false000144438000014443802024-11-112024-11-11
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): November 11, 2024 |
NEVRO CORP.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-36715 |
56-2568057 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
1800 Bridge Parkway |
|
Redwood City, California |
|
94065 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (650) 251-0005 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, $0.001 par value per share |
|
NVRO |
|
The New York Stock Exchange |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On November 11, 2024, Nevro Corp. (“Nevro” or the “Company”) issued a press release relating to its financial results for the three months ended September 30, 2024. The full text of the press release is furnished herewith as Exhibit 99.1.
The Company also hosted a conference call and webcast on November 11, 2024 to discus its results for the three months ended September 30, 2024 and its full-year 2024 guidance. Supplemental slides referenced during the conference call and webcast were available on the Company’s website for viewing by participants. A transcript of the conference call and webcast together with the supplemental slides are furnished herewith as Exhibit 99.2 and Exhibit 99.3, respectively, and are incorporated herein by reference.
Item 7.01. Regulation FD Disclosure.
As noted in Item 2.02 of this report, the Company hosted a conference call and webcast on November 11, 2024, to discuss the Company’s financial results for the three months ended September 30, 2024, and full-year 2024 guidance as reported in the Company’s November 11, 2024 press release. A copy of the press release, which contains additional information regarding how to access the conference call and webcast and how to listen to a recorded playback, is furnished herewith as Exhibit 99.1. A transcript of the conference call and webcast together with supplemental slides referenced during the conference call and webcast are furnished herewith as Exhibit 99.2 and Exhibit 99.3, respectively, and are incorporated herein by reference.
* * * *
The information furnished pursuant to Item 2.02 and Item 7.01 of this Current Report on Form 8-K and Exhibit 99.1, Exhibit 99.2 and Exhibit 99.3, attached hereto shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in Item 2.02 and Item 7.01 above, including Exhibit 99.1, Exhibit 99.2 and Exhibit 99.3, shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
NEVRO CORP. |
|
|
|
|
Date: |
November 14, 2024 |
By: |
/s/ Roderick H. MacLeod |
|
|
|
Roderick H. MacLeod
Chief Financial Officer |
Nevro Reports Third-Quarter 2024 Financial Results
Reaffirms Full-Year 2024 Revenue Guidance and
Raises Full-Year 2024 Adjusted EBITDA Guidance
REDWOOD CITY, California – November 11, 2024 – Nevro Corp. (NYSE: NVRO), a global medical device company that is delivering comprehensive, life-changing solutions for the treatment of chronic pain, today reported its third-quarter 2024 financial results, reaffirmed its full-year 2024 revenue guidance and raised its full-year 2024 adjusted EBITDA guidance.
“Our worldwide revenue and adjusted EBITDA came in better than we anticipated in the third quarter of 2024. In addition, our cash position reflects the benefits from our restructurings earlier this year as well as our focus on working capital management,” said Kevin Thornal, Nevro’s CEO. “We continue to make improvements in our commercial execution and allocation of marketing resources to address ongoing market challenges and return to top-line growth.”
“We are excited about our recent limited market launch of HFX AdaptivAI™, the only artificial intelligence (AI)-driven technology in spinal cord stimulation, which delivers responsive and personalized pain relief in real time. We anticipate the full market release of HFX AdaptivAI by the end of November,” continued Thornal. “We are also thrilled to receive regulatory approval to now offer our HFX iQ system in CE-marked countries in Europe. Our team worked tirelessly to achieve this milestone, and we look forward to the limited market release of HFX iQ in select regions of Europe in the fourth quarter of 2024, followed by a full market release in 2025.”
“In addition, we continue to explore strategic options to accelerate our growth, diversify our product portfolio and deliver shareholder value,” said Thornal. “While this process is ongoing and we are in discussions, we remain focused on our strategy to become the leading provider of treatment options with the most diversified, differentiated and innovative product portfolio in the pain management space.”
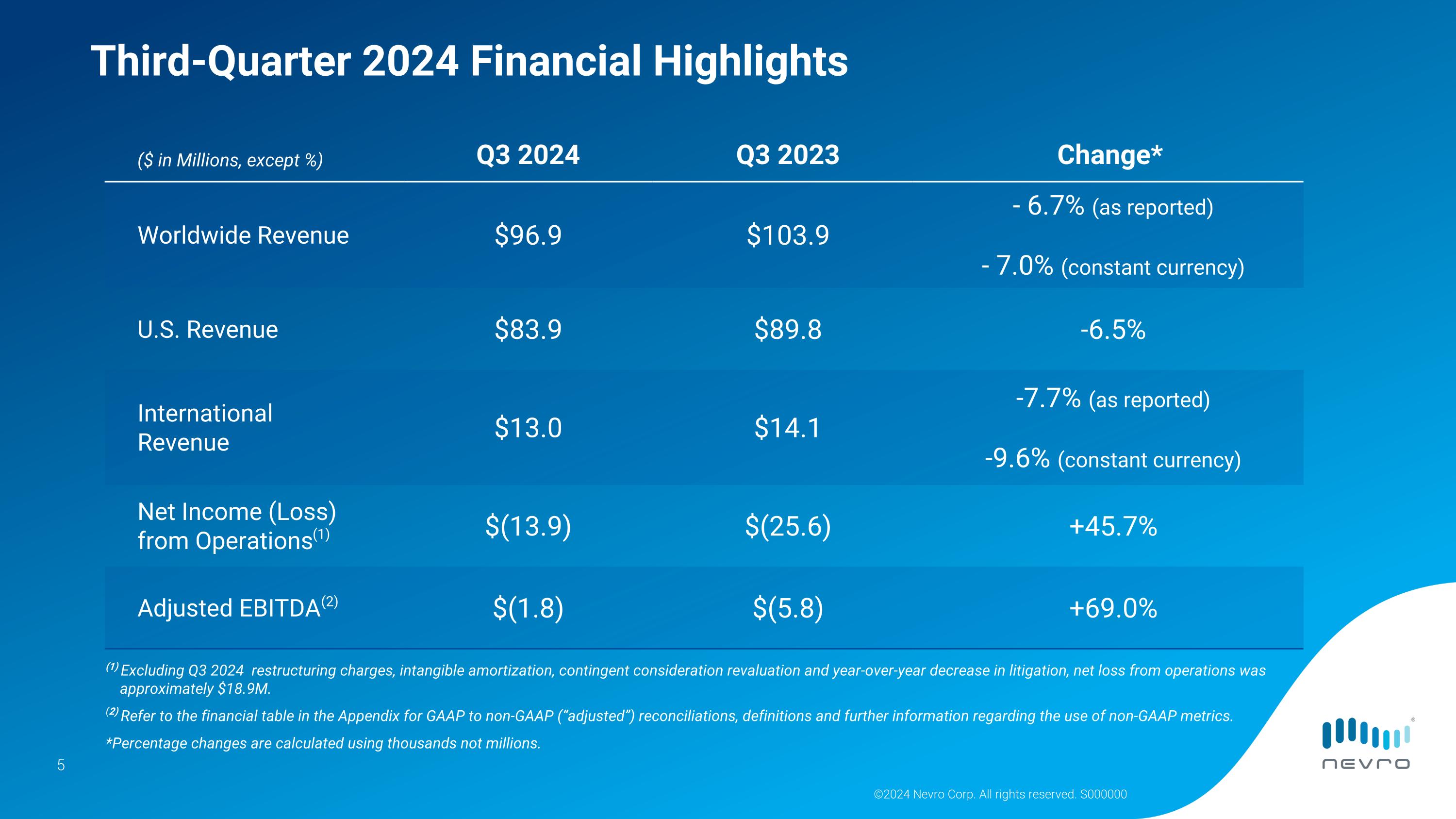
Third-Quarter 2024 Financial and Recent Business Highlights
(As compared with third-quarter 2023)
•Worldwide revenue was $96.9 million, down 6.7% as reported and 7.0% on a constant currency basis.
U.S. revenue was $83.9 million, down 6.5%.
International revenue was $13.0 million, down 7.7% as reported and 9.6% on a constant currency basis.
•U.S. trial procedures decreased 15.2%.
•Net loss from operations was $13.9 million; adjusted EBITDA was negative $1.8 million. Refer to the financial table at the end of this release for GAAP to non-GAAP reconciliations, definitions and further information regarding the use of non-GAAP metrics.
•Cash, cash equivalents and short-term investments totaled $277.0 million as of September 30, 2024, increasing $3.3 million from June 30, 2024. The increase reflects the benefits from the company’s restructuring efforts in the first half of 2024 and disciplined working capital management.
•On September 24, 2024, Nevro announced the US Food and Drug Administration approval and limited market release of HFX iQ AdaptivAI, a responsive, personalized pain management platform powering the HFX iQ spinal
cord stimulation (SCS) system. The company anticipates a full market release of HFX AdaptivAI in the U.S. in the fourth quarter of 2024.
•On October 29, 2024, Nevro announced the publication of new data in the Journal of Pain Research demonstrating significant, durable pain relief and long-term and clinically meaningful reductions in hemoglobin A1c (HbA1c) and weight in study participants with painful diabetic neuropathy (PDN) and Type 2 diabetes who received 10kHz high-frequency spinal cord stimulation (SCS) therapy.
•Nevro received regulatory approval to sell its HFX iQ system in CE-marked countries in the European Union and expects to begin the limited market release in select regions of Europe in the fourth quarter of 2024 with the full market release planned for the first quarter of 2025.
•Comparative biomechanical data on Nevro1™, a novel posterior integrated single-cage system has been accepted for publication in Medical Devices: Evidence and Research. Nevro1 was found to provide equivalent and superior motion reduction respectively with a less invasive and less destructive approach while providing the largest surface area for fusion.
Third-Quarter 2024 Financial Results
Worldwide revenue for the third quarter of 2024 was $96.9 million, a decrease of 6.7% as reported and 7.0% on a constant currency basis, compared with $103.9 million in the third quarter of 2023. The year-over-year decrease was primarily the result of softness in the U.S. SCS market and competitive pressures during the quarter as well as commercial execution.
U.S. revenue in the third quarter of 2024 was $83.9 million, a decrease of approximately 6.5% compared with $89.8 million in the prior year period. U.S. permanent implant procedures decreased 9.6% compared with the third quarter of 2023, and U.S. trial procedures decreased 15.2% compared with the third quarter of 2023 due to the same factors that affected revenue in the third quarter.
International revenue in the third quarter of 2024 was $13.0 million compared with $14.1 million in the third quarter of 2023, a decrease of approximately 7.7% as reported and 9.6% on a constant currency basis. The decline in revenue was primarily due to the continued short-term impact of negative SCS-related media reports in Australia that resulted in the postponement and cancellation of cases as well the ongoing impact of healthcare reform in Germany that caused a delay in procedures in the third quarter of 2024
Gross profit for the third quarter of 2024 was $64.6 million compared with $69.5 million in the third quarter of 2023. Gross margin in the third quarter of 2024 was 66.7% compared with 66.9% in the third quarter of 2023.
Operating expenses for the third quarter of 2024 were $78.5 million compared with $95.1 million for the year-ago period and includes restructuring charges, intangible amortization, contingent consideration revaluations, and a year-over-year reduction in litigation-related expenses. Excluding these items, operating expenses in the third quarter of 2024 decreased by approximately $11.6 million, or 12.2%, compared with the prior-year quarter, reflecting the benefits from the company's January and May 2024 restructurings and continued disciplined expense management efforts in the current-year quarter.
Litigation-related legal expenses were a credit of $0.6 million for the third quarter of 2024 compared with $4.3 million of costs for the third quarter of 2023. The year-over-year decrease was primarily due to the resolution and final payment of the company’s legal disputes with the Mayo Clinic and Flathead Partners.
Net loss from operations for the third quarter of 2024 was $13.9 million, or approximately $18.9 million excluding restructuring charges, intangible amortization, contingent consideration revaluations, and year-over-year decrease in litigation-related expenses. Net loss from operations in the third quarter of 2023 was $25.6 million.
Adjusted EBITDA for the third quarter of 2024 was a loss of $1.8 million compared with a loss of $5.8 million for the third quarter of 2023. Adjusted EBITDA excludes interest, taxes and non-cash items such as stock-based compensation and depreciation and amortization, as well as litigation-related expenses, restructuring and supplier
contract renegotiation charges, and other adjustments. Refer to the financial table at the end of this release for GAAP to adjusted (non-GAAP) reconciliations.
Cash, cash equivalents and short-term investments totaled $277.0 million as of September 30, 2024, an increase of $3.3 million from June 30, 2024. The increase was primarily the result of net cash provided from operations.
Full-Year 2024 Financial Guidance
Based on its third-quarter 2024 performance and outlook for the remainder of this year, Nevro continues to expect its full-year 2024 worldwide revenue to be in the range of approximately $400 million to $405 million. The company is raising its full-year 2024 adjusted EBITDA guidance to a range of negative $18 million to negative $16 million from its previous guidance range of negative $20 million to negative $18 million. Nevro’s full-year 2024 guidance assumes that its U.S. SCS trialing growth rate in the fourth quarter of 2024 does not improve from the third quarter of 2024.
