COBENFY represents the first new
pharmacological approach to treat schizophrenia in decades, with a
mechanism of action distinct from current therapies
Approval is supported by data from the
EMERGENT clinical program demonstrating statistically significant
reductions of schizophrenia symptoms
The safety and tolerability profile of
COBENFY has been established across acute and long-term trials in
schizophrenia
Bristol Myers Squibb (NYSE: BMY) today announced that the U.S.
Food and Drug Administration (FDA) has approved COBENFY™
(xanomeline and trospium chloride), an oral medication for the
treatment of schizophrenia in adults.1 COBENFY represents the first
new class of medicine in several decades and introduces a
fundamentally new approach to treating schizophrenia by selectively
targeting M1 and M4 receptors in the brain without blocking D2
receptors.2,3,4
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20240925382351/en/
Product Image (Photo: Bristol Myers
Squibb)
“Today’s landmark approval of our first-in-class treatment for
schizophrenia marks an important milestone for the community, where
after more than 30 years, there is now an entirely new
pharmacological approach for schizophrenia — one that has the
potential to change the treatment paradigm,” said Chris Boerner,
PhD, board chair and chief executive officer at Bristol Myers
Squibb. “As we reenter the field of neuropsychiatry, we are
dedicated to changing the conversation around serious mental
illness, beginning with today’s approval in schizophrenia.”
Schizophrenia is a persistent and often disabling mental illness
affecting how a person thinks, feels and behaves.5 It is estimated
to impact approximately 2.8 million people in the United States.6
Symptoms typically first appear in early adulthood and present
differently in each person, making symptoms difficult to diagnose
and manage.6 While the current standard of care can be effective in
managing symptoms of schizophrenia, up to 60% of people experience
inadequate improvement in symptoms or intolerable side effects
during therapy.7
“For people living with schizophrenia, it's often difficult to
find a treatment that works for them. Having a variety of treatment
options gives patients and healthcare providers the tools to help
manage this serious condition,” said Gordon Lavigne, chief
executive officer of the Schizophrenia & Psychosis Action
Alliance. “People living with schizophrenia want and deserve more.
Today's approval provides a new option as people with schizophrenia
move forward with proper support to rebuild their lives.”
The FDA approval of COBENFY is supported by data from the
EMERGENT clinical program, which includes three placebo-controlled
efficacy and safety trials and two open-label trials evaluating the
long-term safety and tolerability of COBENFY for up to one year. In
the Phase 3 EMERGENT-2 and EMERGENT-3 trials, COBENFY met its
primary endpoint, demonstrating statistically significant
reductions of schizophrenia symptoms compared to placebo, as
measured by the Positive and Negative Syndrome Scale (PANSS) total
score change from baseline to week five. COBENFY demonstrated a
9.6-point reduction (-21.2 COBENFY vs. -11.6 placebo, p<0.0001)
and an 8.4-point reduction (-20.6 COBENFY vs. -12.2 placebo;
p<0.0001) in PANSS total score compared to placebo at week five
in EMERGENT-2 and EMERGENT-3, respectively. In EMERGENT-2, COBENFY
demonstrated a statistically significant improvement in illness
from baseline to week five, as measured by the Clinical Global
Impression-Severity (CGI-S) score, a secondary endpoint in the
trial.1
The safety and tolerability profile of COBENFY has been
established across acute and long-term trials. In the Phase 3
EMERGENT-2 and EMERGENT-3 trials, the most common adverse reactions
(≥5% and at least twice placebo) were nausea, dyspepsia,
constipation, vomiting, hypertension, abdominal pain, diarrhea,
tachycardia, dizziness and gastroesophageal reflux disease.1
COBENFY does not have atypical antipsychotic class warnings and
precautions and does not have a boxed warning.
“Due to its heterogeneous nature, schizophrenia is not a
one-size-fits-all condition, and people often find themselves in a
cycle of discontinuing and switching therapies,” said Rishi Kakar,
MD, chief scientific officer and medical director at Segal Trials
and investigator in the EMERGENT program. “The approval of COBENFY
is a transformative moment in the treatment of schizophrenia
because, historically, medicines approved to treat schizophrenia
have relied on the same primary pathways in the brain. By
leveraging a novel pathway, COBENFY offers a new option to manage
this challenging condition.”
The Company today also announced the launch of COBENFY Cares™, a
program designed to support patients who have been prescribed
COBENFY. Patients will be able to enroll in the COBENFY Cares
program in late October corresponding with product availability.
The COBENFY Cares phone number is 1-877-COBENFY.
