Filed Pursuant to Rule 424(b)(5)
Registration No. 333-270657
The information in this preliminary
prospectus supplement is not complete and may be changed. A registration statement relating to these securities has been filed with the
Securities and Exchange Commission and is effective. This preliminary prospectus supplement and the accompanying prospectus are not an
offer to sell these securities and we are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale
is not permitted.
SUBJECT TO COMPLETION, DATED
FEBRUARY 4, 2025
PRELIMINARY PROSPECTUS SUPPLEMENT
(to Prospectus dated March 29, 2023)

Shares of Common stock
We are offering shares of our common stock, par
value $0.00001 per share, in this offering.
Our common stock is listed
on The Nasdaq Capital Market under the symbol “NVCT”. On February 3, 2025 the last reported sale price of our common stock
on The Nasdaq Capital Market was $6.80 per share.
Investing in our common
stock involves a high degree of risk. See “Risk Factors” beginning on page S-5 of this prospectus supplement and in the
documents incorporated by reference into this prospectus supplement.
Neither the Securities
and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy
or accuracy of this prospectus supplement or the accompanying prospectus. Any representation to the contrary is a criminal offense.
| | |
| Per share | |
| Total | |
| Public offering price | |
$ | | |
$ | | |
| Underwriting discounts and commissions | |
$ | | |
$ | | |
| Proceeds to Nuvectis, before expenses | |
$ | | |
$ | | |
We have granted the underwriter an option for a
period of 30 days to purchase an additional shares of our common stock, at the public offering price, less the underwriting discount.
If the underwriter exercises the option in full, the total underwriting discounts and commissions payable by us will be $ , and the total
proceeds to us, before expenses will be $ .
Delivery of the shares of common stock is expected
to be made on or about , 2025.
Sole Bookrunner
Lucid Capital Markets, LLC
Prospectus Supplement dated , 2025
Table of contents
We have not authorized
any other person to provide any information other than that contained or incorporated by reference into this prospectus supplement, the
accompanying prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We take no
responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. You should
not assume that the information contained in this prospectus supplement or the accompanying prospectus is accurate as of any date other
than the date of this prospectus supplement or the accompanying prospectus, or that information contained in any document incorporated
or deemed to be incorporated by reference is accurate as of any date other than the date of that document. Our business, financial condition,
results of operations and prospects may have changed since that date. You should read this prospectus supplement, the accompanying prospectus,
the documents incorporated by reference in this prospectus supplement and the accompanying prospectus, and any free writing prospectus
that we have authorized for use in connection with this offering, in their entirety before making an investment decision. You should also
read and consider the information in the documents to which we have referred you in the sections of this prospectus supplement entitled
“Where You Can Find More Information” and “Incorporation of Certain Information by Reference.”
About
this prospectus supplement
This document is in two parts.
The first part is this prospectus supplement, which describes the specific terms of this offering of common stock and also adds to and
updates information contained in the accompanying prospectus and the documents incorporated by reference in this prospectus supplement
and the accompanying prospectus. The second part, the accompanying prospectus, dated March 29, 2023, provides more general information,
some of which may not apply to this offering. Generally, when we refer to this prospectus supplement, we are referring to both parts of
this document combined. To the extent there is a conflict between the information contained in this prospectus supplement and the information
contained in the accompanying prospectus or any document incorporated by reference that was filed with the U.S. Securities and Exchange
Commission, or SEC, before the date of this prospectus supplement, you should rely on the information in this prospectus supplement; provided
that if any statement in one of these documents is inconsistent with a statement in another document having a later date—for example,
a document incorporated by reference in the accompanying prospectus—the statement in the document having the later date modifies
or supersedes the earlier statement.
We further note that the representations,
warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference herein
were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among
the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations,
warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should
not be relied on as accurately representing the current state of our affairs.
We are offering to sell, and
seeking offers to buy, shares of our common stock only in jurisdictions where such offers and sales are permitted. The distribution of
this prospectus supplement and the accompanying prospectus and the offering of the common stock in certain jurisdictions may be restricted
by law. Persons outside the United States who come into possession of this prospectus supplement and the accompanying prospectus must
inform themselves about, and observe any restrictions relating to, the offering of the common stock and the distribution of this prospectus
supplement and the accompanying prospectus outside the United States. This prospectus supplement and the accompanying prospectus do not
constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by
this prospectus supplement and the accompanying prospectus by any person in any jurisdiction in which it is unlawful for such person to
make such an offer or solicitation.
Unless otherwise stated, all
references in this prospectus supplement to “we,” “us,” “our,” “Nuvectis,” the “Company”
and similar designations refer to Nuvectis Pharma, Inc. This prospectus supplement contains trademarks and trade names of Nuvectis Pharma,
Inc., including our name and logo. Other service marks, trademarks and trade names referred to in this document are the property of their
respective owners.
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights information contained
elsewhere or incorporated by reference in this prospectus supplement and the accompanying prospectus and in the documents we incorporate
by reference. This summary does not contain all of the information that you should consider before deciding to invest in our common stock.
You should read this entire prospectus supplement and the accompanying prospectus carefully, including the information referred to in
the section entitled “Risk Factors” beginning on page S-5 of this prospectus supplement, as well as the other documents
that we incorporate by reference into this prospectus supplement and the accompanying base prospectus, including our financial statements
and the exhibits to the registration statement of which this prospectus supplement and the accompanying base prospectus is a part.
Overview
We are a biopharmaceutical company focused on
the development of innovative precision medicines for the treatment of serious conditions of unmet medical need in oncology.
NXP800 (GCN2 Kinase Activator)
We have licensed exclusive world-wide development
and commercial rights to NXP800, an oral, small molecule discovered at the Institute of Cancer Research (“ICR”)
in London, England.
In preclinical studies, treatment with NXP800
inhibited tumor growth in xenograft models of human ovarian, endometrial and gastric cancers, in which, a genetic mutation in the AT-rich
interactive domain-containing protein 1A (“ARID1a”) gene was present, potentially rendering ARID1a as a biomarker for treatment
sensitivity, thereby offering a potential strategy for patient enrichment. Based on this work, we have begun to clinically investigate
NXP800 in platinum-resistant ARID1a-mutated ovarian carcinoma, a type of cancer which is primarily comprised of two histologies, ovarian
clear cell carcinoma (“OCCC”) and ovarian endometrioid carcinoma (“OEC”). We are investigating the utility of
ARID1a deficiency as a potential patient selection marker in additional tumor types. The genetic screening for the mutations in ARID1a
can be detected using a commercially available next generation sequencing-based in vitro diagnostic tests, which are routinely utilized
in the clinic for cancer patients. In addition, in preclinical studies, treatment with NXP800 inhibited tumor growth in patient-derived
xenograft ("PDX") models of cholangiocarcinoma.
In December 2021, the Phase 1 study was initiated
in the United Kingdom and is comprised of two parts: dose-escalation (Phase 1a), followed by an expansion phase (Phase 1b). In the Phase
1a, the safety, tolerability and pharmacokinetic properties of NXP800 were evaluated in patients with advanced solid tumors to identify
a dose and dosing schedule for Phase 1b. The Phase 1b portion of the study, which was initiated in the second quarter of 2023, is evaluating
the safety and preliminary anti-tumor activity of NXP800 in patients with platinum-resistant, ARID1a-mutated ovarian carcinoma.
In June 2022, the Investigational New Drug (“IND”)
application for NXP800 was cleared by the U.S. Food and Drug Administration (“FDA”), including the Phase 1 clinical trial
protocol. In December 2022, we announced that the FDA granted Fast Track Designation status to the development program of NXP800 for the
treatment of platinum-resistant, ARID1a-mutated ovarian carcinoma.
In August 2023, we announced that the FDA granted
Orphan Drug Designation to NXP800 for the treatment of patients with cholangiocarcinoma.
In December 2023, we announced a collaboration
with Mayo Clinic to conduct an investigator-sponsored clinical trial in patients with cholangiocarcinoma.
In August 2024, we announced that the FDA granted
Orphan Drug Designation to NXP800 for the treatment of patients with ARID1a-deficient ovarian, fallopian tube, and primary peritoneal
cancers.
NXP900 (SRC/YES1 Kinase Inhibitor)
We have licensed exclusive world-wide development
and commercial rights to NXP900, a SRC Family Kinase (“SFK”) inhibitor that potently inhibits the c-Src (“SRC”)
and YES1 kinases. NXP900 was discovered at the University of Edinburgh, Scotland.
SRC is aberrantly activated in many cancer types,
including solid tumors such as breast, colon, prostate, and ovarian, while remaining predominantly inactive in non-cancerous cells. Increased
SRC activity is generally associated with late-stage cancers, metastatic potential and resistance to therapy, and correlates with poor
clinical prognosis. YES1 gene amplification has been reported to be implicated in several tumors including lung, head and neck, bladder
and esophageal cancers. In addition, YES1 directly phosphorylates and activates the Yes-associated protein (“YAP1”),
the main effector of the Hippo pathway, which has been identified as a promoter of drug resistance, cancer progression, and metastasis
in several cancer types, including squamous cell, mesothelioma and papillary kidney cancers.
In vivo, treatment with NXP900 inhibited primary
and metastatic tumor growth in xenograft models of breast, cervical, esophageal, head and neck and medulloblastoma cancers, and demonstrated
on-target pharmacodynamic effects. Furthermore, it has been found that YES1 gene amplification is a key mechanism of resistance to Epidermal
Growth Factor Receptor (“EGFR”), Human Epidermal Growth Factor Receptor 2 (“HER2”) and Anaplastic Lymphoma Kinase
(“ALK”). A peer reviewed study published in Nature Communications (not sponsored by the Company) in April 2022 demonstrated
that NXP900 was able to re-sensitize resistant non-small cell lung cancer (“NSCLC”) cells to osimertinib (the active ingredient
in Tagrisso®), the leading EGFR inhibitor used for the treatment of EGFR mutation-positive NSCLC, when used in combination with osimertinib.
These findings were reproduced and expanded by us in cell line models, demonstrating statistically significant synergies in combination
with osimertinib and, separately, with the ALK inhibitor alectinib, (the active ingredient in Alecensa®).
In May 2023, the FDA cleared our IND for NXP900,
which includes the Phase 1 clinical trial protocol.
The Phase 1 study was initiated in September 2023
and is comprised of two parts: dose-escalation (Phase 1a), to be followed by an expansion phase (Phase 1b). In the ongoing Phase 1a, the
safety, tolerability and pharmacokinetic properties of NXP900 in patients with advanced solid tumors are being assessed to identify a
dose and dosing schedule for Phase 1b.
Company information
We were incorporated under the laws of the State
of Delaware in July 2020. Our principal executive office is located in Fort Lee, New Jersey and our telephone number is (201) 614-3150.
Our website address is www.nuvectis.com. We are
not including the information on our website as a part of, nor incorporating it by reference into, this prospectus supplement.
THE OFFERING
| Common stock offered by us |
|
shares |
| |
|
|
| Common stock to be outstanding after the offering |
|
shares (or shares if the underwriter exercises its over-allotment option in full) |
| |
|
|
| Option to purchase additional shares |
|
We have granted the underwriter an option to purchase additional shares of our common stock. This option is exercisable, in whole or in part, for a period of 30 days from the date of this prospectus supplement. |
| |
|
|
| Use of proceeds |
|
We intend to use the net proceeds from this offering to continue to advance the development programs of NXP800 and NXP900 or any future product candidate, hiring of additional personnel, capital expenditures, costs of operating as a public company and other general corporate purposes. |
| |
|
|
| |
|
See “Use of Proceeds” on page S-15 of this prospectus supplement. |
| |
|
|
| Risk factors |
|
See “Risk Factors” beginning on page S-5 and, our “Risk Factors” section beginning on page 17 of our Annual Report on Form 10-K for the year ended December 31, 2023, and our “Risk Factors” section beginning on page 26 of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2024, which is incorporated by reference herein, for a discussion of factors that you should consider before investing in our common stock. |
| |
|
|
| Nasdaq Capital Market symbol |
|
NVCT |
The number of shares of
our common stock outstanding after this offering is based on 18,985,324 actual shares of our common stock outstanding as of
September 30, 2024, and excludes, as of such date:
| · | 348,281 shares of our common stock issuable upon the exercise
of stock options, at a weighted-average exercise price of $4.59 per share, of which 333,281 shares were vested as of such date; and |
| · | 725,311 shares of our common stock available for future issuance
under our 2021 Global Equity Incentive Plan. |
Risk
Factors
An investment in our common stock involves
a high degree of risk. Before deciding whether to invest in our common stock, you should consider carefully the risks discussed below,
together with other information in this prospectus supplement, the accompanying prospectus, the information and documents incorporated
by reference including the section “Risk Factors” beginning on page 17 of our Annual Report on Form 10-K for the year ended December 31, 2023 and beginning on page 26 of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2024, which
is incorporated by reference herein, and in any free writing prospectus that we have authorized for use in connection with this offering.
The risks described below may not be the only ones relating to our company. Additional risks that we currently believe are immaterial
or risks not currently known to us may also impair our business and operations. Our business, results of operation, financial condition,
cash flow and prospects and the trading price of our common stock could be harmed as a result of any of these risks, and investors may
lose all or part of their investment.
Risks related to our financial position and capital requirements
Our limited operating history may make it difficult for you to
evaluate the success of our business to date and to assess our future viability.