Nevro has not provided a quantitative reconciliation of forecasted adjusted EBITDA to forecasted net income (loss) within this press release because the company is unable, without making unreasonable efforts, to calculate certain reconciling items with confidence. For more information regarding the non-GAAP financial measures discussed in this press release, please see the financial table at the end of this release for GAAP to non-GAAP reconciliations, definitions and further information regarding the use of non-GAAP metrics.
Conference Call and Webcast
Nevro will host a conference call and webcast today beginning at 1:30 p.m. PDT (4:30 p.m. EDT) to discuss its financial results. The live webcast and replay of the conference call will be available in the Investor Relations section of the company’s website at Events & Presentation. The webcast can be accessed 10 minutes prior to the conference call start time.
For those parties that do not have internet access, the conference call can be accessed by calling one of the below telephone numbers and providing conference ID 5980028:
U.S. domestic participant dial-in number (toll-free): 1-(888) 596-4144
International participant dial-in number: 1-(646) 968-2525
Internet Posting of Information
Nevro routinely posts information that may be important to investors in the "Investor Relations" section of its website at www.nevro.com. The company encourages investors and potential investors to consult the Nevro website regularly for important information about Nevro.
About Nevro
Headquartered in Redwood City, California, Nevro is a global medical device company focused on delivering comprehensive, life-changing solutions that continue to set the standard for enduring patient outcomes in chronic pain treatment. The company started with a simple mission to help more patients suffering from debilitating pain and developed its proprietary 10 kHz Therapy™, an evidence-based, non-pharmacologic innovation that has impacted the lives of more than 115,000 patients globally. Nevro's comprehensive HFX™ spinal cord stimulation (SCS) platform includes the Senza® SCS system and support services for the treatment of chronic pain of the trunk and limb and painful diabetic neuropathy.
Nevro recently added a minimally invasive treatment option for patients in the U.S. suffering from chronic sacroiliac joint ("SI joint") pain and now provides the most comprehensive portfolio of products in the SI joint fusion space, designed to meet the preferences of physicians and varying patient needs in order to improve outcomes and quality of life for patients. Senza®, Senza II®, Senza Omnia™, and HFX iQ are the only SCS systems
that deliver Nevro's proprietary 10 kHz Therapy. Nevro's unique support services provide every patient with an HFX Coach™ throughout their pain relief journey and every physician with Nevrocloud™ insights for enhanced patient and practice management.
SENZA, SENZA II, SENZA OMNIA, OMNIA, HF10, the HF10 logo, 10 kHz Therapy, HFX, the HFX logo, HFX iQ, the HFX iQ logo, HFX Algorithm, HFX CONNECT, the HFX Connect logo, HFX ACCESS, the HFX Access logo, HFX COACH, the HFX Coach logo, Nevrocloud, RELIEF MULTIPLIED, HFX AdaptivAI, the X logo, NEVRO, and the NEVRO logo are trademarks or registered trademarks of Nevro Corp. Patents covering Senza HFX iQ and other Nevro products are listed at Nevro.com/patents. Bluetooth® and the Bluetooth symbol are registered trademarks of their respective owners.
To learn more about Nevro, connect with us on LinkedIn, X, Facebook, and Instagram.
Forward-Looking Statements
In addition to historical information, this press release contains forward-looking statements reflecting the current beliefs and expectations of the company’s management, made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including: our full-year 2024 financial guidance; our belief that the actions we have taken and intend to take will further position us for a return to growth, success in the marketplace, profitability and shareholder value creation; our belief that execution improvements and our reallocation of marketing resources will allow us to return to sustainable growth; our belief that the market release of HFX AdaptivAI™ in U.S. and HFX iQ™ in Europe will successfully drive sales; our belief that evaluating and/or engaging in strategic opportunities will help us diversify and grow our business, which we believe may position us to accelerate our goals of profitability and maximizing shareholder value; and our beliefs with regards to the SCS market and factors impacting our results, including the duration in which those factors will continue to impact our results; and our beliefs in the catalysts for long-term growth. These forward-looking statements are based upon information that is currently available to us or our current expectations, speak only as of the date hereof, and are subject to numerous risks and uncertainties, including our ability to successfully commercialize our products; our ability to manufacture our products to meet demand; the level and availability of third-party payor reimbursement for our products; our ability to effectively manage our anticipated growth and the costs and expenses of operating our business; our ability to protect our intellectual property rights and proprietary technologies; our ability to operate our business without infringing the intellectual property rights and proprietary technology of third parties; competition in our industry; additional capital and credit availability; our ability to successfully integrate any additive acquisitions we may make, including our acquisition of Vyrsa Technologies; our ability to attract and retain qualified personnel; our ability to accurately forecast financial and operating results; our ability to successfully evaluate and execute on potential strategic opportunities; and product liability claims. These factors, together with those that are described in greater detail in our Annual Report on Form 10-K filed on February 23, 2024, as well as any reports that we may file with the Securities and Exchange Commission in the future, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. We expressly disclaim any obligation, except as required by law, or undertaking to update or revise any such forward-looking statements. Nevro's operating results for the period ending September 30, 2024, are not necessarily indicative of the company’s operating results for any future periods.
Investor and Media Contact:
Angie McCabe
Vice President, Investor Relations & Corporate Communications
angeline.mccabe@nevro.com
Nevro Corp.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended |
|
|
|
September 30, |
|
|
|
2024 |
|
|
2023 |
|
|
|
(unaudited) |
|
Revenue |
|
$ |
96,910 |
|
|
$ |
103,862 |
|
Cost of revenue |
|
|
32,296 |
|
|
|
34,346 |
|
Gross profit |
|
|
64,614 |
|
|
|
69,516 |
|
Operating expenses: |
|
|
|
|
|
|
Research and development |
|
|
10,579 |
|
|
|
13,923 |
|
Sales, general and administrative |
|
|
68,471 |
|
|
|
81,152 |
|
Amortization of intangibles |
|
|
737 |
|
|
|
— |
|
Change in fair value of contingent consideration |
|
|
(1,307 |
) |
|
|
— |
|
Total operating expenses |
|
|
78,480 |
|
|
|
95,075 |
|
Loss from operations |
|
|
(13,866 |
) |
|
|
(25,559 |
) |
Other income (expense): |
|
|
|
|
|
|
Interest income (expense), net |
|
|
(3,782 |
) |
|
|
1,976 |
|
Change in fair market value of warrants |
|
|
3,438 |
|
|
|
— |
|
Other income (expense), net |
|
|
(854 |
) |
|
|
234 |
|
Loss before income taxes |
|
|
(15,064 |
) |
|
|
(23,349 |
) |
Provision for income taxes |
|
|
280 |
|
|
|
130 |
|
Net loss |
|
|
(15,344 |
) |
|
|
(23,479 |
) |
Changes in foreign currency translation adjustment |
|
|
1,337 |
|
|
|
(765 |
) |
Changes in unrealized gains (losses) on short-term investments |
|
|
1,239 |
|
|
|
470 |
|
Net change in other comprehensive income (loss) |
|
|
2,576 |
|
|
|
(295 |
) |
Comprehensive loss |
|
$ |
(12,768 |
) |
|
$ |
(23,774 |
) |
Net loss per share, basic and diluted |
|
$ |
(0.41 |
) |
|
$ |
(0.65 |
) |
Weighted average shares used to compute
net loss per share |
|
|
37,324,907 |
|
|
|
36,142,255 |
|
Nevro Corp.
Condensed Consolidated Balance Sheets
(in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
September 30, |
|
|
December 31, |
|
|
|
2024 |
|
|
2023 |
|
|
|
(unaudited) |
|
|
|
|
Assets |
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
71,982 |
|
|
$ |
104,217 |
|
Short-term investments |
|
|
205,056 |
|
|
|
218,506 |
|
Accounts receivable, net |
|
|
70,601 |
|
|
|
79,377 |
|
Inventories, net |
|
|
120,412 |
|
|
|
118,676 |
|
Prepaid expenses and other current assets |
|
|
11,189 |
|
|
|
10,145 |
|
Total current assets |
|
|
479,240 |
|
|
|
530,921 |
|
Property and equipment, net |
|
|
24,928 |
|
|
|
24,568 |
|
Operating lease assets |
|
|
21,776 |
|
|
|
8,944 |
|
Goodwill |
|
|
38,209 |
|
|
|
38,164 |
|
Other intangible assets, net |
|
|
25,144 |
|
|
|
27,354 |
|
Other assets |
|
|
5,745 |
|
|
|
5,156 |
|
Restricted cash |
|
|
606 |
|
|
|
606 |
|
Total assets |
|
$ |
595,648 |
|
|
$ |
635,713 |
|
Liabilities and stockholders’ equity |
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
Accounts payable |
|
$ |
20,309 |
|
|
$ |
22,520 |
|
Accrued liabilities and other |
|
|
34,943 |
|
|
|
45,297 |
|
Short-term debt |
|
|
37,906 |
|
|
|
— |
|
Contingent liabilities, current portion |
|
|
1,912 |
|
|
|
9,836 |
|
Other current liabilities |
|
|
343 |
|
|
|
5,722 |
|
Total current liabilities |
|
|
95,413 |
|
|
|
83,375 |
|
Long-term debt |
|
|
184,364 |
|
|
|
211,471 |
|
Long-term operating lease liabilities |
|
|
24,321 |
|
|
|
4,634 |
|
Contingent liabilities, non-current portion |
|
|
13,501 |
|
|
|
12,257 |
|
Warrant liability |
|
|
2,238 |
|
|
|
28,739 |
|
Other long-term liabilities |
|
|
2,168 |
|
|
|
2,092 |
|
Total liabilities |
|
|
322,005 |
|
|
|
342,568 |
|
Stockholders’ equity |
|
|
|
|
|
|
Common stock, $0.001 par value, 290,000,000 shares authorized;
38,111,415 and 37,044,390 shares issued at September 30, 2024
and December 31, 2023, respectively; 37,439,555 and 36,361,474
shares outstanding at September 30, 2024 and December 31,
2023, respectively |
|
|
37 |
|
|
|
36 |
|
Additional paid-in capital |
|
|
1,031,899 |
|
|
|
992,762 |
|
Accumulated other comprehensive income (loss) |
|
|
1,445 |
|
|
|
(243 |
) |
Accumulated deficit |
|
|
(759,738 |
) |
|
|
(699,410 |
) |
Total stockholders’ equity |
|
|
273,643 |
|
|
|
293,145 |
|
Total liabilities and stockholders’ equity |
|
$ |
595,648 |
|
|
$ |
635,713 |
|
Nevro Corp.
GAAP to Non-GAAP Adjusted EBITDA Reconciliation
(unaudited)
(in thousands)
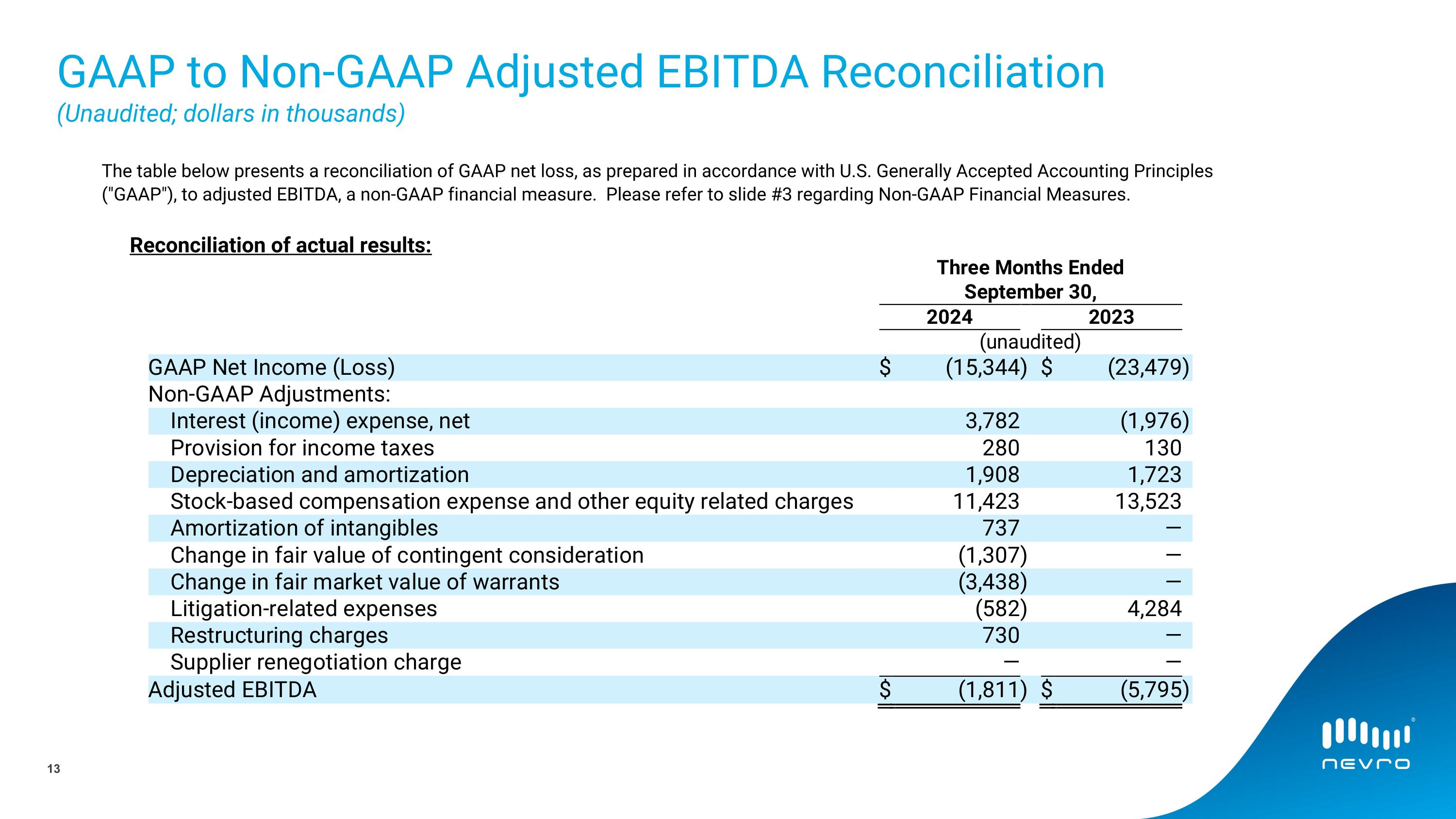
The following table presents a reconciliation of GAAP net loss, as prepared in accordance with U.S. Generally Accepted Accounting Principles ("GAAP"), to adjusted EBITDA, a non-GAAP financial measure.
