| PROSPECTUS |
|
Filed Pursuant to Rule 424(b)(3)
Registration No. 333-275375 |

Aditxt,
Inc.
Up to 264,516 shares of Common Stock
Pursuant to this prospectus, the
selling stockholders identified herein (the “Selling Stockholders”) are offering on a resale basis an aggregate of up to 264,516
shares of common stock of Aditxt, Inc. (the “Company,” “we,” “us” or our”), par value $0.001
per share (the “Common Stock”) consisting of (a) up to an aggregate of 79,268 shares of Common Stock that are issuable upon
exercise of warrants (the “Warrants”) purchased pursuant to securities purchase agreements by and between us and one of the
Selling Stockholders, dated, April 20, 2023 (the “April Purchase Agreement”), (b) up to an aggregate of 164,063 shares of
Common Stock that are issuable upon conversion of a secured promissory note (the “Note”) issued pursuant to a securities purchase
agreement by and between us and one of the Selling Stockholders, dated July 24, 2023 (the “July Purchase Agreement”), and
(c) 21,185 shares of common stock that were issued to certain of the Selling Stockholders as commitment shares, including 17,278 shares
that were issued pursuant to the July Purchase Agreement and 3,907 shares that were issued pursuant to a securities purchase agreement
dated July 3, 2023.
We
will not receive any of the proceeds from the sale by the Selling Stockholders of the Common Stock. Upon any exercise of the Warrants
by payment of cash, however, we will receive the exercise price of the Warrants, which, if exercised in cash with respect to the 79,268
shares of Common Stock offered hereby, would result in gross proceeds to us of approximately $2.7 million. However, we cannot predict
when and in what amounts or if the Warrants will be exercised by payments of cash and it is possible that the Warrants may expire and
never be exercised, in which case we would not receive any cash proceeds.
The
Selling Stockholders may sell or otherwise dispose of the Common Stock covered by this prospectus in a number of different ways and at
varying prices. We provide more information about how the Selling Stockholders may sell or otherwise dispose of the Common Stock covered
by this prospectus in the section entitled “Plan of Distribution” on page 47. Discounts, concessions, commissions and similar
selling expenses attributable to the sale of Common Stock covered by this prospectus will be borne by the Selling Stockholders. We will
pay all expenses (other than discounts, concessions, commissions and similar selling expenses) relating to the registration of the Common
Stock with the Securities and Exchange Commission (the “SEC”).
Our
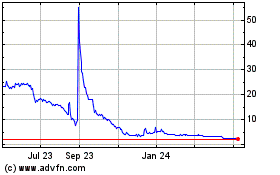
common stock is listed on The Nasdaq Capital Market under the symbol “ADTX”. On November 6, 2023, the closing price as reported
on The Nasdaq Capital Market was $6.15 per share.
We
are an “emerging growth company” under the federal securities laws and, as such, are subject to reduced public company reporting
requirements.
Investing
in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 15 of this prospectus.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is November 13, 2023
TABLE OF CONTENTS
ABOUT
THIS PROSPECTUS
This prospectus relates to the
resale by the Selling Stockholders identified in this prospectus under the caption “Selling Stockholders,” from time to time,
of up to an aggregate of 264,516 shares of Common Stock. We are not selling any shares of Common Stock under this prospectus, and we will
not receive any proceeds from the sale of shares of Common Stock offered hereby by the Selling Stockholders, although we may receive cash
from the exercise of the Warrants.
You
should rely only on the information provided in this prospectus, including any information incorporated by reference. We have not authorized
anyone to provide you with any other information and we take no responsibility for, and can provide no assurances as to the reliability
of, any other information that others may give you. The information contained in this prospectus speaks only as of the date set forth
on the cover page and may not reflect subsequent changes in our business, financial condition, results of operations and prospects.
We
are not, and the Selling Stockholders are not, making offers to sell these securities in any jurisdiction in which an offer or solicitation
is not authorized or permitted or in which the person making such offer or solicitation is not qualified to do so or to any person to
whom it is unlawful to make such an offer or solicitation. You should read this prospectus, including any information incorporated by
reference, in its entirety before making an investment decision. You should also read and consider the information in the documents to
which we have referred you in the sections entitled “Where You Can Find More Information” and “Incorporation of Certain
Information by Reference.”
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus contains forward-looking statements, which reflect the views of our management with respect to future events and financial
performance. These forward-looking statements are subject to a number of uncertainties and other factors that could cause actual results
to differ materially from such statements. Forward-looking statements are identified by words such as “anticipates,” “believes,”
“estimates,” “expects,” “intends,” “plans,” “projects,” “targets,”
and similar expressions. Such forward-looking statements may be contained in the sections “Risk Factors,” and “Business,”
among other places in this prospectus. Readers are cautioned not to place undue reliance on these forward-looking statements, which are
based on the information available to management at this time and which speak only as of this date. We undertake no obligation to update
or revise any forward-looking statements, whether as a result of new information, future events or otherwise. For a discussion of some
of the factors that may cause actual results to differ materially from those suggested by the forward-looking statements, please read
carefully the information under “Risk Factors.”
The
identification in this document of factors that may affect future performance and the accuracy of forward-looking statements is meant
to be illustrative and by no means exhaustive. All forward-looking statements should be evaluated with the understanding of their inherent
uncertainty. You may rely only on the information contained in this prospectus.
We
have not authorized anyone to provide information different from that contained in this prospectus. Neither the delivery of this prospectus
nor the sale of our common stock means that information contained in this prospectus is correct after the date of this prospectus. This
prospectus is not an offer to sell or solicitation of an offer to buy these securities in any circumstances under which the offer or
solicitation is unlawful.
PROSPECTUS
SUMMARY
This
summary highlights certain information about us, this offering and selected information contained elsewhere in this prospectus and in
the documents incorporated by reference. This summary is not complete and does not contain all the information that you should consider
before deciding whether to invest in our securities. For a more complete understanding of our company and this offering, we encourage
you to read and consider carefully the more detailed information contained in or incorporated by reference in this prospectus, including
the information contained under the heading “Risk Factors” beginning on page 15 of this prospectus.
Overview
and Mission
We
believe the world needs—and deserves—a new approach to innovating that harnesses the power of large groups of stakeholders
who work together to ensure that the most promising innovations make it into the hands of people who need them most.
We
were incorporated in the State of Delaware on September 28, 2017, and our headquarters are in Richmond, Virginia. The company was founded
with a mission of bringing stakeholders together, to transform promising innovations into products and services that could address some
of the most challenging needs. The socialization of innovation through engaging stakeholders in every aspect of it, is key to transforming
more innovations, more rapidly, and more efficiently.
At
inception, the first innovation we took on was an immune modulation technology titled ADI/Adimune with a focus on prolonging life and
enhancing life quality of patients that have undergone organ transplants. Since then, we expanded our portfolio of innovations, and we
continue to evaluate a variety of promising health innovations.
Our
Model
Aditxt
is not about a single idea or a single molecule. It is about making sure the right innovation is made possible. Our business model has
three main components as follows:
| (1) | Securing
an Innovation: Our process begins with identifying and securing innovations through licensing or acquisition of an innovation asset.
Assets come from a variety of sources including research institutions, government agencies, and private organizations. |
| (2) | Growing
an Innovation: Once an innovation is secured, we surround it with activation resources that take a systemized approach to bringing that
idea to life. Our activation resources include innovation, operations, commercialization, finance, content and engagement, personnel,
and administration. |
| (3) | Monetizing
an Innovation: Our goal is for each innovation to become commercial-stage and financially and operationally self-sustainable, to create
shareholder value. |
We
engage various stakeholders for each of our programs on every level. This includes identifying researchers and research institution partners,
such as Stanford University; leading health institutions to get critical trials underway, such as Mayo Clinic; manufacturing partners
who enable us to take innovations from preclinical to clinical; municipalities and governments, such as the city of Richmond and the
state of Virginia and public health agencies who work with us to launch our program, Pearsanta’s laboratory; and thousands of shareholders
around the globe. We seek to enable promising innovation to become purposeful products that have the power to change lives.
Our
Value Proposition
We
believe that far too often, promising treatment or technology does not reach commercialization due to lack of expertise, key resources,
or efficiency. As a result, potentially life-changing and lifesaving treatments are not available to the individuals who so desperately
need them.
Aditxt
seeks to bring the holistic concept of an efficient, socialized ecosystem for advancing and accelerating innovations. Our process: We
seek to license or acquire promising innovations. We will then form and build out a subsidiary around each innovation and support the
subsidiaries through innovation, operation, commercialization, content and engagement, finance, personnel, and administration to thrive
and grow as a successful, monetizable business.
Since
our inception, we have built infrastructure consisting of innovation, operation, commercialization, content and engagement, finance,
personnel, and administration, to support the rapid transformation of untapped innovations. Each of the main components of our infrastructure
has established global access to partnerships with industry leaders, top-rated research and medical institutions, universities, manufacturing
and distribution companies, and critical infrastructure such as CLIA-certified state-of-the art labs and GMP manufacturing.
The
Shifting Landscape of Innovation
Innovation
in general, and health innovations specifically, require significant resources. The convergence of biotech, high-tech, and media offers
new possibilities of accelerating breakthrough innovations faster and more efficiently. This approach reflects our mission of “Making
Promising Innovations Possible, Together”.
People
deserve innovative solutions, which have never been more within reach. We believe the best idea, best product and the best solution will
come from creating an ecosystem where all stakeholders, such as vendors, customers, municipalities, and shareholders contribute. When
we disrupt the way we’re innovating, through our collaborative model, we believe we can move faster and more efficiently to activate
viable solutions that have the potential to make a measurable impact.
Our
Growth Strategy
We
believe that the era of precision and personalized medicine is here and that people around the globe would benefit from health diagnostics
and treatments that more accurately pinpoint the problems and more precisely treat the condition. In addition to our current programs,
Adimune and Pearsanta, we look to bring in future health innovations in the areas of software and AI, medical devices, therapeutics,
and other technologies that take a fundamentally different approach to health because they prioritize personalized precision medicine,
timely disease root cause analysis, and targeted treatments.
Year
over year, we plan to continue building our infrastructure and securing more personalized and precision health innovations that align
with our mission. These opportunities may come in different forms such as IP, an early-stage company, or a late-stage company. We will
continue to scale our systemized approach to the innovation process, making large-scale automation and enterprise systems available to
our portfolio companies at every stage of their growth. Specifically, certain subsidiaries will need to grow through further M&A
activities, operational infrastructure implementation, and development or acquisition of critical technologies.
Our
Team
Aditxt
is led by an entrepreneurial team with passion for transforming promising innovations into successful businesses. Our leadership come
from a variety of different industries, with collective expertise in founding startup innovation companies, developing and marketing
biopharmaceutical and diagnostic products, designing clinical trials, manufacturing, and management of private and public companies.
We have deep experience in identifying and accessing promising health innovations and developing them into products and services with
the ability to scale. We understand the capital markets, both public and private, as well as M&A and facilitating complex IPOs.
The
following are profiles of three subsidiaries we have formed, including the terms of the intellectual property licenses that have been
sublicensed from Aditxt to help build each of the businesses.
THE
ADITXT PROGRAMS
ADIMUNE,
INC.
Formed
in January 2023, Adimune™, Inc. (“Adimune”) is focused on leading our immune modulation therapeutic programs. Adimune’s
proprietary immune modulation product Apoptotic DNA Immunotherapy™, or ADI-100™, utilizes a novel approach that mimics the
way our bodies naturally induce tolerance to our own tissues. It includes two DNA molecules designed to deliver signals to induce tolerance.
ADI-100 has been successfully tested in several preclinical models (e.g., skin grafting, psoriasis, type 1 diabetes, multiple sclerosis).
In
May 2023, Adimune entered into a clinical trial agreement with Mayo Clinic to advance clinical studies targeting autoimmune diseases
of the central nervous system (“CNS”) with the initial focus on the rare, but debilitating, autoimmune disease Stiff Person
Syndrome (“SPS”). According to the National Organization of Rare Diseases, the exact incidence and prevalence of SPS is unknown;
however, one estimate places the incidence at approximately one in one million individuals in the general population.
Pending
approval by the International Review Board, a human trial for SPS is expected get underway in the second half of 2023 or the first half
of 2024 with enrollment of 10-15 patients, some of whom may also have type 1 diabetes. ADI-100 will initially be tested for safety and
efficacy. ADI-100 is designed to tolerize against an antigen known as glutamic acid decarboxylase (“GAD”), which is implicated
in type-1 diabetes, psoriasis, and in many autoimmune diseases of the CNS.
Background
The
discovery of immunosuppressive (anti-rejection and monoclonal) drugs over 40 years ago has made possible life-saving organ transplantation
procedures and blocking of unwanted immune responses in autoimmune diseases. However, immune suppression leads to significant undesirable
side effects, such as increased susceptibility to life-threatening infections and cancers, because it indiscriminately and broadly suppresses
immune function throughout the body. While the use of these drugs has been justifiable because they prevent or delay organ rejection,
their use for treatment of autoimmune diseases and allergies may not be acceptable because of the aforementioned side effects. Furthermore,
often transplanted organs ultimately fail despite the use of immune suppression, and about 40% of transplanted organs survive no more
than five years.
Through
Aditxt, Adimune has the right of use to the exclusive worldwide license for commercializing ADI nucleic acid-based technology (which
is currently at the pre-clinical stage) from Loma Linda University. ADI uses a novel approach that mimics the way the body naturally
induces tolerance to our own tissues (“therapeutically induced immune tolerance”). While immune suppression requires continuous
administration to prevent rejection of a transplanted organ, induction of tolerance has the potential to retrain the immune system to
accept the organ for longer periods of time. ADI may allow patients to live with transplanted organs with significantly reduced immune
suppression. ADI is a technology platform which we believe can be engineered to address a wide variety of indications.
Advantages
ADI™
is a nucleic acid-based technology (e.g., DNA-based), which we believe selectively suppresses only those immune cells involved
in attacking or rejecting self and transplanted tissues and organs. It does so by tapping into the body’s natural process of cell
turnover (i.e., apoptosis) to retrain the immune system to stop unwanted attacks on self or transplanted tissues. Apoptosis is a natural
process used by the body to clear dying cells and to allow recognition and tolerance to self-tissues. ADI triggers this process by enabling
the cells of the immune system to recognize the targeted tissues as “self.” Conceptually, it is designed to retrain the immune
system to accept the tissues, similar to how natural apoptosis reminds our immune system to be tolerant to our own “self”
tissues.
While
various groups have promoted tolerance through cell therapies and ex vivo manipulation of patient cells (i.e., takes
place outside the body), to our knowledge, we will be unique in our approach of using in-body induction of apoptosis to promote tolerance
to specific tissues. In addition, ADI treatment itself will not require additional hospitalization but only an injection of minute
amounts of the therapeutic drug into the skin.
Moreover,
preclinical studies have demonstrated that ADI treatment significantly and substantially prolongs graft survival, in addition to successfully
“reversing” other established immune-mediated inflammatory processes.
License
Agreement with Loma Linda University (“LLU”)
On
March 15, 2018, we entered into a License Agreement with LLU, which was subsequently amended on July 1, 2020. Pursuant to the LLU License
Agreement, we obtained the exclusive royalty-bearing worldwide license to all intellectual property, including patents, technical information,
trade secrets, proprietary rights, technology, know-how, data, formulas, drawings, and specifications, owned or controlled by LLU and/or
any of its affiliates (the “LLU Patent and Technology Rights”) and related to therapy for immune-mediated inflammatory diseases
(the Adi™ technology). In consideration for the LLU License Agreement, we issued 625 shares of common stock to LLU.
PEARSANTA,
INC.
Formed
in January 2023, our subsidiary Pearsanta™, Inc. (“Pearsanta”) seeks to take personalized medicine to a whole new level
by delivering “Health by the Numbers.” Since its founding, Pearsanta has been building the platform for enabling our vision
of lab quality testing, anytime, anywhere. Our plan for Pearsanta’s platform is for it to be the transactional backbone for sample
collection, sample processing (on- and off-site), and reporting. This will require the development and convergence of multiple components
developed by Pearsanta, or through transactions with third parties, including collection devices, “lab-on-a-chip” technologies,
Lab Developed Test (LDT) assays, a data-driven analysis engine, and telemedicine. According to a comprehensive research report by Market
Research Future, the clinical and consumer diagnostic market is estimated to hit $429.3 billion by 2030.
We
believe that timely and personalized testing enables far more informed treatment decisions. Pearsanta’s platform is being developed
as a seamless digital healthcare solution. This platform will integrate at-location sample collection, Point-of-Care (“POC”)
and LDT assays, and an analytical reporting engine, with telemedicine-enabled visits with licensed physicians to review test results
and, if necessary, order a prescription. Pearsanta’s goal of extending its platform to enable consumers to monitor their health
more proactively as the goal is to provide a more complete picture about someone’s dynamic health status, factoring in genetic
makeup and their response to medication. The POC component of Pearsanta would enable diagnostic testing at-home, at work, in pharmacies,
and more to generate results quickly so that an individual can access necessary treatment faster. With certain infections, prescribing
the most effective treatment according to one’s numbers can prevent hospital emergency room admissions and potentially life-threatening
consequences.
Examples
of indication-focused tests for the Test2Treat platform will include the evaluation for advanced urinary tract infections (“UTIs”),
COVID-19/flu/respiratory syncytial virus, sexually transmitted infections, gut health, pharmacogenomics (i.e., how your genes affect
the way your body responds to certain therapeutics), and sepsis. We believe that these offerings are novel and needed as the current
standard of care using broad spectrum antibiotic treatment can be ineffective and potentially life-threatening. For example, improperly
prescribed antibiotics may approach 50% of outpatient cases. Further, according to an article published in Physician’s Weekly,
only 1% of board-certified critical care medicine physicians are trained in infectious disease.
Licensed
Technologies – AditxtScoreTM
We
intend to sublicense to Pearsanta an exclusive worldwide sub-license for commercializing the AditxtScore™ technology which provides
a personalized comprehensive profile of the immune system. AditxtScore is intended to detect individual immune responses to viruses,
bacteria, peptides, drugs, supplements, bone marrow and solid organ transplants, and cancer. It has broad applicability to many other
agents of clinical interest impacting the immune system, including those not yet identified such as emerging infectious agents.
AditxtScore
is being designed to enable individuals and their healthcare providers to understand, manage and monitor their immune profiles and to
stay informed about attacks on or by their immune system. We believe AditxtScore can also assist the medical community and individuals
by being able to anticipate the immune system’s potential response to viruses, bacteria, allergens, and foreign tissues such as
transplanted organs. This technology may be able to serve as a warning signal, thereby allowing for more time to respond appropriately.
Its advantages include the ability to provide simple, rapid, accurate, high throughput assays that can be multiplexed to determine the
immune status with respect to several factors simultaneously, in approximately 3-16 hours. In addition, it can determine and differentiate
between distinct types of cellular and humoral immune responses (e.g., T and B cells and other cell types). It also provides for simultaneous
monitoring of cell activation and levels of cytokine release (i.e., cytokine storms).
