
Innovation Series 2023 November 7, 2023 9:00 AM – 1:00 PM ET

1 Welcome & Introductory Remarks Ryan Richardson Chief Strategy Officer

This Slide Presentation Includes Forward-Looking Statements 3 This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: BioNTech's expected revenues and net profit related to sales of BioNTech's COVID-19 vaccine, referred to as COMIRNATY® where approved for use under full or conditional marketing authorization, in territories controlled by BioNTech's collaboration partners, particularly for those figures that are derived from preliminary estimates provided by BioNTech's partners; the rate and degree of market acceptance of BioNTech's COVID-19 vaccine and, if approved, BioNTech's investigational medicines; expectations regarding anticipated changes in COVID-19 vaccine demand, including changes to the ordering environment, seasonality and expected regulatory recommendations to adapt vaccines to address new variants or sublineages; the initiation, timing, progress, results, and cost of BioNTech's research and development programs, including those relating to additional formulations of BioNTech's COVID-19 vaccine, and BioNTech's current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work and the availability of results; our expectations with respect to our intellectual property; the impact of the Company’s collaboration and licensing agreements; the development of sustainable vaccine production and supply solutions and the nature and feasibility of these solutions; and BioNTech's estimates of commercial and other revenues, cost of sales, research and development expenses, sales and marketing expenses, general and administrative expenses, capital expenditures, income taxes, net profit, cash, cash equivalents and security investments, shares outstanding and cash outflows and share consideration. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this presentation are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond BioNTech’s control, and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to: BioNTech's pricing and coverage negotiations with governmental authorities, private health insurers and other third-party payors after BioNTech's initial sales to national governments; the future commercial demand and medical need for initial or booster doses of a COVID-19 vaccine; competition from other COVID-19 vaccines or related to BioNTech's other product candidates, including those with different mechanisms of action and different manufacturing and distribution constraints, on the basis of, among other things, efficacy, cost, convenience of storage and distribution, breadth of approved use, side-effect profile and durability of immune response; the timing of and BioNTech's ability to obtain and maintain regulatory approval for BioNTech's product candidates; the ability of BioNTech’s COVID-19 vaccines to prevent COVID-19 caused by emerging virus variants; BioNTech's and its counterparties’ ability to manage and source necessary energy resources; BioNTech's ability to identify research opportunities and discover and develop investigational medicines; the ability and willingness of BioNTech's third-party collaborators to continue research and development activities relating to BioNTech's development candidates and investigational medicines; the impact of the COVID-19 pandemic on BioNTech's development programs, supply chain, collaborators and financial performance; unforeseen safety issues and claims for potential personal injury or death arising from the use of BioNTech's COVID-19 vaccine and other products and product candidates developed or manufactured by BioNTech; BioNTech's and its collaborators’ ability to commercialize and market BioNTech's COVID-19 vaccine and, if approved, its product candidates; BioNTech's ability to manage its development and expansion; regulatory developments in the United States and other countries; BioNTech's ability to effectively scale BioNTech's production capabilities and manufacture BioNTech's products, including BioNTech's target COVID-19 vaccine production levels, and BioNTech's product candidates; risks relating to the global financial system and markets; and other factors not known to BioNTech at this time. You should review the risks and uncertainties described under the heading “Risk Factors” in BioNTech’s Report on Form 6-K for the period ended September 30, 2023 and in subsequent filings made by BioNTech with the SEC, which are available on the SEC’s website at https://www.sec.gov/. Except as required by law, BioNTech disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation in the event of new information, future developments or otherwise. These forward-looking statements are based on BioNTech’s current expectations and speak only as of the date hereof.

Innovation Series 2023 Agenda 1 Welcome and Introductory Remarks 9:00 AM 2 The BioNTech Approach to Innovation 9:05 AM 3 AI Capabilities and Projects 9:25 AM 4 Our Multi-Platform Oncology Strategy 9:35 AM 5 Our Growth Strategy 10:00 AM Break (10 mins) 6 Novel Backbones: Next-Generation ADCs and Immunomodulators 10:35 AM 7 Solid Tumor Cell Therapy 12:00 AM 8 mRNA Cancer Vaccines 12:15 PM 9 Path to Value Creation 12:30 PM 10 Closing Remarks and Q&A 12:40 PM

Innovation Series 2023 – BioNTech Team 5 Prof. Ugur Sahin, M.D. Chief Executive Officer, Co-founder Karim Beguir Chief Executive Officer, InstaDeep Prof. Ilhan Celik, M.D. Vice President, Clinical Development Prof. Özlem Türeci, M.D. Chief Medical Officer, Co-founder Ryan Richardson Chief Strategy Officer Michael Wenger, M.D. Vice President, Clinical Development

2 The BioNTech Approach to Innovation Prof. Ugur Sahin, M.D. CEO and Co-founder

We Made History 7 1. Nature 589, 16-18 (2021); 2. Measured by sales recorded for a single product in a single year (>$40 billion combined of direct sales recorded by Pfizer or BioNTech in both 2021 and 2022); 3. Cumulative doses shipped in the years 2021 and 2022. 4. COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet. 2022. >4 billion doses of BNT162b2 shipped >170 countries and territories3 Millions of cases of severe illness or death likely averted Trillions of dollars of global economic impact4 The fastest vaccine development in the history of medicine1 The strongest launch of any pharmaceutical product2 Saved lives and impacted the global economy

Innovation at scale A Global Immunotherapy Leader 1. As of October 1, 2023; 2. As of October 24, 2023. Healthcare and social responsibility Innovative and diversified pipeline Leadership in COVID-19 vaccines development Building and expanding a long- term and successful COVID-19 franchise Developing an innovative pipeline with a focus on oncology and infectious disease Aiming to establish a dedicated multi-product oncology company Contributing to democratizing access to novel medicines around the globe 8 >60%1 market share 112 ongoing phase 2 and 3 trials 40%1 of doses delivered to low- and middle-income countries in 2023 >5,7001 employees globally

BioNTech Today 1. As of 31 October, 2023. R&D = research and development; HQ = headquarters United Kingdom London Rwanda Kigali BioNTainer (under construction) Austria Vienna Germany (HQ + 6 sites) Commercial- & clinical-scale mRNA Clinical-scale cell therapy Singapore mRNA commercial manufacturing U.S. Cambridge Gaithersburg Clinical-scale cell therapy China Shanghai Turkey Istanbul BioNTech locations 9 Australia Victoria BioNTainer (planned) >80 different nationalities 36 average age >50 % are female >5,7001 professionals globally Founded in 2008 InstaDeep offices

Cell types Harnessing the Full Power of the Immune System to Fight Human Diseases 10 Macrophage NK cell Dendritic cell Cell migration Removal of diseased cells Healing Cell-cell communication B-cellT-cell Function Cancer Autoimmune diseases Neurodegenerative diseases Cardiovascular disease Infectious diseases Inflammatory diseases Diseases The human immune system plays a central role in >80% of human diseases Ability to kill targeted cells or pathogens with high precision Hundreds of billions of cells Impacts the function of every organ system in the body Potential for long-term memory NK cell = natural killer cell

Focused on Five Innovation Pillars 11 Deep understanding of the immune system Multi-platform innovation engine Manufacturing and automation Target discovery and characterization Digital & AI/ML AI = artificial intelligence; ML = machine learning.

Multi-Technology Innovation Engine 12 1. mRNA encoded cancer-targeting antibodies and cytokines. CAR = chimeric antigen receptor; TLR = toll-like receptor; TCR = T cell receptor; Abs = antibodies; STING = stimulator of interferon genes. Individualized therapiesEnabling technology ADCs * SELECTIVE TLR-7 AGONISM TARGETED CANCER THERAPIES RIBOLYSIN Precision antibacterials OFF-THE- SHELF mRNA CANCER VACCINES FixVac INFECTIOUS DISEASES VACCINES Prophylactic and therapeutic vaccines INDIVIDUA- LIZED mRNA CANCER VACCINES iNeST INDIVIDUALIZED TCR-THERAPY INDIVIDUALIZED EX VIVO T CELL THERAPY SOLID TUMOR CAR-T Ideal CAR-T- cell targets MULTI- TARGET TCR CARVac mRNA vaccine boosted CAR-T-cells STING AGONISTS LIPID FORMULATIONS IMMUNO- THERAPY TARGET DISCOVERY SMALL MOLECULES NEXT-GEN IMMUNO- MODULATORS Mono and bispecific Abs RIBO- LOGICALS1 RiboCytokines RiboMabs mRNA ENCODED HUMABODIES PROTEIN- BASED THERAPIES CELL & GENE THERAPIES mRNA TECHNOLOGY Multi-technology-driven approach rooted in deep fundamental understanding of biology, immunology and medical need Build novel platforms with the ability to produce multiple product candidates Open up new combination opportunities which leverage synergistic modes of action Enable and accelerate individualization of treatment Leverage AI-powered drug discovery, design and development Core principles of our technology strategy Drug class Program Artificial Intelligence, Machine Learning and Computational Medicine Internal capabilities including InstaDeep New product candidates added in 2023

mRNA 2023: A Broad Technology Toolbox 13 Holtkamp et al. Blood 2006; Kuhn et al. Gene Therapy 2010; Sahin, Türeci & Kariko Nat Drug Discovery 2014; Vogel et al. Mol Therapy 2018; Beissert et al. Mol Therapy 2020. mRNA formats Local Tissue-specific Systemic Lipoplex nanoparticles Lipid nanoparticles Polymer nanoparticles Uridine mRNA Pseudo-uridine mRNA Self-amplifying mRNA Trans-amplifying mRNA Nanoparticles Vaccines (e.g. cancer, inf. disease, autoimmunity) Antibodies (e.g. receptor blockade) Signaling molecules (e.g. cytokine) Enzymes (e.g. CRISPR/CAS) Transcription factors (e.g. Yamanaka factors) Encoded drugs A30-L-A70Cap UTRORFUTR A30-L-A70Cap UTRORFUTR vUTR A30-L-A70Cap AntigenSGPORFvUTR A30-L-A70Cap UTRReplicaseUTR vUTR A30-L-A70Cap ORFvUTR Circular RNA, chemically synthesized mRNA Multimodal optimization of mRNA potency and performance over decades (> 10,000x)

Our Innovation Approach To Manufacturing Challenges 14 GMP = Good Manufacturing Practice BioNTech Manufacturing Facility in Marburg Annual has manufactured mRNA drug substance for 1.6 billion doses Delivery at Large Scale Tailoring & Customization Democratizing access to novel technologies Digitized manufacturing of individualized mRNA vaccines Turnaround time 4-6 week BioNTainer: Mobile GMP manufacturing units

AI’s Unprecedented Impact on Science and Medicine 15 The New England journal of medicine vol. 388,13 (2023): 1201-1208. Nature 605, 551–560 (2022). Nature 596, 583–589 (2021). AI, artificial intelligence; ML, machine learning; LLMs, large language models; AGI, artificial general intelligence. Improvements in the ability to process data over 50 years, allows machine learning to progress, and expected to continuously improve Prediction of protein structure is near experimental accuracy by AlphaFold2. De novo protein design solutions introduced AGI is expected to impact medical education and clinical inquiry, beyond public health and hospital operations Speed up clinical trials through more efficient recruitment and matching of study participants and more comprehensive analyses of the data Create synthetic control groups by matching historical data to target trial enrollment criteria Accelerate drug discovery including de novo molecular design and optimization and structure-based drug design AlphaFold2 – structure prediction Rosetta - de novo protein design Increasing storage capacity Improving speed Advances in Computing Power & Algorithms AGI expected to arrive in 2024 – 2029 Bioapplication supported by Data Explosion Leap in LLMs/ Reinforcement Learning

Our Goals for AI 16 AI = artificial intelligence; ML = machine learning; mRNA = messenger ribonucleic acid; TCR = T cell receptor-engineered; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. Optimize mRNA structure and function SARS-CoV-2 variant monitoring and immunogen design Customized and synthetic endolysins for infectious diseases Discovery and de novo design of TCR Discovery and optimized design of antibodies Fully scalable, automated engineering of RiboCytokines And RiboMabs Protein design Lead structure Super-fast genome analysis Individualized mutanome analysis Neoantigen prediction Automated and digitalized manufacturing Powered by data and cutting-edge AI & ML technologies 1. 2. 3. 4. AI enabled drug and target discovery Personalized medicines

Accelerate and Enhance BioNTech’s AI Vision 17 AI = artificial intelligence; ML = machine learning. Fully leverage the power of computational science & AI Implementation strategy • Provide high-quality designs to develop next-generation products with a more efficacious or safer profile • Speed up workflows to develop novel therapeutics & vaccine product candidates • Scale up our capability by fully digitalized automation throughout the whole drug discovery, e.g., high-throughput sequencing, target identification, candidate design and optimization, clinical development and manufacturing & Successful collaboration over past three years Define high priority projects Ensure close teamwork at project level Keep integrity of InstaDeep

3 AI Capabilities and Projects Karim Beguir CEO, InstaDeep

Our AI Capabilities 19 From AI researchers to ML engineers and ML Ops experts, our team has critical size, depth, and a differentiated ability to attract talents in EMEA. Our proprietary GPU cluster in the UK (500 petaflops expected 2024), is optimized for high performance computing and fully managed by our Aichor software platform. Strong contributor to major AI conferences (NeurIPS, ICLR etc.), workshops and journals. 25 publications in 2023, in ML for Biology and AI Decision-Making. Proprietary high-efficiency libraries for advanced Large Language Model (LLM) training, supporting R&D efforts and biology- focused generative AI. Distributed, scalable reinforcement Learning (RL) and combinatorial optimization algorithms. 5 reference JAX frameworks released. Pioneer in Quantum Machine Learning incl. publications in Nature journals, collaborations (NPL, Cambridge, IBM) and commercial partnerships. Converting technology powered by our AI innovation into user-friendly, scalable software products integrated with our compute infrastructure and the Cloud. Physically realistic representations of complex environments, optimized for speed, including GPU-accelerated Molecular Dynamics in biology. 300+ AI Experts Supercomputing Assets AI Research Capabilities Frontier LLMs Large Scale Optimization Quantum Machine Learning Software Productization Simulation Expertise AI = artificial intelligence; ML = machine learning; EMEA = Europe, Middle East, India & Africa; GPU = Graphics Processing Unit; NeurIPS = Neural Information Processing System; ICLR = International Conference on Learning Representations; NPL = National Physical Laboratory.

End-to-End Therapeutics Platform Powered by AI 20 We apply our AI capabilities at the forefront of the design of potential cancer therapies and infectious disease vaccines Crossfunctional learnings across the research themes are shared Target identification mRNA optimization Gene synthesis Functional validation Developing LLMs for RNA translation prediction 36% success rate improvement on oligonucleotide assembly More than 8x speedup on immune response detection Enhanced neoepitope selection algorithms in terms of positive predictive value (PPV) BioNDeep Developing state-of-the-art foundational large language models for DNA AI-designed vaccines competitive with human expert designs Immune recognition modelling in sequence and structure space Synergistic approach designed to improve BioNTech's personalized immunotherapy platform AI = artificial intelligence; LLM = large language model.

Gene Synthesis 21 1. Results from April 2022 internal evaluation; data on file. PCR = polymerase chain reaction; AI = artificial intelligence. DNA is the language of biology, and the starting material for a huge range of bioproducts. Creation of long DNA molecules is complex. Assembly PCR builds complete molecules from carefully designed fragments. However, failure is common and costly. Our AI optimization algorithms improve the success rate of this process by 36 absolute percentage points over the industry standard. Our innovation has been embedded into a software platform that unlocks BioNTech’s capacity for large scale experiments, reducing failure rates by ~5x and increasing successful design throughput by 68 percentage points over the same hardware. Designed DNA DNA fragments Designed DNA Oncology Immunology Protein replacement therapiesIntuitive software platform Synthesis success rate1 BioNTech Industry standard

Functional Validation 22 Data on file. AI = artificial intelligence; EDA = electronic design automation. The ELISpot project streamlines the categorization of experimental results by classifying them into one of three distinct outcomes: those showing no immune response, those exhibiting a positive immune response, and those that are not evaluable. We built an AI product to offer a superior and reliable alternative to traditional manual labeling methods, enhancing accuracy and efficiency of ELISpot assessments. Overall process optimization: ● AI evaluates 97% of experiments, leaving only 3% for experts to review Efficiency improvements: ● Manual process: 8x faster within the ELISpot app ● Full AI automation: 40x faster AI classification accuracy: ● Our AI product: 98% ● Human-level performance: 90% ● Previous tool: 73% Time to evaluate a batch of experiments [hr] AI-powered platform for ELISpot experiments classification Accuracy

Nucleotide Transformer: State-of-the-Art LLM for DNA 23 Dalla-Torre et al. 2023, https://doi.org/10.1101/2023.01.11.523679 LLM = large language model. The Nucleotide Transformer is our collection of language models tailored for DNA developed in collaboration with TUM and Nvidia. The models have been trained on reference genomes from more than 850 species at large scale and are currently the state-of-the-art LLM for genomics. They have been evaluated against many competitors on a large range of tasks including splice site prediction, enhancer activity prediction and epigenetic marks predictions. Comparison to DeepSTARR Stark lab, Nature Genetics Comparison to other LLMs for genomics Enformer, DeepMind, Nature Methods HyenaDNA, Stanford, NeurIPS Comparison to SpliceAI Illumina, Cell Landscape of the tasks performed by the nucleotide transformer from chromatin accessibility, to splice site detection and deleteriousness prediction

4 Our Multi- Platform Oncology Strategy Prof. Ugur Sahin, M.D. CEO and Co-founder

Intraindividual variability & intratumoral heterogeneity driving evasion and secondary resistance mechanism Root Cause of Cancer Treatment Failure Cancer cells Genetically diverse & adaptable5-20 Years – up to 10,000 mutations Mutations DNA Mutation Healthy Cell Mutations Mutations Mutations Mutations Individual patients 25

Our Oncology Strategy 26 Strategy Portfolio strategy covering compound classes with synergistic mechanism of actions • Immunomodulators • Targeted therapies • Personalized mRNA vaccines Programs across a wide range of solid tumors and stages of treatment Programs with first-in-class and / or best-in-class potential Unique therapeutic combinations Vision Address the continuum of cancer treatment Bring novel therapies to cancer patients and establish new treatment paradigms Open up novel options to combine platforms and therapies

Towards a Potentially Curative Approach to Cancer: Differentiated Combinations of Multiplatform Assets Space for curative approaches Immunomodulators Novel checkpoint inhibitors cytokines, immune agonists mRNA vaccines Targeted therapy ADCs, CAR-T, TCR-T, Small molecules SynergySynergy Synergy Immunomodulators • We built a modality agnostic armamentarium to focus on the most relevant and crucial IO pathways • Targeting different but complementary players in the complex cancer immunity cycle to promote a thorough and durable anti-tumoral effect mRNA cancer vaccines • Eliminate polyclonal residual disease with individualized vaccines for potential long-term impact • Polyspecific activity by targeting multiple antigens at once Targeted therapy • Potent and precise therapies to rapidly reduce tumor burden • Efficacy across the entire disease continuum including late lines 27 CAR = chimeric antigen receptor; ADC = antibody-drug conjugate; IO = immuno-oncology; TCR-T = T-cell receptor engineered T cell.

