false
0001701541
0001701541
2024-01-04
2024-01-04
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
The UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant
to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
January 4, 2024
BLACK DIAMOND THERAPEUTICS, INC.
(Exact name of registrant as specified in its
charter)
| |
|
|
| Delaware |
001-39200 |
81-4254660 |
| (State or other jurisdiction |
(Commission |
(I.R.S. Employer |
| of incorporation) |
File Number) |
Identification No.) |
| One
Main Street, 14th Floor |
|
|
| Cambridge, Massachusetts |
|
02141 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
(617) 252-0848
(Registrant’s telephone number, including
area code)
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation to the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b)
of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which
registered |
| Common Stock, $0.0001 par value per share |
BDTX |
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth
company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange
Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 13(a) of the Exchange Act.
| Item 7.01. | Regulation FD Disclosure. |
On January 4, 2024, Black Diamond Therapeutics,
Inc. (the “Company”) issued a press release titled, “Black Diamond Therapeutics Announces Corporate Update
and Expected 2024 Milestones” and updated its corporate presentation for use in meetings with investors, analysts and others.
A copy of the press release and a copy of the corporate presentation are furnished as Exhibits 99.1 and 99.2, respectively, to this Current
Report on the Form 8-K.
The information furnished under this Item
7.01, including Exhibits 99.1 and 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange
Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section nor shall it
be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except as expressly set forth
by specific reference in such a filing.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURE
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
BLACK DIAMOND THERAPEUTICS, INC. |
| |
|
|
| |
|
|
| Date: January 4, 2024 |
By: |
/s/ Brent Hatzis-Schoch |
| |
Name: |
Brent Hatzis-Schoch |
| |
Title: |
Chief Operating Officer and General Counsel |
Exhibit 99.1

Black Diamond Therapeutics Announces Corporate
Update and Expected 2024 Milestones
FDA feedback on BDTX-1535 enables initiation
of Phase 2 cohort in first-line treatment of non-classical EGFR mutant NSCLC
Fast Track Designation granted for BDTX-1535
as second-line treatment for EGFR mutant/C797S NSCLC
BDTX-1535 Phase 2 results for 2L/3L patients
with EGFR mutant NSCLC expected Q3 2024
BDTX-1535 Phase 1 clinical trial results and
“window of opportunity” data in patients with EGFR mutant GBM expected to be presented at a medical meeting in Q2 2024
BDTX-4933 Phase 1 results in patients with KRAS
mutant NSCLC expected Q4 2024
Existing cash, cash equivalents and investments
expected to be sufficient to fund milestone achievements and operations into Q2 2025
CAMBRIDGE, Mass.,
January 4, 2024 (GLOBE NEWSWIRE) – Black Diamond Therapeutics, Inc. (Nasdaq: BDTX), a clinical-stage oncology
company developing MasterKey therapies that target families of oncogenic mutations in patients with genetically defined cancers, today
provided a corporate update outlining clinical development plans and anticipated corporate milestones for 2024.
“We made significant progress in 2023 and
sharpened our focus on our clinical programs: BDTX-1535 in both EGFR mutant NSCLC and GBM, and BDTX-4933 in KRAS mutant NSCLC,”
said Mark Velleca, M.D., Ph.D., Chief Executive Officer of Black Diamond Therapeutics. “In 2024, we anticipate key readouts from
each of these programs, including Phase 2 data from BDTX-1535 in NSCLC. Moreover, recent FDA feedback enables the enrollment of first-line
NSCLC patients into the Phase 2 trial, reflecting the potential of BDTX-1535 to benefit patients in earlier lines of therapy. Due to disciplined
spend, we expect our cash to be sufficient for this year’s milestones and to extend into the second quarter of 2025.”
Clinical Program Updates/Anticipated 2024 Milestones
BDTX-1535 in patients with Epidermal Growth Factor Receptor (EGFR)
mutant Non-Small Cell Lung Cancer (NSCLC)
| · | Dose escalation results were presented at the
AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2023. Phase 2 data in second/third-line
patients with EFGR mutant NSCLC are expected in the third quarter of 2024. The Company intends to discuss Phase 2 results with the U.S.
Food and Drug Administration (FDA) to finalize a pivotal clinical trial design. |
| · | BDTX-1535 received Fast Track Designation for
the treatment of patients with EGFR mutant C797S-positive NSCLC whose disease has progressed on/after a third-generation EGFR tyrosine
kinase inhibitor (TKI). |
| · | Following End of Phase 1 feedback received from
the FDA in the fourth quarter of 2023, a Phase 2 cohort in first-line patients with non-classical EGFR mutant NSCLC is being initiated. |
| · | The Company is also exploring the potential development
of BDTX-1535 in first-line patients who are post-osimertinib adjuvant treatment. |
BDTX-1535 in patients with EGFR mutant Glioblastoma (GBM)
| · | Following release of top-line Phase 1 data in
December 2023, presentation of Phase 1 trial results is anticipated at a medical meeting in the second quarter of 2024. |
| · | Enrollment is ongoing in a “window of opportunity”
trial sponsored by the Ivy Brain Tumor Center in patients with recurrent glioma who are undergoing a planned resection. Results from this
trial are expected to be presented at a medical meeting in the second quarter of 2024. |
| · | The Company expects that results from the dose
escalation and “window of opportunity” trials will inform the next steps in the GBM development program, including a potential
randomized trial in the first-line setting. |
BDTX-4933 in patients with KRAS mutant NSCLC
| · | BDTX-4933 was designed as a “RAF/RAS clamp”
to target the activated RAF conformation in the context of either RAF or RAS mutations, a mechanism distinct from earlier generation RAF
inhibitors. |
| · | Enrollment in a Phase 1 trial began in September 2023
in patients with KRAS mutant NSCLC. Results from this trial are anticipated in the fourth quarter of 2024. |
About BDTX-1535
BDTX-1535 is an
oral, brain-penetrant MasterKey inhibitor of oncogenic epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer
(NSCLC), including classical driver mutations, families of non-classical driver mutations (e.g., L747P, L718Q), acquired resistance C797S
mutation, and complex mutations. BDTX-1535 is a fourth-generation tyrosine kinase inhibitor (TKI) that potently inhibits, based on preclinical
data, more than 50 oncogenic EGFR mutations expressed across a diverse group of patients with NSCLC in multiple lines of therapy. Based
on preclinical data, BDTX-1535 also inhibits EGFR extracellular domain mutations and alterations commonly expressed in glioblastoma (GBM)
and avoids paradoxical activation observed with earlier generation reversible TKIs. A “window of opportunity” trial of BDTX-1535
in patients with GBM is ongoing (NCT06072586) and a Phase 2 trial is currently ongoing in patients with NSCLC (NCT05256290).
About BDTX-4933
BDTX-4933 is an
oral, brain-penetrant RAF MasterKey inhibitor designed to target oncogenic alterations in KRAS, NRAS and BRAF, while also avoiding paradoxical
activation. In preclinical studies, BDTX-4933 has demonstrated a potential best-in-class profile, showing potent target engagement, inhibition
of MAPK signaling and strong anti-tumor activity/tumor regression across tumor models driven by either KRAS, NRAS or BRAF mutations.
BDTX-4933 also exhibits high central nervous system (CNS) exposure leading to dose-dependent tumor growth inhibition and a survival benefit
in an intracranial tumor model harboring oncogenic BRAF mutation. The ongoing BDTX-4933 Phase 1 clinical trial is currently in dose escalation
with emphasis on KRAS mutant NSCLC patients (NCT05786924).
About Black Diamond Therapeutics
Black Diamond Therapeutics
is a clinical-stage oncology company focused on the development of MasterKey therapies that address families of oncogenic mutations in
clinically validated targets. The Company’s MasterKey therapies are designed to address broad genetically defined patient populations,
overcome resistance, minimize wild-type mediated toxicities, and be brain penetrant to treat CNS disease. The Company is advancing two
clinical-stage programs: BDTX-1535, a brain-penetrant fourth-generation EGFR MasterKey inhibitor targeting EGFR mutant NSCLC and GBM,
and BDTX-4933, a brain-penetrant RAF MasterKey inhibitor targeting KRAS, NRAS and BRAF alterations in solid tumors. For more information,
please visit www.blackdiamondtherapeutics.com.
Forward-Looking Statements
Statements contained in this press release regarding
matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation
Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed
or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding: the continued development
and advancement of BDTX-1535 and BDTX-4933, including the ongoing clinical trials and the timing of clinical updates for BDTX-1535 in
patients with NSCLC and in patients with recurrent GBM, and for Phase 1 clinical trial results for BDTX-4933, the potential of BDTX-1535
to benefit patients with NSCLC in earlier lines of therapy, potential future development plans for BDTX-1535 in NSCLC and GBM, including
in first-line settings, and the Company’s expected cash runway. Any forward-looking statements in this statement are based on management’s
current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ
materially and adversely from those set forth in or implied by such forward-looking statements. Risks that contribute to the uncertain
nature of the forward-looking statements include those risks and uncertainties set forth in its Annual Report on Form 10-K for the
year ended December 31, 2022, filed with the United States Securities and Exchange Commission and in its subsequent filings filed
with the United States Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as
of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances
that exist after the date on which they were made.
Contacts
For Investors:
Mario Corso, Head of Investor Relations, Black Diamond Therapeutics
mcorso@bdtx.com
For Media:
media@bdtx.com
Exhibit 99.2

1 Black Diamond Therapeutics, Inc. Developing MasterKey Therapies to Defeat Cancer Resistance January 2024

2 Forward - Looking Statements Statements contained in this presentation regarding matters that are not historical facts are “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 . Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward - looking statements . Such statements include, but are not limited to, statements regarding : the continued development and advancement of BDTX - 1535 and BDTX - 4933 , including the ongoing clinical trials and the timing of clinical updates for BDTX - 1535 in patients with NSCLC and in patients with recurrent GBM, and for Phase 1 clinical trial results for BDTX - 4933 , the potential of BDTX - 1535 to benefit patients with NSCLC in earlier lines of therapy, potential future development plans for BDTX - 1535 in NSCLC and GBM, including in first - line settings, and the Company’s expected cash runway . Any forward - looking statements in this statement are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward - looking statements . Risks that contribute to the uncertain nature of the forward - looking statements include those risks and uncertainties set forth in its Annual Report on Form 10 - K for the year ended December 31 , 2022 , filed with the United States Securities and Exchange Commission and in its subsequent filings filed with the United States Securities and Exchange Commission . All forward - looking statements contained in this presentation speak only as of the date on which they were made . The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made .