We
are actively involved in the regulatory approval process for AditxtScore assays for clinical use and securing manufacturing, marketing,
and distribution partnerships for application in the various markets. To obtain regulatory approval to use AditxtScore as a clinical
assay, we have conducted validation studies to evaluate its performance in detection of antibodies and plan to continue conducting additional
validation studies for new applications in autoimmune diseases.
Advantages
The
sophistication of the AditxtScore technology includes the following:
| ● | greater
sensitivity/specificity. |
| ● | 20-fold
higher dynamic range, greatly reducing signal to noise compared to conventional assays. |
| ● | ability
to customize assays and multiplex a large number of analytes with speed and efficiency. |
| ● | ability
to test for cellular immune responses (i.e., T and B cells and cytokines). |
| ● | proprietary
reporting algorithm. |
License
Agreement with Leland Stanford Junior University (“Stanford”)
On
February 3, 2020, we entered into an exclusive license agreement (the “February 2020 License Agreement”) with Stanford with
regard to a patent concerning a method for detection and measurement of specific cellular responses. Pursuant to the February 2020 License
Agreement, we received an exclusive worldwide license to Stanford’s patent with regard to use, import, offer, and sale of Licensed
Products (as defined in the agreement). The license to the patented technology is exclusive, including the right to sublicense, beginning
on the effective date of the agreement, and ending when the patent expires. Under the exclusivity agreement, we acknowledged that Stanford
had already granted a non-exclusive license in the Nonexclusive Field of Use, under the Licensed Patents in the Licensed Field of Use
in the Licensed Territory (as those terms are defined in the “February 2020 License Agreement”). However, Stanford agreed
not to grant further licenses under the Licensed Patents in the Licensed Field of Use in the Licensed Territory. On December 29, 2021,
we entered into an amendment to the February 2020 License Agreement which extended our exclusive right to license the technology deployed
in AditxtScoreTM and securing worldwide exclusivity in all fields of use of the licensed technology.
ADIVIR,
INC.
Formed
in April of 2023, Adivir™, Inc., is Aditxt’s most recently formed wholly owned subsidiary, dedicated to the clinical and
commercial development efforts of innovative antiviral products, starting with Favipiravir-based monotreatment or combination therapies.
These products have the potential to address a wide range of infectious diseases, including those that currently lack viable treatment
options.
Background
On
April 18, 2023, we entered into an Asset Purchase Agreement (the “Asset Purchase Agreement”) with Cellvera Global Holdings
LLC (“Cellvera Global”), Cellvera Holdings Ltd. (“BVI Holdco”), Cellvera, Ltd. (“Cellvera Ltd.”),
Cellvera Development LLC (“Cellvera Development” and together with Cellvera Global, BVI Holdco, Cellvera Ltd. and Cellvera
Development (the “Sellers”), AiPharma Group Ltd. (“Seller Owner” and collectively with the Sellers, “Cellvera”),
and the legal representative of Cellvera, pursuant to which, the Company will purchase Cellvera’s 50% ownership interest in G Response
Aid FZE (“GRA”), certain other intellectual property and all goodwill related thereto (the “Acquired Assets”). Unless
expressly stated otherwise herein, capitalized terms used but not defined herein have the meanings ascribed to them in the Asset Purchase
Agreement. Pursuant to the Asset Purchase Agreement, the consideration for the Acquired Assets consists of (A) $24.5 million,
comprised of: (i) the forgiveness of the Company’s $14.5 million loan to Cellvera Global, and (ii) approximately $10 million in
cash, and (B) future revenue sharing payments for a term of seven years. GRA holds an exclusive, worldwide license for the antiviral
medication, Avigan® 200mg, excluding Japan, China and Russia. The other 50% interest in GRA is held by Agility, Inc. (“Agility”).
Additionally,
upon the closing, the Share Exchange Agreement previously entered into as of December 28, 2021, between Cellvera Global Holdings, LLC
f/k/a AiPharma Global Holdings, LLC (together with other affiliates and subsidiaries) and the Company, and all other related agreements
will be terminated.
The
obligations of the Company to consummate the Closing are subject to the satisfaction or waiver, at or prior to the Closing of certain
conditions, including but not limited to, the following:
| (i) | Satisfactory
completion of due diligence; |
| (ii) | Completion
by the Company of financing sufficient to consummate the transactions contemplated by the Asset Purchase Agreement; |
| (iii) | Receipt
by the Company of all required Consents from Governmental Bodies for the Acquisition, including but not limited to, any consents required
to complete the transfer and assignment of Cellvera’s membership interests in GRA; |
| |
(iv) |
Receipt
of executed payoff letters reflecting the amount required to be fully pay all of each of Seller’s and Seller Owner’s
Debt to be paid at Closing; |
| |
(v) |
Receipt
by the Company of a release from Agility; |
| |
(vi) |
Execution
of an agreement acceptable to the Company with respect to the acquisition by the Company of certain intellectual property presently
held by a third party; |
| |
(vii) |
Execution
of an amendment to an asset purchase agreement previously entered into by Cellvera with a third party that effectively grants the
Company the rights to acquire the intellectual property from the third party under such agreement; |
| |
(viii) |
Receipt
of a fairness opinion by the Company with respect to the transactions contemplated by the Asset Purchase Agreement; and |
| |
(ix) |
Receipt
by the Company from the Seller Owner of written consent, whether through its official liquidator or the Board of Directors of Seller
Owner, to the sale and purchase of the Acquired Assets and Assumed Liabilities pursuant to the Assert Purchase Agreement. |
There
can be no assurance that the conditions to closing will be satisfied or that the proposed acquisition will be completed as proposed or
at all.
Our
commitment to building our antiviral portfolio is strategic and timely. We believe that there has never has there been a more important
time to address the growing global need to uncover new treatments or commercialize existing ones that treat life-threatening global viral
infections.
Recent
Developments
Nasdaq
Hearing
As
previously disclosed on a Current Report on Form 8-K filed in May 26, 2023, on May 23, 2023, we received written notice (the “May
Notification Letter”) from Nasdaq that, based upon the stockholders equity reported by the Company in its Form 10-Q for the
period ended March 31, 2023, and as of March 31, 2023, the Company was no longer in compliance with Nasdaq Listing Rule 5550(b)(1), which
requires a company to maintain a minimum of $2,500,000 in stockholders’ equity, a market value of listed securities of at least
$35 million, or net income from continuing operations of $500,000 in the most recently completed fiscal year or in two of the three most
recently completed fiscal years (the “Equity Rule”). The May Notification Letter further provided that the Company had 45
calendar days, or until July 7, 2023, to submit a plan to regain compliance and if the plan is accepted by Nasdaq, an extension of up
to 180 calendar days, or until November 19, 2023 to evidence compliance. On
June 22, 2023, we received a letter from Nasdaq notifying the Company that it has failed to maintain compliance with the minimum bid
price rule in Nasdaq Listing Rule 5550(a)(2) (the “Minimum Bid Price Rule”) as the closing price of Company’s common
stock has remained below $1.00 for over 30 consecutive trading days. On June 29, 2023, we submitted an appeal to Nasdaq, which stayed
the delisting and suspension of our securities pending the decision of the Nasdaq Hearings Panel (the “Panel”) no later than
4:00 p.m. Eastern Time on June 29, 2023. The hearing was held on August 31, 2023, which represented the tenth trading day that the closing
price of our Common Stock was above $1.00 per share. At the hearing, we also presented our views and our plans to regain compliance with
the stockholders’ equity requirement to the Panel.
On
September 15, 2023, we received written notice from Nasdaq that we no longer met the minimum 500,000 publicly held shares requirement
for the Nasdaq Capital Market and we were no longer in compliance with Nasdaq Listing Rule 5550(a)(4) (the “Public Float Rule”).
The letter indicated that the Panel will consider this matter in their decision regarding our continued listing on the Nasdaq Capital
Market. On September 29, 2023, we received written notice from Nasdaq that the Panel had
granted the Company an exception through December 26, 2023, to allow the Company to complete its plan to demonstrate compliance with
the Equity Rule. The October Notification Letter also confirmed that the Company had demonstrated compliance with the Minimum Bid Price
Rule, and it granted the Company an exception through December 26, 2023, to allow the Company to demonstrate compliance with the Public
Float Rule.
Reverse
Stock Split
As
previously disclosed on a Current Report on Form 8-K filed on August 17, 2023, on August 17, 2023, we filed a Certificate of Amendment
to our Amended and Restated Certificate of Amendment with the Secretary of State of the State of Delaware to effect a 1-for-40 reverse
stock split of our shares of Common Stock.
August
2023 Private Placement
As
previously disclosed in a Current Report on Form 8-K filed on September 6, 2023, on August 31, 2023, we entered into a securities purchase
agreement (the “Purchase Agreement”) with an institutional investor for the issuance
and sale in a private placement (the “Private Placement”) of (i) pre-funded warrants (the “Pre-Funded Warrants”)
to purchase up to 1,000,000 shares of the Company’s common stock, par value $0.001 (the “Common Stock”), at an exercise
price of $0.001 per share, and (ii) warrants (the “Common Warrants”) to purchase up to 1,000,000 shares of the Company’s
Common Stock at an exercise price of $10.00 per share.
The
Common Warrants are exercisable immediately upon issuance and have a term of exercise equal to five and one-half years from the date
of issuance. The Pre-Funded Warrants are exercisable immediately and may be exercised at any time until the Pre-Funded Warrants are exercised
in full. A holder of Pre-Funded Warrants or Warrants (together with its affiliates) may not exercise any portion of a warrant to the
extent that the holder would own more than 4.99% (or, at the election of the holder 9.99%) of the Company’s outstanding common
stock immediately after exercise.
In
connection with the Private Placement, we entered into a registration rights agreement (the “Registration Rights Agreement”),
dated as of August 31, 2023, with the investor, pursuant to which the Company agreed to prepare and file a registration statement with
the Securities and Exchange Commission (the “SEC”) registering the resale of the shares of Common Stock underlying the Pre-Funded
Warrants and the Common Warrants no later than 15 days after the date of the Registration Rights Agreement, and to use best efforts to
have the registration statement declared effective as promptly as practical thereafter, and in any event no later than 45 days following
the date of the Registration Rights Agreement (or 75 days following the date of the Registration Rights Agreement in the event of a “full
review” by the SEC).
The
Private Placement closed on September 6, 2023. We received net proceeds from the Private Placement of approximately $9 million, after
deducting placement agent fees and expenses and estimated offering expenses payable by us.
H.C.
Wainwright & Co., LLC (“Wainwright”) served as our exclusive placement agent in connection with the Private Placement,
pursuant to those certain engagement letters, dated as of March 27, 2023 and April 25, 2023, as amended, between the Company and Wainwright
(the “Engagement Letter”). Pursuant to the Engagement Letter, we paid Wainwright (i) a total cash fee equal to 7.75% of the
aggregate gross proceeds of the Private Placement, (ii) a management fee of 1.0% of the aggregate gross proceeds of the Private Placement,
(iii) a non-accountable expense allowance of $50,000, and (iv) $100,000 for legal fees and other out-of-pocket expenses. In addition,
we issued to Wainwright or its designees warrants (the “Placement Agent Warrants”) to purchase up to an aggregate of 60,000
shares of Common Stock at an exercise price equal to $12.50 per share. The Placement Agent Warrants are exercisable immediately upon
issuance and have a term of exercise equal to five and one-half years from the date of issuance.
July
Private Placement
On
July 3, 2023, we entered into a Securities Purchase Agreement (the “First Tranche Securities Purchase Agreement”) with an
accredited investor (the “First Tranche Investor”) pursuant to which we issued and sold a secured promissory note in the
principal amount of $375,000 (the “First Tranche Note”) resulting in gross proceeds of $250,000. In connection with the issuance
of the Note, we issued 3,907 shares of common stock (the “First Tranche Commitment Shares”) as a commitment fee to the investor.
Pursuant to the Securities Purchase Agreement, we are obligated to obtain approval of our shareholders (“Shareholder Approval”)
with respect to the issuance of any securities in connection with the Securities Purchase Agreement and the Note in excess of 19.99%
of our issued and outstanding shares on the closing date, which is equal to 33,791 shares of our common stock. Pursuant to the First
Tranche Securities Purchase Agreement, we also granted piggy-back registration rights to the investor with respect to First Tranche Commitment
Shares and any shares of our common stock issuable upon conversion of the First Tranche Note. In addition, we agreed that we would not
enter into any public or private offering of securities that has rights or benefits superior to the First Tranche Investor without providing
such rights and benefits to the First Tranche investor. Under the First Tranche Note, the First Tranche Investor has the right to require
us to immediately apply up to 25% of the cash proceeds from any source, to repay all or any portion of the outstanding balance of the
First Tranche Note. The First Tranche Note has a maturity date of December 31, 2023 and is convertible following Shareholder Approval
and the occurrence of an Event of Default (as defined in the Note) at a conversion price of $18.00 per share. As of the date of this
prospectus, the First Tranche Note has been fully repaid.
In
connection with the First Tranche Securities Purchase Agreement and the issuance of the First Tranche Note, we and certain of our subsidiaries
also entered into a Security Agreement with the investor (the “First Tranche Security Agreement”) pursuant to which we granted
the investor a security interest in certain Collateral (as defined in the First Tranche Security Agreement) to secure our obligations
under the First Tranche Note. In addition, we entered into a Registration Rights Agreement with the investor (the “First Tranche
Registration Rights Agreement”) pursuant to which we agreed to prepare and file with the U.S. Securities and Exchange Commission
a registration statement covering the resale of the First Tranche Commitment Shares and any shares of our common stock issuable upon
conversion of the First Tranche Note within 120 days of the closing date and to have such registration statement declared effective within
150 days of the closing date.
On
July 24, 2023, we entered into a Securities Purchase Agreement (the “Second Tranche Securities Purchase Agreement”) with
an accredited investor (the “Second Tranche Investor”) pursuant to which the Company issued and sold a secured promissory
note in the principal amount of $2,625,000 (the “Second Tranche Note”) resulting in gross proceeds to the Company of $1,750,000.
In connection with the issuance of the Note, we agreed to issue a total of 27,344 shares of common stock (the “Second Tranche Commitment
Shares”) as a commitment fee to the investor. At the request of the investor, we issued 17,277 Second Tranche Commitment Shares
and will issue the remaining 10,067 Second Tranche Commitment Shares within 120 days, subject to the investor’s discretion. Pursuant
to the Second Tranche Securities Purchase Agreement, we are obligated to obtain approval of our shareholders with respect to the issuance
of any securities in connection with the Second Tranche Securities Purchase Agreement and the Second Tranche Note in excess of 19.99%
of our issued and outstanding shares on the closing date, which is equal to 38,026 shares of the Company’s common stock. Pursuant
to the Second Tranche Securities Purchase Agreement, we also granted piggy-back registration rights to the investor with respect to Second
Tranche Commitment Shares and any shares of our common stock issuable upon conversion of the Second Tranche Note. In addition, we agreed
that we would not enter into any public or private offering of securities that has rights or benefits superior to the Second Tranche
Investor without providing such rights and benefits to the Second Tranche Investor. Under the Second Tranche Note, the Second Tranche
Investor has the right to require us to immediately apply up to 25% of the cash proceeds from any source, to repay all or any portion
of the outstanding balance of the Second Tranche Note. The Second Tranche Note has a maturity date of December 31, 2023 and is convertible
following shareholder approval and the occurrence of an Event of Default (as defined in the Note) at a conversion price of $15.60 per
share.
In
connection with the Second Tranche Securities Purchase Agreement and the issuance of the Second Tranche Note, we and certain of our subsidiaries
also entered into a Security Agreement with the investor (the “Second Tranche Security Agreement”) pursuant to which we granted
the investor a security interest in certain Collateral (as defined in the Second Tranche Security Agreement) to secure its obligations
under the Second Tranche Note. In addition, we entered into a Registration Rights Agreement with the investor (the “Second Tranche
Registration Rights Agreement”) pursuant to which we agreed to prepare and file with the U.S. Securities and Exchange Commission
a registration statement covering the resale of the Second Tranche Commitment Shares and any shares of our common stock issuable upon
conversion of the Second Tranche Note within 90 days of the closing date and to have such registration statement declared effective within
120 days of the closing date.
July
2023 Loan Transactions
On
July 3, 2023, we entered into a Business Loan and Security Agreement (the “July Loan Agreement”) with a commercial funding
source (the “July Lender”), pursuant to which we obtained a loan from the July Lender in the principal amount of $215,000,
which includes origination fees of $10,750 (the “July Loan”). Pursuant to the July Loan Agreement, we granted the Lender
a continuing secondary security interest in certain collateral (as defined in the July Loan Agreement). The total amount of interest
and fees payable by us to the July Lender under the July Loan will be $322,285, which will be repaid in 13 weekly installments of $24,500
with a final payment of $3,785 in the fourteenth week. As of August 18, 2023, the outstanding balance on the July Loan is $311,076. On
August 23, 2023, we entered into a new Business Loan and Security Agreement with the July Lender, pursuant to which we consolidated the
amounts outstanding under the April Loan and the July Loan and obtained a new loan in the principal amount of $1.4 million.
July
2023 Private Placement of Series C Preferred Stock
On
July 11, 2023, we entered into a Subscription and Investment Representation Agreement (the “Subscription Agreement”) with
Amro Albanna, our Chief Executive Officer, who is an accredited investor (the “Purchaser”), pursuant to which we agreed to
issue and sell one (1) share of the Company’s Series C Preferred Stock, par value $0.001 per share (the “Preferred Stock”),
to the Purchaser for $1,000.00 in cash. The sale closed on July 11, 2023.
On
July 11, 2023, we filed a certificate of designation (the “Certificate of Designation”) with the Secretary of State of Delaware,
effective as of the time of filing, designating the rights, preferences, privileges and restrictions of the share of Preferred Stock.
The Certificate of Designation provides that the share of Preferred Stock will have 250,000,000 votes and will vote together with the
outstanding shares of our common stock as a single class exclusively with respect to any proposal to amend the Company’s Amended
and Restated Certificate of Incorporation to effect a reverse stock split of our common stock. The Preferred Stock will be voted, without
action by the holder, on any such proposal in the same proportion as shares of common stock are voted. The Preferred Stock otherwise
has no voting rights except as otherwise required by the General Corporation Law of the State of Delaware.
The
Preferred Stock is not convertible into, or exchangeable for, shares of any other class or series of stock or other securities of the
Company. The Preferred Stock has no rights with respect to any distribution of assets of the Company, including upon a liquidation, bankruptcy,
reorganization, merger, acquisition, sale, dissolution or winding up of the Company, whether voluntarily or involuntarily. The holder
of the Preferred Stock will not be entitled to receive dividends of any kind.
The
outstanding share of Preferred Stock shall be redeemed in whole, but not in part, at any time (i) if such redemption is ordered by the
Board of Directors in its sole discretion or (ii) automatically upon the effectiveness of the amendment to the Certificate of Incorporation
implementing a reverse stock split. Upon such redemption, the holder of the Preferred Stock will receive consideration of $1,000.00 in
cash.
Resignation
of Executive Officer
On
July 21, 2023, Matthew Shatzkes tendered his resignation as Chief Legal Officer, General Counsel and Corporate Secretary of the Company.