Reconciliation of actual results:
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended |
|
|
|
September 30, |
|
|
|
2024 |
|
|
2023 |
|
|
|
(unaudited) |
|
GAAP Net Income (Loss) |
|
$ |
(15,344 |
) |
|
$ |
(23,479 |
) |
Non-GAAP Adjustments: |
|
|
|
|
|
|
Interest (income) expense, net |
|
|
3,782 |
|
|
|
(1,976 |
) |
Provision for income taxes |
|
|
280 |
|
|
|
130 |
|
Depreciation and amortization |
|
|
1,908 |
|
|
|
1,723 |
|
Stock-based compensation expense and other equity related charges |
|
|
11,423 |
|
|
|
13,523 |
|
Amortization of intangibles |
|
|
737 |
|
|
|
— |
|
Change in fair value of contingent consideration |
|
|
(1,307 |
) |
|
|
— |
|
Change in fair market value of warrants |
|
|
(3,438 |
) |
|
|
— |
|
Litigation-related expenses |
|
|
(582 |
) |
|
|
4,284 |
|
Restructuring charges |
|
|
730 |
|
|
|
— |
|
Supplier renegotiation charge |
|
|
— |
|
|
|
— |
|
Adjusted EBITDA |
|
$ |
(1,811 |
) |
|
$ |
(5,795 |
) |
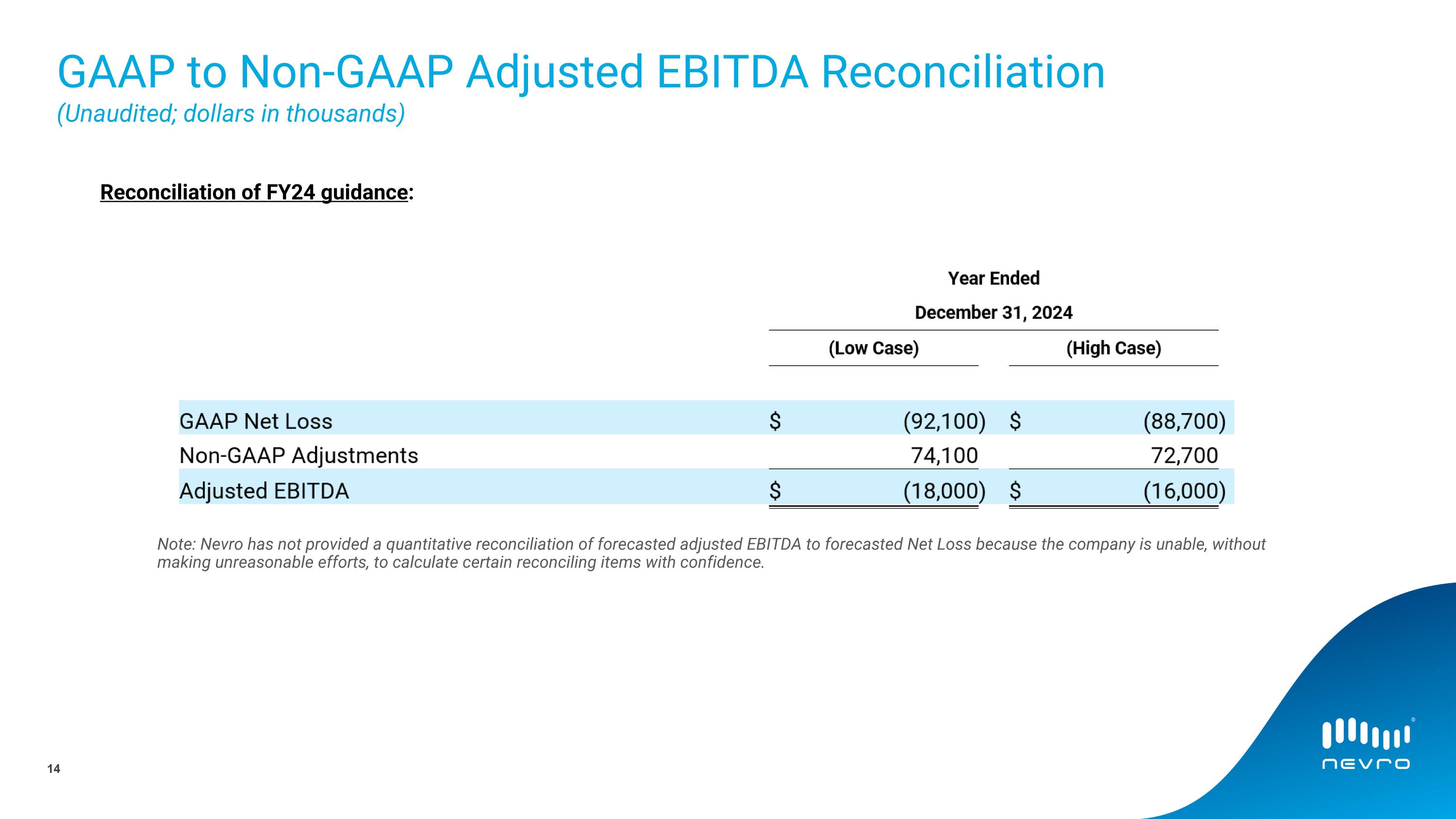
Reconciliation of guidance:
|
|
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
|
December 31, 2024 |
|
|
|
(Low Case) |
|
|
(High Case) |
|
|
|
|
|
|
|
|
GAAP Net Loss |
|
$ |
(92,100 |
) |
|
$ |
(88,700 |
) |
Non-GAAP Adjustments |
|
|
74,100 |
|
|
|
72,700 |
|
Adjusted EBITDA |
|
$ |
(18,000 |
) |
|
$ |
(16,000 |
) |
Management uses certain non-GAAP financial measures, most specifically adjusted EBITDA, as a supplement to GAAP financial measures to further evaluate the company's operating performance period over period, analyze the underlying business trends, assess performance relative to competitors and establish operational objectives.
Management believes it is important to provide investors with the same non-GAAP metrics it uses to evaluate the performance and underlying trends of the company's business operations to facilitate comparisons to its historical operating results and evaluate the effectiveness of its operating strategies. Disclosure of these non-GAAP financial measures also facilitates comparisons of the company's underlying operating performance with other companies in the industry that also supplement their GAAP results with non-GAAP financial measures.
EBITDA is a non-GAAP financial measure, which is calculated by adding interest income and expense, net; provision for income taxes; and depreciation and amortization to net income. In calculating non-GAAP adjusted EBITDA, the company further adjusts for the following items:
•Stock-based compensation expense and other equity-related charges – The company excludes non-cash costs related to the company's stock-based plans, which include stock options, restricted stock units and performance-based restricted stock units as these expenses do not require cash settlement from the company.
•Amortization of intangibles – The company excludes amortization of intangibles from the acquisition of businesses.
•Change in fair value of contingent consideration – The company excludes the changes in the fair value of its contingent consideration liability.
•Change in fair market value of warrants – The company excludes the changes in the fair value of its warrant liability.
•Litigation-related expenses – The company excludes legal and professional fees as well as charges and credits associated with certain legal matters, which management considers not related to the underlying operating performance of the business.
•Restructuring charges – The company excludes charges incurred as a direct result of restructuring programs, such as salaries and other compensation-related expenses.
•Supplier contract renegotiation charge – The company excluded one-time costs associated with the renegotiation of a supplier contract.
Full-year guidance excludes the impact of foreign currency fluctuations.
The non-GAAP financial measure should not be considered in isolation from, or as a replacement for, the most directly comparable GAAP financial measures, as it is not prepared in accordance with U.S. GAAP.
Nevro has not provided a quantitative reconciliation of forecasted adjusted EBITDA to forecasted net income (loss) within this press release because the company is unable, without making unreasonable efforts, to calculate certain reconciling items with confidence. These items include, but are not limited to, stock-based compensation expenses, amortization of intangibles, change in fair value of contingent consideration, change in fair value of warrants, and litigation-related expenses.
Amounts may not add due to rounding and percentages are calculated using thousands not millions.
# # #

©2024 Nevro Corp. All rights reserved. S000000 Third Quarter 2024�Earnings Conference Call November 11, 2024 Exhibit 99.2

©2024 Nevro Corp. All rights reserved. S000000 In addition to historical information, this presentation contains forward-looking statements reflecting the current beliefs and expectations of the company’s management, made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including: our full-year 2024 financial guidance; our belief that the actions we have taken and intend to take will further position us for a return to growth, success in the marketplace, profitability and shareholder value creation; our belief that the expansion of territories led by newly promoted associate sales reps and our reallocating marketing resources will allow us to return to sustainable growth; our belief that the market release of HFX AdaptivAI™ in U.S. and HFX iQ™ in Europe will successfully drive sales; our belief that evaluating and/or engaging in strategic opportunities will help us diversify and grow our business, which we believe may position us to accelerate our goals of profitability and maximizing shareholder value; and our beliefs with regards to the SCS market and factors impacting our results, including the duration in which those factors will continue to impact our results; and our beliefs in the catalysts for long-term growth. These forward-looking statements are based upon information that is currently available to us or our current expectations, speak only as of the date hereof, and are subject to numerous risks and uncertainties, including our ability to successfully commercialize our products; our ability to manufacture our products to meet demand; the level and availability of third-party payor reimbursement for our products; our ability to effectively manage our anticipated growth and the costs and expenses of operating our business; our ability to protect our intellectual property rights and proprietary technologies; our ability to operate our business without infringing the intellectual property rights and proprietary technology of third parties; competition in our industry; additional capital and credit availability; our ability to successfully integrate any future acquisitions we may make, including our acquisition of Vyrsa Technologies; our ability to attract and retain qualified personnel; our ability to accurately forecast financial and operating results; our ability to successfully evaluate and execute on potential strategic opportunities; and product liability claims. These factors, together with those that are described in greater detail in our Annual Report on Form 10-K, as well as any reports that we may file with the Securities and Exchange Commission in the future, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. We expressly disclaim any obligation, except as required by law, or undertaking to update or revise any such forward-looking statements. Nevro's operating results for the third-quarter ended September 30, 2024, are not necessarily indicative of our operating results for any future periods. Forward-Looking Statements

©2024 Nevro Corp. All rights reserved. S000000 Management uses certain non-GAAP financial measures, most specifically Adjusted EBITDA, as a supplement to GAAP financial measures to further evaluate the company’s operating performance period over period, analyze the underlying business trends, assess performance relative to competitors and establish operational objectives. Management believes it is important to provide investors with the same non-GAAP metrics it uses to evaluate the performance and underlying trends of the company’s business operations to facilitate comparisons to its historical operating results and evaluate the effectiveness of its operating strategies. Disclosure of these non-GAAP financial measures also facilitates comparisons of the company’s underlying operating performance with other companies in the industry that also supplement their GAAP results with non-GAAP financial measures. EBITDA is a non-GAAP financial measure, which is calculated by adding interest income and expense, net; provision for income taxes; and depreciation and amortization to net income. In calculating non-GAAP Adjusted EBITDA, the company further adjusts for the following items: Stock-based compensation expense – The company excludes non-cash costs related to the company's stock-based plans, which include stock options, restricted stock units and performance-based restricted stock units as these expenses do not require cash settlement from the company. Amortization of intangibles – The company excludes amortization of intangibles from the acquisition of businesses. Change in fair value of contingent consideration – The company excludes the changes in the fair value of its contingent consideration liability. Change in the fair market value of warrants – The company excludes the changes in the fair market value of its warrant liability, which management considers not related to the underlying operating performance of the business. Litigation-related expenses – The company excludes legal and professional fees as well as charges and credits associated with certain legal matters, which management considers not related to the underlying operating performance of the business. Restructuring charges – The company excludes charges incurred as a direct result of restructuring programs, such as salaries and other compensation-related expenses. Supplier contract renegotiation charge – The company excluded one-time costs associated with the renegotiation of a supplier contract. Full-year guidance excludes the impact of foreign currency fluctuations. The non-GAAP financial measure should not be considered in isolation from, or as a replacement for, the most directly comparable GAAP financial�measures, as it is not prepared in accordance with U.S. GAAP. Non-GAAP Financial Measures

Business Overview Kevin Thornal Chief Executive Officer &� President Financial Overview Rod MacLeod Chief Financial Officer &� Senior Vice President
©2024 Nevro Corp. All rights reserved. S000000 Third-Quarter 2024 Financial Highlights ($ in Millions, except %) Q3 2024 Q3 2023 Change* Worldwide Revenue $96.9 $103.9 - 6.7% (as reported) - 7.0% (constant currency) U.S. Revenue $83.9 $89.8 -6.5% International�Revenue $13.0 $14.1 -7.7% (as reported) -9.6% (constant currency) Net Income (Loss) from Operations(1) $(13.9) $(25.6) +45.7% Adjusted EBITDA(2) $(1.8) $(5.8) +69.0% (1) Excluding Q3 2024 restructuring charges, intangible amortization, contingent consideration revaluation and year-over-year decrease in litigation, net loss from operations was approximately $18.9M. (2) Refer to the financial table in the Appendix for GAAP to non-GAAP (“adjusted”) reconciliations, definitions and further information regarding the use of non-GAAP metrics. *Percentage changes are calculated using thousands not millions.

