Report of Foreign Issuer (6-k)
24 September 2019 - 12:43AM
Edgar (US Regulatory)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
________________
FORM 6-K
________________
REPORT OF FOREIGN PRIVATE ISSUER
Pursuant to Rule 13a-16 or 15d-16
of the Securities Exchange Act of 1934
September 20, 2019
________________
NOVO NORDISK A/S
(Exact name
of Registrant as specified in its charter)
Novo Allé
DK- 2880, Bagsvaerd
Denmark
(Address of principal executive offices)
________________
Indicate by check mark whether the registrant files or will file annual reports under
cover of Form 20-F or Form 40-F
|
Form 20-F [X]
|
Form 40-F [ ]
|
Indicate by check mark whether the registrant by furnishing the information contained
in this Form is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange
Act of 1934.
If “Yes” is marked, indicate below the file number assigned to the registrant
in connection with Rule 12g-32(b):82-________


Rybelsus® (semaglutide
tablets), the first GLP-1 in a tablet approved in the US
Bagsværd, Denmark, 20 September 2019
- Novo Nordisk today announced that the US Food and Drug Administration (FDA) has approved Rybelsus®
(semaglutide tablets), as an adjunct to diet and exercise to improve glycaemic control in adults with type 2 diabetes
mellitus.
Rybelsus®,
the brand name for oral semaglutide in the US, is the first approved glucagon-like peptide-1 (GLP-1) receptor agonist in a tablet.
The approval of Rybelsus® is based on the results from 10 PIONEER clinical
trials which included 9,543 adults with type 2 diabetes. Rybelsus® more effectively
lowered blood sugar than sitagliptin and empagliflozin. Furthermore, treatment with Rybelsus®
resulted in up to 4.4 kg reduction in body weight. Rybelsus®
demonstrated a safe and well-tolerated profile across the PIONEER programme, with the most common adverse event being mild to
moderate nausea which diminished over time.
“We are very excited that we can make
the first oral GLP-1 available in the US and thereby expand the treatment options for adults living with type 2 diabetes,”
said Mads Krogsgaard Thomsen, executive vice president and chief science officer of Novo Nordisk. “Novo Nordisk has a very
long legacy of developing innovative injectable medicines for people living with diabetes and, with the approval of Rybelsus®,
we are now able to bring our innovation into the market for oral antidiabetics.”
Novo Nordisk plans to make Rybelsus®
available to adults with type 2 diabetes in the US in the fourth quarter of 2019.
Conference call
On 23 September at 8 am CEST, corresponding
to 2 am EDT, a conference call for investors will be held. Investors will be able to listen in via a link on the investor section
of novonordisk.com.
About Rybelsus®
Rybelsus®
(oral semaglutide) is an analogue of the naturally occurring hormone glucagon-like peptide-1 (GLP-1). Rybelsus®
is the first and only GLP-1 receptor agonist
Page 2 of 2
(RA) in a tablet. It is administered once daily and is approved
for use in two therapeutic dosages, 7 mg and 14 mg.
Rybelsus®
is currently under review by several regulatory agencies, including the European Medicines Agency and the Japanese
Pharmaceuticals and Medical Devices Agency. Furthermore, Rybelsus® has been
applied for a separate indication with the FDA for the reduction of major adverse cardiovascular events in adults with type 2
diabetes and established cardiovascular disease, with expected review completion by Q1 2020.
Novo Nordisk
is a global healthcare company with more than 95 years of innovation and leadership in diabetes care. This heritage has given
us experience and capabilities that also enable us to help people defeat obesity, haemophilia, growth disorders and other serious
chronic diseases. Headquartered in Denmark, Novo Nordisk employs approximately 41,600 people in 80 countries and markets its products
in more than 170 countries. Novo Nordisk's B shares are listed on Nasdaq Copenhagen (Novo-B). Its ADRs are listed on the New York
Stock Exchange (NVO). For more information, visit novonordisk.com, Facebook, Twitter, LinkedIn, YouTube.
Further information
|
Media:
|
|
|
|
Mette Kruse Danielsen
|
+45 4442 3883
|
mkd@novonordisk.com
|
|
Ken Inchausti (US)
|
+1 609 240 9429
|
kiau@novonordisk.com
|
|
|
|
|
|
Investors:
|
|
|
|
Peter Hugreffe Ankersen
|
+45 3075 9085
|
phak@novonordisk.com
|
|
Valdemar Borum Svarrer
|
+45 3079 0301
|
jvls@novonordisk.com
|
|
Ann Søndermølle Rendbæk
|
+45 3075 2253
|
arnd@novonordisk.com
|
|
Kristoffer Due Berg (US)
|
+1 609 235 2989
|
krdb@novonordisk.com
|
|
Novo Nordisk A/S
Investor Relations
|
Novo Allé
2880 Bagsværd
Denmark
|
Telephone:
+45 4444 8888
|
Internet:
www.novonordisk.com
CVR no:
24 25 67 90
|
|
|
|
Company announcement No 54 / 2019
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has
duly caused this report to be signed on its behalf of the undersigned, thereunto duly authorized.
|
Date: September 23, 2019
|
NOVO NORDISK A/S
Lars Fruergaard Jørgensen
Chief Executive Officer
|
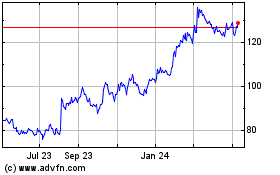
Novo Nordisk (NYSE:NVO)
Historical Stock Chart
From Apr 2024 to May 2024
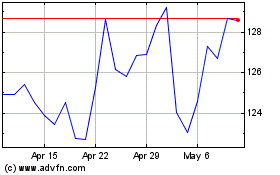
Novo Nordisk (NYSE:NVO)
Historical Stock Chart
From May 2023 to May 2024