In connection with his resignation, we entered into a Separation Agreement and General Release with Mr. Shatzkes (the “Separation
Agreement”). Pursuant to the Separation Agreement, Mr. Shatzkes employment with the Company terminated on August 4, 2023 (the “Termination
Date”). In addition, we agreed to pay Mr. Shatzkes within seven days after the Termination Date: (i) $122,292.32, representing
all accrued salary and wages (inclusive of Base Compensation and earned Subsequent Quarterly Bonus amounts, as those terms are defined
in Mr. Shatzkes employment agreement) (the “Accrued Salary and Wages”), and (ii) $32,575.84, representing Mr. Shatzkes accrued,
but unused paid time off (the “Accrued PTO”). On August 11, 2023, we paid Mr. Shatzkes $64,808. Pursuant to the Separation
Agreement, we also agreed to pay Mr. Shatzkes: (i) $385,000, representing 12 months of Mr. Shatzkes Base Compensation (as that term is
defined in Mr. Shatzkes employment agreement) (the “Severance Base Compensation”), and (ii) $290,000, representing Mr. Shatzkes
Subsequent Year Minimum Bonus (as such term is defined in Mr. Shatzkes employment agreement) (the “Severance Bonus”), on
the 60th day following the Termination Date. In addition, we are required to reimburse Mr. Shatzkes COBRA premium for a period of 12
months and shall cause any restricted stock units granted to Mr. Shatzkes to immediately vest as of the Termination Date.
On
August 15, 2023, we entered into an Amendment to Separation Agreement and General Release with Mr. Shatzkes (the “Separation Agreement
Amendment”). Pursuant to the Separation Agreement Amendment, we are required to pay Mr. Shatzkes, upon the earlier of (i) September
1, 2023 or (ii) two business days following the closing of a capital raise by the Company, an amount equal to $91,060.16, which amount
represents the balance of Mr. Shatzkes’ Accrued Salary and Wages and Accrued PTO plus an additional $1,000 to serve as consideration
for entering into the Separation Agreement Amendment. In addition, under the Separation Agreement Amendment, we are required to pay Mr.
Shatzkes the Severance Base Compensation and the Severance Bonus upon the earlier of (i) the 60th day following the Termination
Date or (ii) two business days following the closing of a capital raise by the Company. We expect to pay the amounts due to Mr. Shatzkes
from the proceeds of our recently completed Private Placement.
Termination
of Letter of Intent
On
August 1, 2023, the Company and Natural State Genomics and Natural State Laboratories mutually agreed to terminate the Amended and Restated
Non-Binding Letter of Intent dated June 12, 2023.
August
2023 Secured Loan Transaction
On
August 23, 2023, we entered into a Business Loan and Security Agreement (the “August Loan Agreement”) with a commercial funding
source (the “August Lender”) pursuant to which we obtained a loan from the August Lender in the principal amount of $1,400,000,
which will satisfy the outstanding balances on loans that we originally obtained From the August Lender in April 2023 and July 2023,
and includes origination fees of $70,000 (the “August Loan”). Pursuant to the August Loan Agreement, we granted the August
Lender a continuing secondary security interest in certain collateral (as defined in the August Loan Agreement). The total amount of
interest and fees payable by us to the August Lender under the August Loan will be $2,079,000, which will be repaid in 21 weekly installments
of $99,000.
April
2023 Registered Direct Offering and Concurrent Private Placement
On
April 20, 2023, we entered into a securities purchase agreement (the “April Purchase Agreement”) with an institutional investor,
pursuant to which the Company agreed to sell to such investor pre-funded warrants (the “Pre-Funded Warrants”) to purchase
up to 39,634 shares of common stock of the Company at a purchase price of $48.76 per Pre-Funded Warrant. The Pre-Funded Warrants (and
shares of common stock underlying the Pre-Funded Warrants) were offered by the Company pursuant to its shelf registration statement on
Form S-3 (File No. 333-257645), which was declared effective by the Securities and Exchange Commission on July 13, 2021.
Concurrently
with the sale of the Pre-Funded Warrants, pursuant to the Purchase Agreement in a concurrent private placement, for each Pre-Funded
Warrant purchased by the investor, such investor received from the Company an unregistered warrant (the “Warrant”) to purchase
two shares of Common Stock. The warrants have an exercise price of $34.40 per share, and are exercisable for a three year period. The
Warrant was issued were made pursuant to Section 4(a)(2) of the Securities Act.
Corporate
Information
We
were incorporated as a Delaware corporation on September 28, 2017. Our principal executive offices are located at 737 N. Fifth Street,
Suite 200 Richmond, VA 23219, and our telephone number is (650) 870-1200.
Our
common stock trades on The Nasdaq Capital Market under the symbol “ADTX.”
THE
OFFERING
Common
Stock to be
offered by the Selling
Stockholders |
|
Up to 264,516 shares of Common
Stock |
| |
|
|
Common
Stock
outstanding prior to this
offering |
|
441,851
shares of Common Stock |
| |
|
|
Common
Stock to be
outstanding after this
offering |
|
685,182 shares of Common Stock, assuming the exercise of
all of the Warrants and the issuance of all shares of Common Stock upon conversion of the Note. |
| Use
of proceeds |
|
We
will not receive any proceeds from the sale of the shares of Common Stock by the Selling Stockholders, except for the Warrant exercise
price paid for the Common Stock offered hereby and issuable upon the exercise of the Warrants. See “Use of Proceeds”
on page 45 of this prospectus. |
| |
|
|
| Risk
factors |
|
See
“Risk Factors” beginning on page 15 of this prospectus, as well as other information included in this prospectus, for
a discussion of factors you should read and consider carefully before investing in our securities. |
| |
|
|
| Nasdaq
Capital Markets symbol |
|
Our
common stock is listed on The Nasdaq Capital Markets under the symbol “ADTX”. There is no established trading market
for the Warrants and we do not expect a trading market to develop. We do not intend to list the Warrants on any securities exchange
or other trading market. Without a trading market, the liquidity of the Warrants will be extremely limited. |
The number of shares of our common stock to be outstanding after this
offering as shown above is based on 441,851 shares outstanding as of November 6, 2023 and excludes as of that date:
| ● | 2,055,924
shares of our common stock issuable upon exercise of warrants, subject to vesting having a weighted average exercise price of $36.95
per share; and |
| ● | 1,127
shares of our common stock issuable upon exercise of outstanding options under our 2017 Equity Incentive Plan or the 2017 Plan, subject
to vesting. |
Except
as otherwise indicated herein, all information in this prospectus assumes no sale of pre-funded warrants, which, if sold, would reduce
the number of shares of common stock that we are offering on a one-for-one basis, no exercise of the warrants or placement agent warrants
issued in this offering, and no exercise of options issued under our Plan or of warrants described above, including the Placement Agent
Warrants.
RISK
FACTORS
An
investment in our securities involves a high degree of risk. This prospectus contains a discussion of the risks applicable to an investment
in our securities. Prior to deciding about investing in our securities, you should carefully consider the specific factors discussed
within this prospectus. The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties
not presently known to us or that we currently deem immaterial may also affect our operations. The occurrence of any of these known or
unknown risks might cause you to lose all or part of your investment in the offered securities.
Risks
Related to Our Common Stock
We
received a written notice from Nasdaq that we have failed to comply with certain listing requirements of the Nasdaq Stock Market, which
could result in our Common Stock being delisted from the Nasdaq Stock Market.
On
May 23, 2023, we received written notice from Nasdaq that, based upon the stockholders equity reported by the Company in its Form
10-Q for the period ended March 31, 2023, and as of March 31, 2023, the Company was no longer in compliance with Nasdaq Listing Rule
5550(b)(1), which requires a company to maintain a minimum of $2,500,000 in stockholders’ equity, a market value of listed securities
of at least $35 million, or net income from continuing operations of $500,000 in the most recently completed fiscal year or in two of
the three most recently completed fiscal years (the “Continued Listing Requirements”). The May notification letter further
provided that the Company has 45 calendar days, or until July 7, 2023, to submit a plan to regain compliance and if the plan is accepted
by Nasdaq, an extension of up to 180 calendar days, or until November 19, 2023 to evidence compliance. On June 22, 2023, we received
a letter from Nasdaq notifying the Company that it has failed to maintain compliance with the minimum bid price rule in Nasdaq Listing
Rule 5550(a)(2) (the “Minimum Bid Price Rule”) as the closing price of Company’s common stock has remained below $1.00
for over 30 consecutive trading days. On June 29, 2023, we submitted an appeal to Nasdaq, which stays the delisting and suspension of
our securities pending the decision of the Nasdaq Hearings Panel (the “Panel”) no later than 4:00 p.m. Eastern Time on June
29, 2023.
On
August 17, 2023, we filed a Certificate of Amendment to our Certificate of Incorporation, as amended, to effect a reverse stock split
at a ratio of one-for-forty (1:40) in order to regain compliance with the Minimum Bid Price Rule. However, we can provide no assurance
that Nasdaq will accept our plan of compliance or grant us any additional time to demonstrate our ability to regain and sustain compliance
with the continued listing requirements over the long term. If we are delisted from Nasdaq, our common stock may be eligible for trading
on an over-the-counter market. If we are not able to obtain a listing on another stock exchange or quotation service for our common stock,
it may be extremely difficult or impossible for stockholders to sell their shares.
The
hearing was held on August 31, 2023, which represented the tenth trading day that the closing price of our Common Stock was above $1.00
per share. At the hearing, we also presented our views and our plans to regain compliance with the stockholders’ equity requirement
to the Panel.
On September 15,
2023, we received written notice from Nasdaq that we no longer met the minimum 500,000 publicly held shares requirement for the Nasdaq
Capital Market and we were no longer in compliance with Nasdaq Listing Rule 5550(a)(4) (the “Public Float Rule”). The letter
indicated that the Panel will consider this matter in their decision regarding our continued listing on the Nasdaq Capital Market. On
September 29, 2023, we received written notice from Nasdaq that the Panel had granted the
Company an exception through December 26, 2023, to allow the Company to complete its plan to demonstrate compliance with the Equity Rule.
The October Notification Letter also confirmed that the Company had demonstrated compliance with the Minimum Bid Price Rule, and it granted
the Company an exception through December 26, 2023, to allow the Company to demonstrate compliance with the Public Float Rule.
If
we are delisted from Nasdaq, but obtain a substitute listing for our common stock, it will likely be on a market with less liquidity,
and therefore experience potentially more price volatility than experienced on Nasdaq. Stockholders may not be able to sell their shares
of common stock on any such substitute market in the quantities, at the times, or at the prices that could potentially be available on
a more liquid trading market. As a result of these factors, if our common stock is delisted from Nasdaq, the value and liquidity of our
common stock, warrants and pre-funded warrants would likely be significantly adversely affected. A delisting of our common stock from
Nasdaq could also adversely affect our ability to obtain financing for our operations and/or result in a loss of confidence by investors,
employees and/or business partners.
We
do not expect to pay dividends in the foreseeable future.
We
do not intend to declare dividends for the foreseeable future, as we anticipate that we will reinvest any and all future earnings in
the development and growth of our business. Therefore, investors will not receive any funds unless they sell their securities, and holders
may be unable to sell their securities on favorable terms or at all. We cannot assure you of a positive return on your investment or
that you will not lose the entire amount of your investment.
Risks
Related to Our Financial Position and Need for Capital
Our
financial situation creates doubt whether we will continue as a going concern.
We
were incorporated in September 2017 and have a limited operating history and our business is subject to all the risks inherent in the
establishment of a new business enterprise. Our likelihood of success must be considered in light of the problems, expenses, difficulties,
complications and delays frequently encountered in connection with development and expansion of a new business enterprise. Since inception,
we have incurred losses and expect to continue to operate at a net loss for at least the next several years as we commence our research
and development efforts, conduct clinical trials, and develop manufacturing, sales, marketing, and distribution capabilities. Our net
loss for the years ended December 31, 2022 and 2021 was $27,649,876 and $46,371,364, respectively, and our accumulated deficit as of
December 31, 2022 was $95,040,362. Our net loss for the six months ended June 30, 2023 and 2022 was $11,666,724 and $11,909,147, respectively,
and our accumulated deficit as of June 30, 2023 was $106,707,086. There can be no assurance that the products under development by us
will be approved for sale in the U.S. or elsewhere. Furthermore, there can be no assurance that if such products are approved, they will
be successfully commercialized, and the extent of our future losses and the timing of our profitability are highly uncertain. If we are
unable to achieve profitability, we may be unable to continue our operations. There can be no assurances that we will be able to achieve
a level of revenues adequate to generate sufficient cash flow from operations or additional financing through private placements, public
offerings and/or bank financing necessary to support our working capital requirements. To the extent that funds generated from any private
placements, public offerings and/or bank financing are insufficient, we will have to raise additional working capital. No assurance can
be given that additional financing will be available, or if available, will be on acceptable terms. These conditions raise substantial
doubt about our ability to continue as a going concern. If adequate working capital is not available, we may be forced to discontinue
operations, which would cause investors to lose their entire investment.
Our
cash and cash equivalents were approximately $500,000 as of November 6, 2023. We will not receive any of the proceeds from the sale by
the Selling Stockholders of the Common Stock. Upon any exercise of the Warrants by payment of cash, however, we will receive the exercise
price of the Warrants, which, if exercised in cash with respect to the 79,268 shares of Common Stock offered hereby, would result in
gross proceeds to us of approximately $2.7 million. However, we cannot predict when and in what amounts or if the Warrants will be exercised
by payments of cash and it is possible that the Warrants may expire and never be exercised, in which case we would not receive any cash
proceeds. There can be no assurance that the Warrants will be exercised for cash, and if they are
not, that it will not have a material adverse effect on our business.
If
we fail to obtain the capital necessary to fund our operations, we will be unable to continue or complete our product development and
you will likely lose your entire investment.
We
will need to continue to seek capital from time to time to continue development of our lead drug candidate beyond our initial combined
Phase I/Iia clinical trial and to acquire and develop other product candidates. Once approved for commercialization, we cannot provide
any assurances that any revenues it may generate in the future will be sufficient to fund our ongoing operations.
Our
business or operations may change in a manner that would consume available funds more rapidly than anticipated and substantial additional
funding may be required to maintain operations, fund expansion, develop new or enhance products, acquire complementary products, business
or technologies, or otherwise respond to competitive pressures and opportunities, such as a change in the regulatory environment or a
change in preferred treatment modalities. In addition, we may need to accelerate the growth of our sales capabilities and distribution
beyond what is currently envisioned, and this would require additional capital. However, we may not be able to secure funding when we
need it or on favorable terms. We may not be able to raise sufficient funds to commercialize the product candidates we intend to develop.
If
we cannot raise adequate funds to satisfy our capital requirements, we will have to delay, scale back or eliminate our research and development
activities, clinical studies, or future operations. We may also be required to obtain funds through arrangements with collaborators,
which arrangements may require us to relinquish rights to certain technologies or products that we otherwise would not consider relinquishing,
including rights to future product candidates or certain major geographic markets. This could result in sharing revenues which we might
otherwise retain for ourselves. Any of these actions may harm our business, financial condition, and results of operations.
The
amount of capital we may need depends on many factors, including the progress, timing and scope of our product development programs;
the progress, timing and scope of our preclinical studies and clinical trials; the time and cost necessary to obtain regulatory approvals;
the time and cost necessary to further develop manufacturing processes and arrange for contract manufacturing; our ability to enter into
and maintain collaborative, licensing and other commercial relationships; and our partners’ commitment of time and resources to
the development and commercialization of our products.
Our
obligations to certain of our creditors are secured by security interests in our assets, so if we default on those obligations, our creditors
could foreclose on some or all of our assets.
Our
obligations to certain of our creditors are secured by security interests in our assets. As of November 6, 2023, approximately $5.0 million
was owed to such secured creditors. Under such agreements, we are required to pay $248,000 on a weekly basis to such creditors. If we
default on our obligations under these agreements, our secured creditors could foreclose on its security interests and liquidate some
or all of these assets, which would harm our financial condition and results of operations and would require us to reduce or cease operations
and possibly seek Bankruptcy Protection.
In
the event we pursue Bankruptcy Protection, we will be subject to the risks and uncertainties associated with such proceedings.
In
the event we file for relief under the United States Bankruptcy Code, our operations, our ability to develop and execute our business
plan and our continuation as a going concern will be subject to the risks and uncertainties associated with bankruptcy proceedings, including,
among others: our ability to execute, confirm and consummate a plan of reorganization; the additional, significant costs of bankruptcy
proceedings and related fees; our ability to obtain sufficient financing to allow us to emerge from bankruptcy and execute our business
plan post-emergence, and our ability to comply with terms and conditions of that financing; our ability to continue our operations in
the ordinary course; our ability to maintain our relationships with our consumers, business partners, counterparties, employees and other
third parties; our ability to obtain, maintain or renew contracts that are critical to our operations on reasonably acceptable terms
and conditions; our ability to attract, motivate and retain key employees; the ability of third parties to use certain limited safe harbor
provisions of the United States Bankruptcy Code to terminate contracts without first seeking Bankruptcy Court approval; the ability of
third parties to force us to into Chapter 7 proceedings rather than Chapter 11 proceedings and the actions and decisions of our stakeholders
and other third parties who have interests in our bankruptcy proceedings that may be inconsistent with our operational and strategic
plans. Any delays in our bankruptcy proceedings would increase the risks of our being unable to reorganize our business and emerge from
bankruptcy proceedings and may increase our costs associated with the bankruptcy process or result in prolonged operational disruption
for us. Also, we would need the prior approval of the bankruptcy court for transactions outside the ordinary course of business during
the course of any bankruptcy proceedings, which may limit our ability to respond timely to certain events or take advantage of certain
opportunities. Because of the risks and uncertainties associated with any bankruptcy proceedings, we cannot accurately predict or quantify
the ultimate impact of events that could occur during any such proceedings. There can be no guarantees that if we seek Bankruptcy Protection
we will emerge from Bankruptcy Protection as a going concern or that holders of our common stock will receive any recovery from any bankruptcy
proceedings.
In
the event we are unable to pursue Bankruptcy Protection under Chapter 11 of the United States Bankruptcy Code, or, if pursued, successfully
emerge from such proceedings, it may be necessary to pursue Bankruptcy Protection under Chapter 7 of the United States Bankruptcy Code
for all or a part of our businesses.
In
the event we are unable to pursue Bankruptcy Protection under Chapter 11 of the United States Bankruptcy Code, or, if pursued, successfully
emerge from such proceedings, it may be necessary for us to pursue Bankruptcy Protection under Chapter 7 of the United States Bankruptcy
Code for all or a part of our businesses. In such event, a Chapter 7 trustee would be appointed or elected to liquidate our assets for
distribution in accordance with the priorities established by the United States Bankruptcy Code. We believe that liquidation under Chapter
7 would result in significantly smaller distributions being made to our stakeholders than those we might obtain under Chapter 11 primarily
because of the likelihood that the assets would have to be sold or otherwise disposed of in a distressed fashion over a short period
of time rather than in a controlled manner and as a going concern.