A CD27 antibody based on the HexaBody technology, specifically engineered to form an antibody hexamer upon binding its target on T cell membranes. BNT313/ GEN10531 Clinical status • Ph1/2 in multiple solid tumors Monospecific antibody with optimized Fc targeting CTLA-4 and selectively depleting tumor-infiltrating Tregs in the TME but not in the periphery due to a pH driven mechanism. BNT316/ ONC-3922 (gotistobart) Clinical status • Ph1/2 in multiple solid tumors • Ph2 in PROC • Ph3 in 2L+ mNSCLC Bispecific antibody to inhibit proliferation of PD1-positive cells. 4-1BB enhances T cell proliferation, T cell effector functions and prevents T cell death. BNT311/ GEN10461 Clinical status • Ph1/2 in multiple solid tumors • Ph2 in mNSCLC • Ph2 in 2L mEC Engagement of CD40 leads to activation and maturation of APCs. 4-1BB enhances T cell proliferation, T cell effector functions and prevents T cell death. BNT312/ GEN10421 Clinical status • Ph1/2 trials in multiple solid tumors Bispecific antibody designed to boost antitumor immune response through EpCAM-dependent 4-1BB agonistic activity. BNT314/ GEN10591 Clinical status • Ph1/2 in multiple solid tumors planned 1. Partnered with Genmab; 2. Partnered with OncoC4; 3. Partnered with Biotheus. CTLA4 = Cytotoxic T-Lymphocyte-Associated Protein 4; CD27, CD40, 4-1BB = members of the tumor necrosis factor receptor superfamily; PD-1 =Programmed cell death protein 1; HER2 = human epidermal growth factor receptor 2; ADCC = Antibody dependent cell-mediated cytotoxicity; ADCP = Antibody dependent cellular phagocytosis; PROC = platinum-resistant ovarian cancer; NSCLC = non-small cell lung cancer; EC = endometrial cancer APC = antigen presenting cells; VEGF = vascular endothelial growth factor; TME = tumor microenvironment; CTx = chemotherapy; LALA = IgG1 variant L234A/L235A. PD-L1 expression or upregulation in tumors may enrich VEGF neutralization into the TME which inhibits angiogenesis. PM80023 Clinical status • Ph1b dose escalation • Ph2a as monotherapy in multiple cancers • Ph2 in combination with CTx in multiple cancers Well-Positioned in Immuno-Oncology with Therapeutic Candidates Across Multiple Tumors Anti-CD27Anti-CTLA4 Anti-4-1BBAnti-PD-L1 Anti-4-1BBAnti CD40 Anti-4-1BBEpCAM Anti-VEGF A Anti-PD-L1 VHH Inert Fc (LALA) Optimized Fc 28

ADCs: The Next Wave of Transformation in Oncology 29 ASCO 2022 Trastuzumab Deruxtecan vs. Chemotherapy, N Engl J Med 2022;387:9-20; Enfortumab Vedotin, + Pembrolizumab vs. Chemotherapy; Powles TB, et al. EV-302/KEYNOTE-A39: Open-label, randomized phase 3 study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC), ESMO Congress 2023. ADC = antibody-drug conjugate; EV = enfortumab vedotin, IO = immuno-oncology. ESMO 2023 standing ovation for EV-302, urothelial cancer ASCO 2022 standing ovation for T-Dxd (Destiny Breast-04), breast cancer ADCs are expected to replace chemotherapy ADC + IO are expected to become a new standard ADC development is practice-changing in oncology Overall survival Risk of death was reduced by 53% in patients who received EV + Pembrolizumab N mOS (95% CI), months Trastuzumab Deruxtecan 373 23.4m (20.0-24.8) Physician‘s Choice 184 16.8 (14.5-20.0) Overall survival Risk of death was reduced by 36% in patients who received Trastuzumab Deruxtecan Hazard ratio for death, 0.64 (95% CI, 0.49- 0.84), p = 0.001) Hazard ratio for death, 0.47 (95% CI, 0.38- 0.58), p < 0.00001) N mOS (95% CI), months EV+P 442 31.5 (25.4-NR) Chemotherapy 444 16.1 (13.9-18.3)

30 ADCs: The Innovation Cycle is Just Beginning Distinguished ADC linker technology • Stability improving safety profile • Higher efficacy • Novel mechanisms of actions • Tumor specific activation • Improved and novel payloads Novel targets and novel epitopes • Targeting broader spectrum of tumors • Higher specificity BioNTech plans to develop ADCs against novel targets Linker • Conjugates the payload to the antibody Antibody • Binds to a specific antigen on the surface of cancer cells Payload • Highly potent cytotoxic compounds BioNTech is driving the development of next-generation ADCs ADC = antibody-drug conjugate. Our deep understanding of ADC targets and immunology distinctively positions us to consolidate and maximize the substantial therapeutic window offered by the next-gen ADC technology

Targeting TROP2, cleavable linker and topoisomerase I inhibitor (P1021) DAR: 4 Targeting B7H3, cleavable linker and topoisomerase I inhibitor (P1021) DAR: 6 Targeting HER3, cleavable linker allows for intracellular and extracellular release of topoisomerase I inhibitor (YL0014) DAR: 8 Targeting HER2, cleavable linker (L101) and topoisomerase I inhibitor (P1003) DAR: 8 Clinical stage ADC Programs 1. Partnered with DualityBio; 2. Partnered with MediLink; The completion of the agreement is subject to customary closing conditions, including clearance under the Hart-Scott-Rodino ("HSR") Antitrust Improvements Act. ADC = antibody-drug conjugates; DAR = drug-to-antibody ratio; HER2/3 = human epidermal growth factor receptor 2/3; TROP2 = trophoblast cell-surface antigen 2; mBC = metastatic breast cancer BNT324/ DB-13111 BNT323/ DB-13031 BNT325/ DB-13051 BNT326/ YL2022 Clinical status • Ph3 in HR+HER2-low mBC • Ph1/2 in multiple solid tumors Clinical status • Ph1/2 in multiple solid tumors Clinical status • Ph1/2 in multiple solid tumors Clinical status • Ph1 in multiple solid tumors HER2 B7H3 TROP2 HER3 31

Towards a Potentially Curative Approach to Cancer: Differentiated Combinations of Multiplatform Assets Space for curative approaches Immunomodulators Novel checkpoint inhibitors cytokines, immune agonists mRNA vaccines Targeted therapy ADCs, CAR-T, TCR-T, Small molecules SynergySynergy Synergy Immunomodulators • We built a modality agnostic armamentarium to focus on the most relevant and crucial IO pathways • Targeting different but complementary players in the complex cancer immunity cycle to promote a thorough and durable anti-tumoral effect mRNA cancer vaccines • Eliminate polyclonal residual disease with individualized vaccines for potential long-term impact • Polyspecific activity by targeting multiple antigens at once Targeted therapy • Potent and precise therapies to rapidly reduce tumor burden • Efficacy across the entire disease continuum including late lines 32 CAR = chimeric antigen receptor; ADC = antibody-drug conjugate; IO = immuno-oncology; TCR-T = T-cell receptor engineered T cell.

mRNA Cancer Vaccines May Become the Next Tangible Transformation in Oncology 33 Individual patient samples (blood and tissue) AI-driven neoantigen prediction On-demand tailored RNA manufacturing Individualized immuno- therapy Mapping of mutations Fixed combination of shared tumor antigens Multi-antigen approach tailored to each indication Neo- antigens Individualized therapy Multiple shared antigens Off-the-shelf therapy Cancer vaccine platforms iNeST1 FixVac individualized Neoantigen-Specific immunoTherapy Fixed Antigen Vaccine ANTIGEN 1 ANTIGEN 2 ANTIGEN 3 ANTIGEN 4 1. iNeST is being developed in collaboration with Genentech, a member of the Roche Group. AI = artificial intelligence.

Potential to Address Numerous Cancer Types Through the Combination of Synergistic Modalities 34 ADC = Antibody-drug conjugate. Multiple combination opportunities Technology toolkitDisclosed phase 2 and 3 indications Cell therapies ADCs Multi-specific immunomodulators mRNA cancer vaccines Non-small lung cancer Melanoma Head and neck cancer Breast cancer Endometrial cancer Colorectal cancer Pancreatic ductal adenocarcinoma Ovarian cancer

Advancing Towards Our Vision . YE = Year end; IND = Investigational new drug. Infectious diseases Globally marketed COVID-19 vaccine franchise Maintain and deepen COVID-19 vaccine leadership Launch multiple oncology products from 2026 onwards Mid-term goalsDriving transformation today Long-term vision Launch next-generation and combination COVID-19 vaccines Approved products across oncology and infectious disease portfolio Cardiovascular diseases Neurodegenerative diseases Autoimmune diseases Oncology Initiating additional registration directed trials YE24 Once in a generation opportunity to potentially transform medicine 35 9 Phase 2 trials 2 Phase 3 trials 8 programs in 9 clinical trials 20 programs in 30 clinical trials Innovation engine producing multiple INDs per year Potential new disease areas

Charting the Course for Tomorrow’s Personalized Precision Medicine 36 AI = Artificial Intelligence. AI & digitally-integrated target & drug discovery and development Individualized treatment platforms to address inter-individual variability Deep genomics & immunology expertise to leverage patient data Automated manufacturing to serve patients on time and globally Tailored on-demand immunotherapies Off-the-shelf drugs Small molecule immunomodulators Antibodies Engineered cell therapies mRNA therapeutics Clinical samples Drug classes Inter- individual variability Antibody-drug conjugates

5 Our Growth Strategy Ryan Richardson Chief Strategy Officer

Our Diversified Model for the Next Phase of Growth 1. Partnered with Pfizer. mRNA = messenger RNA. COVID-191 Immuno-oncology Infectious diseases Drive leadership in COVID-19 vaccine franchise leveraging Pfizer’s global infrastructure Build fully integrated global organization to discover, develop, and commercialize a multi-product portfolio Advance pipeline of innovative mRNA prophylactic and therapeutic vaccine candidates Strategy 38

XBB.1.5-adapted vaccine Effective against multiple variants of concern5 Long-term health consequences Accumulating evidence demonstrates that COVID-19 vaccination reduces long- COVID4 Continuous evolution Ongoing antigenic evolution of SARS-CoV-21,2 Risk remains high For severe COVID-19 in vulnerable populations3 Long-Term Need for Annually Adapted Vaccines Anticipated 39 1. World Health Organization Tracking SARS-CoV-2 variant www.who.int/en/activities/tracking-SARS-CoV-2-variants accessed 30 October 2023; 2. Global Initiative on Sharing All Influenza Data https://gisaid.org/ accessed 30 October 2023; 3. FDA Briefing Document Vaccines and Related Biological Products Advisory Committee Meeting June 15, 2023; 4 Brannock et al, Nature Comm. 2023; 5. Stankov M. V. et al., medRxiv pre-print, 5 October 2023. Annual and/or SEASONAL VACCINATION with variant-adapted vaccines expected for the foreseeable future

Global COVID-19 Vaccine Franchise with Lean Commercial Infrastructure 40 Leveraging partners’ commercial infrastructures for global rollout of Comirnaty Lean commercial organization in Germany and Turkey ~55 person field force in DE ~€45m S&M costs YTD1 Fosun commercial territories1 Pfizer commercial territories BioNTech commercial territories 1. Nine months ended September 30, 2023; 2. Comirnaty is not approved in mainland China. S&M = sales & marketing, YTD = year-to-date. DE = Germany.

Maintained high gross margin Limited sales & marketing expense Reduced R&D expense due to partner cost-sharing Lean Fixed Cost Base of COVID-19 Vaccine Business 41 1. Gross margin average calculated using forecast information for Fully Year 2023 based on assumptions. 2. S&M average calculated using forecast information for Fully Year 2023 based on assumptions. 3. Annual COVID-19 R&D spend as a % of total R&D spend 2021-2023. YTD = year-to-date R&D = Research & Development Average Gross Margin 2021-20231 Average Sales & Marketing expenses 2021-20232 Approximate range of 2021, 2022 and 2023 YTD3 annual COVID-19 R&D spend as a % of total R&D spend >80% ~€60m ~25-45%

COVID-19 Vaccine Market Potential and Mid-term Growth Drivers 42 • Manufacturing base reset to serve endemic market • Shift to commercialization model in some key markets • Expect continued shift to single dose vials and pre-filled syringes • Potential for increased vaccine uptake from combination and next-gen vaccines 2024 2025 Variant adapted vaccines Combination vaccines Next-gen vaccines COVID-19 product franchise expected to remain cash generative

Our Multi-Platform Immuno-Oncology Pipeline Today 43 Phase 1 Phase 1/2 Phase 2 Phase 3 BNT211 (CLDN6) Multiple solid tumors BNT311/GEN10463 (PD-L1x4-1BB) Multiple solid tumors BNT411 (TLR7) Multiple solid tumors BNT312/GEN10423 * (CD40x4-1BB) Multiple solid tumors BNT313/GEN10533 (CD27) Multiple solid tumors BNT316/ONC-392 (gotistobart)4 (CTLA-4) Multiple solid tumors BNT1123 mCRPC & high risk LPC BNT151 (IL-2 variant) Multiple solid tumors BNT142 Multiple CLDN6-pos. adv. solid tumors BNT325/DB-13055 (TROP2) Multiple solid tumors BNT316/ONC-392 (gotistobart)4 (CTLA-4) anti-PD-1/PD-L1 experienced NSCLC BNT323/DB-13035 (HER2) Multiple solid tumors BNT324/DB-13115 (B7H3) Multiple solid tumors BNT323/DB-13035 (HER2) HR+, HER2-low met. breast cancer NEW BNT116 Adv. NSCLC BNT152 + BNT153 (IL-7, IL-2) Multiple solid tumors BNT221 Refractory metastatic melanoma BNT321 (sLea) Metastatic PDAC BNT322/GEN10564 Multiple solid tumors Autogene cevumeran/BNT1221 Multiple solid tumors NEW BNT314//GEN10593 (EpCAMx4-1BB) Multiple solid tumors PLANNED mRNA Antibody Cell therapy Legend Small molecules ADCs BNT326/YL2026 (HER3) Multiple solid tumors NEW BNT311/GEN10463 (PD-L1x4-1BB) R/R met. NSCLC, +/- pembrolizumab BNT1112 aPD(L)1-R/R melanoma, + cemiplimab BNT113 1L rec./met. HPV16+ PDL1+ head and neck cancer, + pembrolizumab Autogene cevumeran/BNT1221 1L adv. melanoma, + pembrolizumab Autogene cevumeran/BNT1221 Adj. ctDNA+ stage II or III CRC BNT1162 1L adv. PD-L1 50% NSCLC, + cemiplimab Autogene cevumeran/BNT1221 Adj. PDAC, + atezolizumab + mFOLFIRINOX NEW BNT316/ONC-392 (gotistobart)4 (CTLA-4) Plat.-R. ovarian cancer, + pembrolizumab BNT316/ONC-392 (gotistobart)4 mCRPC, + radiotherapy PLANNED BNT311/GEN10463 (PD-L1x4-1BB) 2L endometrial cancer, + pembrolizumab NEW 1. Partnered with Genentech, member of Roche Group; 2. Partnered with Regeneron; 3. Partnered with Genmab; 4. Partnered with OncoC4; 5. Partnered with DualityBio; 6. Partnered with MediLink Therapeutics. *Two phase 1/2 clinical trials in patients with solid tumors are ongoing in combination with immune checkpoint inhibitor +/- chemotherapy. NSCLC = non-small cell lung cancer; mCRPC = metastatic castration resistant prostate cancer; LPC = localized prostate cancer; HPV = human papillomavirus; PDAC = pancreatic ductal adenocarcinoma; CRC = colorectal cancer; CLDN = claudin; IL = interleukin; 1L = first line; R/R = relapsed/refractory; HER2/HER3 = human epidermal growth factor 2/3; sLeA = sialyl-Lewis A antigen; TROP2 = tumor-associated calcium transducer 2.

Our Strategy Leverages Partner Organizations and Capabilities 44 Agreements signed in 2023. 1.The total consideration to acquire the remaining InstaDeep shares, excluding the shares already owned by BioNTech, amounts to approximately €500 million in cash, BioNTech shares, and performance-based future milestone payments; AI = artificial intelligence. AcquisitionsR&D funding and partnerships Global strategic partnerships Asset in-licensing and co-development Seven clinical stage product candidates in-licensed in 2023 Cost-sharing and co-development for late-stage assets BioNTech has global commercial rights ex Greater China Funding of up to $90m from CEPI for mRNA vaccine candidates against future outbreaks Eight clinical-stage programs across four partnerships Cost-sharing and co-development across stages Co-commercialize agreements leverage partners’ commercial infrastructure Acquired leading AI company with 300+ bioinformatics and data science workforce for ~€500m1

Translation Active Portfolio Management Approach 45 Access external innovation to accelerate pipeline maturation in a capital-efficient manner Rigorous go/no-go decision-making across all development stages Prioritize lead late-stage programs to accelerate path-to-market Emphasis on demonstration of single agent activity prior to initiation of pivotal trials Plans for at least six programs in 10+ potentially pivotal trials by end of 2024 Seven clinical-stage assets in-licensed this year for ~€500m upfront Our aim is to generate high return on R&D investment Key principles guiding our R&D investments GO NO R&D = Research & Development

Select Oncology Programs to Fuel Our Next Stage of Growth 46 1. Partnered with Genentech, member of Roche Group; 2. Partnered with OncoC4; 3. Partnered with DualityBio; 4. Partnered with Genmab. MoA = mode of action; CTLA-4 = cytotoxic T-lymphocyte-associated protein 4; HER2 = human epidermal growth factor 2; PD1 = programmed cell death protein 1; CD = cluster of differentiation; CLDN6 = claudin 6; CRC = colorectal cancer; PDAC = pancreatic ductal adenocarcinoma; NSCLC = non-small cell lung cancer; R/R = relapsed/recurrent; HR = hormone receptor; adj. = adjuvant; adv. = advanced. Product candidate BNT122/ Autogene cevumeran1 BNT316/ ONC-3922 (gotistobart) BNT323/ DB-13033 BNT311/ GEN10464 BNT312/ GEN10424 BNT211 Target Individual neoantigens CTLA-4 HER2 PD-L1x4-1BB CD40x4-1BB CLDN6 Partner Genentech OncoC4 DualityBio Genmab Genmab - Initial indications 1L Melanoma Adj. CRC Adj. PDAC aPD(L)1-R/R NSCLC 2L+ HR+/HER2- low breast cancer aPD(L)1-R/R NSCLC TBD Adv. CLDN6+ cancers Status Multiple potentially pivotal trials ongoing Ph3 ongoing Ph3 initiated Ph3 planned Pivotal trial TBD Pivotal Ph2 planned for 2024 Planning for multiple oncology launches from 2026 onward Diverse MoAs Each program with potential in multiple indications Mix of partnered and proprietary progr ams

Our Plan is to Build a Specialized Oncology Sales Force in Major Markets 47 Build commercial presence in North America, Europe and other key markets1 Plan to leverage commercial partners for co-commercialization Plan to deploy lean commercial operations with digital enablement Aim to be commercial-ready by end of 2025 1. Other markets not shown.

Time for a 10 minute Break

6 Novel Backbones: Next-Generation ADCs and Immunomodulators Prof. Özlem Türeci, M.D. CMO and Co-founder Prof. Ilhan Celik, M.D. VP, Clinical Development Michael Wenger, M.D. VP, Clinical Development

Leveraging Next-Generation ADCs and IO agents for Transformative Combinations 50 Coleman N.et al. npj Precis. Onc. 2023 Next-Gen ADCs: Targeted cytotoxic agents with untapped potential 1. ADC binds to antigen 2. Internalization of the ADC complex by endocytosis 3. Payload released after linker cleavage Target antigen ADC 4. Cytotoxic effect by payload in the nucleus Bystander cell Targeted Cytotoxicity Bystander killing effect A. Release of drug payload after antigen binding before internalization B. Release of drug payload into the intercellular space due to high drug membrane permeability ADC Next-gen ADCs and IO combos represent a paradigm shift from current chemotherapy and checkpoint inhibitor treatment regimen, which could contribute to curative approaches Next-Gen IO agents: Converging multiple proven MoAs into one molecule BNT311 PM8002 MoA = Mechanism of Action; ADC = antibody-drug conjugate; IO = immuno-oncology; irAE = immune-related adverse event; CTLA-4 = cytotoxic T-lymphocyte-associated Protein 4; PD-L1 = programmed cell death ligand 1 BNT316

ADC Portfolio Constructed with Thoughtful Considerations 1RNAseq data from AACR Project GENIE; 2. Partnered with DualityBio *The completion of the agreement with MediLink is subject to customary closing conditions, including clearance under the Hart-Scott-Rodino Antitrust Improvements Act. ADC = Antibody-drug conjugate; IO = immuno-oncology; MoA = mode of action; HER = human epidermal growth factor receptor; TROP2 = trophoblast cell-surface antigen .UC = Uretherial cancer EC = Endometrial Cancer Target Program Stage Indications Partner Ph1/2 Ph3 HER2 BNT323/DB1303 HR+/HER2-low mBC DualityBio Solid tumors with HER2 expression TROP2 BNT325/DB1305 Solid tumors DualityBio B7H3 BNT324/DB1311 Solid tumors DualityBio HER3 BNT326/YL202 Solid tumors MediLink* Target NSCLC SCLC HER2+ BC HR+ BC TNBC CRC Gastric Ovarian PDAC HNSCC Prostate Other high expression indications HER2 Gynecologic TROP2 B7-H3 UC, EC HER3 High Medium / / Low Very low / No-expression • ADC combinations based non-overlapping tumor antigens and different payload MoAs • ADC + IO to advance towards (neo)adjuvant and frontline settings • Four clinical stage ADCs with broad yet minimal overlapping indication opportunities • Innovative trial design to open leapfrog path • Fast-follower potential in large indications • BNT323/DB-13032 in multiple pivotal studies Expression level by indication1 Advanced asset on path to registration Unique indication selection strategy Wider therapeutic window may enable novel combinations in earlier lines 51

Our Pipeline Holds Potential for Synergistic Drug Combinations IO = immuno-oncology; ADC = antibody-drug conjugates; MoA = Mechanism of Action. ADCs quickly debulk tumors while cancer vaccines meaningfully boost the immune system to eradicate multi-clonal micrometastases hence lifting the long-term survival curve 52 Immunomodulators activate the immune system supporting vaccine-induced tumor- specific T cell responses Complementary and/or potentially synergistic MoA of immunomodulators enhance T cell priming and sustain activation +IO ADC +IO IO +ADC Cancer vaccine +IO Cancer vaccine ADCs deliver cytotoxic drugs directly to cancer cells while immunomodulators activate the immune system to recognize and destroy cancer cells Converging checkpoint inhibition and improved immune cell trafficking and ADC penetration