We are a clinical stage biopharmaceutical company
with a limited operating history. We were incorporated in Delaware in July 2020, and our operations to date have been limited to
organizing and staffing our company, business planning, raising capital, identifying, investigating, licensing and evaluating potential
product candidates, and establishing arrangements with third parties for the manufacture of initial quantities of our lead product candidate
and component materials. Both of our product candidates are in early clinical development. We have not yet demonstrated our ability to
successfully conduct or complete any clinical development program for our drug candidates, obtain marketing approvals, manufacture a commercial-scale
product or arrange for a third party to do so on our behalf, or conduct sales, marketing and distribution activities necessary for successful
product commercialization. Consequently, any predictions about our future success or viability may not be as accurate.
We may need to transition at some point from a
company with a research and development focus to a company capable of supporting commercial activities related to the full product life
cycle. We may not be successful in such a transition.
We have incurred losses since inception and anticipate that we
will continue to incur losses for the foreseeable future. We may never achieve or maintain profitability.
Investment in biopharmaceutical product development
is a highly speculative undertaking and entails substantial upfront capital expenditures and significant risk that our current or potential
future product candidates will fail to demonstrate adequate efficacy or an acceptable safety profile, gain regulatory approval and become
commercially viable. We are still in the early stages of development of our product candidates and initiated our first clinical trial
in December 2021. We have no products approved for commercial sale and have not generated any revenue from product sales to date. We continue
to incur significant research and development and other expenses related to our ongoing operations. In addition, as a business with a
limited operating history, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors,
such as the COVID-19 pandemic.
We have incurred losses in each period since
we commenced operations. Since inception through September 30, 2024, we had an accumulated deficit of approximately $67.0 million.
We expect to continue to incur significant losses for the foreseeable future, and we expect these losses to increase substantially
if and as we continue our research and development efforts for our lead product candidate; conduct preclinical studies and clinical
trials for our current and future product candidates; seek marketing approvals for any current or future product candidate that
successfully completes clinical trials; experience any delays or encounter any issues with any of the above; establish a sales,
marketing and distribution infrastructure and scale-up manufacturing capabilities to commercialize any current or future product
candidates for which we may obtain regulatory approval; obtain, expand, maintain, enforce and protect our intellectual property
portfolio; hire additional clinical, regulatory and scientific personnel; and operate as a public company.
Our pipeline product candidates, NXP800 and NXP900,
are both in clinical development. Both product candidates will require additional preclinical and clinical studies, regulatory review
and approval, substantial investment, access to sufficient clinical and commercial manufacturing capacity and significant marketing efforts
before we can potentially generate any revenue from product sales. To date, we have not generated any revenue from our product candidates.
Our ability to generate revenue will depend on a number of factors, including, but not limited to:
| · | Our ability to timely and successfully complete our preclinical studies and clinical trials, which may
be significantly slower or more costly than anticipated and will depend upon several factors including the performance of third-party
contractors, our ability to enroll patients into the clinical trials and the safety, tolerability and efficacy results generated in preclinical
studies and clinical trials; |
| · | successful submissions of IND applications to the FDA and any additional comparable applications; |
| · | completion of nonclinical studies necessary for the IND or comparable submission, as appropriate; |
| · | whether we are required by the FDA or similar foreign regulatory authorities to conduct additional clinical
trials or other studies to support the approval and commercialization of our current or future product candidates; |
| · | the FDA’s and similar foreign regulatory authorities’ acceptance of the safety, potency, purity,
efficacy and risk to benefit profile of our current or future product candidates; |
| · | the prevalence, duration and severity of side effects or other safety issues experienced with our current
or future product candidates, if any; |
| · | the timely receipt of necessary marketing approvals from the FDA and similar foreign regulatory authorities; |
| · | the actual and perceived availability, cost, risk profile and safety and efficacy of our current or future
product candidates, if approved, relative to existing and future alternative cancer therapies and competitive product candidates and technologies; |
| · | our ability and the ability of third parties with whom we contract to manufacture adequate clinical and
commercial supplies of our current or future product candidates, to remain in good standing with regulatory authorities and to develop,
validate and maintain commercially viable manufacturing processes that are compliant with cGMP; |
| · | our ability to successfully develop a commercial strategy and to commercialize any current or future product
candidate in the United States and internationally, if approved for marketing, reimbursement, sale and distribution in such countries
and territories, whether alone or in collaboration with others; |
| · | patient demand for our current or future product candidates, if approved; and |
| · | our ability to establish and enforce intellectual property rights in and to our current or future product
candidates. |
Many of the factors listed above are beyond our
control and could cause us to experience significant delays or prevent us from obtaining regulatory approvals or commercializing our current
and future product candidates. Even if we can commercialize any current or future product candidates, we may not achieve profitability
soon after generating product sales, if ever.
We will require additional financing to
achieve our goals, and a failure to obtain this capital when needed could force us to delay, limit, reduce or terminate our product development
or commercialization efforts.
Since our inception, most of our resources have
been dedicated to the discovery, acquisition and preclinical and clinical development of our product candidates. We have expended and
believe that we will continue to expend substantial resources for the development of our clinical drug candidates and may expend additional
resources on other product candidates and drug discovery and acquisition efforts. These expenditures will include costs associated with
general administration, facilities, research and development, acquiring new technologies, manufacturing product candidates, conducting
preclinical experiments and clinical trials, applying for regulatory approvals, commercializing any products that might receive approval
for sale, and costs associated with operating as a public company.
We have no significant current
source of income to sustain our present activities, and we do not expect to generate income until, and unless, we obtain approval from
the FDA or other regulatory authorities, and we successfully commercialize one or more of our compounds. As the outcome of our ongoing
and future clinical trials is highly uncertain, our estimates of clinical trial costs necessary to successfully complete the development
and commercialization of our product candidates may differ significantly from our actual costs. In addition, other unanticipated costs
may arise.
As a result of these and other
factors currently unknown to us, we may need to seek additional funds sooner than planned, through public or private equity, debt financings
or other sources, such as strategic partnerships and alliances and licensing arrangements. In addition, we may seek additional capital
due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating
plans.
Our future capital requirements depend on many
factors, including:
| · | the number and characteristics of the product candidates we pursue; |
| · | the scope, progress, results and costs of researching and developing our product candidates, and conducting
preclinical and clinical trials; |
| · | the ability of our product candidates to progress through clinical development successfully; |
| · | the timing of, and the costs involved in, seeking regulatory approvals for our product candidates; |
| · | the cost of commercialization activities if any of our product candidates are approved for sale, including
marketing, sales and distribution costs; |
| · | the cost associated with securing and establishing commercialization and manufacturing capabilities for
our product candidates and any products for which we might receive regulatory approval; |
| · | our ability to establish and maintain strategic partnerships, licensing or other arrangements and the
economic and other terms of such agreements; |
| · | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims,
including litigation costs and the outcome of such litigation; |
| · | the timing, receipt and amount of sales of, or royalties on, our future products, if any; |
| · | our need and ability to hire additional management and scientific, medical, and sales and marketing personnel; |
| · | the effect of competing technological and market developments; and |
| · | our need to implement additional internal systems and infrastructure, including financial and reporting
systems. |
Additional funds may not be available when we need
them, on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to:
| · | delay, limit, reduce or terminate preclinical studies, clinical trials or other research and development
activities for one or more of our product candidates; |
| · | delay, limit, reduce or terminate manufacturing of our product candidates; or |
| · | delay, limit, reduce or terminate our establishment of sales and marketing capabilities or other activities
that may be necessary to commercialize our product candidates and ensure their acceptance by third-party payors and the market. |
Risks Related to This Offering
If you purchase shares of common stock in this offering, you
may suffer immediate dilution of your investment.
If you purchase common stock in this offering,
you will incur immediate and substantial dilution of $ per share, representing the difference between the public offering price of $ per
share, and our as adjusted net tangible book value per share after giving effect to this offering at the public offering price. Moreover,
as of September 30, 2024, there were 333,281 shares subject to outstanding options at a weighted-average exercise price of $4.59 per share.
To the extent that these outstanding options are ultimately exercised or the underwriter exercises its option to purchase additional shares,
you may incur further dilution. For a further description of the dilution you may experience immediately after this offering, see “Dilution.”
Raising additional capital may cause dilution to our existing
stockholders, restrict our operations, or require us to relinquish rights to our current or future technologies or product candidates.
We may seek additional capital through a combination
of public and private equity offerings, ATM facility, debt financings, strategic partnerships and alliances and licensing arrangements.
Any equity or equity-related financing may dilute our stockholders may subject us to restrictive covenants and interest costs. If we obtain
funding through a strategic collaboration or licensing arrangement, we may be required to relinquish our rights to our current product
candidates or any future product candidates that we may develop.
Additional fundraising efforts may divert our
management from their day-to-day activities, which may adversely affect our operations. If we are unable to raise additional capital as
needed or on acceptable terms, we may be required to delay or discontinue any research, development or commercialization programs and
may be unable to expand our operations or otherwise capitalize on our business opportunities. Further, we may be required to seek collaborators
for potential product candidates earlier, or on less favorable terms, than might otherwise be desired, or to relinquish or license our
rights to potential product candidates in markets where we otherwise would seek to pursue development or commercialization. Any of the
above events could significantly harm our business, prospects, financial condition and results of operations and cause the price of our
common stock to decline.
Unstable market and economic conditions
may have serious adverse consequences on our business, financial condition and stock price.
There can be no assurance that deterioration in
credit and financial markets and confidence in economic conditions will not occur. Our general business strategy may be adversely affected
by an economic downturn, a volatile business environment or an unpredictable and unstable market. If equity and credit markets deteriorate,
it may make any necessary equity, debt, or other financing more difficult to secure, more costly, more dilutive, and less favorable to
existing shareholders. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse
effect on our growth strategy, financial performance and stock price and could require us to delay or abandon our business and clinical
development plans. In addition, there is a risk that one or more of our current service providers, manufacturers and other partners may
not survive these difficult economic times, which could directly affect our ability to attain our operating goals on schedule and on budget.
There is a possibility that our stock price may decline, due in part to the volatility of the stock market and the general economic downturn.
We have broad discretion over the use of our cash, cash equivalents
and marketable securities, including the net proceeds we receive in this offering, and may not use them effectively.
Our management has broad discretion to use our
cash, cash equivalents and marketable securities, including the net proceeds we receive in this offering, to fund our operations and could
spend these funds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by our management
to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the
price of our common stock to decline and delay the development of our product candidates. Pending their use to fund operations, we may
invest our cash, cash equivalents and marketable securities in a manner that does not produce income or that loses value.
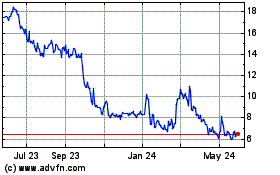
The market price of our common stock may be highly volatile and
our stockholders could incur substantial losses.
The market price of our common stock may be highly
volatile, and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. Since our initial
public offering which occurred in February 2022, the price of our common stock has ranged from $3.08 per share to $20.92 per share. The
stock market in general and the market for biopharmaceutical companies in particular have experienced extreme volatility that has often
been unrelated to the operating performance of particular companies. The market price for our common stock may be influenced by many factors,
including:
| · | results from or delays of clinical trials of our product or product candidates, as well as results of
regulatory reviews relating to the approval of our product candidates; |
| · | our decision to initiate a clinical trial, not to initiate a clinical trial or to terminate an existing
clinical trial; |
| · | our dependence on third parties, including clinical research organizations and contract manufacturing
organizations, trial sites, clinical trial sponsors and clinical investigators; |
| · | our ability to commercialize our approved product candidates; |
| · | the results of our efforts to discover, develop, acquire or in-license additional product candidates or
products; |
| · | new products, product candidates or new uses for existing products or technologies introduced or announced
by our competitors and the timing of these introductions or announcements; |
| · | regulatory or legal developments in the United States and other countries; |
| · | our ability to maintain the license agreements for our product or product candidates; |
| · | developments or disputes concerning patent applications, issued patents or other proprietary rights; |
| · | the recruitment or departure of key scientific or management personnel; |
| · | the level of expenses related to any of our product or product candidates or clinical development programs; |
| · | actual or anticipated changes in estimates as to financial results, development timelines or recommendations
by securities analysts; |
| · | variations in our financial results or those of companies that are perceived to be similar to us; |
| · | sales of common stock by us or our stockholders in the future, as well as the overall trading volume of
our common stock; |
| · | changes in the structure of healthcare payment systems and product pricing restrictions; |
| · | market conditions in the pharmaceutical and biotechnology sectors; |
| · | release or expiration of the underwriter’s post-offering lock-up or other transfer restrictions
on our outstanding common stock; |
| · | general economic, industry and market conditions and other factors that may be unrelated to our operating
performance or the operating performance of our competitors, including changes in market valuations of similar companies; and |
| · | the other factors described in this “Risk Factors” section. |
Risks related to our common stock
We do not know whether an active, liquid
and orderly trading market will develop for our common stock or what the market price of our common stock will be and, as a result, it
may be difficult for you to sell your shares of our common stock.
Prior to the pricing of our initial public offering
on February 4, 2022, there was no public trading market for shares of our common stock and after our IPO the trading price of our common
stock has been volatile. Although our common stock is listed on the Nasdaq Capital Market, an active trading market for our shares is
still developing and may not be sustained in the future. The lack of an active market for our common stock creates volatility in the price
of the stock and may impair investors’ ability to sell their shares at the time they wish to sell them or at a price that they consider
reasonable and may reduce the fair market value of their shares. Further, an inactive market may impair our ability to raise capital by
selling shares of our common stock and to enter into strategic partnerships or acquire companies or products using our shares of common
stock as consideration.
Our principal stockholders and management own a significant percentage
of our stock and will be able to exert significant influence over matters subject to stockholder approval.