©2024 Nevro Corp. All rights reserved. S000000 ” Implementing actions to improve competitive positioning, drive market penetration, return to sustainable top-line growth and continue on path toward profitability Improving commercial execution by expanding territories led by newly promoted associate sales reps and reallocating marketing resources to return to sustainable growth Continued to build and leverage R&D pipeline with limited market releases�of HFX AdaptivAI™ in U.S. and HFX iQ™ in select regions of Europe Building on strong foundation of robust clinical data Cash position increased $3.3 million sequentially and remains strong�with $277.0 million in cash, cash equivalents and short-term investments�on balance sheet Reaffirming FY2024 worldwide revenue guidance and raising FY2024 adjusted EBITDA guidance Continue to explore strategic options to accelerate growth, diversify the product portfolio and deliver shareholder value Third-Quarter 2024 Key Messages

Implementing Actions to Improve Commercial Execution Promoted several new associate sales representatives (ASR) to lead newly created territories in 3Q24 with plan to add new territories in 2025 Expect ramp-up period in 2025 as new ASRs get established in their territories Allows sales team to reach more customers, go deeper with physicians provide high level of customer service and lays foundation for new product introductions Reallocating resources to marketing initiatives to drive patient lead generation and convert higher number of patient leads into spinal cord stimulation (SCS) trials Beginning to see patient interest and response to new DTC advertising campaigns and confident we will see meaningful improvement in SCS trialing activity in 2H2025
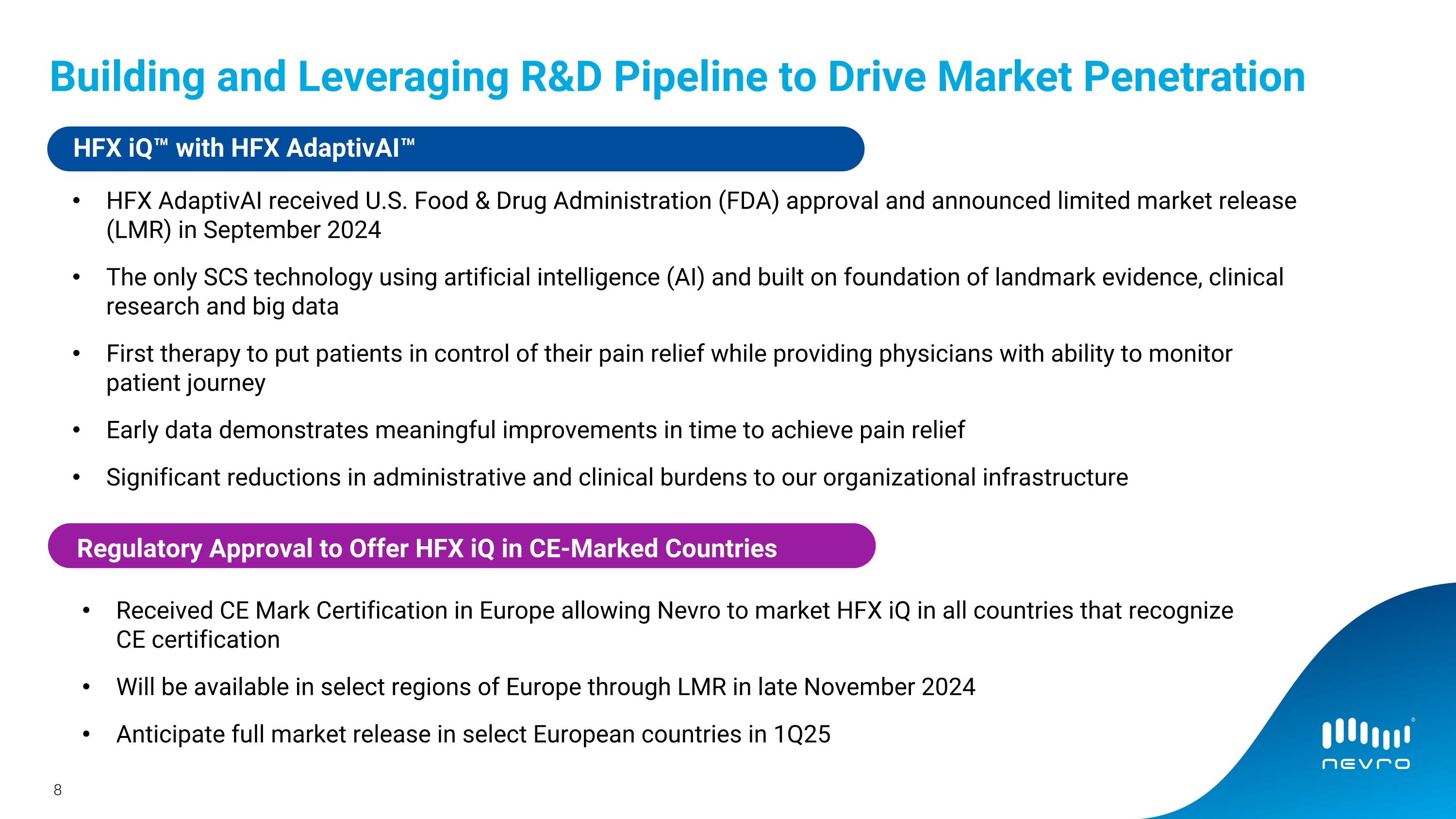
Building and Leveraging R&D Pipeline to Drive Market Penetration HFX AdaptivAI received U.S. Food & Drug Administration (FDA) approval and announced limited market release (LMR) in September 2024 The only SCS technology using artificial intelligence (AI) and built on foundation of landmark evidence, clinical research and big data First therapy to put patients in control of their pain relief while providing physicians with ability to monitor patient journey Early data demonstrates meaningful improvements in time to achieve pain relief Significant reductions in administrative and clinical burdens to our organizational infrastructure Commercial Execution HFX iQ™ with HFX AdaptivAI™ Received CE Mark Certification in Europe allowing Nevro to market HFX iQ in all countries that recognize CE certification Will be available in select regions of Europe through LMR in late November 2024 Anticipate full market release in select European countries in 1Q25 Regulatory Approval to Offer HFX iQ in CE-Marked Countries

Strategy to grow through expanded SCS therapy and alternative therapies earlier in the care continuum Continue to focus on growing painful diabetic neuropathy (PDN) business by educating physicians and raising patient awareness on benefits of SCS therapy New data published in Journal of Pain Medicine demonstrating long-term improvements in pain intensity with�high-frequency SCS therapy First study of SCS to demonstrate long-term, significant and clinically meaningful reductions in HbA1C and weight in participants with PDN and Type 2 diabetes who received high-frequency SCS therapy; initiated feasibility study to specifically evaluate use of 10 kHz therapy to affect HbA1C Senza-Sensory RCT interim analysis ongoing; expect readout in early 2025 Ramping and scaling sacroiliac (SI) joint fusion business Continue to receive positive feedback from physicians who are trained and performing SI joint fusion procedures Comparative biomechanical data on Nevro1™ accepted for publication; Nevro1 was found to provide equivalent �and superior motion reduction respectively with less invasive and less destructive approach while providing�largest surface area for fusion Long-Term Goal:�Become Leading Comprehensive Pain Management Company
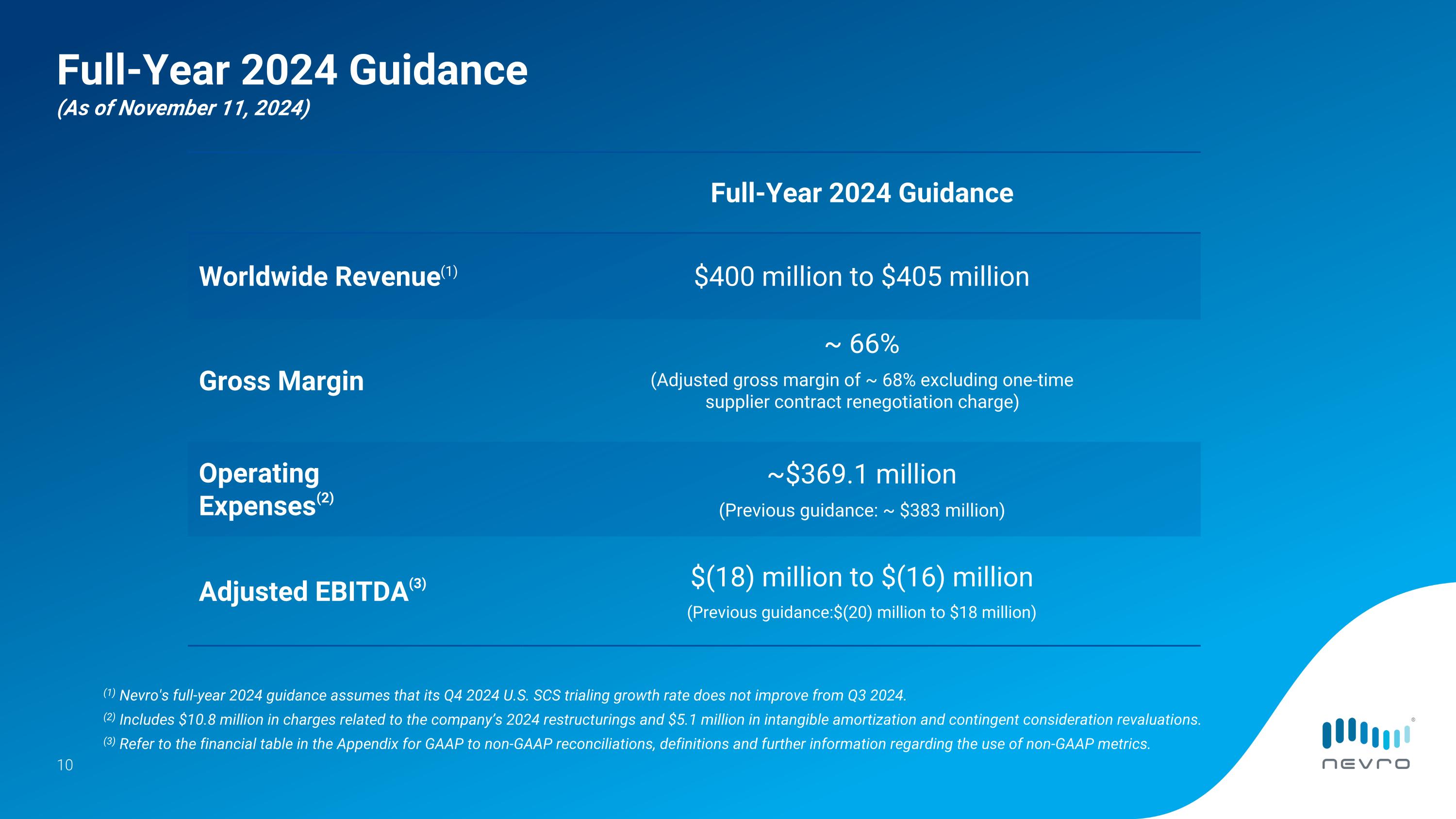
Full-Year 2024 Guidance�(As of November 11, 2024) Full-Year 2024 Guidance Worldwide Revenue(1) $400 million to $405 million Gross Margin ~ 66% (Adjusted gross margin of ~ 68% excluding one-time supplier contract renegotiation charge) Operating�Expenses(2) ~$369.1 million (Previous guidance: ~ $383 million) Adjusted EBITDA(3) $(18) million to $(16) million (Previous guidance:$(20) million to $18 million) (1) Nevro's full-year 2024 guidance assumes that its Q4 2024 U.S. SCS trialing growth rate does not improve from Q3 2024. (2) Includes $10.8 million in charges related to the company’s 2024 restructurings and $5.1 million in intangible amortization and contingent consideration revaluations. (3) Refer to the financial table in the Appendix for GAAP to non-GAAP reconciliations, definitions and further information regarding the use of non-GAAP metrics.