Targeting TROP2, cleavable linker and topoisomerase I inhibitor (P1021) DAR: 4 Targeting B7H3, cleavable linker and topoisomerase I inhibitor (P1021) DAR: 6 Targeting HER3, cleavable linker allows for intracellular and extracellular release of topoisomerase I inhibitor (YL0014) DAR: 8 Targeting HER2, cleavable linker (L101) and topoiso-merase I inhibitor (P1003) DAR: 8 Well-Positioned in Immuno-Oncology with Therapeutic Candidates Across Multiple Tumors 1. Partnered with DualityBio; 2. Partnered with MediLink; The completion of the agreement is subject to customary closing conditions, including clearance under the Hart-Scott-Rodino ("HSR") Antitrust Improvements Act. ADC = antibody-drug conjugates; DAR = drug-to-antibody ratio; HER2/3 = human epidermal growth factor receptor 2/3; TROP2 = trophoblast cell-surface antigen 2; mBC = metastatic breast cancer BNT324/ DB-13111 BNT323/ DB-13031 BNT325/ DB-13051 BNT326/ YL2022 Clinical status • Ph3 in HR+HER2-low mBC • Ph1/2 in multiple solid tumors Clinical status • Ph1/2 in multiple solid tumors Clinical status • Ph1/2 in multiple solid tumors Clinical status • Ph1 in multiple solid tumors HER2 B7H3 TROP2 HER3 53

54 BNT323/DB-13031: A Potentially Best-in-Class HER2-Targeting ADC 1. Partnered with DualityBio; 2. Partnered with Daiichi Sankyo; 3. Partnered with Genentech, member of Roche group. HER2 = human epidermal growth factor receptor 2; DAR = drug-to-antibody ratio; Dxd = deruxtecan; DM1 = mertansine MoA = mechanisms of action; PDX = patient-derived-xenograft; Q3W = Once every 3 weeks. Features of BNT323/DB13031 vs. other HER2-targeting therapies Properties BNT323/DB-13031 Enhertu (Trastuzumab deruxtecan, DS8201)®,2 Kadcyla (trastuzumab emtasine, TDM1)®,3 DAR ~8 ~8 ~3.5 Linker Cleavable Cleavable Non-cleavable Payload MoA Topoisomerase I inhibitor (P1003) Bystander effect Topoisomerase I inhibitor (Dxd) Bystander effect Tubulin inhibitor (DM1) Non-bystander effect Highest non-severely toxic dose* 80 mg/kg, Q3W*3 30 mg/kg, Q3W*3 10 mg/kg, Q3W*4

55 Lin S. et al. Abstract #252. Presented at ORTC-NCI-AACR in 2022. Superior in vitro plasma stability in human plasma Sustained tumor-selective drug release in tumor- bearing mice Efficient bystander killing in tumor cell lines Rapid systemic clearance in monkeys DB-1303 DS-8201* C h a n g e o f D A R f ro m b a s e lin e 120 100 80 60 40 20 0 48 96 144 192 240 288 336 384 Incubation time (hr) C e ll n u m b e r 1x106 8x105 6x105 4x105 2x105 0 V e h ic l e D B -1 3 0 3 0 .3 µ g /m l T -D M 1 ** 0 .3 µ g /m l T -D M 1 ** 0 .1 µ g /m l D B -1 3 0 3 0 .1 µ g /m l HER2 - HER2 + P a yl o a d ( n g /m l) 0 7 14 21 14 28 42352170 Free payload of DS-8201* Free payload of DB-1303 C o n c e n tr a ti o n ( n g /m l) 0 150100 Time (hr) 50 DB-1303 releases payload in serum and tumor Payload Cmax in tumor=22.3 ng/ml Payload Cmax in serum=0.48 ng/ml 1 .1.Partnered with DualityBio. ADC = Antibody-drug conjugate; HER = human epidermal growth factor receptor; cmax = maximum concentration; DAR = Drug antibody ratio. *DS-8201 is an in-house produced analog of DS-8201, Trastuzumab deruxtecan; **Trastuzumab-Emtansin. Payload-Serum Payload-Tumor BNT323/DB-13031: A HER2 ADC With a Potentially Differentiated Profile • A humanized anti-HER2 IgG1 mAb, with a wild-type Fc • A proprietary DNA topoisomerase I inhibitor (P1003) • A maleimide tetrapeptide-based tumor-selectively cleavable linker (L101) • High drug-to-antibody ratio: ~8 Humanized anti-HER2 IgG1 mAb

BNT323/DB-13031: Preclinical Data Show Antitumor Effect and Favorable Safety Profile in HER2 Positive & HER2 Low Tumor Models and Toxicity Studies 56 ToxicityAntitumor effect Lin S. et al. Abstract #252. Presented at EORTC-NCI-AACR in 2022. • BNT323/DB-1303 induced dose-dependent tumor growth inhibition and tumor regression • Potent anti-tumor effect in both HER2 positive and HER2 low tumor models with a wide therapeutic window T u m o r v o lu m e ( m m 3 ) HER2 positive (HER2 2+) PDX tumor model 1000 800 600 400 200 0 7 14 21 28 Days post dosing T u m o r v o lu m e ( m m 3 ) HER2 low (HER2 1+) PDX tumor model 800 600 400 200 0 7 14 21 28 Days post dosing • Toxicity studies2 showed improved toxicity profile compared to published profile of DS- 8201 • Highest non-severely toxic dose: 80mg/kg • BNT323/DB-1303 showed lower risk of causing lung inflammation compared to published profile of DS-8201 • Stable linker and fast clearance may contribute to the improved toxicity profile of BNT323/DB-1303 Vehicle T-DM1, 7mg/kg, single dose DB-1303, 2mg/kg, single dose DB-1303, 7mg/kg, single dose DB-82013, 2mg/kg, single dose DB-82013, 7mg/kg, single dose 1. Partnered with DualityBio. 2. in cynomolgus monkey 3. DS-8201 is an in-house produced analog of DS-8201, Trastuzumab deruxtecan HER = human epidermal growth factor receptor; ILD = interstitial lung disease; PDX = patient-derived xenograft. 3rd generation ADC with improved safety and efficacy may add survival benefit to cancer patients

First-in-Human Trial with BNT323/DB-13031 in Patients with Advanced HER2-Expressing Solid Tumors 57 Phase 1/2a trial design (NCT05150691), multicenter, non-randomized, open-label Hamilton E. et al. TiP #9504. Presented at AACR 2023 1. Partnered with DualityBio. IHC = immunohistochemistry; FIH = First in human; Q3W = every three weeks; DLT = dose limiting toxicity; HER2 = human epidermal growth factor 2; HR = hormone receptor; CRC = colorectal cancer; NSCLC = non-small cell lung cancer; MTD = maximum tolerated dose; RP2D = recommended phase 2 dose; ECOG = Eastern Cooperative Oncology Group; FPI = First patient in; LPO = Last patient out; ISH = in-situ hybridization; NGS = next-generation sequencing. DB-1303 6 mg/kg Q3W DB-1303 7 mg/kg Q3W DB-1303 8 mg/kg Q3W DB-1303 10 mg/kg Q3W DB-1303 12 mg/kg Q3W DB-1303 4.4 mg/kg Q3W DB-1303 2.2 mg/kg Q3W Part 1: Dose escalation (n=88 patients) Part 2a: Dose expansion (n=165 patients) Indications • HER2+ gastric, esophageal or gastroesophageal junction adenocarcinoma, CRC • HR+/HER2-low breast cancer • HER2+ breast cancer • HER2 overexpression and HER2-low endometrial cancer • HER2-mutated NSCLC 3 weeks DLT window Disease progression, withdrawal of consent, unacceptable toxicity (HER2 IHC 3+, IHC 2+, IHC 1+ or ISH +, or HER2 amplification by NGS, or HER2 mutation by NGS) Inclusion criteria • Pretreated advanced or metastatic solid tumors • Histologically confirmed HER2-positive or HER2- expressing cancers • Previous systemic therapies • ECOG PS 0-1 • Adequate organ function Key endpoints Safety, tolerability, pharmacokinetic, preliminary anti-tumor activity at the selected MTD/RP2D Status FPI: Jan 2022 Trial ongoing

BNT323/DB-13031 is Well Tolerated with Low Incidences of Key AEs 58 Phase 1/2a (NCT05150691): Safety Moore K. et al. Presented at ASCO 2023. Abstract #3023. • No DLT observed in all dose levels • Most common TRAEs of grade ≥3: nausea (2.4%), platelet count decreased (3.5%), anemia (5.9%) • No grade 5 TEAEs • Interstitial lung disease occurred in 2 patients (2.4%, grade 1), without any ≥grade 2 • Few patients with neutropenia (10 [11.8%]; grade ≥3 in 1 [1.2%] patients,) and alopecia (3 [3.5%], grade 1) 2.2 mg/kg (n = 1) 4.4 mg/kg (n = 5) 6.0 mg/kg (n = 15) 7.0 mg/kg (n = 29) 8.0 mg/kg (n = 32) 10.0 mg/kg (n = 3) Total (n = 85) Any TEAEs 1 (100.0%) 5 (100.0%) 14 (93.3%) 26 (89.7%) 26 (81.2%) 2 (66.7%) 74 (87.1%) Associated with treatment withdrawal 0 0 0 1 (3.4%) 0 0 1 (1.2%) Associated with treatment dose reduction 0 0 0 2 (6.9%) 1 (3.1%) 0 3 (3.5%) Associated with treatment dose interruption 0 0 4 (26.7%) 8 (27.6%) 5 (15.6%) 0 17 (20.0%) Grade ≥3 0 3 (60.0%) 3 (20.0%) 9 (31.0%) 2 (6.2%) 1 (33.3%) 18 (21.2%) Serious AEs 0 3 (60.0%) 4 (26.7%) 4 (13.8%) 2 (6.2%) 0 13 (15.3%) Treatment-related TEAEs 1 (100.0%) 3 (60.0%) 12 (80.0%) 26 (89.7%) 25 (78.1%) 2 (66.7%) 69 (81.2%) Grade ≥3 0 1 (20.0%) 2 (13.3%) 6 (20.7%) 1 (3.1%) 1 (33.3%) 11 (12.9%) Serious AEs 0 0 2 (13.3%) 0 0 0 2 (2.4%) 1. Partnered with DualityBio. DLT= dose-limiting toxicity. TEAEs: treatment-emergent adverse events. TRAEs: treatment-related adverse events; AEs: adverse events.

BNT323/DB-13031 Demonstrates Encouraging Antitumor Activity in HER2- Expressing Patients 59 1. Partnered with Duality Bio. HER2 = human epidermal growth factor receptor 2; ORR = objective response rate; DCR = disease control rate; IHC = immunohistochemistry; ISH = in situ hybridization; GEJ = gastro oesophageal junction cancer; EsC = esophageal cancer; BC = breast cancer; CRC = colorectal cancer; EC = endometrial cancer; GC = gastric cancer; OC = ovarian cancer; NSCLC = non-small cell lung cancer. Anti-tumor activity in heavily pretreated HER2- expressing patients ORR, % DCR, % All patients (n=52) 44.2 88.5 HER2+ breast cancer (n=26) 50.0 96.2 HER2 low breast cancer (n=13) 38.5 84.6 Dose Level: 3+1+ 2+HER2 IHC Status: -100 -50 0 50 100 2.2 mg/kg 4.4 mg/kg 6 mg/kg 7 mg/kg 8 mg/kg 10 mg/kg B e s t C h a n g e f ro m B a s e li n e ( % ) IS H + IS H + IS H + IS H + IS H + IS H + IS H + G E J B C C R C E s C C R C G C B C B C B C B C B C B C E C B C B C B C E s C B C N S C L C O C B C C R C B C B C B C B C B C B C E C B C B C B C B C B C B C B C B C B C E C B C B C B C B C B C B C B C B C B C O C B C B C Phase 1/2a (NCT05150691): Clinical Efficacy Moore K. et al. Presented at ASCO 2023. Abstract #3023.

Data Support Initiation of a Pivotal Phase 3 Trial Evaluating BNT323/DB-13031 in HER2-Expressing Patients 60 1. Partnered with DualityBio. HER2 = human epidermal growth factor receptor 2; ORR = objective response rate; DCR = disease control rate; FIH = first in human; ADC = antibody-drug conjugate; IHC = immune histochemistry; PD = progressive disease; PR = partial response; SD = stable disease; DLT = dose limiting toxicities; RP2D = recommended Phase 2 dose. Response over time in heavily pretreated HER2-expressing patients treated with different dose levels and HER2 IHC status: Dose Level: 2.2 mg/kg 4.4 mg/kg 6 mg/kg 7 mg/kg 8 mg/kg 10 mg/kg Continue treatment S u b je c ts 100500 150 PD PR SD Response +1 +2 +3 HER2 IHC Status Duration of treatment (days) Phase 1/2a (NCT05150691): Clinical Efficacy Moore K. et al. Presented at ASCO 2023. Abstract #3023.

HR+/HER2neg (70%5 of total breast cancer patients) HR+/HER2 Low (60%6 of HR+/HER2neg Breast Cancer) Early Stage (96%4) Stage IV (4%4) (13K) Advanced/unresectable, Recurrent (95K) Endocrine therapy (ET) +/- CDK4/6 inhibitor (~90%) ~60 % of mBC progress to 2L8 BNT323/DB-1303* and Trastuzumab-Deruxtecan as monotherapy in HR+HER2- low mBC chemotherapy naïve patients Trastuzumab-Deruxtecan Chemotherapy ET therapy/chemotherapy Total diagnosed breast cancer patients in US, UK, EU 4 and Japan: ~708K1-4 Potential future treatment algorithm for patients with adv./met. HR+/HER2-low breast cancer BNT323/DB-1303* Offers Potential to Establish New SoC for Chemotherapy Naïve, HR+/HER2-Low Patients Who Have Limited Therapeutic Options 61 1. American Cancer Society (ACS) 2023 Report; 2. Globocan – Cancer Tomorrow; 3. Cancer.net ASCO; 4. SEER*Stat Research Tool; 5. Putnam Expertise, KOL inputs from SMARTANALYST Syndicated Insights Report and triangulation from published literature; 6. Burstein et al., NEJM 2020; 2557-2570 7. Modi et al., NEJM 2022; Pg 10/12; 8. Market Research, data on file. * Partnered with DualityBio. SoC = standard of care; HR = hormone receptor; HER2 = human epidermal growth factor receptor 2; BC = breast cancer; CDK4/6 = cycline dependent kinase 4/6; 2L = second line; 3 line = third line 3L+ 1L 2L+ Metastatic recurrenc5 Subject to regulatory approvalRelevant patient population

Phase 3 Trial Design BNT323/DB-13031 in Chemotherapy-Naïve Patients with HR+/HER2-Low Breast Cancer 62 1. Partnered with DualityBio; 2. Twelves C. et al. Clinical Breast Cancer. 2022. HR = hormone receptor; HER = human epidermal growth factor; ET = endocrine therapy; ECOG = eastern Cooperative oncology group; IV = intravenous; Q3W = every 3 weeks; RECIST = response evaluation criteria in solid tumors; PFS = progression free survival; OS = overall survival; ORR = objective response rate; DoR = duration of response; DCR = disease control rate; TTR = time to response; PK = pharmacokinetics. * Subjects who have received chemotherapy in the neo-adj. or adj. setting are eligible, as long as they have had a disease-free interval (defined as completion of systemic chemotherapy to diagnosis of adv. or met disease) of >12 months. Inclusion criteria • Adult participants, aged 18 years and older • Documented advanced or metastatic HR+/HER2- low (IHC 1+ or IHC 2+/ISH-) breast cancer • Progressed on at least 2 lines of prior ET or within 6 months of first line ET + CDK4/6 inhibitor in the metastatic setting • No prior chemotherapy for advanced or metastatic breast cancer* • ECOG performance status 0 or 1 Stratification factors • Prior CDK4/6 inhibitor use, HER2 IHC expression, prior taxane use in the non-metastatic setting Randomized patients are treated until: • RECIST 1.1 defined disease progression or • unacceptable toxicity or • withdrawal of consent or • any other criterion for discontinuation is met n=532 R 1:1 Experimental arm: BNT323/DB-13031 8mg/kg IV, Q3W Investigator's choice single agent chemotherapy (paclitaxel or nab-paclitaxel or capecitabine) Key endpoints Primary: PFS Secondary: OS, ORR, DoR, DCR, TTR, safety, tolerability, PK and PRO Open-label, multi-center, randomized Phase 3 trial (NCT06018337) Status Trial initiated in Q3 2023 Historical efficacy chemotherapy in BC patients:2 ORR = 11-36%; mPFS = 3-8 months; mOS = 9-16 months

Unmet Need in Endometrial Cancer 63 1. Sung H, et al. CA: a cancer journal for clinicians. 2021; 2. SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute; 2023 Apr 19. [updated: 2023 Jun 8; cited 2023 Aug 17]. Available from: https://seer.cancer.gov/statistics-network/explorer/. Data source(s): SEER Incidence Data, November 2022 Submission (1975-2020); 3. Makker V, et al. N Engl J Med. 2022; 4. Livasy C A, et al. Gynecol Oncol. 2005; 5. Buza N, et al. Arch Pathol Lab Med. 2021; 6. Santin A D, et al. Am J Obstet Gynecol. 2005. EC = endometrial cancer; HER2 = human epidermal growth factor receptor 2; mPFS = median progression free survival; ORR = objective response rate; UC = uterine carcinosarcoma. The 5-year survival among patients with EC with distant metastases has been reported to be 18%2 In 2020, new EC cases worldwide 1: 417,000+ New deaths caused by EC worldwide 1: 97,000+ The 6th most commonly diagnosed cancer … … and the 4th leading cause of cancer death in women1 Targeted therapies and chemotherapy have had limited efficacy in advanced or recurrent EC after platinum-based chemotherapy3 • In approximately 25%-30% of uterine serous carcinoma (USC)5 • Lenvatinib plus pembrolizumab: ORR, 31.9%; mPFS, 7.2 months3 • Doxorubicin or paclitaxel: ORR, 14.7%; mPFS, 3.8 months3 HER2 protein overexpression and/or gene amplification is present in approximately 17%-38% of EC4 • In approximately 14%-56% of uterine carcinoma4 In patients with USC in the U.S., black women (90%, 9/10) have significantly higher HER2 overexpression than white women (48%, 8/17)6

Efficacy of BNT323/DB-13031 Enables Clear Path to Registration in Heavily Pretreated HER2-Expressing Endometrial Cancer Patients 64 1. Partnered with DualityBio. HER2 = human epidermal growth factor receptor 2; ORR = objective response rate; DCR = disease control rate; FIH = first in human; ADC = antibody-drug conjugate; IHC = immune histo chemistry test; ISH = In situ hybridization; PD = progressive disease; PR = partial response; SD = stable disease. Phase 1/2a FIH study (NCT05150691): Clinical Efficacy Moore K. et al. Presented at ESGO 2023. Abstract # 430 Responsea Dose Escalation Dose Expansion Total (n=17)b 7 mg/kg (n=4)b 8 mg/kg (n=4)b 8 mg/kg (n=9)b Unconfirmed ORR, n (%) 2 (50.0) 4 (100) 4 (44.4) 10 (58.8) Confirmed ORR, n (%) 1 (25.0) 3 (75.0) 0 4 (23.5) Pending confirmation ORR, n (%) 1 (25.0) 1 (25.0) 4 (44.4) 6 (35.3) Unconfirmed DCR, n (%) 4 (100) 4 (100) 8 (88.9) 16 (94.1) a By investigator. b Response-evaluable subjects, which includes subjects with ≥1 postbaseline overall response. • Patients received median 2 lines of prior treatment for their metastatic disease • ~60% of patients had received prior immunotherapy, ~38% of patient had received prior anti-HER2 antibody • Clinical response observed in IHC 1+ patients • 34% of patients had serous carcinoma, ORR 87.5% B e s t C h a n g e f ro m B a s e li n e ( % ) ISH+ ISH+ ISH+ ISH+ ISH+ # Subject 30 0 -30 -60 -90 Dose level 7 mg/kg 8 mg/kg 1+ 2+ 3+HER2 IHC status

Targeting TROP2, cleavable linker and topoisomerase I inhibitor (P1021) DAR: 4 Targeting B7H3, cleavable linker and topoisomerase I inhibitor (P1021) DAR: 6 Targeting HER3, cleavable linker allows for intracellular and extracellular release of topoisomerase I inhibitor (YL0014) DAR: 8 Targeting HER2, cleavable linker (L101) and topoiso-merase I inhibitor (P1003) DAR: 8 Well-Positioned in Immuno-Oncology with Therapeutic Candidates Across Multiple Tumors 1. Partnered with DualityBio; 2. Partnered with MediLink; The completion of the agreement is subject to customary closing conditions, including clearance under the Hart-Scott-Rodino ("HSR") Antitrust Improvements Act. ADC = antibody-drug conjugates; DAR = drug-to-antibody ratio; HER2/3 = human epidermal growth factor receptor 2/3; TROP2 = trophoblast cell-surface antigen 2; mBC = metastatic breast cancer BNT324/ DB-13111 BNT323/ DB-13031 BNT325/ DB-13051 BNT326/ YL2022 Clinical status • Ph3 in HR+HER2-low mBC • Ph1/2 in multiple solid tumors Clinical status • Ph1/2 in multiple solid tumors Clinical status • Ph1/2 in multiple solid tumors Clinical status • Ph1 in multiple solid tumors HER2 B7H3 TROP2 HER3 65

