Nanosonics Sponsors Educational Symposium on Standardizing Ultrasound Probe Disinfection Practices at APIC 2019
11 June 2019 - 11:00PM
Business Wire
Showcases automated trophon2 complete
reprocessing solution for optimized probe decontamination
Nanosonics (ASX: NAN), a leader in infection prevention
solutions, will highlight its proprietary automated trophon®2
complete ultrasound reprocessing system and sponsor an educational
breakfast symposium on standardizing ultrasound probe reprocessing
during the 2019 APIC, June 12-14 in Philadelphia.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20190611005020/en/
Nanosonics' trophon®2 High Level
Disinfection System with a surface ultrasound probe being placed in
the device for decontamination. (Photo: Business Wire)
The symposium, entitled “Standardizing Ultrasound Probe
Reprocessing: The Ultrasound Infection Prevention (IP) Toolkit,”
will be presented by Robert Garcia, a senior infection
preventionist, researcher and consultant for nearly 40 years, and
Betty McGinty, a medical device reprocessing expert and quality
director for a major healthcare system in Atlanta. The focus will
be implementing and supporting reliable and effective reprocessing
of probes throughout hospitals with special emphasis on recent
research, federal guidelines and the successful application of the
Ultrasound IP Toolkit.
The Ultrasound IP Toolkit was developed by industry experts to
help users meet existing evidence-based guidelines and standards.
The toolkit can assist departments, facilities or entire healthcare
systems systematically standardize their ultrasound infection
prevention practices.
“As the rapid increase of ultrasound imaging presents potential
infection control challenges throughout hospitals, appropriate
education is crucial to maintaining patient safety and minimizing
infection transmission,” said Rose Seavey, president of Seavey
Healthcare Consulting and a device reprocessing expert involved in
the development of national standards. Seavey is also a member of
the expert group behind the development of the Ultrasound IP
Toolkit.
“There are areas of procedure awareness that need to be explored
and addressed, including the application of high-level disinfection
(HLD) to surface ultrasound transducers used in invasive procedures
in accordance with Spaulding,” continues Seavey. “The Ultrasound IP
Toolkit was created by a group of concerned infection
preventionists and reprocessing experts to offer a valuable
educational resource designed to help facilities standardize their
ultrasound disinfection practices, create policy and more
effectively prevent infections.”
Ruth M. Carrico, Associate Professor, Division of Infectious
Diseases, University of Louisville School of Medicine, Ky., and
Garcia will present their findings from a recent survey of IPs
regarding a variety of disinfection and use practices for
ultrasound probes during APIC’s Educational Session #3204. In
addition, an observational study on the use and reprocessing of
probes in interventional procedures will be presented.
“In addition to highlighting important resources and strategies
for evidence-based advances in ultrasound infection prevention,
we’re excited to showcase trophon2 at the annual APIC conference,
the largest gathering of infection prevention professionals
worldwide,” said Ken Shaw, president of North America for
Nanosonics. “Our latest innovation in HLD for probes, trophon2 is
widely considered the new standard of care offering a complete
automated reprocessing solution that is proven to be effective
against a wide range of pathogens while helping ensure compliance
with the latest guideline requirements.”
During the APIC annual conference, industry experts will be
stationed in Nanosonics’ booth #709 discussing the Ultrasound IP
Toolkit as well as Nanosonics’ HLD solution. Interactive stations
will feature probe compatibility and the robust testing process
that they go through along with trophon AuditPro, a new data
management service for compliance reporting and audit
readiness.
About trophon* Technology
Nanosonics’ trophon* technology’s high-frequency ultrasonic
vibrations generate a sonically activated, supercharged hydrogen
peroxide (H2O2) mist that inactivates drug resistant pathogens and
spores that cause sexually transmitted infections (STIs) such as
Gonorrhea, HIV and high-risk Human Papillomaviruses (HPV),1 as well
as drug resistant bacteria including MRSA. The trophon systems are
installed in more than 4,000 hospitals and facilities in North
America including all of the top 50 U.S. hospitals** and it’s
estimated that trophon technology is protecting over 60,000
patients daily from the risks of cross-contamination. The device is
validated for use with over 1,000 probes.
About Nanosonics
Nanosonics (ASX:NAN) is a leading medical technology company
headquartered in Sydney, Australia, with its North American
operations based in Indianapolis. Founded in 2001, the company is
one of Australia’s largest medical technology companies and a
recognized leader in its sector of the global infection control
market. More information may be found at www.nanosonics.us
Note to Editors:Standardizing Ultrasound Probe
Reprocessing Breakfast SymposiumTo register:
https://info.nanosonics.com.au/apic2019Friday, June 14, 20196:00 –
7:30 AMRoom 204 ABPhiladelphia Convention Center
*trophon [trophon EPR & trophon2]1. Ryndock E, Robison R,
Meyers C. Susceptibility of HPV16 and 18 to high level
disinfectants indicated for semi-critical ultrasound probes. J Med
Virol. 2016;88(6):1076-80.**US News and World Report.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20190611005020/en/
Amy
Cookamy@amcpublicrelations.nethttps://www.nanosonics.us/news/media-kit/
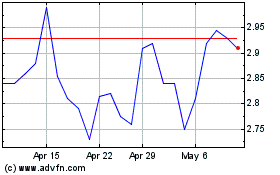
Nanosonics (ASX:NAN)
Historical Stock Chart
From Mar 2025 to Apr 2025
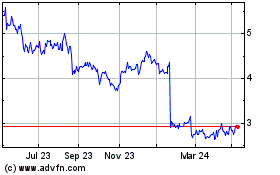
Nanosonics (ASX:NAN)
Historical Stock Chart
From Apr 2024 to Apr 2025