3 Cancer is a Complex and Ever - Evolving Disease Current targeted therapies were designed against a limited subset of oncogenic mutations Increasing adoption of liquid biopsies reveals a broader set of oncogenic mutations We are Developing MasterKey Therapies to Defeat Cancer Resistance MasterKey approach aims to provide one solution for many mutations and expand addressable patient population Our oral therapies are designed so that patients have the opportunity for a longer, healthier, active life
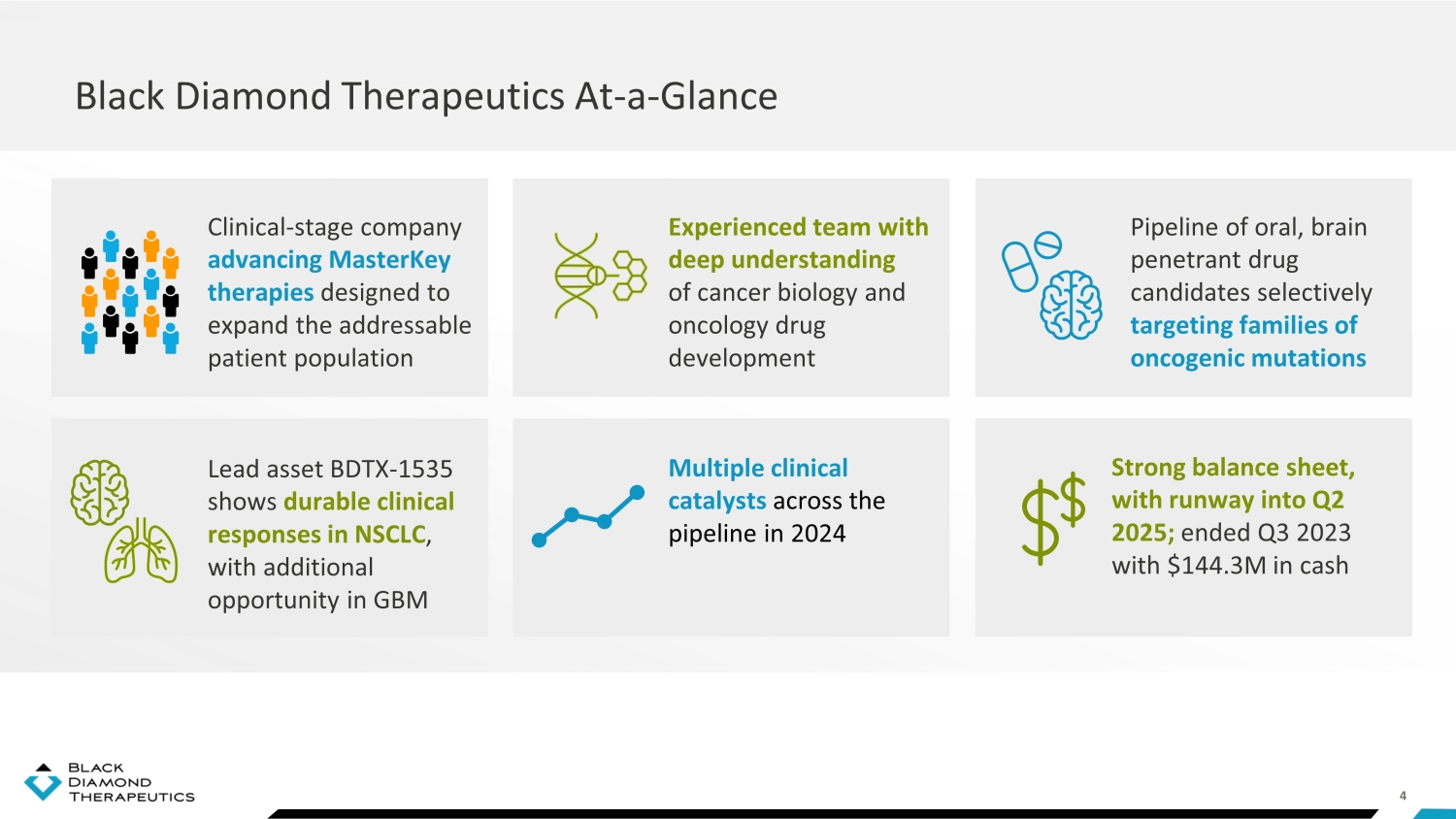
4 Black Diamond Therapeutics At - a - Glance Clinical - stage company advancing MasterKey therapies designed to expand the addressable patient population Experienced team with deep understanding of cancer biology and oncology drug development Lead asset BDTX - 1535 shows durable clinical responses in NSCLC , with additional opportunity in GBM Multiple clinical catalysts across the pipeline in 2024 Pipeline of oral, brain penetrant drug candidates selectively targeting families of oncogenic mutations Strong balance sheet, with runway into Q2 2025; ended Q3 2023 with $144.3M in cash

5 MasterKey : One Solution for Many Mutations Expanded addressable patient population Black Diamond Approach: Targeting families of oncogenic mutations Limited addressable patient population 5 Brain penetrant to treat CNS disease Traditional Approach: Targeting single mutations in individual tumor types Potent against broad mutation families (including drug resistance mutations) Selective targeting to deliver well - tolerated therapies

6 Advancing Wholly Owned Pipeline Across Multiple Oncology Indications Target Drug Candidate Indication Pre - clinical Phase 1 Phase 2 Phase 3 EGFR RAF BDTX - 1535 BDTX - 4933 NSCLC GBM KRAS mutant NSCLC Undisclosed Multiple Solid tumors FGFR2/3 BDTX - 4876 Achondroplasia or solid tumors RAF/RAS mutant solid tumors Phase 2 enrolling with data expected Q3 2024 Phase 1 enrolling data expected Q4 2024 Partnering candidate Partnering candidate Phase 1 and "window of opportunity" data expected Q2 2024 Undisclosed

7 Targets EGFR classical drivers, non - classical drivers, and resistance mutations WT=Wild - Type; MOA=mechanism of action; CNS=Central Nervous System; NSCLC=Non - Small Cell Lung Cancer BDTX - 1535 MasterKey BDTX - 1535: EGFR MasterKey Inhibitor with Clinical Proof - of - Concept Selective for mutations versus WT - EGFR to deliver favorable safety profile Spares WT - EGFR Oral small molecule with covalent MOA for potency and durability Covalent Designed to treat CNS tumors and brain metastases Brain Penetrant Clinical proof - of - concept in NSCLC achieved, promising activity demonstrated in GBM Clinical PoC

8 EGFR Altered Glioblastoma (GBM) EGFR Mutant Non - Small Cell Lung Cancer (NSCLC) Phase 1 topline data demonstrated promising activity Clinical proof - of - concept achieved BDTX - 1535: Clinical Proof - of - Concept Achieved in NSCLC, Promising GBM Data