BNT325/DB-13051 Positioned As a Key Backbone ADC for a Variety of Solid Tumors 66 1. Partnered with DualityBio; 2. Oncotarget. 2015; 6:22496-22512 3. Pathology International. 2020;1–8; 4. Am J Clin Exp Urol. 2021 Feb 15;9(1):73-87. 5. Cancers (Basel). 2022 Sep; 14(17): 4137 TROP-2 = trophoblast cell surface antigen-2; ADC antibody drug conjugate; TNBC = triple negative breast cancer, NSCLC= non-small cell lung cancer; IgG = immunoglobulin G; mAb = monoclonal antibody. Key attributes of BNT325/DB-13051 • Optimized drug-to-antibody ratio: ~4 • Linker highly stable in the circulation • High potency of payload with a short systemic half-life • Bystander antitumor effect Interchain Cysteine Residue Linker-Payload TROP-2 as an ADC target BNT325/DB-13051 and its three components: • Humanized anti-TROP2 IgG1 mAb, with active Fc • Proprietary DNA topoisomerase I inhibitor (P1021) • Cleavable linker Humanized anti-TROP2 IgG1 mAb TNBC2 NSCLC3 TROP2 is highly expressed in a wide range of indications Prostate cancer4 Colorectal cancer5

BNT325/DB13051 - A Potential Best-in-Class TROP2-Targeting ADC 1. Partnered with DualityBio; 2. Zhang Y. et al. Presented at EORTC-NCI-AACR. 2022. 4. Gilead; 5. Daiichi Sankyo; 5. Cheng Y et al. Front. Oncol. 2022; 6. Merck. TROP-2 = trophoblast cell surface antigen-2; ADC = antibody drug conjugate; DAR=Drug-to-antibody ratio; HNSTD=Highest non-severely toxic dose; MoA=Mechanisms of action; PDX=Patient-derived-xenograft; Q3W=Once every 3 weeks. Properties BNT325/DB-13051,2 Trodelvy (Sacituzumab- Govitecan)®,3 Dato-DXd4 SKB2645,6 DAR 4 ~8 ~4 7.4 Linker Cleavable maleimide tetrapeptide linker Hydrolysable (CL2A) Cleavable tetrapeptide- based linker Sulfonyl pyrimidine- CL2A-carbonate (TL033) Payload DNA Topoisomerase inhibitor (P1021) DNA Topoisomerase I inhibitor (SN-38) DNA Topoisomerase I inhibitor (DXd) Belotecan-derivative topoisomerase I inhibitor (KL610023) Payload MoA DNA Topoisomerase inhibitor / Bystander effect DNA Topoisomerase I inhibitor / Bystander effect DNA Topoisomerase I inhibitor / Bystander effect DNA Topoisomerase I inhibitor / Bystander effect HNSTD in Monkey 80 mg/kg Q3W 50 mg/kg 30 mg/kg 50 mg/kg Preclinical comparison BNT325/DB-13051 vs other TROP2-targeting ADCs Zhang Y. et al. Presented at EORTC-NCI-AACR.2022 67

BNT325/DB-13051: Preclinical Data Show Anti-Tumor Effect in TROP2 Positive & Low Tumor Models and a Favorable Toxicity Profile 68 Toxicity dataAntitumor effect Zhang Y. et al. Presented at EORTC-NCI-AACR.2022 • BNT325/DB-1305 induces dose-dependent tumor growth inhibition and tumor regression • Potent anti-tumor effect in TROP2 high and low tumor models with a wide therapeutic window • The HNSTD of BNT325/DB-1305 for cynomolgus monkeys is 80 mg/kg in 6- week repeated-dose toxicity study • Low free payload in circulation may contribute to improved tolerance of BNT325/DB-1305 800 600 400 200 0 0 7 14 21 28 Days after the start of treatment T u m o r v o lu m e ( m m 3 ) Vehicle, once, i.v. DB-1305, 3mpk, once, i.v. DB-1305, 10mpk, once, i.v. DS-1062*, 3mpk, once, i.v. DS-1062*, 10mpk, once, i.v. TGI= 20.22% TGI= 37.77% TGI= 41.45% TGI= 79.89% Trop2-negative CDX Colon-205 (colon cancer) Colon205 tumor xenograft model Tumor volume mean ± SEM 1000400 300 200 100 0 0 7 14 21 28 Days after the start of treatment T u m o r v o lu m e ( m m 3 ) Vehicle, once, i.v. DB-1305, 1mpk, once, i.v. DB-1305, 3mpk, once, i.v. DS-1062*, 1mpk, once, i.v. DS-1062*, 3mpk, once, i.v. TGI= 17,54% TGI= 22.11% TGI= 77.31% TGI= 92.09% Trop2-high CDX MDA-MB-468 (breast cancer) MDA-MB-468 tumor xenograft model Tumor volume mean ± SEM *DS-1062 is an in-house produced analog of Dato deruxtecan 1. Partnered with DualityBio. TROP-2 = trophoblast cell surface antigen-2; CDX = cell-derived xenograft. HNSTD = highest non-severely toxic dose; SEM = standard error of the mean.

First-in-human trial with BNT325/DB-13051 in Patients with Advanced/Metastatic Solid Tumors 1. Partnered with DualityBio. ECOG PS = eastern cooperative oncology group performance status; DL = dose level; Q3W = every three weeks; RP2D = recommended phase 2 dose; HR = hormone recptor; HER2 = human epidermal growth factor receptor 2; NSCLC = non- small cell lung cancer; TNBC = triple negative breast cancer; DLT = dose-limiting toxicity; TEAE = treatment emergent adverse events; SAE = serious adverse events; MTD = maximum tolerated dose; ORR = objective response rate. 69 Phase 1/2 trial design (NCT05438329), multicenter, non-randomized, open-label, n=255 Part 1: Dose escalation Part 2: Dose expansion Indications • NSCLC with actionable genomic alterations • NSCLC without actionable genomic alterations • Ovarian cancer • HR+/HER2-neg breast cancer • TNBC without prior sacituzumab govitecan treatment • TNBC with treatment failure on sacituzumab govitecan RP2D Q3W Disease progression, withdrawal of consent, unacceptable toxicity DB-1305 DL3 Q3W DB-1305 DL4 Q3W DB-1305 DL2 Q3W DB-1305 DL5 Q3W DB-1305 DL1 Q3W Inclusion criteria • Advanced/unresectable, recurrent or metastatic solid tumors • Relapsed or progressed on or after standard systemic treatments • ECOG PS 0-1 • Adequate organ function Key endpoints Primary: Part 1: Assessment of DLT, TEAE, SAE, MTD, RP2D. Phase 2a: TEAEs, SAEs, ORR Secondary: Pharmacokinetic measures Trial ongoing

BNT325/DB-13051 Shows a Manageable Safety Profile 1. Partnered with DualityBio. DLT = dose limiting toxicities; MTD = maximum tolerated dose; TRAE = treatment related adverse event; AE = adverse event; FIH = first in human. ILD = interstitial lung disease. Overall safety • DB-1305 was tolerable and all TRAEs were manageable in dose levels 2 mg/kg and 4 mg/kg • Three patients dosed at 6 mg/kg experienced dose- limiting toxicities (i.e., stomatitis, febrile neutropenia, and white blood cell decrease) • The maximum tolerated dose was established as 5 mg/kg • 1 ILD occurred • No TRAEs led to death 70 Phase 1/2a FIH study (NCT05150691): Safety Marathe O. et al. Presented at ESMO 2023. Poster #689P. 2 mg/kg (n=1) n (%) 4 mg/kg (n=20) n (%) 5 mg/kg (n=17) n (%) 6 mg/kg (n=6) n (%) Total (n=44) n (%) Any TRAEs 0 19 (95.0) 15 (88.2) 6 (100) 41 (93.2) Grade ≥3 1 (100) 13 (65) 6 (35.3) 5 (83.3) 25 (56.8) Serious TRAEs 0 3 (15.0) 4 (23.5) 3 (50.0) 10 (22.7) Lead to dose reduction 0 1 (5.0) 2 (11.8) 3 (50.0) 6 (13.6) Lead to dose interruption 0 6 (30.0) 5 (29.4) 4 (66.7) 15 (34.1) Lead to dose discontinuation 0 1 (5.0) 0 0 1 (2.3) One patient died by suicide on day 18 after first dose and one patient experienced double pneumonia related AE on day 49.

1. Partnered with DualityBio. FIH = first in human; ORR = objective response rate; DCR = disease control rate; NSCLC = non-small cell lung cancer; CRC = colorectal cancer; TNBC = triple-negative breast cancer; GC = gastric cancer; GEJC = gastroesophageal junction cancer. Unconfirmed ORR, % Unconfirmed DCR, % All patients (n=23) 30.4 87.0 NSCLC (n=13) 46.2 92.3 Best tumor response for all patients with post-baseline scans (n=23) Anti-tumor activity in heavily pretreated patients with 3 median prior lines of treatment 71 Dose level 2 mg/kg 4 mg/kg 5 mg/kg 6 mg/kg B e s t C h a n g e f ro m B a s e lin e ( % ) Subject name or identifer 50 0 -50 N S C L C F a llo p ia n t u b e A m p u lla ry N S C L C N S C L C N S C L C N S C L C N S C L C N S C L C N S C L C G C /G E J C N S C L C C R C C R C N S C L C D U O D E N A L A P P E N D IC E A L N S C L C T N B C N S C L C C R C N S C L C C R C BNT325/DB-13051 Demonstrates Promising Antitumor Activity in NSCLC and Other Solid Tumors Phase 1/2 FIH study (NCT05438329): Clinical Efficacy Marathe O. et al. Presented at ESMO 2023. Poster #689P.

ADC Key Takeaways BNT323/DB13031 • Multiple pivotal studies planned BNT324/DB-13111 I BNT325/DB-13051 I BNT326/YL2022 • Ongoing studies will inform potential activity in multiple expansion cohorts and drive future development decisions • Investigate monotherapy or combination regimens 1. Partnered with DualityBio 2. MediLink. ADC= Antibody-drug conjugate. Targeted milestones Strategy • Leverage ADCs as a tool for de-bulking tumor mass to unlock potential in hard-to-treat cancer types • Explore various indication-selection strategies • Leverage ADCs’ wide therapeutic window to enable novel combinations in earlier lines of treatment 73

A CD27 antibody based on the HexaBody technology, specifically engineered to form an antibody hexamer upon binding its target on T cell membranes. BNT313/ GEN10532 Clinical status • Ph1/2 in multiple solid tumors Monospecific antibody with optimized Fc targeting CTLA-4 and selectively depleting tumor-infiltrating Tregs in the TME but not in the periphery due to a pH driven mechanism. BNT316/ ONC-3921 (gotistobart) Clinical status • Ph1/2 in multiple solid tumors • Ph2 in PROC • Ph3 in 2L+ mNSCLC Bispecific antibody to inhibit proliferation of PD1-positive cells. 4-1BB enhances T cell proliferation, T cell effector functions and prevents T cell death. BNT311/ GEN10462 Clinical status • Ph1/2 in multiple solid tumors • Ph2 in mNSCLC • Ph2 in 2L mEC Engagement of CD40 leads to activation and maturation of APCs. 4-1BB enhances T cell proliferation, T cell effector functions and prevents T cell death. BNT312/ GEN10422 Clinical status • Ph1/2 trials in multiple solid tumors Bispecific antibody designed to boost antitumor immune response through EpCAM-dependent 4-1BB agonistic activity. BNT314/ GEN10592 Clinical status • Ph1/2 in multiple solid tumors planned 1. Partnered with OncoC4; 2. Partnered with Genmab; 3. Partnered with Biotheus. CTLA4 = Cytotoxic T-Lymphocyte-Associated Protein 4; CD27, CD40, 4-1BB = members of the tumor necrosis factor receptor superfamily; PD-1 =Programmed cell death protein 1; HER2 = human epidermal growth factor receptor 2; ADCC = Antibody dependent cell-mediated cytotoxicity; ADCP = Antibody dependent cellular phagocytosis; PROC = platinum-resistant ovarian cancer; NSCLC = non-small cell lung cancer; EC = endometrial cancer APC = antigen presenting cells; VEGF = vascular endothelial growth factor; TME = tumor microenvironment; CTx = chemotherapy; LALA = IgG1 variant L234A/L235A. PD-L1 expression or upregulation in tumors may enrich VEGF neutralization into the TME which inhibits angiogenesis. PM80023 Clinical status • Ph1b dose escalation • Ph2a as monotherapy in multiple cancers • Ph2 in combination with CTx in multiple cancers Well-Positioned in Immuno-Oncology with Therapeutic Candidates Across Multiple Tumors Anti-CD27Anti-CTLA4 Anti-4-1BBAnti-PD-L1 Anti-4-1BBAnti CD40 Anti-4-1BBEpCAM Anti-VEGF A Anti-PD-L1 VHH Inert Fc (LALA) Optimized Fc 74

A CD27 antibody based on the HexaBody technology, specifically engineered to form an antibody hexamer upon binding its target on T cell membranes. BNT313/ GEN10532 Clinical status • Ph1/2 in multiple solid tumors Monospecific antibody with optimized Fc targeting CTLA-4 and selectively depleting tumor-infiltrating Tregs in the TME but not in the periphery due to a pH driven mechanism. BNT316/ ONC-3921 (gotistobart) Clinical status • Ph1/2 in multiple solid tumors • Ph2 in PROC • Ph3 in 2L+ mNSCLC Bispecific antibody to inhibit proliferation of PD1-positive cells. 4-1BB enhances T cell proliferation, T cell effector functions and prevents T cell death. BNT311/ GEN10462 Clinical status • Ph1/2 in multiple solid tumors • Ph2 in mNSCLC • Ph2 in 2L mEC Engagement of CD40 leads to activation and maturation of APCs. 4-1BB enhances T cell proliferation, T cell effector functions and prevents T cell death. BNT312/ GEN10422 Clinical status • Ph1/2 trials in multiple solid tumors Bispecific antibody designed to boost antitumor immune response through EpCAM-dependent 4-1BB agonistic activity. BNT314/ GEN10592 Clinical status • Ph1/2 in multiple solid tumors planned 1. Partnered with OncoC4; 2. Partnered with Genmab; 3. Partnered with Biotheus. CTLA4 = Cytotoxic T-Lymphocyte-Associated Protein 4; CD27, CD40, 4-1BB = members of the tumor necrosis factor receptor superfamily; PD-1 =Programmed cell death protein 1; HER2 = human epidermal growth factor receptor 2; ADCC = Antibody dependent cell-mediated cytotoxicity; ADCP = Antibody dependent cellular phagocytosis; PROC = platinum-resistant ovarian cancer; NSCLC = non-small cell lung cancer; EC = endometrial cancer APC = antigen presenting cells; VEGF = vascular endothelial growth factor; TME = tumor microenvironment; CTx = chemotherapy; LALA = IgG1 variant L234A/L235A. PD-L1 expression or upregulation in tumors may enrich VEGF neutralization into the TME which inhibits angiogenesis. PM80023 Clinical status • Ph1b dose escalation • Ph2a as monotherapy in multiple cancers • Ph2 in combination with CTx in multiple cancers Well-Positioned in Immuno-Oncology with Therapeutic Candidates Across Multiple Tumors Anti-CD27Anti-CTLA4 Anti-4-1BBAnti-PD-L1 Anti-4-1BBAnti CD40 Anti-4-1BBEpCAM Anti-VEGF A Anti-PD-L1 VHH Inert Fc (LALA) Optimized Fc 75

Avoiding lysosomal degradation of CTLA-4 for safer and more effective immunotherapy may lead to uncoupling cancer therapeutic effect from immunotherapy-related adverse effects Differentiated Mechanism with Potential to Become Best-in-Class Anti-CTLA-4 Antibody 76 1.Partnered with OncoC4. FcR = fragment crystallizable region, CTLA-4 = cytotoxic T-lymphocyte-associated protein 4, ADCC = antibody-dependent cell-mediated cytotoxicity, ADCP = antibody-dependent cellular phagocytosis BNT316/ONC-392 (gotistobart)1 designed to: • Allow regular recycling and enrichment of antibody and CTLA-4 molecule • Enhance anti-tumor immunity • Reduce immune-related adverse events Endosome pH<6.0 Recycling endosome Recycling pH~6.5 FcR ADCC/ADCP Anti-tumor immunity Autoimmunity C T L A -4 C T L A -4 C T L A -4 C T L A -4 C T L A -4 C T L A -4 C T L A -4 C T L A -4 C T L A -4 C T L A -4 Liu Y. et al. Abstract # 231, SITC 2021. Du et al. Uncoupling therapeutic from immunotherapy-related adverse effects for safer and effective anti-CTLA-4 antibodies in CTLA4 humanized mice. Cell Res. 2018 Apr; 28(4): 416–432. Du et al. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res. 2018 Apr; 28(4): 433–447. MoA designed to allow higher dosing & longer duration of treatment with BNT316/ONC-392 (gotistobart)

Part C: Dose expansion (Hu-Lieskovan et al. Poster #594. Presented at SITC 2022) Part A and B: Dose finding (Li T. et al. Poster #949, Presented at SITC 2021) PRESERVE-001: Phase 1/2 Trial Design and Safety Data 77 1.Partnered with OncoC4. Q3W = every three weeks; MTD = maximum tolerated dose; RP2D = recommended phase 2 dose; DLT = dose-limiting toxicity; TRAE = treatment related adverse event; HNSCC = head and neck squamous cell carcinoma; NSCLC = non-small cell lung cancer; irAE = immune-related adverse event, IO = immuno-oncologic, R/R = relapsed/refractory. Part A: MTD or RP2D for Monotheraphy Part B: MTD or RP2D for combination with pembrolizumab • advanced or metastatic solid tumors with measurable or non- measurable disease • Progression despite standard of care therapy, or no standard therapies exist ONC-392 0.1 mg/kg Q3W ONC-392 0.3 mg/kg Q3W ONC-392 1.0 mg/kg Q3W ONC-392 3.0 mg/kg Q3W ONC-392 10.0 mg/kg Q3W Findings Indications: Monotherapy • Pancreatic cancer • IO naïve NSCLC • IO R/R NSCLC • HNSCC • Triple negative breast cancer • Ovarian cancer • Other multiple solid tumors RP2D Q3W • IO naïve NSCLC • IO R/R NSCLC • IO naïve melanoma • IO R/R melanoma Indications: Combination with pembrolizumab >450 patients treated with BNT316/ONC-392 (gotistobart)1 BNT316/ONC-392 (gotistobart)1 as mono-therapy and in combination with pembrolizumab well tolerated • TRAE manageable, no DLTs, MTD not reached • Monotherapy RP2D: 10 mg/kg, combination RP2D: 6 mg/kg Preliminary data demonstrated lower irAE rate than observed for comparable IO or IO-IO combinations Safety profile allows for higher dosing and longer duration of treatment in monotherapy and in combination with pembrolizumab

BNT316/ONC-392 (gotistobart) (3 or 6mg/kg) in combination with pembrolizumab Hu-Lieskovan et al. Poster #594. Presented at SITC 2022 BNT316/ONC-392 (gotistobart) monotherapy (10mg/kg) in platinum- resistant ovarian cancer patients Hays J et al. Poster #564. Presented at SITC 2022 % C h a n g e f ro m B a s e lin e i n T a rg e t L e s io n s 30% 20% 10% 0% -10% -20% -30% -40% -50% -60% -70% * SDSD SDSDSDPD PD PD PDPDSDSDSDPD PD PD PD PD PD PD PD PD PR CRPRPRPRPR 60% 50% 40% -80% 138% Clinical Efficacy of BNT316/ONC-392 (gotistobart)1 as Single Agent and in Combination in Patients with Multiple Solid Tumors 78 1.Partnered with OncoC4. CR = complete remission; PR = partial response; SD = stable disease; PD = progressive Disease; ORR = objective response rate; DCR = disease control rate, Ipi = Ipilimumab, Nivo = nivolumab, Pem = pemetrexed, Tx = treatment, T-VEC = talimogen laherparepvec, Atezo = atezolizumab, R/R = relapsed/refractory. Best overall response 30% 20% 10% 0% -10% -20% -30% -40% -50% -60% -70% -80% Best overall response 14/28 pts. with clinical activity • CR/PR/SD/PD = 1/5/8/14 • ORR=21%, DCR=50% 8/10 pts. with clinical activity • At 3 mg/kg (6 pts.): 2 PR, 3 SD • At 6 mg/kg (4 pts.): 1 PR, 2 SD BNT316/ONC-392 (gotistobart) (6mg/kg) in combination with pembrolizumab in R/R Melanoma Hu-Lieskovan et al., Poster #594. Presented at SITC 2022 6 pts. with clinical activity • 5 PR, 1 SD % C h a n g e f ro m B a s e lin e i n T a rg e t L e s io n s PD Best overall response % C h a n g e f ro m B a s e lin e i n T a rg e t L e s io n s

PRESERVE-001: Phase 1/2a multicenter, non-randomized, open-label, multiple-dose, FIH trial (NCT04140526) He K. et al. presented at ASCO 2023, Abstract #9024. Data Support Initiation of Pivotal Phase 3 Trial Evaluating BNT316/ONC-392 (gotistobart)1 in CPI-resistant NSCLC 79 Target lesion best overall response (N=27 evaluable) Dosing 10 mg/kg x 2, then 6 mg/kg, q3w (2 pts.: 10 mg/kg x 4, q3w) Target lesion percentage change over time (N=27 evaluable) Dosing; 10 mg/kg x 2, then 6 mg/kg, q3w (2 pts.: 10 mg/kg x 4, q3w) Days from C1D1 -100 -50 0 50 100 0 100 200 300 400 P e rc e n ta g e c h a n g e o f T a rg e t L e s io n s f ro m b a s e lin e Anti-tumor activity observed in ICI-resistant NSCLC patients (n=27) -100 -50 0 50 100 S D S D S D S D C R P R P R P D P D S D P R P D P D S D P D S DP D P R S D S D S D S D P R P D P RP R P D Subject P e rc e n ta g e C h a n g e o f T a rg e t L e s io n s F ro m B a s e lin e Manageable adverse eventsORR: 29.6% (22.2% confirmed & 7.4% unconfirmed) DCR: 70.4% Phase 3 trial in NSCLC ongoing 1.Partnered with OncoC4. CPI = Checkpoint inhibitor; NSCLC = non-small cell lung cancer; FIH = first in human; IO = immuno-oncology; ORR = objective response rate; DCR = disease control rate; pts = patients; q3w = 3-week schedule; C1D1 = Cycle 1 Day 1.

