Aurinia Announces PTAB Has Terminated Inter Partes Review
26 January 2023 - 5:00AM
Business Wire
Aurinia Pharmaceuticals Inc. (NASDAQ: AUPH) (Aurinia) announces
that the Patent Trial and Appeal Board (PTAB) of the United States’
Patent and Trademark Office has terminated the Inter Partes Review
(IPR) it had instituted with respect to Aurinia’s U.S. Patent No.
10,286,036.
About LUPKYNIS LUPKYNIS is the first FDA-approved oral
therapy for LN. LN causes irreversible kidney damage and
significantly increases the risk of kidney failure, cardiac events,
and death. It is one of the most serious and common complications
of the autoimmune disease systemic lupus erythematosus (SLE).
LUPKYNIS is in the United States (U.S.) and across the European
Union (E.U).
About Lupus Nephritis LN is a serious manifestation of
SLE, a chronic and complex autoimmune disease. About
200,000-300,000 people live with SLE in the U.S. and about
one-third of these people are diagnosed with lupus nephritis at the
time of their SLE diagnosis. About 50 percent of all people with
SLE may develop lupus nephritis. If poorly controlled, LN can lead
to permanent and irreversible tissue damage within the kidney.
Black and Asian individuals with SLE are four times more likely to
develop LN and individuals of Hispanic ancestry are approximately
twice as likely to develop the disease when compared with Caucasian
individuals. Black and Hispanic individuals with SLE also tend to
develop LN earlier and have poorer outcomes when compared to
Caucasian individuals.
About Aurinia Aurinia Pharmaceuticals is a fully
integrated biopharmaceutical company focused on delivering
therapies to treat targeted patient populations that are impacted
by serious diseases with a high unmet medical need. In January
2021, the Company introduced LUPKYNIS® (voclosporin), the first
FDA-approved oral therapy for the treatment of adult patients with
active lupus nephritis (LN). The Company’s head office is in
Victoria, British Columbia, its U.S. commercial hub is in
Rockville, Maryland, and the Company focuses its development
efforts globally.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20230125005640/en/
Investor/Media: Aurinia@westwicke.com
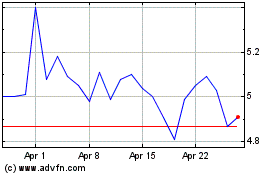
Aurinia Pharmaceuticals (NASDAQ:AUPH)
Historical Stock Chart
From Mar 2024 to Apr 2024
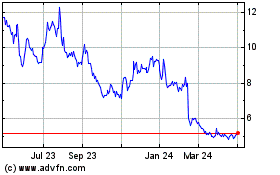
Aurinia Pharmaceuticals (NASDAQ:AUPH)
Historical Stock Chart
From Apr 2023 to Apr 2024