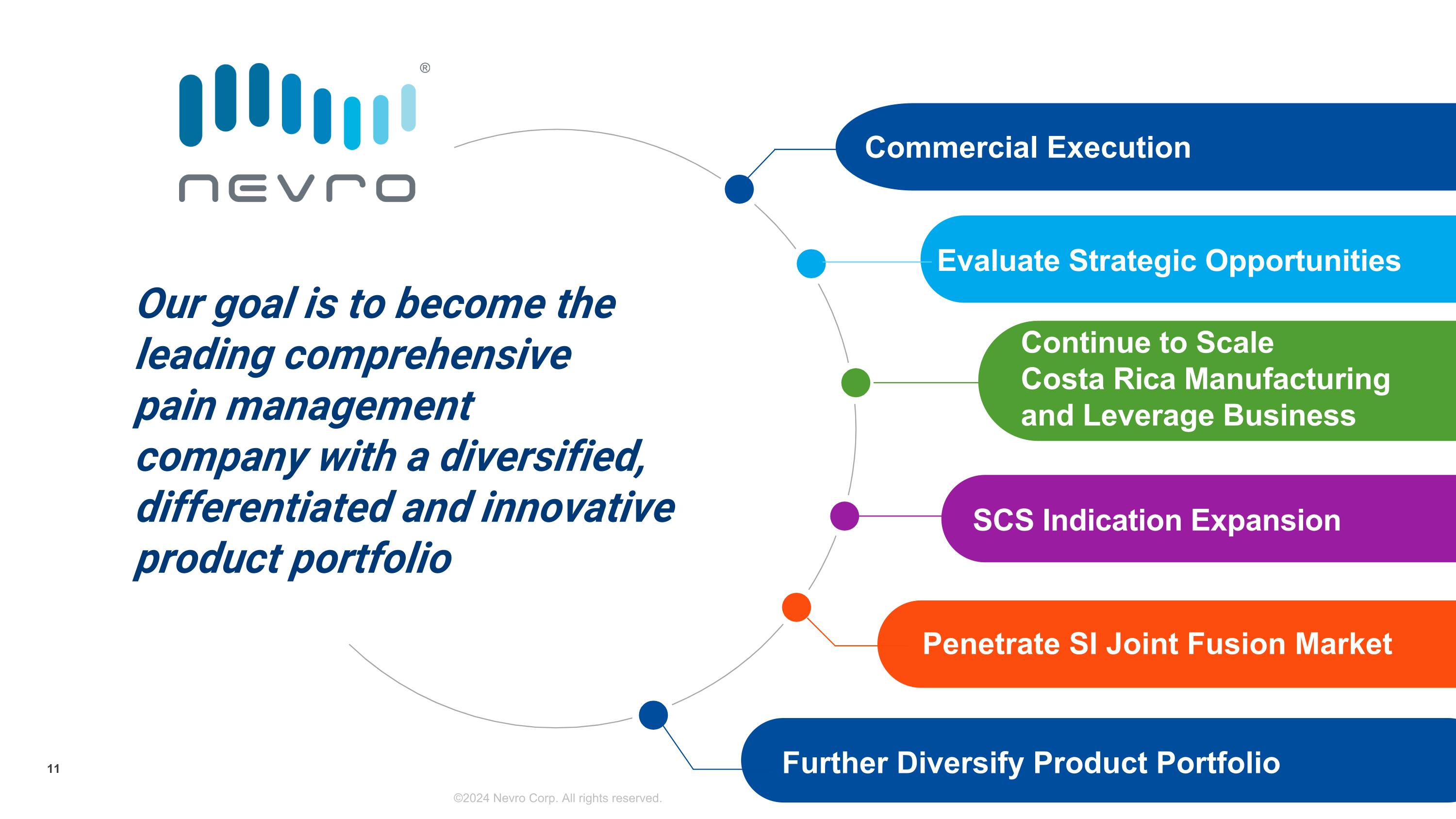
Our goal is to become the leading comprehensive�pain management�company with a diversified, differentiated and innovative product portfolio SCS Indication Expansion Continue to Scale�Costa Rica Manufacturing�and Leverage Business Evaluate Strategic Opportunities Commercial Execution Penetrate SI Joint Fusion Market ©2024 Nevro Corp. All rights reserved. 11 Further Diversify Product Portfolio

Appendix 12
GAAP to Non-GAAP Adjusted EBITDA Reconciliation�(Unaudited; dollars in thousands) The table below presents a reconciliation of GAAP net loss, as prepared in accordance with U.S. Generally Accepted Accounting Principles ("GAAP"), to adjusted EBITDA, a non-GAAP financial measure. Please refer to slide #3 regarding Non-GAAP Financial Measures. Reconciliation of actual results: 13
GAAP to Non-GAAP Adjusted EBITDA Reconciliation�(Unaudited; dollars in thousands) Note: Nevro has not provided a quantitative reconciliation of forecasted adjusted EBITDA to forecasted Net Loss because the company is unable, without making unreasonable efforts, to calculate certain reconciling items with confidence. Reconciliation of FY24 guidance: 14
angeline.mccabe@nevro.com For more information about Nevro, contact: Angie McCabe VICE PRESIDENT, INVESTOR RELATIONS & CORPORATE COMMUNICATIONS Nevro Corp. (NYSE: NVRO) 15

Nevro Corp.
(NYSE: NVRO)
Third-Quarter 2024 Earnings Call Transcript
November 11, 2024
Nevro Corp. Speakers
Angeline C. McCabe
Vice President, Investor Relations & Corporate Communications
Kevin R. Thornal
CEO & President
Roderick H. MacLeod
Senior Vice President & CFO
Operator
Good afternoon. My name is Christa, and I will be your conference operator today. At this time, I would like to welcome everyone to Nevro Corp.'s Third Quarter 2024 Earnings Conference Call and Webcast. Today's conference is being recorded. [Operator Instructions].
I will now turn the call over to Angie McCabe, Nevro's Vice President of Investor Relations and Corporate Communications. Ms. McCabe, please go ahead.
Angeline C. McCabe
Vice President of Investor Relations & Corporate Communications
Thank you. Good afternoon, and welcome to Nevro's Third Quarter 2024 Earnings Conference Call. With me today are Kevin Thornal, our CEO, President; and Rod MacLeod, our Chief Financial Officer.
Before we get started, please note that our earnings release and the supplemental presentation accompanying this call are available on the Events and Presentations page of the Investors section of our corporate website at nevro.com. Also, this call is being broadcast live over the Internet to all interested parties, and an archived copy of this webcast will be available in the Investors section of our corporate website shortly after the conclusion of this call.
I'd like to remind everyone that comments made on today's call may include forward-looking statements within the meaning of federal securities laws. Results could differ materially from those expressed or implied as a result of certain risks and uncertainties. Please refer to Nevro's SEC filings, including our annual report on Form 10-K filed on February 23, 2024, for a detailed presentation of risks.
The forward-looking statements in this call speak only as of today, and the company undertakes no obligation to update or revise any of these statements. In addition, management will refer to adjusted EBITDA, a non-GAAP measure used to help investors understand our ongoing business performance.
Non-GAAP adjusted EBITDA excludes interest, taxes and noncash items such as stock-based compensation and depreciation and amortization as well as litigation-related expenses, restructuring and supplier contract renegotiation charges and other adjustments. Please refer to the financial tables in our press release issued today for reconciliations of GAAP to non-GAAP financial measures.
I will now turn the call over to Kevin Thornal. Kevin?
Kevin R. Thornal
President, CEO & Director
Thank you, Angie, and thank you all for joining us. This afternoon, we issued our third quarter 2024 financial results. In my remarks today, I'll discuss the key factors that drove our financial results for the quarter and share why we remain optimistic about the future of our business and our ability to return to sustainable growth. Rod will then provide details on our third quarter financial results as well as our full year 2024 guidance.
Our results for the third quarter of 2024 compared with the third quarter of 2023 were as follows: worldwide revenue of $96.9 million decreased 6.7% on a reported basis, and U.S. revenue of $83.9 million decreased 6.5%. U.S. spinal cord stimulation or SCS trial procedures declined approximately 15.2%.
Net loss from operations was $13.9 million compared with $25.6 million in the year ago quarter, and adjusted EBITDA was negative $1.8 million compared with a loss of $5.8 million in the year ago quarter, reflecting the actions we took through our restructurings earlier this year and our focus on expense management as we drive toward profitability.
And cash, cash equivalents and short-term investments increased $3.3 million to $277 million as of September 30, 2024, from June 30, 2024. This increase reflects the benefit from our restructurings in the first half of this year and strong working capital management, and as Rod will discuss shortly, we expect our cash position to increase further in the fourth quarter. Importantly, our balance sheet remains strong.
On our second quarter earnings call, we discussed in detail the challenges we are facing in the de novo spinal cord stimulation market as well as opportunities where we can improve our commercial execution. And in the third quarter, we continued implementing actions, including changes to our sales territories as well as reallocating marketing resources to improve our competitive positioning, drive market penetration, return to sustainable top line growth and continue on our path toward profitability.
As we’ve previously discussed, we promoted a number of our associate sales reps or ASRs to lead some newly created territories. In 2025, we plan on continuing to add new territories, many of which will be filled by promoting associate sales reps. There will be a ramp-up period throughout 2025 for these new sales reps as they get established in these territories, and we look forward to fostering their growth. We believe these changes will allow our sales team to reach more customers, go deeper with physicians, provide the high level of service our customers expect, while also laying the foundation for new product introductions.
It is imperative that we achieve sustainable profitability, and one of our largest expenses has always been our commercial efforts. Over the years, we have made adjustments to align our commercial cost structure with our revenue base with the goal of achieving profitable growth and delivering shareholder value. As a reminder, we took more than $30 million in annual run rate expenses out of our cost structure through our 2 restructurings earlier this year.
We had some difficult decisions to make as we went through those processes. As we were evaluating our operating expenses at the beginning of this year to accelerate our path to profitability, we made the decision to reduce our overall direct-to-consumer or DTC advertising spend as it is often difficult to measure the direct return on these investments.
Furthermore, at that time, we believed that our strong referral network combined with our best-in-class clinical data demonstrating the effectiveness of our 10 kHz therapy would be enough to continue driving customer and patient adoption of our unique SCS treatment therapy. However, based on our subsequent analysis, we now know that our DTC advertising efforts had a greater impact on patient lead generation and our U.S. SCS trials than we previously thought.
We also learned that patients had a higher likelihood of moving forward with SCS therapy when we spent more time educating them upfront before referring them to their chosen physician. Accordingly, we began reallocating resources on these initiatives in the middle of the third quarter. At the same time, we are implementing improvements to our DTC advertising approach that are designed to effectively convert a higher number of patient leads into trials.
We are beginning to see patient interest in response to our new DTC advertising campaign and are confident we will see a meaningful improvement in trialing activity in the second half of 2025. While it's still early, we believe the actions we are taking, including reinvesting in our DTC advertising, are beginning to have a positive impact as the rate of decline in U.S. SCS trials in October decreased. We are laser- focused on returning to sustainable growth and are monitoring trends and sales activity on a daily basis.
We continue to build and leverage our R&D pipeline, and we are excited that, in September, we received FDA approval
and announced the limited market release of HFX iQ with HFX AdaptivAI, which we believe further establishes our position as the leading developer of innovative technology using data-driven solutions for pain management.
This is the only SCS technology using artificial intelligence and is built on a strong foundation of landmark evidence, clinical research and big data, leveraging 100 million data points and 10 years of data acquisition, innovation and patient care.
We know that chronic pain is a dynamic, multifaceted condition unique to each individual, therefore therapies must be personalized based on the patient's changing experience of pain. Competitor SCS systems require programming paresthesias to a preferred setting in the physician's office using trial and error methods. This often results in most patients requiring frequent in-office reprogramming visits over many months.
Only HFX AdaptivAI engages with the patient to personalize the delivered SCS therapy using evidence- based algorithms derived from our proprietary data set for specific indications. Through AI, the algorithm adjusts programming in real time using multidimensional metrics of pain relief, sleep quality, function and satisfaction.
It is also important to remember that HFX is a paresthesia-free therapy. Examples of the advanced technology incorporated in HFX AdaptivAI include tailoring dosing over time while maintaining pain relief provides the opportunity to dramatically reduce charging requirements to as few as 6 times per year. This is compared to previous generations of our SCS devices that require some patients to charge once per week, up to 52 times per year.
Proprietary bipole interlacing technology that stores the patient's programming usage and pain relief history to create bespoke programming sequences that brings patients back to pain relief faster or even to improve patients that have already achieved pain relief of greater than 50%.
HFX AdaptivAI is raising the bar on what is considered adequate pain relief. Continuous device-based monitoring of the patient's pain relief state, device performance and usage that automatically alerts our field-based care team to contact the patient to resolve clinical or device performance issues that might arise. Proactive intervention is now a reality.
HFX AdaptivAI is truly a game changer for patients, physicians and Nevro, and importantly closes the loop that matters. Since the limited market release in late September, HFX AdaptivAI is off to a great start and already delivering improved clinical outcomes to patients. Our early data demonstrates that patients are achieving pain relief 41% faster when compared to our prior version, HFX iQ 1.0. Patients start achieving and maintaining meaningful pain relief plus feeling the benefit of our smart power and bipole interlacing additions.
