MeiraGTx Gets $30 Million Milestone Payment From Janssen Pharmaceuticals
28 January 2022 - 1:08AM
Dow Jones News
By Chris Wack
MeiraGTx Holdings PLC said it received a $30 million payment
from Janssen Pharmaceuticals Inc., part of the Janssen
Pharmaceutical Cos. of Johnson & Johnson, for a clinical
milestone in a Phase 3 trial of botaretigene sparoparvovec.
Botaretigene sparoparvovec is an investigational gene therapy
for the treatment of X-linked retinitis pigmentosa. The Phase 3
Lumeos trial is a global study of botaretigene sparoparvovec which
is now dosing participants.
MeiraGTx and Janssen are jointly developing botaretigene
sparoparvovec as part of a broader collaboration to develop and
commercialize gene therapies for the treatment of inherited retinal
diseases.
MeiraGTx remains eligible to receive additional development and
commercial milestones for botaretigene sparoparvovec as well as for
other programs as part of the collaboration agreement.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
January 27, 2022 08:53 ET (13:53 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
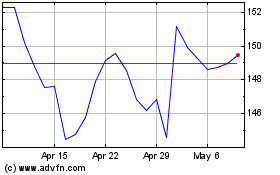
Johnson and Johnson (NYSE:JNJ)
Historical Stock Chart
From Mar 2024 to Apr 2024
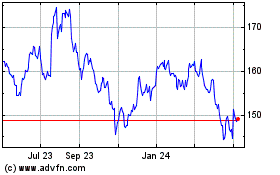
Johnson and Johnson (NYSE:JNJ)
Historical Stock Chart
From Apr 2023 to Apr 2024