Case Report Demonstrates Clinical Response to BNT316/ONC-392 (gotistobart)1 80 1. Partnered with OncoC4. PD-L1 = programmed cell death protein L1; TMB = tumor mutation burden; chemo-RT = chemo-radio therapy;; PET/CT = positron emission tography / computer tomography 64-year-old male Diagnosis Squamous cell carcinoma of lung in Aug 2021, 100 pack years smoking history (quit 15 years ago) Tumor PD-L1 <1%. TMB 4. No actionable mutations. Microsatellite status is stable Prior therapy Initially treated at outside hospital with chemo-RT (weekly paclitaxel and carboplatin), completed in Nov 2021. PET/CT on 12/10/21 showed disease progression with metastases. Started with carboplatin, paclitaxel, ipilimumab and nivolumab; continued progression after 2 cycles of treatment Sites of metastases Spleen and liver February 2022, baseline October 2022July 2022 September 2023 PRESERVE-001: Case report He K. et al. presented at SITC 2023, Abstract #599. Gotistobart, Mar. 7, 2022; active in treatment cycle 25 as of Sep. 2023

Limited 2L Treatment Options Post Immunotherapy in NSCLC 81 1. Kantar CancerMPact Treatment Architecture; 2. Thai AA et al. Lancet. 2021; 3. Markt research, data on file; 4. Partnered with OncoC4. NSCLC = non-small cell lung cancer; IO = immuno oncology; VEGFi = vascular endothelial growth factor inhibitor; TROP-2 = trophoblast cell surface antigen-2; CTLA4 = cytotoxic T-lymphocyte-associated protein 4; ORR = objective response rate; mOS = median overall survival. ~50% with actionable driver mutations2 IO +/- platinum-based chemotherapy ~60 % of mNSCLC w/o driver mutations progress to 2L3 Docetaxel +/- VEGFi IO/IO +/- platinum-based chemotherapy PRESERVE-003 BNT316/ONC- 392 (gotistobart)4 monotherapy Total diagnosed metastatic NSCLC patients in US, UK, EU 4 and Japan: ~375K1 Potential future treatment algorithm – metastatic NSCLC w/o driver mutations 1L 2L+ ~50% without actionable driver mutations (~190K)2 Historic efficacy of docetaxel monotherapy (Garon et al. Lancet. 2014): ORR ~10%; mPFS = 3 months; mOS = 9 months BNT316/ONC-392 (gotistobart) could provide an additional treatment option for 2L NSCLC patients TROP2 ADCs Subject to regulatory approvalRelevant patient population

Phase 3 Trial Evaluating BNT316/ONC-392 (gotistobart)1 in CPI-resistant NSCLC 82 1. Partnered with OncoC4; CPI = Checkpoint inhibitor; NSCLC = Non-small cell lung cancer; PD-1 = Programmed cell death protein 1; IO = immuno-oncology; RESCIST = Response Evaluation Criteria In Solid Tumors; Q3W = once every three weeks; (median)OS = (median) overall survival; ORR = objective response rate; (m)PFS = (median) progression free survival; ECOG = Eastern Cooperative Oncology Group; FPD = first patient dosed. PRESERVE-003 (NCT05671510) Randomized, open-label, active controlled, multi-center Phase 3 trial Stage I (dose-confirmation stage): Assess efficacy and safety of two BNT316/ONC-392 dosing regimens in comparison to docetaxel Stage II: Assess safety and efficacy of BNT316/ONC-392 at the selected dosing regimen versus docetaxel Historic efficacy of docetaxel monotherapy (Garon et al. Lancet. 2014): ORR ~10%; mPFS = 3 months; mOS = 9 months R 1:1 N=480 Inclusion criteria • ≥ 18 years stage IV, metastatic NSCLC • Prior PD-(L)1 +/- platinum-based chemotherapy • Prior IO-IO allowed • RECIST 1.1 measurable lesions R 1:1:1 N=120 BNT316/ONC-392 (gotistobart) 6mg/kg with 2 loading doses of 10mg/kg, Q3W (N=40) BNT316/ONC-392 (gotistobart) 3mg/kg, Q3W (N=40) Docetaxel 75 mg/kg, Q3W (N=40) Docetaxel 75 mg/kg, Q3W (N=240) (N=240) TBD: mg/kg, Q3W Key endpoints Primary: OS Secondary: ORR, PFS, Safety BNT316/ONC-392 (gotistobart) Status Trial actively recruiting

A CD27 antibody based on the HexaBody technology, specifically engineered to form an antibody hexamer upon binding its target on T cell membranes. BNT313/ GEN10532 Clinical status • Ph1/2 in multiple solid tumors Monospecific antibody with optimized Fc targeting CTLA-4 and selectively depleting tumor-infiltrating Tregs in the TME but not in the periphery due to a pH driven mechanism. BNT316/ ONC-3921 (gotistobart) Clinical status • Ph1/2 in multiple solid tumors • Ph2 in PROC • Ph3 in 2L+ mNSCLC Bispecific antibody to inhibit proliferation of PD1-positive cells. 4-1BB enhances T cell proliferation, T cell effector functions and prevents T cell death. BNT311/ GEN10462 Clinical status • Ph1/2 in multiple solid tumors • Ph2 in mNSCLC • Ph2 in 2L mEC Engagement of CD40 leads to activation and maturation of APCs. 4-1BB enhances T cell proliferation, T cell effector functions and prevents T cell death. BNT312/ GEN10422 Clinical status • Ph1/2 trials in multiple solid tumors Bispecific antibody designed to boost antitumor immune response through EpCAM-dependent 4-1BB agonistic activity. BNT314/ GEN10592 Clinical status • Ph1/2 in multiple solid tumors planned 1. Partnered with OncoC4; 2. Partnered with Genmab; 3. Partnered with Biotheus. CTLA4 = Cytotoxic T-Lymphocyte-Associated Protein 4; CD27, CD40, 4-1BB = members of the tumor necrosis factor receptor superfamily; PD-1 =Programmed cell death protein 1; HER2 = human epidermal growth factor receptor 2; ADCC = Antibody dependent cell-mediated cytotoxicity; ADCP = Antibody dependent cellular phagocytosis; PROC = platinum-resistant ovarian cancer; NSCLC = non-small cell lung cancer; EC = endometrial cancer APC = antigen presenting cells; VEGF = vascular endothelial growth factor; TME = tumor microenvironment; CTx = chemotherapy; LALA = IgG1 variant L234A/L235A. PD-L1 expression or upregulation in tumors may enrich VEGF neutralization into the TME which inhibits angiogenesis. PM80023 Clinical status • Ph1b dose escalation • Ph2a as monotherapy in multiple cancers • Ph2 in combination with CTx in multiple cancers Well-Positioned in Immuno-Oncology with Therapeutic Candidates Across Multiple Tumors Anti-CD27Anti-CTLA4 Anti-4-1BBAnti-PD-L1 Anti-4-1BBAnti CD40 Anti-4-1BBEpCAM Anti-VEGF A Anti-PD-L1 VHH Inert Fc (LALA) Optimized Fc 83

BNT311/GEN1046 – Combining Checkpoint Blockade and Conditional T Cell Co-Stimulation 84 1. Partnered with Genmab; Fc = fragment crystallizable region; PD –L1 = programmed cell death ligand 1; PD-1 = programmed cell death protein 1; NK cell = natural killer cell; . Inert Fc, dual targeted 4-1BB co-stimulation that is conditional on PD-L1 binding Novel mechanism that enhances T- and natural killer cell functions BNT311/GEN1046 binding affinity: KD PD-L1: 0.16 nmol/L, 4-1BB: 0.15 nmol/L 4-1BB-expressing cell (eg, T cell) Conditional 4-1BB agonist activity PD-1 PDL-1 4-1BB BNT311/ GEN1046 Checkpoint blockade Enhanced recruitment of immune cells Enhanced NK cells activity Infiltrating immune cells Reactivation of exhausted T cell Enhanced T-cell activation T-cell proliferation and differentiation Enhanced effector T-cell activity PD-1 GEN 1046 PD-L1 4-1BB Perforin Granyme Chemokines Cytokines PD-L1: receptor-ligand expressed on tumor cells that inhibits proliferation of PD1-positive cells, and has a role in immune evasion. Conditional bispecific molecule for two validated targets: 4-1BB: costimulatory tumor necrosis factor expressed on T and NK-cells. Activating the 4-1BB pathway enhances T cell proliferation, T cell effector functions and prevents T cell death. Muik A, et al. Cancer Discov 2022; 12:1248−1345.

BNT311/GEN10461 – Preclinical Data 85 1. Partnered with Genmab. CPI = Checkpoint Inhibitor; PD-L1 = programmed cell death ligand 1; ctrl = control. 4-1BB agonist activity of BNT311/GEN1046 was strictly conditional on PD-L1 binding Muik A, et al. Cancer Discov 2022; 12:1248−1345. P D -1 /P D -L 1 b lo c k a d e ( fo ld i n c re a s e ) 8 6 4 2 0 0.001 0.01 0.1 1 10 100 Antibody concentration (µg/mL) BNT311/GEN1046 Anti–PD-L1 mAb Atezolizumab analoque Isotype ctrl BNT311/GEN1046 blocks the PD-1/PD-L1 axis in the absence of 4-1BB binding, showing that its PD-L1–specific Fab arm also functions as a classic immune CPI BNT311/GEN1046 exhibits antitumor activity in vivo

First-in-Human Trial with BNT311/GEN10461 in Patients with Metastatic or Unresectable Solid Tumors 1. Partnered with Genmab. Q2W = once every three weeks; PD = progressive disease; NSCLC = non-small-cell lung cancer; HNSCC = head and neck squamous-cell cancer; TNBC = triple-negative breast cancer; RECIST = Response Evaluation Criteria In Solid Tumors. Expansion dose 100 mg Q3W Key endpoints Safety, pharmacokinetics, immunogenicity, pharmacodynamics and antitumor activity (RECIST v1.1) Status Recruiting 13 expansion cohorts 86 Phase 2 Dose expansion cohorts: • NSCLC - 1L, monotherapy • NSCLC - 1L, + pembrolizumab • NSCLC (non-squamous) - 1L, +pembrolizumab and chemotherapy • NSCLC (squamous) - 1L, + pembrolizumab and chemotherapy PD-(L)-1 inhibitor pretreated cohorts: • Cervical cancer • Endometrial cancer • HNSCC • TNBC • Urothelial cancer Inclusion criteria • Metastatic or unresectable solid tumors • Patients who are not candidates for standard therapy Phase 1 Dose escalation (N=61) BNT311/GEN1046 IV flat dose Q3W until PD or unacceptable toxicity Phase 1/2a trial design (NCT03917381), multicenter, non-randomized, open-label

Initial Results of BNT311/GEN10461 Monotherapy in Dose Escalation Show a Manageable Safety Profile and Clinical Activity 1. Partnered with Genmab. DLT = dose-limiting toxicity; MTD = maximum tolerated dose; TEAE= treatment-emergent adverse event; TRAE = treatment-related adverse event, TNBC = triple negative breast cancer; NSCLC = non-small cell lung cancer; CPI = checkpoint inhibitor; AST = aspartate transaminase; ALT = alanine transaminase. Dose escalation cohort TEAE's occurring in ≥10% of patients All grades, n (%) Grade ≥3, n (%) Any TRAE 43 (70.5) 17 (27.9) TRAEs in ≥10% patients, by preferred term ALT increased AST increased Hypothyroidism Fatigue 14 (23.0) 13 (21.3) 11 (18.0) 8 (13.1) 5 (8.2) 2 (3.3) 1 (1.6) 1 (1.6) Phase 1/2a FIH trial (NCT03917381): Safety & efficacy, dose escalation monotherapy Garralda E. et al. presented at SITC 2020, Poster #412. 87 In the dose escalation phase, BNT311/GEN10461 demonstrated a manageable safety profile and preliminary clinical activity in a heavily pretreated population with advanced solid tumors: • Disease control achieved in 65.6% (40/61) of patients at a median of 3 months follow-up • 4 early partial responses in TNBC (1), ovarian cancer (1), and CPI pre-treated NSCLC (2) patients • Most AEs were mild to moderate: • TRAEs occurred in 43 (70.5%) patients • Grade 3–4 TRAEs were experienced by 17 (27.9%) patients • MTD was not reached • 6 patients had DLTs; all 6 patients recovered without sequelae Data cut-off: August 31, 2020.

BNT311/GEN10461 Monotherapy Demonstrates Efficacy in Patients with Advanced Solid Tumors Who had Failed PD-(L)1 Treatment including in NSCLC 88 1. Collaboration with Genmab; PD-L1 = programmed cell death ligand 1; NSCLC = non-small cell lung cancer; CPI = checkpoint inhibitor; . Phase 1/2a FIH trial (NCT03917381): Clinical efficacy, 100 mg Q3W monotherapy Ponce Aix S. et al. presented at SITC 2021, Poster #516. B e s t re la ti v e c h a n g e i n s u m o f d ia m e te rs f ro m b a s e li n e , % 40 20 0 -20 -40 PD-L1+ PD-L1- • BNT311/GEN1046 elicits early responses across expansion cohorts of patients who failed prior CPI therapy • Patient selection based on tumoral PD-L1 status and anti–PD-1 combination therapy are being explored and may improve clinical efficacy with GEN1046 Tumor reduction in 7/11 with PD-L1+ tumors Clinical activity by tumor PD-L1 status* in CPI-experienced patients with NSCLC (n=25) Data cut-off: September 21, 2021. *aPD-L1 status based on analysis of fresh tumor biopsy specimens collected following progression on last prior treatment and before first dose of BNT311/GEN1046.

Ongoing Phase 2 Trials Investigating BNT311/GEN10461 as Single Agent and in Combination with Pembrolizumab in NSCLC and Endometrial Cancer 89 1. Partnered with Genmab; 50:50 profit/loss collaboration. NSCLC = non-small cell lung cancer; PD-L1 = programmed cell death ligand 1; FPD = first patient dosed; CPI =check point inhibitor; TPS = tumor proportion score; R/R = relapse/refractory. NSCLC Endometrial cancer Inclusion criteria Stage IV metastatic R/R NSCLC (2L+) PD-L1 TPS ≥1% Prior treatment with an anti-PD-(L) 1 Treatment experienced advanced endometrial carcinoma (2L) Cohort A: CPI naïve Cohort B: CPI-experienced Treatment arms • A: BNT311/GEN1046 monotherapy • B: BNT311/GEN1046 + pembrolizumab (Q3W) • C: BNT311/GEN1046 + pembrolizumab (Q6W) • BNT311/GEN1046 + pembrolizumab Status • Recruiting • FPD December 2021 • Recruiting • FPD projected for November 2023 Engage with health authorities on the design of a pivotal trial in post-IO non-small cell lung cancer Plan to present data at a medical conference in 2024 Next steps NEW

A CD27 antibody based on the HexaBody technology, specifically engineered to form an antibody hexamer upon binding its target on T cell membranes. BNT313/ GEN10532 Clinical status • Ph1/2 in multiple solid tumors Monospecific antibody with optimized Fc targeting CTLA-4 and selectively depleting tumor-infiltrating Tregs in the TME but not in the periphery due to a pH driven mechanism. BNT316/ ONC-3921 (gotistobart) Clinical status • Ph1/2 in multiple solid tumors • Ph2 in PROC • Ph3 in 2L+ mNSCLC Bispecific antibody to inhibit proliferation of PD1-positive cells. 4-1BB enhances T cell proliferation, T cell effector functions and prevents T cell death. BNT311/ GEN10462 Clinical status • Ph1/2 in multiple solid tumors • Ph2 in mNSCLC • Ph2 in 2L mEC Engagement of CD40 leads to activation and maturation of APCs. 4-1BB enhances T cell proliferation, T cell effector functions and prevents T cell death. BNT312/ GEN10422 Clinical status • Ph1/2 trials in multiple solid tumors Bispecific antibody designed to boost antitumor immune response through EpCAM-dependent 4-1BB agonistic activity. BNT314/ GEN10592 Clinical status • Ph1/2 in multiple solid tumors planned 1. Partnered with OncoC4; 2. Partnered with Genmab; 3. Partnered with Biotheus. CTLA4 = Cytotoxic T-Lymphocyte-Associated Protein 4; CD27, CD40, 4-1BB = members of the tumor necrosis factor receptor superfamily; PD-1 =Programmed cell death protein 1; HER2 = human epidermal growth factor receptor 2; ADCC = Antibody dependent cell-mediated cytotoxicity; ADCP = Antibody dependent cellular phagocytosis; PROC = platinum-resistant ovarian cancer; NSCLC = non-small cell lung cancer; EC = endometrial cancer APC = antigen presenting cells; VEGF = vascular endothelial growth factor; TME = tumor microenvironment; CTx = chemotherapy; LALA = IgG1 variant L234A/L235A. PD-L1 expression or upregulation in tumors may enrich VEGF neutralization into the TME which inhibits angiogenesis. PM80023 Clinical status • Ph1b dose escalation • Ph2a as monotherapy in multiple cancers • Ph2 in combination with CTx in multiple cancers Well-Positioned in Immuno-Oncology with Therapeutic Candidates Across Multiple Tumors Anti-CD27Anti-CTLA4 Anti-4-1BBAnti-PD-L1 Anti-4-1BBAnti CD40 Anti-4-1BBEpCAM Anti-VEGF A Anti-PD-L1 VHH Inert Fc (LALA) Optimized Fc 90

BNT312/GEN10421 – Bispecific Antibody Designed to Strengthen T Cell and APC Synapse 91 1. Partnered with Genmab. APC = antigen-presenting cell; Fc = fragment crystallizable region; CD = cluster of differentiation; HLA = human leucocyte antigen; TCR = T-cell receptor; MHC = major histocompatibility complex. Muik A, et al. J Immunother Cancer 2022 CD40: stimulatory receptor primarily expressed on APCs. Engagement of CD40 leads to activation and maturation of APCs “Double-conditional” “dual-agonist” molecule for two preclinically validated targets: 4-1BB: costimulatory tumor necrosis factor expressed on T and NK- cells. Activating the 4-1BB pathway enhances T cell proliferation, T cell effector functions and prevents T cell death Inert Fc to avoid unwanted immune cells crosslinking 4-1BB–expressing cell (eg, T cell) 4-1BB GEN1042 GEN1042 CD40 CD40-expressing APC (eg, dendritic cell Enhanced natural killer (NK) cell activity Expression of costimulatory molecules e.g. HLA-DR, CD86 CD86/HLA-DR GEN 1042 CD40 4-1BB Perforin Granyme TCR-MHC receptors Cytokines CD28/CD80 receptors APC maturation Product of stimulatory cytokines Tumor draining lyph node T cell proliferation and differentiation Enhanced effector T cell activity Inert Fc, double conditional, dual CD40×4-1BB agonist Conditional CD40- stimulation of APC and conditional 4-1BB mediated stimulation of T cellsBNT312/GEN1042 binding affinity: KD CD40 1.0 nmol/L, 4-1BB: 0.17 nmol/L

BNT312/GEN10421 – Double-Conditional Dual-Agonist Molecule 92 R e la ti v e l u m in e s c e n c e u n it s CD40 reporter assay 4-1BB reporter assay In the absence of CD40+ cells, BNT312 does not exhibit any 4-1BB activation In the absence of 4-1BB+ cells, BNT312 does not exhibit any CD40 activation Muik A, et al. J Immuno Ther Cancer 2022; 10:e004322. 1. Partnered with Genmab APC = antigen-presenting cell; CD = cluster of differentiation; bsAb = bispecific antibody.