Importantly, for our shareholders, HFX AdaptivAI is also providing significant reductions in the administrative and clinical burdens to our organizational infrastructure that would otherwise be needed to provide long-term clinical care to these patients. In patients who are now utilizing HFX AdaptivAI, we are seeing an almost 40% reduction in the number of patient calls to our support teams, with an almost 50% reduction in call time duration and a 20% reduction in, in-office patient reprogramming visits when comparing de novo HFX AdaptivAI patients to Omnia, a previous generation of Nevro's non-AI-driven SCS platform.
While we are still in the early days of this product release, we are extremely pleased with both the benefit we are providing to patients and the significant expense reductions we should realize with this product over the long term. This limited market release marks a major milestone not only for our product offering, but the SCS market as well, as it is the first therapy to put patients in control of their pain relief, while at the same time, providing physicians the ability to monitor their patient's pain journey. We look forward to the full U.S. market launch later this month.
We are also thrilled to announce that we recently received regulatory approval to offer HFX iQ in CE Mark countries in the European Union. HFX iQ will be available in select regions of the EU through a limited market release beginning later this month. We anticipate a full market release in the first quarter of 2025. This marks a major milestone in advancing our efforts in the EU, and I want to congratulate our team who worked long and hard to get this approval over the finish line.
As part of our longer-term plan to become a more comprehensive pain management company and drive market penetration in very large and underserved markets over the next few years, we are working to grow our business through expanded SCS indications, next-generation SCS therapy and alternative therapies that are earlier in the care continuum. As we've communicated previously, the PDN market remains underpenetrated at less than 1%. We continue to focus on growing this business by educating referring physicians and raising patient awareness on the benefits of SCS as treatment therapy for these patients.
Building on our strong foundation of robust clinical data, new data was recently published in the Journal of Pain Research demonstrating long-term improvements in pain intensity with our high-frequency SCS therapy. Notably, the analysis is the first study of SCS to demonstrate long-term significant and clinically meaningful reductions in hemoglobin A1c and weight in study participants with PDN and type 2 diabetes who received high-frequency SCS therapy.
It was this data that led us to initiate a feasibility study to specifically evaluate the use of 10 kHz therapy to affect HbA1c. At the 12-month point of this study, we are seeing very encouraging and similar results as with the PDN study, with patients demonstrating significant and clinically meaningful reductions in HbA1c. Our clinical work in this area is very exciting, and there is a signal of the possible metabolic benefits of 10 kHz SCS for diabetic patients.
This clinical work continues to build evidence on the importance of new treatment options, such as 10 kHz SCS therapy for patients that suffer from PDN and other diabetic-related comorbidities. Regarding the SENZA Sensory RCT, the interim analysis is ongoing, and we continue to expect to readout in early 2025. Recall that this trial is designed to more objectively prove the sensory improvements we observed in our initial randomized controlled trial and to obtain an SCS indication beyond just pain.
Depending on the outcome, this may allow for early publication, followed by potential review on specific inclusion of Nevro's proprietary 10 kHz SCS therapy into evidence-based guidelines, particularly those published by the American Diabetes Association. We're excited that our PDN Sensory protocol design abstract has been accepted as an oral presentation at the 2025 NANS Annual Meeting and will be presented by Dr. Erika Petersen, Director of the Section of Functional and Restorative Neurosurgery at UAMS Medical Center.
As we've shared previously, our SCS devices have rechargeable batteries with a very long functional life, yet they will need to be replaced. We began treating a significant number of patients commercially
beginning in late 2015, which means that the IPGs implanted at that time are now nearing end of life. We believe many of our patients, in consultation with their physicians, will want to continue using our best-in-class and clinically proven treatment therapy, and they will now have access to the significant benefits of HFX AdaptivAI technology in treating their chronic pain.
We believe that, over time, a growing number of our implants will be in patients who have already enjoyed effective high-frequency SCS therapy with Nevro technology for many years and whose devices are nearing their natural life of their IPG battery. Remember that we began treating a significant number of patients commercially in late 2015, and now more than 115,000 patients globally are using our 10 kHz SCS therapy.
Our longer-term goal is to become the leading provider of treatment options with the most diversified, differentiated and innovative product portfolio in the pain management space. We continue to ramp and scale our SI joint fusion business and anticipate that it will contribute more meaningfully to our growth beginning next year. SI joint pain is seriously underdiagnosed, and our fusion technology provides a new and simpler approach to this procedure. One of the ways we are educating surgeons and standing out from legacy devices is through the publication of positive clinical data.
We are pleased that comve biomechanical data on Nevro1, our novel posterior integrated single- cage system, has been accepted in a peer-reviewed publication assessing the biomechanical fixation, invasiveness, and fusion characteristics of the Nevro1 SI joint transfixing fusion system as compared to other commercial SI joint transfixing devices. Nevro1 was found to be equivalent or superior to these devices, including the lateral triangular rod system.
The authors conclude that Nevro1 provides a significantly better opportunity for robust SI joint arthrodesis. We continue to receive positive feedback from physicians who are trained and now performing SI joint fusion procedures with Nevro1. We believe that the success of our expanding portfolio of SCS and SI joint products will help create the type of scale, growth, operating leverage and sustainability to unlock the kind of shareholder value that our Board and management team are intent on creating.
With respect to our previously announced exploration of strategic options to accelerate growth, diversify our product portfolio and deliver shareholder value, the activity is ongoing, and we are evaluating several options. Beyond this, we will not provide any further comment or update on the process. In the meantime, we remain laser-focused on executing our strategy and the actions we implemented to return to sustainable growth and drive toward profitability and are excited by the direction of the company as we begin to close out 2024 and head into 2025.
We have the most advanced technology in the space, supported by robust clinical evidence. With our launches of HFX
AdaptivAI in the U.S. and HFX iQ in the EU, the growth opportunity in an underpenetrated SCS market and our diversification into the SI joint fusion space, we have an exciting and compelling path ahead of us.
I'll now turn the call over to Rod for a discussion of our third quarter financial results and guidance for the fourth quarter and full year 2024. Rod?
Roderick H. MacLeod
Senior VP & CFO
Thanks, Kevin, and good afternoon, everyone. To echo Kevin's comments, we are proactively making decisions and reallocating resources to better execute commercially while embarking on a process aimed at accelerating our growth, diversifying our business and creating stockholder value. Note that Q3 2024 had 1 additional day versus prior year.
For the third quarter of 2024 and compared with the year ago period, worldwide revenue decreased 6.7% as reported and 7.0% on a constant currency basis. U.S. trial procedures decreased approximately 15.2%, mainly due to competitive pressures, ongoing softness in the core U.S. SCS market as well as commercial execution. U.S. revenue decreased 6.5% to $83.9 million, primarily due to the aforementioned factors, and international revenue of $13 million decreased 7.7% as reported and 9.6% on a constant currency basis.
As was the case in the second quarter of this year, our international business was primarily affected by the short-term impact of negative SCS-related media reports in Australia, where we have the largest market share that resulted in cases being postponed or canceled as well as the impact of health care reform in Germany that caused a delay in procedures in the current year quarter. The impact from these two factors
did abate somewhat in the third quarter, and we began to see an uptick in procedures in both of these markets.
Gross profit of $64.6 million decreased 7.1% compared with the third quarter of last year. Gross margin was 66.7% in the third quarter, down slightly from the same period a year ago, primarily as a result of a meaningful charge for previously capitalized variances and overhead as we discussed on prior quarter earnings calls. We continued to make progress in shifting additional work towards Costa Rica manufacturing facility to further leverage our investment there and continue on the path towards achieving a long-term gross margin in the mid-70% range and drive toward profitability.
Operating expenses decreased to $78.5 million compared with $95.1 million in the prior year quarter. Excluding restructuring charges, intangible amortization, contingent consideration revaluations and the year-over-year decrease in litigation-related expenses, OpEx was $83.5 million or an approximately 12.2% year-over-year improvement over the year ago period.
This improvement reflects the benefits from our restructuring activity in the first half of this year as well as our continued focus on expense management and operational efficiencies. We also strengthened our cash position in the third quarter. Cash, cash equivalents and short-term investments were $277.0 million at September 30, 2024, increasing $3.3 million from June 30, 2024. This increase reflects the benefits from our two restructurings in the first half of 2024, as well as our focus on working capital management.
Turning now to our 2024 full year guidance. We are maintaining our full year 2024 worldwide revenue guidance of approximately $400 million to $405 million, representing an approximately 5% to 6% decrease from our 2023 worldwide revenue on both a reported and constant currency basis. This guidance takes into account the impact of market challenges that we are facing and reduced DTC marketing spend from earlier this year as well as some impact in the fourth quarter from the two hurricanes that hit the southeast that caused a shortage in IV bags and thus a delay in procedures.
While we are stepping up our commercial execution by optimizing our sales territories and amping up our DTC advertising program, we don't anticipate seeing the benefit from these actions in U.S. SCS trialing growth rates for a few quarters. We continue to expect full year gross margin to be approximately 66% or 68%, excluding the $6 million supplier charge that we discussed on our second quarter call. We continue to see our Costa Rica manufacturing facility produced excellent results, with labor and material costs meeting our expectations for manufactured products.
Additionally, we have continued to move headcount from the United States down to Costa Rica to leverage our investment. As I mentioned earlier in my remarks, we continue to project long-term gross margins
in the mid-70s, assuming pricing holds at current levels. Also, as we previously communicated on our first quarter earnings call, we projected we would see second half gross margin headwinds due to the accounting of inventory variances and overhead that occurred in 2023 as we were transitioning from a heavy reliance on contract manufacturing to bringing manufacturing in-house, therefore gross margin in the fourth quarter of 2024 will be lower than what the business delivered in the first half of this year.
Also note that lower production volumes from decreased sales in 2024 will continue to create some margin headwinds next year. We expect our full year 2024 operating expenses to be approximately $369.1 million, down $20.2 million or down 5.2% from 2023. As we communicated on our second quarter 2024 earnings call, we expect our restructuring efforts to generate savings of more than $25 million in 2024 and full year annualized run rate savings of over $30 million.
We remain laser-focused on maintaining a cost structure aligned with the size of our business and continue to look for additional efficiencies to drive margin improvement. We are also raising our full year 2024 adjusted EBITDA guidance to a range of negative $18 million to negative $16 million from the prior guidance range of negative $20 million to negative $18 million. It is also important to point out that our restructuring efforts and focus on managing working capital should also drive an increase to our cash, cash equivalents and short-term investments in Q4 2024 as well.
While we aren't providing 2025 guidance at this time, as we look ahead to next year, we expect to reap the benefits from the changes to our commercial team and territories to drive growth and market
penetration, increased investment in DTC advertising, continued ramping of our SI joint fusion business and greater revenue contributions from IPG replacements as we move throughout the year.
In closing, as we look to close out this year and ahead to 2025, the entire Nevro team remains committed to executing our strategy to grow and diversify our business and getting us back on the path to profitability and value creation. We will now open the call up for questions.
Question and Answer
Operator
[Operator Instructions]. Your first question comes from the line of Chris Pasquale with Nephron Research.
Christopher Thomas Pasquale
Nephron Research LLC
Congrats on some of the cost-cutting initiatives starting to show through in the reduced OpEx and cash burn, it's nice to see. Kevin, I wanted to ask kind of a big picture question about the outlook for the core SCS business. By our math, your trials, excluding PDN, have been declining now for the better part of the last three years, and the trend this year has been really bigger declines each quarter.
There's competitive noise in there and some launches that have probably impacted share, but it really feels like there is less interest, or at least stagnating interest, in SCS in general as a solution for these patients. You talked about the great work you're doing to develop clinical evidence on the PDN side, on the SI joint side. I'm wondering if you feel a need to maybe generate some new data to really support the use of SCS in the core market, especially given the fact that your technology has evolved quite a bit since the last really big clinical initiative you had for back and leg pain.
Kevin R. Thornal
President, CEO & Director
Yes, Chris, thanks for the question and for the shout-outs there with our restructuring cost. Look, we looked at all of our competitor data. We do the same analysis you guys do on what's going on in the market. And as you know, historically, this SCS market is traditionally volatile as new products launch back and forth between competitors and what have you.
In addition, we're still focused on creating that clinical data. We've done so both in the PDN side as well as nonsurgical back pain, which is a new indication that we did just a few years ago as well. That will take some time to be able to ramp that up, and that is in the core back and the leg space. If you also recall, in
the second quarter earnings call, we talked about those MIS procedures that are often in front of SCS, and those procedures continue to grow. And we believe that those patients will continue to get back to SCS to complete their full pain management journey.
So, with that, I think that when you look at our direct-to-consumer spend, we talked about it on the call, we turned that down. We saw an effect. That was not just in PDN, but also in our core back and leg market. So as we turn that dial back up and really begin to be more focused on those efforts and nurturing those patients, we're actually seeing a return of trials in all those areas. And so we have to control what we control, and we're excited about HFX AdaptivAI and our launch there to be able to compete against our bigger competitors as well.
Christopher Thomas Pasquale
Nephron Research LLC
Do you have any plans to capture the impact of HFX AdaptivAI in a way that's comprehensive and can generate some publications to put that in front of physicians?