Upon
dissolution of our Company, you may not recoup all or any portion of your investment.
In
the event of a liquidation, dissolution or winding-up of our Company, whether voluntary or involuntary, our assets would be used to pay
all of our debts and liabilities, and only thereafter would any remaining assets be distributed to our stockholders, subject to rights
of the holders of the Preferred Stock, if any, on a pro rata basis. There can be no assurance that we will have assets
available from which to pay any amounts to our stockholders upon such a liquidation, dissolution or winding-up. In such an event, you
would lose all of your investment.
We
will need to raise substantial additional capital, which may not be available on acceptable terms, or at all. Failure to obtain this
necessary capital when needed may force us to delay, limit or terminate our product development efforts or cease operations.
We
do not expect that our current cash position will be sufficient to fund our current operations for the next 12 months. Our operating
plan may change because of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through
public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements and other
collaborations, strategic alliances and licensing arrangements or a combination of these approaches. In any event, we will require additional
capital to obtain regulatory approval for, and to commercialize, our product candidates. Raising funds in the current economic environment
may present additional challenges. Even if we believe we have sufficient funds for our current or future operating plans, we may seek
additional capital if market conditions are favorable or if we have specific strategic considerations.
Any
additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to
develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient
amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights
of our stockholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may
cause the market price of our shares to decline. The sale of additional equity or convertible securities may dilute our existing stockholders.
The incurrence of indebtedness would result in increased fixed payment obligations, and we may be required to agree to certain restrictive
covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual
property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required
to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable and
we may be required to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to
us, any of which may have a material adverse effect on our business, operating results and prospects.
If
we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay, or discontinue one or more of
our research or development programs or the commercialization of any product candidate or be unable to expand our operations or otherwise
capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of
operations.
Even
if we can raise additional funding, we may be required to do so on terms that are dilutive to you.
The
capital markets have been unpredictable in the past for unprofitable companies such as ours. In addition, it is generally difficult for
development stage companies to raise capital under current market conditions. The amount of capital that a company such as ours is able
to raise often depends on variables that are beyond our control. As a result, we may not be able to secure financing on terms attractive
to us, or at all. If we can consummate a financing arrangement, the amount raised may not be sufficient to meet our future needs. If
adequate funds are not available on acceptable terms, or at all, our business, including our results of operations, financial condition
and our continued viability will be materially adversely affected.
Risks
Related to Product Development, Regulatory Approval, Manufacturing and Commercialization
The
regulatory approval process is expensive, time-consuming, and uncertain and may prevent us from obtaining approvals for the commercialization
of our future product candidates, if any.
We
will not be permitted to market our product candidates in the United States until we receive approval from the FDA, or in any foreign
countries until we receive the requisite approval from corresponding agencies in such countries. The testing, manufacturing, labeling,
approval, selling, marketing and distribution of health and life science-related products are subject to extensive regulation, which
regulations differ from country to country.
Successfully
completing our clinical program and obtaining approval of a Biologics License Application (“BLA”) is a complex, lengthy,
expensive and uncertain process, and the FDA or other applicable foreign regulator may delay, limit or deny approval of our product candidates
for many reasons, including, among others, because:
| ● | we
may not be able to demonstrate that our product candidates are safe and effective in treating patients to the satisfaction of the FDA
or foreign regulator; |
| ● | the
results of our clinical trials may not meet the level of statistical or clinical significance required by the FDA or foreign regulator
for marketing approval; |
| ● | the
FDA or foreign regulator may disagree with the number, design, size, conduct or implementation of our clinical trials; |
| ● | the
FDA or foreign regulator may require that we conduct additional clinical trials; |
| ● | the
FDA or foreign regulator may not approve the formulation, labeling or specifications of our product candidates; |
| ● | the
contract research organizations (CROs) and other contractors that we may retain to conduct our clinical trials may take actions outside
of our control that materially adversely impact our clinical trials; |
| ● | the
FDA or foreign regulator may find the data from preclinical studies and clinical trials insufficient to demonstrate that our product
candidate(s) are safe and effective for their proposed indications; |
| ● | the
FDA or foreign regulator may disagree with our interpretation of data from our preclinical studies and clinical trials; |
| ● | the
FDA or foreign regulator may not accept data generated at our clinical trial sites or may disagree with us over whether to accept efficacy
results from clinical trial sites outside the United States or outside the EU, as applicable, where the standard of care is potentially
different from that in the United States or in the EU, as applicable; |
| ● | if
and when our BLAs or foreign equivalents are submitted to the applicable regulatory authorities, such agencies may have difficulties
scheduling the necessary review meetings in a timely manner, may recommend against approval of our application or may recommend or require,
as a condition of approval, additional preclinical studies or clinical trials, limitations on approved labeling or distribution and use
restrictions; |
| ● | the
FDA or foreign regulator may require development of a Risk Evaluation and Mitigation Strategy (REMS), which would use risk minimization
strategies to ensure that the benefits of certain prescription drugs outweigh their risks, as a condition of approval or post-approval; |
| ● | the
FDA or other applicable foreign regulatory agencies may not approve the manufacturing processes or facilities of third-party manufacturers
with which we contract; or |
| ● | the
FDA or the other applicable foreign regulatory agencies may change their approval policies or adopt new regulations. |
We
may encounter substantial delays in completing our clinical studies which in turn will require additional costs, or we may fail to demonstrate
adequate safety and efficacy to the satisfaction of applicable regulatory authorities.
It
is difficult to predict if or when any of our product candidates, will prove safe or effective in humans or will receive regulatory
approval. Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct extensive
clinical studies to demonstrate the safety and efficacy of the product candidates in humans. Clinical testing is expensive, time-consuming,
and uncertain as to outcome. We cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if
at all. A failure of one or more clinical studies can occur at any stage of testing. Events that may prevent successful or timely completion
of clinical development include:
| |
● |
delays
in reaching, or failing to reach, a consensus with regulatory agencies on study design; |
| |
● |
delays
in reaching, or failing to reach, agreement on acceptable terms with a sufficient number of prospective contract research organizations
(“CROs”) and clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly
among different CROs and trial sites; |
| |
|
|
| |
● |
delays
in obtaining required Institutional Review Board (“IRB”) or Ethics Committee (“EC”) approval at each clinical
study site; |
| |
|
|
| |
● |
delays
in recruiting a sufficient number of suitable patients to participate in our clinical studies including, but not limited to, recruitment
challenges due to COVID-19; |
| |
|
|
| |
● |
imposition
of a clinical hold by regulatory agencies, after an inspection of our clinical study operations or study sites; |
| |
|
|
| |
● |
failure
by our CROs, other third parties or us to adhere to the clinical study, regulatory or legal requirements; |
| |
|
|
| |
● |
failure
to perform in accordance with the FDA’s good clinical practices (“GCP”) or applicable regulatory guidelines in
other countries; |
| |
|
|
| |
● |
delays
in the testing, validation, manufacturing, and delivery of sufficient quantities of our product candidates to the clinical sites; |
| |
|
|
| |
● |
delays
in having patients’ complete participation in a study or return for post-treatment follow-up; |
| |
|
|
| |
● |
clinical
study sites or patients dropping out of a study; |
| |
|
|
| |
● |
delay
or failure to address any patient safety concerns that arise during the course of a trial; |
| |
|
|
| |
● |
unanticipated
costs or increases in costs of clinical trials of our product candidates; |
| |
|
|
| |
● |
occurrence
of serious adverse events associated with the product candidates that are viewed to outweigh their potential benefits; or |
| |
|
|
| |
● |
changes
in regulatory requirements and guidance that require amending or submitting new clinical protocols. |
We
could also encounter delays if a clinical trial is suspended or terminated by us, by the IRBs or Ecs of the institutions in which such
trials are being conducted, by an independent Safety Review Board (“SRB”) for such trial or by the FDA, European Medicines
Agency (“EMA”), or other regulatory authorities. Such authorities may suspend or terminate a clinical trial due
to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols,
inspection of the clinical trial operations or trial site by the FDA, EMA, or other regulatory authorities resulting in the imposition
of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in
governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial.
Any
inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability
to generate revenues from product sales, regulatory and commercialization milestones, and royalties. In addition, if we make manufacturing
or formulation changes to our product candidates, we may need to conduct additional studies to bridge our modified product candidates
to earlier versions.
Clinical
study delays could also shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow
our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates. In
addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development
and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may significantly
harm our business, financial condition, and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement
or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
The
outcome of preclinical studies and early clinical trials may not be predictive of the success of later clinical trials, and interim results
of a clinical trial do not necessarily predict final results. Further, preclinical and clinical data are often susceptible to various
interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical
studies and clinical trials have, nonetheless, failed to obtain marketing approval. If the results of our clinical studies are inconclusive
or if there are safety concerns or adverse events associated with our other product candidates, we may:
| |
● |
be
delayed in obtaining marketing approval for our product candidates, if approved at all; |
| |
|
|
| |
● |
obtain
approval for indications or patient populations that are not as broad as intended or desired; |
| |
|
|
| |
● |
obtain
approval with labeling that includes significant use or distribution restrictions or safety warnings; |
| |
|
|
| |
● |
be
required to change the way the product is administered; |
| |
|
|
| |
● |
be
required to perform additional clinical studies to support approval or be subject to additional post-marketing testing requirements; |
| |
|
|
| |
● |
have
regulatory authorities withdraw their approval of a product or impose restrictions on its distribution in the form of a modified
risk evaluation and mitigation strategy; |
| |
|
|
| |
● |
be
sued; or |
| |
|
|
| |
● |
experience
damage to our reputation. |
If
we, ours collaborators, or our contract manufacturing organizations (“CMOs”) fail to comply with applicable regulatory requirements
at any stage during the regulatory process, such noncompliance could result in, among other things delays in the approval of applications
or supplements to approved applications; refusal of a regulatory authority, including the FDA, to review pending market approval applications
or supplements to approved applications; warning letters; fines; import and/or export restrictions; product recalls or seizures; injunctions;
total or partial suspension of production; civil penalties; withdrawals of previously approved marketing applications or licenses; recommendations
by the FDA or other regulatory authorities against governmental contracts; and/or criminal prosecutions.
Additionally,
our product candidates could potentially cause other adverse events that have not yet been predicted. The inclusion of ill patients in
our clinical studies may result in deaths or other adverse medical events due to other therapies or medications that such patients may
be using. As described above, any of these events could prevent us from achieving or maintaining market acceptance of our product candidates
and impair our ability to commercialize our products.
We
may not be able to meet requirements for the chemistry, manufacturing, and control of our drug product candidates.
To
receive approval of our products by the FDA and comparable foreign regulatory authorities, we must show that we and our contract manufacturing
partners are able to characterize, control and manufacture our drug products safely and in accordance with regulatory requirements. This
includes synthesizing the active ingredient, developing an acceptable formulation, performing tests to adequately characterize the formulated
product, documenting a repeatable manufacturing process, and demonstrating that our drug products meet stability requirements. Meeting
these chemistry, manufacturing and control (“CMC”) requirements is a complex task that requires specialized expertise. If
we are not able to meet the CMC requirements, we may not be successful in getting our products approved.
Enrollment
and retention of patients in clinical trials is an expensive and time-consuming process and could be made more difficult or rendered
impossible by multiple factors outside our control.
We
may encounter delays or difficulties in enrolling, or be unable to enroll, a sufficient number of patients to complete any of our clinical
trials on its current timelines, or at all, and even once enrolled we may be unable to retain a sufficient number of patients to complete
any of our trials. Enrollment in our clinical trials may be slower than we anticipate, leading to delays in our development timelines.
For example, we may face difficulty enrolling or maintaining a sufficient number of patients in our clinical trials due to the existing
alternative treatments approved for any of our targeted indications as patients may decline to enroll or decide to withdraw from our
clinical trials due to the risk of receiving placebo. Patient enrollment and retention in clinical trials depends on many factors, including
the size of the patient population, the nature of the trial protocol, our ability to recruit clinical trial investigators with the appropriate
competencies and experience, the existing body of safety and efficacy data with respect to the study drug, the number and nature of competing
treatments and ongoing clinical trials of competing drugs for the same indication, the proximity of patients to clinical sites, the eligibility
criteria for the trial and the proportion of patients screened that meets those criteria, our ability to obtain and maintain patient
consents, and our ability to successfully complete prerequisite studies before enrolling certain patient populations.
Furthermore,
any negative results or new safety signals we may report in clinical trials of our product candidates may make it difficult or impossible
to recruit and retain patients in other clinical trials. Similarly, negative results reported by our competitors about their drug candidates
may negatively affect patient recruitment in our clinical trials. Also, marketing authorization of competitors in this same class of
drugs may impair our ability to enroll patients into our clinical trials, delaying or potentially preventing it from completing recruitment
of one or more of our trials.
Delays
or failures in planned patient enrollment or retention may result in increased costs, program delays or both, which could have a harmful
effect on our ability to develop our product candidates or could render further development impossible. In addition, we expect to rely
on CROs and clinical trial sites to ensure proper and timely conduct of our future clinical trials, and, while we intend to enter into
agreements governing their services, we will be limited in our ability to compel their actual performance.
If
our future pre-clinical development or future clinical Phase I/II studies are unsuccessful, we may be unable to obtain regulatory approval
of, or commercialize, our product candidates on a timely basis or at all.
The
successful completion of pre-clinical development and multiple clinical trials is critical to the success of our future products. If
the pre-clinical development and clinical trials are unsuccessful or produce inconsistent results or unanticipated adverse side effects,
or if we are unable to collect reliable data, regulatory approval of our products could be delayed or not given and as a result we may
be unable to commercialize our products. Generally, we expect to engage third parties such as consultants, universities or other collaboration
partners to conduct clinical trials on our behalf. Incompatible practices or misapplication of our products by these third parties could
impair the success of our clinical trials.
Even
if we receive regulatory approval for any of our product candidates, we may not be able to successfully commercialize the product and
the revenue that we generate from their sales, if any, may be limited.
If
approved for marketing, the commercial success of our product candidates will depend upon each product’s acceptance by the medical
community, including physicians, patients, and health care payors. The degree of market acceptance for any of our product candidates
will depend on a number of factors, including:
| |
● |
demonstration
of clinical safety and efficacy; |
| |
|
|
| |
● |
relative
convenience, dosing burden and ease of administration; |
| |
|
|
| |
● |
the
prevalence and severity of any adverse effects; |
| |
|
|
| |
● |
the
willingness of physicians to prescribe our product candidates, and the target patient population to try new therapies; |
| |
|
|
| |
● |
efficacy
of our product candidates compared to competing products; |
| |
|
|
| |
● |
the
introduction of any new products that may in the future become available targeting indications for which our product candidates may
be approved; |
| |
|
|
| |
● |
new
procedures or therapies that may reduce the incidences of any of the indications in which our product candidates may show utility; |
| |
● |
pricing
and cost-effectiveness; |
| |
|
|
| |
● |
the
inclusion or omission of our product candidates in applicable therapeutic and vaccine guidelines; |
| |
|
|
| |
● |
the
effectiveness of our own or any future collaborators’ sales and marketing strategies; |
| |
|
|
| |
● |
limitations
or warnings contained in approved labeling from regulatory authorities; |
| |
|
|
| |
● |
our
ability to obtain and maintain sufficient third-party coverage or reimbursement from government health care programs, including Medicare
and Medicaid, private health insurers and other third-party payors or to receive the necessary pricing approvals from government
bodies regulating the pricing and usage of therapeutics; and |
| |
|
|
| |
● |
the
willingness of patients to pay out-of-pocket in the absence of third-party coverage or reimbursement or government pricing approvals. |
If
any of our product candidates are approved, but do not achieve an adequate level of acceptance by physicians, health care payors, and
patients, we may not generate sufficient revenues and we may not be able to achieve or sustain profitability. Our efforts to educate
the medical community and third-party payors on the benefits of our product candidates may require significant resources and may never
be successful.
In
addition, even if we obtain regulatory approvals, the timing or scope of any approvals may prohibit or reduce our ability to commercialize
our product candidates successfully. For example, if the approval process takes too long, we may miss market opportunities and give other
companies the ability to develop competing products or establish market dominance. Any regulatory approval we ultimately obtain may be
limited or subject to restrictions or post-approval commitments that render our product candidates not commercially viable. For example,
regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, may grant approval
contingent on the performance of costly post-marketing clinical trials, or may approve any of our product candidates with a label that
does not include the labeling claims necessary or desirable for the successful commercialization for that indication. Further, the FDA
or comparable foreign regulatory authorities may place conditions on approvals or require risk management plans or a Risk Evaluation
and Mitigation Strategy (“REMS”) to assure the safe use of the drug. If the FDA or applicable foreign regulatory agency concludes
a REMS is needed, the sponsor of the BLA must submit a proposed REMS; the regulatory agencies will not approve the BLA without an approved
REMS, if required. A REMS could include medication guides, physician communication plans, or elements to assure safe use, such as restricted
distribution methods, patient registries and other risk minimization tools. The regulatory agencies may also require a REMS for approved
product when new safety information emerges. Any of these limitations on approval or marketing could restrict the commercial promotion,
distribution, prescription or dispensing of our product candidates. Moreover, product approvals may be withdrawn for non-compliance with
regulatory standards or if problems occur following the initial marketing of the product. Any of the foregoing scenarios could materially
harm the commercial success of our product candidates.
Adverse
events involving our products may lead the FDA or applicable foreign regulatory agency to delay or deny clearance for our products or
result in product recalls that could harm our reputation, business and financial results.
Once
a product receives regulatory clearance or approval, the agency has the authority to require the recall of commercialized products in
the event of adverse side effects, material deficiencies or defects in design or manufacture. The authority to require a recall must
be based on a regulatory finding that there is a reasonable probability that the product would cause serious injury or death. Manufacturers
may, under their own initiative, recall a product if any material deficiency in a product is found. A government-mandated or voluntary
recall by us or one of our distributors could occur because of adverse side effects, impurities or other product contamination, manufacturing
errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial
resources and have an adverse effect on our financial condition and results of operations. The regulatory agencies require that certain
classifications of recalls be reported to them within ten (10) working days after the recall is initiated. Companies are required to
maintain certain records of recalls, even if they are not reportable to the regulatory agency. We may initiate voluntary recalls involving
our products in the future that we determine do not require notification of the regulatory agencies. If the regulatory agency disagrees
with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation
with customers and negatively affect our sales. In addition, the regulatory agency could take enforcement action for failing to report
the recalls when they were conducted.
The
in-licensing of technologies and the successful testing and early development of technologies in the laboratory may not be indicative
of future results and may not result in commercially viable technologies or products. Further, our future products may have to be modified
from their originally conceived versions in order to reach or be successful in the market.
Positive
results from laboratory testing and early developmental successes, may not be predictive of future successful development, commercialization
and sales results and should not be relied upon as evidence that products developed from our technologies will become commercially viable
and successful. Further, the products we plan to develop in the future may have to be significantly modified from their originally conceived
versions in order for us to control costs, compete with similar products, receive market acceptance, meet specific development and commercialization
timeframes, avoid potential infringement of the proprietary rights of others, or otherwise succeed in developing our business and earning
ongoing revenues. This can be a costly and resource draining activity. What appear to be promising technologies when we license them
may not lead to viable technologies or products, or to commercial success.