About Schizophrenia
Schizophrenia is a persistent and often disabling mental illness
impacting how a person thinks, feels and behaves. There are three
symptom domains of schizophrenia, which include positive symptoms
(e.g., hallucinations, delusions, disordered thinking and speech),
negative symptoms (e.g., lack of motivation, lack of emotional
expression/flat affect, social withdrawal) and cognitive
dysfunction (e.g., impaired attention, deficits in memory,
concentration and decision-making).5 The symptoms of schizophrenia
can affect all areas of people’s lives, making it difficult to
maintain employment, live independently and manage
relationships.8,9 Schizophrenia affects nearly 24 million people
worldwide, including 2.8 million people in the United States, and
is one of the top 15 leading causes of disability
worldwide.6,10,11
About COBENFY™ (xanomeline and trospium chloride)
COBENFY™ (xanomeline and trospium chloride), formerly KarXT, is
an oral medication for the treatment of schizophrenia in adults.
COBENFY combines xanomeline, a dual M1- and M4-preferring
muscarinic receptor agonist, with trospium chloride, a muscarinic
receptor antagonist that does not appreciably cross the blood-brain
barrier, primarily acting in peripheral tissues. While the exact
mechanism of action of COBENFY is unknown, its efficacy is thought
to be due to the agonist activity of xanomeline at M1 and M4
muscarinic acetylcholine receptors in the central nervous
system.
About EMERGENT Clinical Program
The EMERGENT clinical program evaluating COBENFY for the
treatment of schizophrenia in adults includes three
placebo-controlled efficacy and safety studies, including the Phase
3 EMERGENT-2 and EMERGENT-3 trials, and two open-label studies
evaluating the long-term safety and tolerability of COBENFY for up
to one year.
The Phase 3 EMERGENT-2 and EMERGENT-3 trials were five-week,
inpatient trials that evaluated the efficacy, safety and
tolerability of COBENFY compared to placebo in adults with
schizophrenia. In both trials, COBENFY met its primary endpoint,
demonstrating statistically significant reductions of schizophrenia
symptoms compared to placebo as measured by the Positive and
Negative Syndrome Scale (PANSS) total score change from baseline to
week five.
COBENFY demonstrated a 9.6-point reduction (-21.2 COBENFY vs.
-11.6 placebo, p<0.0001) and an 8.4-point reduction (-20.6
COBENFY vs. -12.2 placebo; p<0.0001) in PANSS total score
compared to placebo at week five in EMERGENT-2 and EMERGENT-3,
respectively. In EMERGENT-2, COBENFY demonstrated a statistically
significant 0.6 change (-1.2 COBENFY vs. -0.7 placebo; p<0.0001)
in the Clinical Global Impression-Severity (CGI-S) score compared
to placebo at week five, a secondary endpoint in the trial.
The most common adverse reactions (≥5% and at least twice
placebo) of COBENFY compared to placebo were nausea (19% vs. 4%),
dyspepsia (18% vs 5%), constipation (17% vs 7%), vomiting (15% vs
1%), hypertension (11% vs 2%), abdominal pain (8% vs 4%), diarrhea
(6% vs 2%), tachycardia (5% vs 2%), dizziness (5% vs 2%) and
gastroesophageal reflux disease (5% vs. <1%).
INDICATION
COBENFY™ (xanomeline and trospium chloride) is indicated for the
treatment of schizophrenia in adults.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
COBENFY is contraindicated in patients with:
- urinary retention
- moderate (Child-Pugh Class B) or severe (Child-Pugh Class C)
hepatic impairment
- gastric retention
- history of hypersensitivity to COBENFY or trospium chloride.
Angioedema has been reported with COBENFY and trospium
chloride.
- untreated narrow-angle glaucoma
WARNINGS AND PRECAUTIONS
Risk of Urinary Retention: COBENFY can cause urinary
retention. Geriatric patients and patients with clinically
significant bladder outlet obstruction and incomplete bladder
emptying (e.g., patients with benign prostatic hyperplasia (BPH),
diabetic cystopathy) may be at increased risk of urinary
retention.
COBENFY is contraindicated in patients with pre-existing urinary
retention and is not recommended in patients with moderate or
severe renal impairment.
In patients taking COBENFY, monitor for symptoms of urinary
retention, including urinary hesitancy, weak stream, incomplete
bladder emptying, and dysuria. Instruct patients to be aware of the
risk and promptly report symptoms of urinary retention to their
healthcare provider. Urinary retention is a known risk factor for
urinary tract infections. In patients with symptoms of urinary
retention, consider reducing the dose of COBENFY, discontinuing
COBENFY, or referring patients for urologic evaluation as
clinically indicated.
Risk of Use in Patients with Hepatic Impairment: Patients
with hepatic impairment have higher systemic exposures of
xanomeline, a component of COBENFY, compared to patients with
normal hepatic function, which may result in increased incidence of
COBENFY-related adverse reactions.