9 BDTX - 1535: Summary of NSCLC Data
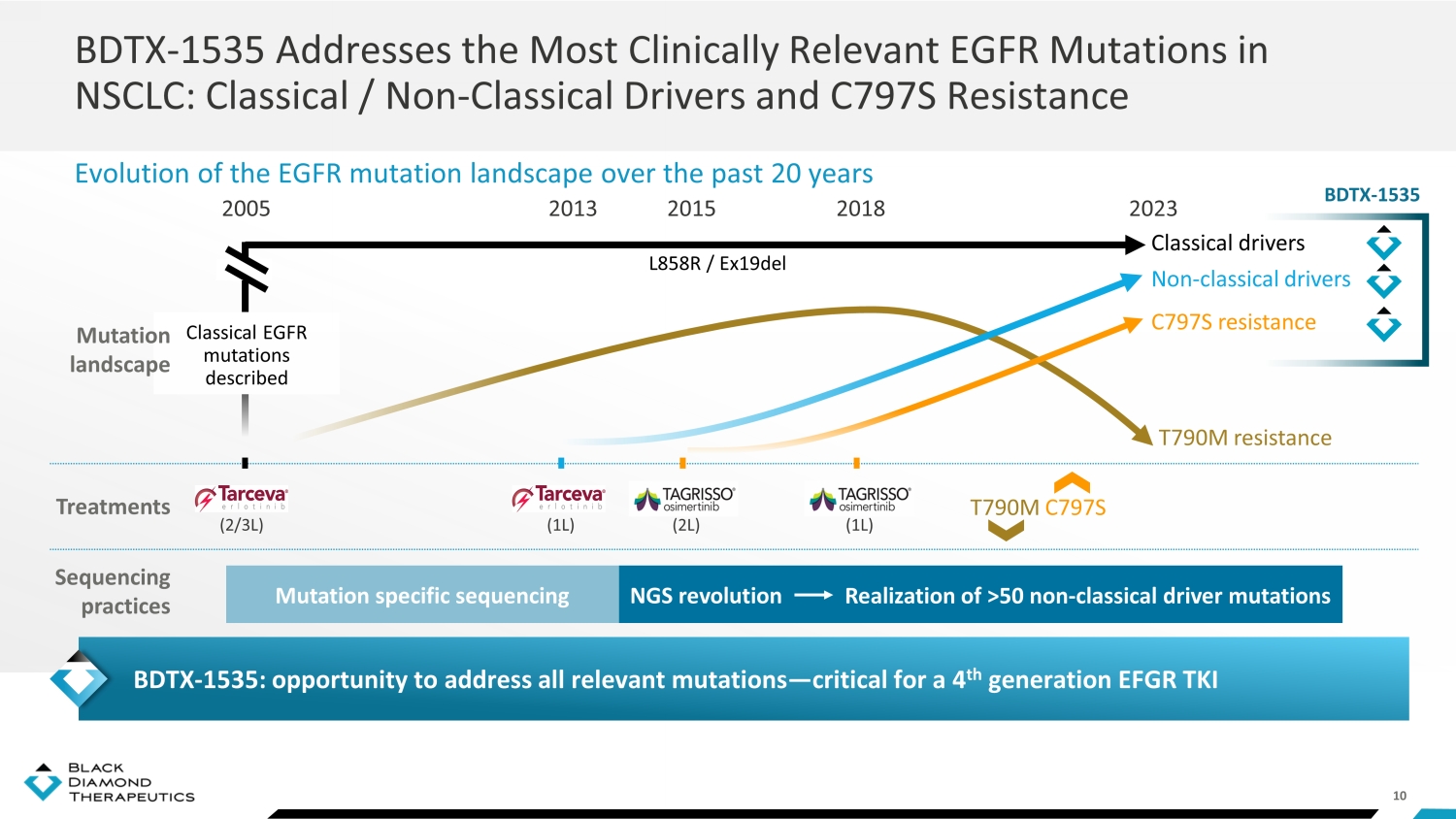
10 BDTX - 1535 Addresses the Most Clinically Relevant EGFR Mutations in NSCLC: Classical / Non - Classical Drivers and C797S Resistance Evolution of the EGFR mutation landscape over the past 20 years NGS revolution Mutation specific sequencing 2005 1G EGFR T (2/3L) 1G EGFR T (1L) 2013 3G EGFR TKI (2L) 2015 3G EGFR TKI (1L) 2018 2023 T790M resistance C797S resistance Non - classical drivers T790M C797S Classical drivers L858R / Ex19del R ealization of >50 non - classical driver mutations Treatments Sequencing practices Classical EGFR mutations described Mutation landscape BDTX - 1535: opportunity to address all relevant mutations — critical for a 4 th generation EFGR TKI BDTX - 1535
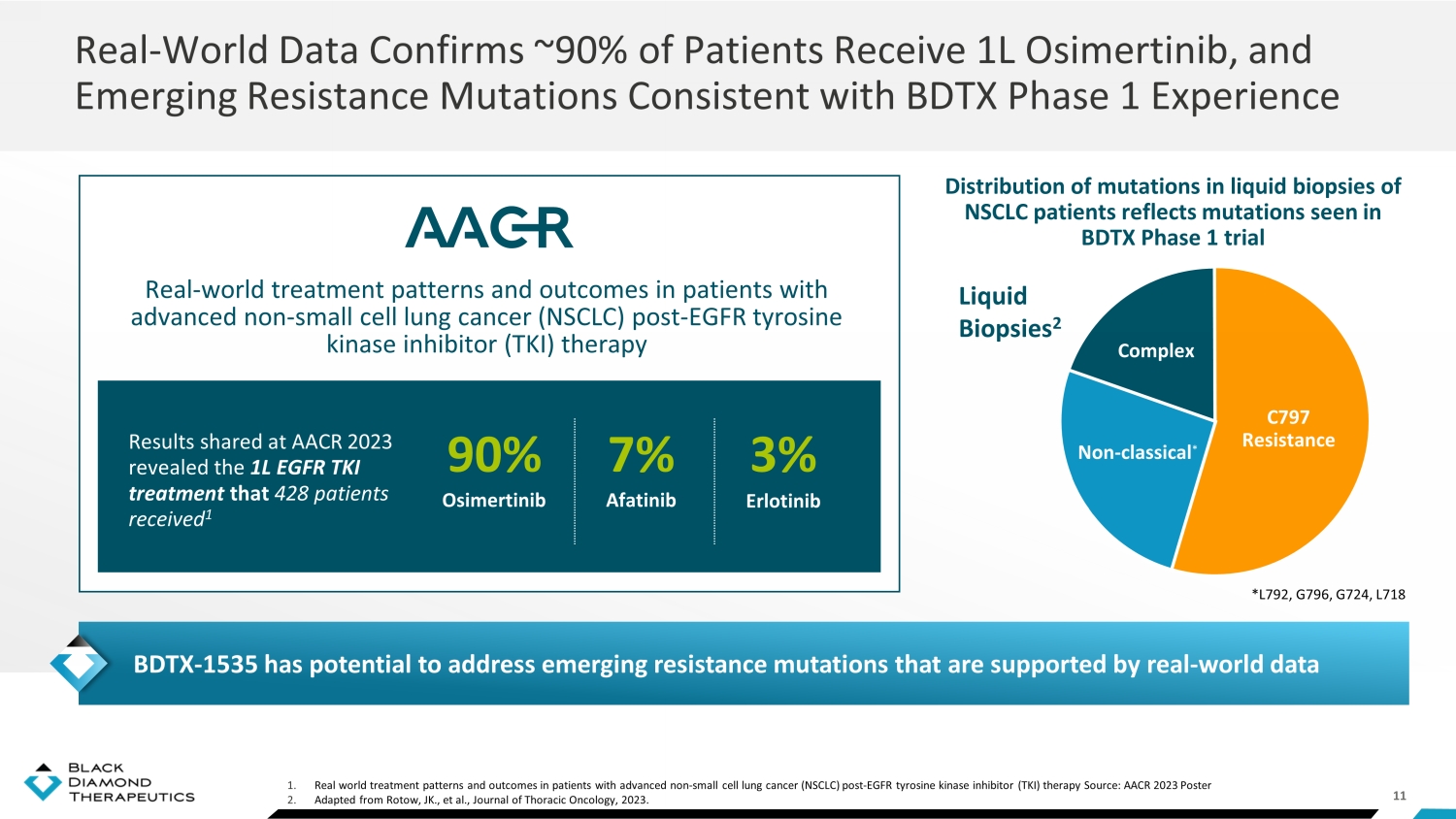
11 1. Real world treatment patterns and outcomes in patients with advanced non - small cell lung cancer (NSCLC) post - EGFR tyrosine kinas e inhibitor (TKI) therapy Source: AACR 2023 Poster 2. Adapted from Rotow , JK., et al., Journal of Thoracic Oncology, 2023. Real - World Data Confirms ~90% of Patients Receive 1L Osimertinib, and Emerging Resistance Mutations Consistent with BDTX Phase 1 Experience Distribution of mutations in liquid biopsies of NSCLC patients reflects mutations seen in BDTX Phase 1 trial Results shared at AACR 2023 revealed the 1L EGFR TKI treatment that 428 patients received 1 Real - world treatment patterns and outcomes in patients with advanced non - small cell lung cancer (NSCLC) post - EGFR tyrosine kinase inhibitor (TKI) therapy 90% Osimertinib 7% Afatinib 3% Erlotinib Liquid Biopsies 2 Complex Non - classical * C797 Resistance BDTX - 1535 has potential to address emerging resistance mutations that are supported by real - world data *L792, G796, G724, L718
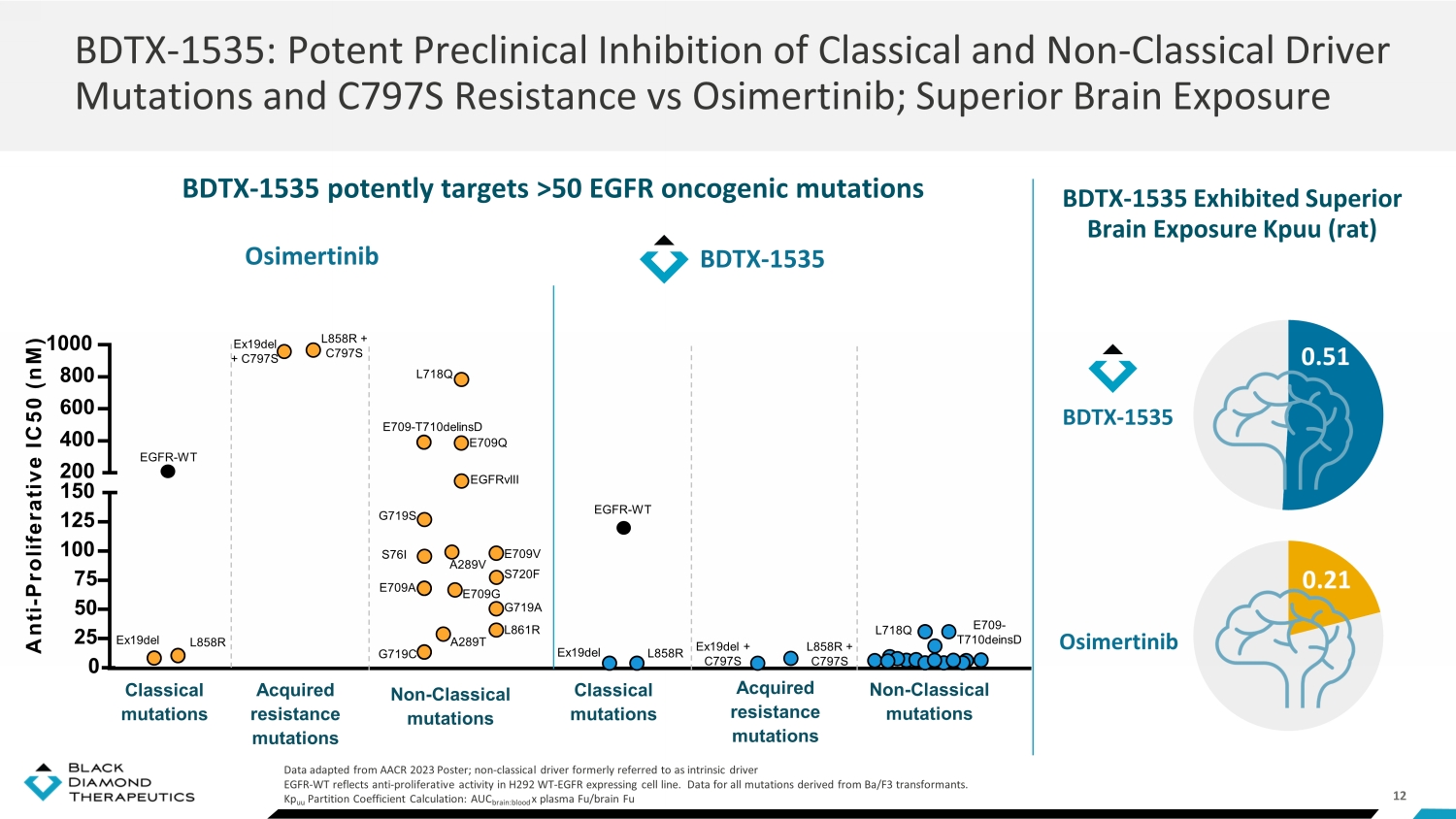
12 BDTX - 1535 potently targets >50 EGFR oncogenic mutations BDTX - 1535: Potent Preclinical Inhibition of Classical and Non - Classical Driver Mutations and C797S Resistance vs Osimertinib; Superior Brain Exposure Data adapted from AACR 2023 Poster; non - classical driver formerly referred to as intrinsic driver EGFR - WT reflects anti - proliferative activity in H292 WT - EGFR expressing cell line. Data for all mutations derived from Ba/F3 tr ansformants. Kp uu Partition Coefficient Calculation: AUC brain:blood x plasma Fu/brain Fu BDTX - 1535 Exhibited Superior Brain Exposure Kpuu (rat) BDTX - 1535 0.51 0.21 Osimertinib 0 25 50 75 100 125 150 200 400 600 800 1000 A n t i - P r o l i f e r a t i v e I C 5 0 ( n M ) BDTX - 1535 A289T A289V EGFRvIII L858R + C797S Ex19del + C797S L718Q Acquired resistance mutations L858R Ex19del EGFR - WT L858R Ex19del Classical mutations EGFR - WT E709 - T710delinsD E709A E709G E709Q E709V G719A G719S G719C L861R S720F Non - Classical mutations S76I Acquired resistance mutations Classical mutations Non - Classical mutations L858R + C797S Ex19del + C797S Osimertinib L718Q E709 - T710deinsD