As of September 30, 2024, our executive officers,
directors, and 5% stockholders beneficially owned approximately 49% of our voting stock and anticipate that the same group will hold
a significant portion of our outstanding voting stock for the foreseeable future. These stockholders will have the ability to influence
us through their ownership position. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock.
Provisions in our certificate of incorporation
and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts
by our stockholders to replace or remove our current management.
Provisions in our certificate of incorporation
and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider
favorable, including transactions in which they might otherwise receive a premium for their shares. These provisions could also limit
the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of
our common stock. Among other things, these provisions:
| · | establish a classified board of directors such that not all members of the board are elected at one time; |
| · | allow the authorized number of our directors to be changed only by resolution of our board of directors; |
| · | limit the manner in which stockholders can remove directors from the board; |
| · | establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings; |
| · | limit who may call special stockholder meetings and the matters transacted at such meetings; and |
| · | authorize our board of directors to issue preferred stock without stockholder approval, which could be
used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively
preventing acquisitions that have not been approved by our board of directors. |
Moreover, because we are incorporated in Delaware, we are governed
by the provisions of Section 203 of the Delaware General Corporation Law, which in general, prohibits a publicly held Delaware corporation
from engaging in a “business combination” with an “interested stockholder” for a three-year period following the
time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. Under
Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of
the following conditions:
| · | before the stockholder became interested, our board of directors approved either the business combination
or the transaction which resulted in the stockholder becoming an interested stockholder; |
| · | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder,
the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced,
excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee
stock plans, in some instances, but not the outstanding voting stock owned by the interested stockholder; or |
| · | at or after the time the stockholder became interested, the business combination was approved by our board
of directors and authorized at an annual or special meeting of the stockholders by the affirmative vote of a majority of the outstanding
voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination
to include:
| · | any merger or consolidation involving the corporation and the interested stockholder; |
| · | any sale, transfer, lease, pledge, exchange, mortgage or other disposition involving the interested stockholder of 10% or more of
the assets of the corporation; |
| · | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation
to the interested stockholder; or |
| · | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided
by or through the corporation. |
In general, Section 203 defines an interested
stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity
or person affiliated with or controlling or controlled by the entity or person. Any provision in our corporate charter or our bylaws or
Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive
a premium for their shares of our common stock and could also affect the price that some investors are willing to pay for our common stock.
We incur significant increased costs as a result of operating
as a public company, and our management will be required to devote substantial time to compliance activities and initiatives.
As a public company, we incur significant legal,
accounting, and other expenses that we did not incur as a private company. We are now subject to the reporting requirements of the Securities
Exchange Act of 1934, as amended, which requires, among other things, that we file with the SEC, annual, quarterly, and current reports
with respect to our business and financial condition. In addition, the Sarbanes-Oxley Act of 2002 (“SOX”), as well
as rules subsequently adopted by the SEC and the Nasdaq Capital Market to implement provisions of SOX, impose significant requirements
on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate
governance practices.
Moreover, these rules and regulations will increase
our legal and financial compliance costs and make some activities more time-consuming and costly. For example, these rules and regulations
make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept
reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be
more difficult for us to attract and retain qualified persons to serve on our Board of Directors, our Board committees or as executive
officers.
If we fail to maintain an effective system
of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result,
stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of
our common stock.
Effective internal controls over financial reporting
are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed
to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation, could
cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley
Act, or the subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls
over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial
statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose
confidence in our reported financial information, which could have a negative effect on the trading price of our common stock.
We do not intend to pay dividends on our common stock in the
foreseeable future, so any returns will be limited to the value of our stock, which may be volatile.
We plan to retain future earnings for the development,
operation, and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any
return to stockholders will be limited to the appreciation of their stock, which may never occur. Further, the trading price of our common
stock is likely to be highly volatile and may be subject to wide fluctuations in response to various factors, some of which are beyond
our control. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating
performance.
If equity research analysts do not publish
research or reports about our business or if they publish negative evaluations of or downgrade our common stock, the price of our common
stock could decline.
The trading market for our common stock relies
in part on the research and reports that equity research analysts publish about us or our business. We do not control these analysts.
We may never obtain research coverage by industry or financial analysts. If no or few analysts publish research reports on the Company
or if analysts publish negative research reports about the Company, our stock price may significantly decline.
Special
cautionary notice regarding forward-looking statements
Certain matters discussed
in this prospectus supplement and the accompanying prospectus, including matters discussed under the caption “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K and our Quarterly
Reports on Form 10-Q, each as incorporated by reference herein, may contain forward-looking statements within the meaning of Section
27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”),
including, without limitation, statements regarding our expectations, beliefs, intentions or future strategies that are signified by
the words “expect,” “anticipate,” “intend,” “believe,” “may,” “plan,”
“will,” “could,” “estimate,” “seek” or similar language. All forward-looking statements
included in this document are based on information available to us on the date hereof and we assume no obligation to update any such
forward-looking statements. For such forward-looking statements, we claim the protection of the safe harbor for forward-looking statements
contained in the Private Securities Litigation Reform Act of 1995. Our business and financial performance are subject to substantial
risks and uncertainties. Actual results could differ materially from those projected in the forward-looking statements. In evaluating
our business, you should carefully consider the information set forth under the heading “Risk Factors” herein and in our
Annual Report on Form 10-K for the year ended December 31, 2023 and our Quarterly Report on Form 10-Q for the quarter ended September 30, 2024. As used below, the words “we,” “us” and “our” may refer to Nuvectis Pharma, Inc.
Some of the factors that we believe could cause
actual results to differ from those anticipated or predicted include:
| · | the success and timing of our clinical trials for our product candidates, including risks relating to
regulatory authority approval, institutional review board approval, scientific review committee approval, site initiation, patient accrual,
trial results including safety and efficacy, and the relevance of trial results for potentially viable regulatory pathways and commercialization
efforts; |
| · | the accuracy of our estimates regarding expenses, future income or revenue, capital requirements and needs
for additional financing; |
| · | our ability to obtain additional funding; |
| · | the possibility that results of clinical trials are not predictive of safety and efficacy results of our
products or product candidates in broader or additional patient populations; |
| · | our ability to adhere to ongoing compliance requirements of all health authorities to which we are subject,
in the U.S. and abroad; |
| · | our ability to obtain and maintain adequate reimbursement for our products; |
| · | product quality, efficacy or safety concerns resulting in product recalls or regulatory action; |
| · | the risk that estimates regarding the number of patients with the diseases that our products and product
candidates are designed to treat are inaccurate, do not predict, or are not reflective of actual numbers; |
| · | our products not gaining acceptance among patients, providers and/or third-party payors, including governmental
agencies, for certain approved indications, due to cost or otherwise; |
| · | the adequacy, and outcomes, of our pharmacovigilance and drug safety reporting processes, and reports,
in the U.S., Europe and other regions; |
| · | the loss of key management, scientific, or other personnel; |
| · | the success and timing of any regulatory filings for our product candidates; |
| · | changes in regulations in the U.S., Europe and other regions; |
| · | new products, new product candidates or new uses for existing products or technologies introduced or announced,
by our competitors, and the timing of these introductions or announcements; |
| · | our available cash and investments; |
| · | our ability to obtain and maintain intellectual property protection for our product candidates; |
| · | delays, interruptions, or failures in the manufacture, supply and distribution of our products and/or
our product candidates; |
| · | our ability to maintain the license agreements for our products and product candidates; |
| · | the ability of our third-party manufacturers to manufacture and supply our products, and the performance
of, and our reliance on, our third-party manufacturers and suppliers; |
| · | the performance of our third-party vendors, including clinical research organizations, clinical trial
sponsors, and clinical trial investigators; |
| · | the success of our preclinical, non-clinical, and pre-investigational new drug, or pre-IND and IND efforts,
efforts; |
| · | our ability to gain access to products we may plan to use in combination studies; and |
| · | our ability to form corporate partnerships, should that be an avenue we choose to pursue. |
Use
of proceeds
We estimate that the net proceeds
from our issuance and sale of shares of our common stock in this offering will be approximately $ million, after deducting underwriting
discounts and commissions and estimated offering expenses payable by us. If the underwriter exercises the option to purchase an additional
shares of our common stock in full, we estimate that the net proceeds from this offering will be approximately $ million, after deducting
estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to use the net proceeds
from this offering to continue to advance the development programs of NXP800 and NXP900 or any future product candidate, hiring of additional
personnel, capital expenditures, costs of operating as a public company and other general corporate purposes.
This expected use of the net
proceeds from this offering represents our intentions based upon our current plans and business conditions. The amounts and timing of
our actual expenditures may vary significantly depending on numerous factors, including the progress of our development efforts, the status
of and results from clinical trials, as well as any collaborations that we may enter into with third parties for our product candidates,
and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this
offering.
While we do not expect that
the net proceeds from this offering and our existing cash, cash equivalents and short-term investments will be sufficient to enable us
to fund the completion of development of any product candidates we may develop, we do believe that our existing cash, cash equivalents, and short-term
investments, after this offering, will be sufficient to cover our cash flow requirements for at least the next two years. We have based
this estimate on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect.
Pending our use of the net
proceeds from this offering as described above, we intend to invest the net proceeds in a variety of capital preservation investments,
including short-term, investment grade, interest bearing instruments and U.S. government securities.
Dividend
policy
We have never declared or paid any cash dividends
on our common stock and do not anticipate paying any cash dividends in the foreseeable future. Any future determination to pay dividends
will be at the sole discretion of our board of directors.
Capitalization
The following table sets forth our capitalization as of September 30,
2024:
| · | on an as adjusted basis to reflect the sale of the shares of common stock offered by us in this offering
(assuming no exercise of the underwriter’s option to purchase additional shares) after deducting underwriting discounts and commissions
and estimated offering expenses payable by us. |
You should read this table together with our financial
statements and related notes and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
in our Annual Report on Form 10-K for the year ended December 31, 2023 and our Quarterly Report on Form 10-Q for the quarter ended September
30, 2024.
| |
|
September 30, 2024 |
|
| (in thousands, except share data) |
|
Actual |
|
As adjusted |
|
| Cash, cash equivalents and short-term investments |
|
$ |
17,169 |
|
|
|
| Stockholders’ equity: |
|
|
|
|
|
| Common stock, $0.00001 par value per share, 60,000,000 shares authorized; 18,985,324 shares actual and shares as adjusted, issued and outstanding |
|
* |
|
|
|
| Additional paid-in capital |
|
77,988 |
|
|
|
| Accumulated other comprehensive income |
|
- |
|
|
|
| Accumulated deficit |
|
(66,997 |
) |
|
|
| Total stockholders’ equity |
|
10,991 |
|
|
|
| Total capitalization |
|
$ |
10,991 |
|
|
|
* Represents an amount lower than $1 USD.
The table above excludes the following shares as of September 30, 2024:
| · | 348,281 shares of our common stock issuable upon the exercise of stock options, at a weighted-average exercise
price of $4.59 per share, of which 333,281 shares were vested as of such date; and |
| · | 725,311 shares of our common stock available for future issuance under our 2021 Global Equity
Incentive Plan. |
Dilution
If you invest in our common
stock in this offering, your ownership interest will be diluted immediately to the extent of the difference between the offering price
per share of our common stock and the as adjusted net tangible book value per share of our common stock after the offering.
Our historical net tangible
book value as of September 30, 2024 was $11.0 million, or $0.58 per share of our common stock. Historical net tangible book value per share
represents the amount of our total tangible assets less total liabilities, divided by the number of shares of our common stock outstanding.
After giving effect to the
sale of shares of common stock by us, at a public offering price of $ per share, less the underwriting discounts and commissions and estimated
offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2024 would have been $ million, or $ per
share. This represents an immediate increase in net tangible book value per share of $ to existing stockholders and immediate dilution
of $ in as adjusted net tangible book value per share to new investors purchasing common stock in this offering.
Dilution per share to new
investors is determined by subtracting as adjusted net tangible book value per share after this offering from the offering price per share
paid by new investors. The following table illustrates this dilution on a per share basis.
| Public Offering Price Per Share |
|
|
|
$ |
|
|
| Historical Net Tangible Book Value Per Share as of September 30, 2024 |
|
$ |
0.58 |
|
|
|
| Increase in Net Tangible Book Value Per Share Attributable to New Investors |
|
|
|
|
|
| As Adjusted Net Tangible Book Value Per Share After the Offering |
|
|
|
|
|
| Dilution Per Share to New Investors |
|
|
|
$ |
|
|
| |
|
|
|
|
|
|
|
If the underwriter exercises
its option to purchase additional shares in this offering in full, the as adjusted net tangible book value after the offering would be
$ per share, the increase in as adjusted net tangible book value per share to existing stockholders would be $ and the dilution per share
to new investors would be $ per share, in each case after deducting underwriting discounts and estimated offering expenses payable by
us. If any shares are issued upon exercise of outstanding options at exercise prices below the public offering price in this offering,
you will experience further dilution.
The table above excludes the following shares as
of September 30, 2024:
| · | 348,281 shares of our common stock issuable upon the exercise
of stock options, at a weighted-average exercise price of $4.59 per share, of which 333,281 shares were vested as of such date; and |
| · | 725,311 shares of our common stock available for future issuance
under our 2021 Global Equity Incentive Plan. |
Material
U.S. federal income tax consequences to non-U.S. holders
The following is a summary
of the material United States federal income tax consequences relating to the acquisition, ownership and disposition of our common stock
as of the date hereof. This summary deals only with our common stock that is held as a capital asset within the meaning of Section 1221
of the Internal Revenue Code of 1986, as amended (the “Code”) by a “non-U.S. holder” (as defined below).