Complying
with numerous regulations pertaining to our business is an expensive and time-consuming process, and any failure to comply could result
in substantial penalties.
We
are subject to the Clinical Laboratory Improvement Amendment of 1988, or CLIA, which is a federal law regulating clinical laboratories
that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention, or treatment
of disease. Our clinical laboratory is in Richmond, Virginia and must be certified under CLIA for us to perform testing on human specimens.
CLIA is intended to ensure the quality and reliability of clinical laboratories in the United States by mandating specific standards
in the areas of personnel qualifications, administration, and participation in proficiency testing, patient test management, quality
control, quality assurance and inspections. We currently hold a CLIA certificate to perform high-complexity testing. Laboratories performing
high complexity testing are required to meet more stringent requirements than laboratories performing fewer complex tests. CLIA regulations
require clinical laboratories like ours to comply with various operational, personnel, facilities administration, quality, and proficiency
testing requirements intended to ensure that testing services are accurate, reliable, and timely. CLIA certification is a prerequisite
for reimbursement eligibility for services provided to state and federal health care program beneficiaries. CLIA is user-fee funded.
Therefore, all costs of administering the program must be covered by the regulated facilities, including certification and survey costs.
To renew this certificate, we are subject to survey and inspection every two years. Moreover, CLIA inspectors may make periodic inspections
of our clinical laboratory outside of the renewal process. The failure to comply with CLIA requirements can result in enforcement actions,
including the revocation, suspension, or limitation of our CLIA certificate of compliance, as well as a directed plan of correction,
state on-site monitoring, civil money penalties, civil injunctive suit and/or criminal penalties. We must maintain CLIA compliance and
certification to be eligible to bill for assays provided to Medicare beneficiaries. If we were to be found out of compliance with CLIA
program requirements and subjected to sanctions, our business and reputation could be harmed. Even if it were possible for us to bring
our laboratory back into compliance, we could incur significant expenses and potentially lose revenue in doing so.
Additionally,
certain states require laboratory licenses to test specimens from patients in those states or received from ordering physicians in those
states. We may also be subject to regulation in foreign jurisdictions if we seek to expand international distribution of our assays outside
the United States.
If
we were to lose our CLIA certification or state laboratory licenses, whether because of a revocation, suspension or limitation, we would
no longer be able to offer our assays (including our AditxtScore™ platform), which would limit our revenues and harm our business.
If we were to lose, or fail to obtain, a license in any other state where we are required to hold a license, we would not be able to
test specimens from those states.
Our
AditxtScore™ tests are currently being offered as a LDTs. Should the FDA disagree that AditxtScore™ tests are LDTs, if our
LDTs do not receive the required emergency use authorizations, or if the FDA’s regulatory approach to LDTs should change in the
future, our commercialization strategy may be adversely affected, which would negatively affect our results of operations and financial
condition.
The
FDA has historically asserted its authority to regulate Laboratory Developed Tests (LDTs) as medical devices under the Federal Food,
Drug, and Cosmetic Act (the “FDCA”), but it has generally exercised enforcement discretion regarding LDTs.
This means that even though the FDA believes it can impose regulatory requirements on LDTs, such as requirements to obtain premarket
approval, de novo classification, or clearance of LDTs, it has generally chosen not to enforce those requirements. The
FDA has, on occasion, sent warning letters to laboratories offering LDTs that the agency believed were not eligible for enforcement discretion
because of how they were developed, validated, performed or marketed and consequent risks to the public.
The
FDA considers an LDT to be a test that is developed, validated, and performed within a single laboratory. We are providing AditxtScore™
as a service as a Laboratory Developed Test (LDT) to assess immunity status to COVID-19. Our AditxtScore™ tests are currently
manufactured in our Mountain View, CA facility and performed in our Richmond, VA facility. If the FDA believes that the AditxtScore™
is not regulated as an LDT, we may be forced to stop performing AditxtScore™ while we worked to obtain the appropriate FDA
authorizations which could negative affect our business, results of operations and financial condition.
On
November 15, 2021, FDA revised its guidance document titled “Policy for Coronavirus Disease-2019 Tests During the Public Health
Emergency (Revised)” (“FDA COVID-19 Testing Guidance”) to require all COVID-19 diagnostic assays conducted as LDTs
to apply for EUA authorization within a 60-day period from the revised guidance’s issuance date. The FDA COVID-19 Testing
Guidance states that FDA does not intend to object to continued offering of LDTs that are the subject of submitted EUA requests while
FDA reviews the EUA requests. The FDA COVID-19 Testing Guidance further states that if FDA declines to issue an EUA or otherwise decides
not to authorize a test for any reason, including a determination that there is a lack of adequate data to support authorization, FDA
generally expects developers to cease marketing and offering their test within 15 calendar days. Moreover, if FDA identifies a significant
problem or concern with a test, based either on the provided information or external reports, FDA generally expects the developer to
take appropriate steps to address such problems, which could include a recall of the test and/or notification concerning corrected test
reports indicating prior test results may not be accurate. We have submitted EUA requests for our SARS-CoV-2 LDTs, and the
applications are pending before FDA. There can be no assurance that the EUA requests that we submitted for our SARS-CoV-2 LDTs
will be granted on a timely basis or at all. If FDA declines to issue a EUAs for our SARS-CoV-2 LDTs, we may be required to cease
marketing the tests and our business, results of operations and financial condition could be negatively affected. Regardless of if our
EUA applications are granted by FDA, we may recall, replace, or make corrections to our LDTs if we become aware of a product concern,
which could negatively impact manufacturing, supply, and customer relationships, and may result in adverse regulatory action, including
revision or revocation of an EUA.
In
addition, there have been numerous legislative proposals to clarify the FDA’s regulatory authority over medical devices. These
include two bills reintroduced in 2021: the VALID Act, which would expressly grant the FDA authority to regulate LDTs under a risk-based
framework; and the VITAL Act, which would assign LDTs to regulation solely under CLIA and would direct CMS to update its CLIA regulations.
We cannot predict if either of these bills will be enacted in their current (or any other) form and cannot quantify the effect of these
bills on our business. In the meantime, the regulation by the FDA of LDTs remains uncertain. If FDA premarket review, classification
or approval is required for AditxtScore™, our laboratory could be forced to stop performing AditxtScore™ while we worked
to obtain the appropriate FDA authorizations which could negative affect our business, results of operations and financial condition.
We
are subject to various governmental regulations relating to the labeling, marketing and sale of our products.
Both
before and after a product is commercially released, we have ongoing responsibilities under regulations promulgated by the FDA, the Federal
Trade Commission, and similar U.S. and foreign regulations governing product labeling and advertising, distribution, sale, and marketing
of our products.
Manufacturers
of medical devices are permitted to promote products solely for the uses and indications set forth in the device’s authorization.
A number of enforcement actions have been taken against manufacturers that promote products for “off-label” uses
(i.e., uses that are not described in the device’s authorization), including actions alleging that claims submitted to government
healthcare programs for reimbursement of products that were promoted for “off-label” uses are fraudulent in violation
of the Federal False Claims Act or other federal and state statutes and that the submission of those claims was caused by off-label promotion.
The failure to comply with prohibitions on “off-label” promotion can result in significant monetary penalties,
revocation or suspension of a company’s business license, suspension of sales of certain products, product recalls, civil or criminal
sanctions, exclusion from participating in federal healthcare programs, or other enforcement actions. In the United States, allegations
of such wrongful conduct could also result in a corporate integrity agreement with the U.S. government that imposes significant administrative
obligations and costs.
We
and our employees and contractors are subject, directly, or indirectly, to federal, state and foreign healthcare fraud and abuse laws,
including false claims laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Our
operations are subject to various federal, state, and foreign fraud and abuse laws. These laws may constrain our operations, including
the financial arrangements and relationships through which we market, sell and distribute our products.
U.S.
federal and state laws that affect our ability to operate include, but are not limited to:
| |
●
|
the
federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting,
receiving, offering or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly,
in cash or in kind in return for, the purchase, recommendation, leasing or furnishing of an item or service reimbursable under a
federal healthcare program, such as the Medicare and Medicaid programs; |
| |
●
|
federal
physician self-referral law, which prohibits a physician from referring a patient to an entity with which the physician (or an immediate
family member) has a financial relationship, for the furnishing of certain designated health services for which payment may be made
by Medicare or Medicaid, unless an exception applies; |
| |
●
|
federal
civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals, or entities
from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid, or other government
payers that are false or fraudulent; |
| |
●
|
Section
242 of HIPAA codified at 18 U.S.C. § 1347, which created new federal criminal statutes that prohibit a person from knowingly
and willfully executing a scheme or from making false or fraudulent statements to defraud any healthcare benefit program (i.e., public
or private); |
| |
●
|
federal
transparency laws, including the Physician Payments Sunshine Act which requires the tracking and disclosure to the federal government
by pharmaceutical and medical device manufacturers of payments and other transfers of value to physicians and teaching hospitals
as well as ownership and investment interests that are held by physicians and their immediate family members; and |
| |
●
|
state
law equivalents of each of these federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed
by any third-party payer, including commercial insurers; state laws that require pharmaceutical and medical device companies to comply
with their industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government
or otherwise restrict certain payments that may be made to healthcare providers and other potential referral sources; state laws
that require drug and medical device manufacturers to report information related to payments and other transfers of value to physicians
and other healthcare providers or marketing expenditures; state laws that prohibit giving gifts to licensed healthcare professionals;
and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each
other in significant ways and may not have the same effect, thus complicating compliance efforts in certain circumstances, such as
specific disease states. |
In
particular, activities and arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent
fraud, waste and other abusive practices. These laws and regulations may restrict or prohibit a wide range of activities or other arrangements
related to the development, marketing or promotion of products, including pricing and discounting of products, provision of customer
incentives, provision of reimbursement support, other customer support services, provision of sales commissions or other incentives to
employees and independent contractors and other interactions with healthcare practitioners, other healthcare providers and patients.
Because
of the breadth of these laws and the narrow scope of the statutory or regulatory exceptions and safe harbors available, our business
activities could be challenged under one or more of these laws.
Government
expectations and industry best practices for compliance continue to evolve and past activities may not always be consistent with current
industry best practices. Further, there is a lack of government guidance as to whether various industry practices comply with these laws,
and government interpretations of these laws continue to evolve, all of which create compliance uncertainties. Any non-compliance could
result in regulatory sanctions, criminal or civil liability and serious harm to our reputation. It is not always possible to identify
and deter misconduct concerning applicable laws, regulations, guidelines, policies and standards, and the precautions we take to detect
and prevent this activity may not be effective in preventing such conduct, mitigating risks, or reducing the chance of governmental investigations
or other actions or lawsuits stemming from a failure to comply with these laws or regulations.
If
a government entity opens an investigation into possible violations of any of these laws (which may include the issuance of subpoenas
or civil investigative demands), we would have to expend significant resources to defend ourselves against the allegations. Allegations
that we, our officers, or our employees violated any one of these laws can be made by individuals called “whistleblowers”
who may be our employees, customers, competitors or other parties. Government policy is to encourage individuals to become whistleblowers
and file a complaint in federal court alleging wrongful conduct. The government is required to investigate all of these complaints and
decide whether to intervene. If the government intervenes and we are required to pay money back to the government, the whistleblower,
as a reward, is awarded a percentage of the collection. If the government declines to intervene, the whistleblower may proceed on their
own and, if they are successful, they will receive a percentage of any judgment or settlement amount the company is required to pay.
The government may also initiate an investigation on its own. Such actions could have a significant impact on our business, including
the imposition of significant fines, and other sanctions that may materially impair our ability to run a profitable business. In particular,
if our operations are found to be in violation of any of the laws described above or if we agree to settle with the government without
admitting to any wrongful conduct or if we are found to be in violation of any other governmental regulations that apply to us, we, our
officers and employees may be subject to sanctions, including civil and criminal penalties, damages, fines, exclusion from participation
in government health care programs, such as Medicare and Medicaid, imprisonment, the curtailment or restructuring of our operations and
the imposition of a corporate integrity agreement, any of which could adversely affect our business, results of operations and financial
condition.
Risks
Related to our Company and our Business
Our
technology is subject to licenses from LLU and Stanford, each of which are revocable in certain circumstances, including in the event
we do not achieve certain payments and milestone deadlines. Without these licenses, we may not be able to continue to develop our product
candidates.
The
LLU License Agreement may be terminated by LLU in the event of a breach by us of any non-payment provision (including the provision that
requires us to meet certain deadlines for milestone events (each, a “Milestone Deadline”)) not cured within 90 days after
delivery of written notice by LLU. Additional Milestone Deadlines include: (i) the requirement to have regulatory approval of an IND
application to initiate first-in-human clinical trials on or before March 31, 2022, (ii) the completion of first-in-human (phase I/II)
clinical trials by March 31, 2024, (iii) the completion of Phase III clinical trials by March 31, 2026 and (iv) biologic licensing approval
(BLA) by the FDA by March 31, 2027. If the LLU License Agreement were to be terminated by LLU, we would lose our most significant asset
and may no longer be able to develop our product candidates, which would have a material adverse effect on our operations.
The
February 2020 License Agreement with Stanford may be terminated by Stanford if we (i) are delinquent on any report or payments; (ii)
are not diligently developing and commercializing Licensed Product (as defined in the February 2020 License Agreement); (iii) miss a
milestone described in the agreement; (iv) are in breach of any other provision of the agreement; or (v) if we provide a false report
to Stanford. The Termination discussed above will take effect only upon 30 days written notice by Stanford unless we remedy the breach
within a 30-day cure period. If the February 2020 License Agreement were to be terminated by Stanford, we would lose a significant asset
and may no longer be able to develop our product candidates, which would have a material adverse effect on our operations.
Our
results of operations will be affected by the level of royalty and milestone payments that we are required to pay to third parties.
The
LLU License Agreement and February 2020 License Agreement with Stanford each require us to remit royalty payments and meet certain performance
milestones related to in-licensed intellectual property. Any failure on our part to pay royalties owed or meet milestones could lead
to us losing rights under our licenses and could thereby adversely affect our business. As our product sales increase, we may, from time-to-time,
disagree with our third-party collaborators as to the appropriate royalties owed and the resolution of such disputes may be costly and
may consume management’s time. Furthermore, we may enter into additional license agreements in the future, which may also include
royalty payments.
We
face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully
than we do.
The
development and commercialization of drugs is highly competitive. We compete with a variety of multinational pharmaceutical companies
and specialized biotechnology companies, as well as products and processes being developed at universities and other research institutions.
Our competitors have developed, are developing or will develop product candidates and processes competitive with our product candidates.
Competitive therapeutic treatments include those that have already been approved and accepted by the medical community and any new treatments
that may enter the market. We believe that a significant number of products are currently available, under development, and may become
commercially available in the future, for the treatment of indications for which we may try to develop product candidates.
More
established companies may have a competitive advantage over us due to their greater size, cash flows and institutional experience. Compared
to us, many of our competitors may have significantly greater financial, technical and human resources. As a result of these factors,
our competitors may have an advantage in marketing their approved products and may obtain regulatory approval of their product candidates
before we are able to, which may limit our ability to develop or commercialize our product candidates. Our competitors may also develop
drugs that are safer, more effective, more widely used and less expensive than ours, and may also be more successful than us in manufacturing
and marketing their products.
Mergers
and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller
number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through
collaborative arrangements with large and established companies. These companies compete with us in recruiting and retaining qualified
scientific, management and commercial personnel, establishing clinical trial sites and subject registration for clinical trials, as well
as in acquiring technologies complementary to, or necessary for, our programs.
Our
technologies and products under development, and our business, may fail if we are not able to successfully commercialize them and ultimately
generate significant revenues as a result.
Successful
development of technologies and our product candidates will require significant additional investment, including costs associated with
additional development, completing trials and obtaining regulatory approval, as well as the ability to manufacture or have others manufacture
our products in sufficient quantities at acceptable costs while also preserving product quality. Difficulties often encountered in scaling
up production include problems involving production yields, quality control and assurance, shortage of qualified personnel, production
costs and process controls. In addition, we are subject to inherent risks associated with new technologies and products. These risks
include the possibility that any of our technologies or future products may:
| |
● |
be
ineffective or less effective than anticipated; |
| |
● |
fail
to receive necessary regulatory approvals; |
| |
● |
be
difficult to competitively price relative to alternative solutions; |
| |
● |
be
harmful to consumers or the environment; |
| |
● |
be
difficult to manufacture on an economically viable scale; |
| |
● |
be
subject to supply chain constraints for raw materials; |
| |
● |
fail
to be developed and accepted by the market prior to the successful marketing of alternative products by competitors; |
| |
● |
be
difficult to market because of infringement on the proprietary rights of third parties; or |
| |
● |
be
too expensive for commercial use. |
Furthermore,
we may be faced with lengthy market partner or distributor evaluation and approval processes. Consequently, we may incur substantial
expenses and devote significant management effort to customize products for market partner or distributor acceptance, though there can
be no assurance of such acceptance. As a result, we cannot accurately predict the volume or timing of any future sales.
Customers
may not adopt our products quickly, or at all.
Customers
in the sector in which we operate can be generally cautious in their adoption of new products and technologies. In addition, given the
relative novelty of our future planned products (including our AditxtScore™ platform), customers of those products may require
education regarding their utility and use, which may delay their adoption. There can be no assurance that customers will adopt our products
quickly, or at all.
The
significant level of competition in the markets for our products developed in the future may result in pricing pressure, reduced margins
or the inability of our future products to achieve market acceptance.
The
markets for our future products are intensely competitive and rapidly changing. We may be unable to compete successfully, which may result
in price reductions, reduced margins and the inability to achieve market acceptance for our products.
Our
competitors may have longer operating histories, significantly greater resources, greater brand recognition and large customer bases
than we do. As a result, they may be able to devote greater resources to the manufacture, promotion or sale of their products, receive
greater resources and support from market partners and independent distributors, initiate or withstand substantial price competition
or more readily take advantage of acquisition or other opportunities.
We
rely on third parties for the distribution of our current and future products, including our AditxtScore™ platform. If these
parties do not distribute our products in a satisfactory or timely manner, in sufficient quantities or at an acceptable cost, our sales
and development efforts could be delayed or otherwise negatively affected.
We
rely on third parties for the distribution of our current and future products, including our AditxtScore™ platform. Our reliance
on third parties to distribute products may present significant risks to us, including the risk that should any of these third parties
fail to adequately distribute our products and services to end consumers and other market participants, our business may be materially
harmed. Additionally, if we need to enter into agreements for the distribution of our future products with other third parties, there
can be no assurance we will be able to do so on favorable terms, if at all.
We
may rely on third parties to produce our future products. If these parties do not produce our products at a satisfactory quality,
in a timely manner, in sufficient quantities or at an acceptable cost, our sales and development efforts could be delayed or otherwise
negatively affected.