13 BDTX - 1535 Phase 1 Dose Escalation: Summary Mutation Matched Phase 1 Study Inclusion Criteria Once - daily dosing delivers sufficient exposure to inhibit EGFR mutations M anageable EGFR TKI safety profile at 200 mg (similar to osimertinib ) Radiographic responses and durable anti - tumor activity across multiple mutation families ctDNA reduction confirms loss of mutant alleles, which is predictive of clinical benefit 1 Phase 2 data expected Q3 2024 NSCLC Key Data T akeaways • Primary objective: PK and safety • Secondary objective: Anti - tumor activity Dose Escalation Completed: 15 mg QD to 400 mg QD 15 mg QD 25 mg QD 50 mg QD 100 mg QD 200 mg QD 300 mg QD 400 mg QD Recurrent GBM Cohort EGFR alterations at resection/diagnosis Wild - type isocitrate dehydrogenase (IDH) Recurrent NSCLC Cohort EGFR mutations at the time of progression: – Non - classical driver, OR – Acquired resistance C797S Progression after EGFR TKI Exclusion of EGFR T790M, Ex20ins, KRAS mutations, cMET amplification • Target coverage and clinical activity at ≥ 100 mg, MTD at 300 mg • Phase 2 in 2L/3L NSCLC enrolling at 100 mg QD and 200 mg QD 1. Thompson, JC., et al., British Journal of Cancer, 2023.

14 BDTX - 1535 - 101 Phase 1 Dose Escalation Patient Characteristics NSCLC GBM 0 2 4 6 8 10 12 14 16 15 25 50 100 200 300 400 Patients enrolled Dose, mg QD NSCLC GBM Patient Enrollment Patient Characteristics All Treated Age, mean (range) 64 (46, 81) Female 18 (67%) ECOG PS 0 7 (26%) 1 20 (74%) Prior lines of therapies median (min, max) 2 (1, 9) Prior anti - cancer agents EGFR TKI 27 (100%) Chemo 19 (70%) Anti - angiogenic or CPIs 11 (41%) HER3 - ADC 2 (7%) Prior EGFR TKIs Osimertinib 23 (85%) 1 st line treatment 17 (74%) Erlotinib or gefitinib 9 (33%) Afatinib 3 (11%) Dacomitinib 1 (4%) BLU - 701 1 (4%) All Treated Age, mean (range) 58.7 (41, 85) Female 10 (37%) Karnofsky PS 90 4 (15%) 80 12 (44%) 70 5 (19%) 60 3 (11%) Prior lines of therapies median (min, max) 2 (1, 4) Prior anti - cancer agents TMZ 26 (96%) Anti - angiogenic or CPIs 11 (41%) Chemo 7 (26%) Data from Poster at EORTC/AACR/NCI International Conference on Molecular Targets and Cancer Therapeutics October 2023; 1 GBM updated November 2023 1

15 15 - 50 m(N=10) 100 mg (N=5) 200 mg (N=12) 3 00 mg (N=15) 400 mg (N= 12 ) Treatment emergent adverse events (TEAEs) occurring in ≥6% patients; All patients in 300 mg cohort received rash prophylaxis Rash group terms: rash, rash maculo - papular, dermatitis acneiform Dose Escalation Treatment Emergent Adverse Events Related to BDTX - 1535: Well - Tolerated Profile All subjects (N=54) Percent prevalence TEAE 0 20 40 60 80 100 ALT increased Dehydration Skin fissures Weight… Hypokalaemia Palmar-plantar… Vomiting Pruritus Decreased… Fatigue Nausea Paronychia Stomatitis Diarrhoea Rash All grade events Grade 3 events 0 20 40 60 80 100 0 20 40 60 80 100 0 20 40 60 80 100 0 20 40 60 80 100 0 20 40 60 80 100 No Grade 4 AEs were reported • No dose limiting toxicity (DLTs) were observed at ≤200 mg • Maximum tolerated dose is 300mg QD Dose reductions: 1 (8%) pt at 200 mg QD; 5 (33%) pts at 300 mg QD Data from Poster at EORTC/AACR/NCI International Conference on Molecular Targets and Cancer Therapeutics October 2023

16 Data from Poster at EORTC/AACR/NCI International Conference on Molecular Targets and Cancer Therapeutics October 2023 **IC 50 of EGFR alterations in GBM is average IC 50 of most prevalent EGFR mutations tested in BaF3 cells *IC 50 of EGFR C797S is average of IC 50 of Exon19del/C797S and L858R/ C797S mutations tested in Ba/F3 cells BDTX - 1535 Linear Plasma PK Profile Supports Daily Dosing To Achieve 24 - Hour Target Coverage Steady State C1D15 mean ( ± SD) Day 1 C1D1 mean ( ± SD) Mean plasma concentration - time profile of BDTX - 1535 0 4 8 12 16 20 24 1 10 100 Time post-dose (hours) M e a n P l a s m a B D T X - 1 5 3 5 C o n c . ( n g / m L ) 25 mg (n=2) 50 mg (n=7) 100 mg (n=5) 200 mg (n=15) 300 mg (n=11) 400 mg (n=12) 0 4 8 12 16 20 24 1 10 100 Time post-dose (hours) M e a n P l a s m a B D T X - 1 5 3 5 C o n c . ( n g / m L ) 25 mg (n=2) 50 mg (n=6) 100 mg (n=4) 200 mg (n=12) 300 mg (n=11) 400 mg (n=9) IC 50 of wild - type EGFR IC 50 of EGFR C797S in NSCLC* IC 50 of EGFR alterations in GBM ** • Target blockade based on preclinical IC50 was achieved at BDTX - 1535 ≥ 100 mg QD • Exposure was dose proportional with half - life ~15 hours to support daily dosing • Clinical anti - tumor activity observed at ≥ 100 mg QD
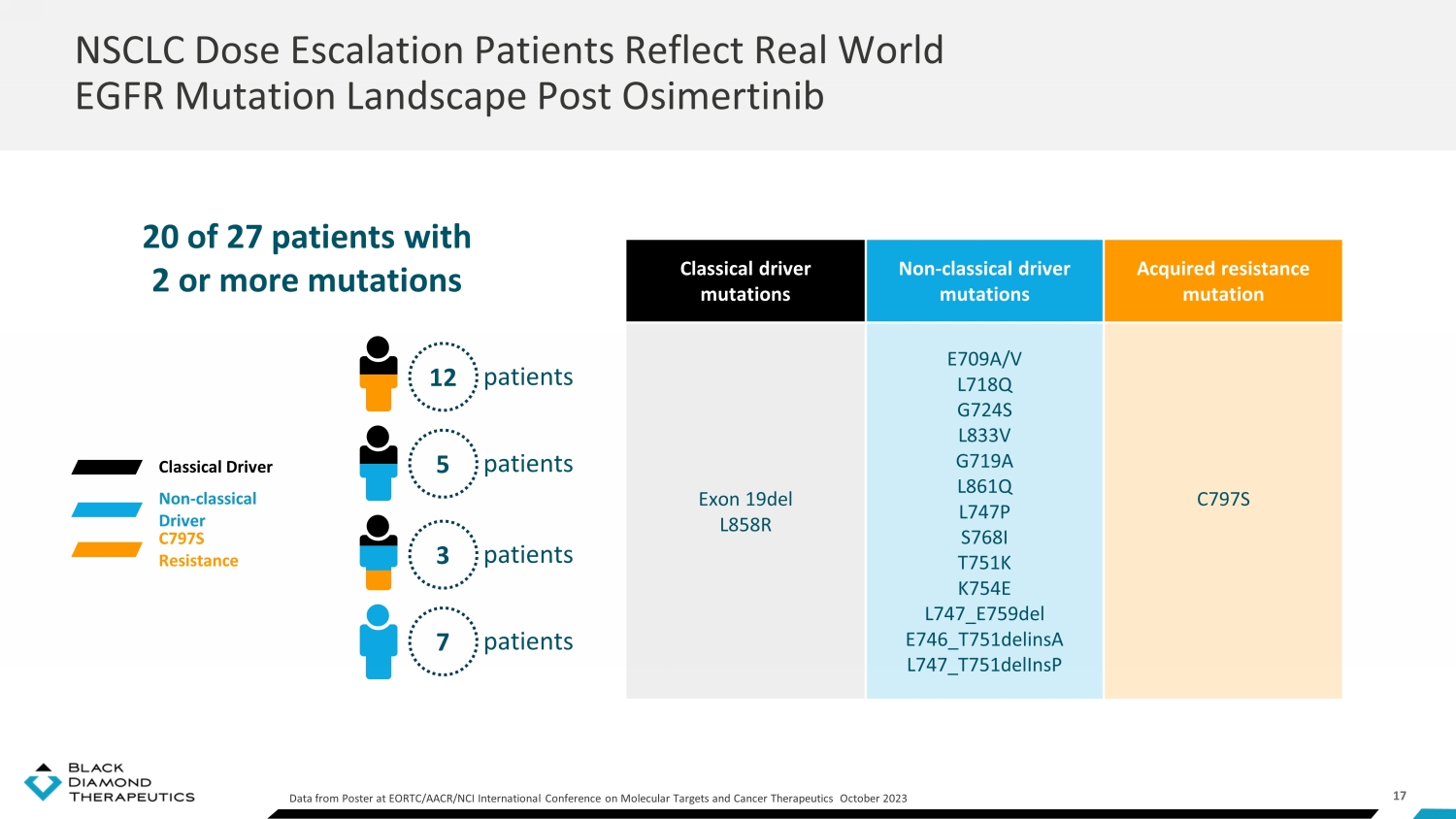
17 NSCLC Dose Escalation Patients Reflect Real World EGFR Mutation Landscape Post Osimertinib Data from Poster at EORTC/AACR/NCI International Conference on Molecular Targets and Cancer Therapeutics October 2023 20 of 27 patients with 2 or more mutations Classical driver mutations Non - classical driver mutations Acquired resistance mutation Exon 19del L858R E709A/V L718Q G724S L833V G719A L861Q L747P S768I T751K K754E L747_E759del E746_T751delinsA L747_T751delInsP C797S Classical Driver Non - classical Driver C797S Resistance patients 12 patients 5 patients 3 patients 7