For purposes of this summary,
a “non-U.S. holder” means a beneficial owner of our common stock (other than a partnership or any other entity treated as
a partnership for United States federal income tax purposes) that is not for United States federal income tax purposes any of the following:
| · | an individual citizen or resident of the United States; |
| · | a corporation (or any other entity treated as a corporation
for United States federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the
District of Columbia; |
| · | an estate the income of which is subject to United States federal
income taxation regardless of its source; or |
| · | a trust if it (1) is subject to the primary supervision of a
court within the United States and one or more United States persons have the authority to control all substantial decisions of the trust
or (2) has a valid election in effect under applicable United States Treasury regulations (“Treasury Regulations”) to be
treated as a United States person. |
This summary is based upon
provisions of the Code and Treasury Regulations, administrative rulings and judicial decisions currently in effect, all as of the date
hereof and all subject to change at any time or to different interpretation including by the Internal Revenue Service (“IRS”),
any such change or differing interpretation may be applied retroactively in a manner that could adversely effect a non-U.S. holder. This
summary does not address all aspects of United States federal taxation, including any non-income taxation, and does not address any foreign,
state, local or other tax considerations that may be relevant to non-U.S. holders in light of their personal circumstances. In addition,
this summary does not represent a detailed description of the United States federal income tax consequences applicable to non-U.S. holders
that are subject to special treatment under the United States federal income tax laws (including, without limitation, a non-U.S. holder
that is a United States expatriate, “controlled foreign corporation,” “passive foreign investment company,” “real
estate investment trust,” “regulated investment company,” dealer in securities or currencies, financial institution,
tax-exempt entity, insurance company, person holding our common stock as part of a hedging, integrated, conversion or constructive sale
transaction or a straddle, trader in securities that elects to use a mark-to-market method of accounting, person liable for the alternative
minimum tax, person who acquired our common stock as compensation for services, or a partnership or other pass-through entity, or partner
in a partnership or beneficial owner of a pass-through entity that holds our common stock for United States federal income tax purposes).
We cannot provide assurance that a change in law will not alter significantly the tax considerations that we describe in this summary.
If a partnership (or other
entity treated as a partnership for U.S. federal income tax purposes) holds our common stock, the tax treatment of a partner (or owner
treated as a partner) will generally depend upon the status of the partner and the activities of the partnership. Non-U.S. holders that
are, for U.S. federal tax purposes, partners of a partnership holding our common stock should consult their tax advisors.
Non-U.S. holders considering
the purchase of our common stock should consult their own tax advisors concerning the particular United States federal income and estate
tax consequences of the ownership of our common stock, as well as the consequences arising under the laws of any other taxing jurisdiction.
Distribution on our common stock
Distributions paid on our
common stock will be taxable as dividends to the extent paid out of current or accumulated earnings and profits, as determined under United
States federal income tax principles. Amounts not treated as dividends for United States federal income tax purposes will constitute a
return of capital and first be applied against and reduce a non-U.S. holder’s adjusted tax basis in its common stock, but not below
zero. Any excess will be treated as capital gain and will be treated as described below under “Gain on disposition of our common
stock” Dividends paid to a non-U.S. holder of our common stock generally will be subject to United States federal withholding tax
at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. However, dividends that are effectively connected
with the conduct of a trade or business within the United States by the non-U.S. holder (and, if required by an applicable income tax
treaty, are attributable to a United States permanent establishment) are not subject to United States federal withholding tax, provided
certain certification and disclosure requirements are satisfied. Instead, such dividends are subject to United States federal income tax
on a net income basis in the same manner as if the non-U.S. holder were a “United States person” as defined in the Code. Any
such effectively connected dividends received by a foreign corporation may, under certain circumstances, be subject to an additional “branch
profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty.
A non-U.S. holder who wishes
to claim the benefit of an applicable treaty rate and avoid backup withholding, as discussed below, for dividends will be required (a)
to complete IRS Form W-8BEN or W-8BEN-E (or other applicable form) and certify under penalty of perjury that it is not a “United
States person” as defined in the Code and is eligible for treaty benefits or (b) if the common stock is held through certain foreign
intermediaries, to satisfy the relevant certification requirements of applicable Treasury Regulations. Special certification and other
requirements apply to certain non-U.S. holders that are pass-through entities rather than corporations or individuals.
A non-U.S. holder eligible
for a reduced rate of United States withholding tax pursuant to an income tax treaty may obtain a refund of any excess amounts withheld
by filing an appropriate claim for refund with the IRS. Dividend distributions to non-U.S. holders would also be subject to the rules
concerning backup withholding and FATCA, discussed below.
Gain on disposition of our common stock
Subject to the discussions
below under the headings “Information reporting and backup withholding” and “FATCA withholding requirements,”
any gain realized on the disposition of our common stock by a non-U.S. holder generally will not be subject to United States federal income
tax unless:
| · | the gain is effectively connected with a trade or business of
the non-U.S. holder in the United States (and, if required by an applicable income tax treaty, is attributable to a United States permanent
establishment of the non-U.S. holder); |
| · | the non-U.S. holder is an individual who is present in the United
States for 183 days or more in the taxable year of that disposition, and certain other conditions are met; or |
| · | we are or have been a “United States real property holding
corporation” for United States federal income tax purposes at any time during the shorter of the five-year period ending on the
date of the disposition or such non-U.S. holder’s holding period for our common stock and such non-U.S. holder held (at any time
during the shorter of the five-year period ending on the date of the disposition or such non-U.S. holder’s holding period) more
than 5% of our common stock. |
An individual non-U.S. holder
described in the first bullet point immediately above will be subject to tax on the net gain derived from the sale under regular graduated
United States federal income tax rates. If a non-U.S. holder that is a foreign corporation falls under the first bullet point immediately
above, it will be subject to tax on its net gain in the same manner as if it were a “United States person” as defined in the
Code and, in addition, may under certain circumstances be subject to a branch profits tax equal to 30% of its effectively connected earnings
and profits or at such lower rate as may be specified by an applicable income tax treaty.
Non-U.S. holders described
in the second bullet above should consult their tax advisors regarding the U.S. federal income tax consequences of the disposition of
our common stock. A non-U.S. holder described in the second bullet point above is generally expected to be subject to U.S. federal income
tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on gain realized upon the sale or other taxable
disposition of our common stock, which may be offset by United States source capital losses of the non-U.S. holder (even though the individual
is not considered a resident of the United States), provided the non-U.S. holder has timely filed U.S. federal income tax returns with
respect to such losses.
We believe we have not been
and are not currently a “United States real property holding corporation” for United States federal income tax purposes; however,
no assurance can be given that we are not or will not become one in the future. If, however, we are or become a “United States real
property holding corporation,” so long as our common stock continues to be regularly traded on an established securities market
(as such terms are defined in applicable Treasury Regulations), only a non-U.S. holder who holds or held (at any time during the shorter
of the five-year period ending on the date of disposition or the non-U.S. holder’s holding period) more than 5% of our common stock
will be subject to United States federal income tax on the disposition of the common stock. Non-U.S. holders should consult their own
tax advisors about the consequences that could result if we are, or become a “United States real property holding corporation.”
Information reporting and backup withholding
Information returns are required
to be filed with the IRS reporting the amount of dividends paid to each non-U.S. holder and the tax withheld with respect to such dividends,
regardless of whether withholding was required. Copies of the information returns reporting such dividends and withholding may also be
made available to the tax authorities in the country in which the non-U.S. holder resides under the provisions of an applicable income
tax treaty.
A non-U.S. holder will be
subject to backup withholding with respect to dividends paid to it unless it certifies under penalty of perjury that it is not a “United
States person” as defined in the Code (and the payor does not have actual knowledge or reason to know that it is a “United
States person” as defined in the Code), or it otherwise establishes an exemption.
Information reporting and,
depending on the circumstances, backup withholding will apply to the proceeds of a sale of our common stock within the United States or
conducted through certain United States-related financial intermediaries, unless the non-U.S. holder certifies under penalty of perjury
that it is not a “United States person” as defined in the Code (and the payor does not have actual knowledge or reason to
know that the beneficial owner is a “United States person” as defined in the Code), or it otherwise establishes an exemption.
Backup withholding is not
an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holder’s
United States federal income tax liability provided the required information is timely furnished to the IRS.
FATCA withholding requirements
Sections 1471 to 1474 of the
Code (such sections, and the Treasury Regulations and administrative guidance issued thereunder, commonly-referred to as FATCA) impose
a 30% United States withholding tax on certain “withholdable payments” made to a “foreign financial institution”
or a “nonfinancial foreign entity.” “Withholdable payments” include payments of dividends (such as amounts treated
as dividends paid with respect to shares of our common stock) in addition to certain other passive income type amounts. In general, if
a non-U.S. holder is a “foreign financial institution”, the 30% withholding tax will apply to withholdable payments made to
it unless it enters into an agreement with the United States Department of Treasury to collect and provide substantial information regarding
its United States account holders, including certain account holders that are foreign entities with United States owners, and to withhold
30% on certain “passthru payments.” If a non-U.S. holder is a “non-financial foreign entity,” FATCA also generally
will impose a withholding tax of 30% on withholdable payments made to it unless it provides the withholding agent with a certification
that it does not have any “substantial United States owners” or a certification identifying its direct and indirect substantial
United States owners. Intergovernmental agreements, to the extent entered into between the United States and a non-U.S. holder’s
resident country, may modify the foregoing requirements and may allow instead the avoidance of any FATCA withholding tax upon the satisfaction
of the requirements of such intergovernmental agreement.
Although withholding under
FATCA would also have applied to payments of gross proceeds from the sale or other disposition of our common stock on or after January
1, 2019, Treasury Regulations proposed in late 2018 eliminate FATCA withholding on payments of gross proceeds entirely. Taxpayers generally
may rely on these proposed Treasury Regulations until final Treasury Regulations are issued.
Non-U.S. holders should consult
their own tax advisors regarding the impact of FATCA on their ownership and disposition of shares of our common stock and the potential
applicability of any intergovernmental agreements.
UNDERWRITING
Pursuant
to the underwriting agreement with Lucid Capital Markets, LLC, as sole underwriter of this offering, we have agreed to issue and sell
to the underwriter, and the underwriter has agreed to purchase from us, at the respective public offering price less the underwriting
discounts and commissions set forth on the cover page of this prospectus supplement, the respective numbers of shares of our common stock
listed opposite its name below, on the closing date, subject to the terms and conditions contained in the underwriting agreement. The
underwriting agreement provides that the obligations of the underwriter are subject to certain customary conditions precedent, representations
and warranties contained therein.
| Underwriter | |
| Number
of
Shares | |
| Lucid Capital Markets, LLC | |
| | |
Pursuant
to the underwriting agreement, the underwriter has agreed to purchase all of the shares of common stock sold under the underwriting
agreement if any of these shares of common stock are purchased, other than those shares covered by the underwriter’s option to purchase
additional shares of common stock. The underwriter has advised us that it does not intend to confirm sales to any account over which it
exercises discretionary authority.
Discounts, Commissions and Expenses
The
underwriter proposes to offer the shares of common stock to the public at the public offering price set forth on the cover page of this
prospectus supplement. The underwriter may offer the shares of common stock to securities dealers at the public offering price less a
concession not in excess of $ per share. If all of the shares of common stock are not sold at the public offering price, the underwriter
may change the offering price and other selling terms.
The
underwriter is offering the shares of common stock subject to its acceptance of the shares of common stock from us and subject to prior
sale. The underwriting agreement provides that the obligations of the underwriter to pay for and accept delivery of the securities offered
by this prospectus supplement are subject to the approval of certain legal matters by their counsel and to certain other conditions specified
in the underwriting agreement.
We
have granted to the underwriter an option, exercisable for 30 days from the date of this prospectus supplement, to purchase up to additional
shares of common stock (up to 15% of the shares of common stock offered in this offering) listed on the cover page of this prospectus
supplement, less underwriting discounts and commissions. However, because our common stock is publicly traded, the underwriter may satisfy
some or all of the over-allotment of shares of our common stock, if any, by purchasing shares in the open market and will have no obligation
to exercise the over-allotment option with respect to our common stock.
The
following table shows the public offering price, underwriting discounts and commissions and proceeds, before expenses and fees, to us.
These amounts are shown assuming both no exercise and full exercise of the underwriter’s option to purchase additional securities.
| |
|
Per Share |
|
Total
without
Option |
|
Total
with
Option |
|
| Public offering price |
|
$ |
|
|
|
|
|
|
| Underwriting discounts and commissions payable by us |
|
$ |
|
|
|
|
|
|
| Proceeds, before expenses and fees, to us |
|
$ |
|
|
|
|
|
|
We
have agreed to pay the underwriter up to $125,000 for fees and expenses of outside legal counsel of the underwriter. We estimate that
the total expenses of the offering, excluding underwriting discounts and commissions, will be approximately $400,000 and are payable by us.
We
have agreed to pay the underwriter a commission equal to 7.0% of the aggregate gross proceeds from the sale of the shares of common stock
in this offering.
Indemnification
We
have agreed to indemnify the underwriter against certain liabilities, including civil liabilities under the Securities Act, or to contribute
to payments that the underwriter may be required to make in respect of those liabilities.
Lock-Up Agreements
We
have agreed with the underwriter to be subject to a lock-up period of 90 days following the date of this prospectus supplement. This means
that, during the applicable lock-up period, we may not issue, enter into any agreement to issue or announce the issuance or proposed issuance
of any shares of common stock or their equivalents, subject to certain exceptions. In addition, each of our directors and executive officers
has entered into a lock-up agreement with the underwriter. Under the lock-up agreements, the directors and executive
officers may not, directly or indirectly, offer, sell, contract to sell, hypothecate, pledge, or otherwise dispose of, shares of our common
stock or securities convertible into or exchangeable for shares our common stock, unless such directors and executive officers obtain
prior written consent of the underwriter for a period of 90 days from the date of this prospectus supplement.
