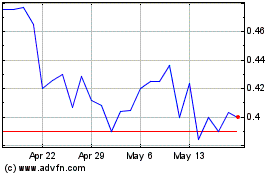
AIM ImmunoTech (AMEX:AIM)
Historical Stock Chart
From Mar 2024 to Mar 2025