18 Adapted from Poster at EORTC/AACR/NCI International Conference on Molecular Targets and Cancer Therapeutics October 2023 Efficacy Evaluable Group Patients receiving starting dose <100mg Discontinuation prior to post - baseline tumor assessment Non - measurable disease No tumor assessment at the time of data cut Ineligible EGFR mutation based on retrospective NGS testing Total NSCLC patients enrolled Total NSCLC patients with eligible EGFR mutations (swimmer plot) Response - evaluable patients (waterfall plot) 27 21 13 3 3 2 4 2 BDTX - 1535 Phase 1 Dose Escalation: 13 Response - Evaluable NSCLC Patients

19 On treatment Osi = Osimertinib; Afa = Afatinib; Gefi = Gefitinib; Daco = Dacomitinib; Erlo = Erlotinib; CPI = Checkpoint inhibitor, C = Chemotherapy; # - mutations were absent on confirmatory test; * uPR =unconfirmed partial response - patient had a PR on a post - baseline scan, but a radiologist was unable to confirm a response on a subsequent scan; this patient remains on study treatme nt without evidence of PD. **% SoD was updated to - 50% from prior data release 24July2023 BDTX - 1535 - 101 clinical data extract Data from Poster at EORTC/AACR/NCI International Conference on Molecular Targets and Cancer Therapeutics October 2023 PR = Partial response SD = Stable disease PD = Progressive di sease Best overall response PD SD SD SD SD SD SD PR PR** uPR * PR PR PR -80 -60 -40 -20 0 20 2061 2029 2027 2059 2019 2026 2041 2035 2053 2058 2020 2054 2015 Best % SoD change Radiographical Responses in Efficacy - Evaluable NSCLC Patients Across All Relevant Mutations Assigned dose level, mg QD 300 400 200 200 200 200 400 400 300 200 200 300 100 EGFR mutation (retrospective central testing) Classical L858R Ex19del L858R# Ex19del Ex19del Ex19del Ex19del L858R L858R L858R Non - classical L833V G719A E709V # G724S S768I E709V L747P L718Q Acquired C797S C797S C797S C797S C797S C797S C797S C797S Prior lines of therapy 1 st line Osi Osi Osi Gefi Osi Osi Erlo CPI Osi Osi Osi C Osi 2 nd line Daco , Osi C C CPI, C C Osi Osi+Gefi C CPI/C Osi >2 line CPI, C Afa C BLU - 701 C C Efficacy - Evaluable Patients 5 cPR , 1 uPR of 13 by RECIST Post - Osimertinib Patients 5 cPR , 1 uPR of 11 by RECIST Adapted from Poster at EORTC/AACR/NCI International Conference on Molecular Targets and Cancer Therapeutics October 2023

20 Assigned Dose (QD) Baseline EGFR Mutation Prior Therapy Response Evaluable Classical Non - classical Acquired 1 st 2 nd >2 line 25 mg Exon 19del C797S Osi SD a 50 mg Exon 19del C797S Osi SD b G719A, L861Q CPI, Afa Osi HER3 - ADC NM a , c 100 mg L858R L718Q, C797S Osi PR d L858R Osi C C No b 200 mg Exon 19del C797S Osi CPI, C SD L747P Osi CPI, C C PR c , f Exon 19del C797S Osi SD Exon 19del G724S C797S Osi SD L858R E709V Osi C uPR e L858R E709V Gefi C SD 300 mg Exon 19del C797S Osi Osi + Gefi BLU - 701 PR c L858R C797S C Osi C PR c L858R, L833V C797S Osi Daco , Osi CPI, C PD c 400 mg Exon 19del C797S Osi C No Exon 19del C797S CPI Osi C PR d E746_T751del insA Osi C NM d G719A Osi C Afa SD c L858R E709A, L718Q Osi No S768I Erlo C SD G719A C Afa No 0 10 20 30 40 50 60 70 80 Physician decision AE AE Withdrawal by subject Withdrawal by subject Withdrawal by subject On treatment PR = Partial response SD = Stable disease PD = Progressive di sease a Dose was increased incrementally to 100 mg QD b Dose was increased incrementally to 200 mg QD c Received more than two prior lines of therapy d Dose was reduced to 300 mg QD e patient had a PR on a post - baseline scan, but a radiologist was unable to confirm a response on a subsequent scan; this patient remains on study treatment without PD f Patient had >20% increase in target lesions at cycle 11, however, continues the study treatment mPFS = 5.5 months for HER3 - ADC (HERTHENA - Lung01, Yu, JCO 2023) or chemotherapy ( KEYNOTE - 789 study of TKI - resistant EGFRm metastatic NCSLC; Yang et al., ASCO 2023) Data from Poster at EORTC/AACR/NCI International Conference on Molecular Targets and Cancer Therapeutics October 2023 Weeks on Treatment BDTX - 1535: Emerging Evidence of Durable Tumor Response in NSCLC

CONFIDENTIAL | 21 -100 -80 -60 -40 -20 0 20 % ctDNA change Assigned Dose, mg QD 200 400 50 100 200 200 200 200 200 400 Retrospective EGFR mutation testing at baseline EGFRm not detected Ex19Del, C797S Ex19Del, C797S L858R L748Q C797S E746_S752 delinsV, C797S Ex19Del, C797S L747P Ex19Del, C797S L858R, E709V EGFR E746_T751 delinsA Status of EGFR mutant VAF at C3D1 (central testing) Classical EGFRm not detected Absent Absent @C5D1 - 58% - 96% Absent Absent Absent C797S Absent Absent @C5D1 Absent Absent Absent Absent Non - Classical Absent Absent Absent Absent ctDNA – circulating tumor DNA; VAF – variant allele frequency; measurable in 10 patients BDTX - 1535 Drives Clearance of Mutant EGFR VAF and Plasma ctDNA 1. Thompson, JC., et al., British Journal of Cancer, 2023 ctDNA = circulating tumor DNA VAF = variant allelic fraction. Eradication of targeted variant alleles and reduction of ctDNA are early predictors of ORR and PFS 1

22 1. L861Q was reported by local testing at screening, but not detected in baseline and post - dose samples by central testing Data from Poster at EORTC/AACR/NCI International Conference on Molecular Targets and Cancer Therapeutics October 2023 ; VAF – variant allelic fraction Clinical Benefit in a Non - Response Evaluable Patient With CNS Disease: Remains on Therapy for > 1 year Absence of EGFR G719A mutant allele in post - baseline ctDNA samples -100 -80 -60 -40 -20 0 % Change in ctDNA VAF at C5D1 from C1D1 Non - Classical Driver G719A Non - Classical Driver L861Q 1 G719A Changes in ctDNA VAF 50 mg 100 mg Treatment duration (weeks) Months From C1D1 - 61 - 54 - 43 - 50 - 37 Carbo + Alimta + Keytruda - 60 - 34 - 5 - 4 - 3 Diagnosis: NSCLC Stage IV Afatinib d/c GI toxicity Brain mets SRS RT Bone and brain mets SRS RT Brain mets SRS RT Osimertinib HER3 ADC D/C Gr 2 pneumonitis Screen Failure QTC>470 RT Cerebellum New brain mets Re - screened QTC 457 Disease History and Prior Therapies - 2 - 1 - 35 BDTX - 1535 Treatment Present Absent Present Absent Present Absent -4 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 Remains on treatment Non - target lesions Non - target Response

23 BDTX - 1535: Phase 2 Trial Enrolling in 1L and 2L/3L NSCLC • 2L/3L patients enrolling at 100 mg QD and 200 mg QD • ORR primary endpoint • Phase 2 data in 2L/3L patients expected Q3 2024 • FDA Fast Track Designation granted for 2L EGFRm/C797S • End of Phase 1 FDA feedback received, 1L cohort initiating Patients with acquired resistance C797S after ≤2 prior lines of therapy with osimertinib as the only prior EGFR TKI Patients with non - classical driver mutations after ≤2 prior lines of therapy with osimertinib as the preferred prior EGFR TKI Cohort 2 n=20 Cohort 1 n=20 Patients with non - classical driver mutations and no prior treatment Cohort 3 n=20 2L/3L 1L