Tail Financing
Until nine months after the closing date of this offering, the Underwriter will be entitled to receive 35% of the economics
of a Tail Financing, defined as gross spread less transaction fees and expenses incurred by the lead book runner(s) for any public or
private offering or other organized capital-raising executed by the Company. In case an investor that was not included in the list of
investors provided by the Company to the Underwriter prior to the closing of this offering participates in a Tail Financing, the Underwriter
will be entitled to a 7% fee of the aggregate gross proceeds for that investor, however, the total fee earned by the Underwriter in a
Tail Financing shall not exceed 35%.
Price Stabilization, Short Positions and
Penalty Bids
In
connection with this offering, the underwriter may engage in stabilizing transactions, overallotment transactions, syndicate covering
transactions and penalty bids in connection with our common stock.
Stabilizing
transactions permit bids to purchase shares of common stock so long as the stabilizing bids do not exceed a specified maximum.
Overallotment
transactions involve sales by the underwriter of shares of common stock in excess of the number of shares the underwriter is obligated
to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered
short position, the number of shares over-allotted by the underwriter is not greater than the number of shares that it may purchase in
the overallotment option. In a naked short position, the number of shares involved is greater than the number of shares in the overallotment
option. The underwriter may close out any short position by exercising its overallotment option and/or purchasing shares in the open market.
Syndicate
covering transactions involve purchases of common stock in the open market after the distribution has been completed in order to cover
syndicate short positions. Such a naked short position would be closed out by buying securities in the open market. A naked short position
is more likely to be created if the underwriter is concerned that there could be downward pressure on the price of the securities in the
open market after pricing that could adversely affect investors who purchase in the offering.
Penalty
bids permit the underwriter to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate
member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. These stabilizing transactions,
syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or
preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock in the open market
may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriter makes any representation
or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may
be effected on The Nasdaq Capital Market, in the over-the-counter market or otherwise and, if commenced, may be discontinued
at any time.
Electronic Distribution
A
prospectus in electronic format may be made available on the websites maintained by the underwriter, if any, participating in this offering
and the underwriter may distribute prospectuses electronically. Other than the prospectus in electronic format, the information on these
websites is not part of this prospectus supplement or the registration statement of which this prospectus supplement forms a part, has
not been approved or endorsed by us or the underwriter, and should not be relied upon by investors.
Other Relationships
From
time to time, the underwriter and its affiliates have provided, and may provide in the future, various advisory, investment and commercial
banking and other services to us in the ordinary course of business, for which it has received and may continue to receive customary fees
and commissions.
Listing on The Nasdaq
Capital Market
Our common stock is quoted
on The Nasdaq Capital Market under the symbol “NVCT.” On February 3, 2025, the closing price of our common stock as
reported on The Nasdaq Capital Market was $6.80 per share.
Offer Restrictions Outside the United States
Other than in the United States,
no action has been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus supplement
in any jurisdiction where action for that purpose is required. The securities offered by this prospectus supplement may not be offered
or sold, directly or indirectly, nor may this prospectus supplement or any other offering material or advertisements in connection with
the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result
in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus supplement
comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus
supplement. This prospectus supplement does not constitute an offer to sell or a solicitation of an offer to buy any securities offered
by this prospectus supplement in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus supplement
is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and
Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian
Corporations Act. Accordingly, (i) the offer of the securities under this prospectus supplement is only made to persons to whom it
is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions
set out in section 708 of the Australian Corporations Act, (ii) this prospectus supplement is made available in Australia only to
those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting
this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under
the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within
12 months after its transfer to the offeree under this prospectus supplement.
Canada
The securities may be sold
in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National
Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in
National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities
must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities
laws. Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages
if this prospectus supplement (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission
or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province
or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province
or territory for particulars of these rights or consult with a legal advisor. Pursuant to section 3A.3 of National Instrument 33-105 Underwriting
Conflicts (NI 33-105), the underwriter is not required to comply with the disclosure requirements of NI33-105 regarding underwriter conflicts
of interest in connection with this offering.
Cayman Islands
No invitation, whether directly
or indirectly, may be made to the public in the Cayman Islands to subscribe for our securities.
European Economic Area — Belgium, Germany, Luxembourg
and Netherlands
The information in this document
has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC (the
“Prospectus Directive”) as implemented in Member States of the European Economic Area (each, a “Relevant Member State”),
from the requirement to produce a prospectus for offers of securities.
An offer to the public of
securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under
the Prospectus Directive as implemented in that Relevant Member State:
| · | to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized
or regulated, whose corporate purpose is solely to invest in securities; |
| · | to any legal entity that has two or more of (i) an average of at least 250 employees during its last
fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated
financial statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated
or consolidated financial statements); |
| · | to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e)
of the Prospectus Directive) subject to obtaining the prior consent of us or any underwriter for any such offer; or |
| · | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that
no such offer of securities shall result in a requirement for the publication by us of a prospectus pursuant to Article 3 of the
Prospectus Directive. |
France
This document is not being
distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning
of Article L.411-1 of the French Monetary and Financial Code (Code Monétaire et Financier) and Articles 211-1 et seq. of the
General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered
or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other
offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly,
may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions
have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account,
as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D.744-1, D.754-1 ;and D.764-1 of the French Monetary
and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs)
acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1; and D.764-1
of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3
of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly)
to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French
Monetary and Financial Code.
Ireland
The information in this document
does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish
regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the
meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities
have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering,
except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural
or legal persons who are not qualified investors.
Israel
The securities offered by
this prospectus supplement have not been approved or disapproved by the Israeli Securities Authority (the “ISA”) nor have
such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel,
absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing
the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion
as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered
by this prospectus supplement is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities
laws and regulations.
Italy
The offering of the securities
in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Società
e la Borsa, “CONSOB”) pursuant to the Italian securities legislation and, accordingly, no offering material relating to the
securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of
Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
| · | to Italian qualified investors, as defined in Article 100 of Decree no.58 by reference to Article 34-ter
of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”);
and |
| · | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of
Decree No. 58 and Article 34-ter of Regulation No. 11971, as amended. |
Any offer, sale or delivery of the securities
or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an
offer from the issuer) under the paragraphs above must be:
| · | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy
in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of
29 October 2007 and any other applicable laws; and |
| · | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable
laws. |
Any subsequent distribution
of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No.
58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result
in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages
suffered by the investors.
Japan
The securities have not been
and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948),
as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities
to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations
promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit
of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may
not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities
is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being
distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal,
within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities
have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any
other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission
(Comissăo do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused
to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a
public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons
who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document
and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been,
and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document
may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to
require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument).
Any offering of securities in Sweden is limited to persons who are “qualified investors” (as
defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the
information contained in it to any other person.
Switzerland
The securities may not be
publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or
regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses
under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of
the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document
nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor
any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority.
In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market
Supervisory Authority (FINMA). This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor
the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental
authority in the United Arab Emirates, nor have we received authorization or licensing from the Central Bank of the United Arab Emirates
or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This
document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including
the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by us.
No offer or invitation to
subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in
this document nor any other document relating to the offer has been delivered for approval to the Financial Conduct Authority in the United
Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)
has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified
investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities
may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances
which do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not be distributed, published
or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement
to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities
has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom
in circumstances in which section 21(1) of FSMA does not apply to us.
In the United Kingdom, this
document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments
falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order
2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth
companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant
persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase
will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any
of its contents.
Legal
matters
The validity of the common stock offered by this
prospectus supplement and the accompanying prospectus will be passed upon for us by Alston & Bird LLP, New York, New York. Certain
legal matters relating to this offering will be passed upon for the underwriter by Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C.,
New York, New York.
Experts
The financial statements
incorporated in this Prospectus by reference to the Annual Report on Form 10-K for the year ended December 31, 2023 have been so incorporated
in reliance on the report of Kesselman & Kesselman C.P.A.s, an independent registered public accounting firm and a PwC member firm,
given on the authority of such firm as experts in accounting and auditing.
Where
you can find more information
This prospectus supplement
and the accompanying prospectus are part of a registration statement on Form S-3 we filed with the SEC and do not contain all of the information
set forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and
the common stock we are offering under this prospectus supplement and the accompanying prospectus, we refer you to the registration statement
and the exhibits and schedules filed as a part of the registration statement. You should rely only on information contained in this prospectus
supplement, the accompanying prospectus and incorporated by reference herein and therein. We have not authorized any person to provide
you with different information. We are not making an offer of these securities in any state where the offer is not permitted. You should
not assume that the information in this prospectus supplement is accurate as of any date other than the date on the front page of this
prospectus supplement, regardless of the time of delivery of this prospectus supplement or any sale of the securities offered by this
prospectus supplement.
We file reports with the SEC
on an annual basis using Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K. The SEC maintains a website that contains
annual, quarterly, and current reports, proxy statements, and other information that issuers (including us) file electronically with the
SEC. The SEC’s website address is http://www.sec.gov. You can also obtain copies of materials we file with the SEC from our internet
website found at www.nuvectis.com. Our stock is quoted on the Nasdaq Capital Market under the symbol “NVCT”.
Incorporation
of certain information by reference
The SEC allows us to “incorporate
by reference” the information we file with them. This means that we can disclose important information to you by referring you to
those documents instead of having to repeat the information in this document. The information incorporated by reference is considered
to be part of this prospectus supplement, and later information that we file with the SEC will automatically update and supersede this
information. We incorporate by reference the documents listed below and any future filings made with the SEC under Sections
13(a), 13(c), 14 or 15(d) of the Exchange Act (1) after the date of the initial registration statement, as amended, and prior
to effectiveness of the registration statement, and (2) after the date of this prospectus and prior to the termination of this offering.
Such information will automatically update and supersede the information contained in this prospectus supplement and the documents listed
below; provided, however, that we are not, unless specifically indicated, incorporating any information furnished under Item 2.02 or Item
7.01 of any current report on Form 8-K, whether listed below or filed in the future, or related exhibits furnished pursuant to Item 9.01
of Form 8-K:
(a) Our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 5, 2024;
(b) Our Quarterly Reports on Form 10-Q for the quarters ended
March 31, 2024, June 30, 2024, and September 30, 2024, filed with the SEC on May 7, 2024, August 6, 2024, and November 5, 2024, respectively;
(c) Our Current Reports on Form 8-K filed with the SEC on June 13, 2024 and August 29, 2024; and
(d) Description of our common stock contained in Exhibit 4.6, which is contained in our Annual Report on Form 10-K filed with the SEC on March 5, 2024.
A statement contained in a
document incorporated by reference into this prospectus supplement and the accompanying prospectus shall be deemed to be modified or superseded
for purposes of this prospectus supplement and the accompanying prospectus to the extent that a statement contained in this prospectus
supplement, the accompanying prospectus, or in any other subsequently filed document which is also incorporated in this prospectus supplement
and the accompanying prospectus modifies or replaces such statement. Any statements so modified or superseded shall not be deemed, except
as so modified or superseded, to constitute a part of this prospectus supplement and the accompanying prospectus.
We will provide to each person,
including any beneficial owner, to whom a copy of this prospectus supplement is delivered, a copy of any or all of the information that
we have incorporated by reference into this prospectus. We will provide this information upon written or oral request at no cost to the
requester. You may request this information by contacting our corporate headquarters at the following address: 1 Bridge Plaza, Suite 275,
Fort Lee, NJ 07024, Attn: Michael Carson, or by calling (201) 614-3150.
We have not authorized anyone
to provide you with information different from that contained in this prospectus supplement or incorporated by reference in this prospectus
supplement.
PROSPECTUS
$150,000,000
Nuvectis Pharma, Inc.
Common Stock
Preferred Stock
Warrants
Debt Securities
Units
The following are types of
securities that we may offer, issue and sell from time to time, together or separately:
| • | shares of our common stock; |
| • | shares of our preferred stock; |
| • | units consisting of any combination of our common stock, preferred stock, warrants or debt securities. |
We may, from time to time,
offer these securities in amounts, at prices, and on terms determined at the time of offering. We may sell these securities directly to
you through agents, underwriters, or dealers we select. If we use agents, underwriters or dealers to sell these securities, we will name
them and describe their compensation in a prospectus supplement. You should read this prospectus and any prospectus supplement carefully
before you invest.
This prospectus provides a
general description of the securities we may offer. Each time we sell securities, we will provide specific terms of the securities offered
in a supplement to this prospectus. The prospectus supplement may also add, update or change information contained in this prospectus.
You should read this prospectus and the applicable prospectus supplement carefully, together with additional information described under
the heading “Where You Can Find More Information,” before you invest in any securities. This prospectus may not be used to
consummate a sale of securities unless accompanied by the applicable prospectus supplement.
We are an “emerging
growth company” as defined under U.S. federal securities laws and, as such, have elected to comply with reduced public company reporting
requirements. This prospectus complies with the requirements that apply to an issuer that is an emerging growth company.
Our common stock is traded
on the Nasdaq Capital Market under the symbol “NVCT.” On March 7, 2023, the per share closing price of our common stock
as reported on the Nasdaq Capital Market was $11.34 per share.
Investing in our securities
involves certain risks. See “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2022,
which has been filed with the SEC and are incorporated by reference into this prospectus. You should read the entire prospectus carefully
before you make your investment decision.