We
may rely on third parties for the manufacture of our future products. Our reliance on third parties to manufacture our future products
may present significant risks to us, including the following:
| |
● |
reduced
control over delivery schedules, yields and product reliability; |
| |
● |
manufacturing
deviations from internal and regulatory specifications; |
| |
● |
the
failure of a key manufacturer to perform as we require for technical, market or other reasons; |
| |
● |
difficulties
in establishing additional manufacturer relationships if we are presented with the need to transfer our manufacturing process technologies
to them; |
| |
● |
misappropriation
of our intellectual property; and |
| |
● |
other
risks in potentially meeting our product development schedule or satisfying the requirements of our market partners, distributors,
direct customers and end users. |
If
we need to enter into agreements for the manufacturing of our future products, there can be no assurance we will be able to do so on
favorable terms, if at all.
If
we are unable to establish successful relations with third-party market partners or distributors, or these market partners or distributors
do not focus adequate resources on selling our products or are otherwise unsuccessful in selling them, sales of our products may not
develop.
We
anticipate relying on independent market partners and distributors to distribute and assist us with the marketing and sale of our products.
Our future revenue generation and growth will depend in large part on our success in establishing and maintaining this sales and distribution
channel. If our market partners and distributors are unable to sell our products, or receive negative feedback from end users, they may
not continue to purchase or market our products. In addition, there can be no assurance that our market partners and distributors will
focus adequate resources on selling our products to end users or will be successful in selling them. Many of our potential market partners
and distributors are in the business of distributing and sometimes manufacturing other, possibly competing, products. As a result,
these market partners and distributors may perceive our products as a threat to various product lines currently being distributed or
manufactured by them. In addition, these market partners and distributors may earn higher margins by selling competing products or combinations
of competing products. If we are unable to establish successful relationships with independent market partners and distributors, we will
need to further develop our own sales and distribution capabilities, which would be expensive and time-consuming and might not be successful.
If
we are not able to attract and retain highly skilled employees and contractors, we may not be able to implement our business model successfully.
We
will rely upon employees and third-party consultant/contractors to effectively establish, manage and grow our business. Consequently,
we believe that our future viability will depend largely on our ability to attract and retain highly skilled personnel. In order
to do so, we may need to pay higher compensation, fees, and/or other incentives to our employees or consultants than we currently expect,
and such higher compensation payments would have a negative effect on our operating results. Competition for experienced, high-quality
employees, consultants and contractors is intense and we cannot assure that we will be able to recruit and retain such personnel. We
may not be able to hire or retain the necessary personnel to implement our business strategy. Our failure to hire and retain such personnel
could impair our ability to develop new products and manage our business effectively.
The
loss of our management team or other key personnel would have an adverse impact on our future development and impair our ability to succeed.
In
the early stages of development, our business will be significantly dependent on the Company’s management team and other key personnel.
Our success will be particularly dependent upon Mr. Amro Albanna and Dr. Shahrokh Shabahang. The loss of any one of these individuals
or any other future key personnel could have a material adverse effect on the Company and our ability to further execute our intended
business.
The
use of our products may be limited by regulations, and we may be exposed to product liability and remediation claims.
The
use of our planned products may be regulated by various local, state, federal and foreign regulators. Even if we are able to comply
with all such regulations and obtain all necessary registrations, we cannot provide assurance that our future products will not cause
injury to the environment, people, or animals and/or otherwise have unintended adverse consequences, under all circumstances. For example,
our products may be improperly combined with other chemicals or, even when properly combined, our products may be blamed for damage caused
by those other chemicals. The costs of remediation or products liability could materially adversely affect our results, financial condition
and operations.
We
may be held liable for, or incur costs to settle, liability and remediation claims if any products we develop, or any products that use
or incorporate any of our technologies, cause injury or are found unsuitable during product testing, manufacturing, marketing, sale or
use. These risks exist even with respect to products that have received, or may in the future receive, regulatory approval, registration
or clearance for commercial use. We cannot guarantee that we will be able to avoid product liability exposure.
At
the stage customary to do so, we expect to maintain product liability insurance at levels we believe are sufficient and consistent with
industry standards for like companies and products. However, we cannot guarantee that our product liability insurance will be sufficient
to help us avoid product liability-related losses. In the future, it is possible that meaningful insurance coverage may not be available
on commercially reasonable terms or at all. In addition, a product liability claim could result in liability to us greater than our assets
or insurance coverage. Moreover, even if we have adequate insurance coverage, product liability claims or recalls could result in negative
publicity or force us to devote significant time and attention to these matters, which could harm our business.
There
may be limitations on the effectiveness of our internal controls, and a failure of our control systems to prevent error or fraud may
materially harm our Company.
We
do not expect that internal control over financial accounting and disclosure, even if timely and well established, will prevent all error
and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that
the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource
constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control
systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected.
Failure of our control systems to prevent error or fraud could materially adversely affect our business.
COVID-19
may impact our operations.
On
January 30, 2020, the World Health Organization declared the COVID-19 coronavirus outbreak a “Public Health Emergency of International
Concern” and on March 10, 2020, declared it to be a pandemic. Actions taken around the world to help mitigate the spread of the
coronavirus include restrictions on travel, and quarantines in certain areas, and forced closures for certain types of public places
and businesses. The COVID-19 coronavirus and actions taken to mitigate it have had and are expected to continue to have an adverse impact
on the economies and financial markets of many countries, including the geographical area in which the Company operates. While it is
unknown how long these conditions will last and what the complete financial effect will be to the Company, capital raise efforts and
additional development of our technologies may be negatively affected.
Risks
Related to Our Acquisition Strategy
We
may engage in future acquisitions or strategic transactions, including the transaction with Cellvera Global, which may require us to
seek additional financing or financial commitments, increase our expenses and/or present significant distractions to our management.
As
described herein, on April 19, 2023 entered into an Asset Purchase Agreement with Cellvera Global, pursuant to which we agreed to acquire
from Cellvera Ltd a 50% membership interest in GRA, together with certain other intellectual property assets and all goodwill related
thereto. We will need to acquire additional financing to fund our obligations under the Asset Purchase Agreement or to fund other potential
acquisitions or strategic transactions (particularly if the acquired entity is not cash flow positive or does not have significant cash
on hand). Obtaining financing through the issuance or sale of additional equity and/or debt securities, if possible, may not be at favorable
terms and may result in additional dilution to our current stockholders. Additionally, any such transaction may require us to incur non-recurring
or other charges, may increase our near and long-term expenditures and may pose significant integration challenges or disrupt our management
or business, which could adversely affect our operations and financial results. For example, an acquisition or strategic transaction
may entail numerous operational and financial risks, including the risks outlined above and additionally:
| |
● |
exposure
to unknown liabilities; |
| |
● |
disruption
of our business and diversion of our management’s time and attention in order to develop acquired products or technologies; |
| |
● |
higher
than expected acquisition and integration costs; |
| |
● |
write-downs
of assets or goodwill or impairment charges; |
| |
● |
increased
amortization expenses; |
| |
● |
difficulty
and cost in combining the operations and personnel of any acquired businesses with our operations and personnel; |
| |
● |
impairment
of relationships with key suppliers or customers of any acquired businesses due to changes in management and ownership; and |
| |
● |
inability
to retain key employees of any acquired businesses. |
Accordingly,
although there can be no assurance that we will undertake or successfully complete any transactions of the nature described above, and
any transactions that we do complete could have a material adverse effect on our business, results of operations, financial condition
and prospects.
While
we have entered into an Asset Purchase Agreement with Cellvera Global, we cannot assure you that the transaction contemplated by the
Asset Purchase Agreement will be consummated or, that if such transactions are consummated, they will be accretive to stockholder value.
As
described herein, we entered into an Asset Purchase Agreement with Cellvera Global, pursuant to which we agreed to acquire from Cellvera
Ltd a 50% membership interest in GRA, together with certain other intellectual property assets and all goodwill related thereto. We will
be required to obtain additional financing through the sale of additional equity and/or debt securities to fund such obligations under
the Asset Purchase Agreement. We can provide no assurance that we will be successful in securing such financing. In addition, such financing,
if available, may not be on favorable terms and may result in additional dilution to our current stockholders.
The
closing of the transaction contemplated by the Asset Purchase Agreement are subject to the satisfaction or waiver of a number of closing
conditions. There is no guarantee that the conditions to closing will be satisfied. Further, even if all conditions to closing are satisfied,
there is no guarantee that the transaction will be completed in the time frame or in the manner currently anticipated, or that we will
recognize the anticipated benefits of the transaction.
Our
acquisition strategy exposes us to substantial risk.
Our
acquisition of companies is subject to substantial risk, including but not limited to the failure to identify material problems during
due diligence (for which we may not be indemnified post-closing), the risk of over-paying for assets (or not making acquisitions on an
accretive basis), the ability to obtain or retain customers and the risks of entering markets where we have limited experience. While
we perform due diligence on prospective acquisitions, we may not be able to discover all potential operational deficiencies in such entities.
Our
acquisition targets may not perform as expected or the returns from such businesses may not support the financing utilized to acquire
them or maintain them. Furthermore, integration and consolidation of acquired businesses requires substantial human, financial and other
resources and may divert management’s attention from our existing business concerns, disrupt our ongoing business or not be successfully
integrated. Even if we consummate businesses that we believe will be accretive, those businesses may in fact result in a decrease in
revenues as a result of incorrect assumptions in our evaluation of such businesses, unforeseen consequences, or other external events
beyond our control. Furthermore, if we consummate any future acquisitions, our capitalization and results of operations may change significantly,
and stockholders will generally not have the opportunity to evaluate the economic, financial, and other relevant information that we
will consider in determining the application of these funds and other resources. As a result, the consummation of acquisitions may have
a material adverse effect on our business, financial condition, results of operations and cash flows.
We
may experience difficulty as we evaluate, acquire and integrate businesses that we may acquire, which could result in drains on our resources,
including the attention of our management, and disruptions of our on-going business.
We
acquire small to mid-sized businesses in various industry segments. Generally, because such businesses are privately held, we may experience
difficulty in evaluating potential target businesses as much of the information concerning these businesses is not publicly available.
Therefore, our estimates and assumptions used to evaluate the operations, management and market risks with respect to potential target
businesses may be subject to various risks and uncertainties. Further, the time and costs associated with identifying and evaluating
potential target businesses may cause a substantial drain on our resources and may divert our management team’s attention away
from the operations of our businesses for significant periods of time.
In
addition, we may have difficulty effectively integrating and managing acquisitions. The management or improvement of businesses we acquire
may be hindered by a number of factors, including limitations in the standards, controls, procedures and policies implemented in connection
with such acquisitions. Further, the management of an acquired business may involve a substantial reorganization of the business’
operations resulting in the loss of employees and customers or the disruption of our ongoing businesses. We may experience greater than
expected costs or difficulties relating to an acquisition, in which case, we might not achieve the anticipated returns from any particular
acquisition.
We
may not be able to effectively integrate the businesses that we acquire.
Our
ability to realize the anticipated benefits of acquisitions will depend on our ability to integrate those businesses with our own. The
combination of multiple independent businesses is a complex, costly and time-consuming process and there can be no assurance that we
will be able to successfully integrate businesses into our business, or if such integration is successfully accomplished, that such integration
will not be costlier or take longer than presently contemplated. Integration of future acquisitions may include various risks and uncertainties,
including the factors discussed in the paragraph below. If we cannot successfully integrate and manage the businesses within a reasonable
time, we may not be able to realize the potential and anticipated benefits of such acquisitions, which could have a material adverse
effect on our stock price, business, cash flows, results of operations and financial position.
We
will consider acquisitions that we believe will complement, strengthen and enhance our growth. We evaluate opportunities on a preliminary
basis from time to time, but these transactions may not advance beyond the preliminary stages or be completed. Such acquisitions are
subject to various risks and uncertainties, including:
| |
● |
the
inability to integrate effectively the operations, products, technologies and personnel of the acquired companies (some of which
are in diverse geographic regions) and achieve expected synergies; |
| |
● |
the
potential disruption of existing business and diversion of management’s attention from day-to-day operations; |
| |
● |
the
inability to maintain uniform standards, controls, procedures and policies; |
| |
● |
the
need or obligation to divest portions of the acquired companies; |
| |
● |
the
potential failure to identify material problems and liabilities during due diligence review of acquisition targets; |
| |
● |
the
potential failure to obtain sufficient indemnification rights to fully offset possible liabilities associated with acquired businesses;
and |
| |
● |
the
challenges associated with operating in new geographic regions. |
The
integration of our acquisitions may result in significant accounting charges that adversely affect the announced results of our Company.
The
financial results of our Company may be adversely affected by cash expenses and non-cash accounting charges incurred in connection with
our recent acquisitions. In addition to the anticipated cash charges, costs associated with the amortization of intangible assets are
expected. The price of our common stock could decline to the extent our financial results are materially affected by the foregoing charges
or if the foregoing charges are larger than anticipated.
Our
planned acquisitions may result in unexpected consequences to our business and results of operations.
Although
we believe that our planned acquisitions will generally be subject to risks similar to those to which we are subject to in our existing
operations, we may not have discovered all risks applicable to these businesses during the due diligence process. Some of these risks
could produce unexpected and unwanted consequences for us. Undiscovered risks may result in us incurring financial liabilities, which
could be material and have a negative impact on our business operations.
Failure
to manage our growing and changing business could have a material adverse effect on our business, prospects, financial condition, and
results of operations.
As
we grow, we expect to encounter additional challenges to our internal processes, capital commitment process, and acquisition funding
and financing capabilities. Our existing operations, personnel, systems, and internal control may not be adequate to support our growth
and expansion and may require us to make additional unanticipated investments in our infrastructure. To manage the future growth of our
operations, we will be required to improve our administrative, operational, and financial systems, procedures, and controls, and maintain,
expand, train, and manage our growing employee base. If we are unable to manage our growth effectively, we may not be able to take advantage
of market opportunities, execute our business strategies successfully or respond to competitive pressures. As a result, our business,
prospects, financial condition, and results of operations could be materially and adversely affected.
We
face competition for businesses that fit our acquisition strategy and, therefore, we may have to acquire targets at sub-optimal prices
or, alternatively, forego certain acquisition opportunities.
Our
acquisition strategy is focused on the acquisition of small to mid-sized businesses. In pursuing such acquisitions, we expect to face
strong competition from a wide range of other potential purchasers. Although the pool of potential purchasers for such businesses is
typically smaller than for larger businesses, those potential purchasers can be aggressive in their approach to acquiring such businesses.
Furthermore, we expect that we will need to use third-party financing in order to fund some or all of these potential acquisitions, thereby
increasing our acquisition costs. To the extent that other potential purchasers do not need to obtain third-party financing or are able
to obtain such financing on more favorable terms, they may be in a position to be more aggressive with their acquisition proposals. As
a result, in order to be competitive, our acquisition proposals may need to be aggressively priced, including at price levels that exceed
what we originally determined to be fair or appropriate. Alternatively, we may determine that we cannot pursue on a cost-effective basis
what would otherwise be an attractive acquisition opportunity.
We
may not be able to successfully fund acquisitions due to the unavailability of equity or debt financing on acceptable terms, which could
impede the implementation of our acquisition strategy.
We
intend to finance acquisitions primarily through additional debt and equity financings. Because the timing and size of acquisitions cannot
be readily predicted, we may need to be able to obtain funding on short notice to benefit fully from attractive acquisition opportunities.
The sale of additional shares of any class of equity will be subject to market conditions and investor demand for such shares at prices
that may not be in the best interest of our stockholders. The sale of additional equity securities could also result in dilution to our
stockholders. The incurrence of indebtedness would result in increased debt service obligations and could require us to agree to operating
and financial covenants that would restrict our operations. Financing may not be available in amounts or on terms acceptable to us, if
at all. These risks may materially adversely affect our ability to pursue our acquisition strategy.
We
may change our management and acquisition strategies without the consent of our stockholders, which may result in a determination by
us to pursue riskier business activities.
We
may change our strategy at any time without the consent of our stockholders, which may result in our acquiring businesses or assets that
are different from, and possibly riskier than, the strategy described in this prospectus. A change in our strategy may increase our exposure
to interest rate and currency fluctuations, subject us to regulation under the Investment Company Act or subject us to other
risks and uncertainties that affect our operations and profitability.
In
the future, we may seek to enter into credit facilities to help fund our acquisition capital and working capital needs. These credit
facilities may expose us to additional risks associated with leverage and may inhibit our operating flexibility.
We
may seek to enter into credit facilities with third-party lenders to help fund our acquisitions. Such credit facilities will likely require
us to pay a commitment fee on the undrawn amount and will likely contain a number of affirmative and restrictive covenants. If we violate
any such covenants, our lenders could accelerate the maturity of any debt outstanding. Such debt may be secured by our assets, including
the stock we may own in businesses that we acquire. Our ability to meet our debt service obligations may be affected by events beyond
our control and will depend primarily upon cash produced by businesses that we currently manage and may acquire in the future and distributed
or paid to us. Any failure to comply with the terms of our indebtedness may have a material adverse effect on our financial condition.
In
addition, we expect that such credit facilities will bear interest at floating rates which will generally change as interest rates change.
We will bear the risk that the rates that we are charged by our lenders will increase faster than we can grow the cash flow from our
businesses or businesses that we may acquire in the future, which could reduce profitability, materially adversely affect our ability
to service our debt, cause us to breach covenants contained in our third-party credit facilities and reduce cash flow available for distribution.
If,
in the future, we cease to control and operate our businesses or other businesses that we acquire in the future or engage in certain
other activities, we may be deemed to be an investment company under the Investment Company Act.
We
have the ability to make investments in businesses that we will not operate or control. If we make significant investments in businesses
that we do not operate or control, or that we cease to operate or control, or if we commence certain investment-related activities, we
may be deemed to be an investment company under the Investment Company Act. Our decision to sell a business will be based upon financial,
operating and other considerations rather than a plan to complete a sale of a business within any specific time frame. If we were deemed
to be an investment company, we would either have to register as an investment company under the Investment Company Act, obtain
exemptive relief from the Securities and Exchange Commission, or the SEC, or modify our investments or organizational structure or our
contract rights to fall outside the definition of an investment company. Registering as an investment company could, among other things,
materially adversely affect our financial condition, business and results of operations, materially limit our ability to borrow funds
or engage in other transactions involving leverage and require us to add directors who are independent of us and otherwise will subject
us to additional regulation that will be costly and time-consuming.
If
intangible assets and goodwill that we recorded in connection with our acquisitions become impaired, we may have to take significant
charges against earnings.
In
connection with the accounting for our completed acquisitions, we may be required to record a significant amount of intangible assets,
including developed technology, in-process research and development, and customer relationships relating to the acquired product lines,
and goodwill. Under generally accepted accounting principles in the United States, we must assess, at least annually and potentially
more frequently, whether the value of indefinite-lived intangible assets and goodwill have been impaired. Intangible assets and goodwill
are assessed for impairment in the event of an impairment indicator. Any reduction or impairment of the value of intangible assets and
goodwill will result in a charge against earnings, which could materially adversely affect our results of operations and shareholders’
equity in future periods.
Risks
Relating to Our Intellectual Property Rights
The
failure to obtain or maintain patents, licensing agreements and other intellectual property could materially impact our ability to compete
effectively.