24 BDTX - 1535 Well Positioned Across All Lines of Therapy for EGFRm NSCLC Classical Driver Mutations (L858R, Del19) ~ 85% of patients Non - classical Driver Mutations (G719X, S786I, etc.) ~13% of patients ~32 - 42k 2027 Estimated EGFRm Incidence (G7) ~56 - 74k ~46 - 61k Resectable / Adjuvant Setting Stage I - IIIA Metastatic 1st Line Metastatic 2+ Line BDTX - 1535 2L BDTX - 1535 Chemo Osimertinib Osimertinib (off - label) +/ - Chemo/IO Afatinib (label limited to 3 mutations) Adjuvant Osimertinib BDTX - 1535 Chemo 2L BDTX - 1535 Chemo 2L BDTX - 1535 Adjuvant BDTX - 1535 Ongoing BDTX trial Standard of Care Potential BDTX market References: Kantar Treatment Architecture; Global Data; Data Monitor Pharma Intelligence; SEER/ NCi data; Zhang et al., Oncotarget 2016

25 BDTX - 1535: Opportunity in Glioblastoma

26 1. Saadeh, F., et al., The International Journal of Biological Markers, 2018 . 2. Real world evidence data from Tempus Labs a nal ysis of 2,540 GBM patient samples Treatment of EGFR - Driven GBM Requires Inhibition of Complex EGFR Mutations: Potent Preclinical Inhibition by BDTX - 1535 Of all ECD - driven tumors were complex, containing 2 or more ECD mutations Of complex ECD - driven tumors were highly heterogeneous, with 3 or more ECD mutations 12% 2 40% 2 32% 2 52% 1 Of all GBM samples had EGFR extracellular - domain (ECD) mutations, according to real - world evidence Of all GBM samples carry oncogenic EGFR alterations GBM patients in the US are diagnosed each year with EGFR mutations that have been shown in preclinical studies to be inhibited by BDTX - 1535 ~7,000

27 BDTX - 1535 Demonstrates Potent Preclinical Inhibition of Oncogenic GBM EGFR Alterations vs. Osimertinib and Superior Brain Exposure BDTX - 1535 Exhibits Superior Brain Exposure Kpuu (rat) BDTX - 1535 0.51 0.21 Osimertinib Amplification Truncations Variants EGFR - AMP EGFR - AMP EGFR vIII EGFR vII EGFR vVI EGFR - C231F EGFR - C595S EGFR - S645C EGFR - A289V EGFR - WT Amplification Truncations Variants vIII vII vVI C231F C595S S645C A289V 0 25 50 75 100 125 150 200 400 600 800 1000 Anti - proliferative IC50 (nM) EGFR - WT reflects anti - proliferative activity in H292 WT - EGFR expressing cell line. Data for all mutations derived from Ba/F3 tr ansformants. Kp uu Partition Coefficient Calculation: AUC brain:blood x plasma Fu/brain Fu BDTX - 1535 Osimertinib EGFR - WT

28 BDTX - 1535: Potential to Overcome Limitations of Prior Attempts to Drug EGFR in GBM Lessons From Past Failures BDTX - 1535 MOA=mechanism of action; CNS=Central Nervous System; WT=Wild - Type; GBM=Glioblastoma Multiforme; NSCLC=Non - Small Cell Lung Cancer Potent MasterKey inhibition of co - occurring EGFR alterations and amplification Heterogenic expression of EGFR oncogenic alterations within tumors Covalent MOA and no paradoxical activation Paradoxical activation of EGFR GBM oncogenes induced by reversible inhibitors Spares WT - EGFR in normal cells while retaining potent activity against EGFR alterations Poor tolerability driven by on target WT - EGFR activity Designed to be brain penetrant to treat CNS tumors Low brain exposure due to a lack of CNS penetrance

29 1. Schäfer, J. Transl . Med. 2019; 2. Wang, Nat. Genet. 2016; 3. Johnson, Science 2014; 4. López , Acta Neuropathol . 2019; 5. Weller, Neuro - Oncology 2013; 6. Audureau , J. Neurooncol . 2018; EGFR Driver Status Often Evolves During Treatment Fresh biopsy tissue used for testing Up - to - date test results guide treatment Treatment matches tumor alterations Opportunity for BDTX - 1535 in Newly Diagnosed Patients BDTX - 1535 Opportunity in Newly Diagnosed EGFRm GBM Patients GBM Treatment Paradigm Newly Diagnosed Recurrence Temozolomide + Radiation Fresh biopsy EGFR status characterized Tumor evolves with time and treatment 1 - 4 EGFR alteration status may change in up to 40% of cases 1,2 Fresh biopsy not available for majority of recurrent patients 5,6 Treatment not matched to mutational profile BDTX - 1535 Opportunity BDTX - 1535 Ph1 Complete

30 Promising Clinical Activity in Heavily Pre - treated GBM Patients From Dose Escalation Portion of Phase 1 Study x Well tolerated , with favorable plasma PK x Of 22 efficacy evaluable heavily pre - treated GBM patients • 3 patients on therapy longer than 10 months ─ 1 remains on therapy at 15 months (100mg QD), had progressed on TMZ after 3 months • 1 patient on therapy longer than 6 months • 5 patients on therapy longer than 4 months x Of 19 patients with measurable disease assessable by RANO criteria • 1 confirmed partial response (200 mg QD), on therapy > 4 months • 8 patients with stable disease ▪ Recurrent, heavily pre - treated (2L/3L+) ▪ Historical PFS ~ 2 - 4 months ▪ EGFR status not confirmed at dosing RANO: Response Assessment in Neuro - Oncology Dose Escalation
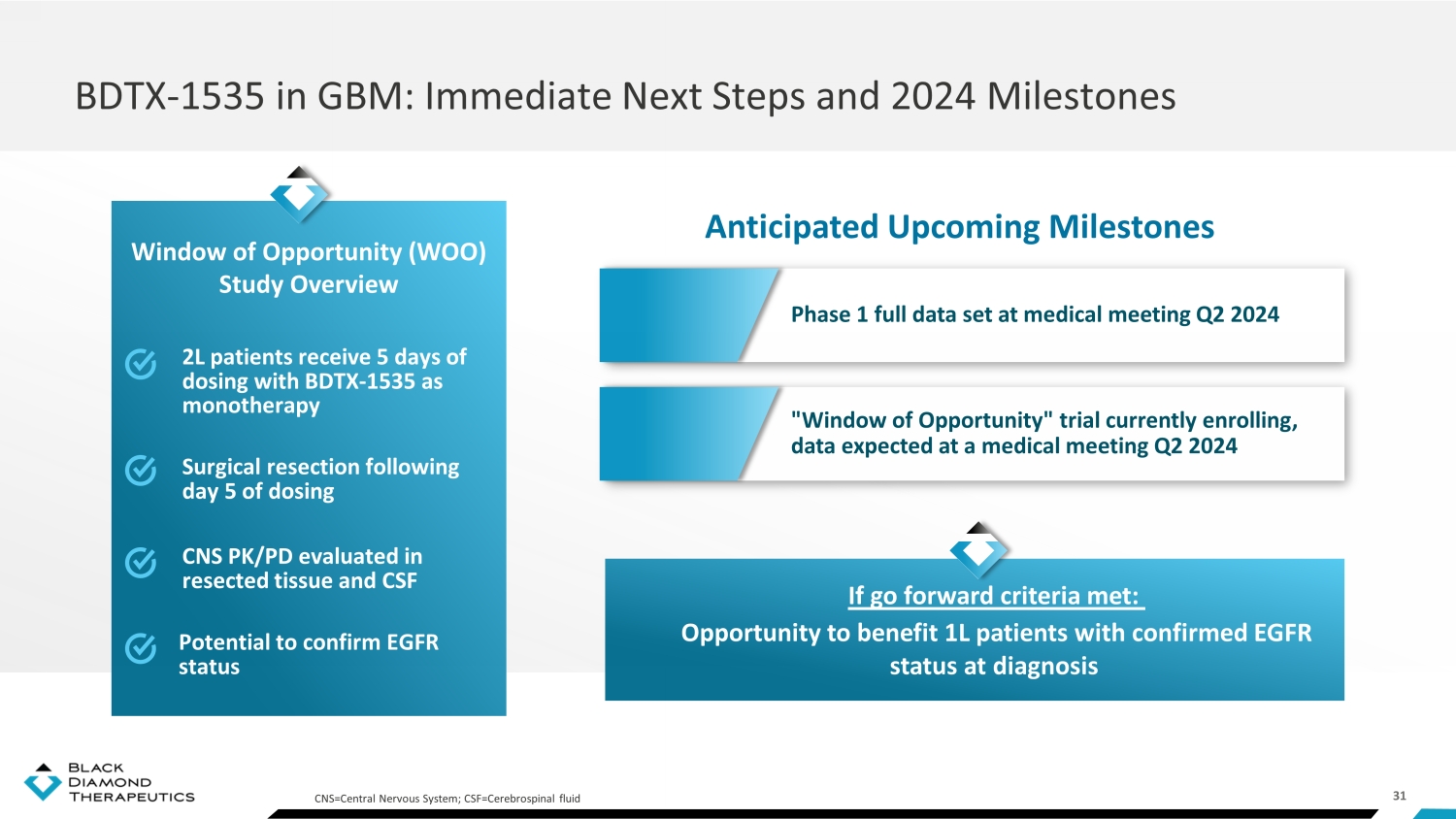
31 BDTX - 1535 in GBM: Immediate Next Steps and 2024 Milestones Anticipated Upcoming Milestones 2L patients receive 5 days of dosing with BDTX - 1535 as monotherapy Surgical resection following day 5 of dosing CNS PK/PD evaluated in resected tissue and CSF Window of Opportunity (WOO) Study Overview CNS=Central Nervous System; CSF=Cerebrospinal fluid Potential to confirm EGFR status Phase 1 full data set at medical meeting Q2 2024 " Window of Opportunity " trial currently enrolling, data expected at a medical meeting Q2 2024 If go forward criteria met: Opportunity to benefit 1L patients with confirmed EGFR status at diagnosis