Neither the Securities
and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy
or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is March 29, 2023.
Table of Contents
ABOUT THIS PROSPECTUS
This prospectus is part of
a “shelf” registration statement that we filed with the United States Securities and Exchange Commission (the “SEC”).
By using a shelf registration statement, we may offer and sell any combination of the securities described a in this prospectus, from
time to time, in one or more offerings. Each time we sell securities, we will provide a prospectus supplement to this prospectus that
contains specific information about the terms of such offering. The prospectus supplement may also add, update or change information contained
in this prospectus. Before purchasing any securities, you should carefully read both this prospectus and any supplement, together with
the additional information incorporated into this prospectus or described under the heading “Where You Can Find More Information.”
You should rely only on the
information contained or incorporated by reference in this prospectus and any prospectus supplement. We have not authorized any other
person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely
on it. We will not make an offer to sell securities in any jurisdiction where the offer or sale is not permitted. You should assume that
the information appearing in this prospectus, as well as information we previously filed with the SEC and have incorporated by reference,
is accurate as of the date on the front cover of this prospectus only, or when such document was filed with the SEC. Our business, financial
condition, results of operations and prospects may have changed since the relevant date.
We will not use this prospectus
to offer and sell securities unless it is accompanied by a prospectus supplement that more fully describes the terms of the offering.
When we refer to “Nuvectis,”
“we,” “our,” “us” and the “Company” in this prospectus, we mean Nuvectis Pharma, Inc.,
unless otherwise specified. When we refer to “you,” we mean the potential holders of the applicable series of securities.
Solely
for convenience, tradenames referred to in this prospectus, the accompanying prospectus and the documents incorporated by reference may
appear without the ® or TM symbol,
but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our
rights or that the applicable owner will not assert its rights, to these tradenames.
NUVECTIS PHARMA, INC.
Overview
We are a biopharmaceutical
company focused on the development of innovative precision medicines for the treatment of serious conditions of unmet medical need in
oncology. We seek to develop drug candidates in the precision medicine space, and our processes for selection and clinical development
of drug candidates is -based on scientific insights into cancer-promoting factors, as well as on our understanding of the clinical landscape
and regulatory requirements.
Products Under Development
NXP800 – Our Lead Product Candidate
In May 2021,
we licensed exclusive world-wide commercial rights to NXP800, a novel orally bioavailable small molecule that was discovered in a screen
for Heat Shock Factor 1 (“HSF1”) pathway inhibitors; NXP800 was discovered at the Institute for Cancer Research (“ICR”)
in London, England. Our license agreement with the ICR is subject to certain milestone and royalty payments. For additional information
see section “NXP800 License Agreement.” In December 2022, NXP800 received Fast Track Designation from the U.S. Food and
Drug Administration (“FDA”) for treatment of Platinum Resistant, AT-Rich Interaction Domain (“ARID1a”)-Mutated
Ovarian Carcinoma.
Scientific Background:
In preclinical studies,
treatment with NXP800 inhibited tumor growth in xenografts of ovarian cancer that harbored a loss of function mutation in the AT-Rich
Interaction Domain (“ARID1a”) gene. Based on this work, we plan to evaluate the safety and efficacy of NXP800 in ARID1a-mutated
ovarian carcinoma, which is a cancer type comprised primarily of two histologies: ovarian clear cell carcinoma (“OCCC”) and
endometrioid ovarian carcinoma (“EOC”), and to investigate the use of ARID1a mutations as a potential patient selection marker
for additional types of cancer. The genetic screening for mutations in the ARID1a gene is included in commercially available Next Generation
Sequencing (“NGS”) kits.
NXP800 Clinical Development Plan
A comprehensive
preclinical data package supported the approval of the Clinical Trial Application (“CTA”) by the Medicines and Healthcare
Regulatory Agency (“MHRA”) in the United Kingdom, and the Investigational New Drug (“IND”) Application submission
by the FDA. In December 2021, we announced the commencement of the Phase 1 study for NXP800. The Phase 1 study is comprised of two
parts: dose-escalation Phase 1a, and an expansion Phase 1b. In the ongoing Phase 1a, we have been evaluating the safety and tolerability
of NXP800 in patients with advanced solid tumors to identify potential a doses and dosing schedule for the Phase 1b. The Phase 1a is nearing
completion and the Phase 1b is expected to begin in 1H 2023. In the Phase 1b, the safety and preliminary anti-tumor activity of NXP800
will be initially evaluated in in women with platinum-resistant, ARID1a-mutated OCCC and EOC. In December 2022, we announced that
the FDA granted Fast Track Designation status to the NXP800 for the treatment of patients with platinum-resistant development program
in platinum resistant, ARID1a-mutated ovarian carcinoma. Moreover, we recently announced that the European Network of Gynecological Oncology
Trial Groups (“ENGOT”) and the GOG Foundation, Inc. (“GOG-F”), the world's premier gynecology oncology clinical
trials consortia, will lead the Phase 1b clinical trial in ARID1a-mutated ovarian carcinoma. Additional cohorts/trials in patients with
other types of solid tumors may also be explored based on emerging data.
Addressing an Unmet Need in Clear
Cell Ovarian Cancer and Advanced-stage Endometrioid Ovarian Carcinoma
We are investigating
plan to initially investigate NXP800 as treatment for platinum-resistant, ARID1a- mutated ovarian carcinoma, which is a cancer type comprised
primarily of two histologies: OCCC and EOC.
OCCC is highly malignant,
difficult to treat, and has a very poor survival rate due to frequent recurrence after surgery and first-line treatment. First-line treatment
consists of platinum-based chemotherapy (“PBC”), for which the reported response rate in relapse/refractory, platinum resistant
patients is 1%, demonstrating a clear and dire need for a new treatment option for women with OCCC. OCCC represents approximately 10%
of all ovarian cancer cases in the United States, with an annual incidence of approximately 2,200 patients.
EOC also represents
approximately 10% of all diagnosed ovarian cancer cases. If diagnosed at an early-stage, EOC can often be resected. However, if diagnosed
at later stages, these tumors have a substantially worse prognosis. Advanced, platinum-refractory, endometrioid cancer in the United States
represents approximately 30% of the endometrioid ovarian cancer segment. In this ovarian subset the progression-free survival at three
years for women diagnosed with stage III/IV disease is a dismal 20% for stage III and 0% for stage IV, representing a clear unmet medical
need.
OCCC and EOC are
subtypes of epithelial ovarian carcinoma whose clinical characteristics are distinct from those of high-grade serous ovarian carcinoma.
They exhibit a unique biological profile that is markedly different from those of other histologic types. The relative prevalence of OCCC
and EOC among women with ovarian cancer is higher in East Asia (for example, approximately 25% and 19% in Japan for OCCC and EOC, respectively),
than in Europe and the United States (approximately 10% for each indication).
NXP900
In August 2021,
we licensed worldwide commercial rights to NXP900 from the University of Edinburgh in Scotland. NXP900 is a targeted-therapy, small molecule
drug candidate that inhibits the Proto-oncogene c-Src (“SRC”) and YES1 kinases. We have completed the IND-enabling studies
and plan to submit an IND application with the FDA, or an equivalent submission with a foreign agency, in order to begin a Phase 1a dose-escalation
study of NXP900 in solid tumors in 1H 2023. Subsequently, upon successful completion of the dose-escalation study, we plan to conduct
a Phase 1b clinical trial to investigate NXP900 in solid tumors where the SRC and/or YES1 pathways are overactivated and implicated in
the disease etiology.
Scientific Background
SRC as an Anti-Cancer Target
SRC is aberrantly
activated in many cancer types, including solid tumor cancers such as breast, colon, prostate, pancreatic and ovarian cancers, while remaining
predominantly inactive in non-cancerous cells. Increased SRC activity is generally associated with late-stage cancers, metastatic potential
and resistance to therapies, and correlates with poor clinical prognosis. To date no kinase inhibitor has been approved for the treatment
of SRC-active solid tumor malignancies.
YES1 as an Anti-Cancer Target
YES1
is a nonreceptor tyrosine kinase that belongs to the SRC family of kinases and controls multiple cancer signaling pathways. YES1 is
amplified and overexpressed in many tumor types, where it promotes cell proliferation, survival, and invasiveness. In addition,
YES1 directly phosphorylates and activates the Yes-associated protein, the main
effector of the Hippo pathway, which has been identified as a promoter of drug resistance, cancer progression, and metastasis in several
cancer types, including squamous cell, mesothelioma and papillary kidney cancers.
NXP900’s Novel Mechanism of
Action
SRC pathway activation
is regulated by a switch between inactive and active conformations. The inactive conformation of SRC family kinases is associated with
lack of membrane binding, lack of phosphorylation of the activation loop, and characterized by a “closed conformation.” The
active “open” conformation allows for the binding of SRC to signaling partners and enables full activation of the pathway
via SRC’s kinase catalytic activity and the scaffolding property.
NXP900 is a targeted-therapy
that inhibits the SRC and YES1 kinases. Unlike the approved and clinical-stage kinase inhibitors that inhibit only the catalytic (enzymatic)
activity of SRC, NXP900 induces and locks SRC in its native inactive conformation, by inhibiting both the catalytic and scaffolding functions
of the kinase, thus preventing phosphorylation and complex formation with its primary partners. NXP900 is also highly selective, a property
typically associated with an improved therapeutic window.
In vivo, single-agent treatment with
NXP900 inhibited primary and metastatic tumor growth in xenograft models of breast, cervical, esophageal, head and neck cancers and medulloblastoma,
and demonstrated on-target pharmacodynamic effects. NXP900’s unique mechanism of action translated into substantial single-agent
induced tumor regression in several in vivo xenograft models. Moreover, publications in the scientific literature outlined opportunities
to potentially reverse resistance to osimertinib (Tagrisso) in non-small cell lung cancer and enzalutamide (Xtandi) in metastatic, castration
resistant prostate cancer, in combination with these agents, validating the importance of NXP900’s key targets, YES1 and SRC kinases,
in these disease settings.
Gene amplification
of the site containing the YES1 gene has been reported in clinical samples in several tumors including lung, head and neck, bladder and
esophageal cancers. YES1-dependent oncogenic transformation has also been reported, suggesting that YES1 plays a key role in these solid
tumors. The transforming ability of YES1 has been demonstrated via several experimental methods, for example down-regulating YES1 by short
hairpin RNA (shRNA) significantly inhibited cell growth in several malignancies, including colon carcinoma, rhabdomyosarcoma, and basal-like
breast cancer suggesting YES1 may play a key role in these solid tumors. Furthermore, it has been found that YES1 gene amplification is
a key mechanism of resistance to Epidermal Growth Factor Receptor, Alk and Human Epidermal growth factor Receptor 2 inhibitors.
There are no YES1 inhibitors that are FDA approved or currently in
clinical development. We plan to conduct additional in vivo studies to better understand the effects of YES1 inhibition in solid tumors
driven by YES1 overexpression or gene amplification.
Our Company
We were incorporated under the laws of the State
of Delaware in July 2020. Our principal executive office is located at 1 Bridge Plaza, Suite 275, Fort Lee, NJ 07024 and our
telephone number is (201) 614-3150.
| |
| THE OFFERING |
| |
|
| Use of Proceeds |
We intend to use the net proceeds of any offering as set forth
in the applicable prospectus supplement. |
| |
|
| Nasdaq Capital Market Symbol |
NVCT |
| |
|
RISK FACTORS
An investment in our securities involves a high
degree of risk. The prospectus supplement applicable to each offering of our securities will contain a discussion of the risks applicable
to an investment in our securities. Prior to making a decision about investing in our securities, you should carefully consider the specific
factors discussed under the heading “Risk Factors” in the applicable prospectus supplement, together with all of the other
information contained or incorporated by reference in the prospectus supplement or appearing or incorporated by reference in this prospectus.
You should also consider the risks, uncertainties and assumptions discussed in the “Risk Factors” section of our most recent
Annual Report on Form 10-K, which is incorporated herein by reference, and may be amended, supplemented or superseded from time to time
by other reports we file with the SEC in the future. Each of the referenced risks and uncertainties could adversely affect our business,
operating results and financial condition, as well as adversely affect the value of an investment in our securities.
FORWARD-LOOKING STATEMENTS
This prospectus includes statements
that are, or may be deemed, “forward-looking statements.” In some cases, these forward-looking statements can be identified
by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,”
“expects,” “plans,” “intends,” “may,” “could,” “would,” “might,”
“will,” “should,” “approximately” or, in each case, their negative or other variations thereon or
comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout
this prospectus and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning,
among other things, our history of net operating losses and uncertainty regarding our ability to obtain capital and achieve profitability,
our ability to develop and commercialize our product candidates, our ability to advance our development programs, enroll our trials, and
achieve clinical endpoints, our ability to use or expand our technology to build a pipeline of product candidates, our ability to obtain
and maintain regulatory approval of our product candidates and comply with ongoing regulatory requirements, our ability to successfully
operate in a competitive industry and gain market acceptance by physician, provider, patient, and payor communities, our reliance on third
parties, unstable economic or market conditions, and our ability to obtain and adequately protect intellectual property rights for our
product candidates.
By their nature, forward-looking
statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific
developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines
than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus,
we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial
condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements
contained in this prospectus. In addition, even if our results of operations, financial condition and liquidity, and the development of
the industry in which we operate are consistent with the forward-looking statements contained in this prospectus, they may not be predictive
of results or developments in future periods. The forward-looking statements contained in this prospectus reflect our views and assumptions
only as of the date of this prospectus. Except as required by law, we assume no responsibility for updating any forward-looking statements.