BNT312/GEN10421 Shows Higher Ability to Promote DC Maturation vs either Monoclonal Antibody or their Combination 93 0 20 40 60 Antibody concentration (μg/mL) % H L A -D R + /C D 8 6 + o f to ta l D C p o p u la ti o n Isotype ctrl bsAb-CD40×ctrl bsAb-ctrl×4-1BB bsAb-CD40×ctrl + bsAb-ctrl×4-1BB Fc inert mitazalimab analog Fc inert urelumab analog DuoBody-CD40×4-1BB 1. Partnered with Genmab Measured by flow cytometry. Data from one donor are shown. Dotted line shows percentage of HLA-DR+CD86+ DCs in DC-T-cell cultures in the absence of treatment. DC = dendritic cell; HLA = human leucocyte antigen; CD = cluster if differentiation; bsAb = bispecific antibody; Fc = fragment crystallizable region Muik A, et al. J Immuno Ther Cancer 2022; 10:e004322. DC maturation

Data from Dose Escalation of BNT312/GEN10421 in Patients with Metastatic or Unresectable Solid Tumors 94 1. Partnered with Genmab; a. Starts with an accelerated titration phase consisting of single-patient cohorts followed by larger cohorts informed by the modified continuous reassessment method and escalation with overdose control design; b. CTor MRI: every 6wk for 50 wk, and every 12 wk thereafter. CNS = central nervous system; RECIST = Response Evaluation Criteria In Solid Tumors; ECOG PS = Eastern Cooperative Oncology Group performance status ; MTD = maximum tolerated dose; RP2D = recommended phase 2 dose; PK = pharmacokinetic. Phase 1/2a trial design (NCT04083599), multicenter, non-randomized, open-label: Dose escalationa Johnson M, et al. J Immunother Cancer. 2021;9(suppl2):A525. Abstract 493. Inclusion criteria • Age ≥18y • Histologically or cytologically confirmed, metastatic or unresectable, non-CNS solid tumor • Not candidate for standard therapy • Measurable disease according to RECIST v1.1b • ECOG PS 0–1 • Aqeduate renal, hepatic, and hematologic function Flat-dose IV BNT312/GEN1042 administered in 21-d cycles until disease progression/unacceptable toxicity Key endpoints Primary: MTD, RP2D Secondary: Safety (tolerability), Antitumor activity by RECIST v1.1; PK, Immunogenicity Exploratory: Pharmacodynamics (safety biomarkers), Biomakers for response, Antitumor activity by iRECIST Data cutoff: August 27, 2021 0.1 mg (n=1) 0.3 mg (n=1) 1 mg (n=2) 3 mg (n=4) 10 mg (n=6) 30 mg (n=9) 60 mg (n=9) 100 mg (n=9) 200 mg (n=9) 400 mg (n=3) Expansion dose

• Disease control rate 50% • 2 patients with confirmed PR (melanoma, neuroendocrine lung cancer) 100mg Q3W was identified as the expansion dose BNT312/GEN10421 Shows Manageable Safety Profile and Encouraging Clinical Activity in a Heavily Pretreated Heterogenous Patient Population 95 1. Partnered with Genmab. DLT`= dose limiting toxicity; MTD = maximum tolerated dose; CRS = cytokine release syndrome; PD = progressive disease; SD = stable disease; PR = partial response; NE = not evaluable; NA = npt applicable. Safety as a single agent: Dose escalation (n=50) Antitumor activity as a single agent: Dose escalation (n=50) • 1 DLT (grade 4 transaminase elevation at 200 mg) that resolved with corticosteroids • MTD not reached • No drug-related grade ≥3 thrombocytopenia or CRS • No treatment-related deaths 75 50 25 0 -25 -50 -75 -100 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 63 66 69 72 75C h a n g e i n s u m o f d ia m e te rs f ro m b a s lin e , % N=50 +20% -30% Best confirmed overall response Overall response NA/NE PD SD PR NE PD SD PR Johnson M, et al. J Immunother Cancer. 2021;9(suppl2):A525. Abstract 493. Study week

96 Dose Expansion of BNT312/GEN10421 in Patients with Metastatic or Unresectable Solid Tumors Phase 1/2 trial designs (NCT04083599, NCT05491317), open-label, multi-center, open-label Melero et al. Presented at ESMO-IO 2022. Poster#692. Inclusion criteria • Selected metastatic or unresectable solid tumors • Measurable disease (per RECIST v1.1) • ECOG PS 0–1 • Adequate renal, hepatic, and bone marrow function • No prior therapy for metastatic diseases and no prior anti-PD(L)1 or other checkpoint inhibitor therapy Expansion cohorts - combination Two trials recruiting for expansion cohorts Key endpoints Primary: DLT, ORR per RECIST v1.1 Secondary: DOR, DCR, PFS, AEs, PK/PD Status 1. Partnered with Genmab. 5-FU, 5-fluorouracil; AEs, adverse events; Carbo, carboplatin; Cis, cisplatin; DCR, disease control rate; DLT, dose-limiting toxicity; DOR, duration of response; ECOG PS, Eastern Cooperative Oncology Group performance status; GEM, gemcitabine; Gr, grade; HNSCC, head and neck squamous cell carcinoma; MTD, maximum tolerated dose; nab-PAC, nab-paclitaxel; NSCLC, non-small cell lung cancer; ORR, overall response rate; PD, progressive disease; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PEM, pembrolizumab; PFS, progression-free survival; PK/PD, pharmacokinetics/pharmacodynamics; Q3W, every 3 weeks; RECIST, Response Evaluation Criteria in Solid Tumours. BNT312/GEN1046 + pembrolizumab: 1L Melanoma 1L NSCLC PD-L1+ TPS 1–49% 1L NSCLC PD-L1+ TPS ≥50% 1L HNSCC PD-L1+ CPS ≥1 BNT312/GEN1046 + pembrolizumab+ chemotherapy: 1L HNSCC PD-L1+ CPS ≥1 1L NSCLC squamous 1L NSCLC non-squamous 1L Pancreatic ductal adenocarcinoma Expansion dose 100 mg Q3W

Safety Run-in Results of BNT312/GEN10421 in Combination with Pembrolizumab and SoC Chemotherapy Show Favorable Safety Profile Data cut-off: October 2, 2022. 1. Partnered with Genmab; 50:50 profit/loss collaboration. DLT = dose-limiting toxicity; MTD = maximum tolerated dose; AE = adverse event. Transaminase elevation includes the preferred terms: alanine aminotransferase increased and aspartate aminotransferase increased. Rash includes the preferred terms: rash and rash maculo-popular. Fatigue includes asthenia and fatigue. 97 • In combination with pembrolizumab +/- SoC chemotherapy BNT312/GEN1042 was well tolerated across a wide range of dose levels • 100mg was selected for dose expansion phase BNT312/GEN1042 (NCT04083599): Safety Melero et al. Presented at ESMO-IO 2022. Poster#692. Treatment-related adverse events in ≥10% No DLTs were observed during the safety run-in AEs were primarily grade 1/2 Immune-related AEs were as expected and manageable Transaminase elevations resolved with corticosteroids GEN1042 + pembro (n=24) GEN1042 + pembro + SoC CTx (n=26) 8.3 8.3 12.5 12.5 4.2 12.5 4.2 4.2 4.2 4.2 4.2 8.3 4.2 0 5 10 15 20 Nausea Pyrexia Fatigue Rash Transaminase elevation Pruritus Grade 1 Grade 2 Grade 3 Grade 4 7.7 7.7 11.5 11.5 19.2 3.8 3.8 3.8 15.4 11.5 3.8 3.8 3.8 7.7 3.8 0 10 20 30 40 Nausea Pyrexia Fatigue Rash Transaminase elevation Pruritus % of patients % of patients 16.7% 16.7% 16.7% 16.7% 12.5% 12.5% 34.6% 23.1% 19.2% 15.4% 15.4% 11.5%

Safety Run-in Results of BNT312/GEN10421 in Combination with Pembrolizumab and SoC Chemotherapy Show Preliminary Activity in Patients with HNSCC 1. Partnered with Genmab. HNSCC = Head and neck squamous cell carcinomas; PD-L1 = programmed cell death ligand 1; PR = partial response; CR = complete response; HPV = human papillomavirus. • Deep responses in 4/4 evaluable patients with advanced/metastatic HNSCC • Responses were seen in tumors with both low and high PD-L1 expression; all 4 patients were HPV negative BNT312/GEN1042 (NCT04083599): Efficacy Melero et al. Presented at ESMO-IO 2022. Poster#692. B e s t c h a n g e f ro m b a s e li n e i n s u m o f le s io n d ia m e te rs ( % ) 0 -20 -40 -60 -80 -100 PR PR CR CR 0 0 12 18 24 30 -25 -50 -75 -100 6 P e rc e n t c h a n g e f ro m b a s e li n e in s u m o f le s io n d ia m e te rs Best confirmed Overall response PR CR 98 Study week Data readout of expansion cohorts of Phase1/2 trial planned for 2024 Next steps Data cut-off date: October 3, 2022.

A CD27 antibody based on the HexaBody technology, specifically engineered to form an antibody hexamer upon binding its target on T cell membranes. BNT313/ GEN10531 Clinical status • Ph1/2 in multiple solid tumors Monospecific antibody with optimized Fc targeting CTLA-4 and selectively depleting tumor-infiltrating Tregs in the TME but not in the periphery due to a pH driven mechanism. BNT316/ ONC-3922 (gotistobart) Clinical status • Ph1/2 in multiple solid tumors • Ph2 in PROC • Ph3 in 2L+ mNSCLC Bispecific antibody to inhibit proliferation of PD1-positive cells. 4-1BB enhances T cell proliferation, T cell effector functions and prevents T cell death. BNT311/ GEN10461 Clinical status • Ph1/2 in multiple solid tumors • Ph2 in mNSCLC • Ph2 in 2L mEC Engagement of CD40 leads to activation and maturation of APCs. 4-1BB enhances T cell proliferation, T cell effector functions and prevents T cell death. BNT312/ GEN10421 Clinical status • Ph1/2 trials in multiple solid tumors Bispecific antibody designed to boost antitumor immune response through EpCAM-dependent 4-1BB agonistic activity. BNT314/ GEN10591 Clinical status • Ph1/2 in multiple solid tumors planned 1. Partnered with Genmab; 2. Partnered with OncoC4; 3. Partnered with Biotheus. CTLA4 = Cytotoxic T-Lymphocyte-Associated Protein 4; CD27, CD40, 4-1BB = members of the tumor necrosis factor receptor superfamily; PD-1 =Programmed cell death protein 1; HER2 = human epidermal growth factor receptor 2; ADCC = Antibody dependent cell-mediated cytotoxicity; ADCP = Antibody dependent cellular phagocytosis; PROC = platinum-resistant ovarian cancer; NSCLC = non-small cell lung cancer; EC = endometrial cancer APC = antigen presenting cells; VEGF = vascular endothelial growth factor; TME = tumor microenvironment; CTx = chemotherapy; LALA = IgG1 variant L234A/L235A. PD-L1 expression or upregulation in tumors may enrich VEGF neutralization into the TME which inhibits angiogenesis. PM80023 Clinical status • Ph1b dose escalation • Ph2a as monotherapy in multiple cancers • Ph2 in combination with CTx in multiple cancers Well-Positioned in Immuno-Oncology with Therapeutic Candidates Across Multiple Tumors Anti-CD27Anti-CTLA4 Anti-4-1BBAnti-PD-L1 Anti-4-1BBAnti CD40 Anti-4-1BBEpCAM Anti-VEGF A Anti-PD-L1 VHH Inert Fc (LALA) Optimized Fc 99

PM80021 – A Next-Gen IO Agent that Combines Two Clinically Validated MoA 100 1. Partnered with Biotheus. MoA = Mode of Action TME = Tumor Microenvironment 2. The MoA graph generated by Biorender.com “Two in one” MoA synergies with ADCs Dual blockade of PD-L1 and VEGF-A have been proven synergistic • Compelling profile with over 500 patients treated to date • Monotherapy activity and synergy in combination therapy observed in early clinical studies • Encouraging safety profile vs PDL1 + VEGF inhibition or PD1 alone Protein binding activity (KD) for human • PD-L1: 5.5 nM • VEGF-A: <0.4 nM PM8002

Reversion of multi-level immune-suppressive effects of VEGF Reversion of tumor-angiogenesis promoting effects of VEGF Anti-VEGF Treatment Impacts Tumor Vasculature and Tumor Microenvironment 101 Sourced from https://www.creativebiolabs.net/bevacizumab-overview.htm VEGF(R) = vascular endothelial growth factor (receptor); DC = dendritic cell; Treg = regulatory T cells; CXCL = chemokine (C-X-C motif) ligand; IFN = interferon; CD3 = cluster of differentiation 3; MSDC = myeloid derived suppressor cells. 1. Downregulating T-cell activation via inhibition of DC maturation 2. Reducing T-cell tumor infiltration 3. Increasing inhibitory cells such as myeloid derived suppressor cells (MDSCs) and regulatory T cells (Tregs) in the tumor microenvironment 1 3 2 1 2 3 Hegde P. et al. Seminars in Cancer Biology. 2018.

Anti-VEGF is a Validated Mechanism Approved in or Shown Clinical Activity in a Wide Range of Tumors 102 1L OC/TFC/PPC 2L+ OC/FTC/PPC Platinum Resistant 2L OC/FTC/PPC Platinum Sensitive 1L NSq NSCLC Driver Gene WT 1L NSCLC EGFRm+Lung Gynae- cology 2L+ NSCLC Cervical Cancer 1L NSq NSCLC RCC 1L HCC 2L CRC GBMBC HER2- 1/2L CRC Breast Gastroin testinal Genitouri nary Others Mono Combo Mono Combo + 5FU based chemo + FOLFIRI + FOLFOX 3L CRC + Trifluridine /Tipiracil + Interferon alfaMaintenance + Paclitaxel + PLD + Topotecan Maintenance + Atezolizumab Cervical Cancer PD- L1+ 2L+ GC/GEJ + Paclitaxel 2L HCC Advanced BTC + Erlotinib (Maintenance) NEN + Temsirolimus + FOLFOX Melanoma + Temsirolimus + Temozolomide SCLC Unapproved Approved + Carboplatin/Paclitaxel + Atezolizumab/ Carboplatin / Paclitaxel +Paclitaxel/Cisplatin + Paclitaxel/Topotecan +Paclitaxel/Carboplatin + Paclitaxel / Carboplatin + Gemcitabine / Carboplatin + Pembrolizumab / Paclitaxel based chemo + Erlotinib + Docetaxel + Capecitabine + Docetaxel + Cisplatin/Irinotecan RCC= Renal Cell Carcinoma; OC=Ovarian Cancer; TFC= Fallopian Tube Cancer; PPC=Primary Peritoneal Cancer: NSCLC=Non-small Cell Lung Cancer; BTC=Biliary Tract Cancer; SCLC=Small Cell Lung Cancer; BC=Breast Cancer; HCC=Hepatocellular Carcinoma; MPM=Malignant Pleural Mesothelioma; NEN=Neuroendocrine Neoplasm, GBM=Glioblastoma, CRC=Colorectal Cancer, GC/GEJ=Gastric /Gastro-Esophageal Junction Cancer; PLD: Pegylated liposomal doxorubicin, Anti-VEGF includes bevacizumab and ramucirumab. Legend: Clinical Activity Demonstrated

PM8002 Mono and Combo Have Been Investigated in 10+ Indications in More Than 500 Patients 103 2L+ Cervical Cancer nccRCC 2L+ ccRCC PROC 2L+ PSOC 2L+ NSCLC EGFRm Advanced BTC 1L MPM 2L SCLC 1L TNBC 1L HCC Mono Combo Lung Breast Gynaeco logy Gastroin testinal Genitour inary Others (+ Paclitaxel) (+ nab-Paclitaxel) (+ FOLFOX4) (+ Pemetrexed / Platinum) Mono Combo (+ Pemetrexed / Carboplatin) 1L SCLC (+ Etoposide / Platinum) Mucosal Melanoma 2L+ Endometrial Cancer 1L NSCLC Driver Gene WT, PD-L1+ 2L NEN (+ FORFIRI) nccRCC=Non-Clear Cell Renal Cell Carcinoma; RCC= Renal Cell Carcinoma; PROC=Platinum-resistant Ovarian Cancer; PSOC=Platinum-sensitive Ovarian Cancer; NSCLC=Non-small Cell Lung Cancer; BTC=Biliary Tract Cancer; SCLC=Small Cell Lung Cancer; TNBC=Triple-negative Breast Cancer; HCC=Hepatocellular Carcinoma; MPM=Malignant Pleural Mesothelioma; NEN=Neuroendocrine Neoplasm. Legend: Ongoing studies with PM8002

PM80021 Monotherapy in Patients with Advanced Solid Tumors 1. Partnered with Biotheus. Trial registration: ChiCTR2000040552. QxW = every x weeks; RP2D = recommended phase 2 dose; ECOG PS = ORR = objective response rate; ECOG PS= eastern cooperative oncology group performance status. Phase 1/2 trial design, open-label, monotherapy 104 Part 1: Dose Escalation Part 2: Dose expansion Indications • Mucosal melanoma • Ovarian cancer • Endometrial cancer • Cervical cancer • Renal cell cancer • Non-small cell lung cancer • Hepatocellular carcinoma • Small cell lung cancer • Others RP2D 20 mg/kg Q2W and 30 mg/kg Q3W Disease progression, withdrawal of consent, unacceptable toxicity Inclusion criteria • Advanced or metastatic tumors • Age 18-75 years • ECOG PS 0-1 • Adequate organ function • Exclude evidence of significant bleeding and coagulation disorder or other significant bleeding risk Primary endpoints: adverse events according to CTCAE5.0 and ORR per RECIST1.1 Secondary endpoint: testing for anti-drug antibodies (ADA) Key endpoints: Dose levels from 1 mg/kg Q2W to 45 mg/kg Q3W were evaluated in 310 patients

PM80021 Monotherapy Shows Encouraging Antitumor Activity and Safety Profile in Patients with Advanced Solid Tumors in a Phase 1/2 Trial 105 1. Partnered with Biotheus. ORR = objective response rate; DCR = disease control rate, DoR = duration of response; PFS = progression free survival; PD = progressive disease; SD = stable disease; PR = partial response; CR = complete response. PM8002 in Ph1/2: Clinical activity of monotherapy Ye Guo et al. Presented at ASCO 2023. Poster#378 Best tumor response for evaluable patients (n=254): • ECOG PS 1: ~62% • Prior # received ≥1 anticancer therapies: ~76% • Prior IO therapy: ~ 5% -100 -80 -60 -40 -20 0 20 40 60 80 100 PD SD PR CR Efficacy evaluable patients, n 254 ORR, % 16.1 DCR, % 74.4 -100 -80 -60 -40 -20 0 20 40 60 80 100 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Study Months C h a n g e f ro m B a s e li n e ( % ) Efficacy evaluable patients, n 254 Median DoR, months 7.4 Median PFS, months 5.6

PM80021 Monotherapy is Well Tolerated in Patients with Advanced Solid Tumors in a Phase 1/2 Trial 106 1. Partnered with Biotheus. TRAE = treatment related adverse event, SAE = serious adverse event. PM8002 in Ph1/2: Safety for monotherapy Ye Guo et al. Presented at ASCO 2023. Poster#378 All TRAEs, n (%) 239 (77.1%) TRAE ≥3, n (%) 64 (20.6) SAE, n (%) 35 (11.3) TRAE leading to dose discontinuation, n (%) 17 (5.5) • 1 grade 4 event: anemia • No grade 5 events PD-L1 expression in tumors may enrich and focus VEGF neutralization into the TME sparing normal tissue and leading to reduced systemic toxicity Blocking VEGF inhibits the development of angiogenesis impeding tumor growth Ph1b/2 dose expansion monotherapy and Ph2 chemotherapy combi ation trials ongoing for multiple indications in China IND accepted for further studies in the US TRAE ≥10% of patients All grades, n (%) Grade 3, n (%) Aspartate aminotransferase increased 42 (13.5) 2 (0.6) Alanine aminotransferase increased 39 (12.6) 1 (0.3) Hypercholesteremia 38 (12.3) 0 Hypoalbuminemia 35 (11.3) 0 Hypertriglyceridemia 31 (10) 2 (0.6) Proteinuria 82 (26.5) 4 (1.3) Hypertension 60 (19.4) 20 (6.5) Hypothyroidism 34 (11) 0 Anemia 32 (10.3) 0

PM80021 in Combination with Paclitaxel as Second Line Treatment for SCLC 107 1. Partnered with Biotheus; 2. As of September 08, 2023. Small Cell Lung Cancer = Small Cell Lung Cancer ECOG PS= eastern cooperative oncology group performance status. ORR = Overall response rate; DCR = Disease control rate; TRAE = treatment-related adverse events; DoR = Durability of Response PFS = Progression Free Survival OS = Overall Survival qxw = every X week(s). Inclusion criteria • Patients with advanced SCLC who progressed after platinum-based chemotherapy with or without checkpoint inhibitors • Age ≥ 18 years • ECOG PS 0-1 • Adequate organ function n=99 Key endpoints Status • 48 patients enrolled2, recruiting ongoing Primary: • ORR per RECIST1.1 • TRAEs incidence and severity Secondary endpoint: DCR, DoR, PFS and OS Phase 2 trial, open-label, single-arm combination (NCT05879068) PM8002 30mg/kg + paclitaxel IV Q3W for 5 cycles PM8002 30mg/kg as maintenance treatment Disease progression, withdrawal of consent, unacceptable toxicity