32 BDTX - 1535 GBM Development Path Designed for Sequential De - Risking 2L patients receive 5 days of dosing with BDTX - 1535 as monotherapy Window of Opportunity (WOO) Study Overview CNS=Central Nervous System; CSF=Cerebrospinal fluid Dose Escalation PK + EGFR Status Registration potential ▪ Recurrent heavily pre - treated (2/3L+) ▪ EGFR alterations are not confirmed (present at initial diagnosis) Confirm safety Confirm brain exposure Confirm 1L efficacy ▪ Recurrent (2L) ▪ Potential to confirm EGFR alterations ▪ Newly diagnosed (1L ) vs. TMZ ▪ EGFR alterations are confirmed Evaluate clinical activity in recurrent settings Phase 1 completed "Window of Opportunity" Randomized Trial BDTX - 1535 GBM Development Path Designed for Sequential De - Risking

33 BDTX - 1535 Summary: NSCLC and GBM Opportunities Robust near - term commercial opportunity in 2L and 1L NSCLC Emerging potential in GBM Differentiated profile F irst and potential best - in - class 4 th gen EGFR TKI Robust clinical POC in a heavily pre - treated Phase 1 NSCLC population • 5 cPR + 1 uPR out of 13 efficacy - evaluable patients • Durable responses and clinical evidence of CNS anti - tumor activity • Well tolerated with manageable (similar to osimertinib ), on - target EGFR TKI AEs • Phase 2 enrolling, data expected in Q3 2024 Clear differentiation against standard of care and emerging treatment options • WT - EGFR sparing with favorable clinical tolerability vs chemo/ADC - based combos • Brain penetrant to address CNS disease • Highly potent against all major, clinically relevant EGFR mutations • Once daily oral administration Real - world evidence 1,2 demonstrates a growing commercial opportunity in NSCLC • 2L: P ost osimertinib – C797S , classical/non - classical drivers and complex mutations • 1L: Non - classical driver mutations • 1L: Post - osimertinib adjuvant therapy Potential in 1L GBM with EGFR alterations (>50% of all newly diagnosed GBM) Leading Position Large Markets Compelling Asset 1. Real world treatment patterns and outcomes in patients with advanced non - small cell lung cancer (NSCLC) post - EGFR tyrosine kinas e inhibitor (TKI) therapy Source: AACR 2023 Poster 2. Rotow, JK., et al., Journal of Thoracic Oncology, 2023.

BDTX - 4933: Potential Best - in - Class Brain - Penetrant RAF MasterKey Inhibitor

35 Potent preclinical inhibition of BRAF, KRAS, and NRAS alterations (“RAF - RAS clamp”) BDTX - 4933 MasterKey BDTX - 4933: Oral, Brain - Penetrant, RAF MasterKey Inhibitor Designed to target oncogenic conformation of RAF and avoid paradoxical activation Unique Pharmacology Designed to be brain penetrant to treat CNS disease Brain Penetrant Phase 1 data expected Q4 2024 Status
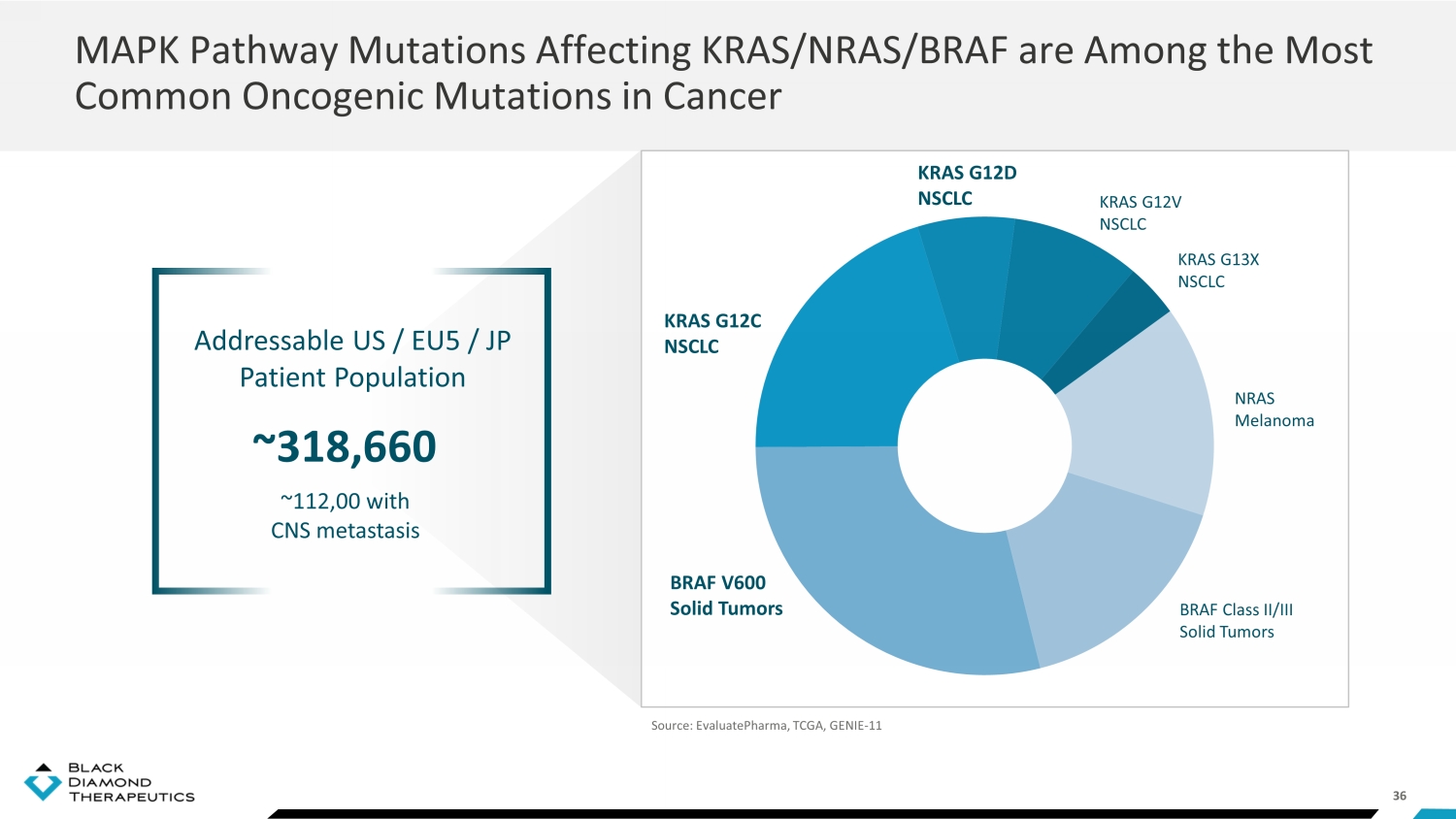
36 MAPK Pathway Mutations Affecting KRAS/NRAS/BRAF are Among the Most Common Oncogenic Mutations in Cancer BRAF Class II/III Solid Tumors BRAF V600 Solid Tumors KRAS G12D NSCLC KRAS G12V NSCLC KRAS G13X NSCLC KRAS G12C NSCLC Source: EvaluatePharma, TCGA, GENIE - 11 NRAS Melanoma Addressable US / EU5 / JP Patient Population ~318,660 ~112,00 with CNS metastasis