We qualify all of our forward-looking statements by these cautionary statements. In addition, with respect to all of our forward-looking
statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform
Act of 1995.
WHERE YOU CAN FIND MORE INFORMATION
We
file reports with the SEC on an annual basis using Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K.
The SEC maintains a website that contains annual, quarterly, and current reports, proxy statements, and other information that issuers
(including us) file electronically with the SEC. The SEC’s website address is http://www.sec.gov. You can also obtain copies
of materials we file with the SEC from our internet website found at www.nuvectis.com. Our stock is quoted on the Nasdaq Capital Market
under the symbol “NVCT”.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate
by reference” the information we file with them. This means that we can disclose important information to you by referring you to
those documents instead of having to repeat the information in this document. The information incorporated by reference is considered
to be part of this prospectus, and later information that we file with the SEC will automatically update and supersede this information.
We incorporate by reference the documents listed below and any future filings made with the SEC under Sections 13(a), 13(c), 14 or
15(d) of the Exchange Act (1) after the date of the initial registration statement, as amended, and prior to effectiveness of
the registration statement, and (2) after the date of this prospectus and prior to the termination of this offering. Such information
will automatically update and supersede the information contained in this prospectus and the documents listed below; provided, however,
that we are not, unless specifically indicated, incorporating any information furnished under Item 2.02 or Item 7.01 of any
current report on Form 8-K, whether listed below or filed in the future, or related exhibits furnished pursuant to Item 9.01
of Form 8-K:
We will provide to each person, including any beneficial
owner, to whom a copy of this prospectus is delivered, a copy of any or all of the information that we have incorporated by reference
into this prospectus. We will provide this information upon written or oral request at no cost to the requester. You may request this
information by contacting our corporate headquarters at the following address: 1 Bridge Plaza, Suite 275, Fort Lee, NJ 07024, Attn:
Ron Bentsur, or by calling (201) 614-3150.
DESCRIPTION OF SECURITIES WE MAY OFFER
This prospectus contains summary
descriptions of the securities we may offer from time to time. These summary descriptions are not meant to be complete descriptions of
each security. The particular terms of any security will be described in the related prospectus supplement.
DESCRIPTION OF CAPITAL STOCK
The
following descriptions are summaries of the material terms of our amended and restated certificate of incorporation, as amended (“certificate
of incorporation”), and our amended and restated bylaws (“bylaws”). The following summaries may not be complete
and are subject to, and qualified by reference to, the terms and provisions of our certificate of incorporation and our bylaws. You should
refer to, and read this summary together with, our certificate of incorporation, and our bylaws to review all of the terms of that may
be important to you.
Common Stock
We are authorized to issue
a total of 60,000,000 shares of common stock, par value $0.00001 per share. As of March 1, 2023, we had 14,752,403 actual shares
of our common stock issued and outstanding. All outstanding shares of our common stock are fully paid and nonassessable. Our common stock
is listed on The Nasdaq Capital Market and trades under the symbol “NVCT” and has been publicly traded since February 4,
2022. Prior to that time, there was no public market for our common stock.
Holders
The number of record holders of our 14,752,403 shares
of outstanding common stock as of March 1, 2023 was 40. This number does not include beneficial owners whose shares are held by nominees
in street name.
Dividends
We have never declared or
paid any cash dividends on our common stock and do not anticipate paying any cash dividends in the foreseeable future. Any future determination
to pay dividends will be at the sole discretion of our board of directors.
Voting Rights
Holders of our common stock
are entitled to one vote for each share held on all matters submitted to a vote of stockholders and do not have cumulative voting rights.
An election of directors by our stockholders will be determined by a plurality of the votes cast by the stockholders entitled to vote
on the election. Holders of common stock are entitled to receive proportionately any dividends as may be declared by our board of directors
in its sole discretion, subject to any preferential dividend rights or other rights of outstanding preferred stock, if any.
Liquidation and Dissolution
In the event of our liquidation
or dissolution, the holders of common stock are entitled to receive proportionately all assets available for distribution to stockholders
after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of common
stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of common stock
are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate
and issue in the future.
Preferred Stock
We currently do not have any
shares of preferred stock outstanding, and we have no present plan to issue any shares of preferred stock. Our board of directors has
the authority, without further action by our stockholders, to issue up to 5,000,000 shares of preferred stock in one or more series and
to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights,
conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting,
or the designation of, such series, any or all of which may be greater than the rights of common stock. The issuance of our preferred
stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments
and payments upon our liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing
a change in control of our company or other corporate action.
Anti-Takeover Provisions
Our certificate of incorporation
and bylaws include a number of provisions that may have the effect of delaying, deferring or preventing another party from acquiring control
of us and encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our board
of directors rather than pursue non-negotiated takeover attempts. These provisions include the items described below.
Board composition and filling vacancies
Our certificate of incorporation
provides for the division of our board of directors into three classes serving staggered three-year terms, with one class being elected
each year. Our certificate of incorporation also provides that directors may be removed only for cause and then only by the affirmative
vote of the holders of two-thirds of the shares then entitled to vote at an election of directors. Furthermore, any vacancy on our board
of directors, however occurring, including a vacancy resulting from an increase in the size of our board, may only be filled by the affirmative
vote of a majority of our directors then in office even if less than a quorum. The classification of directors, together with the limitations
on removal of directors and treatment of vacancies, has the effect of making it more difficult for stockholders to change the composition
of our board of directors.
No written consent of stockholders
Our certificate of incorporation
provides that all stockholder actions are required to be taken by a vote of the stockholders at an annual or special meeting, and that
stockholders may not take any action by written consent in lieu of a meeting. This limit may lengthen the amount of time required to take
stockholder actions and would prevent the amendment of our bylaws or removal of directors by our stockholders without holding a meeting
of stockholders.
Meetings of stockholders
Our certificate of incorporation
and bylaws provide that only a majority of the members of our board of directors then in office may call special meetings of stockholders
and only those matters set forth in the notice of the special meeting may be considered or acted upon at a special meeting of stockholders.
Our bylaws limit the business that may be conducted at an annual meeting of stockholders to those matters properly brought before the
meeting.
Advance notice requirements
Our bylaws establish advance
notice procedures with regard to stockholder proposals relating to the nomination of candidates for election as directors or new business
to be brought before meetings of our stockholders. These procedures provide that notice of stockholder proposals must be timely given
in writing to our corporate secretary prior to the meeting at which the action is to be taken. Generally, to be timely, notice must be
received at our principal executive offices not less than 90 days nor more than 120 days prior to the first anniversary date
of the annual meeting for the preceding year. Our bylaws specify the requirements as to form and content of all stockholders’ notices.
These requirements may preclude stockholders from bringing matters before the stockholders at an annual or special meeting.
Amendment to certificate of incorporation and bylaws
Any amendment of our certificate
of incorporation must first be approved by a majority of our board of directors, and if required by law or our certificate of incorporation,
must thereafter be approved by two-thirds of the outstanding shares entitled to vote on the amendment and two-thirds of the outstanding
shares of each class entitled to vote thereon as a class. Our bylaws may be amended by the affirmative vote of a majority of the directors
then in office, subject to any limitations set forth in the bylaws; and may also be amended by the affirmative vote of two-thirds of the
outstanding shares entitled to vote on the amendment, or, if our board of directors recommends that the stockholders approve the amendment,
by the affirmative vote of two-thirds of the outstanding shares entitled to vote on the amendment, in each case voting together as a single
class.
Undesignated preferred stock
Our certificate of incorporation
provides for 5,000,000 authorized shares of preferred stock. The existence of authorized but unissued shares of preferred stock may enable
our board of directors to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise.
For example, if in the due exercise of its fiduciary obligations, our board of directors were to determine that a takeover proposal is
not in the best interests of our stockholders, our board of directors could cause shares of preferred stock to be issued without stockholder
approval in one or more private offerings or other transactions that might dilute the voting or other rights of the proposed acquirer
or insurgent stockholder or stockholder group. In this regard, our certificate of incorporation grants our board of directors broad power
to establish the rights and preferences of authorized and unissued shares of preferred stock. The issuance of shares of preferred stock
could decrease the amount of earnings and assets available for distribution to holders of shares of common stock. The issuance may also
adversely affect the rights and powers, including voting rights, of these holders and may have the effect of delaying, deterring or preventing
a change in control of us.
Delaware anti-takeover statute
We are subject to the provisions
of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly held Delaware corporation
from engaging in a “business combination” with an “interested stockholder” for a three-year period following the
time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. Under
Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of
the following conditions:
| • | before the stockholder became interested, our board of directors approved either the business combination
or the transaction which resulted in the stockholder becoming an interested stockholder; |
| • | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder,
the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced,
excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee
stock plans, in some instances, but not the outstanding voting stock owned by the interested stockholder; or |
| • | at or after the time the stockholder became interested, the business combination was approved by our board
of directors and authorized at an annual or special meeting of the stockholders by the affirmative vote of a majority of the outstanding
voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination
to include:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, lease, pledge, exchange, mortgage or other disposition involving the interested stockholder
of 10% or more of the assets of the corporation; |
| • | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of
any stock of the corporation to the interested stockholder; or |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided
by or through the corporation. |
In general, Section 203 defines an interested
stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity
or person affiliated with or controlling or controlled by the entity or person.
Choice of forum
Our bylaws provide that the
Court of Chancery of the State of Delaware will be the exclusive forum for state law claims for (i) any derivative action or proceeding
brought on our behalf, (ii) any action asserting a breach of fiduciary duty by one or more of our directors, officers or employees,
(iii) any action asserting a claim against us arising pursuant to the Delaware General Corporation Law or (iv) any action asserting
a claim against us that is governed by the internal affairs doctrine, in each case subject to the Court of Chancery having personal jurisdiction
over the indispensable parties named as defendants therein (the “Delaware Forum Provision”); provided, however, that this
forum provision will not apply to any causes of action arising under the Exchange Act or the Securities Act which, unless we consent in
writing to the selection of an alternative forum, are required to be brought exclusively in federal district courts of the United States
of America in accordance with our certificate of incorporation. In addition, our bylaws provide that any person or entity purchasing or
otherwise acquiring any interest in shares of our common stock is deemed to have notice of and consented to the Delaware Forum Provision.
We recognize that the Delaware Forum Provision in our bylaws may impose additional litigation costs on stockholders in pursuing any such
claims, particularly if the stockholders do not reside in or near the State of Delaware. Additionally, the Delaware Forum Provision may
limit our stockholders’ ability to bring a claim in a judicial forum that they find favorable for disputes with us or our directors,
officers or employees, which may discourage such lawsuits against us and our directors, officers and employees. The Court of Chancery
of the State of Delaware may also reach different judgments or results than would other courts, including courts where a stockholder considering
an action may be located or would otherwise choose to bring the action, and such judgments may be more or less favorable to us than our
stockholders.
Transfer Agent
The transfer agent and registrar
for our common stock is American Stock Transfer & Trust Company, LLC.
Listing
Our common stock is listed
on the Nasdaq Capital Market under the symbol “NVCT”.
DESCRIPTION OF WARRANTS
We may issue warrants to purchase
shares of our common stock, preferred stock, or debt securities in one or more series together with other securities or separately, as
described in each applicable prospectus supplement. The prospectus supplement relating to any warrants we offer will include specific
terms relating to the offering. These terms will include some or all of the following:
| • | the title of the warrants; |
| • | the aggregate number of warrants offered; |
| • | the designation, amount and terms of the securities for which the warrants are exercisable and the procedures and conditions relating
to the exercise of such warrants; |
| • | the exercise price of the warrants; |
| • | the dates or periods during which the warrants are exercisable; |
| • | the designation and terms of other securities, if any, with which the warrants are to be issued and the number of warrants issued
with each such security; |
| • | if the warrants are issued as a unit with another security, the date on and after which the warrants and the other security will be
separately transferable; |
| • | any provisions for the adjustment of the number or amount of securities receivable upon the exercise of the warrants or the exercise
price of the warrants; |
| • | the price or prices at which the securities purchasable upon exercise of the warrants may be purchased; |
| • | the form of consideration that may be used to exercise the warrants; |
| • | the date on which the right to exercise the warrants shall commence and the date on which the right will expire; |
| • | if the exercise price is not payable in U.S. dollars, the foreign currency, currency unit or composite currency in which the exercise
price is denominated; |
| • | any minimum or maximum amount of warrants that may be exercised at any one time; |
| • | the terms of any mandatory or option call provisions; |
| • | any terms relating to the modification of the warrants; |
| • | any terms, procedures and limitations relating to the transferability, exchange or exercise of the warrants; and |
| • | any other specific terms of the warrants. |
DESCRIPTION OF DEBT SECURITIES
We may offer debt securities,
in one or more series, which may be senior, subordinated or junior subordinated and may be convertible. Unless otherwise specified in
the applicable prospectus supplement, our debt securities will be issued in one or more series under an indenture to be entered into between
us and a trustee. We will issue the debt securities offered by this prospectus and any accompanying prospectus supplement under an indenture
to be entered into between us and the trustee identified in the applicable prospectus supplement. The terms of the debt securities will
include those stated in the indenture and those made part of the indenture by reference to the Trust Indenture Act of 1939, as in effect
on the date of the indenture. We have filed a copy of the form of indenture as an exhibit to the registration statement in which this
prospectus is included. The indenture will be subject to and governed by the terms of the Trust Indenture Act of 1939.