In
order for our business to be viable and to compete effectively, we need to develop and maintain, and we will heavily rely on, a proprietary
position with respect to our technologies and intellectual property. However, there are significant risks associated with our actual
or proposed intellectual property. The risks and uncertainties that we face with respect to our rights principally include the following:
| |
● |
pending
patent applications, we have filed or will file may not result in issued patents or may take longer than we expect to result in issued
patents; |
| |
● |
we
may be subject to interference proceedings; |
| |
● |
we
may be subject to reexamination proceedings; |
| |
● |
we
may be subject to post grant review proceedings; |
| |
● |
we
may be subject to inter partes review proceedings; |
| |
● |
we
may be subject to derivation proceedings; |
| |
● |
we
may be subject to opposition proceedings in the U.S. or in foreign countries; |
| |
● |
any
patents that are issued to us may not provide meaningful protection; |
| |
● |
we
may not be able to develop additional proprietary technologies that are patentable; |
| |
● |
other
companies may challenge patents licensed or issued to us; |
| |
● |
other
companies may have independently developed and patented (or may in the future independently develop and patent) similar or alternative
technologies, or duplicate our technologies; |
| |
● |
other
companies may design around technologies we have licensed or developed; |
| |
● |
enforcement
of patents is complex, uncertain, and very expensive and we may not be able to secure, enforce and defend our patents; and |
| |
● |
if
we were to ever seek to enforce our patents in ligation, there is some risk that they could be deemed invalid, not infringed, or
unenforceable. |
We
cannot be certain that any patents will be issued because of any pending or future applications, or that any patents, once issued, will
provide us with adequate protection from competing products. For example, issued patents may be circumvented or challenged, declared
invalid or unenforceable, or narrowed in scope. In addition, since publication of discoveries in scientific or patent literature often
lags actual discoveries, we cannot be certain that we or our licensors were the first to invent or to file patent applications covering
them.
It
is also possible that others may have or may obtain issued patents that could prevent us from commercializing our products or require
us to obtain licenses requiring the payment of significant fees or royalties to enable us to conduct our business. There is no guarantee
that such licenses will be available based on commercially reasonable terms. As to those patents that we have licensed, our rights depend
on maintaining our obligations to the licensor under the applicable license agreement, and we may be unable to do so.
If
we are unable to obtain and maintain patent protection for our products, or if the scope of the patent protection obtained is not sufficiently
broad, competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize
our products could be impaired.
The
patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent
applications at a reasonable cost, in a timely manner, or in all jurisdictions. It is also possible that we will fail to identify patentable
aspects of our development output before it is too late to obtain patent protection.
The
patent position of life science companies generally is highly uncertain, involves complex legal and factual questions and has in past
years been the subject of much litigation. In addition, the laws of foreign countries may not protect our rights to the same extent as
the laws of the United States and we may fail to seek or obtain patent protection in all major markets. For example, unlike the U.S.,
European patent law restricts the patentability of methods of treatment of the human body. Our pending and future patent applications
may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others
from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in
the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection, even post-grant.
Recent
patent reform legislation has increased the uncertainties and costs surrounding the prosecution of patent applications and the enforcement
or defense of issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law.
The Leahy-Smith Act includes a number of significant changes to United States patent law. These include provisions that affect the way
patent applications are prosecuted and may also affect patent litigation. The U.S. Patent and Trademark Office, or USPTO, recently developed
new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated
with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Accordingly, it is
not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation
could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our
issued patents, all of which could have a material adverse effect on our business and financial condition.
Moreover,
we may be subject to a third-party pre-issuance submission of prior art to the USPTO, or become involved in opposition, derivation, reexamination, inter
partes review, post-grant review or interference proceedings challenging our patent rights (whether licensed or otherwise held)
or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of,
or invalidate, our patent rights (whether licensed or otherwise held), allow third parties to commercialize our technology or products
and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing
third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications (whether
licensed or otherwise held) is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize
current or future product candidates.
Even
if our patent applications (whether licensed or otherwise held) result in the issuance of patents, they may not issue in a form that
will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive
advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies
or products in a non-infringing manner.
The
issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our licensed or owned patents may
be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom
to operate or in patent claims being narrowed, invalidated, or held unenforceable, in whole or in part, which could limit our ability
to stop others from using or commercializing similar or identical products, or limit the duration of the patent protection of our products.
Given the amount of time required for the development, testing and regulatory review of new life science product candidates, patents
protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our intellectual property
rights portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We
may become involved in lawsuits to protect or enforce our intellectual property rights, which could be expensive, time-consuming, and
ultimately unsuccessful.
Competitors
may infringe our intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims,
which can be expensive and time-consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims
against us alleging that we infringe their intellectual property or that our intellectual property is invalid or unenforceable. In addition,
in a patent infringement proceeding, a court may decide that a licensed or owned patent of ours is invalid or unenforceable, in whole
or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds
that our patents do not cover that technology. Moreover, lawsuits to protect or enforce our intellectual property rights could be expensive,
time-consuming, and ultimately unsuccessful.
Third
parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would
be uncertain.
Our
commercial success depends upon our ability to develop, manufacture, market and sell our product candidates without infringing the proprietary
rights of third parties. There is considerable intellectual property litigation in the life sciences industry. We cannot guarantee that
our product candidates will not infringe third-party patents or other proprietary rights. We may become party to, or threatened with,
future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, including inter
partes review, interference, or derivation proceedings before the USPTO and similar bodies in other countries. Third parties
may assert infringement claims against us based on existing intellectual property rights and intellectual property rights that may be
granted in the future.
If
we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third
party to continue developing and marketing our products. However, we may not be able to obtain any required license on commercially reasonable
terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same
technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product.
In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have
willfully infringed a patent. A finding of infringement could prevent us from commercializing our product candidates or force us to cease
some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information
or trade secrets of third parties could have a similar negative impact on our business.
Obtaining
and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements
imposed by governmental patent agencies, and our own patent protection could be reduced or eliminated for noncompliance with these requirements.
Periodic
maintenance fees and annuities on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over
the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural,
documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases
be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance
can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the
relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but
are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly
legalize and submit formal documents. In such an event, our competitors might be able to enter our markets, which could have a material
adverse effect on our business.
We
may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property or claiming
ownership of what we regard as our own intellectual property.
Certain
employees and contractors were previously employed at universities or other companies, including potential competitors. Although we try
to ensure that our employees and contractors do not use the proprietary information or know-how of others in their work for us, we may
be subject to claims that these employees or we have used or disclosed intellectual property, including trade secrets or other proprietary
information, of any such employee’s former employer. Litigation may be necessary to defend against these claims, and any such litigation
could have an unfavorable outcome.
In
addition, while it is our policy to require our employees and contractors who may be involved in the development of intellectual property
to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party
who in fact develops intellectual property that we regard as our own. Our and their assignment agreements may not be self-executing or
may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine
the ownership of what we regard as our intellectual property.
If
we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property
rights or personnel. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial
costs and adverse results, and be a distraction to management.
Some
intellectual property which we own or have licensed may have been discovered through government funded programs such as, for example,
the government funded programs referenced in intellectual property licensed under the LLU License Agreement, and thus may be subject
to federal regulations such as “march-in” rights, certain reporting requirements, and a preference for United States industry.
Compliance with such regulations may limit our exclusive rights, subject us to expenditure of resources with respect to reporting requirements
and limit our ability to contract with non-U.S. manufacturers.
Some
of the intellectual property rights we own or have licensed have been generated using United States government funding and may therefore
be subject to certain federal regulations. As a result, the United States government may have certain rights to intellectual property
embodied in our current or future products and product candidates pursuant to the Bayh-Dole Act of 1980. These United States government
rights in certain inventions developed under a government-funded program include a non-exclusive, non-transferable, irrevocable worldwide
license to use inventions for any governmental purpose. In addition, the United States government has the right to require us to grant
exclusive, partially exclusive, or non-exclusive licenses to any of these inventions to a third party if it determines that: (i) adequate
steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs;
or (iii) government action is necessary to meet requirements for public use under federal regulations (also referred to as “march-in
rights”). The United States government also has the right to take title to these inventions if we fail to disclose the invention
to the government and fail to file an application to register the intellectual property within specified time limits. In addition, the
United States government may acquire title to these inventions in any country in which a patent application is not filed within specified
time limits. Intellectual property generated under a government funded program is also subject to certain reporting requirements, compliance
with which may require us to expend substantial resources. In addition, the United States government requires that any products embodying
the subject invention or produced using the subject invention be manufactured substantially in the United States. The manufacturing preference
requirement can be waived if the owner of the intellectual property can show that reasonable but unsuccessful efforts have been made
to grant licenses on similar terms to potential licensees that would be likely to manufacture substantially in the United States or that
under the circumstances domestic manufacture is not commercially feasible. This preference for United States manufacturers may limit
our ability to contract with non-U.S. product manufacturers for products covered by such intellectual property. Any exercise by the government
of any of the foregoing rights could harm our competitive position, business, financial condition, results of operations and prospects.
Intellectual
property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even
if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant
expenses and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public
announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors
perceive these results to be negative, it could have an adverse effect on the price of our common stock. Such litigation or proceedings
could increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution
activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our
competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater
financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could compromise
our ability to compete in the marketplace.
We
may spend considerable resources developing and maintaining patents, licensing agreements and other intellectual property that may later
be abandoned or may otherwise never result in products brought to market.
Not
all technologies and candidate products that initially show potential as the basis for future products ultimately meet the rigors of
our development process and as a result may be abandoned and/or never otherwise result in products brought to market. In some cases,
prior to abandonment we may be required to incur significant costs developing and maintaining intellectual property and/or maintaining
license agreements and our business could be harmed by such costs.
We
rely on information technology, and if we are unable to protect against service interruptions, data corruption, cyber-based attacks or
network security breaches, our operations could be disrupted, and our business could be negatively affected.
We
rely on information technology networks and systems to process, transmit and store electronic and financial information; to coordinate
our business; and to communicate within our Company and with customers, suppliers, partners and other third parties. These information
technology systems may be susceptible to damage, disruptions or shutdowns, hardware or software failures, power outages, computer viruses,
cyber-attacks, telecommunication failures, user errors or catastrophic events. If our information technology systems suffer severe damage,
disruption or shutdown, and our business continuity plans do not effectively resolve the issues in a timely manner, our operations could
be disrupted, and our business could be negatively affected. In addition, cyber-attacks could lead to potential unauthorized access and
disclosure of confidential information, and data loss and corruption. There is no assurance that we will not experience these service
interruptions or cyber-attacks in the future.
On
September 15, 2023, we received written notice from Nasdaq that we no longer met the minimum 500,000 publicly held shares requirement
for the Nasdaq Capital Market and we were no longer in compliance with Nasdaq Listing Rule 5550(a)(4) (the “Public Float Rule”).
The letter indicated that the Panel will consider this matter in their decision regarding our continued listing on the Nasdaq Capital
Market. On September 29, 2023, we received written notice from Nasdaq that the Panel had
granted the Company an exception through December 26, 2023, to allow the Company to complete its plan to demonstrate compliance with
the Equity Rule. The October Notification Letter also confirmed that the Company had demonstrated compliance with the Minimum Bid Price
Rule, and it granted the Company an exception through December 26, 2023, to allow the Company to demonstrate compliance with the Public
Float Rule.
Limitation
of Liability and Indemnification of Management.
The
Delaware General Corporation Law and the Company’s Amended and Restated Certificate of Incorporation provide for the limitation
of the liability of directors for monetary damages. Such provisions may discourage shareholders from bringing a lawsuit against directors
for breaches of fiduciary duty and may also have the effect of reducing the likelihood of derivative litigation against directors and
officers even though such action, if successful, might otherwise be a benefit to the Company’s shareholders. In addition,
a shareholder’s investment in the Company may be adversely affected to the extent that costs of settlement and damage awards against
the Company’s officers or directors are paid by the Company pursuant to such provisions. Additionally, in accordance with Delaware
law and the Company’s Amended and Restated Certificate of Incorporation, the Company shall indemnify, hold harmless and provide
advancement of expenses, to the fullest extent permitted by applicable law, directors, officers, employees, and agents that are made
a party or threatened to be made a party to legal proceedings by reason of the fact that such parties were working at the request of
the Company. We direct you to the Company’s Amended and Restated Certificate of Incorporation for more information.
Anti-takeover
provisions under Delaware law could discourage, delay or prevent a change in control of our Company and could affect the trading price
of our securities.
We
are a Delaware corporation, and the anti-takeover provisions of the Delaware General Corporation Law may discourage, delay or prevent
a change in control by prohibiting us from engaging in a business combination with an interested stockholder for a period of three years
after the person becomes an interested stockholder, even if a change in control would be beneficial to our existing stockholders.
Our
management team is required to devote substantial time to public company compliance initiatives.
As
a publicly reporting company, we incur significant legal, accounting, and other expenses. Our management and other personnel devote a
substantial amount of time to comply with our reporting obligations. Moreover, these reporting obligations increase our legal and financial
compliance costs and make some activities more time-consuming and costly.
Failure
to develop our internal controls over financial reporting as we grow could have an adverse impact on us.
As
our Company matures, we will need to develop our current internal control systems and procedures to manage our growth. We are required
to establish and maintain appropriate internal controls over financial reporting. Failure to establish appropriate controls, or any failure
of those controls once established, could adversely impact our public disclosures regarding our business, financial condition, or results
of operations. In addition, management’s assessment of internal controls over financial reporting may identify weaknesses and conditions
that need to be addressed in our internal controls over financial reporting or other matters that may raise concerns for investors. Any
actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting, disclosure
of management’s assessment of our internal controls over financial reporting or disclosure of our public accounting firm’s
attestation to or report on management’s assessment of our internal controls over financial reporting may have an adverse impact
on the price of our common stock.
We
could issue “blank check” preferred stock without stockholder approval with the effect of diluting interests of
then-current stockholders and impairing their voting rights, and provisions in our charter documents and under Delaware law could discourage
a takeover that stockholders may consider favorable.
Our
Amended and Restated Certificate of Incorporation provides for the authorization to issue up to 3,000,000 shares of “blank check” preferred
stock with designations, rights and preferences as may be determined from time to time by our board of directors. Our board of directors
is empowered, without stockholder approval, to issue one or more series of preferred stock with dividend, liquidation, conversion, voting
or other rights which could dilute the interest of, or impair the voting power of, our common stockholders. The issuance of a series
of preferred stock could be used as a method of discouraging, delaying, or preventing a change in control. For example, it would be possible
for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt
to change control of our company. In addition, advanced notice is required prior to stockholder proposals, which might further delay
a change of control.
Our
Amended and Restated Certificate of Incorporation provides that the Court of Chancery of the State of Delaware will be the sole and exclusive
forum for substantially all disputes between the Company and its stockholders, which could limit stockholders’ ability to
obtain a favorable judicial forum for disputes with the Company or its directors, officers or employees.
Our
Amended and Restated Certificate of Incorporation provides that unless the Company consents in writing to the selection of an alternative
forum, the State of Delaware is the sole and exclusive forum for: (i) any derivative action or proceeding brought on behalf of the
Company, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the Company
to the Company or the Company’s stockholders, (iii) any action asserting a claim against the Company, its directors, officers or
employees arising pursuant to any provision of the Delaware General Corporation Law (the “DGCL”) or our Amended
and Restated Certificate of Incorporation or the Company’s Amended and Restated Bylaws, or (iv) any action asserting a claim against
the Company, its directors, officers, employees or agents governed by the internal affairs doctrine, except for, as to each of (i) through
(iv) above, any claim as to which the Court of Chancery determines that there is an indispensable party not subject to the jurisdiction
of the Court of Chancery (and the indispensable party does not consent to the personal jurisdiction of the Court of Chancery within ten
days following such determination), which is vested in the exclusive jurisdiction of a court or forum other than the Court of Chancery,
or for which the Court of Chancery does not have subject matter jurisdiction. This exclusive forum provision would not apply to suits
brought to enforce any liability or duty created by the Securities Act or the Exchange Act or any other claim for which the federal courts
have exclusive jurisdiction. To the extent that any such claims may be based upon federal law claims, Section 27 of the Exchange Act
creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules
and regulations thereunder.
Section
22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability
created by the Securities Act or the rules and regulations thereunder. However, our Amended and Restated Bylaws contain a federal forum
provision which provides that unless the Company consents in writing to the selection of an alternative forum, the federal district courts
of the United States of America will be the exclusive forum for the resolution of any complaint asserting a cause of action arising under
the Securities Act. Any person or entity purchasing or otherwise acquiring any interest in shares of capital stock of the Corporation
are deemed to have notice of and consented to this provision. The Supreme Court of Delaware has held that this type of exclusive federal
forum provision is enforceable. There may be uncertainty, however, as to whether courts of other jurisdictions would enforce such a provision,
if applicable.
These
choice of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes
with the Company or its directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers
and other employees. Alternatively, if a court were to find our choice of forum provisions contained in either our Amended and Restated
Certificate of Incorporation or Amended and Restated Bylaws to be inapplicable or unenforceable in an action, we may incur additional
costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations, and financial
condition.
We
are an “emerging growth company” and will be able to avail ourselves of reduced disclosure requirements applicable to emerging
growth companies, which could make our common stock less attractive to investors.
We
are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”),
and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies
that are not “emerging growth companies” including not being required to comply with the auditor attestation requirements
of Section 404(b) of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports
and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder
approval of any golden parachute payments not previously approved. In addition, pursuant to Section 107 of the JOBS Act, as an “emerging
growth company” we intend to take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities
Act, for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption
of certain accounting standards until those standards would otherwise apply to private companies. As a result, our financial statements
may not be comparable to those of companies that comply with public company effective dates for complying with new or revised accounting
standards.
We
cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find
our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may
be more volatile. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.”
We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have
total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary
of the date of the completion of our initial public offering; (iii) the date on which we have issued more than $1 billion in
nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under
the rules of the SEC.
USE
OF PROCEEDS
We
will not receive any of the proceeds from the sale by the Selling Stockholders of the Common Stock. Upon any exercise of the Warrants
by payment of cash, however, we will receive the exercise price of the Warrants, which, if exercised in cash with respect to the 79,268
shares of Common Stock offered hereby, would result in gross proceeds to us of approximately $2.7 million. However, we cannot predict
when and in what amounts or if the Warrants will be exercised by payments of cash and it is possible that the Warrants may expire and
never be exercised, in which case we would not receive any cash proceeds.
DIVIDEND
POLICY
We
have never paid cash dividends on our Common Stock and we do not anticipate paying cash dividends in the foreseeable future, but intend
to retain our capital resources for reinvestment in our business. Any future determination to pay cash dividends on our Common Stock
will be at the discretion of our board of directors and will be dependent upon our financial condition, results of operations, capital
requirements and other factors as the board of directors deems relevant.
DETERMINATION
OF THE OFFERING PRICE
The
prices at which the shares of Common Stock covered by this prospectus may actually be sold will be determined by the prevailing public
market price for shares of our Common Stock or by negotiations between the Selling Stockholders and buyers of our Common Stock in private
transactions or as otherwise described in “Plan of Distribution.”