37 1. Zhang, W., Cell Res. (2002); 2. Yuan, J., J Hematol Oncol 13, 113 (2020); 3. Yao Z, Cancer Cell (2015); 4. Karoulia Z, Cancer Cell (2016); 5. J. Wang, Pharmacol. Res. 129, 414 – 423 (2018); 6. H. Ellens, Drug Metab. Dispos. 45, 646 – 656 (2017); 7. R. K. Mittapalli, J. Pharmacol. Exp. Ther. 342, 33 – 40 (2012); 8. R. K. Mittapalli, J. Pharmac ol. Exp. Ther. 344, 655 – 664 (2013); 9. Belum VR, Ann Oncol. (2015); 10. Su F., N Engl J Med. (2012); 11. Hatzivassiliou G, Nature. (2010); 12. Poulikakos PI., Nature, (2010) Opportunity for BDTX - 4933 BRAF Alterations Drive Oncogenesis Through Hyperactivation of the RAS - MAP Kinase Pathway: Multiple Opportunities for BDTX - 4933
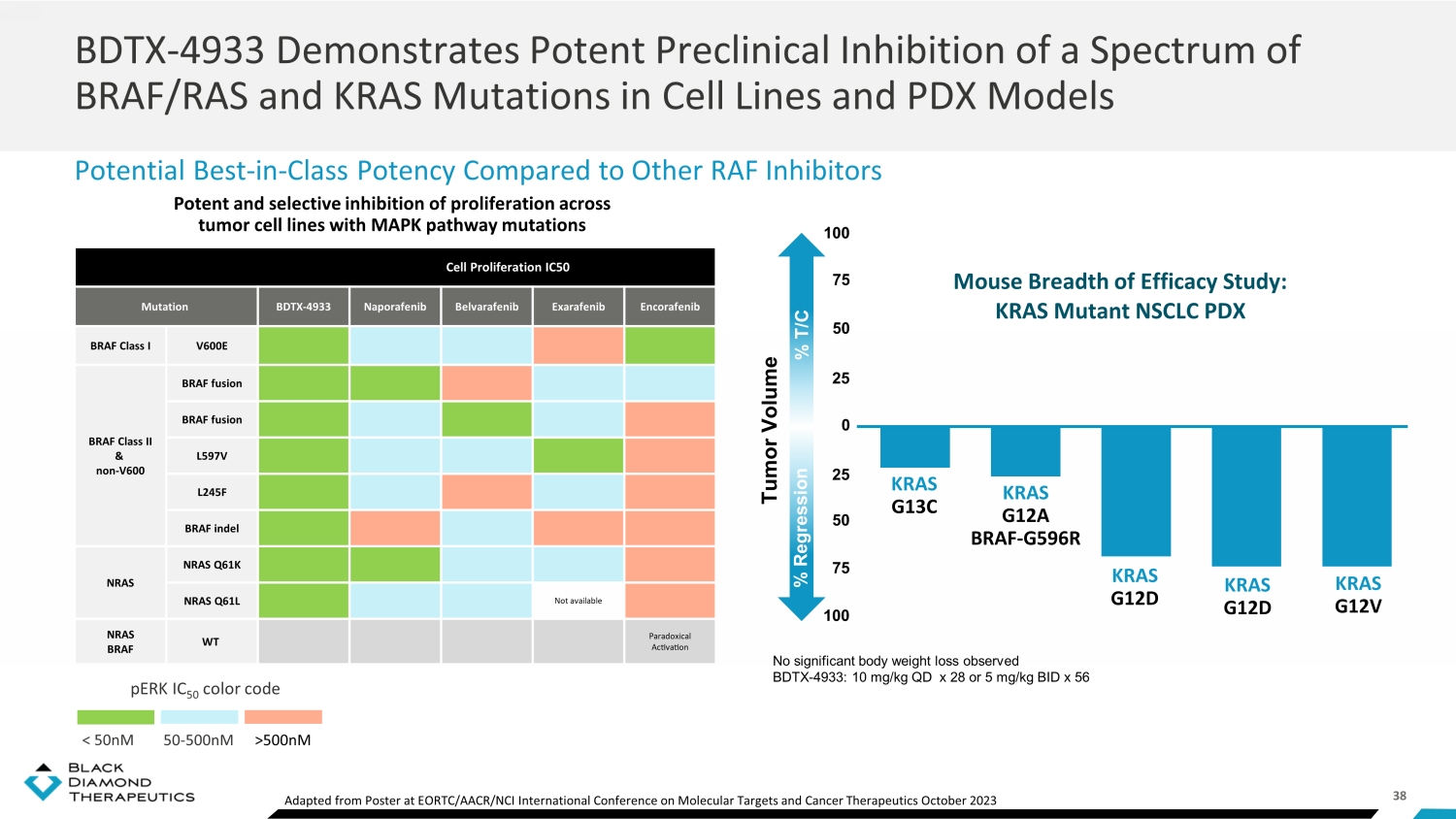
38 Cell Proliferation IC50 Mutation BDTX - 4933 Naporafenib Belvarafenib Exarafenib Encorafenib BRAF Class I V600E BRAF Class II & non - V600 BRAF fusion BRAF fusion L597V L245F BRAF indel NRAS NRAS Q61K NRAS Q61L Not available NRAS BRAF WT Paradoxical Activation Potent and selective inhibition of proliferation across tumor cell lines with MAPK pathway mutations pERK IC 50 color code < 50 nM 50 - 500nM >500nM BDTX - 4933 Demonstrates Potent Preclinical Inhibition of a Spectrum of BRAF/RAS and KRAS Mutations in Cell Lines and PDX Models Potential Best - in - Class Potency Compared to Other RAF Inhibitors No significant body weight loss observed BDTX - 4933: 10 mg/kg QD x 28 or 5 mg/kg BID x 56 Mouse Breadth of Efficacy Study: KRAS Mutant NSCLC PDX % Regression % T/C Tumor Volume 100 100 75 75 50 50 25 25 0 KRAS G13C KRAS G12A BRAF - G596R KRAS G12D KRAS G12D KRAS G12V Adapted from Poster at EORTC/AACR/NCI International Conference on Molecular Targets and Cancer Therapeutics October 2023

39 RP2D • Bayesian optimal interval design • Mutations and alterations confirmed by local tests BDTX - 4933: Focused, Biomarker - Driven Phase 1 Trial Initiated Data Anticipated in Q4 2024 NSCLC with KRAS mutations/BRAF alterations Dose Escalation
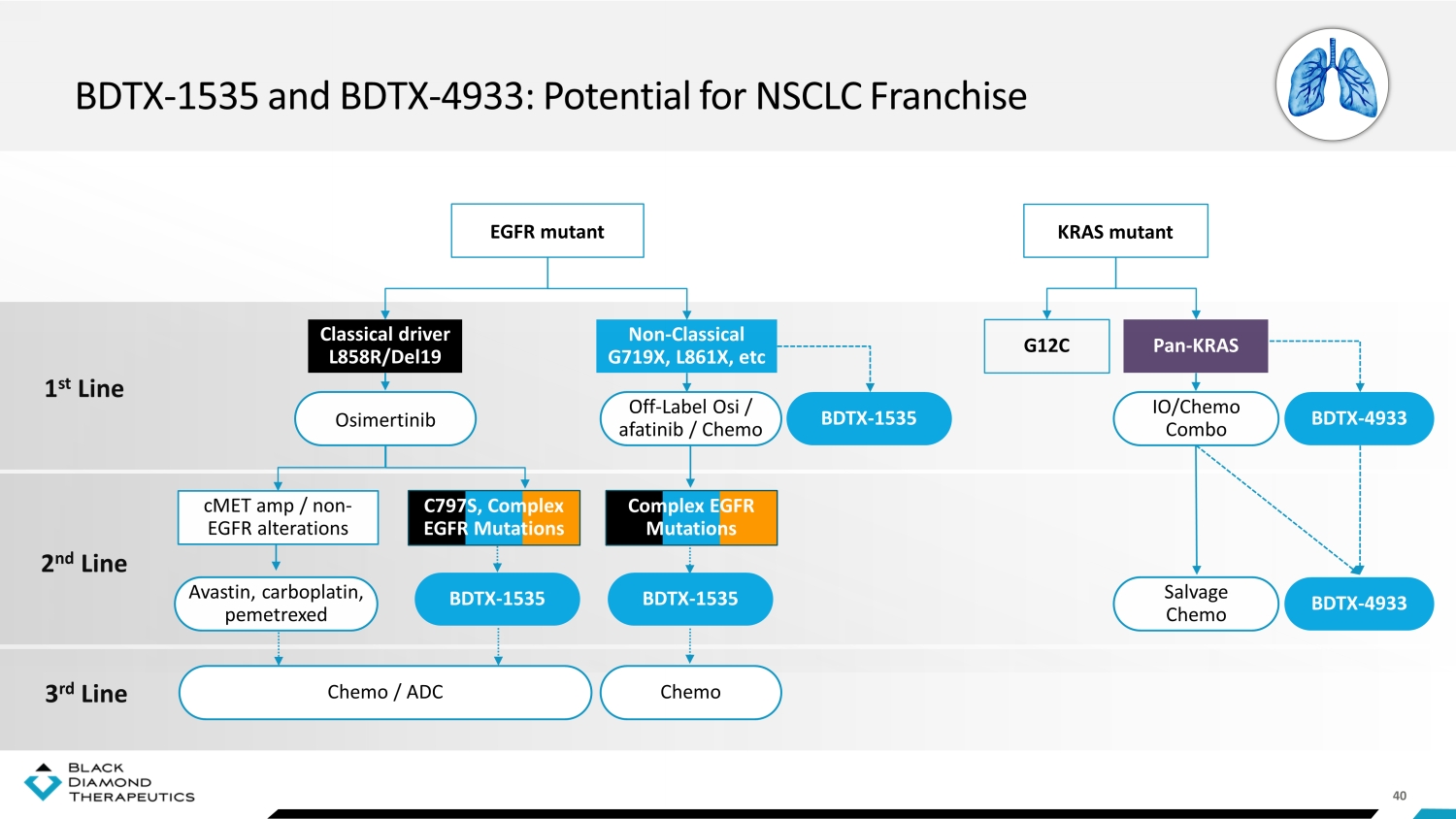
40 BDTX - 1535 and BDTX - 4933: Potential for NSCLC Franchise EGFR mutant 1 st Line 2 nd Line 3 rd Line Pan - KRAS KRAS mutant G12C Non - Classical G719X, L861X, etc Classical driver L858R/Del19 cMET amp / non - EGFR alterations Complex EGFR Mutations C797S, Complex EGFR Mutations Off - Label Osi / afatinib / Chemo BDTX - 1535 IO/Chemo Combo BDTX - 4933 Salvage Chemo BDTX - 4933 BDTX - 1535 BDTX - 1535 Chemo Chemo / ADC Avastin, carboplatin, pemetrexed Osimertinib

41 Anticipated 2024 Key Milestones Q1 Q2 Q3 Q4 Phase 2 initiation in 1L non - classical patients Phase 2 results in 2L/3L patients Potential pivotal trial initiation in 2L patients Potential randomized trial in newly diagnosed patients Phase 1 results BDTX - 1535 EGFRm NSCLC BDTX - 4933 KRASm NSCLC BDTX - 1535 EGFRm GBM Phase 1 results and “window of opportunity” data at medical meeting Financial Summary as of September 30, 2023 Cash runway into Q2 2025 $144.4m

Thank You Partnership: partnership@bdtx.com Investors: investors@bdtx.com Media: media@bdtx.com
v3.23.4
Cover
|
Jan. 04, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 04, 2024
|
| Entity File Number |
001-39200
|
| Entity Registrant Name |
BLACK DIAMOND THERAPEUTICS, INC.
|
| Entity Central Index Key |
0001701541
|
| Entity Tax Identification Number |
81-4254660
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
One
Main Street, 14th Floor
|
| Entity Address, City or Town |
Cambridge
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02141
|
| City Area Code |
617
|
| Local Phone Number |
252-0848
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.0001 par value per share
|
| Trading Symbol |
BDTX
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Black Diamond Therapeutics (NASDAQ:BDTX)
Historical Stock Chart
From Mar 2024 to Apr 2024
Black Diamond Therapeutics (NASDAQ:BDTX)
Historical Stock Chart
From Apr 2023 to Apr 2024