COBENFY is contraindicated in patients with moderate or severe
hepatic impairment. COBENFY is not recommended in patients with
mild hepatic impairment.
Assess liver enzymes prior to initiating COBENFY and as
clinically indicated during treatment.
Risk of Use in Patients with Biliary Disease: In clinical
studies with COBENFY, transient increases in liver enzymes with
rapid decline occurred, consistent with transient biliary
obstruction due to biliary contraction and possible gallstone
passage.
COBENFY is not recommended for patients with active biliary
disease such as symptomatic gallstones. Assess liver enzymes and
bilirubin prior to initiating COBENFY and as clinically indicated
during treatment. The occurrence of symptoms such as dyspepsia,
nausea, vomiting, or upper abdominal pain should prompt assessment
for gallbladder disorders, biliary disorders, and pancreatitis, as
clinically indicated.
Discontinue COBENFY in the presence of signs or symptoms of
substantial liver injury such as jaundice, pruritus, or alanine
aminotransferase levels more than five times the upper limit of
normal or five times baseline values.
Decreased Gastrointestinal Motility: COBENFY contains
trospium chloride. Trospium chloride, like other antimuscarinic
agents, may decrease gastrointestinal motility. Administer COBENFY
with caution in patients with gastrointestinal obstructive
disorders because of the risk of gastric retention. Use COBENFY
with caution in patients with conditions such as ulcerative
colitis, intestinal atony, and myasthenia gravis.
Risk of Angioedema: Angioedema of the face, lips, tongue,
and/or larynx has been reported with COBENFY and trospium chloride,
a component of COBENFY. In one case, angioedema occurred after the
first dose of trospium chloride. Angioedema associated with upper
airway swelling may be life-threatening. If involvement of the
tongue, hypopharynx, or larynx occurs, discontinue COBENFY and
initiate appropriate therapy and/or measures necessary to ensure a
patent airway. COBENFY is contraindicated in patients with a
history of hypersensitivity to trospium chloride.
Risk of Use in Patients with Narrow-angle Glaucoma:
Pupillary dilation may occur due to the anticholinergic effects of
COBENFY. This may trigger an acute angle closure attack in patients
with anatomically narrow angles. In patients known to have
anatomically narrow angles, COBENFY should only be used if the
potential benefits outweigh the risks and with careful
monitoring.
Increases in Heart Rate: COBENFY can increase heart rate.
Assess heart rate at baseline and as clinically indicated during
treatment with COBENFY.
Anticholinergic Adverse Reactions in Patients with Renal
Impairment: Trospium chloride, a component of COBENFY, is
substantially excreted by the kidney. COBENFY is not recommended in
patients with moderate or severe renal impairment (estimated
glomerular filtration rate (eGFR) <60 mL/min). Systemic exposure
of trospium chloride is higher in patients with moderate and severe
renal impairment. Therefore, anticholinergic adverse reactions
(including dry mouth, constipation, dyspepsia, urinary tract
infection, and urinary retention) are expected to be greater in
patients with moderate and severe renal impairment.
Central Nervous System Effects: Trospium chloride, a
component of COBENFY, is associated with anticholinergic central
nervous system (CNS) effects. A variety of CNS anticholinergic
effects have been reported with trospium chloride, including
dizziness, confusion, hallucinations, and somnolence. Monitor
patients for signs of anticholinergic CNS effects, particularly
after beginning treatment or increasing the dose. Advise patients
not to drive or operate heavy machinery until they know how COBENFY
affects them. If a patient experiences anticholinergic CNS effects,
consider dose reduction or drug discontinuation.
Most Common Adverse Reactions (≥5% and at least twice
placebo): nausea, dyspepsia, constipation, vomiting,
hypertension, abdominal pain, diarrhea, tachycardia, dizziness, and
gastroesophageal reflux disease.
Use in Specific Populations:
- Moderate or Severe Renal Impairment: Not recommended
- Mild Hepatic Impairment: Not recommended
COBENFY (xanomeline and trospium chloride) is available in
50mg/20mg, 100mg/20mg, and 125mg/30mg capsules.
Please see U.S. Full Prescribing Information,
including Patient Information.
About Bristol Myers Squibb
Bristol Myers Squibb is a global biopharmaceutical company whose
mission is to discover, develop and deliver innovative medicines
that help patients prevail over serious diseases. For more
information about Bristol Myers Squibb, visit us at BMS.com or
follow us on LinkedIn, Twitter, YouTube, Facebook and
Instagram.