Response Efficacy Evaluation Population ITT (n=36) IO-naïve (n=22) IO- treated (n=14) ORR, % 61.1 72.7 42.9 DCR, % 86.1 81.8 92.9 Phase 2 (NCT05879068): clinical activity of PM8002 in combination with paclitaxel Ying Cheng et al. Presented at ESMO 2023. Poster#1992P PM80021 Combined with Paclitaxel Shows Encouraging Antitumor Activity as Second Line Therapy in Patients with SCLC 108 1. Partnered with Biotheus; SCLC = small cell lung cancer; IO = immuno oncology; ORR = objective response rate; DCR = disease control rate, DoR = duration of response; PFS = progression free survival; CTFI = chemotherapy-free interval; TTP = time to progression; PD = progressive disease; SD = stable disease; PR = partial response; CR = complete response -90 -70 -50 -30 -10 10 30 50 70 PD SD PR CR - - - + + - + ○ + + √ ○ + + ○ - + + + + - √ ○ - - - - - + - ○ - - - ○ - - √ + - √ ○ - - - + - - IO-naïve + IO-treated ○ CTFI < 30 days √ TTP < 3 months -90 -70 -50 -30 -10 10 30 50 70 90 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 PD PR SD CR On Treatment Study Months C h a n g e f ro m B a s e li n e ( % ) Response Efficacy Evaluation Population ITT (n=36) IO-naïve (n=22) IO- treated (n=14) mPFS, m 5.5 5.9 3.9 mDoR, m 10.0 10.0 2.6 • ECOG PS 1: ~62% • Prior # received ≥1 anticancer therapies: ~46%

PM8002 Combined with Paclitaxel Shows Acceptable Toxicity as Second Line Therapy in Patients with SCLC 109 1. Partnered with Biotheus. TRAE = treatment related adverse event, SAE = serious adverse event. Phase 2, open-label, single-arm, trial (NCT05879068) Ying Cheng et al. Presented at ESMO 2023. Poster#1992P PD-L1 expression in tumors may enrich and focus VEGF neutralization into the TME sparing normal tissue and leading to reduced systemic toxicity Blocking VEGF inhibits the development of angiogenesis impeding tumor growth Study week Phase 2 trial ongoing with near-term plans to enter Phase 3 trials Next steps N=48 n (%) All TRAEs 45 (93.8) TRAE ≥3 30 (62.5) SAE 16 (33.3) TRAE leading to dose discontinuation 1 (2.1) TRAE ≥10% of patients All grades, n (%) Grade 3, n (%) Grade 4, n (%) Grade 5, n (%) Neutropenia 23 (47.9) 15 (31.3) 7 (14.6) 0 Leukopenia 23 (47.9) 10 (20.8) 2 (4.2) 0 Decreased platelet count 12 (25.0) 1 (2.1) 0 0 Anemia 11 (22.9) 0 0 0 Proteinuria 9 (18.8) 2 (4.2) 0 0 Pneumonitis 6 (12.5) 0 0 1 (2.1)

Significant Tumor Shrinkage in Patients Treated by PM8002 as Monotherapy and in Combination with Chemotherapy 110 Data on file.1L/1L = First Line, Second Line Week 32 Lesion diameter: 0 mm 28.4 mm 0 mm 1L TNBC: PM8002 + nab-paclitaxel 2L SCLC: PM8002 + paclitaxel Base line Lesion diameter: 44.5mm Week 18 Lesion diameter: 8.5mm EGFR-TKI treated NSCLC: PM8002 monotherapy Base line Lesion diameter: 16.8mm Week 19 Lesion diameter: 6.2mm Base line Lesion diameter: 40.8mm Week 18 Lesion diameter: 5.0mm Base line Lesion diameter: 30.9mm Week 18 Lesion diameter: 5.0mm IO-treated Base line Lesion diameter: 28.4 mm L e s io n 1 L e s io n 2 IO-naïve

PM8002 Safety Profile Appears Favorable with Regard to AEs and irAEs Related to its Two Targets 111 Literature research, cross-trial comparison; Data on file. Anti-PD-1 includes pembrolizumab and nivolumab, anti-PD-L1 includes atezolizumab, anti-VEGF is bevacizumab, VEGF TKI includes lenvatinib and axitinib.. Anti-PD-1 includes pembrolizumab and nivolumab, anti-PD-L1 includes tezolizumab, anti-VEGF is bevacizumab, VEGF TKI includes lenvatinib and axitinib.

Immunomodulators: Key Takeaways BNT316/ONC-392 (gotistobart)1 • Additional data readouts planned in 2024 • Potential registrational trials planned in 2024 and beyond BNT311/GEN104062 • Engage with health authorities on the design of a pivotal trial in post-IO non-small cell lung cancer • Plan to present data at a medical conference in 2024 BNT312/GEN104212 • Provide a clinical data and pivotal development plan update next year 1. Partnered with OncoC4. 2. Partnered with Genmab Targeted Milestones Strategy • Leverage our next-generation immunomodulators to unlock potential in novel patient populations • Potential to act as an improved backbone for novel combinations 112

7 Solid Tumor Cell Therapy

Solid Cancers Pose a Special Challenge for CAR-T cells 114 Permanent accessible antigen Blood vessel # C A R -T time threshold Lack of peripheral antigen Tumor # C A R -T time threshold 0 5 10 15 20 25 CAIX (12) CEA (21) EGFR (11) ErbB2/Her2 (20) Fr-a (14) GD2 (19) IL-13Ra2 (3) L1-CAM (6) Mesothelium (2) MUC1 (1) PSMA (5) VEGFR-2 (23) CR/PR SD PD/NR/NE Liquid tumors Solid tumors CR/PR SD PD/NR/NE 0 3020 100 120 140 160 180 200 220 240 Target+ cells CAR T cell Number of treated patients A n ti g e n s o f s o li d t u m o rs Number of treated patients Best clinical outcome, target antigen CD19 (n=243) Best clinical outcome CR = complete response; NE = not evaluable; NR = no response; PD = progressive disease; PR = partial response; SD = stable disease. Hartmann et al., EMBO MM. 2017. Hartmann et al., EMBO MM. 2017.

Frequencies of CLDN6 expression in high medical need cancers 115 Reinhard, Rengstl et al. Science 2020 Ovary Endometrium Lung Testis Healthy tissue Tumor Indication CLDN6+ CLDN6high Testicular Cancer* 93 % 90-93 % Ovarian Cancer* 56 % 25-30 % Uterine Cancer* 23 % 10-15 % Lung Cancer** 11 % 2-5 % Gastric Cancer*** 9 % 2-5 % * Majority of subtypes ** Primarily adeno and large cell cancer *** α-fetoprotein+ subtype CLDN6high 50% of tumor cells expressing ≥2+ CLDN6 protein (IHC)

Potent 2nd generation CAR with high sensitivity and specificity Reinhard K, et al. Science 2020, 367:446–453 BNT211: A CLDN6 CAR-T-Cell Therapy + CLDN6-Encoding CARVac that Enhances Expansion and Persistence of the Infused CAR-T Cells ACT = adoptive cell transfer; APC = antigen-presenting cell; CAR = chimeric antigen receptor; CARVac = CAR-T cell-amplifying RNA vaccine; CLDN6 = claudin 6. αCLDN6 scFv CD8 hinge 4-1BB CD3ζ L ys is [ % ] C L D N 3 C L D N 4 C L D N 6 C L D N 9 80 60 40 20 0 Liposomes Full-length CLDN6 RNA CARVac IV Combined with CARVac (CAR-T cell amplifying RNA vaccine) to target APCs, Reinhard K, et al. Science 2020, 367:446–453; Kranz LM, et al. Nature 2016; 534:396–401 CLDN6 CAR-T GFP T cells 0 500 1,000 1,500 -10 5 0 5 10 15 20 25 T u m o r v o lu m e [ m m 3 ] Days after ACT ± 116

BNT211-01: Phase 1/2a, FIH, Open-Label, Multicenter, Dose Escalation Trial in R/R Advanced CLDN6+ Solid Tumors (NCT04503278) 117 Data cut-off: 10 Sep 2023. * Crossover to combination not indicated. CAR = chimeric antigen receptor; CARVac = CAR T-cell amplifying RNA vaccine; CLDN6 = claudin-6; DCR = disease control rate; DL = dose level; DLT = dose-limiting toxicity; DoR = duration of response; ECOG PS = Eastern Cooperative Oncology Group performance status; ORR = objective response rate; PFS = progression-free survival; RECIST = response evaluation criteria in solid tumors; R/R = relapsed/refractory. DL2 n=9 + fixed CARVac DL1 n=4 + fixed CARVac DL2 n=6 1×108 CAR-T DL1 n=3 1×107 CAR-T Monotherapy Combination* DL2 n=13 1×108 CAR-T DL1 n=4 1×107 CAR-T Monotherapy Combination DL3 n=7 2-5×108 CAR-T DL0 n=2 1×106 CAR-T • Escalating doses of CLDN6 CAR-T cells ± CLDN6 CARVac • Lymphodepletion prior to CAR-T cell infusion on Day 1 (DLTs assessed for 28 days) • CLDN6 CARVac fixed dose repeatedly after CAR T transfer Assessments: Efficacy assessments Q6W (RECIST v1.1) & tumor marker monitoring Phase I dose escalation (manual product): Completed Phase I dose escalation with an (automated product): Ongoing Inclusion criteria ≥50% tumor cells with 2+/3+ CLDN6 positivity (immunohistochemistry) Measurable disease per RECIST v1.1 or elevated tumor marker ECOG PS 0–1 DL2 n=14 + fixed CARVac DL1 n=4 + fixed CARVac DL3 n=0 + fixed CARVac Key endpoints Primary: Safety and tolerability, DLTs Secondary: Immunogenicity, ORR, DCR, DoR, PFS Dosing: ESMO 2022 (n=22) ESMO 2023 (n=44) Mackensen et al. Nature Medicine. 2023. Data published in Mackensen et al. Nature Medicine. 2023.

Clinical Benefit Seen in Patients with Manual Manufacturing Process 118 LD= lymphodepletion; CR = complete response; PR = partial response; SD = stable disease; PD = progressive disease. Phase 1/2 FIH study (NCT04503278): Clinical activity of BNT211 +/- CARVac Mackensen et al. Nat. Med. 2023.

Case Report Demonstrates Clinical Response to BNT211 119 HDCT = high-dose chemotherapy; ASCT = autologous hematopoietic stemm cell transplant. Diagnosis Mixed germ cell tumor; 80% tumor cells with ≥2 + CLDN6 membrane staining positivity. Prior Therapy • Heavily pretreated (5 lines of chemotherapy in total) including cisplatinum-based chemotherapy, HDCT/ASCT gemcitabine/oxaliplatin/paclitaxel, multiple surgeries and radiotherapy • 5 years later after the 3rd line CTx with HDCT carboplatine/etoposide late disease relapse (teratoma and yolk-sac tumor) • Another relapse of a yolk-sac tumor component prior to trial entry, for the first time with multiple lung metastases • Rapidly progressing disease at accrual: 37% target sum increase between screening and ACT Sites of Metastases Lung Baseline 6 weeks post ACT Case report Mackensen et al. Nature Medicine. 2023. 12 weeks post ACT 18 weeks post ACT 52 weeks post ACT

BNT211-01: CAR T Cell-Dose-Dependent Adverse Event Profile, Dose Evaluation Ongoing to Determine RP2D Data cut-off: 10 Sep 2023. 1 Cohort includes 3 patients dosed with 5×107 CAR-T. 2 Cohort includes 1 patient that did not reach full dose (2×107) and 1 patient treated that received full dose after 50% reduced lymphodepletion. 3 Other indications: 4 patients with lung cancer (different subtypes), 3 with desmoplastic round cell tumors, 2 with esophageal cancer, 1 with endometrial carcinoma and 1 with sinonasal carcinoma. 4 Crossover of patients is not indicated, as option was enabled by safety review committee decision after dose decision for monotherapy cohort without impacting efficacy read out. 5 Most TEAEs ≥G3 were attributed to CAR-T IMP (27/30). Most frequent TEAEs were laboratory findings (43.2%) including decreased blood cell counts, elevated liver function tests as well as levels of bilirubin and ferritin. Accordingly, cytopenia (25%) together with immune system (7%) and hepatobi liary disorders (5%) were reported frequently. 6 Most frequent non- related TESAEs were infections. 7 DLTs include 2 cases of pancytopenia, 1 case of hemophagocytic lymphohistiocytosis and 1 case of liver toxicity together with sepsis. 8 CRS was limited to G1-2 for 21/23 patients with 1 G3 and 1 G4 event. 9 Neurotoxicity was mild and self-limiting in 2 patients. 10 Most patient deaths (11/12) were related to disease progression and 1 patient died from sepsis. Values given as median (range). CAR = chimeric antigen receptor; CLDN6 = claudin-6; CRS = cytokine release syndrome; DL = dose level; DLT = dose-limiting toxicity; G = Grade; ICANS = immune effector cell-associated neurotoxicity syndrome; IMP = investigational medicinal product; TESAE = treatment-emergent (serious) adverse event. Phase 1/2 FIH study (NCT04503278): Baseline characteristics and safety (automated process) Haanen J. et al. Presented at ESMO 2023. Abstract #LBA35. Cohort DL0 (n=2) DL1 (n=4) DL1 + CARVac (n=4) DL2 (n=13)1 DL2 + CARVac (n=14)2 DL3 (n=7) Total (n=44) Patient baseline characteristics Age, years 55.5 (50–61) 54.5 (36–62) 51.0 (42–65) 45.0 (30–69) 48.0 (26–60) 50.5 (29–63) 48.0 (26–69) Gender, male/female 1/1 3/1 2/2 7/6 8/6 4/3 25/19 Indication, n Epithelial ovarian cancer (EOC) Germ cell tumor (GCT) Other indications3 1 1 0 1 0 3 2 1 1 6 5 2 5 6 3 2 3 2 17 16 11 CLDN6 2+/3+ cells, % 82.5 (80–85) 97.5 (80–100) 97.5 (50–100) 95.0 (80–100) 100 (70–100) 80.0 (50–100) 95 (50–100) Prior treatment lines 3.0 (2–4) 4.0 (3–7) 4.0 (2–9) 4.0 (2–7) 4.0 (2–9) 3.5 (2-6) 4.0 (2–9) Treatment and safety outcome Duration of follow-up, days 321.5 (242- 401) 44.5 (22-87) 90.5 (13-189) 71.5 (30-317) 120.5 (9-199) 90 (44-121) 94.5 (9-401) CARVac injections4, n NA NA 3 (1-5) NA 4 (1-7) NA 4 (1-7) Patients with TEAEs ≥G3 related to IMPs5, n 1 1 1 12 9 6 30 Patients with TESAEs related to IMPs6, n 1 0 0 4 4 5 14 Patients with DLTs7, n 0 0 0 1 2 1 4 Patients with CRS8, n 1 0 2 6 9 5 23 Patients with ICANS9, n 0 0 0 1 1 0 2 Deaths10, n 1 3 2 2 4 0 12 120

BNT211-01: Signals of Activity at All Dose Levels Data cut-off: 10 Sep 2023. Waterfall plot showing best percent change from baseline in sum of target lesion diameters for patients treated with CLDN6 CAR-T (N = 38). One patient died prior to first assessment (NR = not reached) and BOR was defined as PD. * Patients had non-measurable disease per RECIST 1.1 and BOR was assessed by tumor marker response. ** Patient achieved complete response after surgical removal of tumors. Response data was pending for 6 patients at the data cutoff. Dotted lines show standard response evaluation criteria used to determine objective tumor response for target lesions per RECIST 1.1 (CR = –100%, PR = 30 to –100%, SD = –30 to 20%, and PD = 20% or higher). Graph contains additional data from 5 patients entered manually into the database following the data cut-off date that was not available in formal outputs. BOR = best overall response; CR = complete response; DCR = disease control rate; DL = dose level; EOC = epithelial ovarian cancer; GCT = germ cell tumor; PD = progressive disease; ORR = objective response rate; PR = partial response; SD = stable disease. CLDN6 CAR-T <DL2 DL2 >DL2 Total Safety evaluable patients, n 10 27 7 44 Efficacy evaluable patients, n 9 22 7 38 Patients with PR/CR, n 1 13 3 17 Patients with SD, n 1 8 2 11 Patients with PD, n 7 1 2 10 ORR, % 11.1 59.1 42.9 44.7 DCR, % 22.2 95.5 71.4 73.7 C h a n g e i n t a rg e t s u m [ % ] 60 30 0 -30 -60 -90 GCT EOC Others NR * * DL 1 1 2 0 3 2 2 1 1 3 2 2 1 1 0 2 3 3 2 2 2 2 3 2 2 2 3 2 2 2 1 2 2 2 3 2 2 2 BOR PD PD PD PD PD SD SD PD PD PR PR SD PD PD SD SD SD SD SD SD PR PR SD PR SD PR PD SD PR PR PR PR PR PR PR PR PR CR ** Best response and change in target sum (all DLs ± CLDN6 CARVac ) Phase 1/2 FIH study (NCT04503278): Efficacy at all dose levels Haanen J. et al. Presented at ESMO 2023. Abstract #LBA35. 121

BNT211-01: Encouraging Signals of Activity at Dose Level 2 122 Data cut-off: 10 Sep 2023. Waterfall plot showing best percent change from baseline in sum of target lesion diameters and spider plot showing percent change in target sum from baseline over time for patients treated with CLDN6 CAR-T ± CLDN6 CARVac at DL2 (N = 22). * Patient had non-measurable disease per RECIST 1.1 and BOR was assessed by tumor marker response. ** Patient achieved complete response after surgical removal of tumors. Response data was pending for 5 patients at the data cut-off. Dotted lines show standard response evaluation criteria used to determine objective tumor response for target lesions per RECIST 1.1 (CR = –100%, PR = 30 to –100%, SD = –30 to 20%, and PD = 20% or higher). Graphs contains additional data entered manually into the database following the data cut-off date that was not available in formal outputs. BOR = best overall response; CR = complete response; DCR = disease control rate; DL = dose level; EOC = epithelial ovarian cancer; GCT = germ cell tumor; PD = progressive disease; ORR = objective response rate; PR = partial response; SD = stable disease. CLDN6 CAR-T <DL2 DL2 >DL2 Total Safety evaluable patients, n 10 27 7 44 Efficacy evaluable patients, n 9 22 7 38 Patients with PR/CR, n 1 13 3 17 Patients with SD, n 1 8 2 11 Patients with PD, n 7 1 2 10 ORR, % 11.1 59.1 42.9 44.7 DCR, % 22.2 95.5 71.4 73.7 Days post-infusionGCT EOC Others C h a n g e i n t a rg e t s u m [ % ] C h a n g e i n t a rg e t s u m [ % ] 60 30 0 -30 -60 -90 * ** 60 40 0 -20 -60 -100 20 -40 -80 0 50 100 150 200 Best response and change in target sum (DL2 only± CLDN6 CARVac ) Phase 1/2 FIH study (NCT04503278): Efficacy at all dose levels Haanen J. et al. Presented at ESMO 2023. Abstract #LBA35.
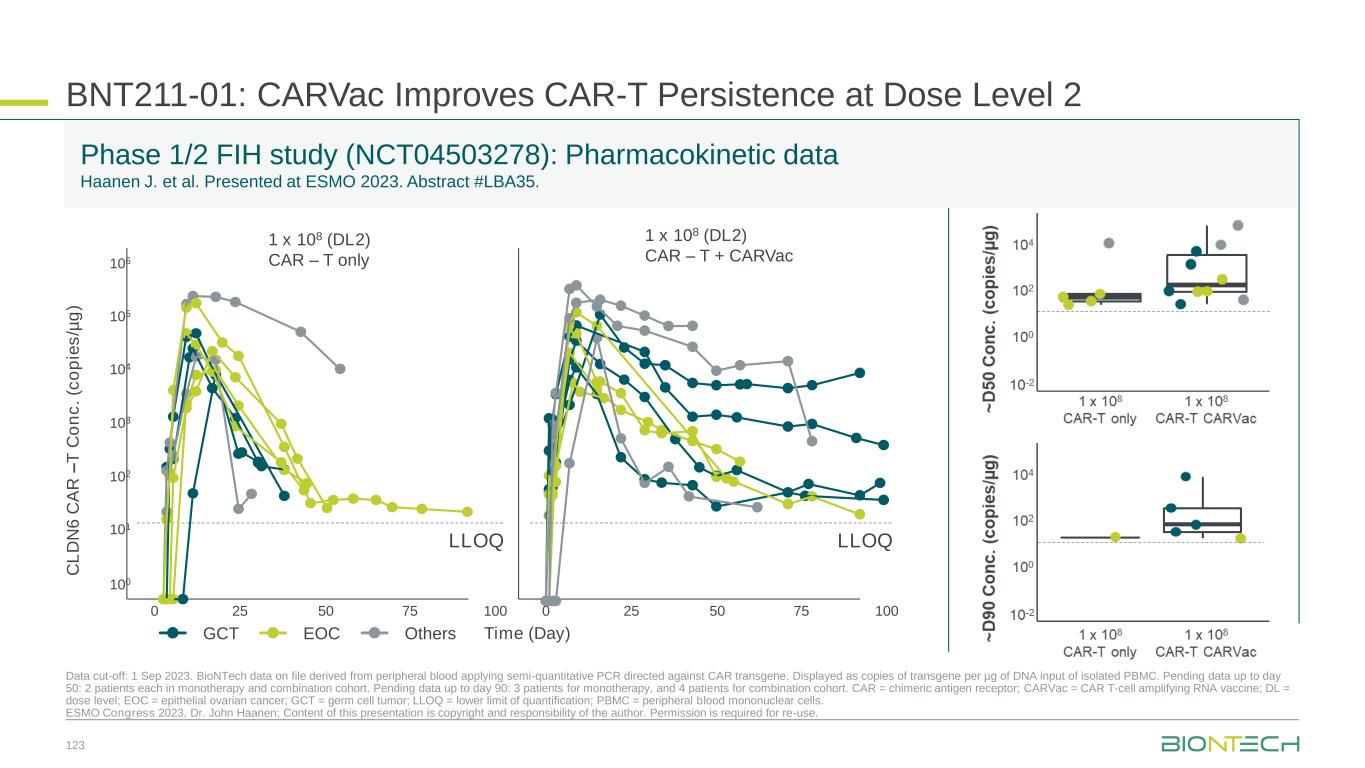
BNT211-01: CARVac Improves CAR-T Persistence at Dose Level 2 Data cut-off: 1 Sep 2023. BioNTech data on file derived from peripheral blood applying semi-quantitative PCR directed against CAR transgene. Displayed as copies of transgene per µg of DNA input of isolated PBMC. Pending data up to day 50: 2 patients each in monotherapy and combination cohort. Pending data up to day 90: 3 patients for monotherapy, and 4 patients for combination cohort. CAR = chimeric antigen receptor; CARVac = CAR T-cell amplifying RNA vaccine; DL = dose level; EOC = epithelial ovarian cancer; GCT = germ cell tumor; LLOQ = lower limit of quantification; PBMC = peripheral blood mononuclear cells. ESMO Congress 2023, Dr. John Haanen; Content of this presentation is copyright and responsibility of the author. Permission is required for re-use. Phase 1/2 FIH study (NCT04503278): Pharmacokinetic data Haanen J. et al. Presented at ESMO 2023. Abstract #LBA35. 1 x 108 (DL2) CAR – T only 1 x 108 (DL2) CAR – T + CARVac C L D N 6 C A R – T C o n c . (c o p ie s /µ g ) Time (Day)GCT EOC Others 106 105 104 103 102 101 100 0 25 50 75 100 0 25 50 75 100 LLOQLLOQ 123