Kevin R. Thornal
President, CEO & Director
Yes. We always look at what we can do in real-world data to be able to bring new points out there. And as you heard during our call, we talked about statistics of what we're already seeing in our limited market release, and we believe some of those physicians that we're involved there would love to do investor- initiated trials there. And so we do want to continue our focus on clinical trials as we always have to bring out important data.
And not only does this bring clinical data and benefits for the patients, benefits for the physicians because they're getting less phone calls because the patients are actually getting back to pain relief really quickly automatically from the algorithm, but also we're seeing less of a burden on our internal staff on reaching out to those patients because they're getting back to that pain relief on their own using the algorithm and just answering 4 easy questions today. So we're excited about it bringing not only clinical value but also commercial value and quite often going to be some cost avoidance and cost savings for us as an organization as well.
Operator
Your next question comes from the line of Shagun Singh with RBC Capital Markets.
Shagun Singh Chadha
RBC Capital Markets, Research Division
I guess I just wanted to get back to U.S. trials down 15%, and you called out competition, SCS market, execution, and then you did call out the DTC impact that it sounds like it will take up to second half of '25 to really yield some results.
Can you just elaborate on each of those buckets? Where are you seeing more pressure versus the other? And I guess just as a follow-up, can you elaborate on your U.S. sales force dynamics? How much of the heavy lifting is behind you? What still needs to be done as we look into 2025? That would be helpful.
Kevin R. Thornal
President, CEO & Director
Sure. Yes, I'll start with the last one first. Our commercial efforts in the U.S., as you recall, in 2023, we made quite a bit of structural changes and reporting line changes and brought on a lot of new people. Most of those were in entry-level type sales positions called associate sales reps, and that's why now those reps have been out in the field for 1.5 years and are ready to take on their own territories.
That was planned all along so that they can hit the ground running much quicker than someone who's coming from outside of the spinal cord stimulation market. Most of those are taking over expansion territories that are within the territory in which they were providing assistance over the last 1.5 years. So they know the physicians, they know the doctors that are in that area and areas where they can go out and convert competitive business as well.
If you go through and look at what components were the headwinds of that 15.2%, there's one thing just to remember here. As our replacements continue to grow and be more part of our overall business, you will see a disassociation between trialing and revenue because you do not do a trial when you're replacing the IPG. I just wanted to make that point. So you can still see revenue growth despite some pressure on trials.
The second is DTC. These are really hard things to measure. We have some great Board members that sit on companies that also spend a lot on direct-to-consumer advertising, and it's always difficult to tell how much of an impact they're having or not having an impact until you increase it or you're decreasing and see the effects of that. And so we made that decision back earlier in 2024 to turn it down a bit.
It takes a little bit of time before you see the effects on that. But as soon as we did, we were now able to realize that it does make an impact to returning that volume back up. And we are already seeing the benefits of that in the early days since we turned the volume back up. So it's really difficult to try to put a percentage of what is providing that headwind, but we do believe that all of our changes and all of our responses to that will reverse that trend.
Operator
Your next question comes from the line of Robbie Marcus with JPMorgan.
Robert Justin Marcus
JPMorgan Chase & Co, Research Division
Congratulations on some pretty impressive cost cuts here. And maybe to that point, I wanted to ask on R&D spend. It came down pretty meaningfully, about 1/3. It's the lowest, I believe, outside of the depths of COVID since 2017.
How do we balance sort of the cost cutting initiative versus future innovation here? And maybe you could give us a little insight into what was cut, how it was cut and how you think about this impacting forward growth? And then I have a follow-up.
Roderick H. MacLeod
Senior VP & CFO
Sure. Yes, this is Rod. I'll take that. There's a couple of things to keep in mind here. We always want to be investing in what's going to be driving future growth, and we really protect those investments that are going to provide an ROI. I think what you're seeing here is focus in the organization towards those projects that do have an ROI associated with them.
Also note that there's some clinical spending that goes on in that category, and we saw some reduction or some focus in that area as well. So we've got a bias towards growth. Kevin and I, our entire careers have been about driving growth in the business, and we're really going to try to drive that focus in an organization towards those projects that can generate revenue returns over the next couple of years.
Kevin R. Thornal
President, CEO & Director
And I'll add a little bit here, Rob, it's Kevin. On that clinical side, you will see some [boluses] up or down just based upon the enrollment and the timing. And so we obviously had a couple of clinical trials roll off and come to completion, and we have our Sensory trial been going, but it's at the preplanned midterm stop. And so you'll see reduction in spending as that occurs.
So that will go up or down, and that's in our R&D line. And as Rod mentioned, we are still focused on the diversification of that product portfolio, along with spinal cord stimulation next-generation devices. As you can see with HFX AdaptivAI, that's not the last one. We'll continue to focus on innovation there, along with getting us into new markets that are already established in the pain management space today.
Robert Justin Marcus
JPMorgan Chase & Co, Research Division
Great. And it's been touched on in some questions already, but maybe just help us understand. This year, you cut expenses, you cut headcount and sales came down, and we're going to see probably one of the flattest third to fourth quarters the company has ever had coming up now.
Especially I imagine headcount exiting the year is going to be lower by a good amount than during the year, and I know you're adding some territories next year, but how do we think about the plan to grow revenues in '25, despite some pretty meaningful expense and likely headcount cuts throughout '24? So I imagine you're going to have tough comps at the beginning of next year on that count. Just help us understand how you can balance a lot less spending with growth.
Roderick H. MacLeod
Senior VP & CFO
Yes. This is Rod again. It's a good question. I mean as you look at the $30-plus million that we've pulled out on an annualized basis, I think we got probably close to 90% of it right. When you do make those sort of reductions, you are going to find a couple of areas where, like Kevin mentioned on DTC, where maybe we turned down the volume a little bit too much. I think as we sit here right now, we're confident that we have a much better sense as to what is going to drive that revenue, what has impacted that revenue.
We feel confident that we can close that gap that we saw in Q3 there, with the cost structure that we have in place. As Kevin mentioned, we're dialing up the DTC. We're making some key investments in certain parts of the commercial execution and the commercial organization to help drive that growth into the
future. So I think we got the majority of it right, but there were definitely a couple of areas where it may have had a little bit of an impact on the business.
Kevin R. Thornal
President, CEO & Director
Yes, it's Kevin here. Just to add in, too, one of the exciting portions and the reason we put it in the prepared remarks around HFX AdaptivAI is the ability to grow without needing to add 1:1 ratios with that new patient population that continues to expand, right? As we implant more new patients, that means we have more patients to take care of.
However, those new patients that start on HFX AdaptivAI and those that will eventually be replaced with HFX AdaptivAI as their old IPGs sort of go to their end-of-life or end of their battery, we need less people to be able to take care of those patients. That's what AI does across all the industries, right? It's making those changes automatically that used to be people that used to have to do a lot of that work. And so as we expand in the future, technology will allow us to do that as well.
Operator
Your next question comes from the line of Matt Taylor with Jefferies.
Matthew Charles Taylor
Jefferies LLC, Research Division
I just wanted to be clear about the comments on trialing because you said that ramping back of DTC would improve them, the trial trends, by second half '25. But are we expecting the next few quarters for the trialing trends to be similar to what we saw in Q3? And then could you provide any color on the quantum of DTC spending that you toggle down and up? It sounds like you said 90%, right? So is it 10% of the $30 million that you're toggling back up?
Kevin R. Thornal
President, CEO & Director
No, we can't really do that math on the expanding. The good news is, in today's world, getting to our patient population is pretty targeted, and we even got better now than we did before by the channels in which we can find these patients easier.
The reason why there's a little delay, there is obviously, as these patients call in to learn more about this exciting therapy to help them out, specifically with their back and leg pain or painful diabetic neuropathy, what we've learned is we need to nurture those patients and educate them before we hand them off to their preferred physician. And we've seen a really good increase of those referrals and those leads going into consultations with their preferred physician.
And that typically takes 4 to 6 months after they have their initial consultation before they would have the trial that would lead to a permanent implant. So that's why it's going to take a little bit of time as the patient flow starts coming back in. As far as what we're spending on DTC, we've never put that number out there before, but we're able to do so, and it wouldn't have an effect on the $30 million that we said is going to be saved this next year.
Roderick H. MacLeod
Senior VP & CFO
Matt, just to pile on there a little bit. So in some of our press release, our slides, we said that for Q4, we're not commenting on 2025 at this point. But for Q4, we did -- we assume the trialing doesn't improve. And as Kevin mentioned, we still believe that our $30 million of annualized cost reductions are in place, and we'll continue to see some benefit of that. But kind of to your math, it is not a huge investment, but it's something that we -- once we realized that it was having an effect, we were able to turn on and get that investment flow going pretty quickly.
Operator
Your next question comes from the line of Joanne Wuensch with Citi.
Anthony Occhiogrosso
This is Anthony on for Joanne. I guess your gross margin guidance and EBITDA guidance for the year, that implies a pretty big step-up in OpEx in the fourth quarter. Is that all related to DTC and expanding sales force? Or is there anything else in there that we should keep in mind?
Roderick H. MacLeod
Senior VP & CFO
Yes, I can take that. First of all, we had a pretty strong Q3 from an adjusted EBITDA perspective, and some of that was related to the revenue over performance versus where we guided to as well as some spend reductions as well as some timing on some spend.
So from a timing perspective, some of that timing is pushing into Q4, and there are some additional investments, some commercial-related, some related to HFX AdaptivAI, that are impacting the P&L. From a timing perspective, some of those we thought would have hit in Q3, but it looks like they're going to be more of a Q4 expense. So it's primarily timing, in terms of that shift really from Q3 to Q4.
Anthony Occhiogrosso
Okay. That's helpful. And then just one on Vyrsa. Can you talk about how that integration is going and if that's performing to internal expectations so far?
Kevin R. Thornal
President, CEO & Director
Thanks, Anthony. Yes, so we're still in the integration process. As a reminder, it was a small spin-out of a small spine company there, and they handed us great technology, but we had to build up a lot of the
things needed to be able to fully launch that, one of which is the tray that you need perform the procedure that need to be turned and flipped back out. We now are finally at full capacity there so that we're able to cover any kind of the trials that's ongoing.
One of the things we talked about before is that we are changing the paradigm for where those patients are treated. Traditionally, they've been treated by neurosurgeons and orthopedic surgeons because of the procedures that were commercially available were pretty intensive. And if you needed some help, if you did something wrong during that procedure, you needed to have a little bit different skills than the physicians that we normally call on.
Now with this posterior approach, it's opened up a new world for the interventionalist to do this procedure, and we spend a lot of time training physicians for the first time. And so instead of them referring those physicians to neurosurgeons like they had in the past, they're now able to keep those patients in their own practice, but that's going to take them some time to be able to build up that patient flow and that patient base that stays within their practice.
So we're liking how it's progressing. Obviously, we always want more than what you have right now, but we're in line with where we want to go as far as the training, the number of physicians that are trained, how they're building their practices and our rep's ability to learn and be able to upscale to be able to be proficient in covering those cases. And we expect that it will have a more meaningful impact as we said, as we head into 2025.
Operator
Your next question comes from the line of Brandon Vazquez with William Blair.
Brandon Vazquez
William Blair & Company L.L.C., Research Division
First, I just wanted to focus on the P&L. You guys have done a great job here taking some costs out. I guess the question being now as we go to '25-plus, is kind of the next leg of improvements in profitabilitygoing to be coming from just reaccelerating the top line? Or is there another leg of OpEx that might come out next year as you guys look towards moving even closer to breakeven and eventually profitability?
Roderick H. MacLeod
Senior VP & CFO
Brandon, this is Rod. I'll take that. As we continue to run this business, we continue to look for areas where we can drive focus in the business and get more efficient in what we're doing, right size the business for the size of that particular aspect of the business. So we still have some projects in areas that we're going after to drive efficiencies in this business, and we'll continue to do that on a go-forward basis.
And additionally, we'll continue to go after working capital. We saw pretty good size reductions in inventories and receivables in the third quarter, and that's going to be a strong part of our offense is we're really trying to drive this business towards a place where we control our own destiny, both from a profit and a cash flow perspective.
Kevin R. Thornal
President, CEO & Director
And I'll just add on to that, Brandon. As we talked about in the prepared remarks, we also are moving some -- the people down to Costa Rica, so they can be closer to the manufacturing plant when they're manufacturing engineering and people like that as well, and so that will continue to have some benefits that we don't fully realize yet.
And then as I mentioned earlier on another question, we really believe that using AI, not only in product but across the
organization, will give us opportunities for us to become more efficient, and allow us to grow our top line without having to add a 1:1 ratio, if you will, of people to be able to take care of our large and growing installed patient base.
And so that will be a cost avoidance and allow us to be able to do more with less people, because HFX AdaptivAI is doing a lot of that work right now, or in previous generations, a person would be left to do to reprogram. And that additional work that they were doing doesn't really give meaningful reimbursement to the physician nor zero reimbursement for us at Nevro or revenue for us at Nevro. So it's not only a cost avoidance, but allows us to do more meaningful things that actually can drive the top line.
Brandon Vazquez
William Blair & Company L.L.C., Research Division
Got it. And Kevin, on that last point that you were making, I think with HFX iQ, even you are already starting to lower some of the barriers or some of the pain points of programming and managing these patients. So maybe can you walk us through a little bit of like what's the patient workflow look like for an iQ versus an HFX AdaptivAI patient when they're coming in and then later down the road, just so that we can understand a little bit of what's the incremental pain points here that HFX AdaptivAI can really take the next leg on?