Cautionary Statement Regarding
Forward-Looking Statements
This press release contains “forward-looking statements” within
the meaning of the Private Securities Litigation Reform Act of 1995
regarding, among other things, the research, development and
commercialization of pharmaceutical products. All statements that
are not statements of historical facts are, or may be deemed to be,
forward-looking statements. Such forward-looking statements are
based on current expectations and projections about our future
financial results, goals, plans and objectives and involve inherent
risks, assumptions and uncertainties, including internal or
external factors that could delay, divert or change any of them in
the next several years, that are difficult to predict, may be
beyond our control and could cause our future financial results,
goals, plans and objectives to differ materially from those
expressed in, or implied by, the statements. These risks,
assumptions, uncertainties and other factors include, among others,
whether COBENFY (xanomeline and trospium chloride) for the
indication described in this release will be commercially
successful, any marketing approvals, if granted, may have
significant limitations on their use, and that continued approval
of COBENFY for such indication described in this release may be
contingent upon verification and description of clinical benefit in
confirmatory trials. No forward-looking statement can be
guaranteed. Forward-looking statements in this press release should
be evaluated together with the many risks and uncertainties that
affect Bristol Myers Squibb’s business and market, particularly
those identified in the cautionary statement and risk factors
discussion in Bristol Myers Squibb’s Annual Report on Form 10-K for
the year ended December 31, 2023, as updated by our subsequent
Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and
other filings with the Securities and Exchange Commission. The
forward-looking statements included in this document are made only
as of the date of this document and except as otherwise required by
applicable law, Bristol Myers Squibb undertakes no obligation to
publicly update or revise any forward-looking statement, whether as
a result of new information, future events, changed circumstances
or otherwise.
REFERENCES
- COBENFY Prescribing Information. COBENFY U.S. Product
Information. September 2024. Princeton, N.J.: Bristol Myers Squibb
Company.
- Kaul I, Sawchak S, Walling DP, et al. Efficacy and safety of
xanomeline-trospium chloride in schizophrenia: a randomized
clinical trial. JAMA Psychiatry. 2024;81(8):749–756.
doi:10.1001/jamapsychiatry.2024.0785
- Nucifora FC, Mihaljevic M, Lee BJ, Sawa A. Clozapine as a model
for antipsychotic development. Neurotherapeutics.
2017;14(3):750-761. doi: 10.1007/s13311-017-0552-9
- Kaul I, Sawchak S, Correll CU, et al. Efficacy and safety of
the muscarinic receptor agonist KarXT (xanomeline-trospium) in
schizophrenia (EMERGENT-2) in the USA: results from a randomised,
double-blind, placebo-controlled, flexible-dose phase 3 trial.
Lancet. 2024;403(10422):160-170. doi:
10.1016/S0140-6736(23)02190-6
- Schizophrenia. National Institute of Mental Health. Accessed
August 5, 2024.
https://www.nimh.nih.gov/health/topics/schizophrenia#part_145430
- Schizophrenia Fact Sheet. Treatment Advocacy Center. Accessed
August 5, 2024.
https://www.treatmentadvocacycenter.org/reports_publications/schizophrenia-fact-sheet
- Patel KR, Cherian J, Gohill K, et al. Schizophrenia: overview
and treatment options. P T. 2014;39(9):638-645. Accessed August 5,
2024. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4159061
- Duman ZC, Tuncer GZ, Sari, A, et al. Views of Individuals
Diagnosed with Schizophrenia on Working Life: A Qualitative Study.
Journal of Psychiatric Nursing. 2021;12(4):341-349.
doi:10.14744/phd.2021.80947
- Sahu KK, Intervening Negative Impact of Stigma on Employability
of a Person with Schizophrenia Through Social Case Work. J.
Psychosoc. Rehabil. Ment. Health. 2015;2(6):87-95.
doi:10.1007/s40737-015-0029-2
- Schizophrenia. World Health Organization. January 10, 2022.
Accessed August 5, 2024.
https://www.who.int/news-room/fact-sheets/detail/schizophrenia
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and
national incidence, prevalence, and years lived with disability for
328 diseases and injuries for 195 countries, 1990-2016: a
systematic analysis for the Global Burden of Disease Study 2016.
Lancet. 2017 Sep 16;390(10100):1211-1259. doi:
10.1016/S0140-6736(17)32154-2
corporatefinancial-news
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240925382351/en/
Media Inquiries: media@bms.com Investors:
investor.relations@bms.com
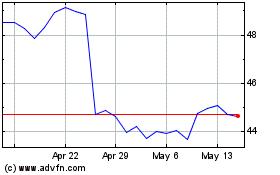
Bristol Myers Squibb (NYSE:BMY)
Historical Stock Chart
From Jan 2025 to Feb 2025
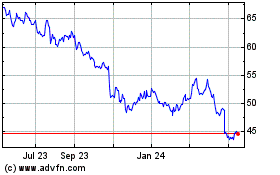
Bristol Myers Squibb (NYSE:BMY)
Historical Stock Chart
From Feb 2024 to Feb 2025