SELLING
STOCKHOLDERS
The
Common Stock being offered by the Selling Stockholders are those issuable to the Selling Stockholders, upon exercise of the Warrants.
For additional information regarding the issuances of those securities, see “Prospectus Summary—Recent Developments—July
Private Placement” and “Prospectus Summary—Recent Developments—April 2023 Registered Direct Offering and
Concurrent Private Placement” above. We are registering the shares of Common Stock in order to permit the Selling Stockholders
to offer the shares for resale from time to time. Except for the ownership of the Warrants and as noted below, the Selling Stockholders
have not had any material relationship with us within the past three years.
The
table below lists the Selling Stockholders and other information regarding the beneficial ownership of the shares of Common Stock by
each of the Selling Stockholders. The second column lists the number of shares of Common Stock beneficially owned by each Selling Stockholder,
based on its ownership of the shares of Common Stock and Warrants, as of November 6, 2023, assuming exercise of the Warrants held by
the Selling Stockholders on that date, without regard to any limitations on exercises.
The
third column lists the shares of Common Stock being offered by this prospectus by the Selling Stockholders.
In
accordance with the terms of a registration rights agreement with the Selling Stockholders, this prospectus generally covers the resale
of the maximum number of shares of Common Stock issuable upon exercise of the Warrants, determined as if the outstanding Warrants were
exercised in full as of the trading day immediately preceding the date this registration statement was initially filed with the SEC,
each as of the trading day immediately preceding the applicable date of determination and all subject to adjustment as provided in the
registration rights agreement, without regard to any limitations on the exercise of the Warrants. The fourth column assumes the sale
of all of the shares offered by the Selling Stockholders pursuant to this prospectus.
Under
the terms of the Warrants, a Selling Stockholder may not exercise the Warrants to the extent such exercise would cause such Selling Stockholder,
together with its affiliates and attribution parties, to beneficially own a number of shares of Common Stock which would exceed 4.99%
of our then outstanding Common Stock following such exercise, excluding for purposes of such determination shares of Common Stock issuable
upon exercise of such Warrants which have not been exercised. The number of shares in the second and fourth columns do not reflect this
limitation. The Selling Stockholders may sell all, some or none of their shares in this offering. See “Plan of Distribution.”
| Name of Selling Stockholder | |
Number of
Shares
of Common
Stock
Beneficially
Owned
Prior to
Offering(1) | | |
Maximum
Number of
Shares
of Common
Stock
to be Sold
in this
Offering | | |
Number of
Shares
of Common
Stock
Beneficially
Owned After
Offering | | |
Percentage
of Shares
Beneficially
Owned after
Offering(1) | |
| Walleye Opportunities Master Fund Ltd.(2) | |
| 194,531 | | |
| 181,341 | | |
| 13,190 | | |
| 1.9 | % |
| Sabby Volatility Warrant Master Fund, Ltd.(3) | |
| 101,068 | | |
| 79,268 | | |
| 21,800 | | |
| 3.2 | % |
| FirstFire Global Opportunities Fund, LLC(4) | |
| 3,907 | | |
| 3,907 | | |
| - | | |
| - | % |
| (1) |
The
ability to exercise the Warrants held by the Selling Stockholders is subject to a beneficial ownership limitation that, at the time
of initial issuance of the Warrants was capped at 4.99% beneficial ownership of the Company’s issued and outstanding Common
Stock (post-exercise). These beneficial ownership limitations may be adjusted up or down, subject to providing advanced notice to
the Company. Beneficial ownership as reflected in the selling stockholder table reflects the total number of shares potentially issuable
underlying the Warrants, and does not give effect
to these beneficial ownership limitations. Accordingly, actual beneficial ownership, as calculated in accordance with Section 13(d)
and Rule 13d-3 thereunder may be lower than as reflected in the table. |
| (2) |
Consists of: (1) 27,343 shares of common stock, (2) 164,063 shares of common stock issuable upon the conversion of the convertible note issued to Walleye Opportunities Master Fund Ltd. (the “Master Fund”) on July 24, 2023, and (3) 3,125 shares of common stock underlying warrants held by the Master Fund. The securities are directly held by the Master Fund, a Cayman Islands exempted company, and may be deemed to be indirectly beneficially owned by Walleye Capital LLC (“Walleye Capital”), as the investment manager of the Master Fund. Walleye Capital disclaims beneficial ownership of the securities. The address of the Master Fund is c/o Walleye Opportunities Master Fund Ltd., 2800 Niagara Lane North, Plymouth, MN 55447. |
| (3) |
Sabby
Management, LLC is the investment manager of Sabby Volatility Warrant Master Fund, Ltd. (“Sabby”) and shares voting and investment
power with respect to these shares in this capacity. As manager of Sabby Management, LLC, Hal Mintz also shares voting and investment
power on behalf of Sabby. Each of Sabby Management, LLC and Mr. Mintz disclaims beneficial ownership over the securities listed except
to the extent of their pecuniary interest therein. |
| (4) |
Mr.
Eli Fireman has sole voting and dispositive power over the shares held by FirstFire Global Opportunities Fund,
LLC. |
Plan
of Distribution
Each
Selling Stockholder of the securities and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any
or all of their securities covered hereby on the principal Trading Market or any other stock exchange, market or trading facility on
which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. A Selling Stockholder may
use any one or more of the following methods when selling securities:
| |
● |
ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| |
● |
block
trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block
as principal to facilitate the transaction; |
| |
● |
purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| |
● |
an
exchange distribution in accordance with the rules of the applicable exchange; |
| |
● |
privately
negotiated transactions; |
| |
● |
settlement
of short sales; |
| |
● |
in
transactions through broker-dealers that agree with the Selling Stockholders to sell a specified number of such securities at a stipulated
price per security; |
| |
● |
through
the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| |
● |
a
combination of any such methods of sale; or |
| |
● |
any
other method permitted pursuant to applicable law. |
The
Selling Stockholders may also sell securities under Rule 144 or any other exemption from registration under the Securities Act of 1933,
as amended (the “Securities Act”), if available, rather than under this prospectus. Broker-dealers engaged by the Selling
Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from
the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be
negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction not in excess of a customary
brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown in compliance
with FINRA Rule 2121.
In
connection with the sale of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers
or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they
assume. The Selling Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan
or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option
or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the
delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer
or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The
Selling Stockholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters”
within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers
or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts
under the Securities Act. Each Selling Stockholder has informed us that it does not have any written or oral agreement or understanding,
directly or indirectly, with any person to distribute the securities.
We
are required to pay certain fees and expenses incurred by us incident to the registration of the securities. We have agreed to indemnify
the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We
agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholders
without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for
us to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect
or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar
effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state
securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered
or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is
complied with.
Under
applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously
engage in market making activities with respect to the Common Stock for the applicable restricted period, as defined in Regulation M,
prior to the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the
Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the
Common Stock by the Selling Stockholders or any other person. We will make copies of this prospectus available to the Selling Stockholders
and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including
by compliance with Rule 172 under the Securities Act).
DESCRIPTION
OF CAPITAL STOCK
Common
Stock
General
Matters
The
following description summarizes the most important terms of our capital stock. Because it is only a summary of the provisions of our
certificate of incorporation, as amended (the “Certificate of Incorporation”), and bylaws, as amended (the “Bylaws”),
it does not contain all of the information that may be important to you. For a complete description of the matters set forth in this
“Description of Capital Stock,” you should refer to our Certificate of Incorporation and Bylaws, each of which are included
as exhibits to the registration statement of which this prospectus is a part, and to the applicable provisions of Delaware law.
As
of November 6, 2023, our authorized capital stock consisted of 100,000,000 shares of Common Stock and 3,000,000 shares of preferred stock,
$0.001 par value per share.
Common
Stock
Voting
The
holders of our common stock are entitled to one vote for each share held on all matters to be voted on by the Company’s stockholders.
There shall be no cumulative voting.
Dividends
The
holders of shares of our common stock are entitled to dividends when and as declared by the Board from funds legally available therefor
if, as and when determined by the Board of Directors of the Company in their sole discretion, subject to provisions of law, and any provision
of the Company’s Amended and Restated Certificate of Incorporation, as amended from time to time. There are no preemptive, conversion
or redemption privileges, nor sinking fund provisions with respect to the common stock.
Liquidation
In
the event of any voluntary or involuntary liquidation, dissolution or winding up of our affairs, the holders of our common stock will
be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of or provision for
all of our debts and other liabilities.
Fully
Paid and Non-assessable
All
outstanding shares of common stock are duly authorized, validly issued, fully paid and non-assessable.
Preferred
Stock
We
are authorized to issue up to 3,000,000 shares of preferred stock. This preferred stock may be issued in one or more series, the terms
of which may be determined at the time of issuance by our board of directors without further action by stockholders. The terms of any
series of preferred stock may include voting rights (including the right to vote as a series on particular matters), preferences as to
dividend, liquidation, conversion and redemption rights and sinking fund provisions. No preferred stock is currently outstanding. The
issuance of any preferred stock could materially adversely affect the rights of the holders of our common stock, and therefore, reduce
the value of our common stock and the Notes. In particular, specific rights granted to future holders of preferred stock could be used
to restrict our ability to merge with, or sell our assets to, a third party and thereby preserve control by the present management.
Exclusive
Forum
Our
Amended and Restated Certificate of Incorporation provides that unless the Company consents in writing to the selection of an alternative
forum, the State of Delaware is the sole and exclusive forum for: (i) any derivative action or proceeding brought on behalf of the
Company, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the Company
to the Company or the Company’s stockholders, (iii) any action asserting a claim against the Company, its directors, officers or
employees arising pursuant to any provision of the DGCL or our Amended and Restated Certificate of Incorporation or the Amended and Restated
Bylaws, or (iv) any action asserting a claim against the Company, its directors, officers, employees or agents governed by the internal
affairs doctrine, except for, as to each of (i) through (iv) above, any claim as to which the Court of Chancery determines that there
is an indispensable party not subject to the jurisdiction of the Court of Chancery (and the indispensable party does not consent to the
personal jurisdiction of the Court of Chancery within ten days following such determination), which is vested in the exclusive jurisdiction
of a court or forum other than the Court of Chancery, or for which the Court of Chancery does not have subject matter jurisdiction.
Additionally,
our Amended and Restated Bylaws provide that unless the Company consents in writing to the selection of an alternative forum, the federal
district courts of the United States of America will be the exclusive forum for the resolution of any complaint asserting a cause of
action arising under the Securities Act. Any person or entity purchasing or otherwise acquiring any interest in shares of capital stock
of the Corporation are deemed to have notice of and consented to this provision. The Supreme Court of Delaware has held that this type
of exclusive federal forum provision is enforceable. There may be uncertainty, however, as to whether courts of other jurisdictions would
enforce such a provision, if applicable.
Transfer
Agent
The
transfer agent and registrar for our common stock is VStock Transfer, LLC.
Changes
in Authorized Number
The
number of authorized shares of common stock may be increased or decreased subject to the Company’s legal commitments at any time
and from time to time to issue them, by the affirmative vote of the holders of a majority of the stock of the Company entitled to vote.
Delaware
Anti-Takeover Statute
We
may become subject to Section 203 of the Delaware General Corporation Law, which prohibits persons deemed to be “interested stockholders”
from engaging in a “business combination” with a publicly held Delaware corporation for three years following the date these
persons become interested stockholders unless the business combination is, or the transaction in which the person became an interested
stockholder was, approved in a prescribed manner or another prescribed exception applies. Generally, an “interested stockholder”
is a person who, together with affiliates and associates, owns, or within three years prior to the determination of interested stockholder
status did own, 15% or more of a corporation’s voting stock. Generally, a “business combination” includes a merger,
asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. The existence of this provision
may have an anti-takeover effect with respect to transactions not approved in advance by the Board of Directors. A Delaware corporation
may “opt out” of these provisions with an express provision in its original certificate of incorporation or an express provision
in its certificate of incorporation or bylaws resulting from a stockholders’ amendment approved by at least a majority of the outstanding
voting shares. We have not opted out of these provisions. As a result, mergers or other takeover or change in control attempts of us
may be discouraged or prevented.
The
Amended and Restated Bylaws establish an advance notice procedure for stockholder proposals to be brought before an annual meeting of
our stockholders, including proposed nominations of persons for election to our board of directors. At an annual meeting, stockholders
may only consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of
our board of directors. Stockholders may also consider a proposal or nomination by a person who was a stockholder at the time of giving
notice and at the time of the meeting, who is entitled to vote at the meeting and who has complied with the notice requirements of the
Amended and Restated Bylaws in all respects. The Amended and Restated Bylaws do not give our board of directors the power to approve
or disapprove stockholder nominations of candidates or proposals regarding other business to be conducted at a special or annual meeting
of our stockholders. However, the Amended and Restated Bylaws may have the effect of precluding the conduct of certain business at a
meeting if the proper procedures are not followed. These provisions may also discourage or deter a potential acquirer from conducting
a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our company.
The
Amended and Restated Bylaws provide that a special meeting of our stockholders may be called only by our Chairman or by resolution adopted
by a majority of our board of directors. Because our stockholders do not have the right to call a special meeting, a stockholder could
not force stockholder consideration of a proposal over the opposition of our board of directors by calling a special meeting of stockholders
prior to such time as a majority of our board of directors, the chairperson of our board of directors, the president or the chief executive
officer believed the matter should be considered or until the next annual meeting provided that the requestor met the
notice requirements. The restriction on the ability of stockholders to call a special meeting means that a proposal to replace our board
of directors also could be delayed until the next annual meeting.
Transfer
Agent and Registrar
The
transfer agent and registrar for our Common Stock is VStock Transfer LLC. The transfer agent and registrar’s address is 18 Lafayette
Place, Woodmere, NY 11598.
Listing
Our
shares of common stock are listed on The Nasdaq Capital Market under the symbol “ADTX.”
LEGAL
MATTERS
Sheppard,
Mullin, Richter & Hampton LLP, New York, New York, will pass upon the validity of the shares of our common stock offered hereby.
EXPERTS
dbbmckennon,
an independent registered public accounting firm, has audited our financial statements included in our Annual Report on Form 10-K for
the year ended December 31, 2022, as set forth in their report, which includes an explanatory paragraph as to our ability to continue
as a going concern, dated April 17, 2023, which is incorporated by reference in this prospectus and elsewhere in the registration statement.
Our financial statements are incorporated by reference in reliance on dbbmckennon report, given on the authority of such firm
as experts in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities offered by this
prospectus. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth
in the registration statement, some of which is contained in exhibits to the registration statement as permitted by the rules and regulations
of the SEC. For further information with respect to us and our securities, we refer you to the registration statement, including the
exhibits filed as a part of the registration statement. Statements contained in this prospectus concerning the contents of any contract
or any other document is not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement,
please see the copy of the contract or document that has been filed. Each statement is this prospectus relating to a contract or document
filed as an exhibit is qualified in all respects by the filed exhibit. We are subject to the informational requirements of the Exchange
Act and in accordance therewith file annual, quarterly and current reports, proxy statements and other information with the SEC. The
SEC maintains an Internet website that contains reports, proxy statements and other information about issuers, like us, that file electronically
with the SEC. The address of that website is www.sec.gov. The registration statement and the documents referred to below
under “Incorporation of Documents By Reference” are also available on our website, www.astrotechcorp.com. We have not
incorporated by reference into this prospectus the information on our website, and you should not consider it to be a part of this prospectus.
INCORPORATION
OF DOCUMENTS BY REFERENCE
This
prospectus is part of the registration statement but the registration statement includes and incorporates by reference additional information
and exhibits. The SEC permits us to “incorporate by reference” the information contained in documents we file with the SEC,
which means that we can disclose important information to you by referring you to those documents rather than by including them in this
prospectus. Information that is incorporated by reference is considered to be part of this prospectus and you should read it with the
same care that you read this prospectus. Information that we file later with the SEC will automatically update and supersede the information
that is either contained, or incorporated by reference, in this prospectus, and will be considered to be a part of this prospectus from
the date those documents are filed. We have filed with the SEC, and incorporate by reference in this prospectus:
| |
● |
Current
Reports on Form 8-K, filed with the SEC on January 6, 2023, January 20, 2023, February 24, 2023, March 21, 2023, April 7, 2023, April 17, 2023, April 24, 2023, April 25, 2023, April 28, 2023, May 26, 2023, May 31, 2023, June 16, 2023, June 21, 2023, June 30, 2023,
July 7, 2023, July 14, 2023, July 27, 2023, July 28, 2023, August 16, 2023, August 17, 2023, August 18, 2023, August 21, 2023, August 28, 2023, September 6, 2023, September 21, 2023, October 5, 2023 and October 11, 2023; |
| |
● |
Annual
Report on Form 10-K for the year ended December 31, 2022 originally filed with the SEC on April 17, 2023 and amended on April 28, 2023; |
| |
● |
Quarterly
Report on Form 10-Q for the three months ended March 31, 2023 filed with the SEC on May 15, 2023; |
| |
● |
Quarterly
Report on Form 10-Q for the six months ended June 30, 2023 filed with the SEC on August 14, 2023; |
| |
● |
Proxy
Statement on Schedule 14A filed on July 20, 2023, as amended; and |
| |
● |
the
description of our common stock and our preferred stock contained in our Registration Statement on Form 8-A12B/A filed with the Commission
on June 17, 2020, and any amendments or reports filed updating such description. |
In
addition, all documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934,
as amended, prior to the termination of the offering (excluding any information furnished rather than filed) shall be deemed to be incorporated
by reference into this prospectus.
Notwithstanding
the statements in the preceding paragraphs, no document, report or exhibit (or portion of any of the foregoing) or any other information
that we have “furnished” to the SEC pursuant to the Securities Exchange Act of 1934, as amended shall be incorporated by
reference into this prospectus.
We
will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference in this
prospectus, including exhibits to these documents. You should direct any requests for documents to:
Aditxt,
Inc.
737
N. Fifth Street, Suite 200
Richmond,
VA 23219
Phone:
(650) 870-1200
You
also may access these filings on our website at http://www.aditxt.com. We do not incorporate the information on our website into this
prospectus or any supplement to this prospectus and you should not consider any information on, or that can be accessed through, our
website as part of this prospectus or any supplement to this prospectus (other than those filings with the SEC that we specifically incorporate
by reference into this prospectus or any supplement to this prospectus).
Any
statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified,
superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes
or replaces such statement. Any statement contained herein or in any document incorporated or deemed to be incorporated by reference
shall be deemed to be modified or superseded for purposes of the registration statement of which this prospectus forms a part to the
extent that a statement contained in any other subsequently filed document which also is or is deemed to be incorporated by reference
modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed to constitute a part of the registration
statement of which this prospectus forms a part, except as so modified or superseded.
Aditxt,
Inc.
Up to 264,516 Shares of Common Stock
PROSPECTUS
November 13,
2023
Aditxt (NASDAQ:ADTX)
Historical Stock Chart
From Apr 2024 to May 2024
Aditxt (NASDAQ:ADTX)
Historical Stock Chart
From May 2023 to May 2024