Pharmacokinetics: CARVac improved CAR-T persistence with sustained, ongoing detection up to 100 days in several patients at DL2 Safety: Manageable AE profile. Dose-dependent AE profile further evaluation of safety via backfilling into dose level several cohorts BNT211 Key Takeaway Messages 124 CAR = chimeric antigen receptors; CARVac = CAR T-cell amplifying RNA vaccine; CLDN6 = claudin-6; DCR = disease control rate; DL = dose level; GCT = germ cell tumor; ORR = objective response rate; RPD2 = recommended Phase II dose. ✓ Efficacy: Encouraging signs of activity with 13 responses in 22 evaluable patients at DL2 (ORR 59%, DCR 95%)✓ Outlook: Determination of RP2D for CLDN6 CAR-T cells ongoing✓ ✓

CAR T-Cells Outlook • No curative treatment options for R/R GCT post salvage cisplatin-based chemotherapy regimens1 • Lack of new developments in the past decades • Checkpoint inhibitors failed in these patients2 1. Feldman, et al. Cancer 2012; 2. Adra, et al. Ann Oncol 2018; 3. Mackensen, et al. Nature Medicine. 2023; 4. Haanen, et al. Presented at ESMO 2023 (LBA35). PoC = Proof of Concept; Unmet medical need in R/R germ cell tumors (GCT) CAR-T cell strategy Achievements: • Presented PoC data for BNT211 in CLDN6+ indications Near-term strategy: • Aim to establish CLDN6 as proven target in solid tumors • Aim to establish first CAR T-cell therapy in first solid tumor indication (R/R GCT) Mid- to long-term strategy: • Explore expansion into other solid tumor indications A pivotal trial in R/R GCT is planned to be initiated in 2024 EMA PRIME designation in testicular cancer Published data showing anti-tumor efficacy among multiple CLDN6+ tumor types3,4 125

8 mRNA Cancer Vaccines Prof. Özlem Türeci, M.D. CMO and Co-founder

Uridine-based mRNA-LPX Vaccines for Systemic Delivery and Induction of Potent Polyspecific Immune Responses against Cancer 127 Kranz LM, et al. Nature 2016; 534:396–401; Lopez J, et al. AACR Annual Meeting 2020; Oral presentation CT301. RNA-LPX = RNA+lipoplex; CD = cluster of differentiation; TLR = toll-like receptor; NF = necrosis factor; MHC = major histocompatibility complex; TCR = T cell receptor; TAP = transporter associated with antigen processing.

mRNA Cancer Vaccines May Enable Highly Specific and Potent Activation of the Immune System Against Shared Tumor Antigens or Individual Neoantigens 128 Individual patient samples (blood and tissue) AI-driven neoantigen prediction On-demand tailored RNA manufacturing Individualized immuno- therapy Mapping of mutations Fixed combination of shared tumor antigens Multi-antigen approach tailored to each indication Neo- antigens Individualized therapy Multiple shared antigens Off-the-shelf therapy Cancer vaccine platforms iNeST1 FixVac individualized Neoantigen-Specific immunoTherapy Fixed Antigen Vaccine ANTIGEN 1 ANTIGEN 2 ANTIGEN 3 ANTIGEN 4 1. iNeST is being developed in collaboration with Genentech, a member of the Roche Group. mRNA = messenger RNA; AI = artificial intelligence.

Growing Portfolio of Cancer Vaccine Candidates Across Multiple Solid Tumors 129 1. Partnered with Genentech, member of Roche Group; 2. Sponsored by Regeneron. iNeST = individualized NeoAntigen Specific Immunotherapy;1L = first line; R/R = relapsed/refractory; CRC = colorectal cancer; PDAC = pancreatic ductal adenocarcinoma; HPV = human papillomavirus; HNSCC = head and neck squamous carcinoma; NSCLC = non-small cell lung cancer; ADT = androgen deprivation therapy; CTx = Chemotherapy. iNeST1 FixVac Multiple Solid Tumors CRC Adjuvant PDAC Prostate CancerMelanoma HPV16+ HNSCC NSCLCMelanoma 1L R/R 1LNeo-adj, mCRR/R 1L, 2L+ Six ongoing Phase 2 trials with cancer vaccine candidates in multiple disease settings Ph 2 study is ongoing Data presented from investigator- initiated Ph 1 study at ASCO 2022 and published (Rojas et al. Nature.2023) Ph 2 started in Q4 2023 Ph 1 data presented Ph 1/2 is ongoing Ph 2 study is ongoing Ph 2 study is ongoing Published data from Ph1 (Sahin et al. Nature.2020) Ph 1 basket study is ongoing Ph 2 in 1L NSCLC started in Q3 20232 Ph 2 enrollment completed Analysis of PFS as primary endpoint will be triggered event-based and defines when we will report results Autogene cevumeran/ BNT122 + Atezolizumab Autogene cevumeran/ BNT122 Monotherapy Autogene cevumeran/ BNT122 + 1x Atezolizumab BNT112 Monotherapy & + Cemiplimab + ADT BNT111 +/- Cemiplimab Pembrolizumab +/- BNT113 BNT116 Monotherapy & Cemiplimab or CTx Autogene cevumeran/ BNT122 + Pembrolizumab

Our Strategy for Potential Leadership in mRNA Cancer Vaccines 130 Aim to establish commercial manufacturing capacity Aim to establish BioNTech commercial manufacturing facility Aim to increase clinical manufacturing capability Continue to decrease manufacturing time Moving to fully automatic platform to further reduce cycle time Continue to advance pipeline Aim to initiate additional late-stage clinical trials in the adjuvant setting Continue to improve neoantigen selection Further improving AI / ML capabilities, improving analytics of clinical samples through high-throughput sequencing and genomics technology development AI = artificial intelligence; ML = machine learning.

First-in-Human Phase 1 Study with an Intranodal Version of Our individualized mRNA Neoantigen Vaccine Sahin et al. Nature. 2017. 13 patients with stage IIIa-c (6 patients), IV (7 patients) melanoma treated 131 Evaluating the safety, tolerability & immunogenicity of intra- nodal administration of an individualized neoantigen- specific mRNA vaccine with or without initial treatment with NY-ESO- 1/tyrosinase vaccine in patients with advanced melanoma (NCT01684241)

post-IVS CD8+ T cells ELISPOT Long Term Persistence of Vaccine Induced T cell Responses Induced by Intra- Nodal Vaccination with a Naked Individualized mRNA-base Neoantigen Vaccine Neoantigen T cells persist for more than 4 yearsex vivo Multimer staining 0 100 200 300 400 0.0 0.1 0.2 0.3 1600 1700 Days after treatment start % m u lt im e r+ o f C D 8 + T c e lls UTP6(H137Y) YSNKPALW / HLA-B*5701 CLINT1(T472I) VSKILPSTW / HLA-B*5701 COX7A2(A84V) GVADVLLYR / HLA-A*1101 Years after treatment start 4.5 COX7A2(A84V) GVADVLLYR / HLA-A*1101 CLINT1(T472I) VSKILPSTW / HLA-B*5701 UTP6(H137Y) YSNKPALW / HLA-B*5701 0.015 0.055 0.064 4.5 Years after treatment start 132 Türeci, presented at CICON2023.

6-Year Passive Follow Up of Patients After Intranodal Vaccination with a Naked Individualized mRNA-based Neoantigen Vaccine Passive follow up for 6 years 133 Sahin et al. Nature. 2017, Türeci, presented at CICON23

Exploiting Somatic Cancer Mutations for mRNA-LPX based Neoantigen Vaccines Vormehr et al., Curr Opin Immunol 39:14-22 (2016). MSI = microsatelite instability; MSI-h = microsatellite instability high; non-synonymous single nucleotide variant. MSS = microsatellite stable; LPX = lipoplex 134

Triple Negative Breast Cancer Pancreatic Ductal Adenocarcinoma High Unmet Medical Need in Early-Stage Cancer Indications 135 CPI = Checkpoint inhibitor; pCR = pathological complete response; CRC = colorectal cancer, TNBC = triple negative breast cancer; PDAC = pancreatic ductal adenocarcinoma. 1. Oettle, H. et al. JAMA 2013; 2. Neoptolemos, J. P. et al. NEJM 2004. Colorectal Cancer 69−75% relapse rate within 5 years after adjuvant therapy • To become the 2nd leading cause of cancer-related death in the US by 2030 • 5-yr survival rates after resection alone is ~10%1,2 • CPI resistant due to low mutation burden and consecutively few mutation-derived neoantigens 35-45% relapse rate within 4 years after adjuvant therapy 20-35% relapse rate within 4 years after adjuvant therapy • Neoadjuvant treatment regimens combining chemo + pembro increase the number of patients reaching pCR • Poor prognosis for patients not reaching pCR after neo-adjuvant treatment • 5-year survival rates of locoregional disease is ~70% • ctDNA is a marker for minimal residual disease and thus can identify patients at high risk of disease recurrence • In ctDNA-positive, Stage 2 (high risk) and Stage 3 CRC post adjuvant chemotherapy, duration of disease-free Phase 1 trial completed in adj. PDAC Randomized Phase 2 trial started Phase 1 trial completed in post (neo) adjuvant TNBC Randomized Phase 2 trial initiated and recruiting

Exploratory Phase 1 Trial of BNT122 in TNBC Patients Post (Neo-)Adjuvant Treatment 136 TNBC = triple-negative breast cancer; TAA = tumor-associated antigen; UTR = untranslated region; ORF = open reading frame; MITD = MHC I-targeting domain. Trial design Inclusion criteria Invasive adenocarcinoma TNBC (pT1cB0M0 - any TanyNM0) Screening • > 5 neoantigens identified (neoantigen vaccine) • (Neo)adjuvant chemotherapy (and radiotherapy) • No recurrence of breast cancer prior to treatment start Treatment • Optional: Non-mutated TAA vaccine treatment • Neoantigen vaccine treatment Screening Non-mutated TAA vaccine Optional (up to 6) Neoantigen Vaccine T cells: Day of vacination 1 15 29 50 64 1 15 29 50 64 Optional

Induction of Persistent Neoantigen-Specific Immune Responses in Patients with TNBC Treated with BNT122 in the Post (Neo-)Adjuvant Setting 137 0 50 0 10 00 P01 P02 P05 P06 P07 P08 P09 P10 P11 P12 P13 P14 2, 00 0 3, 00 0 4, 00 0 5, 00 0 Mean Spot count Ex vivo IFNγ ELISpot counts Induced T cell responses were both of high magnitude and persistent up to 600 days CD8 H EA TR 2 (R 4 7 Q ) H LA M u lt im er P P P 1 R 1 5 B (S 2 7 8 T) H LA M u lt im er Day -7 Day 71 (+7) P01 0.10 0.16 5.66 10.34 0 100 200 0 5 10 15 300 600 Days after treatment start P e r c e n t o f C D 8 + P01 HEATR2(R47Q) Multimer+ IFN and TNF IFN + 0 100 200 0 2 4 6 300 600 Days after treatment start P e r c e n t o f C D 8 + P01 PPP1R15B(S278T) Multimer+ IFNand TNF IFN+ P01 HEATR2(R47Q) PP1R15B(S278T) Days after treatment start Days after treatm n star Day 7 Türeci, presented at CICON2023. TNBC = triple-negative breast cancer; HLA = human leukocyte antigen; IFN = interferon; TNF = tumor necrosis factor.

BNT122/Autogene Cevumeran1 in Adjuvant Pancreatic Ductal Adenocarcinoma 139 1. Partnered with Genentech, member of Roche Group; 2. Rojas et al. Nature. 2023. mFOLFIRINOX = modified FOLFIRINOX; PDAC = pancreatic ductal adenocarcinoma; q2w = every 2 weeks. Active, not recruiting Investigator-initiated single-center study (MSKCC) Data published in Nature (Rojas et al. 2023) Primary: Safety, immunogenicity, feasibility 18-month recurrence-free survival (RFS) BNT122 8 priming doses Atezolizumab 1 dose BNT122 1 booster dose mFOLFIRINOX 12 q2w cycles Surgery Week 0 Week 6 Weeks 9–17 Weeks 21–43 Week 46 Follow-up Custom manufacture of BNT122; up to 20 neoantigens from tumor sample Inclusion criteria Surgically resectable PDAC • No borderline resectable • No locally advanced or metastatic • No neoadjuvant therapy ≥5 neoantigens StatusKey endpoints Phase 1, open-label, investigator-initiated trial (NCT04161755)

Autogene Cevumeran/BNT1221 Induces Immune Responses in Adjuvant Pancreatic Cancer 140 1. Partnered with Genentech, member of Roche Group. BNT122 induces functional neoantigen-specific T cells Rojas et al. Nature. 2023 Half of all the patients who received the vaccine mount neoantigen-specific de novo T cell responses against at least one vaccine neoantigen Immunogenic Non-immunogenic No data N u m b e r o f n e o a n ti g e n s i n a u to g e n e c e v u m e ra n 20 15 10 5 0 Patient Responders (n=8) Non-responders (n=8) 10 29 25 25 5 6 14 1 3 19 4 20 9 18 23 28 R0/R1 R0 R0 R0 R1 R0 R0 R0 R1 R0 R1 R0 R0 R0 R0 R0 R0 Vaccine-expanded T cells are durable and persist for up to 2 years Vaccine-expanded T cells persist despite mFOLFIRINOX treatment Surgery Atezolizumab Autogene cevumeran mFOLFIRINOX P e rc e n ta g e o f a ll b lo o d c e ll s P e rc e n ta g e o f a ll b lo o d c e ll s

Autogene Cevumeran/BNT1221 Demonstrates Clinical Activity in Adjuvant Pancreatic Cancer 141 1. Partnered with Genentech, member of Roche Group. PDAC = Pancreatic ductal adenocarcinoma; OS = overall survival, RFS = relapse-free survival. O S ( % ) 10 0 50 0 0 6 12 At risk 19 18 15 18 9 24 4 30 0 Month R F S ( % ) 100 50 0 0 6 12 At risk 19 16 14 18 8 24 2 30 0 Month N=19 Median follow-up: 18.0 month BNT122 vaccine response correlates with delayed PDAC recurrence Rojas et al. Nature. 2023

BNT122/Autogene Cevumeran1 Investigated in a Phase 2 Randomized Trial vs SoC in Resected PDAC 142 1. Partnered with Genentech, member of Roche Group. SoC = standard of care; PDAC = pancreatic ductal adenocarcinoma; ; CT = computer tomography CTx = chemotherapy. Key endpoints Primary: DFS Secondary: DFS rates, OS, OS rates and safety Status • Recruitment ongoing • FPD October 2023 Inclusion criteria Patients with resected PDAC No prior systemic anti-cancer treatment for PDAC No evidence of disease after surgery Screening Part A Determine 5 neo-epitopes from blood and tumor samples for custom manufacture of BNT122 Screening Part B Confirm patient eligibility based IN/EX criteria n=260 R 1:1 Treatment phases and dosing schedules During the study, patients are monitored at scheduled intervals until recurrence of PDAC, occurrence of new cancers, or unacceptable toxicity, whichever occurs first. Randomization 6-12 weeks following surgery Arm 1 Autogene cevumeran + atezolizumab + mFOLFIRINOX Arm 2 mFOLFIRINOX IMCODE003: Phase 2, open-label, multicenter, randomized trial (NCT05968326) Resection margin, nodal involvement Stratification factors

Personalized mRNA Cancer Vaccines: Key Takeaways 143 We aim to bring personalized cancer vaccines into the adjuvant treatment setting for multiple cancer indications including tumors with low mutational burden and cold tumor types Low tumor mass, with residual cancer cells Tumor resistance mechanisms not fully established Healthier immune system allows for functional T cell responses Low Mutational Burden High unmet need, not addressed by approved immunotherapies Demonstrated ability to generate durable de novo neoantigen specific poly-epitope T cell responses in multiple cold tumor types Adjuvant Setting Rationale:

9 Path to Value Creation Ryan Richardson Chief Strategy Officer

Strategic Outlook 145 1. Partnered with Pfizer. COVID-191 Immuno-oncology Infectious diseases Strategy Advance commercial franchise into combination and next- generation vaccines Execute pivotal trials and launch multiple products from 2026 onwards Initiate first late-stage development programs Planned Next- Stage Drive leadership in COVID-19 vaccine franchise leveraging Pfizer’s global infrastructure Build fully integrated global organization to discover, develop and commercialize a multi- product portfolio Advance pipeline of innovative mRNA prophylactic and therapeutic vaccine candidates

Cash position1 COVID-19 Vaccine Franchise Oncology Pipeline Infectious Disease Pipeline Strategic Vision for 2030 146 1. As of September 30, 2023; 2. Figure is pre-tax. €17bn cash €2bn trade receivables2 Interest income Market-leading vaccine Expanding late-stage pipeline Early-stage ex-COVID-19 pipeline Strong balance sheet Multi-vaccine portfolio Multiple commercial products and novel late-stage pipeline First approved products and late-stage pipeline Today 2030 Vision Key value drivers Diversified, cashflow-generating multi-product portfolio Cashflow generating

Path to Sustained Long-term Growth 147 1. As of September 30, 2023. S&M = sales & marketing; BD = business development; M&A = mergers & acquisitions. 2025-20282023 2024 • Expect to be profitable if full-year 2023 revenue guidance is achieved • ~€1bn investment in BD/M&A • Cash position of ~€17bn1 • Increase oncology R&D investment in pivotal trials • Maintain lean SG&A cost base • Continue active BD/M&A strategy • Maintain strong balance sheet Goal of sustainable strategic growth through: • Multiple new product approvals • Revenue growth from first oncology launches and combination vaccines • Profitable and cashflow positive • Maintain strong balance sheet

Path to Value Creation 148 Increase investment in R&D with a focus on pivotal trials Continued BD and M&A with a focus on synergistic assets Build oncology commercial capabilities leveraging partners in select regions Commercialize multiple new products in infectious disease and oncology Value for Shareholders, patients & society

Innovation Series 2023 THANK YOU Contact us at investors@biontech.de