The following description
briefly sets forth certain general terms and provisions of the debt securities that we may offer. The particular terms of the debt securities
offered by any prospectus supplement and the extent, if any, to which these general provisions may apply to the debt securities, will
be described in the related prospectus supplement. Accordingly, for a description of the terms of a particular issue of debt securities,
reference must be made to both the related prospectus supplement and to the following description.
Debt Securities
The aggregate principal amount
of debt securities that may be issued under the indenture is unlimited. The debt securities may be issued in one or more series as may
be authorized from time to time pursuant to a supplemental indenture entered into between us and the trustee or an order delivered by
us to the trustee. For each series of debt securities we offer, a prospectus supplement accompanying this prospectus will describe the
following terms and conditions of the series of debt securities that we are offering, to the extent applicable:
| • | title and aggregate principal amount; |
| • | whether the debt securities will be senior, subordinated or junior subordinated; |
| • | any limit on the amount that may be issued; |
| • | applicable subordination provisions, if any; |
| • | provisions regarding whether the debt securities will be convertible or exchangeable into other securities or property of the Company
or any other person; |
| • | percentage or percentages of principal amount at which the debt securities will be issued; |
| • | interest rate(s) or the method for determining the interest rate(s); |
| • | whether interest on the debt securities will be payable in cash or additional debt securities of the same series; |
| • | dates on which interest will accrue or the method for determining dates on which interest will accrue and dates on which interest
will be payable; |
| • | whether the amount of payment of principal of, premium, if any, or interest on the debt securities may be determined with reference
to an index, formula or other method; |
| • | redemption, repurchase or early repayment provisions, including our obligation or right to redeem, purchase or repay debt securities
under a sinking fund, amortization or analogous provision; |
| • | if other than the debt securities’ principal amount, the portion of the principal amount of the debt securities that will be
payable upon declaration of acceleration of the maturity; |
| • | a discussion of certain material U.S. federal income tax considerations applicable to the debt securities; |
| • | amount of discount or premium, if any, with which the debt securities will be issued, including whether the debt securities will be
issued as “original issue discount” securities; |
| • | the place or places where the principal of, premium, if any, and interest on the debt securities will be payable; |
| • | where the debt securities may be presented for registration of transfer, exchange or conversion; |
| • | the place or places where notices and demands to or upon the Company in respect of the debt securities may be made; |
| • | whether the debt securities will be issued in whole or in part in the form of one or more global securities; |
| • | if the debt securities will be issued in whole or in part in the form of a book-entry security, the depository or its nominee with
respect to the debt securities and the circumstances under which the book-entry security may be registered for transfer or exchange or
authenticated and delivered in the name of a person other than the depository or its nominee; |
| • | whether a temporary security is to be issued with respect to such series and whether any interest payable prior to the issuance of
definitive securities of the series will be credited to the account of the persons entitled thereto; |
| • | the terms upon which beneficial interests in a temporary global security may be exchanged in whole or in part for beneficial interests
in a definitive global security or for individual definitive securities; |
| • | the guarantors, if any, of the debt securities, and the extent of the guarantees and any additions or changes to permit or facilitate
guarantees of such debt securities; |
| • | any covenants applicable to the particular debt securities being issued; |
| • | any defaults and events of default applicable to the debt securities, including the remedies available in connection therewith; |
| • | currency, currencies or currency units in which the purchase price for, the principal of and any premium and any interest on, such
debt securities will be payable; |
| • | time period within which, the manner in which and the terms and conditions upon which the Company or the purchaser of the debt securities
can select the payment currency; |
| • | securities exchange(s) on which the debt securities will be listed, if any; |
| • | whether any underwriter(s) will act as market maker(s) for the debt securities; |
| • | extent to which a secondary market for the debt securities is expected to develop; |
| • | provisions relating to defeasance; |
| • | provisions relating to satisfaction and discharge of the indenture; |
| • | any restrictions or conditions on the transferability of the debt securities; |
| • | provisions relating to the modification of the indenture both with and without the consent of holders of debt securities issued under
the indenture; |
| • | any addition or change in the provisions related to compensation and reimbursement of the trustee; |
| • | provisions, if any, granting special rights to holders upon the occurrence of specified events; |
| • | whether the debt securities will be secured or unsecured, and, if secured, the terms upon which the debt securities will be secured
and any other additions or changes relating to such security; and |
| • | any other terms of the debt securities that are not inconsistent with the provisions of the Trust Indenture Act (but may modify, amend,
supplement or delete any of the terms of the indenture with respect to such series of debt securities). |
General
One or more series of debt
securities may be sold as “original issue discount” securities. These debt securities would be sold at a substantial discount
below their stated principal amount, bearing no interest or interest at a rate which at the time of issuance is below market rates. One
or more series of debt securities may be variable rate debt securities that may be exchanged for fixed rate debt securities.
United States federal income
tax consequences and special considerations, if any, applicable to any such series will be described in the applicable prospectus supplement.
Debt securities may be issued
where the amount of principal and/or interest payable is determined by reference to one or more currency exchange rates, commodity prices,
equity indices or other factors. Holders of such debt securities may receive a principal amount or a payment of interest that is greater
than or less than the amount of principal or interest otherwise payable on such dates, depending upon the value of the applicable currencies,
commodities, equity indices or other factors. Information as to the methods for determining the amount of principal or interest, if any,
payable on any date, the currencies, commodities, equity indices or other factors to which the amount payable on such date is linked and
certain additional United States federal income tax considerations will be set forth in the applicable prospectus supplement.
The term “debt securities”
includes debt securities denominated in U.S. dollars or, if specified in the applicable prospectus supplement, in any other freely transferable
currency or units based on or relating to foreign currencies.
We expect most debt securities
to be issued in fully registered form without coupons and in denominations of $2,000 and any integral multiples thereof. Subject to the
limitations provided in the indenture and in the prospectus supplement, debt securities that are issued in registered form may be transferred
or exchanged at the principal corporate trust office of the trustee, without the payment of any service charge, other than any tax or
other governmental charge payable in connection therewith.
Global Securities
The debt securities of a series
may be issued in whole or in part in the form of one or more global securities that will be deposited with, or on behalf of, a depositary
identified in the prospectus supplement. Global securities will be issued in registered form and in either temporary or definitive form.
Unless and until it is exchanged in whole or in part for the individual debt securities, a global security may not be transferred except
as a whole by the depositary for such global security to a nominee of such depositary or by a nominee of such depositary to such depositary
or another nominee of such depositary or by such depositary or any such nominee to a successor of such depositary or a nominee of such
successor. The specific terms of the depositary arrangement with respect to any debt securities of a series and the rights of and limitations
upon owners of beneficial interests in a global security will be described in the applicable prospectus supplement.
Governing Law
The indenture and the debt
securities shall be construed in accordance with and governed by the laws of the State of New York.
DESCRIPTION OF UNITS
We may issue units comprised
of one or more of the other securities described in this prospectus in any combination, as described in the applicable prospectus supplement.
Each unit will be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of
a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may
provide that the securities included in the unit may not be held or transferred separately at any time or at any time before a specified
date.
We may evidence units by unit
certificates that we issue under a separate agreement. We may issue the units under a unit agreement between us and one or more unit agents.
If we elect to enter into a unit agreement with a unit agent, the unit agent will act solely as our agent in connection with the units
and will not assume any obligation or relationship of agency or trust for or with any registered holders of units or beneficial owners
of units. We will indicate the name and address and other information regarding the unit agent in the applicable prospectus supplement
relating to a particular series of units if we elect to use a unit agent.
We will describe in the applicable prospectus supplement
the terms of the series of units being offered, including:
| • | the designation and material terms of the units and of the securities comprising the units, including whether and under what circumstances
those securities may be held or transferred separately; |
| • | any material provisions of the governing unit agreement that differ from those described herein; and |
| • | any material provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the
units. |
The other provisions regarding
our common stock, preferred stock, warrants and debt securities as described in this section will apply to each unit to the extent such
unit consists of shares of our common stock, preferred stock, warrants and/or debt securities.
PLAN OF DISTRIBUTION
We may sell the securities covered in this prospectus
from time to time in one or more of the following ways:
| • | through underwriters or dealers; |
| • | in short or long transactions; |
| • | directly to one or more purchasers; |
| • | through registered direct offerings; |
| • | as part of a collaboration with a third party; |
| • | through at-the-market issuances; |
| • | in privately negotiated transactions; or |
| • | through a combination of any of these methods of sale. |
Each time that we use this
prospectus to sell securities, we will also provide a prospectus supplement that contains the specific terms of the offering. The prospectus
supplement will set forth the terms of the offering of the securities, including the following, as applicable:
| • | the name or names of any underwriters, dealers or agents and the amounts of any securities underwritten or purchased by each of them; |
| • | the purchase price of the securities being offered and the proceeds to us and any discounts, commissions or concessions allowed or
reallowed or paid to dealers; |
| • | any options under which underwriters may purchase additional securities from us; and |
| • | any security exchanges on which the securities may be listed. |
The purchase price and any discounts or concessions
allowed or reallowed or paid to dealers may be changed from time to time.
If
underwriters are used in the sale of any securities, the securities will be acquired by the underwriters for their own account and may
be resold from time to time in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying
prices determined at the time of sale. The securities may be either offered to the public through underwriting syndicates represented
by managing underwriters, or directly by underwriters. Generally, the underwriters’ obligations to purchase the securities
will be subject to certain conditions precedent. The underwriters will be obligated to purchase all of the securities if they purchase
any of the securities.
We may sell the securities
through agents from time to time. The prospectus supplement will name any agent involved in the offer or sale of the securities and any
commissions we pay to them. Generally, any agent will be acting on a best efforts basis for the period of its appointment.
We may authorize underwriters,
dealers or agents to solicit offers by certain purchasers to purchase the securities from us at the public offering price set forth in
the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future.
The contracts will be subject only to those conditions set forth in the prospectus supplement, and the prospectus supplement will set
forth any commissions we pay for solicitation of these contracts.
Agents and underwriters may
be entitled to indemnification by us against certain civil liabilities, including liabilities under the Securities Act of 1933, as amended,
or to contribution with respect to payments which the agents or underwriters may be required to make in respect thereof. Agents and underwriters
may be customers of, engage in transactions with, or perform services for us in the ordinary course of business.
We may enter into derivative
transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions.
If the applicable prospectus supplement indicates, in connection with those derivatives, the third parties may sell securities covered
by this prospectus and the applicable prospectus supplement, including in short sale transactions. If so, the third party may use securities
pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of securities, and may use
securities received from us in settlement of those derivatives to close out any related open borrowings of securities. The third party
in such sale transactions will be an underwriter and will be identified in the applicable prospectus supplement (or a post-effective amendment).
We may also use underwriters or such other third parties with whom we have a material relationship. We will describe the nature of any
such relationship in the applicable prospectus supplement.
In compliance with the guidelines
of the Financial Industry Regulatory Authority, Inc., or FINRA, the maximum compensation to be received by a FINRA member or independent
broker-dealer may not exceed 8% of the offering proceeds. It is anticipated that the maximum compensation to be received in any particular
offering of securities will be less than this amount.
At-the-Market Offerings
Upon written instruction from
us, a sales agent party to a distribution agency agreement with us will use its commercially reasonable efforts to sell on our behalf,
as our agent, the shares of common stock offered as agreed upon by us and the sales agent. We will designate the maximum amount of shares
of common stock to be sold through the sales agent, on a daily basis or otherwise as we and the sales agent agree. Subject to the terms
and conditions of the applicable distribution agency agreement, the sales agent will use its commercially reasonable efforts to sell,
as our sales agent and on our behalf, all of the designated shares of common stock. We may instruct the sales agent not to sell shares
of common stock if the sales cannot be effected at or above the price designated by us in any such instruction. We may suspend the offering
of shares of common stock under any distribution agency agreement by notifying the sales agent. Likewise, the sales agent may suspend
the offering of shares of common stock under the applicable distribution agency agreement by notifying us of such suspension.
We also may sell shares to
the sales agent as principal for its own account at a price agreed upon at the time of sale. If we sell shares to the sales agent as principal,
we will enter into a separate agreement setting forth the terms of such transaction.
The offering of common stock
pursuant to a distribution agency agreement will terminate upon the earlier of (1) the sale of all shares of common stock subject
to the distribution agency agreement or (2) the termination of the distribution agency agreement by us or by the sales agent.
Sales agents under our distribution
agency agreements may make sales in privately negotiated transactions and/or any other method permitted by law, including sales deemed
to be an “at-the-market” offering as defined in Rule 415 promulgated under the Securities Act, sales made directly on
the Nasdaq Capital Market, the existing trading market for our common stock, or sales made to or through a market maker other than on
an exchange. The name of any such underwriter or agent involved in the offer and sale of our common stock, the amounts underwritten, and
the nature of its obligations to take our common stock will be described in the applicable prospectus supplement.
LEGAL MATTERS
The legality and validity of the securities offered
from time to time under this prospectus will be passed upon by Alston & Bird LLP, New York, New York.
EXPERTS
The financial statements incorporated
in this Prospectus by reference to the Annual Report on Form 10-K for the year ended December 31, 2022 have been so incorporated
in reliance on the report of Kesselman & Kesselman, Certified Public Accountants (Isr.), a member firm of PricewaterhouseCoopers
International Limited, an independent registered public accounting firm, given on the authority of such firm as experts in auditing and
accounting.
Shares of Common stock

Prospectus
supplement
Sole Bookrunner
Lucid Capital Markets, LLC
, 2025
Nuvectis Pharma (NASDAQ:NVCT)
Historical Stock Chart
From Jan 2025 to Feb 2025
Nuvectis Pharma (NASDAQ:NVCT)
Historical Stock Chart
From Feb 2024 to Feb 2025