Kevin R. Thornal
President, CEO & Director
iQ was the first AI-powered, big data-driven device, but we didn't put AI in the name because we weren't using the full capabilities of our AI power. And so now we're up to over 100 million data points that we can leverage. And so the HFX AdaptivAI now gives us a full range of capabilities in all of the programming available on our devices, high frequency, low frequency, iQ mode. There's a ton of different modes.
And I don't want to miss this point because I said it pretty quickly in the prepared remarks. One of the biggest differences here is because the algorithm is changing the therapy quickly, we're now able to dial that in and likely reduce, for many patients, the burden of the energy use of the battery of the device.
And so you think about someone who maybe was on an older generation device that needed to charge once per week because their IPG was still set on that initial first higher-power type waveform. Now over time, as we get to dial in and personalize that therapy, we see patients now that are only going to need to charge six times per year. So that's a massive decrease in the burden of charging and something that we're really excited about in this new HFX AdaptivAI that wasn't available in the iQ system previously.
Operator
Your next question comes from the line of Bill Plovanic with Canaccord.
William John Plovanic
Canaccord Genuity Corp., Research Division
Just first off, I was wondering if you could help quantify the magnitude of the changes for the territory managers as you promote these? Maybe from probably the low point of right after the changes you made earlier this year in cost cuts and then kind of where you expect to be next year? Are you increasing it 10%, 20% or 30%? And then I have a follow-up.
Kevin R. Thornal
President, CEO & Director
Yes. We haven't given a number yet, but there was quite a few people that we hired in 2023. There were assistant sales reps or therapy consultants that are now ready to take on their territory. And just -- Bill, you've been watching the story for a long time. So in past, sometimes, we would add territories that were pretty much brand-new dirt that had 0 revenue, and you put in a high-priced sales rep to sort of grow that business. Even the best of the best takes years to be able to build up a sizable business and converting those physicians from competitors.
So the approach since 2023 was you put in these assistant sales reps in territories that have a full line sales rep that is already proficient and been in the space for a while, where they get to learn from them and go to the outreaching areas, if you will, from that -- where that sales rep is located today. And so a lot of these ASRs are taking over a piece of dirt that they know that is sort of adjacent to the territory in which they have today. So we believe that they'll be more effective sooner than just pulling somebody off the street, but we've not given any numbers for how many that we've added.
William John Plovanic
Canaccord Genuity Corp., Research Division
Okay. And then just on the HFX AdaptivAI product. As you start full launch of that, you had the HFX iQ. Historically, you've seen a price premium for new products as you bring them to market. How should we think about that as you go full launch on HFX AdaptivAI?
Kevin R. Thornal
President, CEO & Director
Yes. A lot of these things are done through contracts, and it takes some time for us to get through additional products on contract. So we're just now having those conversations. We couldn't do it until we received FDA approval, of course. So we're still just a few weeks into that. But where applicable, obviously, we'll always be able to drive a premium for new technology if it brings value to those physicians. But it's really early right now to try to give an ASP increase because it's still going to be starting off as a smaller portion of our total implants.
Operator
Your next question comes from the line of Suraj Kalia with Oppenheimer.
Suraj Kalia
Oppenheimer & Co. Inc., Research Division
I'll ask both of my questions upfront. So Kevin, you mentioned HFX AdaptivAI. And if I heard correctly, 20% reduction in office visits, 41% sooner reduction in pain relative to HFX 1.0. Maybe you can just give us how does that compare to other platforms to the extent that you can talk about it.
And also, how should we think about the robustness of the 100 million data points that you have referenced in your prepared remarks? And the second question, if I may quickly, Kevin, what percent of systems, Nevro systems, are HF10 versus the more modular waveforms?
Kevin R. Thornal
President, CEO & Director
Thanks, Suraj. It's HFX AdaptivAI, not Adaptive IQ, just want to make sure. I sort of blended those together in talking. And look, we've compared against our previous version, HFX AdaptivAI versus HFX iQ, in those brand- new patients and seeing how quickly they get to pain relief. And so that was that 41% number. And then when you look at our 40% reduction of patient visits, 50% reduction in call time and 20% reduction in, in- office patient reprogramming, that's versus Omnia, which was our previous version before iQ that did not have any AI-powered technology in that.
And so we have not done any head-to-head against any of the competitors out there because, again, we're still in limited market release. And so, we'll continue to provide some of those data points as we get into a full market release starting later in this month. As far as percentage of HF10 versus the other, we exited the quarter with...
Roderick H. MacLeod
Senior VP & CFO
Yes. So, we -- Q3 was about 68% of our mix of iQ product. And if you're specifically talking about HF10, almost all of our patients exclusively use the HF10 therapy versus any of the other wavelengths. In fact, it's probably north of 95%.
Operator
Your next question comes from the line of Richard Newitter with Truist Securities.
Richard Samuel Newitter
Truist Securities, Inc., Research Division
Maybe just on the first, going back to the DTC. Just curious, is it -- am I reading this right that if you're expecting a back half 25% impact from turning the switch back on midway through 3Q, is that a longer time frame to see the ROI from that investment in DTC versus what happened when you turned it off midway through 1Q hitting in the third quarter? Maybe just -- are they equal time frames? Or why would it be longer on the back end?
Kevin R. Thornal
President, CEO & Director
Yes. So, as we said earlier, what you do when you turn it back on, you get patients that see the ads and they say, "Wow, this is great technology. I want to learn more about it." They have a call to action, which goes into a call center that's within our team, a great team, by the way, these HFX coaches that we have that take care of the patients. So, I want to give a shout out to the great work that they're doing.
And then they talk to them numerous times to get them along the pathway towards getting referred to their physician of choice. And once they do, we see those referrals turn into consultations because we know when they get booked in to see the physician. After they're booked to see the physician, it's going to take four to six months for those patients to then go through that care continuum to make sure they're worked up and ready for the procedure. So that's a little bit where you see the delay.
As far as on the front end, right, when you're doing direct-to-consumer, you have months and months of those patients that are part of the -- it's still part of the process. And so, when we turned it down, you
don't see an immediate impact of that because there's still patients within that process. And so as soon as we saw it start to dip down as those patients were out of the funnel, that's when we turned it back on.
And as we said in the prepared remarks, that was in the middle of the third quarter is when we started reinvestment. And as we also said, in October, we already have seen a flow of those patients back in, and already to consultation. It will take some time before they go to a trial and into a perm procedure.
Richard Samuel Newitter
Truist Securities, Inc., Research Division
Okay. Kevin, what gives you confidence that you haven't -- I mean, congratulations on the work you've made on rightsizing the P&L, but what gives you confidence that you haven't cut too far into muscle and that it's limited to the DTC area that perhaps you went a little too far?
And then just one follow-up would just be any commentary that you can provide on kind of the 4Q as a jump-off point in '25 for expenses? And anything we should be considering as we refine our '25 models based on where the consensus sits?
Kevin R. Thornal
President, CEO & Director
Yes. As Rod said, we've got about 90% of it right. What we missed was the DTC. The other areas, while it's never exciting or fun or even doesn't feel good as a human being to have to make readjustments and refocus teams and restructure like that, we do believe we were very thoughtful in how we went about that.
And really focusing on those areas that will have a bigger impact on our revenue and profit over the next strategic plan period of time versus maybe some of those expenditures, that would be great. Possible home run or moonshot that could turn into something maybe 15, 20 years from now. Obviously, we had to make the hard decisions to say, ‘Hey, let's focus on the ones that are more “bird in the hand” that are great projects that we should continue to fund.’
So we believe we did a good job on trying not to affect the top line. In fact, as Rod mentioned, we are investing into our
sales channel now as we expand these territories. We have new products that are launching, and we're educating more physicians than we ever have on our courses. And so we still feel good about our commercial efforts as far as an investment perspective. As far as expenses as we head into 2025, I'll let Rod answer that.
Roderick H. MacLeod
Senior VP & CFO
Yes. We're not providing any '25 guidance at this point. What I'd say is similar to what we spoke about earlier in that we're going to continue to look at areas of the business where we can drive efficiencies, both internally. And also, as it relates to products, as Kevin mentioned, HFX AdaptivAI helps drive a lot of efficiencies in our overall commercial model.
So, we've still got some work to do. We've got some areas that we're going after. We continue to leverage our Costa Rica investment, both from a manufacturing as well as kind of more of a back-office perspective, and we'll continue to look at those as we go into 2025 and the years thereafter.
Operator
Your next question comes from the line of David Rescott with Baird.
David Kenneth Rescott
Robert W. Baird & Co. Incorporated, Research Division
Great. Two on the HFX AdaptivAI platform, and I'll ask them both upfront. The improvement on the reduction in patient visits and time that the patients are on the phone of the rep maybe, with the HFX AdaptivAI program, I think sounds pretty promising. And so, my first question is how replicable do you think this becomes as you roll this out to a broader number of patients that are out there when you compare that to those that are in the earlier launch versus those when you have expanded to a higher number of reps or patients out there?
And then the second piece, it feels like one of the bigger kind of pain points, again, has been around the higher reprogramming rates versus what you see from other kind of stimulation technologies. So what I'm wondering, more on the HFX AdaptivAI platform side, is if this is the technology that maybe could level the playing field, or not level the playing field, but I guess, just bring up your margins or bring up your -- I guess you have better margins, more rep time when you have this type of technology out in the field.
Kevin R. Thornal
President, CEO & Director
Yes. We believe it will, and we're seeing it in the early patients. And while we're not telling you how many patients we've implanted during the limited market release, it's aplenty. The in is very large, and so it gives us a high degree of confidence that it will have these impacts across a broader installed base, if you will.
Also, don't forget, when we launched iQ, we talked about it being a platform technology that will allow us as the only company to be able to go backwards compatible for patients that would like to upgrade just the software portion of the device. And so, a portion of those patients that have been on HFX AdaptivAI that we've seen these stats are patients that had the first generation 1.0.
We've brought them back in and downloaded the new software for HFX AdaptivAI on their older IPG, and we're seeing those benefits from where they were just a week before to where they are a week afterwards.
And so it gives us a high degree of confidence that not only in de novo, brand-new patients install with HFX AdaptivAI, but those that we've also upgraded, we've seen those benefits.
And as far as reprogramming rates, yes, those are some of the biggest cost burdens on us as an organization as well as physicians because it involves drive time, it involves trying to find a time on the physician's calendar, and you sort of have to meet three people all at once to be able to make that reprogramming happen, whereas now it's happening in the cloud behind the scenes.
One of the other things that we were excited about that we saw is in our systems, we get alerted when patients aren't doing well. And so this is for the first time that we see proactive management where it allows our people that are on a phone to reach out to those patients and be able to rectify anything that needs to be rectified without them calling into us or into their physician. And proactive taking care of patients is always more cost effective than waiting for something to happen. So we're excited about it.
Operator
Thank you. And that does conclude the question-and-answer session. And with that, that does conclude today's conference call. Thank you for your participation, and you may now disconnect.
[END]
Forward-Looking Statements
In addition to historical information, this transcript contains forward-looking statements reflecting the current beliefs and expectations of the company’s management, made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including: our full-year 2024 financial guidance; our belief that the actions we have taken and intend to take will further position us for a return to growth, success in the marketplace, profitability and shareholder value creation; our belief that execution improvements and our reallocation of marketing resources will allow us to return to sustainable growth; our belief that the market release of HFX AdaptivAI™ in U.S. and HFX iQ™ in Europe will successfully drive sales; our belief that evaluating and/or engaging in strategic opportunities will help us diversify and grow our business, which we believe may position us to accelerate our goals of profitability and maximizing shareholder value; and our beliefs with regards to the SCS market and factors impacting our results, including the duration in which those factors will continue to impact our results; and our beliefs in the catalysts for long-term growth. These forward-looking statements are based upon information that is currently available to us or our current expectations, speak only as of the date hereof, and are subject to numerous risks and uncertainties, including our ability to successfully commercialize our products; our ability to manufacture our products to meet demand; the level and availability of third-party payor reimbursement for our products; our ability to effectively manage our anticipated growth and the costs and expenses of operating our business; our ability to protect our intellectual property rights and proprietary technologies; our ability to operate our business without infringing the intellectual property rights and proprietary technology of third parties; competition in our industry; additional capital and credit availability; our ability to successfully integrate any additive acquisitions we may make, including our acquisition of Vyrsa Technologies; our ability to attract and retain qualified personnel; our ability to accurately forecast financial and operating results; our ability to successfully evaluate and execute on potential strategic opportunities; and product liability claims. These factors, together with those that are described in greater detail in our Annual Report on Form 10-K filed on February 23, 2024, as well as any reports that we may file with the Securities and Exchange Commission in the future, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. We expressly disclaim any obligation, except as required by law, or undertaking to update or revise any such forward-looking statements. Nevro's operating results for the period ending September 30, 2024, are not necessarily indicative of the company’s operating results for any future periods.
# # #
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Nevro (NYSE:NVRO)
Historical Stock Chart
From Oct 2024 to Nov 2024
Nevro (NYSE:NVRO)
Historical Stock Chart
From Nov 2023 to Nov 2024